Abstract
Tegument is a unique structure of herpesvirus, which surrounds the capsid and interacts with the envelope. Morphogenesis of gammaherpesvirus is poorly understood due to lack of efficient lytic replication for Epstein-Barr virus and Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8, which are etiologically associated with several types of human malignancies. Murine gammaherpesvirus 68 (MHV-68) is genetically related to the human gammaherpesviruses and presents an excellent model for studying de novo lytic replication of gammaherpesviruses. MHV-68 open reading frame 33 (ORF33) is conserved among Alpha-, Beta-, and Gammaherpesvirinae subfamilies. However, the specific role of ORF33 in gammaherpesvirus replication has not yet been characterized. We describe here that ORF33 is a true late gene and encodes a tegument protein. By constructing an ORF33-null MHV-68 mutant, we demonstrated that ORF33 is not required for viral DNA replication, early and late gene expression, viral DNA packaging or capsid assembly but is required for virion morphogenesis and egress. Although the ORF33-null virus was deficient in release of infectious virions, partially tegumented capsids produced by the ORF33-null mutant accumulated in the cytoplasm, containing conserved capsid proteins, ORF52 tegument protein, but virtually no ORF45 tegument protein and the 65-kDa glycoprotein B. Finally, we found that the defect of ORF33-null MHV-68 could be rescued by providing ORF33 in trans or in an ORF33-null revertant virus. Taken together, our results indicate that ORF33 is a tegument protein required for viral lytic replication and functions in virion morphogenesis and egress.
Gammaherpesviruses are associated with tumorigenesis. Like other herpesviruses, they are characterized as having two distinct stages in their life cycle: lytic replication and latency (15, 16, 18, 21, 54). Latency provides the viruses with advantages to escape host immune surveillance and to establish lifelong persistent infection and contributes to transformation and development of malignancies. However, it is through lytic replication that viruses propagate and transmit among hosts to maintain viral reservoirs. Both viral latency and lytic replication play important roles in tumorigenesis. The gammaherpesvirus subfamily includes Epstein-Barr virus (EBV), Kaposi's sarcoma-associated herpesvirus (KSHV)/human herpesvirus 8 and murine gammaherpesvirus 68 (MHV-68), among others. EBV is associated with Burkitt's lymphoma, nasopharyngeal carcinoma, Hodgkin's disease, and lymphoproliferative diseases in immunodeficient patients (28). KSHV is etiologically linked with Kaposi's sarcoma, primary effusion lymphoma, and multicentric Castleman's disease (11-13, 22, 52). Neither in vivo nor in vitro studies of EBV and KSHV are convenient due to their propensity to establish latency in cell culture and their limited host ranges.
MHV-68 is genetically related to these two human gammaherpesviruses, especially to KSHV, based on the alignment of their genomic sequences and other biological properties (55). As a natural pathogen of wild rodents, MHV-68 also infects laboratory mice (6, 40, 46) and replicates to a high titer in a variety of fibroblast and epithelial cell lines. These advantages make MHV-68 an excellent model for studying the lytic replication of gammaherpesviruses in vitro and certain aspects of virus-host interactions in vivo. In addition, the MHV-68 genome has been cloned as a bacterial artificial chromosome (BAC) that can propagate in Escherichia coli (1, 2, 36, 51), making it convenient to study the function of each open reading frame (ORF) by genetic methods. Exploring the functions of MHV-68 ORFs will likely shed light on the functions of their homologues in human gammaherpesviruses.
Gammaherpesviral particles have a characteristic multilayered architecture. An infectious virion contains a double-stranded DNA genome, an icosahedral capsid shell, a thick, proteinaceous tegument compartment, and a lipid bilayer envelope spiked with glycoproteins (14, 30, 47, 49). As a unique structure of herpesviruses, the tegument plays important roles in multiple aspects of the viral life cycle, including virion assembly and egress (38, 48, 53), translocation of nucleocapsids into the nucleus, transactivation of viral immediate-early genes, and modulation of host cell gene expression, innate immunity, and signal transduction (9, 10, 23, 60). Some components of MHV-68 tegument have been identified by a mass spectrometric study (8), and the functions of some tegument proteins have been revealed, such as ORF45, ORF52, and ORF75c (7, 24, 29).
MHV-68 ORF33 is conserved among Alpha-, Beta-, and Gammaherpesvirinae subfamilies. Its homologues include human herpes simplex virus type 1 (HSV-1) UL16, human herpes simplex virus type 2 (HSV-2) UL16, human cytomegalovirus (HCMV) UL94, EBV BGLF2, KSHV ORF33, and rhesus monkey rhadinovirus (RRV) ORF33. HSV-1 UL16 has been identified as a tegument protein and may function in viral DNA packaging, virion assembly, budding, and egress (5, 32, 35, 41, 44). HCMV UL94 is a virion associated protein and might function in virion assembly and budding (31, 57). EBV BGLF2, KSHV ORF33, and RRV ORF33 are also virion-associated proteins, but their functions are not clear (26, 43, 59). The mass spectrometric study of MHV-68 did not identify ORF33 as a virion component (8), although ORF33 is found to be essential for viral lytic replication by transposon mutagenesis of the MHV-68 genome cloned as a BAC (51). However, insertion of the 1.2-kbp Mu transposon in that study may influence the expression of ORFs approximate to ORF33. Consequently, the role ORF33 plays in viral replication needs to be confirmed, preferably through site-directed mutagenesis. Whether ORF33 is a tegument protein and the exact viral replication stage in which it functions also need to be investigated.
We determined that MHV-68 ORF33 encodes a tegument protein and is expressed with true late kinetics. To explore the function of ORF33 in viral lytic phase, we used site-directed mutagenesis and generated an ORF33-null mutant, taking advantage of the MHV-68 BAC system. We showed that the ORF33-null mutant is capable of viral DNA replication, early and late gene expression, capsid assembly, and DNA packaging, but incapable of virion release. The defect of ORF33-null mutant can be rescued in trans by an ORF33 expression plasmid.
MATERIALS AND METHODS
Cells, viruses, and plaque assays.
BHK-21 cells and 293T cells were grown in Dulbecco modified Eagle medium (DMEM) containing 10% fetal bovine serum. Wild-type (WT) MHV-68 was originally obtained from Ren Sun (University of California, Los Angeles), and the working stock was generated by infecting BHK-21 cells at a multiplicity of infection (MOI) of 0.02 PFU per cell. For the generation of WT MHV-68 BAC (WT BAC) virus and the revertant of ORF33-null mutant (33STOP.R), 293T cells were transfected with WT BAC or 33STOP.R DNAs by the calcium phosphate method. At 5 days posttransfection, the supernatant was harvested and cleared of large cellular debris by centrifugation (1,500 × g, 15 min, 4°C). To infect BHK-21 cells, the viral inoculum in DMEM was incubated with cells for 1 h with occasional swirling. The inoculum was removed and replaced with fresh DMEM plus 10% fetal bovine serum. For experiments involving cycloheximide (CHX; Sigma), cells were treated at a concentration of 100 or 200 μg/ml at 1 h prior to, during, and after viral inoculation until they were harvested. For experiments using phosphonoacetic acid (PAA; Sigma), cells were treated at a concentration of 200 μg/ml after viral inoculation until they were harvested.
The titer of WT MHV-68 was measured by a plaque assay, as described previously (58). Briefly, monolayer of BHK-21 cells were infected with virus for 1 h and overlaid with 1% methylcellulose (Sigma) for 5 days. Plaques were then counted to determine the titer.
RNA extraction and Northern blotting analysis.
Total RNA was extracted from BHK-21 cells infected with MHV-68 at an MOI of 5 at different time points using TRIzol reagent (Invitrogen). A 15-μg portion of each RNA sample was denatured and then separated on a 1.2% agarose gel containing 2.2 M formaldehyde in the MOPS buffer (20 mM morpholinepropanesulfonic acid [MOPS], 2 mM sodium acetate, and 1 mM EDTA). A RL6000 RNA ladder (Takara) was loaded onto the gel as the size standards. RNAs on the gel were transferred to a charged nylon membrane (Amersham Pharmacia Biotech). The membrane was UV cross-linked, prehybridized, and hybridized at 65°C in 0.5 M K2HPO4 (pH 7.2) containing 7% sodium dodecyl sulfate (SDS), 1 mM EDTA, 1% bovine serum albumin, and 50 μg of salmon sperm DNA/ml. The probe for ORF33 was generated by a digoxigenin (DIG) kit using DIG deoxynucleoside triphosphates (dNTPs), with a 0.8-kb fragment of MHV-68 genomic DNA (nucleotides [nt] 49691 to 50503) as a template. The probe for 28S rRNA was generated by end labeling DNA oligonucleotides with [α-32P]dCTP. The membrane was then washed at 65°C with 30 mM sodium citrate tribasic dehydrate (pH 7.0) containing 0.3 M NaCl and 0.1% SDS once, followed by washing with 1.5 mM sodium citrate tribasic dehydrate (pH 7.0) containing 15 mM NaCl and 0.1% SDS. Chemiluminescence was detected by adding CSPD onto the membrane, which was then exposed to an X-ray film. Radioactivity was detected with a Typhoon imaging system. Before rehybridization with a different probe, the membrane was stripped at 37°C with 0.2 M NaOH containing 0.1% SDS for 15 min twice to remove the previous probe.
Plasmid construction.
The full-length MHV-68 ORF33 sequence was amplified by PCR using the primers 33-01 and 33-02 (Table 1) and was cloned into pET-30b(+) (Novagen) via the KpnI and XhoI sites to construct pET-30b-33 for expression of His-tagged ORF33 in E. coli strain Rosetta (DE3).
TABLE 1.
Primers used in this study
| Primer | Sequence (5′ to 3′) | Positiona |
|---|---|---|
| 33-01 | CAGGTACCGCAACCAAAGAAGATATAAT | 49592 |
| 33-02 | ATCTCGAGTCAACAAGAATGTAAGTGTG | 50553 |
| 33-03 | TAGGAATTCGGATGGCAACCAAAGAAGATATAA | 49589 |
| 33-04 | TGGCTTAAGTACCATGGACTACAAAGACGA | |
| 33-05 | TCACTCGAGGAGGAGGAGGATCCGGAGGAGGAGGATCCATGGCAACCAAAGAAGATAT | 49589 |
| 33-06 | TATGGTACCCGCCACATCACCACTTTGTAC | |
| 33-07 | GTGCATGCATCCACGCTGTTCAAAATAGATT | 49109 |
| 33-08 | GCAGGATCCTCAATTAATCAATGTAAAGACCTTCTTTCTGC | 49664 |
| 33-09 | TACATTGATTAATTGAGGATCCTGCACCACGTCCATTTCA | 49685 |
| 33-10 | GGTGTGCATGCTACCCATCTCCAGTAGCATCAG | 50244 |
| 33-11 | GTGCATGCATACCTCAGTCCCACCCGTCA | 48670 |
| 33-12 | GGTGTGCTAGCAAGAACATCGCCCACCAGC | 51609 |
| 65-01 | GTCAGGGCCCAGTCCGTA | 94119 |
| 65-02 | TGGCCCTCTACCTTCTGTTGA | 94165 |
5′ nucleotide position on the MHV-68 genome (U97553.2). Boldfacing indicates stop codons, and italics indicate restriction enzyme sites.
