Abstract
Members of the P4 subfamily of P-type ATPases are believed to catalyze transport of phospholipids across cellular bilayers. However, most P-type ATPases pump small cations or metal ions, and atomic structures revealed a transport mechanism that is conserved throughout the family. Hence, a challenging problem is to understand how this mechanism is adapted in P4-ATPases to flip phospholipids. P4-ATPases form heteromeric complexes with Cdc50 proteins. The primary role of these additional polypeptides is unknown. Here, we show that the affinity of yeast P4-ATPase Drs2p for its Cdc50-binding partner fluctuates during the transport cycle, with the strongest interaction occurring at a point where the enzyme is loaded with phospholipid ligand. We also find that specific interactions with Cdc50p are required to render the ATPase competent for phosphorylation at the catalytically important aspartate residue. Our data indicate that Cdc50 proteins are integral components of the P4-ATPase transport machinery. Thus, acquisition of these subunits may have been a crucial step in the evolution of flippases from a family of cation pumps.
P-type ATPases form a large family of membrane pumps that are transiently autophosphorylated at a conserved aspartate residue, hence the designation P-type. Prominent examples include the Ca2+-ATPase SERCA,4 which pumps Ca2+ from the cytosol into the lumen of the sarcoplasmic reticulum of skeletal muscle cells (1), and the Na+/K+-ATPase, which generates the electrochemical gradients for sodium and potassium that are vital to animal cells (2). Transport is accomplished by cyclic changes between two main enzyme conformations, E1 and E2, during which the ATPase is phosphorylated by ATP at the aspartate residue and subsequently dephosphorylated. These processes are coupled to vectorial transport and counter-transport by a controlled opening and closing of cytoplasmic and exoplasmic pathways, which give access to the ion-binding sites that are buried inside the membrane-spanning region of the pump (3). A host of crystal structures of the Ca2+ pump SERCA in well defined states of the reaction cycle revealed important aspects of the transport mechanism (4, 5). Sequence homology and structures of other ATPases show that this mechanism rests on principles and structural elements that apply to all P-type ATPases (6–8).
Although P-type ATPases usually pump small cations or metal ions, members of the P4 subfamily form a notable exception. A growing body of evidence indicates that P4-ATPases catalyze phospholipid transport and create membrane lipid asymmetry (9–11). This process contributes to a multitude of cellular functions, including membrane vesiculation, cell division, and life span. The yeast Saccharomyces cerevisiae contains five P4-ATPases, namely Dnf1p and Dnf2p at the plasma membrane, Drs2p and Dnf3p in the trans-Golgi network, and Neo1p in an endosomal compartment (12–14). Removal of Dnf1p and Dnf2p abolishes inward translocation of 12-(N-methyl-N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl))-labeled analogs of phosphatidylethanolamine (PE), phosphatidylserine (PS), and phosphatidylcholine (PC) and causes an aberrant exposure of endogenous aminophospholipids at the cell surface (13, 15). Trans-Golgi membranes isolated from a yeast strain that lacks the Dnf proteins and contains a temperature-sensitive drs2 allele display a defect in 12-(N-methyl-N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl))-PS translocation when shifted to the non-permissive temperature (16). The latter finding provides strong evidence that Drs2p is directly coupled to flippase activity, and subsequent studies showed that Drs2p, together with Dnf3p, are required for maintaining PE asymmetry in post-Golgi secretory vesicles (17).
Although no P4-ATPase has been shown to display flippase activity in reconstitution experiments with purified enzyme, the relationship of P4-ATPases to flippase activity and lipid asymmetry has gained further support from functional studies in various other organisms, including parasites (18), plants (19), worms (20), and mice (21). Besides a common domain organization, P4-ATPases display a clear sequence homology with cation-transporting P-type pumps. Shared sequence motifs include the canonical phosphorylation site in the P domain, the nucleotide-binding site in the N domain, and a TGES-related sequence in the A domain (22). This implies that P4-ATPases and cation pumps use the same mechanism to couple ATP hydrolysis to ligand transport. Phospholipid transport by P4-ATPases would correspond to counter-transport of H+ ions by the Ca2+ pump and of K+ ions by the Na+/K+-ATPase as the direction of movement is from the exoplasmic to the cytoplasmic leaflet. During the reaction cycle of cation pumps, access to the ion-binding pocket alternates between the two sides of the membrane, with the ions becoming temporarily occluded after each ion binding event (23). How this mechanism is adapted in P4-ATPases to translocate phospholipids is unclear. Flippases must provide a sizeable hydrophilic pathway for the polar headgroup to pass through the membrane as well as accommodate the hydrophobic nature of the lipid backbone. Whether P4-ATPases alone are sufficient to accomplish this task is not known.
Recent studies revealed that P4-ATPases form complexes with members of the Cdc50 protein family (24). Cdc50 proteins consist of two membrane spans and a large, N-glycosylated ectodomain with one or more conserved disulfide bonds (25). The yeast family members Cdc50p, Lem3p, and Crf1p can be co-immunoprecipitated with Drs2p, Dnf1p/Dnf2p, and Dnf3p, respectively. Formation of these complexes is required for proper expression and endoplasmic reticulum (ER) export of either partner (24, 26) so that mutation of one member of the complex phenocopies mutations in the other (15, 25). This behavior in yeast is mirrored in other organisms; Ld Ros3, a Lem3p homolog in Leishmania parasites, is needed for proper trafficking of the P4-ATPase Ld MT (18), whereas the human P4-ATPase ATP8B1 requires a Cdc50p homolog, CDC50A, for ER exit and delivery to the plasma membrane (27). Moreover, the Arabidopsis P4-ATPase ALA3 requires its Cdc50-binding partner ALIS1 to complement the lipid transport defect at the plasma membrane in a Δdnf1Δdnf2Δdrs2 yeast mutant (19).
Together, the above findings indicate that Cdc50 subunits are indispensable for a proper functioning of P4-ATPases and that it is the combination of the two that yields a physiologically active transporter. However, these studies have not clarified the primary function of the Cdc50 polypeptide in the complex. Here, we provide the first evidence that Cdc50 subunits play a crucial role in the P4-ATPase reaction cycle. Using a genetic reporter system, we find that P4-ATPase-Cdc50 interactions are dynamic and tightly coupled to the ATPase reaction cycle. Moreover, by characterizing the enzymatic properties of a purified P4-ATPase-Cdc50 complex, we show that catalytic activity relies on direct and specific interactions between the subunit and transporter.
EXPERIMENTAL PROCEDURES
Reagents, Plasmids, and Yeast Strains
All yeast strains, plasmids, antibodies, and reagents used in this study are described in the supplemental data.
Purification of Drs2p-Cdc50p Complex
Yeast Δcdc50 mutant strain GL001 was co-transfected with pH2-DRS2 and pCDC50-Myc, and then grown at 30 °C to midlogarithmic phase (0.5–1.0 A600) in selective synthetic dextrose (SD) medium using a 14-liter fermentor (New Brunswick Scientific). Cells were washed, resuspended in ice-cold TES lysis buffer (50 mm Tris-HCl, pH 7.5, 1 mm EDTA, 0.6 m sorbitol) containing protease inhibitors (28) at a density of 100 A600/ml, and then broken by vortexing with glass beads. The lysate was clarified by centrifugation at 700 gav (5 min, 4 °C). Membranes were collected by centrifugation at 100,000 gav (1 h, 4 °C), resuspended in storage buffer (20 mm Hepes-KOH, pH 7.2, 0.3 m sucrose) to a protein concentration of 10–20 mg/ml, snap-frozen in liquid N2, and stored at −80 °C. Protein concentration was measured using the BCA protein assay kit (Pierce). For detergent extraction, membranes were resuspended at 2 mg/ml protein in S-buffer (50 mm Hepes-KOH, pH 8.0, 0.1 m NaCl, 20% glycerol, 5 mm MgCl2, 1 mm phenylmethylsulfonyl fluoride, protease inhibitors, 0.5% digitonin), allowed to shake gently at 4 °C for 75 min, and then cleared of insoluble material by centrifugation at 100,000 gav (1 h, 4 °C).
