Abstract
In the developing brain, cell migration is a crucial process for structural organization, and is therefore highly regulated to allow the correct formation of complex networks, wiring neurons, and glia. In the early postnatal brain, late developmental processes such as the production and migration of astrocyte and oligodendrocyte progenitors still occur. Although the brain is completely formed and structured few weeks after birth, it maintains a degree of plasticity throughout life, including axonal remodeling, synaptogenesis, but also neural cell birth, migration and integration. The subventricular zone (SVZ) and the dentate gyrus of the hippocampus (DG) are the two main neurogenic niches in the adult brain. Neural stem cells reside in these structures and produce progenitors that migrate toward their ultimate location: the olfactory bulb and granular cell layer of the DG respectively. The aim of this review is to synthesize the increasing information concerning the organization, regulation and function of cell migration in a mature brain. In a normal brain, protein involved in cell-cell or cell-matrix interactions together with secreted proteins acting as chemoattractant or chemorepellant play key roles in the regulation of neural progenitor cell migration. In addition, recent data suggest that gliomas arise from the transformation of neural stem cells or progenitor cells and that glioma cell infiltration recapitulates key aspects of glial progenitor migration. Thus, we will consider glioma migration in the context of progenitor migration. Finally, many observations show that brain lesions and neurological diseases trigger neural stem/progenitor cell activation and migration towards altered structures. The factors involved in such cell migration/recruitment are just beginning to be understood. Inflammation which has long been considered as thoroughly disastrous for brain repair is now known to produce some positive effects on stem/progenitor cell recruitment via the regulation of growth factor signaling and the secretion of a number of chemoattractant cytokines. This knowledge is crucial for the development of new therapeutic strategies. One of these strategies could consist in increasing the mobilization of endogenous progenitor cells that could replace lost cells and improve functional recovery.
Introduction: from development to the adult brain
The development of a structure as complex as the brain from the embryo to the adult organism is a continued source of fascination for neurobiologists. Successive, overlapping phases occur during brain development: first, the proliferation of embryonic neural stem cells in the ventricular neuroepithelium produces growth of the structure, second, a neurogenic phase takes place and gives rise to cortical neurons, third, gliogenesis occurs, and finally myelination, axon pruning, synaptic stabilization, and apoptosis complete brain maturation. A critical issue is the appropriate integration of the different cell types to form mature brain structures. The origins of cells in the ventricular zone but their final destinations in distant gray and white matter mean that cells have to migrate from their places of birth to their final positions. This migration uses environmental cues like substrates, chemoattractive/chemorepulsive factors, and detachment/stop signals. Although some of these factors have been identified, many remain to be discovered. This is a major challenge since migration disorders during brain development lead to major neurological diseases such as epilepsy, mental retardation and gross motor impairment (Aicardi, 1994; Chevassus-au-Louis and Represa, 1999; Einstein and Ben-Hur, 2008; Leuner et al., 2007).
Two main migration modes are described during development: radial and tangential migration (for review see (Kriegstein and Noctor, 2004). Neural stem cells of the ventricular zone (VZ) extend long processes from the ventricular wall to the pial surface of the cortex. These cells not only generate projection neurons but they also provide tracks along which immature neurons migrate into the cortex. This type of migration of pyramidal neurons is called radial migration because cells migrate perpendicular to the ventricular surface. Migration of different types of pyramidal neurons to defined positions finally results in the characteristic laminar organization of the cortex. In contrast, most of the cortical interneurons arise in the ventral forebrain, in the ganglionic eminences, and migrate tangentially, parallel to white matter tracts before they turn and migrate radially into the cortex (Marin and Rubenstein, 2003)
At birth, neurogenesis is largely complete, with a few notable exceptions. In rodents, for example, the subventricular zone (SVZ) continues to generate large numbers of olfactory interneurons during postnatal development (and in the adult). The newly formed neuroblasts migrate from the SVZ to the olfactory bulb via the rostral migratory stream. Gliogenesis also persists in the SVZ during the early postnatal period, generating astrocytes and oligodendrocytes. Astrocyte and oligodendrocyte progenitors (OPCs) migrate from the subventricular zone (SVZ) into the overlying white matter and cortex and into deep gray nuclei. The latter differentiate into myelinating oligodendrocytes, although a fraction remains as a pool of immature OPCs (see below).
In the adult brain a limited amount of neural cell migration continues, although it is still not clear what functions this serves. Two main structures are involved: the SVZ, lining the lateral ventricle, and the dentate gyrus (DG) of the hippocampus. Stem cells in these discrete areas keep producing progenitors that migrate and give rise largely to neurons, throughout life. The extent of neurogenesis and cell migration differs between these two structures, the SVZ largely exceeding the DG both in the numbers of cells generated and in the distance cells must migrate. Indeed, in rodents 30,000 to 80,000 SVZ-derived newborn cells are generated everyday in the olfactory bulb (OB), representing around 1% of total granule cell population (Kaplan et al., 1985; Peterson, 2002) vs only 9,000 in the DG (i.e., 0.03% of total dentate neuronal population) (Cameron and McKay, 2001; Kempermann et al., 1997). Neuronal progenitors born in the SVZ migrate in a characterized “tangential” migration, i.e., forming homophilic chains along several mm toward the OB in a well-defined pathway, the rostral migratory stream (RMS), whereas DG progenitors do not exit the hippocampal structure and only migrate a few μm along radial fibers from the subgranular layer to the granular layer of the DG. Nevertheless, in both cases, newborn neurons integrate into the pre-existing neuronal networks and become functionally active (van Praag et al., 2002); (Carleton et al., 2003; Mizrahi, 2007). Continuous neurogenesis appears to be important for the maintenance and renewal of the olfactory bulb interneurons as well as for the refinement of the dentate gyrus circuitry involved in hippocampal-dependent memory (Imayoshi et al., 2008).
While this review will focus on rodent and human studies, it is important to note that adult neurogenesis and cell migration is more widespread in other types of vertebrates, such as amphibians and reptiles (Garcia-Verdugo et al., 2002; Kaslin et al., 2008). This may explain, at least in part, the robust capacity for these animals to repair injuries to their central nervous systems (Font et al., 2001).
It is yet not clear why newly added neurons are not born directly in the place they need to reside. Progenitor cell migration may provide an additional level of control for cell positioning; the maintenance of stem cell niches also represent a potential source of cells for brain repair, but it may be costly for the organism and it also requires specific features that restrict the structures where they can persist; this implies that cells need to be able to migrate from these discrete niches to their final destination.
During pathological processes, such as in a variety of neurological diseases, brain reactivity occurs in spontaneous attempts at protection and/or repair. These pathologies result in further cell migration not observed in the resting adult brain. In addition, the cancer cells of gliomas migrate widely through the CNS, in some ways recapitulating the migration of normal progenitor cells during development. Interestingly, in recent years, increasing evidence suggests that brain tumors may originate from transfomation of either adult neural stem or glial progenitor cells (for review see Jackson and Alvarez-Buylla, 2008 and Canoll and Goldman, 2008).
In this review, we will synthesize the current knowledge of neural cell migration in the postnatal brain in normal as well as pathological conditions (excluding non-neural cells such as microglia and inflammatory invading cells from the immune system), trying to understand why migratory capabilities are maintained in the mature brain and how cell migration in the adult brain could be used for repair.
1- Birth and movement of progenitors: migration routes in the normal postnatal brain
1.1- Cell migration in the early postnatal period
An extensive amount of cell migration occurs in the first few postnatal weeks of the rodent brain. Much of this is by glial progenitors, since most neuronal migration has been completed by this time, with a few exceptions. Two of the exceptions, olfactory bulb interneuron generation in the forebrain SVZ and migration along the RMS, and dentate gyrus granule cell generation and migration in the hippocampus, continue to occur throughout adult life (see below). Another exception is the cerebellum, where granular cell genesis persists during early postnatal development. The external granule cell layer, located at the cerebellar surface, will provide the granule cell population by radial migration (Altman and Bayer, 1997). This neuronal migration has been proposed to occur along radial glial fibers (Rakic et al., 1990). However, glial involvement in granule neuronal migration does not seem to represent the main option, since migration often happens independently of glial processes; granule neurons use both vertical and tangential routes, migrating in close association with each other along faciculated fibers of migratory neurons to reach the internal granule cell layer (Hager et al., 1995; Liesi et al., 2003).
The patterns and dynamics of neuronal migration in the RMS and dentate gyrus in the early postnatal brain appear to be very similar to that seen in the adult SVZ. This is described in detail below (section 1.2). Therefore, in this section we will focus on the glial migration in the early postnatal brain.
1.1.1- Glial Progenitor migration
Glial progenitors migrate from the forebrain SVZ into neighboring structures, including white matter, neocortex, striatum, and hippocampus, where they differentiate into astrocytes and oligodendrocytes (Levison and Goldman, 1993; Luskin and McDermott, 1994; Suzuki and Goldman, 2003; Navarro-Quiroga et al., 2006). The forebrain SVZ at this time is a large zone of highly proliferative and migratory cells. The SVZ is largest at the dorsal and lateral borders of the lateral ventricle. Glial progenitors show distinct pathways of emigration from the SVZ and migration to their destinations. Those emigrating dorsally exit in a radial direction. They either stop migrating in the overlying white matter, or continue through the white matter into the cortex. The pathways of migration appear to follow the orientation of radial glial processes. Thus, those progenitors that migrate into the dorsal cortex do so along a radial pathway, while those that migrate into lateral cortex follow the white matter around laterally before changing direction and migrating radially into the neocortex (Fig. 1A). Lateral cortex glia also provides a substrate for lateral migration. It appears, therefore, that radial glia supports the migration of glial progenitors. In vitro studies suggest that radial glia contributes a permissive pathway along which migratory progenitors can travel and that contact with radial glia keeps progenitors in an immature, migratory state (Goldman et al., 1997).
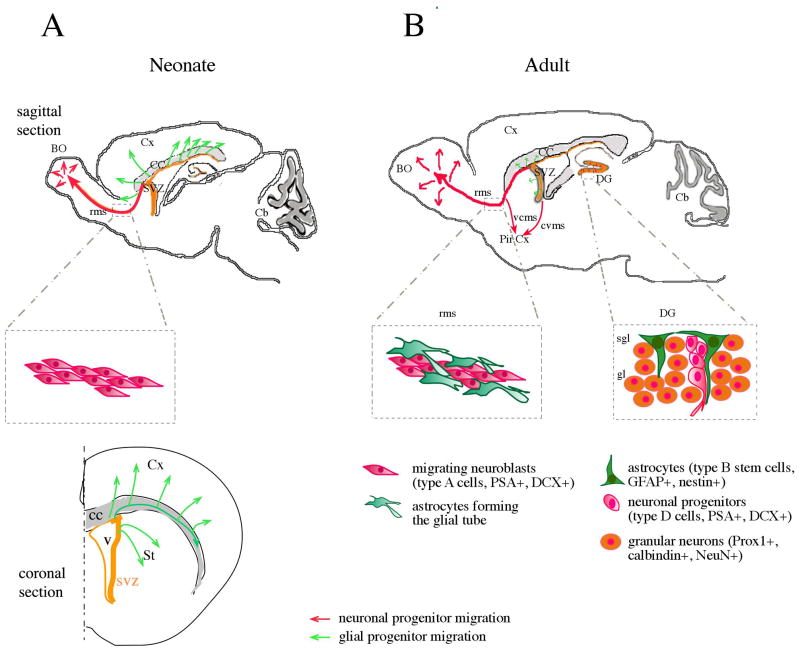
Figure 1. schematic representation of neural progenitor cell migration in neonate (sagittal and coronal sections) and adult rodent brain (sagittal section).
(A) In the newborn, the anterior part of the ventricle collapse to form the rostral migratory stream (rms). Neuronal progenitors (in red) travel from the subventricular zone (SVZ) to the olfactory bulb (OB) along the rms (in absence of astrocytic tube), whereas glial progenitors (in green) migrate caudally toward the corpus callosum (CC) and the cortex (Cx) where they will give rise to astrocytes and oligodendrocytes. (B) In the adult, neuronal migration in the rms toward the OB proceeds by tangential homophilic migration inside astrocytic tubes. Neuroblasts can also migrate along white matter fiber tracts to reach the piriform cortex (Pir Cx) via the caudoventral or ventrocaudal migratory streams (cvms, vcms). In the SVZ, a small population of oligodendrocyte progenitors can still migrate into the corpus callosum and striatum where they generate myelinating oligodendrocytes. In the dentate gyrus (DG), migration is limited since only few tens micrometers separate the birth of progenitors in the subgranular layer (sgl) from their final location in the granular layer (gl). Adapted from Suzuki and Goldman (2003).
Glial progenitors migrate along other substrates as well, however. For example, those that cross the corpus callosum from one hemisphere to the other travel parallel to axons, but in no particular register with radial glia (Kakita et al., 2003). This migration along white matter tracts is recapitulated in glioma migration (see below). In addition, we have seen glial progenitors migrating along blood vessels (P. Canoll, unpublished observations), a migratory track also taken commonly by glioma cells (see 1.2-).
Glia cells, in contrast to projection neurons, continue to proliferate during their migration. Real-time imaging of progenitors in acute brain slices reveals that they stop migrating just prior to mitosis. Mitosis itself takes about one hour, after which the two offspring resume migrating, often in opposite directions. Thus, a single glial progenitor can give rise to a clonal population that can colonize multiple levels of the white matter, cortex, and striatum (Zerlin et al., 2004). In sum, glial progenitor migration does not appear to give rise to specific cell types in specific cortical laminae, as does neuronal migration; rather progenitor proliferation generates a large population of cells that migrate and colonize large territories of forebrain.
Time-lapse microscopy has revealed that neural and glial progenitors migrate in a saltatory manner that consists of 2 distinct but coordinated steps: 1) The cells send out a long leading process in the direction of migration. The leading process extends and retracts with relatively rapid dynamics, occasionally branching or turning, as if responding to attractive and repulsive cues in the local environment. 2) The cell body moves in intermittent bursts that are separated by periods with little or no movement (Kakita and Goldman, 1999; Bellion et al., 2005; Schaar and McConnell, 2005; Tsai et al., 2007; Beadle et al., 2008). Recent studies have shown that myosin II is needed for forward movement of the cell body (Schaar and McConnell, 2005; Tsai et al., 2007), and it has been proposed that myosin II provides the contractile force needed to push the cell body and nucleus through tight spaces in the extracellular environment of the brain (Beadle et al., 2008).
Little is known about the specific molecules that promote or inhibit glial progenitor migration, and much of the literature refers to tissue culture and not in vivo models. In addition, far more is known about migration molecules expressed by OPCs than those by astrocyte progenitors. Nevertheless, the presence of several molecules is well established, and functional studies suggest important regulatory pathways.
1.1.2- Molecules involved in glial progenitor migration
Growth Factors
Oligodendrocyte progenitor cells express PDGFR-alpha, and PDGF stimulates OPC migration and proliferation and inhibits their differentiation. We have recently shown that exposing glial progenitors to high levels of exogenous PDGF (100 ng/ml) will stimulate their migration and proliferation in acute slice cultures of neonatal forbrain (Assanah et al., submitted). PDGF may also be a chemoattractant for OPC, as these cells migrate towards higher concentrations of PDGF in a transfilter migration assay (Armstrong et al., 1990). However, it has not yet been determined if the directionality of OPC migration is regulated by PDGF gradients in the early postnatal forebrain.
EGF signaling has been primarily investigated in the embryonic CNS, but postnatal glial progenitors continue to express EGFR (Ivkovic et al., 2008). Progenitors gradually down-regulate this receptor as they mature. However, when OPCs are forced to express EGFR (wild type) constitutively, they remain immature, proliferative, and migratory (Ivkovic, et al., 2008). Similarly, the expression of the EGFRvIII mutant in glial progenitors will also inhibit differentiation and promote migration (Aguirre et al., 2005). These observations suggest that EGFR signaling is sufficient to inhibit glial differentiation. Whether the EGFR-expressing progenitors remain migratory because they remain immature, or whether EGFR signaling has direct effects on migratory machinery is not yet clear. However, EGFR signaling in cancer cells is sufficient to promote an epithelial to mesenchymal transition, in which non-migratory cells with epithelial contacts lose those contacts and become migratory (for review see Guarino et al., 2007).
Integrins
OPCs express alphaV integrin along with a series of sequentially expressed beta integrins during their differentiation (Blaschuk et al., 2000). AlphaV integrin is important for migration since interfering with integrin function inhibits migration in cultures. Integrin function has been linked to glutamate activation of glutamate receptors on OPCs, through a protein complex that contains proteolipid protein (PLP), alphaV integrin, and the AMPA receptor (Gudz et al., 2006). However, OPCs that do not express PLP can migrate normally, at least in this culture system, and glutamate does not drive migration. These data suggest that glutamate can promote OPC migration via a mechanism that links glutamate receptors to PLP and integrins, but that OPCs have other molecular pathways in addition. Since integrins are activated in part by binding extracellular matrix molecules, we should eventually determine which molecules on radial glial, axonal, and or blood vessel substrates are critical for integrin-mediated progenitor migration. How integrin complexes are linked to underlying cytoskeletal elements to promote glial migration is not yet known, although there are well-established links to Ras and a variety of other GTPases, which eventually converge on actin dynamics.
Purinergic receptors
Purinergic receptors have been linked to glial migration. Both ATP and ADP stimulate OPC migration in culture, activating P2X(7) and P2Y(1) receptors, respectively (Agresti et al., 2005). Astrocytes also express purinergic receptors. Data from astrocytoma lines and primary rodent astrocytes in culture suggest that P2Y(2) receptors associate with alphaV integrins to activate GTPases and myosin-light chain phosphorylation in stimulating astrocyte and astrocytoma migration along UTP gradients (Liao et al., 2007; Wang et al. 2005). Finally, stimulation of P2Y receptors on astrocyte progenitor cells emigrating from neurosphere cultures promotes the outgrowth of these cells (Streidinger et al., 2007; Striedinger and Scemes, 2008). Purinergic receptor activation in astrocytes leads to transient increases in intracellular free calcium, the frequency and magnitude of which regulate progenitor migration. Whether these receptors play an important role in astrocyte progenitor migration in vivo remains unclear, but it should be possible using slice systems to determine the effects of purinergic receptor activation in a more physiological environment.
Chemotactic/Chemorepellant Regulation of Oligodendrocyte Migration
The netrin system provides the best-known example of how attractant and repellant signals regulate the dispersal of OPCs. OPCs express DCC and Unc5, two netrin receptors. In the developing spinal cord a ventral source of netrin serves to repel OPCs from their initial, ventral localization towards more dorsal areas (Tsai et al., 2003). In fact, in netrin-null mice, ventrally-located OPCs do not migrate (Tsai et al, 2006). The migration of OPCs in the developing optic nerve is also regulated by the netrin system, although Spassky et al. (2002) consider netrin a chemoattractant rather than a repellant. These authors suggest that interactions between semaphorins and the semaphorin receptors, neuropilin-1 and –2, which are found on OPCs, can inhibit migration by chemorepellant effects. The presence of netrin-1 and semaphorin 3a at the optic chiasm (Sugimoto et al., 2001) raises the possibility that focal sources along the optic nerve pathway may guide OPC migration in the newborn rat. In this work, netrin 1 showed a repellant effect on a subset of glial progenitors.
Polysialylated neural cell adhesion molecule (PSA-NCAM)
Like neuronal progenitors in the RMS (see 2.1-), glial progenitors also express PSA-NCAM. However, unlike neuroblasts in the RMS, glial progenitors do not migrate in chains. Nevertheless, removing the PSA moieties from NCAM decreased the migration rate of early oligodendrocyte progenitors in culture (Decker et al., 2000). Blocking integrins also reduced migration, suggesting that multiple molecules, or the interactions among them and with substrates, all contribute to OPC migration.
1.2- Cell migration in the adult brain
In the normal adult brain, cell migration is mainly restricted to the migration of neuronal precursors in the DG (Kaplan and Bell, 1984; Cameron et al., 1993; Seki, 2002) and of neuroblasts in the RMS, from the SVZ to the OB (Luskin, 1993; Lois and Alvarez-Buylla, 1994). Gliogenesis also continues throughout adult life and glial progenitors represent the largest population of cycling cells in the adult brain (Roy et al., 1999; Dawson et al., 2003, Assanah et al., 2006). There is evidence that the adult SVZ continues to generate small numbers of OPCs that migrate into the corpus callosum, striatum and fimbria fornix (Menn et al., 2006). However, the vast majority of adult glial progenitors reside outside the neurogenic niches (DG and SVZ) and under normal circumstances these resident adult glial progenitors and their progeny do not migrate, although they can be stimulated to migrate under pathological conditions (discussed in section 3). The remainder of this section will focus on the migration of neuronal precursors that occurs in the normal adult brain.
1.2.1- The dentate gyrus
The adult DG is a relatively homogenous structure, mainly composed of granule neurons, and a small population of basket cells and glia. During embryogenesis, granule neurons of the DG stem from progenitor cells originating in the neuroepithelium lining the lateral ventricle (Altman and Bayer, 1990), and probably migrating radially across the developing hippocampus (Rickmann et al., 1987). Progenitors then remain in the adult DG and establish a secondary germinative area in the subgranular layer. This subgranular layer is defined as a narrow band of about 50 μm in depth between the inner border of the granular layer and the hilus. The cellular and architectural composition of this germinative area strongly influences the migratory behavior of neuronal precursors within this structure. The subgranular layer is composed of two types of cells (Seri et al., 2001): 1- astrocytes (or B cells), which display a long radial process through the granular layer, and a tangential extension lining the inner border of the granular layer, and 2- immature cells (or D cells) produced by B cell divisions. The D cells, or transient progenitors, divide and generate neuronal precursors (Fig. 1B). These precursors express doublecortin (DCX) (Brown et al., 2003) as well as PSA-NCAM (Seki and Arai, 1993), two molecules highly associated with the migrating potential of immature neural cells. Astrocytes, D cells and neural precursors form clusters of cells favoring cell-cell interactions (Seki, 2002). Neuronal precursors migrate short distances to penetrate into the base of the granular cell layer. During this dispersion, it seems that migrating neuroblasts first move tangentially through the subgranular layer, leaving a trailing process in the proliferative area, and then extend radially-orientated dendrites and migrate radially (Seki et al., 2007). Thus, it is possible that the plexus formed by PSA-positive dendritic processes of immature neurons, GFAP-positive radial fibers, and vasculature (Palmer et al., 2000) provides a microenvironment suitable for proliferation, differentiation and migration. Once in the granular layer, neuronal precursors differentiate into granule neurons (Cameron et al., 1993), establish synaptic contacts (Markakis and Gage, 1999) and contribute to functional plasticity of the hippocampus (Bruel-Jungerman et al., 2005; Snyder et al., 2001; van Praag et al., 2002; Wang et al., 2000).
1.2.2- The subventricular zone
In the mature brain, the SVZ represents the main stem cell niche. In contrast to progenitors in the DG, the neuronal progenitors produced in the SVZ need to migrate long distances along the RMS to reach their final destination in the OB.
The adult SVZ, also referred to as subependymal layer, is a remnant of the primitive germinal layers but continues to generate neurons throughout life. Although the SVZ is commonly considered an area of the adult brain fully retaining embryonic features, substantial differences do exist in the morphological and molecular composition of its perinatal and adult counterparts (Peretto et al., 2005). For instance, the prenatal and early postnatal SVZ contains an open olfactory ventricle which closes after birth in rodents, giving rise to the RMS, which will constitute the “highway” for neuronal progenitors en route to the OB. The radial glial cells, identified as the embryonic neural stem cells, disappear from the SVZ in the early postnatal period and give rise to astrocytes, located in cortex and white matter as well as at borders of the SVZ, oligodendrocytes and olfactory interneurons (Malatesta et al., 2000; Noctor et al., 2001; Tramontin et al., 2003; Ventura and Goldman, 2007). The stem cell-like properties of SVZ astrocytes were first demonstrated by Doetsch et al., 1999, and have been supported by subsequent studies (Capela and Temple, 2002; Chiasson et al., 1999; Garcia et al., 2004; Laywell et al., 2000).
The cellular composition of the adult rodent SVZ has been well characterized (Doetsch et al., 1999; Doetsch et al., 1997): a monolayer of ciliated ependymal cells (type E cells) separates the SVZ from the lumen of the ventricle. Astrocytes (type B1 cells), the cell bodies of which are located in the subependymal region, have an apical ending at the ventricular surface and a long basal process that terminates on blood vessels and also often enwraps chains of neuroblasts (Mirzadeh et al., 2008). Both apical and basal processes may participate in the control of proliferation, differentiation and migration, by integrating signals from the blood and the cerebrospinal fluid (CSF) and also by physical guidance of migrating neuroblasts. Type B cells give rise to transit amplifying cells, type C cells. These in turn produce neuronal progenitors (type A cells) that proliferate and migrate along the RMS (Lois et al., 1996). Another characteristic feature of the SVZ is a rich vasculature and the presence of an extravascular basal lamina that extends from blood vessels to ependymal cells (Tavazoie et al., 2008; Shen et al., 2008). The extracellular matrix constituting this basal lamina contacts all cell types present in the SVZ (Mercier et al., 2002), and could play important roles in the regulation of adult neurogenesis and cell migration. Furthermore, a recent study showed that in the dorsal SVZ, the neuroblast chains appear to be built around blood vessels that run in the orientation of migration, raising the interesting possibility that these blood vessels are playing a role in guiding neuroblast migration towards the olfactory bulb (Shen et al., 2008). Finally, although microglia represents only 1.5% of the total SVZ cell population, recent work suggests a crucial role of microglia in neurogenesis regulation in cooperation with T lymphocytes (Ziv et al., 2006).
