Abstract
Background
The production of peroxide and superoxide is an inevitable consequence of aerobic metabolism, and while these particular 'reactive oxygen species' (ROSs) can exhibit a number of biological effects, they are not of themselves excessively reactive and thus they are not especially damaging at physiological concentrations. However, their reactions with poorly liganded iron species can lead to the catalytic production of the very reactive and dangerous hydroxyl radical, which is exceptionally damaging, and a major cause of chronic inflammation.
Review
We review the considerable and wide-ranging evidence for the involvement of this combination of (su)peroxide and poorly liganded iron in a large number of physiological and indeed pathological processes and inflammatory disorders, especially those involving the progressive degradation of cellular and organismal performance. These diseases share a great many similarities and thus might be considered to have a common cause (i.e. iron-catalysed free radical and especially hydroxyl radical generation).
The studies reviewed include those focused on a series of cardiovascular, metabolic and neurological diseases, where iron can be found at the sites of plaques and lesions, as well as studies showing the significance of iron to aging and longevity. The effective chelation of iron by natural or synthetic ligands is thus of major physiological (and potentially therapeutic) importance. As systems properties, we need to recognise that physiological observables have multiple molecular causes, and studying them in isolation leads to inconsistent patterns of apparent causality when it is the simultaneous combination of multiple factors that is responsible.
This explains, for instance, the decidedly mixed effects of antioxidants that have been observed, since in some circumstances (especially the presence of poorly liganded iron) molecules that are nominally antioxidants can actually act as pro-oxidants. The reduction of redox stress thus requires suitable levels of both antioxidants and effective iron chelators. Some polyphenolic antioxidants may serve both roles.
Understanding the exact speciation and liganding of iron in all its states is thus crucial to separating its various pro- and anti-inflammatory activities. Redox stress, innate immunity and pro- (and some anti-)inflammatory cytokines are linked in particular via signalling pathways involving NF-kappaB and p38, with the oxidative roles of iron here seemingly involved upstream of the IkappaB kinase (IKK) reaction. In a number of cases it is possible to identify mechanisms by which ROSs and poorly liganded iron act synergistically and autocatalytically, leading to 'runaway' reactions that are hard to control unless one tackles multiple sites of action simultaneously. Some molecules such as statins and erythropoietin, not traditionally associated with anti-inflammatory activity, do indeed have 'pleiotropic' anti-inflammatory effects that may be of benefit here.
Conclusion
Overall we argue, by synthesising a widely dispersed literature, that the role of poorly liganded iron has been rather underappreciated in the past, and that in combination with peroxide and superoxide its activity underpins the behaviour of a great many physiological processes that degrade over time. Understanding these requires an integrative, systems-level approach that may lead to novel therapeutic targets.
Background and preamble
The 'balkanisation' of the literature is in part due to the amount of it (some 25,000 journals with presently 2.5 million peer-reviewed papers per year, i.e. ~5 per minute [1]), with a number http://www.nlm.nih.gov/bsd/medline_cit_counts_yr_pub.html increasing by something approaching 2 per minute at PubMed/Medline alone. In addition, the disconnect between the papers in the literature (usually as pdf files) and the metadata describing them (author, journal, year, pages, etc) is acute and badly needs filling [2]. Without solving this problem, and without automation of the processes of reading, interpreting and exploiting this literature and its metadata in a digital format, we cannot make use of the existing tools for text mining and natural language processing (e.g. [3-5]), for joining disparate concepts [6], for literature-based discovery (e.g. [7-11], and for studies of bibliometrics [12,13], literature dynamics [14], knowledge domains [15], detecting republication [16] and so on. Until we recognise these possibilities we are unlikely to seek to realise them.
The present article (and see [17] for a preprint) serves to show some of the benefits than can accrue from a more overarching view of the otherwise highly disparate literature in a particular domain (see also [18]), but was done 'the hard way', i.e. with a few bibliographic and bibliometric tools but without the kind of automation implied above. For the record, the main tools used (see a review in [2]) were Web of Knowledge and Scopus for literature and citation searching, supplemented by Google Scholar. Some use was also made of ARROWSMITH [6,19,20] and GOPubMed [21], as well as various workflows in the Taverna environment [22-26], including the BioAID_DiseaseDiscovery workflow http://www.myexperiment.org/workflows/72 written by Marco Roos. Citations and attendant metadata were stored in Endnote (latterly version X).
Introduction
Even under 'normal' conditions, as well as during ischaemia when tissue oxygenation levels are low, the redox poise of the mitochondrial respiratory chain is such that the normally complete four-electron reduction of dioxygen to water is also accompanied by the production, at considerable rates (ca 1–4% of O2 reduced), of partially reduced forms of dioxygen such as hydrogen peroxide and superoxide (e.g. [27-45]). These 1- and 2-electron reductions of O2 are necessarily exacerbated when the redox poise of the b-type cytochromes is low, for instance when substrate supplies are in excess or when the terminal electron acceptor O2 is abnormally low due to hypoxia or ischaemia. Various other oxygenases, oxidases and peroxidases can also lead directly to the production of such 'reduced' forms of dioxygen in vivo (e.g. [46-48]), with H2O2 from xanthine oxidase being especially implicated in ischaemia/reperfusion injury (e.g. [47,49-54]). These molecules (peroxide and superoxide) can cause or contribute to various kinds of oxidative stress. However, this is mainly not in fact because they can react directly with tissue components themselves, since they are comparatively non-toxic, cells have well-known means of dealing with them [55], and they are even used in cellular signalling (e.g. [56-60]). Much more importantly, it is because they can react with other particular species to create far more reactive and damaging products such as hydroxyl radicals, with all these agents nevertheless being known collectively (and indiscriminately) as reactive oxygen species (ROSs). Possibly the commonest means by which such much more damaging species, in particular the hydroxyl radical, are created is by reaction with unliganded or incompletely liganded iron ions [61-63]. The themes of this review are thus (i) that it is this combination of poorly liganded iron species, coupled to the natural production of ROSs, that is especially damaging, (ii) that the role of iron has received far less attention than has the general concept of ROSs, albeit the large literature that we review, and (iii) that this basic combination underpins a great many (and often similar) physiological changes leading to a variety of disease manifestations, and in particular those where the development of the disease is manifestly progressive and degenerative.
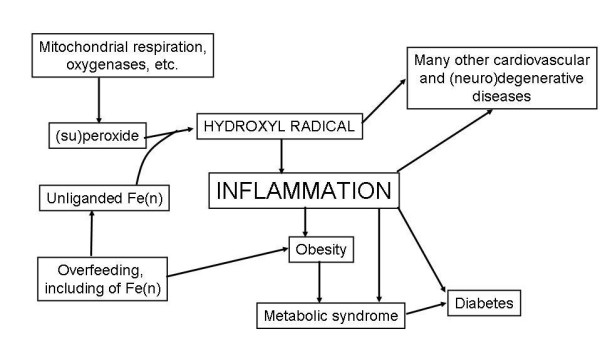
An overview of the structure of the review is given in Fig 1, in the form of a 'mind map' [64]. The main literature review for this meta-analysis was completed on June 30th, 2008, with some updates being added following the refereeing process.
Figure 1.
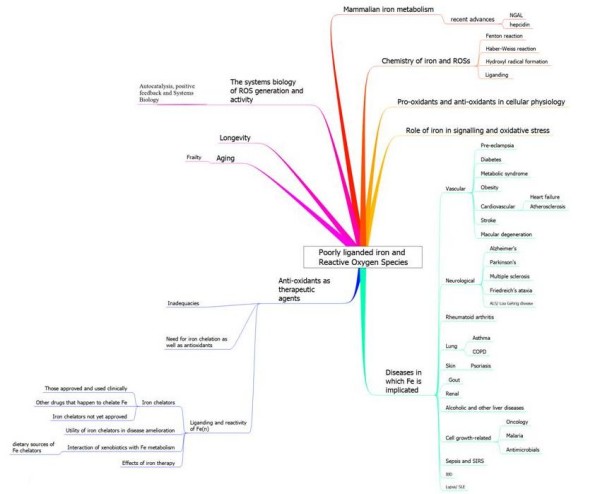
An overview of this article, set out in the form of a 'mind map' [64].
Some relevant chemistry of iron and reduced forms of oxygen
While superoxide and peroxide are the proximate forms of incomplete O2 reduction in biology, a reaction catalysed by the enzyme superoxide dismutase [65] serves to equilibrate superoxide and peroxide:
| 2O2•- + 2H+ → H2O2 + O2 | (1) |
Arguably the most important reaction of hydrogen peroxide with (free or poorly liganded) Fe(II) is the Fenton reaction [66], leading to the very reactive and damaging hydroxyl radical (OH•)
| Fe(II) + H2O2 → Fe(III) + OH- + OH• | (2) |
Superoxide can also react with ferric iron in the Haber-Weiss reaction [67] to produce Fe(II) again, thereby effecting redox cycling:
| O2•- + Fe(III) → O2 + Fe(II) | (3) |
Ascorbate can replace O2•- within the cell for reducing the Fe(III) to Fe(II) [68]. Further reactions, that are not the real focus here, follow from the ability of hydroxyl radicals and indeed Fe(n) directly to interact with many biological macro- and small molecules, especially including DNA, proteins and unsaturated lipids. Thus [69-73], Fe(II) and certain Fe(II) chelates react with lipid hydroperoxides (ROOH), as they do with hydrogen peroxide, splitting the O--O bond. This gives RO•, an alkoxyl radical, which can also abstract H• from polyunsaturated fatty acids and from hydroperoxides. The resulting peroxyl radicals ROO• can continue propagation of lipid peroxidation. Oxidative stress also leads to considerable DNA damage [74-76] and to the polymerisation and denaturation of proteins [77-79] and proteolipids that can together form insoluble structures typically known as lipofucsin (see e.g. [80,81]) or indeed plaques. Such plaques can also entrap the catalysts of their formation, and thereby point them up. Some of the evidence for these is described below. Many small molecule metabolic markers for this kind of oxidative stress induced by the hydroxyl radical and other 'reactive oxygen species' (ROSs) are known [43,82-89], and include 8-oxo-guanine [90-94], 8-hydroxy guanine [95], 8-hydroxy-2'-deoxy-guanosine [96,97], 8-oxo-GTP [98], 4-hydroxy-2-hexenal [99], 4-hydroxy-nonenal [100], 4-hydroperoxy-2-nonenal, various isoprostanes [101-107], 7-keto-cholesterol [108], many other cholesterol derivatives [109], malondialdehyde [110], neopterin [111], nitrotyrosine [112-115] and thymidine glycol [116,117]. Note that the trivial names in common use for this kind of metabolite are not helpful and may even be ambiguous or misleading, and it is desirable (e.g. [118]) to refer to such molecules using terminology that relates them either to molecules identified in persistent curated datbases [119] such as ChEBI [120] or KEGG [121], or better to describe them via database-independent encodings such as SMILES [122] or InChI [123-128] strings. (There are other oxidative markers that may be less direct, such as the ratio of 6-keto-prostaglandin F1α to thromboxane B2 [129], but these are not our focus here.)
Overall, it is in fact well established that the interactions between 'iron' sensu lato and partly reduced forms of oxygen can lead to the production of the very damaging hydroxyl radical (e.g. [43,130-139]), and that this radical in particular probably underpins or mediates many higher-level manifestations of tissue damage, disease, organ failure and ultimately death[36,137,140-143]. While the role of ROSs generally in these processes has been widely discussed, the general recognition of the importance of inadequately liganded iron in each of them has perhaps been less than fully appreciated. One of our tasks here will therefore be to stress this role of 'iron', and to assess the various means of chelating 'iron' such that it does not in fact do this. (Throughout we use 'iron' to refer to forms of Fe(n, n > 0) with unspecified ligands, though we absolutely stress that it is the exact speciation and liganding that determines the reactivity of 'iron' in catalysing reactions such as that of hydroxyl radical formation, and indeed its bioavailability generally – inadequate liganding of iron in the required forms can be a cause of anaemia even if the total amount of 'iron' is plentiful.)
For completeness we note the reactions catalysed by superoxide dismutase
| 2O2•- + 2H+ → O2 + H2O2 | (4) |
and by catalase
| H2O2 → H2O + 1/2 O2 | (5) |
These together, were their activity in the relevant locations sufficiently great, might serve to remove (su)peroxide from cells completely.
In addition to reactive oxygen species there are ions such as the perferryl ion (Fe-O) [144] and reactive nitrogen species [60,145-147]. These latter are mainly formed from the natural radical NO, an important inflammatory mediator [148], with peroxynitrite production (from the reaction of NO and superoxide) [46,149-154] leading to nitrotyrosine [112], or nitro-fatty acid [155,156] or protein cystein nitrosylation [157,158] being a common means of their detection downstream. Other toxic products of the reactions of NO include NO2, N2O3, and S-nitrosothiols [159], and the sequelae of some of these may also involve iron [160].
Overall, we recognise that these kinds of inflammatory, oxidative stress-related reactions are accumulative and somewhat irreversible [161], that they are consequently age-related, and (see [162-165] and later), and that most diseases and causes of mortality that are prevalent in the developed world are in this sense largely manifestations of this kind of aging.
Ligands and siderophores
As well as the reactions described above, ferrous ions will react with oxygen under aerobic conditions to produce ferric ions, and in natural environments there is little to stop this. Consequently, and because these reflect fundamental physicochemical properties of such ions, the problems of both solubility and toxicity were faced by bacteria (and indeed fungi [166-169]) long ago in evolution, and were solved by their creation and excretion of (mainly ferric-)iron chelators known as siderophores [170-189] (and for haemophores see [190]). These typically have extremely tight binding constants (Kf > 1030 [191]) and can solubilise and sequester iron such that it can be internalised via suitable transporter molecules within the bacterial plasma membrane [192]. Bacterial and fungal siderophores usually form hexadentate octahedral complexes with ferric iron and typically employ hydroxamates, α-hydroxycarboxylates and catechols as extremely effective Fe3+ ligands [182]. Since bacterial growth requires iron, it is unsurprising that siderophores are effectively virulence factors (e.g. [174,193-196]). While upwards of 500 microbial siderophores have been identified [182], with new ones still appearing (some via genomic analyses, e.g. [197]), and with the most common one in medical use, desferrioxamine or DFO, being such a bacterial product (see below), it is an astonishing fact that no human siderophore has been chemically identified, even though such activities were detected nearly 30 years ago [198,199] (see also [200-205]). As noted by Kaplan [206], "a discovery that mammals produce siderophores would lead to an epochal change in the paradigm of mammalian iron homeostasis." To this end, some recent events have begun to change matters, and our overall knowledge of the regulation of iron metabolism, considerably.
Mammalian iron metabolism
The total body iron in an adult male is 3000 to 4000 mg and the daily iron requirement for erythropoiesis, the major 'sink', is about 20 mg [207]. However, the loss of iron in a typical adult male is very small [208,209] and can be met by absorbing just 1 – 2 mg of iron per day [210,211]. The careful conservation and recycling of iron – mainly from degrading erythrocytes – is in fact essential because typical human diets contain just enough iron to replace the small losses, although when dietary iron is more abundant, absorption must be (and is) attenuated since higher levels than necessary lead to iron overload and many distressing sequelae contingent on the radical production described above.
A variety of aspects of mammalian iron metabolism have been reviewed in detail elsewhere (e.g. [134,139,195,212-241]), including a series on 'iron imports' [242-248], and for our present purposes (Fig 2) mainly involves the intestinal (mainly duodenal) uptake of Fe(II) (produced from Fe(III) using a luminal ferrireductase) via a divalent metal ion transporter DMT1/DCT1/NRAMP [249,250] and its subsequent binding as Fe(III) to transferrin (Tf). The intestinal uptake of haem (heme) occurs via the heme carrier protein-1 (HCP1) [251] and it is thereby internalized, while the iron in heme is liberated by heme oxygenase-1 (HO1) [252-254]. Haem is synthesised in many tissues, especially liver and erythroid cells [255]. Vesicular routes of intestinal transfer may also occur [256,257]. Low MW cytoplasmic chelators such as citrate can bind iron fairly weakly and thereby contribute to a labile iron pool (LIP) in the cytoplasm and especially the lysosomes and mitochondria (see [258-262]), while ferritin [263] too can bind cytoplasmic iron (via a chaperone [264]) and is seen as a good overall marker of iron status [265-267]. Iron(II) is subsequently exported through the basolateral membrane of the enterocyte by ferroportin-1 (FPN1) [268-270]. Ferroportin may also contribute to uptake in enterocytes [271]. Fe(III) may then be produced by hephaestin (Hp) [272] before it is bound by transferrin (Tf), which is the main but not sole means of binding Fe(III) when it is transported through the circulation, with major iron storage taking place in the liver. Similar processes occur in the peripheral tissues, with significant transfer of iron from transferrin occurring via the transferrin receptor [273].
Figure 2.
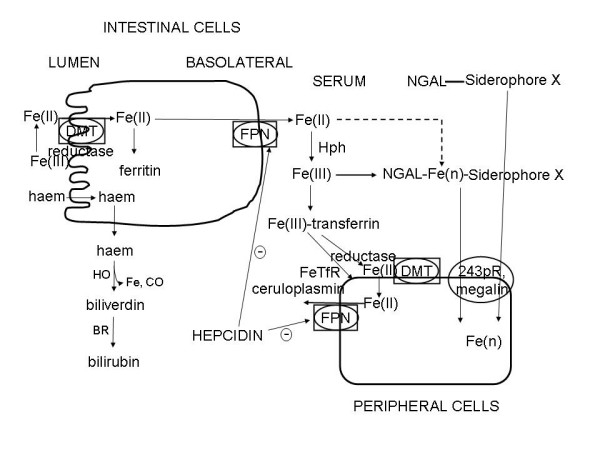
Schematic overview of the main elements considered to participate in mammalian iron metabolism.
'Free' haem appears in the circulation (it may have a signalling role [274]) and elsewhere largely because of erythrocyte degradation, and it can also greatly amplify the cellular damage caused by ROSs [275], and its degradation pathway via haem oxygenase [276,277] to biliverdin and then using biliverdin reductase to form bilirubin generates 'free' (and potentially redox-active) iron. It would appear, not least because biliverdin has powerful antioxidant properties, that haem oxygenase is more protective than damaging [253,278-282], even though one of the products of its reaction is Fe that must eventually be liganded (or e.g. incorporated into ferritin). (Another product is the gas CO, that has been proposed as a measure of oxidative stress in the lung [283].)
All of the above obviously ignores both some important aspects of the speciation and liganding of iron, as well as the tissue distribution of the specific proteins involved – for which latter global information will shortly emerge [284] (http://www.proteinatlas.org/ and see later). It also ignores any discussion on the genetic regulation of iron metabolism (e.g. [285-288]), which is not our main focus.
However, one molecule in particular, hepcidin, has recently emerged as a 'master regulator' of regulation at the physiological level, and we describe some of these new developments.
Hepcidin
In the liver and elsewhere, many aspects of iron metabolism are regulated by a recently discovered 25-amino acid polypeptide called hepcidin [207,241,245,271,289-327] that acts in part as a negative regulator of iron efflux [328] by causing the internalisation of ferroportin [329-333]. Hepcidin is produced, partly under the regulation of a receptor called hemojuvelin (e.g. [334]), via an 84-aa precursor called pre-pro-hepcidin and a 60 mer called pro-hepcidin [304,335,336] although the active agent is considered to be the 25 mer referred to above, and with the inactive precursors appearing not to be useful markers [337,338].
Strikingly, anaemia and anoxia both suppress hepcidin production [245,339,340] (Fig 3), such that just while superoxide production is being enhanced by the anoxia there is more iron being absorbed from the intestine and effluxed into the circulation. In view of the inter-reactivity of superoxide and iron this could be anticipated to enhance free radical formation, leading to a positive feedback loop in which the problems are amplified: ischaemia/anoxia changes Fe(n) distribution leading to differential reactivity with the products of anoxia and thus further free radical production. However, hepcidin is overexpressed in inflammatory disease and is an early inflammatory marker [245,341-345]. Its expression is positively controlled inter alia by SMAD4, and loss of hepatic SMAD4 is thus associated with dramatically decreased expression of hepcidin in liver and increased duodenal expression of a variety of genes involved in intestinal iron absorption, including Dcytb, DMT1 and ferroportin, leading to iron overload [346]. STAT3 is another positive effector of hepcidin expression [347,348], and ROSs inhibit this effect [349], thereby creating a link between ROSs and Fe metabolism. To understand the exact roles of hepcidin in iron metabolism, it is going to be especially important to understand where it is expressed; fortunately, such studies are beginning to emerge [350].
Figure 3.
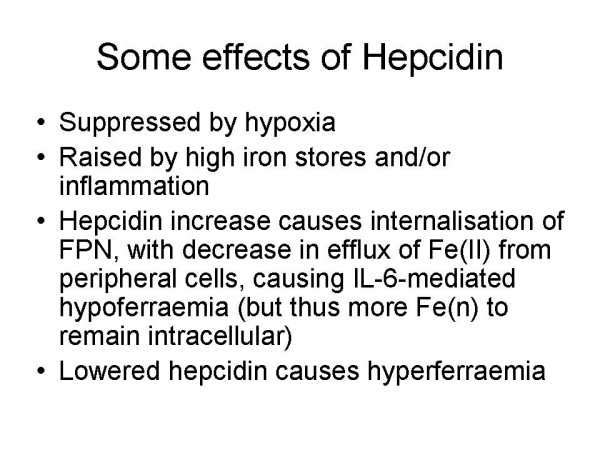
Some effects of hepcidin, summarizing the fact that hypoxic condition can suppress it and thus lead to hyperferraemia. Since hypoxic conditions can also lead to ROS production the hypoxia-mediated regulation of hepcidin can have especially damaging effects.
Overall there is a complex interplay between positive and negative regulation and the organismal distribution of iron caused by changes in hepcidin concentration [351], with in many cases the hypoxic response (decreased hepcidin) seeming to dominate that due to inflammation (increased hepcidin) even when iron levels are high [352,353]. Specifically, lowered hepcidin causes hyperferraemia. Hepcidin is also activated by p53 [354], and may play a role in the degradation of atherosclerotic plaques [355]. Another recently discovered protein that is crucially involved in human iron metabolism is NGAL or siderocalin, and while there is some evidence for their co-regulation [356], they have normally been studied separately.
NGAL (also known as lipocalin-2 or siderocalin)
Lipocalins [357] are a diverse group of ligand-binding proteins that share a conserved structure even in the absence of significant sequence conservation. This core structure includes an eight-stranded anti- parallel β barrel that defines a calyx, or cup-shaped structure, enclosing the ligand binding site.
NGAL – neutrophil gelatinase-associated lipocalin – is a 21 kDal glycoprotein first isolated by Kjeldsen and colleagues in 1993 [358]. Synonyms include lipocalin 2, siderocalin, Lcn2, α2-microglobulin-related protein or neu-related lipocalin (in rats) [359,360] and (in mice) 24p3 or uterocalin [361]. Although lipocalins are well known to be involved in the sequestration and transport of a variety of ligands, the natural ligand of NGAL (as is the case with many lipocalins) was not initially known even in terms of its chemical class. This changed with the seminal paper of Goetz and colleagues [362] (and see [206]) who purified recombinant NGAL from a particular strain of E. coli and found that its structure contained a negatively charged ferric siderophore with a subnanomolar dissociation constant that it had extracted from its bacterial host, and that the apo form of this molecule could also act as a potent bacteriostatic agent by sequestering iron (see also [363-367]). A companion paper [368] showed that the iron-delivering activity was expressed in mammalian cells. The structure of NGAL is now known [369] and one of its interaction partners is a matrix metalloproteinase [370] to which it can presumably donate a metal ion and in the complex decrease its degradation [371].
The finding that NGAL was one of the most highly expressed proteins following ischaemia-reperfusion injury in kidney cells [372-374], and prognostic of kidney damage long before the more traditional marker creatinine was raised significantly, has led to considerable interest in this protein, especially as a marker of renal injury [375-389], and perhaps as a therapeutic [375]. Devireddy and colleagues [390] identified a receptor that internalizes 24p3, and internalization of iron bound to 24p3 prevents apoptosis. In contrast, internalization of the apo form of 24p3 that does not contain iron led to cellular iron efflux and apoptosis via the proapoptotic protein Bim [391]. In humans the megalin receptor can bind siderocalin (and its siderophore payload) and mediate its intracellular uptake [392]. Oxidative stress can also induce its expression [393], and it is protective against it [394].
Exogenously administered NGAL also markedly upregulates heme oxygenase-1, a proven multifunctional protective agent in experimental Acute Kidney Injury (AKI) that is thought to work by limiting iron uptake, promoting intracellular iron release, enhancing production of antioxidants such as biliverdin and carbon monoxide, and inducing the cell cycle regulatory protein p21 [279,395,396]. Because of this multifaceted protective action, NGAL has emerged as a prime therapeutic target in ischaemic AKI [379].
Structural and direct binding studies have suggested that siderocalin tends (although not exclusively) to bind catecholate-type ligands, rather than hydroxamate- or carboxylate-based siderophores, at least when tested with microbially derived siderophores [362,363,365] (but cf. [369] for claims, disputed [360] and not now accepted, as to the binding of bacterially derived formyl peptides!). The role of NGAL, as a siderophore-binding agent, is thus consistent with the widespread recognition that iron-induced radical generation is intimately involved in a variety of renal and other diseases [397,398]. However, while it is certainly the case that siderocalin can reduce the virulence of bacteria when it binds the relevant bacterial siderophores [362-367] and that bacteria can 'evade' this by synthesising siderophores that siderocalin cannot bind (e.g. [186,187,399-401]), it is questionable whether the only role of siderocalin lies in fact in its antibacterial activity. Rather we would suggest that its main role is in sequestrating iron via a human siderophore to stop inappropriately liganded iron from producing damaging oxygen radicals. Consistent with this iron-liganding role for human biology is the fact that the tissue most highly expressing NGAL under normal conditions is bone marrow [360,402], the site of erythropoiesis. The liganding can be extensive; as Goetz and colleagues [362] note, "During inflammation, concentrations of NGAL can increase to levels, with concentrations approaching 20–30 nM in the serum [403], presumably adequate to bind all available iron as ferric siderophore complexes".
Significant changes in NGAL expression have also been observed, for instance, during kinase-mediated signalling [404,405], in cardiovascular disease [406-409] and in cancer [410-412].
These findings on the kidney and the role of NGAL, together with the important knowledge that its chief ligand is probably an unknown human siderophore (Figs 2, 4), thus lead us to consider the role of this system (and unliganded iron generally) in a whole series of other diseases that all share many characteristics of oxidative stress and inflammation (see also [413]). A similar thesis, albeit with comparatively little stress on iron, is the leitmotif of Finch's recent detailed monograph [163]. The theme of these sections is thus to stress the fact that while the role of ROSs in general in such syndromes has been pointed up previously, that of iron as a major culprit has not so generally yet been stressed, notwithstanding that there is in fact a great deal of pertinent literature that we here highlight as the focus of this review.
Figure 4.
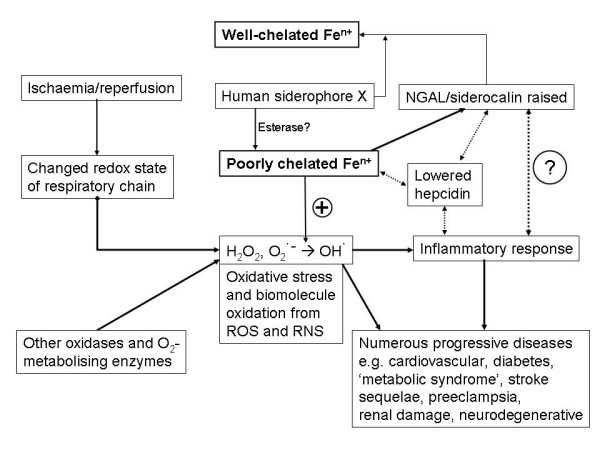
Overview of the roles of ischaemia, ROSs, poorly liganded iron and the iron metabolism regulators HGAL and hepicidin in effecting inflammation as a physiological level.
Some disease manifestations in which iron may be implicated
Preeclampsia (PE)
Another important disease that shares many of the same properties (or at least sequelae) of renal impairment, and may have the same fundamental aetiology, is pre-eclampsia. This is the most significant cause of morbidity and mortality in pregnant women [414]. The chief clinical manifestations at time of diagnosis are a raised blood pressure (hypertension) [415] and proteinuria, together with raised creatinine, consistent with the reversible existence (since it is relieved upon delivery of the baby) of renal impairment. However, prognostic markers that might manifest early in pregnancy are lacking, and would be highly desirable. There is widespread agreement [416] that a poor connection of the placenta to the uterus leads to ischaemia and thus oxidative stress, with a substantial involvement of apoptosis during the placental remodelling [417-423]. Since preeclampsia-like syndromes can be induced in pregnant animals by surgical restriction of the uteroplacental blood supply [424], it is presumed that blood-borne agents arising from the ischaemic placenta are the cause of the generalized endothelial cell damage and inflammatory responses that give rise to the symptoms of hypertension, proteinuria, and sudden oedema characteristic of preeclampsia [70]. Indeed, many studies implicate oxidative stress as a substantial contributor to this [425-489], while some have noted the importance of iron status [70,133,450,490-511], and so far as is known the transporters of iron in the placenta are similar to those in other cells [512]. Oxidative stress of this type is of course inflammatory in nature and inflammation is observed in PE [472,476,484,486,513-519]. We suggest strongly that it is the combination of inadequately liganded Fe(II or III) and superoxide/peroxide leading to OH• formation that is the chief mechanistic cause of the downstream events that manifest in PE, and that appropriate removal by liganding/chelation or otherwise of these ions would prove of therapeutic benefit. (Iron status has also been implicated in other pregnancy and neonatal disorders [520-524].) There is evidence too for the involvement of the radical NO [456,525].
We note that it is quite common nevertheless for iron to be prescribed during pregnancy, especially during its latter stages [526,527], and that this does of course lead to oxidative stress [528,529].
Oxidative stress is caused both by the initial rate of production of superoxide and the rate of their conversion into OH• radicals. The former can be induced by hypoxic conditions such as occur at high altitude, and one prediction, that is borne out [487,530], is that PE should therefore be more prevalent at high altitude. Erythropoietin may be a marker for oxidative stress in pre-eclampsia [531].
Regarding the second stage, predictions include that PE should be more common in those suffering from diseases of iron metabolism. Although such mothers are of course less well a priori, this prediction is borne out for α-thalassemia [532,533] although not, interestingly, for haemochromatosis [534]. We note in this context that thalassaemia not only predisposes towards PE but is known in general to cause hepcidin to decrease and NGAL to increase [352,353,356,535], with consequent and inevitable iron dysregulation.
Another prediction is then that hepcidin should be changed in pre-eclampsia. Although no serum measurements have been reported to date, it is of extreme interest that – while they took it to be an antimicrobial peptide rather than an iron regulator – a recent study by Knox and colleagues of placental gene expression in a mouse model of PE showed that hepcidin expression increased by a greater factor than that of any other gene save one [536], consistent with the view that major changes in the regulation of iron liganding and metabolism underpin PE.
Finally, we note that NGAL is significantly implicated in pregnancy, and was even named uterocalin in mice to reflect its high expression in the uterus [361,537-539]. A very recent study [540] suggests that it may be a useful second trimester biomarker for pre-eclampsia.
Diabetes
Type 2 diabetes and insulin resistance are known complications of pregnancy (e.g. [541-545]), and also predispose towards PE. In a similar vein, various types of pregnancy-related intrauterine growth restriction predispose towards diabetes in later life [546,547], pointing up the progressive nature of these syndromes. Metabolic biomarkers for the one can thus be predictive of the other [548], consistent with a common cause. Certainly ROSs are known to play a substantive role in both insulin resistance [549-556] and in a variety of diabetic sequelae [95,557-559], and mitochondrial dysfunction may be an early step in this [560]. Some anti-diabetic drugs, such as the 'glitazones' that are considered to act on Peroxisome Proliferator Activated Receptor (PPAR)γ, may also act by decreasing ROS production (e.g. [561-565]), and are even active aganst cerebral ischaemia and stroke [566-569]. As with most if not all of the other diseases we review here, studies of pro-inflammatory markers (such as TNF-α, IL-1 and C-reactive protein [570]) during the development of diabetes show its aetiology to be inflammatory in nature [553,571-590]. Iron 'excess' is also a known feature of gestational diabetes [591-593], and is a clear risk factor for the disease even in 'normal' populations [594-603], and indeed diabetes is a classical consequence of iron overloading as seen in hereditary haemochromatosis [604]. Serum ferritin and body iron stores are strongly associated with diabetes [603,605-609], including prospectively [610], while changes in visfatin are also intimately involved in changes of iron metabolism (with pro-hepcidin being elevated) [611]. Most importantly, lipocalin 2 (siderocalin/NGAL) is strongly associated with the development of diabetes [612,613]. Lowering iron improves insulin sensitivity [598,614], and metallothionein is also protective [615-619]. There seems little doubt that iron status is a major determinant of the development of type 2 diabetes [620].
Non-transferrin-bound iron is also considerably elevated in type 2 diabetes [621], and this too is exacerbated by vitamin C. Iron metabolism is substantially deranged in type 2 diabetes and the metabolism of glucose (a reducing sugar) interacts significantly with iron metabolism [598]. Iron is also strongly implicated in non-alcoholic steatohepatitis, considered an early marker of insulin resistance [622-624]. Well-known diabetic complications include retinopathies, and it is noteworthy that elevated levels of ferritin can lead to cataract formation [625,626].
The metabolic syndrome
Although some of its origins may be pre-natal [547], many of the features of these diseases are also seen in the (so-called) Metabolic Syndrome [627-631]. Thus, serum ferritin is also related to insulin resistance [606,632,633] and iron levels are raised [624,634,635]. Of course diabetes and the Metabolic Syndrome are also closely coupled, so it is reasonable that features observed in the one may be observed during the development of the other. The metabolic syndrome is also an independent indicator for chronic kidney disease [636] and may be related to liver steatosis [637]. Metabolic disorders of this type too are closely intertwined with inflammation [575,581,587,638], that is of course stimulated by ROSs whose generation is increased by high-fat diets [639]. Thus, our role here is to point up the existence of a considerable body of more-than-circumstantial evidence that here too the progressive and damaging nature of these diseases may be caused, in part, by inappropriately chelated iron.
Obesity
"As previously pointed out by Booth et al. [640], 100% of the increase in the prevalence of Type 2 diabetes and obesity in the United States during the latter half of the 20th century must be attributed to a changing environment interacting with genes, because 0% of the human genome has changed during this time period." [629]
It is well known that there has been a staggering increase in the prevalence of obesity, diabetes, and especially type 2 diabetes, in the last 50 years or so, and that this increase is expected to continue (e.g. [641-643] and http://www.who.int/diabetes/). Equally, it is now well known that obesity, metabolic syndrome, diabetes and cardiovascular diseases are all more or less related to each other [643], and the question arises here as to whether dysfunctional iron metabolism might be a feature of each of them. In the case of obesity per se, however, we see no major evidence as yet for a causative role of deranged iron metabolism or chelation in causing obesity. Indeed, while they are related [644], what little evidence there is [645,646] suggests that the converse may be true, i.e. that changes in iron metabolism might be consequent upon obesity (possibly via peroxide generation [639]). Importantly, considerable evidence suggests that obesity and inflammation are significantly related [163,486,575,581,642,647-664], not least because adipocytes produce and release various adipokines including pro-inflammatory cytokines such as IL-6 and TNF-α [575,649,650,665-671]. It is likely that it is the combination of overfeeding-induced obesity and inflammation (partly induced by the obesity itself [672]) that leads to diabetes [673]. Certainly there is evidence for increased ROS production in obese mice, possibly mediated in part via the fatty acid-induced activation of NAPH oxidase [674], while obesity is linked [675,676] to urinary levels of 8-epi-PGF2α, a well established marker of oxidative stress (qv). Fig 5 summarises the above in a manner that stresses the roles of iron, overfeeding and inflammation in the genesis of these processes, and notes that interference in several of these steps is likely to be required to limit their progression to best advantage.
Figure 5.
Role of inflammation caused by hydroxyl radical formation in the interactive development of obesity, the metabolic syndrome and diabetes. Intervention at multiple steps is likely to be most beneficial in alleviating this kind of progression.
Hypertension
As well its significance in pre-eclampsia (see above), hypertension is a well known risk factor for many cardiovascular and related disease (e.g. [677]), and there is considerable evidence that its underlying cause is inflammatory in nature [678-686], is related to the metabolic syndrome and obesity (e.g. [648,687-691]), and may be mediated mainly via ROSs [692]. There is evidence that some of its sequelae may be mediated via iron [693,694].
Cardiovascular diseases
It is well known that elevated iron stores can predispose to coronary artery disease and thence myocardial infarction. The 'iron hypothesis' of the benefits of some iron depletion due to menstruation was devised to account for the lowering of heart-disease risk in young women (that disappears in those post-menopause) and was proposed by Jerome Sullivan in 1981 [695-698] (and see also [699,700]). (In this sense, the lack of menstruation during pregnancy would predispose to a comparative abundance of iron, as is indeed found – see above.) It is of particular interest that the well-known adverse vascular effects of homocysteine (in inhibiting flow-mediated dilatation) are in fact iron-dependent [701-703], and that reducing homocysteine (e.g. by folate supplementation) in the absence of lowering iron has shown no clinical benefit to date [704], thereby suggestion iron mediation. By contrast, iron stores represent an established risk factor for cardiovascular disease [705].
Of course many factors such as lipid levels, stress, smoking and so are well-known risk factors for cardiovascular, coronary artery disease and related diseases. Indeed kidney disease is well established as a risk factor for cardiovascular disease [706-708] (and indeed stroke [709]), all consistent with their having in part a common cause – we believe inflammation). Our purpose here, within the spirit of this review, is to indicate the evidence for the involvement of inappropriately chelated iron in cardiovascular diseases too. There is no doubt that the iron-mediated causal chain of ischaemia → (su)peroxide → OH• radical formation occurs during the development of heart disease, especially during reperfusion injury [710-713], and suitable iron chelators inhibit this [714,715] (see also [716,717], and for thalassaemia [718]). Iron is also involved in the protection that can be produced by ischaemic preconditioning [719,720]. Erythropoietin, a hormone with multiple effects that may involve iron metabolism, is also protective [721,722].
Heart failure
The sequelae of heart failure are complex, and involve a chronic and continuing worsening of a variety of physiological properties. ROSs are certainly involved here, since allopurinol (a potent inhibitor of xanthine oxidase) improves prognosis considerably [723], and uric acid is a well known biomarker for heart failure (see e.g. [724,725]). Biopyrrins, degradation products of bilirubin and thus markers of oxidative stress are also considerably increased [726]. Anaemia is a common occurrence (and risk factor) in heart failure [727-729], again implying a role for dysregulated iron metabolism and a need to understand the exact speciation of iron in chronic anaemias linked to inflammatory diseases [730].
It is next on the formation of atherosclerotic plaques that our attention is here focussed.
Atherosclerosis
Atherosclerosis is a progressive inflammatory disease[731-762] characterized by the accumulation of both oxidised lipids and various fibrous elements in arteries, often as plaques [763,764]. Iron and oxidised lipids are both found in atherosclerotic lesions [141,765-777], and iron depletion by dietary or other means delays this [778-782]. There is a correlation between iron status and atherosclerosis [766,776,783-794], evidently caused in part by the known ability of poorly liganded iron to effect lipid [765,784] and protein peroxidation, and by the effects of primed neutrophils [795] and transferrin [758]. In this context, exogenous ferric iron is deleterious to endothelial function [796], while iron chelation improves it [797-800]. However, phlebotomy provided no clinical benefit here [801]. Note that iron levels in plaques correlate with the amount of oxidised proteins therein [771], and that in one study [767], the EPR-detectable iron (essentially Fe(III)) in atherosclerotic tissue was seventeen times greater than that in the equivalent healthy tissue; this is not a small effect. (Iron, as part of the general ROS cascade, has also been implicated in gallstone formation, where melatonin has proved protective [802,803].)
Statins, typically developed on the basis of their ability to inhibit the enzyme HMG-CoA redutase and thus decrease serum cholesterol, are well established to have benefits in terms of decreasing the adverse events of various types of cardiovascular disease [804], albeit that in many populations (e.g. [805-807]) cholesterol alone is a poor predictor of cardiovascular disease, especially in the normal range. However, a known target of statins different from HMGCoA reductase is the β2-integrin leukocyte function antigen-1 (LFA-1) [808,809] and in this context, it is important to note that the clinical benefits of the statins are certainly not due solely to their cholesterol-lowering ability via the inhibition of HMG-CoA reductase (see e.g. [163,281,650,807,808,810-840]), and different statins can cause a variety of distinct expression profiles [841] that are inconsistent with a unitary mode of action. The apparent paradox [842] that lipid-lowering statins do indeed exhibit epidemiological disease-lowering benefit, while having little effect on plaques, is arguably well explained, especially within the context of the present review, via their additional anti-inflammatory effects [817,819,843,163,807,823-825,830,836,837-892], acting upstream of the nuclear transcription factor NF-κB (and see later). It is also extremely relevant, for instance, that some statins have metal chelating properties [893].
It has been pointed out that many measures of iron stress are inappropriate, since it is only the redox-active form of iron that is likely involved in oxidative stress. Serum ferritin is considered by some to be the most reliable marker of iron status in general [894], although it is not well correlated with iron distribution in the heart [895], for instance. What is clear, however, from the above is that deranged iron metabolism is intimately and causally involved in the formation of atherosclerotic lesions, and that appropriate iron chelation can help both to prevent and to reverse this.
Iron status is also closely involved in other chronic vascular diseases, and in the behaviour of wounds [896-899].
Stroke
Stroke is caused by ischaemia, leading to inflammation [900] and to the formation of ROS and other damaging free radicals [901] in the brain (which is high in metal ions [902]), and is exacerbated by existing inflammation – see e.g. [903,904]. Thus, another prediction is that iron excess should also aggravate the sequelae of stroke, and that appropriate chelation or free radical trapping agents should mitigate these effects. These predictions are indeed borne out [138,905-914]. It is also of considerable interest that plasma NGAL levels are increased in stroke [406]; it is noteworthy that this can be seen in plasma despite the localised origin of the disease.
A variety of other studies have shown the beneficial treatments in stroke models of anti-inflammatory and antioxidant treatment, i.e. treatments that lower the amount of ROSs (e.g. [915-922]), as well as of preconditioning [923]. Given its role in iron metabolism, it is of considerable interest that erythropoietin also seems to be very effective in protecting against brain ischaemia/reperfusion injury and stroke [924-939], by a mechanism independent of erythropoiesis [940-942], and one that appears to involve anti-inflammatory activity [943].
Alzheimer's, Parkinson's and other major neurodegenerative diseases
Oxidative stress and inflammation are early events of neurodegenerative diseases [920,944-957] such as Alzheimer's disease (e.g. [958-973]), where plaque formation precedes neurodegeneration [974]. Iron (and in some cases copper) is also strongly implicated in a variety of neurodegenerative diseases [944,981,945,958,962,950,917,278,43,285,972,141,975-1087].
Indeed Thompson and colleagues comment [136] that "The underlying pathogenic event in oxidative stress is cellular iron mismanagement" and stress that "Multiple lines of evidence implicate redox-active transition metals, such as iron and copper, as mediators of oxidative stress and ROS production in neurodegenerative diseases". There is also ample evidence for its presence in the plaques characteristic of Alzheimer's disease [1004,1013,1027,1088], just as in those of atherosclerosis (see above). Note too that iron can catalyse the oxidation of dopamine to a quinine form that can bind covalently to and then aggregate proteins [1089]. Kostoff [1090] has used a very interesting literature-based discovery approach to highlight the role of oxidative stress in the development of Parkinson's disease.
Other papers highlight the role of iron in multiple sclerosis [899,991,1091-1098] and in prion diseases [967,1099,1100]. However, a particularly clear example of iron-mediated neurodegeneration is given by the sequelae consequent upon lesions in a protein known as frataxin involved in the disease Friedreich's ataxia (FA).
Friedreich's ataxia
A number of repiratory chain components contain non-heme iron, and the question arises as to how they acquire it [1101]. Frataxin is a mitochondrial iron chaperone protein [1102-1109], involved in the safe insertion of Fe(II) during the production of Fe-S centres in the mitochondrial respiratory chain [1110]. As are some other aspects of iron metabolism [1111], it is highly conserved in eukaryotes from yeast to humans [1112,1113], a fact that made the unravelling of its function considerably easier [1114-1123]. Friedreich's ataxia (FA) is a neurodegenerative disorder that arises from a genetic deficit of frataxin activity, whether by a missense mutation or, much more commonly, via the addition of GAA trinucleotide repeats [1107,1124-1127]. As well as the neurodegeneration and measurable iron deposition, clinical symptoms include cardiac hypertrophy [1128] and (pre-)diabetes [1129], consistent with the general thesis described here that all are in part manifestations of iron dysregulation, and in which suitable chelation may be beneficial [1130,1131] (but cf. [1132]).
ROSs are undoubtedly involved in FA [1133,1134], specifically via Fenton chemistry [1135,1136], since the attenuation of H2O2 production (but not of superoxide) [1110] ameliorates the disease [1137]. The deficit in frataxin causes both an increase in ROS (H2O2) production via the mitochondrial electron transport deficiency [1138] as well as a dysregulation in iron metabolism, potentially a very damaging synergistic combination (see later). Elements of its (in)activity that are seen as paradoxical [1139] are in fact easily explained when one recognises that it is the combination of free iron with H2O2 that is especially damaging. The neonatally lethal GRACILE syndrome is also caused by a failure of iron chaperoning into mitochondrial complex III due to mutations in the BCS1L gene [1140,1141].
Amyotrophic lateral sclerosis (ALS) or Lou Gehrig's disease
ALS is another progressive inflammatory [1142] disease in which motor neuron death causes irreversible wasting of skeletal muscles. It has largely defied efforts to uncover the genetic basis of any predisposition [1143], save for a very clear association with defects in a Cu/Zn superoxide dismutase [33,1144-1146] that can obviously lead to an increase in the steady-state levels of superoxide (and hence hydroxyl radical formation). There is also significant evidence for the involvement of iron [1147,1148]. Drug therapies have to date shown rather limited benefits, and more in mouse models of Cu/Zn SOD deficiencies than in humans, though iron chelation therapy does not seem to have figured heavily, and it is recognised that combination therapies might offer better prognoses [1149].
Aging
Aging or senescence is defined as a decline in performance and fitness with advancing age [1150]. Iron stores tend to increase with age [1151-1155], partly due to dietary reasons [1156] (and see [1157,1158]), as does anaemia [1159,1160]. So too does the expression of NGAL/Lcn2/siderocalin, a process that can be reversed by melatonin [1161]. Mainstream theories of aging [163,165,1162-1180] recognise the relationship between progressive inflammation, cellular damage and repair and the higher-level manifestations of the aging process, and ('the free radical theory of aging' [1163,1181]) ROSs are of course strongly implicated as partial contributors to the aging process (e.g. [31,43,980,1163,1182-1206]). Needless to say, not least because of the low steady-state net rate of generation of the various ROSs [1207], few studies have managed to be very specific mechanistically [1208], but it should be clear that all ROSs are not created equal and we need here to concentrate mainly on the 'nasty' parts of ROS metabolism, and in particular on the hydroxyl radical as generated via poorly liganded iron and on peroxynitrite, and to have the greatest effects we need to inhibit both their generation and their reactivity (see Systems Biology section, below). The iron content of cells also increases as cells age normally [1209]. As many diseases increase with age, probably via mechanisms highlighted herein, treating aging can thereby treat disease [162], and it is important to recognise that most 'diseases' are in fact consequences of aging (despite the considerably greater historical focus on the former).
Frailty
One issue of aging is not that just it happens but that it can manifest in a series of essentially undesirable physiological changes, referred to as frailty [1210], in which ROSs have also been strongly implicated. Indeed, there are many parts of physiology and metabolism that lose functionality during aging (e.g. the cardiovascular system [1211] and of course cognitive function [1212]), and iron metabolism is known to change considerably as humans age [980,1213], with anaemia a typical accompaniment of aging [1214]. The question then arises as to how much of this deranged iron metabolism is causal in accelerated aging, and this is not easy to state at this time. However, lowering iron does increase the lifespan of Drosophila [1215] and yeast [1105]. At all events, the purpose of this rather brief section is to point out to researchers in aging, frailty and gerontology generally the relevance of inadequately controlled iron metabolism as a major part of ROS-induced injuries that may accelerate the aging process.
Longevity
Although aging and longevity are not of course the same thing, and longevity is not a 'disease', studies of aging are often performed with the intention of improving our understanding of longevity [1216], and certainly longevity is linked to age-related disease [1217]. However, the longevity of many organisms can be varied by the manipulation of any number of diseases or processes. This said, caloric or dietary restriction is a well-known contributor to longevity (indeed the only reliable one in pretty well all species [1218-1223] – although possibly not H. sapiens [1180]), and appears to act at the root of the processes involved [1224]. Caloric restriction appears to be associated with a considerably lowered rate of production of ROSs and accrual of ROS-induced damage [1207,1225-1228], and this is to be expected on general grounds in that a restriction of substrate supply will make the redox poise of mitochondria [1229] more oxiding and thereby minimise the amount of 1- and 2-electron reductions of O2 to form peroxide and superoxide. It is therefore also of great interest that caloric restriction also benefits iron status [1230] and that it is this improved iron status that in part promotes longevity [1231]. Caloric restriction has also been shown to effect a differential stress response between normal and tumour cells [1232], although this study did not look at relative iron status.
Antioxidants can also influence lifespan. Thus (rather high doses of) melatonin extended the lifespan (and stress resistance) of Drosophila [1191,1233-1235] while that of Caenorhadbitis elegans could be extended by mimics [1236] of SOD and catalase [1187], and by a variety of antioxidant and other pharmacological agents [1237].
As an example, let us consider C. elegans. Mutants with a decreased activity of the insulin-like growth factor signalling pathway (e.g. daf2 mutants that have a greater amount of the DAF16 FOXO-like transcription factor) can live for nearly twice as long as wild types [1238,1239] and produce more catalase, superoxide dismutase (sod-3) [1240] and glutathione-S-transferase.
Overall, it is the potent combination of oxidative stress, already leading to damaging peroxides and radicals, and its catalysis and further reactions caused by inappropriately chelated iron, that causes a 'double whammy'. Indeed, iron, copper and H2O2 have been referred to as the 'toxic triad' [1032]. While there is comparatively little that we can do about the production of superoxide and peroxide, we can (by pharmacological or dietary means) try and improve the speciation of iron ions.
Rheumatoid arthritis
One disease whose aetiology is well known to be bound up with ROSs is rheumatoid arthritis (RA) [36,1241-1246]. What is known of the role of iron metabolism? Generally an overall low iron status – anaemia – is a characteristic of rheumatoid arthritis [1247-1251], whereas by contrast iron is elevated in the synovial fluid of arthritic joints [1252-1255]. This suggests a significant derangement of iron metabolism in RA as well, and a mechanism [1256-1259] in which elevated superoxide liberates free iron from ferritin in synovial fluid (and elsewhere [1260]), thereby catalysing further the damaging production of hydroxyl radicals. This autocatalytic process (Fig 6) is, even in principle, especially destructive (and may account for species differences in sensitivity to iron loading [1261]). Note that erythrocytes when oxidized can also release free iron [524]. Natural antioxidants such as vitamin E are also lowered [1262]. There is some evidence that appropriate iron chelators can ameliorate the symptoms of RA [1263], though membrane-impermeant chelators such as desferroxamine cannot [1264].
Figure 6.
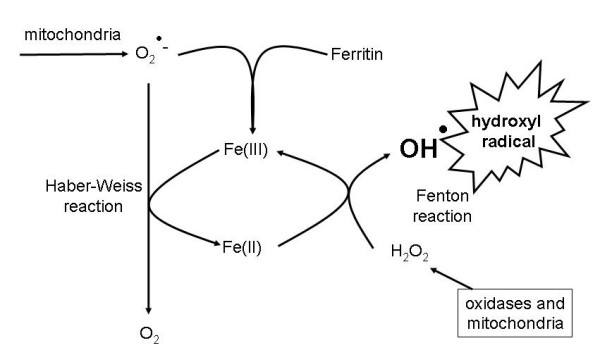
Catalysis and autocatalysis in the Haber-Weiss and Fenton reactions leading to the production of the hydroxyl radical, including the liberation by superoxide of free iron from ferritin.
One interesting feature of RA is that in 75% of women it is strongly ameliorated during pregnancy [1265-1267]; although the multifactorial nature of this observation has made a mechanistic interpretation difficult, from everything that we have seen so far it would be surprising if changes in iron metabolism were not strongly involved.
An interesting related feature is the 'restless legs syndrome' [1268-1270], that is often associated with iron deficiency and pregnancy. Serum transferrin receptor seems to be a rather sensitive measure of this iron deficiency [273,1271,1272]. There are relationships with other syndromes that we discuss here, such as cardiovascular disease [1273], but in the present review it is of especial interest that a dysregulation of iron metabolism appears to play a significant role [1274]
Lupus (Systemic Lupus Erythematosus)
Lupus, or Systemic Lupus Erythematosus (SLE) [1275,1276] describes a syndrome, somewhat related to arthritis and rheumatism, of broadly auto-immunological or inflammatory origin, and with a large variety of manifestations (e.g. [1277-1285]), but characterised in particular by fatigue [1286], often as a result of anaemia. This of course points to a certain level of iron dysregulation (of any number of causes), and there is certain some evidence for this [1274,1287]. Thus, while anaemia is a feature of the disease, serum ferritin may be raised in SLE [1288], and some of the usual lipid markers of oxidative stress (that can be a result of hydroxyl radical production catalysed by poorly liganded iron) are also present [1289].
There is also a very interesting linkage between SLE and vitamin D metabolism [1290], something that has also come up in relation to the statins and atherosclerosis ([832,1291], but cf. [1292]), and indeed it there is evidence that statins may be of benefit in the treatment and even reversal of lupus [1293,1294]. Indeed, from a much more general point of view, there is precedent for these kinds of linkages being used to uncover unknown mediators in disease states that may be worth pursuing (e.g. [6,19,20,1295,1296]).
Asthma
Asthma is a well-known inflammatory disease, and has been linked with ROS generation [1297] catalysed by iron [276,1298-1300].
Inflammatory bowel diseases (IBD)
By definition, IBD such as Crohn's disease and ulcerative colitis are inflammatory diseases, and while the inflammation and ROS production are well established here, their origins are somewhat uncertain [1301-1304]. They are frequently accompanied by anaemia, implying a derangement in iron absorption and/or metabolism [1305,1306], and very probably absorption [1307,1308]. The anaemia may be monitored by ferritin and transferrin receptor levels, and its correction is possible by iron supplementation plus erythropoietin [1309-1315]. One may suppose that some of the issues here relate to iron speciation, that is usually not measured in these studies.
Age-related macular degeneration
Age-related macular degeneration (AMD) [1316] is now the leading cause of blindness and visual disability in the elderly in developed countries [1317-1320]. Many components of atherosclerotic plaques have also been demonstrated in drusen [1321], a characteristic of AMD and, as here, it is reasonable to propose a common mechanism of pathogenesis between AMD and atherosclerosis. Retinal iron levels increase with age [1322], iron is significantly implicated in AMD [1323-1329], and iron chelation may help to reverse the process [1330]. Dietary antioxidants are also protective [1331]. The source of the iron appears to be excess angiogenesis and leakage from blood vessels catalysed by VEGF, and a PEGylated aptamer [1332-1334] against VEGF (pegaptanib) or a monoclonal antibody (ranibizumab) have shown significant promise in the treatment of macular degeneration [1335-1340]. Plausibly a combination therapy with one of these plus a suitable iron chelator might be even more effective.
Psoriasis
Psoriasis is an anflammatory disease in which the production of free radicals and ROSs are strongly implicated [1341-1343]. Here too there is clear evidence for the involvement of a deranged iron metabolism [1343-1345]. Early attempts at therapy with a series of unusual iron chelators (that unfortunately had side effects) [1346] do not seem to have been followed up.
Gout
Gout is another important inflammatory disease, characterised by the accumulation of uric acid [1347]. There is considerable evidence that this too is a disease of iron overload, and that uric acid accumulation – as both an antioxidant and an iron chelator [1348] – is in response to the iron overload [1349,1350] and with highly beneficial remission of gouty symptoms occurring on depletion of iron by phlebotomy [1351].
Alcoholic and other forms of liver disease
It is known that with chronic excess, either iron or alcohol alone may individually injure the liver and other organs, and that in combination, each exaggerates the adverse effects of the other. Specifically, in alcoholic liver disease, both iron and alcohol contribute to the production of hepatic fibrosis [1352-1358]. Iron overload is well known to lead to hepatotoxicity [1359-1362] and liver cancer [1363,1364], and lowering or chelating it is protective [1365,1366]. Hepcidin may be involved here [1367].
Chronic obstructive pulmonary disorder (COPD) and related lung diseases
Chronic obstructive pulmonary disease (COPD) is a progressive and chronic disease which is characterised by an apparently inexorable decline in respiratory function, exercise capacity, and health status. It is also characterised by periods in which the symptoms are considerably exacerbated [1368-1371]. Such an acute exacerbation of COPD (AECOPD) is defined [1372] as "a sustained worsening of the patient's condition from the stable state and beyond normal day to day variations, that is acute in onset and necessitates a change in regular medication in a patient with underlying COPD". Smoking is a major source of free radicals (and indeed metals [1373]), and is a major cause of COPD. Consequently, there is considerable evidence for the evidence for the involvement of inflammation and ROSs in both the 'stable' and 'exacerbated' stages [1374-1381].
Needless to say, there is also considerable evidence for the significance of exposure to iron [1382] (as well as exposure to other toxic metals [1383]) in the development of COPD and other lung diseases [1384]. Haem oxygenase also appears to be a significant culprit [280], and lung (lavage) iron is increased [1385] while transferrin levels can be considerably lower [1386].
Other lung diseases in which ROS and iron have been implicated include Adult Respiratory Distress Syndrome [1387-1390] and cystic fibrosis [1391].
Smoking
Tobacco smoke contains many unpleasant and carcinogenic compounds, and that tobacco smoking is a leading cause of carcinoma of the lung and indeed of other organs has become well known since the pioneering epidemiological studies of Doll, Peto and colleagues (e.g. [1392-1395]). Our purpose here is to point out that many of the particles associated with smoking (and also ingested from other sources) are heavily laden with micro-particulate iron, which, as a major catalyst of hydroxyl radical production, undoubtedly is a substantial contributor as well (see e.g. [1373,1384,1396-1400]).
Cancer and oncology
In addition to the issues of smoking, the development of cancer can certainly contain an inflammatory component (e.g. [1401-1425]), and indeed the long-term use of prophylactic anti-inflammatory aspirin lowers colon cancer incidence by 40% (age-adjusted relative risk = 0.6) [1426] (though note that this may have other side-effects [1427]). (The well-known association between infectious agents such as H. pylori [1428-1430] and e.g. bowel cancer is probably initiated by chronic inflammation.) Given that cells require iron, restricting its supply can also limit the growth of cells, including tumour cells [1431-1442]. Conversely the iron carrier NGAL is overexpressed in tumours [410,411,1443], a process mediated via NF-κB [1444] (and see later). Further, the roles of iron, not least in the mutagenic effects of metal-catalysed Fenton chemistry, are also of significance in promoting oncogenesis [1445-1461]. Iron chelators [1438,1462] are thus a doubly attractive component of anti-cancer therapeutics. The mutagenic, carcinogenic and disease-causing actions of asbestos and related fibres may also be due in significant measure to the ability of the Fe(n) that they contain to catalyse hydroxyl radical production [1463-1475], while there seem to be complex relations between the likelihood of apoptosis and the differential concentrations of superoxide and H2O2 [1476,1477]. Overall, it is becoming increasingly widely recognised that anti-inflammatory agents have a role to play in the treatment of cancers; we would suggest that iron chelation may be a useful component of such treatments.
Malaria
Just as do tumour cells, the malarial parasite Plasmodium falciparum requires considerable iron for growth, and there is evidence that lowering the amount of available iron provides a promising route to antimalarials (e.g. [1478-1494], but cf. [1495]). Note in this context that iron-catalysed radical formation is also significantly involved in the antimalarial (i.e. cytotoxic) mode of action of artemisinin [1494,1496-1504], and this reaction is in fact inhibited by iron chelators [1505] such that a combined artemisinin-chelator therapy would be contraindicated.
Antimicrobials
Lower down the evolutionary scale, and as presaged earlier in the section on bacterial siderophores, microbes require iron for growth, its presence may be limiting even at the scale of global CO2 fixation [1506-1509], its excess can in some circumstances [1510] correlate with infectivity or virulence (see above and e.g. [180,193,1493,1511-1531]), and its chelation in a form not available to bacteria offers a route to at least a bacteriostatic kind of antibiotic or to novel therapies based on the lowering of iron available to microbes by using hepcidin [1532] or NGAL [1533]. Iron chelators are also inhibitory to trypanosomes [1534,1535], and changes in iron metabolism are also associated with viral infections [1536].
Sepsis leading to organ failure and death: severe inflammatory response syndrome
It is well known that one consequence of bacterial infection (sepsis) can be septic shock, that this can be mimicked by the Gram-negative bacterial outer membrane component LPS (lipopolysaccharide), and that in the worst cases this leads via multiple organ failure to death. However, whatever LPS does it is quite independent of the present of viable (i.e. growing or culturable – see [1537,1538]) bacteria as the same phenomena leading to multiple organ failure (MOF) are seen without infection [1539,1540]. Consequently, the recognition of a series of symptoms contingent on this initial inflammatory response has led to the development of the idea of a Systemic Inflammatory Response Syndrome (SIRS) [1541-1551] that leads to the MOF, both via apoptosis [1552] and necrosis [1553,1554]. There is by now little doubt that these phenomena too are associated with the hyperproduction of ROSs [1546,1551,1555-1570]. Circulating free iron is raised in sepsis and related conditions [1400,1571-1573]. Direct assays of oxidant induced cell death indicate that most 'free' iron is concentrated in lysosomes [1574-1577], that its decompartmentation is substantially involved [1570], and that its chelation can thus prevent cell death [1578-1581].
Many circulating inflammatory factors have been identified as important in the development of septic shock, including cytokines such as Tissue Necrosis Factor (TNF) [1582], and cellular responses via the Toll-Like Receptor are clearly involved in this process [1583,1584]. However, we would argue that since antibodies against TNF do not inhibit the sequelae of septic shock such as multiple organ failure, the truly damaging agents are caused elsewhere and are likely to involve the iron-mediated production of damaging hydroxyl radicals (see also [1563]).
In this regard, it is especially interesting that the antioxidant melatonin is particularly effective in preventing septic shock [1585-1587], and a variety of suitable antioxidants have shown potential here [1573,1588-1591], notably in combination with iron chelators [1592,1593] (and see also [1594,1595]). As with quite a number of the indications given above, a further link with Fe metabolism is seen in the protective effects of erythropoietin [1596,1597].
Pro- and anti-oxidants and their contributions to cellular physiology
A very great many cellular metabolites are redox active within the range of redox potentials realistically accessible to biology (including some molecules such as proline [1598] that are not commonly considered to be redox-active), and it is not our purpose here to list them extensively. Not only their redox potential and status but even their absolute amounts can have profound effects on metabolism [1599]). Our chief point here is that it is the intersection of iron metabolism and oxygen reduction that needs to be the focus, with the 'iron'-catalysed production of hydroxyl radical being the nexus, with the standard redox potential of a redox couple per se being less significant in absolute terms, and the redox potential that a particular redox couple 'feels' being dependent in a complex manner on a variety of thermodynamic and kinetic factors [1229]. Thus, although ascorbate is 'reducing' and an 'antioxidant', its reaction with O2, especially when catalysed by Fe(II), produces superoxide and thence OH• radicals that may be pro-oxidant. It is this kind of stepwise multi-electron-transfer phenomenon that explains the otherwise possibly puzzling observation of the oxidant-induced reduction of respiratory chain components (see e.g. [1600,1601]). Consequently, it is extremely unwise to make pronouncements on the role of 'ROSs' without being quite explicit about which ones are meant.
Thus anything – even an antioxidant – that e.g. by reaction with O2 produces superoxide, peroxide and hydroxyl radicals will turn out to be a pro-oxidant if the flux to superoxide and in particular to hydroxyl radicals is stimulated. Thermodynamically, the 1-electron reduction by ascorbate of dioxygen is disfavoured, with the 2-electron reduction to peroxide being the thermodynamically preferred route. However, such reactions are heavily restricted kinetically in the absence of any catalysts [130]. It is an unfortunate fact that the oxygen-mediated "autoxidation" of ascorbate does in fact occur at considerable rates when it is accelerated by the presence of iron or other transition metal ions [130,1602-1606]. In a similar vein, 'free' or inadequately liganded Fe(II) catalyses the production of hydroxyl radicals from oxygen plus a variety of natural biomolecules, including adrenaline (epinephrine) [1607], haemin [1608], and even peptides such as the amyloid-β involved in the development of Alzheimer's disease [1009,1027]. Dietary antioxidants (see below) can therefore act in complex and synergistic ways depending on iron status [1609]. In this regard, the idea of using elemental iron plus ascorbate in food supplements [1610] does not seem a good one.
It should be noted that there are also occasions, e.g. in the decomposition of refractory polymers such as lignin, where such radical production is involved beneficially [1611].
Finally, a variety of molecules can trap hydroxyl radicals, including hippurate [1612], melatonin [1233,1585,1586,1613-1631] and salicylate [1632].
Antioxidants as therapeutic agents? Should we be including iron chelators in such clinical trials?
Given the wide recognition of the importance of ROSs in a variety of diseases as described above, many investigators have considered the use of known antioxidants such as vitamins C (ascorbate) and E (α-tocopherol) in preventative therapy. Although there have been some successes (e.g. [1633]), the results have generally been decidedly mixed, with little clinical benefit (or even actual disbenefit) following from their administration [692,1588,1634-1639], e.g. for ALS [1640], atherosclerosis [106,764], cardiovascular disease [1641-1645], neuroprotection [1646], macular degeneration [1647], pre-eclampsia [1648-1652], critical care medicine [1653], aging [1165,1168,1654,1655], lung disease [1656], elective surgery involving ischaemia-reperfusion [1657], all-cause mortality [1637,1658], etc. One interpretation for these disappointing results, that is consistent with the general theme of this review, involves the recognition that a variety of antioxidants can in fact act as pro-oxidants and thus actually promote the production of OH• radicals in the presence of inappropriately or inadequately liganded Fe(II) [130,528,1602,1604,1659,1660]. (One might also comment that the intracellular location of the antioxidants may be an issue, and that the view that targeting them to mitochondria may well have considerable merit [1661-1663].) Thus any predictions about the utility or otherwise of antioxidants need to take into account the amount of 'free' iron present. In particular, we would suggest that future trials of this type might beneficially include appropriate iron chelators, whether alone or with antioxidants.
Liganding and reactivity of Fe(n)
Given the damage that iron-mediated OH• radical can create, the question arises as to whether appropriate chelators can inhibit this by inhibiting the production of OH•, and while the answer is 'yes' the interpretation of the relevant experiments is not always as clear cut as one would wish [43]. This is because the OH• radical is so reactive that its production is normally assessed by addition of the putative chelator and observation of its effect on the rate of reaction of a target molecule such as salicylate with the OH• generated. The ability of a chelator to inhibit such a reaction can then occur not only via a reduction in the rate of OH• production but by trapping the OH• itself, as well as by other mechanisms [1664]. This said, there is little doubt that iron chelators can be highly protective, and it is many ways very surprising that their use is not more widespread.
We begin by noting that the reactivity of iron does vary greatly depending upon its liganding environment [71]. Cheng et al. state [72] "Oxygen ligands prefer Fe(III); thus, the reduction potential of the iron is decreased. Conversely, nitrogen and sulfur ligands stabilize Fe(II); thus, the reduction potential of the iron is increased. Therefore, chelators with oxygen ligands, such as citrate, promote the oxidation of Fe(II) to Fe(III), while chelators that contain nitrogen ligands, such as phenanthroline, inhibit the oxidation of Fe(II). Many chelators, such as EDTA and Desferal (DFO), will bind both Fe(II) and Fe- (III); however, the stability constants are much greater for the Fe(III)-chelator complexes. Therefore, these chelators will bind Fe(II) and subsequently promote the oxidation of the Fe(II) to Fe(III) with the concomitant reduction of molecular oxygen to partially reduced oxygen species. Since the maximal coordination number of iron is six, the hexadentate chelators can provide more consistently inert complexes due to their ability to completely saturate the coordination sphere of the iron atom and, consequently, deactivate the "free iron" completely. For example, DFO is a very effective antioxidant in clinical application because of its potential to markedly decrease the redox activity of iron [137]." However, it is not easy to make hexadentate ligands orally active [1665].
Iron typically can coordinate 6 ligands in an octahedral arrangement. Preferential chelation of the Fe(II) or the Fe(III) form necessarily changes its redox potential as a result of Le Chatelier's principle, and from Marcus theory [1666-1669] the rate of outer-sphere electron transfer reactions is typically related to differences in the free energy change, i.e. the differences in redox potentials of the interacting partners. In addition, it widely recognised that [137] "The tight binding of low molecular {weight} chelators via coordinating ligands such as O, N, S to iron blocks the iron's ability to catalyze redox reactions. Since the maximal coordination number of iron is six, it is often argued that the hexadentate chelators can provide more consistently inert complexes due to their ability to completely saturate the coordination sphere of the Fe atom. Consequently, a chelator molecule that binds to all six sites of the Fe ion completely deactivates the "free iron". Such chelators are termed "hexidentate" {sic}, of which desferrioxamine is an example. There are many Fe chelators that inhibit the reactions of Fe, oxygen, and their metabolites. For example, desferrioxamine ... (DFO) markedly decreases the redox activity of Fe(III) and is a very effective antioxidant through its ability to bind Fe."
By contrast, bidentate or tridentate chelators that bind to only 2 or 3 of the available iron chelation sites, especially when they bind to both Fe(II) and Fe(III), can in fact catalyse redox cycling and thereby promote free radical generation [1437,1665,1670,1671]. Thus, the most potent iron chelators will normally be hexadentate (but may consequently strip iron from iron-containing enzyme and thereby have deleterious side effects). Bi- or tri-dentate ligands should therefore be at saturating concentrations for maximum effect.
Generally, the harder ligands that favour Fe(III) involve O whereas softer ligands that bind Fe(II) involve N and S. The type of ligand also influences the absorption spectrum of the ferric form of the chelator, such that conclusions can be drawn about the types of group involved in the complex. These charge transfer bands that appear on ligand binding are at around 340 nm for carboxylates, around 425 nm for trishydroxamates, 470 nm for bis-hydroxamates, 515 nm for monohydroxamates, around 480 nm for tris-catecholates, 560 nm for bis-catecholates and 680 nm for mono-catecholates [173]. In addition, for tris-bidentate complexes the complex can, on an octahedral arrangement, have two different configurations, a left-handed propeller, termed the Λ-configuration, and a righthanded propeller, the Δ-configuration [173].
Iron chelators – those approved and used clinically
A number of reviews (e.g. [1437,1438,1672-1678]) cover aspects of iron chelators that have had or may have utility clinically.
Whitnall and Richardson [1062] list a number of useful features of an experimentally (and clinically) useful iron chelator. Thus, "A compound suitable for the treatment of neurodegenerative disease should possess a number of qualities, namely (1) strong affinity for FeIII, (2) low molecular weight, (3) lipophilicity high enough to accommodate permeation of cell membranes and the BBB, (4) oral activity, and (5) minimal toxicity [1062]. Also, partly because there are few trivalent ions other than Fe(III) that the body actually needs, the major synthetic focus has been on the design of FeIII-selective chelators which feature "hard" oxygen donor atoms. Additionally, under aerobic conditions, high-affinity FeIII chelators will tend to chelate FeII to facilitate autoxidation, such that high-affinity FeIII-selective compounds will beneficially bind both FeIII and FeII under most physiological conditions" [1062]. (Note that liophilicity per se may not be relevant, as drugs require carriers to cross membranes [18], and promiscuity and off-target effects increase with lipophilicity [1679].)
Desferrioxamines are nonpeptide hydroxamate siderophores composed of alternating dicarboxylic acid and diamine units. linked by amide bonds. They are produced by many Streptomyces species [1680]. Desferrioxamine B is a linear (acyclic) substance produced (industrially) by the actinobacterium Streptomyces pilosus [1681], and is widely used as an iron chelator for the prevention and treatment of the effects of iron overload. It is commercially available as desferal (desferrioxamine methane sulphonate), also known as deferoxamine in the USA. It has been very effective in the treatment of a number of diseases, leading to the view that such molecules should have considerable therapeutic potential. A significant disadvantage of DFO is that it does not seem to cross the intestine intact (despite the rather catholic substrate specificity of intestinal peptide transporters [1682-1685]) and must therefore be given intravenously or subcutaneously. By contrast, another chelator known as Deferriprone or L1 does appear to cross cell membranes, but it is only bidentate.
Those with approval for clinical use are few in number and we deal with them first. Table 1 compares them with the 'ideal' properties of a clinically useful iron chelator, while Fig 7 gives the structure of the three most common, viz. desferal (deferoxamine), ferriprox (L1 or deferiprone) and exjade (ICL670 or deferasirox). (Dexrazoxane, a hexadentate chelator, is also marketed [1686].)
Table 1.
Comparison of the main available iron chelators to an ideal chelation drug (modified from [2469])
| "Ideal chelator" | Deferoxamine | Deferiprone | Deferasirox | |
| Route of administration | Oral | Parenteral, usually subcutaneous or intravenous | Oral | Oral |
| Plasma half-life | Long enough to give constant protection from labile plasma iron | Short (minutes); requires constant delivery | Moderate (< 2 hours). Requires at least 3-times-per-day dosing | Long, 8–16 hours; remains in plasma at 24 h |
| Therapeutic index | High | High at moderate doses in iron-overloaded subjects | Idiosyncratic side effects are most important | Probably high in iron overloaded subjects* |
| Molar iron chelating efficiency; charge of iron (III) complex | High, uncharged | High (hexadentate); charged | Low (bidentate); uncharged | Moderate (tridentate); uncharged |
| Important side effects | None or only in iron-depleted subjects | Auditory and retinal toxicity; effects on bones and growth; potential lung toxicity, all at high doses; local skin reactions at infusion sites | Rare but severe agranulocytosis; mild neutropenia; common abdominal discomfort; erosive arthritis | Abdominal discomfort; rash or mild diarrhoea upon initiation of therapy; mild increased creatinine level |
| Ability to chelate intracellular cardiac and other tissue iron in humans | High | Probably lower than deferiprone and deferasirox (it is not clear why) | High in clinical and in in vitro studies | Insufficient clinical data available; promising in laboratory studies |
*Nephrotoxicity that has been observed in non-iron-loaded animals has been minimal in iron-overloaded humans, but effectiveness is demonstrated only at higher end of tested doses, as discussed in [1693].
Figure 7.
Some iron chelators that are in clinical use (left hand side) or that have been proposed.
Desferal (deferoxamine) is the most used chelator for historical reasons. It is hexadentate but is not orally bioavailable. Ferriprox (deferiprone) is a bidentate ligand (1,2-dimethyl, 3-hydroxypyridin-4-one). It is orally bioavailable although comparatively high doses are required, and it postdates desferal. "Deferiprone has high affinity for iron and interacts with almost all the iron pools at the molecular, cellular, tissue and organ levels. Doses of 50–120 mg/kg/day appear to be effective in bringing patients to negative iron balance" [1687]. It can have somewhat better properties than desferal [1688]. Finally, Exjade (ICL670) (deferasirox) [1689-1702] is the most recent chelator approved for clinical use, and is tridentate. It is orally active, and there is a large bibliography at http://www.exjade.com/utils/bibliography.jsp?disclaimer=Y. The recommended initial daily dose of Exjade is 20 mg/kg body weight.
It is clear from Table 1 that in the time evolution from deferoxamine through deferiprone to deferasirox there has been a noticeable improvement in the general properties of iron chelators, although there are few published data on the quantitative structure-activity relationships of candidate molecules that might allow one to design future ones rationally. What is certainly clear is that there is a trade-off in properties, and that appropriate chelators will keep iron levels intermediate, i.e. not too low and not too high (a 'Goldilocks' strategy, if you will), and that hexadentate molecules may correspondingly be too tightly binding and strip iron from important molecules that need it. What is particularly important, as well as a good plasma half-life, is the ability to cross cell membranes, as this is necessary both for oral administration and for ensuring that the chelator in question actually accesses the intracellular 'free' iron pools of interest. Which carriers are used for this in humans in vivo is presently uncertain [18,1703].
Drugs that have been approved for clinical use for other purposes, but that also happen to be iron chelators
The high investment of time, money and intellectual activity necessary to get a drug approved clinically has led to a number of strategies to exploit those that already have been approved and are thus considered 'safe'. One such strategy is the combination therapy of approved drugs that can yet serve for novel indications (e.g. [1704-1708]). Another strategy is to look for antioxidant or iron-binding chemical motifs in drugs that have already been approved for other purposes [1709] (or to measure such properties directly).
Clioquinol (CQ) [1062,1674,1710,1711] (Fig 7) is one existing (anti-parasitic) drug that has been proposed for use as an iron chelator, as it contains the known iron-chelating 8-hydroxyquinoline moiety. It has indeed enjoyed some success in this role. However, clioquinol toxicity has been reported if it is used over an extended period [1712] and this may be due to the formation of a Zn-clioquinol chelate [1713].
A particular attraction of such existing drugs is that they are likely to have favourable pharmacokinetics and pharmacodynamics, and in particular are likely to be cell-permeable. Note that despite a widespread assumption that lipophilicity or log P is sufficient to account for drug distribution this is not in fact the case, as there are hundreds of natural transporters that drugs can use (e.g. [18,1714]). For instance, the iron-chelating 8-hydroxy quinoline motif contained in molecules such as clioquinol is also present in the tryptophan catabolite xanthurenic acid (Fig 7), and it is likely that transmembrane transport of the synthetic drug molecule occurs via natural carriers whose 'normal' role is to transport endogenous but structurally related molecules [18,1703].
Iron chelators that have been studied but not yet approved
Given the importance of the field, many academic investigators have sought to develop their own iron chelators that might exhibit the desirable properties listed above. One class of molecule includes isonicotinylhydrazones. Thus, pyridoxal isonicotinyl hydrazone (PIH) [1672,1715-1720] is a promising molecule (also proposed in anti-cancer therapy), although it is hydrolysed both in vivo and in vitro [1721]. Other analogues include salicylaldehyde (SIH) [1722] and 2-hydroxy-1-napthylaldehyde (NIH) isonicotinyl hydrazones. PIH was disclosed before being patented, and is thus seen as having no pharmaceutical (company) interest. Various other derivatives are therefore being considered [1035,1462], including pyrazinylketone isonicotinoyl hydrazones [1723].
A variety of 8-hydroxyquinolines (8HQs) [1724] have been considered, although as with other bidentate and tridentate ligands that cannot necessarily effect complete liganding of iron there is always a danger that inadequate concentrations might be pro-oxidant (e.g. [1725,1726]). One molecule, VK-28, combines various pertinent moieties and has shown some promise in the treatment of neurological disorders [1727-1730]. This strategy of combining drug elements that can hit multiple targets ('polypharmacology' [807,1731-1734]) has much to commend it, including on theoretical grounds, and we discuss these in the section on systems biology below. Another 8HQ that has elicited interest is O-trensox [1431,1735-1742].
Other ligands or motifs that might be considered include di-2-pyridylketone-4,4,-dimethyl-3-thiosemicarbazone (Dp44mT), that has been shown to be effective against tumours [1743], 2,2'-dipyridyl, 1,10-phenanthroline [1744,1745], 2-benzoylpyridine thiosemicarbazones [1746] and thiohydrazones [1747]. HBED (Fig 7) (N,N'-bis-(2-hydroxybenzyl)ethylenediamine-N,N'-diacetic acid) forms a 1:1 complex with Fe(III) but is probably only tetradentate [1748]. It seems not to be very orally active [1749] but may be more effective than is DFO [1750-1752]. Poly-hydroxylated 1,4-naphthoquinones occur as sea urchin pigments and have shown protective effects [1753].
Continuing the theme of polypharmacology, R-(α)-lipoic acid [1754-1757] is also an antioxidant, that may in addition act by stimulating other anti-oxidant pathways [1758]. Finally, one interesting area is that of prochelators (e.g. [1759]) in which the oxidant itself triggers the formation of a chelator able to inhibit the Fenton reaction.
Utility of iron chelators in disease amelioration
Therapeutic uses of iron chelators have been widely and usefully reviewed (e.g. [1437,1489,1676,1760-1767]). Many problems remain, such as bioavailability, mis-dosing [1768] (too little iron as well as too much of it can be bad), toxicity, selectivity and so on, and their design is consequently highly non-trivial [1665,1670]. Nevertheless, iron chelators have demonstrated therapeutic benefits in Alzheimer's [1674,1710,1769-1771], Parkinson's [1037,1729,1772], cold-induced brain injury [1773,1774], coronary disease [714,797], renal diseases [1775], various kinds of infection [1763] and of course in iron overload diseases [1762,1767].
As mentioned above, one interesting strategy is to devise chelators that are only activated by oxidative stress [1760,1776-1779]. Another is to seek to combine different kinds of functionality in the same molecule. To this end, Youdim and colleagues in particular have developed a series of multifunctional 8-hydroxyquinoline [1740] derivatives that are effective bidentate iron chelators and that seem to show considerable promise in the treatment of a variety of neurodegenerative diseases [1037,1727-1729,1780-1784] (see also US Patent 20060234927). In this case the antioxidative mechanism is clearly via chelation since such (8-hydroxyquinoline) molecules are poor scavengers of radicals directly [1785], a fact that also makes them useful scientific tools. As bidentate ligands they cross both cell membranes and the BBB fairly easily (though lipophilicity per se seems not to be important for the biological activity of 8-hydroxyquinoline chelators [1737,1739]). Importantly, the comparatively weak bidentate binders seem not to have major long-term effects if used carefully [1483,1762,1786,1787]
Interaction of xenobiotics with iron metabolism
As Cherny and colleagues point out [1710], there are many US Pharmacopaeia-registered drugs that, while not being termed chelators, do in fact have both chelating properties and favourable toxicity profiles. Thus we need to recognise potentially both positive and negative interactions between drugs in general and iron metabolism. Any drug that can bind iron can also catalyse the formation of free radicals. Thus, gentamicin can form a gentamicin-iron complex that can lead to toxic symptoms such as hearing loss; this is reversed by iron chelators [1788,1789]. Existing drugs other than iron chelators may also have effects on iron metabolism [1790], and iron can catalyse their oxidation [1791]. It is not, of course, news that drugs have multiple effects. In this context, we reiterate that some statins, for instance, have chelating properties [893].
Other toxicants might also mediate their damaging effects through iron-catalysed radical formation [1792,1793]. This in addition to the well-known iron-catalysed, radical-mediated mechanism of toxicity of the viologens such as diquat and paraquat [1794-1799] (whose herbicidal activity is in fact inhibited by iron chelators [1800]) and of adriamycin [1801,1802]. As mentioned above, the carcinogenic action of asbestos may also be due to the ability of the Fe(n) that it contains to catalyse hydroxyl radical production [1469,1475], while carcinogenic mycotoxins such as aflatoxin may interact synergistically with iron [1803].
Dietary sources of iron chelators
There is also a considerable and positive role for nutrients in terms of their chelation of iron. Indeed, polyphenolic compounds, many of which have known health benefits [1804-1813], are widely used as food antioxidants [1814,1815]. There is of course considerable epidemiological evidence for the benefits of consuming fruit and vegetables that are likely to contain such antioxidants (e.g. [1816-1819]), and – although possibly a minimum – this has been popularised as the 'five a day' message (e.g. http://www.fruitsandveggiesmatter.gov/ and http://www.5aday.nhs.uk/). Even though elements of the 'Mediterranean' diet that are considered to be beneficial are usually assumed to be so on the basis of their antioxidant capabilities (but cf. [1820]), many of the polyphenolic compounds (e.g. flavones, isoflavones, stilbenes, flavanones, catechins (flavan-3-ols), chalcones, tannins and anthocyanidins) [1821-1828] so implicated may also act to chelate iron as well [1073,1829-1843]. This is reasonable given that many of these polyphenols and flavonoid compounds [1821,1844-1853] have groups such as the catechol moiety that are part of the known iron-binding elements of microbial siderophores. Examples include flavones such as quercetin [914,1813,1829,1854-1864], rutin [1829,1857,1858,1865,1866], baicalin [1860,1867], curcumin [1813,1868-1872], kolaviron [1873], flavonol [1874], floranol [1875], xanthones such as mangiferin [1876-1879], morin [1876], catechins [1073,1807,1838,1854,1880,1881] and theaflavins [1882], as well as procyanidins [1835,1883] and melatonin [1628,1884-1887]. However, the celebrated (trans-)-resveratrol molecule [1888-1902] may act mainly via other pathways.
A considerable number of studies with non-purified dietary constituents containing the above polyphenolic components have also shown promise in inhibiting diseases in which oxidative stress is implicated [1825,1903-1906]. For instance in stroke and related neuronal aging and stress conditions, preventative activity can be found in blueberries [1907-1913] (and see [1914]), Ginkgo biloba extract (EGb 761) [1910,1915,1916], grapes [1917], green tea [1807,1918-1921], Mangifera indica extract [1879], strawberries [1907], spinach [1907] and Crataegus [922], while combinations of some these components ('protandim') have been claimed to reduce ROS levels by stimulating the production of catalase and SOD [1922]. As with pharmaceutical drugs [18,1923-1925], there are significant problems with bioavailability [1926,1927], although the necessary measurements are starting to come forward [1804,1809,1926-1932]. There is now increasing evidence for the mechanisms with which these dietary components and related natural products and derivatives (often with anti-inflammatory, anti-mutagenic or anti-carcinogenic properties) interact with well recognised cellular signalling pathways (e.g. [1402,1935,1895,1896,1410,1413,1913,1900,1933-1990]).
Role of iron-generated ROSs in cellular signalling and oxidative stress
Thus, although this is not the focus of the present more physiologically based review, we recognise that many of relationships between ROSs and oxidative stress and overt progressive diseases may be mediated via the inflammatory signalling pathways involved in 'innate immunity' [900,1991-1993]. NF-κB is an important transcription factor, and the NF-κB system is intimately involved in this signalling [588,672,719,1408,1409,1454,1994-2019].
In the NF-κB system (e.g. [2020-2024]) (Fig 8), NF-κB is normally held inactive in the cytoplasm by binding to an inhibitor IκB (often IκBα). Pro-inflammatory cytokines such as TNF-α, LPS [2025-2030] and IL-1 [2031] act by binding to receptors at the cell surface and initiating signalling pathways that lead to the activation of a particular kinase, IκB kinase or IKK. This kinase phosphorylates the IκB causing it to be released (and ubiquitinated and degraded by the proteasome), allowing the NF-κB to be translocated to the nucleus where it can activate as many as 300 genes. Simple models of the NF-κB system show the main control elements [2032,2033] and their synergistic interaction [2034]. The NF-κB system is implicated in apoptosis [2035,2036], aging [1199], and in diseases such as cancer [1405,1444,1454,1808,2037-2040], arthritis [2040-2043] and a variety of other diseases [2044]. Antioxidants such as vitamin E [552,2017] and melatonin [2045-2049] are at least partially protective. Oxidative stress seems to act upstream of IKK [2014], on IκBα directly [2050] and in the p38 MAP kinase pathway [1993,2014,2051], and there is also evidence that at least some of the statins act on the PI3K-akt and NF-κB pathways too [819,883,2052-2060]. A considerable number of inhibitors of the NF-κB system exist [2055], many exhibiting cross-reactivity [1734].
Figure 8.
An overview of cellular signaling using the NF-κB and p38 systems. Note that some of the extracellular effectors that mediate NF-κB activation are themselves produced and secreted as a result of the activation, potentially creating an autocatalytic system.
The induction of NF-κB by ROSs appears to involve a coupling via the glutathione system [2007,2035,2036,2061-2078] (and see also [2079,2080]).
A variety of studies have shown that iron is involved in these signalling processes [1839,1996,2015,2081-2085], probably again acting upstream of the IKK [557,2083,2086,2087].
Interestingly, there is interplay between the NF-κB pathway and the regulation of NGAL [404,405,2088,2089], ferritin [2030] and hepcidin [2090], presumably acting as a negative feedback as the cell tries to control and ligand its free Fe(n) in the face of oxidative stress caused by the release of free iron [2091].
The systems biology of ROS generation and activity
It is not news that most major changes in physiological states have multigenic or multifactorial origins (e.g. [2092,2093]). This means, as an inevitable consequence, that we need to recognize that their observation requires a systems approach, and that most diseases are therefore in fact to be seen as systems or network diseases [631,2094-2102]. Changes in individual reaction steps (or even single manipulations) can change the levels of scores or hundreds of transcripts [2103], proteins [2104] or metabolites [725]. In this regard, small molecule (metabolomics) measurements have especial advantages for capturing network organisation, including on theoretical grounds [2105-2112].
If we consider just one variable of present relevance, the quantity of hydroxyl radical, the amount that is able to react with proteins, lipids and DNA is clearly determined by a huge number of reactions, whether directly or otherwise – not only the concentrations of reagents and enzyme that directly catalyse its formation and destruction but by everything else that affects their concentration and activity, and so on. This is of course well established in biology in the formalism of metabolic control analysis (MCA) (see e.g. [2113-2120]), and was recognized over 30 years ago in Morris' prescient review on anaerobes [2121]. Modern systems theories of aging (e.g. [1175,1180,2120,2122] and above) (Fig 9) also recognize physiological progression as being determined in terms of a balance between 'good' and 'bad' factors. MCA and related formalisms can be seen as theories of sensitivity analysis, which in many cases can be normalized such that an overall output function can be described quantitatively in terms of the relative contributions of each of its component steps (e.g. [2123-2127]). In MCA the normalized local sensitivities are known as control coefficients, and the sum of the concentration-control coefficients = 0, in other words in the steady state the rate of production and consumption of a particular entity is in balance and all reactions can contribute to it to some degree. The concentration-control coefficient describes this degree quantitatively. It is now possible to produce appropriate quantitative representations of metabolic networks using quite sparse kinds of information (in fact just the stoichiometry and network structure [2128]), and thereby provide initial estimates for more sophisticated fitting algorithms (e.g. [2129-2132]. Indeed, the analysis of the properties and behaviour of networks is at the core of modern systems biology (e.g. [2095,2133-2140]).
Figure 9.
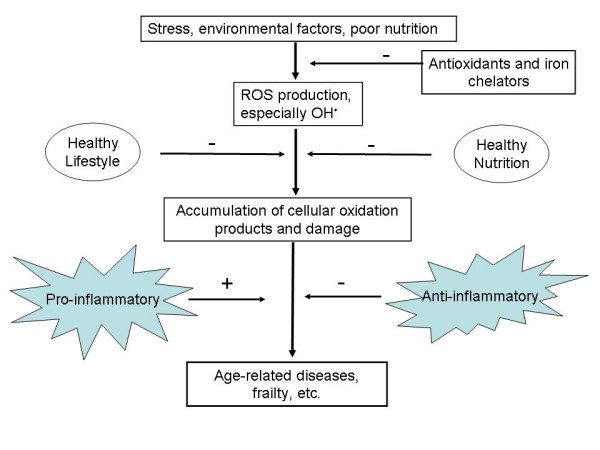
General view of the role of iron, antioxidants and ROSs in aging and degenerative processes. Some of the decay may be ameliorated by lifestyle and dietary means. Based in part on [1175].
A corollary of such considerations is that to decrease the amount of damage caused by OH• (or any other) radicals we need both to decrease their production and increase their removal to harmless substances [2141], and that on general grounds [1706-1708,2142] such a strategy (for instance of combining a cell-permeable iron chelator with a cell-permeable antioxidant) might be expected to give a synergistic response. Even determining the means of cell permeability and tissue distribution turns out to be a systems biology problem in which we need to know the nature and activity of all the carriers that are involved [18,1703,1714,2143]. At all events, it is undoubtedly the case that the steady-state rate of production of a molecule such as the hydroxyl radical is controlled or affected by a considerable number of steps. These minimally include the multiple reactions of the mitochondrial respiratory chain and the various oxidases producing superoxide and peroxide, the activities of catalase and SOD enzymes that together can remove them, protective reactions such as heat-shock proteins, and most pertinently to the present review a large number of reactions involved in the metabolism and safe liganding of iron that help determine the rate at which OH• is produced.
It is also pertinent to enquire as to why we are now seeing so many of these progressive diseases, and as to what may be their causes. Undoubtedly the simple fact of improved longevity is one [165] as damage accumulates. However, we note that anything that decreases the amount of unliganded iron, such as decreasing the total dietary iron intake e.g. from red meat, must be helpful [1156,2144].
Anti-inflammatory cytokines; the example of erythropoietin
We have above adduced considerable evidence that decreasing the amount of hydroxyl radical by any means is valuable, whether by removing initially generated ROSs such as superoxide and peroxide or by chelating poorly liganded iron in a way that stops these ROSs forming the hydroxyl radical. While pro-inflammatory cytokines can themselves increase ROS production and modulate the activities of signaling pathways such as NF-κB and p38, there are also anti-inflammatory cytokines. A particularly interesting example is that of erythropoietin (also discussed above as being protective in a number of iron-mediated diseases).
Erythropoietin was originally recognized via its role in erythropoiesis [2145-2147] (hence its name, of course), but it has become evident that it has many other roles, and in particular it is observed phenomenologically that erythropoietin (and non-erthyropoetic derivatives) is protective in a number of inflammatory conditions that accompany many diseases such as those listed above [940,942,2148-2155]. These included cardiovascular disease [721,722,2156-2175], stroke and other related neurological diseases [924-926,928,929,933,937,938,2155,2176-2204], diabetic neuropathy [2205], kidney injury [2173,2206-2212], intestinal injury [2213] and shock (both septic and non-septic) [1596,1597,2214].
The question then arises as to how it is doing this mechanistically, and the proximate answer is that it (and other anti-inflammatory agents, e.g. [1808,2215,2216]) seem to act via many of the same signalling pathways as do inflammatory agents [943,2150,2217-2226]. There is evidence that it can help maintain superoxide dismutase activity [2214,2227], invoke haem oxygenase [2228], and in particular – from the perspective of this review – that it may remove poorly liganded iron [2229] and interact with hydroxyl radical directly [2230-2233].
It is notable that appropriate levels of erythropoietin appear not only to be efficacious but to be safe, even in pregnancy [2234-2241]. Erythropoietin may itself be a marker of hypoxia and oxidative stress in pregnancy [531,2242-2245], consistent with a view that the body is attempting to deal with these problems by creating anti-inflammatory cytokines.
Hypoxia-inducible factor (HIF)
Although I am mainly not concentrating on genetic regulatory aspects in this review, the HIF [2246,2247] does deserve some mention, since many of the syndromes described above are accompanied by hypoxia, and this causes levels of the HIF to increase. HIF is a transcription factor that can activate a considerable number of genes, including VEGF [1951,2246-2250]. In contrast to the constitutive expression of HIF-1α, HIF-1β protein levels are regulated in response to the cellular oxygen concentration [2251]. The active HIF is the HIF-1αβ heterodimer [2252]. HIF couples anoxia to innate immunity via the NF-κB system [2253]. In particular, HIF effects (via hepcidin) the mobilisation or iron and can cause the expression of inflammatory cytokines such as IL-1, IL-6 and TNF-α [2254-2256] under conditions (hypoxia) where superoxide and peroxide production are likely to be increased, and consequently increases sepsis (in that HIF-knockout mice are resistant to LPS-induced sepsis [2254,2255]). By contrast, induction of HIF (and the genes that it activates) can effect neuroprotection [2252,2257]. HIF also appears to have a significant role in placental development, and defective HIF expression may be involved in pre-eclampsia and intra-uterine growth retardation [435,2246,2258]. Qutub and colleagues provide useful models [2259,2260] of HIF activation under a variety of conditions of iron, O2, 2-oxoglutarate and other factors.
Autocatalysis, positive feedback and Systems Biology
What has emerged in recent years is a recognition that the structure (i.e. topology) of the modules of metabolic and signalling networks, somewhat independent of the individual activities of their components, can have a profound controlling influence on their behaviour (e.g. [2107,2134,2135,2261,2262]). Classically, negative feedback structures are considered to confer stability, while positive feedbacks tend to have the opposite effect. However, negative feedbacks with delay loops can cause oscillations [2022,2109] while some kinds of positive feedback loops can confer stability [2262,2263]. However, there is no doubt that structures in which a damaging agent causes the production of a second damaging agent that itself catalyses the production of the first or a separate damaging agent can exhibit a runaway kind of damage. This is exactly what can happen with iron and superoxide since Fe(n) can be liberated from ferritin by superoxide radicals and then catalyse the production of further hydroxyl radical by increasing the amount of free iron (Fig 6). A similar effect can occur with Fe-S proteins in SOD-deficiency [1148], with the degradation of mitochondria by radical damage leading to further production of radicals [28,30,2264], and the effects of oxidative stress on iron storage [2265]. This again illustrates the importance of acting at multiple points in a network to control these kinds of damage. Exactly the same is true of the IL-1 and TNF-α systems in which IL-1 or TNF-α (oxidative stress) acting on one cell can effect the secretion of further IL-1/TNF-α that can act on adjacent cells (Fig 8), of the hypoxia-dependent increase in both ROSs and serum iron mediated by hepcidin (Fig 3), the autocatalytic synergy between overfeeding, inflammation and (pre-)diabetes, and of the peroxide/iron pair that are liberated when frataxin is deficient (see above). It is these kinds of synergistic effects and autocatalytic cycles that are the hallmark of the major and progressive effects on human physiology that are seen in these kinds of system. Indeed, one might comment that such multi-site and autocatalytic effects are required to overwhelm normal defences precisely because human metabolic and signalling systems are 'robust' systems that have evolved topologies that are resistant or robust to parameter changes (see e.g. [2266-2291]).
Predictive biology
It is often considered (e.g. [2292,2293]) that a desirable feature of a scientific idea is its ability to make useful predictions, and while this is not in fact a particularly well founded philosophical principle, it probably is of value to set out a couple of 'predictions' that follow from the present analysis. One prominent feature of the above is the primacy of the iron-catalysed production of the damaging hydroxyl radical, and thus a test of the involvement of these kinds of reactions in the various physiological and pathological states to which we allude is the prediction that they should be accompanied by markers of oxidative stress characteristic of reactions of endogenous metabolites with the hydroxyl radical. While it is not that easy to disentangle the complex reactions of ROSs with biomolecules [43,1055], at least the following appear to be a result of reactions involving OH• [2294,2295]: 8-oxo-2'-deoxyguanosine (oxo8dG) [98,2296,2297], 8-oxo-7,8- dihydro-2'-deoxyguanosine [90,92] and thymine glycol [92,2294].
