Abstract
Chlamydia trachomatis genome is predicted to encode a type III secretion system consisting of more than forty open reading frames (ORFs). To test whether these ORFs are expressed and immunogenic during chlamydial infection in humans, we expressed 55 chlamydial ORFs covering all putative type III secretion components plus control molecules as fusion proteins and measured the reactivity of these fusion proteins with antibodies from patients infected with C. trachomatis in the urogenital tract (24 antisera) or in the ocular tissue (8 antisera). Forty-five of the 55 proteins were recognized by at least one of the 32 human antisera, suggesting that these proteins are both expressed and immunogenic during chlamydial infection in humans. Tarp, a putative type III secretion effector protein, was identified as a novel immunodominant antigen due to its reactivity with the human antisera at high frequency and titer. The expression and immunogenicity of Tarp were confirmed in cell culture and mouse systems. Tarp was mainly associated with the infectious form of chlamydial organisms and became undetectable between 13 and 24 hours during the infection cycle in cell culture. Mice intravaginally infected with C. muridarum developed Tarp-specific humoral and cellular immune responses. More importantly, immunization of mice with Tarp induced Th1-dominant immunity that significantly reduced the shedding of live organisms from the lower genital tract and attenuated inflammatory pathologies in the fallopian tube tissues. These observations have demonstrated that Tarp, an immunodominant antigen identified by human antisera, can induce protective immunity against chlamydial infection and pathology in mice.
Keywords: Tarp, Chlamydia trachomatis type III secretion, Human antibodies, Vaccine, Mouse urogenital tract infection, Inflammatory pathologies
Introduction
Infection with Chlamydia trachomatis, a species of obligate intracellular bacterial pathogens, imposes serious health problems in humans. The C. trachomatis organisms are categorized into 4 biovars based on their tissue tropism, including the trachoma biovar that infects human ocular epithelial cells [1], the genital biovar that infects human urogenital tract epithelial tissues, potentially leading to complications such as ectopic pregnancy and infertility [2, 3], the lymphogranuloma venereum biovar that can cause systemic infections in humans [4–6], and the murine biovar that is also called mouse pneumonitis agent (designated as MoPn) and now classified as a new species C. muridarum. Although the C. muridarum organisms cause no known diseases in humans, these organisms have been and are still extensively used to study C. trachomatis pathogenesis and immunology in mouse models [7–14]. This is because the C. muridarum-induced genital tract pathologies in mice closely resemble those in the human genital tracts induced by C. trachomatis infection [15, 16]. Using this mouse model, it has been demonstrated that the CD4+ T helper cell (Th1)-dominant and IFNγ-dependent immunity is a major host protective determinant for controlling chlamydial infection [17] although antibodies and other immune components may also contribute to the host resistance to chlamydial infection [18–20]. However, due to insufficient knowledge on the protective determinants from the chlamydial organisms, no effective C. trachomatis vaccines are available.
Despite the diversity in their tissue tropism, all C. trachomatis organisms share a very similar genome [21–23] and a common intracellular biphasic growth cycle [24, 25]. C. trachomatis can invade epithelial cells via an induced phagocytic mechanism in the form of elementary body (EB) that are infectious but metabolically inert. The EB-laden vacuole not only resists the fusion with lysosomes but also supports chlamydial replication. The intravacuolar EB can rapidly differentiate into reticulate bodies (RBs) that are metabolically active but non-infectious. After replication within cytoplasmic vacuoles (also termed inclusions), the progeny RBs can differentiate back into EBs for spreading to the adjacent cells. Since the bacterial type III secretion system is known to inject virulence factors into host cells [26–28], tremendous effort has been made to identify the chlamydial type III secretion apparatus and putative effector molecules. C. trachomatis genome encodes ~50 open reading frames (ORFs) that may be secreted into the inclusion membrane (designated as inclusion membrane protein or Inc; ref: [29, 30] and >40 ORFs that share homology with gram negative bacterial type III secretion-related proteins [23, 31]. Most of the type III secretion-related ORFs are scattered along the entire chlamydial genome in 10 different operons [31, 32]. Due to lack of genetic tools for manipulating chlamydial genomes, the chlamydial type III secretion systems have been studied using indirect approaches, including localization of Chlamydia-secreted proteins in Chlamydia-infected cells [33–38], chlamydial protein secretability in heterologous type III secretion systems [39–42], susceptibility to inhibitors known to inhibit other bacterial type III secretion function [43, 44], biochemical characterization [45–47] and various functional assays in cell-free or surrogate systems [38, 48–50]. These efforts have been productive in generating information for understanding chlamydial pathogenic mechanisms. For example, a putative chlamydial type III secretion effector molecule designated as translocated actin-recruiting phosphoprotein or Tarp has been found to play a critical role in chlamydial invasion of the nonphagocytic epithelial cells by targeting host small GTPases and inducing polymerization of actin molecules [51–57].
To test whether the chlamydial type III secretion-related components are expressed and immunogenic during chlamydial infection in humans, we expressed 55 chlamydial fusion proteins covering all putative type III secretion-related apparatus components, chaperones and effectors and reacted these fusion proteins with antibodies from patients with C. trachomatis infection in the urogenital tract or ocular tissues. Although chlamydial Inc proteins are putative type III secretion effectors [39], only a few Incs were included in the current study. This is because all the C. trachomatis Inc proteins have been previously analyzed with human antisera [35]. In the current study, we found that 45 of the 55 proteins were specifically recognized by at least one of the human antisera tested, suggesting that these proteins are both expressed and immunogenic during chlamydial infection in humans. Importantly, we identified two novel immunodominant proteins recognized by 50% or more human antisera with significantly high titers and they were CT456 (Tarp) & CT673 (Pkn5, a putative serine/theronine kinase). Since Tarp was more immunodominant than Pkn5 in C. trachomatis-infected humans, we further evaluated the immunogenicity of Tarp in a mouse urogenital tract infection model. Immunization of mice with Tarp induced protective immunity that significantly reduced both chlamydial infection and inflammatory pathologies, suggesting that the reactivity with C. trachomatis-infected human antisera can be used to guide the search for C. trachomatis vaccine candidate antigens.
Materials and Methods
1. Chlamydial organisms and chlamydial infection in cell culture
C. trachomatis serovar D or C. muridarum Nigg strain (also called MoPn) organisms were grown, purified and titrated as previously described [58]. Aliquots of the organisms were stored at −80°C till use. HeLa cells (ATCC, Manassas, VA 20108) were maintained in DMEM (GIBCO BRL, Rockville, MD) with 10% fetal calf serum (FCS; GIBCO BRL) at 37°C in an incubator supplied with 5% CO2. To prepare chlamydial infection samples for various assays, HeLa cells grown in tissue culture flasks or on glass coverslips in 24 well plates were pretreated with DMEM containing 30μg/ml of DEAE-Dextran (Sigma, St Luis, MO) for 10 min. After the DEAE-Dextran solution was removed, chlamydial organisms diluted in DMEM were added to the monolayers and allowed to attach to the cell monolayers for 2 hours at 37°C. The infected cells were continuously cultured in DMEM with 10% FCS and 2 μg/ml of cycloheximide (Sigma) and processed at various time points after infection as indicated in individual experiments. The infectious dose was pre-titrated and an infection rate of ~50% or less was applied to samples for immunofluorescence assays or 90% or higher for Western blot assays or making whole cell lysates.
2. Prokaryotic expression of chlamydial fusion proteins, protein purification and antibody production
The open reading frames (ORFs) coding for 52 putative type III secretion-related proteins and three control proteins from C. trachomatis serovar D genome (http://stdgen.northwestern.edu) were selected for the current study. They are CT087-091, CT557-564, CT576-579, CT663-674, CT716-719 & CT860-863 [32], CT083, CT373, CT550, CT606.1, CT642, CT652.1, CT712, CT738 & CT848 [40], CT119, CT529 & CT813 [35, 39], CT847 [48], CT456 [51] and the control proteins CT110 (HSP60), CT681 (MOMP) & CT858 (CPAF). These selected ORFs were cloned into pGEX vectors (Amersham Pharmacia Biotech, Inc., Piscataway, NJ) and expressed as fusion proteins with glutathione-s-transferase (GST) fused to the N-terminus of the chlamydial proteins. Tarp was also cloned from C. muridarum genome [(ORF TC0741, ref: [21], NCBI protein accession# AAF39550, http://www.ncbi.nlm.nih.gov/sites/entrez]. Most of the ORFs were cloned as full length with the following exceptions: CT090 was cloned from the amino acid glycine residue at the 389th position (G389) to the stop codon, CT562 from A149 to stop, CT564 from G117 to stop, CT578 from the start codon to T261, CT579 from the start codon to G216, CT642 from M133 to stop, CT664 from G545 to stop, CT669 from S149 to stop, CT674 from M107 to stop, CT860 from S106- stop, CT861 from M292 to stop, CT863 from G138 to stop. These cloning position variations were made to overcome difficulties in fusion protein expression. Expression of the fusion proteins was induced with isopropyl-beta-D-thiogalactoside (IPTG; Invitrogen, Carlsbad, CA) and the fusion proteins were extracted by lysing the bacteria via sonication in a Triton-X100 lysis buffer (1%TritonX-100, 1mM PMSF, 75 units/ml of Aprotinin, 20 mM Leupeptin and 1.6 mM Pepstatin). After a high-speed centrifugation to remove debris, the fusion protein-containing supernatants were either directly used in various assays or further purified using glutathione-conjugated agarose beads (Pharmacia). While some of the bead-bound fusion proteins were used to deplete antigen-specific antibodies from antiserum samples, others were used to purify soluble proteins by cleavage with a precision protease (Pharmacia). The freed chlamydial protein was concentrated via centricon (Millipore, Billerica, MA) and used to immunize mice for antibody production or evaluating protective immunity as described previously [59–64]. The spleen cells were harvested from mice immunized with purified serovar D Tarp protein and used for generating monoclonal antibodies (mAb) and an IgG1 mAb (clone R2H1.2) was generated and used in the current study.
3. Enzyme-Linked ImmunoSorbent Assay (ELISA)
For measuring chlamydial fusion protein reactivity with human or mouse antisera, a protein array ELISA was used as described elsewhere [35, 37, 59, 65, 66]. Twenty-four human antisera were collected from women diagnosed with C. trachomatis urogenital infection (San Antonio STI clinic, designated as positive STI sera) and 8 human antisera from individuals with trachoma (Gambia, designated as trachoma positive sera) as well as eight human sera collected from individuals without chlamydial infection (San Antonio STI clinic, negative sera) were used in the current study. These human sera were collected under the appropriate IRB protocols and no patient ID and other related information were available to the staff involved in the current study. Antisera from the C. muridarum-infected mice were collected at the specified time points as indicated in the individual experiments. For carrying out the protein array ELISA, bacterial lysates containing the GST fusion proteins were directly added to the 96 well microplates coated with glutathione (Pierce, Rockford, IL) to allow GST to interact with glutathione. After washing to remove excess fusion proteins and blocking with 2.5% nonfat milk (in phosphate-buffered solution), individual serum samples after the appropriate dilution were applied to the antigen-immobilized microplates. The serum antibody binding was detected with a goat anti-human IgG or mouse IgG or different mouse Ig isotypes conjugated with HRP (Jackson ImmunoResearch Laboratories) in combination with the soluble substrate 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulforic acid) diammonium salt (ABTS; Sigma) and quantitated by reading the absorbance (OD) at 405 nm using a microplate reader (Molecular Devices Corporation, Sunnyvale, CA). To reduce background binding, all human serum samples were pre-absorbed with lysates made from XL-1blue bacteria expressing GST alone. In some experiments, the human serum samples were preabsorbed with either C. trachomatis serovar D-infected HeLa or normal HeLa cell lysates while in others, mouse antisera were also measured against C. muridarum-infected HeLa cell lysates. In the latter case, the lysates were used to coat 96 well ELISA microplates (Nunc, Rochester, NY) as the antigens.
