Abstract
Mouse models have been developed to simulate several relevant human traits associated with alcohol use and dependence. However, the neurophysiological substrates regulating these traits remain to be completely elucidated. We have previously demonstrated that differences in the event-related potential (ERP) responses can be found that distinguish high-alcohol preferring from low alcohol preferring mice that resemble difference seen in human studies of individuals with high and low risk for alcohol dependence. Recently, evidence of genes that affect event-related oscillations (EROs) and the risk for alcohol dependence has emerged, however, to date EROs have not been evaluated in genetic mouse models of high and low alcohol preference. Therefore, the objective of the present study was to characterize EROs in mouse models of high (B6 and HAP-1 mice) and low (D2 and LAP-1 mice) alcohol preference. A time-frequency representation method was used to determine delta, theta and alpha/beta ERO energy and the degree of phase variation in these mouse models. The present results suggest that the decrease in P3 amplitudes previously shown in B6 mice, compared to D2 mice, is related to reductions in evoked delta ERO energy and delta and theta phase locking. In contrast, the increase in P1 amplitudes reported in HAP-1 mice, compared to LAP mice, are associated with increases in evoked theta ERO energy. These studies suggest that differences in delta and theta ERO measures in mice mirror changes observed between groups at high- and low-risk for alcoholism where changes in EROs were found to be more significant than group differences in P3 amplitudes, further suggesting that ERO measures are more stable endophenotypes in the study of alcohol dependence. Further studies are needed to determine the relationship between expression of these neurophysiological endophenotypes and the genetic profile of these mouse models.
Keywords: HAP-1 mice, LAP-1 mice, C57BL/6 mice, DBA/2 mice, EEG, neuroendophenotype
Animal models of alcoholism have an advantage in that they allow for the control of a number of characteristics of the animal’s genetic background, prior drug exposure, and to a large extent, the environment (for review, see Rodd et al., 2004; Lovinger and Crabbe, 2005; Bell et al., 2006; Bennett et al., 2006; Sanchis-Segura and Spanagel, 2006). Notably, genetic selection studies have established high drinking lines of mice and rats (see Bell et al., 2006; Green and Grahame, 2008). Mice have been increasingly used to examine the neurobiology of alcohol dependence and comparisons between several inbred mouse strains have shown significant differences in commonly studied phenotypes related to alcohol dependence (for review, see Bennett et al., 2006; Crabbe, 2008). For instance, the inbred mouse strains C57BL/6 (B6) and DBA/2J (D2) exhibit significant differences in their susceptibility to alcohol withdrawal (Buck et al., 1997; Metten and Crabbe, 2005). B6 mice have shown a low level of withdrawal severity, whereas D2 mice exhibited extreme withdrawal severity. Moreover, voluntary alcohol intake differed significantly between B6 and D2 mice. While B6 mice showed high alcohol preference (~16 g/kg/day), D2 mice exhibited low alcohol preference (~0.2 g/kg/day) (Phillips et al., 1994; Rodriguez et al., 1995; Ruf et al., 2004).
The genetics of alcohol consumption have also been studied in mice selectively bred for high and low alcohol preference. After 10 breeding generations, high alcohol preference (HAP-1) mice consumed greater than 10 g/kg alcohol/day and low alcohol preference (LAP-1) mice consumed little more than 2 g/kg alcohol/day (Grahame et al., 1999b). Consistent with findings obtained in low-alcohol preferring D2 mice (Ruf et al., 2004), LAP-1 mice showed greater alcohol withdrawal severity than HAP-1 mice following acute alcohol exposure (Chester et al., 2003).
The study of neurophysiological endophenotypes in genetic mouse models with well-differentiated alcohol-related phenotypes could be an additional tool for identifying susceptibility genes for alcohol dependence. There is considerable support to develop and study mice models that simulate several relevant human endophenotypic behavioral traits associated with alcohol-related phenotypes (Bennett et al., 2006); however, the neurophysiological substrates regulating these traits remain largely unknown. We have previously characterized the electrophysiological profile of mice with high and low alcohol preference [B6 vs. D2 mice (Ehlers and Somes, 2002) and HAP-1 vs. LAP-1 mice (Slawecki et al., 2003)]. These initial electrophysiological studies provided two basic findings. Firstly, B6 mice were found to have significantly lower amplitude of the N1a, N1b and P3 event-related potential (ERP) components, when compared to the D2 strain (Ehlers and Somes, 2002). This finding is similar to what has been reported for human subjects at differing risk for alcohol dependence (for review, see Porjesz et al., 2005). Secondly, it was found that that HAP-1 mice have significantly larger amplitude of the P1 ERP component, when compared to LAP-1 mice (Slawecki et al., 2003), a finding different from that reported in the human literature. These findings suggest that genetic mouse models of increased alcohol consumption exhibit different neurophysiological profiles.
In recent years, there has been considerable debate as to whether the neural basis of ERP generation originate from an additive, evoked activation of neural assemblies independent of ongoing EEG, or generated by phase resetting of ongoing EEG oscillations in response to sensory input (for review, see Sauseng et al., 2007; Rangaswamy and Porjesz, 2008). There is evidence to suggest that both models may play a role in the basis of the ERP. In fact, it has been proposed that some ERP components, including the P3 component, arise from superimposed event-related oscillations (EROs) induced by sensory or cognitive processes that influence the dynamics of EEG rhythms (e.g. Yordanova and Kolev, 1996; Karakas et al., 2000; Demiralp et al., 2001). EROs are estimated by a decomposition of the EEG signal into phase and magnitude information for a range of frequencies and then changes in those frequencies are characterized over a millisecond time scale with respect to task events. EROs have been demonstrated to be sensitive measures of both normal and abnormal cognitive functioning in humans (see Bressler and Freeman, 1980; Klimesch et al., 1994, 1997; Schurmann et al., 1997, 2001; Doppelmayer et al., 1998; Gevins et al., 1998; Basar et al., 1999, 2001a, 2001b, 2001c; Kopell et al., 2000). Additionally, these oscillations have been linked to several relevant genes associated with alcohol dependence phenotypes (Edenberg et al., 2004, Jones et al., 2004, 2006b; Porjesz et al., 2005; Begleiter and Porjesz, 2006; Rangaswamy et al., 2007a, 2007b).
Studies have demonstrated that delta and theta EROs are the primary contributors to the P3 ERP component (Stampfer and Basar, 1985; Basar-Eroglu et al., 1992; Yordanova and Kolev, 1996; Basar et al., 1999; Karakas et al., 2000; Demiralp et al., 2001; Schurmann et al., 2001). More recently, it was demonstrated that adolescent offspring of alcohol dependent individuals (high-risk group) showed a reduction in parietal P3 amplitude and in cortical delta and theta ERO power during the temporal window of the P3 response, compared to age-matched controls (low-risk group) (Rangaswamy et al., 2007a). This study concluded that since differences in delta and theta ERO energy between high- and low-risk groups are more significant than group differences in P3 amplitudes, ERO measures are more stable endophenotypes in the study of alcohol dependence (Rangaswamy et al., 2007a).
Reductions in P3 amplitude have been related not only to decreased cortical ERO energy but also to higher phase variability and weaker phase locking. It has been shown that the reduction of P3 amplitude during retrieval of a working memory task is associated to decreased delta ERO power and increased phase variability (Schack and Klimesch, 2002). In addition to their role generating the P3 ERP component, evoked oscillations have been also shown to play a role in the generation of other ERP components, including the P1-N1 complex. There is evidence to suggest that alpha and theta evoked power and phase locking plays an important role in the generation of the P1-N1 complex (Klimesch et al., 2004). However, whether differences in ERO energy and phase variability play a role in the different neurophysiological profiles previously reported in mouse models of increased alcohol consumption remains unclear.
In view of the evidence from human studies of genes linked to EROs (Porjesz et al., 2005), characterizing the relationship between an alcohol preference phenotype and changes in EROs in genetic mouse models could provide valuable information of specific changes in brain oscillatory activity that have been shown to be under genetic control, however, to date EROs have not been evaluated in these models. The purpose of the present study was to extend our initial analyses of neurophysiological endophenotypes in these genetic mouse models of high (B6 and HAP-1) and low (D2 and LAP-1) alcohol preference in the alcohol-naive state by determining the relationship between their alcohol preference phenotype and cortical oscillatory activity in response to an auditory oddball paradigm. In this study, we investigated cortical oscillatory activity in the delta, theta, and alpha/beta frequency ranges, within the temporal windows of the P1, N1a, N1b and P3 responses, which we previously showed to distinguish these models in the alcohol-naive state (Ehlers and Somes, 2002, Slawecki et al., 2003). ERO and PLI analyses were accomplished from the same datasets that were used to generate the ERP data reported in previous publications (Ehlers and Somes, 2002, Slawecki et al., 2003). We hypothesize that differences in P3 and N1 amplitudes previously reported in B6 and D2 mice in the alcohol-naive state (Ehlers and Somes, 2002) are associated to differences in delta and theta ERO energy and theta and alpha/beta ERO energy, respectively. In contrast, differences in P1 amplitude previously reported in HAP-1 and LAP-1 mice in the alcohol-naive state (Slawecki et al., 2003) are associated to differences in alpha/beta ERO energy.
EXPERIMENTAL PROCEDURES
Animals
In the first study, 66 male mice, 29 of the C57BL/6 strain (B6) and 37 of DBA/2 strain (D2) were used. Mice were obtained from The Scripps Research Institute breeding facility, weighing between 19 and 30 g. In the second study, 52 male mice, 15 HAP-1 mice, 18 LAP-1 mice and 19 HS/Ibg mice were used. HAP-1, LAP-1 and HS/Ibg mice were obtained from Indiana University and were from the 20th breeding generation of the first replicate line of HAP and LAP mice, as previously described (Slawecki et al., 2003). HS/Ibg mice were obtained from the Institute for Behavioral Genetics at the University of Colorado and served as the progenitor stock (Grahame et al., 1999b). They were from the 67th breeding generation. All mice were housed 3–4 per cage in controlled temperature and lighting conditions, with food and water ad libitum for the duration of the study. Detailed description of the environmental conditions of mice from both studies can be found elsewhere (Ehlers and Somes, 2002, Slawecki et al., 2003). The work described herein adheres to the guidelines stipulated in the NIH Guide for the Care and Use of Laboratory Animals (NIH publication No. 80–23, revised 1996) and was reviewed and approved by The Scripps Research Institute’s Institutional Animal Care and Use Committee.
Surgical procedure
Mice were implanted with stainless steel screws (Small Parts, Miami Lakes, FL; size: 000 × 120 × 1/16th) in three sites in the calvarium under halothane anesthesia (1–2% in O2). Screws were placed in the skull overlying the frontal cortex (anterioposterior (AP): + 1.7 mm, mediolateral (ML): ± 1.5 mm; area M1, primary motor) and the parietal cortex (AP: −2.0 mm, ML: ± 2.0 mm; area PPtA, posterior parietal association area) (Franklin and Paxinos, 1997). A third screw, which was grounded during recording, was placed posterior to lambda in the skull overlying the cerebellum. After surgery mice were then returned to their home cages and allowed 3–9 days to recover before the beginning of electrophysiological studies. Further details about the surgical procedures have been described previously (Ehlers and Somes, 2002, Slawecki et al., 2003).
Electrophysiological recording procedures
Electroencephalograms (EEG) and ERPs were recorded using Microtech™ recording cables. ERPs were collected from two monopolar leads (frontal and parietal cortices) referenced to a grounded electrode overlying the cerebellum (i.e., frontal cortex-ground, parietal cortex-ground). EEG signals were recorded on a polygraph, with a band pass of 0.3–35 Hz with a 60-Hz notch filter in. The signals were amplified (75 μV/mm) and the EEG, as well as the calibration signals, were transferred from the Nihon-Kohden polygraph on-line to a Macintosh computer, which also controlled the presentation of the auditory stimuli. ERP trials were digitized at a rate of 256 Hz. Potential artifacts identified by computer software were excluded only after visual analysis of raw EEG. In the first study, B6 and D2 mice were approximately between 10 and 14 weeks of age at the time of the electrophysiological recordings. In the second study, HAP-1, LAP-1 and HS/Ibg mice were initially maintained in a quarantine facility for 7 weeks. They were approximately between 13 and 18 weeks of age at the time of the electrophysiological recording. These studies have been described previously (Ehlers and Somes, 2002, Slawecki et al., 2003).
General procedures
EEG and ERP recordings were collected in one recording session in a sound-attenuated and electrically grounded BRS/LVE recording chamber (90 × 90 × 85 cm). The auditory ERP session consisted of 312 individual tone presentations or trials. A three-tone auditory ‘oddball’ paradigm that has been developed to directly model studies employed in humans was used (Putnam and Roth, 1990, Kaneko et al., 1996). Three tone types were presented: a standard tone (1000 Hz square wave, 70 dB, 84% probability), a rare tone (2000 Hz square wave, 85 dB, 10% probability), and a noise tone (white noise, 100 dB, 6% probability). For study 2, the standard tone generated was 75 dB. All tones were presented for 20 ms with rise and fall times of < 1 ms. Individual trials were 1000 ms in duration (100 ms pre-stimulus + 900 ms post-stimulus) and were separated by variable intervals ranging from 500 to 1000 ms. At the conclusion of the testing, all mice were euthanized using CO2 asphyxiation. Further details about the auditory ERP sessions were described previously (Ehlers and Somes, 2002: Slawecki et al., 2003).
