Abstract
Mosquitoes transmit numerous diseases that continue to be an enormous burden on public health worldwide. Transgenic mosquitoes impervious to vector-borne pathogens, in concert with vector control and drug and vaccine development, comprise an arsenal of means anticipated to defeat mosquito-spread diseases in the future. Mosquito transgenesis allows tissue-specific manipulation of their major immune pathways and enhances the ability to study mosquito–pathogen interactions. Here, we report the generation of two independent transgenic strains of Aedes aegypti overexpressing the NF-κB transcriptional factor REL2, a homologue of Drosophila Relish, which is shown to be under the control of the vitellogenin promoter in the mosquito fat body after a blood meal. We show that this REL2 overexpression in the fat body results in transcriptional activation of Defensins A, C, and D, and Cecropins A and N, as well as translation and secretion of Defensin A protein into the hemolymph. We also demonstrate that induction of REL2 results in the increased resistance of the mosquito to tested Gram-negative and Gram-positive bacteria. Importantly, induction of transgenic REL2 leads to the significant decrease in susceptibility of Ae. aegypti to Plasmodium gallinaceum infection. Consistently, RNAi knockdown of REL2 in wild-type mosquitoes results in a delay in Defensin A and Cecropin A expression in response to infection and in increased susceptibility to both bacteria and P. gallinaceum. Moreover, our transgenic assays demonstrate that the N-terminus of the mosquito REL2, which includes the His/Gln-rich and Serine-rich regions, plays a role in its transactivation properties.
Keywords: Mosquito, transgenesis, antimicrobial peptides, Plasmodium, Relish, Rel transactivation domain
1. Introduction
Despite attempts at intervention, malaria continues to kill more than 2 million people annually, worldwide. Lack of an effective anti-malarial vaccine, growing drug resistance of the malaria parasite, and increasing insecticide resistance of the mosquito vector all contribute to the intransigence of this problem. It is therefore necessary to find alternative ways to control mosquito-spread diseases. Transgenic mosquitoes that are resistant to the malaria parasite, vector control, and drug and vaccine development comprise an arsenal of methods anticipated to defeat malaria in the future. Transgenic modification of the mosquito vector requires profound knowledge of mosquito immunity, which can be achieved through detailed studies using model experimental systems.
The insect immune system consists of structural, cellular, and humoral components (Lemaitre and Hoffmann, 2007). The humoral innate immunity in insects is controlled by signal transduction pathways that resemble immune pathways of mammals (Hoffmann and Reichhart, 2002; Hoffmann, 2003; Lemaitre and Hoffmann, 2007). In both insects and mammals, pattern recognition receptors (PRRs) recognize pathogens and participate in the activation of signaling immune pathways. Such activation results in the nuclear translocation of NF-κB/REL transcriptional factors. Finally, NF-κB proteins induce an array of genes encoding immune effector molecules, including antimicrobial peptides (AMPs) (Ghosh et al., 1998; Baeuerle and Baltimore, 1996; Hoffmann and Reichhart, 2002; Hoffmann, 2003; Lemaitre and Hoffmann, 2007). Two well-characterized immune signaling pathways lead to the activation of NF-κB factors in Drosophila: Toll and Immune-deficiency (Imd). The Toll pathway, responding to fungi and most Gram-positive bacteria, is similar to mammalian Toll-like receptors (TLRs) and IL-1 receptor signaling pathways. The Imd pathway responds mostly to Gram-negative bacteria and is similar to the mammalian tumor-necrosis factor (TNF) immune pathway (Hoffmann, 2003). Signaling through the Toll pathway leads to activation and nuclear translocation of NF-κB factors Dorsal and Dif. Signaling through the Imd pathway results in cleavage and nuclear translocation of the third NF-κB transcriptional factor, Relish (Hedengren et al., 1999; Stöven et al., 2000). Drosophila Relish has been shown to be a major regulator of AMPs. These NF-κB factors induce the transcription of all 21 AMPs encoded in the Drosophila genome (Lemaitre and Hoffmann, 2007). Additionally, Relish synergistically cooperates with Dif in transcriptional activation of AMPs (Han and Ip, 1999; Tanji et al., 2007).
Sequencing and annotation of the Anopheles gambiae (Holt et al., 2002; Christophides et al., 2002) and Aedes aegypti (Nene et al., 2007; Waterhouse et. al., 2007) genomes have revealed that mosquitoes lack the NF-κB factor Dif. Therefore, they rely on two NF-κB factors: Dorsal orthologue REL1 and Relish orthologue REL2. These mosquito REL factors are involved in immunity, and several studies have shown that signaling through REL1 and REL2 in the mosquito activates AMPs (predominantly Cecropins and Defensins) and anti-Plasmodium molecules such as CTL-1, LRIM-1, and TEP-1 (Osta et al., 2004; Meister et al, 2005; Shin et al., 2005; Frolet et al, 2006; Xi et al., 2008).
Ae. aegypti REL2 has three isoforms that are products of an alternative splicing of the Rel2 gene (Shin et al., 2002). The predominant isoform of the Aedes REL2 (REL2-Long) contains the His/Gln (Q/H)-rich and SRR regions, the REL-homology (RHD and IPT) domains, the inhibitor IκB-like ankyrin domain, and the Death domain. This form is similar to Drosophila Relish and the mammalian p105 and p100 Rel/NF-κB transcription factors. The second (REL2-Short) isoform is similar to REL2-Long, although it lacks the ankyrin and Death domains. The third (IκB-type) transcript has an IκB-like domain intact, but lacks most of the N-terminal sequence and RHD domain. An. gambiae Rel2 is similar to Ae. aegypti Rel2; however, it gives rise to only two isoforms—Long (Full) and Short (Meister et al, 2005). Interestingly, two Relish isoforms with similar features to mosquito REL2-Long (Full) and REL2-Short isoforms have also been identified in the silkworm Bombyx mori (Tanaka et al., 2007).
In An. gambiae, REL2 is involved in regulating expression of Cecropin1, Cecropin3, and Gambicin, and also the Plasmodium antagonist LRIM1. Moreover, Anopheles REL2 has been implicated in the mosquito defense against Gram-negative and Gram-positive bacteria, and the rodent malaria parasite Plasmodium berghei (Meister et al., 2005). These authors further showed that the Anopheles REL2-F (Long) and REL2-S (Short) isoforms are involved in resistance against the Gram-positive Staphylococcus aureus and the Gram-negative Escherichia coli bacteria, respectively. The specific roles of Ae. aegypti REL2 isoforms still remain unknown.
Mosquito transformation has become a valuable tool in deciphering anti-pathogen immunity. Several prior studies have reported successful mosquito transformations using transposable elements that allow incorporation of genes of interest into the mosquito genome under control of tissue-specific promoters (Coates et al., 1998; Jasinskiene et al., 1998; Moreira et al., 2000; Kokoza et al., 2000; Kokoza et al., 2001a; Shin et al., 2003, Ito et al., 2004; Kim et al., 2004; Bian et al., 2005; Franz et al., 2006; Smith et al., 2007). Fat-body-specific Vitellogenin (Vg) is one of the best studied promoters, allowing strong tissue specificity and blood-meal-controlled expression of the genes of interest (Kokoza et al., 2000; Kokoza et al., 2001b; Nirmala et al., 2006). Previously, we successfully created a transgenic Ae. aegypti mosquito that overexpressed an N-terminally truncated form of REL2-Short in the fat body after a blood meal (Shin et al., 2003). It demonstrated a Relish Immune Deficient (RIMD) phenotype, in which the expression of Defensin A and Cecropin A in response to bacterial infection was abolished, and the mosquitoes were highly susceptible to Gram-negative bacteria. Thus, the essential role of Aedes REL2 in AMP production and antibacterial defense was proven functionally (Shin et al., 2003).
In this study, we employed mosquito transgenesis to generate two Ae. aegypti independent transgenic strains overexpressing REL2 in the mosquito fat body after a blood meal (PBM) to further study properties of REL2 in the mosquito Ae. aegypti. We used the complete REL2-Short isoform for the transgenic construct, because it lacks the IκB ankyrin domain and is therefore available for nuclear translocation and transcription initiation without additional cleavage. Here, we report the effect of overexpression of the REL2-Short isoform, under the Vg promoter PBM, on the expression of AMPs, and antimicrobial and anti-Plasmodium resistance. Furthermore, we evaluated the results obtained through studying the transgenic phenotype by conducting REL2 RNAi study in wild-type mosquitoes. To our knowledge, this is the first report to describe effects of transgenic overexpression of REL2 in the adult mosquito in vivo.
2. Materials and methods
2.1. Mosquito rearing
The Ae. aegypti wild-type UGAL/Rockefeller strain and two transgenic strains, REL2-A and REL2-B (REL2 gain-of-function strains), were maintained in a laboratory culture at 27°C and 85% humidity. Larvae were provided with standardized larvae diet “mosquito chow”, consisting of ground and sifted rat food, yeast, and lactalbumin in a ratio of 1:1:1 by weight (Hays and Raikhel, 1990). Adult mosquitoes were fed on 10% glucose and water. For all experiments, 4-day-old previtellogenic females fed blood on White Leghorn chickens.
2.2. Transformation plasmid and germ-line transformation
For the mosquito germ-line transformation, we used the pBac[3×P3-EGFP, afm] transformation vector containing the eye-specific promoter (3×P3) in front of the TATA box and enhanced green fluorescent protein (EGFP), as described by Horn and Wimmer (2000). The 2.14-kb full length of the REL2-Short isoform was sub-cloned between the Vg promoter and SV40 polyadenylation element. This construct was introduced into the pBac[3×P3-EGFP, afm] vector at the unique AscI cloning site. The resulting plasmid pBac[3×P3-EGFP, afm, Vg-REL2] was used to inject into mosquito embryos. Ae. aegypti germ-line transformation was carried out as described by Kokoza et al. (2001a). Of the 1000 embryos injected, 100 live F0 mosquitoes were recovered, and 18 female and 38 male mosquito families were organized. Then, the F1 progeny was examined for green fluorescent glowing in the eyes. Two EGFP-expressing mosquitoes from two different families were found, crossed to wild-type mosquitoes, and gave rise to two independent transgenic mosquito lines. Therefore, the germ-line transformation resulted in establishing two independent transgenic REL2-gain-of-function mosquito lines called REL2-A and REL2-B.
2.3. Southern blot analysis
Genomic DNA was extracted from UGAL (wild type), REL2-A, and REL2-B mosquitoes using the DNeasy Tissue kit (Qiagen; Valencia, CA, USA), according to the manufacturer’s instructions. For each mosquito strain, 15 μg genomic DNA was digested using EcoR1 (Roche, Indianapolis, IN, USA) and AscI (NEB, Ipswich, MA, USA). DNA was precipitated, restored in 20 μl of water, and loaded on a 1% agarose gel for DNA band separation. After transfer to a nitrocellulose membrane, the DNA was hybridized with the DNA probes for right vector arm, left vector arm for EcoRI-restricted DNA, and the internal SV-40 polyadenylation region for AscI-restricted DNA.
