Introduction
The clinical role of Cardiovascular Magnetic Resonance (CMR) continues to expand1, supported by ongoing technological advances that have shortened acquisition times while maintaining and often improving image quality. New applications of CMR in cardiovascular imaging continue to emerge and results from larger clinical trials are beginning to define the role of CMR in a range of clinical scenarios. Current accepted indications for CMR include the assessment of congenital heart disease, the great vessels, acquired myocardial and pericardial disease and chronic coronary artery disease (CAD)1. The role of CMR in the assessment of acute coronary syndromes (ACS) is less well established. However, evidence is accumulating that CMR provides often unique information in chest pain syndromes that can aid the detection and differential diagnosis of ACS, guide clinical decision-making and improve risk-stratification after an event.
Following a review of the relevant CMR methodology, this article presents the current evidence for CMR in ACS and gives an outlook of future developments.
CMR methods
The following CMR methods are most commonly used for the assessment of ACS (see Figure 1) and can be incorporated into a clinical protocol that can be performed within an hour.
Figure 1.
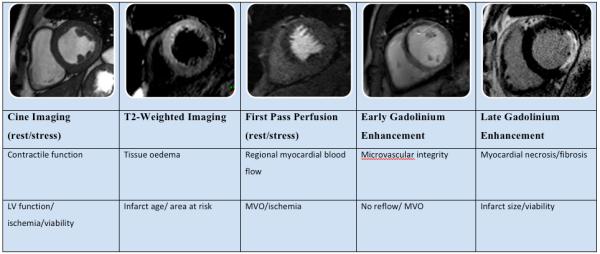
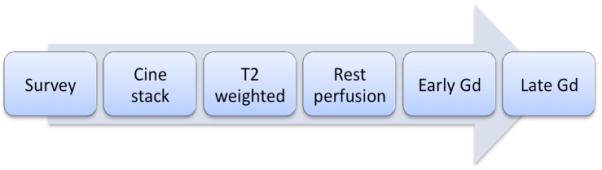
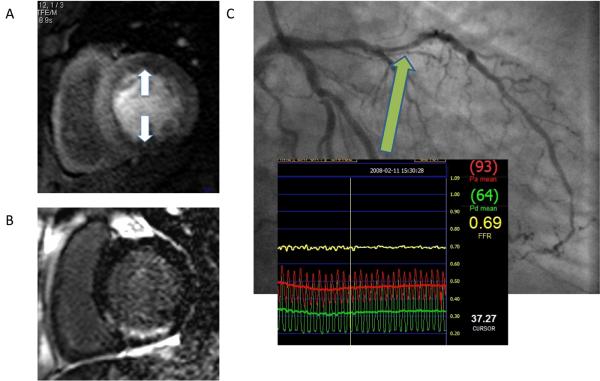
Figure 1a. CMR methods for assessment of ACS. The figure shows short axis views (of different patients) illustrating the different imaging techniques used, their morphological correlates main clinical application.
Figure 1b. These methods can be integrated into a suggested CMR protocol that provides a comprehensive assessment of ACS patients and can be performed in less than 1 hour. Gd = gadolinium.
Cine Imaging
The assessment of global and regional left ventricular (LV) and right ventricular (RV) function by CMR is typically based on a cine data set aligned in the true LV short axis that covers the heart in 10-12 consecutive two-dimensional slices2. Alternatively, three-dimensional cine data sets covering the entire heart in a single breath-hold can be acquired3, 4. In addition to its high tissue contrast, the main advantage of CMR over other imaging modalities is that imaging planes can be freely and reproducibly defined. Consequently, CMR is the most accurate and reproducible imaging modality for the assessment of global ventricular volumes and function 5, 6. In addition, regional contractile function can be assessed either by visual interpretation of cine loops7, 8 or by measuring wall motion, thickening and strain using myocardial tagging methods9, 10. Myocardial tagging during low dose dobutamine stress has been used to measure parameters of diastolic dysfunction such as the time to peak untwist which may identify coronary stenosis11,12. Following AMI, low dose dobutamine cine CMR can be used to predict viability and functional recovery13-16. High dose dobutamine stress CMR has high diagnostic accuracy to identify inducible LV wall motion abnormalities indicative of flow-limiting coronary stenosis17-19.
First Pass Myocardial perfusion
Current first pass myocardial perfusion CMR methods track the passage of a bolus of a T1-shortening contrast agent injected into a peripheral vein20, 21. Data acquired during intravenous vasodilator-stress (most commonly with adenosine) delineate relatively underperfused regions associated with myocardial ischemia. The spatial resolution of CMR myocardial perfusion imaging of 2 to 3 mm is vastly superior to other imaging modalities, so that subendocardial ischemia can be more reliably identified. Recent developments have seen further improvements in spatial resolution to around 1 mm in the imaging plane22 and acquisition at 3-Tesla promises improved signal to noise ratio and diagnostic yield23, 24. Both of these developments should continue to enhance the value of CMR perfusion assessment. The interpretation of CMR myocardial perfusion studies in clinical practice is most commonly visual, but quantitative approaches that measure characteristics of myocardial signal intensity profiles are available21, 25-28 and have been validated against x-ray angiography, SPECT and PET29-31. The recent MR-IMPACT study in 234 patients reported improved detection of coronary stenosis by CMR compared with SPECT in the first multi-centre, multi-vendor comparison32. In the context of ACS, myocardial perfusion CMR imaging can be used to delineate microvascular obstruction and ischaemia, as described in subsequent sections.
Early and late gadolinium enhancement
Following acute ischemic injury, the myocardial distribution volume of extra-cellular gadolinium-based contrast agents is increased because of the presence of sarcrolemmal disintegration and abnormal wash-out kinetics. In chronic myocardial infarction, the presence of fibrotic tissue increases the distribution volume of the contrast agents. The resulting differences in contrast distribution between normal and injured myocardium can be delineated with T1-sensitive inversion-recovery CMR methods. Imaging within the first few minutes after contrast administration is the method of choice to delineate microvascular obstruction (MVO), which prevents contrast delivery to the infarct core and thus results in low signal on T1-weighted imaging33. Acutely injured and chronically infarcted tissue without MVO on the other hand retains contrast agent and therefore appears bright34-37. The preferred imaging time for scar is between 10 and 20 minutes after contrast agent administration, when the differences between scar, normal myocardium and blood pool are maximal. This method is referred to in the literature variably as late gadolinium enhanced CMR (the currently preferred term), late-contrast enhanced, delayed contrast-enhanced or hyperenhancement CMR. It has become the reference standard for the in vivo assessment of myocardial viability because of its very high spatial definition and high contrast to normal myocardium, which allows a detailed assessment of the spatial distribution of scar. Because of its high spatial resolution late gadolinium enhanced CMR can detect infarction in as little as 1ml of tissue, substantially less than other in vivo methods. The technique has been extensively validated in animal models showing excellent agreement with histology and has been applied in numerous recent human studies33-38. Most notably, it was shown that CMR is more sensitive in detecting subendocardial MI than SPECT or PET and in chronic CAD that the extent of scar on CMR predicts the potential for functional recovery after revascularisation 39-41. Figure 2 shows a case example of early and late gadolinium enhancement following acute MI.
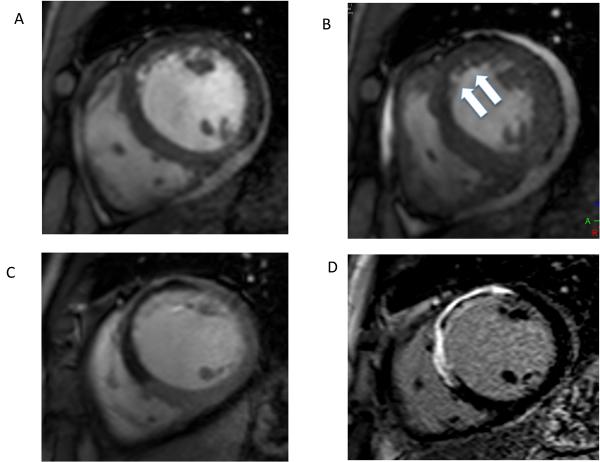
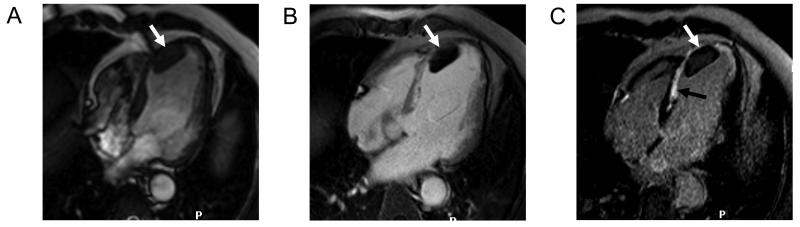
Figure 2.
Diastolic (A) and systolic (B) frame from a cine CMR study acquired at the mid-ventricular level in short axis orientation in a patient with a recent anterior wall acute STEMI. The arrows in B demonstrate the akinetic anterior wall with no systolic thickening compared to the other myocardial segments. Panel 1C is an early gadolinium enhancement image taken 2 minutes after injection of a gadolinium-containing contrast agent and shows a large region of MVO (dark areas) in the anterior wall. The late gadolinium enhancement image acquired 15 minutes after contrast application in panel 1D shows an extensive region of infarction as demarked by the bright white regions of hyperenhancement with a core of MVO still visible. Such extensive transmural infarction with a high burden of MVO suggests that functional recovery in this region is unlikely.
