Abstract
The Alpha-Tocopherol, Beta-Carotene Cancer Prevention (ATBC) Study (1985–1993) recruited 29,133 Finnish male cigarette smokers, finding that vitamin E supplementation had no overall effect on mortality. The authors of this paper found that the effect of vitamin E on respiratory infections in ATBC Study participants was modified by age, smoking, and dietary vitamin C intake; therefore, they examined whether the effect of vitamin E supplementation on mortality is modified by the same variables. During a median follow-up time of 6.1 years, 3,571 deaths occurred. Age and dietary vitamin C intake had a second-order interaction with vitamin E supplementation of 50 mg/day. Among participants with a dietary vitamin C intake above the median of 90 mg/day, vitamin E increased mortality among those aged 50–62 years by 19% (95% confidence interval: 5, 35), whereas vitamin E decreased mortality among those aged 66–69 years by 41% (95% CI: −56, −21). Vitamin E had no effect on participants who had a dietary vitamin C intake below the median. Smoking quantity did not modify the effect of vitamin E. This study provides strong evidence that the effect of vitamin E supplementation on mortality varies between different population groups. Further study is needed to confirm this heterogeneity.
Keywords: aging, antioxidants, oxidative stress, population characteristics, randomized controlled trial, smoking, survival rate
Taking vitamin E supplements is a common practice, particularly among older people. In the United States, about a quarter of adults aged 60 years or older take supplements containing vitamin E (1). Such a common habit makes the health effects of this practice an important public health issue: does vitamin E supplementation improve health or not?
The rationale behind taking the lipid-soluble antioxidant vitamin E is to protect against oxidative stress, which contributes to the aging processes and may affect longevity (2, 3). However, 3 meta-analyses of randomized trials found that vitamin E supplementation did not reduce mortality, implying that vitamin E does not lead to universal systemic benefits against the processes that lead to chronic disease (4–6).
Vitamin E is a major lipid-soluble antioxidant, whereas vitamin C is a major water-soluble antioxidant. They interact in vitro and in vivo (7–11). Smoking increases the plasma α-tocopherol disappearance rate, which is normalized by vitamin C supplementation (10). Thus, since smoking seems to modify the interaction of these 2 antioxidants, intake of vitamin C is particularly important when examining the effect of vitamin E on smokers.
The Alpha-Tocopherol, Beta-Carotene Cancer Prevention (ATBC) Study was a randomized, double-blind, placebo-controlled trial that examined the effects of vitamin E and β-carotene on lung cancer in male smokers (12, 13). Previously, we found significant heterogeneity in the effects of vitamin E supplementation in the ATBC Study; vitamin E increased the risk of tuberculosis for heavy smokers with high dietary vitamin C intake but had no effect on other participants (14). The effect of vitamin E on common cold and pneumonia risk was modified by age and smoking (11, 15–19).
In the ATBC Study, vitamin E supplementation had no overall effect on mortality (12). In this study, we tested the hypothesis that the variables that modify the effect of vitamin E supplementation on respiratory infections would also modify the effect of vitamin E on mortality. We did not presume that changes in the incidence of respiratory infections would significantly affect overall mortality, but that changes in respiratory infections may reflect nonspecific systemic effects of vitamin E that might also affect mortality.
MATERIALS AND METHODS
Participants
The design and methods of the ATBC Study examining the effects of vitamin E (dl-α-tocopheryl acetate, 50 mg/day) and β-carotene (20 mg/day) on the incidence of lung cancer and other cancers have been described earlier (12, 13). The ATBC Study is registered at the website www.ClinicalTrials.gov under the identifier NCT00342992.
In brief, male participants aged 50–69 years had to smoke 5 or more cigarettes per day at entry to be eligible, and those enrolled in the trial (N = 29,133) were randomized to 1 of 4 intervention arms and were administered placebo, vitamin E, β-carotene, or vitamin E + β-carotene; a 2 × 2 factorial design was used. Compliance with supplementation was high: some 90% of the participants took more than 90% of their prescribed capsules during their active participation in the trial; there were no differences in capsule consumption among the intervention groups (13). Supplementation increased the serum level of α-tocopherol by 50% compared with baseline levels (12, 13). The intervention continued until April 30, 1993. The trial was approved by the institutional review boards, and all participants gave written informed consent.
Baseline characteristics
Before randomization at baseline, the men completed questionnaires on their medical and smoking histories and general background characteristics, and their weight was measured. A detailed dietary history questionnaire provided data regarding vitamin C, vitamin E, fruit, vegetable, and berry consumption (20, 21). The validity of the dietary history questionnaire was assessed by comparing it with the food consumption records of 190 participants for 12 separate 2-day periods distributed evenly over 6 months. Classified by the dietary history questionnaire, 74%–76% of participants categorized by food consumption records were in the same vitamin C intake quintile or in the within-one-quintile category (20). Dietary data were not available for 2,022 of the 29,133 participants.
Outcome and follow-up time
Deaths were identified by using the National Death Registry, as previously described (12). In the database we analyzed was one death additional to those in the 1994 report.
Follow-up time for each participant began from the day of randomization and continued until death or the end of the trial. The median follow-up time for the participants in the present analysis was 6.1 years, and there was a total of 169,731 person-years of observation.
