Abstract
Oxidative stress occurs when the level of prooxidants exceeds the level of antioxidants in cells resulting in oxidation of cellular components and consequent loss of cellular function. Oxidative stress is implicated in wide range of age related disorders including Alzheimer's disease, Parkinson's disease amyotrophic lateral sclerosis (ALS), Huntington's disease and the aging process itself (Lin and Beal, 2006). In the anterior segment of the eye, oxidative stress has been linked to lens cataract (Truscott, 2005) and glaucoma (Tezel, 2006) while in the posterior segment of the eye oxidative stress has been associated with macular degeneration (Hollyfield et al., 2008). Key to many oxidative stress conditions are alterations in the efficiency of mitochondrial respiration resulting in superoxide (O2-) production. Superoxide production precedes subsequent reactions that form potentially more dangerous reactive oxygen species (ROS) species such as the hydroxyl radical (˙OH), hydrogen peroxide (H2O2) and peroxynitrite (OONO-). The major source of ROS in the mitochondria, and in the cell overall, is leakage of electrons from complexes I and III of the electron transport chain. It is estimated that 0.2-2% of oxygen taken up by cells is converted to ROS, through mitochondrial superoxide generation, by the mitochondria (Hansford et al., 1997). Generation of superoxide at complex I and III has been shown to occur at both the matrix side of the inner mitochondrial membrane and the cytosolic side of the membrane (Kakkar and Singh 2007). While exogenous sources of ROS such as UV light, visible light, ionizing radiation, chemotherapeutics, and environmental toxins may contribute to the oxidative milieu, mitochondria are perhaps the most significant contribution to ROS production affecting the aging process. In addition to producing ROS, mitochondria are also a target for ROS which in turn reduces mitochondrial efficiency and leads to the generation of more ROS in a vicious self-destructive cycle. Consequently, the mitochondria have evolved a number of antioxidant and key repair systems to limit the damaging potential of free oxygen radicals and to repair damaged proteins (Figure 1.0). The aging eye appears to be at considerable risk from oxidative stress. This review will outline the potential role of mitochondrial function and redox balance in age-related eye diseases, and detail how the methionine sulfoxide reductase (Msr) protein repair system and other redox systems play key roles in the function and maintenance of the aging eye.
Keywords: Mitochondria, Cataract, Macular Degeneration, Oxidative Stress, Reactive Oxygen Species, Aging, Methionine Sulfoxide Reductase
Introduction
Maintaining the redox balance within the mitochondria is critical for cellular homeostasis since the mitochondria house the energy producing systems of the cell and it is widely recognized that damage to the mitochondria plays a key role in aging and age-related disorders. Production of reactive oxygen species (ROS) species such as the hydroxyl radical (˙OH), singlet oxygen (1O2), hydrogen peroxide (H2O2) and peroxynitrite (OONO-) is finely balanced with sophisticated antioxidant and repair systems located in this complex organelle. Loss of these systems leads to protein oxidations that are hallmarks of many ocular diseases including cataract and retinal degeneration. The two most oxidizable protein amino acids are methionine and cysteine making mitochondrial systems that protect or repair these of particular interest. This review is an attempt to integrate how mitochondrial ROS are altered in the aging eye, along with those protective and repair systems believed to regulate ROS levels in this tissue and how damage to these systems contributes to age-onset eye disease. Given the enormity, complexity and wide ranging importance of these systems we undoubtedly have overlooked many critically important aspects of this area. In particular, redox regulation of signaling systems has not been included. Possible omissions in this regard in no way diminish the importance of these areas and we apologize in advance for any omissions.
ROS and Aging
ROS are believed to arise in cells from exogenous (environmental) and endogenous sources. Exogenous sources of ROS include UV light, visible light, ionizing radiation, chemotherapeutics, and environmental toxins. Endogenous sources include activity of peroxisomes, lipooxygenases, NADH oxidase, cytochrome P450 and of course mitochondrial respiration. In humans, ROS have been implicated in a variety of human diseases including cancer, type II diabetes, arteriosclerosis, chronic inflammatory diseases and ischemia/reperfusion injury (Droge, 2002). ROS or oxidative stress have also been linked to age-related diseases and the aging process itself leading to the proposition of the free radical theory of aging. A significant amount of total research is dedicated to the elucidation of the role of ROS in many diseases but also in the aging process. The mitochondrial theory of aging proposes that ROS production leads to an accumulation of mitochondrial DNA (mtDNA) mutations leading to mitochondrial dysfunction, consequent increased ROS production, cell death and generation of aging diseases. ROS production, increases with decreasing cellular antioxidant capacity during aging (Wei and Lee, 2002). The resulting ROS-induced damage to proteins and lipids is believed to underlie the pathogenesis of a number of age related diseases including Alzheimer's disease, Parkinson's disease, Huntington's disease, amyotrophic lateral sclerosis, hereditary spastic paraplegia, and cerebellar degenerations (Beal, 2005).
To reduce the accumulation of ROS damage and hold off the onset of age-related diseases, caloric restriction has been shown to be the most effective anti aging intervention studied thus far. Restricting food intake of laboratory animals increases both the mean and maximum lifespan and actually slows the progression of age-associated disease (Gredilla and Barja, 2005). It has been demonstrated in a number of models that caloric restriction decreases mitochondrial free radical generation thus decreasing macromolecule and mitochondrial damage (Barja, 2004). Interestingly restriction of methionine intake (40% and 80% restriction) has also been shown to decrease mitochondrial ROS generation and percent free radical leak in rat liver mitochondria (Caro et al., 2008). The same group also reported that protein restriction alone could decrease mitochondrial ROS production and mtDNA damage in rat liver (Sanz et al., 2004). Caloric restriction also retards age related diseases, studies of dietary links to Parkinson's disease and Alzheimer's suggest that individuals with a low calorie intake are at reduced risk (Matteson et al., 2002). Caloric restriction was shown to specifically decrease ROS production at complex I of the electron transport chain (Gredilla et al., 2001) giving further strength to the argument that mitochondrial ROS are major contributors to aging and potentially the development of age-onset diseases.
Mitochondria and ROS
Mitochondria are sometimes referred to as the powerhouses of the cell since they generate most of the cells chemical energy requirement in the form of adenosine triphosphate (ATP). They also have a significant role in regulating apoptosis and necrosis, ROS levels, cellular signaling, control of the cell cycle, and growth and differentiation (Pedersen, 1999). Mitochondria are also involved in calcium uptake and release, production of NADH, synthesis of DNA, RNA and proteins, DNA repair and metabolic pathways. Mitochondria are a double membrane organelle with four distinct compartments, the outer membrane, inner membrane, intermembrane space and the matrix. It is the only organelle apart from the nucleus to contain its own DNA, mtDNA is a circular molecule of just over 16000 base pairs making up 37 genes that encode 13 components of the electron transport chain, and transcription and translational machinery. Oxidation of fuels, such as glucose, generates reducing equivalents that feed into the electron transport chain of the mitochondria (Harper et al., 2004). The electron transport chain generates ATP by oxidative phosphorylation, creating a proton gradient through sequential transfer of electrons donated by reducing equivalents. This complex system is made up of five multi enzyme subunits, NADH dehydrogenase comprises complex I (46 subunits), succinate dehydrogenase is complex II (4 subunits), cytochrome C reductase and cytochrome C oxidase make up complexes III and IV respectively (11 and 13 subunits). Complex V is the ATP synthase (16 subunits) that uses the proton gradient created by the first four complexes to drive phosphorylation of ADP to form the energy rich ATP. Increasingly, mitochondria are thought to play a regulatory role in cell death partly due to its role as a source of ROS and due to the release of cytochrome C and other pro-apoptotic factors that activate caspases and trigger apoptosis. Cytochrome C is a small globular heme containing electron carrier in the electron transport chain of the mitochondria. Its primary role in the electron transport chain is a crucial one, shuttling electrons from Complex III (ubiquinol:cytochrome c reductase) to complex IV (cytochrome oxidase), however, its release from the mitochondria to the cytosol is the initiating factor for the internal apoptotic pathway. The release of cytochrome C is a two step process, initiated by release of the hemoprotein from its binding to cardiolipin at the inner mitochondrial membrane (Ott et al., 2002); this results in a pool of free cytochrome C in the intermembrane space. Subsequent permeabilization of the outer mitochondrial membrane releases cytochrome C into the cytosol where it binds apoptotic peptidase activating factor 1 (APAF1).
Mitochondria and Eye Tissues
Tissues with high energy demands such as muscles, heart, liver, endocrine glands, brain and retina, have higher numbers of mitochondria per cell. Distribution of mitochondria in the lens is associated with its development. The lens is composed of cells that differentiate from an anterior layer of cuboidal epithelia and migrate posteriorly to form elongated lens fiber cells that make up the lens nucleus. During this process fiber cells synthesize high levels of lens crystallins before losing their nuclei and mitochondria. Thus, the single monolayer of epithelial cells that lines the anterior of the lens, are the only lens cells that carry out aerobic metabolism and contain mitochondria aside from newly differentiated fiber cells. The lens is especially susceptible to damage with aging since lens the cells and their cellular proteins are not turned over or replaced in this encapsulated tissue. The proteins at the center of the eye are some of the oldest in the body and obviously susceptible to age related oxidative damage. Damage to the mitochondria of the epithelial cells may result in ROS production that is thought to affect the proteins of the underlying fiber cells.
