Responses to acid have been studied extensively in enteric pathogens, such as Escherichia coli, Vibrio cholerae, and Helicobacter pylori that encounter the extremely low pH (pH 2 to 3) of the stomach during ingestion. In contrast, much less is known about how obligate or facultative intracellular bacterial pathogens like Mycobacterium tuberculosis respond, resist, and persist in the moderately acid environment of the phagosome or phagolysosome. The pH of the macrophage compartment, in which M. tuberculosis resides, ranges from pH 6.2 to 4.5, depending on the activation state of the macrophage (55, 81, 99). Phagosomal acidity may provide a critical cue for adaptation of M. tuberculosis to the host niche. At the same time, to ensure survival for what can be decades, the bacterium must prevent excessive entry of protons into its cytosol and expel them when their concentrations threaten the pH homeostasis that most organisms maintain. Here we review current understanding of the ability of M. tuberculosis to adapt to phagosomal levels of acid. It has been challenging to dissect the role of phagosome acidification in the pathogenesis of tuberculosis, because it is accompanied by and synergizes with other host defenses. Similarly, M. tuberculosis acid resistance mechanisms appear to be cross-protective against other forms of stress, making it difficult to directly relate a defect in acid resistance to impaired virulence. Notwithstanding, the phenomenon is central to the pathogenesis of tuberculosis and thus might offer points of vulnerability that could be exploited by new chemotherapeutics.
ACID AS A HOST DEFENSE
Using indicator dyes that change color in acid, such as litmus and neutral red, Elie Metchnikoff reported in 1905 that acidic reactions occur within phagosomes of guinea pig peritoneal macrophages that have ingested bacteria (61). Likewise, with the use of dyes, Peyton Rous observed acidic compartments within peritoneal exudate cells of rats and mice (74, 75). Noting that the dyes provided imprecise measurements of acidity, Rous speculated that the pH of intracellular compartments might be as low as 3. Following this, a report examining the level of acidity surrounding mycobacteria in macrophage compartments demonstrated that the pH of M. tuberculosis- and Mycobacterium smegmatis- containing phagosomes was in the range of 4.7 to 5.5 (85). Subsequent studies made use of fluorescent pH-sensitive molecules that allowed quantitative measurements of phagosomal acidity. Using fluorescently labeled dextran, which accumulates in lysosomes, it was shown that lysosomes of macrophages ranged in pH from 4.5 to 4.8 (67). The aforementioned observations laid ground for the demonstration that when lysosomes fuse with M. tuberculosis-containing phagosomes in immunologically activated macrophages, the phagolysosomal pH falls to 4.5 to 5.0 (55, 81, 84, 99). Numerous studies have confirmed these observations (reviewed in reference 46). Recently, using fluorescent mannosylated beads, it was reported that the pH of the macrophage phagolysosome falls rapidly, within 15 to 60 min, to a pH slightly below 5.0 (103). Thus, the pH of the macrophage phagolysosome appears to vary with the immunological state of activation of the macrophage and the nature of the phagocytic particle but generally reaches a pH associated with cessation of growth of many facultative intracellular bacterial pathogens in broth culture.
Certainly, excess protons can damage DNA, proteins, and lipids and disrupt biochemical reactions. Whether phagosomal acid is actually a major bactericidal effector mechanism of macrophages is difficult to establish. In 1954, Rene Dubos stated, “The hypothesis that intracellular acidity is one of the causes of the bactericidal effects of phagocytosis is at best a working hypothesis almost devoid of experimental support” (25). In fact, phagosomal acidification is important for the induction of virulence factors in certain pathogens and favors the survival of some. For example, acidification is required for escape of Listeria monocytogenes from phagosomes (9) and induction of virulence proteins in Salmonella enterica serovar Typhii (20). Legionella pneumophila (89) and Coxiella burnetii (40, 59) display more efficient replication in acidic phagosomes. Moreover, efforts to define the microbicidal contribution of phagosomal acidification are complicated by the pleiotropic impact of disrupting this process. Inhibiting acidification likely interferes with the antimicrobial capacity of other host defenses and also probably prevents complete biogenesis of the phagosomal compartment as acidification itself is believed to act as a go signal for phagosome maturation (42).
With these caveats in mind, several studies have demonstrated that interference with acidification of the phagosomal compartment favors the survival of mycobacteria (55). It is tempting to conclude that phagosomal acid itself is bactericidal. However, another interpretation is that the acidity of the phagosome supports and synergizes with additional antibacterial mechanisms of phagocytes, such as acid-dependent lysosomal hydrolases and reactive oxygen intermediates (ROI) and reactive nitrogen intermediates (RNI) (44, 87, 93) (Fig. 1). For example, nitric oxide (NO) is the primary product of inducible nitric oxide synthase (iNOS), an enzyme required for control of experimental tuberculosis in mice (54). In oxygenated aqueous environments, NO rapidly autooxidizes, producing roughly equivalent amounts of nitrite and nitrate. These are not microbicidal, and they diffuse away from the enzyme, both out of the macrophage and presumably into the phagosome. However, the pH of a phagolysosome containing M. tuberculosis in an activated macrophage is close enough to the pKa of nitrous acid (3.8) to allow protonated nitrite (that is, nitrous acid) to sustain its own dismutation, forming NO and another toxic radical, nitrogen dioxide. Thus, nitrite diffusing into an acidified compartment generates another round of bactericidal RNI (53). The phagosomal milieu in the activated macrophage thus resembles to some degree the intragastric environment, whose microbicidal efficiency depends on the combined action of acid and RNI (10, 60, 106). In addition, like many other elements of the innate immune response, phagosomal acid serves to link the innate and adaptive immune systems. In dendritic cells (DCs), regulation of phagosomal pH by phagocyte oxidase (NOX2) is important for T-cell activation. In DCs lacking phagocyte oxidase, enhanced phagosomal acidification results in increased antigen degradation and inefficient cross-presentation of antigen to T cells (56, 79).
FIG. 1.
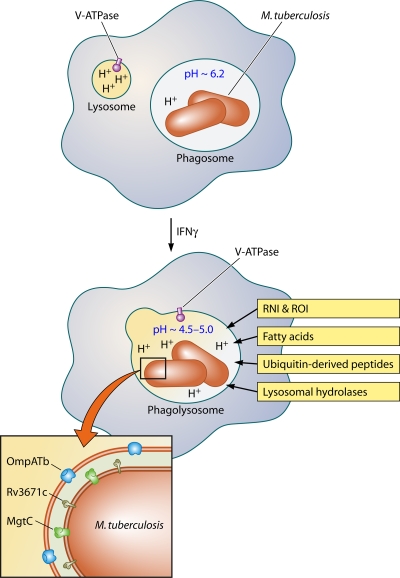
M. tuberculosis inside the macrophage. In resting macrophages, M. tuberculosis impairs phagosome maturation and resides in a mildly acidic compartment. Activation with IFN-γ results in phagosome maturation and phagosome-lysosome fusion. This exposes the bacteria to host-derived stress including protons from the vacuolar ATPase, RNI and ROI, free fatty acids, ubiquitin-derived peptides, and lysosomal hydrolases. M. tuberculosis resists acidification with the help of the Rv3671c-encoded membrane-bound serine protease, the putative magnesium transporter MgtC, and the pore-forming M. tuberculosis outer membrane protein (OmpATb). The exact mechanisms by which these proteins confer acid resistance remain to be identified.
M. TUBERCULOSIS RESIDES IN AN ACIDIC PHAGOSOME
In macrophages that have not yet been immunologically activated, many species of mycobacteria inhibit the fusion of phagosomes with lysosomes and thereby reside in an environment that is only very mildly acidic with a pH of ∼6.2 (55) (Fig. 1). Mycobacterium leprae (84), Mycobacterium avium (88), Mycobacterium bovis BCG (98), and M. tuberculosis (6, 55) all have the ability to prevent maturation of the macrophage phagosome. Lack of acidification of the mycobacterial phagosome is likely due to the absence of the vacuolar proton-ATPase, and many groups have pursued further molecular characterization of this compartment (42, 77). As noted earlier, however, after immunologic activation of the macrophage, such as by exposure to gamma interferon (IFN-γ), the fusion block is relieved and the phagosomal compartment acidifies to pH 4.5 to 5.0 (55, 81, 84, 99) (Fig. 1).
