Abstract
Semiconductor quantum dots and nanoparticles composed of metals, lipids or polymers have emerged with promising applications for early detection and therapy of cancer. Quantum dots with unique optical properties are commonly composed of cadmium contained semiconductors. Cadmium is potentially hazardous, and toxicity of such quantum dots to living cells, and humans, is not yet systematically investigated. Therefore, search for less toxic materials with similar targeting and optical properties is of further interest. Whereas, the investigation of luminescence nanoparticles as light sources for cancer therapy is very interesting. Despite advances in neurosurgery and radiotherapy the prognosis for patients with malignant gliomas has changed little for the last decades. Cancer treatment requires high accuracy in delivering ionizing radiation to reduce toxicity to surrounding tissues. Recently some research has been focused in developing photosensitizing quantum dots for production of radicals upon absorption of visible light. In spite of the fact that visible light is safe, this approach is suitable to treat only superficial tumours. Ionizing radiation (X-rays and gamma rays) penetrate much deeper thus offering a big advantage in treating patients with tumours in internal organs. Such concept of using quantum dots and nanoparticles to yield electrons and radicals in photodynamic and radiation therapies as well their combination is reviewed in this article.
Keywords: Radiation therapy, Radiosensitization, Radioprotection, Photodynamic therapy, Photosensitization, Ionizing radiation, Radicals, Singlet oxygen
1. Introduction
1.1. Quantum dots
Nanomaterials offer great promise in cancer targeting applications. One of them is quantum dots in the size range of 2–100 nm possessing unique tunable optical and targeting properties [1,2]. Although biogenic nanosized particles appear naturally, engineered quantum dots differ because of their crystalline metalloid structure and quantum confinement effect, which occurs at sizes smaller than their Bohr radius of around 1–5 nm [3]. The main goal is to develop small probes with high selectivity, versatility, stability and capacity to penetrate cells and organelles [4]. Despite great enthusiasm in the field, reproducible fabrication and biological difficulties (aggregation, biocompatibility, non-specific binding, toxicity) are the challenges to meet. So far, most of the work on quantum dots is being focused in developing nanocrystals, their bioconjugation for tracking and imaging [5,6]. The possibility to use quantum dots as sensitizers for cancer therapies remains unexplored.
Bioconjugation and imaging applications of quantum dots have been recently well documented in the review by Smith et al. [7] in this journal. Moreover, several other renowned works cover a broad range of aspects from synthesis [8–10], surface modification and doping [11] to multicolour imaging [2,5,12] and cancer targeting [13–15] using quantum dots. Due to superior optical properties quantum dots are being now investigated as laser materials [16]. Therefore, this review will focus on yet scarcely explored aspect of applicability of quantum dots for cancer therapies, namely mechanisms of photosensitization and radiosensitization.
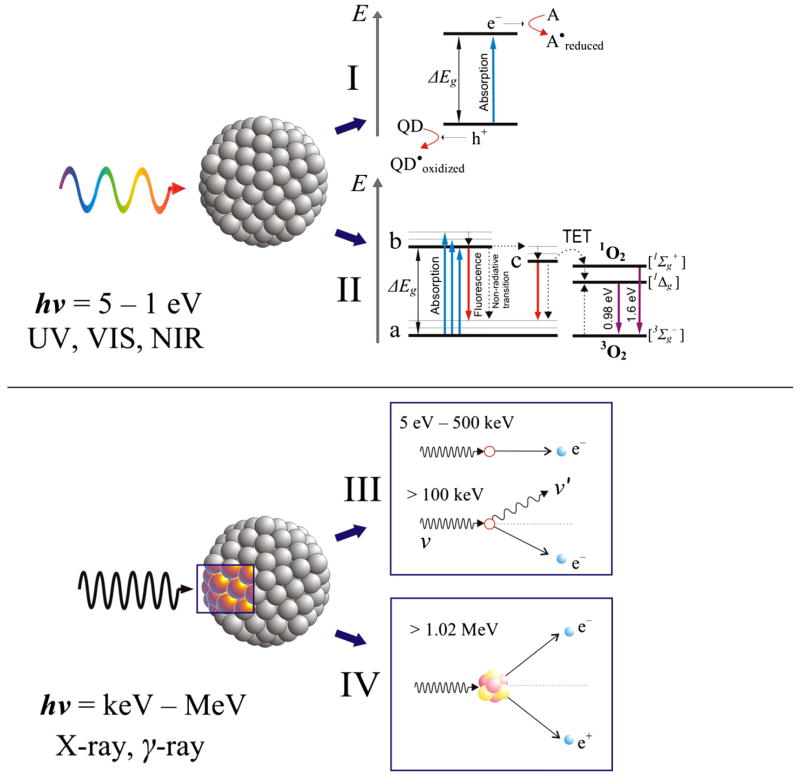
Possessing electronic energy levels in the range of 1–5 eV semiconductor quantum dots can perform as photosensitizers (Fig. 1, I–II) just like organic fluorophores [17,18]. Due to high atom and electron density quantum dots should also act as enhanced absorbers of high-energy photons (X-rays and gamma rays) [19] acting thus as radiosensitizers (Fig. 1, III–IV) and causing localized and targeted damage to cancer cells [20].
Fig. 1.
Schematic presentation of the most likely events occurring when a photon hits a quantum dot. Situation for a low-energy photon (ultraviolet, visible light, near infrared radiation): I) Charge transfer, where QD is a donor and A is an acceptor molecule, ΔEg is the energy gap of the quantum dot, e− and h+ is an electron-hole pair generated by the absorption of the photon, II) Photosensitization, where the quantum dot is promoted from its ground state (a) to a higher energy excited state (b) and then to a “trap” state (c), from which by TET triplet ground-state oxygen (3O2) is promoted into a highly reactive singlet oxygen (1O2). Vibrational levels are depicted for illustrative purpose. Dashed arrows show non-radiative transitions. Situation for a high-energy photon (X-rays and gamma rays): III) Ejection of a high speed electron from an atom constituting the quantum dot due to the photon energy transfer to the electron (photoelectric ionization effect) or Compton scattering, where the incident photon is scattered continuing its voyage with lower energy until next event, IV) Photon annihilation on a nucleus of an atom and generation of an electron-positron pair. The positron will annihilate with a free electron releasing two 0.51 MeV photons, which will further lose their energy through photoelectric effect or Compton scattering. Electrons generated in the events III and IV will induce secondary high speed electrons as well as Auger electrons. Such electrons that succeed to escape into environment will be captured by an acceptor (water, biomolecule, oxygen, nitrogen oxides) in the vicinity of the quantum dot and induce biomolecular radicals, superoxide, hydroxyl radical, peroxynitrite anion or nitric oxide radical.
1.2. Electronic states of quantum dots
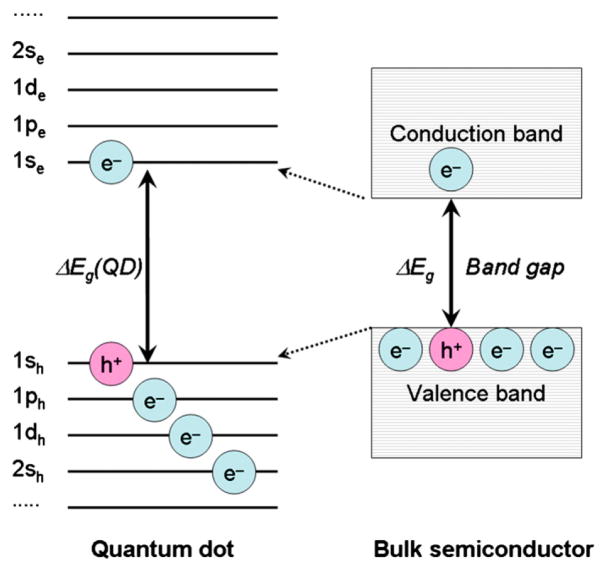
In a bulk semiconductor an energy bandgap between its valence and conductance bands is a fixed factor determined by the nature of the semiconductor material. Sizing down the semiconductor crystal to less than Bohr radius a quantum confinement effect occurs, when electronic excitation states respond to the particle boundaries by adjusting their energy states (Fig. 2). Therefore, the particles that exhibit such effect are termed quantum dots (QD or QDs) [11]. The lowest excited state corresponding to the transition 1Se − 1Sh is commonly referred to as the first exciton state [21]. Decreasing or increasing size of QDs leads to respectively increase or decrease in energy gap: this changes their absorption spectra (appearance colour) and correspondingly shifts their emission wavelength to the blue or red spectral region. The Bohr radius is a characteristic of a material [22]. Three different Bohr radii can be defined:
where m is the rest mass of a particle such as electron (e), hole (h) or electron-hole pair (exciton, exc), m3 is the reduced mass (“effective” inertial mass) of the particle, ε is the material’s dielectric constant, and a0 is the Bohr radius of hydrogen (5.291772×10−11 m). Depending on a radius (R) of a nanocrystal, QDs can be confined in three different regimes: strong confinement (R<ae, ah, aexc), intermediate confinement (ae, ah, <R<aexc) and weak confinement (ah<R<ae, aexc or ae <R<ah, aexc).
Fig. 2.
Simplified diagram illustrating energy levels of a quantum dot compared to its semiconductor material in a bulk crystal. Electrons and holes in the quantum dot and the semiconductor crystal obey Pauli’s exclusion principle when filling the energy states and their positions are shown schematically only. Adapted from Ref. [11].
Solving the Schrödinger equation for a spherical approximation of QDs, the energy gap depends reciprocally on the radius [8]. Due to symmetry of the problem in solving the wave functions [22] the energy levels are then described like atomic orbitals labelled by the quantum numbers αn,l (n =1, 2, 3, etc., l = s, p, d, etc.):
where Eg,0 is an energy bandgap of bulk semiconductor, meh = me × mh/(me + mh) is effective mass of an electron-hole pair (exciton), me and mh are effective masses of an electron and a hole, respectively.
Like Jablonski’s diagram schematically depicting electronic energy levels of organic molecules [23] and photochemical reactions occurring from their excited states [24], a three level model can similarly outline energy states of QDs (Fig. 1, II). The QD level “c” is generally known as a “dark” or “trap” excitonic state since it is populated from the excited state “b” by non-radiative transition [25]. Existence of the “dark” state has been demonstrated by the dependence of luminescence properties of QDs under external magnetic field [26]. Such a “dark” state, comparable by its character to a long-lived triplet state of porphyrins, can generate singlet oxygen [18]. Photoexcited quantum dots and ground-state triplet oxygen have to be in contact to undergo triplet energy transfer (TET), as in the case of conventional PDT photosensitizers [27,28].
Overall, an ideal PDT photosensitizer should possess the following characteristics: it should 1) have a constant composition, 2) be simple to synthesize and easily available, 3) be non-toxic in the absence of light, 4) exhibit target specificity, 5) possess the energy of its excited state higher than 94 kJ/mol (0.97 eV, energy of singlet oxygen), 6) be quickly cleared from the body, 7) possess minimal self-aggregation, and 8) be photostable (no photobleaching). Quantum dots satisfy the first five criteria and are therefore promising new generation photosensitizers [17].
2. Concern about toxicity: quantum dots from non-hazardous materials
2.1. Toxicity of “traditional” quantum dots
Lay and scientific communities are divided into two camps when concerning potential hazards of nanotechnology and its enthusiastic applications [29]. Synthesis of nanoparticles composed of less toxic materials is of great interest. Cadmium is potentially hazardous, and toxicity of such CdSe quantum dots to living cells, and humans, is not yet systematically investigated, and future human applications of CdSe dots are disputable. On the other hand, before nanoparticles composed of non-hazardous materials become available, use of CdSe quantum dots in experiments with cells in vitro might give good indication on how the quantum dot system actually behaves under stimuli such as radiation. Toxicity of “traditional” quantum dots is caused by colloidal instability due to release of Cd2+ with subsequent generation of radicals [30] and malfunction of cellular organelles [31]. Indeed, it has been reported that CdTe quantum dots have much lower toxicity when they are purified by removing the free Cd2+ ions [32]. To prevent cells and tissues from exposure to cadmium, quantum dots are stabilized with a shell. However, after prolonged circulation in the body the shell may deteriorate and thus yielded “naked” quantum dots may induce damage to plasma membrane, mitochondria and nuclei [33]. Cytotoxicity of quantum dots has been recently reviewed in this journal [7,34] and elsewhere [3,35].
2.2. Quantum dots composed of zinc
Synthesis of water-soluble ZnS nanoparticles is available in the literature [36]. Like “traditional” quantum dots, for specific targeting these nanoparticles can be also coated with monoclonal antibodies, aptamers [37], folic acid [38] or amino acids [39]. ZnS nanoparticles are prepared by precipitation method from precursors dissolved in aqueous solution. Resultant nanoparticles form a colloidal suspension. To minimize agglomeration a termination group, e.g. chitosan, methacrylic acid or cysteine, is attached during the synthesis. Polymer molecules adsorbed on the surface of the nanoparticles provide steric hindrance so that osmotic repulsion pushes nanoparticles apart from each other. Doping of the ZnS nanoparticles with Mn2+ [40–43] or Eu2+ [44–46] produces fluorescent nanoparticles.
Furthermore, quantum dots doped with Mn2+ or Eu2+ may have strong luminescence and magnetism that can be potentially used for both fluorescence and magnetic imaging [42,47].
In some cases gamma radiation is used to facilitate colloid formation of ZnS nanoparticles [48].
2.3. Bioactive silicon nanoparticles
Silicon might be a great candidate as the second most abundant element in the earth’s crust. Briefly, initial polycrystalline silicon powder is chemically etched in a mixture of HF–H2O–HNO3 resulting in nanoporous (npSi) material [49]. However, silicon being a dominant semiconductor has not yet been actively developed as biomaterial for cancer therapies. Porosity of silicon can be controlled very precisely with a pore size from less than 2 nm to more than 50 nm. Etched structures retain crystalline backbone with a skeleton covered by silicon hydride (SiHx) surface. It is of great interest as a biodegradable material since the primary degradation product of slowly dissolving silicon is silicic acid (SiOH4), which is its natural form readily eliminated from the body [50]. Surface of such npSi can be further terminated with various functional groups like carboxylate [51] or surfactants [49] to make them hydrophilic and dispersed in aqueous solutions. Property of npSi relevant for biomedical applications has been realized recently, namely ability to induce singlet oxygen under irradiation with visible light [52]. Moreover, observation of luminescence from singlet oxygen was reported for npSi with porphyrins immobilized in its pores [53]. A decade ago it was suggested that polycrystalline silicon might exhibit biological activity by bonding to tissues, behaviour similar to that of bioactive ceramics that might be exploited for implantable devices and biochips [54].
