Abstract
Antagonists of growth hormone-releasing hormone (GHRH) inhibit the proliferation of various human cancers in vitro and in vivo by mechanisms that include apparent direct effects through specific binding sites expressed on tumors and that differ from pituitary human GHRH (hGHRH) receptors. In this study, GHRH antagonist JV-1–38 (20 μg/day per animal s.c.) inhibited the growth of orthotopic CAKI-1 human renal cell carcinoma (RCC) by 83% and inhibited the development of metastases to lung and lymph nodes. Using ligand competition assays with 125I-labeled GHRH antagonist JV-1–42, we demonstrated the presence of specific high-affinity (Kd = 0.25 ± 0.03 nM) binding sites for GHRH with a maximal binding capacity (Bmax) of 70.2 ± 4.1 fmol/mg of membrane protein in CAKI-1 tumors. These receptors bind GHRH antagonists preferentially and display a lower affinity for hGHRH. The binding of 125I-JV-1–42 is not inhibited by vasoactive intestinal peptide (VIP)-related peptides sharing structural homology with hGHRH. The receptors for GHRH antagonists on CAKI-1 tumors are distinct from binding sites detected with 125I-VIP (Kd = 0.89 ± 0.14 nM; Bmax = 183.5 ± 2.6 fmol/mg of protein) and also have different characteristics from GHRH receptors on rat pituitary as documented by the insignificant binding of [His1,125I-Tyr10,Nle27]hGHRH(1–32)NH2. Reverse transcription-PCR revealed the expression of splice variants of hGHRH receptor in CAKI-1 RCC. Biodistribution studies demonstrate an in vivo uptake of 125I-JV-1–42 by the RCC tumor tissue. The presence of specific receptor proteins that bind GHRH antagonists in CAKI-1 RCC supports the view that distinct binding sites that mediate the inhibitory effect of GHRH antagonists are present on various human cancers.
Keywords: growth hormone-releasing hormone antagonists, tumor receptors
Antagonists of growth hormone-releasing hormone (GHRH) were initially developed to block growth hormone (GH) secretion from the pituitary glands, leading to inhibition of insulin-like growth factor I (IGF-I) production in the liver and other tissues (1–6). The reduction in the levels of serum IGF-I could inhibit the proliferation of various cancers dependent on IGF-I, in view of involvement of this growth factor and of IGF-II, which is GH independent, in malignant transformation of cells, tumor progression, and metastasis (1–3). GHRH antagonists were shown to effectively inhibit the in vivo growth of various experimental human cancers, including osteosarcomas, mammary, ovarian and prostatic cancers, renal adenocarcinomas, small-cell lung cancer (SCLC) and non-SCLC, pancreatic and colorectal carcinomas, and malignant gliomas (7–17). The proliferation of some of these human cancers in vitro was also suppressed by GHRH antagonists (7–10, 12–14, 16–18). This finding and the reduction in the concentration of IGF-I and IGF-II and the suppression of the gene expression of IGF-I and -II in the tumors suggested that GHRH antagonists, in addition to indirect action mediated by inhibition of GH–IGF-I axis, might exert direct effects on tumor growth through specific yet-to-be-identified GHRH receptors (3, 19).
The GHRH receptor is a G-protein-coupled transmembrane receptor found predominantly in the pituitary gland, and its mRNA has also been detected in rat placenta, kidney, testis, hypothalamus, and the gastrointestinal tract (20–23). Receptors for other GHRH-related peptides, such as vasoactive intestinal peptide (VIP) and secretin, also belong to the G-protein-linked superfamily and show homology to GHRH receptor proteins (23). Although VIP receptors have been detected in various tumors and could be involved in the regulation of tumor growth (21, 24–26), recent work showed that the antiproliferative effect of GHRH antagonists is exerted through a mechanism independent of VIP receptors (27). When primers for human GHRH (hGHRH) receptor mRNA were used (27), no expression of mRNA was found in LNCaP human prostatic and MiaPaCa-2 human pancreatic cancer cells (27), in accordance with an earlier report on ovarian tumors (28). In addition, specific binding sites for radioligand [His1,125I-Tyr10,Nle27]hGHRH(1–32)NH2 could not be detected in human cancers (14, 17, 27), although this radioligand is widely used for the characterization of pituitary GHRH receptors (29–31). These observations indicate that peptide receptors on human tumors that respond to our GHRH antagonists should be different from the classic pituitary-type GHRH receptors.
In this study, we report the presence of high-affinity binding sites for GHRH antagonists on CAKI-1 human renal cell carcinoma (RCC). The binding characteristics were investigated by ligand competition assays, using 125I-labeled GHRH antagonist JV-1–42 as a specific radioligand. These binding sites appear to be isoforms of hGHRH receptor, as demonstrated by reverse transcription (RT)-PCR, and are also distinct from the VIP receptors. In addition, studies on the in vivo binding of 125I-JV-1–42 to CAKI-1 tumors and the inhibitory effects of a potent GHRH antagonist, JV-1–38, on the orthotopic growth of this human RCC are presented.
Materials and Methods
Peptides and Chemicals.
hGHRH(1–29)NH2, hGHRH(1–44)NH2, and GHRH antagonists JV-1–36, JV-1–38, MZ-5–156, and JV-1–42 ([PhAc-His1,d-Arg2,Phe(4-Cl)6,Arg9,Abu15,Nle27,d-Arg28,Har29]hGHRH(1–29)NH2) [where PhAc is phenylacetyl, Phe(4-Cl) is 4-chlorophenylalanine, Abu is α-aminobutyric acid, Nle is norleucine, and Har is homoarginine], as well as VIP antagonist JV-1–53, were synthesized as described (5, 6, 32). Agonistic analog [His1,Nle27]hGHRH(1–32)NH2, VIP, and pituitary adenylate cyclase-activating polypeptide (PACAP) were obtained from California Peptide Research (Napa, CA). Potent VIP antagonist PG 97–269 was provided by P. Gourlet and P. Robberecht (33). Na125I and 125I-VIP were purchased from Amersham Pharmacia Biotech. All other peptides and chemicals, unless otherwise mentioned, were obtained from Sigma, Bachem, and R & D Systems.