Plasmid pHA-33 was constructed by inserting the full-length MHV-68 ORF33 sequence, amplified using the primers 33-02 and 33-03 (Table 1), into the EcoRI and XhoI sites of the vector pCMV-HA (Clontech).
Plasmid pFLAG-CMV2-33 was constructed by releasing the full-length MHV-68 ORF33 sequence from pET-30b-33 through BglII and XhoI sites and inserting it into the BglII and SalI sites of pFLAG-CMV2 (Sigma). The sequence coding for a FLAG epitope and ORF33 was amplified by using the primers 33-02 and 33-04 (Table 1) and inserted into the vector pcDNA5/TO (Invitrogen) at the AflII and XhoI sites to construct pFLAG-33.
Plasmid pEGFP-33 was constructed by inserting the full-length MHV-68 ORF33 sequence into the XhoI and KpnI sites of pEGFP-C1 (Clontech) with the primers 33-05 and 33-06 (Table 1) to express the green fluorescent protein (GFP)-33 fusion protein.
Construction of recombinant MHV-68 (BAC).
Recombinant MHV-68 BAC plasmids were generated by allelic exchange in E. coli, using procedures described previously (25). BAC sequence was inserted between nt 1838 and 1839 of the viral genome, without disrupting any known genes (7, 25). To construct the shuttle plasmid for generation of ORF33-null MHV-68 BAC (33STOP), a 1.2-kb fragment spanning ORF33 and the flanking sequences was generated by a two-step PCR using the primers 33-07, 33-08, 33-09, and 33-10 (Table 1), in which triple stop codons including a VspI site were introduced into ORF33 (nt 49684 to 49685). The 1.2-kb fragment was cloned into the NsiI and SphI sites of ampicillin (Amp)-resistant plasmid pGS284, harbored in E. coli GS111, and used as the donor strain for allelic exchange with the recipient strain GS500 (recA+) harboring chloramphenicol (Cam)-resistant MHV-68 (BAC). Cointegrates were selected by growth in the presence of Cam (34 μg/ml) and Amp (50 μg/ml), resolved by overnight growth in the presence of Cam alone to ensure the maintenance of the MHV-68 (BAC), and negatively selected against retention of the shuttle plasmid on the LB+Cam plates lacking NaCl, supplemented with 5% sucrose. Colonies were screened for Cam resistance and Amp sensitivity, and the incorporation of stop codons in ORF33 was determined by PCR with the primers 33-07 and 33-10 (Table 1), followed by VspI digestion. To generate the WT revertant of 33STOP (33STOP.R), a 3-kb fragment spanning ORF33 and the flanking sequences was cloned into pGS284 using primers 33-11 and 33-12 (Table 1), cointegrated with GS500 harboring 33STOP as the recipient strain, and resolved.
The inserted DNA fragment in each construct was sequenced to confirm that there were no undesired mutations, deletions, or insertions in the MHV-68 genome sequence.
Southern blotting analysis of WT and recombinant MHV-68 (BAC) DNAs.
BAC DNAs for WT, 33STOP, and 33STOP.R clones were isolated by a plasmid midiprep kit (Qiagen) and Southern blotting was performed by use of a DIG kit (Roche). BAC DNAs were digested with VspI or BglII at 37°C overnight and electrophoresed on a 0.8% agarose gel. The gel was subjected to depurination, denaturation, and neutralization. DNA fragments in the treated gel were transferred to charged nylon membrane (Amersham Pharmacia Biotech), UV cross-linked, probed, and washed according to the manufacturer's protocol. Probes were generated by the DIG kit using DIG dNTPs, with a 1.2-kb fragment of MHV-68 genomic DNA (nt 49109 to 50265) as a template.
Transcomplementation assay.
293T cells seeded in 24-well culture plates were individually transfected with WT BAC, 33STOP, or 33STOP.R DNAs (600 ng/well) plus empty vector (200 ng/well) or 33STOP (600 ng/well) plus plasmid DNA expressing GFP-33 or hemagglutinin (HA)-33 (200 ng/well), by the calcium phosphate method. At the indicated times posttransfection, the supernatant was collected and cleared of debris by centrifugation at 1,500 × g for the plaque assay. Viral DNA in an aliquot of the supernatant was isolated and subjected to a real-time PCR assay to determine the number of released viral genome copies. The cells were harvested to analyze viral lytic protein expression and viral DNA replication.
Determining the titer of 33STOP virus with a reporter cell line.
To determine the titer of ORF33-null MHV-68 generated from transcomplementation, we took advantage of the fact that the promoter of MHV-68 early gene ORF57 can be transactivated by the viral immediate-early protein RTA expressed during viral infection (45) and generated a reporter cell line as follows. LacZ driven by the promoter of MHV-68 ORF57 was cloned into vector pEGFP-C1 upstream of the CMV promoter driving GFP. The resulting plasmid was designated ORF57pLacZ. BHK-21 cells were transfected with ORF57pLacZ using jetPEI (Polyplus) according to the manufacturer's recommendations. At 24 h posttransfection, cells were passaged and selected for stable transformants in medium containing G418 (600 μg/ml). Medium was changed every 4 days until stable cell colonies were observed. Cell colonies were transferred to 24-well plates and expanded. Positive transformants were screened by infection with MHV-68, followed by X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) staining. Positive transformants would express LacZ proteins after MHV-68 infection and turn blue upon X-Gal staining. To determine the titer of 33STOP virus preparation, supernatant was harvested from cells cotransfected with 33STOP and HA-33 expression plasmid and incubated with reporter cells in a series of dilutions. The reporter cells were stained with X-Gal 12 h later, and blue cells were counted to determine the number of infectious virions in the original supernatant.
Viral DNA isolation and analysis by quantitative real-time PCR.
In transcomplementation experiments, whole cellular DNAs of transfected 293T cells were isolated for analyzing viral DNA replication and viral DNAs were isolated from supernatants for analyzing virion release. To isolate the total cellular DNA, transfected cells were harvested, incubated with lysis buffer (10 mM Tris-HCl [pH 8.0], 100 mM NaCl, 25 mM EDTA, 0.5% SDS, 0.1 g of proteinase K/ml, and 25 μg of RNase A/ml) at 50°C overnight, and cell lysates were extracted by equal volume of phenol-chloroform-isoamyl alcohol (25:24:1) twice and chloroform-isoamyl alcohol (24:1) once. DNA was then precipitated by ethanol at room temperature and dissolved in Tris-EDTA buffer (pH 8.0). For extracellular virion DNA isolation, 200 μl of supernatant from each transfection was first treated with 10 μg of DNase I in 1× DNase I buffer (10 mM Tris-HCl [pH 7.5], 2.5 mM MgCl2, and 0.1 mM CaCl2) at 37°C for 30 min, heated at 70°C for 15 min to inactivate DNase I, and incubated with equal volume of 2× lysis buffer. The remaining procedure was similar to that of total cellular DNA isolation, except for precipitation of viral DNA at −20°C for 1 h. To examine viral DNA replication, BHK-21 grown in 12-well plates were infected with either WT BAC, HA-33-rescued 33STOP, or 33STOP.R viruses for 1 h at 4°C at an MOI of 100 genome copies per cell, and total cellular DNAs were isolated at 12, 24, 36, and 48 h postinfection according to the procedures described above. Triplicate real-time PCRs in 20-μl volumes, containing 20% of the extracted virion DNA, 25 ng of the total cellular DNA, or WT BAC DNA standards, were analyzed by using SYBR green and specific primers for ORF65 (65-01 and 65-02, Table 1) by an iCycler instrument (Bio-Rad).
Purification, trypsin, and detergent treatment of viral particles.
MHV-68 virions were purified from the supernatant of MHV-68-infected BHK-21 cell cultures by discontinuous sucrose density gradient ultracentrifugation as described previously (8). Briefly, supernatants were cleared of large debris twice by centrifugation at 4°C and 1,000 × g for 15 min. Extracellular viruses were pelleted by ultracentrifugation through a 7.5% sucrose cushion in a P28S rotor (Hitachi, 4°C, 65,000 × g, 1 h), resuspended in TN buffer (20 mM Tris [pH 7.8], 50 mM NaCl), and purified by 10 to 45% discontinuous sucrose density gradient ultracentrifugation in a P40T rotor (Hitachi, 4°C, 25,000 × g, 4.5 h) with slow acceleration and braking. Content in the bottom half of the tube was saved, and virions were pelleted by ultracentrifugation in a P40T rotor (Hitachi) at 4°C and 65,000 × g for 1 h. Intracellular viral particles were released from pelleted WT BAC/HA, 33STOP/HA or 33STOP/HA-33-cotransfected 293T cells at 4 days posttransfection by rapid liquid N2 freezing and 37°C thawing once in TN buffer. Particle preparations were cleared of insoluble debris by centrifugation at 3,500 × g and ultracentrifuged through a two-step 25 and 50% sucrose cushion at 42,000 × g at 4°C for 2 h. The visible band at the interface was collected, pelleted at 65,000 × g, and resuspended in TN buffer for SDS-polyacrylamide gel electrophoresis (PAGE) and Western blotting analyses. For trypsin and detergent treatment, 20 μl of virion stock was diluted into 200 μl of trypsin buffer (50 mM Tris-HCl [pH 7.5], 150 mM NaCl, 1 mM CaCl2) with or without 1% Triton X-100 supplementation, trypsin was added to a final concentration of 4 μg/ml, and the mixture was incubated at 37°C for 1 h. The reaction was stopped by adding 0.5 mM phenylmethylsulfonyl fluoride and protease inhibitors, and virions were collected by centrifugation at 20,000 × g for 30 min at 4°C.
Dual luciferase assay.
293T cells were seeded into 24-well plates a day before and cotransfected individually with 100 ng of firefly luciferase reporter plasmid driven by promoter of ORF57, ORF26, or ORF65, plus 2 ng of Renilla luciferase reporter plasmid as an internal control. At 24 h after transfection, transfected cells were infected with WT BAC, 33STOP, or 33STOP.R virus at 0.1 infectious unit/cell. At 24 h postinfection, cells were lysed in 100 μl of 1× passive lysis buffer (Promega), and 20 μl of the lysate was assayed for firefly and Renilla luciferase activities by using the luciferase assay system (Promega).
Antibodies, immunoblotting, and indirect immunofluorescence assay.
