Abstract
Human 17β-hydroxysteroid dehydrogenase types 1 and 2 (17βHSD1 and 17βHSD2) regulate estrogen potency by catalyzing the interconversion of estrone (E1) and estradiol (E2) using nicotinamide adenine dinucleotide (phosphate) cofactors NAD(P)(H). In intact cells, 17βHSD1 and 17βHSD2 establish pseudo-equilibria favoring E1 reduction or E2 oxidation, respectively. The vulnerability of these equilibrium steroid distributions to mutations and to altered intracellular cofactor abundance and redox state, however, is not known. We demonstrate that the equilibrium E2/E1 ratio achieved by 17βHSD1 in intact HEK-293 cell lines is progressively reduced from 94:6 to 10:90 after mutagenesis of R38, which interacts with the 2′-phosphate of NADP(H), and by glucose deprivation, which lowers the NADPH/NADP+ ratio. The shift to E2 oxidation parallels changes in apparent Km values for purified 17βHSD1 proteins to favor NAD(H) over NADP(H). In contrast, mutagenesis of E116 (corresponding to R38 in 17βHSD1) and changes in intracellular cofactor ratios do not alter the greater than 90:10 E1/E2 ratio catalyzed by 17βHSD2, and these mutations lower the apparent Km of recombinant 17βHSD2 for NADP(H) only less than 3-fold. We conclude that the equilibrium E1/E2 ratio maintained by human 17βHSD1 in intact cells is governed by NADPH saturation, which is strongly dependent on both R38 and high intracellular NADPH/NADP+ ratios. In contrast, the preference of 17βHSD2 for E2 oxidation strongly resists alteration by genetic and metabolic manipulations. These findings suggest that additional structural features, beyond the lack of a specific arginine residue, disfavor NADPH binding and thus support E2 oxidation by 17βHSD2 in intact cells.
The steady-state estrone-estradiol distribution maintained by human 17βHSD1 in intact cells can be varied from 90% estrone to 90% estradiol by mutagenesis of the cofactor-binding domain and by depletion of intracellular NADPH; in contrast, human 17βHSD2 retains strong preference for estradiol oxidation despite similar manipulations.
The terminal steps of steroid metabolism, both for biosynthesis and catabolism, are dominated by the hydroxysteroid dehydrogenases (HSDs) (1). Multiple genes encoding enzymes with different substrate specificities are expressed in a tissue-specific manner under distinct regulatory mechanisms, and these patterns of regulation and activities allow each HSD to perform its physiological functions (2). In estrogen metabolism, the biosynthesis of estradiol (E2) in the ovary and placenta requires the reduction of estrone (E1) catalyzed by 17βHSD type 1 (17βHSD1) (3,4,5,6). Conversely, peripheral tissues contain the type 2 isoform (17βHSD2), which oxidizes E2 to E1 (7).
The hydride transfer reactions catalyzed by these short-chain dehydrogenase/reductase (SDR) enzymes (8), however, are intrinsically reversible reactions that reach pseudo-equilibrium states in intact cells (9), yet the two enzymes generate radically different steroid distributions in the steady state (6) despite comparable affinities for E1 and E2 (10,11). These steroid distributions appear to be maintained by gradients of the oxidized and reduced forms of the nicotinamide adenine dinucleotide (phosphate) cofactors NAD(P)(H) (12). The cofactor gradients, in turn, are maintained by intermediary metabolism and respiration (13).
Studies of the aldo-keto reductase AKR1C9 (rat liver 3αHSD) (14), which catalyzes the reduction of dihydrotestosterone to more than 95% 5α-androstane-3α,17β-diol in intact cells (15), illustrate these principles. R276 of AKR1C9 forms a salt bridge with the 2′-phosphate of NADPH and thus increases its affinity for NADP(H) over NAD(H) (16,17,18). The magnitude of this directional preference is attenuated by mutation R276M and reversed by mutation R276E, which progressively reduce the affinity of AKR1C9 for NADPH (15,17). Furthermore, glucose deprivation and methylene blue treatments, which progressively deplete cells of NADPH, likewise reduce or reverse the reductive directional preference of AKR1C9 in intact cells (15).
The x-ray crystal structure of human 17βHSD1 shows that R38 (numbering used here includes initiating methionine as residue 1, which is omitted in the historical protein sequence) forms a salt bridge with the 2′-phosphate of NADP(H) (19), analogous to R276 of AKR1C9. Mutation L37D in 17βHSD1 adds a negative charge to the cofactor-binding domain, which similarly reduces the affinity for NADP(H) (10) and inverts the directional preference to oxidation in intact cells (9). The consequences of R38 mutations and of glucose deprivation on the directional preference of 17βHSD1, however, have not been studied, and few studies have attempted to modulate the directional preference of oxidative HSDs (20) such as 17βHSD2, whose structures are poorly characterized. Consequently, we performed a series of experiments to elucidate the factors that govern the steady-state E1/E2 ratio maintained by 17βHSD1 and 17βHSD2 in intact HEK-293 cells.
Materials and Methods
Chemicals and reagents
Radiochemicals, provided and diluted in ethanol, were obtained from PerkinElmer Life Sciences (Waltham, MA): [6,7-3H(N)]E1, 40.4 Ci/mmol; [6,7-3H(N)]E2, 41.3 Ci/mmol; [4-14C]E1, 51.3 mCi/mmol; and [4-14C]E2, 52.0 mCi/mmol. Unlabeled steroids were obtained from Sigma Chemical Co. (St. Louis, MO) or Steraloids (Providence, RI) and dissolved in ethanol. Oligonucleotides were synthesized by Integrated DNA Technologies (Coralville, IA), and enzymes and reagents for DNA modification and purification were obtained from New England Biolabs (Beverly, MA), Promega (Madison, WI), or QIAGEN (Valencia, CA). Other chemicals and reagents were obtained from Thermo-Fisher Scientific (Pittsburgh, PA) or Sigma. Protein determinations used the Coomassie Plus reagent (Pierce, Rockford, IL).
