Abstract
Rationale: Although there have been numerous studies on the development of allergen-induced inflammation, the mechanisms leading to resolution of inflammation remain poorly understood. This represents an important consideration because failure to resolve allergen driven inflammation potentially leads to irreversible airway remodeling, characteristic of chronic asthma.
Objectives: We investigated the resolution of allergic inflammation and identified the factors responsible.
Methods: BALB/c and C57BL/6 mice were sensitized to ovalbumin and challenged through the airways to induce allergic inflammation. Mice were analyzed at 24 hours and 7 days after the final challenge.
Measurements and Main Results: Airway hyperreactivity (AHR) and increased mucus production were present 7 days after the cessation of allergen challenge in BALB/c mice. Persisting AHR correlated with the continued presence of Th2 cells but not eosinophils in the lungs. The role of Th2 cells in maintaining AHR was confirmed using blocking antibodies against T1/ST2, IL-4, and IL-13 during the resolution period. Moreover, AHR in the “Th1 type” C57BL/6 mouse strain was resolved 1 week after allergen challenge, concomitant with clearance of Th2 cells from the lung. Expression of the T1/ST2 ligand, IL-33, also correlated with maintenance of AHR.
Conclusions: We have used blockade of Th2 function and strain differences to show for the first time that resolution of allergic inflammation and AHR may be dependent on the T1/ST2-IL-33 pathway and the presence of Th2 cells, suggesting they are necessary not only for the development of an allergic response but also for its maintenance.
Keywords: Th2 cells, IL-13, IL-4
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Although there have been numerous studies on the development of allergen-induced inflammation, the mechanisms leading to resolution of inflammation remain poorly understood.
What This Study Adds to the Field
We show that Th2 effector cells are associated with the maintenance of allergen-induced inflammation after the cessation of allergen challenge and that resolution of inflammation is dependent on disruption of IL-33–T1/ST2 signaling.
Pulmonary allergic inflammation can be induced in small rodents such as mice and is widely studied as an experimental model for human asthma. One of the most common murine models of allergen-induced airway inflammation involves sensitization of the mouse with a small dose of a protein allergen followed by allergen challenge of the airways to induce pulmonary inflammation (1). This characteristically leads to airway hyperreactivity (AHR), lung eosinophilia, mucus hypersecretion, and increased IgE levels, all features commonly associated with human allergic asthma (2). Sensitization-challenge models provide a system whereby the role of particular cell types or mediators can be studied and have proved useful in identifying important effector mechanisms in allergic pulmonary inflammation. Although the development of allergen-induced airway inflammation has been extensively studied, there have been few studies that address the mechanisms of resolution of allergic inflammation, although this may represent an important therapeutic target for asthma therapy. Eosinophils and Th2 cells have been shown to be important effector cells in human asthma and in murine models of allergen-induced pulmonary inflammation (3–8). However, the relative importance of each cell type in the resolution of inflammation is not clear.
In this study, we investigated resolution of the allergen-induced inflammation in a murine model. Using two different mouse strains, we showed that resolution of AHR and mucus hypersecretion correlated well with clearance of Th2 cells from the lung. Signaling through T1/ST2 has been shown to be crucial for Th2 function, and its ligand, IL-33, induces Th2 responses (8, 9). Blocking IL-33–T1/ST2 signaling using an antibody against T1/ST2 abrogated persisting AHR. Although Th2 cells are required for the development of allergic inflammation, these results suggest that they can contribute to the maintenance of an allergic response after continued allergen provocation via the IL-33–T1/ST2 axis.
METHODS
Induction of Allergen-induced Airway Inflammation
Allergen-induced airway inflammation was induced in female C57BL/6 and BALB/c mice (Harlan, Bicester, United Kingdom) as previously described (10). UK Home Office guidelines for animal welfare based on the Animals (Scientific Procedures) Act 1986 were strictly observed. Mice were killed by exsanguination under terminal anesthesia at 24 hours or 7 days after the final OVA challenge. In selected experiments, mice were treated intravenously with 25 μg anti-T1/ST2 antibody (3E10; a gift from Millennium Pharmaceutical, Cambridge, MA), intraperitoneally with 250 μg rat anti–IL-4 (clone 11B11, generated from hybridoma cell line in house), subcutaneously with 250 μg rabbit anti-mouse IL-13 (CA154_00582 a gift from UCB, Slough UK), with rat Ig (Stratech Scientific, Ltd, Luton, UK) intravenously or intraperitoneally as appropriate or rabbit Ig subcutaneously (101.4, a gift from UCB) on Days 25, 27, and 29. A schematic of this protocol is shown in Figure 1A.
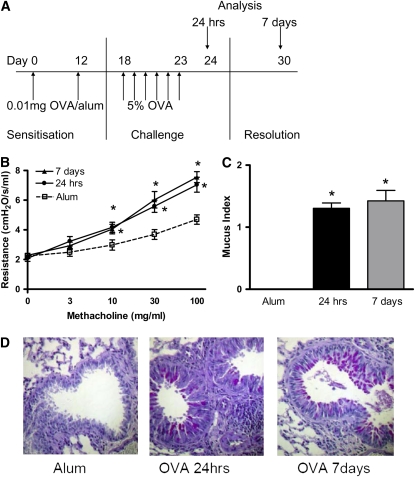
Figure 1.
(A) Airway hyperreactivity (AHR) and mucus production persist 1 week after allergen challenge in BALB/c mice. Airway inflammation was induced in BALB/c mice sensitized with ovalbumin (OVA) in alum on Days 0 and 12. One group of mice received alum instead of OVA/alum and served as the controls. All mice were subjected to daily aerosolized OVA challenge from Days 18 to 23 and were killed on Day 24, 24 hours after the final OVA challenge. A separate group of mice were killed on Day 30, 7 days after the final OVA challenge. (B) AHR was measured as lung resistance in BALB/c mice 24 hours and 7 days after the final OVA challenge in response to increasing concentrations of methacholine (3–100 mg/ml). Lungs were sectioned and stained with Periodic Acid-Schiff (PAS). (C) Sections were scored according to the criteria described in Methods. (D) Representative PAS-stained sections are shown for each group. Data are expressed as mean ± SEM (n = 6–16 mice/group from 1 to 3 independent experiments). *P < 0.05 compared with alum control mice.
Measurement of Airway Hyperreactivity
AHR was measured by changes in lung resistance in anesthetized and tracheostomized mice in response to increasing concentrations of aerosolised methacholine (3–100 mg/ml) using a Buxco system as previously described (12).
