Abstract
Mechanisms of phloem loading in the minor veins of leaves are known for only a few species. We propose that there are a limited number of loading strategies for the primary photoassimilates, sucrose and sugar alcohols. These strategies can be predicted based on thermodynamic and anatomical considerations and identified by autoradiography of veins following uptake of 14C-labeled compounds, analysis of leaf solute composition and concentrations, and plasmodesmatal counting. Experiments on 45 dicotyledonous species identified the predicted loading patterns. Over 50-fold differences in concentrations of sucrose and sugar alcohols in leaves were measured. The cumulative concentrations of transport compounds in leaves correlated with loading mechanisms, a previously unrecognized association. Comparisons of solute concentrations and osmotic potentials of whole leaves suggest that sucrose and sugar alcohols are more concentrated in the cytosol than in the vacuoles of mesophyll cells, thus increasing the driving force for passive loading in species that employ this strategy. Passive loading is more widespread than previously thought, especially in trees. The results indicate that plants have exploited all thermodynamically feasible and structurally compatible loading strategies and that these strategies can be identified with straightforward protocols.
Keywords: plasmodesmata, polymer trap, raffinose, stachyose, sucrose
Phloem loading is the starting point for export of carbohydrates and other nutrients from leaves (1–4). To date, mechanisms of sucrose loading have been established for a relatively small number of species. Less is known about sugar alcohols, which in some plants are present in phloem sap at higher concentrations than sucrose (5–7).
In many species, loading involves an apoplastic step, driven by plasma-membrane transporters and energized by the proton motive force (8). In other plants, loading is symplastic, driven by a downhill concentration gradient from the mesophyll to the phloem and requiring high plasmodesmatal densities (2, 3). In some plants that load symplastically, the process is driven by diffusion alone and is passive (9, 10). In other species that load via the symplast, sucrose from the mesophyll diffuses into specialized companion cells (CCs) in the minor veins (11), known as intermediary cells, and is converted to raffinose and stachyose. These raffinose family oligosaccharides (RFOs) are larger than sucrose and are apparently unable to diffuse back to the mesophyll through the intermediary cell plasmodesmata. The RFOs accumulate in the phloem, a process known as polymer trapping (12), to a combined concentration that is similar to that of sucrose in apoplastic loaders (13, 14). Thus, there are 3 recognized strategies of sucrose loading: Apoplastic and symplastic with or without polymer trapping.
It is reasonable to assume that the anatomical features associated with sucrose loading in a given sieve element-companion cell (SE-CC) complex constrain available strategies of sugar alcohol loading. If sucrose loads apoplastically, sugar alcohol should also load via the apoplast, because there will not be enough plasmodesmata to accommodate symplastic flux. If sucrose loads symplastically, sugar alcohol is expected to follow the same route, because if it were apoplastically loaded it would diffuse back to the mesophyll, generating a futile pump-leak cycle.
To test these ideas we conducted a study of loading characteristics in 45 dicotyledonous species selected to represent different transport carbohydrate profiles, plasmodesmatal frequency distributions, and growth habits. We adopted the plasmodesmatal frequency scheme, and the data, of Gamalei (15), in which plants with high, intermediate, and low plasmodesmatal frequencies in the minor vein phloem are referred to as type 1, type 1–2a, and type 2 respectively. (However, we did not include plants with intermediary cells in the type 1 category, because polymer trapping is a distinct mechanism not shared by other species with high plasmodesmatal counts.) Gamalei's type 2 plants are further subdivided into type 2a with “ordinary” unspecialized CCs, while in type 2b plants the minor vein CCs are transfer cells, with elaborate wall ingrowths that increase plasma membrane surface area and the capacity for sucrose uptake from the apoplast (16).
The results of the survey indicate that loading mechanisms can be determined using relatively simple experimental protocols, that certain combinations of loading strategies are forbidden by incompatible anatomical requirements, and that there is surprising variation (over 50-fold) in the concentrations of transport carbohydrates in leaves that correlates well with loading pathways. In addition, our results indicate that passive flux into the phloem is more common than previously recognized, especially in woody plants.
Results
Autoradiography.
