Abstract
Understanding the transport of hydrophilic proteins across biological membranes continues to be an important undertaking. The general secretory (Sec) pathway in Escherichia coli transports the majority of E. coli proteins from their point of synthesis in the cytoplasm to their sites of final localization, associating sequentially with a number of protein components of the transport machinery. The targeting signals for these substrates must be discriminated from those of proteins transported via other pathways. While targeting signals for each route have common overall characteristics, individual signal peptides vary greatly in their amino acid sequences. How do these diverse signals interact specifically with the proteins that comprise the appropriate transport machinery and, at the same time, avoid targeting to an alternate route? The recent publication of the crystal structures of components of the Sec transport machinery now allows a more thorough consideration of the interactions of signal sequences with these components.
The general secretory (Sec) pathway in Escherichia coli transports the majority of exported E. coli proteins from their point of synthesis in the cytoplasm to their sites of final localization, and it serves as a model system for the Sec pathway of the eukaryotic endoplasmic reticulum (ER1). Preproteins that are secreted across the inner membrane through the Sec system contain a hydrophobic, cleavable signal peptide (Figure 1) that interacts posttranslationally with SecA in the cytoplasm (Figure 2a). The SecA –preprotein complex associates with SecYEG at the membrane where the preprotein travels through the SecYEG pore (Figure 2b).
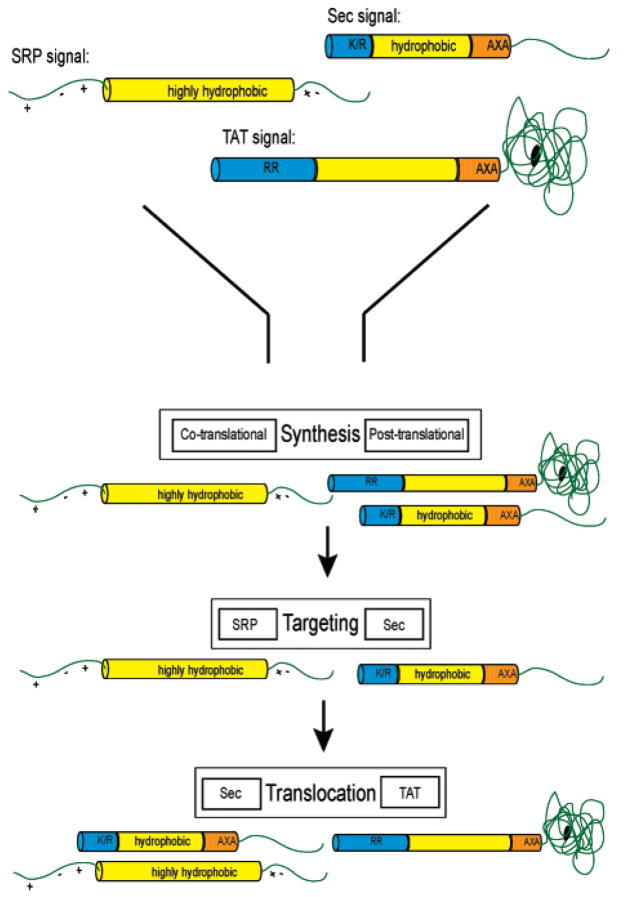
Figure 1.
Classes of bacterial signal peptides. Sec signals are characterized by an amino-terminal positively charged domain of 5–6 residues, followed by a central hydrophobic core of 10–12 residues and a polar region of 6 residues containing the signal peptidase cleavage site. TAT signals contain slightly longer amino-terminal (containing the signature RR) and hydrophobic domains of 10–20 residues each, followed by a similar carboxyl-terminal cleavage region. SRP substrates are typically inner membrane proteins in which a long hydrophobic transmembrane domain acts as a signal anchor and is surrounded by polar regions that ultimately reside in the cytoplasm and periplasm. These signals are discriminated and funneled to the appropriate pathway based on these characteristics at various stages of the preproteins’ export. For example, the long hydrophobic domain of an SRP substrate is detected during synthesis; SRP associates cotranslationally with the nascent chain emerging from the ribosome and directs it to the SecYEG pore. Sec signals are detected posttranslationally and targeted by SecA to the SecYEG translocon. TAT signals are translocated posttranslationally via the TAT translocation channel. Blue, charged amino terminal region; yellow, hydrophobic region; orange, polar cleavage region.
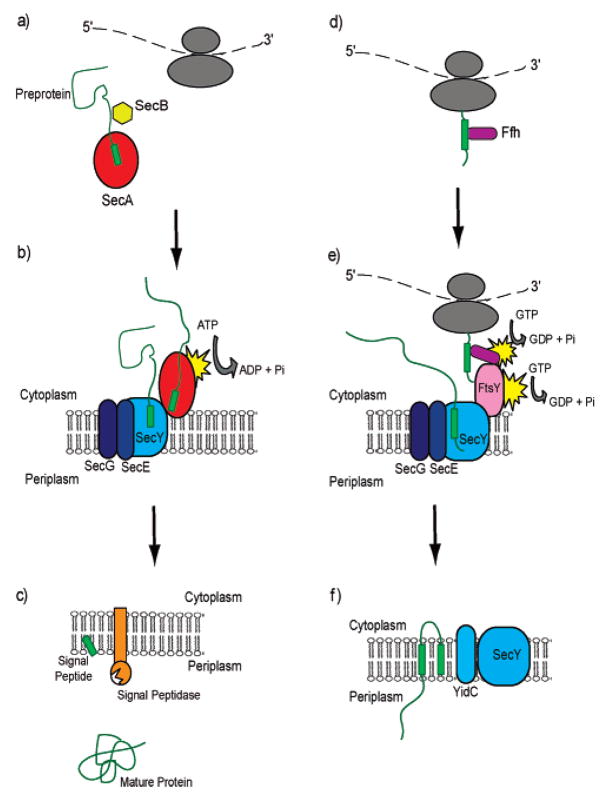
Figure 2.
Schematic representation of signal peptide interactions with components of the Sec machinery during transport. Signal peptides of secretory proteins are bound by SecA (a) in the cytoplasm, delivered to, bound by, and translocated via SecY of the pore (b), and ultimately bind to signal peptidase for cleavage of the signal from the mature protein (c). Inner membrane protein signals are bound by SRP as they emerge from the ribosome (d) and targeted to the membrane. The signal anchor interacts with SecY (e) and translocation occurs via the membrane-embedded SecYEG channel followed by membrane integration via YidC (f).
Following preprotein translocation, signal peptidase cleaves the signal peptide from the mature protein (Figure 2c). We know that a signal peptide is critical for entrance of a preprotein into this pathway yet how signal sequences are recognized and interact specifically with the transport machinery is the subject of intense study.
The lack of primary sequence homology among signal peptides was for some time misleading and the possibility that these peptides were nonetheless endowed with specific recognition elements was not actively considered. Indeed, the “helical hairpin hypothesis” put forth in 1981 (1) emphasized the thermodynamic considerations of moving a hydrophilic protein through a hydrophobic membrane in the absence of specific membrane receptors or transport proteins. More recently, the identification of additional transport routes has required that we rethink the role of the signal peptide. Inner membrane proteins are delivered to SecYEG cotranslationally via the interaction of their highly hydrophobic signal sequences (signal anchor sequences; Figure 1) with the signal recognition particle (SRP) in the cytoplasm (for review, see ref 2; Figure 2d). YidC, a bacterial homolog of mitochondrial Oxa1p that is associated with the Sec translocase (2; Figure 2f), mediates the insertion of both Sec-dependent and Sec-independent inner membrane proteins including some earlier thought to spontaneously insert in the E. coli inner membrane (3). In another route, the TAT (twin arginine translocation) pathway exports folded proteins with an N-terminal signal peptide containing the conserved sequence motif S/TRRXFLK (Figure 1) and includes the transmembrane components, TATA, TATB, and TATC (4). The efficient use of these pathways requires that a cell not simply distinguish cytosolic proteins from secretory proteins but also harbor mechanisms to ensure specific targeting to the proper pathway, to maintain a directional process, and to provide energy transduction to power protein translocation. Intriguingly, utilization of the TAT pathway is dictated by the twin arginine motif preceding the hydrophobic core and a basic residue in the carboxyl terminus of its signal peptide, which serves as a Sec-avoidance signal (5). These findings argue that while signal peptides share global physical properties that facilitate the transport process, these properties are finely tuned for specific interactions and may include critical differences among signal peptide subsets that enhance or inhibit the affinity for one component over another. Consequently, it becomes all the more pressing that we delineate how signal peptides and their corresponding preproteins interface with components of a transport pathway. There is good agreement that at least four components of the Sec pathway interact directly with signal peptides, namely, the SRP, SecA, SecY, and signal peptidase (Figure 3). This perspective focuses on signal peptide recognition by these components.