For generation of polyclonal antibody against MHV-68 ORF33 protein, expression of His-tagged ORF33 protein in log-phase E. coli strain Rosetta (DE3) was induced with IPTG (isopropyl-β-d-thiogalactopyranoside; 1 μg/ml) for 6 h at 30°C, bacteria were lysed by sonication, and His-tagged ORF33 protein was purified by Ni-NTA affinity column according to the manufacturer's instructions. Purified His-33 protein was used to immunize New Zealand White rabbits to generate polyclonal antisera. Anti-gB, anti-ORF26, anti-ORF45, anti-ORF52, anti-ORF65 and anti-MHV-68 sera were kind gifts from Ren Sun (University of California, Los Angeles). Mouse monoclonal antibody M2 against a FLAG epitope and mouse monoclonal antibody against an HA epitope were purchased from Sigma. For Western blotting, samples were heated to 95°C and subjected to SDS-PAGE. Proteins on gels were transferred onto a polyvinylidene difluoride membrane (Millipore) and incubated sequentially with primary antibody and secondary antibody (anti-rabbit or anti-mouse immunoglobulin G conjugated with horseradish peroxidase), and the proteins were detected by the enhanced chemiluminescence system (Millipore). For indirect immunofluorescence assay, 293T cells were transfected with FLAG-33 expression plasmid, applied onto slides, and fixed with 4% paraformaldehyde. The fixed cells were permeabilized with phosphate-buffered saline (PBS) plus 0.2% Triton X-100, incubated sequentially with mouse monoclonal anti-FLAG M2 (1:500) as the primary antibody and with secondary antibody conjugated to fluorescein isothiocyanate (1:100), stained with DAPI (1 μg/ml), and examined by using a confocal microscope (Olympus).
Transmission electron microscopy (TEM).
293T cells were transfected with WT BAC or 33STOP or cotransfected with 33STOP and HA-33 expression plasmid, collected at 4 days posttransfection in PBS, fixed in 2% glutaraldehyde (Sigma) in PBS at 4°C for 12 h, postfixed in 1% OsO4, dehydrated, and embedded in Epon. Approximately 70-nm thin sections were stained with 2% uranyl acetate and 0.3% lead citrate and examined with a 120- or 200-kV electron microscope (Tecnai; FEI).
RESULTS
MHV-68 ORF33 is expressed as a true late gene.
As a first step to characterize MHV-68 ORF33, we expressed and produced recombinant ORF33 protein in an E. coli system. A His6 tag was introduced into the amino terminus to facilitate detection and purification of the recombinant protein. The ORF33 protein expressed from E. coli was detected as a polypeptide of 39 kDa on SDS-PAGE (data not shown).
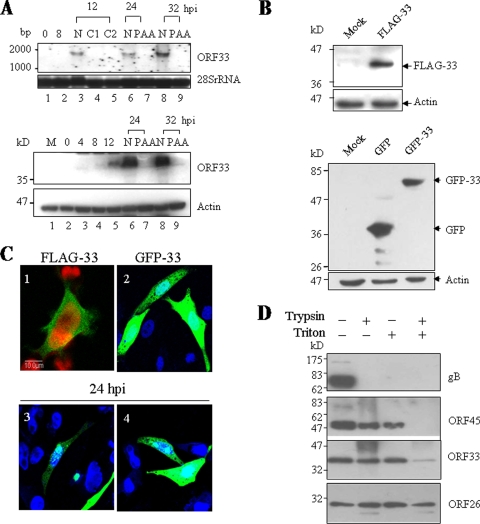
The purified His-tagged ORF33 was used to generate a rabbit polyclonal antibody. To test the titer and specificity of this antibody, 293T or BHK-21 cells infected with MHV-68 were examined by Western blotting analysis. A 36-kDa protein was detected in MHV-68-infected cells but not in mock-infected cells (data not shown). In contrast, no band could be detected from either MHV-68-infected or mock-infected cells by preimmune rabbit serum (data not shown). To determine the expression kinetics of ORF33 during MHV-68 de novo infection, BHK-21 cells were infected at an MOI of 5, and total RNAs were harvested at different time points postinfection. In addition, infections were carried out in the presence of 100 or 200 μg of CHX (a protein synthesis inhibitor)/ml or 200 μg of PAA (a herpesvirus DNA polymerase inhibitor)/ml. The ORF33 transcripts were analyzed by Northern blotting with a 0.8-kb PCR fragment spanning the middle of ORF33 (nt 49691 to 50503) as a probe. As shown in the upper panel of Fig. 1A, a band of 1.6-kb was detected at as early as 12 h postinfection and peaked at 32 h postinfection. However, transcripts of ORF33 could not be detected in the presence of either CHX or PAA. Expression of ORF33 was also analyzed at the protein level by Western blotting using the rabbit polyclonal antibody against ORF33. As shown in the lower panel of Fig. 1A, the amount of ORF33 protein increased till 32 h after infection; however, under 200-μg/ml PAA treatment, expression of ORF33 could not be detected at 24 h or 32 h after infection. Collectively, these results demonstrated that MHV-68 ORF33 is expressed as a true late gene.
FIG. 1.
Characterization of MHV-68 ORF33. (A) MHV-68 ORF33 is expressed as a true late gene. The upper panel shows a Northern blot analysis of ORF33 mRNA from MHV-68-infected BHK-21 cells using a probe for ORF33 coding region. Lanes 1 to 3, lane 6, and lane 8 show samples at 0, 8, 12, 24, and 36 h postinfection, respectively; lane 4, 12 h postinfection plus 100 μg of CHX/ml; lane 5, 12 h postinfection plus 200 μg of CHX/ml; lane 7, 24 h postinfection plus 200 μg of PAA/ml; and lane 9, 32 h postinfection plus 200 μg of PAA/ml. 28S rRNA was used as a loading control. The lower panel shows a Western blot analysis of the ORF33 protein from MHV-68-infected BHK-21 cells using anti-ORF33 polyclonal serum. Lane 1, mock infection; lanes 2 to 6 and lane 8: 0, 4, 8 12, 24, and 32 h postinfection, respectively; lane 7, 24 h postinfection plus PAA treatment; and lane 9, 32 h postinfection plus PAA. β-Actin was used as a loading control. (B) Expression of ORF33 in transfected cells. 293T cells were mock transfected or transfected with pFLAG-33 (the upper panel), pEGFP-C1 vector, or pEGFP-33 (the lower panel). Cells were collected at 48 h after transfection and subjected to Western blotting with anti-FLAG antibody or anti-GFP antibody. Actin was probed as a loading control. (C) Cellular localization of ORF33 in transfected cells. 293T cells were transfected with pFLAG-33 and then analyzed by indirect immunofluorescence assay (panel 1). BHK-21 cells were transfected with pEGFP-33 and then either mock infected (panel 2) or infected with MHV-68 at an MOI of 5 PFU/cell for 24 h (panels 3 and 4). Fluorescence was observed with a confocal laser microscope. (D) Effect of detergent treatment or trypsin digestion on the association of ORF33 with purified virions. The purified virions were treated with trypsin or detergent, as described in Materials and Methods. The tegument-nucleocapsid complexes were analyzed by Western blotting sequentially using anti-gB, anti-ORF45, anti-ORF33, and anti-ORF26 polyclonal antibodies.
MHV-68 ORF33 protein localizes in both the cytoplasm and the nucleus.
To further characterize MHV-68 ORF33, we constructed a plasmid that expresses MHV-68 ORF33 fused to the C terminus of GFP (pEGFP-33) and a plasmid that expresses MHV-68 ORF33 with a FLAG epitope at the N terminus (pFLAG-33). These plasmids were transfected into 293T cells, and protein expression was examined and confirmed by Western blotting with anti-GFP or anti-FLAG monoclonal antibody, respectively (Fig. 1B). To examine the subcellular localization of ORF33, indirect immunofluorescence analysis was performed with 293T cells transiently expressing FLAG-33. Detection with a confocal laser microscope indicated that FLAG-33 was dispersed evenly in both the cytoplasm and the nucleus (Fig. 1C, panel 1). To verify this subcellular location pattern of ORF33, we transfected pEGFP-33 into BHK-21 cells and observed the distribution of GFP-33 similar to that of FLAG-33 (Fig. 1C, panel 2). However, at 24 h after infection of transfected BHK-21 cells with MHV-68, the GFP-33 protein accumulated in some nuclear compartments (Fig. 1C, panels 3 and 4), implying that ORF33 may participate in MHV-68 replication at the step of primary envelopment.
MHV-68 ORF33 encodes a tegument protein.
MHV-68 ORF33 has a 30% amino acid sequence identity to its KSHV homologue (55), which has been identified as a component of purified KSHV virions by mass spectrometry (59). The HSV-1 homologue UL16, EBV homologue BGLF2, RRV homologue ORF33 and HCMV homologue UL94 are also tegument- or virion-associated proteins (26, 35, 41, 43, 57). Therefore, MHV-68 ORF33 is very likely to be a virion protein; however, a mass spectrometric analysis of purified MHV-68 virions did not report ORF33 (8). We thus decided to first determine whether ORF33 is associated with MHV-68 virions before we attempted to investigate the functional role of ORF33 in MHV-68 lytic-phase replication.
Extracellular virions were harvested from MHV-68-infected BHK-21 cell supernatant through gradient ultracentrifugation. Purified virions were lysed, resolved on SDS-PAGE, and analyzed by Western blotting, using the anti-ORF33 polyclonal antibody. A 36-kDa protein was detected in virion lysate (data not shown). This molecular mass is the same as that of ORF33 found in MHV-68-infected BHK-21 cell lysate. After we confirmed the association of ORF33 with purified viral particles, we then determined the trypsin and detergent sensitivity of ORF33 in virions and compared it to known capsid (ORF26), tegument (ORF45), and envelope proteins (glycoprotein B). Purified virions were mock treated or treated with trypsin either in the presence or in the absence of 1% Triton X-100 at 37°C for 1 h. The pelleted phase was analyzed by Western blotting sequentially using anti-gB, anti-ORF45, anti-ORF33, or anti-ORF26 polyclonal antibodies. As shown in Fig. 1D, glycoprotein B was sensitive to trypsin in both the presence and the absence of detergent, whereas ORF26 was not sensitive to trypsin at all. ORF33, as well as ORF45, could be partially degraded by trypsin in the absence of detergent; however, in the presence of detergent, ORF45 was removed completely by trypsin treatment, whereas residual ORF33 protein remained bound to the capsid. This result indicated that ORF33 is located within virions, as a component of the tegument. ORF45 is thought to be associated with both the capsid and the envelope glycoprotein (8). Here, our detergent sensitivity data strongly indicated that ORF33 is also associated with both the capsid and the envelope but binds more strongly to capsid than does ORF45.
As a tegument protein, ORF33 may play important roles in viral particle maturation and release or modulation of microenvironment in infected cells. We next performed a series of experiments to study the functional roles of this tegument protein.
Disruption of ORF33 in a MHV-68 BAC vector.