The aforementioned studies have been conducted with cultured cells. Several lines of evidence suggest that M. tuberculosis also resides within an acidic phagosome during infection of the host. Pyrazinamide, which kills M. tuberculosis in vitro only at acidic pHs, is effective in vivo, suggesting that the pathogen's in vivo environment is acidic as well (104). Additionally, M. tuberculosis-infected lungs are sites of intense T-cell and macrophage activity and are positive for IFN-γ (8) and iNOS, which IFN-γ strongly induces (18, 28, 82, 83), indicating that IFN-γ is functional. Because IFN-γ drives macrophage activation and acidification of M. tuberculosis-containing phagosomes in vitro, it is likely that this also occurs in vivo. Consistent with in vitro evidence, it was demonstrated that in humans coinfected with M. tuberculosis and human immunodeficiency virus, M. tuberculosis resides in phagosomes that fail to fully mature and acidify (64). This may be due to the low levels of IFN-γ in these patients. Moreover, expression of acid-responsive M. tuberculosis genes is increased during infection of macrophages and acid-sensitive mutants of M. tuberculosis are attenuated in vivo, further suggesting that the bacterium encounters and responds to acidity in the host (14, 71, 73, 95).
SURVIVAL OF M. TUBERCULOSIS IN ACID: IN VITRO OBSERVATIONS
In vitro studies of bacteria at low pH are informative because they can indicate whether the bacteria are likely to be acid resistant or sensitive during infection. These studies can also identify bacterial factors that confer protection against low pH and may do so as well in the host environment. Thus, to begin understanding whether M. tuberculosis resists acid in vivo, it is useful to first review survival of M. tuberculosis in acid in vitro. Importantly, however, the interpretation of in vitro studies is complicated by the observations that survival of many bacteria in acid is dependent on the culture conditions, such as bacterial density and composition of the test medium (33). These variables also dramatically influence the survival and growth of mycobacteria at low pH (11, 90, 95).
In general, the fast-growing, saprophytic mycobacteria grow over a wider pH range than the pathogenic, slow-growing mycobacteria (16, 69). This may reflect that the environments in which saprophytic mycobacteria reside, such as soil and water, are often acidic (43). Remarkably, mycobacterial species were found greatly enriched in extremely acidic volcanic rock at pH 1 (100). With humans being its only natural environment and inhalation its most common route of entry into the body, M. tuberculosis does not need to maintain such a high tolerance for acid. Optimal growth of M. tuberculosis in enriched liquid medium (Dubos' or 7H9 medium) is observed at a slightly acid pH, between 5.8 and 6.7. The bacilli display almost no replication at a pH of ≤5.5 (Fig. 2A) (16, 69). At pHs of 5.0 and 4.5, although M. tuberculosis survived at high densities (∼2.5 × 108 CFU/ml), the bacteria were killed dramatically as their density was reduced (Fig. 2A). E. coli also displays greater acid resistance at high densities, and a cell-cell contact-based mechanism appears to be involved (57). Protective factors secreted by E. coli may also play a role in its resistance to acid (76). The in vitro observations of M. tuberculosis in acid invite the speculation that the bacterium might be highly susceptible to the low pH of the phagolysosome, particularly if one chooses to consider the bacterial density (that is, number of bacteria per unit fluid volume) in a phagosome “low.” However, one might also consider the bacterial density in a phagosome to be extremely high. More important, killing of M. tuberculosis at pH 4.5 is greatly influenced by the composition of the medium in a manner that can be considered artifactual (Fig. 2B). Because of their propensity to clump, mycobacteria are commonly grown in detergents to allow for dispersed growth and preparation of relatively uniform bacterial suspensions for experimental studies. When the detergent tyloxapol was used instead of Tween 80 in growth medium acidified to pH 4.5, killing at lower densities was reduced but not eliminated (Fig. 2B). Free fatty acids are toxic to M. tuberculosis, particularly at low pH, and therefore the high susceptibility of the bacterium to low pH in growth medium may be due to the release of oleic acid or other free fatty acids from Tween 80 and albumin, respectively (22, 24, 45, 47, 48, 51). In a simple phosphate-citrate buffer at pH 4.5, M. tuberculosis survived for a prolonged period at a wide range of cell densities (Fig. 2B). It was reported that a variety of strains of M. tuberculosis are resistant to killing at a pH of 4.5 in phosphate-citrate buffer (44). The bacilli are also able to maintain a near neutral intrabacterial pH when placed in phosphate-citrate buffer at pH 4.5, indicating that they are able to counter the entry of protons (95). Therefore, in simple buffer M. tuberculosis resists phagolysosomal concentrations of acid. These studies serve as a reminder that M. tuberculosis' susceptibility to stresses in vitro can be confounded by the model systems utilized.
FIG. 2.
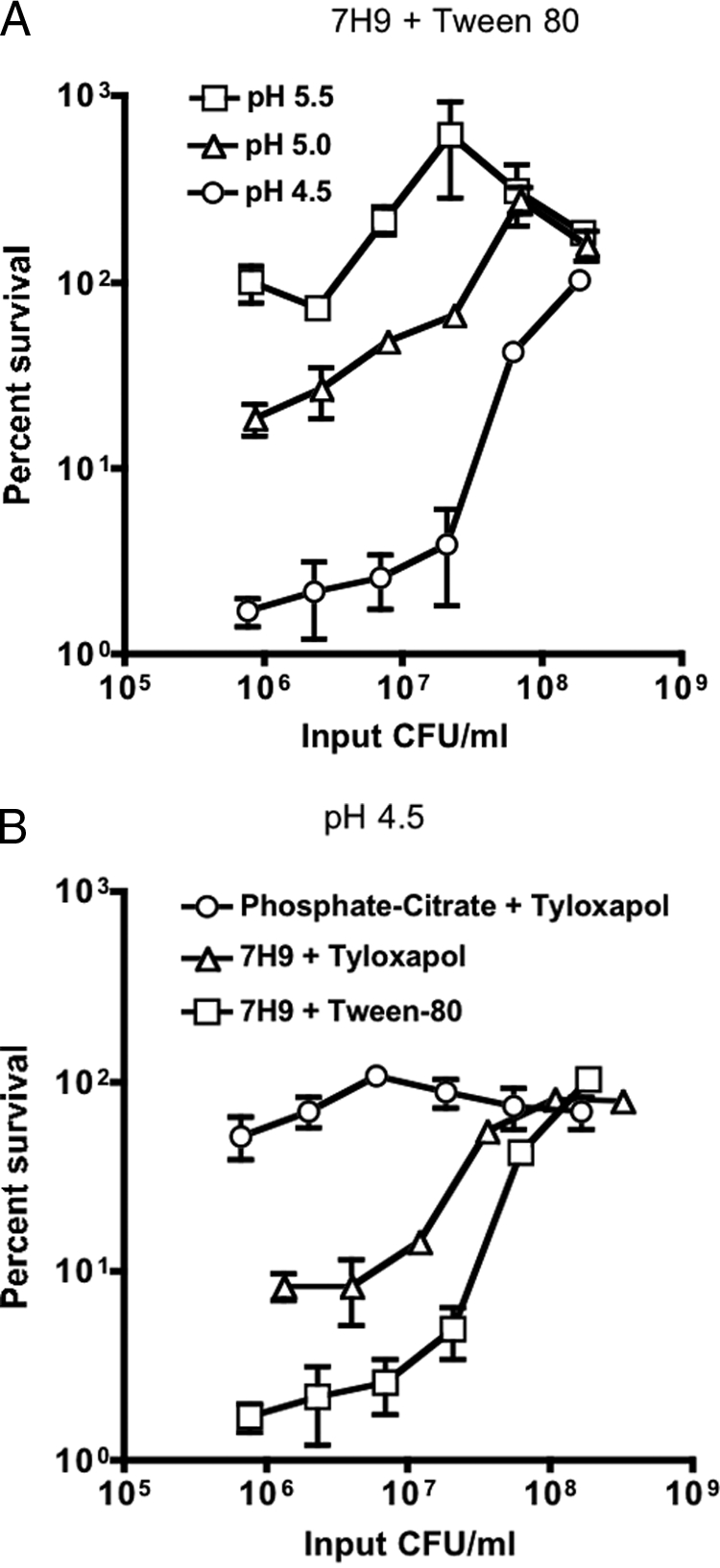
(A) Impact of pH and cell density on survival of M. tuberculosis. Cultures were plated after incubation for 6 days at pH 5.5 (squares), pH 5.0 (triangles), or pH 4.5 (circles) at input densities ranging from 2.5 × 108 to 1 × 106 CFU/ml. Data are means ± standard deviations of triplicate cultures and represent two independent experiments. For the experimental protocol and a description of the media, please see reference 95. In some cases, error bars are too small to visualize. (B) Impact of medium and cell density on survival of M. tuberculosis at pH 4.5. Cultures were plated after incubation for 6 days in 7H9 growth medium containing Tween 80 (squares), 7H9 growth medium containing tyloxapol (triangles), or phosphate citrate buffer containing tyloxapol (circles) at input densities ranging from 2.5 × 108 to 1 × 106 CFU/ml. Data are means ± standard deviations of triplicate cultures and represent two independent experiments. For the experimental protocol and a description of the media, please see reference 95.
