Abstract
Francisella tularensis, an intracellular Gram-negative bacterium, is the causative agent of tularemia and a potential bioweapon. Currently, there is no licensed vaccine against this organism. We have characterized the efficacy of a defined F. tularensis subsp. novicida mutant (ΔiglB) as a live attenuated vaccine against pneumonic tularemia. Replication of the iglB mutant (KKF235) in murine macrophages was significantly lower than the wild type novicida strain U112, and exhibited an LD50 greater than 106 fold (>107 CFU vs <10 CFU) in an intranasal challenge model. Mice immunized with KKF235 intranasally or orally induced robust antigen-specific splenic IFN-γ recall responses, as well as the production of systemic and mucosal antibodies. Intranasal vaccination with KKF235 protected mice from subsequent homotypic challenge with U112 as well as heterotypic challenge with F. tularensis subsp. holarctica (LVS). Moreover, protected animals also exhibited minimal pathological changes compared with mock-vaccinated and challenged animals. The protection conferred by KKF235 vaccination was shown to be highly dependent on endogenous IFN-γ production. Most significantly, oral immunization with KKF235 protected mice from a highly lethal subsp. tularensis (SCHU S4) pulmonary challenge. Collectively, these results further suggest the feasibility of using defined pathogenicity island mutants as live vaccine candidates against pneumonic tularemia.
1. Introduction
Francisella tularensis is a Gram-negative intracellular bacterium, and the causative agent of the zoonotic disease tularemia [1, 2]. Currently, four subspecies of Francisella tularensis are recognized, tularensis (Type A), holarctica (Type B), mediasiatica, and novicida [3, 4]. Type A strains are the most virulent, with an infectious dose as low as 10 colony forming units (CFU) and a high mortality rate of 30-60% among untreated cases of pneumonic tularemia [5]. Because of the high mortality and low infectious dose, F. tularensis was developed as a potential biological weapon by the Soviet Union and the United States [6]. To this end, the live vaccine strain (LVS) derived from F. tularensis subsp. holarctica has been used as a prophylactic vaccine against tularemia [7]. However, LVS has not been licensed for use in humans due to a lack of understanding about the genetic mutations that are responsible for attenuation. F. tularensis subsp. novicida (F. novicida), which causes disease only in immuno-compromised humans but is highly virulent for mice, has been used as a comparative model organism due to the high degree of genetic similarity with Type A organisms (98.1% homology between sequences common to U112 and SCHU S4 [8]) and growth in human macrophages [9, 10]. Moreover, F. novicida has proven to be much more amenable to genetic manipulation [11] than other F. tularensis subspecies, allowing for the identification of a number of attenuating mutations that may be suitable for a live vaccine strain. Given the pressing need to identify putative vaccine candidates, important information on the nature of protective immunity to tularemia may be derived by utilizing defined mutant F. novicida strains as shown previously [12, 13].
To this end, growth of Francisella inside macrophages requires proteins encoded by the Francisella pathogenicity island (FPI) genes [14-16]. The FPI consists of 16-19 genes in a cluster and is found in duplication in most of the sequenced F. tularensis subspecies with the exception of F. novicida U112 [17, 18]. The iglABCD operon in the FPI is highly upregulated following infection in macrophages, and proteins, such as IglC, have been shown to be important for intramacrophage survival and growth of F. novicida and LVS [19, 20]. Certain FPI mutants have been shown to serve as defined vaccine strains against a mouse model of tularemia [12, 15]. To further evaluate the genes within the FPI for defined vaccine strains, we have examined iglB. The iglAB gene homologs are arranged in tandem in several bacterial pathogens, such as Pseudomonus aeruginosa and Vibrio cholerae, as part of a newly defined type VI secretion system [21, 22]. The effectors of this unique secretion system have been identified as putative virulence factors and may induce cytolysis [21, 22]. Moreover, F. novicida IglB has shown to interact with IglA, which is required for intramacrophage growth [16]. Mice vaccinated intranasally (i.n.) with a defined iglB mutant (KKF235) induced significant antigen-specific cellular and humoral responses, and were protected against subsequent Francisella U112 and LVS pulmonary challenge. Furthermore, oral vaccination with KKF235 provides protection against pneumonic infection by the highly virulent Type A SCHU S4 strain.
2. Materials and Methods
2.1 Bacteria
F. novicida U112 was obtained from Dr. Francis Nano (University of Victoria, Canada). Isogenic strain KKF235 (F. novicida ΔiglB::ermC) was generated as described in our previous report [23]. F. tularensis subsp. holarctica LVS (Lot 703-0303-016) was obtained from Dr. R. Lyons at the University of New Mexico. Type A F. tularensis subsp. tularensis (SCHU S4 strain) was obtained from the Centers for Disease Control. Strains were grown at 37°C in Trypticase Soy Broth (TSB) or Trypticase Soy Agar (TSA) (BD Biosciences, San Jose, CA) supplemented with 0.1% L-cysteine (Fisher Sci., Fair Lawn, NJ). All work with the Type A strain was done in a licensed ABSL-3 facility.
2.2 Mice
Four to six-week-old female BALB/c and C57BL/6 mice were obtained from the National Cancer Institute (Bethesda, MD). BALB/c IFN-γ-/- mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Mice were housed at the University of Texas at San Antonio animal facility and all experimental procedures were performed in compliance with the Institutional Animal Care and Use Committee (IACUC) guidelines.
2.3. Intramacrophage growth of KKF235
Murine macrophage-like J774 cells were cultured at 37°C in a 5% CO2 incubator in Dulbecco’s modified Eagle’s medium (DMEM; GIBCO BRL, Grand Islands, N.Y.) supplemented with 10% fetal bovine serum (D-10; HyClone, Logan, Utah). For intramacrophage growth assays, J774 cells were seeded into 96-well microplates at a density of 2×105 cells per well, and incubated for 2 h before infecting with either 2×106 CFU of KKF235 or the wild type U112. The plates were incubated for 2 h to allow for bacterial uptake and further incubated for an additional 1 h with D-10 plus 20 μg/ml of gentamicin (GIBCO) to kill extracellular organisms. At 3 and 24 h, macrophages were lysed with 0.1% deoxycholate and the numbers of intracellular bacteria were determined by dilution plating on TSA + L-cysteine.
