Abstract
We evaluated the safety, reactogenicity and immunogenicity of escalating doses of a new Francisella tularensis Live Vaccine Strain (LVS) lot by scarification (SCAR) or subcutaneously (SQ) in humans. Subjects (N=10/group) received one dose of LVS via SCAR at 105, 107 or 109 cfu/ml or SQ at 102, 103, 104 or 105 cfu/ml; 14 subjects received placebo. All doses/routes were well tolerated. When compared to placebo, vaccination with 107SCAR and 109SCAR resulted in significantly higher serologic response frequencies, as measured by ELISA for IgG, IgM, IgA and microagglutination; whereas vaccination with 105SCAR, 107SCAR 109SCAR and 105SQ elicited a significantly higher interferon-γ response frequency.
Keywords: clinical trial, tularemia vaccine, live vaccine strain, safety and immunogenicity
Introduction
Francisella tularensis (Ft), the causative agent of tularemia, is one of the most infectious organisms to humans: as few as 25 colony forming units can cause significant respiratory disease [1-3]. It also displays a very complex ecology, infecting more than 250 species, with amoebas acting as a potential reservoir [4, 5]. The disease epidemiology in humans is characterized by the presence of disease “hotspots” and by the periodic emergence of disease in areas where disease was previously not recognized, mostly mirroring epizoonotic changes [6, 7].
There are 4 subspecies within the Ft species: tularensis, holarctica, mediasiatica and novicida [8]. The organism is transmitted to humans in a myriad of ways: direct contact with infected animals, ingestion of contaminated water, inhalation of aerosolized organisms or via vectors that include mosquitoes, ticks and flies. Clinical manifestations depend on the route of exposure and the Ft subspecies, with a case fatality rate reaching 30% in untreated cases of typhoidal or respiratory disease [9-11]. Ft subsp. tularensis is the most virulent of the subspecies, causing the most severe disease, albeit with a restricted geographic distribution. The high morbidity and mortality of tularemia, its potential for aerosolization, its low infectious dose and the ease of propagating the organism in vitro have raised concerns about its potential use as a biological weapon. In fact, the USA, USSR and Japan have stockpiled the organism as a weapon in the past, and Ft is classified as a category A select agent by the Centers for Disease Control and Prevention [11-13]. This recent classification has resulted in renewed interest in tularemia vaccines.
Two tularemia vaccines have been studied in humans in the US: the killed vaccine (Foshay) and the live vaccine strain (LVS). Kadull and colleagues immunized individuals with the killed vaccine and, in non-controlled trials, showed limited efficacy in preventing the disease and its severity [14]. The live vaccine was developed in the former Soviet Union from a Ft subsp holarctica strain and was given to millions of individuals to contain outbreaks. In 1956 the Soviet government provided the live vaccine to scientists at Fort Detrick, Maryland. Two colony variants were identified: blue and gray [15]. The blue colony variant was more immunogenic in animals and was designated LVS. The efficacy of Ft LVS was initially evaluated using two routes: inhalation and scarification. The superiority of the Ft LVS over the Foshay vaccine was demonstrated by Saslaw et al who showed that subjects who received LVS by scarification were less likely to develop signs of tularemia following an aerosol challenge; a protection that was later shown to be overcome with increasing the aerosol challenge dose [1, 3]. Hornick et al demonstrated that individuals immunized with 108 LVS organisms via the aerosol route were better protected against a high-dose aerosol challenge with Ft than individuals immunized with LVS via scarification or via a lower dose aerosol [16]. However, due to the logistical constraints of aerosolization, the scarification method was adopted thereafter in the US.
Ft LVS was administered under investigational protocols for many years and was shown to be associated with significant reduction in laboratory-acquired tularemia [17, 18]. A correlate of protection for tularemia has not been identified; however, the literature suggests that the high antibody titers that follow vaccination or infection serve as markers of exposure, while the cell mediated immune response is more closely related to protection [19, 20].
The Ft LVS vaccine was never licensed for use in humans in the US, due to uncertainty about the mechanism of attenuation, concern about reversion to a virulent phenotype and the research-grade production methods. Under a contract from the Joint Vaccine Acquisition Program, Dynport Vaccine Company (DVC) manufactured a new vaccine lot using good manufacturing practices (GMP). Preclinical evaluation of the newly derived lot of Ft LVS in rabbits at escalating dosages of 105 cfu to 109 cfu by the intradermal, subcutaneous (SQ) routes and by scarification (SCAR) demonstrated its safety and immunogenicity as measured by antibody levels [21]. The findings from the preclinical study provided reassurance to proceed with the evaluation of escalating vaccine doses of the new lot in humans using two routes: SCAR and the more quantitative and convenient SQ route.