2.4. Northern blot analysis
Total RNA was extracted from 15 female mosquitoes per time point using the Triazol method (Invitrogen, Carlsbad, CA, USA), according to the manufacturer’s instructions. A total of 5 μg RNA was used for formaldehyde gel electrophoresis followed by Northern blot and successive hybridization with Vg, REL2, Defensin A, and Cecropin A DNA probes. Ribosomal RNA was used as a loading control.
2.5. dsRNA production and RNAi injections
For production of dsRNA, primers against the Ae. aegypti REL2 RHD domain with attached T7 minimal primers were designed. They were chosen so that the PCR product would yield a 500-nucleotide-long fragment. PCR was performed using total cDNA from infected mosquitoes and a High Fidelity Taq Polymerase kit (Invitrogen, Carlsbad, CA, USA). The PCR product was loaded on 1x TBE gel, run for 30 min at 100 mV. The bands of expected size were cut out, and the DNA product was purified using a Qiagen gel extraction kit (Qiagen, Valencia, CA). Non-specific dsRNA against the luciferase gene from firefly (Photinus pyralis) was used as a control. The double-stranded RNA was produced by in vitro transcription with the T7 RNA polymerase using the MEGAscript T7 kit (Ambion, Austin, TX, USA). Approximately, 1 μg dsRNA in 0.5 μl of distilled water was injected into the thorax of 1-day-old CO2-anesthetized female mosquitoes. The dsRNA-treated mosquitoes were allowed to recover for 3 days and then were used for the experiments.
2.6. Reverse transcription and PCR
To study expression dynamics of endogenous REL2 isoforms, REL1, and AMPs in the transgenic mosquitoes, total RNA was extracted from 4-day-old individual transgenic and wild-type mosquitoes—previtellogenic, 12 h and 24 h PBM using the Triazol method (Invitrogen). The RNA from wild-type mosquitoes infected with Enterobacter cloacae and dissected at 24 h post-infection was used as a control. The RNA samples were treated with DNAse (DNAse Amplification grade, Invitrogen), and cDNAs were synthesized using Omniscript Reverse Transcriptase kit (Qiagen). cDNAs from individual mosquitoes were then tested for the presence of an infection marker, Cecropin A, after 30 cycles of PCR. Those mosquito cDNAs that showed no Cecropin A bands of an intensity comparable to the band of the control cDNA were considered to be free from infection and were pooled together. PCR with specific primers was performed under the following conditions: 35 cycles for detection of Cecropins, Diptericin, Gambicin, Holotricin, and Attacin; 30 cycles for the detection of Defensins, REL2-Long and REL2-Short isoforms, transgenic REL2, and REL1.
To evaluate efficiency of the REL2 isoform knockdowns in the mosquitoes treated with dsRNA against REL2, at 4 days after the RNAi treatment, total RNA was extracted from the fat bodies of experimental and control mosquitoes using the Triazol method (Invitrogen). RNA pooled from 12 mosquitoes was used for the experiment. The RNA samples were treated with DNAse (DNAse Amplification grade, Invitrogen), and cDNAs were synthesized as described above. Reverse transcription was performed with the primers specific for REL2-Long and REL2-Short isoforms as follows: REL2-Long forward primer 5’-CGTTCAACAGTCATCATGTCAACAC-3’, REL2-Long reverse primer 5’-CACTTTTTGCATCTCAAAGTAGTTC-3’; REL-Short forward 5’-CGTTCAACAGTCATCATGTCAACAC-3, REL2-Short reverse 5’-GATTCATCGTGTGCTACTCACTTG-3’; REL1-A forward 5’-ACGAAAGAAACGCAAGCCTA-3’, REL2-A reverse 5’-ATCGTTCGTCGCTTCTCAGT-3’.
For the detection of Defensin A and Cecropin A mRNA levels in the infected mosquitoes, RT-PCR was performed with Defensin A- and Cecropin A-specific primers as follows: Defensin A forward 5’-GATCCCGAAAGGACCAACCATGC-3’, Defensin A reverse 5’-ATTCCGGCAGACGCACACCTT-3’; and Cecropin A forward 5’-ATGAACTTCACGAAGTTATTT-3’, Cecropin A reverse 5’-CTTTCTTAGAGCTTTAGCCCC-3’. For all experiments, the PCR products of Actin amplification were used as the PCR amplification and loading control.
2.7. Western blot analysis
Proteins were extracted from mosquitoes’ whole bodies, fat bodies, and ovaries using the Triazol extraction method, according to the manufacturer’s instructions (Invitrogen). For each hemolymph sample, hemolymph from 12 mosquitoes was collected by dissecting 3 female mosquitoes in 21 μl of 1× phosphate buffered saline (PBS) (Cambrex, Charles City, USA) solution with 1× protease inhibitor (Complete Mini, Roche,). Mosquitoes were dissected, and their internal organs washed in the dissection solution. A drop containing the mixture of PBS and the mosquito hemolymph was loaded on QIA shredder spin columns (Qiagen). The procedure was repeated four times, and then the hemolymph from all 12 mosquitoes was centrifuged at 3000-rcf for 1 min. The flow-through from the column was then centrifuged at 500-rcf to separate cell and serum components of the hemolymph. Next, the supernatant was carefully removed and used for Western blot analysis. For both tissue and hemolymph samples, protein concentration was measured using the Bradford method, and 5 mg protein was loaded on NuPAGE 12% Bis-Tris protein gel (Invitrogen). SDS-PAGE and protein transfer were performed using the NuPAGE Western blot system (Invitrogen). The Defensin protein was detected with Anti-DefensinA IgG (Cho et al., 1996), in 1:5000 dilutions. Immunoblots were developed using the SuperSignal West Pico detection system (Pierce, Rockford, IL, USA).
2.8. Survival experiments
To conduct survival experiments for REL2 transgenic mosquitoes, 4-day-old females were blood fed on White Leghorn chickens and then bacteria challenged at 6–10 h PBM. Bacteria Pseudomonas aeruginosa (ATCC 012406/10145) and Enterobacter cloacae were grown in Luria Bertani media (Sigma-Aldrich, St-Louis, MO, USA), and the following concentrations were used for bacterial challenge: P. aeruginosa OD600 = 0.6, E. cloacae OD600 = 2.8. Staphylococcus aureus (ATCC 133011) was grown in Nutrient Broth (Difco 23400), and a culture of S. aureus with OD600 = 4.5 was used for injections. Bacterial infections were done by pricking mosquitoes in the rear part of the abdomen with an acupuncture needle (0.2 × 25mm) dipped into the bacterial culture. A group of 32 blood-fed females was used for each experiment, which was performed at least three times.
To conduct the survival experiments in the REL2 RNAi mosquitoes, the REL2 dsRNA-treated and control iLuc mosquitoes were injected with bacteria Enterobacter cloacae or Enterococcus faecalis on the 4th day-post-RNAi treatment. E. cloacae were grown in Luria Bertani (LB) media (Sigma-Aldrich, St-Louis, MO, USA) to OD600 = 2.8 and then diluted 1:1000 times in the sterile LB media (Sigma). E. faecalis (ATCC 19433) were grown in Brain Heart Infusion (BD 237500) to OD600 = 3.2 and then diluted 1000 times in PBS (Cambrex, East Rutherford, NJ, USA). A total of 0.5 μl of the bacteria-containing LB or PBS was injected into the mosquito thorax. Injections of 0.5 μl sterile LB media or 1× PBS (Cambrex, East Rutherford, NJ, USA) were used as controls. dsRNA-treated mosquitoes were injected on the 4th day post-RNAi treatment. Each experiment with iREL2 and iLUC mosquitoes was performed twice.
2.9. Assessment of Plasmodium gallinaceum oocyst and sporozoite numbers
Plasmodium gallinaceum was maintained by transmission between mosquitoes and White Leghorn chickens. In all experiments, 4- to 7-day-old transgenic mosquitoes or RNAi-treated wild-type mosquitoes on the 4th day post-RNAi treatment were infected by blood feeding on P. gallinaceum-infected chickens with a 9-day-old infection. At 7 days post-infection, mosquito midguts were dissected, stained with 1% mercurochrome (Sigma), and the oocyst number was calculated. To calculate the effect of REL2 overexpression on sporozoite number, mosquitoes were infected with P. gallinaceum (as described above) and given a second naïve blood meal at 7 days post-infection to reactivate Vg-driven REL2. After 14 days post-infection, mosquito salivary glands were dissected, and the sporozoite number was calculated using phase-contrast optics.
2.10. Statistical analyses
The statistical investigation of mosquito survival rates after bacterial infections was performed by means of Cox analysis utilizing SAS version 9.6 software (SAS Institute Inc., Cary, NC).
For the oocyst number assessment experiments, either the Student’s t-test or Mann-Whitney-Wilcoxon test (Wilcoxon test) was used to analyze statistical differences between the wild-type and transgenic mosquitoes. The choice was made based on the results of normality test for the oocyst number distribution. For normal distributions, the Student’s t-test was used; for those violating the normality assumption, the non-parametric Wilcoxon test was used. Both tests were performed using statistical analysis software JMP7 SAS (SAS Institute Inc., Cary, NC).
3. Results
3.1. Generation of two transgenic mosquito strains expressing REL2 in the fat body PBM
To study the role of REL2 in mosquito immunity, we have created two transgenic mosquito strains in which the full length of the REL2-Short isoform—which includes the His/Gln (Q/H)-rich and SRR putative transactivation domain(s) as well as the REL-homology (RHD and IPT) domain—is incorporated into the mosquito genome under the control of the Vg promoter. A transformation vector pBac[3×P3_EGFP, afm] containing the Vg-REL2-Short isoform and SV40 polyadenylation region was used for mosquito germ-line transformation (Fig. 1A). The two transgenic mosquito strains, named REL2-A and REL2-B, were established as a result of the germ-line transformation and distinguished by means of green fluorescent glowing in the eyes at larval, pupal, and adult stages (not shown).
Figure 1.