2.4. Carbon dots
Another candidate is a ubiquitous element carbon. Recently, Sun and coworkers [55] found and reported carbon-based QDs or carbon dots, which are nanoscale carbon particles with simple surface passivation to exhibit strong photoluminescence in both solution and the solid state. The emission spectral features and properties of the available carbon dots are comparable to those of surface-oxidized silicon nanocrystals. The starting pristine carbon nanoparticles can be produced from the laser ablation of a graphite target in inert atmosphere under reduced pressure. The results from Raman spectroscopy, transmission electron microscopy (TEM), and X-ray diffraction (XRD) analyses all suggest that these carbon nanoparticles are largely amorphous. The carbon nanoparticles are by themselves non-emissive either in the solid state or in suspensions. However, upon simple surface passivation by attaching organic molecules, the particles become soluble in common organic solvents and/or water depending upon the attached functionalities. The microscopy results show that these soluble surface-modified carbon nanoparticles are well dispersed as individual particles with diameters of a few nanometers.
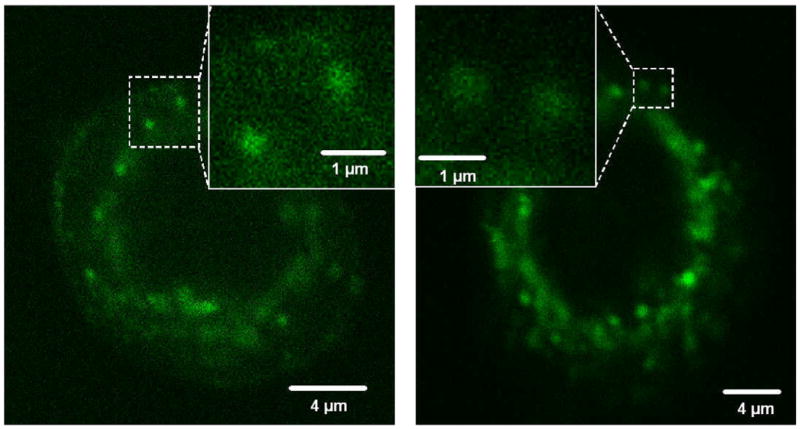
The surface passivation makes carbon nanoparticles into carbon dots, which exhibit bright and colourful photoluminescent in the visible to the near infrared [56]. Mechanistically, the photoluminescence has been attributed to the presence of surface energy traps, likely related to the abundant surface defect sites that become emissive upon passivation. In addition, the surface emissive sites of the carbon dots are likely quantum confined in the sense that a large surface-to-volume ratio is required for the strong photoluminescence. In fact, larger carbon particles (30–50 nm in diameter) with the same surface passivation are much less luminescent. Carbon dots are biocompatible, and they are non-toxic according to preliminary in vivo studies in mice. Carbon dots have also been conjugated with biological and bioactive species, including antibodies and molecules that target specific receptors, for optical bioimaging of cancer cells and tissues (Fig. 3). Other potential bioapplications of carbon dots include their uses in the early detection and therapy of breast cancers, for which various levels of investigations are in progress. Carbon nanotubes functionalized with antibodies have been shown to effectively bind to breast cancer cells [57,58].
Fig. 3.
Fluorescence microscopy detection of PPEI-EI-functionalized carbon dots in MCF-7 cells with one-photon (458 nm) and two-photon (800 nm) excitations. Shown in the insets are signals likely associated with single carbon dots.
2.5. Role of endocytosis
Quantum dots are taken up by endocytosis and are retained in lysosomes [59]. This is confirmed by localization pattern of quantum dots in perinuclear region, similarly to that observed in immunofluorescence staining of lysosomal membrane protein LAMP-2 [60]. To enhance endocytosis quantum dots can be designed with cell penetrating peptides (CPPs) [61], which consist of less than 30 amino acids and may be cationic or amphipathic [62]. As drug delivery vehicles CPPs must be small and simple to synthesize, able to be coupled to cargoes without losing their properties, selective, stable and non-toxic. Penetration through systems that are difficult to cross has been achieved, such as blood–brain barrier. Mechanisms of internalization of CPPs are not yet completely understood. Permeation of CPPs through cellular membranes was observed through endocytosis as well at low temperatures (0–4 °C) or in the presence of endocytosis inhibitors. In addition, inverted micelle mechanism is suggested where positively charged peptides interact with negatively charged head groups of phospholipids converting part of cellular membrane into an inverted micelle [63]. Peptides of distinctive sequences target different enzymes like caspase-1, thrombin, collagenase or chymotrysin [64].
Another strategy would be targeting receptor-mediated endocytosis [12]. Quantum dots bound to specific peptidic receptors can be more effectively internalized [65]. Intracellular accumulation of quantum dots may also be enhanced by exploiting the fact that cancer cells possess more folate receptors since it is important for cells that rapidly divide. Such folate-receptor mediated delivery of quantum dots has been recently demonstrated in the literature for quantum dots [66] and the ones entrapped in liposomes [67].
Lysosomal localization may not be advantageous during PDT or radiotherapy since radical generation may be limited to lysosomes. To enhance their intracellular radical generating ability, escape of quantum dots into cytosol must be stimulated. This can be done by irradiation with UV [59] or blue light [68]. For in vivo situation, UV and blue light has limited tissue penetration depth. Therefore, cells can be loaded with quantum dots together with dipeptides such as ala-ala, ser-tyr or tyr-ala, which have been shown to induce lysosomal rupture due to osmotic imbalance [69].
2.6. Monitoring toxicity in real time using novel impedance technique
Interaction of mammalian cells with surfaces and focus on the kinetic aspects of this phenomenon is of great interest for science. Cell attachment is an important parameter to assess cancer cell potential for metastasis and tumour healing caused by their dynamic interaction with substrates and drugs. Most commonly cell behaviour is studied by imposing an effect to attached cells and quantifying cell density, morphology and number resisting to this treatment by microscopic or ultrastructural techniques. Non-destructive methods to monitor cell responses in real time are also available. Array of microelecrodes in chambers is used to record electrophysiological activity of cells [70,71]. Bioelectrical potential of primary isolated epithelial cells was studied to determine whether the cells can maintain epithelial structure and function when isolated from mesenchymal framework [72]. In another study, cell-to-cell communications were studied by injecting a dye Lucifer yellow to a single cell through a microelectrode: electrical characteristics of the cells were measured with the electrode and transfer of the dye from the single cell to its neighbours by cell-to-cell gap junctions was observed in cell monolayers [73]. The method that applies external electrical field to sense cell spreading upon artificial surfaces in real time is referred to as Electric Cell-substrate Impedance Sensing (ECIS) with frequencies 400 Hz, 4 kHz and 40 kHz being used in most experiments [74]. Although the ECIS technique has been first described by Giaever and Keese [75], it can still be considered novel. When a cell attaches to a small gold electrode, it reduces the detecting area in contact with culture medium and measured electric impedance increases because the cell membranes gradually block the current flow. Amount and type of protein coating the gold surface of the electrode are one factor effecting cell attachment, and protein type is significant [76]. When cells cover a gold electrode, various cell lines will attach at different rates; this in turn will be affected by exposing the cells to a treatment. From the impedance changes, several important properties of the cell layer can be determined such as barrier function, membrane capacitance, cell proliferation, motility and motion [77]. Use of ECIS to study cytotoxicity to chemical compounds experiences growing interest [78,79]. Dynamics of cell invasiveness can also be studied looking at how cells with different metastatic potentials affect confluent monolayer of for example endothelial cells [80]. Measurements using ECIS of repopulation of mechanically disrupted cells in culture have been as well suggested as wound healing assay [81].
The ECIS technique is becoming now a well-established technique to study chemical and physical factors and other dynamic processes [82]. Recently the first paper on the use of this technique to assess toxicity effects of quantum dots was published [83]. Concentration to achieve 50% inhibition was determined in fibroblast V79 cells for free metals and quantum dots: around 6 μM for Cd, 98 μM for Te, 140 μM for Zn, 154 nm for red CdSe/ZnS and 240 nM for green CdSe/ZnS [83]. For the cadmium selenide and telluride quantum dots, toxicity could be assigned to release of free cadmium. The quantum dots synthesized with indium gallium phosphide and gold nanoparticles were not cytotoxic for concentrations studied up to 200 nM and 45 μM, respectively [83]. No more studies for live monitoring of cell behaviour with quantum dots using ECIS assay have been reported so far. We are presently performing photolytic ECIS studies using CdSe/ZnS quantum dots to see whether exposure of cancer cells to the quantum dots and light leads to photodynamic impairment or stimulation of the cell growth and/or adherence.
2.7. Sensing cancer stem cells with quantum dots
Cancer stem cells are a population of cells that possess self-renewal property. Recently it has been hypothesized that cancer therapies might be more efficient if cancer stem cells are targeted [84]. After radiation therapy resultant living cancer cells could be labelled with quantum dots and thus stained. Tolerance of such clones to repeated radiation treatments could be a measure of stem cell population. Tracking stem cells with superparamagnetic iron oxide nanoparticles were first suggested [85]. Detection of mesenchymal stem cells (MSC) with quantum dots has been recently reported [86]. However, the authors observed decreased detection by flow-cytometry of MSC labelled with quantum dots as cells proliferated in time. Long-term labelling of stem cells with quantum dots is suggested to study proliferation and tissue regeneration [87].
2.8. The “other” quantum dots
Recently, novel luminescent probes are coming to light, which are believed to be more biocompatible than traditional quantum dots. As synthesized, quantum dots possess hydrophobic surface. To make quantum dots water soluble, their surface is modified with polar terminal groups or molecules.
Encapsulation of quantum dots in phospholipid micelles for biological imaging has been proposed [88]. Lipid structure of such nanoparticles can be easily exploited in bioconjugation of various targeting molecules to diversity of functional groups (NH2, SH, COOH, biotin, maleimide) available in phospholipids [89]. In a recent study, so-called lipodots were synthesized, where quantum dots were entrapped in a bilayer of phospholipid vesicles [67]. Such folate-targeted lipodots were used to target lymphoma cells with upregulated folate receptor, while colon carcinoma cells not expressing folate receptor did not uptake the lipodots [67]. Zeta potential and fluorimetry titration were used to study adsorption of quantum dots on the surface of such unilamellar vesicles forming stable hybrid giant vesicles [90].
Novel flodots composed of silica nanoparticles doped with a dye consisting of luminescent organic and inorganic molecules dispersed inside silica matrix have been reported [91].
Nanotransducers for conversion of two or more incident photons to a higher energy photon is another example of potential clinical use of nanocrystals [34]. Such upconverting nanoparticles (UCNs) composed of NaYF4 doped with Yb3+ and Er3+ in a polymeric coating will allow treatment at deeper locations [92].
Design of QDs based on bioluminescence energy transfer (BRET) has been suggested. It relies on QDs conjugated with fluorescent protein luciferase [93] or halotagluciferase [94] as energy donor. This concept solves light penetration issue. However, it suffers yet from few problems like administration of a foreign protein and a foreign enzyme substrate, which may have immunogenic potential, and dependence of light generation on enzyme distribution, i.e. extravasation from vasculature and tissue perfusion [95].
Encapsulation of nanoparticles by polymers, phospholipids or inorganic shell prevents dissociation and provides anchor for biomolecule conjugation. Stable functionalization of QDs is still a challenge. Gold nanoparticles were encapsulated by amphiphilic diblock copolymers such as polystyrene, polyglutamic acid and polyacrylic acid producing small (4.8–6.6 nm) and stable polymer micelles [96].
Silica-shelled QDs represent probably the most attractive alternative. Silica is bioinert materials while enabling incorporation of a variety of substances. Silica spheres can accommodate fluorescent and paramagnetic nanoparticles and administered without significant effect on physiological characteristics [97].
3. Quantum dots as photosensitizers for cancer therapy
3.1. Principles of photosensitization
Since the first demonstration of the photodynamic action by Raab and von Tappeiner in 1897–1900 [98,99], great effort has been devoted towards the development of agents, which exhibit specific light absorption and tissue distribution properties. Photodynamic therapy (PDT) is a treatment modality that uses a photosensitizer, usually a porphyrin type pigment that preferentially localizes in target tissue, followed by exposure to visible light. Number of scientific publications has been increasing exponentially after the first report in 1972 [100]. PDT has recently become an approved treatment modality for some types of cancer and skin disorders [101]. However, only few, Photofrin, Photosan, Foscan, Photogem, Photohem, Levulan, Metvix [102], of many experimentally investigated photosensitizers have received regulatory approval and for a small number of diseases [103].
Since the first definitive observations by Herzberg in 1931 [104] singlet oxygen is now recognised as the main intermediate in photosensitized cell killing [105–107]. Photosensitized oxidation induces pathological effects such as cell damage, mutations, cell death and photooxidation of cell constituents such as proteins and DNA. Singlet oxygen can also be generated by amino acids and proteins absorbing UV [108] or directly by infrared radiation [109,110]. Singlet oxygen besides acts as intermediate for activation or impairment of cell signalling pathways by producing oxidation products, which are either positive or negative regulators [111]. Closely related non-pathological redox reactions take place during photosynthesis [112]. Singlet oxygen (1O2) is not a free radical, but an electronically excited form of a dioxygen molecule [113,114], which has high reactivity with biomolecules [115,116]. The destruction of biomolecules however is limited to the diffusion length of singlet oxygen, which is estimated to be less than 0.1 μm [117,118]. Therefore, the localization of a photosensitizer is of crucial importance in targeting specific organelles by PDT [119]. It is important to notice that sensitization is not only important in photodynamic cancer therapy, but also in initiation of diseases. In 1959 Dr. Harman was the first to propose the free radical theory of aging and diseases: the cells’ ability to produce adequate amounts of antioxidants is determined by age, inheritance, nutrition and stress. While for many years his theory was rejected, Harman’s ideas have been validated in 1980–1990s [120].
3.2. Generation of singlet oxygen by quantum dots
In PDT, cells are selectively killed by first chemically targeting them, ideally at the molecular level, with a photosensitizing agent. Next, the tissue is illuminated, and the photosensitizer generates singlet oxygen, which is highly cytotoxic. PDT is potentially useful because of its specificity: only cells in close proximity to the photosensitizer are affected, and the photosensitizer is not cytotoxic until illuminated, allowing excess unbound reagent to be cleared from the system. This specificity could be especially valuable in targeting tumour cells without harming the surrounding tissue. One photosensitizing agent, Photofrin®, has been approved for clinical use, but other agents are still being investigated. QD conjugates may have some advantages, in particular because of the large absorbance cross-section and the tunable optical properties.
The combination of semiconductor quantum dots with PDT photosensitizers enables the use of an excitation wavelength where the photosensitizer alone does not absorb [18]. This was the first demonstration of CdSe QD-based Förster Resonance Energy Transfer (FRET) to facilitate excitation of a phthalocyanine photosensitizer (Pc4), which is known to generate reactive singlet oxygen with quantum yield around 43%. The 77% measured FRET efficiency is encouraging; however, the 488 nm excitation used to excite the quantum dots will not penetrate deep into tissue, and no cell studies have been reported as yet. Since quantum dots exhibit broad absorption spectra, their conjugation to a PDT photosensitizer provides flexibility to utilize variable excitation wavelengths to activate the photosensitizer molecule. These authors have also demonstrated for the first time the emission of singlet oxygen at 1270 nm generated by the energy transfer from QDs alone under excitation at 488 nm with singlet oxygen quantum yield around 5% in toluene [18]. In another study, CdTe QD and aluminium tetrasulfophthalocyanine (QD-AlSPc) nanocomposites generated singlet oxygen with quantum yield around 15% compared to 1% of the QDs alone in D2O [121]. However, it was still lower compared to that of AlSPc alone (36%). The determined FRET efficiency of QD-AlSPc nanocomposites was around 58% [122].