The retina is the most oxygen consuming tissue in the body with consumption level around 50% higher than the brain or kidneys (Rattner and Nathans, 2006). In the retina, mitochondria are found throughout but the highest number of mitochondria per cell is found in the photoreceptors.
Mitochondrial Diseases
Over 100 mutations in mtDNA have been identified in various tissues in aged individuals leading to defects in respiratory function. Mutations can affect specific proteins of the respiratory chain or the synthesis of mitochondrial proteins by mutations in any of the genes coding for necessary RNAs. Mitochondrial diseases result from defects in respiration and oxidative phosphorylation, which lead to decreased ATP synthesis and increased production of ROS. Point mutations in particular can result in diseases ranging from myopathies to multisystem disorders (DiMauro and Schon, 2003). One of the most common mtDNA mutations is the 4977-bp deletion, which was first observed in chronic progressive external opthalmoplegia among other syndromes (Wei and Lee, 2002). Mutations in tRNALeu(UUR) gene are associated with the mitochondrial encephalomyopathy, lactic acidosis and strokelike episodes syndrome (MELAS). Mutations in the tRNALys gene are present in 80-90% of patients with myoclonus epilepsy with ragged red fibers (MERRF) syndrome.
In the eye, Leber's hereditary optic neuropathy (LHON) is considered to be the most prevalent mitochondrial disorder. It arises from point mutations in mtDNA (18 allelic variants are known) leading to selective loss of retinal ganglion cells, central vision loss and optic neuropathy. The point mutations in LHON affect complex I of the electron transport chain, decreasing energy production, increasing oxidative stress and resulting in mitochondrial dysfunction.
Mutations in nuclear DNA (nDNA) may affect subunits of complexes I and II but not the other complexes of the electron transport chain. Those mutation affecting complexes I and II may results in severe neurodegenerative diseases, Leukodystrophy or Leigh's syndrome. In the case of mutations in complex II, paragangliomas and pheochromocytomas may be found. Defects in complex III, IV and V may results from mutations in nDNA encoding proteins responsible for assembly or insertion of cofactors (DiMauro and Schon, 2003).
Mitochondrial Reducing Systems
Reducing systems in the mitochondria employ electron donors such as glutathione (GSH), thioredoxin (Trx), NADPH, NADH, FADH2 and certain amino acids. Reducing equivalents act as important electron donors in order to maintain the redox status of a number of essential proteins and aid antioxidant enzyme systems. In the eye, GSH is a primary protectant of lens, cornea, and retina against ROS induced damage (Ganea and Harding, 2006). The eye lens in particular contains high levels of reduced GSH, in cataractous lenses GSH has been shown to be depleted by up to 60% (Spector, 1995) while GSH levels have been shown to decrease with age particularly in the nucleus of the lens (Bova et al., 2001).
In all cells, GSH is maintained in its reduced form rather than its oxidized form (GSSG) by glutathione reductase in an NADPH dependent manner to prevent mixed disulphide formation and to act as an available electron donor for peroxidases. The mitochondrion, along with a number of organelles, maintains its own GSH pool which has been shown to be essential for cell survival (Reed, 2004). Oxidation or depletion of reduced GSH affects the redox status of the mitochondria and leaves the cell sensitive to apoptotic stimuli. Thioredoxin proteins (Trx) are small thiol proteins that have an active-site dithiol that reduces protein disulphides, leaving an internal disulphide on thioredoxin (Arner and Holmgren, 2000). Thioredoxin 2 is mitochondrial-specific and it acts as an important reducing equivalent for many protective and repair systems in the eye. Thioredoxin, like GSH, must also be maintained in its reduced state, the enzyme thioredoxin reductase reduces thioredoxin in a NADPH dependent reaction. NADPH, the source of which is the pentose phosphate pathway (PPP), also acts as a reducing agent for glutathione reductase, which maintains GSH in its reduced state. Under conditions of oxidative stress the PPP can increase NADPH production making it of key importance in cellular redox control (Giblin et al., 1981). NADH and FADH are formed during glycolysis, fatty acid oxidation and the citric acid cycle and are funneled into the electron transport chain of the mitochondria to act as electron donors. NADH transfers electrons to Complex I of the electron transport chain while FADH2 transfers to complex II during the oxidation of succinate to fumarate.
Methionine (Levine et al., 1996) and cysteine residues are thought to act as antioxidants by directly scavenging ROS; both amino acids are particularly susceptible to oxidative stress; however, unlike most protein oxidations, these modifications are reversible. Free and protein bound methionine can be cycled between the oxidized and reduced forms with the aid of the Msr enzymes (Levine et al., 1996). Protein bound cysteine residues are known to be key players in redox sensing and regulation (Holmgren et al., 2005), and there are a number of repair systems for disulphide bonds, cycling of cysteine residues between oxidized and reduced forms. Ciorba et al. (1997) suggested that the function of the shaker potassium channel was modulated by oxidation and reduction of a key methionine residue by MsrA. In this way, methionine and cysteine residues may become sinks for ROS without permanent adverse consequences for protein and cellular function.
Mitochondrial protective and repair systems
Mitochondrial protection and repair is mediated by the reducing agents detailed above, primary antioxidants and chaperones, antioxidant enzymes and specific protein repair systems. In the mitochondria all of the systems work in concert to protect against ROS-induced damage.
In the eye, primary antioxidants that directly scavenge ROS, such as Vitamin C and E and the carotenoids are well studied. Although in vivo and in vitro animal studies have linked antioxidants and cataract, data showing a protective effect of antioxidant vitamin supplementation is mostly observational. In a randomized double blind trial using vitamin E supplementation to reduce cataract risk the results did not support vitamin supplementation as a valid therapy (McNeil et al., 2004). However, a study of multivitamin Centrum suggested that daily use may be of benefit in delaying onset of lens opacities (Milton et al, 2006).
Plasma concentrations of the carotenoids lutein and zeaxanthin have been studied in association with age related macular degeneration (ARMD), these supported the view that zeaxanthin may help protect against ARMD (Gale et al., 2003). However, in 2006 Trumbo and Ellwood published an evaluation of data concerning lutein and zeaxanthin. They concluded that no credible evidence exists to support intake of lutein or zeaxanthin or both to protect against either ARMD or cataract. Similarly a meta-analysis of supplementation trials by Chong et al. (2007) indicated that there was insufficient evidence to support the use of antioxidant supplementation for prevention of ARMD. Klein et al., in 2008 showed that an individual's response to AREDS supplements may be related to CFH genotype. This is one of the few pharmacogenetic studies to suggest interaction between genotype and treatment. Current evidence does not appear to support the use of antioxidant vitamin supplements to prevent AMD, although more studies are required particularly in light of the Klein study for genetic variation. Another meta-analysis and review of supplementation trials by Evans (2008) suggests that people with AMD, or early signs of the disease, may experience some benefit from taking supplements as used in the age-related eye disease study (AREDS) trial. AREDS compared four treatment groups: antioxidants (vitamin C 500mg, vitamin E 400 IU, and b-carotene 15mg)/day, zinc (zinc oxide 80mg and cupric oxide 2mg), antioxidants plus zinc and a placebo group. It would seem that use of antioxidant supplementation to guard against age related cataract remains controversial, while AREDs type supplementation may help decrease progression in ARMD.
In addition to free radical scavengers chaperones are also believed to be essential for mitochondrial function and to play important roles in eye disease. The mitochondrion contains two specific molecular chaperones, Hsp 70 and Hsp 60. While Hsp 60 appears to be particularly involved in protecting against protein misfolding, the house-keeping functions of the Hsp70 chaperones include transport of proteins between cellular compartments, degradation of unstable and misfolded proteins, prevention and dissolution of protein complexes, folding and refolding of proteins, uncoating of clathrin coated vesicles, and control of regulatory proteins (Daugaard et al., 2007). These important proteins may provide a means of protecting damaged protein in the aging eye and may play a role in the pathogenesis of a number of maculopathies where protein aggregation appears to be the cause (see maculopathies).
In addition to the large heat shock proteins, small heat shock proteins are also believed to play key roles. The small heat shock protein α-crystallin, which is the major protein of the mammalian lens, is an aggregate assembled from two polypeptides (αA and αB). Each polypeptide has a molecular weight around 20,000 kDa and these structural proteins are known to act as molecular chaperones (Horwitz, 1992). Both proteins appear to have extensive non-lens roles (Nagineni and Bhat, 1992). Brady et al., (1997) showed that in αA crystallin knockout mice the lenses were smaller than wild type and developed opacification in the nucleus of the lens that became widespread with age. In the retina, studies on RPE from wild type and αA and αB knockout mice showed that both proteins were localized to the mitochondria and that a lack of alpha crystallins rendered RPE more susceptible to apoptosis following oxidative stress (Yaung et al., 2007). It may be that a loss of crystallin chaperone activity during oxidative stress may lead to RPE death and contribute to the pathogenesis of macular degeneration.
