Summary
Knowledge of the processes by which epilepsy is generated (epileptogenesis) is incomplete and has been a topic of major research efforts. Animal models can inform us about these processes. We focus on the distinguishing features of epileptogenesis in the developing brain and model prolonged febrile seizures (FS) that are associated with human temporal lobe epilepsy. In the animal model of FS, epileptogenesis occurs in ~35% of rats. Unlike the majority of acquired epileptogeneses in adults, this process early in life (in the febrile seizures model as well as in several others) does not require “damage” (cell death). Rather, epileptogenesis early in life involves molecular mechanisms including seizure-evoked, long-lasting alterations of the expression of receptors and ion channels. Whereas transient changes in gene expression programs are common after early-life seizures, enduring effects, such as found after experimental FS, are associated with epileptogenesis. The ability of FS to generate long-lasting molecular changes and epilepsy suggests that mechanisms, including cytokine activation that are intrinsic to FS generation, may play a role also in the epileptogenic consequences of these seizures
Keywords: Epilepsy, Febrile seizures, Gene expression, Damage, Epileptogenesis, Excitotoxicity, Ion channels, Hyperpolarization, Ih, Limbic system, Interleukin, Cytokine, Hippocampus, Neonatal
Knowledge of the processes by which epilepsy is generated (epileptogenesis) is incomplete, and has been a topic of major research efforts, as well as of this WONOEP meeting. In the case of acquired epilepsies, epileptogenesis has classically been divided into three phases: the inciting event (analogous to a mutation of an important gene or perhaps a cerebral malformation in the case of genetic epilepsies), the silent period when epileptogenesis takes place, and, finally, the onset of spontaneous recurrent seizures. In models of acquired epilepsy in the mature brain, the inciting event is typically seizures/status epilepticus (SE), trauma, or stroke. It provokes cell death (“damage”), with associated formation of new synapses of surviving neurons, and a functional reorganization of the previously normal circuit into an “epileptic” one (e.g., Sloviter, 1994; Pitkanen and Sutula, 2002). These events, i.e., loss of vulnerable neurons and the maladaptive rewiring of neuronal connections, precede the onset of epilepsy, and have generally been considered to contribute to the epileptogenic process. Indeed, one or more of these events are often considered a requirement to the development of epilepsy (Coulter and De Lorenzo, 1999; Mathern et al., 2002; Pitkanen and Sutula, 2002; Nadler, 2003)
It has often been assumed that epileptogenesis in the immature brain, including the two first postnatal weeks in the rodent (roughly equivalent to the neonatal and infancy periods of brain development in the human, Avishai-Eliner et al., 2002), employs similar mechanisms. Therefore, a major effort has been devoted to studying whether inciting seizures or SE evokes cell death in neonatal/infant brain. Studies have focused specifically on seizure-vulnerable regions including amygdala (Baram and Ribak, 1995; Toth et al., 1998), the hippocampal formation (e.g., Sperber et al., 1991; Holmes et al., 1998; Sankar et al., 1998; Toth et al., 1998; Koh et al., 1999; Dube et al., 2001; Haas et al., 2001; Lee et al., 2001; Bender et al., 2003; Raol et al., 2003) limbic cortices (Fernandes et al., 1999), and thalamus (Bertram and Scott, 2000, Kubova et al., 2001). However, the majority of studies over the past decade have failed to reveal substantial seizure-provoked neuronal loss in these regions, suggesting that a direct application of the principles of epileptogenesis derived from the mature limbic system to the developmental limbic circuit may not provide us with satisfactory answers about epileptogenesis in the developing brain (Baram et al., 2002; Baram, 2003).