T2-weighted imaging
Myocardial edema is a feature of many forms of acute myocardial injury that are associated with inflammation. Edema alters myocardial T2-relaxation and can therefore be detected with T2-weighted CMR imaging42. Following acute myocardial infarction, T2-weighted CMR can be used to delineate the ischemic risk region, which typically extends beyond the scar (see Figure 3). This is discussed in greater detail later. However, both the relatively small contrast-to-noise ratio between edematous and normal myocardium (around 2 to 3) and artefacts from slow flowing blood at the subendocardial border can make interpretation of T2-weighted images more challenging than other CMR methods, although recent methodological developments promise to improve these limitations43.
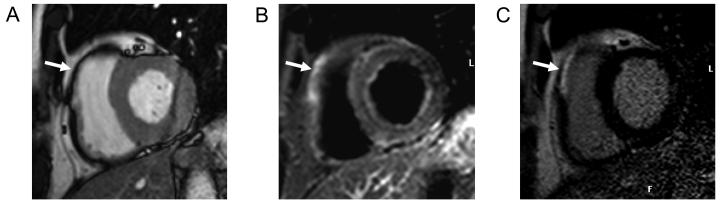
Figure 3.
CMR images taken 24 hours after primary percutaneous intervention in a patient with a lateral STEMI. Panel A shows the T2 weighted images revealing a large area of edema that represents the region of “threatened myocardium” and is clearly larger that the area of late gadolinium enhancement shown in panel D. Resting first pass perfusion in Panel B shows a region of microvascular obstruction in the subendocardial region at the infarct core that closely correlates with the early gadolinium enhanced images (panel C).
Coronary MR angiography
CMR imaging can be used to delineate coronary morphology and detect at least proximal coronary stenosis44. Coronary MR angiography, however, is rarely used in ACS, where invasive angiography is a routine test and non-invasive coronary imaging has little to add to the diagnostic process.
In summary, CMR offers a wide range of tools that can be utilized for the detection, differential diagnosis and management of patients with acute and chronic manifestations of CAD. Data acquisition times for most CMR methods continue to be reduced, allowing multi-parametric assessment in a single imaging session. In the following sections the established and evolving clinical applications of CMR in ACS will be discussed.
Detecting and differentiating ACS
Detection of ACS
Patients with suspected ACS are increasingly managed with early interventional strategies45. However, in low-risk patients or in the presence of concomitant medical problems that increase the risk of complications from cardiac catheterization, an initial non-invasive functional test may be preferred45. In this context, CMR presents an attractive alternative to established diagnostic methods. A study by Kwong and colleagues suggested that CMR imaging may more accurately identify ACS than conventional markers46. In 161 consecutive patients presenting to the emergency room with cardiac chest pain but no evidence for MI, CMR was performed within 12 hours of presentation. The CMR protocol comprised myocardial perfusion at rest, cine imaging and late gadolinium enhancement. The study reports a sensitivity and specificity of 84% and 85%, respectively, of CMR for detecting subsequent ACS defined as 70% coronary stenosis or positive stress test within 8 weeks of the index event. Detection of regional wall motion abnormalities was the most powerful part of the CMR study in this setting where perfusion abnormalities may be normal in between episodes of pain and infarction may not yet be established. CMR was more sensitive than ECG, troponin and the Thrombolysis in Myocardial Infarction (TIMI) risk score and was the strongest predictor of ACS on multivariate logistic regression analysis.
CMR is also helpful in differentiating acute from chronic MI by combining late gadolinium enhancement with T2-weighted imaging, which will delineate the edema associated with acute infarction. In a study of 73 patients with acute and chronic MI by Abdel Aty and colleagues, CMR was 96% sensitive in differentiating acute from chronic MI42. The incremental value of T2-weighted imaging for the detection of ACS was the subject of a recent study of 64 consecutive patients47 presenting with chest pain to the emergency room with negative cardiac enzymes and no ECG changes suggestive of coronary ischemia. Adding T2-weighted imaging and left ventricular wall thickness measurements to a core CMR protocol of cine and late gadolinium enhancement imaging increased the specificity, positive predictive value, and overall accuracy in detecting ACS from 84% to 96%, 55% to 85%, and 84% to 93%, respectively. CMR provided incremental value in the detection of ACS over and above traditional risk stratification with the changes detected by CMR occurring before the rise in cardiac enzymes (6±12 hours).
As well as detecting early changes following ACS, CMR is useful in the setting of delayed presentations where cardiac markers may have returned to normal, while abnormalities on T2-weighted CMR can persist for several weeks48.
Differential diagnosis of ACS
CMR can facilitate the differential diagnosis of ACS, in particular in the context of a normal coronary angiogram. In a study of 27 patients with troponin-positive chest pain and normal x-ray coronary angiogram, half showed subepicardial or mid-wall late gadolinium enhancement, suggesting myocarditis, while the other half demonstrated subendocardial or transmural enhancement typical of myocardial infarction49. Another study of 61 patients presenting with troponin positive chest pain and normal coronary angiography showed that contrast enhanced CMR was able to identify a cause in 65% of cases with the commonest being myocarditis (50%), followed by myocardial infarction and cardiomyopathy50. Laissy and colleagues came to similar results in a study of 55 patients, which included 24 patients with clinically suspected myocarditis and normal coronary angiography and 31 with a history of atypical myocardial infarction and coronary stenosis51. In all but one patient with myocarditis, CMR perfusion was normal and late gadolinium enhanced CMR showed either epicardial or diffuse non-segmental enhancement, while MI patients showed typical endocardial enhancement. The pattern of CMR abnormalities in myocarditis may even predict long-term outcome52. Figure 4 shows an example of enhancement in a patient with myocarditis.
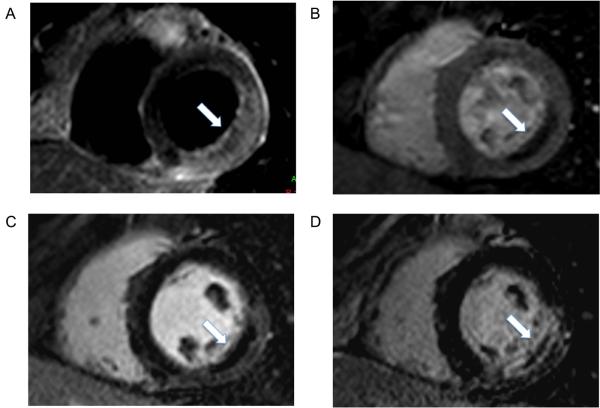
Figure 4.
CMR images from a 49 year-old female presenting with chest pain and breathlessness. Cardiac serum biomarkers were raised and an ECG showed widespread T wave inversion. Coronary angiography showed normal coronary arteries with no evidence for coronary atheroma. CMR shows a regional wall motion abnormality predominantly in the inferior segments (panel A diastole, panel B systole). Late gadolinium enhancement images (panel C) show extensive epicardial hyperenhacement in the inferior and near transmural enhancement in the lateral segments. Based on all available results, a clinical diagnosis of myocarditis was made. The patient declined cardiac biopsy.
Tako Tsubo cardiomyopathy, or apical ballooning syndrome, is a syndrome with distinctive features such as acute chest pain and shortness of breath, ST-segment elevation on electrocardiogram and release of cardiac enzymes. Consequently it can mimic acute myocardial infarction (AMI) at clinical presentation. The diagnosis is usually suspected during invasive coronary angiography, which typically reveals non-obstructed coronary arteries and an apical wall motion abnormality crossing coronary supply territories. CMR can reliably make the diagnosis of abnormal apical contraction that characterizes the syndrome and late gadolinium enhanced CMR shows absence of myocardial necrosis in this syndrome and reliably predicts recovery (Figure 5) 53, 54. A study by Eitel et al demonstrated the ability of CMR to distinguish acute apical myocardial infarction from apical ballooning syndrome without infarction and myocarditis in patients with angiographically normal coronary arteries and characteristic wall motion abnormalities55.
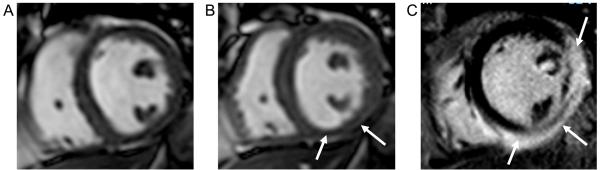
Figure 5.
CMR images from a 52 year old female presenting with an episode of severe chest pain associated with marked anterior ST elevation and raised serum biomarkers. Coronary angiography revealed normal coronary arteries with no atheromatous disease. An LV angiogram showed a marked apical wall motion abnormality, raising the suspicion of Tako-Tsubo syndrome. CMR confirmed the typical diastolic apical “ballooning” on cine images (panels A and B) and absence of scar on late gadolinium enhanced images (panel C).
In summary, CMR may be a useful and accurate test to detect the presence of ACS and can be considered as an additional diagnostic tool to differentiate ACS from chronic MI and from disease entities with similar clinical presentations.
Management of ACS
Non ST elevation ACS
Current guidelines for the management of non-ST elevation ACS recommend that low risk patients with normal biomarkers should undergo a stress test (nuclear perfusion imaging or stress echocardiography) within 72 hours as an alternative to inpatient admission45. Plein et al showed that CMR can also be used safely in the context of ACS56. A CMR study incorporating cine imaging, rest and stress perfusion, coronary MRA and late gadolinium enhancement was performed within 2-5 days of non-ST elevation ACS in 72 patients. CMR reliably predicted the presence of coronary stenosis requiring revascularisation on subsequent X-ray coronary angiography, in particular when several of the CMR modules were interpreted in combination (sensitivity 96%, specificity 83%). Furthermore, this was superior to the prediction based on the TIMI risk score. Figure 6 gives a case example of a patient presenting with a biomarker-negative ACS, in whom CMR identified unknown previous MI and inducible ischemia in two separate coronary territories.
Figure 6.