Event-related oscillations (ERO) and phase locking index (PLI) analyses
ERO and PLI analyses were accomplished from the same datasets that were used to generate the ERP data reported in previous publications (Ehlers and Somes, 2002, Slawecki et al., 2003). Data from each trial generated by the stimuli were processed by a time-frequency analysis algorithm, which utilizes the S-transform (Stockwell et al., 1996), a generalization of the Gabor transform (Gabor, 1946), defined as:
The S-transform resembles a continuous wavelet but it uses scaled Gaussian windows which are not, strictly, wavelets. The equation for calculation of the S transform of discrete time series h(kT) at time jT and frequency n/NT is where T is the sample period of the discrete time series, j is the sample index, N is the number of samples in the time series, n is the frequency index, and H[ ] is the Fourier spectrum of the discrete time series. The computer code we use is based closely on a C language S-transform subroutine available from the NIMH MEG Core Facility web site (http://kurage.nimh.nih.gov/meglab/). The defining equation of the S-transform is a convolution integral in continuous time. The method we use is equivalent to the finite discrete time version of this, but for computational efficiency, multiplications in frequency domain are used rather than convolution in time domain. The inputs to the S-transform are real, but the outputs are complex. We use the magnitudes squared of the time-frequency output values, discarding the corresponding phase angles.
The PLI was calculated to measure phase variability in relation to stimulus onset, as previously described (Schack and Klimesch, 2002; Klimesch et al., 2004). The PLI, which ranges between zero and one, is a measure of phase resetting (Schack and Klimesch, 2002). An increased PLI indicates less phase variability and stronger phase locking to the onset, whereas a reduced PLI indicates higher phase variability and weaker phase locking (Schack and Klimesch, 2002).
Implicit in this and related methods is the assumption that the trial being analyzed is preceded by and followed by identical trials. To reduce consequences of resulting discontinuities, we use a cosine window over the initial and final 100 msec of the input time series of each trial. The output of the transform for each stimuli and electrode site was calculated by averaging the individual trials containing the time-frequency energy distributions. To quantify S transform magnitudes, a region of interest (ROI) was identified by specifying the band of frequencies and the time interval contained in the rectangular ROI. ERO energy was determined as the measure of the energy values in the ROI. PLI was determined as the peak amplitude of the PLI in the ROI. These analyses are similar to what has been previously described (Schack and Klimesch, 2002; Jones et al., 2004). Baseline corrected post-stimulus activity (900 ms) was calculated by subtracting pre-stimulus ERO energy values (100 ms) from the post-stimulus ROI values, as previously described (Padmanabhapillai et al., 2006b). The ROI frequencies were: delta (1–4 Hz), theta (4–8 Hz) and alpha/beta (8–35 Hz). The ROI time intervals correspond to the P1/N1a ERP component (0 – 50 ms), N1b/P3a components (50 – 350 ms) and the P3b component (350 – 800 ms). The duration of time windows for delta, theta and alpha/beta frequencies were as follow: 50 ms for the P1/N1a ERP component, 300 ms for the N1b/P3a component and 450 ms for the P3b component. ROI frequencies and time intervals are consistent with our previous ERP studies (Ehlers and Somes, 2002, Slawecki et al., 2003).
Statistical Analysis
Statistical analyses were performed by using SPSS for the Macintosh (SPSS, Inc., Chicago, IL). Brain regions were assessed independently. Data analyses were performed on ERO energy and PLI for the ROIs in response to standard, rare and noise tones. Group (B6 vs D2 or HAP-1 vs. LAP-1 vs. HS/Ibg) was assessed as a between subject variable. Tone (standard, rare and noise) was assessed as within subject repeated measures. To assess between strain differences, two-way repeated measures ANOVA were used. For all repeated measures analyses, Greenhouse-Geisser corrected P-values were reported to account for violations of sphericity. When appropriate, post hoc analysis of two-way repeated measures ANOVA (Group × Tone) utilized one-way ANOVA to assess strain differences. Post hoc analysis of two-way repeated measures ANOVA (Tone) utilized pairwise comparisons. Post hoc analysis of one-way ANOVA (Group) utilized the Least Square Difference (LSD) test. For these analyses, P-value was set at P < 0.05 to determine the levels of statistical significance. Independent one-way ANOVA were used to assess baseline differences in ERO energy between strains. To correct for multiple comparisons of ROIs, P-value was set at P < 0.01 to determine the levels of statistical significance.
RESULTS
The present study extended our initial analyses of neurophysiological endophenotypes in genetic mouse models of high and low alcohol preference by characterizing changes in ERO mean energy and the degree of phase variability in the frontal and parietal cortices. Changes in ERO energy and PLI were estimated for time-frequency ROIs derived using standard, rare and noise auditory oddball data. Presentation of auditory oddball stimuli produced changes in ERO mean energy in the frontal and parietal cortices. While these changes were observed in all frequency bands studied (delta, theta and alpha/beta), they were dependent on the type of auditory stimuli used to generate the ERP (standard vs. rare vs. noise). Our findings also suggest that changes in ERO mean energy were dependent on the cortical region studied (frontal vs. parietal). Moreover, the present results provide evidence of strain differences in ERO mean energy and PLI of selected frequency bands within time windows corresponding to ERP components previously shown to be changed in these models of alcohol preference.
ERO mean energy and phase locking in B6 and D2 mice
Effect of tone type on ERO energy and PLI
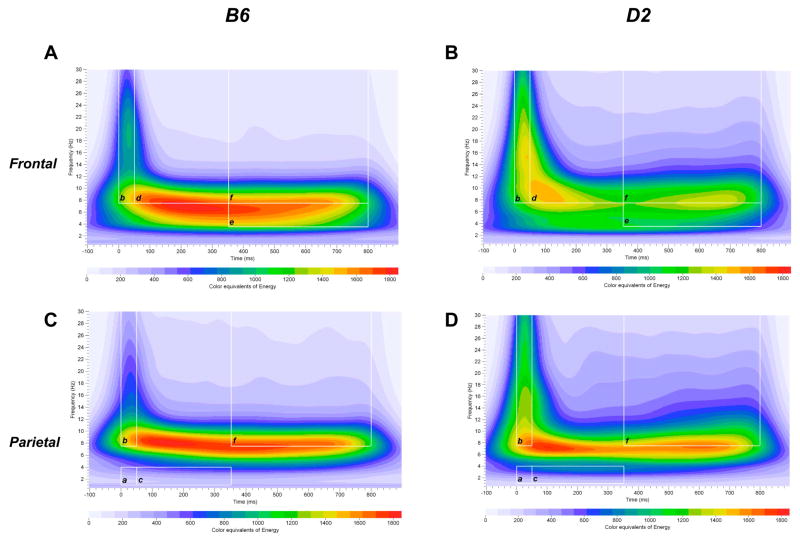
Fig. 1A–D illustrates a grand mean S-transform time-frequency representation of the rare auditory oddball data in the frontal and parietal cortices obtained from B6 and D2 mice. Visual inspection of the time-frequency representation of the EROs in Fig. 1A–D show changes in ERO energy following presentation of the rare tone. As shown in Fig. 1A–B, an increase in ERO energy in the delta, theta and alpha/beta frequency bands is observed following presentation of the rare tone (vs. baseline). Changes in ERO energy were also observed following presentation of standard and noise tones (data not shown). Differences in ERO energy among the three auditory stimuli were observed in the frontal and parietal cortices when collapsing across B6 and D2 groups.
Figure 1.
Time-frequency representation of evoked delta, theta and alpha/beta bands energy distribution of rare stimuli in the frontal and parietal cortices in B6 and D2 mice. Time-frequency responses of evoked theta (e) and alpha/beta (b, d, f) bands energy distribution to rare stimuli in B6 (A) and D2 (B) mice in the frontal cortex. Time-frequency responses of evoked delta (a, c) and alpha/beta (b, f) bands energy distribution to rare stimuli in B6 (C) and D2 (D) mice in the parietal cortex. Time-frequency ROI windows used were 0 – 50 ms, 50 – 350 ms and 350 – 800 ms (white squares).
Two-way repeated measures ANOVA with ERO energy as dependent variable revealed a significant main effect of Tone in the delta (0–50 ms and 350–800 ms time windows), theta (0–50 ms time window) and alpha/beta (0–50 ms, 50–350 ms and 350–800 ms time windows) frequency bands in the frontal cortex (Table 1). Repeated measures ANOVA also showed a significant main effect of Tone in the delta (50–350 ms and 350–800 ms time windows), theta (50–350 ms and 350–800 ms time windows) and alpha/beta (0–50 ms and 350–800 ms time windows) bands in the parietal cortex (Table 1). Post hoc pairwise comparisons showed lower ERO mean energy in the frontal and parietal cortices in the delta and theta frequency bands in response to noise tones (vs. standard and/or rare tones) during the 0–50 ms, 50–350 ms and 350–800 ms time windows (Table 1). Lower ERO energy in the parietal delta and theta bands (50–350 ms and 350–800 ms time window) was observed in response to rare tones, compared to standard tones (Table 1). Pairwise analyses indicated lower ERO mean energy in the frontal and parietal alpha/beta frequency band in response to standard and rare tones (vs. noise) during the 0–50 ms time window (Table 1). Lower ERO energy in the frontal and parietal alpha/beta bands (0–50 ms time windows) were also observed in response to standard tones, compared to rare tones (Table 1). In contrast, lower ERO energy in the frontal and parietal alpha/beta band (350–800 ms) was observed in response to noise tones, compared to standard tones (Table 1).
Table 1.
Tone Main Effects of ERO energy in B6 and D2 mice
| Standard Tone | Rare Tone | Noise Tone | Tone Effects | |
|---|---|---|---|---|
| Frontal | ||||
| 0–50 ms | ||||
| Delta Band | −51.1 ± 2.8 | −52.1 ± 3.0 | −57.6 ± 3.4& | F(1.5,96.1)=8.9, P<0.005 |
| Theta Band | −74.8 ± 4.3 | −73.7 ± 4.4 | −84.2 ± 5.3& | F(1.7,108.8)=7.9, P<0.005 |
| Alpha/Beta Band | 332.6 ± 25.6 | 457.3 ± 31.4@ | 676.1 ± 43.8& | F(1.8,112.8)=54.3, P<0.001 |
| 50–350 ms | ||||
| Delta Band | 432.4 ± 30.2 | 428.8 ± 31.7 | 423.3 ± 27.6 | F(1.4,89.0)=0.25, NS |
| Theta Band | 1288.6 ± 70.4 | 1309.6 ± 79.0 | 1369.2 ± 87.9 | F(1.5,95.1)=2.9, NS |
| Alpha/Beta Band | 2804.9 ± 116.2 | 2868.9 ± 138.1 | 2976.8 ± 138.5 | F(1.8,116.6)=3.7, P<0.05 |
| 350–800 ms | ||||
| Delta Band | 661.9 ± 46.9** | 658.3 ± 51.1 | 601.7 ± 40.6 | F(1.3,84.1)=5.7, P<0.05 |
| Theta Band | 1874.3 ± 101.9 | 1940.4 ± 110.5 | 1815.5 ± 128.7 | F(1.5,95.1)=3.3, NS |
| Alpha/Beta Band | 3778.8 ± 173.4 | 3659.4 ± 194.9 | 3245.3 ± 195.7& | F(1.5,98.6)=16.5, P<0.001 |
| Parietal | ||||
| 0–50 ms | ||||
| Delta Band | −42.4 ± 2.2 | −40.9 ± 2.4 | −39.4 ± 2.2 | F(1.8,112.8)=1.7, NS |
| Theta Band | −73.9 ± 3.4 | −76.9 ± 3.9 | −76.2 ± 3.9 | F(1.7,109.8)=0.6, NS |
| Alpha/Beta Band | 200.9 ± 19.0 | 266.9 ± 24.3@ | 382.2 ± 35.0& | F(1.6,103.7)=23.7, P<0.001 |
| 50–350 ms | ||||
| Delta Band | 311.0 ± 17.3 | 286.6 ± 16.5@ | 260.4 ± 15.1& | F(1.6,105.4)=13.7, P<0.001 |
| Theta Band | 1098.7 ± 49.2 | 1050.9 ± 49.7@ | 929.0 ± 44.0& | F(1.6,105.5)=27.8, P<0.001 |
| Alpha/Beta Band | 2915.9 ± 119.6 | 2896.5 ± 132.6 | 2904.7 ± 128.2 | F(1.8,112.6)=0.04, NS |
| 350–800 ms | ||||
| Delta Band | 472.7 ± 26.2 | 425.4 ± 23.3@ | 381.3 ± 21.8& | F(1.9,120.4)=22.9, P<0.001 |
| Theta Band | 1700.8 ± 78.9 | 1691.9 ± 82.5 | 1519.1 ± 83.2& | F(1.9,123.6)=11.0, P<0.001 |
| Alpha/Beta Band | 4424.9 ± 187.7** | 4225.5 ± 185.6 | 3942.8 ± 205.0 | F(1.8,113.4)=9.2, P<0.001 |
Values are mean ± SEM. Represent statistically significant difference,
standard vs. noise tones;
noise vs. standard and rare tones;
rare vs. standard tones; (post hoc pairwise comparisons, P < 0.05).
Two-way repeated measures ANOVA with PLI as dependent variable revealed a significant main effect of Tone in the delta, theta and alpha/beta frequency bands (0–50 ms, 50–350 ms and 350–800 ms time windows) in the frontal and parietal cortices, when collapsing across B6 and D2 groups (Supplementary table 1). Post hoc pairwise comparisons showed larger PLI in response to noise tones (vs. standard and/or rare tones) during the 0–50 ms, 50–350 ms and 350–800 ms time windows in the frontal and parietal delta, theta and alpha/beta bands. Larger PLI was also observed in response to rare tones (vs. standard tones) (Supplementary table 1).
