Abstract
Bilateral adrenocortical hyperplasia (BAH) is the second most common cause of corticotropin-independent Cushing syndrome (CS). Genetic forms of BAH have been associated with complex syndromes such as Carney Complex and McCune Albright syndrome or may present as isolated micronodular adrenocortical disease (iMAD) usually in children and young adults with CS. A genome-wide association study identified inactivating phosphodiesterase (PDE) 11A (PDE11A) sequencing defects as low-penetrance predisposing factors for iMAD and related abnormalities; we also described a mutation (c.914A>C/H305P) in cAMP-specific PDE8B, in a patient with iMAD. In this study we further characterize this mutation; we also found a novel PDE8B isoform, highly expressed in the adrenal gland. This mutation is shown to significantly affect the ability of the protein to degrade cAMP in vitro. Tumor tissues from patients with iMAD and no mutations in the coding PDE8B sequence or any other related genes (PRKAR1A, PDE11A) showed down-regulated PDE8B expression (compared to normal adrenal cortex). Pde8b is detectable in the adrenal gland of newborn mice and is widely expressed in other mouse tissues. We conclude that PDE8B is another PDE gene linked to iMAD; it is a candidate causative gene for other adrenocortical lesions linked to the cAMP-signaling pathway, and possibly for tumors in other tissues.
Keywords: adrenal gland, Cushing syndrome, protein kinase A, cyclic AMP, phosphodiesterases
Introduction
Micronodular adrenocortical disease (MAD) is a distinct type of corticotropin- independent bilateral adrenocortical hyperplasia (BAH) and is associated with Cushing syndrome (CS) in children and young adults.1 MAD is characterized by multiple nodules, capsular deficits that often result in extra-adrenal cortical excrescences, and hyperplasia of the main cortical tissue; the adrenal medulla is normal in this condition.1–3 A rare variant of MAD is heavily pigmented due to the accumulation of lipofuscin in cortical cells; this form is known as primary pigmented nodular adrenocortical disease (PPNAD).1, 3 All forms of BAH have been linked to abnormalities of the cyclic AMP (cAMP)-signaling pathway4–6: GNAS is mutated in BAH in the context of McCune-Albright syndrome4 and PRKAR1A mutations cause sporadic and familial PPNAD, the latter as part of Carney complex.5
Recently, a single-nucleotide polymorphism (SNP)-based genome-wide association (GWA) study identified inactivating phosphodiesterase (PDE) 11A (PDE11A) sequencing defects in 7 out of 17 patients with iMAD.7 PDE11A encodes a dual-specificity PDE that degrades both cAMP and cGMP and is expressed in a number of endocrine tissues, including the adrenal cortex.7–11 Adrenocortical hyperplasia in the presence of a partially inactivated PDE is likely to be caused by high cellular cAMP levels in a manner analogous to endocrine tumor formation in McCune- Albright syndrome.7, 12
The GWA study identified a number of other chromosomal loci as potentially linked to the development of iMAD. Among them, the chromosomal locus 5q13 containing the gene for a cAMP-specific PDE, PDE8B, was the second most favored by all analyses, including loss-of-heterozygosity (LOH) and transmission-disequilibrium-testing (TDT) (7, supplemental data available at http://www.nature.com/ng/archive/suppinfo/index.html). PDE8B was also shown to have significantly higher expression in the adrenal gland compared to all other cAMP-specific PDEs, including PDE1A, PDE4A, PDE4B, PDE4C, PDE4D, PDE7A and PDE9A (7, supplemental data). We then identified a single germline PDE8B missense substitution (c.914A>C/H305P) in a patient with iMAD that we reported as a brief case report.13
In the present report, we expand this observation on the identified PDE8B mutation and this gene’s involvement in adrenal hyperplasias; we characterized further the mutation and identified a novel PDE8B isoform which is expressed in adrenocortical and other tissues, and, finally, examined PDE8B expression in the developing and adult mouse tissues.
Materials and Methods
Subjects and their samples
The institutional review boards of NICHD, NIH, approved the genetic investigation of patients with adrenocortical tumors under NICHD protocols 95-CH-0059 and 00-CH-0160 after the patients had given informed consent; 22 unrelated pediatric patients with isolated bilateral adrenocortical hyperplasia were included in this study, including the 17 patients described previously.7 The affected individuals ranged in age from 1 to 14, with an average age of 6.2 years. Patients who were diagnosed with CS by standard clinical criteria and testing underwent adrenalectomy. The controls included in this study have been described elsewhere.7, 14
DNA, RNA, protein extraction and sequence analysis
Blood and tissue samples were collected, as previously described.14 DNA, RNA and proteins were extracted from whole blood and cell cultures using standard procedures.
All the primes used for the PCR-amplification of the PDE8B coding regions and exon-intron junctions are listed in Table 1. After the amplification the PCR products were agarose gel purified (Minelute, Qiagen) and bi-directionally sequenced on 3130xl Genetic Analyzer (Applied Biosystems). Sequences were analyzed using Vector NTI 10 software (Invitrogen).
Table 1.
