Abstract
Background
A sizeable minority of taxa is successful in areas prone to submergence. Many such plants elongate with increased vigour when underwater. This helps to restore contact with the aerial environment by shortening the duration of inundation. Poorly adapted species are usually incapable of this underwater escape.
Scope
Evidence implicating ethylene as the principal factor initiating fast underwater elongation by leaves or stems is evaluated comprehensively along with its interactions with other hormones and gases. These interactions make up a sequence of events that link the perception of submergence to a prompt acceleration of extension. The review encompasses whole plant physiology, cell biology and molecular genetics. It includes assessments of how submergence threatens plant life and of the extent to which the submergence escape demonstrably improves the likelihood of survival.
Conclusions
Experimental testing over many years establishes ethylene-promoted underwater extension as one of the most convincing examples of hormone-mediated stress adaptation by plants. The research has utilized a wide range of species that includes numerous angiosperms, a fern and a liverwort. It has also benefited from detailed physiological and molecular studies of underwater elongation by rice (Oryza sativa) and the marsh dock (Rumex palustris). Despite complexities and interactions, the work reveals that the signal transduction pathway is initiated by the simple expediency of physical entrapment of ethylene within growing cells by a covering of water.
Key words: Adaptation, aquatic plants, ethylene, flooding, growth, hypoxia, plant hormones, signal transduction, stress, submergence, review, rice
INTRODUCTION
Submergence in flood water, especially if stagnant or slow moving, is highly damaging to the majority of plant species and can prove fatal. But, in most wetlands and flood-prone margins of rivers and lakes, etc., a thriving and surprisingly diverse community of plants is usually to be found. These communities play a vital ecological role in stabilizing banks and filtering eroded soils, minerals and pollutants (Lytle et al., 1996) and in nurturing much aquatic animal life. The wetland ecosystems they sustain are, therefore, of considerable social, economic and historical significance (Maltby, 1991) and many large wetlands are high profile conservation areas protected under the international Ramsar Convention on Wetlands (Anonymous, 2007). Furthermore, in the humid tropics, where, at certain times of the year, monsoon rain creates near-aquatic environments, highly productive rice farming provides 50–80 % of the calories consumed by the expanding local human population (Hossain, 1995). Most of this rice is produced in flooded paddy fields, managed flood-prone lowlands or in areas susceptible to uncontrolled flooding where other crops would fail. Such ecological and agricultural perspectives amplify any purely botanically based curiosity in aquatic and amphibious species that springs from their inherent physiological distinctiveness and the success they achieve in the face of a highly challenging stress.
The attributes of plants well-adapted to submergence incorporate elements both of resilience and of escape (Jackson, 2006). A strong submergence-induced elongation is one of the most widespread escape mechanisms and one that helps submerged individuals regain or retain contact with the aerial environment on which they depend (Arber, 1920). This positive escape is thought to be mediated by the gaseous hormone ethylene (Osborne, 1984; Jackson, 1985; Ridge, 1987; Voesenek et al., 2004). Such a mechanism seemed implausible 30–40 years ago when ethylene was first becoming widely recognized as one of the major plant hormones. At that time, the adoption of sensitive flame ionization gas chromatography (Meigh, 1959) was helping to establish ethylene as a normal product of plants possessing hormonal significance. However, this research was set against a typecasting of ethylene as a powerful inhibitor of shoot elongation (Pratt and Goeschl, 1969). That prevailing view of ethylene's negative role in extension growth was rooted in an old air pollution history (Knight and Crocker, 1913) and reinforced by influential studies linking growth inhibition to over-generous applications of auxin or ethylene to plants or their excised parts (Burg and Burg, 1966; Ridge and Osborne, 1970). These findings temporarily eclipsed a small early ethylene literature that presaged wider recognition of ethylene's ability to stimulate cell growth at small (ppm, v/v) concentrations. For example, in the first half of the last century, ethylene was reported to promote the cell expansion that underpins epinastic leaf curvature (Crocker et al., 1932), cell hypertrophy (Wallace, 1926), axillary bud sprouting, seed germination (Vacha and Harvey, 1927) and leaf abscission (Denny, 1924). By the early 1970s, a more direct impetus to test the involvement of ethylene in submergence-accelerated elongation (Musgrave et al., 1972) came from three contemporary sources. The first was McComb's report of a successful simulation of submergence-induced stem extension of Callitriche stagnalis by smearing apical rosettes with petroleum jelly (McComb, 1965). This raised the question of which gases were being excluded from the plant or trapped within it that might explain the fast growth response. McComb (1965) eliminated CO2, O2 and N2 and suspected water vapour but left the question otherwise unanswered. The second was a serendipitous observation that ethylene was capable of stimulating vigorous extension by peduncles of Ecballium elaterium fruit prior to their explosive abscission (Jackson et al., 1972). The third was a report that small concentrations of ethylene accumulating in sealed vessels containing growing rice seedlings stimulated coleoptile elongation (Ku et al., 1970). Although these tests with rice were done only in the dark, the authors appeared to have reproduced submergence-promoted growth by trapping the plant's own ethylene in the enclosing glassware. The possibility that flood water might also do this was clear once it became recognized that ethylene is only modestly soluble in water (approx. 126 mL dissolves in 1 L at 20 °C) (McAuliff, 1966) and diffuses almost 10 000 times more slowly in water than it does in air (Burg and Burg, 1965).
The remainder of this article discusses experimental evidence that ethylene is indeed the primary instigator of fast underwater extension in many aquatic and amphibious species and describes how ethylene interacts with other gases, hormones and additional factors to bring about the effect. The supporting evidence is used to explore the underlying physiology or molecular biology, and to point out some ecological implications of the phenomenon. It begins by summarizing how submergence, especially in non-flowing water, is sufficiently threatening to warrant an escape mechanism.
WHAT ARE SUBMERGED PLANTS ESCAPING FROM?
It is difficult to envisage a more biologically benign molecule than water. Its many life sustaining properties extend from being a major co-substrate in photosynthesis to an incompressibility in liquid form that makes for efficient turgor-driven cell expansion. Nevertheless, a potentially life-threatening physical property of water is the high impedance it presents to gas diffusion, most notably to CO2 and O2. The diffusion coefficient for CO2 is 0·159 cm2 s−1 in air but only 1·86 × 10−5 cm2 s−1 in water at 20 °C, a difference of 8548. The diffusion coefficient for O2 is 0·201 cm2 s−1 in air but only 2·1 × 10−5 cm2 s−1 in water at 20 °C, a difference of 9571 (Armstrong, 1979). In the case of O2, low solubility in water reduces the concentration approx. 33-fold compared with in air. This is a further a problem for submerged plants. The combined effect of low diffusivity and solubility of O2 raises the barrier to diffusion from the air–water interface into water approx. 320 000 times compared with that presented by a gas-phase alone (Armstrong and Drew, 2002).
Impeded diffusion on this scale inevitably interferes severely with the rapid influxes of O2 and CO2 associated with normal respiration and photosynthesis (Poorter et al., 1990). Slow CO2 influxes inhibit photosynthesis strongly which is a problem for the plant since most of each day's photosynthate is need to fuel aerobic respiration (Poorter et al. 1990). Although CO2 from the air is very soluble in water, permitting dissolved concentrations to approach atmospheric levels (Sisler and Wood, 1988) this is insufficient to compensate for the low diffusivity. The problem is compounded by a thick boundary layer of static water adhering to the surface of submerged leaves (Smith and Walker, 1980). This is in the order of 100–500 µm thick rather than the single figure values typical for gaseous unstirred layers overlying plants in air. Such thick static layers impede inward entry into the plant by cutting down any convective contribution to gas replenishment at the plant surface and blunting concentration gradients that drive diffusion into the plant. To overcome these barriers to CO2 influx, water needs to be stirred and sparged vigorously with percent concentrations of CO2 rather than the approx. 360 ppm of CO2 of ambient air (Setter et al., 1989). However, percent levels of CO2 are not to be found in flood water in the field (Setter et al., 1987). In addition to CO2-supply problems, photosynthesis underwater suffers from a shading effect by the floodwater that cuts intensity by reflecting back a percentage of surface irradiance and by absorption that increases logarithmically with depth, even when the water is clear (Madsen, 1993).
The resulting suppression of carbon assimilation from these various effects not only suppresses photosynthesis but minimizes the generation of O2 by photosynthetic photolysis of water. Modest generation of O2 by re-fixing of respiratory CO2 remains possible if light intensity is sufficient, but this is a short-term expediency that relies upon stored carbohydrates rather than external air to supply the CO2. A lack of photosynthetic O2 (Waters et al., 1989) adds to major restrictions on oxygenation from external sources imposed by the slow rate of diffusion of aerial O2 through the intervening flood water to the plant and by its low solubility in water (approx. 0·28 mol m−3 at 25 °C). The extent to which oxygen-deficient conditions set-in as a consequence of an impeded O2 supply is greatest in the dark. For example, in plants submerged about 1 h before light is turned off, internal concentrations of O2 then fall to 50 % of normal just 300 µm below the petiole epidermis within the next 45 min (Rijnders et al., 2000). The depressing effects on internal O2 will be even more severe in roots or rhizomes, since their O2-consumming apices are situated more remotely from external O2, because their external soil-based environment is likely to be anaerobic and because they are unable to generate O2 by photosynthesis. Once restricted gas fluxes into mitochondria reduce O2 concentrations sufficiently, severe O2 deficiency will inevitably inhibit rates of aerobic respiration and a range of energy-dependent growth and maintenance processes. Relationships between consumption rate, flux rate and surface geometry of non-photosynthetic tissues have been outlined elsewhere (Jackson, 2005). A halving of respiration can be expected when concentrations fall to approx. 0·14 mmol m−3 O2 at the site of mitochondrial cytochrome oxidase (concentration in air is approx. 8·31 mol m−3). However, the concentrations immediately external to the plant bringing this about will be very much larger (Armstrong and Gaynard, 1976). These aspects have been considered in detail by others (Armstrong and Drew, 2002).
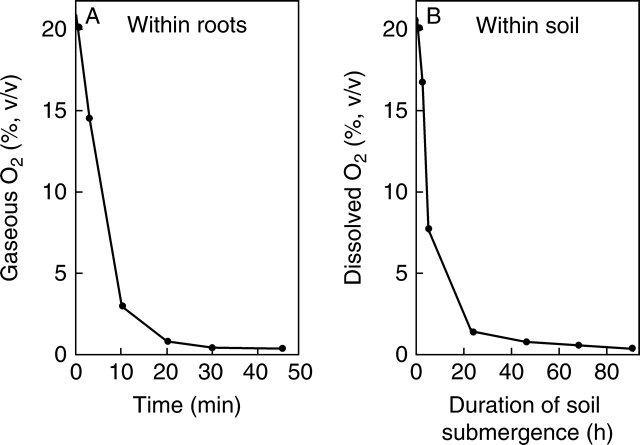
In some flooding-intolerant species, restricting submergence to the soil-based root system (waterlogging) is sufficiently stressful to damage the whole plant severely and can even prove fatal (Jackson, 2004). This arises because of whole-plant dependence on the roots. The amount of O2 stored within the roots is very small and is consumed by respiration within a few minutes (Fig. 1A) in the absence of compensating internal oxygenation via aerenchyma (Voesenek et al., 1999). Similarly, soil O2 is exhausted by bacterial respiration (Fig. 1B). By contrast, in flooding-intolerant land plants, gas space development (i.e. aerenchyma) is usually plentiful (Justin and Armstrong, 1987) and this creates an internal pathway for substantial amounts of root and soil aeration. The flow is driven by diffusion (and sometimes a diffusion-related pressure-driven mass flow) via what has become known as the Schnorkel effect (Kordan, 1974). It is particularly effective when only the soil and roots are submerged (Jackson and Armstrong, 1999). If the water deepens to cover more and more of the shoot, this internal aeration in wetland plants becomes increasingly suppressed as the source of aerial O2 is increasingly denied. It is almost entirely extinguished by complete submergence (Laan et al., 1996). Thus, without a successful escape brought about by faster upward elongation, a combined lack of photosynthetic production of respirable substrates and an inhibition of aerobic respiration by a restricted O2 supply leads, sooner or later, to an energy crisis that compromises the maintenance of cell integrity and encourages potentially fatal cytoplasmic acidosis (Jackson, 2006). Lack of radial oxygen transfer from roots and rhizomes into the soil also leads to a chemically reduced rhizosphere and phyllosphere that is characterized by toxins such as ferrous iron, sulfides and short chain fatty acids that may penetrate the tissues to a damaging extent (Armstrong et al., 2006).
Fig. 1.
(A) Respiratory depletion of the small internal reserve of gaseous O2 in non-aerenchymatous roots of wheat is illustrated. Excised roots were submerged in oil to prevent entry of atmospheric O2 and gas extracted from them for O2 analysis at intervals over 45 min (from Wiedenroth and Jackson, 1993). (B) Time course of respiratory depletion of dissolved O2 in waterlogged soil in terms of decreasing equilibrium concentrations (Jackson and Campbell, 1976).
Despite all the above, it would be wrong to believe that the causes of plant failure from submergence are entirely understood. For example, in the field, floodwater is rarely O2-free and it takes surprisingly little O2 to support some growth or prolong survival, at least in roots (Armstrong and Webb, 1985; Brailsford et al., 1993). Furthermore, photosynthetic fixation of respiratory CO2 by green shoots, even at low light intensities, can generate enough O2 to suppress ethanolic fermentation, with almost all the O2 generated being utilized in aerobic respiration (Boamfa et al., 2003). Despite such activity, submergence damage still takes place. Thus, a severe, anaerobically based energy crisis is not always necessary for submergence injury. In support of this, there is evidence that partial O2 shortage leading to hypoxia rather than anoxia can damage rice or tomato plants even though O2 supply is sufficient to prevent almost all anaerobic metabolism and fermentation (Boamfa et al., 2003; Gharbi et al., 2007). A possible explanation is that, during submergence and when internal O2 decreases to small but not extinguished concentrations, reactive oxygen species (e.g. superoxide radicals) are formed that damage membranes by initiating peroxidative chain reactions targeting polyunsaturated fatty acids such as linolenic acid. This is micro-aerobic generation of reactive oxygen species rather than the more widely reported post-anoxic generation. Such ideas are emerging from non-destructive analysis of volatile emissions from submergence-tolerant and -intolerant forms of rice. Here, better survival of micro-aerobic submergence has been associated with a more effective disposal of free radicals, perhaps via the synthesis of sub-lethal amounts of acetaldehyde from ethanol that involves catalase and H2O2 (Boamfa et al., 2005; Santosa et al., 2007).
It would also be incorrect to believe that escape by elongation is the only mechanism favouring survival of submergence by well-adapted species. The widespread need for an enhancement of the longitudinally interconnected gas pathway (aerenchyma formation) to redistribute O2 from emerged parts of the plant has already been mentioned. Roots with aerenchyma often exhibit a slow rate of radial leakage of O2 that appears to be the result of suberization of a sub-epidermal exodermis (Soukup et al., 2007). In some cases, a decrease in radial loss of O2 from aerenchyma (i.e. less leakage) appears to be submergence-inducible, as in roots of rice (Insalud et al., 2006). Internal aeration via aerenchyma can sometimes be supplemented by gas transport through a pith cavity (where this is present) or via longitudinal bubbles of gas such as those clinging to the outside of leaves of submerged rice plants (Raskin and Kende, 1985; Beckett et al., 1988).