ERO differences between B6 and D2 mice: Baseline activity
No differences were observed in baseline ERO mean energy in the frontal delta and theta bands between B6 and D2 mice (Supplementary table 2). In contrast, baseline ERO mean energy in the frontal alpha/beta band was lower in B6 than in D2 mice in response to standard and rare tones (Supplementary table 2). Baseline ERO energy in the parietal delta, theta and alpha/beta bands was lower in B6 than in D2 mice in response to standard tones. Lower baseline ERO energy in the parietal theta and alpha/beta bands was also observed in B6 mice in response to rare tones and in the parietal theta band in response to noise tones (Supplementary table 2).
ERO differences between B6 and D2 mice: ERO energy and PLI in the 0–50 ms window
ERO Energy
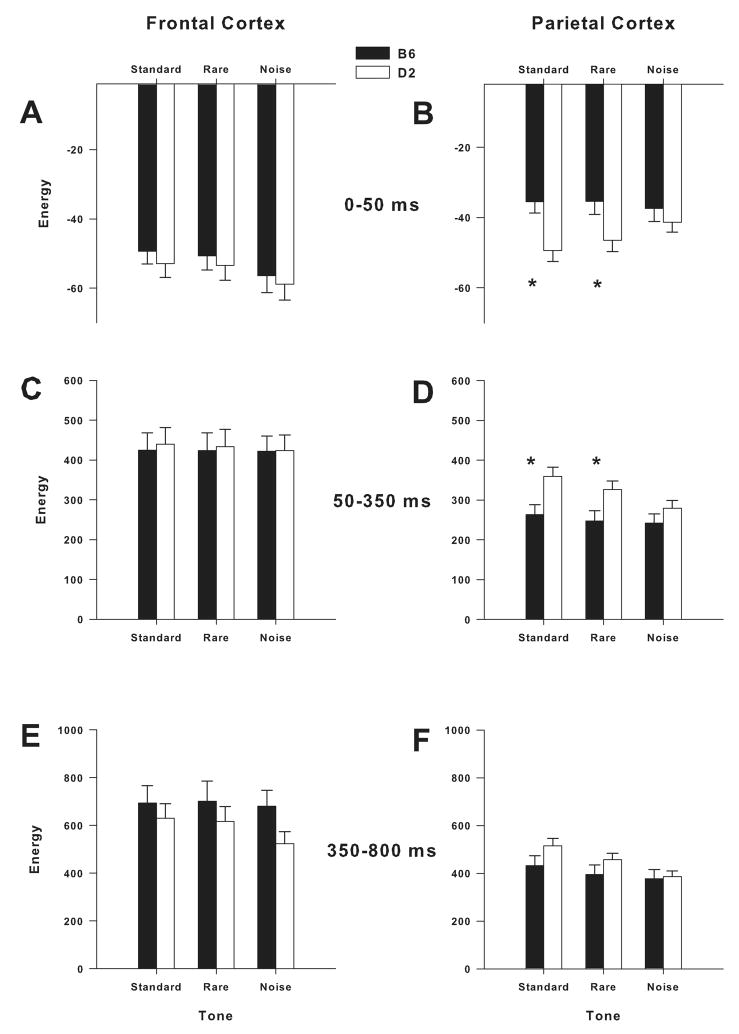
Significant Group × Tone interaction was observed in the delta band in the parietal cortex [F(1.8, 112.8) = 4.8; P < 0.05]. Group × Tone interaction in the delta band was not significant in the frontal cortex (Fig. 2A). Post hoc assessment revealed that the reduction of parietal delta ERO energy in response to standard [F(1,65) = 9.6; P < 0.005] and rare [F(1,65) = 5.2; P < 0.05] tones is attenuated in B6 mice, compared to D2 mice (Fig. 2B).
Figure 2.
Mean amplitude values of ERO energy for delta bands in response to standard, rare and noise stimuli in the frontal and parietal cortices. B6 mice showed attenuation of post-stimulus decrease in delta ERO energy in the 0–50 ms time window in response to standard and rare tones in the parietal cortex (B). B6 mice showed lower delta ERO energy than D2 mice in the 50–350 ms time window in response to standard and rare tones in the parietal cortex (D). * = Significant differences between B6 and D2 mice (P < 0.05).
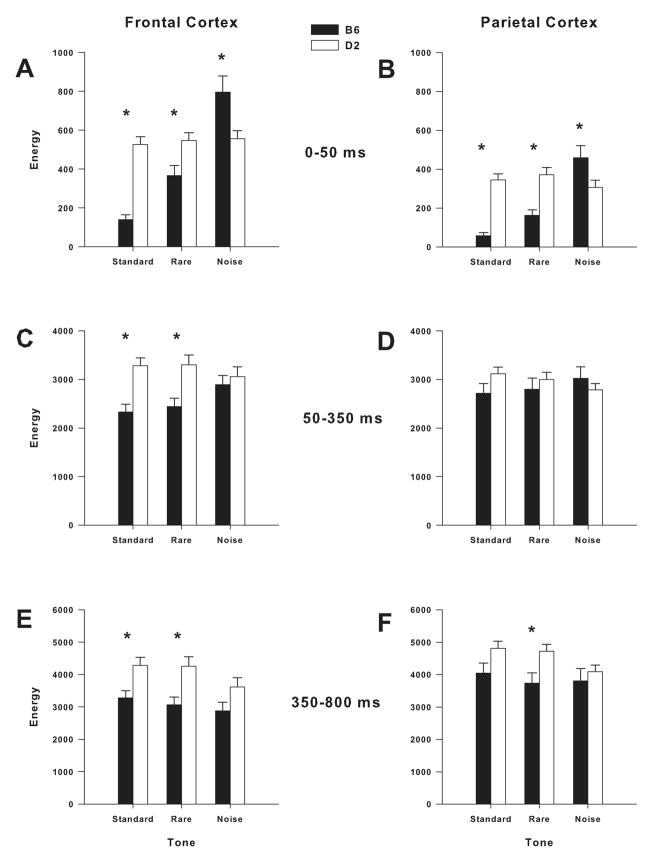
Group × Tone interaction in the theta band was significant in the frontal cortex [F(1.7, 108.8) = 5.8; P < 0.01], but not in the parietal cortex. Post hoc assessment revealed that B6 mice had higher ERO energy in the frontal theta band in response to noise tones [F(1,65) = 4.1, P < 0.05]. Significant Group × Tone interaction was found in the alpha/beta band in the frontal and parietal cortices [frontal: F(1.8, 112.8) = 45.6; P < 0.001; parietal: F(1.6, 103.7) = 38.5; P < 0.001]. Post hoc assessment revealed that B6 mice had lower ERO energy in the frontal alpha/beta band in response to standard [F(1,65) = 57.2; P < 0.001] and rare [F(1,65) = 8.3; P < 0.01] tones (Fig. 3A). Post hoc analyses also revealed that B6 mice had lower ERO energy in the parietal alpha/beta band in response to standard [F(1,65) = 57.5; P < 0.001] and rare [F(1,65) = 18.4; P < 0.001] tones (Fig. 3B). In contrast, post hoc assessment showed that B6 mice had higher ERO energy than D2 mice in the frontal and parietal cortices in response to noise tones [frontal: F(1, 65) = 7.4; P < 0.01; parietal: F(1,65) = 4.7; P < 0.05] (Figs. 3A–B). Figure 1A–D illustrates grand-averaged ERO time-frequency representations of the rare tones for the delta (a: parietal cortex) and alpha/beta (b: frontal and parietal cortices) frequency bands.
Figure 3.
Mean amplitude values of ERO energy for evoked alpha/beta band in response to standard, rare and noise tones in frontal and parietal cortices. B6 mice showed lower ERO energy than D2 mice in the alpha/beta band in response to standard and rare tones in the frontal cortex (A: 0–50 ms; C: 50–350 ms; E: 350–800 ms). B6 mice showed lower ERO energy than D2 mice in the alpha/beta band in response to standard (B: 0–50 ms) and rare (B: 0–50 ms; F: 350–800 ms) in the parietal cortex. In contrast, Bt mice showed higher ERO energy than D2 mice in response to noise tones in the frontal (A: 050 ms) and parietal (B: 0–50 ms) cortices. * = Significant differences between B6 and D2 mice (P<0.05).
PLI
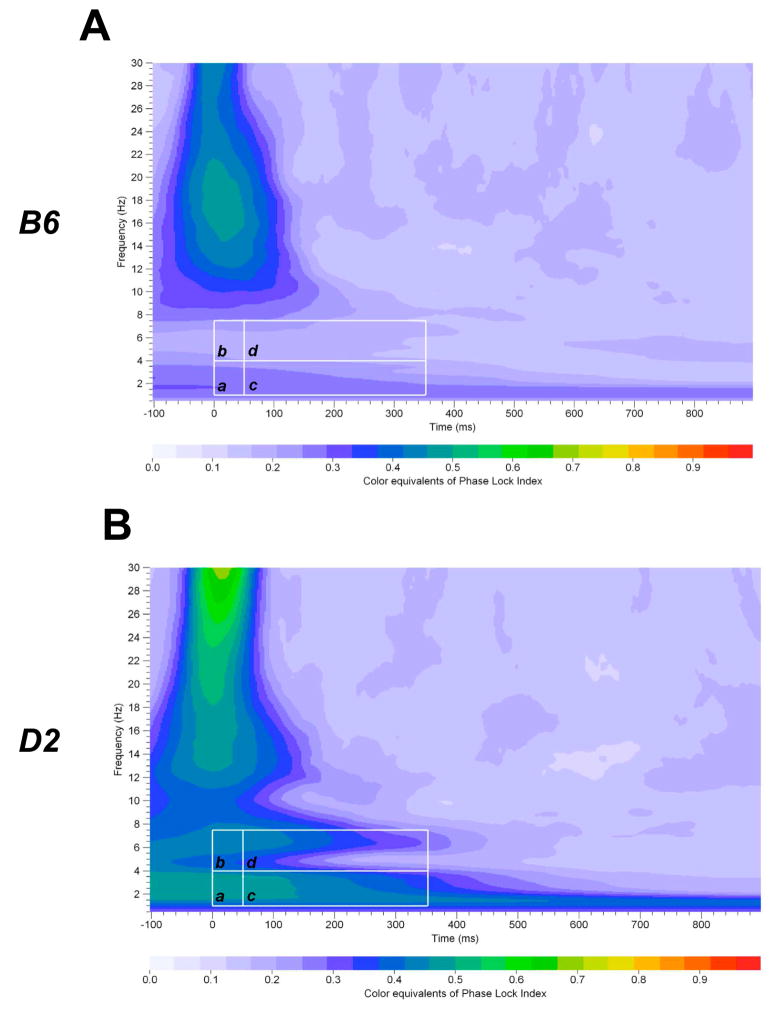
Significant Group × Tone interaction was observed in the delta and theta bands in the frontal and parietal cortices (Table 2). Post hoc assessment revealed that B6 mice had lower PLI in the frontal and parietal delta and theta bands in response to standard, rare and noise tones (Table 2). Post hoc analyses also showed that B6 mice had lower PLI in the frontal and parietal alpha/beta band in response to standard tones and in the parietal alpha/beta band in response to rare tones (Data not shown). In contrast, B6 mice showed higher PLI in the frontal alpha/beta band in response to noise tones (Data not shown). Figure 4A–B illustrates grand-averaged PLI time-frequency representations of the rare tones for the parietal delta (a) and theta (b) frequency bands.
Table 2.
PLI for Delta and Theta bands in B6 and D2 mice during the 0–50 ms and 50–350 ms time windows
| Group | Standard Tone | Rare Tone | Noise Tone | Tone Effects | |
|---|---|---|---|---|---|
| Frontal | |||||
| 0–50 ms | |||||
| Delta Band | B6 | 0.173 ± 0.015 | 0.371 ± 0.021 | 0.525 ± 0.028 | F(1.8,114.7) = 7.4, P < 0.005 |
| D2 | 0.438 ± 0.027* | 0.533 ± 0.025* | 0.677 ± 0.022* | ||
| Theta Band | B6 | 0.176 ± 0.014 | 0.379 ± 0.020 | 0.547 ± 0.024 | F(1.8,113.5) = 8.5, P < 0.005 |
| D2 | 0.435 ± 0.027* | 0.537 ± 0.024* | 0.686 ± 0.021* | ||
| 50–350 ms | |||||
| Delta Band | B6 | 0.181 ± 0.013 | 0.323 ± 0.018 | 0.455 ± 0.025 | F(1.8,115.5) = 0.8, NS |
| D2 | 0.354 ± 0.025 | 0.468 ± 0.018 | 0.587 ± 0.026 | ||
| Theta Band | B6 | 0.194 ± 0.011 | 0.353 ± 0.018 | 0.510 ± 0.022 | F(1.9,122.7) = 2.4, NS |
| D2 | 0.361 ± 0.022 | 0.483 ± 0.017 | 0.609 ± 0.022 | ||
| Parietal | |||||
| 0–50 ms | |||||
| Delta Band | B6 | 0.216 ± 0.019 | 0.393 ± 0.018 | 0.484 ± 0.028 | F(1.5,95.1) = 7.4, P < 0.005 |
| D2 | 0.485 ± 0.024* | 0.561 ± 0.021* | 0.619 ± 0.028* | ||
| Theta Band | B6 | 0.216 ± 0.019 | 0.404 ± 0.017 | 0.493 ± 0.028 | F(1.5,96.1) = 7.5, P < 0.005 |
| D2 | 0.480 ± 0.024* | 0.562 ± 0.021* | 0.628 ± 0.027* | ||
| 50–350 ms | |||||
| Delta Band | B6 | 0.185 ± 0.014 | 0.331 ± 0.020 | 0.465 ± 0.021 | F(1.8,114.3) = 11.6, P < 0.001 |
| D2 | 0.463 ± 0.024* | 0.546 ± 0.024* | 0.580 ± 0.026* | ||
| Theta Band | B6 | 0.189 ± 0.014 | 0.362 ± 0.018 | 0.498 ± 0.018 | F(1.8,114.7) = 15.5, P < 0.001 |
| D2 | 0.461 ± 0.024* | 0.552 ± 0.023* | 0.588 ± 0.026* |
Values are mean ± SEM. Represent statistically significant difference,
post hoc one-way ANOVA, P < 0.05.