A critical mitochondrial protective and repair system for the eye and other tissues employs repair enzymes that operate on protein sulfhydryl groups sensitive to oxidative stress. These moieties can easily conjugate with nonprotein thiols (S-thiolation) to form protein-thiol mixed disulphides. A number of systems exist to combat and/or repair this type of oxidative damage. The glutathione (GSH) system, consisting of reduced GSH, oxidized glutathione (GSSG) and a number of related enzymes, is the main redox control system of the cell. The GSH system detoxifies H2O2, dehydroascorbic acid and lipid peroxides and maintains protein thiols in a reduced state. Glutathione peroxidase reduces H2O2 to water with the concomitant oxidation of GSH to GSSG; GSH is maintained in its reduced form by the enzyme glutathione reductase in an NADPH dependent reaction. A reduction in glutathione peroxidase and glutathione reductase activities has been observed in cataractous lenses, but this may be a consequence of lens injury rather than the cause (Ganea and Harding, 2006). Another vital system acting on free and mixed disulphides is the thioltransferase system which uses reduced GSH for reduction of protein thiols to prevent disulphide bond formation and potentially protein aggregation. Mitochondrial thioltransferase or glutaredoxin 2 has been shown to protect against disruption of the mitochondrial transmembrane potential during oxidative stress in lens epithelial cells (Ferando et al., 2006). Xing and Lou (2003) showed that overexpression of thioltransferase in HLE-B3 cells enhanced protection against H2O2 induced oxidative stress. They also showed that cellular thioltransferase activity was inhibited by addition of cadmium to the medium.
The mitochondrial thioredoxin system consists of thioredoxin 2 (Trx2) and thioredoxin reductase 2 (TrxR2) and also works to maintain mitochondrial proteins in their reduced state. Mitochondrial Trx 2 haploinsufficiency (a single functional copy of a gene, where insufficient product is produced) in mice was shown to reduce ATP production and increase ROS production (Perez et al., 2008). In addition, absence of Trx2 causes early embryonic lethality in mice (Nonn et al., 2003).
A major form of oxidative stress is initiated by transition metals in Fenton-type reactions that generate OH radicals (Fridovich, 1997). One key system to detoxify free metals is metallothioneins. Human exposure to heavy metal comes from a number of sources including cigarette smoke, air pollution, industrial waste, emissions from fossil fuels, leaching from landfills, fertilizers, corrosion of plumbing (Artic Monitoring and Assessment programme, 1988 and Ruffett et al., 1992) and green leafy vegetables (Chunilall, 2004). Since cigarette smoke has been linked with cataractogenesis (Leske et al, 1991), detoxification of heavy metals such as cadmium may be important in delaying onset of cataract. Hawse et al (2006) showed that overexpression of metallothionein IIa (MTIIa) in lens epithelial cells protected against cadmium induced oxidative stress. In addition, they showed that MTIIa could play a role in regulating expression of other antioxidant enzymes, further enhancing protection in lens cells and potentially delaying onset of lens opacity.
Antioxidant enzymes present in the mitochondria work in concert with the reducing and protein repair systems and many have been shown to be crucial for protection against ROS mediated damage and cell death in the eye. MnSOD (SOD 2) in the mitochondrial matrix converts O2- generated by the electron transport chain to hydrogen peroxide. Up and down-regulation of SOD 2 has been shown to be important for protection of lens epithelial cells against oxidative stress (Matsui et al., 2003). CuZnSOD (SOD 1) is present in the intermembrane space in some cells types, where it also converts O2- to H2O2 permitting further diffusion into the cytosol (Ott et al., 2007). Overexpression of SOD 1 in whole lens has been shown to prevent H2O2-mediated damage in the lens and thus was proposed to help prevent cataract, although this overexpression was not mitochondrial specific (Lin et al., 2005). Unlu and Koc, (2007) showed that in yeast, deletion of three genes, SOD1, SOD2, and CCS1 (Copper chaperone for superoxide dismutase), shortened the life span, indicating the potential of these enzymes in the aging process. Mice deficient in SOD 1 have features typical of AMD, older mice exhibited drusen accumulation, thickened Bruch's membrane and choroidal neovascularization (Imamura et al., 2006). SOD 2 was shown to protect against oxidation induced apoptosis in RPE cells from wild type, hemizygous and heterozygous SOD 2 mice (Kasahara, et al., 2005). Reddy et al. (2004) found that in SOD 2 deficient lens epithelial cells challenged with superoxide there were dramatic mitochondrial changes, cytochrome C leakage, caspase 3 activation and increased apoptotic death.
Since H2O2 is a relatively stable and readily diffusible molecule a number of systems exist to limit its damaging potential. These include catalase, glutathione peroxidase and the peroxiredoxins. Mitochondrial localization of catalase appears to be restricted to heart mitochondria, however, overexpression of catalase targeted to mitochondria in mice extended lifespan and delayed cataract formation (Schriner et al., 2005) indicating the importance of eliminating the toxic effects of H2O2 in lens cells. Peroxiredoxins use redox active cysteines to reduce and detoxify H2O2, peroxynitrite and a wide range of organic hydroperoxides (Wood et al., 2003). The peroxiredoxin III enzyme was found to be redox sensitive in lens cells and is localized to human lens and mitochondria of lens epithelial cells (Lee et al., 2007). These systems along with their potential role in ocular disease are outlined in Table 1.0.
Table 1.0. Summary of Systems Discussed.
| Mitochondrial system | Disease | Association | References |
|---|---|---|---|
| Reducing Systems | |||
| GSH | Cataract | Depletion | Spector, 1995; Bova et al., 2001 |
| Calvin et al., 1991 | |||
| Glutathione peroxidase | Cataract | Decreased activity | Ganea and Harding, 2006 |
| Glutathione reductase | Cataract | Decreased activity | Ganea and Harding, 2006 |
| Methionine residues | Cataract | Oxidation | Garner and Spector, 1980 |
| Cysteine residues | Cataract | Oxidation | Garner and Spector, 1980 |
| Antioxidant Systems | |||
| Vitamin C | Cataract and AMD | Supplementation? | McNeill et al., 2004 |
| Vitamin E | Cataract and AMD | Supplementation? | Trumbo and Ellwood, 2006 |
| Carotenoids | Cataract and AMD | Supplementation? | Gale et al., 2003 |
| Chong et al., 2007 | |||
| MnSOD (SOD2) | Cataract | Overexpression | Matsui et al., 2003 |
| Depletion | Unlu and Koc, 2007; Reddy et al., 2004 | ||
| CuZnSOD (SOD1) | AMD | Overexpression | Kasahara et, 2005 |
| Cataract | Overexpression | Lin et al., 2005 | |
| AMD | Depletion | Imamura et al., 2006 | |
| Peroxiredoxin | Cataract | Lee et al., 2007 | |
| Catalase | Cataract | Overexpression | Schriner et al, 2005 |
| Chaperones | |||
| Alpha A crystallin | Cataract | Knockouts | Brady et al, 1997 |
| Alpha B Crystallin | Cataract and AMD | Knockouts | Yaung et al., 2007 |
| Repair and Degradation | |||
| Thioltransferase (glutaredoxin 2) | Cataract | Overexpression | Fernando et al., 2006; Xing and Lou, 2003 |
| MsrA | Cataract | Overexpression | Kantorow et al., 2004 |
| Depletion | Marchetti et al., 2006 | ||
| AMD | Depletion | Lee et al., 2006 | |
| Sreekumar et al 2005 | |||
| MsrB | Cataract | Depletion | Marchetti et al., 2005 |
| Metallothionein | Cataract | Overexpression | Hawse et al., 2006 |
| Proteasome | Cataract | Decreased activity | Zetterberg et al., 2003 |
| Cataract | Oxidation associated with progression | Petersen et al., 2007 | |
| AMD | Ethen et al., 2007 | ||
The Methionine sulfoxide reductase repair system
Msrs are a family of thioredoxin dependent oxidoreductases that reduce methionine sulfoxide back to its reduced form methionine. Two classes of Msrs are known; MsrA and MsrB which act on S- and R- epimers of methionine sulfoxide (MSO) respectively. Repair of oxidized methionine has been shown to protect against oxidative stress in a number of cells, oxidation of methionine may lead to significant changes in protein structure and functions. Eight targets for MsrA have been reported including ribosomal protein L12 (Brot et al., 1981), α-1-proteinase inhibitor (Abrams et al., 1981), calmodulin (Sun et al., 1999), Ffh protein in E.coli (Ezraty et al., 2004), HIV-2 protease (Davies, et al., 2000), shaker potassium channel (Ciorba et al., 1997), Hsp 21 (Gustavssson et al., 2002) and recently α-synuclein (Liu et al., 2008) but it is thought that many more targets exist.
MsrA has been linked to the aging process, it is has been shown to extend lifespan in animals. MsrA knockout mice have been shown to have a 40% reduction in lifespan compared to wild type (Moskovitz, et al., 2001) while over expression of MsrA in Drosophila malanogaster was shown to increase lifespan by up to 70% (Ruan et al., 2002). Over-expression of MsrA has also been shown to increase resistance to oxidative stress in WI-38 SV40 fibroblasts (Picot et al., 2005) and yeast and human T cells (Moskovitz et al., 1998). MsrA repair has been shown to protect against hypoxia/reoxygenation in neuronal cells (Yermolaieva et al., 2004) and cardiac myocytes (Prentice et al., 2007) and in MsrA knockout mice elevated levels of brain pathologies have been observed (Pal et al., 2007), possibly due to an accumulation of oxidatively modified proteins that would otherwise have been efficiently repaired by MsrA.