CELL DEATH AND EPILEPTOGENESIS IN THE IMMATURE BRAIN
As mentioned above, during the first 2–3 postnatal weeks in rodent models, SE and severe seizures have generally been found to cause little cell death. This has been shown for limbic seizures generated by kainic acid (Nitecka et al., 1984), flurothyl (Holmes et al., 1998), hypoxia (Jensen et al., 1992; Sanchez et al., 2001), hyperthermia (Toth et al., 1998; Bender et al., 2003; Dube et al., 2006), or tetanus toxin (Lee et al., 2001). Although modest cell deathwas found in the pilocarpine (Sankar et al., 1998; Kubova et al., 2001) and corticotropin-releasing hormone (CRH) models (Baram and Ribak, 1995; Brunson et al., 2001), the general absence of neuronal death in early-life seizure models indicates that it is not required for seizure-induced functional hippocampal deficits (Sperber et al., 1991; Holmes et al., 1998; Haas et al., 2001; Lee et al., 2001; Bender et al., 2003; Chang et al., 2003; Raol et al., 2003; Dube et al., 2006). It should be noted that initially, this lack of neuronal death was considered indicative of a relative absence of sequelae of experimental seizures on the function of the immature limbic network, particularly because the typical synaptic reorganization (“sprouting”) associated with cell death in the adult was sparse early in life (Sperber et al., 1991; Holmes et al., 1999; Bender et al., 2003; Raol et al., 2003). However, more recently, hippocampal dysfunction after early-life seizures has been recognized in some experimental models, manifest by limbic cognitive deficits (Lee et al., 2001; Faverjon et al., 2002; Chang et al., 2003). This dissociation between seizure-evoked “damage” and seizure-induced functional defects has contributed to the increasingly recognized concept that cell death may not be required for functional injury to the developing hippocampal/limbic network (Baram et al., 2002; Holmes, 2005). The same principle has been found to be true also for epileptogenesis during the first few postnatal weeks (Lee et al., 2001; Raol et al., 2003; Dube et al., 2006).
It should be emphasized that developmental processes, including maturation of neuronal connectivity and the cellular machinery for interneuronal communication undergoes rapid changes during the first few postnatal weeks in the rodent (e.g., Baram and Jensen, 2000; Avishai-Eliner et al., 2002; Ben-Ari, 2002; Jensen and Sanchez, 2002; Swann, 2004). Therefore, when discussing the concept of the “immature” brain, precision about the specific age involved is warranted. This applies to comparing among animal studies carried out at different postnatal ages, and even more so when comparing rodent models to the human neonate, infant, and child.
Several studies have attempted to correlate the timing of human and rodent brain development (e.g., Dobbing and Sands, 1979; Clancy et al. 2001; see references in Avishai-Eliner et al., 2002). They generally have concluded that even comparative analyses of specific regions such as the hippocampal formation cannot provide precise correlation of human and rodent “ages.” However, careful comparisons suggest that the majority of structural and functional milestones occurring during the first week of life in rat hippocampus take place during the third-trimester gestational period of the human. Using the same approach, the first year of human life might correspond roughly to postnatal days 7–14 in the rat, whereas “early preschool years” might correlate with the rat’s third week of postnatal life (Avishai-Eliner et al., 2002). With this in mind, the following discussion of epileptogenesis during the neonatal-infancy period is focused primarily on the first two postnatal weeks in rodent models.
IF CELL DEATH (“DAMAGE”) IS NOT REQUIRED FOR EPILEPTOGENESIS EARLY IN LIFE, THEN WHAT ARE THE CRITICAL MECHANISMS INVOLVED IN THIS PROCESS?
Clearly, understanding the mechanisms by which early-life epilepsy develops would be very helpful because it would permit design of selective preventative or interventional strategies. Whereas available possibilities such as global interruption of excitatory mechanisms can block seizure-evoked excitotoxic processes, this approach may not be suitable to the developing brain because it interferes with normal neuronal function. Defining specific mechanisms for epileptogenesis or functional injury early in life will permit the design of selective blockers, ideally without disruption of central nervous system (CNS) maturation and function. Also evident is the fact that this enormously important problem will not be solved in human studies because preexisting factors in individual infants cannot be controlled, and direct, controlled mechanistic analyses cannot be undertaken in infants and children. In this regard, immature rodent models offer important advantages so that careful design and analysis of immature animal experiments can produce important information about the nature and mechanisms of “acquired” epileptogenesis.
As mentioned above, neuronal death is unlikely to be required for the epileptogenic process early in life. The same is true for synaptic reorganization (Bender et al., 2003; Raol et al., 2003) as well as enhanced neurogenesis, a phenomenon implicated in the consequences of both adult limbic seizures and potentially epileptogenesis (Parent et al., 1997; McCabe et al., 2001; Bender et al., 2003). Indeed, available evidence suggests that structural alterations, such as death, birth, or altered branching of neurons, do not provide a foundation for epileptogenesis in the developing brain.