SURVIVAL OF M. TUBERCULOSIS IN ACID: OBSERVATIONS IN THE MACROPHAGE
As M. tuberculosis causes a chronic infection that often persists for the lifetime of its host, it is likely that at least some proportion of the bacteria is effectively resistant to the level of acid in the phagolysosome. However, some experimental data suggest that M. tuberculosis might be sensitive to host acid not only because it displays a narrow pH optimum for growth in vitro (see above), but also because the bacterium's growth is restricted by IFN-γ-activated macrophages that have acidified their phagosomes (55). However, besides inducing acidification of phagosomes, as noted above, IFN-γ activates many other pathways of mycobacterial control (26, 65).
In order to examine survival of M. tuberculosis in phagolysosomes, M. tuberculosis was coated with serum, which resulted in delivery of the bacilli directly to the phagolysosome via Fc receptor-mediated phagocytosis. The bacilli survived and even replicated slightly in phagolysosomes (5). In another study, macrophages were coinfected with Coxiella burnetii and M. tuberculosis so that both organisms colocalized to acidic vacuoles; the growth of M. tuberculosis was only minimally restricted in coinfected macrophages, suggesting that they can tolerate the phagolysosomal acidic milieu (35). In the coinfection studies, survival of M. tuberculosis was similar to that of M. avium, a mycobacterial species that can infect both through inhalation and the oral route and can survive a gastrointestinal pH of 2 for at least 2 h (11). Also, M. tuberculosis is not killed within IFN-γ-activated macrophages that lack iNOS, an important mediator of mycobacterial control; because iNOS−/− macrophages that have been activated by IFN-γ retain the ability to acidify their phagosomes, it appears that M. tuberculosis can resist killing by low pH in macrophages (55). Additionally, M. tuberculosis mutants that are unable to prevent phagosome-lysosome fusion and therefore localize to phagolysosomes are not necessarily compromised for survival in macrophages, further suggesting that the bacterium is likely acid resistant (38, 52, 68, 72, 86). In support of this notion, M. tuberculosis is able to maintain its intrabacterial pH at near neutral during infection of IFN-γ-activated macrophages (95). However, in LRG-47-deficient macrophages that are unable to completely acidify their phagosomes after activation with IFN-γ, M. tuberculosis exhibits enhanced survival (55). It must be noted that these phagosomes do not mature completely after macrophage activation (55), and it is likely that other antimicrobial effectors, such as free fatty acids (2, 94) or ubiquitin-derived peptides (4), are not delivered to the mycobacterial phagosome in LRG-47-deficient macrophages. It remains difficult to single out the impact that acid may be having on the bacteria; however, the aforementioned studies do suggest that acid may not directly be potently mycobactericidal and M. tuberculosis likely becomes sensitive to low pH in combination with other antimicrobial factors.
THE ROLE OF THE CELL ENVELOPE IN ACID RESISTANCE
The physical structure and molecular composition of bacterial cell envelopes act as an effective primary barrier against the entry of protons. If protons do enter the bacterial cytosol, an array of mechanisms regulates pH within the cell. Proton pumps, production of ammonia, amino acid decarboxylation, cell envelope modification, macromolecule protection, and cell density all contribute to maintenance of intrabacterial pH and survival of bacteria in acid, as reviewed comprehensively in references 13, 30, and 31. At an external pH of 5, the internal pH of M tuberculosis H37Ra was close to 7, indicating that mycobacteria are able to maintain a neutral internal pH in an acidic environment (105), and other studies have confirmed this observation in M. smegmatis and virulent M. tuberculosis (70, 95).
In the early 1900s, Metchnikoff speculated that the waxy M. tuberculosis cell wall serves as an important guard against acid stress present in phagocytes (61). M. tuberculosis has a lipid rich cell wall that consists of a typical bilayered plasma membrane followed by a layer of peptidoglycan-arabinogalactan covalently linked to mycolic acids that can be up to 90 carbon atoms in length. Recent work has demonstrated the existence of an additional outer lipid bilayer surrounding mycobacteria (41, 107). This complex cell envelope acts as a formidable permeability barrier for antibacterial effectors, including protons. Indeed, studies examining the physiology of mycobacteria at low pH indicate that the cell wall plays a critical role in resistance to acid. A large number of the few M. tuberculosis and M. smegmatis acid-sensitive mutants identified so far have defects in genes involved in cell wall functions (92, 95, 96), and many cell wall or lipid biosynthesis genes are transcriptionally regulated upon exposure to low pH (29, 73, 80). In addition, mycobacteria do not appear to display a classical acid tolerance response, in which prior exposure to mildly acidic conditions protects the bacteria in a more acidic environment. Preadaptation of M. smegmatis to a pH of 5.0 only conferred two- to threefold protection to a pH challenge of 3.0 (66). This level of protection is relatively low compared to that of enteric bacteria, which can display a 1,000- to 10,000-fold increase in survival after preadaptation in mildly acidic medium (32, 49). We have not observed an acid tolerance response in M. tuberculosis either (unpublished observation), and it may be that mycobacteria mainly rely upon intrinsic acid defenses, including their cell wall, for survival at suboptimal pH.
ACID-SENSITIVE M. TUBERCULOSIS MUTANTS
Few acid-sensitive M. tuberculosis mutants have been identified, and the mechanisms by which the deficient gene products confer acid resistance have not been elucidated. M. tuberculosis lacking MgtC, a putative magnesium transporter, was attenuated for growth in vitro at a mildly acidic pH of 6.25, but only at low Mg2+ concentrations (14). The MgtC mutant was also attenuated for growth in macrophages and mice, suggesting that Mg2+ acquisition may become important when M. tuberculosis is exposed to the low pH of the phagosomal compartment (14). It has been proposed that Mg2+ may be required in acid for the maintenance of cell envelope integrity, as a cofactor for enzymes that become important during acid stress or for the function of a Mg2+-dependent proton ATPase involved in extruding cytosolic protons (21). However, Salmonella's MgtC does not appear to function as a transporter of Mg2+, but may be involved in regulating membrane potential, perhaps by activating a cation-translocating P-type ATPase (39, 63). However, this evidence is based on heterologous expression of S. enterica serovar Typhimurium MgtC in Xenopus laevis oocytes (39). In E. coli, an MgtC family member has also been implicated in acid resistance (57). Future work is required to delineate whether MgtC acts as a Mg2+ transporter, activates P-type ATPases (3), or plays some other yet to be identified role.