In the eye, much of the work carried out on Msrs has been in the lens. Kantorow et al. (2004) localized MsrA to all regions of the human lens and demonstrated that overexpression of MsrA in lens epithelial cells could protect against H2O2 mediated oxidative stress. Silencing of the MsrA gene using siRNA increased sensitivity to H2O2 mediated oxidative stress in lens cells, interestingly the decrease in MsrA levels lead to decreased viability even in the absence of oxidative stress. Viability in this work was measured using the MTS assay which measures the activity of mitochondrial enzymes indicating that MsrA is important in protecting mitochondrial function in the lens cell. Marchetti et al., (2006) carried out further studies on the effect of silencing the MsrA gene. They showed that decreased MsrA levels not only reduced cell viability but also allowed increased ROS production in the cell and a decrease in mitochondrial membrane potential even in the absence of oxidative stress, these effects were augmented by TBHP-induced oxidative stress. This clearly points to an important role for MsrA in mitochondrial function of lens cells. Additional work on MsrB enzymes indicated that 40% of Msr activity in the lens was attributable to MsrB and remainder to MsrA; they also localized the three MsrB enzymes in lens cells. The three MsrB genes exhibited asymmetric expression patterns between different lens sublocations, including lens fibers and were also shown to be important in protection against tBHP-induced oxidative stress (Marchetti et al., 2005).
In the monkey retina MsrA message is found mainly in the macular RPE-choroid region while its activity was found predominantly in the soluble fraction of neural retina and RPE-choroid (Lee et al., 2006). The MsrA protein was found throughout the retina but not in the mitochondria of photoreceptor cells, possibly leaving these cells susceptible to oxidative stress and contributing to ARMD. As with epithelial cells, silencing of the MsrA message rendered RPE cells more susceptible to tBHP-induced oxidative stress, further stressing the importance of MsrA to the eye. Another group has also looked at the role of Msrs in H2O2-induced oxidative stress in the retina. Sreekumar et al., (2005) demonstrated that in RPE cells exposed to varying doses of H2O2 and found that gene expression of MsrA and MsrB2 increased in dose and time-dependent manner. Silencing of the MsrA gene resulted in caspase 3 activation in RPE cells and decreased cell viability. This data is in agreement with Lee's and further points to an important role for Msrs in prevention of lens and retinal disease.
The Proteasome
The proteasome is responsible for protein turnover and removal of irretrievably damaged proteins; it deals with proteolysis of oxidized proteins in the cytosol and nucleus while the lon protease, an ATP-stimulated mitochondrial matrix protein, is responsible for degradation of such proteins in the mitochondrial matrix. There are three enzymatic activities associated with the proteasome: postglutamyl peptide hydrolysing (PGPH), trypsin-like and chymotrypsin-like cleavage. Proteasome activities have been shown to be decreased in lenses with nuclear opacities possibly due to an accumulation of oxidized proteins that exceed the capacity of the proteasome system (Zetterberg et al, 2003).
In lens cells Petersen et al. (2007) showed that H2O2 induced oxidative stress could inhibit all three peptidases of the proteasome, in addition the oxidised form of GSH GSSG did the same but reduced GSH stimulated chymotrypsin-like and peptidylglutamyl peptidase activities in HLEC lysates. In the retina, proteasomal chymotrypsin-like activity was found to increase in neurosensory retina with AMD progression, indicating a possible retinal response to local inflammation or oxidative stress (Ethen et al., 2007). Lon protease expression and activity are known to decline with age, this includes the proteasome DNA binding and molecular chaperone activities that protect complex I assembly in the mitochondria (Ngo and Davies, 2007).
Age related changes in proteasome activities may contribute to the pathogenesis of diseases involving abnormal accumulation of oxidized proteins including cataract and maculopathies.
Mitochondrial ROS Systems and Cataract
Cataract, the opacification of the crystalline lens, is one of the leading causes of blindness in the world. Already over 50% of Americans over the age of 65 are affected and this is expected to increase over the next 10 years as the population ages, in fact it is estimated that 30.1 million Americans will have cataracts by 2020 (Center for Disease Control and Prevention, 2006). Cataracts therefore present a significant health problem in the US and place a major burden on the economy.
Aging is by far the greatest risk factor for non-congenital cataract formation (Truscott, 2005), but other factors include smoking, steroid use, myopia, diet (Leske et al., 1991) and exposure to sunlight (Taylor et al, 1988). Cataracts result from a change in lens cell protein or lens cell structure which is essentially a breakdown of lens cell microarchitecture. The short range packing of the crystallins, which make up over 90% of the soluble lens, is important for maintenance of lens transparency. To achieve this crystallins must exist in a homogenous state. The lens depends on redox balance to maintain transparency and considerable evidence points to mitochondrial dysfunction and ROS imbalance in the etiology of age related cataract.
ROS induced damage in the lens cell may consist of oxidation of proteins, DNA damage and/or lipid peroxidation, all of which have been implicated in cataractogensis. To remain transparent, the centre of the lens must continuously maintain thiol groups in a nearly 100% reduced state (Lou, 2003 and Truscott, 2005). Cataract is also characterized by an increase in oxidized protein methionines with age (Truscott, 2005). Oxidation of α-crystallin is linked to cataractogenesis, in particular oxidation of cysteine residues (Garner and Spector, 1980). Aggregation of lens crystallin has been demonstrated by Simpanya et al. (2005) in guinea pigs treated with hyperbaric oxygen (higher than atmospheric oxygen tension) three times weekly for 7 months. In this study HPLC-isolated aggregates contained αA- but not αB-crystallin, which is devoid of -SH groups and thus does not participate in disulfide cross-linking. The eye lens in particular contains high levels of reduced GSH which maintains protein thiol groups in a reduced form. It is believed that formation of disulphide bonds leads to cross-linking and protein aggregation, as previously mentioned aggregation of oxidized alpha crystallin is thought to play a major role in cataractogenesis. In cataractous lenses, GSH has been shown to be depleted by up to 60% (Spector, 1995). Depletion of GSH or a shift in the GSH/GSSG ratio in favor of the oxidized form has been associated with cataract, Calvin et al., (1991) showed rapid deterioration of lens fibers in mouse pups treated with a GSH biosynthesis inhibitor.
Age-related accumulation of oxidized proteins, in some cases specific amino acids, reflects age-related increases in rates of ROS generation, decreases in antioxidant activities and losses in the capacity to degrade oxidized proteins (Stadtman, 2006). Methionine oxidation increases with age in a number of aging models while aging is associated with loss of Msr activity in a number of animal tissues (Stadtman et al., 2005). Garner and Spector (1980) found that 60% or more methionines were oxidized in cataractous lenses. They also found that most cysteines were in the disulphide from. Oxidation of methionines and cysteines appears to make a major contribution to the development of cataract and this highlights the importance of the Msr enzymes and the various reducing systems involved in a role in age-dependent cataract formation.
Mitochondrial ROS Systems and Maculopathy
Maculopathies are a diverse group of blinding disorders characterized by loss of central vision associated with retinal pigmented epithelium (RPE) atrophy with or without choroidal neovascularization. Age related macular degeneration (AMD) accounts for 50% of all cases of blindness in the United States of America and Western Europe (Resnikoff et al., 2004). This degenerative disease progresses from waxy retinal deposits called drusen to neovascularization and retinal hemorrhage, eventually resulting in a total loss of central vision The RPE layer of cuboidal epithelial cells lies between the photoreceptor cells and Bruch's membrane, its chief role is to serve the photoreceptor cells. The space in-between the RPE and photoreceptors is filled with the interphotoreceptor matrix (IPM). The IPM is essential for the interaction between RPE and photoreceptors to allow exchange of nutrients, signalling molecules and metabolic end products. Bruch's membrane separates the RPE from the network of blood vessels called the choroid, the choroid supplies oxygen and nutrients to the RPE and therefore the photoreceptors. By exchanging nutrients and clearing out waste products the RPE serves as housekeeper to the photoreceptor cells. Equally important roles for the RPE include re-isomersiation of retinal to 11-cis retinol and phagocytosis of the shed outer segments of the photoreceptor (Wimmers et al., 2007).
Drusen, a wax like substance, accumulates between the RPE and Bruch's membrane in both normal aging and to a greater extent in AMD. Abnormal accumulation of drusen has also been associated with other maculopathies and its composition has been analyzed by a number of groups for clues to a common pathway. Epidemiological studies show that risk factors for AMD include family history, aging and cigarette smoking (Rattner and Nathans, 2006). The prevalence of AMD increases dramatically with age, and both photo-oxidation and inflammation have been implicated in the pathogenic mechanism for AMD (Hejtmancik et al., 2006), in addition the risk associated with cigarette smoking is thought to imply a role for oxidative stress in AMD. Variants in the gene for complement factor H (CFH) and the genes PLEKHA1/LOC387715/HTRA1, Factor B (BF) and complement component 2 (C2) have been implicated as major risk or protective factors for the development of AMD (Montezuma et al., 2007).
