Abstract
Background
Prenatal exposure to cocaine can impede normal brain development triggering a range of neuroanatomical and behavioral anomalies that are evident throughout life. Mouse models have been especially helpful in delineating neuro-teratogenic consequences following prenatal exposure tococaine. The present study employed a mouse model to investigate alterations in D1 dopamine receptor signaling and downstream immediate-early gene induction in the striatum of mice exposed to cocaine in utero.
Methods
Basal, forskolin- and D1 receptor agonist-induced cAMP levels were measured ex vivo in the adult male striatum in mice exposed to cocaine in utero. Further studies assessed cocaine-induced zif 268 and homer 1 expression in the striatum of juvenile (P15), adolescent (P36), and adult (P60) male mice.
Results
The D1 dopamine receptor agonist SKF82958 induced significantly higher levels of cAMP in adult male mice treated with cocaine in utero compared to saline controls. No effects of the prenatal treatment were found for cAMP formation induced by forskolin. Following an acute cocaine challenge (15 mg/kg, i.p.), these mice showed greater induction of zif 268 and homer 1, an effect that was most robust in the medial part of the mid-level striatum and became more pronounced with increasing age.
Conclusions
Together these findings indicate abnormally enhanced D1 receptor signal transduction in adult mice following prenatal cocaine exposure. Such changes in dopamine receptor signaling may underlie aspects of long-lasting neuro-teratogenic effects evident in some humans following in utero exposure to cocaine, and identify the striatum as one target potentially vulnerable to gestational cocaine exposure.
Keywords: Immediate-Early Genes, Prenatal cocaine, Brain development, D1, zif 268, homer 1
Introduction
Exposure to cocaine in utero continues to be a major epidemiological concern with estimates of 45,000 (1) to 375,000 (2) affected infants born each year. Roughly $352 million are spent annually in special education programs for these children in the US (3), and health care costs are estimated to be double that of a non-exposed infant (4). Despite the existing controversy over the definitive effects of prenatal cocaine (see (3)) accruing evidence identifying neuro-behavioral deficits in a subset of exposed offspring reinforce the need for further research aimed towards understanding mechanisms contributing to such long-term sequelae.
Numerous studies reporting findings from exposed offspring have linked prenatal cocaine exposure with microcephaly (5), low birth weight and preterm births (6), altered or delayed motor development (7), global hypertonia and coarse tremor (8), reduced cognitive development (9), and a subtle reduction in IQ (3) in a subset of exposed offspring. However, the data remains elusive as to the independent contribution of prenatal cocaine vs. confounding variables such as poly-drug use, poor prenatal; care, adverse living situations, and compromised maternal health in contributing to adverse postnatal neuro-behavioral outcomes. To control for some of these variables we created an animal model of prenatal cocaine exposure which has provided support for the role of cocaine independently contributing to some but not all of the clinical findings (reviewed in (10)). Of note,, rodents exposed to cocaine in utero display an altered response to drugs of abuse when tested as adults including impaired conditioned place preference (11,12), increased acquisition to (13,14) and enhanced reinforcing ability of cocaine (13,14) in self-administration tests, augmented brain stimulation reward following administration of cocaine and D1 agonists (15), and enhanced stereotypy during cocaine induced behavioral sensitization (16).
The psychostimulant cocaine is a potent, indirect dopaminergic agonist, which inhibits reuptake by the dopamine transporter to increase synaptic dopamine levels (17). Dopamine acting on D1 receptors activates the cAMP pathway and cAMP mediated gene expression. A significant amount of literature details the long-term effects of postnatal cocaine exposure on the dopamine system in rodents (18,19) reviewed in (20,21). However, much less is known about the long-term effects of prenatal exposure to cocaine. Evidence suggests decreased dopamine transporter activity (22,23), and enhanced D1 (24,25) and D2 (23) dopamine receptor levels/function in the striatum or midbrain of prenatally exposed animals. However, evidence that prenatal cocaine attenuates D1 receptor signaling has also been found (26,27). Differences in the species studied, as well as the route, dose and gestational timing of cocaine utilized may contribute to such discrepant data. However, it is now well established that prenatal pharmacological manipulation of the embryonic dopamine system via cocaine seems to interrupt normal development possibly leading to an altered neuro-behavioral phenotype.
To date few studies have characterized alterations in gene expression in the brain of mice prenatally exposed to cocaine. Of special interest are genes encoding proteins that regulate neuronal plasticity including transcription factors (immediate-early genes, IEGs) such as c-fos and zif 268 (28), and synaptic plasticity factors such as Homer proteins (29,30). The striatum is among the brain regions that show the most pronounced changes in gene expression following psychostimulant treatments (31,32). The striatum is part of anatomically distinct cortico-basal ganglia-cortical circuits that mediate planning and execution of motor functions and goal-directed behaviors (33–36). Striatal dysfunction is implicated in a variety of neuro-psychiatric disorders (37–39), including drug addiction (40,41). Genes induced in striatal neurons by systemic psychostimulant administration include c-fos (42,43), zif 268 (44) and homer 1a (45,46). Such gene induction is principally mediated by D1-like receptors; however, D2-like receptors additionally modulate such effects (47,48). Notably, psychostimulant-induced gene regulation is abolished by D1 receptor blockade (42,44,49,50), or by targeted deletion of D1 receptors (51–54).
The present study investigated the effects of prenatal cocaine exposure on dopamine receptor signaling. We assessed D1 receptor signaling by first examining basal, forskolin-, and D1 receptor agonist-regulated cAMP levels in the striatum of adult mice exposed to cocaine in utero. This was followed by investigation of cocaine-induced expression of two IEGs, zif 268 and homer 1, in the striatum of mice exposed to cocaine in utero, at three developmentally distinct ages, P15, P36 and P60, which correspond to juvenile, adolescent and adult age, respectively.
Methods and Materials
Prenatal Cocaine Treatment
Prenatal treatments were accomplished as previously described (55). Briefly, timed-pregnant Swiss Webster dams (Taconic Labs, New York) were assigned to one of two treatment groups to receive twice-daily subcutaneous (s.c.) injections (at 7:00 AM and 7:00 PM) from E8–E17, inclusive, of cocaine HCl (Sigma-Aldrich, St. Louis, MO, 20 mg/kg/injection, s.c., dissolved in saline, 2 mg/ml; PCOC40), or 0.9% saline (PSAL). In the adenylyl cyclase experiment two additional treatment groups were included; PCOC20 (2 daily injections of 10 mg/kg cocaine, s.c. in saline, 1mg/ml), and saline pair-fed (SPF), which were injected with 0.9% saline twice daily, but received a restricted diet to control for the anorectic effect of cocaine. Neither of these control groups was significantly different from the PSAL group regarding fetal growth (see supplemental data) or adenylyl cyclase activity, so offspring were not used in further studies.
All pups were surrogate fostered to control dams (Black Swiss Webster; Taconic Labs, New York), which had delivered within the previous 48 hours. To avoid the problem of ‘oversampling’ (56), no more than two offspring per litter were used.
Adenylyl Cyclase Measurement
Adult (P75-90) mice were exposed briefly to CO2, decapitated and the dorsal striatum (caudate-putamen) was rapidly dissected on ice. Adenylyl cyclase activity was measured as previously described (19). Briefly, crude membranes were immediately prepared from the striatum of individual animals. Tissue homogenates (20–35 μg protein) were incubated in 10 mM imidizole (pH 7.4), 10 mM theophylline, 6 mM MgSO4, 0.6 mM EGTA, 1.5 mM ATP, and 0.01 mM GTP in the absence or presence of 10 nM – 10 uM SKF82958 or 10 uM forskolin in triplicate for 5 minutes at 30°C. Adenylyl cyclase activity was terminated by placing the tubes into boiling H2O for 2 minutes. The amount of cAMP formed was determined by a 3H]cAMP binding protein assay (57). 3H]cAMP (4 nM) in citrate-phosphate buffer (pH 5.0) followed by binding protein prepared from bovine adrenal glands was added to each sample. Standards for quantification were prepared with tissue containing known amounts of cAMP (0.5 – 20 pmol). A 90-minute competition reaction for the binding protein between formed cAMP and 3H]cAMP was incubated at 4°C to reach equilibrium, and terminated with charcoal and centrifugation to separate the free cAMP from that bound to the binding protein. Aliquots of the supernatants were assayed for radioactivity by liquid scintillation spectrometry using CytoScint Scintillation Fluid (ICN Biomedicals, CA). Radioactivity counts were converted to pmol of cAMP protein by comparison to the standard curve. Protein concentrations were determined using a modification of the Lowry procedure (58).
Behavioral Testing
Horizontal locomotion was assessed in three different cohorts of male mice were tested at three distinct ages, postnatal day (P) 15, P36, and P60. On the test day, mice were placed individually in the test box (30 by 30 centimeter activity box with 16 photocells on each side connected to a computer, MedAssociates, Georgia, VT) for a 15-minute habituation period, removed from the box, injected with saline or cocaine (i.p., 15 mg/kg in saline,) and returned to their respective box for 30 minutes. Immediately following, mice were decapitated, their brains removed and rapidly frozen at −30°C in isopentane for in situ hybridization histochemistry.
