Abstract
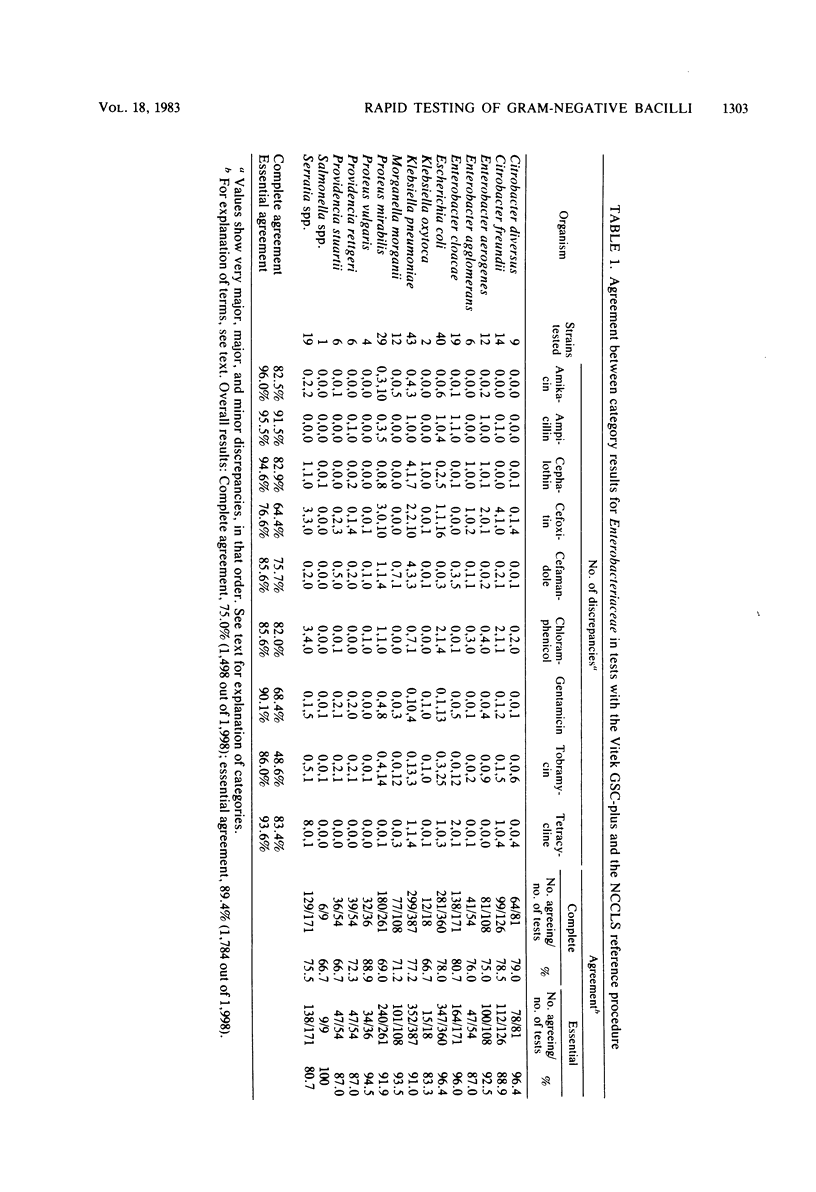
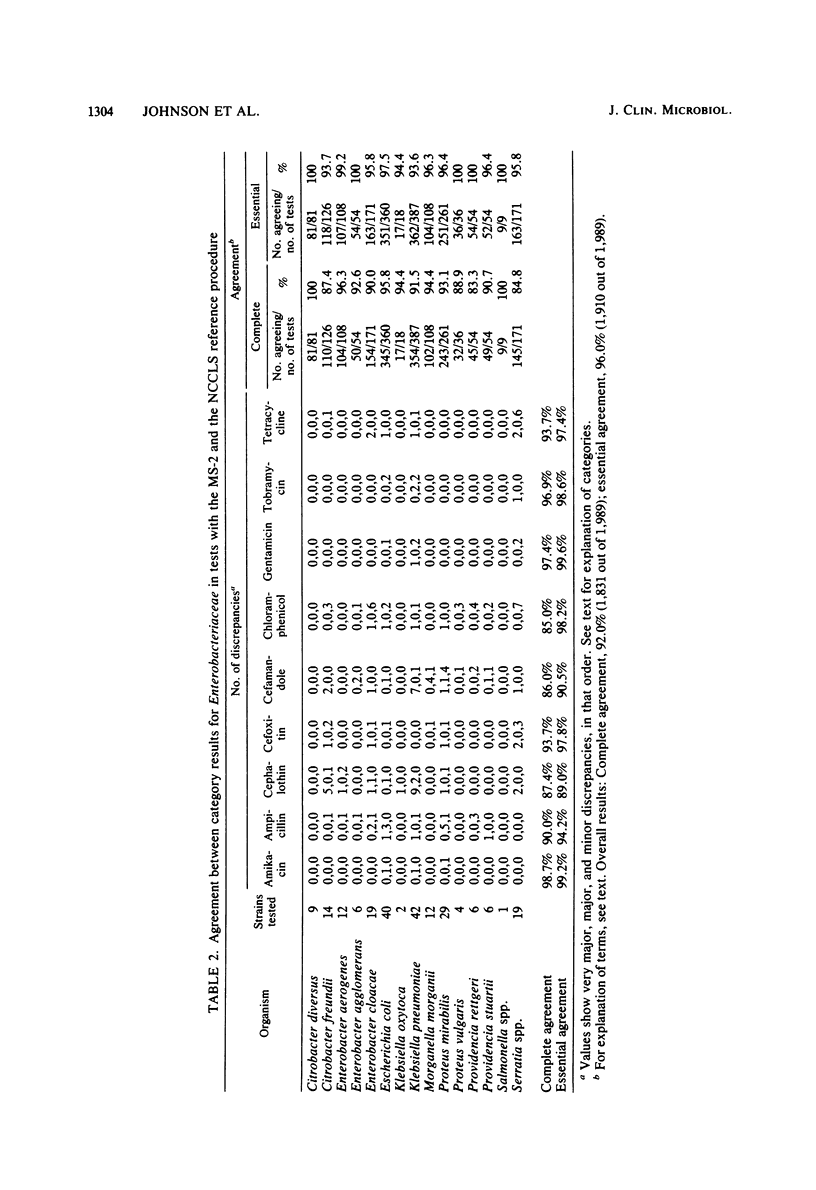
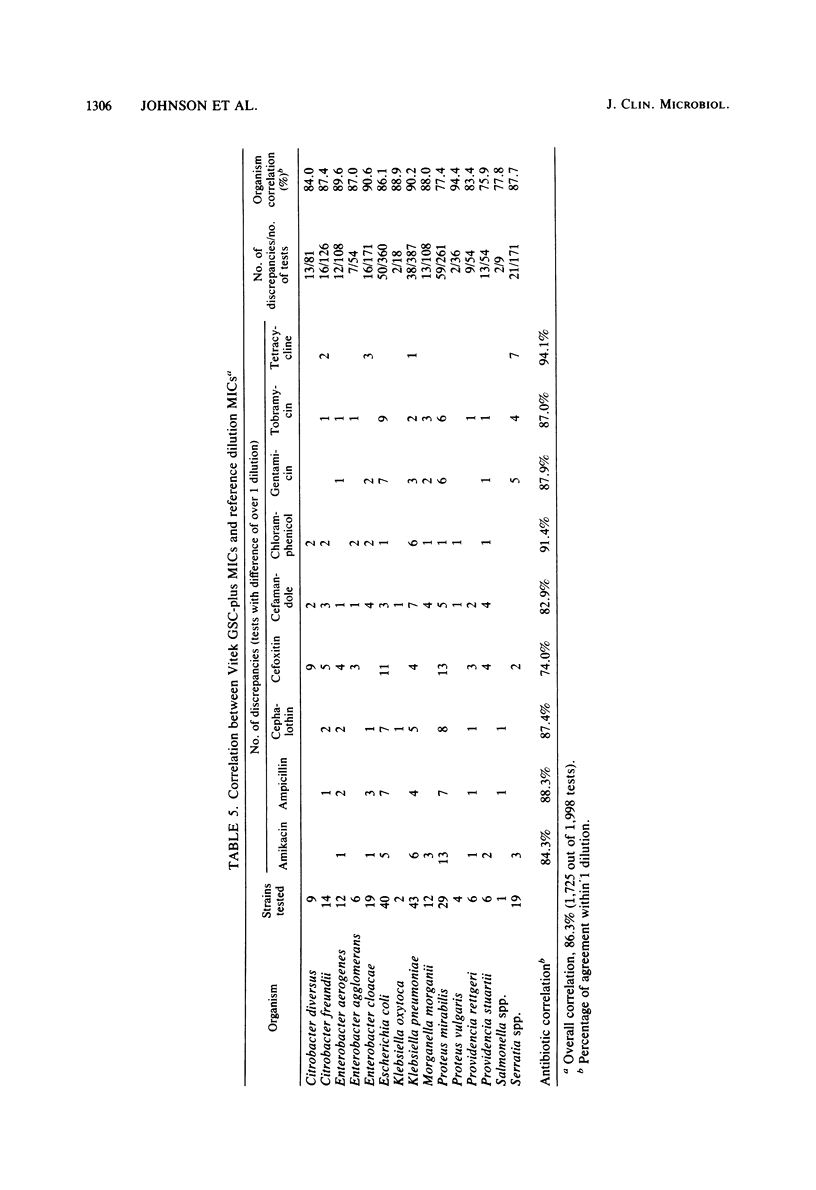
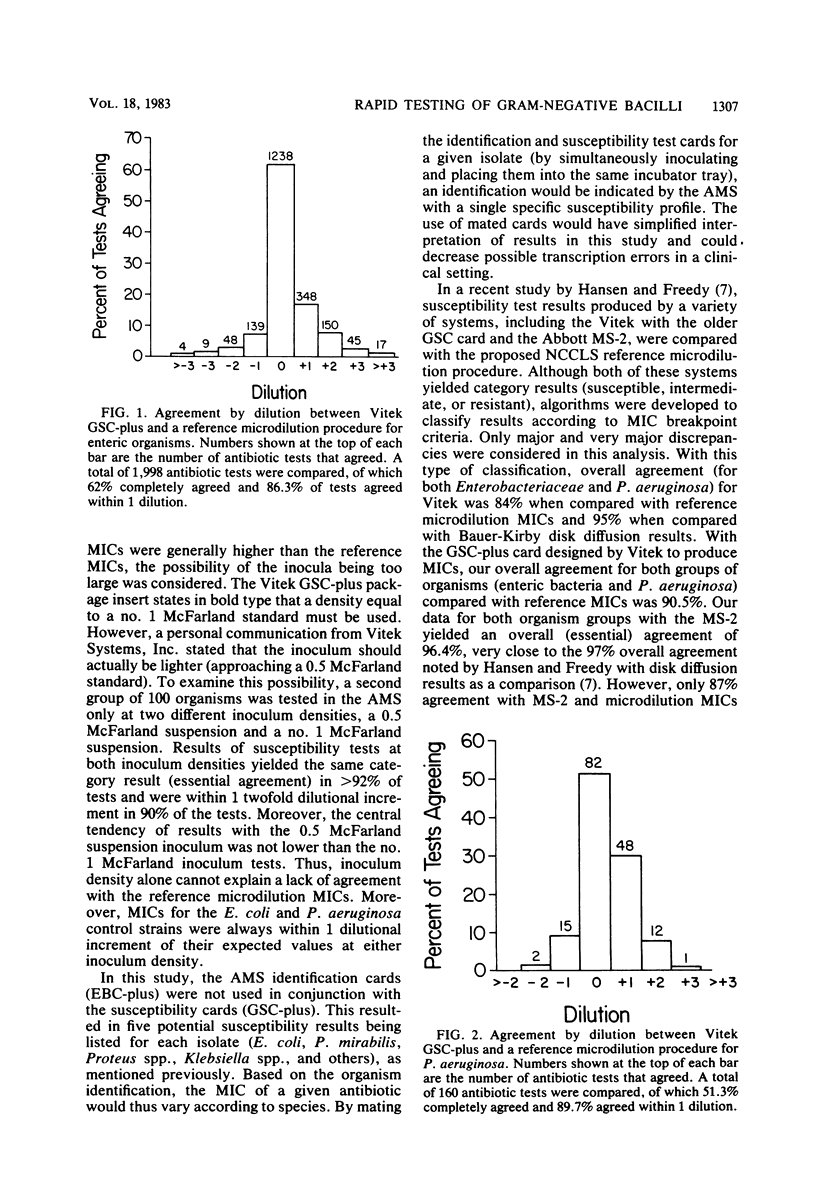
The Vitek AutoMicrobic System with GSC-plus cards and the Abbott MS-2 system were tested in parallel and the results were compared directly with those of a reference microdilution minimal inhibitory concentration (MIC) procedure on a group of 262 clinical isolates of the family Enterobacteriaceae and of Pseudomonas aeruginosa. Results of both systems were compared with the reference MIC for category agreement, and in addition, the Vitek MICs were compared with those obtained by the reference procedure. The Vitek system provided an essential category correlation of 89.4% for enteric bacteria and 97.0% for P. aeruginosa. Vitek MICs agreed within 1 twofold dilutional increment for 86.3% of the enteric bacteria tested and for 96.2% of the P. aeruginosa isolates. The Abbott MS-2 essential categoric agreement was 92.0% for enteric bacteria and 92.4% for P. aeruginosa. If only aminoglycosides or carbenicillin were considered for