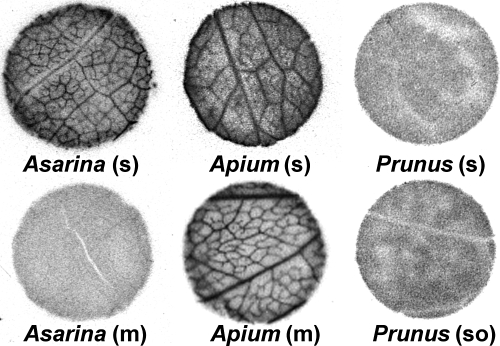
If sucrose or sugar alcohols actively accumulate in the phloem, the minor veins should be visible in autoradiographs following exposure of the tissue to these exogenous 14C-labeled compounds. In contrast, veins should not be visible in autoradiographs if loading is symplastic and passive, since radiolabeled compounds will diffuse readily between cells through the abundant plasmodesmata, thus precluding the establishment of concentration gradients. To distinguish between these outcomes, discs from abraded leaf tissue were exposed to radiolabeled compounds and autoradiographed. In many species, such as Apium graveolens, exogenous 14C-labeled compounds became concentrated in minor veins (Fig. 1). The contrast between veins and background (interveinal regions) was pronounced in many plants surveyed, but in some, the vein image was more difficult to discern (see Fig. S1 for additional autoradiographs). In leaf discs from other species, such as Prunus laurocerasus, the radiolabel was uniformly distributed, and no minor vein images were seen (Fig. 1). Since the latter result was negative, the experiments were repeated using a variety of abrasion and hand-sectioning techniques (see Materials and Methods). Final results were considered negative only if repeated experiments consistently failed to demonstrate accumulation of radiolabel in minor veins. In separate experiments, leaves of species with uniform distribution of radiolabel were exposed to 14CO2 for 1 h and autoradiographed. Again, no vein pattern was seen in these plants.
Fig. 1.
Autoradiographs of leaf discs from Asarina scandens, Apium graveolens, and Prunus laurocerasus. Discs were cut from abraded tissue and floated on either [14C]sucrose (s), [14C]mannitol (m), or [14C]sorbitol (so) for 1 h, flash-frozen, lyophilized, and autoradiographed. Vein loading is apparent in A. scandens and A. graveolens exposed to [14C]sucrose and A. graveolens exposed to [14C]mannitol, but not to A. scandens exposed to [14C]mannitol or P. laurocerasus exposed to either [14C]sucrose or [14C]sorbitol. Leaf discs are 8 mm in diameter.
There are clear correlations between autoradiographic images and the plasmodesmatal frequencies in the minor veins of these plants, as described by Gamalei (15) (Fig. 2). All species with uniform distribution of [14C]sucrose are either type 1 or type 1–2a (15), with high or intermediate numbers of plasmodesmata, as required if these plants load passively.
Fig. 2.
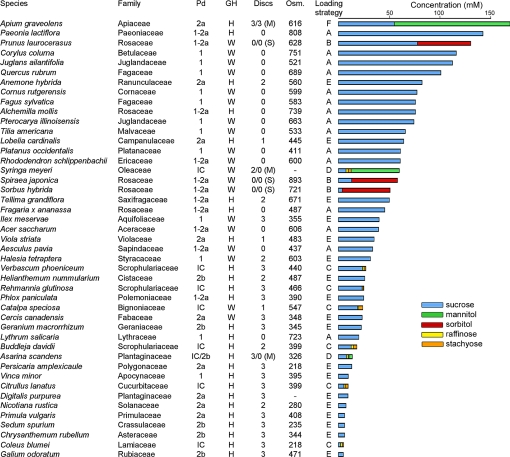
Survey of phloem loading characteristics of 45 species in 36 dicotyledonous families. Plasmodesmatal frequencies (Pd) are from Gamalei's data set (15) and correspond to his companion cell types: 1, abundant; 1–2a, intermediate; 2, low. Type 2a species have smooth cells walls; type 2b have transfer cell wall ingrowths. IC, intermediary cells, a specialized companion cell type with highly abundant plasmodesmata. Growth habit (GH) is either herbaceous (H) or woody (W). (Discs) refers to autoradiographs of leaf discs, with images of minor veins ranging from high contrast, 3; to low contrast, 1; or no minor vein images, 0. Unless otherwise indicated, leaf discs were exposed to [14C]sucrose. In 6 species, another set of discs was exposed to either [14C]mannitol (M) or [14C]sorbitol (S). For example, veins were apparent in autoradiographs of Syringa meyeri leaf discs exposed to [14C]sucrose, but not to [14C]mannitol. Osmolalities (Osm) refer to expressed leaf sap in units of mOsm·kg-1. (-) indicates that leaf sap could not be obtained. Loading strategies are illustrated in Fig. 4. Cumulative concentrations of transport compounds are presented in the bar graph.