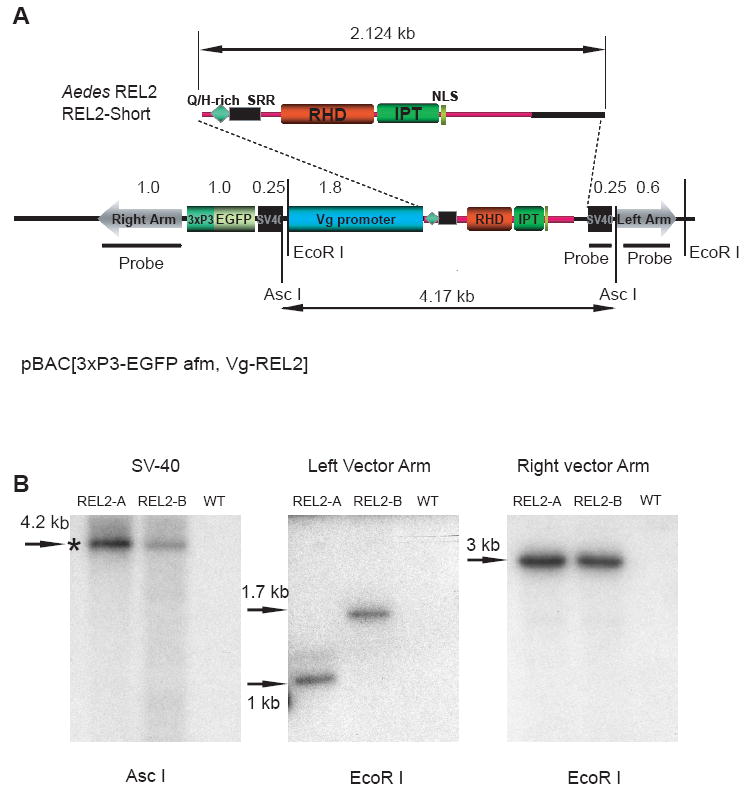
Generation of two independent transgenic strains of Aedes aegypti—REL2-A and REL2-B
(A) Schematic representation of the pBac[3×P3, EGFP afm,Vg-REL2] transformation plasmid used in germ-line transformation. The restriction sites of the restrictases used for DNA digestion are indicated; positions of the probes for right and left vector arms as well as SV40 polyadenylation region are indicated by thick black lines; double-headed arrows indicate the sizes of the REL2-Short isoform (2.124 kb) and the pBAC[3×P3-EGFP afm, Vg-REL2] construct; Q/H, glutamine/histidine-rich region, SRR, serine-rich region, RHD and IPT are parts of the REL DNA-binding domain; NLS, nuclear localization signal.
(B) Southern blot analysis of the genomic DNA extracted from REL2-A and REL2-B transgenic mosquito strains and the wild-type strain. Genomic DNA was isolated, digested using EcoR I and ASC I, and hybridized with probes specific to the internal SV40 polyadenylation region, the left and right vector arms; WT—the wild-type parental strain; asterisk indicates the band of expected size.
To investigate the number of transgene copies and the integrity of transgene insertion, we performed Southern blot analysis. Genomic DNA of the transgenic mosquito and wild-type strains was collected, digested with EcoRI and AscI endonucleases, and hybridized with probes to the left arm, internal SV40 polyadenylation region, and right arm of the pBac[3×P3_EGFP, afm] transformation vector. Southern blot hybridization analysis with the probe for the internal SV40 polyadenylation region showed a single band of expected size (4.2 kb) in both transgenic strains, demonstrating the integrity of the transgene incorporation. Hybridization with the probe for the right and left vector arms yielded a single band in each transgenic strain, demonstrating a single transgene incorporation event. The bands for the right vector arm were of about the same size; however, hybridization with the probe for the left vector arm produced single bands of different sizes in two transgenic strains, validating distinct transgene incorporation regions for the two strains (Fig. 1B). Hence, Southern blot analysis confirmed generation of two distinct transgenic mosquito strains, REL2-A and REL2-B, with different sites of transgene incorporations.
3.2. Effect of the transgenic REL2 gene overexpression
To examine the effect of transgenic REL2 gene overexpression, we performed Northern blot analysis with probes for Vg, REL2, and AMP Defensin A. Expression of transgenic REL2 was detected as early as 2 h PBM, with the maximum at 12 h, and a decline by 48 h (Fig. 2A). At 60 and 72 h PBM, Vg and transgenic REL2 were no longer detectable by means of Northern analysis (data not shown). The same REL2 expression pattern was observed for the second transgenic strain (not shown). We also compared levels of the REL2-transgene expression in REL2-A and REL2-B strains at 12 and 24 h PBM. REL2-A mosquitoes appeared to have a higher degree of transgene expression than REL2-B (Fig. S1). Tissue expression of the REL2-transgene in transgenic mosquitoes was specific to the fat body and was not detected in ovaries or midgut (Fig. 2B). Thus, expression of transgenic REL2 depends on the activation of the Vg promoter, and occurs in a similar stage- and tissue-specific manner as the endogenous Vg gene.
Figure 2.
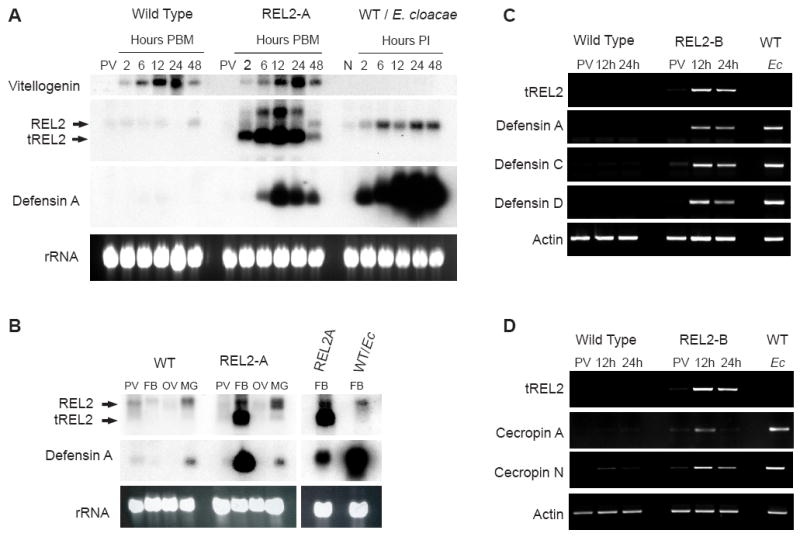
Blood-meal-activated expression of transgenic REL2 and Defensin and Cecropin families of AMP in the transgenic Ae. aegypti mosquito strains, REL2-A and REL2-B.
(A) Northern blot analysis: expression profiles of Vitellogenin, endogenous REL2, transgenic REL2, and Defensin A in the fat body of the REL2-A transgenic mosquitoes. Fat bodies were collected from wild-type (WT) and REL2-A mosquitoes: previtellogenic (PV) and blood-fed mosquitoes at 2, 6, 12, 24, and 48 h post blood meal (PBM). WT mosquitoes were injected with E. cloacae, and the fat bodies were collected from non-infected mosquitoes (n) and infected mosquitoes at 2, 6, 12, 24, and 48 h post-infection (PI). Total RNA was extracted and Northern blot analysis performed using the probes for Vg, REL2 RHD domain, and Defensin A; arrows indicate bands for endogenous REL2—REL2 and transgenic REL2—tREL2, (rRNA) ribosomal RNA—loading control.
(B) Northern blot analysis: fat-body-specific expression of transgenic REL2 and Defensin A in REL2-A mosquitoes. Total bodies were collected from previtellogenic (PV) and blood-fed wild-type (WT) and REL2-A mosquitoes at 24 h post blood meal (PBM). Mosquito fat bodies (FB), ovaries (OV), and midguts (MG) were collected from blood-fed mosquitoes at 24 h PBM. Total RNA was extracted, and Northern analysis was performed with the probes for REL2 RHD domain and Defensin A; FB from WT mosquitoes injected with E. cloacae (Ec) at 24 h post-infection were used as a control of Defensin A expression; REL2—endogenous REL2, tREL2—transgenic REL2.
(C) Blood-meal-activated expression of transgenic REL2 and Defensins A, C, and D; and
(D) blood-meal-activated expression of transgenic REL2 and Cecropin A and N in the transgenic REL2-B strain (RT-PCR analysis). Fat bodies were collected from previtellogenic (PV) and blood-fed mosquitoes at 12 and 24 h post blood meal; fat bodies collected from wild-type (WT) mosquitoes injected with E. cloacae (Ec) at 24 h post-infection were used as a control. Total RNA was extracted and treated with DNase I, and cDNA was synthesized and used as a template for RT-PCR with primers specific for transgenic REL2, Defensin A, C, D, and also Cecropin A and N.
In the transgenic mosquitoes, blood-meal-dependent expression of the transgenic REL2-Short isoform resulted in transcription of Defensin A, specifically in the mosquito fat body (Fig. 2A and B). Defensin A transcripts were detectable at 6 h PBM, reached a maximum at 12 h, and declined by 48 h. As for Vg and transgenic REL2, Defensin A mRNA transcripts were no longer detectable at 60 and 72 h PBM (data not shown). These dynamics of Defensin A transcripts were congruent with the expression profile of transgenic REL2, and with that of the Vg gene (Fig. 2A). However, in the bacteria-challenged wild-type mosquitoes, the dynamics were different: in E. cloacae-challenged mosquitoes, a sharp increase in Defensin A mRNA was detected at 2 h post-infection, increased over the course of infection, and remained at high levels 48 h post-infection, probably because of the persistence of the bacterial pathogen (Fig. 2A).
In order to evaluate whether transgenic overexpression of REL2-Short affects the expression of endogenous REL2 isoforms and REL1 (the main NF-κB factor of the mosquito Toll pathway) in the fat body PBM, total RNA was extracted from blood-fed wild-type and transgenic mosquitoes at 12 and 24 h PBM, cDNA was produced as described in Materials and Methods, and PCR was performed using primers specific to REL2 isoforms and the REL1-A isoform of REL1. No conclusive difference in the expression of REL2-Long or IκB between wild-type and REL2 transgenics in response to a blood meal was noted. In contrast, we detected a considerable increase in the level of the REL2-Short transcript in the REL2 transgenics, likely due to the fact that REL2-Short primers recognized both endogenous REL2-S and transgenic REL2-S. The REL1A transcript appeared to be slightly enhanced in transgenic mosquitoes PBM (Fig. S2).
3.4. Effect of the transgenic REL2 gene on AMP expression
Sequencing and annotation of the Ae. aegypti genome revealed that it contains four different Defensins, ten different Cecropins, and also Diptericin, Attacin, Gambicin, and Holotricin genes (Nene et al., 2007; Waterhouse et al., 2007). To study downstream targets of mosquito REL2, we designed specific primers for all AMP genes listed above and performed RT-PCR with cDNA from the transgenic and wild-type mosquitoes. We used previtellogenic and vitellogenic mosquitoes at 12 and 24 h PBM. Our data show that three of four Defensins encoded in the Ae. aegypti genome—Defensins A, C, and D—were expressed at the detectable levels as a result of transgenic REL2 overexpression, with a maximum expression at 12 h, and a decline at 24 h PBM. The relative levels of Defensins A, C, and D expression at 12 h PBM in transgenic mosquitoes were similar to those of bacterial-induced expression of these Defensins in control mosquitoes at 24 h post-infection (Fig. 2C).
Of ten Cecropins encoded in the Ae. aegypti genome, we could detect that only Cecropins A and N showed an increase in expression in response to a blood meal. Cecropin A was expressed in transgenic mosquitoes 12 h PBM. At 12 and 24 h PBM, Cecropin N was expressed at noticeably higher levels in the transgenic mosquitoes than in wild type (Fig. 2D). Diptericin, Gambicin, Attacin, and Holotricin transcripts were increased in bacteria-challenged mosquitoes, although were not detected in response to a blood meal in either the transgenics or uninfected control wild-type mosquitoes (data not shown).