Blood Removal and Serum Isolation
Mice were bled under terminal anesthesia by cardiac puncture. Approximately 0.5 to 1.0 ml of blood was collected from each mouse. Blood was allowed to clot overnight at 4°C. Blood samples were centrifuged at 500 × g for 10 minutes. Serum was removed and stored at −20°C.
Cell Recovery
Leukocytes were isolated and quantified from the airway lumen (BAL) and lung tissue as previously described (11).
Assessment of Mucus Production
After removal from the animal, one lobe of lung was inflated with PBS. Lungs were fixed in 10% normal buffered formalin. Paraffin-embedded sections (4 μm) were stained with Periodic Acid-Schiff (PAS). Goblet cells were counted on PAS-stained sections using an arbitrary scoring system as previously described (12).
Staining of Leukocytes for Flow Cytometric Analysis
Antibodies for mouse CD4 and mouse T1/ST2 were purchased from BD Biosciences (Oxford, UK) and Morwell Diagnostics (Zurich, Switzerland). BAL and lung digest cells were stained as previously described (13). CD4+T1/ST2+ lymphocyte cell numbers were calculated as shown in Figure E1 in the online supplement.
Measurement of IgE Levels
Levels of total IgE were measured in serum by ELISA using paired antibodies according to the manufacturer's instructions (BD Biosciences). Levels of OVA-specific IgE were measured in serum by ELISA as described previously (14).
Cytokine and Chemokine Analysis
Cytokines were analyzed in BAL supernatants. Paired antibodies for murine IL-4, IFN-γ (BD Biosciences), IL-5 (Endogen, Buckingham UK), CCL11/eotaxin, CCL22/MDC, CCL17/TARC and CCL1/TCA-3 (R&D Systems, Abingdon, UK) were used in standardized sandwich ELISAs according to the manufacturer's protocol. ELISA kits to measure IL-13 and IL-33 were purchased from R&D Systems, and used according to the manufacturer's protocol.
Data Analysis
Data are expressed as mean ± SEM. Statistical significance between groups was tested using a Mann-Whitney U Test. A P value of < 0.05 was considered significant. Graph generation and statistical analysis was performed by using Prism v4.00 software (GraphPad, San Diego, CA).
RESULTS
Allergen-induced AHR and Mucus Hypersecretion Persist in BALB/c Mice after Cessation of Allergen Challenge
AHR is characteristic of the pulmonary response to inhaled allergen in sensitized mice, and therefore AHR was measured by direct measurements of lung resistance in anaethetized and tracheostomized mice 24 hours after the final serial OVA challenge in BALB/c mice. OVA-sensitized BALB/c mice had significant AHR compared with alum control mice when measured 24 hours after the last allergen challenge (Figure 1B). Furthermore, AHR persisted for 7 days after the final allergen challenge.
Increased mucus production occurs after allergen sensitization and challenge due to goblet cell hyperplasia. Analysis of PAS-stained sections revealed mucus hypersecretion in the lungs of OVA-sensitized BALB/c mice 24 hours after the final challenge (Figures 1C and 1D). Mucus production was similarly increased 7 days after the cessation of allergen challenge.
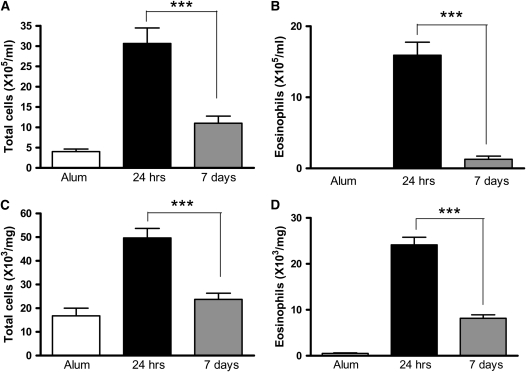
Lung Eosinophilia Is Resolved 1 Week after Cessation of Allergen Challenge
After allergen challenge, leukocytes are recruited to the lung in sensitized mice. Of these leukocytes, the eosinophil is the predominant cell type, and some studies correlate eosinophils with AHR and mucus secretion (5). We therefore determined total leukocyte and eosinophil numbers in the lungs of BALB/c mice 24 hours and 7 days after allergen challenge to establish if persistent eosinophilia could account for continuing AHR and goblet cell hyperplasia. The number and phenotype of the infiltrating leukocytes (Table E1A) were determined in the airway lumen by performing BAL. Although eosinophil numbers were substantially elevated in OVA-sensitized mice 24 hours after allergen challenge, numbers significantly declined 7 days after challenge (Figures 2A and 2B).
Figure 2.
Bronchoalveolar lavage (BAL) and lung leukocyte numbers decline after cessation of allergen challenge. (A and B) BAL and (C and D) lung tissue digest cells were isolated from BALB/c mice. Eosinophil numbers were determined by differential counts of BAL and lung digest cytospins. Data are expressed as mean ± SEM (n = 8 to 10 mice/group from two independent experiments). ***P < 0.001 comparing OVA-sensitized mice at 24 hours and 7 days after challenge.
Leukocyte numbers in the lung parenchyma (Table E1B) were determined by digesting the lung tissue with collagenase and DNase. Total leukocytes and eosinophils were also increased in the lung tissue in OVA-sensitized mice 24 hours after challenge, and, as in the BAL, had significantly decreased 7 days after challenge (Figures 2C and 2D).
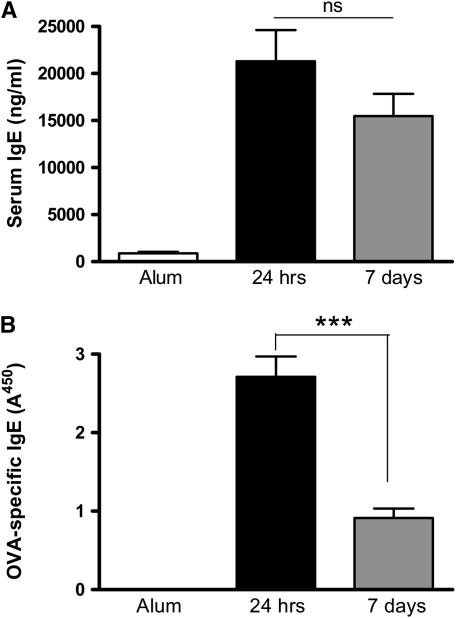
IgE Levels Persist after Cessation of Allergen Challenge although Allergen-Specific IgE Declines
Increased levels of IgE are characteristic of an allergic pulmonary response, and there is evidence that IgE may contribute to AHR in some settings (15, 16). We therefore determined serum levels of total IgE and OVA-specific IgE. Serum IgE and OVA-specific IgE were significantly elevated in OVA-sensitized mice 24 hours and 7 days after the final allergen challenge (Figures 3A and 3B). However, as seen with the eosinophil numbers, OVA-specific IgE levels declined between 24 hours and 7 days after cessation of allergen challenge, although total serum IgE remained similarly elevated at both time points.