The cytokines in the supernatants of the in vitro stimulated lymphocyte cultures were measured using standard cytokine ELISA kits as instructed by the manufacturer and described previously [14, 67]. The commercially available ELISA kits [mouse IFN-γ kit (cat# DY485), IL-4 (cat# DY404) and IL-5 (cat# DY405)] were all obtained from R&D Systems, Inc (Minneapolis, MN). Briefly, splenocytes were harvested from mice with or without MoPn infection and stimulated in vitro with antigens including UV-inactivated MoPn EBs, purified GST-Tarp (TC0741) and GST alone for 3 days. The culture supernatants were collected for cytokine measurements using 96 well ELISA microplates precoated with the corresponding capture antibodies. The capture antibody-bound cytokines were detected with biotin-conjugated antibodies and horseradish peroxidase (HRP)-conjugated Avidin. The cytokine concentrations were calculated based on absorbance values, cytokine standards and sample dilution factors and expressed as ng or pg per ml.
4. Immunofluorescence assay
HeLa cells grown on coverslips were fixed with 2% paraformaldehyde dissolved in PBS for 30 min at room temperature, followed by permeabilization with 1% saponin (Sigma) for an additional 1h. After washing and blocking, the cell samples were subjected to various combinations of antibody and chemical staining. Hoechst (blue, Sigma) was used to visualize nuclear DNA. A rabbit anti-CT395 antibody (unpublished data) plus a goat anti-rabbit IgG conjugated with Cy2 (green; Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) were used to visualize chlamydial inclusions. The mouse antibodies raised with either C. muridarum infection or Tarp immunization plus a goat anti-mouse IgG conjugated with Cy3 (red; Jackson ImmunoResearch) were used to visualize the corresponding antigens. In some cases, the mouse primary antibodies were pre-absorbed with either the corresponding fusion proteins immobilized onto agarose beads or C. muridarum-infected HeLa cell lysates prior to staining cell samples. The cell samples after the appropriate immuno-labeling were used for image analysis and acquisition with an Olympus AX-70 fluorescence microscope equipped with multiple filter sets (Olympus, Melville, NY) as described previously [38, 58, 68, 69]. Briefly, the multi-color-labeled samples were exposed under a given filter set at a time and the single color images were acquired using a Hamamatsu digital camera. The single color images were then superimposed with the software SimplePCI to display multi-colors. All microscopic images were processed using the Adobe Photoshop program (Adobe Systems, San Jose, CA).
5. Western blot assay
The Western blot assay was carried out as described elsewhere [69–73]. Briefly, either the C. trachomatis serovar D GST-Tarp fusion protein, purified serovar D organisms (RB or EB) or HeLa cells with or without serovar D infection were solublized in 2% SDS sample buffer and loaded to SDS polyacrylamide gel wells. After electrophoresis, the proteins were transferred to nitrocellulose membranes and the blots were detected with primary antibodies, including the mouse mAb R2H1.2 against serovar D Tarp (see above), mAb 100a against CPAF [38], mAb AC15 (Sigma) against human β-actin and a rabbit anti-CT681 (serovar D MOMP, unpublished data). The primary antibody binding was probed with an HRP (horse radish peroxidase)-conjugated secondary antibody and visualized with an enhanced chemiluminescence (ECL) kit (Santa Cruz Biotechnology, Inc., Santa Cruz, CA).
6. Mouse immunization and urogenital tract infection
Female Balb/c mice were purchased at the age of 3 to 4 weeks old from Charles River Laboratories, Inc. (Wilmington, MA) and divided into 3 different groups with 10 in each group for immunization. The first group was immunized with adjuvant alone [CpG emulsified in equal volume of incomplete Freund’s adjuvant (IFA)], the second with the purified C. muridarum Tarp (ORF TC0471) emulsified in the CpG-IFA adjuvant and the third with MoPn organisms in the same adjuvant mix. The CpG with a sequence of 5-TCC.ATG.ACG.TTC.CTG.ACG.TT-3′ (all nucleotides are phosphorothioate-modified at the 3′ internucleotide linkage) was purchased from Integrated DNA Technologies (IDT, Coralville, IA) and the IFA from Sigma-Aldrich (St. Louis, MO). We used the CpG-IFA as adjuvant because it has been previously shown to induce Th1 dominant immune responses [74]. The C. muridarum Tarp was purified by cleaving the Tarp protein off from the GST-fusion proteins immobilized onto the agarose beads. A dose of 100 μg of Tarp plus 10μg of CpG in 50ul of PBS emulsified in an equal volume of IFA was used for each injection. For the third group of mice, the Tarp protein was replaced with 1 × 105 IFUs (inclusion forming units) of C. muridarum organisms. All mice were immunized via intramuscular injection (im, with half of the immunogen mix injected into the muscle tissue in each of the hind legs) for a total of three times with an interval of 3 weeks between the first and second injections and 9 days between the second and third injections. One of the mice in the second group died during the immunization. Thirty days after the third immunization, each mouse was inoculated intravaginally with 1 × 104 IFUs of live C. muridarum organisms in 20μl of SPG (sucrose-phosphate-glutamate buffer consisting of 218mM sucrose, 3.76mM KH2PO4, 7.1mM K2HPO4, 4.9mM glutamate, pH 7.2). Five days prior to infection, each mouse was injected with 2.5mg Depo-provera (Pharmacia Upjohn, Kalamazoo, MI) subcutaneously to synchronize menstrual cycle and increase mouse susceptibility to chlamydial infection. One day before the infection, mice were bled from the tail for monitoring antibody responses induced by the immunization.
7. Monitoring mouse shedding of live chlamydial organisms
To monitor live organism shedding, vaginal swabs were taken on different days after the intravaginal infection (once every 3 days until two consecutive negative detection results were obtained from the same mouse). Each swab was soaked in 500μl of SPG and vortexed. The chlamydial organisms released into the supernatants were titrated on HeLa cell monolayers in duplicates as described previously [14]. Briefly, serially diluted swab samples were inoculated onto HeLa cell monolayers grown on coverslips in 24 well plates. After incubation for 24 hours in the presence of 2μg/ml cycloheximide, the cultures were processed for immunofluorescence assay as described above. The inclusions were counted under a fluorescence microscope. Five random fields were counted per coverslip. For coverslips with less than one IFU (inclusion forming unit) per field, the entire coverslips were counted. Coverslips showing obvious cytotoxicity of HeLa cells were excluded. The total number of IFUs per swab was calculated based on the number of IFUs per field, number of fields per coverslip, dilution factors and inoculation and total sample volumes. An average was taken from the serially diluted and duplicate samples for any given swab. The calculated total number of IFUs/swab was converted into log10 and the log10 IFUs were used to calculate means and standard deviation for each group at each time point.
8. Evaluating mouse genital tract tissue pathologies and Histological scoring
Mice were sacrificed 60 days after infection and the mouse urogenital tract tissues were isolated. Before the tissues were removed from mice, an in situ gross examination was performed for evidence of hydrosalpinx formation and any other abnormalities. The excised tissues were then fixed in 10% neutral formalin and embedded in paraffin and serially sectioned longitudinally (with 5 μm/each section). Efforts were made to include cervix, both uterine horns and oviducts as well as lumenal structures of each tissue in each section. The sections were stained with hematoxylin and eosin (H&E) as described elsewhere [15]. The H&E stained sections were assessed by a board certified pathologist (I-T.Y) blinded to mouse treatment and scored for severity of inflammation and pathologies based on the modified schemes established previously [14, 15, 75, 76]. The uterine horns and fallopian tubes were scored separately. Scoring for dilatation of uterine horn or fallopian tube: 0, no significant dilatation; 1, mild dilatation of a single cross section; 2, one to three dilated cross sections; 3, more than three dilated cross sections; and 4, confluent pronounced dilation. Scoring for inflammatory cell infiltrates (at the chronic stage of infection, the infiltrates mainly contain mononuclear cells): 0, no significant infiltration; 1, infiltration at a single focus; 2, infiltration at two to four foci; 3, infiltration at more than four foci; and 4, confluent infiltration. Scores assigned to individual mice were calculated into means ± standard errors for each group of animals (n = 9 to 10).
9. Statistical analysis
ANOVA Test (http://www.physics.csbsju.edu/stats/anova.html) was performed to analyze data from multiple groups and a two-tailed Student t test (Microsoft Excel) to compare the means between two groups. A Chi squared test was used for comparing the rate of incidence between two groups.