3.5. Blood-meal-activated production of Defensin A peptide in REL2 transgenic mosquitoes
Next, we studied the effect of transgenic REL2 overexpression on Defensin A protein dynamics. To analyze the tissue-specific expression of Defensin A, total bodies, fat bodies, and ovaries were collected from transgenic and wild-type female mosquitoes before blood meal and 24 h after blood feeding. Total protein was then extracted, and Western blot analysis performed using antibodies against Ae. aegypti Defensin A. The results showed the presence of Defensin A in transgenic female mosquitoes 24 h PBM. This AMP was detected in the transgenic mosquito fat body. No Defensin protein was detected in the mosquito ovaries, indicating tissue specificity of production of this AMP in the REL2 transgenic mosquitoes (Fig. 3A).
Figure 3.
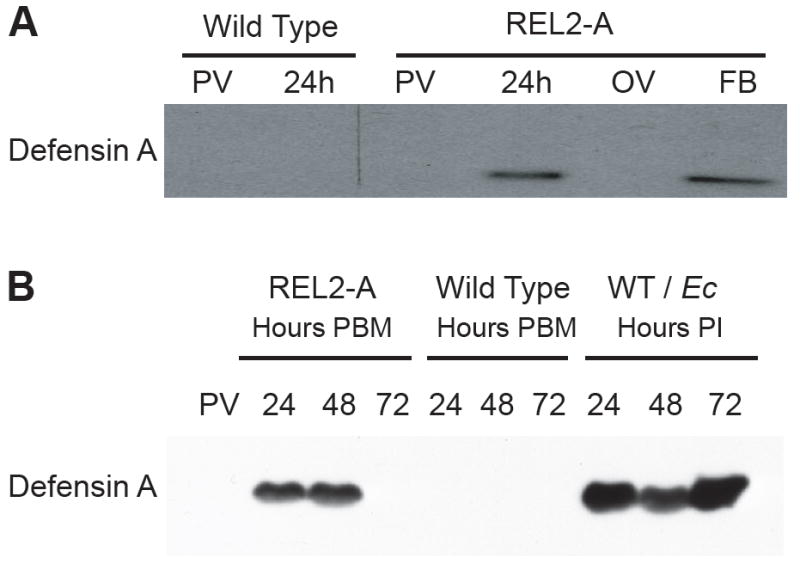
Blood-meal-activated Defensin A protein in the fat bodies (FB) and hemolymph of REL2 transgenic mosquitoes. (A) Blood-meal-activated Defensin A in the FB. Total bodies of previtellogenic (PV) and blood-fed mosquitoes at 24 h post blood meal (PBM), as well as FB and ovaries (OV) of blood-fed REL2-A were used for protein extraction. A 10-μg aliquot of total protein was used per lane for SDS-PAGE, and Western blot analysis was performed using the anti-Defensin A antibodies. (B) Blood-meal-activated secretion of Defensin A peptide in the hemolymph of transgenic mosquitoes. Wild-type and REL2-A mosquitoes were blood fed, and control PV wild-type mosquitoes were injected with E. cloacae; hemolymph was collected as described in Materials and Methods at 24, 48, and 72 h PBM and at the same time points post-infection (PI). Protein (10 μg) was loaded per lane for SDS-PAGE, and Western analysis was performed using the antibodies against Defensin A. WT/Ec—wild-type mosquitoes injected with E. cloacae.
To examine whether Defensin was secreted into the hemolymph of transgenic mosquitoes, we collected hemolymph from previtellogenic or blood-fed transgenic females at 24, 48, and 72 h PBM, and from bacteria-challenged wild-type mosquitoes at 24, 48, and 72 h post-infection. Western blot analysis indicated the presence of Defensin peptide in the hemolymph of transgenic mosquitoes at 24 and 48 h PBM (Fig. 3B). Interestingly, the presence of the blood-meal-induced Defensin in the hemolymph of transgenic REL2 mosquitoes is relatively short compared with that due to infection. At 72 h PBM, Defensin was no longer detected in the hemolymph of transgenic mosquitoes; however, it was still abundantly present in that of bacterially challenged wild-type mosquitoes (Fig. 3B). The disappearance of Defensin A protein from the transgenic mosquito hemolymph after 48 h PBM is consistent with the dynamics of Vg, transgenic REL2, and Defensin A mRNA expression, and is most probably due to the intrinsic properties of the Vg promoter. Thus, it is most likely that other AMPs activated in the fat body of REL2 transgenic mosquitoes are also secreted into the hemolymph.
3.6. REL2 RNAi-mediated knockdown negatively affects Defensin A and Cecropin A expression
To validate whether the observed downstream effects of the transgenic REL2 signaling were due to the transgene function, we used RNAi to knockdown REL2-Long and REL2-Short isoforms in wild-type mosquitoes and studied the effect on Defensin A and Cecropin A expression. dsRNA against the REL2 RHD domain, which is the common region for REL2-Long and REL2-Short, was used for the knockdowns. Wild-type mosquitoes were treated with either dsRNA against RHD domain (iRHD) or control non-specific dsRNA against Luciferase gene (iLuc). On the 4th day after RNAi treatment, the isoform knockdown was confirmed using RT-PCR with REL2-Long- and REL2-Short-specific primers (Fig. 4A). RNAi-treated mosquitoes were then injected with E. cloacae. Total RNA was collected from both naïve, uninfected RNAi-treated mosquitoes and those infected at 1, 6, and 24 h post-bacterial challenge. Following this, RT-PCR was performed using Defensin A- and Cecropin A-specific primers. The results showed that REL2 knockdown led to more than a 6-h delay in Defensin A and Cecropin A expression in bacteria-infected mosquitoes (Fig. 4B and 4C). Thus, combined results of transgenic REL2 overexpression and REL2 RNAi treatment further suggested that this mosquito NF-κB plays a key role in regulation of AMP expression.
Figure 4.

RNAi knockdowns of REL2-Long and REL2-Short result in the delayed expression of AMPs Defensin A and Cecropin A in wild-type, E. cloacae-infected mosquitoes. (A) Expression of REL2-Long and REL2-Short in the REL2 dsRNA-treated mosquitoes. (B and C) Delayed expression of Defensin A (B) and Cecropin A (C) in response to the bacterial challenge in RNAi-treated mosquitoes. iRHD—mosquitoes treated with dsRNA against REL2 RHD domain; iLuc—mosquitoes treated with dsRNA against luciferase gene. N—naïve, non-treated wild-type mosquitoes (neither RNAi nor bacteria treatments); Ecl and E. coli—wild-type mosquitoes not treated with dsRNA, at 24 h post-bacterial infection: Ecl—E. cloacae injections E. coli—E. coli injections. Actin amplification represents the loading control for both iRHD and iLuc mosquitoes, where equal amplification of actin was observed in all experiments.
3.7. Survivorship of REL2 transgenic mosquitoes to different bacterial pathogens
To study the effect of REL2 overexpression on the resistance of mosquitoes to different bacterial pathogens, we performed survival experiments for transgenics and wild-type mosquitoes using three different types of bacteria: two Gram-negative bacteria, P. aeruginosa and E. cloacae; and the Lys-type Gram-positive bacterium S. aureus. P. aeruginosa is an opportunistic human pathogen, although deadly to mosquitoes, and was shown to suppress AMP expression in infected fruit flies (Apidianakis et al., 2005). E. cloacae is moderately pathogenic to mosquitoes and elicits prominent induction of immune markers in response to infection (Hedengren-Olcott et al., 2004). S. aureus is a mild mosquito pathogen, the susceptibility to which highly increases in mosquitoes lacking Defensin or REL2 (Blandin et al., 2002; Meister et al., 2005). For the survival experiments, we used 4-day-old mosquitoes and injected them with bacteria at 6–10 h PBM. The results of our experiments indicated that transgenic REL2-A mosquitoes were more resistant to P. aeruginosa, E. cloacae, and S. aureus than wild-type mosquitoes (Fig. 5A, B, and C). For P. aeruginosa, the transgenics exhibited a 4- to 6-h delay in 50% mortality, and for E. cloacae and S. aureus the transgenics showed a 10–20% increase in mosquito survival. Similar survival profiles were observed for the REL2-B transgenic strain (data not shown). Cox analysis demonstrated a significant difference in the mortality rates between REL2-A transgenics and wild-type mosquitoes for P. aeruginosa, E. cloacae, and S. aureus with a P value < 0.05. We speculate that the increased resistance of the transgenics to bacterial pathogens is most probably due to the presence of Defensins and putatively other antimicrobial factors in the hemolymph of transgenic mosquitoes before the introduction of bacteria.
Figure 5.
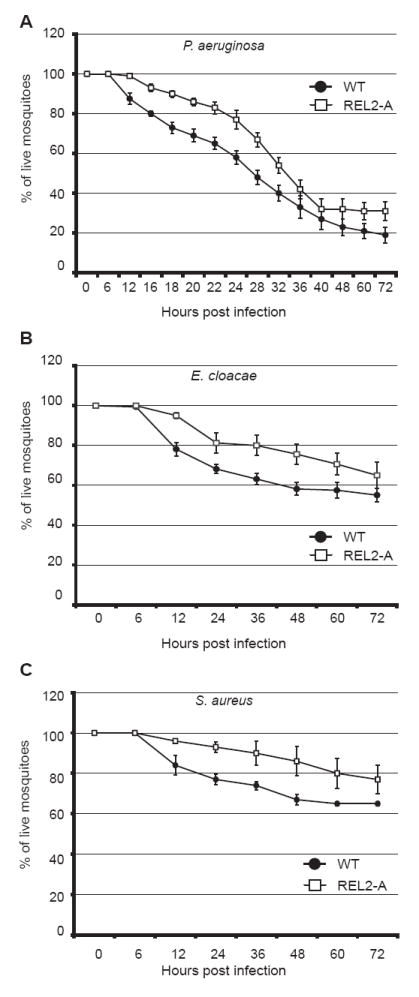
Increase in survival of REL2 transgenics to Gram-negative and Gram-positive bacteria Wild-type and transgenic mosquitoes were blood fed and injected with bacteria between 6 and 12 h post blood meal. Survival was monitored by counting the number of live mosquitoes.
