Abstract
The inhibitory effects of somatostatin have been well documented for many physiological processes. The action of somatostatin is through G-protein-coupled receptor-mediated second-messenger signaling, which in turn affects other downstream targets including ion channels. In the retina, somatostatin is released from a specific class of amacrine cells. Here we report that there was a circadian phase-dependent effect of somatostatin-14 (SS14) on the L-type voltage-gated calcium channels (L-VGCCs) in cultured chicken cone photoreceptors, and our study reveals that this process is dependent on intracellular calcium stores. Application of 500 nM SS14 for 2 h caused a decrease in L-VGCC currents only during the subjective night but not the subjective day. We then explored the cellular mechanisms underlying the circadian phase-dependent effect of SS14. The inhibitory effect of SS14 on L-VGCCs was mediated through the pertussis-toxin-sensitive G-protein-dependent somatostatin receptor 2 (sst2). Activation of sst2 by SS14 further activated downstream signaling involving phospholipase C and intracellular calcium stores. Mobilization of intracellular Ca2+ was required for somatostatin induced inhibition of photoreceptor L-VGCCs, suggesting that somatostatin plays an important role in the modulation of photoreceptor physiology.
INTRODUCTION
Somatostatin (SS), also known as somatotropin release-inhibiting factor, is a peptide hormone originally found in the hypothalamus that inhibits the release of growth hormone from the pituitary gland (Brazeau et al. 1973). SS is well known for its broad inhibitory effects in many physiological processes such as in secretion and cell proliferation and is present in the endocrine, gastroinstestinal, immune, and nervous systems (Weckbecker et al. 2003). In the CNS, SS serves as an inhibitory neuromodulator, which has made it a potential therapeutic target to control hyperexcitability in epilepsy and other neuronal diseases (Weckbecker et al. 2003). In the retina, SS has neuroprotective properties against ischemia and diabetic retinopathy (Cervia et al. 2008a; Thermos 2003). Nevertheless, the function of SS in the retina largely remains unknown, and the molecular mechanisms underlying the effects of somatostatin in the retina are unidentified.
SS is synthesized and released from a subpopulation of amacrine cells known as enkephalin-, neurotensin-, and SS-like immunoreactive (ENSLI) cells in the chicken retina (Yang et al. 1997). There are two biologically active forms of SS, somatostatin-14 (SS14) and the N-terminal extended somatostatin-28 (SS28), and both forms are present in the retina (Ishimoto et al. 1986; Yang et al. 1997). While there are five classes of SS receptors (sst1-5; Hoyer et al. 1995), only four of these, sst2-5, are present in the chicken retina with sst2 as the most abundant (Chen et al. 2007). Both SS14 and SS28 bind to all SS receptor subtypes with varying affinities (Dryer et al. 1991; Rohrer et al. 1998). Even though SS has broad inhibitory effects in the nervous system, its actions in the retina appear to be more complex. SS enhances light-evoked activity of retinal ganglion cells (Adolph 1989; Zalutsky and Miller 1990), modulates ion channels in photoreceptors (Akopian et al. 2000; Chen et al. 2007), and affects neurotransmission in the retina that lead to changes in electroretinogram amplitudes (Cervia et al. 2008b; Dal Monte et al. 2003; Kouvidi et al. 2006; Petrucci et al. 2001; Zalutsky and Miller 1990).
Photoreceptors are nonspiking neurons, and the continuous release of glutamate in the dark is a result of depolarization-evoked activation of L-type voltage-gated calcium channels (L-VGCCs) (Barnes and Kelly 2002). There is a circadian regulation of L-VGCCs in bipolar cells (Hull et al. 2006) and cone photoreceptors (Ko et al. 2007, 2009), in which the L-VGCC current amplitudes and densities are greater at night. Somatostatin decreases L-VGCC currents in various neuronal tissues, including the pituitary gland (Chen et al. 1990), ciliary ganglion (Dryer et al. 1991), and cerebral cortex (Wang et al. 1990). In rod bipolar cell terminals, SS inhibits calcium influx through L-VGCCs (Johnson et al. 2001). Interestingly, the content of SS is under circadian control in the rodent retina (Peinado et al. 1990), and this rhythm correlates with the activities of ENSLI cells with a high sustained rate of activity in the dark and a low sustained rate of activity in the light (Morgan et al. 1994). Hence we postulated that there could be a circadian phase-dependent effect of SS in the modulation of L-VGCCs in retinal photoreceptors. In the present study, we examined the circadian phase-dependent effect of SS on L-VGCCs in cultured chicken cone photoreceptors. Because SS14 and SS28 have different effects in the retina (Chen et al. 2007), we focused on the actions of SS14 in this study. We found that SS14 decreased the L-VGCC current density only during the subjective night, and the effect of SS14 on L-VGCCs was mediated through the pertussis toxin (PTX)-sensitive G-protein-coupled receptor sst2. Specifically, the action of SS on L-VGCCs required the activation of phospholipase C (PLC) and the mobilization of intracellular Ca2+.
METHODS
Cell cultures and circadian entrainment
Fertilized eggs (Gallus gallus) were obtained from the Poultry Science Department, Texas A&M University (College Station, TX). Chicken retinas were dissociated at embryonic day 12 (E12) and cultured for 6–7 days as described previously (Ko et al. 2007). Cultures were prepared in the presence of 40 ng/ml ciliary neurotrophic factor (R&D Systems, Minneapolis, MN) and 10% heat-inactivated horse serum. Cell culture incubators (maintained at 39°C and 5% CO2) were equipped with lights and timers, which allowed for the entrainment of retinal circadian oscillators to 12 h:12 h light-dark (LD) cycles in vitro. Zeitgeber time (ZT 0) zero was designated as the time when the lights turned on, and ZT 12 was the time when the lights went off. The following experiments were performed on the second day of constant darkness (DD), after 6 days of prior entrainment to LD cycles.
Electrophysiology
Electrophysiological recordings were performed at circadian time (CT) 4–7 or CT 16–19 on the second day of DD. Whole cell patch-clamp configuration of L-VGCC current recordings were carried out in an external solution containing the following (in mM): 110 NaCl, 10 BaCl2, 0.4 MgCl2, 5.3 KCl, 20 TEACl, 10 HEPES, and 5.6 glucose, pH 7.4 with NaOH. The pipette solution was (in mM) 135 Cs acetate, 10 CsCl, 2 MgCl2, 0.1 CaCl2, 1.1 EGTA, and 10 HEPES, pH 7.4 adjusted with CsOH. Both perforated and mechanically captured patches were used in this study. For perforated patches, beta-escin was prepared as a 25 mmol/l stock solution in water and added to the pipette solution to yield a final concentration of 25 μmol/l. Recordings were made only from cells with elongated cell bodies with one or more prominent oil droplets because these are the hallmarks of avian cone photoreceptors (Adler et al. 1984; Gleason et al. 1992; GoldSmith et al. 1984; Hart et al. 2006; Johnston and Hudson 1976; Lopez et al. 2005). Currents were recorded at room temperature (23°C) using an Axopatch 200B amplifier (Axon Instruments/Molecular Devices, Union City, CA). Signals were low-pass filtered at 2 kHz and digitized at 5 kHz with Digidata 1440A interface and pCLAMP 10.0 software (Axon Instruments). Currents were leak subtracted. After Gigaohm seals formed, the electrode capacitance was compensated. Current–voltage (I–V) relations were elicited from a holding potential of –65 mV in 200-ms steps (5 s between steps) to test potentials over a range of –20 to +60 mV in 10 mV increments. The maximum currents were obtained when the steps depolarized to 0 ∼10 mV. The membrane capacitance, series resistance, and input resistance of the recorded photoreceptors were measured by applying a 5 mV (100 ms) depolarizing voltage step from a holding potential of –65 mV. Cells with an input resistance <1 GΩ were discarded. The membrane capacitance reading was used as the value for whole cell capacitance. The current densities (pA/pF) were obtained by dividing the current amplitudes by the membrane capacitances. To achieve better controls, we always compared groups by alternating recordings among the same set of cultures during the same period.
