Abstract
Peptides derived from the heptad repeat 2 (HR2) region of the HIV fusogenic protein gp41 are potent inhibitors of viral infection, and one of them, enfuvirtide, is used for the treatment of therapy-experienced AIDS patients. The mechanism of action of these peptides is binding to a critical intermediate along the virus–cell fusion pathway, and accordingly, increasing the affinity for the intermediate yields more potent inhibitors. We took a different approach, namely to increase the potency of the HR2 peptide inhibitor C34 by targeting it to the cell compartment where fusion occurs, and we show here that a simple, yet powerful way to accomplish this is attachment of a cholesterol group. C34 derivatized with cholesterol (C34-Chol) shows dramatically increased antiviral potency on a panel of primary isolates, with IC90 values 15- to 300-fold lower than enfuvirtide and the second-generation inhibitor T1249, making C34-Chol the most potent HIV fusion inhibitor to date. Consistent with its anticipated mechanism of action, the antiviral activity of C34-Chol is unusually persistent: washing target cells after incubation with C34-Chol, but before triggering fusion, increases IC50 only 7-fold, relative to a 400-fold increase observed for C34. Moreover, derivatization with cholesterol extends the half-life of the peptide in vivo. In the mouse, s.c. administration of 3.5 mg/kg C34-Chol yields a plasma concentration 24 h after injection >300-fold higher than the measured IC90 values. Because the fusion machinery targeted by C34-Chol is similar in several other enveloped viruses, we believe that these findings may be of general utility.
Keywords: antiretroviral drug, enveloped viruses, lipid rafts, peptide therapeutic
More than 33 million people are infected with HIV worldwide (www.unaids.org). Because of the persisting difficulties in developing a vaccine against HIV (1), the discovery of drugs to treat infected people and of prophylactic agents to prevent HIV infection remains a critical medical need. Despite considerable progress on this front, an increasing number of patients ultimately become resistant to highly-active antiretroviral therapy (HAART) because of the emergence of variants that are resistant to current treatment regimens (2). Moreover, patients may become infected with virus already resistant to multiple drugs (3), highlighting the need for continuous development of novel agents.
The majority of approved HIV drugs belong to the reverse transcriptase inhibitor and protease inhibitor classes (www.fda.gov), whereas very few exploit alternative mechanisms. One such mechanism is interfering with fusion of viral and cellular membranes, as exemplified by the fusion inhibitor (FI) enfuvirtide (2, 4).
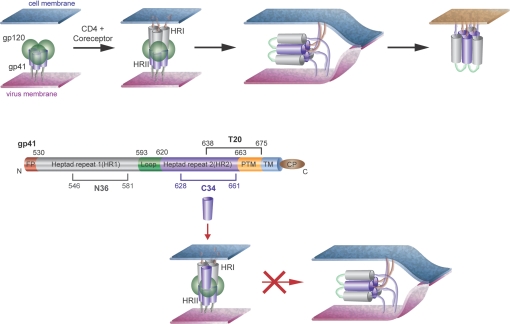
The current view of the chain of events leading to HIV entry is schematically depicted in Fig. 1. Briefly, binding of the gp120 subunit of the trimeric envelope glycoprotein to the CD4 receptor and the chemokine coreceptor (CXCR4 or CCR5) triggers a conformational change in the unmasked subunit gp41, where two regions, the N-terminal heptad repeat 1 (HR1), and the C-terminal heptad repeat 2 (HR2) become separated in the so-called prehairpin intermediate, which bridges the viral and cell membranes (5). In the prehairpin structure, HR1 forms a trimeric coiled-coil, onto which HR2 folds to form a 6-helical bundle, whose formation drives the two membranes in close apposition, ultimately leading to their fusion. Structural details have been obtained of 6-helix bundle formed by the HR1 peptide N36 and the HR2 peptide C34 (6). In this view, inhibitors that bind to the prehairpin intermediate and prevent its transition to the 6-helical bundle inhibit viral entry. Such is indeed the case for C34, T20 (4), which spans part of the HR2 region and the sequence downstream (Fig. 1), and several other FI-targeting HR1 or HR2, including peptides, proteins, and small molecules (7–21).
Fig. 1.
Currently accepted model for HIV fusion and for the mechanism of action of HR2-derived peptide inhibitors. (Top) Binding of the gp120 subunit (green) of the trimeric envelope glycoprotein to the CD4 receptor and the chemokine coreceptor (CXCR4 or CCR5) triggers a conformational change in the unmasked subunit gp41 (gray and violet), where two regions, the N-terminal HR1 (gray), and the C-terminal HR2 (violet) become separated in the so-called “hairpin intermediate,” which bridges the viral and cell membranes. Collapse of the hairpin intermediate into a 6-helix bundle drives viral–cell membrane fusion. (Middle) Schematic representation of gp41, using the same color code for HR1 and HR2. (Bottom) MOA of HR2-derived FI. C34 binds the HR1 coiled-coil and prevents transition to the 6-helix bundle. It is apparent from the figure that C34 must bind to HR1 in antiparallel orientation. The boundaries of the gp41 regions encompassed by C34 and T20 are indicated (FP, fusion peptide; PTM, proximal to the transmembrane region; TM, transmembrane region; CP, cytoplasmic region).
