Abstract
The primary function of the eye lens is to focus light on the retina. The major proteins in the lens—a, b, and g-crystallins—are constantly subjected to age-related changes such as oxidation, deamidation, truncation, glycation, and methylation. Such age-related modifications are cumulative and affect crystallin structure and function. With time, the modified crystallins aggregate, causing the lens to increasingly scatter light on the retina instead of focusing light on it and causing the lens to lose its transparency gradually and become opaque. Age-related lens opacity, or cataract, is the major cause of blindness worldwide. We review deamidation, and glycation that occur in the lenses during aging keeping in mind the structural and functional changes that these modifications bring about in the proteins. In addition, we review proteolysis and discuss recent observations on how crystallin fragments generated in vivo, through their anti-chaperone activity may cause crystallin aggregation in aging lenses. We also review hyperbaric oxygen treatment induced guinea pig and ‘humanized’ ascorbate transporting mouse models as suitable options for studies on age-related changes in lens proteins.
Keywords: lens crystallins, aging, lens opacity, chaperones, deamidation, glycation, oxidation, peptides
1. Introduction
The composition and architecture of the eye lens, with its elongated fiber cells filled with stable long-lived proteins, allows the lens to focus light on the retina. Although the lens has protective mechanisms to preserve its function throughout the life span of an individual, with aging these mechanisms begin to deteriorate and the lens begins to accumulate modified proteins. This failure leads to gradual aggregation of the lens proteins, with a concomitant loss in the optical quality of the lens, and ultimately leads to opacity, or cataract [1–8]. There are several excellent reviews in the literature that describe both the physiology and biochemistry of the lens and cataract formation [7–18]. These reviews provide well-founded accounts of the mechanisms of lens development, lens morphogenesis, lens protein oxidation, presbyopia, and the biology of lens capsule. The purpose of this review is to emphasize the recent findings on age-related changes in lens crystallins and how such changes might affect the structure and function of lens crystallins and modulate aggregation of crystallins, a hallmark of aged and cataract lenses.
2. Lens development, anatomy and physiology
2.1. The embryonic lens
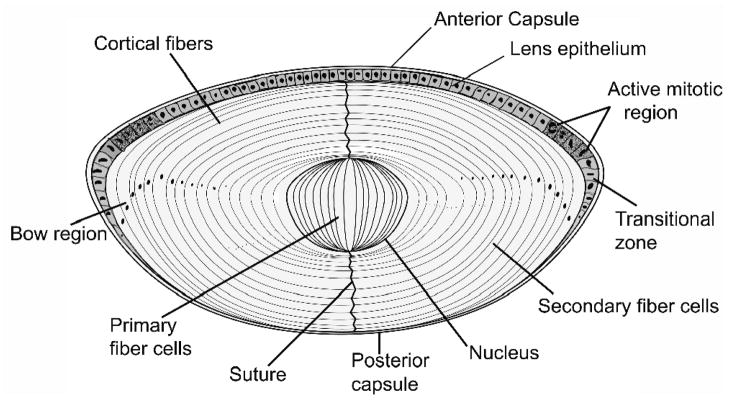
The eye lens is an a vascular tissue encapsulated in a collagenous basement membrane-like material composed of a single layer of epithelial cells on the anterior subcapsular surface [19]. The lens derives all of its nutrients and oxygen from the aqueous humor and vitreous body. At the equatorial zone the epithelial cells begin to differentiate, elongating to become fiber cells, during which time they lose the organelles, and begin synthesizing large quantities of the structural proteins called crystallins. This process continues throughout life, though at a slower pace, with the younger/newer fiber cells pushing the older fibers to the center (nuclear) region of the lens (Fig. 1). Although the lens has a limited number of cells and cell types, its development is complex [5, 12]. Lens development begins with the invagination of lens placode toward the optic cup to become the lens pit. The invagination process continues, the pit closes and a lens vesicle is formed in humans by embryonic day 33. Following this, the differentiating epithelial cells start filling the vesicle until the whole cavity is obliterated with fiber cells by the end of seventh week. The ‘first formed’ fiber cells that occupy the center of the lens become the embryonic nucleus [12, 19]. Lens development is not complete without the removal of all potential light-scattering organelles from the fiber cells, which is accomplished in a programmed process that involves proteases [15, 20]. The embryonic spherical lens, measuring about 0.35 mm in diameter initially, quickly begins to grow to an elliptical organ of about 35 mg by birth. Studies of human lenses ranging in age from 6 months to 99 years show that the lens actually grows in two phases, an “asymptotic phase” during the prenatal and early childhood periods and a linear phase during the rest of the life span [5, 21]. Although it was once thought that lenses of males are heavier than lenses of females, new data do not support this notion [21]. The best estimate of human lens weight can be obtained by the expression, W=1.38Ab+149exp^[exp^(1.6−3Ac)], where W is lens weight in mg, Ab is post-natal age in years and Ac is the time since conception in years [21]. Because the lens is composed of a range of fibers representing different ages, it is an attractive tissue for studying the effects of aging on protein structure and function [4].
Fig. 1. A drawing of lens showing the single layer of epithelial cells covering the anterior cross section.
The elongated fiber cells are directly in contact with epithelial layer in the anterior region, whereas they make contact with capsule in the posterior region. In the lens bow region the cells differentiate, elongate, lose their organelles and begin to form newly differentiated fiber cells.
2.2 The lens capsule and epithelium
The lens capsule is a specialized basement membrane with lamellar structure formed anteriorly by epithelial cells and posteriorly by fiber cells. Collagen type IV, laminin, entactin, heparin sulfate proteoglycan, and fibronectin constitute the major components of the capsule and contribute to the molding of lens shape [5]. The lens capsule, which is about 4 mm in thickness at the birth, grows throughout life but as age advances, its thickness reaches as high as 30 mm at the peripheral region of the anterior capsule [8]. The mechanical properties of the lens capsule decrease with age, which can be attributed to the changes occurring in the capsule collagen. All aspects of the lens capsule have been described in an excellent, recently published review [17]. A single layer of polygonal cuboidal epithelial cells cover the lens anteriorly and can be demarcated into two regions, namely, the non-dividing central epithelium and dividing [germinative] epithelium, which in turn differentiate at the bow region (Fig. 1). The lens epithelial cells possess the remarkable feature of dividing and differentiating into new fiber cells throughout life but this process diminishes with age. The lens epithelium is the major site of transport, metabolism and detoxification, since the lens fiber cells derive the bulk of their nutrients through the epithelial cells. An age-related decrease in epithelial density has been documented [22]. However, it has not been established that low epithelial cell density contributes to the development of cataract [23]. The epithelial cells are known to provide the lens with the first line of defense against oxidative insults [8, 14]. Because the integrity of the epithelial layer is critical to normal lens physiology, epithelial cell death by apoptosis or any abnormalities in the cytoskeleton proteins in these cells are detrimental to the underlying fiber cells. Results of the studies of the effects of different metabolites and drugs on the epithelial cultures (primary or transformed) have been inconclusive in elucidating the exact role of the epithelial cells in inducing age-related changes of the lens because the cell culture system does not replicate the gradient of cellular and metabolic differences observed in lens [7, 8, 24]. A recent review [14] on lens epithelia has summarized the role of aA- and aB- in lens epithelia and has highlighted that the a-crystallin expression is critical for the survival, growth and proliferation of this group of cells.
3. Lens Proteins and Their Role in Lens Aging
3.1. Lens water-soluble and water-insoluble proteins
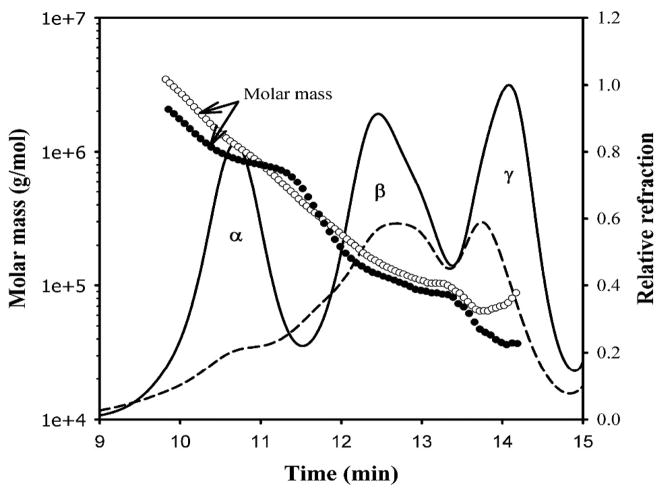
The human lens is composed of three main types of proteins, a-, b- and g-crystallins, which account for nearly 90 percent of the total lens proteins [4, 11]. Homogenization of human lens in water or buffer and centrifugation yields two fractions: a water-soluble (WS) fraction and a water-insoluble (WIS) fraction [4, 5]. As much as 50 percent of the lens proteins in the aged (>50 yrs) human eye enters the WIS fraction [3, 25], and WIS proteins are known to increase with aging [4, 5]. Although it has been known for over 30 years that almost all of the a-crystallin present in the nuclear region of +40-year-old lenses exists in the WIS fraction, [3], the reasons for the insolubilization of a-crystallin in the nuclear region are only now becoming clear. Compared with the WS fraction, the WIS fraction contains a relatively larger proportion of the modified and cross-linked crystallins. In addition, the WIS fraction also contains lens fiber cell membranes and cytoskeleton proteins [5, 26]. The crystallins in the WIS fraction are the primary light-scattering aggregates in aging lenses. Studies show that in human lens, the nuclear region, the oldest part of the lens, has the highest percentage of WIS proteins and the proteins from the nuclear region also exhibit the highest degree of modifications and light-scattering properties [5, 6, 26–35]. Analyses of the WS protein fraction by size exclusion chromatography and dynamic light-scattering methods show that aged lenses have a significant reduction in the WS protein fraction as compared to young lenses (Fig. 2). The crystallins in the human lens WS fraction can be categorized into three major peaks: a-, b- and g-crystallins (Fig. 2). The mass distribution indicates that the aged lens contains WS protein aggregates as large as 3500 kDa, as compared to 2000 kDa aggregates in the young lens [36]. The bulk of the WS protein in aged lenses (that is, 70 years old) is comprised of b- and g-crystallins, whereas in a 19-year-old lens, a-, b- and g-crystallin are present in the WS fraction in comparable concentrations [36].
Fig. 2. Mass distribution in the water-soluble fraction of young (19 years, solid line and filled circle) and aged (83 years, broken line and open circle) human lenses using multi-angle light scattering.
The samples (250 mg of proteins) were injected into a TSK G5000PWXL size exclusion column attached to a Shimadzu high pressure liquid chromatograph system with refractive index detector. The eluting proteins were analyzed with the help of Wyatt multi-angle light scattering and quasi-elastic light scattering detectors. The mobile phase contained 0.05 M sodium phosphate and 0.15 m sodium chloride, pH 7.2. The mass was determined using ASTRA software from Wyatt. Reproduced with permission from the J. of Biol. Chem. (2008) 283 8477–8485.
3.2. a-Crystallinand its A and B subunits
Of the three classes of crystallins, a -crystallin is the predominant type (Table 1) and is composed of two types of subunits, A and B, which noncovalently associate to form aggregates with an average molecular weight of 800 kDa [11]. The aA-crystallin subunit is more prevalent than the aB- subunit, in a ratio varying from 3:1 to 2:1 in different regions of the lens. The primary structures of aA- and aB-crystallin subunits exhibit a high degree of homology between them and to small heat shock proteins [sHSPs] because of the conserved a -crystallin domain in these proteins [9, 11]. The secondary structure of a-crystallin subunits is primarily in the form of b- sheets [9]. The tertiary and quaternary structures of a-crystallin are not known. The a A-crystallin domain is thought to have an immunoglobulin-like folding pattern [11]. Nuclear magnetic resonance studies show that the C-terminal regions of aA- and a B-crystallins are more flexible than the N-terminal regions [37]. The a-crystallin molecule is a dynamic oligomer, with the subunits dissociating and reassociating constantly. For example, in four hours time, all subunits in a a -crystallin molecule exchange with a new set of subunits [38]. This subunit exchange property is also implicated in the α-crystallin chaperone activity. Several studies have shown that the subunit exchange rate is affected by specific mutations in a -crystallin subunits [39, 40] or is altered after truncations [40, 41] and age-related modifications [40, 42, 43]. Both in vivo and in vitro studies to understand the molecular basis of cataract-causing crystallin mutations and age-related cataract show that loss of chaperone activity and increased protein aggregation are responsible for the lens pathology [44–47]. Delineation of the three-dimensional structure of the major constituent of lens proteins, a -crystallin, has thus far eluded scientific investigation. However, the availability of structures of sHSP 16.1 [48] and 16.5 proteins [49], which have a high sequence homology to aB- and a A- sequences and structures allows researchers to build structural models on which to base valid structure-function predictions [11, 50]. The use of site-directed mutagenesis, Cryo-EM and NMR techniques in combination should allow the researchers to build high-resolution structures for aA- and aB-crystallin oligomers.
Table 1.
The Human Lens Crystallins
| Crystallin | No. of Residues | Monomer [kDa] | SwissPort No |
|---|---|---|---|
| a-crystallins | |||
| aA | 173 | 19909 | P02489 |
| aB | 175 | 20159 | P02511 |
| b-crystallins | |||
| bA1 | 198 | 23191 | P05813 |
| bA2 | 196 | 21964 | P53672 |
| bA3 | 215 | 25150 | P05813 |
| bA4 | 195 | 22234 | P53673 |
| bB1 | 251 | 27892 | P53674 |
| bB2 | 204 | 23249 | P43320 |
| bB3 | 211 | 24230 | P26998 |
| g-crystallins* | |||
| gS | 177 | 20875 | P22914 |
| gA | 173 | 20761 | P11844 |
| gB | 174 | 20776 | P07316 |
| gC | 173 | 20747 | P07315 |
| gD | 173 | 20607 | P07320 |
gE and gF- are not expressed in humans whereas these genes are expressed in bovine lenses
3.3. €β-crystallin and g -crystallin
Both b- and g -crystallins are comprised of several proteins related by sequence and structure [11]. The b-crystallins include four acidic and three basic subunits that have molecular masses in the range of 22 – 28 kDa, whereas γ-crystallins have 7 different types of subunits of ~20kDa [Table 1]. The gS-crystallin elutes ahead of the rest of g-crystallins during size exclusion chromatography although all g-crystallins are considered to be monomeric in nature. In human lenses, the gD- and gE-crystallins are not expressed, whereas they are found in bovine lenses. The primary structures of all 3 classes of crystallins have been known for some time, as have the three-dimensional structures of β- and g-crystallins. A recent review provides a good discussion of the structures of b- and g -crystallins [11].
A primary function of crystallins is to focus light on the retina by maintaining the necessary refractive characteristics and clarity of the lens. Lens proteins undergo very little turnover, but they do undergo various changes during aging and cataractogenesis, including increased crystallin proteolysis, fragmentation and aggregation. Binding of denatured b- or g-crystallins to either of the a -crystallin subunits affects the subunit exchange [51]. Proper subunit interaction between crystallins is necessary to prevent the formation of light-scattering aggregates of crystallins. Age-related changes may affect the short-range interactions [52] among a-, b- and g -crystallins that are critical for lens transparency. Equilibrium dialysis studies show that an age-related decrease occurs in the interactions between g-crystallin and a -crystallin [53]. The changes in the interactions may lead to increased homoaggregation of proteins and localized fluctuations in protein densities sufficient to cause light scattering [53, 54]. The formation of protein aggregates of crystallins, either by this mechanism or other mechanisms, could be the first event in the development of cataracts.