Methods and Definitions
Subjects
Study participants were healthy 18-40 year old adults. We excluded subjects on the basis of any of the following: pregnancy, inability or unwillingness to use acceptable methods of contraception, current or recent use of antibiotics or immunomodulatory agents, history of splenectomy, abnormal laboratory values, history of or current drug abuse, history of or current severe mental illness, receipt of blood or blood products in the 3 months prior to enrollment, receipt of a live vaccine 30 days or a non-replicating vaccine 14 days prior to enrollment, previous vaccination against tularemia, previous treatment for tularemia, previous exposure to tularin, hypersensitivity to gelatin, sensitivity to tetracyclines or streptomycin, history of significant medical illnesses, history of anaphylaxis or serious adverse event following immunizations, acute febrile illness within a week of enrollment, positive serology for human immunodeficiency virus, hepatitis C virus or hepatitis B surface antigen, participation in other investigational protocols, significant contact with infants, pregnant women or immunosuppressed individuals that is expected to occur in the 14 days after vaccination or as long as skin lesions persist, history of significant skin disease (eczema, psoriasis, or keloid), presence of prosthetic implants or history of cancer.
Vaccine
The vaccine was manufactured at Cambrex Bio Science, Baltimore, under contract with DVC. The vaccine cell suspension was mixed with formulation buffer (10 mM potassium phosphate, 10% sucrose and 1.3% gelatin) and filled into 1 mL single dose vials and lyophilized. The final product was stored at -20± 10°C. Prior to the inoculation of subjects, the lyophilized vaccine was mixed with 0.25 mL of sterile water for injection yielding an approximate concentration of 109 cfu/mL. Serial tenfold dilutions of the reconstituted product with sterile saline were performed to reach the desired concentration of the vaccine. The achieved concentration was confirmed by plating a 100μl of the final product on duplicate Glucose Cysteine Blood Agar plates. At all the tested vaccine concentrations, the colony counts on plating were in agreement with the target dose level.
Study design
The study was a single center, placebo-controlled, randomized, dose-escalating study to assess the safety of Ft LVS vaccine administered once via one of two routes: SCAR or SQ. The vaccine doses were: 105 cfu/ml SCAR (105SCAR), 107 cfu/ml SCAR (107SCAR), 109 cfu/ml SCAR (109SCAR), 102 cfu/ml SQ (102SQ), 103 cfu/ml SQ (103SQ), 104 cfu/ml SQ (104SQ) or 105 cfu/ml SQ (105SQ).
Each group consisted of 12 subjects randomized to receive the vaccine at the designated dosage and route or placebo using the same route at a 5:1 ratio. The study groups were enrolled sequentially, beginning with SCAR groups. Dosage escalation proceeded following approval of the Safety Monitoring Committee. For the SQ groups, we screened each dosage level for safety in 2 subjects for a period of 2 weeks before enrolling the rest of the group.
Study procedures
After signing a consent form and passing a test of understanding, subjects were screened by review of inclusion and exclusion criteria, medical history, targeted physical examination, blood tests, pregnancy test (for females) and urinalysis. Subjects were randomized to placebo or vaccine in a group using an R program (R Foundation for Statistical Computing, Vienna, Austria). For subjects who received the vaccine by SCAR, a droplet of either LVS or saline was applied to the acetone-wiped volar aspect of the forearm after allowing the skin to air dry. A sterile bifurcated needle was used to puncture the skin 15 times through the droplet. For subjects in the SQ groups, 0.1 mL of vaccine or saline was administered in the deltoid area. Subjects were observed for 30 minutes after injection, and were asked to return for in-clinic examination 1, 2, 3, 7, 10, 14 and 28 days post injection. The subjects maintained a diary for 35 days after injection to record their temperature, symptoms and use of medications. Subjects were followed for a total of 6 months post injection for serious adverse events. Blood and urine samples to assess vaccine safety were obtained on the day of injection and 7, 14 and 28 days post injection. Blood samples for culture and PCR to detect Ft were obtained 1, 2, 3, 7, 10, 14 and 28 days after injection. Swabs from non-epithelialized skin lesions were obtained for Ft detection (culture and PCR). Solicited and unsolicited adverse events (AE) were graded on a scale from 0 to 4, where 0= no symptoms, 1= easily tolerated symptoms, 2= symptoms that interfere with activity, 3= incapacitating symptoms and 4= life threatening symptoms. An AE was considered serious (SAE) if it was fatal or life threatening, was associated with hospitalization or prolongation of hospitalization or resulted in congenital anomaly or permanent disability. Injection site reactions to the vaccine were graded according to size. Erythema and swelling lesions measuring < 30 mm were considered grade 1, lesions measuring 30- <120 mm were considered grade 2, and lesions measuring 120 mm or more were considered grade 3. Induration measuring <15 mm was considered grade 1, 15-<30 mm induration was considered grade 2, and ≥30 mm induration was considered grade 3.
PCR for Ft detection
In addition to blood culture using BACTEC 9420, detection of Ft was performed by targeting fopA and tul4, two genes that encode outer membrane proteins. Primers specific for Ft were paired with fluorescently-labeled hydrolysis probes for each gene, and the targets were amplified and detected using real-time PCR on the Roche LightCycler (Roche Diagnostics, Indianapolis, IN).