Another set of predictions from the systems biology perspective [1704-1708,1731,2138,2291,2298-2310] is that combinations of chemical agents (or manipulations such as those of transcription factors that affect multiple steps in a pathway [1906] or modulation of multiple gene products [2311], or both [2312,2313]) will be far more efficacious, for instance in modulating iron-catalysed oxidative stress and its sequelae, than will be the use of 'magic bullet' single agents. Such combinations of 'causes' do not have to be guessed a priori but can be obtained via inferencing techniques (e.g. [2314-2320]) – for a recent example see [2321]. The nonlinear behaviour of biochemical networks also serves to explain the bell-shaped dose-response curves underpinning the hormesis [2322-2326] often observed.
Iron-mediated oxidative stress is arguably the cause of much of the inflammation typically observed in biological systems, often further mediated via pro-inflammatory cytokines. Another major prediction that comes from the above then is that molecules that are anti-inflammatory, whether widely recognised as such or not, should have beneficial effects in syndromes for which they have not necessarily been tested. An obvious set of candidates in this regard are to be found among the statins, since it is now clear that they have important anti-inflammatory properties (see above). Thus, there are already indications that as well as their established benefits in cardiovascular disease (e.g. [804,2327]) they may exert benefit in a huge variety of syndromes [838], including sepsis [839,2060,2328-2340], heart failure [2341], pain perception [2342], lupus and related diseases [1293,2343], diabetes [877,2344], rheumatoid arthritis [866,869,890,2345-2350], kidney disease [2351-2353], inflammatory skin disease [2354], emphysema [2355], ischaemia-reperfusion injury [2356], stroke [864,872,2357-2364], traumatic brain injury [2365-2367], neurodegenerative diseases [860-862,920,1294,2059,2368-2384], neurotoxicity [2385] and cancer [2386-2400].
Concluding remarks and quo vadis
"Actually, the orgy of fact extraction in which everybody is currently engaged has, like most consumer economies, accumulated a vast debt. This is a debt of theory and some of us are soon going to have an exciting time paying it back – with interest, I hope." [2401].
"But one thing is certain: to understand the whole you must look at the whole" [2402]
"If you are not thinking about your experiments on a whole-genome level you are going to be a dinosaur". J. Stamatoyannopoulos, quoted in [2403].
While it is less common for scientists to publish 'negative' results ('there was no effect of some agent on some process'), and there has been a tendency to seek to falsify specific hypotheses rather than to paint a big picture [2404], there is no doubt that the sheer weight of positive evidence can be persuasive in leading one to a view. As Bertrand Russell put it [2405], "When one admits that nothing is certain one must, I think, also admit that some things are much more nearly certain than others." However, as mentioned above, the huge volume of scientific activity has in many ways led to a 'balkanisation' of the literature [2406] in which scientists deal with the problem of the deluge of published papers by necessarily ignoring most of them. This is no longer realistic (nor necessary) in an age of post-genomics, the internet, Web 2.0 and systems biology, and when we are starting to move to integrative (if distributed) models of organisms (including humans) at a whole organ, genome or whole organism scale [118,2135,2407-2416]. The 'digital human' is thus an important research goal [2410,2415-2417]. Expression profiling atlases are becoming increasingly widespread (e.g. [2418-2423]), and one can anticipate using these straightforwardly to extend these 'generalised' (sub)cellular network models in a tissue-specific manner [2424]. With the ability to exchange models of biochemical networks in a principled way [2425-2428], when they are marked up appropriately (e.g. [3,4]), we can expect to begin to reason about them automatically [118,2429], such that we may soon look forward to an era in which we can recognise the commonalities across a variety of different subfields – a specific message of the present overview. Thus, while iron and metabolism should be considered in the context of other processes that may be contributing to the disorders discussed, and it is evident that they are intimately involved in many disease processes, therapies derived for one of the inflammatory diseases listed above may well have benefit in some of the others where their underlying 'causes' are the same. The 'mass collaboration' agenda (e.g. [2430-2435]), in which dispersed agents contribute their different skills to the solution of a complex problem, may well help this happen effectively. Developments in distributed workflow technology, such as the Taverna [22-26,2436,2437]http://taverna.sourceforge.net/ and the myExperiment [2438]http://www.myexperiment.org/ environments, represent an intellectually important approach. Important too to this endeavour will be Open Access initiatives [1,2439,2440] and institutional and other repositories [2441,2442] of full-text papers. This will help to build an accurate picture of the biochemical networks operating in both normal and diseased states (and see [2443]), preferably marked up semantically as in [118], and hence, by modelling them [2263], where best to consider interventions. In order to develop and exploit this distributed approach, it will also be necessary for those generating them to make their data and metadata available (preferably in a semantically marked up way), probably as Web Services (e.g. [24,2444-2452]), and to give greater scientific weight to those involved in bio-curation [119] as they will be an increasingly important part of the scientific landscape. Modern sequencing instruments, for instance (e.g. [2453-2457]) are generating quality data at truly enormous rates [2458], and innovative but computationally demanding algorithms are required to deal with them (e.g. [2459]). In particular, however, we need tools for manipulating and visualising biochemical models [2110,2410,2429,2460]. As well as storing these models (e.g. as SBML or CellML [2461]) in a file format, it is also convenient to store them in a database format, such as the B-net database developed by Mendes and colleagues [118,2462]. Federated annotation protocols such as the Distributed Annotaton Scheme (see e.g. [2463]) allow data from heterogeneous sources to be combined, while other integrative/distributed architectures such as ONDEX [2464] and Utopia [2465-2468] are similarly showing considerable promise for integrative systems biology.
Given the widely dispersed communities that have been referenced herein, and the future requirement for integrating knowledge generated throughput the world, a programme for understanding the combinatorial roles of poorly liganded iron and reactive oxygen species in the aetiology of many diseases, as set out in the above, appears to be prototypical for the kinds of new approaches to doing science that we may anticipate in the eras of Web 2.0 and the Semantic Web.
Abbreviations
AECOPD: Acute Exacerbation of Chronic Obstructive Pulmonary Disorder; AKI: Acute Kidney Injury; ALS: Amyotrophic Lateral Sclerosis; AMD: Age-related Macular Degeneration; BBB: Blood-brain barrier; ChEBI: Chemical Entities of Biological Interest; CQ: Clioquinol; COPD: Chronic Obstructive Pulmonary Disorder; DFO: Desferrioxamine; Dp44mT: di-2-pyridylketone-4,4,-dimethyl-3-thiosemicarbazone; EDTA: Ethylenediamine-tetraacetic Acid; FA: Friedrich's ataxia; FPN1: Ferroportin-1; HBED: N,N'-bis-(2-hydroxybenzyl)ethylenediamine-N,N'-diacetic acid; HCP1: Heme Carrier Protein-1; HIF: Hypoxia-Inducible Factor; HO1: Heme Oxygenase-1; HMG-CoA: Hydroxymethyl glutaryl Coenzyme A; Hp: Hephaestin; 8HQs: A variety of 8-hydroxyquinolines; IBD: Inflammatory Bowel Disease; InChI: International Chemical Identifier; IKK: IκB kinase; KEGG: Kyoto Encyclopedia of Genes and Genomes; L1: Deferriprone; LFA-1: Leukocyte Function Antigen-1; LIP: Labile Iron Pool; LIPID: Long-Term Intervention with Pravastatin in Ischaemic Disease; LPS: Lipopolysaccharide; MOF: Multiple organ failure; NF-κB: Nuclear Factor κB; NGAL: Neutrophil Gelatinase-Associated Lipocalin (Also known as lipocalin-2 or siderocalin); NIH: 2-hydroxy-1-napthylaldehyde isonicotinyl hydrazone; PE: Pre-eclampsia; PIH: Pyridoxal isonicotinyl hydrazone; PPAR: Peroxisome Proliferator Activated Receptor; RA: Rheumatoid Arthritis; ROS: Reactive Oxygen Species; SBML: Systems Biology Markup Language; SIH: Salicylaldehyde isonicotinyl hydrazone; SIRS: Systemic Inflammatory Response Syndrome; SLE: Systemic Lupus Erythematosus; SMILES: Simplified Molecular Input Line Entry Specification; SOD: Superoxide Dismutase; Tf: Transferrin; TNF: Tissue Necrosis Factor; VEGF: Vascular Endothelial Growth Factor.
Competing interests
The author declares that he has no competing interests.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Acknowledgments
Acknowledgements
I thank Jon Barasch (Columbia University) for drawing my attention to the role of NGAL in human physiology, and Phil Baker, David Brough and Louise Kenny for many further useful and enjoyable discussions. I thank Katya Tarasova for considerable assistance with literature gathering and Julie Cowley for assistance with the proofs. Much of my work when this was written has been supported by the UK BBSRC and EPSRC, for which I am most grateful, and I am also a recipient of funding from the British Heart Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. This is a contribution from the Manchester Centre for Integrative Systems Biology http://www.mcisb.org.
References
- Harnad S, Brody T, Vallieres F, Carr L, Hitchcock S, Gingras Y, Oppenheim C, Hajjem C, Hilf ER. The access/impact problem and the green and gold roads to open access: An update. Serials Review. 2008;34:36–40. [Google Scholar]
- Hull D, Pettifer SR, Kell DB. Defrosting the digital library: bibliographic tools for the next generation web. PLoS Comput Biol. 2008;4:e1000204. doi: 10.1371/journal.pcbi.1000204. doi:10.1371/journal.pcbi.1000204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ananiadou S, McNaught J. Text mining in biology and biomedicine. Artech House, London; 2006. [Google Scholar]
- Ananiadou S, Kell DB, Tsujii Ji. Text Mining and its potential applications in Systems Biology. Trends Biotechnol. 2006;24:571–579. doi: 10.1016/j.tibtech.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Jensen LJ, Saric J, Bork P. Literature mining for the biologist: from information retrieval to biological discovery. Nat Rev Genet. 2006;7:119–29. doi: 10.1038/nrg1768. [DOI] [PubMed] [Google Scholar]
- Smalheiser NR. The Arrowsmith project: 2005 status report. LNCS. 2005;3735:26–43. [Google Scholar]
- Hristovski D, Peterlin B, Mitchell JA, Humphrey SM. Using literature-based discovery to identify disease candidate genes. Int J Med Inform. 2005;74:289–98. doi: 10.1016/j.ijmedinf.2004.04.024. [DOI] [PubMed] [Google Scholar]
- Kostoff RN. Systematic acceleration of radical discovery and innovation in science and technology. Technol Forecasting Soc Change. 2006;73:923–936. [Google Scholar]
- Yetisgen-Yildiz M, Pratt W. Using statistical and knowledge-based approaches for literature-based discovery. J Biomed Informatics. 2006;39:600–611. doi: 10.1016/j.jbi.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Kostoff RN. Validating discovery in literature-based discovery. J Biomed Inform. 2007;40:448–50. doi: 10.1016/j.jbi.2007.05.001. author reply 450–2. [DOI] [PubMed] [Google Scholar]
- Kostoff RN, Briggs MB, Solka JL, Rushenberg RL. Literature-related discovery (LRD): Methodology. Technol Forecast Soc Change. 2008.
- Cronin B. Bibliometrics and beyond: Some thoughts on web-based citation analysis. Journal of Information Science. 2001;27:1–7. [Google Scholar]
- Kasztler A, Leitner KH. Bibliometric analysis and visualisation of intellectual capital. J Universal Comp Sci. 2002;8:516–525. [Google Scholar]
- Tabah AN. Literature dynamics: Studies on growth, diffusion, and epidemics. Annu Rev Inf Sci Technol. 1999;34:249–286. [Google Scholar]
- Börner K, Chen CM, Boyack KW. Visualizing knowledge domains. Annual Review of Information Science and Technology. 2003;37:179–255. [Google Scholar]
- Errami M, Hicks JM, Fisher W, Trusty D, Wren JD, Long TC, Garner HR. Déjà vu: a study of duplicate citations in Medline. Bioinformatics. 2008;24:243–9. doi: 10.1093/bioinformatics/btm574. [DOI] [PubMed] [Google Scholar]
- Kell DB. Iron behaving badly: inappropriate iron chelation as a major contributor to the aetiology of vascular and other progressive inflammatory and degenerative diseases. 2008. http://arxiv.org/ftp/arxiv/papers/0808/0808.1371.pdf [DOI] [PMC free article] [PubMed]
- Dobson PD, Kell DB. Carrier-mediated cellular uptake of pharmaceutical drugs: an exception or the rule? Nat Rev Drug Discov. 2008;7:205–220. doi: 10.1038/nrd2438. [DOI] [PubMed] [Google Scholar]
- Smalheiser NR, Torvik VI, Bischoff-Grethe A, Burhans LB, Gabriel M, Homayouni R, Kashef A, Martone ME, Perkins GA, Price DL, Talk AC, West R. Collaborative development of the Arrowsmith two node search interface designed for laboratory investigators. J Biomed Discov Collab. 2006;1:8. doi: 10.1186/1747-5333-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalheiser NR, Swanson DR. Using ARROWSMITH: a computer-assisted approach to formulating and assessing scientific hypotheses. Comput Methods Programs Biomed. 1998;57:149–53. doi: 10.1016/s0169-2607(98)00033-9. [DOI] [PubMed] [Google Scholar]
- Doms A, Schroeder M. GoPubMed: exploring PubMed with the Gene Ontology. Nucleic Acids Res. 2005;33:W783–6. doi: 10.1093/nar/gki470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oinn T, Addis M, Ferris J, Marvin D, Senger M, Greenwood M, Carver T, Glover K, Pocock MR, Wipat A, Li P. Taverna: a tool for the composition and enactment of bioinformatics workflows. Bioinformatics. 2004;20:3045–54. doi: 10.1093/bioinformatics/bth361. [DOI] [PubMed] [Google Scholar]
- Oinn T, Li P, Kell DB, Goble C, Goderis A, Greenwood M, Hull D, Stevens R, Turi D, Zhao J. Taverna/myGrid: aligning a workflow system with the life sciences community. In: Taylor IJ, Deelman E, Gannon DB, Shields M, editor. Workflows for e-Science: scientific workflows for Grids. Springer, Guildford; 2007. pp. 300–319. [Google Scholar]
- Hull D, Wolstencroft K, Stevens R, Goble C, Pocock MR, Li P, Oinn T. Taverna: a tool for building and running workflows of services. Nucleic Acids Res. 2006;34:W729–32. doi: 10.1093/nar/gkl320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oinn T, Greenwood M, Addis M, Alpdemir MN, Ferris J, Glover K, Goble C, Goderis A, Hull D, Marvin D, Li P, Lord P, Pocock MR, Senger M, Stevens R, Wipat A, Wroe C. Taverna: lessons in creating a workflow environment for the life sciences. Concurrency and Computation: Practice & Experience. 2006;18:1067–1100. [Google Scholar]
- Goble C, Wolstencroft K, Goderis A, Hull D, Zhao J, Alper P, Lord P, Wroe C, Belhajjame K, Turi D, Stevens R, Oinn T, De Roure D. Knowledge discovery for biology with Taverna: producing and consuming semantics in the Web of Science. In: Baker CJO, Cheung K-H, editor. Semantic Web: revolutionising knowledge discovery in the life sciences. Springer, New York; 2007. [Google Scholar]
- Chance B, Sies H, Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev. 1979;59:527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- Lenaz G. Role of mitochondria in oxidative stress and ageing. Biochim Biophys Acta. 1998;1366:53–67. doi: 10.1016/s0005-2728(98)00120-0. [DOI] [PubMed] [Google Scholar]
- Barja G. Mitochondrial oxygen radical generation and leak: sites of production in states 4 and 3, organ specificity, and relation to aging and longevity. J Bioenerg Biomembr. 1999;31:347–66. doi: 10.1023/a:1005427919188. [DOI] [PubMed] [Google Scholar]
- Kowaltowski AJ, Vercesi AE. Mitochondrial damage induced by conditions of oxidative stress. Free Radic Biol Med. 1999;26:463–71. doi: 10.1016/s0891-5849(98)00216-0. [DOI] [PubMed] [Google Scholar]
- Cadenas E, Davies KJ. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic Biol Med. 2000;29:222–30. doi: 10.1016/s0891-5849(00)00317-8. [DOI] [PubMed] [Google Scholar]
- Lenaz G, D'Aurelio M, Merlo Pich M, Genova ML, Ventura B, Bovina C, Formiggini G, Parenti Castelli G. Mitochondrial bioenergetics in aging. Biochim Biophys Acta. 2000;1459:397–404. doi: 10.1016/s0005-2728(00)00177-8. [DOI] [PubMed] [Google Scholar]
- Raha S, Robinson BH. Mitochondria, oxygen free radicals, disease and ageing. Trends Biochem Sci. 2000;25:502–8. doi: 10.1016/s0968-0004(00)01674-1. [DOI] [PubMed] [Google Scholar]
- Lenaz G. The mitochondrial production of reactive oxygen species: mechanisms and implications in human pathology. IUBMB Life. 2001;52:159–64. doi: 10.1080/15216540152845957. [DOI] [PubMed] [Google Scholar]
- Raha S, Robinson BH. Mitochondria, oxygen free radicals, and apoptosis. Am J Med Genet. 2001;106:62–70. doi: 10.1002/ajmg.1398. [DOI] [PubMed] [Google Scholar]
- Dröge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- Taylor ER, Hurrell F, Shannon RJ, Lin TK, Hirst J, Murphy MP. Reversible glutathionylation of complex I increases mitochondrial superoxide formation. J Biol Chem. 2003;278:19603–10. doi: 10.1074/jbc.M209359200. [DOI] [PubMed] [Google Scholar]
- Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. 2003;552:335–44. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam-Vizi V. Production of reactive oxygen species in brain mitochondria: Contribution by electron transport chain and non-electron transport chain sources. Antioxidants & Redox Signaling. 2005;7:1140–1149. doi: 10.1089/ars.2005.7.1140. [DOI] [PubMed] [Google Scholar]
- Andreyev AY, Kushnareva YE, Starkov AA. Mitochondrial metabolism of reactive oxygen species. Biochemistry (Mosc) 2005;70:200–14. doi: 10.1007/s10541-005-0102-7. [DOI] [PubMed] [Google Scholar]
- Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Adam-Vizi V, Chinopoulos C. Bioenergetics and the formation of mitochondrial reactive oxygen species. Trends Pharmacol Sci. 2006;27:639–45. doi: 10.1016/j.tips.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. 4. Oxford University Press, Oxford; 2006. [Google Scholar]
- Orrenius S, Gogvadze A, Zhivotovsky B. Mitochondrial oxidative stress: Implications for cell death. Annual Review of Pharmacology and Toxicology. 2007;47:143–183. doi: 10.1146/annurev.pharmtox.47.120505.105122. [DOI] [PubMed] [Google Scholar]
- Fato R, Bergamini C, Leoni S, Strocchi P, Lenaz G. Generation of reactive oxygen species by mitochondrial complex I: implications in neurodegeneration. Neurochem Res. 2008;33:2487–501. doi: 10.1007/s11064-008-9747-0. [DOI] [PubMed] [Google Scholar]
- Babior BM. Phagocytes and oxidative stress. Am J Med. 2000;109:33–44. doi: 10.1016/s0002-9343(00)00481-2. [DOI] [PubMed] [Google Scholar]
- Cave AC, Brewer AC, Narayanapanicker A, Ray R, Grieve DJ, Walker S, Shah AM. NADPH oxidases in cardiovascular health and disease. Antioxidants & Redox Signaling. 2006;8:691–728. doi: 10.1089/ars.2006.8.691. [DOI] [PubMed] [Google Scholar]
- Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- Brown JM, Terada LS, Grosso MA, Whitmann GJ, Velasco SE, Patt A, Harken AH, Repine JE. Xanthine oxidase produces hydrogen peroxide which contributes to reperfusion injury of ischemic, isolated, perfused rat hearts. J Clin Invest. 1988;81:1297–1301. doi: 10.1172/JCI113448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger DN. Role of xanthine oxidase and granulocytes in ischemia reperfusion injury. Amer J Physiol. 1988;255:H1269–H1275. doi: 10.1152/ajpheart.1988.255.6.H1269. [DOI] [PubMed] [Google Scholar]
- Linas SL, Whittenburg D, Repine JE. Role of xanthine oxidase in ischemia reperfusion injury. Amer J Physiol. 1990;258:F711–F716. doi: 10.1152/ajprenal.1990.258.3.F711. [DOI] [PubMed] [Google Scholar]
- Thompson-Gorman SL, Zweier JL. Evaluation of the role of xanthine oxidase in myocardial reperfusion injury. J Biol Chem. 1990;265:6656–6663. [PubMed] [Google Scholar]
- Adkins WK, Taylor AE. Role of xanthine oxidase and neutrophils in ischemia-reperfusion injury in rabbit lung. J Appl Physiol. 1990;69:2012–2018. doi: 10.1152/jappl.1990.69.6.2012. [DOI] [PubMed] [Google Scholar]
- Müller MJ, Vollmar B, Friedl HP, Menger MD. Xanthine oxidase and superoxide radicals in portal triad crossclamping-induced microvascular reperfusion injury of the liver. Free Radic Biol Med. 1996;21:189–97. doi: 10.1016/0891-5849(96)00028-7. [DOI] [PubMed] [Google Scholar]
- Imlay JA. Cellular defenses against superoxide and hydrogen peroxide. Annu Rev Biochem. 2008;77:755–76. doi: 10.1146/annurev.biochem.77.061606.161055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaresan M, Yu ZX, Ferrans VJ, Irani K, Finkel T. Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science. 1995;270:296–9. doi: 10.1126/science.270.5234.296. [DOI] [PubMed] [Google Scholar]
- Finkel T. Oxidant signals and oxidative stress. Curr Opin Cell Biol. 2003;15:247–54. doi: 10.1016/s0955-0674(03)00002-4. [DOI] [PubMed] [Google Scholar]
- Rhee SG. Cell signaling. H2O2, a necessary evil for cell signaling. Science. 2006;312:1882–3. doi: 10.1126/science.1130481. [DOI] [PubMed] [Google Scholar]
- Miller EW, Tulyathan O, Isacoff EY, Chang CJ. Molecular imaging of hydrogen peroxide produced for cell signaling. Nat Chem Biol. 2007;3:263–7. doi: 10.1038/nchembio871. [DOI] [PubMed] [Google Scholar]
- Forman HJ, Fukuto JM, Miller T, Zhang H, Rinna A, Levy S. The chemistry of cell signaling by reactive oxygen and nitrogen species and 4-hydroxynonenal. Arch Biochem Biophys. 2008;477:183–95. doi: 10.1016/j.abb.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JM. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J. 1984;219:1–14. doi: 10.1042/bj2190001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JMC. Role of free radicals and catalytic metal ions in human disease – an overview. Meth Enzymol. 1990;186:1–85. doi: 10.1016/0076-6879(90)86093-b. [DOI] [PubMed] [Google Scholar]
- Galaris D, Pantopoulos K. Oxidative stress and iron homeostasis: mechanistic and health aspects. Crit Rev Clin Lab Sci. 2008;45:1–23. doi: 10.1080/10408360701713104. [DOI] [PubMed] [Google Scholar]
- Buzan T. How to mind map. Thorsons, London; 2002. [Google Scholar]
- Fridovich I. Superoxide radical and superoxide dismutases. Annu Rev Biochem. 1995;64:97–112. doi: 10.1146/annurev.bi.64.070195.000525. [DOI] [PubMed] [Google Scholar]
- Wardman P, Candeias LP. Fenton chemistry: An introduction. Rad Res. 1996;145:523–531. [PubMed] [Google Scholar]
- Kehrer JP. The Haber-Weiss reaction and mechanisms of toxicity. Toxicology. 2000;149:43–50. doi: 10.1016/s0300-483x(00)00231-6. [DOI] [PubMed] [Google Scholar]
- Hershko C, Weatherall DJ. Iron-chelating therapy. Crit Rev Clin Lab Sci. 1988;26:303–45. doi: 10.3109/10408368809105894. [DOI] [PubMed] [Google Scholar]
- Minotti G. Sources and role of iron in lipid peroxidation. Chem Res Toxicol. 1993;6:134–146. doi: 10.1021/tx00032a001. [DOI] [PubMed] [Google Scholar]
- Rayman MP, Barlis J, Evans RW, Redman CW, King LJ. Abnormal iron parameters in the pregnancy syndrome preeclampsia. Am J Obstet Gynecol. 2002;187:412–8. doi: 10.1067/mob.2002.123895. [DOI] [PubMed] [Google Scholar]
- Welch KD, Davis TZ, Van Eden ME, Aust SD. Deleterious iron-mediated oxidation of biomolecules. Free Radic Biol Med. 2002;32:577–83. doi: 10.1016/s0891-5849(02)00760-8. [DOI] [PubMed] [Google Scholar]
- Cheng Z, Li Y. What is responsible for the initiating chemistry of iron-mediated lipid peroxidation: an update. Chem Rev. 2007;107:748–66. doi: 10.1021/cr040077w. [DOI] [PubMed] [Google Scholar]
- Tsukahara H. Biomarkers for oxidative stress: Clinical application in pediatric medicine. Curr Med Chem. 2007;14:339–351. doi: 10.2174/092986707779941177. [DOI] [PubMed] [Google Scholar]
- Evans MD, Dizdaroglu M, Cooke MS. Oxidative DNA damage and disease: induction, repair and significance. Mut Res. 2004;567:1–61. doi: 10.1016/j.mrrev.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Valko M, Izakovic M, Mazur M, Rhodes CJ, Telser J. Role of oxygen radicals in DNA damage and cancer incidence. Mol Cell Biochem. 2004;266:37–56. doi: 10.1023/b:mcbi.0000049134.69131.89. [DOI] [PubMed] [Google Scholar]
- David SS, O'Shea VL, Kundu S. Base-excision repair of oxidative DNA damage. Nature. 2007;447:941–50. doi: 10.1038/nature05978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtman ER, Oliver CN. Metal-catalyzed oxidation of proteins – physiological consequences. J Biol Chem. 1991;266:2005–2008. [PubMed] [Google Scholar]
- Davies MJ, Fu S, Wang H, Dean RT. Stable markers of oxidant damage to proteins and their application in the study of human disease. Free Radic Biol Med. 1999;27:1151–63. doi: 10.1016/s0891-5849(99)00206-3. [DOI] [PubMed] [Google Scholar]
- Davies MJ. The oxidative environment and protein damage. Biochim Biophys Acta. 2005;1703:93–109. doi: 10.1016/j.bbapap.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Terman A, Brunk UT. Lipofuscin. Int J Biochem Cell Biol. 2004;36:1400–1404. doi: 10.1016/j.biocel.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Jung T, Bader N, Grune T. Lipofuscin: formation, distribution, and metabolic consequences. Ann N Y Acad Sci. 2007;1119:97–111. doi: 10.1196/annals.1404.008. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Free radicals and antioxidants: a personal view. Nutr Rev. 1994;52:253–65. doi: 10.1111/j.1753-4887.1994.tb01453.x. [DOI] [PubMed] [Google Scholar]
- De Zwart LL, Meerman JHN, Commandeur JNM, Vermeulen NPE. Biomarkers of free radical damage applications in experimental animals and in humans. Free Rad Biol Med. 1999;26:202–226. doi: 10.1016/s0891-5849(98)00196-8. [DOI] [PubMed] [Google Scholar]
- Kohen R, Nyska A. Oxidation of biological systems: oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol Pathol. 2002;30:620–50. doi: 10.1080/01926230290166724. [DOI] [PubMed] [Google Scholar]
- Tarpey MM, Wink DA, Grisham MB. Methods for detection of reactive metabolites of oxygen and nitrogen: in vitro and in vivo considerations. Am J Physiol Regul Integr Comp Physiol. 2004;286:R431–44. doi: 10.1152/ajpregu.00361.2003. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Whiteman M. Measuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and what do the results mean? Br J Pharmacol. 2004;142:231–55. doi: 10.1038/sj.bjp.0705776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherubini A, Ruggiero C, Polidori MC, Mecocci P. Potential markers of oxidative stress in stroke. Free Radical Biology and Medicine. 2005;39:841–852. doi: 10.1016/j.freeradbiomed.2005.06.025. [DOI] [PubMed] [Google Scholar]
- Shulaev V, Oliver DJ. Metabolic and proteomic markers for oxidative stress. New tools for reactive oxygen species research. Plant Physiol. 2006;141:367–72. doi: 10.1104/pp.106.077925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serkova NJ, Reisdorph NA, van Patot MCT. Metabolic markers of hypoxia: Systems biology application in biomedicine. Toxicology Mechanisms and Methods. 2008;18:81–95. doi: 10.1080/15376510701795769. [DOI] [PubMed] [Google Scholar]
- Aust AE, Eveleigh JF. Mechanisms of DNA oxidation. Proc Soc Exp Biol Med. 1999;222:246–52. doi: 10.1046/j.1525-1373.1999.d01-141.x. [DOI] [PubMed] [Google Scholar]
- Cooke MS, Lunec J, Evans MD. Progress in the analysis of urinary oxidative DNA damage. Free Radic Biol Med. 2002;33:1601–1614. doi: 10.1016/s0891-5849(02)01146-2. [DOI] [PubMed] [Google Scholar]
- Dizdaroglu M, Jaruga P, Birincioglu M, Rodriguez H. Free radical-induced damage to DNA: Mechanisms and measurement. Free Radical Biology and Medicine. 2002;32:1102–1115. doi: 10.1016/s0891-5849(02)00826-2. [DOI] [PubMed] [Google Scholar]
- Neeley WL, Essigmann JM. Mechanisms of formation, genotoxicity, and mutation of guanine oxidation products. Chem Res Toxicol. 2006;19:491–505. doi: 10.1021/tx0600043. [DOI] [PubMed] [Google Scholar]
- Hwang ES, Bowen PE. DNA damage, a biomarker of carcinogenesis: Its measurement and modulation by diet and environment. Crit Rev Food Sci Nutrition. 2007;47:27–50. doi: 10.1080/10408390600550299. [DOI] [PubMed] [Google Scholar]
- Shin CS, Moon BS, Park KS, Kim SY, Park SJ, Chung MH, Lee HK. Serum 8-hydroxy-guanine levels are increased in diabetic patients. Diabetes Care. 2001;24:733–7. doi: 10.2337/diacare.24.4.733. [DOI] [PubMed] [Google Scholar]
- Loft S, Fischer-Nielsen A, Jeding IB, Vistisen K, Poulsen HE. 8-Hydroxydeoxyguanosine as a urinary biomarker of oxidative DNA damage. J Toxicol Environ Health. 1993;40:391–404. doi: 10.1080/15287399309531806. [DOI] [PubMed] [Google Scholar]
- Migliore L, Fontana I, Colognato R, Coppede F, Siciliano G, Murri L. Searching for the role and the most suitable biomarkers of oxidative stress in Alzheimer's disease and in other neurodegenerative diseases. Neurobiol Aging. 2005;26:587–95. doi: 10.1016/j.neurobiolaging.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Bolin C, Cardozo-Pelaez F. Assessing biomarkers of oxidative stress: Analysis of guanosine and oxidized guanosine nucleotide triphosphates by high performance liquid chromatography with electrochemical detection. J Chromatogr B Analyt Technol Biomed Life Sci. 2007 doi: 10.1016/j.jchromb.2007.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada S, Funada T, Shibata N, Kobayashi M, Kawai Y, Tatsuda E, Furuhata A, Uchida K. Protein-bound 4-hydroxy-2-hexenal as a marker of oxidized n-3 polyunsaturated fatty acids. J Lipid Res. 2004;45:626–634. doi: 10.1194/jlr.M300376-JLR200. [DOI] [PubMed] [Google Scholar]
- Petersen DR, Doorn JA. Reactions of 4-hydroxynonenal with proteins and cellular targets. Free Radic Biol Med. 2004;37:937–45. doi: 10.1016/j.freeradbiomed.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Montuschi P, Ciabattoni G, Paredi P, Pantelidis P, du Bois RM, Kharitonov SA, Barnes PJ. 8-Isoprostane as a biomarker of oxidative stress in interstitial lung diseases. Amer J Resp Crit Care Med. 1998;158:1524–1527. doi: 10.1164/ajrccm.158.5.9803102. [DOI] [PubMed] [Google Scholar]
- Montuschi P, Collins JV, Ciabattoni G, Lazzeri N, Corradi M, Kharitonov SA, Barnes PJ. Exhaled 8-isoprostane as an in vivo biomarker of lung oxidative stress in patients with COPD and healthy smokers. Amer J Resp Crit Care Med. 2000;162:1175–1177. doi: 10.1164/ajrccm.162.3.2001063. [DOI] [PubMed] [Google Scholar]
- Schwedhelm E, Boger RH. Application of gas chromatography-mass spectrometry for analysis of isoprostanes: their role in cardiovascular disease. Clin Chem Lab Med. 2003;41:1552–61. doi: 10.1515/CCLM.2003.238. [DOI] [PubMed] [Google Scholar]
- Davì G, Falco A, Patrono C. Determinants of F2-isoprostane biosynthesis and inhibition in man. Chem Phys Lipids. 2004;128:149–63. doi: 10.1016/j.chemphyslip.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Montuschi P, Barnes PJ, Roberts LJ., 2nd Isoprostanes: markers and mediators of oxidative stress. FASEB J. 2004;18:1791–800. doi: 10.1096/fj.04-2330rev. [DOI] [PubMed] [Google Scholar]
- Morrow JD. Quantification of isoprostanes as indices of oxidant stress and the risk of atherosclerosis in humans. Arterioscler Thromb Vasc Biol. 2005;25:279–86. doi: 10.1161/01.ATV.0000152605.64964.c0. [DOI] [PubMed] [Google Scholar]
- Montuschi P, Barnes P, Roberts LJ., 2nd Insights into oxidative stress: the isoprostanes. Curr Med Chem. 2007;14:703–17. doi: 10.2174/092986707780059607. [DOI] [PubMed] [Google Scholar]
- Mori TA, Croft KD, Puddey IB, Beilin LJ. Analysis of native and oxidized low-density lipoprotein oxysterols using gas chromatography mass spectrometry with selective ion monitoring. Redox Report. 1996;2:25–34. doi: 10.1080/13510002.1996.11747023. [DOI] [PubMed] [Google Scholar]
- Murphy RC, Johnson KM. Cholesterol, reactive oxygen species, and the formation of biologically active mediators. J Biol Chem. 2008;283:15521–5. doi: 10.1074/jbc.R700049200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janero DR. Malondialdehyde and thiobarbituric acid reactivity as diagnostic indexes of lipid peroxidation and peroxidative tissue injury. Free Radic Biol Med. 1990;9:515–540. doi: 10.1016/0891-5849(90)90131-2. [DOI] [PubMed] [Google Scholar]
- Gieseg SP, Crone EM, Flavall EA, Amit Z. Potential to inhibit growth of atherosclerotic plaque development through modulation of macrophage neopterin/7,8-dihydroneopterin synthesis. Br J Pharmacol. 2008;153:627–35. doi: 10.1038/sj.bjp.0707408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herce-Pagliai C, Kotecha S, Shuker DEG. Analytical methods for 3-nitrotyrosine as a marker of exposure to reactive nitrogen species: A review. Nitric Oxide-Biology and Chemistry. 1998;2:324–336. doi: 10.1006/niox.1998.0192. [DOI] [PubMed] [Google Scholar]
- Bian K, Gao Z, Weisbrodt N, Murad F. The nature of heme/iron-induced protein tyrosine nitration. Proc Natl Acad Sci USA. 2003;100:5712–7. doi: 10.1073/pnas.0931291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan MW. A review of approaches to the analysis of 3-nitrotyrosine. Amino Acids. 2003;25:351–361. doi: 10.1007/s00726-003-0022-z. [DOI] [PubMed] [Google Scholar]
- Ryberg H, Caidahl K. Chromatographic and mass spectrometric methods for quantitative determination of 3-nitrotyrosine in biological samples and their application to human samples. J Chromatogr B. 2007;851:160–171. doi: 10.1016/j.jchromb.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Cao EH, Wang JJ. Oxidative Damage to DNA – Levels of Thymine Glycol and Thymidine Glycol in Neoplastic Human Urines. Carcinogenesis. 1993;14:1359–1362. doi: 10.1093/carcin/14.7.1359. [DOI] [PubMed] [Google Scholar]
- Makropoulos W, Kocher K, Heintz B, Schwarz ER, Mertens PR, Stefanidis I. Urinary thymidine glycol as a biomarker for oxidative stress after kidney transplantation. Renal Failure. 2000;22:499–510. doi: 10.1081/jdi-100100891. [DOI] [PubMed] [Google Scholar]
- Herrgård MJ, Swainston N, Dobson P, Dunn WB, Arga KY, Arvas M, Blüthgen N, Borger S, Costenoble R, Heinemann M, Hucka M, Le Novère N, Li P, Liebermeister W, Mo ML, Oliveira AP, Petranovic D, Pettifer S, Simeonidis E, Smallbone K, Spasić I, Weichart D, Brent R, Broomhead DS, Westerhoff HV, Kirdar B, Penttilä M, Klipp E, Palsson BØ, Sauer U, Oliver SG, Mendes P, Nielsen J, Kell DB. A consensus yeast metabolic network obtained from a community approach to systems biology. Nature Biotechnol. 2008;26:1155–1160. doi: 10.1038/nbt1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe D, Costanzo M, Fey P, Gojobori T, Hannick L, Hide W, Hill DP, Kania R, Schaeffer M, St Pierre S, Twigger S, White O, Yon Rhee S. Big data: The future of biocuration. Nature. 2008;455:47–50. doi: 10.1038/455047a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooksbank C, Cameron G, Thornton J. The European Bioinformatics Institute's data resources: towards systems biology. Nucleic Acids Res. 2005;33:D46–53. doi: 10.1093/nar/gki026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanehisa M, Goto S, Hattori M, Aoki-Kinoshita KF, Itoh M, Kawashima S, Katayama T, Araki M, Hirakawa M. From genomics to chemical genomics: new developments in KEGG. Nucleic Acids Res. 2006;34:D354–7. doi: 10.1093/nar/gkj102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weininger D. SMILES, a chemical language and information system .1. Introduction to methodology and encoding rules. J Chem Inf Comput Sci. 1988;28:31–36. [Google Scholar]
- Stein SE, Heller SR, Tchekhovski D. An open standard for chemical structure representation – the IUPAC chemical identifier. Proc 2003 Nimes Int Chem Info Conf. 2003. pp. 131–143.
- Coles SJ, Day NE, Murray-Rust P, Rzepa HS, Zhang Y. Enhancement of the chemical semantic web through the use of InChI identifiers. Organic & Biomolecular Chemistry. 2005;3:1832–1834. doi: 10.1039/b502828k. [DOI] [PubMed] [Google Scholar]
- Casher O, Rzepa HS. SemanticEye: a semantic web application to rationalize and enhance chemical electronic publishing. J Chem Inf Model. 2006;46:2396–411. doi: 10.1021/ci060139e. [DOI] [PubMed] [Google Scholar]
- Monge A, Arrault A, Marot C, Morin-Allory L. Managing, profiling and analyzing a library of 2.6 million compounds gathered from 32 chemical providers. Mol Divers. 2006;10:389–403. doi: 10.1007/s11030-006-9033-5. [DOI] [PubMed] [Google Scholar]
- Knox C, Shrivastava S, Sothard P, Eisner R, Wishart DS. Biospider: a web server for automating metabolome annotations. Pac Symp Biocomputing. 2007;12:145–156. [PubMed] [Google Scholar]
- Klinger R, Kolářik C, Fluck J, Hofmann-Apitius M, Friedrich CM. Detection of IUPAC and IUPAC-like chemical names. Bioinformatics. 2008;24:i268–76. doi: 10.1093/bioinformatics/btn181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavarria ME, Lara-Gonzalez L, Gonzalez-Gleason A, Garcia-Paleta Y, Vital-Reyes VS, Reyes A. Prostacyclin/thromboxane early changes in pregnancies that are complicated by preeclampsia. Am J Obstet Gynecol. 2003;188:986–92. doi: 10.1067/mob.2003.203. [DOI] [PubMed] [Google Scholar]
- Miller DM, Buettner GR, Aust SD. Transition metals as catalysts of "autoxidation" reactions. Free Radic Biol Med. 1990;8:95–108. doi: 10.1016/0891-5849(90)90148-c. [DOI] [PubMed] [Google Scholar]
- Stohs SJ, Bagchi D. Oxidative mechanisms in the toxicity of metal ions. Free Rad Biol Med. 1995;18:321–336. doi: 10.1016/0891-5849(94)00159-h. [DOI] [PubMed] [Google Scholar]
- Meneghini R. Iron homeostasis, oxidative stress, and DNA damage. Free Radical Biology and Medicine. 1997;23:783–792. doi: 10.1016/s0891-5849(97)00016-6. [DOI] [PubMed] [Google Scholar]
- Hubel CA. Dyslipidemia, iron, and oxidative stress in preeclampsia: assessment of maternal and feto-placental interactions. Semin Reprod Endocrinol. 1998;16:75–92. doi: 10.1055/s-2007-1016255. [DOI] [PubMed] [Google Scholar]
- Aisen P, Enns C, Wessling-Resnick M. Chemistry and biology of eukaryotic iron metabolism. Int J Biochem Cell Biol. 2001;33:940–959. doi: 10.1016/s1357-2725(01)00063-2. [DOI] [PubMed] [Google Scholar]
- Reddy MB, Clark L. Iron, oxidative stress, and disease risk. Nutr Rev. 2004;62:120–4. doi: 10.1111/j.1753-4887.2004.tb00033.x. [DOI] [PubMed] [Google Scholar]
- Thompson KJ, Shoham S, Connor JR. Iron and neurodegenerative disorders. Brain Res Bull. 2001;55:155–64. doi: 10.1016/s0361-9230(01)00510-x. [DOI] [PubMed] [Google Scholar]
- Valko M, Morris H, Cronin MTD. Metals, toxicity and oxidative stress. Curr Med Chem. 2005;12:1161–1208. doi: 10.2174/0929867053764635. [DOI] [PubMed] [Google Scholar]
- Carbonell T, Rama R. Iron, oxidative stress and early neurological deterioration in ischemic stroke. Current Medicinal Chemistry. 2007;14:857–874. doi: 10.2174/092986707780363014. [DOI] [PubMed] [Google Scholar]
- Dunn LL, Rahmanto YS, Richardson DR. Iron uptake and metabolism in the new millennium. Trends Cell Biol. 2007;17:93–100. doi: 10.1016/j.tcb.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Weinberg ED. Exposing the hidden dangers of iron: what every medical professional should know about the impact of iron on the disease process. Cumberland House; 2004. [Google Scholar]
- Brewer GJ. Iron and copper toxicity in diseases of aging, particularly atherosclerosis and Alzheimer's disease. Exp Biol Med. 2007;232:323–335. [PubMed] [Google Scholar]
- Valko M, Leibfritz D, Moncol J, Cronin MTD, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Winterbourn CC. Reconciling the chemistry and biology of reactive oxygen species. Nat Chem Biol. 2008;4:278–86. doi: 10.1038/nchembio.85. [DOI] [PubMed] [Google Scholar]
- Qian SY, Buettner GR. Iron and dioxygen chemistry is an important route to initiation of biological free radical oxidations: an electron paramagnetic resonance spin trapping study. Free Radic Biol Med. 1999;26:1447–56. doi: 10.1016/s0891-5849(99)00002-7. [DOI] [PubMed] [Google Scholar]
- Brown GC, Borutaite V. Nitric oxide, mitochondria, and cell death. IUBMB Life. 2001;52:189–95. doi: 10.1080/15216540152845993. [DOI] [PubMed] [Google Scholar]
- Gomes A, Fernandes E, Lima J. Use of fluorescence probes for detection of reactive nitrogen species: A review. J Fluorescence. 2006;16:119–139. doi: 10.1007/s10895-005-0030-3. [DOI] [PubMed] [Google Scholar]
- Yokoyama H, Kuroiwa H, Yano R, Araki T. Targeting reactive oxygen species, reactive nitrogen species and inflammation in MPTP neurotoxicity and Parkinson's disease. Neurol Sci. 2008;29:293–301. doi: 10.1007/s10072-008-0986-2. [DOI] [PubMed] [Google Scholar]
- Moncada S, Palmer RMJ, Higgs EA. Nitric-Oxide – Physiology, Pathophysiology, and Pharmacology. Pharmacological Reviews. 1991;43:109–142. [PubMed] [Google Scholar]
- Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci USA. 1990;87:1620–4. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radi R, Cassina A, Hodara R, Quijano C, Castro L. Peroxynitrite reactions and formation in mitochondria. Free Radic Biol Med. 2002;33:1451–64. doi: 10.1016/s0891-5849(02)01111-5. [DOI] [PubMed] [Google Scholar]
- Rubbo H, O'Donnell V. Nitric oxide, peroxynitrite and lipoxygenase in atherogenesis: mechanistic insights. Toxicology. 2005;208:305–17. doi: 10.1016/j.tox.2004.11.019. [DOI] [PubMed] [Google Scholar]
- Zimmet JM, Hare JM. Nitroso-redox interactions in the cardiovascular system. Circulation. 2006;114:1531–44. doi: 10.1161/CIRCULATIONAHA.105.605519. [DOI] [PubMed] [Google Scholar]
- Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlovic R, Santaniello E. Peroxynitrite and nitrosoperoxycarbonate, a tightly connected oxidizing-nitrating couple in the reactive nitrogen-oxygen species family: new perspectives for protection from radical-promoted injury by flavonoids. J Pharm Pharmacol. 2007;59:1687–95. doi: 10.1211/jpp.59.12.0011. [DOI] [PubMed] [Google Scholar]
- Aslan M, Ryan TM, Adler B, Townes TM, Parks DA, Thompson JA, Tousson A, Gladwin MT, Patel RP, Tarpey MM, Batinic-Haberle I, White CR, Freeman BA. Oxygen radical inhibition of nitric oxide-dependent vascular function in sickle cell disease. Proc Natl Acad Sci. 2001;98:15215–15220. doi: 10.1073/pnas.221292098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell VB, Freeman BA. Interactions between nitric oxide and lipid oxidation pathways – Implications for vascular disease. Circulation Research. 2001;88:12–21. doi: 10.1161/01.res.88.1.12. [DOI] [PubMed] [Google Scholar]
- Lancaster JR., Jr Protein cysteine thiol nitrosation: maker or marker of reactive nitrogen species-induced nonerythroid cellular signaling? Nitric Oxide. 2008;19:68–72. doi: 10.1016/j.niox.2008.04.028. [DOI] [PubMed] [Google Scholar]
- Vaz SM, Augusto O. Inhibition of myeloperoxidase-mediated protein nitration by tempol: Kinetics, mechanism, and implications. Proc Natl Acad Sci USA. 2008;105:8191–6. doi: 10.1073/pnas.0708211105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GC, Bal-Price A. Inflammatory neurodegeneration mediated by nitric oxide, glutamate, and mitochondria. Mol Neurobiol. 2003;27:325–55. doi: 10.1385/MN:27:3:325. [DOI] [PubMed] [Google Scholar]
- Kim YM, Chung HT, Simmons RL, Billiar TR. Cellular non-heme iron content is a determinant of nitric oxide-mediated apoptosis, necrosis, and caspase inhibition. J Biol Chem. 2000;275:10954–61. doi: 10.1074/jbc.275.15.10954. [DOI] [PubMed] [Google Scholar]
- Galli SJ, Tsai M, Piliponsky AM. The development of allergic inflammation. Nature. 2008;454:445–54. doi: 10.1038/nature07204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel T. Radical medicine: treating ageing to cure disease. Nat Rev Mol Cell Biol. 2005;6:971–6. doi: 10.1038/nrm1763. [DOI] [PubMed] [Google Scholar]
- Finch CE. The biology of human longevity: inflammation, nutrition and aging in the evolution of lifespans. Academic Press, Amsterdam; 2007. [Google Scholar]
- Serrano M, Blasco MA. Cancer and ageing: convergent and divergent mechanisms. Nat Rev Mol Cell Biol. 2007;8:715–22. doi: 10.1038/nrm2242. [DOI] [PubMed] [Google Scholar]
- Brown GM. The living end: the future of death, aging and immortality. Macmillan, London; 2008. [Google Scholar]
- Renshaw JC, Robson GD, Trinci APJ, Wiebe MG, Livens FR, Collison D, Taylor RJ. Fungal siderophores: structures, functions and applications. Mycol Res. 2002;106:1123–1142. [Google Scholar]
- Haas H. Molecular genetics of fungal siderophore biosynthesis and uptake: the role of siderophores in iron uptake and storage. Applied Microbiology and Biotechnology. 2003;62:316–330. doi: 10.1007/s00253-003-1335-2. [DOI] [PubMed] [Google Scholar]
- Haas H, Eisendle M, Turgeon BG. Siderophores in fungal physiology and virulence. Annu Rev Phytopathol. 2008;46:149–87. doi: 10.1146/annurev.phyto.45.062806.094338. [DOI] [PubMed] [Google Scholar]
- Johnson L. Iron and siderophores in fungal-host interactions. Mycol Res. 2008;112:170–83. doi: 10.1016/j.mycres.2007.11.012. [DOI] [PubMed] [Google Scholar]
- Raymond KN, Muller G, Matzanke BF. Complexation of iron by siderophores – a review of their solution and structural chemistry and biological function. Top Curr Chem. 1984;123:49–102. [Google Scholar]
- Guerinot ML. Microbial iron transport. Annu Rev Microbiol. 1994;48:743–72. doi: 10.1146/annurev.mi.48.100194.003523. [DOI] [PubMed] [Google Scholar]
- Jurado RL. Iron, infections, and anemia of inflammation. Clin Infect Dis. 1997;25:888–95. doi: 10.1086/515549. [DOI] [PubMed] [Google Scholar]
- Drechsel H, Jung G. Peptide siderophores. Journal of Peptide Science. 1998;4:147–181. doi: 10.1002/(SICI)1099-1387(199805)4:3%3C147::AID-PSC136%3E3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Ratledge C, Dover LG. Iron metabolism in pathogenic bacteria. Annu Rev Microbiol. 2000;54:881–941. doi: 10.1146/annurev.micro.54.1.881. [DOI] [PubMed] [Google Scholar]
- Boukhalfa H, Crumbliss AL. Chemical aspects of siderophore mediated iron transport. Biometals. 2002;15:325–339. doi: 10.1023/a:1020218608266. [DOI] [PubMed] [Google Scholar]
- Braun V, Braun M. Active transport of iron and siderophore antibiotics. Curr Opin Microbiol. 2002;5:194–201. doi: 10.1016/s1369-5274(02)00298-9. [DOI] [PubMed] [Google Scholar]
- Brown JS, Holden DW. Iron acquisition by Gram-positive bacterial pathogens. Microbes Infect. 2002;4:1149–56. doi: 10.1016/s1286-4579(02)01640-4. [DOI] [PubMed] [Google Scholar]
- Cornelis P, Matthijs S. Diversity of siderophore-mediated iron uptake systems in fluorescent pseudomonads: not only pyoverdines. Environ Microbiol. 2002;4:787–798. doi: 10.1046/j.1462-2920.2002.00369.x. [DOI] [PubMed] [Google Scholar]
- Crosa JH, Walsh CT. Genetics and assembly line enzymology of siderophore biosynthesis in bacteria. Microbiology and Molecular Biology Reviews. 2002;66:223. doi: 10.1128/MMBR.66.2.223-249.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx JJ. Iron and infection: competition between host and microbes for a precious element. Best Pract Res Clin Haematol. 2002;15:411–26. [PubMed] [Google Scholar]
- Winkelmann G. Microbial siderophore-mediated transport. Biochem Soc Trans. 2002;30:691–6. doi: 10.1042/bst0300691. [DOI] [PubMed] [Google Scholar]
- Andrews SC, Robinson AK, Rodriguez-Quinones F. Bacterial iron homeostasis. FEMS Microbiol Rev. 2003;27:215–37. doi: 10.1016/S0168-6445(03)00055-X. [DOI] [PubMed] [Google Scholar]
- Faraldo-Gómez JD, Sansom MSP. Acquisition of siderophores in Gram-negative bacteria. Nat Rev Mol Cell Biol. 2003;4:105–116. doi: 10.1038/nrm1015. [DOI] [PubMed] [Google Scholar]
- Raymond KN, Dertz EA, Kim SS. Enterobactin: an archetype for microbial iron transport. Proc Natl Acad Sci USA. 2003;100:3584–8. doi: 10.1073/pnas.0630018100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandersman C, Delepelaire P. Bacterial iron sources: From siderophores to hemophores. Annual Review of Microbiology. 2004;58:611–647. doi: 10.1146/annurev.micro.58.030603.123811. [DOI] [PubMed] [Google Scholar]
- Abergel RJ, Wilson MK, Arceneaux JE, Hoette TM, Strong RK, Byers BR, Raymond KN. Anthrax pathogen evades the mammalian immune system through stealth siderophore production. Proc Natl Acad Sci USA. 2006;103:18499–503. doi: 10.1073/pnas.0607055103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischbach MA, Lin HN, Liu DR, Walsh CT. How pathogenic bacteria evade mammalian sabotage in the battle for iron. Nature Chemical Biology. 2006;2:132–138. doi: 10.1038/nchembio771. [DOI] [PubMed] [Google Scholar]
- Grass G. Iron transport in Escherichia coli: All has not been said and done. Biometals. 2006;19:159–172. doi: 10.1007/s10534-005-4341-2. [DOI] [PubMed] [Google Scholar]
- Miethke M, Marahiel MA. Siderophore-based iron acquisition and pathogen control. Microbiol Mol Biol Rev. 2007;71:413–51. doi: 10.1128/MMBR.00012-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genco CA, Dixon DW. Emerging strategies in microbial haem capture. Molecular Microbiology. 2001;39:1–11. doi: 10.1046/j.1365-2958.2001.02231.x. [DOI] [PubMed] [Google Scholar]
- Loomis LD, Raymond KN. Solution equilibria of enterobactin and metal enterobactin complexes. Inorganic Chemistry. 1991;30:906–911. [Google Scholar]
- Stintzi A, Barnes C, Xu J, Raymond KN. Microbial iron transport via a siderophore shuttle: a membrane ion transport paradigm. Proc Natl Acad Sci USA. 2000;97:10691–6. doi: 10.1073/pnas.200318797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun V. Iron uptake mechanisms and their regulation in pathogenic bacteria. Int J Med Microbiol. 2001;291:67–79. doi: 10.1078/1438-4221-00103. [DOI] [PubMed] [Google Scholar]
- Cendrowski S, MacArthur W, Hanna P. Bacillus anthracis requires siderophore biosynthesis for growth in macrophages and mouse virulence. Mol Microbiol. 2004;51:407–417. doi: 10.1046/j.1365-2958.2003.03861.x. [DOI] [PubMed] [Google Scholar]
- Schaible ME, Kaufmann SHE. Iron and microbial infection. Nat Rev Microbiol. 2004;2:946–953. doi: 10.1038/nrmicro1046. [DOI] [PubMed] [Google Scholar]
- Ong ST, Ho JZS, Ho B, Ding JL. Iron-withholding strategy in innate immunity. Immunobiology. 2006;211:295–314. doi: 10.1016/j.imbio.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Challis GL, Ravel J. Coelichelin, a new peptide siderophore encoded by the Streptomyces coelicolor genome: structure prediction from the sequence of its non-ribosomal peptide synthetase. FEMS Microbiol Lett. 2000;187:111–4. doi: 10.1111/j.1574-6968.2000.tb09145.x. [DOI] [PubMed] [Google Scholar]
- Fernandez-Pol JA. Isolation and characterization of a siderophore-like growth factor from mutants of SV40-transformed cells adapted to picolinic acid. Cell. 1978;14:489–99. doi: 10.1016/0092-8674(78)90235-0. [DOI] [PubMed] [Google Scholar]
- Jones RL, Peterson CM, Grady RW, Cerami A. Low molecular weight iron-binding factor from mammalian tissue that potentiates bacterial growth. J Exp Med. 1980;151:418–28. doi: 10.1084/jem.151.2.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primosigh JV, Thomas ED. Studies on the partition of iron in bone marrow cells. J Clin Invest. 1968;47:1473–82. doi: 10.1172/JCI105841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs A. Low molecular weight intracellular iron transport compounds. Blood. 1977;50:433–9. [PubMed] [Google Scholar]
- Hershko C, Graham G, Bates GW, Rachmilewitz EA. Non-specific serum iron in thalassaemia: an abnormal serum iron fraction of potential toxicity. Br J Haematol. 1978;40:255–63. doi: 10.1111/j.1365-2141.1978.tb03662.x. [DOI] [PubMed] [Google Scholar]
- Graham G, Bates GW, Rachmilewitz EA, Hershko C. Nonspecific serum iron in thalassemia: quantitation and chemical reactivity. Am J Hematol. 1979;6:207–17. doi: 10.1002/ajh.2830060305. [DOI] [PubMed] [Google Scholar]
- Craven CM, Alexander J, Eldridge M, Kushner JP, Bernstein S, Kaplan J. Tissue distribution and clearance kinetics of non-transferrin-bound iron in the hypotransferrinemic mouse: a rodent model for hemochromatosis. Proc Natl Acad Sci. 1987;84:3457–61. doi: 10.1073/pnas.84.10.3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontecave M, Pierre JL. Iron metabolism – the low molecular mass iron pool. Biology of Metals. 1991;4:133–135. doi: 10.1007/BF01141302. [DOI] [PubMed] [Google Scholar]
- Kaplan J. Mechanisms of cellular iron acquisition: another iron in the fire. Cell. 2002;111:603–6. doi: 10.1016/s0092-8674(02)01164-9. [DOI] [PubMed] [Google Scholar]
- Ganz T. Hepcidin, a key regulator of iron metabolism and mediator of anemia of inflammation. Blood. 2003;102:783–788. doi: 10.1182/blood-2003-03-0672. [DOI] [PubMed] [Google Scholar]
- Finch C. Regulators of iron balance in humans. Blood. 1994;84:1697–702. [PubMed] [Google Scholar]
- Kohgo Y, Ikuta K, Ohtake T, Torimoto Y, Kato J. Body iron metabolism and pathophysiology of iron overload. Int J Hematol. 2008;88:7–15. doi: 10.1007/s12185-008-0120-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crichton RR, Ward RJ. Iron metabolism–new perspectives in view. Biochemistry. 1992;31:11255–64. doi: 10.1021/bi00161a001. [DOI] [PubMed] [Google Scholar]
- Andrews NC. Medical progress: Disorders of iron metabolism. New England Journal of Medicine. 1999;341:1986–1995. doi: 10.1056/NEJM199912233412607. [DOI] [PubMed] [Google Scholar]
- Beard JL, Dawson H, Piñero DJ. Iron metabolism: a comprehensive review. Nutr Rev. 1996;54:295–317. doi: 10.1111/j.1753-4887.1996.tb03794.x. [DOI] [PubMed] [Google Scholar]
- Lieu PT, Heiskala M, Peterson PA, Yang Y. The roles of iron in health and disease. Mol Aspects Med. 2001;22:1–87. doi: 10.1016/s0098-2997(00)00006-6. [DOI] [PubMed] [Google Scholar]
- Chung JY, Wessling-Resnick M. Molecular mechanisms and regulation of iron transport. Crit Rev Clin Lab Sci. 2003;40:151–182. doi: 10.1080/713609332. [DOI] [PubMed] [Google Scholar]
- Frazer DM, Anderson GJ. The orchestration of body iron intake: how and where do enterocytes receive their cues? Blood Cells Molecules and Diseases. 2003;30:288–297. doi: 10.1016/s1079-9796(03)00039-1. [DOI] [PubMed] [Google Scholar]
- Knutson M, Wessling-Resnick M. Iron metabolism in the reticuloendothelial system. Crit Rev Biochem Mol Biol. 2003;38:61–88. doi: 10.1080/713609210. [DOI] [PubMed] [Google Scholar]
- McArdle HJ, Danzeisen R, Fosset C, Gambling L. The role of the placenta in iron transfer from mother to fetus and the relationship between iron status and fetal outcome. Biometals. 2003;16:161–7. doi: 10.1023/a:1020714915767. [DOI] [PubMed] [Google Scholar]
- Hentze MW, Muckenthaler MU, Andrews NC. Balancing acts: molecular control of mammalian iron metabolism. Cell. 2004;117:285–97. doi: 10.1016/s0092-8674(04)00343-5. [DOI] [PubMed] [Google Scholar]
- Andrews NC. Molecular control of iron metabolism. Best Pract Res Clin Haematol. 2005;18:159–69. doi: 10.1016/j.beha.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Papanikolaou G, Pantopoulos K. Iron metabolism and toxicity. Toxicol Appl Pharmacol. 2005;202:199–211. doi: 10.1016/j.taap.2004.06.021. [DOI] [PubMed] [Google Scholar]
- Taketani S. Aquisition, mobilization and utilization of cellular iron and heme: Endless findings and growing evidence of tight regulation. Tohoku J Exp Med. 2005;205:297–318. doi: 10.1620/tjem.205.297. [DOI] [PubMed] [Google Scholar]
- Donovan A, Roy CN, Andrews NC. The ins and outs of iron homeostasis. Physiology (Bethesda) 2006;21:115–23. doi: 10.1152/physiol.00052.2005. [DOI] [PubMed] [Google Scholar]
- Ganz T, Nemeth E. Regulation of iron acquisition and iron distribution in mammals. Biochim Biophys Acta. 2006;1763:690–699. doi: 10.1016/j.bbamcr.2006.03.014. [DOI] [PubMed] [Google Scholar]
- Huang XP, O'Brien PJ, Templeton DM. Mitochondrial involvement in genetically determined transition metal toxicity I. Iron toxicity. Chemico-Biological Interactions. 2006;163:68–76. doi: 10.1016/j.cbi.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Nairz M, Weiss G. Molecular and clinical aspects of iron homeostasis: from anemia to hemochromatosis. Wiener Klinische Wochenschrift. 2006;118:442–462. doi: 10.1007/s00508-006-0653-7. [DOI] [PubMed] [Google Scholar]
- Anderson GJ. Mechanisms of iron loading and toxicity. Am J Hematol. 2007;82:1128–31. doi: 10.1002/ajh.21075. [DOI] [PubMed] [Google Scholar]
- Andrews NC, Schmidt PJ. Iron homeostasis. Annu Rev Physiol. 2007;69:69–85. doi: 10.1146/annurev.physiol.69.031905.164337. [DOI] [PubMed] [Google Scholar]
- Harrison-Findik DD. Role of alcohol in the regulation of iron metabolism. World J Gastroenterol. 2007;13:4925–30. doi: 10.3748/wjg.v13.i37.4925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BC, Beard JL, Gibson JN, Unger EL, Allen RP, McCarthy KA, Earley CJ. Systems genetic analysis of peripheral iron parameters in the mouse. Am J Physiol Regul Integr Comp Physiol. 2007;293:R116–24. doi: 10.1152/ajpregu.00608.2006. [DOI] [PubMed] [Google Scholar]
- Mithen R. Effect of genotype on micronutrient absorption and metabolism: a review of iron, copper, iodine and selenium, and folates. Int J Vitam Nutr Res. 2007;77:205–16. doi: 10.1024/0300-9831.77.3.205. [DOI] [PubMed] [Google Scholar]
- Vallerio LG. Mammalian iron metabolism. Toxicology Mechanisms and Methods. 2007;17:497–517. doi: 10.1080/15376510701556690. [DOI] [PubMed] [Google Scholar]
- Andrews NC. Forging a field: the golden age of iron biology. Blood. 2008;112:219–30. doi: 10.1182/blood-2007-12-077388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard J, Han O. Systemic iron status. Biochim Biophys Acta. 2008. [DOI] [PubMed]
- Deugnier Y, Brissot P, Loréal O. Iron and the liver: update 2008. J Hepatol. 2008;48:S113–23. doi: 10.1016/j.jhep.2008.01.014. [DOI] [PubMed] [Google Scholar]
- Ganz T. Iron homeostasis: fitting the puzzle pieces together. Cell Metab. 2008;7:288–90. doi: 10.1016/j.cmet.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Handelman GJ, Levin NW. Iron and anemia in human biology: a review of mechanisms. Heart Fail Rev. 2008;13:393–404. doi: 10.1007/s10741-008-9086-x. [DOI] [PubMed] [Google Scholar]
- Hower V, Mendes P, Torti FM, Laubenbacher R, Akman S, Shulaev V, Torti SV. A general map of iron metabolism and tissue-specific subnetworks. Mol Biosyst. 2008. [DOI] [PMC free article] [PubMed]
- Kemna EH, Tjalsma H, Willems HL, Swinkels DW. Hepcidin: from discovery to differential diagnosis. Haematologica. 2008;93:90–7. doi: 10.3324/haematol.11705. [DOI] [PubMed] [Google Scholar]
- Kong W, Duan X, Shi Z, Chang Y. Iron metabolism in the mononuclear phagocyte system. Progr Nat Sci. 2008;18:1197–1202. [Google Scholar]
- Levi S, Rovida E. The role of iron in mitochondrial function. Biochim Biophys Acta. 2008 doi: 10.1016/j.bbagen.2008.09.008. [DOI] [PubMed] [Google Scholar]
- Wrighting DM, Andrews NC. Iron homeostasis and erythropoiesis. Red Cell Development. 2008;82:141–167. doi: 10.1016/S0070-2153(07)00006-3. [DOI] [PubMed] [Google Scholar]
- Frazer DM, Anderson GJ. Iron Imports. I. Intestinal iron absorption and its regulation. Amer J Physiol. 2005;289:G631–G635. doi: 10.1152/ajpgi.00220.2005. [DOI] [PubMed] [Google Scholar]
- Mackenzie B, Garrick MD. Iron Imports. II. Iron uptake at the apical membrane in the intestine. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2005;289:G981–G986. doi: 10.1152/ajpgi.00363.2005. [DOI] [PubMed] [Google Scholar]
- Wessling-Resnick M. Iron Imports. III. Transfer of iron from the mucosa into circulation. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2006;290:G1–G6. doi: 10.1152/ajpgi.00415.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganz T, Nemeth E. Iron imports. IV. Hepcidin and regulation of body iron metabolism. Amer J Physiol. 2006;290:G199–G203. doi: 10.1152/ajpgi.00412.2005. [DOI] [PubMed] [Google Scholar]
- Fleming RE, Britton RS. Iron Imports. VI. HFE and regulation of intestinal iron absorption. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2006;290:G590–G594. doi: 10.1152/ajpgi.00486.2005. [DOI] [PubMed] [Google Scholar]
- Ma YX, Yeh M, Yeh KY, Glass J. Iron imports. V. Transport of iron through the intestinal epithelium. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2006;290:G417–G422. doi: 10.1152/ajpgi.00489.2005. [DOI] [PubMed] [Google Scholar]
- De Domenico I, Ward DM, Kaplan J. Regulation of iron acquisition and storage: consequences for iron-linked disorders. Nature Reviews Molecular Cell Biology. 2008;9:72–81. doi: 10.1038/nrm2295. [DOI] [PubMed] [Google Scholar]
- Gunshin H, Mackenzie B, Berger UV, Gunshin Y, Romero MF, Boron WF, Nussberger S, Gollan JL, Hediger MA. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature. 1997;388:482–8. doi: 10.1038/41343. [DOI] [PubMed] [Google Scholar]
- Forbes JR, Gros P. Divalent-metal transport by NRAMP proteins at the interface of host-pathogen interactions. Trends Microbiol. 2001;9:397–403. doi: 10.1016/s0966-842x(01)02098-4. [DOI] [PubMed] [Google Scholar]
- Shayeghi M, Latunde-Dada GO, Oakhill JS, Laftah AH, Takeuchi K, Halliday N, Khan Y, Warley A, McCann FE, Hider RC, Frazer DM, Anderson GJ, Vulpe CD, Simpson RJ, McKie AT. Identification of an intestinal heme transporter. Cell. 2005;122:789–801. doi: 10.1016/j.cell.2005.06.025. [DOI] [PubMed] [Google Scholar]
- Ryter SW, Alam J, Choi AM. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol Rev. 2006;86:583–650. doi: 10.1152/physrev.00011.2005. [DOI] [PubMed] [Google Scholar]
- Vítek L, Schwertner HA. The heme catabolic pathway and its protective effects on oxidative stress-mediated diseases. Adv Clin Chem. 2007;43:1–57. doi: 10.1016/s0065-2423(06)43001-8. [DOI] [PubMed] [Google Scholar]
- Abraham NG, Kappas A. Pharmacological and clinical aspects of heme oxygenase. Pharmacol Rev. 2008;60:79–127. doi: 10.1124/pr.107.07104. [DOI] [PubMed] [Google Scholar]
- Ponka P. Tissue-specific regulation of iron metabolism and heme synthesis: Distinct control mechanisms in erythroid cells. Blood. 1997;89:1–25. [PubMed] [Google Scholar]
- Linder MC, Mortya M, Whon A, Kassa A, Gilley C. Vesicular transport of Fe and interaction with other metal ions in polarized Caco2 Cell monolayers. Biological Research. 2006;39:143–156. doi: 10.4067/s0716-97602006000100016. [DOI] [PubMed] [Google Scholar]
- Moriya M, Linder MC. Vesicular transport and apotransferrin in intestinal iron absorption, as shown in the Caco-2 cell model. Amer J Physiol. 2006;290:G301–G309. doi: 10.1152/ajpgi.00029.2005. [DOI] [PubMed] [Google Scholar]
- Petrat F, de Groot H, Sustmann R, Rauen U. The chelatable iron pool in living cells: A methodically defined quantity. Biological Chemistry. 2002;383:489–502. doi: 10.1515/BC.2002.051. [DOI] [PubMed] [Google Scholar]
- Tenopoulou M, Kurz T, Doulias PT, Galaris D, Brunk UT. Does the calcein-AM method assay the total cellular 'labile iron pool' or only a fraction of it? Biochem J. 2007;403:261–6. doi: 10.1042/BJ20061840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shvartsman M, Kikkeri R, Shanzer A, Cabantchik ZI. Non-transferrin-bound iron reaches mitochondria by a chelator-inaccessible mechanism: biological and clinical implications. Am J Physiol Cell Physiol. 2007;293:C1383–94. doi: 10.1152/ajpcell.00054.2007. [DOI] [PubMed] [Google Scholar]
- Breuer W, Shvartsman M, Cabantchik ZI. Intracellular labile iron. Int J Biochem Cell Biol. 2008;40:350–4. doi: 10.1016/j.biocel.2007.03.010. [DOI] [PubMed] [Google Scholar]
- Fakih S, Podinovskaia M, Kong X, Collins HL, Schaible UE, Hider RC. Targeting the lysosome: fluorescent iron(III) chelators to selectively monitor endosomal/lysosomal labile iron pools. J Med Chem. 2008;51:4539–52. doi: 10.1021/jm8001247. [DOI] [PubMed] [Google Scholar]
- Arosio P, Ingrassia R, Cavadini P. Ferritins: A family of molecules for iron storage, antioxidation and more. Biochim Biophys Acta. 2008. [DOI] [PubMed]
- Shi H, Bencze KZ, Stemmler TL, Philpott CC. A cytosolic iron chaperone that delivers iron to ferritin. Science. 2008;320:1207–10. doi: 10.1126/science.1157643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallberg L, Hulthen L. Perspectives on iron absorption. Blood Cells Molecules and Diseases. 2002;29:562–573. doi: 10.1006/bcmd.2002.0603. [DOI] [PubMed] [Google Scholar]
- Arosio P, Levi S. Ferritin, iron homeostasis, and oxidative damage. Free Radic Biol Med. 2002;33:457–63. doi: 10.1016/s0891-5849(02)00842-0. [DOI] [PubMed] [Google Scholar]
- Lee DH, Zacharski LR, Jacobs DR., Jr Comparison of the serum ferritin and percentage of transferrin saturation as exposure markers of iron-driven oxidative stress-related disease outcomes. Am Heart J. 2006;151:1247 e1–7. doi: 10.1016/j.ahj.2006.03.009. [DOI] [PubMed] [Google Scholar]
- McKie AT, Barlow DJ. The SLC40 basolateral iron transporter family (IREG1/ferroportin/MTP1) Pflugers Arch. 2004;447:801–6. doi: 10.1007/s00424-003-1102-3. [DOI] [PubMed] [Google Scholar]
- Donovan A, Lima CA, Pinkus JL, Pinkus GS, Zon LI, Robine S, Andrews NC. The iron exporter ferroportin/Slc40a1 is essential for iron homeostasis. Cell Metabolism. 2005;1:191–200. doi: 10.1016/j.cmet.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Ganz T. Cellular iron: ferroportin is the only way out. Cell Metab. 2005;1:155–7. doi: 10.1016/j.cmet.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Oates PS. The role of hepcidin and ferroportin in iron absorption. Histol Histopathol. 2007;22:791–804. doi: 10.14670/HH-22.791. [DOI] [PubMed] [Google Scholar]
- Vulpe CD, Kuo YM, Murphy TL, Cowley L, Askwith C, Libina N, Gitschier J, Anderson GJ. Hephaestin, a ceruloplasmin homologue implicated in intestinal iron transport, is defective in the sla mouse. Nature Genetics. 1999;21:195–199. doi: 10.1038/5979. [DOI] [PubMed] [Google Scholar]
- Worwood M. Serum transferrin receptor assays and their application. Annals of Clinical Biochemistry. 2002;39:221–230. doi: 10.1258/0004563021902152. [DOI] [PubMed] [Google Scholar]
- Mense SM, Zhang L. Herne: a versatile signaling molecule controlling the activities of diverse regulators ranging from transcription factors to MAP kinases. Cell Res. 2006;16:681–692. doi: 10.1038/sj.cr.7310086. [DOI] [PubMed] [Google Scholar]
- Balla J, Vercellotti GM, Nath K, Yachie A, Nagy E, Eaton JW, Balla G. Haem, haem oxygenase and ferritin in vascular endothelial cell injury. Nephrology Dialysis Transplantation. 2003;18:8–12. doi: 10.1093/ndt/gfg1034. [DOI] [PubMed] [Google Scholar]
- Choi AM, Alam J. Heme oxygenase-1: function, regulation, and implication of a novel stress-inducible protein in oxidant-induced lung injury. Am J Respir Cell Mol Biol. 1996;15:9–19. doi: 10.1165/ajrcmb.15.1.8679227. [DOI] [PubMed] [Google Scholar]
- Foresti R, Green CJ, Motterlini R. Generation of bile pigments by haem oxygenase: a refined cellular strategy in response to stressful insults. Biochem Soc Symp. 2004;71:177–192. doi: 10.1042/bss0710177. [DOI] [PubMed] [Google Scholar]
- Poon HF, Calabrese V, Scapagnini G, Butterfield DA. Free radicals: key to brain aging and heme oxygenase as a cellular response to oxidative stress. J Gerontol A Biol Sci Med Sci. 2004;59:478–93. doi: 10.1093/gerona/59.5.m478. [DOI] [PubMed] [Google Scholar]
- Akagi R, Takahashi T, Sassa S. Cytoprotective effects of heme oxygenase in acute renal failure. Cellular Stress Responses in Renal Diseases, Contrib Nephrol. 2005;148:70–85. doi: 10.1159/000086044. [DOI] [PubMed] [Google Scholar]
- Fredenburgh LE, Perrella MA, Mitsialis SA. The role of heme oxygenase-1 in pulmonary disease. Amer J Resp Cell Mol Biol. 2007;36:158–165. doi: 10.1165/rcmb.2006-0331TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gueler F, Park JK, Rong S, Kirsch T, Lindschau C, Zheng W, Elger M, Fiebeler A, Fliser D, Luft FC, Haller H. Statins attenuate ischemia-reperfusion injury by inducing heme oxygenase-1 in infiltrating macrophages. Am J Pathol. 2007;170:1192–1199. doi: 10.2353/ajpath.2007.060782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi S. Human heme oxygenase-1 deficiency: a lesson on serendipity in the discovery of the novel disease. Pediatr Int. 2007;49:125–32. doi: 10.1111/j.1442-200X.2007.02353.x. [DOI] [PubMed] [Google Scholar]
- Horváth I, Donnelly LE, Kiss A, Paredi P, Kharitonov SA, Barnes PJ. Raised levels of exhaled carbon monoxide are associated with an increased expression of heme oxygenase-1 in airway macrophages in asthma: a new marker of oxidative stress. Thorax. 1998;53:668–72. doi: 10.1136/thx.53.8.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlen M, Bjorling E, Agaton C, Szigyarto CA, Amini B, Andersen E, Andersson AC, Angelidou P, Asplund A, Asplund C, Berglund L, Bergstrom K, Brumer H, Cerjan D, Ekstrom M, Elobeid A, Eriksson C, Fagerberg L, Falk R, Fall J, Forsberg M, Bjorklund MG, Gumbel K, Halimi A, Hallin I, Hamsten C, Hansson M, Hedhammar M, Hercules G, Kampf C, Larsson K, Lindskog M, Lodewyckx W, Lund J, Lundeberg J, Magnusson K, Malm E, Nilsson P, Odling J, Oksvold P, Olsson I, Oster E, Ottosson J, Paavilainen L, Persson A, Rimini R, Rockberg J, Runeson M, Sivertsson A, Skollermo A, Steen J, Stenvall M, Sterky F, Stromberg S, Sundberg M, Tegel H, Tourle S, Wahlund E, Walden A, Wan J, Wernerus H, Westberg J, Wester K, Wrethagen U, Xu LL, Hober S, Ponten F. A human protein atlas for normal and cancer tissues based on antibody proteomics. Mol Cell Proteomics. 2005;4:1920–32. doi: 10.1074/mcp.M500279-MCP200. [DOI] [PubMed] [Google Scholar]
- Lee DW, Andersen JK. Role of HIF-1 in iron regulation: potential therapeutic strategy for neurodegenerative disorders. Curr Mol Med. 2006;6:883–893. doi: 10.2174/156652406779010849. [DOI] [PubMed] [Google Scholar]
- Rouault TA. The role of iron regulatory proteins in mammalian iron homeostasis and disease. Nature Chem Biol. 2006;2:406–414. doi: 10.1038/nchembio807. [DOI] [PubMed] [Google Scholar]
- Wallander ML, Leibold EA, Eisenstein RS. Molecular control of vertebrate iron homeostasis by iron regulatory proteins. Biochim Biophys Acta. 2006;1763:668–689. doi: 10.1016/j.bbamcr.2006.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Roth RA, LaPres JJ. Hypoxia, drug therapy and toxicity. Pharmacology & Therapeutics. 2007;113:229–246. doi: 10.1016/j.pharmthera.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Pigeon C, Ilyin G, Courselaud B, Leroyer P, Turlin B, Brissot P, Loreal O. A new mouse liver-specific gene, encoding a protein homologous to human antimicrobial peptide hepcidin, is overexpressed during iron overload. J Biol Chem. 2001;276:7811–7819. doi: 10.1074/jbc.M008923200. [DOI] [PubMed] [Google Scholar]
- Fleming RE, Sly WS. Hepcidin: A putative iron-regulatory hormone relevant to hereditary hemochromatosis and the anemia of chronic disease. Proc Nal Acad Sci. 2001;98:8160–8162. doi: 10.1073/pnas.161296298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolas G, Bennoun M, Devaux I, Beaumont C, Grandchamp B, Kahn A, Vaulont S. Lack of hepcidin gene expression and severe tissue iron overload in upstream stimulatory factor 2 (USF2) knockout mice. Proc Natl Acad Sci. 2001;98:8780–8785. doi: 10.1073/pnas.151179498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein DA, Roy CN, Fleming MD, Loda MF, Wolfsdorf JI, Andrews NC. Inappropriate expression of hepcidin is associated with iron refractory anemia: implications for the anemia of chronic disease. Blood. 2002;100:3776–81. doi: 10.1182/blood-2002-04-1260. [DOI] [PubMed] [Google Scholar]
- Bridle KR, Frazer DM, Wilkins SJ, Dixon JL, Purdie DM, Crawford DHG, Subramaniam VN, Powell LW, Anderson GJ, Ramm GA. Disrupted hepcidin regulation in HFE-associated haemochromatosis and the liver as a regulator of body iron homoeostasis. Lancet. 2003;361:669–673. doi: 10.1016/S0140-6736(03)12602-5. [DOI] [PubMed] [Google Scholar]
- Nicolas G, Viatte L, Lou DQ, Bennoun M, Beaumont C, Kahn A, Andrews NC, Vaulont S. Constitutive hepcidin expression prevents iron overload in a mouse model of hemochromatosis. Nature Genetics. 2003;34:97–101. doi: 10.1038/ng1150. [DOI] [PubMed] [Google Scholar]
- Roetto A, Papanikolaou G, Politou M, Alberti F, Girelli D, Christakis J, Loukopoulos D, Camaschella C. Mutant antimicrobial peptide hepcidin is associated with severe juvenile hemochromatosis. Nature Genetics. 2003;33:21–22. doi: 10.1038/ng1053. [DOI] [PubMed] [Google Scholar]
- Deicher R, Horl WH. Hepcidin: a molecular link between inflammation and anaemia. Nephrol Dial Transplant. 2004;19:521–4. doi: 10.1093/ndt/gfg560. [DOI] [PubMed] [Google Scholar]
- Leong WI, Lönnerdal B. Hepcidin, the recently identified peptide that appears to regulate iron absorption. J Nutr. 2004;134:1–4. doi: 10.1093/jn/134.1.1. [DOI] [PubMed] [Google Scholar]
- Robson KJ. Hepcidin and its role in iron absorption. Gut. 2004;53:617–9. doi: 10.1136/gut.2003.027631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camaschella C. Understanding iron homeostasis through genetic analysis of hemochromatosis and related disorders. Blood. 2005;106:3710–7. doi: 10.1182/blood-2005-05-1857. [DOI] [PubMed] [Google Scholar]
- Loréal O, Haziza-Pigeon C, Troadec MB, Detivaud L, Turlin B, Courselaud B, Ilyin G, Brissot P. Hepcidin in iron metabolism. Curr Protein Pept Sci. 2005;6:279–91. doi: 10.2174/1389203054065392. [DOI] [PubMed] [Google Scholar]
- Roetto A, Camaschella C. New insights into iron homeostasis through the study of non-HFE hereditary haemochromatosis. Best Pract Res Clin Haematol. 2005;18:235–50. doi: 10.1016/j.beha.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Roy CN, Andrews NC. Anemia of inflammation: the hepcidin link. Curr Opin Hematol. 2005;12:107–11. doi: 10.1097/00062752-200503000-00001. [DOI] [PubMed] [Google Scholar]
- Beutler E. Hemochromatosis: Genetics and pathophysiology. Annu Rev Med. 2006;57:331–347. doi: 10.1146/annurev.med.57.121304.131310. [DOI] [PubMed] [Google Scholar]
- Deicher R, Hörl WH. New insights into the regulation of iron homeostasis. Eur J Clin Invest. 2006;36:301–309. doi: 10.1111/j.1365-2362.2006.01633.x. [DOI] [PubMed] [Google Scholar]
- Farnaud S, Patel A, Evans RW. Modelling of a metal-containing hepcidin. Biometals. 2006;19:527–533. doi: 10.1007/s10534-005-5883-z. [DOI] [PubMed] [Google Scholar]
- Ganz T. Hepcidin – A peptide hormone at the interface of innate immunity and iron metabolism. Antimicrobial Peptides and Human Disease, Current Topics in Microbiology and Immunology. 2006;306:183–198. doi: 10.1007/3-540-29916-5_7. [DOI] [PubMed] [Google Scholar]
- Harrison-Findik DD, Schafer D, Klein E, Timchenko NA, Kulaksiz H, Clemens D, Fein E, Andriopoulos B, Pantopoulos K, Gollan J. Alcohol metabolism-mediated oxidative stress down-regulates hepcidin transcription and leads to increased duodenal iron transporter expression. J Biol Chem. 2006;281:22974–22982. doi: 10.1074/jbc.M602098200. [DOI] [PubMed] [Google Scholar]
- Hugman A. Hepcidin: an important new regulator of iron homeostasis. Clin Lab Haematol. 2006;28:75–83. doi: 10.1111/j.1365-2257.2006.00768.x. [DOI] [PubMed] [Google Scholar]
- Mena NP, Esparza AL, Núñez MT. Regulation of transepithelial transport of iron by hepcidin. Biol Res. 2006;39:191–193. doi: 10.4067/s0716-97602006000100022. [DOI] [PubMed] [Google Scholar]
- Mok H, Mlodnicka AE, Hentze MW, Muckenthaler M, Schumacher A. The molecular circuitry regulating the switch between iron deficiency and overload in mice. J Biol Chem. 2006;281:7946–51. doi: 10.1074/jbc.M509857200. [DOI] [PubMed] [Google Scholar]
- Nemeth E, Ganz T. Regulation of iron metabolism by hepcidin. Annual Review of Nutrition. 2006;26:323–342. doi: 10.1146/annurev.nutr.26.061505.111303. [DOI] [PubMed] [Google Scholar]
- Atanasiu V, Manolescu B, Stoian I. Hepcidin – central regulator of iron metabolism. European Journal of Haematology. 2007;78:1–10. doi: 10.1111/j.1600-0609.2006.00772.x. [DOI] [PubMed] [Google Scholar]
- Malyszko J, Mysliwiec M. Hepcidin in anemia and inflammation in chronic kidney disease. Kidney & Blood Pressure Research. 2007;30:15–30. doi: 10.1159/000098522. [DOI] [PubMed] [Google Scholar]
- Oates PS, Ahmed U. Molecular regulation of hepatic expression of iron regulatory hormone hepcidin. J Gastroenterol Hepatol. 2007;22:1378–87. doi: 10.1111/j.1440-1746.2007.04950.x. [DOI] [PubMed] [Google Scholar]
- Barisani D, Pelucchi S, Mariani R, Galimberti S, Trombini P, Fumagalli D, Meneveri R, Nemeth E, Ganz T, Piperno A. Hepcidin and iron-related gene expression in subjects with Dysmetabolic Hepatic Iron Overload. J Hepatol. 2008;49:123–33. doi: 10.1016/j.jhep.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Camaschella C, Silvestri L. New and old players in the hepcidin pathway. Haematologica. 2008;93:1441–4. doi: 10.3324/haematol.13724. [DOI] [PubMed] [Google Scholar]
- De Domenico I, Nemeth E, Nelson JM, Phillips JD, Ajioka RS, Kay MS, Kushner JP, Ganz T, Ward DM, Kaplan J. The hepcidin-binding site on ferroportin is evolutionarily conserved. Cell Metab. 2008;8:146–56. doi: 10.1016/j.cmet.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Farnaud S, Rapisarda C, Bui T, Drake A, Cammack R, Evans RW. Identification of an iron-hepcidin complex. Biochem J. 2008;413:553–7. doi: 10.1042/BJ20080406. [DOI] [PubMed] [Google Scholar]
- Kartikasari AE, Roelofs R, Schaeps RM, Kemna EH, Peters WH, Swinkels DW, Tjalsma H. Secretion of bioactive hepcidin-25 by liver cells correlates with its gene transcription and points towards synergism between iron and inflammation signaling pathways. Biochim Biophys Acta. 2008;1784:2029–37. doi: 10.1016/j.bbapap.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Muckenthaler MU, Galy B, Hentze MW. Systemic iron homeostasis and the iron-responsive element/iron-regulatory protein (IRE/IRP) regulatory network. Annu Rev Nutr. 2008;28:197–213. doi: 10.1146/annurev.nutr.28.061807.155521. [DOI] [PubMed] [Google Scholar]
- Nemeth E. Iron regulation and erythropoiesis. Curr Opin Hematol. 2008;15:169–75. doi: 10.1097/MOH.0b013e3282f73335. [DOI] [PubMed] [Google Scholar]
- Nishina S, Hino K, Korenaga M, Vecchi C, Pietrangelo A, Mizukami Y, Furutani T, Sakai A, Okuda M, Hidaka I, Okita K, Sakaida I. Hepatitis C virus-induced reactive oxygen species raise hepatic iron level in mice by reducing hepcidin transcription. Gastroenterology. 2008;134:226–38. doi: 10.1053/j.gastro.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Peeling P, Dawson B, Goodman C, Landers G, Trinder D. Athletic induced iron deficiency: new insights into the role of inflammation, cytokines and hormones. Eur J Appl Physiol. 2008;103:381–91. doi: 10.1007/s00421-008-0726-6. [DOI] [PubMed] [Google Scholar]
- Swinkels DW, Girelli D, Laarakkers C, Kroot J, Campostrini N, Kemna EH, Tjalsma H. Advances in quantitative hepcidin measurements by time-of-flight mass spectrometry. PLoS ONE. 2008;3:e2706. doi: 10.1371/journal.pone.0002706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swinkels DW, Wetzels JF. Hepcidin: a new tool in the management of anaemia in patients with chronic kidney disease? Nephrol Dial Transplant. 2008;23:2450–3. doi: 10.1093/ndt/gfn267. [DOI] [PubMed] [Google Scholar]
- Toblli JE, Cao G, Rivas C, Kulaksiz H. Heart and iron deficiency anaemia in rats with renal insufficiency: The role of hepcidin. Nephrology (Carlton) 2008;13:636–645. doi: 10.1111/j.1440-1797.2008.01019.x. [DOI] [PubMed] [Google Scholar]
- Trinder D, Ayonrinde OT, Olynyk JK. HCV, iron, and oxidative stress: the new choreography of hepcidin. Gastroenterology. 2008;134:348–51. doi: 10.1053/j.gastro.2007.11.048. [DOI] [PubMed] [Google Scholar]
- Knutson MD, Oukka M, Koss LM, Aydemir F, Wessling-Resnick M. Iron release from macrophages after erythrophagocytosis is up-regulated by ferroportin 1 overexpression and down-regulated by hepcidin. Proc Natl Acad Sci USA. 2005;102:1324–8. doi: 10.1073/pnas.0409409102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, Ganz T, Kaplan J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090–2093. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- Delaby C, Pilard N, Goncalves AS, Beaumont C, Canonne-Hergaux F. Presence of the iron exporter ferroportin at the plasma membrane of macrophages is enhanced by iron loading and down-regulated by hepcidin. Blood. 2005;106:3979–84. doi: 10.1182/blood-2005-06-2398. [DOI] [PubMed] [Google Scholar]
- Ganz T. Hepcidin – a regulator of intestinal iron absorption and iron recycling by macrophages. Best Practice & Research Clinical Haematology. 2005;18:171–182. doi: 10.1016/j.beha.2004.08.020. [DOI] [PubMed] [Google Scholar]
- Papanikolaou G, Tzilianos M, Christakis JI, Bogdanos D, Tsimirika K, MacFarlane J, Goldberg YP, Sakellaropoulos N, Ganz T, Nemeth E. Hepcidin in iron overload disorders. Blood. 2005;105:4103–4105. doi: 10.1182/blood-2004-12-4844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesbordes-Brion JC, Viatte L, Bennoun M, Lou DQ, Ramey G, Houbron C, Hamard G, Kahn A, Vaulont S. Targeted disruption of the hepcidin 1 gene results in severe hemochromatosis. Blood. 2006;108:1402–1405. doi: 10.1182/blood-2006-02-003376. [DOI] [PubMed] [Google Scholar]
- Anderson GJ, Frazer DM. Iron metabolism meets signal transduction. Nature Genetics. 2006;38:503–504. doi: 10.1038/ng0506-503. [DOI] [PubMed] [Google Scholar]
- Kulaksiz H, Gehrke SG, Janetzko A, Rost D, Bruckner T, Kallinowski B, Stremmel W. Pro-hepcidin: expression and cell specific localisation in the liver and its regulation in hereditary haemochromatosis, chronic renal insufficiency, and renal anaemia. Gut. 2004;53:735–743. doi: 10.1136/gut.2003.022863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulaksiz H, Theilig F, Bachmann S, Gehrke SG, Rost D, Janetzko A, Cetin Y, Stremmel W. The iron-regulatory peptide hormone hepcidin: expression and cellular localization in the mammalian kidney. Journal of Endocrinology. 2005;184:361–370. doi: 10.1677/joe.1.05729. [DOI] [PubMed] [Google Scholar]
- Tiker F, Celik B, Tarcan A, Kilicdag H, Ozbek N, Gurakan B. Serum pro-hepcidin levels and relationships with iron parameters in healthy preterm and term newborns. Pediatr Hematol Oncol. 2006;23:293–297. doi: 10.1080/08880010600629213. [DOI] [PubMed] [Google Scholar]
- Roe MA, Spinks C, Heath ALM, Harvey LJ, Foxall R, Wimperis J, Wolf C, Fairweather-Tait SJ. Serum prohepcidin concentration: no association with iron absorption in healthy men; and no relationship with iron status in men carrying. HFE mutations, hereditary haemochromatosis patients undergoing phlebotomy treatment, or pregnant women. Br J Nutr. 2007;97:544–549. doi: 10.1017/S0007114507336829. [DOI] [PubMed] [Google Scholar]
- Nicolas G, Chauvet C, Viatte L, Danan JL, Bigard X, Devaux I, Beaumont C, Kahn A, Vaulont S. The gene encoding the iron regulatory peptide hepcidin is regulated by anemia, hypoxia, and inflammation. J Clin Invest. 2002;110:1037–1044. doi: 10.1172/JCI15686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pak M, Lopez MA, Gabayan V, Ganz T, Rivera S. Suppression of hepcidin during anemia requires erythropoietic activity. Blood. 2006;108:3730–5. doi: 10.1182/blood-2006-06-028787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth E, Valore EV, Territo M, Schiller G, Lichtenstein A, Ganz T. Hepcidin, a putative mediator of anemia of inflammation, is a type II acute-phase protein. Blood. 2003;101:2461–2463. doi: 10.1182/blood-2002-10-3235. [DOI] [PubMed] [Google Scholar]
- Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, Pedersen BK, Ganz T. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004;113:1271–1276. doi: 10.1172/JCI20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemna E, Pickkers P, Nemeth E, Hoeven H van der, Swinkels D. Time-course analysis of hepcidin, serum iron, and plasma cytokine levels in humans injected with LPS. Blood. 2005;106:1864–1866. doi: 10.1182/blood-2005-03-1159. [DOI] [PubMed] [Google Scholar]
- Fleming RE. Iron and inflammation: cross-talk between pathways regulating hepcidin. J Mol Med. 2008;86:491–4. doi: 10.1007/s00109-008-0349-8. [DOI] [PubMed] [Google Scholar]
- Malyszko J, Malyszko JS, Pawlak K, Drozdowska-Rams L, Brzosko S, Mysliwiec M. Hepcidin is linked to anemia and inflammation in peritoneal dialysis patients. Perit Dial Int. 2008;28:418–21. [PubMed] [Google Scholar]
- Wang RH, Li CL, Xu XL, Zheng Y, Xiao CY, Zerfas P, Cooperman S, Eckhaus M, Rouault T, Mishra L, Deng CX. A role of SMAD4 in iron metabolism through the positive regulation of hepicidin expression. Cell Metabolism. 2005;2:399–409. doi: 10.1016/j.cmet.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Falzacappa MVV, Spasic MV, Kessler R, Stolte J, Hentze MW, Muckenthaler MU. STAT3 mediates hepatic hepcidin expression and its inflammatory stimulation. Blood. 2007;109:353–358. doi: 10.1182/blood-2006-07-033969. [DOI] [PubMed] [Google Scholar]
- Pietrangelo A, Dierssen U, Valli L, Garuti C, Rump A, Corradini E, Ernst M, Klein C, Trautwein C. STAT3 is required for IL-6-gp130-dependent activation of hepcidin in vivo. Gastroenterol. 2007;132:294–300. doi: 10.1053/j.gastro.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Choi SO, Cho YS, Kim HL, Park JW. ROS mediate the hypoxic repression of the hepcidin gene by inhibiting C/EBP alpha and STAT-3. Biochem Biophys Res Comm. 2007;356:312–317. doi: 10.1016/j.bbrc.2007.02.137. [DOI] [PubMed] [Google Scholar]
- Flanagan JM, Truksa J, Peng HF, Lee P, Beutler E. In vivo imaging of hepcidin promoter stimulation by iron and inflammation. Blood Cells Mols Dis. 2007;38:253–257. doi: 10.1016/j.bcmd.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Détivaud L, Nemeth E, Boudjema K, Turlin B, Troadec MB, Leroyer P, Ropert M, Jacquelinet S, Courselaud B, Ganz T, Brissot P, Loréal O. Hepcidin levels in humans are correlated with hepatic iron stores, hemoglobin levels, and hepatic function. Blood. 2005;106:746–748. doi: 10.1182/blood-2004-12-4855. [DOI] [PubMed] [Google Scholar]
- Weizer-Stern O, Adamsky K, Amariglio N, Rachmilewitz E, Breda L, Rivella S, Rechavi G. mRNA expression of iron regulatory genes in beta-thalassemia intermedia and beta-thalassemia major mouse models. Amer J Hematol. 2006;81:479–483. doi: 10.1002/ajh.20549. [DOI] [PubMed] [Google Scholar]
- Weizer-Stern O, Adamsky K, Amariglio N, Levin C, Koren A, Breuer W, Rachmilewitz E, Breda L, Rivella S, Cabantchik ZI, Rechavi G. Downregulation of hepcidin and haemojuvelin expression in the hepatocyte cell-line HepG2 induced by thalassaemic sera. Br J Haematol. 2006;135:129–138. doi: 10.1111/j.1365-2141.2006.06258.x. [DOI] [PubMed] [Google Scholar]
- Weizer-Stern O, Adamsky K, Margalit O, Ashur-Fabian O, Givol D, Amariglio N, Rechavi G. Hepcidin, a key regulator of iron metabolism, is transcriptionally activated by p53. Br J Haematol. 2007;138:253–62. doi: 10.1111/j.1365-2141.2007.06638.x. [DOI] [PubMed] [Google Scholar]
- Sullivan JL. Macrophage iron, hepcidin, and atherosclerotic plaque stability. Exp Biol Med (Maywood) 2007;232:1014–20. doi: 10.3181/0703-MR-54. [DOI] [PubMed] [Google Scholar]
- Adamsky K, Weizer O, Amariglio N, Breda L, Harmelin A, Rivella S, Rachmilewitz E, Rechavi G. Decreased hepcidin mRNA expression in thalassemic mice. Br J Haematol. 2004;124:123–124. doi: 10.1046/j.1365-2141.2003.04734.x. [DOI] [PubMed] [Google Scholar]
- Flower DR, North AC, Sansom CE. The lipocalin protein family: structural and sequence overview. Biochim Biophys Acta. 2000;1482:9–24. doi: 10.1016/s0167-4838(00)00148-5. [DOI] [PubMed] [Google Scholar]
- Kjeldsen L, Johnsen AH, Sengelov H, Borregaard N. Isolation and primary structure of NGAL, a novel protein associated with human neutrophil gelatinase. J Biol Chem. 1993;268:10425–32. [PubMed] [Google Scholar]
- Stoesz SP, Gould MN. Overexpression of neu-related lipocalin (NRL) in neu-initiated but not ras or chemically initiated rat mammary carcinomas. Oncogene. 1995;11:2233–41. [PubMed] [Google Scholar]
- Kjeldsen L, Cowland JB, Borregaard N. Human neutrophil gelatinase-associated lipocalin and homologous proteins in rat and mouse. Biochim Biophys Acta. 2000;1482:272–83. doi: 10.1016/s0167-4838(00)00152-7. [DOI] [PubMed] [Google Scholar]
- Liu Q, Ryon J, Nilsen-Hamilton M. Uterocalin: a mouse acute phase protein expressed in the uterus around birth. Mol Reprod Dev. 1997;46:507–14. doi: 10.1002/(SICI)1098-2795(199704)46:4<507::AID-MRD9>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Goetz DH, Holmes MA, Borregaard N, Bluhm ME, Raymond KN, Strong RK. The neutrophil lipocalin NGAL is a bacteriostatic agent that interferes with siderophore-mediated iron acquisition. Mol Cell. 2002;10:1033–43. doi: 10.1016/s1097-2765(02)00708-6. [DOI] [PubMed] [Google Scholar]
- Flo TH, Smith KD, Sato S, Rodriguez DJ, Holmes MA, Strong RK, Akira S, Aderem A. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature. 2004;432:917–21. doi: 10.1038/nature03104. [DOI] [PubMed] [Google Scholar]
- Fluckinger M, Haas H, Merschak P, Glasgow BJ, Redl B. Human tear lipocalin exhibits antimicrobial activity by scavenging microbial siderophores. Antimicrob Agents Chemother. 2004;48:3367–72. doi: 10.1128/AAC.48.9.3367-3372.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes MA, Paulsene W, Jide X, Ratledge C, Strong RK. Siderocalin (Lcn 2) also binds carboxymycobactins, potentially defending against mycobacterial infections through iron sequestration. Structure. 2005;13:29–41. doi: 10.1016/j.str.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Berger T, Togawa A, Duncan GS, Elia AJ, You-Ten A, Wakeham A, Fong HE, Cheung CC, Mak TW. Lipocalin 2-deficient mice exhibit increased sensitivity to Escherichia coli infection but not to ischemia-reperfusion injury. Proc Natl Acad Sci USA. 2006;103:1834–9. doi: 10.1073/pnas.0510847103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borregaard N, Cowland JB. Neutrophil gelatinase-associated lipocalin, a siderophore-binding eukaryotic protein. Biometals. 2006;19:211–215. doi: 10.1007/s10534-005-3251-7. [DOI] [PubMed] [Google Scholar]
- Yang J, Goetz D, Li JY, Wang W, Mori K, Setlik D, Du T, Erdjument-Bromage H, Tempst P, Strong R, Barasch J. An iron delivery pathway mediated by a lipocalin. Mol Cell. 2002;10:1045–56. doi: 10.1016/s1097-2765(02)00710-4. [DOI] [PubMed] [Google Scholar]
- Coles M, Diercks T, Muehlenweg B, Bartsch S, Zolzer V, Tschesche H, Kessler H. The solution structure and dynamics of human neutrophil gelatinase-associated lipocalin. J Mol Biol. 1999;289:139–57. doi: 10.1006/jmbi.1999.2755. [DOI] [PubMed] [Google Scholar]
- Tschesche H, Zolzer V, Triebel S, Bartsch S. The human neutrophil lipocalin supports the allosteric activation of matrix metalloproteinases. Eur J Biochem. 2001;268:1918–1928. doi: 10.1046/j.1432-1327.2001.02066.x. [DOI] [PubMed] [Google Scholar]
- Yan L, Borregaard N, Kjeldsen L, Moses MA. The high molecular weight urinary matrix metalloproteinase (MMP) activity is a complex of gelatinase B/MMP-9 and neutrophil gelatinase-associated lipocalin (NGAL). Modulation of MMP-9 activity by NGAL. J Biol Chem. 2001;276:37258–65. doi: 10.1074/jbc.M106089200. [DOI] [PubMed] [Google Scholar]
- Devarajan P, Mishra J, Supavekin S, Patterson LT, Steven Potter S. Gene expression in early ischemic renal injury: clues towards pathogenesis, biomarker discovery, and novel therapeutics. Mol Genet Metab. 2003;80:365–76. doi: 10.1016/j.ymgme.2003.09.012. [DOI] [PubMed] [Google Scholar]
- Mishra J, Ma Q, Prada A, Mitsnefes M, Zahedi K, Yang J, Barasch J, Devarajan P. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol. 2003;14:2534–43. doi: 10.1097/01.asn.0000088027.54400.c6. [DOI] [PubMed] [Google Scholar]
- Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, Ruff SM, Zahedi K, Shao M, Bean J, Mori K, Barasch J, Devarajan P. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365:1231–8. doi: 10.1016/S0140-6736(05)74811-X. [DOI] [PubMed] [Google Scholar]
- Mori K, Lee HT, Rapoport D, Drexler IR, Foster K, Yang J, Schmidt-Ott KM, Chen X, Li JY, Weiss S, Mishra J, Cheema FH, Markowitz G, Suganami T, Sawai K, Mukoyama M, Kunis C, D'Agati V, Devarajan P, Barasch J. Endocytic delivery of lipoccalin-siderophore-iron complex rescues the kidney from ischemia-reperfusion injury. Journal of Clinical Investigation. 2005;115:610–621. doi: 10.1172/JCI23056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herget-Rosenthal S. One step forward in the early detection of acute renal failure. Lancet. 2005;365:1205–1206. doi: 10.1016/S0140-6736(05)74787-5. [DOI] [PubMed] [Google Scholar]
- Trachtman H, Christen E, Cnaan A, Patrick J, Mai V, Mishra J, Jain A, Bullington N, Devarajan P. Urinary neutrophil gelatinase-associated lipocalcin in D+HUS: a novel marker of renal injury. Pediatric Nephrology. 2006;21:989–994. doi: 10.1007/s00467-006-0146-y. [DOI] [PubMed] [Google Scholar]