The successful use of enfuvirtide in the clinic has stimulated efforts to develop more potent peptides that are also active on enfuvirtide-resistant strains (22, 23). Because C34 shows essentially no helical structure before folding onto HR1 to form the 6-helix bundle, HR2-derived peptides have been designed with enhanced helical structure in solution, which translates into greater strength of association with the HR1 coiled-coil and greater antiviral potency (8–10, 24). Hybrids between C34 and T20 have also been pursued because the membrane-proximal region of gp41, included in T20, is beneficial for half-life (11). Although these peptides form 6-helix bundles with much increased stability with respect to C34, there appears to be a limit to the increase in potency that can be achieved by this route, with antiviral activity reaching a plateau over a wide range of bundle stability (8).
We took a different approach, namely to increase the potency of C34 by targeting it to the cell compartment where fusion occurs, through introduction of a membrane anchor in the form of a cholesterol group (C34-Chol).
Results and Discussion
Design of C34-Chol.
The general advantage of targeting a drug to a membrane to increase its binding affinity toward membrane-bound receptors has long been recognized (25–28). More than 2 decades ago, Schwyzer (26–28) proposed that natural peptide hormones including opioids, tachykinins, and melanocortins exploit this mechanism by having in their sequence an “address” region responsible for membrane association, which is complemented by a “message” region, specific for the receptor type.
For peptide FI, and T20 in particular, a number of examples document the advantage of this strategy. For example, a construct including T20, a short linker, and a transmembrane (TM) domain, was a more powerful inhibitor than the same construct lacking the TM domain (29, 30). Importantly, mutations in the membrane-proximal region of T20, which completely inactivated the free peptide, did not reduce the potency of the membrane-anchored one (29). It was also reported that addition of a C-terminal octyl group to T20 significantly increased its inhibitory potency. As in the previous example, octylation could rescue the activity of the inactive mutant, in which the C-terminal residues GNWF were replaced by ANAA. Importantly, the position of the octyl group was critical because N-terminal derivatization had no effect on antiviral potency (31), in line with the need for an antiparallel orientation with respect to HR1 (see Fig. 1).
However, despite the recognition of the correlation between lipid binding and antiviral activity in FI, no systematic effort has been carried out so far to develop an optimized lipid anchor. To this aim, we have chosen C34 instead of T20 because the former has more potent antiviral activity in vitro, whereas the latter already includes a hydrophobic C-terminal segment that drives insertion into lipid membranes (32–36).
We hypothesized that a cholesterol group would be the most appropriate type of lipid anchor because of the role that cholesterol-enriched lipid rafts play in HIV fusion. A substantial body of evidence supports the importance of lipid rafts and cholesterol in HIV entry (37, 38). The composition of the lipid membrane of HIV is different from the composition of the host cell membrane, being particularly enriched in cholesterol and sphingomyelin (39–41). This lipid composition likely results from fusion occurring in high-order complexes in confined areas of the interacting viral–host membranes (36). Accordingly, cholesterol and sphingolipids are often laterally segregated in membrane microdomains known as “lipid rafts” (36). A number of transmembrane proteins and receptors are particularly enriched in lipid rafts, and these include CD4, the primary receptor for HIV (41). In addition, gp41 associates with caveolin-1 (42), the structural protein component of a subset of lipid rafts known as caveolae (43). Because caveolin-1 is a cholesterol-binding protein (44), cholesterol is enriched in caveolae together with HIV-1. Finally, the increased ability to partition into cholesterol-rich membranes has been proposed recently as the reason behind the increased clinical efficacy of the second-generation inhibitor T1249 with respect to T20 (32).
In addition to being appropriate for HIV, cholesterol is an ideal anchor to localize stably a peptide to a lipid membrane. There is a general relationship between the degree of hydrophobic modification and the stability of membrane insertion (45). Quasi-irreversible binding requires the presence of two long-chain anchors in the molecule, for example, palmitoyl and farnesyl, or hexadecyl and farnesyl (46). The same quasi-irreversible binding is achieved with a single cholesteryl moiety (47). The use of cholesterol for membrane targeting of a peptide has just been described to increase the potency of a transition-state β-secretase inhibitor (48).
In summary, addition of a cholesterol group to a FI should increase its potency by two complementary mechanisms: (i) generic increase of affinity for membranes, and (ii) specific enrichment in the lipid rafts, where HIV–cell fusion occurs.
Based on the above considerations, we prepared a derivative of C34 (C34-Chol) with a cholesterol group attached, via a thioether bond, to the side chain of a cysteine residue added to the C terminus. To allow for flexibility between the lipid anchor and the C34 sequence, a Gly-Ser-Gly spacer was inserted between the two. Importantly, the current model for HIV fusion indicates the need for an antiparallel arrangement of the N- and C-domain peptides (C34 is derived from the latter) and dictates that the position of a membrane anchor should be at the C terminus of C34: N-terminal derivatization would interfere with binding to the N-peptide (see Fig. 1).