Figure 4.
Time-frequency representation of evoked delta, theta and alpha/beta bands PLI distribution of rare stimuli in the parietal cortex in B6 and D2 mice. Time-frequency responses of evoked delta (a, c) and theta (b, d) bands PLI distribution to rare stimuli in B6 (A) and D2 (B) mice in the parietal cortex. Time-frequency ROI windows used were 0 – 50 ms and 50 – 350 ms (white squares).
ERO differences between B6 and D2 mice: ERO energy in the 50–350 ms window
ERO Energy
Significant Group × Tone interaction was observed in the delta band in the parietal cortex [F(1.6, 105.4) = 4.8; P < 0.05]. Group × Tone interaction in the delta band was not significant in the frontal cortex (Fig. 2C). Post hoc assessment revealed that B6 mice had lower ERO energy in the parietal delta band in response to standard [F(1,65) = 7.7; P < 0.01] and rare [F(1,65) = 5.7; P < 0.05] tones (Fig. 2D).
Group × Tone interaction in the theta band was not significant in the frontal and parietal cortices. In contrast, significant Group × Tone interaction was found in the alpha/beta band in the frontal and parietal cortices [frontal: F(1.8, 116.6) = 22.6; P < 0.001; parietal: F(1.8, 112.6) = 10.7; P < 0.001]. Post hoc assessment revealed that B6 mice had lower ERO energy in the frontal alpha/beta band in response to standard [F(1,65) = 16.7; P < 0.001] and rare [F(1,65) = 9.7; P < 0.005] tones (Fig. 3C). In contrast, post hoc analyses revealed no significant differences in parietal alpha/beta band ERO energy between B6 and D2 mice (Fig. 3D). Figure 1A–D illustrates grand-averaged ERO time-frequency representations of the rare tones for the delta (c: parietal cortex) and alpha/beta (d: frontal cortex) frequency bands.
PLI
Significant Group × Tone interaction was observed in the delta and theta band in the parietal, but not in the frontal cortex (Table 2). Post hoc assessment revealed that B6 mice had lower PLI in the parietal delta and theta bands in response to standard, rare and noise tones (Table 2). Post hoc analyses also showed that B6 mice had lower PLI in the frontal and parietal alpha/beta band in response to standard tones and in the parietal alpha/beta band in response to rare tones (Data not shown). In contrast, B6 mice showed higher PLI in the frontal alpha/beta band in response to noise tones (Data not shown). Figure 4A–B illustrates grand-averaged PLI time-frequency representations of the rare tones for the parietal delta (c) and theta (d) frequency bands.
ERO differences between B6 and D2 mice: ERO energy in the 350–800 ms window
ERO Energy
Group × Tone interaction in the delta band was significant in the parietal cortex [F(1.9, 120.4) = 3.8; P < 0.05], but not in the frontal cortex (Figs. 2E–F). However, post hoc assessment revealed no significant differences in ERO energy in the parietal delta band between B6 and D2 mice (Fig. 2F). Group × Tone interaction in the theta band was significant in the frontal cortex [F(1.5, 95.1) = 7.3; P < 0.005], but not in the parietal cortex. Post hoc assessment revealed that B6 mice had higher ERO energy in the frontal theta band in response to standard [F(1,65) = 4.4; P < 0.05], rare [F(1,65) = 7.3; P < 0.01] and noise [F(1,65) = 9.7; P < 0.005] tones.
Significant Group × Tone interaction was found in the alpha/beta band in the parietal cortex [F(1.8, 113.4) = 5.1; P = 0.01], but not in the frontal cortex (Figs. 3E–F). Post hoc assessment revealed that B6 mice had lower ERO energy in the parietal alpha/beta band in response to standard [F(1,65) = 4.2; P < 0.05] and rare [F(1,65) = 7.1; P = 0.01] tones (Fig. 3F). Figure 1A–B illustrates grand-averaged ERO time-frequency representations of the rare tones for the theta (e) and alpha/beta (f) frequency bands in the frontal cortex.
PLI
Significant Group × Tone interaction was observed in the delta and theta bands in the parietal and frontal cortices [delta: frontal: F(1.8, 116.7) = 5.4; P < 0.01; parietal: F(1.5, 98.0) = 5.6; P = 0.01; theta: frontal: F(1.8, 115.4) = 5.3; P < 0.01; parietal: F(1.8, 112.6) = 10.7; P = 0.01]. Post hoc assessment revealed that B6 mice had lower PLI in the parietal and frontal delta band in response to standard, rare and noise tones (Data not shown). Post hoc analyses also showed that B6 mice had lower PLI in the frontal and parietal theta band in response to standard tones and rare tones (Data not shown). In contrast, Group × Tone interaction was not significant in the alpha/beta band in the frontal and parietal cortices.
ERO mean energy and phase locking in HAP-1, LAP-1 and HS/Ibg mice
Effect of tone type on ERO energy and PLI
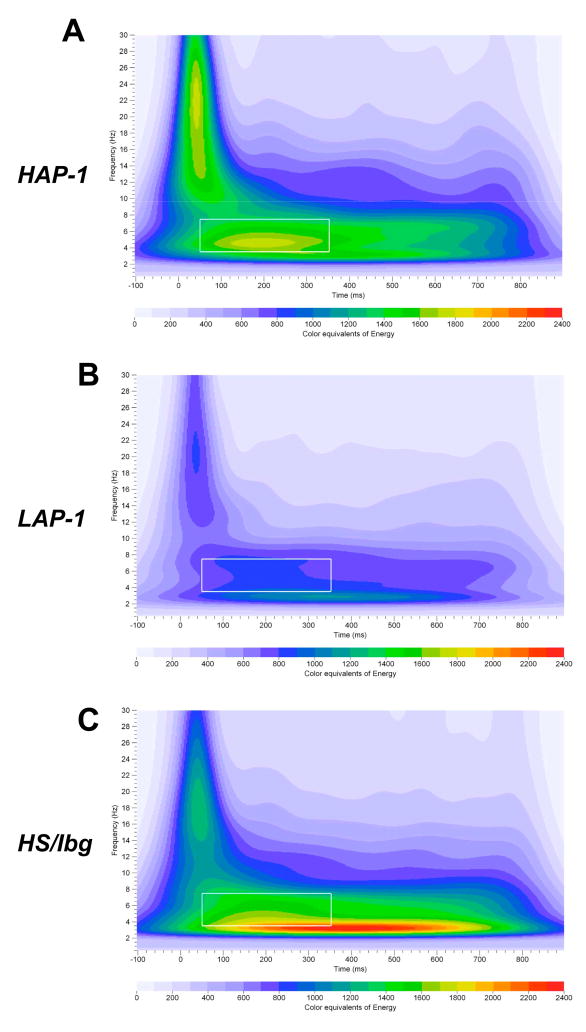
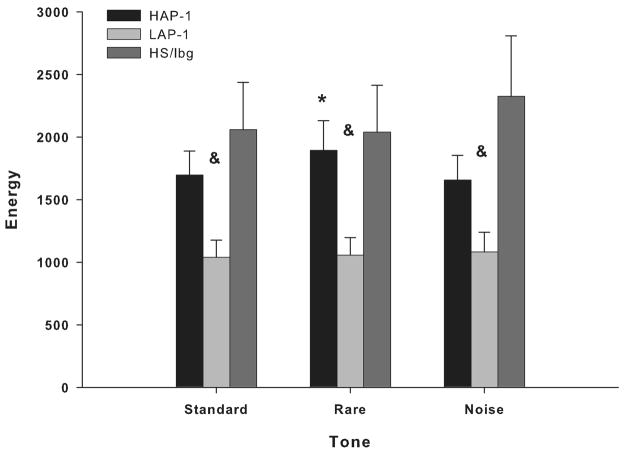
Fig. 5A–C illustrates a grand mean S-transform time-frequency representation of the rare auditory oddball data in the frontal cortex obtained from HAP-1, LAP-1 and HS/Ibg mice. Visual inspection of the time-frequency representation of the EROs in Fig. 5A–C show changes in ERO energy following presentation of the rare tone. As shown in Fig. 5A–C, an increase in ERO energy in the delta, theta and alpha/beta frequency bands is observed following presentation of the rare tone (vs. baseline). Changes in ERO energy were also observed following presentation of standard and noise tones (data not shown). Differences in ERO energy among the three auditory stimuli were observed in the frontal and parietal cortices when collapsing across HAP-1, LAP-1 and HS/Ibg groups. Two-way repeated measures ANOVA with ERO energy as dependent variable revealed a significant main effect of Tone in the delta (0–50 ms, 50–350 ms and 350–800 ms time windows), theta (0–50 ms and 350–800 ms time windows) and alpha/beta (0–50 ms, 50–350 ms and 350–800 ms time windows) frequency bands in the frontal cortex (Table 3). Repeated measures ANOVA also showed a significant main effect of Tone in the delta and theta (50–350 ms and 350–800 ms time windows) bands and in the alpha/beta (0–50 ms and 350–800 ms time windows) band in the parietal cortex (Table 3). Post hoc pairwise comparisons showed lower ERO mean energy in the frontal and parietal delta band in response to noise tones (vs. standard and/or rare tones) during the 0–50 ms, 50–350 ms and 350–800 ms time windows (Table 3). Higher ERO energy in the frontal theta (0–50 ms time window) band and frontal and parietal alpha/beta (0–50 ms and 50–350 ms time windows) band was observed in response to noise tones (vs. standard and/or rare tones) (Table 3). In contrast, lower ERO energy in the frontal alpha/beta band (350–800 ms time window) and parietal theta and alpha/beta bands (50–350 ms and 350–800 ms time windows) was observed in response to noise tones, compared to standard and rare tones (Table 3).
Figure 5.
Time-frequency representation of evoked delta, theta and alpha/beta bands energy distribution of rare stimuli in the frontal cortex in HAP-1, LAP-1 and HS/Ibg mice. Time-frequency responses of evoked theta band energy distribution to rare stimuli in HAP-1 (A), LAP-1 (B) and HS/Ibg (C) mice in the frontal cortex. Time-frequency ROI windows used was 50 – 350 ms (white squares).
Table 3.
Tone Main Effects of ERO energy in HAP-1, LAP-1 and HS/Ibg mice
| Standard Tone | Rare Tone | Noise Tone | Tone Effects | |
|---|---|---|---|---|
| Frontal | ||||
| 0–50 ms | ||||
| Delta Band | 107.0 ± 12.6 | 108.8 ± 12.4 | 92.2 ± 8.5& | F(1.3,64.6)=7.5, P<0.005 |
| Theta Band | 213.4 ± 22.2** | 223.3 ± 22.0 | 244.3 ± 28.3 | F(1.3,64.5)=6.1, P<0.05 |
| Alpha/Beta Band | 1226.9 ± 78.0* | 1371.4 ± 88.2 | 1555.3 ± 136.5 | F(1.4,68.7)=6.0, P<0.01 |
| 50–350 ms | ||||
| Delta Band | 829.7 ± 104.5 | 829.5 ± 102.1 | 678.0 ± 70.2& | F(1.3,62.7)=12.1, P<0.001 |
| Theta Band | 1598.0 ± 157.0 | 1662.3 ± 161.5 | 1686.4 ± 194.6 | F(1.5,71.5)=1.5, NS |
| Alpha/Beta Band | 3856.9 ± 226.9 | 4074.1 ± 248.3@ | 4451.4 ± 293.5& | F(1.5,74.9)=13.3, P<0.001 |
| 350–800 ms | ||||
| Delta Band | 1192.4 ± 159.8 | 1178.2 ± 151.3 | 963.0 ± 122.9& | F(1.5,73.6)=23.4, P<0.001 |
| Theta Band | 2127.0 ± 202.5 | 2140.8 ± 209.8 | 1958.5 ± 241.3& | F(1.5,75.5)=7.8, P<0.005 |
| Alpha/Beta Band | 4458.3 ± 275.9 | 4326.2 ± 276.8 | 3537.6 ± 235.8& | F(1.5,75.1)=46.5, P<0.001 |
| Parietal | ||||
| 0–50 ms | ||||
| Delta Band | 92.8 ± 5.9 | 94.8 ± 6.4 | 90.1 ± 6.2 | F(1.5,74.2)=1.2, NS |
| Theta Band | 283.5 ± 18.6 | 285.2 ± 19.6 | 274.2 ± 18.4 | F(1.5,75.8)=0.7, NS |
| Alpha/Beta Band | 1751.2 ± 122.5* | 1898.8 ± 143.1 | 2032.8 ± 166.1 | F(1.4,66.9)=5.2, P<0.05 |
| 50–350 ms | ||||
| Delta Band | 682.5 ± 43.6 | 685.8 ± 46.0 | 591.6 ± 39.6& | F(1.6,76.2)=12.6, P<0.001 |
| Theta Band | 2210.5 ± 144.6 | 2160.1 ± 143.3 | 1859.5 ± 129.2& | F(1.4,68.5)=20.1, P<0.001 |
| Alpha/Beta Band | 6743.1 ± 448.0 | 6743.5 ± 460.7 | 6804.2 ± 478.9 | F(1.3,61.5)=0.1, NS |
| 350–800 ms | ||||
| Delta Band | 984.8 ± 63.2 | 971.0 ± 67.4 | 729.4 ± 45.6& | F(1.6,79.3)=37.1, P<0.001 |
| Theta Band | 3276.1 ± 221.6 | 3178.8 ± 207.9 | 2691.6 ± 200.2& | F(1.6,80.2)=34.7, P<0.001 |
| Alpha/Beta Band | 8811.1 ± 623.1 | 8545.8 ± 657.8 | 5751.1 ± 450.8& | F(1.3,65.6)=49.4, P<0.001 |
Values are mean ± SEM. Represent statistically significant difference,
standard vs. rare and noise tones;
standard vs. noise tones;
noise vs. standard and rare tones;
rare vs. standard tones; (post hoc pairwise comparisons, P < 0.05).
