Abstract
The role of growth hormone (GH) in amphibian metamorphosis is ambiguous based on experiments in which mammalian GH was administered to tadpoles and frogs. We have reexamined the effects of GH by producing transgenic Xenopus laevis that overexpress the cDNA encoding X. laevis GH. These transgenic tadpoles take the same length of time to reach metamorphosis as control tadpoles, but the transgenic tadpoles are twice as large. After metamorphosis, the transgenic frogs grow at a greatly accelerated rate and develop skeletal abnormalities reminiscent of acromegaly. The transgenic frogs are larger than mature frogs in a few months and die in about 1 year. At as early as 10 months of age, the males have mature sperm. We conclude that the growth-promoting effects of GH in this amphibian closely resemble those described for mammals. Although excess GH increases the size of the tadpole, it does not alter the developmental programs involved in metamorphosis.
The function of growth hormone (GH) in vertebrates is mainly associated with the stimulation of body growth and metabolism (1). However, its role in amphibian metamorphosis has remained ambiguous. Amphibian pituitary glands were reported to contain a growth-promoting hormone or hormones, because hypophysectomy dramatically slows the growth rate and because implanting the pituitary gland at an ectopic site can restore the growth rate (2). The determination of the identity of this growth-promoting hormone was pursued by injecting mammalian hormones into intact or hypophysectomized tadpoles and frogs. The effect of mammalian GH in amphibians is controversial. Mammalian prolactin (PRL) was reported to have a greater growth-promoting effect than mammalian GH in tadpoles, whereas mammalian GH was said to be more potent in juvenile frogs (3–5). In some experiments, the effect of GH was attributed to contamination of the GH preparation with PRL (3). This attribution led to the suggestion that PRL is the growth-promoting hormone in tadpoles, whereas GH accounts for growth in juvenile frogs of the same species (2). However, these interpretations were confounded by the questionable purity of mammalian hormone preparations and their unknown specificity for the amphibian hormone receptors.
In this study, we cloned full-length Xenopus laevis GH (xGH) and xGH receptor (xGHR) cDNAs and tested the specificity of X. laevis and mammalian hormones for xGHR. We show that overexpression of xGH, but not X. laevis PRL (xPRL), has dramatic growth-promoting effects in tadpoles and frogs. The reported growth-promoting effect of mammalian PRL may be explained by the hormone's cross-reaction with the xGHR.
Materials and Methods
Cloning of xGH cDNA.
The 5′ region of xGH was obtained by rapid amplification of cDNA ends (6). Total RNA purified from frog pituitaries by Trizol Reagent (Life Technologies, Grand Island, NY) was used for the synthesis of first-strand cDNA. Anchor primer is a 10:1 mixture of 5′-GTCGACATCGATCTCGAG-3′ and 5′-GTCGACATCGATCTCGAG(T)18-3′. For xGH-A, gene-specific primer 1 (GSP1) is 5′-GCGCTCTAGATTAAATGGTGCAGTTGCTTTC-3′. GSP2 is 5′-TGACTTTTAGGTAGGTCTCC-3′. GSP3 is 5′-GCATTCTAGATTCTTGAAGCAGGACAGAAGGC-3′. For xGH-B, GSP1 is 5′-TGGTAGGTCAGGGGATATGG-3′, and GSP2 is 5′-GCAAACTTGTGTTTACCAGC-3′. The PCR product was cloned into pCR2.1 by TA cloning (Invitrogen) or pBluescript (Stratagene) with restriction sites (XhoI and XbaI) within the primers. After obtaining the sequence of the 5′ region of xGH by rapid amplification of cDNA ends, primers were designed to amplify the coding region of xGH-A by reverse transcription–PCR from total pituitary gland RNA. The primers were 5′-CCATGGATCCCACATGGCTACAGGGTTCTGC-3′ and 5′-GCGCTCTAGATTAAATGGTGCAGTTGCTTTC-3′. The amplified fragment was cloned into the BamHI and XbaI sites of the pCS2+ vector (7). The plasmid pCEP-xGH was made by amplifying the coding region of xGH with primers 5′-CCATGCTAGCACAGCCACCATGGCTACAGGGTTCTGCTC-3′ and 5′-CGTGCTCGAGTTAAATGGTGCAGTTGCTTTC-3′ and cloning the amplified fragment into the NheI and XhoI sites of pCEP4 (Invitrogen). The plasmid pREP-xPRL was made by amplifying the coding region of xPRL (8) with primers 5′-CGATGCTAGCATAATGATTGATCCGATGGAC-3′ and 5′-GAGAGGATCCTAACAGTTGCTGTCATG-3′ and cloning the amplified fragment into the NheI and BamHI sites of pREP4 (Invitrogen).