Measurement of antibody levels by ELISA and microagglutination
Serum IgM, IgG and IgA specific for Ft LVS and Schu-S4 were measured by ELISA as previously described [21]. Horseradish Peroxidase (HRP)-labeled goat anti-human IgG (MP Biomedical, Solon, OH), IgM (Sigma. St. Louis, MO) and IgA (Jackson ImmunoResearch, West Grove, PA) diluted 1:2,000-1:5,000 in PBS 0.05% Tween 20, 10% dry milk, were used as conjugates. Titers were calculated through linear regression as the inverse of the serum dilution that produced an absorbance value of 0.2 above the blank, and expressed in ELISA Units per ml (EU/ml).
Microagglutination titers were measured as previously described [21]. Serum samples diluted two-fold (starting 1:20) were incubated overnight at room temperature with stained Ft antigen (National Diagnostic Systems Division, USAMRIID) in 96-well V bottom plates. A rabbit antiserum specific for Ft and a human calibrated positive control were tested with the experimental samples. A positive response was defined as a fourfold rise in antibody titers (ELISA or microagglutination) from baseline to any time point after vaccination.
Real time interferon-γ measurements
Interferon-γ (IFN-γ) assays were performed using a modified Quantiferon ELISA method (Cellestis, Victoria Australia). Heparinized whole blood was used (1 ml per assay), and supernatants were harvested after 24 hours at 37°C and tested in duplicate via ELISA as previously reported [22]. A standard curve of IFN-γ was used to obtain International Units/ml. Test conditions were unstimulated, Ft LVS and Ft Schu-S4 strains at 3 concentrations (108 cfu/ml, 107 cfu/ml, 106 cfu/ml), heat killed Coxiella burnetti as a negative control (at 108 cfu/ml) and phytohemagglutinin (PHA) as a positive control. Due to the considerable variation among subjects in the amounts of IFN-γ produced, data were expressed relative to the PHA-positive control to normalize IFN-γ secretion. A positive response to vaccine was defined as a twofold rise in IFN-γ levels on days 14 or 28 post vaccination.
Results
Study subjects
We screened 137 subjects and enrolled 88 subjects: 12 in each of the protocol-designated groups (total of 84 subjects) and four subjects who were given the study injection intradermally in error (3 received the vaccine at 103 cfu/ml and 1 received placebo). These additional subjects were followed for the study duration and no safety issues were identified; their data are not included in this article. Three subjects were lost to follow up after having completed at least 3 months of follow up. There were no significant differences among the study groups with respect to gender, ethnicity, race or age. Thirty nine (46.4%) of the subjects were male, 67 (79.8%) were of non-Hispanic ethnicity, 63 (75.9%) were white, 7 (8.3%) were black, 10 (11.9%) were Asian and 4 (4.8%) were multiracial.
Vaccine reactogenicity
Headache and fatigue were the most common systemic solicited AE experienced by study subjects across all groups (Table 1). The percentages of subjects who experienced any solicited AE were similar across the treatment groups. Six subjects reported “rash” on their diary card: on further questioning four described the injection site lesion as “rash” and two had recurrence of a pre-existing heat rash. Injection site erythema and induration were common across study groups (Table 1); however subjects in the 109SCAR, 104SQ and 105SQ tended to report lesions of larger diameter than other groups. Of note, it took longer for the erythema to heal in the SCAR groups: on day 9, more than 90% of subjects in the SCAR group continued to report erythema, compared to 1 subject from all the SQ groups. Skin lesions were generally described as a macule or papule with the exception of 7 subjects in the 109SCAR group who had a vesicle/pustule and 1 subject in the 105SQ group who had a vesicle. Five subjects developed papular satellite lesions surrounding the injection site, 1-2 lesions per subject. These subjects belonged to groups 105SCAR, 107SCAR, 109SCAR (2 subjects) and 104SQ (Figure 1). Five subjects had lymphangitis: 4 subjects in the 109SCAR group and 1 subject in the 105SQ group (Figure 1).
Table 1.
Number of subjects experiencing solicited adverse events by treatment group.
