Abstract
Through T cell receptors (TCRs), T cells can detect and respond to very small numbers of foreign peptides among a huge number of self-peptides presented by major histocompatibility complexes (pMHCs) on the surface of antigen-presenting cells (APCs). How T cells achieve such remarkable sensitivity and specificity through pMHC-TCR binding is an intensively pursued issue in immunology today; the key question is how pMHC-TCR binding initiates, or triggers, a signal from TCRs. Multiple competing models have been proposed, none of which fully explains the sensitivity and specificity of TCR triggering. What has been omitted from existing theories is that the pMHC-TCR interaction at the T cell/APC interface must be under constant mechanical stress, due to the dynamic nature of cell-cell interaction. Taking this condition into consideration, we propose the receptor deformation model of TCR triggering. In this model, TCR signaling is initiated by conformational changes of the TCR/CD3 complex, induced by a pulling force originating from the cytoskeleton and transmitted through pMHC-TCR binding interactions with enough strength to resist rupture. By introducing mechanical force into a model of T cell signal initiation, the receptor deformation model provides potential mechanistic solutions to the sensitivity and specificity of TCR triggering.—Ma, Z., Janmey, P. A., Finkel, T. H. The receptor deformation model of TCR triggering.
Keywords: T cell receptor, signaling, TCR signal initiation, cytoskeleton, mechanical force
Using T cell receptors (TCRs) as sensors, T cells patrol mammalian lymphoid organs and scan the surface of antigen-presenting cells (APCs) for the presence of specific agonist peptides presented by major histocompatibility complexes (pMHCs). Encounter with agonist peptides triggers a TCR signal that eventually leads to the activation of T cells and the execution of defensive measures, such as cytokine production and direct target cell killing. Despite its critical importance, the mechanism of TCR triggering, i.e., how engagement of TCRs by agonist pMHCs initiates TCR signaling, is still unclear.
The TCR triggering puzzle involves more than the question of how binding of agonist pMHC to the TCR induces structural changes of the TCR/CD3 complex to facilitate the first steps of T cell signaling, e.g., tyrosine phosphorylation of CD3 components. APCs process many different self- and foreign proteins indiscriminately and, hence, present a huge variety of peptides on the surface. Therefore, for a given T cell with a unique type of TCR, agonist peptides are usually presented by only a small proportion of MHCs, if by any at all. Effective defense against pathogens demands that T cells detect low numbers of agonist peptides, which are usually derived from pathogen proteins. Indeed, increasing evidence shows that T cells can detect as few as 1–10 agonist pMHCs among a huge number of self-pMHCs (1-3). This remarkable capability dictates that a solution to the TCR triggering puzzle must answer three related questions. 1) Mechanism: how does pMHC-TCR interaction change the TCR/CD3 to a structure more favorable for signal initiation? 2) Sensitivity: How do T cells achieve such a high sensitivity to agonist pMHCs? 3) Specificity: How do TCRs discriminate agonist pMHCs from self-pMHCs, which are structurally very similar?
Here, we investigate an aspect of the pMHC-TCR interaction that has thus far been overlooked, i.e., the mechanical stress exerted on a pMHC-TCR binding pair by cytoskeletal forces at the dynamic T cell/APC interface. Taking this factor into consideration, we propose the receptor deformation model of TCR triggering. In this model, TCR is triggered by conformational changes induced by a mechanical force originating from the cytoskeleton, transferred through pMHC-TCR interactions with enough strength to resist rupture under force. We believe this new model provides a unique perspective in addressing all three aspects—mechanism, sensitivity, and specificity—of the TCR triggering puzzle.
THE TWO-DIMENSIONAL (2D) pMHC-TCR INTERACTION
In a sharp contrast to the T cell's remarkable sensitivity to agonist pMHCs on APCs, it has been well documented that soluble monomeric pMHCs do not trigger the TCR, even at very high concentrations (4-7). This finding strongly suggests that TCR triggering depends on the physical environment of the pMHC-TCR interaction.
Both TCRs and pMHCs are membrane anchored, and their interaction takes place at the T cell/APC interface in a 2D fashion. For membrane-anchored pMHCs and TCRs to interact, the two plasma membranes must be aligned at a distance similar to the combined length of pMHC and TCR (∼15 nm) (8). The alignment is achieved through adhesion molecules, probably in a stepwise fashion. First, large adhesion molecules, such as ICAM-1 and LFA-1, bring two membranes close to each other. This, in turn, facilitates the binding between smaller adhesion molecules, such as CD2 and CD48, which are similar in size to pMHC and TCR (9). Consequently, compared to three-dimensional (3D) interactions between receptors and soluble ligands, 2D interactions have different kinetic features (10). In terms of the on-rate (kon), while surface anchoring improves kon by orienting the binding interfaces of the ligands and receptors, ligand-receptor encounter relies on the motion of cells or cell membranes. In 3D interactions, the off-rate (koff) is controlled only by thermal agitation. In 2D interactions, however, the koff may be heavily influenced by mechanical forces exerted on the binding pair when membrane alignment is altered. Although the importance of the 2D kon has been taken into account by van der Merwe in his kinetic-segregation model (discussed below), the effect of mechanical force on koff has been omitted in models of TCR triggering proposed so far.