4. Role of Crystallins in Lens Transparency and Lens Opacity
4.1. α-Crystallin chaperone activity
There is general agreement that in addition to the structural role of a -crystallin in the lens, it also functions as a chaperone [11, 44, 55, 56]. Studies of total lens homogenates as well as of whole lens also argue in favor of the chaperone function of a -crystallin in the lens [55, 57]. The aA-knockout studies as well as analysis of congenital cataracts with aA- and aB-crystallin mutations also support the role of these two subunits in a -crystallin chaperone activity [46, 47, 58–60]. a -Crystallin chaperone activity (or that of its subunits) can suppress the aggregation of proteins denatured by oxidation, heat, and other stressors [55]. Chaperone activity is susceptible to modulation by low-molecular-weight compounds such as adenosine triphosphate (ATP) and glutathione [61, 62]. Concentrations of ATP and glutathione are known to change with aging [10, 63]. Glycation, ultraviolet irradiation and deamidation results in loss of a -crystallin chaperone activity [40, 42, 43, 64]. Glycation from uncontrolled diabetes and exposure to ultraviolet light are known to increase the cataract risk [64, 65]. The factors that suppress a -crystallin chaperone function are thought to accelerate aggregation of other crystallins that are undergoing age-related modifications and losing their native structure. Consistent with this hypothesis, decreased chaperone activity, increased crystallin aggregation, light scattering and loss of lens transparency have indeed been demonstrated in aged human lenses [66].
Both direct and indirect evidence suggests that multiple regions in a -crystallin subunits may be involved in chaperone activity [67–69]. The structural changes in the modified crystallins are likely to 1) mask the chaperone site and prevent chaperone action, 2) interfere with aA- and aB-crystallin subunit interactions, or 3) make the chaperone protein “hyperactive,” resulting in a chaperone-substrate complex that is unstable and aggregates rapidly [45, 70]. The structure of a -crystallin apparently must remain dynamic, with subunits constantly disassociating and reassociating, in order for chaperone activity to be maintained. For example, crystallin cross-linking studies show that when subunit dissociation is restricted, chaperone-like activity decreases [66, 71]. Conversely, cleavage of a -crystallin intersubunit cross-links results in recovery of chaperone activity [66], indicating that the dynamic nature of the a-crystallin structure needs to be maintained in order to exert maximal chaperone activity. Therefore, increased crosslinking of a -crystallin, which is known to occur in the aging lens [4], may be a contributing factor in the loss of chaperone activity as the lens ages.
4.2. Post-translational modification of crystallins
Studies have shown that lens crystallins undergo extensive modifications [4, 33–35, 72–76]. Recent advances in analytical methods have allowed the identification of several new modification sites. The major modifications include deamidation, truncation, Cys-methylation, phosphorylation, oxidation, acetylation, carbamylation, and glycation [26, 34, 35, 64, 77–84]. Various modifications and modification sites are summarized in the Table 2. While a number of studies have aimed at identifying the modifications and the sites of these modifications, few studies have attempted to measure the extent of the modifications. Most studies have compared the level of modifications in young and aged lenses or in young and aged, cataract lenses. In some instances, the modifications were identified in pooled lens samples, but the values in pooled lens samples do not reflect the sample-to-sample variations generally observed in individual human lens samples. For these reasons one can see inconsistency in some of the modifications reported in the literature. This is obvious from the references in Table 2 which shows that deamidation or oxidation or phosphorylation at specific sites were not discovered by each and every analysis.
Table 2.
Post-translational/age-related modifications in human lens crystallins
| Crystallin | Modification | References | ||
|---|---|---|---|---|
| aA | Deamidation Q6, Q50, Q90, N101, Q104, N123, Q147 | [32–34,87,88,144,198–200] | ||
| Oxidation M1, W9, Y18, Y34, M138, C142, | [32,33,75,84] | |||
| Phoshorylation T13, S45, S122, T140 | [32,34,75,144,199] | |||
| Methylation R21, K88 | [75] | |||
| Acetylation M1, K70, K78, K145 | [34,75,144, 201–203] | |||
| Disulphide C131–C142 | [84] | |||
| Other C131, S162, Unknown S20, H79 | [34,84,144,204,205] | |||
|
| ||||
| aB | Deamidation Q25, N78, Q108, N146 | [32–35,76,144,173] | ||
| Oxidation M1, H7, H7, W9, Y48, W60, M68, | [32–35,75,144,173] | |||
| Phosphorylation S19, S21, S43, S45, S53, S59, S76, | [32,34,75,144,199,206] | |||
| Methylation R22, R50 | [75] | |||
| Acetylation M1, K92, | [32–34,75,144,201,207] | |||
| Other H83, T170, K175 | [34,144,204,205,208,209] | |||
|
| ||||
| βA2 | Phosphorylation S30 | [210] | ||
| € | Acetylation S1 | [34,210] | ||
|
| ||||
| bA3/A1 | Deamidation Q38, N40, Q42, N54, N62, N103, N120, N133, Q164, Q172, Q180, Q203, Q206 | [33–35,102,144,173,211] | ||
| Oxidation M46, W96, W99, M111, M126, M151, M161, W168, C52, H201 | [34,35,75,84,144,173,211] | |||
| Phosphorylation T127, S160 | ||||
| Methylation C82, C117, R137, C185 | [75] | |||
| Acetylation M1, A1[A18], K122, K125, K131, | [34,75,143,144,203] | |||
| [34,75,144,201] | ||||
|
| ||||
| βA4 | Deamidation Q62, Q64, Q65, N82, N113 | [34,144,211] | ||
| Oxidation M13, W79, W148 | [34,211] | |||
| Phosphorylation S34, T43 | [75] | |||
| Acetylation T1 | [34,144,201] | |||
| Other C165 | [84] | |||
|
| ||||
| bB1 | Deamidation N15, N57, N67, Q105, N107, N124, Q146, N157, N161, Q196, Q204, N216, Q222, Q224, Q226, Q235 | [33–35,102,144,173,211] | ||
| Oxidation W100, M112, W123, W126, M136, W192, W215, W218, M225, | [34,75,144,211] | |||
| Phosphorylation S9, T11, | ||||
| Methylation R229, R230, K234 | [75] | |||
| Acetylation S1, K5, K159, | [75] | |||
| Other S151, C79, H214 | [34,75,201] | |||
| [144,212] | ||||
|
| ||||
| bB2 | Deamidation N115, Q146, Q154, Q162, Q182, Q184 | [34,144,201,211] | ||
| Oxidation W58, M121, W150, M192C37 | [34,75,84] | |||
| Phosphorylation T117 | [75] | |||
| Methylation K41, K67, K120 | [75] | |||
| Acetylation A1, K75, K120 | [34,144,201,203,211] | |||
|
| ||||
| bB3 | Deamidation N155 | [34,144] | ||
| Oxidation M129, C39, C45 | [75,84] | |||
| Phosphorylation Y29 | [213] | |||
| Methylation K128 | [75] | |||
| Acetylation M1, A2, K128 | [34,144,201] | |||
|
| ||||
| γB | Deamidation Q66 | [34,144] | ||
| Oxidation W68, M69, M102 | [34,144] | |||
| Phosphorylation Y62, Y65 | [75] | |||
| Methylation C22, C79 | [34,142] | |||
| Carbamylation 1G | [142] | |||
|
| ||||
| gC | Deamidation N24, Q66, | [34,144] | ||
| Oxidation Y55, W68, M69, M101/102, W130 | [34,75,144] | |||
| Phosphorylation Y62, Y65 | [75] | |||
| Methylation C22, C79 | [34,142,144] | |||
| Carbamylation 1G | [142] | |||
| Disulfide C22–C32, C78–C79 | [33] | |||
|
| ||||
| gD | Deamidation Q12, N49, N160 | [34,144] | ||
| Oxidation Y45, W156 | [75,144] | |||
| Methylation C110 | [34,142,144] | |||
| Carbamylation G1 | [34,142] | |||
| Disulphide C18–C32, C108–C110 | [33] | |||
|
| ||||
| gS | Deamidation N14, Q16, N53, Q63, Q70, N76, Q92, Q120, N143, Q170 | [33,34,87,88,93,144] | ||
| Oxidation M58, M73, M118, M123, M136 | [33,34,75,144] | |||
| Phosphorylation S89 | [34, 35,75,141,144] | |||
| Methylation K6, C24, C26, | [35] | |||
| Acetylation S1 | [34,201] | |||
| Disulphide C22–C24, C114–C129 | [33] | |||
| Others C82, C129, R19 | [35,212] | |||
4.3. Deamidation of crystallin affects both structure and function
While several studies have reported deamidation of a-, b- and g-crystallins isolated from young, aged and cataract lenses, the results are not always consistent with respect to deamidation sites in these proteins [33–35]. Crystallins isolated from young, noncataract lenses also contain significant deamidations at specific sites, and studies have shown that the amount of deamidation at these sites increases with age [34, 35, 85–90]. In addition, crystallins isolated from aged and cataract lenses show deamination at additional sites and in higher levels [34, 87, 89]. However, the specific role of deamidation in lens protein aggregation and cataractogenesis is not fully understood because the deamidated proteins also often have other modifications such as truncation, glycation, and oxidation [33–35]. In one study deamidation of lens proteins was more frequent at glutamine sites than at asparagine sites, [34] whereas the reverse was true in another study [35]. Different levels of transglutaminase activity in the lenses used in these studies may in part, explain the discrepancy, since it has been reported that transglutaminase present in lens can also deaminate glutamine [91].
Several studies were carried out recently to determine the role of deamidation at the molecular level [40, 42, 43, 92–94]. Investigators have taken advantage of the chaperone-like activity of aA- and aB-crystallins to determine the effect of modifications on the function of a-crystallin subunits. Deamidation introduces a negative charge to a protein, and protein deamidation causes changes in protein tertiary structure and, in turn, affects structural and functional properties [42, 94]. Deamidation of specific residues in human lenses is higher in the water-insoluble [WI] proteins than in the water-soluble [WS] proteins [34]. This observation suggests that deamidation might be leading to structural instability, which in turn causes insolubilization of a protein. The cataract-specific deamidation of N-143 in γS-crystallin further supports this possibility [95]. A study of age-related deamidation of αA- and αB-crystallins examined the relative effects of deamidation αA-N101D, αA-N123D, or αA-N101/123D on the structural and functional properties of the these crystallins [40, 42]. Far-ultraviolet (UV) circular dichroism spectral analyses generally showed an increase in the β-sheet contents in αA mutants with deamidation. Intrinsic tryptophan (Trp) and total fluorescence spectral studies suggested altered microenvironments in the deamidated αA- mutants. ANS [8-anilino-1-naphthalenesulfate] binding study showed generally increased fluorescence, with a blue shift on deletion of the N-terminal domain in the deamidated mutant proteins. Overall, it was found that, relative to wild-type (WT)- αA- homomers, the mutant proteins exhibited major structural and functional changes. The maximum decrease in chaperone activity in homomers occurred upon deamidation at the N123 residue. These authors showed that deamidation alone exerted a greater effect on chaperone activity than the deletion of the N-terminal domain or C-terminal extension of αA-crystallin. There is evidence for in vivo truncation of a A-crystallin at both C- and N-terminal regions [9, 33, 96]. Interestingly, a previous study also showed that the loss of chaperone activity in the N123 deamidated a A- crystallin was substantially restored when the protein was truncated at either the C- or N-terminal. In the absence of deamidation, N- and C-terminal truncation was found to lead to decreased chaperone activity [40]. The chaperone activity of the deamidated aA- and aB-crystallins at other sites and with combination of truncation at either C- or N-terminal gave mixed results. Therefore the net contributions of deamination and truncations to decreased chaperone activity in aging lenses is not known because aA -crystallin is deamidated at a number of sites and a significant portion of the protein also exists in truncated form [9, 34]. In addition, in vitro studies show that deamidation also affects the structure-function of a B-crystallin [43]. Deamidation of N146 but not of N78 in human αB-crystallin has profound effects on the structural and functional properties of the protein [43]. The heteroaggregates of wild-type aA- or a B-crystallins and deamidated forms of these two crystallins have decreased hydrophobicity, increased oligomer size, altered structure and decreased chaperone activity [42, 43]. These results, obtained with recombinant proteins, are in contrast to an earlier report in which increased hydrophobicity was observed in high molecular weight crystallin aggregates from lenses that generally have deamidated aA- and a B-crystallins [34, 35, 56]. It should also be pointed out that while earlier studies appeared to establish a correlation between hydrophobicity and chaperone activity of a -crystallin [97, 98], subsequent studies show that this is not always the case with several mutants of aA- and a B-crystallins [99, 100]. An explanation for this discrepancy is yet to find its way into literature.
The lack of easily measurable biological activity in b- and g- crystallins has hindered the precise measurement of the effects of age-dependent modifications on these groups of crystallins. To circumvent this limitation, attempts have been made to correlate the modifications to the structure and stability of b- and g-crystallins. Since deamidation is thought to be a major age-related modification in b-crystallins, deamidated mimics of bB1- [92], bB2- [94] and bA3-crystallins [101] have been studied in an effort to understand age-related changes in β-crystallins. These crystallins undergo in vivo deamidation and the extent of deamidation is higher in aged and cataract lenses [34]. Human bB1 Gln204Glu was found to be less stable than the wild-type protein [92]. In a related study, deamidation of bB1Gln146 was also found to be a destabilizing reaction [102]. The deamidation of bB2, the most abundant human β-crystallin, was studied at Q162 and Q70 by creating a double mutant Q70E/Q162E [94]. The recombinant protein failed to refold after urea induced-unfolding, suggesting that alteration in the charges at the monomer-monomer interface destabilizes the protein. In support of this view, the authors also found changes in the tertiary structure of the protein following deamidation. Deamidation of bA3-crystallin at Q180 in the C-terminal domain and at Q85 in the N-terminal domain was found to lead to destabilization and aggregation of the protein. Deamidated bA3-crystallin showed significant changes in the tertiary structure, whereas the difference between the secondary structure of the wild-type proteins and that of mutant proteins was minimal. In another study the role of several surface Gln and Asn residues in the stability of bA3-crystallin was investigated. Deamidation at the C-terminal domain (N133 and N155) was found to have greater effect on the protein structure than deamidation at N-terminal residues (Q42 and N54) [101]. Furthermore, deamidation also resulted in aggregation of bA3-crystallins and in interaction between βA3- and bB1-crystallin subunits. The data support the hypothesis that in vivo deamidation may affect the stability of the protein and that age-related accumulation of deamidated β-crystallins may contribute to crystallin insolubilization in vivo.