Site-directed mutagenesis
The human 17βHSD1 and 17βHSD2 cDNAs were subcloned into vectors pcDNA3 and V10 (pYeDP10, gift from Dr. Denis Pompon, Gif-sur-Yvette, France) using BglII and EcoRI or BamHI and EcoRI palindromes, respectively, appended immediately 5′ of the start codons and 3′ of the stop codons. Mutations were introduced by overlapping PCR using plasmids V10-17βHSD1 and V10-17βHSD2 as templates and terminal oligonucleotides pPGKS2 and tPGKAS1 (see supplemental Table 1, published as supplemental data on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org). The 11βHSD2-17βHSD2-E116G chimera was prepared by overlapping PCR taking advantage of the common Pro-Val at residues 79–80 in both enzymes using the oligonucleotides in supplemental Table 1. Plasmid pcDNA3-11βHSD2 (gift of Drs. Perrin White and Anil Agarwal, University of Texas Southwestern) was used as template for the N-terminal portion of the chimera, whereas pcDNA3-17βHSD2-E116G was used as template for the C-terminal portion of the chimera, with T7 and SP6 used as terminal oligonucleotides. The mutant cDNAs were generated and subcloned into plasmids pcDNA3 and V10 using the same restriction sites as for the wild-type cDNAs. The 17βHSD1 cDNA was also subcloned into plasmid pLW01 (gift of Dr. Lucy Waskell, University of Michigan) using the 5′ NcoI-BamHI fragment from pRC/CMV-17βHSD1 (gift from Dr. Stefan Andersson, University of Texas Southwestern) followed by the 3′ BamHI-EcoRI fragment from plasmid pcDNA3-17βHSD1. All constructs were sequenced to confirm that only the intended mutations were introduced.
Cell culture, transfection, and cell line preparation
HEK-293 cells [American Type Culture Collection (Manassas, VA) ATCC no. CRL-1573] were grown in complete medium (DMEM containing 4.5 g/liter glucose, 10% fetal bovine serum, 100 IU/ml penicillin, and 0.1 mg/ml streptomycin; Mediatech, Herndon, VA) at 37 C with 5% CO2. Cells were seeded into six-well plates at 50% confluence and transfected with 1 μg plasmid per well using FuGENE6 (Roche Diagnostics, Indianapolis, IN), and metabolism experiments were performed the next day (9). The stable line expressing wild-type 17βHSD1 was prepared by transfecting cells in 10-cm dishes and clone selection with G418 (Research Products International, Mount Prospect, IL) in 15-cm plates as described (15). Chinese hamster ovary stably transfected with the polyoma large T antigen (CHOP) cells (gift from Drs. Annika Lindqvist and Stefan Andersson) were cultured and transfected in the same manner.
Metabolism of single substrates
Transfected cells were incubated with 2 ml fresh medium containing 300,000 cpm [3H]steroid plus unlabeled carrier steroid to yield 0.1 μm final concentration as standard assay conditions. The plate was returned to the incubator, and 0.5-ml aliquots of medium were removed at 2, 4, and 8 h. Each aliquot was extracted with 1 ml ethyl acetate/isooctane (1:1), evaporated, mixed with 50 nmol unlabeled E1 and E2, and separated by thin-layer chromatography (Whatman, Kent, UK) with 3:1 chloroform/ethyl acetate as described (9). The regions of the plates containing E1 and E2 were identified with iodine vapor, excised, and quantitated by liquid scintillation counting using Budget-Solve (Research Products International). Experiments were performed in complete (high-glucose) medium or in glucose-free medium with 10 mm 2-deoxyglucose, and equilibrium steroid distributions were determined by fitting the data to exponential growth curves using Origin version 7.5 (OriginLab Corp., Northampton, MA) and setting t = ∞ (9,15). An aliquot of the medium containing the labeled steroid was always analyzed at the end of each experiment to confirm the purity and stability of the radiochemicals used.
Intrinsic rates of half-reactions at equilibrium
The cell line expressing wild-type 17βHSD1 was incubated with medium containing [3H]E1 and [14C]E2 in the relative mass proportions found at equilibrium in 2 ml complete medium and in glucose-free medium with 2-deoxyglucose (see supplemental Table 2 and supplemental Methods). Aliquots of medium (0.5 ml) were removed at intervals, extracted, chromatographed, and quantitated by differential liquid scintillation counting as described (9). The averaged data were plotted as a function of time, and first-order exponential decay curves were fit to the data. Initial rates, which were much greater than background conversion rates, were calculated from the first derivatives at t = 0 (9,15).
Expression of 17βHSD2 in yeast, microsome preparation, and enzyme assay
Yeast strain W303B was transformed with plasmid V10-17βHSD2, wild-type and mutations, using the lithium acetate method as described (21). Clones were selected on dropout medium plates and grown in 500 ml liquid minimal medium to about 1 OD. Yeast cells were lysed with zymolase treatment, and microsomes were prepared as described (22). Assays included 20 μg microsomal protein and 10 μm [3H]steroid in 0.2 ml 50 mm potassium phosphate buffer (pH 7.4). After preincubation at 30 C for 2 min, varying concentrations of NAD(P)+ were added, and the reaction was returned to 30 C for 3–8 min before extraction with 0.5 ml 1:1 ethyl acetate/isooctane, chromatography, and quantitation (21). Kinetic constants Km and Vmax were obtained fitting the means from triplicate experiments to the Michaelis-Menten equation using Origin 7.5 (22).
