Abstract
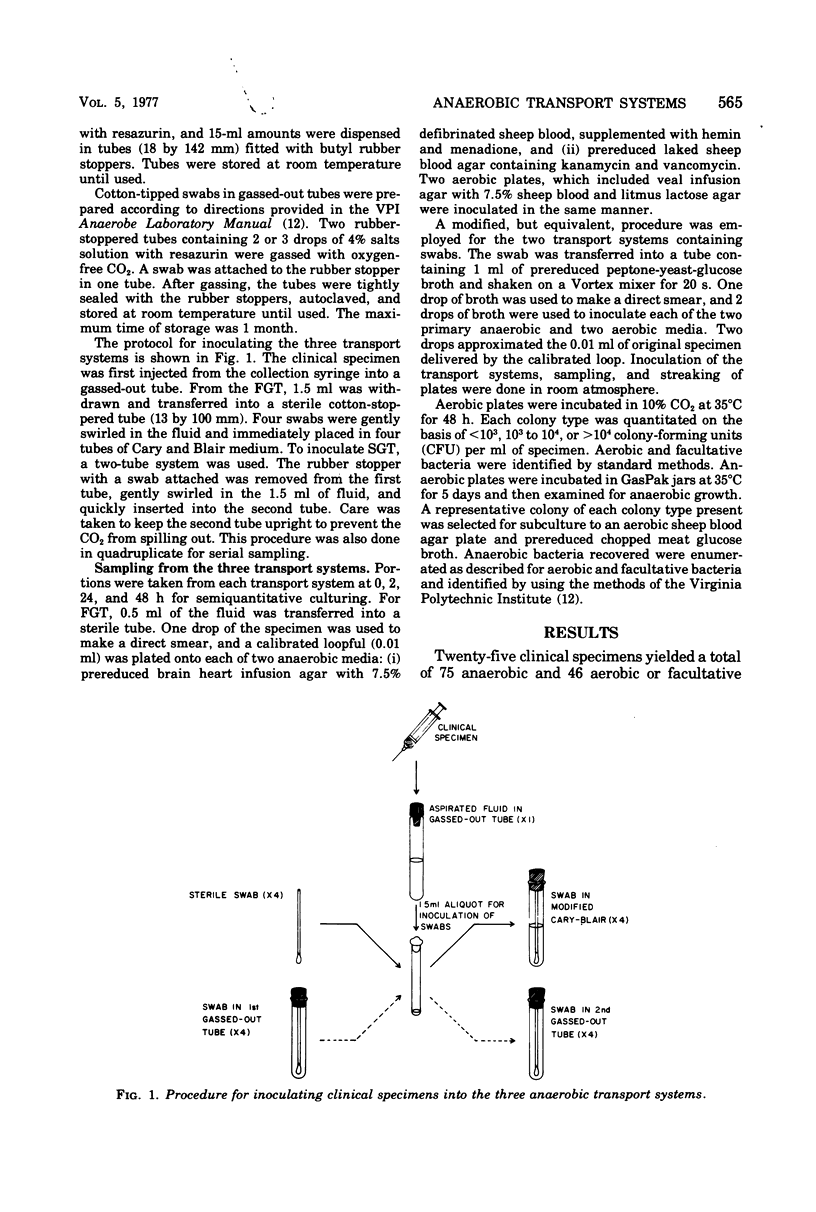
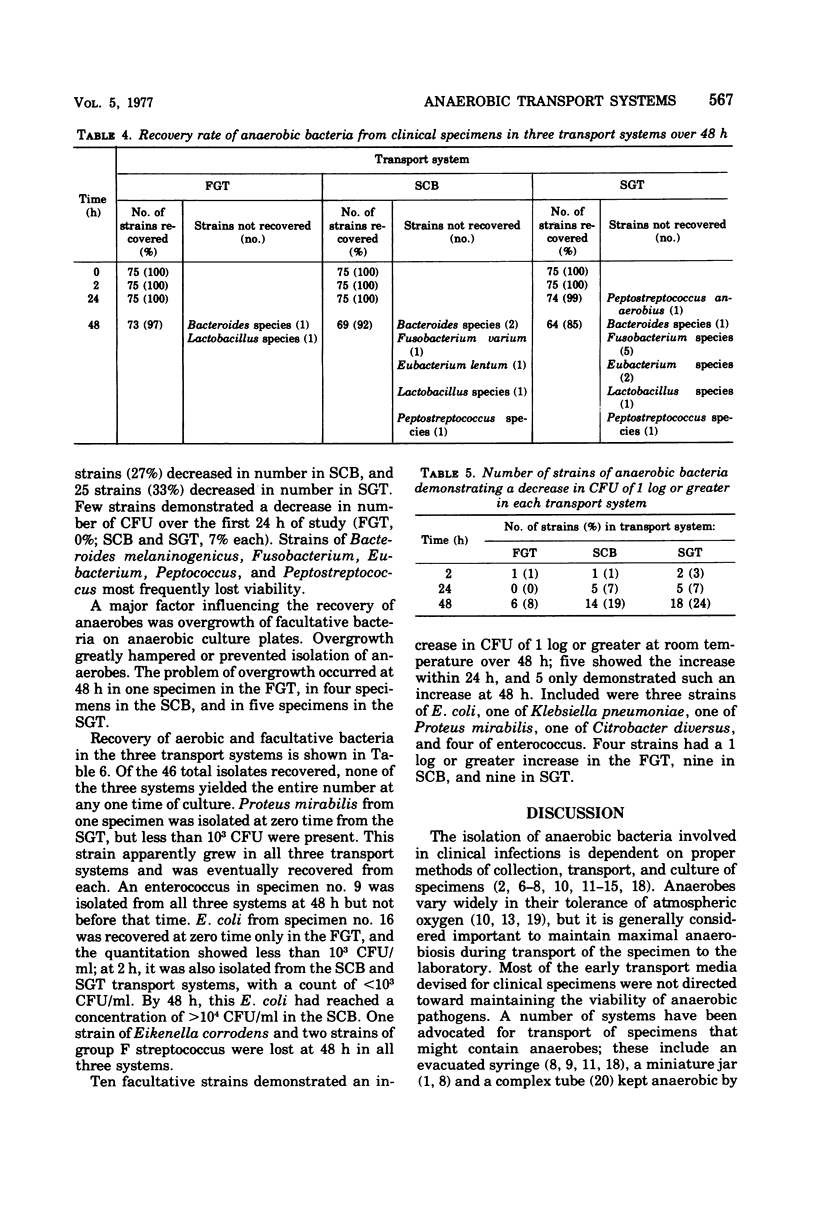
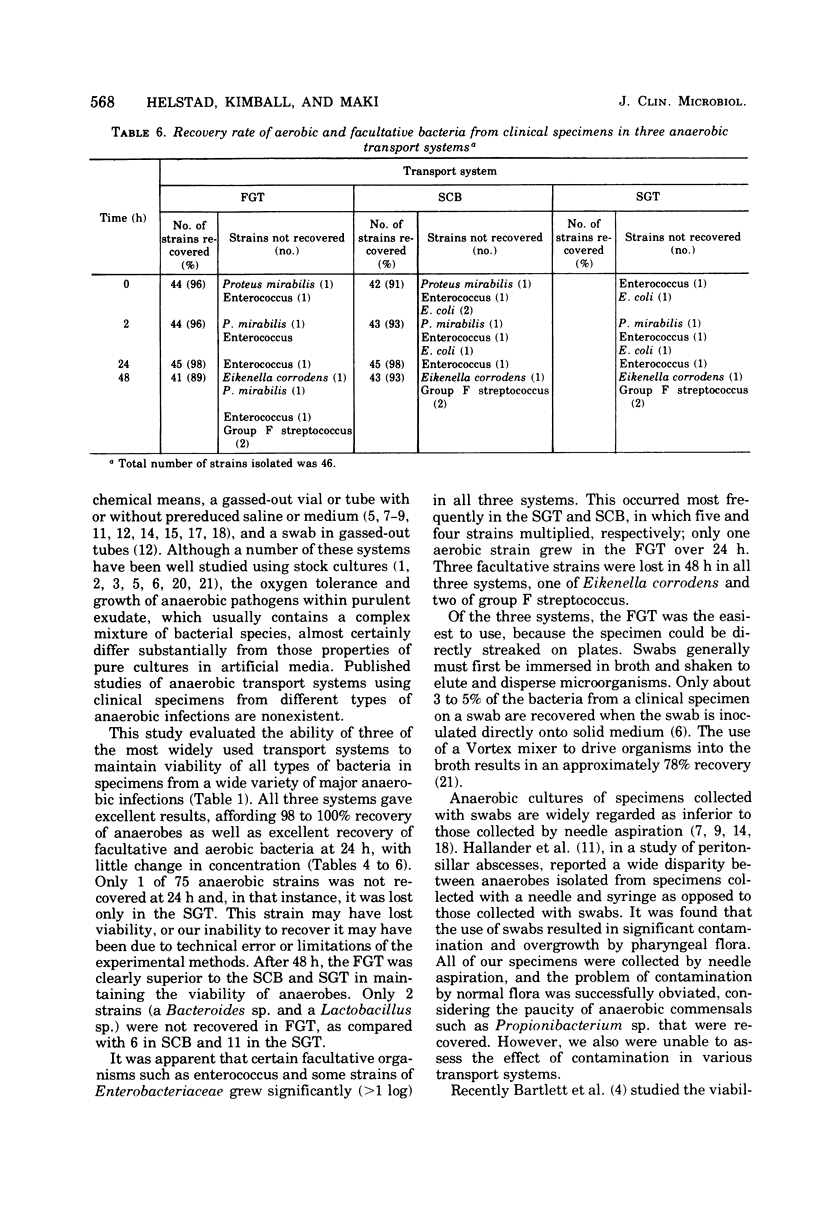
With aspirated specimens from clinical infections, we evaluated the recovery of anaerobic, aerobic, and facultative bacteria in three widely used transport systems: (i) aspirated fluid in a gassed-out tube (FGT), (ii) swab in modified Cary and Blair transport medium (SCB), and (iii) swab in a gassed-out tube (SGT). Transport tubes were held at 25 degrees C and semiquantitatively sampled at 0, 2, 24, and 48 h. Twenty-five clinical specimens yielded 75 anaerobic strains and 43 isolates of facultative and 3 of aerobic bacteria. Only one anaerobic isolate was not recovered in the first 24 h, and then, only in the SGT. At 48 h, 73 anaerobic strains (97%) were recovered in the FGT, 69 (92%) in the SCB, and 64 (85%) in the SGT. Two problems hindered the recovery of anaerobes in the SCB and SGT systems: first die-off of organisms, as evidenced by a decrease in colony-forming units of 20 strains (27%) in the SCB and 25 strains (33%) in the SGT, as compared with 7 strains (9%) in the FGT, over 48 h; and second, overgrowth of facultative bacteria, more frequent with SCB and SGT. The FGT method was clearly superior at 48 h to the SCB and SGT systems in this study and is recommended as the preferred method for transporting specimens for anaerobic culture.