Detergent-solubilized H2-Drs2p was purified in two steps, starting with Ni2+-NTA affinity chromatography. To this end, 500 μl of prewashed Ni2+-NTA beads (Qiagen) was added to 35 ml of membrane extract and gently shaken at 4 °C for 2 h. The beads were then transferred to a column and washed with 40 volumes of Buffer 1 (50 mm Hepes-KOH, pH 7.2, 0.2 m NaCl, 20% glycerol, 5 mm MgCl2, 0.05% digitonin, 0.2 mg/ml dioleoylphosphatidyl choline/dioleoylphosphatidic acid, 10 mm imidazole) followed by 30 volumes of Buffer 2 (Buffer 1 containing 0.1 m NaCl and 25 mm imidazole). H2-Drs2p was eluted with 5× 400 μl of Elution Buffer 1 (Buffer 1 containing 10 mm NaCl and 250 mm imidazole). The eluate was mixed with 100 μl of α-HA mAb-coupled agarose beads (mAb HA-7, Sigma) and then gently shaken for 2 h at 4 °C. The beads were washed 4 × 15 volumes of Buffer 1 (without imidazole). H2-Drs2p was eluted with 2× 400 μl of Elution Buffer 2 (50 mm MOPS-Tris, pH 6.5, 100 mm KCl, 20% glycerol, 5 MgCl2, 0.05% digitonin, 3 mg/ml HA3 peptide) at 30 °C. To purify 100% subunit-bound H2-Drs2p, the Ni2+-NTA eluate was mixed with 100 μl of α-Myc mAb-coupled agarose beads (mAb 9E10, Santa Cruz Biotechnology) and then eluted in Elution Buffer 2 containing 3 mg/ml Myc3 peptide, as above. Fractions were subjected to SDS-PAGE and immunoblotting, as described (13). Purity was checked by silver staining on 8% Laemmli gels. Quantification of H2-Drs2p was performed with a GS-700 densitometer on Coomassie Blue-stained gels, using known amounts of the Ca2+-ATPase SERCA1a as a standard and Molecular Analyst software (Bio-Rad).
Phosphorylation Assay
Phosphorylation from [γ-32P]ATP was performed as described (29, 30) on purified H2-Drs2p preincubated with 1 mg/ml dioleoylphosphatidyl choline/dioleoylphosphatidic acid (9/1) in MOPS buffer (50 mm MOPS-Tris, pH 7.0, 100 mm KCl, 5 mm MgCl2, 0.05% digitonin) for 1 h at 4 °C under gentle agitation. One μg/ml H2-Drs2p (∼0.1 μg/reaction) in MOPS buffer supplemented with 6 units/ml pyruvate kinase was incubated at 4 °C for 50 s with 2 μm [γ-32P]ATP (6 mCi/μmol) in the presence or absence of 1 mm orthovanadate (VO4). Dephosphorylation was initiated at 30 °C by the addition of 5 mm cold ATP or ADP and 0.1 mg/ml PS in MOPS buffer. For phosphorylation of the Ca2+-ATPase SERCA1a, rabbit muscle sarcoplasmic vesicles (Ref. 31; provided by Dr. P. Champeil, CEA Saclay, France) were incubated at 4 °C in MOPS buffer at 0.7 μg/ml ATPase in the presence of either 5 mm EGTA (to chelate free Ca2+) or 20 μm CaCl2. The reaction was started by the addition of 2 μm [γ-32P]ATP. Dephosphorylation was triggered at 30 °C by the addition of 5 mm cold ATP. All reactions were stopped by acid quenching with trichloroacetic acid and H3PO4, added to a final concentration of 16% and 5 mm, respectively, and incubated on ice for 30 min. After centrifugation at 18,000 gav (4 °C, 25 min), protein pellets were washed with 7% trichloroacetic acid and 0.5 mm H3PO4, resuspended in 40 μl of SDS sample buffer (2% SDS, 10 mm EDTA, 150 mm Tris-HCl, pH 6.8, 16% (v/v) glycerol, 0.8 m β-mercaptoethanol, 0.04% bromphenol blue), and then loaded onto acidic Sarkadi-type gels for electrophoretic separation. Gels were stained with Coomassie Blue R-250 and dried exposed to a phosphor screen, as described (29). Radioactivity was revealed with a STORM 860 PhosphorImager (Amersham Biosciences) and quantified by comparison with known amounts of [γ-32P]ATP using the Quantity One software (Bio-Rad). Variations in the amount of precipitated protein among different samples were corrected for by the amount of pyruvate kinase detected on gel after Coomassie Blue staining.
Split Ubiquitin Assay
The mating-based split ubiquitin assay of Obrdlik et al. (32) was used. Constructs containing C terminus of ubiquitin (Cub) were made by in vivo recombination in the yeast strain THY.AP4 (MATa ura3 leu2 lexA::lacZ::trp1 lexA::HIS3 lexA::ADE2). Constructs containing the N terminus of ubiquitin (Nub) were made by in vivo recombination in the yeast strain THY.AP5 (MATα URA3 leu2 trp1 his3 loxP::ade2). Transporter-Cub strains were constructed in THY.AP4 as described (Basic Protocol 1 in Ref. 33) by in vivo recombination between the linearized single copy vector pMETYCgate and PCR fragments, which were generated with pHusion DNA polymerase using pH2-DRS2, pH2-DNF1, or yeast genomic DNA (DNF3) as template and the primer sets listed in supplemental Table S1. The resulting transporter-Cub constructs were reisolated and sequenced to verify fidelity of recombination. Mutants were constructed using the megaprimer method (41). Subunit-Nub strains were constructed in THY.AP5 by in vivo recombination between linearized low copy vector pNXgate33-3HA or high copy vector pNXgate21-3HA and PCR fragments, which were generated as above. Interaction assays were carried out beginning with matings between Cub and Nub strains on YEPD plates, which were then replicated on SD-Leu-Trp plates to select for diploids. The diploids were then replicated on SD-Leu-Trp-His-Ade plates to test for growth. Sensitivity of these growth assays was determined by Met-controlled expression of the Cub construct. Growth assays were carried out on plates containing 150 μm Met.
For quantitative assays of β-galactosidase expression, diploids were grown overnight in SD-Leu-Trp suspension cultures at 30 °C to an A600 between 0.5 and 1.2. One A600 of each culture was centrifuged, permeabilized in 100 μl of YPER permeabilization reagent (Pierce Chemical, Rockford, IL) for 20 min at room temperature, and then combined with 1 ml of Z buffer (60 mm Na2HPO4, 40 mm NaH2PO4, 10 mm KCl, 1 mm MgSO4, pH 7.0) containing 4 mg/ml freshly added o-nitrophenyl-β-d-galactopyranoside. After incubation at 30 °C for 10 min, the assay mixture was sedimented, and the A420 of the supernatant was measured to obtain specific activity for β-galactosidase.
RESULTS
Purification of the Drs2p-Cdc50p Complex
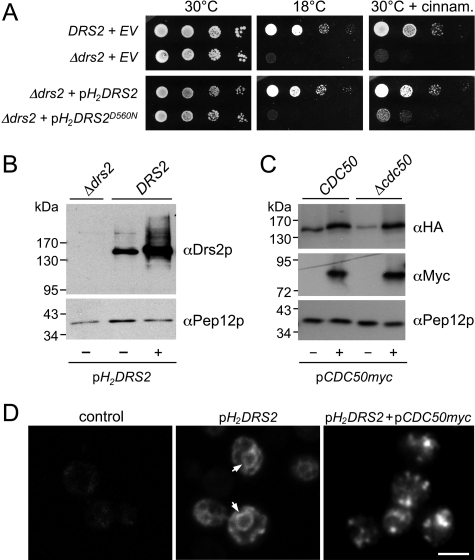
To facilitate purification of a Drs2p-Cdc50p complex for analysis of its enzymatic properties, the DRS2 coding region was tagged at the N terminus with 10 histidines and a triple HA epitope and expressed from a multicopy vector. Tagged DRS2 (H2-DRS2) retained its activity in vivo because it could restore growth of a Δdrs2 mutant at 18 °C and suppress its hypersensitivity to the PE-binding peptide cinnamycin (Fig. 1A). H2-Drs2p was expressed at about 20-fold higher levels than endogenous Drs2p (Fig. 1B). Deletion of CDC50 caused a 5-fold drop in H2-Drs2p levels, whereas expression of Myc-tagged CDC50 from a multicopy vector (CDC50-Myc) restored the level of H2-Drs2p in the Δcdc50 mutant (Fig. 1C). H2-Drs2p expressed in wild type cells primarily localized to the ER, yet co-expression of CDC50-Myc allowed a substantial portion of H2-Drs2p to reach the Golgi complex (Fig. 1D). These data are consistent with previous studies indicating that Cdc50p is required for proper expression and ER export of Drs2p (15, 24). Myc-tagged CDC50 was also able to restore growth of a Δcdc50 mutant at 18 °C (data not shown), indicating that it retained biological activity.
FIGURE 1.