Two-way repeated measures ANOVA with PLI as dependent variable revealed a significant main effect of Tone in the delta, theta and alpha/beta (0–50 ms, 50–350 ms and 350–800 ms time windows) frequency bands in the frontal and parietal cortices, when collapsing across HAP-1, LAP-1 and HS/Ibg groups (Supplementary table 3). Post hoc pairwise comparisons showed larger PLI in response to noise tones (vs. standard and/or rare tones) during the 0–50 ms, 50–350 ms and 350–800 ms time windows in the frontal and parietal delta, theta and alpha/beta bands. Larger PLI was also observed in response to rare tones, compared to standard tones (Supplementary table 3).
ERO differences among HAP-1, LAP-1 and HS/Ibg mice: Baseline activity
No differences were observed in baseline ERO mean energy in the frontal and parietal delta, theta and alpha/beta bands among HAP-1, LAP-1 and HS/Ibg mice (Supplementary table 4).
ERO differences between HAP-1, LAP-1 and HS/Ibg mice: ERO energy and PLI in the 0–50 ms window
ERO Energy
Group × Tone interaction in the delta, theta and alpha/beta bands in the 0 to 50 ms time window were not significant in the frontal and parietal cortices among HAP-1, LAP-1 and HS/Ibg mice.
PLI
Significant Group × Tone interaction was observed in the theta band in the frontal [F(3.3, 80.4) = 4.5; P < 0.005], but not in the parietal cortex. Post hoc assessment revealed that HAP-1 mice had higher PLI than HS/Ibg mice in response to standard tones (Data not shown). No Group × Tone interaction was observed in the delta and alpha/beta bands in the frontal and parietal cortices (Data not shown). Supplementary figure 1A–C illustrates grand-averaged PLI time-frequency representations of the rare tones for HAP-1, LAP-1 and HS/Ibg mice.
ERO differences between HAP-1, LAP-1 and HS/Ibg mice: ERO energy and PLI in the 50–350 ms window
ERO Energy
Group × Tone interaction in the delta and alpha/beta bands were not significant in the frontal and parietal cortices. In contrast, significant Group × Tone interaction was found in the theta band in the frontal cortex [F(2.9, 71.5) = 4.1; P = 0.01] (Fig. 6), but not parietal cortex. Post hoc assessment revealed that HAP-1 mice had higher ERO energy than LAP-1 mice in the frontal theta band in response to rare tones [F(2,51) = 3.8; P < 0.05] (Fig. 6). Post hoc analyses revealed that LAP-1 mice had lower ERO energy than HS/Ibg mice in frontal theta band in response to standard [F(2,51) = 3.9; P < 0.05], rare [F(2,51) = 3.8; P < 0.05] and noise [F(2,51) = 3.7; P < 0.05] tones (Fig. 6). Figure 5A–C illustrates grand-averaged ERO time-frequency representations of the rare tones for the theta frequency band.
Figure 6.
Mean amplitude values of ERO energy for theta bands in response to standard, rare and noise stimuli in the frontal cortex. LAP-1 mice showed lower ERO energy than HAP-1 mice in the 350–800 ms time window in the theta band in response to rare tones. LAP-1 mice showed lower ERO energy than HS/Ibg mice in response to standard, rare and noise tones. * = Significant differences between HAP-1 and LAP-1 mice (P < 0.05). & = Significant differences between HS/Ibg and LAP-1 mice (P < 0.05).
PLI
Significant Group × Tone interaction was observed in the delta band in the frontal (Table 4), but not in the parietal cortex. Post hoc assessment revealed that HS/Ibg mice had lower PLI than HAP-1 and LAP-1 mice in response to rare tones (Table 4). No Group × Tone interaction was observed in the theta and alpha/beta bands in the frontal and parietal cortices (Table 4). Supplementary figure 1A–C illustrates grand-averaged PLI time-frequency representations of the rare tones for HAP-1, LAP-1 and HS/Ibg mice.
Table 4.
PLI for Delta, Theta and Alpha/Beta bands HAP, LAP and HS/Ibg mice during the 50–350 ms time window
| Group | Standard Tone | Rare Tone | Noise Tone | Tone Effects | |
|---|---|---|---|---|---|
| Frontal | |||||
| 50–350 ms | |||||
| Delta Band | HAP | 0.294 ± 0.029 | 0.411 ± 0.036 | 0.443 ± 0.033 | F(3.2,77.2) = 3.4, P < 0.05 |
| LAP | 0.275 ± 0.023 | 0.419 ± 0.032 | 0.530 ± 0.043 | ||
| HS/Ibg | 0.223 ± 0.019 | 0.313 ± 0.025& | 0.512 ± 0.041 | ||
| Theta Band | HAP | 0.280 ± 0.020 | 0.407 ± 0.030 | 0.455 ± 0.038 | F(3.3,80.91) = 2.6, NS |
| LAP | 0.238 ± 0.021 | 0.370 ± 0.029 | 0.534 ± 0.041 | ||
| HS/Ibg | 0.215 ± 0.020 | 0.341 ± 0.019 | 0.475 ± 0.026 | ||
| Alpha/Beta Band | HAP | 0.717 ± 0.023 | 0.764 ± 0.035 | 0.801 ± 0.025 | F(3.9,94.5) = 1.8, NS |
| LAP | 0.646 ± 0.028 | 0.682 ± 0.033 | 0.798 ± 0.023 | ||
| HS/Ibg | 0.536 ± 0.041 | 0.647 ± 0.038 | 0.707 ± 0.034 |
Values are mean ± SEM. Represent statistically significant difference,
HS/Ibg vs. HAP and LAP (post hoc LSD test, P < 0.05).
ERO differences between HAP-1, LAP-1 and HS/Ibg mice: ERO energy and PLI in the 350–800 ms window
ERO Energy
Group × Tone interaction in the delta, theta and alpha/beta bands in the 0 to 50 ms time window were not significant in the frontal and parietal cortices among HAP-1, LAP-1 and HS/Ibg mice.
PLI
Significant Group × Tone interaction was observed in the delta band in the frontal [F(3.0, 73.6) = 3.1; P < 0.05], but not in the parietal cortex. Post hoc assessment revealed no significant differences between HAP-1, LAP-1 and HS/Ibg mice in response to standard, rare and noise tones (Data not shown). No Group × Tone interaction was observed in the theta and alpha/beta bands in the frontal and parietal cortices (Data not shown).
DISCUSSION
Substantial evidence has emerged to suggest that brain oscillations represent neurophysiological correlates of human information processing and cognitive function (Basar et al., 1999; Karakas et al., 2000). More recently, emphasis has been placed on understanding their genetic basis and their potential regulatory role on neural function. As a result, brain oscillations have been proposed to be endophenotypes for complex genetic disorders, including drug addiction and psychiatric disorders (for reviews, see Begleiter and Porjesz, 2006). The Collaborative Study on the Genetics of Alcoholism (COGA) has employed brain oscillations as endophenotypes to search for genes involved in alcohol dependence. Findings from their studies have identified several genes that increase the susceptibility for risk of alcohol dependence. Those studies have also achieved significant progress in identifying EROs associated with generating the P3 component and several genes potentially involved in their regulation (for reviews, see Porjesz et al., 2005; Begleiter and Porjesz, 2006; Rangaswamy and Porjesz, 2008).
Recent research efforts have focused on studying genes that may regulate EROs associated with increased susceptibility to alcohol dependence. Studies have shown that alcohol dependent individuals manifest significantly less evoked theta and delta ERO power than age-matched controls (Jones et al., 2006a). Rangaswamy et al. (2007a) showed that adolescent offspring of alcohol dependent individuals have reduced delta and theta ERO power. Findings from those studies suggest that a decrease in theta and delta EROs may antecede the development of alcohol dependence. Studies have shown significant linkage and association between frontal theta ERO power and the cholinergic muscarinic receptor gene (CHRM2) on chromosome 7 (Jones et al., 2004, 2006b). There is also evidence to suggest a significant association between the parietal delta ERO power and CHRM2 (Jones et al., 2004, 2006b). However, the relationship between an alcohol preference phenotype and changes in EROs are not well understood in genetic mouse models of high and low alcohol preference. The present study sought to examine whether EROs generated by auditory stimuli differentiated mice differing in traits known to influence their amount of alcohol consumption.
Effects of a passive three-tone auditory ‘oddball’ paradigm on ERO energy and PLI
Several laboratories have provided ample evidence to suggest that ERP components, including a late positivity, can be obtained from mice (see Ehlers and Somes, 2002; Siegel et al., 2003; Umbricht et al., 2005). Like most ERP studies using rodents as subjects a “passive” auditory oddball paradigm has been used to generate ERPs in mice. The advantages of a passive paradigm are that it can be administered to human and other animal subjects without extensive prior training, and it does not require the subject to respond to the stimuli. These passive paradigms have been efficiently used in mouse studies where large numbers of subjects are necessary for instance, in the screening of drugs or testing line/sex differences (Slawecki et al., 2003; Connolly et al., 2004; Maxwell et al., 2004; Amann et al., 2008; Ehrlichman et al., 2008; Gandal et al., 2008; Rudnick et al., 2009).
B6 and D2 mice
While ERPs have been successfully recorded in a number of animal species the use of ERO technology to study brain function in animal models has been less applied (Schurmann et al., 2000). The present studies extend and confirm findings demonstrating that EROs can be recorded in mice. EROs have been previously reported in the delta, theta and alpha/beta ranges in response to auditory stimuli that were impacted by differences in stimulus characteristics (Ehlers and Criado, in press). In that study oscillations in the 7.5–40 Hz frequencies were found to be significantly affected in the 0–50 msec time range in response to differences in tone frequency. Whereas, changes in tone loudness produced changes in oscillations in the 7.5–40 Hz frequencies in the 350–800 msec range. In a separate study, evoked gamma oscillations were reported in mice that were enhanced by nicotine and blocked by pretreatment with mecamylamine (Phillips et al., 2007).
Our previous studies in B6 and D2 mice showed that the mouse P3 and N1 amplitudes were significantly larger in response to the rare tones, compared to the standard tones (Ehlers and Somes, 2002). Our present findings showed that ERO energy was not significantly greater in response to the rare tones, compared to the standard tones. However, PLI values in the 0–50 ms and 50–350 ms time window were significantly higher in rare tones, compared to standard tones. These findings suggest that an increase in phase locking and reduction in phase variability could be responsible for the larger P3 and N1 ERP amplitudes in response to the rare tones.
HAP-1, LAP-1 and HS/Ibg mice
We previously showed that, collapsed across lines, frontal N1a and N1b amplitudes increased as a function of the tone loudness. In general, frontal and parietal N1a and N1b amplitudes were greater when the noise tone was presented, compared to the rare and standard tones (Slawecki, et al., 2003). The present findings showed greater frontal theta and alpha/beta ERO energy in response to noise tones during the 0–50 ms and 50–350 ms time windows, compared to standard tones. PLI values in the 0–50 ms and 50–350 ms time windows were significantly higher in noise tones, compared to standard and rare tones. These findings suggest that an increase in phase locking and reduction in phase variability could be responsible for the larger N1a and N1b amplitudes in response to the noise tones.
ERO and PLI analyses in B6 and D2 mice
We previously demonstrated that B6 and D2 strains did not differ in overall EEG amplitudes (Ehlers and Somes, 2002). However, B6 mice showed significantly lower amplitude in the N1 and P3 ERP components in frontal and parietal cortices, when compared to the D2 strain. These findings suggested that B6 mice exhibited lower ‘responsivity’ to neurosensory stimuli and further confirmed that decreased P3 amplitude in this mouse model is associated with increased risk for enhanced alcohol consumption. The N1 component consists of the N1a component, a large negative component that peaks between 20 and 30 ms, and the N1b component, a broader negative component that peaks between 80 and 90 ms. The P3 component is a broad positive wave that peaks between 200 and 300 ms (Ehlers and Somes, 2002). Findings from the present study indicate that lower evoked alpha/beta band energy in the 0 to 50 ms time window, in response to standard and rare tones, may mediate the reduction in N1a amplitudes in B6 mice, compared to D2 mice. Consistent with their reduced N1a amplitudes (Ehlers and Somes, 2002), lower evoked alpha/beta band energy in B6 mice were observed in both frontal and parietal cortices. The negative values of the baseline-corrected delta ERO energy in the 0 to 50 ms time window suggest a significant reduction, below baseline ERO energy levels, in frontal and delta ERO energy following presentation of all auditory tones (0 to 50 ms time window). Compared to D2 mice, B6 mice showed a significant attenuation of the reduction in evoked parietal delta band in the 0 to 50 ms time window. While the functional significance of these findings is presently unclear, attenuation of this time-dependent inhibitory effect on delta ERO energy may be also contributing to the reduced N1a amplitudes in the parietal cortex of B6 mice.