2.3. Determination of LD50 and bacterial dissemination of KKF235
BALB/c mice (6-8 animals/group) were anesthetized with 3% isoflurane using a rodent anesthesia machine (Harvard Apparatus, Holliston, MA) and then i.n. challenged with escalating doses (104, 105, 106 or 107 CFU) of KKF235 or with 103 CFU of U112 in 25μl of phosphate buffered saline (PBS). Animals were monitored for morbidity and mortality. To determine the bacterial dissemination profile of KKF235, BALB/c mice were challenged i.n. with 106 CFU of KKF235 and groups of mice (n=3) were euthanized on days 3, 6, 9, 12 and 15 post-challenge. Lungs, liver, and spleen were removed and the numbers of bacteria in the homogenized tissues were determined by dilution plating on TSA + L-cysteine.
2.4. Immunization and challenge
Four weeks following a single i.n. KKF235 immunization, BALB/c mice were challenged i.n. with approximately 100 LD50 (1000 CFU) of wild-type U112 or 10 LD50 (3×104 CFU) of LVS. For SCHU S4 challenge experiments, mice were vaccinated orally, a vaccination route that we [24] and others [25] have recently characterized, using a 22 gauge, 25 mm long, 1.25 mm tip feeding needle (Fine Science Tools Inc., Foster City, CA) [26] with either 103 CFU of KKF235 in 200 μl of phosphate buffered saline (PBS) or mock-immunized with PBS alone. Some mice received a second oral vaccination boost (103 CFU) of KKF235 three weeks after the first inoculation. Vaccinated mice were rested for 3 weeks and challenged i.n. with 25 or 50 LD50 of SCHU S4 (LD50 <10 CFU [27, 28]) in 25 μl PBS. The actual CFU administered in each experiment was determined by serial dilution-plating of the inoculum on TSA + L-cysteine. Animals were monitored daily for morbidity and mortality.
2.5. Spleen culture for antigen-specific cytokine induction
Mice were mock immunized with PBS or immunized with KKF235 i.n. (106 CFU) or orally (103 CFU). At day 14 after immunization, spleens were collected from mice and single cell suspensions (106 cells/well) were prepared and cultured for 72 h in D-10 with or without UV-inactivated KKF235 (105 CFU). Cells also were cultured with the unrelated antigen hen egg lysozyme (HEL). Culture supernatants were removed for IFN-γ and IL-4 analysis using BD OptEIA™ kits (BD Pharmingen, San Diego, CA), according to the manufacturer’s instructions and as described previously [29].
2.6. Determination of antibody and isotype levels by ELISA
Sera were prepared from KKF235-vaccinated mice at days 14 and 28 after i.n. immunization, or at day 21 after final oral vaccination. Collection of bronchoalveolar lavage (BAL) fluid from mice was performed as previously described [24]. Briefly, the lungs were lavaged two times with Hanks Balanced Salt Solution using a 0.58 mm (outer diameter) polyethylene catheter (Becton Dickinson, Sparks, MD). The recovered BAL fluid (1ml) was centrifuged at 9,500 × G for 7 min at 4°C, and the supernatant was stored at -70°C until use. For analysis of fecal supernatants, 0.1 gm of fresh fecal pellets was collected, dissolved in 1ml of PBS containing protease inhibitor cocktail (Roche Applied Science, Indianapolis, IN), and supernatants collected by centrifugation. Microtiter plates were coated overnight with 106 CFU of UV-inactivated KKF235 in sodium bicarbonate buffer (pH 9.5), washed with PBS containing 0.3 % Brij-35 (Sigma-Aldrich, St. Louis, MO), and blocked for 1 h at room temperature with PBS containing 10 % fetal bovine serum. Serial dilutions of serum (starting at 1:50 dilution), or undiluted fecal supernatants and BAL fluids, were added to wells and incubated at room temperature for 2 h. The plates were then washed and incubated for an additional 1 h with goat anti-mouse total Ig, IgG1, IgG2a, IgA or IgM conjugated to HRP (Southern Biotechnology Associates, Birmingham, AL). The plates were then washed and ABTS peroxidase substrate 1 component (KPL, Gaithersburg, MD) was added for color development. The absorbance at 405 nm was measured using an ELISA microplate reader (Biotek Instruments), and reciprocal serum dilutions corresponding to 50 % maximal binding were used to obtain titers. No binding of immune sera and fecal samples was observed when the plates were coated with the unrelated antigen HEL (1 μg/ well).
2.7. Histology
Mice were euthanized and 10 % neutral formalin was injected into the lungs via the trachea. The lungs and livers were removed, fixed in formalin and embedded in paraffin blocks. Serial horizontal sections (5 μm) were prepared, stained with hematoxylin and eosin (H&E), visualized using a Zeiss Axioskop 2 Plus research microscope, and representative images acquired using an Axiocam digital camera (Zeiss, Thornwood, NY).
2.8. Statistical analyses
SigmaStat (Systat Software Inc., San Jose, CA) was used to perform all tests of significance. Statistical analyses for survival experiments were performed using the Kaplan-Meier test. The Student’s t test was used to determine differences in antibody production. All data are reported as mean ± standard deviation from each experimental animal group and are representative of at least two independent experiments.