Primers used for PDE8B sequencing
| Exon# | Forward primer sequence (5′ – 3′) | Reverse primer sequence (5′ – 3′) |
|---|---|---|
| 1 | gaggaagatggcccaaaag | gctcccatcatctccacaaa |
| 2 | agtgcacacggtggcataat | gagccgagactgagcctcta |
| 3 | gctgggattacaggcatgag | ggagagagaaatctacaggaagga |
| 4 | tcctaatccacaagggcatc | aacaaacaaaacccccaaag |
| 5 | ctgctggagctttctctgct | gtcctggggcttaatttcct |
| 6 | caagcactttgaacaccttga | tgccattcattgcctgttta |
| 7 | ttgggagacatcagcattca | tcagtattctttgcacagcttga |
| 8 | cttcctacggggcacaca | ccagattcacttgagttccaaa |
| 9 | aggcattgggaaatgtaacg | gctcatactggcatttcaagc |
| 10 | atgtgtgggctctgtgtgaa | aaagatttgtcagaggaaccaaa |
| 11 | tggtgtatgtctttcatctgttca | tgccataaaggaagattcaagg |
| 12 | gcccggcctaatgtttattt | ccctccaaagagacgacaaa |
| 13 | ctcctgcctccaaagttcc | ctctcacagaacccgcttg |
| 14 | tcttttggattctgggcata | cagcctcatggaaagacaaa |
| 15 | atcccagttctccacgttct | tgcaattcttactctaactgtgctc |
| 16 | cttccagctagtcccatttga | caggggccatagtctctctg |
| 17 | cccctgtgtgtgtgaagcta | tggcagaaaggtccatgtc |
| 18 | cgcctctccacatctaggtc | gaactcactgaagaccaaatgagat |
| 19 | ctggggaaaatggaatagca | ttcatccccaaggaaaacaa |
| 20 | acaggtggtgacagggactt | aaggaaccagaagcctaccc |
| 21 | aggcttgagagtgggaaggt | ctgctggggatgcaaagaat |
| 22A | gatcccaaacttgtcccaga | CAGGATGACAGCAGAGCAAA* |
| 22B | GTTTGAGGCTTCCATCTGACA | AGCCATAGAGGCTGTGAAGC |
| 22C | CATCTCCCAGGATGGTGACT | ACTTGGTGTAGTGCCTCGTG |
| 22D | CTTCTGGCCGATGGTATGAC | ccctatcccctcccaaaat |
5′RACE
Human adrenal total RNA (Ambion) was subjected to RNA ligase-mediated 5′ RACE (RLMRACE) using the GeneRacer (Invitrogen) kit according to the manufacturer’s instructions. Briefly, 1 μg total RNA was reverse transcribed with Superscript III RT (Invitrogen), and cDNA was subjected to two successive rounds of PCR with Accuprime Taq polymerase (Invitrogen) using primer R3: CACTGGGGTGATCTTCACG-TGCTGTTGG and nested primer R2: CCTGCCACTCCTTTCCCTTCTTGATGCAT. The resulting amplimer was TA-subcloned and fully sequenced.
Generation of PDE8B c.914C>A substitution bearing constructs
Wild type PDE8B1 (Origene) was used to first generate the isoform that was identified in the adrenal gland. The endonucleases EcoRI and ApaI were used according to the manufacturer instructions (New England Biolabs). The first exon was excited from PDE8B1 and replaced with the newly identified exonic sequence from our RACE products. Further, c.914C>A substitution was introduced using overlapping PCR with the following primers:
F1: AGTGTTTACAGCATTAGATCACTGTC;
R1: CAGACAGAGCCTCCTTCATTCAGATAT;
F2: TATCTGAATGAAGGAGGCTC-TGTCTG;
R2: ATCTCTAGAACTCTGTCCAAGGCTTC.
Transfection experiments were performed as previously described.14 Cyclic AMP levels were measured cAMP-Glo Assay (Promega) as recommended by the manufacturer.
Mouse expression studies
Pde8B mice expression studies were performed in collaboration with Phylogeny (www.phylogenyinc.com). Tissues were obtained from e9.5, e15.5, p1, p10, and adult mice. Frozen tissues were cut into 8- to 10-micron sections, fixed for 60 minutes in cold acetone, air-dried, and then stored at room temperature. The antibody used in this study (rabbit polyclonal antibody to human PDE8B synthetic peptide) was purchased from Abcam (Cat. No. ab14621). This antibody has no cross-reactivity with PDE8A or any other PDE family members. Slides were washed in PBS and then incubated with antibody and revealed according to peroxidase technique. To detect non-specific immunoreactivity, normal rabbit serum was used as a control.
To perform IHC, the sections were hydrated in PBS (from Invitrogen 10X, pH 7.2), blocked in TBST (Tris-Buffered Saline with Tween 20, Dako Cat. No. S3006) for 5 minutes, peroxidase-blocked (0.03% H2O2 in diH2O) for 5 minutes, and rinsed (20 dips) in PBS. PDE8B antibody diluted 1:50 with antibody diluent (Dako Cat. No. S0809) was applied to the sections for 60 minutes at room temperature then rinsed with PBS for 5 minutes. Avidin/biotin blocking solution (Dako Cat. No. 0590) was applied for 20 minutes followed by a PBS rinse for 5 minutes. Goat anti-rabbit antibody (Vector BA-1000) was applied at a dilution of 1:200 for 20 minutes at room temperature followed by a rinse with PBS for 5 minutes. The detection reagent Vectastain Elite (Vector PK-6100) was applied for 30 minutes and DAB (Dako Cat. No. K3468) for 5 minutes at room temperature. This procedure produced brown immunostaining within cell cytoplasm or around cells. The sections were rinsed in diH2O, counterstained with hematoxylin, rinsed again in diH2O, dehydrated in a series of alcohols and xylene, and then coverslipped. All sections were counterstained with hematoxylin and photographed under brightfield illumination.