In a few cases, submergence tolerance is known where submergence does not stimulate shoot elongation or even suppresses it, e.g. in the ‘Turlough’ form of Ranunculus repens (Lynn and Waldren, 2003) and the ‘FR13A’ form of indica rice (Jackson et al., 1987). These plants must therefore possess features of enhanced resilience to submergence per se. These may include ethylene-enhanced formation of adventitious roots (Laan et al., 1996; Lorbiecke and Sauter, 1999) and improved photosynthetic ability by new leaves formed underwater (Mommer and Visser, 2005) arising from changes in leaf shape and internal anatomy. An example of the latter is the induction of thin lathe-shaped leaves replacing the thicker ovoid aerial leaves in Callitriche stagnalis (Jones, 1955). However, it must be emphasized that direct experimental testing of the effectiveness of these morphogenetic changes in enhancing survival is mostly lacking.
Subtle metabolic acclimations to O2 or CO2 shortage that reduce energy demands while retaining key cell maintenance processes especially membrane function (Geigenberger, 2003; Greenway and Gibbs, 2003) or enhance photosynthetic performance (Bowes, 1987) may also make a contribution to underwater survival. More overtly, there are several plants such as coleoptiles of rice (Kordan, 1976) and barnyard grass (Pearce and Jackson, 1991), and shoots of Potamogeton pectinatus (Summers and Jackson, 1996) and Schoenoplectus lacustris (Barclay and Crawford, 1982) that not only tolerate anaerobiosis for long periods (days, weeks or months) but also elongate under these conditions, sometimes at a much accelerated rate (the anaerobic escape; Jackson, 2006), despite the large demands for respirable substrates imposed by anaerobic respiration and by the faster growth itself. This anaerobic escape does not involve ethylene since both ethylene biosynthesis (Pearce et al., 1992) and action (Horton, 1991) are halted by anoxia. In view of the contribution of resilience in the face of oxygen shortage, it is not surprising that availability of starch and other respirable reserves that substitute for the absence of photosynthetic carbon gain are frequently linked to an ability to survive total submergence (Yamada, 1959; Nabben et al., 1999; Groeneveld and Voesenek, 2003). Accordingly, survival of darkness alone has sometimes been positively related to ability to tolerate complete submergence (Yamada, 1959). The key role in the survival process for assimilate supply from stores is also illustrated by the greater damage inflicted on rice seedlings if submergence starts just after the normal night break rather than starting after several hours in the light when sugars have accumulated (Ram et al., 2002). The ability in rice to recover from submergence has also been linked to slower consumption of carbohydrate during submergence leaving larger reserves to support re-growth when water levels recede to uncover the plant once more (Das et al., 2005; Fukao et al., 2006).
SURVIVAL VALUE OF THE UNDERWATER ESCAPE
This is a complex issue and is best viewed in the context of a trade-off between costs and potential benefits (Ridge, 1987). For accelerated upward elongation to save the plant, the water must not be so deep that escape is beyond the growth capacity of the particular species or stage of growth. Plants presumably must resurface before metabolic costs of the faster growth exhaust respirable reserves and before damaging processes such as ethylene-promoted leaf senescence (Jackson et al., 1987; Banga et al., 1997) or free radical action (Santosa et al., 2007) intervene. This interpretation is supported by the results of experimental manipulations of rice seedling leaf extension in water that is too deep for re-emergence. They show that fatalities rise when futile underwater extension growth by rice seedlings is promoted with gibberellic acid and reduced when it is suppressed with a gibberellin biosynthesis inhibitor (Setter and Laureles, 1996). A recent ecological survey in the Rhine floodplain gives strong correlative support to the view that, in the locations with appropriate water depths, fast underwater elongation is associated with successful resurfacing and with species success. It reveals that strong underwater elongation is typical of species inhabiting slow-draining sites where shallow pools remain in place for up to about 120 d year−1 (Voesenek et al., 2004). Rumex maritimus, a plant that elongates vigorously underwater, is typical of such species. Without this growth, and the escape it achieves, the plants die within 2 weeks (Van der Sman et al., 1991). Similarly, in the absence of successful resurfacing, escapist Ranunculus sceleratus dies at least as quickly as non-escapist Ranunculus bulbosus (He et al., 1999) in association with severely compromised root aeration status (Laan et al., 1989). The Rhine delta survey also showed that areas, where flooding is too deep for a successful underwater escape, are occupied by species that adopt a short annual life cycle and survive as seeds rather than as plants in the flooding season (Voesenek et al., 2004). In rice, survival of especially deep water is associated with contrasting strategies depending on the ecotype involved. Deepwater and floating rice varieties have evolved an especially vigorous and prolonged capacity to elongate the stems of maturing plants when submerged. This ability much exceeds that of other types of rice. The response is key to their survival since the plants soon die unless some foliage remains above water or contact with air is promptly renewed (Vergara et al., 1976). On the other hand, a suppression of elongation is an outstanding feature of lines of rice (FR13A and its derivatives) that, as seedlings, survive total submergence for longer than other types of rice. This is an adaptation to a prevalence of water that is too deep for the submergence escape to be effective. FR13A is derived from an old Indian farmer variety (Dhullaputia) (Mackill, 1986). This variety was formally grown in submergence-prone Orissa where the main crop, covering over 85 % of all rice grown there, is produced during the south-west monsoon. Overall, the evidence highlights a considerable dependency on successful escape for long-term survival and encourages the view that the cost of rapid growth is rewarded by survival but only if resurfacing can be achieved in time.
A further factor to consider in assessing survival value of the escape process is the changed physical and physiological state of the successfully re-emerged plant. This must not overly compromise its ability to grow, compete and reproduce thereafter. For example, the resulting taller posture may make the plants more vulnerable to grazing. For example, Canada geese preferentially graze on recently de-submerged Plantago major (I. Ridge, The Open University, UK, unpubl. obs.). A similar effect has also been observed for species of Rumex (Voesenek and Blom, 1989). In rice, the prospects for re-emerged plants are sometimes compromised by a tendency to keel over (‘lodge’) and lie horizontally in the wet mud (Zeigler and Puckridge, 1995). This tendency has been linked to decreases in silicon deposition in cell walls weakening the stem base (Rose-John and Kende, 1984). Recovery from lodging is sometimes achieved by gravity-mediated ‘kneeing’. This involves an asymetric expansion by stem nodes that returns the shoot nearer to the upright position (Catling, 1992). Evidence from Echinochloa colonum, an annual grass often found and along the banks of lakes and rivers in tropical wetlands, shows kneeing to be under hormonal regulation with a possible involvement of ethylene (Wright et al., 1978).
In Ranunculus repens (Ridge, 1987), Rumex palustris (Voesenek et al., 1993), Ranunculus pygmaeus (Horton, 1992) and many other wetland species, the fast extension rates of submerged plants are sustained even after re-emergence. This can propel the lamina well above water level (Fig. 2). This emergent habit (see later section ‘Before and After Ancillary Processes’) allows a seemingly strong competitive position to be quickly established thereby increasing the chances of ultimately achieving at least some setting of seed (Van der Sman et al., 1991).
Fig. 2.

Fast underwater elongation is maintained above the water line in many emergent wetland species, such as Caltha palustris.
EVIDENCE SUPPORTING ETHYLENE AS KEY INITIATOR OF FAST UNDERWATER ELONGATION
Several strands of work with a wide variety of species together make a convincing case for ethylene mediation in underwater elongation. Formal criteria (akin to the pathologists' Koch's Postulates) for implicating a hormone in an environmental response (Jacobs, 1959; Jackson, 1987) are considered below and comprise elements of ‘Duplication’; ‘Correlation’; ‘Deletion and re-instatement’; ‘Relevance to lower levels of organization’; and ‘Taxonomic generality’. These criteria are comprehensively satisfied by the experimental results available, making this one of the strongest cases linking an environmental stress response to hormone action and one that is demonstrably at work in both the natural and agricultural worlds. This approach also acts as a convenient vehicle for exploring different aspects of ethylene mediation in the submergence escape.
Correlation between internal ethylene concentration and fast underwater shoot extension
A central strand of evidence needed to support the case for hormone initiation of an environmentally induced process is that the hormone increases to physiologically active levels in advance and/or coincidentally with the developmental change itself. Almost all plant species synthesize ethylene (an exception being Potamogeton pectinatus; Summers et al., 1996). Ethylene is known to be physiologically active when applied in concentrations above about 0·001 ppm (v/v) (1 ppm ethylene in air = 1 µL L−1; 0·1 Pa; 0·041 µmol L−1) with maximal effects often being elicited within the range 1–10 ppm. Musgrave et al. (1972) concluded that in submerged Callitriche platycarpa the floodwater entraps such physiologically active concentrations of ethylene. This conclusion was based on the presence of approx. 1 ppm ethylene in photosynthetically generated gas bubbles at the time fast underwater elongation was proceeding. This interpretation was confirmed using vacuum extraction of internal gas from Ranunculus sceleratus (Samarakoon and Horton, 1984) where 0·4 ppm was measured in <4 h under water, and from deep-water rice where about 1 ppm accumulates within the first 5 h of submergence (Raskin and Kende, 1984a; Rose-John and Kende, 1985). The advent of highly sensitive laser-based photoacoustics allowed internal ethylene concentrations to be quantified with greater precision and in a way that diffusional losses to the water are avoided. In this way, Voesenek et al. (1993b) established that 24 h underwater raises ethylene concentrations in Rumex palustris to 6 ppm. This is 100 times the amount present in non-submerged plants. Inevitably, such an accumulation can also occur in species that fail to elongate when submerged (Banga et al., 1997). Nevertheless, the various demonstrations of ethylene accumulation in concert with fast underwater elongation of well-adapted species leave little doubt that submergence can lift internal ethylene to active levels in time to explain the faster growth. Based on finding that ethylene builds-up to >1 ppm within 1 h of submerging Rumex palustris plants and that 0·1 ppm of applied ethylene is needed for a half-maximal response, Banga et al. (1997) conclude that enough internal ethylene accumulates in 10 min submergence to be growth-active. This is well in advance of the approx. 90 min before the faster elongation begins underwater in this species (Benschop et al., 2005).
In Callitriche platycarpa, de-submergence slows elongation and this can be rejuvenated by a further period of submergence (Jackson, 1982). This suggests an ethylene-mediated response based on an alternating build-up and venting of entrapped ethylene. The venting has been quantified in Rumex palustris by photoacoustic measurements of ethylene emissions from freshly de-submerged plants (Voesenek et al., 1993). They show that most of the internally trapped gas is released from the plant to the aerial environment within 1 min.
Ethylene biosynthesis underwater
Demonstrations of enhanced accumulation of ethylene within submerged plants raises the question of whether this is entirely attributable to entrapment of basal ethylene production by the covering water or whether enhanced biosynthesis also makes a contribution. The answer depends on the species and duration of submergence. In deep-water rice, the partial O2 shortage and CO2 enrichment that stems experience at the base of the shoot system while it is partially submerged stimulate increased stem ethylene production about 4-fold. This faster synthesis is association with increases in the ethylene precursor 1-aminocyclopropane-1-carboxylic acid (ACC) (Raskin and Kende, 1984a) commencing within 2 h (Cohen and Kende, 1987) and with a delayed increase in mRNA coding for one member of a family of ACC synthase enzymes that generate ACC from its precursor S-adenosylmethionine (Zarembinski and Theologis, 1997). Expression of a gene coding for the enzyme ACC oxidase that catalyses the conversion of ACC to ethylene also increases within 4 h (Mekhedov and Kende, 1996). However, stimulation of ethylene formation by shoots suffering partial O2 shortage is an uncommon phenomenon generally, although several examples in roots are known (Jackson et al., 1984). More usual is a slowing of biosynthesis by low O2 (Jackson et al., 1984) probably because the last step in ethylene production (ACC oxidase) that converts ACC to ethylene is O2-dependant, and also subject to inhibition by ethylene itself (Bleecker et al., 1987). Thus, submergence of Rumex palustris and Rumex acetosa slows rather than accelerates their ethylene production (Banga et al., 1996b). This suppression takes place despite a rise in activity of ACC synthase, ACC production and an enhanced accumulation of mRNA coding for ACC oxidase genes detectable within 1–2 h of submergence (Vriezen et al., 1999). Even in rice, the coleoptiles and small seedlings do not make more ethylene when partially O2 deficient (Ku et al., 1970; Pearce et al., 1992; but see Fukao et al., 2006), even though transcript levels coding for ACC synthase (OS-ACS5) rise within 1 h of imposing submergence (Zhou et al., 2001). The resulting accumulations of ACC may have an adaptive value by lowering the Km for O2 of the ACC oxidase enzyme to below 5 % (v/v) (Yip et al., 1988) thereby limiting the extent to which biosynthesis is inhibited by O2 shortage. Claims for hypoxia-stimulated ethylene production in rice, other than by the stem, may be misplaced. Most papers claiming this actually report ethylene production in air as a recovery from a preceding hypoxic or anoxic episode and not ethylene production during the stress itself (Khan et al., 1987; Zhou et al., 2001; Fukao and Bailey-Serres, 2004). This post-stress ethylene, presumably formed from the previously accumulated and unused ACC, cannot be involved in regulating underwater elongation. However, it may well be relevant to regulating growth once contact with air is regained and the O2 supply returns to normal (see the section entitled ‘Before and after ancillary processes’ below).
None of these biosynthetic details apply to the aquatic fern Regnellidium diphyllum and the liverwort Riella helicophylla. These plants accumulate ethylene underwater and respond with faster elongation but, unlike angiosperms, they do not derive their ethylene from methionine or ACC and their biosynthetic rates remain remarkably constant under a range of treatments. The mystery pathway does not include an O2-requiring step (Osborne et al., 1996). These findings reinforce the notion that it is trapping rather than modulation of biosynthetic rate that ensures concentrations of ethylene in submerged plants attain growth-active levels.
Duplication
A second vital strand of evidence supporting ethylene involvement is the extent to which ethylene treatment at physiological concentrations (approx. ≤10 ppm) duplicates the submergence effect. Ideally, reaction times to applied ethylene should be shorter than those to submergence. In Callitriche platycarpa, 12 ppm induces a reaction within 30 min, while the submergence response may take approx. 60 min (Musgrave et al., 1972). Similarly, upright petioles of Nymphoides peltata respond to ethylene within 20–80 min with younger leaves being the more responsive to submergence or applied ethylene (Ridge and Amarasinghe, 1984). Similar lag times to ethylene have been reported for Regnellidium diphyllum petioles (rachis) (20 min) by Musgrave and Walters (1974). In deep-water rice stems, 60 min of ethylene treatment is needed before extension rates increase where 180–220 min are needed for submergence to do this (Rose-John and Kende, 1985). Further support for the notion that ethylene regulates shoot extension comes from observations of a prompt slowing of elongation by de-submergence, a reversibility readily reproduced by withdrawing ethylene from plants previous exposed to the gas in air.