Three of the species that were unable to accumulate [14C]sucrose in the veins also transport sorbitol (Prunus laurocerasus, Sorbus hybrida, and Spiraea japonica). None of these plants accumulated [14C]sorbitol in the minor veins. (See Fig. 1 for autoradiographs of P. laurocerasus discs.) Leaf discs from plants with intermediary cells accumulated radiolabel when exposed to [14C]sucrose, but when leaf discs from the mannitol-transporting species among them (Asarina scandens and Syringa meyeri) were floated on [14C]mannitol, no vein images were detected. (See Fig. 1 for autoradiographs of A. scandens discs.) All species with low plasmodesmatal counts (type 2a or 2b) accumulated [14C]sucrose in minor veins, and when Apium graveolens, the 1 species in this group that transports sugar alcohol (mannitol), was exposed to [14C]mannitol, veins were apparent in autoradiographs (Fig. 1).
Carbohydrates and Osmolalities.
High concentrations of transport compounds in leaves should be a feature of plants that load passively, whereas this is not a requirement in species that load actively. Sucrose, raffinose, stachyose, mannitol, and sorbitol were analyzed in leaf extracts by HPLC, and concentrations were calculated based on leaf water content, assuming no compartmentation between or within cells. Concentrations differed markedly between species (Fig. 2). Sucrose levels ranged from 2.2 mM in Coleus blumei to 142 mM in Paeonia lactiflora. Sugar alcohol levels ranged from 3.2 mM (mannitol) in Asarina scandens to 114 mM (mannitol) in Apium graveolens. Raffinose and stachyose levels were 4 mM or less. Osmolalities of expressed leaf sap ranged from 218 mOsm kg-1 in Coleus blumei to 893 mOsm kg-1 in Spiraea japonica (Fig. 2). Carbohydrate concentrations are plotted as percentages, with statistical analysis, in Fig. S2.
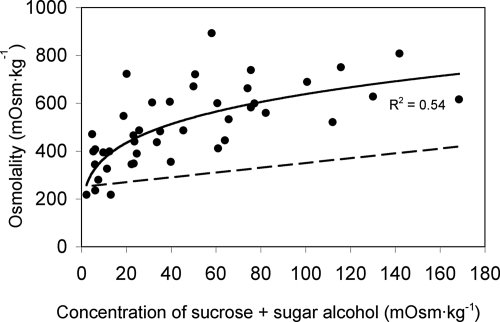
Since passive loading is driven by concentration gradients that are at their highest in the cytosol of mesophyll cells, we explored the concept, developed by Heldt and his colleagues (17), that sucrose and sugar alcohols are compartmentalized preferentially in that location. We reasoned that if the concentrations of these compounds are higher in the cytosol than in the vacuole, then additional osmoticum must be present in the vacuole to balance the water potential of the 2 compartments. As expected, leaf osmolalities were higher in species with higher concentrations of transport compounds (Fig. 3). (The same curve is drawn in Fig. S3, with genus names assigned to each data point.) The percentage of osmolalities accounted for by the transport compounds ranged from 1.0% in Galium odoratum to 27% in Apium graveolens. To determine if the increased leaf osmolality in different species was due solely to the additional transport compounds, a theoretical curve was drawn assuming a basal leaf sap osmolality of 250 mM (the approximate extrapolation of the data in Fig. 3 to zero) plus the combined osmolality of sucrose and sugar alcohols (Figs. 3). This theoretical curve clearly predicts lower values of leaf sap osmolality than measured values, indicating that there is additional osmoticum that cannot be accounted for by the transport compounds alone.
Fig. 3.
The combined concentrations of sucrose and sugar alcohol in leaf tissue of different species (calculated as osmolalities) plotted against leaf sap osmolality. The dashed line is the theoretical osmolality of the leaf sap if it were the sum of a basal value of 250 mOsm plus the sucrose and sugar alcohol alone.
The concentrations of transport compounds in leaves correlate with the qualitative assessment of plasmodesmatal frequencies provided by Gamalei (15). Almost all type 1–2a and type 1 species—those with intermediate or high numbers of plasmodesmata—have high concentrations of transport sugars. In this respect, we see no difference between Gamalei's type 1 and type 1–2a. With some exceptions (see Discussion), type 2a and 2b species, and plants with intermediary cells, have low concentrations of transport sugars. As noted previously by Gamalei (15), types 1 and 1–2a plants are primarily woody, while type 2 plants are primarily herbaceous.