In Situ Hybridization Histochemistry
Cryostat-cut coronal sections (12 μm) through the striatum were thaw-mounted onto glass slides (Superfrost/Plus, Daigger, Wheeling, IL), dried on a slide warmer and stored at −20°C. The sections were then fixed in 4% paraformaldehyde/0.9% saline for 10 minutes at room temperature, incubated in a fresh solution of 0.25% acetic anhydride in 0.1M triethanolamine/0.9% saline (pH 8.0) for 10 minutes, dehydrated, defatted for 2 × 5 minutes in chloroform, rehydrated, and air-dried. Slides were stored at −30°C until hybridization.
Oligonucleotide probes (48-mers; Life Technologies, Rockville, MD) were labeled with 35[S]-dATP (59). The probes had the following sequence: zif 268, complementary to bases 1757–1804, GenBank accession number M28844; homer 1, bases 674–721, AF093257. The homer 1 probe was a pan probe, targeting the beginning of the transcript, in order to produce a more robust signal (60).
Labeled probe (~3 × 106 cpm) in 100 μl of hybridization buffer was added to each slide (61). The sections were coverslipped and incubated at 37°C overnight. After incubation, the slides were first rinsed in 1X saline citrate (150mM sodium chloride, 15 mM sodium citrate), followed by three washes (20 minutes each) in 2X saline citrate/50% formamide at 40°C, and two washes (30 minutes each) in 1X saline citrate at room temperature. After a brief water rinse, the sections were air-dried and then apposed to X-ray film (BioMax MR-2, Kodak) for 4–10 days.
Analysis of Autoradiograms
Gene expression was assessed in sections from rostral (approximately at +1.5 mm rostral to bregma; (62,63), middle (+0.8), and caudal striatal levels (−0.2) (Fig. 1). Hybridization signals on film autoradiograms were measured by densitometry (NIH Image, Wayne Rasband, NIMH) across the total striatum on all 3 levels, as well as, on the rostral level, in medial and lateral striatal sectors and in the nucleus accumbens core, and medial and lateral shell, and on the middle level, in medial, central, lateral and ventral sectors (Fig. 1). The film autoradiograms were captured using a light table (Northern Light, Imaging Research, St. Catharines, Ontario, Canada) and a Sony CCD camera (Imaging Research). “Mean density” values of corresponding regions in the two hemispheres were averaged after correcting for background by subtracting mean density measured over white matter (corpus callosum). The illustrations of film autoradiograms (Fig. 4) are computer-generated images, and are contrast-enhanced when necessary.
Figure 1.
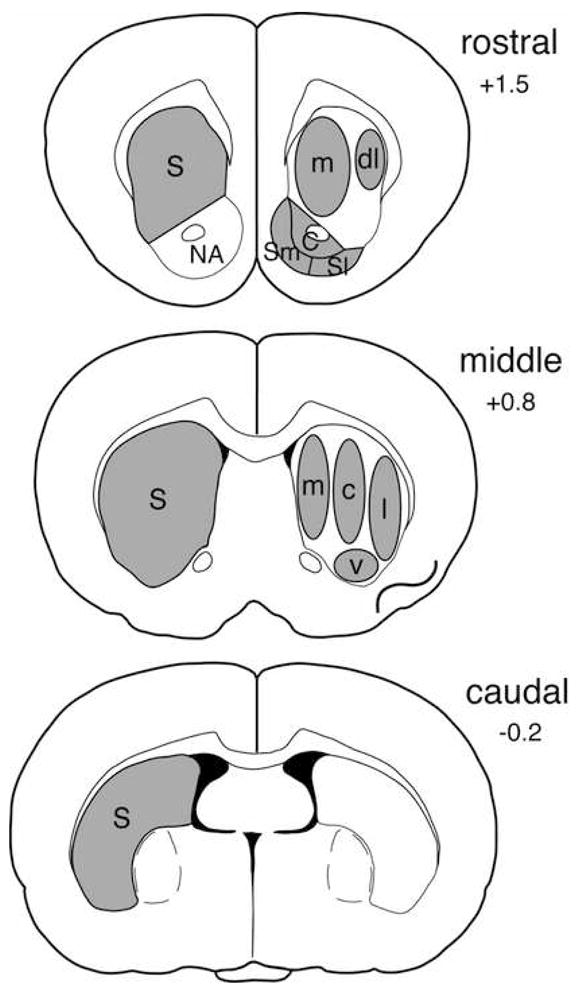
Schematic illustration of sampling areas used to measure gene expression on rostral, middle and caudal striatal levels. Total striatal areas (S, left) and striatal and nucleus accumbens (NA) subregions (right) are shown. Striatum: m, medial; dl, dorsolateral; c, central; l, lateral; v, ventral; Nucleus accumbens: C, core; Sm, medial shell; Sl, lateral shell.
Figure 4.
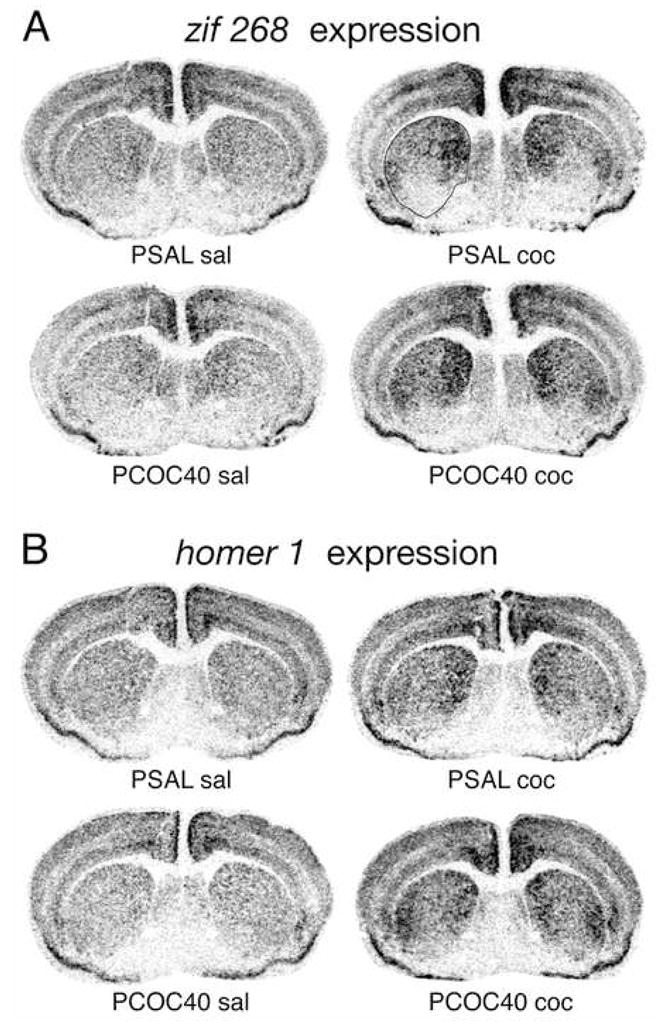
Prenatal cocaine exposure enhanced cocaine-induced zif 268 (A) and homer 1 expression (B) in the striatum of adult mice. Illustrations of film autoradiograms depict gene expression in coronal sections from the middle striatal level in mice that received a saline (sal) or cocaine (15 mg/kg; coc) challenge injection at postnatal day (P) 60, following prenatal exposure to saline (PSAL) or cocaine (40 mg/kg/day; PCOC40). Maximal hybridization signal is black.
Statistical Analysis
Treatment effects were determined by one- or two-factor ANOVAs, followed by Fisher PLSD (gestational data), Tukey’s, or Newman-Keuls post hoc tests (Statistica, StatSoft, Tulsa, OK).
All experimental protocols were approved by the Massachusetts General Hospital Subcommittee on Research Animal Care and were in accordance with NIH and EEC directives for animal studies.
Results
Adenylyl cyclase activity
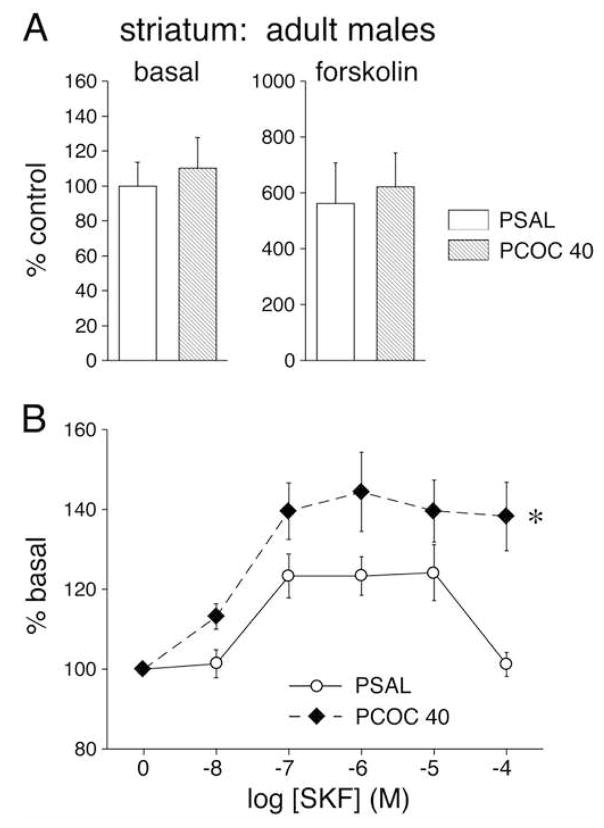
Adenylyl cyclase activity was measured under basal conditions and following the addition of forskolin or the selective D1 receptor full agonist SKF82958 in the striatum of adult male PSAL, PCOC20, PCOC40 and SPF mice. Basal adenylyl cyclase activity was not significantly different between prenatal treatment groups including PSAL, PCOC20, PCOC40 and SPF (p > 0.05; Figure 2). Forskolin stimulated adenylyl cyclase activity by six- to eight-fold in all animals, and there were no significant differences between prenatal treatment groups (p > 0.05; Figure 2). SKF82958 produced an increase in cAMP formation at concentrations of 10 nM to 100 uM (Figure 2). Two-factor ANOVA revealed significant main effects of concentration (p < 0.001) and treatment factors (p < 0.05) as well as a concentration-treatment interaction (p < 0.05). Tukey post-hoc analysis revealed that adult male PCOC40 mice showed significantly greater stimulation of adenylyl cyclase activity by the D1 receptor agonist as compared with PSAL mice (p < 0.05; Figure 2).