H2-tagged Drs2p is functional. A, serial dilutions of wild type (DRS2) and Δdrs2 mutant strains transfected with empty vector (EV), pH2DRS2 or pH2DRS2D560N were spotted onto normal SD plates or SD plates containing 10 μm cinnamycin (cinnam.) and then incubated at the indicated temperature for 4 days. B, wild type (DRS2) and Δdrs2 mutant strains transfected with empty vector (−) or pH2DRS2 (+) were analyzed by immunoblotting using α-Drs2p and α-Pep12p antibodies. C, wild type and Δcdc50 mutant strains co-transfected with pH2DRS2 and empty vector (−) or pCDC50myc (+) were analyzed by immunoblotting using α-HA, α-Myc, and α-Pep12p antibodies. D, wild type strains transfected with empty vector (control), pH2DRS2 alone, or pH2DRS2 and pCDC50myc were analyzed by immunofluorescence microscopy using a rat monoclonal α-HA antibody (13). Note that H2-Drs2p expressed alone primarily localizes to the ER, as indicated by the nuclear envelope staining (arrowheads). H2-Drs2p co-expressed with Cdc50p-Myc displays a punctuate staining pattern, characteristic of the yeast Golgi. Bar, 10 μm.
The Drs2p-Cdc50p complex was purified from a Δcdc50 mutant co-expressing H2-Drs2p and Cdc50p-Myc. Among various detergents tested, digitonin was the mildest detergent that proved effective at solubilizing H2-Drs2p and Cdc50p-Myc (supplemental Fig. S1) while giving a good recovery of purified protein (data not shown). Digitonin-solubilized membranes were subjected to Ni2+-NTA chromatography, and fractions containing the peak of H2-Drs2p (ENTA) were pooled and passed over α-HA antibody-coupled agarose beads. H2-Drs2p was eluted from the α-HA beads with a triple HA peptide (EHA). On a silver-stained gel, EHA contained two clear bands. One had the expected molecular mass (165 kDa) for H2-Drs2p (Fig. 2A); this band reacted with both α-HA (Fig. 2B) and α-Drs2p antibodies (data not shown). The second band had the expected molecular mass (80 kDa) for Cdc50p-Myc, and its identity was confirmed by immunoblotting (Fig. 2B) and mass spectrometry (data not shown). In contrast, another yeast Cdc50 family member, Lem3p, could not be detected in the purified fraction (Fig. 2B). The recovery of Cdc50p-Myc in EHA was strictly dependent on co-expression with H2-Drs2p (Fig. 2C). Thus, H2-Drs2p and Cdc50p-Myc can be co-purified in a specific protein complex that survives detergent solubilization and tandem affinity chromatography.
FIGURE 2.
Purification of the Drs2p-Cdc50p complex. Digitonin-solubilized membranes prepared from a Δcdc50 mutant strain co-transfected with pCDC50myc and pH2DRS2 were subjected to Ni2+-NTA and α-HA immunoaffinity chromatography, as described under “Experimental Procedures.” A, the following fractions were loaded onto an 8% SDS-PAGE and subjected to silver staining: T, total membranes; SN, solubilized membrane fraction; P, insoluble membrane fraction; FTNTA, flow-through Ni2+-NTA beads; ENTA, Ni2+-NTA eluate; FTHA, flow-through α-HA beads; WHA, washing step; EHA, α-HA bead eluate. B, fractions were analyzed by immunoblotting using α-HA, α-Myc, and α-Lem3p antibodies for detection of pH2-Drs2p, Cdc50p-Myc, and Lem3p, respectively. ENTA and FTHA fractions were diluted 6-fold, and the EHA fraction were diluted 12-fold with respect to the other fractions. C, Ni2+-NTA chromatography of membrane extracts from Δcdc50 mutant strains transfected with pCDC50myc and pH2DRS2 (+) or pCDC50myc alone (−). SN and ENTA fractions were analyzed by immunoblotting as in B.
The Purified Drs2p-Cdc50p Complex Undergoes VO4-sensitive Phosphorylation from ATP
The EHA fraction containing the purified Drs2p-Cdc50p complex showed an ATPase activity that was sensitive to orthovanadate (VO4), a potent inhibitor of P-type ATPases (34). Although the VO4-sensitive ATPase activity was stimulated by the addition of PS, the prime substrate of Drs2p (16, 17), it was similarly stimulated by the non-substrate lipid PC (supplemental Fig. S2). To test whether this activity was in fact due to Drs2p, we purified an enzyme-dead version of Drs2p and analyzed the corresponding EHA fraction for ATPase activity. P-type ATPases form an aspartyl-phosphate intermediate as an essential step in the reaction cycle (35). The aspartate at position 560 (Asp-560) in Drs2p is part of the DKTGTLT signature sequence and is modified to form the aspartyl-phosphate intermediate (see below). However, an EHA fraction containing purified H2-Drs2p in which this aspartate is mutated to asparagine (D560N) still showed a prominent VO4-sensitive and lipid-stimulated ATPase activity (supplemental Fig. S2; data not shown). This result indicates that the bulk of the ATPase activity present in EHA is unrelated to Drs2p; the identity of the contaminating enzyme remains to be established.
As an alternative approach to analyzing the catalytic properties of the purified Drs2p-Cdc50p complex, we next investigated its ability to form a phosphoenzyme intermediate from ATP. The EHA fraction was incubated with 2 μm [γ-32P]ATP in the presence of Mg2+ and then fractionated by gel electrophoresis. Autoradiography showed that the incubation led to phosphorylation of H2-Drs2p (Fig. 3A). Although this phosphorylation was only partially inhibited by VO4, all of the VO4-sensitive phosphorylation was abolished in the D560N mutant (Fig. 3B). This indicates that wild type Drs2p co-purified with Cdc50p undergoes VO4-sensitive phosphorylation from ATP at Asp-560. The VO4-insensitive phosphorylation suggests that the enzyme is also phosphorylated at sites other than Asp-560; whether this involves the flippase kinases Fpk1p or Fpk2p (36) remains to be established. For the remainder of this study, formation of the phosphoenzyme was monitored by analyzing the VO4-sensitive phosphorylation of Drs2p. Phosphoenzyme formation was abolished by the addition of N-ethylmaleimide (NEM; Fig. 3C), a well established inhibitor of the aminophospholipid translocase activity (37). The addition of Ca2+ or EGTA had no effect. This is in contrast to the Ca2+-ATPase SERCA, which requires binding of Ca2+ to form the phosphoenzyme intermediate (Fig. 3D).
FIGURE 3.
The purified Drs2p-Cdc50p complex undergoes VO4-sensitive phosphorylation from ATP. A, H2-Drs2p and H2-Drs2pD560N were tandem affinity-purified as in Fig. 2A and then labeled with 2 μm [γ-32P]ATP at 4 °C for 50 s in the absence or presence of 1 mm VO4. Phosphoenzyme formation was detected by autoradiography (32P) and determined for the same amounts of protein (WB). B, quantification of 32P labeling of H2-Drs2p and H2-Drs2pD560N. Data are presented as means ± S.D. (error bars) of three independent experiments. C, effect of N-ethylmaleimide (NEM) (100 μm) on 32P labeling of H2-Drs2p. D, effect of Ca2+ (0.1 mm) and EGTA (5 mm) on 32P labeling of H2-Drs2p and the Ca2+-ATPase SERCA. E, VO4-sensitive 32P-labeling kinetics of H2-Drs2p in different lipid mixtures (0.1 mg/ml PC/PA or PC/PA/PS). The reaction was stopped at the indicated times by acid quenching. F, after 32P labeling of H2-Drs2p in the presence of 0.1 mg/ml PC/PA (50 s, 4 °C), dephosphorylation was triggered by the addition of 5 mm cold ATP and 0.1 mg/ml PS and monitored after 60 s at 30 °C. Error bars indicate S.D.
Formation of the Drs2p phosphoenzyme intermediate reached a maximum within 20 s after the addition of [γ-32P]ATP at 4 °C (Fig. 3E). This rate of phosphorylation was close to that observed for SERCA in the presence of Ca2+ (supplemental Fig. S3). The stoichiometry of phosphorylated catalytic sites measured for SERCA was ∼100 mmol/mol of enzyme (supplemental Fig. S3). For Drs2p purified and assayed in the presence of PC and phosphatidic acid (PA) as the only exogenously added lipids, the stoichiometry of phosphorylation was substantially lower: ∼7.5 mmol/mol of enzyme (Fig. 3E). The addition of PS to the exogenous lipid mixture slightly increased the stoichiometry to ∼9 mmol/mol of enzyme (Fig. 3E). As summarized in the four-step catalytic cycle displayed in Fig. 7A, PS binding would be required for Drs2p to dephosphorylate, analogous to the role of protons in dephosphorylation of SERCA (22). The addition of excess cold ATP triggered a fast (t½ < 2 s) and complete release of 32P from the phosphoenzyme intermediate of SERCA (supplemental Fig. S3). In contrast, only a small fraction (maximum 15%) of the phosphoenzyme intermediate of Drs2p could be dephosphorylated in the presence of PS (Fig. 3F). Hence, PS alone is not sufficient for a fast and complete dephosphorylation of purified Drs2p.