Radioiodination.
Iodinated derivatives of GHRH antagonist JV-1–42 were prepared by the Chloramine-T method as described (31) and purified by HPLC. The fractions corresponding to the mono-iodinated compound and identified by elution position, radioactivity, and UV peak were stored at −70°C for the in vitro and in vivo receptor studies. Because phosphate buffer precipitates JV-1–42 this buffer was avoided during radioiodination as well as collection and storage of 125I-labeled fractions of JV-1–42. Radioiodinated derivatives of [His1,Nle27]hGHRH(1–32)NH2 were prepared as described previously (31).
Preparation of Cell Membranes and Radioligand Binding Studies.
Preparation of membranes from 3 × 108 cells grown in culture and from xenografts of CAKI-1 RCC was performed as described previously (10, 31, 34). GHRH receptor binding was carried out as reported (31), using in vitro ligand competition assays based on binding of 125I-JV-1–42 and [His1,125I-Tyr10,Nle27]hGHRH(1–32)NH2 as radioligands to membrane fractions of CAKI-1 RCC. In brief, for saturation binding analyses, membrane homogenates containing 40–160 μg of protein were incubated in duplicate or triplicate with at least six concentrations of 125I-labeled peptide, ranging from 0.005 to 0.4 nM in the presence or absence of excess unlabeled peptide (1 μM) in a total volume of 300 μl of binding buffer (50 mM Tris⋅HCl/5 mM EDTA/5 mM MgCl2/1% BSA/30 μg/ml bacitracin, pH 7.4) supplemented with protease inhibitors as mentioned above. In the displacement experiments, which also included the specificity experiments, 50- to 150-μg portions of membrane homogenates were incubated in duplicate or triplicate with 60,000–80,000 cpm of radioligand and increasing concentrations (10−12 to 10−6 M) of nonradioactive peptides as competitors. We observed that adsorption of radiolabeled JV-1–42 to borosilicate glass, polystyrene, and polypropylene is relatively high (>30%) and irreversible. Thus, binding reactions were performed in silane-treated polypropylene tubes, where the nonspecific binding of 125I-JV-1–42 to the assay tubes was less than 5%. After 1 h of incubation at room temperature, the tubes were immersed in ice-water, 250 μl of the suspension was transferred into cold silane-treated polypropylene Microfuge tubes (Sigma), and centrifuged at 12,000 × g for 2 min (Beckman J2–21 M) at 4°C, and the supernatant was removed. The pellet was washed and the bottoms of the tubes were cut off and their radioactivities were measured in a γ counter. Receptor binding of VIP was also performed with an in vitro ligand competition assay based on binding of 125I-VIP to tumor membrane homogenates as described (35). Incubation and binding conditions were as in the GHRH receptor studies, but the reactions were terminated by adding 250 μl of ice-cold binding buffer to the polypropylene tubes, and the bound ligand was separated from free ligand by centrifugation.
RT-PCR Analysis of Human GHRH Receptor Splicing Variants in CAKI-1 Human RCC.
Poly(A)+ RNA was isolated from human pituitary adenoma (as control tissue) and CAKI-1 RCC, then reverse transcribed and amplified by RT-PCR using the reagents and protocol of the GeneAmp RNA PCR Core kit (Perkin–Elmer) as described by Rekasi et al. (36). Briefly, RT reaction was performed in a final volume of 20 μl containing 1 μg of poly(A)+ RNA, 2.5 μM oligo(dT), 1 mM each dNTP, 1× PCR buffer, 5 mM MgCl2, 1 unit/μl RNase inhibitor, and 2.5 units/μl Moloney murine leukemia virus reverse transcriptase. Five microliters of the RT reaction was used for each PCR amplification with three sets of primers that would amplify a common fragment of cDNAs of both human pituitary GHRH receptors and its splice variant-1 (SV1) (E6/E12), a fragment of cDNA of SV1 only (I3–1/E12), or a fragment of cDNA of human pituitary GHRH receptor only (E1/E8) (see sequences and location of gene-specific primers in ref. 36). The PCR mixture included 1× PCR buffer, 2 mM MgCl2, 1.0 μM each primer, and 2.5 units/100 μl AmpliTaq DNA polymerase in a 25-μl volume. A secondary PCR was subsequently carried out by using 5 μl of diluted primary PCR product, 1.0 μM each nested primer (E7/E8 for E6/E12 product, I3–2/E8 for I3–1/E12 product, and E3/E4 for E1/E8 product, respectively) in a total volume of 25 μl (see cycle profiles in ref. 36). The secondary PCR products were electrophoresed on a 1.5% agarose gel, stained with 0.5 μg/ml ethidium bromide, visualized under UV light followed by scanning of the gel (AlphaImager 2200 Documentation and Analysis System, Alpha Innotech, San Leandro, CA). The various GHRH receptor splice variants were purified from the gel by using a NucleoTrap Gel Extraction Kit (CLONTECH) and sequenced.
Biodistribution of JV-1–42.