Several groups have already demonstrated cytotoxicity of QDs under exposure to UV radiation [17,123]. Photosensitization by QDs in biological environments certainly warrants more investigations. This is further supported by studies showing singlet oxygen generation by quantum dot-organic dye nanocomposites: QD conjugates with mesotetra(4-sulfonatophenyl)porphine (TSPP) [124], chlorine e6 and rose Bengal [125]. Singlet oxygen generation was shown for mixtures of QDs and photosensitizers with different quantum yields of singlet oxygen generation [126], where the authors reported that extent of singlet oxygen enhancement is inversely proportional to the quantum yield of singlet oxygen generation. In the most recent study, strand breaks and damage to nucleobases in DNA were induced by photosensitization of QDs complexed with plasmid DNA [127].
For two-photon PDT energy-transferring organically modified silica nanoparticles were recently reported [128]. The authors concluded that their approach of two-photon induced intraparticle FRET, based on the use of two-photon fluorescent aggregates as a donor and a photosensitizing drug as an acceptor, offers a simple and proper methodology for developing formulations of drug-carrier nanoassemblies applicable in two-photon activated PDT.
3.3. Generation of other radical species by quantum dots
Besides singlet oxygen, quantum dots generate other reactive oxygen and nitrogen species. We have recently conducted a series of studies with red-absorbing quantum dots and dihydrorhodamine 123, which is a reagent to monitor generation of hydrogen peroxide (H2O2) and peroxynitrite (ONOO−) [129,130]. The solutions were exposed to red light (632 nm) that matches the first exciton peak of the QDs. Dihydrorhodamine 123 was rapidly oxidized to a green fluorescent product rhodamine 123 within few minutes of the red light exposure [68]. It is firmly established in the literature that peroxynitrite does not yield singlet oxygen [131–133]. Bandgap of quantum dots is also potentially enough to reduce oxygen to superoxide ( ) or oxidize water or hydroxide ions to hydroxyl radical (•OH) [134].
Exposure of prostate carcinoma Du145 cell cultures to blue light leads to increase in QD fluorescence [68]. Such photoactivation of QD fluorescence has been recently demonstrated for HeLa and BHK cells in vitro in another study [59]. This shows that a lot more QDs are endocytosed than can initially be judged by fluorescence microscopy (Fig. 4). Moreover, fluorescence increase after light exposure might be a direct indication of lysosomal breakage induced by radicals generated by quantum dots. Such effect of photochemical internalization is known for some traditional photosensitizers. During endocytosis a photosensitizer is transported to endosomes and then to late lysosomes [135]. High local concentrations of the photosensitizer in the lysosomes result in its aggregation and fluorescence quenching. Upon light exposure, photosensitization is induced, which leads to destruction of the lysosomes and release of the photosensitizer into cytosol. In biological environments generation of radicals occurs in a concert; once a certain radical is formed it rapidly decomposes or dissociates into other radical species. Krinsky presented such a complex diagram of chemical, enzymatic and photochemical generation of reactive oxygen species [136,137]. Our data [68] and that found in the literature so far [134] show that there is enough evidence to say that QDs generate radical species other than singlet oxygen during irradiation with ultraviolet and visible light. Whether the generation of these radicals by QDs is enough to be applied in cancer therapy, it remains to be studied.
Fig. 4.
Enhancement of fluorescence of quantum dots (8 nM QD655-carboxyl terminated) endocytosed in Du145 cells (5×105 cells/ml). A) Fluorescence microscopy photograph (objective 40×) taken within 0–1 min of observation after around 24 h incubation. Nuclei are stained with Hoechst 33342. B) The same spot on the culture dish photographed under the same conditions after 5 min irradiation with the microscope excitation light (395–440 nm). Red fluorescence appears while the Hoechst dye is photobleached during irradiation. C) Fluorescence photograph taken at lower magnification (10×) highlights the red fluorescent spot caused by the irradiation using the 40× objective. The scale bars are 100 μm.
Enhancement of QD fluorescence was also seen in pure organic and aqueous solutions of QDs exposed to ultraviolet radiation for extended periods, up to 750 min [138]. Such enhancement was possibly attributed to free radical hypothesis for water-soluble QDs (“oxygen passivation” of the QD surface), and surface chemistry for QDs in organic solvents (improvement of defects, surface passivation, structural changes in nanocrystal lattice).
Studies of toxicity of QDs are in an early phase [7]. It is assumed that QDs exposed to air or ultraviolet radiation release Cd2+ and Se2+ ions that may generate oxidative stress in living cells [30,139]. Some photoeffects occurring in semiconductor nanocrystals can be learned from the investigations of solar cells. Absorption of light leads to electron transfer reactions at QD-liquid interface [140] or at mesoscopic CdSe–TiO2 films [141]. Semiconductor QDs under exposure to ultraviolet radiation develop excitons (e− and h+ pair) [11]. Charge transfer between QDs and neighbouring molecules will compete with radiative and non-radiative decay, and energy transfer [142]. Formation of electron-hole pair occurs during photocatalyst action in the sunlight of TiO2 in the form of mineral anatase, which possess energy bandgap of around 3 eV (around 400 nm) [143]. Generated •OH reacts with adsorbed water or organic molecules: even saturated hydrocarbons are known to be oxidized into CO2 and H2O [143]. Photoexcited generation of and/or •OH in aqueous solutions and photoactivation of cytochrome P450BSβ adsorbed on the surface of QD/P450BSβ nanohybrids was observed [140]. Redox-specific labelling of cells using QD-dopamine conjugates have been demonstrated in cells expressing human D2 dopamine receptors: upon ultraviolet irradiation, oxidation of dopamine was observed [123]. Recently anodic electrochemiluminescence of quantum dots [144] and photoelectro-chemical detection of superoxide radicals by quantum dots has been described [145]. Increased generation of nitric oxide during photolysis of water was increased when CdSe/ZnS quantum dots were present [146]. These are evidences so far for electron and/or energy transfer from QDs to nearby (bio)molecules capable of generating singlet oxygen and other radical species.
4. Quantum dots and nanoparticles as radiosensitizers for cancer therapy
4.1. Principles of radiotherapy
One of the major obstacles in curing cancer with cytostatics is the development of drug resistance. Radiation treatment of both glioma and prostate cancer requires high accuracy in delivering ionizing radiation to minimize toxicity to normal surrounding tissues.
Since its first reported medical use in 1896 radiotherapy is still the most common and efficient cancer treatment [147]. Principle of radiotherapy of cancer is its irradiation with high-energy radiation to destroy malignant cells in a treated volume. Radiation may act directly on a target by ionizing or exciting it and initiating chain of events leading to biological changes. Indirect action is when radiation interacts with other atoms or molecules to produce free radicals that diffuse and damage critical cellular targets [147]. There are two types of ionizing radiation, which usage depends on disease and its location: high-energy electromagnetic waves (X-rays and gamma rays) that ionize cellular compartments or water molecules and release high-speed electrons from the atoms, and particle radiation consisting of alpha or beta particles, electrons, neutrons or protons acting mainly directly on a target [148]. Neutrons being uncharged particles are highly penetrating compared to charged particles. Since water constitutes around 75–90% in biological systems, ionizing radiation cause damage to biomolecules mainly through radiolysis of water. Interaction of radiation with water knocks out an electron e− and produces a series of free or ion radicals such as hydroxyl OH•, hydrogen H •, water H2O+, H3O+, superoxide . Regardless of radiation source (linear accelerator, cobalt-60, cesium-137, iridium-192), the ionizing radiation damages a number of intracellular components with DNA being a primary target of these generated radicals [149]. Hydroxyl radical is considered to be the most reactive one inducing lipid peroxidation [150], and its properties have been well documented by radiation chemists [151]. In recent years damage to cellular membrane resulting from production of reactive oxygen species by ionizing radiation has been recognised to mediate apoptosis [152]. Gamma radiation induces oxidative damage to phosphatidylcholine liposomes and changes permeability of the lipid bilayer [153]. In spite of being carcinogenic, low-dose radiation is widely used in diagnostic radiology. However, number of computed tomography screenings is exponentially increasing for the last two decades, so benefit/risk balance of diagnostic X-rays and possible creation of future public health problems has been questioned recently [154].
Despite advances in neurosurgery and radiotherapy the prognosis for patients with malignant gliomas has changed little for the last decades [155]. Management of prostate cancer has progressed during the last decade due to improving radiation dose delivery and technological advancement from two-dimensional to three-dimensional imaging [156]. Evolution of radiation therapy techniques has added to improved survival rates in lung cancer, which is the second most commonly diagnosed cancer in the United States [157]. However, radiotherapy requires continuous advancements in chemotherapeutic targeting and radiation delivery technology. Treatment of lung cancer has a need of compensation for respiratory motion to minimize radiation dose to healthy lung tissues. Both glioma and prostate cancer require high accuracy in delivering ionizing radiation to minimize damage to surrounding tissues.
4.2. Radiosensitization by quantum dots and nanoparticles
Radiation sensitizers are drugs that enhance the effects of single or fractionated radiation therapy. There is considerably increasing interest in effective dose enhancement of ionizing radiation by particles [158]. Metallic nanoparticles can be used as adjuncts to radiotherapy. Proliferative, cytostatic and cytolytic activities of gamma radiation in combination with metallic nanoparticles in cancer cells are an emerging field. Tightly packed nanoparticles produced of metals should provide enhanced interaction cross-section with gamma photons. Thus, metallic nanoparticles may increase therapeutic efficiency of radiotherapy by selectively scattering and/or absorbing X-rays and gamma rays causing localized damage to DNA and other targeted organelles of cancer cells and thus decreasing total radiation dose to minimize side effects of ionizing radiation on cancer patients [19,20]. Dose enhancement is believed to occur due to transport of photoelectrons scattered on these heavy metals [158]. This approach of using nanomaterial radiosensitizers (NMRS) is termed Nanoparticle Enhanced X-ray Therapy (NEXT) [159,160].
Potential candidates could be different quantum dots and nanoparticles (though most of them not tested yet with ionizing radiation and listed as possible candidates): CaF [161], LaF [162], ZnS [163] or ZnO [164] quantum dots, carbon dots [55], liposomes loaded with hypoxic radiosensitizer ethanidazole [165,166] or paclitaxel [166,167], DNA cross-linker cisplatin [168], nanoparticle-photosensitizer conjugates [2], silicon nanoparticles [52], liposomes loaded with antioxidants such as catechines [169,170], theanines [171,172] and curcumin [173,174], liposomes with entrapped radiosensitizers such as porphyrins [175–177].
Polyphenol extracts from tea represent a special interest due to their dual behaviour: being strong antioxidants [150] these polyphenols exhibit also prooxidant activity in chemosensitization and radiosensitization [178].
Recently some research has been focused in developing photosensitizing quantum dots [17]. Such light-activated eradication of tumours is also based on production of radicals upon absorption of visible light by such chromophore (quantum dot). In spite of the fact that this type of electromagnetic radiation, unlike gamma rays, is harmless, such photosensitization approach is suitable to treat only superficial tumours due to limited penetration of visible light. X-rays and gamma rays penetrate much deeper thus offering a big advantage in treating patients with nodular or deep tumours. Due to high atom and electron density metallic nanoparticles should act as enhanced scatters and/or absorbers of X-rays and gamma radiation [19,20] acting thus as radiosensitizers and causing localized and targeted damage to cancer cells. Such concept of using chemicals and radiation to damage DNA is Auger therapy or photon activated therapy (PAT) [20], where so-called Auger-electron emitters usually iodine (I-123 or I-125) complexes [179,180], are embedded in DNA to yield electrons and radicals around the DNA [181]. Lately, radiosensitization of DNA by gold nanoparticles of the size 1–12 nm bombarded with high-energy electrons resulted in generation of low-energy electrons by the gold nanoparticles, which induced increased DNA damage [182]. In another study, gold microspheres of the size 1.5–3.0 μm have been studied for selective dose enhancement of X-rays: in the presence of 1% gold particles dose enhancement factors ranged at about 1.36–1.54 at survival rate 10% [158]. In this work, preliminary attempts in tumour model resulted in modest radiosensitization by gold particles.
4.3. Radioprotection by nanoparticles
Radiolysis of water generates a series of its radical decomposition products that inactivate enzymes and damage cellular lipids, proteins and DNA. Postirradiation protection is another approach to reduce or reverse deleterious effects after exposure to ionizing radiation [174]. After World War II there was a great interest in developing chemicals, so-called radioprotectors, to protect humans from harmful effects of radiation. Already in 1949 it was shown that rats pretreated with the amino acid cysteine were protected against lethal doses of X-rays [183]. Later it has been found that supplementation with antioxidants [184], selenium compounds [185] and a cytoprotective adjuvant amifostine [186,187] alters cell radiosensitivity.
Recent studies also show radioprotective effect of various nanoparticles. Amifostine is not effective when given orally. However, amifostine containing copolymer nanoparticles, prepared using spray drying technique, have been shown to protect mice against injury induced by whole body gamma irradiation after oral administration of the nanoparticles [188]. When encapsulated in transferrin-coupled liposomes neuroprotective agent citicoline exhibits radioprotective effect in human ovarian adenocarcinoma cells overexpressing transferrin receptor. Such effect is considerably smaller in endothelial cells. However, free citicoline was found to be less radioprotective in the ovarian adenocarcinoma cells than in the endothelial cells [189]. Carbon nanoparticles also exhibit radioprotective effect. Fullerenes (C60) diminished toxicity of radiation on zebra fish embryos by reducing generation of reactive oxygen species [190]. Fulleronols (C60[OH]X) protected unicellular eukaryotes organisms against gamma radiation [191]. Cerium oxide nanoparticles (nanoceria) increased longevity of cells by reducing hydrogen peroxide and ultraviolet radiation induced injury [192]. Autoregenerative reaction cycle Ce3+ ⇔ Ce4+ occurs on the surface of the ceria nanoparticles: changing oxidation state from Ce3+ to Ce4+ might scavenge free radicals produced by radiation [192].
4.4. Conversion of high-energy radiation rays into visible light photons
A new concept of scintillation detectors is an emerging example of interaction of ionizing radiation with quantum dots. Several metal chalcogenides such as ZnS, CdSe/ZnS are known to be highly efficient scintillators. The combination of sol–gel technique with inorganic nanocrystalline quantum dots will be a potential method in preparing novel scintillating devices [193]. It is expected that nanosized quantum dots will provide more electron-hole pairs, and simultaneously emitted visible light photons, per absorbed incident gamma photon compared to that of inorganic crystals currently used in scintillation detectors [194]. However, scintillation mechanisms of quantum dots are not yet completely understood. Advantage of using quantum dots as detectors of ionizing radiation is the possibility of tuning their bandgap according to the size of quantum dots. Solubility of quantum dots in both polar and non-polar solvents can also be controlled. So far, such scintillation conversion of high-energy radiation into visible light by quantum dots has been reported for gamma photons [194], alpha particles [193,195] and short wavelength ultraviolet [196,197].