FIGURE 7.
Drs2p-Cdc50p interactions are tied to the ATPase reaction cycle. A, schematic representation of P2A and P4-ATPase reaction cycles. Residues important for E1 → E1P, E1P → E2P, and E2P → E2 transitions in the P2A ATPase SERCA1a and the corresponding residues in the P4-ATPase Drs2p are indicated (⊥). B, split ubiquitin growth assay reporting DRS2-Cub, DRS2D560N-Cub, DRS2E342Q-Cub, and DRS2G341L-Cub interactions with Nub-CDC50 subunits. C, modulation of Cub fusion expression by methionine was performed as in Fig. 6C. D, split ubiquitin β-galactosidase (β-gal.) assay, performed as in Fig. 6D. Data shown are representative of two independent experiments. E, mutation of Asp-560, Gly-341, or Glu-342 causes loss of Drs2p function in vivo. Serial dilutions of a Δdrs2 mutant strain transfected with pEV-Cub (empty vector (EV) control), pDRS2-Cub, pDRS2D560N–Cub, pDRS2G341L–Cub, or pDRS2E342Q were spotted onto SD plates and incubated at 30 or 18 °C for 4 days.
Phosphoenzyme Formation of Drs2p Is Dependent on Cdc50p
As only a minor fraction of purified Drs2p undergoes phosphorylation and because so little of the phosphoenzyme intermediate formed can be dephosphorylated, we wondered whether the enzyme had lost a co-factor required for catalytic activity. A possible candidate for such a co-factor is Cdc50p, and we therefore quantified the fraction of Drs2p molecules that were associated with Cdc50p after purification. To this end, the ENTA eluate containing partially purified Drs2p was subjected to immunoaffinity chromatography with α-Myc antibody-coupled agarose beads to capture all of the Cdc50p-bound enzymes. Immunoblot analysis of the flow-through (Fig. 4A, FTMYC) and eluate (EMYC) showed that all of the Cdc50p, but only ∼10% of the purified Drs2p molecules, were recovered in the Cdc50p-containing eluate (Fig. 4). This indicates that only 10% of the purified Drs2p molecules was bound to Cdc50p. We then used combinations of Ni2+-NTA, α-HA, and α-Myc affinity chromatography to purify Drs2p in two forms, namely one in which all of the enzyme was bound to Cdc50p (∼100% Cdc50p-bound) and one in which the enzyme was devoid of Cdc50p (∼0.5% Cdc50p-bound; Fig. 4A). When incubated with [γ-32P]ATP in the absence of PS, Cdc50p-bound Drs2p formed a phosphoenzyme intermediate with a stoichiometry of ∼24 mmol/mol of enzyme. In the monomeric Drs2p fraction, on the other hand, less than 4 mmol/mol of enzyme was capable of conversion to the phosphoenzyme intermediate (Fig. 4B). These results indicate that Drs2p phosphoenzyme formation is critically dependent on association with Cdc50p.
FIGURE 4.
Formation of the Drs2p phosphoenzyme intermediate is dependent on Cdc50p. A, to purify 100% Cdc50p-bound and monomeric Drs2p, the Ni2+-NTA eluate (ENTA) fraction obtained in Fig. 2A was subjected to a second affinity purification step in which α-Myc antibody-coupled beads were used to capture all Drs2p molecules associated with Cdc50p-Myc. The flow-through (FTMYC) contained primarily monomeric Drs2p (less than 1% Cdc50p-bound). The molecules bound to the α-Myc-coupled beads were eluted using a triple Myc peptide, yielding 100% Cdc50p-bound Drs2p (EMYC). That this elution step did not dissociate the complex was verified by a subsequent binding step to Ni2+-NTA beads. All fractions were analyzed by immunoblotting, using α-HA and α-Myc antibodies. FTHA, flow-through α-HA beads; EHA, α-HA bead eluate; FTNTA, flow-through Ni2+-NTA beads. B, equal amounts of 100% Cdc50p-bound and monomeric H2-Drs2p were labeled with 2 μm [γ-32P]ATP at 4 °C for 50 s in the absence or presence of 1 mm VO4. The VO4-sensitive phosphoenzyme formation is reported on the y axis. Data presented are means ± S.D. (error bars) of three independent experiments. C, dephosphorylation of 100% Cdc50p-bound Drs2p, triggered by the addition of either 5 mm ATP or 5 mm ADP as described in the legend for Fig. 3. Error bars) indicate S.D.
We next tested whether the presence of Cdc50p is sufficient to ensure dephosphorylation of Drs2p. As shown in Fig. 4C, the addition of cold ATP did not substantially reduce the level of the phosphoenzyme intermediate formed in the 100% Cdc50p-bound Drs2p fraction, even if the incubation mixtures contained PS, which would be essential to complete this step. This result implies that the bulk of the phosphorylatable Drs2p molecules associated with Cdc50p is unable to progress through the reaction cycle. The phosphoenzyme intermediate of P-type ATPases normally undergoes a conformational transition from a high energy E1P to a lower energy E2P form (see Fig. 7A). Although the E1P form can readily undergo back reaction with ADP to reform ATP, the E2P form can no longer transfer the enzyme-bound phosphate back to ADP (38, 39). As shown in Fig. 4C, only a small fraction (maximum 5%) of the Cdc50p-bound phosphoenzyme could react with ADP. From this we conclude that the bulk of the Drs2p-Cdc50p complex entering the ATPase reaction cycle is readily processed from E1 to E1P and from E1P to E2P but then gets stuck at the E2P stage. In sum, although the Drs2p fraction associated with Cdc50p is enriched for molecules capable of phosphoenzyme formation, Cdc50p binding by itself is not sufficient to allow subsequent dephosphorylation of the enzyme.
Cdc50p Contributes Directly and Specifically to the Reaction Cycle of Drs2p
Loss of CDC50 caused a substantial reduction in Drs2p protein levels (Fig. 1, A and B). Expression of CDC50-Myc in the Δcdc50 mutant restored Drs2p levels back to wild type, whereas expression of closely related CRF1-Myc did not. Surprisingly, expression of LEM3-Myc was very effective in restoring Drs2p expression, whereas overexpression of untagged LEM3 had little impact (Fig. 5, A and B). Moreover, although Lem3p lacked any obvious affinity for Drs2p (Fig. 2B), Lem3p-Myc could bind Drs2p with an affinity similar to that of Cdc50p-Myc as comparable amounts of these proteins were recovered following tandem affinity purification of Drs2p (Fig. 5, C and D). The C-terminal cytosolic tail of native Cdc50p is 19 residues longer than that of Lem3p and rich in negatively charged residues. It is feasible that these charged residues contribute to the binding affinity for Drs2p and that tagging Lem3p with the negatively charged Myc epitope creates a C terminus mimicking that of Cdc50p. These findings provided an opportunity to purify Drs2p in complex with a non-native binding partner, Lem3p-Myc, and compare its enzymatic properties with that of the native Drs2p-Cdc50p complex. As shown in Fig. 5, E and F, the ability of the Drs2p-Lem3p-Myc complex to undergo phosphoenzyme formation was essentially abolished (<2 mmol EP/mol against ∼24 mmol EP/mol Drs2p for the native complex). Importantly, these results indicate that although binding of Cdc50p seems to stabilize the enzyme, this stabilizing effect is not how the subunit contributes to the reaction cycle of Drs2p; in addition to binding, specific interactions between the two proteins are necessary to generate enzyme molecules capable of phosphorylation.
FIGURE 5.
Cdc50p contributes directly and specifically to Drs2p phosphoenzyme formation. A, wild type (CDC50) and Δcdc50 mutant strains co-transfected with pH2-DRS2 and pCDC50myc, pCRF1myc, pLEM3myc, pLEM3, or empty vector were analyzed by immunoblotting as indicated. B, quantification of H2-Drs2p levels in transfected wild type and Δcdc50 mutant strains under A. Data presented are means ± S.D. (error bars) of four independent experiments. EV, empty vector. C, H2-Drs2p was co-expressed with either Lem3p-Myc or Cdc50p-Myc in a Δcdc50 mutant strain and then subjected to tandem affinity purification as in Fig. 2A. Following elution from α-HA-coupled beads (EHA), the purified complexes were bound to Ni2+-NTA beads. Unbound (FTNTA) and NTA-bound (BNTA) fractions were collected and analyzed by immunoblotting using α-HA and α-Myc antibodies. D, EHA eluates were subjected to α-Myc immunoaffinity chromatography as in Fig. 4A to purify 100% Cdc50 subunit-bound Drs2p. EMYC, eluate fraction. E, equal amounts of 100% Cdc50 subunit-bound Drs2p were labeled with 2 μm [γ-32P]ATP as in Fig. 3A. F, quantification of VO4-sensitive phosphoenzyme formation in the Drs2p-Cdc50p and Drs2p-Lem3p complexes. Data presented are means ± S.D. (error bars) of three independent experiments.