B6 mice also exhibited reduced delta ERO energy in the 50 to 350 ms time window in the parietal cortex. Since decreased N1b and P3 amplitudes were previously reported in B6 mice (Ehlers and Somes, 2002), lower energy in the delta frequency band may mediate reduced N1b and P3 amplitudes in B6 mice. The present results also demonstrated reduced delta and theta PLI in B6 mice, compared to D2 mice. These findings suggest that decreased N1b and P3 amplitudes are associated with reduced delta and theta PLI. Interestingly, our results also demonstrated higher energy in the evoked frontal theta band in the 350–800 ms time window of B6 mice, compared to D2 mice. Further study is needed to characterize the functional significance of this increase in the energy of the evoked frontal theta band.
Genetic sensitivity to convulsants during alcohol withdrawal has been studied in different mouse strains to determine the neurochemical mechanisms regulating the symptoms of alcohol withdrawal associated with each strain. For instance, while high-alcohol preferring B6 and low-alcohol preferring D2 mice have been shown to exhibit significant differences in voluntary alcohol intake (Metten and Crabbe, 2005), these strains also differ in their susceptibility to alcohol withdrawal. B6 mice have shown low levels of withdrawal severity, whereas D2 mice exhibit high levels of withdrawal severity (Ruf et al., 2004). Moreover, studies by Metten and Crabbe found that, compared to D2 mice, B6 mice were also less sensitive to administration of bicuculline during alcohol withdrawal (Metten and Crabbe, 2005). This reduced sensitivity in B6 mice was also observed with other pro-convulsant drugs acting on GABAA receptors, including tert-butyl-bicyclo-2,2,2-phosphorothionate (TBPS) and 5-(2-cyclohexylidene-ethyl)-5-ethyl barbituric acid (CHEB) (Metten and Crabbe, 2005). These findings led Metten and Crabbe to suggest that B6 mice have deficits in GABAergic receptor function (Metten and Crabbe, 2005).
The present study did not test whether deficits in GABAergic receptor function are responsible for the lower energy in the evoked frontal alpha/beta band observed in the 0 to 50 ms, 50 to 350 ms and 350 to 800 ms time windows in B6 mice, compared to D2 mice. However, there is evidence suggesting that deficits in GABAergic receptors are associated with beta oscillations and with an increased risk for developing alcohol dependence. For instance, inhibitory GABAergic interneurons may play an important role generating high-frequency EEG rhythms such as beta and gamma oscillations (Whittington et al., 2000). Evidence from human studies suggests deficits in GABA/benzodizepine receptors in the brains of individuals at risk for alcohol dependence (Volkow et al., 1995). Consistent with these findings, Porjesz and colleagues reported significant linkage and linkage disequilibrium between a GABAA receptor gene on chromosome 4 and beta oscillations (Porjesz et al., 2002). Moreover, a GABAA receptor gene encoding the alpha-2 subunit has also been associated with beta oscillations and DSM-IV-TR diagnosis of alcohol dependence (Edenberg et al., 2004). Studies have also shown that gamma oscillations, which are also regulated by GABAergic mechanisms (Whittington et al., 2000), are attenuated in individuals diagnosed with alcohol dependence and at risk for alcohol dependence (Padmanabhapillai et al, 2006a, 2006b). More recently, attenuated evoked-beta band oscillations have been reported in alcohol dependent individuals (Rangaswamy et al., 2007b). While these findings suggest that deficits in beta oscillations and GABAA receptor function are associated with increased risk of alcohol dependence, future studies in B6 and D2 mice could provide a better understanding of the relationship between GABAA receptor function, beta oscillations and the risk of alcohol dependence.
B6 mice have been also shown to be less susceptible than D2 mice to the proconvulsant effects of the glutamate agonist N-methyl-D-aspartate (NMDA) and the potassium channel antagonist 4-aminopyridine (Kosobud and Crabbe, 1990; Metten and Crabbe, 2005). These data suggest that, compared to D2 mice, B6 mice have deficits in neural mechanisms mediating inhibitory (e.g., GABAergic system) and excitatory (e.g., NMDA and potassium channel functions) neurotransmission in the CNS. Moreover, microdialysis studies have also shown that B6 mice exhibit lower basal levels of acetylcholine (ACh) in the hippocampus than D2 mice (Imperato et al., 1996). Evidence from human studies has implicated genes regulating glutamatergic and cholinergic function with delta and theta oscillatory activity in individuals at risk of alcohol dependence (for review, see Rangaswamy and Porjesz, 2008). However, further research is needed to determine the relationship between these neurotransmitter systems, cortical oscillatory activity and alcohol preference in B6 and D2 mice.
Previous studies using the taste conditioning procedure have shown that B6 and D2 mice also differ in the taste sensitivity to alcohol (Risinger and Cunningham, 1995). Quantitative train locus (QTL) analyses in different strains of mice, including B6 and D2, have identified several chromosomal regions containing genes that may play a role in the development of alcohol-induced conditioned taste aversion (Risinger and Cunningham, 1998). These studies found taste conditioning QTLs on different chromosomes, including chromosome 7 that encode CHRM2. These finding are consistent with differences in cholinergic function between B6 and D2 mice (Imperato et al., 1996). Evidence of significant linkage and association between frontal theta ERO power and CHRM2 has also been shown in humans (Jones et al., 2004, 2006b). However, the relationship among cortical ERO energy, alcohol taste and CHRM2 in B6 and D2 mice is still unclear. Findings from the present study showed that baseline ERO energy in the frontal alpha/beta band and in the parietal delta, theta and alpha/beta bands were significantly reduced in B6 mice, compared to D2 mice. Studies have shown behavioral differences between B6 and D2 mice (Crawley et al., 1997; Mayeda and Hofstetter, 1999). Findings from these studies indicated that B6 mice showed higher levels of motor activity and better performance in the Morris water maze and contextual fear conditioning tasks than D2 mice. In contrast, while B6 mice showed greater baseline startle responses than D2 mice, they showed reduced levels of anxiety-like behaviors and prepulse inhibition (PPI) than D2 mice (Crawley et al., 1997; DuBois et al., 2006). Whether the neural mechanisms mediating behavioral differences between B6 and D2 mice play a role in the strain-dependent differences in cortical ERO energy remain unclear. While ERO and PLI analyses were performed with baseline-corrected values, future studies are needed to determine whether strain-dependent behavioral activity and basal levels of ERO activity and phase locking play a role in the different neurophysiological profile found between B6 and D2 mice.
ERO and PLI analyses in HAP-1, LAP-1 and HS/Ibg mice
Studies have shown that while HAP-1 mice consume two to three times more alcohol than LAP-1 mice (Grahame et al., 1999a), LAP-1 mice showed greater alcohol withdrawal severity following acute alcohol exposure (Chester et al., 2003). These differences between HAP-1 and LAP-1 mice are consistent with differences in alcohol consumption between B6 and D2 mice. However, the electrophysiological profile distinguishing HAP-1 from LAP-1 mice (Slawecki et al., 2003) differs from our earlier results characterizing B6 and D2 mice (Ehlers and Somes, 2002). We previously showed that HAP-1 mice had significantly larger amplitude in the P1 ERP component compared to LAP-1 and HS/Ibg mice (Slawecki et al., 2003). This increased P1 amplitude in the frontal cortex was one of the neurophysiological variables that differentiated HAP-1 from LAP-1 mice. In addition, we showed that EEG peak frequency was lower in LAP-1 mice in the 2–4 Hz and 8–16 Hz EEG bands of the frontal cortex, compared to HAP-1 mice (Slawecki et al., 2003). In contrast, average cortical EEG power was not different between HAP-1 and LAP1 mice (Slawecki et al., 2003). These electrophysiological findings suggest that increased P1 amplitude and increased delta and alpha EEG peak frequencies are the most likely variables indexing susceptibility to high alcohol consumption and preference in ‘high risk” HAP-1 mice compared to ‘low risk’ LAP-1 mice. The P1 component is a large positive component that peaks between 45 and 60 ms (Slawecki et al., 2003). In our original ERP study (Slawecki et al., 2003), assessment of the neurophysiological profile in HS/Ibg mice allowed for the determination of which line, the HAP-1 or the LAP-1, deviated most from the progenitor stock. These initial studies found that HAP-1 mice consumed more alcohol and had higher alcohol preference than LAP-1 and HS/Ibg mice (Slawecki et al., 2003). P1 amplitude was greater in HAP-1 mice than in LAP-1 and HS/Ibg mice (Slawecki et al., 2003). In contrast, the present study found that HAP-1, LAP-1 and HS/Ibg mice showed no significant differences in ERO energy in the delta, theta and alpha/beta bands in the 0 to 50 ms time window. However, we found that HAP-1 mice showed higher theta ERO energy than LAP-1 mice in the 50–350 ms time window, in response to rare tones. In contrast, HAP-1 and LAP-1 mice showed no significant differences in theta PLI. Differences in P1 amplitude between HAP-1 and LAP-1 mice were observed in all three tones (Slawecki et al., 2003), whereas differences in theta ERO energy between HAP-1 and LAP-1 were only observed in response to rare tones.
The amplitude of the P1 component has been associated with increased arousal and attention (Coull, 1998). Studies in high-alcohol-drinking (HAD) and low-alcohol-drinking (LAD) rats from replicate line 2 (HAD-2 and LAD-2) have also shown that HAD-2 rats have increased P1 amplitude compared to LAD-2 rats (Katner et al., 2002). We previously speculated that increased P1 amplitude in HAP-1 mice, compared to LAP-1 mice, could indicate an increased level of arousal or attention in HAP-1 mice (Slawecki et al., 2003). However, this hypothesis is not supported by the fact that alpha/beta EROs, which have been shown to reflect attentional demands (Klimesch et al., 1998), were not different between HAP-1 and LAP-1 mice in the present study. Interestingly, findings from our previous study also suggested that HAP-1 mice showed a significantly higher frontal EEG peak alpha frequency than LAP-1 and HS/Ibg mice (Slawecki et al., 2003). Consistent with the increase in P1 amplitude, these findings were not observed in the parietal cortex. Whether the higher frontal EEG peak alpha frequency played a role in the increase in P1 amplitude in HAP-1 mice remains unclear.
Previous studies have shown behavioral differences between HAP-1 and LAP-1 mice (Grahame et al., 2000; Chester and Barrenha, 2007). While HAP-1 mice showed greater baseline startle responses and PPI than LAP-1 mice, both strains showed similar baseline levels of motor activity. However, since findings from the present study showed that baseline energy ERO activity was not significantly different among HAP-1, LAP-1 and HS/Ibg mice, these different phenotypes do not account for the findings in the present study. Studies by Chester and colleagues (2003) using the taste conditioning procedure showed that HAP-1 and LAP-1 mice also differ in the taste sensitivity to alcohol. QTL analyses in HAP-1 and LAP-1 mice have identified several chromosomal regions containing genes that may play a role in alcohol preference (Bice et al., 2006, 2008). Initial studies in HAP-1 and LAP-1 mice found a QTL on chromosome 9 near the gene that encodes the dopamine D2 receptor, Drd2 (Bice et al., 2006). More recently, Bice et al. (2008) found that alcohol-naive HAP-1 mice showed significantly reduced levels of Drd2 mRNA expression in the nucleus accumbens and hippocampus, compared to LAP-1 mice. These findings are consistent with previous observations by Belknap and Atkins (2001) and Cunningham (1995) of a QTL near the Drd2 locus on chromosome 9 influencing alcohol consumption and conditioned-place preference. While those findings support the hypothesis that lower expression levels of D2 receptors may increase the risk to alcohol and drug use (e.g., Volkow et al., 1999), it is still unclear whether differences in the neurophysiological profile in HAP-1 and LAP-1 mice might be associated with any of these QTLs.
CONCLUSION
Considerable progress has been made to develop mouse and rat models that simulate several relevant human endophenotypic behavioral traits associated with alcohol-related problems. Our initial electrophysiological studies found that ERP responses that distinguish high-alcohol preferring B6 mice from low-alcohol preferring D2 mice (decreased N1 and P3 amplitudes) differ from the ERP responses that distinguish HAP-1 from LAP-1 mice (increased P1 amplitude) (Ehlers and Somes, 2002, Slawecki et al., 2003). The present study extended our initial analyses in these genetic mouse models and found that the decrease in P3 amplitudes previously shown in B6 mice, compared to D2 mice, is related to reductions in evoked delta ERO energy and delta and theta phase locking. We found no evidence supporting our hypothesis that reductions in theta ERO energy are associated with reduced P3 amplitudes in B6 and D2 mice. Our data also suggest that the increase in P1 amplitudes reported in HAP-1 mice, compared to LAP mice, is associated with increases in evoked theta ERO energy, not in alpha/beta ERO energy. These studies suggest that differences in delta and theta ERO measures in mice mirror changes observed between groups at high- and low-risk for alcoholism where changes in EROs were found to be more significant than group differences in P3 amplitudes, further suggesting that ERO measures are more stable endophenotypes in the study of alcohol dependence. Further studies are needed to determine the relationship between the expression of these neurophysiological endophenotypes and the genetic profile of these mouse models. Understanding the relationship between evoked oscillatory activity, phase resetting and ERP responses in mouse models with high and low alcohol preference may provide insight into the brain processes underlying susceptibility to alcohol dependence.