Immunoblot analysis
Chicken embryos from E12 were entrained in LD cycles for 7 days in ovo. On the last day, retinas were dissected, dissociated, and cultured in DD for 2 day. On the second day of DD, cultured cells were treated with either vehicle (control) or SS14 (500 nM) for 2 h at CT 3 and 15. At CT 5 and CT 17, cells were harvested, washed in ice-cold PBS, and lysed in RIPA buffer, and samples were collected and prepared as described previously (Ko et al. 2007, 2009). Samples were separated on 10% SDS-PAGE gels and transferred to nitrocellulose membranes. The primary antibodies used in this study were anti-VGCCα1D (Alomone, Jerusalem, Israel) and a polyclonal antibody insensitive to the phosphorylation state of Erk (total Erk, used for internal control and loading control, Santa Cruz Biochemicals, Santa Cruz, CA). Blots were visualized using appropriate secondary antibodies conjugated to horseradish peroxidase (Cell Signaling Technology, Danvers, MA) and an ECL detection system (Pierce, Rockford, IL). All measurements were repeated five to six times.
Calcium imaging
Following culture of dissociated E12 retinal cells on coverglass chambered slides for 24 h under 12:12 h LD cycles, cells from the dark phase were loaded for 1 h with 3.0 μM Fluo-4 AM at 37°C in serum and phenol red-free medium and then washed with the same medium prior to measurement of cytosolic Ca2+. A Stallion Imaging workstation, equipped with a Zeiss Axiovert 200M microscope (Carl Zeiss Microimaging, Thornwood, NY) and slidebook software (Intelligent Imaging Innovation, Denver, CO), was used with a ×63 water objective 1.2 NA for acquiring images. Cellular Ca2+ levels were visualized with the cell-permanent probe Fluo-4 AM (Gee et al. 2000). Fluo-4 AM fluorescence was generated in the cells by argon laser excitation at 488 nm and fluorescence emission was monitored at 530 nm. Selected areas were scanned 5 times at 10 s intervals to establish basal calcium levels, and scanning continued for ∼650 s. Thapsigargin was then added and scanning continued for another 600 s. At least two to five cells per field were analyzed. Two experiments were performed on different days.
Chemicals and statistical analysis
SS14 was obtained from American Peptide (Sunnyvale, CA) and Sigma-Aldrich (St. Louis, MO). MK-678 was from Tocris (Ellisville, MO). Cyanamid-154806 was from Sigma-Aldrich. PTX and BAPTA-AM were from Calbiochem (Gibbstown, NJ). ET-18-OCH3 was from A.G. Scientific (San Diego, CA). Thapsigargin and Fluo-4 AM were from Invitrogen (Carlsbad, CA).
All of the data were presented as means ± SE. Student's t-test or one-way ANOVA followed by Tukey's post hoc test for unbalanced n was used for statistical analysis. Throughout, *P < 0.05 was regarded as significant.
RESULTS
SS14 inhibits L-type VGCCs during the subjective night through sst2 receptors
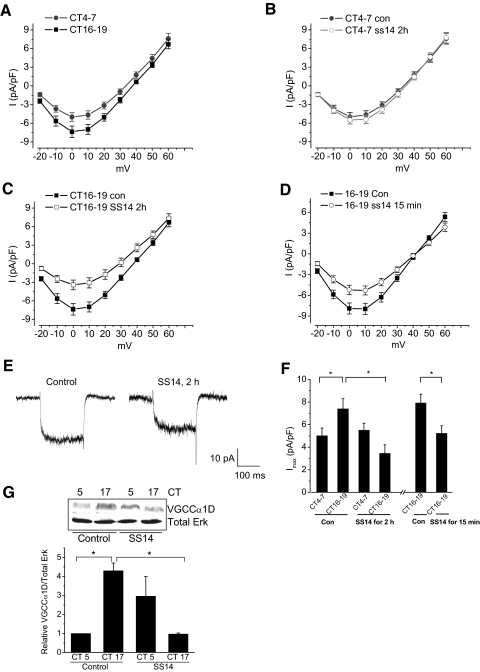
Chicken retinal cells were cultured from E12 and entrained to 12:12 h light-dark (LD) cycles for 5–6 days in vitro and then kept in constant darkness (DD) for 2 days. On the second day of DD, whole cell patch recordings were performed from cone photoreceptors during the subjective day at CT 4–7 or the subjective night (CT 16–19). Over 95% of cone photoreceptors express L-type VGCCs at this embryonic stage (equivalent of E18-E19) (Gleason et al. 1992). As described previously, there is a circadian regulation of L-VGCCs in chicken cone photoreceptors (Ko et al. 2007, 2009), and the maximal L-VGCC current density elicited at a voltage between 0 and 10 mV is significantly larger (*P < 0.05) when cells are recorded during the subjective night than during the subjective day (Fig. 1, A–F) (Ko et al. 2007, 2009). The circadian rhythm of L-VGCC current density is in part attributed to the circadian regulation of mRNA levels and protein expression of the L-VGCCα1D subunit, which peak during the subjective night (at CT 16–17), as well as channel protein trafficking and membrane retention (Ko et al. 2007, 2009; Shi et al. 2009).
FIG. 1.
There is a circadian phase-dependent modulation of cone L-type voltage-gated calcium channels (L-VGCCs) by somatostain-14 (SS14). The current density-voltage (I-V) relationship was obtained from whole cell recordings on the 2nd day of constant darkness (DD). A: the L-VGCC currents were larger during the subjective night [circadian time (CT) 16–19; solid square] than during the subjective day (CT 4–7; solid circle). B: treatment with SS14 (500 nM) for 2 h prior to recordings (○) did not have significant effects on the current density during the day. C: treatment with SS14 for 2 h (□) significantly decreased the L-VGCC current density during the night compared with the control (▪). D: treatment with SS14 for 15 min prior to recordings (○) significantly inhibited the L-VGCCs during the subjective night. E: 2 representative traces recorded during the subjective night from the control (con) and SS14-treated (SS14 2h) groups show currents elicited from −65 to 0 mV. The maximum currents were obtained when the steps depolarized to 0 ∼10 mV. F: treatment with SS14 for 2 h or 15 min prior to recordings significantly decreased the maximum L-VGCC current density (Imax) elicited from −65 to 0 mV during the subjective night. The maximum current density of voltage step to 0 mV: control CT 4–7 (con CT 4–7): 5.0 ± 0.7 pA/pF, n = 24; control CT 16–19 (con CT 16–19): 7.4 ± 0.9 pA/pF, n = 21; treatment with SS14 for 2 h (SS14 for 2 h) CT 4–7: 5.5 ± 0.6 pA/pF, n = 26; SS14 for 2 h CT 16–19:3.4 ± 0.8 pA/pF, n = 26; treatment for 15 min control (con) CT 16–19: 7.9 ± 0.8 pA/pF, n = 14; treatment with SS14 for 15 min (SS14 for 15 min) CT 16–19: 5.2 ± 0.7 pA/pF, n = 15, * P < 0.05. G: the protein levels of L-VGCCα1D in cultures harvested during the subjective night (CT 17) were significantly higher than during the subjective day (CT 5) in controls. Treatment with SS14 (500 nM) for 2 h significantly decreased the L-VGCCα1D during the subjective night, *, P < 0.05.
We observed that SS14 evoked a phase-dependent modulation of L-VGCCs in chicken cone photoreceptors. Application of SS14 (500 nM) for 2 h or 15 min prior to patch recordings caused a significant decrease in L-VGCC current density during the subjective night (CT 16–19; Fig. 1, C–F) but not during the subjective day (CT 4–7; Fig. 1, B and F). The 2-h treatment also decreased retinal L-VGCCα1D subunits during the subjective night (Fig. 1G). Even though our Western blot data showed a slight increase in L-VGCCα1D expression during the day after a 2 h treatment with SS14, we did not observe a significant increase in L-VGCC current density following this treatment during the day. Treatment with SS14 for 2 h did not shift the voltage-current relationship because the maximal currents were still elicited at 0–10 mV (Fig. 1, C and D). Hence our data suggested that there was a circadian phase-dependent effect of SS14 in which SS14 decreased L-VGCC activity only during the subjective night through a significant reduction of L-VGCCα1D subunits. Because chicken retinas express SS receptors 2–5 (sst 2–5) by embryonic day 11 with the sst2 receptor as the most abundant (Chen et al. 2007), we next examined whether the inhibitory effect of SS14 on L-VGCCs was mediated through sst2.