All deamidation effect studies were carried out using ‘mimics’ at 100 percent concentration. However, in vivo, the crystallins are only partially deamidated and the same crystallins have other modifications. From the studies on aA-crystallin, which has truncation and deamidation, we know that multiple post-translational modifications may lead to unpredictable effect [40]. Therefore, from ‘in vitro’ studies it is not clear whether the true impact of in vivo deamidation at a specific site can be determined. A transgenic approach involving expression of deamidated forms of crystallins may offer an opportunity to test the in vivo effect of deamidation at specific sites.
4.4. Advanced glycation endproducts (AGE) affect crystallin structure-function
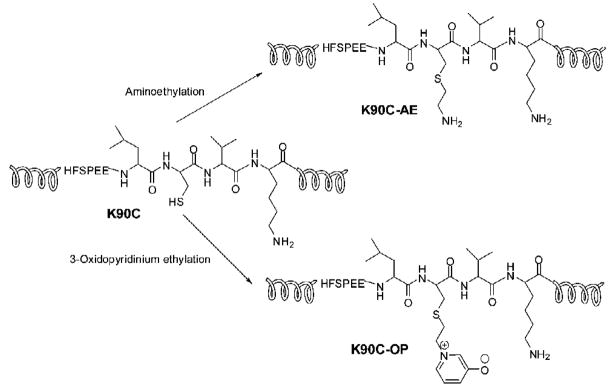
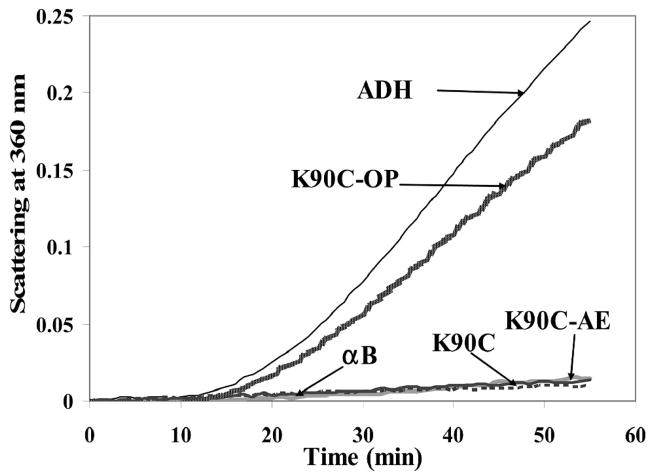
Modification of crystallins by dicarbonyls and sugar derivatives is well documented [64, 80, 83, 103–111]. Evidence is increasing that ascorabate oxidation products have a role in in vivo glycation [80, 110, 111]. Table 3 lists glycation modifications and cross-links isolated from aged and cataract human lenses. The readers should refer to the citations for the levels of AGE’s in lens under different conditions. In general, the concentrations of most of the AGE’s increases with age. While some glycation reactions involve direct or simple modification of arginine (Arg), lysine (Lys) and cysteine (Cys) residues, other glycation reactions generate crosslinks that affect the dynamic state of a-crystallin. Estimates are that <1% of the total Lys and Arg in crystallins are modified in vivo [112]. The vast majority of the modifications involving Lys and Arg are brought about by sugars or their oxidation products [80, 83, 105, 113–116]. While a strong correlation between advanced glycation endproducts (AGE) formation in lens crystallins and overall deterioration of lens transparency was established years ago, the precise changes that occur in lens crystallin structure after modifications have only recently been elucidated [56, 117–119]. A recent study showed that modification of aB-crystallin at a single site is sufficient to alter its structure-function [118]. In this study the authors used a cysteine mutant of aB-crystallin (aBK90C) and site-specific modification of the Cys to get an analog of the AGE OP-lysine [2-Ammonio-6-[3-oxidopyridinium-1-yl]hexanoate] at K90 site of the protein (Fig. 3). Lys 90 is near the chaperone site of aB-crystallin [120]. OP-lysine modification is one of the most abundant AGE modifications in both aged and cataractous lenses [121]. As a control, aBK90C was subjected to aminoethylation to obtain aBK90C-AE. The control protein used in this study retained the positive charge in the mutant protein that ordinarily is lost during the mutation. The authors found that the aBK90C-OP-lysine has diminished chaperone activity (Fig. 4), decreased intrinsic tryptophan fluorescence and bis-ANS binding. The modified protein, however, exhibited no significant alteration in secondary, tertiary and quaternary structures. On the other hand, the control protein aBK90C-AE behaved like wild-type aB-crystallin with respect chaperone activity and structure. The aBK90C-OP study showed, for the first time, that the introduction of a single AGE near the chaperone site diminishes anti-aggregation properties of αB-crystallin, even when the changes in the protein structure are minor. In other studies, extensive modification of aA- and aB-crystallins with glycating agents was shown to lead to aggregation of the crystallins and decreased chaperone activity [56, 117, 119, 122]. The mapping of glycation sites in aA- and aB-crystallin has shown differential susceptibility of Lys and Arg residues [123]. The susceptibility of the residues to glycation and the influence of neighboring residues are attributed to the different glycation rate. Glycation reactions culminating in crosslinks between a-crystallin subunits may also be affecting the chaperone activity by affecting the dynamic state of the protein required for chaperone activity [66, 71]. Since under physiological conditions glycation reactions continue to occur in vivo, it can be argued that with aging, the modifications begin to accumulate and reduce the chaperone function of a-crystallin.
Table 3.
Advanced glycation end products [AGE] in aging lenses
| AGES | Ref |
|---|---|
| Hydroimidazolone [isomer 1, MG-H1] | [109] |
| Hydroimidazolone [isomer 2, MG-H2] | [109] |
| Hydroimidazolone [isomer 3, MG-H3] | [109] |
| Argpyrimidine | [108] |
| Tetrahydropyrimidine | [109] |
| Ne-[1-carboxyethyl]lysine [CEL] | [83,109] |
| Carboxymethyl lysine [CML] | [214] |
| Vesperlysine | [214,215] |
| 1,3-di[Ne -lysino]-4-methyl-imidazolium [MOLD] | [82,109] |
| Glyoxal lysine dimer [GOLD] | [82,113] |
| Pentosidine | [80] |
| 1-(5-Amino-5-carboxypentyl)-4-(5-amino-5-carboxypentylamino)-3-hydroxy-2,3-dihydropyridinium (K2P) | [216] |
| Ne-Fructosyl-lysine | [217] |
| Furosine | [214] |
| Pyrraline | [105] |
| 2-Ammonio-6-(3-oxidopyridinium-1-yl)hexanoate (OP-lysine) | [121] |
| Glucosepane | [213] |
For structures of these AGE’s see the references
Fig. 3. Schematic representation of the modification of aB-crystallin-K90C to K90C-AE [aminoethylation] and K90C-OP [3-oxidopyridinium ethylation].
Reproduced with permission from Biochemistry (2008) 46, 14682–92.
Fig. 4. Aggregation assay with wild-type aB-crystallin, K90C, K90C-AE, and K90C-OP using alcohol dehydrogenase [ADH] as client protein.
The assays were carried out as described earlier [120] using 1:10 w/w ratio of aB-crystallin (native or modified) and ADH. Reproduced with permission from Biochemistry (2008) 46, 14682–92.
While most glycation studies show a decrease in the chaperone activity of a-crystallin following glycation, [117, 119] one study revealed that modification of a-crystallin with a low concentration of methylglyoxal enhances chaperone activity, to the tune of 40 – 60 percent [124, 125]. The chaperone sites involved in the binding of increased amounts of client (substrate) proteins are yet to be delineated. Immunochemical studies have established that methylglyoxal modification does indeed occur in normal lenses and that these modifications accumulate with aging of lens proteins [83, 109, 113]. Limited modification of a-crystallin with methylglyoxal perturbs the protein structure and increases the surface hydrophobicity [124]. Increased surface hydrophobicity has been shown to correlate with increased chaperone activity of a-crystallin [97]. Methylglyoxal reacts with Arg, Cys and Lys to produce stable adducts and cross-links and is one of the glycating dicarbonyls in the lens [83, 109, 113, 125]. It has been shown that Cys residues in aA-crystallin are not involved in methylglyoxal-mediated enhancement of chaperone activity [126]. The neutralization of the positive charge on Arg by methylglyoxal is apparently responsible for the enhanced chaperone activity of a-crystallin [127]. Additionally, the methylglyoxal reaction was also found to block glycation of a-crystallin by ascorbate and ribose. The increased chaperone activity after modification with methylglyoxal prompted the investigators to propose that “low levels of methylglyoxal might help the lens remain transparent during aging” [125]. It is yet to be determined whether the methylglyoxal effect can be sustained if crystallins are also modified by other mechanisms, such as deamidation, phosphorylation or truncations, which are continuously occurring in vivo [34]. Ascorbate oxidation product erythrulose is a powerful glycating agent and there is some evidence for its involvement in lens crystallin glycation [110, 114, 115]. The glycation-induced aggregation of crystallins similar to the aggregation seen in aged lens has been shown by several studies [80, 116, 128].
4.5. Oxidation, phosphorylation and other modifications affect crystallin structure-function
Oxidation is considered to be a major physiological challenge to lens proteins, and recent reviews have discussed the role of oxidation in cataract [16, 18, 129, 130]. The lens appears to be subjected to increasing oxidative stress with aging because of the progressively weakening antioxidant system. The decline in the antioxidant activities of the lens is manifested by decreased glutathione levels, reduced efficiency of enzymes involved in restoring reduced glutathione levels, the accumulation of proteins with disulphides and mixed disulphides, and oxidation of Met residues [4, 5, 8, 33–35]. While aA-crystallin has only two Cys residues susceptible to oxidation, b- and g-crystallins have several Cys residues that are susceptible to oxidation (Table 2). Although Trp and His are susceptible to oxidation, strong evidence is lacking for their oxidation in aging lenses, yet these amino acids are oxidized in advanced cataracts [129]. Free radicals – hydroxyl radical, superoxide and singlet oxygen— are the likely oxidizing species in vivo [131–134]. To study the role of α-crystallin oxidation in lens aging, investigators have generated protein aggregates in vitro, similar to those found in aged lenses, by oxidation of SH- groups in aA-, b-and g-crystallins. Oxidation of aA- and aB-crystallin has been shown to lead to structural changes and loss of chaperone activity [135]. Methionine oxidation in aging lens has been linked to cataractogenesis [136]. Recently loss of chaperone activity in aA-crystallin due to oxidation of Met was demonstrated and this loss was reversed by methionine sulfoxide reductase [137]. Lens crystallins have several phosphorylation sites (Table 2), and some studies have shown that phoshorylation can modulate chaperone activity of a-crystallins [138–140]. There is no evidence of age-related changes in the extent of phosphorylation of crystallins, but one study demonstrated that aB-crystallin from cataract lens was not phosphorylated [84]. The significance of this absence of phosphorylated αB-crystallin is yet to be determined. Methylation of Cys residues in crystallins has been reported in several studies [34, 141–144] but age-related changes in this reaction have not been adequately elucidated. It has been hypothesized that Cys methylation may be a protective mechanism to prevent disulphide formation and aggregation of crystallins [145]. In vivo modification of human lens crystallins by 3-hydroxykynurenine glucoside, kynurenine and 3-hydroxykynurenine has been reported [112, 146–148]. Since kynurenine reacted with His83 at the chaperone site of aB-crystallin it was proposed that this reaction might affect the chaperone activity in aging lenses [146]. However, the structural consequence of this or other modifications with kynurenine is yet to be determined. In addition, modification of crystallins by n-formylkynurenine has been documented in lens [149] and this modification may also result in both structural and functional alterations of crystallins.
4.6. Proteolysis in lens—from morphogenesis to aging
Lens proteases play a critical role during lens development and morphogenesis. Several proteases have been isolated from lenses [150–161]. In addition to these proteases, the lens also contains a whole set of proteases involved in organelle degradation [15, 20, 162–164]. The ubiquitin-dependent proteolytic pathway studied in lens is involved in degradation of oxidatively modified proteins [165, 166]. However, due to age-related decrease in ubiquitin conjugation activity the oxidatively modified crystallins accumulate in lens, primarily in the nuclear region. This in turn is believed to be responsible, at least, in part to age-related protein aggregation and light scattering [166]. Recent studies have shown that inhibition of the proteosome activity involved in differentiation of lens fiber cells prevents normal maturation of fiber cells [164]. Calpains have been implicated in the maturation of rodent lens crystallins [167] and in the development of cataract in sheep [168] but their role in human cataractogenesis is yet to be established, primarily because of the presence of a calpain inhibitor in the lens that is at a several fold higher concentration than calpain itself [154, 169, 170]. The presence of lens proteins cleaved at specific sites during aging [9, 171] provides credence to the statement that, although protease activity begins to decline in lens fibers as they mature, the lens has appreciable levels of protease and peptidase activity [158, 172]. The major bonds cleaved in vivo are on the carboxyl side of Asn101, Asp151, Ser168 and Ser169 of the A chain and on the carboxyl side of Thr170 of the B chain of bovine a-crystallin [9, 171]. The other truncation observed in aA-crystallin is due to cleavage at the carboxyl side of Ser172, [33] and the amount of cleaved aA- and aB-crystallin is high in the WIS fraction. One study found that in aged human lenses, aA-crystallin is truncated by 3 residues and aB is truncated by 1 or 6 residues at the N-terminus [96]. Mass spectrometric analysis of WS and WIS fractions has identified several of the previously known truncations as well as a few additional ones [26, 34, 96, 173]. But the protease[s] responsible for α-crystallin truncation at multiple sites has not been identified. Truncation of bB1-, bA3/A1- and bB2-crystallins in vivo has been reported based on the identification of several truncated and cleaved b- and g-crystallins in both WS and WIS fractions of human lenses [26, 173]. Since truncation affects the stability of bB2-crystallin in vitro, [94] it is hypothesized that in vivo truncation of the protein may be responsible for its aggregation with aging.
4.7. Truncation effect on oligomerization of crystallin subunits and the chaperone activity of crystallin
The lens WIS fraction of crystallin, which contains mostly a-crystallin aggregates (with some b- and g-crystallins, [3, 26, 30] is known to have increased amounts of C-terminally truncated aA- and aB-crystallins as compared to the WS fraction [30, 31, 174]. Recent studies have shown that C-terminal truncation affects oligomerization and subunit exchange, [41] as demonstrated by recombinant human aA151 and wild-type aA-crystallins. The aA151 form is found among the several truncated forms of crystallins in aged lenses [11, 40]. In our study of the effects of truncation on oligomerization of crystallin subunits, we mixed aA151 with wild-type proteins in different proportions and found a concentration-dependent increase in the oligomeric mass for the hetero-oligomer (Table 4). At an aA: aA151 ratio of 3:1, the mass increased 2-fold compared to the mass for wild-type protein mixtures. Incubation of aA and aA151 in 2:1 ratio led to hetero-oligomers that were ~ 3 times larger than oligomers formed from aA-wild-type protein. Since aA151 aggregated by itself, we could not reliably measure the size of the oligomer. These results, along with others, [46, 175–177] suggest that truncated form[s] of aA- and aB-crystallin may hold the key to the formation of larger aggregates in vivo. Fluorescence resonance energy transfer [FRET] experiments involving labeled aA- and aB- crystallins and truncated forms of these proteins showed that truncation affects aA- and aB-crystallin subunit exchange [40, 41]. The demonstration of the abnormal behavior of truncated crystallins is highly significant since it provides insight into mechanisms by which cataract is formed in the aB-mutation involving the genesis of a stop codon at 151 position of aB-crystallin [46]. C-terminal truncation of aA- and aB-crystallin with removal of 1–16 amino acids also affects the chaperone activity of these two subunits [175, 176]. It is likely that the truncation partially removes the sequence in these subunits that is responsible for keeping the chaperone-substrate complexes in soluble form.
