Abstract
The proliferation of various tumors is inhibited by the antagonists of growth hormone-releasing hormone (GHRH) in vitro and in vivo, but the receptors mediating the effects of GHRH antagonists have not been identified so far. Using an approach based on PCR, we detected two major splice variants (SVs) of mRNA for human GHRH receptor (GHRH-R) in human cancer cell lines, including LNCaP prostatic, MiaPaCa-2 pancreatic, MDA-MB-468 breast, OV-1063 ovarian, and H-69 small-cell lung carcinomas. In addition, high-affinity, low-capacity binding sites for GHRH antagonists were found on the membranes of cancer cell lines such as MiaPaCa-2 that are negative for the vasoactive intestinal peptide/pituitary adenylate cyclase-activating polypeptide receptor (VPAC-R) or lines such as LNCaP that are positive for VPAC-R. Sequence analysis of cDNAs revealed that the first three exons in SV1 and SV2 are replaced by a fragment of retained intron 3 having a new putative in-frame start codon. The rest of the coding region of SV1 is identical to that of human pituitary GHRH-R, whereas in SV2 exon 7 is spliced out, resulting in a 1-nt upstream frameshift, which leads to a premature stop codon in exon 8. The intronic sequence may encode a distinct 25-aa fragment of the N-terminal extracellular domain, which could serve as a proposed signal peptide. The continuation of the deduced protein sequence coded by exons 4–13 in SV1 is identical to that of pituitary GHRH-R. SV2 may encode a GHRH-R isoform truncated after the second transmembrane domain. Thus SVs of GHRH-Rs have now been identified in human extrapituitary cells. The findings support the view that distinct receptors are expressed on human cancer cells, which may mediate the antiproliferative effect of GHRH antagonists.
Keywords: alternative splicing, cancer therapy
Antagonistic analogs of growth hormone-releasing hormone (GHRH) are promising new antitumor agents effective against a wide range of experimental cancers (1, 2). GHRH antagonists synthesized in this laboratory (3–5) inhibit the growth of human osteosarcomas (6), glioblastomas (7), small-cell lung carcinomas (SCLCs) and non-SCLCs (8), and renal (9), prostatic (10), pancreatic (11), colorectal (12), ovarian (13), and breast cancers (14) xenografted into nude mice. In vivo, these analogs may inhibit tumor proliferation by acting indirectly through the suppression of pituitary GH/hepatic insulin-like growth factor-I axis (1, 2, 6, 9, 10) or by direct effects on cancer cells (1, 2, 7, 8, 10–14). Antiproliferative activity of GHRH antagonists in vitro, in cultures of various cancer cell lines, under conditions that clearly exclude indirect endocrine effects, demonstrate that these compounds directly influence neoplastic cells (6–9, 11–16). The receptors mediating the direct antiproliferative effect of GHRH antagonists have not been identified so far, and the expression of classic pituitary type GHRH receptors (GHRH-Rs) on tumor cells cannot be detected by conventional reverse transcription (RT)–PCR (16) or radioligand assays (14, 16). Although GHRH antagonists may interact with receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide (VPAC-Rs), expressed in many cancers (16–18), we recently reported antiproliferative effects of GHRH antagonistic analogs independent of VPAC-R (16). Thus we demonstrated that the growth of both VPAC-R-negative human pancreatic (MiaPaCa-2) and VPAC-R-positive human prostatic (LNCaP) cancer cell lines can be inhibited by antagonists selective for GHRH-R (5), but not by those selective for VPAC-R (19). Consequently, it is likely that unknown receptors, which are distinct from both VPAC-R and the classic pituitary GHRH-R, might be involved in the antiproliferative mechanism of GHRH antagonists.
To date, the presence of splice variants (SVs) of GHRH-R has been detected only in human pituitary adenomas (20, 21) and in patients with mutations of the GHRH-R gene (22–25). However, alternatively spliced variants of GHRH-R have not been found so far in human extrapituitary neoplastic tissues. This prompted us to investigate various cancer cell lines for the expression of possible isoforms of GHRH-R, which may mediate the antiproliferative effects of GHRH antagonists.
In this study, using an approach based on PCR, we isolated and sequenced cDNAs encoding four previously undescribed SVs of human GHRH-R expressed in various human normal and malignant cells, together with slight traces of the full-length pituitary type GHRH-R. The binding sites for a GHRH antagonist on both VPAC-R-negative human pancreatic (MiaPaCa-2) and VPAC-R-positive human prostatic (LNCaP) cancer cell lines are also reported.
Materials and Methods
Tissue Cultures.
Human LNCaP (prostatic), MiaPaCa-2 (pancreatic), H-69 (SCLC), MDA-MB-468 (breast), and OV-1063 (ovarian) human cancer cell lines were obtained from the American Type Culture Collection and maintained in cultures as described (15, 16).
Rapid Amplification of cDNA Ends (RACE) and Sequencing of Human GHRH-R SV1.