Results
PDE8B mutation analysis in patients with iMAD and CS
A total of 22 subjects with iMAD and CS were studied, of whom 18 were Caucasians, 2 African-Americans, one Hispanic and one South Asian (from Pakistan). All samples were negative for PRKAR1A, MYH8 and GNAS mutations (data not shown). This cohort included the 17 patients screened for the presence of PDE11A-inactivating mutations and has been described previously.7 The single base substitution (c.914A>T/H305P) that was reported elsewhere,13 was found in the linker region between the PAS (period, aryl-hydrocarbon receptor nuclear translocator (ARNT), and single minded) and the catalytic domain of PDE8B in a female patient with CS (CAR 559.03). As we reported elsewhere, the patient had presented in early childhood with obesity, hypercholesterolemia and growth abnormalities due to iMAD;13 she had inherited the mutation from her father (CAR 559.01), who was not known to have CS, but whose subsequent investigation revealed a phenotype consistent with mild iMAD: he had abnormal midnight cortisol levels,13 obesity, hypertension, and on computed tomography, mild BAH, more prominent on the left adrenal gland (Figure 1). The c.914A>T PDE8B mutation has not been found in 1030 unrelated control individuals from a variety of populations, including 285 controls from a mostly Latin-American-based population sample (Coriell Institute; http://www.coriell.org/) and 745 individuals enrolled in the New York Cancer Project (NYCP) study.14
Figure 1.
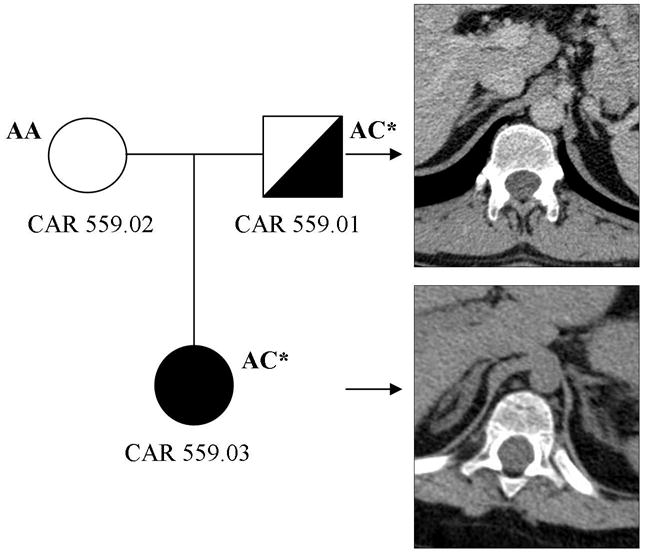
Computed tomography (CT) of the adrenal gland of our patient (CAR 559.03) and her father (CAR 559.01) who is also carrier of the H305P mutation in PDE8B.
In vitro studies of the effect of H305P mutation; novel PDE8B isoform
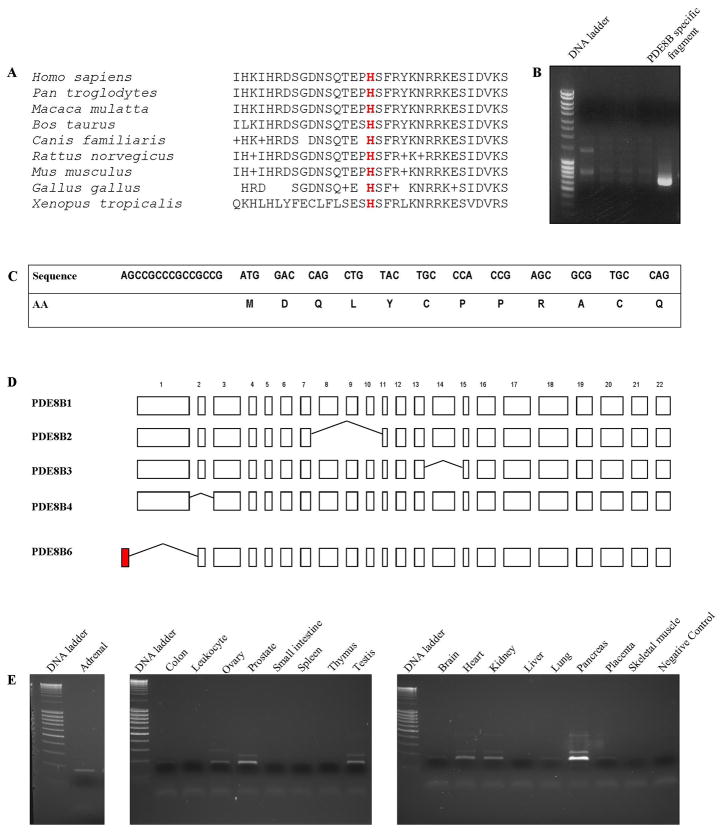
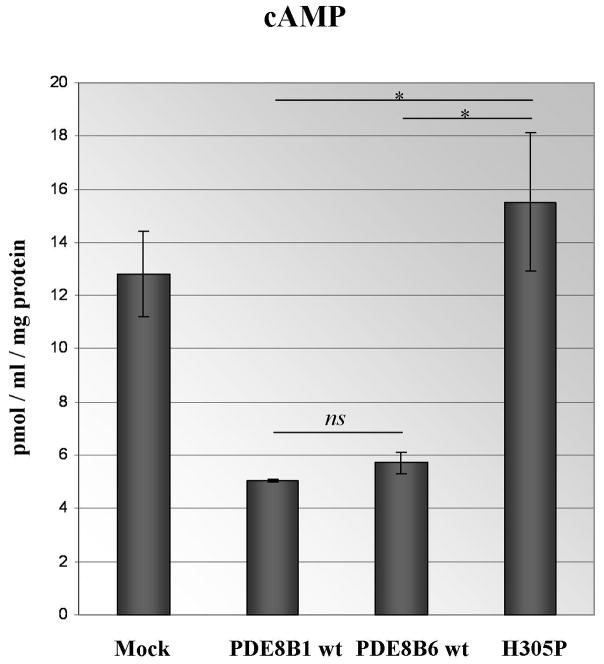
The H305P mutation affects an evolutionary conserved residue of the PDE8B protein (Figure 2A). To estimate the effect on the protein function, we performed in vitro studies on HEK293 cells: the c.914A>T substitution led to significantly higher cAMP level in cells transfected with the PDE8B bearing the substitution.13
Figure 2.
