Abstract
The putative catalytic domain (residues 81–401) of a predicted tomato protein with similarity to 4-diphosphocytidyl-2-C-methyl-d-erythritol kinase of Escherichia coli was expressed in a recombinant E. coli strain. The protein was purified to homogeneity and was shown to catalyze the phosphorylation of the position 2 hydroxy group of 4-diphosphocytidyl-2-C-methyl-d-erythritol at a rate of 33 μmol⋅mg−1⋅min−1. The structure of the reaction product, 4-diphosphocytidyl-2-C-methyl-d-erythritol 2-phosphate, was established by NMR spectroscopy. Divalent metal ions, preferably Mg2+, are required for activity. Neither the tomato enzyme nor the E. coli ortholog catalyzes the phosphorylation of isopentenyl monophosphate.
The mevalonate pathway of terpenoid biosynthesis has been elucidated by Bloch, Cornforth, Lynen, and their coworkers working with yeast and animal cells (for reviews, see refs. 1–4). Subsequent studies by numerous research groups incorrectly attributed the biosynthesis of numerous plant terpenoids to a mevalonate origin (for reviews, see refs. 5–7).
The existence of a second terpenoid pathway was established relatively recently by studies on certain bacteria by the research groups of Arigoni and Rohmer (refs. 8 and 9; for reviews, see refs. 5–7). Arigoni and his group provided the first demonstration that both terpenoid pathways are operative in plants (10, 11). Ever since, it has been confirmed that the mevalonate pathway affords sterols and the alternative pathway affords a wide variety of monoterpenes, diterpenes, and carotenoids (for reviews, see refs. 5–7).
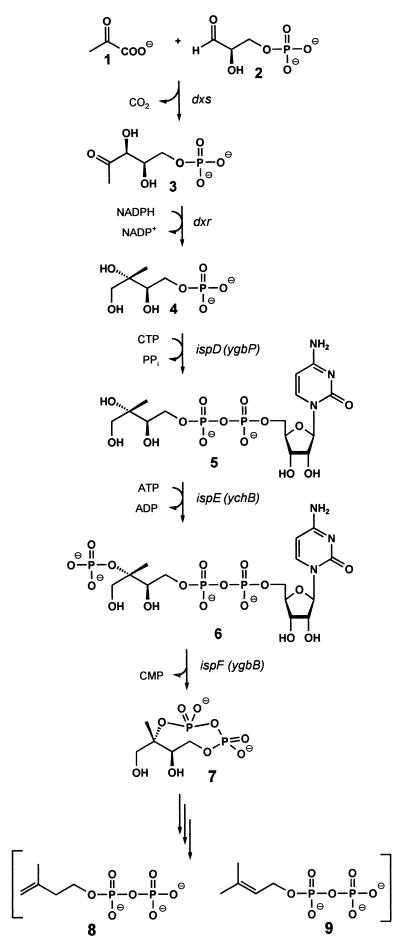
Based on the observation that 1-deoxy-d-xylulose could be incorporated into terpenoids via the alternative pathway (9, 11, 12), enzymes catalyzing the formation of 1-deoxy-d-xylulose 5-phosphate (3) by condensation of glyceraldehyde 3-phosphate (1) with pyruvate (2) under release of carbon dioxide were obtained from Escherichia coli and from plants (Fig. 1) (13–18). Subsequent work showed that 1-deoxy-d-xylulose 5-phosphate can be converted into 2-C-methyl-d-erythritol 4-phosphate (4) by reductoisomerases shown to be present in plants, certain bacteria, and in Plasmodium falciparum (19–22).
Figure 1.
The deoxyxylulose phosphate pathway of isoprenoid biosynthesis.
We have found recently that 2-C-methyl-d-erythritol 4-phosphate can be converted into a cyclic 2,4-diphosphate (7) by the consecutive action of three E. coli proteins specified by the ispDEF genes (Fig. 1) (23–25). In this reaction sequence, 4-diphosphocytidyl-2-C-methyl-d-erythritol kinase specified by the ispE gene (formerly designated ychB) of E. coli has been shown to catalyze the phosphorylation of 4-diphosphocytidyl-2-C-methyl-d-erythritol yielding 4-diphosphocytidyl-2-C-methyl-d-erythritol 2-phosphate (6). On the other hand, Croteau and Lange reported that partially purified YchB protein of E. coli and its putative ortholog from Mentha piperita catalyze the formation of isopentenyl pyrophosphate (8) from isopentenyl monophosphate, albeit at extremely low rates of 178 ± 81 and 1.43 pmol⋅g−1⋅s−1, respectively (26).