Total RNA of cultured LNCaP human prostatic cancer cells was extracted according to the Tri Reagent protocol (Sigma) as described (16). Poly(A)+ RNA was purified from total RNA by using oligo(dT)-cellulose (MicroPolyAPure mRNA Isolation Kit, Ambion). The concentration of mRNA was determined by spectrophotometric analysis at 260 and 280 nm. The 5′- and 3′-RACE-Ready cDNA were synthesized by using the SMART RACE cDNA Amplification Kit (CLONTECH) (26, 27). Briefly, the synthesis of cDNA for 5′-RACE was performed in a final volume of 10 μl containing 1 μg of poly(A)+ RNA, 1 μM modified lock-docking oligo(dT) primer (5′-RACE cDNA synthesis primer: 5′-(T)25N−1N-3′, where N = A, C, G, or T and N−1 = A, G, or C), 1 μM SMART II oligonucleotide (5′-AAG CAG TGG TAA CAA CGC AGA GTA CGC GGG-3′), 1× first-strand buffer, 2 mM DTT, 1 mM each dNTP, and 20 units/μl SuperScript II Moloney murine leukemia virus (MMLV) RNase H− reverse transcriptase (GIBCO/BRL). The terminal stretch of dG residues of the SMART II oligonucleotide was annealed to the dC-rich cDNA tail synthesized by reverse transcriptase and served as an extended template for RT. After the enzyme switched templates from the mRNA molecule to the SMART II oligonucleotide, a complete cDNA copy of the original RNA was synthesized with the additional SMART II oligonucleotide sequence at the end. The synthesis of 3′-RACE cDNA was conducted by using the RT procedure described above, but with another special oligo(dT) primer [1 μM, 3′-RACE cDNA synthesis primer: 5′-AAG CAG TGG TAA CAA CGC AGA GTA C(T)30N−1N-3′] and without the SMART II oligonucleotide. This primer also includes the lock-docking nucleotide positions and has a portion of the SMART II oligonucleotide sequence at its 5′ end. The 5′- and 3′-RACE-Ready cDNAs containing the incorporated SMART II oligonucleotide sequence were diluted 1:25 with 10 mM Tricine–KOH, pH 8.5/1 mM EDTA and used in the subsequent amplifications. The primary PCR included 1.25 μl of 5′- or 3′-RACE-Ready cDNA, 1× universal primer mix (long: 5′-CTA ATA CGA CTC ACT ATA GGG CAA GCA GTG GTA ACA ACG CAG AGT-3′; short: 5′-CTA ATA CGA CTC ACT ATA GGG C-3′) that recognized the SMART II oligonucleotide sequence, 0.2 μM human GHRH-R gene-specific primer (E8 for 5′-RACE-Ready cDNA and E6 for 3′-RACE-Ready cDNA, respectively) (Table 1), 1× Advantage 2 PCR buffer, 0.2 mM each of dNTPs and 1× Advantage 2 Polymerase Mix containing AdvanTaq DNA polymerase, a proofreading polymerase, and TaqStart Antibody to provide automatic hot-start PCR (CLONTECH) in a total volume of 25 μl. The sequences of all GHRH-R gene-specific primers synthesized by GIBCO/BRL used in the PCR are shown in Table 1. To improve the specificity of RACE, a touchdown PCR was performed in a GeneAmp PCR System 2400 (Perkin–Elmer) with the following cycle profile: 5 cycles of 94°C for 5 sec and 72°C for 2 min, and 5 cycles of 94°C for 5 sec, 70°C for 10 sec, and 72°C for 2 min, followed by 20 cycles of 94°C for 5 sec, 68°C for 10 sec, and 72°C for 2 min. The primary PCR product was diluted 1:50 with Tricine/EDTA buffer. To further increase the specificity and sensitivity of amplification, secondary PCR was subsequently carried out with 5 μl of primary PCR product, 0.4 μM nested universal primer (5′-AAG CAG TGG TAA CAA CGC AGA GT-3′), and 0.4 μM nested gene-specific primer (E7 for 3′-RACE product and a primer complementary with E7 for 5′-RACE product) in a total volume of 25 μl with the cycle profile described above. The PCR products were purified by using Concert Rapid PCR Purification System (GIBCO/BRL), and the sequence of both strands was determined at least three times by cycle sequencing using AmpliTaq DNA polymerase FS with an ABI Prism model 377 fluorescent sequencer (Applied Biosystems) with appropriate oligonucleotide primers (GIBCO/BRL) by Research Genetics (Huntsville, AL).
Screening for Human GHRH-R SVs in Various Human Cancer Cells and Normal Tissues.
Total RNA of human pituitary adenoma cells and poly(A)+ RNA of various cultured human cancer cells was isolated as described above. The total RNA of human normal hepatic, prostatic, and pancreatic tissues was purchased from CLONTECH. One microgram of total or poly(A)+ RNA was reverse transcribed and then amplified by using the reagents and protocol of the GeneAmp RNA PCR Core kit (Perkin–Elmer). RT reaction was performed in a final volume of 20 μl containing 2.5 μM oligo(dT), 1 mM each dNTP, 1× PCR buffer, 5 mM MgCl2, 1 unit/μl RNase inhibitor, and 2.5 units/μl MMLV reverse transcriptase. One-fourth (5 μl) of the RT reaction was used for each PCR amplification with three primer sets that would amplify: (i) a common fragment of cDNAs of both human full-length GHRH-R and its SV1 (primers E6 and E12), (ii) a fragment beginning with a part of intron 3 found only in the cDNA of GHRH-R SV1 (primers I 3-1 and E12), or (iii) a fragment beginning with a part of exon 1 found only in the cDNA of full-length GHRH-R (primers E1 and E8) (Table 1). The PCR included 1× PCR buffer, 2 mM MgCl2, 1.0 μM each primer, and 2.5 units/100 μl AmpliTaq DNA polymerase in a volume of 25 μl. The PCR amplification was conducted in a GeneAmp PCR System 2400 (Perkin–Elmer) with the following cycle profile: 95°C for 180 sec, followed by 40 cycles of 95°C for 30 sec, 60°C for 30 sec, and 72°C for 45 sec. After the last cycle, there was a final extension for 7 min at 72°C. The primary PCR product was diluted 1:50 with Tricine/EDTA buffer and secondary PCR was subsequently carried out with 5 μl of the primary PCR product, 1.0 μM each nested primer (E7/E8 for E6/E12 product, I 3-2/E8 for I 3-1/E12 product, and E3/E4 for E1/E8 product) in a total volume of 25 μl with the same cycle profile as described above, but in the case of E6/E12 product and I 3-1/E12 product with 20 cycles; and for E1/E8 product 10 cycles (pituitary adenoma) or 20 cycles (other cells). The secondary PCR products were electrophoresed on 1.5% agarose gel, stained with 0.5 μg/ml ethidium bromide, and visualized under UV light. The various GHRH-R splice variants were purified from the gel by using a NucleoTrap Gel Extraction Kit (CLONTECH) and sequenced as described above.