Supplementary Material
Acknowledgments
Supported in part by National Institute on Alcoholism and Alcohol Abuse grant AA006059 and AA014339 and by the Stein Endowment fund. The computer programs were written by Dr. James Havstad. The authors thank Derek Wills, Evelyn Phillips, Phil Lau and Jennifer Roth for assistance in analyses, and Shirley Sanchez for help in editing.
Abbreviations
- DSM-IV-TR
Diagnostic and Statistical Manual of Mental Disorders IV-Text Revision
- CNS
Central Nervous System
- B6
C57BL/6
- D2
DBA/2J
- HAP-1
high alcohol preference-1
- LAP-1
low alcohol preference-1
- g/kg
grams per kilogram
- ERP
event-related potential
- ERO
event-related oscillation
- EEG
electroencephalogram
- dB
decibels
- Hz
Hertz
- ms
milliseconds
- NIMH
National Institute of Mental Health
- MEG
magnetoencephalography
- ROI
region of interest
- ANOVA
analysis of variance
- TBPS
tert-butyl-bicyclo-2,2,2-phosphorothionate
- CHEB
5-(2-cyclohexylidene-ethyl)-5-ethyl barbituric acid
- GABA
γ-aminobutyric acid
- NMDA
N-methyl-D-aspartate
- HAD
high alcohol drinking
- LAD
low alcohol drinking
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amann LC, Phillips JM, Halene TB, Siegel SJ. Male and female mice differ for baseline and nicotine-induced event related potentials. Behav Neurosci. 2008;122:982–990. doi: 10.1037/a0012995. [DOI] [PubMed] [Google Scholar]
- Basar-Eroglu C, Basar E, Demiralp T, Schurmann M. P300-response: possible psychophysiological correlates in delta and theta frequency channels. A review. Int J Psychophysiol. 1992;13:161–179. doi: 10.1016/0167-8760(92)90055-g. [DOI] [PubMed] [Google Scholar]
- Basar E, Basar-Eroglu C, Karakas S, Schurmann M. Are cognitive processes manifested in event-related gamma, alpha, theta and delta oscillations in the EEG? Neurosci Lett. 1999;259:165–168. doi: 10.1016/s0304-3940(98)00934-3. [DOI] [PubMed] [Google Scholar]
- Basar E, Basar-Eroglu C, Karakas S, Schurmann M. Gamma, alpha, delta, and theta oscillations govern cognitive processes. Int J Psychophysiol. 2001a;39:241–248. doi: 10.1016/s0167-8760(00)00145-8. [DOI] [PubMed] [Google Scholar]
- Basar E, Schurmann M, Sakowitz O. The selectively distributed theta system: functions. Int J Psychophysiol. 2001b;39:197–212. doi: 10.1016/s0167-8760(00)00141-0. [DOI] [PubMed] [Google Scholar]
- Basar E, Schurmann M, Basar-Eroglu C, Demiralp T. Selectively distributed gamma band system of the brain. Int J Psychophysiol. 2001c;39:129–135. doi: 10.1016/s0167-8760(00)00136-7. [DOI] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B. Genetics of human brain oscillations. Int J Psychophysiol. 2006;60:162–171. doi: 10.1016/j.ijpsycho.2005.12.013. [DOI] [PubMed] [Google Scholar]
- Belknap JK, Atkins AL. The replicability of QTLs for murine alcohol preference drinking behavior across eight independent studies. Mamm Genome. 2001;12:893–899. doi: 10.1007/s00335-001-2074-2. [DOI] [PubMed] [Google Scholar]
- Bell RL, Rodd ZA, Lumeng L, Murphy JM, McBride WJ. The alcohol-preferring P rat and animal models of excessive alcohol drinking. Addict Biol. 2006;11:270–288. doi: 10.1111/j.1369-1600.2005.00029.x. [DOI] [PubMed] [Google Scholar]
- Bennett B, Downing C, Parker C, Johnson TE. Mouse genetic models in alcohol research. Trends Genet. 2006;22:367–374. doi: 10.1016/j.tig.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Bice PJ, Foroud T, Carr LG, Zhang L, Liu L, Grahame NJ, Lumeng L, Li TK, Belknap JK. Identification of QTLs influencing alcohol preference in the High Alcohol Preferring (HAP) and Low Alcohol Preferring (LAP) mouse lines. Behav Genet. 2006;36:248–260. doi: 10.1007/s10519-005-9019-6. [DOI] [PubMed] [Google Scholar]
- Bice PJ, Liang T, Zhang L, Strother WN, Carr LG. Drd2 expression in the high alcohol- preferring and low alcohol-preferring mice. Mamm Genome. 2008;19:69–76. doi: 10.1007/s00335-007-9089-2. [DOI] [PubMed] [Google Scholar]
- Bressler SL, Freeman WJ. Frequency analysis of olfactory system EEG in cat, rabbit, and rat. Electroencephalogr Clin Neurophysiol. 1980;50:19–24. doi: 10.1016/0013-4694(80)90319-3. [DOI] [PubMed] [Google Scholar]
- Buck KJ, Metten P, Belknap JK, Crabbe JC. Quantitative trait loci involved in genetic predisposition to acute alcohol withdrawal in mice. J Neurosci. 1997;17:3946–3955. doi: 10.1523/JNEUROSCI.17-10-03946.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chester JA, Lumeng L, Li TK, Grahame NJ. High- and low-alcohol-preferring mice show differences in conditioned taste aversion to alcohol. Alcohol Clin Exp Res. 2003;27:12–18. doi: 10.1097/01.ALC.0000046340.06154.9F. [DOI] [PubMed] [Google Scholar]
- Chester JA, Barrenha GD. Acoustic startle at baseline and during acute alcohol withdrawal in replicate mouse lines selectively bred for high or low alcohol preference. Alcohol Clin Exp Res. 2007;31:1633–1644. doi: 10.1111/j.1530-0277.2007.00462.x. [DOI] [PubMed] [Google Scholar]
- Connolly PM, Maxwell C, Liang Y, Kahn JB, Kanes SJ, Abel T, Gur RE, Turetsky BI, Siegel SJ. The effects of ketamine vary among inbred mouse strains and mimic schizophrenia for the P80, but not P20 or N40 auditory ERP components. Neurochem Res. 2004;29:1179–1188. doi: 10.1023/b:nere.0000023605.68408.fb. [DOI] [PubMed] [Google Scholar]
- Coull JT. Neural correlates of attention and arousal: insights from electrophysiology, functional neuroimaging and psychopharmacology. Prog Neurobiol. 1998;55:343–361. doi: 10.1016/s0301-0082(98)00011-2. [DOI] [PubMed] [Google Scholar]
- Crabbe JC. Neurogenetic studies of alcohol addiction. Philos Trans R Soc Lond B Biol Sci. 2008;363:3201–3211. doi: 10.1098/rstb.2008.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley JN, Belknap JK, Collins A, Crabbe JC, Frankel W, Henderson N, Hitzemann RJ, Maxson SC, Miner LL, Silva AJ, Wehner JM, Wynshaw-Boris A, Paylor R. Behavioral phenotypes of inbred mouse strains: implications and recommendations for molecular studies. Psychopharmacology Berl. 1997;132:107–124. doi: 10.1007/s002130050327. [DOI] [PubMed] [Google Scholar]
- Cunningham CL. Localization of genes influencing ethanol-induced conditioned place preference and locomotor activity in BXD recombinant inbred mice. Psychopharmacology (Berl) 1995;120:28–41. doi: 10.1007/BF02246142. [DOI] [PubMed] [Google Scholar]
- Demiralp T, Ademoglu A, Comerchero M, Polich J. Wavelet analysis of P3a and P3b. Brain Topogr. 2001;13:251–267. doi: 10.1023/a:1011102628306. [DOI] [PubMed] [Google Scholar]
- Doppelmayr M, Klimesch W, Schwaiger J, Auinger P, Winkler T. Theta synchronization in the human EEG and episodic retrieval. Neurosci Lett. 1998;257:41–44. doi: 10.1016/s0304-3940(98)00805-2. [DOI] [PubMed] [Google Scholar]
- DuBois DW, Perlegas A, Floyd DW, Weiner JL, McCool BA. Distinct functional characteristics of the lateral/basolateral amygdala GABAergic system in C57BL/6J and DBA/2J mice. J Pharmacol Exp Ther. 2006;318:629–640. doi: 10.1124/jpet.105.100552. [DOI] [PubMed] [Google Scholar]
- Edenberg HJ, Dick DM, Xuei X, Tian H, Almasy L, Bauer LO, Crowe RR, Goate A, Hesselbrock V, Jones K, Kwon J, Li TK, Nurnberger JI, Jr, O’Connor SJ, Reich T, Rice J, Schuckit MA, Porjesz B, Foroud T, Begleiter H. Variations in GABRA2, encoding the alpha 2 subunit of the GABA(A) receptor, are associated with alcohol dependence and with brain oscillations. Am J Hum Genet. 2004;74:705–714. doi: 10.1086/383283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Somes C. Long latency event-related potentials in mice: effects of stimulus characteristics and strain. Brain Res. 2002;957:117–128. doi: 10.1016/s0006-8993(02)03612-0. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Criado JR. Event-related oscillations in mice:effects of stimulus characteristics. J Neurosci Methods. 2009 doi: 10.1016/j.jneumeth.2009.04.015. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlichman RS, Maxwell CR, Majumdar S, Siegel SJ. Deviance-elicited changes in event-related potentials are attenuated by ketamine in mice. J Cogn Neurosci. 2008;20:1403–1414. doi: 10.1162/jocn.2008.20097. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. New York: Academic Press; 1997. [Google Scholar]
- Gabor D. Theory of Communication. J Inst Elec Eng. 1946;93:429–457. [Google Scholar]
- Gandal MJ, Ehrlichman RS, Rudnick ND, Siegel SJ. A novel electrophysiological model of chemotherapy-induced cognitive impairments in mice. Neuroscience. 2008;157:95–104. doi: 10.1016/j.neuroscience.2008.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gevins A, Smith ME, Leong H, McEvoy L, Whitfield S, Du R, Rush G. Monitoring working memory load during computer-based tasks with EEG pattern recognition methods. Hum Factors. 1998;40:79–91. doi: 10.1518/001872098779480578. [DOI] [PubMed] [Google Scholar]
- Grahame NJ, Li TK, Lumeng L. Limited access alcohol drinking in high- and low- alcohol preferring selected lines of mice. Alcohol Clin Exp Res. 1999a;23:1015–1022. [PubMed] [Google Scholar]
- Grahame NJ, Li TK, Lumeng L. Selective breeding for high and low alcohol preference in mice. Behav Genet. 1999b;29:47–57. doi: 10.1023/a:1021489922751. [DOI] [PubMed] [Google Scholar]
- Grahame NJ, Rodd-Henricks K, Li TK, Lumeng L. Ethanol locomotor sensitization, but not tolerance correlates with selection for alcohol preference in high- and low-alcohol preferring mice. Psychopharmacology (Berl) 2000;151:252–260. doi: 10.1007/s002130000388. [DOI] [PubMed] [Google Scholar]
- Green AS, Grahame NJ. Ethanol drinking in rodents: is free-choice drinking related to the reinforcing effects of ethanol? Alcohol. 2008;42:1–11. doi: 10.1016/j.alcohol.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imperato A, Obinu MC, Mascia MS, Casu MA, Zocchi A, Cabib S, Puglisi-Allegra S. Strain-dependent effects of dopamine agonists on acetylcholine release in the hippocampus: an in vivo study in mice. Neuroscience. 1996;70:653–660. doi: 10.1016/s0306-4522(96)83004-1. [DOI] [PubMed] [Google Scholar]
- Jones KA, Porjesz B, Almasy L, Bierut L, Goate A, Wang JC, Dick DM, Hinrichs A, Kwon J, Rice JP, Rohrbaugh J, Stock H, Wu W, Bauer LO, Chorlian DB, Crowe RR, Edenberg HJ, Foroud T, Hesselbrock V, Kuperman S, Nurnberger J, Jr, O’Connor SJ, Schuckit MA, Stimus AT, Tischfield JA, Reich T, Begleiter H. Linkage and linkage disequilibrium of evoked EEG oscillations with CHRM2 receptor gene polymorphisms: implications for human brain dynamics and cognition. Int J Psychophysiol. 2004;53:75–90. doi: 10.1016/j.ijpsycho.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Jones KA, Porjesz B, Chorlian D, Rangaswamy M, Kamarajan C, Padmanabhapillai A, Stimus A, Begleiter H. S-transform time-frequency analysis of P300 reveals deficits in individuals diagnosed with alcoholism. Clin Neurophysiol. 2006a;117:2128–2143. doi: 10.1016/j.clinph.2006.02.028. [DOI] [PubMed] [Google Scholar]
- Jones KA, Porjesz B, Almasy L, Bierut L, Dick D, Goate A, Hinrichs A, Rice JP, Wang JC, Bauer LO, Crowe R, Foroud T, Hesselbrock V, Kuperman S, Nurnberger J, Jr, O’Connor SJ, Rohrbaugh J, Schuckit MA, Tischfield J, Edenberg HJ, Begleiter H. A cholinergic receptor gene (CHRM2) affects event-related oscillations. Behav Genet. 2006b;36:627–639. doi: 10.1007/s10519-006-9075-6. [DOI] [PubMed] [Google Scholar]
- Kaneko WM, Ehlers CL, Phillips EL, Riley EP. Auditory event-related potentials in fetal alcohol syndrome and Down’s syndrome children. Alcohol Clin Exp Res. 1996;20:35–42. doi: 10.1111/j.1530-0277.1996.tb01040.x. [DOI] [PubMed] [Google Scholar]