- Dent CL, Ma Q, Dastrala S, Bennett M, Mitsnefes MM, Barasch J, Devarajan P. Plasma neutrophil gelatinase-associated lipocalin predicts acute kidney injury, morbidity and mortality after pediatric cardiac surgery: a prospective uncontrolled cohort study. Critical Care. 2007;11:R127. doi: 10.1186/cc6192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devarajan P. Update on mechanisms of ischemic acute kidney injury. Journal of the American Society of Nephrology. 2006;17:1503–1520. doi: 10.1681/ASN.2006010017. [DOI] [PubMed] [Google Scholar]
- Schmidt-Ott KM, Mori K, Kalandadze A, Li JY, Paragas N, Nicholas T, Devarajan P, Barasch J. Neutrophil gelatinase-associated lipocalin-mediated iron traffic in kidney epithelia. Curr Opin Nephrol Hypertens. 2006;15:442–9. doi: 10.1097/01.mnh.0000232886.81142.58. [DOI] [PubMed] [Google Scholar]
- Schmidt-Ott KM, Mori K, Li JY, Kalandadze A, Cohen DJ, Devarajan P, Barasch J. Dual action of neutrophil gelatinase-associated lipocalin. J Am Soc Nephrol. 2007;18:407–13. doi: 10.1681/ASN.2006080882. [DOI] [PubMed] [Google Scholar]
- Mori K, Nakao K. Neutrophil gelatinase-associated lipocalin as the real-time indicator of active kidney damage. Kidney Int. 2007;71:967–70. doi: 10.1038/sj.ki.5002165. [DOI] [PubMed] [Google Scholar]
- Mitsnefes MM, Kathman TS, Mishra J, Kartal J, Khoury PR, Nickolas TL, Barasch J, Devarajan P. Serum neutrophil gelatinase-associated lipocalin as a marker of renal function in children with chronic kidney disease. Pediatr Nephrol. 2007;22:101–8. doi: 10.1007/s00467-006-0244-x. [DOI] [PubMed] [Google Scholar]
- Zappitelli M, Washburn KK, Arikan AA, Loftis L, Ma Q, Devarajan P, Parikh CR, Goldstein SL. Urine neutrophil gelatinase-associated lipocalin is an early marker of acute kidney injury in critically ill children: a prospective cohort study. Critical Care. 2007;11 doi: 10.1186/cc6089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molitoris BA, Melnikov VY, Okusa MD, Himmelfarb J. Technology Insight: biomarker development in acute kidney injury–what can we anticipate? Nat Clin Pract Nephrol. 2008;4:154–65. doi: 10.1038/ncpneph0723. [DOI] [PubMed] [Google Scholar]
- Nickolas TL, O'Rourke MJ, Yang J, Sise ME, Canetta PA, Barasch N, Buchen C, Khan F, Mori K, Giglio J, Devarajan P, Barasch J. Sensitivity and specificity of a single emergency department measurement of urinary neutrophil gelatinase-associated lipocalin for diagnosing acute kidney injury. Ann Intern Med. 2008;148:810–9. doi: 10.7326/0003-4819-148-11-200806030-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malyszko J, Bachorzewska-Gajewska H, Sitniewska E, Malyszko JS, Poniatowski B, Dobrzycki S. Serum neutrophil gelatinase-associated lipocalin as a marker of renal function in non-diabetic patients with stage 2–4 chronic kidney disease. Ren Fail. 2008;30:625–8. doi: 10.1080/08860220802134607. [DOI] [PubMed] [Google Scholar]
- Devarajan P. NGAL in acute kidney injury: from serendipity to utility. Am J Kidney Dis. 2008;52:395–9. doi: 10.1053/j.ajkd.2008.07.008. [DOI] [PubMed] [Google Scholar]
- Devarajan P. Neutrophil gelatinase-associated lipocalin (NGAL): a new marker of kidney disease. Scand J Clin Lab Invest Suppl. 2008;241:89–94. doi: 10.1080/00365510802150158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devireddy LR, Gazin C, Zhu X, Green MR. A cell-surface receptor for lipocalin 24p3 selectively mediates apoptosis and iron uptake. Cell. 2005;123:1293–305. doi: 10.1016/j.cell.2005.10.027. [DOI] [PubMed] [Google Scholar]
- Richardson DR. 24p3 and its receptor: dawn of a new iron age? Cell. 2005;123:1175–7. doi: 10.1016/j.cell.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Hvidberg V, Jacobsen C, Strong RK, Cowland JB, Moestrup SK, Borregaard N. The endocytic receptor megalin binds the iron transporting neutrophil-gelatinase-associated lipocalin with high affinity and mediates its cellular uptake. FEBS Lett. 2005;579:773–7. doi: 10.1016/j.febslet.2004.12.031. [DOI] [PubMed] [Google Scholar]
- Roudkenar MH, Kuwahara Y, Baba T, Roushandeh AM, Ebishima S, Abe S, Ohkubo Y, Fukumoto M. Oxidative stress induced lipocalin 2 gene expression: Addressing its expression under the harmful conditions. Journal of Radiation Research. 2007;48:39–44. doi: 10.1269/jrr.06057. [DOI] [PubMed] [Google Scholar]
- Roudkenar MH, Halabian R, Ghasemipour Z, Roushandeh AM, Rouhbakhsh M, Nekogoftar M, Kuwahara Y, Fukumoto M, Shokrgozar MA. Neutrophil Gelatinase-associated Lipocalin acts as a protective factor against H2O2 toxicity. Arch Med Res. 2008;39:560–6. doi: 10.1016/j.arcmed.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Gueler F, Gwinner W, Schwarz A, Haller H. Long-term effects of acute ischemia and reperfusion injury. Kidney International. 2004;66:523–527. doi: 10.1111/j.1523-1755.2004.761_11.x. [DOI] [PubMed] [Google Scholar]
- Sikorski EM, Hock T, Hill-Kapturczak N, Agarwal A. The story so far: molecular regulation of the heme oxygenase-1 gene in renal injury. Am J Physiol. 2004;286:F425–F441. doi: 10.1152/ajprenal.00297.2003. [DOI] [PubMed] [Google Scholar]
- Shah S. Role of iron in progressive renal disease. Am J Kidney Dis. 2001;37:S30–3. doi: 10.1053/ajkd.2001.20736. [DOI] [PubMed] [Google Scholar]
- Aigner F, Maier HT, Schwelberger HG, Wallnofer EA, Amberger A, Obrist P, Berger T, Mak TW, Maglione M, Margreiter R, Schneeberger S, Troppmair J. Lipocalin-2 regulates the inflammatory response during ischemia and reperfusion of the transplanted heart. Amer J Transplantation. 2007;7:779–788. doi: 10.1111/j.1600-6143.2006.01723.x. [DOI] [PubMed] [Google Scholar]
- Abergel RJ, Moore EG, Strong RK, Raymond KN. Microbial evasion of the immune system: structural modifications of enterobactin impair siderocalin recognition. J Am Chem Soc. 2006;128:10998–9. doi: 10.1021/ja062476+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischbach MA, Lin H, Zhou L, Yu Y, Abergel RJ, Liu DR, Raymond KN, Wanner BL, Strong RK, Walsh CT, Aderem A, Smith KD. The pathogen-associated iroA gene cluster mediates bacterial evasion of lipocalin 2. Proc Natl Acad Sci USA. 2006;103:16502–7. doi: 10.1073/pnas.0604636103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abergel RJ, Clifton MC, Pizarro JC, Warner JA, Shuh DK, Strong RK, Raymond KN. The siderocalin/enterobactin interaction: a link between mammalian immunity and bacterial iron transport. J Am Chem Soc. 2008;130:11524–34. doi: 10.1021/ja803524w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowland JB, Borregaard N. Molecular characterization and pattern of tissue expression of the gene for neutrophil gelatinase-associated lipocalin from humans. Genomics. 1997;45:17–23. doi: 10.1006/geno.1997.4896. [DOI] [PubMed] [Google Scholar]
- Xu S, Venge P. Lipocalins as biochemical markers of disease. Biochim Biophys Acta. 2000;1482:298–307. doi: 10.1016/s0167-4838(00)00163-1. [DOI] [PubMed] [Google Scholar]
- Cowland JB, Sorensen OE, Sehested M, Borregaard N. Neutrophil gelatinase-associated lipocalin is up-regulated in human epithelial cells by IL-1 beta, but not by TNF-alpha. J Immunol. 2003;171:6630–9. doi: 10.4049/jimmunol.171.12.6630. [DOI] [PubMed] [Google Scholar]
- Cowland JB, Muta T, Borregaard N. IL-1beta-specific up-regulation of neutrophil gelatinase-associated lipocalin is controlled by IkappaB-zeta. J Immunol. 2006;176:5559–66. doi: 10.4049/jimmunol.176.9.5559. [DOI] [PubMed] [Google Scholar]
- Elneihoum AM, Falke P, Axelsson L, Lundberg E, Lindgarde F, Ohlsson K. Leukocyte activation detected by increased plasma levels of inflammatory mediators in patients with ischemic cerebrovascular diseases. Stroke. 1996;27:1734–8. doi: 10.1161/01.str.27.10.1734. [DOI] [PubMed] [Google Scholar]
- Elneihoum AM, Falke P, Hedblad B, Lindgarde F, Ohlsson K. Leukocyte activation in atherosclerosis: correlation with risk factors. Atherosclerosis. 1997;131:79–84. doi: 10.1016/s0021-9150(96)06077-7. [DOI] [PubMed] [Google Scholar]
- Falke P, Elneihoum AM, Ohlsson K. Leukocyte activation: relation to cardiovascular mortality after cerebrovascular ischemia. Cerebrovasc Dis. 2000;10:97–101. doi: 10.1159/000016037. [DOI] [PubMed] [Google Scholar]
- Hemdahl AL, Gabrielsen A, Zhu C, Eriksson P, Hedin U, Kastrup J, Thoren P, Hansson GK. Expression of neutrophil gelatinase-associated lipocalin in atherosclerosis and myocardial infarction. Arterioscler Thromb Vasc Biol. 2006;26:136–42. doi: 10.1161/01.ATV.0000193567.88685.f4. [DOI] [PubMed] [Google Scholar]
- Stoesz SP, Friedl A, Haag JD, Lindstrom MJ, Clark GM, Gould MN. Heterogeneous expression of the lipocalin NGAL in primary breast cancers. Int J Cancer. 1998;79:565–72. doi: 10.1002/(sici)1097-0215(19981218)79:6<565::aid-ijc3>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Tong Z, Wu X, Ovcharenko D, Zhu J, Chen CS, Kehrer JP. Neutrophil gelatinase-associated lipocalin as a survival factor. Biochem J. 2005;391:441–8. doi: 10.1042/BJ20051020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong Z, Kunnumakkara AB, Wang H, Matsuo Y, Diagaradjane P, Harikumar KB, Ramachandran V, Sung B, Chakraborty A, Bresalier RS, Logsdon C, Aggarwal BB, Krishnan S, Guha S. Neutrophil gelatinase-associated lipocalin: a novel suppressor of invasion and angiogenesis in pancreatic cancer. Cancer Res. 2008;68:6100–8. doi: 10.1158/0008-5472.CAN-08-0540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg ED. Iron out-of-balance: a risk factor for acute and chronic diseases. Hemoglobin. 2008;32:117–22. doi: 10.1080/03630260701680805. [DOI] [PubMed] [Google Scholar]
- CESDI . 5th Annual Report. Maternal and Child Health Research Consortium; 1998. Confidential Enquiry into Stillbirths and Deaths in Infancy. [Google Scholar]
- LaMarca BD, Gilbert J, Granger JP. Recent progress toward the understanding of the pathophysiology of hypertension during preeclampsia. Hypertension. 2008;51:982–8. doi: 10.1161/HYPERTENSIONAHA.107.108837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman CW, Sargent IL. Placental debris, oxidative stress and pre-eclampsia. Placenta. 2000;21:597–602. doi: 10.1053/plac.2000.0560. [DOI] [PubMed] [Google Scholar]
- DiFederico E, Genbacev O, Fisher SJ. Preeclampsia is associated with widespread apoptosis of placental cytotrophoblasts within the uterine wall. Am J Pathol. 1999;155:293–301. doi: 10.1016/S0002-9440(10)65123-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung DN, Smith SC, To KF, Sahota DS, Baker PN. Increased placental apoptosis in pregnancies complicated by preeclampsia. Am J Obstet Gynecol. 2001;184:1249–50. doi: 10.1067/mob.2001.112906. [DOI] [PubMed] [Google Scholar]
- Huppertz B, Kingdom JC. Apoptosis in the trophoblast–role of apoptosis in placental morphogenesis. J Soc Gynecol Investig. 2004;11:353–62. doi: 10.1016/j.jsgi.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Harris LK, Keogh RJ, Wareing M, Baker PN, Cartwright JE, Aplin JD, Whitley GS. Invasive trophoblasts stimulate vascular smooth muscle cell apoptosis by a fas ligand-dependent mechanism. Am J Pathol. 2006;169:1863–74. doi: 10.2353/ajpath.2006.060265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heazell AE, Moll SJ, Jones CJ, Baker PN, Crocker IP. Formation of syncytial knots is increased by hyperoxia, hypoxia and reactive oxygen species. Placenta. 2007;28:S33–40. doi: 10.1016/j.placenta.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Keogh RJ, Harris LK, Freeman A, Baker PN, Aplin JD, Whitley GS, Cartwright JE. Fetal-derived trophoblast use the apoptotic cytokine tumor necrosis factor-alpha-related apoptosis-inducing ligand to induce smooth muscle cell death. Circ Res. 2007;100:834–41. doi: 10.1161/01.RES.0000261352.81736.37. [DOI] [PubMed] [Google Scholar]
- Heazell AE, Lacey HA, Jones CJ, Huppertz B, Baker PN, Crocker IP. Effects of oxygen on cell turnover and expression of regulators of apoptosis in human placental trophoblast. Placenta. 2008;29:175–86. doi: 10.1016/j.placenta.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Combs CA, Katz MA, Kitzmiller JL, Brescia RJ. Experimental preeclampsia produced by chronic constriction of the lower aorta: validation with longitudinal blood pressure measurements in conscious rhesus monkeys. Am J Obstet Gynecol. 1993;169:215–23. doi: 10.1016/0002-9378(93)90171-e. [DOI] [PubMed] [Google Scholar]
- Wisdom SJ, Wilson R, McKillop JH, Walker JJ. Antioxidant systems in normal pregnancy and in pregnancy-induced hypertension. Am J Obs Gynecol. 1991;165:1701–1704. doi: 10.1016/0002-9378(91)90018-m. [DOI] [PubMed] [Google Scholar]
- Genbacev O, Joslin R, Damsky CH, Polliotti BM, Fisher SJ. Hypoxia alters early gestation human cytotrophoblast differentiation/invasion in vitro and models the placental defects that occur in preeclampsia. J Clin Invest. 1996;97:540–50. doi: 10.1172/JCI118447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benyo DF, Miles TM, Conrad KP. Hypoxia stimulates cytokine production by villous explants from the human placenta. J Clin Endocrinol Metab. 1997;82:1582–8. doi: 10.1210/jcem.82.5.3916. [DOI] [PubMed] [Google Scholar]
- Conrad KP, Benyo DF. Placental cytokines and the pathogenesis of preeclampsia. Am J Reprod Immunol. 1997;37:240–9. doi: 10.1111/j.1600-0897.1997.tb00222.x. [DOI] [PubMed] [Google Scholar]
- Lefevre G, Berkane N, Uzan S, Etienne J. Preeclampsia and oxygen free radicals. Annales De Biologie Clinique. 1997;55:443–450. [PubMed] [Google Scholar]
- Conrad KP, Miles TM, Benyo DF. Circulating levels of immunoreactive cytokines in women with preeclampsia. Am J Reprod Immunol. 1998;40:102–11. doi: 10.1111/j.1600-0897.1998.tb00398.x. [DOI] [PubMed] [Google Scholar]
- Davidge ST. Oxidative stress and altered endothelial cell function in preeclampsia. Semin Reprod Endocrinol. 1998;16:65–73. doi: 10.1055/s-2007-1016254. [DOI] [PubMed] [Google Scholar]
- Mise H, Sagawa N, Matsumoto T, Yura S, Nanno H, Itoh H, Mori T, Masuzaki H, Hosoda K, Ogawa Y, Nakao K. Augmented placental production of leptin in preeclampsia: possible involvement of placental hypoxia. J Clin Endocrinol Metab. 1998;83:3225–9. doi: 10.1210/jcem.83.9.5117. [DOI] [PubMed] [Google Scholar]
- Walker JJ. Antioxidants and inflammatory cell response in preeclampsia. Semin Reprod Endocrinol. 1998;16:47–55. doi: 10.1055/s-2007-1016252. [DOI] [PubMed] [Google Scholar]
- Walsh SW. Maternal-placental interactions of oxidative stress and antioxidants in preeclampsia. Seminars in Reproductive Endocrinology. 1998;16:93–104. doi: 10.1055/s-2007-1016256. [DOI] [PubMed] [Google Scholar]
- Caniggia I, Grisaru-Gravnosky S, Kuliszewsky M, Post M, Lye SJ. Inhibition of TGF-beta 3 restores the invasive capability of extravillous trophoblasts in preeclamptic pregnancies. J Clin Invest. 1999;103:1641–50. doi: 10.1172/JCI6380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel CA. Oxidative stress in the pathogenesis of preeclampsia. Proc Soc Exp Biol Med. 1999;222:222–35. doi: 10.1177/153537029922200305. [DOI] [PubMed] [Google Scholar]
- Rinehart BK, Terrone DA, Lagoo-Deenadayalan S, Barber WH, Hale EA, Martin JN, Jr, Bennett WA. Expression of the placental cytokines tumor necrosis factor alpha, interleukin 1beta, and interleukin 10 is increased in preeclampsia. Am J Obstet Gynecol. 1999;181:915–20. doi: 10.1016/s0002-9378(99)70325-x. [DOI] [PubMed] [Google Scholar]
- Caniggia I, Mostachfi H, Winter J, Gassmann M, Lye SJ, Kuliszewski M, Post M. Hypoxia-inducible factor-1 mediates the biological effects of oxygen on human trophoblast differentiation through TGFbeta(3) J Clin Invest. 2000;105:577–87. doi: 10.1172/JCI8316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharb S. Total free radical trapping antioxidant potential in pre-eclampsia. Int J Gynaecol Obstet. 2000;69:23–6. doi: 10.1016/s0020-7292(99)00198-8. [DOI] [PubMed] [Google Scholar]
- Tálosi G, Endreffy E, Túri S, Németh I. Molecular and genetic aspects of preeclampsia: State of the art. Mol Genet Metab. 2000;71:565–572. doi: 10.1006/mgme.2000.3099. [DOI] [PubMed] [Google Scholar]
- Walsh SW, Vaughan JE, Wang Y, Roberts LJ., 2nd Placental isoprostane is significantly increased in preeclampsia. FASEB J. 2000;14:1289–96. doi: 10.1096/fj.14.10.1289. [DOI] [PubMed] [Google Scholar]
- Wolf M, Kettyle E, Sandler L, Ecker JL, Roberts J, Thadhani R. Obesity and preeclampsia: the potential role of inflammation. Obstet Gynecol. 2001;98:757–62. doi: 10.1016/s0029-7844(01)01551-4. [DOI] [PubMed] [Google Scholar]
- Benyo DF, Smarason A, Redman CW, Sims C, Conrad KP. Expression of inflammatory cytokines in placentas from women with preeclampsia. J Clin Endocrinol Metab. 2001;86:2505–12. doi: 10.1210/jcem.86.6.7585. [DOI] [PubMed] [Google Scholar]
- Hung TH, Skepper JN, Burton GJ. In vitro ischemia-reperfusion injury in term human placenta as a model for oxidative stress in pathological pregnancies. Am J Pathol. 2001;159:1031–43. doi: 10.1016/S0002-9440(10)61778-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Jaramillo P, Casas JP, Serrano N. Preeclampsia: from epidemiological observations to molecular mechanisms. Braz J Med Biol Res. 2001;34:1227–35. doi: 10.1590/s0100-879x2001001000001. [DOI] [PubMed] [Google Scholar]
- Malek A, Sager R, Schneider H. Effect of hypoxia, oxidative stress and lipopolysaccharides on the release of prostaglandins and cytokines from human term placental explants. Placenta. 2001;22:S45–50. doi: 10.1053/plac.2001.0635. [DOI] [PubMed] [Google Scholar]
- Gitto E, Reiter RJ, Karbownik M, Tan DX, Gitto P, Barberi S, Barberi I. Causes of oxidative stress in the pre- and perinatal period. Biology of the Neonate. 2002;81:146–157. doi: 10.1159/000051527. [DOI] [PubMed] [Google Scholar]
- Granger JP, Alexander BT, Llinas MT, Bennett WA, Khalil RA. Pathophysiology of preeclampsia: linking placental ischemia/hypoxia with microvascular dysfunction. Microcirculation. 2002;9:147–60. doi: 10.1038/sj.mn.7800137. [DOI] [PubMed] [Google Scholar]
- Roberts JM, Lain KY. Recent Insights into the pathogenesis of pre-eclampsia. Placenta. 2002;23:359–72. doi: 10.1053/plac.2002.0819. [DOI] [PubMed] [Google Scholar]
- Vaughan JE, Walsh SW. Oxidative stress reproduces placental abnormalities of preeclampsia. Hypertens Preg. 2002;21:205–223. doi: 10.1081/PRG-120015848. [DOI] [PubMed] [Google Scholar]
- Burton GJ, Jauniaux E. Placental oxidative stress: from miscarriage to preeclampsia. J Soc Gynecol Investig. 2004;11:342–352. doi: 10.1016/j.jsgi.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Connors N, Merrill D. Antioxidants for prevention of preterm delivery. Clin Obs Gynecol. 2004;47:822–832. doi: 10.1097/01.grf.0000141431.23642.fc. [DOI] [PubMed] [Google Scholar]
- Raijmakers MT, Peters WH, Steegers EA, Poston L. Amino thiols, detoxification and oxidative stress in pre-eclampsia and other disorders of pregnancy. Curr Pharm Des. 2005;11:711–34. doi: 10.2174/1381612053381837. [DOI] [PubMed] [Google Scholar]
- Redman CW, Sargent IL. Pre-eclampsia, the placenta and the maternal systemic inflammatory response–a review. Placenta. 2003;24:S21–7. doi: 10.1053/plac.2002.0930. [DOI] [PubMed] [Google Scholar]
- Downing JW, Ramasubramanian R, Johnson RF, Minzter BH, Paschall RL, Sundell HW, Engelhardt B, Lewis R. Hypothesis: selective phosphodiesterase-5 inhibition improves outcome in preeclampsia. Med Hypotheses. 2004;63:1057–64. doi: 10.1016/j.mehy.2004.03.042. [DOI] [PubMed] [Google Scholar]
- Myatt L, Cui XL. Oxidative stress in the placenta. Histochem Cell Biol. 2004;122:369–382. doi: 10.1007/s00418-004-0677-x. [DOI] [PubMed] [Google Scholar]
- Touyz RM, Schiffrin EL. Reactive oxygen species in vascular biology: implications in hypertension. Histochem Cell Biol. 2004;122:339–52. doi: 10.1007/s00418-004-0696-7. [DOI] [PubMed] [Google Scholar]
- Touyz RM. Reactive oxygen species, vascular oxidative stress, and redox signaling in hypertension: what is the clinical significance? Hypertension. 2004;44:248–52. doi: 10.1161/01.HYP.0000138070.47616.9d. [DOI] [PubMed] [Google Scholar]
- Tsukahara H, Ohta N, Sato S, Hiraoka M, Shukunami K, Uchiyama M, Kawakami H, Sekine K, Mayumi M. Concentrations of pentosidine, an advanced glycation end-product, in umbilical cord blood. Free Radic Res. 2004;38:691–5. doi: 10.1080/1071576042000220256. [DOI] [PubMed] [Google Scholar]
- Bdolah Y, Karumanchi SA, Sachs BP. Recent advances in understanding of preeclampsia. Croat Med J. 2005;46:728–36. [PubMed] [Google Scholar]
- Biondi C, Pavan B, Lunghi L, Florini S, Vesce F. The role and modulation of the oxidative balance in pregnancy. Curr Pharmaceut Design. 2005;11:2075–2089. doi: 10.2174/1381612054065747. [DOI] [PubMed] [Google Scholar]
- Engin-Üstün Y, Üstün Y, Kamaci M, Şekeroğlu R. Maternal serum ceruloplasmin in preectampsia. Int J Gynecol Obs. 2005;89:51–52. doi: 10.1016/j.ijgo.2005.01.024. [DOI] [PubMed] [Google Scholar]
- Gupta S, Agarwal A, Sharma RK. The role of placental oxidative stress and lipid peroxidation in preeclampsia. Obstet Gynecol Surv. 2005;60:807–16. doi: 10.1097/01.ogx.0000193879.79268.59. [DOI] [PubMed] [Google Scholar]
- Noris M, Perico N, Remuzzi G. Mechanisms of disease: pre-eclampsia. Nature Clin Pract Nephrol. 2005;1:98–114. doi: 10.1038/ncpneph0035. [DOI] [PubMed] [Google Scholar]
- Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;308:1592–1594. doi: 10.1126/science.1111726. [DOI] [PubMed] [Google Scholar]
- Roberts JM, Gammill HS. Preeclampsia: recent insights. Hypertension. 2005;46:1243–9. doi: 10.1161/01.HYP.0000188408.49896.c5. [DOI] [PubMed] [Google Scholar]
- Rodrigo R, Parra M, Bosco C, Fernandez V, Barja P, Guajardo J, Messina R. Pathophysiological basis for the prophylaxis of preeclampsia through early supplementation with antioxidant vitamins. Pharmacology & Therapeutics. 2005;107:177–197. doi: 10.1016/j.pharmthera.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Scholl TO, Leskiw M, Chen X, Sims M, Stein TP. Oxidative stress, diet, and the etiology of preeclampsia. Am J Clin Nutr. 2005;81:1390–6. doi: 10.1093/ajcn/81.6.1390. [DOI] [PubMed] [Google Scholar]
- Soleymanlou N, Jurisica I, Nevo O, Ietta F, Zhang X, Zamudio S, Post M, Caniggia I. Molecular evidence of placental hypoxia in preeclampsia. J Clin Endocrinol Metab. 2005;90:4299–4308. doi: 10.1210/jc.2005-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinnato JA, Livingston JC. Prevention of preeclampsia with antioxidants: Evidence from randomized trials. Clinical Obstetrics and Gynecology. 2005;48:416–429. doi: 10.1097/01.grf.0000160312.74983.f3. [DOI] [PubMed] [Google Scholar]
- Blackburn S. Free radicals in perinatal and neonatal care, part 2 – Oxidative stress during the perinatal and neonatal period. Journal of Perinatal & Neonatal Nursing. 2006;20:125–127. doi: 10.1097/00005237-200604000-00005. [DOI] [PubMed] [Google Scholar]
- Borzychowski AM, Sargent IL, Redman CW. Inflammation and pre-eclampsia. Semin Fetal Neonatal Med. 2006;11:309–16. doi: 10.1016/j.siny.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Hauguel-de Mouzon S, Guerre-Millo M. The placenta cytokine network and inflammatory signals. Placenta. 2006;27:794–8. doi: 10.1016/j.placenta.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Perkins AV. Endogenous anti-oxidants in pregnancy and preeclampsia. Aus New Zeal J Obs Gynaecol. 2006;46:77–83. doi: 10.1111/j.1479-828X.2006.00532.x. [DOI] [PubMed] [Google Scholar]
- Rogers MS, Wang CC, Tam WH, Li CY, Chu KO, Chu CY. Oxidative stress in midpregnancy as a predictor of gestational hypertension and pre-eclampsia. BJOG. 2006;113:1053–9. doi: 10.1111/j.1471-0528.2006.01026.x. [DOI] [PubMed] [Google Scholar]
- Sankaralingam S, Arenas IA, Lalu MM, Davidge ST. Preeclampsia: current understanding of the molecular basis of vascular dysfunction. Expert Rev Mol Med. 2006;8:1–20. doi: 10.1017/S1462399406010465. [DOI] [PubMed] [Google Scholar]
- Sargent IL, Borzychowski AM, Redman CW. Immunoregulation in normal pregnancy and pre-eclampsia: an overview. Reprod Biomed Online. 2006;13:680–6. doi: 10.1016/s1472-6483(10)60659-1. [DOI] [PubMed] [Google Scholar]
- Sharma JB, Sharma A, Bahadur A, Vimala N, Satyam A, Mittal S. Oxidative stress markers and antioxidant levels in normal pregnancy and pre-eclampsia. Int J Gynecol Obs. 2006;94:23–27. doi: 10.1016/j.ijgo.2006.03.025. [DOI] [PubMed] [Google Scholar]
- Webster RP, Brockman D, Myatt L. Nitration of p38 MAPK in the placenta: association of nitration with reduced catalytic activity of p38 MAPK in pre-eclampsia. Molecular Human Reproduction. 2006;12:677–685. doi: 10.1093/molehr/gal071. [DOI] [PubMed] [Google Scholar]
- Burdon C, Mann C, Cindrova-Davies T, Ferguson-Smith AC, Burton GJ. Oxidative stress and the induction of cyclooxygenase enzymes and apoptosis in the murine placenta. Placenta. 2007;28:724–33. doi: 10.1016/j.placenta.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker I. Gabor Than Award Lecture 2006: pre-eclampsia and villous trophoblast turnover: perspectives and possibilities. Placenta. 2007;28:S4–13. doi: 10.1016/j.placenta.2007.01.016. [DOI] [PubMed] [Google Scholar]
- Keelan JA, Mitchell MD. Placental cytokines and preeclampsia. Front Biosci. 2007;12:2706–27. doi: 10.2741/2266. [DOI] [PubMed] [Google Scholar]
- Milczarek R, Sokolowska E, Hallmann A, Klimek J. The NADPH- and iron-dependent lipid peroxidation in human placental microsomes. Molecular and Cellular Biochemistry. 2007;295:105–111. doi: 10.1007/s11010-006-9279-3. [DOI] [PubMed] [Google Scholar]
- Schiessl B. Inflammatory response in preeclampsia. Mol Aspects Med. 2007;28:210–9. doi: 10.1016/j.mam.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Shah TJ, Walsh SW. Activation of NF-kappaB and expression of COX-2 in association with neutrophil infiltration in systemic vascular tissue of women with preeclampsia. Am J Obstet Gynecol. 2007;196:48 e1–8. doi: 10.1016/j.ajog.2006.08.038. [DOI] [PubMed] [Google Scholar]
- Walsh SW. Obesity: a risk factor for preeclampsia. Trends Endocrinol Metab. 2007;18:365–70. doi: 10.1016/j.tem.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Zamudio S. High-altitude hypoxia and preeclampsia. Frontiers in Bioscience. 2007;12:2967–2977. doi: 10.2741/2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert JS, Ryan MJ, LaMarca BB, Sedeek M, Murphy SR, Granger JP. Pathophysiology of hypertension during preeclampsia: linking placental ischemia with endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2008;294:H541–50. doi: 10.1152/ajpheart.01113.2007. [DOI] [PubMed] [Google Scholar]
- Robinson NJ, Wareing M, Hudson NK, Blankley RT, Baker PN, Aplin JD, Crocker IP. Oxygen and the liberation of placental factors responsible for vascular compromise. Lab Invest. 2008. [DOI] [PubMed]
- Entman SS, Richardson LD, Killam AP. Elevated serum ferritin in the altered ferrokinetics of toxemia of pregnancy. AmJ Obs Gynecol. 1982;144:418–422. doi: 10.1016/0002-9378(82)90247-2. [DOI] [PubMed] [Google Scholar]
- Entman SS, Richardson LD. Clinical applications of the altered iron kinetics of toxemia of pregnancy. Am J Obs Gynecol. 1983;146:568–574. doi: 10.1016/0002-9378(83)90804-9. [DOI] [PubMed] [Google Scholar]
- Entman SS, Richardson LD, Killam AP. Altered ferrokinetics in toxemia of pregnancy – a possible indicator of decreased red cell survival. Clin Exp Hypertens B. 1983;2:171–178. doi: 10.3109/10641958309023469. [DOI] [PubMed] [Google Scholar]
- Entman SS, Kambam JR, Bradley CA, Cousar JB. Increased levels of carboxyhemoglobin and serum iron as an indicator of increased red cell turnover in preeclampsia. Am J Obstet Gynecol. 1987;156:1169–73. doi: 10.1016/0002-9378(87)90134-7. [DOI] [PubMed] [Google Scholar]
- Samuels P, Main EK, Mennuti MT, Gabbe SG. The origin of increased serum iron in pregnancy-induced hypertension. Am J Obstet Gynecol. 1987;157:721–5. doi: 10.1016/s0002-9378(87)80037-6. [DOI] [PubMed] [Google Scholar]
- Lindheimer MD, Katz AI. Preeclampsia – Patho-physiology, diagnosis, and management. Annual Review of Medicine. 1989;40:233–250. doi: 10.1146/annurev.me.40.020189.001313. [DOI] [PubMed] [Google Scholar]
- Raman L, Pawashe AB, Yasodhara P. Hyperferritinemia in pregnancy induced hypertension and eclampsia. J Postgrad Med. 1992;38:65–7. [PubMed] [Google Scholar]
- Das SS, Dhall GI, Dhall K, Dash S. Significance of serum iron levels as a biochemical marker in pregnancy-induced hypertension. Int J Gynecol Obs. 1994;45:3–9. doi: 10.1016/0020-7292(94)90758-7. [DOI] [PubMed] [Google Scholar]
- Hubel CA, Kozlov AV, Kagan VE, Evans RW, Davidge ST, McLaughlin MK, Roberts JM. Decreased transferrin and increased transferrin saturation in sera of women with preeclampsia: implications for oxidative stress. Am J Obstet Gynecol. 1996;175:692–700. doi: 10.1053/ob.1996.v175.a74252. [DOI] [PubMed] [Google Scholar]
- Sizoo BB, Paarlberg MM, Bouman AA, Dekker GA. The role of serum iron levels in diagnostic hypertensive disorders in pregnancy. Hypertension in Pregnancy. 1997;16:425–433. [Google Scholar]
- Vitoratos N, Salamalekis E, Dalamaga N, Kassanos D, Creatsas G. Defective antioxidant mechanisms via changes in serum ceruloplasmin and total iron binding capacity of serum in women with pre-eclampsia. Eur J Obstet Gynecol Reprod Biol. 1999;84:63–7. doi: 10.1016/s0301-2115(98)00261-9. [DOI] [PubMed] [Google Scholar]
- Lao TT, Tam KF, Chan LY. Third trimester iron status and pregnancy outcome in non-anaemic women; pregnancy unfavourably affected by maternal iron excess. Hum Reprod. 2000;15:1843–8. doi: 10.1093/humrep/15.8.1843. [DOI] [PubMed] [Google Scholar]
- Waterstone M, Bewley S, Wolfe C. Incidence and predictors of severe obstetric morbidity: case-control study. BMJ. 2001;322:1089–93. doi: 10.1136/bmj.322.7294.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello G, Parretti E, Cioni R, Lagozio C, Mealli F, Pratesi M. Individual longitudinal patterns in biochemical and hematological markers for the early prediction of pre-eclampsia. J Matern Fetal Neonatal Med. 2002;11:93–9. doi: 10.1080/jmf.11.2.93.99. [DOI] [PubMed] [Google Scholar]
- Casanueva E, Viteri FE. Iron and oxidative stress in pregnancy. J Nutr. 2003;133:1700S–1708S. doi: 10.1093/jn/133.5.1700S. [DOI] [PubMed] [Google Scholar]
- Hubel CA, Bodnar LM, Many A, Harger G, Ness RB, Roberts JM. Nonglycosylated ferritin predominates in the circulation of women with preeclampsia but not intrauterine growth restriction. Clin Chem. 2004;50:948–951. doi: 10.1373/clinchem.2003.030932. [DOI] [PubMed] [Google Scholar]
- Norwitz ER, Tsen LC, Park JS, Fitzpatrick PA, Dorfman DM, Saade GR, Buhimschi CS, Buhimschi IA. Discriminatory proteomic biomarker analysis identifies free hemoglobin in the cerebrospinal fluid of women with severe preeclampsia. Amer J Obs Gynecol. 2005;193:957–964. doi: 10.1016/j.ajog.2005.06.055. [DOI] [PubMed] [Google Scholar]
- Scholl TO. Iron status during pregnancy: setting the stage for mother and infant. Am J Clin Nutr. 2005;81:1218S–1222S. doi: 10.1093/ajcn/81.5.1218. [DOI] [PubMed] [Google Scholar]
- Serdar Z, Gür E, Develioğlu O. Serum iron and copper status and oxidative stress in severe and mild preeclampsia. Cell Biochem Funct. 2006;24:209–15. doi: 10.1002/cbf.1235. [DOI] [PubMed] [Google Scholar]
- Smith TG, Robbins PA. Iron, pre-eclampsia and hypoxia-inducible factor. Bjog. 2007;114:1581–2. doi: 10.1111/j.1471-0528.2007.01490.x. [DOI] [PubMed] [Google Scholar]
- Ziaei S, Norrozi M, Faghihzadeh S, Jafarbegloo E. A randomised placebo-controlled trial to determine the effect of iron supplementation on pregnancy outcome in pregnant women with haemoglobin > or = 13.2 g/dl. BJOG. 2007;114:684–8. doi: 10.1111/j.1471-0528.2007.01325.x. [DOI] [PubMed] [Google Scholar]
- Guller S, Buhimschi CS, Ma YY, Huang ST, Yang L, Kuczynski E, Zambrano E, Lockwood CJ, Buhimschi IA. Placental expression of ceruloplasmin in pregnancies complicated by severe preeclampsia. Lab Invest. 2008;88:1057–67. doi: 10.1038/labinvest.2008.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambling L, Danzeisen R, Gair S, Lea RG, Charania Z, Solanky N, Joory KD, Srai SKS, McArdle HJ. Effect of iron deficiency on placental transfer of iron and expression of iron transport proteins in vivo and in vitro. Biochem J. 2001;356:883–889. doi: 10.1042/0264-6021:3560883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman DJ, McManus F, Brown EA, Cherry L, Norrie J, Ramsay JE, Clark P, Walker ID, Sattar N, Greer IA. Short- and long-term changes in plasma inflammatory markers associated with preeclampsia. Hypertension. 2004;44:708–14. doi: 10.1161/01.HYP.0000143849.67254.ca. [DOI] [PubMed] [Google Scholar]
- Bodnar LM, Ness RB, Harger GF, Roberts JM. Inflammation and triglycerides partially mediate the effect of prepregnancy body mass index on the risk of preeclampsia. Am J Epidemiol. 2005;162:1198–206. doi: 10.1093/aje/kwi334. [DOI] [PubMed] [Google Scholar]
- Braekke K, Holthe MR, Harsem NK, Fagerhol MK, Staff AC. Calprotectin, a marker of inflammation, is elevated in the maternal but not in the fetal circulation in preeclampsia. Am J Obstet Gynecol. 2005;193:227–33. doi: 10.1016/j.ajog.2004.11.055. [DOI] [PubMed] [Google Scholar]
- Hu W, Wang H, Wang Z, Huang H, Dong M. Elevated serum levels of interleukin-15 and interleukin-16 in preeclampsia. J Reprod Immunol. 2007;73:166–71. doi: 10.1016/j.jri.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Keelan JA, Mitchell MD. Cytokines, hypoxia, and preeclampsia. J Soc Gynecol Investig. 2005;12:385–7. doi: 10.1016/j.jsgi.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Elovitz MA. Anti-inflammatory interventions in pregnancy: now and the future. Semin Fetal Neonatal Med. 2006;11:327–32. doi: 10.1016/j.siny.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Paternoster DM, Fantinato S, Stella A, Nanhorngue KN, Milani M, Plebani M, Nicolini U, Girolami A. C-reactive protein in hypertensive disorders in pregnancy. Clin Appl Thromb Hemost. 2006;12:330–7. doi: 10.1177/1076029606291382. [DOI] [PubMed] [Google Scholar]
- Dorrepaal CA, Berger HM, Benders M, vanZoerenGrobben D, VanDeBor M, VanBel F. Nonprotein-bound iron in postasphyxial reperfusion injury of the newborn. Pediatrics. 1996;98:883–889. [PubMed] [Google Scholar]
- Goldenberg RL, Tamura T, DuBard M, Johnston KE, Copper RL, Neggers Y. Plasma ferritin and pregnancy outcome. Am J Obs Gynecol. 1996;175:1356–1359. doi: 10.1016/s0002-9378(96)70054-6. [DOI] [PubMed] [Google Scholar]
- Tamura T, Goldenberg RL, Johnston KE, Cliver SP, Hickey CA. Serum ferritin: A predictor of early spontaneous preterm delivery. Obs Gynecol. 1996;87:360–365. doi: 10.1016/0029-7844(95)00437-8. [DOI] [PubMed] [Google Scholar]
- Buhimschi IA, Buhimschi CS, Weiner CP. Protective effect of N-acetylcysteine against fetal death and preterm labor induced by maternal inflammation. American Journal of Obstetrics and Gynecology. 2003;188:203–208. doi: 10.1067/mob.2003.112. [DOI] [PubMed] [Google Scholar]
- Ciccoli L, Rossi V, Leoncini S, Signorini C, Paffetti P, Bracci R, Buonocore G, Comporti M. Iron release in erythrocytes and plasma non protein-bound iron in hypoxic and non hypoxic newborns. Free Radical Research. 2003;37:51–58. doi: 10.1080/1071576021000032122. [DOI] [PubMed] [Google Scholar]
- Buhimschi IA, Saade GR, Chwalisz K, Garfield RE. The nitric oxide pathway in pre-eclampsia: pathophysiological implications. Human Reproduction Update. 1998;4:25–42. doi: 10.1093/humupd/4.1.25. [DOI] [PubMed] [Google Scholar]
- Milman N. Iron and pregnancy – a delicate balance. Annals of Hematology. 2006;85:559–565. doi: 10.1007/s00277-006-0108-2. [DOI] [PubMed] [Google Scholar]
- Milman N. Iron prophylaxis in pregnancy-general or individual and in which dose? Annals of Hematology. 2006;85:821–828. doi: 10.1007/s00277-006-0145-x. [DOI] [PubMed] [Google Scholar]
- Lachili B, Hininger I, Faure H, Arnaud J, Richard MJ, Favier A, Roussel AM. Increased lipid peroxidation in pregnant women after iron and vitamin C supplementation. Biol Trace Elem Res. 2001;83:103–10. doi: 10.1385/BTER:83:2:103. [DOI] [PubMed] [Google Scholar]
- Devrim E, Tarhan I, Ergüder IB, Durak I. Oxidant/antioxidant status of placenta, blood, and cord blood samples from pregnant women supplemented with iron. J Soc Gynecol Investig. 2006;13:502–5. doi: 10.1016/j.jsgi.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Keyes LE, Armaza JF, Niermeyer S, Vargas E, Young DA, Moore LG. Intrauterine growth restriction, preeclampsia, and intrauterine mortality at high altitude in Bolivia. Pediatric Research. 2003;54:20–25. doi: 10.1203/01.PDR.0000069846.64389.DC. [DOI] [PubMed] [Google Scholar]
- Teramo KA, Hiilesmaa VK, Schwartz R, Clemons GK, Widness JA. Amniotic fluid and cord plasma erythropoietin levels in pregnancies complicated by preeclampsia, pregnancy-induced hypertension and chronic hypertension. J Perinat Med. 2004;32:240–7. doi: 10.1515/JPM.2004.045. [DOI] [PubMed] [Google Scholar]
- Liang ST, Wong VC, So WW, Ma HK, Chan V, Todd D. Homozygous alpha-thalassaemia: clinical presentation, diagnosis and management. A review of 46 cases. Br J Obstet Gynaecol. 1985;92:680–4. doi: 10.1111/j.1471-0528.1985.tb01447.x. [DOI] [PubMed] [Google Scholar]
- Tungwiwat W, Fucharoen S, Fucharoen G, Ratanasiri T, Sanchaisuriya K. Development and application of a real-time quantitative PCR for prenatal detection of fetal alpha(0)-thalassemia from maternal plasma. Ann N Y Acad Sci. 2006;1075:103–7. doi: 10.1196/annals.1368.013. [DOI] [PubMed] [Google Scholar]
- Senden IP, de Groot CJ, Steegers EA, Bertina RM, Swinkels DW. Preeclampsia and the C282Y mutation in the hemochromatosis (HFE) gene. Clin Chem. 2004;50:973–4. doi: 10.1373/clinchem.2004.031591. [DOI] [PubMed] [Google Scholar]
- Quek L, Thein SL. Molecular therapies in beta-thalassaemia. British Journal of Haematology. 2007;136:353–365. doi: 10.1111/j.1365-2141.2006.06408.x. [DOI] [PubMed] [Google Scholar]
- Knox KS, Baker JC. Genome-wide expression profiling of placentas in the p57Kip2 model of pre-eclampsia. Mol Hum Reprod. 2007;13:251–63. doi: 10.1093/molehr/gal116. [DOI] [PubMed] [Google Scholar]
- Suire S, Stewart F, Beauchamp J, Kennedy MW. Uterocalin, a lipocalin provisioning the preattachment equine conceptus: fatty acid and retinol binding properties, and structural characterization. Biochem J. 2001;356:369–76. doi: 10.1042/0264-6021:3560369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryon J, Bendickson L, Nilsen-Hamilton M. High expression in involuting reproductive tissues of uterocalin/24p3, a lipocalin and acute phase protein. Biochem J. 2002;367:271–7. doi: 10.1042/BJ20020026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsen-Hamilton M, Liu Q, Ryon J, Bendickson L, Lepont P, Chang Q. Tissue involution and the acute phase response. Ann N Y Acad Sci. 2003;995:94–108. doi: 10.1111/j.1749-6632.2003.tb03213.x. [DOI] [PubMed] [Google Scholar]
- D'Anna R, Baviera G, Giordano D, Todarello G, Corrado F, Buemi M. Second trimester neutrophil gelatinase-associated lipocalin as a potential prediagnostic marker of preeclampsia. Acta Obstet Gynecol Scand. 2008. pp. 1–4. [DOI] [PubMed]
- Coughlan MT, Permezel M, Georgiou HM, Rice GE. Repression of oxidant-induced nuclear factor-kappaB activity mediates placental cytokine responses in gestational diabetes. J Clin Endocrinol Metab. 2004;89:3585–94. doi: 10.1210/jc.2003-031953. [DOI] [PubMed] [Google Scholar]
- Coughlan MT, Vervaart PP, Permezel M, Georgiou HM, Rice GE. Altered placental oxidative stress status in gestational diabetes mellitus. Placenta. 2004;25:78–84. doi: 10.1016/S0143-4004(03)00183-8. [DOI] [PubMed] [Google Scholar]
- King JC. Maternal obesity, metabolism and pregnancy outcomes. Annu Rev Nutrition. 2006;26:271–291. doi: 10.1146/annurev.nutr.24.012003.132249. [DOI] [PubMed] [Google Scholar]
- Mazar RM, Srinivas SK, Sammel MD, Andrela CM, Elovitz MA. Metabolic score as a novel approach to assessing preeclampsia risk. Am J Obstet Gynecol. 2007;197:411 e1–5. doi: 10.1016/j.ajog.2007.07.014. [DOI] [PubMed] [Google Scholar]
- Rademacher TW, Gumaa K, Scioscia M. Preeclampsia, insulin signalling and immunological dysfunction: a fetal, maternal or placental disorder? J Reprod Immunol. 2007;76:78–84. doi: 10.1016/j.jri.2007.03.019. [DOI] [PubMed] [Google Scholar]
- Hales CN, Ozanne SE. For Debate: Fetal and early postnatal growth restriction lead to diabetes, the metabolic syndrome and renal failure. Diabetologia. 2003;46:1013–1019. doi: 10.1007/s00125-003-1131-7. [DOI] [PubMed] [Google Scholar]
- McMillen IC, Robinson JS. Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiol Rev. 2005;85:571–633. doi: 10.1152/physrev.00053.2003. [DOI] [PubMed] [Google Scholar]
- Williams PJ, Gumaa K, Scioscia M, Redman CW, Rademacher TW. Inositol phosphoglycan P-type in preeclampsia: a novel marker? Hypertension. 2007;49:84–9. doi: 10.1161/01.HYP.0000251301.12357.ba. [DOI] [PubMed] [Google Scholar]
- West IC. Radicals and oxidative stress in diabetes. Diabet Med. 2000;17:171–80. doi: 10.1046/j.1464-5491.2000.00259.x. [DOI] [PubMed] [Google Scholar]
- Lipinski B. Pathophysiology of oxidative stress in diabetes mellitus. J Diabetes Complications. 2001;15:203–10. doi: 10.1016/s1056-8727(01)00143-x. [DOI] [PubMed] [Google Scholar]
- Maddux BA, See W, Lawrence JC, Jr, Goldfine AL, Goldfine ID, Evans JL. Protection against oxidative stress-induced insulin resistance in rat L6 muscle cells by mircomolar concentrations of alpha-lipoic acid. Diabetes. 2001;50:404–10. doi: 10.2337/diabetes.50.2.404. [DOI] [PubMed] [Google Scholar]
- Evans JL, Maddux BA, Goldfine ID. The molecular basis for oxidative stress-induced insulin resistance. Antioxid Redox Signal. 2005;7:1040–52. doi: 10.1089/ars.2005.7.1040. [DOI] [PubMed] [Google Scholar]
- Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440:944–8. doi: 10.1038/nature04634. [DOI] [PubMed] [Google Scholar]
- Meigs JB, Larson MG, Fox CS, Keaney JF, Jr, Vasan RS, Benjamin EJ. Association of oxidative stress, insulin resistance, and diabetes risk phenotypes: the Framingham Offspring Study. Diabetes Care. 2007;30:2529–35. doi: 10.2337/dc07-0817. [DOI] [PubMed] [Google Scholar]
- Shah S, Iqbal M, Karam J, Salifu M, McFarlane SI. Oxidative stress, glucose metabolism, and the prevention of type 2 diabetes: Pathophysiological insights. Antioxid Redox Signal. 2007;9:911–929. doi: 10.1089/ars.2007.1629. [DOI] [PubMed] [Google Scholar]
- Choi SW, Benzie IF, Ma SW, Strain JJ, Hannigan BM. Acute hyperglycemia and oxidative stress: direct cause and effect? Free Radic Biol Med. 2008;44:1217–31. doi: 10.1016/j.freeradbiomed.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes HP, Giardino I, Brownlee M. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787–90. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Oxidative stress and stress-activated signaling pathways: A unifying hypothesis of type 2 diabetes. Endocrine Reviews. 2002;23:599–622. doi: 10.1210/er.2001-0039. [DOI] [PubMed] [Google Scholar]
- Niedowicz DM, Daleke DL. The role of oxidative stress in diabetic complications. Cell Biochem Biophys. 2005;43:289–330. doi: 10.1385/CBB:43:2:289. [DOI] [PubMed] [Google Scholar]
- Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes. 2002;51:2944–50. doi: 10.2337/diabetes.51.10.2944. [DOI] [PubMed] [Google Scholar]
- Inoue I, Katayama S, Takahashi K, Negishi K, Miyazaki T, Sonoda M, Komoda T. Troglitazone has a scavenging effect on reactive oxygen species. Biochem Biophys Res Commun. 1997;235:113–6. doi: 10.1006/bbrc.1997.6512. [DOI] [PubMed] [Google Scholar]
- Fukui T, Noma T, Mizushige K, Aki Y, Kimura S, Abe Y. Dietary troglitazone decreases oxidative stress in early stage type II diabetic rats. Life Sci. 2000;66:2043–9. doi: 10.1016/s0024-3205(00)00531-2. [DOI] [PubMed] [Google Scholar]
- Bao Y, Jia RH, Yuan J, Li J. Rosiglitazone ameliorates diabetic nephropathy by inhibiting reactive oxygen species and its downstream-signaling pathways. Pharmacology. 2007;80:57–64. doi: 10.1159/000103232. [DOI] [PubMed] [Google Scholar]
- Jung TW, Lee JY, Shim WS, Kang ES, Kim SK, Ahn CW, Lee HC, Cha BS. Rosiglitazone protects human neuroblastoma SH-SY5Y cells against MPP+ induced cytotoxicity via inhibition of mitochondrial dysfunction and ROS production. J Neurol Sci. 2007;253:53–60. doi: 10.1016/j.jns.2006.11.020. [DOI] [PubMed] [Google Scholar]
- Jung Y, Song S, Choi C. Peroxisome proliferator activated receptor gamma agonists suppress TNFalpha-induced ICAM-1 expression by endothelial cells in a manner potentially dependent on inhibition of reactive oxygen species. Immunol Lett. 2008;117:63–9. doi: 10.1016/j.imlet.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Allahtavakoli M, Shabanzadeh AP, Sadr SS, Parviz M, Djahanguiri B. Rosiglitazone, a peroxisome proliferator-activated receptor-gamma ligand, reduces infarction volume and neurological deficits in an embolic model of stroke. Clin Exp Pharmacol Physiol. 2006;33:1052–8. doi: 10.1111/j.1440-1681.2006.04486.x. [DOI] [PubMed] [Google Scholar]
- Collino M, Aragno M, Mastrocola R, Benetti E, Gallicchio M, Dianzani C, Danni O, Thiemermann C, Fantozzi R. Oxidative stress and inflammatory response evoked by transient cerebral ischemia/reperfusion: effects of the PPAR-alpha agonist WY14643. Free Radic Biol Med. 2006;41:579–89. doi: 10.1016/j.freeradbiomed.2006.04.030. [DOI] [PubMed] [Google Scholar]
- Collino M, Aragno M, Mastrocola R, Gallicchio M, Rosa AC, Dianzani C, Danni O, Thiemermann C, Fantozzi R. Modulation of the oxidative stress and inflammatory response by PPAR-gamma agonists in the hippocampus of rats exposed to cerebral ischemia/reperfusion. Eur J Pharmacol. 2006;530:70–80. doi: 10.1016/j.ejphar.2005.11.049. [DOI] [PubMed] [Google Scholar]
- Allahtavakoli M, Shabanzadeh A, Roohbakhsh A, Pourshanazari A. Combination therapy of rosiglitazone, a peroxisome proliferator-activated receptor-gamma ligand, and NMDA receptor antagonist (MK-801) on experimental embolic stroke in rats. Basic Clin Pharmacol Toxicol. 2007;101:309–14. doi: 10.1111/j.1742-7843.2007.00127.x. [DOI] [PubMed] [Google Scholar]
- Wessel J, Moratorio G, Rao F, Mahata M, Zhang L, Greene W, Rana BK, Kennedy BP, Khandrika S, Huang P, Lillie EO, Shih PA, Smith DW, Wen G, Hamilton BA, Ziegler MG, Witztum JL, Schork NJ, Schmid-Schonbein GW, O'Connor DT. C-reactive protein, an 'intermediate phenotype' for inflammation: human twin studies reveal heritability, association with blood pressure and the metabolic syndrome, and the influence of common polymorphism at catecholaminergic/beta-adrenergic pathway loci. J Hypertens. 2007;25:329–43. doi: 10.1097/HJH.0b013e328011753e. [DOI] [PubMed] [Google Scholar]
- Baynes JW, Thorpe SR. Role of oxidative stress in diabetic complications: a new perspective on an old paradigm. Diabetes. 1999;48:1–9. doi: 10.2337/diabetes.48.1.1. [DOI] [PubMed] [Google Scholar]
- Hu FB, Meigs JB, Li TY, Rifai N, Manson JE. Inflammatory markers and risk of developing type 2 diabetes in women. Diabetes. 2004;53:693–700. doi: 10.2337/diabetes.53.3.693. [DOI] [PubMed] [Google Scholar]
- Pickup JC. Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes. Diabetes Care. 2004;27:813–23. doi: 10.2337/diacare.27.3.813. [DOI] [PubMed] [Google Scholar]
- Kolb H, Mandrup-Poulsen T. An immune origin of type 2 diabetes? Diabetologia. 2005;48:1038–50. doi: 10.1007/s00125-005-1764-9. [DOI] [PubMed] [Google Scholar]
- Lee YH, Pratley RE. The evolving role of inflammation in obesity and the metabolic syndrome. Curr Diab Rep. 2005;5:70–5. doi: 10.1007/s11892-005-0071-7. [DOI] [PubMed] [Google Scholar]
- Tataranni PA, Ortega E. A burning question: does an adipokine-induced activation of the immune system mediate the effect of overnutrition on type 2 diabetes? Diabetes. 2005;54:917–27. doi: 10.2337/diabetes.54.4.917. [DOI] [PubMed] [Google Scholar]
- Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111–9. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandraki K, Piperi C, Kalofoutis C, Singh J, Alaveras A, Kalofoutis A. Inflammatory process in type 2 diabetes: The role of cytokines. Ann N Y Acad Sci. 2006;1084:89–117. doi: 10.1196/annals.1372.039. [DOI] [PubMed] [Google Scholar]
- Chen H. Cellular inflammatory responses: novel insights for obesity and insulin resistance. Pharmacol Res. 2006;53:469–77. doi: 10.1016/j.phrs.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Herder C, Peltonen M, Koenig W, Kraft I, Muller-Scholze S, Martin S, Lakka T, Ilanne-Parikka P, Eriksson JG, Hamalainen H, Keinanen-Kiukaanniemi S, Valle TT, Uusitupa M, Lindstrom J, Kolb H, Tuomilehto J. Systemic immune mediators and lifestyle changes in the prevention of type 2 diabetes: results from the Finnish Diabetes Prevention Study. Diabetes. 2006;55:2340–6. doi: 10.2337/db05-1320. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–7. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- Kempf K, Rose B, Herder C, Kleophas U, Martin S, Kolb H. Inflammation in metabolic syndrome and type 2 diabetes: Impact of dietary glucose. Ann N Y Acad Sci. 2006;1084:30–48. doi: 10.1196/annals.1372.012. [DOI] [PubMed] [Google Scholar]
- Neels JG, Olefsky JM. Inflamed fat: what starts the fire? J Clin Invest. 2006;116:33–5. doi: 10.1172/JCI27280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, Vaddi K, Charo I, Leibel RL, Ferrante AW., Jr CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest. 2006;116:115–24. doi: 10.1172/JCI24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zozulinska D, Wierusz-Wysocka B. Type 2 diabetes mellitus as inflammatory disease. Diabetes Research and Clinical Practice. 2006;74:S12–S16. [Google Scholar]
- Pradhan A. Obesity, metabolic syndrome, and type 2 diabetes: inflammatory basis of glucose metabolic disorders. Nutr Rev. 2007;65:S152–6. doi: 10.1111/j.1753-4887.2007.tb00354.x. [DOI] [PubMed] [Google Scholar]
- Shoelson SE, Herrero L, Naaz A. Obesity, inflammation, and insulin resistance. Gastroenterology. 2007;132:2169–2180. doi: 10.1053/j.gastro.2007.03.059. [DOI] [PubMed] [Google Scholar]
- Thorand B, Baumert J, Kolb H, Meisinger C, Chambless L, Koenig W, Herder C. Sex differences in the prediction of type 2 diabetes by inflammatory markers: results from the MONICA/KORA Augsburg case-cohort study, 1984–2002. Diabetes Care. 2007;30:854–60. doi: 10.2337/dc06-1693. [DOI] [PubMed] [Google Scholar]
- Wei Y, Chen K, Whaley-Connell AT, Stump CS, Ibdah JA, Sowers JR. Skeletal muscle insulin resistance: role of inflammatory cytokines and reactive oxygen species. Am J Physiol Regul Integr Comp Physiol. 2008;294:R673–80. doi: 10.1152/ajpregu.00561.2007. [DOI] [PubMed] [Google Scholar]
- Lao TT, Chan PL, Tam KF. Gestational diabetes mellitus in the last trimester – a feature of maternal iron excess? Diabet Med. 2001;18:218–23. doi: 10.1046/j.1464-5491.2001.00453.x. [DOI] [PubMed] [Google Scholar]
- Lao TT, Tse KY, Chan LY, Tam KF, Ho LF. NBsAg carrier status and the association between gestational diabetes with increased serum ferritin concentration in Chinese women. Diabetes Care. 2003;26:3011–3016. doi: 10.2337/diacare.26.11.3011. [DOI] [PubMed] [Google Scholar]
- Chen X, Scholl TO, Stein TP. Association of elevated serum ferritin levels and the risk of gestational diabetes mellitus in pregnant women: The Camden study. Diabetes Care. 2006;29:1077–82. doi: 10.2337/diacare.2951077. [DOI] [PubMed] [Google Scholar]
- Oberley LW. Free radicals and diabetes. Free Radic Biol Med. 1988;5:113–24. doi: 10.1016/0891-5849(88)90036-6. [DOI] [PubMed] [Google Scholar]
- Wolff SP. Diabetes mellitus and free radicals. Free radicals, transition metals and oxidative stress in the aetiology of diabetes mellitus and complications. Br Med Bull. 1993;49:642–52. doi: 10.1093/oxfordjournals.bmb.a072637. [DOI] [PubMed] [Google Scholar]
- Tuomainen TP, Nyyssonen K, Salonen R, Tervahauta A, Korpela H, Lakka T, Kaplan GA, Salonen JT. Body iron stores are associated with serum insulin and blood glucose concentrations. Population study in 1,013 eastern Finnish men. Diabetes Care. 1997;20:426–8. doi: 10.2337/diacare.20.3.426. [DOI] [PubMed] [Google Scholar]
- Salonen JT, Tuomainen TP, Nyyssonen K, Lakka HM, Punnonen K. Relation between iron stores and non-insulin dependent diabetes in men: case-control study. BMJ. 1998;317:727. doi: 10.1136/bmj.317.7160.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Real JM, López-Bermejo A, Ricart W. Cross-talk between iron metabolism and diabetes. Diabetes. 2002;51:2348–54. doi: 10.2337/diabetes.51.8.2348. [DOI] [PubMed] [Google Scholar]
- Fernández-Real JM, Ricart W. Insulin resistance and chronic cardiovascular inflammatory syndrome. Endocr Rev. 2003;24:278–301. doi: 10.1210/er.2002-0010. [DOI] [PubMed] [Google Scholar]
- Wilson JG, Lindquist JH, Grambow SC, Crook ED, Maher JF. Potential role of increased iron stores in diabetes. Am J Med Sci. 2003;325:332–9. doi: 10.1097/00000441-200306000-00004. [DOI] [PubMed] [Google Scholar]
- Lee DH, Folsom AR, Jacobs DR., Jr Dietary iron intake and Type 2 diabetes incidence in postmenopausal women: the Iowa Women's Health Study. Diabetologia. 2004;47:185–94. doi: 10.1007/s00125-003-1307-1. [DOI] [PubMed] [Google Scholar]
- Kazi TG, Afridi HI, Kazi N, Jamali MK, Arain MB, Jalbani N, Kandhro GA. Copper, chromium, manganese, iron, nickel, and zinc levels in biological samples of diabetes mellitus patients. Biol Trace Elem Res. 2008;122:1–18. doi: 10.1007/s12011-007-8062-y. [DOI] [PubMed] [Google Scholar]
- Rajpathak SN, Crandall JP, Wylie-Rosett J, Kabat GC, Rohan TE, Hu FB. The role of iron in type 2 diabetes in humans. Biochim Biophys Acta. 2008 doi: 10.1016/j.bbagen.2008.04.005. [DOI] [PubMed] [Google Scholar]
- Adams PC, Kertesz AE, Valberg LS. Clinical presentation of hemochromatosis: a changing scene. Am J Med. 1991;90:445–9. [PubMed] [Google Scholar]
- Kaye TB, Guay AT, Simonson DC. Non-insulin-dependent diabetes mellitus and elevated serum ferritin level. J Diabetes Complic. 1993;7:246–249. doi: 10.1016/0891-6632(93)90008-5. [DOI] [PubMed] [Google Scholar]
- Fernández-Real JM, Ricart-Engel W, Arroyo E, Balanca R, Casamitjana-Abella R, Cabrero D, Fernandez-Castaner M, Soler J. Serum ferritin as a component of the insulin resistance syndrome. Diabetes Care. 1998;21:62–68. doi: 10.2337/diacare.21.1.62. [DOI] [PubMed] [Google Scholar]
- Ford ES, Cogswell ME. Diabetes and serum ferritin concentration among U.S. adults. Diabetes Care. 1999;22:1978–83. doi: 10.2337/diacare.22.12.1978. [DOI] [PubMed] [Google Scholar]
- Jiang R, Manson JE, Meigs JB, Ma J, Rifai N, Hu FB. Body iron stores in relation to risk of type 2 diabetes in apparently healthy women. JAMA. 2004;291:711–7. doi: 10.1001/jama.291.6.711. [DOI] [PubMed] [Google Scholar]
- Mojiminiyi OA, Marouf R, Abdella NA. Body iron stores in relation to the metabolic syndrome, glycemic control and complications in female patients with type 2 diabetes. Nutr Metab Cardiovasc Dis. 2008;18:559–66. doi: 10.1016/j.numecd.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Jehn ML, Guallar E, Clark JM, Couper D, Duncan BB, Ballantyne CM, Hoogeveen RC, Harris ZL, Pankow JS. A prospective study of plasma ferritin level and incident diabetes: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Epidemiol. 2007;165:1047–54. doi: 10.1093/aje/kwk093. [DOI] [PubMed] [Google Scholar]
- Fernández-Real JM, Moreno JM, Chico B, Lopez-Bermejo A, Ricart W. Circulating visfatin is associated with parameters of iron metabolism in subjects with altered glucose tolerance. Diabetes Care. 2007;30:616–21. doi: 10.2337/dc06-1581. [DOI] [PubMed] [Google Scholar]
- Wang Y, Lam KSL, Kraegen EW, Sweeney G, Zhang JL, Tso AWK, Chow WS, Wat NMS, Xu JY, Hoo RLC, Xu AM. Lipocalin-2 is an inflammatory marker closely associated with obesity, insulin resistance, and hyperglycemia in humans. Clinical Chemistry. 2007;53:34–41. doi: 10.1373/clinchem.2006.075614. [DOI] [PubMed] [Google Scholar]
- van Dam RM, Hu FB. Lipocalins and insulin resistance: Etiological role of retinol-binding protein 4 and lipocalin-2? Clinical Chemistry. 2007;53:5–7. doi: 10.1373/clinchem.2006.080432. [DOI] [PubMed] [Google Scholar]
- Hua NW, Stoohs RA, Facchini FS. Low iron status and enhanced insulin sensitivity in lacto-ovo vegetarians. Br J Nutr. 2001;86:515–9. doi: 10.1079/bjn2001421. [DOI] [PubMed] [Google Scholar]
- Liang Q, Carlson EC, Donthi RV, Kralik PM, Shen X, Epstein PN. Overexpression of metallothionein reduces diabetic cardiomyopathy. Diabetes. 2002;51:174–81. doi: 10.2337/diabetes.51.1.174. [DOI] [PubMed] [Google Scholar]
- Ye G, Metreveli NS, Ren J, Epstein PN. Metallothionein prevents diabetes-induced deficits in cardiomyocytes by inhibiting reactive oxygen species production. Diabetes. 2003;52:777–83. doi: 10.2337/diabetes.52.3.777. [DOI] [PubMed] [Google Scholar]
- Cai L, Wang J, Li Y, Sun X, Wang L, Zhou Z, Kang YJ. Inhibition of superoxide generation and associated nitrosative damage is involved in metallothionein prevention of diabetic cardiomyopathy. Diabetes. 2005;54:1829–37. doi: 10.2337/diabetes.54.6.1829. [DOI] [PubMed] [Google Scholar]
- Islam MS, Loots du T. Diabetes, metallothionein, and zinc interactions: a review. Biofactors. 2007;29:203–12. doi: 10.1002/biof.5520290404. [DOI] [PubMed] [Google Scholar]
- Li X, Cai L, Feng W. Diabetes and metallothionein. Mini Rev Med Chem. 2007;7:761–8. doi: 10.2174/138955707781024490. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Li XK, Wang Y, Cai L. The role of zinc, copper and iron in the pathogenesis of diabetes and diabetic complications: therapeutic effects by chelators. Hemoglobin. 2008;32:135–45. doi: 10.1080/03630260701727077. [DOI] [PubMed] [Google Scholar]
- Lee DH, Liu DY, Jacobs DR, Jr, Shin HR, Song K, Lee IK, Kim B, Hider RC. Common presence of non-transferrin-bound iron among patients with type 2 diabetes. Diabetes Care. 2006;29:1090–5. doi: 10.2337/diacare.2951090. [DOI] [PubMed] [Google Scholar]
- Chittum HS, Champney WS. Erythromycin inhibits the assembly of the large ribosomal subunit in growing Escherichia coli cells. Curr Microbiol. 1995;30:273–9. doi: 10.1007/BF00295501. [DOI] [PubMed] [Google Scholar]
- Facchini FS, Hua NW, Stoohs RA. Effect of iron depletion in carbohydrate-intolerant patients with clinical evidence of nonalcoholic fatty liver disease. Gastroenterology. 2002;122:931–9. doi: 10.1053/gast.2002.32403. [DOI] [PubMed] [Google Scholar]
- Machado M, Cortez-Pinto H. Nash, insulin resistance and iron. Liver International. 2006;26:1159–1162. doi: 10.1111/j.1478-3231.2006.01394.x. [DOI] [PubMed] [Google Scholar]
- Allerson CR, Cazzola M, Rouault TA. Clinical severity and thermodynamic effects of iron-responsive element mutations in hereditary hyperferritinemia-cataract syndrome. Journal of Biological Chemistry. 1999;274:26439–26447. doi: 10.1074/jbc.274.37.26439. [DOI] [PubMed] [Google Scholar]
- Beaumont C, Leneuve P, Devaux I, Scoazec JY, Berthier M, Loiseau MN, Grandchamp B, Bonneau D. Mutation in the Iron-Responsive Element of the L-Ferritin Messenger-Rna in a Family with Dominant Hyperferritinemia and Cataract. Nature Genetics. 1995;11:444–446. doi: 10.1038/ng1295-444. [DOI] [PubMed] [Google Scholar]
- Dandona P, Aljada A, Chaudhuri A, Mohanty P, Garg R. Metabolic syndrome – A comprehensive perspective based on interactions between obesity, diabetes, and inflammation. Circulation. 2005;111:1448–1454. doi: 10.1161/01.CIR.0000158483.13093.9D. [DOI] [PubMed] [Google Scholar]
- Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–28. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- Roberts CK, Barnard RJ. Effects of exercise and diet on chronic disease. J Appl Physiol. 2005;98:3–30. doi: 10.1152/japplphysiol.00852.2004. [DOI] [PubMed] [Google Scholar]
- Nicolson GL. Metabolic syndrome and mitochondrial function: Molecular replacement and antioxidant supplements to prevent membrane peroxidation and restore mitochondrial function. Journal of Cellular Biochemistry. 2007;100:1352–1369. doi: 10.1002/jcb.21247. [DOI] [PubMed] [Google Scholar]
- Yudkin JS. Insulin resistance and the metabolic syndrome–or the pitfalls of epidemiology. Diabetologia. 2007;50:1576–86. doi: 10.1007/s00125-007-0711-3. [DOI] [PubMed] [Google Scholar]
- Jehn M, Clark JM, Guallar E. Serum ferritin and risk of the metabolic syndrome in U.S. adults. Diabetes Care. 2004;27:2422–8. doi: 10.2337/diacare.27.10.2422. [DOI] [PubMed] [Google Scholar]
- Wrede CE, Buettner R, Bollheimer LC, Scholmerich J, Palitzsch KD, Hellerbrand C. Association between serum ferritin and the insulin resistance syndrome in a representative population. Eur J Endocrinol. 2006;154:333–340. doi: 10.1530/eje.1.02083. [DOI] [PubMed] [Google Scholar]
- Bozzini C, Girelli D, Olivieri O, Martinelli N, Bassi A, De Matteis G, Tenuti I, Lotto V, Friso S, Pizzolo F, Corrocher R. Prevalence of body iron excess in the metabolic syndrome. Diabetes Care. 2005;28:2061–3. doi: 10.2337/diacare.28.8.2061. [DOI] [PubMed] [Google Scholar]
- Choi KM, Lee KW, Kim HY, Seo JA, Kim SG, Kim NH, Choi DS, Baik SH. Association among serum ferritin, alanine aminotransferase levels, and metabolic syndrome in Korean postmenopausal women. Metabolism. 2005;54:1510–4. doi: 10.1016/j.metabol.2005.05.018. [DOI] [PubMed] [Google Scholar]
- Kurella M, Lo JC, Chertow GM. Metabolic syndrome and the risk for chronic kidney disease among nondiabetic adults. J Am Soc Nephrol. 2005;16:2134–2140. doi: 10.1681/ASN.2005010106. [DOI] [PubMed] [Google Scholar]
- den Boer M, Voshol PJ, Kuipers F, Havekes LM, Romijn JA. Hepatic steatosis: a mediator of the metabolic syndrome. Lessons from animal models. Arterioscler Thromb Vasc Biol. 2004;24:644–9. doi: 10.1161/01.ATV.0000116217.57583.6e. [DOI] [PubMed] [Google Scholar]
- Mittra S, Bansal VS, Bhatnagar PK. From a glucocentric to a lipocentric approach towards metabolic syndrome. Drug Discov Today. 2008;13:211–8. doi: 10.1016/j.drudis.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Roberts CK, Barnard RJ, Sindhu RK, Jurczak M, Ehdaie A, Vaziri ND. Oxidative stress and dysregulation of NAD(P)H oxidase and antioxidant enzymes in diet-induced metabolic syndrome. Metabolism. 2006;55:928–34. doi: 10.1016/j.metabol.2006.02.022. [DOI] [PubMed] [Google Scholar]
- Booth FW, Gordon SE, Carlson CJ, Hamilton MT. Waging war on modern chronic diseases: primary prevention through exercise biology. J Appl Physiol. 2000;88:774–87. doi: 10.1152/jappl.2000.88.2.774. [DOI] [PubMed] [Google Scholar]
- Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–53. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840–6. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- Walker CG, Zariwala MG, Holness MJ, Sugden MC. Diet, obesity and diabetes: a current update. Clin Sci (Lond) 2007;112:93–111. doi: 10.1042/CS20060150. [DOI] [PubMed] [Google Scholar]
- Lecube A, Hernandez C, Pelegri D, Simo R. Factors accounting for high ferritin levels in obesity. Int J Obes (Lond) 2008 doi: 10.1038/ijo.2008.154. [DOI] [PubMed] [Google Scholar]
- Fricker J, Lemoel G, Apfelbaum M. Obesity and iron status in menstruating women. Am J Clin Nutrition. 1990;52:863–866. doi: 10.1093/ajcn/52.5.863. [DOI] [PubMed] [Google Scholar]
- Farahani P, Chiu S, Bowlus CL, Boffelli D, Lee E, Fisler JS, Krauss RM, Warden CH. Obesity in BSB mice is correlated with expression of genes for iron homeostasis and leptin. Obesity Research. 2004;12:191–204. doi: 10.1038/oby.2004.26. [DOI] [PubMed] [Google Scholar]
- Das UN. Is obesity an inflammatory condition? Nutrition. 2001;17:953–66. doi: 10.1016/s0899-9007(01)00672-4. [DOI] [PubMed] [Google Scholar]
- Hall JE, Crook ED, Jones DW, Wofford MR, Dubbert PM. Mechanisms of obesity-associated cardiovascular and renal disease. Am J Med Sci. 2002;324:127–37. doi: 10.1097/00000441-200209000-00003. [DOI] [PubMed] [Google Scholar]
- Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr. 2004;92:347–55. doi: 10.1079/bjn20041213. [DOI] [PubMed] [Google Scholar]
- Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ Res. 2005;96:939–49. doi: 10.1161/01.RES.0000163635.62927.34. [DOI] [PubMed] [Google Scholar]
- Hukshorn CJ, Lindeman JH, Toet KH, Saris WH, Eilers PH, Westerterp-Plantenga MS, Kooistra T. Leptin and the proinflammatory state associated with human obesity. J Clin Endocrinol Metab. 2004;89:1773–8. doi: 10.1210/jc.2003-030803. [DOI] [PubMed] [Google Scholar]
- Toni R, Malaguti A, Castorina S, Roti E, Lechan RM. New paradigms in neuroendocrinology: relationships between obesity, systemic inflammation and the neuroendocrine system. J Endocrinol Invest. 2004;27:182–6. doi: 10.1007/BF03346266. [DOI] [PubMed] [Google Scholar]
- Koh KK, Han SH, Quon MJ. Inflammatory markers and the metabolic syndrome: insights from therapeutic interventions. J Am Coll Cardiol. 2005;46:1978–85. doi: 10.1016/j.jacc.2005.06.082. [DOI] [PubMed] [Google Scholar]
- Bastard JP, Maachi M, Lagathu C, Kim MJ, Caron M, Vidal H, Capeau J, Feve B. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw. 2006;17:4–12. [PubMed] [Google Scholar]
- Kapiotis S, Holzer G, Schaller G, Haumer M, Widhalm H, Weghuber D, Jilma B, Roggla G, Wolzt M, Widhalm K, Wagner OF. A proinflammatory state is detectable in obese children and is accompanied by functional and morphological vascular changes. Arterioscler Thromb Vasc Biol. 2006;26:2541–6. doi: 10.1161/01.ATV.0000245795.08139.70. [DOI] [PubMed] [Google Scholar]
- Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006;6:772–83. doi: 10.1038/nri1937. [DOI] [PubMed] [Google Scholar]
- Vincent HK, Taylor AG. Biomarkers and potential mechanisms of obesity-induced oxidant stress in humans. Int J Obes (Lond) 2006;30:400–18. doi: 10.1038/sj.ijo.0803177. [DOI] [PubMed] [Google Scholar]
- Wang B, Trayhurn P. Acute and prolonged effects of TNF-alpha on the expression and secretion of inflammation-related adipokines by human adipocytes differentiated in culture. Pflugers Arch. 2006;452:418–27. doi: 10.1007/s00424-006-0055-8. [DOI] [PubMed] [Google Scholar]
- Chandalia M, Abate N. Metabolic complications of obesity: inflated or inflamed? J Diabetes Complications. 2007;21:128–36. doi: 10.1016/j.jdiacomp.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–84. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Zhu J, Lum PY, Yang X, Pinto S, Macneil DJ, Zhang C, Lamb J, Edwards S, Sieberts SK, Leonardson A, Castellini LW, Wang S, Champy MF, Zhang B, Emilsson V, Doss S, Ghazalpour A, Horvath S, Drake TA, Lusis AJ, Schadt EE. Variations in DNA elucidate molecular networks that cause disease. Nature. 2008;452:429–435. doi: 10.1038/nature06757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung J, Nguyen AK, Henstridge DC, Holmes AG, Chan MH, Mesa JL, Lancaster GI, Southgate RJ, Bruce CR, Duffy SJ, Horvath I, Mestril R, Watt MJ, Hooper PL, Kingwell BA, Vigh L, Hevener A, Febbraio MA. HSP72 protects against obesity-induced insulin resistance. Proc Natl Acad Sci USA. 2008;105:1739–44. doi: 10.1073/pnas.0705799105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emilsson V, Thorleifsson G, Zhang B, Leonardson AS, Zink F, Zhu J, Carlson S, Helgason A, Walters GB, Gunnarsdottir S, Mouy M, Steinthorsdottir V, Eiriksdottir GH, Bjornsdottir G, Reynisdottir I, Gudbjartsson D, Helgadottir A, Jonasdottir A, Jonasdottir A, Styrkarsdottir U, Gretarsdottir S, Magnusson KP, Stefansson H, Fossdal R, Kristjansson K, Gislason HG, Stefansson T, Leifsson BG, Thorsteinsdottir U, Lamb JR, Gulcher JR, Reitman ML, Kong A, Schadt EE, Stefansson K. Genetics of gene expression and its effect on disease. Nature. 2008. [DOI] [PubMed]
- Wärnberg J, Marcos A. Low-grade inflammation and the metabolic syndrome in children and adolescents. Curr Opin Lipidol. 2008;19:11–5. doi: 10.1097/MOL.0b013e3282f4096b. [DOI] [PubMed] [Google Scholar]
- Rajala MW, Scherer PE. Minireview: The adipocyte–at the crossroads of energy homeostasis, inflammation, and atherosclerosis. Endocrinology. 2003;144:3765–73. doi: 10.1210/en.2003-0580. [DOI] [PubMed] [Google Scholar]
- Vendrell J, Broch M, Vilarrasa N, Molina A, Gomez JM, Gutierrez C, Simon I, Soler J, Richart C. Resistin, adiponectin, ghrelin, leptin, and proinflammatory cytokines: relationships in obesity. Obes Res. 2004;12:962–71. doi: 10.1038/oby.2004.118. [DOI] [PubMed] [Google Scholar]
- Arner P. Insulin resistance in type 2 diabetes – role of the adipokines. Curr Mol Med. 2005;5:333–9. doi: 10.2174/1566524053766022. [DOI] [PubMed] [Google Scholar]
- Hutley L, Prins JB. Fat as an endocrine organ: relationship to the metabolic syndrome. Am J Med Sci. 2005;330:280–9. doi: 10.1097/00000441-200512000-00005. [DOI] [PubMed] [Google Scholar]
- Kougias P, Chai H, Lin PH, Yao Q, Lumsden AB, Chen C. Effects of adipocyte-derived cytokines on endothelial functions: implication of vascular disease. J Surg Res. 2005;126:121–9. doi: 10.1016/j.jss.2004.12.023. [DOI] [PubMed] [Google Scholar]
- Lau DC, Dhillon B, Yan H, Szmitko PE, Verma S. Adipokines: molecular links between obesity and atheroslcerosis. Am J Physiol Heart Circ Physiol. 2005;288:H2031–41. doi: 10.1152/ajpheart.01058.2004. [DOI] [PubMed] [Google Scholar]
- Rosen ED, Spiegelman BM. Adipocytes as regulators of energy balance and glucose homeostasis. Nature. 2006;444:847–53. doi: 10.1038/nature05483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim F, Pham M, Luttrell I, Bannerman DD, Tupper J, Thaler J, Hawn TR, Raines EW, Schwartz MW. Toll-like receptor-4 mediates vascular inflammation and insulin resistance in diet-induced obesity. Circ Res. 2007;100:1589–96. doi: 10.1161/CIRCRESAHA.106.142851. [DOI] [PubMed] [Google Scholar]
- Shah PK. Innate immune pathway links obesity to insulin resistance. Circ Res. 2007;100:1531–3. doi: 10.1161/CIRCRESAHA.107.101104. [DOI] [PubMed] [Google Scholar]
- Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752–61. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keaney JF, Larson MG, Jr, Vasan RS, Wilson PW, Lipinska I, Corey D, Massaro JM, Sutherland P, Vita JA, Benjamin EJ. Obesity and systemic oxidative stress: clinical correlates of oxidative stress in the Framingham Study. Arterioscler Thromb Vasc Biol. 2003;23:434–9. doi: 10.1161/01.ATV.0000058402.34138.11. [DOI] [PubMed] [Google Scholar]
- Higdon JV, Frei B. Obesity and oxidative stress: a direct link to CVD? Arterioscler Thromb Vasc Biol. 2003;23:365–7. doi: 10.1161/01.ATV.0000063608.43095.E2. [DOI] [PubMed] [Google Scholar]
- Whitworth JA. 2003 World Health Organization (WHO)/International Society of Hypertension (ISH) statement on management of hypertension. J Hypertens. 2003;21:1983–92. doi: 10.1097/00004872-200311000-00002. [DOI] [PubMed] [Google Scholar]
- Tuder RM, Voelkel NF. Pulmonary hypertension and inflammation. J Lab Clin Med. 1998;132:16–24. doi: 10.1016/s0022-2143(98)90020-8. [DOI] [PubMed] [Google Scholar]
- Intengan HD, Schiffrin EL. Vascular remodeling in hypertension: roles of apoptosis, inflammation, and fibrosis. Hypertension. 2001;38:581–7. doi: 10.1161/hy09t1.096249. [DOI] [PubMed] [Google Scholar]
- Grundy SM. Inflammation, hypertension, and the metabolic syndrome. JAMA. 2003;290:3000–2. doi: 10.1001/jama.290.22.3000. [DOI] [PubMed] [Google Scholar]
- Virdis A, Schiffrin EL. Vascular inflammation: a role in vascular disease in hypertension? Curr Opin Nephrol Hypertens. 2003;12:181–7. doi: 10.1097/00041552-200303000-00009. [DOI] [PubMed] [Google Scholar]
- Boos CJ, Lip GY. Elevated high-sensitive C-reactive protein, large arterial stiffness and atherosclerosis: a relationship between inflammation and hypertension? J Hum Hypertens. 2005;19:511–3. doi: 10.1038/sj.jhh.1001858. [DOI] [PubMed] [Google Scholar]
- Varughese GI, Lip GY. Hypertension in patients with type-II diabetes: relation to urinary albumin excretion, endothelial function and inflammation. J Hum Hypertens. 2005;19:421–4. doi: 10.1038/sj.jhh.1001833. [DOI] [PubMed] [Google Scholar]
- Li JJ. Inflammation in hypertension: primary evidence. Chin Med J (Engl) 2006;119:1215–21. [PubMed] [Google Scholar]
- Savoia C, Schiffrin EL. Inflammation in hypertension. Curr Opin Nephrol Hypertens. 2006;15:152–8. doi: 10.1097/01.mnh.0000203189.57513.76. [DOI] [PubMed] [Google Scholar]
- Savoia C, Schiffrin EL. Vascular inflammation in hypertension and diabetes: molecular mechanisms and therapeutic interventions. Clin Sci (Lond) 2007;112:375–84. doi: 10.1042/CS20060247. [DOI] [PubMed] [Google Scholar]
- Kannel WB, Brand N, Skinner JJ, Jr, Dawber TR, McNamara PM. The relation of adiposity to blood pressure and development of hypertension. The Framingham study. Ann Intern Med. 1967;67:48–59. doi: 10.7326/0003-4819-67-1-48. [DOI] [PubMed] [Google Scholar]
- Mark AL, Correia M, Morgan DA, Shaffer RA, Haynes WG. State-of-the-art-lecture: Obesity-induced hypertension: new concepts from the emerging biology of obesity. Hypertension. 1999;33:537–41. doi: 10.1161/01.hyp.33.1.537. [DOI] [PubMed] [Google Scholar]
- Aizawa-Abe M, Ogawa Y, Masuzaki H, Ebihara K, Satoh N, Iwai H, Matsuoka N, Hayashi T, Hosoda K, Inoue G, Yoshimasa Y, Nakao K. Pathophysiological role of leptin in obesity-related hypertension. J Clin Invest. 2000;105:1243–52. doi: 10.1172/JCI8341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JE. The kidney, hypertension, and obesity. Hypertension. 2003;41:625–33. doi: 10.1161/01.HYP.0000052314.95497.78. [DOI] [PubMed] [Google Scholar]
- Yanai H, Tomono Y, Ito K, Furutani N, Yoshida H, Tada N. The underlying mechanisms for development of hypertension in the metabolic syndrome. Nutr J. 2008;7:10. doi: 10.1186/1475-2891-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaziri ND, Rodriguez-Iturbe B. Mechanisms of disease: oxidative stress and inflammation in the pathogenesis of hypertension. Nat Clin Pract Nephrol. 2006;2:582–93. doi: 10.1038/ncpneph0283. [DOI] [PubMed] [Google Scholar]
- Yakobson MG, Antonov AR, Golovatyuk AV, Efremov AV, Markel AL, Yakobson GS. Iron content and parameters of blood antioxidant activity in rats with hereditary arterial hypertension during experimental myocardial infarction. Bull Exp Biol Med. 2001;132:1041–4. doi: 10.1023/a:1017900121039. [DOI] [PubMed] [Google Scholar]
- Peterson JR, Sharma RV, Davisson RL. Reactive oxygen species in the neuropathogenesis of hypertension. Curr Hypertens Rep. 2006;8:232–41. doi: 10.1007/s11906-006-0056-1. [DOI] [PubMed] [Google Scholar]
- Sullivan JL. Iron and the sex difference in heart-disease risk. Lancet. 1981;1:1293–1294. doi: 10.1016/s0140-6736(81)92463-6. [DOI] [PubMed] [Google Scholar]
- Sullivan JL. Misconceptions in the debate on the iron hypothesis. Journal of Nutritional Biochemistry. 2001;12:33–37. doi: 10.1016/s0955-2863(00)00142-x. [DOI] [PubMed] [Google Scholar]
- Sullivan JL. Stored iron and vascular reactivity. Arteriosclerosis Thrombosis and Vascular Biology. 2005;25:1532–1535. doi: 10.1161/01.ATV.0000174124.20147.22. [DOI] [PubMed] [Google Scholar]
- Sullivan JL. The big idea: the coxib crisis iron, aspirin and heart disease risk revisited. J R Soc Med. 2007;100:346–9. doi: 10.1258/jrsm.100.7.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers DG. The iron hypothesis: does iron play a role in atherosclerosis? Transfusion. 2000;40:1023–1029. doi: 10.1046/j.1537-2995.2000.40081023.x. [DOI] [PubMed] [Google Scholar]
- Yuan XM, Li W. The iron hypothesis of atherosclerosis and its clinical impact. Ann Med. 2003;35:578–91. doi: 10.1080/07853890310016342. [DOI] [PubMed] [Google Scholar]
- Zheng H, Dimayuga C, Hudaihed A, Katz SD. Effect of dexrazoxane on homocysteine-induced endothelial dysfunction in normal subjects. Arterioscler Thromb Vasc Biol. 2002;22:E15–8. doi: 10.1161/01.atv.0000023187.25914.5b. [DOI] [PubMed] [Google Scholar]
- Zheng H, Huang X, Zhang Q, Katz SD. Iron sucrose augments homocysteine-induced endothelial dysfunction in normal subjects. Kidney Int. 2006;69:679–84. doi: 10.1038/sj.ki.5000046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan JL. Is homocysteine an iron-dependent cardiovascular risk factor? Kidney Int. 2006;69:642–4. doi: 10.1038/sj.ki.5000229. [DOI] [PubMed] [Google Scholar]
- Clarke R. Homocysteine-lowering trials for prevention of heart disease and stroke. Semin Vasc Med. 2005;5:215–22. doi: 10.1055/s-2005-872407. [DOI] [PubMed] [Google Scholar]
- Tuomainen TP, Punnonen K, Nyyssonen K, Salonen JT. Association between body iron stores and the risk of acute myocardial infarction in men. Circulation. 1998;97:1461–6. doi: 10.1161/01.cir.97.15.1461. [DOI] [PubMed] [Google Scholar]
- Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, Parfrey P, Pfeffer M, Raij L, Spinosa DJ, Wilson PW. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation. 2003;108:2154–69. doi: 10.1161/01.CIR.0000095676.90936.80. [DOI] [PubMed] [Google Scholar]
- Oberg BP, McMenamin E, Lucas FL, McMonagle E, Morrow J, Ikizler TA, Himmelfarb J. Increased prevalence of oxidant stress and inflammation in patients with moderate to severe chronic kidney disease. Kidney Int. 2004;65:1009–16. doi: 10.1111/j.1523-1755.2004.00465.x. [DOI] [PubMed] [Google Scholar]
- Vaziri ND. Roles of oxidative stress and antioxidant therapy in chronic kidney disease and hypertension. Curr Opin Nephrol Hypertens. 2004;13:93–9. doi: 10.1097/00041552-200401000-00013. [DOI] [PubMed] [Google Scholar]
- Abramson JL, Jurkovitz CT, Vaccarino V, Weintraub WS, McClellan W. Chronic kidney disease, anemia, and incident stroke in a middle-aged, community-based population: the ARIC Study. Kidney Int. 2003;64:610–5. doi: 10.1046/j.1523-1755.2003.00109.x. [DOI] [PubMed] [Google Scholar]
- Smith JK, Carden DL, Grisham MB, Granger DN, Korthuis RJ. Role of iron in postischemic microvascular injury. Am J Physiol. 1989;256:H1472–7. doi: 10.1152/ajpheart.1989.256.5.H1472. [DOI] [PubMed] [Google Scholar]
- Kukreja RC, Hess ML. The oxygen free radical system: from equations through membrane protein interactions to cardiovascular injury and protection. Cardiovasc Res. 1992;26:641–655. doi: 10.1093/cvr/26.7.641. [DOI] [PubMed] [Google Scholar]
- Lesnefsky E, Ye J. Exogenous intracellular, but not extracellular, iron augments myocardial reperfusion injury. Amer J Physiol. 1994;266:H384–H392. doi: 10.1152/ajpheart.1994.266.2.H384. [DOI] [PubMed] [Google Scholar]
- Berenshtein E, Mayer B, Goldberg C, Kitrossky N, Chevion M. Patterns of mobilization of copper and iron following myocardial ischemia: Possible predictive criteria for tissue injury. J Mol Cell Cardiol. 1997;29:3025–3034. doi: 10.1006/jmcc.1997.0535. [DOI] [PubMed] [Google Scholar]
- Horwitz LD, Sherman NA, Kong YN, Pike AW, Gobin J, Fennessey PV, Horwitz MA. Lipophilic siderophores Mycobacterium tuberculosis prevent cardiac reperfusion injury. Proc Natl Acad Sci. 1998;95:5263–5268. doi: 10.1073/pnas.95.9.5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz LD, Rosenthal EA. Iron-mediated cardiovascular injury. Vasc Med. 1999;4:93–9. doi: 10.1177/1358836X9900400207. [DOI] [PubMed] [Google Scholar]
- Pucheu S, Coudray C, Tresallet N, Favier A, Deleiris J. Effect of iron overload in the isolated ischemic and reperfused rat heart. Cardiovascular Drugs and Therapy. 1993;7:701–711. doi: 10.1007/BF00877824. [DOI] [PubMed] [Google Scholar]
- Amersi F, Dulkanchainun T, Nelson SK, Farmer DG, Kato H, Zaky J, Melinek J, Shaw GD, Kupiec-Weglinski JW, Horwitz LD, Horwitz MA, Busuttil RW. Novel iron chelator in combination with a P-selectin antagonist prevents ischemia/reperfusion injury in a rat liver model. Transplantation. 2001;71:112–118. doi: 10.1097/00007890-200101150-00018. [DOI] [PubMed] [Google Scholar]
- Mamtani M, Kulkarni H. Influence of iron chelators on myocardial iron and cardiac function in transfusion-dependent thalassaemia: a systematic review and meta-analysis. Br J Haematol. 2008;141:882–90. doi: 10.1111/j.1365-2141.2008.07122.x. [DOI] [PubMed] [Google Scholar]
- Ravati A, Ahlemeyer B, Becker A, Klumpp S, Krieglstein J. Preconditioning-induced neuroprotection is mediated by reactive oxygen species and activation of the transcription factor nuclear factor-kappa B. Journal of Neurochemistry. 2001;78:909–919. doi: 10.1046/j.1471-4159.2001.00463.x. [DOI] [PubMed] [Google Scholar]
- Berenshtein E, Vaisman B, Goldberg-Langerman C, Kitrossky N, Konijn AM, Chevion M. Roles of ferritin and iron in ischemic preconditioning of the heart. Mol Cell Biochem. 2002;234:283–292. [PubMed] [Google Scholar]
- Calvillo L, Latini R, Kajstura J, Leri A, Anversa P, Ghezzi P, Salio M, Cerami A, Brines M. Recombinant human erythropoietin protects the myocardium from ischemia-reperfusion injury and promotes beneficial remodeling. ProcNatl Acad Sci. 2003;100:4802–4806. doi: 10.1073/pnas.0630444100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsa CJ, Matsumoto A, Kim J, Riel RU, Pascal LS, Walton GB, Thompson RB, Petrofski JA, Annex BH, Stamler JS, Koch WJ. A novel protective effect of erythropoietin in the infarcted heart. J Clin Invest. 2003;112:999–1007. doi: 10.1172/JCI18200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engberding N, Spiekermann S, Schaefer A, Heineke A, Wiencke A, Muller M, Fuchs M, Hilfiker-Kleiner D, Hornig B, Drexler H, Landmesser U. Allopurinol attenuates left ventricular remodeling and dysfunction after experimental myocardial infarction: a new action for an old drug? Circulation. 2004;110:2175–9. doi: 10.1161/01.CIR.0000144303.24894.1C. [DOI] [PubMed] [Google Scholar]
- Reyes AJ. Cardiovascular drugs and serum uric acid. Cardiovasc Drugs Ther. 2003;17:397–414. doi: 10.1023/b:card.0000015855.02485.e3. [DOI] [PubMed] [Google Scholar]
- Dunn WB, Broadhurst DI, Sasalu D, Buch M, McDowell G, Spasic I, Ellis DI, Brooks N, Kell DB, Neyses L. Serum metabolomics reveals many novel metabolic markers of heart failure, including pseudouridine and 2-oxoglutarate. Metabolomics. 2007;3:413–426. [Google Scholar]
- Hokamaki J, Kawano H, Yoshimura M, Soejima H, Miyamoto S, Kajiwara I, Kojima S, Sakamoto T, Sugiyama S, Hirai N, Shimomura H, Nagayoshi Y, Tsujita K, Shioji I, Sasaki S, Ogawa H. Urinary biopyrrins levels are elevated in relation to severity of heart failure. J Am Coll Cardiol. 2004;43:1880–5. doi: 10.1016/j.jacc.2004.01.028. [DOI] [PubMed] [Google Scholar]
- Mozaffarian D, Nye R, Levy WC. Anemia predicts mortality in severe heart failure: the prospective randomized amlodipine survival evaluation (PRAISE) J Am Coll Cardiol. 2003;41:1933–9. doi: 10.1016/s0735-1097(03)00425-x. [DOI] [PubMed] [Google Scholar]
- Felker GM, Adams KF, Jr, Gattis WA, O'Connor CM. Anemia as a risk factor and therapeutic target in heart failure. J Am Coll Cardiol. 2004;44:959–66. doi: 10.1016/j.jacc.2004.05.070. [DOI] [PubMed] [Google Scholar]
- Bolger AP, Bartlett FR, Penston HS, O'Leary J, Pollock N, Kaprielian R, Chapman CM. Intravenous iron alone for the treatment of anemia in patients with chronic heart failure. J Am Coll Cardiol. 2006;48:1225–7. doi: 10.1016/j.jacc.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med. 2005;352:1011–23. doi: 10.1056/NEJMra041809. [DOI] [PubMed] [Google Scholar]
- Kunsch C, Medford RM. Oxidative stress as a regulator of gene expression in the vasculature. Circ Res. 1999;85:753–66. doi: 10.1161/01.res.85.8.753. [DOI] [PubMed] [Google Scholar]
- Ross R. Atherosclerosis: an inflammatory disease. N Engl J Med. 1999;340:115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- Blake GJ, Ridker PM. Novel clinical markers of vascular wall inflammation. Circ Res. 2001;89:763–71. doi: 10.1161/hh2101.099270. [DOI] [PubMed] [Google Scholar]
- Hansson GK. Immune mechanisms in atherosclerosis. Arterioscler Thromb Vasc Biol. 2001;21:1876–90. doi: 10.1161/hq1201.100220. [DOI] [PubMed] [Google Scholar]
- Forrester JS. Prevention of plaque rupture: a new paradigm of therapy. Ann Intern Med. 2002;137:823–33. doi: 10.7326/0003-4819-137-10-200211190-00012. [DOI] [PubMed] [Google Scholar]
- Himmelfarb J, Stenvinkel P, Ikizler TA, Hakim RM. The elephant in uremia: oxidant stress as a unifying concept of cardiovascular disease in uremia. Kidney Int. 2002;62:1524–38. doi: 10.1046/j.1523-1755.2002.00600.x. [DOI] [PubMed] [Google Scholar]
- Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–74. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–43. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- Young JL, Libby P, Schonbeck U. Cytokines in the pathogenesis of atherosclerosis. Thromb Haemost. 2002;88:554–67. [PubMed] [Google Scholar]
- Binder CJ, Chang MK, Shaw PX, Miller YI, Hartvigsen K, Dewan A, Witztum JL. Innate and acquired immunity in atherogenesis. Nat Med. 2002;8:1218–26. doi: 10.1038/nm1102-1218. [DOI] [PubMed] [Google Scholar]
- Altman R. Risk factors in coronary atherosclerosis athero-inflammation: the meeting point. Thromb J. 2003;1:4. doi: 10.1186/1477-9560-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer JH, Allayee H, Dwyer KM, Fan J, Wu H, Mar R, Lusis AJ, Mehrabian M. Arachidonate 5-lipoxygenase promoter genotype, dietary arachidonic acid, and atherosclerosis. N Engl J Med. 2004;350:29–37. doi: 10.1056/NEJMoa025079. [DOI] [PubMed] [Google Scholar]
- Forrester JS. Common ancestors: chronic progressive diseases have the same pathogenesis. Clin Cardiol. 2004;27:186–90. doi: 10.1002/clc.4960270403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti R, Gotto AM, Jr, Hajjar DP. Inflammation in atherosclerosis and implications for therapy. Circulation. 2004;109:III20–6. doi: 10.1161/01.CIR.0000131514.71167.2e. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Brown NJ, Vaughan DE, Harrison DG, Mehta JL. Established and emerging plasma biomarkers in the prediction of first atherothrombotic events. Circulation. 2004;109:6–19. doi: 10.1161/01.CIR.0000133444.17867.56. [DOI] [PubMed] [Google Scholar]
- van Oostrom AJ, van Wijk J, Cabezas MC. Lipaemia, inflammation and atherosclerosis: novel opportunities in the understanding and treatment of atherosclerosis. Drugs. 2004;64:19–41. doi: 10.2165/00003495-200464002-00004. [DOI] [PubMed] [Google Scholar]
- Willerson JT, Ridker PM. Inflammation as a cardiovascular risk factor. Circulation. 2004;109:II2–10. doi: 10.1161/01.CIR.0000129535.04194.38. [DOI] [PubMed] [Google Scholar]
- Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–95. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- Madamanchi NR, Hakim ZS, Runge MS. Oxidative stress in atherogenesis and arterial thrombosis: the disconnect between cellular studies and clinical outcomes. J Thromb Haemost. 2005;3:254–67. doi: 10.1111/j.1538-7836.2004.01085.x. [DOI] [PubMed] [Google Scholar]
- Madamanchi NR, Vendrov A, Runge MS. Oxidative stress and vascular disease. Arterioscler Thromb Vasc Biol. 2005;25:29–38. doi: 10.1161/01.ATV.0000150649.39934.13. [DOI] [PubMed] [Google Scholar]
- Mullenix PS, Andersen CA, Starnes BW. Atherosclerosis as inflammation. Ann Vasc Surg. 2005;19:130–8. doi: 10.1007/s10016-004-0153-z. [DOI] [PubMed] [Google Scholar]
- Nigro J, Osman N, Dart AM, Little PJ. Insulin resistance and atherosclerosis. Endocr Rev. 2006;27:242–59. doi: 10.1210/er.2005-0007. [DOI] [PubMed] [Google Scholar]
- Taqueti VR, Mitchell RN, Lichtman AH. Protecting the pump: controlling myocardial inflammatory responses. Annu Rev Physiol. 2006;68:67–95. doi: 10.1146/annurev.physiol.68.040104.124611. [DOI] [PubMed] [Google Scholar]
- Tedgui A, Mallat Z. Cytokines in atherosclerosis: pathogenic and regulatory pathways. Physiol Rev. 2006;86:515–81. doi: 10.1152/physrev.00024.2005. [DOI] [PubMed] [Google Scholar]
- Grainger DJ. TGF-beta and atherosclerosis in man. Cardiovasc Res. 2007;74:213–22. doi: 10.1016/j.cardiores.2007.02.022. [DOI] [PubMed] [Google Scholar]
- Popa C, Netea MG, van Riel PL, Meer JW van der, Stalenhoef AF. The role of TNF-alpha in chronic inflammatory conditions, intermediary metabolism, and cardiovascular risk. J Lipid Res. 2007;48:751–62. doi: 10.1194/jlr.R600021-JLR200. [DOI] [PubMed] [Google Scholar]
- Schleicher E, Friess U. Oxidative stress, AGE, and atherosclerosis. Kidney Int Suppl. 2007:S17–26. doi: 10.1038/sj.ki.5002382. [DOI] [PubMed] [Google Scholar]
- Kibel A, Belovari T, Drenjančević-Perić I. The role of transferrin in atherosclerosis. Med Hypotheses. 2008;70:793–7. doi: 10.1016/j.mehy.2007.07.039. [DOI] [PubMed] [Google Scholar]
- Packard RR, Libby P. Inflammation in atherosclerosis: from vascular biology to biomarker discovery and risk prediction. Clin Chem. 2008;54:24–38. doi: 10.1373/clinchem.2007.097360. [DOI] [PubMed] [Google Scholar]
- Rader DJ, Daugherty A. Translating molecular discoveries into new therapies for atherosclerosis. Nature. 2008;451:904–13. doi: 10.1038/nature06796. [DOI] [PubMed] [Google Scholar]
- Tan KT, Lip GY. Imaging of the unstable plaque. Int J Cardiol. 2008;127:157–65. doi: 10.1016/j.ijcard.2007.11.054. [DOI] [PubMed] [Google Scholar]
- van Leuven SI, Franssen R, Kastelein JJ, Levi M, Stroes ES, Tak PP. Systemic inflammation as a risk factor for atherothrombosis. Rheumatology (Oxford) 2008;47:3–7. doi: 10.1093/rheumatology/kem202. [DOI] [PubMed] [Google Scholar]
- Lusis AJ. Atherosclerosis. Nature. 2000;407:233–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker R, Keaney JF. Role of oxidative modifications in atherosclerosis. Physiol Rev. 2004;84:1381–1478. doi: 10.1152/physrev.00047.2003. [DOI] [PubMed] [Google Scholar]
- Smith C, Mitchinson MJ, Aruoma OI, Halliwell B. Stimulation of lipid peroxidation and hydroxyl radical generation by the contents of human atherosclerotic lesions. Biochem J. 1992;286:901–905. doi: 10.1042/bj2860901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishna G, Rooke TW, Cooper LT. Iron and peripheral arterial disease: revisiting the iron hypothesis in a different light. Vasc Med. 2003;8:203–210. doi: 10.1191/1358863x03vm493ra. [DOI] [PubMed] [Google Scholar]
- Stadler N, Lindner RA, Davies MJ. Direct detection and quantification of transition metal ions in human atherosclerotic plaques: evidence for the presence of elevated levels of iron and copper. Arterioscler Thromb Vasc Biol. 2004;24:949–54. doi: 10.1161/01.ATV.0000124892.90999.cb. [DOI] [PubMed] [Google Scholar]
- Wolff B, Volzke H, Ludemann J, Robinson D, Vogelgesang D, Staudt A, Kessler C, Dahm JB, John U, Felix SB. Association between high serum ferritin levels and carotid atherosclerosis in the study of health in Pomerania (SHIP) Stroke. 2004;35:453–7. doi: 10.1161/01.STR.0000114875.31599.1C. [DOI] [PubMed] [Google Scholar]
- Roijers RB, Dutta RK, Mutsaers PHA, Gijbels MJJ, de Winther MPJ, de Goeij JJM, Vusse GJ van der. Spatial correlation of trace elements with morphological features of atherosclerotic plaques. Nuclear Instruments & Methods in Physics Research Section B-Beam Interactions with Materials and Atoms. 2005;231:239–244. [Google Scholar]
- Stocker R, Keaney JF. New insights on oxidative stress in the artery wall. Journal of Thrombosis and Haemostasis. 2005;3:1825–1834. doi: 10.1111/j.1538-7836.2005.01370.x. [DOI] [PubMed] [Google Scholar]
- Stanley N, Stadler N, Woods AA, Bannon PG, Davies MJ. Concentrations of iron correlate with the extent of protein, but not lipid, oxidation in advanced human atherosclerotic lesions. Free Radical Biology and Medicine. 2006;40:1636–1643. doi: 10.1016/j.freeradbiomed.2005.12.031. [DOI] [PubMed] [Google Scholar]
- Watt F, Rajendran R, Ren MQ, Tan BKH, Halliwell B. A nuclear microscopy study of trace elements Ca, Fe, Zn and Cu in atherosclerosis. Nucl Instr Meth Phys Res B. 2006;249:646–652. [Google Scholar]
- Fernandes de Godoy M, Takakura IT, Machado RD, Grassi LV, Nogueira PR. Serum ferritin and obstructive coronary artery disease: Angiographic correlation. Arquivos Brasileiros De Cardiologia. 2007;88:430–433. doi: 10.1590/s0066-782x2007000400011. [DOI] [PubMed] [Google Scholar]
- Rajendran R, Ren M, Ning P, Tan Kwong Huat B, Halliwell B, Watt F. Promotion of atherogenesis by copper or iron–which is more likely? Biochem Biophys Res Commun. 2007;353:6–10. doi: 10.1016/j.bbrc.2006.11.038. [DOI] [PubMed] [Google Scholar]
- Kazi TG, Afridi HI, Kazi N, Jamali MK, Arain MB, Sarfraz RA, Jalbani N, Ansari R, Shah AQ, Memon AU, Khandhro GA. Distribution of zinc, copper and iron in biological samples of Pakistani myocardial infarction (1st, 2nd and 3rd heart attack) patients and controls. Clin Chim Acta. 2008;389:114–9. doi: 10.1016/j.cca.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Sullivan JL. Iron in arterial plaque: A modifiable risk factor for atherosclerosis. Biochim Biophys Acta. 2008 doi: 10.1016/j.bbagen.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Yuan XM, Li W. Iron involvement in multiple signaling pathways of atherosclerosis: a revisited hypothesis. Curr Med Chem. 2008;15:2157–72. doi: 10.2174/092986708785747634. [DOI] [PubMed] [Google Scholar]
- Matthews AJ, Vercellotti GM, Menchaca HJ, Bloch PH, Michalek VN, Marker PH, Murar J, Buchwald H. Iron and atherosclerosis: inhibition by the iron chelator deferiprone (L1) J Surg Res. 1997;73:35–40. doi: 10.1006/jsre.1997.5180. [DOI] [PubMed] [Google Scholar]