Figure 3.
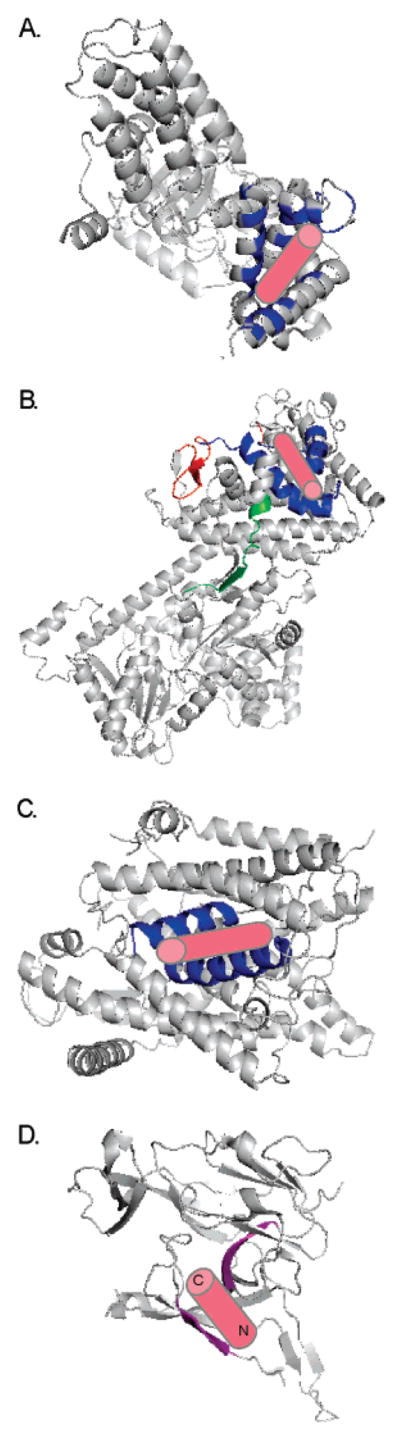
Models based on the crystal structures of Sec transport components with the predicted location of bound signal peptide (pink cylinder) illustrated: (A) Ffh from Thermus aquaticus (PDB ID 2FFH). Conserved residues that line the hydrophobic groove of the M-domain and are implicated in signal peptide binding are shown in blue. This region was entirely disordered in the E. coli structure (113) suggesting flexibility inherent in binding a variety of sequences. (B) SecA from Bacillus subtilis (PDB ID 1M6N). The residues proposed to bind signal peptide are shown in red (PPXD; 68), green (“stem” of PBD; 70), and blue (SPBG; 71). The signal peptide is shown as predicted in ref 71; the SPBG overlaps with the PPXD and is adjacent to the PBD “stem”. (C) SecYEβ from Methanococcus jannaschii (PDB ID 1RHZ). Cross-linking data suggest that helices two and seven (shown in blue) interact with the signal peptide (93, 94). (D) Catalytic domain of E. coli signal peptidase (PDB ID 1KN9). Molecular modeling suggested hydrogen bonding of the signal peptide to β-sheets (shown in purple) that line the shallow substrate binding pocket (99).
Signal Peptides Direct the Preprotein into the Appropriate Pathway
An amino-terminal signal peptide earmarks a cytoplasmic preprotein for transport and plays a fundamental role in determining the appropriate pathway for exported proteins (Figure 1). The signal peptides of preproteins to be transported via the Sec pathway in bacteria, archaea, and eukaryotic ER and thylakoids all exhibit the same general pattern of overall sequence features although they lack primary sequence homology even within species. Slight variations in length, charge, and hydrophobicity distinguish the signals from different organisms, but side chain properties required for cleavage by signal peptidase are conserved throughout (6).
In E. coli, Sec signal peptides are composed of an amino-terminal region of 5–6 residues containing at least one positive charge, followed by a central hydrophobic core of 10–15 residues, and a 6-residue carboxyl terminus in which residues at the −3 and −1 positions have small side chains. The importance of these traits in promoting efficient transport in vivo has been described in numerous studies. Signal sequences containing a negative or net zero charge in the amino terminus result in decreased rates of transport (7, 8) that can be rescued by increased hydrophobicity of the core region (9–11). Disruption of the hydrophobic core by insertion of a polar or charged residue also results in secretion defects (12–15). This region must maintain a minimum “hydrophobic density” to function efficiently (16–18) although a core that is too short, regardless of its hydrophobicity, is nonfunctional (19, 20). A correlation was also shown between in vivo function of signal sequences and the α-helical content of the hydrophobic core of the corresponding synthetic signal peptides (21–23). Many of the signal sequence mutations described above, including charged residues in the hydrophobic core and a lack of charge in the amino terminus, result in severe processing defects in vivo yet have no effect on in vitro processing by signal peptidase (24). However, a strict requirement for cleavage by signal peptidase is small neutral side chains at positions −1 and −3 in the carboxyl region; this has been verified by both statistical (25) and site-directed mutagenesis studies (26–29).
While the importance of certain characteristics of the signal sequence in promoting efficient preprotein transport has long been recognized, as have mutations in SecA and SecY that suppress signal sequence defects, the molecular details of how the features of the signal peptide interface with the transport machinery remain a mystery. The amino-terminal positive charge may be important for electrostatic interactions with the negatively charged phospholipids that comprise the E. coli inner membrane (30, 31) or for promoting the association with SecA (9, 32). However, this association of the preprotein with SecA and then the SecYEG channel is likely driven by the hydrophobic core region. Finally, the specific residue requirements near the signal peptidase cleavage site reflect the enzyme’s substrate specificity. Recent structural data on individual components of the Sec transport pathway provide additional information for understanding the dynamic process of protein translocation. However, without a high-resolution structure of the preprotein in complex with these components, the mechanistic details of the interactions remain unclear.
Signal Recognition Particle Targets Inner Membrane Protein Localization
In eukaryotes, the signal recognition particle (SRP) pathway is well-characterized as the major transport pathway in the endoplasmic reticulum, transporting its preproteins cotranslationally. The SRP is composed of RNA and peptide components, the number of which vary among organisms. E. coli SRP is composed of a 4.5S RNA and a single polypeptide, initially termed P48 (33) or Ffh (for fifty-four homologue; it is homologous to SRP54, a peptide component of the ER SRP; 34). A ribosome-bound nascent chain is transported via Ffh to FtsY (the E. coli SRP receptor) on the cytoplasmic membrane where it then interacts with the SecYEG channel for preprotein translocation. Whether a signal targets its protein to SRP or to SecA (Figure 2) appears to depend on the hydrophobicity of the core region of the signal; the highly hydrophobic inner membrane protein signals associate with SRP, while the less hydrophobic signals of secretory proteins utilize SecA (35). Cross-linking studies showed that increasingly hydrophobic signals interacted more strongly with Ffh (36); similarly, highly hydrophobic signals were able to outcompete less hydrophobic ones, precluding these precursors from the Sec pathway under conditions in which Ffh was limited (37). It is also possible that both positive charge and hydrophobicity of the targeting signal play a role in preprotein recognition by Ffh, and increased hydrophobicity can compensate for a lack of positive charge in binding SRP (38). The helical propensity of the hydrophobic region of the signal peptide may also play a role in recognition by Ffh (39). Fluorescence spectroscopy analysis indicates that the Kd for SRP binding of ribosome–nascent chain complexes containing different signal sequences is below 1 nM (40).