- Karakas S, Erzengin OU, Basar E. A new strategy involving multiple cognitive paradigms demonstrates that ERP components are determined by the superposition of oscillatory responses. Clin Neuropathol. 2000;111:1719–1732. doi: 10.1016/s1388-2457(00)00418-1. [DOI] [PubMed] [Google Scholar]
- Katner SN, Slawecki CJ, Ehlers CL. Neurophysiological profiles of replicate line 2 high-alcohol-drinking (HAD-2) and low-alcohol-drinking (LAD-2) rats. Alcohol Clin Exp Res. 2002;26:1669–1677. doi: 10.1097/01.ALC.0000036286.55213.D8. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Schimke H, Schwaiger J. Episodic and semantic memory: an analysis in the EEG theta and alpha band. Electroencephalogr Clin Neurophysiol. 1994;91:428–441. doi: 10.1016/0013-4694(94)90164-3. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Schimke H, Ripper B. Theta synchronization and alpha desynchronization in a memory task. Psychophysiology. 1997;34:169–176. doi: 10.1111/j.1469-8986.1997.tb02128.x. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Russegger H, Pachinger T, Schwaiger J. Induced alpha band power changes in the human EEG and attention. Neurosci Lett. 1998;244:73–76. doi: 10.1016/s0304-3940(98)00122-0. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Schack B, Schabus M, Doppelmayr M, Gruber W, Sauseng P. Phase-locked alpha and theta oscillations generate the P1-N1 complex and are related to memory performance. Brain Res Cogn Brain Res. 2004;19:302–316. doi: 10.1016/j.cogbrainres.2003.11.016. [DOI] [PubMed] [Google Scholar]
- Kopell N, Ermentrout GB, Whittington MA, Traub RD. Gamma rhythms and beta rhythms have different synchronization properties. Proc Natl Acad Sci U S A. 2000;97:1867–1872. doi: 10.1073/pnas.97.4.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosobud AE, Crabbe JC. Genetic correlations among inbred strain sensitivities to convulsions induced by 9 convulsant drugs. Brain Res. 1990;526:8–16. doi: 10.1016/0006-8993(90)90243-5. [DOI] [PubMed] [Google Scholar]
- Lovinger DM, Crabbe JC. Laboratory models of alcoholism: treatment target identification and insight into mechanisms. Nat Neurosci. 2005;8:1471–1480. doi: 10.1038/nn1581. [DOI] [PubMed] [Google Scholar]
- Maxwell CR, Liang Y, Weightman BD, Kanes SJ, Abel T, Gur RE, Turetsky BI, Bilker WB, Lenox RH, Siegel SJ. Effects of chronic olanzapine and haloperidol differ on the mouse N1 auditory evoked potential. Neuropsychopharmacology. 2004;29:739–746. doi: 10.1038/sj.npp.1300376. [DOI] [PubMed] [Google Scholar]
- Mayeda AR, Hofstetter JR. A QTL for the genetic variance in free-running period and level of locomotor activity between inbred strains of mice. Behav Genet. 1999;29:171–176. doi: 10.1023/a:1021639901679. [DOI] [PubMed] [Google Scholar]
- Metten P, Crabbe JC. Alcohol withdrawal severity in inbred mouse (Mus musculus) strains. Behav Neurosci. 2005;119:911–925. doi: 10.1037/0735-7044.119.4.911. [DOI] [PubMed] [Google Scholar]
- Padmanabhapillai A, Porjesz B, Ranganathan M, Jones KA, Chorlian DB, Tang Y, Kamarajan C, Rangaswamy M, Stimus A, Begleiter H. Suppression of early evoked gamma band response in male alcoholics during a visual oddball task. Int J Psychophysiol. 2006a;60:15–26. doi: 10.1016/j.ijpsycho.2005.03.026. [DOI] [PubMed] [Google Scholar]
- Padmanabhapillai A, Tang Y, Ranganathan M, Rangaswamy M, Jones KA, Chorlian DB, Kamarajan C, Stimus A, Kuperman S, Rohrbaugh J, O’Connor SJ, Bauer LO, Schuckit MA, Begleiter H, Porjesz B. Evoked gamma band response in male adolescent subjects at high risk for alcoholism during a visual oddball task. Int J Psychophysiol. 2006b;62:262–271. doi: 10.1016/j.ijpsycho.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Phillips JM, Ehrlichman RS, Siegel SJ. Mecamylamine blocks nicotine-induced enhancement of the P20 auditory event-related potential and evoked gamma. Neuroscience. 2007;144:1314–1323. doi: 10.1016/j.neuroscience.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips TJ, Crabbe JC, Metten P, Belknap JK. Localization of genes affecting alcohol drinking in mice. Alcohol Clin Exp Res. 1994;18:931–941. doi: 10.1111/j.1530-0277.1994.tb00062.x. [DOI] [PubMed] [Google Scholar]
- Porjesz B, Almasy L, Edenberg HJ, Wang K, Chorlian DB, Foroud T, Goate A, Rice JP, O’Connor SJ, Rohrbaugh J, Kuperman S, Bauer LO, Crowe RR, Schuckit MA, Hesselbrock V, Conneally PM, Tischfield JA, Li TK, Reich T, Begleiter H. Linkage disequilibrium between the beta frequency of the human EEG and a GABAA receptor gene locus. Proc Natl Acad Sci U S A. 2002;99:3729–3733. doi: 10.1073/pnas.052716399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porjesz B, Rangaswamy M, Kamarajan C, Jones KA, Padmanabhapillai A, Begleiter H. The utility of neurophysiological markers in the study of alcoholism. Clin Neuropathol. 2005;116:993–1018. doi: 10.1016/j.clinph.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Putnam LE, Roth WT. Effects of stimulus repetition, duration, and rise time on startle blink and automatically elicited P300. Psychophysiology. 1990;27:275–297. doi: 10.1111/j.1469-8986.1990.tb00383.x. [DOI] [PubMed] [Google Scholar]
- Rangaswamy M, Jones KA, Porjesz B, Chorlian DB, Padmanabhapillai A, Kamarajan C, Kuperman S, Rohrbaugh J, O’Connor SJ, Bauer LO, Schuckit MA, Begleiter H. Delta and theta oscillations as risk markers in adolescent offspring of alcoholics. Int J Psychophysiol. 2007a;63:3–15. doi: 10.1016/j.ijpsycho.2006.10.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangaswamy M, Porjesz B, Chorlian D, Bodis-Wollner I. NIDA 2007 Mini Convention: Frontiers in Addiction Research, Poster Session, San Diego, CA. Bethesda, MD: National Institute on Drug Abuse, National Institutes of Health, U.S. Department of Health and Human Services; 2007b. Attenuate beta band oscillations to onset and offset of visual stimuli in alcohol dependence: Indication of compromised circuitry. [Google Scholar]
- Rangaswamy M, Porjesz B. Uncovering genes for cognitive (dys)function and predisposition for alcoholism spectrum disorders: a review of human brain oscillations as effective endophenotypes. Brain Res. 2008;1235:153–171. doi: 10.1016/j.brainres.2008.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risinger FO, Cunningham CL. Genetic differences in ethanol-induced conditioned taste aversion after ethanol preexposure. Alcohol. 1995;12:535–539. doi: 10.1016/0741-8329(95)00040-2. [DOI] [PubMed] [Google Scholar]
- Risinger FO, Cunningham CL. Ethanol-induced conditioned taste aversion in BXD recombinant inbred mice. Alcohol Clin Exp Res. 1998;22:1234–1244. [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, Sable HJ, Murphy JM, McBride WJ. Recent advances in animal models of alcohol craving and relapse. Pharmacol Biochem Behav. 2004;79:439–450. doi: 10.1016/j.pbb.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Rodriguez LA, Plomin R, Blizard DA, Jones BC, McClearn GE. Alcohol acceptance, preference, and sensitivity in mice. II. Quantitative trait loci mapping analysis using BXD recombinant inbred strains. Alcohol Clin Exp Res. 1995;19:367–373. doi: 10.1111/j.1530-0277.1995.tb01517.x. [DOI] [PubMed] [Google Scholar]
- Rudnick ND, Koehler C, Picciotto MR, Siegel SJ. Role of beta2-containing nicotinic acetylcholine receptors in auditory event-related potentials. Psychopharmacology (Berl) 2009;202:745–751. doi: 10.1007/s00213-008-1358-6. [DOI] [PubMed] [Google Scholar]
- Ruf C, Carosone-Link P, Springett J, Bennett B. Confirmation and genetic dissection of a major quantitative trait locus for alcohol preference drinking. Alcohol Clin Exp Res. 2004;28:1613–1621. doi: 10.1097/01.alc.0000145693.58448.95. [DOI] [PubMed] [Google Scholar]
- Sanchis-Segura C, Spanagel R. Behavioural assessment of drug reinforcement and addictive features in rodents: an overview. Addict Biol. 2006;11:2–38. doi: 10.1111/j.1369-1600.2006.00012.x. [DOI] [PubMed] [Google Scholar]
- Sauseng P, Klimesch W, Gruber WR, Hanslmayr S, Freunberger R, Doppelmayr M. Are event-related potential components generated by phase resetting of brain oscillations? A critical discussion. Neuroscience. 2007;146:1435–1444. doi: 10.1016/j.neuroscience.2007.03.014. [DOI] [PubMed] [Google Scholar]
- Schack B, Klimesch W. Frequency characteristics of evoked and oscillatory electroencephalic activity in a human memory scanning task. Neurosci Lett. 2002;331:107–110. doi: 10.1016/s0304-3940(02)00846-7. [DOI] [PubMed] [Google Scholar]
- Schurmann M, Basar-Eroglu C, Basar E. Gamma responses in the EEG: elementary signals with multiple functional correlates. Neuroreport. 1997;8:531–534. doi: 10.1097/00001756-199701200-00030. [DOI] [PubMed] [Google Scholar]
- Schurmann M, Demiralp T, Basar E, Basar EC. Electroencephalogram alpha (8–15 Hz) responses to visual stimuli in cat cortex, thalamus, and hippocampus: a distributed alpha network? Neurosci Lett. 2000;292:175–178. doi: 10.1016/s0304-3940(00)01456-7. [DOI] [PubMed] [Google Scholar]
- Schurmann M, Basar-Eroglu C, Kolev V, Basar E. Delta responses and cognitive processing: single-trial evaluations of human visual P300. Int J Psychophysiol. 2001;39:229–239. doi: 10.1016/s0167-8760(00)00144-6. [DOI] [PubMed] [Google Scholar]
- Siegel SJ, Connolly P, Liang Y, Lenox RH, Gur RE, Bilker WB, Kanes SJ, Turetsky BI. Effects of strain, novelty, and NMDA blockade on auditory-evoked potentials in mice. Neuropsychopharmacology. 2003;28:675–682. doi: 10.1038/sj.npp.1300087. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ, Grahame NJ, Roth J, Katner SN, Ehlers CL. EEG and ERP profiles in the high alcohol preferring (HAP) and low alcohol preferring (LAP) mice: relationship to ethanol preference. Brain Res. 2003;961:243–254. doi: 10.1016/s0006-8993(02)03959-8. [DOI] [PubMed] [Google Scholar]
- Stampfer HG, Basar E. Does frequency analysis lead to better understanding of human event related potentials. Int J Neurosci. 1985;26:181–196. doi: 10.3109/00207458508985616. [DOI] [PubMed] [Google Scholar]
- Stockwell RG, Mansinha L, Lowe RP. Localization of the complex spectrum: The S Transform. IEEE Trans on Signal Processing. 1996;44:998–1001. [Google Scholar]
- Umbricht D, Vyssotki D, Latanov A, Nitsch R, Lipp HP. Deviance-related electrophysiological activity in mice: is there mismatch negativity in mice? Clin Neurophysiol. 2005;116:353–363. doi: 10.1016/j.clinph.2004.08.015. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Begleiter H, Hitzemann R, Pappas N, Burr G, Pascani K, Wong C, Fowler JS, Wolf AP. Regional brain metabolic response to lorazepam in subjects at risk for alcoholism. Alcohol Clin Exp Res. 1995;19:510–516. doi: 10.1111/j.1530-0277.1995.tb01539.x. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Gifford A, Hitzemann R, Ding YS, Pappas N. Prediction of reinforcing responses to psychostimulants in humans by brain dopamine D2 receptor levels. Am J Psychiatry. 1999;156:1440–1443. doi: 10.1176/ajp.156.9.1440. [DOI] [PubMed] [Google Scholar]
- Whittington MA, Traub RD, Kopell N, Ermentrout B, Buhl EH. Inhibition-based rhythms: experimental and mathematical observations on network dynamics. Int J Psychophysiol. 2000;38:315–336. doi: 10.1016/s0167-8760(00)00173-2. [DOI] [PubMed] [Google Scholar]
- Yordanova J, Kolev V. Brain theta response predicts P300 latency in children. Neuroreport. 1996;8:277–280. doi: 10.1097/00001756-199612200-00055. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.