Table 4.
Effect of truncation on the oligomeric mass of aA-crystallin
| Molar mass at the peak apex Mw [g/mol] | Hydrodynamic radius | |
|---|---|---|
| a A | 7.50 ± 0.02 e5 | 8.0 ± 0.2 |
| aA: a A151 [3:1] | 14.0 ± 0.04 e5 | 9.6 ± 0.2 |
| aA: a A151 [2:1] | 20.0 ± 0.01 e5 | 11.8 ± 0.3 |
4.8. Low molecular weight crystallin fragments in aging human lens
We have previously shown that the amount of crystallin fragments in older lens fibers, as well as in the WIS fraction, is greater than the amount in younger lens fiber cells and in the WS fraction [152, 157]. The amount of degraded crystallin [2.8 to 18 kDa] can be as much as 20% in aged/cataract lenses [78, 178]. Crystallin truncation and fragmentation is also seen in bovine lens tissue imaging studies [179, 180]. While other investigators have focused on truncated crystallins that have lost few amino acids [74, 175, 176, 181] or that are in 2.8 to 18 kDa range [78, 174, 178], we have been investigating the low-molecular-weight crystallin fragments and have isolated and identified in human lenses over 25 different crystallin fragments of less than 3.5 kDa. (Table 2 in ref [36]). Among the crystallin fragments we have identified, several are derived from aA-, aB- and bB3-crystallins, including residues from aA- and aB-crystallin subunit interaction regions [68, 182, 183] or chaperone sites [67, 68, 120]. We found that the total amount of crystallin fragments is 3- to 4-fold higher in aged lenses. Further, the concentration of peptides is higher in the nuclear region of the lens which represents the aged portion of the lens. The nuclear region of the lens, which has the highest WIS protein content [5], also was found to have the greatest amount of peptides or shorter crystallins [152, 157]. This was also illustrated by protein-image analysis studies. When whole-lens cross sections were subjected to MALDI imaging [179, 180] to determine the relative distribution of specific crystallin fragments in a given lens, once again the low molecular weight crystallin fragments were found in increasing quantities in the nuclear region (Fig. 5 for representative distribution of 3–5 kDa peptides). At present, it is not known how these peptides are formed, nor is it known why they are not completely hydrolyzed in vivo since the lens has appreciable levels of aminopeptidase activity [152, 172]. These observations raise important questions: What is the role of crystallin fragments in high molecular weight aggregate formation? What are the effects of crystallin-derived peptides on chaperone activity of a-crystallin? What is the effect of peptides on a-crystallin subunit interaction since subunit interaction has a role in the chaperone activity of a-crystallin?
Fig. 5. MALDIimaging of a 17- and a 73-year-old human lens.
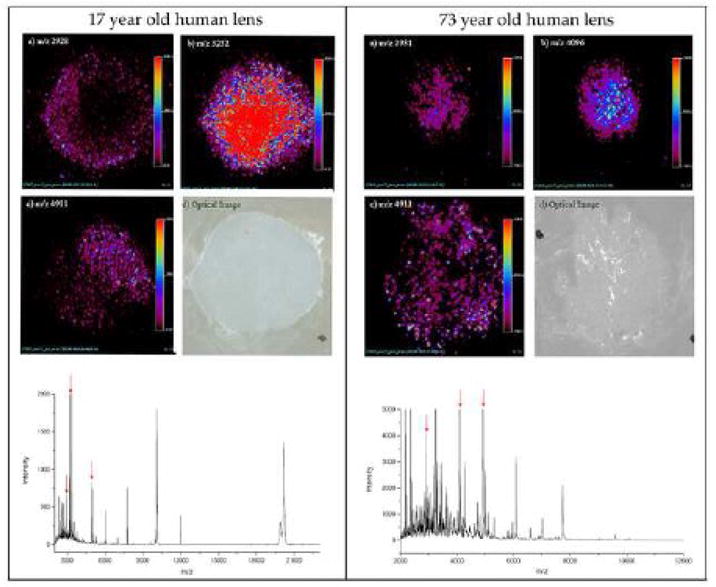
The three ion images correspond to three distinct molecular species measured from a 12 micron section. The three ion images shown in 17-year-old human lens have m/z values of 2928, 3252, and 4911. The three ion images shown in 73-year-old human lens have m/z values of 2931, 4096, and 4911. The optical image [d] of the tissue specimen shown was captured following application of sinapinic acid matrix by spray coating. The data were compiled into Analyze 7.5 format after baseline correction. A 2-D ion density map (image) for specific ion constructed is shown. The procedure allows the construction of images for the specific mass signal detected from the surface of the section, theoretically offering an opportunity to generate hundreds of images, each representing the distribution of the specific ionized species from a single imaging mass spectrometry experiment. Average mass spectrum collected from the surface of the lens samples are shown in the bottom of the figure. Notice the abundance of ions in the spectrum for 73-year-old human lens. Red arrows denote the ions highlighted in figures. Data in this Figure is unpublished results from authors study.
4.9. Anti-chaperone activity of crystallin fragments and a -crystallin aggregation
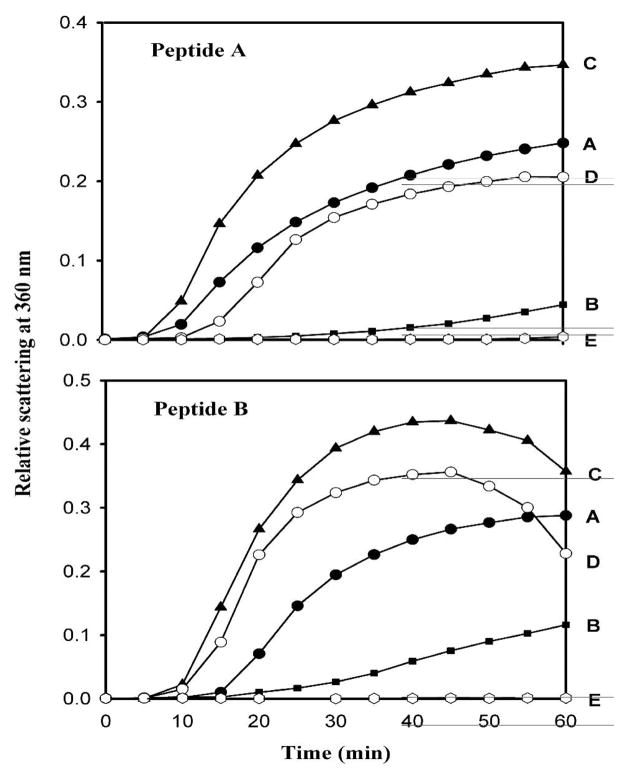
The age-related accumulations of crystallin breakdown products are believed to have a role in the loss of lens transparency and the eventual development of lens opacity [184]. Earlier we proposed that crystallin fragments generated from the oxidized crystallins in aging lenses may enhance the aggregation of denaturing proteins and interfere with the chaperone activity of a-crystallin [185]. In support of this hypothesis, it was shown that trypsin digestion of in vitro oxidized b-crystallin generates a peptide that has anti-chaperone activity [185]. The oxidized peptide binding enhanced the aggregation of denaturing bL- and g-crystallins [186, 187]. The potential role of in vivo- generated crystallin fragments in masking the chaperone activity was investigated using both synthetic a-crystallin fragments and crystallin fragments isolated from aged human lenses [36]. The studies showed that denaturing b- and g-crystallins aggregate in the presence of peptides from human lenses. A detailed analysis of the interaction of 3 representative peptides —aB-1–18 (MDIAIHHPWIRRPFFPFH), bA3/A1-102–117 (SNAYHIERLMSFRPIC) and gS-167–178 (SPAVQSFRRIVE)— with denaturing proteins showed their ability to enhance protein aggregation and inhibit chaperone activity of a-crystallin (Fig. 6). aB1–18 and bA3/A1102–117 were found to enhance the aggregation of denaturing proteins, whereas gS-167–178 did not affect protein aggregation. Additionally, both aB and bA3/A1 peptides showed chaperone inhibition activity [36, 188]. Crosslinking studies have confirmed the interaction of bA3/A1102–117 at the chaperone site of aB-crystallin [188]. This study is the first to show that a sequence within bA3/A1-crystallin may become an anti-chaperone peptide (a peptide interfering in chaperone activity) when it is removed from its parent protein. This study is the first report of in vivo accumulating peptides in aging lenses that may be, in part, responsible for in vivo protein aggregation and loss of chaperone activity. Additionally, other peptides present in lenses, originating from the subunit interaction regions, may affect native protein subunit interactions and short-range order interactions [52, 54] required to maintain lens transparency.
Fig. 6. Inhibition of chaperone-like activity of a-crystallin against bL-crystallin by peptide A and B.
Peptide A: aB-1MDIAIHHPWRRPFFPFH18; Peptide B: bA3/A159SNAYHIERLMSFRPIC74Top, anti-chaperone activity of Peptide A. Bottom, anti-chaperone activity of peptide B. A, 100 mg of bL-crystallin; B, bL-crystallin + 25 mg of aA-crystallin; C, bL-crystallin + 60 mg of peptide; D, bL-crystallin + aA-crystallin + peptide; E, peptide alone (60 mg). The aggregation assay was carried out as described earlier [185]. Reproduced with permission from the Journal of Biol. Chem. (2008) 283 8477–8485.
5. Pathway of Crystallin Fragmentation and Aggregation in Lens Aging
5.1 Pathways for age-related crystallin aggregation and thedevelopment of opacity
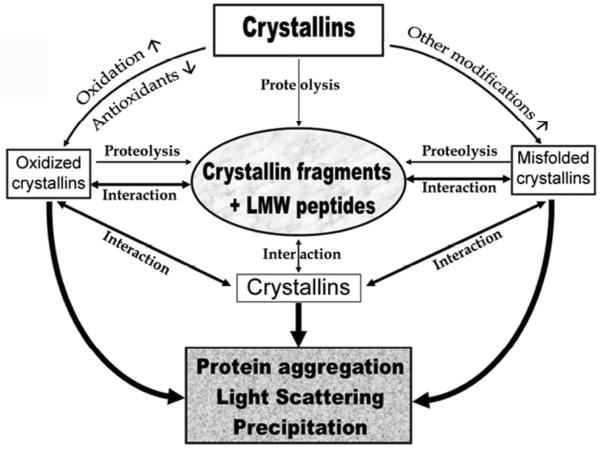
The age-related increase in crystallin aggregation and loss of lens transparency is well documented [6, 8, 11]. Aging of lens crystallins is accompanied by proteolysis and several other post-translational modifications in both native protein and truncated crystallins. As shown in the Fig. 7, these changes are proposed to be the driving force that induces conformational change in crystallins. At the same time, crystallin modifications and truncations affect the chaperone function of a-crystallin. These events lead to further aggregation of b- and g-crystallins and masking of the chaperone sites in a-crystallin by the anti-chaperone peptides. In this scheme crystallin fragments accumulating in the lens during aging occupy the central position in the pathway for lens opacification due to their ability to induce aggregation and precipitation of aA- and aB-crystallin and their anti-chaperone activity [36, 188]. Chaperone activity of aA- and aB-crystallin is believed to be responsible for maintaining lens transparency [55, 57]. In animal models disruption of chaperone activity has been shown to result in lens protein aggregation and opacity. While the scheme in Fig. 7 does not include various risk factors for post-translational modification of crystallins, [16, 64, 65, 83, 129, 136] it is assumed that during lifetime an individual is exposed to most of the risks, albeit at low level.
Figure 7. Schematic representation of the proposed role of crystallin fragments in lenticular aging and cataractogenesis.
Oxidizing and modifying reactions/factors are not shown for simplicity.
5.2. Animal models for studies of lens aging
Studies of the physiology and biochemistry of the lens show that the age-related changes taking place in the lens occur in an orderly way and at a slow pace over the lifetime of an individual, whether it is a human or an animal. The in vivo changes accumulate, and the aged lens constitutes a collection of these changes. Therefore, it is very difficult, if not impossible, to replicate these changes in a shorter time frame under laboratory conditions using what is considered to be physiological concentrations of reagents. Immortalized lens epithelial cells, primary culture cells and lens organ cultures have been used to study the age-related lens changes. The results of such studies, however, often come under criticism because 1) the studies require the use of 10- to 100-fold more reagents, 2) there is a significant difference in the protein profile of immortalized and primary lens epithelial cells [24] and 3) the epithelial cells do not satisfy the functional obligations as the cells in contact with fiber cells.
Guinea pig lens is similar in most aspects to human lens and may serve as a good model for investigating the mechanisms of lens opacification and cataract formation. Long-term hyperbaric oxygen treatment is used to induce nuclear lens opacity in the guinea pig in an attempt to replicate the development of lens opacity in the human lens [189, 190]. Analysis of hyperbaric oxygen-treated lenses shows glutathione loss, increased protein degradation, protein aggregation and insolubilization, protein cross linking, membrane abnormalities, etc, features like the changes observed in the aging human lens [189–192]. For these reasons it appears that this model is suitable to study the molecular mechanisms of age-related lens opacification and cataract. It remains to be seen whether crystallin fragments accumulate in hyperbaric oxygen-treated lenses. The existence of such peptides in the guinea pig lens should allow investigation of the contribution of the peptides in age-related crystallin aggregation
Many of the age-related protein modifications occurring in human lenses can be replicated by incubation of lens proteins with ascorbate and oxygen [103, 114–116, 121, 134, 193]. In vitro studies have shown that ascorbate oxidation products are involved in these reactions [115, 193–195]. Based on these observations, it has been proposed that high concentrations of ascorbic acid and its oxidation products are responsible, in part, for the age-related changes in human lenses. However, the role of ascorbate oxidation products in lens aging cannot be tested in rodent models because these animals have very low vitamin C levels. To overcome this problem, a transgenic mouse model has been developed in which vitamin C transporter is overexpressed in lens epithelium and cortical fiber cells [110]. The lenses of these transgenic mice have high levels of vitamin C and its oxidation products. Analysis of the lenses show the accumulation of several AGEs identical to those found in aging human lens. In addition, the lenses of aged transgenic mice show the yellow discoloration characteristic of older human lenses [110, 111, 196]. It is yet to be determined whether the transgenic lenses show increased crystallin oxidation, proteolysis and crystallin aggregation. If they do, the mouse lens over-expressing vitamin C transporter would be a good model to study the human lens aging and to test the potential of drugs to delay the lens aging.