Statistical models
We estimated the effect of vitamin E supplementation on mortality through proportional hazards regression models. We calculated the risk ratio and the 95% confidence interval of the risk ratio by using the PROC PHREG procedure in SAS software (release 8.2; SAS Institute, Inc., Cary, North Carolina). The 2 × 2 factorial design of the trial permitted assessment of the effect of vitamin E independent of β-carotene after confirming no statistical interaction between the agents. Thus, we compared the trial participants administered vitamin E with those not receiving vitamin E (no-vitamin-E participants). We did not analyze the effects of β-carotene in this study. Regarding supplementation, we carried out the analyses following the intention-to-treat principle. Because deaths were identified in the National Death Registry, which registers all deaths occurring in Finland, loss-to-follow-up is insignificant.
To test the statistical significance of interaction between vitamin E supplementation and potential modifying factors, we first added the supplementation and modifying factor to the regression model. The statistical significance of the interaction was thereafter calculated from the change in −2 × log(likelihood) when the interaction term of vitamin E supplementation and the modifying factor was added to the model.
In our subgroup analyses, we split dietary vitamin E and C levels, and the residual of fruit, vegetable, and berry consumption, at rounded levels close to the medians. Dietary vitamin C was also used as a continuous variable because interaction with a continuous variable refutes the possibility that dichotomizing dietary vitamin C intake might cause a spurious interaction; to decrease the skewness of the distribution, the statistical model included the logarithm of dietary vitamin C intake.
Nelson-Aalen cumulative hazard functions were constructed by using the STATA sts program (release 9.1; Stata Corporation, College Station, Texas). Two-tailed P values were used.
Examination of the specificity of effect modification by vitamin C
The major sources of vitamin C in the diet of study participants were fruit, vegetables, and berries, on average 58% of dietary vitamin C originating from these foods. Total intake of fruit, vegetables, and berries was strongly correlated with the calculated vitamin C intake (r = 0.88). Thus, it is possible that an association with dietary vitamin C is a statistical artifact reflecting other substances in these foods or the lifestyle related to eating these foods. To examine the possible role of dietary compounds other than vitamin C in these foods, we calculated the residual of fruit, vegetables, and berries intake by using linear regression to model fruit, vegetables, and berries as a function of dietary vitamin C, as previously (22). As designed, the residual of fruit, vegetables, and berries intake has no correlation with dietary vitamin C. We assumed that any other putative compound that might interact with vitamin E supplementation has no perfect correlation with vitamin C and, therefore, that variation in the other compound remains as variation in the residual of fruit, vegetables, and berries intake, which was split at 0 g/day, close to the median. High residual of fruit, vegetables, and berries intake (above zero) indicates that the participant with a given vitamin C level consumes more than the average quantity of fruit, vegetables, and berries, whereas low residual of fruit, vegetables, and berries intake (below zero) indicates less-than-average intake of these food classes.
RESULTS
During the 169,731 person-years of follow-up of the ATBC Study participants, 3,571 deaths occurred, equivalent to 21.0 deaths per 1,000 person-years. The deaths were equally divided between the vitamin E and no-vitamin-E groups: 1,801 vs. 1,770, corresponding to a risk ratio of 1.02 (95% confidence interval (CI): 0.95, 1.09).
Dietary vitamin C intake did not modify the effect of vitamin E supplementation. For participants with a vitamin C intake of less than 90 mg/day, the effect of vitamin E on mortality was a risk ratio of 1.00 (95% CI: 0.92, 1.11); for those with a higher vitamin C intake, the effect was a risk ratio of 1.04 (95% CI: 0.94, 1.16). Age did not modify the effect of vitamin E (P = 0.06; interaction between vitamin E and age as a continuous variable).
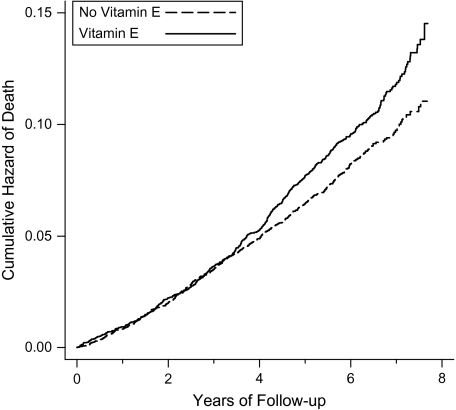
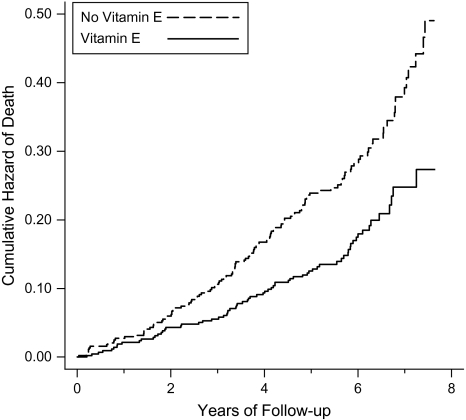
We found that vitamin E supplementation had a second-order interaction with dietary vitamin C and age (Table 1). Vitamin E did not affect mortality for participants with low vitamin C intake. However, for participants with high dietary vitamin C intake, the effect of vitamin E depended on age, so that it increased mortality in the young (aged <63 years) participants by 19% but reduced mortality in the old (aged ≥66 years) participants by 41% (Table 1, Figures 1 and 2).