Figure 2.
Basal, forskolin- and D1 receptor agonist, SKF82958, stimulated adenylyl cyclase activity in the striatum of adult mice prenatally exposed to cocaine. Values (mean ± SEM) are given for males (N=8–9) that were exposed in utero to saline (PSAL) or cocaine (40 mg/kg/day, PCOC40). Data are expressed in percent of saline controls. A. Neither basal nor forskolin-stimulated adenylyl cyclase activity was significantly different between treatment groups. B. PCOC40 mice showed significantly higher D1 receptor-stimulated adenylyl cyclase activity than the PSAL control group (* P<0.05).
Behavioral effects
Three-way ANOVA revealed a significant main effect of postnatal treatment (coc vs. sal; F(1,94)= 79.924, p < 0.0001) on locomotion in all three age groups, which was more pronounced at P36 and P60 (p < 0.0001) than at P15 (p < 0.01), but no main effect of prenatal treatment at any age studied (Figure 3). For both PCOC and PSAL groups post hoc analyses revealed significantly higher locomotor activity in cocaine-treated mice compared to saline-treated controls at P36 and P60 (p < 0.001), but not at P15.
Figure 3.
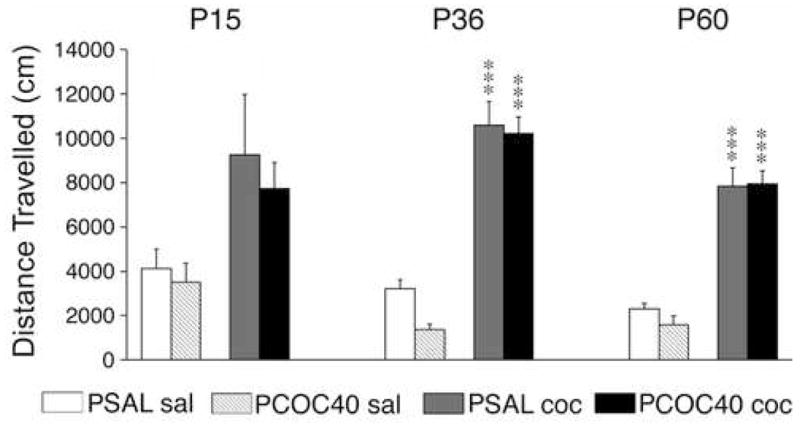
Effects of prenatal cocaine exposure on basal and cocaine-induced locomotor activity at different ages. Distance traveled (mean±SEM) during a 30-min test is given for male mice that received an injection of saline (sal) or cocaine (15 mg/kg; coc) on postnatal (P) days 15, 36 or 60, following prenatal exposure to saline (PSAL) or cocaine (40 mg/kg/day; PCOC40). While the cocaine challenge increased locomotion at all 3 ages, no significant main effect of the prenatal treatment was observed. *** P<0.001, vs. respective saline controls.
Cocaine-induced zif 268 expression in the striatum and nucleus accumbens
Basal zif 268 expression (i.e., in PSAL mice) in the striatum was relatively low, but showed a medial-to-lateral gradient with higher levels medially, in all three age groups (Table 1). Cocaine challenge-induced zif 268 expression displayed distinct regional patterns throughout the striatum. Induction of zif 268 was most pronounced on the middle striatal level, slightly lower on the rostral level, but was minimal in the caudal striatum (Table 1). In both the rostral and middle striatum, zif 268 mRNA levels were considerably higher medially than laterally after the cocaine challenge. However, due to the medial-lateral gradient in basal expression, the drug-induced increase tended to be greater laterally. On all levels, zif 268 induction was less pronounced or absent in ventral striatal regions, including the nucleus accumbens (Table 1).
Table 1.
Effects of prenatal cocaine treatment on the postnatal expression of zif 268.
Mean density values (mean±SEM) measured in different striatal regions on rostral, middle and caudal levels are given for mice that were prenatally exposed to saline (PSAL) or cocaine (PCOC) and tested at postnatal days (P) 15, P36 or P60 after an injection of saline (sal) or 15 mg/kg cocaine (coc). Responses to the cocaine challenge are also shown in percentage of values in respective saline controls (%). Striatum (S): m, medial; dl, dorsolateral; c, central; l, lateral; v, ventral; tot, total. Nucleus accumbens (NA): C, core; Sm, medial shell; Sl, lateral shell.
| age | area | PSALsal | PCOCsal | PSALcoc | PCOCcoc | PSALcoc(%) | PCOCcoc(%) |
|---|---|---|---|---|---|---|---|
| P15 | rostral |
||||||
| S m | 54.5±2.5 | 53.4±2.3 | 78.7±2.8*** | 79.0±3.5*** | 144.2±5.1 | 147.8±6.5 | |
| S dl | 47.3±1.7 | 44.8±1.3 | 60.9±1.8*** | 58.7±2.6*** | 128.9±3.7 | 131.3±5.8 | |
| S tot | 48.1±1.8 | 47.1±1.4 | 68.0±1.7*** | 67.2±3.0*** | 141.1±3.6 | 142.6±6.3 | |
| NA C | 38.0±2.6 | 40.6±2.8 | 57.6±4.1** | 58.2±4.5** | 151.3±10.7 | 143.3±11.2 | |
| NA Sm | 35.1±1.2 | 36.3±1.0 | 40.5±3.3 | 37.0±2.6 | 115.4±9.4 | 101.8±7.1 | |
| NA Sl | 27.0±1.0 | 27.3±1.3 | 36.0±3.8 | 31.6±3.2 | 133.4±14.2 | 115.6±11.7 | |
|
|
|||||||
| middle |
|||||||
| S m | 56.7±3.6 | 52.6±2.6 | 80.2±3.5*** | 78.9±3.0*** | 141.4±6.2 | 150.1±5.8 | |
| S c | 42.8±1.9 | 39.8±1.6 | 64.8±3.0*** | 63.7±4.8*** | 151.2±7.1 | 160.1±12.1 | |
| S l | 40.1±1.7 | 40.5±2.9 | 56.4±3.7** | 52.0±4.1* | 140.4±9.1 | 128.4±10.0 | |
| S v | 20.8±1.7 | 24.3±2.4 | 27.7±2.8 | 25.4±3.5 | 132.9±13.4 | 104.8±14.5 | |
| S tot | 40.7±1.9 | 36.8±2.3 | 57.8±1.7*** | 51.9±1.9*** # | 142.0±4.1 | 141.3±5.3 | |
|
|
|||||||
| caudal |
|||||||
| S tot | 31.3±1.8 | 32.4±2.1 | 39.1±2.5 | 33.9±2.6 | 124.7±7.9 | 104.7±7.9 | |
|
| |||||||
| P36 | rostral |
||||||
| S m | 55.8±3.1 | 52.8±1.9 | 79.7±2.4*** | 77.9±2.3*** | 142.8±4.2 | 147.3±4.3 | |
| S dl | 43.5±2.7 | 42.6±2.7 | 53.8±1.2** | 52.6±2.0** | 123.6±2.8 | 123.5±4.7 | |
| S tot | 48.1±2.7 | 45.9±2.0 | 65.0±2.0*** | 63.4±2.2*** | 135.2±4.2 | 138.1±4.9 | |
| NA C | 26.5±3.3 | 21.6±2.0 | 36.7±3.2* | 34.3±2.3* | 138.8±12.0 | 158.4±10.6 | |
| NA Sm | 31.3±2.3 | 25.6±1.9 | 35.9±2.7 | 34.5±2.1* | 114.7±8.6 | 134.4±8.2 | |
| NA Sl | 19.1±1.6 | 18.0±0.9 | 25.7±1.9* | 26.4±2.4* | 134.7±9.8 | 146.5±13.6 | |
|
|
|||||||
| middle |
|||||||
| S m | 57.6±3.3 | 51.3±2.0 | 83.0±1.8*** | 81.7±3.0*** | 143.9±3.1 | 159.1±5.9# | |
| S c | 41.0±2.9 | 38.2±2.4 | 57.5±2.1*** | 54.8±2.8*** | 140.4±5.2 | 143.5±7.2 | |
| S l | 32.9±1.9 | 33.7±2.8 | 56.9±1.9*** | 51.7±3.4*** | 172.9±5.9 | 153.4±10.2 | |
| S v | 19.3±2.3 | 15.3±1.5 | 22.2±2.7 | 17.3±2.3 | 115.0±14.1 | 113.0±15.1 | |
| S tot | 39.0±2.3 | 36.3±2.0 | 57.0±1.9*** | 54.4±2.6*** | 146.2±4.9 | 150.0±7.1 | |
|
|
|||||||
| caudal |
|||||||