In vitro studies using the neurosphere assay (Gritti et al., 2002) as well as more recent in vivo analysis (Mendoza-Torreblanca et al., 2008; Alonso et al., 2008) suggest that cells with stem-like characteristics are not confined to the SVZ, but are also present along the RMS. These studies furthermore indicate that cells with stem-like potential endowed with different functional characteristics occur at different levels of the SVZ–RMS pathway: cells located in the proximal part of the RMS generate more oligodendrocytes whereas cells in the distal part give rise by a majority to periglomerular interneurons (Hack et al., 2005; Mendoza-Torreblanca et al., 2008).
Neuronal progenitors (expressing mCD24, βIII tubulin, DCX and PSA-NCAM) migrate along the RMS in what has been described as a tangential “chain migration” (Fig. 1B). This migration occurs without the aid of radial glial or axonal substrates; the cells are closely associated in chains and use homophilic migration (Doetsch and Alvarez-Buylla, 1996; Lois et al., 1996). Within the chains, neuroblasts keep proliferating (Lois et al., 1996; Luskin 1998). This homophilic migration has been suggested to be a particularly efficient mode of migration (Bovetti et al., 2007a). Several in vitro and in vivo studies have attempted to measure migration velocities of neuronal progenitors along the RMS. However, the results are somewhat disparate, ranging from 20 to 100 μm/hr (Bolteus and Bordey, 2004; Davenne et al., 2005; De Marchis et al., 2001; Lois and Alvarez-Buylla, 1994). These discrepancies may in fact reflect the heterogeneous nature of neuroblasts. A recent study using two-photon time-lapse microscopy of brain slices from postnatal and adult eGFP mice suggests that a bipolar morphology was not predictive of cell motility, and that all migrating cells are not DCX-positive (Nam et al., 2007). It is also now known that cell migration is not constant but rather irregular with periods of inactivity and exploratory behaviors (Davenne et al., 2005; Nam et al., 2007). Interestingly, this study also demonstrated the occurrence of dorsoventral migration throughout the striatal SVZ by contrast to the caudorostral migration observed in the dorsal SVZ (Nam et al., 2007). The development of new methods of non-invasive visualization of endogenous progenitor cell migration by micron-sized iron oxide particles labeling and magnetic resonance imaging (Shapiro et al., 2007) might help increase our understanding of such migration processes.
Migrating neuronal progenitors are ensheathed within the RMS by a meshwork of specialized GFAP-positive astrocytes (Lois and Alvarez-Buylla, 1994; Lois et al., 1996). These astrocytes (type B2 cells) are organized in glial tubes that compartmentalize the SVZ in longitudinal channels and that mark the borders with the surrounding brain parenchyma (Fig. 1B). These astroglial tubes contain chains of migrating neuroblasts orientated tangentially. Although the close physical interaction between the neuronal progenitors and the tunnel of astrocytes invites speculation that the astrocytes might guide migration, there is no evidence that these GFAP-positive ensheathing cells are necessary to direct neuronal progenitor cell migration in the RMS. Indeed, when explants of SVZ are cultured in a three-dimensional matrix, cells with a neuronal phenotype migrate rapidly away from the explants in close association with each other, in the absence of GFAP-positive astrocytes or other glia (Lim and Alvarez-Buylla, 1999; Wichterle et al., 1997). Interestingly, the astrocyte tube is absent in the neonate brain and appears only 2 to 3 weeks after birth. In the neonate neuroblasts migrate more individually, progressively shifting to chain migration. Chain formation is not directly linked to glial tube assembly since it usually precedes complete glial ensheathment (Peretto et al., 2005). These observations suggest that this astrocytic rearrangement may not be essential for cell migration itself, but rather a means to allow an adaptation of embryonic/postnatal modes of cell migration to the less permissive mature nervous tissue environment.
Yet other studies suggest that the tube of astrocytes regulates the speed of migration of neuronal progenitors by controlling the degree of GABA receptor activation through the expression of GABA transporters (Bolteus and Bordey, 2004). Astrocytes also produce a protein called MIA (migration-inducing activity), which induces neuronal migration by initiating and enhancing the movement of neuronal progenitors (Mason et al., 2001). Altogether, these studies contribute to acknowledge the multifaceted role of astrocytes in the SVZ-RMS system: stem cells, migration guide and/or substrate, boundary between tissue compartments…
Once they arrive in the OB, migrating neuronal progenitors detach from the chain and migrate radially into the granule and periglomerular cell layers of the OB where they differentiate into GABAergic and dopaminergic interneurons (Belluzzi et al., 2003; Carleton et al., 2003; Doetsch and Alvarez-Buylla, 1996). Little is known about the mechanisms governing radial migration of neuronal progenitors within the OB. Although in development radial glia cells are present in the OB, they are absent in the adult and there are no glial or axonal processes that span the entire depth of the OB from the center to the edge and that could guide the cells. Recent work shows that cells traversing the deep granule cell layer use blood vessels as migratory scaffolds (Bovetti et al., 2007b). By analogy to the neurophilic migration along axons and gliophilic migration along radial processes, the authors called this new mode of migration “vasophilic migration”. Note that early glial progenitors and glioma cells can also migrate along blood vessels.
The assertion that neurogenesis and cell migration occur in only two restricted brain regions in the adult brain, the DG and the SVZ, should be modulated by recent findings indicating that white matter (Takemura, 2005) and discrete cortical areas could be sites of secondary neurogenesis. Indeed, the amygdala and the piriform cortex, which represents the major part of the olfactory cortex, receiving olfactory bulb projection axons, also display newly-generated neurons in the adult brain of several mammalian species, including rodents, rabbits and non-human primates (Bernier et al., 2002; Luzzati et al., 2003; Pekcec et al., 2006; Shapiro et al., 2007). Using BrdU, phenotypic markers as well as DiI tracing (Shapiro et al., 2007) have suggested that the newly-generated neurons of the adult piriform cortex arise from the SVZ of the lateral ventricle, migrate through subcortical white and gray matter structures to reach the piriform cortex, and some of these cells differentiate and survive in the piriform cortex. This migration route has been called by the authors the “caudoventral migratory stream” because it arises from the caudal portion of the lateral ventricle and extends ventrally. These data are consistent with the recent observation of extensive dorsoventral migration in the ventral regions of the lateral ventricle (Nam et al., 2007) that could contribute to this migration pathway. However, these results have been challenged by recent findings showing that new projection neurons in the piriform cortex rather arise in situ from local PDGFRα/NG2 positive cells (Rivers et al., 2008). The dorso-ventral migration observed by Nam et al. may therefore rather correspond to the ventral migratory stream sending neurons to the island of Calleja and olfactory tubercules (De Marchis et al., 2004). Although DCX+/BrdU+ progenitors with bipolar morphology have been observed migrating either individually or in discontinuous chains within white matter regions, the precise mode of migration through these structures toward the piriform cortex of these progenitors is not known. Interestingly, grafting studies of mouse and human neural progenitors into the adult rodent brain revealed that, except for the RMS, subcortical white matter tracts represent the major permissive structure for cell migration in the adult brain (Englund et al., 2002; Tabar et al., 2005; Cayre et al., 2006). Again, this white matter migration recapitulates migratory directions taken by some glial progenitors in the neonatal brain and is a prominent pathway for the migration of glioma cells (see below).
Most of the studies describing the migration of neuronal progenitors in the adult brain refer to studies in rodents. Is there similar migration in the human brain? This is a legitimate question in regard to the functional significance and potential therapeutic applications of this process. First considered as developmental vestige only present in lower species, adult neurogenesis was finally demonstrated in humans in 1998 by Ericksson and colleagues (Eriksson et al., 1998). They studied the brains of cancer patients, who had been intravenously injected with BrdU for prognosis purposes, and found that new neurons were produced in the dentate gyrus of adult humans and that some SVZ cells were labeled with BrdU, confirming the presence of two germinative zones well identified in rodents and non-human primates. However, although the adult human SVZ is now accepted to be a reservoir of neural stem cells (Quinones-Hinojosa et al., 2006; Sanai et al., 2004), a controversy persists regarding the existence of an RMS toward the olfactory bulb in humans: the group of Alvarez-Buylla first described the presence in the adult human SVZ of a ribbon of proliferative astrocytes but did not find chains of migrating neuroblasts or an RMS (Sanai et al., 2004). In contrast, Curtis et al. (2007a) interpreted brain sections as revealing the presence of a lateral ventricular extension reaching the OB in the adult human brain, and inferred that this extension may contain progenitors that migrate and become mature neurons in the olfactory bulb. This discrepancy is still a matter of debate; technical considerations could explain this inconsistency, and the existence of a human RMS illustrates a continuum and the presence of common biological processes among mammals (Curtis et al., 2007b). Others maintain that the current evidence is not strong enough, even though the potential interest of such discovery would be obvious (Sanai et al., 2007). In either case, it is clear that the production of new neurons in the SVZ and the extensive migration along the RMS that occurs in the adult rodent brain far exceed that seen in the adult human brain, and may therefore impede the therapeutic potential of endogenous neural stem cells. This likely relates to the high rate of turnover of interneurons in the rodents’ disproportionately large olfactory bulbs. In a sense, this mirrors the robust neurogenesis that occurs in the forebrains of adult songbirds, where new neurons migrate to and become incorporated into brain regions that control songs. One might propose that patterns of cell proliferation and migration in the adult human brain should be tailored to meet the demands of cell replacement that are required to maintain our unique cognitive functions.
2- Factors involved in the regulation of progenitor cell migration in the normal adult brain
A fundamental issue concerning adult migration is to understand the intrinsic and extracellular cues that allow the persistence of migratory progenitors. Increasing evidence argues for the involvement of developmental signals that are maintained in restricted adult brain structures. It thus appears that the adult brain uses developmental strategies in order to maintain a higher degree of plasticity permissive for cell remodeling. In the adult as in development, orientated neuronal migration requires the cooperative actions of attachment, repellant and guidance signals, and migrating cells need to be equipped with proteins conferring them with the intrinsic capacity to migrate.
2.1- PSA-NCAM
As mentioned above, SVZ-derived neuroblasts are able to migrate in chains in a homophilic mode, each neuroblast using neighboring neuroblasts as substrates for migration. Polysialylated NCAM (PSA-NCAM) was among the first molecules found to regulate chain migration in the RMS (Chazal et al., 2000; Hu et al., 1996; Ono et al., 1994). Indeed, PSA-NCAM is highly expressed during development, regulating cell-cell interactions (Kiss and Rougon, 1997; Rutishauser and Landmesser, 1996). In the adult central nervous system, PSA-NCAM expression is downregulated and only persists in discrete areas that keep the ability to undergo structural and functional plasticity (Seki and Arai, 1993; Theodosis et al., 1991): more specifically it is present on type A cells of the SVZ and RMS, and newborn neurons in the dentate gyrus; it disappears from the cell surface after neuronal differentiation. Chemical (endoneuraminidase) or genetic (NCAM knock out mice) approaches (Hu et al., 1996; Chazal et al., 2000) show that PSA-NCAM positively regulates cell migration in both neurogenic areas in the adult brain. In the absence of PSA, although migration is still present, it is defective, in that the neuronal progenitor chains don’t form properly (Hu, 2000) and cells accumulate in the SVZ. Interestingly, in newborns PSA-NCAM is very low in the RMS and at this time chain migration does not occur, the progenitors migrating as single cells (Hu, 2000), suggesting that PSA is not necessary per se for cell migration but allows or promotes chain migration. Although the exact mechanisms are not completely deciphered yet, it is proposed that the presence of PSA on NCAM acts as an anti-adhesive, therefore facilitating the slippage of the cells on one another during the process of homophilic migration.
2.2- Doublecortin (DCX)
DCX is another protein expressed by migrating neuroblasts in the adult brain (Brown et al., 2003; Nacher et al., 2001; Yang et al., 2004). DCX is a developmentally regulated, microtubule-associated protein required for neuronal migration. Its absence during development leads to cortical and subcortical heterotopias and lissencephaly syndrome (des Portes et al., 1998; Francis et al., 1999). DCX is an essential player in microtubule polymerization and stabilization (Moores et al., 2004), and is thus important in regulating microtubule dynamics during cell migration and mitosis. In vitro studies have shown that DCX inhibition by RNAi reduced cell migration from neurospheres (Ocbina et al., 2006). Surprisingly, adult mutant mice lacking DCX show no major defect in cortical lamination, but their RMS is greatly enlarged and newly formed neurons do not migrate properly toward the OB. These defects mainly result in a decrease of periglomerular interneurons in the olfactory bulb without affecting the population of granular internuerons. Migrating progenitors lacking DCX exhibit a multipolar phenotype instead of the typical bipolar shape and undergo long and frequent migratory pauses due to defects in nuclear translocation (Koizumi et al., 2006). Neuroblast migration along the RMS is characterized by a stereotypic behavior: the cell first extends a leading process orientated toward the direction of migration, and then the forward movement of the centrosome allows/promotes nuclear translocation; finally the trailing process detaches and repositions itself as the cell soma moves forward. Koizumi et al. (2006) demonstrated that DCX is required to mediate the coupling between the centrosome and the nucleus during this translocation process. However, not all migrating progenitors in the SVZ are DCX-positive (Nam et al., 2007) suggesting that DCX is not indispensable for cell migration. Other proteins are likely involved. LIS1 and cytoplasmic dynein, the microtubule motor, are key players in both radial and tangential migration of neural progenitors (Tsai et al., 2007). It is important to note that genetic loss of either DCX or LIS1 causes severe developmental brain abnormalities due to neuronal migration defects. Ezrin, a membrane-cytoskeletal linker protein, is highly expressed in the glial tubes of the RMS together with β-catenin, suggesting a potential role of these proteins in signaling processes regulating cell migration (Cleary et al., 2006).
2.3- Extracellular matrix molecules and interactions
Migration in the relatively non-permissive adult brain requires modification of the microenvironment surrounding the migrating cell. Numerous extracellular matrix (ECM) molecules, such as tenascin-C, chondroitin sulfate proteoglycans and laminin are present along the RMS (Jankovski and Sotelo, 1996; Murase and Horwitz, 2002; Thomas et al., 1996). Matrix metalloproteases (MMPs) cleave ECM components and thereby modify the extracellular environment. MMPs are expressed along the SVZ-OB pathway, and one of them (MT5-MMP) is produced by the neuroblasts themselves. Interestingly, MMP inhibition reduces tangential migration in neonates, where migration in the RMS still proceeds individually, but not in adults where chain migration is functional. MMP inhibition also inhibits radial migration both in neonates and adults, suggesting that MMPs are important for individual cell migration (Bovetti et al., 2007a). The fact that MMPs are dispensable for chain migration is consistent with the relative isolation of chain migrating progenitors within glial tubes and with the efficacy of this homophilic neuronal migration. Proteins containing a disintegrin and metalloprotease domain (ADAMs) are transmembrane proteins with metalloprotease, integrin-binding, intracellular signaling and cell adhesion activities. Yang et al. (2005) showed that ADAM21 is expressed in adult rodent brain by ependyma and SVZ cells with long basal processes that contact blood vessels, and are intermingled with neuroblasts in the RMS. This observation suggested a potential role of ADAM21 in neurogenesis and neuroblast migration, supported by its possible interaction with integrins.
Indeed, integrins constitute an important family of heterodimeric cell surface proteins that bind to ECM components and to counter-receptors on adjacent cells. Several subunits of integrins are present in the postnatal and adult SVZ and the use of blocking antibodies against a6 or β1 integrins strongly perturb migration and translocation (Emsley and Hagg, 2003). A recent study confirmed that β1 integrin and its laminin ligands promote the formation of chains in the adult RMS and also provided evidence that β1 integrins are required for the maintenance of glial tube integrity (Belvindrah et al., 2007). VLDLR, ApoER2 and Dab1 have also been identified as major components of the cellular machinery that supports the formation of the chains (Andrade et al., 2007). These receptors and adaptor are usually implied in Reelin signaling, but in this case it seems to be a Reelin-independent process, F-Spondin being here a potential ligand (Andrade et al., 2007).
2.4- Tyrosine kinase receptors (Eph-Ephrin interactions, ErbBR and neuregulin interactions)
The Eph family of tyrosine kinase receptors and their transmembrane Ephrin ligands are also expressed in the adult SVZ. During development, Eph/Ephrin interactions are involved in axon guidance, neural crest cell migration and formation of angiogenic capillary plexi. Conover and colleagues (2000) showed that blocking Eph/Ephrin interactions in the adult brain results in highly increased SVZ astrocyte proliferation together with disrupted neuroblast migration. Another tyrosine kinase receptor closely related to EGF receptor, the ErbB4 receptor and its ligands neuregulins (NRG1 and NRG2), has been involved in SVZ cell proliferation and in the chain migration process in the adult SVZ/RMS: in ErbB4 mutants, neuroblasts migrate as misorientated cell clusters or as individual cells probably as a result of altered cell adhesion (Anton et al., 2004; Ghashghaei et al., 2006). Similarly, EGF receptor activation induces both SVZ cell proliferation (Craig et al., 1996; Kuhn et al., 1997) and progenitor cell migration (Aguirre et al., 2005). Altogether these studies show that cell surface proteins and secreted substances regulate the activity of cell surface receptors that control cell-cell and cell-environment interactions necessary for adapted cell migration.
2.5- Chemoattractive and chemorepulsive signals
Concentration gradients of chemoattractant and chemorepulsive molecules participate in the directionally-orientated rostral migration of neuronal progenitors in the postnatal RMS. The first suggestion that structures surrounding the SVZ could provide repellant cues to direct neuroblast migration was based on co-cultures of explants from SVZ and septum showing that chains of cells migrated out of the SVZ explant in a direction away from the septal explant (Wu et al., 1999). Slit-1 and Slit-2 are expressed in the postnatal septum and choroid plexus, and replacing septal explants by HEK cells producing Slit produced the same repulsive effects as the septal explants themselves (Hu, 1999; Ward et al., 2003; Wu et al., 1999). The Slit receptors Robo2 and Robo3 are expressed in the SVZ and RMS, thus showing a complementary pattern of expression to that of Slit (Marillat et al., 2002), and adult mice lacking Slit1/2 exhibit altered migration with chains of SVZ-derived progenitors migrating caudally toward the corpus callosum (Nguyen-Ba-Charvet et al., 2004). The interaction between Slit and Robo is enhanced by cell-surface heparan sulfate, and the repulsive activity of Slit on neuroblast migration is abolished when heparan sulfate is removed from the cell surface (Hu, 2001). Slit activity also seems to be regulated by MIA, the migration inducing activity factor secreted by the glial tube. In the absence of MIA, Slit exhibits an inhibitory effect on cell migration without any repellant activity (Mason et al., 2001). However, these observations were not reproduced by Ward et al., 2003, who confirmed that Slit is primarily a repellant of migrating neurons. Indeed, Slit signaling leads to a stereotypical sequence of morphological events, starting with the extension of a new process from the previous trailing edge, followed by centrosome reorientation to the base of this new process and finally nuclear translocation toward this new direction. The atypical protein kinase C (PKCz) and glycogen synthase kinase 3β (GSK3β), two proteins belonging to evolutionary conserved pathways involved in cell polarity, are necessary for Slit-induced reorientation of the centrosome and reversal of neuronal polarity (Higginbotham et al., 2006). Besides, the discovery that type A and C cells of the SVZ could also secrete Slit, and migration experiments from Slit1−/− explants led to the hypothesis that Slit may also act cell autonomously (Nguyen-Ba-Charvet et al., 2004). How Slit expression is regulated is not clear, although there is evidence that sonic hedgehog promotes Slit expression. Thus, in mice lacking Hedgehog signaling, Slit1 expression appeared greatly reduced (Balordi and Fishell, 2007). Sonic hedgehog may coordinate several stem and progenitor cell behaviors, since sonic hedgehog signaling appears to be necessary for the maintenance of stem cells in the adult SVZ and also for neuroblast migration in the RMS.
Recently a study using transgenic mice Tg737orpk with ependymal cilia defects suggested that the beating of ependymal cilia orientates the cerebrospinal fluid (CSF) flow and is responsible for maintenance of a gradient of Slit concentration and consequently for the forward migration of neuroblasts (Sawamoto et al., 2006). However, this interpretation is controversial since the flow is directed from caudal to rostral and therefore should invert the Slit gradient (Gotz and Stricker, 2006). These authors rather support the hypothesis that cilia defects present in these transgenic mice may perturb the ability of ependymal cells to perceive signals from the CSF and consequently alter the communication between these cells and migrating neuroblasts.
Attractant molecules have also been identified that could contribute to rostral migration of neuronal progenitors along the RMS. Chemokines such as stromal cell-derived factor-1 (SDF-1/CXCL12) and its receptor CXCR4, which are potent chemoattractants of leukocytes, are also expressed in the developing brain, where they play a major role in interneuron migration (Tiveron et al., 2006; Li et al., 2008). In the postnatal brain, CXCR4 is expressed in the neurogenic regions (Tran et al., 2007) and in vitro CXCL12 administration directly induces progenitor chemotaxis (Xu et al., 2007). However, the physiological role of this cytokine in neuroblast migration in the healthy adult brain remains uncertain; its involvement in the migration/recruitment of SVZ progenitors after brain insults is much more documented (Li and Ransohoff, 2008). The morphogen sonic hedgehog, which is expressed in the SVZ, also displays chemoattractant properties on SVZ progenitors and has been proposed to regulate the departure of neuroblasts from SVZ to the RMS (Angot et al., 2008), adding a level of regulation to the system.
Despite initial observations indicating that the OB was not a determinant for neuroblast migration along the RMS (Kirschenbaum et al., 1999), more recent quantitative work showed that the removal of the OB significantly reduced the migration of SVZ cells in the RMS and suggested the release of chemoattractant molecules by the glomerular layer of the OB (Liu and Rao, 2003). Murase and Horwitz (2002) proposed that a netrin-like molecule and its receptor DCC (deleted in colorectal carcinoma) could present such a chemoattractant activity. However, Netrin-1 expression tends to disappear a few days after birth, suggesting that other signals should be present in adults. In the past years, several secreted proteins have been identified that could play a role in attraction of migrating progenitors toward the OB. One such molecule is prokineticin2, produced by the granular and periglomerular layers of the OB. In vitro, it orientates the migration of neuronal progenitors through the activation of a type G protein coupled receptor. Kineticin 2 receptor-deficient mice exhibit a reduced OB and an enlarged RMS, suggesting abnormal progenitor cell migration and/or detachment (Ng et al., 2005; Prosser et al., 2007). During development, the transcription factors Dlx1 and Dlx2 regulate tangential migration to the OB presumably via the modulation of Erb4, Slit1, prokineticine 2 and Robo2 expression (Long et al., 2007), but their implication in the adult has not been investigated.
The secreted isoform of neuregulin1 may also function as a chemoattractant/motogenic factor on migrating neuroblasts (Anton et al., 2004; Ghashghaei et al., 2006).
Two neurotrophic factors, glial cell line-derived neurotrophic factor (GDNF) and brain derived neurotrophic factor (BDNF), contribute to the maintenance of oriented neuronal migration toward the OB. Indeed, although in the SVZ-RMS-OB system GDNF production is almost restricted to the OB, the protein is subsequently distributed along the RMS, in a distribution pattern coincident with its GPI-anchored receptor GFRa1 (Paratcha et al., 2006). Furthermore, explants experiments showed that GDNF attracted RMS-derived but not SVZ-derived progenitors. Altogether, these results are consistent with a role of GDNF as chemoattractant for migrating neuroblasts from the SVZ to the OB. This effect is mediated through NCAM and cyclin dependent kinase 5 (Paratcha et al., 2006). Similarly, BDNF is expressed with a rostro-caudal gradient along the SVZ-RMS-OB compatible with a chemoattractant effect and in vitro it triggers both motogenic and chemotactic actions on neuroblasts (Chiaramello et al., 2007). CREB activation via phosphatidylinositol 3-kinase and mitogen activated protein kinase signaling is required for BDNF-induced chemoattraction (Chiaramello et al., 2007). These studies reveal new functions of trophic factors in the SVZ system, in which a variety of protein kinase pathways are involved to induce neuroblast migration.