Table 1.
Effect of Vitamin E Supplementation on Mortality by Age and Dietary Vitamin C Intake, Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study, 1985–1993
| Age at Baseline | Vitamin C |
Test for Vitamin C Interaction (P Value) | |
| <90 mg/day (n = 13,567)a | ≥90 mg/day (n = 13,544)a | ||
| 50–62 years (n = 22,413) | |||
| Risk ratiob | 1.00 | 1.19 | 0.048 |
| 95% confidence interval | 0.90, 1.13 | 1.05, 1.35 | |
| Deathsc | 614/616 | 552/469 | |
| 63–65 years (n = 2,761) | |||
| Risk ratiob | 0.95 | 0.89 | 0.7 |
| 95% confidence interval | 0.75, 1.20 | 0.68, 1.17 | |
| Deathsc | 142/139 | 106/110 | |
| 66–69 years (n = 1,937) | |||
| Risk ratiob | 1.07 | 0.59 | 0.002 |
| 95% confidence interval | 0.84, 1.36 | 0.44, 0.79 | |
| Deathsc | 139/137 | 71/124 | |
| Test for interaction; age as a continuous variable (P value)d | 0.4 | 0.0003 | |
Information on dietary vitamin C intake was missing for 2,022 participants, with 177 deaths of vitamin-E and 175 deaths of no-vitamin-E participants.
Proportional hazards regression model comparing participants who received vitamin E with those who did not.
Number of deaths of vitamin-E participants/number of deaths of no-vitamin-E participants.
The second-order interaction term between vitamin E supplementation, dietary vitamin C, and age improved the regression model by χ2(1 df) = 10.1, P = 0.0015. The uniformity of the vitamin E effect was also tested by adding a dummy variable for vitamin E effect in 5 groups of the table, allowing each of the 6 groups its own vitamin E supplementation effect. The regression model was improved by χ2(5 df) = 22.2, P = 0.0005 compared with the model with a uniform vitamin E effect. Adding the vitamin E effect to only those groups aged 50–62 and 66–69 years with high vitamin C intake led to similar improvement in the regression model, by χ2(2 df) = 21.0 compared with the model with a uniform vitamin E effect.
Figure 1.
Effect of vitamin E supplementation on mortality among participants aged 50–62 years with a dietary vitamin C intake of >90 mg/day (n = 11,448 with 1,021 deaths), Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study, 1985–1993. Nelson-Aalen cumulative hazard functions for the vitamin-E and no-vitamin-E groups are shown. Each step indicates 1 death. For the difference between the 2 groups, log-rank-test P = 0.006. The number of participants with follow-up time of ≥7 years was 2,316; the curves are cut at 7.8 years because the number of participants declined abruptly thereafter. The possibility of a lag period was examined by adding a different risk ratio term for vitamin E effect starting at variable time points. The best improvement in the regression model was achieved by adding the second vitamin E effect starting at 3.3 person-years, which improved the regression model by χ2(1 df) = 7.1, P = 0.007. This model gives risk ratios of 0.99 (95% confidence interval: 0.82, 1.19) during the first 3.3 years and 1.38 (95% confidence interval: 1.17, 1.63) thereafter.
Figure 2.
Effect of vitamin E supplementation on mortality among participants aged 66–69 years with a dietary vitamin C intake of >90 mg/day (n = 872 with 195 deaths), Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study, 1985–1993. Nelson-Aalen cumulative hazard functions for the vitamin-E and no-vitamin-E groups are shown. Each step indicates 1 death. For the difference between the 2 groups, log-rank-test P = 0.0003. The number of participants with follow-up time of ≥7 years was 128; the curves are cut at 7.8 years because the number of participants declined abruptly thereafter. The possibility of a lag period was examined by adding 2 different risk ratio terms for vitamin E effect starting at variable time points because the 2 curves diverge at the initiation of supplementation and at about 2 years. The best improvement in the regression model was achieved by adding the second vitamin E effect starting at 0.3 person-years and the third risk ratio starting from 1.9 years, which improved the regression model by χ2(2 df) = 5.2, P = 0.073. This model gives risk ratios of 0.15 (95% confidence interval: 0.02, 1.2) during the first 0.3 years, 1.04 (95% confidence interval: 0.53, 2.04) during the period 0.3–1.9 years, and 0.54 (95% confidence interval: 0.39, 0.76) thereafter. During the first 0.3 years of follow-up, there were 5 deaths in the β-carotene arm, 2 deaths in the placebo arm, 1 death in the vitamin E arm, and no deaths in the vitamin E + β-carotene arm.
Division of participants into the age categories shown in Table 1 was based on the inspection of data, but the findings were not sensitive to the cutpoints of age. For young participants with high vitamin C intake, the point estimate of the vitamin E effect was very similar in narrower age categories: risk ratio = 1.17 (95% CI: 0.93, 1.48; 297 deaths), risk ratio = 1.25 (95% CI: 1.02, 1.53; 371 deaths), and risk ratio = 1.13 (95% CI: 0.92, 1.40; 353 deaths) in the groups aged 50–54, 55–58, and 59–62 years, respectively, which justified combining these age groups. In participants aged 66–69 years with high dietary vitamin C intake, vitamin E had the same effect in 2-year categories: risk ratio = 0.58 (95% CI: 0.39, 0.86; 113 deaths) and risk ratio = 0.58 (95% CI: 0.37, 0.91; 82 deaths) in the groups aged 66–67 and 68–69 years, respectively.