Figure 3.
Peripheral and local humoral responses decline after cessation of allergen challenge. Levels of (A) total IgE and (B) OVA-specific IgE were measured in the serum of BALB/c mice by ELISA. Data are expressed as mean ± SEM (n = 8–10 mice/group from two independent experiments). ***P < 0.001 comparing OVA-sensitized mice at 24 hours and 7 days after challenge.
Th2 Cells and IL-4 Persist in the Airway Lumen and Lung Tissue of OVA-sensitized Mice 1 Week after Allergen Challenge
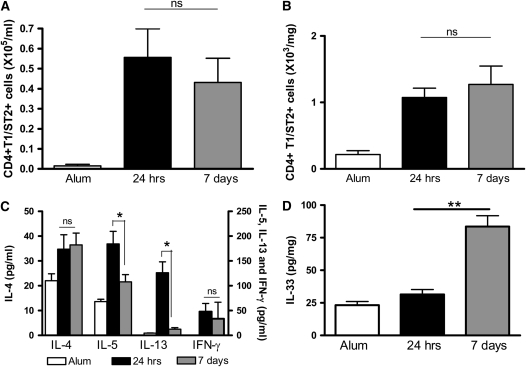
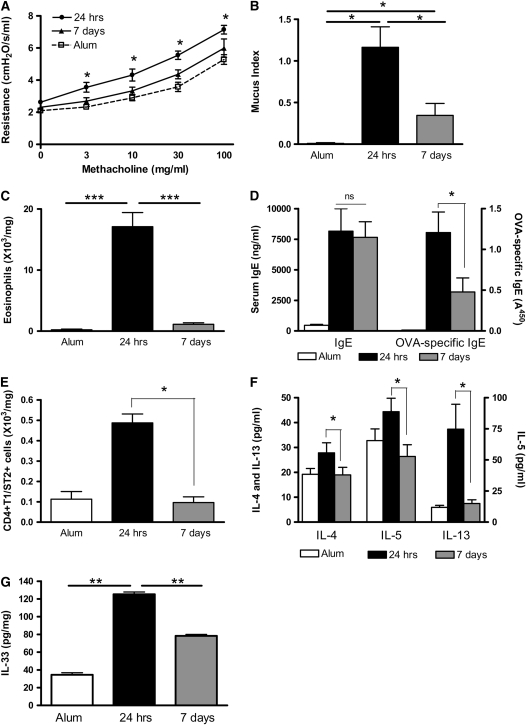
Th2 cells are critical effector cells in the pulmonary allergic response, and therefore the presence of Th2 cells in the lung could account for the persistence of AHR. Th2 cell numbers were determined in the BAL and lung tissue by co-staining cells with CD4 and the Th2 surrogate marker, T1/ST2 (13, 17, 18). There was an increase in Th2 cell numbers in the airway lumen and lung of OVA-sensitized BALB/c mice compared with unsensitized control mice 24 hours after the final OVA challenge (Figures 4A and 4B). Furthermore, Th2 cells remain similarly elevated 7 days after cessation of allergen challenge.
Figure 4.
T1/ST2+ Th2 cells and IL-4 persist in the airway lumen and lung of OVA-sensitized mice 1 week after allergen challenge. (A) BAL and (B) lung tissue digest cells were isolated from BALB/c mice. CD4+T1/ST2+ Th2 cell numbers were determined 24 hours or 7 days after the final OVA challenge by antibody staining and flow cytometric analysis. (C) IL-4, IL-5, and IL-13 levels were measured in BAL fluid by ELISA. (D) IL-33 levels were measured in lung tissue by ELISA. Values are expressed as mean ± SEM (n = 4–12 mice/group from two independent experiments).*P < 0.05 and **P < 0.01 comparing OVA-sensitized mice at 24 hours and 7 days after challenge.
We also determined cytokine levels in the BAL fluid (BALF). We found that the Th2 cytokines IL-4, IL-5, and IL-13 were elevated 24 hours after challenge (Figure 4C). One week after challenge, IL-5 and IL-13 levels had significantly declined, but, in contrast to IL-5 and IL-13 expression, IL-4 levels were maintained for at least 7 days after challenge (Figure 4C). Expression of the Th1 cytokine IFN-γ was determined and was not elevated in OVA-sensitized mice at either time point compared with alum control mice (data not shown). We measured levels of the T1/ST2 ligand IL-33 in lung tissue from allergic mice and found that levels were only elevated at 7 days after the final challenge (Figure 4D).
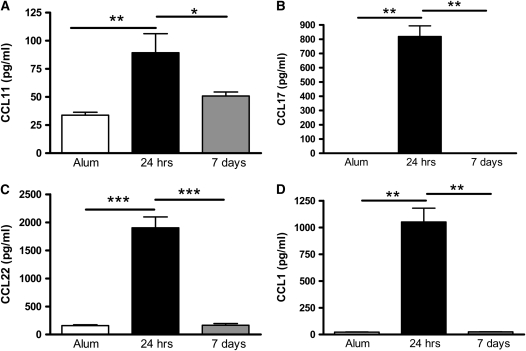
The chemokines CCL11/eotaxin, CCL17/TARC, CCL22/MDC, and CCL1/TCA-3 are potentially involved in Th2 cell recruitment during an allergic response (19–21); hence, they could be responsible for the persistence of Th2 cells in the lung 1 week after OVA challenge. CCL11, CCL17, CCL22, and CCL1 levels were increased in OVA-sensitized mice 24 hours after the final OVA challenge (Figures 5A–5D). However, chemokine levels had declined 7 days after cessation of allergen challenge, suggesting that Th2 cells are no longer being actively recruited to the lung by these chemokines (Figures 5A–5D).
Figure 5.
Expression of Th2-attracting chemokines declines 1 week after the final OVA challenge. Levels of (A) CCL11/eotaxin, (B) CCL17/TARC, (C) CCL22/MDC, and (D) CCL1/TCA-3 were determined in BAL supernatant by ELISA in BALB/c mice 24 hours and 7 days after the final allergen challenge. Data are expressed as mean ± SEM (n = 8–10 mice/group from two independent experiments). *P < 0.05; **P < 0.01; ***P < 0.001.