Cloning of xGHR.
Degenerate primers corresponding to well conserved regions of GHR between mammalian and avian species were used to amplify tadpole tail cDNA that had been reverse transcribed from RNA. The primers were 5′-CCWCCWGTNCCWGTNCCWAARATHAARGG-3′ (W = A + T; N = A + T + C + G; R = A + G; H = A + T + C), which corresponds to the conserved peptide PPVPVPKIKG, and 5′-TCDATRTCCAGYTCDATRAAYTCNACCCA-3′ (D = G + A + T; R = A + G; Y = C + T; N = A + G + T + C), which corresponds to the conserved peptide WVEFIELDID. The PCR condition was 3 cycles of 94°C for 30s, 55°C for 1 min, and 72°C for 3 min, followed by 30 cycles of 94°C for 30 s, 60°C for 1 min, and 72°C for 2 min. The hot-start PCR technique was applied by using the AmpliWax PCR Gem 100 wax bead (Perkin–Elmer). The amplified fragment was cloned, sequenced, and used to screen a tail cDNA phage library (9). The coding region of xGHR was amplified by PCR with the full-length cDNA as template with primers 5′-GCGCAGATCTACAGCCACCATGGCTTACTGGCTATTCTGG-3′ and 5′-CGGCCTCGAGCTACTTCATGACTTTGTTTAC-3′ and cloned into the BglII and XhoI sites of pCSGFP3 (a gift of Enrique Amaya, Wellcome/CRC Institute, Cambridge, U.K.) replacing green fluorescent protein.
Northern Blotting.
Total RNA was purified from whole tadpoles of different stages by using Trizol Reagent (Life Technologies). mRNA was then selected from total RNA by using oligo(dT) cellulose (Amersham Pharmacia) following a standard procedure (9). mRNAs from each tadpole stage (20 μg) were fractionated by electrophoresis through a 1% formaldehyde agarose gel and transferred to a Nytran Plus membrane (Schleicher & Schuell). RNA was cross-linked to the membrane by UV irradiation. 32P-labeled DNA probes were prepared by hexamer oligonucleotide random priming. Blot hybridization was carried out as described (9). Blots were reused after stripping the probe by boiling for 5 min in an excess of buffer (10 mM Tris, pH 7.5/1 mM EDTA/1% SDS).
Cell Culture and Transient Transfection.
X. laevis XLA cultured cells were grown at 25°C in 70% (vol/vol) L-15 medium supplemented with 10% (vol/vol) FBS. Subconfluent cells were cotransfected with a β-casein/luciferase reporter and with constructs expressing STAT5A1, β-galactosidase, and either xGHR or xPRL receptor (xPRLR). Hormones were added to the culture medium 24 h after transfection, and the cells were cultured for another 36 h. Luciferase activity was assayed in cell extracts and normalized to β-galactosidase activity. The control response (Fig. 1, bar 1) had all components except the hormone. Because purified xGH and xPRL were not available, they were prepared by transfecting human embryonic kidney fibroblast 293 cells with DNA constructs expressing xGH or xPRL. The hormones were secreted into the culture medium, and their concentrations were determined by Western blotting by using xGH or xPRL standards prepared from Escherichia coli overexpressing xGH or xPRL. This conditioned medium was used in the transient transfection assay.