| S tot | 27.0±2.3 | 24.5±2.0 | 32.1±2.2 | 30.4±1.4 | 118.8±8.1 | 123.9±5.9 | |
|
| |||||||
| P60 | rostral |
||||||
| S m | 59.8±3.2 | 56.1±3.2 | 76.6±2.4*** | 73.5±2.2*** | 128.0±3.9 | 131.0±3.9 | |
| S dl | 49.5±3.6 | 46.9±2.2 | 56.4±2.2 | 51.6±1.4 | 114.0±4.4 | 110.0±3.0 | |
| S tot | 51.3±2.7 | 48.8±3.0 | 64.3±1.4*** | 59.8±1.6** | 125.3±2.7 | 122.5±3.3 | |
| NA C | 39.9±3.8 | 33.3±3.1 | 47.6±2.5 | 42.5±2.0 | 119.2±6.3 | 127.6±6.1 | |
| NA Sm | 39.4±2.2 | 37.6±2.4 | 43.0±2.1 | 36.1±1.6 | 109.1±5.2 | 96.1±4.4 | |
| NA Sl | 30.8±3.1 | 32.1±1.5 | 32.9±2.9 | 26.6±1.9 | 106.9±9.3 | 82.8±5.9# | |
|
|
|||||||
| middle |
|||||||
| S m | 61.8±2.2 | 55.7±4.1 | 79.9±1.7*** | 79.7±1.7*** | 129.2±2.8 | 143.1±3.0## | |
| S c | 43.9±2.8 | 39.8±3.5 | 53.2±3.0 | 53.0±2.0* | 121.2±6.8 | 133.2±5.1 | |
| S l | 38.3±2.8 | 33.1±2.8 | 54.2±5.0** | 59.6±2.4*** | 141.6±12.9 | 180.0±7.1# | |
| S v | 23.5±2.4 | 16.9±3.0 | 22.5±1.7 | 23.4±2.4 | 95.7±7.1 | 138.2±14.3# | |
| S tot | 43.9±2.0 | 38.6±3.0 | 54.3±2.1** | 55.4±1.3*** | 123.7±4.7 | 143.6±3.5## | |
|
|
|||||||
| caudal |
|||||||
| S tot | 32.5±0.9 | 29.7±1.4 | 37.8±2.0 | 35.7±2.2 | 116.3±6.2 | 120.3±7.4 | |
p < 0.001,
p < 0.01,
p < 0.05, vs. respective saline control;
p < 0.01,
p < 0.05, vs. PSALcoc.
These expression patterns were in part age-dependent. Overall, younger animals tended to show a greater zif 268 response than adults. For example, in the nucleus accumbens, at P15, there was a significant increase in zif 268 expression after cocaine injection in the core (p < 0.01, both prenatal treatments), whereas at P36, the response just attained statistical significance (p < 0.05; core and shell), and at P60, no significant challenge effects were found in these subdivisions of the nucleus accumbens. The spatial pattern of zif 268 expression shifted with increasing age in the dorsal striatum as well. While stable in the medial part at all ages studied, zif 268 induction in the rostral lateral striatum gradually diminished from P15 to P60 (Table 1). Moreover, on the middle level, the zif 268 response was statistically more robust in the central than in the lateral part of the striatum at P15, but gradually reversed to become more robust laterally at P60 (Figure 4).
Effects of prenatal cocaine treatment on zif 268 expression were restricted to the middle striatum; these effects were maximal in the medial part and increased with age (Figure 5; Table 1). Overall, mice prenatally exposed to cocaine vs. saline, tended to show reduced zif 268 mRNA levels following injection with saline (i.e., PCOC sal vs. PSAL sal), although this effect did not reach statistical significance. Because of this somewhat reduced basal expression, effects of the cocaine challenge were best revealed by the relative magnitude of the zif 268 response (expressed as % of basal levels). No statistically significant effects of prenatal cocaine were seen at P15, with the one exception of a smaller challenge response (p < 0.05) in the total middle striatum in animals prenatally exposed to cocaine (Table 1; Figure 5). In contrast, at P36, there was a significantly increased relative zif 268 response in the medial part of the middle striatum in animals prenatally exposed to cocaine (PCOC coc vs. PSAL coc, p < 0.05; Table 1). No other effects were seen at this age. At P60, a statistically more robust (p < 0.01) increase was found in the medial striatum. In addition, increased zif 268 induction (p < 0.05) was also present in the lateral and ventral striatum, and the increased response thus reached statistical significance also for the total striatum on the middle level (p < 0.01; Table 1; Figure 5). In contrast, a decreased zif 268 response (p < 0.05) was seen in the lateral shell of the nucleus accumbens (Table 1).
Figure 5.
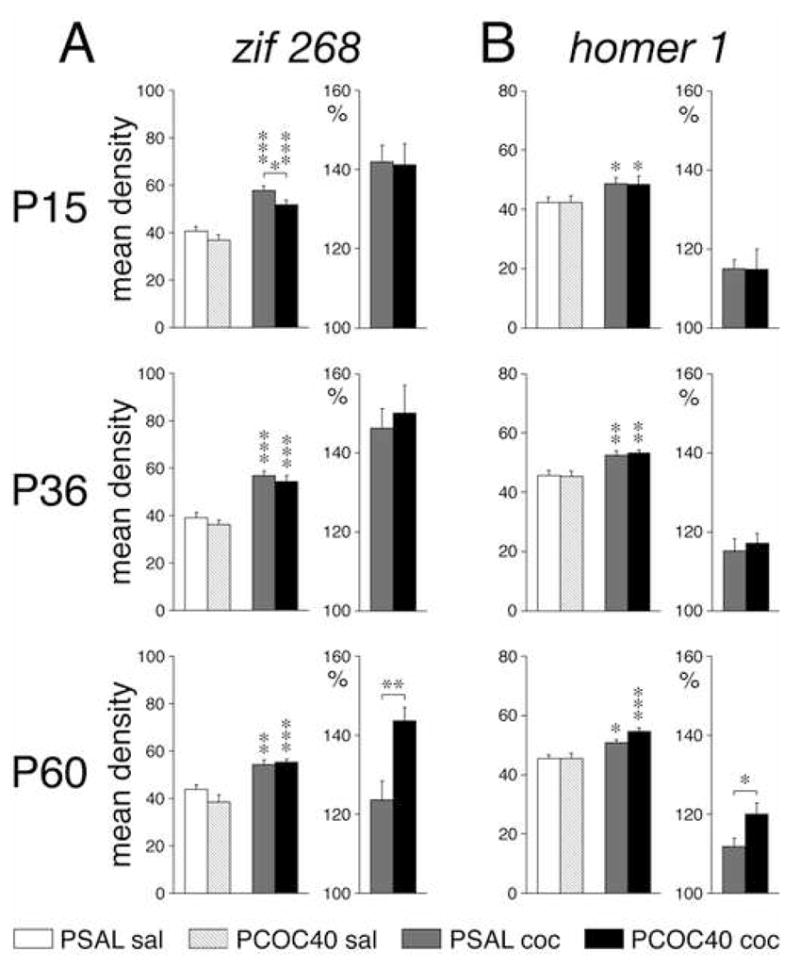
Effects of prenatal cocaine exposure on zif 268 (A) and homer 1 expression (B) in the striatum at different ages. Mean density values (mean±SEM, arbitrary units) measured across the total striatum at the middle level are shown for mice that received a saline (sal) or cocaine (coc) challenge injection at postnatal day (P) 15, P36, or P60, following prenatal exposure to saline (PSAL) or cocaine (40 mg/kg/day; PCOC40). Cocaine challenge-induced gene expression is also given in percent (%) of expression in the respective saline control (right). PCOC40 mice showed enhanced induction of zif 268 and homer 1 by the cocaine challenge at P60. * P<0.05, ** P<0.01, *** P<0.001, vs. saline control or as indicated.
Cocaine-induced homer 1 expression in the striatum and nucleus accumbens
Basal homer 1 expression in the striatum was also low, comparable to zif 268 expression (Figure 4). After the cocaine challenge homer 1 expression was increased, showing similar regional patterns as zif 268 induction, but this increase was generally less robust than that for zif 268, especially in younger animals. Cocaine-induced homer 1 expression was also more pronounced in the medial than the lateral striatum, on all striatal levels and at all ages examined. On the middle level, a similar, but less distinct, expression shift from more central than lateral at P15 to more lateral than central at P60 was apparent. In the ventral striatum, minimal or no effects of the cocaine challenge on homer 1 expression were seen. In the core and in the lateral shell of the nucleus accumbens, there was increased expression, which just reached statistical significance (p < 0.05), in PCOC coc and PSAL coc mice, respectively, at P36 (Table 2).