P. aeruginosa isolates, the essential category agreement was 92.5% for the Vitek and 93.3% for the MS-2. The majority of MS-2 category errors (13 of 19) with P. aeruginosa involved gentamicin results on isolates whose reference MICs were 8 micrograms/ml and whose MS-2 results were susceptible (MIC less than or equal to 4 micrograms/ml). Retesting of the P. aeruginosa isolates in calcium-supplemented MS-2 broth increased the essential agreement for the aminoglycosides to 97.5%.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldridge C., Jones P. W., Gibson S., Lanham J., Meyer M., Vannest R., Charles R. Automated microbiological detection/identification system. J Clin Microbiol. 1977 Oct;6(4):406–413. doi: 10.1128/jcm.6.4.406-413.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes W. G., Green L. R., Talley R. L. Clinical evaluation of automated antibiotic susceptibility testing with the MS-2 system. J Clin Microbiol. 1980 Oct;12(4):527–532. doi: 10.1128/jcm.12.4.527-532.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer A. W., Kirby W. M., Sherris J. C., Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966 Apr;45(4):493–496. [PubMed] [Google Scholar]
- Hansen S. L., Freedy P. K. Concurrent comparability of automated systems and commercially prepared microdilution trays for susceptibility testing. J Clin Microbiol. 1983 May;17(5):878–886. doi: 10.1128/jcm.17.5.878-886.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. N. Antimicrobial susceptibility testing (AST): a review of changing trends, quality control guidelines, test accuracy, and recommendation for the testing of beta-lactam drugs. Diagn Microbiol Infect Dis. 1983 Mar;1(1):1–24. doi: 10.1016/0732-8893(83)90028-7. [DOI] [PubMed] [Google Scholar]
- Kelly M. T., Latimer J. M., Balfour L. C. Comparison of three automated systems for antimicrobial susceptibility testing of gram-negative bacilli. J Clin Microbiol. 1982 May;15(5):902–905. doi: 10.1128/jcm.15.5.902-905.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampe M. F., Aitken C. L., Dennis P. G., Forsythe P. S., Patrick K. E., Schoenknecht F. D., Sherris J. C. Relationship of early readings of minimal inhibitory concentrations to the results of overnight tests. Antimicrob Agents Chemother. 1975 Oct;8(4):429–433. doi: 10.1128/aac.8.4.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray P. R., Jorgensen J. H. Quantitative susceptibility test methods in major United States medical centers. Antimicrob Agents Chemother. 1981 Jul;20(1):66–70. doi: 10.1128/aac.20.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornsberry C., Anhalt J. P., Washington J. A., 2nd, McCarthy L. R., Schoenknecht F. D., Sherris J. C., Spencer H. J. Clinical laboratory evaluation of the Abbott MS-2 automated antimicrobial susceptibility testing system: report of a collaborative study. J Clin Microbiol. 1980 Sep;12(3):375–390. doi: 10.1128/jcm.12.3.375-390.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornsberry C., Gavan T. L., Sherris J. C., Balows A., Matsen J. M., Sabath L. D., Schoenknecht F., Thrupp L. D., Washington J. A., 2nd Laboratory evaluation of a rapid, automatic susceptibility testing system: report of a collaborative study. Antimicrob Agents Chemother. 1975 Apr;7(4):466–480. doi: 10.1128/aac.7.4.466. [DOI] [PMC free article] [PubMed] [Google Scholar]