3. RESULTS
3.1 In vitro and in vivo replication of KKF235
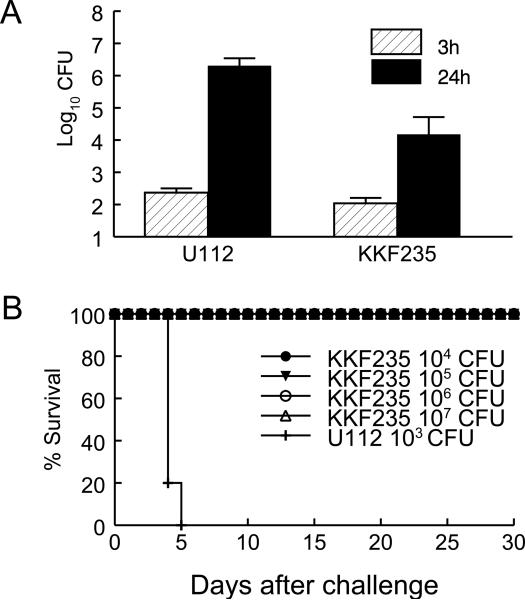
KKF235, an iglB mutant of F. novicida, was constructed using a targeted mutagenesis strategy [23]. J774 mouse macrophages were infected with the mutant and the wild type U112 strain, and bacteria recovered at 3 and 24 h. As shown in Fig. 1A, U112 exhibited a 4 log10 increase from 3 to 24 h in contrast to KKF235, which increased 2 log10 under the same conditions. To examine the virulence of KKF235 in vivo, groups of BALB/c mice were challenged i.n. with escalating inocula (104, 105, 106 or 107 CFU) of KKF235 and monitored for survival and weight loss. These analyses revealed that KKF235 was highly attenuated, with all animals surviving the i.n. challenge dose up to the highest dose tested (107 CFU, Fig. 1B). Moreover, none of the challenged animals exhibited signs of clinical illness, including only minimal loss of initial body weight (data not shown). In comparison, all mice challenged i.n. with the wild type U112 strain (1000 CFU; ∼100 LD50) succumbed to the infection by day 5. To determine the ability of KKF235 to replicate in vivo, BALB/c mice were challenged (106 CFU) i.n., and lungs, livers and spleens were collected from the infected mice at three day intervals, and the numbers of recovered bacteria from homogenized tissues were determined by dilution plating. As shown in Fig. 2, there was increasing replication of KKF235 in the lungs up to the first 9 days (approx. 8 × 107 CFU), with a significant reduction in burden noted by day 15 (< 3.2 log10 CFU). The organism also disseminated to the liver and spleen as early as 3 days after i.n. challenge. However, there were lower numbers (2-3 log10) of KKF235 recovered from the liver and spleen than from the lungs throughout the period of observation. Most of the recoverable bacteria from all organs were greatly diminished by 21 days after challenge (data not shown). Together, these analyses reveal that KKF235 had reduced intramacrophage replication and was highly attenuated in vivo, with an i.n. LD50 dose greater than 107 CFU, compared to the wild type U112 strain (LD50 about 10 CFU).
Fig. 1.
The F. novicida iglB mutant KKF235 is attenuated. (A) Intramacrophage growth of KKF235. Murine macrophage line J774 cells were infected with KKF235 or its parental strain (U112), using a MOI of 10. The numbers of viable bacteria in the macrophages were measured at 3 and 24 h post-challenge. (B) LD50 of KKF235. Groups of BALB/c mice (female, 4-6 weeks old, n=5) were challenged i.n. with 104, 105, 106 or 107 CFU of KKF235, or 1000 CFU of U112, to determine the LD50 of the mutant. Mice were observed for morbidity and mortality through day 30.
Fig. 2.
Growth and clearance of KKF235 in target organs after i.n. vaccination. BALB/c mice were challenged with 106 CFU of KKF235 and bacterial burdens were determined for the lungs, livers and spleens of mice (n=3) at each time point indicated. * one of the 3 examined mice showed no detectable bacterial burden.
3.2 Intranasal vaccination with KKF235 protected mice against homotypic pulmonary U112 challenge
To evaluate the efficacy of KKF235 in protecting mice against virulent U112 i.n. challenge, BALB/c mice were vaccinated i.n. with 106 CFU of KKF235. The animals were rested for 30 days and challenged i.n. with 103 CFU (100 LD50) of U112. Vaccination with KKF235 conferred complete protection (100% survival) against otherwise lethal pulmonary challenge with U112 during the observation period of 30 days (Fig. 3A). Moreover, there was minimal loss of body weight in the vaccinated mice following challenge (data not shown). In contrast, mock (PBS) vaccinated mice succumbed to the infection by day 5, with marked loss of body weight. We further assessed the immunity generated by i.n. KKF235 immunization. Sera from KKF235-vaccinated mice were analyzed for antibody profiles at 14 and 28 days after initial vaccination. As shown in Fig. 3B, mice immunized with KKF235 produced significant amounts of serum antibody at day 14 after priming, and the titers were further increased at day 28 compared to mock-vaccinated animals (titer <100) (data not shown; p < 0.01). Isotype analyses indicated both Th1- (IgG2a) and Th2- (IgG1) type antibodies were produced in mice after the KKF235 immunization. No KKF235-specific antibody was detected in mock-vaccinated mice and all sera showed no reactivity to the unrelated protein HEL (data not shown). Within the site of pulmonary challenge, significant levels of respiratory antibodies were detected in KKF235 immunized mice (Fig. 3C). Vaccinated mice exhibited elevated total antibody responses, which consist of both IgG1 and IgG2a. Moreover, there also was significant induction of IgA and IgM within the respiratory secretions.
Fig. 3.
Intranasal immunization with KKF 235 protected mice against homotypic F. novicida challenge. BALB/c mice received a single immunizing dose (106 CFU) of KKF235 or PBS (as a mock control). Mice were rested for 30 days, then challenged i.n. with 1000 CFU of U112 (∼100 LD50). Mice were monitored for survival (A). Difference in survival between KKF235- and mock- vaccinated mice was significant at a p<0.005. Sera (B) were collected at 14 and 28 days, and BAL fluid (C) collected at 28 days after immunization and used to determine the levels of anti-KKF235 specific antibody. Differences in antibody binding in the sera between between KKF235- and mock-vaccinated mice (titer < 100; data not shown) were significant at a p value of <0.01. Differences in antibody binding between KKF235- and mock-vaccinated mice were significant at a p value of <0.0001 for Total Ab, IgG1 and IgG2a, <0.005 for IgA, and <0.0005 for IgM in the BAL.