(A) Decreased susceptibility of REL2-A transgenics to E. cloacae infection. Combined results of five independent experiments; total number of mosquitoes used was 155; error bars represent standard errors; P values < 0.05 (Cox analysis)
(B) Decreased susceptibility of REL2-A transgenics to P. aeruginosa infection. Combined results of six independent experiments; total number of mosquitoes used was 192; error bars represent standard errors; P values < 0.05 (Cox analysis)
(C) Decreased susceptibility of REL2-A transgenics to S. aureus infection. Combined results of three independent experiments; total number of mosquitoes used was 96; error bars represent standard errors; P values < 0.05 (Cox analysis)
3.8. REL2 RNAi-mediated deletion results in the increased susceptibility of the mosquitoes to bacterial pathogens
Next, we investigated the effect of RNAi-mediated REL2 knockdown on the mosquito resistance to bacterial pathogens of different Gram types. We used the Gram-negative bacteria Enterobacter cloacae and the Gram-positive bacteria Enterococcus faecalis, which is a mild mosquito pathogen (Hillyer et al., 2004) that contains Lys-type peptidoglycans and activates the Toll immune pathway in Drosophila (Ferrandon et al., 2007). Our results demonstrated that the knockdown of REL2 resulted in an increase in mosquito mortality to both tested bacteria (Fig. 6A and B). In iRHD-treated mosquitoes, the injections with E. cloacae caused 50% mosquito mortality at 24 h post-infection and, by 36 h, all iRHD-treated mosquitoes were dead. At the same time, about 40% of those treated with iLuc were still alive 5 days after bacterial challenge (Fig. 6A). The injections with E. faecalis resulted in 50% iRHD mosquito mortality at 72 h post-infection, and all iRHD mosquitoes were dead by day 5 post-infection; about 80% of infected iLuc mosquitoes, however, were still alive at this time point (Fig. 6B). These results are consistent with findings in the REL2 transgenic mosquitoes, where overexpression of REL2-Short had led to an increase in resistance to Gram-negative and Gram-positive bacteria. Although dsREl2 knockdown revealed only weak phenotypes with respect to Defensin A and Cecropin A expression (Fig. 4), the susceptibility to bacterial infections was strong (Fig. 6). This suggests that the decrease in Rel2 expression by iREL2 might lead to a reduced induction of other AMPs, warranting further investigation of this system in the future. Recently, a microarray-based gene expression assay in Ae. aegypti has revealed that 35 genes are induced as a result of Caspar depletion, a negative regulator of REL2 (Xi et al., 2008). Among genes induced by Caspar silencing were TEP13 and the AMPs Defensin E and gambicin.
Figure 6.
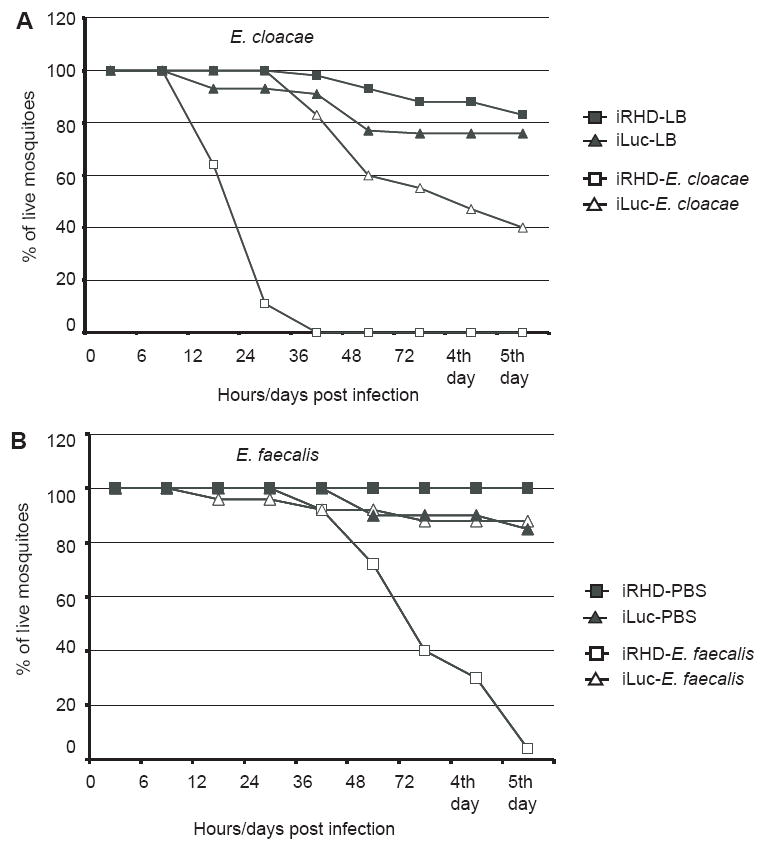
REL2 RNAi-mediated knockdown results in the increased susceptibility to Gram-negative and Gram-positive bacteria REL2 sdRNA-treated mosquitoes were injected with bacteria on the 4th day after RNAi treatment. Survival was monitored by counting the number of live mosquitoes. (A) Increased susceptibility of iRHD-treated mosquitoes to E. cloacae infection. Combined results of two independent experiments. (B) Increased susceptibility of iRHD mosquitoes to E. faecalis infection; combined results of two independent experiments. iRHD—mosquitoes treated with dsRNA against REL2 RHD domain; iLuc—mosquitoes treated with non-specific dsRNA against luciferase gene; LB—mosquitoes injected with sterile LB media.
3.9. Increased resistance of REL2 transgenic mosquitoes to Plasmodium gallinaceum
To examine the effect of overexpression of REL2 on development of the malaria parasite P. gallinaceum in its vector host Ae. aegypti, we carried out a Plasmodium oocyst and sporozoite number study. To address the oocyst development in the mosquito midgut, we infected REL2-A, REL2-B, and the wild-type mosquitoes with P. gallinaceum by allowing them to feed on P. gallinaceum-infected chickens. At 7 days post-infection, mosquito midguts were dissected and stained with mercurochrome, and the oocyst number was counted. The results showed that under average infection conditions REL2-A had significantly fewer oocysts than wild-type mosquitoes: most REL2-A mosquitoes had between 0 and 10 oocysts relative to the wild-type mosquitoes which had more than 40 oocysts per midgut (Student’s t-test, P < 0.05) (Fig. 7A and B). Similar results demonstrating a significant reduction in oocyst number were obtained for the REL2-B mosquito strain (Wilcoxon test, P < 0.05) (Fig. 7C and D).
Figure 7.
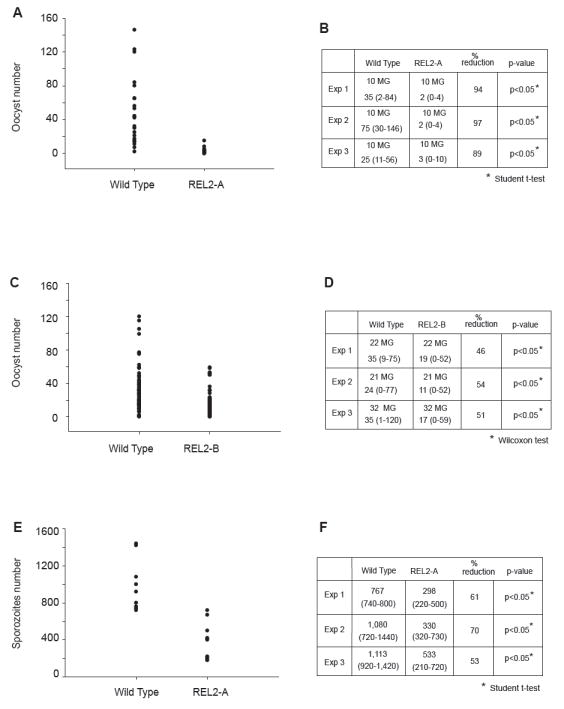
Increased resistance of REL2 transgenic Ae. aegypti to P. gallinaceum infection. (A) and (C) Decreased number of P. gallinaceum oocysts in the midguts of REL2-A and REL2-B mosquitoes, respectively. (B) and (D) Numeric representation of the graphs in (A) and (C), respectively; average oocyst number for REL2-A, REL2-B, and wild-type mosquitoes represents the average range of number of oocysts per experiment (shown in parentheses); number of midguts used per experiment is indicated; (MG) midguts. (E) and (F) Sporozoite number was significantly reduced in REL2-A mosquitoes: (C) P. gallinaceum sporozoite number in the REL2-A mosquitoes; (D) numeric representation of the graph in (C); the table reflects average number of sporozoites and the range is indicated in parentheses.
To study sporozoite development in the transgenic REL2, we first infected mosquitoes with P. gallinaceum. At 7 days post-infection, we gave the mosquitoes a second naïve blood meal to reactivate transgenic REL2 expression. At 14 days after infection, mosquito salivary glands were dissected, and the sporozoite number was calculated. The experiments were repeated three times, and the results demonstrated a significant decrease in sporozoite number in REL2-A mosquitoes (Student’s t-test, P < 0.05) when compared with the wild-type (Fig. 7E and F). Thus, these data showed that overexpression of REL2 hinders development of P. gallinaceum.
3.10. REL2 is required for the resistance against Plasmodium gallinaceum
Finally, to study the effect of REL2 RNAi-mediated deficiency on P. gallinaceum development, we conducted oocyst number experiments in the REL2 RNAi-treated mosquitoes. In this trial, on the 4th day post-RNAi treatment, REL2 RNAi and control iLuc mosquitoes were infected by blood feeding on P. gallinaceum-infected chickens. The oocyst number was counted on the 7th day post-infectious blood meal, as described above. Our results indicated that REL2 RNAi-treated mosquitoes were significantly more susceptible to P. gallinaceum infection than control iLuc mosquitoes (Wilcoxon tests, P < 0.05) (Fig. 8A and B). Thus, combined results of REL2 overexpression and REL2 knockdowns suggest an important role for REL2 in Ae. aegypti anti-Plasmodium immunity.
Figure 8.
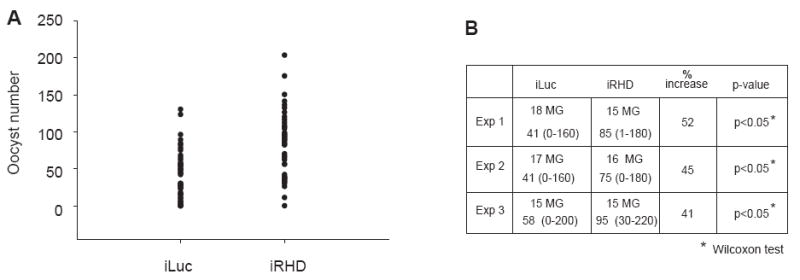
REL2 RNAi-mediated knockdown results in the increased susceptibility of Ae. aegypti to P. gallinaceum infection. (A) Increased number of P. gallinaceum oocysts in the midguts of iRHD-treated mosquitoes versus control iLuc mosquitoes (P < 0.05 Wilcoxon test). (B) Numeric representation of the graph in (A), average oocyst number for REL2 RNAi (iREL2), and control Luciferase RNAi (iLuc) mosquitoes represents the average range of number of oocysts per experiment (shown in parentheses). Number of midguts used per experiment is indicated; (MG) midguts.
4. Discussion
In this study, we used mosquito transgenesis and RNA interference to further decipher the role of mosquito REL2 in antibacterial and anti-Plasmodium immunity of Ae. aegypti. First, we generated and characterized two independent transgenic mosquito strains of Ae. aegypti, in which the REL2-Short isoform of NF-κB transcriptional factor REL2 was overexpressed in the mosquito fat body under control of the Vg promoter. We have presented molecular and phenotypic characterization of these strains, as well as gene expression analysis, and have reported the effect of REL2 overexpression on both AMP synthesis in the adult mosquito and mosquito resistance to bacterial and P. gallinaceum infection. Second, we used RNAi to study the effect of REL2 knockdowns on AMP expression and resistance to bacterial and Plasmodium pathogens.