THE DYNAMIC T CELL/APC INTERACTION
T cells are highly mobile by nature. In tissue culture, T cells are constantly polarized and move at high speed by an amoeboid walk on artificial lipid bilayers or plastic surfaces (11-13). Their locomotion is spontaneous and random in direction, although it can be influenced by chemokine cues (14). On a 2D surface, the rapid migration comes to a halt when the T cell encounters an APC presenting agonist peptides (11, 12, 15), and a stable interaction, for hours, with the same APC was shown to be necessary for T cell activation (11). This stable interaction does not mean, however, that the T cell/APC interaction is completely static. In fact, the cognate interaction between a T cell and an APC is dynamic, with active T cell morphological changes and T cell movement involved in interacting with different areas of the same APC (11, 13, 16). In fact, in a more physiological environmental setting, T cell activation was shown to be a much more dynamic process. Using T cells and dendritic cells (DCs) cultured in a 3D collagen matrix, Gunzer et al. (17) observed that T cells migrate vigorously and interact with DCs presenting agonist peptides in a transient and serial manner, with a median interaction time of 7 to 12 min. The dynamic nature of T cell/APC interaction was recently confirmed in vivo by using two-photon microscopy (18, 19). In the cortical region of lymph nodes, T cells were observed to move randomly at high speeds (11 μm/min on average), in an apparently autonomous fashion. In the presence of specific antigen, during the first 2 h of encounter, T cells make a series of brief interactions (11 to 12 min), engaging and disengaging DCs at an average speed of 5.4 μm/min. Even after 2 h, T cells move with a velocity of 2.6 μm/min within a dynamic cluster formed with APCs. Therefore, T cell activation appears to be the result of a series of dynamic interactions with APCs, accompanied by very active T cell motility.
The importance of the physical dynamics of T cell/APC interaction in TCR triggering is highlighted by the indispensable role of functional actin cytoskeleton in this process. Actin filaments control cell morphology and plasticity and provide the mechanical force necessary for motility (20). Treatment of T cells with the actin depolymerizing agent cytochalasin D inhibits T cell locomotion and blocks T cell calcium flux stimulated by APCs (11). Myosin II, a type of myosin motor that moves along actin filaments while consuming ATP, has also been shown to be necessary for T cell polarization and motility (21).
THE DYNAMIC T CELL/APC INTERFACE AND THE STABILITY OF pMHC-TCR INTERACTION
The dynamic interaction between T cells and APCs observed in vivo (18, 19) strongly suggests that the membrane alignment at the interface is highly unstable. Indeed, under the electron microscope, close alignment permitting pMHC-TCR interaction exists as small patches of varying sizes (87–300 nm in dimension on average), randomly scattered across the interface (8). It is difficult to conceive that during the dynamic T cell/APC interaction, the alignment in these small areas can be stably maintained with the nanometer-scale precision required for interactions between pMHCs and TCRs. Rather, it is more likely that membrane alignment undergoes constant remodeling, driven by the alternating dominance of adhesion and detaching forces necessary for T cell motility and active scanning of the APC surface.
Unstable membrane alignment should have a significant impact on the stability of pMHC-TCR interaction. By anchoring receptors on a surface and monitoring soluble ligand binding, the average half-life (t1/2) of pMHC-TCR binding measured by surface plasmon resonance is ∼10 s (22). Half-life measured in this 3D setting reflects intrinsic pMHC-TCR binding stability. At the 2D interface between T cells and APCs, however, the stability is likely to be heavily influenced, if not dominated, by membrane movements and the forces behind these movements. In the total absence of membrane movements, i.e., when the close membrane alignment suitable for pMHC-TCR interaction is precisely and stably maintained, the spontaneous dissociation should be slower than in a 3D setting, due to the inability of the receptors and ligands to freely diffuse in the third dimension. As discussed above, however, such a scenario is highly improbable at the dynamic T cell/APC interface. Instead, it is much more likely that membrane detachment pulls apart the pMHC-TCR binding at a rate determined by the speed of locomotion. Moreover, because the koff (t1/2=ln2/koff) of ligand-receptor binding increases exponentially with a disengaging force (23), the stability of pMHC-TCR binding should be significantly reduced by a detaching force, even if the membranes are not completely separated.
The speed of membrane detachment supports our hypothesis that force-induced unbinding of pMHC-TCR interaction is likely to be the dominant means of dissociation. T cells move at a speed of 2.6–5.4 μm/min, or 43–90 nm/s in vivo (18, 19), suggesting that the speed of membrane detachment, which can be considered as steps of cell locomotion, should be at least as fast. At this speed, two membranes would double the 15 nm close alignment distance in less than 1 s, before pMHC-TCR spontaneously dissociates. In addition, the detaching force is generated by the movement of myosins along actin filaments (24), which can achieve a velocity as high as 3.6 μm/s (25).