It might be noted that epileptogenesis that is independent from neuronal death has been found in several typically genetic epilepsy models in the mature brain (e.g., GAERS, WAG/Rij; Budde et al., 2005). Little cell death is also found after kindling (Cavazos et al., 1994). The concept supported by converging studies on epileptogenesis in the developing brain supports enduring functional changes, manifest (at least partly) by altered programs of gene expression, as the foundation of the epileptogenic process.
EPILEPTOGENESIS WITHOUT CELL DEATH, BUT WITH PERSISTENT CHANGES IN GENE EXPRESSION
Recent work has demonstrated epileptogenesis in several infant rodent models. The first, a model of human childhood prolonged febrile seizures (FS), has been studied extensively (Toth et al., 1998; Chen et al., 1999; Dube et al., 2000; Chen et al., 2001; Brewster et al. 2002; Bender et al., 2003; Brewster et al., 2005; Dube et al., 2005a, 2005b, 2006). In this model, the inciting seizures do evoke epileptogenesis (Dube et al., 2006). Remarkably, excitotoxicity (cell death) does not accompany the epileptogenic process, indicating that cell death is not required for epilepsy generation (Toth et al., 1998; Bender et al., 2003; Dube et al., 2006). In other words, the developmental FS model dissociates proepileptogenic processes from excitotoxicity.
Occurrence of spontaneous seizures (epilepsy) has also been found after developmental SE induced by lithiumpilocarpine (Raol et al., 2003) and tetanus toxin (Anderson et al., 1999; Lee et al., 2001). In these models (including the FS model), changes occurring at the molecular/functional level, such as alterations in neurotransmitter receptors (Sanchez et al., 2001; Zhang et al., 2004) or voltage-gated channels (Chen et al., 2001; Brewster et al., 2002, 2005), rather than cell death, may be the critical mediators of epileptogenesis. Thus, a hallmark of the FS and the pilocarpine-model is the persistence (for weeks and months) of seizure-evoked perturbation in gene expression programs (Brewster et al., 2002; Zhang et al., 2004), and it may be that the model-specific impact on these programs is the distinguishing feature between developmental seizures that provoke epileptogenesis from seizures that do not. For instance, both experimental FS and other seizures (e.g., kainic acid-evoked) alter the expression of the hyperpolarization-activated, cyclic nucleotide-gated (HCN) ion channels in hippocampus (Brewster et al., 2002) via a calcium-mediated mechanism (Richichi et al., 2005). However, whereas changes in hippocampal HCN channel expression are transient after kainate-induced seizures, they endure after experimental FS and render the hippocampal network in the affected animals permanently hyperexcitable (Dube et al., 2000; Chen et al., 2001; Brewster et al., 2002, 2005).
WHAT IS SO SPECIFIC ABOUT THE FS MODEL?
An understanding of mechanisms that may interact to render the developing brain hyperexcitable, and subsequently epileptic, is only beginning to emerge. Findings in the FS model have recently shed light on a potential role of cytokines, and particularly of interleukin-1β (IL-1β; Dube et al., 2005b) in the epileptogenic process. Both fever and hyperthermia induce the synthesis and release of IL-1β in the brain (Haveman et al., 1996; Gatti et al., 2002; Heida and Pittman, 2005). Indeed, IL-1β is required for the generation of hyperthermic seizures in the FS model (Dube et al., 2005b) because the temperature threshold for hyperthermia-induced seizure induction was significantly increased in mice that lack the IL-1βR1-gene. Interestingly, IL-1β does not seem to be released or involved in other experimental seizure types during development (Rizzi et al., 2003). Thus, the seizure-evoked actions of IL-1β on hippocampal neurons are a distinguishing feature of the FS model compared to other early-life seizure models.