6. Concluding Remarks
The lens is a unique tissue with long-lived proteins, called crystallins, classified into three groups. The lens grows throughout the lifetime of an individual, and significant changes occur in the structure and function of lens crystallins. Various modifications, such as deamidation, truncation, oxidation, glycation, and methylation, lead to structural changes in the crystallins. These mechanisms play a major role in converting the largely soluble pool of crystallins into the largely insoluble pool with aging. Studies suggest that the same modifications decrease the chaperone activity of a-crystallin. Diminishing chaperone activity and altered short-range order interactions likely contribute to the protein aggregation in the lens. The inability of the lens to remove or repair the damaged crystallins results in the accumulation and aggregation of the modified proteins. Recent studies show that crystallin fragments accumulating in the lens may have a role in lens protein insolubilization. The formation of aggregates capable of light scattering is the starting point for cataractogenesis. Of the several animal models available to study lens protein changes and cataractogenesis, hyperbaric oxygen treatment of guinea pig seems to be the only model that can replicate many of the age-related changes occurring in human lenses. The newer, ‘humanized’ mouse lens with ascorbate transporter appears to be a promising model to study age-related crystallin modification.
It seems that protein modification and aggregation is the hallmark of lens aging, and controlling these two events may be a key to slowing the aging process in the lens. The protein modification is directly linked to the depleting antioxidative mechanisms. It has been proposed that by midlife, a “barrier” [197] is created in human lens that impedes the free movement of antioxidant molecules from the periphery of the lens, where they are in high concentrations, to the nuclear region, where they are most needed. However, the molecular mechanism for formation of this barrier is not fully understood. Methods that would delay the formation of the “barrier” should allow the lens to remain transparent well into the later years of life. At this time it is not known how and why the crystallin fragments with anti-chaperone activity accumulate in aging lenses. The development of methods to control the anti-chaperone peptide formation should provide a means to delay the lens crystallin aggregation by prolonging the chaperone activity of a-crystallin.
Acknowledgments
The work in our laboratory is supported by grants from NIH, University of Missouri Prime Funds and an unrestricted award from Research to Prevent Blindness to the Department of Ophthalmology of the University of Missouri. We thank Sharon Morey for her editorial assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dilley KJ, Pirie A. Changes to the proteins of the human lens nucleus in cataract. Exp Eye Res. 1974;19:59–72. doi: 10.1016/0014-4835(74)90073-6. [DOI] [PubMed] [Google Scholar]
- 2.Spector A, Li S, Sigelman J. Age-dependent changes in the molecular size of human lens proteins and their relationship to light scatter. Invest Ophthalmol. 1974;13:795–798. [PubMed] [Google Scholar]
- 3.Roy D, Spector A. Absence of low-molecular-weight alpha crystallin in nuclear region of old human lenses. Proc Natl Acad Sci USA. 1976;73:3484–3487. doi: 10.1073/pnas.73.10.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bloemendal H. The lens proteins. In: Bloemendal H, editor. Molecular and Cellular Biology of the Eye Lens. New York: John Willey & Sons; 1981. pp. 1–49. [Google Scholar]
- 5.Jaffe NS, Horwitz J. Lens and Cataract. In: Podos SM, Yanoff M, editors. Text Book of Ophthalmology. Vol. 3. Gower Med. Publishing; New York: 1991. [Google Scholar]
- 6.Benedek GB. Cataract as a protein condensation disease: the Proctor Lecture. Invest Ophthalmol Vis Sci. 1997;38:1911–1921. [PubMed] [Google Scholar]
- 7.Duncan G, Wormstone IM, Davies PD. The aging human lens: structure, growth, and physiological behaviour. Br J Ophthalmol. 1997;81:818–823. doi: 10.1136/bjo.81.10.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alio JL, Anania A, Sagnelli P. The aging of the lens. In: Cavallotti CAP, Cerulli L, editors. Age-related changes of the human eye. Humana Press; Totowa, NJ, USA: 2008. pp. 61–131. [Google Scholar]
- 9.Groenen PJ, Merck KB, de Jong WW, Bloemendal H. Structure and modifications of the junior chaperone alpha-crystallin. From lens transparency to molecular pathology. Eur J Biochem. 1994;225:1–19. doi: 10.1111/j.1432-1033.1994.00001.x. [DOI] [PubMed] [Google Scholar]
- 10.Giblin FJ. Glutathione: a vital lens antioxidant. J Ocul Pharmacol Ther. 2000;16:121–135. doi: 10.1089/jop.2000.16.121. [DOI] [PubMed] [Google Scholar]
- 11.Bloemendal H, de Jong W, Jaenicke R, Lubsen NH, Slingsby C, Tardieu A. Ageing and vision: structure, stability and function of lens crystallins. Prog Biophys Mol Biol. 2004;86:407–485. doi: 10.1016/j.pbiomolbio.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 12.Lovicu FJ, Robinson ML. In: Development of the ocular lens. Lovicu FJ, Robinson ML, editors. Cambridge University Press; 2004. [Google Scholar]
- 13.Salvi SM, Akhtar S, Currie Z. Ageing changes in the eye. Postgrad Med J. 2006;82:581–587. doi: 10.1136/pgmj.2005.040857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andley UP. The lens epithelium: focus on the expression and function of the alpha-crystallin chaperones. Int J Biochem Cell Biol. 2008;40:317–323. doi: 10.1016/j.biocel.2007.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bassnett S. On the mechanism of organelle degradation in the vertebrate lens. Exp Eye Res. 2009;88:133–139. doi: 10.1016/j.exer.2008.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brennan LA, Kantorow M. Mitochondrial function and redox control in the aging eye: role of MsrA and other repair systems in cataract and macular degenerations. Exp Eye Res. 2009;88:195–203. doi: 10.1016/j.exer.2008.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Danysh BP, Duncan MK. The lens capsule. Exp Eye Res. 2009;88:151–164. doi: 10.1016/j.exer.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berthoud VM, Beyer EC. Oxidative stress, lens gap junctions, and cataracts. Antioxid Redox Signal. 2009;11:339–353. doi: 10.1089/ars.2008.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuszak JR. Embryology and anotomy of the lens. In: Tasman W, Jaeger EA, editors. Clinical Ophthalmology. J.B. Lippincott; Philadelphia: 1990. pp. 1–9. [Google Scholar]
- 20.Bassnett S. Lens organelle degradation. Exp Eye Res. 2002;74:1–6. doi: 10.1006/exer.2001.1111. [DOI] [PubMed] [Google Scholar]
- 21.Augusteyn RC. Growth of the human eye lens. Mol Vis. 2007;13:252–257. [PMC free article] [PubMed] [Google Scholar]
- 22.Balaram M, Tung WH, Kuszak JR, Ayaki M, Shinohara T, Chylack LT., Jr Noncontact specular microscopy of human lens epithelium. Invest Ophthalmol Vis Sci. 2000;41:474–481. [PubMed] [Google Scholar]
- 23.Harocopos GJ, Alvares KM, Kolker AE, Beebe DC. Human age-related cataract and lens epithelial cell death. Invest Ophthalmol Vis Sci. 1998;39:2696–2706. [PubMed] [Google Scholar]
- 24.Wang-Su ST, McCormack AL, Yang S, et al. Proteome analysis of lens epithelia, fibers, and the HLE B-3 cell line. Invest Ophthalmol Vis Sci. 2003;44:4829–4836. doi: 10.1167/iovs.03-0556. [DOI] [PubMed] [Google Scholar]
- 25.McFall-Ngai MJ, Ding LL, Takemoto LJ, Horwitz J. Spatial and temporal mapping of the age-related changes in human lens crystallins. Exp Eye Res. 1985;41:745–758. doi: 10.1016/0014-4835(85)90183-6. [DOI] [PubMed] [Google Scholar]
- 26.Harrington V, Srivastava OP, Kirk M. Proteomic analysis of water insoluble proteins from normal and cataractous human lenses. Mol Vis. 2007;13:1680–1694. [PubMed] [Google Scholar]
- 27.Jedziniak JA, Kinoshita JH, Yates EM, Hocker LO, Benedek GB. On the presence and mechanism of formation of heavy molecular weight aggregates in human normal and cataractous lenses. Exp Eye Res. 1973;15:185–192. doi: 10.1016/0014-4835(73)90118-8. [DOI] [PubMed] [Google Scholar]
- 28.Benedek GB, Chylack LT, Jr, Libondi T, Magnante P, Pennett M. Quantitative detection of the molecular changes associated with early cataractogenesis in the living human lens using quasielastic light scattering. Curr Eye Res. 1987;6:1421–1432. doi: 10.3109/02713688709044506. [DOI] [PubMed] [Google Scholar]
- 29.Ortwerth BJ, Olesen PR. Studies on the nature of the water-insoluble fraction from aged bovine lenses. Exp Eye Res. 1989;48:605–619. doi: 10.1016/0014-4835(89)90003-1. [DOI] [PubMed] [Google Scholar]
- 30.Ortwerth BJ, Olesen PR. Studies on the solubilization of the water-insoluble fraction from human lens and cataract. Exp Eye Res. 1992;55:777–783. doi: 10.1016/0014-4835(92)90004-c. [DOI] [PubMed] [Google Scholar]
- 31.Ortwerth BJ, Sharma KK, Olesen PR. The effect of urea on the aggregate state and elastase inhibitor activity of the water-insoluble fraction from bovine and human lens. Exp Eye Res. 1992;54:573–581. doi: 10.1016/0014-4835(92)90136-g. [DOI] [PubMed] [Google Scholar]
- 32.Lund AL, Smith JB, Smith DL. Modifications of the water-insoluble human lens alpha-crystallins. Exp Eye Res. 1996;63:661–672. doi: 10.1006/exer.1996.0160. [DOI] [PubMed] [Google Scholar]
- 33.Hanson SR, Hasan A, Smith DL, Smith JB. The major in vivo modifications of the human water-insoluble lens crystallins are disulfide bonds, deamidation, methionine oxidation and backbone cleavage. Exp Eye Res. 2000;71:195–207. doi: 10.1006/exer.2000.0868. [DOI] [PubMed] [Google Scholar]
- 34.Wilmarth PA, Tanner S, Dasari S, et al. Age-related changes in human crystallins determined from comparative analysis of post-translational modifications in young and aged lens: does deamidation contribute to crystallin insolubility? J Proteome Res. 2006;5:2554–2566. doi: 10.1021/pr050473a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hains PG, Truscott RJ. Post-translational modifications in the nuclear region of young, aged, and cataract human lenses. J Proteome Res. 2007;6:3935–3943. doi: 10.1021/pr070138h. [DOI] [PubMed] [Google Scholar]
- 36.Santhoshkumar P, Udupa P, Murugesan R, Sharma KK. Significance of interactions of low molecular weight crystallin fragments in lens aging and cataract formation. J Biol Chem. 2008;283:8477–8485. doi: 10.1074/jbc.M705876200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carver JA, Lindner RA. NMR spectroscopy of alpha-crystallin. Insights into the structure, interactions and chaperone action of small heat-shock proteins. Int J Biol Macromol. 1998;22:197–209. doi: 10.1016/s0141-8130(98)00017-8. [DOI] [PubMed] [Google Scholar]
- 38.Sun TX, Liang JJ. Intermolecular exchange and stabilization of recombinant human alphaA- and alphaB-crystallin. J Biol Chem. 1998;273:286–290. doi: 10.1074/jbc.273.1.286. [DOI] [PubMed] [Google Scholar]
- 39.Sreelakshmi Y, Sharma KK. Recognition sequence 2 (residues 60–71) plays a role in oligomerization and exchange dynamics of alphaB-crystallin. Biochemistry. 2005;44:12245–12252. doi: 10.1021/bi051005h. [DOI] [PubMed] [Google Scholar]
- 40.Chaves JM, Srivastava K, Gupta R, Srivastava OP. Structural and functional roles of deamidation and/or truncation of N- or C-termini in human alpha A-crystallin. Biochemistry. 2008;47:10069–10083. doi: 10.1021/bi8001902. [DOI] [PubMed] [Google Scholar]
- 41.Aziz A, Santhoshkumar P, Sharma KK, Abraham EC. Cleavage of the C-terminal serine of human alphaA-crystallin produces alphaA1-172 with increased chaperone activity and oligomeric size. Biochemistry. 2007;46:2510–2519. doi: 10.1021/bi0618722. [DOI] [PubMed] [Google Scholar]
- 42.Gupta R, Srivastava OP. Deamidation affects structural and functional properties of human alphaA-crystallin and its oligomerization with alphaB-crystallin. J Biol Chem. 2004;279:44258–44269. doi: 10.1074/jbc.M405648200. [DOI] [PubMed] [Google Scholar]
- 43.Gupta R, Srivastava OP. Effect of deamidation of asparagine 146 on functional and structural properties of human lens alphaB-crystallin. Invest Ophthalmol Vis Sci. 2004;45:206–214. doi: 10.1167/iovs.03-0720. [DOI] [PubMed] [Google Scholar]
- 44.Horwitz J. Alpha-crystallin. Exp Eye Res. 2003;76:145–153. doi: 10.1016/s0014-4835(02)00278-6. [DOI] [PubMed] [Google Scholar]
- 45.Koteiche HA, McHaourab HS. Mechanism of a hereditary cataract phenotype. Mutations in alphaA-crystallin activate substrate binding. J Biol Chem. 2006;281:14273–14279. doi: 10.1074/jbc.M512938200. [DOI] [PubMed] [Google Scholar]
- 46.Hayes VH, Devlin G, Quinlan RA. Truncation of alphaB-crystallin by the myopathy-causing Q151X mutation significantly destabilizes the protein leading to aggregate formation in transfected cells. J Biol Chem. 2008;283:10500–10512. doi: 10.1074/jbc.M706453200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xi JH, Bai F, Gross J, Townsend RR, Menko AS, Andley UP. Mechanism of small heat shock protein function in vivo: a knock-in mouse model demonstrates that the R49C mutation in alpha A-crystallin enhances protein insolubility and cell death. J Biol Chem. 2008;283:5801–5814. doi: 10.1074/jbc.M708704200. [DOI] [PubMed] [Google Scholar]