A) Conservation of His at position 305 of PDE across different species; B) Gel electrophoresis on the products generated by 5′ RACE; the adrenal specific fragment that was further used to identify the 5′ end of the novel isoform is shown; C) Nucleotide and amino acid sequence of the novel exon; D) PDE8B isoforms generated by alternative splicing; the newly identified exon is shown in red; E) expression of the novel isoform across panel of 17 normal human tissues.
During our attempts to amplify the whole open reading frame (ORF) of PDE8B from adrenal tissue we identified the presence of a shorter than the previously published isoform with different 5′ end. We performed 5′RACE and isolated a distinct, novel isoform (Figure 2B). The novel isoform includes one additional 5′ located exon of 53 bp that contains an in-frame initiation codon, accompanied by classical Kozak motif; the deduced protein sequence of 12 AA is presented at Figure 2C. This exon was located 30,473 bp 5′ of the known transcription start site and its upstream sequences displayed clear promoter and regulatory features. Similarly to the already known transcription initiation site, a CpG island was located between positions −245 and 454 of the newly identified initiator codon. In contrast to all the known PDE8B isoforms, the novel isoform skips the currently recognized exon 1, known as the longest exon of the gene (Figure 2D). Sequencing of the newly identified exon in our 22 patients did not reveal any alterations from the published genomic sequence (data not shown). Quantitative PCR showed highest expression of the novel isoform in the pancreas, followed by prostate, testis, heart, kidney, ovary and adrenal (Figure 2E). The ability of the novel isoform to degrade cAMP was similar to PDE8B1, as shown by our transfection experiments on HEK293 cells; this was compared with the wild-type, known ORF and the mutant c.914A>T form (Figure 3).
Figure 3.
In vitro experiments with PDE8B ORF construct; asterisk indicates significance of the observed difference between the wild type and mutant constructs (P<0.05).
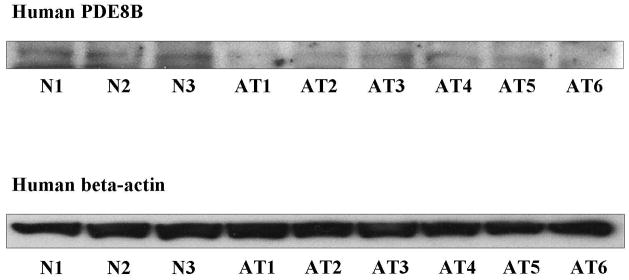
PDE8B expression in normal and a series of adrenocortical tumors
We assessed PDE8B expression in a panel of 3 normal and 6 adrenocortical tumor samples using Western blot analysis. These samples have been screened for CTNNB1, GNAS, PDE11A and PDE8B sequence defects and none was found (data not shown). All 6 samples contained lower PDE8B protein levels despite the absence of mutations in the coding sequence of PDE8B (Figure 4). Interestingly, quantitative PCR on the same adrenocortical samples did not show differential PDE8B expression between normal and tumor tissues (data not shown).
Figure 4.
Western blot analysis of PDE8B in 3 normal (N) and 6 tumor adrenocortical (AT) tissue samples from patients with no mutations in the coding sequence of PDE8B. Lower protein levels in the tumor samples compared to the normal tissues were observd. The protein expression is controlled by beta-actin.
Pde8b protein expression in mice
We studied Pde8B protein expression in embryonic and adult mice; a summary of Pde8B distribution at stages e15.5, p1, p10 and p77 is presented in Table 2. Pde8B was undetectable at stage e9.5 in the embryo, but occurs in e15.5 mouse tissues (Figure 5A). At this stage, Pde8B was found primarily in the embryonic adipose tissue primordium forming the future hibernation gland that is located in the space between the scapulae (Figure 5B). Little Pde8B was found in the e15.5 liver and heart ventricle (Figure 5, C and D respectively). Pde8B distribution formed islets around groups of liver cells and between the heart ventricle muscle fibers; this type of extracellular labeling was not seen in other regions.