SRP54 (and Ffh) contain an NG domain that binds GTP, and an M domain that has been shown by cross-linking (41, 42) and recently cryo-EM (43) to directly bind the signal peptide of a nascent chain. The M domain is defined by its high content of methionine residues, and the hydrophobic groove in this domain is thought to provide the signal sequence binding pocket. Consistent with this view, the crystal structure of Ffh from Thermus aquaticus (44; Figure 3A) depicts a hydrophobic binding groove of sufficient size and hydrophobicity to accommodate a variety of signal sequences.
SecA Provides the Energy for Preprotein Translocation
While SRP functions cotranslationally, SecA delivers preproteins posttranslationally to the SecYEG channel. SecA may interact with the preprotein alone or in complex with SecB. SecB is a cytoplasmic, transport-dedicated chaperone that maintains the preprotein in an unfolded state in the cytoplasm by interacting with the mature portion of the preprotein rather than the signal sequence and thus can assist SecA in targeting the preprotein to SecYEG (45). SecA becomes peripherally associated with the cytoplasmic membrane and provides the energy, via ATP hydrolysis, to power preprotein translocation. SecA may also function as a cytoplasmic chaperone, preventing preproteins without signal sequences from entering the transport pathway (46). SecA is present only in prokaryotes and in the chloroplasts of plants. In the eukaryotic ER transport system, the ATPase, BiP, may play a similar role but on the trans side of the membrane, acting in the ER lumen to assist the preprotein in completion of translocation as it emerges from the translocon (47).
SecA is likely dimeric in the cytoplasm, but there is considerable debate as to whether it remains dimeric throughout its reaction cycle. Several studies provide evidence that lipids (48, 49), SecY (50), and signal peptides (48, 51) cause SecA to dissociate into monomers whereas other studies indicate that SecA remains dimeric (52–56). Furthermore, crystal structures of SecA from several species, including Mycobacterium tuberculosis (57), E. coli (58), and Bacillus subtilis (59, Figure 3B), suggest a dimeric form. A more “open” form of monomeric SecA has also been crystallized from B. subtilis (60).
SecA drives preprotein translocation and its ATPase activity is stimulated by the preprotein (61) and by the signal peptide region alone (62). Consistent with the in vivo work described above, a synthetic signal peptide with limited core region hydrophobicity stimulated SecA ATPase activity, provided sufficient positive charge was present in the amino terminus (63).
Helicity and charge of a signal peptide was shown in NMR analyses to be important for peptide binding to SecA (64), and this association occurred with a Kd ≈ 10−5 M. This indicates that SecA’s affinity for targeting signals is significantly weaker than is that of SRP (<1 nM). This difference in affinity may be appropriate given the cellular concentration of SecA (5 μM in cell; 48) versus SRP (10 nM in mammalian cytosol; 65). It may also have functional significance. SRP is the first transport component to interface with a nascent chain. It should have high affinity for its substrates so that they are not incorrectly released into the cytosol and degraded. If, as has been proposed (46), SecA has a chaperone function in the cytosol, binding and release of its substrates is required; therefore, a high Kd is expected.
SecA can discriminate among targeting signals to direct only appropriate substrates to the SecYEG channel. SecA ATPase activity is preferentially stimulated by Sec-dependent signal peptides relative to TAT or Sec-independent ones (66). Furthermore, chloroplast SecA discriminates thylakoid Sec-dependent signals from those of the ΔpH-dependent pathway and from E. coli Sec signals (67).
Different regions of SecA have been proposed as potential preprotein binding sites (Figure 3B). A cross-linking study using deletion mutants of SecA suggested residues of the preprotein cross-linking domain (PPXD, residues 267–340) were responsible for preprotein binding (68). More recently, a similar approach pointed to the involvement of a substrate specificity domain (SSD, residues 219–244), based on a loss of synthetic peptide binding to a SecA variant with this fragment deleted (69), and the possibility that a region of the preprotein binding domain (PBD, residues 233–365) largely overlapping the PPXD binds the remainder of the preprotein (70). However when such large regions of the molecule are removed, key elements of the native structure may be lost. A recent study used intact E. coli SecA photolabeled with a synthetic signal peptide and subsequently cleaved at a unique protease site to delineate the region of binding (71) and identified a 53 residue signal peptide binding groove (SPBG) comprising residues 269–322. The crystal structure of B. subtilis SecA suggested a deep groove between the PPXD and the helical scaffold and wing domains (HSD/HWD) as a good candidate for peptide binding (60). This groove extends C-terminally from the SPBG and may provide a site in which the remainder of the preprotein resides.
SecYEG: The Proteinaceous Pore through the Membrane
The conserved heterotrimeric membrane protein complexes, SecYEG and Sec61αβγ, form the protein-conducting channel across the cytoplasmic membrane in prokaryotes and the ER membrane in eukaryotes, respectively (47). Some evidence suggests that like its eukaryotic counterpart, SecYEG may oligomerize during channel formation when protein translocation is initiated and then disassemble upon completion of translocation (72). On the other hand, the recent crystal structure of Methanococcus jannaschii SecYEβ (Figure 3C; homologous to E. coli SecYEG) suggests that a single heterotrimer, as opposed to an oligomer, may function as the conducting channel for protein translocation (73). Similarly for E. coli, although a dimer of SecYEG may exist during preprotein translocation, a single heterotrimer of SecYEG likely functions as the active channel during transport (74, 75). Freeze–fracture experiments showed the increased presence of dimers (and tetramers) of E. coli SecYEG in lipid bilayers in the presence of the translocation ligands, SecA, ATP, and preprotein (76).
SecY, a polytopic membrane protein with 10 transmembrane segments, represents the central component of the translocation channel, and it interacts directly with SecA, SecE, SecG, SecDFYajC, and the preprotein (77). SecY shares sequence homology and similar topology with Sec61α/ Sec61p, while SecE is homologous to Sec61γ/Sss1p in mammals and yeast, respectively (78). SecG is not homologous to eukaryotic Sec61β and is not required for SecA-dependent precursor translocation in proteoliposomes, where only SecY and SecE are required (79). However, SecG stimulates preprotein translocation probably by regulating membrane cycling of SecA (80).
Suppressors of signal sequence defects have been found in SecY (prlA), SecE (prlG), SecG (prlH) and SecA (prlD). Of these, most commonly isolated suppressors are in prlA and are clustered in transmembrane regions seven and ten (TM7 and TM10) and the plug domain (TM2a, previously referred to as periplasmic loop 1 (P1)) (81). It was initially thought that these suppressor mutations restored the recognition of a defective signal sequence (82); however, the fact that certain prl strains can efficiently transport both periplasmic and outer membrane proteins that lack a signal sequence, combined with the lack of allele specificity between signal sequence mutations and prl suppressors, refutes this hypothesis (83–85). PrlA suppressors were also shown to relieve the requirement for the proton-motive force during translocation of preproteins with folded domains (86) and are thought to result in a weaker association among the subunits of the SecYEG complex (87). These studies indicate that prlA mutations cause an increase in the conformational flexibility of the Sec channel rather than a decrease in signal peptide recognition and selectivity. Moreover, studies with prlA4 strains indicate that these suppressors stabilize SecA–SecY binding during initiation of translocation (81). On the other hand, it was proposed that some prlA suppressors accelerate the deinsertion of SecA, the rate-limiting step of translocation (88). A comprehensive analysis of characterized prlA mutations localize all to the channel interior, the plug region thought to play a role in channel gating, or the hydrophobic constriction at the pore’s center (89). It appears that these mutations all act by either stabilizing the open state of the channel or destabilizing the closed state (73, 89). Recently, a phenotype like that of prl mutants was displayed by a SecY in which the plug region was deleted (90, 91).