| 105 SCAR (N=10) |
107 SCAR (N=10) |
109 SCAR (N=10) |
102 SQ (N=10) |
103 SQ (N=10) |
104 SQ (N=10) |
105 SQ (N=10) |
Placebo (N=14) |
|
|---|---|---|---|---|---|---|---|---|
| Systemic | ||||||||
| Fever | ||||||||
| Any | 0 | 0 | 1 | 0 | 2 | 0 | 0 | 1 |
| Grade ≥2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Feverishness | ||||||||
| Any | 2 | 3 | 1 | 3 | 3 | 2 | 4 | 3 |
| Grade ≥2 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 |
| Myalgia | ||||||||
| Any | 1 | 6 | 2 | 3 | 4 | 4 | 6 | 4 |
| Grade ≥2 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 |
| Chills | ||||||||
| Any | 1 | 2 | 2 | 1 | 2 | 0 | 2 | 2 |
| Grade ≥2 | 0 | 2 | 1 | 0 | 1 | 0 | 0 | 1 |
| Headaches | ||||||||
| Any | 4 | 7 | 4 | 7 | 5 | 6 | 6 | 10 |
| Grade ≥2 | 2 | 2 | 2 | 4 | 2 | 3 | 1 | 5 |
| Nausea | ||||||||
| Any | 0 | 2 | 0 | 2 | 3 | 3 | 4 | 1 |
| Grade ≥2 | 0 | 0 | 0 | 0 | 1 | 2 | 1 | 0 |
| Fatigue | ||||||||
| Any | 4 | 4 | 6 | 5 | 7 | 7 | 8 | 9 |
| Grade ≥2 | 0 | 2 | 6 | 1 | 2 | 4 | 3 | 3 |
| Axillary pain | ||||||||
| Any | 0 | 0 | 1 | 0 | 1 | 2 | 1 | 0 |
| Grade ≥2 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Axillary swelling | ||||||||
| Any | 0 | 0 | 1 | 1 | 0 | 1 | 2 | 0 |
| Grade ≥2 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Joint pain | ||||||||
| Any | 0 | 1 | 2 | 1 | 0 | 2 | 5 | 0 |
| Grade ≥2 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Rash | ||||||||
| Any | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 |
| Grade ≥2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Local | ||||||||
| Pain/tenderness | ||||||||
| Any | 6 | 3 | 6 | 1 | 2 | 10 | 9 | 2 |
| Grade ≥2 | 0 | 0 | 2 | 0 | 0 | 1 | 2 | 0 |
| Itching | ||||||||
| Any | 5 | 5 | 7 | 0 | 1 | 3 | 5 | 1 |
| Grade ≥2 | 0 | 0 | 1 | 0 | 0 | 0 | 2 | 0 |
| Erythema | ||||||||
| Any | 10 | 10 | 10 | 8 | 6 | 9 | 10 | 9 |
| Grade ≥2 | 1 | 1 | 10 | 0 | 0 | 4 | 8 | 1 |
| Induration/Swelling | ||||||||
| Any | 10 | 10 | 10 | 6 | 4 | 9 | 10 | 7 |
| Grade ≥2 | 0 | 1 | 9 | 0 | 0 | 8 | 9 | 0 |
Notes. For erythema and induration: we used the maximum reported diameter on the diary card or during the in-clinic examination.
Figure 1.
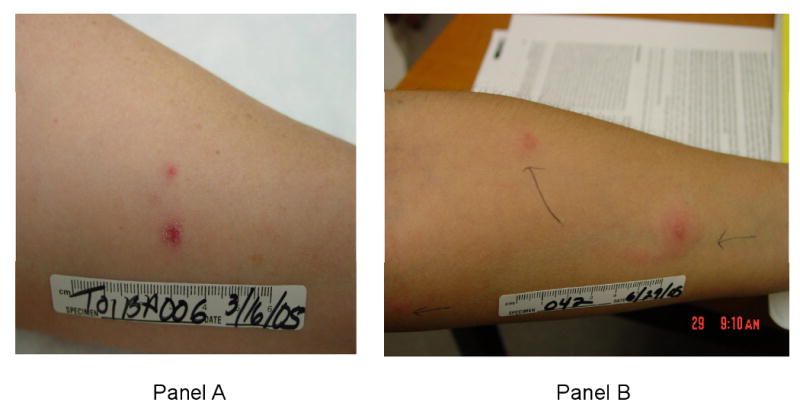
Panel A: Photograph of a subject in the 105SCAR group with a satellite lesion, 28 days post injection. Overall, satellite lesions developed 1-28 days post injection and resolved within 5-24 days. Panel B: Photograph of a subject in the 109SCAR group with lymphangitis and a satellite lesion, 1 day post injection. Overall, lymphangitis developed 1-7 days post vaccination and resolved within 1-4 days.
There were 133 unsolicited AE, of which 21 were considered associated with vaccination; all were mild in severity. One SAE was reported during: a 24- year old woman developed dizziness 10 weeks after injection with placebo. She was diagnosed with transient ischemic attacks secondary to paradoxical emboli through a patent foramen ovale that she failed to disclose upon enrollment. Three subjects who received the vaccine at 107SCAR, 102SQ and 104SQ had elevations in serum creatine kinase and aspartate aminotransferase that were graded as severe, 30, 1 and 15 days post injection, respectively. All three subjects reported heavy exertion prior to the blood draw, and the enzymes normalized on repeat evaluations.
Bacterial shedding
Ft was not detected in any blood sample. Five subjects, all in the 109SCAR group had a skin swab positive by PCR for Ft one day post vaccination, and 3 out of the 5 subjects had a positive culture as well. Two of these 5 subjects had Ft-detected by PCR from the swab two days after the injection. Lesions were epithelialized and healing thereafter.
Serologic responses
We tested the serologic response to the vaccine pre- and 14, 28, 84 and 180 days post-vaccination. The experiments were performed using Ft LVS and Schu-S4 as antigens and the results were almost identical. We present the data using the LVS antigen. When compared to placebo recipients, subjects in the 107SCAR and 109SCAR groups had significantly higher response frequencies after vaccination, as measured by ELISA for IgG, IgM, IgA and microagglutination. Response frequencies in the SQ groups were not significantly different from placebo, except for subjects in the 104SQ group who had a significantly higher IgM and microagglutination response frequency than placebo (Table 2). Similarly, vaccination by the SQ route did not result in antibody levels that were significantly different from placebo. However, vaccination by 107SCAR and 109SCAR resulted in IgA and IgG levels that were significantly higher than placebo at 28 days post vaccination (Figure 2).