Male athymic nude mice (Ncr nu/nu; Frederick Cancer Research and Development Center of the National Cancer Institute, Frederick, MD), approximately 6 weeks old on arrival, were housed and fed as described (10, 34). The CAKI-1 human RCC cell line (American Type Culture Collection) was maintained in culture and used to initiate growth of s.c. xenografts in donor animals as described (10, 34). Radiolabeled JV-1–42 (approximately 2 μCi; 1 μCi = 37kBq) was injected i.v. into the jugular vein of 8 nude mice bearing s.c. xenografts of CAKI-1 RCC. Animals were killed 3 min (n = 4) and 60 min (n = 4) after the injection. Body fluids and organs were dissected and weighed, and radioactivities were measured in a γ counter (Micromedic Systems, Huntsville, AL).
Inhibition of Tumor Growth in Vivo.
Male nude mice were implanted orthotopically with 10 mg of minced CAKI-1 tumor tissue suspended in 0.02 ml of RPMI medium 1640 under a capsule of the left kidney as described previously (34). One week later, animals were randomly divided into two groups for treatment with JV-1–38 (20 μg/day s.c., n = 8) or vehicle solution (0.1% DMSO in 10% aqueous propylene glycol, n = 7). Mice were euthanized after 30 days and tumors specimens were excised. Tumor weight was calculated by subtracting the weight of the right (normal) kidney from the weight of the left kidney implanted with tumor. Mice were carefully checked under a dissecting microscope for the presence of gross metastases in lymph nodes and lungs. All animal studies were conducted in accordance with institutional ethical guidelines for the care and use of experimental animals.
Data Analysis.
Specific ligand binding capacities and affinities were calculated by the Ligand-PC computerized curve-fitting program of Munson and Rodbard (37) as modified by McPherson (38). To determine the types of receptor binding, equilibrium dissociation constants (Kd values), and the maximal binding capacity of receptors (Bmax), receptor binding data were also analyzed by the Scatchard method (39). The differences between groups were assessed with the Student two-tailed t test. P < 0.05 was considered statistically significant.
Results
GHRH Receptor Binding Studies.
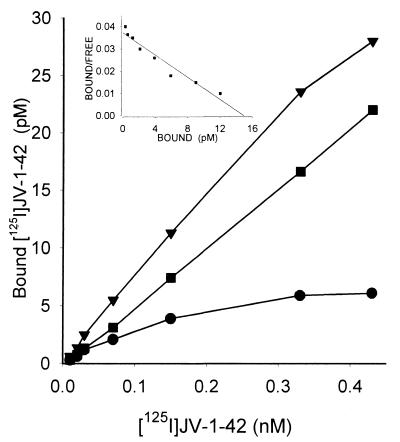
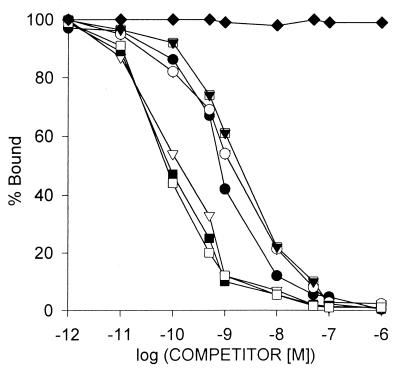
The presence of specific GHRH binding sites and binding characteristics of radiolabeled GHRH antagonist JV-1–42 to membrane receptors on CAKI-1 human RCC were determined by ligand competition assays. We found that accurate results could be obtained at room temperature when GHRH binding reached equilibrium in about 60 min and within a range of 30–180 μg of membrane protein (Fig. 1). Analyses of the binding data revealed that the one-site model provided the best fit, indicating the presence of one class of high-affinity low-capacity GHRH receptors in membranes of CAKI-1 tumors. The single class of binding sites had a mean dissociation constant (Kd) of 0.25 ± 0.03 nM with a mean maximal binding capacity (Bmax) of 70.2 ± 4.1 fmol/mg of membrane protein as determined by computerized nonlinear curve-fitting and the Scatchard plot analyses of the GHRH binding data (Fig. 1) in CAKI-1 tumors. The binding of 125I-JV-1–42 was found to be saturable, reversible, time- and temperature-dependent, and linear with protein concentration in CAKI-1 human RCC examined (some data not shown). There were no significant differences in the binding characteristics of receptors for GHRH in the membrane fractions prepared from cells cultured in medium or from tumor xenografts. The specificity of GHRH binding was demonstrated by competitive binding experiments using several peptides structurally related or unrelated to GHRH (Fig. 2). The binding of radiolabeled JV-1–42 was completely displaced by increasing concentrations (10−12 to 10−6 M) of GHRH and its analogs, whereas none of the structurally and functionally unrelated peptides tested such as luteinizing hormone-releasing hormone, epidermal growth factor, [Tyr11]somatostatin-14, [Tyr4]bombesin, and IGF-I inhibited the binding of 125I-JV-1–42. GHRH antagonists JV-1–36, JV-1–38, and MZ-5–156 displayed the highest binding affinity to GHRH receptors in membranes of CAKI-1 RCC (Fig. 2). Their affinity was more than one order of magnitude higher than that of native human GHRH or its N-terminal fragment hGHRH(1–29)NH2 and agonistic analog [His1,Nle27]hGHRH(1–32)NH2. Peptides of the VIP–glucagon–PACAP family, including VIP receptor antagonists JV-1–53 and PG 97–269, did not compete with the radioligand at concentrations as high as 1 μM (Fig. 2).
Figure 1.
Representative example of the saturation of 125I-JV-1–42 binding to CAKI-1 tumor membrane fraction. The total (▾), nonspecific (■), and specific (●) binding is plotted as a function of radiolabeled peptide concentration. Specific 125I-JV-1–42 binding (●) was experimentally determined as the difference between total binding and nonspecific binding in parallel assays in the absence and presence of 1 μM unlabeled JV-1–42. (Inset) Representative Scatchard plot derived from specific 125I-JV-1–42 binding to the membrane fraction isolated from CAKI-1 human RCC. Specific binding was determined as described above. Each point represents the mean of four experiments, done in triplicate.