There seemed to be an approximately 3-year lag period before vitamin E started to increase the mortality of participants aged 50–62 years with high vitamin C intake (Figure 1). During the first 3 years, vitamin E had no effect on mortality, but thereafter it increased mortality by 38%. Inclusion of the lag period in the regression model led to significant improvement in the model. The survival curves for participants aged 66–69 years suggested a 2-year lag period (Figure 2).
The main food sources of vitamin C are fruit, vegetables, and berries. Thus, modification of the vitamin E effect by dietary vitamin C might be explained by high levels of other substances in these foods. Residual fruit, vegetables, and berry intake (refer to the Materials and Methods section) did not modify the effect of vitamin E in participants aged 50–62 years (Table 2), indicating that other substances in these foods do not explain the effect modification by vitamin C. The vitamin E effect was modified by vitamin C as a continuous variable, indicating that the cutpoint for dichotomization was not crucial to the finding. Dietary vitamin E intake and β-carotene supplementation did not modify the effect of vitamin E supplementation. In the participants aged 66–69 years, residual fruit, vegetables, and berry intake; dietary vitamin E intake; and β-carotene supplementation did not modify the effect of vitamin E (Table 3).
Table 2.
Specificity of Vitamin C in Modifying the Effect of Vitamin E Supplementation on the Mortality of Participants Aged 50–62 Years, Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study, 1985–1993
| Subgroup | No. of Participants | Vitamin E |
No Vitamin E |
Risk Ratioa | 95% Confidence Interval | Test of Interaction (P Value) | ||
| No. of Deaths | Rateb | No. of Deaths | Rateb | |||||
| All | 24,000 | 1,292 | 18.4 | 1,202 | 17.0 | 1.08 | 1.00, 1.17 | |
| Dietary vitamin Cc | ||||||||
| <90 mg/day | 10,965 | 614 | 19.2 | 616 | 19.1 | 1.00 | 0.90, 1.13 | 0.048d |
| ≥90 mg/day | 11,448 | 552 | 16.3 | 469 | 13.7 | 1.19 | 1.05, 1.35 | |
| Residual of fruit, vegetables, and berriese | ||||||||
| <0 g/day | 11,839 | 638 | 18.5 | 575 | 16.5 | 1.11 | 1.00, 1.25 | 0.5 |
| ≥0 g/day | 10,574 | 528 | 16.9 | 510 | 16.1 | 1.05 | 0.93, 1.19 | |
| Dietary vitamin Ec | ||||||||
| <10 mg/day | 9,295 | 516 | 19.1 | 499 | 18.2 | 1.05 | 0.92, 1.19 | 0.5 |
| ≥10 mg/day | 13,118 | 650 | 16.8 | 586 | 15.0 | 1.11 | 1.00, 1.25 | |
| β-Carotene | ||||||||
| No | 12,041 | 617 | 17.5 | 567 | 15.9 | 1.10 | 0.98, 1.23 | 0.7 |
| Yes | 11,959 | 675 | 19.3 | 635 | 18.1 | 1.06 | 0.95, 1.19 | |
Proportional hazards regression model comparing participants who received vitamin E with those who did not.
Number of deaths per 1,000 person-years.
Information on dietary vitamins C and E intake was missing for 1,587 participants, with 126 deaths of vitamin-E and 117 deaths of no-vitamin-E participants.
Dietary vitamin C as a continuous variable: test for vitamin E interaction, P = 0.011.
Difference between an individual's intake and the mean intake with a given dietary vitamin C intake; refer to the Materials and Methods section of the text. Information on fruit, vegetables, and berries intake was missing for 1,587 participants, with 126 deaths of vitamin-E and 117 deaths of no-vitamin-E participants.
Table 3.
Specificity of Vitamin C in Modifying the Effect of Vitamin E Supplementation on the Mortality of Participants Aged 66–69 Years, Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study, 1985–1993
| Subgroup | No. of Participants | Vitamin E |
No Vitamin E |
Risk Ratioa | 95% Confidence Interval | Test of Interaction (P Value) | ||
| No. of Deaths | Rateb | No. of Deaths | Rateb | |||||
| All | 2,140 | 237 | 41.6 | 291 | 48.6 | 0.87 | 0.73, 1.03 | |
| Dietary vitamin Cc | ||||||||
| <90 mg/day | 1,065 | 139 | 49.6 | 137 | 46.4 | 1.07 | 0.84, 1.36 | 0.002d |
| ≥90 mg/day | 872 | 71 | 29.6 | 124 | 50.8 | 0.59 | 0.44, 0.79 | |
| Residual of fruit, vegetables, and berriese | ||||||||
| <0 g/day | 992 | 111 | 41.3 | 127 | 47.9 | 0.88 | 0.68, 1.13 | 0.7 |
| ≥0 g/day | 945 | 99 | 39.3 | 134 | 48.9 | 0.81 | 0.62, 1.05 | |
| Dietary vitamin Ec | ||||||||
| <10 mg/day | 1,057 | 123 | 43.8 | 144 | 49.7 | 0.89 | 0.69, 1.13 | 0.5 |
| ≥10 mg/day | 880 | 87 | 36.4 | 117 | 46.9 | 0.78 | 0.59, 1.03 | |
| β-Carotene | ||||||||
| No | 1,070 | 122 | 41.5 | 143 | 49.6 | 0.85 | 0.66, 1.08 | 0.8 |
| Yes | 1,070 | 115 | 41.7 | 148 | 47.6 | 0.89 | 0.69, 1.13 | |
Proportional hazards regression model comparing participants who received vitamin E with those who did not.