Structure–Activity Relationship for Cholesterol Derivatization.
Table 1 shows the sequence of C34-Chol and a number of control peptides used in our studies. These include the control C34-Acm, where the extra cysteine was capped by alkylation with iodoacetamide; Chol-C34, where cholesterol was attached to the N terminus of C34, to investigate the positional dependence of derivatization on activity; C34-Pam, where the lipid moiety was a C16 palmitoyl group instead of cholesterol; and the derivative of enfuvirtide (T20) with cholesterol.
Table 1.
Sequence of peptides used in this study
| Peptide | Sequencelo* |
|---|---|
| C34† | |
| C34-Acm | C34-GlySerGly-Cys(CH2CONH2) |
| C34-Chol | C34-GlySerGly-Cys(Chol) |
| Chol-C34 | Cys(Chol)-GlySerGly-C34 |
| C34-Pam | C34-GlySerGly-Lys(Pam) |
| T20‡ | |
| T20-Chol | T20-GlySerGly-Cys(Chol) |
*All peptides N-terminal acetyl and C-terminal carboxyamide. Chol, cholesterol; Lys(Pam), Lys(Nε-palmitoyl).
†The sequence of C34 is WMEWDREINNYTSLIHSLIEESQNQQEKNEQELL.
‡The sequence of T20 (enfuvirtide) is YTSLIHSLIEESQNQQEKNEQELLELDKWASLWNWF.
The antiviral activity of these peptides in the single-cycle infectivity assay against the HIV strain HXB2 is shown in Table 2. The results are fully consistent with the design: (i) Addition of cholesterol to C34 increases its antiviral potency 50-fold over C34 and the control peptide C34-Acm. (ii) Addition of cholesterol at the N terminus instead of the C terminus is detrimental to antiviral activity (50-fold decrease compared with underivatized C34), likely resulting from the contrasting orientation needs of cholesterol and C34 for binding to the cell membrane and the hairpin intermediate, respectively (see Fig. 1). (iii) A different lipid group like palmitic acid does not have the same effect as cholesterol: C34-Pam has activity comparable with that of underivatized C34. This would be consistent with both the reduced affinity for membranes of the fatty acid versus cholesterol and the different tendency of fatty acids and cholesterol to partition in raft microdomains (49). (iv) The potency-enhancing effect observed with C34 does not occur with T20, whose lipophilic segment already has intrinsic affinity for a lipid membrane (32): when cholesterol is added C-terminally to T20, the effect is actually to decrease antiviral activity, perhaps because of interference between the two lipophilic moieties.
Table 2.
Antiviral potency of C34-Chol and related peptides
| Peptide* | IC50 viral infectivity, pM |
|---|---|
| C34 | 205 ± 59 |
| C34-Acm | 270 ± 88 |
| C34-Chol | 4 ± 1 |
| Chol-C34 | 9,515 ± 3,172 |
| C34-Pam | 713 ± 305 |
| T20 | 692 ± 196 |
| T20-Chol | 3,726 ± 1,196 |
*Sequence as in Table 1.
Notably, in the only other reported example of cholesterol tethering to a peptide, the same absolute need for C-terminal versus N-terminal linkage of cholesterol was observed, and palmitoyl derivatization was inferior to cholesterol in promoting biological activity (48).
C34-Chol Is the Most Potent HIV FI Known to Date.
We next examined the breadth of the antiviral response of C34-Chol (Table 3). When tested on a variety of viral strains, including primary isolates, C34-Chol showed widespread response with comparable IC50 in all strains. Across the whole panel, C34-Chol was ≈50-fold more potent than C34 or C34-Acm.
Table 3.
Antiviral potency of C34-Chol and controls against multiple HIV-1 isolates
| HIV isolate | IC50 viral infectivity, pM |
||
|---|---|---|---|
| C34-Chol | C34-Acm | C34 | |
| HXB2 | 8 ± 2 | 227 ± 104 | 141 ± 59 |
| BAL | 9 ± 5 | 344 ± 82 | 273 ± 75 |
| NL4-3 | 6 ± 2 | 173 ± 20 | 292 ± 67 |
| MN-1 | 21 ± 11 | 1,003 ± 184 | 866 ± 367 |
| 89.6 | 34 ± 12 | 6,022 ± 480 | 3,912 ± 1,429 |
| R8 | 15 ± 8 | 516 ± 119 | 314 ± 35 |
| SHIV* | 9 ± 5 | 1,271 ± 301 | 694 ± 353 |
| VSVG† | NA‡ | NA‡ | NA‡ |
The antiviral potency was determined in a single-cycle infectivity assay. All data were from three independent experiments and are expressed as mean ± SEM.
* Strain sf162p3.
†VSVG is a control virus with no HIV envelope.
‡NA, not active.