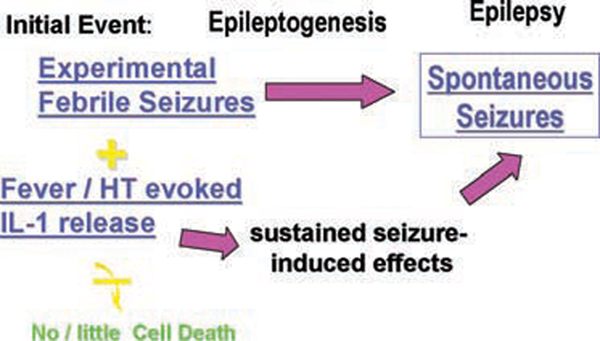
Several lines of evidence suggest that IL-1β release could promote hyperexcitability (Vezzani et al., 1999, 2000; reviewed in Vezzani and Baram, 2006), particularly in hippocampus, where IL-1β receptors (IL-1βR1) are expressed in high density (Takao et al., 1990; Ban et al., 1991; Nishiyori et al., 1997). IL-1β binding to its receptor triggers a cascade of intracellular messengers (Viviani et al., 2003), exerting genomic effects via MAP kinase and NF-κB signaling. Therefore, it is attractive to hypothesize that the genomic actions of this cytokine contribute to the enduring alterations in gene expression that underlie the epileptogenic process following these seizures. This notion is schematized in Fig. 1: the effects of the seizures themselves on gene expression (i.e., activity-dependent plasticity) are compounded by the genomic actions of interleukins. Combined, these processes result in enduring changes of the expression of critical molecules, including receptors and ion channels that promote epileptogenesis.
FIG. 1.
A schematic representation of a hypothetical scenario for epileptogenesis following prolonged experimental febrile seizures. The initial, inciting “insult” in this case consists of two elements: First, the experimental seizures, that, similar to other early life seizures, provoke transient changes in the expression of several ion channel and receptor genes (top fuchsia arrow). Second, the release of endogenous interleukins, a phenomenon that appears not to occur in chemical-induced seizures provoked prior to postnatal day 14–15 in the rodent. Interleukins, in turn, act via several molecular cascades to influence gene expression (bottom fuchsia arrow). Because interleukin expression and genomic actions appear to be sustained, the alteration in gene expression described above endure, and promote epileptogenesis. Bottom yellow arrow, with a “strikeout” bar, denotes relative absence of neuronal death associated with these processes.
In conclusion, information from numerous and diverse developmental models of epilepsy indicate that epileptogenesis early in life has unique features. These data make a compelling case for the notion that cell death is not required for epileptogenesis. In contrast, the enhanced plasticity of gene expression programs early in life (Welberg et al., 2001; Baram, 2003; Fenoglio et al., 2006) may allow seizures to alter expression of critical genes (receptors, channels) in an enduring manner, facilitating conversion of a “normal” developing limbic circuit to an “epileptic” one.
Acknowledgments
The editorial assistance of M. Hinojosa is appreciated. The authors’ research has been supported by National Institutes of Health grants NS28912, NS35439, NS39307/MH31356, by EFA (Milken Foundation) and AES.
REFERENCES
- Anderson AE, Hrachovy RA, Antalffy BA, Armstrong DL, Swann JW. A chronic focal epilepsy with mossy fiber sprouting follows recurrent seizures induced by intrahippocampal tetanus toxin injection in infant rats. Neuroscience. 1999;92:73–82. doi: 10.1016/s0306-4522(98)00746-5. [DOI] [PubMed] [Google Scholar]
- Avishai-Eliner S, Brunson KL, Sandman CA, Baram TZ. Stressed out? Or in (utero) Trends Neurosci. 2002;25:518–524. doi: 10.1016/s0166-2236(02)02241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ban E, Milon G, Prudhomme N, Fillion G, Haour F. Receptors for interleukin-1 (alpha and beta) in mouse brain: mapping and neuronal localization in hippocampus. Neuroscience. 1991;43:21–30. doi: 10.1016/0306-4522(91)90412-h. [DOI] [PubMed] [Google Scholar]
