Abstract
Zinc is an essential biological trace element. It is required for the structure or function of over 300 proteins, and is increasingly recognized for its role in cell signaling. However, high concentrations of zinc have cytotoxic effects, and overexposure to zinc can cause pain and inflammation through unknown mechanisms. Here we show that zinc excites nociceptive somatosensory neurons and causes nociception in mice through TRPA1, a cation channel previously shown to mediate the pungency of wasabi and cinnamon through cysteine-modification. Zinc activates TRPA1 through a novel mechanism that requires zinc influx through TRPA1 channels and subsequent activation via specific intracellular cysteine and histidine residues. TRPA1 is highly sensitive to intracellular zinc, as low nanomolar concentrations activate TRPA1 and modulate its sensitivity. These findings identify TRPA1 as a major target for the sensory effects of zinc, and support an emerging role for zinc as a signaling molecule that can modulate sensory transmission.
Introduction
Zinc is an indispensable heavy metal in the human body. It is mostly bound to proteins and enzymes serving as an essential catalytic, co-catalytic, and structural element 1. Furthermore, free (unbound) zinc acts as a signaling molecule affecting both neuronal and non-neuronal systems 2. Consequently zinc is an essential component of our daily food intake and zinc deficiency can lead to a variety of pathological symptoms. However, zinc is also known to have cytotoxic potential, and endogenous zinc distribution is tightly regulated by efficient homeostatic mechanisms 1, 2. Indeed, exposure to excessive zinc can be harmful and have pathological consequences. For example, exposure through ingestion can cause symptoms including nausea, and gastric pain 3. Zinc exposure can also occur as a consequence of inhalation, as zinc is a common constituent of particulate air pollution, and is an occupational toxin present in welding fumes and smoke bombs. Inhaled zinc causes airway irritation; and in severe cases, zinc fume fever - a disease characterized by pulmonary inflammation and flu-like symptoms caused by the water-soluble fraction of inhaled zinc oxide 3-6. The mechanism behind the pain/irritation and inflammation associated with zinc toxicity is unknown. Noxious levels of zinc could cause irritation by upregulation of inflammatory mediators and subsequent activation of somatosensory neurons 4, 6, 7. Alternatively, zinc could interact directly with nociceptive sensory nerve fibers, initiating pain and neurogenic inflammation
Sensory neurons inform the central nervous system of the thermal, mechanical and chemical conditions of the skin and internal organs. Noxious conditions such as extreme temperatures, tissue damage, or noxious chemicals are detected by a subpopulation of sensory neurons, the so-called nociceptors, which upon excitation signal pain and induce neurogenic inflammation. Recent studies have shown that TRPV1 and TRPA1, both cation channels of the transient receptor potential (TRP) family, are expressed in nociceptive neurons where they function as polymodal receptors for noxious stimuli 8. Specifically, TRPV1 functions as a receptor for noxious hot temperatures (<42° C) and capsaicin (1), the pungent ingredient in chilli peppers 9. TRPA1 is the receptor for mustard oil (2), cinnamaldehyde (3), and various other pungent phytochemicals, and has been proposed as a receptor for noxious cold (<17°C)10-16. Conditions of inflammation and injury can sensitize nociceptive neurons through various mechanisms, including the upregulation of endogenous TRP channel agonists and modulators. Inversely, TRP channel agonists can cause neurogenic inflammation by causing excitation of nociceptive sensory neurons and subsequent neurogenic release of inflammatory peptides 8.
Here we tested the hypothesis that zinc toxicity may involve direct excitation of somatosensory neurons. We show that zinc excites nociceptive sensory neurons and causes pain/irritation through activation of TRPA1 via a unique mechanism, suggesting that a neurogenic mechanism underlies at least part of the sensory symptoms associated with zinc toxicity.
Results
Zinc activates sensory neurons through TRPA1
We used calcium imaging to ask whether zinc could activate cultured sensory neurons from mouse dorsal root ganglia (DRG). Indeed, approximately 15 % of the neurons exhibited increases in calcium levels upon exposure to zinc (Fig. 1a). Approximately 80% of the zinc-responsive neurons also responded to a subsequent application of mustard oil, a TRPA1 agonist. This suggests that zinc acts on a subset of nociceptive neurons 17. To identify the mechanism by which zinc causes calcium influx in sensory neurons, we used a candidate approach and asked whether TRP ion channels reported to be expressed in sensory neurons could account for the observed zinc activation. No activation was observed for TRPV1, TRPV2, TRPV3, TRPV4 or TRPM8 at zinc concentrations between 0.3 μM and 3 mM when assayed by calcium imaging of HEK293 cell line transiently-expressing these ion channels (data not shown). In contrast, zinc potently activated TRPA1, and this activation was inhibited by ruthenium red (4), a known TRPA1 blocker (Fig. 1b and data not shown). TRPA1 activation was observed at zinc concentrations as low as 300 nM, with an EC50 of approximately 2 μM. However, at concentrations higher than 300 μM, zinc also exhibited an inhibitory effect on TRPA1 (data not shown), suggesting that there might be a low affinity inhibitory site for zinc in TRPA1 18. We also tested for activation by another heavy metal with similar properties as zinc, i.e. cadmium. Similar to zinc, cadmium is a potent TRPA1 agonist (EC50 of ∼2 μM, data not show) while no activation of TRPV1, TRPV2, TRPV3, TRPV4 or TRPM8 was observed.
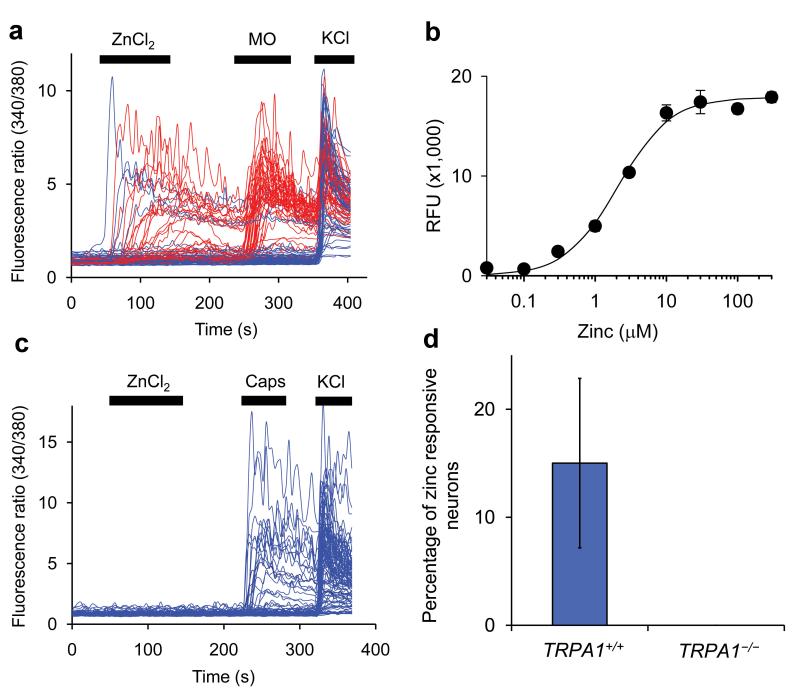
Figure 1. Zinc activates sensory neurons through TRPA1.
(a) Ratiometric calcium imaging of cultured mouse dorsal root ganglia neurons. Each trace corresponds to fluorescence in a single neuron. Neurons were exposed to 30 μM ZnCl2, 100 μM mustard oil and 100 mM KCl for the indicated times (black bars). DRG neurons were isolated from TRPA1+/+ mice facilitating a direct comparison to Fig. 1c. Mustard oil responsive neurons are shown in red. 21% of the neurons responded to ZnCl2 and 48% to mustard oil (b) Zinc dose response relationship on HEK293 cells expressing TRPA1, measured using FLIPR assay. Data points indicate average (± SEM) of fluorescence increase in 4 wells. The EC50 (± sem) was determined at 2.3 ± 0.3 μM. (c) Calcium imaging of TRPA1-/- DRG neurons, exposed to 30 μM ZnCl2, 1 μM capsaicin and 100 mM KCl for the indicated times. Note the absence of zinc responsive neurons. (d) Summary of zinc responses in neurons isolated from TRPA1+/+ and TRPA1-/- mice. Average % (± stdev) of three (TRPA1+/+) and two (TRPA1-/-) individual preps are shown. In total 1900 TRPA1+/+ neurons were tested among which 243 responded to ZnCl2. Out of 1141 TRPA1-/- neurons tested 0 responded to ZnCl2.