Number of deaths per 1,000 person-years.
Information on dietary vitamins C and E intake was missing for 203 participants, with 27 deaths of vitamin-E and 30 deaths of no-vitamin-E participants.
Dietary vitamin C as a continuous variable: test for vitamin E interaction, P = 0.002.
Difference between an individual's intake and the mean intake with a given dietary vitamin C intake; refer to the Materials and Methods section of the text. Information on fruit, vegetables, and berries intake was missing for 203 participants, with 27 deaths of vitamin-E and 30 deaths of no-vitamin-E participants.
Smoking had a marginally significant modification effect on the participants aged 66–69 years with high vitamin C intake (P = 0.051). The decrease in mortality with vitamin E supplementation was more evident for those who smoked less than a pack of cigarettes per day (risk ratio (RR) = 0.44, 95% CI: 0.28, 0.68; 89 deaths) compared with a pack or more (RR = 0.78, 95% CI: 0.53, 1.15; 106 deaths). For the participants aged 50–62 years with high vitamin C intake, the effect was more apparent for those who smoked a pack of cigarettes or more per day (RR = 1.22, 95% CI: 1.05, 1.42; 689 deaths) than for those who smoked less (RR = 1.12, 95% CI: 0.90, 1.39; 332 deaths), but the confidence intervals overlapped broadly and the difference was not statistically significant (P = 0.5 in heterogeneity test).
Of the participants aged 66–69 years with high vitamin C intake, 16.7% of the vitamin-E participants died during follow-up, whereas 27.7% of the no-vitamin-E participants died. Thus, based on the baseline cohort, the number needed to treat to prevent one death during follow-up was 9.1 in this subgroup. On the other hand, the cumulative hazard estimates over the 8-year follow-up (Figure 2) indicate mortality of 28% in the vitamin-E group and 49% in the no-vitamin-E group, corresponding to the number needed to treat of 4.8 over an 8-year supplementation period.
DISCUSSION
Vitamin E supplementation had no overall effect on mortality among the ATBC Study participants, yet we found strong evidence of heterogeneity between subgroups. Vitamin E increased, decreased, or had no effect on mortality depending on age and vitamin C intake. The numerical estimates calculated for the subgroups are less essential than the strong evidence of heterogeneity. When the effect of vitamin E depends on the characteristics of people, it seems obvious that the estimates of intervention effect obtained from one study cannot be confidently generalized to other population groups.
Although we divided the participants into several subgroups, the multiple comparison problem did not seem to be a concern in our study. First, the subgroup heterogeneity in the 6 groups defined by age and dietary vitamin C intake was significant even when we allowed each of the 6 subgroups to have its own vitamin E effect (Table 1). Second, our current subgroup analyses tested our earlier subgroup findings for respiratory infection outcomes in the ATBC Study (11, 14–19).
Our findings have several important implications. Subgroup analysis has been discouraged because it can lead to false-positive findings due to the multiple comparison problem (23–25). It has even been argued that “believing that a treatment effect exists in one stratum of patients, even though no overall significant treatment effect exists, is a common error” (24, p. 15). Furthermore, large trials are set up after years of deliberation by experts, and it is assumed that all relevant knowledge is therefore incorporated into the study plans (25). However, biology is complex, and it seems unlikely that all important biologic knowledge could ever be taken into account properly when setting up a pragmatic controlled trial. A single estimate of treatment effect calculated for a large population can be misleading if it is thought to be valid for all participants, as shown in our work. The overall estimate for all ATBC Study participants, risk ratio = 1.02, is inconsistent with our subgroup findings for nearly half of all participants, that is, the young and old with high dietary vitamin C intake.
Our current study and earlier subgroup analyses of the ATBC Study suggest that subgroup analyses of large trial databases should be encouraged even if there is no overall effect in the study population, keeping in mind the possibility of spurious findings from multiple testing. Given the long-term commitment of participants and the resources invested, it might even be considered a moral imperative to analyze the large trial databases as extensively as possible rather than simply to calculate an overall effect. Evidently, subgroup findings should be considered cautiously, and the interpretation of P values must be related to the number of subgroup analyses being carried out. Nevertheless, our subgroup findings in the ATBC Study point out the need for further research on vitamin E, whereas the overall estimate of effect suggests that no further studies would have been worthwhile. We concur with Feinstein's concern (26) that anti-subgroup doctrines have become so entrenched that they often hamper investigation of socially and biologically sound subgroups with public health relevance.