However, there are situations where the vigour of the ethylene-induced elongation fails to match that brought about by submergence. Such discrepancies have been explained in terms of other features of submerged conditions that modify the ethylene response, or possibly its rate of production. For example, slow gas diffusion in floodwater not only entraps ethylene but enables respiration to drive-down internal O2 to levels (e.g. 3 % v/v) that enhance ethylene action via a sensitization to ethylene. Such sensitization is apparent in rice coleoptiles (Ku et al., 1970) and in R. palustris petioles (Voesenek et al., 1997). In the latter case, the ethylene concentration needed to stimulate elongation to half maximum is reduced by 3 % O2 from 0·26 ppm to 0·04 ppm. Any accumulated CO2 in non-photosynthesizing tissue (e.g. 6 % v/v) can also have elongation-promoting promoting effects (Suge and Kusanagi, 1975; Metraux and Kende, 1984; Raskin and Kende, 1984a). Thus, for ethylene treatment to stimulate rice stems to elongate as fast as submerged ones they also require concomitant exposure to about 3 % (v/v) O2 and 6 % (v/v) CO2. In petioles of the aquatic fern Regnellidium diphyllum (Musgrave and Walters, 1974) and in Nymphoides pelatata (Ridge and Amarasinghe, 1984) only when ethylene treatment in air is coupled with a longitudinally applied physical tension to simulate underwater buoyant tension does the application of gas reproduce the full submergence effect. The available evidence largely eliminates shading (McComb, 1965; but see Mommer et al., 2005), water pressure and depth, or rate of water flow (Banga et al., 1995) as additional factors interacting with ethylene underwater. Thus, the overall weight of evidence indicates a substantial amount of duplication of the submergence effect by ethylene and convincing explanations are available for instances where ethylene applications fail to reproduce fully the submergence effect.
In a few cases, fast underwater elongation cannot readily be linked with ethylene mediation. For example, Potomageton pecinatus is constitutively unable to synthesize ethylene and yet elongates rapidly when submerged. It has, seemingly, evolved alternative mechanisms for responding to inundation that involve complete or partial O2 deficiency or to increased CO2 as stimulating signals (Summers and Jackson, 1996). Rice coleoptiles (but not other organs of rice) can also elongate quickly in the complete absence of O2 (Costes and Vartapetian, 1978). Similarly, coleoptiles of the rice-mimic Echinochloa oryzicola sustain vigorous elongation irrespective of ethylene, O2 or CO2 levels (Pearce and Jackson, 1991), while petiole elongation by Rumex acetosa is promoted by submergence independently of ethylene once synthesis of the growth-retarding hormone abscisic acid (ABA) is artificially suppressed (Benschop et al., 2005). Thus, although ethylene is not the only signal plants use to sense they are submerged and to initiate their escape, its influence predominates (see also the subsection entitled ‘Taxonomic diversity’).
Relevance to lower levels of organization
If ethylene is indeed the principal signal initiating fast underwater growth, ethylene treatment should also reproduce cellular, metabolic and molecular changes that are thought to underpin the faster elongation.
Cell elongation and division
Submergence not only accelerates elongation rate of stems or leaves but increases their final length. This can simply be the outcome of a similar number of cells being longer than air-grown controls at the end of their growth phase with little or no change in the number of growing cells. This is the situation for Callitriche platycarpa (Musgrave et al., 1972), older petioles of Rumex palustris (Voesenek et al., 1990) and Regnellidium diphyllum (Ridge and Amarasinghe, 1984), Hydrocharis morsus-ranae and Ranunculus sceleratus (Cookson and Osborne, 1978) where the submergence/ethylene effect on organ length is convincingly explained by increased cell lengths. In addition to greater cell extension, cell division can also be involved in the underwater escape, especially in immature stems and leaves where the proportional increase in final cell length can be less than that of the elongated organ itself. This implies that more cells are being made available for elongation (Funke and Bartels, 1937). In Nymphoides peltata (the Limnanthemum nymphoides of Funke and Bartels), submergence induces a 2·4-fold increase in longitudinal cell count along the petiolar epidermis of the youngest leaf after 16 d of submergence. In Regnellidium diphyllum, this stimulation of cell division could be reproduced, in part, by 10 ppm ethylene given to air-grown plants (Ridge and Amarasinghe, 1984); the shortfall possibly being due to a lack of buoyant tension. Much of the enhanced cell production in the very youngest leaves of submerged Ranunculus pygmaeus can also be duplicated by exogenous ethylene (Horton, 1992). Studies of Ranunculus repens and Nymphoides peltata by Ridge (1987) lead to the conclusion that leaves just emerging underwater elongate faster almost exclusively because cells are dividing faster, middle-aged leaves elongate faster by a combination of faster division and cell extension, while older leaves extend underwater almost exclusively by cell enlargement. Applications of ethylene in air reproduced this pattern in principle, but quantitative discrepancies suggest that other submergence-related factors need to be included to obtain the full response. For example, Metraux and Kende (1984) found that 1 ppm ethylene reproduced the full cell division response only in the company of low O2 and high CO2. In this way, they successfully simulated the faster cell production and cell extension induced by submergence in intercalary meristems of deep-water rice. DNA labelling with [3H]thymidine indicated a doubling of the length of the meristematic region. This may well be the outcome of ethylene initiation of gibberellin-dependant promotion of DNA synthesis of nuclei in G1 linked to activation of cyclin-dependent protein kinase genes (Fabian et al., 2000). However, while their expression and transcript accumulation can be induced by submergence or applications of GA within 2 h, experimental confirmation that ethylene can bring about this up-regulation does not appear to have been reported.
Cell wall changes
Faster or more prolonged cell expansion in plants is often the outcome of increased wall extensibility that imparts a reduction in mechanical resistance to expansion-promoting hydrostatic forces (turgor). These forces are generated by water diffusing across the plasma membrane into cells down a gradient in water potential created mainly by a greater concentration of dissolved solutes within the cell than outside. The Lockart equation summarizes key aspects of these relationships and can be simplified for submerged plants to:
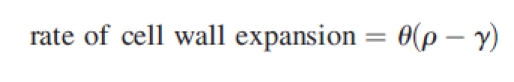 |
where θ = a wall yield coefficient (i.e. a measure of extensibility), ρ = cell turgor and γ = the minimum turgor needed to start wall extension. The contribution of increased extensibility is, seemingly, of overriding importance in submerged plants because the concentration of osmotically active solutes in their cells and the resulting turgor-generating potential (the driving force for cell expansion) can decease rather than increase (Ridge and Osborne, 1989). This presumably occurs because of loss of photosynthetically generated sucrose, and solute dilution by faster cell expansion. This loss in driving force must, necessarily, be more than compensated for by a decrease in the resistance to extension by cell walls if faster growth is to be achieved. This could be brought about by changes such as loss of lignification in outer cell layers of deep-water rice stems and increases in β-glucan (Sauter and Kende, 1992), by reorientation of microtubules as cells leave the meristem thereby favouring the oblique orientation of cellulose microfibrils thought to favour elongation (Sauter et al., 1993). The significance of the loss of wall peroxidase activity reported in submerged Ranunculus sceleratus (Horton, 1993) is uncertain since this could be expected to stiffen cell walls by lowering production of cell wall-loosening hydroxyl radicals (Schweikert et al., 2002) but also lengthen the duration of cell elongation by slowing cross-linking between wall components or restricting lignification (MacAdam et al., 1992). Two other factors likely to promote wall extensibility are a lowering of apoplastic pH and an increase in the non-enzymic wall protein expansin. This protein is thought to promote wall extensibility by breaking hydrogen bonds between hemicellulose and cellulose (McQueen-Mason and Rochange, 1999). There is ample evidence that treating non-submerged plants or freshly excised petiole segments with ethylene can reproduce the effects of submergence on extensibility and wall acidification in Nymphoides pelatata (Malone and Ridge, 1983; Ridge and Osborne, 1989) and Ranunculus sceleratus (Craker et al., 1978), although some contrary evidence is available for rice coleoptiles (Ishizawa and Esashi, 1984). These positive findings have been extended by studies of internodes of deep-water rice (Kutschera and Kende, 1988) and petioles of Rumex palustris. In deep-water rice internodes, amounts of transcript of the expansin gene Os-EXP4 increase before the rate of underwater elongation picks-up (Cho and Kende, 1997), and expansin protein has been found in thick cell walls of the internodal epidermis, the likely growth-limiting layer (Cho and Kende, 1998). Unfortunately, duplication of these submergence effects in deep-water rice by applying ethylene has not been reported. In Rumex palustris (Vreeburg et al., 2005), however, there is clear evidence of submergence-enhanced proton secretion, greater acid-inducible wall extensibility (a measure of expansin activity), higher levels of expansin protein and amount of transcript for one of the 13 expansin genes (RpEXPA1) known in this species. All this takes place within the first 4–6 h when faster elongation is underway. It is suppressed if ethylene action is inhibited with 1-MCP (Vreeburg et al., 2005). The effects on expansin mRNA, at least, have been shown to be reproducible in Rumex palustris by applying ethylene (Vriezen et al., 2000) while, tellingly, closely related Rumex acetosa cannot respond to ethylene or to submergence in this way. Ethylene also successfully reproduces the impact of submergence on petiole extensibility in the fern Regnellidium diphyllum (Cookson and Osborne, 1979). Although wall acidification has been reported to play no part in stimulating extensibility in submerged or ethylene-treated plants of this fern (Ridge and Osborne, 1989), later work (Kim et al., 2000) shows submergence and ethylene not only promoting expression of an expansin gene (RD-EXP1) but also enhancing in vitro extensibility when sections are assayed in acidic buffer (pH 4·5). Overall, it appears that ethylene-stimulated cell wall extensibility mediated, in part, by wall acidification, enhanced expansin levels and transcriptional activation of one or two key members of the expansin gene family is an integral part of the submergence escape and one that may predate the divergence of angiosperms from ferns. However, to see increases in expansin gene transcription as the ultimate effect of ethylene that explains the faster cell extension would be an over-interpretation. It is only one of several, mostly unknown other changes also needed for the faster growth. This is clear from results showing that additions of ABA or an inhibitor of gibberellin biosynthesis fail to suppress submergence-induced expression of expansin genes (Vreeburg et al., 2005) even though underwater extension is strongly inhibited.
Biochemical changes
Faster elongation under water is demanding of energy and solutes (Ridge and Osborne, 1989) and it is not surprising that in rice (Yamada, 1959) and Rumex palustris, it has been linked to a set of biochemical changes favouring release of respirable substrates from starch. Results show a downward spiral in the amounts of starch during submergence (Emes et al., 1988; Groeneveld and Voesenek, 2003). Thus, in the absence of vigorous photosynthesis, starch breakdown holds the key to sustaining underwater consumption of respirable sugars. Accordingly, Raskin and Kende (1984b) report a linear loss in starch content from the internodes of deep-water rice during 90 h of fast underwater growth in association with much increased amolytic enzyme activity. This is readily reproduced by applying 1 ppm ethylene, the starch degradation being ascribed mainly to α-amylase activity (Smith et al., 1987).
Ethylene reception
Several examples of duplication of the effect of submergence by ethylene at the molecular level will emerge in later sections of this article. To illustrate the point here, the enigmatic enhancement of expression of genes coding for putative ethylene receptor proteins is considered. The foundation of this work lies in mutation studies of Arabidopsis thaliana that identified proteins embedded in the endoplasmic reticulum as ethylene receptors with sequence domains similar to the so-called two-component signal transducing proteins identified in bacteria and yeast. These findings revealed that, for ethylene to act in Arabidopsis thaliana, it must first bind to receptors coded for by a 5-member family of such genes [ETR1 (ethylene receptor 1) and ERS1 (ethylene sensor 1) ETR2, ERS2 and EIN4 (ethylene insensitive 4) (Hua et al., 1998)]. The binding of ethylene to the N-terminal domain of receptor protein coded for by these genes is believed to de-activate a histidine kinase present at the C-terminus. This, in turn, may regulate downstream components of the signal transduction pathway starting with CTR1 (constitutive triple response 1), a mitogen-activated serine/threonine protein kinase kinase kinase related to the RAF kinases (Kieber et al., 1993). Something similar is now thought to apply to most plant species (Chen et al., 2005), although some uncertainties remain (Cho and Yoo, 2007). When a homologue (RP-ERS1) of Arabidopsis thaliana ERS was cloned from Rumex palustris, its expression in petioles, measured by blot hybridizations, was found to double within 1 h and be up-regulated 8-fold by 24 h submergence, with similarly prompt decreases when plants were de-submerged. The up-regulation could be reproduced by 5 ppm ethylene when augmented by low O2 concentration (5 %, v/v) that simulates likely O2 levels in true submergence (Vriezen et al., 1997). In rice, too, a putative ethylene receptor gene (Os-ERL1) homologous to Arabidopsis thaliana ETR2 and EIN4 is up-regulated by submergence or by ethylene within 3 h (Watanabe et al., 2004). Low O2 applied without extra ethylene is itself also growth-promoting in Rumex palustris and also up-regulates RP-ERS1. Thus we have a clear example of ethylene reproducing the effect of submergence on the timing and extent of expression of a gene coding for a key receptor protein involved in ethylene action. Examples of ethylene increasing the levels of message coding for its own receptor are also known in ripening apples and peaches where the ethylene action inhibitor 1-methylcyclopropene (1-MCP) slows ethylene-promoted ripening while depressing receptor mRNA levels (Dal Cin et al., 2006). Paradoxically, genetic analyses in Arabidopsis thaliana indicate ethylene receptors are negative regulators of downstream events (Chen et al., 2005). Thus, in the absence of ethylene occupancy, ethylene receptor proteins actively suppress subsequent steps leading to gene activation and growth effects. More ethylene-induced receptors in submergence plants would, logically, lead to a self-induced loss of sensitivity to ethylene rather than its amplification. Clearly, there is much still to learn here but it may be advantageous for the plants to temper the overall ethylene response when submerged if this limits senescence-promoting effects of ethylene while allowing some of the more sensitive growth promotion to survive (Banga et al., 1997). It is also possible that up-regulation of ethylene receptors by ethylene may also help to shut down ethylene action when, later, plants are desubmerged and ethylene vacates receptor proteins and is vented to the atmosphere (more suppressing receptors in action as a result).
Deletion and re-instatement
One key experimental approach to establishing the impact of a supposed hormonal signal is to try and suppress the response by eliminating the hormonal signal and checking the specificity of the suppressor by recovering the response by reintroducing the hormonal signal. A variant on this is to reduce or eliminate the responsiveness of the plant to the signal. Such deletion and re-instatement tests are often made using chemical inhibitors of ethylene biosynthesis such as 1-aminoethoxyvinylglycine (AVG) or cobalt chloride (Co), or with chemical inhibitors of ethylene action such as silver nitrate (Ag) or the gaseous cyclic olefine1-MCP. Experiments with mutations that interfere with ethylene biosynthesis or action also fall into this category, although opportunities are rare amongst wetland species.