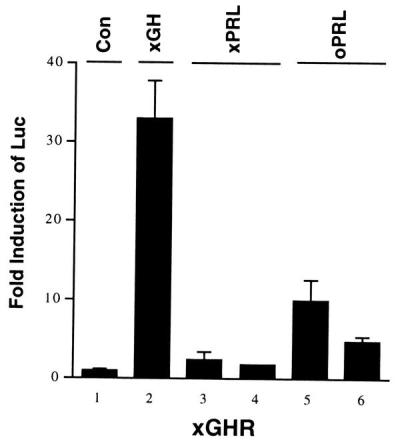
Figure 1.
Specificity of different hormones for xGHR as determined by a transient transfection assay (see Materials and Methods). Luciferase reporter activity was assayed and normalized to β-galactosidase activity. Each column represents the mean of triplicate samples, and the error bars represent standard deviation. The hormone concentrations used are no hormone/control (Con; bar 1); 50 ng/ml (bar 2); 500 ng/ml (bar 3); 250 ng/ml (bar 4); 500 ng/ml (bar 5); and 250 ng/ml (bar 6). oPRL, ovine PRL.
Human embryonic kidney fibroblast 293 cells were grown in DMEM supplemented with 10% (vol/vol) FBS. Subconfluent cells were transfected with pCEP-xGH or pREP-xPRL by using Lipofectamine Reagent (Life Technologies), and conditioned medium was collected 48 h after transfection. For stable transfection, cells were selected for 2 weeks in DMEM/10% (vol/vol) FBS with 100 μg/ml hygromycin and 200 μg/ml geneticin. After the selection, cells were grown in DMEM/10% (vol/vol) FBS or cultured in DMEM for a few days when they reached high density and conditioned medium was collected and assayed.
Transgenesis.
Transgenesis procedures, the identification of transgenic animals, and tadpole-raising conditions have been described (10, 11). X. laevis tadpoles were staged according to the criteria of Nieuwkoop and Faber (12). Stage 59 is the onset of metamorphic climax.
X-Ray Radiography.
The x-ray examinations of frogs were carried out by using the MX-20 Specimen Radiography System (Faxitron X-Ray, Wheeling, IL).
Bone and Cartilage Staining.
The tadpoles were fixed and stained as described (13).
Results
Cloning of xGH and xGHR cDNAs.
To study the effect of the homologous hormone, we cloned the full-length xGH cDNA by rapid amplification of cDNA ends by using the partial cDNA sequence available (14). Like many genes in X. laevis, there are two genomic copies of the GH gene (xGH-A and xGH-B). xGH-A and B encode precursor proteins of 214 and 208 amino acids, respectively. They are 77% identical to each other and 60–70% identical to GH of other species. xGH-B has a six internal amino acid deletions when compared with xGH-A. We used xGH-A for subsequent experiments.
To test the relative specificity of different hormones, we cloned full-length xGHR cDNA. A DNA fragment was amplified from tadpole tail cDNA by PCR with degenerate primers corresponding to well conserved regions of GHR between mammalian and avian species and was cloned and sequenced. This fragment was used to screen a X. laevis tail cDNA phage library. A 2.5-kilobase full-length cDNA clone was identified that encodes 603 amino acid residues and has overall identity of 47–51% with mammalian GHRs. xGHR shares the highly conserved region that includes positionally conserved cysteine residues and the proline-rich box 1 region (15). xGH mRNA is detectable in the pituitary gland throughout tadpole development with peak synthesis at the onset of metamorphic climax. It rises again after metamorphosis (8). xGHR mRNA is expressed uniformly at all stages of tadpole development. There is no significant difference of expression at different stages in the whole tadpole (data not shown).
Specificity of Different Hormones.
