Abstract
HIV-1 eradication from infected individuals has not been achieved with the use of highly active antiretroviral therapy (HAART) for a prolonged period of time. The cellular reservoir for HIV-1 in resting memory CD4+ T cells remains a major obstacle to viral elimination. The reservoir does not decay significantly over long periods of time but is able to release replication-competent HIV-1 upon cell activation. Residual ongoing viral replication may likely occur in many patients because low levels of virus can be detected in plasma by sensitive assays and transient episodes of viremia, or HIV-1 blips, are often observed in patients even with successful viral suppression for many years. Here we review our current knowledge of the factors contributing to viral persistence, the latent reservoir, and blips, and mathematical models developed to explore them and their relationships. We show how mathematical modeling can help improve our understanding of HIV-1 dynamics in patients on HAART and of the quantitative events underlying HIV-1 latency, reservoir stability, low-level viremic persistence, and emergence of intermittent viral blips. We also discuss treatment implications related to these studies.
Keywords: HIV-1, HAART, low-level viremia, latency, viral reservoirs, blips, mathematical models
1 Introduction
The advent of potent combination antiretroviral therapy has resulted in a substantial reduction in the incidence of HIV-1-related morbidity and mortality [151]. Highly active antiretroviral therapy (HAART) based on the administration of at least three different drugs from two or more classes (e.g., two nucleoside reverse transcriptase inhibitors (NRTI) combined with either a protease inhibitor or a non-nucleoside reverse transcriptase inhibitor (NNRTI)) has proved extremely effective in suppressing the plasma viral load1 of most HIV-1-infected patients to below the limit of viral detection (e.g., 50 RNA copies/mL) of standard assays [35] (Figure 1). Since viral replication is directly linked to CD4+ T cell2 depletion, viral evolution and disease progression [144], the viral decline in the presence of combination therapy has profound clinical significance.
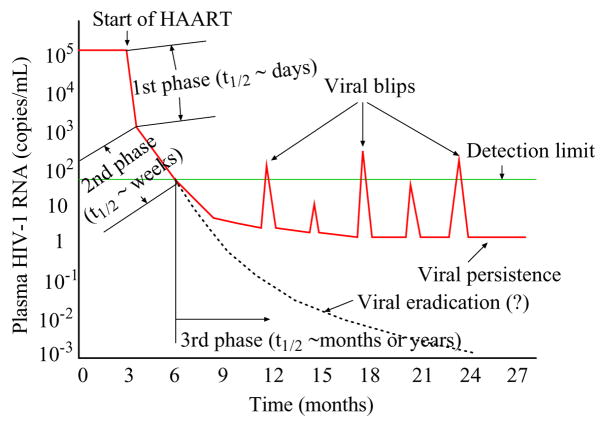
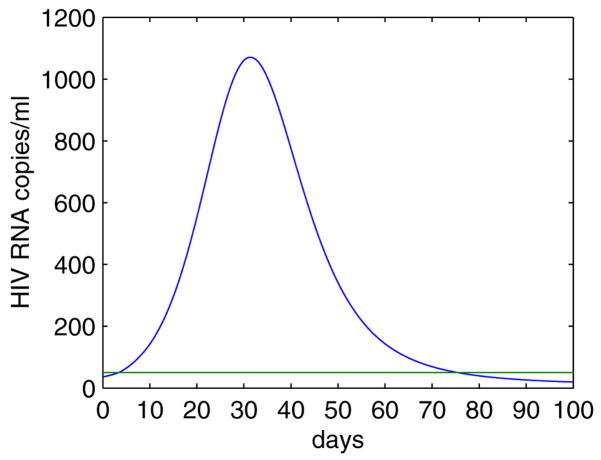
Figure 1.
The plasma viral load remains at a relatively constant level during chronic infection before initiation of HAART. Following treatment the plasma RNA level undergoes a multiphasic decay and declines to below 50 copies/mL after 3–6 months. However, virus cannot be eradicated with current antiretroviral therapy. A low level of viremia persists in patients with apparently suppressive treatment for many years. A number of patients experience intermittent viral blips, with transient HIV-1 RNA levels above the detection limit.
Over the past decades, many mathematical models, both deterministic and stochastic, have been developed to study HIV-1 infection and drug treatment. Many of these models, and particularly those developed before the mid-1990s, focused on the decline of CD4+ T cells [173], partially due to lack of accurate methods that could measure the number of virus particles in blood. The development of rapid and sensitive polymerase chain reaction (PCR)-based methods that can quantify genomic viral RNA molecules (each virus particle contains two RNA molecules) has proven to be significant in understanding HIV-1 viral load and facilitated the study of the host-pathogen interaction in HIV-1 infection by modeling. Seminal experimental studies by Ho et al. [91], Wei et al. [223] and modeling results by Perelson et al. [174] suggested that both free virus and productively infected cells have rapid turnover. It was estimated that more than 1010 virions are produced every day in an untreated patient with chronic HIV-1 infection [174]. These results clarified that HIV-1 is not a “slow” virus and that it can replicate rapidly. The observation that HIV viral loads assume a relatively constant level in patients during chronic infection, due to a balance between rapid viral production and rapid viral clearance, allowed one to calculate viral production rates from the rate of viral clearance observed during potent antiretroviral therapy. More importantly, these results suggested that drug resistant mutations are very common in the viral genome because of the large HIV-1 turnover rate, and that the failure of antiretroviral drugs, when used as monotherapy, is an inevitable consequence of the rapid HIV-1 replication. In this article, we will start with a review of a few basic models used to study viral infection and estimate parameters that govern viral production and clearance. We show how mathematical models combined with experimental results revealed a number of different time scales, from hours to days to weeks to months, and biological processes underlying them during HIV-1 infection. These results have made significant contributions to our understanding of HIV-1 dynamics and drug therapy.
Eradication of HIV-1 from infected individuals is the ultimate goal of antiretroviral therapeutic interventions. However, this possibility seems unlikely at present despite the great deal of progress that has been made in developing potent antiretroviral drugs and in understanding the molecular biology of HIV-1 replication (see reviews in [8, 90, 136, 181, 190, 206, 214]). Although HAART has proved extremely effective in reducing the viral load in HIV-infected patients to below 50 RNA copies/mL [35, 213], the detection limit of current standard assays, a low level of viremia can be detected in plasma by more sensitive assays even after years of treatment [51, 164, 165]. Moreover, a number of patients experience transient episodes of detectable viremia, or blips, even when the viral load has been suppressed to below the limit of detection for many years [67, 81, 147, 207]. These phenomena indicate that residual ongoing viral replication3 is very likely to continue in many patients on HAART.
HIV-1 can establish a state of latent infection in resting memory CD4+ T cells4 [19, 26]. These latently infected cells are capable of escaping from viral cytopathic effects5 and host immune mechanisms due to very low levels of HIV-1 messenger RNA (mRNA) and proteins they express [85, 119]. Because of the nature of memory CD4+ T cells [210], they remain in the resting state in the presence of potent combination therapy for a very long period of time [30, 56, 229]. However, they can produce new virus when stimulated by relevant antigen [23]. Thus, a viral rebound seems inevitable when therapy is withdrawn.
Determining the decay rate of the latent reservoir6 remains an important issue since it is directly related to the possibility and the time needed for the antiretroviral regimens currently in use to cure the infection. Estimates of the half-life7 of the latent reservoir are quite divergent, ranging from about 6 months [27, 185, 235] to 44 months [55, 202]. Therefore, combination treatment over as long as 73 years might be required for eradication of the latent reservoir [55]. Considering drug toxicities and medical tolerance, emergence of drug resistance, and treatment cost, such a lifetime therapy may not be reasonable for HIV-infected patients. Devising efficient strategies to accelerate the decay of the latent reservoir is a prerequisite for viral eradication [190].