Table 2.
Effects of prenatal cocaine treatment on the postnatal expression of homer 1.
Mean density values (mean±SEM) measured in different striatal regions on rostral, middle and caudal levels are given for mice that were prenatally exposed to saline (PSAL) or cocaine (PCOC) and tested at postnatal days (P) 15, P36 or P60 after an injection of saline (sal) or 15 mg/kg cocaine (coc). Responses to the cocaine challenge are also shown in percentage of values in respective saline controls (%). Striatum (S): m, medial; dl, dorsolateral; c, central; l, lateral; v, ventral; tot, total. Nucleus accumbens (NA): C, core; Sm, medial shell; Sl, lateral shell.
| age | area | PSALsal | PCOCsal | PSALcoc | PCOCcoc | PSALcoc(%) | PCOCcoc(%) |
|---|---|---|---|---|---|---|---|
| P15 | rostral |
||||||
| S m | 46.1±0.7 | 44.2±1.5 | 53.1±1.4* | 54.9±2.8** | 115.1±3.1 | 124.2±6.3 | |
| S dl | 46.1±1.4 | 45.9±1.2 | 49.1±1.5 | 51.0±2.2 | 106.5±3.2 | 111.0±4.8 | |
| S tot | 44.5±0.8 | 42.7±1.0 | 48.7±1.2 | 51.9±2.5** | 109.4±2.8 | 121.7±5.9 | |
| NA C | 35.7±1.8 | 32.7±1.8 | 36.4±2.4 | 39.6±2.4 | 101.8±6.8 | 120.9±7.4 | |
| NA Sm | 33.6±2.1 | 32.5±2.2 | 29.2±2.3 | 29.7±1.8 | 86.8±7.0 | 91.2±5.4 | |
| NA Sl | 35.0±2.1 | 37.7±2.5 | 35.3±2.1 | 32.8±2.5 | 100.8±6.1 | 86.9±6.5 | |
|
|
|||||||
| middle |
|||||||
| S m | 43.1±0.9 | 40.6±1.1 | 53.1±1.8*** | 53.8±2.3*** | 123.0±4.3 | 132.5±5.8 | |
| S c | 45.4±0.6 | 44.8±1.2 | 52.7±1.6* | 53.1±2.6** | 116.1±3.5 | 118.5±5.7 | |
| S l | 50.7±0.9 | 50.8±1.7 | 55.7±1.6 | 52.7±2.2 | 109.7±3.2 | 103.8±4.2 | |
| S v | 27.3±1.2 | 29.7±1.9 | 30.5±1.8 | 30.4±1.3 | 111.7±6.6 | 102.4±4.3 | |
| S tot | 42.3±0.7 | 42.2±1.4 | 48.7±1.0* | 48.5±2.1* | 115.0±2.4 | 114.9±5.0 | |
|
|
|||||||
| caudal |
|||||||
| S tot | 33.8±1.4 | 33.5±1.0 | 36.0±2.2 | 34.6±2.1 | 106.4±6.4 | 103.4±6.4 | |
|
| |||||||
| P36 | rostral |
||||||
| S m | 47.7±1.3 | 47.9±1.3 | 56.9±1.2*** | 59.5±1.1*** | 119.2±2.4 | 124.3±2.3 | |
| S dl | 48.9±1.5 | 51.1±1.6 | 53.2±1.3 | 54.7±1.5 | 108.7±2.8 | 107.0±2.9 | |
| S tot | 47.4±1.2 | 47.0±1.2 | 53.0±1.0** | 55.4±1.2*** | 111.9±2.1 | 117.8±2.5 | |
| NA C | 33.4±1.3 | 30.2±1.8 | 37.9±1.9 | 36.2±1.6* | 113.5±5.8 | 120.0±5.3 | |
| NA Sm | 33.8±1.5 | 31.4±1.7 | 35.4±2.2 | 33.0±1.8 | 104.7±6.4 | 105.0±5.7 | |
| NA Sl | 30.8±3.3 | 35.7±1.8 | 38.9±2.1* | 40.1±1.4 | 126.2±6.7 | 112.1±3.8 | |
|
|
|||||||
| middle |
|||||||
| S m | 49.6±1.7 | 47.2±1.4 | 59.7±1.1*** | 63.0±1.8*** | 120.2±2.2 | 133.3±3.8## | |
| S c | 46.4±1.9 | 45.9±1.6 | 51.0±1.7 | 52.5±1.3* | 110.0±3.7 | 114.3±2.9 | |
| S l | 50.2±2.2 | 51.9±2.0 | 58.9±2.2* | 58.2±1.9* | 117.3±4.3 | 112.0±3.7 | |
| S v | 29.9±2.3 | 31.5±2.0 | 31.2±0.7 | 30.9±0.6 | 104.3±2.3 | 98.1±1.9 | |
| S tot | 45.6±1.8 | 45.4±1.7 | 52.5±1.5** | 53.1±1.2** | 115.1±3.2 | 117.0±2.6 | |
|
|
|||||||
| caudal |
|||||||
| S tot | 35.9±1.8 | 34.7±1.6 | 38.0±1.5 | 40.3±1.1 | 105.8±4.3 | 115.9±3.3 | |
|
| |||||||
| P60 | rostral |
||||||
| S m | 51.4±0.9 | 51.8±1.3 | 59.8±0.8*** | 62.3±0.5*** | 116.3±1.5 | 120.2±1.1 | |
| S dl | 50.9±2.6 | 51.5±2.4 | 54.4±1.9 | 57.3±1.7 | 106.9±3.7 | 111.2±3.3 | |
| S tot | 50.5±1.1 | 50.5±1.1 | 54.6±0.7** | 57.4±0.6*** # | 108.2±1.5 | 113.6±1.3# | |
| NA C | 41.2±2.6 | 43.3±3.6 | 41.4±1.4 | 43.3±1.8 | 100.4±3.4 | 100.0±4.3 | |
| NA Sm | 41.2±1.9 | 41.5±2.3 | 40.1±1.3 | 39.6±2.0 | 97.3±3.0 | 95.4±4.9 | |
| NA Sl | 39.9±2.5 | 43.5±1.8 | 43.1±1.8 | 43.3±2.4 | 107.9±4.5 | 99.5±5.6 | |
|
|
|||||||
| middle |
|||||||
| S m | 52.3±2.0 | 50.4±2.7 | 64.6±1.8*** | 66.6±1.8*** | 123.6±3.4 | 132.2±3.5 | |
| S c | 44.8±1.5 | 44.4±1.9 | 49.2±1.3 | 51.1±1.8* | 109.9±2.8 | 115.2±4.0 | |
| S l | 49.7±2.1 | 48.3±1.8 | 57.1±2.2* | 62.9±2.8** | 114.8±4.5 | 130.1±5.9 | |
| S v | 29.9±2.4 | 35.3±1.6 | 30.9±1.9 | 37.5±1.3# | 103.2±6.4 | 106.2±3.7 | |
| S tot | 45.5±1.4 | 45.6±1.7 | 50.9±1.0* | 54.7±1.3*** | 111.8±2.2 | 120.0±2.9# | |
|
|
|||||||
| caudal |
|||||||
| S tot | 36.8±1.5 | 37.1±1.6 | 41.3±0.5 | 42.3±1.6* | 112.3±1.4 | 114.0±4.2 | |
P < 0.001,
p < 0.01,
p < 0.05, vs. respective saline control;
p < 0.01,
p < 0.05, vs. PSALcoc.
Effects of prenatal cocaine treatment on homer 1 expression were similar to those on zif 268 expression, including the age-dependent expression pattern. No effects of the prenatal treatment were seen in P15 animals. In contrast, at P36, animals prenatally exposed to cocaine vs. saline displayed a significantly greater homer 1 response to the cocaine challenge in the medial part of the middle striatum (PCOC coc vs. PSAL coc, % increase, p < 0.01; Table 2). Moreover, at P60, mice prenatally exposed to cocaine showed a significantly enhanced homer 1 response in the rostral striatum (total, p < 0.05, absolute and relative increase; Table 2) and in the middle striatum (total, p < 0.05, relative; ventral, p < 0.05, absolute; Figure 5, Table 2).
Discussion
In this study we provide evidence for an enhanced D1 dopamine receptor agonist-induced cAMP response in the striatum of adult male, but not female (see Supplemental Materials) mice prenatally exposed to cocaine compared to controls. Furthermore we show an age-dependent augmentation of prenatal cocaine treatment on cocaine-induced striatal IEG expression beginning at adolescence and becoming more robust into adulthood.