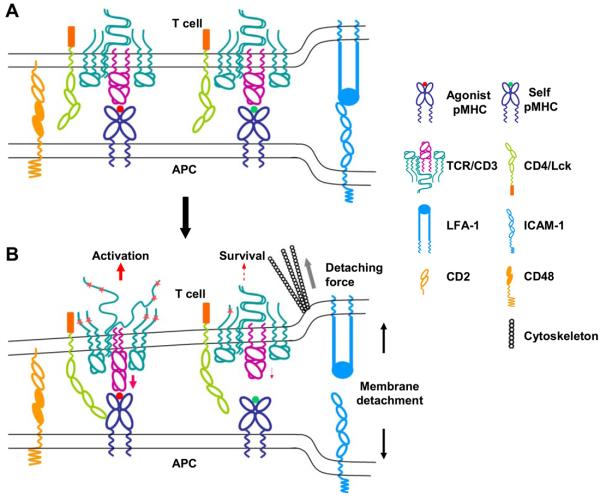
Therefore, pMHC-TCR binding at the dynamic T cell/APC interface may be highly unstable. If this is the case, in the absence of stable ligand-receptor interaction, what is it that triggers TCR? In the receptor deformation model described below (Fig. 1), we argue that it is the cytoskeletal force inherent in T cell motility that drives TCR triggering.
Figure 1.
The schematics of the receptor deformation model of TCR triggering. A) Interaction of pMHCs and TCRs at the close membrane-membrane contact site of the T cell/APC interface. Large adhesion molecules such as LFA-1 and ICAM-1 bring together the cell membranes, which are further aligned by small adhesion molecules such as CD2 and CD48, to a distance permitting pMHC-TCR interaction. The interaction between agonist pMHC and TCR per se does not initiate TCR signaling. B) Forces generated from the cytoskeleton, which drive active T cell movement relative to APC and T cell morphological changes, detach the T cell plasma membrane from the APC. Part of the force is delivered to the TCR/CD3 complex through pMHC-TCR binding. Binding between agonist pMHC and TCR is strong enough to deliver a force that deforms the TCR/CD3 complex to a conformation or configuration that can initiate an activation signal. Weak binding between self-pMHC and TCR breaks before such a force can be delivered. Such a binding could, however, deliver a weak force that causes minor receptor deformation, leading to a survival signal. The detaching force could also deform the MHC molecule to a conformation that binds coreceptors with higher affinity, or directly deform the coreceptors to alter the activity of associated tyrosine kinase Lck.
HOW THE RECEPTOR DEFORMATION MODEL WORKS
In this model, at the dynamic T cell/APC interface, TCR interacts with pMHC when and where a sufficiently close membrane-membrane contact is formed. pMHC-TCR binding per se, however, does not trigger TCR. Instead, a signal is initiated when the binding pair is pulled by a detaching force originating from the T cell actin cytoskeleton, a force originally intended to detach the membrane-membrane contact for T cell movement. The pulling force may induce structural changes that favor TCR signal initiation in the following ways. First, the pulling force may induce a conformational change, or deformation, of the αβ TCR, which is then transferred to the intracellular domains of the CD3 complex through interactions between their extracellular and transmembrane domains (26, 27). Alternatively, if the αβ TCR is rigid, the pulling force may deform the local lipid bilayer and cause changes in the relative positioning and orientation of the components of the TCR/CD3 complex. Third, the pulling force may induce conformational changes of the core-ceptor (CD4 or CD8) binding domains of the MHC molecule and lead to better recruitment or conformational change of the coreceptor. Fourth, the pulling force may directly deform the coreceptor. The deformation may be transmitted to the associated Lck kinase and alter its function or its orientation relative to the CD3 complex. These changes initiate TCR signaling by increased access to and phosphorylation of immunoreceptor tyrosine-based activation motifs (ITAMs) of the CD3 complex by the coreceptor-associated tyrosine kinase, Lck. Minor receptor deformation may be induced by endogenous pMHCs to generate a survival signal. To initiate an activating signal, however, a deformation threshold may have to be exceeded through forces that can only be delivered via stronger agonist pMHC-TCR binding. An alternative type of force is a pushing force on TCR, which could also induce conformational changes. Although we think this is less likely, due to its lack of specificity, we cannot rule out a role in TCR triggering.
HOW THE RECEPTOR DEFORMATION MODEL EXPLAINS THE HIGH SENSITIVITY AND SPECIFICITY OF TCR TRIGGERING
T cell activation by agonist pMHC is dose-dependent. Microscopy studies show that a single agonist pMHC induces only a very weak and transient increase of intracellular calcium (3). This finding suggests that, although a single agonist pMHC can induce TCR signaling, accumulation of signaling from multiple pMHC-TCR interactions is necessary to reach the threshold for T cell activation. How do T cells accumulate signals from as few as one agonist pMHC on an APC? Valitutti et al. (28) proposed that a single agonist pMHC may serially trigger multiple TCRs. If this is the case, in order to accumulate signals from multiple TCRs within a given period of time, rapid disengagement of already-triggered TCRs should be favored. Moreover, since a triggered signal may be short-lived, efficient temporal accumulation of signals would benefit from fast serial triggering. Without external forces, however, TCR binds agonist pMHC with a t1/2 of 10 to 50 s (29). Although the half-life seems relatively short compared with other high-affinity bindings, it may still be too long for T cell activation via serial triggering, since downstream signaling events such as high-level calcium flux (11, 30) and ERK activation (2) are seen within seconds to a few minutes after T cell/APC contact. By introducing an external mechanical force, the receptor deformation model fits well with the serial triggering mechanism, incorporating a disengaging force that would significantly shorten the pMHC-TCR binding time. This, together with T cell motility, would lead to rapid serial triggering of multiple neighboring TCRs by a single agonist pMHC, resulting in highly efficient temporal and spatial accumulation of TCR signaling.