CMR images from a patient with previous stents to the RCA and LAD 2 years earlier who presented to the emergency room with troponin-negative chest pain. CMR was performed within 24 hours of admission. Panel A shows inferior and anterior ischemia on adenosine-stress perfusion imaging. Panel B shows late gadolinium enhanced images with a region of subendocardial gadolinium enhancement in the inferior wall suggesting old myocardial infarction that was not previously known about. T2-weighted images were normal (not shown). Subsequent coronary angiography revealed tight in-stent restenosis in the RCA and significant flow-limiting disease in the LAD (panel C) as assessed by a pressure wire during hyperaemic conditions (fractional flow reserve 0.69). Both lesions were stented successfully and the patient was discharged home.
Ingkanisorn et al. showed subsequently that CMR adds significant prognostic value in predicting future diagnosis of CAD, MI, or death over clinical risk factors57. They studied 135 troponin-negative patients presenting to the emergency room with chest pain using adenosine stress perfusion CMR. CMR had 100% sensitivity and 93% specificity to predict the development of CAD at one-year follow-up.
ST-elevation MI
Following acute ST-elevation MI (STEMI) patients may not receive definitive revascularisation at the time of initial presentation for a variety of reasons, including late presentation, concomitant medical problems that exclude reperfusion strategies or widespread or complex coronary disease that is not suitable for percutaneous revascularisation. The detection and quantification of scar by late gadolinium enhanced CMR is increasingly used to guide revascularisation decisions in these patients in particular. In a group of 50 patients with myocardial infarction, of which 6 were studied within two weeks of infarction, Kim et al. showed that late gadolinium enhanced CMR predicts reversible myocardial dysfunction. Ninety percent of the myocardial segments studied that contained hyperenhancement between 51% and 75% of tissue and virtually all of those with transmural infarction did not improve after revascularization. Conversely, 256 out of 339 (78%) of hypokinetic segments containing no hyperenhancement had improved contractility after revascularization 34. A subsequent study by Nijveldt et al. in 60 patients with recent AMI confirmed that segments with >75% transmural enhancement are unlikely to function completely at follow-up, while in about half of the segments with less than 25% transmural enhancement function improved completely58. These results were supported by Bodi et al. who showed that the amount of viable myocardium on CMR predicts functional recovery in thrombolysed STEMI patients who receive post-hoc revacularisation59.
In patients receiving thrombolytic therapy for the treatment of acute STEMI, functional assessment with exercise testing or a pharmacological imaging study is recommended for low risk patients before discharge from hospital60. Greenwood and colleagues showed in a small sample of 35 patients that CMR imaging with adenosine stress perfusion, viability and function assessment can be safely performed within a few days of acute STEMI and is more accurate than an exercise tolerance test for detecting residual ischemia61.
Complications of ACS
In addition to supporting management decision-making in ACS, CMR reliably detects important complications of AMI. Ventricular aneurysms and ventricular septum defects are clearly identified on cine images. With late gadolinium enhanced CMR, these pathologies can be further characterized in the context of the acute injury and may aid planning of surgical or percutaenous procedures 62. Early or late gadolinium enhanced imaging is also very useful to identify LV thrombus 63, 64 (Figure 7).
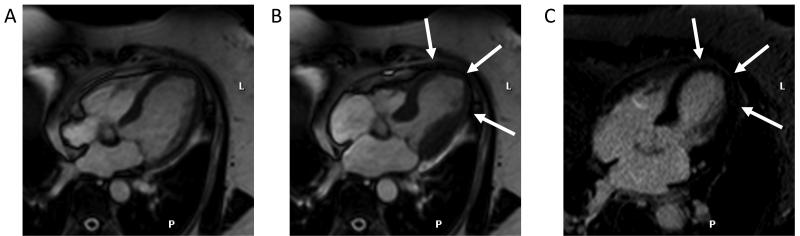
Figure 7.
CMR images in four-chamber orientation from a patient with previous antero-septal myocardial infarction. The diastolic frame from a cine CMR study (panel A) shows thinning of the interventricular septum with a mass lesion at the endocardial surface (arrow). Early gadolinium enhanced images (panel B) show that the lesion does not take up contrast. Late gadolinium enhanced images (panel C) delineate the extent of myocardial infarction (black arrow) as hyperenhancement and show that the lesion remains unenhanced (white arrow), suggesting a left ventricular thrombus.
In summary, there is early evidence that using CMR in ACS patients is safe and provides a comprehensive assessment of the sequelae of AMI that can help to guide patient management. CMR has the benefit over other imaging modalities of providing the most accurate information on cardiac morphology, function and scar. In addition, CMR can determine the presence of residual myocardial ischemia and reliably detects complications associated with ACS.
Risk-stratification after ACS
In patients with known or suspected coronary artery disease CMR has comparable predictive value for future adverse cardiac events with nuclear scintigraphy or stress echocardiography65. Even small amounts of scar on late gadolinium enhanced CMR provide incremental prognostic value beyond the usual clinical, angiographic, and functional predictors66. In the context of ACS, several CMR measures are associated with prognosis (see Table 1).
Table 1.
Prognostic value of CMR imaging following ACS.
| Study | Year | n | Initial CMR Scan |
Follow up | CMR method | Main parameter examined |
Outcome | P value |
|---|---|---|---|---|---|---|---|---|
| Wu et al67 | 1998 | 44 | 10±6 days post AMI |
6mth CMR; clinical 16±5mth |
1st pass perfusion |
MVO | Predicts poor LV recovery with ↑MACE |
<0.01 |
| Taylor et al68 |
2004 | 20 | 24 hrs post PPCI | 3mth CMR | 1st pass perfusion & LGE |
MVO; infarct transmurality (>75%) |
Predicts poor LV recovery |
0.02; 0.048 |
| Bodi et al59 |
2005 | 40 | 1 week post AMI |
6mth CMR | Dobutamine stress & LGE |
↓dobutamine response; infarct transmurality (>50%) |
Predicts poor LV recovery |
<0.0001 |
| Hombach et al69 |
2005 | 110 | 6.1±2.2 days post AMI |
225±92 days CMR & clinical |
1st pass perfusion & LGE |
MVO; infarct transmurality (>75%) |
Predicts poor LV recovery with ↑MACE |
0.0074 |
| Baks et al70 |
2006 | 22 | 5 days post PPCI |
5mth CMR | 1st pass perfusion |
MVO | Predicts poor LV recovery |
0.006 |
| Tarantini et al71 |
2006 | 76 | 6±2 days post PPCI |
6±2mth transthoracic echo |
LGE | In farct transmurality (>75%) |
Predicts poor LV recovery |
<0.001 |
| Ingkanison et al57 |
2006 | 135 | <72 hrs post trop negative chest pain |
1yr clinical | Adenosine stress prefusion |
Reversible ischaemia | Highly accurate to predict CAD, MI, death |
<0.0001 |
| Larose et al72 |
2007 | 147 | >30 days post AMI |
Clinical median 17mth (6- 53mth) |
Cine & LGE | RV function (EF<40%) |
↑mortality independent of LV function |
<0.003 |
| Roes et al73 |
2007 | 231 | >3mths post AMI |
1.7yrs clinical | LGE | In farct size | Predicts mortality | 0.005 |
| Wu et al74 | 2008 | 122 | 1 week post AMI |
3mth CMR; clinical 2yrs |
LGE | Acute infarct size (>18% LV) |
↑MACE | <0.05 |
AMI = acute myocardial infacrtion, PPCI = primary percutaneous coronary intervention, LGE = late gadolinium enhancement, LV = left ventricle, RV = right ventricle, CAD = coronary artery disease, MACE = major adverse cardiovascular event, trop = troponin.
Infarct size
Infarct size measured by late gadolinium enhancement is directly associated with outcome. Tarantini and colleagues71 showed in 76 patients with acute revascularized MI that the amount of transmural necrosis on late gadolinium enhanced CMR predicted adverse LV remodelling, with significant additional predictive value to infarct size and microvascular obstruction. These findings were confirmed by Roes et al who showed in 231 patients with healed myocardial infarction that infarct size on late gadolinium enhanced CMR was a stronger predictor of all-cause mortality than LVEF and LV volumes. Transmurality of infarction was also associated with worse outcome73. Wu et al showed that acute infarct size as determined by CMR, which was independent of LV stunning and loading, directly relates to LV remodeling and is a stronger predictor of future events than measures of LV systolic performance74.
Peri-infarct zone
On late gadolinium enhanced CMR images, a border zone of intermediate signal can be observed between the infarct and surrounding tissue. This peri-infarct zone may reflect partial volume or partial myocardial necrosis and edema. A study by Kwong et al. suggested that the extent of the peri-infarct zone is an independent predictor of post-MI mortality. In 144 patients with documented coronary artery disease and previous MI, they found that the amount of myocardium exhibiting an intermediate signal on late gadolinium enhanced CMR images was a strong predictor of all-cause mortality75. A subsequent study suggested that one mechanism for this increased mortality may be that the peri-infarct zone is a substrate for arrhythmias76. CMR data on scar and peri-infarct zone could thus prove useful in the future to identify patients for implantable cardioverter/defibrillator and cardiac resynchronisation therapy (CRT)77, 78.
Microvascular obstruction
Early contrast-enhanced CMR can reliably demonstrate the presence and extent of MVO following reperfused AMI. MVO results in poor tissue perfusion despite restoration of epicardial blood flow to the infarcted region. It is the consequence of clogging of the small myocardial arterioles with embolic debris, acute inflammation, platelet aggregation and vasospasm. Studies have shown that the presence and extent of MVO following AMI as measured by early gadolinium enhancement CMR is associated with adverse ventricular remodelling16, 68, 79-81 and clinical outcome that is independent of the infarct size67, 69. Baks et al examined patients with CMR soon after primary percutaneous revascularisation for STEMI and again at five months. They found that dysfunctional segments without MVO had early increased wall thickness and late partial functional recovery compared to segments with MVO that showed late wall thinning and no functional recovery 70. Nijveldt et al demonstrated that MVO had incremental diagnostic value over transmurality of infarction in particular in segments with 75-100% transmural enhancement58.