Discussion
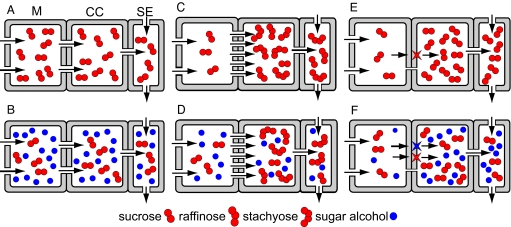
Starting from thermodynamic and anatomical principles, we considered the possible mechanisms and combinations of mechanisms by which sucrose and sugar alcohols could theoretically be loaded into the minor vein phloem, based on available transport pathways. Three pathways and mechanisms are known for sucrose loading, depending on the type of CC and available transport routes. These are illustrated in Fig. 4: Symplastic and passive (A); symplastic followed by polymer trapping (C); and transporter-driven via the apoplast (E). Feasible mechanisms for sugar alcohol loading in these CC types, in addition to sucrose loading, are also illustrated in Fig. 4: Symplastic and passive (B and D) and transporter-driven via the apoplast (F). In no case have we invoked both apoplastic and symplastic pathways in the same SE-CC complex because the presence of a highly conductive symplastic route would lead to a futile pump-leak system for apoplastically loaded solute. The strategies suggested in Fig. 4 are tentatively assigned to individual species in Fig. 2.
Fig. 4.
Hypothetical phloem loading strategies for sucrose (A, C, E) and sucrose plus sugar alcohols (B, D, F). Represented cell types are mesophyll (M), companion cell (CC), and sieve element (SE). Sucrose loading may be passive, requiring abundant plasmodesmata (gaps in walls) and high sucrose concentrations in the cytosol of mesophyll cells (A and B), or energized, either by polymer trapping (C and D) or by active transport from the apoplast mediated by sucrose transporters (red stars on membranes) (E and F). Sugar alcohol may load passively through available plasmodesmata (B and D) or from the apoplast, mediated by specific transporters (blue star) (F).
Passive Loading.
Passive flux of sucrose or sugar alcohol from mesophyll cells to the SEs of minor veins, without a concentrating step (Fig. 4A, B, and D), has gone almost unrecognized as a loading mechanism, perhaps due to the tacit assumption that phloem loading invariably results in the active accumulation of transport compounds in the SE-CC complex. In most studies of symplastic loading, the species selected have been plants with intermediary cells, which synthesize and accumulate RFOs to high concentrations. Much less attention has been paid to species with high plasmodesmatal numbers that do not transport RFOs and have no such concentrating capacity. Furthermore, sugar alcohols have received much less attention than sucrose in loading studies. Chemical inhibition of sucrose transporters by p-chloromercuribenzenesulfonic acid (PCMBS) has commonly been used to distinguish between apoplastic and symplastic loading, but PCMBS does not affect all sugar alcohol transporters (see discussion in reference 10), and this experimental strategy does not distinguish between passive and polymer-trapping strategies, in which loading is independent of transporters. For these reasons, and the complicating fact that PCMBS can potentially affect solute flux indirectly by inhibiting aquaporins (18), we have chosen not to use this approach in the present study.
Eschrich and Fromm (19) conducted autoradiographic experiments on several non-RFO plants, with and without high plasmodesmatal numbers, and suggested that there is another loading pathway beside the apoplastic route. However, they apparently did not abrade the leaf tissue, which may have led to false negative results in certain cases. In our experiments we have found that a variety of abrasion methods, including physical removal of the epidermis, must be used to confirm negative results, especially in thick leaves. Eschrich and Fromm (19) did not speculate on alternate mechanisms and did not distinguish between energized and passive flux.
Another reason that passive loading has not been properly recognized is that relatively few studies have been conducted on woody species, in which this mechanism appears to be most common. Because the possibility of passive loading—which requires high sugar and/or sugar alcohol concentrations in the mesophyll—has been understudied, the correlation between loading mechanisms and solute concentrations in leaves has gone unnoticed.
Plants that load passively should have 3 common characteristics. First, since there is no concentrating step, high levels of transport carbohydrates in mesophyll cells are needed to maintain elevated solute concentrations, and hydrostatic pressure, in the phloem. Second, plasmodesmata must be sufficiently numerous to accommodate flux. Third, symplastic continuity between the mesophyll and phloem should render these plants incapable of accumulating radiolabeled compounds in the minor veins. Even if exogenous sugars are loaded into the phloem, they will diffuse through plasmodesmata into the surrounding mesophyll (9, 10). Fifteen of the species examined, from 11 families, have these characteristics and transport sucrose alone (Figs. 2 and 4A). Three species in the Rosaceae also have these characteristics and transport sorbitol in addition to sucrose (Figs. 2 and 4B).