AHR Is Resolved 1 Week after Allergen Challenge in C57BL/6 Mice Concomitant with Clearance of Th2 Cells from the Lung
To establish that persistence of Th2 cells in OVA-sensitized BALB/c mice leads to continued AHR, we induced AHR and inflammation in C57BL/6 mice and compared resolution in this strain. C57BL/6 mice are considered to be more prone to mount Th1 responses compared with the BALB/c, which tends to develop more Th2-biased responses (22). We found that OVA-sensitized C57BL/6 mice developed significant AHR 24 hours after allergen challenge (Figure 6A). However, in contrast to the BALB/c mouse, AHR in C57BL/6 mice had returned to baseline values 7 days after challenge. Mucus production was increased in the airways of OVA-sensitized mice 24 hours and 1 week after challenge, although, unlike in the BALB/c mice, it declined between 24 hours and 7 days (Figure 6B). Eosinophil numbers were increased in the BAL and lung tissue of OVA-sensitized C57BL/6 mice 24 hours after challenge but reduced by 7 days (Figure 6C; BAL data not shown). IgE and OVA-specific IgE levels were elevated in the serum of OVA-sensitized C57BL/6 mice 24 hours and 7 days after allergen challenge (Figure 6D). IgE levels remained similarly elevated 7 days after cessation of allergen challenge, although OVA-specific IgE levels had begun to decrease by this point (Figure 6D). We also determined numbers of T1/ST2+ T cells in the BAL and lung tissue of C57BL/6 mice and found that, although numbers were increased 24 hours after challenge, they declined by 7 days after allergen challenge down to baseline. This correlated with decreased AHR (Figure 6E; BAL data not shown). Levels of the Th2 cytokines IL-4, IL-5, and IL-13 were also increased in BALF 24 hours after OVA challenge but were significantly decreased after 7 days after challenge (Figure 6F). There was also a significant decrease in levels of the T1/ST2 ligand IL-33 at 7 days after allergen challenge compared with 24 hours (Figure 6G).
Figure 6.
AHR and Th2 cells do not persist in C57BL/6 mice 1 week after challenge. C57BL/6 mice were sensitized and challenged with OVA. (A) AHR was assessed by measuring lung resistance in response to increasing concentrations of methacholine (3–100 mg/ml). (B) Mucus secretion in PAS-stained lung sections were scored according to the criteria described in Methods. (C) Lung tissue eosinophils were determined by differential counts of lung digest cytospins. (D) Total serum IgE and OVA-specific IgE levels were measured by ELISA. (E) Lung tissue CD4+T1/ST2+ Th2 cells were quantified by antibody staining and flow cytometric analysis. (F) Th2 cytokine levels in BAL fluid and (G) IL-33 levels in lung tissue were measured by ELISA. Data are expressed as mean ± SEM (n = 8 to 10 mice/group from two independent experiments). *P < 0.05 and **P < 0.01 compared with alum control mice (A and B) or comparing OVA-sensitized mice at 24 hours and 7 days after challenge (C–F).
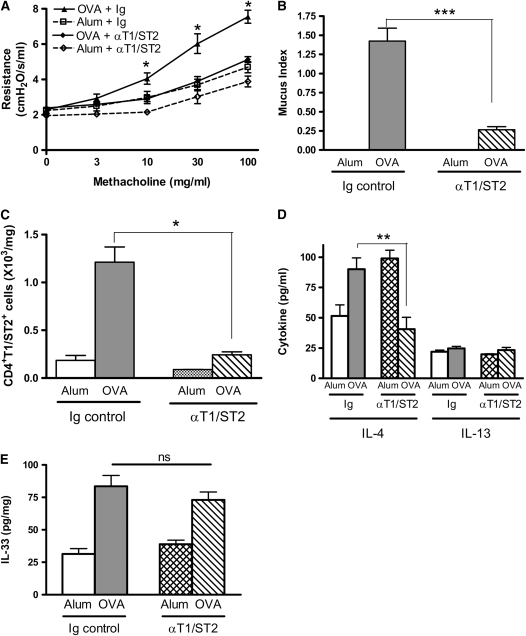
Persistence of AHR Is Abrogated by Blockade of T1/ST2
To define a functional role for T1/ST2+ T cells in the persistence of AHR, we administered a monoclonal antibody against T1/ST2 (8, 17) to BALB/c mice during the resolution phase of the model only. This antibody has previously been shown to abrogate Th2 cell function in vitro and in vivo. Anti-T1/ST2 antibody treatment during the postchallenge period reduced AHR to levels indistinguishable from alum control mice (Figures 7A and 7B). Moreover, although mucus production as assessed on PAS-stained sections was observed in OVA-sensitized anti-T1/ST2 antibody treated mice compared with alum control mice, it was significantly decreased in comparison to OVA-sensitized Ig-treated mice (Figure 7C). In addition, T1/ST2+ T cells numbers present in the BAL and lung tissue (Figure 7D and data not shown) were significantly attenuated by anti-T1/ST2 antibody. Because this is not a depleting antibody (8), this suggests that the Th2 cells may contribute toward their continued presence in the lung in the BALB/c mice. We therefore measured lung expression of IL-4 because this cytokine was elevated 1 week after allergen challenge and found that anti-T1/ST2 antibody significantly reduced the expression of IL-4 (Figure 7E). In contrast, IL-13 levels were low and unchanged by anti-T1/ST2 antibody treatment. Overall, these data emphasize the critical role played by T1/ST2+ T cells in the persistence of AHR after cessation of allergen challenge and suggest that they contribute to their own maintenance through release of IL-4.
Figure 7.
Blockade of T1/ST2–IL-33 pathway reduces persistent AHR and mucus production in BALB/c mice. BALB/c mice were treated with anti-T1/ST2 antibody or rat Ig control antibody during the resolution phase (Days 25, 27, and 30). Airway inflammation and AHR were measured 7 days after the final OVA challenge. (A) AHR was assessed by measuring lung resistance in response to increasing concentrations of methacholine (3–100 mg/ml). (B) Mucus secretion was assessed in PAS-stained lung sections. (C) CD4+T1/ST2+ Th2 cell numbers were determined by antibody staining and flow cytometric analysis. (D) Th2 cytokine expression in BAL supernatant and (E) IL-33 in lung tissue were determined by ELISA. Data are expressed as mean ± SEM (n = 4–6 mice/group). *P < 0.05, **P < 0.01, and ***P < 0.001 compared with alum control mice (A) or comparing OVA-sensitized mice treated with anti-T1/ST2 antibody with those treated with control Ig (B–E).