Enhanced D1 receptor signaling
The current data provide evidence for an enhanced cAMP response to the selective D1 receptor full agonist SKF82958 in the striatum of adult male, mice exposed prenatally to cocaine vs. saline. We have previously shown that D1 receptor-stimulated adenylyl cyclase activity in mice normally increases with age peaking at P20 (64). In that study mice exposed to cocaine in utero exhibited a decrease in D1 receptor-stimulated adenylyl cyclase activity in the striatum at E18, 24 hours following the final exposure to cocaine, which was a result of a significant decrease in the expression of adenylyl under basal conditions. The present study, which demonstrated an increase in D1 receptor-stimulated adenylyl cyclase activity in the striatum of adult mice, with no significant difference evident under basal conditions, suggests that the normal ontogeny of D1 receptor signaling evident postnatally is exaggerated following prenatal cocaine exposure. P15, the earliest time at which we assessed cocaine-induced IEG induction coincides with the time by which basal and foskolin-induced adenyly cyclase activity have reached adult levels (64). The age-dependent increase in cocaine-induced gene expression we observed in mice prenatally exposed to cocaine vs. saline at P15, P36, and P60 thus may require a functionally mature signaling pathway to demonstrate the full effects of that exposure. The exaggerated and persistent increase in D1 receptor-mediated intracellular signaling is a potential mechanism contributing to other behavioral and neuro-anatomical anomalies we and others have observed in adult mice following prenatal exposure to cocaine (e.g., 13,14). Despite such persistent neuro-adaptations it is clear that prenatal cocaine treatment does not affect the acute locomotor response to cocaine, similar to what has previously been seen at various postnatal ages (16,79,80).
Persistent deficits in striatal D1 receptor signaling have been identified in a rabbit model of gestational cocaine exposure. Rabbits exposed to cocaine in utero displayed a functional uncoupling of D1 receptors from G alpha s, without any changes in the concentration of G alpha s, D2 coupling, or D1 receptor antagonist binding (26,65). Furthermore, a recent report (66) demonstrated a decrease in D1 receptor membrane expression in the striatum of preadolescent rabbits prenatally exposed to cocaine, a novel finding which may provide mechanistic insights into the reduction in G alpha s coupling seen in that model. At the level of D1 receptor-activated signal transduction, Zhen et al. (67) have demonstrated an increase in phosphorylation of DARPP-32 at Thr 34, an inhibitor of protein phosphatase 1 (PP1), and an increase in D1 receptor phosphorylation in the striatum of rabbits prenatally exposed to cocaine. They speculated that decreased PP1 activity leads to increased phosphorylation and increased internalization of D1 receptors, which they hypothesized leads to decreased D1 receptor signal transduction (26). Differences in the species studied, as well as the route, dose and gestational timing of cocaine utilized may contribute to the discrepancies between the present data obtained in mice and that published in the rabbit model.
Altered gene expression in the striatum
Our results show that male PCOC40 mice displayed greater induction of the IEGs zif 268 and homer 1 in the striatum after a cocaine challenge injection. This effect was age-dependent; it emerged during adolescence (P36) and was maximal in adults (P60). Regionally, this enhanced gene induction was most robust in the middle striatum and was maximal in the medial part. These findings are consistent with enhanced D1 receptor signaling in the striatum of these mice.
The present study assessed changes in gene regulation in different functional domains of the striatum. We used a topographical mapping technique based on cortical input patterns (34,68) that was developed for the rat (69,70) and simplified for the mouse. While limited anatomical studies in the mouse (e.g., 71) indicate that the overall topographical organization of the cortico-striatal projections is similar to that in the rat, the definitive association of the present gene regulation effects with particular functional domains in the mouse will need confirmation.
Our most important finding is the age-dependent effect of prenatal cocaine treatment on IEG induction in the striatum. Consistent with greater D1 receptor-stimulated cAMP formation, the present study demonstrates that cocaine exposure in utero produces a long-term enhancement of IEG induction by cocaine. This effect was most pronounced at P60 and most robust in the middle striatum, and maximal in, but not limited to, the medial sector, which receives inputs from prelimbic and anterior cingulate cortex in rat (69) and mouse (73). This abnormal gene induction was best revealed when the challenge response was expressed relative to basal expression, especially for zif 268, as basal zif 268 mRNA levels tended to be reduced in mice exposed prenatally to cocaine compared to saline-injected controls. A suppression of basal zif 268 expression in the striatum by repeated cocaine treatment has been demonstrated before, although this suppression was found after repeated treatment in adults and recovered within 2–3 days of the treatment (74).
The enhanced induction of striatal zif 268 and homer 1 provides a potential mechanism for altered plasticity in striatal neurons. Zif 268 encodes a zinc finger transcription factor that regulates a number of genes and has been implicated in a variety of neuroplastic processes (28). Homer 1 is a member of a family of scaffolding proteins (Homer/Vesl proteins) of the postsynaptic density that cluster and traffic glutamate receptors, link these to internal calcium stores, and play a role in various mechanisms of synapse structuring and plasticity (29,30,75). The homer 1 signal rapidly induced by psychostimulants (45,70,76) and other treatments mostly reflects the IEG isoform homer 1a (60). This truncated homer 1 splice variant appears to act as a dominant negative regulator of glutamate synapse strength (77), by promoting disassembly and turnover of the signaling complex (29,30). This is seen as a necessary stabilizing process (78) during activity-dependent synaptic plasticity (77). Enhanced induction of these plasticity-associated molecules suggests that prenatal cocaine exposure renders these animals more susceptible to cocaine-induced neuroplasticity. There is the potential for an abnormal regulation in striatal synaptic growth and transmission related to altered induction of homer 1 isoforms (77), and electrophysiological consequences related to altered zif 268 induction (see 28).
Overall the current study provides evidence for abnormally increased striatal D1 dopamine receptor activity following prenatal cocaine exposure as shown by an enhanced D1 receptor agonist-induced cAMP response and increased striatal expression of the synaptic plasticity factor, Homer 1, and transcription factor, Zif 268. Further research is necessary to evaluate the mechanisms underlying the persistent augmentation of striatal D1 receptor signaling, and its potential contribution to some of the neuro-behavioral deficits observed in preclinical models as well as in a subset of infants, children, adolescents and adults following prenatal exposure to cocaine, and identify the striatum as one target potentially vulnerable to gestational cocaine exposure.
Supplementary Material
Acknowledgments
The authors would like to thank Dr. Anjali Rajadhyaksha for her assistance and expert advice in preparation of this manuscript. This work was supported by NIDA grants DA08648 and DA00354 (BEK); DA11261 (HS); DA09580 (EMU); NS41871 (MEE).
Footnotes
Financial Disclosures
Mr. Tropea reported no biomedical financial interests or potential conflicts of interest. Mr. Guerriero reported no biomedical financial interests or potential conflicts of interest. Mr. Willuhn reported no biomedical financial interests or potential conflicts of interest. Dr. Ehrlich reported no biomedical financial interests or potential conflicts of interest. Dr. Unterwald reported no biomedical financial interests or potential conflicts of interest. Dr. Steiner reported no biomedical financial interests or potential conflicts of interest. Dr. Kosofsky reported no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.National Institute of Drug Abuse. National Pregnancy and Health Survey. Rockville, MD: National Institute of Health.; 1996. Drug Use Among Women Delivering Livebirths: 1992. Vol Publication No. 96-3819. [Google Scholar]
- 2.United States General Office of Accounting. Report to the Chairman, Committee on Finance, US Senate, Drug Exposed Infants. Washington, DC: US GOA; 1990. A Generation at Risk. [Google Scholar]
- 3.Lester BM, LaGasse LL, Seifer R. Cocaine exposure and children: the meaning of subtle effects. Science. 1998;282:633–634. doi: 10.1126/science.282.5389.633. [DOI] [PubMed] [Google Scholar]