Further analysis of the antiviral potency of C34-Chol is shown in Table 4, where the antiviral activity of C34-Chol is compared, in two separate experiments, with underivatized C34, and with T20 and the second-generation FI T1249 (32, 50), which is active against most enfuvirtide-resistant strains (23). Comparison of the IC90 values, a stringent measure of antiviral potency, across a panel of HIV primary isolates from multiple subtypes shows that, depending on the strain tested, C34-Chol is 25- to 100-fold more potent than C34, 50- to 400-fold more potent than enfuvirtide, and 15- to 300-fold more potent than T1249. Overall, C34-Chol is the most potent HIV FI known to date.
Table 4.
Comparison of antiviral potency of C34-Chol versus C34, T20, and the second-generation inhibitor T1249
| HIV isolate | IC90 viral infectivity, nM |
||||
|---|---|---|---|---|---|
| Experiment 1 |
Experiment 2 |
||||
| C34-Chol | C34 | C34-Chol | T20 | T1249 | |
| DJ258 | 0.20 | 4.8 | 0.015 | 7.6 | 4.5 |
| JRFL | 0.16 | 20.0 | 0.36 | 20 | 8.3 |
| NL4-3 | 0.16 | 5.4 | 0.09 | 6.1 | 2.6 |
| CC 7/86 | 1.0 | 22.0 | 0.08 | 32 | 24 |
| 94ZW103 | 0.41 | 5.3 | 0.10 | 11 | 1.9 |
| UG270 | 1.50 | 51.0 | 0.38 | 53 | 5.2 |
| CM235 | 1.80 | 20.0 | 0.46 | 61 | 11 |
| BZ162 | 0.37 | 6.1 | 0.10 | 9.2 | 2.2 |
Mechanism of Action (MOA) of C34-Chol.
As pointed out, a number of peptide FI were designed successfully based on the hypothesis that greater strength of association with the HR1 would translate into greater antiviral potency (8–10, 24). Based on the hypothesized MOA, this should not be the case for C34-Chol. Table 5 shows the relative ability of C34-Chol and related peptides to bind to the HR1 trimeric coiled-coil, by using a described competition assay (14). C34-Chol, which inhibits HIV fusion 50-fold better than C34 (Table 2), has 20-fold lower affinity for HR1 than C34. This lower affinity is likely caused by steric hindrance of the lipid group because C34-Acm binds slightly better then C34, whereas derivatization with palmitic acid or cholesterol yields comparable results. Interestingly, when cholesterol is attached to the N terminus of C34, inhibition of HR1 binding is further increased.
Table 5.
In vitro binding to 5-helix of C34-Chol and related peptides
| Peptide* | IC50 competitive binding to 5H, nM |
|---|---|
| C34 | 1 ± 5 |
| C34-Acm | 0.2 ± 0.01 |
| C34-Chol | 19 ± 6 |
| Chol-C34 | 120 ± 53 |
| C34-Pam | 21 ± 5 |
| T20 | NA† |
| T20-Chol | NA† |
*Sequence as in Table 1.
†NA, not active.
We then looked for evidence that C34-Chol accumulates at the target cell membrane, which in our hypothesis is key to its MOA. We performed an experiment where the peptide and its controls were incubated with P4-2/R5 cells at 37 °C for 1 h, followed by thorough washing to remove any unbound peptide, and by addition of HIV-HXB2, to initiate infection. After 48 h, the antiviral activities of the residual peptides that survive the washing steps were measured. As shown in Table 6, the washing step dramatically reduced the antiviral potency of C34 and C34-Acm, consistent with the knowledge that these agents need to be present at the time of HIV membrane fusion (51). However, washing only induced a 7-fold shift in the IC50 of C34-Chol. Our interpretation [illustrated in supporting information (SI) Fig. S1] is that C34-Chol binds to the raft compartments of the membrane during the incubation step, and because of its high affinity for this compartment, it is not removed during the washing step. When infection is initiated by addition of the virus, C34-Chol is thus available for dominant-negative interference with 6-helix bundle formation.
Table 6.
Antiviral potency of C34-Chol is retained when preincubated with target cells followed by wash
| Peptide* | IC50 viral infectivity, pM† | |
|---|---|---|
| No wash | With wash | |
| C34-Chol | 7 | 50 |
| C34-Acm | 194 | 109,610 |
| C34 | 313 | 116,930 |
Antiviral potency was determined as before.
*Sequence as in Table 1.
†Data are from a single experiment, representative of 3 repeats.
This property makes C34-Chol particularly attractive for use in a topical microbicide to block sexual transmission of HIV (52). Preferred agents for this use inhibit early steps in the HIV cycle, like attachment, fusion, and entry, to prevent integration of the viral genome into chromosomal DNA (53). Successful use of entry inhibitors to block vaginal transmission of HIV in rhesus macaques has been reported (54–56). It has been shown recently that C34 can completely block HIV infection of Langerhans cells when continuously present before, during, and after exposure of cells to virus, but only partially when the peptide is not present during incubation with virus (51). In this setting, C34-Chol, which is retained at the site of action after washing, should be a superior agent to C34.
Effect of Lipid Derivatization on the Pharmacokinetics of C34-Chol.