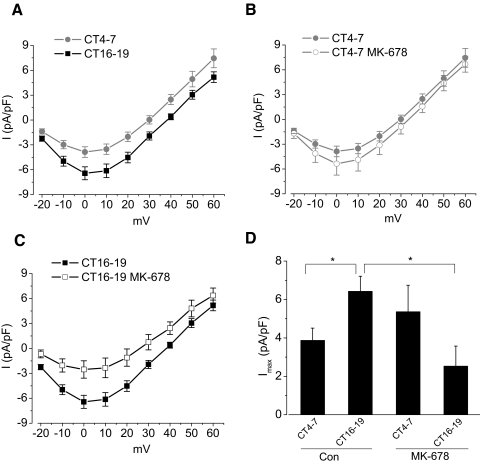
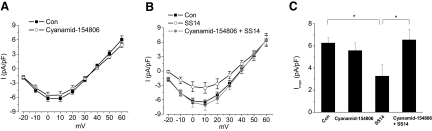
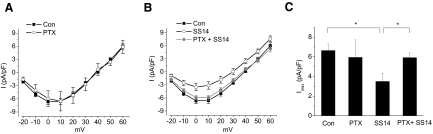
A 2-h treatment with MK-678 (100 nM), an sst2 preferred agonist, evoked a phase-dependent modulation of L-VGCCs (Fig. 2) and mimicked the effect of SS14 (C and D). MK-678 also caused a slight increase in L-VGCC current density during the subjective day (Fig. 2, B and D). However, in the presence of the sst2 antagonist cyanamid-154806 (1 mM), the inhibitory effect of SS14 on L-VGCCs during the subjective night was blocked (Fig. 3, B and C), while cyanamid-154806 itself did not affect L-VGCC current density (Fig. 3, A and C). Thus our data indicated that the inhibitory effect of SS14 on L-VGCCs during the subjective night was mediated through sst2 receptors. While somatostatin receptors can couple with both PTX-sensitive and –insensitive G proteins (Weckbecker et al. 2003), it was not clear whether the SS14 effect we observed was mediated through a PTX-sensitive G protein. We found that treatment with PTX (200 ng/ml) for 20–24 h prior to recordings blocked the inhibitory effect of SS14 on L-VGCCs (Fig. 4, B and C), but PTX did not interfere with calcium influx through the L-VGCCs (A and C). These data indicated that the effect of SS14 on cone L-VGCCs was through the sst2 receptor that coupled with a PTX-sensitive G protein. Because receptor-coupled G proteins activate multiple downstream intracellular signaling pathways, we subsequently investigated which of these pathways mediated the inhibitory effect of SS14 on L-VGCCs in cone photoreceptors.
FIG. 2.
The somatostatin receptor 2 (sst2) agonist, MK-678, mimics the effect of SS14. A: L-VGCC current densities were larger when photoreceptors were recorded during the subjective night (CT 16–19; ▪) than during the subjective day (CT 4–7; •). B and C: application of MK-678 (100 nM) for 2 h prior to recordings had no effect during the subjective day (CT 4–7; ○; B) but caused a significant decrease in L-VGCC current density during the subjective night (CT 16–19; □; C). D: MK-678 significantly inhibited the maximum L-VGCC current density (Imax) during the subjective night but not the subjective day. Control (Con) CT 4–7: 3.9 ± 0.7 pA/pF, n = 11; control CT 16–19: 6.4 ± 0.8 pA/pF, n = 13; MK-678 CT 4–7: 5.4 ± 1.4 pA/pF, n = 13; MK-678 CT 16–19: 2.5 ± 1.0 pA/pF, n = 8. *P < 0.05.
FIG. 3.
The inhibitory effect of SS14 during the subjective night is mediated through somatostatin receptor 2 (sst2). A: application of the sst2 antagonist cyanamid-154806 (1 mM) for 2 h (○) had no effect on L-VGCCs compared with control (▪). B: treatment with SS14 for 2 h (○) significantly decreased L-VGCC current density, while cyanamid-154806 reversed the inhibitory effect of SS14 on L-VGCC current density (•). C: SS14 significantly decreased the maximum current density of L-VGCCs. Treatment with cyanamid-154806 did not have any effect on the maximum L-VGCC current density (Imax), but it significantly reversed the inhibitory effect of SS14 on L-VGCC maximum current density. Control (Con): 6.2 ± 0.6 pA/pF, n = 15; cyanamid-154806: 5.6 ± 0.7 pA/pF, n = 15; SS14: 3.3 ± 1.0 pA/pF, n = 9; cyanamid-154806 + SS14: 6.5 ± 1.0 pA/pF, n = 13.
FIG. 4.
The inhibitory effect of SS14 on L-VGCCs during the subjective night is mediated through the PTX-sensitive G protein. Cells were treated with PTX (200 ng/ml) for 20–24 h prior to recordings. A: treatment with PTX did not affect L-VGCC current density. B: treatment with SS14 for 2 h (○) decreased L-VGCC current density, but in the presence of PTX, SS14 did not affect L-VGCCs (•). C: treatment with PTX reversed the inhibitory effect of SS14 on the maximum L-VGCC current density (Imax). Control (Con): 6.6 ± 0.7 pA/pF, n = 15; PTX alone (PTX): 5.9 ± 1.8 pA/pF, n = 5; SS14 for 2 h (SS14): 3.5 ± 0.8 pA/pF, n = 22; treatment with SS14 in the presence of PTX (PTX + SS14): 6.0 ± 0.5 pA/pF, n = 15.
Intracellular calcium mobilization is required for somatostatin-14 inhibition of L-VGCCs
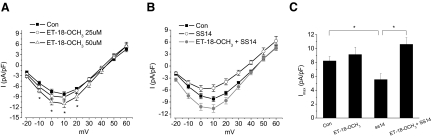
In chicken cone photoreceptors, somatostatin stimulates phospholipase C (PLC) activation (Chen et al. 2007), so we examined whether the effect of SS14 on L-VGCCs was mediated through PLC. Treatment with 1-O-octadecyl-2-O-methyl-sn-glycero-3-phosphorylcholine (ET-18-OCH3), an inhibitor of phosphatidylinositol-specific PLCs, for 2 h increased the cone L-VGCC current density in a dose-dependent manner. At 50 μM, ET-18-OCH3 significantly (*P < 0.05) increased the L-VGCC current density compared with the control during the subjective night but did not affect L-VGCC channel properties at a lower concentration (25 μM, Fig. 5 A). Interestingly, 25 μM ET-18-OCH3 was sufficient to reverse the inhibitory effect of SS14 on L-VGCC currents (Fig. 5B). We tested another PLC inhibitor, U73122, which showed similar results (data not shown). At 1 μM, U73122 did not affect L-VGCC channel properties but effectively reversed the inhibitory effect of SS14 on L-VGCC currents, whereas at 5 μM, U73122 alone significantly increased the L-VGCC current density compared with the control during the subjective night (data not shown). Therefore the inhibitory effect of SS14 on L-VGCCs in cone photoreceptors was mediated through the activation of PLC.
FIG. 5.
Inhibition of PLC reverse the inhibitory effect of SS14 on L-VGCCs during the subjective night. A: there was a dose-dependent effect of ET-18-OCH3, a phospholipase C (PLC) inhibitor, on L-VGCCs. Treatment with 25 μM ET-18-OCH3 for 2 h (○) did not have a significant effect on L-VGCC current density, but 50 μM ET-18-OCH3 (▵) increased L-VGCC current density (*P < 0.05). B: the inhibitory effect of SS14 (○) on L-VGCCs was reversed by 25 μM ET-18-OCH3 (ET-18-OCH3+SS14, •). C: treatment with 25 μM ET-18-OCH3 alone (ET-18-OCH3) had no significant effect on the maximum L-VGCC current density (Imax), but it significantly reversed the inhibitory effect of SS14 (ET-18-OCH3 + SS14). Control (Con): 7.5 ± 0.7 pA/pF, n = 12; 25 μM ET-18-OCH3 alone (ET-18-OCH3): 8.8 ± 1 pA/pF, n = 9; SS14: 5.6 ± 0.8 pA/pF, n = 16; treatment with SS14 in the presence of ET-18-OCH3 (SS14 + ET-18-OCH3): 10.2 ± 0.9 pA/pF, n = 13; *P < 0.05.