Expression of 17βHSD1 in Escherichia coli, purification, and enzyme assay
E. coli strain C41(DE3) (Overexpress, Lucigen, Middleton, WI) were transformed with plasmid pLW01-17βHSD1 and selected on plates of Luria-Bertani broth containing 50 μg/ml ampicillin. Clones were precultured in 2 ml broth the next morning at 37 C until about 1 OD and stored at 4 C. Within 3 d, 1 liter terrific broth with ampicillin was inoculated with 1 ml of the preculture and grown at 37 C until about 0.8 OD. The cultures were cooled to room temperature and induced with 0.4 mm isopropyl-thio-β-galactoside followed by shaking at 18 C for 12–18 h. Cells were harvested by centrifugation, frozen, and lysed in 10 mm potassium phosphate buffer with 20% glycerol and 1 mm EDTA (buffer 1) with a cell disruptor (EmulsiFlex C-5; Avestin, Ottowa, Canada). After centrifugation, glycerol was added to the supernatant to 50% vol/vol, and the solution was heated at 65 C for 40 min and centrifuged. The supernatant was diluted with buffer 1 to 20% glycerol and mixed with of Reactive Red agarose (Sigma) (2 ml resin per liter culture) overnight at 4 C. The resin was loaded into a column and washed with 25 ml buffer 1, then 8 ml buffer 1 with 0.2 m KCl, and again with 10 ml buffer 1. The purified protein was eluted with at least two batch washes of 5 ml 0.4 mm NADP+ (or NAD+ for mutation R38D) in buffer 1 at 30–37 C for 2 h. The protein was concentrated with centrifugation concentrators (Amicon) if necessary and showed with specific activity for E2 oxidation of 3 μmol/min · mg protein. Kinetic assays and data analyses were performed as described for yeast microsomes using 0.8–2 μg enzyme per assay.
Results
Equilibrium steroid distributions and rates for wild-type 17βHSD1 and R38 mutations in HEK-293 cells
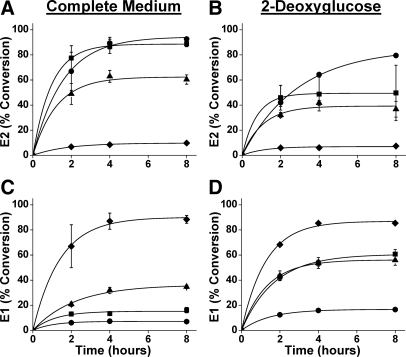
The 17βHSD1 mutations R38K, R38G, and R38D were generated to transition the positively charged guanidinium of R38 to a negatively charged carboxylate, and the consequences of these mutations on equilibrium steroid distributions was assessed in intact HEK-293 cells. Cells expressing wild-type 17βHSD1 metabolized 0.1 μm E1 greater than 90% to E2 as previously found in our hands (9). Mutations R38K, R38G, and R38D attenuated the reductive preference of 17βHSD1 in a graded fashion, to 88, 62, and 10%, respectively, and experiments using [3H]E2 as substrate gave similar final steroid distributions (Fig. 1, A and C, and top of Table 1). Glucose deprivation with 2-deoxyglucose, to lower the intracellular NADPH/NADP+ ratio, reduced the equilibrium E2 abundance, with the greatest effect for the R38K and R38G mutations (Fig. 1, B and D, and top of Table 1). Experiments starting with E1 and E2 gave similar final equilibrium steroid distributions.
Figure 1.
Metabolism of E1 and E2 in HEK-293 cells expressing human 17βHSD1 or R38 mutations. E1 (0.1 μm) conversion to E2 by cells expressing wild-type 17βHSD1 (circles) or mutations R38K (squares), R38G (triangles), and R38D (diamonds) in high-glucose medium (A) or 2-deoxyglucose (B) is shown as a function of time. Data points are means ± sd of three or more assays, and curves are best fits of the data to the function: ƒ(t) = A(1 − e−kt). E2 (0.1 μm) conversion to E1 by the same cells in high-glucose medium (C) or 2-deoxyglucose (D) is shown using the same symbols and fitting procedure. Equilibrium steroid distributions, derived by setting t = ∞, are summarized in Table 1 (r2 > 0.98 except R38K in B, r2 = 0.91).
Table 1.
Steroid distributions at pseudo-equilibrium in HEK-293 and CHOP cells
| Enzyme | E1→E2 (%)
|
E2→E1 (%)
|
||||||
|---|---|---|---|---|---|---|---|---|
| High glucose
|
2-Deoxyglucose
|
High glucose
|
2-Deoxyglucose
|
|||||
| E1 | E2 | E1 | E2 | E1 | E2 | E1 | E2 | |
| HEK-293 cells | ||||||||
| 17βHSD1 | 6 | 94 | 15 | 85 | 7 | 93 | 17 | 83 |
| R38K | 12 | 88 | 64 | 36 | 15 | 85 | 61 | 39 |
| R38G | 38 | 62 | 61 | 39 | 36 | 67 | 56 | 44 |
| R38D | 90 | 10 | 93 | 7 | 90 | 10 | 87 | 13 |
| 17βHSD2 | 96 | 4 | ||||||
| E116D | 95 | 5 | ||||||
| E116G | 93 | 7 | ||||||
| E116R | 96 | 4 | ||||||
| E116G+N117R | 96 | 4 | ||||||
| N-11βHSD2-C-17βHSD2 chimera | ||||||||
| E116G | 87 | 13 | ||||||
| CHOP cells | ||||||||
| 17βHSD1 | 10 | 90 | 32 | 68 | 17 | 83 | 68 | 32 |
| R38G | 43 | 57 | 59 | 41 | 45 | 55 | 61 | 39 |
| R38D | 82 | 18 | 88 | 12 | ||||
Experiments using wild-type 17βHSD1 and the informative mutations R38G and R38D were repeated with CHOP cells, which have higher endogenous oxidative (E2→E1) activity. Results with CHOP cells qualitatively confirmed conclusions using HEK-293 cells. The proportion of E2 in the steady state was highest for wild-type 17βHSD1, intermediate for mutation R38G, and lowest for R38D, with generally lower E2 than in HEK-293 cells, probably due to higher background activity (see supplemental Fig. 1 and bottom of Table 1). Glucose deprivation with 2-deoxyglucose further reduced the proportion of E2 as well (bottom of Table 1).