- Ponraj D, Makjanic J, Thong PSP, Tan BKH, Watt F. The onset of atherosclerotic lesion formation in hypercholesterolemic rabbits is delayed by iron depletion. FEBS Lett. 1999;459:218–222. doi: 10.1016/s0014-5793(99)01199-0. [DOI] [PubMed] [Google Scholar]
- Lee TS, Shiao MS, Pan CC, Chau LY. Iron-deficient diet reduces atherosclerotic lesions in ApoE-deficient mice. Circulation. 1999;99:1222–1229. doi: 10.1161/01.cir.99.9.1222. [DOI] [PubMed] [Google Scholar]
- Ren MQ, Rajendran R, Pan N, Tan BKH, Ong WY, Watt F, Halliwell B. The iron chelator desferrioxamine inhibits atherosclerotic lesion development and decreases lesion iron concentrations in the cholesterol-fed rabbit. Free Radical Biology and Medicine. 2005;38:1206–1211. doi: 10.1016/j.freeradbiomed.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Ferrara DE, Taylor WR. Iron chelation and vascular function: in search of the mechanisms. Arterioscler Thromb Vasc Biol. 2005;25:2235–7. doi: 10.1161/01.ATV.0000189303.45609.1f. [DOI] [PubMed] [Google Scholar]
- Kiechl S, Willeit J, Egger G, Poewe W, Oberhollenzer F. Body iron stores and the risk of carotid atherosclerosis: prospective results from the Bruneck study. Circulation. 1997;96:3300–7. doi: 10.1161/01.cir.96.10.3300. [DOI] [PubMed] [Google Scholar]
- de Valk B, Marx JJM. Iron, atherosclerosis, and ischemic heart disease. Arch Internal Med. 1999;159:1542–1548. doi: 10.1001/archinte.159.14.1542. [DOI] [PubMed] [Google Scholar]
- Zacharski LR, Chow B, Lavori PW, Howes PS, Bell MR, DiTommaso MA, Carnegie NM, Bech F, Amidi M, Muluk S. The Iron (Fe) and Atherosclerosis Study (FeAST): A pilot study of reduction of body iron stores in atherosclerotic peripheral vascular disease. American Heart Journal. 2000;139:337–345. doi: 10.1067/mhj.2000.102909. [DOI] [PubMed] [Google Scholar]
- Day SM, Duquaine D, Mundada LV, Menon RG, Khan BV, Rajagopalan S, Fay WP. Chronic iron administration increases vascular oxidative stress and accelerates arterial thrombosis. Circulation. 2003;107:2601–2606. doi: 10.1161/01.CIR.0000066910.02844.D0. [DOI] [PubMed] [Google Scholar]
- Ren MQ, Watt F, Huat BTK, Halliwell B. Trace elemental distributions in induced atherosclerotic lesions using nuclear microscopy. Nuclear Instr Meth Phys Res B. 2003;210:336–342. [Google Scholar]
- Minqin R, Watt F, Huat BTK, Halliwell B. Correlation of iron and zinc levels with lesion depth in newly formed atherosclerotic lesions. Free Radical Biology and Medicine. 2003;34:746–752. doi: 10.1016/s0891-5849(02)01427-2. [DOI] [PubMed] [Google Scholar]
- You SA, Archacki SR, Angheloiu G, Moravec CS, Rao S, Kinter M, Topol EJ, Wang Q. Proteomic approach to coronary atherosclerosis shows ferritin light chain as a significant marker: evidence consistent with iron hypothesis in atherosclerosis. Physiol Genomics. 2003;13:25–30. doi: 10.1152/physiolgenomics.00124.2002. [DOI] [PubMed] [Google Scholar]
- Zacharski LR, Gerhard GS. Atherosclerosis: a manifestation of chronic iron toxicity? Vascular Medicine. 2003;8:153–155. doi: 10.1191/1358863x03vm492ed. [DOI] [PubMed] [Google Scholar]
- Kallianpur AR. Iron and oxidative injury – A commentary on "Fatty acid-mediated iron translocation: A synergistic mechanism of oxidative injury" by D. Yao et al. Free Radical Biology and Medicine. 2005;39:1305–1309. doi: 10.1016/j.freeradbiomed.2005.07.014. [DOI] [PubMed] [Google Scholar]
- You SA, Wang Q. Ferritin in atherosclerosis. Clin Chim Acta. 2005;357:1–16. doi: 10.1016/j.cccn.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Lapenna D, Pierdomenico SD, Ciofani G, Ucchino S, Neri M, Giamberardino MA, Cuccurullo F. Association of body iron stores with low molecular weight iron and oxidant damage of human atherosclerotic plaques. Free Rad Biol Med. 2007;42:492–498. doi: 10.1016/j.freeradbiomed.2006.11.014. [DOI] [PubMed] [Google Scholar]
- Marx JJ, Kartikasari AE, Georgiou NA. Can iron chelators influence the progression of atherosclerosis? Hemoglobin. 2008;32:123–34. doi: 10.1080/03630260701726871. [DOI] [PubMed] [Google Scholar]
- Paulsson J, Dadfar E, Held C, Jacobson SH, Lundahl J. Activation of peripheral and in vivo transmigrated neutrophils in patients with stable coronary artery disease. Atherosclerosis. 2007;192:328–34. doi: 10.1016/j.atherosclerosis.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Rooyakkers TM, Stroes ES, Kooistra MP, van Faassen EE, Hider RC, Rabelink TJ, Marx JJ. Ferric saccharate induces oxygen radical stress and endothelial dysfunction in vivo. Eur J Clin Invest. 2002;32:9–16. doi: 10.1046/j.1365-2362.2002.0320s1009.x. [DOI] [PubMed] [Google Scholar]
- Duffy SJ, Biegelsen ES, Holbrook M, Russell JD, Gokce N, Keaney JF, Jr, Vita JA. Iron chelation improves endothelial function in patients with coronary artery disease. Circulation. 2001;103:2799–804. doi: 10.1161/01.cir.103.23.2799. [DOI] [PubMed] [Google Scholar]
- Ishizaka N, Saito K, Mori I, Matsuzaki G, Ohno M, Nagai R. Iron chelation suppresses ferritin upregulation and attenuates vascular dysfunction in the aorta of angiotensin II-infused rats. Arterioscler Thromb Vasc Biol. 2005;25:2282–8. doi: 10.1161/01.ATV.0000181763.57495.2b. [DOI] [PubMed] [Google Scholar]
- Saito K, Ishizaka N, Aizawa T, Sata M, Iso-o N, Noiri E, Mori I, Ohno M, Nagai R. Iron chelation and a free radical scavenger suppress angiotensin II-induced upregulation of TGF-beta1 in the heart. Am J Physiol Heart Circ Physiol. 2005;288:H1836–43. doi: 10.1152/ajpheart.00679.2004. [DOI] [PubMed] [Google Scholar]
- Thomas SR, Schulz E, Keaney JF. Hydrogen peroxide restrains endothelium-derived nitric oxide bioactivity – Role for iron-dependent oxidative stress. Free Radical Biology and Medicine. 2006;41:681–688. doi: 10.1016/j.freeradbiomed.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Zacharski LR, Chow BK, Howes PS, Shamayeva G, Baron JA, Dalman RL, Malenka DJ, Ozaki CK, Lavori PW. Reduction of iron stores and cardiovascular outcomes in patients with peripheral arterial disease – A randomized controlled trial. JAMA. 2007;297:603–610. doi: 10.1001/jama.297.6.603. [DOI] [PubMed] [Google Scholar]
- Shiesh SC, Chen CY, Lin XZ, Liu ZA, Tsao HC. Melatonin prevents pigment gallstone formation induced by bile duct ligation in guinea pigs. Hepatology. 2000;32:455–60. doi: 10.1053/jhep.2000.16332. [DOI] [PubMed] [Google Scholar]
- Koppisetti S, Jenigiri B, Terron MP, Tengattini S, Tamura H, Flores LJ, Tan DX, Reiter RJ. Reactive oxygen species and the hypomotility of the gall bladder as targets for the treatment of gallstones with melatonin: a review. Dig Dis Sci. 2008;53:2592–603. doi: 10.1007/s10620-007-0195-5. [DOI] [PubMed] [Google Scholar]
- Robinson JG. Models for describing relations among the various statin drugs, low-density lipoprotein cholesterol lowering, pleiotropic effects, and cardiovascular risk. Am J Cardiol. 2008;101:1009–15. doi: 10.1016/j.amjcard.2007.11.060. [DOI] [PubMed] [Google Scholar]
- Prentice RL, Langer RD, Stefanick ML, Howard BV, Pettinger M, Anderson GL, Barad D, Curb JD, Kotchen J, Kuller L, Limacher M, Wactawski-Wende J. Combined analysis of Women's Health Initiative observational and clinical trial data on postmenopausal hormone treatment and cardiovascular disease. Am J Epidemiol. 2006;163:589–99. doi: 10.1093/aje/kwj079. [DOI] [PubMed] [Google Scholar]
- Couzin J. Cholesterol veers off script. Science. 2008;322:220–3. doi: 10.1126/science.322.5899.220. [DOI] [PubMed] [Google Scholar]
- Peterson RT. Chemical biology and the limits of reductionism. Nat Chem Biol. 2008;4:635–8. doi: 10.1038/nchembio1108-635. [DOI] [PubMed] [Google Scholar]
- Weitz-Schmidt G, Welzenbach K, Brinkmann V, Kamata T, Kallen J, Bruns C, Cottens S, Takada Y, Hommel U. Statins selectively inhibit leukocyte function antigen-1 by binding to a novel regulatory integrin site. Nat Med. 2001;7:687–92. doi: 10.1038/89058. [DOI] [PubMed] [Google Scholar]
- Arkin MR, Wells JA. Small-molecule inhibitors of protein-protein interactions: progressing towards the dream. Nat Rev Drug Discov. 2004;3:301–17. doi: 10.1038/nrd1343. [DOI] [PubMed] [Google Scholar]
- Bellosta S, Ferri N, Arnaboldi L, Bernini F, Paoletti R, Corsini A. Pleiotropic effects of statins in atherosclerosis and diabetes. Diabetes Care. 2000;23:B72–U5. [PubMed] [Google Scholar]
- Kwak B, Mulhaupt F, Myit S, Mach F. Statins as a newly recognized type of immunomodulator. Nat Med. 2000;6:1399–402. doi: 10.1038/82219. [DOI] [PubMed] [Google Scholar]
- Albert MA, Danielson E, Rifai N, Ridker PM. Effect of statin therapy on C-reactive protein levels: the pravastatin inflammation/CRP evaluation (PRINCE): a randomized trial and cohort study. JAMA. 2001;286:64–70. doi: 10.1001/jama.286.1.64. [DOI] [PubMed] [Google Scholar]
- LaRosa JC. Pleiotropic effects of statins and their clinical significance. American Journal of Cardiology. 2001;88:291. doi: 10.1016/s0002-9149(01)01643-5. [DOI] [PubMed] [Google Scholar]
- Takemoto M, Node K, Nakagami H, Liao YL, Grimm M, Takemoto Y, Kitakaze M, Liao JK. Statins as antioxidant therapy for preventing cardiac myocyte hypertrophy. Journal of Clinical Investigation. 2001;108:1429–1437. doi: 10.1172/JCI13350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao JK. Beyond lipid lowering: the role of statins in vascular protection. Int J Cardiol. 2002;86:5–18. doi: 10.1016/s0167-5273(02)00195-x. [DOI] [PubMed] [Google Scholar]
- McFarlane SI, Muniyappa R, Francisco R, Sowers JR. Clinical review 145 – Pleiotropic effects of statins: Lipid reduction and beyond. J Clin Endocrinol Metab. 2002;87:1451–1458. doi: 10.1210/jcem.87.4.8412. [DOI] [PubMed] [Google Scholar]
- Undas A, Brozek J, Musial J. Anti-inflammatory and antithrombotic effects of statins in the management of coronary artery disease. Clin Lab. 2002;48:287–96. [PubMed] [Google Scholar]
- Bonetti PO, Lerman LO, Napoli C, Lerman A. Statin effects beyond lipid lowering–are they clinically relevant? Eur Heart J. 2003;24:225–48. doi: 10.1016/s0195-668x(02)00419-0. [DOI] [PubMed] [Google Scholar]
- Dichtl W, Dulak J, Frick M, Alber HF, Schwarzacher SP, Ares MPS, Nilsson J, Pachinger O, Weidinger F. HMG-CoA reductase inhibitors regulate inflammatory transcription factors in human endothelial and vascular smooth muscle cells. Arteriosclerosis Thrombosis and Vascular Biology. 2003;23:58–63. doi: 10.1161/01.atv.0000043456.48735.20. [DOI] [PubMed] [Google Scholar]
- Mulhaupt F, Matter CM, Kwak BR, Pelli G, Veillard NR, Burger F, Graber P, Luscher TF, Mach F. Statins (HMG-CoA reductase inhibitors) reduce CD40 expression in human vascular cells. Cardiovasc Res. 2003;59:755–66. doi: 10.1016/s0008-6363(03)00515-7. [DOI] [PubMed] [Google Scholar]
- Wierzbicki AS, Poston R, Ferro A. The lipid and non-lipid effects of statins. Pharmacol Ther. 2003;99:95–112. doi: 10.1016/s0163-7258(03)00055-x. [DOI] [PubMed] [Google Scholar]
- Davignon J. Beneficial cardiovascular pleiotropic effects of statins. Circulation. 2004;109:39–43. doi: 10.1161/01.CIR.0000131517.20177.5a. [DOI] [PubMed] [Google Scholar]
- Halcox JP, Deanfield JE. Beyond the laboratory: clinical implications for statin pleiotropy. Circulation. 2004;109:II42–8. doi: 10.1161/01.CIR.0000129500.29229.92. [DOI] [PubMed] [Google Scholar]
- Liao JK. Statins: potent vascular anti-inflammatory agents. Int J Clin Pract Suppl. 2004:41–8. doi: 10.1111/j.1368-504x.2004.00375.x. [DOI] [PubMed] [Google Scholar]
- Schönbeck U, Libby P. Inflammation, immunity, and HMG-CoA reductase inhibitors – Statins as antiinflammatory agents? Circulation. 2004;109:18–26. doi: 10.1161/01.CIR.0000129505.34151.23. [DOI] [PubMed] [Google Scholar]
- Liao JK, Laufs U. Pleiotropic effects of statins. Annu Rev Pharmacol Toxicol. 2005;45:89–118. doi: 10.1146/annurev.pharmtox.45.120403.095748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elrod JW, Lefer DJ. The effects of statins on endothelium, inflammation and cardioprotection. Drug News Perspect. 2005;18:229–36. doi: 10.1358/dnp.2005.18.4.908656. [DOI] [PubMed] [Google Scholar]
- Robinson JG, Smith B, Maheshwari N, Schrott H. Pleiotropic effects of statins: Benefit beyond cholesterol reduction? A meta-regression analysis. J Amer Coll Cardiol. 2005;46:1855–1862. doi: 10.1016/j.jacc.2005.05.085. [DOI] [PubMed] [Google Scholar]
- Tsubouchi H, Inoguchi T, Sonta T, Sato N, Sekiguchi N, Kobayashi K, Sumimoto H, Utsumi H, Nawata H. Statin attenuates high glucose-induced and diabetes-induced oxidative stress in vitro and in vivo evaluated by electron spin resonance measurement. Free Radical Biology and Medicine. 2005;39:444–452. doi: 10.1016/j.freeradbiomed.2005.03.031. [DOI] [PubMed] [Google Scholar]
- Beckman JA, Creager MA. The nonlipid effects of statins on endothelial function. Trends Cardiovasc Med. 2006;16:156–62. doi: 10.1016/j.tcm.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Carloni S, Mazzoni E, Cimino M, De Simoni MG, Perego C, Scopa C, Balduini W. Simvastatin reduces caspase-3 activation and inflammatory markers induced by hypoxia-ischemia in the newborn rat. Neurobiol Dis. 2006;21:119–26. doi: 10.1016/j.nbd.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Grimes DS. Are statins analogues of vitamin D? Lancet. 2006;368:83–6. doi: 10.1016/S0140-6736(06)68971-X. [DOI] [PubMed] [Google Scholar]
- Paumelle R, Blanquart C, Briand O, Barbier O, Duhem C, Woerly G, Percevault F, Fruchart JC, Dombrowicz D, Glineur C, Staels B. Acute antiinflammatory properties of statins involve peroxisome proliferator-activated receptor-alpha via inhibition of the protein kinase C signaling pathway. Circ Res. 2006;98:361–9. doi: 10.1161/01.RES.0000202706.70992.95. [DOI] [PubMed] [Google Scholar]
- Alegret M, Silvestre JS. Pleiotropic effects of statins and related pharmacological experimental approaches. Timely Top Med Cardiovasc Dis. 2007;11:E10. [PubMed] [Google Scholar]
- Farooqui AA, Ong WY, Horrocks LA, Chen P, Farooqui T. Comparison of biochemical effects of statins and fish oil in brain: the battle of the titans. Brain Res Rev. 2007;56:443–71. doi: 10.1016/j.brainresrev.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Kuipers HF, Elsen PJ van den. Immunomodulation by statins: inhibition of cholesterol vs. isoprenoid biosynthesis. Biomed Pharmacother. 2007;61:400–7. doi: 10.1016/j.biopha.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Lahera V, Goicoechea M, de Vinuesa SG, Miana M, de las Heras N, Cachofeiro V, Luno J. Endothelial dysfunction, oxidative stress and inflammation in atherosclerosis: beneficial effects of statins. Curr Med Chem. 2007;14:243–8. doi: 10.2174/092986707779313381. [DOI] [PubMed] [Google Scholar]
- Paraskevas KI, Tzovaras AA, Briana DD, Mikhailidis DP. Emerging indications for statins: a pluripotent family of agents with several potential applications. Curr Pharm Des. 2007;13:3622–36. doi: 10.2174/138161207782794194. [DOI] [PubMed] [Google Scholar]
- Terblanche M, Almog Y, Rosenson R, Smith TS, Hackam DG. Statins and sepsis: multiple modifications at multiple levels. Lancet Infectious Diseases. 2007;7:358–368. doi: 10.1016/S1473-3099(07)70111-1. [DOI] [PubMed] [Google Scholar]
- Wang CY, Liu PY, Liao JK. Pleiotropic effects of statin therapy: molecular mechanisms and clinical results. Trends Mol Med. 2008;14:37–44. doi: 10.1016/j.molmed.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner BK, Kitami T, Gilbert TJ, Peck D, Ramanathan A, Schreiber SL, Golub TR, Mootha VK. Large-scale chemical dissection of mitochondrial function. Nat Biotechnol. 2008;26:343–351. doi: 10.1038/nbt1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P, Aikawa M. Stabilization of atherosclerotic plaques: new mechanisms and clinical targets. Nat Med. 2002;8:1257–62. doi: 10.1038/nm1102-1257. [DOI] [PubMed] [Google Scholar]
- Blake GJ, Ridker PM. Are statins anti-inflammatory? Curr Control Trials Cardiovasc Med. 2000;1:161–165. doi: 10.1186/cvm-1-3-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diomede L, Albani D, Sottocorno M, Donati MB, Bianchi M, Fruscella P, Salmona M. In vivo anti-inflammatory effect of statins is mediated by nonsterol mevalonate products. Arterioscler Thromb Vasc Biol. 2001;21:1327–32. doi: 10.1161/hq0801.094222. [DOI] [PubMed] [Google Scholar]
- Laufs U, Gertz K, Dirnagl U, Bohm M, Nickenig G, Endres M. Rosuvastatin, a new HMG-CoA reductase inhibitor, upregulates endothelial nitric oxide synthase and protects from ischemic stroke in mice. Brain Res. 2002;942:23–30. doi: 10.1016/s0006-8993(02)02649-5. [DOI] [PubMed] [Google Scholar]
- Neuhaus O, Strasser-Fuchs S, Fazekas F, Kieseier BC, Niederwieser G, Hartung HP, Archelos JJ. Statins as immunomodulators: comparison with interferon-beta 1b in MS. Neurology. 2002;59:990–7. doi: 10.1212/wnl.59.7.990. [DOI] [PubMed] [Google Scholar]
- Rasmussen LM, Hansen PR, Nabipour MT, Olesen P, Kristiansen MT, Ledet T. Diverse effects of inhibition of 3-hydroxy-3-methylglutaryl-CoA reductase on the expression of VCAM-1 and E-selectin in endothelial cells. Biochem J. 2001;360:363–70. doi: 10.1042/0264-6021:3600363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto M, Liao JK. Pleiotropic effects of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitors. Arterioscler Thromb Vasc Biol. 2001;21:1712–9. doi: 10.1161/hq1101.098486. [DOI] [PubMed] [Google Scholar]
- Sukhova GK, Williams JK, Libby P. Statins reduce inflammation in atheroma of nonhuman primates independent of effects on serum cholesterol. Arterioscler Thromb Vasc Biol. 2002;22:1452–8. doi: 10.1161/01.atv.0000030360.72503.56. [DOI] [PubMed] [Google Scholar]
- Scalia R, Stalker TJ. Microcirculation as a target for the anti-inflammatory properties of statins. Microcirculation. 2002;9:431–42. doi: 10.1038/sj.mn.7800168. [DOI] [PubMed] [Google Scholar]
- Weitz-Schmidt G. Statins as anti-inflammatory agents. Trends Pharmacol Sci. 2002;23:482–6. doi: 10.1016/s0165-6147(02)02077-1. [DOI] [PubMed] [Google Scholar]
- Youssef S, Stuve O, Patarroyo JC, Ruiz PJ, Radosevich JL, Hur EM, Bravo M, Mitchell DJ, Sobel RA, Steinman L, Zamvil SS. The HMG-CoA reductase inhibitor, atorvastatin, promotes a Th2 bias and reverses paralysis in central nervous system autoimmune disease. Nature. 2002;420:78–84. doi: 10.1038/nature01158. [DOI] [PubMed] [Google Scholar]
- Balk EM, Lau J, Goudas LC, Jordan HS, Kupelnick B, Kim LU, Karas RH. Effects of statins on nonlipid serum markers associated with cardiovascular disease: a systematic review. Ann Intern Med. 2003;139:670–82. doi: 10.7326/0003-4819-139-8-200310210-00011. [DOI] [PubMed] [Google Scholar]
- Blanco-Colio LM, Tuñon J, Martin-Ventura JL, Egido J. Anti-inflammatory and immunomodulatory effects of statins. Kidney Int. 2003;63:12–23. doi: 10.1046/j.1523-1755.2003.00744.x. [DOI] [PubMed] [Google Scholar]
- Kwak BR, Mulhaupt F, Mach F. Atherosclerosis: anti-inflammatory and immunomodulatory activities of statins. Autoimmun Rev. 2003;2:332–8. doi: 10.1016/s1568-9972(03)00049-1. [DOI] [PubMed] [Google Scholar]
- Mason JC. Statins and their role in vascular protection. Clin Sci (Lond) 2003;105:251–66. doi: 10.1042/CS20030148. [DOI] [PubMed] [Google Scholar]
- Naidu BV, Woolley SM, Farivar AS, Thomas R, Fraga C, Mulligan MS. Simvastatin ameliorates injury in an experimental model of lung ischemia-reperfusion. J Thorac Cardiovasc Surg. 2003;126:482–9. doi: 10.1016/s0022-5223(03)00699-8. [DOI] [PubMed] [Google Scholar]
- Pate GE, Tahir MN, Murphy RT, Foley JB. Anti-inflammatory effects of statins in patients with aortic stenosis. J Cardiovasc Pharmacol Ther. 2003;8:201–6. doi: 10.1177/107424840300800305. [DOI] [PubMed] [Google Scholar]
- Shishehbor MH, Aviles RJ, Brennan ML, Fu XM, Goormastic M, Pearce GL, Gokce N, Keaney JF, Penn MS, Sprecher DL, Vita JA, Hazen SL. Association of nitrotyrosine levels with cardiovascular disease and modulation by statin therapy. JAMA. 2003;289:1675–1680. doi: 10.1001/jama.289.13.1675. [DOI] [PubMed] [Google Scholar]
- Stüve O, Youssef S, Dunn S, Slavin AJ, Steinman L, Zamvil SS. The potential therapeutic role of statins in central nervous system autoimmune disorders. Cell Mol Life Sci. 2003;60:2483–91. doi: 10.1007/s00018-003-3146-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stüve O, Prod'homme T, Slavin A, Youssef S, Dunn S, Steinman L, Zamvil SS. Statins and their potential targets in multiple sclerosis therapy. Expert Opin Ther Targets. 2003;7:613–22. doi: 10.1517/14728222.7.5.613. [DOI] [PubMed] [Google Scholar]
- Stüve O, Youssef S, Steinman L, Zamvil SS. Statins as potential therapeutic agents in neuroinflammatory disorders. Curr Opin Neurol. 2003;16:393–401. doi: 10.1097/01.wco.0000073942.19076.d1. [DOI] [PubMed] [Google Scholar]
- Durant R, Klouche K, Delbosc S, Morena M, Amigues L, Beraud JJ, Canaud B, Cristol JP. Superoxide anion overproduction in sepsis: effects of vitamin e and simvastatin. Shock. 2004;22:34–9. doi: 10.1097/01.shk.0000129197.46212.7e. [DOI] [PubMed] [Google Scholar]
- Endres M, Laufs U. Effects of statins on endothelium and signaling mechanisms. Stroke. 2004;35:2708–11. doi: 10.1161/01.STR.0000143319.73503.38. [DOI] [PubMed] [Google Scholar]
- Kunkel EJ, Dea M, Ebens A, Hytopoulos E, Melrose J, Nguyen D, Ota KS, Plavec I, Wang Y, Watson SR, Butcher EC, Berg EL. An integrative biology approach for analysis of drug action in models of human vascular inflammation. FASEB J. 2004;18:1279–81. doi: 10.1096/fj.04-1538fje. [DOI] [PubMed] [Google Scholar]
- McCarey DW, McInnes IB, Madhok R, Hampson R, Scherbakov O, Ford I, Capell HA, Sattar N. Trial of Atorvastatin in Rheumatoid Arthritis (TARA): double-blind, randomised placebo-controlled trial. Lancet. 2004;363:2015–21. doi: 10.1016/S0140-6736(04)16449-0. [DOI] [PubMed] [Google Scholar]
- Rosenson RS. Statins in atherosclerosis: lipid-lowering agents with antioxidant capabilities. Atherosclerosis. 2004;173:1–12. doi: 10.1016/S0021-9150(03)00239-9. [DOI] [PubMed] [Google Scholar]
- McInnes IB, McCarey DW, Sattar N. Do statins offer therapeutic potential in inflammatory arthritis? Ann Rheum Dis. 2004;63:1535–7. doi: 10.1136/ard.2004.022061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffens S, Mach F. Anti-inflammatory properties of statins. Semin Vasc Med. 2004;4:417–22. doi: 10.1055/s-2004-869599. [DOI] [PubMed] [Google Scholar]
- Stoll LL, McCormick ML, Denning GM, Weintraub NL. Antioxidant effects of statins. Drugs Today (Barc) 2004;40:975–90. doi: 10.1358/dot.2004.40.12.872573. [DOI] [PubMed] [Google Scholar]
- Arnaud C, Braunersreuther V, Mach F. Toward immunomodulatory and anti-inflammatory properties of statins. Trends Cardiovasc Med. 2005;15:202–6. doi: 10.1016/j.tcm.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Endres M. Statins and stroke. J Cereb Blood Flow Metab. 2005;25:1093–110. doi: 10.1038/sj.jcbfm.9600116. [DOI] [PubMed] [Google Scholar]
- Gaugler MH, Vereycken-Holler V, Squiban C, Vandamme M, Vozenin-Brotons MC, Benderitter M. Pravastatin limits endothelial activation after irradiation and decreases the resulting inflammatory and thrombotic responses. Radiat Res. 2005;163:479–87. doi: 10.1667/rr3302. [DOI] [PubMed] [Google Scholar]
- Jain MK, Ridker PM. Anti-inflammatory effects of statins: clinical evidence and basic mechanisms. Nat Rev Drug Discov. 2005;4:977–87. doi: 10.1038/nrd1901. [DOI] [PubMed] [Google Scholar]
- Macin SM, Perna ER, Farias EF, Franciosi V, Cialzeta JR, Brizuela M, Medina F, Tajer C, Doval H, Badaracco R. Atorvastatin has an important acute anti-inflammatory effect in patients with acute coronary syndrome: results of a randomized, double-blind, placebo-controlled study. Am Heart J. 2005;149:451–7. doi: 10.1016/j.ahj.2004.07.041. [DOI] [PubMed] [Google Scholar]
- Methe H, Kim JO, Kofler S, Nabauer M, Weis M. Statins decrease Toll-like receptor 4 expression and downstream signaling in human CD14+ monocytes. Arterioscler Thromb Vasc Biol. 2005;25:1439–45. doi: 10.1161/01.ATV.0000168410.44722.86. [DOI] [PubMed] [Google Scholar]
- Schultz Johansen J, Harris AK, Rychly DJ, Ergul A. Oxidative stress and the use of antioxidants in diabetes: linking basic science to clinical practice. Cardiovasc Diabetol. 2005;4:5. doi: 10.1186/1475-2840-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonelli M, Sacks F, Pfeffer M, Jhangri GS, Curhan G. Biomarkers of inflammation and progression of chronic kidney disease. Kidney Int. 2005;68:237–45. doi: 10.1111/j.1523-1755.2005.00398.x. [DOI] [PubMed] [Google Scholar]
- Abeles AM, Pillinger MH. Statins as antiinflammatory and immunomodulatory agents: a future in rheumatologic therapy? Arthritis Rheum. 2006;54:393–407. doi: 10.1002/art.21521. [DOI] [PubMed] [Google Scholar]
- Endres M. Statins: potential new indications in inflammatory conditions. Atheroscler Suppl. 2006;7:31–5. doi: 10.1016/j.atherosclerosissup.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Yang J, Li XP, Zhao SP, Li J, Li JD, Xie XM. The effect of different doses of fluvastatin on inflammatory markers in the early phase of acute coronary syndrome. Clin Chim Acta. 2006;368:183–7. doi: 10.1016/j.cca.2005.12.029. [DOI] [PubMed] [Google Scholar]
- Ahn KS, Sethi G, Aggarwal BB. Simvastatin potentiates TNF-alpha-induced apoptosis through the down-regulation of NF-kappaB-dependent antiapoptotic gene products: role of IkappaBalpha kinase and TGF-beta-activated kinase-1. J Immunol. 2007;178:2507–16. doi: 10.4049/jimmunol.178.4.2507. [DOI] [PubMed] [Google Scholar]
- Becker RC. Off-target properties of pharmacotherapy and the importance of mechanistic investigations in early clinical phase drug development. J Thromb Thrombolysis. 2007;23:159–61. doi: 10.1007/s11239-006-0007-3. [DOI] [PubMed] [Google Scholar]
- Greenwood J, Mason JC. Statins and the vascular endothelial inflammatory response. Trends Immunol. 2007;28:88–98. doi: 10.1016/j.it.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JJ, Zheng X, Li J. Statins may be beneficial for patients with slow coronary flow syndrome due to its anti-inflammatory property. Med Hypotheses. 2007;69:333–7. doi: 10.1016/j.mehy.2006.09.070. [DOI] [PubMed] [Google Scholar]
- Sandek A, Utchill S, Rauchhaus M. The endotoxin-lipoprotein hypothesis – an update. Archives of Medical Science. 2007;3:S81–S90. [Google Scholar]
- Siffrin V, Brandt AU, Herz J, Zipp F. New insights into adaptive immunity in chronic neuroinflammation. Adv Immunol. 2007;96:1–40. doi: 10.1016/S0065-2776(07)96001-0. [DOI] [PubMed] [Google Scholar]
- Souza-Costa DC, Sandrim VC, Lopes LF, Gerlach RF, Rego EM, Tanus-Santos JE. Anti-inflammatory effects of atorvastatin: modulation by the T-786C polymorphism in the endothelial nitric oxide synthase gene. Atherosclerosis. 2007;193:438–44. doi: 10.1016/j.atherosclerosis.2006.07.020. [DOI] [PubMed] [Google Scholar]
- Hernández-Romero MdC, Argüelles S, Villaran RF, de Pablos RM, Delgado-Cortés MJ, Santiago M, Herrera AJ, Cano J, Machado A. Simvastatin prevents the inflammatory process and the dopaminergic degeneration induced by the intranigral injection of lipopolysaccharide. J Neurochem. 2008;105:445–59. doi: 10.1111/j.1471-4159.2007.05148.x. [DOI] [PubMed] [Google Scholar]
- Paraskevas KI. Statin treatment for rheumatoid arthritis: a promising novel indication. Clin Rheumatol. 2008;27:281–7. doi: 10.1007/s10067-007-0806-8. [DOI] [PubMed] [Google Scholar]
- Prinz V, Laufs U, Gertz K, Kronenberg G, Balkaya M, Leithner C, Lindauer U, Endres M. Intravenous rosuvastatin for acute stroke treatment: an animal study. Stroke. 2008;39:433–8. doi: 10.1161/STROKEAHA.107.492470. [DOI] [PubMed] [Google Scholar]
- Xu SZ, Zhong W, Watson NM, Dickerson E, Wake JD, Lindow SW, Newton CJ, Atkin SL. Fluvastatin reduces oxidative damage in human vascular endothelial cells by upregulating Bcl-2. J Thromb Haemost. 2008;6:692–700. doi: 10.1111/j.1538-7836.2008.02913.x. [DOI] [PubMed] [Google Scholar]
- Dombrecht EJ, De Tollenaere CB, Aerts K, Cos P, Schuerwegh AJ, Bridts CH, Van Offel JF, Ebo DG, Stevens WJ, De Clerck LS. Antioxidant effect of bisphosphonates and simvastatin on chondrocyte lipid peroxidation. Biochem Biophys Res Commun. 2006;348:459–64. doi: 10.1016/j.bbrc.2006.07.075. [DOI] [PubMed] [Google Scholar]
- Lee DH, Zacharski LR, Jacobs DR. Comparison of the serum ferritin and percentage of transferrin saturation as exposure markers of iron-driven oxidative stress-related disease outcomes. American Heart Journal. 2006;151 doi: 10.1016/j.ahj.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Anderson LJ, Holden S, Davis B, Prescott E, Charrier CC, Bunce NH, Firmin DN, Wonke B, Porter J, Walker JM, Pennell DJ. Cardiovascular T2-star (T2*) magnetic resonance for the early diagnosis of myocardial iron overload. Eur Heart J. 2001;22:2171–2179. doi: 10.1053/euhj.2001.2822. [DOI] [PubMed] [Google Scholar]
- Yeoh-Ellerton S, Stacey MC. Iron and 8-isoprostane levels in acute and chronic wounds. J Invest Dermatol. 2003;121:918–925. doi: 10.1046/j.1523-1747.2003.12471.x. [DOI] [PubMed] [Google Scholar]
- Bergan JJ, Schmid-Schonbein GW, Smith PDC, Nicolaides AN, Boisseau MR, Eklof B. Mechanisms of disease: Chronic venous disease. New England Journal of Medicine. 2006;355:488–498. doi: 10.1056/NEJMra055289. [DOI] [PubMed] [Google Scholar]
- Zamboni P, Izzo M, Fogato L, Carandina S, Lanzara V. Urine hemosiderin: a novel marker to assess the severity of chronic venous disease. J Vasc Surg. 2003;37:132–6. doi: 10.1067/mva.2003.64. [DOI] [PubMed] [Google Scholar]
- Zamboni P. The Big Idea: Iron-dependent inflammation in venous disease and proposed parallels in multiple sclerosis. J R Soc Med. 2006;99:589–593. doi: 10.1258/jrsm.99.11.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas SM, Rothwell NJ, Gibson RM. The role of inflammation in CNS injury and disease. Br J Pharmacol. 2006;147:S232–40. doi: 10.1038/sj.bjp.0706400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewén A, Matz P, Chan PH. Free radical pathways in CNS injury. J Neurotrauma. 2000;17:871–90. doi: 10.1089/neu.2000.17.871. [DOI] [PubMed] [Google Scholar]
- Que EL, Domaille DW, Chang CJ. Metals in neurobiology: probing their chemistry and biology with molecular imaging. Chem Rev. 2008;108:1517–49. doi: 10.1021/cr078203u. [DOI] [PubMed] [Google Scholar]
- Kent TA, Soukup VM, Fabian RH. Heterogeneity affecting outcome from acute stroke therapy: making reperfusion worse. Stroke. 2001;32:2318–27. doi: 10.1161/hs1001.096588. [DOI] [PubMed] [Google Scholar]
- Emsley HC, Tyrrell PJ. Inflammation and infection in clinical stroke. J Cereb Blood Flow Metab. 2002;22:1399–419. doi: 10.1097/01.WCB.0000037880.62590.28. [DOI] [PubMed] [Google Scholar]
- Patt A, Horesh IR, Berger EM, Harken AH, Repine JE. Iron depletion or chelation reduces ischemia/reperfusion-induced edema in gerbil brains. J Pediatr Surg. 1990;25:224–7. doi: 10.1016/0022-3468(90)90407-z. [DOI] [PubMed] [Google Scholar]
- Dávalos A, Fernandezreal JM, Ricart W, Soler S, Molins A, Planas E, Genis D. Iron-related damage in acute ischemic stroke. Stroke. 1994;25:1543–1546. doi: 10.1161/01.str.25.8.1543. [DOI] [PubMed] [Google Scholar]
- Bishop GM, Robinson SR. Quantitative analysis of cell death and ferritin expression in response to cortical iron: implications for hypoxia-ischemia and stroke. Brain Research. 2001;907:175–187. doi: 10.1016/s0006-8993(01)02303-4. [DOI] [PubMed] [Google Scholar]
- Demougeot C, Van Hoecke M, Bertrand N, Prigent-Tessier A, Mossiat C, Beley A, Marie C. Cytoprotective efficacy and mechanisms of the liposoluble iron chelator 2,2 '-dipyridyl in the rat photothrombotic ischemic stroke model. J Pharm Exp Therapeut. 2004;311:1080–1087. doi: 10.1124/jpet.104.072744. [DOI] [PubMed] [Google Scholar]
- Mehta SH, Webb RC, Ergul A, Tawak A, Dorrance AM. Neuroprotection by tempol in a model of iron-induced oxidative stress in acute ischemic stroke. Am J Physiol. 2004;286:R283–R288. doi: 10.1152/ajpregu.00446.2002. [DOI] [PubMed] [Google Scholar]
- Selim MH, Ratan RR. The role of iron neurotoxicity in ischemic stroke. Ageing Research Reviews. 2004;3:345–353. doi: 10.1016/j.arr.2004.04.001. [DOI] [PubMed] [Google Scholar]
- van der ADL, Grobbee DE, Roest M, Marx JJM, Voorbij HA, Schouw YT van der. Serum ferritin is a risk factor for stroke in postmenopausal women. Stroke. 2005;36:1637–1641. doi: 10.1161/01.STR.0000173172.82880.72. [DOI] [PubMed] [Google Scholar]
- Hua Y, Keep RF, Hoff JT, Xi G. Brain injury after intracerebral hemorrhage: the role of thrombin and iron. Stroke. 2007;38:759–62. doi: 10.1161/01.STR.0000247868.97078.10. [DOI] [PubMed] [Google Scholar]
- Millan M, Sobrino T, Castellanos M, Nombela F, Arenillas JF, Riva E, Cristobo I, Garcia MM, Vivancos J, Serena J, Moro MA, Castillo J, Dávalos A. Increased body iron stores are associated with poor outcome after thrombolytic treatment in acute stroke. Stroke. 2007;38:90–95. doi: 10.1161/01.STR.0000251798.25803.e0. [DOI] [PubMed] [Google Scholar]
- Slemmer JE, Shacka JJ, Sweeney MI, Weber JT. Antioxidants and free radical scavengers for the treatment of stroke, traumatic brain injury and aging. Curr Med Chem. 2008;15:404–14. doi: 10.2174/092986708783497337. [DOI] [PubMed] [Google Scholar]
- Baker K, Marcus CB, Huffman K, Kruk H, Malfroy B, Doctrow SR. Synthetic combined superoxide dismutase/catalase mimetics are protective as a delayed treatment in a rat stroke model: a key role for reactive oxygen species in ischemic brain injury. J Pharmacol Exp Ther. 1998;284:215–21. [PubMed] [Google Scholar]
- Sun AY, Chen YM. Oxidative stress and neurodegenerative disorders. Journal of Biomedical Science. 1998;5:401–414. doi: 10.1007/BF02255928. [DOI] [PubMed] [Google Scholar]
- Pong K. Oxidative stress in neurodegenerative diseases: therapeutic implications for superoxide dismutase mimetics. Expert Opinion on Biological Therapy. 2003;3:127–139. doi: 10.1517/14712598.3.1.127. [DOI] [PubMed] [Google Scholar]
- Bastianetto S, Quirion R. Natural antioxidants and neurodegenerative diseases. Frontiers in Bioscience. 2004;9:3447–3452. doi: 10.2741/1493. [DOI] [PubMed] [Google Scholar]
- Warner DS, Sheng HX, Batinic-Haberle I. Oxidants, antioxidants and the ischemic brain. J Exp Biol. 2004;207:3221–3231. doi: 10.1242/jeb.01022. [DOI] [PubMed] [Google Scholar]
- Craft JM, Watterson DM, Van Eldik LJ. Neuroinflammation: a potential therapeutic target. Expert Opin Ther Targets. 2005;9:887–900. doi: 10.1517/14728222.9.5.887. [DOI] [PubMed] [Google Scholar]
- Margaill I, Plotkine M, Lerouet D. Antioxidant strategies in the treatment of stroke. Free Radical Biology and Medicine. 2005;39:429–443. doi: 10.1016/j.freeradbiomed.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Zhao BL. Natural antioxidants for neurodegenerative diseases. Mol Neurobiol. 2005;31:283–293. doi: 10.1385/MN:31:1-3:283. [DOI] [PubMed] [Google Scholar]
- Obrenovitch TP. Molecular physiology of preconditioning-induced brain tolerance to ischemia. Physiol Rev. 2008;88:211–47. doi: 10.1152/physrev.00039.2006. [DOI] [PubMed] [Google Scholar]
- Sirén AL, Fratelli M, Brines M, Goemans C, Casagrande S, Lewczuk P, Keenan S, Gleiter C, Pasquali C, Capobianco A, Mennini T, Heumann R, Cerami A, Ehrenreich H, Ghezzi P. Erythropoietin prevents neuronal apoptosis after cerebral ischemia and metabolic stress. Proc Natl Acad Sci USA. 2001;98:4044–9. doi: 10.1073/pnas.051606598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brines ML, Ghezzi P, Keenan S, Agnello D, de Lanerolle NC, Cerami C, Itri LM, Cerami A. Erythropoietin crosses the blood-brain barrier to protect against experimental brain injury. Proc Natl Acad Sci USA. 2000;97:10526–31. doi: 10.1073/pnas.97.19.10526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Digicaylioglu M, Lipton SA. Erythropoietin-mediated neuroprotection involves cross-talk between Jak2 and NF-kappaB signalling cascades. Nature. 2001;412:641–7. doi: 10.1038/35088074. [DOI] [PubMed] [Google Scholar]
- Dawson TM. Preconditioning-mediated neuroprotection through erythropoietin? Lancet. 2002;359:96–7. doi: 10.1016/S0140-6736(02)07335-X. [DOI] [PubMed] [Google Scholar]
- Ehrenreich H, Hasselblatt M, Dembowski C, Cepek L, Lewczuk P, Stiefel M, Rustenbeck HH, Breiter N, Jacob S, Knerlich F, Bohn M, Poser W, Ruther E, Kochen M, Gefeller O, Gleiter C, Wessel TC, De Ryck M, Itri L, Prange H, Cerami A, Brines M, Sirén AL. Erythropoietin therapy for acute stroke is both safe and beneficial. Mol Med. 2002;8:495–505. [PMC free article] [PubMed] [Google Scholar]
- Leker RR, Shohami E. Cerebral ischemia and trauma-different etiologies yet similar mechanisms: neuroprotective opportunities. Brain Res Rev. 2002;39:55–73. doi: 10.1016/s0165-0173(02)00157-1. [DOI] [PubMed] [Google Scholar]
- Aydin A, Genc K, Akhisaroglu M, Yorukoglu K, Gokmen N, Gonullu E. Erythropoietin exerts neuroprotective effect in neonatal rat model of hypoxic-ischemic brain injury. Brain Dev. 2003;25:494–8. doi: 10.1016/s0387-7604(03)00039-1. [DOI] [PubMed] [Google Scholar]
- Kumral A, Ozer E, Yilmaz O, Akhisaroglu M, Gokmen N, Duman N, Ulukus C, Genc S, Ozkan H. Neuroprotective effect of erythropoietin on hypoxic-ischemic brain injury in neonatal rats. Biol Neonate. 2003;83:224–8. doi: 10.1159/000068926. [DOI] [PubMed] [Google Scholar]
- Matsushita H, Johnston MV, Lange MS, Wilson MA. Protective effect of erythropoietin in neonatal hypoxic ischemia in mice. Neuroreport. 2003;14:1757–61. doi: 10.1097/00001756-200309150-00020. [DOI] [PubMed] [Google Scholar]
- Grasso G, Sfacteria A, Cerami A, Brines M. Erythropoietin as a tissue-protective cytokine in brain injury: what do we know and where do we go? Neuroscientist. 2004;10:93–8. doi: 10.1177/1073858403259187. [DOI] [PubMed] [Google Scholar]
- Sun Y, Zhou C, Polk P, Nanda A, Zhang JH. Mechanisms of erythropoietin-induced brain protection in neonatal hypoxia-ischemia rat model. J Cereb Blood Flow Metab. 2004;24:259–70. doi: 10.1097/01.WCB.0000110049.43905.AC. [DOI] [PubMed] [Google Scholar]
- Wang CH, Liang CL, Huang LT, Liu JK, Hung PH, Sun A, Hung KS. Single intravenous injection of naked plasmid DNA encoding erythropoietin provides neuroprotection in hypoxia-ischemia rats. Biochem Biophys Res Commun. 2004;314:1064–71. doi: 10.1016/j.bbrc.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Bailey DM, Robach P, Thomsen JJ, Lundby C. Erythropoietin depletes iron stores: antioxidant neuroprotection for ischemic stroke? Stroke. 2006;37:2453. doi: 10.1161/01.STR.0000239787.92203.16. [DOI] [PubMed] [Google Scholar]
- Grasso G, Sfacteria A, Meli F, Passalacqua M, Fodale V, Buemi M, Giambartino F, Iacopino DG, Tomasello F. The role of erythropoietin in neuroprotection: therapeutic perspectives. Drug News Perspect. 2007;20:315–20. doi: 10.1358/dnp.2007.20.5.1120219. [DOI] [PubMed] [Google Scholar]
- McPherson RJ, Juul SE. Recent trends in erythropoietin-mediated neuroprotection. Int J Dev Neurosci. 2008;26:103–11. doi: 10.1016/j.ijdevneu.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sola A, Peng H, Rogido M, Wen TC. Animal models of neonatal stroke and response to erythropoietin and cardiotrophin-1. Int J Dev Neurosci. 2008;26:27–35. doi: 10.1016/j.ijdevneu.2007.08.016. [DOI] [PubMed] [Google Scholar]
- Erbayraktar S, Yilmaz O, Gokmen N, Brines M. Erythropoietin is a multifunctional tissue-protective cytokine. Curr Hematol Rep. 2003;2:465–70. [PubMed] [Google Scholar]
- Leist M, Ghezzi P, Grasso G, Bianchi R, Villa P, Fratelli M, Savino C, Bianchi M, Nielsen J, Gerwien J, Kallunki P, Larsen AK, Helboe L, Christensen S, Pedersen LO, Nielsen M, Torup L, Sager T, Sfacteria A, Erbayraktar S, Erbayraktar Z, Gokmen N, Yilmaz O, Cerami-Hand C, Xie QW, Coleman T, Cerami A, Brines M. Derivatives of erythropoietin that are tissue protective but not erythropoietic. Science. 2004;305:239–42. doi: 10.1126/science.1098313. [DOI] [PubMed] [Google Scholar]
- Arcasoy MO. The non-haematopoietic biological effects of erythropoietin. Br J Haematol. 2008;141:14–31. doi: 10.1111/j.1365-2141.2008.07014.x. [DOI] [PubMed] [Google Scholar]
- Agnello D, Bigini P, Villa P, Mennini T, Cerami A, Brines ML, Ghezzi P. Erythropoietin exerts an anti-inflammatory effect on the CNS in a model of experimental autoimmune encephalomyelitis. Brain Res. 2002;952:128–34. doi: 10.1016/s0006-8993(02)03239-0. [DOI] [PubMed] [Google Scholar]
- Fahn S, Cohen G. The oxidant stress hypothesis in Parkinson's disease: evidence supporting it. Ann Neurol. 1992;32:804–12. doi: 10.1002/ana.410320616. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Reactive oxygen species and the central nervous system. J Neurochem. 1992;59:1609–1623. doi: 10.1111/j.1471-4159.1992.tb10990.x. [DOI] [PubMed] [Google Scholar]
- Coyle JT, Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science. 1993;262:689–95. doi: 10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- Behl C, Davis JB, Lesley R, Schubert D. Hydrogen peroxide mediates amyloid beta protein toxicity. Cell. 1994;77:817–27. doi: 10.1016/0092-8674(94)90131-7. [DOI] [PubMed] [Google Scholar]
- Good PF, Werner P, Hsu A, Olanow CW, Perl DP. Evidence of neuronal oxidative damage in Alzheimer's disease. Am J Pathol. 1996;149:21–8. [PMC free article] [PubMed] [Google Scholar]
- Jenner P, Olanow CW. Oxidative stress and the pathogenesis of Parkinson's disease. Neurology. 1996;47:S161–70. doi: 10.1212/wnl.47.6_suppl_3.161s. [DOI] [PubMed] [Google Scholar]
- Simonian NA, Coyle JT. Oxidative stress in neurodegenerative diseases. Annu Rev Pharmacol Toxicol. 1996;36:83–106. doi: 10.1146/annurev.pa.36.040196.000503. [DOI] [PubMed] [Google Scholar]
- Yoritaka A, Hattori N, Uchida K, Tanaka M, Stadtman ER, Mizuno Y. Immunohistochemical detection of 4-hydroxynonenal protein adducts in Parkinson disease. Proc Natl Acad Sci USA. 1996;93:2696–701. doi: 10.1073/pnas.93.7.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packer L, Tritschler HJ, Wessel K. Neuroprotection by the metabolic antioxidant alpha-lipoic acid. Free Radic Biol Med. 1997;22:359–78. doi: 10.1016/s0891-5849(96)00269-9. [DOI] [PubMed] [Google Scholar]
- Gao HM, Liu B, Zhang W, Hong JS. Novel anti-inflammatory therapy for Parkinson's disease. Trends Pharmacol Sci. 2003;24:395–401. doi: 10.1016/S0165-6147(03)00176-7. [DOI] [PubMed] [Google Scholar]
- Mariani E, Polidori MC, Cherubini A, Mecocci P. Oxidative stress in brain aging, neurodegenerative and vascular diseases: an overview. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;827:65–75. doi: 10.1016/j.jchromb.2005.04.023. [DOI] [PubMed] [Google Scholar]
- Zeevalk GD, Bernard LP, Song C, Gluck M, Ehrhart J. Mitochondrial inhibition and oxidative stress: reciprocating players in neurodegeneration. Antioxid Redox Signal. 2005;7:1117–39. doi: 10.1089/ars.2005.7.1117. [DOI] [PubMed] [Google Scholar]
- Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–95. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- Trushina E, McMurray CT. Oxidative stress and mitochondrial dysfunction in neurodegenerative diseases. Neuroscience. 2007;145:1233–48. doi: 10.1016/j.neuroscience.2006.10.056. [DOI] [PubMed] [Google Scholar]
- Benzi G, Moretti A. Are reactive oxygen species involved in Alzheimer's disease? Neurobiol Aging. 1995;16:661–74. doi: 10.1016/0197-4580(95)00066-n. [DOI] [PubMed] [Google Scholar]
- Hensley K, Hall N, Subramaniam R, Cole P, Harris M, Aksenov M, Aksenova M, Gabbita SP, Wu JF, Carney JM, et al. Brain regional correspondence between Alzheimer's disease histopathology and biomarkers of protein oxidation. J Neurochem. 1995;65:2146–56. doi: 10.1046/j.1471-4159.1995.65052146.x. [DOI] [PubMed] [Google Scholar]
- Markesbery WR. Oxidative stress hypothesis in Alzheimer's disease. Free Radic Biol Med. 1997;23:134–47. doi: 10.1016/s0891-5849(96)00629-6. [DOI] [PubMed] [Google Scholar]
- Markesbery WR, Lovell MA. 4-hydroxynonenal, a product of lipid peroxidation, is increased in the brain in Alzheimer's disease. Neurobiol Aging. 1998;19:33–6. doi: 10.1016/s0197-4580(98)00009-8. [DOI] [PubMed] [Google Scholar]
- Cassarino DS, Bennett JP., Jr An evaluation of the role of mitochondria in neurodegenerative diseases: mitochondrial mutations and oxidative pathology, protective nuclear responses, and cell death in neurodegeneration. Brain Res Brain Res Rev. 1999;29:1–25. doi: 10.1016/s0165-0173(98)00046-0. [DOI] [PubMed] [Google Scholar]
- Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, Finch CE, Frautschy S, Griffin WS, Hampel H, Hull M, Landreth G, Lue L, Mrak R, Mackenzie IR, McGeer PL, O'Banion MK, Pachter J, Pasinetti G, Plata-Salaman C, Rogers J, Rydel R, Shen Y, Streit W, Strohmeyer R, Tooyoma I, Van Muiswinkel FL, Veerhuis R, Walker D, Webster S, Wegrzyniak B, Wenk G, Wyss-Coray T. Inflammation and Alzheimer's disease. Neurobiol Aging. 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunomura A, Perry G, Aliev G, Hirai K, Takeda A, Balraj EK, Jones PK, Ghanbari H, Wataya T, Shimohama S, Chiba S, Atwood CS, Petersen RB, Smith MA. Oxidative damage is the earliest event in Alzheimer disease. J Neuropathol Exp Neurol. 2001;60:759–767. doi: 10.1093/jnen/60.8.759. [DOI] [PubMed] [Google Scholar]
- McGeer PL, McGeer EG. Inflammation, autotoxicity and Alzheimer disease. Neurobiol Aging. 2001;22:799–809. doi: 10.1016/s0197-4580(01)00289-5. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Lauderback CM. Lipid peroxidation and protein oxidation in Alzheimer's disease brain: potential causes and consequences involving amyloid beta-peptide-associated free radical oxidative stress. Free Radic Biol Med. 2002;32:1050–60. doi: 10.1016/s0891-5849(02)00794-3. [DOI] [PubMed] [Google Scholar]
- Eikelenboom P, Bate C, Van Gool WA, Hoozemans JJ, Rozemuller JM, Veerhuis R, Williams A. Neuroinflammation in Alzheimer's disease and prion disease. Glia. 2002;40:232–9. doi: 10.1002/glia.10146. [DOI] [PubMed] [Google Scholar]
- Suh YH, Checler F. Amyloid precursor protein, presenilins, and alpha-synuclein: molecular pathogenesis and pharmacological applications in Alzheimer's disease. Pharmacol Rev. 2002;54:469–525. doi: 10.1124/pr.54.3.469. [DOI] [PubMed] [Google Scholar]
- McGeer EG, McGeer PL. Inflammatory processes in Alzheimer's disease. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:741–9. doi: 10.1016/S0278-5846(03)00124-6. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Perluigi M, Sultana R. Oxidative stress in Alzheimer's disease brain: new insights from redox proteomics. Eur J Pharmacol. 2006;545:39–50. doi: 10.1016/j.ejphar.2006.06.026. [DOI] [PubMed] [Google Scholar]
- Jenner P. Oxidative stress in Parkinson's disease. Ann Neurol. 2003;53:S26–36. doi: 10.1002/ana.10483. [DOI] [PubMed] [Google Scholar]
- Nunomura A, Castellani RJ, Zhu X, Moreira PI, Perry G, Smith MA. Involvement of oxidative stress in Alzheimer disease. J Neuropathol Exp Neurol. 2006;65:631–41. doi: 10.1097/01.jnen.0000228136.58062.bf. [DOI] [PubMed] [Google Scholar]
- Licastro F, Porcellini E, Caruso C, Lio D, Corder EH. Genetic risk profiles for Alzheimer's disease: integration of APOE genotype and variants that up-regulate inflammation. Neurobiol Aging. 2007;28:1637–43. doi: 10.1016/j.neurobiolaging.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Meyer-Luehmann M, Spires-Jones TL, Prada C, Garcia-Alloza M, de Calignon A, Rozkalne A, Koenigsknecht-Talboo J, Holtzman DM, Bacskai BJ, Hyman BT. Rapid appearance and local toxicity of amyloid-beta plaques in a mouse model of Alzheimer's disease. Nature. 2008;451:720–4. doi: 10.1038/nature06616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman L. Alzheimer's disease; a clinico-pathologic analysis of twenty-three cases with a theory on pathogenesis. J Nerv Ment Dis. 1953;118:97–130. [PubMed] [Google Scholar]
- Dexter DT, Wells FR, Lees AJ, Agid F, Agid Y, Jenner P, Marsden CD. Increased nigral iron content and alterations in other metal ions occurring in brain in Parkinsons disease. J Neurochem. 1989;52:1830–1836. doi: 10.1111/j.1471-4159.1989.tb07264.x. [DOI] [PubMed] [Google Scholar]
- Riederer P, Sofic E, Rausch WD, Schmidt B, Reynolds GP, Jellinger K, Youdim MB. Transition metals, ferritin, glutathione, and ascorbic acid in parkinsonian brains. J Neurochem. 1989;52:515–20. doi: 10.1111/j.1471-4159.1989.tb09150.x. [DOI] [PubMed] [Google Scholar]
- Jellinger K, Paulus W, Grundke-Iqbal I, Riederer P, Youdim MB. Brain iron and ferritin in Parkinson's and Alzheimer's diseases. J Neural Transm Park Dis Dement Sect. 1990;2:327–40. doi: 10.1007/BF02252926. [DOI] [PubMed] [Google Scholar]
- O'Brien PJ. Molecular mechanisms of quinone cytotoxicity. Chemico-Biol Interact. 1991;80:1–41. doi: 10.1016/0009-2797(91)90029-7. [DOI] [PubMed] [Google Scholar]
- Connor JR, Snyder BS, Beard JL, Fine RE, Mufson EJ. Regional distribution of iron and iron-regulatory proteins in the brain in aging and Alzheimers disease. J Neuroscie Res. 1992;31:327–335. doi: 10.1002/jnr.490310214. [DOI] [PubMed] [Google Scholar]
- Good PF, Perl DP, Bierer LM, Schmeidler J. Selective accumulation of aluminum and iron in the neurofibrillary tangles of Alzheimer's disease: a laser microprobe (LAMMA) study. Ann Neurol. 1992;31:286–92. doi: 10.1002/ana.410310310. [DOI] [PubMed] [Google Scholar]
- Montgomery EB., Jr Heavy metals and the etiology of Parkinson's disease and other movement disorders. Toxicology. 1995;97:3–9. doi: 10.1016/0300-483x(94)02962-t. [DOI] [PubMed] [Google Scholar]
- Koeppen AH. The history of iron in the brain. J Neurol Sci. 1995;134:1–9. doi: 10.1016/0022-510x(95)00202-d. [DOI] [PubMed] [Google Scholar]
- Deibel MA, Ehmann WD, Markesbery WR. Copper, iron, and zinc imbalances in severely degenerated brain regions in Alzheimer's disease: possible relation to oxidative stress. J Neurol Sci. 1996;143:137–42. doi: 10.1016/s0022-510x(96)00203-1. [DOI] [PubMed] [Google Scholar]
- Gorell JM, Johnson CC, Rybicki BA, Peterson EL, Kortsha GX, Brown GG, Richardson RJ. Occupational exposures to metals as risk factors for Parkinson's disease. Neurology. 1997;48:650–8. doi: 10.1212/wnl.48.3.650. [DOI] [PubMed] [Google Scholar]
- Koeppen AH. A brief history of brain iron research. J Neurol Sci. 2003;207:95–7. doi: 10.1016/s0022-510x(02)00429-x. [DOI] [PubMed] [Google Scholar]
- Youdim MBH, Benshachar D, Riederer P. The possible role of iron in the etiopathology of Parkinsons disease. Movement Disorders. 1993;8:1–12. doi: 10.1002/mds.870080102. [DOI] [PubMed] [Google Scholar]
- Gerlach M, Benshachar D, Riederer P, Youdim MBH. Altered brain metabolism of iron as a cause of neurodegenerative diseases. J Neurochem. 1994;63:793–807. doi: 10.1046/j.1471-4159.1994.63030793.x. [DOI] [PubMed] [Google Scholar]
- Götz ME, Künig G, Riederer P, Youdim MBH. Oxidative stress – free radical production in neural degeneration. Pharmacol Therapeut. 1994;63:37–122. doi: 10.1016/0163-7258(94)90055-8. [DOI] [PubMed] [Google Scholar]
- Smith MA, Perry G, Richey PL, Sayre LM, Anderson VE, Beal MF, Kowall N. Oxidative damage in Alzheimer's. Nature. 1996;382:120–121. doi: 10.1038/382120b0. [DOI] [PubMed] [Google Scholar]
- LeVine SM. Iron deposits in multiple sclerosis and Alzheimer's disease brains. Brain Research. 1997;760:298–303. doi: 10.1016/s0006-8993(97)00470-8. [DOI] [PubMed] [Google Scholar]
- Smith MA, Harris PLR, Sayre LM, Perry G. Iron accumulation in Alzheimer disease is a source of redox-generated free radicals. Proc Natl Acad Sci. 1997;94:9866–9868. doi: 10.1073/pnas.94.18.9866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovell MA, Robertson JD, Teesdale WJ, Campbell JL, Markesbery WR. Copper, iron and zinc in Alzheimer's disease senile plaques. J Neurol Sci. 1998;158:47–52. doi: 10.1016/s0022-510x(98)00092-6. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Cummings J, Perlman S, Hance DB, Mintz J. Increased basal ganglia iron levels in Huntington disease. Arch Neurol. 1999;56:569–74. doi: 10.1001/archneur.56.5.569. [DOI] [PubMed] [Google Scholar]
- Huang XD, Cuajungco MP, Atwood CS, Hartshorn MA, Tyndall JDA, Hanson GR, Stokes KC, Leopold M, Multhaup G, Goldstein LE, Scarpa RC, Saunders AJ, Lim J, Moir RD, Glabe C, Bowden EF, Masters CL, Fairlie DP, Tanzi RE, Bush AI. Cu(II) potentiation of Alzheimer A beta neurotoxicity – Correlation with cell-free hydrogen peroxide production and metal reduction. J Biol Chem. 1999;274:37111–37116. doi: 10.1074/jbc.274.52.37111. [DOI] [PubMed] [Google Scholar]
- Jellinger KA. The role of iron in neurodegeneration – Prospects for pharmacotherapy of Parkinson's disease. Drugs & Aging. 1999;14:115–140. doi: 10.2165/00002512-199914020-00004. [DOI] [PubMed] [Google Scholar]
- Olanow CW, Tatton WG. Etiology and pathogenesis of Parkinson's disease. Annu Rev Neurosci. 1999;22:123–144. doi: 10.1146/annurev.neuro.22.1.123. [DOI] [PubMed] [Google Scholar]
- Sayre LM, Perry G, Smith MA. Redox metals and neurodegenerative disease. Current Opinion in Chemical Biology. 1999;3:220–225. doi: 10.1016/S1367-5931(99)80035-0. [DOI] [PubMed] [Google Scholar]
- Bush AI. Metals and neuroscience. Curr Opin Chem Biol. 2000;4:184–91. doi: 10.1016/s1367-5931(99)00073-3. [DOI] [PubMed] [Google Scholar]
- Chiueh CC, Andoh T, Lai AR, Lai E, Krishna G. Neuroprotective strategies in Parkinson's disease: protection against progressive nigral damage induced by free radicals. Neurotox Res. 2000;2:293–310. doi: 10.1007/BF03033799. [DOI] [PubMed] [Google Scholar]
- Christen Y. Oxidative stress and Alzheimer disease. American Journal of Clinical Nutrition. 2000;71:621S–629S. doi: 10.1093/ajcn/71.2.621s. [DOI] [PubMed] [Google Scholar]
- Floor E. Iron as a vulnerability factor in nigrostriatal degeneration in aging and Parkinson's disease. Cell Mol Biol (Noisy-le-grand) 2000;46:709–20. [PubMed] [Google Scholar]
- Smith MA, Rottkamp CA, Nunomura A, Raina AK, Perry G. Oxidative stress in Alzheimer's disease. Biochim Biophys Acta. 2000;1502:139–44. doi: 10.1016/s0925-4439(00)00040-5. [DOI] [PubMed] [Google Scholar]
- Varadarajan S, Yatin S, Aksenova M, Butterfield DA. Alzheimer's amyloid beta-peptide-associated free radical oxidative stress and neurotoxicity. J Struct Biol. 2000;130:184–208. doi: 10.1006/jsbi.2000.4274. [DOI] [PubMed] [Google Scholar]
- Berg D, Gerlach M, Youdim MBH, Double KL, Zecca L, Riederer P, Becker G. Brain iron pathways and their relevance to Parkinson's disease. J Neurochem. 2001;79:225–236. doi: 10.1046/j.1471-4159.2001.00608.x. [DOI] [PubMed] [Google Scholar]
- Blum D, Torch S, Lambeng N, Nissou MF, Benabid AL, Sadoul R, Verna JM. Molecular pathways involved in the neurotoxicity of 6-OHDA, dopamine and MPTP: contribution to the apoptotic theory in Parkinson's disease. Progress in Neurobiology. 2001;65:135–172. doi: 10.1016/s0301-0082(01)00003-x. [DOI] [PubMed] [Google Scholar]
- Campbell A, Smith MA, Sayre LM, Bondy SC, Perry G. Mechanisms by which metals promote events connected to neurodegenerative diseases. Brain Research Bulletin. 2001;55:125–132. doi: 10.1016/s0361-9230(01)00455-5. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Role of free radicals in the neurodegenerative diseases – Therapeutic implications for antioxidant treatment. Drugs & Aging. 2001;18:685–716. doi: 10.2165/00002512-200118090-00004. [DOI] [PubMed] [Google Scholar]
- HaMai D, Bondy SC, Becaria A, Campbell A. The chemistry of transition metals in relation to their potential role in neurodegenerative processes. Curr Top Med Chem. 2001;1:541–51. doi: 10.2174/1568026013394796. [DOI] [PubMed] [Google Scholar]
- Rottkamp CA, Raina AK, Zhu XW, Gaier E, Bush AI, Atwood CS, Chevion M, Perry G, Smith MA. Redox-active iron mediates amyloid-beta toxicity. Free Rad Biol Med. 2001;30:447–450. doi: 10.1016/s0891-5849(00)00494-9. [DOI] [PubMed] [Google Scholar]
- Sayre LM, Smith MA, Perry G. Chemistry and biochemistry of oxidative stress in neurodegenerative disease. Current Medicinal Chemistry. 2001;8:721–738. doi: 10.2174/0929867013372922. [DOI] [PubMed] [Google Scholar]
- Bharath S, Hsu M, Kaur D, Rajagopalan S, Andersen JK. Glutathione, iron and Parkinson's disease. Biochem Pharmacol. 2002;64:1037–48. doi: 10.1016/s0006-2952(02)01174-7. [DOI] [PubMed] [Google Scholar]
- Bishop GM, Robinson SR, Liu Q, Perry G, Atwood CS, Smith MA. Iron: a pathological mediator of Alzheimer disease? Dev Neurosci. 2002;24:184–7. doi: 10.1159/000065696. [DOI] [PubMed] [Google Scholar]
- Giasson BI, Ischiropoulos H, Lee VM, Trojanowski JQ. The relationship between oxidative/nitrative stress and pathological inclusions in Alzheimer's and Parkinson's diseases. Free Radic Biol Med. 2002;32:1264–75. doi: 10.1016/s0891-5849(02)00804-3. [DOI] [PubMed] [Google Scholar]
- Gnjec A, Fonte JA, Atwood C, Martins RN. Transition metal chelator therapy – A potential treatment for Alzheimer's disease? Frontiers in Bioscience. 2002;7:D1016–D1023. doi: 10.2741/gnjec. [DOI] [PubMed] [Google Scholar]
- Perry G, Sayre LM, Atwood CS, Castellani RJ, Cash AD, Rottkamp CA, Smith MA. The role of iron and copper in the aetiology of neurodegenerative disorders – Therapeutic implications. CNS Drugs. 2002;16:339–352. doi: 10.2165/00023210-200216050-00006. [DOI] [PubMed] [Google Scholar]
- Rao AV, Balachandran B. Role of oxidative stress and antioxidants in neurodegenerative diseases. Nutritional Neuroscience. 2002;5:291–309. doi: 10.1080/1028415021000033767. [DOI] [PubMed] [Google Scholar]
- Burdo JR, Connor JR. Brain iron uptake and homeostatic mechanisms: An overview. Biometals. 2003;16:63–75. doi: 10.1023/a:1020718718550. [DOI] [PubMed] [Google Scholar]
- Bush AI. The metallobiology of Alzheimer's disease. Trends Neurosci. 2003;26:207–214. doi: 10.1016/S0166-2236(03)00067-5. [DOI] [PubMed] [Google Scholar]
- Finefrock AE, Bush AI, Doraiswamy PM. Current status of metals as therapeutic targets in Alzheimer's disease. J Am Geriatr Soc. 2003;51:1143–8. doi: 10.1046/j.1532-5415.2003.51368.x. [DOI] [PubMed] [Google Scholar]
- Ke Y, Qian ZM. Iron misregulation in the brain: a primary cause of neurodegenerative disorders. Lancet Neurol. 2003;2:246–253. doi: 10.1016/s1474-4422(03)00353-3. [DOI] [PubMed] [Google Scholar]
- Barnham KJ, Masters CL, Bush AI. Neurodegenerative diseases and oxidative stress. Nat Rev Drug Discov. 2004;3:205–14. doi: 10.1038/nrd1330. [DOI] [PubMed] [Google Scholar]
- Bondy SC, Lahiri DK, Perreau VM, Sharman KZ, Campbell A, Zhou J, Sharman EH. Retardation of brain aging by chronic treatment with melatonin. Ann N Y Acad Sci. 2004;1035:197–215. doi: 10.1196/annals.1332.013. [DOI] [PubMed] [Google Scholar]
- Casadesus G, Smith MA, Zhu X, Aliev G, Cash AD, Honda K, Petersen RB, Perry G. Alzheimer disease: evidence for a central pathogenic role of iron-mediated reactive oxygen species. J Alzheimers Dis. 2004;6:165–9. doi: 10.3233/jad-2004-6208. [DOI] [PubMed] [Google Scholar]
- Doraiswamy PM, Finefrock AE. Metals in our minds: therapeutic implications for neurodegenerative disorders. Lancet Neurology. 2004;3:431–434. doi: 10.1016/S1474-4422(04)00809-9. [DOI] [PubMed] [Google Scholar]
- Gotz ME, Double K, Gerlach M, Youdim MBH, Riederer P. The relevance of iron in the pathogenesis of Parkinson's disease. Redox-Active Metals in Neurological Disorders, Annals of the New York Academy of Sciences. 2004;1012:193–208. doi: 10.1196/annals.1306.017. [DOI] [PubMed] [Google Scholar]
- Huang X, Moir RD, Tanzi RE, Bush AI, Rogers JT. Redox-active metals, oxidative stress, and Alzheimer's disease pathology. Ann N Y Acad Sci. 2004;1012:153–63. doi: 10.1196/annals.1306.012. [DOI] [PubMed] [Google Scholar]
- Kalivendi SV, Cunningham S, Kotamraju S, Joseph J, Hillard CJ, Kalyanaraman B. alpha-synuclein up-regulation and aggregation during MPP+-induced apoptosis in neuroblastoma cells – Intermediacy of transferrin receptor iron and hydrogen peroxide. Journal of Biological Chemistry. 2004;279:15240–15247. doi: 10.1074/jbc.M312497200. [DOI] [PubMed] [Google Scholar]
- Kaur D, Andersen J. Does cellular iron dysregulation play a causative role in Parkinson's disease? Ageing Res Rev. 2004;3:327–343. doi: 10.1016/j.arr.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Metal-catalyzed disruption of membrane protein and lipid signaling in the pathogenesis of neurodegenerative disorders. Ann N Y Acad Sci. 2004;1012:37–50. doi: 10.1196/annals.1306.004. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Pathways towards and away from Alzheimer's disease. Nature. 2004;430:631–9. doi: 10.1038/nature02621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milton NGN. Role of hydrogen peroxide in the aetiology of Alzheimer's disease – Implications for treatment. Drugs & Aging. 2004;21:81–100. doi: 10.2165/00002512-200421020-00002. [DOI] [PubMed] [Google Scholar]
- Moos T, Morgan EH. The metabolism of neuronal iron and its pathogenic role in neurological disease – Review. Ann NY Acad Sci. 2004;1012:14–26. doi: 10.1196/annals.1306.002. [DOI] [PubMed] [Google Scholar]
- Poon HF, Calabrese V, Scapagnini G, Butterfield DA. Free radicals and brain aging. Clin Geriatr Med. 2004;20:329–59. doi: 10.1016/j.cger.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Richardson DR. Novel chelators for central nervous system disorders that involve alterations in the metabolism of iron and other metal ions. Ann N Y Acad Sci. 2004;1012:326–41. doi: 10.1196/annals.1306.026. [DOI] [PubMed] [Google Scholar]
- Warner DS, Sheng H, Batinić-Haberle I. Oxidants, antioxidants and the ischemic brain. J Exp Biol. 2004;207:3221–31. doi: 10.1242/jeb.01022. [DOI] [PubMed] [Google Scholar]
- Youdim MBH, Fridkin M, Zheng H. Novel bifunctional drugs targeting monoamine oxidase inhibition and iron chelation as an approach to neuroprotection in Parkinson's disease and other neurodegenerative diseases. J Neural Transmission. 2004;111:1455–1471. doi: 10.1007/s00702-004-0143-x. [DOI] [PubMed] [Google Scholar]
- Zecca L, Youdim MBH, Riederer P, Connor JR, Crichton RR. Iron, brain ageing and neurodegenerative disorders. Nat Rev Neurosci. 2004;5:863–873. doi: 10.1038/nrn1537. [DOI] [PubMed] [Google Scholar]
- Zhu X, Raina AK, Lee HG, Casadesus G, Smith MA, Perry G. Oxidative stress signalling in Alzheimer's disease. Brain Res. 2004;1000:32–9. doi: 10.1016/j.brainres.2004.01.012. [DOI] [PubMed] [Google Scholar]
- Calabrese V, Lodi R, Tonon C, D'Agata V, Sapienza M, Scapagnini G, Mangiameli A, Pennisi G, Stella AMG, Butterfield DA. Oxidative stress, mitochondrial dysfunction and cellular stress response in Friedreich's ataxia. Journal of the Neurological Sciences. 2005;233:145–162. doi: 10.1016/j.jns.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Gaeta A, Hider RC. The crucial role of metal ions in neurodegeneration: the basis for a promising therapeutic strategy. Br J Pharmacol. 2005;146:1041–59. doi: 10.1038/sj.bjp.0706416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hald A, Lotharius J. Oxidative stress and inflammation in Parkinson's disease: Is there a causal link? Exp Neurol. 2005;193:279–290. doi: 10.1016/j.expneurol.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Honda K, Smith MA, Zhu X, Baus D, Merrick WC, Tartakoff AM, Hattier T, Harris PL, Siedlak SL, Fujioka H, Liu Q, Moreira PI, Miller FP, Nunomura A, Shimohama S, Perry G. Ribosomal RNA in Alzheimer disease is oxidized by bound redox-active iron. J Biol Chem. 2005;280:20978–86. doi: 10.1074/jbc.M500526200. [DOI] [PubMed] [Google Scholar]
- Ide-Ektessabi A, Rabionet M. The role of trace metallic elements in neurodegenerative disorders: quantitative analysis using XRF and XANES spectroscopy. Anal Sci. 2005;21:885–92. doi: 10.2116/analsci.21.885. [DOI] [PubMed] [Google Scholar]
- Liu G, Garrett MR, Men P, Zhu XW, Perry G, Smith MA. Nanoparticle and other metal chelation therapeutics in Alzheimer disease. Biochimica Et Biophysica Acta-Molecular Basis of Disease. 2005;1741:246–252. doi: 10.1016/j.bbadis.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Maynard CJ, Bush AI, Masters CL, Cappai R, Li QX. Metals and amyloid-beta in Alzheimer's disease. Int J Exp Pathol. 2005;86:147–159. doi: 10.1111/j.0959-9673.2005.00434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong WY, Farooqui AA. Iron, neuroinflammation, and Alzheimer's disease. J Alzheimers Dis. 2005;8:183–200. doi: 10.3233/jad-2005-8211. [DOI] [PubMed] [Google Scholar]
- Adlard PA, Bush AI. Metals and Alzheimer's disease. J Alzheimers Dis. 2006;10:145–163. doi: 10.3233/jad-2006-102-303. [DOI] [PubMed] [Google Scholar]
- Aracena P, Aguirre P, Munoz P, Nunez MT. Iron and glutathione at the crossroad of redox metabolism in neurons. Biol Res. 2006;39:157–65. doi: 10.4067/s0716-97602006000100017. [DOI] [PubMed] [Google Scholar]
- Berg D, Hochstrasser H. Iron metabolism in parkinsonian syndromes. Movement Disorders. 2006;21:1299–1310. doi: 10.1002/mds.21020. [DOI] [PubMed] [Google Scholar]
- Collingwood J, Dobson J. Mapping and characterization of iron compounds in Alzheimer's tissue. J Alzheimers Dis. 2006;10:215–222. doi: 10.3233/jad-2006-102-308. [DOI] [PubMed] [Google Scholar]
- Fasano M, Bergamasco B, Lopiano L. Modifications of the iron-neuromelanin system in Parkinson's disease. J Neurochem. 2006;96:909–916. doi: 10.1111/j.1471-4159.2005.03638.x. [DOI] [PubMed] [Google Scholar]
- Gaggelli E, Kozlowski H, Valensin D, Valensin G. Copper homeostasis and neurodegenerative disorders (Alzheimer's, prion, and Parkinson's diseases and amyotrophic lateral sclerosis) Chem Rev. 2006;106:1995–2044. doi: 10.1021/cr040410w. [DOI] [PubMed] [Google Scholar]
- Gerlach M, Double KL, Youdim MBH, Riederer P. Potential sources of increased iron in the substantia nigra of parkinsonian patients. J Neural Transmission – Suppl. 2006. pp. 133–142. [DOI] [PubMed]
- Halliwell B. Oxidative stress and neurodegeneration: where are we now? Journal of Neurochemistry. 2006;97:1634–1658. doi: 10.1111/j.1471-4159.2006.03907.x. [DOI] [PubMed] [Google Scholar]
- Huber A, Stuchbury G, Burkle A, Burnell J, Munch G. Neuroprotective therapies for Alzheimer's disease. Curr Pharmaceut Des. 2006;12:705–717. doi: 10.2174/138161206775474251. [DOI] [PubMed] [Google Scholar]
- Liu GJ, Huang WD, Moir RD, Vanderburg CR, Lai B, Peng ZC, Tanzi RE, Rogers JT, Huang XD. Metal exposure and Alzheimer's pathogenesis. J Struct Biol. 2006;155:45–51. doi: 10.1016/j.jsb.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Lee DW, Andersen JK, Kaur D. Iron dysregulation and neurodegeneration – The molecular connection. Molecular Interventions. 2006;6:89–97. doi: 10.1124/mi.6.2.6. [DOI] [PubMed] [Google Scholar]
- Mancuso M, Coppede F, Migliore L, Siciliano G, Murri L. Mitochondrial dysfunction, oxidative stress and neurodegeneration. Journal of Alzheimers Disease. 2006;10:59–73. doi: 10.3233/jad-2006-10110. [DOI] [PubMed] [Google Scholar]
- Mosley RL, Benner EJ, Kadiu I, Thomas M, Boska MD, Hasan K, Laurie C, Gendelman HE. Neuroinflammation, oxidative stress, and the pathogenesis of Parkinson's disease. Clinical Neuroscience Research. 2006;6:261–281. doi: 10.1016/j.cnr.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana C, Bellefqih S, Laval JY, Guerquin-Kern JL, Wu TD, Avila J, Ferrer I, Arranz R, Patino C. Study of the localization of iron, ferritin, and hemosiderin in Alzheimer's disease hippocampus by analytical microscopy at the subcellular level. J Struct Biol. 2006;153:42–54. doi: 10.1016/j.jsb.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Whitnall M, Richardson DR. Iron: a new target for pharmacological intervention in neurodegenerative diseases. Semin Pediatr Neurol. 2006;13:186–97. doi: 10.1016/j.spen.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Zhang HY. Same causes, same cures. Biochem Biophys Res Commun. 2006;351:578–581. doi: 10.1016/j.bbrc.2006.10.086. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Tishler TA, Lu PH, Villablanca P, Altshuler LL, Carter M, Huang D, Edwards N, Mintz J. Brain ferritin iron may influence age- and gender-related risks of neurodegeneration. Neurobiol Aging. 2007;28:414–23. doi: 10.1016/j.neurobiolaging.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Bondy SC, Sharman EH. Melatonin and the aging brain. Neurochem Int. 2007;50:571–80. doi: 10.1016/j.neuint.2006.12.014. [DOI] [PubMed] [Google Scholar]
- Cass WA, Grondin R, Andersen AH, Zhang Z, Hardy PA, Hussey-Andersen LK, Rayens WS, Gerhardt GA, Gash DM. Iron accumulation in the striatum predicts aging-related decline in motor function in rhesus monkeys. Neurobiol Aging. 2007;28:258–71. doi: 10.1016/j.neurobiolaging.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Castellani RJ, Moreira PI, Liu G, Dobson J, Perry G, Smith MA, Zhu X. Iron: the Redox-active center of oxidative stress in Alzheimer disease. Neurochem Res. 2007;32:1640–5. doi: 10.1007/s11064-007-9360-7. [DOI] [PubMed] [Google Scholar]
- Crouch PJ, White AR, Bush AI. The modulation of metal bio-availability as a therapeutic strategy for the treatment of Alzheimer's disease. FEBS J. 2007;274:3775–83. doi: 10.1111/j.1742-4658.2007.05918.x. [DOI] [PubMed] [Google Scholar]
- Donnelly PS, Xiao Z, Wedd AG. Copper and Alzheimer's disease. Curr Opin Chem Biol. 2007;11:128–33. doi: 10.1016/j.cbpa.2007.01.678. [DOI] [PubMed] [Google Scholar]
- Gaasch JA, Lockman PR, Geldenhuys WJ, Allen DD, Schyf CJ Van der. Brain iron toxicity: differential responses of astrocytes, neurons, and endothelial cells. Neurochem Res. 2007;32:1196–208. doi: 10.1007/s11064-007-9290-4. [DOI] [PubMed] [Google Scholar]
- Ke Y, Qian ZM. Brain iron metabolism: neurobiology and neurochemistry. Prog Neurobiol. 2007;83:149–73. doi: 10.1016/j.pneurobio.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Mancuso C, Scapagnini G, Curro D, Stella AMG, De Marco C, Butterfield DA, Calabrese V. Mitochondrial dysfunction, free radical generation and cellular stress response in neurodegenerative disorders. Frontiers in Bioscience. 2007;12:1107–1123. doi: 10.2741/2130. [DOI] [PubMed] [Google Scholar]
- Mandel S, Amit T, Bar-Am O, Youdim MB. Iron dysregulation in Alzheimer's disease: multimodal brain permeable iron chelating drugs, possessing neuroprotective-neurorescue and amyloid precursor protein-processing regulatory activities as therapeutic agents. Prog Neurobiol. 2007;82:348–60. doi: 10.1016/j.pneurobio.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Molina-Holgado F, Hider RC, Gaeta A, Williams R, Francis P. Metals ions and neurodegeneration. Biometals. 2007;20:639–54. doi: 10.1007/s10534-006-9033-z. [DOI] [PubMed] [Google Scholar]
- Petersen RB, Nunomura A, Lee HG, Casadesus G, Perry G, Smith MA, Zhu X. Signal transduction cascades associated with oxidative stress in Alzheimer's disease. J Alzheimers Dis. 2007;11:143–52. doi: 10.3233/jad-2007-11202. [DOI] [PubMed] [Google Scholar]
- Reynolds A, Laurie C, Mosley RL, Gendelman HE. Oxidative stress and the pathogenesis of neurodegenerative disorders. Int Rev Neurobiol. 2007;82:297–325. doi: 10.1016/S0074-7742(07)82016-2. [DOI] [PubMed] [Google Scholar]
- Singh C, Ahmad I, Kumar A. Pesticides and metals induced Parkinson's disease: involvement of free radicals and oxidative stress. Cell Mol Biol (Noisy-le-grand) 2007;53:19–28. [PubMed] [Google Scholar]
- Stankiewicz J, Panter SS, Neema M, Arora A, Batt CE, Bakshi R. Iron in chronic brain disorders: imaging and neurotherapeutic implications. Neurotherapeutics. 2007;4:371–86. doi: 10.1016/j.nurt.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Su B, Wang X, Smith MA, Perry G. Causes of oxidative stress in Alzheimer disease. Cell Mol Life Sci. 2007;64:2202–10. doi: 10.1007/s00018-007-7218-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amit T, Avramovich-Tirosh Y, Youdim MB, Mandel S. Targeting multiple Alzheimer's disease etiologies with multimodal neuroprotective and neurorestorative iron chelators. FASEB J. 2008;22:1296–305. doi: 10.1096/fj.07-8627rev. [DOI] [PubMed] [Google Scholar]
- Bush AI, Curtain CC. Twenty years of metallo-neurobiology: where to now? Eur Biophys J. 2008;37:241–5. doi: 10.1007/s00249-007-0228-1. [DOI] [PubMed] [Google Scholar]
- Drechsel DA, Patel M. Role of reactive oxygen species in the neurotoxicity of environmental agents implicated in Parkinson's disease. Free Radic Biol Med. 2008;44:1873–86. doi: 10.1016/j.freeradbiomed.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankhurst Q, Hautot D, Khan N, Dobson J. Increased levels of magnetic iron compounds in Alzheimer's disease. J Alzheimers Dis. 2008;13:49–52. doi: 10.3233/jad-2008-13105. [DOI] [PubMed] [Google Scholar]
- Rogers JT, Bush AI, Cho HH, Smith DH, Thomson AM, Friedlich AL, Lahiri DK, Leedman PJ, Huang X, Cahill CM. Iron and the translation of the amyloid precursor protein (APP) and ferritin mRNAs: riboregulation against neural oxidative damage in Alzheimer's disease. Biochem Soc Trans. 2008;36:1282–7. doi: 10.1042/BST0361282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvestri L, Camaschella C. A potential pathogenetic role of iron in Alzheimer's Disease. J Cell Mol Med. 2008;12:1548–1550. doi: 10.1111/j.1582-4934.2008.00356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder AM, Connor JR. Iron, the substantia nigra and related neurological disorders. Biochim Biophys Acta. 2008 doi: 10.1016/j.bbagen.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Szczerbowska-Boruchowska M. X-ray fluorescence spectrometry, an analytical tool in neurochemical research. X-Ray Spectrometry. 2008;37:21–31. [Google Scholar]
- Sayre LM, Perry G, Harris PLR, Liu YH, Schubert KA, Smith MA. In situ oxidative catalysis by neurofibrillary tangles and senile plaques in Alzheimer's disease: A central role for bound transition metals. Journal of Neurochemistry. 2000;74:270–279. doi: 10.1046/j.1471-4159.2000.0740270.x. [DOI] [PubMed] [Google Scholar]
- Vélez-Pardo C, Del Río MJ, Verschueren H, Ebinger G, Vauquelin G. Dopamine and iron induce apoptosis in PC12 cells. Pharmacol Toxicol. 1997;80:76–84. doi: 10.1111/j.1600-0773.1997.tb00287.x. [DOI] [PubMed] [Google Scholar]
- Kostoff RN, Briggs MB. Literature-Related Discovery (LRD): potential treatments for Parkinson's disease. Technol Forecast Soc Change. 2007. [DOI] [PMC free article] [PubMed]
- LeVine SM, Chakrabarty A. The role of iron in the pathogenesis of experimental allergic encephalomyelitis and multiple sclerosis. Redox-Active Metals in Neurological Disorders, Annals of the New York Academy of Sciences. 2004;1012:252–266. doi: 10.1196/annals.1306.021. [DOI] [PubMed] [Google Scholar]
- Kotze MJ, de Villiers JNP, Rooney RN, Grobbelaar JJ, Mansvelt EPG, Bouwens CSH, Carr J, Stander I, du Plessis L. Analysis of the NRAMP1 gene implicated in iron transport: Association with multiple sclerosis and age effects. Blood Cells Molecules and Diseases. 2001;27:44–53. doi: 10.1006/bcmd.2000.0349. [DOI] [PubMed] [Google Scholar]
- LeVine SM, Lynch SG, Ou CN, Wulser MJ, Tam E, Boo N. Ferritin, transferrin and iron concentrations in the cerebrospinal fluid of multiple sclerosis patients. Brain Research. 1999;821:511–515. doi: 10.1016/s0006-8993(98)01360-2. [DOI] [PubMed] [Google Scholar]
- Valberg LS, Flanagan PR, Kertesz A, Ebers GC. Abnormalities in Iron-Metabolism in Multiple-Sclerosis. Canadian Journal of Neurological Sciences. 1989;16:184–186. doi: 10.1017/s0317167100028869. [DOI] [PubMed] [Google Scholar]
- Adams CWM. Perivascular Iron Deposition and Other Vascular Damage in Multiple-Sclerosis. Journal of Neurology Neurosurgery and Psychiatry. 1988;51:260–265. doi: 10.1136/jnnp.51.2.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drayer B, Burger P, Hurwitz B, Dawson D, Cain J. Reduced Signal Intensity on Mr Images of Thalamus and Putamen in Multiple-Sclerosis – Increased Iron Content. American Journal of Neuroradiology. 1987;8:413–419. doi: 10.2214/ajr.149.2.357. [DOI] [PubMed] [Google Scholar]
- Craelius W, Migdal MW, Luessenhop CP, Sugar A, Mihalakis I. Iron Deposits Surrounding Multiple-Sclerosis Plaques. Archives of Pathology & Laboratory Medicine. 1982;106:397–399. [PubMed] [Google Scholar]
- Ge Y, Jensen JH, Lu H, Helpern JA, Miles L, Inglese M, Babb JS, Herbert J, Grossman RI. Quantitative assessment of iron accumulation in the deep gray matter of multiple sclerosis by magnetic field correlation imaging. AJNR Am J Neuroradiol. 2007;28:1639–44. doi: 10.3174/ajnr.A0646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim NH, Choi JK, Jeong BH, Kim JI, Kwon MS, Carp RI, Kim YS. Effect of transition metals (Mn, Cu, Fe) and deoxycholic acid (DA) on the conversion of PrPC to PrPres. FASEB J. 2005;19:783–5. doi: 10.1096/fj.04-2117fje. [DOI] [PubMed] [Google Scholar]
- Basu S, Mohan ML, Luo X, Kundu B, Kong Q, Singh N. Modulation of proteinase K-resistant prion protein in cells and infectious brain homogenate by redox iron: implications for prion replication and disease pathogenesis. Mol Biol Cell. 2007;18:3302–12. doi: 10.1091/mbc.E07-04-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lill R, Muhlenhoff U. Maturation of iron-sulfur proteins in eukaryotes: mechanisms, connected processes, and diseases. Annu Rev Biochem. 2008;77:669–700. doi: 10.1146/annurev.biochem.76.052705.162653. [DOI] [PubMed] [Google Scholar]
- Cavadini P, O'Neill HA, Benada O, Isaya G. Assembly and iron-binding properties of human frataxin, the protein deficient in Friedreich ataxia. Hum Mol Genet. 2002;11:217–27. doi: 10.1093/hmg/11.3.217. [DOI] [PubMed] [Google Scholar]
- Napier I, Ponka P, Richardson DR. Iron trafficking in the mitochondrion: novel pathways revealed by disease. Blood. 2005;105:1867–74. doi: 10.1182/blood-2004-10-3856. [DOI] [PubMed] [Google Scholar]
- O'Neill HA, Gakh O, Park S, Cui J, Mooney SM, Sampson M, Ferreira GC, Isaya G. Assembly of human frataxin is a mechanism for detoxifying redox-active iron. Biochemistry. 2005;44:537–45. doi: 10.1021/bi048459j. [DOI] [PubMed] [Google Scholar]
- Gakh O, Park S, Liu G, Macomber L, Imlay JA, Ferreira GC, Isaya G. Mitochondrial iron detoxification is a primary function of frataxin that limits oxidative damage and preserves cell longevity. Human Molecular Genetics. 2006;15:467–479. doi: 10.1093/hmg/ddi461. [DOI] [PubMed] [Google Scholar]
- Napoli E, Taroni F, Cortopassi GA. Frataxin, iron-sulfur clusters, heme, ROS, and aging. Antioxid Redox Signal. 2006;8:506–16. doi: 10.1089/ars.2006.8.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babady NE, Carelle N, Wells RD, Rouault TA, Hirano M, Lynch DR, Delatycki MB, Wilson RB, Isaya G, Puccio H. Advancements in the pathophysiology of Friedreich's Ataxia and new prospects for treatments. Mol Genet Metab. 2007;92:23–35. doi: 10.1016/j.ymgme.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, Cortopassi G. Frataxin knockdown causes loss of cytoplasmic iron-sulfur cluster functions, redox alterations and induction of heme transcripts. Arch Biochem Biophys. 2007;457:111–22. doi: 10.1016/j.abb.2006.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanella I, Derosas M, Corrado M, Cocco E, Cavadini P, Biasiotto G, Poli M, Verardi R, Arosio P. The effects of frataxin silencing in HeLa cells are rescued by the expression of human mitochondrial ferritin. Biochim Biophys Acta. 2008;1782:90–8. doi: 10.1016/j.bbadis.2007.11.006. [DOI] [PubMed] [Google Scholar]
- Anderson PR, Kirby K, Hilliker AJ, Phillips JP. RNAi-mediated suppression of the mitochondrial iron chaperone, frataxin, in Drosophila. Hum Mol Genet. 2005;14:3397–405. doi: 10.1093/hmg/ddi367. [DOI] [PubMed] [Google Scholar]
- Jo WJ, Loguinov A, Chang M, Wintz H, Nislow C, Arkin AP, Giaever G, Vulpe CD. Identification of genes involved in the toxic response of Saccharomyces cerevisiae against iron and copper overload by parallel analysis of deletion mutants. Toxicol Sci. 2008;101:140–51. doi: 10.1093/toxsci/kfm226. [DOI] [PubMed] [Google Scholar]
- Gibson TJ, Koonin EV, Musco G, Pastore A, Bork P. Friedreich's ataxia protein: phylogenetic evidence for mitochondrial dysfunction. Trends Neurosci. 1996;19:465–8. doi: 10.1016/S0166-2236(96)20054-2. [DOI] [PubMed] [Google Scholar]
- Koutnikova H, Campuzano V, Foury F, Dolle P, Cazzalini O, Koenig M. Studies of human, mouse and yeast homologues indicate a mitochondrial function for frataxin. Nat Genet. 1997;16:345–51. doi: 10.1038/ng0897-345. [DOI] [PubMed] [Google Scholar]
- Foury F. Human genetic diseases: A cross-talk between man and yeast. Gene. 1997;195:1–10. doi: 10.1016/s0378-1119(97)00140-6. [DOI] [PubMed] [Google Scholar]
- Foury F, Cazzalini O. Deletion of the yeast homologue of the human gene associated with Friedreich's ataxia elicits iron accumulation in mitochondria. FEBS Lett. 1997;411:373–377. doi: 10.1016/s0014-5793(97)00734-5. [DOI] [PubMed] [Google Scholar]
- Cavadini P, Gellera C, Patel PI, Isaya G. Human frataxin maintains mitochondrial iron homeostasis in Saccharomyces cerevisiae. Hum Mol Genet. 2000;9:2523–30. doi: 10.1093/hmg/9.17.2523. [DOI] [PubMed] [Google Scholar]
- Becker E, Richardson DR. Frataxin: its role in iron metabolism and the pathogenesis of Friedreich's ataxia. Int J Biochem Cell Biol. 2001;33:1–10. doi: 10.1016/s1357-2725(00)00067-4. [DOI] [PubMed] [Google Scholar]
- Foury F, Talibi D. Mitochondrial control of iron homeostasis. A genome wide analysis of gene expression in a yeast frataxin-deficient strain. J Biol Chem. 2001;276:7762–8. doi: 10.1074/jbc.M005804200. [DOI] [PubMed] [Google Scholar]
- De Freitas J, Wintz H, Kim JH, Poynton H, Fox T, Vulpe C. Yeast, a model organism for iron and copper metabolism studies. Biometals. 2003;16:185–197. doi: 10.1023/a:1020771000746. [DOI] [PubMed] [Google Scholar]
- González-Cabo P, Vázquez-Manrique RP, García-Gimeno MA, Sanz P, Palau F. Frataxin interacts functionally with mitochondrial electron transport chain proteins. Hum Mol Genet. 2005;14:2091–8. doi: 10.1093/hmg/ddi214. [DOI] [PubMed] [Google Scholar]
- Irazusta V, Cabiscol E, Reverter-Branchat G, Ros J, Tamarit J. Manganese is the link between frataxin and iron-sulfur deficiency in the yeast model of Friedreich ataxia. J Biol Chem. 2006;281:12227–32. doi: 10.1074/jbc.M511649200. [DOI] [PubMed] [Google Scholar]
- Vázquez-Manrique RP, González-Cabo P, Ros S, Aziz H, Baylis HA, Palau F. Reduction of Caenorhabditis elegans frataxin increases sensitivity to oxidative stress, reduces lifespan, and causes lethality in a mitochondrial complex II mutant. Faseb J. 2006;20:172–4. doi: 10.1096/fj.05-4212fje. [DOI] [PubMed] [Google Scholar]
- Irazusta V, Moreno-Cermeno A, Cabiscol E, Ros J, Tamarit J. Major targets of iron-induced protein oxidative damage in frataxin-deficient yeasts are magnesium-binding proteins. Free Radic Biol Med. 2008 doi: 10.1016/j.freeradbiomed.2008.01.014. [DOI] [PubMed] [Google Scholar]
- Campuzano V, Montermini L, Molto MD, Pianese L, Cossee M, Cavalcanti F, Monros E, Rodius F, Duclos F, Monticelli A, Zara F, Canizares J, Koutnikova H, Bidichandani SI, Gellera C, Brice A, Trouillas P, De Michele G, Filla A, De Frutos R, Palau F, Patel PI, Di Donato S, Mandel JL, Cocozza S, Koenig M, Pandolfo M. Friedreich's ataxia: autosomal recessive disease caused by an intronic GAA triplet repeat expansion. Science. 1996;271:1423–7. doi: 10.1126/science.271.5254.1423. [DOI] [PubMed] [Google Scholar]
- Palau F. Friedreich's ataxia and frataxin: molecular genetics, evolution and pathogenesis (Review) Int J Mol Med. 2001;7:581–9. doi: 10.3892/ijmm.7.6.581. [DOI] [PubMed] [Google Scholar]
- Patel PI, Isaya G. Friedreich ataxia: from GAA triplet-repeat expansion to frataxin deficiency. Am J Hum Genet. 2001;69:15–24. doi: 10.1086/321283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RB. Frataxin and frataxin deficiency in Friedreich's ataxia. J Neurol Sci. 2003;207:103–5. doi: 10.1016/s0022-510x(02)00432-x. [DOI] [PubMed] [Google Scholar]
- Michael S, Petrocine SV, Qian J, Lamarche JB, Knutson MD, Garrick MD, Koeppen AH. Iron and iron-responsive proteins in the cardiomyopathy of Friedreich's ataxia. Cerebellum. 2006;5:257–67. doi: 10.1080/14734220600913246. [DOI] [PubMed] [Google Scholar]
- Rötig A, Sidi D, Munnich A, Rustin P. Molecular insights into Friedreich's ataxia and antioxidant-based therapies. Trends Mol Med. 2002;8:221–4. doi: 10.1016/s1471-4914(02)02330-4. [DOI] [PubMed] [Google Scholar]
- Boddaert N, Le Quan Sang KH, Rotig A, Leroy-Willig A, Gallet S, Brunelle F, Sidi D, Thalabard JC, Munnich A, Cabantchik ZI. Selective iron chelation in Friedreich ataxia: biologic and clinical implications. Blood. 2007;110:401–8. doi: 10.1182/blood-2006-12-065433. [DOI] [PubMed] [Google Scholar]
- Sohn YS, Breuer W, Munnich A, Cabantchik ZI. Redistribution of accumulated cell iron: a modality of chelation with therapeutic implications. Blood. 2008;111:1690–9. doi: 10.1182/blood-2007-07-102335. [DOI] [PubMed] [Google Scholar]
- Besarab A, Bolton WK, Browne JK, Egrie JC, Nissenson AR, Okamoto DM, Schwab SJ, Goodkin DA. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med. 1998;339:584–90. doi: 10.1056/NEJM199808273390903. [DOI] [PubMed] [Google Scholar]
- Campuzano V, Montermini L, Lutz Y, Cova L, Hindelang C, Jiralerspong S, Trottier Y, Kish SJ, Faucheux B, Trouillas P, Authier FJ, Durr A, Mandel JL, Vescovi A, Pandolfo M, Koenig M. Frataxin is reduced in Friedreich ataxia patients and is associated with mitochondrial membranes. Hum Mol Genet. 1997;6:1771–80. doi: 10.1093/hmg/6.11.1771. [DOI] [PubMed] [Google Scholar]
- Wong A, Yang J, Cavadini P, Gellera C, Lonnerdal B, Taroni F, Cortopassi G. The Friedreich's ataxia mutation confers cellular sensitivity to oxidant stress which is rescued by chelators of iron and calcium and inhibitors of apoptosis. Hum Mol Genet. 1999;8:425–30. doi: 10.1093/hmg/8.3.425. [DOI] [PubMed] [Google Scholar]
- Park S, Gakh O, Mooney SM, Isaya G. The ferroxidase activity of yeast frataxin. J Biol Chem. 2002;277:38589–95. doi: 10.1074/jbc.M206711200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm B, Bistrich U, Schranzhofer M, Sarsero JP, Rauen U, Scheiber-Mojdehkar B, de Groot H, Ioannou P, Petrat F. Friedreich's ataxia, no changes in mitochondrial labile iron in human lymphoblasts and fibroblasts: a decrease in antioxidative capacity? J Biol Chem. 2005;280:6701–8. doi: 10.1074/jbc.M408717200. [DOI] [PubMed] [Google Scholar]
- Anderson PR, Kirby K, Orr WC, Hilliker AJ, Phillips JP. Hydrogen peroxide scavenging rescues frataxin deficiency in a Drosophila model of Friedreich's ataxia. Proc Natl Acad Sci USA. 2008;105:611–6. doi: 10.1073/pnas.0709691105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RB, Roof DM. Respiratory deficiency due to loss of mitochondrial DNA in yeast lacking the frataxin homologue. Nat Genet. 1997;16:352–7. doi: 10.1038/ng0897-352. [DOI] [PubMed] [Google Scholar]
- Seznec H, Simon D, Bouton C, Reutenauer L, Hertzog A, Golik P, Procaccio V, Patel M, Drapier JC, Koenig M, Puccio H. Friedreich ataxia: the oxidative stress paradox. Hum Mol Genet. 2005;14:463–74. doi: 10.1093/hmg/ddi042. [DOI] [PubMed] [Google Scholar]
- Visapää I, Fellman V, Vesa J, Dasvarma A, Hutton JL, Kumar V, Payne GS, Makarow M, Van Coster R, Taylor RW, Turnbull DM, Suomalainen A, Peltonen L. GRACILE syndrome, a lethal metabolic disorder with iron overload, is caused by a point mutation in BCS1L. Am J Hum Genet. 2002;71:863–76. doi: 10.1086/342773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellman V, Lemmela S, Sajantila A, Pihko H, Jarvela I. Screening of BCS1L mutations in severe neonatal disorders suspicious for mitochondrial cause. J Hum Genet. 2008;53:554–8. doi: 10.1007/s10038-008-0284-0. [DOI] [PubMed] [Google Scholar]
- McGeer PL, McGeer EG. Inflammatory processes in amyotrophic lateral sclerosis. Muscle Nerve. 2002;26:459–70. doi: 10.1002/mus.10191. [DOI] [PubMed] [Google Scholar]
- Garber K. The elusive ALS genes. Science. 2008;319:20. doi: 10.1126/science.319.5859.20. [DOI] [PubMed] [Google Scholar]
- Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, Donaldson D, Goto J, O'Regan JP, Deng HX, et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362:59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- Kruman II, Pedersen WA, Springer JE, Mattson MP. ALS-linked Cu/Zn-SOD mutation increases vulnerability of motor neurons to excitotoxicity by a mechanism involving increased oxidative stress and perturbed calcium homeostasis. Exp Neurol. 1999;160:28–39. doi: 10.1006/exnr.1999.7190. [DOI] [PubMed] [Google Scholar]
- Bruijn LI, Miller TM, Cleveland DW. Unraveling the mechanisms involved in motor neuron degeneration in ALS. Annu Rev Neurosci. 2004;27:723–49. doi: 10.1146/annurev.neuro.27.070203.144244. [DOI] [PubMed] [Google Scholar]
- Kasarskis EJ, Tandon L, Lovell MA, Ehmann WD. Aluminum, calcium, and iron in the spinal cord of patients with sporadic amyotrophic lateral sclerosis using laser microprobe mass spectroscopy: a preliminary study. J Neurol Sci. 1995;130:203–8. doi: 10.1016/0022-510x(95)00037-3. [DOI] [PubMed] [Google Scholar]
- Carri MT, Ferri A, Cozzolino M, Calabrese L, Rotilio G. Neurodegeneration in amyotrophic lateral sclerosis: the role of oxidative stress and altered homeostasis of metals. Brain Res Bull. 2003;61:365–74. doi: 10.1016/s0361-9230(03)00179-5. [DOI] [PubMed] [Google Scholar]
- Carri MT, Grignaschi G, Bendotti C. Targets in ALS: designing multidrug therapies. Trends Pharmacol Sci. 2006;27:267–73. doi: 10.1016/j.tips.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Hughes KA, Reynolds RM. Evolutionary and mechanistic theories of aging. Annual Review of Entomology. 2005;50:421–445. doi: 10.1146/annurev.ento.50.071803.130409. [DOI] [PubMed] [Google Scholar]
- Zacharski LR, Ornstein DL, Woloshin S, Schwartz LM. Association of age, sex, and race with body iron stores in adults: analysis of NHANES III data. Am Heart J. 2000;140:98–104. doi: 10.1067/mhj.2000.106646. [DOI] [PubMed] [Google Scholar]
- Ritchie RF, Palomaki GE, Neveux LM, Navolotskaia O, Ledue TB, Craig WY. Reference distributions for serum iron and transferrin saturation: a comparison of a large cohort to the world's literature. J Clin Lab Anal. 2002;16:246–52. doi: 10.1002/jcla.10047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie RF, Palomaki GE, Neveux LM, Navolotskaia O, Ledue TB, Craig WY. Reference distributions for serum iron and transferrin saturation: a practical, simple, and clinically relevant approach in a large cohort. J Clin Lab Anal. 2002;16:237–45. doi: 10.1002/jcla.10048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JM, Hankinson SE, Stampfer MJ, Rifai N, Willett WC, Ma J. Body iron stores and their determinants in healthy postmenopausal US women. Am J Clin Nutr. 2003;78:1160–7. doi: 10.1093/ajcn/78.6.1160. [DOI] [PubMed] [Google Scholar]
- Doulias PT, Vlachou C, Boudouri C, Kanavaros P, Siamopoulos KC, Galaris D. Flow cytometric estimation of 'labile iron pool' in human white blood cells reveals a positive association with ageing. Free Radic Res. 2008;42:253–9. doi: 10.1080/10715760801911649. [DOI] [PubMed] [Google Scholar]
- Fleming DJ, Tucker KL, Jacques PF, Dallal GE, Wilson PW, Wood RJ. Dietary factors associated with the risk of high iron stores in the elderly Framingham Heart Study cohort. Am J Clin Nutr. 2002;76:1375–84. doi: 10.1093/ajcn/76.6.1375. [DOI] [PubMed] [Google Scholar]
- Beard J. Dietary iron intakes and elevated iron stores in the elderly: is it time to abandon the set-point hypothesis of regulation of iron absorption? Am J Clin Nutr. 2002;76:1189–90. doi: 10.1093/ajcn/76.6.1189. [DOI] [PubMed] [Google Scholar]
- Cade JE, Moreton JA, O'Hara B, Greenwood DC, Moor J, Burley VJ, Kukalizch K, Bishop DT, Worwood M. Diet and genetic factors associated with iron status in middle-aged women. Am J Clin Nutr. 2005;82:813–20. doi: 10.1093/ajcn/82.4.813. [DOI] [PubMed] [Google Scholar]
- Guralnik JM, Eisenstaedt RS, Ferrucci L, Klein HG, Woodman RC. Prevalence of anemia in persons 65 years and older in the United States: evidence for a high rate of unexplained anemia. Blood. 2004;104:2263–8. doi: 10.1182/blood-2004-05-1812. [DOI] [PubMed] [Google Scholar]
- Price EA. Aging and erythropoiesis: current state of knowledge. Blood Cells Mol Dis. 2008;41:158–65. doi: 10.1016/j.bcmd.2008.04.005. [DOI] [PubMed] [Google Scholar]
- Sharman EH, Bondy SC, Sharman KG, Lahiri D, Cotman CW, Perreau VM. Effects of melatonin and age on gene expression in mouse CNS using microarray analysis. Neurochemistry International. 2007;50:336–344. doi: 10.1016/j.neuint.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood TBL. Evolution of Aging. Nature. 1977;270:301–304. doi: 10.1038/270301a0. [DOI] [PubMed] [Google Scholar]
- Beckman KB, Ames BN. The free radical theory of aging matures. Physiol Rev. 1998;78:547–81. doi: 10.1152/physrev.1998.78.2.547. [DOI] [PubMed] [Google Scholar]
- Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–47. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- Sohal RS, Mockett RJ, Orr WC. Current issues concerning the role of oxidative stress in aging: a perspective. Results Probl Cell Differ. 2000;29:45–66. doi: 10.1007/978-3-540-48003-7_3. [DOI] [PubMed] [Google Scholar]
- Cutler RG, Rodriguez H. Critical reviews of oxidative stress and aging: advances in basic science, diagnostics and intervention. World Scientific, Singapore. 2002.