4.5. Nanoparticle-based X-ray-induced photodynamic therapy
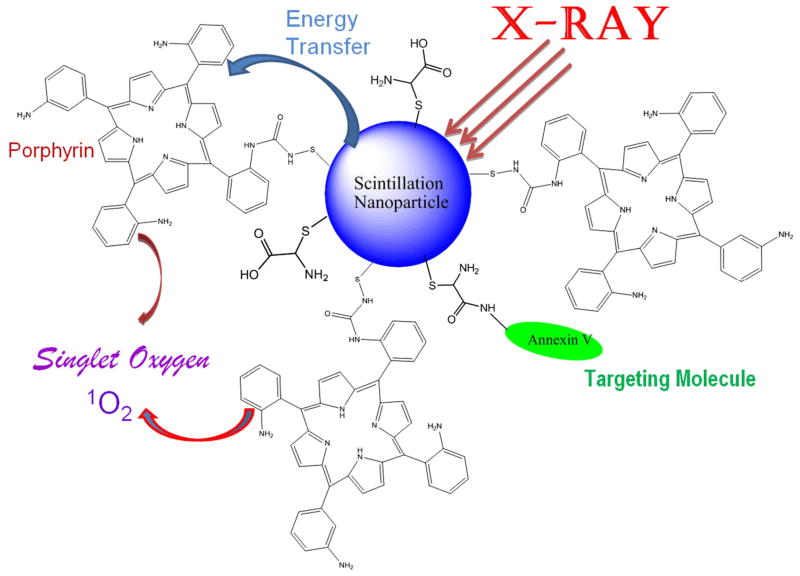
Light delivery is still a challenging issue for deep cancer treatment by PDT. Only near infrared light in the range of 700–1100 nm can penetrate deep into the tissue because most tissue chromophores, including oxyhemoglobin, deoxyhemoglobin, melanin, and fat, absorb weakly in the near infrared window [198]. Unfortunately, most available photosensitizers have absorption bands at wavelengths shorter than 700 nm. Porphyrins exhibit weak absorption maxima around 630 nm, and chlorines and bacteriochlorins have strong absorption maxima at around 650 and 710 nm, respectively.
All porphyrin-derived PDT compounds have a strong absorption band near 400 nm called the Soret band [199]. This means that the excitation in the UV-blue band is much more efficient for photodynamic therapy. Unfortunately, attempting to activate PDT through absorption at the Soret band is not practical because blue light has minimal penetration into tissue and even weaker satellite absorption peaks (Q-bands) between 600 nm and 800 nm, which are used for the treatment. To solve this problem, a novel approach to deep cancer treatment has been proposed by the combination of radiotherapy and PDT employing scintillating nanoparticles [200]. In this new concept of cancer treatment, photosensitizers such as porphyrins are conjugated to X-ray luminescent nanoparticles. When the nanoparticle-photosensitizer conjugates are targeted to the tumour and stimulated by X-rays during radiotherapy, the particles will generate light to activate the photosensitizers for PDT (Fig. 5). Therefore, the radiation and photodynamic therapies are combined and occur simultaneously, and the tumour destruction will be more efficient. More importantly, it can be used for deep tumour treatment as X-rays penetrate deep into the body, as required for prostate cancer. The advantages of the new PDT-radiation therapy are: 1) two effective treatments (radiation therapy and PDT) are combined; 2) the modality is capable and efficient for deep cancer treatment; 3) the risk of radiation damage to healthy tissue is lower; and 4) the treatment is simple, relatively inexpensive and efficient.
Fig. 5.
Schematic presentation of the nanoparticle-based X-ray-induced PDT. Under ionizing radiation a nanoparticle starts to scintillate transferring its energy into a conjugated porphyrin molecule, which then generates singlet oxygen necessary to produce photosensitizing effect. This methodology will help to treat nodular and deeper tumours due to higher penetrating capacity of X-rays and gamma rays compared to that of visible light commonly used in PDT.
To serve as PDT agents, nanoparticles selected for photosensitizer enhancement must meet the following requirements:
The nanoparticle emission spectrum must match the photosensitizer’s absorption spectrum. Matching the nanoparticle emission band with the absorption wavelength of the photosensitizer guarantees efficient activation of the photosensitizers and production of singlet oxygen. For example, if Photofrin is used, the nanoparticle must emit at approximately 400 nm to match the absorption band of Photofrin.
The nanoparticles must have high luminescence efficiency, i.e., excitation by an energy source should result in strong emission by the nanoparticle. For example, if a scintillation nanoparticle is used, luminescence is produced as a result of excitation by radiation (scintillation luminescence), and the nanoparticle should emit strongly if excited by X-rays and other forms of radiation.
The nanoparticles must be easily attached to or linked with photosensitizers.
The nanoparticles must be non-toxic, water soluble, and stable in biological environments.
Matching the nanoparticle’s emission spectrum to the photosensitizer’s absorption spectrum is easily achieved because a nanoparticle’s emission spectrum is dependent on the diameter of the nanoparticle. Thus, to meet the first requirement, the nanoparticles used are selected according to a size that provides a matching emission spectrum. Due to the greater electron-hole overlap, nanoparticles have greater luminescence quantum efficiency than conventional fluorophores and phosphors. This increased level of quantum efficiency yields greater oscillator strength and, as a consequence, enhanced luminescence efficiency. For this reason, nanoparticles satisfy the requirement for strong luminescence efficiency. Finally, satisfying the requirement for biocompatibility necessitates selecting nanoparticles of an appropriate composition or modifying the nanoparticles with coatings or attachments that enhance biocompatibility.
The above concept has been investigated by Chen et al. [201]. In these pilot studies, the authors have demonstrated that scintillation nanoparticles like LaF3:Tb3+ can be used to activate photosensitizers to generate singlet oxygen for PDT [162]. The energy transfer from LaF3: Tb3+ nanoparticles to meso-tetra(4-carboxyphenyl) porphine (MTCP) was measured by fluorescence quenching technique and lifetime changes. The transfer efficiency is typically measured using the relative fluorescence intensity of the donor (scintillation nanoparticles), in the absence (FD) and presence (FDA) of acceptor (photosensitizers):
The transfer efficiencies are estimated to be approximately 68, 52, and 50% for the emission bands at 542, 584 and 621 nm, respectively. This leads to an average transfer efficiency of 56.7% in the LaF3:Tb3+-MTCP conjugate system [162].
As shown in Fig. 6, a small amount of singlet oxygen was generated from MTCP by X-ray irradiation, while the singlet oxygen generation increased rapidly when MTCP was conjugated with LaF3:Tb3+ nanoparticles [162]. This phenomenon can be attributed to the low stopping power of MTCP to X-rays. LaF3:Tb3+ can be excited by X-rays to generate green emissions, where the MTCP absorbs strongly. Therefore, when MTCP is conjugated with LaF3:Tb3+ particles, it can be excited by X-rays to generate singlet oxygen due to the excitation by the blue-green emission light from the LaF3:Tb3+ nanoparticles. The luminescence of anthracenedipropionic acid (ADPA) started to be quenched at the X-ray dose of 0.22 Gy (0.5 min), indicating the generation of singlet oxygen. At the dose of 13.2 Gy (30 min), the luminescence was quenched almost 100%. This manifests that the generation of singlet oxygen by X-ray irradiation in the nanoparticle-MTCP system is efficient and the preliminary results are intriguing.
Fig. 6.
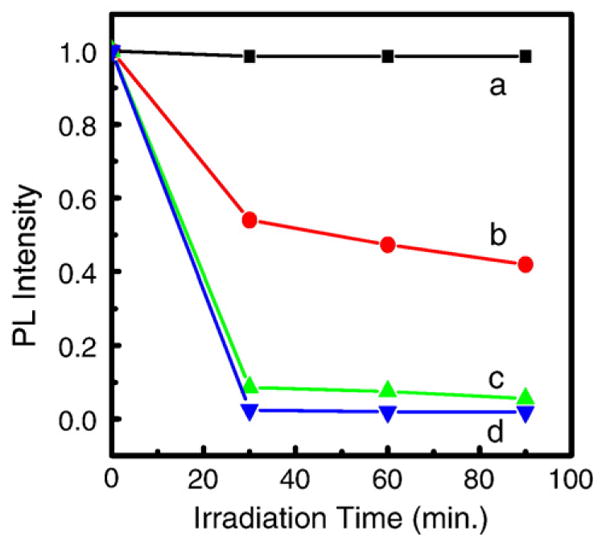
Quenching of ADPA photoluminescence (PL) with X-ray irradiation in water (a), ADPA in MTCP solution (b), ADPA in LaF3:Tb3+-MTCP conjugate (c) and ADPA in LaF3:Tb3+-MTCP-folic acid conjugate (d). Reprinted with permission from Ref. [162] © 2008 American Institute of Physics.
Chen et al. are trying to improve the nanoparticle-photosensitizer conjugates for practical application by: 1) improving the luminescence efficiency by core–shell structures; 2) improving the energy transfer rate by optimizing the spectral match between the nanoparticles and the photosensitizers; and 3) improving the biocompatibility by coating with ZnO and CaF2. For example, the average energy transfer rate in the LaF3:Tb3+-MTCP conjugates is 56.7% [162] while for ZnO-MTAP (meso-(o-amino-phenyl) porphine) conjugates the energy transfer is around 89% [164]. The dark toxicity and phototoxicity of ZnO-MTCAP conjugates in comparison with pure ZnO nanoparticles and MTCP using ovarian tumour cells are shown in Fig. 7 [164]. No toxicity was observed from pure ZnO nanoparticles either in dark or stimulated by the 365 nm UV light. However, phototoxicity was observed in ZnO-MTCP conjugates and their phototoxicity stimulated at 365 nm is much intense that MTCP alone. This could be attributed to the strong absorption of ZnO nanoparticles at 365 nm and the efficient energy transfer to the porphyrin [164].
Fig. 7.
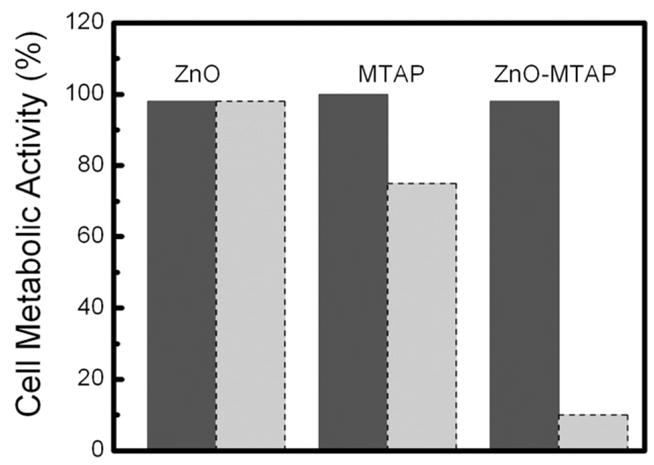
Cytotoxicity (left bars for each sample) and phototoxicity (right bars for each sample) of 3×10−5 M ZnO, 3×10−5 M MTCP and 3×10−5 M ZnO-MTCP on NIH-OVCAR-3 cells. Reprinted with permission from Ref. [164] © 2008 American Institute of Physics.
4.6. Nanoparticles as hypoxic radiosensitizers
Radiotherapy not always eradicates cancer cells completely due to the presence of resistant hypoxic cells. This phenomenon known as radioresistance [202] was already reported almost 100 years ago [203]. The level at which hypoxia causes radioresistance is less than 0.5% pO2 [203], a value similar to that reported as critical in PDTof cancer cells [204]. Synergistic effect of the combination of gamma radiation together with paclitaxel and etanidazole, which are known hypoxic radiosensitizers, has been reported [166]. This area has emerged as an interesting approach to treat hypoxic tumour cells by specific modification of tumour radiosensitivity. The mechanism(s) by which etanidazole acts as a chemo- and radiosensitizer are not known. The most important effects may involve interaction of its metabolites with the alkylating agent in the vicinity of DNA (see references therein [205]).
Nanoparticles could be also exploited to challenge radioresistance since it could be expected that recoil energy occurring during interaction of such nanoparticle with a gamma photon would transfer energy or electrons into neighbouring biomolecules and damage them without presence of oxygen, i.e. without generation of oxygen radicals. To deliver large drug loads intracellularly, liposomes loaded with etanidazole can be exploited [165].
4.7. Radiosensitization with nanoparticles loaded with antioxidants
Antioxidants act as free radical scavengers and thus protect from damage to DNA and other organelles. Biological activities of antioxidants include antithrombotic, vasodilatory and anticarcinogenic effects, as well as anti-inflammatory, antiallergic, antiulcer, antibacterial and antiviral properties [206]. Flavonoids are effective antioxidants from plants and protect cells from free radicals and lipid peroxidation because of their radical scavenging and metal ion chelating properties [150]. In addition to flavonoids, amino acid theanine present in green tea is also reported to exhibit biological activity, including anticarcinogenic effects [172].
Potential anticarcinogenic, anti-inflammatory and reduction in radiation cytotoxicity effects of antioxidants have been of interest for the last 50 years. Antioxidants may prevent radiation toxicity by reducing direct effects, and minimizing indirect effect such as long-term oxidative damage. Molecules that are not primarily antioxidants can also act as radiation protectors by modify cell proliferation and signalling events through altering activity of growth factors, cytokines and DNA repair [207]. Both radiation enhancing and protecting effects have been reported in the literature. Combined effect of X-radiation with antioxidant epigallocatechin from green tea extract to enhance apoptosis of cancer cells has been reported [169]. On the other hand, another study shows protection of DNA damage from gamma radiation by epicatechin [170]. Theanine has been also shown to enhance antitumour activity of chemotherapeutic agents [171]. Anthocyanin-rich extracts have a protective role in colon carcinogenesis with multiple mechanisms of action [208].
Therefore, it remains of great interest to study whether for example supplementation with antioxidants might be beneficial during cancer therapy or not. Polyphenols show wide variability in their bioavailability [209]. A direct and target delivery is desirable, since it would allow the treatment of local pathologies without significant systemic side effects. In addition, antioxidants might degrade, become hazardous by oxidation reactions and change their original physical properties. Polyphenols have been entrapped in particulate drug carriers such as nanospheres, nanocapsules or liposomes to modify their biodistribution and target them to the site of action [210]. Nanoparticles with encapsulated catechins have been produced by spray drying to improve their bioavailability and resistance to oxygen and light [211]. Previous works have reported that a greater amount of catechins was delivered into solid tumour by liposomes than by its aqueous solution [212]. Drug release rate and vesicle size of liposomes may influence drug deposition in tumour tissues. Liposome-encapsulated antioxidants (α-tocopherol, L-ascorbic acid and their mixture) have been demonstrated in vivo to prevent oxidative attack during cerebral ischemia and reperfusion [213].