The specificity of xGH, xPRL, and oPRL to activate gene expression by way of xGHR was tested by a transient transfection assay (16). X. laevis XLA cultured cells were cotransfected with a β-casein/luciferase reporter and constructs expressing STAT5A1, β-galactosidase, and xGHR. After adding GH or PRL to the culture medium, the luciferase activity was assayed. Because the β-casein/luciferase reporter gave a better induction by xGH than did the typical GH responsive serine protease inhibitor/luciferase reporter (17) in XLA cells (data not shown), β-casein/luciferase reporter was used. Purified xGH and xPRL were not available; thus, they were prepared by transfecting human embryonic kidney fibroblast 293 cells with DNA constructs expressing xGH or xPRL, resulting in the hormone being secreted into the culture medium. Their concentrations were determined by Western blotting by using a xGH or xPRL standard prepared from E. coli overexpressing xGH or xPRL (data not shown). This conditioned medium was used in the assay described above. Induction of the reporter activity over the control (no hormone added) depended on transfected xGHR cDNA. As shown in Fig. 1, xGH binds and activates xGHR at a concentration of 50 ng/ml. xPRL does not activate xGHR significantly even at a concentration of 500 ng/ml. Interestingly, oPRL, the hormone that has been administered most frequently to tadpoles in earlier studies, cross-reacts with xGHR significantly at a concentration of 250 ng/ml or higher.
Overexpression of xGH.
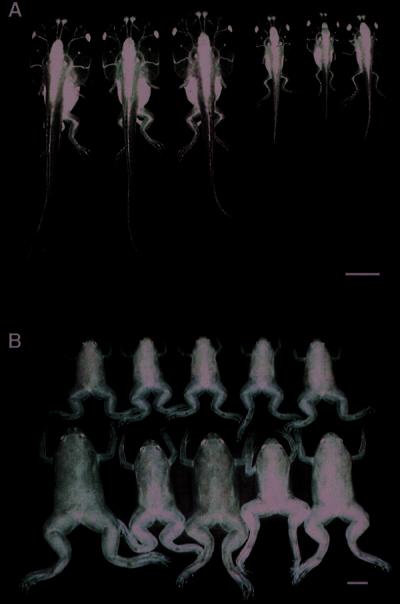
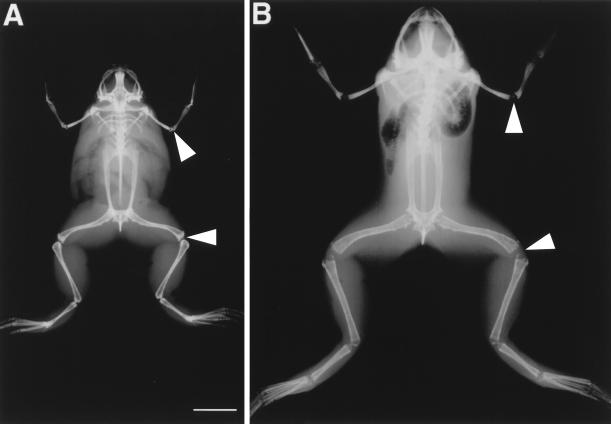
We produced transgenic X. laevis overexpressing xGH driven by a cytomegalovirus promoter. Transgenic tadpoles grew much larger than their nontransgenic siblings (Fig. 2A). After metamorphosis, the frogs were raised to adulthood. These frogs had voracious appetites and grew much faster than the control frogs. They developed skeletal defects such as disproportionally large heads and feet and deformed vertebral columns (Figs. 3 and 4). The width/thickness of the epiphyseal cartilage at the ends of long bones is another growth-related parameter. Growth ceases when the epiphyses ossify as revealed by x-ray radiography. To date, F0 frogs have died at about 1 year of age and have been examined by x-ray. All these transgenic frogs were larger than the largest control frogs, and the transgenic frogs had open epiphyses (Fig. 4). The males had mature sperm, and a single female had immature ovaries. Sperm from a 1-year-old transgenic F0 male frog was used to fertilize eggs of wild-type females, and the effect of xGH on metamorphosis was analyzed in F1 tadpoles. As early as 2 weeks after fertilization (about stage 48), transgenic tadpoles were larger than nontransgenics (see figure 4 of ref. 18). The size difference continued to increase to metamorphic climax (Fig. 2A). The heads of tadpoles overexpressing xGH have a characteristic square shape mainly because of the increased number and size of gill arches revealed by a bone-cartilage staining (Fig. 5), but the entire tadpole is larger in weight and size. There was no significant difference in the time that it took transgenics and nontransgenics to reach stage 59, the climax of metamorphosis (Fig. 6). At that stage, transgenic tadpoles weighed about twice as much as nontransgenics (Table 1). After stage 59, each tadpole was put into a separate container, and the time to complete metamorphosis was recorded. Transgenic tadpoles took 13 days, on average, to complete metamorphosis compared with 9 days for nontransgenic siblings. After metamorphosis, the body weight of transgenic and nontransgenic froglets was monitored every week for a total of 6 weeks. Transgenic frogs grew faster and, on average, weighed three times the weight of control frogs by the end of the sixth week after metamorphosis (Fig. 2B and Table 1).