M. tuberculosis OmpA (OmpATb), a pore-forming protein or porin, is also important for acid resistance and virulence (71). Transcription of ompATb was induced at pH 5.5, and an ompATb mutant was attenuated for growth at pH 5.5 in vitro in macrophages and in mice (71). The mechanism by which OmpATb confers acid resistance is unknown. Work on the pore-forming activity of OmpATb in lipid bilayers has indicated that the channel is pH sensitive and has a propensity to close at low pH (62). Closing of OmpATb may be an adaptive mechanism used by M. tuberculosis to survive in the low pH of the phagosome, and the channel's increased expression at low pH may compensate for its reduced activity in acid. The precise role of OmpATb in transporting molecular factors at low pH remains to be determined.
In a screen of 10,100 M. tuberculosis transposon mutants for mutants hypersensitive to pH 4.5, 21 genes were identified whose disruption conferred sensitivity to low pH (95, 96). All 21 acid-sensitive mutants exhibited growth similar to wild type at near neutral pH. Fifteen of the 21 mutants were deficient in genes annotated to be involved in cell wall functions, of which several are possibly involved in the biosynthesis of peptidoglycan or the cell wall lipid lipoarabinomannan (Rv2052c, Rv2136c, Rv2224c, ppm1, and ponA2) (19, 95, 96). Several M. tuberculosis acid-sensitive mutants also displayed increased sensitivity to cell-wall-damaging stress, such as lipophilic antibiotics and a detergent, suggesting that their cell walls were possibly compromised (96). M. tuberculosis mutants deficient in homologs of genes shown to be involved in low-pH resistance in M. smegmatis were not isolated (92). In a screen of 5,000 transposon mutants, 8 M. smegmatis acid-sensitive mutants identified were disrupted in genes predicted to be involved in phosphonate/phosphite transport, methionine biosynthesis, and lipid biosynthesis; several genes of unknown function were also identified (92). It is possible that M. tuberculosis mutants in homologous genes were not represented in the screened library or that those genes are not expressed under the conditions studied or are redundant in their function. However, cell wall biosynthesis pathways seem to be required for acid resistance in both mycobacterial species. Furthermore, mutants of M. tuberculosis genes annotated to be involved in mechanisms used by gram-negative and positive bacteria to resist acid, such as potassium-proton antiporters, amino acid decarboxylases, and FoF1 ATPases were not isolated (30). To our knowledge, many pathways that are important for acid resistance and intrabacterial pH homeostasis in gram-negative and gram-positive bacteria have not been identified to play a role in mycobacterial acid resistance. This is likely due to the general paucity of work in this area. Additionally, acid resistance systems may not function in an analogous fashion in facultative intracellular pathogens like mycobacteria that modulate endosomal maturation. However, some mechanisms that protect from acidification seem to be conserved between bacterial pathogens. For example, ammonia produced from urea by an H. pylori urease neutralizes gastric acid and is important for survival of the bacterium in the low pH of the stomach (78). In mycobacteria, it has been proposed that ammonia generated by the mycobacterial urease is involved in neutralizing phagosomal pH and inhibiting phagosome-lysosome fusion (37, 38).
To identify mutants whose acid sensitivity was independent of medium components, the 21 acid-sensitive mutants were counterscreened in other media. Only two mutants, those disrupted in Rv3671c and Rv2136c, were hypersensitive in a simple phosphate citrate buffer at pH 4.5. These two mutants failed to maintain intrabacterial pH in acid in vitro and in IFN-γ-activated macrophages, and their growth was severely attenuated in mice (95, 96). Thus, intrabacterial pH homeostasis is important for virulence of M. tuberculosis. Because acid promotes the activity of numerous host defenses, such as lysosomal hydrolases and ROI and RNI, the marked attenuation of these mutants in vivo is probably due to the synergistic interaction of phagosomal acid with other macrophage products (Fig. 1). Some of the acid-sensitive mutants were also hypersensitive to oxidative and nitrosative stress and heat shock in vitro (96). In Salmonella and E. coli, low pH induces oxidative and heat shock regulons (34, 58), and in M. tuberculosis the sigma factors SigB and SigH, which are induced by oxidative and heat stress, are also induced by acid (73). These observations indicate that there is considerable overlap between low pH and oxidative and heat stress responses and resistance pathways may be cross-protective both in vitro and in vivo.
The mechanisms by which Rv3671c and Rv2136c protect against acid and support virulence remain to be identified. Rv2136c encodes a homolog of Escherichia coli BacA (19). BacA, which has been renamed UppP, is an undecaprenol pyrophosphate phosphatase involved in peptidoglycan biosynthesis (27). A Streptococcus mutans strain deficient in an undecaprenol kinase was also sensitive to acid, supporting that peptidoglycan biosynthesis is required for acid resistance (50, 102). However, survival of the M. tuberculosis Rv2136c mutant at low pH was not restored by a wild-type copy of the gene; therefore, other mutations on the genome may be contributing to its acid sensitivity (96). Rv3671c is a membrane-bound serine protease that, in contrast to the HtrA membrane serine proteases, lacks a PDZ protein-protein interaction domain (95; unpublished data). Rv3671c may protect M. tuberculosis against acid by modifying the bacterial cell envelope, regulating protein or lipid quality control, and/or serving in signaling pathways that help the bacterium resist extracellular stress.
GENE EXPRESSION OF M. TUBERCULOSIS IN ACID
Genes regulated in M. tuberculosis at pH 5.5 were identified using DNA microarrays (29). Several genes involved in fatty acid metabolism, such as icl (isocitrate lyase), and many with homology to nonribosomal peptide synthetases and polyketide synthases were induced in acid. Among other genes, the kas/FASII operon, which is involved in mycolic acid biosynthesis, was repressed upon exposure to acid. Thus, M. tuberculosis responds transcriptionally to acid in vitro, and this may mimic M. tuberculosis's adaptation to the acid conditions of the phagosome. However, these and other gene expression studies were conducted in a medium containing the detergent Tween 80, which as mentioned above can be hydrolyzed to release the free fatty acid oleic acid. Therefore, the induction of some of these genes, particularly those involved in fatty acid metabolism, may in fact be a response to other components of the medium, such as Tween 80, along with, in synergy with, or even instead of acid. To examine the importance of acid-induced genes in virulence, a deletion mutant of an operon (Rv3083-Rv3089) that was highly induced (17- to 33-fold) upon acid exposure was constructed (17). The mutant was not attenuated at pH 5.5 in vitro; however, it was deficient for growth in macrophages (17). Whether attenuation of the mutant in macrophages was due to susceptibility to phagosomal acid or to some other aspect of the host environment remains unknown.
In another study, an in vivo expression promoter trap system was used to identify the promoter regions of two genes activated at pH 4.5, lipF and Rv0834c (80). LipF is annotated as a lipase/esterase, and Rv0834c encodes a PE-PGRS (proline-glutamic acid-polymorphic GC-rich repetitive sequence) family protein. PE-PGRS proteins are only present in pathogenic mycobacteria, and their function remains largely unknown. The authors postulate that lipF may serve to hydrolyze toxic fatty acids present in macrophages during infection or may modify the cell wall of the bacterium. The promoters of lipF and Rv0834c were not induced in resting or IFN-γ-activated J774 cells (80); however, lipF transcription was induced in early phagosomes of bone marrow-derived mouse macrophages (73). A M. tuberculosis lipF transposon mutant was attenuated for growth in mouse lungs (15). Whether the lipF mutant is sensitive to acid in vitro has not been reported.