- 48.Kim KK, Kim R, Kim SH. Crystal structure of a small heat-shock protein. Nature. 1998;394:595–599. doi: 10.1038/29106. [DOI] [PubMed] [Google Scholar]
- 49.van Montfort RL, Basha E, Friedrich KL, Slingsby C, Vierling E. Crystal structure and assembly of a eukaryotic small heat shock protein. Nat Struct Biol. 2001;8:1025–1030. doi: 10.1038/nsb722. [DOI] [PubMed] [Google Scholar]
- 50.Horwitz J, Bova MP, Ding LL, Haley DA, Stewart PL. Lens alpha-crystallin: function and structure. Eye. 1999;13:403–408. doi: 10.1038/eye.1999.114. [DOI] [PubMed] [Google Scholar]
- 51.Putilina T, Skouri-Panet F, Prat K, Lubsen NH, Tardieu A. Subunit exchange demonstrates a differential chaperone activity of calf alpha-crystallin toward beta LOW- and individual gamma-crystallins. J Biol Chem. 2003;278:13747–13756. doi: 10.1074/jbc.M208157200. [DOI] [PubMed] [Google Scholar]
- 52.Delaye M, Tardieu A. Short-range order of crystallin proteins accounts for eye lens transparency. Nature. 1983;302:415–417. doi: 10.1038/302415a0. [DOI] [PubMed] [Google Scholar]
- 53.Ponce A, Sorensen C, Takemoto L. Role of short-range protein interactions in lens opacifications. Mol Vis. 2006;12:879–884. [PubMed] [Google Scholar]
- 54.Takemoto L, Sorensen CM. Protein-protein interactions and lens transparency. Exp Eye Res. 2008;87:496–501. doi: 10.1016/j.exer.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Horwitz J. Alpha-crystallin can function as a molecular chaperone. Proc Natl Acad Sci USA. 1992;89:10449–10453. doi: 10.1073/pnas.89.21.10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Harding JJ. Viewing molecular mechanisms of ageing through a lens. Ageing Res Rev. 2002;1:465–479. doi: 10.1016/s1568-1637(02)00012-0. [DOI] [PubMed] [Google Scholar]
- 57.Rao PV, Huang QL, Horwitz J, Zigler JS., Jr Evidence that alpha-crystallin prevents non-specific protein aggregation in the intact eye lens. Biochim Biophys Acta. 1995;1245:439–447. doi: 10.1016/0304-4165(95)00125-5. [DOI] [PubMed] [Google Scholar]
- 58.Brady JP, Garland D, Duglas-Tabor Y, Robison WG, Jr, Groome A, Wawrousek EF. Targeted disruption of the mouse alpha A-crystallin gene induces cataract and cytoplasmic inclusion bodies containing the small heat shock protein alpha B-crystallin. Proc Natl Acad Sci USA. 1997;94:884–889. doi: 10.1073/pnas.94.3.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brady JP, Garland DL, Green DE, Tamm ER, Giblin FJ, Wawrousek EF. AlphaB-crystallin in lens development and muscle integrity: a gene knockout approach. Invest Ophthalmol Vis Sci. 2001;42:2924–2934. [PubMed] [Google Scholar]
- 60.Andley UP, Hamilton PD, Ravi N. Mechanism of insolubilization by a single-point mutation in alphaA-crystallin linked with hereditary human cataracts. Biochemistry. 2008;47:9697–9706. doi: 10.1021/bi800594t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Clark JI, Huang QL. Modulation of the chaperone-like activity of bovine alpha-crystallin. Proc Natl Acad Sci USA. 1996;93:15185–15189. doi: 10.1073/pnas.93.26.15185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Muchowski PJ, Clark JI. ATP-enhanced molecular chaperone functions of the small heat shock protein human alphaB crystallin. Proc Natl Acad Sci USA. 1998;95:1004–1009. doi: 10.1073/pnas.95.3.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Maraini G, Santori M, Carta F. Modifications of adenosine triphosphate and of some enzymatic activities during the development of human senile cataract. Exp Eye Res. 1967;6:126–129. doi: 10.1016/s0014-4835(67)80063-0. [DOI] [PubMed] [Google Scholar]
- 64.Chiou SH, Chylack LT, Jr, Bunn HF, Kinoshita JH. Role of nonenzymatic glycosylation in experimental cataract formation. Biochem Biophys Res Commun. 1980;95:894–901. doi: 10.1016/0006-291x(80)90871-2. [DOI] [PubMed] [Google Scholar]
- 65.Robman L, Taylor H. External factors in the development of cataract. Eye. 2005;19:1074–1082. doi: 10.1038/sj.eye.6701964. [DOI] [PubMed] [Google Scholar]
- 66.Sharma KK, Ortwerth BJ. Effect of cross-linking on the chaperone-like function of alpha crystallin. Exp Eye Res. 1995;61:413–421. doi: 10.1016/s0014-4835(05)80136-8. [DOI] [PubMed] [Google Scholar]
- 67.Sharma KK, Kumar RS, Kumar GS, Quinn PT. Synthesis and characterization of a peptide identified as a functional element in alphaA-crystallin. J Biol Chem. 2000;275:3767–3771. doi: 10.1074/jbc.275.6.3767. [DOI] [PubMed] [Google Scholar]
- 68.Ghosh JG, Estrada MR, Clark JI. Interactive domains for chaperone activity in the small heat shock protein, human alphaB crystallin. Biochemistry. 2005;44:14854–14869. doi: 10.1021/bi0503910. [DOI] [PubMed] [Google Scholar]
- 69.Ghosh JG, Houck SA, Clark JI. Interactive sequences in the molecular chaperone, human alphaB crystallin modulate the fibrillation of amyloidogenic proteins. Int J Biochem Cell Biol. 2008;40:954–967. doi: 10.1016/j.biocel.2007.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Murugesan R, Santhoshkumar P, Sharma KK. Cataract-causing alphaAG98R mutant shows substrate-dependent chaperone activity. Mol Vis. 2007;13:2301–2309. [PubMed] [Google Scholar]
- 71.Murugesan R, Santhoshkumar P, Sharma KK. Role of alphaBI5 and alphaBT162 residues in subunit interaction during oligomerization of alphaB-crystallin. Mol Vis. 2008;14:1835–1844. [PMC free article] [PubMed] [Google Scholar]
- 72.Horwitz J, Hansen JS, Cheung CC, et al. Presence of low molecular weight polypeptides in human brunescent cataracts. Biochem Biophys Res Commun. 1983;113:65–71. doi: 10.1016/0006-291x(83)90432-1. [DOI] [PubMed] [Google Scholar]
- 73.Takemoto L, Horwitz J, Emmons T. Oxidation of the N-terminal methionine of lens alpha-A crystallin. Curr Eye Res. 1992;11:651–655. doi: 10.3109/02713689209000738. [DOI] [PubMed] [Google Scholar]
- 74.Takemoto LJ. Identification of the in vivo truncation sites at the C-terminal region of alpha-A crystallin from aged bovine and human lens. Curr Eye Res. 1995;14:837–841. doi: 10.3109/02713689508995806. [DOI] [PubMed] [Google Scholar]
- 75.MacCoss MJ, McDonald WH, Saraf A, 3rd, et al. Shotgun identification of protein modifications from protein complexes and lens tissue. Proc Natl Acad Sci USA. 2002;99:7900–7905. doi: 10.1073/pnas.122231399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Srivastava OP, Srivastava K. Existence of deamidated alphaB-crystallin fragments in normal and cataractous human lenses. Mol Vis. 2003;9:110–118. [PubMed] [Google Scholar]
- 77.Swamy MS, Abraham EC. Lens protein composition, glycation and high molecular weight aggregation in aging rats. Invest Ophthalmol Vis Sci. 1987;28:1693–1701. [PubMed] [Google Scholar]
- 78.Srivastava OP. Age-related increase in concentration and aggregation of degraded polypeptides in human lenses. Exp Eye Res. 1988;47:525–543. doi: 10.1016/0014-4835(88)90092-9. [DOI] [PubMed] [Google Scholar]
- 79.Dunn JA, Patrick JS, Thorpe SR, Baynes JW. Oxidation of glycated proteins: age-dependent accumulation of N epsilon-(carboxymethyl)lysine in lens proteins. Biochemistry. 1989;28:9464–9468. doi: 10.1021/bi00450a033. [DOI] [PubMed] [Google Scholar]
- 80.Nagaraj RH, Sell DR, Prabhakaram M, Ortwerth BJ, Monnier VM. High correlation between pentosidine protein crosslinks and pigmentation implicates ascorbate oxidation in human lens senescence and cataractogenesis. Proc Natl Acad Sci USA. 1991;88:10257–10261. doi: 10.1073/pnas.88.22.10257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Swamy MS, Tsai C, Abraham A, Abraham EC. Glycation mediated lens crystallin aggregation and cross-linking by various sugars and sugar phosphates in vitro. Exp Eye Res. 1993;56:177–185. doi: 10.1006/exer.1993.1025. [DOI] [PubMed] [Google Scholar]
- 82.Nagaraj RH, Shipanova IN, Faust FM. Protein cross-linking by the Maillard reaction. Isolation, characterization, and in vivo detection of a lysine-lysine cross-link derived from methylglyoxal. J Biol Chem. 1996;271:19338–19345. doi: 10.1074/jbc.271.32.19338. [DOI] [PubMed] [Google Scholar]
- 83.Ahmed MU, Brinkmann Frye E, Degenhardt TP, Thorpe SR, Baynes JW. N-epsilon-(carboxyethyl)lysine, a product of the chemical modification of proteins by methylglyoxal, increases with age in human lens proteins. Biochem J. 1997;324(Pt 2):565–570. doi: 10.1042/bj3240565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hains PG, Truscott RJ. Proteomic analysis of the oxidation of cysteine residues in human age-related nuclear cataract lenses. Biochim Biophys Acta. 2008;1784:1959–1964. doi: 10.1016/j.bbapap.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 85.Lampi KJ, Ma Z, Hanson SR, et al. Age-related changes in human lens crystallins identified by two-dimensional electrophoresis and mass spectrometry. Exp Eye Res. 1998;67:31–43. doi: 10.1006/exer.1998.0481. [DOI] [PubMed] [Google Scholar]
- 86.Takemoto L, Boyle D. Deamidation of specific glutamine residues from alpha-A crystallin during aging of the human lens. Biochemistry. 1998;37:13681–13685. doi: 10.1021/bi981542k. [DOI] [PubMed] [Google Scholar]
- 87.Takemoto LJ. Quantitation of asparagine-101 deamidation from alpha-A crystallin during aging of the human lens. Curr Eye Res. 1998;17:247–250. doi: 10.1076/ceyr.17.3.247.5218. [DOI] [PubMed] [Google Scholar]
- 88.Takemoto L. Increased deamidation of asparagine-101 from alpha-A crystallin in the high molecular weight aggregate of the normal human lens. Exp Eye Res. 1999;68:641–645. doi: 10.1006/exer.1999.0668. [DOI] [PubMed] [Google Scholar]
- 89.Takemoto L, Boyle D. Increased deamidation of asparagine during human senile cataractogenesis. Mol Vis. 2000;6:164–168. [PubMed] [Google Scholar]
- 90.Takemoto L. Deamidation of Asn-143 of gamma S crystallin from protein aggregates of the human lens. Curr Eye Res. 2001;22:148–153. doi: 10.1076/ceyr.22.2.148.5524. [DOI] [PubMed] [Google Scholar]
- 91.Murthy SN, Velasco PT, Lorand L. Properties of purified lens transglutaminase and regulation of its transamidase/crosslinking activity by GTP. Exp Eye Res. 1998;67:273–281. doi: 10.1006/exer.1998.0509. [DOI] [PubMed] [Google Scholar]
- 92.Lampi KJ, Oxford JT, Bachinger HP, Shearer TR, David LL, Kapfer DM. Deamidation of human beta B1 alters the elongated structure of the dimer. Exp Eye Res. 2001;72:279–288. doi: 10.1006/exer.2000.0950. [DOI] [PubMed] [Google Scholar]
- 93.Flaugh SL, Mills IA, King J. Glutamine deamidation destabilizes human gammaD-crystallin and lowers the kinetic barrier to unfolding. J Biol Chem. 2006;281:30782–30793. doi: 10.1074/jbc.M603882200. [DOI] [PubMed] [Google Scholar]
- 94.Lampi KJ, Amyx KK, Ahmann P, Steel EA. Deamidation in human lens betaB2-crystallin destabilizes the dimer. Biochemistry. 2006;45:3146–3153. doi: 10.1021/bi052051k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lapko VN, Purkiss AG, Smith DL, Smith JB. Deamidation in human gamma S-crystallin from cataractous lenses is influenced by surface exposure. Biochemistry. 2002;41:8638–8648. doi: 10.1021/bi015924t. [DOI] [PubMed] [Google Scholar]
- 96.Kamei A, Iwase H, Masuda K. Cleavage of amino acid residue(s) from the N-terminal region of alpha A- and alpha B-crystallins in human crystalline lens during aging. Biochem Biophys Res Commun. 1997;231:373–378. doi: 10.1006/bbrc.1997.6105. [DOI] [PubMed] [Google Scholar]
- 97.Das KP, Surewicz WK. Temperature-induced exposure of hydrophobic surfaces and its effect on the chaperone activity of alpha-crystallin. FEBS Lett. 1995;369:321–325. doi: 10.1016/0014-5793(95)00775-5. [DOI] [PubMed] [Google Scholar]
- 98.Srinivas V, Raman B, Rao KS, Ramakrishna T, Rao Ch M. Structural perturbation and enhancement of the chaperone-like activity of alpha-crystallin by arginine hydrochloride. Protein Sci. 2003;12:1262–1270. doi: 10.1110/ps.0302003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Santhoshkumar P, Sharma KK. Phe71 is essential for chaperone-like function in alpha A-crystallin. J Biol Chem. 2001;276:47094–47099. doi: 10.1074/jbc.M107737200. [DOI] [PubMed] [Google Scholar]
- 100.Reddy GB, Kumar PA, Kumar MS. Chaperone-like activity and hydrophobicity of alpha-crystallin. IUBMB Life. 2006;58:632–641. doi: 10.1080/15216540601010096. [DOI] [PubMed] [Google Scholar]
- 101.Takata T, Oxford JT, Brandon TR, Lampi KJ. Deamidation alters the structure and decreases the stability of human lens betaA3-crystallin. Biochemistry. 2007;46:8861–8871. doi: 10.1021/bi700487q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Harms MJ, Wilmarth PA, Kapfer DM, et al. Laser light-scattering evidence for an altered association of beta B1-crystallin deamidated in the connecting peptide. Protein Sci. 2004;13:678–686. doi: 10.1110/ps.03427504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ortwerth BJ, Feather MS, Olesen PR. The precipitation and cross-linking of lens crystallins by ascorbic acid. Exp Eye Res. 1988;47:155–168. doi: 10.1016/0014-4835(88)90032-2. [DOI] [PubMed] [Google Scholar]
- 104.Sell DR, Monnier VM. Structure elucidation of a senescence cross-link from human extracellular matrix. Implication of pentoses in the aging process. J Biol Chem. 1989;264:21597–21602. [PubMed] [Google Scholar]
- 105.Nagaraj RH, Sady C. The presence of a glucose-derived Maillard reaction product in the human lens. FEBS Lett. 1996;382:234–238. doi: 10.1016/0014-5793(96)00142-1. [DOI] [PubMed] [Google Scholar]