- Baram TZ, Ribak CE. Peptide-induced infant status epilepticus causes neuronal death and synaptic reorganization. NeuroReport. 1995;6:277–280. doi: 10.1097/00001756-199501000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baram TZ, Jensen FE. Developmental seizures induced by common early-life insults: short- and long-term effects on seizure susceptibility. Ment Retard Dev Disabil Res Rev. 2000;6:253–257. doi: 10.1002/1098-2779(2000)6:4<253::AID-MRDD4>3.0.CO;2-P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baram TZ, Eghbal-Ahmadi M, Bender RA. Is neuronal death required for seizure-induced epileptogenesis in the immature brain? Prog Brain Res. 2002;135:365–375. doi: 10.1016/S0079-6123(02)35033-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baram TZ. Long-term neuroplasticity and functional consequences of single versus recurrent early-life seizures. Ann Neurol. 2003;54:701–705. doi: 10.1002/ana.10833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y. Excitatory actions of GABA during development: the nature of the nurture. Nat Rev Neurosci. 2002;3:728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- Bender RA, Dube C, Gonzalez-Vega R, Mina EW, Baram TZ. Mossy fiber plasticity and enhanced hippocampal excitability, without hippocampal cell loss or altered neurogenesis, in an animal model of prolonged febrile seizures. Hippocampus. 2003;13:357–370. doi: 10.1002/hipo.10089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertram EH, Scott C. The pathological substrate of limbic epilepsy: neuronal loss in the medial dorsal thalamic nucleus as the consistent change. Epilepsia. 2000;41 Suppl 6:S3–S8. doi: 10.1111/j.1528-1157.2000.tb01548.x. [DOI] [PubMed] [Google Scholar]
- Brewster A, Bender RA, Chen Y, Dube C, Eghbal-Ahmadi M, Baram TZ. Developmental febrile seizures modulate hippocampal gene expression of hyperpolarization-activated channels in an isoform and cell-specific manner. J Neurosci. 2002;22:4591–4599. doi: 10.1523/JNEUROSCI.22-11-04591.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewster AL, Bernard JA, Gall CM, Baram TZ. Formation of heteromeric hyperpolarization-activated cyclic nucleotide-gated (HCN) channels in the hippocampus is regulated by developmental seizures. Neurobiol Dis. 2005;19:200–207. doi: 10.1016/j.nbd.2004.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunson KL, Eghbal-Ahmadi M, Bender RA, Chen Y, Baram TZ. Long-term progressive hippocampal cell loss and dysfunction induced by early-life administration of corticotropin releasing hormone re-produce the effects of early-life stress. Proc Natl Acad Sci U S A. 2001;98:8856–8861. doi: 10.1073/pnas.151224898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budde T, Caputi L, Kanyshkova T, Staak R, Abrahamczik C, Munsch T, Pape HC. Impaired regulation of thalamic pacemaker channels through an imbalance of subunit expression in absence epilepsy. J Neurosci. 2005;25:9871–9882. doi: 10.1523/JNEUROSCI.2590-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavazos JE, Das I, Sutula TP. Neuronal loss induced in limbic pathways by kindling: evidence for induction of hippocampal sclerosis by repeated brief seizures. J Neurosci. 1994;14:3106–3121. doi: 10.1523/JNEUROSCI.14-05-03106.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YC, Huang AM, Kuo YM, Wang ST, Chang YY, Huang CC. Febrile seizures impair memory and cAMP-response element binding protein activation. Ann Neurol. 2003;4:706–718. doi: 10.1002/ana.10789. [DOI] [PubMed] [Google Scholar]
- Chen K, Baram TZ, Soltesz I. Febrile seizures in the developing brain result in persistent modification of neuronal excitability in limbic circuits. Nat Med. 1999;5:888–894. doi: 10.1038/11330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Aradi I, Thon N, Eghbal-Ahmadi M, Baram TZ, Soltesz I. Persistently modified h-channels after complex febrile seizures convert the seizure-induced enhancement of inhibition to hyperexcitability. Nat Med. 2001;7:331–337. doi: 10.1038/85480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy B, Darlington RB, Finlay BL. Translating developmental time across mammalian species. Neuroscience. 2001;105:7–17. doi: 10.1016/s0306-4522(01)00171-3. [DOI] [PubMed] [Google Scholar]