We will review our current understanding of the factors that contribute to viral persistence, the latent reservoir persistence and viral blips. We discuss recent models proposed to study virus dynamics in patients on potent combination treatment and to explore possible mechanisms underlying low-level viral persistence, stability of the latent reservoir, and occurrence of intermittent viral blips. These models offer a quantitative investigation of the influence of ongoing viral replication on the viral load dynamics and the latent reservoir decay characteristics observed in HAART-treated patients. Finally, we discuss related treatment implications for clinical practice.
2 Multiphasic viral decay
Quantitative analysis of HIV-1 replication in vivo has made significant contributions to our understanding of AIDS pathogenesis and antiretroviral treatment (reviewed in [57, 170]). After a few months of HIV-1 infection, the plasma virus usually attains a viral set-point8, ranging from 102 to 107 copies/mL [180] in different patients, that persists for years [89]. At the set-point, viral production and clearance in infected individuals must be in equilibrium. Even though quantitative methods like RT-PCR can quantify viral load in HIV-1 infection, they cannot determine whether virus is produced quickly or slowly. Work by Ho et al. [91] and Wei et al. [223] revealed that drug-induced perturbations of the steady-state viral loads would provide information on HIV-1 production and clearance and on CD4+ T cell turnover. Based on further models developed by Perelson et al. [171, 174] and others [10, 11, 41, 125, 148, 156, 160, 179, 187, 198, 199, 211], significant progress has been made in understanding important features of HIV-1 dynamics and their clinical implications for antiretroviral treatment.
Analysis of the viral decay following initiation of potent combination drug therapy has suggested that the plasma viral load declines in multiple distinct phases (Fig. 1, see reviews in [57, 109, 170, 206]).
2.1 The first phase
The first studies that provide kinetic information on virus and CD4+ lymphocyte turnover in vivo were carried out by perturbing the viral set-point in patients during the asymptomatic phase of HIV infection with antiretroviral drugs, such as the protease inhibitors ritonavir [91] or saquinavir [223], as well as the reverse transcriptase (RT) inhibitor nevirapine [223]. If viral production is completely blocked by inhibitors, then the subsequent viral decay reveals the clearance rate of free virus. If it is not completely blocked, then the rate of viral decay will also depend on the death rate of productively infected cells and the effectiveness of the drug [170]. Administration of different antiretroviral agents resulted in a rapid decline (~ 2 log) in plasma virus levels in the first two weeks [91, 223], showing that the decline was not dependent on a particular drug but rather was the effect of blocking HIV replication in any manner. Further, this observation suggested that to maintain a set-point viral load there must be rapid production of the plasma virus and infection of CD4+ T cells. Thus, the replication of HIV-1 in vivo is a dynamic process involving continuous rounds of de novo virus infection and reproduction.
Perelson et al. [174] and Wei et al. [223] developed models to further analyze the HIV-1 viral load data collected from patients after the administration of a protease inhibitor. They studied two separate processes that contribute to the viral decay observed after drug administration: the clearance of free virus from plasma and the loss of productively infected cells. The model for viral dynamics can be described by the following equations:
| (1) |
where T(t), T*(t) and V(t) denote the concentration of uninfected CD4+ T cells, productively infected cells, and free virus at time t, respectively. λ represents the recruitment rate of uninfected T cells, d is the per capita death rate of uninfected cells, k is the rate constant at which uninfected cells are infected by free virus. Here the infection is modeled by a commonly used “mass action” term, kVT. δ is the per capita death rate of infected cells, N (burst size) is the total number of virus particles produced by a productively infected cell during its lifetime, and c is the clearance rate of virus. Therefore, Nδ, which is N divided by the cell life-span, 1/δ, gives the per capita viral production rate.
RT inhibitors can effectively block RT’s enzymatic function and prevent completion of synthesis of the viral DNA from HIV-1 RNA. Thus, the infection rate k is reduced by a quantity, (1 − εRT), where εRT is the efficacy of RT inhibitors and 0 ≤ εRT ≤ 1. Protease inhibitors prevent HIV protease from cleaving the HIV polyprotein into functional units, causing infected cells to produce immature virus particles that are noninfectious. Thus, only a part, (1 − εPI), of newly produced virus is infectious, where εPI is the protease inhibitor efficacy and similarly, 0 ≤ εPI ≤ 1.
Considering the effects of both RT and protease inhibitors, model (1) can be modified to:
| (2) |
where VI and VNI are the concentration of infectious and non-infectious virus, respectively. V = VI +VNI is the total amount of virus.
If one assumes that only a 100% effective protease inhibitor (εPI = 1, εRT = 0) is administered to an infected individual at quasi steady state (QSS) with initial viral load, V0, and that the uninfected target cells remain approximately at a constant level, T0, over the time period of interest, then the viral load at time t can be solved from Eq. (2) [174]:
| (3) |
Using nonlinear regression analysis, the parameters, c and δ, could be estimated by fitting Eq. (3) to plasma HIV-1 RNA data9 (data are shown in Table 1) [174]. The estimates of c, with a mean of 3 day−1, were similar for different patients, suggesting that the plasma virus has an intrinsic constant decay rate. The corresponding half-life (t1/2 = ln 2/c) of plasma virus is very short, with a mean of ~ 6 hours. Estimates of δ had a mean of 0.5 day−1, with a corresponding mean half-life of 1.6 days. These estimates give upper bounds for the half-lives of the virus and productively infected cells (i.e., the estimated values of c and δ are minimal estimates) because therapy in reality is not 100% effective and additional viral clearance and/or loss of virus-producing cells is required to account for the residual viral replication. Assumption of QSS and 100% drug effectiveness allowed solution with only c and δ. Whether these values of c and δ are also relevant during primary infection is still unknown.
Table 1.
Plasma viral load data (HIV-1 RNA copies/mL) from Perelson et al. [174]
| Time (day)* | Pt 102 | Pt 103 | Pt 104 | Pt 105 | Pt 107 |
|---|---|---|---|---|---|
| 0 | 610,000 | 19,000 | 100,000 | 1,022,000 | 160,000 |
| 0.083 | 410,000 | 18,000 | 95,000 | 1,079,000 | 240,000 |
| 0.166 | 460,000 | 34,000 | 160,000 | 2,148,000 | 260,000 |
| 0.25 | 560,000 | 34,000 | 150,000 | 1,043,000 | 230,000 |
| 0.5 | 480,000 | 32,000 | 120,000 | 2,170,000 | 240,000 |
| 0.75 | 720,000 | 38,000 | 120,000 | 1,538,000 | 180,000 |
| 1 | 460,000 | 28,000 | 110,000 | 3,098,000 | 200,000 |
| 1.25 | 510,000 | 25,000 | 110,000 | 2,262,000 | 180,000 |
| 1.5 | 300,000 | 18,000 | 73,000 | 1,315,000 | 120,000 |
| 1.75 | 420,000 | 33,000 | 82,000 | 1,200,000 | 140,000 |
| 2 | 250,000 | 18,000 | 66,600 | 980,000 | 150,000 |
| 3 | 290,000 | 11,000 | 35,000 | 710,000 | 67,000 |
| 4 | 250,000 | 5,400 | 22,000 | 500,000 | 54,000 |
| 5 | 190,000 | 1,600 | 21,000 | 280,000 | 31,000 |
| 6 | 110,000 | 1,400 | 16,000 | 170,000 | 16,000 |
| 7 | - | - | 4,800 | 89,000 | 11,000 |
These results had enormous implications for HIV-1 pathogenesis and antiretroviral treatment. Using the estimate of c and the pretreatment viral concentration, V0, it was estimated that more than 1010 virions were cleared daily. Therefore, at the steady state the same number of virions were produced and released into the extracellular fluid in the average mid-stage HIV-1-infected untreated patient per day [174]. Production of a large amount of virus [16, 76, 93] and the short half-life of productively infected cells suggest that a large number of CD4+ T cells are actively infected daily. Due to the highly error-prone reverse transcription10 from HIV-1 RNA to DNA, not only does every possible single mutation arise [33, 172], but ~ 1% of all possible double mutations can be generated daily [172]. High replication and high mutability of HIV-1 have greatly advanced our understanding of the evolution of drug resistance mutations and viral escape from immune responses. When drugs are administered individually, virological failure (treatment fails to achieve successful viral suppression to below the limit of detection) seems inevitable because of the rapid emergence of drug resistant virus variants in patients. Recent reviews on HIV-1 drug resistance can be found in [31, 189]. Mathematical models have been proposed to study the development of drug resistance during treatment, see [12, 106, 110, 142, 153, 161, 188, 191, 192, 193, 208, 209, 222, 230] and references cited therein.