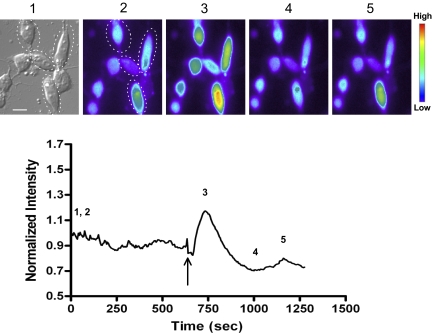
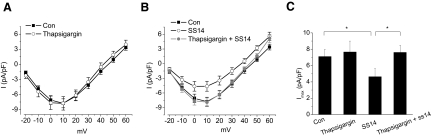
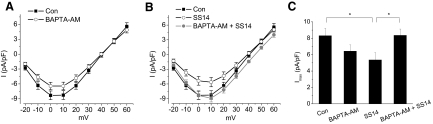
Activation of PLC hydrolyzes membrane phospholipid phosphatidylinositol 4,5-biphosphate (PIP2) into inositol 1,4,5-triphosphate (IP3) and diacylglycerol, and IP3 further binds to its receptors that leads to Ca2+ release from IP3-sensitive intracellular calcium stores (Berridge et al. 2000). Thus we further explored whether increased intracellular Ca2+ played a role in the effect of SS14 on L-VGCCs. We first verified the existence of intracellular calcium stores in chicken cone photoreceptors by calcium imaging. Dark adapted retinal cells were loaded for 1 h with 3.0 μM Fluo-4 AM at 37°C in serum and phenol red-free medium and then washed with the same medium prior to measuring cytosolic Ca2+. Treatment with thapsigargin, an inhibitor of endoplasmic reticulum Ca2+ ATPase (Fischer et al. 1998), caused a sudden surge of intracellular Ca2+ followed by complete depletion of Ca2+ from intracellular stores (Fig. 6). The calcium-imaging result indicated the presence of intracellular calcium stores in chicken cone photoreceptors. We found that SS14 inhibition of L-VGCCs during the subjective night was completely blocked by thapsigargin (5 μM; Fig. 7, B and C), but thapsigargin alone did not affect the L-VGCCs (A and C). Furthermore, a 2-h incubation with BAPTA-AM, a membrane-permeable Ca2+ chealator, showed the same blockade of the SS14 effect on L-VGCCs as thapsigarin during the subjective night (Fig. 8, B and C), while BAPTA-AM alone only slightly decreased the L-VGCC current (A and C). These results indicated that Ca2+ release from intracellular stores was required for the inhibitory effect of SS14 on cone L-VGCCs during the subjective night.
FIG. 6.
Intracellular calcium stores are present in cultured chicken cone photoreceptors. Dark-adapted cultured retinal cells were loaded with Fluo-4 AM. Treatment with thapsigargin triggered a surge of intracellular calcium followed by depletion of the intracellular calcium. Top: an example of a photoreceptor DIC image as well as 4 different fluo-4 images at different time points (2–5) following thapsigargin addition. Only elongated cone photoreceptors with oil droplets (as in circles) were analyzed. Bar = 3 μm. Bottom: an average from a group of photoreceptors. The arrow indicates the time when thapsigargin was applied. The normalized intensity represents the ratio of fluo-4 fluorescence intensity at any time point to basal fluo-4 fluorescence intensity (at time 0).
FIG. 7.
The inhibitory effect of SS14 on L-VGCCs during the subjective night requires Ca2+ from intracellular stores. A: treatment with thapsigargin (in 0.05% DMSO) for 2 h (5 μM; ○) had no effect on the L-VGCC currents. B: treatment with SS14 for 2 h in the presence of thapsigargin (thapsigargin + SS14, •) reversed the inhibitory effect of SS14 (○) on L-VGCCs. C: SS14 significantly decreased the maximum L-VGCC current density (Imax), but thapsigarin reversed the inhibitory effect of SS14. Control (Con): 7.1 ± 0.9 pA/pF, n = 7; thapsigargin alone for 2 h (thapsigargin): 7.7 ± 1.3 pA/pF, n = 10; SS14 for 2 h (SS14): 4.6 ± 1.0 pA/pF, n = 8; treatment with SS14 in the presence of thapsigargin (SS14 + thapsigargin): 7.6 ± 0.9 pA/pF, n = 11; *P < 0.05.
FIG. 8.
The inhibitory effect of SS14 on L-VGCCs during the subjective night requires intracellular Ca2+. A: the Ca2+ chealator BAPTA-AM (13 μM; ○) significantly decreased L-VGCC current density. B: Treatment with SS14 for 2 h (○) decreased L-VGCC currents, but BAPTA-AM reversed the inhibitory effect of SS14 on L-VGCCs (BAPTA-AM + SS14; •). C: the inhibitory effect of SS14 on the maximum L-VGCC current density was reversed by BAPTA-AM. Treatment with BAPTA-AM alone for 2 h significantly decreased the maximum L-VGCC current density (Imax). Treatment with 0.1% DMSO as controls (Con): 8.3 ± 0.9 pA/pF, n = 17; 13 μM BAPTA-AM dissolved in 0.1% DMSO (BAPTA-AM): 6.4 ± 0.8 pA/pF, n = 21; treatment with SS14 for 2 h (SS14): 5.4 ± 0.9 pA/pF, n = 11; treatment with SS14 in the presence of BAPTA-AM (SS14 + BAPTA-AM): 8.4 ± 0.8 pA/pF, n = 16; *P < 0.05.
DISCUSSION
In this study, we report that there was a circadian phase-dependent inhibitory effect of SS on L-VGCCs in cultured cone photoreceptors. Somatostatin caused a significant decrease in the L-VGCC current density as well as L-VGCCα1D subunit expression during the subjective night but not the subjective day. The inhibitory effect of SS was through the sst2 receptor that coupled with a PTX-sensitive G protein. Among the subtypes of SS receptors, sst2 is the most abundant in the chicken retina (Chen et al. 2007) and is also expressed in salamander, rat, and human cone photoreceptors (Akopian et al. 2000; Grigoryan et al. 2003; Helboe et al. 1997; Johnson et al. 1999; Vasilaki et al. 2001). In the retina, sst2 couples to both PTX–sensitive (Akopian et al. 2000; Pavan et al. 2004; Vasilaki et al. 2003) and -insensitive G proteins (Chen et al. 2007). The complex intracellular signaling elicited by SS not only relies on different SS receptors coupling to different G proteins, it also depends on the coupling between different SS receptors (Pavan et al. 2004). Interestingly, in the salamander retina, somatostatin elicits an increase of L-VGCC currents in cone photoreceptors but a decrease in rod photoreceptors (Akopian et al. 2000). Our data showed that SS14 slightly increased the protein expression of the L-VGCCα1D subunit at CT 5, and the sst2 agonist MK-678 also slightly increased the L-VGCC current density at CT 4–7 (Fig. 1 and 2). However, we did not observe an increase in L-VGCC current density by SS14 during the subjective day (CT 4–7). Because our recording data were the average across at least three circadian hours, it is possible that SS14 would have a more profound effect during the subjective day but at a different time period (i.e., CT 2–5 or CT 6–9). Currently, we are investigating the phase-dependent effect of SS14 during the subjective day at different time periods. In this report, we only focused on the effect of SS14 on L-VGCCs and the underlying signaling pathway in cone photoreceptors during the subjective night.
Activation of SS receptors further activates PLC (Akbar et al. 1994; Chen et al. 2007; Ho et al. 2001; Johansen et al. 2001; Murthy et al. 2004; Siffert et al. 1995), and all human SS receptor subtypes can stimulate different PLC isoforms (Akbar et al. 1994). We found that the inhibitory effect of SS on L-VGCCs was through activation of PLC. Interestingly, at a lower concentration, a PLC inhibitor was sufficient to reverse the effect of SS14 without affecting L-VGCC current density. However, at a higher concentration, the PLC inhibitor itself increased L-VGCC current density during the subjective night. There are two possible explanations. At higher concentrations, the PLC inhibitor might have some nonspecific effects that caused an increase in L-VGCC current density. However, we used two structurally different PLC inhibitors, ET-18-OCH3 and U73122, and both had similar effects on L-VGCCs. The higher concentrations used for both PLC inhibitors were within pharmacological ranges applied in other published studies on the inhibitory effects of PLC. Hence we exclude the possibility of nonspecific PLC inhibitor effects at higher concentrations on L-VGCCs. Alternatively, it is possible that there is a basal level of PLC activity in chicken cone photoreceptors at night, which might have slightly dampened the L-VGCC currents. The inhibitory effect of SS14 on L-VGCCs could be through a further activation of PLC.