5. Conclusions
Up-to-date literature supports the possibility of using QDs for light induced photooxidation via both Type II mechanism (generation of singlet oxygen) and Type I mechanism (generation of other reactive oxygen species and radicals) [214,215]. However, this is only true for solutions and cells in vitro. It is therefore of further interest to select QDs that generate appropriate radicals and target fatal cellular sites, and to apply such selected candidates for photodynamic cancer therapy in systems in vivo.
Gold nanoparticles with silica shell have recently been described as biocompatible contrast agents possessing increased absorption of X-rays [216]. Metallic nanoparticles may increase therapeutic efficiency of radiotherapy by such selective scattering and/or absorbtion of ionizing radiation and thus allowing to decrease radiation dose and minimize side effects. Nanoparticles containing antioxidants have also been shown as effective adjuvants for radiotherapy. Scintillating nanoparticles are being investigated as light source for PDT for deep cancer treatment. Furthermore, radioprotection by nanoparticles to reduce or reverse deleterious effects of ionizing radiation also warrants further investigations.
Acknowledgments
P. Juzenas is grateful for the funding received from the Research Council of Norway (researcher grant for P.J. and fellowship for R.G., project 182058) and the Norwegian Radium Hospital Research Foundation (fellowship for N.G., project FU0802). W. Chen would like to thank the support from the Startup and LERR Funds from UTA, the NSF and DHS joint program (CBET-0736172), DOD HDTRA1-08-P-0034 and the DOD Congressionally Directed Medical Research Programs (W81XWH-08-1-0450). Y.-P. Sun would like to acknowledge the support by the U.S. Department of Defense Breast Cancer Research Program and the National Institute of Health. M. Coelho is grateful for the funding received from FCT (project PTDC/BIO/69359/2006) and EU project (Nanocapsules with Functionalized Surfaces and Walls), and to Prof. H. Möhwald (Max-Planck Institute of Colloids and Interfaces) for scientific discussions.
Footnotes
Other uses, including reproduction and distribution, or selling or licensing copies, or posting to personal, institutional or third party websites are prohibited.
In most cases authors are permitted to post their version of the article (e.g. in Word or Tex form) to their personal website or institutional repository. Authors requiring further information regarding Elsevier’s archiving and manuscript policies are encouraged to visit: http://www.elsevier.com/copyright
This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution and sharing with colleagues.
References
- 1.Samia AC, Dayal S, Burda C. Quantum dot-based energy transfer: perspectives and potential for applications in photodynamic therapy. Photochem Photobiol. 2006;82:617–625. doi: 10.1562/2005-05-11-IR-525. [DOI] [PubMed] [Google Scholar]
- 2.Chen W. Nanoparticle fluorescence based technology for biological applications. J Nanosci Nanotechnol. 2008;8:1019–1051. doi: 10.1166/jnn.2008.301. [DOI] [PubMed] [Google Scholar]
- 3.Hardman R. A toxicologic review of quantum dots: toxicity depends on physicochemical and environmental factors. Environ Health Perspect. 2006;114:165–172. doi: 10.1289/ehp.8284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jovin TM. Quantum dots finally come of age. Nat Biotechnol. 2003;21:32–33. doi: 10.1038/nbt0103-32. [DOI] [PubMed] [Google Scholar]
- 5.Smith AM, Gao X, Nie S. Quantum dot nanocrystals for in vivo molecular and cellular imaging. Photochem Photobiol. 2004;80:377–385. doi: 10.1562/0031-8655(2004)080<0377:QDNFIV>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 6.Ferrari M. Cancer nanotechnology: opportunities and challenges. Nat Rev, Cancer. 2005;5:161–171. doi: 10.1038/nrc1566. [DOI] [PubMed] [Google Scholar]
- 7.Smith AM, Duan H, Mohs AM, Nie S. Bioconjugated quantum dots for in vivo molecular and cellular imaging. Adv Drug Deliv Rev. 2008;60:1226–1240. doi: 10.1016/j.addr.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kippeny T, Swafford LA, Rosenthal SJ. Semiconductor nanocrystals: a powerful visual aid for introducing the particle in a box. J Chem Educ. 2002;79:1094–1100. [Google Scholar]
- 9.Yu WW, Peng X. Formation of high-quality CdS and other II–VI semiconductor nanocrystals in noncoordinating solvents: tunable reactivity of monomers. Angew Chem Int, Ed Engl. 2002;41:2368–2371. doi: 10.1002/1521-3773(20020703)41:13<2368::AID-ANIE2368>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 10.Boatman EM, Lisensky GC. A safer, easier, faster synthesis for CdSe quantum dot nanocrystals. J Chem Educ. 2005;82:1697–1699. [Google Scholar]
- 11.Hollingsworth JA, Klimov VI. Soft chemical synthesis and manipulation of semiconductor nanocrystals. In: Klimov VI, editor. Semiconductor and Metal Nanocrystals: Synthesis and Electronic and Optical Properties. Marcel Dekker; New York: 2004. pp. 1–64. Basel. [Google Scholar]
- 12.Jaiswal JK, Mattoussi H, Mauro JM, Simon SM. Long-term multiple color imaging of live cells using quantum dot bioconjugates. Nat Biotechnol. 2003;21:47–51. doi: 10.1038/nbt767. [DOI] [PubMed] [Google Scholar]
- 13.Chen GY, Yao SQ. Lighting up cancer cells with “dots”. Lancet. 2004;364:2001–2003. doi: 10.1016/S0140-6736(04)17527-2. [DOI] [PubMed] [Google Scholar]
- 14.Gao X, Cui Y, Levenson RM, Chung LW, Nie S. In vivo cancer targeting and imaging with semiconductor quantum dots. Nat Biotechnol. 2004;22:969–976. doi: 10.1038/nbt994. [DOI] [PubMed] [Google Scholar]
- 15.Chen W, Sun XK. Luminescent nanoparticles for biological applications: Imaging, therapy, and targeting strategies. In: Chen X, editor. Recent Advances of Bioconjugation Chemistry in Molecular Imaging. Research Signpost; Kerala: (in press) [Google Scholar]
- 16.Doose S. Optical amplification from single excitons in colloidal quantum dots. Small. 2007;3:1856–1858. doi: 10.1002/smll.200700478. [DOI] [PubMed] [Google Scholar]
- 17.Bakalova R, Ohba H, Zhelev Z, Ishikawa M, Baba Y. Quantum dots as photosensitizers? Nat Biotechnol. 2004;22:1360–1361. doi: 10.1038/nbt1104-1360. [DOI] [PubMed] [Google Scholar]
- 18.Samia AC, Chen X, Burda C. Semiconductor quantum dots for photodynamic therapy. J Am Chem Soc. 2003;125:15736–15737. doi: 10.1021/ja0386905. [DOI] [PubMed] [Google Scholar]
- 19.Park YS, Liz-Marzan LM, Kasuya A, Kobayashi Y, Nagao D, Konno M, Mamykin S, Dmytruk A, Takeda M, Ohuchi N. X-ray absorption of gold nanoparticles with thin silica shell. J Nanosci Nanotechnol. 2006;6:3503–3506. [PubMed] [Google Scholar]
- 20.Carter JD, Cheng NN, Qu Y, Suarez GD, Guo T. Nanoscale energy deposition by X-ray absorbing nanostructures. J Phys Chem, B. 2007;111:11622–11625. doi: 10.1021/jp075253u. [DOI] [PubMed] [Google Scholar]
- 21.Bawendi MG, Wilson WL, Rothberg L, Carroll PJ, Jedju TM, Steigerwald ML, Brus LE. Electronic structure and photoexcited-carrier dynamics in nanometer-size CdSe clusters. Phys Rev Lett. 1990;65:1623–1626. doi: 10.1103/PhysRevLett.65.1623. [DOI] [PubMed] [Google Scholar]
- 22.Norris DJ. Electronic structurein semiconductor nanocrystals. In: Klimov VI, editor. Semiconductor and Metal Nanocrystals: Synthesis and Electronic and Optical Properties. Marcel Dekker; New York: 2004. pp. 65–102. Basel. [Google Scholar]
- 23.Lakowicz JR. Introduction to fluorescence, principles of fluorescence spectroscopy. Springer Science+Business Media, LLC; New York: 2006. pp. 1–26. [Google Scholar]
- 24.Juzeniene A, Nielsen KP, Moan J. Biophysical aspects of photodynamic therapy. J Environ Pathol Toxicol Oncol. 2006;25:7–28. doi: 10.1615/jenvironpatholtoxicoloncol.v25.i1-2.20. [DOI] [PubMed] [Google Scholar]
- 25.Bawendi MG, Carroll PJ, Wilson WL, Brus LE. Luminescence properties of CdSe quantum crystallites: resonance between interior and surface localized states. J Chem Phys. 1992;96:946–954. [Google Scholar]
- 26.Nirmal M, Norris DJ, Kuno M, Bawendi MG, Efros AL, Rosen M. Observation of the “dark exciton” in CdSe quantum dots. Phys Rev Lett. 1995;75:3728–3731. doi: 10.1103/PhysRevLett.75.3728. [DOI] [PubMed] [Google Scholar]
- 27.Schweitzer C, Schmidt R. Physical mechanisms of generation and deactivation of singlet oxygen. Chem Rev. 2003;103:1685–1757. doi: 10.1021/cr010371d. [DOI] [PubMed] [Google Scholar]
- 28.Krasnovsky AA., Jr Primary mechanisms of photoactivation of molecular oxygen. History of development and the modern status of research. Biochemistry (Mosc) 2007;72:1065–1080. doi: 10.1134/s0006297907100057. [DOI] [PubMed] [Google Scholar]
- 29.Maysinger D, Lovric J. Quantum dots and other fluorescent nanoparticles: quo vadis in the cell? Adv Exp Med Biol. 2007;620:156–167. doi: 10.1007/978-0-387-76713-0_12. [DOI] [PubMed] [Google Scholar]
- 30.Tsay JM, Michalet X. New light on quantum dot cytotoxicity. Chem Biol. 2005;12:1159–1161. doi: 10.1016/j.chembiol.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 31.Lovric J, Bazzi HS, Cuie Y, Fortin GR, Winnik FM, Maysinger D. Differences in subcellular distribution and toxicity of green and red emitting CdTe quantum dots. J Mol Med. 2005;83:377–385. doi: 10.1007/s00109-004-0629-x. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Y, Chen W, Zhang J, Liu J, Chen G, Pope C. In vitro and in vivo toxicity of CdTe nanoparticles. J Nanosci Nanotechnol. 2007;7:497–503. doi: 10.1166/jnn.2007.125. [DOI] [PubMed] [Google Scholar]
- 33.Lovric J, Cho SJ, Winnik FM, Maysinger D. Unmodified cadmium telluride quantum dots induce reactive oxygen species formation leading to multiple organelle damage and cell death. Chem Biol. 2005;12:1227–1234. doi: 10.1016/j.chembiol.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 34.Chatterjee DK, Fong LS, Zhang Y. Nanoparticles in photodynamic therapy: an emerging paradigm. Adv Drug Deliv Rev. 2008;60:1627–1638. doi: 10.1016/j.addr.2008.08.003. (in press) [DOI] [PubMed] [Google Scholar]
- 35.Lewinski N, Colvin V, Drezek R. Cytotoxicity of nanoparticles. Small. 2008;4:26–49. doi: 10.1002/smll.200700595. [DOI] [PubMed] [Google Scholar]
- 36.Lee JW, Cho K, Kim H, Kim JH, Park B, Noh T, Kim SH, Kim S. Photoluminescence characteristics of Mn- and Pr-doped ZnS nanoparticles optically annealed with UV illumination. Jpn J Appl Phys. 2005;44:7694–7697. [Google Scholar]
- 37.Dwarakanath S, Bruno JG, Shastry A, Phillips T, John AA, Kumar A, Stephenson LD. Quantum dot-antibody and aptamer conjugates shift fluorescence upon binding bacteria. Biochem Biophys Res Commun. 2004;325:739–743. doi: 10.1016/j.bbrc.2004.10.099. [DOI] [PubMed] [Google Scholar]
- 38.Zhao X, Li H, Lee RJ. Targeted drug delivery via folate receptors. Expert Opin Drug Deliv. 2008;5:309–319. doi: 10.1517/17425247.5.3.309. [DOI] [PubMed] [Google Scholar]
- 39.Ai X, Xu Q, Jones M, Song Q, Ding SY, Ellingson RJ, Himmel M, Rumbles G. Photophysics of (CdSe)ZnS colloidal quantum dots in an aqueous environment stabilized with amino acids and genetically-modified proteins. Photochem Photobiol Sci. 2007;6:1027–1033. doi: 10.1039/b706471c. [DOI] [PubMed] [Google Scholar]
- 40.Chen W, Sammynaiken R, Huang Y. Luminescence enhancement of ZnS:Mn nanoclusters in zeolite. J Appl Phys. 2000;88:5188–5193. [Google Scholar]
- 41.Chen W, Joly AG, Zhang JZ. Up-conversion luminescence of Mn2+ in ZnS:Mn2+ nanoparticles. Phys Rev, B. 2001;64:1–4. doi: 10.1166/jnn.2001.049. 041202. [DOI] [PubMed] [Google Scholar]
- 42.Tsujii N, Kitazawa H, Kido G. Magnetic properties of Mn- and Eu-doped ZnS nanocrystals. J Appl Phys. 2003;93:6957–6959. [Google Scholar]
- 43.Quan Z, Wang Z, Yang P, Lin J, Fang J. Synthesis and characterization of high-quality ZnS, ZnS:Mn2+, and ZnS:Mn2+/ZnS (core/shell) luminescent nanocrystals. Inorg Chem. 2007;46:1354–1360. doi: 10.1021/ic061917n. [DOI] [PubMed] [Google Scholar]
- 44.Chen W, Malm JO, Zwiller V, Huang Y, Liu S, Wallenberg R, Bovin JO, Samuelson L. Energy structure and fluorescence of Eu2+ in ZnS:Eu nanoparticles. Phys Rev B. 2000;61:11021–11024. [Google Scholar]
- 45.Chen W, Malm JO, Zwiller V, Wallenberg R, Bovin JO. Size dependence of Eu2+ fluorescence in ZnS:Eu2+ nanoparticles. J Appl Phys. 2001;89:2671–2675. [Google Scholar]
- 46.Chen W, Joly AG, Malm JO, Bovin JO. Upconversion luminescence of Eu3+ and Mn2+ in ZnS:Mn2+, Eu3+ codoped nanoparticles. J Appl Phys. 2004;95:667–672. [Google Scholar]