2.2 The second phase
Clinical studies showed that simultaneous administration with multiple antiretroviral drugs would overcome the emergence of drug resistance when a single agent was used and lead to a significantly prolonged benefit [72, 79]. Perelson et al. [171] analyzed changes in viral loads of eight HIV-1-infected patients after initiation of treatment with combined antiretroviral agents and found that each patient responded with a similar pattern of viral decline: an initial rapid exponential decline followed by a slower second-phase viral decay with a half-life of 1 – 4 weeks. The plasma HIV-1 RNA levels in all patients dropped below 1000 copies/mL by 8 weeks of treatment, and below 50 copies/mL at week 16–20, demonstrating the potency of combination drug therapy and the lack of emergence of drug resistance during the study period.
The nature of the cellular or anatomical compartments that are responsible for the second phase viral decay remains obscure and several cell populations or sanctuary sites might contribute. One possibility is the existence of a long-lived population of productively infected cells [171], such as infected macrophages, which are less susceptible to viral cytopathic effects than are CD4+ T cells [92]. Other sources include, but are not restricted to, infected CD4+ T cells in a latent state [236], which can be activated to produce virus when encountering specific antigen, and release of the virus trapped in tissue reservoirs, for example, on the surface of follicular dendritic cells (FDCs) [86, 87, 88]. The contribution of these sources to the plasma virus in untreated patients is minor but becomes influential when combination therapy is administered and the infection of cells that produce most of the virions in plasma is largely blocked.
An extended mathematical model was proposed to analyze the observed two-phase viral load decay [171]. The model incorporated into the basic model (1) additional sources that could contribute to the plasma virus, such as long-lived productively infected cells and activation of latently infected cells, to explain the second-phase viral decline. Let M* be the population of long-lived infected cells and L latently infected cells, the model before therapy is described by the following equations:
| (4) |
where cells, M, which upon infection with a rate, kM, become long-lived infected cells, which produce virus at a rate p and are lost with a rate μM. Latently infected cells, L, which are generated with a rate fk, smaller than k by a factor f < 1, die with a rate dL, and are activated into productively infected cells with a rate a, giving a total rate constant of loss μL = a + dL. When used to fit short-term data, the level of susceptible CD4+ T cells was assumed to be constant over the study period [171], and hence no equation was needed for T.
In the presence of RT and protease inhibitors, Eq. (4) can be modified as in Eq. (2). Assuming that both RT and protease inhibitors are 100% effective, and that the level of target cells remains constant at value T0, the viral level after drug therapy can be solved [171]:
| (5) |
where
and the level of infected cells in blood is given by
| (6) |
Simultaneously fitting V(t) and I(t) to the plasma virus and PBMC 11 infectivity data showed that the loss of long-lived infected cells (t1/2 of 1 – 4 weeks) was a major contributor to the second phase of viral decay, whereas the activation of latently infected cells was only a minor source [171].
The above-mentioned models were extremely useful and popular, but not unique, in explaining the multiple viral decay observed in patients after initiation of potent combination treatment. For example, De Boer [37] and Rodin Porrata (personal communication) suggested that models with an eclipse phase can generate the second phase, but with length of eclipse phase if it is sufficiently long rather than lifespan of productively infected cells determining the second phase decay. Grossman et al. [68, 69] and Bucy [13] also developed different models to study viral dynamics. The underlying mechanisms for these models are reviewed in [181]. However, there are technical problems with the analysis of the Grossman model [156], and its results can not be relied on, although its suggestion that the rate of cell death may not follow a simple first order process is worthy of further study. In vitro labeling studies by Hodgkin and colleagues [66, 83] and models of carboxyfluorescin diacetate succinimidyl ester (CFSE) labeling studies [38, 42, 122, 182] support the notion of pursuing more complex laws of lymphocyte dynamics. This has yet to be done in the context of HIV dynamics.
2.3 The third phase: eradication?
After several months of HAART, most patients, particularly those who have not taken any antiretroviral medicines previously, achieve viral loads that are below the detection limit of current standard assays [72, 79, 171]. These observations raised the hope that prolonged potent antiretroviral treatment in those patients who have suppressed their viral loads to very low levels would eradicate HIV-1 from both short and long-lived compartments. Perelson and his colleagues [171] were the first to make such predictions based on the second-phase viral decay observed in the eight patients who took combination antiretroviral drugs. They predicted that, even if the initial number of chronically infected cells was as large as 1012 cells, which is larger than the estimated number of CD4+ T cells (~ 2 × 1011 cells [237]), the virus could be completely eliminated from these two compartments after ~3 years of treatment. However, this prediction was made with the assumption that antiretroviral regimen is 100% effective and more importantly, no additional viral compartments or sanctuary sites exist. Now it is clear that other long-lived viral reservoirs exist (see below), rendering eradication of HIV-1 unattainable at present. An excellent review of the cell types and anatomical sites that may serve as potential reservoirs for HIV-1 is given in [8].
3 Low-level viremic persistence
Although potent combination therapy can suppress the viral loads in many patients to below the detection limit of present assays, 50 RNA copies/mL, this does not imply that virus production has been completely stopped by the therapy. On the contrary, in many patients with suppressed plasma viral levels for a prolonged time, a low level of viremia can still be detected by more sensitive assays that can quantify HIV-1 RNA down to one or a few copies/mL [51, 164, 165]. This phase with plasma viral levels below 50 copies/mL has been referred to as the third phase [57]. The dynamics and sources of residual viremia in the third phase are of great interest because they are related to the issue of whether viral eradication can be achieved.
The factors contributing to this low-level viremic persistence have not been well characterized. It is possible that antiretroviral therapy is not completely suppressive and virus replication is still occurring [8, 197], albeit at low levels. Another possibility is that even if therapy is fully suppressive, HIV-1 establishes a state of latent infection in resting memory CD4+ T cells (see Section 4) [19, 26, 30, 56, 229], and a small number of virus particles are continuously released from the reservoir by activation of these latently infected cells [23]. A more reasonable scenario is that both contribute to viral persistence —ongoing viral replication replenishes the latent reservoir while the latent reservoir releases virus that fuels ongoing active viral replication. Further understanding of these factors and their relative contributions is critical for the goal of eventual viral elimination.
3.1 Ongoing viral replication
A number of studies have suggested that residual viral replication continues in patients under potent combination therapy even when the viral loads have been suppressed to below the limit of detection for a long time [17, 28, 29, 62, 115, 168]. This low-level ongoing virus replication involves generation of new infected cells through de novo infection from the virus released from other infected cells. The evidence for ongoing replication during HAART comes from the detection of unintegrated proviral DNA12, both linear [30] and circularized [200, 201], cell-associated HIV RNA13 [63, 155, 168, 235, 238], and the isolation of replication-competent virus14 [30, 56, 229] from both PBMC and seminal cells [51, 234]. However, care must be taken in comparing studies, as newer drugs may be more potent than older ones, patient compliance with therapy may vary, and the existence of drug resistance mutations may be different for different drug regimens and patient’s prior drug exposure.
Another line of evidence for residual viral replication with some forms of HAART is that treatment intensification can lead to better viral suppression and acceleration of the HIV-1 decay rate [82, 186]. In one study, the decay rate of the latent reservoir increased and the frequency of intermittent viremia decreased in 5 patients who underwent treatment intensification compared with 5 patients with comparable baseline characteristics who remained on standard combination therapy [186]. The result suggested that ongoing virus replication during standard antiretroviral therapy is at least partially due to the inadequate antiviral potency of some regimens. In another cohort of 14 patients whose HIV RNA levels had been sustained at < 50 copies/mL for more than 5 years, Havlir et al. [82] added the RT inhibitor abacavir to the regimen and found HIV plasma RNA levels declined rapidly and reached a new lower steady state that persisted over 5 years, suggesting that productive infection contributes to residual ongoing viremia and can be further inhibited with treatment intensification.