Apart from humoral immunity, antigen-specific IFN-γ production has previously been shown to be a crucial component of protective immunity against F. tularensis [29-31]. Thus, we examined whether vaccination with the KKF235 strain induces antigen-specific cell-mediated responses. Mice were i.n. vaccinated with KKF235 (106 CFU), then 14 days later spleen cells were analyzed for F. novicida-induced cytokine recall responses. As shown in Fig. 4A, the spleen cells stimulated with UV-inactivated KKF235 induced an appreciable IFN-γ response in culture, but a negligible IL-4 induction (data not shown). Cells from mock (PBS)-vaccinated animals had no cytokine responses upon recall with KKF235. In addition, there was no recall response to the unrelated control antigen HEL in cells from vaccinated animals. IFN-γ is a known isotype switch factor for IgG2a production [32] and has been shown to be important for protective immunity against F. tularensis [12]. To determine the contribution of IFN-γ in protection mediated by KKF235, BALB/c IFN-γ-/- and IFN-γ+/+ mice were immunized i.n. with 106 CFU of KKF235 and then challenged with the wild type strain U112. Notably, the vaccinated IFN-γ-/- mice all survived the immunization with KKF235, with no overt symptoms of disease, indicating the highly attenuated nature of the ΔiglB strain. When vaccinated mice were challenged i.n. 30 days later with 100 LD50 (1000 CFU) of the wild-type U112 strain, it was found that all of the IFN-γ-/- vaccinated mice succumbed within 8-9 days, whereas all of the IFN-γ+/+ mice were completely (100%) protected against this lethal challenge dose (Fig. 4B). These results indicate that IFN-γ-dependent mechanisms play a pivotal role in shaping protective anti-Francisella immunity conferred by KKF235 vaccination. Collectively, the results demonstrate that i.n. vaccination with KKF235 induces systemic and respiratory antibody responses, as well as robust cell-mediated immunity, and provided protection against U112 challenge via IFN-γ-dependent mechanisms.
Fig. 4.
Protective immunity induced by KKF235 immunization is IFN-γdependent. (A) Spleens were collected from KKF235 i.n. immunized BALB/c mice, single cells prepared, and assayed for KKF235 induced IFN-γ with UV-inactivated KKF235 (105 CFU). HEL, an unrelated antigen, was used as a control stimulus. (B) BALB/c IFN-γ-/- mice and IFN γ+/+ mice (n=5) were vaccinated i.n. with KKF235 (106 CFU) or mock vaccinated with PBS. Animals were challenged 30 days later with 1000 CFU of U112 (∼100 LD50). All animals were monitored daily for survival. Difference in survival between KKF235 vaccinated IFN γ+/+ and IFN γ-/- mice was significant at p < 0.005.
3.3 KKF235 vaccination reduced inflammation in subsequentially challenged mice
The efficacy of protective immunity conferred by KKF235 vaccination also was evaluated by histological analyses (Fig. 5). Lung sections from mice vaccinated i.n. with 106 CFU of KKF235 prior to challenge (Day 0, Panel e) revealed open air spaces with normal pulmonary architecture and no obvious evidence of histopathological changes, similar to the case for mock (PBS)-treated and unchallenged mice (Panel a). Moreover, the livers of KKF235 vaccinated mice (Panel g) also displayed no apparent histological changes and were similar to that of mock-treated and unchallenged mice (Panel c), collectively suggesting that the defined-vaccine strain (KKF235) induced no overt symptoms of disease. Three days after i.n. WT (U112) challenge, lungs from mock immunized mice (Panel b) had intense inflammatory cellular infiltration in the peribronchiolar and perivascular areas, including the presence of peribonchiolar cuffing. The infiltrates were composed of both mononuclear and polymorphonuclear leukocytes, and consisted of pyknotic and fragmented nuclei indicating necrosis, collectively suggesting the presence of a necrotizing bronchopneumonia. Moreover, the livers from these challenged mock-vaccinated mice also displayed foci of cellular infiltration, with mononuclear and polymorphonuclear cells and fragmented nuclei indicating necrotic changes, both within the parenchyma and around the hepatic vasculature (Panel d). All of the mock-vaccinated mice succumbed to the infection by approximately five days post-challenge as shown previously (Fig. 3A). In comparison, KKF235 vaccinated and challenged mice displayed milder inflammatory changes in both the lungs and the livers. Specifically, at three days after i.n. WT (U112) challenge, lungs from KKF235 vaccinated mice displayed foci of peribonchiolar and perivascular inflammatory infiltration, which were relatively smaller than those found in the mock-vaccinated animals, and were composed predominantly of mononuclear lymphocytes (Panel f). Additionally, the bronchiolar lumen was clear and did not display the severity of inflammatory changes seen when compared with similarly challenged mock-immunized animals. The livers from KKF235 vaccinated and challenged mice on day 3 displayed small foci of inflammatory cellular infiltration in the parenchyma and around the hepatic vasculature, which were composed predominantly of mononuclear lymphocytes and displayed minimal fragmented nuclei and necrotic changes (Panel h).
Fig. 5.
Intranasal vaccination with KKF235 (106 CFU) induces protection against pulmonary and liver histopathological changes following F novicida U112 challenge. Lungs and livers were removed from BALB/c mice, prepared for histological study, and stained with hematoxylin and eosin. Inflammatory cells (black arrows) were evident in the lungs and livers of mock-vaccinated U112 challenged mice (day 3), but reduced in KKF235-vaccinated and challenged mice (days 3, 15, and 30). Bronchioles (B); Vasculature (V).
Since vaccinated mice were completely protected and did not succumb to the infection, we also examined the histopathology in the lungs and livers of these animals at 15 and 30 days after pulmonary U112 challenge. It was found that the inflammatory changes observed in both the lungs (Panel i) and livers (Panel k) on day 15 were similar to those at day 3 after KKF235 vaccination and challenge, with a predominance of lymphocytes and minimal indications of necrosis. At day 30 after challenge, the lungs (Panel j) of KKF235 vaccinated mice appeared normal with minimal pathological changes, and the inflammation had largely resolved in the liver (Panel l) with the exception of a few pin-point foci of lymphocytic infiltrates seen within the parenchyma. These results suggest that vaccination with the F. tularensis ΔiglB strain induces robust cellular immune responses early after i.n. U112 challenge, thereby effectively controlling the infection and promoting survival.