The retina is metabolically a highly active area leading to generation of ROS as a byproduct of a number of processes (Beatty et al., 2000). Oxygen consumption by the retina is much greater than by any other tissue, leading to leakage from the electron transport chain of the mitochondria as described above. The retina is subject to high levels of cumulative irradiation. Photoreceptor outer segment membranes are rich in polyunsaturated fatty acids (PUFAs); oxidation of PUFAs can initiate a cytotoxic chain-reaction (Beatty et al., 2000). RPE are the most actively phagocytozing cells in the body. This process may lead to generation of ROS, although some work suggests that the process is different to that of macrophages (Irschick, et al., 2004). As the RPE ages, oxidation of lipids and other cellular components result in an accumulation of indigestible material lipofuscin in the lysosomes, leading to their enlargement and formation of lipofuscin granules. These closely parallel Drusen formation in time and distribution in the retina (Hejtmancik et al., 2006). ARPE19 cells exposed to oxidized LDL responded by altering transcription of genes related to lipid metabolism, oxidative stress, inflammation and apoptosis (Yamada et al., 2008). In addition oxidative damage to mtDNA in RPE cells has also been implicated in the mechanism for RPE aging and AMD (Liang and Godley, 2003).
A number of monogenic diseases that cause early onset macular degeneration are also important in this category. They include Sorsby's fundus dystrophy (SFD), malattia leventinese (ML, also known as Doyne Honeycomb retinal dystrophy), autosomal dominant and recessive Stargardt macular dystrophies and Best macular dystrophy. The genes and proteins involved in these diseases are known but the exact mechanism for each disease remains elusive. Because of the close phenotypic similarities with AMD each disease also provides an opportunity to elucidate a common mechanism for macular degeneration. Similarities of the above diseases with AMD include death of RPE cells, thickening of Bruch's membrane, accumulation of drusen and progressive vision loss. SFD and ML diseases involve mutations in proteins that are responsible for regulation of the extracellular matrix. In SFD point mutations, eight of which have been identified, in the Timp-3 gene result in unpaired cysteines in the c-terminal end of the expressed protein. ML arises from a single Arg345Trp mutation in Fibulin 3, also an extracellular protein with a number of EGF-like repeats, leading to misfolding of the protein (Timpl et al., 2003). SFD is characterized by thickening of Bruch's membrane, deposition of lipofuscin like material, atrophy of the RPE and subretinal neovascularisation. A similar phenotype is observed in ML with significant Drusen deposits between Bruch's membrane and the RPE, both Timp-3 and Fibulin 3 have been identified in Drusen but neither makes up a significant component (Crabb et al., 2002, Mamorstein et al., 2002). Since Timp-3 and Fibulin 3 are binding partners (Klenotic et al., 2004) an abnormal association of the two due to mutation in either protein may underlie the cause of both diseases however little work has been carried out in this area. Autosomal recessive Stargardt disease is caused by mutations in a photoreceptor specific ABC transporter (ABCR or ABC4) while dominant Stargardt-like disease is caused by mutations in the enzyme for long-chain fatty acid synthesis (ELOVL4). Best macular dystrophy is autosomal disorder, that arises from mutations in the gene VMD2 which codes for bestrophin a transmembrane protein that appears to be part of a novel family of chloride channels (Sun et al., 2002). Common to all macular degenerative diseases is the accumulation of drusen or lipofuscin type material that may underlie the pathogenesis of the disorders by somehow creating a barrier that prevents flow of nutrients between and choroid and the photoreceptor cells. It is still unclear however how the varied mutations in inherited macular degenerations lead to drusen accumulation and ultimately loss of vision.
Summary
In summary oxidation and reduction reactions clearly play a significant role in pathogenesis of eye disease. Multiple mitochondrial antioxidant and repair systems work in concert to maintain transparency in the lens and retinal function. It is clear that oxidation and cross-linking of proteins has a significant role to play in the pathogenesis of cataracts, AMD and some of the monogenic maculopathies. Aging of the eye is characterized by increased ROS, decreased antioxidant capability and loss of the ability to degrade or remove oxidized proteins. An accumulation of oxidized protein aggregates leads to loss of cell function, apoptosis and necrosis. Further studies characterizing those mitochondrial and other cellular systems that prevent or repair oxidative stress damage are likely to provide insight into the etiology of these diseases and the development of therapies to treat them.
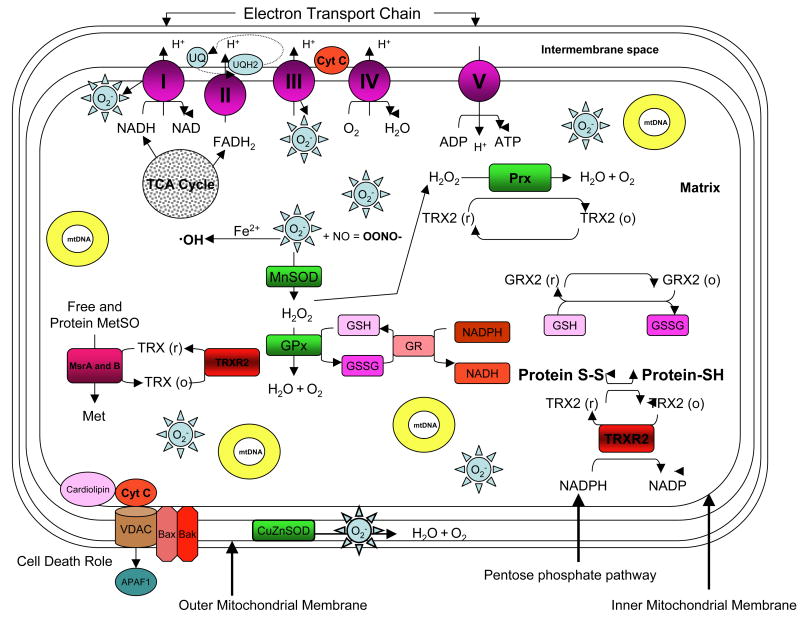
Figure 1.
Overview of the systems involved in mitochondrial ROS production and protection. The process begins with leakage of superoxide (O2-) from complexes I and III of the electron transport chain. All other process depicted function to reduce or repair the damaging potential of this and other subsequently formed oxygen radicals. UQ – Ubiquinone, NO - nitric oxide, OONO- - Peroxynitrite, TCA cycle – Tricarboxylic acid cycle, Prx – Peroxiredoxin, TRX(2) – Thioredoxin, TRXR2 – Thioredoxin 2 reductase, mtDNA – mitochondrial DNA, H2O2 – hydrogen peroxide, GRX2 – glutaredoxin, MetSO – methionine sulfoxide, Met – methionine, GPx – glutathione peroxidase, MnSOD – Manganese superoxide dismutase, CuZnSOD – Copper Zinc superoxide dismutase, GSH – Glutathione, GSSG – oxidized glutathione, OH – Hydroxyl radical, Fe2+ - Iron, Protein S-S – protein disulphide bonds, Protein S-H – repaired thiol groups, Cyt C – cytochrome c, VDAC – voltage dependent anion channel, APAF1 – apoptotic peptidase activating factor 1, Bax and Bak – pro apoptotic factors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Lisa A. Brennan, Biomedical Sciences Department, Charles E. Schmidt College of Biomedical Science, Florida Atlantic University, Boca Raton, FL, Phone: (561) 297-2918, Fax: (561) 297-2221, lbrenna6@fau.edu.
Marc Kantorow, Biomedical Sciences Department, Charles E. Schmidt College of Biomedical Science, Florida Atlantic University, Boca Raton, FL, Phone: (561) 297-2910, Fax: (561) 297-2221, mkantoro@fau.edu.