M. tuberculosis alters gene expression in response to the low pH of the macrophage phagosome (73). To define the phagosomal acid-regulated transcriptome, gene induction of intracellular M. tuberculosis was compared in macrophages that were untreated or treated with the vacuolar ATPase inhibitor concanamycin A, which prevents phagosome acidification. Twenty-four concanamycin A-sensitive genes were identified, representing the acid-responsive M. tuberculosis transcriptome. Many of these acid response genes, including lipF, belong to the regulon of the two-component system PhoP. PhoP is involved in regulation of lipid biosynthesis genes in M. tuberculosis (36, 101), suggesting that the mycobacterial lipidome plays an important role in acid resistance (73). In the enterobacteria Salmonella and E. coli, PhoP also acts as a pH response regulator (7, 31). The 24 acid-induced genes of M. tuberculosis overlapped to a large degree with those induced by pH 5.5 and 6.5 in vitro, indicating that even mild acidification serves as a signal for adaptation of M. tuberculosis to the environment of the early phagosome (73). It will be interesting to examine the function of these genes in the mycobacterial response to acid and whether their absence attenuates survival in the acidic phagosome. M. tuberculosis possesses proteins whose activity is optimal at acidic pH (1, 91); however, mutants in genes encoding these proteins were not attenuated during mouse infections (12, 23). Nonetheless, delineation of gene and protein networks involved in the low-pH response provides important insights into mycobacterial virulence strategies and may reveal novel targets for chemotherapy.
CONCLUDING REMARKS
Systems involved in cytosolic pH homeostasis are critical for the survival of all organisms and are particularly important for those that are exposed to high concentrations of extracellular protons during their life cycles, including during infection of hosts. Upon inhalation into the lung, M. tuberculosis is engulfed by macrophages into phagosomes. Macrophages contain acidic lysosomes that are involved in the digestion and clearance of invading microorganisms. Although M. tuberculosis can block phagosome-lysosome fusion, this process is set in motion once macrophages have been activated by IFN-γ (55, 81, 99). In vitro, in the absence of detergents or albumin, both of which can release fatty acids at low pH, M. tuberculosis is able to maintain its intrabacterial pH and survive at a pH of 4.5. Similarly, M. tuberculosis can maintain its intrabacterial pH and survive in the acidic phagolysosomes of activated macrophages. Therefore, it is likely that this pathogen is able to tolerate the acidity of the phagolysosome during chronic infection of its host. It can be proposed that M. tuberculosis resists acid in the macrophage by two means: one is to restrict fusion of phagosomes with lysosomes, and the second is to resist killing within the acidic phagolysosomal compartment after phagosome-lysosome fusion.
Several mechanisms used by M. tuberculosis to prevent fusion of phagosomes with lysosomes have recently been identified (97). However, much less is known about how M. tuberculosis is able to resist the acidic environment of the arrested phagosome or the mature phagolysosome. The identification of acid-regulated genes, as well as M. tuberculosis proteins whose activity is increased at low pH, indicates that the low pH of the phagosome is an important cue for adaptation within the host niche and that the bacterium is equipped to cope with this stress (1, 73, 91). Once within the low-pH environment, acid resistance mechanisms become critical for survival. Accordingly, several mutants of M. tuberculosis that are acid sensitive in vitro are attenuated in animal models of infection (14, 71, 95, 96). The mycobacterial cell wall appears to play a critical role in survival at low pH, but the molecular mechanisms need to be understood, and additional pathways involved in mycobacterial acid resistance remain to be identified. Knowledge of these intracellular survival mechanisms is likely to lead to the identification of new drug targets and development of chemotherapeutics for the treatment of tuberculosis.
Acknowledgments
Work by the authors was funded by the I. T. Hirschl Trust (S.E.) and NIH P01 grant AI056293 (C.F.N.). The Department of Microbiology and Immunology acknowledges the support of the William Randolph Hearst Foundation.
We thank the anonymous reviewers for constructive comments.
Footnotes
Published ahead of print on 22 May 2009.
REFERENCES
- 1.Agranoff, D., I. M. Monahan, J. A. Mangan, P. D. Butcher, and S. Krishna. 1999. Mycobacterium tuberculosis expresses a novel pH-dependent divalent cation transporter belonging to the Nramp family. J. Exp. Med. 190717-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akaki, T., H. Tomioka, T. Shimizu, S. Dekio, and K. Sato. 2000. Comparative roles of free fatty acids with reactive nitrogen intermediates and reactive oxygen intermediates in expression of the anti-microbial activity of macrophages against Mycobacterium tuberculosis. Clin. Exp. Immunol. 121302-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alix, E., and A. B. Blanc-Potard. 2007. MgtC: a key player in intramacrophage survival. Trends Microbiol. 15252-256. [DOI] [PubMed] [Google Scholar]
- 4.Alonso, S., K. Pethe, D. G. Russell, and G. E. Purdy. 2007. Lysosomal killing of Mycobacterium mediated by ubiquitin-derived peptides is enhanced by autophagy. Proc. Natl. Acad. Sci. USA 1046031-6036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armstrong, J. A., and P. D. Hart. 1975. Phagosome-lysosome interactions in cultured macrophages infected with virulent tubercle bacilli. Reversal of the usual nonfusion pattern and observations on bacterial survival. J. Exp. Med. 1421-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Armstrong, J. A., and P. D. Hart. 1971. Response of cultured macrophages to Mycobacterium tuberculosis, with observations on fusion of lysosomes with phagosomes. J. Exp. Med. 134713-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Audia, J. P., C. C. Webb, and J. W. Foster. 2001. Breaking through the acid barrier: an orchestrated response to proton stress by enteric bacteria. Int. J. Med. Microbiol. 29197-106. [DOI] [PubMed] [Google Scholar]
- 8.Barnes, P. F., S. J. Fong, P. J. Brennan, P. E. Twomey, A. Mazumder, and R. L. Modlin. 1990. Local production of tumor necrosis factor and IFN-gamma in tuberculous pleuritis. J. Immunol. 145149-154. [PubMed] [Google Scholar]
- 9.Beauregard, K. E., K. D. Lee, R. J. Collier, and J. A. Swanson. 1997. pH-dependent perforation of macrophage phagosomes by listeriolysin O from Listeria monocytogenes. J. Exp. Med. 1861159-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benjamin, N., F. O'Driscoll, H. Dougall, C. Duncan, L. Smith, M. Golden, and H. McKenzie. 1994. Stomach NO synthesis. Nature 368502. [DOI] [PubMed] [Google Scholar]
- 11.Bodmer, T., E. Miltner, and L. E. Bermudez. 2000. Mycobacterium avium resists exposure to the acidic conditions of the stomach. FEMS Microbiol. Lett. 18245-49. [DOI] [PubMed] [Google Scholar]
- 12.Boechat, N., B. Lagier-Roger, S. Petit, Y. Bordat, J. Rauzier, A. J. Hance, B. Gicquel, and J.-M. Reyrat. 2002. Disruption of the gene homologous to mammalian Nramp1 in Mycobacterium tuberculosis does not affect virulence in mice. Infect. Immun. 704124-4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Booth, I. R. 1985. Regulation of cytoplasmic pH in bacteria. Microbiol. Rev. 49359-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buchmeier, N., A. Blanc-Potard, S. Ehrt, D. Piddington, L. Riley, and E. A. Groisman. 2000. A parallel intraphagosomal survival strategy shared by Mycobacterium tuberculosis and Salmonella enterica. Mol. Microbiol. 351375-1382. [DOI] [PubMed] [Google Scholar]