In addition to a very low number of agonist pMHCs, T cells are also faced with the challenge of distinguishing these from a huge number of structurally very similar self-pMHCs. What are the binding characteristics that determine specificity? Data show that, in general, the potency of pMHC to trigger TCR is inversely correlated with the koff of the pMHC-TCR binding measured by surface plasmon resonance (29, 31). Because t1/2 = ln2/koff, it has been argued that stable binding favors TCR triggering. However, as discussed earlier, the stability of the pMHC-TCR interaction taking place in a 2D setting between two closely ap-posed membranes is likely to be determined by dynamic membrane alignments rather than by koff measured in a 3D setting under zero force. Also, as described above, stable pMHC-TCR binding works against efficient TCR serial triggering.
In the receptor deformation model, we postulate that the outcome of a particular pMHC-TCR binding is determined by how much force this binding can deliver to the TCR/CD3 complex before it is broken. In other words, TCR binding strength under force determines whether a pMHC is an agonist and how potent it is. The parameter that describes TCR binding strength under force is the “rupture force”, i.e., the amount of force required to break the binding. The question then becomes whether this direct correlation between pMHC potency and rupture force can be reconciled with the observed inverse correlation between pMHC potency and zero force koff. To reconcile them, an inverse correlation between rupture force and zero force koff needs to be established. Interestingly, such a correlation has been found when antigen-antibody bindings with varying zero force koff were subjected to pulling and their rupture forces were recorded by atomic force microscope (32). Therefore, the proposition that pMHC specificity is defined by pMHC-TCR binding strength under force is consistent with existing experimental data on pMHC-TCR binding kinetics and pMHC potency.
THE IMMUNOLOGICAL SYNAPSE: A DYNAMIC MACHINE DRIVING SUSTAINED SIGNALING?
The concept of the immunological synapse is not very clear. We define it as the interface between a T cell and an APC where pMHC-TCR interaction and information transfer take place. In vitro, especially when a lipid bilayer-based artificial APC is used, segregation of TCR and the adhesion molecule LFA-1 lead to the formation of a bull's-eye pattern, with TCR at the center, surrounded by LFA-1 (33, 34). The formation of this particular pattern has not been confirmed in vivo, and its role is still unclear (35). What is very clear, however, is that the immunological synapse is a highly dynamic structure (34, 36). In terms of morphology, on encountering an APC with agonist peptides, the T cell stops migration and spreads its leading edge to maximize the contact area with the APC. At the molecular level, TCR/CD3 complexes actively translocate from the periphery to the center of the contact area (34, 36). What is interesting is that, after reaching maximum contact, TCR signaling occurs mainly at the periphery of the contact area, where membrane appears to ruffle the most actively (36). Based on the receptor deformation model, we postulate that the initial encounter of the first, or the first few, agonist pMHCs by a T cell while scanning an APC triggers a weak signal that is not sufficient to activate the T cell but enough to induce a stop signal to allow this APC to be probed further. This weak signal also induces active actin cytoskeleton rearrangements (37, 38), not only to maximize the contact area but also to drive active membrane movement and ruffling. These actions help probe more pMHCs on the APC surface and facilitate TCR serial triggering. The persistent membrane movement at the periphery of the synapse sustains signaling. TCRs that have been triggered are transported to the center of the synapse, probably for internalization and recycling, as proposed previously (35, 36, 39). Therefore, in the receptor deformation model, forces from the actin cytoskeleton drive all stages of the TCR triggering process. Forces for T cell locomotion drive the initial triggering, which signals actin cytoskeleton to rearrange in a way that purposefully facilitates serial triggering and sustains the triggering of TCR.
COMPARISON WITH EXISTING MODELS
TCR crosslinking by agonist-agonist pMHCs was among the first models proposed for TCR triggering. This theory, however, suffers from the lack of clear evidence for the presence of MHC dimers on the APC surface. The chance of agonist-agonist dimer formation is particularly small when agonist peptides occupy only a small proportion of surface MHCs. Based on experimental data showing that some endogenous pMHCs enhance TCR triggering by agonist pMHCs, it was recently proposed that TCRs are triggered by a “pseudodimer” of an agonist pMHC and an endogenous pMHC (40, 41). This model, however, is contradicted by other studies showing that endogenous pMHCs do not contribute to TCR triggering (2, 42). Neither crosslinking model explains the high sensitivity and specificity of TCR triggering. In particular, the pseudodimer model suggests that endogenous pMHCs work synergistically with agonist pMHCs to achieve high sensitivity to agonist pMHC, an argument that seems irreconcilable with how TCRs distinguish endogenous and agonist pMHCs.