- 47.Morgan NY, English S, Chen W, Chernomordik V, Russo A, Smith PD, Gandjbakhche A. Real time in vivo non-invasive optical imaging using near-infrared fluorescent quantum dots. Acad Radiol. 2005;12:313–323. doi: 10.1016/j.acra.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 48.Yin Y, Xu X, Ge X, Lu Y, Zhang Z. Synthesis and characterization of ZnS colloidal particles via gamma-radiation. Radiat Phys Chem. 1999;55:353–356. [Google Scholar]
- 49.Chirvony A, Chyrvonaya A, Ovejero J, Matveeva E, Goller B, Kovalev D, Huygens A, de Witte P. Surfactant-modified hydrophilic nanostructured porous silicon for the photosensitized formation of singlet oxygen in water. Adv Mater. 2007;19:2967–2972. [Google Scholar]
- 50.Canham LT. Porous silicon as a therapeutic biomaterial. Microtech Med Biol. 2000:109–112. [Google Scholar]
- 51.Lee S, Cho WJ. Water-soluble, carboxyl-modified silicon nanoparticles. NSTI-Nanotech. 2005;1:218–221. [Google Scholar]
- 52.Kovalev D, Fujii M. Silicon nanocrystals: photosensitizers for oxygen molecules. Adv Mater. 2005;17:2531–2544. [Google Scholar]
- 53.Parkhutik V, Chirvony V, Matveyeva E. Optical properties of porphyrin molecules immobilized in nanoporous silicon. Biomol Eng. 2007;24:71–73. doi: 10.1016/j.bioeng.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 54.Canham LT, Reeves CL, King DO, Branfield PJ, Crabb JG, Ward MCL. Bioactive polycrystalline silicon. Adv Mater. 1996;8:850–852. [Google Scholar]
- 55.Sun YP, Zhou B, Lin Y, Wang W, Fernando KA, Pathak P, Meziani MJ, Harruff BA, Wang X, Wang H, Luo PG, Yang H, Kose ME, Chen B, Veca LM, Xie SY. Quantum-sized carbon dots for bright and colorful photoluminescence. J Am Chem Soc. 2006;128:7756–7757. doi: 10.1021/ja062677d. [DOI] [PubMed] [Google Scholar]
- 56.Cao L, Wang X, Meziani MJ, Lu F, Wang H, Luo PG, Lin Y, Harruff BA, Veca LM, Murray D, Xie SY, Sun YP. Carbon dots for multiphoton bioimaging. J Am Chem Soc. 2007;129:11318–11319. doi: 10.1021/ja073527l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sirdeshmukh R, Teker K, Panchapakesan B. Functionalization of carbon nanotubes with antibodies for breast cancer detection applications. Proc MEMS, NANO Smart Syst. 2004:48–53. [Google Scholar]
- 58.Teker K, Sirdeshmukh R, Panchapakesan B. Antibody functionalization of carbon nanotubes for breast cancer applications. Proc IEEE Sensors. 2004;2:814–817. [Google Scholar]
- 59.Silver J, Ou W. Photoactivation of quantum dot fluorescence following endocytosis. Nano Lett. 2005;5:1445–1449. doi: 10.1021/nl050808n. [DOI] [PubMed] [Google Scholar]
- 60.Saftig P. Lysosomal membrane proteins. In: Saftig P, editor. Lysosomes, Eurekah, Landes Bioscience. Springer Science and Business Media; New York: 2005. pp. 37–49. Georgetown. [Google Scholar]
- 61.Zhou M, Ghosh I. Quantum dots and peptides: a bright future together. Biopolymers. 2007;88:325–339. doi: 10.1002/bip.20655. [DOI] [PubMed] [Google Scholar]
- 62.Patel LN, Zaro JL, Shen WC. Cell penetrating peptides: intracellular pathways and pharmaceutical perspectives. Pharm Res. 2007;24:1977–1992. doi: 10.1007/s11095-007-9303-7. [DOI] [PubMed] [Google Scholar]
- 63.Zorko M, Langel U. Cell-penetrating peptides: mechanism and kinetics of cargo delivery. Adv Drug Deliv Rev. 2005;57:529–545. doi: 10.1016/j.addr.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 64.Medintz IL, Clapp AR, Brunel FM, Tiefenbrunn T, Uyeda HT, Chang EL, Deschamps JR, Dawson PE, Mattoussi H. Proteolytic activity monitored by fluorescence resonance energy transfer through quantum-dot-peptide conjugates. Nat Mater. 2006;5:581–589. doi: 10.1038/nmat1676. [DOI] [PubMed] [Google Scholar]
- 65.Delehanty JB, Medintz IL, Pons T, Brunel FM, Dawson PE, Mattoussi H. Self-assembled quantum dot-peptide bioconjugates for selective intracellular delivery. Bioconjug Chem. 2006;17:920–927. doi: 10.1021/bc060044i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bharali DJ, Lucey DW, Jayakumar H, Pudavar HE, Prasad PN. Folate-receptor-mediated delivery of InP quantum dots for bioimaging using confocal and two-photon microscopy. J Am Chem Soc. 2005;127:11364–11371. doi: 10.1021/ja051455x. [DOI] [PubMed] [Google Scholar]
- 67.Schroeder JE, Shweky I, Shmeeda H, Banin U, Gabizon A. Folate-mediated tumor cell uptake of quantum dots entrapped in lipid nanoparticles. J Control Release. 2007;124:28–34. doi: 10.1016/j.jconrel.2007.08.028. [DOI] [PubMed] [Google Scholar]
- 68.Juzenas P, Generalov R, Juzeniene A, Moan J. Generation of nitrogen oxide and oxygen radicals by quantum dots. J Biomed Nanotech. (in press) [Google Scholar]
- 69.Bird SJ, Lloyd JB. Mechanism of lysosome rupture by dipeptides. Cell Biochem Funct. 1995;13:79–83. doi: 10.1002/cbf.290130203. [DOI] [PubMed] [Google Scholar]
- 70.Thomas CA, Springer PA, Loeb GE, Berwald-Netter Y, Okun LM. A miniature microelectrode array to monitor the bioelectric activity of cultured cells. Exp Cell Res. 1972;74:61–66. doi: 10.1016/0014-4827(72)90481-8. [DOI] [PubMed] [Google Scholar]
- 71.Connolly P, Clark P, Curtis AS, Dow JA, Wilkinson CD. An extracellular microelectrode array for monitoring electrogenic cells in culture. Biosens Bioelectron. 1990;5:223–234. doi: 10.1016/0956-5663(90)80011-2. [DOI] [PubMed] [Google Scholar]
- 72.Misfeldt DS, Hamamoto ST, Pitelka DR. Transepithelial transport in cell culture. Proc Natl Acad Sci U S A. 1976;73:1212–1216. doi: 10.1073/pnas.73.4.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cereijido M, Robbins E, Sabatini DD, Stefani E. Cell-to-cell communication in monolayers of epithelioid cells (MDCK) as a function of the age of the monolayer. J Membr Biol. 1984;81:41–48. doi: 10.1007/BF01868808. [DOI] [PubMed] [Google Scholar]
- 74.Wegener J, Keese CR, Giaever I. Electric cell-substrate impedance sensing (ECIS) as a noninvasive means to monitor the kinetics of cell spreading to artificial surfaces. Exp Cell Res. 2000;259:158–166. doi: 10.1006/excr.2000.4919. [DOI] [PubMed] [Google Scholar]
- 75.Giaever I, Keese CR. Monitoring fibroblast behavior in tissue culture with an applied electric field. Proc Natl Acad Sci U S A. 1984;81:3761–3764. doi: 10.1073/pnas.81.12.3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xiao C, Lachance B, Sunahara G, Luong JH. An in-depth analysis of electric cell-substrate impedance sensing to study the attachment and spreading of mammalian cells. Anal Chem. 2002;74:1333–1339. doi: 10.1021/ac011104a. [DOI] [PubMed] [Google Scholar]
- 77.Giaever I, Keese CR. A morphological biosensor for mammalian cells. Nature. 1993;366:591–592. doi: 10.1038/366591a0. [DOI] [PubMed] [Google Scholar]
- 78.Xiao C, Luong JH. Assessment of cytotoxicity by emerging impedance spectroscopy. Toxicol Appl Pharmacol. 2005;206:102–112. doi: 10.1016/j.taap.2004.10.025. [DOI] [PubMed] [Google Scholar]
- 79.Ceriotti L, Ponti J, Colpo P, Sabbioni E, Rossi F. Assessment of cytotoxicity by impedance spectroscopy. Biosens Bioelectron. 2007;22:3057–3063. doi: 10.1016/j.bios.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 80.Keese CR, Bhawe K, Wegener J, Giaever I. Real-time impedance assay to follow the invasive activities of metastatic cells in culture. Biotechniques. 2002;33:842–850. doi: 10.2144/02334rr01. [DOI] [PubMed] [Google Scholar]
- 81.Keese CR, Wegener J, Walker SR, Giaever I. Electrical wound-healing assay for cells in vitro. Proc Natl Acad Sci U S A. 2004;101:1554–1559. doi: 10.1073/pnas.0307588100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.De Blasio BF, Laane M, Walmann T, Giaever I. Combining optical and electrical impedance techniques for quantitative measurement of confluence in MDCK-I cell cultures. Biotechniques. 2004;36:650–662. doi: 10.2144/04364RR01. [DOI] [PubMed] [Google Scholar]
- 83.Male KB, Lachance B, Hrapovic S, Sunahara G, Luong JH. Assessment of cytotoxicity of quantum dots and gold nanoparticles using cell-based impedance spectroscopy. Anal Chem. 2008;80:5487–5493. doi: 10.1021/ac8004555. [DOI] [PubMed] [Google Scholar]
- 84.Wei C, Guomin W, Yujun L, Ruizhe Q. Cancer stem-like cells in human prostate carcinoma cells DU145: the seeds of the cell line? Cancer Biother. 2007;6:763–768. doi: 10.4161/cbt.6.5.3996. [DOI] [PubMed] [Google Scholar]
- 85.Jain KK. Applications of nanobiotechnology in clinical diagnostics. Clin Chem. 2007;53:2002–2009. doi: 10.1373/clinchem.2007.090795. [DOI] [PubMed] [Google Scholar]
- 86.Muller-Borer BJ, Collins MC, Gunst PR, Cascio WE, Kypson AP. Quantum dot labeling of mesenchymal stem cells. J Nanobiotechnology. 2007;5:9. doi: 10.1186/1477-3155-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shah BS, Clark PA, Moioli EK, Stroscio MA, Mao JJ. Labeling of mesenchymal stem cells by bioconjugated quantum dots. Nano Lett. 2007;7:3071–3079. doi: 10.1021/nl071547f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dubertret B, Skourides P, Norris DJ, Noireaux V, Brivanlou AH, Libchaber A. In vivo imaging of quantum dots encapsulated in phospholipid micelles. Science. 2002;298:1759–1762. doi: 10.1126/science.1077194. [DOI] [PubMed] [Google Scholar]
- 89.Carion O, Mahler B, Pons T, Dubertret B. Synthesis, encapsulation, purification and coupling of single quantum dots in phospholipid micelles for their use in cellular and in vivo imaging. Nat Protoc. 2007;2:2383–2390. doi: 10.1038/nprot.2007.351. [DOI] [PubMed] [Google Scholar]
- 90.Dif A, Henry E, Artzner F, Baudy-Floc’h M, Schmutz M, Dahan M, Marchi-Artzner V. Interaction between water-soluble peptidic CdSe/ZnS nanocrystals and membranes: formation of hybrid vesicles and condensed lamellar phases. J Am Chem Soc. 2008;130:8289–8296. doi: 10.1021/ja711378g. [DOI] [PubMed] [Google Scholar]
- 91.Yao G, Wang L, Wu Y, Smith J, Xu J, Zhao W, Lee E, Tan W. FloDots: luminescent nanoparticles. Anal Bioanal Chem. 2006;385:518–524. doi: 10.1007/s00216-006-0452-z. [DOI] [PubMed] [Google Scholar]
- 92.Chatterjee DK, Yong Z. Upconverting nanoparticles as nanotransducers for photodynamic therapy in cancer cells. Nanomed. 2008;3:73–82. doi: 10.2217/17435889.3.1.73. [DOI] [PubMed] [Google Scholar]
- 93.So MK, Xu C, Loening AM, Gambhir SS, Rao J. Self-illuminating quantum dot conjugates for in vivo imaging. Nat Biotechnol. 2006;24:339–343. doi: 10.1038/nbt1188. [DOI] [PubMed] [Google Scholar]
- 94.Zhang Y, So MK, Loening AM, Yao H, Gambhir SS, Rao J. HaloTag protein-mediated site-specific conjugation of bioluminescent proteins to quantum dots. Angew Chem, Int Ed Engl. 2006;45:4936–4940. doi: 10.1002/anie.200601197. [DOI] [PubMed] [Google Scholar]
- 95.Frangioni JV. Self-illuminating quantum dots light the way. Nat Biotechnol. 2006;24:326–328. doi: 10.1038/nbt0306-326. [DOI] [PubMed] [Google Scholar]
- 96.Chen HY, Abraham S, Mendenhall J, Delamarre SC, Smith K, Kim I, Batt CA. Encapsulation of single small gold nanoparticles by diblock copolymers. Chem Phys Chem. 2008;9:388–392. doi: 10.1002/cphc.200700598. [DOI] [PubMed] [Google Scholar]
- 97.Bakalova R, Zhelev Z, Aoki I, Masamoto K, Mileva M, Obata T, Higuchi M, Gadjeva V, Kanno I. Multimodal silica-shelled quantum dots: direct intracellular delivery, photosensitization, toxic, and microcirculation effects. Bioconjug Chem. 2008;19:1135–1142. doi: 10.1021/bc700431c. [DOI] [PubMed] [Google Scholar]
- 98.Spikes JD. Photodynamic action: from paramecium to photochemotherapy12. Photochem Photobiol. 1997;65S:142–147. [Google Scholar]
- 99.Moan J, Peng Q. An outline of the hundred-year history of PDT. Anticancer Res. 2003;23:3591–3600. [PubMed] [Google Scholar]
- 100.Diamond I, Granelli SG, McDonagh AF, Nielsen S, Wilson CB, Jaenicke R. Photodynamic therapy of malignant tumours. Lancet. 1972;2:1175–1177. doi: 10.1016/s0140-6736(72)92596-2. [DOI] [PubMed] [Google Scholar]
- 101.Dougherty TJ, Gomer CJ, Henderson BW, Jori G, Kessel D, Korbelik M, Moan J, Peng Q. Photodynamic therapy. J Natl Cancer Inst. 1998;90:889–905. doi: 10.1093/jnci/90.12.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Juarranz A, Jaen P, Sanz-Rodriguez F, Cuevas J, Gonzalez S. Photodynamic therapy of cancer. Basic principles and applications. Clin Transl Oncol. 2008;10:148–154. doi: 10.1007/s12094-008-0172-2. [DOI] [PubMed] [Google Scholar]
- 103.Dolmans DE, Fukumura D, Jain RK. Photodynamic therapy for cancer. Nat Rev, Cancer. 2003;3:380–387. doi: 10.1038/nrc1071. [DOI] [PubMed] [Google Scholar]
- 104.Wasserman HH, Murray RW. Singlet Oxygen. Academic Press; New York: San Francisco, London: 1979. [Google Scholar]