Activation of PLC elicits a production of inositol trisphosphate (IP3) and diacylglycerol, and IP3 then causes Ca2+ release from IP3-sensitive intracellular calcium stores (Berridge et al. 2000; Hirata et al. 1985). There is a species- and spatial- dependent distribution of different intracellular calcium stores. Caffeine, a modulator of ryanodine receptor operant intracellular stores, readily evokes an intracellular Ca2+ rise in the inner segments of rods but not cones in tiger salamanders (Krizaj et al. 2003). While ryanodine receptor operant intracellular stores are more dominantly present in rod photoreceptors in rodents at both the outer and inner segments (Krizaj 2005; Shoshan-Barmatz et al. 2007), IP3 receptor operant intracellular stores are typically more prevalent in cone photoreceptors as well as at the synaptic terminals and inner segments of various vertebrate species (Peng et al. 1991; Wang et al. 1999). We found that thapsigargin (10 μM), an inhibitor of endoplasmic reticulum Ca2+ ATPase, evoked a robust surge of intracellular Ca2+ followed by its complete depletion, which provided evidence for the existence of intracellular calcium stores in chicken cone photoreceptors. Somatostatin has been shown to induce calcium release from intracellular calcium stores in different cell types such as pituitary cells and neuroblastoma × glioma NG108-15 cells (Akbar et al. 1994; Nunn et al. 2004; Rhie et al. 2003; Romoser et al. 2001; Siehler et al. 2005; Siffert et al. 1995). Here, we showed that the inhibitory effect of SS14 on L-VGCCs was blocked by both the Ca2+ chelator BAPTA-AM and thapsigargin. Hence, we demonstrated that through activation of PLC, SS triggered the release of calcium from intracellular stores that in turn decreased L-VGCC currents in cone photoreceptors.
Calcium-dependent inhibition of L-VGCCs (by elevated intracellular Ca2+), also known as calcium-induced calcium inactivation, has been demonstrated in various cells (Brehm and Eckert 1978; Peterson et al. 1999). There are two possible feedback regulatory mechanisms for calcium-dependent inhibition of L-VGCCs. When Ca2+ enters through L-VGCCs, it binds to calmodulin and calmodulin-like proteins (Lee et al. 2007; Peterson et al. 1999; Tippens and Lee 2007) and subsequently activates calcium-dependent kinases (Lee et al. 2007). Phosphorylation of L-VGCC subunits by calcium-dependent kinases can directly decrease L-VGCC currents and alter channel gating kinetics (Calin-Jageman and Lee 2008; Lee et al. 2007). However, activation of calcium-dependent kinases can also further elicit Ca2+ release from intracellular calcium stores, and possibly through other mechanisms, that ultimately lead to the inhibition of VGCCs in the plasma membrane (Alvarez et al. 1991; Fischer et al. 1998; Mohr et al. 1995; Thayer et al. 1988). Our Western blot data indicated that the protein level of L-VGCCα1D was downregulated by treatment with SS14 for 2 h. We postulate that calcium released from the intracellular stores might trigger downstream signaling that ultimately affects the protein synthesis of L-VGCCα1D and causes a decrease in L-VGCCs. Such calcium-dependent inhibition of L-VGCCs in cone photoreceptors would have an impact on photoreceptor physiology that is waiting to be explored.
We showed that SS14 inhibited L-VGCCs during the subjective night but not during the subjective day in chicken cone photoreceptors. A similar circadian phase-dependent result was also observed in cGMP-gated channels (CNGCs) (Chen et al. 2007), in which SS14 decreased the sensitivity of CNGCs to cGMP during the subjective night but had no effect during the subjective day. Together, Chen et al. (2007) and our results demonstrate that SS14 inhibits Ca2+ influx through both CNGCs and L-VGCCs in a circadian-dependent manner. While CNGCs are essential for phototransduction, L-VGCCs are indispensable in other aspects of photoreceptor physiology including synaptic transmission and excitability, and therefore SS14 may have a tremendous impact on the modulation of visual processes.
In the retina, the content of SS is affected by exposure to light. The content of SS is higher at night in the rat retina (Peinado et al. 1990), while a greater intracellular somatostatin-like immunoreactivity is observed in bipolar synaptic terminals during prolonged exposure to light in the chicken retina (Ishimoto et al. 1986). In the chicken retina, ENSLI amacrine cells that contain SS are more active in the dark phase (Morgan and Boelen 1996). Currently, it is not known whether the content of SS is under circadian control, and the physiological range of SS concentration is not clear in the chicken retina. It will be critical to re-evaluate the circadian regulation of SS content and release in the retina across different species, so the circadian phase-dependent effect of SS and its physiological significance and meaning can be properly interpreted. We observed inhibitory effects of SS14 on L-VGCCs only during the subjective night, but not during the day. This observation is interesting. Because there are four SS receptor subtypes expressed in the chicken retina, it is possible that some are regulated in a circadian manner while others are not. The effect of SS during the day might be mediated through a different SS receptor, coupled to a different G protein, leading to a different signaling pathway. We are currently investigating this hypothesis. Because visual systems have to anticipate daily changes in ambient illumination, the circadian oscillators in the retina provide a mechanism for visual systems to initiate more sustained adaptive changes throughout the course of a day (Cahill and Besharse 1993; Green and Besharse 2004). Somatostatin, along with dopamine and melatonin, has been proposed to serve as part of a “dark-light switch” within the retina neuronal circuitry in the diurnal changes of retinal physiology and function (Morgan and Boelen 1996). Therefore somatostatin plays an important role in fine-tuning light sensitivity of photoreceptors.
There are other factors that could contribute to the modulation of retina L-VGCC circadian rhythms in vivo. For example, dopamine levels in the retina are antiphase to those of melatonin (Adachi et al. 1998). Through D2 receptors, dopamine has differential effects on the L-VGCCs in rods and cones of salamander retinas (Stella and Thoreson 2000). Dopamine can entrain the photoreceptor circadian oscillators, induce light-like phase shifts, and contribute to circadian rhythms of rod-cone dominance (Hasegawa and Cahill 1999; Manglapus et al. 1998, 1999; Steenhard and Besharse 2000; Wang and Mangel 1996; Witkovsky et al. 1988). Dopamine is also known to modulate cGMP-gated cation channels of cone photoreceptors in a circadian phase-dependent manner (Ko et al. 2003). Therefore it is possible that dopamine may contribute to a circadian phase-dependent modulation of L-VGCC activities in cone photoreceptors. Another possible factor is the circadian regulation of retinal pH (Dmitriev and Mangel 2000, 2001, 2004; Harsanyi and Mangel 1993). In both fish and rabbit retinas, there is an increase in pH difference between the retina and superfusate at night in an in vitro whole retina preparation. The pH changes in the retina correlate to overall retinal energy metabolism and excitability measured by electroretinograms (ERGs) (Dmitriev and Mangel 2004). Because voltage-gated calcium channels, including L-types, are very sensitive to changes in extracellular pH (Iijima et al. 1986; Konnerth et al. 1987; Krafte and Kass 1988; Prod'hom et al. 1987), circadian rhythms in retinal pH may contribute to the modulation of L-VGCCs. Our in vitro dissociated retina culture system is a reduced system, as such the ability to simulate an in vivo environment is limited, and it is impossible to investigate all factors that potentially could contribute to the modulation of circadian oscillators in photoreceptors at once. Nonetheless, retinal photoreceptors possess self-sustained circadian oscillators that function independently in the absence of other retinal inputs (Cahill and Besharse 1993; Ko et al. 2001; Thomas et al. 1993). These photoreceptor oscillators lead to morphological, physiological, biochemical, and molecular changes that ultimately regulate photoreceptor function and physiology in a circadian fashion (Green and Besharse 2004), while other extracellular factors can further contribute to the modulation of photoreceptor oscillators, as well as stabilize the overall circadian rhythms of the retina.