Receptor Binding.
Preparation of membrane fractions of human prostatic (LNCaP) and pancreatic (MiaPaCa-2) cancer cells was carried out as reported (16, 28, 29). Receptor binding of GHRH was performed with in vitro ligand competition assay based on the binding of the radiolabeled GHRH antagonist JV-1–42 (5) to membrane fractions of the cancer cells [for details see Materials and Methods in the preceding publication by Halmos et al. (29)]. The type of receptor binding, the dissociation constant (Kd), and the maximal binding capacity (Bmax) of receptors were determined as described (29).
Results and Discussion
RACE and Nucleotide Sequence Analysis of Human GHRH-R SV1 Isolated from LNCaP Human Prostatic Cancer Cells.
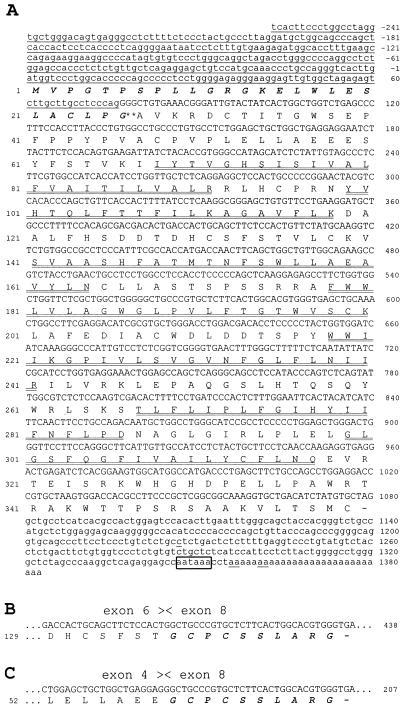
The complete cDNA of human GHRH-R SV1, isolated from LNCaP human prostatic cancer cells, was amplified by RACE-PCR. The 5′ and 3′ ends of SV1 were amplified separately, but with a short overlap, which allowed a proper joining into a single nucleotide sequence that spanned the entire coding region. The cDNA sequence of SV1, together with its deduced amino acid sequence, is shown in Fig. 1A. A comparison of the cDNA sequences showed that the major part of the nucleotide sequence (nucleotides 77–1383) of SV1 had more than 99% identity with the corresponding sequence of pituitary GHRH-R cDNA derived from exon 4–13 (30–32) (Figs. 1A and 2C). However, the first 334 nucleotides of SV1 were completely different. A blast search (33) revealed that the distinct sequence at the 5′ end of cDNA was identical to the 3′ end of intron 3 found in the human GHRH-R gene (¶, 34, 35). The nucleotide sequences of SV1 and the full-length GHRH-R diverge precisely at the splice site, implying that the 5′ end of the cDNA of SV1 is not an amplification artifact, but is derived from an intronic sequence of the gene, whereas the sequence of the first three exons is missing (these data are also supported by the screening data from PCR using primers E1/E8 then E3/E4, which is discussed below). The termination of sequencing at the end of the SMART II oligonucleotide (data not shown) suggests that the complete 5′-RACE product was sequenced. The sequence of intron 3 possessing a CAG consensus motif at position 74–76 has been retained in the acceptor splice site of exon 4 (GAA TCT) (34), instead of splicing of the donor site of exon 3 (GAG TCA G) (34) to this acceptor site. This intronic sequence of SV1 has four ATG codons, but only the last ATG (nucleotides 1–3) occurs in an adequate context (environment) to meet the criteria (YNNatgG, where Y is a pyrimidine) for being a putative translation initiation site (36) and resides in-frame with the published sequence of pituitary GHRH-R (30–32). When this ATG was used as a start codon, the cDNA of SV1 showed an ORF of 1,080 bp, which was 100% identical to that of the pituitary GHRH-R downstream from the boundary of intron 3–exon 4 (1,004 bp). The 3′ noncoding region of SV1 differs from that of pituitary GHRH-R only in five nucleotides at positions 1227, 1356, 1357, 1361, and 1362. This region ends with a poly(A)+ tail starting at position 1356 and contains a polyadenylation signal of AATAAA. The other three upstream out-of-frame ATG codons are in a less favorable environment and run into upstream terminator codons after a short distance. These small ORFs apparently retained the potential to reinitiate the translation of the same mRNA at initiation codons located further downstream, in agreement with the data reported previously (37). However, the efficiency of translation of the major downstream ORF (1,080 bp) is probably reduced (36).
Figure 1.