2.6- Detachment signals
Once they reach the OB, migrating neuroblasts undergo a switch from homophilic tangential to unicellular, radial mode migration. Three main signals have been shown to participate to this switch: Reelin, Tenascin R and Prokineticin2. Reelin is a secreted glycoprotein that is crucial during cortical development for proper inside-out lamination of the cortex (D’Arcangelo et al., 1995). In the adult, it is downregulated but remains highly expressed in the OB and DG, the two neurogenic niches as well as the cerebellum (Ramos-Moreno et al., 2006). In the OB, Reelin is secreted by mitral cells and acts as a detachment signal on chains of migrating neuroblasts: when Reelin is added to SVZ explants, cells start to migrate individually. Furthermore, in transgenic mice lacking Reelin cells accumulate at the center of the OB and do not migrate radially (Hack et al., 2002). Reelin-dependent detachment of migrating neurons is mediated via the activation of the ERK pathway and PI3K, signaling through downstream Dab1 phosphorylation (Simo et al., 2007). In contrast, a Reelin-independent activation of Dab1 has been described in the SVZ that would promote chain formation in the RMS (Andrade et al., 2007).
The extracellular matrix glycoprotein Tenascin-R is not detectable in the SVZ or RMS but it is expressed in the granular layer of the OB, and acts as a detachment signal necessary and sufficient for the recruitment of neuroblasts from the RMS to the OB (Saghatelyan et al., 2004). Interestingly, sensory inputs regulate the expression of Tenascin-R in the OB, suggesting an activity-dependant incorporation of newborn neurons into the bulb. Prokineticin2, mentioned above as a chemoattractant molecule seems to be also involved in the detachment of neuroblasts from the chains once arrived in the OB (Ng et al., 2005).
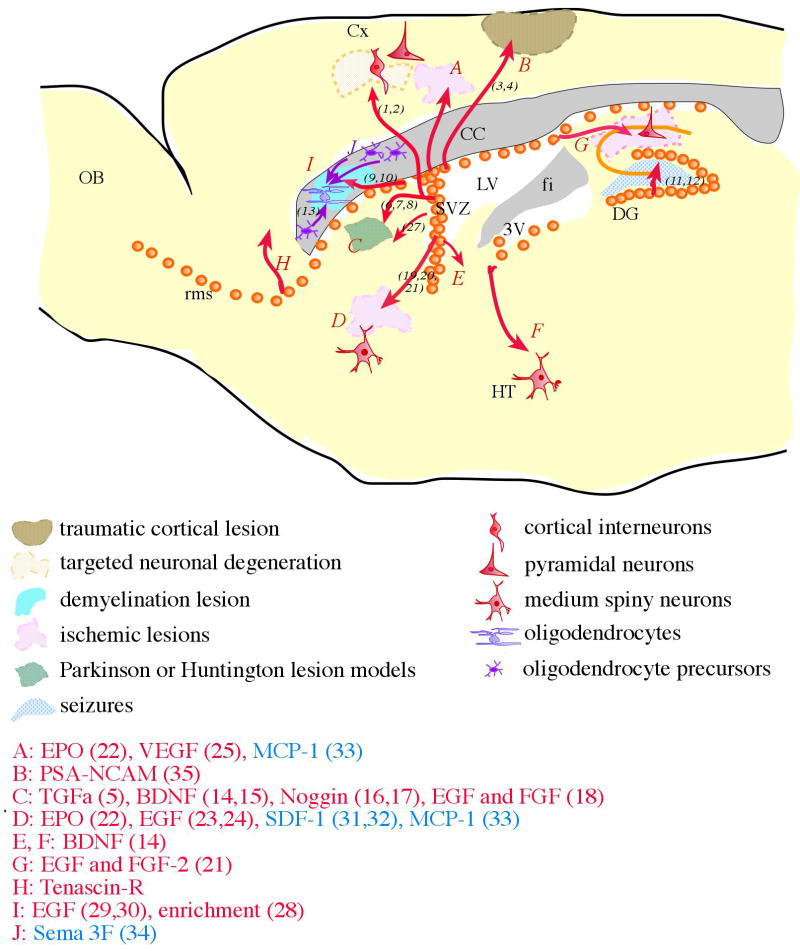
Altogether, the analysis of progenitor cell migration in the postnatal brain reveals that most mechanisms involved in migration (repulsive, attractant, permissive, motogenic factors) are shared between developmental and adult stages, and underlines that proper migration relies on the integration of all the environmental cues signaling in a cooperative manner (see recapitulation in table 1).
Table 1.
factors involved in neuroblast migration from the SVZ to the OB in the normal adult brain.
| family | molecules | expression | interactors/mechanisms of action | effects | representative references | |
|---|---|---|---|---|---|---|
| microtubule-associated protein | DCX | Type A cells SVZ/RMS | microtubule polymerisation | nuclear translocation | Ocbina et al., 2006; Koizumi et al., 2006 | |
| cell-cell or cell-matrix interactions | Immunoglobulin superfamily | PSA-NCAM | Type A cells SVZ/RMS | reduction of adhesive properties | facilitation of cell translocation in the RMS | Ono et al., 1994; Hu et al., 1996; Chazal et al., 2000 |
| extracellular matrix proteins | tenascin C | SVZ, RMS, blood vessels | interact with a6β1 integrin | permissive factors for chain migration; provide appropriate levels of adhesion to propel cell motility | Thomas et al., 1996, Jancovski and Sotelo, 1996, Murase and Horwitz, 2002 | |
| chondroitin sulfate proteoglycans | ||||||
| laminin | ||||||
| matrix metalloproteases | MT5-MMP | along the SVZ-OB pathway | ECM cleavage | necessary for individual cell migration | Bovetti et al., 2007b | |
| ADAMs | ADAM21 | ependymal cells and a population of SVZ cells | interaction with integrins | promote chain formation, required for glial tube integrity | Yang et al., 2005 | |
| Integrins | a6β1 integrin | SVZ and RMS | binds to ECM, ADAMs and laminin | Jacques et al., 1998; Murase and Horwitz, 2002; Emsley and Hagg, 2003; Belvindrah et al., 2007 | ||
| VLDLR, ApoER2, Dab1 | SVZ and RMS | Independant of Reelin; potential ligand: F-Spondin? | required for chain formation | Andrade et al., 2007 | ||
| Tyrosine kinase receptors | EphB | SVZ cells, notably astrocytes | binds ephrin B | regulation of SVZ cell proliferation necessary for proper organization of neuroblasts into chains | Conover et al., 2000 | |
| Erb4 | Type A cells SVZ/RMS | binds the membrane-bound NRG1 type III | required for proper assembly of the RMS and orientated cell migration toward the OB | Anton et al., 2004; Ghashghaei et al., 2006 | ||
| secreted proteins 1-chemorepulsion | Slit 1/2 | septum, choroid plexus, SVZ cells | bind to Robo 2/3 action via PKCz and GSKb | emigration from SVZ | Wu et al., 1999; Hu et al., 1999; Ngyuen-Charvet et al., 2004; Higginbotham et al., 2006 | |
| Shh | SVZ stem cells | via Slit expression regulation | Balordi and Fishell, 2007; Angot et al., 2008 | |||
| MIA | glial tubes | interacts with Slit/Robo | motogenic factor | Balordi et al., 2007 | ||
| secreted proteins2-chemoattraction | cytokine | SDF-1 | lingand? CXCR4 expressed in SVZ/RMS cells | binds to CXCR4 | chemoattraction | Tran et al., 2007; Xu et al., 2007 |
| netrin 1 ? | mitral cells in OB | binds to DCC | gradient for chemoattraction | Murase and Horwitz 2002 | ||
| prokineticin 2 | granular and periglomerular layers of OB | acts through G protein-coupled receptors PKR1 and PKR2 | chemoattraction and detachment | Ng et al., 2005; Prosser et al., 2007 | ||
| NRG1 type I/II | Type A cells SVZ/RMS | binds to ErbB4 tyrosine kinase receptor | chemoattractant/motogenic factor | Anton et al., 2004; Ghashghaei et al., 2006 | ||
| growth factors | GDNF | produced in the OB and then distributed along the RMS | binds to the GPI-anchored receptor GFRa1 and increases cdk5 activity. Also interacts with NCAM | chemoattraction | Paratcha et al., 2006 | |
| BDNF | from SVZ to OB with increasing gradient | via PI3K and MAPK pathways | chemoattractant/motogenic factor | Chiaramello et al., 2007 | ||
| secreted proteins 3-detachment signal | Reelin | mitral cells in the OB | binds to VLDLR and ApoER2; signal via erk and PI3K pathways | cell detachment; change from tangential to radial migration | Hack et al., 2002; Simo et al., 2007 | |
| Tenascin R | granular layer of the OB | ? | Saghatelyan et al., 2004 | |||
| prokineticin 2 | granular and periglomerular layers of OB | acts through G protein-coupled receptors PKR1 and PKR2 | Ng et al., 2005; Prosser et al., 2007 |
3- Cell migration in the pathological brain
3.1- Relationship between brain patholologies, adult neurogenesis and cell migration
3.1.1- Psychiatric disorders
In the last few years, a number of studies have examined the impact of neurological pathologies on neurogenesis niches in the adult brain. They lead to the conclusion that cell proliferation and cell migration in the SVZ and DG are almost always disturbed in the diseased brain, including models of psychiatric disorders, neurodegenerative diseases or traumatic injuries. However, it is not clear whether these modifications of neurogenesis are the causes or the consequences of brain pathologies (Thompson et al., 2008).
In schizophrenia, hippocampal stem cell proliferation, measured by Ki67 immunostaining, is impaired in patients (Reif et al., 2006) and cell proliferation impaired in animal models (Liu et al., 2006). Interestingly, several molecules (such as reelin, NRG1 and its receptor Erb4) that play crucial roles in cell migration and positioning in the developing brain are disrupted in schizophrenia patients (Eastwood and Harrison, 2006; Fatemi, 2005; Norton et al., 2006; Stefansson et al., 2003). Furthermore, downregulation of Disrupted-in-Schizophrenia 1 (DISC1), a schizophrenia susceptibility gene, results in mispositioning of newborn granule cells in the dentate gyrus due to accelerated differentiation and neuronal integration (Duan et al., 2007). A current hypothesis is that abnormalities of migration of newborn progenitors and precocious differentiation lead to aberrant circuit formation, contributing to increased excitability and emergence of such psychiatric diseases. This points out that not only developmental cell migration disruption but also alteration of adult brain cell migration can result in neurological disorders.
3.1.2- Neurodegenerative diseases
The results obtained when studying human diseases and animal models are not always consistent. For instance, in patients with Alzheimer’s disease (AD), hippocampal neurogenesis is increased (Jin et al., 2004b); however, it is decreased in most transgenic mouse models of this pathology except from one model expressing a mutant form of the human amyloid precursor protein (Feng et al., 2001; Jin et al., 2004a; Verret et al., 2007; Wen et al., 2002; Zhang et al., 2007a). Associative memory and olfaction are both affected early in AD, and therefore altered neurogenesis in the DG and OB has been proposed to contribute to the development of the disease. However, at the present time, the exact relationship between neurogenesis and AD is not clear (Galvan and Bredesen, 2007). In Huntington’s disease the results are more consistent. Progenitor cell proliferation is enhanced in the subependemal layer of patients as compared to controls and this is positively correlated with the severity of the symptoms (Curtis et al., 2003). Similarly, the SVZ is expanded and cell proliferation is increased in the R6/2 mouse model of Huntington, and some progenitors exit the SVZ/RMS to migrate toward the striatum (Batista et al., 2006). In a rat model of this disease using quinolinic acid injection into the striatum, analogous results were obtained (Tattersfield et al., 2004). Conversely, patients with Parkinson’s disease, in which dopaminergic transmission is deficient in the striatum, show reduced cell proliferation, both in the subependymal layer and in the dentate gyrus (Hoglinger et al., 2004), which is consistent with the fact that dopamine positively regulates adult neurogenesis (Baker et al., 2004; Borta and Hoglinger, 2007; Freundlieb et al., 2006; Liu et al., 2006). In the 6-hydroxydopamine (6-OHDA) induced rodent model of Parkinson, a reduction of SVZ cell proliferation is also observed, together with the redirection of some neuroblasts toward the lesioned striatum (Baker et al., 2004; Hoglinger et al., 2004; Winner et al., 2008). This migration toward the striatum is constantly found in diverse studies (Baker et al., 2004; Cooper and Isacson, 2004; Fallon et al., 2000; Hoglinger et al., 2004; Winner et al., 2008), although the fate of these progenitors once in the striatum is a source of debate. Fallon and colleagues first claimed that dopaminergic differentiation of newborn neurons occurred in the striatum of 6-OHDA rats, whereas other laboratories showed only glial but no neuronal differentiation of these striatum migrating progenitor cells (Cameron and McKay, 2001; Cooper and Isacson, 2004; Winner et al., 2008). Therefore, the functional role of ectopic migration of progenitors from the SVZ to surrounding structures remains unclear, as does its participation in brain repair.
3.1.3- Epilepsy
The relationship between adult hippocampal neurogenesis and the profound remodeling of the hippocampus that occurs during temporal lobe epilepsy (reorganization of mossy fibers, granule cell dispersion, ectopic granule cells) in humans as well as in animal models has been extensively analyzed. Dentate gyrus neurogenesis is stimulated by pilocarpin-induced seizures and ectopic neuroblasts are observed in the hilus (Parent et al., 1997; Scharfman et al., 2000), but blocking cell proliferation by irradiation does not prevent aberrant mossy fiber remodeling (Parent et al., 1999), suggesting that adult granule cells mainly contribute to seizure-induced network reorganization. Nevertheless, pilocarpine-induced ectopic migration of granular cells may result from the loss of reelin expressing interneurons that play a role in normal progenitor cell migration in the DG (Gong et al., 2007). Ectopic granule neurons in the hilus are hyperexcitable and may contribute to seizure generation and propagation (Parent, 2007). Pilocarpine also expands SVZ by increasing cell proliferation and leads to premature exit of progenitors from the RMS to the striatum, corpus callosum, hippocampus and cortex (Parent and Lowenstein, 2002; Parent et al., 2006). These progenitors give rise to astrocytes and oligodendrocytes in these structures (Parent et al., 2006). In the human epileptic brain, increased cell proliferation in the DG and SVZ has also been observed (Blumcke et al., 2001; Gonzalez-Martinez et al., 2007). Furthermore, migration from the SVZ to adjacent white matter and neocortex, with even some neuronal differentiation, was reported in brain slices removed from epileptic patients and grown in organotypic culture (Gonzalez-Martinez et al., 2007). However these observations are challenged by a recent work performed on hippocampal biopsies of patients with temporal lobe epilepsy, suggesting that epileptic activity does not stimulate neurogenesis in the human dentate gyrus and that granule cell dispersion does not result from ectopic newborn neuron migration (Fahrner et al., 2007).
3.1.4- Ischemia
Progenitor cell reactivity and migration from the neurogenic niches of the lesioned adult brain have been more thoroughly examined in ischemia models (for review see Miles and Kernie, 2006), even if similar observations have been reported in other kinds of brain insults such as cortical trauma, neuronal targeted apoptosis or demyelination (Chen et al., 2004; Gould and Tanapat, 1997; Magavi et al., 2000; Nait-Oumesmar et al., 1999; Nait-Oumesmar et al., 2007; Picard-Riera et al., 2002; Ramaswamy et al., 2005; Sun et al., 2005; Sundholm-Peters et al., 2005; Szele and Chesselet, 1996; Urrea et al., 2007). In rodents, global ischemia leads to the proliferation of DG and posterior periventricular progenitors together with migration toward the damaged CA1 region (Liu et al., 1998; Nakatomi et al., 2002), whereas focal ischemia triggers SVZ cell proliferation and migration toward the striatum (Arvidsson et al., 2002; Jin et al., 2003; Zhang et al., 2001b). It is noteworthy that neuroblasts keep dividing while they migrate toward the ischemic striatum (Zhang et al., 2007). The increased number of SVZ progenitors is partly due to a higher proportion of symmetric (vs asymmetric) division in ischemic SVZ (Zhang et al., 2004b). Recently, the same team showed that ependymal and/or subependymal cells are also induced to proliferate and give rise to radial glia that contribute to neural progenitor expansion and support neuroblast migration (Zhang et al., 2007b). Although increased cell proliferation is transient (few weeks), the SVZ appears altered morphologically and functionally over a longer term (Kokaia et al., 2006). Indeed, ectopic cell migration of neuroblasts toward the striatum is observed for over a year. In the healthy brain, the glial tubes surrounding the migrating progenitors probably strongly participate to their restriction within the RMS, acting as a physical barrier in the same way as the astrocytic scar prevents axonal regeneration after a spinal cord lesion (White and Jakeman, 2008). After an ischemic episode, ectopic migration might be facilitated by an infarct-induced breakdown of the astrocytic physical barrier between the SVZ and striatum, and can occur either via chain-like structures (Zhang et al., 2004b) or individually (Winner et al., 2008). Migrating cells form aggregates when they reach the periphery of the lesion, and then start to disperse (Zhang et al., 2005) and differentiate notably into medium spiny neurons (Arvidsson et al., 2002; Teramoto et al., 2003). Interestingly, although neurogenesis is considerably decreased during aging, (Kuhn et al., 1996; Leuner et al., 2007; Luo et al., 2006), it seems that the size of ischemic lesion, but not the age of the animal, determines the degree of activation and recruitment of new striatal neurons (Darsalia et al., 2005; Kokaia et al., 2006). These results suggest that the aging SVZ and DG remain responsive to ischemia-induced cell proliferation and migration.
Recent post-mortem analysis of human brains showed that ischemic injuries are associated with increased cell proliferation in the SVZ, but no evident sign of cell migration toward adjacent parenchyma was detected (Macas et al., 2006), perhaps due to too short a delay between the attack and the death of the patient. However, limited migration was also mentioned in non-human primates, where cells do not deviate from their normal migratory pathway toward the OB after ischemic insult (Tonchev et al., 2005). This could suggest more limited capacity of cell migration in the primate brains. Yet, ectopic migration in white matter and neocortex has been described in organotypic slice cultures from human epileptic brain (Gonzalez-Martinez et al., 2007). Unfortunately, even when migration occurs, the survival rate of newly formed striatal neurons is very low, probably due to the high degree of inflammation within and around the lesion. It has been estimated that only 0.2% of dead striatal neurons are replaced by this recruitment process (Arvidsson et al., 2002), and even if they express markers of interneurons such as parvalbumin or NPY, or markers of medium spiny neurons such as pbx and DARPP-32 (Collin et al., 2005), there is as yet little solid proof of their functional integration in the neuronal circuitry. Notably, Nakatomi and colleagues (2002) used electrophysiological and behavioral experiments to demonstrate newborn neuron functionality. At the present time, it is still uncertain how this process of striatal neurogenesis contributes to functional recovery.
3.2- Oligodendrocyte progenitor migration
In addition to neuronal progenitors, glial progenitors also have the potential to migrate through the adult central nervous system. Indeed, a population of oligodendrocyte progenitors (OPCs) remains in the adult brain and spinal cord, widely distributed throughout the grey and white matter (for review, see McTigue and Tripathi, 2008). Some of this population is composed of NG2-positive, PDGFRa-positive Nkx2.2- positive cells (Dawson et al., 2003; Reynolds and Hardy, 1997), while others are not NG2+ and may be further along in the oligodendrocyte differentiation pathway (Mason and Goldman, 2002). The NG2 proteoglycan expression identifies a heterogenous population of cells in the adult CNS (Nishiyama et al., 2002) including OPCs. NG2 expressed by OPCs participates to the migratory properties of these cells. Integrins and chondroitin sulfate glycosaminoglycan cooperate with NG2 to trigger a cascade of signaling pathways and phosphorylation of intracellular and cytosqueletal proteins finally mediating cytosqueletal reorganization and motility (Karram et al., 2005). In the adult resting brain, these cells do not migrate, but will differentiate into myelinating oligodendrocytes, presumably to replace oligodendrocytes that have died in the normal turnover. In experimental models of demyelination, they start to actively proliferate, migrate and differentiate to replenish lost oligodendrocytes, often leading to spontaneous repair (Blakemore and Keirstead, 1999; Gensert and Goldman, 1997; Glezer et al., 2006; Levine and Reynolds, 1999; Watanabe et al., 2002). The degree to which OPCs migrate is not clear, however. In some experimental cases, remyelination is accomplished by OPCs close to the site of the lesion. OPCs activation is not exclusively restricted to response to specific demyelination pathologies but is also observed in a large variety of CNS injuries (McTigue and Tripathi, 2008). After focal ischemia, OPCs and oligodendrocytes are depleted in the peri-infarct area, and myelin density is decreased. This is followed by the activation of surrounding OPCs and their migration toward the lesion leading to the restoration of the oligodendrocyte pool and remyelination (Tanaka et al., 2003). Also, acute inflammation induced by LPS injection, without any demyelination, is sufficient to trigger OPC activation (Glezer et al., 2006).
3.3- Migration of glioma cells
Diffusely infiltrating gliomas, including glioblastomas, astrocytomas, and oligodendrogliomas, are the most common types of primary brain tumors. In these tumors the glioma cells have a remarkable ability to migrate long distances through brain tissue, making complete surgical resection impossible. Glioma growth patterns were carefully studied in the 1930’s by the Belgian pathologist Hans-Joachim Scherer. He noted that the patterns of glioma infiltration and migration were not random, but rather glioma cells tended to migrate along certain preferred paths, such as myelinated fiber tracts, the subpial surface, and blood vessels (Scherer, 1940). The resulting growth patterns are referred to as ‘secondary structures of Scherer’. These patterns of migration strikingly resemble the patterns of migration that glial progenitors take during normal brain development (Kakita and Goldman, 1999). Furthermore, special stains which highlight cellular morphology reveal that many infiltrating glioma have a unipolar or bipolar morphology with a prominent leading process that resembles the morphology of migrating glial progenitors (Suzuki et al., 2002). Thus, it appears that glioma infiltration may recapitulate normal glial migration, albeit to a pathological extent. This raises the interesting possibility that glial progenitor migration and glioma infiltration are regulated by similar mechanisms (discussed below).
The idea that glioma infiltration recapitulates glial progenitor migration is strongly supported by recent studies looking at migration in animal models of glioma. Infecting glial progenitors with a retrovirus that expresses PDGF and GFP induces the formation of diffusely infiltrating brain tumors that closely resemble glioblastoma. The cells infiltrate the brain by migrating along the same routes taken by glial progenitors during brain development. For example, tracking the distribution and fate of cells infected by a GFP expressing retrovirus that had been injected into the neonatal SVZ showed that glial progenitors disperse widely through the subcortical white matter and a small number will cross the corpus callosum into the contralateral hemisphere (Kakita et al., 2003). By comparison, when PDGF-IRES-GFP expressing retroviruses were injected into the neonatal SVZ, tumors formed at the injection site and GFP+ cells showed extensive infiltration into cortex and subcortical white matter, with many cells crossing the corpus callosum into the contralateral hemisphere (Assanah et al., submitted).
Animal models also show the perivascular migration that is seen in human gliomas. When C6 glioma cells (and RG2 glioma cells) are transplanted into neonatal rat brain the cells infiltrate the brain by migrating along the abluminal surface of host blood vessels (Farin et al., 2006). The migrating glioma cells proliferate en route leading to the gradual encasement of the host blood vessels in a dense collar of tumor cells. Interestingly, cell division often occurs at vascular branch points, suggesting that there is something unique about the branch points that triggers cells to enter mitosis. The tendency of glioma cells to stop and divide at vascular branch points may also recapitulate a normal developmental process. Several recent studies have shown that clusters of proliferating neural stem cells occupy a vascular niche, located between endothelial cells and astrocyte end feet (Tavazoie et al., 2008, Shen et al., 2008). These clusters also contain proliferating endothelial precursors and are commonly found at vascular branch points or the terminus of capillary sprouts, suggesting an active site of angiogenesis (Horner and Palmer, 2003; Palmer et al., 2000; Wurmser et al., 2004). More recently it has been proposed that glioma cells with stem cell-like properties are maintained in, and perhaps confined to, a perivascular niche (Ignatova et al., 2002; Singh et al., 2003, 2004; Galli et al., 2004). In support of this idea, it has recently been shown that blocking tumor induced angiogenesis with antibodies against VEGF will not only reduce proliferative vessels, but will also deplete the tumor of stem-like cells.
3.4- Mechanisms regulating cell migration in the diseased brain
3.4.1- Progenitor cell migration
In order to be able to use endogenous progenitor cell recruitment for brain repair, it is important to understand the mechanisms that control induced migration in the lesioned brain. Several mechanisms and migratory tracks have been proposed for the guidance of migrating neural progenitors toward brain lesions: 1- along myelinated fiber tracts: emigrating progenitors from the SVZ can reach the cortex contralateral to the lesion using white matter fiber tracts of the corpus callosum (Goings et al., 2004; Ramaswamy et al., 2005; Sundholm-Peters et al., 2005). These observations are in agreement with migratory behavior of neural progenitors following a graft in the adult healthy brain (Cayre et al., 2006; Englund et al., 2002; Tabar et al., 2005); 2- along radial processes: many studies have pointed out the appearance of vimentin-positive astrocytes closely surrounding multiple types of brain injuries (Alonso, 2001; Calvo et al., 1991; Janeczko, 1991, 1993; Schiffer et al., 1986; Takamiya et al., 1988). Considering the localization of these vimentin-expressing astrocytes, between the lesion and the corpus callosum, and the similar properties of radial glia and vimentin-positive reactive astrocytes (Mori et al., 2005), it has been suggested that this cell population may serve as a conduit for migrating progenitors after brain lesion (Wang et al., 2004a); 3- along blood vessels: neuroblasts appear to migrate closely associated to capillaries present in the adjacent parenchyma following stroke-induced angiogenesis (Thored et al., 2007; Yamashita et al., 2006). Activated endothelial cells secrete MMPs, which facilitate cell migration (Wang et al., 2006). The vasculature could serve not only as a guide for migration, but also provide support for survival and differentiation, and thus long-term neurogenesis in non-neurogenic structures. This would be in agreement with the well-known participation of vascularization in neurogenic niches of the adult brain (Louissaint et al., 2002; Palmer et al., 2000; Shen et al., 2004).