GRANTS
The Image Analysis Laboratory has been supported in part by National Institutes of Health grants S10RR-022532, P42ES-004917, and P30ES-009106. This work was supported by National Institutes of Health RO1EY-017452 to G. Ko.
Acknowledgments
We thank Drs. Ottorino Belluzzi (University of Ferrara, Italy), Liangwei Gong (University of Illinois at Chicago), and Jianrong Li (Texas A&M University) for their critical reading and fruitful comments on the manuscript. We thank Dr. Robert Burghardt, Director of the Image Analysis Laboratory, College of Veterinary Medicine and Biomedical Sciences at Texas A&M University for assistance in using the imaging facility for calcium imaging.
REFERENCES
- Adachi et al. 1998.Adachi A, Nogi T, Ebihara S. Phase-relationship and mutual effects between circadian rhythms of ocular melatonin and dopamine in the pigeon. Brain Res 792: 361–369, 1998. [DOI] [PubMed] [Google Scholar]
- Adler et al. 1984.Adler R, Lindsey JD, Elsner CL. Expression of cone-like properties by chick embryo neural retina cells in glial-free monolayer cultures. J Cell Biol 99: 1173–1178, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolph 1989.Adolph AR. Pharmacological actions of peptides and indoleamines on turtle retinal ganglion cells. Vis Neurosci 3: 411–423, 1989. [DOI] [PubMed] [Google Scholar]
- Akbar et al. 1994.Akbar M, Okajima F, Tomura H, Majid MA, Yamada Y, Seino S, Kondo Y. Phospholipase C activation and Ca2+ mobilization by cloned human somatostatin receptor subtypes 1–5, in transfected COS-7 cells. FEBS Lett 348: 192–196, 1994. [DOI] [PubMed] [Google Scholar]
- Akopian et al. 2000.Akopian A, Johnson J, Gabriel R, Brecha N, Witkovsky P. Somatostatin modulates voltage-gated K(+) and Ca(2+) currents in rod and cone photoreceptors of the salamander retina. J Neurosci 20: 929–936, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez et al. 1991.Alvarez J, Montero M, Garcia-Sancho J. Cytochrome P-450 may link intracellular Ca2+ stores with plasma membrane Ca2+ influx. Biochem J 274: 193–197, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes and Kelly 2002.Barnes S, Kelly ME. Calcium channels at the photoreceptor synapse. Adv Exp Med Biol 514: 465–476, 2002. [DOI] [PubMed] [Google Scholar]
- Berridge et al. 2000.Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol 1: 11–21, 2000. [DOI] [PubMed] [Google Scholar]
- Brazeau et al. 1973.Brazeau P, Vale W, Burgus R, Ling N, Butcher M, Rivier J, Guillemin R. Hypothalamic polypeptide that inhibits the secretion of immunoreactive pituitary growth hormone. Science 179: 77–79, 1973. [DOI] [PubMed] [Google Scholar]
- Brehm and Eckert 1978.Brehm P, Eckert R. Calcium entry leads to inactivation of calcium channel in Paramecium. Science 202: 1203–1206, 1978. [DOI] [PubMed] [Google Scholar]
- Cahill and Besharse 1993.Cahill GM, Besharse JC. Circadian clock functions localized in xenopus retinal photoreceptors. Neuron 10: 573–577, 1993. [DOI] [PubMed] [Google Scholar]
- Calin-Jageman and Lee 2008.Calin-Jageman I, Lee A. Ca(v)1 L-type Ca2+ channel signaling complexes in neurons. J Neurochem 105: 573–583, 2008. [DOI] [PubMed] [Google Scholar]
- Cervia et al. 2008a.Cervia D, Casini G, Bagnoli P. Physiology and pathology of somatostatin in the mammalian retina: a current view. Mol Cell Endocrinol 286: 112–122, 2008a. [DOI] [PubMed] [Google Scholar]
- Cervia et al. 2008b.Cervia D, Martini D, Ristori C, Catalani E, Timperio AM, Bagnoli P, Casini G. Modulation of the neuronal response to ischaemia by somatostatin analogues in wild-type and knock-out mouse retinas. J Neurochem 106: 2224–2235, 2008b. [DOI] [PubMed] [Google Scholar]
- Chen et al. 1990.Chen C, Zhang J, Vincent JD, Israel JM. Somatostatin increases voltage-dependent potassium currents in rat somatotrophs. Am J Physiol Cell Physiol 259: C854–861, 1990. [DOI] [PubMed] [Google Scholar]
- Chen et al. 2007.Chen SK, Ko GY, Dryer SE. Somatostatin peptides produce multiple effects on gating properties of native cone photoreceptor cGMP-gated channels that depend on circadian phase and previous illumination. J Neurosci 27: 12168–12175, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Monte et al. 2003.Dal Monte M, Petrucci C, Vasilaki A, Cervia D, Grouselle D, Epelbaum J, Kreienkamp HJ, Richter D, Hoyer D, Bagnoli P. Genetic deletion of somatostatin receptor 1 alters somatostatinergic transmission in the mouse retina. Neuropharmacology 45: 1080–1092, 2003. [DOI] [PubMed] [Google Scholar]
- Dmitriev and Mangel 2000.Dmitriev AV, Mangel SC. A circadian clock regulates the pH of the fish retina. J Physiol 522 Pt 1: 77–82, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dmitriev and Mangel 2001.Dmitriev AV, Mangel SC. Circadian clock regulation of pH in the rabbit retina. J Neurosci 21: 2897–2902, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dmitriev et al. 2004.Dmitriev AV, Mangel SC. Retinal pH reflects retinal energy metabolism in the day and night. J Neurophysiol 91: 2404–2412, 2004. [DOI] [PubMed] [Google Scholar]
- Dryer et al. 1991.Dryer SE, Dourado MM, Wisgirda ME. Properties of Ca2+ currents in acutely dissociated neurons of the chick ciliary ganglion: inhibition by somatostatin-14 and somatostatin-28. Neuroscience 44: 663–672, 1991. [DOI] [PubMed] [Google Scholar]
- Fischer et al. 1998.Fischer MJ, Paulussen JJ, de Mol NJ, Janssen LH. Dual effect of the anti-allergic astemizole on Ca2+ fluxes in rat basophilic leukemia (RBL-2H3) cells: release of Ca2+ from intracellular stores and inhibition of Ca2+ release-activated Ca2+ influx. Biochem Pharmacol 55: 1255–1262, 1998. [DOI] [PubMed] [Google Scholar]
- Gee et al. 2000.Gee KR, Brown KA, Chen WN, Bishop-Stewart J, Gray D, Johnson I. Chemical and physiological characterization of fluo-4 Ca(2+)-indicator dyes. Cell Calcium 27: 97–106, 2000. [DOI] [PubMed] [Google Scholar]
- Gleason et al. 1992.Gleason E, Mobbs P, Nuccitelli R, Wilson M. Development of functional calcium channels in cultured avian photoreceptors. Vis Neurosci 8: 315–327, 1992. [DOI] [PubMed] [Google Scholar]
- Goldsmith et al. 1984.Goldsmith TH, Collins JS, Licht S. The cone oil droplets of avian retinas. Vision Res 24: 1661–1671, 1984. [DOI] [PubMed] [Google Scholar]
- Green and Besharse 2004.Green CB, Besharse JC. Retinal circadian clocks and control of retinal physiology. J Biol Rhythms 19: 91–102, 2004. [DOI] [PubMed] [Google Scholar]
- Grigoryan et al. 2003.Grigoryan EN, Vasilaki A, Mastrodimou N, Thermos K. Somatostatin receptor immunoreactivity in the eye of the adult newt (Pleurodeles waltlii Michan). Neurosci Lett 337: 143–146, 2003. [DOI] [PubMed] [Google Scholar]
- Harsanyi and Mangel 1993.Harsanyi K, Mangel SC. Modulation of cone to horizontal cell transmission by calcium and pH in the fish retina. Vis Neurosci 10: 81–91, 1993. [DOI] [PubMed] [Google Scholar]
- Hart et al. 2006.Hart NS, Lisney TJ, Collin SP. Cone photoreceptor oil droplet pigmentation is affected by ambient light intensity. J Exp Biol 209: 4776–4787, 2006. [DOI] [PubMed] [Google Scholar]