- Herndon LA, Schmeissner PJ, Dudaronek JM, Brown PA, Listner KM, Sakano Y, Paupard MC, Hall DH, Driscoll M. Stochastic and genetic factors influence tissue-specific decline in ageing C. elegans. Nature. 2002;419:808–14. doi: 10.1038/nature01135. [DOI] [PubMed] [Google Scholar]
- Sohal RS, Mockett RJ, Orr WC. Mechanisms of aging: an appraisal of the oxidative stress hypothesis. Free Radic Biol Med. 2002;33:575–86. doi: 10.1016/s0891-5849(02)00886-9. [DOI] [PubMed] [Google Scholar]
- Partridge L, Gems D. Mechanisms of ageing: public or private? Nat Rev Genet. 2002;3:165–75. doi: 10.1038/nrg753. [DOI] [PubMed] [Google Scholar]
- Hekimi S, Guarente L. Genetics and the specificity of the aging process. Science. 2003;299:1351–4. doi: 10.1126/science.1082358. [DOI] [PubMed] [Google Scholar]
- Krabbe KS, Pedersen M, Bruunsgaard H. Inflammatory mediators in the elderly. Exp Gerontol. 2004;39:687–99. doi: 10.1016/j.exger.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Longo VD, Finch CE. Evolutionary medicine: from dwarf model systems to healthy centenarians? Science. 2003;299:1342–6. doi: 10.1126/science.1077991. [DOI] [PubMed] [Google Scholar]
- McGeer PL, McGeer EG. Inflammation and the degenerative diseases of aging. Ann N Y Acad Sci. 2004;1035:104–16. doi: 10.1196/annals.1332.007. [DOI] [PubMed] [Google Scholar]
- Afanas'ev IB. Free radical mechanisms of aging processes under physiological conditions. Biogerontology. 2005;6:283–90. doi: 10.1007/s10522-005-2626-z. [DOI] [PubMed] [Google Scholar]
- Kirkwood TB. Understanding the odd science of aging. Cell. 2005;120:437–47. doi: 10.1016/j.cell.2005.01.027. [DOI] [PubMed] [Google Scholar]
- Tuppo EE, Arias HR. The role of inflammation in Alzheimer's disease. Int J Biochem Cell Biol. 2005;37:289–305. doi: 10.1016/j.biocel.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Partridge L, Gems D. Beyond the evolutionary theory of ageing, from functional genomics to evo-gero. Trends Ecol Evol. 2006;21:334–40. doi: 10.1016/j.tree.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Salvioli S, Capri M, Valensin S, Tieri P, Monti D, Ottaviani E, Franceschi C. Inflamm-aging, cytokines and aging: state of the art, new hypotheses on the role of mitochondria and new perspectives from systems biology. Curr Pharm Des. 2006;12:3161–71. doi: 10.2174/138161206777947470. [DOI] [PubMed] [Google Scholar]
- Franco OH, Kirkwood TB, Powell JR, Catt M, Goodwin J, Ordovas JM, Ouderaa F van der. Ten commandments for the future of ageing research in the UK: a vision for action. BMC Geriatr. 2007;7:10. doi: 10.1186/1471-2318-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood TB. A systematic look at an old problem. Nature. 2008;451:644–7. doi: 10.1038/451644a. [DOI] [PubMed] [Google Scholar]
- Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- Ames BN, Shigenaga MK, Hagen TM. Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci USA. 1993;90:7915–22. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin GM, Austad SN, Johnson TE. Genetic analysis of ageing: role of oxidative damage and environmental stresses. Nat Genet. 1996;13:25–34. doi: 10.1038/ng0596-25. [DOI] [PubMed] [Google Scholar]
- Ishii N, Fujii M, Hartman PS, Tsuda M, Yasuda K, Senoo-Matsuda N, Yanase S, Ayusawa D, Suzuki K. A mutation in succinate dehydrogenase cytochrome b causes oxidative stress and ageing in nematodes. Nature. 1998;394:694–7. doi: 10.1038/29331. [DOI] [PubMed] [Google Scholar]
- Fukagawa NK. Aging: is oxidative stress a marker or is it causal? Proc Soc Exp Biol Med. 1999;222:293–8. doi: 10.1046/j.1525-1373.1999.d01-146.x. [DOI] [PubMed] [Google Scholar]
- Johnson FB, Sinclair DA, Guarente L. Molecular biology of aging. Cell. 1999;96:291–302. doi: 10.1016/s0092-8674(00)80567-x. [DOI] [PubMed] [Google Scholar]
- Melov S, Ravenscroft J, Malik S, Gill MS, Walker DW, Clayton PE, Wallace DC, Malfroy B, Doctrow SR, Lithgow GJ. Extension of life-span with superoxide dismutase/catalase mimetics. Science. 2000;289:1567–9. doi: 10.1126/science.289.5484.1567. [DOI] [PubMed] [Google Scholar]
- Hamilton ML, Van Remmen H, Drake JA, Yang H, Guo ZM, Kewitt K, Walter CA, Richardson A. Does oxidative damage to DNA increase with age? Proc Natl Acad Sci USA. 2001;98:10469–74. doi: 10.1073/pnas.171202698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Zglinicki T, Bürkle A, Kirkwood TBL. Stress, DNA damage and ageing – an integrative approach. Exp Gerontol. 2001;36:1049–1062. doi: 10.1016/s0531-5565(01)00111-5. [DOI] [PubMed] [Google Scholar]
- Barja G. Rate of generation of oxidative stress-related damage and animal longevity. Free Radical Biology and Medicine. 2002;33:1167–1172. doi: 10.1016/s0891-5849(02)00910-3. [DOI] [PubMed] [Google Scholar]
- Bonilla E, Medina-Leendertz S, Diaz S. Extension of life span and stress resistance of Drosophila melanogaster by long-term supplementation with melatonin. Exp Gerontol. 2002;37:629–38. doi: 10.1016/s0531-5565(01)00229-7. [DOI] [PubMed] [Google Scholar]
- Golden TR, Hinerfeld DA, Melov S. Oxidative stress and aging: beyond correlation. Aging Cell. 2002;1:117–23. doi: 10.1046/j.1474-9728.2002.00015.x. [DOI] [PubMed] [Google Scholar]
- Hagen TM. Oxidative stress, redox imbalance, and the aging process. Antioxidants & Redox Signaling. 2003;5:503–506. doi: 10.1089/152308603770310149. [DOI] [PubMed] [Google Scholar]
- Karanjawala ZE, Murphy N, Hinton DR, Hsieh CL, Lieber MR. Oxygen metabolism causes chromosome breaks and is associated with the neuronal apoptosis observed in DNA double-strand break repair mutants. Curr Biol. 2002;12:397–402. doi: 10.1016/s0960-9822(02)00684-x. [DOI] [PubMed] [Google Scholar]
- Melov S. Animal models of oxidative stress, aging, and therapeutic antioxidant interventions. Int J Biochem Cell Biol. 2002;34:1395–400. doi: 10.1016/s1357-2725(02)00086-9. [DOI] [PubMed] [Google Scholar]
- Barros MH, Bandy B, Tahara EB, Kowaltowski AJ. Higher respiratory activity decreases mitochondrial reactive oxygen release and increases life span in Saccharomyces cerevisiae. Journal of Biological Chemistry. 2004;279:49883–49888. doi: 10.1074/jbc.M408918200. [DOI] [PubMed] [Google Scholar]
- Gredilla R, Barja G. Minireview: the role of oxidative stress in relation to caloric restriction and longevity. Endocrinology. 2005;146:3713–7. doi: 10.1210/en.2005-0378. [DOI] [PubMed] [Google Scholar]
- Blagosklonny MV. Aging and immortality: quasi-programmed senescence and its pharmacologic inhibition. Cell Cycle. 2006;5:2087–102. doi: 10.4161/cc.5.18.3288. [DOI] [PubMed] [Google Scholar]
- Chung HY, Sung B, Jung KJ, Zou Y, Yu BP. The molecular inflammatory process in aging. Antioxid Redox Signal. 2006;8:572–81. doi: 10.1089/ars.2006.8.572. [DOI] [PubMed] [Google Scholar]
- Sedensky MM, Morgan PG. Mitochondrial respiration and reactive oxygen species in C. elegans. Exp Gerontol. 2006;41:957–67. doi: 10.1016/j.exger.2006.06.056. [DOI] [PubMed] [Google Scholar]
- Terman A, Brunk UT. Oxidative stress, accumulation of biological 'garbage', and aging. Antioxidants & Redox Signaling. 2006;8:197–204. doi: 10.1089/ars.2006.8.197. [DOI] [PubMed] [Google Scholar]
- Yu BP, Chung HY. Adaptive mechanisms to oxidative stress during aging. Mechanisms of Ageing and Development. 2006;127:436–443. doi: 10.1016/j.mad.2006.01.023. [DOI] [PubMed] [Google Scholar]
- Chakravarti B, Chakravarti DN. Oxidative modification of proteins: Age-related changes. Gerontology. 2007;53:128–139. doi: 10.1159/000097865. [DOI] [PubMed] [Google Scholar]
- Kim SK. Common aging pathways in worms, flies, mice and humans. J Exp Biol. 2007;210:1607–12. doi: 10.1242/jeb.004887. [DOI] [PubMed] [Google Scholar]
- Kregel KC, Zhang HJ. An integrated view of oxidative stress in aging: basic mechanisms, functional effects, and pathological considerations. Am J Physiol Regul Integr Comp Physiol. 2007;292:R18–36. doi: 10.1152/ajpregu.00327.2006. [DOI] [PubMed] [Google Scholar]
- Nijnik A, Woodbine L, Marchetti C, Dawson S, Lambe T, Liu C, Rodrigues NP, Crockford TL, Cabuy E, Vindigni A, Enver T, Bell JI, Slijepcevic P, Goodnow CC, Jeggo PA, Cornall RJ. DNA repair is limiting for haematopoietic stem cells during ageing. Nature. 2007;447:686–90. doi: 10.1038/nature05875. [DOI] [PubMed] [Google Scholar]
- Sohal RS, Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardon MC. Stress and ageing interactions: a paradox in the context of shared etiological and physiopathological processes. Brain Res Rev. 2007;54:251–73. doi: 10.1016/j.brainresrev.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Killilea DW, Atamna H, Liao C, Ames BN. Iron accumulation during cellular senescence in human fibroblasts in vitro. Antioxid Redox Signal. 2003;5:507–16. doi: 10.1089/152308603770310158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamerman D. Toward an understanding of frailty. Ann Int Med. 1999;130:945–950. doi: 10.7326/0003-4819-130-11-199906010-00022. [DOI] [PubMed] [Google Scholar]
- Docherty JR. Cardiovascular responses in aging – a review. Pharmacol Rev. 1990;42:103–125. [PubMed] [Google Scholar]
- Dröge W, Schipper HM. Oxidative stress and aberrant signaling in aging and cognitive decline. Aging Cell. 2007;6:361–370. doi: 10.1111/j.1474-9726.2007.00294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massie HR, Aiello VR, Banziger V. Iron accumulation and lipid peroxidation in aging C57bl/6j mice. Exp Gerontol. 1983;18:277–285. doi: 10.1016/0531-5565(83)90038-4. [DOI] [PubMed] [Google Scholar]
- Ershler WB. Biological interactions of aging and anemia: A focus on cytokines. J Amer Geriatr Soc. 2003;51:S18–S21. doi: 10.1046/j.1532-5415.51.3s.2.x. [DOI] [PubMed] [Google Scholar]
- Massie HR, Aiello VR, Williams TR. Inhibition of iron absorption prolongs the lifespan of Drosophila. Mech Ageing Dev. 1993;67:227–237. doi: 10.1016/0047-6374(93)90001-8. [DOI] [PubMed] [Google Scholar]
- Rattan SIS. Aging, anti-aging, and hormesis. Mechanisms of Ageing and Development. 2004;125:285–289. doi: 10.1016/j.mad.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Kenyon C. The plasticity of aging: insights from long-lived mutants. Cell. 2005;120:449–60. doi: 10.1016/j.cell.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Lee CK, Klopp RG, Weindruch R, Prolla TA. Gene expression profile of aging and its retardation by caloric restriction. Science. 1999;285:1390–3. doi: 10.1126/science.285.5432.1390. [DOI] [PubMed] [Google Scholar]
- Bordone L, Guarente L. Calorie restriction, SIRT1 and metabolism: understanding longevity. Nat Rev Mol Cell Biol. 2005;6:298–305. doi: 10.1038/nrm1616. [DOI] [PubMed] [Google Scholar]
- Taguchi A, Wartschow LM, White MF. Brain IRS2 signaling coordinates life span and nutrient homeostasis. Science. 2007;317:369–72. doi: 10.1126/science.1142179. [DOI] [PubMed] [Google Scholar]
- Hulbert AJ, Pamplona R, Buffenstein R, Buttemer WA. Life and death: metabolic rate, membrane composition, and life span of animals. Physiol Rev. 2007;87:1175–213. doi: 10.1152/physrev.00047.2006. [DOI] [PubMed] [Google Scholar]
- Kennedy BK, Steffen KK, Kaeberlein M. Ruminations on dietary restriction and aging. Cell Mol Life Sci. 2007;64:1323–8. doi: 10.1007/s00018-007-6470-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mair W, Dillin A. Aging and survival: the genetics of life span extension by dietary restriction. Annu Rev Biochem. 2008;77:727–54. doi: 10.1146/annurev.biochem.77.061206.171059. [DOI] [PubMed] [Google Scholar]
- Piper MD, Partridge L. Dietary restriction in Drosophila: delayed aging or experimental artefact? PLoS Genet. 2007;3:e57. doi: 10.1371/journal.pgen.0030057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weindruch R, Sohal RS. Caloric intake and aging. New England Journal of Medicine. 1997;337:986–994. doi: 10.1056/NEJM199710023371407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lass A, Sohal BH, Weindruch R, Forster MJ, Sohal RS. Caloric restriction prevents age-associated accrual of oxidative damage to mouse skeletal muscle mitochondria. Free Radical Biology and Medicine. 1998;25:1089–1097. doi: 10.1016/s0891-5849(98)00144-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masoro EJ. Caloric restriction and aging: an update. Exp Gerontol. 2000;35:299–305. doi: 10.1016/s0531-5565(00)00084-x. [DOI] [PubMed] [Google Scholar]
- Thrasivoulou C, Soubeyre V, Ridha H, Giuliani D, Giaroni C, Michael GJ, Saffrey MJ, Cowen T. Reactive oxygen species, dietary restriction and neurotrophic factors in age-related loss of myenteric neurons. Aging Cell. 2006;5:247–57. doi: 10.1111/j.1474-9726.2006.00214.x. [DOI] [PubMed] [Google Scholar]
- Walz D. Thermodynamics of oxidation-reduction reactions and its application to bioenergetics. Biochim Biophys Acta. 1979;505:279–353. doi: 10.1016/0304-4173(79)90007-7. [DOI] [PubMed] [Google Scholar]
- Cook CI, Yu BP. Iron accumulation in aging: modulation by dietary restriction. Mechanisms of Ageing and Development. 1998;102:1–13. doi: 10.1016/s0047-6374(98)00005-0. [DOI] [PubMed] [Google Scholar]
- Reverter-Branchat G, Cabiscol E, Tamarit J, Ros J. Oxidative damage to specific proteins in replicative and chronological-aged Saccharomyces cerevisiae – Common targets and prevention by calorie restriction. J Biol Chem. 2004;279:31983–31989. doi: 10.1074/jbc.M404849200. [DOI] [PubMed] [Google Scholar]
- Raffaghello L, Lee C, Safdie FM, Wei M, Madia F, Bianchi G, Longo VD. Starvation-dependent differential stress resistance protects normal but not cancer cells against high-dose chemotherapy. Proc Natl Acad Sci USA. 2008;105:8215–20. doi: 10.1073/pnas.0708100105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anisimov VN. Effects of exogenous melatonin–a review. Toxicol Pathol. 2003;31:589–603. doi: 10.1080/01926230390257885. [DOI] [PubMed] [Google Scholar]
- Poeggeler B. Melatonin, aging, and age-related diseases: perspectives for prevention, intervention, and therapy. Endocrine. 2005;27:201–12. doi: 10.1385/ENDO:27:2:201. [DOI] [PubMed] [Google Scholar]
- Bonilla E, Medina-Leendertz S, Villalobos V, Molero L, Bohorquez A. Paraquat-induced oxidative stress in Drosophila melanogaster: Effects of melatonin, glutathione, serotonin, minocycline, lipoic acid and ascorbic acid. Neurochemical Research. 2006;31:1425–1432. doi: 10.1007/s11064-006-9194-8. [DOI] [PubMed] [Google Scholar]
- Salvemini D, Riley DP, Cuzzocrea S. SOD mimetics are coming of age. Nature Reviews Drug Discovery. 2002;1:367–374. doi: 10.1038/nrd796. [DOI] [PubMed] [Google Scholar]
- Lithgow GJ, Gill MS, Olsen A, Sampayo JN. Pharmacological intervention in invertebrate aging. Ageing Res Rev. 2005;27:213–223. doi: 10.1007/s11357-005-3625-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy CT, McCarroll SA, Bargmann CI, Fraser A, Kamath RS, Ahringer J, Li H, Kenyon C. Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature. 2003;424:277–83. doi: 10.1038/nature01789. [DOI] [PubMed] [Google Scholar]
- Dong MQ, Venable JD, Au N, Xu T, Park SK, Cociorva D, Johnson JR, Dillin A, Yates JR., 3rd Quantitative mass spectrometry identifies insulin signaling targets in C. elegans. Science. 2007;317:660–3. doi: 10.1126/science.1139952. [DOI] [PubMed] [Google Scholar]
- Honda Y, Honda S. The daf-2 gene network for longevity regulates oxidative stress resistance and Mn-superoxide dismutase gene expression in Caenorhabditis elegans. FASEB J. 1999;13:1385–93. [PubMed] [Google Scholar]
- Bauerova K, Bezek S. Role of reactive oxygen and nitrogen species in etiopathogenesis of rheumatoid arthritis. General Physiology and Biophysics. 1999;18:15–20. [PubMed] [Google Scholar]
- Aruoma OI. Free radicals, oxidative stress, and antioxidants in human health and disease. J Amer Oil Chemists Soc. 1998;75:199–212. doi: 10.1007/s11746-998-0032-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mapp PI, Grootveld MC, Blake DR. Hypoxia, oxidative stress and rheumatoid arthritis. British Medical Bulletin. 1995;51:419–436. doi: 10.1093/oxfordjournals.bmb.a072970. [DOI] [PubMed] [Google Scholar]
- Gracy RW, Talent JM, Kong Y, Conrad CC. Reactive oxygen species: the unavoidable environmental insult? Mut Res. 1999;428:17–22. doi: 10.1016/s1383-5742(99)00027-7. [DOI] [PubMed] [Google Scholar]
- Henrotin YE, Bruckner P, Pujol JPL. The role of reactive oxygen species in homeostasis and degradation of cartilage. Osteoarthritis and Cartilage. 2003;11:747–755. doi: 10.1016/s1063-4584(03)00150-x. [DOI] [PubMed] [Google Scholar]
- Griffiths HR. ROS as signalling molecules in T cells – evidence for abnormal redox signalling in the autoimmune disease, rheumatoid arthritis. Redox Report. 2005;10:273–280. doi: 10.1179/135100005X83680. [DOI] [PubMed] [Google Scholar]
- Weinstein IM. A Correlative Study of the Erythrokinetics and Disturbances in Iron Metabolism Associated with the Anemia of Rheumatoid Arthritis. Blood. 1959;14:950–966. [PubMed] [Google Scholar]
- Bentley DP, Williams P. Serum ferritin concentration as an index of storage iron in rheumatoid arthritis. J Clin Pathol. 1974;27:786–788. doi: 10.1136/jcp.27.10.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen TM, Hansen NE, Birgens HS, Holund B, Lorenzen I. Serum Ferritin and the Assessment of Iron-Deficiency in Rheumatoid-Arthritis. Scandinavian Journal of Rheumatology. 1983;12:353–359. doi: 10.3109/03009748309099740. [DOI] [PubMed] [Google Scholar]
- Weber J, Werre JM, Julius HW, Marx JJ. Decreased iron absorption in patients with active rheumatoid arthritis, with and without iron deficiency. Ann Rheum Dis. 1988;47:404–9. doi: 10.1136/ard.47.5.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vreugdenhil G, Wognum AW, Vaneijk HG, Swaak AJG. Anemia in Rheumatoid-Arthritis – the Role of Iron, Vitamin-B12, and Folic-Acid Deficiency, and Erythropoietin Responsiveness. Annals of the Rheumatic Diseases. 1990;49:93–98. doi: 10.1136/ard.49.2.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muirden KD, Senator GB. Iron in synovial membrane in rheumatoid arthritis and other joint diseases. Ann Rheumat Dis. 1968;27:38. doi: 10.1136/ard.27.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley D, Gutteridge JMC, Blake D, Farr M, Halliwell B. Lipid peroxidation in rheumatoid arthritis – thiobarbituric acid-reactive material and catalytic iron salts in synovial fluid from rheumatoid patients. Clin Sci. 1984;66:691–695. doi: 10.1042/cs0660691. [DOI] [PubMed] [Google Scholar]
- Biemond P, Swaak AJG, Vaneijk HG, Koster JF. Intraarticular ferritin-bound iron in rheumatoid arthritis – a factor that increases oxygen free radical-induced tissue destruction. Arthritis and Rheumatism. 1986;29:1187–1193. doi: 10.1002/art.1780291002. [DOI] [PubMed] [Google Scholar]
- Lipiński P, Drapier JC. Interplay between ferritin metabolism, reactive oxygen species and nitric oxide. J Biol Inorg Chem. 1997;2:559–566. [Google Scholar]
- Biemond P, Swaak AJG, Vaneijk HG, Koster JF. Superoxide Dependent Iron Release from Ferritin in Inflammatory Diseases. Free Radical Biology and Medicine. 1988;4:185–198. doi: 10.1016/0891-5849(88)90026-3. [DOI] [PubMed] [Google Scholar]
- Biemond P, Swaak AJG, Beindorff CM, Koster JF. Superoxide-Dependent and Superoxide-Independent Mechanisms of Iron Mobilization from Ferritin by Xanthine-Oxidase – Implications for Oxygen-Free-Radical-Induced Tissue Destruction During Ischemia and Inflammation. Biochemical Journal. 1986;239:169–173. doi: 10.1042/bj2390169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biemond P, Swaak AJG, Penders JMA, Beindorff CM, Koster JF. Superoxide Production by Polymorphonuclear Leukocytes in Rheumatoid-Arthritis and Osteoarthritis – Invivo Inhibition by the Antirheumatic Drug Piroxicam Due to Interference with the Activation of the Nadph-Oxidase. Annals of the Rheumatic Diseases. 1986;45:249–255. doi: 10.1136/ard.45.3.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biemond P, Vaneijk HG, Swaak AJG, Koster JF. Iron Mobilization from Ferritin by Superoxide Derived from Stimulated Polymorphonuclear Leukocytes – Possible Mechanism in Inflammation Diseases. Journal of Clinical Investigation. 1984;73:1576–1579. doi: 10.1172/JCI111364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mladĕnka P, Šimůnek T, Hübl M, Hrdina R. The role of reactive oxygen and nitrogen species in cellular iron metabolism.". Free Radic Res. 2006;40:263–72. doi: 10.1080/10715760500511484. [DOI] [PubMed] [Google Scholar]
- Harris LR, Cake MH, Macey DJ. Iron release from ferritin and its sensitivity to superoxide ions differs among vertebrates. Biochem J. 1994;301:385–389. doi: 10.1042/bj3010385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairburn K, Grootveld M, Ward RJ, Abiuka C, Kus M, Williams RB, Winyard PG, Blake DR. Alpha-Tocopherol, Lipids and Lipoproteins in Knee-Joint Synovial-Fluid and Serum from Patients with Inflammatory Joint Disease. Clinical Science. 1992;83:657–664. doi: 10.1042/cs0830657. [DOI] [PubMed] [Google Scholar]
- Dombrecht EJ, Cos P, Berghe D Vanden, Van Offel JF, Schuerwegh AJ, Bridts CH, Stevens WJ, De Clerck LS. Selective in vitro antioxidant properties of bisphosphonates. Biochem Biophys Res Commun. 2004;314:675–80. doi: 10.1016/j.bbrc.2003.12.149. [DOI] [PubMed] [Google Scholar]
- Ostrakhovitch EA, Afanas'ev IB. Oxidative stress in rheumatoid arthritis leukocytes: suppression by rutin and other antioxidants and chelators. Biochem Pharmacol. 2001;62:743–746. doi: 10.1016/s0006-2952(01)00707-9. [DOI] [PubMed] [Google Scholar]
- Hazes JMW, Dijkmans BAC, Vandenbroucke JP, Devries RRP, Cats A. Pregnancy and the risk of developing rheumatoid arthritis. Arthritis and Rheumatism. 1990;33:1770–1775. doi: 10.1002/art.1780331203. [DOI] [PubMed] [Google Scholar]
- Østensen M, Husby G. A prospective clinical study of the effect of pregnancy on rheumatoid arthritis and ankylosing spondylitis. Arthritis and Rheumatism. 1983;26:1155–1159. doi: 10.1002/art.1780260915. [DOI] [PubMed] [Google Scholar]
- Da Silva JAP, Spector TD. The role of pregnancy in the course and etiology of rheumatoid arthritis. Clin Rheumat. 1992;11:189–194. doi: 10.1007/BF02207955. [DOI] [PubMed] [Google Scholar]
- O'Keeffe ST. Restless legs syndrome – A review. Archives of Internal Medicine. 1996;156:243–248. [PubMed] [Google Scholar]
- Ondo W, Tan EK, Mansoor J. Rheumatologic serologies in secondary restless legs syndrome. Movement Disorders. 2000;15:321–323. doi: 10.1002/1531-8257(200003)15:2<321::aid-mds1019>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Trenkwalder C, Paulus W, Walters AS. The restless legs syndrome. Lancet Neurology. 2005;4:465–475. doi: 10.1016/S1474-4422(05)70139-3. [DOI] [PubMed] [Google Scholar]
- Feelders RA, Kuiper-Kramer EPA, van Eijk HG. Structure, function and clinical significance of transferrin receptors. Clinical Chemistry and Laboratory Medicine. 1999;37:1–10. doi: 10.1515/CCLM.1999.001. [DOI] [PubMed] [Google Scholar]
- Ahluwalia N. Diagnostic utility of serum transferrin receptors measurement in assessing iron status. Nutrition Reviews. 1998;56:133–141. doi: 10.1111/j.1753-4887.1998.tb01738.x. [DOI] [PubMed] [Google Scholar]
- Frostegård J. SLE, atherosclerosis and cardiovascular disease. J Intern Med. 2005;257:485–95. doi: 10.1111/j.1365-2796.2005.01502.x. [DOI] [PubMed] [Google Scholar]
- Mascitelli L, Pezzetta F. High iron stores and cardiovascular disease in systemic lupus erythematosus. J Intern Med. 2005;258:584. doi: 10.1111/j.1365-2796.2005.01577.x. [DOI] [PubMed] [Google Scholar]
- Harvey AM, Shulman LE, Tumulty PA, Conley CL, Schoenrich EH. Systemic lupus erythematosus: review of the literature and clinical analysis of 138 cases. Medicine (Baltimore) 1954;33:291–437. [PubMed] [Google Scholar]
- Johnson RT, Richardson EP. The neurological manifestations of systemic lupus erythematosus. Medicine (Baltimore) 1968;47:337–69. doi: 10.1097/00005792-196807000-00002. [DOI] [PubMed] [Google Scholar]
- Feng PH, Cheah PS, Lee YK. Mortality in systemic lupus erythematosus: a 10-year review. Br Med J. 1973;4:772–4. doi: 10.1136/bmj.4.5895.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthay RA, Schwarz MI, Petty TL, Stanford RE, Gupta RC, Sahn SA, Steigerwald JC. Pulmonary manifestations of systemic lupus erythematosus: review of twelve cases of acute lupus pneumonitis. Medicine (Baltimore) 1975;54:397–409. doi: 10.1097/00005792-197509000-00003. [DOI] [PubMed] [Google Scholar]
- O'Loughlin S, Schroeter AL, Jordon RE. Chronic urticaria-like lesions in systemic lupus erythematosus. A review of 12 cases. Arch Dermatol. 1978;114:879–83. [PubMed] [Google Scholar]
- Hoffman BI, Katz WA. The gastrointestinal manifestations of systemic lupus erythematosus: a review of the literature. Semin Arthritis Rheum. 1980;9:237–47. doi: 10.1016/0049-0172(80)90016-5. [DOI] [PubMed] [Google Scholar]
- Reynolds JC, Inman RD, Kimberly RP, Chuong JH, Kovacs JE, Walsh MB. Acute pancreatitis in systemic lupus erythematosus: report of twenty cases and a review of the literature. Medicine (Baltimore) 1982;61:25–32. doi: 10.1097/00005792-198201000-00003. [DOI] [PubMed] [Google Scholar]
- Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, Schaller JG, Talal N, Winchester RJ. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–7. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- Levine SR, Joseph R, D'Andrea G, Welch KM. Migraine and the lupus anticoagulant. Case reports and review of the literature. Cephalalgia. 1987;7:93–9. doi: 10.1046/j.1468-2982.1987.0702093.x. [DOI] [PubMed] [Google Scholar]
- Crowson AN, Magro C. The cutaneous pathology of lupus erythematosus: a review. J Cutan Pathol. 2001;28:1–23. doi: 10.1034/j.1600-0560.2001.280101.x. [DOI] [PubMed] [Google Scholar]
- Karrar A, Sequeira W, Block JA. Coronary artery disease in systemic lupus erythematosus: A review of the literature. Semin Arthritis Rheum. 2001;30:436–43. doi: 10.1053/sarh.2001.23498. [DOI] [PubMed] [Google Scholar]
- Ad hoc committee on systemic lupus erythematosus response criteria for fatigue Measurement of fatigue in systemic lupus erythematosus: a systematic review. Arthritis Rheum. 2007;57:1348–57. doi: 10.1002/art.23113. [DOI] [PubMed] [Google Scholar]
- Zhang X, Jin M, Wu H, Nadasdy T, Nadasdy G, Harris N, Green-Church K, Nagaraja H, Birmingham DJ, Yu CY, Hebert LA, Rovin BH. Biomarkers of lupus nephritis determined by serial urine proteomics. Kidney Int. 2008;74:799–807. doi: 10.1038/ki.2008.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim MK, Lee CK, Ju YS, Cho YS, Lee MS, Yoo B, Moon HB. Serum ferritin as a serologic marker of activity in systemic lupus erythematosus. Rheumatol Int. 2001;20:89–93. doi: 10.1007/s002960000083. [DOI] [PubMed] [Google Scholar]
- Abou-Raya A, el-Hallous D, Fayed H. 8-Isoprostaglandin F2 alpha: a potential index of lipid peroxidation in systemic lupus erythematosus. Clin Invest Med. 2004;27:306–11. [PubMed] [Google Scholar]
- Cutolo M, Otsa K. Review: vitamin D, immunity and lupus. Lupus. 2008;17:6–10. doi: 10.1177/0961203307085879. [DOI] [PubMed] [Google Scholar]
- Li HF. Are statins analogues of vitamin D? Lancet. 2006;368:1233–4. doi: 10.1016/S0140-6736(06)69508-1. [DOI] [PubMed] [Google Scholar]
- Wu-Wong JR, Nakane M, Ma J, Ju TR. Statins do not directly activate vitamin D receptor. J Thromb Haemost. 2007;5:415–6. doi: 10.1111/j.1538-7836.2007.02316.x. [DOI] [PubMed] [Google Scholar]
- Lawman S, Mauri C, Jury EC, Cook HT, Ehrenstein MR. Atorvastatin inhibits autoreactive B cell activation and delays lupus development in New Zealand black/white F1 mice. J Immunol. 2004;173:7641–6. doi: 10.4049/jimmunol.173.12.7641. [DOI] [PubMed] [Google Scholar]
- Jury EC, Isenberg DA, Mauri C, Ehrenstein MR. Atorvastatin restores Lck expression and lipid raft-associated signaling in T cells from patients with systemic lupus erythematosus. J Immunol. 2006;177:7416–22. doi: 10.4049/jimmunol.177.10.7416. [DOI] [PubMed] [Google Scholar]
- Swanson DR. Undiscovered public knowledge. Library Quarterly. 1986;56:103–118. [Google Scholar]
- Swanson DR, Smalheiser NR, Torvik VI. Ranking indirect connections in literature-based discovery: The role of medical subject headings. J Amer Soc Inf Sci Technol. 2006;57:1427–1439. [Google Scholar]
- Andreadis AA, Hazen SL, Comhair SAA, Erzurum SC. Oxidative and nitrosative events in asthma. Free Radical Biology and Medicine. 2003;35:213–225. doi: 10.1016/s0891-5849(03)00278-8. [DOI] [PubMed] [Google Scholar]
- Greene LS. Asthma and oxidant stress – nutritional, environmental, and genetic risk factors. J Amer Coll Nutrition. 1995;14:317–324. doi: 10.1080/07315724.1995.10718516. [DOI] [PubMed] [Google Scholar]
- Han JY, Takeshita K, Utsumi H. Noninvasive detection of hydroxyl radical generation in lung by diesel exhaust particles. Free Radic Biol Med. 2001;30:516–25. doi: 10.1016/s0891-5849(00)00501-3. [DOI] [PubMed] [Google Scholar]
- Kocyigit A, Armutcu F, Gurel A, Ermis B. Alterations in plasma essential trace elements selenium, manganese, zinc, copper, and iron concentrations and the possible role of these elements on oxidative status in patients with childhood asthma. Biological Trace Element Research. 2004;97:31–41. doi: 10.1385/BTER:97:1:31. [DOI] [PubMed] [Google Scholar]
- Lih-Brody L, Powell SR, Collier KP, Reddy GM, Cerchia R, Kahn E, Weissman GS, Katz S, Floyd RA, McKinley MJ, Fisher SE, Mullin GE. Increased oxidative stress and decreased antioxidant defenses in mucosa of inflammatory bowel disease. Dig Dis Sci. 1996;41:2078–86. doi: 10.1007/BF02093613. [DOI] [PubMed] [Google Scholar]
- Kruidenier L, Verspaget HW. Review article: oxidative stress as a pathogenic factor in inflammatory bowel disease–radicals or ridiculous? Aliment Pharmacol Ther. 2002;16:1997–2015. doi: 10.1046/j.1365-2036.2002.01378.x. [DOI] [PubMed] [Google Scholar]
- Kruidenier L, Kuiper I, Lamers CB, Verspaget HW. Intestinal oxidative damage in inflammatory bowel disease: semi-quantification, localization, and association with mucosal antioxidants. J Pathol. 2003;201:28–36. doi: 10.1002/path.1409. [DOI] [PubMed] [Google Scholar]
- Martin AR, Villegas I, La Casa C, de la Lastra CA. Resveratrol, a polyphenol found in grapes, suppresses oxidative damage and stimulates apoptosis during early colonic inflammation in rats. Biochem Pharmacol. 2004;67:1399–410. doi: 10.1016/j.bcp.2003.12.024. [DOI] [PubMed] [Google Scholar]
- Seril DN, Liao J, Yang GY, Yang CS. Oxidative stress and ulcerative colitis-associated carcinogenesis: studies in humans and animal models. Carcinogenesis. 2003;24:353–62. doi: 10.1093/carcin/24.3.353. [DOI] [PubMed] [Google Scholar]
- Gisbert JP, Gomollón F. Common misconceptions in the diagnosis and management of anemia in inflammatory bowel disease. Am J Gastroenterol. 2008;103:1299–307. doi: 10.1111/j.1572-0241.2008.01846.x. [DOI] [PubMed] [Google Scholar]
- Semrin G, Fishman DS, Bousvaros A, Zholudev A, Saunders AC, Correia CE, Nemeth E, Grand RJ, Weinstein DA. Impaired intestinal iron absorption in Crohn's disease correlates with disease activity and markers of inflammation. Inflamm Bowel Dis. 2006;12:1101–6. doi: 10.1097/01.mib.0000235097.86360.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaahl MG, Winter TA, Warnich L, Kotze MJ. The -237C-->T promoter polymorphism of the SLC11A1 gene is associated with a protective effect in relation to inflammatory bowel disease in the South African population. Int J Colorectal Dis. 2006;21:402–8. doi: 10.1007/s00384-005-0019-z. [DOI] [PubMed] [Google Scholar]
- Bartels U, Pedersen NS, Jarnum S. Iron absorption and serum ferritin in chronic inflammatory bowel disease. Scand J Gastroenterol. 1978;13:649–56. doi: 10.3109/00365527809181777. [DOI] [PubMed] [Google Scholar]
- Thomson AB, Brust R, Ali MA, Mant MJ, Valberg LS. Iron deficiency in inflammatory bowel disease. Diagnostic efficacy of serum ferritin. Am J Dig Dis. 1978;23:705–9. doi: 10.1007/BF01072356. [DOI] [PubMed] [Google Scholar]
- Oldenburg B, Koningsberger JC, Van Berge Henegouwen GP, Van Asbeck BS, Marx JJ. Iron and inflammatory bowel disease. Aliment Pharmacol Ther. 2001;15:429–38. doi: 10.1046/j.1365-2036.2001.00930.x. [DOI] [PubMed] [Google Scholar]
- Gasche C, Lomer MC, Cavill I, Weiss G. Iron, anaemia, and inflammatory bowel diseases. Gut. 2004;53:1190–7. doi: 10.1136/gut.2003.035758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Silva AD, Tsironi E, Feakins RM, Rampton DS. Efficacy and tolerability of oral iron therapy in inflammatory bowel disease: a prospective, comparative trial. Aliment Pharmacol Ther. 2005;22:1097–105. doi: 10.1111/j.1365-2036.2005.02700.x. [DOI] [PubMed] [Google Scholar]
- Guagnozzi D, Severi C, Ialongo P, Viscido A, Patrizi F, Testino G, Vannella L, Labriola R, Strom R, Caprilli R. Ferritin as a simple indicator of iron deficiency in anemic IBD patients. Inflamm Bowel Dis. 2006;12:150–1. doi: 10.1097/01.MIB.0000199223.27595.e3. [DOI] [PubMed] [Google Scholar]
- Gasche C, Berstad A, Befrits R, Beglinger C, Dignass A, Erichsen K, Gomollon F, Hjortswang H, Koutroubakis I, Kulnigg S, Oldenburg B, Rampton D, Schroeder O, Stein J, Travis S, Van Assche G. Guidelines on the diagnosis and management of iron deficiency and anemia in inflammatory bowel diseases. Inflamm Bowel Dis. 2007;13:1545–53. doi: 10.1002/ibd.20285. [DOI] [PubMed] [Google Scholar]
- Rattner A, Nathans J. Macular degeneration: recent advances and therapeutic opportunities. Nat Rev Neurosci. 2006;7:860–72. doi: 10.1038/nrn2007. [DOI] [PubMed] [Google Scholar]
- Tielsch JM, Javitt JC, Coleman A, Katz J, Sommer A. The prevalence of blindness and visual impairment among nursing home residents in Baltimore. N Engl J Med. 1995;332:1205–9. doi: 10.1056/NEJM199505043321806. [DOI] [PubMed] [Google Scholar]
- Harvey PT. Common eye diseases of elderly people: identifying and treating causes of vision loss. Gerontology. 2003;49:1–11. doi: 10.1159/000066507. [DOI] [PubMed] [Google Scholar]
- Fine SL. Age-related macular degeneration 1969–2004: a 35-year personal perspective. Am J Ophthalmol. 2005;139:405–20. doi: 10.1016/j.ajo.2004.11.050. [DOI] [PubMed] [Google Scholar]
- Lotery A, Trump D. Progress in defining the molecular biology of age related macular degeneration. Hum Genet. 2007;122:219–36. doi: 10.1007/s00439-007-0406-3. [DOI] [PubMed] [Google Scholar]
- Anderson DH, Mullins RF, Hageman GS, Johnson LV. A role for local inflammation in the formation of drusen in the aging eye. Am J Ophthalmol. 2002;134:411–31. doi: 10.1016/s0002-9394(02)01624-0. [DOI] [PubMed] [Google Scholar]
- Hahn P, Ying GS, Beard J, Dunaief JL. Iron levels in human retina: sex difference and increase with age. Neuroreport. 2006;17:1803–6. doi: 10.1097/WNR.0b013e3280107776. [DOI] [PubMed] [Google Scholar]
- Cai J, Nelson KC, Wu M, Sternberg P, Jr, Jones DP. Oxidative damage and protection of the RPE. Prog Retin Eye Res. 2000;19:205–21. doi: 10.1016/s1350-9462(99)00009-9. [DOI] [PubMed] [Google Scholar]
- Richer S, Rudy D, Statkute L, Karofty K, Frankowski J. Serum iron, transferrin saturation, ferritin, and dietary data in age-related macular degeneration. Am J Ther. 2002;9:25–8. doi: 10.1097/00045391-200201000-00006. [DOI] [PubMed] [Google Scholar]
- Hahn P, Milam AH, Dunaief JL. Maculas affected by age-related macular degeneration contain increased chelatable iron in the retinal pigment epithelium and Bruch's membrane. Arch Ophthalmol. 2003;121:1099–105. doi: 10.1001/archopht.121.8.1099. [DOI] [PubMed] [Google Scholar]
- Hahn P, Qian Y, Dentchev T, Chen L, Beard J, Harris ZL, Dunaief JL. Disruption of ceruloplasmin and hephaestin in mice causes retinal iron overload and retinal degeneration with features of age-related macular degeneration. Proc Natl Acad Sci USA. 2004;101:13850–5. doi: 10.1073/pnas.0405146101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunaief JL. Iron induced oxidative damage as a potential factor in age-related macular degeneration: The Cogan Lecture. Invest Ophthalmol Vis Sci. 2006;47:4660–4664. doi: 10.1167/iovs.06-0568. [DOI] [PubMed] [Google Scholar]
- He X, Hahn P, Iacovelli J, Wong R, King C, Bhisitkul R, Massaro-Giordano M, Dunaief JL. Iron homeostasis and toxicity in retinal degeneration. Prog Retin Eye Res. 2007;26:649–73. doi: 10.1016/j.preteyeres.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong RW, Richa DC, Hahn P, Green WR, Dunaief JL. Iron toxicity as a potential factor in AMD. Retina. 2007;27:997–1003. doi: 10.1097/IAE.0b013e318074c290. [DOI] [PubMed] [Google Scholar]
- Voloboueva LA, Killilea DW, Atamna H, Ames BN. N-tert-butyl hydroxylamine, a mitochondrial antioxidant, protects human retinal pigment epithelial cells from iron overload: relevance to macular degeneration. FASEB J. 2007;21:4077–86. doi: 10.1096/fj.07-8396com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan JS, Wang JJ, Flood V, Rochtchina E, Smith W, Mitchell P. Dietary antioxidants and the long-term incidence of age-related macular degeneration: the Blue Mountains Eye Study. Ophthalmology. 2008;115:334–41. doi: 10.1016/j.ophtha.2007.03.083. [DOI] [PubMed] [Google Scholar]
- Wilson DS, Szostak JW. In vitro selection of functional nucleic acids. Annu Rev Biochem. 1999;68:611–647. doi: 10.1146/annurev.biochem.68.1.611. [DOI] [PubMed] [Google Scholar]
- Nimjee SM, Rusconi CP, Sullenger BA. Aptamers: an emerging class of therapeutics. Annu Rev Med. 2005;56:555–83. doi: 10.1146/annurev.med.56.062904.144915. [DOI] [PubMed] [Google Scholar]
- Famulok M, Hartig JS, Mayer G. Functional aptamers and aptazymes in biotechnology, diagnostics, and therapy. Chem Rev. 2007;107:3715–43. doi: 10.1021/cr0306743. [DOI] [PubMed] [Google Scholar]
- Chapman JA, Beckey C. Pegaptanib: a novel approach to ocular neovascularization. Ann Pharmacother. 2006;40:1322–6. doi: 10.1345/aph.1G604. [DOI] [PubMed] [Google Scholar]
- Kourlas H, Schiller DS. Pegaptanib sodium for the treatment of neovascular age-related macular degeneration: a review. Clin Ther. 2006;28:36–44. doi: 10.1016/j.clinthera.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Zhou B, Wang B. Pegaptanib for the treatment of age-related macular degeneration. Exp Eye Res. 2006;83:615–9. doi: 10.1016/j.exer.2006.02.010. [DOI] [PubMed] [Google Scholar]
- Virgili G, Do DV, Bressler NM, Menchini U. New therapies for neovascular age-related macular degeneration: critical appraisal of the current evidence. Acta Ophthalmol Scand. 2007;85:6–20. doi: 10.1111/j.1600-0420.2006.00711.x. [DOI] [PubMed] [Google Scholar]
- Takeda AL, Colquitt J, Clegg AJ, Jones J. Pegaptanib and ranibizumab for neovascular age-related macular degeneration: a systematic review. Br J Ophthalmol. 2007;91:1177–82. doi: 10.1136/bjo.2007.118562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apte RS. Pegaptanib sodium for the treatment of age-related macular degeneration. Expert Opin Pharmacother. 2008;9:499–508. doi: 10.1517/14656566.9.3.499. [DOI] [PubMed] [Google Scholar]
- Trenam CW, Dabbagh AJ, Morris CJ, Blake DR. Skin inflammation induced by reactive oxygen species (ROS): an in vivo model. Br J Dermatol. 1991;125:325–9. doi: 10.1111/j.1365-2133.1991.tb14165.x. [DOI] [PubMed] [Google Scholar]
- Trouba KJ, Hamadeh HK, Amin RP, Germolec DR. Oxidative stress and its role in skin disease. Antioxidants & Redox Signaling. 2002;4:665–673. doi: 10.1089/15230860260220175. [DOI] [PubMed] [Google Scholar]
- Wojas-Pelc A, Marcinkiewicz J. What is a role of haeme oxygenase-1 in psoriasis? Current concepts of pathogenesis. Int J Exp Pathol. 2007;88:95–102. doi: 10.1111/j.1365-2613.2006.00505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trenam CW, Blake DR, Morris CJ. Skin inflammation – reactive oxygen species and the role of iron. J Invest Dermatol. 1992;99:675–682. doi: 10.1111/1523-1747.ep12613740. [DOI] [PubMed] [Google Scholar]
- Trenam CW, Dabbagh AJ, Blake DR, Morris CJ. The role of iron in an acute model of skin inflammation induced by reactive oxygen species (ROS) Br J Dermatol. 1992;126:250–256. doi: 10.1111/j.1365-2133.1992.tb00654.x. [DOI] [PubMed] [Google Scholar]
- Hider RC, Singh S, Porter JB. Iron chelating agents with clinical potential. Proc R Soc Ed B. 1992;99:137–168. [Google Scholar]
- Kim KY, Schumacher HR, Hunsche E, Wertheimer AI, Kong SX. A literature review of the epidemiology and treatment of acute gout. Clinical Therapeutics. 2003;25:1593–1617. doi: 10.1016/s0149-2918(03)80158-3. [DOI] [PubMed] [Google Scholar]
- Davies KJ, Sevanian A, Muakkassah-Kelly SF, Hochstein P. Uric acid-iron ion complexes. A new aspect of the antioxidant functions of uric acid. Biochem J. 1986;235:747–54. doi: 10.1042/bj2350747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghio AJ, Kennedy TP, Rao G, Cooke CL, Miller MJ, Hoidal JR. Complexation of iron cation by sodium urate crystals and gouty inflammation. Arch Biochem Biophys. 1994;313:215–221. doi: 10.1006/abbi.1994.1379. [DOI] [PubMed] [Google Scholar]
- Ghio AJ, Ford ES, Kennedy TP, Hoidal JR. The association between serum ferritin and uric acid in humans. Free Radical Research. 2005;39:337–342. doi: 10.1080/10715760400026088. [DOI] [PubMed] [Google Scholar]
- Facchini FS. Near-iron deficiency-induced remission of gouty arthritis. Rheumatology. 2003;42:1550–1555. doi: 10.1093/rheumatology/keg402. [DOI] [PubMed] [Google Scholar]
- Poli R, Langdon WB. Proc 3rd Conf Genetic Programming GP'98. Morgan Kaufmann, Madison, WI; 1998. On the search properties of different crossover operators in genetic programming; pp. 293–301. [Google Scholar]
- Brittenham GM. Iron chelators and iron toxicity. Alcohol. 2003;30:151–8. doi: 10.1016/s0741-8329(03)00101-0. [DOI] [PubMed] [Google Scholar]
- Tung BY, Emond MJ, Bronner MP, Raaka SD, Cotler SJ, Kowdley KV. Hepatitis C, iron status, and disease severity: relationship with HFE mutations. Gastroenterology. 2003;124:318–26. doi: 10.1053/gast.2003.50046. [DOI] [PubMed] [Google Scholar]
- Corradini E, Ferrara F, Pietrangelo A. Iron and the liver. Pediatr Endocrinol Rev. 2004;2:245–8. [PubMed] [Google Scholar]
- Rigamonti C, Andorno S, Maduli E, Capelli F, Boldorini R, Sartori M. Gender and liver fibrosis in chronic hepatitis: the role of iron status. Aliment Pharmacol Ther. 2005;21:1445–51. doi: 10.1111/j.1365-2036.2005.02517.x. [DOI] [PubMed] [Google Scholar]
- Philippe MA, Ruddell RG, Ramm GA. Role of iron in hepatic fibrosis: one piece in the puzzle. World J Gastroenterol. 2007;13:4746–54. doi: 10.3748/wjg.v13.i35.4746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohgo Y, Ohtake T, Ikuta K, Suzuki Y, Torimoto Y, Kato J. Dysregulation of systemic iron metabolism in alcoholic liver diseases. J Gastroenterol Hepatol. 2008;23:S78–81. doi: 10.1111/j.1440-1746.2007.05290.x. [DOI] [PubMed] [Google Scholar]
- Ramm GA, Ruddell RG. Hepatotoxicity of iron overload: Mechanisms of iron-induced hepatic fibrogenesis. Seminars in Liver Disease. 2005;25:433–449. doi: 10.1055/s-2005-923315. [DOI] [PubMed] [Google Scholar]
- McCullough KD, Bartfay WJ. The dose-dependent effects of chronic iron overload on the production of oxygen free radicals and vitamin E concentrations in the liver of a murine model. Biol Res Nurs. 2007;8:300–4. doi: 10.1177/109980040629873. [DOI] [PubMed] [Google Scholar]
- Petrak J, Myslivcova D, Man P, Cmejla R, Cmejlova J, Vyoral D, Elleder M, Vulpe CD. Proteomic analysis of hepatic iron overload in mice suggests dysregulation of urea cycle, impairment of fatty acid oxidation, and changes in the methylation cycle. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1490–8. doi: 10.1152/ajpgi.00455.2006. [DOI] [PubMed] [Google Scholar]
- Aigner E, Theurl I, Theurl M, Lederer D, Haufe H, Dietze O, Strasser M, Datz C, Weiss G. Pathways underlying iron accumulation in human nonalcoholic fatty liver disease. Am J Clin Nutr. 2008;87:1374–83. doi: 10.1093/ajcn/87.5.1374. [DOI] [PubMed] [Google Scholar]
- Chen CJ, Yu MW, Liaw YF. Epidemiological characteristics and risk factors of hepatocellular carcinoma. J Gastroenterol Hepatol. 1997;12:S294–308. doi: 10.1111/j.1440-1746.1997.tb00513.x. [DOI] [PubMed] [Google Scholar]
- Asare GA, Mossanda KS, Kew MC, Paterson AC, Kahler-Venter CP, Siziba K. Hepatocellular carcinoma caused by iron overload: A possible mechanism of direct hepatocarcinogenicity. Toxicology. 2006;219:41–52. doi: 10.1016/j.tox.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Kato J, Kobune M, Nakamura T, Kuroiwa G, Takada K, Takimoto R, Sato Y, Fujikawa K, Takahashi M, Takayama T, Ikeda T, Niitsu Y. Normalization of elevated hepatic 8-hydroxy-2'-deoxyguanosine levels in chronic hepatitis C patients by phlebotomy and low iron diet. Cancer Res. 2001;61:8697–702. [PubMed] [Google Scholar]
- Ritter C, Reinke A, Andrades M, Martins MR, Rocha J, Menna-Barreto S, Quevedo J, Moreira JC, Dal-Pizzol F. Protective effect of N-acetylcysteine and deferoxamine on carbon tetrachloride-induced acute hepatic failure in rats. Crit Care Med. 2004;32:2079–83. doi: 10.1097/01.ccm.0000142699.54266.d9. [DOI] [PubMed] [Google Scholar]
- Bridle K, Cheung TK, Murphy T, Walters M, Anderson G, Crawford DG, Fletcher LM. Hepcidin is down-regulated in alcoholic liver injury: implications for the pathogenesis of alcoholic liver disease. Alcohol Clin Exp Res. 2006;30:106–12. doi: 10.1111/j.1530-0277.2006.00002.x. [DOI] [PubMed] [Google Scholar]
- Antó JM, Vermeire P, Vestbo J, Sunyer J. Epidemiology of chronic obstructive pulmonary disease. European Respiratory Journal. 2001;17:982–994. doi: 10.1183/09031936.01.17509820. [DOI] [PubMed] [Google Scholar]
- White AJ, Gompertz S, Stockley RA. Chronic obstructive pulmonary disease .6: The aetiology of exacerbations of chronic obstructive pulmonary disease. Thorax. 2003;58:73–80. doi: 10.1136/thorax.58.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsoumakidou M, Siafakas NM. Novel insights into the aetiology and pathophysiology of increased airway inflammation during COPD exacerbations. Respiratory Research. 2006;7 doi: 10.1186/1465-9921-7-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapey E, Stockley RA. COPD exacerbations 2: Aetiology. Thorax. 2006;61:250–258. doi: 10.1136/thx.2005.041822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Roisin R. Toward a consensus definition for COPD exacerbations. Chest. 2000;117:398S–401S. doi: 10.1378/chest.117.5_suppl_2.398s. [DOI] [PubMed] [Google Scholar]
- Weinberg ED. Tobacco smoke iron: an initiator/promoter of multiple diseases. Biometals. 2008 doi: 10.1007/s10534-008-9156-5. [DOI] [PubMed] [Google Scholar]
- Rahman I, van Schadewijk AA, Crowther AJ, Hiemstra PS, Stolk J, MacNee W, De Boer WI. 4-Hydroxy-2-nonenal, a specific lipid peroxidation product, is elevated in lungs of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;166:490–5. doi: 10.1164/rccm.2110101. [DOI] [PubMed] [Google Scholar]
- Langen RCJ, Korn SH, Wouters EFM. ROS in the local and systemic pathogenesis of COPD. Free Rad Biol Med. 2003;35:226–235. doi: 10.1016/s0891-5849(03)00316-2. [DOI] [PubMed] [Google Scholar]
- Barnes PJ. Mediators of chronic obstructive pulmonary disease. Pharmacol Rev. 2004;56:515–548. doi: 10.1124/pr.56.4.2. [DOI] [PubMed] [Google Scholar]
- Santos MC, Oliveira AL, Viegas-Crespo AM, Vicente L, Barreiros A, Monteiro P, Pinheiro T, De Almeida AB. Systemic markers of the redox balance in chronic obstructive pulmonary disease. Biomarkers. 2004;9:461–469. doi: 10.1080/13547500400024768. [DOI] [PubMed] [Google Scholar]
- Kinnula VL. Focus on antioxidant enzymes and antioxidant strategies in smoking related airway diseases. Thorax. 2005;60:693–700. doi: 10.1136/thx.2004.037473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rytila P, Rehn T, Ilumets H, Rouhos A, Sovijarvi A, Myllarniemi M, Kinnula VL. Increased oxidative stress in asymptomatic current chronic smokers and GOLD stage 0 COPD. Respiratory Research. 2006;7 doi: 10.1186/1465-9921-7-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson CS, Koch LG, Britton SL. Aerobic capacity, oxidant stress, and chronic obstructive pulmonary disease – A new take on an old hypothesis. Pharmacol Therapeut. 2006;110:71–82. doi: 10.1016/j.pharmthera.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Kinnula VL, Ilumets H, Myllarnlemi M, Sovijarvi A, Rytila P. 8-Isoprostane as a marker of oxidative stress in nonsymptomatic cigarette smokers and COPD. European Respiratory Journal. 2007;29:51–55. doi: 10.1183/09031936.00023606. [DOI] [PubMed] [Google Scholar]
- Huang X, Li WH, Attfield MD, Nadas A, Frenkel K, Finkelman RB. Mapping and prediction of coal workers' pneumoconiosis with bioavailable iron content in the bituminous coals. Environmental Health Perspectives. 2005;113:964–968. doi: 10.1289/ehp.7679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutti A, Corradi M, Goldoni M, Vettori MV, Bernard A, Apostoli P. Exhaled metallic elements and serum pneumoproteins in asymptomatic smokers and patients with COPD or asthma. Chest. 2006;129:1288–1297. doi: 10.1378/chest.129.5.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter JD, Ghio AJ, Samet JM, Devlin RB. Cytokine production by human airway epithelial cells after exposure to an air pollution particle is metal-dependent. Toxicol Appl Pharmacol. 1997;146:180–8. doi: 10.1006/taap.1997.8254. [DOI] [PubMed] [Google Scholar]
- Corhay JL, Weber G, Bury T, Mariz S, Roelandts I, Radermecker MF. Iron content in human alveolar macrophages. Eur Resp J. 1992;5:804–809. [PubMed] [Google Scholar]
- Stites SW, Nelson ME, Wesselius LJ. Transferrin concentrations in serum and lower respiratory tract fluid of mechanically ventilated patients with COPD or ARDS. Chest. 1995;107:1681–1685. doi: 10.1378/chest.107.6.1681. [DOI] [PubMed] [Google Scholar]
- Southorn PA, Powis G. Free radicals in medicine .2. involvement in human disease. Mayo Clinic Proceedings. 1988;63:390–408. doi: 10.1016/s0025-6196(12)64862-9. [DOI] [PubMed] [Google Scholar]
- Quinlan GJ, Lamb NJ, Tilley R, Evans TW, Gutteridge JMC. Plasma hypoxanthine levels in ARDS: Implications for oxidative stress, morbidity, and mortality. Amer J Resp Crit Care Med. 1997;155:479–484. doi: 10.1164/ajrccm.155.2.9032182. [DOI] [PubMed] [Google Scholar]
- Quinlan GJ, Evans TW, Gutteridge JMC. Oxidative damage to plasma proteins in adult respiratory distress syndrome. Free Radical Research. 1994;20:289–298. doi: 10.3109/10715769409145628. [DOI] [PubMed] [Google Scholar]
- Gutteridge JMC, Quinlan GJ. Antioxidant protection against organic and inorganic oxygen radicals by normal human plasma – the important primary role for iron-binding and iron-oxidizing proteins. Biochim Biophys Acta. 1993;1156:144–150. doi: 10.1016/0304-4165(93)90129-v. [DOI] [PubMed] [Google Scholar]
- Reid DW, Lam QT, Schneider H, Walters EH. Airway iron and iron-regulatory cytokines in cystic fibrosis. European Respiratory Journal. 2004;24:286–291. doi: 10.1183/09031936.04.00104803. [DOI] [PubMed] [Google Scholar]
- Doll R, Hill AB. Smoking and carcinoma of the lung; preliminary report. Br Med J. 1950;2:739–48. doi: 10.1136/bmj.2.4682.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peto R, Darby S, Deo H, Silcocks P, Whitley E, Doll R. Smoking, smoking cessation, and lung cancer in the UK since 1950: combination of national statistics with two case-control studies. BMJ. 2000;321:323–9. doi: 10.1136/bmj.321.7257.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doll R, Hill AB. The mortality of doctors in relation to their smoking habits: a preliminary report. 1954. BMJ. 2004;328:1529–33. doi: 10.1136/bmj.328.7455.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doll R, Peto R, Boreham J, Sutherland I. Mortality from cancer in relation to smoking: 50 years observations on British doctors. Br J Cancer. 2005;92:426–9. doi: 10.1038/sj.bjc.6602359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AB, Bohling T, Heires A, Linder J, Rennard SI. Lower respiratory tract iron burden is increased in association with cigarette smoking. J Lab Clin Med. 1991;117:493–9. [PubMed] [Google Scholar]
- Dreher KL, Jaskot RH, Lehmann JR, Richards JH, McGee JK, Ghio AJ, Costa DL. Soluble transition metals mediate residual oil fly ash induced acute lung injury. J Toxicol Environ Health. 1997;50:285–305. [PubMed] [Google Scholar]
- Mateos F, Brock JH, Perez-Arellano JL. Iron metabolism in the lower respiratory tract. Thorax. 1998;53:594–600. doi: 10.1136/thx.53.7.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boelaert JR, Gomes MS, Gordeuk VR. Smoking, iron, and tuberculosis. Lancet. 2003;362:1243–4. doi: 10.1016/S0140-6736(03)14529-1. [DOI] [PubMed] [Google Scholar]
- Ghio AJ, Carter JD, Richards JH, Richer LD, Grissom CK, Elstad MR. Iron and iron-related proteins in the lower respiratory tract of patients with acute respiratory distress syndrome. Crit Care Med. 2003;31:395–400. doi: 10.1097/01.CCM.0000050284.35609.97. [DOI] [PubMed] [Google Scholar]
- Ernst P. Review article: the role of inflammation in the pathogenesis of gastric cancer. Aliment Pharmacol Ther. 1999;13:13–18. doi: 10.1046/j.1365-2036.1999.00003.x. [DOI] [PubMed] [Google Scholar]
- Kelloff GJ, Crowell JA, Steele VE, Lubet RA, Malone WA, Boone CW, Kopelovich L, Hawk ET, Lieberman R, Lawrence JA, Ali I, Viner JL, Sigman CC. Progress in cancer chemoprevention: development of diet-derived chemopreventive agents. J Nutr. 2000;130:467S–471S. doi: 10.1093/jn/130.2.467S. [DOI] [PubMed] [Google Scholar]
- Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–45. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggarwal BB. Nuclear factor-kappaB: the enemy within. Cancer Cell. 2004;6:203–8. doi: 10.1016/j.ccr.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Vakkila J, Lotze MT. Inflammation and necrosis promote tumour growth. Nat Rev Immunol. 2004;4:641–8. doi: 10.1038/nri1415. [DOI] [PubMed] [Google Scholar]
- Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;7:211–7. doi: 10.1016/j.ccr.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749–59. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- Li Q, Withoff S, Verma IM. Inflammation-associated cancer: NF-kappaB is the lynchpin. Trends Immunol. 2005;26:318–25. doi: 10.1016/j.it.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Steele VE, Kelloff GJ. Development of cancer chemopreventive drugs based on mechanistic approaches. Mutat Res. 2005;591:16–23. doi: 10.1016/j.mrfmmm.2005.04.018. [DOI] [PubMed] [Google Scholar]
- Aggarwal BB, Shishodia S, Sandur SK, Pandey MK, Sethi G. Inflammation and cancer: how hot is the link? Biochem Pharmacol. 2006;72:1605–21. doi: 10.1016/j.bcp.2006.06.029. [DOI] [PubMed] [Google Scholar]
- Karin M. NF-kappaB and cancer: mechanisms and targets. Mol Carcinog. 2006;45:355–61. doi: 10.1002/mc.20217. [DOI] [PubMed] [Google Scholar]
- Kelloff GJ, Lippman SM, Dannenberg AJ, Sigman CC, Pearce HL, Reid BJ, Szabo E, Jordan VC, Spitz MR, Mills GB, Papadimitrakopoulou VA, Lotan R, Aggarwal BB, Bresalier RS, Kim J, Arun B, Lu KH, Thomas ME, Rhodes HE, Brewer MA, Follen M, Shin DM, Parnes HL, Siegfried JM, Evans AA, Blot WJ, Chow WH, Blount PL, Maley CC, Wang KK, Lam S, Lee JJ, Dubinett SM, Engstrom PF, Meyskens FL, Jr, O'Shaughnessy J, Hawk ET, Levin B, Nelson WG, Hong WK. Progress in chemoprevention drug development: the promise of molecular biomarkers for prevention of intraepithelial neoplasia and cancer–a plan to move forward. Clin Cancer Res. 2006;12:3661–97. doi: 10.1158/1078-0432.CCR-06-1104. [DOI] [PubMed] [Google Scholar]
- Rakoff-Nahoum S. Why cancer and inflammation? Yale J Biol Med. 2006;79:123–30. [PMC free article] [PubMed] [Google Scholar]
- Yoshimura A. Signal transduction of inflammatory cytokines and tumor development. Cancer Sci. 2006;97:439–47. doi: 10.1111/j.1349-7006.2006.00197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain SP, Harris CC. Inflammation and cancer: an ancient link with novel potentials. Int J Cancer. 2007;121:2373–80. doi: 10.1002/ijc.23173. [DOI] [PubMed] [Google Scholar]
- Allavena P, Garlanda C, Borrello MG, Sica A, Mantovani A. Pathways connecting inflammation and cancer. Curr Opin Genet Dev. 2008;18:3–10. doi: 10.1016/j.gde.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Borrello MG, Degl'Innocenti D, Pierotti MA. Inflammation and cancer: the oncogene-driven connection. Cancer Lett. 2008;267:262–70. doi: 10.1016/j.canlet.2008.03.060. [DOI] [PubMed] [Google Scholar]
- Haverkamp J, Charbonneau B, Ratliff TL. Prostate inflammation and its potential impact on prostate cancer: a current review. J Cell Biochem. 2008;103:1344–53. doi: 10.1002/jcb.21536. [DOI] [PubMed] [Google Scholar]
- Ishikawa K, Takenaga K, Akimoto M, Koshikawa N, Yamaguchi A, Imanishi H, Nakada K, Honma Y, Hayashi J. ROS-generating mitochondrial DNA mutations can regulate tumor cell metastasis. Science. 2008;320:661–4. doi: 10.1126/science.1156906. [DOI] [PubMed] [Google Scholar]
- Karin M. The IkappaB kinase – a bridge between inflammation and cancer. Cell Res. 2008;18:334–42. doi: 10.1038/cr.2008.30. [DOI] [PubMed] [Google Scholar]
- Maeda S, Omata M. Inflammation and cancer: role of nuclear factor-kappaB activation. Cancer Sci. 2008;99:836–42. doi: 10.1111/j.1349-7006.2008.00763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–44. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- Sica A, Allavena P, Mantovani A. Cancer related inflammation: the macrophage connection. Cancer Lett. 2008;267:204–15. doi: 10.1016/j.canlet.2008.03.028. [DOI] [PubMed] [Google Scholar]
- Vasto S, Carruba G, Lio D, Colonna-Romano G, Di Bona D, Candore G, Caruso C. Inflammation, ageing and cancer. Mech Ageing Dev. 2008 doi: 10.1016/j.mad.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Baron JA, Sandler RS. Nonsteroidal anti-inflammatory drugs and cancer prevention. Annu Rev Med. 2000;51:511–23. doi: 10.1146/annurev.med.51.1.511. [DOI] [PubMed] [Google Scholar]
- Rostom A, Dube C, Lewin G, Tsertsvadze A, Barrowman N, Code C, Sampson M, Moher D. Nonsteroidal anti-inflammatory drugs and cyclooxygenase-2 inhibitors for primary prevention of colorectal cancer: a systematic review prepared for the U.S. Preventive Services Task Force. Ann Intern Med. 2007;146:376–89. doi: 10.7326/0003-4819-146-5-200703060-00010. [DOI] [PubMed] [Google Scholar]
- Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1:1311–1315. doi: 10.1016/s0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- Davey HM, Kell DB. Flow cytometry and cell sorting of heterogeneous microbial populations: the importance of single-cell analysis. Microbiol Rev. 1996;60:641–696. doi: 10.1128/mr.60.4.641-696.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall B. Helicobacter connections. ChemMedChem. 2006;1:783–802. doi: 10.1002/cmdc.200600153. [DOI] [PubMed] [Google Scholar]
- Rakba N, Aouad F, Henry C, Caris C, Morel I, Baret P, Pierre JL, Brissot P, Ward RJ, Lescoat G, Crichton RR. Iron mobilisation and cellular protection by a new synthetic chelator O-Trensox. Biochem Pharmacol. 1998;55:1797–806. doi: 10.1016/s0006-2952(98)00009-4. [DOI] [PubMed] [Google Scholar]
- Le NTV, Richardson DR. The role of iron in cell cycle progression and the proliferation of neoplastic cells. Biochimica Et Biophysica Acta-Reviews on Cancer. 2002;1603:31–46. doi: 10.1016/s0304-419x(02)00068-9. [DOI] [PubMed] [Google Scholar]
- Buss JL, Torti FM, Torti SV. The role of iron chelation in cancer therapy. Curr Med Chem. 2003;10:1021–34. doi: 10.2174/0929867033457638. [DOI] [PubMed] [Google Scholar]
- Lovejoy DB, Richardson DR. Iron chelators as anti-neoplastic agents: Current developments and promise of the PIH class of chelators. Current Medicinal Chemistry. 2003;10:1035–1049. doi: 10.2174/0929867033457557. [DOI] [PubMed] [Google Scholar]
- Buss JL, Greene BT, Turner J, Torti FM, Torti SV. Iron chelators in cancer chemotherapy. Curr Top Med Chem. 2004;4:1623–35. doi: 10.2174/1568026043387269. [DOI] [PubMed] [Google Scholar]
- Hanai J, Mammoto T, Seth P, Mori K, Karumanchi SA, Barasch J, Sukhatme VP. Lipocalin 2 diminishes invasiveness and metastasis of Ras-transformed cells. J Biol Chem. 2005;280:13641–7. doi: 10.1074/jbc.M413047200. [DOI] [PubMed] [Google Scholar]
- Kalinowski DS, Richardson DR. The evolution of iron chelators for the treatment of iron overload disease and cancer. Pharmacol Rev. 2005;57:547–83. doi: 10.1124/pr.57.4.2. [DOI] [PubMed] [Google Scholar]
- Pahl PMB, Horwitz LD. Cell permeable iron chelators as potential cancer chemotherapeutic agents. Cancer Invest. 2005;23:683–691. doi: 10.1080/07357900500359976. [DOI] [PubMed] [Google Scholar]
- Nie GJ, Chen GH, Sheftel AD, Pantopoulos K, Ponka P. In vivo tumor growth is inhibited by cytosolic iron deprivation caused by the expression of mitochondrial ferritin. Blood. 2006;108:2428–2434. doi: 10.1182/blood-2006-04-018341. [DOI] [PubMed] [Google Scholar]
- Edgren G, Nyren O, Melbye M. Cancer as a ferrotoxic disease: are we getting hard stainless evidence? J Natl Cancer Inst. 2008;100:976–7. doi: 10.1093/jnci/djn225. [DOI] [PubMed] [Google Scholar]
- Huang X. Does iron have a role in breast cancer? Lancet Oncol. 2008;9:803–7. doi: 10.1016/S1470-2045(08)70200-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharski LR, Chow BK, Howes PS, Shamayeva G, Baron JA, Dalman RL, Malenka DJ, Ozaki CK, Lavori PW. Decreased cancer risk after iron reduction in patients with peripheral arterial disease: results from a randomized trial. J Natl Cancer Inst. 2008;100:996–1002. doi: 10.1093/jnci/djn209. [DOI] [PubMed] [Google Scholar]
- Lim R, Ahmed N, Borregaard N, Riley C, Wafai R, Thompson EW, Quinn MA, Rice GE. Neutrophil gelatinase-associated lipocalin (NGAL) an early-screening biomarker for ovarian cancer: NGAL is associated with epidermal growth factor-induced epithelio-mesenchymal transition. Int J Cancer. 2007;120:2426–2434. doi: 10.1002/ijc.22352. [DOI] [PubMed] [Google Scholar]
- Iannetti A, Pacifico F, Acquaviva R, Lavorgna A, Crescenzi E, Vascotto C, Tell G, Salzano AM, Scaloni A, Vuttariello E, Chiappetta G, Formisano S, Leonardi A. The neutrophil gelatinase-associated lipocalin (NGAL), a NF-{kappa}B-regulated gene, is a survival factor for thyroid neoplastic cells. Proc Natl Acad Sci USA. 2008;105:14058–63. doi: 10.1073/pnas.0710846105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson RL, Davis FG, Sutter E, Sobin LH, Kikendall JW, Bowen P. Body iron stores and risk of colonic neoplasia. J Natl Cancer Inst. 1994;86:455–60. doi: 10.1093/jnci/86.6.455. [DOI] [PubMed] [Google Scholar]
- Okada S. Iron-induced tissue damage and cancer: The role of reactive oxygen species-free radicals. Pathol Int. 1996;46:311–332. doi: 10.1111/j.1440-1827.1996.tb03617.x. [DOI] [PubMed] [Google Scholar]
- Toyokuni S. Iron-induced carcinogenesis: The role of redox regulation. Free Radical Biology and Medicine. 1996;20:553–566. doi: 10.1016/0891-5849(95)02111-6. [DOI] [PubMed] [Google Scholar]
- Weinberg ED. The role of iron in cancer. Eur J Cancer Prev. 1996;5:19–36. [PubMed] [Google Scholar]
- Weinberg ED. Iron loading and disease surveillance. Emerg Infect Dis. 1999;5:346–52. doi: 10.3201/eid0503.990305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Núñez MT, Tapia V, Toyokuni S, Okada S. Iron-induced oxidative damage in colon carcinoma (Caco-2) cells. Free Radic Res. 2001;34:57–68. doi: 10.1080/10715760100300061. [DOI] [PubMed] [Google Scholar]
- Glei M, Latunde-Dada GO, Klinder A, Becker TW, Hermann U, Voigt K, Pool-Zobel BL. Iron-overload induces oxidative DNA damage in the human colon carcinoma cell line HT29 clone 19A. Mutat Res. 2002;519:151–61. doi: 10.1016/s1383-5718(02)00135-3. [DOI] [PubMed] [Google Scholar]
- Toyokuni S. Iron and carcinogenesis: from Fenton reaction to target genes. Redox Rep. 2002;7:189–97. doi: 10.1179/135100002125000596. [DOI] [PubMed] [Google Scholar]
- Deugnier Y. Iron and liver cancer. Alcohol. 2003;30:145–150. doi: 10.1016/s0741-8329(03)00129-0. [DOI] [PubMed] [Google Scholar]
- Klaunig JE, Kamendulis LM. The role of oxidative stress in carcinogenesis. Annu Rev Pharmacol Toxicol. 2004;44:239–67. doi: 10.1146/annurev.pharmtox.44.101802.121851. [DOI] [PubMed] [Google Scholar]
- Storz P. Reactive oxygen species in tumor progression. Front Biosci. 2005;10:1881–96. doi: 10.2741/1667. [DOI] [PubMed] [Google Scholar]
- Lee SK, Lee JJ, Lee HJ, Lee J, Jeon BH, Jun CD, Lee SK, Kim EC. Iron chelator-induced growth arrest and cytochrome c-dependent apoptosis in immortalized and malignant oral keratinocytes. Journal of Oral Pathology & Medicine. 2006;35:218–226. doi: 10.1111/j.1600-0714.2006.00415.x. [DOI] [PubMed] [Google Scholar]
- Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chemico-Biological Interactions. 2006;160:1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Wu J, Eckard J, Chen H, Costa M, Frenkel K, Huang X. Altered iron homeostasis involvement in arsenite-mediated cell transformation. Free Radic Biol Med. 2006;40:444–52. doi: 10.1016/j.freeradbiomed.2005.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallianpur AR, Lee SA, Gao YT, Lu W, Zheng Y, Ruan ZX, Dai Q, Gu K, Shu XO, Zheng W. Dietary animal-derived iron and fat intake and breast cancer risk in the Shanghai Breast Cancer Study. Breast Cancer Res Treat. 2008;107:123–32. doi: 10.1007/s10549-007-9538-3. [DOI] [PubMed] [Google Scholar]
- Prá D, Franke SI, Giulian R, Yoneama ML, Dias JF, Erdtmann B, Henriques JA. Genotoxicity and mutagenicity of iron and copper in mice. Biometals. 2008;21:289–97. doi: 10.1007/s10534-007-9118-3. [DOI] [PubMed] [Google Scholar]
- Yang WS, Stockwell BR. Synthetic lethal screening identifies compounds activating iron-dependent, nonapoptotic cell death in oncogenic-RAS-harboring cancer cells. Chem Biol. 2008;15:234–45. doi: 10.1016/j.chembiol.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson DR, Kalinowski DS, Lau S, Jansson PJ, Lovejoy DB. Cancer cell iron metabolism and the development of potent iron chelators as anti-tumour agents. Biochim Biophys Acta. 2008. [DOI] [PubMed]
- Weitzman SA, Graceffa P. Asbestos catalyzes hydroxyl and superoxide radical generation from hydrogen peroxide. Arch Biochem Biophys. 1984;228:373–6. doi: 10.1016/0003-9861(84)90078-x. [DOI] [PubMed] [Google Scholar]
- Gulumian M, van Wyk JA. Hydroxyl radical production in the presence of fibres by a Fenton-type reaction. Chem Biol Interact. 1987;62:89–97. doi: 10.1016/0009-2797(87)90081-0. [DOI] [PubMed] [Google Scholar]
- Mossman BT, Marsh JP, Shatos MA, Doherty J, Gilbert R, Hill S. Implication of active oxygen species as second messengers of asbestos toxicity. Drug Chem Toxicol. 1987;10:157–80. doi: 10.3109/01480548709042587. [DOI] [PubMed] [Google Scholar]
- Shatos MA, Doherty JM, Marsh JP, Mossman BT. Prevention of asbestos-induced cell death in rat lung fibroblasts and alveolar macrophages by scavengers of active oxygen species. Environ Res. 1987;44:103–16. doi: 10.1016/s0013-9351(87)80090-7. [DOI] [PubMed] [Google Scholar]
- Kamp DW, Graceffa P, Pryor WA, Weitzman SA. The role of free radicals in asbestos-induced diseases. Free Radic Biol Med. 1992;12:293–315. doi: 10.1016/0891-5849(92)90117-y. [DOI] [PubMed] [Google Scholar]
- Quinlan TR, Marsh JP, Janssen YM, Borm PA, Mossman BT. Oxygen radicals and asbestos-mediated disease. Environ Health Perspect. 1994;102:107–10. doi: 10.1289/ehp.94102s10107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy JA, Aust AE. Iron in asbestos chemistry and carcinogenicity. Chem Rev. 1995;95:97–118. [Google Scholar]
- Gilmour PS, Brown DM, Beswick PH, MacNee W, Rahman I, Donaldson K. Free radical activity of industrial fibers: role of iron in oxidative stress and activation of transcription factors. Environ Health Perspect. 1997;105:1313–7. doi: 10.1289/ehp.97105s51313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Governa M, Amati M, Fontana S, Visona I, Botta GC, Mollo F, Bellis D, Bo P. Role of iron in asbestos-body-induced oxidant radical generation. J Toxicol Environ Health A. 1999;58:279–87. doi: 10.1080/009841099157241. [DOI] [PubMed] [Google Scholar]
- Kamp DW, Weitzman SA. The molecular basis of asbestos induced lung injury. Thorax. 1999;54:638–52. doi: 10.1136/thx.54.7.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu A, Wu LJ, Santella RM, Hei TK. Role of oxyradicals in mutagenicity and DNA damage induced by crocidolite asbestos in mammalian cells. Cancer Res. 1999;59:5922–6. [PubMed] [Google Scholar]
- Aljandali A, Pollack H, Yeldandi A, Li Y, Weitzman SA, Kamp DW. Asbestos causes apoptosis in alveolar epithelial cells: role of iron-induced free radicals. J Lab Clin Med. 2001;137:330–9. doi: 10.1067/mlc.2001.114826. [DOI] [PubMed] [Google Scholar]
- Gazzano E, Turci F, Foresti E, Putzu MG, Aldieri E, Silvagno F, Lesci IG, Tomatis M, Riganti C, Romano C, Fubini B, Roveri N, Ghigo D. Iron-loaded synthetic chrysotile: a new model solid for studying the role of iron in asbestos toxicity. Chem Res Toxicol. 2007;20:380–7. doi: 10.1021/tx600354f. [DOI] [PubMed] [Google Scholar]
- Pervaiz S. Pro-oxidant milieu blunts scissors: Insight into tumor progression, drug resistance, and novel druggable targets. Curr Pharmaceut Des. 2006;12:4469–4477. doi: 10.2174/138161206779010503. [DOI] [PubMed] [Google Scholar]
- Perrone GG, Tan SX, Dawes IW. Reactive oxygen species and yeast apoptosis. Biochim Biophys Acta. 2008;1783:1354–68. doi: 10.1016/j.bbamcr.2008.01.023. [DOI] [PubMed] [Google Scholar]
- Scheibel LW, Adler A. Anti-malarial activity of selected aromatic chelators. Mol Pharmacol. 1980;18:320–325. [PubMed] [Google Scholar]
- Scheibel LW, Rodriguez S. Antimalarial activity of selected aromatic chelators V. Localization of 59Fe in Plasmodium falciparum in the presence of oxines. Prog Clin Biol Res. 1989;313:119–49. [PubMed] [Google Scholar]
- Hershko C, Gordeuk VR, Thuma PE, Theanacho EN, Spira DT, Hider RC, Peto TEA, Brittenham GM. The antimalaria effect of iron chelators – studies in animal models and in humans with mild Falciparum malaria. J Inorg Biochem. 1992;47:267–277. doi: 10.1016/0162-0134(92)84072-u. [DOI] [PubMed] [Google Scholar]
- Gordeuk V, Thuma P, Brittenham G, McLaren C, Parry D, Backenstose A, Biemba G, Msiska R, Holmes L, McKinley E, Vargas L, Gilkeson R, Poltera AA. Effect of iron chelation therapy on recovery from deep coma in children with cerebral malaria. New Engl J Med. 1992;327:1473–1477. doi: 10.1056/NEJM199211193272101. [DOI] [PubMed] [Google Scholar]
- Gordeuk VR, Thuma PE, Brittenham GM, Biemba G, Zulu S, Simwanza G, Kalense P, Mhango A, Parry D, Poltera AA, Aikawa M. Iron chelation as a chemotherapeutic strategy for Falciparum malaria. Amer J Trop Med Hyg. 1993;48:193–197. doi: 10.4269/ajtmh.1993.48.193. [DOI] [PubMed] [Google Scholar]
- Voest EE, Vreugdenhil G, Marx JJM. Iron-chelating agents in non-iron overload conditions. Ann Internal Med. 1994;120:490–499. doi: 10.7326/0003-4819-120-6-199403150-00008. [DOI] [PubMed] [Google Scholar]
- Hider RC, Liu ZD. The treatment of malaria with iron chelators. J Pharm Pharmacol. 1997;49:59–64. [Google Scholar]
- Cabantchik ZI, Moody-Haupt S, Gordeuk VR. Iron chelators as anti-infectives; malaria as a paradigm. FEMS Immunol Med Microbiol. 1999;26:289–98. doi: 10.1111/j.1574-695X.1999.tb01401.x. [DOI] [PubMed] [Google Scholar]
- Mabeza GF, Loyevsky M, Gordeuk VR, Weiss G. Iron chelation therapy for malaria: A review. Pharmacology & Therapeutics. 1999;81:53–75. doi: 10.1016/s0163-7258(98)00037-0. [DOI] [PubMed] [Google Scholar]
- Fritsche G, Larcher C, Schennach H, Weiss G. Regulatory interactions between iron and nitric oxide metabolism for immune defense against Plasmodium falciparum infection. J Infect Dis. 2001;183:1388–94. doi: 10.1086/319860. [DOI] [PubMed] [Google Scholar]
- Pradines B, Rolain JM, Ramiandrasoa F, Fusai T, Mosnier J, Rogier C, Daries W, Baret E, Kunesch G, Le Bras J, Parzy D. Iron chelators as antimalarial agents: in vitro activity of dicatecholate against Plasmodium falciparum. J Antimicrob Chemother. 2002;50:177–87. doi: 10.1093/jac/dkf104. [DOI] [PubMed] [Google Scholar]
- Birch N, Wang X, Chong HS. Iron chelators as therapeutic iron depletion agents. Exp Opin Therapeut Pat. 2006;16:1533–1556. [Google Scholar]
- Golenser J, Domb A, Mordechai-Daniel T, Leshem B, Luty A, Kremsner P. Iron chelators: correlation between effects on Plasmodium spp. and immune functions. J Parasitol. 2006;92:170–7. doi: 10.1645/GE-3517.1. [DOI] [PubMed] [Google Scholar]
- Melnyk P, Leroux V, Sergheraert C, Grellier P. Design, synthesis and in vitro antimalarial activity of an acylhydrazone library. Bioorg Med Chem Lett. 2006;16:31–35. doi: 10.1016/j.bmcl.2005.09.058. [DOI] [PubMed] [Google Scholar]
- Pradines B, Tall A, Ramiandrasoa FR, Spiegel A, Sokhna C, Fusai T, Mosnier J, Daries W, Trape JF, Kunesch G, Parzy D, Rogier C. In vitro activity of iron-binding compounds against Senegalese isolates of Plasmodium falciparum. J Antimicrobial Chemotherapy. 2006;57:1093–1099. doi: 10.1093/jac/dkl117. [DOI] [PubMed] [Google Scholar]
- Doherty CP. Host-pathogen interactions: the role of iron. J Nutr. 2007;137:1341–4. doi: 10.1093/jn/137.5.1341. [DOI] [PubMed] [Google Scholar]
- Vangapandu S, Jain M, Kaur K, Patil P, Patel SR, Jain R. Recent advances in antimalarial drug development. Medicinal Research Reviews. 2007;27:65–107. doi: 10.1002/med.20062. [DOI] [PubMed] [Google Scholar]
- Thuma PE, Mabeza GF, Biemba G, Bhat GJ, McLaren CE, Moyo VM, Zulu S, Khumalo H, Mabeza P, M'Hango A, Parry D, Poltera AA, Brittenham GM, Gordeuk VR. Effect of iron chelation therapy on mortality in Zambian children with cerebral malaria. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1998;92:214–218. doi: 10.1016/s0035-9203(98)90753-2. [DOI] [PubMed] [Google Scholar]
- Meshnick SR, Yang YZ, Lima V, Kuypers F, Kamchonwongpaisan S, Yuthavong Y. Iron-dependent free radical generation from the antimalarial agent artemisinin (qinghaosu) Antimicrob Agents Chemother. 1993;37:1108–14. doi: 10.1128/aac.37.5.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamchonwongpaisan S, Meshnick SR. The mode of action of the antimalarial artemisinin and its derivatives. Gen Pharmacol. 1996;27:587–92. doi: 10.1016/0306-3623(95)02047-0. [DOI] [PubMed] [Google Scholar]
- Meshnick SR, Taylor TE, Kamchonwongpaisan S. Artemisinin and the antimalarial endoperoxides: from herbal remedy to targeted chemotherapy. Microbiol Rev. 1996;60:301–15. doi: 10.1128/mr.60.2.301-315.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YK, Yue ZY, Wu YL. Interaction of Qinghaosu (artemisinin) with cysteine sulfhydryl mediated by traces of non-heme iron. Angewandte Chemie-International Edition. 1999;38:2580–2582. doi: 10.1002/(sici)1521-3773(19990903)38:17<2580::aid-anie2580>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Li W, Mo W, Shen D, Sun L, Wang J, Lu S, Gitschier JM, Zhou B. Yeast model uncovers dual roles of mitochondria in action of artemisinin. PLoS Genet. 2005;1:e36. doi: 10.1371/journal.pgen.0010036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efferth T, Benakis A, Romero MR, Tomicic M, Rauh R, Steinbach D, Hafer R, Stamminger T, Oesch F, Kaina B, Marschall M. Enhancement of cytotoxicity of artemisinins toward cancer cells by ferrous iron. Free Radic Biol Med. 2004;37:998–1009. doi: 10.1016/j.freeradbiomed.2004.06.023. [DOI] [PubMed] [Google Scholar]
- Haynes RK, Chan WC, Lung CM, Uhlemann AC, Eckstein U, Taramelli D, Parapini S, Monti D, Krishna S. The Fe2+-mediated decomposition, PfATP6 Binding, and antimalarial activities of artemisone and other artemisinins: the unlikelihood of C-centered radicals as bioactive intermediates. Chem Med Chem. 2007;2:1480–1497. doi: 10.1002/cmdc.200700108. [DOI] [PubMed] [Google Scholar]
- Nakase I, Lai H, Singh NP, Sasaki T. Anticancer properties of artemisinin derivatives and their targeted delivery by transferrin conjugation. Int J Pharm. 2008;354:28–33. doi: 10.1016/j.ijpharm.2007.09.003. [DOI] [PubMed] [Google Scholar]
- White NJ. Qinghaosu (artemisinin): the price of success. Science. 2008;320:330–4. doi: 10.1126/science.1155165. [DOI] [PubMed] [Google Scholar]
- Eckstein-Ludwig U, Webb RJ, Van Goethem ID, East JM, Lee AG, Kimura M, O'Neill PM, Bray PG, Ward SA, Krishna S. Artemisinins target the SERCA of Plasmodium falciparum. Nature. 2003;424:957–61. doi: 10.1038/nature01813. [DOI] [PubMed] [Google Scholar]
- Boyd PW, Jickells T, Law CS, Blain S, Boyle EA, Buesseler KO, Coale KH, Cullen JJ, de Baar HJ, Follows M, Harvey M, Lancelot C, Levasseur M, Owens NP, Pollard R, Rivkin RB, Sarmiento J, Schoemann V, Smetacek V, Takeda S, Tsuda A, Turner S, Watson AJ. Mesoscale iron enrichment experiments 1993–2005: synthesis and future directions. Science. 2007;315:612–7. doi: 10.1126/science.1131669. [DOI] [PubMed] [Google Scholar]
- Blain S, Queguiner B, Armand L, Belviso S, Bombled B, Bopp L, Bowie A, Brunet C, Brussaard C, Carlotti F, Christaki U, Corbiere A, Durand I, Ebersbach F, Fuda JL, Garcia N, Gerringa L, Griffiths B, Guigue C, Guillerm C, Jacquet S, Jeandel C, Laan P, Lefevre D, Lo Monaco C, Malits A, Mosseri J, Obernosterer I, Park YH, Picheral M, Pondaven P, Remenyi T, Sandroni V, Sarthou G, Savoye N, Scouarnec L, Souhaut M, Thuiller D, Timmermans K, Trull T, Uitz J, van Beek P, Veldhuis M, Vincent D, Viollier E, Vong L, Wagener T. Effect of natural iron fertilization on carbon sequestration in the Southern Ocean. Nature. 2007;446:1070–4. doi: 10.1038/nature05700. [DOI] [PubMed] [Google Scholar]
- Boyd PW. Biogeochemistry: iron findings. Nature. 2007;446:989–91. doi: 10.1038/446989a. [DOI] [PubMed] [Google Scholar]
- Watson AJ, Bakker DC, Ridgwell AJ, Boyd PW, Law CS. Effect of iron supply on Southern Ocean CO2 uptake and implications for glacial atmospheric CO2. Nature. 2000;407:730–3. doi: 10.1038/35037561. [DOI] [PubMed] [Google Scholar]