Considering the autoradiography data, note that vein images were obtained from every type 2 species (Fig. 2). This strict correlation strongly supports the use of this technique in studying transport mechanisms. On this basis, it is reasonable to use the absence of vein images in autoradiographs as an indicator of passive loading, bearing in mind that it constitutes negative evidence only and should be corroborated by other means.
It is also reasonable to expect plants that load passively to have high concentrations of transport compounds in leaves. The data in Fig. 2 support that conclusion. While there is overlap between the groups, species that yield negative results in vein imaging experiments clearly tend to have higher concentrations of transport compounds than plants that produce visible vein images.
The correlation between autoradiographic results and sugar levels are complicated by compartmentation issues. It is important to realize that the concentrations recorded in Fig. 2 were calculated on the assumption of uniform distributions within the lamina. This assumption ignores compartmentation between the mesophyll and phloem. However, the phloem constitutes such a small percentage of leaf volume (13, 20) that the concentrations of sucrose and sugar alcohol as recorded reflect mesophyll cell values reasonably well. More important is compartmentation between the cytosol and vacuole of mesophyll cells, because it is the cytosolic concentration that drives flux through plasmodesmata.
By non-aqueous fractionation, it has been shown that the concentrations of sucrose and mannitol are considerably higher in the cytosol than in the vacuole of mesophyll cells in several herbaceous species (21, and references therein). We reasoned that if this asymmetric distribution is a common phenomenon, the osmolality of leaf sap in plants with higher levels of sucrose and sugar alcohol should be greater than accounted for by these transport carbohydrates alone, because additional osmoticum (e.g., monosaccharides and ions) will be needed in the vacuole to balance the water potential of the 2 compartments. That this is the case, as shown in Fig. 3 and Fig. S3, for a broad range of unrelated species with differing growth habits suggests that the compartmentation model developed by Heldt and his colleagues (17) is valid in the general sense. Therefore, the driving force for passive flux into the phloem is probably much higher than indicated by the concentrations recorded in Fig. 2. As an example, the combined sucrose and sorbitol concentration in the cytosol of mesophyll cells in peach leaves, as measured by non-aqueous fractionation, is ≈400 mM (21). This asymmetric distribution could explain how Lythrum salicaria can load passively with a relatively low overall leaf sucrose concentration (20.1 mM). Note that the osmolality of the leaf sap in L. salicaria is very high (723 mM), consistent with a strong asymmetry toward sucrose in the cytosol. Given the same subcellular fractional volumes as Nicotiana tabacum (22), the sucrose concentration in the cytosol of L. salicaria mesophyll cells could be as high as 476 mM.
Polymer Trapping.
Species that use the polymer trap mechanism are easily identified by the presence of RFOs in leaf extracts, and intermediary cells in minor veins (23). Six of the surveyed species transport sucrose and RFOs alone (Figs. 2 and 4C), and 2 additionally transport mannitol (Figs. 2 and 4D). RFO concentrations are low in the leaf extracts (Fig. 2), because these sugars are confined to the small intermediary cells where they are synthesized. In the RFO plant Alonsoa meridionalis, the combined concentration of raffinose and stachyose in the leaf is only 2.1 mM, but in the phloem sap it is 391 mM (14). Sucrose concentrations in RFO plants are also low. Although sucrose diffuses into the intermediary cells from the mesophyll, its concentration need not be high, since inside the intermediary cell the sucrose is converted to RFOs, lowering the concentration further. It is the difference in concentrations between the mesophyll and phloem that motivates flux, not the absolute levels in the mesophyll.
Since intermediary cells have numerous plasmodesmata that link them to the bundle sheath, there is an uninterrupted symplastic pathway into the phloem. Therefore it is reasonable to predict that sugar alcohols enter the phloem symplastically in RFO plants because sugar alcohols are smaller than sucrose and should pass through the intermediary cell plasmodesmata easily (Fig. 4D). This reasoning is consistent with autoradiographic results indicating that, in Asarina scandens and Syringa meyeri, [14C]sucrose accumulates in the minor veins, but [14C]mannitol does not (10) (Fig. 2). These autoradiographic results indicating passive loading of sugar alcohol are particularly compelling because [14C]sucrose acts as a positive control.