- Hasegawa and Cahill 1999.Hasegawa M, Cahill GM. A role for cyclic AMP in entrainment of the circadian oscillator in Xenopus retinal photoreceptors by dopamine but not by light. J Neurochem 72: 1812–1820, 1999. [DOI] [PubMed] [Google Scholar]
- Helboe et al. 1997.Helboe L, Moller M, Norregaard L, Schiodt M, Stidsen CE. Development of selective antibodies against the human somatostatin receptor subtypes sst1-sst5. Brain Res Mol Brain Res 49: 82–88, 1997. [DOI] [PubMed] [Google Scholar]
- Hirata et al. 1985.Hirata M, Kukita M, Sasaguri T, Suematsu E, Hashimoto T, Koga T. Increase in Ca2+ permeability of intracellular Ca2+ store membrane of saponin-treated guinea pig peritoneal macrophages by inositol 1,4,5-trisphosphate. J Biochem 97: 1575–1582, 1985. [DOI] [PubMed] [Google Scholar]
- Ho et al. 2001.Ho MK, Yung LY, Chan JS, Chan JH, Wong CS, Wong YH. Galpha(14) links a variety of G(i)- and G(s)-coupled receptors to the stimulation of phospholipase C. Br J Pharmacol 132: 1431–1440, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer et al. 1995.Hoyer D, Bell GI, Berelowitz M, Epelbaum J, Feniuk W, Humphrey PP, O'Carroll AM, Patel YC, Schonbrunn A, Taylor JE, Reìsìne T. Classification and nomenclature of somatostatin receptors. Trends Pharmacol Sci 16: 86–88, 1995. [DOI] [PubMed] [Google Scholar]
- Hull et al. 2006.Hull C, Studholme K, Yazulla S, von Gersdorff H. Diurnal changes in exocytosis and the number of synaptic ribbons at active zones of an ON-type bipolar cell terminal. J Neurophysiol 96: 2025–2033, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iijima et al. 1986.Iijima T, Ciani S, Hagiwara S. Effects of the external pH on Ca channels: experimental studies and theoretical considerations using a two-site, two-ion model. Proc Natl Acad Sci USA 83: 654–658, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimoto et al. 1986.Ishimoto I, Millar T, Chubb IW, Morgan IG. Somatostatin-immunoreactive amacrine cells of chicken retina: retinal mosaic, ultrastructural features, and light-driven variations in peptide metabolism. Neuroscience 17: 1217–1233, 1986. [DOI] [PubMed] [Google Scholar]
- Johansen et al. 2001.Johansen PW, Lund HW, Gordeladze JO. Specific combinations of G-protein subunits discriminate hormonal signalling in rat pituitary (GH(3)) cells in culture. Cell Signal 13: 251–256, 2001. [DOI] [PubMed] [Google Scholar]
- Johnson et al. 2001.Johnson J, Caravelli ML, Brecha NC. Somatostatin inhibits calcium influx into rat rod bipolar cell axonal terminals. Vis Neurosci 18: 101–108, 2001. [DOI] [PubMed] [Google Scholar]
- Johnson et al. 1999.Johnson J, Wu V, Wong H, Walsh JH, Brecha NC. Somatostatin receptor subtype 2A expression in the rat retina. Neuroscience 94: 675–683, 1999. [DOI] [PubMed] [Google Scholar]
- Johnston and Hudson 1976.Johnston D, Hudson RA. Isolation and composition of the carotenoid-containing oil droplets from cone photoreceptors. Biochim Biophys Acta 424: 235–245, 1976. [DOI] [PubMed] [Google Scholar]
- Ko et al. 2001.Ko GY, Ko ML, Dryer SE. Circadian regulation of cGMP-gated cationic channels of chick retinal cones. Erk MAP kinase and Ca2+/calmodulin-dependent protein kinase II. Neuron 29: 255–266, 2001. [DOI] [PubMed] [Google Scholar]
- Ko et al. 2003.Ko GY, Ko ML, Dryer SE. Circadian phase-dependent modulation of cGMP-gated channels of cone photoreceptors by dopamine and D2 agonist. J Neurosci 23: 3145–3153, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko et al. 2009.Ko ML, Jian K, Shi L, Ko GY. Phosphatidylinositol 3 kinase-Akt signaling serves as a circadian output in the retina. J Neurochem 108: 1607–1620, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko et al. 2007.Ko ML, Liu Y, Dryer SE, Ko GY. The expression of L-type voltage-gated calcium channels in retinal photoreceptors is under circadian control. J Neurochem 103: 784–792, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konnerth et al. 1987.Konnerth A, Lux HD, Morad M. Proton-induced transformation of calcium channel in chick dorsal root ganglion cells. J Physiol 386: 603–633, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouvidi et al. 2006.Kouvidi E, Papadopoulou-Daifoti Z, Thermos K. Somatostatin modulates dopamine release in rat retina. Neurosci Lett 391: 82–86, 2006. [DOI] [PubMed] [Google Scholar]
- Krafte and Kass 1988.Krafte DS, Kass RS. Hydrogen ion modulation of Ca channel current in cardiac ventricular cells. Evidence for multiple mechanisms. J Gen Physiol 91: 641–657, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krizaj 2005.Krizaj D. Compartmentalization of calcium entry pathways in mouse rods. Eur J Neurosci 22: 3292–3296, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krizaj et al. 2003.Krizaj D, Lai FA, Copenhagen DR. Ryanodine stores and calcium regulation in the inner segments of salamander rods and cones. J Physiol 547: 761–774, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee et al. 2007.Lee A, Jimenez A, Cui G, Haeseleer F. Phosphorylation of the Ca2+-binding protein CaBP4 by protein kinase C zeta in photoreceptors. J Neurosci 27: 12743–12754, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez et al. 2005.Lopez R, Lopez-Gallardo M, Busturia I, Anezary L, Prada C. Spatial and temporal patterns of growth and differentiation of cone oil droplets in the chick retina. J Neurosci Res 79: 401–411, 2005. [DOI] [PubMed] [Google Scholar]
- Manglapus et al. 1999.Manglapus MK, Iuvone PM, Underwood H, Pierce ME, Barlow RB. Dopamine mediates circadian rhythms of rod-cone dominance in the Japanese quail retina. J Neurosci 19: 4132–4141, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manglapus et al. 1998.Manglapus MK, Uchiyama H, Buelow NF, Barlow RB. Circadian rhythms of rod-cone dominance in the Japanese quail retina. J Neurosci 18: 4775–4784, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr et al. 1995.Mohr FC, Alojipan SV, Dunston SK, Pessah IN. The delta isomer of hexachlorocyclohexane induces rapid release of the myo-inositol-1,4,5-trisphosphate-sensitive Ca2+ store and blocks capacitative Ca2+ entry in rat basophilic leukemia cells. Mol Pharmacol 48: 512–522, 1995. [PubMed] [Google Scholar]
- Morgan and Boelen 1996.Morgan IG, Boelen MK. A retinal dark-light switch: a review of the evidence. Vis Neurosci 13: 399–409, 1996. [DOI] [PubMed] [Google Scholar]
- Morgan et al. 1994.Morgan IG, Wellard JW, Boelen MK. A role for the enkephalin-immunoreactive amacrine cells of the chicken retina in adaptation to light and dark. Neurosci Lett 174: 64–66, 1994. [DOI] [PubMed] [Google Scholar]
- Murthy et al. 2004.Murthy KS, Zhou H, Huang J, Pentyala SN. Activation of PLC-delta1 by Gi/o-coupled receptor agonists. Am J Physiol Cell Physiol 287: C1679–1687, 2004. [DOI] [PubMed] [Google Scholar]