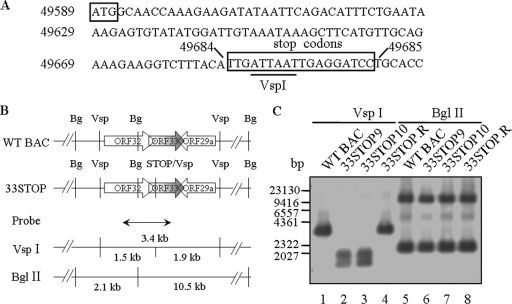
Although ORF33 was identified as essential for viral lytic replication by signature-tagged transposon mutagenesis (51), insertion of the 1.2-kbp Mu transposon may have disrupted the expression of genes proximal to the ORF33 locus. Therefore, to precisely study the role of ORF33 in MHV-68 lytic replication, we constructed an ORF33-null MHV-68 (BAC) mutant (33STOP) through site-specific mutagenesis. We inserted triple-frame translation termination codons between nt 49684 and 49685 on the viral genome (94 nt downstream of the translation start codon for ORF33) (Fig. 2A). A VspI site was also included in the triple stop codon sequences to facilitate screening of positive clones. 33STOP clones were selected as described in Materials and Methods and analyzed by VspI or BglII digestion, electrophoresis, ethidium staining (data not shown), and Southern blotting with a 1.2-kb probe corresponding to nt 49109 to 50264 on MHV-68 genome. The probe and predicted fragments from restriction enzyme digestion in the region of ORF33 and its flanking ORFs are shown in Fig. 2B. In VspI digests, one 3.4-kb band from WT MHV-68 BAC (WT BAC) was detected by the probe (Fig. 2C, lane 1), whereas two bands (1.5 and 1.9-kb) from two independent clones of 33STOP (33STOP9 and 33STOP10) were detected by the probe (Fig. 2C, lanes 2 and 3). In BglII digests, the two independent clones of 33STOP generated two bands (2.1 and 10.5-kb) as detected by the probe, exhibiting the same pattern as WT BAC (Fig. 2C, lanes 5, 6, and 7). No other rearrangement was detected in these two clones of 33STOP compared to WT BAC. We thus concluded that the tripe stop codon mutation was successfully inserted into the MHV-68 ORF33 region. For all further experiments, we chose to use 33STOP10 (hereafter referred simply as 33STOP). A revertant of 33STOP, or simply 33STOP.R, was generated from 33STOP by allelic exchange, replacing the ORF33-STOP with a WT copy of ORF33. In restriction enzyme digestion and Southern blotting analysis, 33STOP.R BAC exhibited same fragment patterns as WT BAC (Fig. 2C, lanes 4 and 8).
FIG. 2.
Construction and analysis of ORF33-null MHV-68 (BAC) DNAs. (A) Nucleotide sequence of the region containing the mutation in the ORF33-null MHV-68 mutant. The genome coordinates are given to the left of the nucleotide sequence and on top of the nucleotides between which mutations were introduced. The ORF33 ATG and the three inserted nonsense and frameshift mutations are boxed, and the introduced VspI site is underlined. (B) Schematic representation of the ORF33 locus and its flanking ORFs on the WT BAC or 33STOP genome, the position of a probe (nt 49109 to 50264), and the predicted restriction fragments detected by the probe in Southern blotting analysis. (C) Southern blotting analysis of WT BAC and 33STOP DNAs. Various BAC DNAs were digested with VspI or BglII as indicated at the top of the panel, electrophoresed, blotted, and hybridized with the DIG-labeled probe. Positions of a λ/HindIII DNA ladder run on the same gel are indicated to the left of the panel.
33STOP is deficient in MHV-68 lytic replication but can be rescued by expression of ORF33 in trans.
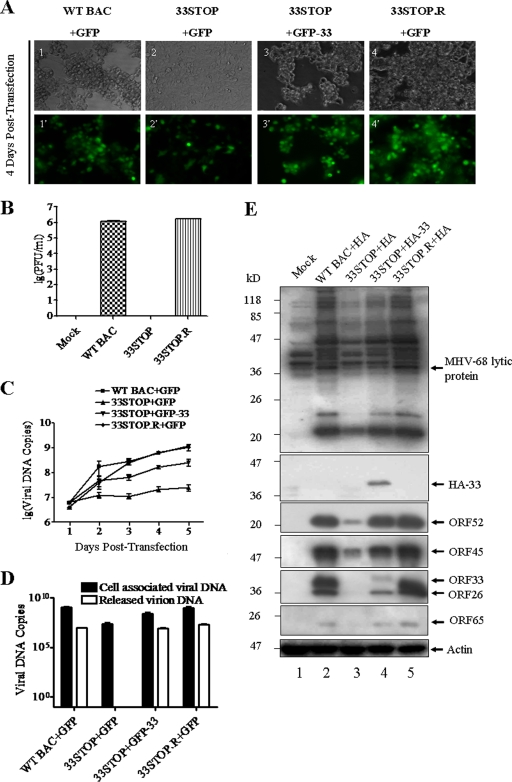
In order to explore whether ORF33 is required for MHV-68 lytic replication, we transfected WT BAC, 33STOP, or 33STOP.R into 293T cells and examined their ability to establish productive infection. Cells transfected with WT BAC or 33STOP.R plus the GFP expression vector showed a severe cytopathic effect (CPE) at 4 days posttransfection, whereas no obvious CPE was detected in cells transfected with 33STOP plus the GFP expression vector (Fig. 3A). To further verify that the deficiency of 33STOP in producing virus was caused by the null mutation of ORF33, we cotransfected 33STOP with the GFP-33 fusion expression plasmid to test complementation. Cells showed high levels of CPE and GFP expression at 4 days posttransfection (Fig. 3A, panels 3 and 3′), indicating that GFP-33 could successfully complement 33STOP in trans. To quantitate virus production, supernatants from each transfection were collected for plaque assays. As shown in Fig. 3B, titers of virus produced by WT BAC or 33STOP.R-transfection reached ca. 1.5 × 106 PFU/ml, but the supernatant from 33STOP transfection was unable to form plaques on susceptible BHK-21 cells. Two more rounds of passage of the supernatants harvested from 33STOP-transfected cultures did not result in the development of CPE, suggesting that no infectious virus was produced and released into the supernatant.
FIG. 3.
Deficiency in and transcomplementation of viral replication for ORF33-null mutant. (A) 293T cells were transfected with WT BAC plus plasmid pEGFP-C1 (panels 1 and 1′), 33STOP plus pEGFP-C1 (panels 2 and 2′), 33STOP plus pEGFP-33 (panels 3 and 3′), or 33STOP.R plus pEGFP-C1 (panels 4 and 4′). Cell morphology and GFP fluorescence were visualized under a fluorescence microscope at 4 days posttransfection, as indicated to the left. (B) Deficiency in infectious virus production by 33STOP. Supernatants from 293T cells transfected with various BAC DNAs plus pEGFP-C1 or pGFP-33 were collected at 4 days posttransfection. Virus titers were determined by plaque assays in BHK-21 cells in duplicates. Error bars represent standard deviations from three independent experiments. (C) 293T cells were transfected with various BAC DNAs plus pEGFP-C1 or pGFP-33. At the indicated time points, total cellular DNA was isolated and analyzed by a real-time PCR assay using primers specific to ORF65 sequences. The copy number of viral genomes was normalized to 25 ng of total cellular DNA. Error bars represent the standard deviations from three independent experiments. (D) The deficiency of 33STOP in virion release can be rescued in trans. Extracellular virions DNAs and total cellular DNAs were separately isolated from 293T cultures transfected with various BAC DNAs plus pEGFP-C1 or pEGFP-33 at 5 days posttransfection. The copy number of viral genomes was determined by a real-time PCR assay using primers specific to ORF65. The copy number of cell associated viral genomes was normalized to 25 ng of total cellular DNA, and the copy number of extracellular viral genomes was expressed as viral genome copies per 1 ml of supernatant. Error bars represent the standard deviations from three independent experiments. (E) 293T cells were individually transfected with WT BAC plus plasmid pCMV-HA, 33STOP plus pCMV-HA, 33STOP plus pHA-33, or 33STOP.R plus pCMV-HA. At 4 days posttransfection, the cells were lysed and probed by Western blotting with polyclonal anti-MHV-68 serum, anti-ORF26, anti-ORF65, anti-ORF33, anti-ORF45, or anti-ORF52 antibodies or a monoclonal anti-HA antibody. Actin was probed as a loading control.
To quantitate the efficiency of complementation, we harvested cell-associated viruses produced from 293T cells transfected with various combinations of BAC DNA and expression plasmid at different time points and assayed the viral genome copies by a real-time PCR assay using primers specific for ORF65. As shown in Fig. 3C, at 5 days posttransfection, the cell-associated viral genome copies from both WT BAC and 33STOP.R samples increased ∼200-fold, whereas viral DNA copies of 33STOP increased less than 5-fold. However, cotransfection with pEGFP-33 partially restored the viral lytic replication of 33STOP, as evidenced by a 40-fold increase in the copy number of cell-associated 33STOP DNAs. We also quantitated and compared the genome copies of both cell-associated and extracellular viral DNAs at 5 days posttransfection. As shown in Fig. 3D, the genome copy number of cell associated 33STOP was ∼50-fold lower than that of the WT BAC or 33STOP.R. No virus genome was detected in supernatant from 33STOP-transfected cell culture after DNase I treatment of supernatant, whereas the genome copy number of released WT BAC or 33STOP.R virions was more than 107. The results showed obvious deficiency of 33STOP in virion release compared to WT BAC and 33STOP.R. As expected, cotransfection of 33STOP with GFP-33 restored the release of 33STOP virions to a level comparable to that of WT BAC and 33STOP.R.
Since the size of GFP is relatively big for ORF33, we also tested the ability of an HA-tagged ORF33 expression plasmid to rescue 33STOP. As previously described, 293T cells were transfected with various BAC DNAs plus expression plasmids. Five days later, each group of transfected cells was harvested and subjected to Western blotting with polyclonal anti-MHV-68 serum, antisera to various virion proteins, or anti-HA monoclonal antibody. Fewer lytic antigens were detected in lysates from cells transfected with 33STOP than those transfected with WT BAC or 33STOP.R (Fig. 3E, lanes 2, 3, and 5). However, providing HA-33 in trans increased lytic antigens expression in 33STOP-transfected sample (Fig. 3E, lane 4).
In order to exclude the possibility that inadvertent reversion arose during transcomplementation assay, we used viruses from the supernatants generated in trans-complementation assay to infect fresh BHK-21 cells at an MOI of 500 genome copies per cell. BHK-21 cells infected with WT BAC or 33STOP.R showed severe CPE at 2 days postinfection, whereas BHK-21 cells infected with 33STOP complemented with GFP-33 or HA-33 did not exhibit obvious CPE (data not shown). To further examine whether homologous recombination occurred between 33STOP DNA and the GFP-33 or HA-33 expression plasmid, total cellular DNAs were isolated from BHK-21 cells infected with various virus preparations and analyzed by PCR, using primers specific for the probe fragment used for determining the insertion of triple stop codons previously. These PCR products were digested by VspI, and the resulting patterns were as predicted, indicating that no DNA recombination had occurred (data not shown). Therefore, the 33STOP virions were not generated from inadvertent reversion but from protein complementation. These results strongly indicate that 33STOP is deficient for viral lytic replication but can be rescued by providing ORF33 in trans (GFP-33 or HA-33).
ORF33 is not required for MHV-68 DNA replication.