- 105.Fuchs J, Thiele J. The role of oxygen in cutaneous photodynamic therapy. Free Radic Biol Med. 1998;24:835–847. doi: 10.1016/s0891-5849(97)00370-5. [DOI] [PubMed] [Google Scholar]
- 106.Niedre M, Patterson MS, Wilson BC. Direct near-infrared luminescence detection of singlet oxygen generated by photodynamic therapy in cells in vitro and tissues in vivo. Photochem Photobiol. 2002;75:382–391. doi: 10.1562/0031-8655(2002)075<0382:DNILDO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 107.DeRosa MC, Crutchley RJ. Photosensitized singlet oxygen and its applications. Coord Chem Rev. 2002;233–234:351–371. [Google Scholar]
- 108.Chin KK, Trevithick-Sutton CC, McCallum J, Jockusch S, Turro NJ, Scaiano JC, Foote CS, Garcia-Garibay MA. Quantitative determination of singlet oxygen generated by excited state aromatic amino acids, proteins, and immunoglobulins. J Am Chem Soc. 2008;130:6912–6913. doi: 10.1021/ja800926v. [DOI] [PubMed] [Google Scholar]
- 109.Krasnovsky AA, Jr, Drozdova NN, Ivanov AV, Ambartsumian RV. Activation of molecular oxygen by infrared laser radiation in pigment-free aerobic systems. Biochemistry (Mosc) 2003;68:963–966. doi: 10.1023/a:1026052310563. [DOI] [PubMed] [Google Scholar]
- 110.Jockusch S, Turro NJ, Thompson EK, Gouterman M, Callis JB, Khalil GE. Singlet molecular oxygen by direct excitation. Photochem Photobiol Sci. 2008;7:235–239. doi: 10.1039/b714286b. [DOI] [PubMed] [Google Scholar]
- 111.Klotz LO, Kroncke KD, Sies H. Singlet oxygen-induced signaling effects in mammalian cells. Photochem Photobiol Sci. 2003;2:88–94. doi: 10.1039/b210750c. [DOI] [PubMed] [Google Scholar]
- 112.Foote CS. Mechanisms of photosensitized oxidation. There are several different types of photosensitized oxidation which may be important in biological systems. Science. 1968;162:963–970. doi: 10.1126/science.162.3857.963. [DOI] [PubMed] [Google Scholar]
- 113.Kasha M, Brabham DE. Singlet oxygen electronic structure and photosensitization. In: Wasserman HH, Murray RW, editors. Singlet Oxygen. Academic Press; New York: San Francisco, London: 1979. pp. 1–33. [Google Scholar]
- 114.Weldon D, Poulsen TD, Mikkelsen KV, Ogilby PR. Singlet sigma: the “other” singlet oxygen in solution. Photochem Photobiol. 1999;70:369–379. [Google Scholar]
- 115.Schmidt R. Photosensitized generation of singlet oxygen. Photochem Photobiol. 2006;82:1161–1177. doi: 10.1562/2006-03-03-IR-833. [DOI] [PubMed] [Google Scholar]
- 116.Juzenas P, Moan J. Singlet oxygen in photosensitization. J Environ Pathol Toxicol Oncol. 2006;25:29–50. doi: 10.1615/jenvironpatholtoxicoloncol.v25.i1-2.30. [DOI] [PubMed] [Google Scholar]
- 117.Kochevar IE. Singlet oxygen signaling: from intimate to global. Sci STKE. 2004;2004:e7. doi: 10.1126/stke.2212004pe7. [DOI] [PubMed] [Google Scholar]
- 118.Moan J. On the diffusion length of singlet oxygen in cells and tissues. J Photochem Photobiol, B: Biol. 1990;6:343–344. [Google Scholar]
- 119.Clo E, Snyder JW, Ogilby PR, Gothelf KV. Control and selectivity of photosensitized singlet oxygen production: challenges in complex biological systems. Chembiochem. 2007;8:475–481. doi: 10.1002/cbic.200600454. [DOI] [PubMed] [Google Scholar]
- 120.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 121.Ma J, Chen JY, Idowu M, Nyokong T. Generation of singlet oxygen via the composites of water-soluble thiol-capped CdTe quantum dots-sulfonated aluminum phthalocyanines. J Phys Chem, B. 2008;112:4465–4469. doi: 10.1021/jp711537j. [DOI] [PubMed] [Google Scholar]
- 122.Idowu M, Chen JY, Nyokong T. Photoinduced energy transfer between water-soluble CdTe quantum dots and aluminium tetrasulfonated phthalocyanine. New J Chem. 2008;32:290–296. [Google Scholar]
- 123.Clarke SJ, Hollmann CA, Zhang Z, Suffern D, Bradforth SE, Dimitrijevic NM, Minarik WG, Nadeau JL. Photophysics of dopamine-modified quantum dots and effects on biological systems. Nat Mater. 2006;5:409–417. doi: 10.1038/nmat1631. [DOI] [PubMed] [Google Scholar]
- 124.Shi L, Hernandez B, Selke M. Singlet oxygen generation from water-soluble quantum dot-organic dye nanocomposites. J Am Chem Soc. 2006;128:6278–6279. doi: 10.1021/ja057959c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tsay JM, Trzoss M, Shi L, Kong X, Selke M, Jung ME, Weiss S. Singlet oxygen production by Peptide-coated quantum dot-photosensitizer conjugates. J Am Chem Soc. 2007;129:6865–6871. doi: 10.1021/ja070713i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhang Y, Aslan K, Previte MJ, Geddes CD. Plasmonic engineering of singlet oxygen generation. Proc Natl Acad Sci U S A. 2008;105:1798–1802. doi: 10.1073/pnas.0709501105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Anas A, Akita H, Harashima H, Itoh T, Ishikawa M, Biju V. Photosensitized breakage and damage of DNA by CdSe–ZnS quantum dots. J Phys Chem, B. 2008;112:10005–10011. doi: 10.1021/jp8018606. [DOI] [PubMed] [Google Scholar]
- 128.Kim S, Ohulchanskyy TY, Pudavar HE, Pandey RK, Prasad PN. Organically modified silica nanoparticles co-encapsulating photosensitizing drug and aggregation-enhanced two-photon absorbing fluorescent dye aggregates for two-photon photodynamic therapy. J Am Chem Soc. 2007;129:2669–2675. doi: 10.1021/ja0680257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Crow JP. Dichlorodihydrofluorescein and dihydrorhodamine 123 are sensitive indicators of peroxynitrite in vitro: implications for intracellular measurement of reactive nitrogen and oxygen species. Nitric Oxide. 1997;1:145–157. doi: 10.1006/niox.1996.0113. [DOI] [PubMed] [Google Scholar]
- 130.Wrona M, Patel K, Wardman P. Reactivity of 2′,7′-dichlorodihydrofluorescein and dihydrorhodamine 123 and their oxidized forms toward carbonate, nitrogen dioxide, and hydroxyl radicals. Free Radic Biol Med. 2005;38:262–270. doi: 10.1016/j.freeradbiomed.2004.10.022. [DOI] [PubMed] [Google Scholar]
- 131.Khan AU, Kovacic D, Kolbanovskiy A, Desai M, Frenkel K, Geacintov NE. The decomposition of peroxynitrite to nitroxyl anion (NO−) and singlet oxygen in aqueous solution. Proc Natl Acad Sci U S A. 2000;97:2984–2989. doi: 10.1073/pnas.050587297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Martinez GR, Di MP, Bonini MG, Augusto O, Briviba K, Sies H, Maurer P, Rothlisberger U, Herold S, Koppenol WH. Peroxynitrite does not decompose to singlet oxygen ((1)Delta (g)O(2)) andnitroxyl (NO(−)) Proc Natl Acad Sci U S A. 2000;97:10307–10312. doi: 10.1073/pnas.190256897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Merenyi G, Lind J, Czapski G, Goldstein S. The decomposition of peroxynitrite does not yield nitroxyl anion and singlet oxygen. Proc Natl Acad Sci U S A. 2000;97:8216–8218. doi: 10.1073/pnas.150238197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ipe BI, Lehnig M, Niemeyer CM. On the generation of free radical species from quantum dots. Small. 2005;1:706–709. doi: 10.1002/smll.200500105. [DOI] [PubMed] [Google Scholar]
- 135.Berg K, Selbo PK, Prasmickaite L, Tjelle TE, Sandvig K, Moan J, Gaudernack G, Fodstad O, Kjolsrud S, Anholt H, Rodal GH, Rodal SK, Hogset A. Photochemical internalization: a novel technology for delivery of macromolecules into cytosol. Cancer Res. 1999;59:1180–1183. [PubMed] [Google Scholar]
- 136.Krinsky NI. Biological roles of singlet oxygen. In: Wasserman HH, Murray RW, editors. Singlet Oxygen. Academic Press; New York: San Francisco, London: 1979. pp. 597–641. [Google Scholar]
- 137.Min DB, Boff JM. Chemistry and reaction of singlet oxygen in foods 3. Compr Rev Food Sci Food Saf. 2002;1:58–72. doi: 10.1111/j.1541-4337.2002.tb00007.x. [DOI] [PubMed] [Google Scholar]
- 138.Zhelev Z, Jose R, Nagase T, Ohba H, Bakalova R, Ishikawa M, Baba Y. Enhancement of the photoluminescence of CdSe quantum dots during long-term UV-irradiation: privilege or fault in life science research? J Photochem Photobiol B: Biol. 2004;75:99–105. doi: 10.1016/j.jphotobiol.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 139.Derfus AM, Chan WCW, Bhatia SN. Probing the cytotoxicity of semiconductor quantum dots. Nano Lett. 2004;4:11–18. doi: 10.1021/nl0347334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ipe BI, Niemeyer CM. Nanohybrids composed of quantum dots and cytochrome P450 as photocatalysts. Angew Chem, Int Ed Engl. 2006;45:504–507. doi: 10.1002/anie.200503084. [DOI] [PubMed] [Google Scholar]
- 141.Robel I, Subramanian V, Kuno M, Kamat PV. Quantum dot solar cells. Harvesting light energy with CdSe nanocrystals molecularly linked to mesoscopic TiO2 films. J Am Chem Soc. 2006;128:2385–2393. doi: 10.1021/ja056494n. [DOI] [PubMed] [Google Scholar]
- 142.Ginger DS, Greenham NC. Electrical properties of semiconductor nanocrystals. In: Klimov VI, editor. Semiconductor and Metal Nanocrystals: Synthesis and Electronic and Optical Properties. Marcel Dekker; New York: 2004. pp. 239–288. Basel. [Google Scholar]
- 143.Maestro P, Chagny M, Jobert PP, Van Damme H, Berthier S. Optics. In: Brechignac C, Houdy P, Lahmani M, editors. Nanomaterials and Nanochemistry. Belin; Springer-Verlag: Belin; Berlin, Heidelberg: 2006. pp. 633–659. [Google Scholar]
- 144.Liu X, Jiang H, Lei J, Ju H. Anodic electrochemiluminescence of CdTe quantum dots and its energy transfer for detection of catechol derivatives. Anal Chem. 2007;79:8055–8060. doi: 10.1021/ac070927i. [DOI] [PubMed] [Google Scholar]
- 145.Stoll C, Gehring C, Schubert K, Zanella M, Parak WJ, Lisdat F. Photoelectrochemical signal chain based on quantum dots on gold-sensitive to superoxide radicals in solution. Biosens Bioelectron. 2008;24:260–265. doi: 10.1016/j.bios.2008.03.039. [DOI] [PubMed] [Google Scholar]
- 146.Neuman D, Ostrowski AD, Mikhailovsky AA, Absalonson RO, Strouse GF, Ford PC. Quantum dot fluorescence quenching pathways with Cr(III) complexes. photosensitized NO production from trans-Cr(cyclam)(ONO)2+ J Am Chem Soc. 2008;130:168–175. doi: 10.1021/ja074164s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hall EJ, Giaccia AJ. Radiobiology for the Radiologist. Lippincott Williams & Wilkins; Philadelphia: Baltimore, New York, London, Buenos Aires, Hong Kong, Sydney, Tokyo: 2006. [Google Scholar]
- 148.Hogle WP. The state of the art in radiation therapy. Semin Oncol Nurs. 2006;22:212–220. doi: 10.1016/j.soncn.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 149.Halliwell B, Aruoma OI. DNA damage by oxygen-derived species. Its mechanism and measurement in mammalian systems. FEBS Lett. 1991;281:9–19. doi: 10.1016/0014-5793(91)80347-6. [DOI] [PubMed] [Google Scholar]
- 150.Kandaswami C, Middleton E., Jr Free radical scavenging and antioxidant activity of plant flavonoids. Adv Exp Med Biol. 1994;366:351–376. doi: 10.1007/978-1-4615-1833-4_25. [DOI] [PubMed] [Google Scholar]
- 151.Halliwell B. Oxygen radicals: a commonsense look at their nature and medical importance. Med Biol. 1984;62:71–77. [PubMed] [Google Scholar]
- 152.Mishra KP. Cell membrane oxidative damage induced by gamma-radiation and apoptotic sensitivity. J Environ Pathol Toxicol Oncol. 2004;23:61–66. doi: 10.1615/jenvpathtoxoncol.v23.i1.60. [DOI] [PubMed] [Google Scholar]
- 153.Marathe D, Mishra KP. Radiation-induced changes in permeability in unilamellar phospholipid liposomes. Radiat Res. 2002;157:685–692. doi: 10.1667/0033-7587(2002)157[0685:ricipi]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 154.Hall EJ, Brenner DJ. Cancer risks from diagnostic radiology. Br J Radiol. 2008;81:362–378. doi: 10.1259/bjr/01948454. [DOI] [PubMed] [Google Scholar]
- 155.Soffietti R, Leoncini B, Ruda R. New developments in the treatment of malignant gliomas. Expert Rev Neurother. 2007;7:1313–1326. doi: 10.1586/14737175.7.10.1313. [DOI] [PubMed] [Google Scholar]
- 156.Speight JL, Roach M., III Advances in the treatment of localized prostate cancer: the role of anatomic and functional imaging in men managed with radiotherapy. J Clin Oncol. 2007;25:987–995. doi: 10.1200/JCO.2006.10.3218. [DOI] [PubMed] [Google Scholar]
- 157.Haas ML. Advances in radiation therapy for lung cancer. Semin Oncol Nurs. 2008;24:34–40. doi: 10.1016/j.soncn.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 158.Herold DM, Das IJ, Stobbe CC, Iyer RV, Chapman JD. Gold microspheres: a selective technique for producing biologically effective dose enhancement. Int J Radiat Biol. 2000;76:1357–1364. doi: 10.1080/09553000050151637. [DOI] [PubMed] [Google Scholar]
- 159.Guo T. Nanoparticle radiosensitizers. 2006 WO2006037081. [Google Scholar]