P4-ATPase-Cdc50 Interactions Are Sensitive to Whether the P4-ATPase Can Be Phosphorylated
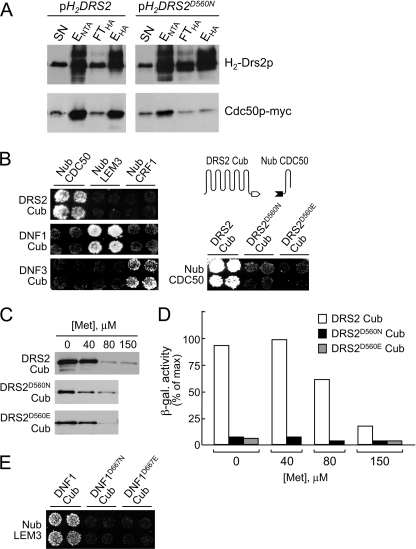
When the D560N variant of Drs2p was purified, we noticed that the recovery of Cdc50p co-purifying with Drs2pD560N was substantially lower than the recovery during purification of wild type Drs2p (Fig. 6A). This finding could not be ascribed to a difference in Cdc50p expression levels. Instead, it appeared that the D560N mutation affected the ability of Drs2p to bind Cdc50p. To ascertain that this loss of interaction also occurs in vivo, we employed the split ubiquitin system. This system has been used in a variety of contexts to study membrane protein interactions (32, 40). When two proteins carrying the C-terminal Cub and N-terminal Nub halves of ubiquitin interact, a complete ubiquitin can be formed. This causes the proteolytic release of a transcription factor that activates reporter genes required for growth on selective medium and for expression of β-galactosidase, whose activity can be measured quantitatively. As shown in Fig. 6B, an interaction between Drs2p and Cdc50p could be detected by the growth assay when Cub and Nub moieties were fused to the C terminus of Drs2p and the N terminus of Cdc50p, respectively. This interaction was specific because a similar interaction was not observed with two other tagged Cdc50 family members, Nub-LEM3 and Nub-CRF1. Conversely, Nub-LEM3 and Nub-CRF1 showed specific interactions with DNF1-Cub and DNF3-Cub, respectively (Fig. 6B). These data indicate that each Cdc50 subunit interacts with a different P4-ATPase in vivo, a conclusion consistent with results from in vitro interaction assays (24, 26).
FIGURE 6.
Mutation of active site residue Asp-560 affects Drs2p-Cdc50p complex stability. A, membrane extracts from Δcdc50 mutant strains co-transfected with pCDC50myc and pH2DRS2 or pH2DRS2D560N were subjected to tandem affinity purification as in Fig. 2A. Fractions were immunoblotted using α-HA and α-Myc antibodies for detection of H2-Drs2p and Cdc50p-Myc, respectively. SN, solubilized membrane fraction; ENTA, Ni2+-NTA eluate; FTHA, flow-through α-HA beads; EHA, α-HA bead eluate. B, split ubiquitin growth assay reporting interactions between wild type DRS2-Cub, DNF1-Cub, DNF3-Cub, or mutant DRS2D560E-Cub and their cognate Nub-CDC50 subunits. C, modulation of Cub fusion expression by varying the Met concentration in the medium. Membrane extracts from equal amounts of cells were immunoblotted using an antibody directed against the PLV moiety of the Cub fusion. D, quantitative measurement of Nub-CDC50 interactions with DRS2-Cub, DRS2D560N-Cub, or DRS2E342Q-Cub in cells grown at the indicated Met concentration. Data shown are representative of two independent experiments. β-gal., β-galactosidase. E, split ubiquitin growth assay reporting interactions of Nub-LEM3 with DNF1-Cub, DNF1D667N-Cub, and DNF1D667E-Cub.
We then applied the split ubiquitin assay to assess the interaction of Cdc50p with the D560N mutant of Drs2p. As shown in Fig. 6B, with this pair, virtually no growth was seen on the selective medium, suggesting that an intact Asp-560 residue is required for stability of the Drs2p-Cdc50p complex in vivo. Swapping Asp-560 for glutamate also abolished interaction of Drs2p with Cdc50p, indicating that Drs2p-Cdc50p complex formation is not the result of an electrostatic interaction between the two proteins at this position. These observations were confirmed by quantitative measurements of β-galactosidase activity. Cells expressing DRS2-Cub with the D560N or D560E mutation contained at least 10-fold lower levels of β-galactosidase activity than cells expressing wild type DRS2-Cub (Fig. 6D). Although immunoblotting revealed that the D560N and D560E mutants are expressed at lower levels than the wild type protein (Fig. 6C), this did not account for all of the differences in interaction. Transcription of the Cub fusions is under the control of a Met promoter, and so their expression levels can be adjusted by varying the methionine concentration in the medium. As shown in Fig. 6D, the level of β-galactosidase activity in cells expressing wild type DRS2-Cub is much higher than in cells expressing the mutants, even when the amount of the wild type protein is substantially lower than that of the mutant. The same behavior was observed for the D560E mutant.
The above results indicate that the interaction between Drs2p and Cdc50p is sensitive to whether Drs2p can be phosphorylated. To investigate whether this feature reflects a more general property of P4-ATPase-subunit complexes, we mutated the catalytic aspartate residue in Dnf1p and analyzed the ability of the mutant transporter to associate with its cognate subunit, Lem3p. As shown in Fig. 6E, mutation of the catalytic aspartate in Dnf1p had the same effect as observed for Drs2p: a substantial reduction in interaction between transporter and subunit. These findings suggest that a phosphorylatable active site is a common prerequisite for stability of P4-ATPase-Cdc50 complexes in vivo and that the interaction between transporter and subunit may fluctuate during the reaction cycle.
Drs2p-Cdc50p Interactions Are Coupled to the ATPase Reaction Cycle
Although the above results argue that a phosphorylated intermediate of Drs2p interacts most strongly with Cdc50p, they do not define which phosphorylated intermediate that forms during the reaction cycle corresponds to the high affinity interactor. A simplified scheme of the P-type ATPase reaction cycle is shown in Fig. 7A. The mechanism of ATP-dependent phosphorylation and dephosphorylation appears to be conserved in all P-type ATPases (6, 22). In particular, phosphorylation of the catalytic aspartate residue results in a phosphorylated and ADP-bound form of the enzyme, which is denoted E1P. Release of the ADP clears a binding site for the TGES loop in the A domain of the enzyme. A rearrangement of the A domain puts the loop in place and shifts the enzyme to a phosphorylated form, E2P, with a low affinity for the cytoplasmic ligand (Ca2+ for SERCA, unknown for P4-ATPases) and a clear exit path for that ligand to the exoplasmic side (4). The TGES loop itself is formed by a tight turn in the polypeptide chain at the conserved glycine in the TGES sequence. Mutation of this glycine to a bulky leucine residue (G182L in SERCA) leaves the phosphorylation reaction that creates E1P largely unaffected but blocks the transition from E1P to E2P, trapping the enzyme in the E1P form (42). To determine whether this phosphorylated form of Drs2p interacts strongly with Cdc50p, we generated the corresponding G341L mutant in Drs2p-Cub and investigated its interaction with Cdc50p in vivo by the split ubiquitin assay. As shown in Fig. 7, B and D, this mutant showed no evidence of interaction either by growth on selective medium or by β-galactosidase activity. This loss of interaction could not be ascribed to differences in Drs2p expression (Fig. 7, C and D). Hence, the interaction phenotype of Drs2p blocked at the E1 or E1P step of the reaction cycle appeared to be the same, suggesting that Cdc50p does not interact strongly with those conformations of the enzyme in which the ligand-binding sites are open to the cytoplasmic side.