References
- Abrams WR, Weinbaum G, Weissbach L, Weissbach L, Brot N. Enzymatic reduction of oxidized a-1-proteinase inhibitor restores biological activity. Proc Natl Acad Sci U S A. 1981;78(12):7483–7486. doi: 10.1073/pnas.78.12.7483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arctic Monitoring and Assessment Programme. Arctic Pollution Issues. Oslo: Arctic Monitoring and Assessment Programme; 1998. [Google Scholar]
- Arner ES, Holmgren A. Physiological functions of thioredoxin and thioredoxin reductase. Eur J Biochem. 2000;267(20):6102–6109. doi: 10.1046/j.1432-1327.2000.01701.x. [DOI] [PubMed] [Google Scholar]
- Barja G. Free radicals and aging. Trends in Neurosciences. 2004;27:595–600. doi: 10.1016/j.tins.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Beal MF. Mitochondria take center stage in aging and neurodegeneration. Ann Neurol. 2005;58:495–505. doi: 10.1002/ana.20624. [DOI] [PubMed] [Google Scholar]
- Beatty S, Koh H, Henson D, Boulton M. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Survey of Ophthalmology. 2000;45(2):115–134. doi: 10.1016/s0039-6257(00)00140-5. [DOI] [PubMed] [Google Scholar]
- Bova LM, Sweeney MH, Jamie JF, Truscott RJ. Major changes in human ocular UV protection with age. Invest Ophthalmol Vis Sci. 2001;42(1):200–5. [PubMed] [Google Scholar]
- Brady JP, Garland D, Duglas-Tabor Y, Robison WG, Groome A, Wawrousek EF. Targeted disruption of the mouse αA-crystallin gene indices cataract and cytoplamsic inclusion bodies containing the small heat shock protein αB-crystallin. Proc Natl Acad Sci U S A. 1997;94:884–889. doi: 10.1073/pnas.94.3.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brot N, Weissbach L, Werth J, Weissbach H. Enzymatic reduction of protein bound methionine. Proc Natl Acad Sci U S A. 1981;78:2155–2158. doi: 10.1073/pnas.78.4.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvin HI, Medvedosvsky C, David JC, Broglio TM, Hess JL, Fu SCJ, Worgul BV. Rapid deterioration of lens fibers in GSH-depleted mouse pups. Invest Ophthalmol Vis Sci. 1991;32(6):1916–1924. [PubMed] [Google Scholar]
- Caro P, Gomez J, Lopez-Torres M, Sanchez I, Naudi A, Jove M, Pamplona R, Barja G. Forty percent and eighty percent methionine restriction decrease mitochondrial ROS generation and oxidative stress in rat liver. Biogerontology. 2008;9(3):183–196. doi: 10.1007/s10522-008-9130-1. [DOI] [PubMed] [Google Scholar]
- Chaudhuri A, Van Remmen H, Richardson A, Ikeno Y. Thioredoxin 2 haploinsufficiency in mice results in impaired mitochondrial function and increased oxidative stress. Free Radic Biol Med. 2008;44:882–892. doi: 10.1016/j.freeradbiomed.2007.11.018. [DOI] [PubMed] [Google Scholar]
- Chong EWT, Wong TY, Kreis AJ, Simpson JA, Guymer RH. Dietary antioxidants and primary prevention of age related macular degeneration: systematic review and meta-analysis. BMJ. 2006;335(7623):755–762. doi: 10.1136/bmj.39350.500428.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chunilall V, Kindness A, Jonnalagadda B. Heavy metal uptake by spinach leaves grown on contaminated soils with lead, mercury, cadmium and nickel. Journal of Environmental Science and Health. 2004;B39:473–481. doi: 10.1081/pfc-120035931. [DOI] [PubMed] [Google Scholar]
- Ciorba MA, Hennemann SH, Weissbach H, Brot N, Hoshi T. Modulation of potassium channel function by methionine oxidation and reduction. Proc Natl Acad Sci U S A. 1997;94:9932–9937. doi: 10.1073/pnas.94.18.9932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabb JW, Miyagi M, Gu X, Shadrach K, West KA, Sakaguchi H, Kamei M, Hasan A, Yan L, Rayborn ME, Salomon RG, Hollyfield JG. Drusen proteome analysis: An approach to the etiology of age-related macular degeneration. Proc Natl Acad Sci U S A. 2002;99(23):14682–14687. doi: 10.1073/pnas.222551899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugaard M, Rohde M, Jaattela M. The heat shock protein 70 family: Highly homologous proteins with overlapping and distinct functions. FEBS Lett. 2007;581:3702–3710. doi: 10.1016/j.febslet.2007.05.039. [DOI] [PubMed] [Google Scholar]
- Davies DA, Newcomb FM, Moskovitz J, Wingfield PT, Stahl SJ, Kaufman J, Fales HM, Levine RL, Yarchoan R. Hiv-2 protease is inactivated after oxidation at the dimer surface and activity can be partly restored with methionine sulphoxide reductase. Biochem J. 2000;346:305–311. [PMC free article] [PubMed] [Google Scholar]
- DiMauro S, Schon EA. Mitochondrial respiratory-chain diseases. The New England Journal of Medicine. 2003;348:2656–2668. doi: 10.1056/NEJMra022567. [DOI] [PubMed] [Google Scholar]
- Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- Ethen CM, Hussong SA, Reilly C, Feng X, Olsen TW, Ferrington DA. Transformation of the proteasome with age-related macular degeneration. FEBS Lett. 2007;581:885–890. doi: 10.1016/j.febslet.2007.01.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans J. Antioxidant supplements to prevent or slow down the progression of AMD: a systemic review and meta-analysis. Eye. 2008 doi: 10.1038/eye.2008.100. In press doi: 10.1038/eye.2008.100. [DOI] [PubMed] [Google Scholar]
- Ezraty B, Grimaud R, El Hassouni M, Moinier D, Barras F. Methionine sulfoxide reductases protect Ffh from oxidative damages in Escherichia coli. EMBO J. 2004;223(8):1868–1877. doi: 10.1038/sj.emboj.7600172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferando MR, Lechner JM, Lofgren S, Gladyshev VN, Lou MF. Mitochondrial thioltransferase (glutaredoxin 2) has GSH-dependent and thioredoxin reductase-dependent peroxidase activities in vitro and in lens epithelial cells. FASEB J. 2006;20:E2240–E2248. doi: 10.1096/fj.06-5919fje. [DOI] [PubMed] [Google Scholar]
- Fridovich I. Superoxide anion radical (O2·-), superoxide dismutases and related matters. J Biol Chem. 1997;272(30):18515–18517. doi: 10.1074/jbc.272.30.18515. [DOI] [PubMed] [Google Scholar]
- Gale CR, Hall NF, Phillips DIW, Martyn CN. Lutein and Zeaxanthin Status and Risk of Age-Related Macular Degeneration. Invest Ophthalmol Vis Sci. 2003;44(6):2461–2465. doi: 10.1167/iovs.02-0929. [DOI] [PubMed] [Google Scholar]
- Ganea E, Harding JJ. Glutathione-related enzymes and the eye. Curr Eye Res. 2006;31:1–11. doi: 10.1080/02713680500477347. [DOI] [PubMed] [Google Scholar]
- Garner MG, Spector A. Selective oxidation of cysteine and methionine in normal and senile cataractous lenses. Proc Natl Acad Sci U S A. 1980;77(3):1274–1277. doi: 10.1073/pnas.77.3.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giblin FJ, Nies DE, Reddy VN. Stimulation of the hexose monophosphate shunt in rabbit lens in response to the oxidation of glutathione. Exp Eye Res. 1981;33(3):289–298. doi: 10.1016/s0014-4835(81)80052-8. [DOI] [PubMed] [Google Scholar]
- Gredilla R, Sanz A, Lopez-Torres M, Barja G. Caloric restriction decreases mitochondrial free radical generation at complex I and lowers oxidative damage to mitochondrial DNA in the rat heart. FASEB J. 2001;15(9):1589–1591. doi: 10.1096/fj.00-0764fje. [DOI] [PubMed] [Google Scholar]
- Gredilla R, Barja G. Minireview: The role of oxidative stress in relation to caloric restriction and longevity. Endocrinology. 2005;146(9):3713–3717. doi: 10.1210/en.2005-0378. [DOI] [PubMed] [Google Scholar]
- Gustavsson N, Kokke BP, Harndahl U, Silow M, Bechtold U, Poghosyan Z, Murphy D, Boelens WC, Sundby C. A peptide methionine sulfoxide reductase highly expressed in photosynthetic tissue in Arabidopsis thaliana can protect the chaperone-like activity of a chloroplast-localized small heat shock protein. Plant J. 2002;29:545–553. doi: 10.1046/j.1365-313x.2002.029005545.x. [DOI] [PubMed] [Google Scholar]
- Hansford RG, Hogue BA, Mildazien V. Dependence of H2O2 formation by rat heart mitochondria on substrate availability and donor age. J Bioenerg Biomembr. 1997;29:89–95. doi: 10.1023/a:1022420007908. [DOI] [PubMed] [Google Scholar]
- Harper MW, Bevilacqua L, Hagopian K, Weindruch R, Ramsey JJ. Ageing, oxidative stress, and mitochondrial uncoupling. Acta Physiol Scand. 2004;182:321–331. doi: 10.1111/j.1365-201X.2004.01370.x. [DOI] [PubMed] [Google Scholar]
- Hawse JR, Padgaonkar VA, Leverenz VR, Pellica SE, Kantorow M, Giblin FJ. The role of metallothionein IIa in defending lens epithelial cells against cadmium and TBHP induced oxidative stress. Mol Vis. 2006;12:342–349. [PMC free article] [PubMed] [Google Scholar]
- Hejtmancik JF, Kantorow M, Iwata T. Models of Age-Related Vision Problem. In: Conn PM, editor. Handbook of Models for Human Aging. Elsevier Academic Press; London, UK: 2006. pp. 813–828. [Google Scholar]