Structure of cDNA and protein of the human GHRH-R isoforms. The figure shows nucleotide sequence and deduced amino acid sequence of the large ORF of the human GHRH-R SV1 (A) and the C terminus of SV2 (B) and SV4 (C) from LNCaP human prostate cancer cells (SV1, SV2) and human pituitary adenoma (SV4). The amino acids (standard one-letter symbols) are enumerated at left, and the nucleotides are numbered at right. In SV1 the nucleotide sequence of the fragment of retained intron 3 and the 3′ noncoding region is shown in lowercase. The single underlines mark the nucleotides that are different from those of pituitary GHRH-R. The amino acids in boldface italic type denote a putative signal peptide, and the probable cleavage site between Gly-26 and Ala-27 is indicated by **. The seven transmembrane regions are indicated by double underlines. The potential polyadenylation signal is in a box. The alternative splicing sites in SV2 and SV4 are labeled by arrowheads. The presence of intronic nucleotide sequence shows that SV1, SV2, and SV4 are distinct from pituitary GHRH-R in the first 334 nucleotides (nucleotides −258 to +76) (A). Nucleotides 406–435 (B) and 175–204 (C) may encode a similar amino acid sequence (in boldface italic type) in SV2 and SV4, respectively, which differ from SV1 and pituitary GHRH-R.
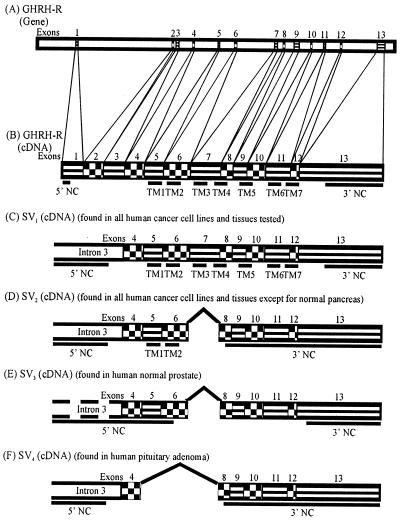
Figure 2.
Schematic drawing of exon–intron structure of the GHRH-R gene (A); and cDNA structures of the full-length pituitary GHRH-R (B), GHRH-R SV1 (C), SV2 (D), SV3 (E), and SV4 (F). The thick underlines indicate the 5′ and 3′ noncoding regions (NC) as well as the transmembrane domains (TM). The blank areas connecting the underlines represent the other receptor protein domains. Designations similar to those of Gaylinn et al. (35) are used with additional data (¶, 34).
Screening for SVs of Human GHRH-R in Various Human Normal Tissues and Cancer Cell Lines, and Characterization of cDNA Sequences.
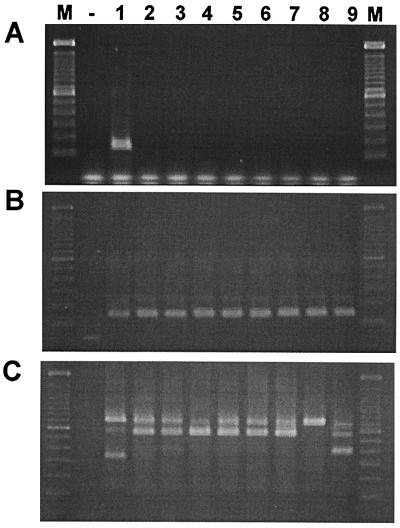
In a search for the expression of GHRH-R splice variants in various human normal and cancer cells, we performed RT-PCR with three different primer sets based on the cDNA sequence of the full-length pituitary GHRH-R (30–32) and SV1 isolated from LNCaP prostate cancer cells. Because multiple products were obtained in the primary PCR, including the PCR product of a proper size (data not shown), a secondary PCR was carried out to increase the specificity of the amplification. When sense primers designed for the first three exons of human pituitary GHRH-R gene (primer set: E1/E8 followed by E3/E4) (Table 1) were used, a single 144-bp PCR product was amplified only in pituitary adenoma after 20 cycles of nested PCR (Fig. 3A). However, even after a longer amplification (40 cycles), only slight traces of this PCR product were detected in the human cancer and normal cells tested (data not shown). In contrast, we were able to find a single 147-bp PCR product in all of the cells tested (Fig. 3B) with primers corresponding to common sequences of cDNAs of both human pituitary GHRH-R and SV1 (primer set: E6/E12 then E7/E8). When sense primers complementary to intron 3 (primer set I 3-1/E12 then I 3-2/E8) were used, two distinct PCR products (720-bp and 566-bp bands) were amplified in all of the cells screened, with the exception of normal pancreas, where only the 720-bp PCR product was detected (Fig. 3C). In addition, a 335-bp and a 390-bp band were observed in the pituitary adenoma and in the normal prostate, respectively (Fig. 3C). Sequence analyses of these four PCR fragments revealed different major ORFs, the longest of which (720 bp) was identical with that of SV1 isolated from LNCaP prostate cancer cells (Figs. 1A and 2C). All of the splice variants possess the retained intronic sequence at their 5′ end as found in SV1, with the exception of the 390-bp SV3, which has a shorter fragment (Fig. 2). In addition, all of the splice variants lack the first three exons. This is also supported by PCR using sense primers for the first three exons of human GHRH-R gene (primers E1/E8 followed by E3/E4), because only a single PCR product was detected even after 40 cycles (data not shown). This cDNA fragment is identical with the PCR product found in pituitary adenoma after 10 cycles of amplification, corresponding to a fragment of the cDNA of full-length GHRH-R. If the first three exons were present in the 5′ end of splice variants, longer PCR products containing the intronic sequence would have been amplified also. In the 566-bp SV2 and 390-bp SV3, exon 7 is spliced out of the RNA, whereas in the 335-bp SV4, exons 5, 6, and 7 are missing (Fig. 1 B and C and Fig. 2 D–F). The fact that the sequence divergence ends at the intron–exon or exon–exon boundary strongly argues against the possibility of amplification artifacts. Because only fragments of intronic sequence were found in SV3, which lacks the proposed translation initiation site, the putative start codon was shifted 355 nt downstream to exon 6. This out-of-frame start codon and the lack of exon 7 result in a distinct ORF, which terminates on exon 13 (Fig. 2E). In SV2 and SV4, the lack of exon 5, 6, and/or 7 results in a 1-nt upstream frameshift in their ORF, leading to a premature TGA stop codon in exon 8 (Fig. 1 B and C, Fig. 2 D and F).