Figure 2.
Transgenic F1 tadpoles and frogs from a cross of a transgenic F0 male overexpressing xGH with a wild-type female. (A) Stage 59 tadpoles. The left three tadpoles are transgenics, and the right three are sibling controls. (B) Sibling froglets 6 weeks after metamorphosis. The upper five are controls, and the lower five are transgenics. The scale bars equal 1 cm.
Figure 3.
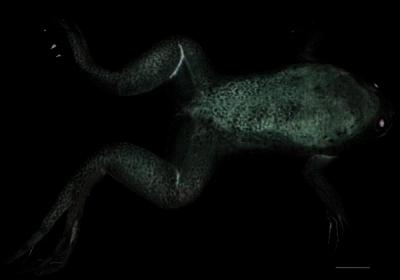
An 8-month-old transgenic frog overexpressing xGH. Note the disproportionally large head and feet, protruding lower jaw, and bent spine. The scale bar equals 2 cm.
Figure 4.
X-ray radiography of a large normal adult male frog (A) and a male transgenic frog overexpressing xGH at 1 year of age (B). The epiphyses (arrows) in the transgenic frog are open. The scale bar equals 2 cm.
Figure 5.
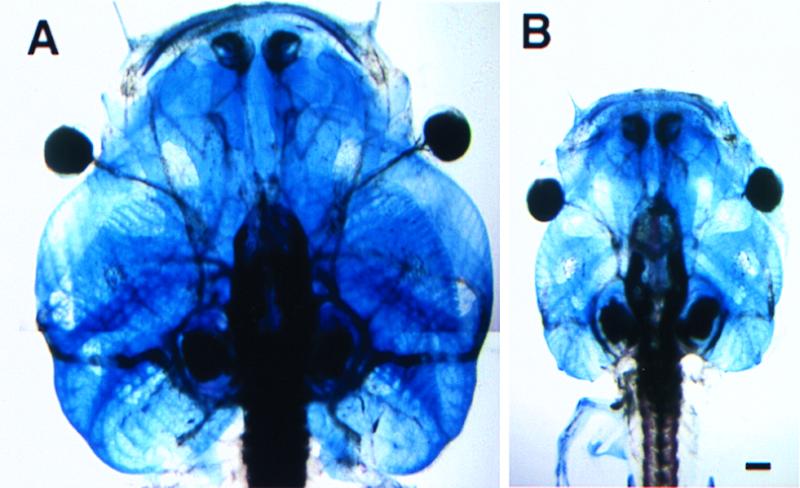
Cartilages of tadpole heads. (A) Stage 59 transgenic tadpole overexpressing xGH. (B) Stage 59 nontransgenic sibling. The scale bar equals 1 mm.
Figure 6.