Results
1. Human antibody recognition of putative chlamydial type III secretion-related proteins identifies Tarp and Pkn5 as novel immunodominant antigens
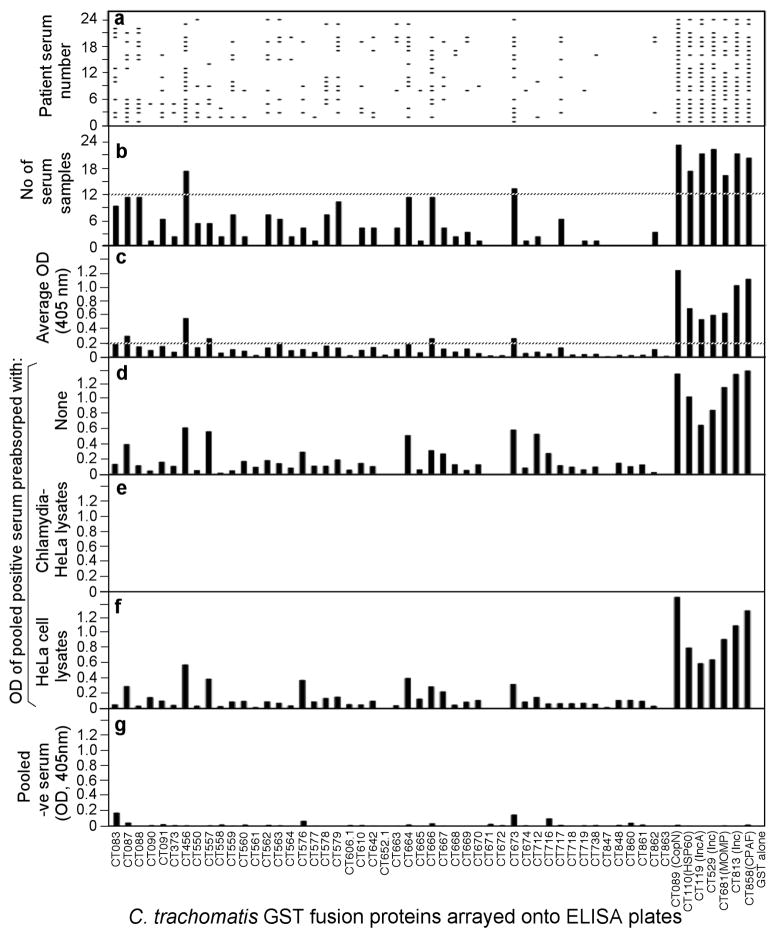
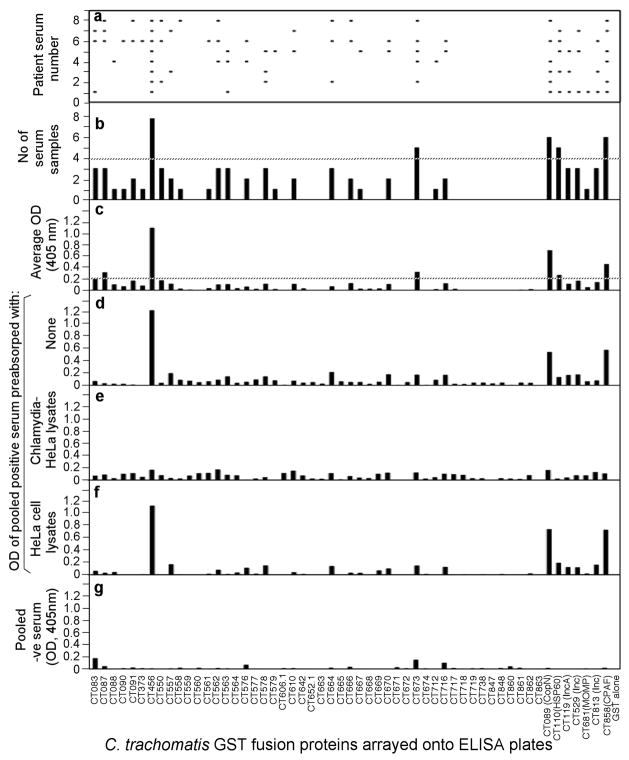
We expressed 52 ORFs related to the chlamydial type III secretion system and three chlamydial control antigens (MOMP, HSP60 & CPAF) as GST fusion proteins and reacted these 55 fusion proteins with 24 antisera from women urogenitally infected with C. trachomatis (STI antisera, Fig. 1) and 8 from trachoma patients (trachoma antisera, Fig. 2). We found that 45 of the 55 fusion proteins positively reacted with one or more human antisera, suggesting that the 45 chlamydial proteins were both expressed and immunogenic during chlamydial infection in humans. The fusion proteins that reacted with the human antibodies with both positive titers (average OD= or > 0.2) and high frequency (= or > 50% of the 32 antisera) were defined as immunodominant antigens [63, 77]. Nine of the 45 human antibody-reactive proteins met the immunodominance requirements, including CT089 (CopN), CT110 (HSP60), CT119 (Inc A), CT456 (Tarp), CT529 (Inc), CT673 (Pkn5), CT681 (MOMP), CT813 (Inc) & CT858 (CPAF) (see Table 1). Tarp & Pkn5 are novel immunodominant antigens uncovered for the first time in the current study. The remaining 7 antigens were previously shown to be immunodominant by using STI antisera [35, 66, 77]. The lack of recognition of MOMP from C. trachomatis serovar D by trachoma antisera might be due to MOMP sequence variation between the genital serovar D and the ocular serovars [78]. The trachoma antisera predominantly recognized the conserved antigens CPAF and HSP60. However, although the three Inc proteins CT119, 529 and 813 are also highly conserved between serovar D and the ocular serovars [23, 79], only three of the 8 trachoma antisera positively recognized these Inc proteins (Fig. 2, panel C), suggesting that the Inc proteins were not always dominantly presented to the host immune system during C. trachomatis infection in human ocular tissues despite the fact that the Inc proteins are immunodominant during human urogenital infection with C. trachomatis [35]. Nevertheless, both the STI and trachoma sera predominantly recognized Tarp (with a positive recognition frequency of 70% and 100% by STI and trachoma antisera respectively) and Pkn5 (54% and 63% respectively; Table 1). These results are consistent with the fact that Tarp and Pkn5 are conserved between different C. trachomatis serovars and suggest that these two antigens are highly immunogenic in humans with C. trachomatis infection regardless of the site of infection (in either the urogenital or ocular epithelial tissues).
Fig. 1. Reactivity of 24 STI antisera with 55 GST-chlamydial fusion proteins.
Each of the 24 human antibodies (displayed along the Y-axis of panel a) after 1:500 dilution was reacted with each of the 55 GST fusion proteins (listed along the X-axis) immobilized onto the 96 well microplates. The human antibody binding was detected with a secondary goat anti-human IgG antibody conjugated with HRP plus a soluble substrate. The results were expressed as OD readings obtained at the wavelength of 405nm. Any given reaction with an OD reading 4 fold above the value from the control well (GST alone-coated well) was determined positive, which was represented with a horizontal bar in panel a. The total number of human serum samples that positively recognized a given fusion protein was summarized in panel b. The dotted line marked those fusion proteins recognized by 12 or more human antisera. The average OD readings calculated by dividing the total OD values by 24 were displayed in panel c. The dotted line marked those fusion proteins with an average OD of 0.2 or above. The 24 human serum samples were pooled at an equal ratio and assayed against each of the fusion proteins at a dilution of 1:200 and the OD values were displayed along the Y-axis of panel d. The pooled serum sample was also subjected to pre-absorption with either C. trachomatis serovar D-infected (panel e) or HeLa cell alone (f) lysates prior to reacting with the fusion proteins. Finally a pooled serum from 8 negative individuals failed to react with the fusion proteins (panel g).
Fig. 2. Reactivity of 8 trachoma antisera with 55 GST-chlamydial fusion proteins.
The experiment was carried out and the data presented in the exactly same way as described in Fig. 1 legend except that the antisera tested were from 8 trachoma patients.
Table 1. Summary of human antibody recognition of chlamydial fusion proteins.
antibody reactivity.
| Antigen groupa | Gene name | Function and related information | Frequency of reactivity with antisera: | ||
|---|---|---|---|---|---|
| STIb (24)c | Trachoma (8) | ||||
| Proteins predominantly recognized by human antisera in the current study | New antigens (Not published) | CT456 | Tarp, a chlamydial translocated actin recruiting phosphoprotein, injected into host cell cytosol to facilitate chlamydial invasion of nonphagocytic epithelial cells by targeting host small GTPases and inducing F-actin reorganization | +d (17)e | + (8) |
| CT673 | Pkn5, possible serine/threonine kinase secretable by Salmonella T3SS | + (13) | + (5) | ||
| Antigens also identified in previously published studies | CT089 | CopN, a negative regulator of chlamydial T3SS, immunization with CopN can induce partial protection against C. pneumoniae airway infection [77] | + (23) | + (6) | |
| CT110 | HSP60 [77] | + (17) | + (5) | ||
| CT119 | Inclusion membrane protein A (IncA) [35] | + (19) | − (3) | ||
| CT529 | Inc | + (20) | − (3) | ||
| CT681 | MOMP from serovar D [77] | + (16) | − (1) | ||
| CT813 | Inc | + (21) | − (3) | ||
| CT858 | CPAF, a chlamydial protease involved in immune evasion, able to induce partial protection against C. trachomatis urogenital infection [66, 84] | + (21) | + (6) | ||
| Proteins not recognized by any human antisera | CT606.1 | 79AA, Hypothetical protein | − (0) | − (0) | |
| CT652.1 | 59AA, Hypothetical protein | − (0) | − (0) | ||
| CT671 | 283AA, Hypothetical protein | − (0) | − (0) | ||
| CT672 | FliN flagellar motor switch domain | − (0) | − (0) | ||
| CT718 | Related to FliI & FliF | − (0) | − (0) | ||
| CT847 | 172AA, Hypothetical protein | − (0) | − (0) | ||
| CT848 | 168AA, Hypothetical protein | − (0) | − (0) | ||
| CT860 | Predicted to be CopD2 | − (0) | − (0) | ||
| CT861 | Predicted to be CopB2 | − (0) | − (0) | ||
| CT863 | 482AA, Hypothetical protein | − (0) | − (0) | ||
Ag=antigen, the 55 chlamydial antigens were categorized into 4 different groups based on their reactivity with human antisera and only three groups were listed in the table. The antigens that were positively recognized by one or more human antisera but not immunodominant were not listed.
STI stands for patients with C. trachomatis infection in the urogenital tract while “trachoma” stands for patients with the infection in ocular epithelial tissues.
A total of 24 STI and 8 trachoma antisera were used in the reactivity measurement.
“+” indicates that the antigen was immunodominantly recognized by the human antibody samples while “−” for nondominant or no recognition.
The number of positively reactive antisera for each antigen was listed in the bracket.
2. Tarp is synthesized at the late stage of chlamydial infection and associated with the infectious form of the chlamydial organisms
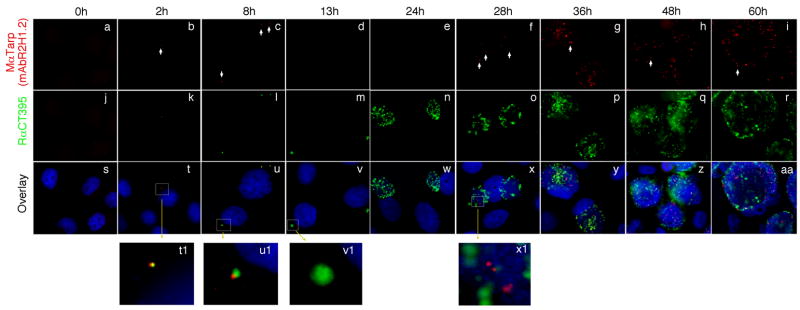
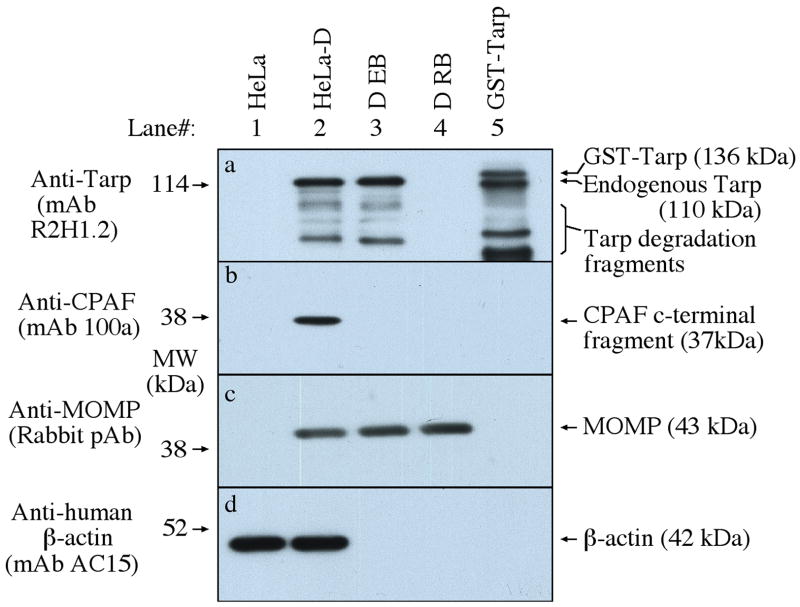
The predominant recognition of Tarp by human antisera suggests that Tarp is expressed during C. trachomatis infection in humans. However, it is not known when Tarp protein is available for host immune recognition during the chlamydial infection cycle. The secretion [51] and phosphorylation [51, 53, 54]} of Tarp and its actin nucleation function [55, 57] have been well characterized in cell culture systems. Here, we monitored the Tarp protein expression along the chlamydial infection cycle in cell culture system using a Tarp-specific antibody (Fig. 3). Tarp was detected in the first 8 hours and no longer detectable by 13 hours after infection. Although chlamydial inclusions became very obvious at 24 hours after infection, Tarp remained undetectable. However, by 28 hours after infection, Tarp started to reappear and progressively increased as the incubation continued. The late expression of Tarp protein is consistent with previous observations that Tarp, as one of the type III secretion effector molecule, is encoded by a late gene activated during RB to EB conversion [44, 80–82]. However, it is not clear whether Tarp protein detected for first 8 hours after chlamydial infection is contributed solely by the preexisting Tarp incorporated into EBs during the previous cycle of infection or also involves new biosynthesis. Nevertheless, the current data clearly showed that no Tarp protein was detectable between 13 and 24 hours after infection, during which the chlamydial inclusions are enriched in RBs. Indeed, Tarp protein was only detected in purified EBs but not RBs on a Western blot (Fig. 4), suggesting that Tarp protein, once synthesized in RBs, is rapidly incorporated into EBs.