Figure 2.
Ligand-binding specificity of GHRH antagonist JV-1–42 binding. Competition for binding of radioligand 125I-JV-1–42 to membrane fractions of CAKI-1 human RCC was determined in the presence of increasing concentrations of JV-1–36 (■), JV-1–38 (□), MZ-5–156 (▿), hGHRH(1–44)NH2 (○), hGHRH(1–29)NH2 (●), and [His1,Nle27]hGHRH(1–32)NH2 ( ). VIP (♦) as well as glucagon, PACAP, JV-1–53, and PG 97–269 and other unrelated peptides, such as luteinizing hormone-releasing hormone, epidermal growth factor, [Tyr11]somatostatin-14, [Tyr4]bombesin, and IGF-I, did not displace the radioligand (data not shown). One hundred percent specific binding is defined as difference between binding in absence and in presence of 10−5 M JV-1–42. Each data point represents mean of at least two experiments, done in duplicate or triplicate.
). VIP (♦) as well as glucagon, PACAP, JV-1–53, and PG 97–269 and other unrelated peptides, such as luteinizing hormone-releasing hormone, epidermal growth factor, [Tyr11]somatostatin-14, [Tyr4]bombesin, and IGF-I, did not displace the radioligand (data not shown). One hundred percent specific binding is defined as difference between binding in absence and in presence of 10−5 M JV-1–42. Each data point represents mean of at least two experiments, done in duplicate or triplicate.
Ligand competition assays using 125I-labeled GHRH agonist [His1,Nle27]hGHRH(1–32)NH2 as radioligand revealed a weak receptor binding in membrane fractions of CAKI-1 RCC. Because the signal was very low, the binding characteristics could not be calculated by curve-fitting programs.
VIP Receptor Binding Studies.
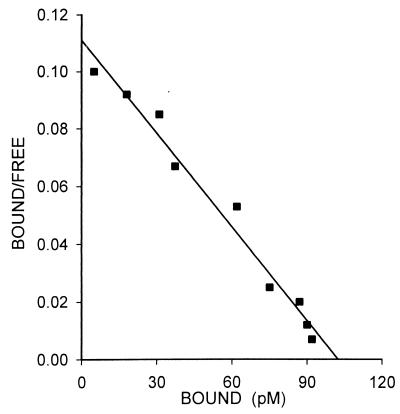
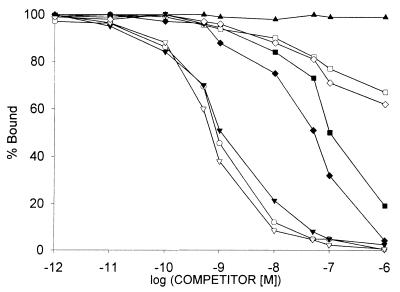
High-affinity binding sites for VIP were also found in the membrane fractions of xenografts of CAKI-1 RCC. Radiolabeled VIP was bound to one class of binding sites with a mean Kd of 0.89 ± 0.14 nM and a mean Bmax of 183.5 ± 2.6 fmol/mg of protein (Fig. 3). The binding of 125I-VIP was found to be reversible, time- and temperature-dependent, and linear with the membrane protein concentration (data not shown). To study the specificity of receptors for VIP, heterologous displacement experiments were also performed. VIP and its specific antagonists JV-1–53 and PG 97–269 showed the highest binding affinity to VIP receptors on CAKI-1 cancer cells (Fig. 4). GHRH antagonists JV-1–36 and JV-1–38 displayed around two orders of magnitude lower affinity than the VIP-related peptides. The other two GHRH antagonists tested, JV-1–42 and MZ-5–156, had negligible binding affinity for VIP receptors (Fig. 4). Native hGHRH, hGHRH(1–29)NH2, and GHRH agonistic analog [His1,Nle27]hGHRH(1–32)NH2 as well as glucagon did not show binding to VIP receptors. Finally, none of the structurally or functionally unrelated peptides tested could compete with the binding of radiolabeled VIP on CAKI-1 tumors.
Figure 3.
Representative Scatchard plot derived from specific 125I-VIP binding to the membrane fraction isolated from CAKI-1 human RCC. Specific binding was determined as described in the legend of Fig. 1. Each point represents the mean of three experiments, done in triplicate.
Figure 4.
Ligand-binding specificity of VIP binding. Competition for binding of radioligand 125I-VIP to membrane fractions of CAKI-1 human RCC was determined in the presence of increasing concentrations of VIP (○), JV-1–53 (▾), PG 97–269 (▿), JV-1–36 (■), JV-1–38 (♦), JV-1–42 (⋄), and MZ-5–156 (□). hGHRH(1–44)NH2 (▴), hGHRH(1–29)NH2, [His1,Nle27]hGHRH(1–32)NH2, and glucagon as well as unrelated peptides, such as luteinizing hormone-releasing hormone, epidermal growth factor, [Tyr11]somatostatin-14, [Tyr4]bombesin, and IGF-I, did not displace the radioligand (data not shown). One hundred percent specific binding is defined as the difference between binding in absence and in presence of 10−5 M VIP. Each data point represents mean of at least two experiments, done in duplicate or triplicate.
Detection of hGHRH Receptor mRNA in CAKI-1 Tumors.