Other evidence for ongoing replication includes the occurrence of occasional viral measurements above the limit of detection of conventional assays [81, 186, 217], and changes in HIV-1 proviral sequences in PBMCs [235]. Ongoing replication is also found in other non-plasma compartments during HAART (reviewed in [111]), such as the central nervous system (CNS) [118].
Ongoing virus replication during therapy should inevitably lead to selection of drug resistant mutants. However, studies on viral evolution by analysis of clonal sequences have demonstrated mixed results. Some studies have detected drug resistance mutations in individuals on highly suppressive therapy [62, 73, 137, 139, 167], whereas other studies have not found resistance mutations [6, 74, 107, 166]. The discrepancies between these studies may be due to sampling differences [126] but some other factors, such as local drug concentration [106] and the level of target cells, may also affect the development of drug resistance in patients on HAART [111].
Mathematical models have been developed to describe sustained, low-level production of virus under potent antiretroviral drugs. Although earlier models have achieved great success in analyzing experimental data on viral loads from the first two phase viral decline, they cannot describe long-term virus dynamics in patients who received effective combination therapy. Many models, as surveyed by Callaway and Perelson [14], cannot robustly produce a steady state viral load below the limits of detectability. Before we address the shortcoming of the basic model (2) and its variations in describing low-level viremia, we note one can reduce model (2) to a simpler form using the fact that the ratio of infectious to noninfectious virus is nearly a constant after a short time following initiation of treatment [194]:
| (7) |
where V = VI + VNI is the total viral load and ε is the overall drug efficacy, which is defined as ε = 1 − (1 − εRT)(1 − εPI).
There is only one possible positive steady state of the viral load:
| (8) |
It is clear that V̄ > 0 if and only if the overall drug efficacy ε is less than a “critical drug efficacy”, εc, above which virus is predicted to be eradicated. εc is given by
| (9) |
The derivative of V̄ with respect to ε is , whose absolute value is very large when ε increases to approach εc [10, 14]. This implies that the steady state viral load is very sensitive to small changes of the drug efficacy ε around εc. Thus, if the model reflects reality, then many patients should have cleared the virus, contrary to what has been observed in the clinic.
Two classes of models were shown not to exhibit extreme sensitivity of the steady state viral load to changes in drug efficacy [14]. In the first model, the death rate of productively infected cells, δ, is assumed to be dependent on the infected cell density. This form is motivated by the notion that infected cells could be cleared by the immune response at a rate proportional to the density of infected cells. Thus, if the death rate of infected cells, δ, is a function of the density of immune effector cells, E, and E is a function of the density of infected cells, T*, then as suggested by Holte et al. [94], a simple way to achieve a density-dependent infected cell death rate is via a power-law function, for example, δ(T*) = δ′T*ω, where ω governs the size of the immune effect on the death rate. Holte et al. [94] further showed that using ω > 0 gave a better fit to viral load decay obtained from children on HAART than the standard model with ω = 0. With this power-law function, the T* and V equations in the basic model (Eq. (7)) can be modified to: dT*/dt = (1 − ε)kVT − δ′T*ωT* and dV/dt = pvT* − cV. Since the model includes a density-dependent infected cell death rate, the viral production rate is decoupled from the cell death rate and virus is assumed to be produced at a constant rate, pv. Other laws for the death rate of infected cells may also give more realistic behavior, but this has not been explored.
There is only one possible positive equilibrium. The steady state viral load is given by V̄ = pvT̄*/c, where T̄* is determined by the equation
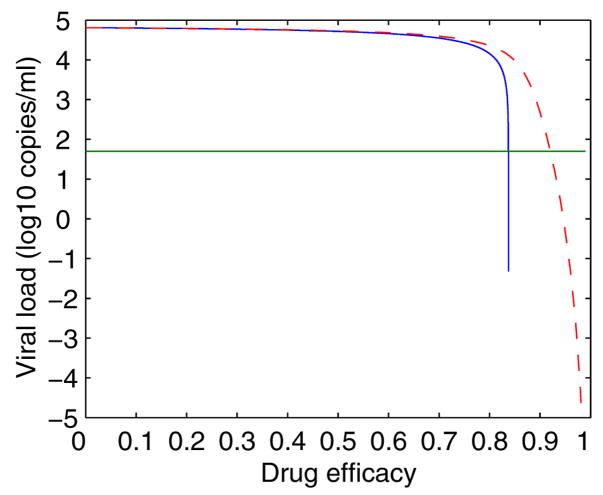
Callaway and Perelson [14] noted that while the steady state viral load in the basic model is very sensitive to changes in drug efficacy, particularly when drug efficacy approaches εc, in the model with a density-dependent infected cell death rate this does not occur (Fig. 2).
Figure 2.
The steady state viral load vs drug efficacy for both the basic model (Eq. (7), solid line) and the model with density-dependent infected cell death (dashed line). The values of parameters are [14]: λ = 104 mL−1 day−1, k = 8 × 10−7 mL day−1, c = 13 day−1, δ = 0.7 day−1, N = 100, d = 0.01 day−1, ω = 0.1, pv = 70 day−1. δ′ is chosen to be 0.274 day−1(mL/cell)ω such that both models have the same initial viral load when ε = 0. The basic model is much more sensitive to changes in drug efficacy, particularly when ε is close to the critical drug threshold (~0.85). The green horizontal line represents the detection limit.
Another class of models in which antiretroviral drugs have different effects in two distinct compartments are also able to model low-level steady state viral loads robustly [14]. The model considers the infection process to occur in two compartments, with one regarded as a drug sanctuary15, and can be described by the following equations:
| (10) |
where Ti, , and Vi represent the concentration of target cells, short-lived infected cells, long-lived chronically infected cells, and free virus, respectively, where i = 1 in the main compartment and i = 2 in the drug sanctuary. The drug efficacy in the sanctuary is reduced by a factor f. The two compartments are coupled by allowing transport of virus between them, which is governed by two rate constants D1 and D2. A subpopulation of chronically infected cells are included in the model because otherwise the viral load may become unreasonably low before it approaches the steady state. A fraction (ϕ) of infection events result in long-lived or chronically infected cells. These chronically infected cells die with a rate constant, μ, and each produces NC virus particles during its lifetime.
There is only one nontrivial equilibrium point of Eq. (10). The steady state viral load is plotted as a function of drug efficacy in Fig. 3. The dashed line describes the two-compartment model and the solid line describes the corresponding one-compartment (the main compartment) model. Because of transport from the sanctuary site to the main compartment, the virus in the main compartment cannot be eradicated even with 100% drug efficacy there. In fact, the dashed curve becomes concave up from concave down near the point of the critical drug efficacy (~0.8) in the one-compartment model, suggesting that the steady state viral load in the two-compartment model is not sensitive to small changes in drug efficacy.
Figure 3.

The steady state viral load vs drug efficacy for the two-compartment model (Eq. (10), dashed line) and its corresponding one-compartment model (solid line). The values of parameters are [14]: f = 0.45, ϕ = 0.195, μ = 0.07 day−1, NT = 100, NC = 4.11, D1 = 0.1048 day−1, D2 = 19.66 day−1. The other parameter values are the same as in Fig. 2. The two-compartment model is less sensitive to changes in drug efficacy than the one-compartment model. The green horizontal line represents the detection limit.
A similar model that accounts for heterogeneous drug responsiveness in two types of target cells cocirculating in a single compartment was also shown capable of simulating the persistence of residual HIV replication in the face of potent drug treatment [14]. This points in the direction of improving drug efficacy in all cell types and physiological compartments in order to improve the chance of ultimately eradicating virus from infected individuals.