Fig. 3. Time course expression of Tarp.
HeLa cells infected with C. trachomatis serovar D organisms were processed at various time points after infection as indicated on top of the figure. The infected cell monolayers were immunostained with a mouse anti-Tarp mAb plus a goat anti-mouse IgG conjugated with Cy3 (red) and a rabbit anti-chlamydial organism antibody plus a goat anti-rabbit IgG conjugated with Cy2 (green) and a DNA dye (blue). Please note that Tarp was detected during the first 8 hours after infection (panels t1 & u1) and disappeared (panels v1 & w) until 28 hours after infection (×1). As incubation continued, more Tarp-positive granules were detected (panels g to i).
Fig. 4. Detection of Tarp in purified EBs but not RBs.
HeLa cells with (HeLa-D) or without (HeLa) C. trachomatis D organisms, purified EBs or RBs along with the fusion protein GST-Tarp (from serovar D) were resolved in a SDS polyacrylamide gel. The gel-separated protein bands were blotted onto nitrocellulose membrane for Western blot detection of chlamydial proteins including Tarp, CPAF, MOMP and human b-actin with antibodies listed along the left side of the figure. Tarp was detected in both the whole infected cell lysate and purified EB but not RB samples. Tarp protein always displayed degradation fragments regardless of the source as indicated along the right side of the panel a.
3. Mice infected with MoPn develop Tarp-specific humoral and cellular immune responses
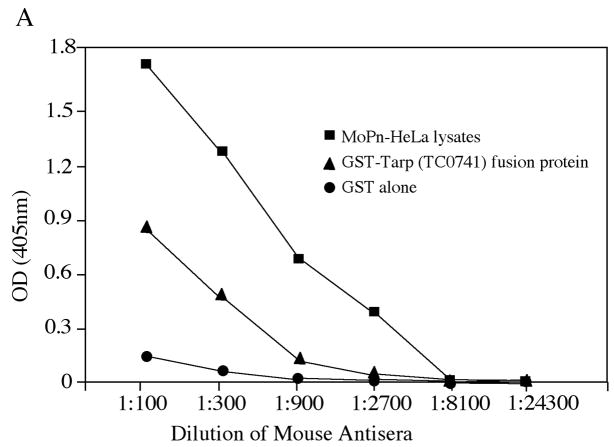
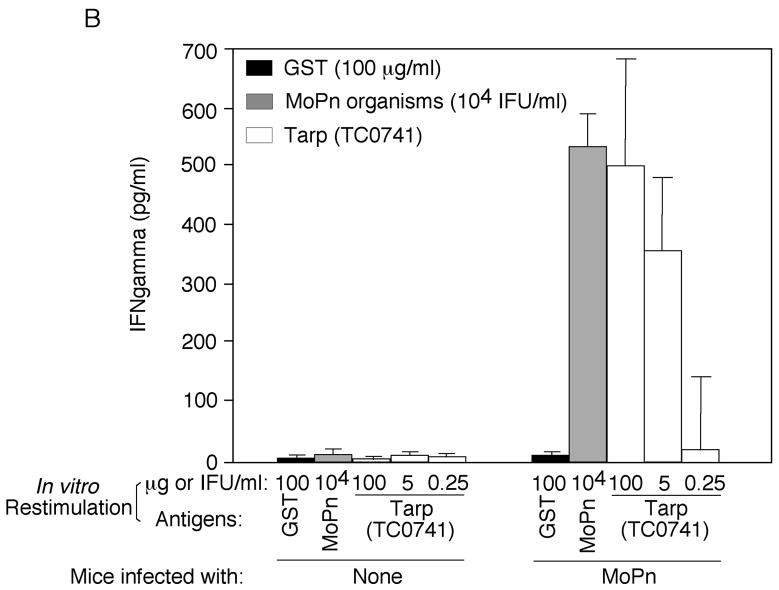
The observations that Tarp is immunodominantly recognized by human antibodies and associated with the EB forms of C. trachomatis have inspired us to ask whether Tarp can be used as a vaccine candidate antigen. Before we evaluated the protection efficacy of Tarp in a mouse urogenital tract infection model, we tested whether mice infected with C. muridarum, a chlamydial species (also designated as MoPn) that can productively infect mouse urogenital tracts and has been extensively used to study C. trachomatis immunology and pathogenesis, can develop immune responses to Tarp. Thirty days after mice were infected with MoPn organisms via intravaginal infection, mouse sera and spleen cells were collected for measuring Tarp-specific antibody and T cell responses respectively. The infected mice developed antibodies that recognized both MoPn-derived antigens and Tarp fusion proteins but not the fusion tag GST alone (Fig. 5A). It is worth noting that the Tarp from MoPn was always used for experiments-related to MoPn infection since serovar D Tarp only shares 43% amino acid identity with Tarp encoded by MoPn [21, 23]. The MoPn-primed T cells were also recalled by Tarp fusion proteins but not the fusion tag alone (Fig. 5B). These observations together have demonstrated that Tarp antigen is efficiently presented to the murine immune system during MoPn infection in the urogenital tracts as it is during human infection. The next question is whether Tarp alone is immunogenic.
Fig. 5. Induction of Tarp-specific immune responses by MoPn intravaginal infection.
Mice were intravaginally infected with or without MoPn organisms (1 × 104 IFU) as described in the method and material section and 30 days after infection, both sera were collected for antibody measurements (A) and spleen cells for in vitro stimulation assay (B). For antibody measurement, the pooled serum samples from each group were serially diluted as shown along the X-axis and assayed against MoPn-HeLa lysates (filled square), GST-Tarp fusion protein (filled triangle) or GST alone (filled circle) in ELISA. The results were expressed as OD values as shown along the Y-axis. Please note that the mouse antisera positively reacted with both MoPn antigens and Tarp but not GST alone. For monitoring cellular response, the spleen lymphocytes harvested from the mice were re-stimulated in vitro with the following antigens as listed along the X-axis: UV-inactivated MoPn EB organisms at 1 × 104 IFUs/well, GST-Tarp fusion protein at the concentrations of 100, 5 & 0.25ug/ml and GST alone at 100ug/ml. Three days after the stimulation, the culture supernatants were collected for IFNg detection in an ELISA and the results were expressed as pg/ml as listed along the Y-axis.
4. Immunization with Tarp induces MoPn-specific immune responses
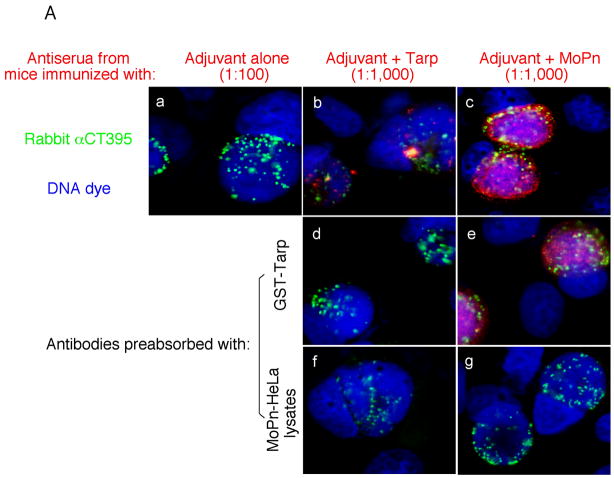
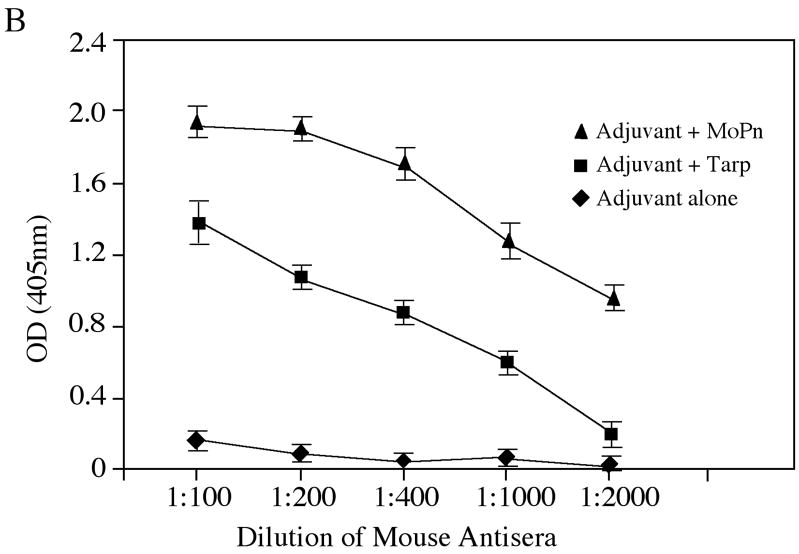
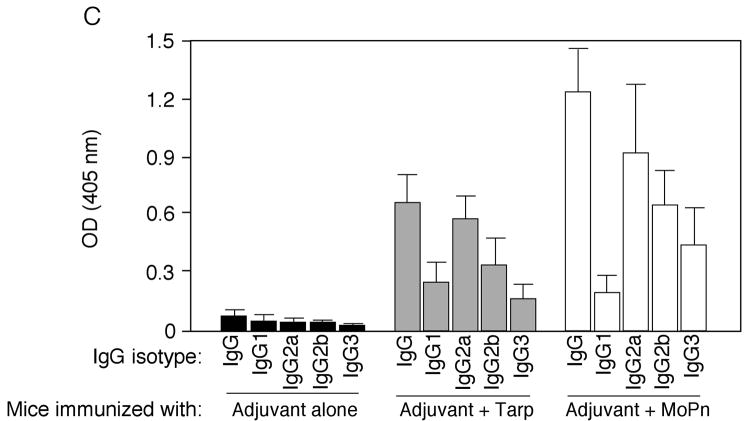
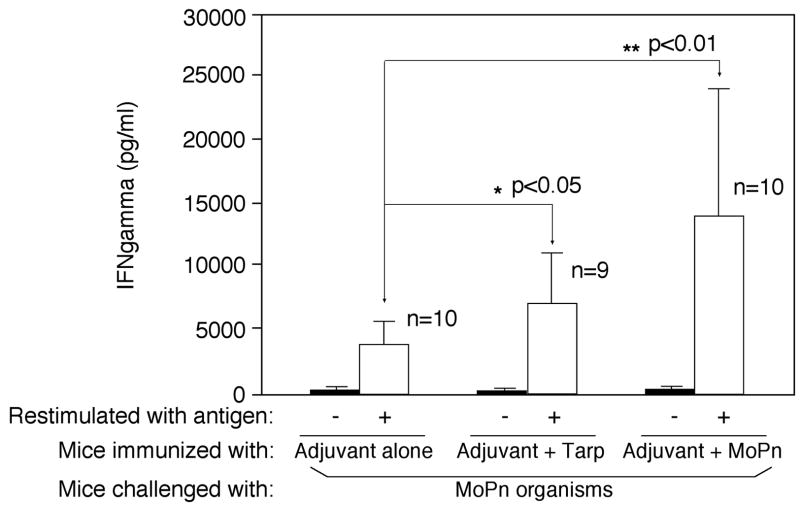
To evaluate the immunogenicity of Tarp, mice were immunized with Tarp plus adjuvant or adjuvant alone. The intact MoPn organisms similarly emulsified in the same adjuvant were used as a positive control. Sixty days after the immunization, mice were bled for monitoring MoPn-specific antibody responses (Fig. 6). Immunization with either MoPn or Tarp but not adjuvant alone induced antibodies against MoPn endogenous antigens as demonstrated in an immunofluorescence assay (Fig. 6A). The antisera induced by MoPn or Tarp but not adjuvant alone visualized antigens in MoPn inclusions. More importantly, the anti-Tarp antibody labeling was blocked by either the immunogen GST-Tarp or MoPn-infected cell lysates while the anti-MoPn antibody labeling was effectively blocked by MoPn-infected cell lysates. We further quantitated the titers of the MoPn antigen-reactive antibodies by reacting the serially diluted antisera with MoPn-infected HeLa lysates in an ELISA (Fig. 6B), which independently confirmed that immunization with either MoPn or Tarp induced high titers of antibodies that specifically recognized MoPn endogenous antigens. We further isotyped the mouse IgG antibodies and found that immunization with either Tarp or MoPn induced more IgG2a than IgG1 (Fig. 6C), suggesting that both antigens provoked a Th1-dominant response, which is known to be required for clearing chlamydial urogenital infection in mice [17, 83]. Since IFNγ is a critical Th1 effector molecule for clearing chlamydial infection, we evaluated the immunized mice for their ability to develop an IFNγ-dominant and chlamydial antigen-specific T cell response sixty days after MoPn challenge infection. When the mouse spleen cells were re-stimulated with MoPn antigens in vitro, we found that the spleen cells from mice immunized with either MoPn or Tarp produced significantly higher levels of IFNγ than those from adjuvant alone immunization (Fig. 7). No IL-4 and only minimum levels of IL-5 were detected from the spleen cell culture supernatants of the immunized mice (data not shown). These results further strengthened the conclusion that immunization with Tarp induced a Th1-dominant immune response.