Three meta-analyses of controlled trials found no effect of vitamin E supplementation on mortality (4–6). Combining several large trials leads to estimates with narrow confidence intervals; however, pooling is based on the assumption of the same universal effect in all populations of the combined studies. Our strong evidence of heterogeneity in the ATBC Study refutes the assumption of a uniform effect and casts doubt on the validity of the pooled estimates of the meta-analyses. Although the pooled estimates reject the proposal that vitamin E supplementation would be beneficial for wide-ranging population groups, our study suggests that important subgroups being harmed or benefiting from vitamin E may be hidden in the misleadingly narrow confidence intervals.
The recently published Physicians’ Health Study II found no overall effect of vitamin E and C supplementation on mortality (27). However, the lack of average effect does not conflict with our findings of heterogeneity because vitamin E had no overall effect in the ATBC Study either. The number of deaths in the ATBC Study (n = 3,571) was twice that in the Physicians’ Health Study II (n = 1,661); consequently, there is more statistical power to analyze potential subgroup differences in the ATBC Study. Furthermore, ATBC Study participants were recruited from the general population, so there is greater potential for analyzing heterogeneity compared with the Physicians’ Health Study II focused on a single upper-class profession.
Our strongest findings concern vitamin E supplementation because the division of participants into vitamin-E and no-vitamin-E groups was random. The evidence of heterogeneity of the vitamin E effect is strong irrespective of the specificity of vitamin C as the modifier. Nevertheless, by using the residual of fruit, vegetables, and berries, which has no correlation with dietary vitamin C, we were able to show that modification of the vitamin E effect is not explained by other substances in these foods, suggesting that vitamin C is the specific substance explaining the modification. Furthermore, the subgroup divisions in our current study were based on the effects of vitamin E on respiratory infections, and vitamin C has also affected respiratory infections in certain controlled trials, showing that its physiologic effects are not limited to preventing scurvy (28, 29). Finally, the interaction between vitamins E and C is well established (7–11). These arguments support the possibility that vitamin C specifically causes the modification of the vitamin E effect, although our vitamin C intake levels were based on observational data.
All ATBC Study participants were smokers, and no direct conclusions can be drawn for nonsmokers. The benefit of vitamin E supplementation on respiratory infections was previously more evident in ATBC Study participants who smoked less, and the harm was more apparent in those who smoked more (14, 15, 18, 19). We saw similar trends in the effect of vitamin E on mortality, but the modification of the vitamin E effect caused by smoking quantity was not statistically significant. Nevertheless, the greater reduction in mortality among old participants with high vitamin C intake who smoked less suggests that vitamin E supplementation might affect mortality among older male nonsmokers as well.
Since vitamin E is a fat-soluble substance and it may take months before tissue levels are substantially elevated (30, 31), short trials may be uninformative. In the current study, there seemed to be 2- and 3-year lag periods before vitamin E started to decrease or increase mortality (Figures 1 and 2). These lag periods are consistent with a delay in the effects of the fat-soluble vitamin. Nevertheless, the maximum of 8 years of follow-up in the ATBC Study was long enough to observe that vitamin E supplementation does have effects. On the other hand, the risk of tuberculosis increased significantly within a year of supplementation being initiated (14) and the risk of pneumonia in participants who exercised in their leisure time decreased without a lag (16), so not all effects of vitamin E are delayed.
It has been suggested that the vitamin E doses in the randomized trials were too low to show any effect (32), and high doses in some trials increased mortality (5). Given our observations that 50 mg/day of vitamin E caused significant increase and decrease in mortality in the ATBC Study population, depending on the characteristics of participants, no justification exists for claiming that the vitamin E doses in randomized trials have been too low. Our study also suggests that it may be primarily subject characteristics and not dose of vitamin E that determines whether vitamin E causes harm.
Many people take vitamin E supplements because they believe that the vitamin protects them against oxidative stress, which has a role in the aging processes (2, 3). Our findings among the older ATBC Study participants are consistent with the concept that the antioxidant vitamins C and E can counteract oxidative stress processes in old people under some conditions. Assuming that the benefit of vitamin E supplementation in old people is conditional on a high intake of vitamin C, it seems possible that old people with low dietary vitamin C might benefit from a combination of the 2. Therefore, the most informative type of further study would be a factorial design with vitamins E and C on old people with low dietary vitamin C intake.
Finally, the US nutritional recommendations and a recent review consider that vitamin E is safe in doses up to 1,000 mg/day (33, 34). Our finding that 50 mg/day of vitamin E significantly increased mortality among half of the male smokers in the ATBC Study aged 50–62 years contradicts the universal safety of 1,000 mg/day of vitamin E. Previously, we found that 50 mg/day of vitamin E significantly increased the risk of the common cold, pneumonia, and tuberculosis in subgroups of the ATBC Study, also indicating that such a low dose can cause harmful effects in some people (14, 18, 19).