To summarize, the model with density-dependent infected cell death is a simple way to reduce the sensitivity of viral loads to changes in drug efficacy. However, we do not know the accurate relationship between the death rate and the concentration of infected cells. Models with different types of target cells or cell compartments are more biologically feasible, although they involve more parameters.
3.2 Virus released from latent reservoirs
Release of virus by activation of latently infected cells in latent reservoirs may also contribute to low-level persistent viremia. Direct evidence of proviral latency came from the isolation of resting memory CD4+ T cells with stably integrated HIV-1 DNA16 [26]. In these latently infected cells virus production is extinguished because of the absence of active forms of the host transcriptional activators that are believed to be necessary for HIV-1 gene expression [119]. Hence, latently infected cells are almost indistinguishable from uninfected CD4+ T cells. The virus persists in the resting cell as integrated DNA, unaffected by antiretroviral drugs. When the cell encounters an antigen that it recognizes or is activated by cytokines or other stimuli, upregulation of viral gene expression results in active viral production. The establishment, dynamics and nature of the latent reservoir that consists of latently infected CD4+ T cells in the setting of HAART will be discussed in detail in Section 4. Here we review the evidence that low-level persistent viremia may originate from the latent reservoir and discuss developments of mathematical models that describe low-level viremic persistence by considering latent cell activation.
In the majority of patients who received uninterrupted antiviral therapy and who remained aviremic for up to 9.1 years, Chun et al. [29] detected substantially higher levels of HIV proviral DNA in activated CD4+ T cells than resting CD4+ T cells. Considering the short half-life of activated CD4+ T cells and phylogenetic analysis of viral sequences isolated from resting and activated CD4+ T cells, they suggested that virions released due to activation of latently infected resting CD4+ T cells might spread to and infect neighboring activated as well as resting CD4+ T cells. In this way, activation of latently infected resting CD4+ T cells, most likely as a consequence of normal immunologic responses to various relevant antigens, routine vaccination [212], or induction of cytokines [23], may offer a plausible mechanism to explain HIV-1 persistence in infected individuals receiving effective therapy.
Eighteen HIV-infected patients, including seven children and 11 adults, were enrolled in another longitudinal study of HIV-1 reservoirs [84]. Many of them achieved viral loads below 50 RNA copies/mL after treatment. Among the patients with prior nonsuppressive therapy, mutations were found but they were resistant to drugs used in the pre-HAART treatment. These resistance profiles suggest that low-level viremia may not lead to development of new resistance mutations, and drug resistant HIV-1 variants may result from archival, pre-HAART virus that has been deposited in the latent reservoir [84]. Genetic analyses of low-level plasma HIV-1 in six of children were further performed to fully characterize the persistent viremia and the evolution of resistance mutations in the protease gene [176]. Despite the frequent use of nelfinavir, a drug that has a low mutational barrier to resistance, over a long observation period, accumulation of mutations in protease was not observed. Extensive commingling of protease sequences from plasma virus and replication-competent HIV-1 recovered from resting CD4+ T cells indicated that the low-level plasma virus is very likely to originate from the latent reservoir [176].
Study of rebounding virus upon cessation of suppressive drug therapy also provides a useful way to characterize the low-level persistent viremia in suppressed individuals because continuously produced virus is most likely to rebound when treatment is stopped. HIV-1 quasispecies17 that rebound following a single interruption of HAART were found to be similar to the viral quasispecies present before initiation of therapy, suggesting that rebounding plasma viremia might originate from archived virus present in the pool of resting CD4+ T cells [97]. This is supported by the observation of rapid reappearance of wild-type virus in a number of patients with drug resistant variants after interruption of treatment [43, 44, 146, 221]. The replacement of drug resistant variants by the wild-type virus was abrupt and fast, implying that it was a consequence of reappearance of archived wild-type virus from the latent reservoir rather than the reversal of mutations from resistant viral variants [159]. Zhang et al. [233] reported on the genetic characterization of rebounding virus in eight patients with plasma viral load below the limit of detection for about three years. The rebound virus in five patients who did not show evidence of viral replication was genetically identical to sequences isolated from the latent reservoir. In two patients with some degree of residual viral replication, the rebound virus was genetically different from the latent reservoir virus, corresponding instead to minor viral variants detected during the course of treatment in lymphoid tissues. This supports the idea that in cases with apparently complete HIV-1 suppression by HAART, low-level viremia persistence may originate from activation of virus from the latent reservoir. Whereas in patients with incomplete suppression, the low-level virus is likely triggered by low-level ongoing replication [158].
Another study by Davey et al. [36] suggested that the rebound virus shortly after therapy interruption might not originate from the pool of latently infected, resting CD4+ T cells in patients who had achieved suppressed viral loads over prolonged periods. They did not find an obvious correlation between the kinetics of viral rebound in plasma upon cessation of HAART and the size of the latent reservoir before discontinuation of the therapy [36]. The sources contributing to the early rebounding virus after cessation of therapy were further studied by Chun et al. [21]. The rebounding virus in the majority of patients was genetically distinct from both the cell-associated HIV RNA and the replication-competent virus within the pool of latently infected CD4+ T cells. This indicates that resting CD4+ T cells do not account entirely for the early viral rebound and other HIV-1 reservoirs may exist in the setting of HAART. This result is also supported by genetic characterization of rebounding plasma HIV-1 during multiple interruptions of HAART [53].
Mathematical models have been developed to consider activation of latently infected cells and examine whether the activation is responsible for the observed low-level persistent viremia. A basic model that includes latent cell activation is [171]
| (11) |
where η is the fraction of infections that result in latency rather than active production of HIV particles. aL is the rate at which latently infected cells become activated. dT and dL represent the death rate of susceptible target cells and latently infected cells, respectively.
There is a single nontrivial positive equilibrium of model (11):
| (12) |
Similarly, V̄ > 0 if and only if the efficacy ε is less than a critical efficacy, εc, which is given by
| (13) |
By the same arguments as in Section 3.1 we can establish that the steady state viral load is very sensitive to small changes in drug efficacy and thus it is difficult to obtain robust low-level viremic persistence during therapy with Eq. (11).
Rong and Perelson have recently extended this model by considering asymmetric cell division [194] or programmed expansion and contraction [195] of latently infected cells upon exposure to antigen, and showed that the low-level viral load during HAART could be maintained solely by latent cell activation. These models will be discussed in detail in Section 5.
Taken together, several factors, including residual viral replication in the presence of HAART, virus released from the reservoir of latently infected CD4+ T cells, and other possible fluid, anatomical and cellular compartments that have reduced drug penetrance, may all contribute to low-level viremic persistence. Quantitative understanding of these sources and their relative contributions would provide valuable information that could potentially allow the design of more efficient treatment strategies.
4 Latent reservoir
Because latently infected CD4+ cells can rekindle productive viral infection when treatment is withdrawn and because they have a very slow decay rate in the majority of patients on HAART [56, 185, 202, 235], the latent reservoir has been considered as a major obstacle to viral eradication (see reviews in [8, 25]).
4.1 Establishment during primary infection
Why an HIV-infected CD4+ T cell becomes latent remains largely obscure. A few molecular mechanisms have been proposed to explain latency (reviewed in [119]), for example, inaccessibility of integrated proviruses to the transcriptional machinery [103], absence of host transcriptional factors [52, 154] or HIV-1 Tat [105], an important protein that upregulates HIV-1 gene expression. Since latent viral genomes have been shown to reside within the introns of actively transcribed genes [80], the first mechanism seems less likely [119]. Weinberger et al. [224] presented an integrated experimental and computational study of an HIV-1 model vector, and showed that Tat fluctuations could generate distinct phenotypes analogous to productive and latent viral infection.
Another critical issue related to HIV-1 latency is how early the latent reservoir is established during the course of HIV-1 infection. Chun et al. [22] demonstrated that initiation of HAART in patients as early as 10 days after the onset of symptoms of primary infection did not prevent generation of a latent reservoir. There was no significant correlation between the frequency of resting CD4+ T cells harboring integrated HIV-1 DNA and the time of initiation of treatment, suggesting that once plasma viremia is present and virus is disseminated to lymphoid organs, the latent reservoir has already been established.