If phosphorylation is required for robust interaction with Cdc50p and if the E1P conformation does not interact strongly, then some other phosphorylated intermediate of Drs2p must interact with Cdc50p. In the reaction cycle, transformation of the E1P to E2P form makes the ligand-binding sites of the enzyme accessible from the exoplasmic side. Because the E2P conformation has low affinity for the cytoplasmic ligand, this ligand dissociates, and the exoplasmic ligand (H+ for SERCA, a phospholipid for P4-ATPases) is taken up (Fig. 7A). Binding and occlusion of this ligand allows the enzyme to be dephosphorylated, giving rise to the E2 form. In this dephosphorylation reaction, the conserved glutamate in the TGES sequence activates the water molecule that reacts with the aspartyl phosphate, and substitution of a glutamine for this glutamate (E183Q in SERCA) blocks dephosphorylation, leaving the enzyme stuck in the E2P conformation (42). To investigate whether Cdc50p interacts with this conformation, we generated the corresponding E342Q mutation in Drs2p and tested the interaction of the mutant protein with Cdc50p in vivo. As shown in Fig. 7, B and D, the E342Q mutant interacted strongly with Cdc50p, as evidenced both by growth on selective medium and by development of β-galactosidase activity. Together, these results suggest that the E2P form of Drs2p interacts strongly with Cdc50p.
The logic of the experiments described above depends on two assumptions. The first is that Cub-modified Drs2p is enzymatically active. The other is that the mutations that were introduced are capable of blocking the reaction cycle of the modified enzyme. To test these assumptions, we took advantage of the fact that inactivation of Drs2p results in a cold-sensitive growth phenotype (Fig. 1A). As shown in Fig. 7E, when Drs2-Cub was introduced into a Δdrs2 strain, it was capable of restoring growth at 18 °C. In contrast, introduction of any of the mutated Drs2-Cub proteins failed to restore cold-sensitive growth, indicating that all were blocked in the reaction cycle, including the E342Q mutant that interacts strongly with Cdc50p. Taken together, these results indicate that Cdc50p preferentially interacts with the E2P phosphoenzyme intermediate of Drs2p, and hence, that Drs2p-Cdc50p interactions are tied to the ATPase reaction cycle.
DISCUSSION
Although P4-ATPases are thought to catalyze phospholipid transport across cellular bilayers, their kinship to cation-transporting P-type pumps has raised doubts on whether these enzymes alone are sufficient to mediate flippase activity. P4-ATPases form complexes with members of the Cdc50 protein family. The primary role of this additional polypeptide is not known. Here, we provide two lines of evidence indicating that Cdc50 subunits directly participate in the P4-ATPase transport reaction. First, we show that association of the Cdc50 subunit with the ATPase fluctuates during the reaction cycle, with the strongest interaction occurring at or near a point where the enzyme would be loaded with phospholipid ligand. Second, we find that specific interactions with the Cdc50 subunit are vital to render the enzyme competent for phosphorylation. Collectively, our data suggest that Cdc50 proteins are part of the catalytic mechanism of transport by P4-ATPases.
P4-ATPase-Cdc50 Interactions Are Dynamic
A first indication that Cdc50 interactions are an element of the P4-ATPase reaction cycle is the fact that the interaction is sensitive to whether the enzyme is able to carry out the sequence of reactions required for transport. Mutation of the aspartate (Asp-560 in Drs2) that is phosphorylated during the reaction cycle dramatically alters the interaction measured in vivo by the split ubiquitin assay. The substantial changes in ATPase conformation that occur during the reaction cycle might have generated this result by making the two ubiquitin halves sterically inaccessible to each other in the non-phosphorylated intermediates. However, the reduced yield of the Cdc50 polypeptide co-purifying with the mutated ATPase is direct physical evidence for a reduced affinity. Moreover, the reduction in affinity is accompanied by an apparent instability of the ATPase, which is also observed when the subunit is deleted. Together, the data suggest that the Cdc50 protein binds most tightly to a phosphorylated conformation of the ATPase and less tightly to non-phosphorylated forms.
Because the reaction mechanism of P-type ATPases is well understood from studies of the Ca2+-ATPase SERCA, it is possible by mutational analysis to refine this conclusion. The phosphorylated E1P form of the enzyme is converted to an E2P form, with an open pathway to the exoplasmic side that allows Ca2+ ions to escape (4). This conversion results from docking of the TGES loop of the A domain near the phosphorylated aspartate after release of the ADP. This tight loop can be rendered non-functional by substitution of a bulky leucine for the conserved glycine in the TGES sequence. This mutation blocks the conformational change from E1P to E2P without significantly degrading the ability of the enzyme to phosphorylate itself (42). The fact that the Cdc50 subunit interacts weakly with the analogous G341L mutant in Drs2 implies that a strong interaction does not depend on phosphorylation itself. Rather, phosphorylation is required for the enzyme to reach another step in the reaction cycle. In SERCA, the conserved glutamate in the TGES sequence is the critical catalytic residue in the dephosphorylation reaction. Mutation of this residue to the sterically similar, but chemically insufficient, glutamine blocks the dephosphorylation step that converts the E2P to the E2 form of the enzyme and thus blocks the cycle with the enzyme in the E2P conformation (42, 43). As shown here, introducing the analogous E342Q mutation in Drs2 yields a protein that binds strongly to Cdc50. Moreover, this mutant is enzymatically inactive. This indicates that tight interactions with the Cdc50 subunit are not lost in mutants because the cycle is blocked but rather because of where the cycle is blocked.
The observation that a mutational block in phosphorylation of Dnf1p leads to a similar reduction in affinity for its binding partner Lem3p implies that a dynamic interaction of subunit and transporter during the reaction cycle is general to P4-ATPases. So what feature of the transport reaction, common to this class, might require a subunit? P4-ATPases are thought to catalyze transport of phospholipids, a substrate that is quite different from the small, spherical metal ions that are the usual substrates of P-type pumps. Moreover, the substrate of P4-ATPases is transported toward the cytoplasmic side of the membrane, in contrast to most other P-type ATPases, which pump their principal substrates toward the exoplasmic side. This raises the possibility that the Cdc50 subunit participates in the cytoplasmically directed transport step. For instance, by donating two additional membrane helices, the subunit may help form a specific phospholipid-binding site or complete a pathway for translocation of this bulky substrate. Alternatively, the large ectodomain of Cdc50 proteins may be required for occlusion of the bound phospholipid (Fig. 8A). Both possibilities are consistent with our finding that the subunit interacts most strongly with the E2P conformation, the form of the enzyme that would be loaded with phospholipid ligand. The possibility that there is a role for the subunit in the occlusion step finds additional support from our recent observation that the ectodomain of Cdc50 proteins harbors a high affinity and reaction cycle-sensitive P4-ATPase-binding site.5
FIGURE 8.
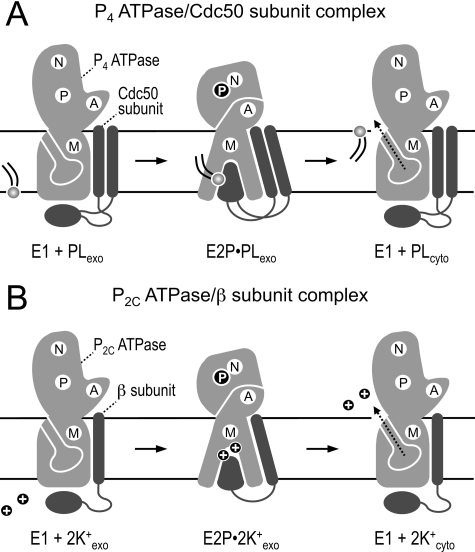
Model of reaction cycle-dependent transporter-subunit rearrangements in P2- and P2C-ATPase complexes. A and B, schematics of the E1 and E2P forms of P4 (A) and P2C-ATPases (B) in complex with their accessory subunit (Cdc50 subunit for P4-ATPases; β subunit for P2C-ATPases). Movements of the three cytoplasmic domains (P, N, and A) and intramembrane region (M) of the ATPase are accompanied by changes in ATPase-subunit binding affinity. The E1 form, which has a relatively low affinity for the subunit, is accessible for ligands from the cytosol (unknown for P4-ATPases; Na+ or H+ for P2C-ATPases). Binding of ligand promotes phosphorylation of E1 to create E1P (not shown). During conversion of E1P to E2P, the ligands are discharged to the exoplasmic side, and counter-transported ligands (phospholipid for P4-ATPases; K+ for P2C-ATPases) can now bind. This is accompanied by a tighter association of the ATPase to the subunit, presumably involving a high affinity interaction between the ATPase and the large ectodomain of the subunit (49, 53).5 The latter may contribute to formation of the ligand-binding site or serve as a lid to close access to the ligand-binding site from the exoplasmic side. Failure to achieve this specific arrangement may block dephosphorylation of E2P, hence preventing the ATPase from continuing successfully through the remainder of the cycle. During reversion to the E1 state, counter-transported ligands are released to the cytoplasmic side. See “Discussion” for further details. PL, phospholipid; N, nucleotide-binding domain; P, phosphorylation domain; A, actuator domain; M, intramembrane domain.