Signal peptide binding to SecYEG is thought to initiate translocation through the channel by destabilizing interactions that maintain the plug in the pore. This results in plug movement away from the pore for tanslocation of the preprotein through its center. Initial studies by Osborne and Silhavy (92) suggested that TM7 of SecY, where most of the prlA alleles are clustered, is the site of signal sequence recognition, and crystal structure evidence also suggests that the signal sequence binds to TMs 2b (the portion of TM2 not involved in formation of the plug) and 7 of SecY of the M. jannaschii SecYEβ complex (73; Figure 3C). A small hinge movement between TMs 5 and 6 would create a pore of sufficient dimensions to allow insertion of the preprotein as a loop. This hinge movement could vary to allow TMs 2b and 7 to orient as needed to accommodate different signal peptides. Direct cross-linking between E. coli SecY and a synthetic signal peptide demonstrated that the binding was primarily to regions of the protein containing transmembrane domains 7 and 2b (93). This is consistent with work on Sec61p showing intercalation of prepro-α-factor into TMs 2 and 7 (94).
Signal Peptidase Catalyzes the Final Step in Protein Maturation
Protein translocation is accompanied by cleavage of the signal peptide by signal peptidase, and although the cleavage event is not required for translocation, uncleaved precursors remain membrane bound with the uncleaved signal peptide acting as a membrane anchor (16). The ER membrane, the inner mitochondrial membrane, chloroplasts, and the bacterial cytoplasmic membrane all contain type I signal peptidase (SPase I) (95). In addition to SPase I, which cleaves most signal peptides, E. coli has a type II signal peptidase that is associated with the Sec pathway and cleaves signal peptides from lipid-modified proteins (96). The signal peptidase complex (SPC) in the ER is composed of five membrane-associated polypeptides, of which two share a weak homology to bacterial SPase I (96). Bacterial SPase I can cleave ER signals, and SPC can cleave bacterial signal sequences (97). Similarly, thylakoid processing peptidase and bacterial SPase I have identical substrate specificities (98).
E. coli SPase I is a membrane bound serine protease with two transmembrane segments; both the N- and C-termini are located in the periplasmic space, and its active site is located in the large C-terminal domain (99). The active enzyme lacking its transmembrane segments has been crystallized with (99, 100) and without (101) a bound inhibitor. The crystal structure of the apoenzyme (101; Figure 3D) was used to model the signal peptide binding site based, in part, on the enzyme structure with inhibitor bound (99). Current models indicate that only the cleavage region is in intimate contact with SPase I, leaving open the possibility that the remainder of the signal peptide is still associated with the transport machinery at the time of cleavage.
SPase I is a product of the lepB gene that exists as a single copy with approximately 1000 SPase I molecules per cell (102). In addition to Sec signal peptides, SPase I cleaves the M13 procoat signal peptide (28), which requires YidC for transport (103), and is believed to process signals for the TAT pathway (104). TAT signals are, on average, less hydrophobic and 14 amino acids longer than Sec signals (Figure 1). It is not clear whether SPase I interfaces with both the Sec and TAT pathways, either through active recruitment by the respective translocon or by passive recognition, or whether multiple copies of the enzyme allow individual peptidases to be dedicated to one transport route.
For the cleavage event to occur, specific requirements with regard to amino acid composition, topological alignment, and conformation of the cleavage region are required. The small neutral side chains at positions −1 and −3 of the signal peptide cleavage region are required because they occupy two shallow binding clefts lined with hydrophobic residues (the proposed S1 and S3 binding sites) (Figure 3D) in the region of Ser90 and Lys145, the catalytic dyad (99). Proper presentation of the cleavage region to the signal peptidase at the surface of the lipid bilayer is essential for cleavage. Cleavage regions ranging from three to nine residues are processed efficiently, whereas the extent of cleavage drops markedly thereafter with no processing observed for signal peptides with a 13 residue long C-region (105). Such a long linker might extend too far into the periplasm, removing the cleavage site of the precursor from proximity with the enzyme’s active site thus making interaction with the signal peptidase difficult.
Extending the signal peptide hydrophobic core length of a secretory protein to 20 leucines leads to translocation without cleavage (16) and results in the hydrophobic core functioning as a signal anchor probably positioned in the translocase in a way that precludes its access to the SPase I. Similarly in eukaryotes, signal peptides with polyleucine hydrophobic cores of up to 17 residues and wild-type cleavage regions are cleaved by signal peptidase, while signals with 20 to 26 residues in the core are not (106). This suggests that perhaps signal peptides are accessible to SPase I while signal anchors are not because the length of their respective hydrophobic cores causes them to be positioned differently in the translocase; in the case of signal anchors, their position in the translocon may be critical to allow for their lateral exit.
The propensity to have a helix-breaking residue (proline or glycine) at the hydrophobic core/cleavage boundary of both prokaryotic and eukaryotic signal peptides led to the suggestion that the cleavage region forms a β-turn structure, which is required for transport (107, 108). However, mutational analysis showed that the proline at −6 of the alkaline phosphatase signal peptide is not essential and can be replaced by non-turn-forming amino acids (27). Studies modeling the pro-OmpA signal peptide bound to signal peptidase reveal that the interaction likely involves the cleavage region in an extended chain conformation forming hydrogen bonds with residues lining the signal sequence binding site (101; Figure 3D). Sequence mutations of the cleavage region coupled with molecular modeling of the alkaline phosphatase signal peptide onto the active site of SPase I suggested that the cleavage region can assume at least two conformations and that the hydrophobic core is not in a fixed position relative to SPase I (109).
The enzyme may act as a free agent in the lipid bilayer, and its transmembrane domains may not come in contact with the core of the signal peptide. Supporting this hypothesis is the finding that a truncated form of SPase I that lacks the membrane spanning domains is capable of proper processing of its substrate (24). Interestingly, SPC in the ER associates with and can be cross-linked to Sec61α (ER homolog to SecY), where ribosome binding to Sec61α leads to recruitment of the SPC to the translocase (110). No experimental evidence exists thus far that demonstrates a similar association of bacterial SPase with SecY.
Rapoport and co-workers (110) suggest that it would be more enzymatically efficient for SPC that is associated with the translocase to cleave the signal peptide just as it emerges from the translocation channel. Yet, when SPase I is presented with two WT-signal peptides in tandem on the same protein, it cleaves both with almost the same efficiency; when their hydrophobicities differ, the more hydrophobic signal is preferentially cleaved (111) suggesting that its location is not an overriding factor. A study by Josefsson and Randall (112) lends experimental support to the notion that cleavage of the signal peptide occurs at a later stage of the transport process. By using in vivo labeling of precursor maltose binding protein and quantitation of the nascent chains that were processed, they determined that for cotranslational processing 80% of the polypeptide chain is synthesized before signal peptide cleavage takes place suggesting that much of the preprotein has emerged from the translocon prior to signal peptide cleavage.
Conclusions
The properties of signal peptides play a critical role in directing preproteins to the appropriate export pathway. Despite the variety that exists among the sequences of signal peptides, the interactions they maintain with the components of the Sec transport pathway are relatively specific. This specificity is imparted not through primary sequence but via features such as degree of hydrophobicity, length, and flanking charge. The recent publication of the crystal structures of several components of the transport machinery now allows the examination of signal peptide recognition sites on each. These sites are as different as the peptides that interact with them, and no single binding motif is evident although some themes emerge. Not surprisingly, all include a hydrophobic groove that should be accessible to the signal peptide. Each recognition site has features that provide the flexibility to bind a range of signal peptide substrates. Consistent with this idea, the signal peptide binding domains of the E. coli SRP and SecA were not well resolved in crystals (58, 113). The binding site on SRP is lined with amino acid side chains, the so-called methionine bristles, which are flexible and can be readily reoriented (44). TM2 and TM7 of SecY, which contribute to the signal peptide binding surface, have sufficient flexibility to adapt their separation and orientation to accommodate different sequences (73). More precision is found in the SPase I site with respect to the S1 and S3 cavities that interact with the −1 and −3 signal peptide residues, but the remainder of the binding groove is adaptable to binding a variety of residues (99). These sites are all appropriate for binding the signal peptide of a translocating polypeptide that interacts only transiently with each component. Once a preprotein is targeted to the Sec pathway, interactions with successive components need not require particularly high affinity since the effective concentration of the preprotein in the translocon will be high and because release and movement through the relay system is important. Undoubtedly spatial and temporal factors also impact recognition, release, and the order of each binding event. Elucidating these complexities for the multicomponent system is needed to understand the dynamic aspects of protein transport.