4.2 Decay characteristics of the latent reservoir
The decay characteristics of the latent reservoir remain controversial. Estimates of its half-life have been quite divergent. Finzi et al. [55] estimated the mean half-life of the reservoir in chronically infected patients to be ~44 months. Thus, even with a conservative estimate that the latent reservoir consists of only 105 cells, more than 60 years of treatment would be required to eradicate this compartment in most patients. In fact, the mean slope of the decay in some patients was not statistically different from zero, suggesting that the reservoir in these patients may not decay in the setting of HAART.
A more rapid decay rate was reported by Zhang et al. [235] in a subset of acute seroconvertors who started combination therapy within 90 days of infection. Eight men were selected from over 100 subjects enrolled in the clinical trial because they had been fully compliant with the prescribed antiretroviral therapy and plasma viremia was suppressed to below 50 copies/mL after 5 months of treatment. Using two independent approaches, they estimated that the half-life of the latent, replication-competent virus in resting CD4+ cells was approximately 6 months. In a recent study, Chun et al. [27] obtained an even shorter half-life of ~4.6 months in patients who initiated antiretroviral therapy early in infection. This result suggested that it would take up to 7.7 years of therapy to completely eradicate the reservoir in these patients.
The apparent discrepancy between the half-life estimates was addressed in the study of Ramratnam et al. [185]. With a quantitative micro-culture assay, they demonstrated that the latent reservoir decayed with a mean half-life of about 6 months in patients who consistently maintained plasma virus of less than 50 copies/mL, whereas in those patients who experienced intermittent episodes of viremia the decay was much slower. Thus, the latent reservoir persistence is likely to some extent due to the continual replenishment by active viral replication. In addition, many other factors, such as unintentional selection of patients with rapid decay of the reservoir, the duration of follow-up studies, and differences in the assay sensitivities, may also strongly impact the estimate of the decay rate of the reservoir [202].
The above estimates assume that long-lived infected cells belong to a homogeneous population exhibiting exponential decay characteristics. However, Strain et al. [216] suggested that the decay rate of latently infected cells could decelerate during treatment. They analyzed the decay kinetics of HIV-1 DNA and of cell-associated infectivity, and estimated that the latent reservoir had a median half-life of 20 weeks during the first year of therapy, whereas the decay slowed significantly during the subsequent 3 years with a median half-life of 70 weeks. Furthermore, it seems that the deceleration is continuing. The decelerating decay of the latent reservoir can be explained by the heterogeneity in the activation rate of latently infected cells [108, 150, 215]. Cells specific for frequently encountered antigens may be preferentially activated and quickly removed from the reservoir during the first year, whereas cells specific for rarely encountered antigens may persist without activation or be activated slowly in subsequent years.
A simple mathematical model was employed by Muller et al. [150] to study the decay characteristics of viral load by considering the heterogeneity of latent cell activation after prolonged fully suppressive therapy. Let L(t) be latently infected cells, which die at rate dL and are activated at rate a into the productively infected class, T*. The model reads:
| (14) |
Since the dynamics of latently infected cells are much slower than those of productively infected cells, Muller et al. [150] assumed the latter to be a quasi-steady state. Setting the second equation to zero, one has T*(t) = (a/δ)L(t). Similarly, as virus turns over much faster than productively infected cells [174], the viral load will follow the latter as a constant ratio: V(t) = (p/c)T*(t). Therefore, the viral load maintained by activation of latently infected cells is
Considering the heterogeneity in the activation from the latent to productive infection, they assumed that the latently infected cell pool had an initial distribution of activation rates. Using a continuous range of activation rates, instead of a single fixed value, the above viral load equation changes to
where L0(a) represents the initial distribution of latently infected cells with respect to the activation rate, which has the maximum value, amax.
To obtain the total viral load from initiation of therapy, they also incorporated other virus-producing cell populations into the model. From the results given in [171], they obtained the total viral load
| (15) |
where and M0 represent the initial population of productively infected cells and long-lived infected cells, respectively. pM and δM are the virus production rate and death rate, respectively, of the latter cells.
Plotting V (t) according to (15) shows a triphasic decline in which the third phase reflects the long tail of the decelerating viral production by activation of latently infected cells [150]. This suggests that the population of latently infected cells will gradually shift toward cells with a small probability of activation, i.e., cells specific for increasingly rare antigens. In this regard, the progressively decelerating clearance of latently infected cells can explain the observed persistence and long-term dynamics of HIV-1 during effective therapy. Notice that a triphasic decay can also be formed from (5) if δ, μM and μL are well separated.
Kim and Perelson [108] developed another model that studies the decay characteristics of the latent reservoir. Their model includes two novel features. First, as suggested by the observations in [216], the model assumes that the rate of latent cell activation decreases with time during antiretroviral therapy, leaving behind cells that are specific for increasingly rare antigens and less frequently activated. Second, the model includes bystander proliferation18 of latently infected cells without transitioning them into active viral production, which contributes to the maintenance of the latent reservoir. The model is described as follows:
| (16) |
where a(t) is the activation rate that exponentially decays with the deceleration rate ω, from the initial value, a0, to a minimum value, amin. r represents the net effect of bystander proliferation and death, i.e., r = pbs − dL, where pbs is the bystander proliferation rate and dL is the death rate of latently infected cells.
From the L(t) equation in (16), T cell bystander proliferation, latent cell activation, and ongoing viral replication (determined by drug efficacy) all impact the decay of the latent reservoir. In the case of perfect treatment (ε = 1), the final size of the latent reservoir is fully determined by the relationship between the net proliferation rate, r, and the minimum activation rate, amin. If amin is assumed to be zero, then the pool size of the latent reservoir keeps increasing when r > 0, stabilizes to a steady state when r = 0, and is always decreasing when r < 0. However, even if the proliferation rate r is slightly greater than zero, the model still cannot maintain low-level viremia (Fig. 4). This can be explained analytically from the T* equation in (16). As ε = 1 and a(t) → amin = 0, the asymptotic behavior of productively infected cells is determined by the equation: dT*/dt = −δT*, which yields T* → 0 and thereby V → 0. In order to simulate the persistence of both low-level viremia and the latent reservoir, the minimum activation rate, amin, has to be greater than zero (Fig. 5). In the scenario of amin > 0, the size of the latent reservoir keeps on increasing when r > amin, approaches a steady state when r = amin (Fig. 5), and decreases when r < amin. The viral load can be maintained at a low level as long as aminL = δT*.
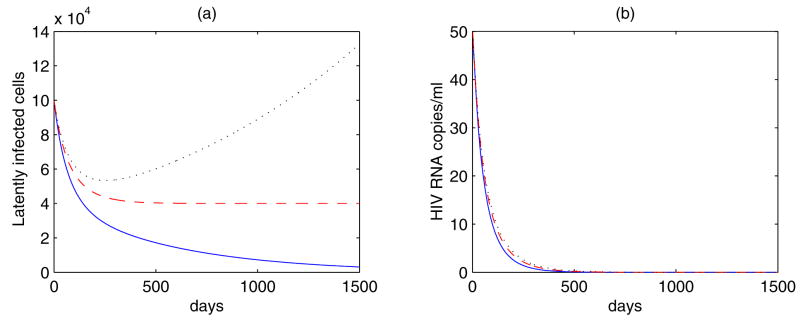
Figure 4.
Simulations of Eq. (16) with amin = 0 and ε = 1. The net proliferation rate r varies: r = −0.00171 day−1 (blue solid line); r = 0 (red dashed line); r = 0.0008 day−1 (black dotted line). The other parameter values are [108]: ω = 9.39 × 10−3 day−1, η = 3 ×10−6, k = 2.4 ×10−8 mL day−1, T0 = 595 cells/μl, N = 2 × 104, δ = 1 day−1, c = 23 day−1. (a) The pool size of latently infected cells increases as r > 0, decreases as r < 0, and stabilizes to a steady state as r = 0. (b) In each case, the viral load decreases to zero. Thus, low-level viremic persistence cannot be generated by Eq. (16) with amin = 0 and ε = 1.