For both transgenic strains of Ae. aegypti—REL2-A and REL2-B—there was a single site for transgene incorporation, and the incorporated vector constructs were intact. Also, REL2-A and REL2-B transgenics showed distinct regions of the transgene incorporation in their genomes. Together, this knowledge allowed us to rule out the position effect of the transgenes and interpret the results of the experiments as direct effects of the transgene function. Both REL2-A and REL2-B transgenic mosquitoes demonstrated tissue-specific expression of transgenic REL2 in the fat body PBM. The pattern of the transgene expression was consistent with the dynamics of Vg gene expression (Kokoza et al, 2000; 2001b). Transgenic REL2 transcripts were detected at time points that corresponded to those of Vg transcripts, confirming blood-meal-dependent activation of the transgenic REL2.
Previously, we have shown that transgenic overexpression of REL2 lacking the N-terminal Q/H rich and SRR region results in an immune-deficient (RIMD) phenotype in the mosquito Ae. aegypti (Shin et al., 2003). This RIMD transgenic mosquito exhibited significant reduction in expression of Defensin A and Cecropin A and was highly sensitive to infection with Gram-negative bacteria. In our present work, the full-length REL2-Short isoform that includes an N-terminus with the Q/H rich and SRR region was overexpressed under the control of the Vg promoter in two transgenic mosquito strains. Transgenic overexpression of the complete REL2-Short isoform resulted in an enhanced expression of several AMPs and increased antimicrobial and anti-Plasmodium resistance. Hence, the N-terminal region that includes Q/H rich and SRR sequences plays a major role in transactivation by the REL2 NF-κB transcription factor. Shin et al. (2002) have suggested the transactivation function of this REL2 region based on the sequence similarly with glutamine-rich transcriptional activation domains of some transcription factors, which are also associated with histidine-rich stretches. Drosophila Dorsal contains an N-terminal Rel homology domain (RHD), which is responsible for DNA binding and regulated nuclear import, and a C-terminal domain (CTD) that contains activation and repression motifs. Mutational experiments, however, have revealed that its N-terminal domain also plays an active role in transcriptional regulation (Jia et al., 2002). Likewise, transcriptional activation analysis has shown that both the N and C termini of RelB, a member of the Rel family of transcription factors, are required for full gene transactivation (Dobrzanski et al., 1993). More detailed functional studies are needed to decipher composition of transactivation domains in the mosquito REL2.
In the Ae. aegypti genome, 18 AMP genes were annotated, including four defensin genes: Defensins A, C, D, and E (Waterhouse et al., 2007). We studied the effect of REL2 overexpression on Defensin gene family expression. Our results showed that Defensins A, C, and D were expressed in the fat bodies of the transgenic mosquitoes in response to a blood meal. Moreover, Defensin A protein was detected in the fat body tissue and mosquito hemolymph of these transgenic mosquitoes PBM, independently of bacterial challenge. The presence of Defensin A in the hemolymph was dependent on transgenic REL2 activation, since a decrease in transgenic REL2 expression at later time points after the blood meal resulted in Defensin A diminishing in the hemolymph. All presented observations suggest that, in Ae. aegypti, REL2 is a major contributor to the induction of Defensin family genes in the fat body.
Sequencing and annotation of the Ae. aegypti genome revealed a high diversity of the Cecropin gene family. The Aedes genome encodes ten cecropin genes, whereas A. gambiae possesses only four and D. melanogaster five (Waterhouse et al., 2007). In our study, we investigated the input of REL2 signaling on the expression of the cecropin gene family. Of ten cecropins encoded in the Ae. aegypti genome, only Cecropins A and N demonstrated a modest level of transcriptional activation in response to REL2 expression. Moreover, we were unable to detect expression of Attacin, Gambicin, and Holotricin in REL2 transgenic mosquitoes activated by a blood meal. This suggests that, in Ae. aegypti, REL2 short isoform signaling alone is not sufficient for transcriptional activation of most genes in the Cecropin gene family or of other AMPs.
There are several possible explanations for such differential AMP gene expression in response to REL2-Short overexpression. The first originates from the nature of NF-κB factors that must form dimers for DNA binding and activation of transcription (Baeuerle and Baltimore, 1996). In Drosophila melanogaster, Toll and IMD pathways synergistically activate an innate immune response. Constitutive activation of Toll and PGRP-LC/IMD mimics the synergistic stimulation. In addition, RNA interference assays and promoter analyses demonstrated that cooperation of different NF-κB-related transcription factors mediates the synergy. In Drosophila cell lines, Relish/Relish homodimers cause modest levels of Cecropin and Attacin expression, while Relish/Dif heterodimers enhance significantly the transcription of these AMPs (Han and Ip, 1999; Tanji et al., 2007). In our transgenic system, in which REL2 is activated by the Vg promoter, bypassing the entire immune response, only REL2/REL2 homodimers were expected to be available for κB site binding in the promoters of responsive genes. Thus, it is possible that, in Ae. aegypti, REL2/REL2 homodimer binding to κB sites in the promoters of Defensins A, C, and D is sufficient to initiate transcription; REL1/REL2 heterodimer formation, however, might be necessary for the transcriptional activation of other immune factors. In addition, a general requirement for JNK signaling in the expression of AMPs has recently been demonstrated by the expression of a JNK inhibitor and induction of JNK loss-of-function clones in immune responsive tissue of Drosophila (Delaney et al., 2006). Moreover, full activation of the Drosophila CecropinA1 gene requires simultaneous function of NF-κB factors and a yet-unknown factor R1 (Uvel and Engström, 2003; 2007).
REL2 transgenics exhibited increased resistance to Gram-negative and Gram-positive bacteria. Previously, the key role of REL2 in the mosquito Defensin A and Cecropin A production as well as defense against Gram-negative bacteria has been demonstrated by transgenic overexpression of a non-functional form of REL2 in Ae. aegypti (Shin et al., 2003). In An. gambiae, dsRNA knockdown of REL2 resulted in suppression of Defensin and Cecropin 1 expression and in dramatically increased susceptibility of the mosquito to Gram-negative and Gram-positive bacteria (Meister et al., 2005). In congruence with these findings, in our study, overexpression of REL2 resulted in increased resistance of Ae. aegypti to two Gram-negative bacteria, P. aeruginosa and E. cloacae, which are strong mosquito pathogens. In An. gambiae, REL2 has been implicated in the mosquito defense against the Gram-positive bacteria S. aureus (Meister et al., 2005). Our results indicated that overexpression of REL2 increases resistance of mosquitoes to S. aureus, suggesting involvement of REL2 in the defense against Gram-positive bacteria. This was further supported by the results of the survival tests in REL2 RNAi-treated mosquitoes, in which a dramatic increase in mosquito mortality was observed in response to bacterial infection by either Gram-negative or Gram-positive bacteria.
REL2-overexpressing transgenics have increased resistance to P. gallinaceum. On average, we observed a decrease of 90% and 50%, for REL2-A and REL2-B, respectively, in oocyst number in the midguts of the transgenics when compared with that in the wild type. Also, the transgenics exhibited a significant decrease in sporozoite number in the salivary gland when the Plasmodium-infected mosquitoes were given the second naïve blood meal on the 7th day post-infection to re-activate transgenic REL2 expression. The importance of REL2 for anti-Plasmodium immunity was further supported by the oocyst number assessment experiments in REL2 RNAi mosquitoes, in which REL2 RNAi mosquitoes had significantly higher oocyst numbers than the control RNAi insects. Likewise, RNAi depletion of REL2 in An. gambiae has been shown to significantly increase susceptibility of the mosquito to P. berghei, indicating involvement of REL2 in anti-malaria defense (Meister, et al., 2005). It is possible that the increased resistance of REL2-A and REL2-B transgenic Ae. aegypti mosquitoes to Plasmodium was due to toxicity of Defensins and Cecropins to Plasmodium oocysts and sporozoites. In early experiments, it was found that Defensins from dragon fly (Aeschna cyanea) and flesh fly (Phormia terranovae) inhibited the development of P. gallinaceum oocysts and sporozoites (Shahabuddin et al., 1998). Indeed, transgenic overexpression of both Defensin A and Cecropin A in Ae. aegypti results in nearly complete inhibition of Plasmodium development and interruption of its transmission (Ahmed, Kokoza and Raikhel, unpublished results). However, we cannot exclude the possibility that increased resistance of REL2 transgenic to P. gallinaceum was also due to the production of other anti-Plasmodium factors that are controlled by REL2.
Transgenesis and RNA interference are indispensable tools, permitting in-depth study of the molecular mechanisms underlying biological processes. Our findings contribute to current knowledge of the role of NF-κB factor REL2 in mosquito innate immunity.
Supplementary Material
Figure S1: Levels of the transgenic REL2 and Defensin A expression in REL2-A and REL2-B transgenic mosquito strains. Northern analysis: RNA was collected from REL2-A and REL2-B transgenic mosquitoes, previtellogenic (PV), and 12 and 24 h post blood meal (PBM); wild-type mosquitoes injected with E. cloacae 24 h post-injection (WT/Ecl) were used as a control. Northern blot analysis was performed using the probes for Vg and REL2 RHD domain; arrows indicate bands for endogenous REL2—REL2 and transgenic REL2—tREL2, (rRNA) ribosomal RNA—loading control.
Figure S2: The expression of endogenous isoforms of REL2 and REL1 in the REL2 transgenics in response to a blood meal in transgenic mosquitoes with blood-meal activated transgenic REL2-Short. (A) Primers designed for detection of the endogenous REL2-isoforms expression in REL2 transgenic mosquitoes after a blood meal. For detection of the REL2-Long isoform, the forward primer was designed to the region flanking the junction of exons 1 and 2. This region is specific to the REL2-Long and REL2-Short isoforms. The reverse primer was chosen to include the last three nucleotides of REL2-Long ORF and the beginning of the 3’UTR region. This primer is specific to both REL2-Long and IκB isoforms, since REL2-Long and IκB isoforms share an identical 3-UTR region. Such a primer design allowed specific amplification of REL2-Long. The PCR product obtained from use of these primers yielded a band of about 3 kb, which corresponds to the length of REL2-Long isoform (3.2 kb). For detection of the IκB isoform, the forward primer was chosen in the region flanking the junction between exons 1 and 5. This region is specific to the IκB isoform. The reverse primer used was the same as that used for amplification of REL2-Long. The PCR product obtained from use of these primers yielded a band of about 2.2 kb, which corresponds to the length of the IκB isoform (2.7) without exon 1. To detect the REL2-Short isoform, we used the same forward primer as was used for the amplification of REL2-Long. The reverse primer was chosen for the region unique for REL2-Short, i.e., the last three nucleotides of ORF and the 3’-UTR region. The PCR product obtained from use of these primers yielded a band of about 2 kb, which corresponds to the length of REL2-Short isoform (2.1 kb). Primers used for detection of the endogenous REL2-Short isoform also amplify the transgenic REL2-Short isoform because we overexpressed the full length of the REL2-Short isoform and, therefore, the transgenic and endogenous REL2-Short isoforms have identical nucleotide sequence. To detect the transgenic transcript, a pair of primers consisting of forward primer from the 3’ region of Vg promoter sequence and the reverse from REL2 exon 2 was used. The arrows indicate the position of the detection primers. Numbers represent the exons of REL2; specific 3’UTR of REL2-Short is shown in red.