Fig. 6. Induction of MoPn-specific immune responses by Tarp immunization.
Sera were collected from three different groups of mice immunized with either the adjuvant alone (a total of 10 mice), adjuvant plus Tarp (9 mice) or plus MoPn (10 mice) on day 29 after the last immunization. The sera from each group were pooled and used to detect the endogenous antigens in MoPn-infected cells using an immunofluorescence assay (A) or ELISA (B). For the immunofluorescence assay, the MoPn-infected cells were costained with the mouse antisera (red) as indicated on top of the figure, a rabbit anti-chlamydial organism antibody (aCT395; green) and a DNA dye (blue). To confirm the staining specificity, the antisera from the Tarp and MoPn-immunized groups were preabsorbed with either GST-Tarp or MoPn-infected cell lysates prior to the staining as indicated at the left side of the figure (panels d–g). For ELISA titration of the antibody titers, the MoPn-infected HeLa cell lysates were coated onto microplates and the serially diluted antiserum samples pooled from each group of mice (shown along the X-axis) were applied to the plates. The antibody binding was detected and expressed as OD values as shown along the Y-axis. The ELISA was repeated three times with duplicates in each. (C) The IgG antibodies from each individual mouse of the three groups (adjuvant alone, solid bar; adjuvant + Tarp, gray bar; adjuvant + MoPn, open bar) were further isotyped using the ELISA as described in the legend of panel B above. All mouse antisera were diluted 1:1000 and the titers of the total IgG and each IgG isotype as indicated along the X-axis were measured as OD values at 405nm as displayed along the Y-axis. The results were expressed as mean plus standard deviation.
Fig. 7. Induction of MoPn-specific cellular immune responses by Tarp immunization.
The three groups of mice described in the legend of Fig. 6 were intravaginally infected with MoPn on day 30 after the final immunization and 60 days after the infection, the mouse spleen lymphocytes were collected for monitoring cellular responses to MoPn antigens by using an in vitro restimulation plus cytokine detection approach as described in Fig. 5B legend. The lymphocyte cultures from the three different groups of mice were stimulated with (open bar) or without (solid bar) UV-inactivated MoPn EB organisms at 1 × 106 IFUs/well as indicated along the X-axis. Three days after the stimulation, the culture supernatants were collected for IFNg detection in an ELISA and the results were expressed as pg/ml as listed along the Y-axis (mean ± SD). The number of mice in each group was indicated in the figure. Note that although all three groups of mice produced significant levels of IFNg due to MoPn infection, immunization with either MoPn or Tarp significantly enhanced IFNg production upon MoPn restimulation.
5. Tarp-induced immunity reduces MoPn organism shedding from mouse lower genital tract and prevents inflammatory pathologies in the upper genital tract
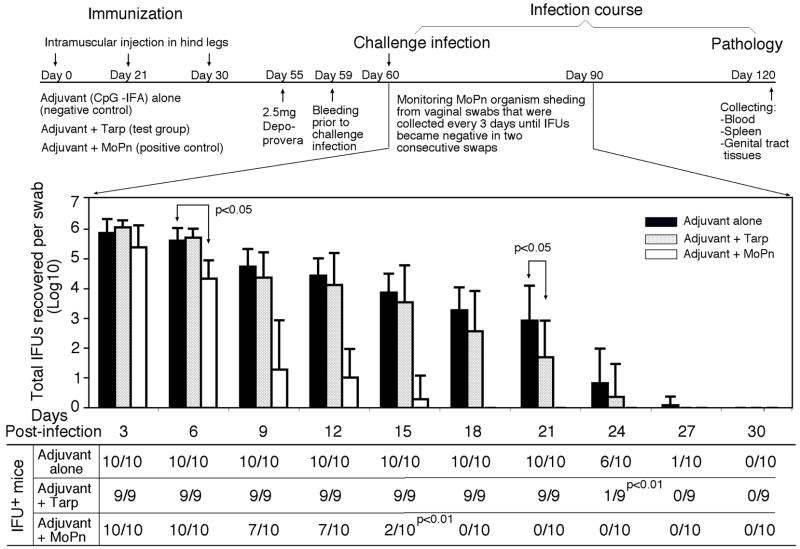
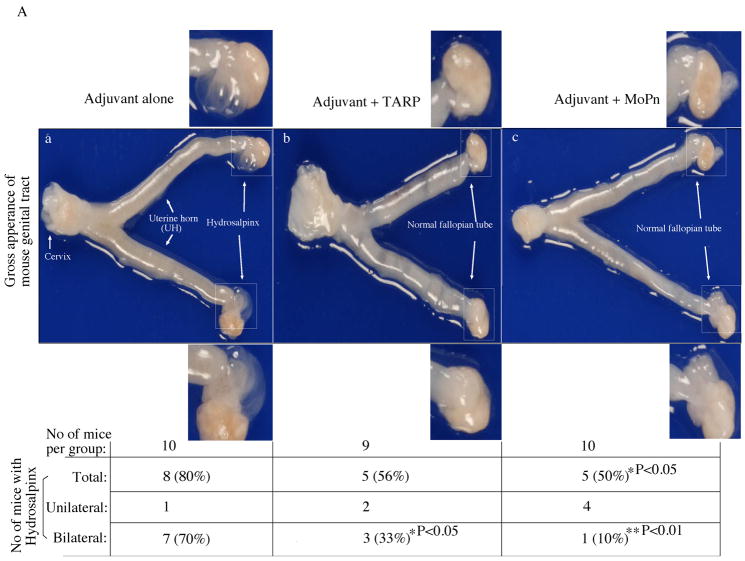
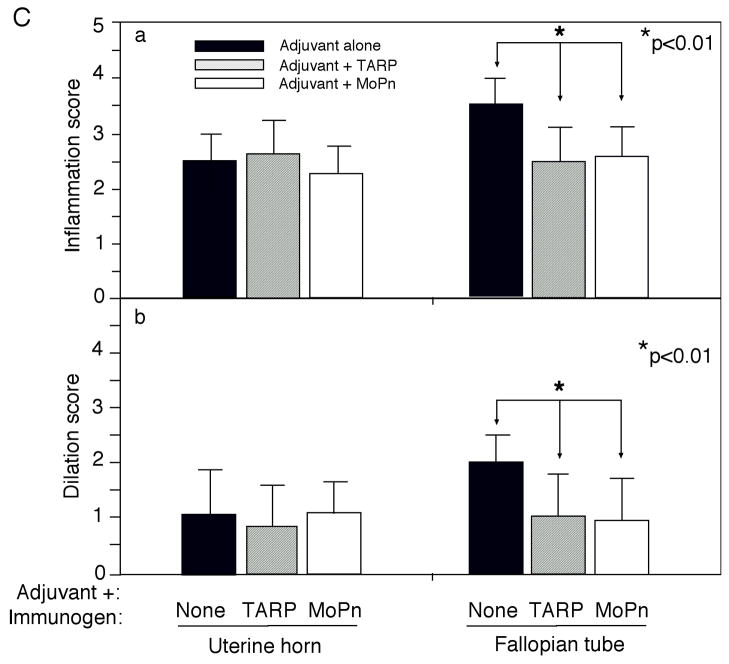
To test whether the Tarp-induced Th1 dominant immune responses can promote the clearance of MoPn infection, we monitored the MoPn organism shedding by periodically collecting vaginal swabs following the MoPn intravaginal infection (Fig. 8). Live MoPn organisms were detected in the vaginal swabs collected from the adjuvant alone group of mice up to 27 days after infection, which represents a typical infection time course in Balb/c mice infected with MoPn [64, 76, 84]. Immunization with the intact MoPn organisms shortened the infection time course to 15 days with a significant reduction in the infectious titers from the vaginal swabs within the first week after infection. Importantly, immunization with Tarp resulted in a significant reduction in the vaginal shedding of live organisms on day 21 and most of the Tarp-immunized mice cleared infection by day 24 post infection. Thus, Tarp-induced immunity enhanced the resolution of MoPn infection from the mouse lower genital tracts although not as potently as the intact organism-induced immunity. We further evaluated the effect of Tarp immunization on inflammatory pathologies in mouse upper genital tract. Sixty days after the intravaginal challenge infection, mice were sacrificed and mouse genital tract tissues were collected for pathology evaluation. Hydrosalpinx is a hallmark of inflammatory pathology in the fallopian tube regions induced by chlamydial infection, which can be identified by visual inspection of the mouse genital tissues. As shown in Fig. 9A, 8 of the 10 mice treated with adjuvant alone developed obvious hydrosalpinx in either both sides (bilateral, 7 mice) or a single side (unilateral, 1 mouse) of the mouse fallopian tubes. However, only 5 out of the 9 Tarp-immunized and 10 MoPn-immunized mice developed hydrosalpinx respectively. Importantly, the incidence of bilateral hydrosalpinx in Tarp-immunized (33%) or MoPn-immunized (10%) mice is significantly lower than that in the adjuvant alone-immunized mice (70%). After gross appearance assessment, we further evaluated the severity of inflammation microscopically using histology sections (Fig. 9B). Both the inflammatory cell infiltration and luminal dilatation were blindly semi-quantitated using a scale of 1 to 4 (Fig. 9C). There was no significant difference in either inflammation or lumenal dilatation scores in the uterine horn tissues between the three groups of mice. However, mice immunized with either Tarp or MoPn displayed significantly lower scores in both inflammatory cell infiltration and lumenal dilatation than the control group of mice, suggesting that the immunity induced by either Tarp or MoPn can reduce the inflammatory damages in the mouse oviducts.
Fig. 8. Effect of Tarp immunization on live chlamydial organism shedding following chlamydial infection.