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attebery H. R., Finegold S. M. A miniature anaerobic jar for tissue transport or for cultivation of anaerobes. Am J Clin Pathol. 1970 Mar;53(3):383–388. doi: 10.1093/ajcp/53.3.383. [DOI] [PubMed] [Google Scholar]
- Attebery H. R., Finegold S. M. Combined screw-cap and rubber-stopper closure for Hungate tubes (pre-reduced anaerobically sterilized roll tubes and liquid media). Appl Microbiol. 1969 Oct;18(4):558–561. doi: 10.1128/am.18.4.558-561.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry A. L., Fay G. D., Sauer R. L. Efficiency of a transport medium for the recovery of aerobic and anaerobic bacteria from applicator swabs. Appl Microbiol. 1972 Jul;24(1):31–33. doi: 10.1128/am.24.1.31-33.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett J. G., Sullivan-Sigler N., Louie T. J., Gorbach S. L. Anaerobes survive in clinical specimens despite delayed processing. J Clin Microbiol. 1976 Feb;3(2):133–136. doi: 10.1128/jcm.3.2.133-136.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow A. W., Cunningham P. J., Guze L. B. Survival of anaerobic and aerobic bacteria in a nonsupportive gassed transport system. J Clin Microbiol. 1976 Feb;3(2):128–132. doi: 10.1128/jcm.3.2.128-132.