We next tested if TRPA1 is required for zinc responses in DRGs. Sensory neurons from TRPA1-/- mice were completely unresponsive to zinc, but retained responsiveness to capsaicin (Fig. 1c,d). These results indicate that TRPA1 is the sole target for zinc-induced sensory neuron responses as assayed by calcium imaging.
It was observed that mustard oil application caused a larger number of neurons to respond than zinc, suggesting that not all TRPA1 expressing neurons respond to zinc (Fig. 1a). A similar phenomenon was observed when comparing the TRPA1 specific agonists cinnamaldehyde to mustard oil (more neurons responding to mustard oil)11.
Zinc induces nociception through TRPA1
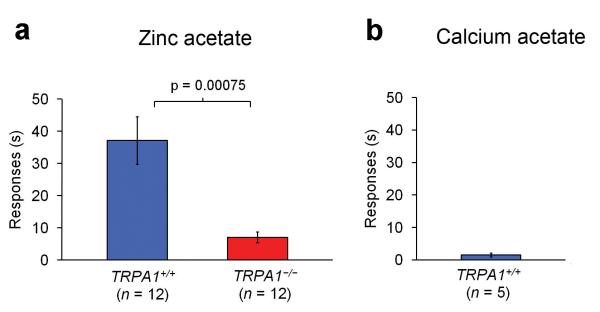
We next asked whether TRPA1 is involved in the pain/irritation effects of zinc in vivo. In spite of the well-documented property of zinc as an irritant in humans, to our knowledge the direct nociceptive effect of zinc in mice had not been tested. In agreement with the described noxious effects of zinc, injection of 30 mM zinc acetate in mouse hindpaw caused licking and flicking of the injected paw indicative of irritation and/or pain (Fig. 2a). In a control experiment no responses were observed in mice injected with 30 mM calcium acetate indicating that the behavior is mediated by zinc rather than the counter ion acetate (Fig. 2b). TRPA1-/- mice displayed a dramatically attenuated zinc-induced nociception. These results indicate that TRPA1 is required for the irritation/pain symptoms associated with excess zinc exposure.
Figure 2. TRPA1 mediates zinc-induced nocifensive behavior.
Zinc acetate (a) or calcium acetate (b) at a concentration of 30 mM was injected in the hindpaw of wildtype and TRPA1-/- mice, and nocifensive responses (paw flicking and licking) was recorded over a period of 5 min. (see methods for details).
The use of millimolar zinc concentrations in these experiments is consistent with other nocifensive behavioral studies involving TRPA1 agonists such as formaldehyde (5), iodoacetamide (6), and mustard oil, for which solutions of 66 mM (0.2%), 15 mM and 75 mM respectively have been used, even though in vitro experiments indicate micromolar EC50 values 16, 19, 20. Possibly the differences between in-vivo and in-vitro potency of these compounds reflects their pharmacokinetic properties or the route of exposure.
Zinc activates TRPA1 via an intracellular site
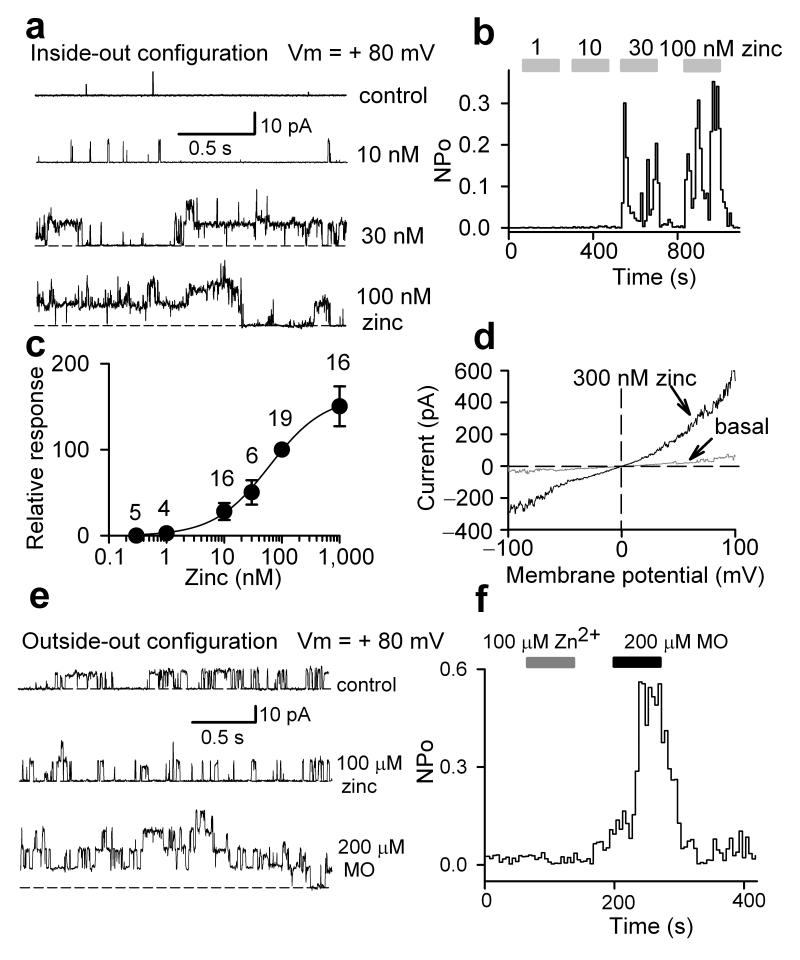
We next set out to characterize the mechanism by which zinc activates TRPA1. First we asked whether activation required intracellular components or is membrane-delimited. To answer this question we measured zinc induced currents in cell-detached membrane patches of HEK293 cells expressing TRPA1. Using the inside-out configuration, we perfused zinc onto the intracellular face of the membrane which induced a reversible and concentration-dependent TRPA1 activation (Fig. 3a-d). Dose-response experiments revealed that intracellular zinc at a concentration as low as 10 nM activated TRPA1, with an EC50 of approximately 50 nM (Fig. 3a-c). Therefore, TRPA1 is much more sensitive to zinc, when assayed intracellularly. Channel activity was inhibited by ruthenium red and camphor (7), two known TRPA1 blockers, and was not observed in control patches without TRPA1 10, 21 (Supplementary Fig. 1, and data not shown). We also observed that 10 nM zinc augments TRPA1 activation by calcium, providing further evidence that low levels of zinc modulate TRPA1 activity (Supplementary Fig. 2 online). These results suggest that zinc activates TRPA1 in a membrane delimited fashion. Zinc activated TRPA1 channels at both positive and negative membrane potentials in the inside-out configuration (Fig. 3d). On the other hand, zinc (100 nM) applied to inside out patches from untransfected HEK293 cells failed to evoke channel activities in 5 out of 5 patches (data not shown).
Figure 3. Zinc activates TRPA1 through intracellular sites.
(a) TRPA1 channel activity in an excised inside-out patch in response to indicated concentrations of ZnCl2. EGTA (1 mM) was included in the pipette solution. (b) Histogram illustrates zinc-induced single channel open probability in an excised inside out patch as shown in (a). (c) Concentration-response curve of zinc-evoked increase of single channel open probability of TRPA1 in excised inside out patches. All points were normalized to the effect of 100 nM zinc. The curve shown is the best fit of the data to the logistic equation, y = Emax/[1 + (x/EC50) -n], where Emax is the maximal response, EC50 is the agonist concentration producing 50% of the maximal response, and n is the slope factor. Numbers of patches tested at each concentration are indicated. (d) Zinc activated TRPA1 channels at both positive and negative membrane potentials in an excised patch with inside-out patch configuration. Voltage ramps of 200 ms to +100 mV after a brief (20 ms) step to -100 mV from holding potential of 0 mV were applied every second in the absence (basal) and presence of 300 nM zinc. (e) TRPA1 channel activity in an excised outside-out patch in response to ZnCl2 and mustard oil (MO). No activity is observed in response to zinc (n = 6). EGTA (1 mM) was included in the pipette solution. (f) Histogram illustrates the time course and effect of zinc and MO on single channel open probability of TRPA1 as shown in (d).