- Collins HL. Withholding iron as a cellular defence mechanism–friend or foe? Eur J Immunol. 2008;38:1803–6. doi: 10.1002/eji.200838505. [DOI] [PubMed] [Google Scholar]
- Weinberg ED. Iron and susceptibility to infectious disease. Science. 1974;184:952–6. doi: 10.1126/science.184.4140.952. [DOI] [PubMed] [Google Scholar]
- Weinberg ED. Iron and infection. Microbiol Rev. 1978;42:45–66. doi: 10.1128/mr.42.1.45-66.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershko C, Peto TEA, Weatherall DJ. Iron and Infection. British Medical Journal. 1988;296:660–664. [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer SJ. Iron and infection: the clinical evidence. Acta Paediatr Scand Suppl. 1989;361:53–62. doi: 10.1111/apa.1989.78.s361.53. [DOI] [PubMed] [Google Scholar]
- Otto BR, Verweij vanVught A, Maclaren DM. Transferrins and heme compounds as iron sources for pathogenic bacteria. Critical Reviews in Microbiology. 1992;18:217–233. doi: 10.3109/10408419209114559. [DOI] [PubMed] [Google Scholar]
- Vasil ML, Ochsner UA. The response of Pseudomonas aeruginosa to iron: genetics, biochemistry and virulence. Mol Microbiol. 1999;34:399–413. doi: 10.1046/j.1365-2958.1999.01586.x. [DOI] [PubMed] [Google Scholar]
- Bullen J, Griffiths E, Rogers H, Ward G. Sepsis: the critical role of iron. Microbes Infect. 2000;2:409–15. doi: 10.1016/s1286-4579(00)00326-9. [DOI] [PubMed] [Google Scholar]
- Law D. Virulence factors of Escherichia coli O157 and other Shiga toxin-producing E. coli. J Appl Microbiol. 2000;88:729–45. doi: 10.1046/j.1365-2672.2000.01031.x. [DOI] [PubMed] [Google Scholar]
- van Asbeck BS, Georgiou NA, Bruggen T van der, Oudshoorn M, Nottet HS, Marx JJ. Anti-HIV effect of iron chelators: different mechanisms involved. J Clin Virol. 2001;20:141–7. doi: 10.1016/s1386-6532(00)00122-0. [DOI] [PubMed] [Google Scholar]
- Gordeuk VR, Delanghe JR, Langlois MR, Boelaert JR. Iron status and the outcome of HIV infection: an overview. J Clin Virol. 2001;20:111–5. doi: 10.1016/s1386-6532(00)00134-7. [DOI] [PubMed] [Google Scholar]
- Rodriguez GM, Smith I. Mechanisms of iron regulation in mycobacteria: role in physiology and virulence. Mol Microbiol. 2003;47:1485–94. doi: 10.1046/j.1365-2958.2003.03384.x. [DOI] [PubMed] [Google Scholar]
- Bullen JJ, Rogers HJ, Spalding PB, Ward CG. Iron and infection: the heart of the matter. FEMS Immunol Med Microbiol. 2005;43:325–30. doi: 10.1016/j.femsim.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Bullen JJ, Rogers HJ, Spalding PB, Ward CG. Natural resistance, iron and infection: a challenge for clinical medicine. J Med Microbiol. 2006;55:251–8. doi: 10.1099/jmm.0.46386-0. [DOI] [PubMed] [Google Scholar]
- Maresso AW, Schneewind O. Iron acquisition and transport in Staphylococcus aureus. Biometals. 2006;19:193–203. doi: 10.1007/s10534-005-4863-7. [DOI] [PubMed] [Google Scholar]
- McDermid JM, Prentice AM. Iron and infection: effects of host iron status and the iron-regulatory genes haptoglobin and NRAMP1 (SLC11A1) on host-pathogen interactions in tuberculosis and HIV. Clin Sci (Lond) 2006;110:503–24. doi: 10.1042/CS20050273. [DOI] [PubMed] [Google Scholar]
- Meyer D. Iron chelation as therapy for HIV and Mycobacterium tuberculosis co-infection under conditions of iron overload. Curr Pharm Des. 2006;12:1943–7. doi: 10.2174/138161206777442164. [DOI] [PubMed] [Google Scholar]
- Sritharan M. Iron and bacterial virulence. Indian J Med Microbiol. 2006;24:163–4. [PubMed] [Google Scholar]
- Boelaert JR, Vandecasteele SJ, Appelberg R, Gordeuk VR. The effect of the host's iron status on tuberculosis. J Infect Dis. 2007;195:1745–53. doi: 10.1086/518040. [DOI] [PubMed] [Google Scholar]
- Maynor L, Brophy DF. Risk of infection with intravenous iron therapy. Ann Pharmacother. 2007;41:1476–80. doi: 10.1345/aph.1K187. [DOI] [PubMed] [Google Scholar]
- Basaraba RJ, Bielefeldt-Ohmann H, Eschelbach EK, Reisenhauer C, Tolnay AE, Taraba LC, Shanley CA, Smith EA, Bedwell CL, Chlipala EA, Orme IM. Increased expression of host iron-binding proteins precedes iron accumulation and calcification of primary lung lesions in experimental tuberculosis in the guinea pig. Tuberculosis (Edinb) 2008;88:69–79. doi: 10.1016/j.tube.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg ED. Iron availability and infection. Biochim Biophys Acta. 2008 doi: 10.1016/j.bbagen.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Ashrafian H. Hepcidin: the missing link between hemochromatosis and infections. Infect Immun. 2003;71:6693–700. doi: 10.1128/IAI.71.12.6693-6700.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiga H, Nishimura J, Kuwata H, Okuyama M, Matsumoto S, Sato S, Matsumoto M, Akira S, Yoshikai Y, Honda K, Yamamoto M, Takeda K. Lipocalin 2-dependent inhibition of mycobacterial growth in alveolar epithelium. J Immunol. 2008;181:8521–7. doi: 10.4049/jimmunol.181.12.8521. [DOI] [PubMed] [Google Scholar]
- Breidbach T, Scory S, Krauth-Siegel RL, Steverding D. Growth inhibition of bloodstream forms of Trypanosoma brucei by the iron chelator deferoxamine. Int J Parasitol. 2002;32:473–479. doi: 10.1016/s0020-7519(01)00310-1. [DOI] [PubMed] [Google Scholar]
- Merschjohann K, Steverding D. In vitro growth inhibition of bloodstream forms of Trypanosoma brucei and Trypanosoma congolense by iron chelators. Kinetoplastid Biol Dis. 2006;5:3. doi: 10.1186/1475-9292-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drakesmith H, Prentice A. Viral infection and iron metabolism. Nat Rev Microbiol. 2008;6:541–52. doi: 10.1038/nrmicro1930. [DOI] [PubMed] [Google Scholar]
- Kaprelyants AS, Gottschal JC, Kell DB. Dormancy in non-sporulating bacteria. FEMS Microbiol Rev. 1993;10:271–286. doi: 10.1111/j.1574-6968.1993.tb05871.x. [DOI] [PubMed] [Google Scholar]
- Kell DB, Kaprelyants AS, Weichart DH, Harwood CL, Barer MR. Viability and activity in readily culturable bacteria: a review and discussion of the practical issues. Antonie van Leeuwenhoek. 1998;73:169–187. doi: 10.1023/a:1000664013047. [DOI] [PubMed] [Google Scholar]
- Goris RJ. Mediators of multiple organ failure. Intensive Care Med. 1990;16:S192–6. doi: 10.1007/BF01709699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suntharalingam G, Perry MR, Ward S, Brett SJ, Castello-Cortes A, Brunner MD, Panoskaltsis N. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N Engl J Med. 2006;355:1018–28. doi: 10.1056/NEJMoa063842. [DOI] [PubMed] [Google Scholar]
- Beal AL, Cerra FB. Multiple Organ Failure Syndrome in the 1990s – Systemic Inflammatory Response and Organ Dysfunction. Jama-Journal of the American Medical Association. 1994;271:226–233. [PubMed] [Google Scholar]
- Bolton CF. Sepsis and the systemic inflammatory response syndrome: Neuromuscular manifestations. Critical Care Medicine. 1996;24:1408–1416. doi: 10.1097/00003246-199608000-00022. [DOI] [PubMed] [Google Scholar]
- Bone RC. Toward a theory regarding the pathogenesis of the systemic inflammatory response syndrome: What we do and do not know about cytokine regulation. Critical Care Medicine. 1996;24:163–172. doi: 10.1097/00003246-199601000-00026. [DOI] [PubMed] [Google Scholar]
- Davies MG, Hagen PO. Systemic inflammatory response syndrome. British Journal of Surgery. 1997;84:920–935. doi: 10.1002/bjs.1800840707. [DOI] [PubMed] [Google Scholar]
- Baue AE, Durham R, Faist E. Systemic inflammatory response syndrome (SIRS), multiple organ dysfunction syndrome (MODS), multiple organ failure (MOF): Are we winning the battle? Shock. 1998;10:79–89. doi: 10.1097/00024382-199808000-00001. [DOI] [PubMed] [Google Scholar]
- Gutteridge JMC, Mitchell J. Redox imbalance in the critically ill. British Medical Bulletin. 1999;55:49–75. doi: 10.1258/0007142991902295. [DOI] [PubMed] [Google Scholar]
- Johnson D, Mayers I. Multiple organ dysfunction syndrome: a narrative review. Can J Anaesth. 2001;48:502–9. doi: 10.1007/BF03028318. [DOI] [PubMed] [Google Scholar]
- Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. New England Journal of Medicine. 2003;348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- Keel M, Trentz O. Pathophysiology of polytrauma. Injury. 2005;36:691–709. doi: 10.1016/j.injury.2004.12.037. [DOI] [PubMed] [Google Scholar]
- Nguyen HB, Rivers EP, Abrahamian FM, Moran GJ, Abraham E, Trzeciak S, Huang DT, Osborn T, Stevens D, Talan DA. Severe sepsis and septic shock: Review of the literature and emergency department management guidelines. Ann Emergency Med. 2006;48:28–54. doi: 10.1016/j.annemergmed.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Protti A, Singer M. Bench-to-bedside review: Potential strategies to protect or reverse mitochondrial dysfunction in sepsis-induced organ failure. Critical Care. 2006;10:227–233. doi: 10.1186/cc5014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papathanassoglou EDE, Moynihan JA, Ackerman MH. Does programmed cell death (apoptosis) play a role in the development of multiple organ dysfunction in critically ill patients? A review and a theoretical framework. Critical Care Medicine. 2000;28:537–549. doi: 10.1097/00003246-200002000-00042. [DOI] [PubMed] [Google Scholar]
- Festjens N, Berghe T Vanden, Vandenabeele P. Necrosis, a well-orchestrated form of cell demise: Signalling cascades, important mediators and concomitant immune response. Biochim Biophys Acta. 2006;1757:1371–1387. doi: 10.1016/j.bbabio.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Golstein P, Kroemer G. Cell death by necrosis: towards a molecular definition. Trends in Biochemical Sciences. 2007;32:37–43. doi: 10.1016/j.tibs.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Goode HF, Webster NR. Free radicals and antioxidants in sepsis. Crit Care Med. 1993;21:1770–6. doi: 10.1097/00003246-199311000-00029. [DOI] [PubMed] [Google Scholar]
- Redl H, Gasser H, Schlag G, Marzi I. Involvement of oxygen radicals in shock related cell injury. Br Med Bull. 1993;49:556–65. doi: 10.1093/oxfordjournals.bmb.a072630. [DOI] [PubMed] [Google Scholar]
- Horn KD. Evolving strategies in the treatment of sepsis and systemic inflammatory response syndrome (SIRS) QJM. 1998;91:265–77. doi: 10.1093/qjmed/91.4.265. [DOI] [PubMed] [Google Scholar]
- Bulger EM, Maier RV. Antioxidants in critical illness. Arch Surg. 2001;136:1201–7. doi: 10.1001/archsurg.136.10.1201. [DOI] [PubMed] [Google Scholar]
- Cadenas S, Cadenas AM. Fighting the stranger-antioxidant protection against endotoxin toxicity. Toxicology. 2002;180:45–63. doi: 10.1016/s0300-483x(02)00381-5. [DOI] [PubMed] [Google Scholar]
- Victor VM, Rocha M, De la Fuente M. Immune cells: free radicals and antioxidants in sepsis. Int Immunopharmacol. 2004;4:327–47. doi: 10.1016/j.intimp.2004.01.020. [DOI] [PubMed] [Google Scholar]
- Victor VM, Rocha M, Esplugues JV, De la Fuente M. Role of free radicals in sepsis: Antioxidant therapy. Current Pharmaceutical Design. 2005;11:3141–3158. doi: 10.2174/1381612054864894. [DOI] [PubMed] [Google Scholar]
- Vlessis AA, Goldman RK, Trunkey DD. New concepts in the pathophysiology of oxygen metabolism during sepsis. Br J Surg. 1995;82:870–6. doi: 10.1002/bjs.1800820705. [DOI] [PubMed] [Google Scholar]
- Quinlan GJ, Chen Y, Evans TW, Gutteridge JMC. Iron signalling regulated directly and through oxygen: implications for sepsis and the acute respiratory distress syndrome. Clinical Science. 2001;100:169–182. [PubMed] [Google Scholar]
- Closa D, Folch-Puy E. Oxygen free radicals and the systemic inflammatory response. IUBMB Life. 2004;56:185–191. doi: 10.1080/15216540410001701642. [DOI] [PubMed] [Google Scholar]
- Abdelrahman M, Mazzon E, Bauer M, Bauer I, Delbosc S, Cristol JP, Patel NS, Cuzzocrea S, Thiemermann C. Inhibitors of NADPH oxidase reduce the organ injury in hemorrhagic shock. Shock. 2005;23:107–14. doi: 10.1097/01.shk.0000151028.15377.f7. [DOI] [PubMed] [Google Scholar]
- Javadi P, Buchman TG, Stromberg PE, Turnbull IR, Vyas D, Hotchkiss RS, Karl IE, Coopersmith CM. Iron dysregulation combined with aging prevents sepsis-induced apoptosis. J Surg Res. 2005;128:37–44. doi: 10.1016/j.jss.2005.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crimi E, Sica V, Slutsky AS, Zhang HB, Williams-Ignarro S, Ignarro LJ, Napoli C. Role of oxidative stress in experimental sepsis and multisystem organ dysfunction. Free Radical Research. 2006;40:665–672. doi: 10.1080/10715760600669612. [DOI] [PubMed] [Google Scholar]
- Crimi E, Sica V, Williams-Ignarro S, Zhang H, Slutsky AS, Ignarro LJ, Napoli C. The role of oxidative stress in adult critical care. Free Radic Biol Med. 2006;40:398–406. doi: 10.1016/j.freeradbiomed.2005.10.054. [DOI] [PubMed] [Google Scholar]
- Lemineur T, Deby-Dupont G, Preiser JC. Biomarkers of oxidative stress in critically ill patients: what should be measured, when and how? Curr Op Clin Nutr Metab Care. 2006;9:704–710. doi: 10.1097/01.mco.0000247467.41661.f3. [DOI] [PubMed] [Google Scholar]
- Lagan AL, Melley DD, Evans TW, Quinlan GJ. Pathogenesis of the systemic inflammatory syndrome and acute lung injury: role of iron mobilization and decompartmentalization. Am J Physiol Lung Cell Mol Physiol. 2008;294:L161–74. doi: 10.1152/ajplung.00169.2007. [DOI] [PubMed] [Google Scholar]
- Galley HF, Webster NR. Elevated serum bleomycin-detectable iron concentrations in patients with sepsis syndrome. Intensive Care Med. 1996;22:226–9. doi: 10.1007/BF01712241. [DOI] [PubMed] [Google Scholar]
- Galley HF, Davies MJ, Webster NR. Ascorbyl radical formation in patients with sepsis: effect of ascorbate loading. Free Radic Biol Med. 1996;20:139–43. doi: 10.1016/0891-5849(95)02022-5. [DOI] [PubMed] [Google Scholar]
- Galley HF, Howdle PD, Walker BE, Webster NR. The effects of intravenous antioxidants in patients with septic shock. Free Radic Biol Med. 1997;23:768–74. doi: 10.1016/s0891-5849(97)00059-2. [DOI] [PubMed] [Google Scholar]
- Eaton JW, Qian MW. Molecular bases of cellular iron toxicity. Free Radical Biology and Medicine. 2002;32:833–840. doi: 10.1016/s0891-5849(02)00772-4. [DOI] [PubMed] [Google Scholar]
- Yu Z, Persson HL, Eaton JW, Brunk UT. Intralysosomal iron: a major determinant of oxidant-induced cell death. Free Radic Biol Med. 2003;34:1243–52. doi: 10.1016/s0891-5849(03)00109-6. [DOI] [PubMed] [Google Scholar]
- Guicciardi ME, Leist M, Gores GJ. Lysosomes in cell death. Oncogene. 2004;23:2881–90. doi: 10.1038/sj.onc.1207512. [DOI] [PubMed] [Google Scholar]
- Terman A, Kurz T, Gustafsson B, Brunk UT. Lysosomal labilization. IUBMB Life. 2006;58:531–9. doi: 10.1080/15216540600904885. [DOI] [PubMed] [Google Scholar]
- Vulcano M, Meiss RP, Isturiz MA. Deferoxamine reduces tissue injury and lethality in LPS-treated mice. Int J Immunopharmacol. 2000;22:635–44. doi: 10.1016/s0192-0561(00)00026-6. [DOI] [PubMed] [Google Scholar]
- Cuzzocrea S, Rossi A, Pisano B, Di Paola R, Genovese T, Patel NS, Cuzzocrea E, Ianaro A, Sautebin L, Fulia F, Chatterjee PK, Caputi AP, Thiemermann C. Pyrrolidine dithiocarbamate attenuates the development of organ failure induced by zymosan in mice. Intensive Care Med. 2003;29:2016–25. doi: 10.1007/s00134-003-1887-8. [DOI] [PubMed] [Google Scholar]
- Persson HL, Yu Z, Tirosh O, Eaton JW, Brunk UT. Prevention of oxidant-induced cell death by lysosomotropic iron chelators. Free Radic Biol Med. 2003;34:1295–305. doi: 10.1016/s0891-5849(03)00106-0. [DOI] [PubMed] [Google Scholar]
- Messaris E, Antonakis PT, Memos N, Chatzigianni E, Leandros E, Konstadoulakis MM. Deferoxamine administration in septic animals: improved survival and altered apoptotic gene expression. Int Immunopharmacol. 2004;4:455–9. doi: 10.1016/j.intimp.2004.01.012. [DOI] [PubMed] [Google Scholar]
- Tracey KJ, Cerami A. Tumor Necrosis Factor: a pleiotropic cytokine and therapeutic target. Annu Rev Med. 1994;45:491–503. doi: 10.1146/annurev.med.45.1.491. [DOI] [PubMed] [Google Scholar]
- Opal SM, Huber CE. Bench-to-bedside review: Toll-like receptors and their role in septic shock. Critical Care. 2002;6:125–136. doi: 10.1186/cc1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins P, Cohen J. Toll-like receptors: the key to the stable door? Crit Care. 2002;6:99–101. doi: 10.1186/cc1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds FD, Dauchy R, Blask D, Dietz PA, Lynch D, Zuckerman R. The pineal gland hormone melatonin improves survival in a rat model of sepsis/shock induced by zymosan A. Surgery. 2003;134:474–479. doi: 10.1067/s0039-6060(03)00253-8. [DOI] [PubMed] [Google Scholar]
- Carrillo-Vico A, Lardone PJ, Naji L, Fernández-Santos JM, Martín-Lacave I, Guerrero JM, Calvo JR. Beneficial pleiotropic actions of melatonin in an experimental model of septic shock in mice: regulation of pro-/anti-inflammatory cytokine network, protection against oxidative damage and anti-apoptotic effects. J Pineal Res. 2005;39:400–408. doi: 10.1111/j.1600-079X.2005.00265.x. [DOI] [PubMed] [Google Scholar]
- Escames G, Acuna-Castroviejo D, López LC, Tan DX, Maldonado MD, Sanchez-Hidalgo M, León J, Reiter RJ. Pharmacological utility of melatonin in the treatment of septic shock: experimental and clinical evidence. J Pharm Pharmacol. 2006;58:1153–1165. doi: 10.1211/jpp.58.9.0001. [DOI] [PubMed] [Google Scholar]
- Cuzzocrea S, Riley DP, Caputi AP, Salvemini D. Antioxidant therapy: a new pharmacological approach in shock, inflammation, and ischemia/reperfusion injury. Pharmacol Rev. 2001;53:135–59. [PubMed] [Google Scholar]
- Nathens AB, Neff MJ, Jurkovich GJ, Klotz P, Farver K, Ruzinski JT, Radella F, Garcia I, Maier RV. Randomized, prospective trial of antioxidant supplementation in critically ill surgical patients. Annals of Surgery. 2002;236:814–822. doi: 10.1097/00000658-200212000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuzzocrea S, Thiemermann C, Salvemini D. Potential therapeutic effect of antioxidant therapy in shock and inflammation. Current Medicinal Chemistry. 2004;11:1147–1162. doi: 10.2174/0929867043365396. [DOI] [PubMed] [Google Scholar]
- Di Paola R, Mazzon E, Muia C, Crisafulli C, Genovese T, Di Bella P, Esposito E, Menegazzi M, Meli R, Suzuki H, Cuzzocrea S. Green tea polyphenol extract attenuates zymosan-induced non-septic shock in mice. Shock. 2006;26:402–9. doi: 10.1097/01.shk.0000191379.62897.1d. [DOI] [PubMed] [Google Scholar]
- Ritter C, Andrades ME, Reinke A, Menna-Barreto S, Moreira JC, Dal-Pizzol F. Treatment with N-acetylcysteine plus deferoxamine protects rats against oxidative stress and improves survival in sepsis. Crit Care Med. 2004;32:342–9. doi: 10.1097/01.CCM.0000109454.13145.CA. [DOI] [PubMed] [Google Scholar]
- Ritter C, da Cunha AA, Echer IC, Andrades M, Reinke A, Lucchiari N, Rocha J, Streck EL, Menna-Barreto S, Moreira JC, Dal-Pizzol F. Effects of N-acetylcysteine plus deferoxamine in lipopolysaccharide-induced acute lung injury in the rat. Crit Care Med. 2006;34:471–7. doi: 10.1097/01.ccm.0000199069.19193.89. [DOI] [PubMed] [Google Scholar]
- Pinho RA, Silveira PC, Silva LA, Luiz Streck E, Dal-Pizzol F, JC FM. N-acetylcysteine and deferoxamine reduce pulmonary oxidative stress and inflammation in rats after coal dust exposure. Environ Res. 2005;99:355–60. doi: 10.1016/j.envres.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Teixeira KC, Soares FS, Rocha LG, Silveira PC, Silva LA, Valenca SS, Pizzol FD, Streck EL, Pinho RA. Attenuation of bleomycin-induced lung injury and oxidative stress by N-acetylcysteine plus deferoxamine. Pulm Pharmacol Ther. 2008;21:309–16. doi: 10.1016/j.pupt.2007.07.006. [DOI] [PubMed] [Google Scholar]
- Abdelrahman M, Sharples EJ, McDonald MC, Collin M, Patel NS, Yaqoob MM, Thiemermann C. Erythropoietin attenuates the tissue injury associated with hemorrhagic shock and myocardial ischemia. Shock. 2004;22:63–9. doi: 10.1097/01.shk.00001276869.21260.9d. [DOI] [PubMed] [Google Scholar]
- Cuzzocrea S, Di Paola R, Mazzon E, Patel NS, Genovese T, Muia C, Crisafulli C, Caputi AP, Thiemermann C. Erythropoietin reduces the development of nonseptic shock induced by zymosan in mice. Crit Care Med. 2006;34:1168–77. doi: 10.1097/01.CCM.0000207346.56477.E8. [DOI] [PubMed] [Google Scholar]
- Lovitt RW, Kell DB, Morris JG. Proline reduction by Clostridium sporogenes is coupled to vectorial proton ejection. FEMS Microbiology Letters. 1986;36:269–273. [Google Scholar]
- Kell DB, Mendes P. Snapshots of systems: metabolic control analysis and biotechnology in the post-genomic era. In: Cornish-Bowden A, Cárdenas ML, editor. Technological and Medical Implications of Metabolic Control Analysis. Kluwer Academic Publishers, Dordrecht; 2000. pp. 3–25.http://dbkgroup.org/WhitePapers/mcabio.htm [Google Scholar]
- Wikström MKF. The different cytochrome b components in the respiratory chain of animal mitochondria and their role in electron transport and energy conservation. Biochim Biophys Acta. 1973;301:155–93. doi: 10.1016/0304-4173(73)90003-7. [DOI] [PubMed] [Google Scholar]
- John P, Papa S. Rapid oxygen-induced reduction of b-type cytochromes in Paracoccus denitrificans. FEBS Lett. 1978;85:179–82. doi: 10.1016/0014-5793(78)81275-7. [DOI] [PubMed] [Google Scholar]
- Reif DW. Ferritin as a source of iron for oxidative damage. Free Rad Biol Med. 1992;12:417–427. doi: 10.1016/0891-5849(92)90091-t. [DOI] [PubMed] [Google Scholar]
- Moison RM, Bloemhof FE, Geerdink JA, de Beaufort AJ, Berger HM. The capacity of different infusion fluids to lower the prooxidant activity of plasma iron: an important factor in resuscitation? Transfusion. 2000;40:1346–51. doi: 10.1046/j.1537-2995.2000.40111346.x. [DOI] [PubMed] [Google Scholar]
- Fábián I, Csordás V. Metal ion catalyzed autoxidation reactions: Kinetics and mechanisms. Adv Inorg Chem. 2003;54:395–461. [Google Scholar]
- Duarte TL, Almeida GM, Jones GD. Investigation of the role of extracellular H2O2 and transition metal ions in the genotoxic action of ascorbic acid in cell culture models. Toxicol Lett. 2007;170:57–65. doi: 10.1016/j.toxlet.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Wang S, Geraci G, Kuhlmann MK, Levin NW, Handelman GJ. Chemical reactions of vitamin C with intravenous-iron formulations. Nephrol Dial Transplant. 2008;23:120–5. doi: 10.1093/ndt/gfm557. [DOI] [PubMed] [Google Scholar]
- Yagi K, Ishida N, Komura S, Ohishi N, Kusai M, Kohno M. Generation of hydroxyl radical from linoleic acid hydroperoxide in the presence of epinephrine and iron. Biochem Biophys Res Commun. 1992;183:945–51. doi: 10.1016/s0006-291x(05)80281-5. [DOI] [PubMed] [Google Scholar]
- Everse J, Hsia N. The toxicities of native and modified hemoglobins. Free Radical Biology and Medicine. 1997;22:1075–1099. doi: 10.1016/s0891-5849(96)00499-6. [DOI] [PubMed] [Google Scholar]
- Medina I, Tombo I, Satue-Gracia MT, German JB, Frankel EN. Effects of natural phenolic compounds on the antioxidant activity of lactoferrin in liposomes and oil-in-water emulsions. J Agric Food Chem. 2002;50:2392–9. doi: 10.1021/jf011126y. [DOI] [PubMed] [Google Scholar]
- Hoppe M, Hulthen L, Hallberg L. The relative bioavailability in humans of elemental iron powders for use in food fortification. Eur J Nutrition. 2006;45:37–44. doi: 10.1007/s00394-005-0560-0. [DOI] [PubMed] [Google Scholar]
- Goodell B, Jellison J, Liu J, Daniel G, Paszczynski A, Fekete F, Krishnamurthy S, Jun L, Xu G. Low molecular weight chelators and phenolic compounds isolated from wood decay fungi and their role in the fungal biodegradation of wood. J Biotechnol. 1997;53:133–162. [Google Scholar]
- Mályusz M, Kahler W, Gronow G. Hippurate metabolism as a hydroxyl radical trapping mechanism in the rat kidney. Kidney Blood Press Res. 2001;24:149–58. doi: 10.1159/000054222. [DOI] [PubMed] [Google Scholar]
- Brzezinski A. Melatonin in humans. N Engl J Med. 1997;336:186–95. doi: 10.1056/NEJM199701163360306. [DOI] [PubMed] [Google Scholar]
- Reiter R, Tang L, Garcia JJ, MunozHoyos A. Pharmacological actions of melatonin in oxygen radical pathophysiology. Life Sci. 1997;60:2255–2271. doi: 10.1016/s0024-3205(97)00030-1. [DOI] [PubMed] [Google Scholar]
- Beyer CE, Steketee JD, Saphier D. Antioxidant properties of melatonin – An emerging mystery. Biochem Pharmacol. 1998;56:1265–1272. doi: 10.1016/s0006-2952(98)00180-4. [DOI] [PubMed] [Google Scholar]
- Reiter RJ. Oxidative damage in the central nervous system: protection by melatonin. Progr Neurobiol. 1998;56:359–384. doi: 10.1016/s0301-0082(98)00052-5. [DOI] [PubMed] [Google Scholar]
- Cuzzocrea S, Costantino G, Gitto E, Mazzon E, Fulia F, Serraino I, Cordaro S, Barberi I, De Sarro A, Caputi AP. Protective effects of melatonin in ischemic brain injury. J Pineal Res. 2000;29:217–27. doi: 10.1034/j.1600-0633.2002.290404.x. [DOI] [PubMed] [Google Scholar]
- Karbownik M, Lewinski A, Reiter RJ. Anticarcinogenic actions of melatonin which involve antioxidative processes: comparison with other antioxidants. International Journal of Biochemistry & Cell Biology. 2001;33:735–753. doi: 10.1016/s1357-2725(01)00059-0. [DOI] [PubMed] [Google Scholar]
- Letechipia-Vallejo G, Gonzalez-Burgos I, Cervantes M. Neuroprotective effect of melatonin on brain damage induced by acute global cerebral ischemia in cats. Arch Med Res. 2001;32:186–92. doi: 10.1016/s0188-4409(01)00268-5. [DOI] [PubMed] [Google Scholar]
- Okatani Y, Wakatsuki A, Shinohara K, Taniguchi K, Fukaya T. Melatonin protects against oxidative mitochondrial damage induced in rat placenta by ischemia and reperfusion. J Pineal Res. 2001;31:173–8. doi: 10.1034/j.1600-079x.2001.310212.x. [DOI] [PubMed] [Google Scholar]
- Reiter RJ, Tan DX, Manchester LC, Qi WB. Biochemical reactivity of melatonin with reactive oxygen and nitrogen species – A review of the evidence. Cell Biochemistry and Biophysics. 2001;34:237–256. doi: 10.1385/CBB:34:2:237. [DOI] [PubMed] [Google Scholar]
- Allegra M, Reiter RJ, Tan DX, Gentile C, Tesoriere L, Livrea MA. The chemistry of melatonin's interaction with reactive species. Journal of Pineal Research. 2003;34:1–10. doi: 10.1034/j.1600-079x.2003.02112.x. [DOI] [PubMed] [Google Scholar]
- Cheung RTF. The utility of melatonin in reducing cerebral damage resulting from ischemia and reperfusion. Journal of Pineal Research. 2003;34:153–160. doi: 10.1034/j.1600-079x.2003.00034.x. [DOI] [PubMed] [Google Scholar]
- Okatani Y, Wakatsuki A, Reiter RJ, Enzan H, Miyahara Y. Protective effect of melatonin against mitochondrial injury induced by ischemia and reperfusion of rat liver. Eur J Pharmacol. 2003;469:145–152. doi: 10.1016/s0014-2999(03)01643-1. [DOI] [PubMed] [Google Scholar]
- Pei Z, Pang SF, Cheung RTF. Administration of melatonin after onset of ischemia reduces the volume of cerebral infarction in a rat middle cerebral artery occlusion stroke model. Stroke. 2003;34:770–775. doi: 10.1161/01.STR.0000057460.14810.3E. [DOI] [PubMed] [Google Scholar]
- Reiter RJ, Tan DX. Melatonin: a novel protective agent against oxidative injury of the ischemic/reperfused heart. Cardiovascular Research. 2003;58:10–19. doi: 10.1016/s0008-6363(02)00827-1. [DOI] [PubMed] [Google Scholar]
- Reiter RJ. Melatonin: clinical relevance. Best Pract Res Clin Endocrinol Metab. 2003;17:273–85. doi: 10.1016/s1521-690x(03)00016-2. [DOI] [PubMed] [Google Scholar]
- Weiss JF, Landauer MR. Protection against ionizing radiation by antioxidant nutrients and phytochemicals. Toxicology. 2003;189:1–20. doi: 10.1016/s0300-483x(03)00149-5. [DOI] [PubMed] [Google Scholar]
- Macleod MR, O'Collins T, Horky LL, Howells DW, Donnan GA. Systematic review and meta-analysis of the efficacy of melatonin in experimental stroke. J Pineal Res. 2005;38:35–41. doi: 10.1111/j.1600-079X.2004.00172.x. [DOI] [PubMed] [Google Scholar]
- Srinivasan V, Pandi-Perumal SR, Maestroni GJM, Esquifino AI, Hardeland R, Cardinali DP. Role of melatonin in neurodegenerative diseases. Neurotox Res. 2005;7:293–318. doi: 10.1007/BF03033887. [DOI] [PubMed] [Google Scholar]
- Hardeland R, Pandi-Perumal SR, Cardinali DP. Melatonin. International Journal of Biochemistry & Cell Biology. 2006;38:313–316. doi: 10.1016/j.biocel.2005.08.020. [DOI] [PubMed] [Google Scholar]
- Grootveld M, Halliwell B. Aromatic hydroxylation as a potential measure of hydroxyl-radical formation in vivo. Identification of hydroxylated derivatives of salicylate in human body fluids. Biochem J. 1986;237:499–504. doi: 10.1042/bj2370499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roob JM, Khoschsorur G, Tiran A, Horina JH, Holzer H, Winklhofer-Roob BM. Vitamin E attenuates oxidative stress induced by intravenous iron in patients on hemodialysis. J Am Soc Nephrol. 2000;11:539–49. doi: 10.1681/ASN.V113539. [DOI] [PubMed] [Google Scholar]
- Oldham KM, Bowen PE. Oxidative stress in critical care: is antioxidant supplementation beneficial? J Am Diet Assoc. 1998;98:1001–8. doi: 10.1016/S0002-8223(98)00230-2. [DOI] [PubMed] [Google Scholar]
- Rehman A, Collis CS, Yang M, Kelly M, Diplock AT, Halliwell B, Rice-Evans C. The effects of iron and vitamin C co-supplementation on oxidative damage to DNA in healthy volunteers. Biochem Biophys Res Comm. 1998;246:293–298. doi: 10.1006/bbrc.1998.8592. [DOI] [PubMed] [Google Scholar]
- Gilgun-Sherki Y, Rosenbaum Z, Melamed E, Offen D. Antioxidant therapy in acute central nervous system injury: current state. Pharmacol Rev. 2002;54:271–84. doi: 10.1124/pr.54.2.271. [DOI] [PubMed] [Google Scholar]
- Miller ER, 3rd, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005;142:37–46. doi: 10.7326/0003-4819-142-1-200501040-00110. [DOI] [PubMed] [Google Scholar]
- Rodrigo R, Guichard C, Charles R. Clinical pharmacology and therapeutic use of antioxidant vitamins. Fund Clin Pharmacol. 2007;21:111–127. doi: 10.1111/j.1472-8206.2006.00466.x. [DOI] [PubMed] [Google Scholar]
- Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA. 2007;297:842–57. doi: 10.1001/jama.297.8.842. [DOI] [PubMed] [Google Scholar]
- Orrell RW, Lane RJ, Ross M. Antioxidant treatment for amyotrophic lateral sclerosis/motor neuron disease. Cochrane Database Syst Rev. 2007:CD002829. doi: 10.1002/14651858.CD002829.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens NG, Parsons A, Schofield PM, Kelly F, Cheeseman K, Mitchinson MJ. Randomised controlled trial of vitamin E in patients with coronary disease: Cambridge Heart Antioxidant Study (CHAOS) Lancet. 1996;347:781–6. doi: 10.1016/s0140-6736(96)90866-1. [DOI] [PubMed] [Google Scholar]
- Yusuf S, Dagenais G, Pogue J, Bosch J, Sleight P. Vitamin E supplementation and cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342:154–60. doi: 10.1056/NEJM200001203420302. [DOI] [PubMed] [Google Scholar]
- Lee DH, Folsom AR, Harnack L, Halliwell B, Jacobs DR., Jr Does supplemental vitamin C increase cardiovascular disease risk in women with diabetes? Am J Clin Nutr. 2004;80:1194–200. doi: 10.1093/ajcn/80.5.1194. [DOI] [PubMed] [Google Scholar]
- Williams KJ, Fisher EA. Oxidation, lipoproteins, and atherosclerosis: which is wrong, the antioxidants or the theory? Curr Opin Clin Nutr Metab Care. 2005;8:139–46. doi: 10.1097/00075197-200503000-00006. [DOI] [PubMed] [Google Scholar]
- Papaharalambus CA, Griendling KK. Basic mechanisms of oxidative stress and reactive oxygen species in cardiovascular injury. Trends Cardiovasc Med. 2007;17:48–54. doi: 10.1016/j.tcm.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Collins VE, Macleod MR, Donnan GA, Horky LL, Worp BH van der, Howells DW. 1,026 experimental treatments in acute stroke. Ann Neurol. 2006;59:467–77. doi: 10.1002/ana.20741. [DOI] [PubMed] [Google Scholar]
- Evans JR, Henshaw K. Antioxidant vitamin and mineral supplements for preventing age-related macular degeneration. Cochrane Database Syst Rev. 2008:CD000253. doi: 10.1002/14651858.CD000253.pub2. [DOI] [PubMed] [Google Scholar]
- Jeyabalan A, Caritis SN. Antioxidants and the prevention of preeclampsia – Unresolved issues. N Engl J Med. 2006;354:1841–1843. doi: 10.1056/NEJMe068046. [DOI] [PubMed] [Google Scholar]
- Poston L, Briley AL, Seed PT, Kelly FJ, Shennan AH. Vitamin C and vitamin E in pregnant women at risk for pre-eclampsia (VIP trial): randomised placebo-controlled trial. Lancet. 2006;367:1145–54. doi: 10.1016/S0140-6736(06)68433-X. [DOI] [PubMed] [Google Scholar]
- Rumbold AR, Crowther CA, Haslam RR, Dekker GA, Robinson JS. Vitamins C and E and the risks of preeclampsia and perinatal complications. N Engl J Med. 2006;354:1796–806. doi: 10.1056/NEJMoa054186. [DOI] [PubMed] [Google Scholar]
- Spinnato JA. New therapies in the prevention of preeclampsia. Current Opinion in Obstetrics & Gynecology. 2006;18:601–604. doi: 10.1097/01.gco.0000247393.86968.e6. [DOI] [PubMed] [Google Scholar]
- Rumbold A, Duley L, Crowther CA, Haslam RR. Antioxidants for preventing pre-eclampsia. Cochrane Database Syst Rev. 2008:CD004227. doi: 10.1002/14651858.CD004227.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman NA. Antioxidants in critical care medicine. Env Toxicol Pharmacol. 2001;10:183–188. doi: 10.1016/s1382-6689(01)00082-5. [DOI] [PubMed] [Google Scholar]
- Bayne AC, Sohal RS. Effects of superoxide dismutase/catalase mimetics on life span and oxidative stress resistance in the housefly, Musca domestica. Free Radic Biol Med. 2002;32:1229–34. doi: 10.1016/s0891-5849(02)00849-3. [DOI] [PubMed] [Google Scholar]
- Magwere T, West M, Riyahi K, Murphy MP, Smith RA, Partridge L. The effects of exogenous antioxidants on lifespan and oxidative stress resistance in Drosophila melanogaster. Mech Ageing Dev. 2006;127:356–70. doi: 10.1016/j.mad.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Omenn GS, Goodman GE, Thornquist MD, Balmes J, Cullen MR, Glass A, Keogh JP, Meyskens FL, Valanis B, Williams JH, Barnhart S, Hammar S. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med. 1996;334:1150–5. doi: 10.1056/NEJM199605023341802. [DOI] [PubMed] [Google Scholar]
- Bailey DM, Raman S, McEneny J, Young IS, Parham KL, Hullin DA, Davies B, McKeeman G, McCord JM, Lewis MH. Vitamin C prophylaxis promotes oxidative lipid damage during surgical ischemia-reperfusion. Free Radical Biology and Medicine. 2006;40:591–600. doi: 10.1016/j.freeradbiomed.2005.09.024. [DOI] [PubMed] [Google Scholar]
- Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases. Cochrane Database of Systematic Reviews 2008. 2008. p. CD007176. [DOI] [PubMed]
- Long LH, Clement MV, Halliwell B. Artifacts in cell culture: Rapid generation of hydrogen peroxide on addition of (-)-epigallocatechin, (-)-epigallocatechin gallate, (+)-catechin, and quercetin to commonly used cell culture media. Biochem Biophys Res Comm. 2000;273:50–53. doi: 10.1006/bbrc.2000.2895. [DOI] [PubMed] [Google Scholar]
- Hininger I, Waters R, Osman M, Garrel C, Fernholz K, Roussel AM, Anderson RA. Acute prooxidant effects of vitamin C in EDTA chelation therapy and long-term antioxidant benefits of therapy. Free Rad Biol Med. 2005;38:1565–1570. doi: 10.1016/j.freeradbiomed.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Szewczyk A, Wojtczak L. Mitochondria as a pharmacological target. Pharmacol Rev. 2002;54:101–27. doi: 10.1124/pr.54.1.101. [DOI] [PubMed] [Google Scholar]
- Murphy MP, Smith RA. Targeting antioxidants to mitochondria by conjugation to lipophilic cations. Annu Rev Pharmacol Toxicol. 2007;47:629–56. doi: 10.1146/annurev.pharmtox.47.120505.105110. [DOI] [PubMed] [Google Scholar]
- Jou MJ. Pathophysiological and pharmacological implications of mitochondria-targeted reactive oxygen species generation in astrocytes. Adv Drug Deliv Rev. 2008;60:1512–26. doi: 10.1016/j.addr.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Balcerczyk A, Sowa K, Bartou G. Metal chelators react also with reactive oxygen and nitrogen species. Biochem Biophys Res Comm. 2007;352:522–525. doi: 10.1016/j.bbrc.2006.11.053. [DOI] [PubMed] [Google Scholar]
- Hider RC, Zhou T. The design of orally active iron chelators. Ann N Y Acad Sci. 2005;1054:141–54. doi: 10.1196/annals.1345.017. [DOI] [PubMed] [Google Scholar]
- Marcus RA. Theory of Oxidation-Reduction Reactions Involving Electron Transfer .1. J Chem Phys. 1956;24:966–978. [Google Scholar]
- Marcus RA. Chemical + Electrochemical Electron-Transfer Theory. Annu Rev Phys Chem. 1964;15:155. [Google Scholar]
- Chou M, Creutz C, Sutin N. Rate constants and activation parameters for outer-sphere electron transfer reactions and comparisons with predictions of Marcus theory. JACS. 1977;99:5615–5623. [Google Scholar]
- Marcus RA, Sutin N. Electron transfers in chemistry and biology. Biochim Biophys Acta. 1985;811:265–322. [Google Scholar]
- Liu ZD, Hider RC. Design of iron chelators with therapeutic application. Coord Chem Rev. 2002;232:151–171. [Google Scholar]
- Liu ZD, Hider RC. Design of clinically useful iron(III)-selective chelators. Medicinal Res Rev. 2002;22:26–64. doi: 10.1002/med.1027. [DOI] [PubMed] [Google Scholar]
- Richardson DR, Ponka P. Development of iron chelators to treat iron overload disease and their use as experimental tools to probe intracellular iron metabolism. Am J Hematol. 1998;58:299–305. doi: 10.1002/(sici)1096-8652(199808)58:4<299::aid-ajh9>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Aouad F, Florence A, Zhang Y, Collins F, Henry C, Ward RJ, Crichton RR. Evaluation of new iron chelators and their therapeutic potential. Inorganica Chimica Acta. 2002;339:470–480. [Google Scholar]
- Bush AI. Metal complexing agents as therapies for Alzheimer's disease. Neurobiology of Aging. 2002;23:1031–1038. doi: 10.1016/s0197-4580(02)00120-3. [DOI] [PubMed] [Google Scholar]
- Crichton RR, Florence A, Ward RJ. Aluminium and iron in the brain – prospects for chelation. Coordination Chemistry Reviews. 2002;228:365–371. [Google Scholar]
- Chaston TB, Richardson DR. Iron chelators for the treatment of iron overload disease: relationship between structure, redox activity, and toxicity. Amer J Hematol. 2003;73:200–210. doi: 10.1002/ajh.10348. [DOI] [PubMed] [Google Scholar]
- Crichton RR, Ward RJ. Iron chelators and their therapeutic potential. Metal Ions in Biological Systems, Metal Ions and Their Complexes in Medication, Metal Ions in Biological Systems. 2004;41:185–219. [PubMed] [Google Scholar]
- Bernhardt PV. Coordination chemistry and biology of chelators for the treatment of iron overload disorders. Dalton Trans. 2007:3214–3220. doi: 10.1039/b708133b. [DOI] [PubMed] [Google Scholar]
- Leeson PD, Springthorpe B. The influence of drug-like concepts on decision-making in medicinal chemistry. Nat Rev Drug Discov. 2007;6:881–90. doi: 10.1038/nrd2445. [DOI] [PubMed] [Google Scholar]
- Imbert M, Bechet M, Blondeau R. Comparison of the Main Siderophores Produced by Some Species of Streptomyces. Current Microbiology. 1995;31:129–133. [Google Scholar]
- Flores FJ, Rincón J, Martín JF. Characterization of the iron-regulated desA promoter of Streptomyces pilosus as a system for controlled gene expression in actinomycetes. Microb Cell Fact. 2003;2:5. doi: 10.1186/1475-2859-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catnach SM, Fairclough PD, Hammond SM. Intestinal absorption of peptide drugs: advances in our understanding and clinical implications. Gut. 1994;35:441–4. doi: 10.1136/gut.35.4.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey PD, Boyd CA, Bronk JR, Collier ID, Meredith D, Morgan KM, Temple CS. How to make drugs orally active: a substrate template for peptide transporter PepT1. Angew Chem Int Ed Engl. 2000;39:505–508. doi: 10.1002/(sici)1521-3773(20000204)39:3<505::aid-anie505>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Terada T, Inui K. Peptide transporters: structure, function, regulation and application for drug delivery. Curr Drug Metab. 2004;5:85–94. doi: 10.2174/1389200043489153. [DOI] [PubMed] [Google Scholar]
- Bailey PD, Boyd CA, Collier ID, George JG, Kellett GL, Meredith D, Morgan KM, Pettecrew R, Price RA, Pritchard RG. Conformational and spacial preferences for substrates of PepT1. Chem Commun (Camb) 2005:5352–4. doi: 10.1039/b510697d. [DOI] [PubMed] [Google Scholar]
- Maggio A. Light and shadows in the iron chelation treatment of haematological diseases. Br J Haematol. 2007;138:407–21. doi: 10.1111/j.1365-2141.2007.06666.x. [DOI] [PubMed] [Google Scholar]
- Kontoghiorghes GJ, Neocleous K, Kolnagou A. Benefits and risks of deferiprone in iron overload in Thalassaemia and other conditions: comparison of epidemiological and therapeutic aspects with deferoxamine. Drug Saf. 2003;26:553–84. doi: 10.2165/00002018-200326080-00003. [DOI] [PubMed] [Google Scholar]
- Anderson LJ, Wonke B, Prescott E, Holden S, Walker JM, Pennell DJ. Comparison of effects of oral deferiprone and subcutaneous desferrioxamine on myocardial iron concentrations and ventricular function in beta-thalassaemia. Lancet. 2002;360:516–20. doi: 10.1016/s0140-6736(02)09740-4. [DOI] [PubMed] [Google Scholar]
- Heinz U, Hegetschweiler K, Acklin P, Faller B, Lattmann R, Schnebli HP. 4-[3,5-Bis(2-hydroxyphenyl)-1,2,4-triazol-1-yl]-benzoic acid: a novel efficient and selective iron(III) complexing agent. Angew Chem Int Ed Engl. 1999;38:2568–2570. [PubMed] [Google Scholar]
- Nick H, Acklin P, Lattmann R, Buehlmayer P, Hauffe S, Schupp J, Alberti D. Development of tridentate iron chelators: From desferrithiocin to ICL670. Curr Med Chem. 2003;10:1065–1076. doi: 10.2174/0929867033457610. [DOI] [PubMed] [Google Scholar]
- Nisbet-Brown E, Olivieri NF, Giardina PJ, Grady RW, Neufeld EJ, Sechaud R, Krebs-Brown AJ, Anderson JR, Alberti D, Sizer KC, Nathan DG. Effectiveness and safety of ICL670 in iron-loaded patients with thalassaemia: a randomised, double-blind, placebo-controlled, dose-escalation trial. Lancet. 2003;361:1597–602. doi: 10.1016/S0140-6736(03)13309-0. [DOI] [PubMed] [Google Scholar]
- Kontoghiorghes GJ. Effects of ICL670 (deferasirox) on cardiac iron concentrations. Lancet. 2005;366:804. doi: 10.1016/S0140-6736(05)67205-4. [DOI] [PubMed] [Google Scholar]
- Cappellini MD, Cohen A, Piga A, Bejaoui M, Perrotta S, Agaoglu L, Aydinok Y, Kattamis A, Kilinc Y, Porter J, Capra M, Galanello R, Fattoum S, Drelichman G, Magnano C, Verissimo M, Athanassiou-Metaxa M, Giardina P, Kourakli-Symeonidis A, Janka-Schaub G, Coates T, Vermylen C, Olivieri N, Thuret I, Opitz H, Ressayre-Djaffer C, Marks P, Alberti D. A phase 3 study of deferasirox (ICL670), a once-daily oral iron chelator, in patients with beta-thalassemia. Blood. 2006;107:3455–62. doi: 10.1182/blood-2005-08-3430. [DOI] [PubMed] [Google Scholar]
- Chantrel-Groussard K, Gaboriau F, Pasdeloup N, Havouis R, Nick H, Pierre JL, Brissot P, Lescoat G. The new orally active iron chelator ICL670A exhibits a higher antiproliferative effect in human hepatocyte cultures than O-trensox. Eur J Pharmacol. 2006;541:129–137. doi: 10.1016/j.ejphar.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Glickstein H, El RB, Link G, Breuer W, Konijn AM, Hershko C, Nick H, Cabantchik ZI. Action of chelators in iron-loaded cardiac cells: Accessibility to intracellular labile iron and functional consequences. Blood. 2006;108:3195–203. doi: 10.1182/blood-2006-05-020867. [DOI] [PubMed] [Google Scholar]
- Kontoghiorghes GJ. Future chelation monotherapy and combination therapy strategies in thalassemia and other conditions. comparison of deferiprone deferoxamine, ICL670, GT56-252, L1NAll and starch deferoxamine polymers. Hemoglobin. 2006;30:329–47. doi: 10.1080/03630260600642674. [DOI] [PubMed] [Google Scholar]
- Piga A, Galanello R, Forni GL, Cappellini MD, Origa R, Zappu A, Donato G, Bordone E, Lavagetto A, Zanaboni L, Sechaud R, Hewson N, Ford JM, Opitz H, Alberti D. Randomized phase II trial of deferasirox (Exjade, ICL670), a once-daily, orally-administered iron chelator, in comparison to deferoxamine in thalassemia patients with transfusional iron overload. Haematologica. 2006;91:873–80. [PubMed] [Google Scholar]
- Porter JB. Deferasirox: An effective once-daily orally active iron chelator. Drugs of Today. 2006;42:623–637. doi: 10.1358/dot.2006.42.10.1009901. [DOI] [PubMed] [Google Scholar]
- VanOrden HE, Hagemann TM. Deferasirox–an oral agent for chronic iron overload. Ann Pharmacother. 2006;40:1110–7. doi: 10.1345/aph.1G566. [DOI] [PubMed] [Google Scholar]
- Nick H. Iron chelation, quo vadis? Curr Opin Chem Biol. 2007;11:419–23. doi: 10.1016/j.cbpa.2007.04.025. [DOI] [PubMed] [Google Scholar]
- Yang LP, Keam SJ, Keating GM. Deferasirox: a review of its use in the management of transfusional chronic iron overload. Drugs. 2007;67:2211–30. doi: 10.2165/00003495-200767150-00007. [DOI] [PubMed] [Google Scholar]
- Vermylen C. What is new in iron overload? Eur J Pediatr. 2008;167:377–81. doi: 10.1007/s00431-007-0604-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson PD, Patel Y, Kell DB. "Metabolite-likeness" as a criterion in the design and selection of pharmaceutical drug libraries. Drug Disc Today. 2008. [DOI] [PubMed]
- Borisy AA, Elliott PJ, Hurst NW, Lee MS, Lehar J, Price ER, Serbedzija G, Zimmermann GR, Foley MA, Stockwell BR, Keith CT. Systematic discovery of multicomponent therapeutics. Proc Natl Acad Sci USA. 2003;100:7977–82. doi: 10.1073/pnas.1337088100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith CT, Borisy AA, Stockwell BR. Multicomponent therapeutics for networked systems. Nat Rev Drug Discov. 2005;4:71–8. doi: 10.1038/nrd1609. [DOI] [PubMed] [Google Scholar]
- Costello F, Stuve O, Weber MS, Zamvil SS, Frohman E. Combination therapies for multiple sclerosis: scientific rationale, clinical trials, and clinical practice. Curr Opin Neurol. 2007;20:281–5. doi: 10.1097/WCO.0b013e328122de1b. [DOI] [PubMed] [Google Scholar]
- Lehár J, Zimmermann GR, Krueger AS, Molnar RA, Ledell JT, Heilbut AM, Short GF, 3rd, Giusti LC, Nolan GP, Magid OA, Lee MS, Borisy AA, Stockwell BR, Keith CT. Chemical combination effects predict connectivity in biological systems. Mol Syst Biol. 2007;3:80. doi: 10.1038/msb4100116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann GR, Lehár J, Keith CT. Multi-target therapeutics: when the whole is greater than the sum of the parts. Drug Discov Today. 2007;12:34–42. doi: 10.1016/j.drudis.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Aruoma OI, Halliwell B. The iron-binding and hydroxyl radical scavenging action of anti-inflammatory drugs. Xenobiotica. 1988;18:459–70. doi: 10.3109/00498258809041682. [DOI] [PubMed] [Google Scholar]
- Cherny RA, Atwood CS, Xilinas ME, Gray DN, Jones WD, McLean CA, Barnham KJ, Volitakis I, Fraser FW, Kim YS, Huang XD, Goldstein LE, Moir RD, Lim JT, Beyreuther K, Zheng H, Tanzi RE, Masters CL, Bush AI. Treatment with a copper-zinc chelator markedly and rapidly inhibits beta-amyloid accumulation in Alzheimer's disease transgenic mice. Neuron. 2001;30:665–676. doi: 10.1016/s0896-6273(01)00317-8. [DOI] [PubMed] [Google Scholar]
- Di Vaira M, Bazzicalupi C, Orioli P, Messori L, Bruni B, Zatta P. Clioquinol, a drug for Alzheimer's disease specifically interfering with brain metal metabolism: structural characterization of its zinc(II) and copper(II) complexes. Inorg Chem. 2004;43:3795–7. doi: 10.1021/ic0494051. [DOI] [PubMed] [Google Scholar]
- Shiraki H. The neuropathology of subacute myelo-optico-neuropathy, "SMON", in the humans: –with special reference to the quinoform intoxication. Jpn J Med Sci Biol. 1975;28:101–64. [PubMed] [Google Scholar]
- Arbiser JL, Kraeft SK, van Leeuwen R, Hurwitz SJ, Selig M, Dickersin GR, Flint A, Byers HR, Chen LB. Clioquinol-zinc chelate: a candidate causative agent of subacute myelo-optic neuropathy. Mol Med. 1998;4:665–70. [PMC free article] [PubMed] [Google Scholar]
- Sai Y. Biochemical and molecular pharmacological aspects of transporters as determinants of drug disposition. Drug Metab Pharmacokinet. 2005;20:91–9. doi: 10.2133/dmpk.20.91. [DOI] [PubMed] [Google Scholar]
- Richardson DR, Ponka P. Orally effective iron chelators for the treatment of iron overload disease: the case for a further look at pyridoxal isonicotinoyl hydrazone and its analogs. J Lab Clin Med. 1998;132:351–2. doi: 10.1016/s0022-2143(98)90049-x. [DOI] [PubMed] [Google Scholar]
- Richardson DR, Ponka P. Pyridoxal isonicotinoyl hydrazone and its analogs: potential orally effective iron-chelating agents for the treatment of iron overload disease. J Lab Clin Med. 1998;131:306–15. doi: 10.1016/s0022-2143(98)90180-9. [DOI] [PubMed] [Google Scholar]
- Buss JL, Arduini E, Shephard KC, Ponka P. Lipophilicity of analogs of pyridoxal isonicotinoyl hydrazone (PTH) determines the efflux of iron complexes and toxicity in K562 cells. Biochem Pharmacol. 2003;65:349–360. doi: 10.1016/s0006-2952(02)01551-4. [DOI] [PubMed] [Google Scholar]
- Buss JL, Neuzil J, Gellert N, Weber C, Ponka P. Pyridoxal isonicotinoyl hydrazone analogs induce apoptosis in hematopoietic cells due to their iron-chelating properties. Biochemical Pharmacology. 2003;65:161–172. doi: 10.1016/s0006-2952(02)01512-5. [DOI] [PubMed] [Google Scholar]
- Lovejoy DB, Richardson DR. Iron chelators as anti-neoplastic agents: current developments and promise of the PIH class of chelators. Curr Med Chem. 2003;10:1035–49. doi: 10.2174/0929867033457557. [DOI] [PubMed] [Google Scholar]
- Simunek T, Sterba M, Popelova O, Kaiserova H, Potacova A, Adamcova M, Mazurova Y, Ponka P, Gersl V. Pyridoxal isonicotinoyl hydrazone (PIH) and its analogs as protectants against anthracycline-induced cardiotoxicity. Hemoglobin. 2008;32:207–15. doi: 10.1080/03630260701680276. [DOI] [PubMed] [Google Scholar]
- Buss JL, Ponka P. Hydrolysis of pyridoxal isonicotinoyl hydrazone and its analogs. Biochim Biophys Acta. 2003;1619:177–186. doi: 10.1016/s0304-4165(02)00478-6. [DOI] [PubMed] [Google Scholar]
- Šimůnek T, Boer C, Bouwman RA, Vlasblom R, Versteilen AMG, Štĕrba M, Geršl V, Hrdina R, Poňka P, de Lange JJ, Paulus WJ, Musters RJP. SIH – a novel lipophilic iron chelator – protects H9c2 cardiomyoblasts from oxidative stress-induced mitochondrial injury and cell death. Journal of Molecular and Cellular Cardiology. 2005;39:345–354. doi: 10.1016/j.yjmcc.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Kalinowski DS, Sharpe PC, Bernhardt PV, Richardson DR. Structure-Activity Relationships of Novel Iron Chelators for the Treatment of Iron Overload Disease: The Methyl Pyrazinylketone Isonicotinoyl Hydrazone Series. J Med Chem. 2007. [DOI] [PubMed]
- Mouralian C, Buss JL, Stranix B, Chin J, Ponka P. Mobilization of iron from cells by hydroxyquinoline-based chelators. Biochem Pharmacol. 2005;71:214–222. doi: 10.1016/j.bcp.2005.10.032. [DOI] [PubMed] [Google Scholar]
- Murakami K, Haneda M, Yoshino M. Prooxidant action of xanthurenic acid and quinoline compounds: role of transition metals in the generation of reactive oxygen species and enhanced formation of 8-hydroxy-2'-deoxyguanosine in DNA. Biometals. 2006;19:429–35. doi: 10.1007/s10534-005-4528-6. [DOI] [PubMed] [Google Scholar]
- Murakami K, Ishida K, Watakabe K, Tsubouchi R, Naruse M, Yoshino M. Maltol/iron-mediated apoptosis in HL60 cells: participation of reactive oxygen species. Toxicol Lett. 2006;161:102–7. doi: 10.1016/j.toxlet.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Youdim MBH, Stephenson G, Ben Shachar D. Ironing iron out in Parkinson's disease and other neurodegenerative diseases with iron chelators – A lesson from 6-hydroxydopamine and iron chelators, desferal and VK-28. Redox-Active Metals in Neurological Disorders, Annals of the New York Academy of Sciences. 2004;1012:306–325. doi: 10.1196/annals.1306.025. [DOI] [PubMed] [Google Scholar]
- Ben Shachar D, Kahana N, Kampel V, Warshawsky A, Youdim MBH. Neuroprotection by a novel brain permeable iron chelator, VK-28, against 6-hydroxydopamine lession in rats. Neuropharmacology. 2004;46:254–263. doi: 10.1016/j.neuropharm.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Youdim MBH, Fridkin M, Zheng HL. Bifunctional drug derivatives of MAO-B inhibitor rasagiline and iron chelator VK-28 as a more effective approach to treatment of brain ageing and ageing neurodegenerative diseases. Mech Ageing Dev. 2005;126:317–326. doi: 10.1016/j.mad.2004.08.023. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay S, Huang X, Cho H, Greig NH, Youdim MB, Rogers JT. Metal specificity of an iron-responsive element in Alzheimer's APP mRNA 5 ' untranslated region, tolerance of SH-SY5Y and H4 neural cells to desferrioxamine clioquinol, VK-28, and a piperazine chelator. Journal of Neural Transmission-Supplement. 2006:237–247. doi: 10.1007/978-3-211-33328-0_25. [DOI] [PubMed] [Google Scholar]
- Hopkins AL, Mason JS, Overington JP. Can we rationally design promiscuous drugs? Curr Opin Struct Biol. 2006;16:127–36. doi: 10.1016/j.sbi.2006.01.013. [DOI] [PubMed] [Google Scholar]
- Overington JP, Al-Lazikani B, Hopkins AL. How many drug targets are there? Nat Rev Drug Discov. 2006;5:993–6. doi: 10.1038/nrd2199. [DOI] [PubMed] [Google Scholar]
- Paolini GV, Shapland RH, van Hoorn WP, Mason JS, Hopkins AL. Global mapping of pharmacological space. Nat Biotechnol. 2006;24:805–815. doi: 10.1038/nbt1228. [DOI] [PubMed] [Google Scholar]
- Dey A, Tergaonkar V, Lane DP. Double-edged swords as cancer therapeutics: simultaneously targeting p53 and NF-kappaB pathways. Nat Rev Drug Discov. 2008;7:1031–40. doi: 10.1038/nrd2759. [DOI] [PubMed] [Google Scholar]
- Baret P, Beguin CG, Boukhalfa H, Caris C, Laulhere JP, Pierre JL, Serratrice G. O-Trensox – a promising water-soluble iron chelator (both Fe-III and Fe-II) potentially suitable for plant nutrition and iron chelation therapy. JACS. 1995;117:9760–9761. [Google Scholar]
- Caris C, Baret P, Beguin C, Serratrice G, Pierre JL, Laulhere JP. Metabolization of iron by plant cells using O-Trensox, a high-affinity abiotic iron-chelating agent. Biochem J. 1995;312:879–85. doi: 10.1042/bj3120879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry C, Rakba N, Imbert D, Thomas F, Baret P, Serratrice G, Gaude D, Pierre JL, Ward RJ, Crichton RR, Lescoat G. New 8-hydroxyquinoline and catecholate iron chelators: Influence of their partition coefficient on their biological activity. Biochem Pharmacol. 2001;62:1355–1362. doi: 10.1016/s0006-2952(01)00779-1. [DOI] [PubMed] [Google Scholar]
- Serratrice G, Boukhalfa H, Beguin C, Baret P, Caris C, Pierre JL. O-TRENSOX, a new tripodal iron chelator based on 8-hydroxyquinoline subunits: Thermodynamic and kinetic studies. Inorganic Chemistry. 1997;36:3898–3910. [Google Scholar]
- Imbert D, Baret P, Gaude D, Gautier-Luneau I, Gellon G, Thomas F, Serratrice G, Pierre JL. Hydrophilic and lipophilic iron chelators with the same complexing abilities. Chemistry-a European Journal. 2002;8:1091–1100. doi: 10.1002/1521-3765(20020301)8:5<1091::aid-chem1091>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Pierre JL, Baret P, Serratrice G. Hydroxyquinolines as iron chelators. Curr Med Chem. 2003;10:1077–1084. doi: 10.2174/0929867033457584. [DOI] [PubMed] [Google Scholar]
- Apostol M, Baret P, Serratrice G, Desbrieres J, Putaux JL, Stebe MJ, Expert D, Pierre JL. Self-assembly of an amphiphilic iron(III) chelator: Mimicking iron acquisition in marine bacteria. Angewandte Chemie-International Edition. 2005;44:2580–2582. doi: 10.1002/anie.200462841. [DOI] [PubMed] [Google Scholar]
- d'Hardemare AD, Torelli S, Serratrice G, Pierre JL. Design of iron chelators: Syntheses and iron (III) complexing abilities of tripodal tris-bidentate ligands. Biometals. 2006;19:349–366. doi: 10.1007/s10534-005-2997-2. [DOI] [PubMed] [Google Scholar]
- Whitnall M, Howard J, Ponka P, Richardson DR. A class of iron chelators with a wide spectrum of potent antitumor activity that overcomes resistance to chemotherapeutics. Proc Natl Acad Sci USA. 2006;103:14901–6. doi: 10.1073/pnas.0604979103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello Filho AC, Hoffmann ME, Meneghini R. Cell killing and DNA damage by hydrogen peroxide are mediated by intracellular iron. Biochem J. 1984;218:273–5. doi: 10.1042/bj2180273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsuta M, Iishi H, Baba M, Mikuni T, Narahara H, Uedo N, Yano H. Suppression by iron chelator phenanthroline of sodium chloride-enhanced gastric carcinogenesis induced by N-methyl-N'-nitro-N-nitrosoguanidine in Wistar rats. Cancer Lett. 2003;191:9–16. doi: 10.1016/s0304-3835(01)00797-2. [DOI] [PubMed] [Google Scholar]
- Kalinowski DS, Yu Y, Sharpe PC, Islam M, Liao YT, Lovejoy DB, Kumar N, Bernhardt PV, Richardson DR. Design synthesis, and characterization of novel iron chelators: structure-activity relationships of the 2-benzoylpyridine thiosemicarbazone series and their 3-nitrobenzoyl analogues as potent antitumor agents. J Med Chem. 2007;50:3716–29. doi: 10.1021/jm070445z. [DOI] [PubMed] [Google Scholar]
- Kalinowski DS, Sharpe PC, Bernhardt PV, Richardson DR. Design Synthesis, and Characterization of New Iron Chelators with Anti-Proliferative Activity: Structure-Activity Relationships of Novel Thiohydrazone Analogues. J Med Chem. 2007;50:6212–6225. doi: 10.1021/jm070839q. [DOI] [PubMed] [Google Scholar]
- L'Eplattenier F, Murase I, Martell AE. New multidentate ligands .6. chelating tendencies of N,N'-di(2-hydroxybenzyl) ethylenediamine-N,N'-diacetic acid. JACS. 1967;89:837. [Google Scholar]
- Faller B, Spanka C, Sergejew T, Tschinke V. Improving the oral bioavailability of the iron chelator HBED by breaking the symmetry of the intramolecular H-bond network. J Med Chem. 2000;43:1467–1475. doi: 10.1021/jm990261n. [DOI] [PubMed] [Google Scholar]
- Bergeron RJ, Wiegand J, Brittenham GM. HBED: The continuing development of a potential alternative to deferoxamine for iron-chelating therapy. Blood. 1999;93:370–375. [PubMed] [Google Scholar]
- Samuni AM, Afeworki M, Stein W, Yordanov AT, DeGraff W, Krishna MC, Mitchell JB, Brechbiel MW. Multifunctional antioxidant activity of HBED iron chelator. Free Radical Biology and Medicine. 2001;30:170–177. doi: 10.1016/s0891-5849(00)00459-7. [DOI] [PubMed] [Google Scholar]
- Bergeron RJ, Wiegand J, Brittenham GM. HBED ligand: preclinical studies of a potential alternative to deferoxamine for treatment of chronic iron overload and acute iron poisoning. Blood. 2002;99:3019–3026. doi: 10.1182/blood.v99.8.3019. [DOI] [PubMed] [Google Scholar]
- Lebedev AV, Ivanova MV, Levitsky DO. Iron chelators and free radical scavengers in naturally occurring polyhydroxylated 1,4-naphthoquinones. Hemoglobin. 2008;32:165–79. doi: 10.1080/03630260701700017. [DOI] [PubMed] [Google Scholar]
- Liu JK, Killilea DW, Ames BN. Age-associated mitochondrial oxidative decay: Improvement of carnitine acetyltransferase substrate-binding affinity and activity in brain by feeding old rats acetyl-L-carnitine and/or R-alpha-lipoic acid. Proc Natl Acad Sci. 2002;99:1876–1881. doi: 10.1073/pnas.261709098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh JH, Moreau R, Heath SH, Hagen TM. Dietary supplementation with (R)-alpha-lipoic acid reverses the age-related accumulation of iron and depletion of antioxidants in the rat cerebral cortex. Redox Rep. 2005;10:52–60. doi: 10.1179/135100005X21624. [DOI] [PubMed] [Google Scholar]
- Skibska B, Jozelowicz-Okonkwo G, Goraca A. Protective effects of early administration of alpha-lipoic acid against lipopolysaccharide-induced plasma lipid peroxidation. Pharmacological Reports. 2006;58:399–404. [PubMed] [Google Scholar]
- Holmquist L, Stuchbury G, Berbaum K, Muscat S, Young S, Hager K, Engel J, Munch G. Lipoic acid as a novel treatment for Alzheimer's disease and related dementias. Pharmacology & Therapeutics. 2007;113:154–164. doi: 10.1016/j.pharmthera.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Petersen Shay K, Moreau RF, Smith EJ, Hagen TM. Is alpha-lipoic acid a scavenger of reactive oxygen species in vivo? Evidence for its initiation of stress signaling pathways that promote endogenous antioxidant capacity. IUBMB Life. 2008;60:362–7. doi: 10.1002/iub.40. [DOI] [PubMed] [Google Scholar]
- Wei Y, Guo M. Hydrogen peroxide triggered prochelator activation, subsequent metal chelation, and attenuation of the fenton reaction. Angew Chem Int Ed Engl. 2007;46:4722–5. doi: 10.1002/anie.200604859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galey JB. Potential use of iron chelators against oxidative damage. Adv Pharmacol. 1997;38:167–203. doi: 10.1016/s1054-3589(08)60984-9. [DOI] [PubMed] [Google Scholar]
- Faa G, Crisponi G. Iron chelating agents in clinical practice. Coordination Chemistry Reviews. 1999;184:291–310. [Google Scholar]
- Kontoghiorghes GJ, Pattichi K, Hadjigavriel M, Kolnagou A. Transfusional iron overload and chelation therapy with deferoxamine and deferiprone (L1) Transfusion Science. 2000;23:211–223. doi: 10.1016/s0955-3886(00)00089-8. [DOI] [PubMed] [Google Scholar]
- Emerit J, Beaumont C, Trivin F. Iron metabolism, free radicals, and oxidative injury. Biomed Pharmacother. 2001;55:333–9. doi: 10.1016/s0753-3322(01)00068-3. [DOI] [PubMed] [Google Scholar]
- Tam TF, Leung-Toung R, Li WR, Wang YS, Karimian K, Spino M. Iron chelator research: Past present, and future. Current Medicinal Chemistry. 2003;10:983–995. doi: 10.2174/0929867033457593. [DOI] [PubMed] [Google Scholar]
- Blanuša M, Varnai VM, Piasek M, Kostial K. Chelators as antidotes of metal toxicity: Therapeutic and experimental aspects. Curr Med Chem. 2005;12:2771–2794. doi: 10.2174/092986705774462987. [DOI] [PubMed] [Google Scholar]
- Kontoghiorghes GJ, Kolnagou A. Molecular factors and mechanisms affecting iron and other metal excretion or absorption in health and disease. The role of natural and synthetic chelators. Current Medicinal Chemistry. 2005;12:2695–2709. doi: 10.2174/092986705774463030. [DOI] [PubMed] [Google Scholar]
- Kontoghiorghes GJ. Iron mobilization from transferrin and non-transferrin-bound-iron by deferiprone. Implications in the treatment of thalassemia, anemia of chronic disease, cancer and other conditions. Hemoglobin. 2006;30:183–200. doi: 10.1080/03630260600642450. [DOI] [PubMed] [Google Scholar]
- Golenser J, Domb A, Leshem B, Kremsner P, Luty A. Iron chelators as drugs against malaria pose a potential risk. Redox Rep. 2003;8:268–71. doi: 10.1179/135100003225002880. [DOI] [PubMed] [Google Scholar]
- Cherny RA, Legg JT, McLean CA, Fairlie DP, Huang XD, Atwood CS, Beyreuther K, Tanzi RE, Masters CL, Bush AI. Aqueous dissolution of Alzheimer's disease A beta amyloid deposits by biometal depletion. J Biol Chem. 1999;274:23223–23228. doi: 10.1074/jbc.274.33.23223. [DOI] [PubMed] [Google Scholar]
- Ritchie CW, Bush AI, Mackinnon A, Macfarlane S, Mastwyk M, MacGregor L, Kiers L, Cherny R, Li QX, Tammer A, Carrington D, Mavros C, Volitakis I, Xilinas M, Ames D, Davis S, Volitakis I, Xilinas M, Ames D, Davis S, Beyreuther K, Tanzi RE, Masters CL. Metal-protein attenuation with iodochlorhydroxyquin (clioquinol) targeting A beta amyloid deposition and toxicity in Alzheimer disease – A pilot phase 2 clinical trial. Archives of Neurology. 2003;60:1685–1691. doi: 10.1001/archneur.60.12.1685. [DOI] [PubMed] [Google Scholar]
- Regland B, Lehmann W, Abedini I, Blennow K, Jonsson M, Karlsson I, Sjogren M, Wallin A, Xilinas M, Gottfries CG. Treatment of Alzheimer's disease with clioquinol. Dementia and Geriatric Cognitive Disorders. 2001;12:408–414. doi: 10.1159/000051288. [DOI] [PubMed] [Google Scholar]
- Kaur D, Yantiri F, Rajagopalan S, Kumar J, Mo JO, Boonplueang R, Viswanath V, Jacobs R, Yang L, Beal MF, DiMonte D, Volitaskis I, Ellerby L, Cherny RA, Bush AI, Andersen JK. Genetic or pharmacological iron chelation prevents MPTP-induced neurotoxicity in vivo: A novel therapy for Parkinson's disease. Neuron. 2003;37:899–909. doi: 10.1016/s0896-6273(03)00126-0. [DOI] [PubMed] [Google Scholar]
- Ikeda Y, Ikeda K, Long DM. Comparative study of different iron-chelating agents in cold-induced brain edema. Neurosurgery. 1989;24:820–4. doi: 10.1227/00006123-198906000-00006. [DOI] [PubMed] [Google Scholar]
- Masuda T, Hida H, Kanda Y, Aihara N, Ohta K, Yamada K, Nishino H. Oral administration of metal chelator ameliorates motor dysfunction after a small hemorrhage near the internal capsule in rat. J Neurosci Res. 2007;85:213–222. doi: 10.1002/jnr.21089. [DOI] [PubMed] [Google Scholar]
- De Vries B, Walter SJ, Von Bonsdorff L, Wolfs TGAM, Van Heurn LWE, Parkkinen J, Buurman WA. Reduction of circulating redox-active iron by apotransferrin protects against renal ischemia-reperfusion injury. Transplantation. 2004;77:669–675. doi: 10.1097/01.tp.0000115002.28575.e7. [DOI] [PubMed] [Google Scholar]
- Galey JB, Destree O, Dumats J, Pichaud P, Marche J, Genard S, Bracciolli G, Le Capitaine L, Plessix H, Brambilla L, Cantoni O. Protection of U937 cells against oxidative injury by a novel series of iron chelators. Free Radic Biol Med. 1998;25:881–90. doi: 10.1016/s0891-5849(98)00113-0. [DOI] [PubMed] [Google Scholar]
- Galey JB, Destree O, Dumats J, Genard S, Tachon P. Protection against oxidative damage by iron chelators: effect of lipophilic analogues and prodrugs of N,N'-bis(3,4,5-trimethoxybenzyl)ethylenediamine- N,N'-diacetic acid (OR10141) J Med Chem. 2000;43:1418–21. doi: 10.1021/jm9911635. [DOI] [PubMed] [Google Scholar]
- Galey JB. Recent advances in the design of iron chelators against oxidative damage. Mini Rev Med Chem. 2001;1:233–42. doi: 10.2174/1389557013406846. [DOI] [PubMed] [Google Scholar]
- Charkoudian LK, Pham DM, Franz KJ. A pro-chelator triggered by hydrogen peroxide inhibits iron-promoted hydroxyl radical formation. JACS. 2006;128:12424–12425. doi: 10.1021/ja064806w. [DOI] [PubMed] [Google Scholar]
- Gal S, Zheng H, Fridkin M, Youdim MBH. Novel multifunctional neuroprotective iron chelator-monoamine oxidase inhibitor drugs for neurodegenerative diseases. In vivo selective brain monoamine oxidase inhibition and prevention of MPTP-induced striatal dopamine depletion. Journal of Neurochemistry. 2005;95:79–88. doi: 10.1111/j.1471-4159.2005.03341.x. [DOI] [PubMed] [Google Scholar]
- Zheng H, Weiner LM, Bar-Am O, Epsztejn S, Cabantchik ZI, Warshawsky A, Youdim MB, Fridkin M. Design synthesis, and evaluation of novel bifunctional iron-chelators as potential agents for neuroprotection in Alzheimer's, Parkinson's, and other neurodegenerative diseases. Bioorg Med Chem. 2005;13:773–83. doi: 10.1016/j.bmc.2004.10.037. [DOI] [PubMed] [Google Scholar]
- Zheng HL, Youdim MBH, Weiner LM, Fridkin M. Novel potential neuroprotective agents with both iron chelating and amino acid-based derivatives targeting central nervous system neurons. Biochemical Pharmacology. 2005;70:1642–1652. doi: 10.1016/j.bcp.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Gal S, Fridkin M, Amit T, Zheng H, Youdim MBH. M30, a novel multifunctional neuroprotective drug with potent iron chelating and brain selective monoamine oxidase-ab inhibitory activity for Parkinson's disease. J Neural Transmission – Suppl. 2006. pp. 447–456. [DOI] [PubMed]
- Avramovich-Tirosh Y, Amit T, Bar-Am O, Zheng HL, Fridkin M, Youdim MBH. Therapeutic targets and potential of the novel brain- permeable multifunctional iron chelator-monoamine oxidase inhibitor drug, M-30, for the treatment of Alzheimer's disease. J Neurochem. 2007;100:490–502. doi: 10.1111/j.1471-4159.2006.04258.x. [DOI] [PubMed] [Google Scholar]
- Kayyali R, Pannala AS, Khodr H, Hider RC. Comparative radical scavenging ability of bidentate iron (III) chelators. Biochem Pharmacol. 1998;55:1327–32. doi: 10.1016/s0006-2952(97)00602-3. [DOI] [PubMed] [Google Scholar]
- Kontoghiorghes GJ, Weinberg ED. Iron – mammalian defense systems, mechanisms of disease, and chelation therapy approaches. Blood Rev. 1995;9:33–45. doi: 10.1016/0268-960x(95)90038-1. [DOI] [PubMed] [Google Scholar]
- Gabutti V, Piga A. Results of long-perm iron-chelating therapy. Acta Haematologica. 1996;95:26–36. doi: 10.1159/000203853. [DOI] [PubMed] [Google Scholar]
- Song BB, Anderson DJ, Schacht J. Protection from gentamicin ototoxicity by iron chelators in guinea pig in vivo. Journal of Pharmacology and Experimental Therapeutics. 1997;282:369–377. [PubMed] [Google Scholar]
- Song BB, Schacht J. Variable efficacy of radical scavengers and iron chelators to attenuate gentamicin ototoxicity in guinea pig in vivo. Hearing Research. 1996;94:87–93. doi: 10.1016/0378-5955(96)00003-2. [DOI] [PubMed] [Google Scholar]
- Wetli HA, Buckett PD, Wessling-Resnick M. Small-molecule screening identifies the selanazal drug ebselen as a potent inhibitor of DMT1-mediated iron uptake. Chemistry & Biology. 2006;13:965–972. doi: 10.1016/j.chembiol.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovorka SW, Schoneich C. Oxidative degradation of pharmaceuticals: Theory, mechanisms and inhibition. J Pharm Sci. 2001;90:253–269. doi: 10.1002/1520-6017(200103)90:3<253::aid-jps1>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- England K, Driscoll CO, Cotter TG. ROS and protein oxidation in early stages of cytotoxic drug induced apoptosis. Free Radical Research. 2006;40:1124–1137. doi: 10.1080/10715760600838209. [DOI] [PubMed] [Google Scholar]
- Cha'on U, Valmas N, Collins PJ, Reilly PEB, Hammock BD, Ebert PR. Disruption of iron homeostasis increases phosphine toxicity in Caenorhabditis elegans. Toxicological Sciences. 2007;96:194–201. doi: 10.1093/toxsci/kfl187. [DOI] [PubMed] [Google Scholar]
- Bus JS, Gibson JE, Aust SD. Superoxide-catalyzed and singlet oxygen-catalyzed lipid peroxidation as a possible mechanism for paraquat (methyl viologen) toxicity. Biochem Biophys Res Commun. 1974;58:749–755. doi: 10.1016/s0006-291x(74)80481-x. [DOI] [PubMed] [Google Scholar]
- Doelman CJA, Bast A. Oxygen Radicals in Lung Pathology. Free Rad Biol Med. 1990;9:381–400. doi: 10.1016/0891-5849(90)90015-b. [DOI] [PubMed] [Google Scholar]
- Hippeli S, Elstner EF. Transition metal ion-catalyzed oxygen activation during pathogenic processes. Febs Letters. 1999;443:1–7. doi: 10.1016/s0014-5793(98)01665-2. [DOI] [PubMed] [Google Scholar]
- Jones GM, Vale JA. Mechanisms of toxicity, clinical features, and management of diquat poisoning: A review. J Toxicol. 2000;38:123–128. doi: 10.1081/clt-100100926. [DOI] [PubMed] [Google Scholar]
- Suntres ZE. Role of antioxidants in paraquat toxicity. Toxicology. 2002;180:65–77. doi: 10.1016/s0300-483x(02)00382-7. [DOI] [PubMed] [Google Scholar]
- Abdollahi M, Ranjbar A, Shadnia S, Nikfar S, Rezaie A. Pesticides and oxidative stress: a review. Medical Science Monitor. 2004;10:RA141–RA147. [PubMed] [Google Scholar]
- Rogachev I, Kampel V, Gusis V, Cohen N, Gressel J, Warshawsky A. Synthesis properties, and use of copper-chelating amphiphilic dithiocarbamates as synergists of oxidant-generating herbicides. Pesticide Biochemistry and Physiology. 1998;60:133–145. [Google Scholar]
- Kotamraju S, Chitambar CR, Kalivendi SV, Joseph J, Kalyanaraman B. Transferrin receptor-dependent iron uptake is responsible for doxorubicin-mediated apoptosis in endothelial cells – Role of oxidant-induced iron signaling in apoptosis. Journal of Biological Chemistry. 2002;277:17179–17187. doi: 10.1074/jbc.M111604200. [DOI] [PubMed] [Google Scholar]
- Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L. Anthracyclines: Molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacological Reviews. 2004;56:185–229. doi: 10.1124/pr.56.2.6. [DOI] [PubMed] [Google Scholar]
- Asare GA, Bronz M, Naidoo V, Kew MC. Interactions between aflatoxin B1 and dietary iron overload in hepatic mutagenesis. Toxicology. 2007;234:157–66. doi: 10.1016/j.tox.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Scalbert A, Williamson G. Dietary intake and bioavailability of polyphenols. J Nutr. 2000;130:2073S–85S. doi: 10.1093/jn/130.8.2073S. [DOI] [PubMed] [Google Scholar]
- Reed J. Cranberry flavonoids, atherosclerosis and cardiovascular health. Crit Rev Food Sci Nutr. 2002;42:301–16. doi: 10.1080/10408390209351919. [DOI] [PubMed] [Google Scholar]
- Benzie IF. Evolution of dietary antioxidants. Comp Biochem Physiol A Mol Integr Physiol. 2003;136:113–26. doi: 10.1016/s1095-6433(02)00368-9. [DOI] [PubMed] [Google Scholar]
- Higdon JV, Frei B. Tea catechins and polyphenols: health effects metabolism, and antioxidant functions. Crit Rev Food Sci Nutr. 2003;43:89–143. doi: 10.1080/10408690390826464. [DOI] [PubMed] [Google Scholar]
- Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3:768–80. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- Manach C, Scalbert A, Morand C, Remesy C, Jimenez L. Polyphenols: food sources and bioavailability. Am J Clin Nutr. 2004;79:727–47. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- Manach C, Mazur A, Scalbert A. Polyphenols and prevention of cardiovascular diseases. Curr Opin Lipidol. 2005;16:77–84. doi: 10.1097/00041433-200502000-00013. [DOI] [PubMed] [Google Scholar]
- Scalbert A, Manach C, Morand C, Remesy C, Jimenez L. Dietary polyphenols and the prevention of diseases. Crit Rev Food Sci Nutr. 2005;45:287–306. doi: 10.1080/1040869059096. [DOI] [PubMed] [Google Scholar]
- Nichenametla SN, Taruscio TG, Barney DL, Exon JH. A review of the effects and mechanisms of polyphenolics in cancer. Crit Rev Food Sci Nutr. 2006;46:161–83. doi: 10.1080/10408390591000541. [DOI] [PubMed] [Google Scholar]
- Zhang HY, Yang DP, Tang GY. Multipotent antioxidants: from screening to design. Drug Discov Today. 2006;11:749–54. doi: 10.1016/j.drudis.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Shahidi F, Wanasundara PK. Phenolic antioxidants. Crit Rev Food Sci Nutr. 1992;32:67–103. doi: 10.1080/10408399209527581. [DOI] [PubMed] [Google Scholar]
- Wright JS, Johnson ER, DiLabio GA. Predicting the activity of phenolic antioxidants: theoretical method, analysis of substituent effects, and application to major families of antioxidants. J Am Chem Soc. 2001;123:1173–83. doi: 10.1021/ja002455u. [DOI] [PubMed] [Google Scholar]
- Block G, Patterson B, Subar A. Fruit vegetables, and cancer prevention: a review of the epidemiological evidence. Nutr Cancer. 1992;18:1–29. doi: 10.1080/01635589209514201. [DOI] [PubMed] [Google Scholar]
- Ness AR, Powles JW. Fruit and vegetables, and cardiovascular disease: a review. Int J Epidemiol. 1997;26:1–13. doi: 10.1093/ije/26.1.1. [DOI] [PubMed] [Google Scholar]
- Krauss RM, Eckel RH, Howard B, Appel LJ, Daniels SR, Deckelbaum RJ, Erdman JW, Jr, Kris-Etherton P, Goldberg IJ, Kotchen TA, Lichtenstein AH, Mitch WE, Mullis R, Robinson K, Wylie-Rosett J, St Jeor S, Suttie J, Tribble DL, Bazzarre TL. AHA Dietary Guidelines: revision 2000: A statement for healthcare professionals from the Nutrition Committee of the American Heart Association. Circulation. 2000;102:2284–99. doi: 10.1161/01.cir.102.18.2284. [DOI] [PubMed] [Google Scholar]
- Yang CS, Landau JM, Huang MT, Newmark HL. Inhibition of carcinogenesis by dietary polyphenolic compounds. Annu Rev Nutr. 2001;21:381–406. doi: 10.1146/annurev.nutr.21.1.381. [DOI] [PubMed] [Google Scholar]
- Lotito SB, Frei B. Consumption of flavonoid-rich foods and increased plasma antioxidant capacity in humans: Cause consequence, or epiphenomenon? Free Rad Biol Med. 2006;41:1727–1746. doi: 10.1016/j.freeradbiomed.2006.04.033. [DOI] [PubMed] [Google Scholar]
- Cook NC, Samman S. Flavonoids – Chemistry metabolism, cardioprotective effects, and dietary sources. Journal of Nutritional Biochemistry. 1996;7:66–76. [Google Scholar]
- Bravo L. Polyphenols: chemistry, dietary sources metabolism, and nutritional significance. Nutrition Reviews. 1998;56:317–333. doi: 10.1111/j.1753-4887.1998.tb01670.x. [DOI] [PubMed] [Google Scholar]
- Fang YZ, Yang S, Wu GY. Free radicals antioxidants, and nutrition. Nutrition. 2002;18:872–879. doi: 10.1016/s0899-9007(02)00916-4. [DOI] [PubMed] [Google Scholar]
- Fraga CG, Oteiza PI. Iron toxicity and antioxidant nutrients. Toxicology. 2002;180:23–32. doi: 10.1016/s0300-483x(02)00379-7. [DOI] [PubMed] [Google Scholar]
- Halvorsen BL, Holte K, Myhrstad MC, Barikmo I, Hvattum E, Remberg SF, Wold AB, Haffner K, Baugerod H, Andersen LF, Moskaug O, Jacobs DR, Jr, Blomhoff R. A systematic screening of total antioxidants in dietary plants. J Nutr. 2002;132:461–71. doi: 10.1093/jn/132.3.461. [DOI] [PubMed] [Google Scholar]
- Havsteen BH. The biochemistry and medical significance of the flavonoids. Pharmacol Ther. 2002;96:67–202. doi: 10.1016/s0163-7258(02)00298-x. [DOI] [PubMed] [Google Scholar]
- Vertuani S, Angusti A, Manfredini S. The antioxidants and pro-antioxidants network: an overview. Curr Pharmaceut Design. 2004;10:1677–1694. doi: 10.2174/1381612043384655. [DOI] [PubMed] [Google Scholar]
- Arts ICW, Hollman PCH. Polyphenols and disease risk in epidemiologic studies. American Journal of Clinical Nutrition. 2005;81:317S–325S. doi: 10.1093/ajcn/81.1.317S. [DOI] [PubMed] [Google Scholar]
- Afanas'ev IB, Dorozhko AI, Brodskii AV, Kostyuk VA, Potapovitch AI. Chelating and free radical scavenging mechanisms of inhibitory action of rutin and quercetin in lipid peroxidation. Biochem Pharmacol. 1989;38:1763–9. doi: 10.1016/0006-2952(89)90410-3. [DOI] [PubMed] [Google Scholar]
- Morel I, Lescoat G, Cillard P, Cillard J. Role of flavonoids and iron chelation in antioxidant action. Methods Enzymol. 1994;234:437–43. doi: 10.1016/0076-6879(94)34114-1. [DOI] [PubMed] [Google Scholar]
- Moran JF, Klucas RV, Grayer RJ, Abian J, Becana M. Complexes of iron with phenolic compounds from soybean nodules and other legume tissues: prooxidant and antioxidant properties. Free Radic Biol Med. 1997;22:861–70. doi: 10.1016/s0891-5849(96)00426-1. [DOI] [PubMed] [Google Scholar]
- Yoshino M, Murakami K. Interaction of iron with polyphenolic compounds: application to antioxidant characterization. Anal Biochem. 1998;257:40–4. doi: 10.1006/abio.1997.2522. [DOI] [PubMed] [Google Scholar]
- Grinberg LN, Newmark H, Kitrossky N, Rahamim E, Chevion M, Rachmilewitz EA. Protective effects of tea polyphenols against oxidative damage to red blood cells. Biochemical Pharmacology. 1997;54:973–978. doi: 10.1016/s0006-2952(97)00155-x. [DOI] [PubMed] [Google Scholar]