The three groups of mice described in the legend of Fig. 6 were intravaginally infected with MoPn organisms on day 30 after the final immunization and vaginal swabs were taken along the infection course and on day 60 after infection, all mice were sacrificed (flowchart on top of the figure). As indicated along the X-axis, vaginal swabs were collected every 3 days after MoPn infection for measuring the number of live organisms as inclusion forming units (IFUs). The IFU from each swab was converted into Log10 and the Log10 IFUs were used to calculate mean and standard deviation (mean ± SD) for each mouse group at each time point as presented along the Y-axis. The log10 IFUs were compared between the three groups of mice at each time point using the ANOVA Test and a two-tailed Student t Test was used to further analyze the significance between the Tarp- or MoPn-immunized and the adjuvant alone control group. The number of mice with detectable IFUs from each group and at each time point is listed in the chart at the bottom. The rates of IFU positivity were compared between the immunized and the adjuvant alone groups using a Chitest. The statistically significant differences were indicated in the figure and note that the Tarp immunization significantly reduced the level of IFUs on 21 day and the number of IFU positive mice on day 24 after infection although the MoPn organism immunization was much more robust in shortening the infection time course.
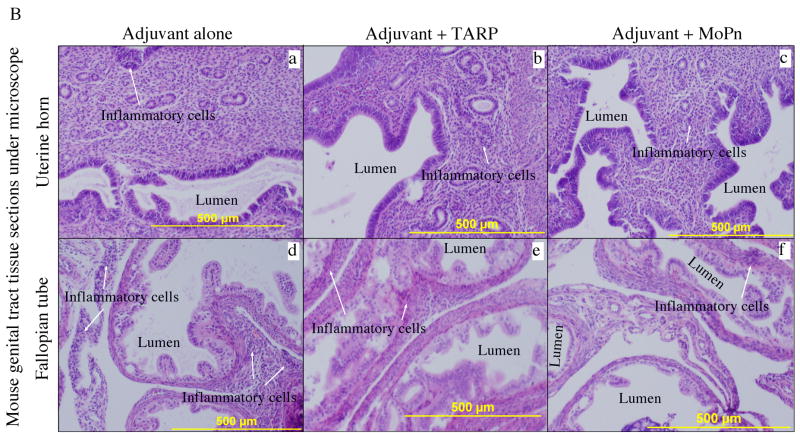
Fig. 9. Effect of Tarp immunization on the development of inflammatory pathologies in the mouse urogenital tract following chlamydial infection.
Sixty days after MoPn infection, mice were sacrificed for pathology observation. (A) The urogenital tract tissues from mice immunized with adjuvant alone (panel a), adjuvant + Tarp (b) or adjuvant + MoPn (c) were examined at the level of gross appearance. Mice with hydrosalpinx in only one (unilateral) or both (bilateral) oviducts were identified and listed in the chart at the bottom of the figure. The incidence of hydrosalpinx was compared between different groups using the Chi-Test and as indicated in the figure, Tarp immunization significantly reduced the rate of bilateral hydrosalpinx comparing to the adjuvant alone group (33% vs. 70%, p<0.05). (B) The urogenital tract tissues were sectioned for microscopic observation of inflammatory pathologies. Representative H&E stained section images covering either the uterine horn (panels a–c) or fallopian tube (d–f) regions were presented from each group of mice. The location of lumen and examples of inflammatory cell infiltration were indicated. Each section was scored for both lumenal dilatation and inflammatory infiltration and the semi-quantitation results were displayed in (C). Inflammation (panel a) and dilatation (panel b) scores assigned to individual mice were used to calculate the means and standard errors for each group as shown along the Y-axis. The mouse and tissue groups were indicated along the X-axis. Solid bar indicates tissue samples from adjuvant alone (n=10), hatched bar for adjuvant + Tarp (n=9) and open bar for adjuvant + MoPn (n=10) groups. ANOVA test was used to analyze differences among different groups and a two-tailed Student t Test for analyzing the differences between Tarp- or MoPn-immunized and adjuvant alone groups. Note that Tarp immunization significantly reduced both the inflammation and lumen dilatation scores in the fallopian tube tissues.
Discussion
Searching for C. trachomatis vaccine candidate antigens has been a long-standing goal in the chlamydial fields. The facts that protective immunity can be induced by bacterial virulence factors [76, 85, 86] and that type III secretion system is specialized in secreting virulence factors have inspired us to search for immunodominant and protective antigens from the chlamydial type III secretion system-related proteins. We expressed all ORFs encoded by the 10 type III secretion-related operons in C. trachomatis serovar D genome [31, 32] and identified Tarp and Pkn5, two putative type III secretion effector molecules [42, 51], as immunodominant antigens using both STI and trachoma patient sera. We further evaluated Tarp for its ability to induce protective immunity in mice because Tarp was recognized by human antisera at higher frequency and with higher titers compared to Pkn5. Immunization with Tarp plus CpG and IFA as adjuvant induced Th1-dominant immune responses that conferred partial protection against both chlamydial infection in the lower genital tracts and inflammatory pathologies in the oviducts.
Although many putative chlamydial type III secretion effector molecules have been identified in cell culture system [36, 39–42, 48], it is not clear whether these molecules are expressed during chlamydial infection in humans. We have used a human antibody reactivity approach to assess whether a given chlamydial protein is present or expressed when humans are infected with C. trachomatis. This is because humans can only make antibodies to chlamydial antigens that are presented to human immune systems during chlamydial infection. We found that 45 of the 55 proteins analyzed were specifically recognized by one or more human antisera (Fig. 1 & 2), suggesting that these 45 proteins are either preexisting in the organisms or newly synthesized or both during chlamydial infection in humans. However, it is difficult to determine whether the remaining 10 chlamydial proteins that failed to react with any human antisera (Table 1) are expressed during chlamydial infection in humans. This is because not every expressed chlamydial protein is immunogenic in humans or accessible to human immune system during chlamydial infection. However, these 10 chlamydial proteins are hypothetical proteins unique to Chlamydia or prokaryotic species and have no significant homology with any proteins from animals or humans. In fact, when these proteins were used to immunize mice, high titers of antibodies were produced (data not shown). Thus, lack of human antibody production against these proteins may not be due to lack of immunogenicity by these proteins. We also realize that lack of reactivity between human antibodies and the chlamydial fusion proteins may also be caused by the difference in conformation between the endogenous and fusion proteins. Thus, alternative strategies are required to increase the detection sensitivity and determine the expression status of these chlamydial proteins in humans.
The predominant recognition of Tarp by human antibodies suggests that Tarp is both highly expressed and efficiently presented to human immune system during chlamydial infection. In cell culture system, Tarp is a pre-existing protein in chlamydial EBs and injected into the host cell upon chlamydial attachment [51]. However, it disappeared between 13 and 24 hours after infection (Fig. 3), during which the chlamydial inclusions are enriched in RBs. Indeed, RBs harvested during this period of time lacked Tarp protein (Fig. 4). The reappearance of Tarp at 28 hours after infection indicates that Tarp is expressed at a late stage of chlamydial infection, which is consistent with the previous observations that some chlamydial type III secretion effector molecules are expressed at the late stage during RB to EB conversion [44, 80–82]. Interestingly, Tarp was accumulated in RBs when a bacterial type III secretion inhibitor C1 was applied to the culture [44], suggesting that a functional type III secretion system may be required for Tarp to integrate into EBs and the RB to EB conversion may depend on Tarp secretion. However, the exact pathway on how Tarp is integrated into EBs requires further studies. Nevertheless, the findings that Tarp is synthesized at the very late stage of chlamydial growth cycle and rapidly packaged into EBs are consistent with its primary function, which is to facilitate the chlamydial EB invasion of new target cells [51–57, 79]. The presence of Tarp on chlamydial EBs during the extracellular stage may partially explain its immunodominance in humans.
Tarp was also found to be highly immunogenic during chlamydial infection in mice (Fig. 5). More importantly, immunization of mice with Tarp induced partial protection against live chlamydial organism shedding from the lower genital tract (Fig. 8) and significant reduction in the inflammatory pathology in the oviducts (Fig. 9). The Tarp-induced protection correlated well with the Th1 dominant immunity in the Tarp-immunized mice, in which, most antigen-specific IgG antibodies were isotyped IgG2a (Fig. 6C) and the T cell responses recalled by chlamydial antigens were dominated by IFNγ production (Fig. 7 & data not shown). However, the Tarp-induced immunity only shortened the time course of live organism shedding detected in the vaginal swab samples by a few days while the whole organism-induced immunity reduced shedding by more than 10 days compared to the adjuvant alone-immunized group (Fig. 8). Importantly, Tarp immunization-induced immunity resulted in a more obvious reduction in inflammatory pathologies in mouse oviducts (Fig. 9). We are aware that our data came from relatively small sample size and there is an inherent variability in quantitating chlamydial infectious titer and urogenital pathology severity. Nevertheless, the apparent discordance between live chlamydial organism shedding from the lower urogenital tract and inflammatory pathologies in the upper tract is interesting. It is generally thought that inflammatory pathologies in the upper tract are due to ascending infection. The amount of live organisms collected from vaginal swabs only indicates the level of shedding of organisms from the lower genital tract. It will be worth testing whether Tarp-induced immunity can selectively block the chlamydial ascending infection, leading to a significant reduction in the mouse upper urogenital tract inflammatory pathologies, without dramatically affecting the infection in the lower tract.