This paper shows that the recombinant catalytic domain of IspE (YchB) protein from tomato (Lycopersicon esculentum) purified to apparent homogeneity phosphorylates 4-diphosphocytidyl-2-C-methyl-d-erythritol at a rate of Vmax = 33 μmol⋅mg−1⋅min−1, whereas isopentenyl monophosphate was not detectably phosphorylated.
Experimental Procedures
Materials.
[4-14C]Isopentenyl pyrophosphate (57.5 μCi⋅μmol−1) was purchased from Dupont/NEN. Oligonucleotides (Table 1) were custom-synthesized by MWG Biotech (Ebersberg, Germany). The preparation of 4-diphosphocytidyl-2-C-methyl-d-erythritol (unlabeled resp. 13C-labeled) will be reported elsewhere. [4-14C]Isopentenyl monophosphate was prepared from a reaction mixture containing 100 mM Tris⋅hydrochloride (pH 9.0), 180 μM [4-14C]isopentenyl pyrophosphate (57.5 μCi⋅μmol−1), and 200 μg of inorganic pyrophosphatase (Sigma) in a total volume of 100 μl. The reaction mixture was incubated for 480 min at 25°C, and [4-14C]isopentenyl monophosphate was purified by preparative TLC (Polygram SIL N-HR, Macherey & Nagel) using acetonitrile/acetone/water (6:1:3, vol/vol/vol).
Table 1.
Oligonucleotides used in this study
| Designation | 5′-sequence-3′ |
|---|---|
| His6a | CATGCACCACCACCACCACCACGCGTCCATGGCCGC |
| His6b | GGCCATGGACGCGTGGTGGTGGTGGTGGTG |
| TM-YCHB-A | GGTACAGACAATTACTTTTGGATTCATC |
| TM-YCHB-B | AAGAGATGGAAGAACTTCAAAGGCAGGAGG |
| TM-YCHB-1 | CTGATTATCAAAGCCCTCAATCTTTATCGTAAAAAGACCGGTACAGACAATTACTTTTGGATTCATC |
| TM-YCHB-2 | GACCGCGGCCAGCAGCAATTACACGTTGTTTTAAACGTTTAAGAGATGGAAGAACTTCAAAGGCAGGAGG |
| TM-YCHB-3 | ACTAATGTTGCTGGCGTTCCACTCGATGAGCGTAATCTGATTATCAAAGCCCTCAATCTTTATCG |
| TM-YCHB-4 | TGTGCTGCCACTACCAGACATGAAGACTGCATCATCATATTGACCGCGGCCAGCCAGCAATTACACG |
| TM-YCHB-5 | AAAATTAAGTTCTCGCTGTCACCATCGAAATCAAAGGATCGTTTATCTACTAATGTTGCTGGCGTTCCACTC |
| TM-YCHB-6 | CATAGACAAATTGTGGCGATCTGGAGAGCCAACACCTACGATTGTGCTGCCACTACCAGACATGAAG |
| TM-YCHB-7 | GACGGTTATCATGATCTGGCGTCTCTCTTTCATGTAATTAGTCTTGGCGATAAAATTAAGTTCTCGCTGTCACC |
| TM-YCHB-8 | TGCTTCTGACAAGAAGACATCTTTGTACTCTTCGTCATCATAGACAAATTGTGGCGGATCTGG |
| TM-YCHB-9 | TTTTCTCCTTGCAAGATTAATGTTTTCCTGCGCATCACAAGCAAACGTGATGACGGTTATCATGATCTGGCGTCTC |
| TM-YCHB-10 | CAACATACCACTCGTTGGCTGGACGAGTGATGAAACTTGCTTCTGACAAGAAGACATCTTTG |
| TM-YCHB-11 | CGTGAAGCTGGTCTTTCACGCCTCACTCTTTTTTCTCCTTGCAAGATTAATGTTTTCCTG |
| TM-YCHB-12 | CAGGTTGATCACCAATAGTGCTACCTGAAACAGGTTCAACATACCACTCGTTGGCTGGACG |
| TM-YCHB-13 | ATAATAGAATTCATTAAAGAGGAGAAATTAACCATGGATCGTGAAGCTGGTCTTTCACGCCTC |
| TM-YCHB-14 | TATTATTATAAGCTTAAGACATGTCAAAAGATGTAGAGAACTCAGGTTGATCACCAATAGTGCTACC |
Bacterial Strains.
Bacterial strains and plasmids used in this study are summarized in Table 2.