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collee J. G., Watt B., Brown R., Johnstone S. The recovery of anaerobic bacteria from swabs. J Hyg (Lond) 1974 Jun;72(3):339–347. doi: 10.1017/s0022172400023561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finegold S. M., Rosenblatt J. E. Practical aspects of anaerobic sepsis. Medicine (Baltimore) 1973 Jul;52(4):311–322. doi: 10.1097/00005792-197307000-00010. [DOI] [PubMed] [Google Scholar]
- Gorbach S. L., Bartlett J. G. Anaerobic infections (third of three parts). N Engl J Med. 1974 Jun 6;290(23):1289–1294. doi: 10.1056/NEJM197406062902305. [DOI] [PubMed] [Google Scholar]
- Hallander H. O., Flodström A., Holmberg K. Influence of the collection and transport of specimens on the recovery of bacteria from peritonsillar abscesses. J Clin Microbiol. 1975 Dec;2(6):504–509. doi: 10.1128/jcm.2.6.504-509.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loesche W. J. Oxygen sensitivity of various anaerobic bacteria. Appl Microbiol. 1969 Nov;18(5):723–727. doi: 10.1128/am.18.5.723-727.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin W. J. Isolation and indentification of anaerobic bacteria in the clinical laboratory. A 2-year experience. Mayo Clin Proc. 1974 May;49(5):300–308. [PubMed] [Google Scholar]