- 160.Praetorius NP, Mandal TK. Engineered nanoparticles in cancer therapy. Rec Pat Drug Deliv Formul. 2007;1:37–51. doi: 10.2174/187221107779814104. [DOI] [PubMed] [Google Scholar]
- 161.Chen W, Westcott SL, Zhang J. Dose dependence of X-ray luminescence from CaF [sub 2]:Eu[sup 2+], Mn[sup 2+] phosphors. Appl Phys Lett. 2007;91:1–3. 211103. [Google Scholar]
- 162.Liu Y, Chen W, Wang Y, Joly AG. Investigation of water-soluble X-ray luminescence nanoparticles for photodynamic activation. Appl Phys Lett. 2008;92:1–3. 043901. [Google Scholar]
- 163.Wang YH, Chen Z, Zhou XQ. Synthesis and photoluminescence of ZnS quantum dots. J Nanosci Nanotechnol. 2008;8:1312–1315. [PubMed] [Google Scholar]
- 164.Liu Y, Zhang Y, Wang S, Pope C, Chen W. Optical behaviors of ZnO-porphyrin conjugates and their potential applications for cancer treatment. Appl Phys Lett. 2008;92:1–3. 143901. [Google Scholar]
- 165.Morilla MJ, Montanari J, Frank F, Malchiodi E, Corral R, Petray P, Romero EL. Etanidazole in pH-sensitive liposomes: design, characterization and in vitro/in vivo anti-Trypanosoma cruzi activity. J Control Release. 2005;103:599–607. doi: 10.1016/j.jconrel.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 166.Jin C, Bai L, Wu H, Tian F, Guo G. Radiosensitization of paclitaxel, etanidazole and paclitaxel+etanidazole nanoparticles on hypoxic human tumor cells in vitro. Biomaterials. 2007;28:3724–3730. doi: 10.1016/j.biomaterials.2007.04.032. [DOI] [PubMed] [Google Scholar]
- 167.Jin C, Bai L, Wu H, Liu J, Guo G, Chen J. Paclitaxel-loaded poly(D,L-lactide-co-glycolide) nanoparticles for radiotherapy in hypoxic human tumor cells in vitro. Cancer Biother. 2008;7 doi: 10.4161/cbt.7.6.5912. [DOI] [PubMed] [Google Scholar]
- 168.Zheng Y, Hunting DJ, Ayotte P, Sanche L. Role of secondary low-energy electrons in the concomitant chemoradiation therapy of cancer. Phys Rev Lett. 2008;100:198101. doi: 10.1103/PhysRevLett.100.198101. [DOI] [PubMed] [Google Scholar]
- 169.Baatout S, Jacquet P, Derradji H, Ooms D, Michaux A, Mergeay M. Study of the combined effect of X-irradiation and epigallocatechingallate (a tea component) on the growth inhibition and induction of apoptosis in human cancer cell lines. Oncol Rep. 2004;12:159–167. [PubMed] [Google Scholar]
- 170.Nair CK, Salvi VP. Protection of DNA from gamma-radiation induced strand breaks by Epicatechin. Mutat Res. 2008;650:48–54. doi: 10.1016/j.mrgentox.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 171.Sugiyama T, Sadzuka Y. Theanine and glutamate transporter inhibitors enhance the antitumor efficacy of chemotherapeutic agents. Biochim Biophys Acta. 2003;1653:47–59. doi: 10.1016/s0304-419x(03)00031-3. [DOI] [PubMed] [Google Scholar]
- 172.Friedman M, Mackey BE, Kim HJ, Lee IS, Lee KR, Lee SU, Kozukue E, Kozukue N. Structure–activity relationships of tea compounds against human cancer cells. J Agric Food Chem. 2007;55:243–253. doi: 10.1021/jf062276h. [DOI] [PubMed] [Google Scholar]
- 173.Chen J, Wanming D, Zhang D, Liu Q, Kang J. Water-soluble antioxidants improve the antioxidant and anticancer activity of low concentrations of curcumin in human leukemia cells. Pharmazie. 2005;60:57–61. [PubMed] [Google Scholar]
- 174.Jagetia GC. Radioprotection and radiosensitization by curcumin. Adv Exp Med Biol. 2007;595:301–320. doi: 10.1007/978-0-387-46401-5_13. [DOI] [PubMed] [Google Scholar]
- 175.Cohen L, Schwartz S. Modification of radiosensitivity by porphyrins. II. Transplanted rhabdomyosarcoma in mice. Cancer Res. 1966;26:1769–1773. [PubMed] [Google Scholar]
- 176.Moan J, Pettersen EO. X-irradiation of human cells in culture in the presence of haematoporphyrin. Int J Radiat Biol Relat Stud Phys Chem Med. 1981;40:107–109. [PubMed] [Google Scholar]
- 177.Schaffer M, Schaffer PM, Corti L, Gardiman M, Sotti G, Hofstetter A, Jori G, Duhmke E. Photofrin as a specific radiosensitizing agent for tumors: studies in comparison to other porphyrins, in an experimental in vivo model. J Photochem Photobiol, B: Biol. 2002;66:157–164. doi: 10.1016/s1011-1344(02)00237-3. [DOI] [PubMed] [Google Scholar]
- 178.Garg AK, Buchholz TA, Aggarwal BB. Chemosensitization and radiosensitization of tumors by plant polyphenols. Antioxid Redox Signal. 2005;7:1630–1647. doi: 10.1089/ars.2005.7.1630. [DOI] [PubMed] [Google Scholar]
- 179.Adelstein SJ, Kassis AI, Bodei L, Mariani G. Radiotoxicity of iodine-125 and other Auger-electron-emitting radionuclides: background to therapy. Cancer Biother Radiopharm. 2003;18:301–316. doi: 10.1089/108497803322285062. [DOI] [PubMed] [Google Scholar]
- 180.Bodei L, Kassis AI, Adelstein SJ, Mariani G. Radionuclide therapy with iodine-125 and other auger-electron-emitting radionuclides: experimental models and clinical applications. Cancer Biother Radiopharm. 2003;18:861–877. doi: 10.1089/108497803322702833. [DOI] [PubMed] [Google Scholar]
- 181.O’Donoghue JA, Wheldon TE. Targeted radiotherapy using Auger electron emitters. Phys Med Biol. 1996;41:1973–1992. doi: 10.1088/0031-9155/41/10/009. [DOI] [PubMed] [Google Scholar]
- 182.Zheng Y, Hunting DJ, Ayotte P, Sanche L. Radiosensitization of DNA by gold nanoparticles irradiated with high-energy electrons. Radiat Res. 2008;169:19–27. doi: 10.1667/RR1080.1. [DOI] [PubMed] [Google Scholar]
- 183.Patt HM, Tyree EB, Straube RL, Smith DE. Cysteine protection against X irradiation. Science. 1949;110:213–214. doi: 10.1126/science.110.2852.213. [DOI] [PubMed] [Google Scholar]
- 184.Kumar KS, Srinivasan V, Toles R, Jobe L, Seed TM. Nutritional approaches to radioprotection: vitamin E. Mil Med. 2002;167:57–59. [PubMed] [Google Scholar]
- 185.Weiss JF, Landauer MR. Radioprotection by antioxidants. Ann N Y Acad Sci. 2000;899:44–60. [PubMed] [Google Scholar]
- 186.Murray D, Altschuler EM, Hunter N, Milas L. Protection by WR-3689 against gamma-ray-induced intestinal damage: comparative effect on clonogenic cell survival, mouse survival, and DNA damage. Radiat Res. 1989;120:339–351. [PubMed] [Google Scholar]
- 187.Capizzi RL, Oster W. Chemoprotective and radioprotective effects of amifostine: an update of clinical trials. Int J Hematol. 2000;72:425–435. [PubMed] [Google Scholar]
- 188.Pamujula S, Kishore V, Rider B, Fermin CD, Graves RA, Agrawal KC, Mandal TK. Radioprotection in mice following oral delivery of amifostine nanoparticles. Int J Radiat Biol. 2005;81:251–257. doi: 10.1080/09553000500103470. [DOI] [PubMed] [Google Scholar]
- 189.Suresh Reddy J, Venkateswarlu V, Koning GA. Radioprotective effect of transferrin targeted citicoline liposomes. J Drug Target. 2006;14:13–19. doi: 10.1080/10611860600613241. [DOI] [PubMed] [Google Scholar]
- 190.Daroczi B, Kari G, McAleer MF, Wolf JC, Rodeck U, Dicker AP. In vivo radioprotection by the fullerene nanoparticle DF-1 as assessed in a zebrafish model. Clin Cancer Res. 2006;12:7086–7091. doi: 10.1158/1078-0432.CCR-06-0514. [DOI] [PubMed] [Google Scholar]
- 191.Zhao Q, Li Y, Xu J, Liu R, Li W. Radioprotection by fullerenols of Stylonychia mytilus exposed to gamma-rays. Int J Radiat Biol. 2005;81:169–175. doi: 10.1080/09553000400029536. [DOI] [PubMed] [Google Scholar]
- 192.Tarnuzzer RW, Colon J, Patil S, Seal S. Vacancy engineered ceria nanostructures for protection from radiation-induced cellular damage. Nano Lett. 2005;5:2573–2577. doi: 10.1021/nl052024f. [DOI] [PubMed] [Google Scholar]
- 193.Dai S, Saengkerdsub S, Im HJ, Stephan AC, Mahurin SM. Nanocrystal-based scintillators for radiation detection. AIP Conf Proc. 2002;632:220–224. [Google Scholar]
- 194.Letant SE, Wang TF. Semiconductor quantum dot scintillation under gamma-ray irradiation. Nano Lett. 2006;6:2877–2880. doi: 10.1021/nl0620942. [DOI] [PubMed] [Google Scholar]
- 195.Letant SE, Wang TF. Study of porous glass doped with quantum dots or laser dyes under alpha irradiation. Appl Phys Lett. 2006;88:103110. [Google Scholar]
- 196.Mutlugun E, Soganci IM, Demir HV. Nanocrystal hybridized scintillators for enhanced detection and imaging on Si platforms in UV. Opt Express. 2007;15:1128–1134. doi: 10.1364/oe.15.001128. [DOI] [PubMed] [Google Scholar]
- 197.Mutlugun E, Soganci IM, Demir HV. Photovoltaic nanocrystal scintillators hybridized on Si solar cells for enhanced conversion efficiency in UV. Opt Express. 2008;16:3537–3545. doi: 10.1364/oe.16.003537. [DOI] [PubMed] [Google Scholar]
- 198.Ntziachristos V, Bremer C, Weissleder R. Fluorescence imaging with near-infrared light: new technological advances that enable in vivo molecular imaging. Eur Radiol. 2003;13:195–208. doi: 10.1007/s00330-002-1524-x. [DOI] [PubMed] [Google Scholar]
- 199.Alexiades-Armenakas M. Laser-mediated photodynamic therapy. Clin Dermatol. 2006;24:16–25. doi: 10.1016/j.clindermatol.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 200.Chen W, Zhang J. Using nanoparticles to enable simultaneous radiation and photodynamic therapies for cancer treatment. J Nanosci Nanotechnol. 2006;6:1159–1166. doi: 10.1166/jnn.2006.327. [DOI] [PubMed] [Google Scholar]
- 201.Liu Y, Chen W, Joly AG, Wang Y, Pope C, Zhang Y, Bovin JO, Sherwood P. Comparison of water-soluble CdTe nanoparticles synthesized in air and in nitrogen. J Phys Chem, B. 2006;110:16992–17000. doi: 10.1021/jp063085k. [DOI] [PubMed] [Google Scholar]
- 202.Coleman CN. Hypoxic cell radiosensitizers: expectations and progress in drug development. Int J Radiat Oncol Biol Phys. 1985;11:323–329. doi: 10.1016/0360-3016(85)90154-3. [DOI] [PubMed] [Google Scholar]
- 203.Overgaard J. Hypoxic radiosensitization: adored and ignored. J Clin Oncol. 2007;25:4066–4074. doi: 10.1200/JCO.2007.12.7878. [DOI] [PubMed] [Google Scholar]
- 204.Moan J, Sommer S. Oxygen dependence of the photosensitizing effect of hematoporphyrin derivative in NHIK 3025 cells. Cancer Res. 1985;45:1608–1610. [PubMed] [Google Scholar]
- 205.Brooks SE, Korbut TT, Dupuis NP, Holden SA, Teicher BA. Cytotoxicity of antitumor platinum complexes with L-buthionine-(R,S)-sulfoximine and/or etanidazole in human carcinoma cell lines sensitive and resistant to cisplatin. Cancer Chemother Pharmacol. 1995;36:431–438. doi: 10.1007/BF00686193. [DOI] [PubMed] [Google Scholar]
- 206.Middleton E, Jr, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol Rev. 2000;52:673–751. [PubMed] [Google Scholar]
- 207.Okunieff P, Swarts S, Keng P, Sun W, Wang W, Kim J, Yang S, Zhang H, Liu C, Williams JP, Huser AK, Zhang L. Antioxidants reduce consequences of radiation exposure. Adv Exp Med Biol. 2008;614:165–178. doi: 10.1007/978-0-387-74911-2_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Lala G, Malik M, Zhao C, He J, Kwon Y, Giusti MM, Magnuson BA. Anthocyanin-rich extracts inhibit multiple biomarkers of colon cancer in rats. Nutr Cancer. 2006;54:84–93. doi: 10.1207/s15327914nc5401_10. [DOI] [PubMed] [Google Scholar]
- 209.Manach C, Williamson G, Morand C, Scalbert A, Remesy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr. 2005;81:230S–242S. doi: 10.1093/ajcn/81.1.230S. [DOI] [PubMed] [Google Scholar]
- 210.Mainardes RM, Silva LP. Drug delivery systems: past, present, and future. Curr Drug Targets. 2004;5:449–455. doi: 10.2174/1389450043345407. [DOI] [PubMed] [Google Scholar]
- 211.Ferreira I, Rocha S, Coelho M. Encapsulation of antioxidants by spray-drying. Eng Trans. 2007;11:713–717. [Google Scholar]
- 212.Fang JY, Hung CF, Hwang TL, Huang YL. Physicochemical characteristics and in vivo deposition of liposome-encapsulated tea catechins by topical and intratumor administrations. J Drug Target. 2005;13:19–27. doi: 10.1080/10611860400015977. [DOI] [PubMed] [Google Scholar]
- 213.Sinha J, Das N, Basu MK. Liposomal antioxidants in combating ischemia–reperfusion injury in rat brain. Biomed Pharmacother. 2001;55:264–271. doi: 10.1016/s0753-3322(01)00060-9. [DOI] [PubMed] [Google Scholar]
- 214.Foote CS. Definition of Type I and Type II photosensitized oxidation. Photochem Photobiol. 1991;54:659. doi: 10.1111/j.1751-1097.1991.tb02071.x. [DOI] [PubMed] [Google Scholar]
- 215.Krasnovsky AA., Jr Photodynamic action and singlet oxygen. Biophys (Mosc) 2004;49:289–306. [Google Scholar]
- 216.Park YS, Kasuya A, Dmytruk A, Yasuto N, Takeda M, Ohuchi N, Sato Y, Tohji K, Uo M, Watari F. Concentrated colloids of silica-encapsulated gold nanoparticles: colloidal stability, cytotoxicity, and X-ray absorption. J Nanosci Nanotechnol. 2007;7:2690–2695. doi: 10.1166/jnn.2007.601. [DOI] [PubMed] [Google Scholar]