It should be noted that in the above experiments zinc was only added to the intracellular buffer. In fact the extracellular (pipette) buffer contained 1 mM EGTA, which strongly chelates zinc (Kd(Zn) ∼1 nM) indicating that TRPA1 is activated specifically by intracellular zinc. In the reciprocal experiment, we used the outside-out configuration to perfuse zinc onto the extracellular face of the membrane while chelating intracellular zinc with 1 mM EGTA. In striking contrast to intracellular zinc, extracellular zinc did not increase single channel activity above basal level, even though a control agonist did, suggesting that extracellular zinc by itself is unable to activate the channel (Fig. 3e-f). These data suggest that zinc activates TRPA1 through an intracellular site(s).
TRPA1 is zinc permeable
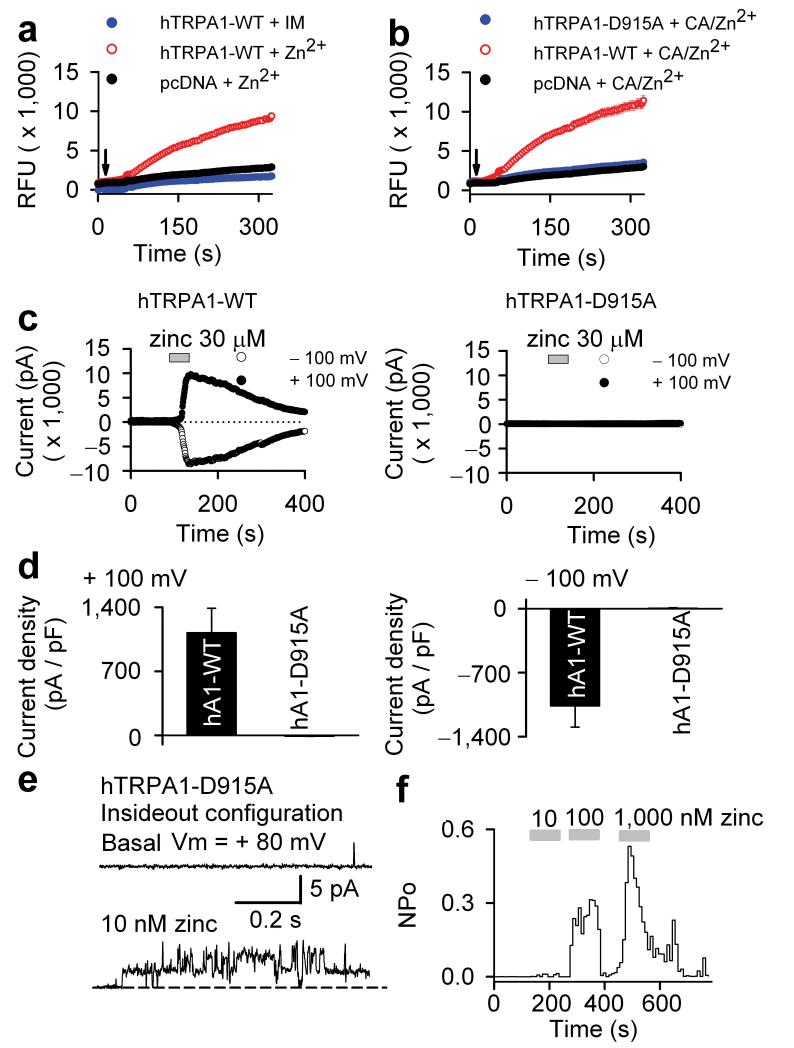
Because zinc is not membrane permeable, the above data raised the question on how extracellularly applied zinc can cause TRPA1 activation in intact HEK293 cells and neurons. One possibility is that zinc enters the cell through active uptake via zinc transporters or through ion channels co-expressed with TRPA1. Given that TRPA1 could be activated by zinc in various cell types (CHO, HEK293, DRG; Fig. 1 and data not shown) we hypothesized that TRPA1 itself might mediate the zinc influx. To explore this, we employed intracellular zinc imaging using the zinc selective fluorophore Fluozin-3 (8) 22. In agreement with our hypothesis, zinc imaging revealed that extracellular application of zinc resulted in significant zinc influx in TRPA1-transfected HEK293 cells, which was absent in cells transfected with vector control (Fig. 4a). No fluorescence increase was observed upon addition of ionomycin (9) indicating that the signal could not have been due to influx of calcium (Fig. 4a).
Figure 4. Zinc permeation through TRPA1 is required for activation by extracellular zinc.
(a) Zinc imaging (Fluozin-3) of wt TRPA1-transfected cells exposed to 30 μM ZnCl2 (red trace) or 2 μM ionomycin (IM, blue trace) at the indicated time (arrow). Vector control-transfected cells were exposed to 30 μM ZnCl2 (black trace). (b) Zinc imaging of cells transfected with wt TRPA1 (red trace), D915A mutant (blue trace) and vector control (black trace). Cells were exposed to 30 μM ZnCl2 in combination with cinnamaldehyde (CA, 1 mM) at indicted time (arrow). (c) Time course of zinc (30 μM)-activated whole-cell currents taken at +100 mV or -100 mV membrane potential in HEK293 cells transfected with wild type TRPA1 or TRPA1-D915A mutant. (d) 30 μM zinc-induced whole-cell current densities at +100 and -100 mV of HEK293 cells transfected with wild type TRPA1 and TRPA1-D915A mutant clones (n = 8 for wild type and n = 7 for TRPA1-D915A mutant). (e) Zinc increased single channel opening in an inside out patch excised from a HEK293 cell transfected with TRPA1-D915A (n = 7). Single channel traces are taken at +80 mV. (f) Histogram illustrates concentration dependent activation of single channels in an excised inside out patch from a TRPA1-D915A mutant expressing HEK293 cell.
To test whether zinc permeation is also a property of natively expressed TRPA1 we assayed intracellular zinc levels in dorsal root ganglia, after exposure to zinc. Strikingly, neurons from TRPA1+/+ mice exhibited increases in Fluozin-3 fluorescence ranging from 1 to ∼8 times the basal level. In contrast, the fluorescence increases in neurons from TRPA1-/- mice were limited largely to less than 2 fold (Supplementary Fig. 3 online). These results suggest that natively-expressed TRPA1 mediates zinc influx. Interestingly, TRPA1 independent mechanisms of zinc influx may also exist since zinc induced moderate fluorescence increases in TRPA1-/- neurons.
These results indicate that TRPA1 can facilitate zinc influx. It is known that TRPA1, like many other TRP channels, exhibits basal channel activity in the absence of any stimulus 23, 24. We therefore proposed a working model in which zinc enters the cell through low level basal activity of TRPA1 channels resulting in subsequent full activation through zinc interaction with intracellular sites. To further test this hypothesis, we generated a mutation in the selectivity filter of TRPA1 known to affect TRP channel permeability to divalent cations 25. This mutant channel (D915A) was functional, but exhibited a lower conductance than the wild type channel, indicative of a mutation affecting the ion permeation pathway (Supplementary Fig. 4a-c online). Interestingly, no zinc influx could be observed in this mutant, even in the presence of saturating concentrations of TRPA1 agonist, cinnamaldehyde. This suggests that TRPA1-D915A abolishes zinc permeation (Fig. 4b). Consistent with this, no activation of TRPA1-D915A mutation by zinc was observed in whole-cell configuration despite the fact that zinc activated large currents at both positive and negative membrane potentials in wild type TRPA1 (Fig. 4c-d). Remarkably, TRPA1-D915A is robustly activated by zinc in inside-out patches, demonstrating that this mutant channel can still be activated by intracellular zinc (Fig. 4e-f). These results suggest that activation by extracellular zinc requires its permeation through TRPA1 to gain access to intracellular sites.
Structural requirements for zinc activation
To further elucidate the mechanism underlying zinc activation, we set out to identify structural elements required for TRPA1 activation by zinc. Detailed insight into protein-zinc interaction sites has come from structural studies on metalloenzymes. These indicate that zinc binding is most commonly mediated by the coordination of multiple side chains from aspartate, glutamate, histidine, and cysteine residues, with the latter two being most prevalent among zinc binding sites 1, 26. Although TRPA1 bears no apparent structural similarity to metalloenzymes, it does contain three N-terminal cytoplasmic cysteine residues that have been implicated in channel activation by electrophilic (cysteine reactive) TRPA1 agonists 27, 28. We therefore asked whether these cysteines are also involved in zinc activation. TRPA1 mutants in which these cysteines were replaced by serines were tested for responses to zinc, cinnamaldehyde (a cysteine-reactive agonist) and flufenamic acid (10; FFA, a non-reactive compound that we recently found to be a potent hTRPA1 agonist). Individual mutation of the cysteines only moderately affected the zinc responses (Supplementary Fig 5a-c; Supplementary Table 1 online). An hTRPA1 channel mutated at all three cysteines responded significantly less to zinc (Supplementary Fig. 5d-f; Supplementary Table 1 online). However, zinc activation was not completely absent. Cinnamaldehyde responses were also significantly reduced as was expected. The non-electrophilic agonist FFA also activated this mutant less potently, suggesting that the loss in zinc activation may in part be due to a more general defect of channel function.