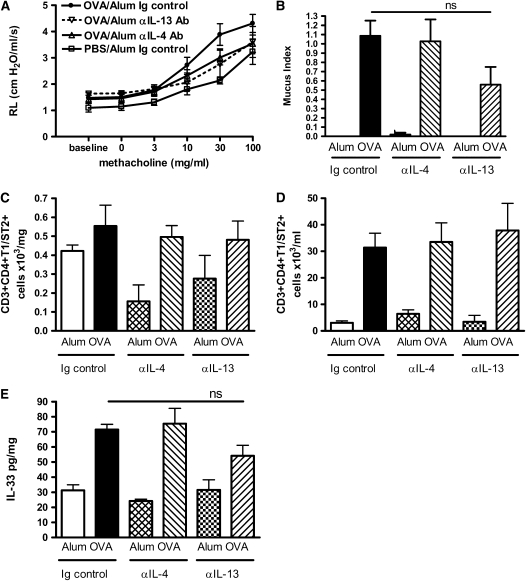
Persistence of AHR Is Partially Blocked by Neutralization of IL-4 or IL-13
To define the role of the classical Th2 cytokines IL-4 and IL-13 in the persistence of AHR, we administered monoclonal antibodies specific for IL-4 or IL-13 to BALB/c mice during the resolution phase of the model only. We found that neither antibody resulted in a complete abrogation of AHR, but both treatments substantially reduced airway resistance, with anti–IL-13 antibody treatment reaching statistical significance (Figure 8A). Anti–IL-4 treatment had no effect on mucus production, but antibodies to IL-13 resulted in a decrease in the number of mucus positive cells (Figure 8B). Neither treatment had an effect on the levels of T1/ST2+ T cells in the lung or the BAL or on expression of Th2 cytokines in the BALF (Figures 8C and 8D and data not shown). Neither treatment had a significant effect on the level of IL-33 detected in the lung tissue (Figure 8E).
Figure 8.
Blockade of IL-4 or IL-13 leads to partial resolution of AHR and mucus production. BALB/c mice were treated with anti–IL-4 antibody, anti–IL-13 antibody, or Ig control antibody during the resolution phase (Days 25, 27, and 30). Airway inflammation and AHR were measured 7 days after the final OVA challenge. (A) AHR was assessed by measuring lung resistance in response to increasing concentrations of methacholine (3–100 mg/ml). (B) Mucus secretion was assessed in PAS-stained lung sections. CD4+T1/ST2+ Th2 cell numbers in (C) lung tissue and (D) BAL were determined by antibody staining and flow cytometric analysis. (E) IL-33 in lung tissue were determined by ELISA. Data are expressed as mean ± SEM (n = 4–7 mice/group). *P < 0.05 and **P < 0.01 comparing anti–IL-13 antibody–treated OVA-challenged mice with Ig control–treated OVA-challenged mice. No statistically significant differences were measured between the OVA-challenged Ig control group compared with anti–IL-4 antibody–treated groups.
DISCUSSION
We have demonstrated that allergen-induced AHR and mucus production persist in BALB/c but not C57BL/6 mice for up to 1 week after the cessation of allergen challenge. Our data suggest that the persistence of AHR in BALB/c mice is due to the continued presence of T1/ST2+ T cells in the lung, implicating these cells as key mediators in the maintenance of AHR
In this study, we tried to address the factors responsible for the persistence of AHR after cessation of allergen challenge. Previously it has been demonstrated that T1/ST2+ T cells are critical for the development of AHR (8). AHR may also be provoked by the transfer of in vitro polarized Th2 cells into naive mice, followed by allergen challenge, showing that they are sufficient to induce AHR (7, 19). Our data also suggest that T1/ST2+ T cells are responsible for the persistence of AHR in BALB/c mice because by 7 days after challenge, T1/ST2+ T cells are still present in the lung at similar levels as at 24 hours after challenge. In contrast, when C57BL/6 mice are used, AHR and lung T1/ST2+ T cells are absent 1 week after allergen challenge, strongly implying a causal link between Th2 cells and AHR. Furthermore, when Th2 cell function is blocked in BALB/c mice during the resolution phase by administration of anti-T1/ST2 antibody, AHR is abrogated. In contrast, neutralization of IL-4 or IL-13 during the resolution period gave only a partial inhibition of AHR. It has previously been shown that antibodies to T1/ST2 can inhibit Th2 function in vitro and in vivo (8, 17, 23, 24), and, although our results differ from some studies (25, 26), they use very different disease models and mouse strains.
The ligand for T1/ST2 was recently described as IL-33, a member of the IL-1 family of inflammatory cytokines (9). To determine the mechanism by which T1/ST2+ cells contribute to the maintenance of AHR, we determined levels of the IL-33 in lungs from BALB/c and C57BL/6 mice at the peak of inflammation at 24 hours and 7 days after the final allergen challenge. C57BL/6 mice had high levels of IL-33 at the peak of inflammation, thereby showing for the first time that IL-33 protein expression is increased in the lung in an allergic setting. However, IL-33 levels were not maintained in the absence of continued allergen challenge in this strain. In direct contrast, BALB/c mice had significantly increased levels of IL-33 only on Day 7, and not at the peak of inflammation. Taken together with the kinetics of T1/ST2+ T cell clearance, our data implicate the T1/ST2–IL-33 signaling axis in the maintenance of AHR, where elevated levels of IL-33 and increased numbers of T1/ST2+ T cells are needed for AHR. In C57BL/6 mice, AHR declines when IL-33 and T1/ST2+ T cell numbers decline. In contrast, in the BALB/c mouse, there are still significant numbers of T1/ST2+ T cells and heightened IL-33 levels resulting in maintenance of AHR. Blockade of T1/ST2 results in a decrease in AHR, even though there is no change in IL-33 levels, because it blocks signaling between ligand and receptor. Other studies have shown that intranasal instillation of IL-33 induces AHR (27), but this is the first time that this cytokine-receptor axis has been implicated in the maintenance of established AHR during an in vivo allergic response.