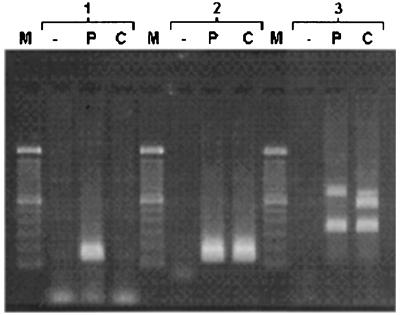
Using primers complementary to a part of the exons 3 and 4 of human pituitary GHRH receptor gene, we obtained a single 144-bp PCR product in pituitary adenoma after 20 cycles (Fig. 5). In CAKI-1 human RCC, only a slight trace of the corresponding band could be detected after 40 cycles. Using primers for amplifying a common fragment of cDNAs of both human pituitary GHRH receptor and its SV1, we detected an intense 147-bp band in both human pituitary adenoma and CAKI-1 human RCC (Fig. 5). Amplification with primers designed for intron 3 and exon 8 yielded a 720-bp, a 566-bp, and a 335-bp PCR product in various proportions in pituitary adenoma and CAKI-1 tumors. Sequence analyses of these three PCR fragments revealed different major open reading frames, which were identical with SV1, SV2, and SV4 of GHRH receptor isolated from other cancer cells (36). All of the splice variants have a retained intronic sequence at their 5′ end and lack the first three exons. The intronic sequence replacing the first three exons gives rise to a truncated N-terminal extracellular domain. The rest of the 720-bp SV1 is identical to pituitary GHRH receptor. In the 566-bp GHRH receptor SV2, exon 7 was spliced out of the RNA, whereas in the 335-bp GHRH receptor SV4, exon 5 and exon 6 were missing in addition. In SV2 and SV4, the lack of exon 5, 6, and/or 7 resulted in a 1-nucleotide upstream frameshift leading to a premature TGA stop codon in exon 8.
Figure 5.
RT-PCR analysis of mRNA of GHRH receptor and its splice variants in human pituitary adenoma and CAKI-1 human RCC. Poly(A)+ RNA was reverse transcribed and amplified in PCR with primers for exon 3–4 (group 1), exon 7–8 (group 2), and intron 3–exon 8 (group 3) of human GHRH receptor gene. The secondary PCR products were separated electrophoretically on 1.5% agarose gels and stained with ethidium bromide. The PCR products were of the expected size of 144 bp (exon 3–4) (group 1); 147 bp (exon 7–8) (group 2); as well as 720 bp, 566 bp, and 335 bp (intron 3–exon 8) (group 3). Lanes: M, 100-bp DNA molecular weight marker; P, human pituitary adenoma; C, CAKI-1 RCC; −, RT-negative control from the mixture of poly(A)+ RNA from human pituitary adenoma and CAKI-1 RCC.
Biodistribution of 125I-JV-1–42.
The biodistribution of 125I-JV-1–42 was determined 3 min and 60 min after the i.v. injection of the radiolabeled peptide into nude mice bearing xenografts of CAKI-1 human RCC. As shown in Table 1, 3 min after the injection, the density of 125I-JV-1–42 in CAKI-1 tumors was moderate as compared with high signals in the lung, liver, kidney, adrenals, and blood. However, in contrast to other organs, the accumulation of 125I-JV-1–42 in the tumors increased significantly at 60 min. The uptake of 125I-JV-1–42 by GHRH receptor-negative organs (skeletal muscle) was low at 3 and 60 min. Similarly, low density of 125I-JV-1–42 detected in brain cortex suggests that radioligand does not cross the blood–brain barrier. The radioactivity in the thyroid gland may reflect the uptake of free 125I.
Table 1.
Biodistribution of 125I-JV-1–42 in nude mice bearing s.c. xenografts of CAKI-1 human RCC
| Organ | Density, cpm/mg wet tissue
|
|
|---|---|---|
| 3 min | 60 min* | |
| Adrenals | 318.4 ± 50.8 | 73.0 ± 11.3† |
| Blood | 171.4 ± 11.2 | 68.9 ± 13.7† |
| Brain cortex | 12.6 ± 1.6 | 3.6 ± 0.8† |
| Kidney | 184.1 ± 12.8 | 93.0 ± 10.7‡ |
| Liver | 493.8 ± 112.8 | 89.9 ± 4.8§ |
| Lung | 1355.2 ± 129.9 | 704.5 ± 35.3‡ |
| Muscle | 7.3 ± 0.6 | 10.2 ± 2.7 NS |
| Small intestine | 33.6 ± 5.8 | 43.9 ± 12.7 NS |
| Thyroid | 71.2 ± 27.7 | 358.0 ± 69.7§ |
| Tumor | 16.0 ± 4.5 | 37.4 ± 6.3§ |
Animals were injected i.v. with approximately 106 cpm of 125I-JV-1–42 and sacrificed after 3 and 60 min. The organs were weighed and their radioactivities were measured in a γ counter as described in the text. Results are presented as mean ± SEM.
Two-tailed Student's t test, 60 min vs. 3 min:
, P < 0.01;
, P < 0.001;
, P < 0.05; NS, nonsignificant.
Inhibition of the Orthotopic Growth and the Development of Metastases of CAKI-1 RCC.
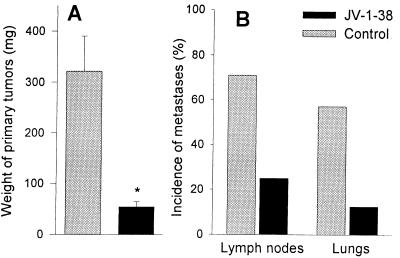
The treatment with daily injections of JV-1–38 was started 1 week after orthotopic implantation of CAKI-1 RCC. Thirty days later the mean weight of orthotopic tumors treated with JV-1–38 was 54.7 ± 9.7 mg, corresponding to an 83% reduction (P < 0.01), as compared with control tumors, which measured 322.3 ± 67.9 mg (Fig. 6A). Five of seven control animals (71%) developed metastases into regional and distant lymph nodes (Fig. 6B), whereas the incidence of lymphatic spread in mice given JV-1–38 was 25% (two of eight). The rate of lung metastases found in control group was 57%, whereas only one of eight animals (12%) treated with JV-1–38 developed metastases to the lungs (Fig. 6B).