Table 2.
IHC Distribution PDE8B
| Tissue | e15.5 (sex not determined) | p1 | p10 | P77 |
|---|---|---|---|---|
| Adipose Tissue Axillae (M) Adipose Tissue Axillae (F) |
+++++/− | +++++/− +++++/− |
+++/− +++/− |
ne ++/− |
| Adipose Tissue Scapulae (M) Adipose Tissue Scapulae (F) |
++/− | +++++/− +++++/− |
+++/− ++++/− |
++/− +++/− |
| Adrenal Gland (M) Adrenal Gland (F) |
ne | +/− +/− |
ne ne |
ne ne |
| Adipose around Blood Vessels (M) Adipose around Blood Vessels (F) |
−/− | ++/− +++/− |
+++/− +++/− |
++/− ++/− |
| Brain (M) Brain (F) |
+/− | +/− +/− |
+/+ +/+ |
ne ne |
| Stomach (M) Stomach (F) |
- | ++/− +++/− |
+/+ +/+ |
ne ne |
| Small intestine (M) Small intestine (F) |
−/− | +++/− +++/− |
++/− +++/− |
ne +++ |
| Heart, Ventricle (M) Heart, Ventricle (F) |
+/− | ++/− ++/− |
+/− +/− |
−/− +/− |
| Kidney (M) Kidney (F) |
++/− | ++/− +++/− |
ne ne |
ne ne |
| Liver (M) Liver (F) |
+++/− | ++/−* ++/−* |
++/− ++/− |
+++/− +++/− |
| Lung, Bronchus (M) Lung, Bronchus (F) |
+/− | +/− +/− |
−/− +/− |
−/− −/− |
| Skeletal Muscles (M) Skeletal Muscles (F) |
+/− | −/− +/− |
+/− +/− |
−/− −/− |
| Pancreatic Islets (M) Pancreatic Islets (F) |
ne | −/− −/− |
−/− −/− |
ne ++/− |
| Spleen (M) Spleen (F) |
ne | +/− ++/− |
ne ne |
ne +/− |
| Testis | +/− | ± | +/− | −/− |
Score: ne not examined, - undetectable, + weak, +++ medium, +++++ strong immunoreaction presented as PDE8B/IgG
Relates to PDE8B localization in embryonic and newborn hepatic tissue in form of islets.
Figure 5.
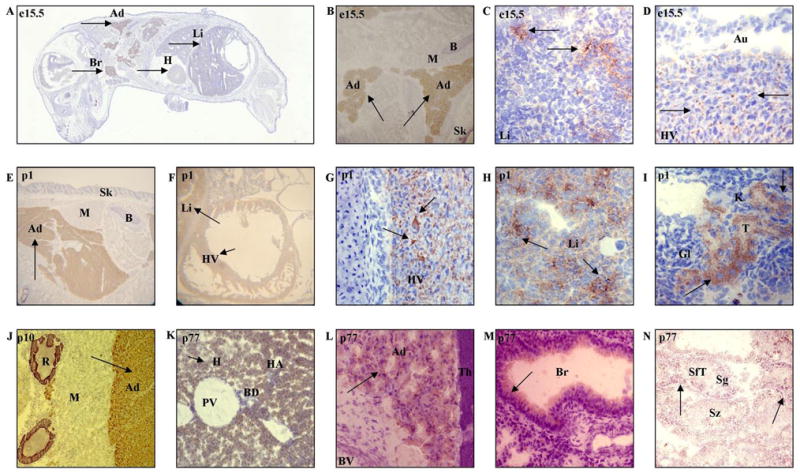
A) Brown PDE8B immunostaining (arrows) in the mouse embryo on day 15.5, seen in a whole body section (Br – brain; H – heart; Li – liver; Ad – adipose tissue, upper arrow indicates the hibernating gland); B) Brown PDE8B immunostaining (arrows) in the adipose tissue; upper arrow indicates the hibernating gland (B – bone; M – skeletal muscle; Sk – skin); C) Immunostaining in embryonic liver showing brown labeling forming the islets in between the liver cells (arrows); D) PDE8B in the heart ventricle (HV) forming small spots between muscle fibers (arrows). The atria (Au) do not show any labeling; E) Brown PDE8B immunostaining in the adipose tissue of the hibernating gland of a newborn mouse, arrow indicates the hibernating gland; F) PDE8B immunostaining (arrows) in the heart ventricle and liver of a newborn mouse; G) The wall of the heart ventricle; PDE8B labeling (arrow) forms a network around the muscle fibers; H) Liver; PDE8B labeling (arrow) seen as a heterogeneous distribution throughout the liver parenchyma; I) Kidney tubules, most likely distal tubules, but not glomeruli, showing PDE8B labeling (arrow); Gl – glomerulus; K – kidney; T – tubules; J) Intense PDE8B immunostaining (arrow) in the adipose tissue; R- rib; K) PDE8B immunostaining (arrow) in the liver perenchyma; blood vessels, including branches of the portal vein and hepatic artery, and bile duct remain unlabeled (BD – bile duct; H – hepatocyte; HA – hepatic artery; PV – portal vein branch); L) PDE8B immunostaining (arrow) in the brown adipose tissue surrounding the thymus; thymus and blood vessels remain unstained (BV – blood vessel; Th – thymus); M) Light PDE8B immunostaining (arrow) in the epithelial cells of the bronchioles (Br – bronchiole); N) PDE8B immunostaining (arrow) in the seminiferous tubules of the adult mouse testis showing spermatogonia to be slightly labeled, spermatozoa seem to be unstained (SfT – seminiferous tubule; Sg – spermatogonia; Sz – spermatozoa).