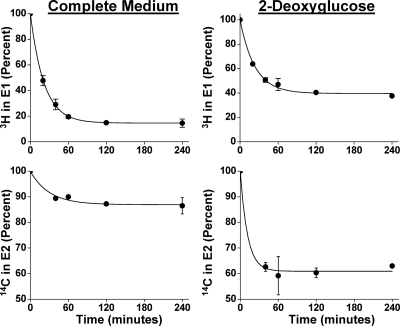
To demonstrate that the reaction remains reversible and rapid during glucose deprivation, double-isotope scrambling experiments were performed (9) to measure the rates of the forward and reverse reactions in the steady state. To assure similar enzyme expression, a stable HEK-293 cell line was generated as for AKR1C9 studies (15). This line established steroid distribution with 80% E2 in complete medium and 60% E2 in 2-deoxyglucose (not shown). The rates of E1 reduction were 28 and 36 pmol/min · well in complete medium and in 2-deoxyglucose, respectively, demonstrating that glucose deprivation does not reduce the intrinsic rate of the reaction in intact cells (Fig. 2). Based on curve-fitting statistics, the estimated rates of E2 oxidation are less accurate than rates of E1 reduction but nonetheless similar at 13 and 75 pmol/min · well in complete medium and in 2-deoxyglucose, respectively. These rate data confirm rapid, bidirectional steroid flux in both media.
Figure 2.
Double-isotope scrambling experiments with HEK-293 cell line stably expressing 17βHSD1. Rates are determined by fitting the mean data from three assays to the exponential decay curve ƒ(t) = Ae−kt + C (r2 > 0.99 for 3H data and >0.97 for 14C data) and determining the first derivatives at t = 0 [ƒ′ (0) = A × K; see supplemental Methods].
Expression, purification, and kinetic constants for 17βHSD1 and R38 mutations
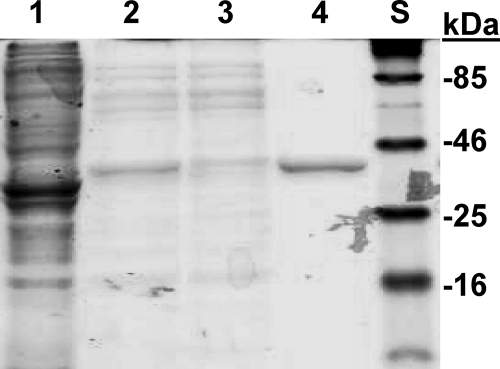
Expression of 17βHSD1 in E. coli and purification using affinity chromatography similar to the procedure for purification from placenta (23) yields up to 11 mg homogeneous protein per liter of culture (Fig. 3), about half the yield obtained previously for wild-type 17βHSD1 using Sf9 cells (24) (see supplemental Table 3), with yields for the R38 mutations 30–50% lower. To use the same assay for all enzymes, the catalytically relevant preferences for NAD(H) and NADP(H) were estimated from the apparent Km values for NAD+ and NADP+ with E2 oxidation. Consistent with previous reports using 17βHSD1 purified from placenta or expressed in Sf9 cells (10), the wild-type enzyme shows apparent Km values of 6 and 48 μm for NADP+ and NAD+, respectively, reflecting a preference for using NADP+ over NAD+. Mutations R38G and R38D raise the apparent Km values for both NAD+ and NADP+, and cofactor preference for mutation R38D is reversed to favor NAD+ (Table 2; see also supplemental Fig. 2). The progressive shifts in equilibrium steroid distributions observed for mutations R38G and R38D correlate with increases in the apparent Km for NADP+. This comparison of data with intact cells and kinetic parameters obtained with purified enzyme explains how the R38 mutations translate changes in cofactor affinities to changes in equilibrium steroid proportions.
Figure 3.
Purification of 17βHSD1 from E. coli. The lanes of a 12% SDS-PAGE gel were stained with Coomassie blue: 1, crude homogenate; 2, after heat treatment; 3, reactive red agarose flow-through; 4, final purified protein; S, standards (molecular weights shown at right).
Table 2.
Kinetic constants [apparent Km for cofactors (micromolar) and Vmax (nanomoles per minute per milligram)] during E2 oxidation for 17βHSD1, 17βHSD2, and mutations
| Enzyme | NAD+
|
NADP+
|
Km NAD+/Km NADP+ | ||||
|---|---|---|---|---|---|---|---|
| Km | Vmax | Vmax/Km | Km | Vmax | Vmax/Km | ||
| 17βHSD1 | 48 | 320 | 6.7 | 6 | 145 | 24 | 8 |
| R38G | 170 | 265 | 1.6 | 25 | 254 | 10 | 7 |
| R38D | 160 | 135 | 0.84 | 415 | 112 | 0.28 | 0.4 |
| 17βHSD2 | 31 | 6 | 0.19 | 17000 | 7 | 0.0004 | 0.002 |
| E116G | 21 | 51 | 2.4 | 7000 | 5 | 0.0007 | 0.003 |
| E116R | 36 | 36 | 1 | 8100 | 2 | 0.0002 | 0.004 |
Equilibrium steroid distributions for wild-type 17βHSD2 and E116 mutations in HEK-293 cells
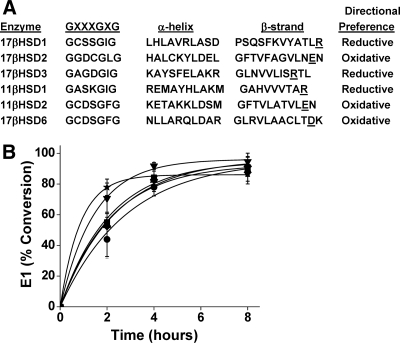
Based on alignments of multiple HSDs, residue E116 of 17βHSD2 is predicted to occupy a similar position as R38 in 17βHSD1 and to interact with the 2′-position of NAD(P)(H) (Fig. 4). The negatively charged carboxylate of E116 might repel the 2′-phosphate of NADP(H) and thus disfavor its binding. Given our results with 17βHSD1, we reasoned that neutralization or reversal of this negative charge would convert 17βHSD2 to a reductive enzyme in intact cells. Consequently, 17βHSD2 mutations E116D, E116G, and E116R were generated to move, neutralize, or reverse the negative charge, respectively. In contrast to expectations, HEK-293 cells expressing all 17βHSD2 mutations oxidized more than 90% of E2 to E1, equivalent to the wild-type enzyme (Fig. 4 and middle of Table 1). Attempts to reduce the NAD+/NADH gradient by adding ethanol or lactate to the medium did not change the equilibrium steroid distributions (not shown).