Table 2.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Genotype or relevant characteristic | Reference or source |
|---|---|---|
| E. coli | ||
| XL1-Blue | RecA1, endA1, gyrA96, thi-1, hsdR17, supE44, relA1, lac, [F′, proAB, lacqZΔM15, Tn10 (ter)] | Ref. 28, Stratagene |
| Plasmids | ||
| pACYC184 | Cloning vector | Ref. 27, New England Biolabs |
| pNCO113 | High copy expression vector | ATCC, PTA-852 |
| pNCO-SB-HIS6-ACYC184 | High copy His-tag expression vector | This study |
| pNCO-HIS6-TM-YCHB | This study |
Construction of Expression Vector pNCO-SB-HIS6-ACYC184.
The plasmid pACYC184 (27) was digested with the restriction enzymes NcoI and BamHI followed by agarose gel electrophoresis. A 2.2-kb NcoI/BamHI DNA fragment was excised from the gel, was purified, and was ligated to the vector pNCO113, which had been treated with the same restriction enzymes. The ligation mixture was electroporated into E. coli XL1-Blue (28) cells, yielding the plasmid pNCO-NB-ACYC184 that was isolated and was treated with NcoI and SacII. A 4.4-kb DNA fragment was isolated and ligated to a double-stranded DNA linker prepared by hybridization of the oligonucleotides His6a and His6b (Table 2). The ligation mixture was electroporated into E. coli of XL1-Blue cells, and the vector DNA was isolated affording the vector pNCO-SB-HIS6-ACYC184.
Construction of an Expression Plasmid.
A section of the pTOM41 sequence from tomato (GenBank accession no. U62773) was amplified by PCR using the oligonucleotides TM-YCHB-A and TM-YCHB-B as primers (Tables 1 and 3) and cDNA from tomato as template. The resulting 447-bp fragment was purified and was extended in both directions to a final size of 1,015 bp by seven consecutive PCR reactions using the respective primer pairs listed in Table 3. The final amplificate was digested with HindIII and NcoI and was ligated into the plasmid pNCO-SB-HIS6-ACYC184, which had been treated with the same restriction enzymes. The resulting plasmid pNCO-HIS6-TM-YCHB was electroporated into E. coli XL1 Blue cells affording the recombinant strain XL1-pNCO-HIS6-TM-YCHB. The DNA sequence of this construct has been deposited in the GenBank database (accession no. AF258339).
Table 3.
Construction of a tomato ispE open reading frame optimized for expression in E. coli
| PCR step | Oligonucleotides | |
|---|---|---|
| 1 | TM-YCHB-1 | TM-YCHB-2 |
| 2 | TM-YCHB-3 | TM-YCHB-4 |
| 3 | TM-YCHB-5 | TM-YCHB-6 |
| 4 | TM-YCHB-7 | TM-YCHB-8 |
| 5 | TM-YCHB-9 | TM-YCHB-10 |
| 6 | TM-YCHB-11 | TM-YCHB-12 |
| 7 | TM-YCHB-13 | TM-YCHB-14 |
DNA Sequence Determination.
DNA was sequenced by the automated dideoxynucleotide method (29) using a 377 Prism sequencer from Perkin–Elmer.
Enzyme Purification.
E. coli strain XL1-pNCO-HIS6-TM-YCHB was grown in Luria–Bertani broth containing 180 mg of ampicillin per liter. The culture was incubated at 37°C with shaking. At an optical density (600 nm) of 0.7, IPTG was added to a final concentration of 2 mM, and the culture was incubated for 5 h. The cells were harvested by centrifugation, were washed with 100 mM Tris⋅hydrochloride (pH 8.0), and were stored at −20°C.
Frozen cell mass (8 g) was thawed in 40 ml of 100 mM Tris⋅hydrochloride (pH 8.0), containing 0.5 M sodium chloride and 20 mM imidazole·hydrochloride. The suspension was subjected to ultrasonic treatment and was centrifuged.
The supernatant was applied to a column of Ni-chelating Sepharose FF (2.6 × 5 cm, Amersham Pharmacia) previously equilibrated with 100 mM Tris⋅hydrochloride (pH 8.0), containing 0.5 M sodium chloride and 20 mM imidazole hydrochloride (flow rate, 3 ml⋅min−1). The column was washed with 150 ml of 100 mM Tris⋅hydrochloride (pH 8.0), containing 0.5 M sodium chloride and 20 mM imidazole hydrochloride. The column was developed with a linear gradient of 20–500 mM imidazole hydrochloride in 130 ml of 100 mM Tris⋅hydrochloride (pH 8.0), containing 0.5 M sodium chloride. Fractions were combined and dialyzed overnight against standard buffer containing 5 mM dithioerythritol and 0.02% sodium azide. The solution was loaded on top of a Mono Q HR column (0.5 × 5 cm, Amersham Pharmacia), which was developed with a linear gradient of 0–0.5 M potassium chloride in 60 ml of 100 mM Tris⋅hydrochloride (pH 8.0), containing 5 mM dithioerythritol and 0.02% sodium azide (flow rate, 2 ml⋅min−1). Fractions were combined and applied to a Superdex 200 column (2.6 × 60 cm, Amersham Pharmacia), which had been equilibrated with 100 mM Tris⋅hydrochloride (pH 8.0), containing 5 mM dithioerythritol, 0.02% sodium aride, and 100 mM NaCl (flow rate, 3 ml⋅min−1). Fractions were combined and concentrated by ultrafiltration.