- Nunn et al. 2004.Nunn C, Cervia D, Langenegger D, Tenaillon L, Bouhelal R, Hoyer D. Comparison of functional profiles at human recombinant somatostatin sst2 receptor: simultaneous determination of intracellular Ca2+ and luciferase expression in CHO-K1 cells. Br J Pharmacol 142: 150–160, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavan et al. 2004.Pavan B, Fiorini S, Dal Monte M, Lunghi L, Biondi C, Bagnoli P, Cervia D. Somatostatin coupling to adenylyl cyclase activity in the mouse retina. Naunyn Schmiedebergs Arch Pharmacol 370: 91–98, 2004. [DOI] [PubMed] [Google Scholar]
- Peinado et al. 1990.Peinado MA, Fajardo N, Hernandez G, Puig-Domingo M, Viader M, Reiter RJ, Webb SM. Immunoreactive somatostatin diurnal rhythms in rat pineal, retina and harderian gland: effects of sex, season, continuous darkness and estrous cycle. J Neural Transm Gen Sect 81: 63–72, 1990. [DOI] [PubMed] [Google Scholar]
- Peng et al. 1991.Peng YW, Sharp AH, Snyder SH, Yau KW. Localization of the inositol 1,4,5-trisphosphate receptor in synaptic terminals in the vertebrate retina. Neuron 6: 525–531, 1991. [DOI] [PubMed] [Google Scholar]
- Peterson et al. 1999.Peterson BZ, DeMaria CD, Adelman JP, Yue DT. Calmodulin is the Ca2+ sensor for Ca2+ -dependent inactivation of L-type calcium channels. Neuron 22: 549–558, 1999. [DOI] [PubMed] [Google Scholar]
- Petrucci et al. 2001.Petrucci C, Resta V, Fieni F, Bigiani A, Bagnoli P. Modulation of potassium current and calcium influx by somatostatin in rod bipolar cells isolated from the rabbit retina via sst2 receptors. Naunyn Schmiedebergs Arch Pharmacol 363: 680–694, 2001. [DOI] [PubMed] [Google Scholar]
- Prod'hom et al. 1987.Prod'hom B, Pietrobon D, Hess P. Direct measurement of proton transfer rates to a group controlling the dihydropyridine-sensitive Ca2+ channel. Nature 329: 243–246, 1987. [DOI] [PubMed] [Google Scholar]
- Rhie et al. 2003.Rhie DJ, Sung JH, Ha US, Kim HJ, Min DS, Hahn SJ, Kim MS, Jo YH, Yoon SH. Endogenous somatostatin receptors mobilize calcium from inositol 1,4,5-trisphosphate-sensitive stores in NG108-15 cells. Brain Res 975: 120–128, 2003. [DOI] [PubMed] [Google Scholar]
- Rohrer et al. 1998.Rohrer SP, Birzin ET, Mosley RT, Berk SC, Hutchins SM, Shen DM, Xiong Y, Hayes EC, Parmar RM, Foor F, Mitra SW, Degrado SJ, Shu M, Klopp JM, Cai SJ, Blake A, Chan WW, Pasternak A, Yang L, Patchett AA, Smith RG, Chapman KT, Schaeffer JM. Rapid identification of subtype-selective agonists of the somatostatin receptor through combinatorial chemistry. Science 282: 737–740, 1998. [DOI] [PubMed] [Google Scholar]
- Romoser et al. 2001.Romoser VA, Graves TK, Wu D, Jiang H, Hinkle PM. Calcium responses to thyrotropin-releasing hormone, gonadotropin-releasing hormone and somatostatin in phospholipase css3 knockout mice. Mol Endocrinol 15: 125–135, 2001. [DOI] [PubMed] [Google Scholar]
- Shi et al. 2009.Shi L, Jian K, Ko ML, Trump D, Ko GY. Retinoschisin, a new binding partner for L-type voltage-gated calcium channels in the retina. J Biol Chem 284: 3966–3975, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoshan-Barmatz et al. 2007.Shoshan-Barmatz V, Zakar M, Shmuelivich F, Nahon E, Vardi N. Retina expresses a novel variant of the ryanodine receptor. Eur J Neurosci 26: 3113–3125, 2007. [DOI] [PubMed] [Google Scholar]
- Siehler et al. 2005.Siehler S, Nunn C, Zupanc GK, Hoyer D. Fish somatostatin sst3 receptor: comparison of radioligand and GTPgammaS binding, adenylate cyclase and phospholipase C activities reveals different agonist-dependent pharmacological signatures. Auton Autacoid Pharmacol 25: 1–16, 2005. [DOI] [PubMed] [Google Scholar]
- Siffert et al. 1995.Siffert W, Rosskopf D, Moritz A, Wieland T, Kaldenberg-Stasch S, Kettler N, Hartung K, Beckmann S, Jakobs KH. Enhanced G protein activation in immortalized lymphoblasts from patients with essential hypertension. J Clin Invest 96: 759–766, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenhard and Besharse 2000.Steenhard BM, Besharse JC. Phase shifting the retinal circadian clock: xPer2 mRNA induction by light and dopamine. J Neurosci 20: 8572–8577, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stella and Thoreson 2000.Stella SL, Thoreson WB. Differential modulation of rod and cone calcium currents in tiger salamander retina by D2 dopamine receptors and cAMP. Eur J Neurosci 12: 3537–3548, 2000. [DOI] [PubMed] [Google Scholar]
- Thayer et al. 1988.Thayer SA, Hirning LD, Miller RJ. The role of caffeine-sensitive calcium stores in the regulation of the intracellular free calcium concentration in rat sympathetic neurons in vitro. Mol Pharmacol 34: 664–673, 1988. [PubMed] [Google Scholar]
- Thermos 2003.Thermos K. Functional mapping of somatostatin receptors in the retina: a review. Vision Res 43: 1805–1815, 2003. [DOI] [PubMed] [Google Scholar]
- Thomas et al. 1993.Thomas KB, Tigges M, Iuvone PM. Melatonin synthesis and circadian tryptophan hydroxylase activity in chicken retina following destruction of serotonin immunoreactive amacrine and bipolar cells by kainic acid. Brain Res 601: 303–307, 1993. [DOI] [PubMed] [Google Scholar]
- Tippens and Lee 2007.Tippens AL, Lee A. Caldendrin, a neuron-specific modulator of Cav/1.2 (L-type) Ca2+ channels. J Biol Chem 282: 8464–8473, 2007. [DOI] [PubMed] [Google Scholar]
- Vasilaki et al. 2001.Vasilaki A, Gardette R, Epelbaum J, Thermos K. NADPH-diaphorase colocalization with somatostatin receptor subtypes sst2A and sst2B in the retina. Invest Ophthalmol Vis Sci 42: 1600–1609, 2001. [PubMed] [Google Scholar]
- Vasilaki et al. 2003.Vasilaki A, Georgoussi Z, Thermos K. Somatostatin receptors (sst2) are coupled to Go and modulate GTPase activity in the rabbit retina. J Neurochem 84: 625–632, 2003. [DOI] [PubMed] [Google Scholar]
- Wang et al. 1990.Wang HL, Reisine T, Dichter M. Somatostatin-14 and somatostatin-28 inhibit calcium currents in rat neocortical neurons. Neuroscience 38: 335–342, 1990. [DOI] [PubMed] [Google Scholar]
- Wang et al. 1999.Wang TL, Sterling P, Vardi N. Localization of type I inositol 1,4,5-triphosphate receptor in the outer segments of mammalian cones. J Neurosci 19: 4221–4228, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang and Mangel 1996.Wang Y, Mangel SC. A circadian clock regulates rod and cone input to fish retinal cone horizontal cells. Proc Natl Acad Sci USA 93: 4655–4660, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weckbecker et al. 2003.Weckbecker G, Lewis I, Albert R, Schmid HA, Hoyer D, Bruns C. Opportunities in somatostatin research: biological, chemical and therapeutic aspects. Nat Rev Drug Discov 2: 999–1017, 2003. [DOI] [PubMed] [Google Scholar]
- Witkovsky et al. 1988.Witkovsky P, Stone S, Besharse JC. Dopamine modifies the balance of rod and cone inputs to horizontal cells of the Xenopus retina. Brain Res 449: 332–336, 1988. [DOI] [PubMed] [Google Scholar]
- Yang et al. 1997.Yang DS, Boelen MK, Morgan IG. Development of the enkephalin-, neurotensin- and somatostatin-like (ENSLI) amacrine cells in the chicken retina. Brain Res Dev Brain Res 101: 57–65, 1997. [DOI] [PubMed] [Google Scholar]
- Zalutsky and Miller 1990.Zalutsky RA, Miller RF. The physiology of somatostatin in the rabbit retina. J Neurosci 10: 383–393, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]