Monomeric agonist pMHCs may trigger TCRs through either TCR conformational change or heterodimerization of the TCR and the CD4 or CD8 coreceptor via simultaneous pMHC binding. In the conventional conformation change model, conformational change is induced by pMHC binding itself, without assistance from external forces. Due to technical challenges, crystallographic observation of structural changes of the intact TCR/CD3 complex as a whole has not been achieved. Nevertheless, the failure of multiple crystallography studies (43) to show global large-scale conformational changes in the TCR extra-cellular domains following engagement of agonistic pMHCs does not support the classical conformational change model. In the absence of external forces, it is also difficult to conceive how a consistent signal-initiating TCR conformational change could be induced by the binding of a huge variety of pMHCs and TCRs. As to the heterodimerization model, although coreceptors have been shown to enhance T cell activation by pMHCs (5), it is uncertain how critical a role coreceptors play in TCR triggering since TCR triggering in vitro and in vivo can occur in the complete absence of CD4 or CD8 (44, 45). Both models favor stable pMHC-TCR interaction, hence, do not offer a good explanation for the sensitivity through TCR serial triggering.
Putting the pMHC-TCR interaction in a 2D context, van der Merwe proposed the kinetic-segregation model (9, 46, 47). This model states that TCR signaling is initiated by the segregation of small and large membrane molecules, and, hence, their respectively associated tyrosine kinases and phosphatases, at small close membrane-membrane contact zones between a T cell and an APC. The resultant net increase of kinase activity within these zones initiates signaling. The spec-ificity of triggering is determined by how long these zones can be maintained, which is, in turn, determined by the t1/2 of pMHC-TCR binding. Disruption of local kinase and phosphatase balance offers a reasonable mechanism for TCR signal initiation. However, although maintaining close contact zones through specific pMHC-TCR binding is possible in the absence of disturbance caused by an external force, it is difficult to conceive how it can be achieved at a dynamic T cell/APC interface, especially when each zone contains only one agonist pMHC, as originally proposed. Because this model relies on stable pMHC-TCR interaction, serial triggering cannot be used to explain the high sensitivity of TCR triggering. If considered in a dynamic setting, this model also has difficulty addressing the specificity of TCR triggering. Recently, agonist pMHCs with elongated extracellular stems were shown to have decreased TCR triggering potency (46). This finding was cited as evidence for the kinetic-segregation model, since elongated pMHCs should result in less segregation of the pMHC-TCR binding pair from large tyrosine phosphatases, such as CD45. Interestingly, these data would also support the TCR deformation model, since elongated pMHCs would require the two opposing membranes to travel a longer distance to exert force on pMHC-TCR binding.
By introducing external mechanical forces, the receptor deformation model provides receptor deformation as the signal initiation mechanism, explains the high sensitivity of TCR triggering with rapid TCR serial engagement, and offers binding strength under force, or rupture force, as the pMHC-TCR binding character that defines pMHC specificity and potency. In terms of what defines a specific pMHC-TCR binding, this new model may represent a new step in the evolution of our understanding of this question, from affinity to 3D kinetics, to 2D kinetics, to dynamic 2D kinetics. Notwithstanding these strengths, the receptor deformation model has its weaknesses. First, it does not explain TCR triggering by soluble multimeric agonist pMHCs (5, 48). In our opinion, it is not surprising that T cells can be activated via a receptor crosslinking mechanism that is used by B cells, given their similarity in evolution, development, antigen receptor structure, and intracellular signaling pathways. We cannot, in fact, exclude the possibility that when agonist pMHCs are present at extremely high levels on APCs, TCR crosslinking is a working mechanism of TCR triggering. In this situation, the chance of two nearby MHC molecules, both presenting agonist peptides, may be reasonably high. This, and the fact that a large proportion of MHC molecules on the APC surface is immobile (49, 50), make it plausible that two monomeric agonist pMHCs could be stably localized in close enough proximity to act as functional dimers that can crosslink TCRs. Given the rarity of agonist pMHCs on APCs under physiological conditions, however, a mechanism that does not rely on TCR crosslinking, such as receptor deformation, is likely to be the main working mechanism. Second, and more importantly, critical elements of the receptor deformation model, such as force-induced structural change of the TCR/CD3 complex and the correlation between pMHC potency and pMHC-TCR binding strength under force, have yet to be directly supported by experimental data.
CONCLUSIONS
We examine the TCR triggering mechanism from the perspective of dynamic T cell/APC interaction and present the receptor deformation model of TCR triggering. In addition to offering alternative explanations to the mechanism, sensitivity, and specificity of TCR triggering, this model is consistent with the dynamics of T cell/APC interaction, the correlation between zero force koff and TCR triggering potency, and the negative impact of elongated pMHCs on TCR triggering. Conformational change in the CD3 complex induced by pMHC-TCR binding has also been documented (51, 52). Given its theoretical merits and testability by currently available technologies, such as atomic force microscopy, we believe this model warrants further investigation.