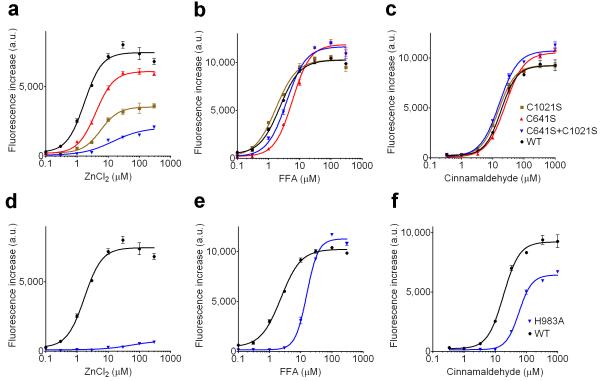
Since the triple-cys mutant still exhibited significant zinc activation, we employed further mutagenesis studies to ask whether other cysteine or histidine residues are required for zinc activation. We thus individually mutated every histidine and cysteine present in hTRPA1 (Supplementary Fig. 6; Supplementary Table 1 and 2 online). This led us to identify C1021S mutant in the c-terminal cytoplasmic region, which shows a partial but highly specific loss of zinc sensitivity (Fig. 5a-c; Supplementary Table 1 online). Combining this mutation with the N-terminal C641S mutation (which among the three N-terminal mutations appeared most selectively affected in its zinc sensitivity) resulted in a mutant that was severely compromised in its responses to zinc while activation by other agonists were not significantly affected (Fig. 5a-c; Supplementary Table 1 online). Among the histidine mutants, H983A caused a severe loss of zinc activation while other agonists could still activate these channels, albeit less potently (Fig. 5d-f). In our mutagenesis experiments, we disregarded mutants which were inactive in response to a non-electrophilic agonist. Thus structural elements required for activation by all agonist types will have been missed.
Figure 5. Role of cysteine and histidine residues in TRPA1 activation by zinc.
Dose response profiles for the indicated TRPA1 mutants in response to cinnamaldehyde (a cysteine reactive TRPA1 agonist), flufenamic acid (FFA, a non-reactive TRPA1 agonist) and ZnCl2 as determined by calcium imaging (FLIPR) (a-c) C1021S (brown squares), C641S (red triangles) and combined C1021S/C641S double mutant (inverted blue triangles) compared to wt TRPA1 (black circles) (d-f) H983A (inverted blue triangles) mutant compared to wt TRPA1 (black circles).
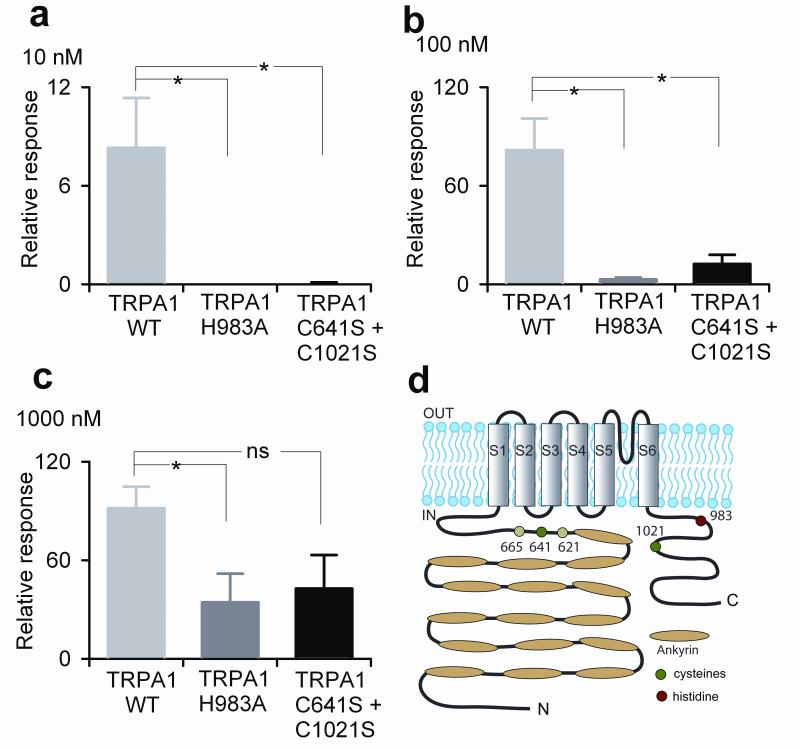
As the above experiments tested responsiveness to extracellular zinc, the loss in zinc activation could be explained by either a defect in zinc influx or a loss of sensitivity to intracellular zinc. Therefore we tested the most zinc-insensitive mutants in inside-out patches. Zinc application to the intracellular side of the membrane showed that both the C641S/C1021S and H983A mutants were severely deficient in intracellular zinc sensitivity (Fig. 6a-c). Importantly, single channel conductance of these mutant channels was similar to wild type TRPA1 (Supplementary Fig. 4 online). Taken together, these results demonstrate that both intracellular cysteine and histidine residues are involved in TRPA1’s sensitivity to intracellular zinc.
Figure 6. H983A and C641S/C1021S double mutant exhibit decreased intracellular zinc sensitivity.
Single channel activity in excised inside-out patches of HEK293 cells expressing wildtype, H983, and C641S/C1021S TRPA1 at holding potential of +80 mV in response to 10 nM (a), 100 nM (b), and 1000 nM (c) ZnCl2. All responses were normalized to the open probability of 500 μM cinnamaldehyde-evoked response in the same patch. N = 7 for wild type, n = 6 for H983 mutant and n = 7 for C641S/C1021S mutant. (d) Cartoon of human TRPA1 with position of histidine and cysteine residues involved in channel activation. Darker green circles indicate cysteines specifically involved in zinc-activation.
Discussion
Overexposure to zinc is often associated with acute irritation and inflammation. However, whether this is due to direct activation of nociceptors or due to indirect consequences of zinc toxicity was not investigated. In this study we find that zinc potently excites nociceptive sensory neurons in a TRPA1 dependent mechanism. Furthermore, zinc induced irritation is dramatically reduced in TRPA1-/- mice. These results suggest that TRPA1 is a major target for sensory symptoms elicited by zinc exposure. Although our data suggest a major role for TRPA1, a minor zinc irritation remained in TRPA1-/- mice, indicating that additional zinc-sensing mechanisms may exist. Furthermore, zinc toxicity with symptoms including inflammation and even fever is likely complex. Although activation of TRPA1 in lung or gut sensory neurons forms an attractive hypothesis to explain at least some of these symptoms, further studies will be required. Given our finding that cadmium is also a TRPA1 agonist, further studies could also test the role of TRPA1 in cadmium toxicity, the symptoms of which appear similar to zinc toxicity including gastrointestinal and respiratory pain/irritation and inflammation29, 30.
The result that TRPA1 acts as a sensor for exogenous zinc raises the question whether TRPA1 can also sense endogenous zinc. Endogenous free zinc ions are increasingly recognized as a signaling molecule and dynamic fluctuations of both intra- and extra-cellular free zinc have been reported. In hippocampal neurons for instance, zinc released from presynaptic vesicles can lead to synaptic concentrations of 10-30 μM that inhibit NMDA (11) and GABA (12) receptor function, and in mast cells intracellular free zinc is upregulated upon IgE receptor (FcepsilonRI) signaling 2, 31. TRPA1 is among the most zinc-sensitive ion channels and receptors presently known, suggesting that TRPA1 might play an important role in sensing endogenous zinc32, 33. Our results show that 10 nM of intracellular zinc is enough to modulate TRPA1 activity. Intracellular free zinc concentrations have been estimated to be in the picomolar to nanomolar range although little is known about variations in concentration in different mammalian cells types 34. Possibly, upregulation of intracellular free zinc could yield concentrations in a range where it could augment TRPA1 function and thus participate in nociception, hyperalgesia, and allodynia. Notably, histochemical stainings of DRG neurons have shown the presence of intracellular zinc, and have documented an increase of this staining upon neuronal injury 35.