Footnotes
This work was supported in part by National Institutes of Health Grant GM37639 (to D.A.K.).
Abbreviations: ER, endoplasmic reticulum; HSD, helical scaffold domain; HWD, helical wing domain; PBD, preprotein binding domain; PPXD, preprotein cross-linking domain; SPBG, signal peptide binding groove; SPC, signal peptidase complex; SRP, signal recognition particle; SSD, substrate specificity domain; TAT, twin arginine translocation.
References
- 1.Engelman DM, Steitz TA. The spontaneous insertion of proteins into and across membranes: the helical hairpin hypothesis. Cell. 1981;23:411–422. doi: 10.1016/0092-8674(81)90136-7. [DOI] [PubMed] [Google Scholar]
- 2.Luirink J, von Heijne G, Houben E, de Gier JW. Biogenesis of inner membrane proteins in Escherichia coli. Annu Rev Microbiol. 2005;59:329–355. doi: 10.1146/annurev.micro.59.030804.121246. [DOI] [PubMed] [Google Scholar]
- 3.Chen M, Samuelson JC, Jiang F, Muller M, Kuhn A, Dalbey RE. Direct interaction of YidC with the Sec-independent Pf3 coat protein during its membrane protein insertion. J Biol Chem. 2002;277:7670–7675. doi: 10.1074/jbc.M110644200. [DOI] [PubMed] [Google Scholar]
- 4.Sargent F, Berks BC, Palmer T. Pathfinders and trailblazers: a prokaryotic targeting system for transport of folded proteins. FEMS Microbiol Lett. 2006;254:198–207. doi: 10.1111/j.1574-6968.2005.00049.x. [DOI] [PubMed] [Google Scholar]
- 5.Blaudeck N, Kreutzenbeck P, Freudl R, Sprenger GA. Genetic analysis of pathway specificity during posttranslational protein translocation across the Escherichia coli plasma membrane. J Bacteriol. 2003;185:2811–2819. doi: 10.1128/JB.185.9.2811-2819.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.von Heijne G, Abrahmsen L. Species-specific variation in signal peptide design. Implications for protein secretion in foreign hosts. FEBS Lett. 1989;244:439–446. doi: 10.1016/0014-5793(89)80579-4. [DOI] [PubMed] [Google Scholar]
- 7.Inouye S, Soberon X, Franceschini T, Nakamura K, Itakura K, Inouye M. Role of positive charge on the amino-terminal region of the signal peptide in protein secretion across the membrane. Proc Natl Acad Sci USA. 1982;79:3438–3441. doi: 10.1073/pnas.79.11.3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vlasuk GP, Inouye S, Ito H, Itakura K, Inouye M. Effects of the complete removal of basic amino acid residues from the signal peptide on secretion of lipoprotein in Escherichia coli. J Biol Chem. 1983;258:7141–7148. [PubMed] [Google Scholar]
- 9.Puziss JW, Fikes JD, Bassford PJ., Jr Analysis of mutational alterations in the hydrophilic segment of the maltose-binding protein signal peptide. J Bacteriol. 1989;171:2303–2311. doi: 10.1128/jb.171.5.2303-2311.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hikita C, Mizushima S. The requirement of a positive charge at the amino terminus can be compensated for by a longer central hydrophobic stretch in the functioning of signal peptides. J Biol Chem. 1992;267:12375–12379. [PubMed] [Google Scholar]
- 11.Izard JW, Rusch SL, Kendall DA. The amino-terminal charge and core region hydrophobicity interdependently contribute to the function of signal sequences. J Biol Chem. 1996;271:21579–21582. doi: 10.1074/jbc.271.35.21579. [DOI] [PubMed] [Google Scholar]
- 12.Bedouelle H, Bassford PJ, Jr, Fowler AV, Zabin I, Beckwith J, Hofnung M. Mutations which alter the function of the signal sequence of the maltose binding protein of Escherichia coli. Nature. 1980;285:78–81. doi: 10.1038/285078a0. [DOI] [PubMed] [Google Scholar]
- 13.Stader J, Benson SA, Silhavy TJ. Kinetic analysis of lamB mutants suggests the signal sequence plays multiple roles in protein export. J Biol Chem. 1986;261:15075–15080. [PubMed] [Google Scholar]
- 14.Kendall DA, Doud SK, Kaiser ET. A comparative analysis of single and multiple residue substitutions in the alkaline phosphatase signal peptide. Biopolymers. 1990;29:139–147. doi: 10.1002/bip.360290119. [DOI] [PubMed] [Google Scholar]
- 15.Goldstein J, Lehnhardt S, Inouye M. In vivo effect of asparagine in the hydrophobic region of the signal sequence. J Biol Chem. 1991;266:14413–14417. [PubMed] [Google Scholar]
- 16.Chou MM, Kendall DA. Polymeric sequences reveal a functional interrelationship between hydrophobicity and length of signal peptides. J Biol Chem. 1990;265:2873–2880. [PubMed] [Google Scholar]
- 17.Rusch SL, Kendall DA. Signal sequences containing multiple aromatic residues. J Mol Biol. 1992;224:77–85. doi: 10.1016/0022-2836(92)90577-7. [DOI] [PubMed] [Google Scholar]
- 18.Doud SK, Chou MM, Kendall DA. Titration of protein transport activity by incremental changes in signal peptide hydrophobicity. Biochemistry. 1993;32:1251–1256. doi: 10.1021/bi00056a008. [DOI] [PubMed] [Google Scholar]
- 19.von Heijne G. Signal sequences. The limits of variation. J Mol Biol. 1985;184:99–105. doi: 10.1016/0022-2836(85)90046-4. [DOI] [PubMed] [Google Scholar]
- 20.Hikita C, Mizushima S. Effects of total hydrophobicity and length of the hydrophobic domain of a signal peptide on in vitro translocation efficiency. J Biol Chem. 1992;267:4882–4888. [PubMed] [Google Scholar]
- 21.Bruch MD, Gierasch LM. Comparison of helix stability in wild-type and mutant LamB signal sequences. J Biol Chem. 1990;265:3851–3858. [PubMed] [Google Scholar]
- 22.Rizo J, Blanco FJ, Kobe B, Bruch MD, Gierasch LM. Conformational behavior of Escherichia coli OmpA signal peptides in membrane mimetic environments. Biochemistry. 1993;32:4881–4894. doi: 10.1021/bi00069a025. [DOI] [PubMed] [Google Scholar]
- 23.Izard JW, Doughty MB, Kendall DA. Physical and conformational properties of synthetic idealized signal sequences parallel their biological function. Biochemistry. 1995;34:9904–9912. doi: 10.1021/bi00031a012. [DOI] [PubMed] [Google Scholar]
- 24.Carlos JL, Paetzel M, Brubaker G, Karla A, Ashwell CM, Lively MO, Cao G, Bullinger P, Dalbey RE. The role of the membrane-spanning domain of type I signal peptidases in substrate cleavage site selection. J Biol Chem. 2000;275:38813–38822. doi: 10.1074/jbc.M007093200. [DOI] [PubMed] [Google Scholar]
- 25.von Heijne G. How signal sequences maintain cleavage specificity. J Mol Biol. 1984;173:243–251. doi: 10.1016/0022-2836(84)90192-x. [DOI] [PubMed] [Google Scholar]
- 26.Fikes JD, Barkocy-Gallagher GA, Klapper DG, Bassford PJ., Jr Maturation of Escherichia coli maltose-binding protein by signal peptidase I in vivo. Sequence requirements for efficient processing and demonstration of an alternate cleavage site. J Biol Chem. 1990;265:3417–3423. [PubMed] [Google Scholar]
- 27.Laforet GA, Kendall DA. Functional limits of conformation, hydrophobicity, and steric constraints in prokaryotic signal peptide cleavage regions. Wild type transport by a simple polymeric signal sequence. J Biol Chem. 1991;266:1326–1334. [PubMed] [Google Scholar]
- 28.Shen LM, Lee JI, Cheng SY, Jutte H, Kuhn A, Dalbey RE. Use of site-directed mutagenesis to define the limits of sequence variation tolerated for processing of the M13 procoat protein by the Escherichia coli leader peptidase. Biochemistry. 1991;30:11775–11781. doi: 10.1021/bi00115a006. [DOI] [PubMed] [Google Scholar]