Besides these mechanisms, inflammation-induced chemoattraction plays a major role in progenitor cell migration after brain lesions (see review Das and Basu, 2008) (Fig. 2). Among the cytokines involved in the inflammation process, MCP-1, SDF-1 and their receptors (CCR2 and CXCR4 respectively) clearly regulate the directed migration of endogenous neural progenitors from SVZ to the ischemic striatum (Belmadani et al., 2006; Imitola et al., 2004; Robin et al., 2006; Yan et al., 2007). Indeed, neural progenitors of the SVZ and DG express the chemokine receptor CXCR4 (Tran et al., 2007; Tran et al., 2004), and SDF-1 expression is highly upregulated in reactive astrocytes, microglia and endothelial cells around the infarct during several weeks after a stroke (Hill et al., 2004; Thored et al., 2006). In vitro, SDF-1 stimulates neural progenitor cell proliferation and promotes chain migration and transmigration (Imitola et al., 2004). In vivo, blocking SDF-1 action with a neutralizing antibody against CXCR4 strongly reduces stroke-induced progenitor migration (Robin et al., 2006), thus convincingly implicating SDF-1/CXCR4 in the recruitment of stem/progenitor cells toward the lesion. Very similar results were obtained with MCP-1 and CCR2 (Belmadani et al., 2006; Yan et al., 2007), supporting the crucial role of cytokines in the chemoattraction of neural progenitors in a lesioned brain. Hypoxia-induced astrocytes upregulate multiple chemokines (SCF, SDF-1, MCP-1) and growth factors (VEGF), which are all involved in neural progenitor cell migration, in a time-dependent manner (Xu et al., 2007). Inflammatory cues seem also to contribute to the “homing” of transplanted neural stem cells toward affected regions of the brain. For instance, human neurospheres grafted in ischemic cortex of a rat exhibit directed migration toward the lesion, while they migrated radially and to a much lower extent in the cortex of healthy animals (Kelly et al., 2004). Similarly, in experimental autoimmune encephalomyelitis, neural stem cells grafted in the lateral ventricle or injected intrathecally or even intravenously are found within the disseminated demyelinated lesions throughout the central nervous system (Pluchino et al., 2003).
Figure 2. Schematic representation of directed neural cell migration in adult pathological rodent brain (sagittal section).
Diverse types of brain injuries trigger neural progenitor redirected migration toward the lesions. Factors involved in this directed cell migration are mentioned for each migration route refered to as letters (A to J); in blue endogenous cytokines expressed by the inflamed parenchyma, in red experimentally administered factors. The corresponding references (in brackets) are listed below. CC: corpus callosum; Cx: cortex; DG: dentate gyrus; fi: fimbria; HT: hypothalamus; LV: lateral ventricle; OB: olfactory bulb; rms: rostral migratory stream; SVZ: subventricular zone; 3V: third ventricle.
(1) Magavi et al. 2000; (2) Chen et al. 2004; (3) Ramaswamy et al. 2005; (4) Sundholm-Peters et al. 2005; (5) Fallon et al. 2000; (6) Cooper & Isacson 2004; (7) Tattersfield et al. 2004; (8) Batista et al. 2006; (9) Nait-Oumesmar et al. 1999; (10) Picard-Riera et al. 2002; (11) Parent et al. 1997; (12) Scharfmann et al. 2000; (13) Gensert et al. 1997; (14) Pencea et al. 2001; (15) Benraiss et al. 2001; (16) Chmielnicki et al. 2004; (17) Cho et al. 2007; (18) Winner et al. 2008; (19) Zhang et al. 2000; (20) Arvidsson et al. 2002; (21) Jin et al. 2003; (22) Wang et al. 2004; (23) Sugiura et al. 2005; (24) Ninomiya et al. 2006; (25) Wang et al. 2007; (26) Nakatomi et al. 2002; (27) Saghatelyan et al. 2004; (28) Magalon et al. 2007; (29) Cantarella et al. 2008; (30) Aguirre et al. 2007; (31) Imitola et al. 2004; (32) Robin et al. 2006; (33) Yan et al. 2007; (34) Williams et al. 2007; (35) El Maarouf et al., 2006
However, inflammatory cues also contribute to decrease survival of these migrating progenitors, and astrocytes contribute to form glial barrier that decrease plasticity and prevent accessibility to the lesion. These observations highlight the dual effect of the complex neuro-inflammatory milieu: on the one hand it is useful by attracting progenitors to appropriate areas to facilitate repair process, but on the other hand it prevents efficient cell replacement by affecting survival abilities of arriving progenitors. Such two-faced properties of inflammation complicate the development of therapeutic strategies in the context of many brain pathologies.
Interestingly, brain tumors also attract neural stem/progenitor cells, but the signals required for such homing are not well understood. A recent study suggests that urokinase plasmogen activator and its receptor expression together with the secretion of cytokines such as IL-6, IL-8 and MCP-1 by solid tumors could play a key role in this process (Gutova et al., 2008). These observations have led to the development of gene delivery strategies by neural stem cells to inhibit tumor growth (Aboody et al., 2008). While these studies have used transplantation of neural stem/progenitor cells or cell lines, the contribution of endogenous SVZ cells to tumor homing seems to be absent or reduced (Staflin et al., 2007). In contrast, there is evidence from animal studies using PDGF expressing retrovirus to induce glioma-like tumors that uninfected glial progenitors that reside in the adult white matter can be recruited to gliomas where they proliferate massively and contribute significantly to tumor growth (Assanah et al., 2006). More recently it has been shown that freshly isolated human glioma cells that have been transplanted into the brains of adult nude rats also have the capacity to recruit endogenous rodent PDGFRa+ glial progenitor cells into the xenograft tumors. The recruited progenitors acquire a malignant phenotype that shows the histological hallmarks of glioblastomas. For this reason, it would be impossible to distinguish recruited progenitors from bona fide glioma cells within the heterogeneous mixture that composes a human glioma specimen. However, using species-specific antibodies in a xenograft system, the authors provide unambiguous evidence of human glioma cells recruiting massive numbers of rodent progenitors. Furthermore, this study showed that recruitment was greatly enhanced by over-expressing PDGF in human glioma cells, further implicating PDGF in the paracrine signaling that mediates progenitor recruitment (Lopez et al., submitted).
Although OPCs represent a population of endogenous progenitors that may contribute to cell replacement and brain repair, at the present time they are primarily studied in the context of demyelinating diseases. Yet, deciphering the mechanisms leading to their activation, migration and differentiation in the pathological brain may open new perspectives for their use in other neurodegenerative diseases. Acute demyelinated lesions usually spontaneously recover some degree of remyelination, whereas chronic or repeated demyelination, as in chronic multiple sclerosis, often leads to incomplete remyelination. The incomplete remyelination appears to be due to a series of factors, including axonal damage, depletion of the local OPCs pool, insufficient migration of OPCs into the demyelinated areas, and differentiation failure. TNFα via TNF receptor 2 promotes the accumulation of proliferating OPCs, which then mature into myelinating oligodendrocytes (Arnett et al., 2001). Also, activation of brain immune response by lipopolysaccharide (LPS) triggers OPC reactivity. LPS-induced microglia activation promotes the recruitment of OPCs in demyelinated areas and is favorable to remyelination via the induction of key transcription factors and prevention of myelin byproducts accumulation (Glezer et al., 2006). Active multiple sclerosis lesions, where inflammatory process is present, show high expression of semaphorins (sema 3A and sema 3F) acting as OPC guidance cues during development but normally down-regulated in adulthood. Therefore these semaphorins have been proposed as good candidates for OPC-induced migration toward demyelinated lesions in the adult brain (Williams et al., 2007). PSA-NCAM up-regulation after demyelinating lesions (Oumesmar et al., 1995) may also contribute to promote OPC migration in the adult brain (Zhang et al., 2004a) but PSA needs to be down-regulated before efficient remyelination occurs (Charles et al., 2000; Fewou et al., 2007). Here again, these observations counterbalance the negative role usually assigned to inflammation in demyelinated diseases.
3.4.2- Mechanisms of glioma migration
Glioma migration-infiltration, like the migration of normal glial progenitors is a complex, multi-step process that requires the coordinated sequence of events that involves dynamic reorganization of the cytoskeleton, regulation of cell adhesion and proteolytic modification of the extracellular environment (see, for example Mohanam et al., 1994; Gillespie e al., 1999, Goldbrunner et al., 1999; Fillmore et al., 2001; Wick et al., 2001; Ernst et al., 2005; Salhai et al., 2008; Beadle et al., 2008). Indeed, the mature brain through which gliomas infiltrate is composed of tightly packed neuronal and glial processes, and the average dimensions of the extracellular space are in the sub-micrometer range (Thorne and Nicholson, 2006), smaller than the larger extracellular space in the developing CNS. This tight apposition of normal processes presents a unique mechanical challenge to migrating glioma cells that must squeeze themselves through the narrow passages of this spatially constrained environment. Gliomas accomplish this migration by a variety of mechanisms. These include the secretion of extracellular matrix material, including tenascin, laminin, fibronectin, and osteopontin, and the expression of MMPs, which presumably act by reshaping cell-matrix interactions to allow the binding and release of cell processes from the extracellular matrix that is essential for migration. Brain tumor cells express integrins, and multiple data converge to propose that, together with ECM components, integrins facilitate the infiltration through normal brain tissue (D’Abaco and Kaye, 2007). Gliomas also express voltage-gated and GABA-responsive chloride channels (Sontheimer, 2003). The efflux of water that follows the efflux of chloride causes the cell to shrink, a process that presumably is required for a cell to squeeze through the extracellular space of the CNS. These channels can be found in glioma cells in situ and at the leading edge of glioma cells in culture (Olsen et al., 2003), indicating that chloride channels allow local volume shifts within tumor cells.
Similar to glial progenitors, migrating glioma cells display transient fluctuations in intracellular free calcium, linked to calcium-permeable AMPA receptors (Ishiuchi et al., 2002). Blocking calcium entry in cultured glioblastoma cells inhibits migration. AMPA receptors, which are activated by glutamate, promote glioma migration, presumably by producing brief increases in intracellular calcium levels. Lyons et al. (2007) point out that glutamate, known to be secreted by gliomas, could have both autocrine and paracrine functions in stimulating surrounding cells via AMPA receptors. Calcium signaling is linked to underlying cytoskeletal rearrangements required for cell motility – specifically how calcium signaling stimulates the migration of glial progenitors and gliomas remains to be determined.
As noted above, myosin II is a critical molecule in the forward movement of the cell body and nucleus during saltatory migration. Treating brain slices with a small molecule inhibitor of myosin II, blebbistatin, effectively blocks the migration of glioma cells (Beadle et al., 2008). Similarly, blebbistatin or RNAi knockdown of myosin II inhibited migration across a 3-micron pore in a transfilter migration assay. In contrast, when glioma cells were placed on a 2-dimensional surface and allowed to migrate into a spatially unrestricted cell-free zone, they did not move in a saltatory manner and they did not require myosin II. This study showed that the cellular mechanisms of glioma migration depend on the mechanical properties of the environment in which the cells must move and highlight the importance of studying migration in a physiologically relevant system.
4- Therapeutic strategies and perspectives
Considering the migratory and potential migratory abilities of stem/progenitor cells, one could propose the mobilization of endogenous stem and progenitor cells as an alternative to grafting. Repair by endogenous cells would overcome the problem of transplant rejection. Despite its status of “immuno-privileged organ”, recent findings demonstrate that the brain remains sensitive to immune attacks via some activated lymphocytes and microglial cells with antigen presenting properties (Wekerle, 2007). Furthermore, neural stem cells express MHC class I proteins (Yin et al., 2008). Although at the present time the compensatory neurogenesis described after various types of brain lesions appears to be modest, such a strategy remains of high interest. The main challenge, therefore, resides in promoting directed mobilization and phenotypic induction of stem/progenitor cells.
4.1- Administration of trophic factors and cytokines to promote SVZ cell mobilisation
A number of trophic factors and cytokines have been tested and delivered to the brain either by viral vectors, infusions or, less invasively, via intranasal administration. Some exhibit interesting properties (Fig. 2). The first evidence that endogenous stem/progenitors could respond to extrinsic factors in order to promote proliferation, directed migration and neuronal replacement in a lesioned brain was given by Fallon et al. (2000), who showed that TGFa infusion into the putamen of a6 -OHDA mouse model of Parkinson disease attracted neuronal progenitors toward the lesion and improved functional deficits. This work raised hope for the development of new therapeutic strategies in Parkinson disease and other neurodegenerative pathologies. Other studies, however, have not engendered as much optimism. For instance, in the same animal model, infusion of FGF-2 and EGF also proved useful in increasing directed neuroblast migration toward the lesioned striatum; however, neuronal differentiation failed to occur, gliogenesis being largely favored (Winner et al., 2008). Nakatomi and colleagues (2002) showed, using a cocktail of FGF-2 and EGF, that trophic factor infusion into the ventricle could enhance neuron replacement and recovery in ischemic hippocampus. Further studies showed that EGF signaling plays a critical role in enhancing SVZ cell mobilization. HB-EGF intraventricular injection after focal ischemia leads to the expansion of transit amplifying cells in the SVZ together with a higher number of mature neurons formation in the ipsilateral striatum/hippocampus as compared to animals injected with control solution; furthermore, it significantly enhanced functional recovery (Ninomiya et al., 2006; Sugiura et al., 2005). In a focal demyelination model, intranasal administration of HB-EGF also triggered SVZ cell proliferation, favored cell dispersion from the RMS, and consequently promoted directed mobilization of stem/progenitor cells toward the demyelinated corpus callosum where they gave rise to oligodendrocytes and astrocytes (Cantarella et al., 2008). In the same model but using another approach by overexpression of EGFR under the control of the CNP promoter, Aguirre and colleagues demonstrated that EGF signaling contributes to enhance proliferation, migration and survival in both SVZ- and white matter- endogenous progenitors, leading to improved remyelination (Aguirre et al., 2007). From these studies, it appears that EGF could stand as an interesting candidate to promote endogenous progenitor recruitment. However, it also presents limitations, two of them being real concerns: 1- it is not favorable to neuronal differentiation and therefore rather may lead to astrocytic differentiation of recruited cells (which could generate more deleterious than beneficial effects), and 2- it may facilitate the development of tumors (Ayuso-Sacido et al., 2006).
Brain derived neurotrophic factor (BDNF) is another growth factor that presents valuable properties for endogenous progenitor mobilization and neuronal replacement. In the healthy brain, intraventricular injection of an adenovirus expressing BDNF triggers increased olfactory neurogenesis, but more interestingly, it also induces the migration of newborn neurons from SVZ to otherwise non-neurogenic regions, such as the neostriatum where they differentiate into medium spiny neurons, (Benraiss et al., 2001; Pencea et al., 2001). BDNF-mediated recruitment of new neurons in the adult neostriatum can be further potentiated by concomitant administration of noggin, an inhibitor of glial differentiation (Chmielnicki et al., 2004). In the pathological brain, BDNF efficiently improves compensatory neurogenesis in the lesioned striatum (Henry et al., 2007). After a stroke, brain levels of BDNF and VEGF can be increased by erythropoietin treatment, thus contributing to angiogenesis, neurogenesis and functional recovery (Wang et al., 2004b). Finally, in a rodent model of Huntington’s disease, the R6/2 transgenic mice, the strategy of BDNF and noggin combined adenoviral injection provided enhanced striatal neuronal replacement with correct projection into the globus pallidus, delayed motor impairment and increased survival of the mice (Cho et al., 2007). This experimental success points out the need to associate strategies targeting different steps of the mobilization process: proliferation, inhibition of glial differentiation, neuronal differentiation and survival.
Considering the migration-facilitating properties of vasculature noted above (physical guide, MMP secretion, survival effects, etc.), angiogenesis represents another putative target to improve mediated mobilization of endogenous progenitor cells. The hematopoeitic cytokine erythropoietin (EPO) is a strong enhancer of angiogenesis. Intraperitoneal injection of EPO in rats with middle cerebral artery occlusion significantly improves functional recovery, and improves neurogenesis and migration of DCX-positive neuronal progenitors toward the ischemic boundary of the cortex (Kolb et al., 2007). EPO concomitantly increases brain VEGF and BDNF titers, suggesting that angiogenesis and neurogenesis effects of EPO may be mediated via these factors (Wang et al., 2004b). In accordance with this hypothesis, Sun et al. (2003) showed that there was improved histological and functional outcome from stroke after VEGF treatment in rats. Similarly, VEGF overexpressing mice exhibit enhanced post-ischemic neurogenesis and neuromigration, with chains of neuroblasts extending from the SVZ to the peri-infarct cortex, together with improved motor recovery (Wang et al., 2007). Therapeutic benefits of nitric oxide (NO) administration after stroke have also been proposed (Zhang et al., 2001a). Indeed, NO donors induce cell proliferation in neurogenic regions of the adult brain, and reduce neurological deficits following middle cerebral artery occlusion. However, these effects have been shown to be mediated via cGMP-increased VEGF and angiogenesis (Zhang et al., 2003). It can be concluded from these studies that VEGF presents interesting properties for brain repair, associating neuroprotection and enhanced migration and neurogenesis.
The extracellular matrix protein Tenascin-R, which is a key player in the detachment of migrating neuroblasts once they reach the OB, may also be useful to promote directed migration toward periventricular structures. Indeed, when grafted cells providing an ectopic source of Tenascin-R are present in the striatum or above the RMS, PSA-NCAM-positive progenitors are rerouted from the SVZ/RMS toward the Tenascin-R producing cells, suggesting potential therapeutic implications (Saghatelyan et al., 2004).
Finally, another strategy consisting in overexpressing PSA-NCAM may be helpful to promote cell migration in the adult brain. Indeed, a recent study showed that embryonic stem cell-derived glial progenitors transduced to overexpress polysialyltransferase are more responsive to chemotropic factors and present enhanced migrating behaviour after grafting in the striatum (Glaser et al., 2007). Furthermore, viral-induced PSA overexpression in astrocytes strongly promotes endogenous progenitor cell recruitment to cortical lesion in adult mice (El Maarouf et al., 2006). Altogether these data suggest that PSA or PSA-mimetic peptides could be interesting molecules for the development of innovating brain repair strategies.
4.2- Environmental enrichment and exercise
A broad number of studies suggest that environment enrichment and exercise enhance brain plasticity. In physiological conditions, running and enrichment increase the levels of growth factors such as BDNF, IGF, NGF, and FGF2 (Carro et al., 2001; Ding et al., 2004; Gomez-Pinilla et al., 1997; Ickes et al., 2000; Pham et al., 1999) and stimulate DG neurogenesis (Kempermann et al., 1997; Kempermann and Gage, 1999; van Praag et al., 1999). In pathological conditions, enrichment may promote neuronal survival and resistance to brain insult (Carro et al., 2001; Young et al., 1999). Functional recovery provided by neural grafts is also improved by enrichment (Dobrossy and Dunnett, 2001, 2005). In addition to these effects, recent studies showed that such enrichment could be used to promote SVZ cell mobilization and directed migration of progenitors toward brain lesions. In a focal model of demyelination as well as in experimental autoimmune encephalomyelitis, environmental enrichment associated with voluntary exercise stimulated SVZ reactivity, increased the number of SVZ-derived cells within demyelinated lesions and accelerated motor recovery (Magalon et al., 2007). Similarly, in the case of stroke, enriched environment increased the number of endogenous DCX-positive migrating neuronal progenitors in the SVZ, and also promoted survival of SVZ grafted cells and their migration toward ischemic areas, leading to improved functional recovery (Hicks et al., 2007). All these arguments plead in favor of the potential use of enrichment and exercise as complementary therapy for brain repair.
4.3- Are there other endogenous cell types usable for brain repair?
NG2-proteoglycan-expressing progenitors are distributed throughout the postnatal CNS and are slowly dividing; they thus represent the largest pool of proliferative progenitors in the postnatal brain. Genetic tracing demonstrated that NG2-positive cells generate oligodendrocytes and also a subpopulation of protoplasmic astrocytes (Zhu et al., 2008). A fraction of this cell population in the neonatal rodent CNS can acquire stem-like properties in cell culture (Belachew et al., 2003). They can form neurospheres in vitro and generate astrocytes, oligodendrocytes and neurons. Considering the distribution, the proliferative and migrating properties and the multipotency of these NG2-positive progenitors, they may represent a major cellular target for the development of endogenous brain repair strategies. However, these hopes are weakened by the observation that only early postnatal but not adult cortical NG2-positive progenitors are able to form renewable and multipotent neurospheres (Buffo et al., 2008). Nevertheless, in the adult rabbit a delayed genesis of neuronal and glial progenitors has been demonstrated from local progenitors in the molecular layer of the cerebellum (Ponti et al., 2008). Uncovering the specificities underlying the potentialities proper to the rabbit cerebellum could be of great help for understanding nervous tissue permissiveness in mammals and for the perspective of modulating widespread adult neurogenesis beyond the highly restricted neurogenic niches. Similar multipotent progenitors have been identified in the adult human brain and isolated from subcortical white matter (Nunes et al., 2003).
A new promising source of multipotent cells has been recently proposed: reactive astrocytes. Upon injury, local astrocytes undergo morphological modifications, with somata and processes hypertrophy, up-regulation of GFAP and re-expression of markers such as vimentin and nestin (Miyake et al., 1988; Wilhelmsson et al., 2006). Using fate mapping approach, it has been demonstrated that a large proportion of quiescent astrocytes re-enter the cell cycle and contribute to the generation of reactive astrocytes after a brain lesion. Even more striking, these reactive astrocytes are able to form self-renewable and multipotent neurospheres when placed in favorable culture medium (Buffo et al., 2008). Thus, brain injury can induce post-mitotic mature astrocytes to divide and acquire stem cell-like properties. Unfortunately, in vivo the parenchymal environment is strongly non-neurogenic and therefore reactive astrocytes do not generate neurons. Further work should be pursued in order to understand how to counteract the non-permissive environment and consequently allow neuronal differentiation of reactive astrocytes. Such strategy could be an alternative way to work around the obstacle of long migration of neural progenitors from neurogenic niches to distant brain lesions.
Conclusion
The recent finding that neural stem cells reside in the adult central nervous system throughout life and present the capacity to migrate through this mature structure raised hope to develop new therapeutic strategies. However, the endogenous progenitor cell mobilization that spontaneously occurs after a lesion or in neurodegenerative pathologies only leads to very low rates of cell replacement. Transplantation strategies can be considered an interesting alternative since (i) white matter tracts appear to be permissive structures for neural progenitor migration and (ii) the injured area produces chemoattractant coumpounds. Beside stem/progenitor cell mobilization and migration, efficient repair will be achieved only if we concomitantly manage to control cell death. Indeed, the vast majority of the recruited progenitors die very quickly and therefore they do not contribute to cell replacement (Arvidsson et al., 2002). In addition to cell replacement, the presence of neural stem cells within lesioned areas may be beneficial through anti-inflammatory, neuroprotective and neurotrophic effects (Ourednik and Ourednik, 2007; Einstein and Ben-Hur, 2008). In the last decade, a vigorous effort has been dedicated to better understand neural stem cell biology, to decipher why stem cells reside in specific “niches” (Ninkovic and Götz, 2007; Riquelme et al., 2008), and to determine which signaling cascades control their proliferation, migration and differentiation. With the prospect of using these cells in pathological conditions to promote brain repair, it is now crucial to address the question of their survival outside of their niches and in pathological environments (Chen and Palmer, 2008). Cooperation between neurobiologists and immunologists will promote solutions to such a challenge.