- Hollyfield JG, Bonilha VL, Rayborn ME, Yang X, Shadrach KG, Lu L, Ufret RL, Aalomen RG, Perez VL. Oxidative damage-induced inflammation initiates age-related macular degeneration. Nat Med. 2008;14(2):194–198. doi: 10.1038/nm1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren A, Johansson C, Berndt C, Lonn ME, Hudemann C, Lillig CH. Thiol redox control via thioredoxin and glutaredoxin systems. Biochem Soc Trans. 2005;33(6):1375–1377. doi: 10.1042/BST0331375. [DOI] [PubMed] [Google Scholar]
- Horwitz J. α-crystallin can function as a molecular chaperone. Proc Natl Acad Sci U S A. 1992;89:10449–10453. doi: 10.1073/pnas.89.21.10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi T. Methionine sulfoxide reductase A protects neuronal cells against brief hypoxia/reoxygenation. Proc Natl Acad Sci U S A. 2004;101(5):1159–1164. doi: 10.1073/pnas.0308215100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura Y, Noda S, Hashizume K, Shinoda K, Yamaguchi M, Uchiyama S, Shimizu T, Mizushima Y, Shirasawa T, Tsubota K. Drusen, choroidal neovascularization, and retinal pigment epithelium dysfunction in SOD1-deficient mice: A model of age-related macular degeneration. Proc Natl Acad Sci U S A. 2006;103(30):11282–11287. doi: 10.1073/pnas.0602131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irschick EU, Sgonc R, Böck G, Wolf H, Fuchs D, Nussbaumer W, Göttinger W, Huemer HP. Retinal pigment epithelial phagocytosis and metabolism differ from those of macrophages. Ophthalmic Res. 2004;36(4):200–10. doi: 10.1159/000078778. [DOI] [PubMed] [Google Scholar]
- Kakkar P, Singh BK. Mitochondria: a hub of redox activities and cellular distress control. Mol Cell Biochem. 2007;305:235–253. doi: 10.1007/s11010-007-9520-8. [DOI] [PubMed] [Google Scholar]
- Kantorow M, Hawse JR, Cowell TL, Benhamed S, Pizarro G, Reddy V, Hejtmancik JF. Methionine sulfoxide reductase A is important for lens cell viability and resistance to oxidative stress. Proc Natl Acad Sci U S A. 2004;101(26):9654–9659. doi: 10.1073/pnas.0403532101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasahara E, Lin L, Ho Y, Reddy VN. SOD2 protects against oxidation-induced apoptosis mouse in retinal pigment epithelium: Implications for age-related macular degeneration. Invest Ophthalmol Vis Sci. 2005;46(9):3426–3434. doi: 10.1167/iovs.05-0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein ML, Francis PJ, Rosner B, Reynolds R, Hamon SC, Schultz DW, Jurg O, Seddon JM. CFH and LOC387715/ARMS2 genotypes and treatment with antioxidants and zinc for age-related macular degeneration. Ophthalmology. 2008 doi: 10.1016/j.ophtha.2008.01.036. In press - doi:10.1016/j.ophtha.2008.01.036. [DOI] [PubMed] [Google Scholar]
- Klenotic PA, Munier FL, Marmorstein LY, Anand-Apte B. Tissue inhibitor of metalloproteinases-3 (TIMP-3) is a binding partner of epithelial growth factor-containing fibulin-like extracellular matrix protein 1 (EFEMP1) J Biol Chem. 2004;279(29):30469–304732. doi: 10.1074/jbc.M403026200. [DOI] [PubMed] [Google Scholar]
- Lee JW, Gordiyenko NV, Marchetti M, Tserentoodol N, Sagher D, Alam S, Weissbach H, Kantorow M, Rodriquez IR. Gene structure, localization, and role in oxidative stress of methionine sulfoxide reductase A (MsrA) in the monkey retina. Exp Eye Res. 2006;82:816–827. doi: 10.1016/j.exer.2005.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W, Wells T, Kantorow M. Localization and H2O2-specific induction of PRDX3 in the eye lens. Mol Vis. 2007;13:1469–74. [PubMed] [Google Scholar]
- Leske MC, Chylack LT, Jr, Wu SY. The Lens Opacities Case-Control Study. Risk factors for cataract Arch Ophthalmol. 1991;109(2):244–251. doi: 10.1001/archopht.1991.01080020090051. [DOI] [PubMed] [Google Scholar]
- Levine RL, Mosoni L, Berlett BS, Stadtman ER. Methionine residues as endogenous antioxidants in proteins. Proc Natl Acad Sci U S A. 1996;93:15036–15040. doi: 10.1073/pnas.93.26.15036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang FQ, Godley BF. Oxidative stress-induced mitochondrial DNA damage in human retinal pigment epithelial cells: a possible mechanism for RPE aging and age-related macular degeneration. Exp Eye Res. 2003;76:397–403. doi: 10.1016/s0014-4835(03)00023-x. [DOI] [PubMed] [Google Scholar]
- Lin D, Barnett M, Grauer L, Robben J, Jewell A, Takemoto L, Takemoto DJ. Expression of superoxide dismutase in whole lens prevents cataract formation. Mol Vis. 2005;11:853–858. [PubMed] [Google Scholar]
- Lin TL, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- Liu F, Hindupur J, Nguyen JL, Ruf KL, Zhu J, Schieler JL, Bonham CC, Wood KV, Davisson VJ, Rochet JC. Methionine sulfoxide reductase A protects dopaminergic cells from parkinson's disease-related insults. Free Radic Biol In Med. 2008 doi: 10.1016/j.freeradbiomed.2008.03.022. In press: Doi:10.1016/j.freeradbiomed.2008.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou MF. Redox Regulation in the Lens. Progress in Retinal and Eye Research. 2003;22:657–682. doi: 10.1016/s1350-9462(03)00050-8. [DOI] [PubMed] [Google Scholar]
- Mamorstein LY, Munier FL, Arsenijevic Y, Schorderet DF, McLaughlin PJ, Chung D, Traboulis E, Marmorstein AD. Aberrant accumulation of EFEMP1 underlies drusen formation in Malattia Leventinese and age-related macular degeneration. Proc Natl Acad Sci U S A. 2002;99(20):13067–13072. doi: 10.1073/pnas.202491599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti MA, Pizzaro GO, Sagher D, Deamics C, Brot N, Hejtmancik JF, Weissbach H, Kantorow M. Methionine sulfoxide reductases B1, B2 and B3 are present in the human lens and confer oxidative stress resistance to lens cells. Invest Ophthalmol Visual Sci. 2005;46:2107–2112. doi: 10.1167/iovs.05-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti MA, Lee W, Cowell TL, Wells TM, Weissbach H, Kantorow M. Silencing of the methionine sulfoxide reductase A gene results in loss of mitochondrial membrane potential and increased ROS production in human lens cells. Exp Eye Res. 2006;83:1281–1286. doi: 10.1016/j.exer.2006.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Duan W, Chan SL, Cheng A, Haughey N, Gary DS, Guo Z, Lee J, Furukawa K. Neuroprotective and neurorestorative signal transduction mechanisms in brain aging: modification by genes, diet and behavior. Neurobiology of Ageing. 2002;23:695–705. doi: 10.1016/s0197-4580(02)00025-8. [DOI] [PubMed] [Google Scholar]
- Matsui H, Lin L, Ho Y, Reddy VN. The effect of up- and down-regulation of MnSOD enzyme on oxidative stress in human lens epithelial cells. Invest Ophthalmol Vis Sci. 2003;44(8):3467–3475. doi: 10.1167/iovs.02-0830. [DOI] [PubMed] [Google Scholar]
- McNeil JJ, Robman L, Tikellis G, Sinclair MI, McCarty CA, Taylor HR. Vitamin E supplementation and cataract: Randomized control trial. Ophthalmology. 2004;111(1):75–84. doi: 10.1016/j.ophtha.2003.04.009. [DOI] [PubMed] [Google Scholar]
- Milton RC, Sperduto RD, Clemons TE, Ferris FL. Centrum use and progression of age-related cataract in the age-related eye disease study: A Propensity score approach. AREDS report No 21. Ophthalmology. 2006;113(8):1264–1270. doi: 10.1016/j.ophtha.2006.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montezuma SR, Sobrin L, Seddon JM. Review of genetics in age-related macular degeneration. Semin Opthalmol. 2007;22(4):229–240. doi: 10.1080/08820530701745140. [DOI] [PubMed] [Google Scholar]
- Moskovitz J, Flescher E, Berlett BS, Azare J, Poston JM, Stadtman ER. Overexpression of peptide-methionine sulfoxide reductase in Saccharomyces cerevisiae and human T cells provides them with high resistance to oxidative stress. Proc Natl Acad Sci U S A. 1998;95(24):14071–5. doi: 10.1073/pnas.95.24.14071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskovitz J, Bar-Noy S, Williams WM, Requena J, Berlett BS, Stadtman ER. Methionine sulfoxide reductase (MsrA) is a regulator of antioxidant defense and lifespan in mammals. Proc Natl Acad Sci U S A. 2001;98(23):12920–12925. doi: 10.1073/pnas.231472998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagineni CN, Bhat SP. Lens fiber cell differentiation and expression of crystallins in co-cultures of human fetal lens epithelial cells and fibroblasts. Exp Eye Res. 1992;54(2):193–200. doi: 10.1016/s0014-4835(05)80208-8. [DOI] [PubMed] [Google Scholar]
- Ngo JK, Davies KJA. Importance of the lon protease in mitochondrial maintenance and the significance of declining lon in aging. Ann N Y Acad Sci. 2007;1119:78–87. doi: 10.1196/annals.1404.015. [DOI] [PubMed] [Google Scholar]