- 29.Karamyshev AL, Karamysheva ZN, Kajava AV, Ksenzenko VN, Nesmeyanova MA. Processing of Escherichia coli alkaline phosphatase: role of the primary structure of the signal peptide cleavage region. J Mol Biol. 1998;277:859–870. doi: 10.1006/jmbi.1997.1617. [DOI] [PubMed] [Google Scholar]
- 30.de Vrije T, Batenburg AM, Jordi W, de Kruijff B. Inhibition of PhoE translocation across Escherichia coli inner-membrane vesicles by synthetic signal peptides suggests an important role of acidic phospholipids in protein translocation. Eur J Biochem. 1989;180:385–392. doi: 10.1111/j.1432-1033.1989.tb14660.x. [DOI] [PubMed] [Google Scholar]
- 31.Phoenix DA, Kusters R, Hikita C, Mizushima S, de Kruijff B. OmpF-Lpp signal sequence mutants with varying charge hydrophobicity ratios provide evidence for a phosphatidylglycerol-signal sequence interaction during protein translocation across the Escherichia coli inner membrane. J Biol Chem. 1993;268:17069–17073. [PubMed] [Google Scholar]
- 32.Akita M, Sasaki S, Matsuyama S, Mizushima S. SecA interacts with secretory proteins by recognizing the positive charge at the amino terminus of the signal peptide in Escherichia coli. J Biol Chem. 1990;265:8164–8169. [PubMed] [Google Scholar]
- 33.Luirink J, High S, Wood H, Giner A, Tollervey D, Dobberstein B. Signal-sequence recognition by an Escherichia coli ribonucleoprotein complex. Nature. 1992;359:741–743. doi: 10.1038/359741a0. [DOI] [PubMed] [Google Scholar]
- 34.Römisch K, Webb J, Herz J, Prehn S, Frank R, Vingron M, Dobberstein B. Homology of 54K protein of signal-recognition particle, docking protein and two E. coli proteins with putative GTP-binding domains. Nature. 1989;340:478–482. doi: 10.1038/340478a0. [DOI] [PubMed] [Google Scholar]
- 35.Lee HC, Bernstein HD. The targeting pathway of Escherichia coli presecretory and integral membrane proteins is specified by the hydrophobicity of the targeting signal. Proc Natl Acad Sci USA. 2001;98:3471–3476. doi: 10.1073/pnas.051484198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Valent QA, de Gier JWL, von Heijne G, Kendall DA, ten Hagen-Jongman CM, Oudega B, Luirink J. Nascent membrane and presecretory proteins synthesized in Escherichia coli associate with signal recognition particle and trigger factor. Mol Microbiol. 1997;25:53–64. doi: 10.1046/j.1365-2958.1997.4431808.x. [DOI] [PubMed] [Google Scholar]
- 37.Kim J, Rusch SL, Luirink J, Kendall DA. Is Ffh required for export of secretory proteins? FEBS Lett. 2001;505:245–248. doi: 10.1016/s0014-5793(01)02784-3. [DOI] [PubMed] [Google Scholar]
- 38.Peterson JH, Woolhead CA, Bernstein HD. Basic amino acids in a distinct subset of signal peptides promote interaction with the signal recognition particle. J Biol Chem. 2003;278:46155–46162. doi: 10.1074/jbc.M309082200. [DOI] [PubMed] [Google Scholar]
- 39.Adams H, Scotti PA, de Cock H, Luirink J, Tommassen J. The presence of a helix breaker in the hydrophobic core of signal sequences of secretory proteins prevents recognition by the signal-recognition particle in Escherichia coli. Eur J Biochem. 2002;269:5564–5571. doi: 10.1046/j.1432-1033.2002.03262.x. [DOI] [PubMed] [Google Scholar]
- 40.Flanagan JJ, Chen JC, Miao Y, Shao Y, Lin J, Bock PE, Johnson AE. Signal recognition particle binds to ribosome-bound signal sequences with fluorescence-detected subnanomolar affinity that does not diminish as the nascent chain lengthens. J Biol Chem. 2003;278:18628–18637. doi: 10.1074/jbc.M300173200. [DOI] [PubMed] [Google Scholar]
- 41.Zopf D, Bernstein HD, Johnson AE, Walter P. The methionine-rich domain of the 54 kd protein subunit of the signal recognition particle contains an RNA binding site and can be cross-linked to a signal sequence. EMBO J. 1990;9:4511–4517. doi: 10.1002/j.1460-2075.1990.tb07902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lütcke H, High S, Römisch K, Ashford AJ, Dobberstein B. The methionine-rich domain of the 54 kDa subunit of signal recognition particle is sufficient for the interaction with signal sequences. EMBO J. 1992;11:1543–1551. doi: 10.1002/j.1460-2075.1992.tb05199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Halic M, Blau M, Becker T, Mielke T, Pool MR, Wild K, Sinning I, Beckmann R. Following the signal sequence from ribosomal tunnel exit to signal recognition particle. Nature. 2006;444:507–511. doi: 10.1038/nature05326. [DOI] [PubMed] [Google Scholar]
- 44.Keenan RJ, Freymann DM, Walter P, Stroud RM. Crystal structure of the signal sequence binding subunit of the signal recognition particle. Cell. 1998;94:181–191. doi: 10.1016/s0092-8674(00)81418-x. [DOI] [PubMed] [Google Scholar]
- 45.Driessen AJM. SecB, a molecular chaperone with two faces. Trends Microbiol. 2001;9:193–196. doi: 10.1016/s0966-842x(01)01980-1. [DOI] [PubMed] [Google Scholar]
- 46.Eser M, Ehrmann M. Sec-dependent quality control of intracellular protein localization. Proc Natl Acad Sci USA. 2003;100:13231–13234. doi: 10.1073/pnas.2234410100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Osborne AR, Rapoport TA, van den Berg B. Protein translocation by the Sec61/SecY channel. Annu ReV Cell Dev Biol. 2005;21:529–550. doi: 10.1146/annurev.cellbio.21.012704.133214. [DOI] [PubMed] [Google Scholar]
- 48.Or E, Navon A, Rapoport T. Dissociation of the dimeric SecA ATPase during protein translocation across the bacterial membrane. EMBO J. 2002;21:4470–4479. doi: 10.1093/emboj/cdf471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Benach J, Chou YT, Fak JJ, Itkin A, Nicolae DD, Smith PC, Wittrock G, Floyd DL, Golsaz CM, Gierasch LM, Hunt JF. Phospholipid-induced monomerization and signal-peptide-induced oligomerization of SecA. J Biol Chem. 2003;278:3628–3638. doi: 10.1074/jbc.M205992200. [DOI] [PubMed] [Google Scholar]
- 50.Duong F. Binding, activation and dissociation of the dimeric SecA ATPase at the dimeric SecYEG translocase. EMBO J. 2003;22:4375–4384. doi: 10.1093/emboj/cdg418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Musial-Siwek M, Rusch SL, Kendall DA. Probing the affinity of SecA for signal peptide in different environments. Biochemistry. 2005;44:13987–13996. doi: 10.1021/bi050882k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Driessen AJM. SecA, the peripheral subunit of the Escherichia coli precursor protein translocase, is functional as a dimer. Biochemistry. 1993;32:13190–13197. doi: 10.1021/bi00211a030. [DOI] [PubMed] [Google Scholar]
- 53.Karamanou S, Sianidis G, Gouridis G, Pozidis C, Papanikolau Y, Papanikou E, Economou A. Escherichia coli SecA truncated at its termini is functional and dimeric. FEBS Lett. 2005;579:1267–1271. doi: 10.1016/j.febslet.2005.01.025. [DOI] [PubMed] [Google Scholar]
- 54.Jilaveanu LB, Zito CR, Oliver D. Dimeric SecA is essential for protein translocation. Proc Natl Acad Sci USA. 2005;102:7511–7516. doi: 10.1073/pnas.0502774102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.de Keyzer J, van der Sluis EO, Spelbrink REJ, Nijstad N, de Kruijff B, Nouwen N, van der Does C, Driessen AJM. Covalently dimerized SecA is functional in protein translocation. J Biol Chem. 2005;280:35255–35260. doi: 10.1074/jbc.M506157200. [DOI] [PubMed] [Google Scholar]