Acknowledgments
This work was supported in part by grants (to G. Zhong) from the US National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Taylor HR, Johnson SL, Schachter J, Caldwell HD, Prendergast RA. Pathogenesis of trachoma: the stimulus for inflammation. J Immunol. 1987 May 1;138(9):3023–7. [PubMed] [Google Scholar]
- 2.Sherman KJ, Daling JR, Stergachis A, Weiss NS, Foy HM, Wang SP, et al. Sexually transmitted diseases and tubal pregnancy. Sex Transm Dis. 1990 Jul-Sep;17(3):115–21. doi: 10.1097/00007435-199007000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Kinnunen AH, Surcel HM, Lehtinen M, Karhukorpi J, Tiitinen A, Halttunen M, et al. HLA DQ alleles and interleukin-10 polymorphism associated with Chlamydia trachomatis-related tubal factor infertility: a case-control study. Hum Reprod. 2002 Aug;17(8):2073–8. doi: 10.1093/humrep/17.8.2073. [DOI] [PubMed] [Google Scholar]
- 4.Bauwens JE, Orlander H, Gomez MP, Lampe M, Morse S, Stamm WE, et al. Epidemic Lymphogranuloma venereum during epidemics of crack cocaine use and HIV infection in the Bahamas. Sex Transm Dis. 2002 May;29(5):253–9. doi: 10.1097/00007435-200205000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Schachter J, Moncada J. Lymphogranuloma venereum: how to turn an endemic disease into an outbreak of a new disease? Start looking. Sex Transm Dis. 2005 Jun;32(6):331–2. doi: 10.1097/01.olq.0000168429.13282.c8. [DOI] [PubMed] [Google Scholar]
- 6.Spaargaren J. Slow epidemic of lymphogranuloma venereum l2b strain. Emerg Infect Dis. 2005 Nov;11(11):1787–8. doi: 10.3201/eid1111.050821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cotter TW, Meng Q, Shen ZL, Zhang YX, Su H, Caldwell HD. Protective efficacy of major outer membrane protein-specific immunoglobulin A (IgA) and IgG monoclonal antibodies in a murine model of Chlamydia trachomatis genital tract infection. Infect Immun. 1995 Dec;63(12):4704–14. doi: 10.1128/iai.63.12.4704-4714.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perry LL, Feilzer K, Hughes S, Caldwell HD. Clearance of Chlamydia trachomatis from the murine genital mucosa does not require perforin-mediated cytolysis or Fas-mediated apoptosis. Infect Immun. 1999 Mar;67(3):1379–85. doi: 10.1128/iai.67.3.1379-1385.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pal S, Peterson EM, de la Maza LM. Vaccination with the Chlamydia trachomatis major outer membrane protein can elicit an immune response as protective as that resulting from inoculation with live bacteria. Infect Immun. 2005 Dec;73(12):8153–60. doi: 10.1128/IAI.73.12.8153-8160.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barteneva N, Theodor I, Peterson EM, de la Maza LM. Role of neutrophils in controlling early stages of a Chlamydia trachomatis infection. Infect Immun. 1996 Nov;64(11):4830–3. doi: 10.1128/iai.64.11.4830-4833.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang D, Yang X, Lu H, Zhong G, Brunham RC. Immunity to Chlamydia trachomatis mouse pneumonitis induced by vaccination with live organisms correlates with early granulocyte-macrophage colony-stimulating factor and interleukin-12 production and with dendritic cell-like maturation. Infect Immun. 1999 Apr;67(4):1606–13. doi: 10.1128/iai.67.4.1606-1613.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu H, Zhong G. Interleukin-12 production is required for chlamydial antigen-pulsed dendritic cells to induce protection against live Chlamydia trachomatis infection. Infect Immun. 1999 Apr;67(4):1763–9. doi: 10.1128/iai.67.4.1763-1769.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murthy AK, Sharma J, Coalson JJ, Zhong G, Arulanandam BP. Chlamydia trachomatis pulmonary infection induces greater inflammatory pathology in immunoglobulin A deficient mice. Cell Immunol. 2004 Jul;230(1):56–64. doi: 10.1016/j.cellimm.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 14.Cheng W, Shivshankar P, Li Z, Chen L, Yeh IT, Zhong G. Caspase-1 contributes to Chlamydia trachomatis-induced upper urogenital tract inflammatory pathologies without affecting the course of infection. Infect Immun. 2008 Feb;76(2):515–22. doi: 10.1128/IAI.01064-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shah AA, Schripsema JH, Imtiaz MT, Sigar IM, Kasimos J, Matos PG, et al. Histopathologic changes related to fibrotic oviduct occlusion after genital tract infection of mice with Chlamydia muridarum. Sex Transm Dis. 2005 Jan;32(1):49–56. doi: 10.1097/01.olq.0000148299.14513.11. [DOI] [PubMed] [Google Scholar]
- 16.Patton DL, Landers DV, Schachter J. Experimental Chlamydia trachomatis salpingitis in mice: initial studies on the characterization of the leukocyte response to chlamydial infection. J Infect Dis. 1989 Jun;159(6):1105–10. doi: 10.1093/infdis/159.6.1105. [DOI] [PubMed] [Google Scholar]
- 17.Morrison RP, Caldwell HD. Immunity to murine chlamydial genital infection. Infect Immun. 2002 Jun;70(6):2741–51. doi: 10.1128/IAI.70.6.2741-2751.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morrison SG, Morrison RP. A predominant role for antibody in acquired immunity to chlamydial genital tract reinfection. J Immunol. 2005 Dec 1;175(11):7536–42. doi: 10.4049/jimmunol.175.11.7536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fling SP, Sutherland RA, Steele LN, Hess B, D’Orazio SE, Maisonneuve J, et al. CD8+ T cells recognize an inclusion membrane-associated protein from the vacuolar pathogen Chlamydia trachomatis. Proc Natl Acad Sci U S A. 2001 Jan 30;98(3):1160–5. doi: 10.1073/pnas.98.3.1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lampe MF, Wilson CB, Bevan MJ, Starnbach MN. Gamma interferon production by cytotoxic T lymphocytes is required for resolution of Chlamydia trachomatis infection. Infect Immun. 1998 Nov;66(11):5457–61. doi: 10.1128/iai.66.11.5457-5461.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Read TD, Brunham RC, Shen C, Gill SR, Heidelberg JF, White O, et al. Genome sequences of Chlamydia trachomatis MoPn and Chlamydia pneumoniae AR39. Nucleic Acids Res. 2000 Mar 15;28(6):1397–406. doi: 10.1093/nar/28.6.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Read TD, Myers GS, Brunham RC, Nelson WC, Paulsen IT, Heidelberg J, et al. Genome sequence of Chlamydophila caviae (Chlamydia psittaci GPIC): examining the role of niche-specific genes in the evolution of the Chlamydiaceae. Nucleic Acids Res. 2003 Apr 15;31(8):2134–47. doi: 10.1093/nar/gkg321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stephens RS, Kalman S, Lammel C, Fan J, Marathe R, Aravind L, et al. Genome sequence of an obligate intracellular pathogen of humans: Chlamydia trachomatis. Science. 1998 Oct 23;282(5389):754–9. doi: 10.1126/science.282.5389.754. [DOI] [PubMed] [Google Scholar]
- 24.Hackstadt T. The diverse habitats of obligate intracellular parasites. Curr Opin Microbiol. 1998 Feb;1(1):82–7. doi: 10.1016/s1369-5274(98)80146-x. [DOI] [PubMed] [Google Scholar]
- 25.Hackstadt T, Fischer ER, Scidmore MA, Rockey DD, Heinzen RA. Origins and functions of the chlamydial inclusion. Trends Microbiol. 1997 Jul;5(7):288–93. doi: 10.1016/S0966-842X(97)01061-5. [DOI] [PubMed] [Google Scholar]
- 26.Stuber K, Frey J, Burnens AP, Kuhnert P. Detection of type III secretion genes as a general indicator of bacterial virulence. Mol Cell Probes. 2003 Feb;17(1):25–32. doi: 10.1016/s0890-8508(02)00108-1. [DOI] [PubMed] [Google Scholar]
- 27.Hueck CJ. Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol Mol Biol Rev. 1998 Jun;62(2):379–433. doi: 10.1128/mmbr.62.2.379-433.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Galan JE, Collmer A. Type III secretion machines: bacterial devices for protein delivery into host cells. Science. 1999 May 21;284(5418):1322–8. doi: 10.1126/science.284.5418.1322. [DOI] [PubMed] [Google Scholar]
- 29.Bannantine JP, Griffiths RS, Viratyosin W, Brown WJ, Rockey DD. A secondary structure motif predictive of protein localization to the chlamydial inclusion membrane. Cell Microbiol. 2000 Feb;2(1):35–47. doi: 10.1046/j.1462-5822.2000.00029.x. [DOI] [PubMed] [Google Scholar]
- 30.Toh H, Miura K, Shirai M, Hattori M. In silico inference of inclusion membrane protein family in obligate intracellular parasites chlamydiae. DNA Res. 2003 Feb 28;10(1):9–17. doi: 10.1093/dnares/10.1.9. [DOI] [PubMed] [Google Scholar]
- 31.Peters J, Wilson DP, Myers G, Timms P, Bavoil PM. Type III secretion a la Chlamydia. Trends Microbiol. 2007 Jun;15(6):241–51. doi: 10.1016/j.tim.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 32.Hefty PS, Stephens RS. Chlamydial type III secretion system is encoded on ten operons preceded by sigma 70-like promoter elements. J Bacteriol. 2007 Jan;189(1):198–206. doi: 10.1128/JB.01034-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rockey DD, Heinzen RA, Hackstadt T. Cloning and characterization of a Chlamydia psittaci gene coding for a protein localized in the inclusion membrane of infected cells. Mol Microbiol. 1995 Feb;15(4):617–26. doi: 10.1111/j.1365-2958.1995.tb02371.x. [DOI] [PubMed] [Google Scholar]
- 34.Rockey DD, Scidmore MA, Bannantine JP, Brown WJ. Proteins in the chlamydial inclusion membrane. Microbes Infect. 2002 Mar;4(3):333–40. doi: 10.1016/s1286-4579(02)01546-0. [DOI] [PubMed] [Google Scholar]
- 35.Li Z, Chen C, Chen D, Wu Y, Zhong Y, Zhong G. Characterization of fifty putative inclusion membrane proteins encoded in the Chlamydia trachomatis genome. Infect Immun. 2008 Jun;76(6):2746–57. doi: 10.1128/IAI.00010-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Valdivia RH. Chlamydia effector proteins and new insights into chlamydial cellular microbiology. Curr Opin Microbiol. 2008 Feb;11(1):53–9. doi: 10.1016/j.mib.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 37.Li Z, Chen D, Zhong Y, Wang S, Zhong G. The chlamydial plasmid-encoded protein pgp3 is secreted into the cytosol of Chlamydia-infected cells. Infect Immun. 2008 Aug;76(8):3415–28. doi: 10.1128/IAI.01377-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhong G, Fan P, Ji H, Dong F, Huang Y. Identification of a chlamydial protease-like activity factor responsible for the degradation of host transcription factors. J Exp Med. 2001 Apr 16;193(8):935–42. doi: 10.1084/jem.193.8.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Subtil A, Parsot C, Dautry-Varsat A. Secretion of predicted Inc proteins of Chlamydia pneumoniae by a heterologous type III machinery. Mol Microbiol. 2001 Feb;39(3):792–800. doi: 10.1046/j.1365-2958.2001.02272.x. [DOI] [PubMed] [Google Scholar]
- 40.Subtil A, Delevoye C, Balana ME, Tastevin L, Perrinet S, Dautry-Varsat A. A directed screen for chlamydial proteins secreted by a type III mechanism identifies a translocated protein and numerous other new candidates. Mol Microbiol. 2005 Jun;56(6):1636–47. doi: 10.1111/j.1365-2958.2005.04647.x. [DOI] [PubMed] [Google Scholar]
- 41.Fields KA, Hackstadt T. Evidence for the secretion of Chlamydia trachomatis CopN by a type III secretion mechanism. Mol Microbiol. 2000 Dec;38(5):1048–60. doi: 10.1046/j.1365-2958.2000.02212.x. [DOI] [PubMed] [Google Scholar]