Area at risk
The area at risk (AAR) is defined as hypoperfused myocardium at the time of an ischemic episode82. Currently the gold-standard for determining the AAR is single photon emission tomography with injection of Technitium-99m tracer into the occluded coronary artery prior to revascularisation83. In the experimental setting, myocardial contrast echo has also been used84.
T2-weighted CMR offers a potentially attractive alternative for the non-invasive measurement of AAR. The increased water content of myocardium following acute ischemia-reperfusion injury85 leads to high signal on T2-weighted images86 and combined with late gadolinium enhancement allows the delineation of areas that are injured but not infarcted following reperfusion87, 88. The areas of T2 enhancement are invariably transmural and subsequently larger than the regions of late gadolinium enhancement89 88 and the difference between the two likely represents myocardial salvage90. The advantages of this method are that such a region can be retrospectively determined days after the acute event87 and without the need for direct injection of agents into the coronary artery at the time of primary reperfusion. So far, no outcome studies based on a CMR assessment of AAR have been published.
Right ventricular infarction
It is well recognized that involvement of the right ventricle (RV) in acute MI is associated with adverse clinical outcome. RV function is difficult to assess reliably with most imaging modalities, while it poses no particular challenge to CMR cine imaging (Figure 8). Kaandorp et al. showed that RV infarction can be detected by late gadolinium enhancement and that the extent of scar tissue is linearly related to the severity of RV dilatation at 6 months follow-up91. Kumar et al. found in 37 patients that acute RV infarction is more frequently detected by late gadolinium enhancement than by ECG and echocardiography92. Finally, Larose et al. have shown that RV ejection fraction measured by CMR is an important predictor of prognosis after AMI72. In 147 consecutive patients studied late after MI, RVEF <40% was strongly associated with mortality (hazard ratio 4.02), independent of patient age, LV infarct size and LVEF.
Figure 8.
CMR images from a patient with acute non-ST elevation myocardial infarction, evidenced by a rise in cardiac enzymes and widespread ST segment changes on an electrocardiogram. Invasive coronary angiography revealed three vessel coronary artery disease with an occluded proximal right ventricular branch of the right coronary artery. A cine CMR image in diastole (panel A) shows a subtle wall motion abnormality in the anterior right ventricular free wall (arrow). The T2-weigthed image (panel B) shows high signal in this area with corresponding high signal on the late gadolinium enhanced image (panel C). There is no evidence for scar in the left ventricle, suggesting isolated right ventricular infarction.
In summary, CMR imaging provides several independent measures of prognosis after ACS that can all be obtained in a single imaging procedure and cannot be assessed equally with other imaging modalities. A potential role for CMR may thus be to offer an improved method for risk stratification in the early post ACS period.
Future perspectives
Owing to ongoing technological advances (faster gradients, multi-channel receiver coils, transform coding to accelerate data acquisition) scan times for many CMR methods continue to be shortened. Until now, this development has usually been invested into obtaining higher quality images (e.g. better spatial resolution, better temporal resolution, better signal to noise ratio). However, given the image quality achieved today, future improvements of imaging speed are likely to be reinvested into shortening scan time for a given CMR method. This speed up can be used to evaluate more patients per time for chronic disease, but also to answer numerous questions within a single short setting for patients with acute coronary syndromes. A likely scenario for MR imaging in these patients will resemble one of the current utilizations of CCT in patients with chest pain– the triple rule out of pulmonary embolism, aortic dissection and myocardial infarction in a single imaging session. Such an approach would look at wall motion abnormalities, edema and resting perfusion first, then use a higher bolus of contrast agent for a time resolved 3D angiography which allows assessment of the anatomy of the pulmonary arteries and the aorta within a single scan and the final assessment of late gadolinium enhancement to visualize scar tissue.
In vivo MRI of advanced plaques in human carotid arteries using contrast-enhanced techniques has identified lipid core with 85% sensitivity and 92% sensitivity93. In addition, thin and ruptured plaques can be identified in vivo by MRI in a high proportion of patients in whom it was subsequently identified on examination of post-atherectomy specimens94. Improving methods95-97 and the introduction of 3-Tesla clinical imaging98 raise the possibility that in the near future such techniques may be applied to the coronary arteries.
More specific techniques to visualize activation within a plaque using targeted (molecular) contrast agents could be used as a potential predictor of plaque rupture, with the potential for identification of specific disease processes in vivo. This has already been demonstrated in animal models for fibrin specific contrast agents, which allow the highlighting of ruptured plaques and thrombosis within the coronary arteries99, 100. Similarly, contrast agents designed to show earlier processes of plaque vulnerability such as myeloperoxidase activity101 and macrophage activation102,103 have been applied in animal models If translated into clinical application, the ability to assess non-invasively plaque activity may overcome a major limitation of the current practice of cardiology.
Which patient with ACS should undergo CMR imaging?
In patients presenting with suspected ACS but no angiographic evidence of coronary artery stenosis, CMR can contribute to the differential diagnosis of ACS from other acute myocardial diseases such as myocarditis.
In low risk patients presenting with suspected ACS, an early stress-perfusion CMR scan can be considered as an alternative non-invasive stress test to risk stratify and discharge patients.
Patients with established myocardial infarction may undergo CMR following diagnostic angiography to determine of the degree of myocardial necrosis and likelihood of recovery to plan further revascularization strategies.
Post-AMI, CMR may also be used to risk stratify patients using LV ejection fraction, infarct size and characteristics and RV involvement. Based on these data, high-risk patients, for example, with poor ventricular function and a large scar burden can be selected for ICD implantation.
A CMR protocol for these clinical scenarios that follows the recent recommendations of the Society of Cardiovascular Magnetic Resonance104 is given in Figure 1b.
Conclusions
CMR imaging is emerging as a versatile diagnostic tool for the management of the patient with suspected or established ACS. It provides additional information over other clinical tests for the detection, differential diagnosis and prognostication after ACS, owing to the high spatial definition and multi-modal data it provides. Future larger studies will determine more fully the role of CMR in the setting of ACS.
Acknowledgments
Funding Sources The authors acknowledge financial support from the Department of Health via the National Institute for Health Research (NIHR) comprehensive Biomedical Research Centre award to Guy's & St Thomas' NHS Foundation Trust in partnership with King's College London.
Dr Lockie is supported by a fellowship from the British Heart Foundation. Dr Plein is support by a Wellcome Trust fellowship (WT078288).
Footnotes
Conflict of Interest Disclosures
Dr Lockie none
Prof Nagel Research grant (Philips Healthcare, Bayer Shering Pharma); speakers bureau (Bayer Shering Pharma, GE Healthcare); consultant advisory board (Philips Healthcare, Bayer Sherling Pharma, GE Healthcare)
Dr Redwood none
Dr Plein Research grant (Wellcome Trust, British Heart Foundation)
References
- 1.Pennell DJ, Sechtem UP, Higgins CB, Manning WJ, Pohost GM, Rademakers FE, van Rossum AC, Shaw LJ, Yucel EK. Clinical indications for cardiovascular magnetic resonance (CMR): Consensus Panel report. Eur Heart J. 2004;25:1940–1965. doi: 10.1016/j.ehj.2004.06.040. [DOI] [PubMed] [Google Scholar]