Although eosinophils have previously been shown to be important in the development of AHR (5), our data suggest that they are not involved in the persistence of AHR after cessation of allergen challenge because lung eosinophil numbers have declined considerably between 24 hours and 7 days after OVA challenge. Although there are still very low levels of eosinophils in the lungs of BALB/c mice 7 days after cessation of allergen challenge, there are also low levels of eosinophils in the C57BL/6 mice, who have resolved AHR. In contrast, Th2 cells are completely cleared from the lungs of C57BL/6 mice 7 days after allergen challenge. Moreover, other studies dispute the involvement of eosinophils in AHR (28, 4, 29). We also suggest that it is not IgE that is responsible for the persistent AHR. Although IgE and OVA-specific IgE are still present in the serum of BALB/c mice 7 days after allergen challenge, levels were significantly decreased. Furthermore, C57BL/6 mice that have no AHR at this time still have elevated IgE levels compared with control mice. Also, a number of studies suggest that IgE is not required for AHR in models using alum as an adjuvant, such as this one (30, 14). Taken together, this evidence implicates Th2 cells rather than eosinophils or IgE in the persistence of AHR in OVA-sensitized BALB/c mice.
Th2 cells are thought to contribute to AHR by the release of cytokines. In particular, IL-13 has been shown to be necessary and sufficient to cause AHR (12, 31, 32). However, although IL-13 was substantially increased 24 hours after challenge in BALB/c mice, levels had declined considerably by 7 days and were unaffected by anti-T1/ST2 antibody treatment. Similarly, blockade of IL-13 during the resolution phase partially abrogated AHR, suggesting that IL-13 is required but not sufficient for the maintenance of AHR after allergen challenge. This is in keeping with a previous study in which neutralization of IL-13 could suppress the development of AHR but could not reverse persisting AHR (33). In contrast, IL-4 is still elevated at Day 7, but, like IL-13, blockade of IL-4 during the resolution phase showed that IL-4 is not alone sufficient to maintain AHR to Day 7. Our data also question Th2 cells as major sources of IL-5 and IL-13 in the lung during an allergic response. Indeed, the numbers of T1/ST2+ T cell numbers in the lung of OVA-sensitized BALB/c mice is similar 24 hours and 7 days after challenge, yet, only IL-4 of the Th2 cytokines remains elevated. It is known that eosinophils can release IL-5 and IL-13, and it may be that these cells and not Th2 cells are the major source of IL-5 and IL-13 during an allergic response in this model (34).
In these studies, we found that blocking T1/ST2 was more effective than blocking individual Th2 cytokines. This could be because more than one cytokine is involved in the maintenance of AHR or due to the expression of T1/ST2 on cell types other than Th2 cells. T1/ST2 is also expressed on mast cells (35), although we would argue against a mast cell role in this setting because development of inflammation and AHR has been shown to be mast cell independent in the model (36). IL-33, the ligand for T1/ST2 has also been shown to activate NK cells, NKT cells, eosinophils, and basophils (37, 38) in vitro, and, although this has not been confirmed in vivo, we cannot rule out a role for one of these cell types in maintaining AHR. However, the presence of a CD4+ T1/ST2-expressing population in the lungs of BALB/c mice, which is absent in C57BL/6 mice 1 week after cessation of allergen challenge, strongly suggests that it is the Th2 cell that is causing AHR to persist.
We attempted to address the reason that Th2 cells persist in the lungs of BALB/c mice after cessation of allergen challenge. During an immune response, Th2 cells are recruited to the lung along chemokine gradients. The chemokines CCL11/eotaxin, CCL17/TARC, CCL22/MDC, and CCL1/TCA-3 have been suggested to be important in recruitment of Th2 cells (19–21). We therefore determined levels of these chemokines in the lung 24 hours and 7 days after allergen challenge. Although CCL11, CCL17, CCL22, and CCL1 were increased 24 hours after challenge, expression had declined to baseline after 7 days. This suggests that the Th2 cells are no longer being recruited to the lung by chemokines at this point. However, we found persisting IL-4 levels 7 days after allergen challenge, and increased lung levels of IL-4 may promote Th2 cell survival within the lung tissue itself because this cytokine is important for driving Th2 cell development (39–41). We also found that blockade of Th2 function reduced numbers of Th2 cells found in the lung and expression of IL-4 after 7 days, suggesting that Th2 cells can contribute toward their maintenance within the lung and that this may occur through the release of cytokines such as IL-4.
In the present study, we demonstrate that persistence of Th2 cells in the lung leads to continuing AHR and mucus hypersecretion 1 week after allergen challenge. We have shown for the first time that genetic differences can affect the kinetics of the resolution of allergic inflammation. In contrast, blockade of IL-4 or IL-13 during the resolution phase only partially affected the resolution of AHR and mucus production and had no correlation with Th2 cells. Little is known about the mechanisms by which allergic inflammation is resolved, although IL-3 has previously been implicated in the prevention of apoptosis of infiltrating leukocytes in the lung tissue, leading to persisting AHR (14). Also, matrix metalloproteinases (MMPs) have been implicated as a critical mediators of resolution of allergic inflammation in the lung (11). Clearance of inflammatory cells is severely delayed in mice lacking MMP-2, leading to increased mortality (42). Increased accumulation of inflammatory cells in the lung parenchyma was associated with an increase in levels of Th2 cytokines. We have also shown previously that MMP-9–deficient mice display increased AHR after allergen challenge, and this was still present 1 week after allergen challenge, although it had declined in wild-type control mice (10). In keeping with the current study, Th2 cells but not eosinophils also persisted in the lungs of MMP-9–deficient mice.
In comparing allergen-induced inflammation and its resolution in C57BL/6 and BALB/c mice, we have uncovered some important differences in resolution of the Th2 response due to genetic background. Although several studies have examined the strain differences in acute allergen-induced inflammation and AHR (43–45), this is the first report that compares resolution of inflammation in two murine strains. This is an important consideration in choosing mouse strain and timing after allergen challenge of analysis of pulmonary inflammation and AHR. We have shown that sensitized C57BL/6 mice develop considerable and significant Th2 type inflammation and AHR in response to allergen challenge but that these responses are not maintained to the degree that they are in BALB/c mice. The kinetics of IL-33 production in the lung varied considerably between the two strains of mice and, together with T1/ST2+ T cells, correlated with the maintenance of AHR. Further study of these genetic differences could therefore enhance our understanding of the molecular pathways involved in resolving allergic inflammation. The data presented here suggest that genetic background may influence the kinetics of the allergic response, and therefore the timing of analysis in a murine model may influence the response measured. Moreover, our results support the increasing evidence that Th2 cells rather than eosinophils are the prinicipal mediators of AHR, again emphasizing their importance as critical effectors of the allergic response and suggesting that modulation of Th2 cell function via the T1/ST2-IL-33 axis could be of therapeutic benefit for patients with allergies.