P4-ATPase-Cdc50 Interactions Are Necessary for Catalytic Activity
The notion that the Cdc50 subunit contributes to the reaction cycle of P4-ATPases is further substantiated by the analysis of the catalytic properties of the purified Drs2p-Cdc50p complex. The two-step purification described here yields a relatively pure population of ATPase molecules, and the substoichiometric recovery of Cdc50p in these preparations is consistent with the proposal that the interaction between the subunit and ATPase is dynamic. A substantial fraction of the enzyme can be phosphorylated in vitro, but the genetic evidence shows that only the phosphorylation that is vanadate-sensitive is linked to the reaction cycle. Because the enzyme as purified contains substoichiometric amounts of the Cdc50 subunit, it is possible to separate the preparation into those molecules that are associated with a subunit and those molecules that are not and then compare their properties. As shown here, the subunit-bound enzyme fraction contains the vast majority of the molecules capable of vanadate-sensitive phosphorylation. This could be due to stabilization of the enzyme by the presence of a binding partner. However, we show that a stabilizing binding partner for Drs2p can also be provided in the form of a Myc-tagged Lem3p, yet this complex is not capable of phosphorylation. Importantly, this demonstrates that specific interactions between subunit and transporter are vital to render the transporter competent for phosphorylation.
What competence does the transporter acquire when associated with its native subunit? One possibility is that the subunit is required for the reaction that converts the ATPase from the E1 to the E1P form. However, as pointed out above, the reaction is vanadate-sensitive, which suggests that the E2 form of the enzyme is relevant to this reaction. Moreover, this model would suggest that the subunit is important for the reaction cycle at a point where it is least strongly associated with the ATPase. An alternative possibility is that the phosphorylation assay measures the fraction of Drs2 molecules that corresponds to, or can reach the E2 form of the enzyme, which then can proceed to the phosphorylation reaction. Hence, the reduced phosphorylation in the fraction that lacks the subunit may reflect the presence of fewer such molecules. This view is consistent with the interaction data because the E2 form of the enzyme is generated from the E2P form, which is the conformation of the enzyme that interacts strongly with the subunit. The fact that more such phosphorylatable molecules are found in the fraction containing the bulk of the subunit would then be consistent with a role for the subunit in the E2P to E2 transition.
The bulk of purified Drs2 molecules that can form a phosphoenzyme intermediate cannot continue successfully through the remainder of the reaction cycle but rather becomes blocked at the E2P step of the reaction. Because these molecules are capable of the first step of the reaction, the enzyme preparation is not denatured in any simple sense. As the fraction capable of phosphorylation is not substantially increased in the presence of PS, the problem is not the absence of a transportable phospholipid substrate. It is feasible that loading of the phospholipid ligand is perturbed by the presence of detergent. Another possibility is that the phosphorylated form is missing a component required to continue through the cycle. This could be the Cdc50 subunit itself, if that molecule would dissociate during the phosphorylation, or it could be another essential, yet unknown, polypeptide or small molecule co-factor.
Analogy between P4-ATPase-Cdc50 Complexes and Oligomeric P2C ATPases
Besides P4-ATPases, only one other subfamily of P-type ATPases is known to have a subunit, namely the P2C subfamily of Na+/K+- and H+/K+-ATPases (44). P2C-ATPases are associated with a β subunit, which is indispensable for their function. The phylogeny of the ATPases suggests that there is no direct evolutionary relationship between Cdc50 and β subunits. The presumption that they evolved independently is supported by the absence of any easily discernable sequence homology between them. However, at the structural level, the two proteins share some similarity in that each has a small cytoplasmic N-terminal domain, linked by a membrane span to a relatively large globular ectodomain with conserved, disulfide bridge-forming cysteine residues (45). In the case of Cdc50 proteins, the ectodomain is linked to another membrane span and a short C-terminal cytoplasmic tail that are absent in the β subunit. Both the Cdc50 subunit and the β subunit are required for export of their respective transporters from the ER (24, 27, 44, 46); in each case, there is no clear evidence that this is the primary role of the subunit. An early suggestion was that the β subunit might be necessary because P2C-ATPases uniquely catalyze counter-transport (toward the cytoplasm) of the K+ ion (44). The fact that P4-ATPases also catalyze counter-transport of phospholipids suggests that the same consideration applies in this case as well (47).
The above suggestion implies that the important interaction of the subunit in either case will be with the form of the enzyme that interacts with the counter-transported species, namely the E2P form. This is the conclusion suggested here for the P4-ATPases, and there are indications that the same holds for P2C-ATPases as well. In the case of the Na+/K+-ATPase, biochemical evidence indicates that disruption of the disulfide bonds in the ectodomain of the β subunit blocks K+ occlusion, and conversely, that binding K+ ions can protect the β subunit from disruption (48, 49). Moreover, disruption of interactions between specific residues within the membrane spans of the Na+/K+ transporter and β subunit results in a shift toward an E1 conformation (50, 51). This suggests that binding of the native β subunit exerts a stabilizing effect on an E2 or E2P conformation. Such stabilizing effects would imply a stronger interaction between the transporter and the subunit at the E2P conformation. Although this prediction has not been tested directly, fluorometric measurements and analysis of tryptic digests have demonstrated that structural rearrangements and movements between transporter and β subunit accompany the binding and translocation of K+ ions (52, 53). Analogous to what we propose for P4-ATPases, these changes likely reflect dynamic transporter-subunit interactions that are tightly linked to the ATPase reaction cycle (Fig. 8B).
In sum, the combination of genetic and biochemical data presented here provides the first evidence that Cdc50 proteins directly participate in the reaction cycle of P4-ATPases. Their function bears a striking similarity to the role of the β subunit in P2C-ATPases. Our findings mark an important step forward in understanding how the conserved transport mechanism of P-type ATPases has been adapted by a family of prime candidate lipid pumps and offer a fresh starting point for the functional reconstitution of these enzymes.
Supplementary Material
Acknowledgment
We thank Philippe Champeil for providing SR vesicles and advice, Ruud Cox for technical assistance, and Thomas Pomorski, Todd Graham, Christopher Grefen, Sean Munro, and Kazuma Tanaka for plasmids or antibodies.
This work was supported by grants from the Fondation Recherge Médicale (to G. L.), the Dutch Organization of Sciences (NWO-CW), the Utrecht High Potential Program (to J. H.), and the National Science Foundation (to P. W.).
This article was selected as a Paper of the Week.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Experimental Procedures, supplemental Table S1, supplemental Figs. S1–S3.
5 C. F. Puts, P. Williamson, and J. C. M. Holthuis, unpublished data.
- SERCA
- sarcoplasmic reticulum Ca2+-ATPase
- ER
- endoplasmic reticulum
- PE
- phosphatidylethanolamine
- PS
- phosphatidylserine
- PC
- phosphatidylcholine
- PA
- phosphatidic acid
- SD
- synthetic dextrose
- TES
- 2-{[2-hydroxy-1,1-bis(hydroxymethyl)ethyl]amino}ethanesulfonic acid
- MOPS
- 4-morpholinepropanesulfonic acid
- mAb
- monoclonal antibody
- HA
- hemagglutinin
- Ni2+-NTA
- Ni2+-nitrilotriacetic acid
- Cub
- C terminus of ubiquitin
- Nub
- N terminus of ubiquitin.