(B) The expression of endogenous isoforms of REL2 and REL1 in the REL2 transgenics in response to a blood meal. Total RNA was collected from REL2 transgenics previtellogenic and 12 and 24 h post blood meal; RNA from E. cloacae-infected wild-type (WT) mosquitoes at 24 h post-infection was used as a control. RT-PCR was performed with primers specific for the endogenous REL2 isoforms and REL1-A; amplification Actin DNA was used as a control. tREL2—transgenic REL2; Long, Short, IκB—isoforms of Ae. aegypti REL2; WT—wild type, WT-Ecl—wild-type mosquitoes infected with E. cloacae; PBM—post blood meal; PI—post-infection.
Acknowledgments
This work was supported by grants 5RO1 AI059492 and 4R37 AI024716 from the National Institutes of Health. We wish to thank Dr. W.L. Cho for his kind gift of anti-Defensin A antibodies, Drs. Sang Woon Shin and Zhen Zou for critical reading of the manuscript, and Ms. E. Cannell for her excellent technical support.
Abbreviations
- AMP
antimicrobial peptides
- Vg
Vitellogenin
- REL2
transcriptional factor REL2, homologue of Drosophila Relish
- EGFP
enhanced green fluorescent protein
- BM
blood meal
- PBM
post blood meal
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Apidianakis Y, Mindrinos MN, Xiao W, Lau GW, Baldini RL. Profiling early infection responses: Pseudomonas aeruginosa eludes host defenses by suppressing antimicrobial peptide gene expression. Proc Natl Acad Sci U S A. 2005;102:2573–2578. doi: 10.1073/pnas.0409588102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeuerle PA, Baltimore D. NF-κB: Ten Years After. Cell. 1996;87:13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- Bian G, Shin SW, Cheon HM, Kokoza V, Raikhel AS. Transgenic alteration of Toll immune pathway in the female mosquito Aedes aegypti. Proc Natl Acad Sci U S A. 2005;102:13568–13573. doi: 10.1073/pnas.0502815102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blandin S, Moita KF, Kocher T, Wilm M, Kafatos FC, Levashina EA. Reverse genetics in the mosquito Anopheles gambiae: targeted disruption of the defensin gene. EMBO Rep. 2002;3:852–856. doi: 10.1093/embo-reports/kvf180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christophides GK, Zdobnov E, Barillas-Mury C, Birney E, Blandin S, Blass C, Brey PT, Collins FH, Danielli A, Dimopoulos G, Hetru C, Hoa NT, Hoffmann JA, Kanzok SM, Letunic I, Levashina EA, Loukeris TG, Lycett G, Meister S, Michel K, Moita LF, Müller HM, Osta MA, Paskewitz SM, Reichhart JM, Rzhetsky A, Troxler L, Vernick KD, Vlachou D, Volz J, von Mering C, Xu J, Zheng L, Bork P, Kafatos FC. Immunity-related genes and gene families in Anopheles gambiae. Science. 2002;298:59–65. doi: 10.1126/science.1077136. [DOI] [PubMed] [Google Scholar]
- Christophides GK, Vlachou D, Kafatos FC. Comparative and functional genomics of the innate immune system in the malaria vector Anopheles gambiae. Immunol Rev. 2004;198:127–148. doi: 10.1111/j.0105-2896.2004.0127.x. [DOI] [PubMed] [Google Scholar]
- Cho WL, Fu YC, Chen CC, Ho CM. Cloning and characterization of cDNAs encoding the antibacterial peptide, defensin A, from the mosquito, Aedes aegypti. Insect Biochem Mol Biol. 1996;26:395–402. doi: 10.1016/0965-1748(95)00108-5. [DOI] [PubMed] [Google Scholar]
- Coates CJ, Jasinskiene N, Miyashiro L, James AA. Mariner transposition and transformation of the yellow fever mosquito, Aedes aegypti. Proc Natl Acad Sci U S A. 1998;95:3748–2751. doi: 10.1073/pnas.95.7.3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney JR, Stöven S, Uvell H, Anderson KV, Engström Y, Mlodzik M. Cooperative control of Drosophila immune responses by the JNK and NF-κB signaling pathway. EMBO J. 2006;25:3068–3077. doi: 10.1038/sj.emboj.7601182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrzanski P, Ryseck RP, Bravo R. Both N- and C-terminal domains of RelB are required for full transactivation: role of the N-terminal leucine zipper-like motif. Mol Cell Biol. 1993;13:1572–1582. doi: 10.1128/mcb.13.3.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrandon D, Imler JL, Hetru C, Hoffmann JA. The Drosophila systemic immune response: sensing and signalling during bacterial and fungal infections. Nat Rev Immunol. 2007;7:862–874. doi: 10.1038/nri2194. [DOI] [PubMed] [Google Scholar]
- Franz AWE, Sanchez-Vargas I, Adelman ZN, Blair CD, Beaty BJ, James AA, Olson KE. Engineering RNA interference-based resistance to dengue virus type 2 in genetically modified Aedes aegypti. Proc Natl Acad Sci U S A. 2006;103:4198–4203. doi: 10.1073/pnas.0600479103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frolet C, Thoma M, Blandin S, Hoffmann JA, Levashina EA. Boosting of NF-κB dependent basal immunity of Anopheles gambiae aborts development of Plasmodium berghei. Immunity. 2006;25:1–9. doi: 10.1016/j.immuni.2006.08.019. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Michael J, May MJ, Kopp EB. NF-κB and REL proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–60. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- Han SZ, Ip YT. Interaction and specificity of Rel-related proteins in regulating Drosophila immunity gene expression. J Biol Chem. 1999;274:21355–21361. doi: 10.1074/jbc.274.30.21355. [DOI] [PubMed] [Google Scholar]
- Hays AR, Raikhel AS. Novel protein produced by the vitellogenic fat body and accumulated in mosquito oocytes. Roux’s Arch Dev Biol. 1990;199:114–121. doi: 10.1007/BF02029559. [DOI] [PubMed] [Google Scholar]
- Hedengren M, Asling B, Dushay MS, Ando I, Ekengren S, Wihlborg M, Hultmark D. Relish, a central factor in the control of humoral but not cellular immunity in Drosophila. Mol Cell. 1999;4:827–837. doi: 10.1016/s1097-2765(00)80392-5. [DOI] [PubMed] [Google Scholar]
- Hedengren-Olcott M, Olcott MC, Mooney DT, Ekengren S, Geller BL, Taylor BJ. Differential activation of the NF-κB-like factors Relish and Dif in Drosophila melanogaster by fungi and Gram-positive bacteria. J Biol Chem. 2004;279:21121–21127. doi: 10.1074/jbc.M313856200. [DOI] [PubMed] [Google Scholar]
- Hillyer JF, Schmidt SL, Christensen BM. The antibacterial innate immune response by the mosquito Aedes aegypti is mediated by hemocytes and independent of Gram type and pathogenicity. Microbes Infect. 2004;6:448–459. doi: 10.1016/j.micinf.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Hoffmann JA. The immune response of Drosophila. Nature. 2003;426:33–38. doi: 10.1038/nature02021. [DOI] [PubMed] [Google Scholar]
- Hoffmann JA, Reichhart JM. Drosophila innate immunity: an evolutionary perspective. Nat Immunol. 2002;3:121–126. doi: 10.1038/ni0202-121. [DOI] [PubMed] [Google Scholar]
- Holt RA, Subramanian GM, Halpern A, Sutton GG, Charlab R, Nusskern DR, Wincker P, Clark AG, Ribeiro JM, Wides R, Salzberg SL, Loftus B, Yandell M, Majoros WH, Rusch DB, Lai Z, Kraft CL, Abril JF, Anthouard V, Arensburger P, Atkinson PW, Baden H, de Berardinis V, Baldwin D, Benes V, Biedler J, Blass C, Bolanos R, Boscus D, Barnstead M, Cai S, Center A, Chaturverdi K, Christophides GK, Chrystal MA, Clamp M, Cravchik A, Curwen V, Dana A, Delcher A, Dew I, Evans CA, Flanigan M, Grundschober-Freimoser A, Friedli L, Gu Z, Guan P, Guigo R, Hillenmeyer ME, Hladun SL, Hogan JR, Hong YS, Hoover J, Jaillon O, Ke Z, Kodira C, Kokoza E, Koutsos A, Letunic I, Levitsky A, Liang Y, Lin JJ, Lobo NF, Lopez JR, Malek JA, McIntosh TC, Meister S, Miller J, Mobarry C, Mongin E, Murphy SD, O’Brochta DA, Pfannkoch C, Qi R, Regier MA, Remington K, Shao H, Sharakhova MV, Sitter CD, Shetty J, Smith TJ, Strong R, Sun J, Thomasova D, Ton LQ, Topalis P, Tu Z, Unger MF, Walenz B, Wang A, Wang J, Wang M, Wang X, Woodford KJ, Wortman JR, Wu M, Yao A, Zdobnov EM, Zhang H, Zhao Q, Zhao S, Zhu SC, Zhimulev I, Coluzzi M, della Torre A, Roth CW, Louis C, Kalush F, Mural RJ, Myers EW, Adams MD, Smith HO, Broder S, Gardner MJ, Fraser CM, Birney E, Bork P, Brey PT, Venter JC, Weissenbach J, Kafatos FC, Collins FH, Hoffman SL. The genome sequence of the malaria mosquito Anopheles gambiae. Science. 2002;298:129–49. doi: 10.1126/science.1076181. [DOI] [PubMed] [Google Scholar]
- Horn C, Wimmer EA. A versatile vector set for animal transgenesis. Dev Genes Evol. 2000;210:630–637. doi: 10.1007/s004270000110. [DOI] [PubMed] [Google Scholar]
- Ito J, Ghosh A, Luciano A, Moreira LA, Ernst A, Wimmer EA, Jacobs-Lorena M. Transgenic anopheline mosquitoes impaired in transmission of a malaria parasite. Nature. 2002;417:452–455. doi: 10.1038/417452a. [DOI] [PubMed] [Google Scholar]
- Jasinskiene N, Coates CJ, Benedict MQ, Cornel AJ, Rafferty CS, James AA, Collins FH. Stable transformation of the yellow fever mosquito, Aedes aegypti, with the Hermes element from the housefly. Proc Natl Acad Sci U S A. 1998;95:3743–3747. doi: 10.1073/pnas.95.7.3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia S, Flores-Saaib RD, Courey AJ. The Dorsal Rel homology domain plays an active role in transcriptional regulation. Mol Cell Biol. 2002;22:5089–5099. doi: 10.1128/MCB.22.14.5089-5099.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim W, Koo H, Richman AM, Seeley D, Vizioli J, Klocko AD, O’Brochta DA. Ectopic expression of a Cecropin Transgene in the human malaria vector mosquito Anopheles gambiae (Diptera: Culicidae): effect on susceptibility to Plasmodium. J Med Entomol. 2004;41:447–455. doi: 10.1603/0022-2585-41.3.447. [DOI] [PubMed] [Google Scholar]
- Kokoza V, Ahmed A, Cho WL, Jasinskiene N, James AA, Raikhel AS. Engineering blood meal-activated systemic immunity in the yellow fever mosquito, Aedes aegypti. Proc Natl Acad Sci U S A. 2000;97:9144–9149. doi: 10.1073/pnas.160258197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokoza V, Ahmed A, Wimmer EA, Raikhel AS. Efficient transformation of the yellow fever mosquito Aedes aegypti using the piggyBac transposable element vector pBac[3×P3-EGFP afm] Insect Biochem Mol Biol. 2001a;31:1137–1143. doi: 10.1016/s0965-1748(01)00120-5. [DOI] [PubMed] [Google Scholar]