REFERENCES
- 1.Sykulev Y, Joo M, Vturina I, Tsomides TJ, Eisen HN. Evidence that a single peptide-MHC complex on a target cell can elicit a cytolytic T cell response. Immunity. 1996;4:565–571. doi: 10.1016/s1074-7613(00)80483-5. [DOI] [PubMed] [Google Scholar]
- 2.Altan-Bonnet G, Germain RN. Modeling T cell antigen discrimination based on feedback control of digital ERK responses. PLoS Biol. 2005;3:1925–1938. doi: 10.1371/journal.pbio.0030356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Irvine DJ, Purbhoo MA, Krogsgaard M, Davis MM. Direct observation of ligand recognition by T cells. Nature. 2002;419:845–849. doi: 10.1038/nature01076. [DOI] [PubMed] [Google Scholar]
- 4.Cochran JR, Cameron TO, Stern LJ. The relationship of MHC-peptide binding and T cell activation probed using chemically defined MHC class II oligomers. Immunity. 2000;12:241–250. doi: 10.1016/s1074-7613(00)80177-6. [DOI] [PubMed] [Google Scholar]
- 5.Boniface JJ, Rabinowitz JD, Wulfing C, Hampl J, Reich Z, Altman JD, Kantor RM, Beeson C, McConnell HM, Davis MM. Initiation of signal transduction through the T cell receptor requires the multivalent engagement of peptide/MHC ligands [corrected] Immunity. 1998;9:459–466. doi: 10.1016/s1074-7613(00)80629-9. [DOI] [PubMed] [Google Scholar]
- 6.Casares S, Zong CS, Radu DL, Miller A, Bona CA, Brumeanu TD. Antigen-specific signaling by a soluble, dimeric peptide/major histocompatibility complex class II/Fc chimera leading to T helper cell type 2 differentiation. J. Exp. Med. 1999;190:543–553. doi: 10.1084/jem.190.4.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stone JD, Stern LJ. CD8 T cells, like CD4 T cells, are triggered by multivalent engagement of TCRs by MHC-peptide ligands but not by monovalent engagement. J. Immunol. 2006;176:1498–1505. doi: 10.4049/jimmunol.176.3.1498. [DOI] [PubMed] [Google Scholar]
- 8.Brossard C, Feuillet V, Schmitt A, Randriamampita C, Romao M, Raposo G, Trautmann A. Multifocal structure of the T cell-dendritic cell synapse. Eur. J. Immunol. 2005;35:1741–1753. doi: 10.1002/eji.200425857. [DOI] [PubMed] [Google Scholar]
- 9.Anton van der Merwe P, Davis SJ, Shaw AS, Dustin ML. Cytoskeletal polarization and redistribution of cell-surface molecules during T cell antigen recognition. Semin. Immunol. 2000;12:5–21. doi: 10.1006/smim.2000.0203. [DOI] [PubMed] [Google Scholar]
- 10.Dustin ML, Bromley SK, Davis MM, Zhu C. Identification of self through two-dimensional chemistry and synapses. Annu. Rev. Cell Dev. Biol. 2001;17:133–157. doi: 10.1146/annurev.cellbio.17.1.133. [DOI] [PubMed] [Google Scholar]
- 11.Valitutti S, Dessing M, Aktories K, Gallati H, Lanzavecchia A. Sustained signaling leading to T cell activation results from prolonged T cell receptor occupancy. Role of T cell actin cytoskeleton. J. Exp. Med. 1995;181:577–584. doi: 10.1084/jem.181.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dustin ML, Bromley SK, Kan Z, Peterson DA, Unanue ER. Antigen receptor engagement delivers a stop signal to migrating T lymphocytes. Proc. Natl. Acad. Sci. U. S. A. 1997;94:3909–3913. doi: 10.1073/pnas.94.8.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Underhill DM, Bassetti M, Rudensky A, Aderem A. Dynamic interactions of macrophages with T cells during antigen presentation. J. Exp. Med. 1999;190:1909–1914. doi: 10.1084/jem.190.12.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bromley SK, Peterson DA, Gunn MD, Dustin ML. Cutting edge: hierarchy of chemokine receptor and TCR signals regulating T cell migration and proliferation. J. Immunol. 2000;165:15–19. doi: 10.4049/jimmunol.165.1.15. [DOI] [PubMed] [Google Scholar]
- 15.Inaba K, Steinman RM. Accessory cell-T lymphocyte interactions. Antigen-dependent and -independent clustering. J. Exp. Med. 1986;163:247–261. doi: 10.1084/jem.163.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Donnadieu E, Bismuth G, Trautmann A. Antigen recognition by helper T cells elicits a sequence of distinct changes of their shape and intracellular calcium. Curr. Biol. 1994;4:584–595. doi: 10.1016/s0960-9822(00)00130-5. [DOI] [PubMed] [Google Scholar]