Active loading of sugar alcohol into intermediary cells is theoretically unlikely, because it would result in a futile pump-leak cycle. However, it must be emphasized that loading strategies as defined here, and as illustrated in Fig. 4, apply only to specific SE-CC complexes. It is possible for a given species to exploit more than 1 strategy if it has more than 1 type of SE-CC complex, so-called “mixed loading” (14, 24). This appears to be the case in some, and perhaps all, RFO plants, because they have “ordinary” CCs in the minor veins in addition to intermediary cells. In Asarina scandens, these CCs are especially large, with extensive transfer cell wall ingrowths, a specialization associated with apoplastic loading (16). Acanthus mollis (Acanthaceae) also has intermediary cells and transfer cells in the minor vein phloem and, on the basis of PCMBS sensitivity, appears to load predominately through the apoplast (25). It is also reasonable to postulate that sugar alcohols are actively loaded into ordinary CCs in some RFO-transporting species, although we have not encountered this combination of loading strategies in our survey.
Since RFO plants generate the driving force for long-distance transport by polymer trapping, and perhaps by additional apoplastic loading, there is no a priori reason that high concentrations of sugar alcohols should be present in the mesophyll to fulfill the same function by diffusion. The mannitol concentration is high in Syringa meyeri, but considerably lower in Asarina scandens (Fig. 2). Thus, it appears that sugar alcohols “hitch-hike” in RFO plants by diffusing into the phloem through already available channels.
Apoplastic Loading.
As demonstrated by autoradiography, many non-RFO species in our study accumulate exogenous [14C]sucrose in minor veins, suggesting symplastic isolation of the phloem complex and active loading from the apoplast (Figs. 2 and 4E). By our reasoning, if a SE-CC complex loads 1 compound from the apoplast, a second loaded compound must follow the same route because the phloem has to be essentially, if not entirely, isolated symplastically from surrounding cells to prevent back diffusion into the mesophyll (Fig. 4F). Consistent with this reasoning, both [14C]sucrose and [14C]mannitol accumulate in the minor veins of Apium graveolens (Fig. 2), and in previous experiments, both [14C]sucrose and [14C]sorbitol accumulated in the minor veins of Plantago major, another plant that loads apoplastically (10).
It is notable that the concentrations of transport compounds are low in many, but not all, of the apoplastic loading species. An exception is Apium graveolens, with the highest combined levels of sucrose and sugar alcohol of any plant in the survey, a feature associated with high salt tolerance in this species (26). The amount of sucrose in leaves correlates, at least to a degree, with contrast in autoradiographic images. Species in which vein images are difficult to discern in autoradiographs are, in most cases, those with moderate to high concentrations of foliar sucrose. It is not clear if differences in vein contrast in autoradiographs are physiologically meaningful or are due to technical difficulties in abrasion or some other aspect of administering labeled compounds. One possibility is that mesophyll cells in species with high foliar sucrose retrieve more exogenous [14C]sucrose than mesophyll cells in species with low foliar sucrose. If so, this would reduce the contrast between the mesophyll and veins. However, if this is the cause of low image contrast, it is not uniform because autoradiographic contrast is high in some species with high leaf sucrose levels, most notably Apium graveolens.
Twenty species in the survey are putative apoplastic loaders based on the presence of veins in autoradiographs and the presence of ordinary CCs rather than intermediary cells in the minor veins. (Asarina scandens is included, because it has both intermediary cells and transfer cells, as described above.) Of these 20 species, 14 have low plasmodesmatal frequencies (types 2a or 2b), as expected. Counterintuitively, the remaining 6 species are type 1 or type 1–2a, with more abundant plasmodesmata, which would seem to preclude the possibility of active phloem loading. The ability of some type 1 plants to actively load from the apoplast has been previously described (27, 28). One reasonable explanation is that, in these species, the plasmodesmata linking CCs to surrounding cells in the minor veins are temporarily or permanently sealed to prevent sucrose leakage.
In this regard, it is worth considering the possibility that loading pathways and mechanisms are flexible in at least some type 1 and type 1–2a species: Passive when the sugar content in the cytosol of mesophyll cells is high, and active when the concentration drops below a threshold value. It is also possible that loading is mixed in the sense that some SE-CC complexes in a vein load actively while others do not. This could help explain the different degrees of contrast in the autoradiographs of certain species. Switching from passive to active modes in plants with sufficient numbers of plasmodesmata may be relatively simple and rapid, given that sucrose transporters turn over rapidly (29) and plasmodesmata are highly dynamic and capable of altering their size exclusion limits in different conditions (30).
Clearly there is much to be learned about the phloem loading strategies of individual species in different physiological, developmental, and ecological contexts. However, we feel that the concepts outlined here, based on fundamental anatomical and thermodynamic principles, provide a reasonable foundation for this discovery process.
Materials and Methods
Plant Material.
Mature leaves were collected from plants growing on the Cornell University campus or grown in a greenhouse. Leaves were collected between 10:00 AM and 2:00 PM during the months of June, July, and August.