Figure 6.
The effect of GHRH antagonist JV-1–38 on growth of primary tumors (A) and development of metastases to the lymph nodes and lung (B) in nude mice implanted orthotopically with CAKI-1 RCC. Treatment with JV-1–38 (20 μg/day s.c.) was started 1 week after implantation and lasted for 30 days. Bars, SE; *, P < 0.01 vs. control.
Discussion
Antagonists of GHRH are promising candidates for anticancer therapy (1–3). GHRH antagonists (4–6) inhibit the growth of various human neoplasms such as prostatic, ovarian, mammary, renal, pancreatic, and colorectal cancers, osteosarcomas, small-cell lung cancer (SCLC) and non-SCLC, and malignant gliomas (7–18). The mechanisms that mediate the antitumor effect of GHRH antagonists in vivo include the suppression of the pituitary GH/hepatic IGF-I axis and inhibition of the autocrine/paracrine production of IGF-I and -II in tumors (3, 7–11, 14, 16–19). It was also shown that GHRH antagonists inhibit cell proliferation, suppress the production of IGF-II, and decrease the telomerase activity in various human cancer cell lines cultured in vitro (8, 9, 11, 13, 14, 18, 40). Some evidence also suggests that GHRH antagonists can suppress tumor growth by mechanisms that are IGF-independent (12, 14). These effects and direct actions on tumoral IGF-II are likely to be mediated through specific yet-to-be-identified GHRH receptors expressed on human tumors.
This study reports the existence of GHRH binding sites in a human cancer. The receptors for GHRH antagonists on CAKI-1 RCC were characterized by radioligand binding studies and found to display saturability, specificity and reversibility, and dependence on time, temperature, and membrane protein concentration. The 125I-labeled GHRH antagonist JV-1–42 binds to a single class of high-affinity (Kd = 0.25 nM), low-capacity binding sites (Bmax = 70.2 fmol/mg of membrane protein) in membrane fractions of CAKI-1 RCC. Ligand displacement studies demonstrated that these receptor proteins recognize GHRH and its analogs in a highly specific manner as ascertained by the evaluation of a variety of unlabeled compounds. However, the affinity of the native human GHRH or its Nterminal fragment hGHRH(1–29)NH2 and agonistic analog [His1,Nle27]hGHRH(1–32)NH2 was more than one order of magnitude lower than that of GHRH antagonists JV-1–36, JV-1–38, and MZ-5–156. GHRH is a member of the family of peptides that includes VIP, glucagon, secretin, gastric inhibitory peptide, and PACAP (3, 23). Although these peptides possess significant amino acid sequence homologies, only GHRH analogs could displace the binding of 125I-JV-1–42. VIP receptor antagonists JV-1–53 and PG 97–269 also did not compete with the radioligand. When 125I-labeled [His1,Nle27]hGHRH(1–32)NH2 was used as radioligand, only very weak receptor binding was detected in CAKI-1 samples. This is in agreement with previous reports that no binding sites are detected on LNCaP human prostatic, MiaPaCa-2 pancreatic, or MDAMB-468 breast cancer cells when radioligand [His1,125I-Tyr10,Nle27]hGHRH(1–32)NH2 (14, 17, 32) is used. In contrast, this agonistic analog of GHRH shows high binding affinity to rat pituitary GHRH receptors and consequently is widely used as a radioligand for the detection and characterization of pituitary GHRH receptors (29–31). Thus, the differences in binding properties of GHRH receptors on pituitary gland and malignant tissues may be detected by using the suitable analogs of GHRH as radioligands. The sequence of JV-1–42 is three amino acid shorter than that of [His1,Nle27]hGHRH(1–32)NH2, and there are also differences at several positions, but the His1 and Nle27 substitutions are present in both ligands (6). GHRH antagonist JV-1–42, and other antagonists, such as JV-1–36, JV-1–38, and MZ-5–156, are based on the sequence of hGHRH(1–29), which is the shortest fully active fragment of the native hGHRH (1–3). The amino acid substitutions present in JV-1–42, such as Phe(4-Cl)6, Arg9, Abu15, Nle27, d-Arg28, and Har29, together with the N-terminal PhAc moiety, markedly increase the binding affinity to the pituitary GHRH receptors, as compared with hGHRH(1–29)NH2 (6). d-Arg2 substitution is responsible for the antagonistic property of the analog (2–6). In addition, JV-1–42 contains His1 in place of Tyr1, and therefore this analog can be selectively radiolabeled on the Tyr10 residue, whereas His1 will not react. RT-PCR analyses revealed the expression of splice variants of hGHRH receptors in CAKI-1 RCC. Similar splice variants were also found in other normal and malignant human extrapituitary cells as presented in detail in the accompanying publication (36). However additional studies are needed for the identification of the protein structure of these GHRH receptor isoforms in human cancers.