- 42.Ho TD, Starnbach MN. The Salmonella enterica serovar typhimurium-encoded type III secretion systems can translocate Chlamydia trachomatis proteins into the cytosol of host cells. Infect Immun. 2005 Feb;73(2):905–11. doi: 10.1128/IAI.73.2.905-911.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Muschiol S, Bailey L, Gylfe A, Sundin C, Hultenby K, Bergstrom S, et al. A small-molecule inhibitor of type III secretion inhibits different stages of the infectious cycle of Chlamydia trachomatis. Proc Natl Acad Sci U S A. 2006 Sep 26;103(39):14566–71. doi: 10.1073/pnas.0606412103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wolf K, Betts HJ, Chellas-Gery B, Hower S, Linton CN, Fields KA. Treatment of Chlamydia trachomatis with a small molecule inhibitor of the Yersinia type III secretion system disrupts progression of the chlamydial developmental cycle. Mol Microbiol. 2006 Sep;61(6):1543–55. doi: 10.1111/j.1365-2958.2006.05347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Betts HJ, Twiggs LE, Sal MS, Wyrick PB, Fields KA. Bioinformatic and biochemical evidence for the identification of the type III secretion system needle protein of Chlamydia trachomatis. J Bacteriol. 2008 Mar;190(5):1680–90. doi: 10.1128/JB.01671-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fields KA, Fischer ER, Mead DJ, Hackstadt T. Analysis of putative Chlamydia trachomatis chaperones Scc2 and Scc3 and their use in the identification of type III secretion substrates. J Bacteriol. 2005 Sep;187(18):6466–78. doi: 10.1128/JB.187.18.6466-6478.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Slepenkin A, de la Maza LM, Peterson EM. Interaction between components of the type III secretion system of Chlamydiaceae. J Bacteriol. 2005 Jan;187(2):473–9. doi: 10.1128/JB.187.2.473-479.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chellas-Gery B, Linton CN, Fields KA. Human GCIP interacts with CT847, a novel Chlamydia trachomatis type III secretion substrate, and is degraded in a tissue-culture infection model. Cell Microbiol. 2007 Oct;9(10):2417–30. doi: 10.1111/j.1462-5822.2007.00970.x. [DOI] [PubMed] [Google Scholar]
- 49.Zhong G, Fan T, Liu L. Chlamydia inhibits interferon gamma-inducible major histocompatibility complex class II expression by degradation of upstream stimulatory factor 1. J Exp Med. 1999 Jun 21;189(12):1931–8. doi: 10.1084/jem.189.12.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhong G, Liu L, Fan T, Fan P, Ji H. Degradation of transcription factor RFX5 during the inhibition of both constitutive and interferon gamma-inducible major histocompatibility complex class I expression in chlamydia-infected cells. J Exp Med. 2000 May 1;191(9):1525–34. doi: 10.1084/jem.191.9.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Clifton DR, Fields KA, Grieshaber SS, Dooley CA, Fischer ER, Mead DJ, et al. A chlamydial type III translocated protein is tyrosine-phosphorylated at the site of entry and associated with recruitment of actin. Proc Natl Acad Sci U S A. 2004 Jul 6;101(27):10166–71. doi: 10.1073/pnas.0402829101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Backert S, Selbach M. Tyrosine-phosphorylated bacterial effector proteins: the enemies within. Trends Microbiol. 2005 Oct;13(10):476–84. doi: 10.1016/j.tim.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 53.Clifton DR, Dooley CA, Grieshaber SS, Carabeo RA, Fields KA, Hackstadt T. Tyrosine phosphorylation of the chlamydial effector protein Tarp is species specific and not required for recruitment of actin. Infect Immun. 2005 Jul;73(7):3860–8. doi: 10.1128/IAI.73.7.3860-3868.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Engel J. Tarp and Arp: How Chlamydia induces its own entry. Proc Natl Acad Sci U S A. 2004 Jul 6;101(27):9947–8. doi: 10.1073/pnas.0403633101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jewett TJ, Fischer ER, Mead DJ, Hackstadt T. Chlamydial TARP is a bacterial nucleator of actin. Proc Natl Acad Sci U S A. 2006 Oct 17;103(42):15599–604. doi: 10.1073/pnas.0603044103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jewett TJ, Dooley CA, Mead DJ, Hackstadt T. Chlamydia trachomatis tarp is phosphorylated by src family tyrosine kinases. Biochem Biophys Res Commun. 2008 Jun 27;371(2):339–44. doi: 10.1016/j.bbrc.2008.04.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lane BJ, Mutchler C, Al Khodor S, Grieshaber SS, Carabeo RA. Chlamydial entry involves TARP binding of guanine nucleotide exchange factors. PLoS Pathog. 2008 Mar;4(3):e1000014. doi: 10.1371/journal.ppat.1000014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Greene W, Xiao Y, Huang Y, McClarty G, Zhong G. Chlamydia-infected cells continue to undergo mitosis and resist induction of apoptosis. Infect Immun. 2004 Jan;72(1):451–60. doi: 10.1128/IAI.72.1.451-460.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhong G, Toth I, Reid R, Brunham RC. Immunogenicity evaluation of a lipidic amino acid-based synthetic peptide vaccine for Chlamydia trachomatis. J Immunol. 1993 Oct 1;151(7):3728–36. [PubMed] [Google Scholar]
- 60.Zhong G, Reis e Sousa C, Germain RN. Production, specificity, and functionality of monoclonal antibodies to specific peptide-major histocompatibility complex class II complexes formed by processing of exogenous protein. Proc Natl Acad Sci U S A. 1997 Dec 9;94(25):13856–61. doi: 10.1073/pnas.94.25.13856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhong G, Berry J, Brunham RC. Antibody recognition of a neutralization epitope on the major outer membrane protein of Chlamydia trachomatis. Infect Immun. 1994 May;62(5):1576–83. doi: 10.1128/iai.62.5.1576-1583.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhong G, Brunham RC. Antibody responses to the chlamydial heat shock proteins hsp60 and hsp70 are H-2 linked. Infect Immun. 1992 Aug;60(8):3143–9. doi: 10.1128/iai.60.8.3143-3149.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhong GM, Brunham RC. Antigenic determinants of the chlamydial major outer membrane protein resolved at a single amino acid level. Infect Immun. 1991 Mar;59(3):1141–7. doi: 10.1128/iai.59.3.1141-1147.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Murthy AK, Cong Y, Murphey C, Guentzel MN, Forsthuber TG, Zhong G, et al. Chlamydial protease-like activity factor induces protective immunity against genital chlamydial infection in transgenic mice that express the human HLA-DR4 allele. Infect Immun. 2006 Dec;74(12):6722–9. doi: 10.1128/IAI.01119-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhong GM, Brunham RC. Immunoaccessible peptide sequences of the major outer membrane protein from Chlamydia trachomatis serovar C. Infect Immun. 1990 Oct;58(10):3438–41. doi: 10.1128/iai.58.10.3438-3441.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sharma J, Bosnic AM, Piper JM, Zhong G. Human antibody responses to a Chlamydia-secreted protease factor. Infect Immun. 2004 Dec;72(12):7164–71. doi: 10.1128/IAI.72.12.7164-7171.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cheng W, Shivshankar P, Zhong Y, Chen D, Li Z, Zhong G. Intracellular interleukin-1alpha mediates interleukin-8 production induced by Chlamydia trachomatis infection via a mechanism independent of type I interleukin-1 receptor. Infect Immun. 2008 Mar;76(3):942–51. doi: 10.1128/IAI.01313-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xiao Y, Zhong Y, Su H, Zhou Z, Chiao P, Zhong G. NF-kappa B activation is not required for Chlamydia trachomatis inhibition of host epithelial cell apoptosis. J Immunol. 2005 Feb 1;174(3):1701–8. doi: 10.4049/jimmunol.174.3.1701. [DOI] [PubMed] [Google Scholar]
- 69.Fan T, Lu H, Hu H, Shi L, McClarty GA, Nance DM, et al. Inhibition of apoptosis in chlamydia-infected cells: blockade of mitochondrial cytochrome c release and caspase activation. J Exp Med. 1998 Feb 16;187(4):487–96. doi: 10.1084/jem.187.4.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhong G, Castellino F, Romagnoli P, Germain RN. Evidence that binding site occupancy is necessary and sufficient for effective major histocompatibility complex (MHC) class II transport through the secretory pathway redefines the primary function of class II-associated invariant chain peptides (CLIP) J Exp Med. 1996 Nov 1;184(5):2061–6. doi: 10.1084/jem.184.5.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sharma J, Dong F, Pirbhai M, Zhong G. Inhibition of proteolytic activity of a chlamydial proteasome/protease-like activity factor by antibodies from humans infected with Chlamydia trachomatis. Infect Immun. 2005 Jul;73(7):4414–9. doi: 10.1128/IAI.73.7.4414-4419.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dong F, Zhong Y, Arulanandam B, Zhong G. Production of a proteolytically active protein, chlamydial protease/proteasome-like activity factor, by five different Chlamydia species. Infect Immun. 2005 Mar;73(3):1868–72. doi: 10.1128/IAI.73.3.1868-1872.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dong F, Pirbhai M, Xiao Y, Zhong Y, Wu Y, Zhong G. Degradation of the proapoptotic proteins Bik, Puma, and Bim with Bcl-2 domain 3 homology in Chlamydia trachomatis-infected cells. Infect Immun. 2005 Mar;73(3):1861–4. doi: 10.1128/IAI.73.3.1861-1864.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chu RS, Targoni OS, Krieg AM, Lehmann PV, Harding CV. CpG oligodeoxynucleotides act as adjuvants that switch on T helper 1 (Th1) immunity. J Exp Med. 1997 Nov 17;186(10):1623–31. doi: 10.1084/jem.186.10.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Imtiaz MT, Schripsema JH, Sigar IM, Kasimos JN, Ramsey KH. Inhibition of matrix metalloproteinases protects mice from ascending infection and chronic disease manifestations resulting from urogenital Chlamydia muridarum infection. Infect Immun. 2006 Oct;74(10):5513–21. doi: 10.1128/IAI.00730-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Murthy AK, Chambers JP, Meier PA, Zhong G, Arulanandam BP. Intranasal vaccination with a secreted chlamydial protein enhances resolution of genital Chlamydia muridarum infection, protects against oviduct pathology, and is highly dependent upon endogenous gamma interferon production. Infect Immun. 2007 Feb;75(2):666–76. doi: 10.1128/IAI.01280-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sharma J, Zhong Y, Dong F, Piper JM, Wang G, Zhong G. Profiling of human antibody responses to Chlamydia trachomatis urogenital tract infection using microplates arrayed with 156 chlamydial fusion proteins. Infect Immun. 2006 Mar;74(3):1490–9. doi: 10.1128/IAI.74.3.1490-1499.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yuan Y, Zhang YX, Watkins NG, Caldwell HD. Nucleotide and deduced amino acid sequences for the four variable domains of the major outer membrane proteins of the 15 Chlamydia trachomatis serovars. Infect Immun. 1989 Apr;57(4):1040–9. doi: 10.1128/iai.57.4.1040-1049.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Carlson JH, Porcella SF, McClarty G, Caldwell HD. Comparative genomic analysis of Chlamydia trachomatis oculotropic and genitotropic strains. Infect Immun. 2005 Oct;73(10):6407–18. doi: 10.1128/IAI.73.10.6407-6418.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fields KA, Mead DJ, Dooley CA, Hackstadt T. Chlamydia trachomatis type III secretion: evidence for a functional apparatus during early-cycle development. Mol Microbiol. 2003 May;48(3):671–83. doi: 10.1046/j.1365-2958.2003.03462.x. [DOI] [PubMed] [Google Scholar]
- 81.Belland RJ, Zhong G, Crane DD, Hogan D, Sturdevant D, Sharma J, et al. Genomic transcriptional profiling of the developmental cycle of Chlamydia trachomatis. Proc Natl Acad Sci U S A. 2003 Jul 8;100(14):8478–83. doi: 10.1073/pnas.1331135100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shaw EI, Dooley CA, Fischer ER, Scidmore MA, Fields KA, Hackstadt T. Three temporal classes of gene expression during the Chlamydia trachomatis developmental cycle. Mol Microbiol. 2000 Aug;37(4):913–25. doi: 10.1046/j.1365-2958.2000.02057.x. [DOI] [PubMed] [Google Scholar]
- 83.Morrison RP, Feilzer K, Tumas DB. Gene knockout mice establish a primary protective role for major histocompatibility complex class II-restricted responses in Chlamydia trachomatis genital tract infection. Infect Immun. 1995 Dec;63(12):4661–8. doi: 10.1128/iai.63.12.4661-4668.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Murphey C, Murthy AK, Meier PA, Neal Guentzel M, Zhong G, Arulanandam BP. The protective efficacy of chlamydial protease-like activity factor vaccination is dependent upon CD4+ T cells. Cell Immunol. 2006 Aug;242(2):110–7. doi: 10.1016/j.cellimm.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Saikh KU, Kissner TL, Dyas B, Tropea JE, Waugh DS, Ulrich RG. Human cytolytic T cell recognition of Yersinia pestis virulence proteins that target innate immune responses. J Infect Dis. 2006 Dec 15;194(12):1753–60. doi: 10.1086/509507. [DOI] [PubMed] [Google Scholar]
- 86.Abe A, Nagamatsu K, Watanabe M. The Bordetella type III secretion system: its application to vaccine development. Microbiol Immunol. 2008;52(2):128–33. doi: 10.1111/j.1348-0421.2008.00028.x. [DOI] [PubMed] [Google Scholar]