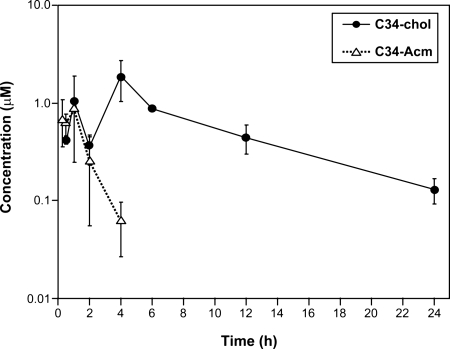
Despite the high antiviral activity, C34 is unsuitable as a therapeutic because of its very short half-life. A favored approach to improve peptide pharmacokinetics is conjugation to lipids (57), which prolongs half-life in circulation through binding to serum proteins. The most typical derivatization is with long-chain fatty acids, whereas derivatization with cholesterol has been little explored (57). However, a recent report on the comparative efficacy in vivo of intravenously administered lipophilic derivatives of siRNA showed that derivatization with cholesterol and related molecules gave the highest biological activity (58). Therefore, we investigated whether derivatization with cholesterol, in addition to providing improved antiviral potency, also extends the half-life of the peptide in vivo. Fig. 2 shows the pharmacokinetics of C34-Chol and C34-Acm when injected s.c. into the mouse at the concentration of 3.5 mg/kg. Derivatization with cholesterol dramatically improved the circulatory half-life of the peptide: whereas C34-Acm was undetectable in plasma after 6 h, ≈130 nM C34-Chol was still detectable in plasma 24 h after injection; this concentration is still >300-fold higher than the IC90 measured against multiple HIV strains (80–360 pM, Table 4).
Fig. 2.
Pharmacokinetics of C34-Chol in mice. C34-Acm or C34-Chol (3.5 mg/kg) in 10 mM glycine buffer containing 10% ethanol was administered s.c. to C57BL/6 mice, and the concentration of peptide was monitored with time. The calculated pharmacokinetic parameters are given in Table S1.
In conclusion, we have shown here that targeting a FI to the membrane compartment where viral fusion occurs is a simple, yet powerful way to improve antiviral potency and that derivatization with cholesterol is a most appropriate way to achieve this goal. Moreover, we show that increased potency comes together with other desirable properties, like accumulation at the site of action and improved pharmacokinetic properties. Because the fusion machinery targeted by C34-Chol is similar in several other enveloped viruses (59), we believe that these findings may be of general utility.
Materials and Methods
Peptide Synthesis.
See details in SI Materials and Methods.
HIV-1 Infectivity Assay.
P4–2/R5 cells (HeLa cells expressing endogenous CXCR4 and stably transfected to express CD4 and CCR5, which also contain an integrated β-galactosidase reporter gene under control of an HIV LTR promoter) maintained in phenol red-free DMEM, 10% FBS, and 1% penicillin/streptomycin were seeded in 96-well plates at 2.5 × 103 cells per well and infected the next day with the HXB2 or other strains of HIV-1 in the presence of the test inhibitory peptides at 37 °C. After a 48-h incubation, cells were lysed, and β-galactosidase was detected by using Gal Screen chemiluminescent substrate (Applied Biosystems) according to the manufacturer's instructions. Data were obtained by using a Dynex luminometer, and IC50 values were calculated by KaleidaGraph.
In one set of experiments where the retention and functioning of C34-Chol, C34-Acm, and C34 on target cell surface were evaluated, each peptide was preincubated with P4-2/R5 cells at 37 °C for 1 h, followed by three washes with culture medium to remove unbound peptides (no wash as control) and addition of HXB2 to initiate infection. After 48 h, the antiviral activities of the residual peptides that survive the washing steps were determined by measuring the β-galactosidase activities within lysed cells as described above.
HR1/5H Peptide Competition Assay. This is a modification of a described assay (14).
Supplementary Material
Acknowledgments.
We thank Gennaro Ciliberto and Daria J. Hazuda for continuous support and critical discussion, Marco Finotto for peptide synthesis, Simone Bufali for the formulation studies, and Manuela Emili for the artwork. This work was supported by National Institutes of Health Grants U19 AI65413 and U19 AI76982 (to T.J.K. and J.P.M.).
Footnotes
Conflict of interest statement: Peter S. Kim and P.I., E.B., N.A.L., Y.-J.W., R.H., M.V., F.B., M.D.M., and A.P. are employees of Merck & Co., Inc.
This article contains supporting information online at www.pnas.org/cgi/content/full/0901007106/DCSupplemental.