- Coulter DA, De Lorenzo RJ. Basic mechanisms of status epilepticus. Adv Neurol. 1999;79:725–733. [PubMed] [Google Scholar]
- Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum Dev. 1979;3:79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- Dube C, Chen K, Eghbal-Ahmadi M, Brunson K, Soltesz I, Baram TZ. Prolonged febrile seizures in the immature rat model enhance hippocampal excitability long-term. Ann Neurol. 2000;47:336–344. [PMC free article] [PubMed] [Google Scholar]
- Dube C, Boyet S, Marescaux C, Nehlig A. Relationship between neuronal loss and interictal glucose metabolism during the chronic phase of the lithium-pilocarpine model of epilepsy in the immature and adult rat. Exp Neurol. 2001;167:227–241. doi: 10.1006/exnr.2000.7561. [DOI] [PubMed] [Google Scholar]
- Dube C, Brunson KL, Eghbal-Ahmadi M, Gonzalez-Vega R, Baram TZ. Endogenous neuropeptide Y prevents recurrence of experimental febrile seizures by increasing seizure threshold. J Mol Neurosci. 2005a;25:275–284. doi: 10.1385/JMN:25:3:275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube C, Vezzani A, Behrens M, Bartfai T, Baram TZ. Interleukin-1beta contributes to the generation of experimental febrile seizures. Ann Neurol. 2005b;57:152–155. doi: 10.1002/ana.20358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube C, Richichi C, Bender RA, Chung G, Litt B, Baram TZ. Temporal lobe epilepsy after experimental prolonged febrile seizures: prospective analysis. Brain. 2006;129:911–922. doi: 10.1093/brain/awl018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faverjon S, Silveira DC, Fu DD, Cha BH, Akman C, Hu Y, Holmes GL. Beneficial effects of enriched environment following status epilepticus in immature rats. Neurology. 2002;59:1356–1364. doi: 10.1212/01.wnl.0000033588.59005.55. [DOI] [PubMed] [Google Scholar]
- Fenoglio KA, Chen Y, Baram TZ. Neuroplasticity of the hypothalamic-pituitary-adrenal axis early in life requires recurrent recruitment of stress-regulating brain regions. J Neurosci. 2006;26:2434–2442. doi: 10.1523/JNEUROSCI.4080-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes MJ, Dube C, Boyet S, Marescaux C, Nehlig A. Correlation between hypermetabolism and neuronal damage during status epilepticus induced by lithium and pilocarpine in immature and adult rats. J Cereb Blood Flow Metab. 1999;19:195–209. doi: 10.1097/00004647-199902000-00011. [DOI] [PubMed] [Google Scholar]
- Gatti S, Vezzani A, Bartfai T. Mechanisms of fever and febrile seizures: putative role of the interleukin-1 system. In: Baram TZ, Shinnar S, editors. Febrile seizures. San Diego, CA: Academic Press; 2002. pp. 169–188. [Google Scholar]
- Haas KF, Sperber EF, Opanashuk LA, Stanton PK, Moshe SL. Resistance of immature hippocampus to morphologic and physiologic alterations following status epilepticus or kindling. Hippocampus. 2001;11:615–625. doi: 10.1002/hipo.1076. [DOI] [PubMed] [Google Scholar]
- Haveman J, Geerdink AG, Rodermond HM. Cytokine production after whole body and localized hyperthermia. Int J Hyperthermia. 1996;12:791–800. doi: 10.3109/02656739609027685. [DOI] [PubMed] [Google Scholar]
- Heida JG, Pittman QJ. Causal links between brain cytokines and experimental febrile convulsions in the rat. Epilepsia. 2005;46:1906–1913. doi: 10.1111/j.1528-1167.2005.00294.x. [DOI] [PubMed] [Google Scholar]
- Holmes GL. Effects of seizures on brain development: lessons from the laboratory. Pediatr Neurol. 2005;33:1–11. doi: 10.1016/j.pediatrneurol.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Holmes GL, Gairsa J-L, Chevassus-Au-Louis N, Ben-Ari Y. Consequences of neonatal seizures in the rat: morphological and behavioral effects. Ann Neurol. 1998;44:845–857. doi: 10.1002/ana.410440602. [DOI] [PubMed] [Google Scholar]