- 15.Camacho, L. R., D. Ensergueix, E. Perez, B. Gicquel, and C. Guilhot. 1999. Identification of a virulence gene cluster of Mycobacterium tuberculosis by signature-tagged transposon mutagenesis. Mol. Microbiol. 34257-267. [DOI] [PubMed] [Google Scholar]
- 16.Chapman, J. S., and J. S. Bernard. 1962. The tolerances of unclassified mycobacteria. I. Limits of pH tolerance. Am. Rev. Respir. Dis. 86582-583. [DOI] [PubMed] [Google Scholar]
- 17.Cheruvu, M., B. B. Plikaytis, and T. M. Shinnick. 2007. The acid-induced operon Rv3083-Rv3089 is required for growth of Mycobacterium tuberculosis in macrophages. Tuberculosis 8712-20. [DOI] [PubMed] [Google Scholar]
- 18.Choi, H. S., P. R. Rai, H. W. Chu, C. Cool, and E. D. Chan. 2002. Analysis of nitric oxide synthase and nitrotyrosine expression in human pulmonary tuberculosis. Am. J. Respir. Crit. Care Med. 166178-186. [DOI] [PubMed] [Google Scholar]
- 19.Cole, S. T., R. Brosch, J. Parkhill, T. Garnier, C. Churcher, D. Harris, S. V. Gordon, K. Eiglmeier, S. Gas, C. E. Barry III, F. Tekaia, K. Badcock, D. Basham, D. Brown, T. Chillingworth, R. Connor, R. Davies, K. Devlin, T. Feltwell, S. Gentles, N. Hamlin, S. Holroyd, T. Hornsby, K. Jagels, A. Krogh, J. McLean, S. Moule, L. Murphy, K. Oliver, J. Osborne, M. A. Quail, M. A. Rajandream, J. Rogers, S. Rutter, K. Seeger, J. Skelton, R. Squares, S. Squares, J. E. Sulston, K. Taylor, S. Whitehead, and B. G. Barrell. 1998. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393537-544. [DOI] [PubMed] [Google Scholar]
- 20.Coombes, B. K., N. F. Brown, Y. Valdez, J. H. Brumell, and B. B. Finlay. 2004. Expression and secretion of Salmonella pathogenicity island-2 virulence genes in response to acidification exhibit differential requirements of a functional type III secretion apparatus and SsaL. J. Biol. Chem. 27949804-49815. [DOI] [PubMed] [Google Scholar]
- 21.Cotter, P. D., and C. Hill. 2003. Surviving the acid test: responses of gram-positive bacteria to low pH. Microbiol. Mol. Biol. Rev. 67429-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davis, B. D., and R. J. Dubos. 1947. The binding of fatty acids by serum albumin, a protective growth factor in bacteriological media. J. Exp. Med. 86215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dittrich, D., C. Keller, S. Ehlers, J. E. Schultz, and P. Sander. 2006. Characterization of a Mycobacterium tuberculosis mutant deficient in pH-sensing adenylate cyclase Rv1264. Int. J. Med. Microbiol. 296563-566. [DOI] [PubMed] [Google Scholar]
- 24.Dubos, R. 1947. The effect of lipids and serum albumin on bacterial growth. J. Exp. Med. 859-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dubos, R. J. 1954. Biochemical determinants of microbial disease, p. 42. Harvard University Press, Cambridge, MA.
- 26.Ehrt, S., D. Schnappinger, S. Bekiranov, J. Drenkow, S. Shi, T. R. Gingeras, T. Gaasterland, G. Schoolnik, and C. Nathan. 2001. Reprogramming of the macrophage transcriptome in response to interferon-gamma and Mycobacterium tuberculosis: signaling roles of nitric oxide synthase-2 and phagocyte oxidase. J. Exp. Med. 1941123-1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.El Ghachi, M., A. Bouhss, D. Blanot, and D. Mengin-Lecreulx. 2004. The bacA gene of Escherichia coli encodes an undecaprenyl pyrophosphate phosphatase activity. J. Biol. Chem. 27930106-30113. [DOI] [PubMed] [Google Scholar]
- 28.Facchetti, F., W. Vermi, S. Fiorentini, M. Chilosi, A. Caruso, M. Duse, L. D. Notarangelo, and R. Badolato. 1999. Expression of inducible nitric oxide synthase in human granulomas and histiocytic reactions. Am. J. Pathol. 154145-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fisher, M. A., B. B. Plikaytis, and T. M. Shinnick. 2002. Microarray analysis of the Mycobacterium tuberculosis transcriptional response to the acidic conditions found in phagosomes. J. Bacteriol. 1844025-4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Foster, J. W. 2004. Escherichia coli acid resistance: tales of an amateur acidophile. Nat. Rev. Microbiol. 2898-907. [DOI] [PubMed] [Google Scholar]
- 31.Foster, J. W. 2000. Microbial responses to acid stress, p. 99-115. In G. Storz and R. Hennge-Aronis (ed.), Bacterial stress responses. ASM Press, Washington, DC.
- 32.Foster, J. W. 1991. Salmonella acid shock proteins are required for the adaptive acid tolerance response. J. Bacteriol. 1736896-6902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Foster, J. W. 1999. When protons attack: microbial strategies of acid adaptation. Curr. Opin. Microbiol. 2170-174. [DOI] [PubMed] [Google Scholar]
- 34.Foster, J. W., and M. P. Spector. 1995. How Salmonella survive against the odds. Annu. Rev. Microbiol. 49145-174. [DOI] [PubMed] [Google Scholar]
- 35.Gomes, M. S., S. Paul, A. L. Moreira, R. Appelberg, M. Rabinovitch, and G. Kaplan. 1999. Survival of Mycobacterium avium and Mycobacterium tuberculosis in acidified vacuoles of murine macrophages. Infect. Immun. 673199-3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gonzalo-Asensio, J., S. Mostowy, J. Harders-Westerveen, K. Huygen, R. Hernandez-Pando, J. Thole, M. Behr, B. Gicquel, and C. Martin. 2008. PhoP: a missing piece in the intricate puzzle of Mycobacterium tuberculosis virulence. PLoS ONE 3e3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gordon, A. H., P. D. Hart, and M. R. Young. 1980. Ammonia inhibits phagosome-lysosome fusion in macrophages. Nature 28679-80. [DOI] [PubMed] [Google Scholar]
- 38.Grode, L., P. Seiler, S. Baumann, J. Hess, V. Brinkmann, A. Nasser Eddine, P. Mann, C. Goosmann, S. Bandermann, D. Smith, G. J. Bancroft, J. M. Reyrat, D. van Soolingen, B. Raupach, and S. H. Kaufmann. 2005. Increased vaccine efficacy against tuberculosis of recombinant Mycobacterium bovis bacille Calmette-Guerin mutants that secrete listeriolysin. J. Clin. Investig. 1152472-2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Günzel, D., L. M. Kucharski, D. G. Kehres, M. F. Romero, and M. E. Maguire. 2006. The MgtC virulence factor of Salmonella enterica serovar Typhimurium activates Na+,K+-ATPase. J. Bacteriol. 1885586-5594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heinzen, R. A., M. A. Scidmore, D. D. Rockey, and T. Hackstadt. 1996. Differential interaction with endocytic and exocytic pathways distinguish parasitophorous vacuoles of Coxiella burnetii and Chlamydia trachomatis. Infect. Immun. 64796-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoffmann, C., A. Leis, M. Niederweis, J. M. Plitzko, and H. Engelhardt. 2008. Disclosure of the mycobacterial outer membrane: cryo-electron tomography and vitreous sections reveal the lipid bilayer structure. Proc. Natl. Acad. Sci. USA 1053963-3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huynh, K. K., and S. Grinstein. 2007. Regulation of vacuolar pH and its modulation by some microbial species. Microbiol. Mol. Biol. Rev. 71452-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iivanainen, E., P. J. Martikainen, P. Vaananen, and M. L. Katila. 1999. Environmental factors affecting the occurrence of mycobacteria in brook sediments. J. Appl. Microbiol. 86673-681. [DOI] [PubMed] [Google Scholar]
- 44.Jackett, P. S., V. R. Aber, and D. B. Lowrie. 1978. Virulence and resistance to superoxide, low pH and hydrogen peroxide among strains of Mycobacterium tuberculosis. J. Gen. Microbiol. 10437-45. [DOI] [PubMed] [Google Scholar]
- 45.Kanai, K., and E. Kondo. 1979. Antibacterial and cytotoxic aspects of long-chain fatty acids as cell surface events: selected topics. Jpn. J. Med. Sci. Biol. 32135-174. [DOI] [PubMed] [Google Scholar]
- 46.Klebanoff, S. J. 2005. Myeloperoxidase: friend and foe. J. Leukoc. Biol. 77598-625. [DOI] [PubMed] [Google Scholar]
- 47.Kondo, E., and K. Kanai. 1976. Further studies on the lethal effect of long-chain fatty acids on mycobacteria. Jpn. J. Med. Sci. Biol. 2925-37. [DOI] [PubMed] [Google Scholar]