- Kokoza V, Martin D, Mienaltowski MJ, Ahmed A, Morton CM, Raikhel AS. Transcriptional regulation of the mosquito Vitellogenin gene via a blood meal-triggered cascade. Gene. 2001b;274:47–65. doi: 10.1016/s0378-1119(01)00602-3. [DOI] [PubMed] [Google Scholar]
- Lemaitre B, Hoffmann J. The host defense of Drosophila melanogaster. Annu Rev Immunol. 2007;25:697–743. doi: 10.1146/annurev.immunol.25.022106.141615. [DOI] [PubMed] [Google Scholar]
- Meister S, Kanzok SM, Zheng X, Luna C, Li T-R, Hoa NT, Clayton JR, White KP, Kafatos FC, Christophides GK, Zheng L. Immune signaling pathways regulating bacterial and malaria parasite infection of the mosquito Anopheles gambiae. Proc Natl Acad Sci U S A. 2005;102:11420–11425. doi: 10.1073/pnas.0504950102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira LA, Ito JA, Ghosh A, Devenport M, Zieler H, Abraham EJ, Crisanti A, Nolan T, Catteruccia F, Jacobs-Lorena M. Bee venom phospholipase inhibits malaria parasite development in transgenic mosquitoes. J Biol Chem. 2002;277:40839–40843. doi: 10.1074/jbc.M206647200. [DOI] [PubMed] [Google Scholar]
- Nene V, Wortman JR, Lawson D, Haas B, Kodira C, Tu ZJ, Loftus B, Xi Z, Megy K, Grabherr M, Ren Q, Zdobnov EM, Lobo NF, Campbell KS, Brown SE, Bonaldo MF, Zhu J, Sinkins SP, Hogenkamp DG, Amedeo P, Arensburger P, Atkinson PW, Bidwell S, Biedler J, Birney E, Bruggner RV, Costas J, Coy MR, Crabtree J, Crawford M, Debruyn B, Decaprio D, Eiglmeier K, Eisenstadt E, El-Dorry H, Gelbart WM, Gomes SL, Hammond M, Hannick LI, Hogan JR, Holmes MH, Jaffe D, Johnston JS, Kennedy RC, Koo H, Kravitz S, Kriventseva EV, Kulp D, Labutti K, Lee E, Li S, Lovin DD, Mao C, Mauceli E, Menck CF, Miller JR, Montgomery P, Mori A, Nascimento AL, Naveira HF, Nusbaum C, O’leary S, Orvis J, Pertea M, Quesneville H, Reidenbach KR, Rogers YH, Roth CW, Schneider JR, Schatz M, Shumway M, Stanke M, Stinson EO, Tubio JM, Vanzee JP, Verjovski-Almeida S, Werner D, White O, Wyder S, Zeng Q, Zhao Q, Zhao Y, Hill CA, Raikhel AS, Soares MB, Knudson DL, Lee NH, Galagan J, Salzberg SL, Paulsen IT, Dimopoulos G, Collins FH, Birren B, Fraser-Liggett CM, Severson DW. Genome sequence of Aedes aegypti, a major arbovirus vector. Science. 2007;316(5832):1718–23. doi: 10.1126/science.1138878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nirmala X, Marinotti O, Sandoval JM, Phin S, Gakhar S, Jasinskiene N, James AA. Functional characterization of the promoter of the vitellogenin gene, AsVg1, of the malaria vector, Anopheles stephensi. Insect Biochem Mol Biol. 2006;36:694–700. doi: 10.1016/j.ibmb.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Osta MA, Christophides GK, Kafatos FC. Effects of mosquito genes on Plasmodium development. Science. 2004;303:2030–2032. doi: 10.1126/science.1091789. [DOI] [PubMed] [Google Scholar]
- Shahabuddin M, Fields I, Bulet F, Hoffman JA, Miller LH. Plasmodium gallinaceum: differential killing of some mosquito stages of the parasite by insect Defensin. Exp Parasitol. 1998;89:103–112. doi: 10.1006/expr.1998.4212. [DOI] [PubMed] [Google Scholar]
- Shin SW, Kokoza V, Ahmed A, Raikhel AS. Characterization of three alternatively spliced isoforms of the Rel/NF-κB transcription factor Relish from the mosquito Aedes aegypti. Proc Natl Acad Sci U S A. 2002;99:9978–9983. doi: 10.1073/pnas.162345999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin SW, Kokoza V, Lobkov I, Raikhel AS. Relish-mediated immune deficiency in the transgenic mosquito Aedes aegypti. Proc Natl Acad Sci U S A. 2003;100:2616–2621. doi: 10.1073/pnas.0537347100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin SW, Kokoza V, Bian G, Cheon HM, Kim YJ, Raikhel AS. REL1, a homologue of Drosophila Dorsal, regulates toll antifungal immune pathway in the female mosquito Aedes aegypti. J Biol Chem. 2005;280:16499–16507. doi: 10.1074/jbc.M500711200. [DOI] [PubMed] [Google Scholar]
- Smith RC, Walter MF, Hice RH, O’Brochta DA, Atkinson PW. Testis-specific expression of the beta2 tubulin promoter of Aedes aegypti and its application as a genetic sex-separation marker. Insect Mol Biol. 2007;16:61–71. doi: 10.1111/j.1365-2583.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- Stöven S, Ando I, Kadalayil L, Engström Y, Hultmark D. Activation of the Drosophila NF-kappaB factor Relish by rapid endoproteolytic cleavage. EMBO Rep. 2000;1:347–52. doi: 10.1093/embo-reports/kvd072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka H, Matsuki H, Furukawa S, Sagisaka A, Kotani E, Mori H, Yamakawa M. Identification and functional analysis of Relish homologs in the silkworm, Bombyx mori. Biochim Biophys Acta. 2007;769:559–568. doi: 10.1016/j.bbaexp.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Tanji T, Hu X, Weber AN, Ip YT. Toll and IMD pathways synergistically activate an innate immune response in Drosophila melanogaster. Mol Cell Biol. 2007;27:4578–4588. doi: 10.1128/MCB.01814-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uvell H, Engström Y. Functional characterization of a novel promoter element required for an innate immune response in Drosophila. Mol Cell Biol. 2003;23:1358–1367. doi: 10.1128/MCB.23.22.8272-8281.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uvell H, Engström Y. A multilayered defense against infection: combinatorial control of insect immune genes. Trends Genet. 2007;23:342–349. doi: 10.1016/j.tig.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Waterhouse RM, Kriventseva EV, Meister S, Xi Z, Alvarez KS, Bartholomay LC, Barillas-Mury C, Bian G, Blandin S, Christensen BM, Dong Y, Jiang H, Kanost MR, Koutsos AC, Levashina EA, Li J, Ligoxygakis P, Maccallum RM, Mayhew GF, Mendes A, Michel K, Osta MA, Paskewitz S, Shin SW, Vlachou D, Wang L, Wei W, Zheng L, Zou Z, Severson DW, Raikhel AS, Kafatos FC, Dimopoulos G, Zdobnov EM, Christophides GK. Evolutionary dynamics of immune-related genes and pathways in disease-vector mosquitoes. Science. 2007;316:1738–1743. doi: 10.1126/science.1139862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi Z, Ramirez JL, Dimopoulos G. The Aedes aegypti toll pathway controls dengue virus infection. PLOS Pathog. 2008;4(7):e1000098. doi: 10.1371/journal.ppat.1000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Levels of the transgenic REL2 and Defensin A expression in REL2-A and REL2-B transgenic mosquito strains. Northern analysis: RNA was collected from REL2-A and REL2-B transgenic mosquitoes, previtellogenic (PV), and 12 and 24 h post blood meal (PBM); wild-type mosquitoes injected with E. cloacae 24 h post-injection (WT/Ecl) were used as a control. Northern blot analysis was performed using the probes for Vg and REL2 RHD domain; arrows indicate bands for endogenous REL2—REL2 and transgenic REL2—tREL2, (rRNA) ribosomal RNA—loading control.
Figure S2: The expression of endogenous isoforms of REL2 and REL1 in the REL2 transgenics in response to a blood meal in transgenic mosquitoes with blood-meal activated transgenic REL2-Short. (A) Primers designed for detection of the endogenous REL2-isoforms expression in REL2 transgenic mosquitoes after a blood meal. For detection of the REL2-Long isoform, the forward primer was designed to the region flanking the junction of exons 1 and 2. This region is specific to the REL2-Long and REL2-Short isoforms. The reverse primer was chosen to include the last three nucleotides of REL2-Long ORF and the beginning of the 3’UTR region. This primer is specific to both REL2-Long and IκB isoforms, since REL2-Long and IκB isoforms share an identical 3-UTR region. Such a primer design allowed specific amplification of REL2-Long. The PCR product obtained from use of these primers yielded a band of about 3 kb, which corresponds to the length of REL2-Long isoform (3.2 kb). For detection of the IκB isoform, the forward primer was chosen in the region flanking the junction between exons 1 and 5. This region is specific to the IκB isoform. The reverse primer used was the same as that used for amplification of REL2-Long. The PCR product obtained from use of these primers yielded a band of about 2.2 kb, which corresponds to the length of the IκB isoform (2.7) without exon 1. To detect the REL2-Short isoform, we used the same forward primer as was used for the amplification of REL2-Long. The reverse primer was chosen for the region unique for REL2-Short, i.e., the last three nucleotides of ORF and the 3’-UTR region. The PCR product obtained from use of these primers yielded a band of about 2 kb, which corresponds to the length of REL2-Short isoform (2.1 kb). Primers used for detection of the endogenous REL2-Short isoform also amplify the transgenic REL2-Short isoform because we overexpressed the full length of the REL2-Short isoform and, therefore, the transgenic and endogenous REL2-Short isoforms have identical nucleotide sequence. To detect the transgenic transcript, a pair of primers consisting of forward primer from the 3’ region of Vg promoter sequence and the reverse from REL2 exon 2 was used. The arrows indicate the position of the detection primers. Numbers represent the exons of REL2; specific 3’UTR of REL2-Short is shown in red.
(B) The expression of endogenous isoforms of REL2 and REL1 in the REL2 transgenics in response to a blood meal. Total RNA was collected from REL2 transgenics previtellogenic and 12 and 24 h post blood meal; RNA from E. cloacae-infected wild-type (WT) mosquitoes at 24 h post-infection was used as a control. RT-PCR was performed with primers specific for the endogenous REL2 isoforms and REL1-A; amplification Actin DNA was used as a control. tREL2—transgenic REL2; Long, Short, IκB—isoforms of Ae. aegypti REL2; WT—wild type, WT-Ecl—wild-type mosquitoes infected with E. cloacae; PBM—post blood meal; PI—post-infection.