- Holmes GL, Sarkisian M, Ben-Ari Y, Chevassus-Au-Louis N. Mossy fiber sprouting after recurrent seizures during early development in rats. J Comp Neurol. 1999 Jan;22:537–553. doi: 10.1002/(sici)1096-9861(19990222)404:4<537::aid-cne9>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Jensen FE, Holmes GL, Lombroso CT, Blume HK, Firkusny IR. Age-dependent changes in long-term seizure susceptibility and behavior after hypoxia in rats. Epilepsia. 1992;33:971–980. doi: 10.1111/j.1528-1157.1992.tb01746.x. [DOI] [PubMed] [Google Scholar]
- Jensen FE, Sanchez RM. Why does the developing brain demonstrate heightened susceptibility to febrile and other seizures? In: Baram TZ, Shinnar S, editors. Febrile seizures. San Diego, CA: Academic Press; 2002. pp. 153–168. [Google Scholar]
- Koh S, Storey TW, Santos TC, Mian AY, Cole AJ. Early-life seizures in rats increase susceptibility to seizure-induced brain injury in adult-hood. Neurology. 1999;53:915–921. doi: 10.1212/wnl.53.5.915. [DOI] [PubMed] [Google Scholar]
- Kubova H, Druga R, Lukasiuk K, Suchomelova L, Haugvicova R, Jirmanova I, Pitkanen A. Status epilepticus causes necrotic damage in the mediodorsal nucleus of the thalamus in immature rats. J Neurosci. 2001;21:3593–3599. doi: 10.1523/JNEUROSCI.21-10-03593.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CL, Hannay J, Hrachovy R, Rashid S, Antalffy B, Swann JW. Spatial learning deficits without hippocampal neuronal loss in a model of early-onset epilepsy. Neuroscience. 2001;107:71–84. doi: 10.1016/s0306-4522(01)00327-x. [DOI] [PubMed] [Google Scholar]
- McCabe BK, Silveira DC, Cilio MR, Cha BH, Liu X, Sogawa Y, Holmes GL. Reduced neurogenesis after neonatal seizures. J Neurosci. 2001;21:2094–2103. doi: 10.1523/JNEUROSCI.21-06-02094.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathern GW, Adelson PD, Cahan LD, Leite JP. Hippocampal neuron damage in human epilepsy: Meyer’s hypothesis revisited. Prog Brain Res. 2002;135:237–252. doi: 10.1016/s0079-6123(02)35023-4. [DOI] [PubMed] [Google Scholar]
- Nadler JV. The recurrent mossy fiber pathway of the epileptic brain. Neurochem Res. 2003;28:1649–1658. doi: 10.1023/a:1026004904199. [DOI] [PubMed] [Google Scholar]
- Nishiyori A, Minami M, Takami S, Sanoh M. Type 2 interleukin-1 receptor mRNA is induced by kainic acid in the rat brain. Mol Brain Res. 1997;50:237–245. doi: 10.1016/s0169-328x(97)00195-2. [DOI] [PubMed] [Google Scholar]
- Nitecka L, Tremblay E, Charton G, Bouillot JP, Berger ML, Ben-Ari Y. Maturation of kainic acid seizure-brain damage syndrome in the rat. II. Histopathological sequelae. Neuroscience. 1984;13:1073–1094. doi: 10.1016/0306-4522(84)90289-6. [DOI] [PubMed] [Google Scholar]
- Parent JM, Yu TW, Leibowitz RT, Geschwind DH, Sloviter RS, Lowenstein DH. Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J Neurosci. 1997;17:3727–3738. doi: 10.1523/JNEUROSCI.17-10-03727.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitkanen A, Sutula TP. Is epilepsy a progressive disorder? Prospects for new therapeutic approaches in temporal-lobe epilepsy. Lancet Neurol. 2002;1:173–181. doi: 10.1016/s1474-4422(02)00073-x. [DOI] [PubMed] [Google Scholar]
- Raol YS, Budreck EC, Brooks-Kayal AR. Epilepsy after early-life seizures can be independent of hippocampal injury. Ann Neurol. 2003;53:503–511. doi: 10.1002/ana.10490. [DOI] [PubMed] [Google Scholar]
- Richichi C, Bender RA, Brewster AL, Simeone T, Yin H, Weiss JH, Baram TZ. CaM Kinase II is required for seizure-evoked reduction of hyperpolarization-activated, cyclic nucleotide-gated (HCN)1 channel expression. Soc Neurosci Abstr. 2005;34:377–416. [Google Scholar]