Abbreviation List
- 6-OHDA
6-hydroxydopamine
- AD
Alzheimer’s disease
- ADAM
Proteins containing a disintegrin and metalloprotease domain
- AMPA
alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- BDNF
brain derived neurotrophic factor
- BrdU
bromodeoxyuridine
- CC
corpus callosum
- CNP
2′,3′-Cyclic Nucleotide 3′-Phosphodiesterase
- CNS
central nervous system
- CREB
cAMP response element-binding
- CSF
cerebrospinal fluid
- CVMS
caudoventral migratory streams
- Cx
cortex
- Dab1
disabled 1
- DCC
deleted in colorectal carcinoma
- DCX
doublecortin
- DG
dentate gyrus
- ECM
extracellular matrix
- EGF
epithelial growth factor
- EGFR
epithelial growth factor receptor
- EPO
erythropoietin
- FGF-2
fibroblast growth factor 2
- Fi
fimbria
- GABA
gamma aminobutyric acid
- GDNF
glial derived neurotrophic factor
- GFAP
glial fibrillary acidic protein
- GFP
green fluorescent protein
- GL
granular layer
- GPI
glycosylphosphatidylinositol
- GSK3β
glycogen synthase kinase 3β
- HB-EGF
heparin binding epithelial growth factor
- IGF
insulin growth factor
- LPS
lipopolysaccharid
- LV
lateral ventricle
- MCP-1
monocyte chemotactic protein-1
- MHC
major histocompatibility complex
- MIA
migration induced-activity
- MMP
matrix metalloprotease
- NGF
nerve growth factor
- NO
nitric oxide
- NRG
neuregulin
- OB
olfactory bulb
- OPC
oligodendrocyte precursor cell
- PDGF
platelet derived groth factor
- PDGFRα
platelet derived groth factor receptor alpha
- PI3K
phosphoinositide 3-kinase
- PKCζ
atypical protein kinase C
- PSA-NCAM
the polysialic form of the neural cell adhesion molecule
- RMS
rostral migratory stream
- SDF-1
stromal derived factor 1
- Sema
semaphorin
- SGL
subgranular layer
- SVZ
subventricular zone
- TGFα
transforming growth factor α
- TNF
tumor necrosis factor
- VCMS
ventrocaudal migratory streams
- VEGF
vascular endothelial growth factor
- VLDLR
very low density lipoprotein receptor
Glossary
- Neural stem cells
Cells that reside in the neural tissue and are multipotent (can give rise to astrocytes, oligodendrocytes and neurons) and have the capacity to self renew (they divide and maintain a pool of cells identical to themselves all life long)
- Neural progenitors
Derive from neural stem cells but present a restricted potentiality(often unipotent). Although they are proliferative cells, they present limited self-renewal potential. Here we used the term “neuroblast” to design a neuronal progenitor, i.e. a neural progenitor restricted to the neuronal lineage
- Neural precursors
immature cells that are engaged in a specific cell lineage. They stop proliferating and give rise to the finally mature cell
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aboody KS, Najbauer J, Danks MK. Stem and progenitor cell-mediated tumor selective gene therapy. Gene therapy. 2008;15:739–752. doi: 10.1038/gt.2008.41. [DOI] [PubMed] [Google Scholar]
- Agresti C, Meomartini ME, Amadio S, Ambrosini E, Volonté C, Aloisi F, Visentin S. ATP regulates oligodendrocyte progenitor migration, proliferation, and differentiation: involvement of metabotropic P2 receptors. Brain Res Brain Res Rev. 2005;48:157–65. doi: 10.1016/j.brainresrev.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Aguirre A, Dupree JL, Mangin JM, Gallo V. A functional role for EGFR signaling in myelination and remyelination. Nat Neurosci. 2007;10:990–1002. doi: 10.1038/nn1938. [DOI] [PubMed] [Google Scholar]
- Aguirre A, Rizvi TA, Ratner N, Gallo V. Overexpression of the epidermal growth factor receptor confers migratory properties to nonmigratory postnatal neural progenitors. J Neurosci. 2005;25:11092–11106. doi: 10.1523/JNEUROSCI.2981-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aicardi J. The place of neuronal migration abnormalities in child neurology. The Canadian journal of neurological sciences. 1994;21:185–193. doi: 10.1017/s0317167100041159. [DOI] [PubMed] [Google Scholar]
- Alonso G. Proliferation of progenitor cells in the adult rat brain correlates with the presence of vimentin-expressing astrocytes. Glia. 2001;34:253–266. doi: 10.1002/glia.1059. [DOI] [PubMed] [Google Scholar]
- Alonso M, Ortega-Pérez I, Grubb MS, Bourgeois JP, Charneau P, Lledo PM. Turning astrocytes from the rostral migratory stream into neurons: a role for the olfactory sensory organ. J Neurosci. 2008;28:11089–102. doi: 10.1523/JNEUROSCI.3713-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman J, Bayer SA. Prolonged sojourn of developing pyramidal cells in the intermediate zone of the hippocampus and their settling in the stratum pyramidale. The Journal of comparative neurology. 1990;301:343–364. doi: 10.1002/cne.903010303. [DOI] [PubMed] [Google Scholar]
- Altman J, Bayer SA. Development of the cerebellar system. CRC Press; Boca Raton: 1997. [Google Scholar]
- Andrade N, Komnenovic V, Blake SM, Jossin Y, Howell B, Goffinet A, Schneider WJ, Nimpf J. ApoER2/VLDL receptor and Dab1 in the rostral migratory stream function in postnatal neuronal migration independently of Reelin. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:8508–8513. doi: 10.1073/pnas.0611391104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angot E, Loulier K, Nguyen-Ba-Charvet KT, Gadeau AP, Ruat M, Traiffort E. Chemoattractive activity of sonic hedgehog in the adult subventricular zone modulates the number of neural precursors reaching the olfactory bulb. Stem cells. 2008;26:2311–2320. doi: 10.1634/stemcells.2008-0297. [DOI] [PubMed] [Google Scholar]
- Anton ES, Ghashghaei HT, Weber JL, McCann C, Fischer TM, Cheung ID, Gassmann M, Messing A, Klein R, Schwab MH, Lloyd KC, Lai C. Receptor tyrosine kinase ErbB4 modulates neuroblast migration and placement in the adult forebrain. Nat Neurosci. 2004;7:1319–1328. doi: 10.1038/nn1345. [DOI] [PubMed] [Google Scholar]
- Armstrong RC, Harvath L, Dubois-Dalcq ME. Type 1 astrocytes and oligodendrocyte-type 2 astrocyte glial progenitors migrate toward distinct molecules. J Neurosci Res. 1990;27:400–7. doi: 10.1002/jnr.490270319. [DOI] [PubMed] [Google Scholar]
- Arnett HA, Mason J, Marino M, Suzuki K, Matsushima GK, Ting JP. TNF alpha promotes proliferation of oligodendrocyte progenitors and remyelination. Nat Neurosci. 2001;4:1116–1122. doi: 10.1038/nn738. [DOI] [PubMed] [Google Scholar]
- Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nature medicine. 2002;8:963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- Assanah M, Lochhead R, Ogden A, Bruce J, Goldman J, Canoll P. Glial progenitors in adult white matter are driven to form malignant gliomas by platelet-derived growth factor-expressing retroviruses. J Neurosci. 2006;26:6781–6790. doi: 10.1523/JNEUROSCI.0514-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayuso-Sacido A, Graham C, Greenfield JP, Boockvar JA. The duality of epidermal growth factor receptor (EGFR) signaling and neural stem cell phenotype: cell enhancer or cell transformer? Current stem cell research & therapy. 2006;1:387–394. doi: 10.2174/157488806778226849. [DOI] [PubMed] [Google Scholar]
- Baker SA, Baker KA, Hagg T. Dopaminergic nigrostriatal projections regulate neural precursor proliferation in the adult mouse subventricular zone. The European journal of neuroscience. 2004;20:575–579. doi: 10.1111/j.1460-9568.2004.03486.x. [DOI] [PubMed] [Google Scholar]
- Balordi F, Fishell G. Hedgehog signaling in the subventricular zone is required for both the maintenance of stem cells and the migration of newborn neurons. J Neurosci. 2007;27:5936–5947. doi: 10.1523/JNEUROSCI.1040-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista CM, Kippin TE, Willaime-Morawek S, Shimabukuro MK, Akamatsu W, van der Kooy D. A progressive and cell non-autonomous increase in striatal neural stem cells in the Huntington’s disease R6/2 mouse. J Neurosci. 2006;26:10452–10460. doi: 10.1523/JNEUROSCI.2850-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beadle C, Assanah MC, Monzo P, Vallee R, Rosenfeld SS, Canoll P. The Role of Myosin II in Glioma Invasion of the Brain. Mol Biol Cell. 2008;19:3357–68. doi: 10.1091/mbc.E08-03-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belachew S, Chittajallu R, Aguirre AA, Yuan X, Kirby M, Anderson S, Gallo V. Postnatal NG2 proteoglycan-expressing progenitor cells are intrinsically multipotent and generate functional neurons. J Cell Biol. 2003;161:169–186. doi: 10.1083/jcb.200210110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellion A, Baudoin JP, Alvarez C, Bornens M, Metin C. Nucleokinesis in tangentially migrating neurons comprises two alternating phases: forward migration of the Golgi/centrosome associated with centrosome splitting and myosin contraction at the rear. Journal of Neuroscience. 2005;25:5691–5699. doi: 10.1523/JNEUROSCI.1030-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belluzzi O, Benedusi M, Ackman J, LoTurco JJ. Electrophysiological differentiation of new neurons in the olfactory bulb. J Neurosci. 2003;23:10411–10418. doi: 10.1523/JNEUROSCI.23-32-10411.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmadani A, Tran PB, Ren D, Miller RJ. Chemokines regulate the migration of neural progenitors to sites of neuroinflammation. J Neurosci. 2006;26:3182–3191. doi: 10.1523/JNEUROSCI.0156-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaschuk KL, Frost EE, ffrench-Constant C. The regulation of proliferation and differentiation in oligodendrocyte progenitor cells by alphaV integrins. Development. 2000;127:1961–9. doi: 10.1242/dev.127.9.1961. [DOI] [PubMed] [Google Scholar]
- Belvindrah R, Hankel S, Walker J, Patton BL, Muller U. Beta1 integrins control the formation of cell chains in the adult rostral migratory stream. J Neurosci. 2007;27:2704–2717. doi: 10.1523/JNEUROSCI.2991-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benraiss A, Chmielnicki E, Lerner K, Roh D, Goldman SA. Adenoviral brain-derived neurotrophic factor induces both neostriatal and olfactory neuronal recruitment from endogenous progenitor cells in the adult forebrain. J Neurosci. 2001;21:6718–6731. doi: 10.1523/JNEUROSCI.21-17-06718.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier PJ, Bedard A, Vinet J, Levesque M, Parent A. Newly generated neurons in the amygdala and adjoining cortex of adult primates. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:11464–11469. doi: 10.1073/pnas.172403999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore WF, Keirstead HS. The origin of remyelinating cells in the central nervous system. J Neuroimmunol. 1999;98:69–76. doi: 10.1016/s0165-5728(99)00083-1. [DOI] [PubMed] [Google Scholar]
- Blumcke I, Schewe JC, Normann S, Brustle O, Schramm J, Elger CE, Wiestler OD. Increase of nestin-immunoreactive neural precursor cells in the dentate gyrus of pediatric patients with early-onset temporal lobe epilepsy. Hippocampus. 2001;11:311–321. doi: 10.1002/hipo.1045. [DOI] [PubMed] [Google Scholar]
- Bolteus AJ, Bordey A. GABA release and uptake regulate neuronal precursor migration in the postnatal subventricular zone. J Neurosci. 2004;24:7623–7631. doi: 10.1523/JNEUROSCI.1999-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borta A, Hoglinger GU. Dopamine and adult neurogenesis. J Neurochem. 2007;100:587–595. doi: 10.1111/j.1471-4159.2006.04241.x. [DOI] [PubMed] [Google Scholar]
- Bovetti S, Bovolin P, Perroteau I, Puche AC. Subventricular zone-derived neuroblast migration to the olfactory bulb is modulated by matrix remodelling. The European journal of neuroscience. 2007a;25:2021–2033. doi: 10.1111/j.1460-9568.2007.05441.x. [DOI] [PubMed] [Google Scholar]
- Bovetti S, Hsieh YC, Bovolin P, Perroteau I, Kazunori T, Puche AC. Blood vessels form a scaffold for neuroblast migration in the adult olfactory bulb. J Neurosci. 2007b;27:5976–5980. doi: 10.1523/JNEUROSCI.0678-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JP, Couillard-Despres S, Cooper-Kuhn CM, Winkler J, Aigner L, Kuhn HG. Transient expression of doublecortin during adult neurogenesis. The Journal of comparative neurology. 2003;467:1–10. doi: 10.1002/cne.10874. [DOI] [PubMed] [Google Scholar]
- Bruel-Jungerman E, Laroche S, Rampon C. New neurons in the dentate gyrus are involved in the expression of enhanced long-term memory following environmental enrichment. The European journal of neuroscience. 2005;21:513–521. doi: 10.1111/j.1460-9568.2005.03875.x. [DOI] [PubMed] [Google Scholar]
- Buffo A, Rite I, Tripathi P, Lepier A, Colak D, Horn AP, Mori T, Gotz M. Origin and progeny of reactive gliosis: A source of multipotent cells in the injured brain. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:3581–3586. doi: 10.1073/pnas.0709002105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo JL, Carbonell AL, Boya J. Co-expression of glial fibrillary acidic protein and vimentin in reactive astrocytes following brain injury in rats. Brain Res. 1991;566:333–336. doi: 10.1016/0006-8993(91)91720-l. [DOI] [PubMed] [Google Scholar]
- Cameron HA, McKay RD. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. The Journal of comparative neurology. 2001;435:406–417. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- Cameron HA, Woolley CS, McEwen BS, Gould E. Differentiation of newly born neurons and glia in the dentate gyrus of the adult rat. Neuroscience. 1993;56:337–344. doi: 10.1016/0306-4522(93)90335-d. [DOI] [PubMed] [Google Scholar]
- Canoll P, Goldman JE. The interface between glial progenitors and gliomas. Acta neuropathologica. 2008;116:465–477. doi: 10.1007/s00401-008-0432-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantarella C, Cayre M, Magalon K, Durbec P. Intranasal HB-EGF administration favors adult SVZ cell mobilization to demyelinated lesions in mouse corpus callosum. Developmental neurobiology. 2008;68:223–236. doi: 10.1002/dneu.20588. [DOI] [PubMed] [Google Scholar]
- Capela A, Temple S. LeX/ssea-1 is expressed by adult mouse CNS stem cells, identifying them as nonependymal. Neuron. 2002;35:865–875. doi: 10.1016/s0896-6273(02)00835-8. [DOI] [PubMed] [Google Scholar]
- Carleton A, Petreanu LT, Lansford R, Alvarez-Buylla A, Lledo PM. Becoming a new neuron in the adult olfactory bulb. Nat Neurosci. 2003;6:507–518. doi: 10.1038/nn1048. [DOI] [PubMed] [Google Scholar]
- Carro E, Trejo JL, Busiguina S, Torres-Aleman I. Circulating insulin-like growth factor I mediates the protective effects of physical exercise against brain insults of different etiology and anatomy. J Neurosci. 2001;21:5678–5684. doi: 10.1523/JNEUROSCI.21-15-05678.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cayre M, Bancila M, Virard I, Borges A, Durbec P. Migrating and myelinating potential of subventricular zone neural progenitor cells in white matter tracts of the adult rodent brain. Molecular and cellular neurosciences. 2006;31:748–758. doi: 10.1016/j.mcn.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Charles P, Hernandez MP, Stankoff B, Aigrot MS, Colin C, Rougon G, Zalc B, Lubetzki C. Negative regulation of central nervous system myelination by polysialylated-neural cell adhesion molecule. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:7585–7590. doi: 10.1073/pnas.100076197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chazal G, Durbec P, Jankovski A, Rougon G, Cremer H. Consequences of neural cell adhesion molecule deficiency on cell migration in the rostral migratory stream of the mouse. J Neurosci. 2000;20:1446–1457. doi: 10.1523/JNEUROSCI.20-04-01446.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Magavi SS, Macklis JD. Neurogenesis of corticospinal motor neurons extending spinal projections in adult mice. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:16357–16362. doi: 10.1073/pnas.0406795101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Palmer TD. Cellular repair of CNS disorders: an immunological perspective. Hum Mol Genet. 2008;17(R1):R84–92. doi: 10.1093/hmg/ddn104. [DOI] [PubMed] [Google Scholar]
- Chevassus-au-Louis N, Represa A. The right neuron at the wrong place: biology of heterotopic neurons in cortical neuronal migration disorders, with special reference to associated pathologies. Cell Mol Life Sci. 1999;55:1206–1215. doi: 10.1007/s000180050367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiaramello S, Dalmasso G, Bezin L, Marcel D, Jourdan F, Peretto P, Fasolo A, De Marchis S. BDNF/TrkB interaction regulates migration of SVZ precursor cells via PI3-K and MAP-K signalling pathways. The European journal of neuroscience. 2007;26:1780–1790. doi: 10.1111/j.1460-9568.2007.05818.x. [DOI] [PubMed] [Google Scholar]
- Chiasson BJ, Tropepe V, Morshead CM, van der Kooy D. Adult mammalian forebrain ependymal and subependymal cells demonstrate proliferative potential, but only subependymal cells have neural stem cell characteristics. J Neurosci. 1999;19:4462–4471. doi: 10.1523/JNEUROSCI.19-11-04462.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chmielnicki E, Benraiss A, Economides AN, Goldman SA. Adenovirally expressed noggin and brain-derived neurotrophic factor cooperate to induce new medium spiny neurons from resident progenitor cells in the adult striatal ventricular zone. J Neurosci. 2004;24:2133–2142. doi: 10.1523/JNEUROSCI.1554-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho SR, Benraiss A, Chmielnicki E, Samdani A, Economides A, Goldman SA. Induction of neostriatal neurogenesis slows disease progression in a transgenic murine model of Huntington disease. The Journal of clinical investigation. 2007;117:2889–2902. doi: 10.1172/JCI31778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary MA, Uboha N, Picciotto MR, Beech RD. Expression of ezrin in glial tubes in the adult subventricular zone and rostral migratory stream. Neuroscience. 2006;143:851–861. doi: 10.1016/j.neuroscience.2006.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin T, Arvidsson A, Kokaia Z, Lindvall O. Quantitative analysis of the generation of different striatal neuronal subtypes in the adult brain following excitotoxic injury. Exp Neurol. 2005;195:71–80. doi: 10.1016/j.expneurol.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Conover JC, Doetsch F, Garcia-Verdugo JM, Gale NW, Yancopoulos GD, Alvarez-Buylla A. Disruption of Eph/ephrin signaling affects migration and proliferation in the adult subventricular zone. Nat Neurosci. 2000;3:1091–1097. doi: 10.1038/80606. [DOI] [PubMed] [Google Scholar]
- Cooper O, Isacson O. Intrastriatal transforming growth factor alpha delivery to a model of Parkinson’s disease induces proliferation and migration of endogenous adult neural progenitor cells without differentiation into dopaminergic neurons. J Neurosci. 2004;24:8924–8931. doi: 10.1523/JNEUROSCI.2344-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig CG, Tropepe V, Morshead CM, Reynolds BA, Weiss S, van der Kooy D. In vivo growth factor expansion of endogenous subependymal neural precursor cell populations in the adult mouse brain. J Neurosci. 1996;16:2649–2658. doi: 10.1523/JNEUROSCI.16-08-02649.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis MA, Kam M, Nannmark U, Anderson MF, Axell MZ, Wikkelso C, Holtas S, van Roon-Mom WM, Bjork-Eriksson T, Nordborg C, Frisen J, Dragunow M, Faull RL, Eriksson PS. Human neuroblasts migrate to the olfactory bulb via a lateral ventricular extension. Science. 2007a;315:1243–1249. doi: 10.1126/science.1136281. [DOI] [PubMed] [Google Scholar]
- Curtis MA, Kam M, Nannmark U, Faull RL, Eriksson PS. Response to comment on “human neuroblasts migrate to the olfactory bulb via a lateral ventricular extension. Science. 2007b;318:392–394. doi: 10.1126/science.1136281. [DOI] [PubMed] [Google Scholar]
- Curtis MA, Penney EB, Pearson AG, van Roon-Mom WM, Butterworth NJ, Dragunow M, Connor B, Faull RL. Increased cell proliferation and neurogenesis in the adult human Huntington’s disease brain. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:9023–9027. doi: 10.1073/pnas.1532244100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Arcangelo G, Miao GG, Chen SC, Soares HD, Morgan JI, Curran T. A protein related to extracellular matrix proteins deleted in the mouse mutant reeler. Nature. 1995;374:719–723. doi: 10.1038/374719a0. [DOI] [PubMed] [Google Scholar]
- D’Abaco GM, Kaye AH. Integrins: molecular determinants of glioma invasion. J Clin Neurosci. 2007;14:1041–8. doi: 10.1016/j.jocn.2007.06.019. [DOI] [PubMed] [Google Scholar]
- Darsalia V, Heldmann U, Lindvall O, Kokaia Z. Stroke-induced neurogenesis in aged brain. Stroke; a journal of cerebral circulation. 2005;36:1790–1795. doi: 10.1161/01.STR.0000173151.36031.be. [DOI] [PubMed] [Google Scholar]
- Das S, Basu A. Inflammation: a new candidate in modulating adult neurogenesis. J Neurosci Res. 2008;86:1199–208. doi: 10.1002/jnr.21585. [DOI] [PubMed] [Google Scholar]
- Davenne M, Custody C, Charneau P, Lledo PM. In vivo imaging of migrating neurons in the Mammalian forebrain. Chemical senses. 2005;30(Suppl 1):i115–i116. doi: 10.1093/chemse/bjh141. [DOI] [PubMed] [Google Scholar]
- Dawson MR, Polito A, Levine JM, Reynolds R. NG2-expressing glial progenitor cells: an abundant and widespread population of cycling cells in the adult rat CNS. Molecular and cellular neurosciences. 2003;24:476–488. doi: 10.1016/s1044-7431(03)00210-0. [DOI] [PubMed] [Google Scholar]
- Decker L, Avellana-Adalid V, Nait-Oumesmar B, Durbec P, Baron-Van Evercooren A. Oligodendrocyte precursor migration and differentiation: combined effects of PSA residues, growth factors, and substrates. Mol Cell Neurosci. 2000;16:422–39. doi: 10.1006/mcne.2000.0885. [DOI] [PubMed] [Google Scholar]
- De Marchis S, Fasolo A, Shipley M, Puche A. Unique neuronal tracers show migration and differentiation of SVZ progenitors in organotypic slices. Journal of neurobiology. 2001;49:326–338. doi: 10.1002/neu.10012. [DOI] [PubMed] [Google Scholar]
- De Marchis S, Fasolo A, Puche AC. Subventricular zone-derived neuronal progenitors migrate into the subcortical forebrain of postnatal mice. J Comp Neurol. 2004;476:290–300. doi: 10.1002/cne.20217. [DOI] [PubMed] [Google Scholar]
- des Portes V, Pinard JM, Billuart P, Vinet MC, Koulakoff A, Carrie A, Gelot A, Dupuis E, Motte J, Berwald-Netter Y, Catala M, Kahn A, Beldjord C, Chelly J. A novel CNS gene required for neuronal migration and involved in X-linked subcortical laminar heterotopia and lissencephaly syndrome. Cell. 1998;92:51–61. doi: 10.1016/s0092-8674(00)80898-3. [DOI] [PubMed] [Google Scholar]
- Dimou L, Simon C, Kirchhoff F, Takebayashi H, Gotz M. Progeny of Olig2-expressing progenitors in the gray and white matter of the adult mouse cerebral cortex. J Neurosci. 2008;28:10434–10442. doi: 10.1523/JNEUROSCI.2831-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding YH, Luan XD, Li J, Rafols JA, Guthinkonda M, Diaz FG, Ding Y. Exercise-induced overexpression of angiogenic factors and reduction of ischemia/reperfusion injury in stroke. Current neurovascular research. 2004;1:411–420. doi: 10.2174/1567202043361875. [DOI] [PubMed] [Google Scholar]
- Dobrossy MD, Dunnett SB. The influence of environment and experience on neural grafts. Nat Rev Neurosci. 2001;2:871–879. doi: 10.1038/35104055. [DOI] [PubMed] [Google Scholar]