Figure 4.
Metabolism of E2 to E1 in HEK-293 cells expressing human 17βHSD2 or E116 mutations. A, Alignment of short-chain dehydrogenases identifies residue E116 is the charged residue corresponding to R38 of 17βHSD1. B, E1 (0.1 μm) conversion to E2 by cells expressing wild-type (circles) or mutations E116D (squares), E116G (triangles), and E116R (inverted triangles), E116G+N117R (diamonds), and chimera N-11βHSD2-C-17βHSD2-E116G (stars) in complete medium is shown as a function of time. Data points are means ± sd of two to five experiments, and curves are best fits of the data to the function: ƒ(t) = A(1 − e−kt). Equilibrium steroid distributions, derived by setting t = ∞, are summarized in Table 1 (r2 > 0.99 for all curves).
We considered two possible explanations for the failure of these mutations to alter the equilibrium steroid distributions. First, the precise positioning of the positive charge might be necessary to favor NADP(H) binding, and previous studies suggest that the conserved arginine of the reductive HSDs may be positioned one residue further from the N terminus than the aspartic or glutamic acids in the oxidative HSDs (25). Second, 17βHSD2 is located in the lumen of the endoplasmic reticulum, which is a strongly oxidative compartment with lower NADPH/NADP+ gradients compared with the cytoplasm (26,27,28). Therefore, we first constructed mutation E116G+N117R to reposition the arginine, but this mutation also retained strong oxidative preference in HEK-293 cells (Fig. 4 and middle of Table 1). Second, we constructed chimera N-11βHSD2-C-17βHSD2-E116G, containing the N-terminal 80 residues of 11βHSD2 and the C-terminal 307 residues of 17βHSD2 mutation E116G, overlapping at the proline-valine motif eight residues N-terminal to the GXXXGXG feature common to both enzymes. This chimera deletes the KYKK endoplasmic reticulum targeting signal at residues 25–28 of 17βHSD2 mutation E116G to reposition the catalytic domain facing the cytoplasm. The oxidative preference of this chimera was minimally reduced from the other 17βHSD2 mutations, extrapolating to 87% E1 (Fig. 4 and middle of Table 1).
Expression of wild-type 17βHSD2 and E116 mutations in yeast and enzyme assays
To quantitatively explain the inability of E116 mutations to alter the directional preference of 17βHSD2, we expressed these cDNAs in yeast and measured the apparent Km values for NADP+ and NAD+ using yeast microsomes. Wild-type 17βHSD2 showed a 500-fold lower apparent Km for NAD+ than for NADP+, similar to the preference observed with purified 17βHSD2 in proteoliposomes (11). Mutations E116G and E116R lowered the apparent Km for NADP+ only slightly, remaining more than 200-fold higher than the apparent Km for NAD+ (Table 2; see also supplemental Fig. 3). Microsomes containing E116 mutations showed a higher Vmax for incubations with NAD+, but differences in enzyme expression could not be excluded. These data show that the strong oxidative preference of 17βHSD2 in intact cells derives primarily from its very high apparent Km value with NADP+, in the millimolar range, but that E116 is not the sole contributor to its cofactor preference.
Discussion
The principal finding of this study is that the strong directional preference of 17βHSD1 for E1 reduction in intact cells is vulnerable to attenuation both by mutations that impair NADPH binding and by glucose deprivation, which partially reduces NADPH regeneration (29). In contrast, we were unable to alter the strong preference of 17βHSD2 for E2 oxidation despite single and double mutations as well as chimerism. Although alignments have suggested that one key residue in the cofactor-binding site of the SDR-type HSDs confers cofactor selectivity (25,30) and thus directional preferences in intact cells (12,13), our data demonstrate that the cofactor-binding site of 17βHSD2 incorporates additional structural features that afford poor affinity for NADP(H) yet maintain high affinity for NAD(H).
In comparing our results for 17βHSD1 herein with similar experiments using AKR1C9 (15), the major difference is the facile attenuation of the strong preference for E1 reduction by 17βHSD1 in intact cells with mutagenesis or glucose deprivation. Whereas glucose deprivation and neutralization of R38 with mutation R38G decreased the fraction of E2 at pseudo-equilibrium in intact cells from 95 to 85 or 62%, respectively (Fig. 1, A and C vs. B and D, and Table 1), similar maneuvers minimally lowered the strong preference of AKR1C9 for dihydrotestosterone reduction (15). These differences can be explained by comparing the relative affinities of the two enzymes for NAD(P)(H). Based on Km or Kd values, AKR1C9 demonstrates a large NADP(H) preference, up to 1000-fold over NAD(H) (17), whereas the same measure of cofactor preference is only 8 for 17βHSD1 (Table 2). AKR1C9 mutation R276M impairs NADP(H) binding over 10-fold (17), but this change is insufficient to reverse the NADP(H) preference or to significantly attenuate the strong directional preference for dihydrotestosterone reduction in intact cells, unless glucose deprivation concomitantly depletes the NADPH/NADP+ gradient (15). This analysis suggests that R276 contributes more to the tenacious NADP(H) binding of AKR1C9 than R38 does to the weaker NADP(H) binding of 17βHSD1. Sequence alignments and structures provide general principles of HSD cofactor utilization (25,30), but the fidelity of directional preferences catalyzed by these HSDs in intact cells appears to vary considerably among specific enzymes.