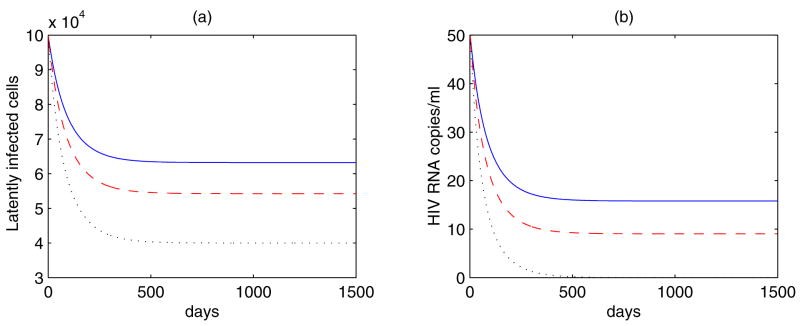
Figure 5.
Simulations of Eq. (16) with amin ≥ 0 and ε = 1. The value of r varies: r = amin = a0/2 (blue solid line); r = amin = a0/3 (red dashed line); r = amin = 0 (black dotted line), where a0 = 8.625 ×10−3 day−1 is the initial activation rate of latently infected cells. The other parameter values are the same as in Fig. 4. Both the viral and latent reservoir persistence can be obtained when r = amin > 0. The larger the value of r, the higher the levels of latently infected cells and viral load.
When treatment is not 100% effective (ε < 1), the analysis becomes complicated. In the case of amin = 0, there exists a critical drug efficacy, εc = 1 − c/((1 − f)N kT0). For ε > εc, both the virus and the latent reservoir decay toward zero. The contribution of ongoing viral replication to the level of latently infected cells remains insignificant. When ε = εc, the viral load approaches a nonzero steady state, whereas the latent cell population does not stabilize unless r < 0. When ε is slightly less than εc, the viral load keeps increasing and the latent reservoir eventually blows up after an initial decline.
These results suggest that ongoing viral replication together with bystander proliferation of latently infected cells is able to describe the extremely slow decay or stability of the latent reservoir and the persistence of low-level viremia during potent treatment. However, the model is still sensitive (e.g., the viral load and the size of the latent reservoir) to the net proliferation rate, r, and the drug efficacy, ε. If viral blips also originate from activation of latently infected CD4+ cells, then this activation will consume the latent reservoir more quickly than what we observed in clinical measurements. In Section 5, recent developments of models that describe viral blips will be discussed. Some models are shown to be robust in maintaining the latent reservoir as well as low-level persistent viremia. More interestingly, they demonstrate the potential to explain the differences between the divergent estimates of the half-life of the latent reservoir in the literature.
4.3 Mechanisms of the reservoir stability
Although latently infected cells are rare in plasma, with a frequency on the order of 1 in 106 resting CD4+ T cells [19], they are of considerable concern because they decay extremely slowly and can be activated to produce virus, which can rekindle infection when treatment is interrupted. The factors that contribute to the remarkable stability of the latent reservoir have not been fully elucidated. As discussed above, a low level of ongoing active viral replication may reseed the latent reservoir in the presence of HAART [29, 185, 215]. Persistence of the latent reservoir could also stem from the intrinsic stability of latently infected cells. The latent reservoir consists at least of a number of resting memory CD4+ T cells [55, 202, 204, 215], whose intermitotic half-life is around 6 months [141, 145]. As memory T cells can be maintained by occasional proliferation in some murine systems [210, 218], latently infected CD4+ cells might reseed the reservoir if they can also undergo such occasional proliferation events upon activation that do not fully turn on HIV-1 gene expression [181]. In this situation, the latent reservoir may decay with a much longer half-life than 6 months. This idea is supported by the model of Kim and Perelson [108] discussed above (Section 4.2). A recent study by Chomont et al. [18] shows that persistence of the latent reservoir can be maintained by homeostatic proliferation, which provides the first evidence for the validity of the model proposed in [108].
Recent evidence from genotypic analysis seems to favor the hypothesis that the reservoir stability comes principally from the intrinsic stability of latently infected cells. Strain et al. [215] exploited a predictable drug resistance mutation in the viral reverse transcriptase active site to track cells infected during suppressive therapy and to identify cells within the latent reservoir replenished by ongoing viral replication. This allowed them to quantify both the intrinsic turnover rate of the reservoir and the extent of its replenishment by residual replication. The result showed that even though ongoing replication can replenish the reservoir, it cannot explain the observation that cells that were recently infected and contained viral DNA bearing mutations were cleared more rapidly than cells that were infected earlier and contained the wild-type codon. Further analysis suggested that the latent reservoir has a heterogeneous and dynamic composition, and that the reservoir stability reflects decreasing decay rates of subpopulations within the latent pool [215]. In the study of Ruff et al. [196], archival wild-type virus was found to persist in resting CD4+ T cells despite selective pressure that favors drug resistant mutants in a cohort of children for a prolonged period of time. In addition, viral sequences isolated from latently infected cells at later time points did not show greater divergence than those isolated from earlier time points, indicating that HIV-1 persists in a latent form in long-lived resting memory CD4+ T cells. In some patients failing HAART, Monie et al. [149] found that resting CD4+ T cells harbored a diverse array of wild-type and archival drug resistant viruses. However, their pol sequences were genetically distinct from contemporaneous plasma virus that consisted of a relatively homogeneous population of drug resistant viruses. These results all support the idea that the reservoir stability results primarily from the intrinsic stability of resting memory CD4+ T cells. The influence of ongoing viral replication on the decay of the latent reservoir remains insignificant, particularly in patients who have suppressed plasma viral loads for long periods of time. However, the precise extent to which residual viral replication reseeds the latent reservoir during HAART has not been fully delineated.
Mathematical modeling has the potential to shed light on the quantitative contribution from ongoing viral replication. Siliciano and his colleagues detected a predominant plasma clone (PPC) of HIV-1 that dominates the residual viremia in some patients on HAART [3]. If the latent reservoir is replenished by viral replication, then this unique sequence would be incorporated into the latent reservoir and be readily detected at later time points since its genotype is different from sequences in resting CD4+ T cells. In this regard, the PPC sequence can serve as a functional label for measuring the rate of replenishment of the latent reservoir in the setting of HAART. They developed a model for the dynamics of the latent reservoir and estimated the maximum daily flow of cells into the latent reservoir [199]. The model includes two variables, latently infected cells containing PPC proviruses (L1) and latently infected cells containing all other proviruses (L2), and is described by the following equations:
| (17) |
where kin is the rate at which free virus enters the latent reservoir, kout is the decay rate of latently infected cells, and f represents the fraction of plasma virus that consists of the PPC.
Introducing K = kin/kout, the above system of equations can be solved explicitly to give
| (18) |
Assuming that the PPC permanently disappears from the plasma after a period of time te, the two subpopulations of latently infected cells at time t > te are governed by the equations:
| (19) |
The corresponding solution for t > te is given by
| (20) |
where L1(te) and L2(te) are solutions (18) evaluated at te. Thus, the fraction of latently infected cells containing the PPC at time t > te, ΦPPC, can be calculated from equations (20) as
where K = kin/kout. The probability that there are m PPC sequences out of n total sequences can be approximated by a binomial distribution:
Using this probability distribution, a maximum likelihood approach was employed to estimate the value of kin from patient data [199]. The magnitude of the maximum daily flow of cells into the latent reservoir was very small compared with the overall latent reservoir size, suggesting that ongoing viral replication is unlikely to significantly influence the decay of the latent reservoir.
5 Intermittent viral blips
Although many infected individuals exhibit sustained low-level viremia on HAART, a number of them have occasional viral load measurements above the detection limit. Such transient episodes of detectable viremia are called “blips” (Fig. 1). Since viral blips are relatively rare events, neither their occurrence timing, frequency, duration, amplitude nor their etiology is well known. With more extensive sampling viral blips are more likely to be identified in the majority of patients at some time.