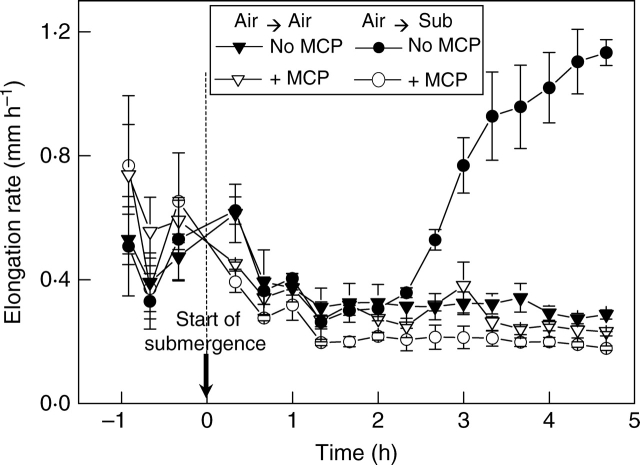
The outcome of deletion and re-instatement experiments is a wealth of evidence backing the notion of ethylene as the prime instigator of accelerated shoot elongation in submerged plants. In early work (Cookson and Osborne, 1978), underwater elongation of Hydrocharis morsus-ranae and Ranunculus sceleratus was retarded successfully with 1,2-amino-4-(2′aminoethoxy)-trans-3-butenoic acid, an ethylene biosynthesis inhibitor that blocks the ACC synthase step. Cookson and Osborne (1978) also substantially overcame the inhibitor's slowing of growth by simultaneously supplying ethylene gas. The specificity of the inhibitor was illustrated further by showing a lack of effect on elongation of Renellidium diphyllum, a species with a different biosynthetic pathway for ethylene that is consequently not susceptible to inhibition at the ACC synthase step with 1,2-amino-4-(2′aminoethoxy)-trans-3-butenoic acid. Demonstrations of chemical repression of ethylene biosynthesis linked to inhibition of underwater shoot extension and its successful reversal with ethylene or ACC have also been obtained for rice (Métraux and Kende, 1983) and Ranunculus sceleratus (Samarakoon et al., 1985). Silver ions inhibit ethylene action (Beyer, 1979) and sub-toxic concentrations of about 10−5 m strongly inhibit underwater elongation in species where this has been tested (Cookson and Osborne, 1978; Jackson, 1982; Banga et al., 1997). Less toxic inhibitors such as norbornadiene and 1-MCP that also interfere with ethylene by binding to its protein receptors (Sisler and Serek, 1997) almost completely abolish the elongation response to submergence in deep-water rice (Bleecker et al., 1987), Rumex palustris (Fig. 3) and also downstream responses to submergence and ethylene such as expression of the RpEXP1 expansin gene and apoplastic acidification (Benschop et al., 2005; Vreeburg et al., 2005). Such responses are wholly in line with the notion of ethylene mediation in submergence-promoted shoot extension.
Fig. 3.
Promotion of petiole elongation by submergence (Sub) in Rumex palustris and its inhibition by 1 ppm of the ethylene-action inhibitor 1-methylcyclopropene (MCP) given 1 h before the start of submergence. Means with standard errors (drawn from Benschop et al., 2005).
Unfortunately, there is no plethora of ethylene biosynthesis or response mutants amongst aquatic or amphibious species to utilize in genetically based deletion and/or reinstatement experiments. However, a small group of related cultivars of indica rice (e.g. FR13A and Kurkaruppan) may fall into this category. These cultivars fail to accelerate their leaf elongation strongly when submerged. The explanation is that leaf extension (and also senescence) is much less sensitive to stimulation by ethylene than it is in most other rice lines (Jackson et al., 1987). A quantitative trait locus (Sub1) for the absence of fast underwater growth has been mapped to near the centromere of chromosome 9 (Nandi et al., 1997; Xu et al., 2000; Toojinda et al., 2003). When this locus from FR13A is introgressed by back-crossing into a normally elongating rice variety such as Thai jasmine rice KDML05, the leaf elongation response to submergence is lost (Siangliw et al., 2003) presumably because ethylene insensitivity has been introduced. In support of this explanation, sequencing of the locus has revealed a cluster of paralogous genes that contain putative ethylene response factor (ERF) domains (Xu et al., 2006) and code for likely DNA binding proteins. One form of one of these genes (Sub1A-1) is dominant and unique to non-elongating varieties derived from or related to FR13A. It is strongly expressed throughout up to 14 d of submergence and its expression is also inducible by applying ethylene to non-submerged plants (Fukao et al., 2006). An apparent consequence of this up-regulation of Sub1A-1 is a suppression of the neighbouring Sub1C gene that, in normal elongating types, is strongly up-regulated by submergence. The picture also holds when air-grown plants are given ethylene rather than submerged. The suppression of Sub1C-1 by ethylene-activated Sub1A-1 is thought to be linked causally to the inhibition of elongation. Thus, it seems that, in the FR13A mutant, one kind of ethylene response (activation of Sub1A-1) leads to the loss of another ethylene-promoted up-regulation (that of Sub1C-1). In submerged plants, this suppression is associated not only with inhibition of leaf elongation but also with retardation of leaf senescence, slowing of starch and sugar consumption, down-regulation of three α-amylase genes, three sucrose synthase genes and five members of a large family of expansin genes (Fukao et al., 2006). Expression of some of these genes in submergence-intolerant japonica plants has been shown to be up-regulated by applying ethylene in air to non-submerged plants unless blocked by the presence of the Sub1A-1 gene. However, exact duplication of the submergence-induced pattern gene expression by applying ethylene alone has proved elusive (Fukao et al., 2006), perhaps because interactions with other aspects of the submerged environment such as low O2 were not incorporated into the experiments. In water that is too deep for fast underwater extension to deliver the submergence escape, a slow or arrested rate of elongation conferred by ethylene insensitivity in FR13A and related lines leads to much increased resilience to several days of total immersion (Setter and Laureles, 1996) as discussed in section ‘Survival value of the underwater escape’ above. This underwater quiescence has been linked to a conservation of respirable resources, less ethylene-induced leaf senescence (reviewed by Jackson and Ram, 2003) and decreased damage from membrane peroxidation by hypoxia-related free radical production (Santosa et al., 2007).
Overall, these experiments with chemical inhibitors of ethylene biosynthesis and with ethylene-insensitive rice cultivars strongly support the view that ethylene action is inextricably involved in the promotion of shoot elongation in plants adopting the submergence escape strategy.
Taxonomic generality
If ethylene-mediation of fast underwater elongation is to be recognized as more than an interesting physiological sideline, its essential details must apply to a wide range of species. This appears to be the case since ethylene treatment to non-submerged plants can reproduce the gross effects of submergence on shoot elongation in a wide variety of taxa that adopt the escape strategy. In early work, Ku et al. (1970) successfully reproduced the submergence-promoted elongation of rice coleoptiles with small additions of ethylene (10 ppm) and Musgrave et al. (1972) demonstrated a similar effect on stems of Calitriche platycarpa and Ranunculus sceleratus. By 1984, Osborne added seven more species to the list (Osborne, 1984) and 3 years later Ridge (1987) identified 21 species that elongate more quickly in response to submergence or ethylene. The list of species is now considerably longer and includes monocots, eudicots, an aquatic fern and a liverwort (Stange and Osborne, 1988). This diverse range of embryo-bearing species suggests a long lineage stretching back to early forms of plant life on the land (Anonymous, 2002). However, while it may be tempting to suggest that key traits for aquatic survival such as the ethylene-mediated underwater escape share the same ancient ancestor, this seems unlikely (Feild and Arens, 2005). Cook (1999) has deduced that colonization of aquatic environments from drier conditions has occurred many times (bryophytes 10–19 times, ferns seven times, angiosperms 205–245 times but never in gymnosperms) and over very large time spans. Although a thorough survey of the ethylene-mediated underwater escape has not been made, the available examples suggest it may well be one of a small number of obligatory developmental options that include aerenchyma formation (evident in 400 million-year-old fossils from the Devonian period) that seem to have re-appeared many times as land plants evolved to re-invade wet places. If true, this is a reflection of the dependability of ethylene as a signal for submergence that possesses the high physiological activity required to trigger fast elongation in small amounts.
The generality of the acceleration of elongation response to ethylene across taxa with highly divergent evolutionary origin thus marks it out as an adaptive phenomenon of broad ecological significance. This taxonomic tailoring to flooded niches is nicely illustrated by comparative studies of closely-related extant species of Rumex. Ethylene treatment to submergence-adapted Rumex palustris and R. maritimus show a positive growth response to the gas and to submergence (Voesenek et al., 2004). In contrast, R. acetosa and R. acetosella that inhabit nearby well-drained sandy areas fail to elongate fast underwater and in response to applied ethylene (Banga et al., 1996a, 1997). An intermediate response was observed in R. crispus (Voesenek and Blom, 1989). It seems clear that presence or absence of the ethylene-mediated escape delineates closely related species that can or cannot cope with the stress of submergence.
Although faster shoot elongation in response to ethylene is, taxonomically, a widely spread adaptation to submergence, it is not the exclusive preserve of aquatic and amphibious plants. Ethylene can promote stem, leaf or petiole elongation in some species not known for their submergence tolerance [e.g. Ecballium elaterium (Jackson et al., 1972), various grass species (Pooviah and Leopold, 1973), Stellaria longipes (Emery et al., 1994)] and hypocotyls of light-grown Arabidopsis thaliana (Smalle et al., 1997). It may also be needed for shade-stimulated shoot elongation (Pierik et al., 2004) in land plants. But these exceptions do little to detract from the widespread and seemingly functional link between the ethylene-mediated escape and success in frequently flooded areas. They also help place the intriguing positive and negative effects of ethylene on organ elongation in a wider conceptual framework involving a combination of competition, environmental influences and genetically determined features (Pierik et al., 2006).
HORMONAL INTERACTIONS
The primary task here is to establish the extent of their necessary involvement of other hormones in ethylene-mediated promotion of elongation by submerged plants. Coverage is still very incomplete because little information is available for cytokinins, brassinosteroids, polyamines, jasmonic acid/methyl jasmonate, salicylic acid and nitric oxide. The present analysis summarizes what is known of the physiological roles of abscisic acid (ABA), gibberellins (GAs) and auxin as they interact with ethylene to influence leaf or stem extension. Much of the work described has been with stems of deep-water rice and petioles of Rumex palustris.
Abscisic acid
ABA is well-known as an inhibitor of elongation (amongst other properties) and normal background amounts appear to stand between ethylene perception and subsequent growth promotion. This block is rapidly removed at the start of submergence, with endogenous ABA in internodes of deep-water rice declining by 75 % within 3 h (Hoffmann-Benning and Kende, 1992). Similar effects have been reported for leaves of rice seedlings (Ram et al., 2002), Scirpus mucronatus (Lee et al., 1996) and petioles of Rumex palustris where much ABA lost is within just 1 h (Benschop et al., 2005). In species such as Rumex acetosa, which does not respond to submergence with faster elongation rates, ABA remains stable during submergence. The fast drop in endogenous ABA therefore seems strongly associated with submergence-escaping plants. The loss of ABA in Rumex palustris and rice is ascribable to ethylene action since it can be reproduced readily by applying ethylene to non-submerged plants and prevented by pre-treating submerged plants with the ethylene action inhibitor 1-MCP. Furthermore, compensating for the loss of ABA by submerged plants with exogenous ABA during 48 h of submergence suppresses underwater elongation. On the other hand, removing the inherent ABA block ahead of time, by supplying the ABA-biosynthesis inhibitor fluoridone, shortens the time-lag for submergence-promoted extension to only 1 h (Benschop, 2004). In rice, at least, ABA may also stand in the way of sustained ethylene biosynthesis by repressing an ACC synthase gene (OS-ACS5) (Van der Straeten et al., 2001). These findings illustrate the ability of ABA to arrest the submergence escape. Its removal is therefore key. Surprisingly perhaps, ABA neither interferes with ethylene/submergence-induced expression of the RPEXP1 expansin gene in Rumex palustris nor with wall acidification, although it is assumed to inhibit wall extensibility. Also, ABA inhibition of growth cannot be explained in terms of desensitizing growing tissue to GA, although repression of GA biosynthesis may come into play after the first few hours underwater (Benschop et al., 2006) – see also subsection on gibberellins below. The basis of the ABA growth suppression thus remains uncertain but the existence, in Arabidopsis thaliana, of shared components of the signalling pathways for ABA and ethylene have led to the suggestion that relief of glucose-mediated repression of starch breakdown and mobilization is involved (Voesenek et al., 2003b).
The rapid decline in endogenous ABA underwater appears to be brought about by promotion of ABA breakdown and some suppression of its synthesis. In support of this, concentrations of phaseic acid (PA), the primary breakdown product of ABA, rise sharply within 1 h of exposing Rumex palustris to ethylene or submergence. Similarly, inhibiting the hydroxylase enzyme that generates PA from ABA by applying 8′acetylene-ABA strongly suppresses underwater growth (Benschop et al., 2005). A similar mechanism may operate in rice where 1 h of submergence increases the expression of one of a group of three genes (OsABA8ox1) demonstrably coding for the ABA hydroxylase protein. The ethylene action inhibitor 1-MCP interferes partially with this up-regulation (Saika et al., 2007). On the biosynthesis side, genes coding for steps in ABA production such as 9-cis-epoxycarotenoid dioxygenase (NCED) are down-regulated by submergence (Benschop et al., 2005; Saika et al., 2007), although the lack of efficacy of ethylene and poor correlation between ABA levels and recovery of NCED expression in de-submerged plants (Benschop et al., 2005) suggest that repression of biosynthesis is less important that promotion of ABA breakdown.
The balance of these findings strongly supports the view that the basal ABA content of air-grown plants is too high for the ethylene-driven underwater escape to proceed and that a fast-acting ethylene-inducible depression of ABA, predominantly brought about by accelerated ABA breakdown, is a prerequisite. It should be made clear that removal of ABA itself is not the instigator of faster elongation – it merely unlocks ethylene-promoted extension. This is apparent from the lack of underwater growth by Rumex palustris plants made ABA deficient with fluridone and, at the same time, rendered ethylene insensitive with 1-MCP (Benschop et al., 2005).
Gibberellins
Some authors consider gibberellin (GA) rather than ethylene to be the principal hormonal regulator of fast underwater elongation (Kende et al., 1998; Sauter, 2000). However, unlike the speed of the ethylene response, speed of response to GA application and withdrawal fails to match closely the kinetics of submergence-promoted growth (Musgrave et al., 1972) making it an unlikely front-line regulator mediating submergence events. Furthermore, observations that GA promotion of elongation in Callitriche platycarpa does not require ethylene while ethylene action normally requires GA (Musgrave et al., 1972) emphasizes the downstream role of GA. Recent work with Rumex palustris treated with a GA biosynthesis inhibitor also highlights the secondary part played by GA (Benschop et al., 2006) since it demonstrates an insensitivity to GA during the first few hours of ethylene-driven underwater extension. However, a crucial involvement of GA in sustaining growth at later times is revealed by this work, although it is important to note that GAs are not always required for submergence/ethylene-induced growth. For example, while buoyant tension and ethylene together induce fast underwater elongation in the fern Regnillidium diphyllum, there appears to be no need for GA (Musgrave and Walters, 1974). For plants other than Regnillidium diphyllum, GAs are perhaps best seen as an essential follow-up component of a complex chain reaction that is initiated by entrapped ethylene but requires GA for prolonged effectiveness. Experimental evidence to support this view is summarized next.
Stimulation of elongation by submergence or ethylene is either partially or totally arrested when inhibitors of GA biosynthesis are applied to Callitriche platycarpa (Musgrave et al., 1972), Ranunculus sceleratus (Samarakoon and Horton, 1983), deep-water rice stem segments (Raskin and Kende, 1984c) and Rumex palustris (Rijnders et al., 1997). In each case, the effect of inhibitors (e.g. AMO1618, tetcyclasis, uniconazole or paclobutrazol) is reversible by exogenous GA3, indicating a useful degree of specificity. GA action does not involve promoting ethylene production. The interaction is seen in terms of ethylene sensitizing the elongating internodes or petioles to GA. In turn, GA action appears responsible for some of the cellular events linked to sustained fast cell elongation and, where appropriate, cell division. In Rumex palustris, however, these GA-dependant events do not appear to include cell wall acidification or increases in expansin levels (Vreeburg et al., 2005) and, in deep-water rice, do not include stimulation of starch-degrading α-amylase activity (Smith et al., 1987) since this can take place in the presence of GA biosynthesis inhibitors. In deep-water rice internodes, consequences of GA action that may support submergence-stimulated growth include enhanced expression of (a) four members of a large family of expansin genes (Lee and Kende, 2001); (b) two cyclin-dependent protein kinases involved in regulating cell division (Fabian et al., 2000) which are central to cell cycle regulation; (c) a transcription factor gene (Os-GRF1) containing a nuclear localization signal (van der Knaap et al., 2000); (d) a receptor-like transmembrane protein kinase, Os-TMK (van der Knaap et al., 1999) and a heterotrimeric protein involved in DNA replication recombination or repair and transcription (van der Knaap et al., 1997). However, the actual involvement of submergence or ethylene in initiating these promotions has not been tested experimentally and is only implied from the positive effects of GA.