Figure 3.
RT-PCR analysis of mRNA of GHRH-R and its SVs in human pituitary adenoma and various human cancerous and normal cells. Poly(A)+ RNA was reverse transcribed and amplified in PCR using primers E3/E4 for exon 3 and 4 (A), primers E7/E8 for exon 7 and 8 (B), and primers I 3-2/E8 for intron 3 and exon 8 (C) of human GHRH-R gene. The secondary PCR products were separated by 1.5% agarose gel electrophoresis and stained with ethidium bromide. The PCR products were of the expected size of 144 bp (exon 3–4) (A); 147 bp (exon 7–8) (B); as well as 720 bp, 566 bp, 390 bp, and 335 bp (intron 3–exon 8). Lanes: M, 100-bp DNA molecular weight marker; −, RT negative control from the mixture of poly(A)+ RNA from human pituitary adenoma and all of the human cancerous and normal cells tested; 1, human pituitary adenoma; 2, LNCaP prostatic cancer; 3, MiaPaCa-2 pancreatic cancer; 4, H-69 SCLC; 5, MDA-MB-468 breast cancer; 6, OV-1063 ovarian cancer; 7, normal liver; 8, normal pancreas; 9, normal prostate.
To study the full-length transcript of GHRH-R SVs, we also performed Northern blot analysis using PCR-generated probes corresponding to different fragments of cDNA of pituitary GHRH-R and/or SV1 (data not shown). With the exception of hybridization with labeled β-actin probe, no signals were detected on blots containing total RNA from human extrapituitary tissues (liver, prostate, pancreas) or from various human cancer cell lines (data not shown). These data suggest that the expression level of these SVs is below the detection limit of Northern blot analysis.
Structure of the Deduced Receptor Proteins.
The ORFs of splice variants putatively encode four distinct protein sequences (SV1, 359 aa; SV2, 145 aa; SV3, 151 aa; and SV4, 68 aa). Because the ORF of SV3 may encode an amino acid sequence completely different from that of the pituitary GHRH-R or SV1, the deduced protein structure of SV3 cannot be considered to be a GHRH-R isoform. No significant similarity to any amino acid sequence deposited in the databanks was found by blast. SV3 was detected only in normal prostate; thus it cannot play a role in mediating the antiproliferative effect of GHRH antagonists, and its structure does not appear in Fig. 1. We assume that in SV1, SV2, and SV4 the intronic sequence downstream from the putative start codon gives rise to a 25-aa fragment of the N-terminal extracellular domain (Figs. 1 and 2). The truncation of the receptor protein probably results in the deletion of the potential N-linked glycosylation site and the first six extracellular cysteine residues present in the pituitary GHRH-R protein (38). In SV1, the 324-aa continuation of the deduced protein sequence coded by exons 4–13 is identical to the pituitary GHRH-R, including a distal fragment of the first extracellular domain, seven putative hydrophobic transmembrane helices separated by small extra- and intracellular loops, and a 43-aa C-terminal intracellular tail (36) (Figs. 1A and 2C). In SV2, only the first two transmembrane domains of the full-length GHRH-R are likely present, because it was truncated downstream from the first extracellular loop by an early stop codon in exon 8 (Figs. 1B and 2D). The lack of any transmembrane domains, intervening loops, and C terminus in the other two GHRH-R isoforms derived from SV3 and SV4 (Fig. 1C and Fig. 2 E and F) implies that they probably do not represent mature receptor proteins expressible on the cell surface.
We also assume that the putative N terminus of SV1 and SV2 receptor proteins, derived almost exclusively from the intronic sequence, may serve as a signal sequence substituting for the missing signal peptide, which may have a proposed cleavage site between Gly-26 and Ala-27 (Fig. 1A). A similar replacement of a signal peptide by a retained intron has been reported previously (39). The proposed 26-aa signal sequence of SV1 and SV2 has hydrophobic residues at positions 16–24, although two polar amino acids, Glu-19 and Ser-20, are inserted, which may attenuate the well-defined hydrophobic region typical of signal peptides (40). Because the structural requirements for membrane translocation presumably depend on overall hydrophobicity, rather than on specific sequence of signal peptides (41), the hydrophobic regions of SV1 identical to the transmembrane domains of full-length GHRH-R are probably sufficient in length for the truncated receptor protein to cross the membrane despite the less hydrophobic character of its signal sequence. SV2 protein, which probably possesses only the truncated N-terminal extracellular domain, the first two transmembrane domains, the first intracellular loop, and most of the first extracellular loop, also might be transported to the cell surface (Fig. 2D). In a previous study an even more truncated GHRH-R has been reported to be expressed on the surface of transfected HeLa T4 cells (42).
Receptor Binding Studies.