- 48.Kondo, E., and K. Kanai. 1972. The lethal effect of long-chain fatty acids on mycobacteria. Jpn. J. Med. Sci. Biol. 251-13. [DOI] [PubMed] [Google Scholar]
- 49.Lin, J., I. S. Lee, J. Frey, J. L. Slonczewski, and J. W. Foster. 1995. Comparative analysis of extreme acid survival in Salmonella typhimurium, Shigella flexneri, and Escherichia coli. J. Bacteriol. 1774097-4104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lis, M., and H. K. Kuramitsu. 2003. The stress-responsive dgk gene from Streptococcus mutans encodes a putative undecaprenol kinase activity. Infect. Immun. 711938-1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lynn, M., A. R. Wilson, and M. Solotorovsky. 1979. Role of bovine serum albumin in the nutrition of Mycobacterium tuberculosis. Appl. Environ. Microbiol. 38806-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.MacGurn, J. A., and J. S. Cox. 2007. A genetic screen for Mycobacterium tuberculosis mutants defective for phagosome maturation arrest identifies components of the ESX-1 secretion system. Infect. Immun. 752668-2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.MacMicking, J., Q. W. Xie, and C. Nathan. 1997. Nitric oxide and macrophage function. Annu. Rev. Immunol. 15323-350. [DOI] [PubMed] [Google Scholar]
- 54.MacMicking, J. D., R. J. North, R. LaCourse, J. S. Mudgett, S. K. Shah, and C. F. Nathan. 1997. Identification of nitric oxide synthase as a protective locus against tuberculosis. Proc. Natl. Acad. Sci. USA 945243-5248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.MacMicking, J. D., G. A. Taylor, and J. D. McKinney. 2003. Immune control of tuberculosis by IFN-gamma-inducible LRG-47. Science 302654-659. [DOI] [PubMed] [Google Scholar]
- 56.Mantegazza, A. R., A. Savina, M. Vermeulen, L. Perez, J. Geffner, O. Hermine, S. D. Rosenzweig, F. Faure, and S. Amigorena. 2008. NADPH oxidase controls phagosomal pH and antigen cross-presentation in human dendritic cells. Blood 1124712-4722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mates, A. K., A. K. Sayed, and J. W. Foster. 2007. Products of the Escherichia coli acid fitness island attenuate metabolite stress at extremely low pH and mediate a cell density-dependent acid resistance. J. Bacteriol. 1892759-2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maurer, L. M., E. Yohannes, S. S. Bondurant, M. Radmacher, and J. L. Slonczewski. 2005. pH regulates genes for flagellar motility, catabolism, and oxidative stress in Escherichia coli K-12. J. Bacteriol. 187304-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maurin, M., A. M. Benoliel, P. Bongrand, and D. Raoult. 1992. Phagolysosomes of Coxiella burnetii-infected cell lines maintain an acidic pH during persistent infection. Infect. Immun. 605013-5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McKnight, G. M., L. M. Smith, R. S. Drummond, C. W. Duncan, M. Golden, and N. Benjamin. 1997. Chemical synthesis of nitric oxide in the stomach from dietary nitrate in humans. Gut 40211-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Metchnikoff, E. 1905. Immunity to infective disease, p. 182. Cambridge University Press, Cambridge, United Kingdom.
- 62.Molle, V., N. Saint, S. Campagna, L. Kremer, E. Lea, P. Draper, and G. Molle. 2006. pH-dependent pore-forming activity of OmpATb from Mycobacterium tuberculosis and characterization of the channel by peptidic dissection. Mol. Microbiol. 61826-837. [DOI] [PubMed] [Google Scholar]
- 63.Moncrief, M. B. C., and M. E. Macguire. 1998. Magnesium and the role of MgtC in growth of Salmonella typhimurium. Infect. Immun. 663802-3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mwandumba, H. C., D. G. Russell, M. H. Nyirenda, J. Anderson, S. A. White, M. E. Molyneux, and S. B. Squire. 2004. Mycobacterium tuberculosis resides in nonacidified vacuoles in endocytically competent alveolar macrophages from patients with tuberculosis and HIV infection. J. Immunol. 1724592-4598. [DOI] [PubMed] [Google Scholar]
- 65.Nathan, C. F., and S. Ehrt. 2004. Nitric oxide in tuberculosis, p. 215-235. In W. N. Rom and S. M. Garay (ed.), Tuberculosis, 2nd ed. Lippincott, Williams & Wilkins, Philadelphia, PA.
- 66.O'Brien, L. M., S. V. Gordon, I. S. Roberts, and P. W. Andrew. 1996. Response of Mycobacterium smegmatis to acid stress. FEMS Microbiol. Lett. 13911-17. [DOI] [PubMed] [Google Scholar]
- 67.Ohkuma, S., and B. Poole. 1978. Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents. Proc. Natl. Acad. Sci. USA 753327-3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pethe, K., D. L. Swenson, S. Alonso, J. Anderson, C. Wang, and D. G. Russell. 2004. Isolation of Mycobacterium tuberculosis mutants defective in the arrest of phagosome maturation. Proc. Natl. Acad. Sci. USA 10113642-13647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Portaels, F., and S. R. Pattyn. 1982. Growth of mycobacteria in relation to the pH of the medium. Ann. Microbiol. (Paris) 133213-221. [PubMed] [Google Scholar]
- 70.Rao, M., T. L. Streur, F. E. Aldwell, and G. M. Cook. 2001. Intracellular pH regulation by Mycobacterium smegmatis and Mycobacterium bovis BCG. Microbiology 1471017-1024. [DOI] [PubMed] [Google Scholar]
- 71.Raynaud, C., K. G. Papavinasasundaram, R. A. Speight, B. Springer, P. Sander, E. C. Bottger, M. J. Colston, and P. Draper. 2002. The functions of OmpATb, a pore-forming protein of Mycobacterium tuberculosis. Mol. Microbiol. 46191-201. [DOI] [PubMed] [Google Scholar]
- 72.Reyrat, J.-M., G. Lopez-Ramirez, C. Ofredo, B. Gicquel, and N. Winter. 1996. Urease activity does not contribute dramatically to persistence of Mycobacterium bovis bacillus Calmette-Guerin. Infect. Immun. 643934-3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rohde, K. H., R. B. Abramovitch, and D. G. Russell. 2007. Mycobacterium tuberculosis invasion of macrophages: linking bacterial gene expression to environmental cues. Cell Host Microbe 2352-364. [DOI] [PubMed] [Google Scholar]
- 74.Rous, P. 1925. The relative reaction within living mammalian tissues. I. General features of vital staining with litmus. J. Exp. Med. 41379-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rous, P. 1925. The relative reaction within living mammalian tissues. II. On the mobilization of acid material within cells, and the reaction as influenced by the cell state. J. Exp. Med. 41399-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rowbury, R. J., N. H. Hussain, and M. Goodson. 1998. Extracellular proteins and other components as obligate intermediates in the induction of a range of acid tolerance and sensitisation responses in Escherichia coli. FEMS Microbiol. Lett. 166283-288. [DOI] [PubMed] [Google Scholar]
- 77.Russell, D. G. 2001. Mycobacterium tuberculosis: here today, and here tomorrow. Nat. Rev. Mol. Cell Biol. 2569-577. [DOI] [PubMed] [Google Scholar]
- 78.Sachs, G., D. L. Weeks, K. Melchers, and D. R. Scott. 2003. The gastric biology of Helicobacter pylori. Annu. Rev. Physiol. 65349-369. [DOI] [PubMed] [Google Scholar]
- 79.Savina, A., C. Jancic, S. Hugues, P. Guermonprez, P. Vargas, I. C. Moura, A. M. Lennon-Dumenil, M. C. Seabra, G. Raposo, and S. Amigorena. 2006. NOX2 controls phagosomal pH to regulate antigen processing during crosspresentation by dendritic cells. Cell 126205-218. [DOI] [PubMed] [Google Scholar]
- 80.Saviola, B., S. C. Woolwine, and W. R. Bishai. 2003. Isolation of acid-inducible genes of Mycobacterium tuberculosis with the use of recombinase-based in vivo expression technology. Infect. Immun. 711379-1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schaible, U. E., S. Sturgill-Koszycki, P. H. Schlesinger, and D. G. Russell. 1998. Cytokine activation leads to acidification and increases maturation of Mycobacterium avium-containing phagosomes in murine macrophages. J. Immunol. 1601290-1296. [PubMed] [Google Scholar]
- 82.Schon, T. 2002. Nitric oxide in tuberculosis and leprosy. Linkoping University, Linkoping, Sweden.