- 106.Frye EB, Degenhardt TP, Thorpe SR, Baynes JW. Role of the Maillard reaction in aging of tissue proteins. Advanced glycation end product-dependent increase in imidazolium cross-links in human lens proteins. J Biol Chem. 1998;273:18714–18719. doi: 10.1074/jbc.273.30.18714. [DOI] [PubMed] [Google Scholar]
- 107.Zarina S, Zhao HR, Abraham EC. Advanced glycation end products in human senile and diabetic cataractous lenses. Mol Cell Biochem. 2000;210:29–34. doi: 10.1023/a:1007015416572. [DOI] [PubMed] [Google Scholar]
- 108.Padayatti PS, Ng AS, Uchida K, Glomb MA, Nagaraj RH. Argpyrimidine, a blue fluorophore in human lens proteins: high levels in brunescent cataractous lenses. Invest Ophthalmol Vis Sci. 2001;42:1299–1304. [PubMed] [Google Scholar]
- 109.Ahmed N, Thornalley PJ, Dawczynski J, et al. Methylglyoxal-derived hydroimidazolone advanced glycation end-products of human lens proteins. Invest Ophthalmol Vis Sci. 2003;44:5287–5292. doi: 10.1167/iovs.03-0573. [DOI] [PubMed] [Google Scholar]
- 110.Fan X, Reneker LW, Obrenovich ME, et al. Vitamin C mediates chemical aging of lens crystallins by the Maillard reaction in a humanized mouse model. Proc Natl Acad Sci USA. 2006;103:16912–16917. doi: 10.1073/pnas.0605101103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fan X, Monnier VM. Inhibition of crystallin ascorbylation by nucleophilic compounds in the hSVCT2 mouse model of lenticular aging. Invest Ophthalmol Vis Sci. 2008;49:4945–4952. doi: 10.1167/iovs.08-1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Korlimbinis A, Aquilina JA, Truscott RJ. Protein-bound UV filters in normal human lenses: the concentration of bound UV filters equals that of free UV filters in the center of older lenses. Invest Ophthalmol Vis Sci. 2007;48:1718–1723. doi: 10.1167/iovs.06-1134. [DOI] [PubMed] [Google Scholar]
- 113.Chellan P, Nagaraj RH. Protein crosslinking by the Maillard reaction: dicarbonyl-derived imidazolium crosslinks in aging and diabetes. Arch Biochem Biophys. 1999;368:98–104. doi: 10.1006/abbi.1999.1291. [DOI] [PubMed] [Google Scholar]
- 114.Cheng R, Lin B, Lee KW, Ortwerth BJ. Similarity of the yellow chromophores isolated from human cataracts with those from ascorbic acid-modified calf lens proteins: evidence for ascorbic acid glycation during cataract formation. Biochim Biophys Acta. 2001;1537:14–26. doi: 10.1016/s0925-4439(01)00051-5. [DOI] [PubMed] [Google Scholar]
- 115.Cheng R, Feng Q, Ortwerth BJ. LC-MS display of the total modified amino acids in cataract lens proteins and in lens proteins glycated by ascorbic acid in vitro. Biochim Biophys Acta. 2006;1762:533–543. doi: 10.1016/j.bbadis.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 116.Linetsky M, Shipova E, Cheng R, Ortwerth BJ. Glycation by ascorbic acid oxidation products leads to the aggregation of lens proteins. Biochim Biophys Acta. 2008;1782:22–34. doi: 10.1016/j.bbadis.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Derham BK, Harding JJ. Effects of modifications of alpha-crystallin on its chaperone and other properties. Biochem J. 2002;364:711–717. doi: 10.1042/BJ20011512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bhattacharyya J, Shipova EV, Santhoshkumar P, Sharma KK, Ortwerth BJ. Effect of a single AGE modification on the structure and chaperone activity of human alphaB-crystallin. Biochemistry. 2007;46:14682–14692. doi: 10.1021/bi701326b. [DOI] [PubMed] [Google Scholar]
- 119.Kumar PA, Kumar MS, Reddy GB. Effect of glycation on alpha-crystallin structure and chaperone-like function. Biochem J. 2007;408:251–258. doi: 10.1042/BJ20070989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bhattacharyya J, Padmanabha Udupa EG, Wang J, Sharma KK. Mini-alphaB-crystallin: a functional element of alphaB-crystallin with chaperone-like activity. Biochemistry. 2006;45:3069–3076. doi: 10.1021/bi0518141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Argirov OK, Lin B, Ortwerth BJ. 2-ammonio-6-(3-oxidopyridinium-1-yl)hexanoate (OP-lysine) is a newly identified advanced glycation end product in cataractous and aged human lenses. J Biol Chem. 2004;279:6487–6495. doi: 10.1074/jbc.M309090200. [DOI] [PubMed] [Google Scholar]
- 122.Datta P, Kallur L, Abraham EC. Reversal of chaperone activity loss of glycated alphaA-crystallin by a crosslink breaker. Mol Cell Biochem. 2008;315:137–142. doi: 10.1007/s11010-008-9797-2. [DOI] [PubMed] [Google Scholar]
- 123.Ortwerth BJ, Slight SH, Prabhakaram M, Sun Y, Smith JB. Site-specific glycation of lens crystallins by ascorbic acid. Biochim Biophys Acta. 1992;1117:207–215. doi: 10.1016/0304-4165(92)90081-5. [DOI] [PubMed] [Google Scholar]
- 124.Nagaraj RH, Oya-Ito T, Padayatti PS, et al. Enhancement of chaperone function of alpha-crystallin by methylglyoxal modification. Biochemistry. 2003;42:10746–10755. doi: 10.1021/bi034541n. [DOI] [PubMed] [Google Scholar]
- 125.Nagaraj RH, Biswas A, Miller A, Oya-Ito T, Bhat M. The other side of the Maillard reaction. Ann N Y Acad Sci. 2008;1126:107–112. doi: 10.1196/annals.1433.045. [DOI] [PubMed] [Google Scholar]
- 126.Kanade SR, Pasupuleti N, Nagaraj RH. Role of cysteine residues in the enhancement of chaperone function in methylglyoxal-modified human alphaA-crystallin. Mol Cell Biochem. 2008 doi: 10.1007/s11010-008-9956-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Biswas A, Goshe J, Miller A, et al. Paradoxical effects of substitution and deletion mutation of Arg56 on the structure and chaperone function of human alphaB-crystallin. Biochemistry. 2007;46:1117–1127. doi: 10.1021/bi061323w. [DOI] [PubMed] [Google Scholar]
- 128.Thampi P, Zarina S, Abraham EC. alpha-Crystallin chaperone function in diabetic rat and human lenses. Mol Cell Biochem. 2002;229:113–118. doi: 10.1023/a:1017980713089. [DOI] [PubMed] [Google Scholar]
- 129.Truscott RJ. Age-related nuclear cataract-oxidation is the key. Exp Eye Res. 2005;80:709–725. doi: 10.1016/j.exer.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 130.Vinson JA. Oxidative stress in cataracts. Pathophysiology. 2006;13:151–162. doi: 10.1016/j.pathophys.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 131.Linetsky M, Ortwerth BJ. The generation of hydrogen peroxide by the UVA irradiation of human lens proteins. Photochem Photobiol. 1995;62:87–93. doi: 10.1111/j.1751-1097.1995.tb05243.x. [DOI] [PubMed] [Google Scholar]
- 132.Linetsky M, James HL, Ortwerth BJ. The generation of superoxide anion by the UVA irradiation of human lens proteins. Exp Eye Res. 1996;63:67–74. doi: 10.1006/exer.1996.0092. [DOI] [PubMed] [Google Scholar]
- 133.Linetsky M, Ortwerth BJ. Quantitation of the singlet oxygen produced by UVA irradiation of human lens proteins. Photochem Photobiol. 1997;65:522–529. doi: 10.1111/j.1751-1097.1997.tb08598.x. [DOI] [PubMed] [Google Scholar]
- 134.Linetsky M, James HL, Ortwerth BJ. Spontaneous generation of superoxide anion by human lens proteins and by calf lens proteins ascorbylated in vitro. Exp Eye Res. 1999;69:239–248. doi: 10.1006/exer.1999.0710. [DOI] [PubMed] [Google Scholar]
- 135.Rajan S, Horn C, Abraham EC. Effect of oxidation of alphaA- and alphaB-crystallins on their structure, oligomerization and chaperone function. Mol Cell Biochem. 2006;288:125–134. doi: 10.1007/s11010-006-9128-4. [DOI] [PubMed] [Google Scholar]
- 136.Spector A. Oxidative stress-induced cataract: mechanism of action. Faseb J. 1995;9:1173–1182. [PubMed] [Google Scholar]
- 137.Kantrow SP, Lee W, Sur A, Cowell T, Giblin F, Brennan L. MSRA repairs lens cytochrome C and alpha-crystallin. Exp Eye Res. 2008;87(Supplement):125. [Google Scholar]
- 138.Aquilina JA, Benesch JL, Ding LL, Yaron O, Horwitz J, Robinson CV. Phosphorylation of alphaB-crystallin alters chaperone function through loss of dimeric substructure. J Biol Chem. 2004;279:28675–28680. doi: 10.1074/jbc.M403348200. [DOI] [PubMed] [Google Scholar]
- 139.Ecroyd H, Meehan S, Horwitz J, et al. Mimicking phosphorylation of alphaB-crystallin affects its chaperone activity. Biochem J. 2007;401:129–141. doi: 10.1042/BJ20060981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ahmad MF, Raman B, Ramakrishna T, Rao Ch M. Effect of phosphorylation on alpha B-crystallin: differences in stability, subunit exchange and chaperone activity of homo and mixed oligomers of alpha B-crystallin and its phosphorylation-mimicking mutant. J Mol Biol. 2008;375:1040–1051. doi: 10.1016/j.jmb.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 141.Lapko VN, Smith DL, Smith JB. S-methylated cysteines in human lens gamma S-crystallins. Biochemistry. 2002;41:14645–14651. doi: 10.1021/bi0267700. [DOI] [PubMed] [Google Scholar]
- 142.Lapko VN, Smith DL, Smith JB. Methylation and carbamylation of human gamma-crystallins. Protein Sci. 2003;12:1762–1774. doi: 10.1110/ps.0305403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lapko VN, Cerny RL, Smith DL, Smith JB. Modifications of human betaA1/betaA3-crystallins include S-methylation, glutathiolation, and truncation. Protein Sci. 2005;14:45–54. doi: 10.1110/ps.04738505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Searle BC, Dasari S, Wilmarth PA, et al. Identification of protein modifications using MS/MS de novo sequencing and the OpenSea alignment algorithm. J Proteome Res. 2005;4:546–554. doi: 10.1021/pr049781j. [DOI] [PubMed] [Google Scholar]
- 145.Srikanthan D, Bateman OA, Purkiss AG, Slingsby C. Sulfur in human crystallins. Exp Eye Res. 2004;79:823–831. doi: 10.1016/j.exer.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 146.Aquilina JA, Truscott RJ. Kynurenine binds to the peptide binding region of the chaperone alphaB-crystallin. Biochem Biophys Res Commun. 2001;285:1107–1113. doi: 10.1006/bbrc.2001.5288. [DOI] [PubMed] [Google Scholar]
- 147.Staniszewska MM, Nagaraj RH. 3-hydroxykynurenine-mediated modification of human lens proteins: structure determination of a major modification using a monoclonal antibody. J Biol Chem. 2005;280:22154–22164. doi: 10.1074/jbc.M501419200. [DOI] [PubMed] [Google Scholar]
- 148.Mizdrak J, Hains PG, Truscott RJ, Jamie JF, Davies MJ. Tryptophan-derived ultraviolet filter compounds covalently bound to lens proteins are photosensitizers of oxidative damage. Free Radic Biol Med. 2008;44:1108–1119. doi: 10.1016/j.freeradbiomed.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 149.Finley EL, Dillon J, Crouch RK, Schey KL. Identification of tryptophan oxidation products in bovine alpha-crystallin. Protein Sci. 1998;7:2391–2397. doi: 10.1002/pro.5560071116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yoshida H, Murachi T, Tsukahara I. Limited proteolysis of bovine lens alpha-crystallin by calpain, a Ca2+-dependent cysteine proteinase, isolated from the same tissue. Biochim Biophys Acta. 1984;798:252–259. doi: 10.1016/0304-4165(84)90313-1. [DOI] [PubMed] [Google Scholar]
- 151.David LL, Shearer TR. Purification of calpain II from rat lens and determination of endogenous substrates. Exp Eye Res. 1986;42:227–238. doi: 10.1016/0014-4835(86)90057-6. [DOI] [PubMed] [Google Scholar]
- 152.Sharma KK, Ortwerth BJ. Aminopeptidase III activity in normal and cataractous lenses. Curr Eye Res. 1986;5:373–380. doi: 10.3109/02713688609025176. [DOI] [PubMed] [Google Scholar]
- 153.Srivastava OP. Characterization of a highly purified membrane proteinase from bovine lens. Exp Eye Res. 1988;46:269–283. doi: 10.1016/s0014-4835(88)80084-8. [DOI] [PubMed] [Google Scholar]
- 154.David LL, Varnum MD, Lampi KJ, Shearer TR. Calpain II in human lens. Invest Ophthalmol Vis Sci. 1989;30:269–275. [PubMed] [Google Scholar]
- 155.Sharma KK, Ortwerth BJ. Bovine lens acylpeptide hydrolase. Purification and characterization of a tetrameric enzyme resistant to urea denaturation and proteolytic inactivation. Eur J Biochem. 1993;216:631–637. doi: 10.1111/j.1432-1033.1993.tb18183.x. [DOI] [PubMed] [Google Scholar]
- 156.Sharma KK, Ortwerth BJ. Purification and characterization of prolyl oligopeptidase from bovine lens. Exp Eye Res. 1994;59:107–115. doi: 10.1006/exer.1994.1086. [DOI] [PubMed] [Google Scholar]
- 157.Sharma KK, Kester K. Peptide hydrolysis in lens: role of leucine aminopeptidase, aminopeptidase III, prolyloligopeptidase and acylpeptidehydrolase. Curr Eye Res. 1996;15:363–369. doi: 10.3109/02713689608995826. [DOI] [PubMed] [Google Scholar]
- 158.Sharma KK, Kester K, Elser N. Identification of new lens protease(s) using peptide substrates having in vivo cleavage sites. Biochem Biophys Res Commun. 1996;218:365–370. doi: 10.1006/bbrc.1996.0064. [DOI] [PubMed] [Google Scholar]
- 159.Srivastava OP, Srivastava K. Characterization of a sodium deoxycholate-activatable proteinase activity associated with betaA3/A1-crystallin of human lenses. Biochim Biophys Acta. 1999;1434:331–346. doi: 10.1016/s0167-4838(99)00183-1. [DOI] [PubMed] [Google Scholar]
- 160.Wang L, Christensen BN, Bhatnagar A, Srivastava SK. Role of calcium-dependent protease(s) in globulization of isolated rat lens cortical fiber cells. Invest Ophthalmol Vis Sci. 2001;42:194–199. [PubMed] [Google Scholar]
- 161.Chaerkady R, Sharma KK. Characterization of a bradykinin-hydrolyzing protease from the bovine lens. Invest Ophthalmol Vis Sci. 2004;45:1214–1223. doi: 10.1167/iovs.03-0769. [DOI] [PubMed] [Google Scholar]