125I-labeled [His1,Nle27]hGHRH(1–32)NH2 (h indicating the human sequence), an accepted radioligand for pituitary GHRH-R assay (28, 43), failed to show binding sites for GHRH on LNCaP prostatic or MiaPaCa-2 pancreatic cancer cells examined in our previous studies (16). In contrast, using a radiolabeled antagonistic analog of GHRH, 125I-JV-1–42 [see Halmos et al. (29) for details], we were able to detect high-affinity, low-capacity binding sites on both VPAC-R-negative MiaPaCa-2 (Kd = 8.58 ± 1.73 nM and Bmax = 277.4 ± 18.7 fmol/mg of membrane protein) and VPAC-R-positive LNCaP (Kd = 0.64 ± 0.02 nM and Bmax = 85.5 ± 5.9 fmol/mg of membrane protein) cancer cells. Parallel studies on CAKI-1 renal cell carcinoma demonstrated that the receptors for GHRH antagonist display a lower binding affinity for human GHRH and its agonistic analogs than for GHRH antagonists (29). These binding sites did not recognize peptides of the vasoactive intestinal peptide/glucagon/secretin family (29). These data suggest that the receptors for GHRH antagonists on tumors are distinct from the pituitary type GHRH-R or VPAC-R. However, additional direct evidence is needed to prove that the binding sites found in the radioligand assay correspond to the amino acid sequences derived from the SVs.
The physiological and pathophysiological relevance of these alternative mRNA species of GHRH-R found in a large variety of normal and cancerous tissues remains to be established. A widespread distribution of GHRH-R has also been observed in various normal rat tissues (44, 45). However, the complete sequences of the mRNA of extrapituitary GHRH-R have not been examined in these studies, and thus, the possible differences between the rat pituitary and extrapituitary receptors are unknown. The structures of the SVs of human GHRH-R reported in this paper are different from those reported earlier (20–25). In a search for the causes of these special, but generally occurring, SVs, we sequenced the genomic DNA of LNCaP prostate cancer cells at the site of splicing, but no mutation was found.
Activating mutations in related G-protein-coupled GHRH-R have been demonstrated in some pituitary tumors (20, 34, 46, 47). The receptors for GHRH antagonists may also play a role in the regulation of cell proliferation. The natural ligand for these receptors and the precise mechanisms of action still have to be elucidated. However various studies indicate that GHRH, in addition to stimulating the release of growth hormone from the pituitary, functions as an autocrine/paracrine growth factor in human breast, ovarian, and endometrial cancers and SCLC (1, 8, 48, 49). Thus the expression of mRNA for GHRH and the presence of biologically active GHRH were observed in human breast, endometrial, and ovarian cancers (48, 49). Similarly, H-69 and H-510A SCLC lines express mRNA for GHRH that apparently is translated into peptide GHRH and then secreted by the cells (8). GHRH(1–29)NH2 stimulates the proliferation of SCLCs in vitro and GHRH antagonist JV-1–36 inhibits it (8). GHRH antagonists also inhibit the growth of SCLC and human breast and ovarian cancers xenografted into nude mice (1, 8, 14). These results suggest that locally produced GHRH can function as a growth factor in various cancers. GHRH antagonists inhibit the in vitro and in vivo growth of cancers that express SVs of GHRH-R and have been demonstrated to be potential antitumor agents (1).
In conclusion, the isolation and sequencing of cDNA encoding receptor SVs of GHRH-R on various tumors shows that they are distinct from the pituitary GHRH receptors. The SVs of GHRH-R found in this study could mediate the antiproliferative effects of GHRH antagonists on various cancers.
Table I.
Human GHRH-R gene-specific primers used in this study
| Primer name* | Location in GHRH-R
|
Direction | Sequence (5′…3′) | |
|---|---|---|---|---|
| Gene† | cDNA‡ | |||
| E1 | 50703–50724 | 79–100 | Sense | TTC TGC GTG TTG AGC CCG TTA C |
| E3 | 55685–55706 | 227–248 | Sense | ATG GGC TGC TGT GCT GGC CAA C |
| I 3-1 | 56183–56204 | Sense | CCT ACT GCC CTT AGG ATG CTG G | |
| I 3-2 | 56262–56283 | Sense | GCA CCT TTG AAG CCA GAG AAG G | |
| E4 | 56504–56525 | 350–371 | Antisense | TAA GGT GGA AAG GGC TCA GAC C |
| E6 | 58622–58643 | 568–589 | Sense | CTC AAG GCG GGA GCT GTG TTC C |
| E7 | 60624–60645 | 681–702 | Sense | CGC CAC CAT GAC CAA CTT CAG C |
| E8 | 61039–61060 | 806–827 | Antisense | CAC GTG CCA GTG AAG AGC ACG G |
| E12 | 63890–63911 | 1156–1177 | Antisense | GCA GTA GAG GAT GGC AAC AAT G |
The primers were named according to the location of their sequences in the human GHRH-R gene (e.g., primer E7 is in exon 7, and I 3 is in intron 3).
From ¶ footnote.
From ref. 32.
Acknowledgments
We thank Dr. Bruce D. Gaylinn (University of Virginia, Charlottesville) and Dr. Rhonda D. Kineman (University of Illinois at Chicago) for critical reading of the manuscript and helpful advice and Ms. Patricia Armatis and Dr. Balazs Csernus for experimental assistance. This work was supported by the Medical Research Service of the Veterans Affairs Department, a CaPCURE Foundation Research Award, and a grant from ASTA Medica AG. (Frankfurt am Main, Germany) to Tulane University School of Medicine (all to A.V.S.). Tulane University has applied for a patent on some of the GHRH antagonists cited in this paper. J. L. Varga and A.V.S. are co-inventors on that patent, but this paper deals with the identification of GHRH receptors on tumors, which is a purely academic project.
Abbreviations
- GHRH
growth hormone-releasing hormone
- GHRH-R
GHRH receptor
- RACE
rapid amplification of cDNA ends
- RT
reverse transcription
- SCLC
small-cell lung carcinoma
- SV
splice variant
- VPAC-R
vasoactive intestinal peptide/pituitary adenylate cyclase-activating polypeptide receptor
Footnotes
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession nos. AF282259, AF282260, AF282261, and AF282262).