3.4 Vaccination with KKF235 protected mice against heterotypic pulmonary LVS and Type A SCHU S4 challenge
It has been documented that LVS (an attenuated strain derived from Type B) immunization can protect against Type A Francisella infection [7, 25, 33, 34]. However, there is no reported study concerning cross subspecies protection induced by vaccination with attenuated subsp. novicida strains. We set out to evaluate the ability of vaccination with KKF235 to confer protective heterotypic immunity against LVS. BALB/c mice were vaccinated i.n. with KKF235 (106 CFU) and challenged i.n. with a lethal dose (3×104 CFU ∼10 LD50) of LVS. As shown in Fig. 6, mice immunized with KKF235 were completely (100% survival) protected from subsequent heterotypic pulmonary LVS challenge. In contrast, mock (PBS) vaccinated mice, succumbed to the infection by day 9. However, a similar i.n. vaccination strategy did not provide protection against the highly virulent Type A strain (data not shown), suggesting that i.n. priming may not be sufficient to control the rapid replication and dissemination of this organism from the respiratory compartment. To this end, we have recently reported [24] that oral LVS vaccination significantly induced mucosal immunity through preferential targeting to intestinal M-cells and protected mice against lethal Type A SCHU S4 pulmonary challenge. Since this route of vaccination seemed more efficacious in inducing protective respiratory responses to Type A challenges, we further examined the ability of oral KKF235 vaccination to induce heterotypic immunity against SCHU S4. Mice were orally vaccinated (103 CFU) with KKF235, rested for three weeks and challenged i.n. with ∼ 25 or 50 LD50 of SCHU S4. It was found that oral KKF235 vaccination conferred a protective response inducing 67% protection in mice challenged i.n. with 25 CFU of SCHU S4 (Fig. 7A). This protection was titratable and observed to wane (10%) upon doubling (52 CFU) of the i.n. challenge inoculum. To determine if the efficacy of KKF235 vaccination could be enhanced by an additional vaccination dose, mice were vaccinated orally, rested for 3 weeks, given an additional oral boost and subsequently challenged after 3 weeks. As shown in Fig. 7A, mice orally primed and boosted (P+B) with an additional dose of KKF235 exhibited a 40% survival to the higher dose i.n. SCHU S4 challenge (52 CFU). As expected, all mock vaccinated (PBS) mice succumbed to SCHU S4 challenge (25 CFU) by day 6.
Fig. 6.
Protective efficacy of KKF235 immunization against heterologous LVS challenge. BALB/c mice received a single immunizing dose (106 CFU) of KKF235 or PBS (mock control). Mice were rested for 30 days and then challenged i.n. with 3×104 CFU of LVS (∼10 LD50). Mice were then monitored for survival. Difference in survival between KKF235- and mock- vaccinated mice was significant at a p<0.001.
Fig. 7.
Protective efficacy of KKF235 immunization against heterologous Type A SCHU S4 challenge. C57BL/6 mice were immunized orally with a single priming (P) dose (106 CFU) or a prime plus booster dose (P+B) of KKF235, or mock immunized with PBS. Mice were rested for 3 weeks and then challenged i.n. with 25 or 52 CFU of SCHU S4. Mice were monitored for survival (A). Difference in survival between KKF235 primed and mock- vaccinated mice when challenged with 25 CFU of SCHU S4 was significant at a p<0.005. Spleen cells from KKF235-primed mice were prepared for IFN-γ recall assay using UV-inactivated KKF235 or HEL (an unrelated antigen) as the stimulus (B). Production of antigen specific intestinal (C) and serum (D) antibodies in the KKF235 primed or primed+boosted (P+B) mice were measured from fecal or blood samples, respectively. Respiratory antibodies (E) in BAL fluid collected from KKF235 vaccinated mice. Differences in antibody binding in sera between KKF235 and mock-vaccinated mice (titer < 100; data not shown) were significant at a p value of <0.01. Differences in antibody binding between KKF235- and mock-vaccinated mice were significant at a p value of <0.0001 for intestinal IgA, and all measured respiratory antibodies except IgM. Levels of intestinal IgA and total antibody between primed and primed plus boosted mice also were significant at a p value of <0.0001.
We further analyzed cellular and humoral immune profiles induced by oral KKF235 vaccination. Mice orally vaccinated with KKF235 induced high amounts of antigen-specific IFN-γ production in isolated spleenocytes, but not with the unrelated antigen HEL (Fig. 7B). In contrast, there was negligible induction of IFN-γ production in cells from mock vaccinated (PBS) mice (data not shown). These data suggest that antigen specific cell mediated responses can be induced following oral KKF235 vaccination in mice. To measure mucosal and serum antibody following oral KKF235 immunization, fecal pellets and sera were collected at day 21 after immunization. Mice receiving a single immunizing dose of KKF235 induced significant amounts of antigen specific intestinal IgA (Fig. 7C) and serum antibodies (Fig. 7D). There were comparable levels of IgG1 and IgG2a produced by a single oral immunization dose. In contrast, mice primed and boosted (P+B) with KKF235, exhibited higher levels of secretory IgA and serum antibodies (Fig. 7C and D). Oral KKF235 immunization also resulted in significant induction of antibody production in the respiratory compartment as shown in Fig. 7E. Orally vaccinated mice exhibited increased total antibody responses including the induction of IgG1, IgG2a, IgA, and to a lesser extend IgM. These results collectively suggest that vaccination with KKF235 induces systemic and mucosal immunity and provides cross protective immunity across distinct Francisella subspp.
4. Discussion
The biological function of IglB is still unknown. However, the tandem arrangement of iglAB genes in many pathogenic bacteria, its location within a secretion system cluster, and the physical interaction of the two proteins have prompted de Burin et. al [16] to propose that IglA and IglB may function as chaperon-like proteins. An iglB transposon mutant of F. novicida has been reported to have defective growth within macrophages, however, the potential polar effect of expression of the immediate downstream IglC protein in that strain is not known [20]. IglC is highly upregulated after the bacteria are phagocytosed by macrophages and is required for bacterial escape from the phagosomes [19, 20, 35]. In this study, we used a non-polar F. novicida iglB mutant (KKF235), which exhibited normal expression of the IglC protein by Western analysis [23], and analyzed the protective efficacy against homotypic and heterotypic Francisella pulmonary challenge.