Table 2.
Number of subjects with a positive serologic or Interferon-γ response by treatment group.
| 105 SCAR (N=10) |
107 SCAR (N=10) |
109 SCAR (N=10) |
102 SQ (N=10) |
103 SQ (N=10) |
104 SQ (N=10) |
105 SQ (N=10) |
Placebo (N=14) |
|
|---|---|---|---|---|---|---|---|---|
| Ig A | 3 | 5 | 8* | 1 | 1 | 3 | 2 | 1 |
| IgG | 3 | 7* | 8* | 2 | 1 | 2 | 1 | 0 |
| IgM | 4* | 4* | 6* | 0 | 1 | 4* | 0 | 0 |
| Microagglutination | 3 | 7* | 8* | 2 | 1 | 4* | 1 | 0 |
| Interferon γ | 9* | 8* | 8* | 4 | 2 | 4 | 7* | 2 |
P < 0.05 when compared to placebo
Note. A positive serologic response is defined as a fourfold or more rise in antibody levels from baseline when tested at 14, 28, 84 or 180 days post injection. A positive Interferon γ response is defined as a twofold or more rise in tularemia-specific interferon-γ release using a quantiferon assay at 14 or 28 days post injection.
Figure 2.
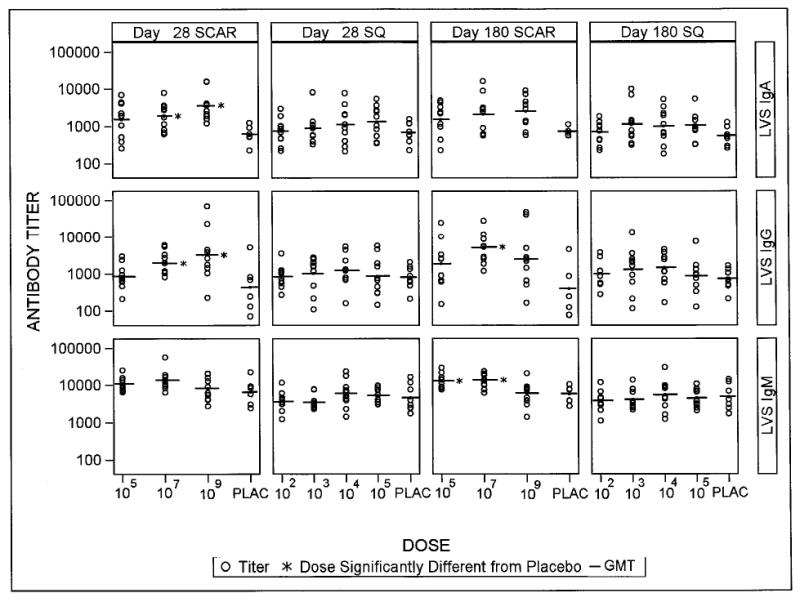
Day 28 and day 180 antibody levels by treatment group. The line represents the geometric mean titer. Dosage groups were compared to placebo using Dunnett's test control for multiple comparisons in a test. * P<0.05.
Interferon-γ release assay
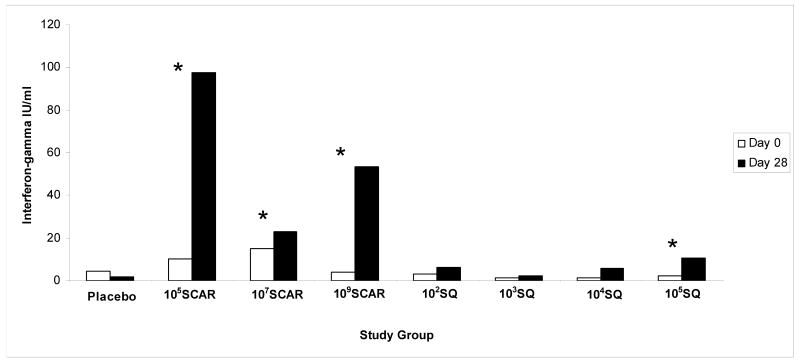
Vaccination with 105SCAR, 107SCAR and 109SCAR elicited a statistically significant higher response frequency when compared to placebo. In contrast, in the SQ cohort, vaccination with 105SQ was the only dosage that elicited a response frequency that was significantly greater than placebo (Table 2). Using a paired t-test, we found that study groups 105SCAR, 107SCAR, 109SCAR and 105SQ had a statistically significant increase in the median IFN-γ levels on day 28 compared to baseline (Figure 3). Of note, there were statistically significant correlations of IFN-γ levels and IgA levels on day 28 (r=0.40, P<0.001) and IgM levels on days 14 and 28 (r=0.33 and r=0.49, respectively; P<0.01) using a Spearman rank correlation test.