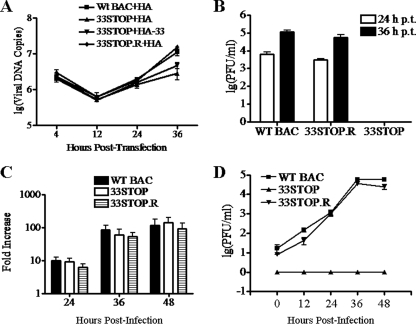
Like that of other characterized herpesviruses, lytic replication of MHV-68 occurs in a cascade fashion: immediate-early and early gene expression, DNA genome replication, late gene expression, and virion assembly and egress. In order to investigate at which step ORF33 is required for MHV-68 lytic replication, we first determined whether knocking out ORF33 would affect viral DNA replication during a single round of lytic replication. 293T cells were transfected with WT BAC plus pCMV-HA, 33STOP plus pCMV-HA, 33STOP plus pHA-33, or 33STOP.R plus pCMV-HA. At the indicated time points, total cellular DNAs were isolated and analyzed by real-time PCR. As shown in Fig. 4A, at 12 h posttransfection, a decrease in viral DNA copy number from all samples was observed. This decrease may have resulted from degradation of the viral genomes before their trafficking into cell nuclei. At 24 h posttransfection, 33STOP viral DNAs increased by similar fold, whether providing the HA-33 protein or not, compared to WT BAC and 33STOP.R viral DNAs. However, it was noted that at 36 h posttransfection, the copy number of WT BAC or 33STOP.R viral DNA copies was obviously higher than that of 33STOP DNA. The difference may be attributed to the deficiency of 33STOP in release of virions, effectively blocking second-round infection and the resulting further amplification of viral genomes observed in WT BAC and 33STOP.R-transfected cell cultures. However, the copy number of 33STOP viral DNA was higher in the presence of HA-33 protein, indicating that expression of HA-33 partially rescued the release of 33STOP virions.
FIG. 4.
ORF33 is not required for MHV-68 lytic DNA replication. (A) Analysis of viral DNA replication in viral genome transfection experiment. 293T cells were transfected with WT BAC plus pCMV-HA, 33STOP plus pCMV-HA, 33STOP plus pHA-33 or 33STOP.R plus pCMV-HA. At the indicated time points posttransfection, total cellular DNA was isolated and analyzed by a real-time PCR assay using primers specific to ORF65. The copy number of viral genomes was normalized to 25 ng of total cellular DNA. (B) Determining virus titers in the supernatant from the transfection experiment. The supernatant from 293T cells transfected with WT BAC, 33STOP, or 33STOP.R plus pCMV-HA was collected at 24 or 36 h posttransfection. Virus titers were determined by plaque assay in BHK-21 cells. (C) Analysis of viral DNA replication in infection experiment. BHK-21 cells were infected with the WT BAC, 33STOP, or 33STOP.R virus at 100 genome copies per cell. At 12, 24, 36, or 48 h postinfection, total DNAs were isolated from the infected cells. The copy number of viral genomes was determined by real-time PCR. The result is presented as the fold increase in viral genome copy numbers at 24, 36, and 48 h postinfection compared to that at 12 h postinfection. (D) Determining the virus titer in the supernatant from the infection experiment. The supernatant from BHK-21 cells infected with WT BAC, 33STOP, or 33STOP.R virus was collected at 0, 12, 24, 36, or 48 h postinfection. Virus titers were determined by plaque assay in BHK-21 cells. No plaque was detected in supernatant from 33STOP infection at any of the time points. Error bars represent the standard deviations from three independent experiments.
To confirm that the differences in viral genome copy numbers among WT BAC, 33STOP.R, and 33STOP observed at 36 h posttransfection was due to multistep (for WT and 33STOP.R) versus single-step (for 33STOP) infection rather than to deficiencies in genome replication in a single round of infection for 33STOP, we measured virus titers from culture supernatants at 24 h and 36 h posttransfection. Titers of extracellular WT BAC and 33STOP.R viruses were up to 6 × 103 and 3 × 103 PFU/ml at 24 h posttransfection and increased to 1.1 × 105 and 5.7 × 104 PFU/ml, respectively, at 36 h posttransfection (Fig. 4B). No plaque was detected in BHK-21 cells incubated with the supernatant from 33STOP plus pCMV-HA transfected-293T cells (Fig. 4B). These results indicated that for either WT BAC or 33STOP.R, progeny virions were released into the medium at 24 h posttransfection, and their infection of new cells (i.e., multistep infection) contributed to the increase in genome copy numbers at 36 h posttransfection.
To further validate the results from transfected genomes, we next assayed viral DNA replication in BHK-21 cells during de novo infection with same amount of infectious virions of WT BAC, 33STOP, or 33STOP.R. Cells were infected with different viruses at 100 genome copies/cell, and total DNAs were isolated from cells at 12, 24, 36, or 48 h postinfection. Quantitation of viral genome copies was again carried out by real-time PCR using primers specific for ORF65. The results revealed that the intracellular genome copy of the WT BAC, 33STOP, and 33STOP.R virus increased by a similar fold at 24, 36, or 48 h postinfection compared to that at 12 h postinfection (Fig. 4C, with genome replication level at 12 h set as 1). To exclude the possibility that reversion of 33STOP had occurred during the experiment and accounted for the increase observed for 33STOP, viruses released from BHK-21 cells infected with WT BAC, 33STOP, or 33STOP.R were titrated. Virus titers at 48 h postinfection were up to 58,000 PFU/ml and 25,000 PFU/ml for WT BAC and 33STOP.R, respectively. However, no plaque was detected in BHK-21 cells infected with supernatant from 33STOP-infected cells (Fig. 4D). Taken together, these data demonstrated that viral DNA replication proceeds normally without a functional ORF33.
ORF33 is not required for MHV-68 early or late gene expression.
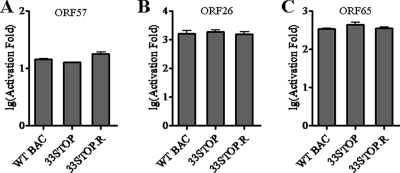
Viral DNA replication depends on immediate-early and early gene expressions. ORF33 is not required for viral DNA replication, suggesting that viral immediate-early and early gene expressions do not rely on ORF33. We next sought to determine whether ORF33 is required for MHV-68 late gene expression by examining viral promoter activities during WT BAC, 33STOP, or 33STOP.R virus infection using a reporter assay. Two late gene promoters (pORI/ORF26pLUC and pORI/ORF65pLUC) were analyzed, and an early gene promoter (ORF57pLUC) was also included in the assay for comparison (3). Since 33STOP virus harvested from supernatants of 33STOP/pHA-33-cotransfected cell cultures cannot form plaques, we determined its “titer” by a reporter cell line as described in Materials and Methods. For comparisons, we examined the titers of WT BAC and 33STOP.R viruses by both the reporter cell line and the standard plaque assay, through which the titer of 33STOP virus determined by the reporter cell line could be converted to the format of PFU/ml. One infectious unit determined by reporter cell line is equal to 60 PFU. 293T cells were transfected individually with each reporter plasmid and subsequently infected with WT BAC, 33STOP, or 33STOP.R viruses at an MOI of 0.1 infectious unit/cell. As shown in Fig. 5, activation of each viral promoter during 33STOP virus infection was similar to that during WT BAC or 33STOP.R virus infections. This result indicated that ORF33 is not required for viral early or late gene expression. Thus, ORF33 may function late in the lytic phase, directly or indirectly in either virion morphogenesis or release.
FIG. 5.
ORF33 is not required for MHV-68 early or late gene expression. ORF33 is not required for viral early or late gene expression. Firefly luciferase reporter constructs driven by ORF57 (A), ORF26 (B), or ORF65 (C) promoter were individually transfected into 293T cells. At 12 h posttransfection, the cells were infected with WT BAC, 33STOP, or 33STOP.R viruses at 0.1 infectious unit/cell. The firefly luciferase activity was measured at 24 h postinfection and was normalized against the Renilla luciferase internal control. The fold activation was calculated by comparing the normalized value of infected sample to that obtained from uninfected sample. Error bars represent standard deviations from three independent experiments.
ORF33 is required for morphogenesis of MHV-68 virions in the cytoplasm.
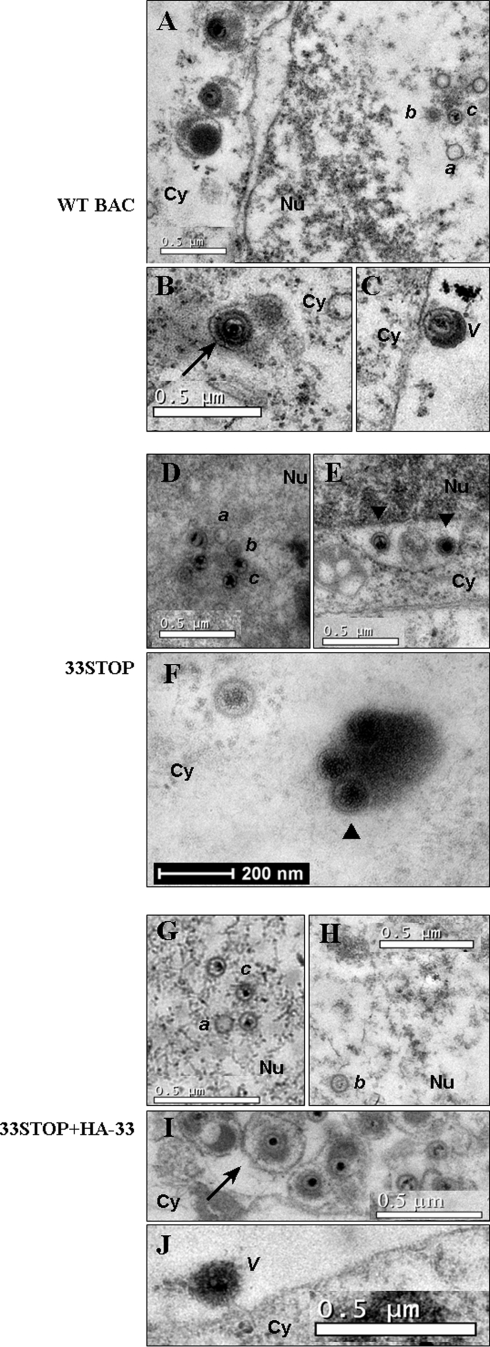
To study the role of ORF33 in virion assembly, maturation, and release, we examined the ultrastructure of cells transfected with WT BAC plus pCMV-HA, 33STOP plus pCMV-HA, or 33STOP plus pHA-33 by thin-section TEM. Virion assembly and egress were examined by noting the existence of nascent viral particles at each stage of virion maturation, according to current models (37, 38): capsid formation and viral DNA packaging in the nucleus, primary envelopment at the inner nuclear membrane and deenvelopment at the outer nuclear membrane, association with tegument proteins and final envelopment in the cytoplasm, and budding of complete virions into Golgi body-derived compartments for egress via the cellular secretory pathway.