These findings raise the question of whether submergence actually promotes GA production, if ethylene action is responsible for this and if any faster synthesis is really needed for enhanced extension growth. Studies with deep-water rice internodes give a positive outcome to the extent that concentrations of GA1 (the active GA in rice) increases four times during the first 3 h of submergence and co-incidentally with a decline in growth-inhibiting ABA (Hoffmann-Benning and Kende, 1992). Although the efficacy of ethylene per se in simulating the effect of submergence on GA levels was not tested in this rice system, application of 5 ppm ethylene to Rumex palustris does replicate the submergence effect by raising levels of the active GA1 and related GAs 2·5-fold within 24 h (Rijnders et al., 1997). The effect is unattainable in closely related R. acetosa which does not elongate faster in response to ethylene, implying a functional role for the enhanced GA content. This view is supported in systems such as petiole extension in Arabidopsis thaliana where a raised synthetic rate for active GAs promotes elongation (Hisamatsu et al., 2005). Further support comes from detailed measurements of growth-active GA1 and transcript levels of RpGA3ox1 which codes for GA 3-oxidase enzyme converting GA20 to GA1. They show increases after 4 h submergence (and by implication after 4 h of raised ethylene), an effect that appears dependant on a prior decrease in endogenous ABA (Benschop et al., 2006). While this up-regulation of GA biosynthesis is 2 h too late to explain the early acceleration of elongation by submergence, intervention with paclobutrazol to suppress the GA rise, inhibits underwater growth strongly at later times. This indicates a need for GA synthesis to support fast growth in the longer term. Gibberellin action in promoting elongation in rice is thought to involve binding to a soluble receptor protein in the nucleus (Ueguchi-Tanaka et al., 2005). Current thinking is that the GA/receptor complex then interacts with growth-retarding DELLA proteins by stimulating their phosphorylation. This, in turn, results in an interaction with a ubiquitin ligase leading to ubiquitylation and a targeted transfer to proteasomes. Here the growth-retarding DELLA is degraded thereby permitting elongation (Alvey and Harberd, 2005) by permitting transcription of genes needed for GA-responses.
Auxin
Despite the famous dictum of ‘Ohne Wuchsstoffe, keine Wachstum’ (‘No auxin, no growth’) that emerged from the work of F. W Went on grass coleoptiles in the 1920s, limited inhibitor-based evidence indicates that auxin may not be needed for ethylene-promoted elongation in rice coleoptiles (Katsura and Suge, 1979). Also, there is, seemingly, no published evidence that auxin is needed for the ethylene response of deep-water rice internodes. However, work with various eudicot species, and with Regnellidium diphyllum, has demonstrated that auxin is needed for the submergence/ethylene response. Thus, fronds of Regnellidium diphyllum soon loose their ability to respond to ethylene once excised from the parent plant, but their fast elongation can be retained by adding indole acetic acid (IAA) (Walters and Osborne, 1979). A similar effect is seen in Nymphoides peltata (Malone and Ridge, 1983; Ridge and Osborne, 1989), where petioles separated for >2 h from their presumed source of endogenous auxin (the lamina) only acidify their walls, become more extensible and elongate in response to ethylene when exogenous auxin is also given. A similar dependence is apparent in Ranunculus sceleratus where lamina removal quickly abolishes ethylene stimulated elongation as does the application of inhibitors of polar auxin transport (Horton and Samarakoon, 1982; Rijnders et al., 1996) unless overridden by exogenous auxin. As with GAs, auxin can stimulate elongation even when ethylene action is blocked, suggesting a role downstream from ethylene, although early inhibitor studies suggest independent modes of action are also possible (Cookson and Osborne, 1978, 1979). In Rumex palustris, submergence has little effect on endogenous IAA after the first day underwater when whole petioles were analysed (Cox et al., 2004) but when outer layers of the petiole (where its extension rate is thought to be controlled) were assayed, a rise of about 125–160 % was seen after only 4 h underwater (Cox et al., 2006). The effect was abolished by removal of the leaf blade which also interfered with the elongation response in the short term. However, over the longer term (48 h), there seems to be little evidence of auxin dependency by ethylene-promoted petiole extension in Rumex palustris, although such a dependency was more apparent in Ranunculus sceleratus (Rijnders et al., 1996). It may be relevant that expression of a submergence/ethylene-inducible expansin gene (RpEXPA1) can be increased by exogenous auxin (Vreeburg et al., 2005). Thus, it seems that some but not all the evidence supports the view that auxin is needed in submergence/ethylene-induced growth especially during the first few hours of submergence.
BEFORE AND AFTER ANCILLARY PROCESSES
Hyponastic growth in Rumex palustris
When first submerged, the elongating leaves of young Rumex palustris plants do not point upwards towards the water surface. Instead, they are almost horizontal and too stiff to bend upwards by much under buoyancy forces. In this near-horizontal position, ethylene-induced elongation is unlikely to lead to rapid re-surfacing. But, futile sideways elongation is avoided as a result of a rapid re-orientation of the leaves to a much more upright stance (Banga et al., 1997; Cox et al., 2003). This upward underwater re-adjustment of leaf angle away from the horizontal (hyponasty) is the outcome of a rapid and highly localized growth stimulation in a short length of tissue, about 20 cells long, positioned strategically on the under side of the petioles of young leaves at their base (Cox et al., 2004). This acts as a lever. The hyponasty begins well before the entire petiole starts to extend. Clever tilting experiments have shown that hyponastic repositioning of the entire leaf is a prerequisite for the main elongation response. This main elongation starts about 40 min after hyponasty raises the angle above the horizontal to 40–50° (Cox et al., 2003). For a time, elongation and further hyponasty proceed in tandem to lengthen the petiole in an evermore upright direction. These findings suggest that the overall elongation response along the petiole during the first day of submergence is subject to a gravity block that is only relieved once the angle exceeds a threshold value of approx. 45°. While the mechanism of this gravity mediation remains unknown, much has been learned about the control of hyponasty itself (Cox et al., 2004). It encapsulates much of what was previously discovered for the overall petiole elongation response based on a similar range of experiments incorporating elements of ‘Duplication’; ‘Correlation’; ‘Deletion and re-instatement’; ‘Relevance to lower levels of organization’. They show the process is largely instigated by ethylene (although a small part of the response may have some other cause), to require a decline in endogenous ABA to unblock the ethylene response, and to incorporate an element of GA dependency, but with little evidence for the involvement of expansins. Auxin plays an important role. Removing its presumed source by excising the leaf lamina, lowers IAA concentrations in the petiole and interferes in the hyponasty. At the petiole base, IAA becomes internally redistributed towards hyponasting cells. This redistribution is probably brought about by gravity-stimulated efflux carriers because application of naphthylphtalamic acid, an inhibitor of auxin efflux, suppresses both the redistribution and the hyponastic growth (Cox et al., 2004). Taken together, these experiments insert a gravity-operated gate between ethylene perception and the main elongation response by the whole petiole. Perhaps what distinguishes the small group of hyponasty cells from the rest of the petiole is an absence of the gravity gate.
Post-emergent stimulation of elongation
Casual inspection of many riverside or lakeside communities reveals a dominance by amphibious species that elongate strongly even after they emerge above the water level (Fig. 2). The extent of emergence has been linked positively to greater subsequent seed production (Van der Sman et al., 1991). These plants are conveniently termed emergent species and contrast with floating types such as Callitriche platycarpa and Regnellidium diphyllum that typically inhabit deeper water. Little is known about the regulation of emergent growth but preliminary work with Rumex indicates a role for ethylene (Voesenek et al., 2003a) even though the gas escapes rapidly once water no longer covers all the shoot (Voesenek et al., 1993). The feature compensating for this loss of entrapped gas appears to be a concomitantly increased rate of ethylene biosynthesis in the shoot that follows submergence or period of oxygen shortage (Van der Sman et al., 1991). Other species also show faster ethylene production after submergence or a period of O2 shortage (Khan et al., 1987; Zhou et al., 2001; Fukao and Bailey-Serres, 2004). In contrast to Rumex palustris, species of Rumex that do not give the submergence escape response (e.g. Rumex acetosa and Rumex thyrsiflorus) are notably slow ethylene producers after de-submergence. In R. palustris, fast post-submergence ethylene production is associated with a prompt rise in ACC content of the shoot (Voesenek et al., 2003a). Some of this ACC is delivered from the root system in the re-started transpiration stream – delivery rates being 3–4 times those of never-submerged control plants. Some of the extra ACC is also generated within the shoot as a consequence of an approx. 3-fold rise in the activity of ACC synthase activity deduced from the increased expression of an ACC synthase gene (RP-ACS1). The instigation of these latter changes in freshly de-submerged plants has been ascribed to the combined effect of O2 enrichment and partial loss of entrapped ethylene. This speculation is based on the observation that up-regulation of RP-ACS1 can be suppressed if plants are de-submerged into a gas mixture of 10 ppm ethylene and 5 % O2 (v/v) rather than into air (Voesenek et al., 2003a).
CONCLUSIONS
Submergence, especially in slow-moving or stagnant water, is a threat to all but the very simplest forms of plant life. Much is now known about the manner in which damage is inflicted, although some uncertainties remain. Escape from submergence, achieved by a speeding-up of shoot elongation, is one of the outstanding characteristics of most aquatic and amphibious plants. The case for invoking water-entrapped ethylene as prime instigator of the submergence escape has strengthened steadily over 35 years. The large number of embryo-bearing taxa to which this applies is impressively large and it is difficult to see the position of ethylene being usurped by some as yet unidentified submergence signal. This is the case even though, in some species, other signals, notably partial lack of O2 or increases in CO2 can serve a similar purpose. The presence or absence of ethylene-mediated growth promotion amongst species that occur naturally in flood-prone areas or in drier parts gives strong support to the view that the ethylene effect is of ecological significance and a seeming benefit to plants commonly subjected to submergence stress. However, although it must be admitted that direct experimental demonstration of its survival value is less than comprehensive, correlative work is supportive. Furthermore, the notion is bolstered by the well-known ability of deep-water and floating rice to survive and yield grain in flood water several metres deep. This is achieved by virtue of a strong ethylene-promoted stem elongation that can be sustained over long periods. Other rice types with less ability to do this fail in deep-water areas. The survival value of submergence escape and its ability to secure seed production is thus illustrated by agricultural practice and holds considerable economic value.
Much has been learned of the cellular events that underlie the ethylene-promoted elongation, especially by detailed studies of internodes of deep-water rice (Oryza sativa), petioles of submergence-tolerant marsh dock (Rumex palustris) and more recently the leaves of small rice seedlings. Thanks to such work, it is increasingly possible to identify some of the many steps that may lie between initial ethylene perception and the faster cell elongation (and sometimes cell division) that constitute the actual growth response. However, putting this information together in one comprehensive model is problematical not least because of difficulties in marrying information from the three systems. Furthermore, maintaining the faster elongation beyond the first few hours may well involve factors not necessarily needed for the initial response (Benschop et al., 2006). The model given in Fig. 4 is thus necessarily a superficial summary but may provide a useful context for assimilating future research findings.
Fig. 4.
Model of the promotion of underwater shoot elongation by ethylene. C2H4 = ethylene; ABA = abscisic acid; GA = gibberellin; RP = Rumex palustris.
Four areas appear to hold particular promise for future work. The first springs from studies of the vexed question of the mechanisms which explain species distribution and co-existence in species-rich habitats. These include a sophisticated mathematical analysis of spatial distribution of about 80 species in two species-rich water meadows in the UK. This revealed a hitherto unrecognized differential sensitivity of species to small differences in the extent of flooding (Silvertown et al., 1999). The work has made a major contribution to understanding how subtle adaptation to microniches defined by their proneness to flooding and drought explains the non-random distribution of species across such meadow terrain. The physiological basis for these finely tuned adaptations to small differences in hydrology is not known but ethylene action is a likely candidate deserving further exploration. The second involves the use of Arabidopsis thaliana (Pierik et al., 2005) to overcome the lack of developmental mutants and practical difficulties in genetic transformation or regeneration that characterize most aquatic and amphibious plants. Success here will depend on the extent to which growth responses to submergence by some lines of Arabidopsis thaliana that resemble those of wetland plants also hold-up at the cellular and molecular level. The third area of promise is molecular study of submergence-tolerant FR13A and related rice lines that fail to elongate their leaves rapidly when underwater. These lines possess an additional mutated gene in the so-called Sub1 locus. In FR13A, this gene is strongly expressed on submergence or ethylene treatment and this expression suppresses a nearby ethylene-responsive paralogous gene. The Sub1 genes are located on chromosome 9 and contain ethylene response factor domains (Fukao et al., 2006; Xu et al., 2006). Clearly, finding which genes and associated physiological and biochemical steps are suppressed by the action of the dominant mutated gene of FR13A should help identify steps needed for the normal positive underwater growth response to ethylene. The fourth area of promise, not taken directly from research on wetland plants, involves the post-transcriptional destabilization of proteins such as DELLA, and other hormone signal transduction proteins, by ubiquitylation and subsequent proteolysis in 26S proteasomes. This work has the potential to integrate the complexities of the signal transduction pathways for ethylene (Potuschak et al., 2003) and the other plant hormones it interacts with (Chen et al., 2005; Djakovic-Petrovic et al., 2007). It seems likely that ethylene, ABA, GA and auxin each operates through activation of the destruction of negatively acting signal transduction proteins (Alvey and Harberd, 2005; Achard et al., 2006; Dreher and Callis, 2007). This commonality may help clarify the interplay of these hormones in the regulation of underwater growth and help understand more of how the ethylene response is amplified after it deactivates its receptor protein at the endoplasmic reticulum.
ACKNOWLEDGEMENTS
I thank Julia Bailey-Serres (University of California, Riverside, USA), Irene Ridge (Open University, Milton Keynes, UK), Rens Voesenek (University of Utrecht, The Netherlands) and William Armstrong (University of Hull, UK) for helpful comments on this article.
LITERATURE CITED
- Achard P, Cheng H, De Grauwe L, Decat J, Schoutteten H, Moritz T, et al. Integration of plant responses to environmentally activated phytohormonal signals. Science. 2006;311:91–94. doi: 10.1126/science.1118642. [DOI] [PubMed] [Google Scholar]
- Alvey L, Harberd NP. DELLA proteins: integrators of multiple plant growth regulatory inputs? Physiologia Plantarum. 2005;123:153–160. [Google Scholar]
- Anonymous. Tree of Life Web Project. 2002. (http://tolweb.org/tree/phylogeny.html. )
- Anonymous. The Ramsar Convention on Wetlands. 2007. www.ramsar.org .