- 162.Weber GF, Menko AS. The canonical intrinsic mitochondrial death pathway has a non-apoptotic role in signaling lens cell differentiation. J Biol Chem. 2005;280:22135–22145. doi: 10.1074/jbc.M414270200. [DOI] [PubMed] [Google Scholar]
- 163.Morozov V, Wawrousek EF. Caspase-dependent secondary lens fiber cell disintegration in alphaA-/alphaB-crystallin double-knockout mice. Development. 2006;133:813–821. doi: 10.1242/dev.02262. [DOI] [PubMed] [Google Scholar]
- 164.Zandy AJ, Bassnett S. Proteolytic mechanisms underlying mitochondrial degradation in the ocular lens. Invest Ophthalmol Vis Sci. 2007;48:293–302. doi: 10.1167/iovs.06-0656. [DOI] [PubMed] [Google Scholar]
- 165.Jahngen JH, Haas AL, Ciechanover A, Blondin J, Eisenhauer D, Taylor A. The eye lens has an active ubiquitin-protein conjugation system. J Biol Chem. 1986;261:13760–13767. [PubMed] [Google Scholar]
- 166.Shang F, Gong X, Palmer HJ, Nowell TR, Jr, Taylor A. Age-related decline in ubiquitin conjugation in response to oxidative stress in the lens. Exp Eye Res. 1997;64:21–30. doi: 10.1006/exer.1996.0176. [DOI] [PubMed] [Google Scholar]
- 167.David LL, Azuma M, Shearer TR. Cataract and the acceleration of calpain-induced beta-crystallin insolubilization occurring during normal maturation of rat lens. Invest Ophthalmol Vis Sci. 1994;35:785–793. [PubMed] [Google Scholar]
- 168.Robertson LJ, David LL, Riviere MA, Wilmarth PA, Muir MS, Morton JD. Susceptibility of ovine lens crystallins to proteolytic cleavage during formation of hereditary cataract. Invest Ophthalmol Vis Sci. 2008;49:1016–1022. doi: 10.1167/iovs.07-0792. [DOI] [PubMed] [Google Scholar]
- 169.Fukiage C, Azuma M, Nakamura Y, Tamada Y, Shearer TR. Calpain-induced light scattering by crystallins from three rodent species. Exp Eye Res. 1997;65:757–770. doi: 10.1006/exer.1997.0381. [DOI] [PubMed] [Google Scholar]
- 170.Fukiage C, Nakajima E, Ma H, Azuma M, Shearer TR. Characterization and regulation of lens-specific calpain Lp82. J Biol Chem. 2002;277:20678–20685. doi: 10.1074/jbc.M200697200. [DOI] [PubMed] [Google Scholar]
- 171.Van Kleef SM, Willems-Thijssen W, Hoenders HJ. Intracellular degradation and deamidation of alpha-crystallin subunits. Eur J Biochem. 1976;66:477–483. doi: 10.1111/j.1432-1033.1976.tb10572.x. [DOI] [PubMed] [Google Scholar]
- 172.Taylor A, Daims M, Lee J, Surgenor T. Identification and quantification of leucine aminopeptidase in aged normal and cataractous human lenses and ability of bovine lens LAP to cleave bovine crystallins. Curr Eye Res. 1982;2:47–56. doi: 10.3109/02713688208998379. [DOI] [PubMed] [Google Scholar]
- 173.Harrington V, McCall S, Huynh S, Srivastava K, Srivastava OP. Crystallins in water soluble-high molecular weight protein fractions and water insoluble protein fractions in aging and cataractous human lenses. Mol Vis. 2004;10:476–489. [PubMed] [Google Scholar]
- 174.Roy D, Spector A. Human insoluble lens protein. II. Isolation and characterization of a 9600 dalton polypeptide. Exp Eye Res. 1978;26:445–459. doi: 10.1016/0014-4835(78)90131-8. [DOI] [PubMed] [Google Scholar]
- 175.Takemoto L, Emmons T, Horwitz J. The C-terminal region of alpha-crystallin: involvement in protection against heat-induced denaturation. Biochem J. 1993;294(Pt 2):435–438. doi: 10.1042/bj2940435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Andley UP, Mathur S, Griest TA, Petrash JM. Cloning, expression, and chaperone-like activity of human alphaA-crystallin. J Biol Chem. 1996;271:31973–31980. doi: 10.1074/jbc.271.50.31973. [DOI] [PubMed] [Google Scholar]
- 177.Zhang X, Dudek EJ, Liu B, et al. Degradation of C-terminal truncated alpha A-crystallins by the ubiquitin-proteasome pathway. Invest Ophthalmol Vis Sci. 2007;48:4200–4208. doi: 10.1167/iovs.07-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Srivastava OP, Srivastava K, Silney C. Levels of crystallin fragments and identification of their origin in water soluble high molecular weight (HMW) proteins of human lenses. Curr Eye Res. 1996;15:511–520. doi: 10.3109/02713689609000762. [DOI] [PubMed] [Google Scholar]
- 179.Han J, Schey KL. MALDI tissue imaging of ocular lens alpha-crystallin. Invest Ophthalmol Vis Sci. 2006;47:2990–2996. doi: 10.1167/iovs.05-1529. [DOI] [PubMed] [Google Scholar]
- 180.Grey AC, Schey KL. Distribution of bovine and rabbit lens alpha-crystallin products by MALDI imaging mass spectrometry. Mol Vis. 2008;14:171–179. [PMC free article] [PubMed] [Google Scholar]
- 181.Thampi P, Hassan A, Smith JB, Abraham EC. Enhanced C-terminal truncation of alphaA- and alphaB-crystallins in diabetic lenses. Invest Ophthalmol Vis Sci. 2002;43:3265–3272. [PubMed] [Google Scholar]
- 182.Sreelakshmi Y, Santhoshkumar P, Bhattacharyya J, Sharma KK. AlphaA-crystallin interacting regions in the small heat shock protein, alphaB-crystallin. Biochemistry. 2004;43:15785–15795. doi: 10.1021/bi048151s. [DOI] [PubMed] [Google Scholar]
- 183.Ghosh JG, Clark JI. Insights into the domains required for dimerization and assembly of human alphaB crystallin. Protein Sci. 2005;14:684–695. doi: 10.1110/ps.041152805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.David LL, Shearer TR. Role of proteolysis in lenses: a review. Lens Eye Toxic Res. 1989;6:725–747. [PubMed] [Google Scholar]
- 185.Senthilkumar R, Chaerkady R, Sharma KK. Identification and properties of anti-chaperone-like peptides derived from oxidized bovine lens betaL-crystallins. J Biol Chem. 2002;277:39136–39143. doi: 10.1074/jbc.M204684200. [DOI] [PubMed] [Google Scholar]
- 186.Udupa EG, Sharma KK. Effect of oxidized betaB3-crystallin peptide on lens betaL-crystallin: interaction with betaB2-crystallin. Invest Ophthalmol Vis Sci. 2005;46:2514–2521. doi: 10.1167/iovs.05-0031. [DOI] [PubMed] [Google Scholar]
- 187.Udupa PE, Sharma KK. Effect of oxidized betaB3-crystallin peptide (152–166) on thermal aggregation of bovine lens gamma-crystallins: identification of peptide interacting sites. Exp Eye Res. 2005;80:185–196. doi: 10.1016/j.exer.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 188.Rao G, Santhoshkumar P, Sharma KK. Anti-chaperone betaA3/A1(102–117) peptide interacting sites in human alphaB-crystallin. Mol Vis. 2008;14:666–674. [PMC free article] [PubMed] [Google Scholar]
- 189.Bantseev V, Oriowo OM, Giblin FJ, Leverenz VR, Trevithick JR, Sivak JG. Effect of hyperbaric oxygen on guinea pig lens optical quality and on the refractive state of the eye. Exp Eye Res. 2004;78:925–931. doi: 10.1016/j.exer.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 190.Simpanya MF, Ansari RR, Suh KI, Leverenz VR, Giblin FJ. Aggregation of lens crystallins in an in vivo hyperbaric oxygen guinea pig model of nuclear cataract: dynamic light-scattering and HPLC analysis. Invest Ophthalmol Vis Sci. 2005;46:4641–4651. doi: 10.1167/iovs.05-0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Padgaonkar VA, Lin LR, Leverenz VR, Rinke A, Reddy VN, Giblin FJ. Hyperbaric oxygen in vivo accelerates the loss of cytoskeletal proteins and MIP26 in guinea pig lens nucleus. Exp Eye Res. 1999;68:493–504. doi: 10.1006/exer.1998.0630. [DOI] [PubMed] [Google Scholar]
- 192.Freel CD, Gilliland KO, Mekeel HE, Giblin FJ, Costello MJ. Ultrastructural characterization and Fourier analysis of fiber cell cytoplasm in the hyperbaric oxygen treated guinea pig lens opacification model. Exp Eye Res. 2003;76:405–415. doi: 10.1016/s0014-4835(03)00004-6. [DOI] [PubMed] [Google Scholar]
- 193.Cheng R, Lin B, Ortwerth BJ. Rate of formation of AGEs during ascorbate glycation and during aging in human lens tissue. Biochim Biophys Acta. 2002;1587:65–74. doi: 10.1016/s0925-4439(02)00069-8. [DOI] [PubMed] [Google Scholar]
- 194.Lee KW, Mossine V, Ortwerth BJ. The relative ability of glucose and ascorbate to glycate and crosslink lens proteins in vitro. Exp Eye Res. 1998;67:95–104. doi: 10.1006/exer.1998.0500. [DOI] [PubMed] [Google Scholar]
- 195.Nagaraj RH, Shamsi FA, Huber B, Pischetsrieder M. Immunochemical detection of oxalate monoalkylamide, an ascorbate-derived Maillard reaction product in the human lens. FEBS Lett. 1999;453:327–330. doi: 10.1016/s0014-5793(99)00571-2. [DOI] [PubMed] [Google Scholar]
- 196.Fan X, Monnier VM. Vitamin C-mediated Maillard reaction in the lens probed in a transgenic-mouse model. Ann NY Acad Sci. 2008;1126:194–200. doi: 10.1196/annals.1433.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Truscott RJ. Age-related nuclear cataract: a lens transport problem. Ophthalmic Res. 2000;32:185–194. doi: 10.1159/000055612. [DOI] [PubMed] [Google Scholar]
- 198.Kramps JA, de Jong WW, Wollensak J, Hoenders HJ. The polypeptide chains of alpha-crystallin from old human eye lenses. Biochim Biophys Acta. 1978;533:487–495. doi: 10.1016/0005-2795(78)90394-x. [DOI] [PubMed] [Google Scholar]
- 199.Miesbauer LR, Zhou X, Yang Z, et al. Post-translational modifications of water-soluble human lens crystallins from young adults. J Biol Chem. 1994;269:12494–12502. [PubMed] [Google Scholar]
- 200.Takemoto L, Boyle D. Deamidation of alpha-A crystallin from nuclei of cataractous and normal human lenses. Mol Vis. 1999;5:2. [PubMed] [Google Scholar]
- 201.Lampi KJ, Ma Z, Shih M, et al. Sequence analysis of betaA3, betaB3, and betaA4 crystallins completes the identification of the major proteins in young human lens. J Biol Chem. 1997;272:2268–2275. doi: 10.1074/jbc.272.4.2268. [DOI] [PubMed] [Google Scholar]
- 202.Lin PP, Barry RC, Smith DL, Smith JB. In vivo acetylation identified at lysine 70 of human lens alphaA-crystallin. Protein Sci. 1998;7:1451–1457. doi: 10.1002/pro.5560070622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Searle BC, Dasari S, Turner M, et al. High-throughput identification of proteins and unanticipated sequence modifications using a mass-based alignment algorithm for MS/MS de novo sequencing results. Anal Chem. 2004;76:2220–2230. doi: 10.1021/ac035258x. [DOI] [PubMed] [Google Scholar]
- 204.Aquilina JA, Truscott RJ. Cysteine is the initial site of modification of alpha-crystallin by kynurenine. Biochem Biophys Res Commun. 2000;276:216–223. doi: 10.1006/bbrc.2000.3461. [DOI] [PubMed] [Google Scholar]
- 205.Chalkley RJ, Burlingame AL. Identification of GlcNAcylation sites of peptides and alpha-crystallin using Q-TOF mass spectrometry. J Am Soc Mass Spectrom. 2001;12:1106–1113. doi: 10.1016/s1044-0305(01)00295-1. [DOI] [PubMed] [Google Scholar]
- 206.Kamei A, Hamaguchi T, Matsuura N, Masuda K. Does post-translational modification influence chaperone-like activity of alpha-crystallin? I. Study on phosphorylation. Biol Pharm Bull. 2001;24:96–99. doi: 10.1248/bpb.24.96. [DOI] [PubMed] [Google Scholar]
- 207.Kamei A, Hamaguchi T, Matsuura N, Iwase H, Masuda K. Post-translational modification of alphaB-crystallin of normal human lens. Biol Pharm Bull. 2000;23:226–230. doi: 10.1248/bpb.23.226. [DOI] [PubMed] [Google Scholar]
- 208.Lin P, Smith DL, Smith JB. In vivo modification of the C-terminal lysine of human lens alphaB-crystallin. Exp Eye Res. 1997;65:673–680. doi: 10.1006/exer.1997.0376. [DOI] [PubMed] [Google Scholar]
- 209.Lapko VN, Smith DL, Smith JB. In vivo carbamylation and acetylation of water-soluble human lens alphaB-crystallin lysine 92. Protein Sci. 2001;10:1130–1136. doi: 10.1110/ps.40901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Lapko VN, Smith DL, Smith JB. Expression of betaA2-crystallin in human lenses. Exp Eye Res. 2003;77:383–385. doi: 10.1016/s0014-4835(03)00156-8. [DOI] [PubMed] [Google Scholar]
- 211.Zhang Z, Smith DL, Smith JB. Human beta-crystallins modified by backbone cleavage, deamidation and oxidation are prone to associate. Exp Eye Res. 2003;77:259–272. doi: 10.1016/s0014-4835(03)00159-3. [DOI] [PubMed] [Google Scholar]
- 212.Aquilina JA, Truscott RJ. Identifying sites of attachment of UV filters to proteins in older human lenses. Biochim Biophys Acta. 2002;1596:6–15. doi: 10.1016/s0167-4838(01)00313-2. [DOI] [PubMed] [Google Scholar]
- 213.Biemel KM, Friedl DA, Lederer MO. Identification and quantification of major maillard cross-links in human serum albumin and lens protein. Evidence for glucosepane as the dominant compound. J Biol Chem. 2002;277:24907–24915. doi: 10.1074/jbc.M202681200. [DOI] [PubMed] [Google Scholar]
- 214.Tessier F, Obrenovich M, Monnier VM. Structure and mechanism of formation of human lens fluorophore LM-1. Relationship to vesperlysine A and the advanced Maillard reaction in aging, diabetes, and cataractogenesis. J Biol Chem. 1999;274:20796–20804. doi: 10.1074/jbc.274.30.20796. [DOI] [PubMed] [Google Scholar]
- 215.Nagaraj RH, Monnier VM. Isolation and characterization of a blue fluorophore from human eye lens crystallins: in vitro formation from Maillard reaction with ascorbate and ribose. Biochim Biophys Acta. 1992;1116:34–42. doi: 10.1016/0304-4165(92)90125-e. [DOI] [PubMed] [Google Scholar]
- 216.Cheng R, Feng Q, Argirov OK, Ortwerth BJ. K2P--a novel cross-link from human lens protein. Ann N Y Acad Sci. 2005;1043:184–194. doi: 10.1196/annals.1333.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Lyons TJ, Silvestri G, Dunn JA, Dyer DG, Baynes JW. Role of glycation in modification of lens crystallins in diabetic and nondiabetic senile cataracts. Diabetes. 1991;40:1010–1015. doi: 10.2337/diab.40.8.1010. [DOI] [PubMed] [Google Scholar]