In nuclei, three herpesvirus capsid species were observed in 33STOP plus pCMV-HA, as well as WT BAC plus pCMV-HA and 33STOP plus pHA-33 transfections (Fig. 6), a finding consistent with the progression of lytic infection to a late stage (38, 42). Empty or A capsids, as well as B capsids containing scaffolding proteins, and viral DNA-filled C capsids were visible (Fig. 6A, D, G, and H). Obviously, capsids in 33STOP plus pCMV-HA transfected cells were capable of budding through the nuclear membrane into the cytoplasm, since we observed primary enveloped virions in the perinuclear space (Fig. 6E) and partially tegumented nucleocapsids in the cytoplasm (Fig. 6F). The primary enveloped virion contained few tegument proteins, with no obvious tegument layer between the nucleocapsid and the envelope, thus appearing smaller than the mature virion. As shown Fig. 6F, several viral particles of 33STOP clustered in the cytoplasm and were surrounded by high-density materials that we thought were tegument proteins, suggesting tegumentation of 33STOP. In WT BAC plus pCMV-HA or 33STOP plus pHA-33 transfected cells, fully enveloped virions within cytoplasmic vesicles (Fig. 6B and I) and extracellular virions associated with the cellular plasma membrane (Fig. 6C and J) were observed, indicating progression of complete viral lytic replication cycle. These mature virions contained, from inside to outside, a high-density nucleic acid core, the icosahedral capsid, a relatively high-density layer of tegument, and an envelope. However, in 33STOP plus pCMV-HA transfected cells, we found no fully enveloped virions within cytoplasmic vesicles, and no extracellular virion associated with the cellular plasma membrane was observed (Fig. 6E and F). These results demonstrated that the null mutation of ORF33 blocked the secondary envelopment of MHV-68 viral particles, which could be rescued by providing ORF33 in trans.
FIG. 6.
ORF33 is required for maturation of MHV-68 virions in the cytoplasm. 293T cells were transfected with WT BAC plus pCMV-HA (A, B, and C), 33STOP plus pCMV-HA (D, E, and F), or 33STOP plus pHA-33 (G, H, I, and J). Approximately 70-nm thin sections were examined at 4 days posttransfection by transmission electron microscopy. Putative A capsid (a), B capsid (b), and C capsid (c) were found in WT BAC plus pCMV-HA, 33STOP plus pCMV-HA and 33STOP plus pHA-33-transfected cell nuclei (Nu) (A, D, G, and H). Primary enveloped virions of 33STOP (▾)were observed in the perinuclear space (E). In the cytoplasm (Cy), viral particles, including enveloped-tegumented virions (→) were observed in WT BAC plus pCMV-HA and 33STOP plus pHA-33 transfectants (B and I), but not in 33STOP plus pCMV-HA transfectant. Partially tegumented capsids (▴) were identified in 33STOP plus pCMV-HA transfectant (F). In the extracellular space, WT BAC plus pCMV-HA and 33STOP plus pHA-33 transfectants contained virions associated with cellular plasma membrane (V) (C and J), whereas no identifiable virions are found in 33STOP plus pCMV-HA transfectant.
To quantify the block in virion assembly that occurs in the absence of ORF33, viral particles at various stages of maturation in electron micrographs were classified and enumerated (Table 2). Particles were classified as nuclear capsids (A, B, and C capsids), cytoplasmic A and B capsids, immature cytoplasmic virions (partially tegumented virions and C capsids in the cytoplasm), or extracellular virions. In 33STOP plus pCMV-HA transfectants, the proportion of nuclear capsids to total particles (0.82) is much higher than that in WT BAC plus pCMV-HA (0.42) or 33STOP plus pHA-33 (0.47) transfectants, whereas the proportion of immature cytoplasmic virions to total particles (0.09) is lower than that in WT BAC plus pCMV-HA (0.38) or 33STOP plus pHA-33 (0.46) transfectants. These observations indicated that without ORF33, the release of nucleocapsids from the nucleus into the cytoplasm was markedly reduced and maturation of virions was arrested at a cytoplasmic stage of partially tegumented nucleocapsids, suggesting that ORF33 participates in both primary envelopment of nucleocapsids and morphogenesis of virions in the cytoplasm prior to virion egress.
TABLE 2.
Distribution of viral particles
| BAC transfection | No. of particlesa | Fraction of total particles of the indicated region
|
|||
|---|---|---|---|---|---|
| Nucleus (all capsids) | Cytoplasm
|
Extracellular virionsc | |||
| A and B capsids | Immature virionsb | ||||
| WT BAC | 161 | 0.42 | 0.07 | 0.38 | 0.13 |
| 33STOP | 142 | 0.82 | 0.09 | 0.09 | 0 |
| 33STOP+HA-33 | 175 | 0.47 | 0.02 | 0.46 | 0.05 |
Total viral and subviral particles enumerated in 10 TEM images at 4 days posttransfection.
Fraction of immature virions, including C capsids in the cytoplasm, of the total numbers of particles.
Fraction representing fully mature extracellular virions.
Protein composition of 33STOP particles, WT BAC virions, and complemented 33STOP virions.
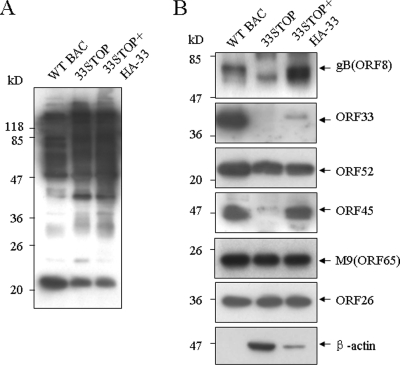
Without ORF33 protein, virions do not mature and egress from the cell, so we were unable to purify infectious virions from culture supernatant from 33STOP plus pCMV-HA transfection. We thus compared the nascent viral particles produced in WT BAC plus pCMV-HA, 33STOP plus pCMV-HA, or 33STOP plus pHA-33 transfected cells. Particles were released from these transfected cells by freeze-thaw treatment, and virion proteins present in WT BAC, 33STOP, or 33STOP+HA-33 intracellular particles were examined by Western blotting. As shown in Fig. 7A, the types and abundances of MHV-68 structural proteins contained in WT BAC particles as detected by the anti-MHV-68 polyclonal serum were different from those in 33STOP particles, which is consistent with the notion of different protein components between mature and immature viral particles.
FIG. 7.
Protein compositions of WT BAC, 33STOP, and complemented 33STOP particles. Viral particles were isolated from 293T cells cotransfected with WT BAC plus pCMV-HA, 33STOP plus pCMV-HA, or 33STOP plus pHA-33 at 4 days posttransfection. Virion proteins of WT BAC, 33STOP, and 33STOP plus HA-33 particles were separated by SDS-PAGE and examined by Western blotting, using polyclonal anti-MHV-68 serum (A), polyclonal antibodies to gB, ORF26, ORF65, ORF33, ORF45 or ORF52, or monoclonal anti-β-actin antibody (B).
To more accurately assess and compare the protein compositions of WT BAC, 33STOP, and 33STOP+HA-33 particles, we used a panel of antibodies against specific viral structural proteins in Western blots (Fig. 7B). As expected, ORF33 protein is present in WT BAC but not in 33STOP particles. Supplying ORF33 in trans resulted in incorporation of HA-33 protein into 33STOP+HA-33 particles but with a much lower efficiency than that in WT BAC particles. The lower incorporation of HA-33 into 33STOP+HA-33 particles may be due to two reasons: (i) the expression level of HA-33 provided for 33STOP in trans is not as high as that of ORF33 in WT BAC transfected cells (Fig. 3E) and (ii) the incorporation of protein provided in trans may be less efficient than that during wild-type virus infection, which is reminiscent of a previous study on ORF45 showing that fewer GFP-45 proteins were incorporated into 45STOP virions in the transcomplementation experiment than wild-type ORF45 proteins in wild-type virions (24). WT BAC, 33STOP and 33STOP+HA-33 particles all contained the conserved herpesvirus capsid proteins ORF26 and ORF65. Although 33STOP particles were blocked from final egress, they were partially tegumented, containing similar amounts of ORF52 tegument protein to that of WT BAC and 33STOP+HA-33 particles. However, unlike WT BAC and 33STOP+HA-33 particles, 33STOP particles did not contain appreciable amount of ORF45 tegument protein, suggesting that ORF33 is required for efficient incorporation of ORF45 into the viral particles. The amounts of ORF45 protein in WT BAC and 33STOP+HA-33 were similar, although the incorporation of HA-33 was much less than that of ORF33 in WT BAC, indicating that ORF33 could help viral particle maturation even at a low level of expression or incorporation. In WT BAC virions, glycoprotein B (envelope glycoprotein) was mainly detected as a 65-kDa protein (33). However, 33STOP+HA-33 particles also contained another form of glycoprotein B with a smaller molecular mass that may be the 55-kDa form described by Lopes et al. (33). Only the smaller form of glycoprotein B existed in 33STOP particles, which is consistent with our TEM result that no enveloped 33STOP viral particles was observed, since the 65-kDa protein is the abundant form of glycoprotein B in mature virions (33).
Interestingly, a higher amount of actin protein was observed in 33STOP particles than in 33STOP+HA-33 particles, whereas no actin protein was detected in WT BAC particles in Western blot analyses. Since studies of extracellular virions of KSHV and EBV have identified actin as a virion protein (26, 59), we next examined the existence of actin in MHV-68 virion by liquid chromatography-tandem mass spectrometry (LC-MS/MS). After an SDS-PAGE analysis of aliquots of purified viral particles, bands of 42-kDa protein in the lane for 33STOP particles and corresponding areas in lanes for extracellular MHV-68 and WT BAC virions were excised, digested with trypsin, and subjected to LC-MS/MS analysis. Peptides corresponding to human β-actin, γ-1-actin, and γ-2-actin were found in extracellular MHV-68 virion and WT BAC virion, and similar peptides were also found in 33STOP particles (data not shown). Therefore, our LC-MS/MS results identified actin as a component in MHV-68 virion, even though we failed to detect any actin in WT BAC virion by Western blotting, which may be due to the lower sensitivity of the assay. Taken together, our results showed that actin exists in MHV-68 virion, and its incorporation into virion increases as a result of the null mutation of ORF33.
DISCUSSION
ORF33 is conserved among the Alpha-, Beta-, and Gammaherpesvirinae subfamilies. Although previous work has revealed some functional roles of the ORF33 homologues in alpha- and betaherpesviruses, none of the gammaherpesviral ORF33 proteins has yet been functionally characterized. In this report, we showed that the MHV-68 ORF33 encodes a 36-kDa tegument protein expressed with true late kinetics during de novo lytic replication. Through functional analysis of a 33STOP mutant, we demonstrated that the ORF33 protein is essential for viral lytic replication and that its null mutation prevents virion budding and egress in cytoplasm without affecting viral gene expression and viral DNA replication. Finally, we found that the defects in 33STOP virus replication can be complemented in trans by MHV-68 ORF33 protein.
Previous DNA array analysis has revealed that transcription of ORF33 during MHV-68 de novo infection is reduced by CHX and PAA treatment (34). We confirmed here that ORF33 is expressed with true late kinetics by both Northern blotting and Western blotting (Fig. 1A). The expression kinetics of ORF33 during MHV-68 de novo infection is consistent with our data demonstrating that ORF33 functions in the late phase of viral lytic replication (Fig. 4, 5, 6, and 7).