- Lopes GKB, Schulman HM, Hermes-Lima M. Polyphenol tannic acid inhibits hydroxyl radical formation from Fenton reaction by complexing ferrous ions. Biochimica Et Biophysica Acta-General Subjects. 1999;1472:142–152. doi: 10.1016/s0304-4165(99)00117-8. [DOI] [PubMed] [Google Scholar]
- Packer L, Rimbach G, Virgili F. Antioxidant activity and biologic properties of a procyanidin-rich extract from pine (Pinus maritima) bark, pycnogenol. Free Rad Biol Med. 1999;27:704–724. doi: 10.1016/s0891-5849(99)00090-8. [DOI] [PubMed] [Google Scholar]
- Robards K, Prenzler PD, Tucker G, Swatsitang P, Glover W. Phenolic compounds and their role in oxidative processes in fruits. Food Chem. 1999;66:401–436. [Google Scholar]
- Middleton E, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacological Reviews. 2000;52:673–751. [PubMed] [Google Scholar]
- Hider RC, Liu ZD, Khodr HH. Metal chelation of polyphenols. Flavonoids and Other Polyphenols, Methods in Enzymology. 2001;335:190–203. doi: 10.1016/s0076-6879(01)35243-6. [DOI] [PubMed] [Google Scholar]
- Levites Y, Youdim MBH, Maor G, Mandel S. Attenuation of 6-hydroxydopamine (6-OHDA)-induced nuclear factor-kappaB (NF-kappa B) activation and cell death by tea extracts in neuronal cultures. Biochemical Pharmacology. 2002;63:21–29. doi: 10.1016/s0006-2952(01)00813-9. [DOI] [PubMed] [Google Scholar]
- Mandel S, Youdim MBH. Catechin polyphenols: Neurodegeneration and neuroprotection in neurodegenerative diseases. Free Radical Biology and Medicine. 2004;37:304–317. doi: 10.1016/j.freeradbiomed.2004.04.012. [DOI] [PubMed] [Google Scholar]
- Mandel S, Weinreb O, Amit T, Youdim MBH. Cell signaling pathways in the neuroprotective actions of the green tea polyphenol (-)-epigallocatechin-3-gallate: implications for neurodegenerative diseases. J Neurochem. 2004;88:1555–1569. doi: 10.1046/j.1471-4159.2003.02291.x. [DOI] [PubMed] [Google Scholar]
- Hague T, Andrews PLR, Barker J, Naughton DP. Dietary chelators as antioxidant enzyme mimetics: implications for dietary intervention in neurodegenerative diseases. Behavioural Pharmacology. 2006;17:425–430. doi: 10.1097/00008877-200609000-00008. [DOI] [PubMed] [Google Scholar]
- Weinreb O, Amit T, Youdim MB. A novel approach of proteomics and transcriptomics to study the mechanism of action of the antioxidant-iron chelator green tea polyphenol (-)-epigallocatechin-3-gallate. Free Radic Biol Med. 2007;43:546–56. doi: 10.1016/j.freeradbiomed.2007.05.011. [DOI] [PubMed] [Google Scholar]
- Rice-Evans CA, Miller NJ, Paganga G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med. 1996;20:933–56. doi: 10.1016/0891-5849(95)02227-9. [DOI] [PubMed] [Google Scholar]
- Rice-Evans CA, Miller J, Paganga G. Antioxidant properties of phenolic compounds. Trends in Plant Science. 1997;2:152–159. [Google Scholar]
- Kähkönen MP, Hopia AI, Vuorela HJ, Rauha JP, Pihlaja K, Kujala TS, Heinonen M. Antioxidant activity of plant extracts containing phenolic compounds. J Agric Food Chem. 1999;47:3954–62. doi: 10.1021/jf990146l. [DOI] [PubMed] [Google Scholar]
- Wang H, Cao GH, Prior RL. Oxygen radical absorbing capacity of anthocyanins. Journal of Agricultural and Food Chemistry. 1997;45:304–309. [Google Scholar]
- Harborne JB, Williams CA. Advances in flavonoid research since 1992. Phytochemistry. 2000;55:481–504. doi: 10.1016/s0031-9422(00)00235-1. [DOI] [PubMed] [Google Scholar]
- Nijveldt RJ, van Nood E, van Hoorn DEC, Boelens PG, van Norren K, van Leeuwen PAM. Flavonoids: a review of probable mechanisms of action and potential applications. American Journal of Clinical Nutrition. 2001;74:418–425. doi: 10.1093/ajcn/74.4.418. [DOI] [PubMed] [Google Scholar]
- Heim KE, Tagliaferro AR, Bobilya DJ. Flavonoid antioxidants: chemistry, metabolism and structure-activity relationships. J Nutr Biochem. 2002;13:572–584. doi: 10.1016/s0955-2863(02)00208-5. [DOI] [PubMed] [Google Scholar]
- Rice-Evans CA, Packer L. Flavonoids in health and disease. 2. Marcel Dekker, New York; 2003. [Google Scholar]
- Robbins RJ. Phenolic acids in foods: an overview of analytical methodology. J Agric Food Chem. 2003;51:2866–87. doi: 10.1021/jf026182t. [DOI] [PubMed] [Google Scholar]
- Sakakibara H, Honda Y, Nakagawa S, Ashida H, Kanazawa K. Simultaneous determination of all polyphenols in vegetables fruits, and teas. J Agric Food Chem. 2003;51:571–81. doi: 10.1021/jf020926l. [DOI] [PubMed] [Google Scholar]
- Morel I, Lescoat G, Cogrel P, Sergent O, Pasdeloup N, Brissot P, Cillard P, Cillard J. Antioxidant and iron-chelating activities of the flavonoids catechin, quercetin and diosmetin on iron-loaded rat hepatocyte cultures. Biochem Pharmacol. 1993;45:13–19. doi: 10.1016/0006-2952(93)90371-3. [DOI] [PubMed] [Google Scholar]
- Ferrali M, Signorini C, Caciotti B, Sugherini L, Ciccoli L, Giachetti D, Comporti M. Protection against oxidative damage of erythrocyte membrane by the flavonoid quercetin and its relation to iron chelating activity. FEBS Lett. 1997;416:123–9. doi: 10.1016/s0014-5793(97)01182-4. [DOI] [PubMed] [Google Scholar]
- Shoskes DA. Effect of bioflavonoids quercetin and curcumin on ischemic renal injury – A new class of renoprotective agents. Transplantation. 1998;66:147–152. doi: 10.1097/00007890-199807270-00001. [DOI] [PubMed] [Google Scholar]
- Kostyuk VA, Potapovich AI. Antiradical and chelating effects in flavonoid protection against silica-induced cell injury. Archives of Biochemistry and Biophysics. 1998;355:43–48. doi: 10.1006/abbi.1998.0708. [DOI] [PubMed] [Google Scholar]
- Aherne SA, O'Brien NM. Mechanism of protection by the flavonoids, quercetin and rutin, against tert-butylhydroperoxide- and menadione induced DNA single strand breaks in Caco-2 cells. Free Radical Biology and Medicine. 2000;29:507–514. doi: 10.1016/s0891-5849(00)00360-9. [DOI] [PubMed] [Google Scholar]
- Cheng IF, Breen K. On the ability of four flavonoids baicilein, luteolin naringenin, and quercetin, to suppress the Fenton reaction of the iron-ATP complex. Biometals. 2000;13:77–83. doi: 10.1023/a:1009229429250. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Li H, Zhao Y, Gao Z. Dietary supplementation of baicalin and quercetin attenuates iron overload induced mouse liver injury. Eur J Pharmacol. 2006;535:263–9. doi: 10.1016/j.ejphar.2006.01.067. [DOI] [PubMed] [Google Scholar]
- Comporti M, Signorini C, Buonocore G, Ciccoli L. Iron release, oxidative stress and erythrocyte ageing. Free Radical Biology and Medicine. 2002;32:568–576. doi: 10.1016/s0891-5849(02)00759-1. [DOI] [PubMed] [Google Scholar]
- Mira L, Fernandez MT, Santos M, Rocha R, Florencio MH, Jennings KR. Interactions of flavonoids with iron and copper ions: A mechanism for their antioxidant activity. Free Radical Research. 2002;36:1199–1208. doi: 10.1080/1071576021000016463. [DOI] [PubMed] [Google Scholar]
- Melidou M, Riganakos K, Galaris D. Protection against nuclear DNA damage offered by flavonoids in cells exposed to hydrogen peroxide: The role of iron chelation. Free Rad Biol Med. 2005;39:1591–1600. doi: 10.1016/j.freeradbiomed.2005.08.009. [DOI] [PubMed] [Google Scholar]
- El Hajji H, Nkhili E, Tomao V, Dangles O. Interactions of quercetin with iron and copper ions: complexation and autoxidation. Free Radic Res. 2006;40:303–20. doi: 10.1080/10715760500484351. [DOI] [PubMed] [Google Scholar]
- Russo A, Acquaviva R, Campisi A, Sorrenti V, Di Giacomo C, Virgata G, Barcellona ML, Vanella A. Bioflavonoids as antiradicals, antioxidants and DNA cleavage protectors. Cell Biology and Toxicology. 2000;16:91–98. doi: 10.1023/a:1007685909018. [DOI] [PubMed] [Google Scholar]
- Kaiserová H, Šimůnek T, Vijgh WJ van der, Bast A, Kvasničková E. Flavonoids as protectors against doxorubicin cardiotoxicity: role of iron chelation, antioxidant activity and inhibition of carbonyl reductase. Biochim Biophys Acta. 2007;1772:1065–74. doi: 10.1016/j.bbadis.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Li H, Gao Z, Xu H. Effects of dietary baicalin supplementation on iron overload-induced mouse liver oxidative injury. Eur J Pharmacol. 2005;509:195–200. doi: 10.1016/j.ejphar.2004.11.060. [DOI] [PubMed] [Google Scholar]
- Borsari M, Ferrari E, Grandi R, Saladini M. Curcuminoids as potential new iron-chelating agents: spectroscopic, polarographic and potentiometric study on their Fe(III) complexing ability. Inorganica Chimica Acta. 2002;328:61–68. [Google Scholar]
- Baum L, Ng A. Curcumin interaction with copper and iron suggests one possible mechanism of action in Alzheimer's disease animal models. J Alzheimers Dis. 2004;6:367–377. doi: 10.3233/jad-2004-6403. [DOI] [PubMed] [Google Scholar]
- Bernabé-Pineda M, Ramírez-Silva MT, Romero-Romo MA, González-Vergara E, Rojas-Hernández A. Spectrophotometric and electrochemical determination of the formation constants of the complexes Curcumin-Fe(Ill)-water and Curcumin-Fe(II)-water. Spectrochim Acta A. 2004;60:1105–1113. doi: 10.1016/S1386-1425(03)00344-5. [DOI] [PubMed] [Google Scholar]
- Jiao Y, Wilkinson J, Pletsch EC, Buss JL, Wang W, Planalp R, Torti FM, Torti SV. Iron chelation in the biological activity of curcumin. Free Radical Biology and Medicine. 2006;40:1152–1160. doi: 10.1016/j.freeradbiomed.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Aggarwal BB, Sundaram C, Malani N, Ichikawa H. Curcumin: The Indian solid gold. Adv Exp Med Biol. 2007;595:1–75. doi: 10.1007/978-0-387-46401-5_1. [DOI] [PubMed] [Google Scholar]
- Farombi EO, Nwaokeafor IA. Anti-oxidant mechanisms of kolaviron: studies on serum lipoprotein oxidation, metal chelation and oxidative membrane damage in rats. Clin Exp Pharmacol Physiol. 2005;32:667–74. doi: 10.1111/j.0305-1870.2005.04248.x. [DOI] [PubMed] [Google Scholar]
- Engelmann MD, Hutcheson R, Cheng IF. Stability of ferric complexes with 3-hydroxyflavone (flavonol), 5,7-dihydroxyflavone (chrysin), and 3', 4'-dihydroxyflavone. J Agric Food Chem. 2005;53:2953–2960. doi: 10.1021/jf048298q. [DOI] [PubMed] [Google Scholar]
- Botelho FV, Alvarez-Leite JI, Lemos VS, Pimenta AM, Calado HD, Matencio T, Miranda CT, Pereira-Maia EC. Physicochemical study of floranol, its copper(II) and iron(III) complexes, and their inhibitory effect on LDL oxidation. J Inorg Biochem. 2007;101:935–43. doi: 10.1016/j.jinorgbio.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Gottlieb M, Leal-Campanario R, Campos-Esparza MR, Sanchez-Gomez MV, Alberdi E, Arranz A, Delgado-Garcia JM, Gruart A, Matute C. Neuroprotection by two polyphenols following excitotoxicity and experimental ischemia. Neurobiol Dis. 2006;23:374–86. doi: 10.1016/j.nbd.2006.03.017. [DOI] [PubMed] [Google Scholar]
- Pardo-Andreu GL, Sanchez-Baldoquin C, Avila-Gonzalez R, Delgado R, Naal Z, Curti C. Fe(III) improves antioxidant and cytoprotecting activities of mangiferin. European Journal of Pharmacology. 2006;547:31–36. doi: 10.1016/j.ejphar.2006.07.040. [DOI] [PubMed] [Google Scholar]
- Pardo-Andreu GL, Cavalheiro RA, Dorta DJ, Naal Z, Delgado R, Vercesi AE, Curti C. Fe(III) shifts the mitochondria permeability transition-eliciting capacity of mangiferin to protection of organelle. J Pharmacol Exp Ther. 2007;320:646–53. doi: 10.1124/jpet.106.112003. [DOI] [PubMed] [Google Scholar]
- Pardo-Andreu GL, Barrios MF, Curti C, Hernandez I, Merino N, Lemus Y, Martinez I, Riano A, Delgado R. Protective effects of Mangifera indica L extract (Vimang), and its major component mangiferin, on iron-induced oxidative damage to rat serum and liver. Pharmacol Res. 2008;57:79–86. doi: 10.1016/j.phrs.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Mandel S, Weinreb O, Reznichenko L, Kalfon L, Amit T. Green tea catechins as brain-permeable, non toxic iron chelators to "iron out iron" from the brain. Journal of Neural Transmission-Supplement. 2006:249–257. doi: 10.1007/978-3-211-33328-0_26. [DOI] [PubMed] [Google Scholar]
- Mandel S, Amit T, Reznichenko L, Weinreb O, Youdim MBH. Green tea catechins as brain-permeable, natural iron chelators-antioxidants for the treatment of neurodegenerative disorders. Molecular Nutrition & Food Research. 2006;50:229–234. doi: 10.1002/mnfr.200500156. [DOI] [PubMed] [Google Scholar]
- Miller NJ, Castelluccio C, Tijburg L, Rice-Evans C. The antioxidant properties of theaflavins and their gallate esters–radical scavengers or metal chelators? FEBS Lett. 1996;392:40–4. doi: 10.1016/0014-5793(96)00780-6. [DOI] [PubMed] [Google Scholar]
- Khokhar S, Apenten RKO. Iron binding characteristics of phenolic compounds: some tentative structure-activity relations. Food Chemistry. 2003;81:133–140. [Google Scholar]
- Limson J, Nyokong T, Daya S. The interaction of melatonin and its precursors with aluminium cadmium, copper iron, lead, and zinc: an adsorptive voltammetric study. J Pineal Res. 1998;24:15–21. doi: 10.1111/j.1600-079x.1998.tb00361.x. [DOI] [PubMed] [Google Scholar]
- Lin AM, Ho LT. Melatonin suppresses iron-induced neurodegeneration in rat brain. Free Radic Biol Med. 2000;28:904–11. doi: 10.1016/s0891-5849(00)00169-6. [DOI] [PubMed] [Google Scholar]
- Gulcin I, Buyukokuroglu ME, Kufrevioglu OI. Metal chelating and hydrogen peroxide scavenging effects of melatonin. J Pineal Res. 2003;34:278–281. doi: 10.1034/j.1600-079x.2003.00042.x. [DOI] [PubMed] [Google Scholar]
- Chwelatiuk E, Wlostowski T, Krasowska A, Bonda E. The effect of orally administered melatonin on tissue accumulation and toxicity of cadmium in mice. Journal of Trace Elements in Medicine and Biology. 2006;19:259–265. doi: 10.1016/j.jtemb.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Belguendouz L, Fremont L, Linard A. Resveratrol inhibits metal ion-dependent and independent peroxidation of porcine low-density lipoproteins. Biochemical Pharmacology. 1997;53:1347–1355. doi: 10.1016/s0006-2952(96)00820-9. [DOI] [PubMed] [Google Scholar]
- Cadenas S, Barja G. Resveratrol melatonin, vitamin E, and PBN protect against renal oxidative DNA damage induced by the kidney carcinogen KBrO3. Free Radic Biol Med. 1999;26:1531–7. doi: 10.1016/s0891-5849(99)00019-2. [DOI] [PubMed] [Google Scholar]
- Hung LM, Chen JK, Huang SS, Lee RS, Su MJ. Cardioprotective effect of resveratrol, a natural antioxidant derived from grapes. Cardiovasc Res. 2000;47:549–55. doi: 10.1016/s0008-6363(00)00102-4. [DOI] [PubMed] [Google Scholar]
- Giovannini L, Migliori M, Longoni BM, Das DK, Bertelli AAE, Panichi SV, Filippi C, Bertelli A. Resveratrol, a polyphenol found in wine, reduces ischemia reperfusion injury in rat kidneys. J Cardiovasc Pharmacol. 2001;37:262–270. doi: 10.1097/00005344-200103000-00004. [DOI] [PubMed] [Google Scholar]
- Huang SS, Tsai MC, Chih CL, Hung LM, Tsai SK. Resveratrol reduction of infarct size in Long-Evans rats subjected to focal cerebral ischemia. Life Sci. 2001;69:1057–65. doi: 10.1016/s0024-3205(01)01195-x. [DOI] [PubMed] [Google Scholar]
- Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–6. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- Leonard SS, Xia C, Jiang BH, Stinefelt B, Klandorf H, Harris GK, Shi X. Resveratrol scavenges reactive oxygen species and effects radical-induced cellular responses. Biochem Biophys Res Commun. 2003;309:1017–26. doi: 10.1016/j.bbrc.2003.08.105. [DOI] [PubMed] [Google Scholar]
- Kundu JK, Surh YJ. Molecular basis of chemoprevention by resveratrol: NF-kappaB and AP-1 as potential targets. Mutat Res. 2004;555:65–80. doi: 10.1016/j.mrfmmm.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Signorelli P, Ghidoni R. Resveratrol as an anticancer nutrient: molecular basis, open questions and promises. J Nutr Biochem. 2005;16:449–66. doi: 10.1016/j.jnutbio.2005.01.017. [DOI] [PubMed] [Google Scholar]
- Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–42. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- Kotha A, Sekharam M, Cilenti L, Siddiquee K, Khaled A, Zervos AS, Carter B, Turkson J, Jove R. Resveratrol inhibits Src and Stat3 signaling and induces the apoptosis of malignant cells containing activated Stat3 protein. Mol Cancer Ther. 2006;5:621–9. doi: 10.1158/1535-7163.MCT-05-0268. [DOI] [PubMed] [Google Scholar]
- Russo GL. Ins and outs of dietary phytochemicals in cancer chemoprevention. Biochem Pharmacol. 2007;74:533–44. doi: 10.1016/j.bcp.2007.02.014. [DOI] [PubMed] [Google Scholar]
- Tsai SK, Hung LM, Fu YT, Cheng H, Nien MW, Liu HY, Zhang FB, Huang SS. Resveratrol neuroprotective effects during focal cerebral ischemia injury via nitric oxide mechanism in rats. J Vasc Surg. 2007;46:346–53. doi: 10.1016/j.jvs.2007.04.044. [DOI] [PubMed] [Google Scholar]
- Gatz SA, Wiesmuller L. Take a break–resveratrol in action on DNA. Carcinogenesis. 2008;29:321–32. doi: 10.1093/carcin/bgm276. [DOI] [PubMed] [Google Scholar]
- Chu YF, Sun J, Wu X, Liu RH. Antioxidant and antiproliferative activities of common vegetables. J Agric Food Chem. 2002;50:6910–6. doi: 10.1021/jf020665f. [DOI] [PubMed] [Google Scholar]
- Afaq F, Saleem M, Krueger CG, Reed JD, Mukhtar H. Anthocyanin- and hydrolyzable tannin-rich pomegranate fruit extract modulates MAPK and NF-kappaB pathways and inhibits skin tumorigenesis in CD-1 mice. Int J Cancer. 2005;113:423–33. doi: 10.1002/ijc.20587. [DOI] [PubMed] [Google Scholar]
- Malik A, Afaq F, Sarfaraz S, Adhami VM, Syed DN, Mukhtar H. Pomegranate fruit juice for chemoprevention and chemotherapy of prostate cancer. Proc Natl Acad Sci USA. 2005;102:14813–8. doi: 10.1073/pnas.0505870102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butelli E, Titta L, Giorgio M, Mock HP, Matros A, Peterek S, Schijlen EG, Hall RD, Bovy AG, Luo J, Martin C. Enrichment of tomato fruit with health-promoting anthocyanins by expression of select transcription factors. Nat Biotechnol. 2008;26:1301–1308. doi: 10.1038/nbt.1506. [DOI] [PubMed] [Google Scholar]
- Joseph JA, Shukitt-Hale B, Denisova NA, Bielinski D, Martin A, McEwen JJ, Bickford PC. Reversals of age-related declines in neuronal signal transduction cognitive, and motor behavioral deficits with blueberry spinach, or strawberry dietary supplementation. Journal of Neuroscience. 1999;19:8114–8121. doi: 10.1523/JNEUROSCI.19-18-08114.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney MI, Kalt W, MacKinnon SL, Ashby J, Gottschall-Pass KT. Feeding rats diets enriched in lowbush blueberries for six weeks decreases ischemia-induced brain damage. Nutritional Neuroscience. 2002;5:427–431. doi: 10.1080/1028415021000055970. [DOI] [PubMed] [Google Scholar]
- Joseph JA, Fisher DR, Bielinski D. Blueberry extract alters oxidative stress-mediated signaling in COS-7 cells transfected with selectively vulnerable muscarinic receptor subtypes. J Alzheimers Dis. 2006;9:35–42. doi: 10.3233/jad-2006-9103. [DOI] [PubMed] [Google Scholar]
- Ramassamy C. Emerging role of polyphenolic compounds in the treatment of neurodegenerative diseases: A review of their intracellular targets. Eur J Pharmacol. 2006;545:51–64. doi: 10.1016/j.ejphar.2006.06.025. [DOI] [PubMed] [Google Scholar]
- Kalt W, Joseph JA, Shukitt-Hale B. Blueberries and human health: a review of current reseach. J Amer Pomol Soc. 2007;61:151–160. [Google Scholar]
- Lau FC, Bielinski DF, Joseph JA. Inhibitory effects of blueberry extract on the production of inflammatory mediators in lipopolysaccharide-activated BV2 microglia. J Neurosci Res. 2007;85:1010–7. doi: 10.1002/jnr.21205. [DOI] [PubMed] [Google Scholar]
- Neto CC. Cranberry and blueberry: evidence for protective effects against cancer and vascular diseases. Mol Nutr Food Res. 2007;51:652–64. doi: 10.1002/mnfr.200600279. [DOI] [PubMed] [Google Scholar]
- McAnulty SR, McAnulty LS, Nieman DC, Dumke CL, Morrow JD, Utter AC, Henson DA, Proulx WR, George GL. Consumption of blueberry polyphenols reduces exercise-induced oxidative stress compared to vitamin C. Nutrition Research. 2004;24:209–221. [Google Scholar]
- Longpré F, Garneau P, Christen Y, Ramassamy C. Protection by EGb 761 against beta-amyloid-induced neurotoxicity: Involvement of NF-kappa B, SIRT1, and MAPKs pathways and inhibition of amyloid fibril formation. Free Rad Biol Med. 2006;41:1781–1794. doi: 10.1016/j.freeradbiomed.2006.08.015. [DOI] [PubMed] [Google Scholar]
- Ramassamy C, Longpré F, Christen Y. Ginkgo biloba extract (EGb 761) in Alzheimer's disease: Is there any evidence? Current Alzheimer Research. 2007;4:253–262. doi: 10.2174/156720507781077304. [DOI] [PubMed] [Google Scholar]
- Wang Q, Simonyi A, Li WL, Sisk BA, Miller RL, MacDonald RS, Lubahn DE, Sun GY, Sun AY. Dietary grape supplement ameliorates cerebral ischemia-induced neuronal death in gerbils. Molecular Nutrition & Food Research. 2005;49:443–451. doi: 10.1002/mnfr.200500019. [DOI] [PubMed] [Google Scholar]
- Curin Y, Andriantsitohaina R. Polyphenols as potential therapeutical agents against cardiovascular diseases. Pharmacological Reports. 2005;57:97–107. [PubMed] [Google Scholar]
- Kuriyama S, Shimazu T, Ohmori K, Kikuchi N, Nakaya N, Nishino Y, Tsubono Y, Tsuji I. Green tea consumption and mortality due to cardiovascular disease cancer, and all causes in Japan – The Ohsaki Study. Jama-Journal of the American Medical Association. 2006;296:1255–1265. doi: 10.1001/jama.296.10.1255. [DOI] [PubMed] [Google Scholar]
- Fraser ML, Mok GS, Lee AH. Green tea and stroke prevention: emerging evidence. Complement Ther Med. 2007;15:46–53. doi: 10.1016/j.ctim.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Ikeda M, Suzuki C, Umegaki K, Saito K, Tabuchi M, Tomita T. Preventive effects of green tea catechins on spontaneous stroke in rats. Medical Science Monitor. 2007;13:BR40–BR45. [PubMed] [Google Scholar]
- Nelson SK, Bose SK, Grunwald GK, Myhill P, McCord JM. The induction of human superoxide dismutase and catalase in vivo: A fundamentally new approach to antioxidant therapy. Free Radical Biology and Medicine. 2006;40:341–347. doi: 10.1016/j.freeradbiomed.2005.08.043. [DOI] [PubMed] [Google Scholar]
- Amidon GL, Lennernas H, Shah VP, Crison JR. A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm Res. 1995;12:413–20. doi: 10.1023/a:1016212804288. [DOI] [PubMed] [Google Scholar]
- Lennernäs H, Abrahamsson B. The use of biopharmaceutic classification of drugs in drug discovery and development: current status and future extension. J Pharm Pharmacol. 2005;57:273–85. doi: 10.1211/0022357055263. [DOI] [PubMed] [Google Scholar]
- Wu CY, Benet LZ. Predicting drug disposition via application of BCS: transport/absorption/elimination interplay and development of a biopharmaceutics drug disposition classification system. Pharm Res. 2005;22:11–23. doi: 10.1007/s11095-004-9004-4. [DOI] [PubMed] [Google Scholar]
- Manach C, Williamson G, Morand C, Scalbert A, Remesy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr. 2005;81:230S–242S. doi: 10.1093/ajcn/81.1.230S. [DOI] [PubMed] [Google Scholar]
- Williamson G, Manach C. Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies. Am J Clin Nutr. 2005;81:243S–255S. doi: 10.1093/ajcn/81.1.243S. [DOI] [PubMed] [Google Scholar]
- Rechner AR, Kuhnle G, Bremner P, Hubbard GP, Moore KP, Rice-Evans CA. The metabolic fate of dietary polyphenols in humans. Free Radic Biol Med. 2002;33:220–35. doi: 10.1016/s0891-5849(02)00877-8. [DOI] [PubMed] [Google Scholar]
- Ross JA, Kasum CM. Dietary flavonoids: bioavailability, metabolic effects, and safety. Annu Rev Nutr. 2002;22:19–34. doi: 10.1146/annurev.nutr.22.111401.144957. [DOI] [PubMed] [Google Scholar]
- Scalbert A, Morand C, Manach C, Remesy C. Absorption and metabolism of polyphenols in the gut and impact on health. Biomedicine & Pharmacotherapy. 2002;56:276–282. doi: 10.1016/s0753-3322(02)00205-6. [DOI] [PubMed] [Google Scholar]
- Passamonti S, Vrhovsek U, Vanzo A, Mattivi F. Fast access of some grape pigments to the brain. J Agric Food Chem. 2005;53:7029–34. doi: 10.1021/jf050565k. [DOI] [PubMed] [Google Scholar]
- Serra H, Mendes T, Bronze MR, Simplicio AL. Prediction of intestinal absorption and metabolism of pharmacologically active flavones and flavanones. Bioorg Med Chem. 2008;16:4009–18. doi: 10.1016/j.bmc.2008.01.028. [DOI] [PubMed] [Google Scholar]
- Heiss E, Herhaus C, Klimo K, Bartsch H, Gerhauser C. Nuclear factor kappa B is a molecular target for sulforaphane-mediated anti-inflammatory mechanisms. J Biol Chem. 2001;276:32008–15. doi: 10.1074/jbc.M104794200. [DOI] [PubMed] [Google Scholar]
- Gerhäuser C, Klimo K, Heiss E, Neumann I, Gamal-Eldeen A, Knauft J, Liu GY, Sitthimonchai S, Frank N. Mechanism-based in vitro screening of potential cancer chemopreventive agents. Mutat Res. 2003;523–524:163–72. doi: 10.1016/s0027-5107(02)00332-9. [DOI] [PubMed] [Google Scholar]
- Manson MM. Cancer prevention – the potential for diet to modulate molecular signalling. Trends Mol Med. 2003;9:11–8. doi: 10.1016/s1471-4914(02)00002-3. [DOI] [PubMed] [Google Scholar]
- Murakami A, Takahashi D, Hagihara K, Koshimizu K, Ohigashi H. Combinatorial effects of nonsteroidal anti-inflammatory drugs and food constituents on production of prostaglandin E2 and tumor necrosis factor-alpha in RAW264.7 murine macrophages. Biosci Biotechnol Biochem. 2003;67:1056–62. doi: 10.1271/bbb.67.1056. [DOI] [PubMed] [Google Scholar]
- Murakami A, Takahashi D, Koshimizu K, Ohigashi H. Synergistic suppression of superoxide and nitric oxide generation from inflammatory cells by combined food factors. Mutat Res. 2003;523–524:151–61. doi: 10.1016/s0027-5107(02)00331-7. [DOI] [PubMed] [Google Scholar]
- Murakami A, Matsumoto K, Koshimizu K, Ohigashi H. Effects of selected food factors with chemopreventive properties on combined lipopolysaccharide- and interferon-gamma-induced IkappaB degradation in RAW264.7 macrophages. Cancer Lett. 2003;195:17–25. doi: 10.1016/s0304-3835(03)00058-2. [DOI] [PubMed] [Google Scholar]
- Surh YJ, Ferguson LR. Dietary and medicinal antimutagens and anticarcinogens: molecular mechanisms and chemopreventive potential–highlights of a symposium. Mutat Res. 2003;523–524:1–8. doi: 10.1016/s0027-5107(02)00343-3. [DOI] [PubMed] [Google Scholar]
- Alcaraz MJ, Vicente AM, Araico A, Dominguez JN, Terencio MC, Ferrandiz ML. Role of nuclear factor-kappaB and heme oxygenase-1 in the mechanism of action of an anti-inflammatory chalcone derivative in RAW 264.7 cells. Br J Pharmacol. 2004;142:1191–9. doi: 10.1038/sj.bjp.0705821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorai T, Aggarwal BB. Role of chemopreventive agents in cancer therapy. Cancer Lett. 2004;215:129–40. doi: 10.1016/j.canlet.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Feng R, Bowman LL, Lu Y, Leonard SS, Shi X, Jiang BH, Castranova V, Vallyathan V, Ding M. Blackberry extracts inhibit activating protein 1 activation and cell transformation by perturbing the mitogenic signaling pathway. Nutr Cancer. 2004;50:80–9. doi: 10.1207/s15327914nc5001_11. [DOI] [PubMed] [Google Scholar]
- Goyarzu P, Malin DH, Lau FC, Taglialatela G, Moon WD, Jennings R, Moy E, Moy D, Lippold S, Shukitt-Hale B, Joseph JA. Blueberry supplemented diet: effects on object recognition memory and nuclear factor-kappa B levels in aged rats. Nutr Neurosci. 2004;7:75–83. doi: 10.1080/10284150410001710410. [DOI] [PubMed] [Google Scholar]
- Jeong WS, Kim IW, Hu R, Kong AN. Modulatory properties of various natural chemopreventive agents on the activation of NF-kappaB signaling pathway. Pharm Res. 2004;21:661–70. doi: 10.1023/b:pham.0000022413.43212.cf. [DOI] [PubMed] [Google Scholar]
- Park OJ, Surh YJ. Chemopreventive potential of epigallocatechin gallate and genistein: evidence from epidemiological and laboratory studies. Toxicol Lett. 2004;150:43–56. doi: 10.1016/j.toxlet.2003.06.001. [DOI] [PubMed] [Google Scholar]
- Pham NA, Jacobberger JW, Schimmer AD, Cao P, Gronda M, Hedley DW. The dietary isothiocyanate sulforaphane targets pathways of apoptosis, cell cycle arrest, and oxidative stress in human pancreatic cancer cells and inhibits tumor growth in severe combined immunodeficient mice. Mol Cancer Ther. 2004;3:1239–48. [PubMed] [Google Scholar]
- Zhang Y. Cancer-preventive isothiocyanates: measurement of human exposure and mechanism of action. Mutat Res. 2004;555:173–90. doi: 10.1016/j.mrfmmm.2004.04.017. [DOI] [PubMed] [Google Scholar]
- Bai SK, Lee SJ, Na HJ, Ha KS, Han JA, Lee H, Kwon YG, Chung CK, Kim YM. Beta-carotene inhibits inflammatory gene expression in lipopolysaccharide-stimulated macrophages by suppressing redox-based NF-kappaB activation. Exp Mol Med. 2005;37:323–34. doi: 10.1038/emm.2005.42. [DOI] [PubMed] [Google Scholar]
- Chen C, Kong AN. Dietary cancer-chemopreventive compounds: from signaling and gene expression to pharmacological effects. Trends Pharmacol Sci. 2005;26:318–26. doi: 10.1016/j.tips.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Kundu JK, Surh YJ. Breaking the relay in deregulated cellular signal transduction as a rationale for chemoprevention with anti-inflammatory phytochemicals. Mutat Res. 2005;591:123–46. doi: 10.1016/j.mrfmmm.2005.04.019. [DOI] [PubMed] [Google Scholar]
- Losso JN, Bawadi HA. Hypoxia inducible factor pathways as targets for functional foods. J Agric Food Chem. 2005;53:3751–68. doi: 10.1021/jf0479719. [DOI] [PubMed] [Google Scholar]
- Ramos S, Alia M, Bravo L, Goya L. Comparative effects of food-derived polyphenols on the viability and apoptosis of a human hepatoma cell line (HepG2) J Agric Food Chem. 2005;53:1271–80. doi: 10.1021/jf0490798. [DOI] [PubMed] [Google Scholar]
- Surh YJ, Kundu JK, Na HK, Lee JS. Redox-sensitive transcription factors as prime targets for chemoprevention with anti-inflammatory and antioxidative phytochemicals. J Nutr. 2005;135:2993S–3001S. doi: 10.1093/jn/135.12.2993S. [DOI] [PubMed] [Google Scholar]
- Xu C, Shen G, Chen C, Gelinas C, Kong AN. Suppression of NF-kappaB and NF-kappaB-regulated gene expression by sulforaphane and PEITC through IkappaBalpha, IKK pathway in human prostate cancer PC-3 cells. Oncogene. 2005;24:4486–95. doi: 10.1038/sj.onc.1208656. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Li J, Tang L. Cancer-preventive isothiocyanates: dichotomous modulators of oxidative stress. Free Radic Biol Med. 2005;38:70–7. doi: 10.1016/j.freeradbiomed.2004.09.033. [DOI] [PubMed] [Google Scholar]
- Wang SY, Feng R, Lu Y, Bowman L, Ding M. Inhibitory effect on activator protein-1, nuclear factor-kappaB, and cell transformation by extracts of strawberries (Fragaria × ananassa Duch.) J Agric Food Chem. 2005;53:4187–93. doi: 10.1021/jf0478049. [DOI] [PubMed] [Google Scholar]
- Aggarwal BB, Shishodia S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem Pharmacol. 2006;71:1397–421. doi: 10.1016/j.bcp.2006.02.009. [DOI] [PubMed] [Google Scholar]
- Brigelius-Flohé R, Banning A. Sulforaphane and selenium, partners in adaptive response and prevention of cancer. Free Radic Res. 2006;40:775–87. doi: 10.1080/10715760600722643. [DOI] [PubMed] [Google Scholar]
- Jakubíkova J, Sedlák J, Bod'o J, Bao Y. Effect of isothiocyanates on nuclear accumulation of NF-kappaB, Nrf2, and thioredoxin in caco-2 cells. J Agric Food Chem. 2006;54:1656–62. doi: 10.1021/jf052717h. [DOI] [PubMed] [Google Scholar]
- Jacob C. A scent of therapy: pharmacological implications of natural products containing redox-active sulfur atoms. Nat Prod Rep. 2006;23:851–63. doi: 10.1039/b609523m. [DOI] [PubMed] [Google Scholar]
- Keum YS, Yu S, Chang PP, Yuan X, Kim JH, Xu C, Han J, Agarwal A, Kong AN. Mechanism of action of sulforaphane: inhibition of p38 mitogen-activated protein kinase isoforms contributing to the induction of antioxidant response element-mediated heme oxygenase-1 in human hepatoma HepG2 cells. Cancer Res. 2006;66:8804–13. doi: 10.1158/0008-5472.CAN-05-3513. [DOI] [PubMed] [Google Scholar]
- Köhle C, Bock KW. Activation of coupled Ah receptor and Nrf2 gene batteries by dietary phytochemicals in relation to chemoprevention. Biochem Pharmacol. 2006;72:795–805. doi: 10.1016/j.bcp.2006.04.017. [DOI] [PubMed] [Google Scholar]
- Kresty LA, Frankel WL, Hammond CD, Baird ME, Mele JM, Stoner GD, Fromkes JJ. Transitioning from preclinical to clinical chemopreventive assessments of lyophilized black raspberries: interim results show berries modulate markers of oxidative stress in Barrett's esophagus patients. Nutr Cancer. 2006;54:148–56. doi: 10.1207/s15327914nc5401_15. [DOI] [PubMed] [Google Scholar]
- Lee KW, Kundu JK, Kim SO, Chun KS, Lee HJ, Surh YJ. Cocoa polyphenols inhibit phorbol ester-induced superoxide anion formation in cultured HL-60 cells and expression of cyclooxygenase-2 and activation of NF-kappaB and MAPKs in mouse skin in vivo. J Nutr. 2006;136:1150–5. doi: 10.1093/jn/136.5.1150. [DOI] [PubMed] [Google Scholar]
- Lu H, Li J, Zhang D, Stoner GD, Huang C. Molecular mechanisms involved in chemoprevention of black raspberry extracts: from transcription factors to their target genes. Nutr Cancer. 2006;54:69–78. doi: 10.1207/s15327914nc5401_8. [DOI] [PubMed] [Google Scholar]
- Martin KR. Targeting apoptosis with dietary bioactive agents. Exp Biol Med. 2006;231:117–29. doi: 10.1177/153537020623100201. [DOI] [PubMed] [Google Scholar]
- Na HK, Surh YJ. Transcriptional regulation via cysteine thiol modification: a novel molecular strategy for chemoprevention and cytoprotection. Mol Carcinog. 2006;45:368–80. doi: 10.1002/mc.20225. [DOI] [PubMed] [Google Scholar]
- Pappa G, Lichtenberg M, Iori R, Barillari J, Bartsch H, Gerhauser C. Comparison of growth inhibition profiles and mechanisms of apoptosis induction in human colon cancer cell lines by isothiocyanates and indoles from Brassicaceae. Mutat Res. 2006;599:76–87. doi: 10.1016/j.mrfmmm.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Sandur SK, Ichikawa H, Sethi G, Ahn KS, Aggarwal BB. Plumbagin (5-hydroxy-2-methyl-1,4-naphthoquinone) suppresses NF-kappaB activation and NF-kappaB-regulated gene products through modulation of p65 and IkappaBalpha kinase activation, leading to potentiation of apoptosis induced by cytokine and chemotherapeutic agents. J Biol Chem. 2006;281:17023–33. doi: 10.1074/jbc.M601595200. [DOI] [PubMed] [Google Scholar]
- Shen G, Xu C, Hu R, Jain MR, Gopalkrishnan A, Nair S, Huang MT, Chan JY, Kong AN. Modulation of nuclear factor E2-related factor 2-mediated gene expression in mice liver and small intestine by cancer chemopreventive agent curcumin. Mol Cancer Ther. 2006;5:39–51. doi: 10.1158/1535-7163.MCT-05-0293. [DOI] [PubMed] [Google Scholar]
- Thangapazham RL, Sharma A, Maheshwari RK. Multiple molecular targets in cancer chemoprevention by curcumin. Aaps J. 2006;8:E443–9. doi: 10.1208/aapsj080352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vina J, Borras C, Gomez-Cabrera MC, Orr WC. Role of reactive oxygen species and (phyto)oestrogens in the modulation of adaptive response to stress. Free Radic Res. 2006;40:111–9. doi: 10.1080/10715760500405778. [DOI] [PubMed] [Google Scholar]
- Davis CD, Milner JA. Biomarkers for diet and cancer prevention research: potentials and challenges. Acta Pharmacol Sin. 2007;28:1262–73. doi: 10.1111/j.1745-7254.2007.00678.x. [DOI] [PubMed] [Google Scholar]
- Higdon JV, Delage B, Williams DE, Dashwood RH. Cruciferous vegetables and human cancer risk: epidemiologic evidence and mechanistic basis. Pharmacol Res. 2007;55:224–36. doi: 10.1016/j.phrs.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber WW, Parzefall W. Thiols and the chemoprevention of cancer. Curr Opin Pharmacol. 2007;7:404–9. doi: 10.1016/j.coph.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Juge N, Mithen RF, Traka M. Molecular basis for chemoprevention by sulforaphane: a comprehensive review. Cell Mol Life Sci. 2007;64:1105–27. doi: 10.1007/s00018-007-6484-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampa M, Nifli AP, Notas G, Castanas E. Polyphenols and cancer cell growth. Rev Physiol Biochem Pharmacol. 2007;159:79–113. doi: 10.1007/112_2006_0702. [DOI] [PubMed] [Google Scholar]
- Kwon KH, Barve A, Yu S, Huang MT, Kong AN. Cancer chemoprevention by phytochemicals: potential molecular targets, biomarkers and animal models. Acta Pharmacol Sin. 2007;28:1409–21. doi: 10.1111/j.1745-7254.2007.00694.x. [DOI] [PubMed] [Google Scholar]
- Liby KT, Yore MM, Sporn MB. Triterpenoids and rexinoids as multifunctional agents for the prevention and treatment of cancer. Nat Rev Cancer. 2007;7:357–69. doi: 10.1038/nrc2129. [DOI] [PubMed] [Google Scholar]
- Murakami A, Ohigashi H. Targeting NOX, INOS and COX-2 in inflammatory cells: chemoprevention using food phytochemicals. Int J Cancer. 2007;121:2357–63. doi: 10.1002/ijc.23161. [DOI] [PubMed] [Google Scholar]
- Nair S, Li W, Kong AN. Natural dietary anti-cancer chemopreventive compounds: redox-mediated differential signaling mechanisms in cytoprotection of normal cells versus cytotoxicity in tumor cells. Acta Pharmacol Sin. 2007;28:459–72. doi: 10.1111/j.1745-7254.2007.00549.x. [DOI] [PubMed] [Google Scholar]
- Neto CC. Cranberry and its phytochemicals: a review of in vitro anticancer studies. J Nutr. 2007;137:186S–193S. doi: 10.1093/jn/137.1.186S. [DOI] [PubMed] [Google Scholar]
- Syed DN, Afaq F, Mukhtar H. Pomegranate derived products for cancer chemoprevention. Semin Cancer Biol. 2007;17:377–85. doi: 10.1016/j.semcancer.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Zafra-Stone S, Yasmin T, Bagchi M, Chatterjee A, Vinson JA, Bagchi D. Berry anthocyanins as novel antioxidants in human health and disease prevention. Mol Nutr Food Res. 2007;51:675–83. doi: 10.1002/mnfr.200700002. [DOI] [PubMed] [Google Scholar]
- Gopalakrishnan A, Tony Kong AN. Anticarcinogenesis by dietary phytochemicals: cytoprotection by Nrf2 in normal cells and cytotoxicity by modulation of transcription factors NF-kappa B and AP-1 in abnormal cancer cells. Food Chem Toxicol. 2008;46:1257–70. doi: 10.1016/j.fct.2007.09.082. [DOI] [PubMed] [Google Scholar]
- Nair S, Hebbar V, Shen G, Gopalakrishnan A, Khor TO, Yu S, Xu C, Kong AN. Synergistic effects of a combination of dietary factors sulforaphane and (-) epigallocatechin-3-gallate in HT-29 AP-1 human colon carcinoma cells. Pharm Res. 2008;25:387–99. doi: 10.1007/s11095-007-9364-7. [DOI] [PubMed] [Google Scholar]
- Neto CC, Amoroso JW, Liberty AM. Anticancer activities of cranberry phytochemicals: An update. Mol Nutr Food Res. 2008 doi: 10.1002/mnfr.200700433. [DOI] [PubMed] [Google Scholar]
- Pan MH, Ghai G, Ho CT. Food bioactives apoptosis, and cancer. Mol Nutr Food Res. 2008;52:43–52. doi: 10.1002/mnfr.200700380. [DOI] [PubMed] [Google Scholar]
- Ramos S. Cancer chemoprevention and chemotherapy: dietary polyphenols and signalling pathways. Mol Nutr Food Res. 2008;52:507–26. doi: 10.1002/mnfr.200700326. [DOI] [PubMed] [Google Scholar]
- Youn HS, Lim HJ, Lee HJ, Hwang D, Yang M, Jeon R, Ryu JH. Garlic (Allium sativum) extract inhibits lipopolysaccharide-induced Toll-like receptor 4 dimerization. Biosci Biotechnol Biochem. 2008;72:368–75. doi: 10.1271/bbb.70434. [DOI] [PubMed] [Google Scholar]
- Dower SK, Qwarnstrom EE. Signalling networks, inflammation and innate immunity. Biochem Soc Trans. 2003;31:1462–71. doi: 10.1042/bst0311462. [DOI] [PubMed] [Google Scholar]
- Leonard SS, Harris GK, Shi XL. Metal-induced oxidative stress and signal transduction. Free Rad Biol Med. 2004;37:1921–1942. doi: 10.1016/j.freeradbiomed.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Matsuzawa A, Saegusa K, Noguchi T, Sadamitsu C, Nishitoh H, Nagai S, Koyasu S, Matsumoto K, Takeda K, Ichijo H. ROS-dependent activation of the TRAF6-ASK1-p38 pathway is selectively required for TLR4-mediated innate immunity. Nat Immunol. 2005;6:587–92. doi: 10.1038/ni1200. [DOI] [PubMed] [Google Scholar]
- Schreck R, Rieber P, Baeuerle PA. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-Kappa-B transcription Factor and HIV-1. EMBO J. 1991;10:2247–2258. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreck R, Albermann K, Baeuerle PA. Nuclear factor kappa-B – an oxidative stress-responsive transcription factor of eukaryotic cells (a Review) Free Rad Res Comm. 1992;17:221–237. doi: 10.3109/10715769209079515. [DOI] [PubMed] [Google Scholar]
- Schreck R, Meier B, Mannel DN, Droge W, Baeuerle PA. Dithiocarbamates as potent inhibitors of nuclear factor kappa B activation in intact cells. J Exp Med. 1992;175:1181–94. doi: 10.1084/jem.175.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan N, Sadeghi K, Reiter RJ, Meltz ML. The neurohormone melatonin inhibits cytokine, mitogen and ionizing radiation induced NF-kappa B. Biochemistry and Molecular Biology International. 1995;37:1063–1070. [PubMed] [Google Scholar]
- Baeuerle PA, Baltimore D. NF-kappa B: ten years after. Cell. 1996;87:13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- Baldwin AS., Jr The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–83. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- Dalton TP, Shertzer HG, Puga A. Regulation of gene expression by reactive oxygen. Annu Rev Pharmacol Toxicol. 1999;39:67–101. doi: 10.1146/annurev.pharmtox.39.1.67. [DOI] [PubMed] [Google Scholar]
- Li C, Browder W, Kao RL. Early activation of transcription factor NF-kappaB during ischemia in perfused rat heart. Am J Physiol. 1999;276:H543–52. doi: 10.1152/ajpheart.1999.276.2.H543. [DOI] [PubMed] [Google Scholar]
- Wang S, Leonard SS, Castranova V, Vallyathan V, Shi X. The role of superoxide radical in TNF-alpha induced NF-kappaB activation. Ann Clin Lab Sci. 1999;29:192–9. [PubMed] [Google Scholar]
- Knight JA. Review: Free radicals, antioxidants, and the immune system. Ann Clin Lab Sci. 2000;30:145–158. [PubMed] [Google Scholar]
- Schoonbroodt S, Piette J. Oxidative stress interference with the nuclear factor-kappa B activation pathways. Biochem Pharmacol. 2000;60:1075–83. doi: 10.1016/s0006-2952(00)00371-3. [DOI] [PubMed] [Google Scholar]
- Forman HJ, Torres M. Reactive oxygen species and cell signaling: respiratory burst in macrophage signaling. Am J Respir Crit Care Med. 2002;166:S4–8. doi: 10.1164/rccm.2206007. [DOI] [PubMed] [Google Scholar]
- Wang SW, Kotamraju S, Konorev E, Kalivendi S, Joseph J, Kalyanaraman B. Activation of nuclear factor-kappa B during doxorubicin-induced apoptosis in endothelial cells and myocytes is pro-apoptotic: the role of hydrogen peroxide. Biochemical Journal. 2002;367:729–740. doi: 10.1042/BJ20020752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad JJ. Science review: Redox and oxygen-sensitive transcription factors in the regulation of oxidant-mediated lung injury: role for nuclear factor-kappaB. Crit Care. 2002;6:481–90. doi: 10.1186/cc1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asehnoune K, Strassheim D, Mitra S, Kim JY, Abraham E. Involvement of reactive oxygen species in Toll-like receptor 4-dependent activation of NF-kappa B. J Immunol. 2004;172:2522–9. doi: 10.4049/jimmunol.172.4.2522. [DOI] [PubMed] [Google Scholar]
- Poli G, Leonarduzzi G, Biasi F, Chiarpotto E. Oxidative stress and cell signalling. Curr Med Chem. 2004;11:1163–1182. doi: 10.2174/0929867043365323. [DOI] [PubMed] [Google Scholar]
- Manna SK, Sarkar S, Barr J, Wise K, Barrera EV, Jejelowo O, Rice-Ficht AC, Ramesh GT. Single-walled carbon nanotube induces oxidative stress and activates nuclear transcription factor-kappaB in human keratinocytes. Nano Lett. 2005;5:1676–84. doi: 10.1021/nl0507966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubici C, Papa S, Dean K, Franzoso G. Mutual cross-talk between reactive oxygen species and nuclear factor-kappa B: molecular basis and biological significance. Oncogene. 2006;25:6731–48. doi: 10.1038/sj.onc.1209936. [DOI] [PubMed] [Google Scholar]
- Bubici C, Papa S, Pham CG, Zazzeroni F, Franzoso G. The NF-kappaB-mediated control of ROS and JNK signaling. Histol Histopathol. 2006;21:69–80. doi: 10.14670/HH-21.69. [DOI] [PubMed] [Google Scholar]
- Gloire G, Legrand-Poels S, Piette J. NF-kappaB activation by reactive oxygen species: fifteen years later. Biochem Pharmacol. 2006;72:1493–505. doi: 10.1016/j.bcp.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Kim JY, Jung KJ, Choi JS, Chung HY. Modulation of the age-related nuclear factor-kappaB (NF-kappaB) pathway by hesperetin. Aging Cell. 2006;5:401–11. doi: 10.1111/j.1474-9726.2006.00233.x. [DOI] [PubMed] [Google Scholar]
- Li LX, Frei B. Iron chelation inhibits NF-kappa B-mediated adhesion molecule expression by inhibiting p22(phox) protein expression and NADPH oxidase activity. Arteriosclerosis Thromb Vasc Biol. 2006;26:2638–2643. doi: 10.1161/01.ATV.0000245820.34238.da. [DOI] [PubMed] [Google Scholar]
- Kramer HF, Goodyear LJ. Exercise MAPK, and NF-kappaB signaling in skeletal muscle. J Appl Physiol. 2007;103:388–95. doi: 10.1152/japplphysiol.00085.2007. [DOI] [PubMed] [Google Scholar]
- Glauert HP. Vitamin E and NF-kappaB activation: a review. Vitam Horm. 2007;76:135–53. doi: 10.1016/S0083-6729(07)76006-5. [DOI] [PubMed] [Google Scholar]
- Perkins ND. Integrating cell-signalling pathways with NF-kappaB and IKK function. Nat Rev Mol Cell Biol. 2007;8:49–62. doi: 10.1038/nrm2083. [DOI] [PubMed] [Google Scholar]
- Uwe S. Anti-inflammatory interventions of NF-kappaB signaling: potential applications and risks. Biochem Pharmacol. 2008;75:1567–79. doi: 10.1016/j.bcp.2007.10.027. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell. 2002;109:S81–96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- Hayden MS, Ghosh S. Signaling to NF-kappaB. Genes Dev. 2004;18:2195–224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- Nelson DE, Ihekwaba AEC, Elliott M, Gibney CA, Foreman BE, Nelson G, See V, Horton CA, Spiller DG, Edwards SW, McDowell HP, Unitt JF, Sullivan E, Grimley R, Benson N, Broomhead DS, Kell DB, White MRH. Oscillations in NF-κB signalling control the dynamics of target gene expression. Science. 2004;306:704–708. doi: 10.1126/science.1099962. [DOI] [PubMed] [Google Scholar]
- Hoffmann A, Baltimore D. Circuitry of nuclear factor kappaB signaling. Immunol Rev. 2006;210:171–86. doi: 10.1111/j.0105-2896.2006.00375.x. [DOI] [PubMed] [Google Scholar]
- Perkins ND, Gilmore TD. Good cop, bad cop: the different faces of NF-kappaB. Cell Death Differ. 2006;13:759–72. doi: 10.1038/sj.cdd.4401838. [DOI] [PubMed] [Google Scholar]
- Chen CC, Wang JK, Lin SB. Antisense oligonucleotides targeting protein kinase C-alpha, -beta I, or -delta but not -eta inhibit lipopolysaccharide-induced nitric oxide synthase expression in RAW 264.7 macrophages: involvement of a nuclear factor kappa B-dependent mechanism. J Immunol. 1998;161:6206–14. [PubMed] [Google Scholar]
- Sanlioglu S, Williams CM, Samavati L, Butler NS, Wang G, McCray PB, Ritchie TC, Jr, Hunninghake GW, Zandi E, Engelhardt JF. Lipopolysaccharide induces Rac1-dependent reactive oxygen species formation and coordinates tumor necrosis factor-alpha secretion through IKK regulation of NF-kappa B. J Biol Chem. 2001;276:30188–98. doi: 10.1074/jbc.M102061200. [DOI] [PubMed] [Google Scholar]
- Hsu HY, Wen MH. Lipopolysaccharide-mediated reactive oxygen species and signal transduction in the regulation of interleukin-1 gene expression. J Biol Chem. 2002;277:22131–9. doi: 10.1074/jbc.M111883200. [DOI] [PubMed] [Google Scholar]
- Macdonald J, Galley HF, Webster NR. Oxidative stress and gene expression in sepsis. Br J Anaesth. 2003;90:221–32. doi: 10.1093/bja/aeg034. [DOI] [PubMed] [Google Scholar]
- Park HS, Jung HY, Park EY, Kim J, Lee WJ, Bae YS. Cutting edge: direct interaction of TLR4 with NAD(P)H oxidase 4 isozyme is essential for lipopolysaccharide-induced production of reactive oxygen species and activation of NF-kappa B. J Immunol. 2004;173:3589–93. doi: 10.4049/jimmunol.173.6.3589. [DOI] [PubMed] [Google Scholar]
- Pham CG, Bubici C, Zazzeroni F, Papa S, Jones J, Alvarez K, Jayawardena S, De Smaele E, Cong R, Beaumont C, Torti FM, Torti SV, Franzoso G. Ferritin heavy chain upregulation by NF-kappaB inhibits TNFalpha-induced apoptosis by suppressing reactive oxygen species. Cell. 2004;119:529–42. doi: 10.1016/j.cell.2004.10.017. [DOI] [PubMed] [Google Scholar]
- Allan SM, Tyrrell PJ, Rothwell NJ. Interleukin-1 and neuronal injury. Nat Rev Immunol. 2005;5:629–40. doi: 10.1038/nri1664. [DOI] [PubMed] [Google Scholar]
- Ihekwaba AEC, Broomhead DS, Grimley R, Benson N, Kell DB. Sensitivity analysis of parameters controlling oscillatory signalling in the NF-κB pathway: the roles of IKK and IκBα. Systems Biology. 2004;1:93–103. doi: 10.1049/sb:20045009. [DOI] [PubMed] [Google Scholar]
- Yue H, Brown M, Knowles J, Wang H, Broomhead DS, Kell DB. Insights into the behaviour of systems biology models from dynamic sensitivity and identifiability analysis: a case study of an NF-kappaB signalling pathway. Mol Biosyst. 2006;2:640–649. doi: 10.1039/b609442b. [DOI] [PubMed] [Google Scholar]
- Ihekwaba AEC, Broomhead DS, Grimley R, Benson N, White MRH, Kell DB. Synergistic control of oscillations in the NF-κB signalling pathway. IEE Systems Biology. 2005;152:153–160. doi: 10.1049/ip-syb:20050050. [DOI] [PubMed] [Google Scholar]
- Mignotte B, Vayssiere JL. Mitochondria and apoptosis. Eur J Biochem. 1998;252:1–15. doi: 10.1046/j.1432-1327.1998.2520001.x. [DOI] [PubMed] [Google Scholar]
- Fleury C, Mignotte B, Vayssiere JL. Mitochondrial reactive oxygen species in cell death signaling. Biochimie. 2002;84:131–41. doi: 10.1016/s0300-9084(02)01369-x. [DOI] [PubMed] [Google Scholar]
- Bharti AC, Aggarwal BB. Nuclear factor-kappa B and cancer: its role in prevention and therapy. Biochem Pharmacol. 2002;64:883–8. doi: 10.1016/s0006-2952(02)01154-1. [DOI] [PubMed] [Google Scholar]
- Richmond A. NF-κB, chemokine gene transcription and tumour growth. Nat Rev Immunol. 2002;2:664–74. doi: 10.1038/nri887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Rigas B. NF-kappaB, inflammation and pancreatic carcinogenesis: NF-kappaB as a chemoprevention target (review) Int J Oncol. 2006;29:185–92. [PubMed] [Google Scholar]
- Calzado MA, Bacher S, Schmitz ML. NF-kappaB inhibitors for the treatment of inflammatory diseases and cancer. Curr Med Chem. 2007;14:367–76. doi: 10.2174/092986707779941113. [DOI] [PubMed] [Google Scholar]
- Roman-Blas JA, Jimenez SA. NF-kappaB as a potential therapeutic target in osteoarthritis and rheumatoid arthritis. Osteoarthritis Cartilage. 2006;14:839–48. doi: 10.1016/j.joca.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Saklatvala J. Inflammatory signaling in cartilage: MAPK and NF-kappaB pathways in chondrocytes and the use of inhibitors for research into pathogenesis and therapy of osteoarthritis. Curr Drug Targets. 2007;8:305–13. doi: 10.2174/138945007779940115. [DOI] [PubMed] [Google Scholar]
- Simmonds RE, Foxwell BM. NF-{kappa}B and its relevance to arthritis and inflammation. Rheumatology (Oxford) 2008 doi: 10.1093/rheumatology/kem298. [DOI] [PubMed] [Google Scholar]
- Smahi A, Courtois G, Rabia SH, Doffinger R, Bodemer C, Munnich A, Casanova JL, Israel A. The NF-kappaB signalling pathway in human diseases: from incontinentia pigmenti to ectodermal dysplasias and immune-deficiency syndromes. Hum Mol Genet. 2002;11:2371–5. doi: 10.1093/hmg/11.20.2371. [DOI] [PubMed] [Google Scholar]
- Lezoualc'h F, Sparapani M, Behl C. N-acetyl-serotonin (normelatonin) and melatonin protect neurons against oxidative challenges and suppress the activity of the transcription factor NF-kappaB. J Pineal Res. 1998;24:168–78. doi: 10.1111/j.1600-079x.1998.tb00530.x. [DOI] [PubMed] [Google Scholar]
- Post A, Holsboer F, Behl C. Induction of NF-kappaB activity during haloperidol-induced oxidative toxicity in clonal hippocampal cells: suppression of NF-kappaB and neuroprotection by antioxidants. J Neurosci. 1998;18:8236–46. doi: 10.1523/JNEUROSCI.18-20-08236.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera J, Nava M, Romero F, Rodriguez-Iturbe B. Melatonin prevents oxidative stress resulting from iron and erythropoietin administration. Am J Kidney Dis. 2001;37:750–7. doi: 10.1016/s0272-6386(01)80124-4. [DOI] [PubMed] [Google Scholar]
- Beni SM, Kohen R, Reiter RJ, Tan DX, Shohami E. Melatonin-induced neuroprotection after closed head injury is associated with increased brain antioxidants and attenuated late-phase activation of NF-kappaB and AP-1. FASEB J. 2004;18:149–51. doi: 10.1096/fj.03-0323fje. [DOI] [PubMed] [Google Scholar]
- Rodriguez MI, Escames G, Lopez LC, Lopez A, Garcia JA, Ortiz F, Acuna-Castroviejo D. Chronic melatonin treatment reduces the age-dependent inflammatory process in senescence-accelerated mice. J Pineal Res. 2007;42:272–9. doi: 10.1111/j.1600-079X.2006.00416.x. [DOI] [PubMed] [Google Scholar]
- Takada Y, Mukhopadhyay A, Kundu GC, Mahabeleshwar GH, Singh S, Aggarwal BB. Hydrogen peroxide activates NF-kappa B through tyrosine phosphorylation of I kappa B alpha and serine phosphorylation of p65: evidence for the involvement of I kappa B alpha kinase and Syk protein-tyrosine kinase. J Biol Chem. 2003;278:24233–41. doi: 10.1074/jbc.M212389200. [DOI] [PubMed] [Google Scholar]
- Hsieh CC, Papaconstantinou J. Thioredoxin-ASK1 complex levels regulate ROS-mediated p38 MAPK pathway activity in livers of aged and long-lived Snell dwarf mice. FASEB J. 2006;20:259–68. doi: 10.1096/fj.05-4376com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter DH, Dimmeler S, Zeiher AM. Effects of statins on endothelium and endothelial progenitor cell recruitment. Semin Vasc Med. 2004;4:385–93. doi: 10.1055/s-2004-869595. [DOI] [PubMed] [Google Scholar]
- Madonna R, Di Napoli P, Massaro M, Grilli A, Felaco M, De Caterina A, Tang D, De Caterina R, Geng YJ. Simvastatin attenuates expression of cytokine-inducible nitric-oxide synthase in embryonic cardiac myoblasts. J Biol Chem. 2005;280:13503–11. doi: 10.1074/jbc.M411859200. [DOI] [PubMed] [Google Scholar]
- Urbich C, Dimmeler S. Risk factors for coronary artery disease, circulating endothelial progenitor cells, and the role of HMG-CoA reductase inhibitors. Kidney Int. 2005;67:1672–6. doi: 10.1111/j.1523-1755.2005.00261.x. [DOI] [PubMed] [Google Scholar]
- Gilmore TD, Herscovitch M. Inhibitors of NF-kappaB signaling: 785 and counting. Oncogene. 2006;25:6887–99. doi: 10.1038/sj.onc.1209982. [DOI] [PubMed] [Google Scholar]
- Lazzerini PE, Lorenzini S, Selvi E, Capecchi PL, Chindamo D, Bisogno S, Ghittoni R, Natale MR, Caporali F, Giuntini S, Marcolongo R, Galeazzi M, Laghi-Pasini F. Simvastatin inhibits cytokine production and nuclear factor-kB activation in interleukin 1beta-stimulated synoviocytes from rheumatoid arthritis patients. Clin Exp Rheumatol. 2007;25:696–700. [PubMed] [Google Scholar]
- Lee JY, Kim JS, Kim JM, Kim N, Jung HC, Song IS. Simvastatin inhibits NF-kappaB signaling in intestinal epithelial cells and ameliorates acute murine colitis. Int Immunopharmacol. 2007;7:241–8. doi: 10.1016/j.intimp.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Lin SJ, Chen YH, Lin FY, Hsieh LY, Wang SH, Lin CY, Wang YC, Ku HH, Chen JW, Chen YL. Pravastatin induces thrombomodulin expression in TNFalpha-treated human aortic endothelial cells by inhibiting Rac1 and Cdc42 translocation and activity. J Cell Biochem. 2007;101:642–53. doi: 10.1002/jcb.21206. [DOI] [PubMed] [Google Scholar]
- Dolga AM, Nijholt IM, Ostroveanu A, Ten Bosch Q, Luiten PG, Eisel UL. Lovastatin induces neuroprotection through tumor necrosis factor receptor 2 signaling pathways. J Alzheimers Dis. 2008;13:111–22. doi: 10.3233/jad-2008-13201. [DOI] [PubMed] [Google Scholar]
- Gao F, Linhartova L, Johnston AM, Thickett DR. Statins and sepsis. British Journal of Anaesthesia. 2008;100:288–298. doi: 10.1093/bja/aem406. [DOI] [PubMed] [Google Scholar]
- Galter D, Mihm S, Dröge W. Distinct effects of glutathione disulphide on the nuclear transcription factor kappa B and the activator protein-1. Eur J Biochem. 1994;221:639–48. doi: 10.1111/j.1432-1033.1994.tb18776.x. [DOI] [PubMed] [Google Scholar]
- Kretz-Remy C, Mehlen P, Mirault ME, Arrigo AP. Inhibition of I kappa B-alpha phosphorylation and degradation and subsequent NF-kappa B activation by glutathione peroxidase overexpression. J Cell Biol. 1996;133:1083–93. doi: 10.1083/jcb.133.5.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Ruiz C, Colell A, Morales A, Kaplowitz N, Fernández-Checa JC. Role of oxidative stress generated from the mitochondrial electron transport chain and mitochondrial glutathione status in loss of mitochondrial function and activation of transcription factor nuclear factor-kappa B: studies with isolated mitochondria and rat hepatocytes. Mol Pharmacol. 1995;48:825–34. [PubMed] [Google Scholar]
- Mihm S, Galter D, Dröge W. Modulation of transcription factor NF kappa B activity by intracellular glutathione levels and by variations of the extracellular cysteine supply. Faseb J. 1995;9:246–52. doi: 10.1096/fasebj.9.2.7781927. [DOI] [PubMed] [Google Scholar]
- Ginn-Pease ME, Whisler RL. Optimal NF kappa B mediated transcriptional responses in Jurkat T cells exposed to oxidative stress are dependent on intracellular glutathione and costimulatory signals. Biochem Biophys Res Commun. 1996;226:695–702. doi: 10.1006/bbrc.1996.1416. [DOI] [PubMed] [Google Scholar]
- Pinkus R, Weiner LM, Daniel V. Role of oxidants and antioxidants in the induction of AP-1, NF-kappaB, and glutathione S-transferase gene expression. J Biol Chem. 1996;271:13422–9. doi: 10.1074/jbc.271.23.13422. [DOI] [PubMed] [Google Scholar]
- Brigelius-Flohé R, Friedrichs B, Maurer S, Schultz M, Streicher R. Interleukin-1-induced nuclear factor kappa B activation is inhibited by overexpression of phospholipid hydroperoxide glutathione peroxidase in a human endothelial cell line. Biochem J. 1997;328:199–203. doi: 10.1042/bj3280199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen CK, Khanna S, Reznick AZ, Roy S, Packer L. Glutathione regulation of tumor necrosis factor-alpha-induced NF-kappa B activation in skeletal muscle-derived L6 cells. Biochem Biophys Res Commun. 1997;237:645–9. doi: 10.1006/bbrc.1997.7206. [DOI] [PubMed] [Google Scholar]
- Cho S, Urata Y, Iida T, Goto S, Yamaguchi M, Sumikawa K, Kondo T. Glutathione downregulates the phosphorylation of I kappa B: autoloop regulation of the NF-kappa B-mediated expression of NF-kappa B subunits by TNF-alpha in mouse vascular endothelial cells. Biochem Biophys Res Commun. 1998;253:104–8. doi: 10.1006/bbrc.1998.9697. [DOI] [PubMed] [Google Scholar]
- Rokutan K, Teshima S, Miyoshi M, Kawai T, Nikawa T, Kishi K. Glutathione depletion inhibits oxidant-induced activation of nuclear factor-kappa B, AP-1, and c-Jun/ATF-2 in cultured guinea-pig gastric epithelial cells. J Gastroenterol. 1998;33:646–55. doi: 10.1007/s005350050151. [DOI] [PubMed] [Google Scholar]
- Fernandez PC, Machado J, Jr, Heussler VT, Botteron C, Palmer GH, Dobbelaere DA. The inhibition of NF-kappaB activation pathways and the induction of apoptosis by dithiocarbamates in T cells are blocked by the glutathione precursor N-acetyl-L-cysteine. Biol Chem. 1999;380:1383–94. doi: 10.1515/BC.1999.178. [DOI] [PubMed] [Google Scholar]
- Haddad JJ, Olver RE, Land SC. Antioxidant/pro-oxidant equilibrium regulates HIF-1alpha and NF-kappa B redox sensitivity. Evidence for inhibition by glutathione oxidation in alveolar epithelial cells. J Biol Chem. 2000;275:21130–9. doi: 10.1074/jbc.M000737200. [DOI] [PubMed] [Google Scholar]
- Haddad JJ, Land SC. O2-evoked regulation of HIF-1alpha and NF-kappaB in perinatal lung epithelium requires glutathione biosynthesis. Am J Physiol Lung Cell Mol Physiol. 2000;278:L492–503. doi: 10.1152/ajplung.2000.278.3.L492. [DOI] [PubMed] [Google Scholar]
- Rahman I, MacNee W. Oxidative stress and regulation of glutathione in lung inflammation. Eur Respir J. 2000;16:534–54. doi: 10.1034/j.1399-3003.2000.016003534.x. [DOI] [PubMed] [Google Scholar]
- Rahman I, MacNee W. Regulation of redox glutathione levels and gene transcription in lung inflammation: therapeutic approaches. Free Radic Biol Med. 2000;28:1405–20. doi: 10.1016/s0891-5849(00)00215-x. [DOI] [PubMed] [Google Scholar]
- Davis W, Jr, Ronai Z, Tew KD. Cellular thiols and reactive oxygen species in drug-induced apoptosis. J Pharmacol Exp Ther. 2001;296:1–6. [PubMed] [Google Scholar]
- Nishi T, Shimizu N, Hiramoto M, Sato I, Yamaguchi Y, Hasegawa M, Aizawa S, Tanaka H, Kataoka K, Watanabe H, Handa H. Spatial redox regulation of a critical cysteine residue of NF-kappa B in vivo. J Biol Chem. 2002;277:44548–56. doi: 10.1074/jbc.M202970200. [DOI] [PubMed] [Google Scholar]
- Biswas SK, McClure D, Jimenez LA, Megson IL, Rahman I. Curcumin induces glutathione biosynthesis and inhibits NF-kappaB activation and interleukin-8 release in alveolar epithelial cells: mechanism of free radical scavenging activity. Antioxid Redox Signal. 2005;7:32–41. doi: 10.1089/ars.2005.7.32. [DOI] [PubMed] [Google Scholar]
- Biswas SK, Newby DE, Rahman I, Megson IL. Depressed glutathione synthesis precedes oxidative stress and atherogenesis in Apo-E(-/-) mice. Biochem Biophys Res Commun. 2005;338:1368–73. doi: 10.1016/j.bbrc.2005.10.098. [DOI] [PubMed] [Google Scholar]
- Rahman I, Biswas SK, Jimenez LA, Torres M, Forman HJ. Glutathione, stress responses, and redox signaling in lung inflammation. Antioxid Redox Signal. 2005;7:42–59. doi: 10.1089/ars.2005.7.42. [DOI] [PubMed] [Google Scholar]
- Cuzzocrea S, Chatterjee PK, Mazzon E, Dugo L, Serraino I, Britti D, Mazzullo G, Caputi AP, Thiemermann C. Pyrrolidine dithiocarbamate attenuates the development of acute and chronic inflammation. Br J Pharmacol. 2002;135:496–510. doi: 10.1038/sj.bjp.0704463. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Desoize B. Metals and metal compounds in carcinogenesis. In Vivo. 2003;17:529–539. [PubMed] [Google Scholar]
- Xiong SG, She HY, Takeuchi H, Han B, Engelhardt JF, Barton CH, Zandi E, Giulivi C, Tsukamoto H. Signaling role of intracellular iron in NF-kappa B activation. Journal of Biological Chemistry. 2003;278:17646–17654. doi: 10.1074/jbc.M210905200. [DOI] [PubMed] [Google Scholar]
- Parodi FE, Mao D, Ennis TL, Bartoli MA, Thompson RW. Suppression of experimental abdominal aortic aneurysms in mice by treatment with pyrrolidine dithiocarbamate, an antioxidant inhibitor of nuclear factor-kappaB. J Vasc Surg. 2005;41:479–89. doi: 10.1016/j.jvs.2004.12.030. [DOI] [PubMed] [Google Scholar]
- Antosiewicz J, Ziolkowski W, Kaczor JJ, Herman-Antosiewicz A. Tumor necrosis factor-alpha-induced reactive oxygen species formation is mediated by JNK1-dependent ferritin degradation and elevation of labile iron pool. Free Radic Biol Med. 2007;43:265–70. doi: 10.1016/j.freeradbiomed.2007.04.023. [DOI] [PubMed] [Google Scholar]
- Vélez-Pardo C, Ospina GG, Del Río MJ. Abeta[25–35] peptide and iron promote apoptosis in lymphocytes by an oxidative stress mechanism: involvement of H2O2, caspase-3, NF-kappaB, p53 and c-Jun. Neurotoxicology. 2002;23:351–65. doi: 10.1016/s0161-813x(02)00081-5. [DOI] [PubMed] [Google Scholar]
- Bisti S, Soteriadou K. Is the reactive oxygen species-dependent-NF-kappaB activation observed in iron-loaded BALB/c mice a key process preventing growth of Leishmania major progeny and tissue-damage? Microbes Infect. 2006;8:1473–82. doi: 10.1016/j.micinf.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Bu DX, Hemdahl AL, Gabrielsen A, Fuxe J, Zhu C, Eriksson P, Yan ZQ. Induction of neutrophil gelatinase-associated lipocalin in vascular injury via activation of nuclear factor-kappaB. Am J Pathol. 2006;169:2245–53. doi: 10.2353/ajpath.2006.050706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer G, Weise S, Kralisch S, Lossner U, Bluher M, Stumvoll M, Fasshauer M. Lipocalin-2 is induced by interleukin-1beta in murine adipocytes in vitro. J Cell Biochem. 2008 doi: 10.1002/jcb.21980. [DOI] [PubMed] [Google Scholar]
- Lee P, Peng H, Gelbart T, Wang L, Beutler E. Regulation of hepcidin transcription by interleukin-1 and interleukin-6. Proc Natl Acad Sci USA. 2005;102:1906–10. doi: 10.1073/pnas.0409808102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvigneau JC, Piskernik C, Haindl S, Kloesch B, Hartl RT, Huttemann M, Lee I, Ebel T, Moldzio R, Gemeiner M, Redl H, Kozlov AV. A novel endotoxin-induced pathway: upregulation of heme oxygenase 1, accumulation of free iron, and free iron-mediated mitochondrial dysfunction. Lab Invest. 2008;88:70–7. doi: 10.1038/labinvest.3700691. [DOI] [PubMed] [Google Scholar]
- Whitcomb DC, Aoun E, Vodovotz Y, Clermont G, Barmada MM. Evaluating disorders with a complex genetics basis. the future roles of meta-analysis and systems biology. Dig Dis Sci. 2005;50:2195–202. doi: 10.1007/s10620-005-3033-7. [DOI] [PubMed] [Google Scholar]
- Xiong M, Feghali-Bostwick CA, Arnett FC, Zhou X. A systems biology approach to genetic studies of complex diseases. FEBS Lett. 2005;579:5325–32. doi: 10.1016/j.febslet.2005.08.058. [DOI] [PubMed] [Google Scholar]
- Ideker T, Galitski T, Hood L. A new approach to decoding life: systems biology. Annu Rev Genomics Hum Genet. 2001;2:343–72. doi: 10.1146/annurev.genom.2.1.343. [DOI] [PubMed] [Google Scholar]
- Barabási A-L, Oltvai ZN. Network biology: understanding the cell's functional organization. Nat Rev Genet. 2004;5:101–113. doi: 10.1038/nrg1272. [DOI] [PubMed] [Google Scholar]
- Morel NM, Holland JM, Greef J van der, Marple EW, Clish C, Loscalzo J, Naylor S. Primer on medical genomics. Part XIV: Introduction to systems biology–a new approach to understanding disease and treatment. Mayo Clin Proc. 2004;79:651–8. doi: 10.4065/79.5.651. [DOI] [PubMed] [Google Scholar]
- Khalil IG, Hill C. Systems biology for cancer. Curr Opin Oncol. 2005;17:44–8. doi: 10.1097/01.cco.0000150951.38222.16. [DOI] [PubMed] [Google Scholar]
- Moore JH, Boczko EM, Summar ML. Connecting the dots between genes biochemistry, and disease susceptibility: systems biology modeling in human genetics. Mol Genet Metab. 2005;84:104–11. doi: 10.1016/j.ymgme.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Hornberg JJ, Bruggeman FJ, Westerhoff HV, Lankelma J. Cancer: a Systems Biology disease. Biosystems. 2006;83:81–90. doi: 10.1016/j.biosystems.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Lemberger T. Systems biology in human health and disease. Mol Syst Biol. 2007;3:136. doi: 10.1038/msb4100175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loscalzo J, Kohane I, Barabasi AL. Human disease classification in the postgenomic era: a complex systems approach to human pathobiology. Mol Syst Biol. 2007;3:124. doi: 10.1038/msb4100163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnackenberg LK, Beger RD. Monitoring the health to disease continuum with global metabolic profiling and systems biology. Pharmacogenomics. 2006;7:1077–86. doi: 10.2217/14622416.7.7.1077. [DOI] [PubMed] [Google Scholar]
- Featherstone DE, Broadie K. Wrestling with pleiotropy: genomic and topological analysis of the yeast gene expression network. Bioessays. 2002;24:267–74. doi: 10.1002/bies.10054. [DOI] [PubMed] [Google Scholar]
- Castrillo JI, Zeef LA, Hoyle DC, Zhang N, Hayes A, Gardner DCJ, Cornell MJ, Petty J, Hakes L, Wardleworth L, Rash B, Brown M, Dunn WB, Broadhurst D, O'Donoghue K, Hester SS, Dunkley TPJ, Hart SR, Swainston N, Li P, Gaskell SJ, Paton NW, Lilley KS, Kell DB, Oliver SG. Growth control of the eukaryote cell: a systems biology study in yeast. J Biol. 2007;6:4. doi: 10.1186/jbiol54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raamsdonk LM, Teusink B, Broadhurst D, Zhang N, Hayes A, Walsh M, Berden JA, Brindle KM, Kell DB, Rowland JJ, Westerhoff HV, van Dam K, Oliver SG. A functional genomics strategy that uses metabolome data to reveal the phenotype of silent mutations. Nature Biotechnol. 2001;19:45–50. doi: 10.1038/83496. [DOI] [PubMed] [Google Scholar]
- Mo ML, Jamshidi N, Palsson BO. A genome-scale, constraint-based approach to systems biology of human metabolism. Mol Biosyst. 2007;3:598–603. doi: 10.1039/b705597h. [DOI] [PubMed] [Google Scholar]
- Kell DB. Metabolomics and systems biology: making sense of the soup. Curr Op Microbiol. 2004;7:296–307. doi: 10.1016/j.mib.2004.04.012. [DOI] [PubMed] [Google Scholar]
- Kell DB, Brown M, Davey HM, Dunn WB, Spasic I, Oliver SG. Metabolic footprinting and Systems Biology: the medium is the message. Nat Rev Microbiol. 2005;3:557–565. doi: 10.1038/nrmicro1177. [DOI] [PubMed] [Google Scholar]
- Kell DB. Metabolomics, modelling and machine learning in systems biology: towards an understanding of the languages of cells. The 2005 Theodor Bücher lecture. FEBS J. 2006;273:873–894. doi: 10.1111/j.1742-4658.2006.05136.x. [DOI] [PubMed] [Google Scholar]
- Kell DB. Systems biology, metabolic modelling and metabolomics in drug discovery and development. Drug Disc Today. 2006;11:1085–1092. doi: 10.1016/j.drudis.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Kell DB. Metabolomic biomarkers: search, discovery and validation. Exp Rev Mol Diagnost. 2007;7:329–333. doi: 10.1586/14737159.7.4.329. [DOI] [PubMed] [Google Scholar]
- Greef J van der, Martin S, Juhasz P, Adourian A, Plasterer T, Verheij ER, McBurney RN. The art and practice of systems biology in medicine: mapping patterns of relationships. J Proteome Res. 2007;6:1540–59. doi: 10.1021/pr0606530. [DOI] [PubMed] [Google Scholar]
- Kacser H, Burns JA. The control of flux. In: Davies DD, editor. Rate Control of Biological Processes Symposium of the Society for Experimental Biology. Vol. 27. Cambridge University Press, Cambridge; 1973. pp. 65–104. [Google Scholar]
- Heinrich R, Rapoport TA. A linear steady-state treatment of enzymatic chains. General properties, control and effector strength. Eur J Biochem. 1974;42:89–95. doi: 10.1111/j.1432-1033.1974.tb03318.x. [DOI] [PubMed] [Google Scholar]
- Kell DB, Westerhoff HV. Metabolic control theory: its role in microbiology and biotechnology. FEMS Microbiol Rev. 1986;39:305–320. [Google Scholar]
- Westerhoff HV, van Dam K. Thermodynamics and control of biological free energy transduction. Elsevier, Amsterdam; 1987. [Google Scholar]
- Westerhoff HV, Kell DB. Matrix method for determining the steps most rate-limiting to metabolic fluxes in biotechnological processes. Biotechnol Bioeng. 1987;30:101–107. doi: 10.1002/bit.260300115. [DOI] [PubMed] [Google Scholar]
- Fell DA. Understanding the control of metabolism. Portland Press, London; 1996. [Google Scholar]
- Heinrich R, Schuster S. The regulation of cellular systems. Chapman & Hall, New York; 1996. [Google Scholar]
- Murphy MP, Partridge L. Toward a control theory analysis of aging. Annu Rev Biochem. 2008;77:777–98. doi: 10.1146/annurev.biochem.77.070606.101605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JG. The physiology of obligate anaerobiosis. Adv Microb Physiol. 1975;12:169–246. [Google Scholar]
- Kirkwood TB, Boys RJ, Gillespie CS, Proctor CJ, Shanley DP, Wilkinson DJ. Towards an e-biology of ageing: integrating theory and data. Nat Rev Mol Cell Biol. 2003;4:243–9. doi: 10.1038/nrm1051. [DOI] [PubMed] [Google Scholar]
- Hofmeyr JH, Westerhoff HV. Building the cellular puzzle: control in multi-level reaction networks. J Theor Biol. 2001;208:261–85. doi: 10.1006/jtbi.2000.2216. [DOI] [PubMed] [Google Scholar]
- Saltelli A, Tarantola S, Campolongo F, Ratto M. Sensitivity analysis in practice: a guide to assessing scientific models. Wiley, New York; 2004. [Google Scholar]
- Sauro HM, Kholodenko BN. Quantitative analysis of signaling networks. Prog Biophys Mol Biol. 2004;86:5–43. doi: 10.1016/j.pbiomolbio.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Lüdtke N, Panzeri S, Brown M, Broomhead DS, Knowles J, Montemurro MA, Kell DB. Information-theoretic Sensitivity Analysis: a general method for credit assignment in complex networks. J Roy Soc Interface. 2008;5:223–235. doi: 10.1098/rsif.2007.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltelli A, Ratto M, Andres T, Campolongo F, Cariboni J, Gatelli D, Saisana M, Tarantola S. Global sensitivity analysis: the primer. WileyBlackwell, New York; 2008. [Google Scholar]
- Smallbone K, Simeonidis E, Broomhead DS, Kell DB. Something from nothing: bridging the gap between constraint-based and kinetic modelling. FEBS J. 2007;274:5576–5585. doi: 10.1111/j.1742-4658.2007.06076.x. [DOI] [PubMed] [Google Scholar]
- Mendes P, Kell DB. Non-linear optimization of biochemical pathways: applications to metabolic engineering and parameter estimation. Bioinformatics. 1998;14:869–883. doi: 10.1093/bioinformatics/14.10.869. [DOI] [PubMed] [Google Scholar]
- Moles CG, Mendes P, Banga JR. Parameter estimation in biochemical pathways: a comparison of global optimization methods. Genome Res. 2003;13:2467–74. doi: 10.1101/gr.1262503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Fernandez M, Mendes P, Banga JR. A hybrid approach for efficient and robust parameter estimation in biochemical pathways. Biosystems. 2006;83:248–65. doi: 10.1016/j.biosystems.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Wilkinson SJ, Benson N, Kell DB. Proximate parameter tuning for biochemical networks with uncertain kinetic parameters. Mol Biosyst. 2008;4:74–97. doi: 10.1039/b707506e. [DOI] [PubMed] [Google Scholar]
- Klipp E, Herwig R, Kowald A, Wierling C, Lehrach H. Systems biology in practice: concepts, implementation and clinical application. Wiley/VCH, Berlin; 2005. [Google Scholar]
- Alon U. An introduction to systems biology: design principles of biological circuits. Chapman and Hall/CRC, London; 2006. [Google Scholar]
- Palsson BØ. Systems biology: properties of reconstructed networks. Cambridge University Press, Cambridge; 2006. [Google Scholar]
- Doyle FJ, 3rd, Stelling J. Systems interface biology. J R Soc Interface. 2006;3:603–16. doi: 10.1098/rsif.2006.0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble D. The music of life: biology beyond genes. Oxford University Press, Oxford; 2006. [Google Scholar]
- Hopkins AL. Network pharmacology: the next paradigm in drug discovery. Nat Chem Biol. 2008;4:682–690. doi: 10.1038/nchembio.118. [DOI] [PubMed] [Google Scholar]
- Rand DA. Mapping global sensitivity of cellular network dynamics: sensitivity heat maps and a global summation law. J R Soc Interface. 2008;5:S59–69. doi: 10.1098/rsif.2008.0084.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagkopoulos I, Liu YC, Tavazoie S. Predictive behavior within microbial genetic networks. Science. 2008;320:1313–7. doi: 10.1126/science.1154456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscoli C, Cuzzocrea S, Riley DP, Zweier JL, Thiemermann C, Wang ZQ, Salvemini D. On the selectivity of superoxide dismutase mimetics and its importance in pharmacological studies. British Journal of Pharmacology. 2003;140:445–460. doi: 10.1038/sj.bjp.0705430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fell DA. Increasing the flux in metabolic pathways: A metabolic control analysis perspective. Biotechnol Bioeng. 1998;58:121–124. doi: 10.1002/(sici)1097-0290(19980420)58:2/3<121::aid-bit2>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Sai Y, Tsuji A. Transporter-mediated drug delivery: recent progress and experimental approaches. Drug Discov Today. 2004;9:712–20. doi: 10.1016/S1359-6446(04)03198-8. [DOI] [PubMed] [Google Scholar]
- Schulze MB, Manson JE, Willett WC, Hu FB. Processed meat intake and incidence of Type 2 diabetes in younger and middle-aged women. Diabetologia. 2003;46:1465–73. doi: 10.1007/s00125-003-1220-7. [DOI] [PubMed] [Google Scholar]
- Miyake T, Kung CK, Goldwasser E. Purification of human erythropoietin. J Biol Chem. 1977;252:5558–64. [PubMed] [Google Scholar]
- Jacobs K, Shoemaker C, Rudersdorf R, Neill SD, Kaufman RJ, Mufson A, Seehra J, Jones SS, Hewick R, Fritsch EF, et al. Isolation and characterization of genomic and cDNA clones of human erythropoietin. Nature. 1985;313:806–10. doi: 10.1038/313806a0. [DOI] [PubMed] [Google Scholar]
- Lin FK, Suggs S, Lin CH, Browne JK, Smalling R, Egrie JC, Chen KK, Fox GM, Martin F, Stabinsky Z, et al. Cloning and expression of the human erythropoietin gene. Proc Natl Acad Sci USA. 1985;82:7580–4. doi: 10.1073/pnas.82.22.7580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenjves ES, Ochoa MS, Cabrera O, Mendez AJ, Kenyon NS, Inverardi L, Ricordi C. Human, nonhuman primate, and rat pancreatic islets express erythropoietin receptors. Transplantation. 2003;75:1356–60. doi: 10.1097/01.TP.0000062862.88375.BD. [DOI] [PubMed] [Google Scholar]
- Ghezzi P, Brines M. Erythropoietin as an antiapoptotic, tissue-protective cytokine. Cell Death Differ. 2004;11:S37–44. doi: 10.1038/sj.cdd.4401450. [DOI] [PubMed] [Google Scholar]
- Brines M, Cerami A. Emerging biological roles for erythropoietin in the nervous system. Nat Rev Neurosci. 2005;6:484–94. doi: 10.1038/nrn1687. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Li F, Maiese K. Oxidative stress in the brain: novel cellular targets that govern survival during neurodegenerative disease. Prog Neurobiol. 2005;75:207–46. doi: 10.1016/j.pneurobio.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Brines M, Cerami A. Discovering erythropoietin's extra-hematopoietic functions: biology and clinical promise. Kidney Int. 2006;70:246–50. doi: 10.1038/sj.ki.5001546. [DOI] [PubMed] [Google Scholar]
- Coleman TR, Westenfelder C, Togel FE, Yang Y, Hu Z, Swenson L, Leuvenink HG, Ploeg RJ, d'Uscio LV, Katusic ZS, Ghezzi P, Zanetti A, Kaushansky K, Fox NE, Cerami A, Brines M. Cytoprotective doses of erythropoietin or carbamylated erythropoietin have markedly different procoagulant and vasoactive activities. Proc Natl Acad Sci USA. 2006;103:5965–70. doi: 10.1073/pnas.0601377103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghezzi P, Mengozzi M. Activities of erythropoietin on tumors: an immunological perspective. Eur J Immunol. 2007;37:1427–30. doi: 10.1002/eji.200737401. [DOI] [PubMed] [Google Scholar]
- Tönges L, Schlachetzki JCM, Weishaupt JH, Bähr M. Hematopoietic cytokines – on the verge of conquering neurology. Current Molecular Medicine. 2007;7:157–170. doi: 10.2174/156652407780059186. [DOI] [PubMed] [Google Scholar]