Autoradiography.
Leaves were abraded to remove the cuticle using either 320-grit carborundum powder or sandpaper, or the cuticle was removed using a razor blade or epidermal peels. Discs were cut under distilled water with a cork borer and floated, abraded side down, on 2 mL solution containing 20 mM Mes-NaOH buffer, pH 5.5, 2 mM CaCl2, and 1 mM of either [14C]sucrose, [14C]sorbitol, or [14C]mannitol (40 kBq mL-1) for 1 h at room temperature. The discs were then rinsed 3 times for 20 min each, flash-frozen with dry ice, freeze-dried, pressed flat, and exposed to Kodak BioMax MR film for 24–48 h.
Carbohydrate and Osmolyte Analysis.
Carbohydrates from 0.5 g (fresh weight) of mature leaf tissue, avoiding large veins and the midrib, were extracted, purified by anion and cation exchange, and analyzed by HPLC as described (9). Replicates from 3 leaves were analyzed. The water content of each species was calculated from fresh and dry weights, and the water content was used to calculate carbohydrate concentrations.
To obtain leaf sap, leaves were kept in the dark for 1 h with the petioles in water. Leaf tissue free of major veins was then placed in a syringe, frozen at -80 °C, thawed, and the sap expressed by depressing the syringe piston. Osmolalities were measured with a Fiske model 110 freezing-point osmometer and are presented as the means of 5 replicates.
Supplementary Material
Acknowledgments.
We thank R. Wayne for the use of the osmometer and W.W. Adams III, B. Demmig-Adams, L. Cheng, A. Jagendorf, E. Reidel, and C. Zhang for providing valuable discussions and careful readings of the manuscript. This work was supported by U.S. Department of Agriculture Grant CSREES 2005-02485.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0902279106/DCSupplemental.
References
- 1.Lalonde S, Tegeder M, Throne-Holst M, Frommer WB, Patrick JW. Phloem loading and unloading of sugars and amino acids. Plant Cell Environ. 2003;26:37–56. [Google Scholar]
- 2.Turgeon R, Ayre BG. Pathways and mechanisms of phloem loading. In: Holbrook NM, Zwieniecki MA, editors. Vascular Transport in Plants. Oxford: Elsevier/Academic; 2005. pp. 45–67. [Google Scholar]
- 3.Schulz A. Role of plasmodesmata in solute loading and unloading. In: Oparka KJ, editor. Plasmodesmata. Oxford: Blackwell; 2005. pp. 135–161. [Google Scholar]
- 4.Turgeon R. Phloem loading: How leaves gain their independence. Bioscience. 2006;56:15–24. [Google Scholar]
- 5.Loescher WH, Everard JD. Regulation of sugar alcohol biosynthesis. In: Leegood RC, Sharkey TD, von Caemmerer S, editors. Photosynthesis: Physiology and Metabolism. Dordrecht, The Netherlands: Kluwer Academic; 2000. pp. 275–299. [Google Scholar]
- 6.Noiraud N, Maurousset L, Lemoine R. Transport of polyols in higher plants. Plant Physiol Biochem. 2001;39:717–728. [Google Scholar]
- 7.Pommerrenig B, Papini-Terzi FS, Sauer N. Differential regulation of sorbitol and sucrose loading into the phloem of Plantago major in response to salt stress. Plant Physiol. 2007;144:1029–1038. doi: 10.1104/pp.106.089151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sauer N. Molecular physiology of higher plant sucrose transporters. FEBS Lett. 2007;581:2309–2317. doi: 10.1016/j.febslet.2007.03.048. [DOI] [PubMed] [Google Scholar]
- 9.Turgeon R, Medville R. The absence of phloem loading in willow leaves. Proc Natl Acad Sci USA. 1998;95:12055–12060. doi: 10.1073/pnas.95.20.12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reidel EJ, Rennie EA, Amiard V, Cheng L, Turgeon R. Phloem loading strategies in three plant species that transport sugar alcohols. Plant Physiol. 2009;149:1601–1608. doi: 10.1104/pp.108.134791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turgeon R, Hepler PK. Symplastic continuity between mesophyll and companion cells in minor veins of mature Cucurbita pepo L. leaves. Planta. 1989;179:24–31. doi: 10.1007/BF00395767. [DOI] [PubMed] [Google Scholar]
- 12.Turgeon R. Symplastic phloem loading and the sink-source transition in leaves: A model. In: Bonnemain J-L, Delrot S, Dainty J, Lucas WJ, editors. Recent Advances in Phloem Transport and Assimilate Compartmentation. Nantes, France: Ouest Editions; 1991. pp. 18–22. [Google Scholar]