- Arber A. Water plants: a study of aquatic angiosperms. Cambridge: Cambridge University Press; 1920. [Google Scholar]
- Armstrong W. Aeration in higher plants. Advances in Botanical Research. 1979;7:601–614. [Google Scholar]
- Armstrong J, Jones RE, Armstrong W. Rhizome phyllosphere oxygenation in Phragmites and other species in relation to redox potential, convective gas flow, submergence and aeration pathways. New Phytologist. 2006;172:719–731. doi: 10.1111/j.1469-8137.2006.01878.x. [DOI] [PubMed] [Google Scholar]
- Armstrong W, Drew MC. Root growth and metabolism under oxygen deficiency. In: Waisel Y, Eshel A, Kafkafi U,, editors. Plant roots: the hidden half. New York, NY: Marcel Dekker; 2002. pp. 729–761. [Google Scholar]
- Armstrong W, Gaynard TJ. The critical oxygen pressures for respiration in intact plants. Physiologia Plantarum. 1976;37:200–206. doi: 10.1111/j.1399-3054.1976.tb03958.x. [DOI] [PubMed] [Google Scholar]
- Armstrong W, Webb T. A critical oxygen pressure for root extension in rice. Journal of Experimental Botany. 1985;36:1573–1582. [Google Scholar]
- Banga M, Blom C, Voesenek L. Flood-induced leaf elongation in Rumex species: effects of water depth and water movements. New Phytologist. 1995;131:191–198. [Google Scholar]
- Banga M, Blom CWPM, Voesenek LACJ. Sensitivity to ethylene: the key factor in submergence-induced shoot elongation of Rumex. Plant, Cell and Environment. 1996;a 19:1423–1430. [Google Scholar]
- Banga M, Slaa EJ, Blom CWPM, Voesenek LACJ. Ethylene biosynthesis and accumulation under drained and submerged conditions: a comparative study of two Rumex species. Plant Physiology. 1996;b 112:229–237. doi: 10.1104/pp.112.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banga M, Bogemann GM, Blom CWPM, Voesenek LACJ. Flooding resistance of Rumex species strongly depends on their response to ethylene: rapid shoot elongation or foliar senescence. Physiologia Plantarum. 1997;99:415–422. [Google Scholar]
- Barclay AM, Crawford RMM. Plant growth and survival under strict anaerobiosis. Journal of Experimental Botany. 1982;33:541–549. [Google Scholar]
- Beckett PM, Armstrong W, Justin SHFW, Armstrong J. On the relative importance of convective and diffusive gas flows in plant aeration. New Phytologist. 1988;110:463–468. [Google Scholar]
- Benschop J. The role of abscisic acid in ethylene-induced elongation. Utrecht: Faculty of Biology, University of Utrecht; 2004. [Google Scholar]
- Benschop JJ, Jackson MB, Guhl K, Vreeburg RAM, Croker SJ, Peeters AJM, et al. Contrasting interactions between ethylene and abscisic acid in Rumex species differing in submergence tolerance. The Plant Journal. 2005;44:756–768. doi: 10.1111/j.1365-313X.2005.02563.x. [DOI] [PubMed] [Google Scholar]
- Benschop JJ, Bou J, Peeters AJM, Wagemaker N, Guhl K, Ward D, et al. Long-term submergence-induced elongation in Rumex palustris requires abscisic acid-dependent biosynthesis of gibberellin. Plant Physiology. 2006;141:1644–1652. doi: 10.1104/pp.106.082636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer EM. Effect of silver ion, carbon dioxide, and oxygen on ethylene action and metabolism. Plant Physiology. 1979;63:169–173. doi: 10.1104/pp.63.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleecker AB, Rosejohn S, Kende H. An evaluation of 2,5-norbornadiene as a reversible inhibitor of ethylene action in deep-water rice. Plant Physiology. 1987;84:395–398. doi: 10.1104/pp.84.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boamfa EI, Ram PC, Jackson MB, Reuss J, Harren FJM. Dynamic aspects of alcoholic fermentation of rice seedlings in response to anaerobiosis and to complete submergence: relationship to submergence tolerance. Annals of Botany. 2003;91:279–290. doi: 10.1093/aob/mcf205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boamfa EI, Veres AH, Ram PC, Jackson MB, Reuss J, Harren FJM. Kinetics of ethanol and acetaldehyde release suggest a role for acetaldehyde production in tolerance of rice seedlings to micro-aerobic conditions. Annals of Botany. 2005;96:727–736. doi: 10.1093/aob/mci224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowes G. Aquatic plant photosynthesis: strategies that enhance carbon gain. In: Crawford RMM,, editor. Plant life in aquatic and amphibious habitats. Oxford: Blackwell Scientific Publications; 1987. pp. 79–98. [Google Scholar]
- Brailsford RW, Voesenek LACJ, Blom CWPM, Smith AR, Hall MA, Jackson MB. Enhanced ethylene production by primary roots of Zea mays L. in response to sub-ambient partial pressures of oxygen. Plant Cell and Environment. 1993;16:1071–1080. [Google Scholar]
- Burg SP, Burg EA. Gas exchange in fruits. Physiologia Plantarum. 1965;18:870–884. [Google Scholar]
- Burg SP, Burg EA. Interaction between auxin and ethylene and its role in plant growth. Proceedings of the National Academy of Sciences of the USA. 1966;55:262–269. doi: 10.1073/pnas.55.2.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catling D. Rice in deep water. London/Manila: Macmillan Press/International Rice Research Institute; 1992. [Google Scholar]
- Chen Y-F, Etheridge N, Schaller GE. Ethylene signal transduction. Annals of Botany. 2005;95:901–915. doi: 10.1093/aob/mci100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho HT, Kende H. Expression of expansin genes is correlated with growth in deepwater rice. The Plant Cell. 1997;9:1661–1671. doi: 10.1105/tpc.9.9.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H-T, Kende H. Tissue localization of expansins in deepwater rice. The Plant Journal. 1998;15:805–812. doi: 10.1046/j.1365-313x.1998.00258.x. [DOI] [PubMed] [Google Scholar]
- Cho Y-H, Yoo S-D. ETHYLENE RESPONSE 1 histidine kinase activity of Arabidopsis promotes plant growth. Plant Physiology. 2007;143:612–616. doi: 10.1104/pp.106.091504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen E, Kende H. In vivo 1-aminocyclopropane-1-carboxylate synthase activity in internodes of deep-water rice: enhancement by submergence and low oxygen levels. Plant Physiology. 1987;84:282–286. doi: 10.1104/pp.84.2.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook CDK. The number and kinds of embryo-bearing plants which have become aquatic: a survey. Perspectives in Plant Ecology, Evolution and Systematics. 1999;2:79–102. [Google Scholar]
- Cookson C, Osborne DJ. The stimulation of cell extension by ethylene and auxin in aquatic plants. Planta. 1978;144:39–47. doi: 10.1007/BF00385005. [DOI] [PubMed] [Google Scholar]
- Cookson C, Osborne DJ. Effect of ethylene and auxin on cell-wall extensibility of the semi-aquatic fern, Regnellidium-diphyllum. Planta. 1979;146:303–307. doi: 10.1007/BF00387802. [DOI] [PubMed] [Google Scholar]
- Costes C, Vartapetian BB. Plant grown in a vacuum: ultrastructure and functions of mitochondria. Plant Science Letters. 1978;11:115–119. [Google Scholar]
- Cox MCH, Millenaar FF, de Jong van Berkel YEM, Peeters AJM, Voesenek LACJ. Plant movement: submergence-induced petiole elongation in Rumex palustris depends on hyponastic growth. Plant Physiology. 2003;132:282–291. doi: 10.1104/pp.102.014548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox MCH, Benschop JJ, Vreeburg RAM, Wagemaker CAM, Moritz T, Peeters AJM, et al. The roles of ethylene, auxin, abscisic acid, and gibberellin in the hyponastic growth of submerged Rumex palustris petioles. Plant Physiology. 2004;136:2948–2960. doi: 10.1104/pp.104.049197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox MCH, Peeters AJM, Voesenek L. The stimulating effects of ethylene and auxin on petiole elongation and on hyponastic curvature are independent processes in submerged Rumex palustris. Plant, Cell and Environment. 2006;29:282–290. doi: 10.1111/j.1365-3040.2005.01420.x. [DOI] [PubMed] [Google Scholar]
- Craker LE, Cookson C, Osborne DJ. Control of proton extrusion and cell elongation by ethylene and auxin in water plant Ranunculus sceleratus. Plant Science Letters. 1978;12:379–385. [Google Scholar]
- Crocker WP, Zimmerman PW, Hitchcock AE. Ethylene-induced epinasty of leaves and the relation of gravity to it. Contributions of the Boyce Thompson Institute for Plant Research. 1932;4:177–218. [Google Scholar]
- Dal Cin V, Rizzini FM, Botton A, Tonutti P. The ethylene biosynthetic and signal transduction pathways are differently affected by 1-MCP in apple and peach fruit. Postharvest Biology and Technology. 2006;42:125–133. [Google Scholar]
- Das KK, Sarkar RK, Ismail AM. Elongation ability and non-structural carbohydrate levels in relation to submergence tolerance in rice. Plant Science. 2005;168:131–136. [Google Scholar]
- Denny FE. Effect of ethylene upon respiration of lemons. Botanical Gazette. 1924;77:322–329. [Google Scholar]
- Djakovic-Petrovic T, de Wit M, Voesenek LACJ, Pierik R. DELLA protein function in growth responses to canopy signals. The Plant Journal. 2007;51:117–126. doi: 10.1111/j.1365-313X.2007.03122.x. [DOI] [PubMed] [Google Scholar]
- Dreher K, Callis J. Ubiquitin, hormones and biotic stress in plants. Annals of Botany. 2007;99:787–822. doi: 10.1093/aob/mcl255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery RJN, Reid DM, Chinnappa CC. Phenotypic plasticity of stem elongation in 2 ecotypes of Stellaria longipes: the role of ethylene and response to wind. Plant, Cell and Environment. 1994;17:691–700. [Google Scholar]
- Emes MJ, Wilkins CP, Smith PA, Kupkanchanakul K, Hawker K, Charlton WA, et al. 1987 International Deepwater Rice Workshop. Manila: International Rice Research Institute; 1988. Starch utilization by deepwater rice during submergence; pp. 319–326. [Google Scholar]
- Fabian T, Lorbiecke R, Umeda M, Sauter M. The cell cycle genes cycA1;1 and cdc2Os-3 are coordinately regulated by gibberellin in planta. Planta. 2000;211:376–383. doi: 10.1007/s004250000295. [DOI] [PubMed] [Google Scholar]
- Feild TS, Arens NC. Form, function and environments of the early angiosperms: merging extant phylogeny and ecophysiology with fossils. New Phytologist. 2005;166:383–408. doi: 10.1111/j.1469-8137.2005.01333.x. [DOI] [PubMed] [Google Scholar]
- Fukao T, Bailey-Serres J. Plant responses to hypoxia – is survival a balancing act? Trends in Plant Science. 2004;9:449–456. doi: 10.1016/j.tplants.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Fukao T, Xu K, Ronald PC, Bailey-Serres J. A variable cluster of ethylene response factor-like genes regulates metabolic and developmental acclimation responses to submergence in rice. The Plant Cell. 2006;18:2021–2034. doi: 10.1105/tpc.106.043000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funke GL, Bartels PM. Observations on the growth of water plants. Biologisch Jaarboek. 1937;4:316–344. [Google Scholar]
- Geigenberger P. Response of plant metabolism to too little oxygen. Current Opinion in Plant Biology. 2003;6:247–256. doi: 10.1016/s1369-5266(03)00038-4. [DOI] [PubMed] [Google Scholar]
- Gharbi I, Ricard B, Rolin D, Maucourt M, Andrieu M-H, Bizid E, et al. Effect of hexokinase activity on tomato root metabolism during prolonged hypoxia. Plant, Cell and Environment. 2007;30:508–517. doi: 10.1111/j.1365-3040.2007.01640.x. [DOI] [PubMed] [Google Scholar]
- Greenway H, Gibbs J. Mechanisms of anoxia tolerance in plants. II. Energy requirements for maintenance and energy distribution to essential processes. Functional Plant Biology. 2003;30:999–1036. doi: 10.1071/PP98096. [DOI] [PubMed] [Google Scholar]
- Groeneveld HW, Voesenek LACJ. Submergence-induced petiole elongation in Rumex palustris is controlled by developmental stage and storage compounds. Plant and Soil. 2003;253:115–123. [Google Scholar]
- He JB, Bögemann GM, van de Steeg MH, Rijnders JGHM, Voesenek LACJ, Blom CWPM. Survival tactics of Ranunculus species in river floodplains. Oecologia. 1999;118:1–8. doi: 10.1007/s004420050696. [DOI] [PubMed] [Google Scholar]
- Hisamatsu T, King RW, Helliwell CA, Koshioka M. The involvement of gibberellin 20-oxidase genes in phytochrome-regulated petiole elongation of Arabidopsis. Plant Physiology. 2005;138:1106–1116. doi: 10.1104/pp.104.059055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann-Benning S, Kende H. On the role of abscisic acid and gibberellin in the regulation of growth in rice. Plant Physiology. 1992;99:1156–1161. doi: 10.1104/pp.99.3.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton RF. The effect of ethylene and other regulators on coleoptile growth of rice under anoxia. Plant Science. 1991;79:57–62. [Google Scholar]
- Horton RF. Submergence-promoted growth of petioles of Ranunculus pygmaeus Wahl. Aquatic Botany. 1992;44:23–30. [Google Scholar]
- Horton RF. Peroxidase, ethylene, and submergence-promoted growth of petioles of Ranunculus sceleratus L. Journal of Plant Physiology. 1993;141:690–693. [Google Scholar]
- Horton RF, Samarakoon AB. Petiole growth in the celery-leaved crowfoot (Ranunculus sceleratus L.): effects of auxin-transport inhibitors. Aquatic Botany. 1982;13:97–104. [Google Scholar]
- Hossain M. Fragile lives in fragile ecosystems. Los Banos, Philippines: International Rice Research Institute; 1995. Sustaining food security for fragile environments in Asia: achievements, challenges, and implications for rice research; pp. 3–23. [Google Scholar]
- Hua J, Sakai H, Nourizadeh S, Chen QG, Bleecker AB, Ecker JR, et al. EIN4 and ERS2 are members of the putative ethylene receptor gene family in Arabidopsis. The Plant Cell. 1998;10:1321–1332. doi: 10.1105/tpc.10.8.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insalud N, Bell RW, Colmer TD, Rerkasem B. Morphological and physiological responses of rice (Oryza sativa) to limited phosphorus supply in aerated and stagnant solution culture. Annals of Botany. 2006;98:995–1004. doi: 10.1093/aob/mcl194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizawa K, Esashi Y. Osmoregulation in rice coleoptile elongation as promoted by cooperation between IAA and ethylene. Plant and Cell Physiology. 1984;25:495–504. [Google Scholar]
- Jackson MB. Ethylene as a growth promoting hormone under flooded conditions. In: Wareing PF, editor. Plant growth substances. London: Academic Press; 1982. pp. 291–301. [Google Scholar]
- Jackson MB. Ethylene and responses of plants to soil waterlogging and submergence. Annual Review of Plant Physiology. 1985;36:145–174. [Google Scholar]
- Jackson MB. A structured evaluation of the involvement of ethylene and abscisic acid in plant responses to aeration stress. In: Hoad GV, Lenton JR, Jackson MB, Atkin RK, editors. Hormone action in plant development – a critical appraisal. London: Butterworths; 1987. pp. 189–199. [Google Scholar]