Supplementary Material
Acknowledgments
The authors thank Lorraine Lawrence for histological sectioning and staining.
Supported by The Wellcome Trust (ref. #057704) (to C.M.L.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200805-666OC on January 29, 2009
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Lloyd CM, Gonzalo JA, Coyle AJ, Gutierrez-Ramos JC. Mouse models of allergic airway disease. Adv Immunol 2001;77:263–295. [DOI] [PubMed] [Google Scholar]
- 2.Bousquet J, Jeffery PK, Busse WW, Johnson M, Vignola AM. Asthma: from bronchoconstriction to airways inflammation and remodeling. Am J Respir Crit Care Med 2000;161:1720–1745. [DOI] [PubMed] [Google Scholar]
- 3.Kay AB. The role of eosinophils in the pathogenesis of asthma. Trends Mol Med 2005;11:148–152. [DOI] [PubMed] [Google Scholar]
- 4.Humbles AA, Lloyd CM, McMillan SJ, Friend DS, Xanthou G, McKenna E, Ghiran S, Gerard NP, Yu C, Orkin SH, et al. A critical role for eosinophils in allergic airways remodeling. Science 2004;305:1776–1779. [DOI] [PubMed] [Google Scholar]
- 5.Lee JJ, Dimina D, Macias MP, Ochkur SI, McGarry MP, O'Neill KR, Protheroe C, Pero R, Nguyen T, Cormier SA, et al. Defining a link with asthma in mice congenitally deficient in eosinophils. Science 2004;305:1773–1776. [DOI] [PubMed] [Google Scholar]
- 6.Robinson DS, Hamid Q, Ying S, Tsicopoulos A, Barkans J, Bentley AM, Corrigan C, Durham SR, Kay AB. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med 1992;326:298–304. [DOI] [PubMed] [Google Scholar]
- 7.Cohn L, Homer RJ, Marinov A, Rankin J, Bottomly K. Induction of airway mucus production by T helper 2 (Th2) cells: a critical role for interleukin 4 in cell recruitment but not mucus production. J Exp Med 1997;186:1737–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coyle AJ, Lloyd C, Tian J, Nguyen T, Erikkson C, Wang L, Ottoson P, Persson P, Delaney T, Lehar S, et al. Crucial role of the interleukin 1 receptor family member T1/ST2 in T helper cell type 2 mediated lung mucosal immune responses. J Exp Med 1999;190:895–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 2005;23:479–490. [DOI] [PubMed] [Google Scholar]
- 10.McMillan SJ, Bishop B, Townsend MJ, McKenzie AN, Lloyd CM. The absence of interleukin 9 does not affect the development of allergen-induced pulmonary inflammation nor airway hyperreactivity. J Exp Med 2002;195:51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McMillan SJ, Kearley J, Campbell JD, Zhu XW, Larbi KY, Shipley JM, Senior RM, Nourshargh S, Lloyd CM. Matrix metalloproteinase-9 deficiency results in enhanced allergen-induced airway inflammation. J Immunol 2004;172:2586–2594. [DOI] [PubMed] [Google Scholar]
- 12.Grunig G, Warnock M, Wakil AE, Venkayya R, Brombacher F, Rennick DM, Sheppard D, Mohrs M, Donaldson DD, Locksley RM, et al. Requirement for IL-13 Independently of IL-4 in Experimental Asthma. Science 1998;282:2261–2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kearley J, Barker JE, Robinson DS, Lloyd CM. Resolution of airway inflammation and hyperreactivity after in vivo transfer of CD4+CD25+ regulatory T cells is interleukin 10 dependent. J Exp Med 2005;202:1539–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lloyd CM, Gonzalo JA, Nguyen T, Delaney T, Tian J, Oettgen H, Coyle AJ, Gutierrez-Ramos JC. Resolution of bronchial hyperresponsiveness and pulmonary inflammation is associated with IL-3 and tissue leukocyte apoptosis. J Immunol 2001;166:2033–2040. [DOI] [PubMed] [Google Scholar]
- 15.Oshiba A, Hamelmann E, Takeda K, Bradley KL, Loader JE, Larsen GL, Gelfand EW. Passive transfer of immediate hypersensitivity and airway hyperresponsiveness by allergen-specific immunoglobulin (Ig) E and IgG1 in mice. J Clin Invest 1996;97:1398–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamelmann E, Tadeda K, Oshiba A, Gelfand EW. Role of IgE in the development of allergic airway inflammation and airway hyperresponsiveness: a murine model. Allergy 1999;54:297–305. [DOI] [PubMed] [Google Scholar]
- 17.Lohning M, Stroehmann A, Coyle AJ, Grogan JL, Lin S, Gutierrez-Ramos JC, Levinson D, Radbruch A, Kamradt T. T1/ST2 is preferentially expressed on murine Th2 cells, independent of interleukin 4, interleukin 5, and interleukin 10, and important for Th2 effector function. Proc Natl Acad Sci USA 1998;95:6930–6935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bishop B, Lloyd CM. CC chemokine ligand 1 promotes recruitment of eosinophils but not Th2 cells during the development of allergic airways disease. J Immunol 2003;170:4810–4817. [DOI] [PubMed] [Google Scholar]
- 19.Lloyd CM, Delaney T, Nguyen T, Tian J, Martinez A, Coyle AJ, Gutierrez-Ramos JC. CC chemokine receptor (CCR)3/eotaxin is followed by CCR4/monocyte-derived chemokine in mediating pulmonary t helper lymphocyte type 2 recruitment after serial antigen challenge in vivo. J Exp Med 2000;191:265–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawasaki S, Takizawa H, Yoneyama H, Nakayama T, Fujisawa R, Izumizaki M, Imai T, Yoshie O, Homma I, Yamamoto K, et al. Intervention of thymus and activation-regulated chemokine attenuates the development of allergic airway inflammation and hyperresponsiveness in mice. J Immunol 2001;166:2055–2062. [DOI] [PubMed] [Google Scholar]