- Rizzi M, Perego C, Aliprandi M, Richichi C, Ravizza T, Colella D, Veliskova J, Moshe SL, De Simoni MG, Vezzani A. Glia activation and cytokine increase in rat hippocampus by kainic acid-induced status epilepticus during postnatal development. Neurobiol Dis. 2003;14:494–503. doi: 10.1016/j.nbd.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Sanchez RM, Koh S, Rio C, Wang C, Lamperti ED, Sharma D, Corfas D, Jensen FE. Decreased glutamate receptor 2 expression and enhanced epileptogenesis in immature rat hippocampus after perinatal hypoxia-induced seizures. J Neurosci. 2001;21:8154–8163. doi: 10.1523/JNEUROSCI.21-20-08154.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankar R, Shin DH, Liu H, Mazarati A, Pereira de Vasconcelos A, Wasterlain CG. Patterns of status epilepticus-induced neuronal injury during development and long-term consequences. J Neurosci. 1998;18:8382–8393. doi: 10.1523/JNEUROSCI.18-20-08382.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloviter RS. The functional organization of the hippocampal dentate gyrus and its relevance to the pathogenesis of temporal lobe epilepsy. Ann Neurol. 1994;35:640–654. doi: 10.1002/ana.410350604. [DOI] [PubMed] [Google Scholar]
- Sperber EF, Haas KZ, Stanton PK, Moshe SL. Resistance of the immature hippocampus to seizure-induced synaptic reorganization. Dev Brain Res. 1991;60:88–93. doi: 10.1016/0165-3806(91)90158-f. [DOI] [PubMed] [Google Scholar]
- Swann JW. The effects of seizures on the connectivity and circuitry of the developing brain. Ment Retard Dev Disabil Res Rev. 2004;10:96–100. doi: 10.1002/mrdd.20018. [DOI] [PubMed] [Google Scholar]
- Takao T, Tracey DE, Mitchell WM, De Souza EB. Interleukin-1 receptors in mouse brain: characterization and neuronal localization. Endocrinology. 1990;127:3070–3078. doi: 10.1210/endo-127-6-3070. [DOI] [PubMed] [Google Scholar]
- Toth Z, Yan XX, Heftoglu S, Ribak CE, Baram TZ. Seizure-induced neuronal injury: vulnerability to febrile seizures in an immature rat model. J Neurosci. 1998;18:4285–4294. doi: 10.1523/JNEUROSCI.18-11-04285.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezzani A, Conti M, De Luigi A, Ravizza T, Moneta D, Marchesi F, De Simoni MG. Interleukin-1beta immunoreactivity and microglia are enhanced in the rat hippocampus by focal kainate application: functional evidence for enhancement of electrographic seizures. J Neurosci. 1999;19:5054–5065. doi: 10.1523/JNEUROSCI.19-12-05054.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezzani A, Moneta D, Conti M, Richichi C, Ravizza T, De Luigi A, De Simoni MG, Sperk G, Andell-Jonsson S, Lundkvist J, Iverfeldt K, Bartfai T. Powerful anticonvulsant action of IL-1 receptor antagonist on intracerebral injection and astrocytic overexpression in mice. Proc Natl Acad Sci U S A. 2000;97:11534–11539. doi: 10.1073/pnas.190206797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezzani A, Baram TZ. New roles for interleukin-1 beta in the mechanisms of epilepsy. Epilepsy Currents. 2007;7:45–50. doi: 10.1111/j.1535-7511.2007.00165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viviani B, Bartesaghi S, Gardoni F, Vezzani A, Behrens MM, Bartfai T, Binaglia M, Corsini E, Di Luca M, Galli CL, Marinovich M. Interleukin-1beta enhances NMDA receptor-mediated intracellular calcium increase through activation of the Src family of kinases. J Neurosci. 2003;23:8692–8700. doi: 10.1523/JNEUROSCI.23-25-08692.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welberg LA, Seckl JR, Holmes MC. Prenatal glucocorticoid programming of brain corticosteroid receptors and corticotrophin-releasing hormone: possible implications for behaviour. Neuroscience. 2001;104:71–79. doi: 10.1016/s0306-4522(01)00065-3. [DOI] [PubMed] [Google Scholar]
- Zhang G, Raol YH, Hsu FC, Brooks-Kayal AR. Long-term alterations in glutamate receptor and transporter expression following early-life seizures are associated with increased seizure susceptibility. J Neurochem. 2004;88:91–101. doi: 10.1046/j.1471-4159.2003.02124.x. [DOI] [PubMed] [Google Scholar]