Figure 3.
Median interferon-γ levels pre injection (day 0) and 28 days post injection. * denotes P<0.05 when comparing the median IFN- γ levels between days 0 and 28.
Injection site reactogenicity and the immune response
The maximum recorded erythema measurement during the 28 days post vaccination correlated significantly with microagglutination titers (P<0.0001), but not with the IgA, IgG and IgM titers; while the maximum recorded induration measurement correlated significantly with the IgA, IgM and microagglutination titers across all groups. Subjects who had a serologic response to vaccination as measured by day-14 and day-28 IgA, IgG and microagglutination titers had significantly larger maximum erythema and induration measurements than those who did not. We found no correlation between the maximum erythema measurements and IFN-γ levels produced in response to vaccination, but found a significant correlation between the maximum induration measurements and IFN-γ levels (P= 0.005).
Discussion
We present comprehensive safety, reactogenicity and immunogenicity data from a phase I clinical trial evaluating the first tularemia LVS vaccine to be produced in accordance with GMP standards. We also compare the safety and immunogenicity of the vaccine administered via two routes: the traditional scarification route and an alternate subcutaneous route.
In general, all the tested dosages and routes were well tolerated. In a minority of the subjects, vaccination resulted in rather unique reactions such as satellite papular lesions or lymphangitis. However, the lesions were self limited and did not interfere with the subjects' daily activities. Systemic reactions to the vaccine were mostly mild to moderate in severity, and occurred at comparable frequencies across treatment groups, including placebo. We found no evidence of bacteremia using traditional culture methods and PCR, despite frequent sampling. Although these results are reassuring with regard to the overall safety and tolerability of the vaccine, generalization of these findings should be done with caution, since the study was not powered for group comparisons. Moreover, the study population consisted of young healthy adults without known significant medical problems.
The immunogenicity of the vaccine was not comparable across treatment groups. Using the “historic” microagglutination assay or an optimized ELISA to measure antibody levels, the groups that received the vaccine by SCAR had significantly higher response frequencies than those that received the vaccine SQ. We also assessed the cell-mediated immunogenicity of the various vaccine doses/routes by IFN-γ production at 14 or 28 days post injection. We found that all SCAR groups and the 105SQ group had a significant antigen-specific rise in IFN-γ post vaccination. In order to strictly compare the 2 routes of the vaccines, we need to estimate the doses delivered by SCAR and SQ. The maximum amount of scarified inoculum is equivalent to the volume picked up on the bifurcated needle or 0.0025 ml; therefore the subjects received no more than 2.5 × 102, 2.5 × 104, and 2.5 × 106 cfu respectively in the 105SCAR, 107SCAR and 109SCAR groups [23]. In the SQ group, subjects were inoculated with 0.1 ml; hence, they have received 10, 102, 103, and 104 cfu in the 102SQ, 103SQ, 104SQ and 105SQ groups, respectively. Delivering the vaccine inoculum by SCAR tended to elicit a higher serologic response frequency, antibody levels and IFN-γ responses than delivering it via the SQ route. Confirmation of this finding will require a larger sample size, including an evaluation of higher SQ doses of the vaccine that are equivalent to the inoculum given by 109 SCAR.
We utilized day 14 and day 28 IFN-γ production as a marker for the cell-mediated response to vaccination. Previous studies showed that IFN-γ is important in the containment of tularemia, especially early after infection. Elkins et al demonstrated that in the first 3-4 weeks after infecting mice with LVS, IFN-γ was critical to the survival of the mammal [24]. Most of the early IFN-γ production is from natural killer cells (NK); a finding corroborated by Fuller et al. who found that 1 day post vaccination of human subjects with LVS there is a strong early upregulation of TCRγδ+ T cells, NK T cells and monocytes with kinetics compatible with a strong innate immune response [25]. However, the long term protection against tularemia is dependent on antigen-specific effector T-cells, and animal data suggest that IFN-γ is necessary but not sufficient for long term protection [19, 26, 27]. Of note, two subjects who received saline placebo had a twofold-rise in IFN-γ levels on subsequent testing. The antigen used in the assay is killed Ft which may have resulted in a decrease in the specificity of the assay. It is known that there may be some cross-reactivity between the antigens of Ft and Brucella, Proteus and Yersinia species which could explain this finding. In our study we found a significant correlation between cellular and humoral immune responses. However, IFN-γ levels positively correlated with IgA and IgM only. It is unclear if a larger sample size, or the use of a more specific antigen could result in a better correlation. These findings underscore the importance of incorporating cellular immune response assays and more specific antigens in future clinical trials.
The relative importance of the humoral and cellular immune responses in protection against tularemia has been debated in the literature. Although the cell mediated immune responses have long been demonstrated to be key effectors in protection against tularemia, new animal data suggest the importance of the interplay between both arms of the effector immune response; albeit that, for Ft subsp tularensis, studies did not identify an important role for antibodies in the host defense [28-30]. However, no definitive correlate of protection has been identified. Ideally, the vaccine dose/route that deserves further investigation should elicit good humoral and cellular immune responses. In our study, the 107SCAR and 109SCAR are the two groups that met this requirement.