- 21.Chensue SW, Lukacs NW, Yang TY, Shang X, Frait KA, Kunkel SL, Kung T, Wiekowski MT, Hedrick JA, Cook DN, et al. Aberrant in vivo T helper type 2 cell response and impaired eosinophil recruitment in CC chemokine receptor 8 knockout mice. J Exp Med 2001;193:573–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heinzel FP, Sadick MD, Holaday BJ, Coffman RL, Locksley RM. Reciprocal expression of interferon gamma or interleukin 4 during the resolution or progression of murine leishmaniasis: evidence for expansion of distinct helper T cell subsets. J Exp Med 1989;169:59–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hayakawa H, Hayakawa M, Kume A, Tominaga S. Soluble ST2 blocks interleukin-33 signaling in allergic airway inflammation. J Biol Chem 2007;282:26369–26380. [DOI] [PubMed] [Google Scholar]
- 24.Kurowska-Stolarska M, Kewin P, Murphy G, Russo RC, Stolarski B, Garcia CC, Komai-Koma M, Pitman N, Li Y, McKenzie AN, et al. IL-33 induces antigen-specific IL-5+ T cells and promotes allergic-induced airway inflammation independent of IL-4. J Immunol 2008;181:4780–4790. [DOI] [PubMed] [Google Scholar]
- 25.Mangan NE, Dasvarma A, McKenzie AN, Fallon PG. T1/ST2 expression on Th2 cells negatively regulates allergic pulmonary inflammation. Eur J Immunol 2007;37:1302–1312. [DOI] [PubMed] [Google Scholar]
- 26.Kropf P, Herath S, Klemenz R, Muller I. Signaling through the T1/ST2 molecule is not necessary for Th2 differentiation but is important for the regulation of type 1 responses in nonhealing Leishmania major infection. Infect Immun 2003;71:1961–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kondo Y, Yoshimoto T, Yasuda K, Futatsugi-Yumikura S, Morimoto M, Hayashi N, Hoshino T, Fujimoto J, Nakanishi K. Administration of IL-33 induces airway hyperresponsiveness and goblet cell hyperplasia in the lungs in the absence of adaptive immune system. Int Immunol 2008;20:791–800. [DOI] [PubMed] [Google Scholar]
- 28.Corry DB, Folkesson HG, Warnock ML, Erle DJ, Matthay MA, Wiener-Kronish JP, Locksley RM. Interleukin 4, but not interleukin 5 or eosinophils, is required in a murine model of acute airway hyperreactivity. J Exp Med 1996;183:109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Siegle JS, Hansbro N, Herbert C, Yang M, Foster PS, Kumar RK. Airway hyperreactivity in exacerbation of chronic asthma is independent of eosinophilic inflammation. Am J Respir Cell Mol Biol 2006;35:565–570. [DOI] [PubMed] [Google Scholar]
- 30.Hamelmann E, Cieslewicz G, Ishizuka T, Schwarze J, Ishizuka T, Joetham A, Heusser CH, Gelfand EW. Anti-interleukin 5 but not anti-IgE prevents airway inflammation and airway hyperresponsiveness. Am J Respir Crit Care Med 1999;160:934–941. [DOI] [PubMed] [Google Scholar]
- 31.Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, Donaldson DD. Interleukin-13: central mediator of allergic asthma. Science 1998;282:2258–2261. [DOI] [PubMed] [Google Scholar]
- 32.Walter DM, McIntire JJ, Berry G, McKenzie ANJ, Donaldson DD, DeKruyff RH, Umetsu DT. Critical role for IL-13 in the development of allergen-induced airway hyperreactivity. J Immunol 2001;167:4668–4675. [DOI] [PubMed] [Google Scholar]
- 33.Leigh R, Ellis R, Wattie J, Donaldson DD, Inman MD. Is interleukin-13 critical in maintaining airway hyperresposiveness in allergen-challenged mice? Am J Respir Crit Care Med 2004;170:851–856. [DOI] [PubMed] [Google Scholar]
- 34.Rothenberg ME, Hogan SP. The Eosinophil. Annu Rev Immunol 2006;24:147–174. [DOI] [PubMed] [Google Scholar]
- 35.Moritz DR, Rodewald HR, Gheyselinck J, Klemenz R. The IL-1 receptor-related T1 antigen is expressed on immature and mature mast cells and on fetal blood mast cell progenitors. J Immunol 1998;161:4866–4874. [PubMed] [Google Scholar]
- 36.Williams CM, Galli SJ. The diverse potential effector and immunoregulatory roles of mast cells in allergic disease. J Allergy Clin Immunol 2000;105:847–859. [DOI] [PubMed] [Google Scholar]
- 37.Pecaric-Petkovic T, Didichenko SA, Kaempfer S, Spiegl N, Dahinden CA. Human basophils and eosinophils are the direct target leukocytes of the novel IL-1-family member IL-33. Blood 2009;113:1396–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smithgall MD, Comeau MR, Yoon BR, Kaufman D, Armitage R, Smith DE. IL-33 amplifies both Th1- and Th2-type responses through its activity on human basophils, allergen-reactive Th2 cells, iNKT and NK cells. Int Immunol 2008;20:1019–1030. [DOI] [PubMed] [Google Scholar]
- 39.Swain SL, Weinberg AD, English M, Huston G. IL-4 directs the development of Th2-like helper effectors. J Immunol 1990;145:3796–3806. [PubMed] [Google Scholar]
- 40.Kopf M, Gros GL, Bachmann M, Lamers MC, Bluethmann H, Kohler G. Disruption of the murine IL-4 gene blocks Th2 cytokine responses. Nature 1993;362:245–248. [DOI] [PubMed] [Google Scholar]
- 41.Constant SL, Brogdon JL, Piggott DA, Herrick CA, Visintin I, Ruddle NH, Bottomly K. Resident lung antigen-presenting cells have the capacity to promote Th2 T cell differentiation in situ. J Clin Invest 2002;110:1441–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Corry DB, Rishi K, Kanellis J, Kiss A, Song Lz LZ, Xu J, Feng L, Werb Z, Kheradmand F. Decreased allergic lung inflammatory cell egression and increased susceptibility to asphyxiation in MMP2-deficiency. Nat Immunol 2002;3:347–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Whitehead GS, Walker JKL, Berman KG, Foster WM, Schwartz DA. Allergen-induced airway disease is mouse strain dependent. Am J Physiol Lung Cell Mol Physiol 2003;285:L32–L42. [DOI] [PubMed] [Google Scholar]
- 44.Brewer JP, Kisselgof AB, Martin TR. Genetic variability in pulmonary physiological, cellular, and antibody responses to antigen in mice. Am J Respir Crit Care Med 1999;160:1150–1156. [DOI] [PubMed] [Google Scholar]
- 45.Wills-Karp M, Ewart S. The genetics of allergen-induced airway hyperresponsiveness in mice. Am J Respir Crit Care Med 1997;156:89S–96S. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.