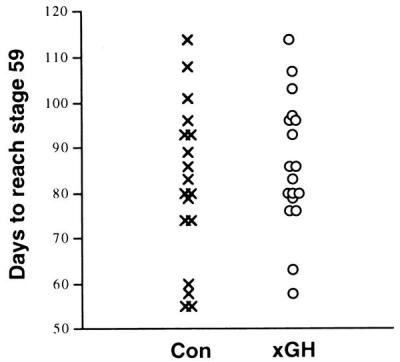
The time it takes transgenic tadpoles to reach stage 59. Transgenic tadpoles overexpressing xGH and sibling controls were raised in the same tank. Animals must be raised together in the same tank because of the effect of even slight differences in rearing conditions on the rate of tadpole development. We sometimes see this large spread in the time that it takes sibling tadpoles raised together to reach the climax of metamorphosis. This large spread means that the growth conditions were suboptimal, but control and transgenics are affected equally. Each point represents one animal.
Table 1.
Body weight of tadpoles and froglets
| Type of tadpole or frog | n | Body weight, g* | P value |
|---|---|---|---|
| Control st.59 | 18 | 0.70 ± 0.14 | <0.001† |
| xGH st.59 | 19 | 1.67 ± 0.58 | |
| Control froglet | 5 | 2.23 ± 0.25 | <0.001† |
| xGH froglet | 5 | 6.38 ± 1.62 | |
| control st.59 | 5 | 1.03 ± 0.18 | 0.002‡ |
| oPRL 1 | 11 | 1.56 ± 0.51 | |
| control st.59 | 4 | 1.25 ± 0.23 | |
| oPRL 2 | 14 | 1.63 ± 0.29 | |
| control st.59 | 6 | 1.27 ± 0.23 | 0.23‡ |
| xPRL 1 | 15 | 1.49 ± 0.40 | |
| control st.59 | 11 | 1.02 ± 0.25 | |
| xPRL 2 | 10 | 1.17 ± 0.35 | |
| control st.59 | 2 | 1.46 ± 0.35 | |
| xPRL 3 | 17 | 1.27 ± 0.32 |
Sibling control and transgenic tadpoles were always raised together in the same tank until stage 59, because the rate of tadpole growth and development is influenced greatly by subtle differences in water quality, light, temperature, tadpole density, and feeding. Differences in weight between control tadpoles raised in separate tanks can be seen in table.
Values are means ± SD.
P values were determined by t tests.
P values were determined by one-factor ANOVA (complete block design).
Overexpression of xPRL and oPRL.
For comparison, the body weights at stage 59 of transgenic tadpoles overexpressing xPRL or oPRL (19) were recorded (Table 1). oPRL transgenic tadpoles weighed about 30–50% more than controls at stage 59. There was no significant difference between the weights of xPRL transgenics and controls, suggesting that the increased weight of oPRL transgenics could reflect the activation of xGHR by oPRL. The oPRL transgenic frogs grow faster than control frogs, but the growth rate is much slower than the frogs overexpressing xGH.
Discussion
Mammalian PRL was reported to stimulate the growth of tadpoles to a greater extent than mammalian GH, whereas mammalian GH was reported to be more effective in juvenile frogs (2), leading to the speculation that tadpoles and frogs use different growth-promoting hormones. In a more recent study, it was shown that mammalian GH accelerates limb growth and development, whereas mammalian PRL stimulates tail growth and increases body weight (20). We show in this study that xGH overexpression stimulates the overall growth of tadpoles. Various parts of the tadpole including limbs and tail are proportionally larger. The increase in gill arches causes a disproportionate increase in the tadpole head. Almost every transgenic frog developed skeletal abnormalities reminiscent of acromegaly in humans.
Although xGH binds to and activates its receptor in a transient transfection assay, it does not cross-react with xPRLR even at high concentrations (19). Therefore, the dramatic growth-promoting effects observed in transgenic tadpoles overexpressing xGH is due to activation mediated by the xGHR. The growth-promoting effects of oPRL observed in earlier studies could reflect its cross-reactivity with xGHR (Fig. 1), particularly when a large amount of oPRL is administered. It is interesting to note that oPRL can bind to the chicken GH receptor (21).