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.180313297.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.180313297
Andrews, S., Dubbelde, C. & Ryan, E. (1998) The sequence of Homo sapiens PAC clone DJ 0877J02, GenBank accession no. AC005155. Available from GenBank, provided by the U.S. National Center for Biotechnology Information under the National Library of Medicine/National Institutes of Health at http://www.ncbi.nlm.gov/.
References
- 1.Schally A V, Varga J L. Trends Endocrinol Metab. 1999;10:383–391. doi: 10.1016/s1043-2760(99)00209-x. [DOI] [PubMed] [Google Scholar]
- 2.Kineman R D. Proc Natl Acad Sci USA. 2000;97:532–534. doi: 10.1073/pnas.97.2.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zarandi M, Horvath J E, Halmos G, Pinski J, Nagy A, Groot K, Rekasi Z, Schally A V. Proc Natl Acad Sci USA. 1994;91:12298–12302. doi: 10.1073/pnas.91.25.12298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zarandi M, Kovacs M, Horvath J E, Toth K, Halmos G, Groot K, Nagy A, Kele Z, Schally A V. Peptides. 1997;18:423–430. doi: 10.1016/s0196-9781(96)00344-0. [DOI] [PubMed] [Google Scholar]
- 5.Varga J L, Schally A V, Csernus V J, Zarandi M, Halmos G, Groot K, Rekasi Z. Proc Natl Acad Sci USA. 1999;96:692–697. doi: 10.1073/pnas.96.2.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pinski J, Schally A V, Groot K, Halmos G, Szepeshazi K, Zarandi M, Armatis P. J Natl Cancer Inst. 1995;87:1787–1794. doi: 10.1093/jnci/87.23.1787. [DOI] [PubMed] [Google Scholar]
- 7.Kiaris H, Schally A V, Varga J L. Neoplasia. 2000;2:242–250. doi: 10.1038/sj.neo.7900074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kiaris H, Schally A V, Varga J L, Groot K, Armatis P. Proc Natl Acad Sci USA. 1999;96:14894–14898. doi: 10.1073/pnas.96.26.14894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jungwirth A, Schally A V, Pinski J, Groot K, Armatis P, Halmos G. Proc Natl Acad Sci USA. 1997;94:5810–5813. doi: 10.1073/pnas.94.11.5810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lamharzi N, Schally A V, Koppan M, Groot K. Proc Natl Acad Sci USA. 1998;95:8864–8868. doi: 10.1073/pnas.95.15.8864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Szepeshazi K, Schally A V, Groot K, Armatis P, Hebert F, Halmos G. Eur J Cancer. 2000;36:128–136. doi: 10.1016/s0959-8049(99)00230-0. [DOI] [PubMed] [Google Scholar]
- 12.Szepeshazi K, Schally A V, Groot K, Armatis P, Halmos G, Hebert F, Szende B, Varga J L, Zarandi M. Br J Cancer. 2000;82:1724–1731. doi: 10.1054/bjoc.2000.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chatzistamou, I., Schally, A. V., Varga, J. L., Groot, K., Armatis, P. & Halmos, G. (2000) J. Clin. Endocrinol. Metab., in press. [DOI] [PubMed]
- 14.Kahan Z, Varga J L, Schally A V, Rekasi Z, Armatis P, Chatzistamou I, Czompoly T, Halmos G. Breast Cancer Res Treat. 2000;60:71–79. doi: 10.1023/a:1006363230990. [DOI] [PubMed] [Google Scholar]
- 15.Csernus V J, Schally A V, Kiaris H, Armatis P. Proc Natl Acad Sci USA. 1999;96:3098–3103. doi: 10.1073/pnas.96.6.3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rekasi Z, Varga J L, Schally A V, Halmos G, Armatis P, Groot K, Czompoly T. Endocrinology. 2000;141:2120–2128. doi: 10.1210/endo.141.6.7511. [DOI] [PubMed] [Google Scholar]
- 17.Reubi J C. J Nucl Med. 1995;36:1846–1853. [PubMed] [Google Scholar]
- 18.Moody T W. Peptides. 1996;17:545–555. doi: 10.1016/0196-9781(95)02148-5. [DOI] [PubMed] [Google Scholar]
- 19.Rekasi Z, Varga J L, Schally A V, Halmos G, Groot K, Czompoly T. Proc Natl Acad Sci USA. 2000;97:1218–1223. doi: 10.1073/pnas.97.3.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang J, Lagace G, Castagne J, Collu R. J Clin Endocrinol Metab. 1995;80:2381–2387. doi: 10.1210/jcem.80.8.7629234. [DOI] [PubMed] [Google Scholar]
- 21.Hashimoto K, Koga M, Motomura T, Kasayama S, Kouhara H, Ohnishi T, Arita N, Hayakawa T, Sato B, Kishimoto T. J Clin Endocrinol Metab. 1995;80:2933–2939. doi: 10.1210/jcem.80.10.7559877. [DOI] [PubMed] [Google Scholar]