- Dobrossy MD, Dunnett SB. Optimising plasticity: environmental and training associated factors in transplant-mediated brain repair. Rev Neurosci. 2005;16:1–21. doi: 10.1515/revneuro.2005.16.1.1. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Alvarez-Buylla A. Network of tangential pathways for neuronal migration in adult mammalian brain. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:14895–14900. doi: 10.1073/pnas.93.25.14895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci. 1997;17:5046–5061. doi: 10.1523/JNEUROSCI.17-13-05046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan X, Chang JH, Ge S, Faulkner RL, Kim JY, Kitabatake Y, Liu XB, Yang CH, Jordan JD, Ma DK, Liu CY, Ganesan S, Cheng HJ, Ming GL, Lu B, Song H. Disrupted-In-Schizophrenia 1 regulates integration of newly generated neurons in the adult brain. Cell. 2007;130:1146–1158. doi: 10.1016/j.cell.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastwood SL, Harrison PJ. Cellular basis of reduced cortical reelin expression in schizophrenia. The American journal of psychiatry. 2006;163:540–542. doi: 10.1176/appi.ajp.163.3.540. [DOI] [PubMed] [Google Scholar]
- Einstein O, Ben-Hur T. The changing face of neural stem cell therapy in neurologic diseases. Archives of neurology. 2008;65:452–456. doi: 10.1001/archneur.65.4.452. [DOI] [PubMed] [Google Scholar]
- El Maarouf A, Petridis AK, Rutishauser U. Use of polysialic acid in repair of the central nervous system. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:16989–16994. doi: 10.1073/pnas.0608036103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley JG, Hagg T. alpha6beta1 integrin directs migration of neuronal precursors in adult mouse forebrain. Exp Neurol. 2003;183:273–285. doi: 10.1016/s0014-4886(03)00209-7. [DOI] [PubMed] [Google Scholar]
- Englund U, Bjorklund A, Wictorin K, Lindvall O, Kokaia M. Grafted neural stem cells develop into functional pyramidal neurons and integrate into host cortical circuitry. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:17089–17094. doi: 10.1073/pnas.252589099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nature medicine. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Ernest NJ, Weaver AK, Van Duyn LB, Sontheimer HW. Relative contribution of chloride channels and transporters to regulatory volume decrease in human glioma cells. Am J Physiol Cell Physiol. 2005;288:C1451–C1461. doi: 10.1152/ajpcell.00503.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahrner A, Kann G, Flubacher A, Heinrich C, Freiman TM, Zentner J, Frotscher M, Haas CA. Granule cell dispersion is not accompanied by enhanced neurogenesis in temporal lobe epilepsy patients. Exp Neurol. 2007;203:320–332. doi: 10.1016/j.expneurol.2006.08.023. [DOI] [PubMed] [Google Scholar]
- Fallon J, Reid S, Kinyamu R, Opole I, Opole R, Baratta J, Korc M, Endo TL, Duong A, Nguyen G, Karkehabadhi M, Twardzik D, Patel S, Loughlin S. In vivo induction of massive proliferation, directed migration, and differentiation of neural cells in the adult mammalian brain. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:14686–14691. doi: 10.1073/pnas.97.26.14686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farin A, Suzuki SO, Weiker M, Goldman JE, Bruce JN, Canoll P. Transplanted glioma cells migrate and proliferate on host brain vasculature: a dynamic analysis. Glia. 2006;53:799–808. doi: 10.1002/glia.20334. [DOI] [PubMed] [Google Scholar]
- Fatemi SH. Reelin glycoprotein in autism and schizophrenia. International review of neurobiology. 2005;71:179–187. doi: 10.1016/s0074-7742(05)71008-4. [DOI] [PubMed] [Google Scholar]
- Feng R, Rampon C, Tang YP, Shrom D, Jin J, Kyin M, Sopher B, Miller MW, Ware CB, Martin GM, Kim SH, Langdon RB, Sisodia SS, Tsien JZ. Deficient neurogenesis in forebrain-specific presenilin-1 knockout mice is associated with reduced clearance of hippocampal memory traces. Neuron. 2001;32:911–926. doi: 10.1016/s0896-6273(01)00523-2. [DOI] [PubMed] [Google Scholar]
- Fewou SN, Ramakrishnan H, Bussow H, Gieselmann V, Eckhardt M. Down-regulation of polysialic acid is required for efficient myelin formation. J Biol Chem. 2007;282:16700–16711. doi: 10.1074/jbc.M610797200. [DOI] [PubMed] [Google Scholar]
- Fillmore HL, VanMeter TE, Broaddus WC. Membrane-type matrix metalloproteinases (MT-MMPs): expression and function during glioma invasion. Journal of Neuro-Oncology. 2001;53:187–202. doi: 10.1023/a:1012213604731. [DOI] [PubMed] [Google Scholar]
- Font E, Desfilis E, Perez-Canellas MM, Garcia-Verdugo JM. Neurogenesis and neuronal regeneration in the adult reptilian brain. Brain, behavior and evolution. 2001;58:276–295. doi: 10.1159/000057570. [DOI] [PubMed] [Google Scholar]
- Francis F, Koulakoff A, Boucher D, Chafey P, Schaar B, Vinet MC, Friocourt G, McDonnell N, Reiner O, Kahn A, McConnell SK, Berwald-Netter Y, Denoulet P, Chelly J. Doublecortin is a developmentally regulated, microtubule-associated protein expressed in migrating and differentiating neurons. Neuron. 1999;23:247–256. doi: 10.1016/s0896-6273(00)80777-1. [DOI] [PubMed] [Google Scholar]
- Freundlieb N, Francois C, Tande D, Oertel WH, Hirsch EC, Hoglinger GU. Dopaminergic substantia nigra neurons project topographically organized to the subventricular zone and stimulate precursor cell proliferation in aged primates. J Neurosci. 2006;26:2321–2325. doi: 10.1523/JNEUROSCI.4859-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli R, Binda E, Orfanelli U, Cipelletti B, Gritti A, De Vitis S, Fiocco R, Foroni C, Dimeco F, Vescovi A. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004;64:7011–7021. doi: 10.1158/0008-5472.CAN-04-1364. [DOI] [PubMed] [Google Scholar]
- Galvan V, Bredesen DE. Neurogenesis in the adult brain: implications for Alzheimer’s disease. CNS & neurological disorders drug targets. 2007;6:303–310. doi: 10.2174/187152707783220938. [DOI] [PubMed] [Google Scholar]
- Garcia AD, Doan NB, Imura T, Bush TG, Sofroniew MV. GFAP-expressing progenitors are the principal source of constitutive neurogenesis in adult mouse forebrain. Nat Neurosci. 2004;7:1233–1241. doi: 10.1038/nn1340. [DOI] [PubMed] [Google Scholar]
- García-Verdugo JM, Ferrón S, Flames N, Collado L, Desfilis E, Font E. The proliferative ventricular zone in adult vertebrates: a comparative study using reptiles, birds, and mammals. Brain Res Bull. 2002;57:765–75. doi: 10.1016/s0361-9230(01)00769-9. [DOI] [PubMed] [Google Scholar]
- Gensert JM, Goldman JE. Endogenous progenitors remyelinate demyelinated axons in the adult CNS. Neuron. 1997;19:197–203. doi: 10.1016/s0896-6273(00)80359-1. [DOI] [PubMed] [Google Scholar]
- Ghashghaei HT, Weber J, Pevny L, Schmid R, Schwab MH, Lloyd KC, Eisenstat DD, Lai C, Anton ES. The role of neuregulin-ErbB4 interactions on the proliferation and organization of cells in the subventricular zone. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:1930–1935. doi: 10.1073/pnas.0510410103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie GY, Soroceanu L, Manning T, Gladson CL, Rosenfeld SS. Glioma migration can be blocked by non-toxic inhibitors of myosin II. Cancer Research. 1999;59:2076–2082. [PubMed] [Google Scholar]
- Glaser T, Brose C, Franceschini I, Hamann K, Smorodchenko A, Zipp F, Dubois-Dalcq M, Brustle O. Neural cell adhesion molecule polysialylation enhances the sensitivity of embryonic stem cell-derived neural precursors to migration guidance cues. Stem Cells. 2007;25:3016–3025. doi: 10.1634/stemcells.2007-0218. [DOI] [PubMed] [Google Scholar]
- Glezer I, Lapointe A, Rivest S. Innate immunity triggers oligodendrocyte progenitor reactivity and confines damages to brain injuries. Faseb J. 2006;20:750–752. doi: 10.1096/fj.05-5234fje. [DOI] [PubMed] [Google Scholar]
- Goings GE, Sahni V, Szele FG. Migration patterns of subventricular zone cells in adult mice change after cerebral cortex injury. Brain Res. 2004;996:213–226. doi: 10.1016/j.brainres.2003.10.034. [DOI] [PubMed] [Google Scholar]
- Goldbrunner RH, Bernstein JJ, Tonn JC. Cell-extracellular matrix interaction in glioma invasion. Acta Neurochirurgica. 1999;141:295–305. doi: 10.1007/s007010050301. [DOI] [PubMed] [Google Scholar]
- Goldman JE, Zerlin M, Newman S, Zhang L, Gensert J. Fate determination and migration of progenitors in the postnatal mammalian CNS. Developmental neuroscience. 1997;19:42–48. doi: 10.1159/000111184. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla F, Dao L, So V. Physical exercise induces FGF-2 and its mRNA in the hippocampus. Brain Res. 1997;764:1–8. doi: 10.1016/s0006-8993(97)00375-2. [DOI] [PubMed] [Google Scholar]
- Gong C, Wang TW, Huang HS, Parent JM. Reelin regulates neuronal progenitor migration in intact and epileptic hippocampus. J Neurosci. 2007;27:1803–1811. doi: 10.1523/JNEUROSCI.3111-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Martinez JA, Bingaman WE, Toms SA, Najm IM. Neurogenesis in the postnatal human epileptic brain. J Neurosurg. 2007;107:628–635. doi: 10.3171/JNS-07/09/0628. [DOI] [PubMed] [Google Scholar]
- Gotz M, Stricker SH. Go with the flow: signaling from the ventricle directs neuroblast migration. Nat Neurosci. 2006;9:470–472. doi: 10.1038/nn0406-470. [DOI] [PubMed] [Google Scholar]
- Gould E, Tanapat P. Lesion-induced proliferation of neuronal progenitors in the dentate gyrus of the adult rat. Neuroscience. 1997;80:427–436. doi: 10.1016/s0306-4522(97)00127-9. [DOI] [PubMed] [Google Scholar]
- Gritti A, Vescovi AL, Galli R. Adult neural stem cells: plasticity and developmental potential. Journal of physiology, Paris. 2002;96:81–90. doi: 10.1016/s0928-4257(01)00083-3. [DOI] [PubMed] [Google Scholar]
- Guarino M, Rubino B, Ballabio G. The role of epithelial-mesenchymal transition in cancer pathology. Pathology. 2007;39:305–18. doi: 10.1080/00313020701329914. [DOI] [PubMed] [Google Scholar]
- Gudz TI, Komuro H, Macklin WB. Glutamate stimulates oligodendrocyte progenitor migration mediated via an alphaV integrin/myelin proteolipid protein complex. J Neurosci. 2006;26:2458–66. doi: 10.1523/JNEUROSCI.4054-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutova M, Najbauer J, Frank RT, Kendall SE, Gevorgyan A, Metz MZ, Guevorkian M, Edmiston M, Zhao D, Glackin CA, Kim SU, Aboody KS. Urokinase plasminogen activator and urokinase plasminogen activator receptor mediate human stem cell tropism to malignant solid tumors. Stem Cells. 2008;26:1406–1413. doi: 10.1634/stemcells.2008-0141. [DOI] [PubMed] [Google Scholar]
- Hack I, Bancila M, Loulier K, Carroll P, Cremer H. Reelin is a detachment signal in tangential chain-migration during postnatal neurogenesis. Nat Neurosci. 2002;5:939–945. doi: 10.1038/nn923. [DOI] [PubMed] [Google Scholar]
- Hack MA, Saghatelyan A, de Chevigny A, Pfeifer A, Ashery-Padan R, Lledo PM, Gotz M. Neuronal fate determinants of adult olfactory bulb neurogenesis. Nat Neurosci. 2005;8:865–872. doi: 10.1038/nn1479. [DOI] [PubMed] [Google Scholar]
- Hager G, Dodt H-U, Zieglgansberger W, Liesi P. A novel form of neuronal movement in the rat cerebellum. J Neurosci Res. 1995;40:207–219. doi: 10.1002/jnr.490400209. [DOI] [PubMed] [Google Scholar]
- Henry RA, Hughes SM, Connor B. AAV-mediated delivery of BDNF augments neurogenesis in the normal and quinolinic acid-lesioned adult rat brain. The European journal of neuroscience. 2007;25:3513–3525. doi: 10.1111/j.1460-9568.2007.05625.x. [DOI] [PubMed] [Google Scholar]
- Hicks AU, Hewlett K, Windle V, Chernenko G, Ploughman M, Jolkkonen J, Weiss S, Corbett D. Enriched environment enhances transplanted subventricular zone stem cell migration and functional recovery after stroke. Neuroscience. 2007;146:31–40. doi: 10.1016/j.neuroscience.2007.01.020. [DOI] [PubMed] [Google Scholar]
- Higginbotham H, Tanaka T, Brinkman BC, Gleeson JG. GSK3beta and PKCzeta function in centrosome localization and process stabilization during Slit-mediated neuronal repolarization. Molecular and cellular neurosciences. 2006;32:118–132. doi: 10.1016/j.mcn.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Hill WD, Hess DC, Martin-Studdard A, Carothers JJ, Zheng J, Hale D, Maeda M, Fagan SC, Carroll JE, Conway SJ. SDF-1 (CXCL12) is upregulated in the ischemic penumbra following stroke: association with bone marrow cell homing to injury. J Neuropathol Exp Neurol. 2004;63:84–96. doi: 10.1093/jnen/63.1.84. [DOI] [PubMed] [Google Scholar]
- Hoglinger GU, Rizk P, Muriel MP, Duyckaerts C, Oertel WH, Caille I, Hirsch EC. Dopamine depletion impairs precursor cell proliferation in Parkinson disease. Nat Neurosci. 2004;7:726–735. doi: 10.1038/nn1265. [DOI] [PubMed] [Google Scholar]
- Hu H. Chemorepulsion of neuronal migration by Slit2 in the developing mammalian forebrain. Neuron. 1999;23:703–711. doi: 10.1016/s0896-6273(01)80029-5. [DOI] [PubMed] [Google Scholar]
- Hu H. Polysialic acid regulates chain formation by migrating olfactory interneuron precursors. Journal of neuroscience research. 2000;61:480–492. doi: 10.1002/1097-4547(20000901)61:5<480::AID-JNR2>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Hu H. Cell-surface heparan sulfate is involved in the repulsive guidance activities of Slit2 protein. Nat Neurosci. 2001;4:695–701. doi: 10.1038/89482. [DOI] [PubMed] [Google Scholar]
- Hu H, Tomasiewicz H, Magnuson T, Rutishauser U. The role of polysialic acid in migration of olfactory bulb interneuron precursors in the subventricular zone. Neuron. 1996;16:735–743. doi: 10.1016/s0896-6273(00)80094-x. [DOI] [PubMed] [Google Scholar]
- Ickes BR, Pham TM, Sanders LA, Albeck DS, Mohammed AH, Granholm AC. Long-term environmental enrichment leads to regional increases in neurotrophin levels in rat brain. Exp Neurol. 2000;164:45–52. doi: 10.1006/exnr.2000.7415. [DOI] [PubMed] [Google Scholar]
- Ignatova TN, Kukekov VG, Laywell ED, Suslov ON, Vrionis FD, Steindler DA. Human cortical glial tumors contain neural stem-like cells expressing astroglial and neuronal markers in vitro. Glia. 2002;39:193–206. doi: 10.1002/glia.10094. [DOI] [PubMed] [Google Scholar]
- Imayoshi I, Sakamoto M, Ohtsuka T, Takao K, Miyakawa T, Yamaguchi M, Mori K, Ikeda T, Itohara S, Kageyama R. Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat Neurosci. 2008;11:1153–61. doi: 10.1038/nn.2185. [DOI] [PubMed] [Google Scholar]
- Imitola J, Raddassi K, Park KI, Mueller FJ, Nieto M, Teng YD, Frenkel D, Li J, Sidman RL, Walsh CA, Snyder EY, Khoury SJ. Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1alpha/CXC chemokine receptor 4 pathway. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:18117–18122. doi: 10.1073/pnas.0408258102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiuchi S, Tsuzuki K, Yoshida Y, Yamada N, Hagimura N, Okado H, Miwa A, Kurihara H, Nakazato Y, Tamura M, Sasaki T, Ozawa S. Blockage of Ca(2+)-permeable AMPA receptors suppresses migration and induces apoptosis in human glioblastoma cells. Nat Med. 2002;8:971–8. doi: 10.1038/nm746. [DOI] [PubMed] [Google Scholar]
- Ivkovic S, Canoll P, Goldman JE. Constitutive EGFR signaling in oligodendrocyte progenitors leads to diffuse hyperplasia in postnatal white matter. J Neurosci. 2008;28:914–22. doi: 10.1523/JNEUROSCI.4327-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson EL, Alvarez-Buylla A. Characterization of adult neural stem cells and their relation to brain tumors. Cells Tissues Organs. 2008;188:212–247. doi: 10.1159/000114541. [DOI] [PubMed] [Google Scholar]
- Jacques TS, Relvas JB, Nishimura S, Pytela R, Edwards GM, Streuli CH, ffrench-Constant C. Neural precursor cell chain migration and division are regulated through different beta1 integrins. Development. 1998;125:3167–77. doi: 10.1242/dev.125.16.3167. [DOI] [PubMed] [Google Scholar]
- Janeczko K. Lesion-induced proliferation of vimentin-positive astrocytes in the mouse cerebral hemisphere. Folia histochemica et cytobiologica/Polish Academy of Sciences, Polish Histochemical and Cytochemical Society. 1991;29:55–57. [PubMed] [Google Scholar]
- Janeczko K. Co-expression of GFAP and vimentin in astrocytes proliferating in response to injury in the mouse cerebral hemisphere. A combined autoradiographic and double immunocytochemical study. Int J Dev Neurosci. 1993;11:139–147. doi: 10.1016/0736-5748(93)90074-n. [DOI] [PubMed] [Google Scholar]
- Jankovski A, Sotelo C. Subventricular zone-olfactory bulb migratory pathway in the adult mouse: cellular composition and specificity as determined by heterochronic and heterotopic transplantation. The Journal of comparative neurology. 1996;371:376–396. doi: 10.1002/(SICI)1096-9861(19960729)371:3<376::AID-CNE3>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Jin K, Galvan V, Xie L, Mao XO, Gorostiza OF, Bredesen DE, Greenberg DA. Enhanced neurogenesis in Alzheimer’s disease transgenic (PDGF-APPSw, Ind) mice. Proceedings of the National Academy of Sciences of the United States of America. 2004a;101:13363–13367. doi: 10.1073/pnas.0403678101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Peel AL, Mao XO, Xie L, Cottrell BA, Henshall DC, Greenberg DA. Increased hippocampal neurogenesis in Alzheimer’s disease. Proceedings of the National Academy of Sciences of the United States of America. 2004b;101:343–347. doi: 10.1073/pnas.2634794100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Sun Y, Xie L, Peel A, Mao XO, Batteur S, Greenberg DA. Directed migration of neuronal precursors into the ischemic cerebral cortex and striatum. Molecular and cellular neurosciences. 2003;24:171–189. doi: 10.1016/s1044-7431(03)00159-3. [DOI] [PubMed] [Google Scholar]
- Kakita A, Goldman JE. Patterns and dynamics of SVZ cell migration in the postnatal forebrain: monitoring living progenitors in slice preparations. Neuron. 1999;23:461–472. doi: 10.1016/s0896-6273(00)80800-4. [DOI] [PubMed] [Google Scholar]
- Kakita A, Zerlin M, Takahashi H, Goldman JE. Some glial progenitors in the neonatal subventricular zone migrate through the corpus callosum to the contralateral cerebral hemisphere. The Journal of comparative neurology. 2003;458:381–388. doi: 10.1002/cne.10597. [DOI] [PubMed] [Google Scholar]
- Kaplan MS, Bell DH. Mitotic neuroblasts in the 9-day-old and 11-month-old rodent hippocampus. J Neurosci. 1984;4:1429–1441. doi: 10.1523/JNEUROSCI.04-06-01429.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan MS, McNelly NA, Hinds JW. Population dynamics of adult-formed granule neurons of the rat olfactory bulb. The Journal of comparative neurology. 1985;239:117–125. doi: 10.1002/cne.902390110. [DOI] [PubMed] [Google Scholar]
- Karram K, Chatterjee N, Trotter J. NG2-expressing cells in the nervous system: role of the proteoglycan in migration and glial-neuron interaction. Journal of anatomy. 2005;207:735–744. doi: 10.1111/j.1469-7580.2005.00461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaslin J, Ganz J, Brand M. Proliferation, neurogenesis and regeneration in the non-mammalian vertebrate brain. Philos Trans R Soc Lond B Biol Sci. 2008;363:101–22. doi: 10.1098/rstb.2006.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly S, Bliss TM, Shah AK, Sun GH, Ma M, Foo WC, Masel J, Yenari MA, Weissman IL, Uchida N, Palmer T, Steinberg GK. Transplanted human fetal neural stem cells survive, migrate, and differentiate in ischemic rat cerebral cortex. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:11839–11844. doi: 10.1073/pnas.0404474101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Gage FH. Experience-dependent regulation of adult hippocampal neurogenesis: effects of long-term stimulation and stimulus withdrawal. Hippocampus. 1999;9:321–332. doi: 10.1002/(SICI)1098-1063(1999)9:3<321::AID-HIPO11>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- Kirschenbaum B, Doetsch F, Lois C, Alvarez-Buylla A. Adult subventricular zone neuronal precursors continue to proliferate and migrate in the absence of the olfactory bulb. J Neurosci. 1999;19:2171–2180. doi: 10.1523/JNEUROSCI.19-06-02171.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss JZ, Rougon G. Cell biology of polysialic acid. Curr Opin Neurobiol. 1997;7:640–646. doi: 10.1016/s0959-4388(97)80083-9. [DOI] [PubMed] [Google Scholar]
- Koizumi H, Higginbotham H, Poon T, Tanaka T, Brinkman BC, Gleeson JG. Doublecortin maintains bipolar shape and nuclear translocation during migration in the adult forebrain. Nat Neurosci. 2006;9:779–786. doi: 10.1038/nn1704. [DOI] [PubMed] [Google Scholar]
- Kokaia Z, Thored P, Arvidsson A, Lindvall O. Regulation of stroke-induced neurogenesis in adult brain--recent scientific progress. Cereb Cortex. 2006;16(Suppl 1):i162–167. doi: 10.1093/cercor/bhj174. [DOI] [PubMed] [Google Scholar]
- Kolb B, Morshead C, Gonzalez C, Kim M, Gregg C, Shingo T, Weiss S. Growth factor-stimulated generation of new cortical tissue and functional recovery after stroke damage to the motor cortex of rats. J Cereb Blood Flow Metab. 2007;27:983–997. doi: 10.1038/sj.jcbfm.9600402. [DOI] [PubMed] [Google Scholar]
- Kriegstein AR, Noctor SC. Patterns of neuronal migration in the embryonic cortex. Trends Neurosci. 2004;27:392–399. doi: 10.1016/j.tins.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn HG, Winkler J, Kempermann G, Thal LJ, Gage FH. Epidermal growth factor and fibroblast growth factor-2 have different effects on neural progenitors in the adult rat brain. J Neurosci. 1997;17:5820–5829. doi: 10.1523/JNEUROSCI.17-15-05820.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laywell ED, Rakic P, Kukekov VG, Holland EC, Steindler DA. Identification of a multipotent astrocytic stem cell in the immature and adult mouse brain. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:13883–13888. doi: 10.1073/pnas.250471697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Kozorovitskiy Y, Gross CG, Gould E. Diminished adult neurogenesis in the marmoset brain precedes old age. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:17169–17173. doi: 10.1073/pnas.0708228104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine JM, Reynolds R. Activation and proliferation of endogenous oligodendrocyte precursor cells during ethidium bromide-induced demyelination. Exp Neurol. 1999;160:333–347. doi: 10.1006/exnr.1999.7224. [DOI] [PubMed] [Google Scholar]
- Levison SW, Goldman JE. Both oligodendrocytes and astrocytes develop from progenitors in the subventricular zone of postnatal rat forebrain. Neuron. 1993;10:201–212. doi: 10.1016/0896-6273(93)90311-e. [DOI] [PubMed] [Google Scholar]
- Li M, Ransohoff RM. Multiple roles of chemokine CXCL12 in the central nervous system: a migration from immunology to neurobiology. Prog Neurobiol. 2008;84:116–131. doi: 10.1016/j.pneurobio.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Z, Seye CI, Weisman GA, Erb L. The P2Y2 nucleotide receptor requires interaction with alpha v integrins to access and activate G12. J Cell Sci. 2007;120:1654–62. doi: 10.1242/jcs.03441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesi P, Akinshola E, Matsuba K, Lange K, Morest K. Cellular migration in the postnatal rat cerebellar cortex: confocal-infrared microscopy and the rapid Golgi method. J Neurosci Res. 2003;72:290–302. doi: 10.1002/jnr.10573. [DOI] [PubMed] [Google Scholar]