- 17.Gunzer M, Schafer A, Borgmann S, Grabbe S, Zanker KS, Brocker EB, Kampgen E, Friedl P. Antigen presentation in extracellular matrix: interactions of T cells with dendritic cells are dynamic, short lived, and sequential. Immunity. 2000;13:323–332. doi: 10.1016/s1074-7613(00)00032-7. [DOI] [PubMed] [Google Scholar]
- 18.Miller MJ, Safrina O, Parker I, Cahalan MD. Imaging the single cell dynamics of CD4+ T cell activation by dendritic cells in lymph nodes. J. Exp. Med. 2004;200:847–856. doi: 10.1084/jem.20041236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mempel TR, Henrickson SE, Von Andrian UH. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature. 2004;427:154–159. doi: 10.1038/nature02238. [DOI] [PubMed] [Google Scholar]
- 20.Vicente-Manzanares M, Sanchez-Madrid F. Role of the cytoskeleton during leukocyte responses. Nat. Rev. Immunol. 2004;4:110–122. doi: 10.1038/nri1268. [DOI] [PubMed] [Google Scholar]
- 21.Jacobelli J, Chmura SA, Buxton DB, Davis MM, Krummel MF. A single class II myosin modulates T cell motility and stopping, but not synapse formation. Nat. Immunol. 2004;5:531–538. doi: 10.1038/ni1065. [DOI] [PubMed] [Google Scholar]
- 22.Van der Merwe PA, Davis SJ. Molecular interactions mediating T cell antigen recognition. Annu. Rev. Immunol. 2003;21:659–684. doi: 10.1146/annurev.immunol.21.120601.141036. [DOI] [PubMed] [Google Scholar]
- 23.Bell GI. Models for the specific adhesion of cells to cells. Science. 1978;200:618–627. doi: 10.1126/science.347575. [DOI] [PubMed] [Google Scholar]
- 24.Lauffenburger DA, Horwitz AF. Cell migration: a physically integrated molecular process. Cell. 1996;84:359–369. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- 25.Oiwa K, Chaen S, Kamitsubo E, Shimmen T, Sugi H. Steady-state force-velocity relation in the ATP-dependent sliding movement of myosin-coated beads on actin cables in vitro studied with a centrifuge microscope. Proc. Natl. Acad. Sci. U. S. A. 1990;87:7893–7897. doi: 10.1073/pnas.87.20.7893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuhns MS, Davis MM, Garcia KC. Deconstructing the form and function of the TCR/CD3 complex. Immunity. 2006;24:133–139. doi: 10.1016/j.immuni.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 27.Levin SE, Weiss A. Twisting tails exposed: the evidence for TCR conformational change. J. Exp. Med. 2005;201:489–492. doi: 10.1084/jem.20050179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Valitutti S, Muller S, Cella M, Padovan E, Lanzavecchia A. Serial triggering of many T-cell receptors by a few peptide-MHC complexes. Nature. 1995;375:148–151. doi: 10.1038/375148a0. [DOI] [PubMed] [Google Scholar]
- 29.Krogsgaard M, Prado N, Adams EJ, He XL, Chow DC, Wilson DB, Garcia KC, Davis MM. Evidence that structural rearrangements and/or flexibility during TCR binding can contribute to T cell activation. Mol. Cell. 2003;12:1367–1378. doi: 10.1016/s1097-2765(03)00474-x. [DOI] [PubMed] [Google Scholar]
- 30.Gray LS, Gnarra JR, Sullivan JA, Mandell GL, Engelhard VH. Spatial and temporal characteristics of the increase in intracellular Ca2+ induced in cytotoxic T lymphocytes by cellular antigen. J. Immunol. 1988;141:2424–2430. [PubMed] [Google Scholar]
- 31.Matsui K, Boniface JJ, Steffner P, Reay PA, Davis MM. Kinetics of T-cell receptor binding to peptide/I-Ek complexes: correlation of the dissociation rate with T-cell responsiveness. Proc. Natl. Acad. Sci. U. S. A. 1994;91:12862–12866. doi: 10.1073/pnas.91.26.12862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwesinger F, Ros R, Strunz T, Anselmetti D, Guntherodt HJ, Honegger A, Jermutus L, Tiefenauer L, Pluck-thun A. Unbinding forces of single antibody-antigen complexes correlate with their thermal dissociation rates. Proc. Natl. Acad. Sci. U. S. A. 2000;97:9972–9977. doi: 10.1073/pnas.97.18.9972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Monks CR, Freiberg BA, Kupfer H, Sciaky N, Kupfer A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 1998;395:82–86. doi: 10.1038/25764. [DOI] [PubMed] [Google Scholar]
- 34.Grakoui A, Bromley SK, Sumen C, Davis MM, Shaw AS, Allen PM, Dustin ML. The immunological synapse: a molecular machine controlling T cell activation. Science. 1999;285:221–227. doi: 10.1126/science.285.5425.221. [DOI] [PubMed] [Google Scholar]