Our study has its limitations: a small number of subjects per group, relatively healthy young vaccine recipients, short duration of follow up and a lack of a correlate of protection for tularemia vaccines. Nonetheless, the statistically significant difference in the immunogenicity observed between the groups allows us with a degree of confidence to recommend 107SCAR and 109SCAR for further evaluation. Given the acceptable tolerability of the SQ route in our study, escalating the dose to 106SQ is reasonable for future studies because, short of a non-replicating vaccine, a quantitative, convenient method of administering Ft LVS vaccine remains an important yet elusive goal.
Acknowledgments
We would like to acknowledge Elaine Tracey (study coordinator), Charles Stager, James Versalovic and Maya Janecki (quantitative and molecular microbiology), Edward Young, Alan Cross and Steven Opal (Safety Monitoring Committee), Gerald Poley, Janet Shimko, Stephen Heyse, Vicki Pierson and Carol Ostrye (National Institutes of Health), Melinda Tibbals, Don Stablein and Robin Cessna (EMMES Corporation), Rosangela Salerno-Goncalves, Mardi Reymann and Lillian Cuberos (Immunology Group, Center for Vaccine Development), Marjorie Darbonne and Jacob Couturier (modified Quantiferon assay) and our study subjects.
Funding for the project was provided by The National Institute of Allergy and Infectious Diseases contracts N01-AI-25465 (Principal Investigator: Wendy A. Keitel) and N01-AI-30028 (Principal Investigator: Marcelo B. Sztein)
Hana El Sahly receives research support from GlaxoSmithKline, Wendy Keitel receives research support from Novartis, Shital Patel receives research support from Novartis and GlaxoSmithKline. Robert Atmar, Janet Wells, Thomas Cate, Martin Ho, Kuo Guo, Marcela Pasetti, Doris Lewis and Marcelo Sztein report no conflict of interest.
Footnotes
The data in this manuscript were presented in part at the Vaccine Congress Meeting, Boston, MA, December 6-9, 2008
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McCrumb FR. Aerosol infection of man with Pasteurella tularensis. Bacteriol Rev. 1961;25:262–7. doi: 10.1128/br.25.3.262-267.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saslaw S, Eigelsbach HT, Prior JA, Wilson HE, Carhart S. Tularemia vaccine study. II. Intracutaneous challenge. Arch Intern Med. 1961;107:689–701. doi: 10.1001/archinte.1961.03620050055006. [DOI] [PubMed] [Google Scholar]
- 3.Saslaw S, Eigelsbach HT, Prior JA, Wilson HE, Carhart S. Tularemia vaccine study. II. Respiratory challenge. Arch Intern Med. 1961;107:702–14. doi: 10.1001/archinte.1961.03620050068007. [DOI] [PubMed] [Google Scholar]
- 4.Mörner T. The ecology of tularaemia. Rev Sci Tech. 1992;11:1123–30. [PubMed] [Google Scholar]
- 5.Abd H, Johansson T, Golovliov A, Sandström G, Forsman M. Survival and growth of Francisella tularensis in Acanthamoeba castellanii. Appl Environ Microbiol. 2003;69:600–6. doi: 10.1128/AEM.69.1.600-606.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whipp MJ, Davis JM, Lum G, de Boer J, Zhou Y, Bearden SW, et al. Characterization of a novicida-like subspecies of Francisella tularensis isolated in Australia. J Med Microbiol. 2003;52:839–42. doi: 10.1099/jmm.0.05245-0. [DOI] [PubMed] [Google Scholar]
- 7.Petersen JM, Schriefer ME. Tularemia: emergence/re-emergence. Vet Res. 2005;36:455–67. doi: 10.1051/vetres:2005006. [DOI] [PubMed] [Google Scholar]
- 8.Oyston PCF, Sjostedt A, Titball RW. Tularaemia: bioterrorism defence renews interest in Francisella Tularensis. Nat Rev Microbiol. 2004;2:967–78. doi: 10.1038/nrmicro1045. [DOI] [PubMed] [Google Scholar]
- 9.Penn RL. Francisella tularensis (tularemia) In: Mandell, Douglas, Bennett, editors. Prinicples And Practice of Infectious Diseases. 6th. Elsevier Inc.; 2005. pp. 2674–85. [Google Scholar]
- 10.Ellis J, Oyston PC, Green M, Titball RW. Tularemia. Clin Microbiol Rev. 2002;15:631–46. doi: 10.1128/CMR.15.4.631-646.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dennis DT, Inglesby TV, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, et al. Tularemia as a biological weapon: medical and public health management. JAMA. 2001;285:2763–73. doi: 10.1001/jama.285.21.2763. [DOI] [PubMed] [Google Scholar]
- 12.Harris S. Japanese biological warfare research on humans: a case study of microbiology and ethics. Ann N Y Acad Sci. 1992;666:21–52. doi: 10.1111/j.1749-6632.1992.tb38021.x. [DOI] [PubMed] [Google Scholar]