- Cai Z, Manalo DJ, Wei G, Rodriguez ER, Fox-Talbot K, Lu H, Zweier JL, Semenza GL. Hearts from rodents exposed to intermittent hypoxia or erythropoietin are protected against ischemia-reperfusion injury. Circulation. 2003;108:79–85. doi: 10.1161/01.CIR.0000078635.89229.8A. [DOI] [PubMed] [Google Scholar]
- Tramontano AF, Muniyappa R, Black AD, Blendea MC, Cohen I, Deng L, Sowers JR, Cutaia MV, El-Sherif N. Erythropoietin protects cardiac myocytes from hypoxia-induced apoptosis through an Akt-dependent pathway. Biochem Biophys Res Commun. 2003;308:990–4. doi: 10.1016/s0006-291x(03)01503-1. [DOI] [PubMed] [Google Scholar]
- Bogoyevitch MA. An update on the cardiac effects of erythropoietin cardioprotection by erythropoietin and the lessons learnt from studies in neuroprotection. Cardiovasc Res. 2004;63:208–16. doi: 10.1016/j.cardiores.2004.03.017. [DOI] [PubMed] [Google Scholar]
- Cai Z, Semenza GL. Phosphatidylinositol-3-kinase signaling is required for erythropoietin-mediated acute protection against myocardial ischemia/reperfusion injury. Circulation. 2004;109:2050–3. doi: 10.1161/01.CIR.0000127954.98131.23. [DOI] [PubMed] [Google Scholar]
- Wright GL, Hanlon P, Amin K, Steenbergen C, Murphy E, Arcasoy MO. Erythropoietin receptor expression in adult rat cardiomyocytes is associated with an acute cardioprotective effect for recombinant erythropoietin during ischemia-reperfusion injury. FASEB J. 2004;18:1031–3. doi: 10.1096/fj.03-1289fje. [DOI] [PubMed] [Google Scholar]
- Fiordaliso F, Chimenti S, Staszewsky L, Bai A, Carlo E, Cuccovillo I, Doni M, Mengozzi M, Tonelli R, Ghezzi P, Coleman T, Brines M, Cerami A, Latini R. A nonerythropoietic derivative of erythropoietin protects the myocardium from ischemia-reperfusion injury. Proc Natl Acad Sci USA. 2005;102:2046–51. doi: 10.1073/pnas.0409329102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon C, Krawczyk M, Paik D, Lakatta EG, Talan MI. Cardioprotection by recombinant human erythropoietin following acute experimental myocardial infarction: dose response and therapeutic window. Cardiovasc Drugs Ther. 2005;19:243–50. doi: 10.1007/s10557-005-3189-6. [DOI] [PubMed] [Google Scholar]
- Rui T, Feng Q, Lei M, Peng T, Zhang J, Xu M, Abel ED, Xenocostas A, Kvietys PR. Erythropoietin prevents the acute myocardial inflammatory response induced by ischemia/reperfusion via induction of AP-1. Cardiovasc Res. 2005;65:719–27. doi: 10.1016/j.cardiores.2004.11.019. [DOI] [PubMed] [Google Scholar]
- Lee ST, Chu K, Sinn DI, Jung KH, Kim EH, Kim SJ, Kim JM, Ko SY, Kim M, Roh JK. Erythropoietin reduces perihematomal inflammation and cell death with eNOS and STAT3 activations in experimental intracerebral hemorrhage. J Neurochem. 2006;96:1728–39. doi: 10.1111/j.1471-4159.2006.03697.x. [DOI] [PubMed] [Google Scholar]
- Li Y, Takemura G, Okada H, Miyata S, Maruyama R, Li L, Higuchi M, Minatoguchi S, Fujiwara T, Fujiwara H. Reduction of inflammatory cytokine expression and oxidative damage by erythropoietin in chronic heart failure. Cardiovasc Res. 2006;71:684–94. doi: 10.1016/j.cardiores.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Li L, Takemura G, Li Y, Miyata S, Esaki M, Okada H, Kanamori H, Khai NC, Maruyama R, Ogino A, Minatoguchi S, Fujiwara T, Fujiwara H. Preventive effect of erythropoietin on cardiac dysfunction in doxorubicin-induced cardiomyopathy. Circulation. 2006;113:535–43. doi: 10.1161/CIRCULATIONAHA.105.568402. [DOI] [PubMed] [Google Scholar]
- Miki T, Miura T, Yano T, Takahashi A, Sakamoto J, Tanno M, Kobayashi H, Ikeda Y, Nishihara M, Naitoh K, Ohori K, Shimamoto K. Alteration in erythropoietin-induced cardioprotective signaling by postinfarct ventricular remodeling. J Pharmacol Exp Ther. 2006;317:68–75. doi: 10.1124/jpet.105.095745. [DOI] [PubMed] [Google Scholar]
- Moon C, Krawczyk M, Paik D, Coleman T, Brines M, Juhaszova M, Sollott SJ, Lakatta EG, Talan MI. Erythropoietin, modified to not stimulate red blood cell production, retains its cardioprotective properties. J Pharmacol Exp Ther. 2006;316:999–1005. doi: 10.1124/jpet.105.094854. [DOI] [PubMed] [Google Scholar]
- Nishihara M, Miura T, Miki T, Sakamoto J, Tanno M, Kobayashi H, Ikeda Y, Ohori K, Takahashi A, Shimamoto K. Erythropoietin affords additional cardioprotection to preconditioned hearts by enhanced phosphorylation of glycogen synthase kinase-3 beta. Am J Physiol Heart Circ Physiol. 2006;291:H748–55. doi: 10.1152/ajpheart.00837.2005. [DOI] [PubMed] [Google Scholar]
- Binbrek AS, Mittal B, Rao KN, Sobel BE. The potential of erythropoietin for conferring cardioprotection complementing reperfusion. Coron Artery Dis. 2007;18:583–5. doi: 10.1097/MCA.0b013e3282ef4ed6. [DOI] [PubMed] [Google Scholar]
- Chen X, Chen Y, Bi Y, Fu N, Shan C, Wang S, Aslam S, Wang PW, Xu J. Preventive cardioprotection of erythropoietin against doxorubicin-induced cardiomyopathy. Cardiovasc Drugs Ther. 2007;21:367–74. doi: 10.1007/s10557-007-6052-0. [DOI] [PubMed] [Google Scholar]
- Gao E, Boucher M, Chuprun JK, Zhou RH, Eckhart AD, Koch WJ. Darbepoetin alfa, a long-acting erythropoietin analog, offers novel and delayed cardioprotection for the ischemic heart. Am J Physiol Heart Circ Physiol. 2007;293:H60–8. doi: 10.1152/ajpheart.00227.2007. [DOI] [PubMed] [Google Scholar]
- Putten K van der, Braam B, Jie KE, Gaillard CA. Mechanisms of Disease: erythropoietin resistance in patients with both heart and kidney failure. Nat Clin Pract Nephrol. 2008;4:47–57. doi: 10.1038/ncpneph0655. [DOI] [PubMed] [Google Scholar]
- Furlani D, Klopsch C, Gabel R, Ugurlucan M, Pittermann E, Klee D, Wagner K, Li W, Wang W, Ong LL, Nizze H, Titze U, Lutzow K, Lendlein A, Steinhoff G, Ma N. Intracardiac erythropoietin injection reveals antiinflammatory potential and improved cardiac functions detected by Forced Swim Test. Transplant Proc. 2008;40:962–6. doi: 10.1016/j.transproceed.2008.03.033. [DOI] [PubMed] [Google Scholar]
- Robey TE, Saiget MK, Reinecke H, Murry CE. Systems approaches to preventing transplanted cell death in cardiac repair. J Mol Cell Cardiol. 2008;45:567–81. doi: 10.1016/j.yjmcc.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirén AL, Ehrenreich H. Erythropoietin–a novel concept for neuroprotection. Eur Arch Psychiatry Clin Neurosci. 2001;251:179–84. doi: 10.1007/s004060170038. [DOI] [PubMed] [Google Scholar]
- Buemi M, Cavallaro E, Floccari F, Sturiale A, Aloisi C, Trimarchi M, Grasso G, Corica F, Frisina N. Erythropoietin and the brain: from neurodevelopment to neuroprotection. Clin Sci (Lond) 2002;103:275–82. doi: 10.1042/cs1030275. [DOI] [PubMed] [Google Scholar]
- Junk AK, Mammis A, Savitz SI, Singh M, Roth S, Malhotra S, Rosenbaum PS, Cerami A, Brines M, Rosenbaum DM. Erythropoietin administration protects retinal neurons from acute ischemia-reperfusion injury. Proc Natl Acad Sci USA. 2002;99:10659–64. doi: 10.1073/pnas.152321399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juul S. Erythropoietin in the central nervous system, and its use to prevent hypoxic-ischemic brain damage. Acta Paediatr Suppl. 2002;91:36–42. doi: 10.1111/j.1651-2227.2002.tb02904.x. [DOI] [PubMed] [Google Scholar]
- Ruscher K, Freyer D, Karsch M, Isaev N, Megow D, Sawitzki B, Priller J, Dirnagl U, Meisel A. Erythropoietin is a paracrine mediator of ischemic tolerance in the brain: evidence from an in vitro model. J Neurosci. 2002;22:10291–301. doi: 10.1523/JNEUROSCI.22-23-10291.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campana WM, Myers RR. Exogenous erythropoietin protects against dorsal root ganglion apoptosis and pain following peripheral nerve injury. Eur J Neurosci. 2003;18:1497–506. doi: 10.1046/j.1460-9568.2003.02875.x. [DOI] [PubMed] [Google Scholar]
- Prass K, Scharff A, Ruscher K, Lowl D, Muselmann C, Victorov I, Kapinya K, Dirnagl U, Meisel A. Hypoxia-induced stroke tolerance in the mouse is mediated by erythropoietin. Stroke. 2003;34:1981–6. doi: 10.1161/01.STR.0000080381.76409.B2. [DOI] [PubMed] [Google Scholar]
- Villa P, Bigini P, Mennini T, Agnello D, Laragione T, Cagnotto A, Viviani B, Marinovich M, Cerami A, Coleman TR, Brines M, Ghezzi P. Erythropoietin selectively attenuates cytokine production and inflammation in cerebral ischemia by targeting neuronal apoptosis. J Exp Med. 2003;198:971–5. doi: 10.1084/jem.20021067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Digicaylioglu M, Garden G, Timberlake S, Fletcher L, Lipton SA. Acute neuroprotective synergy of erythropoietin and insulin-like growth factor I. Proc Natl Acad Sci USA. 2004;101:9855–60. doi: 10.1073/pnas.0403172101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenreich H, Aust C, Krampe H, Jahn H, Jacob S, Herrmann M, Siren AL. Erythropoietin: novel approaches to neuroprotection in human brain disease. Metab Brain Dis. 2004;19:195–206. doi: 10.1023/b:mebr.0000043969.96895.3c. [DOI] [PubMed] [Google Scholar]
- Genc S, Koroglu TF, Genc K. Erythropoietin and the nervous system. Brain Res. 2004;1000:19–31. doi: 10.1016/j.brainres.2003.12.037. [DOI] [PubMed] [Google Scholar]
- Keswani SC, Buldanlioglu U, Fischer A, Reed N, Polley M, Liang H, Zhou C, Jack C, Leitz GJ, Hoke A. A novel endogenous erythropoietin mediated pathway prevents axonal degeneration. Ann Neurol. 2004;56:815–26. doi: 10.1002/ana.20285. [DOI] [PubMed] [Google Scholar]
- Lipton SA. Erythropoietin for neurologic protection and diabetic neuropathy. N Engl J Med. 2004;350:2516–7. doi: 10.1056/NEJMcibr041121. [DOI] [PubMed] [Google Scholar]
- Marti HH. Erythropoietin and the hypoxic brain. J Exp Biol. 2004;207:3233–42. doi: 10.1242/jeb.01049. [DOI] [PubMed] [Google Scholar]
- Prass K, Ruscher K, Karsch M, Isaev N, Megow D, Priller J, Scharff A, Dirnagl U, Meisel A. Desferrioxamine induces delayed tolerance against cerebral ischemia in vivo and in vitro. J Cereb Blood Flow Metab. 2002;22:520–5. doi: 10.1097/00004647-200205000-00003. [DOI] [PubMed] [Google Scholar]
- Diem R, Sättler MB, Merkler D, Demmer I, Maier K, Stadelmann C, Ehrenreich H, Bähr M. Combined therapy with methylprednisolone and erythropoietin in a model of multiple sclerosis. Brain. 2005;128:375–85. doi: 10.1093/brain/awh365. [DOI] [PubMed] [Google Scholar]
- Yatsiv I, Grigoriadis N, Simeonidou C, Stahel PF, Schmidt OI, Alexandrovitch AG, Tsenter J, Shohami E. Erythropoietin is neuroprotective, improves functional recovery, and reduces neuronal apoptosis and inflammation in a rodent model of experimental closed head injury. FASEB J. 2005;19:1701–3. doi: 10.1096/fj.05-3907fje. [DOI] [PubMed] [Google Scholar]
- Savino C, Pedotti R, Baggi F, Ubiali F, Gallo B, Nava S, Bigini P, Barbera S, Fumagalli E, Mennini T, Vezzani A, Rizzi M, Coleman T, Cerami A, Brines M, Ghezzi P, Bianchi R. Delayed administration of erythropoietin and its non-erythropoietic derivatives ameliorates chronic murine autoimmune encephalomyelitis. J Neuroimmunol. 2006;172:27–37. doi: 10.1016/j.jneuroim.2005.10.016. [DOI] [PubMed] [Google Scholar]
- Sirén AL, Radyushkin K, Boretius S, Kammer D, Riechers CC, Natt O, Sargin D, Watanabe T, Sperling S, Michaelis T, Price J, Meyer B, Frahm J, Ehrenreich H. Global brain atrophy after unilateral parietal lesion and its prevention by erythropoietin. Brain. 2006;129:480–9. doi: 10.1093/brain/awh703. [DOI] [PubMed] [Google Scholar]
- Chen G, Shi JX, Hang CH, Xie W, Liu J, Liu X. Inhibitory effect on cerebral inflammatory agents that accompany traumatic brain injury in a rat model: a potential neuroprotective mechanism of recombinant human erythropoietin (rhEPO) Neurosci Lett. 2007;425:177–82. doi: 10.1016/j.neulet.2007.08.022. [DOI] [PubMed] [Google Scholar]
- Cherian L, Goodman JC, Robertson C. Neuroprotection with erythropoietin administration following controlled cortical impact injury in rats. J Pharmacol Exp Ther. 2007;322:789–94. doi: 10.1124/jpet.107.119628. [DOI] [PubMed] [Google Scholar]
- Ehrenreich H, Fischer B, Norra C, Schellenberger F, Stender N, Stiefel M, Siren AL, Paulus W, Nave KA, Gold R, Bartels C. Exploring recombinant human erythropoietin in chronic progressive multiple sclerosis. Brain. 2007;130:2577–88. doi: 10.1093/brain/awm203. [DOI] [PubMed] [Google Scholar]
- Esiri MM. The interplay between inflammation and neurodegeneration in CNS disease. J Neuroimmunol. 2007;184:4–16. doi: 10.1016/j.jneuroim.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Juul S, Felderhoff-Mueser U. Epo and other hematopoietic factors. Semin Fetal Neonatal Med. 2007;12:250–8. doi: 10.1016/j.siny.2007.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lykissas MG, Korompilias AV, Vekris MD, Mitsionis GI, Sakellariou E, Beris AE. The role of erythropoietin in central and peripheral nerve injury. Clin Neurol Neurosurg. 2007;109:639–44. doi: 10.1016/j.clineuro.2007.05.013. [DOI] [PubMed] [Google Scholar]
- Noguchi CT, Asavaritikrai P, Teng R, Jia Y. Role of erythropoietin in the brain. Crit Rev Oncol Hematol. 2007;64:159–71. doi: 10.1016/j.critrevonc.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhang ZG, Rhodes K, Renzi M, Zhang RL, Kapke A, Lu M, Pool C, Heavner G, Chopp M. Post-ischemic treatment with erythropoietin or carbamylated erythropoietin reduces infarction and improves neurological outcome in a rat model of focal cerebral ischemia. Br J Pharmacol. 2007;151:1377–84. doi: 10.1038/sj.bjp.0707285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Shang Y, Sun SG, Liu RG, Yang WQ. Protective effect of erythropoietin against 1-methyl-4-phenylpyridinium-induced neurodegenaration in PC12 cells. Neurosci Bull. 2007;23:156–64. doi: 10.1007/s12264-007-0023-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiese L, Hempel C, Penkowa M, Kirkby N, Kurtzhals JA. Recombinant human erythropoietin increases survival and reduces neuronal apoptosis in a murine model of cerebral malaria. Malar J. 2008;7:3. doi: 10.1186/1475-2875-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi R, Buyukakilli B, Brines M, Savino C, Cavaletti G, Oggioni N, Lauria G, Borgna M, Lombardi R, Cimen B, Comelekoglu U, Kanik A, Tataroglu C, Cerami A, Ghezzi P. Erythropoietin both protects from and reverses experimental diabetic neuropathy. Proc Natl Acad Sci USA. 2004;101:823–8. doi: 10.1073/pnas.0307823100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- İnal M, Kanbak G, Şen S, Akyüz F, Sunal E. Antioxidant status and lipid peroxidation in hemodialysis patients undergoing erythropoietin and erythropoietin-vitamin E combined therapy. Free Radic Res. 1999;31:211–6. doi: 10.1080/10715769900300771. [DOI] [PubMed] [Google Scholar]
- Yang CW, Li C, Jung JY, Shin SJ, Choi BS, Lim SW, Sun BK, Kim YS, Kim J, Chang YS, Bang BK. Preconditioning with erythropoietin protects against subsequent ischemia-reperfusion injury in rat kidney. FASEB J. 2003;17:1754–5. doi: 10.1096/fj.02-1191fje. [DOI] [PubMed] [Google Scholar]
- Patel NS, Sharples EJ, Cuzzocrea S, Chatterjee PK, Britti D, Yaqoob MM, Thiemermann C. Pretreatment with EPO reduces the injury and dysfunction caused by ischemia/reperfusion in the mouse kidney in vivo. Kidney Int. 2004;66:983–9. doi: 10.1111/j.1523-1755.2004.00847.x. [DOI] [PubMed] [Google Scholar]
- Sharples EJ, Patel N, Brown P, Stewart K, Mota-Philipe H, Sheaff M, Kieswich J, Allen D, Harwood S, Raftery M, Thiemermann C, Yaqoob MM. Erythropoietin protects the kidney against the injury and dysfunction caused by ischemia-reperfusion. J Am Soc Nephrol. 2004;15:2115–24. doi: 10.1097/01.ASN.0000135059.67385.5D. [DOI] [PubMed] [Google Scholar]
- Ates E, Yalcin AU, Yilmaz S, Koken T, Tokyol C. Protective effect of erythropoietin on renal ischemia and reperfusion injury. ANZ J Surg. 2005;75:1100–5. doi: 10.1111/j.1445-2197.2005.03612.x. [DOI] [PubMed] [Google Scholar]
- Fliser D, Bahlmann FH, Haller H. EPO: renoprotection beyond anemia correction. Pediatr Nephrol. 2006;21:1785–9. doi: 10.1007/s00467-006-0284-2. [DOI] [PubMed] [Google Scholar]
- Jie KE, Verhaar MC, Cramer MJ, Putten K van der, Gaillard CA, Doevendans PA, Koomans HA, Joles JA, Braam B. Erythropoietin and the cardiorenal syndrome: cellular mechanisms on the cardiorenal connectors. Am J Physiol Renal Physiol. 2006;291:F932–44. doi: 10.1152/ajprenal.00200.2006. [DOI] [PubMed] [Google Scholar]
- Guneli E, Cavdar Z, Islekel H, Sarioglu S, Erbayraktar S, Kiray M, Sokmen S, Yilmaz O, Gokmen N. Erythropoietin protects the intestine against ischemia/reperfusion injury in rats. Mol Med. 2007;13:509–17. doi: 10.2119/2007-00032.Guneli. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra A, Bansal S, Wang W, Falk S, Zolty E, Schrier RW. Erythropoietin ameliorates renal dysfunction during endotoxaemia. Nephrol Dial Transplant. 2007;22:2349–53. doi: 10.1093/ndt/gfm216. [DOI] [PubMed] [Google Scholar]
- Ashikawa K, Majumdar S, Banerjee S, Bharti AC, Shishodia S, Aggarwal BB. Piceatannol inhibits TNF-induced NF-kappaB activation and NF-kappaB-mediated gene expression through suppression of IkappaBalpha kinase and p65 phosphorylation. J Immunol. 2002;169:6490–7. doi: 10.4049/jimmunol.169.11.6490. [DOI] [PubMed] [Google Scholar]
- Paur I, Austenaa LM, Blomhoff R. Extracts of dietary plants are efficient modulators of nuclear factor kappa B. Food Chem Toxicol. 2008;46:1288–97. doi: 10.1016/j.fct.2007.09.103. [DOI] [PubMed] [Google Scholar]
- Wojchowski DM, Gregory RC, Miller CP, Pandit AK, Pircher TJ. Signal transduction in the erythropoietin receptor system. Exp Cell Res. 1999;253:143–56. doi: 10.1006/excr.1999.4673. [DOI] [PubMed] [Google Scholar]
- Coleman T, Brines M. Science review: recombinant human erythropoietin in critical illness: a role beyond anemia? Crit Care. 2004;8:337–41. doi: 10.1186/cc2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon PR, Fu P, Wright GL, Steenbergen C, Arcasoy MO, Murphy E. Mechanisms of erythropoietin-mediated cardioprotection during ischemia-reperfusion injury: role of protein kinase C and phosphatidylinositol 3-kinase signaling. FASEB J. 2005;19:1323–5. doi: 10.1096/fj.04-3545fje. [DOI] [PubMed] [Google Scholar]
- Maiese K, Li F, Chong ZZ. Erythropoietin in the brain: can the promise to protect be fulfilled? Trends Pharmacol Sci. 2004;25:577–83. doi: 10.1016/j.tips.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Li F, Maiese K. Erythropoietin requires NF-kappaB and its nuclear translocation to prevent early and late apoptotic neuronal injury during beta-amyloid toxicity. Curr Neurovasc Res. 2005;2:387–99. doi: 10.2174/156720205774962683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K, Li F, Chong ZZ. New avenues of exploration for erythropoietin. JAMA. 2005;293:90–5. doi: 10.1001/jama.293.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B, Dong GH, Liu H, Wang YQ, Wu HW, Jing H. Recombinant human erythropoietin pretreatment attenuates myocardial infarct size: a possible mechanism involves heat shock Protein 70 and attenuation of nuclear factor-kappaB. Ann Clin Lab Sci. 2005;35:161–8. [PubMed] [Google Scholar]
- Aerbajinai W, Zhu J, Gao Z, Chin K, Rodgers GP. Thalidomide induces gamma-globin gene expression through increased reactive oxygen species-mediated p38 MAPK signaling and histone H4 acetylation in adult erythropoiesis. Blood. 2007;110:2864–71. doi: 10.1182/blood-2007-01-065201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryter SW, Kim HP, Hoetzel A, Park JW, Nakahira K, Wang X, Choi AM. Mechanisms of cell death in oxidative stress. Antioxid Redox Signal. 2007;9:49–89. doi: 10.1089/ars.2007.9.49. [DOI] [PubMed] [Google Scholar]
- Wu Y, Shang Y, Sun S, Liang H, Liu R. Erythropoietin prevents PC12 cells from 1-methyl-4-phenylpyridinium ion-induced apoptosis via the Akt/GSK-3beta/caspase-3 mediated signaling pathway. Apoptosis. 2007;12:1365–75. doi: 10.1007/s10495-007-0065-9. [DOI] [PubMed] [Google Scholar]
- Cavdar C, Camsari T, Semin I, Gonenc S, Acikgoz O. Lipid peroxidation and antioxidant activity in chronic haemodialysis patients treated with recombinant human erythropoietin. Scand J Urol Nephrol. 1997;31:371–5. doi: 10.3109/00365599709030622. [DOI] [PubMed] [Google Scholar]
- Katavetin P, Inagi R, Miyata T, Shao J, Sassa R, Adler S, Eto N, Kato H, Fujita T, Nangaku M. Erythropoietin induces heme oxygenase-1 expression and attenuates oxidative stress. Biochem Biophys Res Commun. 2007;359:928–34. doi: 10.1016/j.bbrc.2007.05.207. [DOI] [PubMed] [Google Scholar]
- Katavetin P, Tungsanga K, Eiam-Ong S, Nangaku M. Antioxidative effects of erythropoietin. Kidney Int Suppl. 2007:S10–5. doi: 10.1038/sj.ki.5002482. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay A, Das Choudhury TD, Basu MK, Datta AG. Effect of Cu2+-ascorbic acid on lipid peroxidation, Mg2+-ATPase activity and spectrin of RBC membrane and reversal by erythropoietin. Mol Cell Biochem. 1992;118:23–30. doi: 10.1007/BF00249691. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay A, Choudhury TD, Bandyopadhyay D, Datta AG. Protective effect of erythropoietin on the oxidative damage of erythrocyte membrane by hydroxyl radical. Biochem Pharmacol. 2000;59:419–25. doi: 10.1016/s0006-2952(99)00277-4. [DOI] [PubMed] [Google Scholar]
- Akisu M, Tuzun S, Arslanoglu S, Yalaz M, Kultursay N. Effect of recombinant human erythropoietin administration on lipid peroxidation and antioxidant enzyme(s) activities in preterm infants. Acta Med Okayama. 2001;55:357–62. doi: 10.18926/AMO/31997. [DOI] [PubMed] [Google Scholar]
- Hirayama A, Nagase S, Gotoh M, Ueda A, Ishizu T, Yoh K, Aoyagi K, Terao J, Koyama A. Reduced serum hydroxyl radical scavenging activity in erythropoietin therapy resistant renal anemia. Free Radic Res. 2002;36:1155–61. doi: 10.1080/1071576021000016418. [DOI] [PubMed] [Google Scholar]
- Yankowitz J, Piraino B, Laifer SA, Frassetto L, Gavin L, Kitzmiller JL, Crombleholme W. Erythropoietin in pregnancies complicated by severe anemia of renal failure. Obstet Gynecol. 1992;80:485–8. [PubMed] [Google Scholar]
- Braga J, Marques R, Branco A, Goncalves J, Lobato L, Pimentel JP, Flores MM, Goncalves E, Jorge CS. Maternal and perinatal implications of the use of human recombinant erythropoietin. Acta Obstet Gynecol Scand. 1996;75:449–3. doi: 10.3109/00016349609033352. [DOI] [PubMed] [Google Scholar]
- Jungers P, Chauveau D. Pregnancy in renal disease. Kidney Int. 1997;52:871–85. doi: 10.1038/ki.1997.408. [DOI] [PubMed] [Google Scholar]
- Vora M, Gruslin A. Erythropoietin in obstetrics. Obstet Gynecol Surv. 1998;53:500–8. doi: 10.1097/00006254-199808000-00023. [DOI] [PubMed] [Google Scholar]
- Breymann C, Visca E, Huch R, Huch A. Efficacy and safety of intravenously administered iron sucrose with and without adjuvant recombinant human erythropoietin for the treatment of resistant iron-deficiency anemia during pregnancy. Am J Obstet Gynecol. 2001;184:662–7. doi: 10.1067/mob.2001.111717. [DOI] [PubMed] [Google Scholar]
- Sifakis S, Angelakis E, Vardaki E, Koumantaki Y, Matalliotakis I, Koumantakis E. Erythropoietin in the treatment of iron deficiency anemia during pregnancy. Gynecol Obstet Invest. 2001;51:150–6. doi: 10.1159/000052914. [DOI] [PubMed] [Google Scholar]
- Sesti F, Ticconi C, Bonifacio S, Piccione E. Preoperative administration of recombinant human erythropoietin in patients undergoing gynecologic surgery. Gynecol Obstet Invest. 2002;54:1–5. doi: 10.1159/000064688. [DOI] [PubMed] [Google Scholar]
- Donato E, Guinot M, Vilar C, Garcia R, Canigral G. rHuEPO in the management of pregnancy complicated by anti-Dib. Transfusion. 2003;43:681–2. doi: 10.1046/j.1537-2995.2003.00370.x. [DOI] [PubMed] [Google Scholar]
- Kaupke CJ, Vaziri ND, Powers DR, Gonzales E. Erythropoietin in preeclampsia. Obstet Gynecol. 1991;78:795–9. [PubMed] [Google Scholar]
- Buescher U, Hertwig K, Wolf C, Dudenhausen JW. Erythropoietin in amniotic fluid as a marker of chronic fetal hypoxia. Int J Gynaecol Obstet. 1998;60:257–63. doi: 10.1016/s0020-7292(98)00011-3. [DOI] [PubMed] [Google Scholar]
- Goldstein JD, Garry DJ, Maulik D. Obstetric conditions and erythropoietin levels. Am J Obstet Gynecol. 2000;182:1055–7. doi: 10.1067/mob.2000.105389. [DOI] [PubMed] [Google Scholar]
- Troeger C, Holzgreve W, Ladewig A, Zhong XY, Hahn S. Examination of maternal plasma erythropoietin and activin A concentrations with regard to circulatory erythroblast levels in normal and preeclamptic pregnancies. Fetal Diagn Ther. 2006;21:156–60. doi: 10.1159/000089068. [DOI] [PubMed] [Google Scholar]
- Semenza GL. HIF-1 and human disease: one highly involved factor. Genes Dev. 2000;14:1983–91. [PubMed] [Google Scholar]
- Semenza GL. Hypoxia-inducible factor 1: oxygen homeostasis and disease pathophysiology. Trends Mol Med. 2001;7:345–50. doi: 10.1016/s1471-4914(01)02090-1. [DOI] [PubMed] [Google Scholar]
- Wenger RH. Cellular adaptation to hypoxia: O2-sensing protein hydroxylases, hypoxia-inducible transcription factors, and O2-regulated gene expression. FASEB J. 2002;16:1151–62. doi: 10.1096/fj.01-0944rev. [DOI] [PubMed] [Google Scholar]
- Pugh CW, Ratcliffe PJ. Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med. 2003;9:677–84. doi: 10.1038/nm0603-677. [DOI] [PubMed] [Google Scholar]
- Haase VH. Hypoxia-inducible factors in the kidney. Amer J Physiol. 2006;291:F271–F281. doi: 10.1152/ajprenal.00071.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon D, Pastore YD, Divoky V, Liu E, Mlodnicka AE, Rainey K, Ponka P, Semenza GL, Schumacher A, Prchal JT. Hypoxia-inducible factor-1 deficiency results in dysregulated erythropoiesis signaling and iron homeostasis in mouse development. J Biol Chem. 2006;281:25703–11. doi: 10.1074/jbc.M602329200. [DOI] [PubMed] [Google Scholar]
- Zhang B, Tanaka J, Yang L, Yang L, Sakanaka M, Hata R, Maeda N, Mitsuda N. Protective effect of vitamin E against focal brain ischemia and neuronal death through induction of target genes of hypoxia-inducible factor-1. Neuroscience. 2004;126:433–440. doi: 10.1016/j.neuroscience.2004.03.057. [DOI] [PubMed] [Google Scholar]
- Rius J, Guma M, Schachtrup C, Akassoglou K, Zinkernagel AS, Nizet V, Johnson RS, Haddad GG, Karin M. NF-kappaB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1alpha. Nature. 2008 doi: 10.1038/nature06905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyssonnaux C, Zinkernagel AS, Schuepbach RA, Rankin E, Vaulont S, Haase VH, Nizet V, Johnson RS. Regulation of iron homeostasis by the hypoxia-inducible transcription factors (HIFs) J Clin Invest. 2007;117:1926–32. doi: 10.1172/JCI31370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyssonnaux C, Cejudo-Martin P, Doedens A, Zinkernagel AS, Johnson RS, Nizet V. Cutting edge: Essential role of hypoxia inducible factor-1alpha in development of lipopolysaccharide-induced sepsis. J Immunol. 2007;178:7516–9. doi: 10.4049/jimmunol.178.12.7516. [DOI] [PubMed] [Google Scholar]
- Peyssonnaux C, Nizet V, Johnson RS. Role of the hypoxia inducible factors HIF in iron metabolism. Cell Cycle. 2008;7:28–32. doi: 10.4161/cc.7.1.5145. [DOI] [PubMed] [Google Scholar]
- Hamrick SE, McQuillen PS, Jiang X, Mu D, Madan A, Ferriero DM. A role for hypoxia-inducible factor-1alpha in desferoxamine neuroprotection. Neurosci Lett. 2005;379:96–100. doi: 10.1016/j.neulet.2004.12.080. [DOI] [PubMed] [Google Scholar]
- Caniggia I, Winter J, Lye SJ, Post M. Oxygen and placental development during the first trimester: implications for the pathophysiology of pre-eclampsia. Placenta. 2000;21:S25–30. doi: 10.1053/plac.1999.0522. [DOI] [PubMed] [Google Scholar]
- Qutub AA, Popel AS. A computational model of intracellular oxygen sensing by hypoxia-inducible factor HIF1 alpha. J Cell Sci. 2006;119:3467–80. doi: 10.1242/jcs.03087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qutub AA, Popel AS. Three autocrine feedback loops determine HIF1 alpha expression in chronic hypoxia. Biochim Biophys Acta. 2007;1773:1511–25. doi: 10.1016/j.bbamcr.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Dassow G, Meir E, Munro EM, Odell GM. The segment polarity network is a robust development module. Nature. 2000;406:188–192. doi: 10.1038/35018085. [DOI] [PubMed] [Google Scholar]
- Brandman O, Ferrell JE, Jr, Li R, Meyer T. Interlinked fast and slow positive feedback loops drive reliable cell decisions. Science. 2005;310:496–8. doi: 10.1126/science.1113834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kell DB, Knowles JD. The role of modeling in systems biology. In: Szallasi Z, Stelling J, Periwal V, editor. System modeling in cellular biology: from concepts to nuts and bolts. MIT Press, Cambridge; 2006. pp. 3–18. [Google Scholar]
- Wei YH, Lee HC. Oxidative stress, mitochondrial DNA mutation, and impairment of antioxidant enzymes in aging. Exp Biol Med (Maywood) 2002;227:671–82. doi: 10.1177/153537020222700901. [DOI] [PubMed] [Google Scholar]
- McCord JM. Iron, free radicals, and oxidative injury. Semin Hematol. 1998;35:5–12. [PubMed] [Google Scholar]
- Barkai N, Leibler S. Robustness in simple biochemical networks. Nature. 1997;387:913–7. doi: 10.1038/43199. [DOI] [PubMed] [Google Scholar]
- Bornholdt S, Sneppen K. Robustness as an evolutionary principle. Proc R Soc B-Biological Sciences. 2000;267:2281–2286. doi: 10.1098/rspb.2000.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn MC, Tohmaz AS, Giroux KJ, Blumenthal GM, Feezor MD, Millington DS. Robustness of MetaNet graph models: Predicting control of urea production in humans. Biosystems. 2002;65:61–78. doi: 10.1016/s0303-2647(02)00002-3. [DOI] [PubMed] [Google Scholar]
- Ma L, Iglesias PA. Quantifying robustness of biochemical network models. BMC Bioinformatics. 2002;3 doi: 10.1186/1471-2105-3-38. http://www.biomedcentral.com/1471-2105/3/38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morohashi M, Winn AE, Borisuk MT, Bolouri H, Doyle J, Kitano H. Robustness as a measure of plausibility in models of biochemical networks. J Theor Biol. 2002;216:19–30. doi: 10.1006/jtbi.2002.2537. [DOI] [PubMed] [Google Scholar]
- Aldana M, Cluzel P. A natural class of robust networks. Proc Natl Acad Sci USA. 2003;100:8710–4. doi: 10.1073/pnas.1536783100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebenhoh O, Heinrich R. Stoichiometric design of metabolic networks: multifunctionality clusters, optimization, weak and strong robustness. Bull Math Biol. 2003;65:323–57. doi: 10.1016/S0092-8240(03)00002-8. [DOI] [PubMed] [Google Scholar]
- Kitano H, Oda K, Kimura T, Matsuoka Y, Csete M, Doyle J, Muramatsu M. Metabolic syndrome and robustness tradeoffs. Diabetes. 2004;53:S6–S15. doi: 10.2337/diabetes.53.suppl_3.s6. [DOI] [PubMed] [Google Scholar]
- Kitano H. Biological robustness. Nat Rev Genet. 2004;5:826–37. doi: 10.1038/nrg1471. [DOI] [PubMed] [Google Scholar]
- Stelling J, Sauer U, Szallasi Z, Doyle FJ, 3rd, Doyle J. Robustness of cellular functions. Cell. 2004;118:675–85. doi: 10.1016/j.cell.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Stelling J, Gilles ED, Doyle FJ., 3rd Robustness properties of circadian clock architectures. Proc Natl Acad Sci USA. 2004;101:13210–5. doi: 10.1073/pnas.0401463101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BS, Wang YC, Wu WS, Li WH. A new measure of the robustness of biochemical networks. Bioinformatics. 2005;21:2698–705. doi: 10.1093/bioinformatics/bti348. [DOI] [PubMed] [Google Scholar]
- Wagner A. Robustness evolvability, and neutrality. FEBS Lett. 2005;579:1772–8. doi: 10.1016/j.febslet.2005.01.063. [DOI] [PubMed] [Google Scholar]
- Wagner A. Circuit topology and the evolution of robustness in two-gene circadian oscillators. Proc Natl Acad Sci USA. 2005;102:11775–80. doi: 10.1073/pnas.0501094102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurata H, El-Samad H, Iwasaki R, Ohtake H, Doyle JC, Grigorova I, Gross CA, Khammash M. Module-based analysis of robustness tradeoffs in the heat shock response system. PLoS Comput Biol. 2006;2:e59. doi: 10.1371/journal.pcbi.0020059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner A. Robustness and evolvability in living systems. Princeton University Press Princeton, NJ; 2005. [Google Scholar]
- Moriya H, Shimizu-Yoshida Y, Kitano H. In vivo robustness analysis of cell division cycle genes in Saccharomyces cerevisiae. PLoS Genet. 2006;2:e111. doi: 10.1371/journal.pgen.0020111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W, Lai L, Ouyang Q, Tang C. Robustness and modular design of the Drosophila segment polarity network. Mol Syst Biol. 2006;2:70. doi: 10.1038/msb4100111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitano H. A robustness-based approach to systems-oriented drug design. Nat Rev Drug Discov. 2007;6:202–210. doi: 10.1038/nrd2195. [DOI] [PubMed] [Google Scholar]
- Vizan P, Mazurek S, Cascante M. Robust metabolic adaptation underlying tumor progression. Metabolomics. 2008;4:1–12. [Google Scholar]
- Jacobsen EW, Cedersund G. Structural robustness of biochemical network models-with application to the oscillatory metabolism of activated neutrophils. IET Syst Biol. 2008;2:39–47. doi: 10.1049/iet-syb:20070008. [DOI] [PubMed] [Google Scholar]
- Tsai TY, Choi YS, Ma W, Pomerening JR, Tang C, Ferrell JE., Jr Robust, tunable biological oscillations from interlinked positive and negative feedback loops. Science. 2008;321:126–9. doi: 10.1126/science.1156951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels BC, Chen YJ, Sethna JP, Gutenkunst RN, Myers CR. Sloppiness robustness, and evolvability in systems biology. Curr Opin Biotechnol. 2008;19:389–95. doi: 10.1016/j.copbio.2008.06.008. [DOI] [PubMed] [Google Scholar]
- Martin OC, Wagner A. Multifunctionality and robustness trade-offs in model genetic circuits. Biophys J. 2008;94:2927–37. doi: 10.1529/biophysj.107.114348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner A. Robustness and evolvability: a paradox resolved. Proc Biol Sci. 2008;275:91–100. doi: 10.1098/rspb.2007.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehár J, Krueger A, Zimmermann G, Borisy A. High-order combination effects and biological robustness. Molecular Systems Biology. 2008;4 doi: 10.1038/msb.2008.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medawar P. Pluto's republic. Oxford University Press, Oxford; 1982. [Google Scholar]
- Gilbert GN, Mulkay M. Opening Pandora's box: a sociological analysis of scientists' discourse. Cambridge University Press, Cambridge; 1984. [Google Scholar]
- Breen AP, Murphy JA. Reactions of oxyl radicals with DNA. Free Radic Biol Med. 1995;18:1033–77. doi: 10.1016/0891-5849(94)00209-3. [DOI] [PubMed] [Google Scholar]
- Burrows CJ, Muller JG. Oxidative nucleobase modifications leading to strand scission. Chem Rev. 1998;98:1109–1152. doi: 10.1021/cr960421s. [DOI] [PubMed] [Google Scholar]
- Bolton JL, Trush MA, Penning TM, Dryhurst G, Monks TJ. Role of quinones in toxicology. Chem Res Toxicol. 2000;13:135–60. doi: 10.1021/tx9902082. [DOI] [PubMed] [Google Scholar]
- Cooke MS, Evans MD, Dizdaroglu M, Lunec J. Oxidative DNA damage: mechanisms mutation, and disease. Faseb J. 2003;17:1195–214. doi: 10.1096/fj.02-0752rev. [DOI] [PubMed] [Google Scholar]
- Haggarty SJ, Clemons PA, Schreiber SL. Chemical genomic profiling of biological networks using graph theory and combinations of small molecule perturbations. J Am Chem Soc. 2003;125:10543–5. doi: 10.1021/ja035413p. [DOI] [PubMed] [Google Scholar]
- Csermely P, Agoston V, Pongor S. The efficiency of multi-target drugs: the network approach might help drug design. Trends Pharmacol Sci. 2005;26:178–82. doi: 10.1016/j.tips.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Ambesi-Impiombato A, di Bernardo D. Computational biology and drug discovery: From single-target to network drugs. Current Bioinformatics. 2006;1:3–13. [Google Scholar]
- Yeh P, Tschumi AI, Kishony R. Functional classification of drugs by properties of their pairwise interactions. Nat Genet. 2006;38:489–94. doi: 10.1038/ng1755. [DOI] [PubMed] [Google Scholar]
- Lee MS, Johansen L, Zhang Y, Wilson A, Keegan M, Avery W, Elliott P, Borisy AA, Keith CT. The novel combination of chlorpromazine and pentamidine exerts synergistic antiproliferative effects through dual mitotic action. Cancer Res. 2007;67:11359–67. doi: 10.1158/0008-5472.CAN-07-2235. [DOI] [PubMed] [Google Scholar]
- Abbott A. Pharmaceutical futures: a fiendish puzzle. Nature. 2008;455:1164–7. doi: 10.1038/4551164a. [DOI] [PubMed] [Google Scholar]
- Henney A, Superti-Furga G. A network solution. Nature. 2008;455:730–1. doi: 10.1038/455730a. [DOI] [PubMed] [Google Scholar]
- Hoon S, Smith AM, Wallace IM, Suresh S, Miranda M, Fung E, Proctor M, Shokat KM, Zhang C, Davis RW, Giaever G, St Onge RP, Nislow C. An integrated platform of genomic assays reveals small-molecule bioactivities. Nat Chem Biol. 2008;4:498–506. doi: 10.1038/nchembio.100. [DOI] [PubMed] [Google Scholar]
- Kvien TK, Fjeld E, Slatkowsky-Christensen B, Nichols M, Zhang Y, Proven A, Mikkelsen K, Palm O, Borisy AA, Lessem J. Efficacy and safety of a novel synergistic drug candidate, CRx-102, in hand osteoarthritis. Ann Rheum Dis. 2008;67:942–8. doi: 10.1136/ard.2007.074401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehár J, Stockwell BR, Giaever G, Nislow C. Combination chemical genetics. Nat Chem Biol. 2008;4:674–81. doi: 10.1038/nchembio.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelander S, Wang W, Nilsson B, She QB, Pratilas C, Rosen N, Gennemark P, Sander C. Models from experiments: combinatorial drug perturbations of cancer cells. Mol Syst Biol. 2008;4:216. doi: 10.1038/msb.2008.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerhoff HV. Systems biology: the grand challenge for European biology. Screening. 2008;9:34–37. [Google Scholar]
- Westerhoff HV, Mosekilde E, Noe CR, Clemensen AM. Integrating systems approaches into pharmaceutical sciences. Eur J Pharm Sci. 2008;35:1–4. doi: 10.1016/j.ejps.2008.05.011. [DOI] [PubMed] [Google Scholar]
- Tong AH, Lesage G, Bader GD, Ding H, Xu H, Xin X, Young J, Berriz GF, Brost RL, Chang M, Chen Y, Cheng X, Chua G, Friesen H, Goldberg DS, Haynes J, Humphries C, He G, Hussein S, Ke L, Krogan N, Li Z, Levinson JN, Lu H, Menard P, Munyana C, Parsons AB, Ryan O, Tonikian R, Roberts T, Sdicu AM, Shapiro J, Sheikh B, Suter B, Wong SL, Zhang LV, Zhu H, Burd CG, Munro S, Sander C, Rine J, Greenblatt J, Peter M, Bretscher A, Bell G, Roth FP, Brown GW, Andrews B, Bussey H, Boone C. Global mapping of the yeast genetic interaction network. Science. 2004;303:808–13. doi: 10.1126/science.1091317. [DOI] [PubMed] [Google Scholar]
- Parsons AB, Brost RL, Ding H, Li Z, Zhang C, Sheikh B, Brown GW, Kane PM, Hughes TR, Boone C. Integration of chemical-genetic and genetic interaction data links bioactive compounds to cellular target pathways. Nat Biotechnol. 2004;22:62–9. doi: 10.1038/nbt919. [DOI] [PubMed] [Google Scholar]
- Parsons AB, Lopez A, Givoni IE, Williams DE, Gray CA, Porter J, Chua G, Sopko R, Brost RL, Ho CH, Wang J, Ketela T, Brenner C, Brill JA, Fernandez GE, Lorenz TC, Payne GS, Ishihara S, Ohya Y, Andrews B, Hughes TR, Frey BJ, Graham TR, Andersen RJ, Boone C. Exploring the mode-of-action of bioactive compounds by chemical-genetic profiling in yeast. Cell. 2006;126:611–25. doi: 10.1016/j.cell.2006.06.040. [DOI] [PubMed] [Google Scholar]
- Pearl J. Probabilistic reasoning in intelligent systems: networks of plausible inference. Morgan Kaufmann, San Francisco; 1988. [Google Scholar]
- Pearl J. Causality: models, reasoning and inference. Cambridge University Press, Cambridge; 2000. [Google Scholar]
- Hastie T, Tibshirani R, Friedman J. The elements of statistical learning: data mining, inference and prediction. Springer-Verlag, Berlin; 2001. [Google Scholar]
- Shipley B. Cause and correlation in biology: a user's guide to path analysis, structural equations and causal inference. Cambridge University Press, Cambridge; 2001. [Google Scholar]
- Mackay DJC. Information theory, inference and learning algorithms. Cambridge University Press, Cambridge; 2003. [Google Scholar]
- Needham CJ, Bradford JR, Bulpitt AJ, Westhead DR. Inference in Bayesian networks. Nat Biotechnol. 2006;24:51–53. doi: 10.1038/nbt0106-51. [DOI] [PubMed] [Google Scholar]
- Jayawardhana B, Kell DB, Rattray M. Bayesian inference of the sites of perturbations in metabolic pathways via Markov Chain Monte Carlo. Bioinformatics. 2008;24:1191–1197. doi: 10.1093/bioinformatics/btn103. [DOI] [PubMed] [Google Scholar]
- Rohr JR, Raffel TR, Sessions SK, Hudson PJ. Understanding the net effects of pesticides on amphibian trematode infections. Ecol Appl. 2008;18:1743–53. doi: 10.1890/07-1429.1. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ. Converging concepts: adaptive response preconditioning, and the Yerkes-Dodson Law are manifestations of hormesis. Ageing Res Rev. 2008;7:8–20. doi: 10.1016/j.arr.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ. Hormesis and medicine. Br J Clin Pharmacol. 2008 doi: 10.1111/j.1365-2125.2008.03243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese E. Hormesis: why it is important to toxicology and toxicologists. Environ Toxicol Chem. 2008. p. 1. [DOI] [PubMed]
- Mattson MP. Hormesis and disease resistance: activation of cellular stress response pathways. Hum Exp Toxicol. 2008;27:155–62. doi: 10.1177/0960327107083417. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Hormesis defined. Ageing Res Rev. 2008;7:1–7. doi: 10.1016/j.arr.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med. 1998;339:1349–57. doi: 10.1056/NEJM199811053391902. [DOI] [PubMed] [Google Scholar]
- Liappis AP, Kan VL, Rochester CG, Simon GL. The effect of statins on mortality in patients with bacteremia. Clin Infect Dis. 2001;33:1352–7. doi: 10.1086/323334. [DOI] [PubMed] [Google Scholar]
- Almog Y, Shefer A, Novack V, Maimon N, Barski L, Eizinger M, Friger M, Zeller L, Danon A. Prior statin therapy is associated with a decreased rate of severe sepsis. Circulation. 2004;110:880–5. doi: 10.1161/01.CIR.0000138932.17956.F1. [DOI] [PubMed] [Google Scholar]
- Merx MW, Liehn EA, Janssens U, Lutticken R, Schrader J, Hanrath P, Weber C. HMG-CoA reductase inhibitor simvastatin profoundly improves survival in a murine model of sepsis. Circulation. 2004;109:2560–5. doi: 10.1161/01.CIR.0000129774.09737.5B. [DOI] [PubMed] [Google Scholar]
- Merx MW, Liehn EA, Graf J, Sandt A van de, Schaltenbrand M, Schrader J, Hanrath P, Weber C. Statin treatment after onset of sepsis in a murine model improves survival. Circulation. 2005;112:117–24. doi: 10.1161/CIRCULATIONAHA.104.502195. [DOI] [PubMed] [Google Scholar]
- Zhang L, Zhang ZG, Ding GL, Jiang Q, Liu X, Meng H, Hozeska A, Zhang C, Li L, Morris D, Zhang RL, Lu M, Chopp M. Multitargeted effects of statin-enhanced thrombolytic therapy for stroke with recombinant human tissue-type plasminogen activator in the rat. Circulation. 2005;112:3486–94. doi: 10.1161/CIRCULATIONAHA.104.516757. [DOI] [PubMed] [Google Scholar]
- Hackam DG, Mamdani M, Li P, Redelmeier DA. Statins and sepsis in patients with cardiovascular disease: a population-based cohort analysis. Lancet. 2006;367:413–8. doi: 10.1016/S0140-6736(06)68041-0. [DOI] [PubMed] [Google Scholar]
- Terblanche M, Almog Y, Rosenson RS, Smith TS, Hackam DG. Statins: panacea for sepsis? Lancet Infect Dis. 2006;6:242–8. doi: 10.1016/S1473-3099(06)70439-X. [DOI] [PubMed] [Google Scholar]
- Thomsen RW, Hundborg HH, Johnsen SP, Pedersen L, Sorensen HT, Schonheyder HC, Lervang HH. Statin use and mortality within 180 days after bacteremia: a population-based cohort study. Crit Care Med. 2006;34:1080–6. doi: 10.1097/01.CCM.0000207345.92928.E4. [DOI] [PubMed] [Google Scholar]
- Aneja R, Fink MP. Promising therapeutic agents for sepsis. Trends Microbiol. 2007;15:31–7. doi: 10.1016/j.tim.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Martin CP, Talbert RL, Burgess DS, Peters JI. Effectiveness of statins in reducing the rate of severe sepsis: a retrospective evaluation. Pharmacotherapy. 2007;27:20–6. doi: 10.1592/phco.27.1.20. [DOI] [PubMed] [Google Scholar]
- Merx MW, Weber C. Sepsis and the heart. Circulation. 2007;116:793–802. doi: 10.1161/CIRCULATIONAHA.106.678359. [DOI] [PubMed] [Google Scholar]
- Schlienger RG, Fedson DS, Jick SS, Jick H, Meier CR. Statins and the risk of pneumonia: a population-based, nested case-control study. Pharmacotherapy. 2007;27:325–32. doi: 10.1592/phco.27.3.325. [DOI] [PubMed] [Google Scholar]
- Falagas ME, Makris GC, Matthaiou DK, Rafailidis PI. Statins for infection and sepsis: a systematic review of the clinical evidence. J Antimicrob Chemother. 2008;61:774–85. doi: 10.1093/jac/dkn019. [DOI] [PubMed] [Google Scholar]
- Lipinski MJ, Abbate A, Fuster V, Vetrovec GW. Drug insight: statins for nonischemic heart failure–evidence and potential mechanisms. Nat Clin Pract Cardiovasc Med. 2007;4:196–205. doi: 10.1038/ncpcardio0855. [DOI] [PubMed] [Google Scholar]
- Santodomingo-Garzón T, Cunha TM, Verri WA, Jr, Valerio DA, Parada CA, Poole S, Ferreira SH, Cunha FQ. Atorvastatin inhibits inflammatory hypernociception. Br J Pharmacol. 2006;149:14–22. doi: 10.1038/sj.bjp.0706836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira GA, Navarro TP, Telles RW, Andrade LE, Sato EI. Atorvastatin therapy improves endothelial-dependent vasodilation in patients with systemic lupus erythematosus: an 8 weeks controlled trial. Rheumatology (Oxford) 2007;46:1560–5. doi: 10.1093/rheumatology/kem186. [DOI] [PubMed] [Google Scholar]
- Rydgren T, Vaarala O, Sandler S. Simvastatin protects against multiple low-dose streptozotocin-induced type 1 diabetes in CD-1 mice and recurrence of disease in nonobese diabetic mice. J Pharmacol Exp Ther. 2007;323:180–5. doi: 10.1124/jpet.107.122655. [DOI] [PubMed] [Google Scholar]
- Leung BP, Sattar N, Crilly A, Prach M, McCarey DW, Payne H, Madhok R, Campbell C, Gracie JA, Liew FY, McInnes IB. A novel anti-inflammatory role for simvastatin in inflammatory arthritis. J Immunol. 2003;170:1524–30. doi: 10.4049/jimmunol.170.3.1524. [DOI] [PubMed] [Google Scholar]
- Sattar N, McCarey DW, Capell H, McInnes IB. Explaining how "high-grade" systemic inflammation accelerates vascular risk in rheumatoid arthritis. Circulation. 2003;108:2957–63. doi: 10.1161/01.CIR.0000099844.31524.05. [DOI] [PubMed] [Google Scholar]
- Barsante MM, Roffe E, Yokoro CM, Tafuri WL, Souza DG, Pinho V, Castro MS, Teixeira MM. Anti-inflammatory and analgesic effects of atorvastatin in a rat model of adjuvant-induced arthritis. Eur J Pharmacol. 2005;516:282–9. doi: 10.1016/j.ejphar.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Dombrecht EJ, Van Offel JF, Bridts CH, Ebo DG, Seynhaeve V, Schuerwegh AJ, Stevens WJ, De Clerck LS. Influence of simvastatin on the production of pro-inflammatory cytokines and nitric oxide by activated human chondrocytes. Clin Exp Rheumatol. 2007;25:534–9. [PubMed] [Google Scholar]
- Haruna Y, Morita Y, Yada T, Satoh M, Fox DA, Kashihara N. Fluvastatin reverses endothelial dysfunction and increased vascular oxidative stress in rat adjuvant-induced arthritis. Arthritis Rheum. 2007;56:1827–35. doi: 10.1002/art.22632. [DOI] [PubMed] [Google Scholar]
- Yamagata T, Kinoshita K, Nozaki Y, Sugiyama M, Ikoma S, Funauchi M. Effects of pravastatin in murine collagen-induced arthritis. Rheumatol Int. 2007;27:631–9. doi: 10.1007/s00296-006-0270-9. [DOI] [PubMed] [Google Scholar]
- Epstein M, Campese VM. Pleiotropic effects of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitors on renal function. Am J Kidney Dis. 2005;45:2–14. doi: 10.1053/j.ajkd.2004.08.040. [DOI] [PubMed] [Google Scholar]
- Yasuda H, Yuen PS, Hu X, Zhou H, Star RA. Simvastatin improves sepsis-induced mortality and acute kidney injury via renal vascular effects. Kidney Int. 2006;69:1535–42. doi: 10.1038/sj.ki.5000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campese VM, Park J. HMG-CoA reductase inhibitors and the kidney. Kidney Int. 2007;71:1215–22. doi: 10.1038/sj.ki.5002174. [DOI] [PubMed] [Google Scholar]
- Otuki MF, Pietrovski EF, Cabrini DA. Topical simvastatin: preclinical evidence for a treatment of skin inflammatory conditions. J Dermatol Sci. 2006;44:45–7. doi: 10.1016/j.jdermsci.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Lee JH, Lee DS, Kim EK, Choe KH, Oh YM, Shim TS, Kim SE, Lee YS, Lee SD. Simvastatin inhibits cigarette smoking-induced emphysema and pulmonary hypertension in rat lungs. Am J Respir Crit Care Med. 2005;172:987–93. doi: 10.1164/rccm.200501-041OC. [DOI] [PubMed] [Google Scholar]
- Pirat A, Zeyneloglu P, Aldemir D, Yücel M, Özen O, Candan S, Arslan G. Pretreatment with simvastatin reduces lung injury related to intestinal ischemia-reperfusion in rats. Anesth Analg. 2006;102:225–32. doi: 10.1213/01.ane.0000189554.41095.98. [DOI] [PubMed] [Google Scholar]
- Endres M, Laufs U, Huang Z, Nakamura T, Huang P, Moskowitz MA, Liao JK. Stroke protection by 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase inhibitors mediated by endothelial nitric oxide synthase. Proc Natl Acad Sci USA. 1998;95:8880–5. doi: 10.1073/pnas.95.15.8880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan CJ, Delanty N. Neuroprotective properties of statins in cerebral ischemia and stroke. Stroke. 1999;30:1969–73. doi: 10.1161/01.str.30.9.1969. [DOI] [PubMed] [Google Scholar]
- Amin-Hanjani S, Stagliano NE, Yamada M, Huang PL, Liao JK, Moskowitz MA. Mevastatin, an HMG-CoA reductase inhibitor, reduces stroke damage and upregulates endothelial nitric oxide synthase in mice. Stroke. 2001;32:980–6. doi: 10.1161/01.str.32.4.980. [DOI] [PubMed] [Google Scholar]
- Sironi L, Cimino M, Guerrini U, Calvio AM, Lodetti B, Asdente M, Balduini W, Paoletti R, Tremoli E. Treatment with statins after induction of focal ischemia in rats reduces the extent of brain damage. Arterioscler Thromb Vasc Biol. 2003;23:322–7. doi: 10.1161/01.atv.0000044458.23905.3b. [DOI] [PubMed] [Google Scholar]
- Endres M, Laufs U, Liao JK, Moskowitz MA. Targeting eNOS for stroke protection. Trends Neurosci. 2004;27:283–9. doi: 10.1016/j.tins.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Amarenco P, Moskowitz MA. The dynamics of statins: from event prevention to neuroprotection. Stroke. 2006;37:294–6. doi: 10.1161/01.STR.0000201856.90105.ab. [DOI] [PubMed] [Google Scholar]
- Amarenco P, Lavallee PC, Mazighi M, Labreuche J. The role of statins in the prevention of stroke. Archives of Medical Science. 2007;3:S109–S114. [Google Scholar]
- DeFaria Yeh D, Waters DD. Preventing and treating stroke and transient ischemic attack. Am J Cardiol. 2008;101:270–3. doi: 10.1016/j.amjcard.2007.07.072. [DOI] [PubMed] [Google Scholar]
- Chen SF, Hung TH, Chen CC, Lin KH, Huang YN, Tsai HC, Wang JY. Lovastatin improves histological and functional outcomes and reduces inflammation after experimental traumatic brain injury. Life Sci. 2007;81:288–98. doi: 10.1016/j.lfs.2007.05.023. [DOI] [PubMed] [Google Scholar]
- Faden AI, Stoica B. Neuroprotection: challenges and opportunities. Arch Neurol. 2007;64:794–800. doi: 10.1001/archneur.64.6.794. [DOI] [PubMed] [Google Scholar]
- Wu H, Lu D, Jiang H, Xiong Y, Qu C, Li B, Mahmood A, Zhou D, Chopp M. Simvastatin-mediated upregulation of VEGF and BDNF, activation of the PI3K/Akt pathway, and increase of neurogenesis are associated with therapeutic improvement after traumatic brain injury. J Neurotrauma. 2008;25:130–9. doi: 10.1089/neu.2007.0369. [DOI] [PubMed] [Google Scholar]
- Jick H, Zornberg GL, Jick SS, Seshadri S, Drachman DA. Statins and the risk of dementia. Lancet. 2000;356:1627–31. doi: 10.1016/s0140-6736(00)03155-x. [DOI] [PubMed] [Google Scholar]
- Wolozin B, Kellman W, Ruosseau P, Celesia GG, Siegel G. Decreased prevalence of Alzheimer disease associated with 3-hydroxy-3-methyglutaryl coenzyme A reductase inhibitors. Arch Neurol. 2000;57:1439–43. doi: 10.1001/archneur.57.10.1439. [DOI] [PubMed] [Google Scholar]
- Fassbender K, Simons M, Bergmann C, Stroick M, Lutjohann D, Keller P, Runz H, Kuhl S, Bertsch T, von Bergmann K, Hennerici M, Beyreuther K, Hartmann T. Simvastatin strongly reduces levels of Alzheimer's disease beta -amyloid peptides Abeta 42 and Abeta 40 in vitro and in vivo. Proc Natl Acad Sci USA. 2001;98:5856–61. doi: 10.1073/pnas.081620098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Refolo LM, Pappolla MA, LaFrancois J, Malester B, Schmidt SD, Thomas-Bryant T, Tint GS, Wang R, Mercken M, Petanceska SS, Duff KE. A cholesterol-lowering drug reduces beta-amyloid pathology in a transgenic mouse model of Alzheimer's disease. Neurobiol Dis. 2001;8:890–9. doi: 10.1006/nbdi.2001.0422. [DOI] [PubMed] [Google Scholar]
- Hajjar I, Schumpert J, Hirth V, Wieland D, Eleazer GP. The impact of the use of statins on the prevalence of dementia and the progression of cognitive impairment. J Gerontol A Biol Sci Med Sci. 2002;57:M414–8. doi: 10.1093/gerona/57.7.m414. [DOI] [PubMed] [Google Scholar]
- Zamvil SS, Steinman L. Cholesterol-lowering statins possess anti-inflammatory activity that might be useful for treatment of MS. Neurology. 2002;59:970–1. doi: 10.1212/wnl.59.7.970. [DOI] [PubMed] [Google Scholar]
- Aktas O, Waiczies S, Smorodchenko A, Dorr J, Seeger B, Prozorovski T, Sallach S, Endres M, Brocke S, Nitsch R, Zipp F. Treatment of relapsing paralysis in experimental encephalomyelitis by targeting Th1 cells through atorvastatin. J Exp Med. 2003;197:725–33. doi: 10.1084/jem.20021425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casserly I, Topol E. Convergence of atherosclerosis and Alzheimer's disease: inflammation cholesterol, and misfolded proteins. Lancet. 2004;363:1139–46. doi: 10.1016/S0140-6736(04)15900-X. [DOI] [PubMed] [Google Scholar]
- Nath N, Giri S, Prasad R, Singh AK, Singh I. Potential targets of 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor for multiple sclerosis therapy. J Immunol. 2004;172:1273–86. doi: 10.4049/jimmunol.172.2.1273. [DOI] [PubMed] [Google Scholar]
- Neuhaus O, Stuve O, Zamvil SS, Hartung HP. Are statins a treatment option for multiple sclerosis? Lancet Neurol. 2004;3:369–71. doi: 10.1016/S1474-4422(04)00770-7. [DOI] [PubMed] [Google Scholar]
- Vollmer T, Key L, Durkalski V, Tyor W, Corboy J, Markovic-Plese S, Preiningerova J, Rizzo M, Singh I. Oral simvastatin treatment in relapsing-remitting multiple sclerosis. Lancet. 2004;363:1607–8. doi: 10.1016/S0140-6736(04)16205-3. [DOI] [PubMed] [Google Scholar]
- Neuhaus O, Stuve O, Zamvil SS, Hartung HP. Evaluation of HMG-CoA reductase inhibitors for multiple sclerosis: opportunities and obstacles. CNS Drugs. 2005;19:833–41. doi: 10.2165/00023210-200519100-00003. [DOI] [PubMed] [Google Scholar]
- Mok SW, Thelen KM, Riemer C, Bamme T, Gultner S, Lutjohann D, Baier M. Simvastatin prolongs survival times in prion infections of the central nervous system. Biochem Biophys Res Commun. 2006;348:697–702. doi: 10.1016/j.bbrc.2006.07.123. [DOI] [PubMed] [Google Scholar]
- Schmeer C, Kretz A, Isenmann S. Statin-mediated protective effects in the central nervous system: general mechanisms and putative role of stress proteins. Restor Neurol Neurosci. 2006;24:79–95. [PubMed] [Google Scholar]
- Wolozin B, Wang SW, Li NC, Lee A, Lee TA, Kazis LE. Simvastatin is associated with a reduced incidence of dementia and Parkinson's disease. BMC Med. 2007;5:20. doi: 10.1186/1741-7015-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalli A, Bolognesi ML, Minarini A, Rosini M, Tumiatti V, Recanatini M, Melchiorre C. Multi-target-directed ligands to combat neurodegenerative diseases. J Med Chem. 2008;51:347–72. doi: 10.1021/jm7009364. [DOI] [PubMed] [Google Scholar]
- Paul F, Waiczies S, Wuerfel J, Bellmann-Strobl J, Dorr J, Waiczies H, Haertle M, Wernecke KD, Volk HD, Aktas O, Zipp F. Oral high-dose atorvastatin treatment in relapsing-remitting multiple sclerosis. PLoS ONE. 2008;3:e1928. doi: 10.1371/journal.pone.0001928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bösel J, Gandor F, Harms C, Synowitz M, Harms U, Djoufack PC, Megow D, Dirnagl U, Hortnagl H, Fink KB, Endres M. Neuroprotective effects of atorvastatin against glutamate-induced excitotoxicity in primary cortical neurones. J Neurochem. 2005;92:1386–98. doi: 10.1111/j.1471-4159.2004.02980.x. [DOI] [PubMed] [Google Scholar]
- Wong WW, Dimitroulakos J, Minden MD, Penn LZ. HMG-CoA reductase inhibitors and the malignant cell: the statin family of drugs as triggers of tumor-specific apoptosis. Leukemia. 2002;16:508–19. doi: 10.1038/sj.leu.2402476. [DOI] [PubMed] [Google Scholar]
- Kaushal V, Kohli M, Mehta P, Mehta JL. Potential anticancer effects of statins: fact or fiction? Endothelium. 2003;10:49–58. doi: 10.1080/10623320303358. [DOI] [PubMed] [Google Scholar]
- Wu J, Wong WW, Khosravi F, Minden MD, Penn LZ. Blocking the Raf/MEK/ERK pathway sensitizes acute myelogenous leukemia cells to lovastatin-induced apoptosis. Cancer Res. 2004;64:6461–8. doi: 10.1158/0008-5472.CAN-04-0866. [DOI] [PubMed] [Google Scholar]
- Demierre MF, Higgins PD, Gruber SB, Hawk E, Lippman SM. Statins and cancer prevention. Nat Rev Cancer. 2005;5:930–42. doi: 10.1038/nrc1751. [DOI] [PubMed] [Google Scholar]
- Fritz G. HMG-CoA reductase inhibitors (statins) as anticancer drugs (review) Int J Oncol. 2005;27:1401–9. [PubMed] [Google Scholar]
- Katz MS. Therapy insight: Potential of statins for cancer chemoprevention and therapy. Nat Clin Pract Oncol. 2005;2:82–9. doi: 10.1038/ncponc0097. [DOI] [PubMed] [Google Scholar]
- Shannon J, Tewoderos S, Garzotto M, Beer TM, Derenick R, Palma A, Farris PE. Statins and prostate cancer risk: a case-control study. Am J Epidemiol. 2005;162:318–25. doi: 10.1093/aje/kwi203. [DOI] [PubMed] [Google Scholar]
- Campbell MJ, Esserman LJ, Zhou Y, Shoemaker M, Lobo M, Borman E, Baehner F, Kumar AS, Adduci K, Marx C, Petricoin EF, Liotta LA, Winters M, Benz S, Benz CC. Breast cancer growth prevention by statins. Cancer Res. 2006;66:8707–14. doi: 10.1158/0008-5472.CAN-05-4061. [DOI] [PubMed] [Google Scholar]
- Cauley JA, McTiernan A, Rodabough RJ, LaCroix A, Bauer DC, Margolis KL, Paskett ED, Vitolins MZ, Furberg CD, Chlebowski RT. Statin use and breast cancer: prospective results from the Women's Health Initiative. J Natl Cancer Inst. 2006;98:700–7. doi: 10.1093/jnci/djj188. [DOI] [PubMed] [Google Scholar]
- Platz EA, Leitzmann MF, Visvanathan K, Rimm EB, Stampfer MJ, Willett WC, Giovannucci E. Statin drugs and risk of advanced prostate cancer. J Natl Cancer Inst. 2006;98:1819–25. doi: 10.1093/jnci/djj499. [DOI] [PubMed] [Google Scholar]
- Jacobs EJ, Rodriguez C, Bain EB, Wang Y, Thun MJ, Calle EE. Cholesterol-lowering drugs and advanced prostate cancer incidence in a large U.S. cohort. Cancer Epidemiol Biomarkers Prev. 2007;16:2213–7. doi: 10.1158/1055-9965.EPI-07-0448. [DOI] [PubMed] [Google Scholar]
- Kodach LL, Bleuming SA, Peppelenbosch MP, Hommes DW, Brink GR van den, Hardwick JC. The effect of statins in colorectal cancer is mediated through the bone morphogenetic protein pathway. Gastroenterology. 2007;133:1272–81. doi: 10.1053/j.gastro.2007.08.021. [DOI] [PubMed] [Google Scholar]
- Farwell WR, Scranton RE, Lawler EV, Lew RA, Brophy MT, Fiore LD, Gaziano JM. The association between statins and cancer incidence in a veterans population. J Natl Cancer Inst. 2008;100:134–9. doi: 10.1093/jnci/djm286. [DOI] [PubMed] [Google Scholar]
- Karp I, Behlouli H, Lelorier J, Pilote L. Statins and cancer risk. Am J Med. 2008;121:302–9. doi: 10.1016/j.amjmed.2007.12.011. [DOI] [PubMed] [Google Scholar]
- Sassano A, Platanias LC. Statins in tumor suppression. Cancer Lett. 2008;260:11–9. doi: 10.1016/j.canlet.2007.11.036. [DOI] [PubMed] [Google Scholar]
- Brenner S. Loose ends. Current Biology, London; 1997. [DOI] [PubMed] [Google Scholar]
- Kacser H. On parts and wholes in metabolism. In: Welch GR, Clegg JS, editor. The organization of cell metabolism. Plenum Press, New York; 1986. pp. 327–337. [Google Scholar]
- Pennisi E. A new window on how genomes work. Science. 2007;316:1120–1. doi: 10.1126/science.316.5828.1120. [DOI] [PubMed] [Google Scholar]
- Kell DB, Oliver SG. Here is the evidence, now what is the hypothesis? The complementary roles of inductive and hypothesis-driven science in the post-genomic era. Bioessays. 2004;26:99–105. doi: 10.1002/bies.10385. [DOI] [PubMed] [Google Scholar]
- Russell B. Am I an atheist or an agnostic? 1947.
- Kostoff RN. Overcoming specialization. Bioscience. 2002;52:937–941. [Google Scholar]
- Noble D. Modeling the heart – from genes to cells to the whole organ. Science. 2002;295:1678–1682. doi: 10.1126/science.1069881. [DOI] [PubMed] [Google Scholar]
- Hunter PJ. The IUPS Physiome Project: a framework for computational physiology. Prog Biophys Mol Biol. 2004;85:551–69. doi: 10.1016/j.pbiomolbio.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Duarte NC, Becker SA, Jamshidi N, Thiele I, Mo ML, Vo TD, Srvivas R, Palsson BØ. Global reconstruction of the human metabolic network based on genomic and bibliomic data. Proc Natl Acad Sci. 2007;104:1777–1782. doi: 10.1073/pnas.0610772104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kell DB. The virtual human: towards a global systems biology of multiscale, distributed biochemical network models. IUBMB Life. 2007;59:689–95. doi: 10.1080/15216540701694252. [DOI] [PubMed] [Google Scholar]
- Ma H, Sorokin A, Mazein A, Selkov A, Selkov E, Demin O, Goryanin I. The Edinburgh human metabolic network reconstruction and its functional analysis. Mol Syst Biol. 2007;3:135. doi: 10.1038/msb4100177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feist AM, Palsson BØ. The growing scope of applications of genome-scale metabolic reconstructions using Escherichia coli. Nat Biotechnol. 2008;26:659–67. doi: 10.1038/nbt1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H, Goryanin I. Human metabolic network reconstruction and its impact on drug discovery and development. Drug Discov Today. 2008;13:402–8. doi: 10.1016/j.drudis.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Jamshidi N, Palsson BØ. Formulating genome-scale kinetic models in the post-genome era. Mol Syst Biol. 2008;4:171. doi: 10.1038/msb.2008.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapworthy G, Viceconti M, Coveney PV, Kohl P. The virtual physiological human: building a framework for computational biomedicine I. Editorial. Philos Transact A Math Phys Eng Sci. 2008;366:2975–8. doi: 10.1098/rsta.2008.0103. [DOI] [PubMed] [Google Scholar]
- Fenner JW, Brook B, Clapworthy G, Coveney PV, Feipel V, Gregersen H, Hose DR, Kohl P, Lawford P, McCormack KM, Pinney D, Thomas SR, Van Sint Jan S, Waters S, Viceconti M. The EuroPhysiome, STEP and a roadmap for the virtual physiological human. Philos Transact A Math Phys Eng Sci. 2008;366:2979–99. doi: 10.1098/rsta.2008.0089. [DOI] [PubMed] [Google Scholar]
- Snoep JL, Bruggeman F, Olivier BG, Westerhoff HV. Towards building the silicon cell: a modular approach. Biosystems. 2006;83:207–16. doi: 10.1016/j.biosystems.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Jongeneel CV, Delorenzi M, Iseli C, Zhou D, Haudenschild CD, Khrebtukova I, Kuznetsov D, Stevenson BJ, Strausberg RL, Simpson AJ, Vasicek TJ. An atlas of human gene expression from massively parallel signature sequencing (MPSS) Genome Res. 2005;15:1007–14. doi: 10.1101/gr.4041005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson A, Hober S, Uhlen M. A human protein atlas based on antibody proteomics. Curr Opin Mol Ther. 2006;8:185–90. [PubMed] [Google Scholar]
- Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, Boe AF, Boguski MS, Brockway KS, Byrnes EJ, Chen L, Chen L, Chen TM, Chin MC, Chong J, Crook BE, Czaplinska A, Dang CN, Datta S, Dee NR, Desaki AL, Desta T, Diep E, Dolbeare TA, Donelan MJ, Dong HW, Dougherty JG, Duncan BJ, Ebbert AJ, Eichele G, Estin LK, Faber C, Facer BA, Fields R, Fischer SR, Fliss TP, Frensley C, Gates SN, Glattfelder KJ, Halverson KR, Hart MR, Hohmann JG, Howell MP, Jeung DP, Johnson RA, Karr PT, Kawal R, Kidney JM, Knapik RH, Kuan CL, Lake JH, Laramee AR, Larsen KD, Lau C, Lemon TA, Liang AJ, Liu Y, Luong LT, Michaels J, Morgan JJ, Morgan RJ, Mortrud MT, Mosqueda NF, Ng LL, Ng R, Orta GJ, Overly CC, Pak TH, Parry SE, Pathak SD, Pearson OC, Puchalski RB, Riley ZL, Rockett HR, Rowland SA, Royall JJ, Ruiz MJ, Sarno NR, Schaffnit K, Shapovalova NV, Sivisay T, Slaughterbeck CR, Smith SC, Smith KA, Smith BI, Sodt AJ, Stewart NN, Stumpf KR, Sunkin SM, Sutram M, Tam A, Teemer CD, Thaller C, Thompson CL, Varnam LR, Visel A, Whitlock RM, Wohnoutka PE, Wolkey CK, Wong VY, et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445:168–76. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- Hober S, Uhlén M. Human protein atlas and the use of microarray technologies. Curr Opin Biotechnol. 2008;19:30–5. doi: 10.1016/j.copbio.2007.11.006. [DOI] [PubMed] [Google Scholar]
- Barbe L, Lundberg E, Oksvold P, Stenius A, Lewin E, Björling E, Asplund A, Ponten F, Brismar H, Uhlén M, Andersson-Svahn H. Toward a confocal subcellular atlas of the human proteome. Mol Cell Proteomics. 2008;7:499–508. doi: 10.1074/mcp.M700325-MCP200. [DOI] [PubMed] [Google Scholar]
- Sultan M, Schulz MH, Richard H, Magen A, Klingenhoff A, Scherf M, Seifert M, Borodina T, Soldatov A, Parkhomchuk D, Schmidt D, O'Keeffe S, Haas S, Vingron M, Lehrach H, Yaspo ML. A global view of gene activity and alternative splicing by deep sequencing of the human transcriptome. Science. 2008;321:956–60. doi: 10.1126/science.1160342. [DOI] [PubMed] [Google Scholar]
- Shlomi T, Cabili MN, Herrgard MJ, Palsson BØ, Ruppin E. Network-based prediction of human tissue-specific metabolism. Nat Biotechnol. 2008;26:1003–10. doi: 10.1038/nbt.1487. [DOI] [PubMed] [Google Scholar]
- Hucka M, Finney A, Sauro HM, Bolouri H, Doyle JC, Kitano H, Arkin AP, Bornstein BJ, Bray D, Cornish-Bowden A, Cuellar AA, Dronov S, Gilles ED, Ginkel M, Gor V, Goryanin II, Hedley WJ, Hodgman TC, Hofmeyr JH, Hunter PJ, Juty NS, Kasberger JL, Kremling A, Kummer U, Le Novere N, Loew LM, Lucio D, Mendes P, Minch E, Mjolsness ED, Nakayama Y, Nelson MR, Nielsen PF, Sakurada T, Schaff JC, Shapiro BE, Shimizu TS, Spence HD, Stelling J, Takahashi K, Tomita M, Wagner J, Wang J. The systems biology markup language (SBML): a medium for representation and exchange of biochemical network models. Bioinformatics. 2003;19:524–31. doi: 10.1093/bioinformatics/btg015. [DOI] [PubMed] [Google Scholar]
- Le Novère N, Finney A, Hucka M, Bhalla US, Campagne F, Collado-Vides J, Crampin EJ, Halstead M, Klipp E, Mendes P, Nielsen P, Sauro H, Shapiro B, Snoep JL, Spence HD, Wanner BL. Minimum information requested in the annotation of biochemical models (MIRIAM) Nat Biotechnol. 2005;23:1509–15. doi: 10.1038/nbt1156. [DOI] [PubMed] [Google Scholar]
- Taylor CF, Field D, Sansone SA, Aerts J, Apweiler R, Ashburner M, Ball CA, Binz PA, Bogue M, Booth T, Brazma A, Brinkman RR, Michael Clark A, Deutsch EW, Fiehn O, Fostel J, Ghazal P, Gibson F, Gray T, Grimes G, Hancock JM, Hardy NW, Hermjakob H, Julian RK, Jr, Kane M, Kettner C, Kinsinger C, Kolker E, Kuiper M, Novere NL, Leebens-Mack J, Lewis SE, Lord P, Mallon AM, Marthandan N, Masuya H, McNally R, Mehrle A, Morrison N, Orchard S, Quackenbush J, Reecy JM, Robertson DG, Rocca-Serra P, Rodriguez H, Rosenfelder H, Santoyo-Lopez J, Scheuermann RH, Schober D, Smith B, Snape J, Stoeckert CJ, Tipton K, Jr, Sterk P, Untergasser A, Vandesompele J, Wiemann S. Promoting coherent minimum reporting guidelines for biological and biomedical investigations: the MIBBI project. Nat Biotechnol. 2008;26:889–96. doi: 10.1038/nbt.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kell DB. Progress being made on standards for use in data sharing. Nature. 2008;456:29. doi: 10.1038/456029c. [DOI] [PubMed] [Google Scholar]
- Kell DB, Mendes P. The markup is the model: reasoning about systems biology models in the Semantic Web era. J Theoret Biol. 2008;252:538–543. doi: 10.1016/j.jtbi.2007.10.023. [DOI] [PubMed] [Google Scholar]
- Surowiecki J. The wisdom of crowds: why the many are smarter than the few. Abacus, London; 2004. [Google Scholar]
- Anderson CM. The long tail: how endless choice is creating unlimited demand. Random House, London; 2006. [Google Scholar]
- Sunstein CR. Infotopia: how many minds produce knowledge. Oxfor University Press, Oxford; 2006. [Google Scholar]
- Tapscott D, Williams A. Wikinomics: how masscollaboration changes everything. New Paradigm; 2007. [Google Scholar]
- Leadbeater C. We-think. Profile Books, London; 2008. [Google Scholar]
- Shirky C. Here comes everybody. Allen Lane, London; 2008. [Google Scholar]
- Li P, Oinn T, Soiland S, Kell DB. Automated manipulation of systems biology models using libSBML within Taverna workflows. Bioinformatics. 2008;24:287–289. doi: 10.1093/bioinformatics/btm578. [DOI] [PubMed] [Google Scholar]
- Li P, Castrillo JI, Velarde G, Wassink I, Soiland-Reyes S, Owen S, Withers D, Oinn T, Pocock MR, Goble CA, Oliver SG, Kell DB. Performing statistical analyses on quantitative data in Taverna workflows: an example using R and maxdBrowse to identify differentially expressed genes from microarray data. BMC Bioinformatics. 2008;9:334. doi: 10.1186/1471-2105-9-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Roure D, Goble C. myExperiment – A Web 2.0 Virtual Research Environment. Proc International Workshop on Virtual Research Environments and Collaborative Work Environments, May 2007, Edinburgh, UK. 2007. http://eprints.ecs.soton.ac.uk/13961/
- Suber P. Open access to the scientific journal literature. J Biol. 2002;1:3. doi: 10.1186/1475-4924-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne PE, Fink JL, Gerstein M. Open access: taking full advantage of the content. PLoS Comput Biol. 2008;4:e1000037. doi: 10.1371/journal.pcbi.1000037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RK. Institutional Repositories: partnering with faculty to enhance scholarly communication. D-Lib Magazine. 2002;8 http://www.dlib.org/dlib/november02/johnson/11johnson.html [Google Scholar]
- Lynch CA. Institutional repositories: Essential infrastructure for scholarship in the digital age. Libr Acad. 2003;3:327–336. [Google Scholar]
- Lee E, Chuang HY, Kim JW, Ideker T, Lee D. Inferring pathway activity toward precise disease classification. PLoS Comput Biol. 2008;4:e1000217. doi: 10.1371/journal.pcbi.1000217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein LD. Integrating biological databases. Nat Rev Genet. 2003;4:337–45. doi: 10.1038/nrg1065. [DOI] [PubMed] [Google Scholar]
- Lord P, Bechhofer S, Wilkinson MD, Schiltz G, Gessler D, Hull D, Goble CA, Stein L. Applying Semantic Web services to bioinformatics: Experiences gained, lessons learnt. LNCS. 2004;3298:350–364. [Google Scholar]
- Curcin V, Ghanem M, Guo Y. Web services in the life sciences. Drug Discov Today. 2005;10:865–71. doi: 10.1016/S1359-6446(05)03481-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neerincx PB, Leunissen JA. Evolution of web services in bioinformatics. Brief Bioinform. 2005;6:178–88. doi: 10.1093/bib/6.2.178. [DOI] [PubMed] [Google Scholar]
- Le Novère N, Bornstein B, Broicher A, Courtot M, Donizelli M, Dharuri H, Li L, Sauro H, Schilstra M, Shapiro B, Snoep JL, Hucka M. BioModels Database: a free, centralized database of curated published, quantitative kinetic models of biochemical and cellular systems. Nucleic Acids Res. 2006;34:D689–91. doi: 10.1093/nar/gkj092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson L, Riuby S. RESTful web services. O'Reilly Sebastopol, CA; 2007. [Google Scholar]
- Fernández JM, Hoffmann R, Valencia A. iHOP web services. Nucleic Acids Res. 2007;35:W21–6. doi: 10.1093/nar/gkm298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein LD. Towards a cyberinfrastructure for the biological sciences: progress, visions and challenges. Nat Rev Genet. 2008;9:678–88. doi: 10.1038/nrg2414. [DOI] [PubMed] [Google Scholar]
- Williams AJ. A perspective of publicly accessible/open-access chemistry databases. Drug Discov Today. 2008;13:495–501. doi: 10.1016/j.drudis.2008.03.017. [DOI] [PubMed] [Google Scholar]
- Margulies M, Egholm M, Altman WE, Attiya S, Bader JS, Bemben LA, Berka J, Braverman MS, Chen YJ, Chen Z, Dewell SB, Du L, Fierro JM, Gomes XV, Godwin BC, He W, Helgesen S, Ho CH, Irzyk GP, Jando SC, Alenquer ML, Jarvie TP, Jirage KB, Kim JB, Knight JR, Lanza JR, Leamon JH, Lefkowitz SM, Lei M, Li J, Lohman KL, Lu H, Makhijani VB, McDade KE, McKenna MP, Myers EW, Nickerson E, Nobile JR, Plant R, Puc BP, Ronan MT, Roth GT, Sarkis GJ, Simons JF, Simpson JW, Srinivasan M, Tartaro KR, Tomasz A, Vogt KA, Volkmer GA, Wang SH, Wang Y, Weiner MP, Yu P, Begley RF, Rothberg JM. Genome sequencing in microfabricated high-density picolitre reactors. Nature. 2005;437:376–380. doi: 10.1038/nature03959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shendure J, Porreca GJ, Reppas NB, Lin X, McCutcheon JP, Rosenbaum AM, Wang MD, Zhang K, Mitra RD, Church GM. Accurate multiplex polony sequencing of an evolved bacterial genome. Science. 2005;309:1728–32. doi: 10.1126/science.1117389. [DOI] [PubMed] [Google Scholar]
- Bentley DR, Balasubramanian S, Swerdlow HP, Smith GP, Milton J, Brown CG, Hall KP, Evers DJ, Barnes CL, Bignell HR, Boutell JM, Bryant J, Carter RJ, Keira Cheetham R, Cox AJ, Ellis DJ, Flatbush MR, Gormley NA, Humphray SJ, Irving LJ, Karbelashvili MS, Kirk SM, Li H, Liu X, Maisinger KS, Murray LJ, Obradovic B, Ost T, Parkinson ML, Pratt MR, Rasolonjatovo IM, Reed MT, Rigatti R, Rodighiero C, Ross MT, Sabot A, Sankar SV, Scally A, Schroth GP, Smith ME, Smith VP, Spiridou A, Torrance PE, Tzonev SS, Vermaas EH, Walter K, Wu X, Zhang L, Alam MD, Anastasi C, Aniebo IC, Bailey DM, Bancarz IR, Banerjee S, Barbour SG, Baybayan PA, Benoit VA, Benson KF, Bevis C, Black PJ, Boodhun A, Brennan JS, Bridgham JA, Brown RC, Brown AA, Buermann DH, Bundu AA, Burrows JC, Carter NP, Castillo N, Chiara ECM, Chang S, Neil Cooley R, Crake NR, Dada OO, Diakoumakos KD, Dominguez-Fernandez B, Earnshaw DJ, Egbujor UC, Elmore DW, Etchin SS, Ewan MR, Fedurco M, Fraser LJ, Fuentes Fajardo KV, Scott Furey W, George D, Gietzen KJ, Goddard CP, Golda GS, Granieri PA, Green DE, Gustafson DL, Hansen NF, Harnish K, Haudenschild CD, Heyer NI, Hims MM, Ho JT, Horgan AM, et al. Accurate whole human genome sequencing using reversible terminator chemistry. Nature. 2008;456:53–9. doi: 10.1038/nature07517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris TD, Buzby PR, Babcock H, Beer E, Bowers J, Braslavsky I, Causey M, Colonell J, Dimeo J, Efcavitch JW, Giladi E, Gill J, Healy J, Jarosz M, Lapen D, Moulton K, Quake SR, Steinmann K, Thayer E, Tyurina A, Ward R, Weiss H, Xie Z. Single-molecule DNA sequencing of a viral genome. Science. 2008;320:106–9. doi: 10.1126/science.1150427. [DOI] [PubMed] [Google Scholar]
- Gupta PK. Single-molecule DNA sequencing technologies for future genomics research. Trends Biotechnol. 2008;26:602–11. doi: 10.1016/j.tibtech.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Kahvejian A, Quackenbush J, Thompson JF. What would you do if you could sequence everything? Nat Biotechnol. 2008;26:1125–33. doi: 10.1038/nbt1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerbino DR, Birney E. Velvet: algorithms for de novo short read assembly using de Bruijn graphs. Genome Res. 2008;18:821–9. doi: 10.1101/gr.074492.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlopoulos GA, Wegener A-L, Schneider R. A survey of visualization tools for biological network analysis. Bio-data mining. 2008;1:12. doi: 10.1186/1756-0381-1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garny A, Nickerson DP, Cooper J, Weber dos Santos R, Miller AK, McKeever S, Nielsen PM, Hunter PJ. CellML and associated tools and techniques. Philos Transact A Math Phys Eng Sci. 2008;366:3017–43. doi: 10.1098/rsta.2008.0094. [DOI] [PubMed] [Google Scholar]
- Li XJ, Brazhnik O, Kamal A, Guo D, Lee C, Hoops S, Mendes P. Databases and visualization for metabolomics. In: Harrigan GG, Goodacre R, editor. Metabolic profiling: its role in biomarker discovery and gene function analysis. Kluwer Academic Publishers, Boston; 2003. pp. 293–309. [Google Scholar]
- Jenkinson AM, Albrecht M, Birney E, Blankenburg H, Down T, Finn RD, Hermjakob H, Hubbard TJ, Jimenez RC, Jones P, Kähäri A, Kulesha E, Macias JR, Reeves GA, Prlić A. Integrating biological data – the Distributed Annotation System. BMC Bioinformatics. 2008;9:S3. doi: 10.1186/1471-2105-9-S8-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler J, Baumbach J, Taubert J, Specht M, Skusa A, Ruegg A, Rawlings C, Verrier P, Philippi S. Graph-based analysis and visualization of experimental results with ONDEX. Bioinformatics. 2006;22:1383–90. doi: 10.1093/bioinformatics/btl081. [DOI] [PubMed] [Google Scholar]
- Pettifer SR, Sinnott JR, Attwood TK. UTOPIA – user-friendly tools for operating informatics applications. Comp Func Genom. 2004;5:56–60. doi: 10.1002/cfg.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott P, Sinnott J, Thorne D, Pettifer S, Attwood T. An architecture for visualisation and interactive analysis of proteins. Proc 4th Int Conf Coordinated and Multiple Views in Exploratory Visualization (CMV06) 2006. pp. 55–68.
- Thorne D, Pettifer S, Attwood T. Integrating Abstract and Physical Molecular Model Interaction. In: Lever L, McDerby M, editor. EG UK Theory and Practice of Computer Graphics (2005) Eurographics Association; 2005. pp. 75–82. [Google Scholar]
- Pettifer S, Wolstencroft K, Alper P, Attwood T, Coletta A, Goble C, Li P, McDermott J, Marsh J, Oinn T, Sinnott J, Thorne D. myGrid and UTOPIA: an integrated approach to enacting and visualising in silico experiments in the life sciences. Proc Conf Data Integration in the Life Sciences. 2007. pp. 59–70.
- Neufeld EJ. Oral chelators deferasirox and deferiprone for transfusional iron overload in thalassemia major: new data, new questions. Blood. 2006;107:3436–41. doi: 10.1182/blood-2006-02-002394. [DOI] [PMC free article] [PubMed] [Google Scholar]