Our results with 17βHSD2 cannot be directly compared with related studies of 11βHSD2, in which mutations of the corresponding E115 were analyzed. First, these studies of 11βHSD2 used cell homogenates and did not determine directional preferences in intact cells (20,31). Second, these investigators assayed hydroxysteroid oxidation at a fixed concentration of cofactor (0.4 mm) and showed that mutations E115S and E115T reduced the ratio of Km values for steroid substrate using NADP+ vs. NAD+ 10-fold (20), whereas mutations E115Q and E115K reduced this ratio only 2-fold (31). The double mutation E115S combined with D91S in the GXXXGXG motif (GGDCGLG, with D91 underlined) had equivalent Vmax and Km and values for steroid in the presence of saturating NAD+ or NADP+; however, Km values for cofactors themselves were not measured.
In addition, we did not mutate residues in and near the GXXXGXG motif, which also interact with cofactors, as was done with 11βHSD2 (20). The crystal structure (PDB accession codes 3CXR and 3CXT) of NADP(H) binding to Streptococcus suis gluconate 5-dehydrogenase (32) illustrates the contribution of residues in the GXXXGXG motif to cofactor binding. In this structure, a tyrosine (underlined in GASYGIG) primarily coordinates with the 2′-phosphate of NADP+, without contribution from residues in the corresponding loop that contains R38 in 17βHSD1 (see supplemental Discussion, supplemental Table 4, and supplemental Fig. 4). This result illustrates the complexity of cofactor binding in HSDs and emphasizes that additional structural data are necessary to understand the cofactor preference of oxidative HSDs such as 11βHSD2 and 17βHSD2. The crystal structure of 17βHSD10 (PDB accession code 1U7T), a human SDR-type HSD with strong oxidative preference, is available but does not contain bound cofactor. Our results with 17βHSD1 mutations R38G and R38D indicate that a 1000-fold increase in affinity for NADP(H) would be required for a 17βHSD2 mutation to favor E1 reduction in intact cells.
The main limitation of this work is that intracellular cofactor concentrations could not be measured directly but were based on previous studies (29). Techniques such as two-photon excitation microscopy have been developed to assay nicotinamide cofactors in intact cells (33), but these methods measure the sum of oxidized and reduced cofactors and require additional measurements to correct for bound and free cofactors (34). In addition, we did not prove that the catalytic domain of chimera N-11βHSD2-C-17βHSD2-E116G was exposed to the cytosol given that a second YKKK motif in the C terminus might still target the enzyme to the endoplasmic reticulum lumen (7). Given the apparent Km values of wild-type 17βHSD2 and the mutations described in this study (Table 2), it is unlikely that this enzyme would show a reductive preference in intact cells regardless of subcellular localization. We used apparent Km rather than Kd to relate a kinetic constant using in vitro assays to equilibrium steroid distributions in intact cells. Because Km qualitatively reflects enzyme saturation relevant to catalysis, these values are reasonable estimates of relative cofactor affinities for this purpose.
Potent endogenous androgens and estrogens are efficiently inactivated in peripheral tissues containing 17βHSD2 (1,7), and our data suggest that this process is unlikely to be influenced by changes in cellular redox state or fuel supply. In contrast, the reduction of E1 to E2 in intact cells catalyzed by 17βHSD1 is vulnerable to attenuation by mutation of R38 and strongly dependent on the cytoplasmic NADPH/NADP+ ratio, which is depleted by reduced glucose supply and diabetes (35) but may be increased by hypoxia (36). Importantly, the impaired E1 reduction by 17βHSD1 in intact cells during NADPH depletion cannot be overcome by increased enzyme expression, because the reaction reaches a reversible pseudo-equilibrium in the steady state (9,12,13). Consequently, ovarian E2 synthesis might be restricted in states of insulin resistance such as polycystic ovary syndrome (37) independent of 17βHSD1 abundance.
In addition, 17βHSD1 is the most important enzyme for local E2 production in breast cancer and remains an important drug target in this disease (38). The high activity of the pentose-phosphate pathway in metastatic breast cancers (39) might maximize E2 synthesis by 17βHSD1 in these tumors, and therapeutic approaches that inhibit this route of glucose metabolism (40) might reduce E2 production as well. Intracellular redox state and intracellular nicotinamide cofactor gradients also influence the activity of transcription factors such as carboxyl terminal binding protein (41), illustrating how glucose metabolism, steroid potency, and transcription factor activity share complex relationships in health and disease (13). The concepts derived from studies of HSDs biochemistry, in turn, might lead to new approaches to the treatment of steroid-dependent diseases by exploiting the interdependence of these pathways.
Supplementary Material
Acknowledgments
We thank Drs. Stefan Andersson and Anil Agarwal for helpful discussions, and we thank Arhana Chattopadhyay for assistance with 17βHSD1 expression and purification.
Footnotes
This work was supported by Grant 6-FY06-620 from the March of Dimes Foundation (to R.J.A.). D.P.S. was a Medical Student Fellow of the Howard Hughes Medical Institute, S.R. was a University of Texas Southwestern medical student research fellow, and S.A. was a University of Texas Southwestern Quantitative and Physical Sciences-Summer Undergraduate Research Fellowship Program student.
Disclosure Summary: The authors have nothing to disclose.
First Published Online June 25, 2009
Abbreviations: AKR, Aldo-keto reductase; CHOP, Chinese hamster ovary cells stably transfected with the polyoma large T antigen; E1, estrone; E2, estradiol; HSD, hydroxysteroid dehydrogenase; NAD(P)(H), nicotine adenine dinucleotide (phosphate), (reduced form); SDR, short-chain dehydrogenase/reductase.