NMR Spectroscopy.
NMR spectra were recorded by using an AVANCE DRX 500 spectrometer from Bruker (Karlsruhe, Germany). The transmitter frequencies were 500.1 MHz and 125.6 MHz for 1H and 13C, respectively. Chemical shifts were referenced to external trimethylsilylpropane sulfonate. Two-dimensional correlation experiments (INADEQUATE) were performed by using xwinnmr software from Bruker.
Assay of 4-Diphosphocytidyl-2-C-Methyl-d-Erythritol Kinase Activity.
Assay mixtures contained 100 mM Tris⋅hydrochloride (pH 8.0), 5 mM DTT, 5 mM MgCl2, 2 mM ATP, 2 mM 4-diphosphocytidyl-2-C-methyl-d-erythritol, and 0.5 μg of protein in a total volume of 100 μl. The mixtures were incubated for 15 min at 37°C. The reaction was terminated by heating at 80°C for 5 min. The samples were centrifuged, and the supernatant was analyzed by HPLC using a column of Multospher 120 RP 18-AQ-5 (4.6 × 250 mm, CS-Chromatographie Service GmbH, Langerwehe, Germany) that had been equilibrated with 10 mM tetra-n-butylammonium hydrogen sulfate at a flow rate of 0.75 ml⋅min−1. The column was washed with 15 ml of 10 mM tetra-n-butylammonium hydrogen sulfate and was developed with a linear gradient of 0–42% (vol/vol) methanol in 45 ml of tetra-n-butylammonium hydrogen sulfate. The effluent was monitored photometrically (270 nm). The retention volumes of 4-diphosphocytidyl-2-C-methyl-d-erythritol and 4-diphosphocytidyl-2-C-methyl-d-erythritol 2-phosphate were 29 and 50 ml, respectively.
Determination of Isopentenyl Monophosphate Kinase Activity.
Assay mixtures contained 100 mM Tris⋅hydrochloride buffer (pH 8.0), 5 mM DTT, 5 mM ATP, 1 mM [4-14C]isopentenyl monophosphate (0.5 μCi⋅μmol−1), and 100 μg of protein in a volume of 100 μl. The mixtures were incubated at 37°C. The reaction was terminated by heating at 80°C for 5 min, and the mixtures were centrifuged. The supernatants were analyzed by reversed phase HPLC using a column of Multospher 120 RP 18-AQ-5 (4.6 × 250 mm) that had been equilibrated with 10 mM tetra-n-butylammonium hydrogen sulfate. The column was developed by a linear gradient of 0–70% (vol/vol) methanol in 30 ml of 10 mM tetra-n-butylammonium hydrogen sulfate (flow rate, 1 ml⋅min−1). The effluent was monitored by a continuous-flow radiodetector (Beta-RAM, Biostep GmbH, Jahnsdorf, Germany). The retention volumes of isopentenyl pyrophosphate and isopentenyl monophosphate were 27 and 24 ml, respectively. Amounts of 25 pmol of isopentenyl pyrophosphate can be detected by this method.
Results
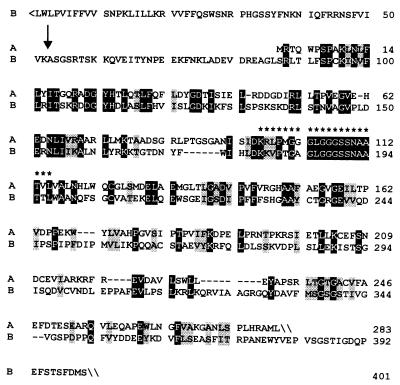
A protein specified by a tomato cDNA (GenBank accession no. U62773) that had been associated with fruit ripening (31) shows considerable similarity with 4-diphosphocytidyl-2-C-methyl-d-erythritol kinase of E. coli (24). More specifically, 30% of the amino residues of the E. coli protein were also found in the predicted tomato protein sequence in equivalent positions. The N-terminal part of the tomato protein had no match in the database and was tentatively interpreted as a plastid targeting sequence with a potential cleavage site between amino acid residues 52 and 53 as predicted by the program chloropv1.1§ (Fig. 2).