- 22.Salvatori R, Hayashida C Y, Aguiar-Oliveira M H, Phillips J A, III, Souza A H O, Gondo R G, Toledo S P A, Conceicao M M, Prince M, Maheshwari H G, et al. J Clin Endocrinol Metab. 1999;84:917–923. doi: 10.1210/jcem.84.3.5599. [DOI] [PubMed] [Google Scholar]
- 23.Wajnrajch M P, Gertner J M, Harbison M D, Chua S C, Jr, Leibel R L. Nat Genet. 1996;12:88–90. doi: 10.1038/ng0196-88. [DOI] [PubMed] [Google Scholar]
- 24.Baumann G, Maheshwari H. Acta Paediatr Suppl. 1997;423:33–38. doi: 10.1111/j.1651-2227.1997.tb18366.x. [DOI] [PubMed] [Google Scholar]
- 25.Netchine I, Talon P, Dastot F, Vitaux F, Goossens M, Amselem S. J Clin Endocrinol Metab. 1998;83:432–436. doi: 10.1210/jcem.83.2.4528. [DOI] [PubMed] [Google Scholar]
- 26.Chenchik A, Zhu Y Y, Diatchenko L, Li R, Hill J, Siebert P D. In: Gene Cloning and Analysis by RT-PCR. Siebert P, Larrick J, editors. Natick, MA: Eaton; 1998. pp. 305–319. [Google Scholar]
- 27.Matz M, Shagin D, Bogdanova E, Britanova O, Lukyanov S, Diatchenko L, Chenchik A. Nucleic Acids Res. 1999;27:1558–1560. doi: 10.1093/nar/27.6.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Halmos G, Rekasi Z, Szoke B, Schally A V. Receptor. 1993;3:87–97. [PubMed] [Google Scholar]
- 29.Halmos G, Schally A V, Varga J L, Plonowski A, Rekasi Z, Czompoly T. Proc Natl Acad Sci USA. 2000;97:10555–10560. doi: 10.1073/pnas.180313097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mayo K E. Mol Endocrinol. 1992;6:1734–1744. doi: 10.1210/mend.6.10.1333056. [DOI] [PubMed] [Google Scholar]
- 31.Lin C, Lin S-C, Chang C-P, Rosenfeld M G. Nature (London) 1992;360:765–768. doi: 10.1038/360765a0. [DOI] [PubMed] [Google Scholar]
- 32.Gaylinn B D, Harrison J K, Zysk J R, Lyons C E, Lynch K R, Thorner M O. Mol Endocrinol. 1993;7:77–84. doi: 10.1210/mend.7.1.7680413. [DOI] [PubMed] [Google Scholar]
- 33.Altschule S F, Madden T L, Schaffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Petersenn S, Rasch A C, Heyens M, Schulte H M. Mol Endocrinol. 1998;12:233–247. doi: 10.1210/mend.12.2.0057. [DOI] [PubMed] [Google Scholar]
- 35.Gaylinn B D. Growth Horm IGF Res. 1999;9:37–44. doi: 10.1016/s1096-6374(99)80008-2. [DOI] [PubMed] [Google Scholar]
- 36.Kozak M. Mamm Genome. 1996;7:563–574. doi: 10.1007/s003359900171. [DOI] [PubMed] [Google Scholar]
- 37.Jackson R J, Kaminski A. RNA. 1995;1:985–1000. [PMC free article] [PubMed] [Google Scholar]
- 38.Gaylinn B D, DeAlmeida V I, Lyons C E, Jr, Wu K C, Mayo K E, Thorner M O. Endocrinology. 1999;140:5066–5074. doi: 10.1210/endo.140.11.7092. [DOI] [PubMed] [Google Scholar]
- 39.Nystedt S, Emilsson K, Wahlestedt C, Sundelin J. Proc Natl Acad Sci USA. 1994;91:9208–9212. doi: 10.1073/pnas.91.20.9208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.von Heijne G. J Mol Biol. 1985;184:99–105. doi: 10.1016/0022-2836(85)90046-4. [DOI] [PubMed] [Google Scholar]
- 41.Chiu I-M, Wang W-P, Lehtoma K. Oncogene. 1990;5:755–762. [PubMed] [Google Scholar]
- 42.DeAlmeida V I, Mayo K E. Mol Endocrinol. 1998;12:750–765. doi: 10.1210/mend.12.5.0102. [DOI] [PubMed] [Google Scholar]
- 43.Seifert H, Perrin M, Rivier J, Vale W. Nature (London) 1985;313:487–489. doi: 10.1038/313487a0. [DOI] [PubMed] [Google Scholar]
- 44.Matsubara S, Sato M, Mizobuchi M, Niimi M, Takahara J. Endocrinology. 1995;136:4147–4150. doi: 10.1210/endo.136.9.7649123. [DOI] [PubMed] [Google Scholar]
- 45.Takahashi T, Okimura Y, Yoshimura K, Shigeyoshi Y, Kaji H, Abe H, Chihara K. Endocrinology. 1995;136:4721–4724. doi: 10.1210/endo.136.10.7664697. [DOI] [PubMed] [Google Scholar]
- 46.Landis C, Masters S, Spada A, Pace A, Bourne H, Vallar L. Nature (London) 1989;340:692–696. doi: 10.1038/340692a0. [DOI] [PubMed] [Google Scholar]
- 47.Burton F H, Hasel K W, Bloom F E, Sutcliffe J G. Nature (London) 1991;350:74–78. doi: 10.1038/350074a0. [DOI] [PubMed] [Google Scholar]
- 48.Kahan Z, Arencibia J M, Csernus V J, Groot K, Kineman R D, Robinson W R, Schally A V. J Clin Endocrinol Metab. 1999;84:582–589. doi: 10.1210/jcem.84.2.5487. [DOI] [PubMed] [Google Scholar]
- 49.Benlot C, Lèvy L, Fontanaud P, Roche A, Rouannet P, Joubert D. J Clin Endocrinol Metab. 1997;82:690–696. doi: 10.1210/jcem.82.2.3754. [DOI] [PubMed] [Google Scholar]