The pituitary GHRH receptor belongs to G-protein-coupled transmembrane receptor superfamily, which includes receptors for VIP and secretin (20–23). VIP and its antagonists can inhibit the proliferation of various human carcinomas in vivo and in vitro through VIP receptors expressed on cancer cells (24–26). Although considerable structural homology exists between GHRH and VIP peptides as well as between their receptors, our recent work shows that the antiproliferative effect of GHRH antagonists such as JV-1–36, JV-1–38, and JV-1–42 is exerted through a mechanism independent of VIP receptors (27). High-affinity receptors for VIP are also present on CAKI-1 RCC, but our work clearly demonstrates that these receptors do not recognize hGHRH and its analogs and display markedly lower or almost negligible binding to GHRH antagonists such as JV-1–42. The biodistribution studies show significant tumor uptake 60 min after the injection of 125I-JV-1–42, confirming in vitro GHRH receptor findings. The increase in uptake of the radioligand in CAKI-1 tumors appears to be mediated by GHRH receptors, since VIP receptor-positive tissues such as lung and small intestine displayed decreasing or unchanged radioactivities.
This study also shows that treatment of orthotopically implanted CAKI-1 RCC with a potent GHRH antagonist, JV-1–38, significantly inhibits the growth of primary and metastatic lesions. This finding is in good agreement with a previous study indicating a suppression of growth of s.c. xenografts of CAKI-1 RCC with an early GHRH antagonist, MZ-4–71 (10). The present results suggest that, in addition to previously demonstrated endocrine action of GHRH antagonists based on inhibition of GH/IGF-I axis, antitumor effects of these analogs can be exerted directly through GHRH receptors expressed by CAKI-1 RCC.
In conclusion, the ligand binding studies described herein, combined with the evidence from the isolation and sequencing of cDNA encoding receptor splice variants reported in the accompanying publication (36), indicate that the receptors on various tumors that bind GHRH antagonists are distinct from the pituitary GHRH receptors.
Acknowledgments
We thank Dr. Bruce D. Gaylinn, University of Virginia, Health Science Center, Charlottesville, VA, and Dr. Rhonda Kineman, University of Illinois at Chicago, for critical reading of the manuscript and helpful advice. We thank Drs. P. Gourlet and P. Robberecht, Université Libre de Bruxelles, Belgium, for VIP antagonist (PG 97–269), Dr. A. Nagy for help with the manuscript, and P. Armatis for experimental assistance. The work described in this paper was supported by the Medical Research Service of the Veterans Affairs Department and by a grant from ASTA Medica, Frankfurt am Main, Germany (all to A.V.S.) to Tulane University School of Medicine. Tulane University has applied for a patent on some of the GHRH antagonists cited in this paper. J.L.V. and A.V.S. are co-inventors on that patent, but this paper deals with the identification of GHRH receptors on tumors, which is a purely academic project.
Abbreviations
- GH
growth hormone
- GHRH
GH-releasing hormone
- hGHRH
human GHRH
- VIP
vasoactive intestinal peptide
- IGF-I
insulin-like growth factor I
- RCC
renal cell carcinoma
- PACAP
pituitary adenylate cyclase-activating polypeptide
- RT
reverse transcription
- SV
splice variant
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.180313097.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.180313097
References
- 1.Schally A V, Comaru-Schally A M. In: Cancer Medicine. 5th Ed. Holland J F, Frei E III, Bast R C Jr, Kufe D W, Pollock R E, Weichselbaum R R, editors. Ontario: Decker; 2000. pp. 715–729. [Google Scholar]
- 2.Schally A V, Kovacs M, Toth K, Comaru-Schally A M. In: Growth Hormone Secretagogues in Clinical Practice. Bercu B B, Walker R F, editors. New York: Dekker; 1998. pp. 145–162. [Google Scholar]
- 3.Schally A V, Varga J L. Trends Endocrinol Metab. 1999;10:383–391. doi: 10.1016/s1043-2760(99)00209-x. [DOI] [PubMed] [Google Scholar]
- 4.Zarandi M, Horvath J E, Halmos G, Pinski J, Nagy A, Groot K, Rekasi Z, Schally A V. Proc Natl Acad Sci USA. 1994;91:12298–12302. doi: 10.1073/pnas.91.25.12298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zarandi M, Kovacs M, Horvath J E, Toth K, Halmos G, Groot K, Nagy A, Kele Z, Schally A V. Peptides. 1997;18:423–430. doi: 10.1016/s0196-9781(96)00344-0. [DOI] [PubMed] [Google Scholar]
- 6.Varga J L, Schally A V, Csernus V J, Zarandi M, Halmos G, Groot K, Rekasi Z. Proc Natl Acad Sci USA. 1999;96:692–697. doi: 10.1073/pnas.96.2.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pinski J, Schally A V, Groot K, Halmos G, Szepeshazi K, Zarandi M, Armatis P. J Natl Cancer Inst. 1995;87:1787–1794. doi: 10.1093/jnci/87.23.1787. [DOI] [PubMed] [Google Scholar]