Figure 2.
Sequence comparison of 4-diphosphocytidyl-2-C-methyl-d-erythritol kinases. A, L. esculentum (GenBank accession no. U62773); B, E. coli (GenBank accession no. AAF29530). Identical residues are shown in inverse contrast; similar residues are shaded. Residues representing a putative ATP binding site are indicated by asterisks. A putative cleavage site of the transit peptide is indicated by an arrow.
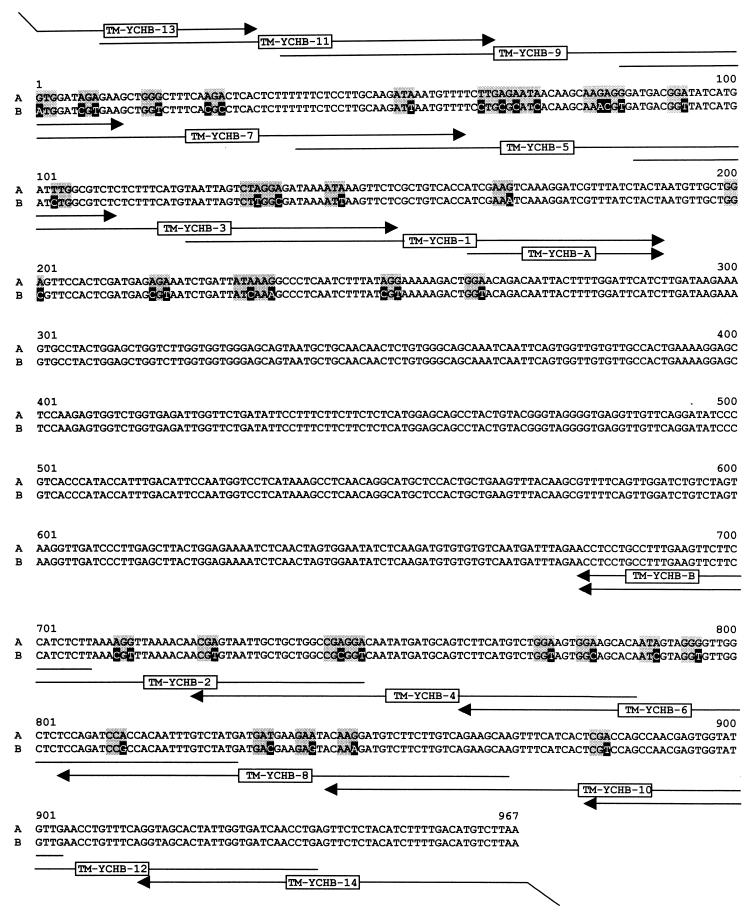
Because the tomato gene comprises a substantial number of codons that are poorly expressed in E. coli, we constructed an ORF that was optimized for expression in E. coli by replacement of 35 codons (Fig. 3). The gene cassette obtained by a sequence of seven consecutive PCR reactions specifies the amino acid residues 81–401 of the tomato gene preceded by the sequence motif MHHHHHAS (for details, see Experimental Procedures). The synthetic ORF comprising unique cutting sites for NcoI and HindIII was ligated into the expression plasmid pNCO-HIS6-TM-YCHB that had been constructed as described in Experimental Procedures.
Figure 3.
Cloning of the catalytic domain of 4-diphosphocytidyl-2-C-methyl-d-erythritol kinase from tomato. A, wild-type cDNA sequence (GenBank accession no. U62773); B, DNA sequence adapted to E. coli. Mutagenized residues are shown in inverse contrast; adapted codons are shaded. Oligonucleotides used for PCR amplifications are indicated by arrows.
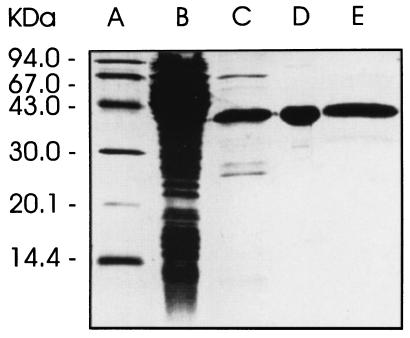
A recombinant E. coli strain harboring that plasmid with the synthetic gene cassette under the control of a T5 promoter and lac operator produced a recombinant protein with an apparent mass of 36.4 kDa accounting for approximately 4% of cell protein. The recombinant protein was obtained in apparently pure form by affinity chromatography on a nickel-chelating column followed by anion exchange and size-exclusion chromatography (Fig. 4). A typical experiment is summarized in Table 4.