REFERENCES
- 1.Ebashi F., Ebashi S. ( 1962) Nature 194, 378– 379 [DOI] [PubMed] [Google Scholar]
- 2.Jorgensen P. L., Hakansson K. O., Karlish S. J. ( 2003) Annu. Rev. Physiol. 65, 817– 849 [DOI] [PubMed] [Google Scholar]
- 3.Møller J. V., Nissen P., Sørensen T. L., le Maire M. ( 2005) Curr. Opin. Struct. Biol. 15, 387– 393 [DOI] [PubMed] [Google Scholar]
- 4.Olesen C., Picard M., Winther A. M., Gyrup C., Morth J. P., Oxvig C., Møller J. V., Nissen P. ( 2007) Nature 450, 1036– 1042 [DOI] [PubMed] [Google Scholar]
- 5.Toyoshima C., Inesi G. ( 2004) Annu. Rev. Biochem. 73, 269– 292 [DOI] [PubMed] [Google Scholar]
- 6.Kühlbrandt W. ( 2004) Nat. Rev. Mol. Cell Biol. 5, 282– 295 [DOI] [PubMed] [Google Scholar]
- 7.Morth J. P., Pedersen B. P., Toustrup-Jensen M. S., Sørensen T. L., Petersen J., Andersen J. P., Vilsen B., Nissen P. ( 2007) Nature 450, 1043– 1049 [DOI] [PubMed] [Google Scholar]
- 8.Pedersen B. P., Buch-Pedersen M. J., Morth J. P., Palmgren M. G., Nissen P. ( 2007) Nature 450, 1111– 1114 [DOI] [PubMed] [Google Scholar]
- 9.Daleke D. L. ( 2007) J. Biol. Chem. 282, 821– 825 [DOI] [PubMed] [Google Scholar]
- 10.Pomorski T., Holthuis J. C., Herrmann A., van Meer G. ( 2004) J. Cell Sci. 117, 805– 813 [DOI] [PubMed] [Google Scholar]
- 11.Tang X., Halleck M. S., Schlegel R. A., Williamson P. ( 1996) Science 272, 1495– 1497 [DOI] [PubMed] [Google Scholar]
- 12.Hua Z., Fatheddin P., Graham T. R. ( 2002) Mol. Biol. Cell 13, 3162– 3177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pomorski T., Lombardi R., Riezman H., Devaux P. F., van Meer G., Holthuis J. C. ( 2003) Mol. Biol. Cell 14, 1240– 1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wicky S., Schwarz H., Singer-Krüger B. ( 2004) Mol. Cell. Biol. 24, 7402– 7418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen S., Wang J., Muthusamy B. P., Liu K., Zare S., Andersen R. J., Graham T. R. ( 2006) Traffic 7, 1503– 1517 [DOI] [PubMed] [Google Scholar]
- 16.Natarajan P., Wang J., Hua Z., Graham T. R. ( 2004) Proc. Natl. Acad. Sci. U.S.A. 101, 10614– 10619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alder-Baerens N., Lisman Q., Luong L., Pomorski T., Holthuis J. C. ( 2006) Mol. Biol. Cell 17, 1632– 1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pérez-Victoria F. J., Sánchez-Cañete M. P., Castanys S., Gamarro F. ( 2006) J. Biol. Chem. 281, 23766– 23775 [DOI] [PubMed] [Google Scholar]
- 19.Poulsen L. R., López-Marqués R. L., McDowell S. C., Okkeri J., Licht D., Schulz A., Pomorski T., Harper J. F., Palmgren M. G. ( 2008) Plant Cell 20, 658– 676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Darland-Ransom M., Wang X., Sun C. L., Mapes J., Gengyo-Ando K., Mitani S., Xue D. ( 2008) Science 320, 528– 531 [DOI] [PubMed] [Google Scholar]
- 21.Paulusma C. C., Groen A., Kunne C., Ho-Mok K. S., Spijkerboer A. L., Rudi de Waart D., Hoek F. J., Vreeling H., Hoeben K. A., van Marle J., Pawlikowska L., Bull L. N., Hofmann A. F., Knisely A. S., Oude Elferink R. P. ( 2006) Hepatology 44, 195– 204 [DOI] [PubMed] [Google Scholar]
- 22.Lenoir G., Williamson P., Holthuis J. C. ( 2007) Curr. Opin. Chem. Biol. 11, 654– 661 [DOI] [PubMed] [Google Scholar]
- 23.Vilsen B., Andersen J. P. ( 1986) Biochim. Biophys. Acta 855, 429– 431 [DOI] [PubMed] [Google Scholar]
- 24.Saito K., Fujimura-Kamada K., Furuta N., Kato U., Umeda M., Tanaka K. ( 2004) Mol. Biol. Cell 15, 3418– 3432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kato U., Emoto K., Fredriksson C., Nakamura H., Ohta A., Kobayashi T., Murakami-Murofushi K., Kobayashi T., Umeda M. ( 2002) J. Biol. Chem. 277, 37855– 37862 [DOI] [PubMed] [Google Scholar]
- 26.Furuta N., Fujimura-Kamada K., Saito K., Yamamoto T., Tanaka K. ( 2007) Mol. Biol. Cell 18, 295– 312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paulusma C. C., Folmer D. E., Ho-Mok K. S., de Waart D. R., Hilarius P. M., Verhoeven A. J., Oude Elferink R. P. ( 2008) Hepatology 47, 268– 278 [DOI] [PubMed] [Google Scholar]
- 28.Holthuis J. C., Nichols B. J., Dhruvakumar S., Pelham H. R. ( 1998) EMBO J. 17, 113– 126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lenoir G., Picard M., Gauron C., Montigny C., Le Maréchal P., Falson P., Le Maire M., Møller J. V., Champeil P. ( 2004) J. Biol. Chem. 279, 9156– 9166 [DOI] [PubMed] [Google Scholar]
- 30.Lenoir G., Picard M., Møller J. V., le Maire M., Champeil P., Falson P. ( 2004) J. Biol. Chem. 279, 32125– 32133 [DOI] [PubMed] [Google Scholar]
- 31.Champeil P., Guillain F., Vénien C., Gingold M. P. ( 1985) Biochemistry 24, 69– 81 [DOI] [PubMed] [Google Scholar]
- 32.Obrdlik P., El-Bakkoury M., Hamacher T., Cappellaro C., Vilarino C., Fleischer C., Ellerbrok H., Kamuzinzi R., Ledent V., Blaudez D., Sanders D., Revuelta J. L., Boles E., André B., Frommer W. B. ( 2004) Proc. Natl. Acad. Sci. U.S.A. 101, 12242– 12247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grefen C., Lalonde S., Obrdlik P. ( 2007) Current Protocols in Neuroscience, pp. 5.27.1– 5.27.41, John Wiley & Sons, Inc., New York: [DOI] [PubMed] [Google Scholar]
- 34.Cantley L. C., Jr., Cantley L. G., Josephson L. ( 1978) J. Biol. Chem. 253, 7361– 7368 [PubMed] [Google Scholar]
- 35.Allen G., Green N. M. ( 1976) FEBS Lett. 63, 188– 192 [DOI] [PubMed] [Google Scholar]
- 36.Nakano K., Yamamoto T., Kishimoto T., Noji T., Tanaka K. ( 2008) Mol. Biol. Cell 19, 1783– 1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Auland M. E., Roufogalis B. D., Devaux P. F., Zachowski A. ( 1994) Proc. Natl. Acad. Sci. U.S.A. 91, 10938– 10942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Meis L., Vianna A. L. ( 1979) Annu. Rev. Biochem. 48, 275– 292 [DOI] [PubMed] [Google Scholar]
- 39.Vilsen B., Andersen J. P., Clarke D. M., MacLennan D. H. ( 1989) J. Biol. Chem. 264, 21024– 21030 [PubMed] [Google Scholar]
- 40.Johnsson N., Varshavsky A. ( 1994) Proc. Natl. Acad. Sci. U.S.A. 91, 10340– 10344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Orr-Weaver T. L., Szostak J. W., Rothstein R. J. ( 1983) Methods Enzymol. 101, 228– 245 [DOI] [PubMed] [Google Scholar]
- 42.Anthonisen A. N., Clausen J. D., Andersen J. P. ( 2006) J. Biol. Chem. 281, 31572– 31582 [DOI] [PubMed] [Google Scholar]
- 43.Clausen J. D., Vilsen B., McIntosh D. B., Einholm A. P., Andersen J. P. ( 2004) Proc. Natl. Acad. Sci. U.S.A. 101, 2776– 2781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Geering K. ( 2001) J. Bioenerg. Biomembr. 33, 425– 438 [DOI] [PubMed] [Google Scholar]
- 45.Poulsen L. R., López-Marqués R. L., Palmgren M. G. ( 2008) Cell. Mol. Life Sci. 65, 3119– 3125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gottardi C. J., Caplan M. J. ( 1993) Science 260, 552– 556 [DOI] [PubMed] [Google Scholar]
- 47.Puts C. F., Holthuis J. C. ( February21, 2009) Biochim. Biophys. Acta doi:10.1016/j.bbalip.2009.02.005 [DOI] [PubMed] [Google Scholar]
- 48.Kawamura M., Ohmizo K., Morohashi M., Nagano K. ( 1985) Biochim. Biophys. Acta 821, 115– 120 [DOI] [PubMed] [Google Scholar]
- 49.Lutsenko S., Kaplan J. H. ( 1993) Biochemistry 32, 6737– 6743 [DOI] [PubMed] [Google Scholar]
- 50.Dürr K. L., Tavraz N. N., Dempski R. E., Bamberg E., Friedrich T. ( 2009) J. Biol. Chem. 284, 3842– 3854 [DOI] [PubMed] [Google Scholar]
- 51.Hasler U., Crambert G., Horisberger J. D., Geering K. ( 2001) J. Biol. Chem. 276, 16356– 16364 [DOI] [PubMed] [Google Scholar]
- 52.Dempski R. E., Friedrich T., Bamberg E. ( 2005) J. Gen. Physiol. 125, 505– 520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lutsenko S., Kaplan J. H. ( 1994) J. Biol. Chem. 269, 4555– 4564 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.