- Lim DA, Alvarez-Buylla A. Interaction between astrocytes and adult subventricular zone precursors stimulates neurogenesis. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:7526–7531. doi: 10.1073/pnas.96.13.7526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Rao Y. Neuronal migration from the forebrain to the olfactory bulb requires a new attractant persistent in the olfactory bulb. J Neurosci. 2003;23:6651–6659. doi: 10.1523/JNEUROSCI.23-16-06651.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Solway K, Messing RO, Sharp FR. Increased neurogenesis in the dentate gyrus after transient global ischemia in gerbils. J Neurosci. 1998;18:7768–7778. doi: 10.1523/JNEUROSCI.18-19-07768.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Suzuki T, Seki T, Namba T, Tanimura A, Arai H. Effects of repeated phencyclidine administration on adult hippocampal neurogenesis in the rat. Synapse (New York, NY. 2006;60:56–68. doi: 10.1002/syn.20275. [DOI] [PubMed] [Google Scholar]
- Lois C, Alvarez-Buylla A. Long-distance neuronal migration in the adult mammalian brain. Science. 1994;264:1145–1148. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]
- Lois C, Garcia-Verdugo JM, Alvarez-Buylla A. Chain migration of neuronal precursors. Science. 1996;271:978–981. doi: 10.1126/science.271.5251.978. [DOI] [PubMed] [Google Scholar]
- Long JE, Garel S, Alvarez-Dolado M, Yoshikawa K, Osumi N, Alvarez-Buylla A, Rubenstein JL. Dlx-dependent and -independent regulation of olfactory bulb interneuron differentiation. J Neurosci. 2007;27:3230–3243. doi: 10.1523/JNEUROSCI.5265-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louissaint A, Jr, Rao S, Leventhal C, Goldman SA. Coordinated interaction of neurogenesis and angiogenesis in the adult songbird brain. Neuron. 2002;34:945–960. doi: 10.1016/s0896-6273(02)00722-5. [DOI] [PubMed] [Google Scholar]
- Luo J, Daniels SB, Lennington JB, Notti RQ, Conover JC. The aging neurogenic subventricular zone. Aging cell. 2006;5:139–152. doi: 10.1111/j.1474-9726.2006.00197.x. [DOI] [PubMed] [Google Scholar]
- Luskin MB. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron. 1993;11:173–189. doi: 10.1016/0896-6273(93)90281-u. [DOI] [PubMed] [Google Scholar]
- Luskin MB, McDermott K. Divergent lineages for oligodendrocytes and astrocytes originating in the neonatal forebrain subventricular zone. Glia. 1994;11:211–226. doi: 10.1002/glia.440110302. [DOI] [PubMed] [Google Scholar]
- Luskin MB. Neuroblasts of the postnatal mammalian forebrain: their phenotype and fate. J Neurobiol. 1998;36:221–33. [PubMed] [Google Scholar]
- Luzzati F, Peretto P, Aimar P, Ponti G, Fasolo A, Bonfanti L. Glia-independent chains of neuroblasts through the subcortical parenchyma of the adult rabbit brain. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:13036–13041. doi: 10.1073/pnas.1735482100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons SA, Chung WJ, Weaver AK, Ogunrinu T, Sontheimer H. Autocrine glutamate signaling promotes glioma cell invasion. Cancer Res. 2007;67:9463–71. doi: 10.1158/0008-5472.CAN-07-2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macas J, Nern C, Plate KH, Momma S. Increased generation of neuronal progenitors after ischemic injury in the aged adult human forebrain. J Neurosci. 2006;26:13114–13119. doi: 10.1523/JNEUROSCI.4667-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magalon K, Cantarella C, Monti G, Cayre M, Durbec P. Enriched environment promotes adult neural progenitor cell mobilization in mouse demyelination models. The European journal of neuroscience. 2007;25:761–771. doi: 10.1111/j.1460-9568.2007.05335.x. [DOI] [PubMed] [Google Scholar]
- Magavi SS, Leavitt BR, Macklis JD. Induction of neurogenesis in the neocortex of adult mice. Nature. 2000;405:951–955. doi: 10.1038/35016083. [DOI] [PubMed] [Google Scholar]
- Malatesta P, Hartfuss E, Gotz M. Isolation of radial glial cells by fluorescent-activated cell sorting reveals a neuronal lineage. Development. 2000;127:5253–5263. doi: 10.1242/dev.127.24.5253. [DOI] [PubMed] [Google Scholar]
- Marillat V, Cases O, Nguyen-Ba-Charvet KT, Tessier-Lavigne M, Sotelo C, Chedotal A. Spatiotemporal expression patterns of slit and robo genes in the rat brain. The Journal of comparative neurology. 2002;442:130–155. doi: 10.1002/cne.10068. [DOI] [PubMed] [Google Scholar]
- Marin O, Rubenstein JL. Cell migration in the forebrain. Annual review of neuroscience. 2003;26:441–483. doi: 10.1146/annurev.neuro.26.041002.131058. [DOI] [PubMed] [Google Scholar]
- Markakis EA, Gage FH. Adult-generated neurons in the dentate gyrus send axonal projections to field CA3 and are surrounded by synaptic vesicles. The Journal of comparative neurology. 1999;406:449–460. [PubMed] [Google Scholar]
- Mason JL, Goldman JE. A2B5+ and O4+ Cycling progenitors in the adult forebrain white matter respond differentially to PDGF-AA, FGF-2, and IGF-1. Molecular and cellular neurosciences. 2002;20:30–42. doi: 10.1006/mcne.2002.1114. [DOI] [PubMed] [Google Scholar]
- McTigue DM, Tripathi RB. The life, death, and replacement of oligodendrocytes in the adult CNS. J Neurochem. 2008;107:1–19. doi: 10.1111/j.1471-4159.2008.05570.x. [DOI] [PubMed] [Google Scholar]
- Mendoza-Torreblanca JG, Martinez-Martinez E, Tapia-Rodriguez M, Ramirez-Hernandez R, Gutierrez-Ospina G. The rostral migratory stream is a neurogenic niche that predominantly engenders periglomerular cells: in vivo evidence in the adult rat brain. Neuroscience research. 2008;60:289–299. doi: 10.1016/j.neures.2007.11.013. [DOI] [PubMed] [Google Scholar]
- Menn B, Garcia-Verdugo JM, Yaschine C, Gonzalez-Perez O, Rowitch D, Alvarez-Buylla A. Origin of oligodendrocytes in the subventricular zone of the adult brain. J Neurosci. 2006;26:7907–7918. doi: 10.1523/JNEUROSCI.1299-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercier F, Kitasako JT, Hatton GI. Anatomy of the brain neurogenic zones revisited: fractones and the fibroblast/macrophage network. The Journal of comparative neurology. 2002;451:170–188. doi: 10.1002/cne.10342. [DOI] [PubMed] [Google Scholar]
- Miles DK, Kernie SG. Activation of neural stem and progenitor cells after brain injury. Prog Brain Res. 2006;157:187–197. doi: 10.1016/s0079-6123(06)57012-8. [DOI] [PubMed] [Google Scholar]
- Mirzadeh Z, Merkle FT, Soriano-Navarro M, Garcia-Verdugo JM, Alvarez-Buylla A. Neural stem cells confer unique pinwheel architecture to the ventricular surface in neurogenic regions of the adult brain. Cell Stem Cell. 2008;3:265–78. doi: 10.1016/j.stem.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake T, Hattori T, Fukuda M, Kitamura T, Fujita S. Quantitative studies on proliferative changes of reactive astrocytes in mouse cerebral cortex. Brain Res. 1988;451:133–138. doi: 10.1016/0006-8993(88)90757-3. [DOI] [PubMed] [Google Scholar]
- Mizrahi A. Dendritic development and plasticity of adult-born neurons in the mouse olfactory bulb. Nat Neurosci. 2007;10:444–452. doi: 10.1038/nn1875. [DOI] [PubMed] [Google Scholar]
- Mohanam S, Sawaya RE, Yamamoto M, Bruner JM, Nicholson GL, Rao JS. Proteolysis and invasiveness of brain tumors: role of urokinase-type plasminogen activator receptor. Journal of Neuro-Oncology. 1994;22:153–60. doi: 10.1007/BF01052890. [DOI] [PubMed] [Google Scholar]
- Moores CA, Perderiset M, Francis F, Chelly J, Houdusse A, Milligan RA. Mechanism of microtubule stabilization by doublecortin. Molecular cell. 2004;14:833–839. doi: 10.1016/j.molcel.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Mori T, Buffo A, Gotz M. The novel roles of glial cells revisited: the contribution of radial glia and astrocytes to neurogenesis. Curr Top Dev Biol. 2005;69:67–99. doi: 10.1016/S0070-2153(05)69004-7. [DOI] [PubMed] [Google Scholar]
- Murase S, Horwitz AF. Deleted in colorectal carcinoma and differentially expressed integrins mediate the directional migration of neural precursors in the rostral migratory stream. J Neurosci. 2002;22:3568–3579. doi: 10.1523/JNEUROSCI.22-09-03568.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacher J, Crespo C, McEwen BS. Doublecortin expression in the adult rat telencephalon. The European journal of neuroscience. 2001;14:629–644. doi: 10.1046/j.0953-816x.2001.01683.x. [DOI] [PubMed] [Google Scholar]
- Nait-Oumesmar B, Decker L, Lachapelle F, Avellana-Adalid V, Bachelin C, Van Evercooren AB. Progenitor cells of the adult mouse subventricular zone proliferate, migrate and differentiate into oligodendrocytes after demyelination. The European journal of neuroscience. 1999;11:4357–4366. doi: 10.1046/j.1460-9568.1999.00873.x. [DOI] [PubMed] [Google Scholar]
- Nait-Oumesmar B, Picard-Riera N, Kerninon C, Decker L, Seilhean D, Hoglinger GU, Hirsch EC, Reynolds R, Baron-Van Evercooren A. Activation of the subventricular zone in multiple sclerosis: evidence for early glial progenitors. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:4694–4699. doi: 10.1073/pnas.0606835104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nait-Oumesmar B, Picard-Riéra N, Kerninon C, Baron-Van Evercooren A. The role of SVZ-derived neural precursors in demyelinating diseases: from animal models to multiple sclerosis. J Neurol Sci. 2008;265:26–31. doi: 10.1016/j.jns.2007.09.032. [DOI] [PubMed] [Google Scholar]
- Nakatomi H, Kuriu T, Okabe S, Yamamoto S, Hatano O, Kawahara N, Tamura A, Kirino T, Nakafuku M. Regeneration of hippocampal pyramidal neurons after ischemic brain injury by recruitment of endogenous neural progenitors. Cell. 2002;110:429–441. doi: 10.1016/s0092-8674(02)00862-0. [DOI] [PubMed] [Google Scholar]
- Nam SC, Kim Y, Dryanovski D, Walker A, Goings G, Woolfrey K, Kang SS, Chu C, Chenn A, Erdelyi F, Szabo G, Hockberger P, Szele FG. Dynamic features of postnatal subventricular zone cell motility: a two-photon time-lapse study. The Journal of Comparative Neurology. 2007;505:190–208. doi: 10.1002/cne.21473. [DOI] [PubMed] [Google Scholar]
- Navarro-Quiroga I, Hernandez-Valdes M, Lin SL, Naegele JR. Postnatal cellular contributions ofr the hippocampus subventricular zone to the dentate gyrus, corpus callosum, fimbria, and cerebral cortex. The Journal of Comparative Neurology. 2006;497:833–845. doi: 10.1002/cne.21037. [DOI] [PubMed] [Google Scholar]
- Ng KL, Li JD, Cheng MY, Leslie FM, Lee AG, Zhou QY. Dependence of olfactory bulb neurogenesis on prokineticin 2 signaling. Science. 2005;308:1923–1927. doi: 10.1126/science.1112103. [DOI] [PubMed] [Google Scholar]
- Nguyen-Ba-Charvet KT, Picard-Riera N, Tessier-Lavigne M, Baron-Van Evercooren A, Sotelo C, Chedotal A. Multiple roles for slits in the control of cell migration in the rostral migratory stream. J Neurosci. 2004;24:1497–1506. doi: 10.1523/JNEUROSCI.4729-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninkovic J, Götz M. Signaling in adult neurogenesis: from stem cell niche to neuronal networks. Curr Opin Neurobiol. 2007;17:338–44. doi: 10.1016/j.conb.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Ninomiya M, Yamashita T, Araki N, Okano H, Sawamoto K. Enhanced neurogenesis in the ischemic striatum following EGF-induced expansion of transit-amplifying cells in the subventricular zone. Neurosci Lett. 2006;403:63–67. doi: 10.1016/j.neulet.2006.04.039. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Watanabe M, Yang Z, Bu J. Identity, distribution, and development of polydendrocytes: NG2-expressing glial cells. J Neurocytol. 2002;31:437–455. doi: 10.1023/a:1025783412651. [DOI] [PubMed] [Google Scholar]
- Nishiyama A. Polydendrocytes: NG2 cells with many roles in development and repair of the CNS. Neuroscientist. 2007;13(1):62–76. doi: 10.1177/1073858406295586. [DOI] [PubMed] [Google Scholar]
- Noctor SC, Flint AC, Weissman TA, Dammerman RS, Kriegstein AR. Neurons derived from radial glial cells establish radial units in neocortex. Nature. 2001;409:714–720. doi: 10.1038/35055553. [DOI] [PubMed] [Google Scholar]
- Norton N, Moskvina V, Morris DW, Bray NJ, Zammit S, Williams NM, Williams HJ, Preece AC, Dwyer S, Wilkinson JC, Spurlock G, Kirov G, Buckland P, Waddington JL, Gill M, Corvin AP, Owen MJ, O’Donovan MC. Evidence that interaction between neuregulin 1 and its receptor erbB4 increases susceptibility to schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:96–101. doi: 10.1002/ajmg.b.30236. [DOI] [PubMed] [Google Scholar]
- Nunes MC, Roy NS, Keyoung HM, Goodman RR, McKhann G, 2nd, Jiang L, Kang J, Nedergaard M, Goldman SA. Identification and isolation of multipotential neural progenitor cells from the subcortical white matter of the adult human brain. Nature medicine. 2003;9:439–447. doi: 10.1038/nm837. [DOI] [PubMed] [Google Scholar]
- Ocbina PJ, Dizon ML, Shin L, Szele FG. Doublecortin is necessary for the migration of adult subventricular zone cells from neurospheres. Molecular and cellular neurosciences. 2006;33:126–135. doi: 10.1016/j.mcn.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Olsen ML, Schade S, Lyons SA, Amaral MD, Sontheimer H. Expression of voltage-gated chloride channels in human glioma cells. J Neurosci. 2003;23:5572–82. doi: 10.1523/JNEUROSCI.23-13-05572.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono K, Tomasiewicz H, Magnuson T, Rutishauser U. N-CAM mutation inhibits tangential neuronal migration and is phenocopied by enzymatic removal of polysialic acid. Neuron. 1994;13:595–609. doi: 10.1016/0896-6273(94)90028-0. [DOI] [PubMed] [Google Scholar]
- Oumesmar BN, Vignais L, Duhamel-Clerin E, Avellana-Adalid V, Rougon G, Baron-Van Evercooren A. Expression of the highly polysialylated neural cell adhesion molecule during postnatal myelination and following chemically induced demyelination of the adult mouse spinal cord. The European journal of neuroscience. 1995;7:480–491. doi: 10.1111/j.1460-9568.1995.tb00344.x. [DOI] [PubMed] [Google Scholar]
- Ourednik V, Ourednik J. Plasticity of the central nervous system and formation of “auxiliary niches” after stem cell grafting: an essay. Cell Transplant. 2007;16:263–71. doi: 10.3727/000000007783464696. [DOI] [PubMed] [Google Scholar]
- Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. The Journal of comparative neurology. 2000;425:479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Paratcha G, Ibanez CF, Ledda F. GDNF is a chemoattractant factor for neuronal precursor cells in the rostral migratory stream. Molecular and cellular neurosciences. 2006;31:505–514. doi: 10.1016/j.mcn.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Parent JM. Adult neurogenesis in the intact and epileptic dentate gyrus. Prog Brain Res. 2007;163:529–540. doi: 10.1016/S0079-6123(07)63028-3. [DOI] [PubMed] [Google Scholar]
- Parent JM, Lowenstein DH. Seizure-induced neurogenesis: are more new neurons good for an adult brain? Prog Brain Res. 2002;135:121–131. doi: 10.1016/S0079-6123(02)35012-X. [DOI] [PubMed] [Google Scholar]
- Parent JM, Tada E, Fike JR, Lowenstein DH. Inhibition of dentate granule cell neurogenesis with brain irradiation does not prevent seizure-induced mossy fiber synaptic reorganization in the rat. J Neurosci. 1999;19:4508–4519. doi: 10.1523/JNEUROSCI.19-11-04508.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent JM, von dem Bussche N, Lowenstein DH. Prolonged seizures recruit caudal subventricular zone glial progenitors into the injured hippocampus. Hippocampus. 2006;16:321–328. doi: 10.1002/hipo.20166. [DOI] [PubMed] [Google Scholar]
- Parent JM, Yu TW, Leibowitz RT, Geschwind DH, Sloviter RS, Lowenstein DH. Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J Neurosci. 1997;17:3727–3738. doi: 10.1523/JNEUROSCI.17-10-03727.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekcec A, Loscher W, Potschka H. Neurogenesis in the adult rat piriform cortex. Neuroreport. 2006;17:571–574. doi: 10.1097/00001756-200604240-00003. [DOI] [PubMed] [Google Scholar]
- Pencea V, Bingaman KD, Wiegand SJ, Luskin MB. Infusion of brain-derived neurotrophic factor into the lateral ventricle of the adult rat leads to new neurons in the parenchyma of the striatum, septum, thalamus, and hypothalamus. J Neurosci. 2001;21:6706–6717. doi: 10.1523/JNEUROSCI.21-17-06706.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peretto P, Giachino C, Aimar P, Fasolo A, Bonfanti L. Chain formation and glial tube assembly in the shift from neonatal to adult subventricular zone of the rodent forebrain. The Journal of comparative neurology. 2005;487:407–427. doi: 10.1002/cne.20576. [DOI] [PubMed] [Google Scholar]
- Peterson DA. Stem cells in brain plasticity and repair. Current opinion in pharmacology. 2002;2:34–42. doi: 10.1016/s1471-4892(01)00118-7. [DOI] [PubMed] [Google Scholar]
- Pham TM, Ickes B, Albeck D, Soderstrom S, Granholm AC, Mohammed AH. Changes in brain nerve growth factor levels and nerve growth factor receptors in rats exposed to environmental enrichment for one year. Neuroscience. 1999;94:279–286. doi: 10.1016/s0306-4522(99)00316-4. [DOI] [PubMed] [Google Scholar]
- Picard-Riera N, Decker L, Delarasse C, Goude K, Nait-Oumesmar B, Liblau R, Pham-Dinh D, Evercooren AB. Experimental autoimmune encephalomyelitis mobilizes neural progenitors from the subventricular zone to undergo oligodendrogenesis in adult mice. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:13211–13216. doi: 10.1073/pnas.192314199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluchino S, Quattrini A, Brambilla E, Gritti A, Salani G, Dina G, Galli R, Del Carro U, Amadio S, Bergami A, Furlan R, Comi G, Vescovi AL, Martino G. Injection of adult neurospheres induces recovery in a chronic model of multiple sclerosis. Nature. 2003;422:688–694. doi: 10.1038/nature01552. [DOI] [PubMed] [Google Scholar]
- Ponti G, Peretto P, Bonfanti L. Genesis of neuronal and glial progenitors in the cerebellar cortex of peripuberal and adult rabbits. PLoS ONE. 2008;3(6):e2366. doi: 10.1371/journal.pone.0002366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosser HM, Bradley A, Caldwell MA. Olfactory bulb hypoplasia in Prokr2 null mice stems from defective neuronal progenitor migration and differentiation. Eur J Neurosci. 2007;12:3339–44. doi: 10.1111/j.1460-9568.2007.05958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinones-Hinojosa A, Sanai N, Soriano-Navarro M, Gonzalez-Perez O, Mirzadeh Z, Gil-Perotin S, Romero-Rodriguez R, Berger MS, Garcia-Verdugo JM, Alvarez-Buylla A. Cellular composition and cytoarchitecture of the adult human subventricular zone: a niche of neural stem cells. The Journal of comparative neurology. 2006;494:415–434. doi: 10.1002/cne.20798. [DOI] [PubMed] [Google Scholar]
- Ramaswamy S, Goings GE, Soderstrom KE, Szele FG, Kozlowski DA. Cellular proliferation and migration following a controlled cortical impact in the mouse. Brain Res. 2005;1053:38–53. doi: 10.1016/j.brainres.2005.06.042. [DOI] [PubMed] [Google Scholar]
- Ramos-Moreno T, Galazo MJ, Porrero C, Martinez-Cerdeno V, Clasca F. Extracellular matrix molecules and synaptic plasticity: immunomapping of intracellular and secreted Reelin in the adult rat brain. The European journal of neuroscience. 2006;23:401–422. doi: 10.1111/j.1460-9568.2005.04567.x. [DOI] [PubMed] [Google Scholar]
- Reif A, Fritzen S, Finger M, Strobel A, Lauer M, Schmitt A, Lesch KP. Neural stem cell proliferation is decreased in schizophrenia, but not in depression. Molecular psychiatry. 2006;11:514–522. doi: 10.1038/sj.mp.4001791. [DOI] [PubMed] [Google Scholar]
- Reynolds R, Hardy R. Oligodendroglial progenitors labeled with the O4 antibody persist in the adult rat cerebral cortex in vivo. Journal of neuroscience research. 1997;47:455–470. doi: 10.1002/(sici)1097-4547(19970301)47:5<455::aid-jnr1>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Rickmann M, Amaral DG, Cowan WM. Organization of radial glial cells during the development of the rat dentate gyrus. The Journal of comparative neurology. 1987;264:449–479. doi: 10.1002/cne.902640403. [DOI] [PubMed] [Google Scholar]
- Riquelme PA, Drapeau E, Doetsch F. Brain micro-ecologies: neural stem cell niches in the adult mammalian brain. Philos Trans R Soc Lond B Biol Sci. 2008;363(1489):123–37. doi: 10.1098/rstb.2006.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivers LE, Young KM, Rizzi M, Jamen F, Psachoulia K, Wade A, Kessaris N, Richardson WD. PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice. Nat Neurosci. 2008;12:1392–401. doi: 10.1038/nn.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robin AM, Zhang ZG, Wang L, Zhang RL, Katakowski M, Zhang L, Wang Y, Zhang C, Chopp M. Stromal cell-derived factor 1alpha mediates neural progenitor cell motility after focal cerebral ischemia. J Cereb Blood Flow Metab. 2006;26:125–134. doi: 10.1038/sj.jcbfm.9600172. [DOI] [PubMed] [Google Scholar]
- Roy NS, Wang S, Harrison-Restelli C, Benraiss A, Fraser RA, Gravel M, Braun PE, Goldman SA. Identification, isolation, and promoter-defined separation of mitotic oligodendrocyte progenitor cells from the adult human subcortical white matter. J Neurosci. 1999;19:9986–9995. doi: 10.1523/JNEUROSCI.19-22-09986.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutishauser U, Landmesser L. Polysialic acid in the vertebrate nervous system: a promoter of plasticity in cell-cell interactions. Trends Neurosci. 1996;19:422–427. doi: 10.1016/0166-2236(96)10041-2. [DOI] [PubMed] [Google Scholar]
- Saghatelyan A, de Chevigny A, Schachner M, Lledo PM. Tenascin-R mediates activity-dependent recruitment of neuroblasts in the adult mouse forebrain. Nat Neurosci. 2004;7:347–356. doi: 10.1038/nn1211. [DOI] [PubMed] [Google Scholar]
- Salhia B, Hwang JH, Smith CA, Nakada M, Rutka F, Symons M, Rutka JT. Role of myosin II activity and the regulation of myosin light chain phosphorylation in astrocytomas. Cell motility and the cytoskeleton. 2008;65:12–24. doi: 10.1002/cm.20240. [DOI] [PubMed] [Google Scholar]
- Sanai N, Berger MS, Garcia-Verdugo JM, Alvarez-Buylla A. Comment on “Human neuroblasts migrate to the olfactory bulb via a lateral ventricular extension”. Science. 2007;318:393. doi: 10.1126/science.318.5849.393a. author reply 393. [DOI] [PubMed] [Google Scholar]
- Sanai N, Tramontin AD, Quinones-Hinojosa A, Barbaro NM, Gupta N, Kunwar S, Lawton MT, McDermott MW, Parsa AT, Manuel-Garcia Verdugo J, Berger MS, Alvarez-Buylla A. Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature. 2004;427:740–744. doi: 10.1038/nature02301. [DOI] [PubMed] [Google Scholar]
- Sawamoto K, Wichterle H, Gonzalez-Perez O, Cholfin JA, Yamada M, Spassky N, Murcia NS, Garcia-Verdugo JM, Marin O, Rubenstein JL, Tessier-Lavigne M, Okano H, Alvarez-Buylla A. New neurons follow the flow of cerebrospinal fluid in the adult brain. Science. 2006;311:629–632. doi: 10.1126/science.1119133. [DOI] [PubMed] [Google Scholar]
- Schaar BT, McConnell SK. Cytoskeletal coordination during neuronal migration. Proc Nat Acad Sci USA. 2005;102:13652–13657. doi: 10.1073/pnas.0506008102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Goodman JH, Sollas AL. Granule-like neurons at the hilar/CA3 border after status epilepticus and their synchrony with area CA3 pyramidal cells: functional implications of seizure-induced neurogenesis. J Neurosci. 2000;20:6144–6158. doi: 10.1523/JNEUROSCI.20-16-06144.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer HD. Cerebral astrocytomas and their derivatives. American Journal of Cancer. 1940;1:159–98. [Google Scholar]