References
- Ghayee HK, Auchus RJ 2007 Basic concepts and recent developments in human steroid hormone biosynthesis. Rev Endocr Metab Disord 8:289–300 [DOI] [PubMed] [Google Scholar]
- Labrie F, Luu-The V, Lin SX, Labrie C, Simard J, Breton R, Bélanger A 1997 The key role of 17β-hydroxysteroid dehydrogenases in sex steroid biology. Steroids 62:148–158 [DOI] [PubMed] [Google Scholar]
- Peltoketo H, Isomaa V, Mäentausta O, Vihko R 1988 Complete amino acid sequence of human placental 17β-hydroxysteroid dehydrogenase deduced from cDNA. FEBS Lett 239:73–77 [DOI] [PubMed] [Google Scholar]
- Gast MJ, Sims HF, Murdock GL, Gast PM, Strauss AW 1989 Isolation and sequencing of a complementary deoxyribonucleic acid clone encoding human placental 17β-estradiol dehydrogenase: identification of the putative cofactor binding site. Am J Obstet Gynecol 161:1726–1731 [DOI] [PubMed] [Google Scholar]
- Luu The V, Labrie C, Zhao HF, Couët J, Lachance Y, Simard J, Leblanc G, Côté J, Bérubé D, Gagné R 1989 Characterization of cDNAs for human estradiol 17β-dehydrogenase and assignment of the gene to chromosome 17: evidence of two mRNA species with distinct 5′ termini in human placenta. Mol Endocrinol 3:1301–1309 [DOI] [PubMed] [Google Scholar]
- Luu-The V, Zhang Y, Poirier D, Labrie F 1995 Characteristics of human types 1, 2 and 3 17β-hydroxysteroid dehydrogenase activities: oxidation/reduction and inhibition. J Steroid Biochem Mol Biol 55:581–587 [DOI] [PubMed] [Google Scholar]
- Wu L, Einstein M, Geissler WM, Chan HK, Elliston KO, Andersson S 1993 Expression cloning and characterization of human 17β-hydroxysteroid dehydrogenase type 2, a microsomal enzyme possessing 20α-hydroxysteroid dehydrogenase activity. J Biol Chem 268:12964–12969 [PubMed] [Google Scholar]
- Jörnvall H, Persson B, Krook M, Atrian S, Gonzàlez-Duarte R, Jeffery J, Ghosh D 1995 Short-chain dehydrogenases/reductases (SDR). Biochemistry 34:6003–6013 [DOI] [PubMed] [Google Scholar]
- Khan N, Sharma KK, Andersson S, Auchus RJ 2004 Human 17β-hydroxysteroid dehydrogenases types 1, 2, and 3 catalyze bi-directional equilibrium reactions, rather than unidirectional metabolism, in HEK-293 cells. Arch Biochem Biophys 429:50–59 [DOI] [PubMed] [Google Scholar]
- Huang YW, Pineau I, Chang HJ, Azzi A, Bellemare V, Laberge S, Lin SX 2001 Critical residues for the specificity of cofactors and substrates in human estrogenic 17β-hydroxysteroid dehydrogenase 1: variants designed from the three-dimensional structure of the enzyme. Mol Endocrinol 15:2010–2020 [DOI] [PubMed] [Google Scholar]
- Lu ML, Huang YW, Lin SX 2002 Purification, reconstitution, and steady-state kinetics of the trans-membrane 17β-hydroxysteroid dehydrogenase 2. J Biol Chem 277:22123–22130 [DOI] [PubMed] [Google Scholar]
- Sherbet DP, Papari-Zareei M, Khan N, Sharma KK, Brandmaier A, Rambally S, Chattopadhyay A, Andersson S, Agarwal AK, Auchus RJ 2007 Cofactors, redox state, and directional preferences of hydroxysteroid dehydrogenases. Mol Cell Endocrinol 265–266:83–88 [DOI] [PubMed] [Google Scholar]
- Agarwal AK, Auchus RJ 2005 Cellular redox state regulates hydroxysteroid dehydrogenase activity and intracellular hormone potency. Endocrinology 146:2531–2538 [DOI] [PubMed] [Google Scholar]
- Cooper WC, Jin Y, Penning TM 2007 Elucidation of a complete kinetic mechanism for a mammalian hydroxysteroid dehydrogenase (HSD) and identification of all enzyme forms on the reaction coordinate: the example of rat liver 3α-HSD (AKR1C9). J Biol Chem 282:33484–33493 [DOI] [PubMed] [Google Scholar]
- Papari-Zareei M, Brandmaier A, Auchus RJ 2006 Arginine 276 controls the directional preference of AKR1C9 (rat liver 3α-hydroxysteroid dehydrogenase) in human embryonic kidney 293 cells. Endocrinology 147:100–107 [DOI] [PubMed] [Google Scholar]
- Hoog SS, Pawlowski JE, Alzari PM, Penning TM, Lewis M 1994 Three-dimensional structure of rat liver 3α-hydroxysteroid/dihydrodiol dehydrogenase: a member of the aldo-keto reductase superfamily. Proc Natl Acad Sci USA 91:2517–2521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratnam K, Ma H, Penning TM 1999 The arginine 276 anchor for NADP(H) dictates fluorescence kinetic transients in 3α-hydroxysteroid dehydrogenase, a representative aldo-keto reductase. Biochemistry 38:7856–7864 [DOI] [PubMed] [Google Scholar]
- Heredia VV, Penning TM 2004 Dissection of the physiological interconversion of 5α-DHT and 3α-diol by rat 3α-HSD via transient kinetics shows that the chemical step is rate-determining: effect of mutating cofactor and substrate-binding pocket residues on catalysis. Biochemistry 43:12028–12037 [DOI] [PubMed] [Google Scholar]
- Ghosh D, Pletnev VZ, Zhu DW, Wawrzak Z, Duax WL, Pangborn W, Labrie F, Lin SX 1995 Structure of human estrogenic 17β-hydroxysteroid dehydrogenase at 2.20 Å resolution. Structure 3:503–513 [DOI] [PubMed] [Google Scholar]
- Arnold P, Tam S, Yan L, Baker ME, Frey FJ, Odermatt A 2003 Glutamate-115 renders specificity of human 11β-hydroxysteroid dehydrogenase type 2 for the cofactor NAD+. Mol Cell Endocrinol 201:177–187 [DOI] [PubMed] [Google Scholar]