In addition to intracellular zinc, extracellular endogenous zinc could activate TRPA1. In analogy to hippocampal neurons, several studies have indicated the existence of zinc containing vesicles in dorsal root ganglia neurons and in layers of the dorsal horn involved in sensory transmission 35-37. Therefore, vesicular zinc release at the spinal projections of the dorsal root ganglia could potentially affect TRPA1 channels present there38. It should be noted here that under some conditions zinc can also have antinociceptive effects suggesting zinc modulation of sensory signaling is likely complex 39-41. Sensory neurons also innervate peripheral targets that have high levels of zinc. Skin, the major innervation target of sensory neurons is for instance highly enriched for zinc, containing 32 times more total zinc than plasma 42. Another intriguing innervation target is the pancreatic islets of Langerhans. Islet beta cells have been shown to co-secrete zinc with insulin, and extracellular concentrations of 0.6-7 μM free zinc have been measured, which would be sufficient for TRPA1 activation and modulation 43, 44. Notably, TRPV1 expressing sensory neurons have recently been shown to innervate the vicinity of islets, and control beta cell stress and islet inflammation in autoimmune diabetes 45. Given that TRPA1 is expressed in TRPV1-expressing neurons, zinc could act as a messenger for a neuroendocrine link between pancreas and sensory neurons. Whether dynamic changes in endogenous zinc can regulate TRPA1 requires further study. Interestingly, modulation by zinc is readily reversible in nature which is in contrast to recently-proposed TRPA1 modulation by reactive endogenous metabolites 8.
Our findings indicate that zinc activation of TRPA1 is reversible and membrane delimited. Although not formal proof, this is consistent with a direct effect of zinc on the channel. Our results further show that TRPA1 activation is mediated via intracellular zinc interaction sites. TRPA1 activation by extracellular zinc thus requires zinc influx. Many TRP channels including TRPA1 exhibit tonic (basal) activity and TRPA1 is shown here to be zinc permeable (but not gated by extracellular zinc, as demonstrated in outside-out patches) 23, 24. Thus our preferred model is that tonic activity of TRPA1 mediates influx of small amounts of zinc which can initiate further activation. Consistent with this model, a D915A mutation in the ion permeation pathway of TRPA1 abolished zinc permeation, and activation by extracellular but not intracellular zinc. This model may explain the large difference in EC50 for intracellular zinc (as measured using inside out patches) and extracellular zinc as measured in intact cells (using FLIPR). In the former experiment intracellular zinc has direct access to the intracellular activation site while in the latter experiment activation depends on accumulation of intracellular zinc which is limited by the rate of influx through TRPA1 on one hand and zinc homeostatic mechanisms present in intact cells on the other (including zinc chelation by intracellular proteins). Thus higher concentrations of extracellular zinc will be required to achieve the same effect as intracellular zinc.
Whether TRPA1-independent mechanisms of zinc influx in sensory neurons, such as through zinc transporters, can also influence zinc activation of TRPA1 remains to be determined. Furthermore, mechanisms of zinc activation may be complicated, and we cannot rule out that full activation may require zinc interactions with both intra- and extra-cellular sites. Irrespective, intracellular action of zinc is a major determinant of TRPA1 activation.
TRPA1 is activated by various reactive and non-reactive chemicals as well as by cold temperatures and in response to receptor-mediated PLC activation 46. The activation of TRPA1 by zinc has given us further insight in the diversity of noxious signals that can activate TRPA1. Zinc-protein interactions have been well studied in metalloproteins suggesting that zinc-binding involves the coordination by 2-4 amino acid side chains, with cysteine and histidine being most prevalent coordinating sites 1, 26. Consistent with this, we identified two cysteine (C641 and C1021) and one histidine (H983) residues that clearly affect zinc sensitivity. The N-terminal C641, which was previously identified as a residue involved in responses to reactive chemicals, showed only a modest requirement for zinc sensitivity. This finding does, however, indicate that zinc sensitivity and cysteine-reactivity may employ partly overlapping mechanisms. The modest deficits in known cysteine mutants led us to identify additional residues involved in zinc activation. The two newly-identified residues (C1021 and H983) reside in the intracellular C-terminal domain of the channel, which has not been previously implicated in TRPA1 activation (Fig. 6d). C1021 displays the most selective requirement for zinc responses, as mutation in this residue shows profound deficits in response to zinc, but not to FFA or cinnamaldehyde. This indicates that zinc activation is at least partly separable from activation by cysteine-modifiers and non-reactive chemicals. H983 is the most robust zinc mutant, severely affecting zinc responses. However, H983 is also partly required for other TRPA1 agonists. Notably, C641 and H983 are conserved between mouse and human TRPA1 and C1021 is a histidine in the mouse sequence suggesting a possible functional conservation. Although mutagenesis studies have to be interpreted with caution, it is tempting to hypothesize that zinc directly binds to these cysteine and histidine residues identified. As the identified residues are in distinct domains of the protein, this raises the question whether the N- and C-termini come together to form a zinc binding site. Alternatively, the different sites could form multiple zinc binding sites. Interestingly, intracellular zinc sites have also been described in voltage gated K+ channels 47.
Our studies show that the activation of TRPA1 by zinc can explain some of the pathological consequences of zinc toxicity. In addition, the activation of TRPA1 by zinc has given us further insight in the diversity of noxious signals that are sensed by TRPA1, and the diversity of mechanisms involved in the activation of this ion channel. Future studies will address how zinc binding or covalent modification of cysteine and histidines translates into TRPA1 ion channel activation. Given the sensitivity of TRPA1 to nanomolar concentrations of zinc, future work will also address if this channel plays a role in sensing endogenous zinc.
Materials and methods
DRG neurons, cell culture and genetic constructs
Dissociation and culturing of mouse DRG neurons was performed as previously described 48. HEK293 cells were grown at 37°C, 5% CO2 in Dulbecco’s minimal essential medium containing 4.5 mg/ml glucose, 10% heat-inactivated fetal bovine serum, 50 units/ml penicillin, and 50 μg/ml streptomycin. For calcium imaging, zinc imaging and electrophysiological studies, cells were transfected with the desired cDNA using Fugene 6.0 (Roche Diagnostics) following manufacturer protocol. cDNA for human TRPA1, rat TRPV1, rat TRPV2, mouse TRPV3, and rat TRPV4, were in the pcDNA3/5-FRT vector. Human TRPM8 was in the pcDNA4-TO vector. All site-directed mutants were generated by QuikChange® II XL Site-Directed Mutagenesis Kit (Stratagene) and verified by DNA sequencing (we used human instead of mouse TRPA1 for all studies described here because mouse mutants showed more pronounced overall channel dysfunction in FLIPR assay)
Calcium- and zinc imaging
For single cell intracellular calcium- and zinc imaging, dorsal root ganglion neurons were isolated and cultured for 24h on polylysine coated coverslips as described 48. For single cell zinc imaging, neurons were incubated for 45-75 min. at 37 °C with 4 μM Fluozin-3-AM and 0.04% pluronic F127 (Invitrogen) in assay buffer (10mM HEPES buffered Hanks’ Balanced Salt Solution: 1,26mM Ca2+, 0.9mM Mg2+, 5.8mM K+, 138.6mM Na+, Invitrogen). After loading cells were washed and constantly perfused with assay buffer. Images of cells (Ex: 490 nm, Em:530 nm) were captured with a cooled CCD camera attached to an inverted microscope (Nikon) and analyzed using MetaFluor software (Universal Imaging Corporation). For single cell calcium imaging a similar procedure was used 10. Briefly, cells were loaded with the ratiometric fluorophore Fluo-2 and imaged (Ex:340 and 380, Em:510) while constantly perfused with assay buffer (10mM HEPES buffered Hanks’ Balanced Salt Solution). ZnCl2 was added to the buffer as indicated and experiments were performed at room temperature
Intracellular calcium imaging of HEK293 cells was performed using a 384-well fluorescent imaging plate reader (FLIPR, Molecular devices) essentially as described 49. Briefly, HEK293 cells were transfected with the desired cDNA (50 ng/well), and seeded in black 384-well clear bottom plates at a density of 8000 cells per well. Two days after transfection cells were washed with FLIPR assay buffer (see below), loaded with Fluo-3 (Invitrogen) according to manufacturer’s protocol, washed again, and placed on FLIPR to measure fluorescence increase upon addition of agonist. For intracellular zinc imaging Fluo-3 was replaced by the zinc specific fluorophore Fluozin-3 (Invitrogen) and 1.25 mM probenecid was included in the assay buffer to block dye leakage observed with this fluorophore. For both Ca2+ and Zn2+ imaging, the FLIPR assay buffer consisted of 20mM HEPES buffered Hanks’ Balanced Salt Solution (Invitrogen; 1,26mM Ca2+, 0.9mM Mg2+, 5.8mM K+, 138.6mM Na+). Dose response curves were fitted to a variable slope sigmoidal dose response curve (PRISM and Sigmaplot) and error bars represent SEM. EC50 values ± SEM are indicated (Sigmaplot).