KKF235 was found to replicate significantly less within J774 macrophages and exhibited an i.n. LD50 dose of at least a million fold higher than the respective wild type U112 strain, indicating a high degree of attenuation. Interestingly, although KKF235 was attenuated, it did disseminate from the lungs to the liver and spleen and was detectable up to 15 days following i.n. inoculation. This extended presence of replicating organisms in vaccinated mice may have been beneficial in adequately priming cellular and humoral responses to confer protective immunity against subsequent pulmonary challenge.
To this end, intranasal vaccination strategies have the advantage of directly targeting the respiratory compartment and hence inducing protective immunity at the primary site of infection. Indeed, reports have shown that aerosol/i.n. vaccination with LVS resulted in better protection than scarification/subcutaneous vaccination against pulmonary Type A challenge in mice, monkeys, and humans [33, 34, 36]. Our results showed that i.n. vaccination with KKF235 fully protected mice against homologous novicida U112, as well as heterologous LVS, respiratory challenge. Robust humoral responses, which consisted of mixed IgG1 and IgG2a serum and respiratory antibody production, as well as antigen-specific IFN-γ induction, was evident after i.n. KKF235 vaccination. The protection conferred by KKF235 vaccination likely may involve both cellular and humoral components, as previously seen with another defined novicida strain (ΔiglC), and include Fc-receptor dependent and independent mechanisms via the participation of IFN-γ producing cells such as NK and T cells [13, 37-39]. To this end, the protective effects were shown to be highly dependent on endogenous IFN-γ production, which may have played a major role in containment of bacteria within the lungs, resulting in minimal systemic dissemination and reduced inflammatory pathology observed in vaccinated animals.
Oral immunization is another effective route to induce both systemic and mucosal immunity. We [24] and others [25] have shown that oral vaccination with LVS protected mice against i.n. F. tularensis Type A SCHU S4 challenge and that protective immunity was mediated cooperatively by CD4+ T cells and antibody, including IgA. Oral KKF235 vaccination induced robust antigen specific cell-mediated and humoral immunity and conferred protection against respiratory SCHU S4 challenge after 3-4 weeks. However, the protective window of long term immunity induced by KKF235 vaccination may wane with time as observed by us [24], and others [40, 41], after LVS immunization. The protective efficacy against SCHU S4 challenge by oral KKF235 immunization, however, could be enhanced by an additional vaccination boost, which may be due to enhanced systemic and mucosal antibody production. This, to our knowledge, is the first report of a defined novicida vaccine strain being able to provide protective immunity against a human virulent Francisella species, which strongly indicates the presence of conserved protective antigens.
The virulence of different F. tularensis subspecies in humans varies; the subsp. tularensis (Type A) is the most virulent while subsp. novicida appears to be essentially avirulent in humans. However, gene homology among these subsp. is high (more than 98%) [8], and immunization with LVS (derived from subsp. holarctica) provides cross subsp. protection against the Type A strain in experimental murine models and human volunteers [7, 42]. Moreover, F. novicida behaves similar to subsp. tularensis within human macrophages [9, 10], and exhibits a high degree of virulence similar to subsp. tularensis via the i.n. route in mice [29, 43]. There also are notable differences between the different subspecies, such the LPS of subsp. novicida being more stimulatory [44-46] and the reported phase variation in the LPS of the Type A and B strains, which is not observed in novicida [44]. Given these differences, we have shown that vaccination with a defined novicida strain can protect animals from a subsequent heterologous LVS challenge and to a lesser degree to Type A challenge. Although the margin of protection by oral KKF235 immunization is within a narrow window (25-52 CFU), these analyses provide pivotal insight into the utility of developing similar defined genetic mutants within the Type A and B strains. Using a recently developed targetron transformation system [47], the ability to knock out genes with multiple copies in Francisella can now be applied to generate iglB mutants in Type A and Type B strains for further evaluation as vaccine candidates against the highly virulent wild type human strains.
Acknowledgment
This project has been funded in part with Federal funds from the National Institute of Allergies and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under Contract No. HHSN266200500040C and Grant PO1 AI057986.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Ellis J, Oyston PC, Green M, Titball RW. Tularemia. Clin Microbiol Rev. 2002;15(4):631–46. doi: 10.1128/CMR.15.4.631-646.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Tarnvik A. Nature of protective immunity to Francisella tularensis. Rev Infect Dis. 1989;11(3):440–51. [PubMed] [Google Scholar]
- [3].Sjostedt A. Francisella. In: Brenner DJ, Staley JT, Garrity GM, editors. Bergey’s Manual of Systematic Bacteriology. The Proteobacteria, PartB. 2nd ed. Springer; New York, NY: 2005. pp. 200–10. [Google Scholar]
- [4].Titball RW, Johansson A, Forsman M. Will the enigma of Francisella tularensis virulence soon be solved? Trends Microbiol. 2003;11(3):118–23. doi: 10.1016/s0966-842x(03)00020-9. [DOI] [PubMed] [Google Scholar]
- [5].Stuart BM, Pullen RL. Tularemic pneumonia: review of American literature and report of 15 additional cases. Am J Medi Sci. 1945;210(2):223–36. [Google Scholar]