- Schiffer D, Giordana MT, Migheli A, Giaccone G, Pezzotta S, Mauro A. Glial fibrillary acidic protein and vimentin in the experimental glial reaction of the rat brain. Brain Res. 1986;374:110–118. doi: 10.1016/0006-8993(86)90399-9. [DOI] [PubMed] [Google Scholar]
- Shen Q, Wang Y, Kokovay E, Lin G, Chuang SM, Goderie SK, Roysam B, Temple S. adult SVZ stem cells lie in a vascular niche: a quantitative analysis of niche cell-cell interactions. Cell Stem Cell. 2008;3:289–300. doi: 10.1016/j.stem.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki T. Hippocampal adult neurogenesis occurs in a microenvironment provided by PSA-NCAM-expressing immature neurons. Journal of neuroscience research. 2002;69:772–783. doi: 10.1002/jnr.10366. [DOI] [PubMed] [Google Scholar]
- Seki T, Arai Y. Highly polysialylated neural cell adhesion molecule (NCAM-H) is expressed by newly generated granule cells in the dentate gyrus of the adult rat. J Neurosci. 1993;13:2351–2358. doi: 10.1523/JNEUROSCI.13-06-02351.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki T, Namba T, Mochizuki H, Onodera M. Clustering, migration, and neurite formation of neural precursor cells in the adult rat hippocampus. The Journal of comparative neurology. 2007;502:275–290. doi: 10.1002/cne.21301. [DOI] [PubMed] [Google Scholar]
- Seri B, Garcia-Verdugo JM, McEwen BS, Alvarez-Buylla A. Astrocytes give rise to new neurons in the adult mammalian hippocampus. J Neurosci. 2001;21:7153–7160. doi: 10.1523/JNEUROSCI.21-18-07153.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro LA, Ng KL, Kinyamu R, Whitaker-Azmitia P, Geisert EE, Blurton-Jones M, Zhou QY, Ribak CE. Origin, migration and fate of newly generated neurons in the adult rodent piriform cortex. Brain structure & function. 2007;212:133–148. doi: 10.1007/s00429-007-0151-3. [DOI] [PubMed] [Google Scholar]
- Shen Q, Goderie SK, Jin L, Karanth N, Sun Y, Abramova N, Vincent P, Pumiglia K, Temple S. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304:1338–1340. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- Simo S, Pujadas L, Segura MF, La Torre A, Del Rio JA, Urena JM, Comella JX, Soriano E. Reelin induces the detachment of postnatal subventricular zone cells and the expression of the Egr-1 through Erk1/2 activation. Cereb Cortex. 2007;17:294–303. doi: 10.1093/cercor/bhj147. [DOI] [PubMed] [Google Scholar]
- Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–5828. [PubMed] [Google Scholar]
- Snyder JS, Kee N, Wojtowicz JM. Effects of adult neurogenesis on synaptic plasticity in the rat dentate gyrus. Journal of neurophysiology. 2001;85:2423–2431. doi: 10.1152/jn.2001.85.6.2423. [DOI] [PubMed] [Google Scholar]
- Sontheimer H. Malignant gliomas: perverting glutamate and ion homeostasis for selective advantage. TRENDS Neurosci. 2003;26:543–549. doi: 10.1016/j.tins.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Spassky N, de Castro F, Le Bras B, Heydon K, Quéraud-LeSaux F, Bloch-Gallego E, Chédotal A, Zalc B, Thomas JL. Directional guidance of oligodendroglial migration by class 3 semaphorins and netrin-1. J Neurosci. 2002;22:5992–6004. doi: 10.1523/JNEUROSCI.22-14-05992.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staflin K, Lindvall M, Zuchner T, Lundberg C. Instructive cross-talk between neural progenitor cells and gliomas. Journal of neuroscience research. 2007;85:2147–2159. doi: 10.1002/jnr.21344. [DOI] [PubMed] [Google Scholar]
- Stefansson H, Sarginson J, Kong A, Yates P, Steinthorsdottir V, Gudfinnsson E, Gunnarsdottir S, Walker N, Petursson H, Crombie C, Ingason A, Gulcher JR, Stefansson K, St Clair D. Association of neuregulin 1 with schizophrenia confirmed in a Scottish population. American journal of human genetics. 2003;72:83–87. doi: 10.1086/345442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striedinger K, Meda P, Scemes E. Exocytosis of ATP from astrocyte progenitors modulates spontaneous Ca2+ oscillations and cell migration. Glia. 2007;55:652–662. doi: 10.1002/glia.20494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striedinger K, Scemes E. Interleukin-1beta affect calcium signaling and in vitro cell migration of astrocyte progenitors. J Neuroimmunol. 2008;196:116–123. doi: 10.1016/j.jneuroim.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto Y, Taniguchi M, Yagi T, Akagi Y, Nojyo Y, Tamamaki N. Guidance of glial precursor cell migration by secreted cues in the developing optic nerve. Development. 2001;128:3321–30. doi: 10.1242/dev.128.17.3321. [DOI] [PubMed] [Google Scholar]
- Sugiura S, Kitagawa K, Tanaka S, Todo K, Omura-Matsuoka E, Sasaki T, Mabuchi T, Matsushita K, Yagita Y, Hori M. Adenovirus-mediated gene transfer of heparin-binding epidermal growth factor-like growth factor enhances neurogenesis and angiogenesis after focal cerebral ischemia in rats. Stroke; a journal of cerebral circulation. 2005;36:859–864. doi: 10.1161/01.STR.0000158905.22871.95. [DOI] [PubMed] [Google Scholar]
- Sun D, Colello RJ, Daugherty WP, Kwon TH, McGinn MJ, Harvey HB, Bullock MR. Cell proliferation and neuronal differentiation in the dentate gyrus in juvenile and adult rats following traumatic brain injury. Journal of neurotrauma. 2005;22:95–105. doi: 10.1089/neu.2005.22.95. [DOI] [PubMed] [Google Scholar]
- Sun Y, Jin K, Xie L, Childs J, Mao XO, Logvinova A, Greenberg DA. VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. The Journal of clinical investigation. 2003;111:1843–1851. doi: 10.1172/JCI17977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundholm-Peters NL, Yang HK, Goings GE, Walker AS, Szele FG. Subventricular zone neuroblasts emigrate toward cortical lesions. J Neuropathol Exp Neurol. 2005;64:1089–1100. doi: 10.1097/01.jnen.0000190066.13312.8f. [DOI] [PubMed] [Google Scholar]
- Suzuki SO, Goldman JE. Multiple cell populations in the early postnatal subventricular zone take distinct migratory pathways: a dynamic study of glial and neuronal progenitor migration. J Neurosci. 2003;23:4240–4250. doi: 10.1523/JNEUROSCI.23-10-04240.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki SO, Kitai R, Llena J, Lee SC, Goldman JE, Shafit-Zagardo B. MAP-2e, a novel MAP-2 isoform, is expressed in gliomas and delineates tumor architecture and patterns of infiltration. J Neuropath Exp Neurol. 2002;61:403–412. doi: 10.1093/jnen/61.5.403. [DOI] [PubMed] [Google Scholar]
- Szele FG, Chesselet MF. Cortical lesions induce an increase in cell number and PSA-NCAM expression in the subventricular zone of adult rats. The Journal of comparative neurology. 1996;368:439–454. doi: 10.1002/(SICI)1096-9861(19960506)368:3<439::AID-CNE9>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Tabar V, Panagiotakos G, Greenberg ED, Chan BK, Sadelain M, Gutin PH, Studer L. Migration and differentiation of neural precursors derived from human embryonic stem cells in the rat brain. Nat Biotechnol. 2005;23:601–606. doi: 10.1038/nbt1088. [DOI] [PubMed] [Google Scholar]
- Takamiya Y, Kohsaka S, Toya S, Otani M, Tsukada Y. Immunohistochemical studies on the proliferation of reactive astrocytes and the expression of cytoskeletal proteins following brain injury in rats. Brain Res. 1988;466:201–210. doi: 10.1016/0165-3806(88)90045-4. [DOI] [PubMed] [Google Scholar]
- Takemura NU. Evidence for neurogenesis within the white matter beneath the temporal neocortex of the adult rat brain. Neuroscience. 2005;134:121–132. doi: 10.1016/j.neuroscience.2005.04.033. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Nogawa S, Suzuki S, Dembo T, Kosakai A. Upregulation of oligodendrocyte progenitor cells associated with restoration of mature oligodendrocytes and myelination in peri-infarct area in the rat brain. Brain Res. 2003;989:172–179. doi: 10.1016/s0006-8993(03)03317-1. [DOI] [PubMed] [Google Scholar]
- Tattersfield AS, Croon RJ, Liu YW, Kells AP, Faull RL, Connor B. Neurogenesis in the striatum of the quinolinic acid lesion model of Huntington’s disease. Neuroscience. 2004;127:319–332. doi: 10.1016/j.neuroscience.2004.04.061. [DOI] [PubMed] [Google Scholar]
- Tavazoie M, Van der Veken L, Silva-Vargas V, Louissaint M, Colonna L, Zaidi B, Garcia-Verdugo JM, Doetsch F. A specialized vascular niche for adult neural stem cells. Cell Stem Cell. 2008;3:279–88. doi: 10.1016/j.stem.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teramoto T, Qiu J, Plumier JC, Moskowitz MA. EGF amplifies the replacement of parvalbumin-expressing striatal interneurons after ischemia. The Journal of clinical investigation. 2003;111:1125–1132. doi: 10.1172/JCI17170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodosis DT, Rougon G, Poulain DA. Retention of embryonic features by an adult neuronal system capable of plasticity: polysialylated neural cell adhesion molecule in the hypothalamo-neurohypophysial system. Proceedings of the National Academy of Sciences of the United States of America. 1991;88:5494–5498. doi: 10.1073/pnas.88.13.5494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas LB, Gates MA, Steindler DA. Young neurons from the adult subependymal zone proliferate and migrate along an astrocyte, extracellular matrix-rich pathway. Glia. 1996;17:1–14. doi: 10.1002/(SICI)1098-1136(199605)17:1<1::AID-GLIA1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Thompson A, Boekhoorn K, Van Dam AM, Lucassen PJ. Changes in adult neurogenesis in neurodegenerative diseases: cause or consequence? Genes, brain, and behavior. 2008;7(Suppl 1):28–42. doi: 10.1111/j.1601-183X.2007.00379.x. [DOI] [PubMed] [Google Scholar]
- Thored P, Arvidsson A, Cacci E, Ahlenius H, Kallur T, Darsalia V, Ekdahl CT, Kokaia Z, Lindvall O. Persistent production of neurons from adult brain stem cells during recovery after stroke. Stem Cells. 2006;24:739–747. doi: 10.1634/stemcells.2005-0281. [DOI] [PubMed] [Google Scholar]
- Thored P, Wood J, Arvidsson A, Cammenga J, Kokaia Z, Lindvall O. Long-term neuroblast migration along blood vessels in an area with transient angiogenesis and increased vascularization after stroke. Stroke; a journal of cerebral circulation. 2007;38:3032–3039. doi: 10.1161/STROKEAHA.107.488445. [DOI] [PubMed] [Google Scholar]
- Tiveron MC, Rossel M, Moepps B, Zhang YL, Seidenfaden R, Favor J, Konig N, Cremer H. Molecular interaction between projection neuron precursors and invading interneurons via stromal-derived factor 1 (CXCL12)/CXCR4 signaling in the cortical subventricular zone/intermediate zone. J Neurosci. 2006;26:13273–13278. doi: 10.1523/JNEUROSCI.4162-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonchev AB, Yamashima T, Sawamoto K, Okano H. Enhanced proliferation of progenitor cells in the subventricular zone and limited neuronal production in the striatum and neocortex of adult macaque monkeys after global cerebral ischemia. Journal of neuroscience research. 2005;81:776–788. doi: 10.1002/jnr.20604. [DOI] [PubMed] [Google Scholar]
- Tramontin AD, Garcia-Verdugo JM, Lim DA, Alvarez-Buylla A. Postnatal development of radial glia and the ventricular zone (VZ): a continuum of the neural stem cell compartment. Cereb Cortex. 2003;13:580–587. doi: 10.1093/cercor/13.6.580. [DOI] [PubMed] [Google Scholar]
- Tran PB, Banisadr G, Ren D, Chenn A, Miller RJ. Chemokine receptor expression by neural progenitor cells in neurogenic regions of mouse brain. The Journal of comparative neurology. 2007;500:1007–1033. doi: 10.1002/cne.21229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran PB, Ren D, Veldhouse TJ, Miller RJ. Chemokine receptors are expressed widely by embryonic and adult neural progenitor cells. Journal of neuroscience research. 2004;76:20–34. doi: 10.1002/jnr.20001. [DOI] [PubMed] [Google Scholar]
- Tsai HH, Macklin WB, Miller RH. Netrin-1 is required for the normal development of spinal cord oligodendrocytes. J Neurosci. 2006;26:1913–22. doi: 10.1523/JNEUROSCI.3571-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai HH, Tessier-Lavigne M, Miller RH. Netrin 1 mediates spinal cord oligodendrocyte precursor dispersal. Development. 2003;130:2095–105. doi: 10.1242/dev.00424. [DOI] [PubMed] [Google Scholar]
- Tsai JW, Bremner KH, Vallee RB. Dual subcellular roles for LIS1 and dynein in radial neuronal migration in live brain tissue. Nat Neurosci. 2007;10:970–979. doi: 10.1038/nn1934. [DOI] [PubMed] [Google Scholar]
- Urrea C, Castellanos DA, Sagen J, Tsoulfas P, Bramlett HM, Dietrich WD. Widespread cellular proliferation and focal neurogenesis after traumatic brain injury in the rat. Restorative neurology and neuroscience. 2007;25:65–76. [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura RE, Goldman JE. Dorsal radial glia generate olfactory bulb interneurons in the postnatal murine brain. J Neurosci. 2007;27:4297–4302. doi: 10.1523/JNEUROSCI.0399-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verret L, Jankowsky JL, Xu GM, Borchelt DR, Rampon C. Alzheimer’s-type amyloidosis in transgenic mice impairs survival of newborn neurons derived from adult hippocampal neurogenesis. J Neurosci. 2007;27:6771–6780. doi: 10.1523/JNEUROSCI.5564-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Bekar LK, Furber K, Walz W. Vimentin-expressing proximal reactive astrocytes correlate with migration rather than proliferation following focal brain injury. Brain Res. 2004a;1024:193–202. doi: 10.1016/j.brainres.2004.07.086. [DOI] [PubMed] [Google Scholar]
- Wang L, Zhang Z, Wang Y, Zhang R, Chopp M. Treatment of stroke with erythropoietin enhances neurogenesis and angiogenesis and improves neurological function in rats. Stroke; a journal of cerebral circulation. 2004b;35:1732–1737. doi: 10.1161/01.STR.0000132196.49028.a4. [DOI] [PubMed] [Google Scholar]
- Wang L, Zhang ZG, Zhang RL, Gregg SR, Hozeska-Solgot A, LeTourneau Y, Wang Y, Chopp M. Matrix metalloproteinase 2 (MMP2) and MMP9 secreted by erythropoietin-activated endothelial cells promote neural progenitor cell migration. J Neurosci. 2006;26:5996–6003. doi: 10.1523/JNEUROSCI.5380-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Scott BW, Wojtowicz JM. Heterogenous properties of dentate granule neurons in the adult rat. Journal of neurobiology. 2000;42:248–257. [PubMed] [Google Scholar]
- Wang Y, Jin K, Mao XO, Xie L, Banwait S, Marti HH, Greenberg DA. VEGF-overexpressing transgenic mice show enhanced post-ischemic neurogenesis and neuromigration. Journal of neuroscience research. 2007;85:740–747. doi: 10.1002/jnr.21169. [DOI] [PubMed] [Google Scholar]
- Ward M, McCann C, DeWulf M, Wu JY, Rao Y. Distinguishing between directional guidance and motility regulation in neuronal migration. J Neurosci. 2003;23:5170–5177. doi: 10.1523/JNEUROSCI.23-12-05170.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Toyama Y, Nishiyama A. Differentiation of proliferated NG2-positive glial progenitor cells in a remyelinating lesion. Journal of neuroscience research. 2002;69:826–836. doi: 10.1002/jnr.10338. [DOI] [PubMed] [Google Scholar]
- Wekerle H. The enigma of arrival: [corrected] the entrance of auto-immune T lymphocytes in central nervous tissues and of their attack against the myelin structures. Comptes rendus biologies. 2007;330:1–12. doi: 10.1016/j.crvi.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Wen PH, Shao X, Shao Z, Hof PR, Wisniewski T, Kelley K, Friedrich VL, Jr, Ho L, Pasinetti GM, Shioi J, Robakis NK, Elder GA. Overexpression of wild type but not an FAD mutant presenilin-1 promotes neurogenesis in the hippocampus of adult mice. Neurobiol Dis. 2002;10:8–19. doi: 10.1006/nbdi.2002.0490. [DOI] [PubMed] [Google Scholar]
- Wichterle H, Garcia-Verdugo JM, Alvarez-Buylla A. Direct evidence for homotypic, glia-independent neuronal migration. Neuron. 1997;18:779–791. doi: 10.1016/s0896-6273(00)80317-7. [DOI] [PubMed] [Google Scholar]
- Wick W, Platten M, Weller M. Glioma cell invasion: regulation of metalloproteinase activity by TGF-beta. Journal of Neuro-Oncology. 2001;53:177–185. doi: 10.1023/a:1012209518843. [DOI] [PubMed] [Google Scholar]
- Wilhelmsson U, Bushong EA, Price DL, Smarr BL, Phung V, Terada M, Ellisman MH, Pekny M. Redefining the concept of reactive astrocytes as cells that remain within their unique domains upon reaction to injury. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:17513–17518. doi: 10.1073/pnas.0602841103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams A, Piaton G, Aigrot MS, Belhadi A, Theaudin M, Petermann F, Thomas JL, Zalc B, Lubetzki C. Semaphorin 3A and 3F: key players in myelin repair in multiple sclerosis? Brain. 2007;130:2554–2565. doi: 10.1093/brain/awm202. [DOI] [PubMed] [Google Scholar]
- Winner B, Couillard-Despres S, Geyer M, Aigner R, Bogdahn U, Aigner L, Kuhn HG, Winkler J. Dopaminergic lesion enhances growth factor-induced striatal neuroblast migration. J Neuropathol Exp Neurol. 2008;67:105–116. doi: 10.1097/nen.0b013e3181630cff. [DOI] [PubMed] [Google Scholar]
- White RE, Jakeman LB. Don’t fence me in: harnessing the beneficial roles of astrocytes for spinal cord repair. Restor Neurol Neurosci. 2008;26:197–214. [PMC free article] [PubMed] [Google Scholar]
- Wu W, Wong K, Chen J, Jiang Z, Dupuis S, Wu JY, Rao Y. Directional guidance of neuronal migration in the olfactory system by the protein Slit [see comments] Nature. 1999;400:331–336. doi: 10.1038/22477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Wang S, Jiang X, Zhao Y, Gao M, Zhang Y, Wang X, Tano K, Kanehara M, Zhang W, Ishida T. Hypoxia-induced astrocytes promote the migration of neural progenitor cells via vascular endothelial factor, stem cell factor, stromal-derived factor-1alpha and monocyte chemoattractant protein-1 upregulation in vitro. Clinical and experimental pharmacology & physiology. 2007;34:624–631. doi: 10.1111/j.1440-1681.2007.04619.x. [DOI] [PubMed] [Google Scholar]
- Yamashita T, Ninomiya M, Hernandez Acosta P, Garcia-Verdugo JM, Sunabori T, Sakaguchi M, Adachi K, Kojima T, Hirota Y, Kawase T, Araki N, Abe K, Okano H, Sawamoto K. Subventricular zone-derived neuroblasts migrate and differentiate into mature neurons in the post-stroke adult striatum. J Neurosci. 2006;26:6627–6636. doi: 10.1523/JNEUROSCI.0149-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan YP, Sailor KA, Lang BT, Park SW, Vemuganti R, Dempsey RJ. Monocyte chemoattractant protein-1 plays a critical role in neuroblast migration after focal cerebral ischemia. J Cereb Blood Flow Metab. 2007;27:1213–1224. doi: 10.1038/sj.jcbfm.9600432. [DOI] [PubMed] [Google Scholar]
- Yang HK, Sundholm-Peters NL, Goings GE, Walker AS, Hyland K, Szele FG. Distribution of doublecortin expressing cells near the lateral ventricles in the adult mouse brain. Journal of neuroscience research. 2004;76:282–295. doi: 10.1002/jnr.20071. [DOI] [PubMed] [Google Scholar]
- Yang P, Baker KA, Hagg T. A disintegrin and metalloprotease 21 (ADAM21) is associated with neurogenesis and axonal growth in developing and adult rodent CNS. The Journal of comparative neurology. 2005;490:163–179. doi: 10.1002/cne.20659. [DOI] [PubMed] [Google Scholar]
- Yin L, Fu SL, Shi GY, Li Y, Jin JQ, Ma ZW, Lu PH. Expression and regulation of major histocompatibility complex on neural stem cells and their lineages. Stem cells and development. 2008;17:53–65. doi: 10.1089/scd.2007.0063. [DOI] [PubMed] [Google Scholar]
- Young D, Lawlor PA, Leone P, Dragunow M, During MJ. Environmental enrichment inhibits spontaneous apoptosis, prevents seizures and is neuroprotective. Nature medicine. 1999;5:448–453. doi: 10.1038/7449. [DOI] [PubMed] [Google Scholar]
- Zerlin M, Milosevic A, Goldman JE. Glial progenitors of the neonatal subventricular zone differentiate asynchronously, leading to spatial dispersion of glial clones and to the persistence of immature glia in the adult mammalian CNS. Dev Biol. 2004;270:200–13. doi: 10.1016/j.ydbio.2004.02.024. [DOI] [PubMed] [Google Scholar]
- Zhang C, McNeil E, Dressler L, Siman R. Long-lasting impairment in hippocampal neurogenesis associated with amyloid deposition in a knock-in mouse model of familial Alzheimer’s disease. Exp Neurol. 2007a;204:77–87. doi: 10.1016/j.expneurol.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Vutskits L, Calaora V, Durbec P, Kiss JZ. A role for the polysialic acid-neural cell adhesion molecule in PDGF-induced chemotaxis of oligodendrocyte precursor cells. J Cell Sci. 2004a;117:93–103. doi: 10.1242/jcs.00827. [DOI] [PubMed] [Google Scholar]
- Zhang R, Wang L, Zhang L, Chen J, Zhu Z, Zhang Z, Chopp M. Nitric oxide enhances angiogenesis via the synthesis of vascular endothelial growth factor and cGMP after stroke in the rat. Circulation research. 2003;92:308–313. doi: 10.1161/01.res.0000056757.93432.8c. [DOI] [PubMed] [Google Scholar]
- Zhang R, Zhang L, Zhang Z, Wang Y, Lu M, Lapointe M, Chopp M. A nitric oxide donor induces neurogenesis and reduces functional deficits after stroke in rats. Ann Neurol. 2001a;50:602–611. doi: 10.1002/ana.1249. [DOI] [PubMed] [Google Scholar]
- Zhang R, Zhang Z, Zhang C, Zhang L, Robin A, Wang Y, Lu M, Chopp M. Stroke transiently increases subventricular zone cell division from asymmetric to symmetric and increases neuronal differentiation in the adult rat. J Neurosci. 2004b;24:5810–5815. doi: 10.1523/JNEUROSCI.1109-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang RL, LeTourneau Y, Gregg SR, Wang Y, Toh Y, Robin AM, Zhang ZG, Chopp M. Neuroblast division during migration toward the ischemic striatum: a study of dynamic migratory and proliferative characteristics of neuroblasts from the subventricular zone. J Neurosci. 2007;27:3157–62. doi: 10.1523/JNEUROSCI.4969-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang RL, Zhang ZG, Chopp M. Neurogenesis in the adult ischemic brain: generation, migration, survival, and restorative therapy. Neuroscientist. 2005;11:408–416. doi: 10.1177/1073858405278865. [DOI] [PubMed] [Google Scholar]
- Zhang RL, Zhang ZG, Wang Y, LeTourneau Y, Liu XS, Zhang X, Gregg SR, Wang L, Chopp M. Stroke induces ependymal cell transformation into radial glia in the subventricular zone of the adult rodent brain. J Cereb Blood Flow Metab. 2007b;27:1201–1212. doi: 10.1038/sj.jcbfm.9600430. [DOI] [PubMed] [Google Scholar]
- Zhang RL, Zhang ZG, Zhang L, Chopp M. Proliferation and differentiation of progenitor cells in the cortex and the subventricular zone in the adult rat after focal cerebral ischemia. Neuroscience. 2001b;105:33–41. doi: 10.1016/s0306-4522(01)00117-8. [DOI] [PubMed] [Google Scholar]
- Zhu X, Bergles DE, Nishiyama A. NG2 cells generate both oligodendrocytes and gray matter astrocytes. Development. 2008;135(1):145–57. doi: 10.1242/dev.004895. [DOI] [PubMed] [Google Scholar]
- Ziv Y, Ron N, Butovsky O, Landa G, Sudai E, Greenberg N, Cohen H, Kipnis J, Schwartz M. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat Neurosci. 2006;9:268–275. doi: 10.1038/nn1629. [DOI] [PubMed] [Google Scholar]