- Jackson MB. The impact of flooding stress on plants and crops. 2004. http://www.plantstress.com/Articles/index.asp . (a website addressing plant environmental stress issues in agriculture, plant physiology, breeding, genetics and biotechnology)
- Jackson MB. Aeration stress in plant tissue cultures. In: Hvoslef-Eide AK, Preil W, editors. Liquid culture systems for in vitro plant propagation. Dordrecht: Kluwer Academic; 2005. pp. 459–473. [Google Scholar]
- Jackson MB. Plant survival in wet environments: resilience and escape mediated by shoot systems. In: Bobbink R, Beltman B, Verhoeven JTA, Whigham DE, editors. Wetlands: functioning, biodiversity, conservation, and restoration. Ecological Studies. Vol. 191. Berlin: Springer-Verlag; 2006. pp. 15–36. [Google Scholar]
- Jackson MB, Armstrong W. Formation of aerenchyma and the process of plant ventilation in relation to soil flooding and submergence. Plant Biology. 1999;1:274–287. [Google Scholar]
- Jackson MB, Campbell DJ. Waterlogging and petiole epinasty in tomato: the role of ethylene and low oxygen. New Phytologist. 1976;76:21–29. [Google Scholar]
- Jackson MB, Ram PC. Physiological and molecular basis of susceptibility and tolerance of rice plants to complete submergence. Annals of Botany. 2003;91:227–241. doi: 10.1093/aob/mcf242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson MB, Morrow IB, Osborne DJ. Abscission and dehiscence in the squirting cucumber, Ecballium elaterium: regulation by ethylene. Canadian Journal of Botany. 1972;50:1465–1471. [Google Scholar]
- Jackson MB, Dobson CM, Herman B, Merryweather A. Modification of 3,5-diiodohydroxybenzoic acid (DIHB) activity and stimulation of ethylene production by small concentrations of ethylene in the root environment. Plant Growth Regulation. 1984;2:251–262. [Google Scholar]
- Jackson MB, Waters I, Setter TL, Greenway H. Injury to rice plants caused by complete submergence; a contribution by ethylene. Journal of Experimental Botany. 1987;38:1826–1838. [Google Scholar]
- Jacobs WP. What substance normally controls a given biological process? 1. Formulation of some rules. Developmental Biology. 1959;1:527–533. [Google Scholar]
- Jones HG. Heterophylly in some species of Callitriche, with especial reference to Callitriche intermedia. Annals of Botany. 1955;19:225–245. [Google Scholar]
- Justin SHFW, Armstrong W. Anatomical characteristics of roots and plant response to soil flooding. New Phytologist. 1987;105:465–495. [Google Scholar]
- Katsura N, Suge H. Does ethylene induce elongation of the rice coleoptile through auxin? Plant and Cell Physiology. 1979;20:1147–1150. [Google Scholar]
- Kende H, van der Knaap E, Cho H-T. Deepwater rice: a model plant to study stem elongation. Plant Physiology. 1998;118:1105–1110. doi: 10.1104/pp.118.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan AA, Thakur R, Akbar M, Hillerislambers D, Seshu DV. Relationship of ethylene production to elongation in deep-water rice. Crop Science. 1987;27:1188–1196. [Google Scholar]
- Kieber JJ, Rothenberg M, Roman G, Feldmann KA, Ecker JR. CTR1, a negative regulator of the ethylene response pathway in arabidopsis, encodes a member of the RAF family of protein-kinases. Cell. 1993;72:427–441. doi: 10.1016/0092-8674(93)90119-b. [DOI] [PubMed] [Google Scholar]
- Kim JH, Cho HT, Kende H. alpha-Expansins in the semiaquatic ferns Marsilea quadrifolia and Regnellidium diphyllum: evolutionary aspects and physiological role in rachis elongation. Planta. 2000;212:85–92. doi: 10.1007/s004250000367. [DOI] [PubMed] [Google Scholar]
- van der Knaap E, Jagoueix S, Kende H. Expression of an ortholog of replication protein A1 (RPA1) is induced by gibberellin in deepwater rice. Proceedings of the National Academy of Sciences of the USA. 1997;94:9979–9983. doi: 10.1073/pnas.94.18.9979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Knaap E, Song WY, Ruan DL, Sauter M, Ronald PC, Kende H. Expression of a gibberellin-induced leucine-rich repeat receptor-like protein kinase in deepwater rice and its interaction with kinase-associated protein phosphatase. Plant Physiology. 1999;120:559–569. doi: 10.1104/pp.120.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Knaap E, Kim JH, Kende H. A novel gibberellin-induced gene from rice and its potential regulatory role in stem growth. Plant Physiology. 2000;122:695–704. doi: 10.1104/pp.122.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight LE, Crocker W. Toxicity of smoke. Botanical Gazette. 1913;55:337–371. [Google Scholar]
- Kordan HA. Patterns of shoot and root growth in rice seedlings germinating under water. Journal of Applied Ecology. 1974;11:685–690. [Google Scholar]
- Kordan HA. Rice seed germination in an in vacuo anaerobic environment. Annals of Botany. 1976;40:385–386. [Google Scholar]
- Ku HS, Suge H, Rappaport L, Pratt HK. Stimulation of rice coleoptile growth by ethylene. Planta. 1970;90:333–339. doi: 10.1007/BF00386385. [DOI] [PubMed] [Google Scholar]
- Kutschera U, Kende H. The biophysical basis of elongation growth in internodes of deepwater rice. Plant Physiology. 1988;88:361–366. doi: 10.1104/pp.88.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laan P, Tosserams M, Blom CWPM, Veen BW. Internal oxygen transport in Rumex species and its significance for respiration under hypoxic conditions. Plant and Soil. 1989;122:39–46. [Google Scholar]
- Lee T-M, Shieh Y-J, Chou C-H. Abscisic acid inhibits shoot elongation of Scirpus mucronatus. Physiologia Plantarum. 1996;97:1–4. [Google Scholar]
- Lee Y, Kende H. Expression of beta-expansins is correlated with internodal elongation in deepwater rice. Plant Physiology. 2001;127:645–654. [PMC free article] [PubMed] [Google Scholar]
- Lorbiecke R, Sauter M. Adventitious root growth and cell-cycle induction in deepwater rice. Plant Physiology. 1999;119:21–30. doi: 10.1104/pp.119.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn DE, Waldren S. Survival of Ranunculus repens L. (creeping buttercup) in an amphibious habitat. Annals of Botany. 2003;91:75–84. doi: 10.1093/aob/mcg011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lytle CM, Lytle FW, Smith BN. Use of XAS to determine the chemical speciation of bioaccumulated manganese in Potamogeton pectinatus. Journal of Environmental Quality. 1996;25:311–316. [Google Scholar]
- McAuliff C. Solubility in water of paraffin cycloparaffin olefin acetylene cycloolefin and aromatic hydrocarbons. Journal of Physical Chemistry. 1966;70:1267–1268. [Google Scholar]
- McComb AJ. The control of elongation in Callitriche shoots by environment and gibberellic acid. Annals of Botany. 1965;29:445–458. [Google Scholar]
- McQueen-Mason SJ, Rochange F. Expansins in plant growth and development: an update on an emerging topic. Plant Biology. 1999;1:19–25. [Google Scholar]
- MacAdam JW, Nelson CJ, Sharp RE. Peroxidase activity in the leaf elongation zone of tall fescue. I. Spatial distribution of ionically bound peroxidase activity in genotypes differing in length of the elongation zone. Plant Physiology. 1992;99:872–878. doi: 10.1104/pp.99.3.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackill DJ. Progress in rainfed lowland rice. Los Baños, Philippines: International Rice Research Institute; 1986. Varietal improvement for rainfed lowland rice in South and Southeast Asia: results of a survey; pp. 115–144. [Google Scholar]
- Madsen TV. Inorganic carbon assimilation and growth of aquatic macrophytes. In: Jackson MB, Black CR, editors. Interacting stresses on plants in a changing climate. Berlin: Springer-Verlag; 1993. pp. 267–285. [Google Scholar]
- Malone M, Ridge I. Ethylene-induced growth and proton excretion in the aquatic plant Nymphoides peltata. Planta. 1983;157:71–73. doi: 10.1007/BF00394542. [DOI] [PubMed] [Google Scholar]
- Maltby E. Wetlands – their status and role in the biosphere. In: Jackson MB, Davies DD, Lambers H, editors. Plant life under oxygen deprivation. Ecology, physiology and biochemistry. The Hague: SPB Academic Publishing; 1991. pp. 3–21. [Google Scholar]
- Meigh DF. The nature of olefines produced by apples. Nature. 1959;184:1072–1073. [Google Scholar]
- Mekhedov SL, Kende H. Submergence enhances expression of a gene encoding 1-aminocyclopropane-1-carboxylate oxidase in deepwater rice. Plant and Cell Physiology. 1996;37:531–537. doi: 10.1093/oxfordjournals.pcp.a028976. [DOI] [PubMed] [Google Scholar]
- Métraux J-P, Kende H. The role of ethylene in the growth response of submerged deep water rice. Plant Physiology. 1983;72:441–446. doi: 10.1104/pp.72.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Métraux J-P, Kende H. The cellular basis of the elongation response in submerged deep-water rice. Planta. 1984;160:73–77. doi: 10.1007/BF00392468. [DOI] [PubMed] [Google Scholar]
- Mommer L, Visser EJW. Underwater photosynthesis in flooded terrestrial plants: a matter of leaf plasticity. Annals of Botany. 2005;96:581–589. doi: 10.1093/aob/mci212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mommer L, de Kroon H, Pierik R, Bogemann GM, Visser EJW. A functional comparison of acclimation to shade and submergence in two terrestrial plant species. New Phytologist. 2005;167:197–206. doi: 10.1111/j.1469-8137.2005.01404.x. [DOI] [PubMed] [Google Scholar]
- Musgrave A, Walters J. Ethylene and buoyancy control rachis elongation of semi-aquatic fern Regnillidium diphyllum. Planta. 1974;121:51–56. doi: 10.1007/BF00384005. [DOI] [PubMed] [Google Scholar]
- Musgrave A, Jackson MB, Ling E. Callitriche stem elongation is controlled by ethylene and gibberellin. Nature New Biology. 1972;238:93–96. [Google Scholar]
- Nabben RHM, Blom CWPM, Voesenek LACJ. Resistance to complete submergence in Rumex species with different life histories: the influence of plant size and light. New Phytologist. 1999;144:313–321. [Google Scholar]
- Nandi S, Subudhi PK, Senadhira D, Manigbas NL, Sen-Mandi S, Huang N. Mapping QTLs for submergence tolerance in rice by AFLP analysis and selective genotyping. Molecular and General Genetics. 1997;255:1–8. doi: 10.1007/s004380050468. [DOI] [PubMed] [Google Scholar]
- Osborne DJ. Ethylene and plants of aquatic and semi-aquatic environments. Plant Growth Regulation. 1984;2:167–185. [Google Scholar]
- Osborne DJ, Walters J, Milborrow BV, Norville A, Stange LMC. Evidence for a non-ACC ethylene biosynthesis pathway in lower plants. Phytochemistry. 1996;42:51–60. [Google Scholar]
- Pearce DME, Jackson MB. Comparison of growth responses of barnyard grass (Echinochloa oryzoides) and rice (Oryza sativa) to submergence, ethylene, carbon dioxide and oxygen shortage. Annals of Botany. 1991;68:201–209. [Google Scholar]
- Pearce DME, Hall KC, Jackson MB. The effects of oxygen, carbon dioxide and ethylene on ethylene biosynthesis in relation to shoot extension in seedlings of rice (Oryza sativa) and barnyard grass (Echinochloa oryzoides) Annals of Botany. 1992;69:441–447. [Google Scholar]
- Pierik R, Cuppens MLC, Voesenek LACJ, Visser EJW. Interactions between ethylene and gibberellins in phytochrome-mediated shade avoidance responses in tobacco. Plant Physiology. 2004;136:2928–2936. doi: 10.1104/pp.104.045120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierik R, Millenaar FF, Peeters AJM, Voesenek LACJ. New perspectives in flooding research: the use of shade avoidance and Arabidopsis thaliana. Annals of Botany. 2005;96:533–540. doi: 10.1093/aob/mci208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierik R, Tholen D, Poorter H, Visser EJW, Voesenek LACJ. The Janus face of ethylene: growth inhibition and stimulation. Trends in Plant Science. 2006;11:176–183. doi: 10.1016/j.tplants.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Poorter H, Remkes C, Lambers H. Carbon and nitrogen economy of 24 wild species differing in relative growth rate. Plant Physiology. 1990;94:621–627. doi: 10.1104/pp.94.2.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pooviah BW, Leopold AC. Effects of ethephon on growth of grasses. Crop Science. 1973;13:755–758. [Google Scholar]
- Potuschak T, Lechner E, Parmentier Y, Yanagisawa S, Grava S, Koncz C, et al. EIN3-dependent regulation of plant ethylene hormone signaling by two Arabidopsis F box proteins: EBF1 and EBF2. Cell. 2003;115:679–689. doi: 10.1016/s0092-8674(03)00968-1. [DOI] [PubMed] [Google Scholar]
- Pratt HK, Goeschl JD. Physiological roles of ethylene in plants. Annual Review of Plant Physiology. 1969;20:541–584. [Google Scholar]
- Ram PC, Singh BB, Singh AK, Ram P, Singh PN, Singh HP, et al. Submergence tolerance in rainfed lowland rice: physiological basis and prospects for cultivar improvement through marker-aided breeding. Field Crops Research. 2002;76:131–152. [Google Scholar]
- Raskin I, Kende H. Regulation of growth in stem sections of deep-water rice. Planta. 1984;a 160:66–72. doi: 10.1007/BF00392467. [DOI] [PubMed] [Google Scholar]
- Raskin I, Kende H. Effect of submergence on translocation, starch content and amylolytic activity in deep-water rice. Planta. 1984;b 162:556–559. doi: 10.1007/BF00399922. [DOI] [PubMed] [Google Scholar]
- Raskin I, Kende H. Role of gibberellin in the growth response of submerged deep water rice. Plant Physiology. 1984;c 76:947–950. doi: 10.1104/pp.76.4.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskin I, Kende H. Mechanism of aeration in rice. Science. 1985;228:327–329. doi: 10.1126/science.228.4697.327. [DOI] [PubMed] [Google Scholar]
- Ridge I. Ethylene and growth control in amphibious plants. In: Crawford RMM, editor. Plant life in aquatic and amphibious habitats. Oxford: Blackwell Scientific Publications; 1987. pp. 53–76. [Google Scholar]
- Ridge I, Amarasinghe I. Ethylene and growth-control in the fringed waterlily (Nymphoides peltata): stimulation of cell-division and interaction with buoyant tension in petioles. Plant Growth Regulation. 1984;2:235–249. [Google Scholar]
- Ridge I, Osborne DJ. Regulation of peroxidase activity by ethylene in Pisum sativum: requirements for protein and RNA synthesis. Journal of Experimental Botany. 1970;21:720–734. [Google Scholar]
- Ridge I, Osborne DJ. Wall extensibility, wall pH and tissue osmolality – significance for auxin and ethylene-enhanced petiole growth in semi-aquatic plants. Plant, Cell and Environment. 1989;12:383–393. [Google Scholar]