- 8.Pinski J, Schally A V, Jungwirth A, Groot K, Halmos G, Armatis P, Zarandi M, Vadillo-Buenfil M. Int J Oncol. 1996;9:1099–1105. doi: 10.3892/ijo.9.6.1099. [DOI] [PubMed] [Google Scholar]
- 9.Jungwirth A, Schally A V, Pinski J, Halmos G, Groot K, Armatis P, Vadillo-Buenfil M. Br J Cancer. 1997;75:1585–1592. doi: 10.1038/bjc.1997.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jungwirth A, Schally A V, Pinski J, Groot K, Armatis P, Halmos G. Proc Natl Acad Sci USA. 1997;94:5810–5813. doi: 10.1073/pnas.94.11.5810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lamharzi N, Schally A V, Koppan M, Groot K. Proc Natl Acad Sci USA. 1998;95:8864–8868. doi: 10.1073/pnas.95.15.8864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kiaris H, Schally A V, Varga J L, Groot K, Armatis P. Proc Natl Acad Sci USA. 1999;96:14894–14898. doi: 10.1073/pnas.96.26.14894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Szepeshazi K, Schally A V, Groot K, Armatis P, Hebert F, Halmos G. Eur J Cancer. 1999;36:128–136. doi: 10.1016/s0959-8049(99)00230-0. [DOI] [PubMed] [Google Scholar]
- 14.Kahan Z, Varga J L, Schally A V, Rekasi Z, Armatis P, Chatzistamou I, Czompoly T, Halmos G. Breast Cancer Res Treat. 2000;60:71–79. doi: 10.1023/a:1006363230990. [DOI] [PubMed] [Google Scholar]
- 15.Kiaris H, Schally A V, Varga J L. Neoplasia. 2000;2:242–250. doi: 10.1038/sj.neo.7900074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Szepeshazi K, Schally A V, Groot K, Armatis P, Halmos G, Hebert F, Szende B, Varga J L, Zarandi M. Br J Cancer. 1999;82:1724–1731. doi: 10.1054/bjoc.2000.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chatzistamou, I., Schally, A. V., Varga, J. L., Groot, K., Armatis, P. & Halmos, G. (2000) J. Clin. Endocrinol. Metab., in press. [DOI] [PubMed]
- 18.Csernus V J, Schally A V, Kiaris H, Armatis P. Proc Natl Acad Sci USA. 1999;96:3098–3103. doi: 10.1073/pnas.96.6.3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kineman R D. Proc Natl Acad Sci USA. 2000;97:532–534. doi: 10.1073/pnas.97.2.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mayo K E. Mol Endocrinol. 1992;6:1734–1744. doi: 10.1210/mend.6.10.1333056. [DOI] [PubMed] [Google Scholar]
- 21.Gaylinn B D, Harrison J K, Zysk J R, Lyons C E, Lynch K R, Thorner M O. Mol Endocrinol. 1993;7:77–84. doi: 10.1210/mend.7.1.7680413. [DOI] [PubMed] [Google Scholar]
- 22.Petersenn S, Rasch A C, Heyens M, Schulte H M. Mol Endocrinol. 1998;12:233–247. doi: 10.1210/mend.12.2.0057. [DOI] [PubMed] [Google Scholar]
- 23.Gaylinn B D. Growth Horm IGF Res. 1999;9:37–44. doi: 10.1016/s1096-6374(99)80008-2. [DOI] [PubMed] [Google Scholar]
- 24.Moody T W, Zia F, Draoui M, Brenneman D E, Fridkin M, Davidson A, Gozes I. Proc Natl Acad Sci USA. 1993;90:4345–4349. doi: 10.1073/pnas.90.10.4345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reubi J C. J Nucl Med. 1995;36:1846–1853. [PubMed] [Google Scholar]
- 26.Maruno K, Absood A, Said S I. Proc Natl Acad Sci USA. 1998;95:14373–14378. doi: 10.1073/pnas.95.24.14373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rekasi Z, Varga J L, Schally A V, Halmos G, Armatis P, Groot K, Czompoly T. Endocrinology. 2000;141:2120–2128. doi: 10.1210/endo.141.6.7511. [DOI] [PubMed] [Google Scholar]
- 28.Tang J, Lagace G, Castagne J, Collu R. J Clin Endocrinol Metab. 1995;80:2381–2387. doi: 10.1210/jcem.80.8.7629234. [DOI] [PubMed] [Google Scholar]
- 29.Seifert H, Perrin M, Rivier J, Vale W. Nature (London) 1985;313:487–489. doi: 10.1038/313487a0. [DOI] [PubMed] [Google Scholar]
- 30.Campbell R M, Lee Y, Rivier J, Heimer E P, Felix A M, Mowles T F. Peptides. 1991;12:569–574. doi: 10.1016/0196-9781(91)90103-v. [DOI] [PubMed] [Google Scholar]
- 31.Halmos G, Rekasi Z, Szoke B, Schally A V. Receptor. 1993;3:87–97. [PubMed] [Google Scholar]
- 32.Rekasi Z, Varga J L, Schally A V, Halmos G, Groot K, Czompoly T. Proc Natl Acad Sci USA. 2000;97:1218–1223. doi: 10.1073/pnas.97.3.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gourlet P, De Neef P, Cnudde J, Waelbroeck M, Robberecht P. Peptides. 1997;18:1555–1560. doi: 10.1016/s0196-9781(97)00230-1. [DOI] [PubMed] [Google Scholar]
- 34.Plonowski A, Schally A V, Nagy A, Hippokratis H, Hebert F, Halmos G. Cancer Res. 1999;60:2996–3001. [PubMed] [Google Scholar]
- 35.Wanke I E, Rorstad O P. Endocrinology. 1990;126:1981–1988. doi: 10.1210/endo-126-4-1981. [DOI] [PubMed] [Google Scholar]
- 36.Rekasi Z, Czompoly T, Schally A V, Halmos G. Proc Natl Acad Sci USA. 2000;97:10561–10566. doi: 10.1073/pnas.180313297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Munson P J, Rodbard D. Anal Biochem. 1980;107:220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- 38.McPherson G A. J Pharmacol Methods. 1985;14:213–228. doi: 10.1016/0160-5402(85)90034-8. [DOI] [PubMed] [Google Scholar]
- 39.Scatchard G. Ann NY Acad Sci. 1949;51:660–675. [Google Scholar]
- 40.Kiaris H, Schally A V. Proc Natl Acad Sci USA. 1999;96:226–231. doi: 10.1073/pnas.96.1.226. [DOI] [PMC free article] [PubMed] [Google Scholar]