- [6].Dennis DT, Inglesby TV, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, et al. Tularemia as a biological weapon: medical and public health management. JAMA. 2001;285(21):2763–73. doi: 10.1001/jama.285.21.2763. [DOI] [PubMed] [Google Scholar]
- [7].Saslaw S, Eigelsbach HT, Prior JA, Wilson HE, Carhart S. Tularemia vaccine study. II. Respiratory challenge. Arch Intern Med. 1961;107:134–46. doi: 10.1001/archinte.1961.03620050068007. [DOI] [PubMed] [Google Scholar]
- [8].Rohmer L, Fong C, Abmayr S, Wasnick M, Freeman TJL, Radey M, et al. Comparison of Francisella tularensis genomes reveals evolutionary events associated with the emergence of human pathogenic strains. Genome Biol. 2007;8(6):R102. doi: 10.1186/gb-2007-8-6-r102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Clemens DL, Lee BY, Horwitz MA. Virulent and avirulent strains of Francisella tularensis prevent acidification and maturation of their phagosomes and escape into the cytoplasm in human macrophages. Infect Immun. 2004;72(6):3204–17. doi: 10.1128/IAI.72.6.3204-3217.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Santic M, Molmeret M, Abu KY. Modulation of biogenesis of the Francisella tularensis subsp. novicida-containing phagosome in quiescent human macrophages and its maturation into a phagolysosome upon activation by IFN-gamma. Cell Microbiol. 2005;7(7):957–67. doi: 10.1111/j.1462-5822.2005.00529.x. [DOI] [PubMed] [Google Scholar]
- [11].Frank DW, Zahrt TC. Genetics and genetic manipulation in Francisella tularensis. Ann N Y Acad Sci. 2007;1105:67–97. doi: 10.1196/annals.1409.008. [DOI] [PubMed] [Google Scholar]
- [12].Pammit MA, Raulie EK, Lauriano CM, Klose KE, Arulanandam BP. Intranasal vaccination with a defined attenuated Francisella novicida strain induces gamma interferon-dependent antibody-mediated protection against tularemia. Infect Immun. 2006;74(4):2063–71. doi: 10.1128/IAI.74.4.2063-2071.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Powell HJ, Cong Y, Yu JJ, Guentzel MN, Berton MT, Klose KE, et al. CD4+ T cells are required during priming but not the effector phase of antibody-mediated IFN-gamma-dependent protective immunity against pulmonary Francisella novicida infection. Immunol Cell Biol. 2008;86(6):515–22. doi: 10.1038/icb.2008.31. [DOI] [PubMed] [Google Scholar]
- [14].Nano FE, Zhang N, Cowley SC, Klose KE, Cheung KK, Roberts MJ, et al. A Francisella tularensis pathogenicity island required for intramacrophage growth. J Bacteriol. 2004;186(19):6430–6. doi: 10.1128/JB.186.19.6430-6436.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ludu JS, de Bruin OM, Duplantis BN, Schmerk CL, Chou AY, Elkins KL, et al. The Francisella pathogenicity island protein PdpD is required for full virulence and associates with homologues of the type VI secretion system. J Bacteriol. 2008;190(13):4584–95. doi: 10.1128/JB.00198-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].de Bruin OM, Ludu JS, Nano FE. The Francisella pathogenicity island protein IglA localizes to the bacterial cytoplasm and is needed for intracellular growth. BMC Microbiol. 2007;7:1. doi: 10.1186/1471-2180-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Barker JR, Klose KE. Molecular and genetic basis of pathogenesis in Francisella tularensis. Ann N Y Acad Sci. 2007;1105:138–59. doi: 10.1196/annals.1409.010. [DOI] [PubMed] [Google Scholar]
- [18].Nano FE, Schmerk C. The Francisella pathogenicity island. Ann N Y Acad Sci. 2007;1105:122–37. doi: 10.1196/annals.1409.000. [DOI] [PubMed] [Google Scholar]
- [19].Golovliov I, Sjostedt A, Mokrievich A, Pavlov V. A method for allelic replacement in Francisella tularensis. FEMS Microbiol Lett. 2003;222(2):273–80. doi: 10.1016/S0378-1097(03)00313-6. [DOI] [PubMed] [Google Scholar]
- [20].Gray CG, Cowley SC, Cheung KK, Nano FE. The identification of five genetic loci of Francisella novicida associated with intracellular growth. FEMS Microbiol Lett. 2002;215(1):53–6. doi: 10.1111/j.1574-6968.2002.tb11369.x. [DOI] [PubMed] [Google Scholar]
- [21].Mougous JD, Cuff ME, Raunser S, Shen A, Zhou M, Gifford CA, et al. A virulence locus of Pseudomonas aeruginosa encodes a protein secretion apparatus. Science. 2006;312(5779):1526–30. doi: 10.1126/science.1128393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Pukatzki S, Ma AT, Sturtevant D, Krastins B, Sarracino D, Nelson WC, et al. Identification of a conserved bacterial protein secretion system in Vibrio cholerae using the Dictyostelium host model system. Proc Natl Acad Sci U S A. 2006;103(5):1528–33. doi: 10.1073/pnas.0510322103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Liu J, Zogaj X, Barker JR, Klose KE. Construction of targeted insertion mutations in Francisella tularensis subsp. novicida. Biotechniques. 2007;43(4):487–90. 92. doi: 10.2144/000112574. [DOI] [PubMed] [Google Scholar]
- [24].Ray HJ, Cong Y, Murthy AK, Selby DM, Klose KE, Barker JR, et al. Oral live vaccine strain-induced protective immunity against pulmonary Francisella tularensis challenge is mediated by CD4+ T cells and antibodies, including immunoglobulin A. Clinical and vaccine immunology: Clin Vaccine Immunol. 2009;16(4):444–52. doi: 10.1128/CVI.00405-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].KuoLee R, Harris G, Conlan JW, Chen WX. Oral immunization of mice with the live vaccine strain (LVS) of Francisella tularensis protects mice against respiratory challenge with virulent type A F. tularensis. Vaccine. 2007;25(19):3781–91. doi: 10.1016/j.vaccine.2007.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hamrick TS, Horton JR, Spears PA, Havell EA, Smoak IW, Orndorff PE. Influence of pregnancy on the pathogenesis of listeriosis in mice inoculated intragastrically. Infect Immun. 2003;71(9):5202–9. doi: 10.1128/IAI.71.9.5202-5209.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Shen H, Chen W, Conlan JW. Susceptibility of various mouse strains to systemically- or aerosol-initiated tularemia by virulent type A Francisella tularensis before and after immunization with the attenuated live vaccine strain of the pathogen. Vaccine. 2004;22(1718):2116–21. doi: 10.1016/j.vaccine.2003.12.003. [DOI] [PubMed] [Google Scholar]