- 4.Behnke M, Eyler FD, Conlon M, Casanova OQ, Woods NS. How fetal cocaine exposure increases neonatal hospital costs. Pediatrics. 1997;99:204–208. doi: 10.1542/peds.99.2.204. [DOI] [PubMed] [Google Scholar]
- 5.Eyler FD, Behnke M, Conlon M, Woods NS, Wobie K. Birth outcome from a prospective, matched study of prenatal crack/cocaine use: II. Interactive and dose effects on neurobehavioral assessment. Pediatrics. 1998;101:237–241. doi: 10.1542/peds.101.2.237. [DOI] [PubMed] [Google Scholar]
- 6.Bada HS, Das A, Bauer CR, Shankaran S, Lester BM, Gard CC, et al. Low birth weight and preterm births: etiologic fraction attributable to prenatal drug exposure. J Perinatol. 2005;25:631–637. doi: 10.1038/sj.jp.7211378. [DOI] [PubMed] [Google Scholar]
- 7.Miller-Loncar C, Lester BM, Seifer R, Lagasse LL, Bauer CR, Shankaran S, et al. Predictors of motor development in children prenatally exposed to cocaine. Neurotoxicol Teratol. 2005;27:213–220. doi: 10.1016/j.ntt.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 8.Chiriboga CA, Brust JC, Bateman D, Hauser WA. Dose-response effect of fetal cocaine exposure on newborn neurologic function. Pediatrics. 1999;103:79–85. doi: 10.1542/peds.103.1.79. [DOI] [PubMed] [Google Scholar]
- 9.Alessandri SM, Bendersky M, Lewis M. Cognitive functioning in 8- to 18-month-old drug-exposed infants. Dev Psychol. 1998;34:565–573. doi: 10.1037//0012-1649.34.3.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kosofsky BE, Wilkins AS. A mouse model of transplacental cocaine exposure. Clinical implications for exposed infants and children. Ann N Y Acad Sci. 1998;846:248–261. [PubMed] [Google Scholar]
- 11.Heyser CJ, Miller JS, Spear NE, Spear LP. Prenatal exposure to cocaine disrupts cocaine-induced conditioned place preference in rats. Neurotoxicol Teratol. 1992;14:57–64. doi: 10.1016/0892-0362(92)90029-a. [DOI] [PubMed] [Google Scholar]
- 12.Malanga CJ, Pejchal M, Kosofsky BE. Prenatal exposure to cocaine alters the developmental conditioned place preference to cocaine in adult mice. doi: 10.1016/j.pbb.2007.06.002. In Preparation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keller RW, Jr, LeFevre R, Raucci J, Carlson JN, Glick SD. Enhanced cocaine self-administration in adult rats prenatally exposed to cocaine. Neurosci Lett. 1996;205:153–156. doi: 10.1016/0304-3940(96)12409-5. [DOI] [PubMed] [Google Scholar]
- 14.Rocha BA, Mead AN, Kosofsky BE. Increased vulnerability to self-administer cocaine in mice prenatally exposed to cocaine. Psychopharmacology (Berl) 2002;163:221–229. doi: 10.1007/s00213-002-1140-0. [DOI] [PubMed] [Google Scholar]
- 15.Malanga CJ, Riday TT, Carlezon WA, Kosofsky BE. Prenatal exposure to cocaine increases the rewarding potency of cocaine and selective dopaminergic agonists in adult mice. Biological Psychiatry. doi: 10.1016/j.biopsych.2007.01.014. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crozatier C, Guerriero RM, Mathieu F, Giros B, Nosten-Bertrand M, Kosofsky BE. Altered cocaine-induced behavioral sensitization in adult mice exposed to cocaine in utero. Brain Res Dev Brain Res. 2003;147:97–105. doi: 10.1016/j.devbrainres.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 17.Wise RA. Neural mechanisms of the reinforcing action of cocaine. NIDA Res Monogr. 1984;50:15–33. [PubMed] [Google Scholar]
- 18.Daws LC, Callaghan PD, Moron JA, Kahlig KM, Shippenberg TS, Javitch JA, Galli A. Cocaine increases dopamine uptake and cell surface expression of dopamine transporters. Biochem Biophys Res Commun. 2002;290:1545–1550. doi: 10.1006/bbrc.2002.6384. [DOI] [PubMed] [Google Scholar]
- 19.Unterwald EM, Fillmore J, Kreek MJ. Chronic repeated cocaine administration increases dopamine D1 receptor-mediated signal transduction. Eur J Pharmacol. 1996;318:31–35. doi: 10.1016/s0014-2999(96)00841-2. [DOI] [PubMed] [Google Scholar]
- 20.Anderson SM, Pierce RC. Cocaine-induced alterations in dopamine receptor signaling: implications for reinforcement and reinstatement. Pharmacol Ther. 2005;106:389–403. doi: 10.1016/j.pharmthera.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 21.Kuhar MJ, Ritz MC, Boja JW. The dopamine hypothesis of the reinforcing properties of cocaine. Trends Neurosci. 1991;14:299–302. doi: 10.1016/0166-2236(91)90141-g. [DOI] [PubMed] [Google Scholar]
- 22.Collins LM, Meyer JS. Prenatal cocaine alters dopamine transporter binding in postnatal day 10 rat striatum. Synapse. 1996;23:335–343. doi: 10.1002/(SICI)1098-2396(199608)23:4<335::AID-SYN12>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 23.Choi SJ, Mazzio E, Kolta MG, Soliman KF. Prenatal cocaine exposure affects postnatal dopaminergic systems in various regions of the rat brain. Ann N Y Acad Sci. 1998;844:293–302. [PubMed] [Google Scholar]
- 24.Dow-Edwards DL, Freed LA, Fico TA. Structural and functional effects of prenatal cocaine exposure in adult rat brain. Brain Res Dev Brain Res. 1990;57:263–268. doi: 10.1016/0165-3806(90)90052-z. [DOI] [PubMed] [Google Scholar]
- 25.Dow-Edwards DL. Long-term neurochemical and neurobehavioral consequences of cocaine use during pregnancy. Ann N Y Acad Sci. 1989;562:280–289. doi: 10.1111/j.1749-6632.1989.tb21026.x. [DOI] [PubMed] [Google Scholar]
- 26.Wang HY, Runyan S, Yadin E, Friedman E. Prenatal exposure to cocaine selectively reduces D1 dopamine receptor-mediated activation of striatal Gs proteins. J Pharmacol Exp Ther. 1995;273:492–498. [PubMed] [Google Scholar]
- 27.Friedman E, Wang HY. Prenatal cocaine exposure alters signal transduction in the brain D1 dopamine receptor system. Ann N Y Acad Sci. 1998;846:238–247. doi: 10.1111/j.1749-6632.1998.tb09741.x. [DOI] [PubMed] [Google Scholar]
- 28.Knapska E, Kaczmarek L. A gene for neuronal plasticity in the mammalian brain: Zif268/Egr-1/NGFI-A/Krox-24/TIS8/ZENK? Prog Neurobiol. 2004;74:183–211. doi: 10.1016/j.pneurobio.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 29.Xiao B, Tu JC, Worley PF. Homer: a link between neural activity and glutamate receptor function. Curr Opin Neurobiol. 2000;10:370–374. doi: 10.1016/s0959-4388(00)00087-8. [DOI] [PubMed] [Google Scholar]
- 30.Thomas U. Modulation of synaptic signalling complexes by Homer proteins. J Neurochem. 2002;81:407–413. doi: 10.1046/j.1471-4159.2002.00869.x. [DOI] [PubMed] [Google Scholar]
- 31.Harlan RE, Garcia MM. Drugs of abuse and immediate-early genes in the forebrain. Mol Neurobiol. 1998;16:221–267. doi: 10.1007/BF02741385. [DOI] [PubMed] [Google Scholar]
- 32.Berke JD, Hyman SE. Addiction, dopamine, and the molecular mechanisms of memory. Neuron. 2000;25:515–532. doi: 10.1016/s0896-6273(00)81056-9. [DOI] [PubMed] [Google Scholar]
- 33.Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- 34.Alexander GE, Crutcher MD, DeLong MR. Basal ganglia-thalamocortical circuits: parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Prog Brain Res. 1990;85:119–146. [PubMed] [Google Scholar]
- 35.Middleton FA, Strick PL. Basal-ganglia ‘projections’ to the prefrontal cortex of the primate. Cereb Cortex. 2002;12:926–935. doi: 10.1093/cercor/12.9.926. [DOI] [PubMed] [Google Scholar]
- 36.Haber SN. The primate basal ganglia: parallel and integrative networks. J Chem Neuroanat. 2003;26:317–330. doi: 10.1016/j.jchemneu.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 37.Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- 38.DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 1990;13:281–285. doi: 10.1016/0166-2236(90)90110-v. [DOI] [PubMed] [Google Scholar]
- 39.Graybiel AM, Canales JJ, Capper-Loup C. Levodopa-induced dyskinesias and dopamine-dependent stereotypies: a new hypothesis. Trends Neurosci. 2000;23:S71–77. doi: 10.1016/s1471-1931(00)00027-6. [DOI] [PubMed] [Google Scholar]
- 40.Hyman SE, Malenka RC. Addiction and the brain: the neurobiology of compulsion and its persistence. Nat Rev Neurosci. 2001;2:695–703. doi: 10.1038/35094560. [DOI] [PubMed] [Google Scholar]
- 41.Nestler EJ. Molecular neurobiology of addiction. Am J Addict. 2001;10:201–217. doi: 10.1080/105504901750532094. [DOI] [PubMed] [Google Scholar]
- 42.Graybiel AM, Moratalla R, Robertson HA. Amphetamine and cocaine induce drug-specific activation of the c-fos gene in striosome-matrix compartments and limbic subdivisions of the striatum. Proc Natl Acad Sci U S A. 1990;87:6912–6916. doi: 10.1073/pnas.87.17.6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Steiner H, Gerfen CR. Cocaine-induced c-fos messenger RNA is inversely related to dynorphin expression in striatum. J Neurosci. 1993;13:5066–5081. doi: 10.1523/JNEUROSCI.13-12-05066.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cole AJ, Bhat RV, Patt C, Worley PF, Baraban JM. D1 dopamine receptor activation of multiple transcription factor genes in rat striatum. J Neurochem. 1992;58:1420–1426. doi: 10.1111/j.1471-4159.1992.tb11358.x. [DOI] [PubMed] [Google Scholar]