Relative to the embryonic stage, a much greater amount of Pde8B was detected in the p1 mouse. Pde8B was labeled in the adipose tissue, especially in the hibernating gland situated between the scapulae (Figure 5E). Also evident was the presence of Pde8B in the heart and liver, stomach mucosa, and in the convoluted tubules of the kidney (Figure 5, F–I). The way the labeling is distributed in these organs (around, rather than within the cells) suggests the presence of Pde8B on cell surfaces. Pde8B was detected on the surface of cardiomyocytes, the epithelial cell apical portion of the stomach, and the brush border surface of the presumptive kidney distal tubules. Alternatively, this pattern of labeling may be attributable to the presence of Pde8B in the interstitial space between cells.
By postnatal day 10, a decrease of Pde8B was observed in many tissues, including the adipose tissue (Figure 5J). Taking into account mass and distribution, however, the adipose tissue still can be considered to contain more Pde8B than any other tissue. Lower Pde8B levels were detected in the heart ventricle tissue.
In the liver, the pattern of Pde8B changed from extracellular in the embryo to intracellular in the adult (Figure 5K). Barely detectable Pde8B was seen in the epithelium of the apical portion of the lung bronchioles (Figure 5L). The pancreas exhibited faint labeling in the endocrine pancreatic islets and even less in the exocrine pancreatic acini (Figure 5M). There was detectable Pde8B in the vas deferens (data not shown). In the male reproductive organs, detectable Pde8B occurred in the seminiferous tubules of the testis, consistent with the distribution of the spermatogonia, but not spermatozoa (Figure 5N).
Discussion
The reported P305H substitution in PDE8B was present in family members with very mild to a clinically significant phenotype in terms of the severity of CS.13 We have reported before similar differences in the phenotype of iMAD:7, 14 it appears that PDE-sequence defects are acting as predisposing factors to the development of nodularity and they are not causative per se. This is consistent with the relatively high (for a rare disease) frequency of PDE11A sequencing defects in the general population,14 although the P305H PDE8B mutation was not found in more than 2000 control chromosomes.
Although the cause of all forms of BAH studied to date seems to be linked to increased cAMP signaling, the histopathological changes in the adrenal glands of patients with the various mutations or functional abnormalities of this pathway differ significantly and overlap only partially.1 PRKAR1A mutations are associated with PPNAD,5 whereas PDE11A and possibly PDE8B mutations seem to predispose to a variety of lesions from isolated (without any other associated tumors) PPNAD to non-pigmented iMAD and other forms of BAH;7, 13, 14 GNAS mutations are associated with the mostly macronodular and clearly nonpigmented form of BAH that one sees in McCune-Albright syndrome.4 The identification of more mutations in these and other PDEs will shed more light to this apparent lack of tight genotype-phenotype correlation which is somewhat unexpected since tissues affected by PPNAD are almost identical histologically.
PDE8B encodes cAMP specific phosphodiesterase with the highest affinity to cAMP among all the known PDEs.15, 16 Although involvement in some environment signals transduction has been suggested by the presence of REC (receiver) and PAS domains, no ligand has yet been identified, and PDE8B’s function remains largely unknown. As in other PDEs, the PDE8B locus is quite complex and encodes multiple isoforms, arising mainly from alternative splicing and displaying tissue specific expression.9, 15–18 We describe here a novel isoform that is highly expressed in endocrine tissues, including the adrenal gland, where it shows the highest expression level compared to other cAMP degrading PDEs. The high adrenal-specific expression levels, its significant affinity to cAMP, and the overall importance of cAMP signaling for adrenocortical tumorigenesis, suggest that PDE8B could be a candidate for further investigations of its role in adrenal (and possible other endocrine) tumors.
We detected decreased PDE8B protein levels in adrenocortical tumor samples that are negative for germline mutations in the gene. The real-time PCR experiments did not show significant alteration of the PDE8B mRNA levels in the same tumors, thus suggesting posttranscriptional events involved in the regulation of expression. The human microRNA databases list four miRNAs that are predicted to target PDE8B: hsa-miR-412, hsa-miRNA-770-5p, hsa-miRNA-520d-5p and hsa-miRNA-493 (www.microRNA.org). All four of them recognize 5′ located regions of this gene that do not overlap with the identified in our study substitution. Ongoing experiments assess their ability to silence PDE8B expression in vitro.
Mouse studies showed that predominant expression of Pde8B in the adipose tissue was detected in both embryonic and adult mouse. At embryonic stage e15.5, Pde8B was labeled primarily in the adipose tissue primordium forming the future hibernation gland. Late in gestation, the hibernation gland accumulates fat to serve as an immediate energy reserve at birth. While the hibernation gland does not yet contain fat at stage e15.5, the presence of Pde8B may indicate that it plays a role in processes related to fat intake and storage. The adipose remained to be the tissue with the highest Pde8B expression in newborn (p1) and adult mice (p10 and p77), although slight decrease in the protein levels have been observed after birth.