In conclusion, our subgroup analyses of the ATBC Study cohort support the conclusions of meta-analysis of controlled trials (4–6), in that vitamin E supplementation seems ineffective or harmful for middle-aged male smokers. Evidently, in people younger than age 65 years, taking vitamin E supplements should be strongly discouraged until clear evidence emerges that some population groups of younger or middle-aged people benefit. On the other hand, our study indicates that vitamin E supplementation may lead to beneficial effects in some subgroups of old people, and this possibility should be investigated by using a factorial design with vitamin C supplementation. Finally, the substantial decrease in mortality with vitamin E supplementation among the older participants with high dietary vitamin C intake raises the question of whether the decrease in overall mortality is attributable to a single cause of death or a few causes, or whether it suggests a general decrease in frailty reflected in lessened mortality from diverse causes.
Acknowledgments
Authors affiliation: Department of Public Health, University of Helsinki, Helsinki, Finland (Harri Hemilä, Jaakko Kaprio).
The authors thank the ATBC Study (the National Public Health Institute, Finland, and the National Cancer Institute, United States) for access to the data.
H. H. planned the study and wrote the draft of the manuscript. J. K. participated in planning the analyses and the critical revision of the manuscript. H. H. had full access to all data in this study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict of interest: none declared.
Glossary
Abbreviations
- ATBC
Alpha-Tocopherol, Beta-Carotene Cancer Prevention
- CI
confidence interval
- RR
risk ratio
References
- 1.Radimer K, Bindewald B, Hughes J, et al. Dietary supplement use by US adults: data from the National Health and Nutrition Examination Survey, 1999–2000. Am J Epidemiol. 2004;160(4):339–349. doi: 10.1093/aje/kwh207. [DOI] [PubMed] [Google Scholar]
- 2.Beckman KB, Ames BN. The free radical theory of aging matures. Physiol Rev. 1998;78(2):547–581. doi: 10.1152/physrev.1998.78.2.547. [DOI] [PubMed] [Google Scholar]
- 3.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408(6809):239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 4.Vivekananthan DP, Penn MS, Sapp SK, et al. Use of antioxidant vitamins for the prevention of cardiovascular disease: meta-analysis of randomised trials. Lancet. 2003;361(9374):2017–2023. doi: 10.1016/S0140-6736(03)13637-9. [DOI] [PubMed] [Google Scholar]
- 5.Miller ER, Pastor-Barriuso R, Dalal D, et al. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005;142(1):37–46. doi: 10.7326/0003-4819-142-1-200501040-00110. (Comments: Ann Intern Med. 2005;143(2):150–158) [DOI] [PubMed] [Google Scholar]
- 6.Bjelakovic G, Nikolova D, Gluud LL, et al. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA. 2007;297(8):842–857. doi: 10.1001/jama.297.8.842. (Comments: JAMA. 2007;298(4):400–403) [DOI] [PubMed] [Google Scholar]
- 7.Packer JE, Slater TF, Willson RL. Direct observation of a free radical interaction between vitamin E and vitamin C. Nature. 1979;278(5706):737–738. doi: 10.1038/278737a0. [DOI] [PubMed] [Google Scholar]
- 8.Tanaka K, Hashimoto T, Tokumaru S, et al. Interactions between vitamin C and vitamin E are observed in tissues of inherently scorbutic rats. J Nutr. 1997;127(10):2060–2064. doi: 10.1093/jn/127.10.2060. [DOI] [PubMed] [Google Scholar]
- 9.Hill KE, Montine TJ, Motley AK, et al. Combined deficiency of vitamins E and C causes paralysis and death in guinea pigs. Am J Clin Nutr. 2003;77(6):1484–1488. doi: 10.1093/ajcn/77.6.1484. [DOI] [PubMed] [Google Scholar]
- 10.Bruno RS, Leonard SW, Atkinson J, et al. Faster plasma vitamin E disappearance in smokers is normalized by vitamin C supplementation. Free Radic Biol Med. 2006;40(4):689–697. doi: 10.1016/j.freeradbiomed.2005.10.051. (Comments: Free Radic Biol Med. 2007;42(4):578–580) [DOI] [PubMed] [Google Scholar]
- 11.Hemilä H. Do Vitamins C and E Affect Respiratory Infections? [dissertation] Helsinki, Finland: University of Helsinki; 2006. 10–11, 56–57. (Available at http://ethesis.helsinki.fi/julkaisut/laa/kansa/vk/hemila/). (Accessed December 15, 2008) [Google Scholar]
- 12.The effect of vitamin E and beta-carotene on the incidence of lung cancer and other cancers in male smokers. The Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study Group. N Engl J Med. 1994;330(15):1029–1035. doi: 10.1056/NEJM199404143301501. [DOI] [PubMed] [Google Scholar]
- 13.The alpha-tocopherol, beta-carotene lung cancer prevention study: design, methods, participant characteristics, and compliance. The ATBC Cancer Prevention Study Group. Ann Epidemiol. 1994;4(1):1–10. doi: 10.1016/1047-2797(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 14.Hemilä H, Kaprio J. Vitamin E supplementation may transiently increase tuberculosis risk in males who smoke heavily and have high dietary vitamin C intake. Br J Nutr. 2008;100(4):896–902. doi: 10.1017/S0007114508923709. (Comments: Br J Nutr. 2009;101(1):145–147) [DOI] [PubMed] [Google Scholar]