Figure 4.
SDS/PAGE. Lanes: A, molecular weight markers; B, crude cell extract of the recombinant E. coli strain XL1-pNCO-HIS6-TM-YCHB; C–E, recombinant catalytic domain of 4-diphosphocytidyl-2-C-methyl-d-erythritol kinase from tomato after chromatography by affinity, anion exchange, and size exclusion, respectively.
Table 4.
Purification of recombinant 4-disphosphocytidyl-2-C-methyl-d-erythritol kinase from tomato
| Step | Total protein, mg | Total activity, μmol⋅min−1 | Specific activity, μmol⋅mg−1⋅min−1 | Yield, % | Purification, fold |
|---|---|---|---|---|---|
| Cell extract | 488 | 683 | 1.4 | 100 | 1 |
| Ni-Sepharose | 17 | 323 | 19 | 47 | 14 |
| Mono Q | 6.4 | 198 | 31 | 29 | 22 |
| Superdex 200 | 1.6 | 53 | 33 | 8 | 24 |
The recombinant protein was shown to catalyze the ATP-dependent phosphorylation of 4-diphosphocytidyl-2-C-methyl-d-erythritol. The specific activity was 33 μmol⋅mg−1⋅min−1 in close similarity with the E. coli protein (Table 5).
Table 5.
Catalytic rates of 4-diphosphocytidyl-2-C-methyl-d-erythritol kinases
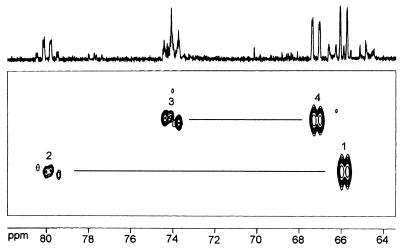
[1,2,2-methyl,3,4-13C5]4-Diphosphocytidyl-2-C-methyl-d-erythritol was used as substrate to determine the structure of the product by NMR spectroscopy. The crude enzyme reaction mixture was analyzed by 13C NMR and INADEQUATE spectroscopy (Fig. 5). Intense 13C-coupled 13C NMR signals were observed at chemical shifts (Table 6) that were attributed previously to 4-diphosphocytidyl-2-C-methyl-d-erythritol 2-phosphate (24). Most notably, the signal at 79.96 ppm signaled the presence of a phosphate moiety at C-2. The assignments were further confirmed by a two-dimensional INADEQUATE spectrum (Fig. 5).
Figure 5.
INADEQUATE spectrum of [1,2,2-methyl,3,4-13C5]4-diphosphocytidyl-2-C-methyl-d-erythritol 2-phosphate. Correlations between C-2 and C-2-methyl, as well as between C-2 and C-3, are outside the displayed spectral range.
Table 6.
13C NMR data of [1,2,3-methyl,3,4-13C5]4-diphosphocytidyl-2-C-methyl-d-erythritol 2-phosphate obtained by incubation of [1,2,2-methyl,3,4-13C5]4-diphosphocytidyl-2-C-methyl-d-erythritol with recombinant 4-diphosphocytidyl-2-C-methyl-d-erythritol kinase from tomato
| Position | Chemical shift, ppm | Coupling constants, Hz
|
|
|---|---|---|---|
| JCC | JPC | ||
| 1 | 65.91 | 40.6 (2) | |
| 2 | 79.96 | 41.6 (1,3,2-Me) | 7.9 (P-2) |
| 2-Methyl | 17.94 | 39.5 (2) | |
| 3 | 74.09 | — | |
| 4 | 67.21 | 42.8 (3) | 4.8 (P-4) |
Recently, Croteau and Lange (26) expressed the ispE gene of E. coli as well as a putative ortholog from peppermint in recombinant E. coli strains. Both proteins were reported to phosphorylate isopentenyl phosphate, albeit at extremely low rates of, respectively, 178 ± 81 and 1.43 pmol⋅g−1⋅s−1.
We used [4-14C]isopentenyl monophosphate to check recombinant 4-diphosphocytidyl-2-C-methyl-d-erythritol kinase from tomato and E. coli for isopentenyl phosphate kinase activity. Both proteins were assayed at high concentrations and in highly purified form, but conversion of isopentenyl phosphate into isopentenyl pyrophosphate could not be detected. The detection limit of the method used was estimated at 1.4 pmol⋅mg−1⋅min−1 (equivalent to 24 pmol⋅g−1⋅s−1).