Besides its role in the energy intake and storage, the adipose tissue has been recently recognized to have an important endocrine function, and leptin has been the first characterized hormone that is secreted by the adipocytes.19 Several studies from the last decade have contributed to the elucidation of the endocrinology of adipose tissue and its secretion of adipokines.20, 21 The early and stable Pde8B expression in mouse adipocytes is in need of further investigation. A recent study explores the stimulatory effect of adipose tissue-derived factors on adrenal steroidogenesis and secretory function.22 An important crosstalk between adipose tissue and adrenocortical function is supported by the study of Lamounier-Zepter et al. on A-ZIP/F1 transgenic mice.23 This and previous studies have suggested that leptin deficiency may be one of the causes for increased adrenal glucocorticoid production.24 Since leptin signaling has been suggested to involve another cAMP specific phosphodiesterase, PDE3B, a similar role could be speculated for Pde8B.25
Apart from the adipose tissue, an early Pde8B labeling was detected in the liver and the heart ventricle. The type of labeling of Pde8B in these two tissues was suggestive for extracellular or membranous localization of the protein. The same localization of Pde8B was observed later at p1 stage in the newborn mouse liver and heart where, however, significantly higher levels of the protein were detected. In addition, at p1 stage Pde8B was detected on the surface of the epithelial cell apical portion of the stomach, and the brush border surface of the presumptive kidney distal tubules. At p10 stage, a slight decrease in the Pde8B expression was detected in almost all of the tissues. Considering ontogeny, differences in the subcellular Pde8B localization were noted, especially the liver. In the embryonic and newborn mouse liver, Pde8B seemed to exhibit a rather membranous (or extracellular) localization pattern, whereas in the adult mouse liver, Pde8B was localized in the cytoplasm. This observation requires further specifically designed subcellular localization studies including in situ hybridization to define the cells which are expressing the gene during the time when extracellular localization is observed.
In conclusion, we describe here the expression studies of a mutation of PDE8B causing iMAD and the identification of a novel expression isoform of this gene that is expressed in the adrenal gland. We also present expression studies for this gene in normal mouse tissues; these studies suggest a wider role of PDE8B in endocrine function.
Acknowledgments
It was supported by US NIH intramural project Z01-HD-000642-04 to C.A.S. and, in part, by Groupement d’Intérêt Scientifique-Institut National de la Santé et de la Recherche Médicale Institut des Maladies Rares and the Plan Hospitalier de Recherche Clinique (AOM 02068) to the Comete Network.
Footnotes
Conflict of Interest Statement
The authors declare that they have no competing financial interests.
References
- 1.Stratakis CA, Boikos SA. Genetics of adrenal tumors associated with Cushing’s syndrome: a new classification for bilateral adrenocortical hyperplasias. Nat Clin Pract Endocrinol Metab. 2007;3:748–757. doi: 10.1038/ncpendmet0648. [DOI] [PubMed] [Google Scholar]
- 2.Gunther DF, Bourdeau I, Matyakhina L, et al. Cyclical Cushing syndrome presenting in infancy: an early form of primary pigmented nodular adrenocortical disease, or a new entity? J Clin Endocrinol Metab. 2004;89:3173–3182. doi: 10.1210/jc.2003-032247. [DOI] [PubMed] [Google Scholar]
- 3.Stratakis CA. Adrenocortical tumors, primary pigmented adrenocortical disease (PPNAD)/Carney complex, and other bilateral hyperplasias: the NIH studies. Horm Metab Res. 2007;39:467–473. doi: 10.1055/s-2007-981477. [DOI] [PubMed] [Google Scholar]
- 4.Weinstein LS, Yu S, Warner DR, Liu J. Endocrine manifestations o stimulatory G protein -subunit mutations and the role of genomic imprinting. Endocr Rev. 2001;22:675–705. doi: 10.1210/edrv.22.5.0439. [DOI] [PubMed] [Google Scholar]
- 5.Kirschner LS, Carney JA, Pack SD, et al. Mutations of the gene encoding the protein kinase A type I-alpha regulatory subunit in patients with the Carney complex. Nat Genet. 2000;26:89–92. doi: 10.1038/79238. [DOI] [PubMed] [Google Scholar]
- 6.Bourdeau I, Stratakis CA. Cyclic AMP-dependent signaling aberrations in macronodular adrenal disease. Ann N Y Acad Sci. 2002;968:240–255. doi: 10.1111/j.1749-6632.2002.tb04339.x. [DOI] [PubMed] [Google Scholar]