Electrophysiological recordings
HEK293 cells were co-transfected with EGFP and wild-type or mutant hTRPA1 constructs in wells of 24-well plates. Transfected HEK293 cells were reseeded on 12 mm round glass coverslips (Warner Instruments) one day after transfection. Whole cell recordings were performed the following day. Recording pipettes were pulled from micropipette glass (Sutter) to 2–4 MΩ when filled with a low buffered internal solution containing 140 mM CsCl, 2.0 mM MgCl2, 0.02 mM EGTA, 10 mM Hepes, pH 7.2. Bathing solution contained 140 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 10 mM glucose, and 10 mM Hepes, pH 7.4. To prevent TRPA1 desensitization, after the formation of whole cell configuration, cells were perfused in the same solution except without addition of calcium. Isolated cells were voltage-clamped in the whole cell mode using an EPC9 (HEKA Instruments Inc) amplifier. Voltage commands were made from the Pulse and PulseFit program, and the currents were recorded at 5 kHz. Voltage ramps of 200 ms to +100 mV after a brief (20 ms) step to -100 mV from holding potential of 0 mV were applied every second. Cells were continuously perfused with the bath solution through a Valve-Bank perfusion system (Automate Scientific). For inside-out and outside-out patches, both the pipette solution and bath solution contained (in mM) 140 CsCl, 1 EGTA, 1 MgCl2, 10 Hepes, pH 7.4. Excised patches were held constantly at desired potentials while compounds were repetitively applied and washed from the bath. Single channel currents were recorded at 10 kHz and filtered at 3 kHz. For quantitative analysis of single channel recording data the Pulse files were converted to Axon Binary Files (ABF Utility, Synaptosoft, Decatur, GA, USA). Data were analyzed and plotted using Clampfit 9.2 (molecular devices). Single-channel events were identified on the basis of the half-amplitude threshold-crossing criteria. P(open) was determined from idealized traces as the ratio of the sum of all open durations to the total trace duration. Values are given as the means ± SEM; n represents the number of measurements. To determine the statistical significance of differences between the means a t test was used. Differences were judged to be statistically significant when P < 0.05. All experiments were performed at 25°C. For Zn2+ or Ca2+-buffered external solutions, total Zn2+ and Ca2+ concentrations were determined using the MaxChelator, available online, according to program instructions.
Behavioral experiment
Nocifensive behavior upon intraplantar hindpaw injection was assayed as described 50. Animals were injected intraplantarly with 20 μl 0.9% saline containing 30 mM zinc- or calcium-acetate (Sigma). The pH of calcium acetate solution was titrated to match the pH of the zinc acetate solution (pH 6.4). Time spent on nocifencive behavior (flicking, licking, and lifting injected paw) was recorded during 5 minutes. Genetically modified animals were tested and compared with their control (wild type) littermates. TRPA1-/- mice were kindly provided by Dr. David Corey 16. All experiments were performed blind with respect to genotype and were conducted with the approval of the The Scripps Research Insitute Animal Research Committee. Students’ T test was used for all statistical calculations. Error bars represent standard error of the mean.
Supplementary Material
Supplemental Figure 1. Ruthenium red and camphor block zinc activation of TRPA1 single channel in inside out patches. (a) Histogram of single channel open probability reveals blockage of zinc-activated single channel activity by 2 mM camphor. (b) Single channel current traces show that zinc increased single channel activity as compared with TRPA1 basal activity. Camphor blocked zinc-evoked response and induced long close state of the channel. All traces were taken at +80 mV. (c) Histogram illustrates the blocking effect of ruthenium red on zinc-evoked TRPA1 single channel activity in an excised inside out patch. (gd) Single channel traces illustrate a reversible block of zinc-evoked single channel openings by 10 \g=m\M ruthenium red. All traces were taken at +80 mV. N = 3 for both camphor and ruthenium block.
Supplemental Figure 2. Zinc modulates calcium activation of TRPA1 in excised inside out patches. (a) TRPA1 activity in an excised inside out patch at holding potential of +80 mV in response to indicated zinc and calcium concentrations. (b) Histogram illustrates the effect of zinc, calcium or co-application of both on single channel open probability of TRPA1. (c) Bar chart illustrates the modulation of zinc and calcium on single channel activity of TRPA1 in excised inside out patches. All values were normalized to the effect of 1 \g=m\M calcium. ** P < 0.01, n = 9.
Supplemental Figure 3. TRPA1-mediated zinc influx as assayed by zinc imaging of cultured mouse dorsal root ganglia neurons. Each trace corresponds to fluorescence (Fluozin-3) in a single neuron. DRG neurons isolated from TRPA1+/+ (a) and TRPA1-/- (b) mice were continuously perfused with assay buffer (1,26 mM Ca2+, 0.9 mM Mg2+, 5.8 mM K+, 138.6 mM Na+) that included 300 \g=m\M ZnCl2 for the indicated time (black bar). (c) The percentage of neurons that exhibited > 3 fold increase in fluorescence upon application of ZnCl2 at 30 and 300 \g=m\M. Number of zinc responsive/total neurons tested are indicated between parentheses.
Supplemental Figure 4. Single channel conductance of wild type TRPA1 and D915A, H983A and C641S/C1021S mutants. (a) Single-channel current amplitudes obtained from Gaussian fit to current histograms as a function of voltage. Error bars are standard errors; straight lines indicate linear fits to the data. The average values of unitary conductance were 177.5 pS (wild-type TRPA1, n = 30), 180.2 pS (H983A, n = 9), and 194.8 pS (C641S/C1021S, n = 12). All data were obtained from inside-out configurations clamped at various membrane potentials. (b-e) Single channel traces taken at referred voltages for wild type and indicated mutant channels with inside-out configuration.
Supplemental Figure 5. Role of cysteine and histidine residues in TRPA1 activation by zinc. Dose response profiles for the indicated TRPA1 mutants in response to cinnamaldehyde (a cysteine reactive TRPA1 agonist), flufenamic acid (FFA, a non-reactive TRPA1 agonist) and ZnCl2 as determined by calcium imaging (FLIPR) (a-c) Individual N-terminal cysteine mutants C621S, C641S, and C665S compared to wt TRPA1. (d-f) Triple cysteine mutant C621S/C641S/C665S compared to wt TRPA1
Supplemental Figure 6. Schematic representation of histidine and cysteine mutants tested for zinc sensitivity. Sequence alignment of human and mouse TRPA1 protein sequences is shown. Colors indicate mutant phenotype and bars indicate putative transmembrane segments. Statistics for agonist sensitivity of individual mutants are presented in supplemental Table 1 and 2. Mutants with a 3 fold increase in EC50 values and/or 60% lower in maximal responses compared with wild type TRPA1 are considered to have decreased zinc or FFA responses.
Supplemental Table 1. EC50 values, Hill coefficients and normalized maximal responses for zinc, FFA and cinnamaldehyde activation of wild type and mutant TRPA1 channels depicted in Figure 5 and Supplemental Figure 5. Maximal responses of all mutants to zinc or FFA are normalized to maximal response evoked by 100 \g=m\M zinc or FFA in wild type TRPA1. Data of mutant TRPA1 were compared with wild type TRPA1 transfected in the same 384 well plates
Supplemental Table 2. EC50 values, Hill coefficients and normalized maximal responses for zinc and FFA activation of wild type and mutant TRPA1 channels. Maximal responses of all mutants to zinc or FFA are normalized to maximal response evoked by 100 \g=m\M zinc or FFA in wild type TRPA1. Data of mutant TRPA1 were compared with wild type TRPA1 transfected in the same 384 well plates.