We identified MHV-68 ORF33 as a virion associated protein by analyzing purified extracellular viral particles. The ORF33 protein is identified as a tegument protein located in between the envelope and the capsid, based on its sensitivity to trypsin and detergent treatment (Fig. 1D). In transfected mammalian cells, the ORF33 protein is distributed evenly in both the cytoplasm and nuclei; however, in the late phase of viral infection, ORF33 appeared to be accumulating in some nucleic compartments (Fig. 1C), similar to HSV-1 UL16 (35). These ORF33 protein-rich compartments might be viral assemblons formed during capsid assembly and maturation (56). These results suggest that ORF33 may participate in primary envelopment after capsid assembly and viral DNA packaging. The role of ORF33 in primary envelopment seems to be nonessential, since the C-type capsid of 33STOP can traffic from the nuclear membrane into cytoplasm (Fig. 6E and F). However, the absence of ORF33 markedly reduced the efficiency of nucleocapsid primary envelopment, since a considerably higher proportion of 33STOP nucleocapsids (0.82) was observed in nuclei than that of WT BAC (0.42) and 33STOP+HA-33 (0.47) nucleocapsids (Table 2).
MHV-68 ORF33 shares 20% similarity in amino acid sequence with HSV-1 UL16. However, unlike MHV-68 ORF33, UL16 is dispensable for viral replication in tissue culture, although virus titer drops as much as a log in the absence of the UL16 protein (4, 5). The interaction between the UL16 protein and the capsid is thought to be dynamic. The UL16 protein attaches to the capsid first in the cytoplasm by interacting with UL21 (27), and then targets the partially tegumented virion to UL11 localized in a Golgi-like compartment (32). The acidic environment of trans-Golgi network derived compartments regulates the association of the UL16 protein with the capsid (35). However, neither UL21 nor UL11 is essential for viral replication, indicating that interactions of UL16 with UL11 or UL21 are redundant. HCMV UL94, which also shares 20% similarity in amino acid sequence with MHV-68 ORF33, is a binding partner for HCMV tegument protein pp28 encoded by UL99. The pp28 protein, accumulating in a stable, juxtanuclear, membranous cytoplasmic structure termed the assembly compartment, is essential for the assembly of infectious HCMV virions. The UL94 mainly localizes in the nucleus when singly expressed and changes its localization from the nucleus to cytoplasm through interacting with pp28. During HCMV infection process, UL94 is found in the nucleus during early infection but later moves to the cytoplasm. Although the specific function of UL94 is not clear, its nuclear localization suggests a role in virion assembly, and the interaction between UL94 and pp28 suggests another role in virion tegumentation and budding, bridging the tegumented capsid and the envelope (31).
We also found that ORF33 interacts with several tegument proteins (data not shown), and Rozen et al. showed that the KSHV ORF33 interacts with the tegument protein ORF52 and glycoproteins gB and gN (50). We hypothesize that, through a series of protein-protein interactions, ORF33 binds to the capsid in the nucleus or cytoplasm first and then targets partially tegumented virions to trans-Golgi network to finally obtain the envelope. In the absence of ORF33 protein, tegumentation by ORF52 seems to occur normally; however, the tegument protein ORF45 and the 65-kDa form of glycoprotein B are not recruited into the assembled viral particle (Fig. 6), suggesting that the progression of virion morphogenesis is blocked at a stage of partially tegumented nucleocapsids. Surprisingly, a smaller form of gB was recruited into 33STOP viral particles and not into WT BAC virions. Although the function and localization of the smaller gB in the 33STOP virion are not clear, it appears that the interaction between the smaller gB and the tegument in immature virions is blocked and/or dissociated by the attachment of ORF33 to the tegument layer. To fully accomplish virion morphogenesis and eventual egress, partially tegumented nucleocapsids may depend on interactions of ORF33 with other viral or cellular proteins to obtain complete tegument and envelope components. Knocking out ORF33 may thus result in low efficiency of virion primary envelopment and failed release of infectious virions.
In addition to virus-encoded proteins, we also identified actin as a component of MHV-68 virion by LC-MS/MS, in contrast to the previous report that no actin was found in MHV-68 virions (8). Surprisingly, in the absence of ORF33, immature virions were restrained in a state interacting with actin (Fig. 7B), since there was a higher amount of actin associated with 33STOP viral particles than that associated with WT BAC virions. In a study of several pseudorabies viruses lacking tegument proteins, actin was also found to be enriched in the virions of these mutant particles (39). In cell nuclei, nucleocapsids undergo movement toward the nuclear envelope, possibly along actin filaments through interacting with motor myosin V (19, 20); however, little is known about the functional role of actin in virion morphogenesis in the cytoplasm. Considering that actin is involved in the herpesvirus nucleocapsid trafficking in the nucleus and vesicle transport in the cytoplasm (17), it is possible that the movement of partially tegumented herpesvirus capsid to the egressing site in the cytoplasm relies on the participation of actin, and the absence of ORF33 prevents the partially tegumented capsid on the actin filaments from migrating into Golgi body-derived vesicles. If so, then the majority of the 33STOP particles would still be attached to the actin filaments when they were purified in our experiments, which could result in a higher abundance of actin in these immature viral particles, as observed in Western blot analyses.
Aside from ORF33, the other functionally characterized MHV-68 tegument protein is ORF52. MHV-68 ORF52 mainly functions in facilitating tegumentation/envelopment of nucleocapsids at a cytoplasmic stage of virion morphogenesis prior to virion egress. However, knocking out ORF52 did not affect the primary envelopment of nucleocapsids (7). Similar to ORF52, ORF33 also plays important roles in virion morphogenesis, but in two stages of viral lytic replication: primary envelopment in the nucleus and tegumentation/envelopment in the cytoplasm, based on the reduced egress of nucleocapsids through the nuclear membrane and failed release of infectious virions in the absence of ORF33 (Fig. 6). Both primary envelopment and secondary envelopment of virions depend on interactions between tegument and membrane proteins; therefore, the localization of ORF33 in both the nucleus and the cytoplasm (Fig. 1C) provides it with the feasibility of participating in these two events. Also, we could not exclude the possibility that the absence of ORF33 only reduced the formation of infectious virions to a level undetectable by electron microscopy. Interactions between ORF33 and other viral proteins may be redundant during high-dose infection, and we will further study whether these interactions are required for viral replication.
In addition, as a tegument protein, ORF33 may also participate in regulation of cellular gene expression and modulation of cellular environment to facilitate viral infection at the early stage of MHV-68 de novo infection. More experiments will be performed to investigate these possibilities.
Acknowledgments
We thank Ren Sun for providing reagents, Lei Sun at the Electron Microscope Imaging Center of the Institute of Biophysics for help with the transmission electron microscope experiments, and Ting-Ting Wu and Vaithilingaraja Arumugaswami and members of the Deng laboratory for helpful discussions.
This study was supported by the National Protein Project (2006CB910901) and NIH grant DE15612.
Footnotes
Published ahead of print on 5 August 2009.
REFERENCES
- 1.Adler, H., M. Messerle, and U. H. Koszinowski. 2001. Virus reconstituted from infectious bacterial artificial chromosome (BAC)-cloned murine gammaherpesvirus 68 acquires wild-type properties in vivo only after excision of BAC vector sequences. J. Virol. 75:5692-5696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adler, H., M. Messerle, M. Wagner, and U. H. Koszinowski. 2000. Cloning and mutagenesis of the murine gammaherpesvirus 68 genome as an infectious bacterial artificial chromosome. J. Virol. 74:6964-6974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arumugaswami, V., T. T. Wu, D. Martinez-Guzman, Q. Jia, H. Deng, N. Reyes, and R. Sun. 2006. ORF18 is a transfactor that is essential for late gene transcription of a gammaherpesvirus. J. Virol. 80:9730-9740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baines, J. D., and B. Roizman. 1992. The cDNA of UL15, a highly conserved herpes simplex virus 1 gene, effectively replaces the two exons of the wild-type virus. J. Virol. 66:5621-5626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baines, J. D., and B. Roizman. 1991. The open reading frames UL3, UL4, UL10, and UL16 are dispensable for the replication of herpes simplex virus 1 in cell culture. J. Virol. 65:938-944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blasdell, K., C. McCracken, A. Morris, A. A. Nash, M. Begon, M. Bennett, and J. P. Stewart. 2003. The wood mouse is a natural host for Murid herpesvirus 4. J. Gen. Virol. 84:111-113. [DOI] [PubMed] [Google Scholar]
- 7.Bortz, E., L. Wang, Q. Jia, T. T. Wu, J. P. Whitelegge, H. Deng, Z. H. Zhou, and R. Sun. 2007. Murine gammaherpesvirus 68 ORF52 encodes a tegument protein required for virion morphogenesis in the cytoplasm. J. Virol. 81:10137-10150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bortz, E., J. P. Whitelegge, Q. Jia, Z. H. Zhou, J. P. Stewart, T. T. Wu, and R. Sun. 2003. Identification of proteins associated with murine gammaherpesvirus 68 virions. J. Virol. 77:13425-13432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bresnahan, W. A., and T. E. Shenk. 2000. UL82 virion protein activates expression of immediate early viral genes in human cytomegalovirus-infected cells. Proc. Natl. Acad. Sci. USA 97:14506-14511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castillo, J. P., and T. F. Kowalik. 2002. Human cytomegalovirus immediate early proteins and cell growth control. Gene 290:19-34. [DOI] [PubMed] [Google Scholar]
- 11.Cesarman, E., Y. Chang, P. S. Moore, J. W. Said, and D. M. Knowles. 1995. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N. Engl. J. Med. 332:1186-1191. [DOI] [PubMed] [Google Scholar]
- 12.Chang, Y., E. Cesarman, M. S. Pessin, F. Lee, J. Culpepper, D. M. Knowles, and P. S. Moore. 1994. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 266:1865-1869. [DOI] [PubMed] [Google Scholar]
- 13.Corbellino, M., L. Poirel, J. T. Aubin, M. Paulli, U. Magrini, G. Bestetti, M. Galli, and C. Parravicini. 1996. The role of human herpesvirus 8 and Epstein-Barr virus in the pathogenesis of giant lymph node hyperplasia (Castleman's disease). Clin. Infect. Dis. 22:1120-1121. [DOI] [PubMed] [Google Scholar]