Discussion
The enzyme specified by the ispE gene of E. coli and its ortholog from tomato phosphorylate the position 2 hydroxy group of 4-diphosphocytidyl-2-C-methyl-d-erythritol at similar rates of 34 and 33 μmol⋅mg−1⋅min−1, respectively.
Recently, Lange and Croteau (26) reported on recombinant IspE proteins from E. coli and peppermint. Using crude enzyme fractions, the authors reported the phosphorylation of isopentenyl phosphate by the E. coli and peppermint enzyme at rates of 178 ± 81 pmol⋅g−1⋅s−1 (i.e., 10.7 ± 4.9 pmol⋅mg−1⋅min−1) and 1.43 pmol⋅g−1⋅s−1 (i.e., 86 fmol⋅mg−1⋅min−1), respectively. From the published electropherograms, it can be estimated that the recombinant proteins had been obtained at ≈0.1–1% purity. With this assumption, the activities reported by Lange and Croteau would translate into hypothetical specific activities of ≈1 and ≈0.1 nmol⋅mg−1⋅min−1 for the enzymes of E. coli and peppermint, respectively.
In light of these observations, we checked 4-diphosphocytidyl-2-C-methyl-d-erythritol kinase of tomato and E. coli for isopentenyl phosphate kinase activity. Even when very high concentrations of the recombinant enzymes were used, we were unable to detect any enzyme activity using radioactive isopentenyl phosphate as substrate. We estimated that the sensitivity of our assay would have been sufficient to detect a specific activity of 1.4 pmol⋅mg−1⋅min−1.
It appears important to note that the 4-diphosphocytidyl-2-C-methyl-d-erythritol kinase activity of the E. coli enzyme exceeds the isopentenyl monophosphate kinase activity by more than five orders of magnitude (according to the data in ref. 26).
It should also be noted that the isopentenyl monophosphate phosphorylation activities reported by Lange and Croteau for the enzymes from E. coli and peppermint translate into approximate turnover numbers of 2 and 0.2 h−1, respectively. It is hardly conceivable that such low catalytic activities could be metabolically relevant.
Moreover, recent data of Kuzuyama et al. confirm that 4-diphosphocytidyl-2-C-methyl-d-erythritol and not isopentenyl monophosphate is the true substrate for the IspE protein of E. coli (30).
Several years ago, it was shown that the mRNA specifying 4-diphosphocytidyl-2-C-methyl-d-erythritol kinase is up-regulated during the ripening process of tomatoes (31). Because carotenoids such as lycopene are massively produced in tomatoes via the deoxyxylulose phosphate pathway, this finding strongly supports the proposed involvement of that protein in exactly that biosynthetic reaction sequence.
Acknowledgments
We thank Katrin Gärtner for assistance and Angelika Werner for expert help with the preparation of the manuscript. This work was supported by the SFB 369 of Deutsche Forschungsgemeinschaft and the Fonds der Chemischen Industrie and the Hans-Fischer-Gesellschaft.
Footnotes
Data deposition: The sequence reported in this paper has been submitted to the GenBank database (accession no. AF263101).
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.140209197.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.140209197
References
- 1.Qureshi N, Porter J W. In: Biosynthesis of Isoprenoid Compounds. Porter J W, Spurgeon S L, editors. Vol. 1. New York: Wiley; 1981. pp. 47–94. [Google Scholar]
- 2.Bloch K. Steroids. 1992;57:378–382. doi: 10.1016/0039-128x(92)90081-j. [DOI] [PubMed] [Google Scholar]
- 3.Bach T J. Lipids. 1995;30:191–202. doi: 10.1007/BF02537822. [DOI] [PubMed] [Google Scholar]
- 4.Bochar D A, Friesen J A, Stauffacher C V, Rodwell V W. In: Comprehensive Natural Product Chemistry. Barton D, Nakanishi K, editors. Vol. 2. Oxford: Pergamon; 1999. pp. 15–44. [Google Scholar]
- 5.Rohmer M. Nat Prod Rep. 1999;16:565–574. doi: 10.1039/a709175c. [DOI] [PubMed] [Google Scholar]
- 6.Lichtenthaler H K. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:47–65. doi: 10.1146/annurev.arplant.50.1.47. [DOI] [PubMed] [Google Scholar]