- 15.Hemilä H, Virtamo J, Albanes D, et al. Vitamin E and beta-carotene supplementation and hospital-treated pneumonia incidence in male smokers. Chest. 2004;125(2):557–565. doi: 10.1378/chest.125.2.557. [DOI] [PubMed] [Google Scholar]
- 16.Hemilä H, Kaprio J, Albanes D, et al. Physical activity and the risk of pneumonia in male smokers administered vitamin E and β-carotene. Int J Sports Med. 2006;27(4):336–341. doi: 10.1055/s-2005-865670. [DOI] [PubMed] [Google Scholar]
- 17.Hemilä H, Kaprio J, Albanes D, et al. Vitamin C, vitamin E, and beta-carotene in relation to common cold incidence in male smokers. Epidemiology. 2002;13(1):32–37. doi: 10.1097/00001648-200201000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Hemilä H, Virtamo J, Albanes D, et al. The effect of vitamin E on common cold incidence is modified by age, smoking and residential neighborhood. J Am Coll Nutr. 2006;25(4):332–339. doi: 10.1080/07315724.2006.10719543. [DOI] [PubMed] [Google Scholar]
- 19.Hemilä H, Kaprio J. Vitamin E supplementation and pneumonia risk in males who initiated smoking at an early age: effect modification by body weight and vitamin C [electronic article] Nutr J. 2008;7(1):33. doi: 10.1186/1475-2891-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pietinen P, Hartman AM, Haapa E, et al. Reproducibility and validity of dietary assessment instruments. I. A self-administered food use questionnaire with a portion size picture booklet. Am J Epidemiol. 1988;128(3):655–666. doi: 10.1093/oxfordjournals.aje.a115013. [DOI] [PubMed] [Google Scholar]
- 21.Ovaskainen ML, Valsta M, Lauronen J. The compilation of food analysis values as a database for dietary studies—the Finnish experience. Food Chem. 1996;57(1):133–136. [Google Scholar]
- 22.Hemilä H, Kaprio J, Pietinen P, et al. Vitamin C and other compounds in vitamin C rich food in relation to risk of tuberculosis in male smokers. Am J Epidemiol. 1999;150(6):632–641. doi: 10.1093/oxfordjournals.aje.a010062. [DOI] [PubMed] [Google Scholar]
- 23.Assmann SF, Pocock SJ, Enos LE, et al. Subgroup analysis and other (mis)uses of baseline data in clinical trials. Lancet. 2000;355(9209):1064–1069. doi: 10.1016/S0140-6736(00)02039-0. [DOI] [PubMed] [Google Scholar]
- 24.Peto R, Pike MC, Armitage P, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. Analysis and examples. Br J Cancer. 1977;35(1):1–39. doi: 10.1038/bjc.1977.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vandenbroucke JP. Observational research, randomised trials, and two views of medical science [electronic article] PLoS Med. 2008;5(3):e67. doi: 10.1371/journal.pmed.0050067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feinstein AR. The problem of cogent subgroups: a clinicostatistical tragedy. J Clin Epidemiol. 1998;51(4):297–299. doi: 10.1016/s0895-4356(98)00004-3. [DOI] [PubMed] [Google Scholar]
- 27.Sesso HD, Buring JE, Christen WG, et al. Vitamins E and C in the prevention of cardiovascular disease in men: the Physicians’ Health Study II randomized controlled trial. JAMA. 2008;300(18):2123–2133. doi: 10.1001/jama.2008.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Douglas RM, Hemilä H. Vitamin C for preventing and treating the common cold [electronic article] PLoS Med. 2005;2(6):e168. doi: 10.1371/journal.pmed.0020168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hemilä H, Louhiala P. Vitamin C may affect lung infections. J R Soc Med. 2007;100(11):495–498. doi: 10.1258/jrsm.100.11.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kitagawa M, Mino M. Effects of elevated d-alpha(RRR)-tocopherol dosage in man. J Nutr Sci Vitaminol (Tokyo) 1989;35(2):133–142. doi: 10.3177/jnsv.35.133. [DOI] [PubMed] [Google Scholar]
- 31.Handelman GJ, Epstein WL, Peerson J, et al. Human adipose alpha-tocopherol and gamma-tocopherol kinetics during and after 1 y of alpha-tocopherol supplementation. Am J Clin Nutr. 1994;59(5):1025–1032. doi: 10.1093/ajcn/59.5.1025. [DOI] [PubMed] [Google Scholar]
- 32.Blumberg JB, Frei B. Why clinical trials of vitamin E and cardiovascular diseases may be fatally flawed. Commentary on “The relationship between dose of vitamin E and suppression of oxidative stress in humans”. Free Radic Biol Med. 2007;43(10):1374–1376. doi: 10.1016/j.freeradbiomed.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 33.Food and Nutrition Board, Institute of Medicine. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Washington, DC: The National Academies Press; 2000. pp. 249–260. (Available at http://www.nap.edu/books/0309069351/html/249.html). (Accessed December 15, 2008) [PubMed] [Google Scholar]
- 34.Hathcock JN, Azzi A, Blumberg J, et al. Vitamins E and C are safe across a broad range of intakes. Am J Clin Nutr. 2005;81(4):736–745. doi: 10.1093/ajcn/81.4.736. (Comments: Am J Clin Nutr. 2005;82(5):1141–1143) [DOI] [PubMed] [Google Scholar]