The expression pattern of xGH mRNA during development (8) showed that the level of xGH drops right before climax, and this level increases again after the completion of metamorphosis. Our results showed that the overexpression of xGH does not delay the time it takes to reach the climax of metamorphosis (Fig. 6). The ensuing climax of metamorphosis leading to a froglet takes about 50% longer in transgenic animals overexpressing xGH. This increased length of time is presumably due to the increased amount of tadpole tissue that must be resorbed or remodeled in these large tadpoles.
GH and PRL have clear and distinct functions in other vertebrates. Dwarf animals that lack functional GH or GHR have been well studied in human, mouse, and chicken (22). These dwarfs invariably have functional PRL arguing against a direct effect of PRL on body growth. Mice lacking either the PRLR (23) or PRL gene (24) grow normally. Overexpression of GH in transgenic mice dramatically increases their growth rate (25, 26), and the only reported effect of PRL overexpression in transgenic mice was enlargement of the prostate gland (27). Although the removal of the pituitary gland decreases tadpole growth, the interpretation of this experiment is complicated by the loss of other pituitary hormones. To our knowledge, there are no reported experiments in which GH was eliminated specifically from an amphibian.
Because the overexpression phenotype in X. laevis closely resembles that of mammals, it seems likely that GH is a key hormone controlling growth in tadpoles and frogs.
Acknowledgments
We are grateful to J. Rosen for STAT5A1 construct, P. A. Kelly for β-casein/luciferase construct, T. Adams for oPRL cDNA, T. Wood for pSPI/Luc construct, S. Kikuyama for xPRL antiserum, C. M. Fan for 293 cells and pCEP4 and pREP4 plasmids, and A. F. Parlow and the National Hormone and Pituitary Program of the National Institute of Diabetes, Digestive, and Kidney Diseases for various mammalian hormones and antisera. We thank A. M. Schreiber for help with statistical analysis and B. F. Remo and T. Liu for excellent technical support. Our colleagues and J. R. Tata provided helpful comments on the manuscript. This research has been performed by H.H. in partial fulfillment of the requirements for the Ph.D. degree at the Johns Hopkins University. This work was supported by grants to D.D.B. from the National Institutes of Health and the G. Harold and Leila Y. Mathers Charitable Trust.
Abbreviations
- GH
growth hormone
- xGH
X. laevis GH
- xGHR
xGH receptor
- PRL
prolactin
- xPRL
X. laevis PRL
- xPRLR
xPRL receptor
- oPRL
ovine PRL
- GSP
gene-specific primer
Footnotes
References
- 1.Harvey S, Scanes C G, Daughaday W H. Growth Hormone. Boca Raton, FL: CRC; 1995. [Google Scholar]
- 2.Dodd M H I, Dodd J M. In: Physiology of the Amphibia. Lofts B, editor. Vol. 3. New York: Academic; 1976. pp. 467–599. [Google Scholar]
- 3.Berman R, Bern H A, Nicoll C S, Strohman R C. J Exp Zool. 1964;156:353–360. doi: 10.1002/jez.1401560311. [DOI] [PubMed] [Google Scholar]
- 4.Bern H A, Nicoll C S, Strohan R C. Proc Soc Exp Biol Med. 1967;126:518–520. doi: 10.3181/00379727-126-32493. [DOI] [PubMed] [Google Scholar]
- 5.Brown P S, Frye B E. Gen Comp Endocrinol. 1969;13:126–138. doi: 10.1016/0016-6480(69)90229-9. [DOI] [PubMed] [Google Scholar]