- 13.Rotz LD, Khan AS, Lillibridge SR, Ostroff SM, Hughes JM. Public health assessment of potential biological terrorism agents. Emerg Infect Dis. 2002;8:225–30. doi: 10.3201/eid0802.010164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kadull PJ, Reames HR, Coriell LL, Foshay L. Studies on tularemia. V. Immunization of man. J Immunol. 1950;65:425–35. [PubMed] [Google Scholar]
- 15.Eigelsbach HT, Downs CM. Prophylactic effectiveness of live and killed tularemia vaccines. I. Production of vaccine and evaluation in the white mouse and guinea pig. J Immunol. 1961;87:415–25. [PubMed] [Google Scholar]
- 16.Hornick RB, Eigelsbach HT. Aerogenic immunization of man with live Tularemia vaccine. Bacteriol Rev. 1966;30:532–8. doi: 10.1128/br.30.3.532-538.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burke DS. Immunization against tularemia: analysis of the effectiveness of live Francisella tularensis vaccine in prevention of laboratory-acquired tularemia. J Infect Dis. 1977;135:55–60. doi: 10.1093/infdis/135.1.55. [DOI] [PubMed] [Google Scholar]
- 18.Rusnak JM, Kortepeter MG, Hawley RJ, Anderson AO, Boudreau E, Eitzen E. Risk of occupationally acquired illnesses from biological threat agents in unvaccinated laboratory workers. Biosecur Bioterror. 2004;2:281–93. doi: 10.1089/bsp.2004.2.281. [DOI] [PubMed] [Google Scholar]
- 19.Tärnvik A. Nature of protective immunity to Francisella tularensis. Rev Infect Dis. 1989;11:440–51. [PubMed] [Google Scholar]
- 20.Wayne Conlan J, Oyston PC. Vaccines against Francisella tularensis. Ann N Y Acad Sci. 2007;1105:325–50. doi: 10.1196/annals.1409.012. [DOI] [PubMed] [Google Scholar]
- 21.Pasetti MF, Cuberos L, Horn TL, Shearer JD, Matthews SJ, House RV, et al. An improved Francisella tularensis live vaccine strain (LVS) is well tolerated and highly immunogenic when administered to rabbits in escalating doses using various immunization routes. Vaccine. 2008;26:1773–85. doi: 10.1016/j.vaccine.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feske M, Nudelman RJ, Medina M, Lew J, Singh M, Couturier J, et al. Enhancement of human antigen-specific memory T-cell responses by interleukin-7 may improve accuracy in diagnosing tuberculosis. Clin Vaccine Immunol. 2008;15:1616–22. doi: 10.1128/CVI.00185-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention (CDC) Recommendations for using smallpox vaccine in a pre-event vaccination program. MMWR Morb Mortal Wkly Rep. 2003;52:1–16. [PubMed] [Google Scholar]
- 24.Elkins KL, Cowley SC, Bosio CM. Innate and adaptive immune responses to an intracellular bacterium, Francisella tularensis live vaccine strain. Microbes Infect. 2003;5:135–42. doi: 10.1016/s1286-4579(02)00084-9. [DOI] [PubMed] [Google Scholar]
- 25.Fuller CL, Brittingham KC, Hepburn MJ, Martin JW, Petitt PL, Pittman PR, et al. Dominance of human innate immune responses in primary Francisella tularensis live vaccine strain vaccination. J Allergy Clin Immunol. 2006;117:1186–8. doi: 10.1016/j.jaci.2006.01.044. [DOI] [PubMed] [Google Scholar]
- 26.Leiby DA, Fortier AH, Crawford RM, Schreiber RD, Nacy CA. In vivo modulation of the murine immune response to Francisella tularensis LVS by administration of anticytokine antibodies. Infect Immun. 1992;60:84–9. doi: 10.1128/iai.60.1.84-89.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sjöstedt A, North RJ, Conlan JW. The requirement of tumour necrosis factor-alpha and interferon-gamma for the expression of protective immunity to secondary murine tularaemia depends on the size of the challenge inoculum. Microbiology. 1996;142:1369–74. doi: 10.1099/13500872-142-6-1369. [DOI] [PubMed] [Google Scholar]
- 28.Koskela P, Herva E. Cell-mediated immunity against Francisella tularensis after natural infection. Scand J Infect Dis. 1980;12:281–7. doi: 10.3109/inf.1980.12.issue-4.08. [DOI] [PubMed] [Google Scholar]
- 29.Tärnvik A, Eriksson M, Sandström G, Sjöstedt A. Francisella tularensis--a model for studies of the immune response to intracellular bacteria in man. Immunology. 1992;76:349–54. [PMC free article] [PubMed] [Google Scholar]
- 30.Kirimanjeswara GS, Golden JM, Bakshi CS, Metzger DW. Prophylactic and therapeutic use of antibodies for protection against respiratory infection with Francisella tularensis. J Immunol. 2007;179:532–9. doi: 10.4049/jimmunol.179.1.532. [DOI] [PubMed] [Google Scholar]