- 35.Cemerski S, Shaw A. Immune synapses in T-cell activation. Curr. Opin. Immunol. 2006;18:298–304. doi: 10.1016/j.coi.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 36.Yokosuka T, Sakata-Sogawa K, Kobayashi W, Hiroshima M, Hashimoto-Tane A, Tokunaga M, Dustin ML, Saito T. Newly generated T cell receptor microclusters initiate and sustain T cell activation by recruitment of Zap70 and SLP-76. Nat. Immunol. 2005;6:1253–1262. doi: 10.1038/ni1272. [DOI] [PubMed] [Google Scholar]
- 37.Bunnell SC, Kapoor V, Trible RP, Zhang W, Samelson LE. Dynamic actin polymerization drives T cell receptor-induced spreading: a role for the signal transduction adaptor LAT. Immunity. 2001;14:315–329. doi: 10.1016/s1074-7613(01)00112-1. [DOI] [PubMed] [Google Scholar]
- 38.Tskvitaria-Fuller I, Rozelle AL, Yin HL, Wulfing C. Regulation of sustained actin dynamics by the TCR and costimulation as a mechanism of receptor localization. J. Immunol. 2003;171:2287–2295. doi: 10.4049/jimmunol.171.5.2287. [DOI] [PubMed] [Google Scholar]
- 39.Wiedemann A, Muller S, Favier B, Penna D, Guiraud M, Delmas C, Champagne E, Valitutti S. T-cell activation is accompanied by an ubiquitination process occurring at the immunological synapse. Immunol. Lett. 2005;98:57–61. doi: 10.1016/j.imlet.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 40.Krogsgaard M, Davis MM. How T cells “see” antigen. Nat. Immunol. 2005;6:239–245. doi: 10.1038/ni1173. [DOI] [PubMed] [Google Scholar]
- 41.Krogsgaard M, Li QJ, Sumen C, Huppa JB, Huse M, Davis MM. Agonist/endogenous peptide-MHC heterodimers drive T cell activation and sensitivity. Nature. 2005;434:238–243. doi: 10.1038/nature03391. [DOI] [PubMed] [Google Scholar]
- 42.Sporri R, Reis e Sousa C. Self peptide/MHC class I complexes have a negligible effect on the response of some CD8+ T cells to foreign antigen. Eur. J. Immunol. 2002;32:3161–3170. doi: 10.1002/1521-4141(200211)32:11<3161::AID-IMMU3161>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 43.Rudolph MG, Stanfield RL, Wilson IA. How TCRs bind MHCs, peptides, and coreceptors. Annu. Rev. Immunol. 2006;24:419–466. doi: 10.1146/annurev.immunol.23.021704.115658. [DOI] [PubMed] [Google Scholar]
- 44.Locksley RM, Reiner SL, Hatam F, Littman DR, Killeen N. Helper T cells without CD4: control of leishmaniasis in CD4-deficient mice. Science. 1993;261:1448–1451. doi: 10.1126/science.8367726. [DOI] [PubMed] [Google Scholar]
- 45.Schilham MW, Fung-Leung WP, Rahemtulla A, Kuendig T, Zhang L, Potter J, Miller RG, Hengartner H, Mak TW. Alloreactive cytotoxic T cells can develop and function in mice lacking both CD4 and CD8. Eur. J. Immunol. 1993;23:1299–1304. doi: 10.1002/eji.1830230617. [DOI] [PubMed] [Google Scholar]
- 46.Choudhuri K, Wiseman D, Brown MH, Gould K, van der Merwe PA. T-cell receptor triggering is critically dependent on the dimensions of its peptide-MHC ligand. Nature. 2005;436:578–582. doi: 10.1038/nature03843. [DOI] [PubMed] [Google Scholar]
- 47.Davis SJ, van der Merwe PA. The kinetic-segregation model: TCR triggering and beyond. Nat. Immunol. 2006;7:803–809. doi: 10.1038/ni1369. [DOI] [PubMed] [Google Scholar]
- 48.Cebecauer M, Guillaume P, Hozak P, Mark S, Everett H, Schneider P, Luescher IF. Soluble MHC-peptide complexes induce rapid death of CD8+ CTL. J. Immunol. 2005;174:6809–6819. doi: 10.4049/jimmunol.174.11.6809. [DOI] [PubMed] [Google Scholar]
- 49.Barisas BG, Wade WF, Jovin TM, Arndt-Jovin D, Roess DA. Dynamics of molecules involved in antigen presentation: effects of fixation. Mol. Immunol. 1999;36:701–708. doi: 10.1016/s0161-5890(99)00091-7. [DOI] [PubMed] [Google Scholar]
- 50.Munnelly HM, Brady CJ, Hagen GM, Wade WF, Roess DA, Barisas BG. Rotational and lateral dynamics of I-A(k) molecules expressing cytoplasmic truncations. Int. Immunol. 2000;12:1319–1328. doi: 10.1093/intimm/12.9.1319. [DOI] [PubMed] [Google Scholar]
- 51.Gil D, Schrum AG, Alarcon B, Palmer E. T cell receptor engagement by peptide-MHC ligands induces a conformational change in the CD3 complex of thymocytes. J. Exp. Med. 2005;201:517–522. doi: 10.1084/jem.20042036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.La Gruta NL, Liu H, Dilioglou S, Rhodes M, Wiest DL, Vignali DA. Architectural changes in the TCR:CD3 complex induced by MHC:peptide ligation. J. Immunol. 2004;172:3662–3669. doi: 10.4049/jimmunol.172.6.3662. [DOI] [PubMed] [Google Scholar]