- Auchus RJ, Lee TC, Miller WL 1998 Cytochrome b5 augments the 17,20 lyase activity of human P450c17 without direct electron transfer. J Biol Chem 273:3158–3165 [DOI] [PubMed] [Google Scholar]
- Sherbet DP, Tiosano D, Kwist KM, Hochberg Z, Auchus RJ 2003 CYP17 mutation E305G causes isolated 17,20-lyase deficiency by selectively altering substrate binding. J Biol Chem 278:48563–48569 [DOI] [PubMed] [Google Scholar]
- Auchus RJ, Covey DF 1986 Mechanism-based inactivation of 17β,20α-hydroxysteroid dehydrogenase by an acetylenic secoestradiol. Biochemistry 25:7295–7300 [DOI] [PubMed] [Google Scholar]
- Breton R, Yang F, Jin JZ, Li B, Labrie F, Lin SX 1994 Human 17β-hydroxysteroid dehydrogenase: overproduction using a baculovirus expression system and characterization. J Steroid Biochem Mol Biol 50:275–282 [DOI] [PubMed] [Google Scholar]
- Duax WL, Pletnev V, Addlagatta A, Bruenn J, Weeks CM 2003 Rational proteomics. I. Fingerprint identification and cofactor specificity in the short-chain oxidoreductase (SCOR) enzyme family. Proteins 53:931–943 [DOI] [PubMed] [Google Scholar]
- Braakman I, Helenius J, Helenius A 1992 Manipulating disulfide bond formation and protein folding in the endoplasmic reticulum. EMBO J 11:1717–1722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bublitz C, Lawler CA 1987 The levels of nicotinamide nucleotides in liver microsomes and their possible significance to the function of hexose phosphate dehydrogenase. Biochem J 245:263–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang C, Sinskey AJ, Lodish HF 1992 Oxidized redox state of glutathione in the endoplasmic reticulum. Science 257:1496-1502 [DOI] [PubMed] [Google Scholar]
- Merker MP, Bongard RD, Kettenhofen NJ, Okamoto Y, Dawson CA 2002 Intracellular redox status affects transplasma membrane electron transport in pulmonary arterial endothelial cells. Am J Physiol Lung Cell Mol Physiol 282:L36–L43 [DOI] [PubMed] [Google Scholar]
- Pletnev VZ, Weeks CM, Duax WL 2004 Rational proteomics. II. Electrostatic nature of cofactor preference in the short-chain oxidoreductase (SCOR) enzyme family. Proteins 57:294–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odermatt A, Dick B, Arnold P, Zaehner T, Plueschke V, Deregibus MN, Repetto H, Frey BM, Frey FJ, Ferrari P 2001 A mutation in the cofactor-binding domain of 11β-hydroxysteroid dehydrogenase type 2 associated with mineralocorticoid hypertension. J Clin Endocrinol Metab 86:1247–1252 [DOI] [PubMed] [Google Scholar]
- Zhang Q, Peng H, Gao F, Liu Y, Cheng H, Thompson J, Gao GF 2009 Structural insight into the catalytic mechanism of gluconate 5-dehydrogenase from Streptococcus suis: crystal structures of the substrate-free and quaternary complex enzymes. Protein Sci 18:294–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson GH, Knobel SM, Arkhammar P, Thastrup O, Piston DW 2000 Separation of the glucose-stimulated cytoplasmic and mitochondrial NAD(P)H responses in pancreatic islet β-cells. Proc Natl Acad Sci USA 97:5203–5207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Piston DW, Goodman RH 2002 Regulation of corepressor function by nuclear NADH. Science 295:1895–1897 [DOI] [PubMed] [Google Scholar]
- Díaz-Flores M, Ibáñez-Hernández MA, Galván RE, Gutiérrez M, Durán-Reyes G, Medina-Navarro R, Pascoe-Lira D, Ortega-Camarillo C, Vilar-Rojas C, Cruz M, Baiza-Gutman LA 2006 Glucose-6-phosphate dehydrogenase activity and NADPH/NADP+ ratio in liver and pancreas are dependent on the severity of hyperglycemia in rat. Life Sci 78:2601–2607 [DOI] [PubMed] [Google Scholar]
- Gupte SA, Okada T, McMurtry IF, Oka M 2006 Role of pentose phosphate pathway-derived NADPH in hypoxic pulmonary vasoconstriction. Pulm Pharmacol Ther 19:303–309 [DOI] [PubMed] [Google Scholar]
- Dunaif A, Segal KR, Futterweit W, Dobrjansky A 1989 Profound peripheral insulin resistance, independent of obesity, in the polycystic ovary syndrome. Diabetes 38:1165–1174 [DOI] [PubMed] [Google Scholar]
- Day JM, Foster PA, Tutill HJ, Parsons MF, Newman SP, Chander SK, Allan GM, Lawrence HR, Vicker N, Potter BV, Reed MJ, Purohit A 2008 17β-Hydroxysteroid dehydrogenase type 1, and not type 12, is a target for endocrine therapy of hormone-dependent breast cancer. Int J Cancer 122:1931–1940 [DOI] [PubMed] [Google Scholar]
- Chen EI, Hewel J, Krueger JS, Tiraby C, Weber MR, Kralli A, Becker K, Yates 3rd JR, Felding-Habermann B 2007 Adaptation of energy metabolism in breast cancer brain metastases. Cancer Res 67:1472–1486 [DOI] [PubMed] [Google Scholar]
- Meadows AL, Kong B, Berdichevsky M, Roy S, Rosiva R, Blanch HW, Clark DS 2008 Metabolic and morphological differences between rapidly proliferating cancerous and normal breast epithelial cells. Biotechnol Prog 24:334–341 [DOI] [PubMed] [Google Scholar]
- Fjeld CC, Birdsong WT, Goodman RH 2003 Differential binding of NAD+ and NADH allows the transcriptional corepressor carboxyl-terminal binding protein to serve as a metabolic sensor. Proc Natl Acad Sci USA 100:9202–9207 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.