Acknowledgements
We thank Jayanti Mathur, Taryn Earley, James Watson and Marina Garrett for excellent technical support, Takashi Miyamoto and Bailong Xiao for supplying mutant TRPA1 constructs, and Tim Jegla and members of the Patapoutian lab for helpful discussions. We also thank the following individuals for generously sharing reagents: M. Caterina for rat TRPV1, N. Prevarskaya for human TRPM8. This research was supported by NIH and Novartis Research Foundation.
References
- 1.Vallee BL, Falchuk KH. The biochemical basis of zinc physiology. Physiol Rev. 1993;73:79–118. doi: 10.1152/physrev.1993.73.1.79. [DOI] [PubMed] [Google Scholar]
- 2.Frederickson CJ, Koh JY, Bush AI. The neurobiology of zinc in health and disease. Nat Rev Neurosci. 2005;6:449–62. doi: 10.1038/nrn1671. [DOI] [PubMed] [Google Scholar]
- 3.Barceloux DG. Zinc. J Toxicol Clin Toxicol. 1999;37:279–92. doi: 10.1081/clt-100102426. [DOI] [PubMed] [Google Scholar]
- 4.Blanc PD, Boushey HA, Wong H, Wintermeyer SF, Bernstein MS. Cytokines in metal fume fever. Am Rev Respir Dis. 1993;147:134–8. doi: 10.1164/ajrccm/147.1.134. [DOI] [PubMed] [Google Scholar]
- 5.Adamson IY, Prieditis H, Hedgecock C, Vincent R. Zinc is the toxic factor in the lung response to an atmospheric particulate sample. Toxicol Appl Pharmacol. 2000;166:111–9. doi: 10.1006/taap.2000.8955. [DOI] [PubMed] [Google Scholar]
- 6.Kuschner WG, D’Alessandro A, Wong H, Blanc PD. Early pulmonary cytokine responses to zinc oxide fume inhalation. Environ Res. 1997;75:7–11. doi: 10.1006/enrs.1997.3765. [DOI] [PubMed] [Google Scholar]
- 7.Ozaktay AC, et al. Effects of interleukin-1 beta, interleukin-6, and tumor necrosis factor on sensitivity of dorsal root ganglion and peripheral receptive fields in rats. Eur Spine J. 2006;15:1529–37. doi: 10.1007/s00586-005-0058-8. [DOI] [PubMed] [Google Scholar]
- 8.Patapoutian A, Tate S, Woolf CJ. Transient receptor potential channels: targeting pain at the source. Nat Rev Drug Discov. 2009;8:55–68. doi: 10.1038/nrd2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caterina MJ, et al. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–24. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 10.Story GM, et al. ANKTM1, a TRP-like Channel Expressed in Nociceptive Neurons, Is Activated by Cold Temperatures. Cell. 2003;112:819–29. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- 11.Bandell M, et al. Noxious Cold Ion Channel TRPA1 Is Activated by Pungent Compounds and Bradykinin. Neuron. 2004;41:849–57. doi: 10.1016/s0896-6273(04)00150-3. [DOI] [PubMed] [Google Scholar]
- 12.Jordt SE, et al. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427:260–5. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- 13.Macpherson LJ, et al. The pungency of garlic: activation of TRPA1 and TRPV1 in response to allicin. Curr Biol. 2005;15:929–34. doi: 10.1016/j.cub.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 14.Obata K, et al. TRPA1 induced in sensory neurons contributes to cold hyperalgesia after inflammation and nerve injury. J Clin Invest. 2005;115:2393–401. doi: 10.1172/JCI25437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bautista DM, et al. TRPA1 Mediates the Inflammatory Actions of Environmental Irritants and Proalgesic Agents. Cell. 2006;124:1269–82. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 16.Kwan KY, Allchorne AJ, Vollrath MA, Christensen A, Zhang DS, Woolf CJ, Corey DP. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron. 2006;50 doi: 10.1016/j.neuron.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 17.Dhaka A, Viswanath V, Patapoutian A. TRP Ion Channels and Temperature Sensation. Annu Rev Neurosci. 2006 doi: 10.1146/annurev.neuro.29.051605.112958. [DOI] [PubMed] [Google Scholar]
- 18.Riera CE, Vogel H, Simon SA, le Coutre J. Artificial sweeteners and salts producing a metallic taste sensation activate TRPV1 receptors. Am J Physiol Regul Integr Comp Physiol. 2007;293:R626–34. doi: 10.1152/ajpregu.00286.2007. [DOI] [PubMed] [Google Scholar]
- 19.Macpherson LJ, et al. An ion channel essential for sensing chemical damage. J Neurosci. 2007;27:11412–5. doi: 10.1523/JNEUROSCI.3600-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McNamara CR, et al. TRPA1 mediates formalin-induced pain. Proc Natl Acad Sci U S A. 2007;104:13525–30. doi: 10.1073/pnas.0705924104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Macpherson LJ, et al. More than cool: Promiscuous relationships of menthol and other sensory compounds. Mol Cell Neurosci. 2006;32:335–43. doi: 10.1016/j.mcn.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 22.Gee KR, Zhou ZL, Ton-That D, Sensi SL, Weiss JH. Measuring zinc in living cells. A new generation of sensitive and selective fluorescent probes. Cell Calcium. 2002;31:245–51. doi: 10.1016/S0143-4160(02)00053-2. [DOI] [PubMed] [Google Scholar]
- 23.Xu H, Delling M, Jun JC, Clapham DE. Oregano, thyme and clove-derived flavors and skin sensitizers activate specific TRP channels. Nat Neurosci. 2006;9:628–35. doi: 10.1038/nn1692. [DOI] [PubMed] [Google Scholar]
- 24.Karashima Y, et al. Bimodal action of menthol on the transient receptor potential channel TRPA1. J Neurosci. 2007;27:9874–84. doi: 10.1523/JNEUROSCI.2221-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang YY, Chang RB, Waters HN, McKemy DD, Liman ER. The nociceptor ion channel TRPA1 is potentiated and inactivated by permeating calcium ions. J Biol Chem. 2008 doi: 10.1074/jbc.M803568200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patel K, Kumar A, Durani S. Analysis of the structural consensus of the zinc coordination centers of metalloprotein structures. Biochim Biophys Acta. 2007;1774:1247–53. doi: 10.1016/j.bbapap.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 27.Macpherson LJ, et al. Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature. 2007;445:541–5. doi: 10.1038/nature05544. [DOI] [PubMed] [Google Scholar]
- 28.Hinman A, Chuang HH, Bautista DM, Julius D. TRP channel activation by reversible covalent modification. Proc Natl Acad Sci U S A. 2006;103:19564–8. doi: 10.1073/pnas.0609598103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barnhart S, Rosenstock L. Cadmium chemical pneumonitis. Chest. 1984;86:789–91. doi: 10.1378/chest.86.5.789. [DOI] [PubMed] [Google Scholar]
- 30.Nordberg G. Excursions of intake above ADI: case study on cadmium. Regul Toxicol Pharmacol. 1999;30:S57–62. doi: 10.1006/rtph.1999.1327. [DOI] [PubMed] [Google Scholar]
- 31.Yamasaki S, et al. Zinc is a novel intracellular second messenger. J Cell Biol. 2007;177:637–45. doi: 10.1083/jcb.200702081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mathie A, Sutton GL, Clarke CE, Veale EL. Zinc and copper: pharmacological probes and endogenous modulators of neuronal excitability. Pharmacol Ther. 2006;111:567–83. doi: 10.1016/j.pharmthera.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 33.Hershfinkel M, Moran A, Grossman N, Sekler I. A zinc-sensing receptor triggers the release of intracellular Ca2+ and regulates ion transport. Proc Natl Acad Sci U S A. 2001;98:11749–54. doi: 10.1073/pnas.201193398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krezel A, Hao Q, Maret W. The zinc/thiolate redox biochemistry of metallothionein and the control of zinc ion fluctuations in cell signaling. Arch Biochem Biophys. 2007;463:188–200. doi: 10.1016/j.abb.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 35.Jo SM, Danscher G, Schroder HD, Suh SW. Depletion of vesicular zinc in dorsal horn of spinal cord causes increased neuropathic pain in mice. Biometals. 2008;21:151–8. doi: 10.1007/s10534-007-9103-x. [DOI] [PubMed] [Google Scholar]