- 14.Dai, W., Q. Jia, E. Bortz, S. Shah, J. Liu, I. Atanasov, X. Li, K. A. Taylor, R. Sun, and Z. H. Zhou. 2008. Unique structures in a tumor herpesvirus revealed by cryo-electron tomography and microscopy. J. Struct. Biol. 161:428-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Decker, L. L., L. D. Klaman, and D. A. Thorley-Lawson. 1996. Detection of the latent form of Epstein-Barr virus DNA in the peripheral blood of healthy individuals. J. Virol. 70:3286-3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Decker, L. L., P. Shankar, G. Khan, R. B. Freeman, B. J. Dezube, J. Lieberman, and D. A. Thorley-Lawson. 1996. The Kaposi sarcoma-associated herpesvirus (KSHV) is present as an intact latent genome in KS tissue but replicates in the peripheral blood mononuclear cells of KS patients. J. Exp. Med. 184:283-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DePina, A. S., and G. M. Langford. 1999. Vesicle transport: the role of actin filaments and myosin motors. Microsc. Res. Tech. 47:93-106. [DOI] [PubMed] [Google Scholar]
- 18.Dupin, N., C. Fisher, P. Kellam, S. Ariad, M. Tulliez, N. Franck, E. van Marck, D. Salmon, I. Gorin, J. P. Escande, R. A. Weiss, K. Alitalo, and C. Boshoff. 1999. Distribution of human herpesvirus-8 latently infected cells in Kaposi's sarcoma, multicentric Castleman's disease, and primary effusion lymphoma. Proc. Natl. Acad. Sci. USA 96:4546-4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Favoreel, H. W., L. W. Enquist, and B. Feierbach. 2007. Actin and Rho GTPases in herpesvirus biology. Trends Microbiol. 15:426-433. [DOI] [PubMed] [Google Scholar]
- 20.Feierbach, B., S. Piccinotti, M. Bisher, W. Denk, and L. W. Enquist. 2006. Alpha-herpesvirus infection induces the formation of nuclear actin filaments. PLoS Pathog. 2:e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flano, E., S. M. Husain, J. T. Sample, D. L. Woodland, and M. A. Blackman. 2000. Latent murine gamma-herpesvirus infection is established in activated B cells, dendritic cells, and macrophages. J. Immunol. 165:1074-1081. [DOI] [PubMed] [Google Scholar]
- 22.Ganem, D. 2006. KSHV infection and the pathogenesis of Kaposi's sarcoma. Annu. Rev. Pathol. 1:273-296. [DOI] [PubMed] [Google Scholar]
- 23.Ishov, A. M., O. V. Vladimirova, and G. G. Maul. 2002. Daxx-mediated accumulation of human cytomegalovirus tegument protein pp71 at ND10 facilitates initiation of viral infection at these nuclear domains. J. Virol. 76:7705-7712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jia, Q., V. Chernishof, E. Bortz, I. McHardy, T. T. Wu, H. I. Liao, and R. Sun. 2005. Murine gammaherpesvirus 68 open reading frame 45 plays an essential role during the immediate-early phase of viral replication. J. Virol. 79:5129-5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jia, Q., T. T. Wu, H. I. Liao, V. Chernishof, and R. Sun. 2004. Murine gammaherpesvirus 68 open reading frame 31 is required for viral replication. J. Virol. 78:6610-6620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johannsen, E., M. Luftig, M. R. Chase, S. Weicksel, E. Cahir-McFarland, D. Illanes, D. Sarracino, and E. Kieff. 2004. Proteins of purified Epstein-Barr virus. Proc. Natl. Acad. Sci. USA 101:16286-16291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klupp, B. G., S. Bottcher, H. Granzow, M. Kopp, and T. C. Mettenleiter. 2005. Complex formation between the UL16 and UL21 tegument proteins of pseudorabies virus. J. Virol. 79:1510-1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kutok, J. L., and F. Wang. 2006. Spectrum of Epstein-Barr virus-associated diseases. Annu. Rev. Pathol. 1:375-404. [DOI] [PubMed] [Google Scholar]
- 29.Ling, P. D., J. Tan, J. Sewatanon, and R. Peng. 2008. Murine gammaherpesvirus 68 open reading frame 75c tegument protein induces the degradation of PML and is essential for production of infectious virus. J. Virol. 82:8000-8012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu, F., and Z. H. Zhou. 2006. Comparative virion structures of human herpesviruses, p. 27-42. In A. Arvin, G. Campadelli-Fiume, P. Moore, et al. (ed.), Human herpesviruses: biology, therapy, and immunoprophylaxis. Cambridge University Press, Cambridge, United Kingdom.
- 31.Liu, Y., Z. Cui, Z. Zhang, H. Wei, Y. Zhou, M. Wang, and X. E. Zhang. 2009. The tegument protein UL94 of human cytomegalovirus as a binding partner for tegument protein pp28 identified by intracellular imaging. Virology 388:68-77. [DOI] [PubMed] [Google Scholar]
- 32.Loomis, J. S., R. J. Courtney, and J. W. Wills. 2003. Binding partners for the UL11 tegument protein of herpes simplex virus type 1. J. Virol. 77:11417-11424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lopes, F. B., S. Colaco, J. S. May, and P. G. Stevenson. 2004. Characterization of murine gammaherpesvirus 68 glycoprotein B. J. Virol. 78:13370-13375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martinez-Guzman, D., T. Rickabaugh, T. T. Wu, H. Brown, S. Cole, M. J. Song, L. Tong, and R. Sun. 2003. Transcription program of murine gammaherpesvirus 68. J. Virol. 77:10488-10503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meckes, D. G., Jr., and J. W. Wills. 2007. Dynamic interactions of the UL16 tegument protein with the capsid of herpes simplex virus. J. Virol. 81:13028-13036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Messerle, M., I. Crnkovic, W. Hammerschmidt, H. Ziegler, and U. H. Koszinowski. 1997. Cloning and mutagenesis of a herpesvirus genome as an infectious bacterial artificial chromosome. Proc. Natl. Acad. Sci. USA 94:14759-14763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mettenleiter, T. C. 2004. Budding events in herpesvirus morphogenesis. Virus Res. 106:167-180. [DOI] [PubMed] [Google Scholar]
- 38.Mettenleiter, T. C. 2002. Herpesvirus assembly and egress. J. Virol. 76:1537-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Michael, K., B. G. Klupp, T. C. Mettenleiter, and A. Karger. 2006. Composition of pseudorabies virus particles lacking tegument protein US3, UL47, or UL49 or envelope glycoprotein E. J. Virol. 80:1332-1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mistrikova, J., and D. Blaskovic. 1985. Ecology of the murine alphaherpesvirus and its isolation from lungs of rodents in cell culture. Acta Virol. 29:312-317. [PubMed] [Google Scholar]
- 41.Nalwanga, D., S. Rempel, B. Roizman, and J. D. Baines. 1996. The UL16 gene product of herpes simplex virus 1 is a virion protein that colocalizes with intranuclear capsid proteins. Virology 226:236-242. [DOI] [PubMed] [Google Scholar]
- 42.Nealon, K., W. W. Newcomb, T. R. Pray, C. S. Craik, J. C. Brown, and D. H. Kedes. 2001. Lytic replication of Kaposi's sarcoma-associated herpesvirus results in the formation of multiple capsid species: isolation and molecular characterization of A, B, and C capsids from a gammaherpesvirus. J. Virol. 75:2866-2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O'Connor, C. M., and D. H. Kedes. 2006. Mass spectrometric analyses of purified rhesus monkey rhadinovirus reveal 33 virion-associated proteins. J. Virol. 80:1574-1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oshima, S., T. Daikoku, S. Shibata, H. Yamada, F. Goshima, and Y. Nishiyama. 1998. Characterization of the UL16 gene product of herpes simplex virus type 2. Arch. Virol. 143:863-880. [DOI] [PubMed] [Google Scholar]
- 45.Pavlova, I., C. Y. Lin, and S. H. Speck. 2005. Murine gammaherpesvirus 68 Rta-dependent activation of the gene 57 promoter. Virology 333:169-179. [DOI] [PubMed] [Google Scholar]
- 46.Rajcani, J., D. Blaskovic, J. Svobodova, F. Ciampor, D. Huckova, and D. Stanekova. 1985. Pathogenesis of acute and persistent murine herpesvirus infection in mice. Acta Virol. 29:51-60. [PubMed] [Google Scholar]
- 47.Rixon, F. J. 1993. Structure and assembly of herpesviruses. Semin. Virol. 4:135-144. [Google Scholar]
- 48.Roizman, B., and D. M. Knipe. 2001. Herpes simplex viruses and their replication, p. 2399-2459. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott-Raven Publishers, Philadelphia, PA.
- 49.Roizman, B., and P. E. Pellett. 2001. Herpesviridae: a brief introduction, p. 2381-2398. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott-Raven Publishers, Philadelphia, PA.
- 50.Rozen, R., N. Sathish, Y. Li, and Y. Yuan. 2008. Virion-wide protein interactions of Kaposi's sarcoma-associated herpesvirus. J. Virol. 82:4742-4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Song, M. J., S. Hwang, W. H. Wong, T. T. Wu, S. Lee, H. I. Liao, and R. Sun. 2005. Identification of viral genes essential for replication of murine gamma-herpesvirus 68 using signature-tagged mutagenesis. Proc. Natl. Acad. Sci. USA 102:3805-3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Soulier, J., L. Grollet, E. Oksenhendler, P. Cacoub, D. Cazals-Hatem, P. Babinet, M. F. d'Agay, J. P. Clauvel, M. Raphael, L. Degos, et al. 1995. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman's disease. Blood 86:1276-1280. [PubMed] [Google Scholar]
- 53.Subak-Sharpe, J. H., and D. J. Dargan. 1998. HSV molecular biology: general aspects of herpes simplex virus molecular biology. Virus Genes 16:239-251. [DOI] [PubMed] [Google Scholar]
- 54.Sunil-Chandra, N. P., S. Efstathiou, and A. A. Nash. 1992. Murine gammaherpesvirus 68 establishes a latent infection in mouse B lymphocytes in vivo. J. Gen. Virol. 73(Pt. 12):3275-3279. [DOI] [PubMed] [Google Scholar]
- 55.Virgin, H. W. T., P. Latreille, P. Wamsley, K. Hallsworth, K. E. Weck, A. J. Dal Canto, and S. H. Speck. 1997. Complete sequence and genomic analysis of murine gammaherpesvirus 68. J. Virol. 71:5894-5904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ward, P. L., W. O. Ogle, and B. Roizman. 1996. Assemblons: nuclear structures defined by aggregation of immature capsids and some tegument proteins of herpes simplex virus 1. J. Virol. 70:4623-4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wing, B. A., G. C. Lee, and E. S. Huang. 1996. The human cytomegalovirus UL94 open reading frame encodes a conserved herpesvirus capsid/tegument-associated virion protein that is expressed with true late kinetics. J. Virol. 70:3339-3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu, T. T., E. J. Usherwood, J. P. Stewart, A. A. Nash, and R. Sun. 2000. Rta of murine gammaherpesvirus 68 reactivates the complete lytic cycle from latency. J. Virol. 74:3659-3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhu, F. X., J. M. Chong, L. Wu, and Y. Yuan. 2005. Virion proteins of Kaposi's sarcoma-associated herpesvirus. J. Virol. 79:800-811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhu, F. X., S. M. King, E. J. Smith, D. E. Levy, and Y. Yuan. 2002. A Kaposi's sarcoma-associated herpesviral protein inhibits virus-mediated induction of type I interferon by blocking IRF-7 phosphorylation and nuclear accumulation. Proc. Natl. Acad. Sci. USA 99:5573-5578. [DOI] [PMC free article] [PubMed] [Google Scholar]