- 2.Barkhausen J, Ruehm SG, Goyen M, Buck T, Laub G, Debatin JF. MR evaluation of ventricular function: true fast imaging with steady-state precession versus fast low-angle shot cine MR imaging: feasibility study. Radiology. 2001;219:264–269. doi: 10.1148/radiology.219.1.r01ap12264. [DOI] [PubMed] [Google Scholar]
- 3.Jahnke C, Paetsch I, Gebker R, Bornstedt A, Fleck E, Nagel E. Accelerated 4D dobutamine stress MR imaging with k-t BLAST: feasibility and diagnostic performance. Radiology. 2006;241:718–728. doi: 10.1148/radiol.2413051522. [DOI] [PubMed] [Google Scholar]
- 4.Jahnke C, Nagel E, Gebker R, Bornstedt A, Schnackenburg B, Kozerke S, Fleck E, Paetsch I. Four-dimensional single breathhold magnetic resonance imaging using kt-BLAST enables reliable assessment of left- and right-ventricular volumes and mass. J Magn Reson Imaging. 2007;25:737–742. doi: 10.1002/jmri.20877. [DOI] [PubMed] [Google Scholar]
- 5.Bellenger NG, Burgess MI, Ray SG, Lahiri A, Coats AJ, Cleland JG, Pennell DJ. Comparison of left ventricular ejection fraction and volumes in heart failure by echocardiography, radionuclide ventriculography and cardiovascular magnetic resonance; are they interchangeable? Eur Heart J. 2000;21:1387–1396. doi: 10.1053/euhj.2000.2011. [DOI] [PubMed] [Google Scholar]
- 6.Semelka RC, Tomei E, Wagner S, Mayo J, Caputo G, O'Sullivan M, Parmley WW, Chatterjee K, Wolfe C, Higgins CB. Interstudy reproducibility of dimensional and functional measurements between cine magnetic resonance studies in the morphologically abnormal left ventricle. Am Heart J. 1990;119:1367–1373. doi: 10.1016/s0002-8703(05)80187-5. [DOI] [PubMed] [Google Scholar]
- 7.Sechtem U, Sommerhoff BA, Markiewicz W, White RD, Cheitlin MD, Higgins CB. Regional left ventricular wall thickening by magnetic resonance imaging: evaluation in normal persons and patients with global and regional dysfunction. Am J Cardiol. 1987;59:145–151. doi: 10.1016/s0002-9149(87)80088-7. [DOI] [PubMed] [Google Scholar]
- 8.Hoffmann R, von Bardeleben S, Kasprzak JD, Borges AC, ten Cate F, Firschke C, Lafitte S, Al-Saadi N, Kuntz-Hehner S, Horstick G, Greis C, Engelhardt M, Vanoverschelde JL, Becher H. Analysis of regional left ventricular function by cineventriculography, cardiac magnetic resonance imaging, and unenhanced and contrast-enhanced echocardiography: a multicenter comparison of methods. J Am Coll Cardiol. 2006;47:121–128. doi: 10.1016/j.jacc.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 9.Zerhouni EA, Parish DM, Rogers WJ, Yang A, Shapiro EP. Human heart: tagging with MR imaging--a method for noninvasive assessment of myocardial motion. Radiology. 1988;169:59–63. doi: 10.1148/radiology.169.1.3420283. [DOI] [PubMed] [Google Scholar]
- 10.Rutz AK, Ryf S, Plein S, Boesiger P, Kozerke S. Accelerated whole-heart 3D CSPAMM for myocardial motion quantification. Magn Reson Med. 2008;59:755–763. doi: 10.1002/mrm.21363. [DOI] [PubMed] [Google Scholar]
- 11.Garot J, Pascal O, Diebold B, Derumeaux G, Gerber BL, Dubois-Rande JL, Lima JA, Gueret P. Alterations of systolic left ventricular twist after acute myocardial infarction. Am J Physiol Heart Circ Physiol. 2002;282:H357–362. doi: 10.1152/ajpheart.00136.2001. [DOI] [PubMed] [Google Scholar]
- 12.Paetsch I, Foll D, Kaluza A, Luechinger R, Stuber M, Bornstedt A, Wahl A, Fleck E, Nagel E. Magnetic resonance stress tagging in ischemic heart disease. Am J Physiol Heart Circ Physiol. 2005;288:H2708–2714. doi: 10.1152/ajpheart.01017.2003. [DOI] [PubMed] [Google Scholar]
- 13.Kramer CM, Rogers WJ, Geskin G, Power TP, Theobald TM, Hu YL, Reichek N. Usefulness of magnetic resonance imaging early after acute myocardial infarction. Am J Cardiol. 1997;80:690–695. doi: 10.1016/s0002-9149(97)00496-7. [DOI] [PubMed] [Google Scholar]
- 14.Geskin G, Kramer CM, Rogers WJ, Theobald TM, Pakstis D, Hu YL, Reichek N. Quantitative assessment of myocardial viability after infarction by dobutamine magnetic resonance tagging. Circulation. 1998;98:217–223. doi: 10.1161/01.cir.98.3.217. [DOI] [PubMed] [Google Scholar]
- 15.Kramer CM, Malkowski MJ, Mankad S, Theobald TM, Pakstis DL, Rogers WJ., Jr Magnetic resonance tagging and echocardiographic response to dobutamine and functional improvement after reperfused myocardial infarction. Am Heart J. 2002;143:1046–1051. doi: 10.1067/mhj.2002.122515. [DOI] [PubMed] [Google Scholar]
- 16.Gerber BL, Rochitte CE, Melin JA, McVeigh ER, Bluemke DA, Wu KC, Becker LC, Lima JA. Microvascular obstruction and left ventricular remodeling early after acute myocardial infarction. Circulation. 2000;101:2734–2741. doi: 10.1161/01.cir.101.23.2734. [DOI] [PubMed] [Google Scholar]
- 17.Baer FM, Voth E, Schneider CA, Theissen P, Schicha H, Sechtem U. Comparison of low-dose dobutamine-gradient-echo magnetic resonance imaging and positron emission tomography with [18F]fluorodeoxyglucose in patients with chronic coronary artery disease. A functional and morphological approach to the detection of residual myocardial viability. Circulation. 1995;91:1006–1015. doi: 10.1161/01.cir.91.4.1006. [DOI] [PubMed] [Google Scholar]
- 18.Paetsch I, Jahnke C, Ferrari VA, Rademakers FE, Pellikka PA, Hundley WG, Poldermans D, Bax JJ, Wegscheider K, Fleck E, Nagel E. Determination of interobserver variability for identifying inducible left ventricular wall motion abnormalities during dobutamine stress magnetic resonance imaging. Eur Heart J. 2006;27:1459–1464. doi: 10.1093/eurheartj/ehi883. [DOI] [PubMed] [Google Scholar]
- 19.Paetsch I, Jahnke C, Wahl A, Gebker R, Neuss M, Fleck E, Nagel E. Comparison of dobutamine stress magnetic resonance, adenosine stress magnetic resonance, and adenosine stress magnetic resonance perfusion. Circulation. 2004;110:835–842. doi: 10.1161/01.CIR.0000138927.00357.FB. [DOI] [PubMed] [Google Scholar]
- 20.Schwitter J. Myocardial perfusion imaging by cardiac magnetic resonance. J Nucl Cardiol. 2006;13:841–854. doi: 10.1016/j.nuclcard.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 21.Schwitter J, Nanz D, Kneifel S, Bertschinger K, Buchi M, Knusel PR, Marincek B, Luscher TF, von Schulthess GK. Assessment of myocardial perfusion in coronary artery disease by magnetic resonance: a comparison with positron emission tomography and coronary angiography. Circulation. 2001;103:2230–2235. doi: 10.1161/01.cir.103.18.2230. [DOI] [PubMed] [Google Scholar]
- 22.Plein S, Ryf S, Schwitter J, Radjenovic A, Boesiger P, Kozerke S. Dynamic contrast-enhanced myocardial perfusion MRI accelerated with k-t sense. Magn Reson Med. 2007;58:777–785. doi: 10.1002/mrm.21381. [DOI] [PubMed] [Google Scholar]
- 23.Gebker R, Jahnke C, Paetsch I, Kelle S, Schnackenburg B, Fleck E, Nagel E. Diagnostic performance of myocardial perfusion MR at 3 T in patients with coronary artery disease. Radiology. 2008;247:57–63. doi: 10.1148/radiol.2471070596. [DOI] [PubMed] [Google Scholar]
- 24.Cheng AS, Pegg TJ, Karamitsos TD, Searle N, Jerosch-Herold M, Choudhury RP, Banning AP, Neubauer S, Robson MD, Selvanayagam JB. Cardiovascular magnetic resonance perfusion imaging at 3-tesla for the detection of coronary artery disease: a comparison with 1.5-tesla. J Am Coll Cardiol. 2007;49:2440–2449. doi: 10.1016/j.jacc.2007.03.028. [DOI] [PubMed] [Google Scholar]
- 25.Fritz-Hansen T, Hove JD, Kofoed KF, Kelbaek H, Larsson HB. Quantification of MRI measured myocardial perfusion reserve in healthy humans: a comparison with positron emission tomography. J Magn Reson Imaging. 2008;27:818–824. doi: 10.1002/jmri.21306. [DOI] [PubMed] [Google Scholar]
- 26.Jerosch-Herold M, Wilke N, Stillman AE. Magnetic resonance quantification of the myocardial perfusion reserve with a Fermi function model for constrained deconvolution. Med Phys. 1998;25:73–84. doi: 10.1118/1.598163. [DOI] [PubMed] [Google Scholar]
- 27.Selvanayagam JB, Jerosch-Herold M, Porto I, Sheridan D, Cheng AS, Petersen SE, Searle N, Channon KM, Banning AP, Neubauer S. Resting myocardial blood flow is impaired in hibernating myocardium: a magnetic resonance study of quantitative perfusion assessment. Circulation. 2005;112:3289–3296. doi: 10.1161/CIRCULATIONAHA.105.549170. [DOI] [PubMed] [Google Scholar]
- 28.Nagel E, Klein C, Paetsch I, Hettwer S, Schnackenburg B, Wegscheider K, Fleck E. Magnetic resonance perfusion measurements for the noninvasive detection of coronary artery disease. Circulation. 2003;108:432–437. doi: 10.1161/01.CIR.0000080915.35024.A9. [DOI] [PubMed] [Google Scholar]