References
- 1.Walker BD, Burton DR. Toward an AIDS vaccine. Science. 2008;320:760–764. doi: 10.1126/science.1152622. [DOI] [PubMed] [Google Scholar]
- 2.Lalezari JP, et al. Enfuvirtide, an HIV-1 fusion inhibitor, for drug-resistant HIV infection in North and South America. N Engl J Med. 2003;348:2175–2185. doi: 10.1056/NEJMoa035026. [DOI] [PubMed] [Google Scholar]
- 3.Little SJ, et al. Antiretroviral-drug resistance among patients recently infected with HIV. N Engl J Med. 2002;347:385–394. doi: 10.1056/NEJMoa013552. [DOI] [PubMed] [Google Scholar]
- 4.Matthews T, et al. Enfuvirtide: The first therapy to inhibit the entry of HIV-1 into host CD4 lymphocytes. Nat Rev Drug Discov. 2004;3:215–225. doi: 10.1038/nrd1331. [DOI] [PubMed] [Google Scholar]
- 5.Eckert DM, Kim PS. Mechanisms of viral membrane fusion and its inhibition. Annu Rev Biochem. 2001;70:777–810. doi: 10.1146/annurev.biochem.70.1.777. [DOI] [PubMed] [Google Scholar]
- 6.Chan DC, Fass D, Berger JM, Kim PS. Core structure of gp41 from the HIV envelope glycoprotein. Cell. 1997;89:263–273. doi: 10.1016/s0092-8674(00)80205-6. [DOI] [PubMed] [Google Scholar]
- 7.Root MJ, Steger HK. HIV-1 gp41 as a target for viral entry inhibition. Curr Pharm Des. 2004;10:1805–1825. doi: 10.2174/1381612043384448. [DOI] [PubMed] [Google Scholar]
- 8.Dwyer JJ, et al. Design of helical, oligomeric HIV-1 fusion inhibitor peptides with potent activity against enfuvirtide-resistant virus. Proc Natl Acad Sci USA. 2007;104:12772–12777. doi: 10.1073/pnas.0701478104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oishi S, et al. Design of a novel HIV-1 fusion inhibitor that displays a minimal interface for binding affinity. J Med Chem. 2008;51:388–391. doi: 10.1021/jm701109d. [DOI] [PubMed] [Google Scholar]
- 10.Otaka A, et al. Remodeling of gp41–C34 peptide leads to highly effective inhibitors of the fusion of HIV-1 with target cells. Angew Chem Int Ed Engl. 2002;41:2937–2940. doi: 10.1002/1521-3773(20020816)41:16<2937::AID-ANIE2937>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 11.He Y, et al. Design and evaluation of sifuvirtide, a novel HIV-1 fusion inhibitor. J Biol Chem. 2008;283:11126–11134. doi: 10.1074/jbc.M800200200. [DOI] [PubMed] [Google Scholar]
- 12.Welch BD, VanDemark AP, Heroux A, Hill CP, Kay MS. Potent D-peptide inhibitors of HIV-1 entry. Proc Natl Acad Sci USA. 2007;104:16828–16833. doi: 10.1073/pnas.0708109104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bianchi E, et al. Covalent stabilization of coiled coils of the HIV gp41 N region yields extremely potent and broad inhibitors of viral infection. Proc Natl Acad Sci USA. 2005;102:12903–12908. doi: 10.1073/pnas.0502449102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller MD, et al. A human monoclonal antibody neutralizes diverse HIV-1 isolates by binding a critical gp41 epitope. Proc Natl Acad Sci USA. 2005;102:14759–14764. doi: 10.1073/pnas.0506927102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luftig MA, et al. Structural basis for HIV-1 neutralization by a gp41 fusion intermediate-directed antibody. Nat Struct Mol Biol. 2006;13:740–747. doi: 10.1038/nsmb1127. [DOI] [PubMed] [Google Scholar]
- 16.Liu S, Wu S, Jiang S. HIV entry inhibitors targeting gp41: From polypeptides to small-molecule compounds. Curr Pharm Des. 2007;13:143–162. doi: 10.2174/138161207779313722. [DOI] [PubMed] [Google Scholar]
- 17.Eckert DM, Kim PS. Design of potent inhibitors of HIV-1 entry from the gp41 N-peptide region. Proc Natl Acad Sci USA. 2001;98:11187–11192. doi: 10.1073/pnas.201392898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Root MJ, Kay MS, Kim PS. Protein design of an HIV-1 entry inhibitor. Science. 2001;291:884–888. doi: 10.1126/science.1057453. [DOI] [PubMed] [Google Scholar]
- 19.Louis JM, Nesheiwat I, Chang L, Clore GM, Bewley CA. Covalent trimers of the internal N-terminal trimeric coiled-coil of gp41 and antibodies directed against them are potent inhibitors of HIV envelope-mediated cell fusion. J Biol Chem. 2003;278:20278–20285. doi: 10.1074/jbc.M301627200. [DOI] [PubMed] [Google Scholar]
- 20.Bewley CA, Louis JM, Ghirlando R, Clore GM. Design of a novel peptide inhibitor of HIV fusion that disrupts the internal trimeric coiled-coil of gp41. J Biol Chem. 2002;277:14238–14245. doi: 10.1074/jbc.M201453200. [DOI] [PubMed] [Google Scholar]