- Rijnders JGHM, Barendse GWM, Blom CWPM, Voesenek LACJ. The contrasting role of auxin in submergence-induced petiole elongation in two species from frequently flooded wetlands. Physiologia Plantarum. 1996;96:467–473. [Google Scholar]
- Rijnders JGHM, Yang YY, Kamiya Y, Takahashi N, Barendse GWM, Blom CWPM, et al. Ethylene enhances gibberellin levels and petiole sensitivity in flooding-tolerant Rumex palustris but not in flooding-intolerant R. acetosa. Planta. 1997;203:20–25. [Google Scholar]
- Rijnders JGHM, Armstrong W, Darwent MJ, Blom CWPM, Voesenek LACJ. The role of oxygen in submergence-induced petiole elongation in Rumex palustris: in situ measurements of oxygen in petioles of intact plants using micro-electrodes. New Phytologist. 2000;147:497–504. doi: 10.1046/j.1469-8137.2000.00727.x. [DOI] [PubMed] [Google Scholar]
- Rose-John S, Kende H. Effect of submergence on the cell-wall composition of deep-water rice internodes. Plant Physiology. 1984;76:106–111. doi: 10.1104/pp.76.1.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose-John S, Kende H. Short-term growth-response of deep-water rice to submergence and ethylene. Plant Science. 1985;38:129–134. [Google Scholar]
- Saika H, Okamoto M, Miyoshi K, Kushiro T, Shinoda S, Jikumaru Y, et al. Ethylene promotes submergence-induced expression of OsABA8ox1, a gene that encodes ABA 8′-hydroxylase in rice. Plant and Cell Physiology. 2007;48:287–298. doi: 10.1093/pcp/pcm003. [DOI] [PubMed] [Google Scholar]
- Samarakoon AB, Horton RF. Petiole growth in Ranunculus sceleratus: the role of growth regulators and the leaf blade. Canadian Journal of Botany. 1983;61:3326–3331. [Google Scholar]
- Samarakoon AB, Horton RF. Petiole growth in Ranunculus sceleratus L.: ethylene synthesis and submergence. Annals of Botany. 1984;54:263–270. [Google Scholar]
- Samarakoon AB, Woodrow L, Horton RF. Ethylene- and submergence-promoted growth in Ranunculus sceleratus L. petioles: the effect of cobalt ions. Aquatic Botany. 1985;21:33–41. [Google Scholar]
- Santosa IE, Ram PC, Boamfa EI, Laarhoven LJJ, Reuss J, Jackson MB, et al. Patterns of peroxidative ethane emission from submerged rice seedlings indicate that damage from reactive oxygen species takes place during submergence and is not necessarily a post-anoxic phenomenon. Planta. 2007;26:193–202. doi: 10.1007/s00425-006-0457-z. [DOI] [PubMed] [Google Scholar]
- Sauter M. Rice in deep water: ‘How to take heed against a sea of troubles. Naturwissenschaften. 2000;87:289–303. doi: 10.1007/s001140050725. [DOI] [PubMed] [Google Scholar]
- Sauter M, Kende H. Levels of beta-glucan and lignin in elongating internodes of deepwater rice. Plant and Cell Physiology. 1992;33:1089–1097. [Google Scholar]
- Sauter M, Seagull RW, Kende H. Internodal elongation and orientation of cellulose microfibrils and microtubules in deep-water rice. Planta. 1993;190:354–362. [Google Scholar]
- Schweikert C, Liszkay A, Schopfer P. Polysaccharide degradation by Fenton reaction- or peroxidase-generated hydroxyl radicals in isolated plant cell walls. Phytochemistry. 2002;61:31–35. doi: 10.1016/s0031-9422(02)00183-8. [DOI] [PubMed] [Google Scholar]
- Setter TL, Laureles EV. The beneficial effect of reduced elongation growth on submergence tolerance of rice. Journal of Experimental Botany. 1996;47:1551–1559. [Google Scholar]
- Setter TL, Kupkanchanakul T, Kupkanchanakul K, Bhekasut P, Wiengweera A, Greenway H. Concentrations of CO2 and O2 in floodwater and in internodal lacunae of floating rice growing at 1–2 metre depths. Plant, Cell and Environment. 1987;10:767–776. [Google Scholar]
- Setter TL, Waters I, Wallace I, Bhekasut P, Greenway H. Submergence of rice. I. Growth and photosynthetic response to CO2 enrichment of floodwater. Australian Journal of Plant Physiology. 1989;16:251–263. [Google Scholar]
- Siangliw M, Toojinda T, Tragoonrung S, Vanavichit A. Thai jasmine rice carrying QTLch9 (SubQTL) is submergence tolerant. Annals of Botany. 2003;91:255–261. doi: 10.1093/aob/mcf123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvertown J, Dodd ME, Gowing DJG, Mountford JO. Hydrologically defined niches reveal a basis for species richness in plant communities. Nature. 1999;400:61–63. [Google Scholar]
- Sisler EC, Serek M. Inhibitors of ethylene responses in plants at the receptor level: recent developments. Physiologia Plantarum. 1997;100:577–582. [Google Scholar]
- Sisler EC, Wood C. Interactions of ethylene and CO2. Physiologia Plantarum. 1988;73:440–444. [Google Scholar]
- Smalle J, Haegman M, Kurepa J, Van Montagu M, Van der Straeten D. Ethylene can stimulate Arabidopsis hypocotyl elongation in the light. Proceedings of the National Academy of Sciences of the USA. 1997;94:2756–2761. doi: 10.1073/pnas.94.6.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith FA, Walker NA. Photosynthesis by aquatic plants: effects of unstirred layers in relation to assimilation of CO2 and and to carbon isotopic discrimination. New Phytologist. 1980;86:245–259. [Google Scholar]
- Smith MA, Jacobsen JV, Kende H. Amylase activity and growth in internodes of deepwater rice. Planta. 1987;172:114–120. doi: 10.1007/BF00403036. [DOI] [PubMed] [Google Scholar]
- Soukup A, Armstrong W, Schreiber L, Franke R, Votrubova O. Apoplastic barriers to radial oxygen loss and solute penetration: a chemical and functional comparison of the exodermis of two wetland species, Phragmites australis and Glyceria maxima. New Phytologist. 2007;173:264–278. doi: 10.1111/j.1469-8137.2006.01907.x. [DOI] [PubMed] [Google Scholar]
- Stange L, Osborne DJ. Cell specificity in auxin-induced and ethylene-induced supergrowth in Riella helicophylla. Planta. 1988;175:341–347. doi: 10.1007/BF00396339. [DOI] [PubMed] [Google Scholar]
- Suge H, Kusanagi T. Ethylene and carbon dioxide: regulation of growth in two perennial aquatic plants, arrowhead and pondweed. Plant and Cell Physiology. 1975;16:65–72. [Google Scholar]
- Summers JE, Jackson MB. Anaerobic promotion of stem extension in Potamogeton pectinatus: roles for carbon dioxide, acidification and hormones. Physiologia Plantarum. 1996;96:615–622. [Google Scholar]
- Summers JE, Voesenek LACJ, Blom CWPM, Lewis MJ, Jackson MB. Potamogeton pectinatus is constitutively incapable of synthesizing ethylene and lacks 1-aminocyclopropane-1-carboxylic acid oxidase. Plant Physiology. 1996;111:901–908. doi: 10.1104/pp.111.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toojinda T, Siangliw M, Tragoonrung S, Vanavichit A. Molecular genetics of submergence tolerance in rice: QTL analysis of key traits. Annals of Botany. 2003;91:243–253. doi: 10.1093/aob/mcf072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueguchi-Tanaka M, Ashikari M, Nakajima M, Itoh H, Katoh E, Kobayashi M, et al. GIBBERELLIN INSENSITIVE DWARF1 encodes a soluble receptor for gibberellin. Annual Review of Ecology and Systematics. 2005;437:693–698. doi: 10.1038/nature04028. [DOI] [PubMed] [Google Scholar]
- Vacha GA, Harvey RB. The use of ethylene, propylene, and similar compounds in breaking the rest period of tubers, bulbs, cuttings, and seeds. Plant Physiology. 1927;2:187–193. doi: 10.1104/pp.2.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Sman AJM, Voesenek LACJ, Blom CWPM, Harren FJM, Reuss J. The role of ethylene in shoot elongation with respect to survival and seed output of flooded Rumex maritimus L. plants. Functional Ecology. 1991;5:304–313. [Google Scholar]
- Van der Straeten D, Zhou ZY, Prinsen E, Van Onckelen HA, Van Montagu MC. A comparative molecular-physiological study of submergence response in lowland and deepwater rice. Plant Physiology. 2001;125:955–968. doi: 10.1104/pp.125.2.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergara BS, Jackson B, De Datta SK. Proceedings of the symposium on climate and rice. Los Banos, Phillipines: International Rice Research Institute; 1976. Deep water rice and its response to deep water stress; pp. 301–319. [Google Scholar]
- Voesenek LACJ, Blom CWPM. Growth-responses of Rumex species in relation to submergence and ethylene. Plant, Cell and Environment. 1989;12:433–439. [Google Scholar]
- Voesenek LACJ, Perik PJM, Blom CWPM, Sassen MMA. Petiole elongation in Rumex species during submergence and ethylene exposure: the relative contributions of cell division and cell expansion. Journal of Plant Growth Regulation. 1990;9:13–17. [Google Scholar]
- Voesenek LACJ, Banga M, Thier RH, Mudde CM, Harren FJM, Barendse GWM, et al. Submergence-induced ethylene synthesis, entrapment and growth in two plant species with contrasting flooding resistances. Plant Physiology. 1993;103:783–791. doi: 10.1104/pp.103.3.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voesenek LACJ, Vriezen WH, Smekens M, Huitink FHM, Bogemann GM, Blom CWPM. Ethylene sensitivity and response sensor expression in petioles of Rumex species at low O2 and high CO2 concentrations. Plant Physiology. 1997;114:1501–1509. doi: 10.1104/pp.114.4.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voesenek LACJ, Armstrong W, Bogemann GM, McDonald MP, Colmer TD. A lack of aerenchyma and high rates of radial oxygen loss from the root base contribute to the waterlogging intolerance of Brassica napus. Australian Journal of Plant Physiology. 1999;26:87–93. [Google Scholar]
- Voesenek LACJ, Jackson MB, Toebes AHW, Huibers W, Vriezen WH, Colmer TD. De-submergence-induced ethylene production in Rumex palustris: regulation and ecophysiological significance. The Plant Journal. 2003;a 33:341–352. doi: 10.1046/j.1365-313x.2003.01632.x. [DOI] [PubMed] [Google Scholar]
- Voesenek LACJ, Benschop JJ, Bou J, Cox MCH, Groenveld HW, Millenaar FF, et al. Interactions between plant hormones regulate submergence-induced shoot elongation in the flooding-tolerant dicot Rumex palustris. Annals of Botany. 2003;b 91:205–211. doi: 10.1093/aob/mcf116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voesenek LACJ, Rijnders JHGM, Peeters AJM, van de Steeg HM, de Kroon H. Plant hormones regulate fast shoot elongation underwater: from genes to communities. Ecology. 2004;85:16–27. [Google Scholar]
- Vreeburg RAM, Benschop JJ, Peeters AJM, Colmer TD, Ammerlaan AHM, Staal M, et al. Ethylene regulates fast apoplastic acidification and expansin A transcription during submergence-induced petiole elongation in Rumex palustris. The Plant Journal. 2005;43:597–610. doi: 10.1111/j.1365-313X.2005.02477.x. [DOI] [PubMed] [Google Scholar]
- Vriezen WH, vanRijn CPE, Voesenek LACJ, Mariani C. A homolog of the Arabidopsis thaliana ERS gene is actively regulated in Rumex palustris upon flooding. The Plant Journal. 1997;11:1265–1271. doi: 10.1046/j.1365-313x.1997.11061265.x. [DOI] [PubMed] [Google Scholar]
- Vriezen WH, Hulzink R, Mariani C, Voesenek LACJ. 1-aminocyclopropane-1-carboxylate oxidase activity limits ethylene biosynthesis in Rumex palustris during submergence. Plant Physiology. 1999;121:189–195. doi: 10.1104/pp.121.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vriezen WH, De Graaf B, Mariani C, Voesenek L. Submergence induces expansin gene expression in flooding-tolerant Rumex palustris and not in flooding-intolerant R. acetosa. Planta. 2000;210:956–963. doi: 10.1007/s004250050703. [DOI] [PubMed] [Google Scholar]
- Wallace RH. The production of intumescences upon apple twigs by ethylene gas. Bulletin of the Torrey Botanical Club. 1926;53:385–402. [Google Scholar]
- Walters J, Osborne DJ. Ethylene and auxin-induced cell growth in relation to auxin transport and metabolism and ethylene production in the semi-aquatic plant, Regnellidium diphyllum. Planta. 1979;V146:309–317. doi: 10.1007/BF00387803. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Saigusa M, Hase S, Hayakawa T, Satoh S. Cloning of a cDNA encoding an ETR2-like protein (Os-ERL1) from deep water rice (Oryza sativa L.) and increase in its mRNA level by submergence, ethylene, and gibberellin treatments. Journal of Experimental Botany. 2004;55:1145–1148. doi: 10.1093/jxb/erh110. [DOI] [PubMed] [Google Scholar]
- Waters I, Armstrong W, Thompson CJ, Setter TL, Adkins S, Gibbs J, et al. Diurnal changes in radial oxygen loss and ethanol metabolism in roots of submerged and non-submerged rice seedlings. New Phytologist. 1989;113:439–451. [Google Scholar]
- Wiedenroth EM, Jackson MB. Depletion of oxygen in roots of wheat and maize by respiration following encasement in olive oil. Annals of Botany. 1993;71:427–430. [Google Scholar]
- Wright M, Mousdale DMA, Osborne DJ. Evidence for a gravity-regulated level of endogenous auxin controlling cell elongation and ethylene production during geotropic bending in grass nodes. Biochemie und Physiologie der Pflanzenphysiologie. 1978;172:581–596. [Google Scholar]
- Xu K, Xu X, Ronald PC, Mackill DJ. A high-resolution linkage map of the vicinity of the rice submergence-tolerance locus Sub1. Molecular and General Genetics. 2000;263:681–689. doi: 10.1007/s004380051217. [DOI] [PubMed] [Google Scholar]
- Xu K, Xu X, Fukao T, Canlas P, Maghirang-Rodriguez R, Heuer S, et al. Sub1A is an ethylene-response-factor-like gene that confers submergence tolerance to rice. Nature. 2006;442:705–708. doi: 10.1038/nature04920. [DOI] [PubMed] [Google Scholar]
- Yamada N. Physiological basis of resistance of rice plant against overhead flooding. Bulletin of the National Institute of Agricultural Sciences, Series D (Plant Physiology, Genetics and Crops in General) 1959;8:1–112. [in Japanese with extensive English summary and captions] [Google Scholar]
- Yip WK, Jiao XZ, Yang SF. Dependence of in vivo ethylene production rate on 1-aminocyclopropane-1-carboxylic acid content and oxygen concentrations. Plant Physiology. 1988;88:553–558. doi: 10.1104/pp.88.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarembinski TI, Theologis A. Expression characteristics of OS-ACS1 and OS-ACS2, two members of the 1-aminocyclopropane- 1-carboxylate synthase gene family in rice (Oryza sativa L. cv Habiganj Aman II) during partial submergence. Plant Molecular Biology. 1997;33:71–77. doi: 10.1023/b:plan.0000009693.26740.c3. [DOI] [PubMed] [Google Scholar]
- Zeigler RS, Puckridge DW. Improving sustainable productivity in rice-based rainfed lowland systems of south and southeast asia. GeoJournal. 1995;35:307–324. [Google Scholar]
- Zhou ZY, Vriezen W, Van Caeneghem W, Van Montagu M, Van der Straeten D. Rapid induction of a novel ACC synthase gene in deepwater rice seedlings upon complete submergence. Euphytica. 2001;121:137–143. [Google Scholar]