- 29.Cullen JH, Horsfield MA, Reek CR, Cherryman GR, Barnett DB, Samani NJ. A myocardial perfusion reserve index in humans using first-pass contrast-enhanced magnetic resonance imaging. J Am Coll Cardiol. 1999;33:1386–1394. doi: 10.1016/s0735-1097(99)00004-2. [DOI] [PubMed] [Google Scholar]
- 30.Al-Saadi N, Nagel E, Gross M, Bornstedt A, Schnackenburg B, Klein C, Klimek W, Oswald H, Fleck E. Noninvasive detection of myocardial ischemia from perfusion reserve based on cardiovascular magnetic resonance. Circulation. 2000;101:1379–1383. doi: 10.1161/01.cir.101.12.1379. [DOI] [PubMed] [Google Scholar]
- 31.Wolff SD, Schwitter J, Coulden R, Friedrich MG, Bluemke DA, Biederman RW, Martin ET, Lansky AJ, Kashanian F, Foo TK, Licato PE, Comeau CR. Myocardial first-pass perfusion magnetic resonance imaging: a multicenter dose-ranging study. Circulation. 2004;110:732–737. doi: 10.1161/01.CIR.0000138106.84335.62. [DOI] [PubMed] [Google Scholar]
- 32.Schwitter J, Wacker CM, van Rossum AC, Lombardi M, Al-Saadi N, Ahlstrom H, Dill T, Larsson HB, Flamm SD, Marquardt M, Johansson L. MR-IMPACT: comparison of perfusion-cardiac magnetic resonance with single-photon emission computed tomography for the detection of coronary artery disease in a multicentre, multivendor, randomized trial. Eur Heart J. 2008;29:480–489. doi: 10.1093/eurheartj/ehm617. [DOI] [PubMed] [Google Scholar]
- 33.Kim RJ, Chen EL, Lima JA, Judd RM. Myocardial Gd-DTPA kinetics determine MRI contrast enhancement and reflect the extent and severity of myocardial injury after acute reperfused infarction. Circulation. 1996;94:3318–3326. doi: 10.1161/01.cir.94.12.3318. [DOI] [PubMed] [Google Scholar]
- 34.Kim RJ, Wu E, Rafael A, Chen EL, Parker MA, Simonetti O, Klocke FJ, Bonow RO, Judd RM. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med. 2000;343:1445–1453. doi: 10.1056/NEJM200011163432003. [DOI] [PubMed] [Google Scholar]
- 35.Judd RM, Lugo-Olivieri CH, Arai M, Kondo T, Croisille P, Lima JA, Mohan V, Becker LC, Zerhouni EA. Physiological basis of myocardial contrast enhancement in fast magnetic resonance images of 2-day-old reperfused canine infarcts. Circulation. 1995;92:1902–1910. doi: 10.1161/01.cir.92.7.1902. [DOI] [PubMed] [Google Scholar]
- 36.Kim RJ, Fieno DS, Parrish TB, Harris K, Chen EL, Simonetti O, Bundy J, Finn JP, Klocke FJ, Judd RM. Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation. 1999;100:1992–2002. doi: 10.1161/01.cir.100.19.1992. [DOI] [PubMed] [Google Scholar]
- 37.Fieno DS, Kim RJ, Chen EL, Lomasney JW, Klocke FJ, Judd RM. Contrast-enhanced magnetic resonance imaging of myocardium at risk: distinction between reversible and irreversible injury throughout infarct healing. J Am Coll Cardiol. 2000;36:1985–1991. doi: 10.1016/s0735-1097(00)00958-x. [DOI] [PubMed] [Google Scholar]
- 38.Wu E, Judd RM, Vargas JD, Klocke FJ, Bonow RO, Kim RJ. Visualisation of presence, location, and transmural extent of healed Q-wave and non-Q-wave myocardial infarction. Lancet. 2001;357:21–28. doi: 10.1016/S0140-6736(00)03567-4. [DOI] [PubMed] [Google Scholar]
- 39.Klein C, Nekolla SG, Bengel FM, Momose M, Sammer A, Haas F, Schnackenburg B, Delius W, Mudra H, Wolfram D, Schwaiger M. Assessment of myocardial viability with contrast-enhanced magnetic resonance imaging: comparison with positron emission tomography. Circulation. 2002;105:162–167. doi: 10.1161/hc0202.102123. [DOI] [PubMed] [Google Scholar]
- 40.Wagner A, Mahrholdt H, Holly TA, Elliott MD, Regenfus M, Parker M, Klocke FJ, Bonow RO, Kim RJ, Judd RM. Contrast-enhanced MRI and routine single photon emission computed tomography (SPECT) perfusion imaging for detection of subendocardial myocardial infarcts: an imaging study. Lancet. 2003;361:374–379. doi: 10.1016/S0140-6736(03)12389-6. [DOI] [PubMed] [Google Scholar]
- 41.Bondarenko O, Beek AM, Nijveldt R, McCann GP, van Dockum WG, Hofman MB, Twisk JW, Visser CA, van Rossum AC. Functional outcome after revascularization in patients with chronic ischemic heart disease: a quantitative late gadolinium enhancement CMR study evaluating transmural scar extent, wall thickness and periprocedural necrosis. J Cardiovasc Magn Reson. 2007;9:815–821. doi: 10.1080/10976640701547335. [DOI] [PubMed] [Google Scholar]
- 42.Abdel-Aty H, Zagrosek A, Schulz-Menger J, Taylor AJ, Messroghli D, Kumar A, Gross M, Dietz R, Friedrich MG. Delayed enhancement and T2-weighted cardiovascular magnetic resonance imaging differentiate acute from chronic myocardial infarction. Circulation. 2004;109:2411–2416. doi: 10.1161/01.CIR.0000127428.10985.C6. [DOI] [PubMed] [Google Scholar]
- 43.Kellman P, Aletras AH, Mancini C, McVeigh ER, Arai AE. T2-prepared SSFP improves diagnostic confidence in edema imaging in acute myocardial infarction compared to turbo spin echo. Magn Reson Med. 2007;57:891–897. doi: 10.1002/mrm.21215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim WY, Danias PG, Stuber M, Flamm SD, Plein S, Nagel E, Langerak SE, Weber OM, Pedersen EM, Schmidt M, Botnar RM, Manning WJ. Coronary magnetic resonance angiography for the detection of coronary stenoses. N Engl J Med. 2001;345:1863–1869. doi: 10.1056/NEJMoa010866. [DOI] [PubMed] [Google Scholar]
- 45.Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE, Jr., Chavey WE, 2nd, Fesmire FM, Hochman JS, Levin TN, Lincoff AM, Peterson ED, Theroux P, Wenger NK, Wright RS, Smith SC, Jr., Jacobs AK, Adams CD, Anderson JL, Antman EM, Halperin JL, Hunt SA, Krumholz HM, Kushner FG, Lytle BW, Nishimura R, Ornato JP, Page RL, Riegel B. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-Elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction) developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. J Am Coll Cardiol. 2007;50:e1–e157. doi: 10.1016/j.jacc.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 46.Kwong RY, Schussheim AE, Rekhraj S, Aletras AH, Geller N, Davis J, Christian TF, Balaban RS, Arai AE. Detecting acute coronary syndrome in the emergency department with cardiac magnetic resonance imaging. Circulation. 2003;107:531–537. doi: 10.1161/01.cir.0000047527.11221.29. [DOI] [PubMed] [Google Scholar]
- 47.Cury RC, Shash K, Nagurney JT, Rosito G, Shapiro MD, Nomura CH, Abbara S, Bamberg F, Ferencik M, Schmidt EJ, Brown DF, Hoffmann U, Brady TJ. Cardiac Magnetic Resonance With T2-Weighted Imaging Improves Detection of Patients With Acute Coronary Syndrome in the Emergency Department. Circulation. 2008;110:234–240. doi: 10.1161/CIRCULATIONAHA.107.740597. [DOI] [PubMed] [Google Scholar]
- 48.Nilsson JC, Nielsen G, Groenning BA, Fritz-Hansen T, Sondergaard L, Jensen GB, Larsson HB. Sustained postinfarction myocardial oedema in humans visualised by magnetic resonance imaging. Heart. 2001;85:639–642. doi: 10.1136/heart.85.6.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Codreanu A, Djaballah W, Angioi M, Ethevenot G, Moulin F, Felblinger J, Sadoul N, Karcher G, Aliot E, Marie PY. Detection of myocarditis by contrast-enhanced MRI in patients presenting with acute coronary syndrome but no coronary stenosis. J Magn Reson Imaging. 2007;25:957–964. doi: 10.1002/jmri.20897. [DOI] [PubMed] [Google Scholar]
- 50.Assomull RG, Lyne JC, Keenan N, Gulati A, Bunce NH, Davies SW, Pennell DJ, Prasad SK. The role of cardiovascular magnetic resonance in patients presenting with chest pain, raised troponin, and unobstructed coronary arteries. Eur Heart J. 2007;28:1242–1249. doi: 10.1093/eurheartj/ehm113. [DOI] [PubMed] [Google Scholar]
- 51.Laissy JP, Hyafil F, Feldman LJ, Juliard JM, Schouman-Claeys E, Steg PG, Faraggi M. Differentiating acute myocardial infarction from myocarditis: diagnostic value of early- and delayed-perfusion cardiac MR imaging. Radiology. 2005;237:75–82. doi: 10.1148/radiol.2371041322. [DOI] [PubMed] [Google Scholar]
- 52.Mahrholdt H, Wagner A, Deluigi CC, Kispert E, Hager S, Meinhardt G, Vogelsberg H, Fritz P, Dippon J, Bock CT, Klingel K, Kandolf R, Sechtem U. Presentation, patterns of myocardial damage, and clinical course of viral myocarditis. Circulation. 2006;114:1581–1590. doi: 10.1161/CIRCULATIONAHA.105.606509. [DOI] [PubMed] [Google Scholar]
- 53.Mitchell JH, Hadden TB, Wilson JM, Achari A, Muthupillai R, Flamm SD. Clinical features and usefulness of cardiac magnetic resonance imaging in assessing myocardial viability and prognosis in Takotsubo cardiomyopathy (transient left ventricular apical ballooning syndrome) Am J Cardiol. 2007;100:296–301. doi: 10.1016/j.amjcard.2007.02.091. [DOI] [PubMed] [Google Scholar]