- 36.Velazquez RA, Cai Y, Shi Q, Larson AA. The distribution of zinc selenite and expression of metallothionein-III mRNA in the spinal cord and dorsal root ganglia of the rat suggest a role for zinc in sensory transmission. J Neurosci. 1999;19:2288–300. doi: 10.1523/JNEUROSCI.19-06-02288.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Danscher G, et al. Inhibitory zinc-enriched terminals in mouse spinal cord. Neuroscience. 2001;105:941–7. doi: 10.1016/s0306-4522(01)00243-3. [DOI] [PubMed] [Google Scholar]
- 38.Kosugi M, Nakatsuka T, Fujita T, Kuroda Y, Kumamoto E. Activation of TRPA1 channel facilitates excitatory synaptic transmission in substantia gelatinosa neurons of the adult rat spinal cord. J Neurosci. 2007;27:4443–51. doi: 10.1523/JNEUROSCI.0557-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Larson AA, Kitto KF. Manipulations of zinc in the spinal cord, by intrathecal injection of zinc chloride, disodium-calcium-EDTA, or dipicolinic acid, alter nociceptive activity in mice. J Pharmacol Exp Ther. 1997;282:1319–25. [PubMed] [Google Scholar]
- 40.Safieh-Garabedian B, et al. Zinc reduces the hyperalgesia and upregulation of NGF and IL-1 beta produced by peripheral inflammation in the rat. Neuropharmacology. 1996;35:599–603. doi: 10.1016/0028-3908(96)84630-2. [DOI] [PubMed] [Google Scholar]
- 41.Nelson MT, et al. Reducing agents sensitize C-type nociceptors by relieving high-affinity zinc inhibition of T-type calcium channels. J Neurosci. 2007;27:8250–60. doi: 10.1523/JNEUROSCI.1800-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.King JC, Shames DM, Woodhouse LR. Zinc homeostasis in humans. J Nutr. 2000;130:1360S–6S. doi: 10.1093/jn/130.5.1360S. [DOI] [PubMed] [Google Scholar]
- 43.Gee KR, Zhou ZL, Qian WJ, Kennedy R. Detection and imaging of zinc secretion from pancreatic beta-cells using a new fluorescent zinc indicator. J Am Chem Soc. 2002;124:776–8. doi: 10.1021/ja011774y. [DOI] [PubMed] [Google Scholar]
- 44.Qian WJ, Aspinwall CA, Battiste MA, Kennedy RT. Detection of secretion from single pancreatic beta-cells using extracellular fluorogenic reactions and confocal fluorescence microscopy. Anal Chem. 2000;72:711–7. doi: 10.1021/ac991085t. [DOI] [PubMed] [Google Scholar]
- 45.Razavi R, et al. TRPV1+ sensory neurons control beta cell stress and islet inflammation in autoimmune diabetes. Cell. 2006;127:1123–35. doi: 10.1016/j.cell.2006.10.038. [DOI] [PubMed] [Google Scholar]
- 46.Bandell M, Macpherson LJ, Patapoutian A. From chills to chilis: mechanisms for thermosensation and chemesthesis via thermoTRPs. Curr Opin Neurobiol. 2007;17:490–7. doi: 10.1016/j.conb.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang G, Strang C, Pfaffinger PJ, Covarrubias M. Zn2+-dependent redox switch in the intracellular T1-T1 interface of a Kv channel. J Biol Chem. 2007;282:13637–47. doi: 10.1074/jbc.M609182200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dhaka A, Murray AN, Mathur J, Earley T, Petrus M, Patapoutian A. TRPM8 is required for cold sensation in mice. Neuron. 2007 doi: 10.1016/j.neuron.2007.02.024. doi:10.1016/j.neuron.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 49.Bandell M, et al. High-throughput random mutagenesis screen reveals TRPM8 residues specifically required for activation by menthol. Nat Neurosci. 2006;9:493–500. doi: 10.1038/nn1665. [DOI] [PubMed] [Google Scholar]
- 50.Petrus M, et al. A role of TRPA1 in mechanical hyperalgesia is revealed by pharmacological inhibition. Mol Pain. 2007;3:40. doi: 10.1186/1744-8069-3-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Ruthenium red and camphor block zinc activation of TRPA1 single channel in inside out patches. (a) Histogram of single channel open probability reveals blockage of zinc-activated single channel activity by 2 mM camphor. (b) Single channel current traces show that zinc increased single channel activity as compared with TRPA1 basal activity. Camphor blocked zinc-evoked response and induced long close state of the channel. All traces were taken at +80 mV. (c) Histogram illustrates the blocking effect of ruthenium red on zinc-evoked TRPA1 single channel activity in an excised inside out patch. (gd) Single channel traces illustrate a reversible block of zinc-evoked single channel openings by 10 \g=m\M ruthenium red. All traces were taken at +80 mV. N = 3 for both camphor and ruthenium block.
Supplemental Figure 2. Zinc modulates calcium activation of TRPA1 in excised inside out patches. (a) TRPA1 activity in an excised inside out patch at holding potential of +80 mV in response to indicated zinc and calcium concentrations. (b) Histogram illustrates the effect of zinc, calcium or co-application of both on single channel open probability of TRPA1. (c) Bar chart illustrates the modulation of zinc and calcium on single channel activity of TRPA1 in excised inside out patches. All values were normalized to the effect of 1 \g=m\M calcium. ** P < 0.01, n = 9.
Supplemental Figure 3. TRPA1-mediated zinc influx as assayed by zinc imaging of cultured mouse dorsal root ganglia neurons. Each trace corresponds to fluorescence (Fluozin-3) in a single neuron. DRG neurons isolated from TRPA1+/+ (a) and TRPA1-/- (b) mice were continuously perfused with assay buffer (1,26 mM Ca2+, 0.9 mM Mg2+, 5.8 mM K+, 138.6 mM Na+) that included 300 \g=m\M ZnCl2 for the indicated time (black bar). (c) The percentage of neurons that exhibited > 3 fold increase in fluorescence upon application of ZnCl2 at 30 and 300 \g=m\M. Number of zinc responsive/total neurons tested are indicated between parentheses.
Supplemental Figure 4. Single channel conductance of wild type TRPA1 and D915A, H983A and C641S/C1021S mutants. (a) Single-channel current amplitudes obtained from Gaussian fit to current histograms as a function of voltage. Error bars are standard errors; straight lines indicate linear fits to the data. The average values of unitary conductance were 177.5 pS (wild-type TRPA1, n = 30), 180.2 pS (H983A, n = 9), and 194.8 pS (C641S/C1021S, n = 12). All data were obtained from inside-out configurations clamped at various membrane potentials. (b-e) Single channel traces taken at referred voltages for wild type and indicated mutant channels with inside-out configuration.
Supplemental Figure 5. Role of cysteine and histidine residues in TRPA1 activation by zinc. Dose response profiles for the indicated TRPA1 mutants in response to cinnamaldehyde (a cysteine reactive TRPA1 agonist), flufenamic acid (FFA, a non-reactive TRPA1 agonist) and ZnCl2 as determined by calcium imaging (FLIPR) (a-c) Individual N-terminal cysteine mutants C621S, C641S, and C665S compared to wt TRPA1. (d-f) Triple cysteine mutant C621S/C641S/C665S compared to wt TRPA1
Supplemental Figure 6. Schematic representation of histidine and cysteine mutants tested for zinc sensitivity. Sequence alignment of human and mouse TRPA1 protein sequences is shown. Colors indicate mutant phenotype and bars indicate putative transmembrane segments. Statistics for agonist sensitivity of individual mutants are presented in supplemental Table 1 and 2. Mutants with a 3 fold increase in EC50 values and/or 60% lower in maximal responses compared with wild type TRPA1 are considered to have decreased zinc or FFA responses.
Supplemental Table 1. EC50 values, Hill coefficients and normalized maximal responses for zinc, FFA and cinnamaldehyde activation of wild type and mutant TRPA1 channels depicted in Figure 5 and Supplemental Figure 5. Maximal responses of all mutants to zinc or FFA are normalized to maximal response evoked by 100 \g=m\M zinc or FFA in wild type TRPA1. Data of mutant TRPA1 were compared with wild type TRPA1 transfected in the same 384 well plates
Supplemental Table 2. EC50 values, Hill coefficients and normalized maximal responses for zinc and FFA activation of wild type and mutant TRPA1 channels. Maximal responses of all mutants to zinc or FFA are normalized to maximal response evoked by 100 \g=m\M zinc or FFA in wild type TRPA1. Data of mutant TRPA1 were compared with wild type TRPA1 transfected in the same 384 well plates.