- 7.Eisenreich W, Schwarz M, Cartayrade A, Arigoni D, Zenk M, Bacher A. Chem Biol. 1998;5:R221–R233. doi: 10.1016/s1074-5521(98)90002-3. [DOI] [PubMed] [Google Scholar]
- 8.Rohmer M, Knani M, Simonin P, Sutter B, Sahm H. Biochem J. 1993;295:517–524. doi: 10.1042/bj2950517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Broers S T J. Ph.D. thesis. Zurich: Eidgenössische Technische Hochschule; 1994. [Google Scholar]
- 10.Schwarz M K. Ph.D. thesis. Zurich: Eidgenössische Technische Hochschule; 1994. [Google Scholar]
- 11.Schwarz M, Arigoni D. In: Comprehensive Natural Product Chemistry. Barton D, Nakanishi K, editors. Vol. 2. Oxford: Pergamon; 1999. pp. 367–399. [Google Scholar]
- 12.Rohmer M, Seemann M, Horbach S, Bringer-Meyer S, Sahm H. J Am Chem Soc. 1996;118:2564–2566. [Google Scholar]
- 13.Sprenger G A, Schörken U, Wiegert T, Grolle S, deGraaf A A, Taylor S V, Begley T P, Bringer-Meyer S, Sahm H. Proc Natl Acad Sci USA. 1997;94:12857–12862. doi: 10.1073/pnas.94.24.12857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lois L M, Campos N, Putra S R, Danielsen K, Rohmer M, Boronat A. Proc Natl Acad Sci USA. 1998;95:2105–2110. doi: 10.1073/pnas.95.5.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller B, Heuser T, Zimmer W. FEBS Lett. 1999;460:485–490. doi: 10.1016/s0014-5793(99)01397-6. [DOI] [PubMed] [Google Scholar]
- 16.Kuzuyama T, Takagi M, Takahashi S, Seto H. J Bacteriol. 2000;182:891–897. doi: 10.1128/jb.182.4.891-897.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lange B M, Wildung M R, McCaskill D, Croteau R. Proc Natl Acad Sci USA. 1998;95:2100–2104. doi: 10.1073/pnas.95.5.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bouvier F, d'Harlingue A, Suire C, Backhaus R A, Camara B. Plant Physiol. 1998;117:1423–1431. doi: 10.1104/pp.117.4.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takahashi S, Kuzuyama T, Watanabe H, Seto H. Proc Natl Acad Sci USA. 1998;95:9879–9884. doi: 10.1073/pnas.95.17.9879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lange B M, Croteau R. Arch Biochem Biophys. 1999;365:170–174. doi: 10.1006/abbi.1999.1168. [DOI] [PubMed] [Google Scholar]
- 21.Schwender J, Müller C, Zeidler J, Lichtenthaler H K. FEBS Lett. 1999;455:140–144. doi: 10.1016/s0014-5793(99)00849-2. [DOI] [PubMed] [Google Scholar]
- 22.Jomaa H, Wiesner J, Sanderbrand S, Altinicicek B, Weidemeyer C, Hintz M, Türbachova I, Eberl M, Zeidler J, Lichtenthaler H K, Soldati D, Beck E. Science. 1999;285:1573–1576. doi: 10.1126/science.285.5433.1573. [DOI] [PubMed] [Google Scholar]
- 23.Rohdich F, Wungsintaweekul J, Fellermeier M, Sagner S, Herz S, Kis K, Eisenreich W, Bacher A, Zenk M H. Proc Natl Acad Sci USA. 1999;96:11758–11763. doi: 10.1073/pnas.96.21.11758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lüttgen H, Rohdich F, Herz S, Wungsintaweekul J, Hecht S, Schuhr C A, Fellermeier M, Sagner S, Zenk M H, Bacher A, Eisenreich W. Proc Natl Acad Sci USA. 2000;97:1062–1067. doi: 10.1073/pnas.97.3.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herz S, Wungsintaweekul J, Schuhr C A, Hecht S, Lüttgen H, Sagner S, Fellermeier M, Eisenreich W, Zenk M H, Bacher A, Rohdich F. Proc Natl Acad Sci USA. 2000;97:2486–2490. doi: 10.1073/pnas.040554697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lange M, Croteau R. Proc Natl Acad Sci USA. 1999;96:13714–13719. doi: 10.1073/pnas.96.24.13714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang A C Y, Cohen S N. J Bacteriol. 1978;134:1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bullock W O, Fernandez J M, Short J M. BioTechniques. 1987;5:376–379. [Google Scholar]
- 29.Sanger F, Nicklen S, Coulson A R. Proc Acad Natl Sci USA. 1977;74:5463–5468. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuzuyama T, Takagi M, Kaneda K, Watanabe H, Dairi T, Seto H. Tetrahedron Lett. 2000;42:2925–2928. [Google Scholar]
- 31.Lawrence D L, Cline K, Moore G A. Plant Mol Biol. 1997;33:483–492. doi: 10.1023/a:1005785321165. [DOI] [PubMed] [Google Scholar]