- 6.Frohman M A, Dush M K, Martin G R. Proc Natl Acad Sci USA. 1988;85:8998–9002. doi: 10.1073/pnas.85.23.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turner D L, Weintraub H. Genes Dev. 1994;8:1434–1447. doi: 10.1101/gad.8.12.1434. [DOI] [PubMed] [Google Scholar]
- 8.Buckbinder L, Brown D D. Proc Natl Acad Sci USA. 1993;90:3820–3824. doi: 10.1073/pnas.90.9.3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd Ed. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 10.Kroll K L, Amaya E. Development (Cambridge, UK) 1996;122:3173–3183. doi: 10.1242/dev.122.10.3173. [DOI] [PubMed] [Google Scholar]
- 11.Huang H, Marsh-Armstrong N, Brown D D. Proc Natl Acad Sci USA. 1999;96:962–967. doi: 10.1073/pnas.96.3.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nieuwkoop P D, Faber J. Normal Table of Xenopus laevis (Daudin) Amsterdam: Elsevier/North-Holland; 1956. [Google Scholar]
- 13.Berry D L, Rose C S, Remo B F, Brown D D. Dev Biol. 1998;203:24–35. doi: 10.1006/dbio.1998.8975. [DOI] [PubMed] [Google Scholar]
- 14.Martens G J, Groenen P J, Braks A A, Bussemakers M J. Nucleic Acids Res. 1989;17:3974. doi: 10.1093/nar/17.10.3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kelly P A, Ali S, Rozakis M, Goujon L, Nagano M, Pellegrini I, Gould D, Djiane J, Edery M, Finidori J, et al. Recent Prog Horm Res. 1993;48:123–164. doi: 10.1016/b978-0-12-571148-7.50009-9. [DOI] [PubMed] [Google Scholar]
- 16.Lesueur L, Edery M, Ali S, Paly J, Kelly P A, Djiane J. Proc Natl Acad Sci USA. 1991;88:824–828. doi: 10.1073/pnas.88.3.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wood T J, Sliva D, Lobie P E, Goullieux F, Mui A L, Groner B, Norstedt G, Haldosen L A. Mol Cell Endocrinol. 1997;130:69–81. doi: 10.1016/s0303-7207(97)00075-0. [DOI] [PubMed] [Google Scholar]
- 18.Marsh-Armstrong N, Huang H, Berry D L, Brown D D. Proc Natl Acad Sci USA. 1999;96:14389–14393. doi: 10.1073/pnas.96.25.14389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang H, Brown D D. Proc Natl Acad Sci USA. 2000;97:195–199. doi: 10.1073/pnas.97.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Delidow B C. J Exp Zool. 1989;249:279–283. doi: 10.1002/jez.1402490306. [DOI] [PubMed] [Google Scholar]
- 21.Krishnan K A, Proudman J A, Bahr J M. Mol Cell Endocrinol. 1989;66:125–134. doi: 10.1016/0303-7207(89)90024-5. [DOI] [PubMed] [Google Scholar]
- 22.Scanes C G, Daughaday W H. In: Growth Hormone. Harvey S, Scanes C G, Daughaday W H, editors. Boca Raton, FL: CRC; 1995. pp. 351–370. [Google Scholar]
- 23.Ormandy C J, Camus A, Barra J, Damotte D, Lucas B, Buteau H, Edery M, Brousse N, Babinet C, Binart N, et al. Genes Dev. 1997;11:167–178. doi: 10.1101/gad.11.2.167. [DOI] [PubMed] [Google Scholar]
- 24.Horseman N D, Zhao W, Montecino-Rodriguez E, Tanaka M, Nakashima K, Engle S J, Smith F, Markoff E, Dorshkind K. EMBO J. 1997;16:6926–6935. doi: 10.1093/emboj/16.23.6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palmiter R D, Brinster R L, Hammer R E, Trumbauer M E, Rosenfeld M G, Birnberg N C, Evans R M. Nature (London) 1982;300:611–615. doi: 10.1038/300611a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palmiter R D, Norstedt G, Gelinas R E, Hammer R E, Brinster R L. Science. 1983;222:809–814. doi: 10.1126/science.6356363. [DOI] [PubMed] [Google Scholar]
- 27.Wennbo H, Kindblom J, Isaksson O G, Tornell J. Endocrinology. 1997;138:4410–4415. doi: 10.1210/endo.138.10.5461. [DOI] [PubMed] [Google Scholar]