- 54.Haghi D, Fluechter S, Suselbeck T, Kaden JJ, Borggrefe M, Papavassiliu T. Cardiovascular magnetic resonance findings in typical versus atypical forms of the acute apical ballooning syndrome (Takotsubo cardiomyopathy) Int J Cardiol. 2007;120:205–211. doi: 10.1016/j.ijcard.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 55.Eitel I, Behrendt F, Schindler K, Kivelitz D, Gutberlet M, Schuler G, Thiele H. Differential diagnosis of suspected apical ballooning syndrome using contrast-enhanced magnetic resonance imaging. Eur Heart J. 2008;29:2651–2659. doi: 10.1093/eurheartj/ehn433. [DOI] [PubMed] [Google Scholar]
- 56.Plein S, Greenwood JP, Ridgway JP, Cranny G, Ball SG, Sivananthan MU. Assessment of non-ST-segment elevation acute coronary syndromes with cardiac magnetic resonance imaging. J Am Coll Cardiol. 2004;44:2173–2181. doi: 10.1016/j.jacc.2004.08.056. [DOI] [PubMed] [Google Scholar]
- 57.Ingkanisorn WP, Kwong RY, Bohme NS, Geller NL, Rhoads KL, Dyke CK, Paterson DI, Syed MA, Aletras AH, Arai AE. Prognosis of negative adenosine stress magnetic resonance in patients presenting to an emergency department with chest pain. J Am Coll Cardiol. 2006;47:1427–1432. doi: 10.1016/j.jacc.2005.11.059. [DOI] [PubMed] [Google Scholar]
- 58.Nijveldt R, Beek AM, Hirsch A, Stoel MG, Hofman MB, Umans VA, Algra PR, Twisk JW, van Rossum AC. Functional recovery after acute myocardial infarction: comparison between angiography, electrocardiography, and cardiovascular magnetic resonance measures of microvascular injury. J Am Coll Cardiol. 2008;52:181–189. doi: 10.1016/j.jacc.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 59.Bodi V, Sanchis J, Lopez-Lereu MP, Losada A, Nunez J, Pellicer M, Bertomeu V, Chorro FJ, Llacer A. Usefulness of a comprehensive cardiovascular magnetic resonance imaging assessment for predicting recovery of left ventricular wall motion in the setting of myocardial stunning. J Am Coll Cardiol. 2005;46:1747–1752. doi: 10.1016/j.jacc.2005.07.039. [DOI] [PubMed] [Google Scholar]
- 60.Antman EM, Hand M, Armstrong PW, Bates ER, Green LA, Halasyamani LK, Hochman JS, Krumholz HM, Lamas GA, Mullany CJ, Pearle DL, Sloan MA, Smith SC, Jr., Anbe DT, Kushner FG, Ornato JP, Pearle DL, Sloan MA, Jacobs AK, Adams CD, Anderson JL, Buller CE, Creager MA, Ettinger SM, Halperin JL, Hunt SA, Lytle BW, Nishimura R, Page RL, Riegel B, Tarkington LG, Yancy CW. 2007 focused update of the ACC/AHA 2004 guidelines for the management of patients with ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2008;51:210–247. doi: 10.1016/j.jacc.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 61.Greenwood JP, Younger JF, Ridgway JP, Sivananthan MU, Ball SG, Plein S. Safety and diagnostic accuracy of stress cardiac magnetic resonance imaging vs exercise tolerance testing early after acute ST elevation myocardial infarction. Heart. 2007;93:1363–1368. doi: 10.1136/hrt.2006.106427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang ZJ, Reddy GP, Gotway MB, Yeh BM, Higgins CB. Cardiovascular shunts: MR imaging evaluation. Radiographics. 2003;23:S181–194. doi: 10.1148/rg.23si035503. [DOI] [PubMed] [Google Scholar]
- 63.Weinsaft JW, Kim HW, Shah DJ, Klem I, Crowley AL, Brosnan R, James OG, Patel MR, Heitner J, Parker M, Velazquez EJ, Steenbergen C, Judd RM, Kim RJ. Detection of left ventricular thrombus by delayed-enhancement cardiovascular magnetic resonance prevalence and markers in patients with systolic dysfunction. J Am Coll Cardiol. 2008;52:148–157. doi: 10.1016/j.jacc.2008.03.041. [DOI] [PubMed] [Google Scholar]
- 64.Mollet NR, Dymarkowski S, Volders W, Wathiong J, Herbots L, Rademakers FE, Bogaert J. Visualization of ventricular thrombi with contrast-enhanced magnetic resonance imaging in patients with ischemic heart disease. Circulation. 2002;106:2873–2876. doi: 10.1161/01.cir.0000044389.51236.91. [DOI] [PubMed] [Google Scholar]
- 65.Jahnke C, Nagel E, Gebker R, Kokocinski T, Kelle S, Manka R, Fleck E, Paetsch I. Prognostic value of cardiac magnetic resonance stress tests: adenosine stress perfusion and dobutamine stress wall motion imaging. Circulation. 2007;115:1769–1776. doi: 10.1161/CIRCULATIONAHA.106.652016. [DOI] [PubMed] [Google Scholar]
- 66.Kwong RY, Chan AK, Brown KA, Chan CW, Reynolds HG, Tsang S, Davis RB. Impact of unrecognized myocardial scar detected by cardiac magnetic resonance imaging on event-free survival in patients presenting with signs or symptoms of coronary artery disease. Circulation. 2006;113:2733–2743. doi: 10.1161/CIRCULATIONAHA.105.570648. [DOI] [PubMed] [Google Scholar]
- 67.Wu KC, Zerhouni EA, Judd RM, Lugo-Olivieri CH, Barouch LA, Schulman SP, Blumenthal RS, Lima JA. Prognostic significance of microvascular obstruction by magnetic resonance imaging in patients with acute myocardial infarction. Circulation. 1998;97:765–772. doi: 10.1161/01.cir.97.8.765. [DOI] [PubMed] [Google Scholar]
- 68.Taylor AJ, Al-Saadi N, Abdel-Aty H, Schulz-Menger J, Messroghli DR, Friedrich MG. Detection of acutely impaired microvascular reperfusion after infarct angioplasty with magnetic resonance imaging. Circulation. 2004;109:2080–2085. doi: 10.1161/01.CIR.0000127812.62277.50. [DOI] [PubMed] [Google Scholar]
- 69.Hombach V, Grebe O, Merkle N, Waldenmaier S, Hoher M, Kochs M, Wohrle J, Kestler HA. Sequelae of acute myocardial infarction regarding cardiac structure and function and their prognostic significance as assessed by magnetic resonance imaging. Eur Heart J. 2005;26:549–557. doi: 10.1093/eurheartj/ehi147. [DOI] [PubMed] [Google Scholar]
- 70.Baks T, van Geuns RJ, Biagini E, Wielopolski P, Mollet NR, Cademartiri F, van der Giessen WJ, Krestin GP, Serruys PW, Duncker DJ, de Feyter PJ. Effects of primary angioplasty for acute myocardial infarction on early and late infarct size and left ventricular wall characteristics. J Am Coll Cardiol. 2006;47:40–44. doi: 10.1016/j.jacc.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 71.Tarantini G, Razzolini R, Cacciavillani L, Bilato C, Sarais C, Corbetti F, Marra MP, Napodano M, Ramondo A, Iliceto S. Influence of transmurality, infarct size, and severe microvascular obstruction on left ventricular remodeling and function after primary coronary angioplasty. Am J Cardiol. 2006;98:1033–1040. doi: 10.1016/j.amjcard.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 72.Larose E, Ganz P, Reynolds HG, Dorbala S, Di Carli MF, Brown KA, Kwong RY. Right ventricular dysfunction assessed by cardiovascular magnetic resonance imaging predicts poor prognosis late after myocardial infarction. J Am Coll Cardiol. 2007;49:855–862. doi: 10.1016/j.jacc.2006.10.056. [DOI] [PubMed] [Google Scholar]
- 73.Roes SD, Kelle S, Kaandorp TA, Kokocinski T, Poldermans D, Lamb HJ, Boersma E, van der Wall EE, Fleck E, de Roos A, Nagel E, Bax JJ. Comparison of myocardial infarct size assessed with contrast-enhanced magnetic resonance imaging and left ventricular function and volumes to predict mortality in patients with healed myocardial infarction. Am J Cardiol. 2007;100:930–936. doi: 10.1016/j.amjcard.2007.04.029. [DOI] [PubMed] [Google Scholar]
- 74.Wu E, Ortiz JT, Tejedor P, Lee DC, Bucciarelli-Ducci C, Kansal P, Carr JC, Holly TA, Lloyd-Jones D, Klocke FJ, Bonow RO. Infarct size by contrast enhanced cardiac magnetic resonance is a stronger predictor of outcomes than left ventricular ejection fraction or end-systolic volume index: prospective cohort study. Heart. 2008;94:730–736. doi: 10.1136/hrt.2007.122622. [DOI] [PubMed] [Google Scholar]
- 75.Yan AT, Shayne AJ, Brown KA, Gupta SN, Chan CW, Luu TM, Di Carli MF, Reynolds HG, Stevenson WG, Kwong RY. Characterization of the peri-infarct zone by contrast-enhanced cardiac magnetic resonance imaging is a powerful predictor of post-myocardial infarction mortality. Circulation. 2006;114:32–39. doi: 10.1161/CIRCULATIONAHA.106.613414. [DOI] [PubMed] [Google Scholar]
- 76.Schmidt A, Azevedo CF, Cheng A, Gupta SN, Bluemke DA, Foo TK, Gerstenblith G, Weiss RG, Marban E, Tomaselli GF, Lima JA, Wu KC. Infarct tissue heterogeneity by magnetic resonance imaging identifies enhanced cardiac arrhythmia susceptibility in patients with left ventricular dysfunction. Circulation. 2007;115:2006–2014. doi: 10.1161/CIRCULATIONAHA.106.653568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ypenburg C, Roes SD, Bleeker GB, Kaandorp TA, de Roos A, Schalij MJ, van der Wall EE, Bax JJ. Effect of total scar burden on contrast-enhanced magnetic resonance imaging on response to cardiac resynchronization therapy. Am J Cardiol. 2007;99:657–660. doi: 10.1016/j.amjcard.2006.09.115. [DOI] [PubMed] [Google Scholar]