- 83.Schon, T., G. Elmberger, Y. Negesse, R. H. Pando, T. Sundqvist, and S. Britton. 2004. Local production of nitric oxide in patients with tuberculosis. Int. J. Tuberc. Lung Dis. 81134-1137. [PubMed] [Google Scholar]
- 84.Sibley, L. D., S. G. Franzblau, and J. L. Krahenbuhl. 1987. Intracellular fate of Mycobacterium leprae in normal and activated mouse macrophages. Infect. Immun. 55680-685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sprick, M. G. 1956. Phagocytosis of M. tuberculosis and M. smegmatis stained with indicator dyes. Am. Rev. Tuberc. 74552-565. [DOI] [PubMed] [Google Scholar]
- 86.Stewart, G. R., J. Patel, B. D. Robertson, A. Rae, and D. B. Young. 2005. Mycobacterial mutants with defective control of phagosomal acidification. PLoS Pathog. 1269-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Stuehr, D. J., and C. F. Nathan. 1989. Nitric oxide. A macrophage product responsible for cytostasis and respiratory inhibition in tumor target cells. J. Exp. Med. 1691543-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sturgill-Koszycki, S., P. H. Schlesinger, P. Chakraborty, P. L. Haddix, H. L. Collins, A. K. Fok, R. D. Allen, S. L. Gluck, J. Heuser, and D. G. Russell. 1994. Lack of acidification in Mycobacterium phagosomes produced by exclusion of the vesicular proton-ATPase. Science 263678-681. [DOI] [PubMed] [Google Scholar]
- 89.Sturgill-Koszycki, S., and M. S. Swanson. 2000. Legionella pneumophila replication vacuoles mature into acidic, endocytic organelles. J. Exp. Med. 1921261-1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sung, N., and M. T. Collins. 2003. Variation in resistance of Mycobacterium paratuberculosis to acid environments as a function of culture medium. Appl. Environ. Microbiol. 696833-6840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tews, I., F. Findeisen, I. Sinning, A. Schultz, J. E. Schultz, and J. U. Linder. 2005. The structure of a pH-sensing mycobacterial adenylyl cyclase holoenzyme. Science 3081020-1023. [DOI] [PubMed] [Google Scholar]
- 92.Tran, S. L., M. Rao, C. Simmers, S. Gebhard, K. Olsson, and G. M. Cook. 2005. Mutants of Mycobacterium smegmatis unable to grow at acidic pH in the presence of the protonophore carbonyl cyanide m-chlorophenylhydrazone. Microbiology 151665-672. [DOI] [PubMed] [Google Scholar]
- 93.Turk, V., B. Turk, and D. Turk. 2001. Lysosomal cysteine proteases: facts and opportunities. EMBO J. 204629-4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vandal, O. H., M. H. Gelb, S. Ehrt, and C. F. Nathan. 2006. Cytosolic phospholipase A2 enzymes are not required by mouse bone marrow-derived macrophages for the control of Mycobacterium tuberculosis in vitro. Infect. Immun. 741751-1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vandal, O. H., L. M. Pierini, D. Schnappinger, C. F. Nathan, and S. Ehrt. 2008. A membrane protein preserves intrabacterial pH in intraphagosomal Mycobacterium tuberculosis. Nat. Med. 14849-854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Vandal, O. H., J. A. Roberts, T. Odaira, D. Schnappinger, C. F. Nathan, and S. Ehrt. 2009. Acid-susceptible mutants of Mycobacterium tuberculosis share hypersusceptibility to cell wall and oxidative stress and to the host environment. J. Bacteriol. 191625-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Vergne, I., J. Chua, S. B. Singh, and V. Deretic. 2004. Cell biology of Mycobacterium tuberculosis phagosome. Annu. Rev. Cell Dev. Biol. 20367-394. [DOI] [PubMed] [Google Scholar]
- 98.Via, L. E., D. Deretic, R. J. Ulmer, N. S. Hibler, L. A. Huber, and V. Deretic. 1997. Arrest of mycobacterial phagosome maturation is caused by a block in vesicle fusion between stages controlled by rab5 and rab7. J. Biol. Chem. 27213326-13331. [DOI] [PubMed] [Google Scholar]
- 99.Via, L. E., R. A. Fratti, M. McFalone, E. Pagan-Ramos, D. Deretic, and V. Deretic. 1998. Effects of cytokines on mycobacterial phagosome maturation. J. Cell Sci. 111897-905. [DOI] [PubMed] [Google Scholar]
- 100.Walker, J. J., J. R. Spear, and N. R. Pace. 2005. Geobiology of a microbial endolithic community in the Yellowstone geothermal environment. Nature 4341011-1014. [DOI] [PubMed] [Google Scholar]
- 101.Walters, S. B., E. Dubnau, I. Kolesnikova, F. Laval, M. Daffe, and I. Smith. 2006. The Mycobacterium tuberculosis PhoPR two-component system regulates genes essential for virulence and complex lipid biosynthesis. Mol. Microbiol. 60312-330. [DOI] [PubMed] [Google Scholar]
- 102.Yamashita, Y., T. Takehara, and H. K. Kuramitsu. 1993. Molecular characterization of a Streptococcus mutans mutant altered in environmental stress responses. J. Bacteriol. 1756220-6228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yates, R. M., A. Hermetter, G. A. Taylor, and D. G. Russell. 2007. Macrophage activation downregulates the degradative capacity of the phagosome. Traffic 8241-250. [DOI] [PubMed] [Google Scholar]
- 104.Zhang, Y., and D. Mitchison. 2003. The curious characteristics of pyrazinamide: a review. Int. J. Tuberc. Lung Dis. 76-21. [PubMed] [Google Scholar]
- 105.Zhang, Y., A. Scorpio, H. Nikaido, and Z. Sun. 1999. Role of acid pH and deficient efflux of pyrazinoic acid in unique susceptibility of Mycobacterium tuberculosis to pyrazinamide. J. Bacteriol. 1812044-2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhu, H., C. A. Hart, D. Sales, and N. B. Roberts. 2006. Bacterial killing in gastric juice—effect of pH and pepsin on Escherichia coli and Helicobacter pylori. J. Med. Microbiol. 551265-1270. [DOI] [PubMed] [Google Scholar]
- 107.Zuber, B., M. Chami, C. Houssin, J. Dubochet, G. Griffiths, and M. Daffe. 2008. Direct visualization of the outer membrane of mycobacteria and corynebacteria in their native state. J. Bacteriol. 1905672-5680. [DOI] [PMC free article] [PubMed] [Google Scholar]