- 56.Jilaveanu LB, Oliver D. SecA dimer cross-linked at its subunit interface is functional for protein translocation. J Bacteriol. 2006;188:335–338. doi: 10.1128/JB.188.1.335-338.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sharma V, Arockiasamy A, Ronning DR, Savva CG, Holzenburg A, Braunstein M, Jacobs WR, Jr, Sacchettini JC. Crystal structure of Mycobacterium tuberculosis SecA, a preprotein translocating ATPase. Proc Natl Acad Sci USA. 2003;100:2243–2248. doi: 10.1073/pnas.0538077100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Papanikolau Y, Papadovasilaki M, Ravelli RBG, McCarthy AA, Cusack S, Economou A, Petratos K. Structure of dimeric SecA, the Escherichia coli preprotein translocase motor. J Mol Biol. 2007;366:1545–1557. doi: 10.1016/j.jmb.2006.12.049. [DOI] [PubMed] [Google Scholar]
- 59.Hunt JF, Weinkauf S, Henry L, Fak JJ, McNicholas P, Oliver DB, Deisenhofer J. Nucleotide control of interdomain interactions in the conformational reaction cycle of SecA. Science. 2002;297:2018–2026. doi: 10.1126/science.1074424. [DOI] [PubMed] [Google Scholar]
- 60.Osborne AR, Clemons WM, Jr, Rapoport TA. A large conformational change of the translocation ATPase SecA. Proc Natl Acad Sci USA. 2004;101:10937–10942. doi: 10.1073/pnas.0401742101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lill R, Dowhan W, Wickner W. The ATPase activity of SecA is regulated by acidic phospholipids, SecY, and leader and mature domains of precursor proteins. Cell. 1990;60:271–280. doi: 10.1016/0092-8674(90)90742-w. [DOI] [PubMed] [Google Scholar]
- 62.Miller A, Wang L, Kendall DA. Synthetic signal peptides specifically recognize SecA and stimulate ATPase activity in the absence of preprotein. J Biol Chem. 1998;273:11409–11412. doi: 10.1074/jbc.273.19.11409. [DOI] [PubMed] [Google Scholar]
- 63.Wang L, Miller A, Kendall DA. Signal peptide determinants of SecA binding and stimulation of ATPase activity. J Biol Chem. 2000;275:10154–10159. doi: 10.1074/jbc.275.14.10154. [DOI] [PubMed] [Google Scholar]
- 64.Chou YT, Gierasch LM. The conformation of a signal peptide bound by Escherichia coli preprotein translocase SecA. J Biol Chem. 2005;280:32753–32760. doi: 10.1074/jbc.M507532200. [DOI] [PubMed] [Google Scholar]
- 65.Siegel V, Walter P. The affinity of signal recognition particle for presecretory proteins is dependent on nascent chain length. EMBO J. 1988;7:1769–1775. doi: 10.1002/j.1460-2075.1988.tb03007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kebir M, Kendall DA. SecA signal peptide substrate specificity. Biochemistry. 2002;41:5573–5580. doi: 10.1021/bi015798t. [DOI] [PubMed] [Google Scholar]
- 67.Sun C, Rusch SL, Kim J, Kendall DA. Chloroplast SecA and Escherichia coli SecA have distinct lipid and signal peptide preferences. J Bacteriol. 2007;189:1171–1175. doi: 10.1128/JB.01589-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kimura E, Akita M, Matsuyama S, Mizushima S. Determination of a region in SecA that interacts with presecretory proteins in Escherichia coli. J Biol Chem. 1991;266:6600–6606. [PubMed] [Google Scholar]
- 69.Baud C, Karamanou S, Sianidis G, Vrontou E, Politou AS, Economou A. Allosteric communication between signal peptides and the SecA protein DEAD motor ATPase domain. J Biol Chem. 2002;277:13724–13731. doi: 10.1074/jbc.M200047200. [DOI] [PubMed] [Google Scholar]
- 70.Papanikou E, Karamanou S, Baud C, Frank M, Sianidis G, Keramisanou D, Kalodimos CG, Kuhn A, Economou A. Identification of the preprotein binding domain of SecA. J Biol Chem. 2005;280:43209–43217. doi: 10.1074/jbc.M509990200. [DOI] [PubMed] [Google Scholar]
- 71.Musial-Siwek M, Rusch SL, Kendall DA. Selective Photoaffinity Labeling Identifies the Signal Peptide Binding Domain on SecA. J Mol Biol. 2007;365:637–648. doi: 10.1016/j.jmb.2006.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Collinson I, Breyton C, Duong F, Tziatzios C, Schubert D, Or E, Rapoport T, Kühlbrandt W. Projection structure and oligomeric properties of a bacterial core protein translocase. EMBO J. 2001;20:2462–2471. doi: 10.1093/emboj/20.10.2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.van den Berg B, Clemons WM, Jr, Collinson I, Modis Y, Hartmann E, Harrison SC, Rapoport TA. X-ray structure of a protein-conducting channel. Nature. 2004;427:36–44. doi: 10.1038/nature02218. [DOI] [PubMed] [Google Scholar]
- 74.Tam PCK, Maillard AP, Chan KKY, Duong F. Investigating the SecY plug movement at the SecYEG translocation channel. EMBO J. 2005;24:3380–3388. doi: 10.1038/sj.emboj.7600804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Osborne AR, Rapoport TA. Protein translocation is mediated by oligomers of the SecY complex with one SecY copy forming the channel. Cell. 2007;129:97–110. doi: 10.1016/j.cell.2007.02.036. [DOI] [PubMed] [Google Scholar]
- 76.Scheuring J, Braun N, Northdurft L, Stumpf M, Veenendaal AKJ, Kol S, van der Does C, Driessen AJM, Weinkauf S. The oligomeric distribution of SecYEG is altered by SecA and translocation ligands. J Mol Biol. 2005;354:358–271. doi: 10.1016/j.jmb.2005.09.058. [DOI] [PubMed] [Google Scholar]
- 77.Veenendaal AKJ, van der Does C, Driessen AJM. The protein-conducting channel SecYEG. Biochim Biophys Acta. 2004;1694:81–95. doi: 10.1016/j.bbamcr.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 78.Meyer TH, Ménétret JF, Breitling R, Miller KR, Akey CW, Rapoport TA. The bacterial SecY/E translocation complex forms channel-like structures similar to those of the eukaryotic Sec61p complex. J Mol Biol. 1999;285:1789–1800. doi: 10.1006/jmbi.1998.2413. [DOI] [PubMed] [Google Scholar]
- 79.Akimaru J, Matsuyama S, Tokuda H, Mizushima S. Reconstitution of a protein translocation system containing purified SecY, SecE and SecA from Escherichia coli. Proc Natl Acad Sci USA. 1991;88:6545–6549. doi: 10.1073/pnas.88.15.6545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Duong F, Wickner W. Distinct catalytic roles of the SecYE, SecG and SecDFyajC subunits of preprotein translocase holoenzyme. EMBO J. 1997;16:2756–2768. doi: 10.1093/emboj/16.10.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.van der Wolk JP, Fekkes P, Boorsma A, Huie JL, Silhavy TJ, Driessen AJ. PrlA4 prevents the rejection of signal sequence defective preproteins by stabilizing the SecA-SecY interaction during the initiation of translocation. EMBO J. 1998;17:3631–3639. doi: 10.1093/emboj/17.13.3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Puziss JW, Strobel SM, Bassford PJ., Jr Export of maltose-binding protein species with altered charge distribution surrounding the signal peptide hydrophobic core in Escherichia coli cells harboring prl suppressor mutations. J Bacteriol. 1992;174:92–101. doi: 10.1128/jb.174.1.92-101.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Derman AI, Puziss JW, Bassford PJ, Jr, Beckwith J. A signal sequence is not required for protein export in prlA mutants of Escherichia coli. EMBO J. 1993;12:879–888. doi: 10.1002/j.1460-2075.1993.tb05728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Flower AM, Doebele RC, Silhavy TJ. PrlA and PrlG suppressors reduce the requirement for signal sequence recognition. J Bacteriol. 1994;176:5607–5614. doi: 10.1128/jb.176.18.5607-5614.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Prinz WA, Spiess C, Ehrmann M, Schierle C, Beckwith J. Targeting of signal sequenceless proteins for export in Escherichia coli with altered protein translocase. EMBO J. 1996;15:5209–5217. [PMC free article] [PubMed] [Google Scholar]