- 78.White JA, Yee R, Yuan X, Krahn A, Skanes A, Parker M, Klein G, Drangova M. Delayed enhancement magnetic resonance imaging predicts response to cardiac resynchronization therapy in patients with intraventricular dyssynchrony. J Am Coll Cardiol. 2006;48:1953–1960. doi: 10.1016/j.jacc.2006.07.046. [DOI] [PubMed] [Google Scholar]
- 79.Choi CJ, Haji-Momenian S, Dimaria JM, Epstein FH, Bove CM, Rogers WJ, Kramer CM. Infarct involution and improved function during healing of acute myocardial infarction: the role of microvascular obstruction. J Cardiovasc Magn Reson. 2004;6:917–925. doi: 10.1081/jcmr-200036206. [DOI] [PubMed] [Google Scholar]
- 80.Rochitte CE, Lima JA, Bluemke DA, Reeder SB, McVeigh ER, Furuta T, Becker LC, Melin JA. Magnitude and time course of microvascular obstruction and tissue injury after acute myocardial infarction. Circulation. 1998;98:1006–1014. doi: 10.1161/01.cir.98.10.1006. [DOI] [PubMed] [Google Scholar]
- 81.Wu KC, Kim RJ, Bluemke DA, Rochitte CE, Zerhouni EA, Becker LC, Lima JA. Quantification and time course of microvascular obstruction by contrast-enhanced echocardiography and magnetic resonance imaging following acute myocardial infarction and reperfusion. J Am Coll Cardiol. 1998;32:1756–1764. doi: 10.1016/s0735-1097(98)00429-x. [DOI] [PubMed] [Google Scholar]
- 82.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 83.Verani MS, Jeroudi MO, Mahmarian JJ, Boyce TM, Borges-Neto S, Patel B, Bolli R. Quantification of myocardial infarction during coronary occlusion and myocardial salvage after reperfusion using cardiac imaging with technetium-99m hexakis 2-methoxyisobutyl isonitrile. J Am Coll Cardiol. 1988;12:1573–1581. doi: 10.1016/s0735-1097(88)80028-7. [DOI] [PubMed] [Google Scholar]
- 84.Kaul S, Pandian NG, Okada RD, Pohost GM, Weyman AE. Contrast echocardiography in acute myocardial ischemia: I. In vivo determination of total left ventricular “area at risk”. J Am Coll Cardiol. 1984;4:1272–1282. doi: 10.1016/s0735-1097(84)80149-7. [DOI] [PubMed] [Google Scholar]
- 85.Reimer KA, Jennings RB. The changing anatomic reference base of evolving myocardial infarction. Underestimation of myocardial collateral blood flow and overestimation of experimental anatomic infarct size due to tissue edema, hemorrhage and acute inflammation. Circulation. 1979;60:866–876. doi: 10.1161/01.cir.60.4.866. [DOI] [PubMed] [Google Scholar]
- 86.Garcia-Dorado D, Oliveras J, Gili J, Sanz E, Perez-Villa F, Barrabes J, Carreras MJ, Solares J, Soler-Soler J. Analysis of myocardial oedema by magnetic resonance imaging early after coronary artery occlusion with or without reperfusion. Cardiovasc Res. 1993;27:1462–1469. doi: 10.1093/cvr/27.8.1462. [DOI] [PubMed] [Google Scholar]
- 87.Aletras AH, Tilak GS, Natanzon A, Hsu LY, Gonzalez FM, Hoyt RF, Jr., Arai AE. Retrospective determination of the area at risk for reperfused acute myocardial infarction with T2-weighted cardiac magnetic resonance imaging: histopathological and displacement encoding with stimulated echoes (DENSE) functional validations. Circulation. 2006;113:1865–1870. doi: 10.1161/CIRCULATIONAHA.105.576025. [DOI] [PubMed] [Google Scholar]
- 88.Friedrich MG, Abdel-Aty H, Taylor A, Schulz-Menger J, Messroghli D, Dietz R. The salvaged area at risk in reperfused acute myocardial infarction as visualized by cardiovascular magnetic resonance. J Am Coll Cardiol. 2008;51:1581–1587. doi: 10.1016/j.jacc.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 89.Li G, Xiang B, Dai G, Shaw A, Liu H, Yang B, Jackson M, Deslauriers R, Tian G. Tissue edema does not change gadolinium-diethylenetriamine pentaacetic acid (Gd-DTPA)-enhanced T1 relaxation times of viable myocardium. J Magn Reson Imaging. 2005;21:744–751. doi: 10.1002/jmri.20330. [DOI] [PubMed] [Google Scholar]
- 90.Pflugfelder PW, Wisenberg G, Prato FS, Turner KL, Carroll SE. Serial imaging of canine myocardial infarction by in vivo nuclear magnetic resonance. J Am Coll Cardiol. 1986;7:843–849. doi: 10.1016/s0735-1097(86)80346-1. [DOI] [PubMed] [Google Scholar]
- 91.Kaandorp TA, Lamb HJ, Poldermans D, Viergever EP, Boersma E, van der Wall EE, de Roos A, Bax JJ. Assessment of right ventricular infarction with contrast-enhanced magnetic resonance imaging. Coron Artery Dis. 2007;18:39–43. doi: 10.1097/MCA.0b013e32801104c1. [DOI] [PubMed] [Google Scholar]
- 92.Kumar A, Abdel-Aty H, Kriedemann I, Schulz-Menger J, Gross CM, Dietz R, Friedrich MG. Contrast-enhanced cardiovascular magnetic resonance imaging of right ventricular infarction. J Am Coll Cardiol. 2006;48:1969–1976. doi: 10.1016/j.jacc.2006.05.078. [DOI] [PubMed] [Google Scholar]
- 93.Yuan C, Mitsumori LM, Ferguson MS, Polissar NL, Echelard D, Ortiz G, Small R, Davies JW, Kerwin WS, Hatsukami TS. In vivo accuracy of multispectral magnetic resonance imaging for identifying lipid-rich necrotic cores and intraplaque hemorrhage in advanced human carotid plaques. Circulation. 2001;104:2051–2056. doi: 10.1161/hc4201.097839. [DOI] [PubMed] [Google Scholar]
- 94.Hatsukami TS, Ross R, Polissar NL, Yuan C. Visualization of fibrous cap thickness and rupture in human atherosclerotic carotid plaque in vivo with high-resolution magnetic resonance imaging. Circulation. 2000;102:959–964. doi: 10.1161/01.cir.102.9.959. [DOI] [PubMed] [Google Scholar]
- 95.Kim WY, Stuber M, Bornert P, Kissinger KV, Manning WJ, Botnar RM. Three-dimensional black-blood cardiac magnetic resonance coronary vessel wall imaging detects positive arterial remodeling in patients with nonsignificant coronary artery disease. Circulation. 2002;106:296–299. doi: 10.1161/01.cir.0000025629.85631.1e. [DOI] [PubMed] [Google Scholar]
- 96.Botnar RM, Stuber M, Kissinger KV, Kim WY, Spuentrup E, Manning WJ. Noninvasive coronary vessel wall and plaque imaging with magnetic resonance imaging. Circulation. 2000;102:2582–2587. doi: 10.1161/01.cir.102.21.2582. [DOI] [PubMed] [Google Scholar]
- 97.Fayad ZA, Fuster V, Fallon JT, Jayasundera T, Worthley SG, Helft G, Aguinaldo JG, Badimon JJ, Sharma SK. Noninvasive in vivo human coronary artery lumen and wall imaging using black-blood magnetic resonance imaging. Circulation. 2000;102:506–510. doi: 10.1161/01.cir.102.5.506. [DOI] [PubMed] [Google Scholar]
- 98.Priest AN, Bansmann PM, Mullerleile K, Adam G. Coronary vessel-wall and lumen imaging using radial k-space acquisition with MRI at 3 Tesla. Eur Radiol. 2007;17:339–346. doi: 10.1007/s00330-006-0368-1. [DOI] [PubMed] [Google Scholar]
- 99.Spuentrup E, Buecker A, Katoh M, Wiethoff AJ, Parsons EC, Jr., Botnar RM, Weisskoff RM, Graham PB, Manning WJ, Gunther RW. Molecular magnetic resonance imaging of coronary thrombosis and pulmonary emboli with a novel fibrin-targeted contrast agent. Circulation. 2005;111:1377–1382. doi: 10.1161/01.CIR.0000158478.29668.9B. [DOI] [PubMed] [Google Scholar]
- 100.Botnar RM, Buecker A, Wiethoff AJ, Parsons EC, Jr., Katoh M, Katsimaglis G, Weisskoff RM, Lauffer RB, Graham PB, Gunther RW, Manning WJ, Spuentrup E. In vivo magnetic resonance imaging of coronary thrombosis using a fibrin-binding molecular magnetic resonance contrast agent. Circulation. 2004;110:1463–1466. doi: 10.1161/01.CIR.0000134960.31304.87. [DOI] [PubMed] [Google Scholar]
- 101.Chen JW, Querol Sans M, Bogdanov A, Jr., Weissleder R. Imaging of myeloperoxidase in mice by using novel amplifiable paramagnetic substrates. Radiology. 2006;240:473–481. doi: 10.1148/radiol.2402050994. [DOI] [PubMed] [Google Scholar]
- 102.Weissleder R, Kelly K, Sun EY, Shtatland T, Josephson L. Cell-specific targeting of nanoparticles by multivalent attachment of small molecules. Nat Biotechnol. 2005;23:1418–1423. doi: 10.1038/nbt1159. [DOI] [PubMed] [Google Scholar]
- 103.Laurent S, Vander Elst L, Fu Y, Muller RN. Synthesis and physicochemical characterization of Gd-DTPA-B(sLex)A, a new MRI contrast agent targeted to inflammation. Bioconjug Chem. 2004;15:99–103. doi: 10.1021/bc034114m. [DOI] [PubMed] [Google Scholar]
- 104.Kramer CF, Barkhausen J, Flamm SD, Kim RJ, Nagel E. Standardized cardiovascular magnetic resonance imaging (CMR) protocols, society for cardiovascular magnetic resonance: board of trustees task force on standardized protocols. Journal of Cardiovascular Magnetic Resonance. 2008;10:35. doi: 10.1186/1532-429X-10-35. [DOI] [PMC free article] [PubMed] [Google Scholar]