- McMinn M. T., Crawford J. J. Recovery of anaerobic microorganisms from clinical specimens in prereduced media versus recovery by routine clinical laboratory methods. Appl Microbiol. 1970 Feb;19(2):207–213. doi: 10.1128/am.19.2.207-213.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore W. E., Cato E. P., Holdeman L. V. Anaerobic bacteria of the gastrointestinal flora and their occurrence in clinical infections. J Infect Dis. 1969 Jun;119(6):641–649. doi: 10.1093/infdis/119.6.641. [DOI] [PubMed] [Google Scholar]
- Rosenblatt J. E., Fallon A., Finegold S. M. Comparison of methods for isolation of anaerobic bacteria from clinical specimens. Appl Microbiol. 1973 Jan;25(1):77–85. doi: 10.1128/am.25.1.77-85.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tally F. P., Stewart P. R., Sutter V. L., Rosenblatt J. E. Oxygen tolerance of fresh clinical anaerobic bacteria. J Clin Microbiol. 1975 Feb;1(2):161–164. doi: 10.1128/jcm.1.2.161-164.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins T. D., Jimenez-Ulate F. Anaerobic specimen transport device. J Clin Microbiol. 1975 Nov;2(5):441–447. doi: 10.1128/jcm.2.5.441-447.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yrios J. W., Balish E., Helstad A., Field C., Inhorn S. Survival of anaerobic and aerobic bacteria on cotton swabs in three transport systems. J Clin Microbiol. 1975 Feb;1(2):196–200. doi: 10.1128/jcm.1.2.196-200.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]