- [28].Twine SM, Shen H, Kelly JF, Chen W, Sjostedt A, Conlan JW. Virulence comparison in mice of distinct isolates of type A Francisella tularensis. Microb Pathog. 2006;40(3):133–8. doi: 10.1016/j.micpath.2005.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Pammit MA, Budhavarapu VN, Raulie EK, Klose KE, Teale JM, Arulanandam BP. Intranasal interleukin-12 treatment promotes antimicrobial clearance and survival in pulmonary Francisella tularensis subsp. novicida infection. Antimicrob Agents Chemother. 2004;48(12):4513–9. doi: 10.1128/AAC.48.12.4513-4519.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Elkins KL, Cowley SC, Bosio CM. Innate and adaptive immune responses to an intracellular bacterium, Francisella tularensis live vaccine strain. Microbes Infect. 2003;5(2):135–42. doi: 10.1016/s1286-4579(02)00084-9. [DOI] [PubMed] [Google Scholar]
- [31].Elkins KL, Cowley SC, Bosio CM. Innate and adaptive immunity to Francisella. Ann N Y Acad Sci. 2007;1105:284–324. doi: 10.1196/annals.1409.014. [DOI] [PubMed] [Google Scholar]
- [32].Collins JT, Dunnick WA. Germline transcripts of the murine Immunoglobulin Gamma-2A gene - structure and induction by IFN-gamma. Intl Immunol. 1993;5(8):885–91. doi: 10.1093/intimm/5.8.885. [DOI] [PubMed] [Google Scholar]
- [33].Eigelsbach HT, Tulis JJ, Overholt EL, Griffith WR. Aerogenic immunization of the monkey and guinea pig with live tularemia vaccine. Proc Soci Exp Biol Med. 1961;108:732–4. doi: 10.3181/00379727-108-27049. [DOI] [PubMed] [Google Scholar]
- [34].Hornick RB, Eigelsbach HT. Aerogenic immunization of man with live tularemia vaccine. Bacteriol Rev. 1966;30(3):532–8. doi: 10.1128/br.30.3.532-538.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Golovliov I, Ericsson M, Sandstrom G, Tarnvik A, Sjostedt A. Identification of proteins of Francisella tularensis induced during growth in macrophages and cloning of the gene encoding a prominently induced 23-kilodalton protein. Infect Immun. 1997;65(6):2183–9. doi: 10.1128/iai.65.6.2183-2189.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Wu TH, Hutt JA, Garrison KA, Berliba LS, Zhou Y, Lyons CR. Intranasal vaccination induces protective immunity against intranasal infection with virulent Francisella tularensis biovar A. Infect Immun. 2005;73(5):2644–54. doi: 10.1128/IAI.73.5.2644-2654.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Bokhari SM, Kim KJ, Pinson DM, Slusser J, Yeh HW, Parmely MJ. NK cells and gamma interferon coordinate the formation and function of hepatic granulomas in mice infected with the Francisella tularensis live vaccine strain. Infect Immun. 2008;76(4):1379–89. doi: 10.1128/IAI.00745-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Fortier AH, Polsinelli T, Green SJ, Nacy CA. Activation of macrophages for destruction of Francisella tularensis: identification of cytokines, effector cells, and effector molecules. Infect Immun. 1992;60(3):817–25. doi: 10.1128/iai.60.3.817-825.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Lindgren H, Shen H, Zingmark C, Golovliov I, Conlan W, Sjostedt A. The resistance of Francisella tularensis strains against reactive nitrogen and oxygen species with special reference to the role of KatG. Infect Immun. 2007;75(3):1303–9. doi: 10.1128/IAI.01717-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Chen W, Shen H, Webb A, KuoLee R, Conlan JW. Tularemia in BALB/c and C57BL/6 mice vaccinated with Francisella tularensis LVS and challenged intradermally, or by aerosol with virulent isolates of the pathogen: protection varies depending on pathogen virulence, route of exposure, and host genetic background. Vaccine. 2003;21(2526):3690–700. doi: 10.1016/s0264-410x(03)00386-4. [DOI] [PubMed] [Google Scholar]
- [41].Conlan WJ, Shen H, Kuolee R, Zhao X, Chen W. Aerosol-, but not intradermal-immunization with the live vaccine strain of Francisella tularensis protects mice against subsequent aerosol challenge with a highly virulent type A strain of the pathogen by an alphabeta T cell- and interferon gamma- dependent mechanism. Vaccine. 2005;23(19):2477–85. doi: 10.1016/j.vaccine.2004.10.034. [DOI] [PubMed] [Google Scholar]
- [42].Eigelsbach HT, Downs CM. Prophylactic effectiveness of live and killed tularemia vaccines. I. Production of vaccine and evaluation in the white mouse and guinea pig. J Immunol. 1961;87:415–24. [PubMed] [Google Scholar]
- [43].Lauriano CM, Barker JR, Yoon SS, Nano FE, Arulanandam BP, Hassett DJ, et al. MglA regulates transcription of virulence factors necessary for Francisella tularensis intraamoebae and intramacrophage survival. Proc Natl Acad Sci U S A. 2004;101(12):4246–9. doi: 10.1073/pnas.0307690101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Cowley SC, Myltseva SV, Nano FE. Phase variation in Francisella tularensis affecting intracellular growth, lipopolysaccharide antigenicity and nitric oxide production. Mol Microbiol. 1996;20(4):867–74. doi: 10.1111/j.1365-2958.1996.tb02524.x. [DOI] [PubMed] [Google Scholar]
- [45].Kieffer TL, Cowley S, Nano FE, Elkins KL. Francisella novicida LPS has greater immunobiological activity in mice than F. tularensis LPS, and contributes to F. novicida murine pathogenesis. Microbes Infect. 2003;5(5):397–403. doi: 10.1016/s1286-4579(03)00052-2. [DOI] [PubMed] [Google Scholar]
- [46].Vinogradov E, Perry MB, Conlan JW. Structural analysis of Francisella tularensis lipopolysaccharide. Eur J Biochem. 2002;269(24):6112–8. doi: 10.1046/j.1432-1033.2002.03321.x. [DOI] [PubMed] [Google Scholar]
- [47].Rodriguez SA, Yu JJ, Davis G, Arulanandam BP, Klose KE. Targeted inactivation of Francisella tularensis genes by group II introns. Appl Environ Microbiol. 2008;74(9):2619–26. doi: 10.1128/AEM.02905-07. [DOI] [PMC free article] [PubMed] [Google Scholar]