- 13.Haritatos E, Keller F, Turgeon R. Raffinose oligosaccharide concentrations measured in individual cell and tissue types in Cucumis melo L. leaves: Implications for phloem loading. Planta. 1996;198:614–622. doi: 10.1007/BF00262649. [DOI] [PubMed] [Google Scholar]
- 14.Voitsekhovskaja OV, et al. Phloem loading in two Scrophulariaceae species. What can drive symplastic flow via plasmodesmata? Plant Physiol. 2006;140:383–395. doi: 10.1104/pp.105.068312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gamalei Y. Structure and function of leaf minor veins in trees and herbs. Trees. 1989;3:96–110. [Google Scholar]
- 16.Wimmers LE, Turgeon R. Transfer cells and solute uptake in minor veins of Pisum sativum leaves. Planta. 1991;186:2–12. doi: 10.1007/BF00201491. [DOI] [PubMed] [Google Scholar]
- 17.Gerhardt R, Heldt HW. Measurement of subcellular metabolite levels in leaves by fractionation of freeze-stopped material in nonaqueous media. Plant Physiol. 1984;75:542–547. doi: 10.1104/pp.75.3.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tyerman SD, Niemietz CM, Bramley H. Plant aquaporins: Multifunctional water and solute channels with expanding roles. Plant Cell Environ. 2002;25:173–194. doi: 10.1046/j.0016-8025.2001.00791.x. [DOI] [PubMed] [Google Scholar]
- 19.Eschrich W, Fromm J. Evidence for two pathways of phloem loading. Physiol Plant. 1994;90:699–707. [Google Scholar]
- 20.Winter H, Lohaus G, Heldt HW. Phloem transport of amino acids in relation to their cytosolic levels in barley leaves. Plant Physiol. 1992;99:996–1004. doi: 10.1104/pp.99.3.996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nadwodnik J, Lohaus G. Subcellular concentrations of sugar alcohols and sugars in relation to phloem translocation in Plantago major, Plantago maritima, Prunus persica, and Apium graveolens. Planta. 2008;227:1079–1089. doi: 10.1007/s00425-007-0682-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heineke D, Wildenberg K, Sonnewald U, Willmitzer L, Heldt HW. Accumulation of hexoses in leaf vacuoles: Studies with transgenic tobacco plants expressing yeast-derived invertase in the cytosol, vacuole or apoplasm. Planta. 1994;194:29–33. [Google Scholar]
- 23.Turgeon R, Beebe DU, Gowan E. The intermediary cell: Minor-vein anatomy and raffinose oligosaccharide synthesis in the Scrophulariaceae. Planta. 1993;191:446–456. [Google Scholar]
- 24.van Bel AJE, Gamalei Y. Multiprogrammed phloem loading. In: Bonnemain J-L, Delrot S, Dainty J, Lucas WJ, editors. Proceedings of the 1990 International Conference on Phloem Transport and Assimilate Compartmentation; Nantes, France: Ouest Editions; 1991. pp. 128–139. [Google Scholar]
- 25.van Bel AJE, Gamalei YV, Ammerlaan A, Bik LPM. Dissimilar phloem loading in leaves with symplasmic or apoplasmic minor-vein configurations. Planta. 1992;186:518–525. doi: 10.1007/BF00198031. [DOI] [PubMed] [Google Scholar]
- 26.Everard JD, Gucci R, Kann SC, Flore JA, Loescher WH. Gas exchange and carbon partitioning in the leaves of celery (Apium graveolens L. ) at various levels of root zone salinity. Plant Physiol. 1994;106:281–292. doi: 10.1104/pp.106.1.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goggin FL, Medville R, Turgeon R. Phloem loading in the tulip tree. Mechanisms and evolutionary implications. Plant Physiol. 2001;125:891–899. doi: 10.1104/pp.125.2.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turgeon R, Medville R. Phloem loading. A reevaluation of the relationship between plasmodesmatal frequencies and loading strategies. Plant Physiol. 2004;136:3795–3803. doi: 10.1104/pp.104.042036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Riesmeier JW, Hirner B, Frommer WB. Potato sucrose transporter expression in minor veins indicates a role in phloem loading. Plant Cell. 1993;5:1591–1598. doi: 10.1105/tpc.5.11.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maule AJ. Plasmodesmata: Structure, function and biogenesis. Curr Opin Plant Biol. 2008;11:680–686. doi: 10.1016/j.pbi.2008.08.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.