- 86.Nouwen N, de Kruijff B, Tommassen J. prlA suppressors in Escherichia coli relieve the proton electrochemical gradient dependency of translocation of wild-type precursors. Proc Natl Acad Sci USA. 1996;93:5953–5957. doi: 10.1073/pnas.93.12.5953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Duong F, Wickner W. The PrlA and PrlG phenotypes are caused by a loosened association among the translocase SecYEG subunits. EMBO J. 1999;18:3263–3270. doi: 10.1093/emboj/18.12.3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nishiyama K, Fukuda A, Morita K, Tokuda H. Membrane deinsertion of SecA underlying proton motive force-dependent stimulation of protein translocation. EMBO J. 1999;18:1049–1058. doi: 10.1093/emboj/18.4.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Smith MA, Clemons WM, Jr, DeMars CJ, Flower AM. Modeling the effects of prl mutations on the Escherichia coli SecY complex. J Bacteriol. 2005;187:6454–6465. doi: 10.1128/JB.187.18.6454-6465.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Maillard AP, Lalani S, Silva F, Belin D, Duong F. Deregulation of the SecYEG translocation channel upon removal of the plug domain. J Biol Chem. 2007;282:1281–1287. doi: 10.1074/jbc.M610060200. [DOI] [PubMed] [Google Scholar]
- 91.Li W, Schulman S, Boyd D, Erlandson K, Beckwith J, Rapoport TA. The plug domain of the SecY protein stabilizes the closed state of the translocation channel and maintains a membrane seal. Mol Cell. 2007;26:511–521. doi: 10.1016/j.molcel.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 92.Osborne RS, Silhavy TJ. PrlA suppressor mutations cluster in regions corresponding to three distinct topological domains. EMBO J. 1993;12:3391–3398. doi: 10.1002/j.1460-2075.1993.tb06013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang L, Miller A, Rusch SL, Kendall DA. Demonstration of a specific Escherichia coli SecY-signal peptide interaction. Biochemistry. 2004;43:13185–13192. doi: 10.1021/bi049485k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Plath K, Mothes W, Wilkinson BM, Stirling CJ, Rapoport TA. Signal sequence recognition in posttranslational protein transport across the yeast ER membrane. Cell. 1998;94:795–807. doi: 10.1016/s0092-8674(00)81738-9. [DOI] [PubMed] [Google Scholar]
- 95.Tuteja R. Type I signal peptidase: an overview. Arch Biochem Biophys. 2005;441:107–111. doi: 10.1016/j.abb.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 96.Paetzel M, Karla A, Strynadka NCJ, Dalbey RE. Signal peptidases. Chem Rev. 2002;102:4549–4579. doi: 10.1021/cr010166y. [DOI] [PubMed] [Google Scholar]
- 97.Watts C, Wickner W, Zimmermann R. M13 procoat and a pre-immunoglobulin share processing specificity but use different membrane receptor mechanisms. Proc Natl Acad Sci USA. 1983;80:2809–2813. doi: 10.1073/pnas.80.10.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Halpin C, Elderfield PD, James HE, Zimmermann R, Dunbar B, Robinson C. The reaction specificities of the thylakoidal processing peptidase and Escherichia coli leader peptidase are identical. EMBO J. 1989;8:3917–3921. doi: 10.1002/j.1460-2075.1989.tb08572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Paetzel M, Dalbey RE, Strynadka NC. Crystal structure of a bacterial signal peptidase in complex with a beta-lactam inhibitor. Nature. 1998;396:186–190. doi: 10.1038/24196. [DOI] [PubMed] [Google Scholar]
- 100.Paetzel M, Goodall JJ, Kania M, Dalbey RE, Page MGP. Crystallographic and biophysical analysis of a bacterial signal peptidase in complex with a lipopeptide-based inhibitor. J Biol Chem. 2004;279:30781–30790. doi: 10.1074/jbc.M401686200. [DOI] [PubMed] [Google Scholar]
- 101.Paetzel M, Dalbey RE, Strynadka NC. Crystal structure of a bacterial signal peptidase apoenzyme: implications for signal peptide binding and the Ser-Lys dyad mechanism. J Biol Chem. 2002;277:9512–9519. doi: 10.1074/jbc.M110983200. [DOI] [PubMed] [Google Scholar]
- 102.van Klompenburg W, Whitley P, Diemel R, von Heijne G, de Kruiff B. A quantitative assay to determine the amount of signal peptidase I in E. coli and the orientation of membrane vesicles. Mol Membr Biol. 1995;12:349–353. doi: 10.3109/09687689509072437. [DOI] [PubMed] [Google Scholar]
- 103.Samuelson JC, Chen M, Jiang F, Moller I, Wiedmann M, Kuhn A, Phillips GJ, Dalbey RE. YidC mediates membrane protein insertion in bacteria. Nature. 2000;406:637–641. doi: 10.1038/35020586. [DOI] [PubMed] [Google Scholar]
- 104.Yahr TL, Wickner W. Functional reconstitution of bacterial Tat translocation in vitro. EMBO J. 2001;20:3472–2479. doi: 10.1093/emboj/20.10.2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jain RG, Rusch SL, Kendall DA. Signal peptide cleavage regions. Functional limits on length and topological implications. J Biol Chem. 1994;269:16305–16310. [PubMed] [Google Scholar]
- 106.Nilsson I, Whitley P, von Heijne G. The COOH-terminal ends of internal signal and signal-anchor sequences are positioned differently in the ER translocase. J Cell Biol. 1994;126:1127–1132. doi: 10.1083/jcb.126.5.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rosenblatt M, Beaudette NV, Fasman GD. Conformational studies of the synthetic precursor-specific region of preproparathyroid hormone. Proc Natl Acad Sci USA. 1980;77:3983–3987. doi: 10.1073/pnas.77.7.3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Perlman D, Halvorson HO. A putative signal peptidase recognition site and sequence in eukaryotic and prokaryotic signal peptides. J Mol Biol. 1983;167:391–409. doi: 10.1016/s0022-2836(83)80341-6. [DOI] [PubMed] [Google Scholar]
- 109.Kajava AV, Zolov SN, Pyatkov KI, Kalinin AE, Nesmeyonova MA. Processing of Escherichia coli alkaline phosphatase. Sequence requirements and possible conformations of the −6 to +4 region of the signal peptide. J Biol Chem. 2002;277:50396–50402. doi: 10.1074/jbc.M205781200. [DOI] [PubMed] [Google Scholar]
- 110.Kalies KU, Rapoport TA, Hartmann E. The beta subunit of the Sec61 complex facilitates cotranslational protein transport and interacts with the signal peptidase during translocation. J Cell Biol. 1998;141:887–894. doi: 10.1083/jcb.141.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chen H, Kim J, Kendall DA. Competition between functional signal peptides demonstrates variation in affinity for the secretion pathway. J Bacteriol. 1996;178:658–6664. doi: 10.1128/jb.178.23.6658-6664.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Josefsson LG, Randall LL. Different exported protein in E. coli show differences in the temporal mode of processing in vivo. Cell. 1981;25:151–157. doi: 10.1016/0092-8674(81)90239-7. [DOI] [PubMed] [Google Scholar]
- 113.Batey RT, Rambo RP, Lucast L, Rha B, Doudna JA. Crystal structure of the ribonucleoprotein core of the signal recognition particle. Science. 2000;287:1232–1239. doi: 10.1126/science.287.5456.1232. [DOI] [PubMed] [Google Scholar]