- 21.Louis JM, Bewley CA, Clore GM. Design and properties of N(CCG)-gp41, a chimeric gp41 molecule with nanomolar HIV fusion inhibitory activity. J Biol Chem. 2001;276:29485–29489. doi: 10.1074/jbc.C100317200. [DOI] [PubMed] [Google Scholar]
- 22.Miller MD, Hazuda DJ. HIV resistance to the fusion inhibitor enfuvirtide: Mechanisms and clinical implications. Drug Resist Update. 2004;7:89–95. doi: 10.1016/j.drup.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 23.Lalezari JP, et al. T-1249 retains potent antiretroviral activity in patients who had experienced virological failure while on an enfuvirtide-containing treatment regimen. J Infect Dis. 2005;191:1155–1163. doi: 10.1086/427993. [DOI] [PubMed] [Google Scholar]
- 24.Judice JK, et al. Inhibition of HIV type 1 infectivity by constrained α-helical peptides: Implications for the viral fusion mechanism. Proc Natl Acad Sci USA. 1997;94:13426–13430. doi: 10.1073/pnas.94.25.13426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao X, Wong TC. Studies of the binding and structure of adrenocorticotropin peptides in membrane mimics by NMR spectroscopy and pulsed-field gradient diffusion. Biophys J. 1998;74:1871–1888. doi: 10.1016/S0006-3495(98)77897-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schwyzer R. How do peptides interact with lipid membranes and how does this affect their biological activity? Braz J Med Biol Res. 1992;25:1077–1089. [PubMed] [Google Scholar]
- 27.Schwyzer R. Peptide–membrane interactions and a new principle in quantitative structure–activity relationships. Biopolymers. 1991;31:785–792. doi: 10.1002/bip.360310624. [DOI] [PubMed] [Google Scholar]
- 28.Schwyzer R. Membrane-assisted molecular mechanism of neurokinin receptor subtype selection. EMBO J. 1987;6:2255–2259. doi: 10.1002/j.1460-2075.1987.tb02498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hildinger M, et al. Membrane-anchored peptide inhibits human immunodeficiency virus entry. J Virol. 2001;75:3038–3042. doi: 10.1128/JVI.75.6.3038-3042.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Egelhofer M, et al. Inhibition of human immunodeficiency virus type 1 entry in cells expressing gp41-derived peptides. J Virol. 2004;78:568–575. doi: 10.1128/JVI.78.2.568-575.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peisajovich SG, Gallo SA, Blumenthal R, Shai Y. C-terminal octylation rescues an inactive T20 mutant: Implications for the mechanism of HIV/simian immunodeficiency virus-induced membrane fusion. J Biol Chem. 2003;278:21012–21017. doi: 10.1074/jbc.M212773200. [DOI] [PubMed] [Google Scholar]
- 32.Veiga AS, Santos NC, Loura LM, Fedorov A, Castanho MA. HIV fusion inhibitor peptide T-1249 is able to insert or adsorb to lipidic bilayers: Putative correlation with improved efficiency. J Am Chem Soc. 2004;126:14758–14763. doi: 10.1021/ja0459882. [DOI] [PubMed] [Google Scholar]
- 33.Barbato G, et al. Structural analysis of the epitope of the anti-HIV antibody 2F5 sheds light into its mechanism of neutralization and HIV fusion. J Mol Biol. 2003;330:1101–1115. doi: 10.1016/s0022-2836(03)00611-9. [DOI] [PubMed] [Google Scholar]
- 34.Biron Z, et al. A monomeric 3(10)-helix is formed in water by a 13-residue peptide representing the neutralizing determinant of HIV-1 on gp41. Biochemistry. 2002;41:12687–12696. doi: 10.1021/bi026261y. [DOI] [PubMed] [Google Scholar]
- 35.Schibli DJ, Montelaro RC, Vogel HJ. The membrane-proximal tryptophan-rich region of the HIV glycoprotein, gp41, forms a well-defined helix in dodecylphosphocholine micelles. Biochemistry. 2001;40:9570–9578. doi: 10.1021/bi010640u. [DOI] [PubMed] [Google Scholar]
- 36.Ono A, Freed EO. Role of lipid rafts in virus replication. Adv Virus Res. 2005;64:311–358. doi: 10.1016/S0065-3527(05)64010-9. [DOI] [PubMed] [Google Scholar]
- 37.Aloia RC, Jensen FC, Curtain CC, Mobley PW, Gordon LM. Lipid composition and fluidity of the human immunodeficiency virus. Proc Natl Acad Sci USA. 1988;85:900–904. doi: 10.1073/pnas.85.3.900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aloia RC, Tian H, Jensen FC. Lipid composition and fluidity of the human immunodeficiency virus envelope and host cell plasma membranes. Proc Natl Acad Sci USA. 1993;90:5181–5185. doi: 10.1073/pnas.90.11.5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bentz J. Membrane fusion mediated by coiled coils: A hypothesis. Biophys J. 2000;78:886–900. doi: 10.1016/S0006-3495(00)76646-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bentz J, Mittal A. Deployment of membrane fusion protein domains during fusion. Cell Biol Int. 2000;24:819–838. doi: 10.1006/cbir.2000.0632. [DOI] [PubMed] [Google Scholar]