- 7.Horvath A, Boikos S, Giatzakis C, et al. A genome-wide scan identifies mutations in the gene encoding phosphodiesterase 11A4 (PDE11A) in individuals with adrenocortical hyperplasia. Nat Genet. 2006;38:794–800. doi: 10.1038/ng1809. [DOI] [PubMed] [Google Scholar]
- 8.Fawcett L, Baxendale R, Stacey P, et al. Molecular cloning and characterization of a distinct human phosphodiesterase gene family: PDE11A. Proc Natl Acad Sci USA. 2000;97:3702–3707. doi: 10.1073/pnas.050585197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yuasa K, Kotera J, Fujishige K, Michibata H, Sasaki T, Omori K. Isolation and characterization of two novel phosphodiesterase PDE11A variants showing unique structure and tissue-specific expression. J Biol Chem. 2000;275:31469–31479. doi: 10.1074/jbc.M003041200. [DOI] [PubMed] [Google Scholar]
- 10.Hetman JM, Robas N, Baxendale R, et al. Cloning and characterization of two splice variants of human phosphodiesterase 11A. Proc Natl Acad Sci USA. 2000;97:12891–12895. doi: 10.1073/pnas.200355397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loughney K, Taylor J, Florio VA. 3′,5′-cyclic nucleotide phosphodiesterase 11A: localization in human tissues. Int J Impot Res. 2005;17:320–325. doi: 10.1038/sj.ijir.3901317. [DOI] [PubMed] [Google Scholar]
- 12.Diaz A, Danon M, Crawford J. McCune-Albright syndrome and disorders due to activating mutations of GNAS1. J Pediatr Endocrinol Metab. 2007;20:853–880. doi: 10.1515/jpem.2007.20.8.853. [DOI] [PubMed] [Google Scholar]
- 13.Horvath A, Mericq V, Stratakis CA. Mutation in PDE8B, a cAMP-specific Phosphodiesterase in Adrenal Hyperplasia. N Engl J Med. 2008 doi: 10.1056/NEJMc0706182. in press. [DOI] [PubMed] [Google Scholar]
- 14.Horvath A, Giatzakis C, Robinson-White A, et al. Adrenal hyperplasia and adenomas are associated with inhibition of phosphodiesterase 11A in carriers of PDE11A sequence variants that are frequent in the population. Cancer Res. 2006;66:11571–11575. doi: 10.1158/0008-5472.CAN-06-2914. [DOI] [PubMed] [Google Scholar]
- 15.Soderling SH, Bayuga SJ, Beavo JA. Cloning and characterization of a cAMP-specific cyclic nucleotide phosphodiesterase. Proc Natl Acad Sci USA. 1998;95:8991–8996. doi: 10.1073/pnas.95.15.8991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hayashi M, Shimada Y, Nishimura Y, Hama T, Tanaka T. Genomic organization, chromosomal localization, and alternative splicing of the human phosphodiesterase 8B gene. Biochem Biophys Res Commun. 2002;297:1253–1258. doi: 10.1016/s0006-291x(02)02371-9. [DOI] [PubMed] [Google Scholar]
- 17.Gamanuma M, Yuasa K, Sasaki T, Sakurai N, Kotera J, Omori K. Comparison of enzymatic characterization and gene organization of cyclic nucleotide phosphodiesterase 8 family in humans. Cell Signal. 2003;15:565–574. doi: 10.1016/s0898-6568(02)00146-8. [DOI] [PubMed] [Google Scholar]
- 18.Conti M, Beavo J. Biochemistry and physiology of cyclic nucleotide phosphodiesterases: essential components in cyclic nucleotide signaling. Annu Rev Biochem. 2007;76:481–511. doi: 10.1146/annurev.biochem.76.060305.150444. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 20.Kim S, Moustaid-Moussa N. Secretory, endocrine and autocrine/paracrine function of the adipocyte. J Nutr. 2000;130:3110S–3115S. doi: 10.1093/jn/130.12.3110S. [DOI] [PubMed] [Google Scholar]
- 21.Trayhurn P, Beattie JH. Physiological role of adipose tissue: white adipose tissue as an endocrine and secretory organ. Proc Nutr Soc. 2001;60:329–339. doi: 10.1079/pns200194. [DOI] [PubMed] [Google Scholar]
- 22.Ehrhart-Bornstein M, Lamounier-Zepter V, Schraven A, et al. Human adipocytes secrete mineralocorticoid-releasing factors. Proc Natl Acad Sci USA. 2003;100:14211–14216. doi: 10.1073/pnas.2336140100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lamounier-Zepter V, Bornstein SR, Kunes J, et al. Adrenocortical changes and arterial hypertension in lipoatrophic A-ZIP/F-1 mice. Mol Cell Endocrinol. 2008;280:39–46. doi: 10.1016/j.mce.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 24.Haluzik M, Dietz KR, Kim JK, et al. Adrenalectomy improves diabetes in A-ZIP/F-1 lipoatrophic mice by increasing both liver and muscle insulin sensitivity. Diabetes. 2002;51:2113–2118. doi: 10.2337/diabetes.51.7.2113. [DOI] [PubMed] [Google Scholar]
- 25.Hsu HT, Chang YC, Chiu YN, Liu CL, Chang KJ, Guo IC. Leptin interferes with adrenocorticotropin/3′,5′-cyclic adenosine monophosphate (cAMP) signaling, possibly through a Janus kinase 2-phosphatidylinositol 3-kinase/Akt-phosphodiesterase 3-cAMP pathway, to down-regulate cholesterol side-chain cleavage cytochrome P450 enzyme in human adrenocortical NCI-H295 cell line. J Clin Endocrinol Metab. 2006;91:2761–2769. doi: 10.1210/jc.2005-2383. [DOI] [PubMed] [Google Scholar]