- Nonn L, Williams RR, Erickson RP, Powis G. The absence of mitochondrial thioredoxin 2 causes massive apoptosis, exencephaly, and early embryonic lethality in homozygous mice. Mol Cell Biol. 2003;23(3):916–22. doi: 10.1128/MCB.23.3.916-922.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott M, Gogvadze V, Orrenius S, Zhivotovsky B. Mitochondrial, oxidative stress and cell death. Apoptosis. 2002;12:913–922. doi: 10.1007/s10495-007-0756-2. [DOI] [PubMed] [Google Scholar]
- Ott M, Robertson JD, Gogvadze V, Zhivotovsky B, Orrenius S. Cytochrome C release from the mitochondria proceeds by a two-step process. Proc Natl Acad Sci U S A. 2007;99(3):1259–1263. doi: 10.1073/pnas.241655498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal R, Oien DB, Ersen FY, Moskovitz J. Elevated levels of brain-pathologies associated with neurodegenerative diseases in the methionine sulfoxide reductase A knockout mouse. Exp Brain Res. 2007;180:765–774. doi: 10.1007/s00221-007-0903-6. [DOI] [PubMed] [Google Scholar]
- Pedersen PL. Mitochondrial events in the life and death of animal cells: A brief overview. J Bioenerg Biomembr. 1999;31(4):291–304. doi: 10.1023/a:1005453700533. [DOI] [PubMed] [Google Scholar]
- Petersen A, Carlsson T, Karlsson JO, Zetterberg M. The proteasome and intracellular redox status: Implications for apoptotic regulation in lens epithelial cells. Curr Eye Res. 2007;32:871–882. doi: 10.1080/02713680701642327. [DOI] [PubMed] [Google Scholar]
- Pérez VI, Lew CM, Cortez LA, Webb CR, Rodriguez M, Liu Y, Qi W, Li Y, Chaudhuri A, Van Remmen H, Richardson A, Ikeno Y. Thioredoxin 2 haploinsufficiency in mice results in impaired mitochondrial function and increased oxidative stress. Free Radic Biol Med. 2008;44(5):882–892. doi: 10.1016/j.freeradbiomed.2007.11.018. [DOI] [PubMed] [Google Scholar]
- Picot CR, Petropoulos I, Perichon M, Moreau M, Nizard C, Friguet B. Overexpression of MsrA protects WI-38 SV40 fibroblasts against H2O2-mediated oxidative stress. Free Radic Biol Med. 2005;39:1332–1241. doi: 10.1016/j.freeradbiomed.2005.06.017. [DOI] [PubMed] [Google Scholar]
- Prentice HM, Moench IA, Rickaway ZT, Dougherty CJ, Webster KA, Weissbach H. MsrA protects cardiac myocytes against hypoxia/reoxygenation induced cell death. Biochem Biophys Res Commun. 2008;366(3):775–8. doi: 10.1016/j.bbrc.2007.12.043. [DOI] [PubMed] [Google Scholar]
- Rattner A, Nathans J. Macular degeneration: recent advances and therapeutic opportunities. Nature. 2006;7:860–872. doi: 10.1038/nrn2007. [DOI] [PubMed] [Google Scholar]
- Reddy VN, Kasahara E, Hiraoka M, Lin LR, Ho YS. Effects of variation in superoxide dismutases (SOD) on oxidative stress and apoptosis in lens epithelium. Exp Eye Res. 2004;79:859–868. doi: 10.1016/j.exer.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Reed DJ. Mitochondrial glutathione and chemically induced stress including ethanol. Drug Metab Rev. 2004;36:569–582. doi: 10.1081/dmr-200033449. [DOI] [PubMed] [Google Scholar]
- Resnikoff S, Pascolini D, Etya'ale D, Kocur I, Pararajasegaram R, Pokharel GP, Mariotti SP. Global data on visual impairment in the year 2002. Bulletin of the World Health Organization. 2004;82:844–51. [PMC free article] [PubMed] [Google Scholar]
- Ruan H, Tang XD, Chen C, Joiner MA, Sun G, Brot N, Weissbach H, Heinemann SH, Linda Iverson L, Wu C, Hoshi T. High-quality life extension by the enzyme peptide methionine sulfoxide reductase. Proc Natl Acad Sci U S A. 2002;99(5):2748–2753. doi: 10.1073/pnas.032671199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffett I, Ayres J, McBride D. Possible chemical pollution. Practitioner. 1992;236:13–6. [PubMed] [Google Scholar]
- Sanz A, Caro P, Ayala V, Portero-Otin M, Pamplona R, Barja G. Methionine restriction decreases mitochondrial oxygen radical generation and leak as well as oxidative damage to mitochondrial DNA and proteins. FASEB J. 2004;20(8):1064–1073. doi: 10.1096/fj.05-5568com. [DOI] [PubMed] [Google Scholar]
- Simpanya MF, Ansari RR, Kwang KI, Leverenz VR, Giblin FJ. Aggregation of lens crystallins in an in vivo hyperbaric oxygen guinea pig model of nuclear cataract: dynamic light-scattering and HPLC analysis. Invest Ophthalmol Vis Sci. 2005;46(12):4641–4651. doi: 10.1167/iovs.05-0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schriner SE, Linford NJ, Martin GM, Treuting P, Ogburn CE, Emond M, Coskun PE, Ladiges W, Wolf N, Van Remmen H, Wallace DC, Rabinovitch PS. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science. 2005;308:1909–1911. doi: 10.1126/science.1106653. [DOI] [PubMed] [Google Scholar]
- Spector A. Oxidative stress-induced cataract: mechanism of action. FASEB J. 1995;9:1173–1182. [PubMed] [Google Scholar]
- Sreekumar PG, Kannan R, Yuang J, Spee CK, Ryan SJ, Hinton DR. Protection from oxidative stress by methionine sulfoxide reductases in RPE cells. Biochem Biophys Res Commun. 2005;334:245–253. doi: 10.1016/j.bbrc.2005.06.081. [DOI] [PubMed] [Google Scholar]
- Stadtman ER, Van Remmem H, Richardson A, Wehr NB, Levine RL. Methionine Oxidation and aging. Biochimica et Biophysica Acta. 2005;1703:135–140. doi: 10.1016/j.bbapap.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Stadtman ER. Protein oxidation and aging. Free Radical Research. 2006;40(12):1250–1258. doi: 10.1080/10715760600918142. [DOI] [PubMed] [Google Scholar]
- Sun H, Gao J, Ferrington DA, Biesiada H, Williams TD, Squier TC. Repair of oxidized calmodulin by methionine sulfoxide reductase restores ability to activate the plasma membrane Ca-ATPase. Biochemistry. 1999;38:105–112. doi: 10.1021/bi981295k. [DOI] [PubMed] [Google Scholar]
- Sun H, Tsunenari T, Yau KW, Nathans J. The vitelliform macular dystrophy protein defines a new family of chloride channels. Proc Natl Acad Sci U S A. 2002;99(6):4008–4013. doi: 10.1073/pnas.052692999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor HK, West SK, Rosenthal FS, Munoz B, Newland HS, Abbey H, Emmett EA. Effect of ultraviolet radiation on cataract formation. N Engl J Med. 1988;319(22):1429–1433. doi: 10.1056/NEJM198812013192201. [DOI] [PubMed] [Google Scholar]
- Tezel G. Oxidative stress in glaucomatous neurodegeneration: mechanisms and consequences. Prog Retin Eye Res. 2006;25:490–513. doi: 10.1016/j.preteyeres.2006.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timpl R, Sasaki T, Kostka G, Chu ML. Fibulins: a versatile family of extracellular matrix proteins. Nat Rev Mol Cell Biol. 2003;4(6):479–89. doi: 10.1038/nrm1130. [DOI] [PubMed] [Google Scholar]
- Trumbo PR, Ellwood KC. Lutein and zeaxanthin intakes and risk of age-related macular degeneration and cataracts: an evaluation using the Food and Drug Administration's evidence-based review system for health claims. Am J Clin Nutr. 2006;84:971–974. doi: 10.1093/ajcn/84.5.971. [DOI] [PubMed] [Google Scholar]
- Truscott RJW. Age-related nuclear cataract – oxidation is the key. Exp Eye Res. 2005;80:709–725. doi: 10.1016/j.exer.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Unlu ES, Koc A. Effects of deleting mitochondrial antioxidant genes on lifespan. Ann NY Acad Sci. 2007;1100:505–509. doi: 10.1196/annals.1395.055. [DOI] [PubMed] [Google Scholar]
- Wei YH, Lee HC. Oxidative stress, Mitochondrial DNA Mutation and Impairment of Antioxidant Enzymes in Aging. Exp Bio Med. 2002;227:671–682. doi: 10.1177/153537020222700901. [DOI] [PubMed] [Google Scholar]
- Wimmers S, Karl MO, Strauss O. Ion channels in the RPE. Prog Retin Eye Res. 2007;26:263–301. doi: 10.1016/j.preteyeres.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Wood ZA, Schroder E, Harris JR, Poole LB. Structure, mechanisms and regulation of peroxiredoxins. Trends in Biochemical Sciences. 2003;28(1):32–40. doi: 10.1016/s0968-0004(02)00003-8. [DOI] [PubMed] [Google Scholar]
- Xing K, Lou MF. The possible physiological function of thioltransferase in cells. FASEB J. 2003;17(14):2088–90. doi: 10.1096/fj.02-1164fje. [DOI] [PubMed] [Google Scholar]
- Yaung J, Jin M, Barron E, Spee C, Wawrousek EF, Kannan R, Hinton DR. α-crystallin distribution in retinal pigment epithelium and effect of gene knockouts on sensitivity to oxidative stress. Mol Vis. 2007;13:566–577. [PMC free article] [PubMed] [Google Scholar]
- Yermolaieva O, Xu R, Schinstock C, Brot N, Weissbach H, Heinemann SH, Hoshi T. Methionine sulfoxide reductase A protects neuronal cells against brief hypoxia/reoxygenation. Proc Natl Acad Sci U S A. 2004;101(5):1159–1164. doi: 10.1073/pnas.0308215100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zetterberg M, Petersen A, Sjostrand J, Karlsson J. Proteasome activity in human lens nuclei and correlation with age, gender and severity of cataract. Curr Eye Res. 2003;27:45–53. doi: 10.1076/ceyr.27.2.45.15457. [DOI] [PubMed] [Google Scholar]
- Improving The Nation's Vision Health: A Coordinated Public Health Approach. Center for Disease Control and Prevention; 2006. [Google Scholar]