- 45.Brakeman PR, Lanahan AA, O’Brien R, Roche K, Barnes CA, Huganir RL, Worley PF. Homer: a protein that selectively binds metabotropic glutamate receptors. Nature. 1997;386:284–288. doi: 10.1038/386284a0. [DOI] [PubMed] [Google Scholar]
- 46.Yano M, Steiner H. Topography of methylphenidate (ritalin)-induced gene regulation in the striatum: differential effects on c-fos, substance P and opioid peptides. Neuropsychopharmacology. 2005;30:901–915. doi: 10.1038/sj.npp.1300613. [DOI] [PubMed] [Google Scholar]
- 47.Paul ML, Graybiel AM, David JC, Robertson HA. D1-like and D2-like dopamine receptors synergistically activate rotation and c-fos expression in the dopamine-depleted striatum in a rat model of Parkinson’s disease. J Neurosci. 1992;12:3729–3742. doi: 10.1523/JNEUROSCI.12-10-03729.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gerfen CR, Keefe KA, Gauda EB. D1 and D2 dopamine receptor function in the striatum: coactivation of D1- and D2-dopamine receptors on separate populations of neurons results in potentiated immediate early gene response in D1-containing neurons. J Neurosci. 1995;15:8167–8176. doi: 10.1523/JNEUROSCI.15-12-08167.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Steiner H, Gerfen CR. Dynorphin opioid inhibition of cocaine-induced, D1 dopamine receptor-mediated immediate-early gene expression in the striatum. J Comp Neurol. 1995;353:200–212. doi: 10.1002/cne.903530204. [DOI] [PubMed] [Google Scholar]
- 50.Yano M, Beverley JA, Steiner H. Inhibition of methylphenidate-induced gene expression in the striatum by local blockade of D1 dopamine receptors: interhemispheric effects. Neuroscience. 2006;140:699–709. doi: 10.1016/j.neuroscience.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 51.Drago J, Gerfen CR, Westphal H, Steiner H. D1 dopamine receptor-deficient mouse: cocaine-induced regulation of immediate-early gene and substance P expression in the striatum. Neuroscience. 1996;74:813–823. doi: 10.1016/0306-4522(96)00145-5. [DOI] [PubMed] [Google Scholar]
- 52.Moratalla R, Xu M, Tonegawa S, Graybiel AM. Cellular responses to psychomotor stimulant and neuroleptic drugs are abnormal in mice lacking the D1 dopamine receptor. Proc Natl Acad Sci U S A. 1996;93:14928–14933. doi: 10.1073/pnas.93.25.14928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang L, Lou D, Jiao H, Zhang D, Wang X, Xia Y, et al. Cocaine-induced intracellular signaling and gene expression are oppositely regulated by the dopamine D1 and D3 receptors. J Neurosci. 2004;24:3344–3354. doi: 10.1523/JNEUROSCI.0060-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang D, Zhang L, Tang Y, Zhang Q, Lou D, Sharp FR, et al. Repeated cocaine administration induces gene expression changes through the dopamine D1 receptors. Neuropsychopharmacology. 2005;30:1443–1454. doi: 10.1038/sj.npp.1300680. [DOI] [PubMed] [Google Scholar]
- 55.Guerriero RM, Rajadhyaksha A, Crozatier C, Giros B, Nosten-Bertrand M, Kosofsky BE. Augmented constitutive CREB expression in the nucleus accumbens and striatum may contribute to the altered behavioral response to cocaine of adult mice exposed to cocaine in utero. Dev Neurosci. 2005;27:235–248. doi: 10.1159/000085997. [DOI] [PubMed] [Google Scholar]
- 56.Holson RR, Pearce B. Principles and pitfalls in the analysis of prenatal treatment effects in multiparous species. Neurotoxicol Teratol. 1992;14:221–228. doi: 10.1016/0892-0362(92)90020-b. [DOI] [PubMed] [Google Scholar]
- 57.Brown BL, Albano JD, Ekins RP, Sgherzi AM. A simple and sensitive saturation assay method for the measurement of adenosine 3′:5′-cyclic monophosphate. Biochem J. 1971;121:561–562. doi: 10.1042/bj1210561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 59.Steiner H, Kitai ST. Regulation of rat cortex function by D1 dopamine receptors in the striatum. J Neurosci. 2000;20:5449–5460. doi: 10.1523/JNEUROSCI.20-14-05449.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bottai D, Guzowski JF, Schwarz MK, Kang SH, Xiao B, Lanahan A, et al. Synaptic activity-induced conversion of intronic to exonic sequence in Homer 1 immediate early gene expression. J Neurosci. 2002;22:167–175. doi: 10.1523/JNEUROSCI.22-01-00167.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Steiner H, Kitai ST. Unilateral striatal dopamine depletion: time-dependent effects on cortical function and behavioural correlates. Eur J Neurosci. 2001;14:1390–1404. doi: 10.1046/j.0953-816x.2001.01756.x. [DOI] [PubMed] [Google Scholar]
- 62.Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. New York: Academic Press; 1998. [Google Scholar]
- 63.Paxinos GWC. The Rat Brain in Stereotaxic coordinates. New York: Academic Press; 1998. [Google Scholar]
- 64.Unterwald EM, Ivkovic S, Cuntapay M, Stroppolo A, Guinea B, Ehrlich ME. Prenatal exposure to cocaine decreases adenylyl cyclase activity in embryonic mouse striatum. Brain Res Dev Brain Res. 2003;147:67–75. doi: 10.1016/s0165-3806(03)00058-0. [DOI] [PubMed] [Google Scholar]
- 65.Jones LB, Stanwood GD, Reinoso BS, Washington RA, Wang HY, Friedman E, Levitt P. In utero cocaine-induced dysfunction of dopamine D1 receptor signaling and abnormal differentiation of cerebral cortical neurons. J Neurosci. 2000;20:4606–4614. doi: 10.1523/JNEUROSCI.20-12-04606.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stanwood GD, Levitt P. Prenatal exposure to cocaine produces unique developmental and long-term adaptive changes in dopamine D1 receptor activity and subcellular distribution. J Neurosci. 2007;27:152–157. doi: 10.1523/JNEUROSCI.4591-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhen X, Torres C, Wang HY, Friedman E. Prenatal exposure to cocaine disrupts D1A dopamine receptor function via selective inhibition of protein phosphatase 1 pathway in rabbit frontal cortex. J Neurosci. 2001;21:9160–9167. doi: 10.1523/JNEUROSCI.21-23-09160.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Groenewegen HJ, Berendse HW, Wolters JG, Lohman AH. The anatomical relationship of the prefrontal cortex with the striatopallidal system, the thalamus and the amygdala: evidence for a parallel organization. Prog Brain Res. 1990;85:95–116. doi: 10.1016/s0079-6123(08)62677-1. discussion 116–118. [DOI] [PubMed] [Google Scholar]
- 69.Willuhn I, Sun W, Steiner H. Topography of cocaine-induced gene regulation in the rat striatum: relationship to cortical inputs and role of behavioural context. Eur J Neurosci. 2003;17:1053–1066. doi: 10.1046/j.1460-9568.2003.02525.x. [DOI] [PubMed] [Google Scholar]
- 70.Yano M, Steiner H. Methylphenidate (Ritalin) induces Homer 1a and zif 268 expression in specific corticostriatal circuits. Neuroscience. 2005;132:855–865. doi: 10.1016/j.neuroscience.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 71.Andersen SL, LeBlanc CJ, Lyss PJ. Maturational increases in c-fos expression in the ascending dopamine systems. Synapse. 2001;41:345–350. doi: 10.1002/syn.1091. [DOI] [PubMed] [Google Scholar]
- 72.Ehrlich ME, Sommer J, Canas E, Unterwald EM. Periadolescent mice show enhanced DeltaFosB upregulation in response to cocaine and amphetamine. J Neurosci. 2002;22:9155–9159. doi: 10.1523/JNEUROSCI.22-21-09155.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nisenbaum LK, Webster SM, Chang SL, McQueeney KD, LoTurco JJ. Early patterning of prelimbic cortical axons to the striatal patch compartment in the neonatal mouse. Dev Neurosci. 1998;20:113–124. doi: 10.1159/000017307. [DOI] [PubMed] [Google Scholar]
- 74.Bhat RV, Cole AJ, Baraban JM. Role of monoamine systems in activation of zif268 by cocaine. J Psychiatry Neurosci. 1992;17:94–102. [PMC free article] [PubMed] [Google Scholar]
- 75.Ehrengruber MU, Kato A, Inokuchi K, Hennou S. Homer/Vesl proteins and their roles in CNS neurons. Mol Neurobiol. 2004;29:213–227. doi: 10.1385/MN:29:3:213. [DOI] [PubMed] [Google Scholar]
- 76.Berke JD, Paletzki RF, Aronson GJ, Hyman SE, Gerfen CR. A complex program of striatal gene expression induced by dopaminergic stimulation. J Neurosci. 1998;18:5301–5310. doi: 10.1523/JNEUROSCI.18-14-05301.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sala C, Futai K, Yamamoto K, Worley PF, Hayashi Y, Sheng M. Inhibition of dendritic spine morphogenesis and synaptic transmission by activity-inducible protein Homer1a. J Neurosci. 2003;23:6327–6337. doi: 10.1523/JNEUROSCI.23-15-06327.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Turrigiano GG, Nelson SB. Hebb and homeostasis in neuronal plasticity. Curr Opin Neurobiol. 2000;10:358–364. doi: 10.1016/s0959-4388(00)00091-x. [DOI] [PubMed] [Google Scholar]
- 79.Kunko PM, Wallace MJ, Robinson SE. Gestational cocaine and ethanol exposure alter spontaneous and cocaine-induced behavior in weanling rats. Pharmacol Biochem Behav. 1996;55:559–564. doi: 10.1016/s0091-3057(96)00283-3. [DOI] [PubMed] [Google Scholar]
- 80.Heyser CJ, Rajachandran L, Spear NE, Spear LP. Responsiveness to cocaine challenge in adult rats following prenatal exposure to cocaine. Psychopharmacology (Berl) 1994;116:45–55. doi: 10.1007/BF02244870. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.