- 41.Chernomordik LV, Frolov VA, Leikina E, Bronk P, Zimmerberg J. The pathway of membrane fusion catalyzed by influenza hemagglutinin: Restriction of lipids, hemifusion, and lipidic fusion pore formation. J Cell Biol. 1998;140:1369–1382. doi: 10.1083/jcb.140.6.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hovanessian AG, et al. The caveolin-1 binding domain of HIV-1 glycoprotein gp41 is an efficient B cell epitope vaccine candidate against virus infection. Immunity. 2004;21:617–627. doi: 10.1016/j.immuni.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 43.Okamoto T, Schlegel A, Scherer PE, Lisanti MP. Caveolins, a family of scaffolding proteins for organizing “preassembled signaling complexes” at the plasma membrane. J Biol Chem. 1998;273:5419–5422. doi: 10.1074/jbc.273.10.5419. [DOI] [PubMed] [Google Scholar]
- 44.Murata M, et al. VIP21/caveolin is a cholesterol-binding protein. Proc Natl Acad Sci USA. 1995;92:10339–10343. doi: 10.1073/pnas.92.22.10339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schelhaas M, et al. Chemoenzymatic synthesis of biotinylated Ras peptides and their use in membrane binding studies of lipidated model proteins by surface plasmon resonance. Chem Eur J. 1999;5:1239–1252. [Google Scholar]
- 46.Bader B, et al. Bioorganic synthesis of lipid-modified proteins for the study of signal transduction. Nature. 2000;403:223–226. doi: 10.1038/35003249. [DOI] [PubMed] [Google Scholar]
- 47.Peters C, Wolf A, Wagner M, Kuhlmann J, Waldmann H. The cholesterol membrane anchor of the Hedgehog protein confers stable membrane association to lipid-modified proteins. Proc Natl Acad Sci USA. 2004;101:8531–8536. doi: 10.1073/pnas.0308449101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rajendran L, et al. Efficient inhibition of the Alzheimer's disease β-secretase by membrane targeting. Science. 2008;320:520–523. doi: 10.1126/science.1156609. [DOI] [PubMed] [Google Scholar]
- 49.Resh MD. Membrane targeting of lipid modified signal transduction proteins. Subcell Biochem. 2004;37:217–232. doi: 10.1007/978-1-4757-5806-1_6. [DOI] [PubMed] [Google Scholar]
- 50.Eron JJ, et al. Short-term safety and antiretroviral activity of T-1249, a second-generation fusion inhibitor of HIV. J Infect Dis. 2004;189:1075–1083. doi: 10.1086/381707. [DOI] [PubMed] [Google Scholar]
- 51.Sugaya M, Hartley O, Root MJ, Blauvelt A. C34, a membrane fusion inhibitor, blocks HIV infection of Langerhans cells and viral transmission to T cells. J Invest Dermatol. 2007;127:1436–1443. doi: 10.1038/sj.jid.5700736. [DOI] [PubMed] [Google Scholar]
- 52.Klasse PJ, Shattock R, Moore JP. Antiretroviral drug-based microbicides to prevent HIV-1 sexual transmission. Annu Rev Med. 2008;59:455–471. doi: 10.1146/annurev.med.59.061206.112737. [DOI] [PubMed] [Google Scholar]
- 53.Lederman MM, Offord RE, Hartley O. Microbicides and other topical strategies to prevent vaginal transmission of HIV. Nat Rev Immunol. 2006;6:371–382. doi: 10.1038/nri1848. [DOI] [PubMed] [Google Scholar]
- 54.Veazey RS, et al. Prevention of virus transmission to macaque monkeys by a vaginally applied monoclonal antibody to HIV-1 gp120. Nat Med. 2003;9:343–346. doi: 10.1038/nm833. [DOI] [PubMed] [Google Scholar]
- 55.Lederman MM, Veazey RS, Offord R, Mosier DE, Dufour J, et al. Prevention of vaginal SHIV transmission in rhesus macaques through inhibition of CCR5. Science. 2004;306:485–487. doi: 10.1126/science.1099288. [DOI] [PubMed] [Google Scholar]
- 56.Veazey RS, et al. Protection of macaques from vaginal SHIV challenge by vaginally delivered inhibitors of virus-cell fusion. Nature. 2005;438:99–102. doi: 10.1038/nature04055. [DOI] [PubMed] [Google Scholar]
- 57.Madsen K, et al. Structure–activity and protraction relationship of long-acting glucagon-like peptide-1 derivatives: Importance of fatty acid length, polarity, and bulkiness. J Med Chem. 2007;50:6126–6132. doi: 10.1021/jm070861j. [DOI] [PubMed] [Google Scholar]
- 58.Wolfrum C, et al. Mechanisms and optimization of in vivo delivery of lipophilic siRNAs. Nat Biotechnol. 2007;25:1149–1157. doi: 10.1038/nbt1339. [DOI] [PubMed] [Google Scholar]
- 59.Gao GF. In: Combating the Threat of Pandemic Influenza. Torrence PF, editor. Hoboken, NJ: Wiley; 2007. pp. 226–246. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.