5.1 Characteristics of viral blips
There are three main features characterizing intermittent viral blips: blip frequency, viral amplitude, and duration of occurrence. Di Mascio et al. [45] examined viral blip time series obtained from 123 HIV-1 infected patients and found that the mean blip frequency was 0.09±0.11/sample, and the mean blip amplitude was 158±132 RNA copies/mL. Although the frequency and amplitude of blips did not increase with longer periods of observation, the frequency was inversely correlated with the CD4+ T cell count at the start of therapy [45]. By comparing the dynamics of blips in two treatment subgroups, they concluded that the blip frequency was roughly two-fold higher in patients treated during chronic infection than in patients treated during acute infection [46]. They also suggested that blips were not isolated random events, but rather extended transient episodes of viremia with a duration of roughly 3 weeks [47]. The amplitudes of blips observed in these patients do not share a common probability distribution, and thus blips may not be interpreted as being due solely to assay variations [169]. They further showed that the blip amplitudes were consistent with random sampling from a profile in which the viral load rises rapidly to a peak, followed by a slower biphasic decay [48].
5.2 Origin of blips
A number of mechanisms have been proposed to explain the occurrence of viral blips. By comparing the proportion of episodes occurring in patients who received or did not receive triple-drug therapy, it has been suggested that intermittent viremia might occur because of higher levels of viral replication [81], including selection of drug resistant variants [34]. Thus, there was concern that viral blips could represent viral evolution [73] through active virus replication and, thereby, signal imminent virological failure [67]. However, in other studies viral blips were not associated with virological failure [81, 138, 147, 207], even though in some cases they were associated with the emergence of new drug resistant HIV variants [34, 131]. By comparing viral sequences derived from transient viremia with sequences from PBMC collected before and during HAART, Tobin et al. [217] showed that viral blips could result from production of virus following immune activation and clonal expansion of latently infected cells. Other mechanisms for the generation of viral blips include antigen-driven target cell activation [60, 62] due to vaccination [75, 100] or opportunistic infections19 [101, 102]. However, viral blips, especially when of low amplitude, can also be the result of laboratory error or statistical variation rather than genuine viral rebound [123, 157]. Nettles et al. [157] examined the characteristics of viral blips by intensive sampling of 10 patients over a period of 90 days. In their sample, blips were common (9 of 10 patients), brief in duration (median, less than 3 days), low in magnitude (median, 79 copies/mL), and poorly reproducible on independent testing. Moreover, blip frequency was not related to illness, vaccination, or drug concentrations. These observations suggest that random biological or statistical variation around a mean viral load less than 50 copies/mL might be responsible for the aberrant viral load measurements they observed. In summary, the nature and clinical significance of viral blips are not well known, some may result from measurement error while others may reflect transient episodes of viral replication.
5.3 Models of viral blips
Recently, a few mathematical models have been developed to describe intermittent viral blips, as well as the extremely slow decay of the latent reservoir. These models mainly consider two types of cell activation: target T cell activation and latently infected cell activation.
5.3.1 Target CD4+ T cell activation
Motivated by the observations that vaccination [75, 100] or opportunistic infection [143] is associated with enhanced viral replication, and the modeling studies of Ferguson et al. [54] and Fraser et al. [59, 60] that antigen-driven T cell proliferation can result in a burst of virus production, Jones and Perelson [101] proposed a mathematical model for immune cell expansion in the presence of pathogen. The model is an extension of the model proposed in [14], in which two co-circulating populations of target cells have different drug penetration and hence one population has reduced drug efficacy. Let A be a pathogen or some other growing antigen that stimulates an immune response. The model that considers antigen-driven target cell activation is given by
| (21) |
where T1 and T2 represent two types of co-circulating CD4+ T cells. In one population (T1), drug efficacy is ε, and in the second population (T2), drug efficacy is reduced by a factor f < 1. As in Eq. (10), the model also includes productively infected cells, and , and chronically infected cells, and .
In Eq. (21), g(A) is an activation function, which depends on the antigen concentration and can be approximated by a type-II functional response:
where a is a maximum T cell activation rate and K4 is a half-saturation concentration of antigen that stimulates a CD4+ cell response.
On exposure to antigen, naive CD8+ T cells20 experience a burst of proliferation, undergoing a programmed cascade of divisions that culminate in the production of mature activated effector cells [104]. Let N0 be the initial naive CD8+ T cells, and Ni the CD8+ cells that have completed i divisions. The following equation describes such T-cell proliferation under antigenic stimulation [101]:
| (22) |
Here pathogen undergoes density-dependent growth governed by a logistic term with a maximum growth rate, r0, and the carrying capacity, Amax. γ is the clearance rate constant for the pathogen due to effector cells, E. Cells are assumed to become effectors after a number of divisions, say, four, and stop proliferating after more divisions, say, eight. Thus, p is the constant proliferation rate for all divisions after the first; d0, d, and dE (d0 < d < dE) are the mortality rate of naive cells, non-effector proliferative cells, effector cells, respectively.
The rate of the first T cell division is antigen-dependent and is described by the function [101]
| (23) |
where p0 is the maximum proliferation rate, K8 is antigen half-saturation for stimulating CD8+ T cells, and n is the Hill coefficient that determines the steepness of the response.
Following exposure to antigen described by Eq. (22), target cell activation (Eq. (21)) is shown capable of generating a transient increase of the viral load (Fig. 6). Thus, transient episodes of viremia can be explained by occasional activation of the immune system by opportunistic infections [101].
Figure 6.
Simulation of the target cell activation model (Eq. (21)). The model can generate a transient viral load increase. The parameter values are [101]: λ1 = 104 mL−1 day−1, λ2 = 56 mL−1 day−1, k1 = 8 × 10−7 mL day−1, k2 = 10−4 mL day−1, ε = 0.9, dT = 0.01 day−1, f = 0.6, ϕ = 0.195, δ = 0.7 day−1, μ = 0.07 day−1, NT = 100, NC = 4.11, c = 23 day−1, a = 0.2 day−1, K4 = 1000 mL−1, r0 = 2 day−1, Amax = 108 mL−1, γ = 10−3 day−1, d0 = 0.01 day−1, p = 2.92 day−1, d = 0.1 day−1, dE = 0.325 day−1, p0 = 1 day−1, n = 1, K8 = 1000 mL−1. The green horizontal line represents the detection limit.
Many earlier models of HIV infection that include the immune response are phenomenological rather than mechanistic. For example, they used simple predator-prey type dynamics to describe the interaction between infected cells and immune cells [160, 226, 227]. Very few models have included detailed T cell responses to antigen. The model (Eqs. (21) and (22)) was built through a series of incrementally more complex models, which capture the main features of CD8+ T cell responses upon encounters with antigen or opportunistic infections and help explain the occurrence of viral blips in HIV-infected patients on effective treatment. Despite its complexity, the model is still a simplification of the processes underlying immune activation. It neither considers CD4+ T cell help, antibody responses, nor distinguishes HIV-specific CD8+ and CD4+ T cells from non-specific T cells. Due to limited space, we do not discuss additional models that include an immune response. The reader is referred to [162, 170, 225, 228] and references cited therein.
5.3.2 Latently infected cell activation: asymmetric division
Two classes of models have recently been developed that study activation of latently infected cells upon stochastic antigenic stimulation in HIV-infected patients on HAART [194, 195]. In the first model [194], latently infected T cells are hypothesized to undergo asymmetric divisions like stem cells [78, 96, 98] or T cells [15] on exposure to their relevant antigen. When a latently infected cell divides, one daughter cell is activated that can produce new virus, whereas the other daughter cell remains in the resting state with varied probability, providing the potential to renew the latent reservoir. To study the influence of asymmetric division on the latent reservoir and viral load, the following model was considered:
| (24) |
where pL represents the probability that a daughter cell remains in the latent state when a latently infected cell divides upon antigen stimulation. After each division, 2pL latently infected cells and 2(1 − pL) activated infected cells are generated. As discussed in Section 3.1, the model includes a density-dependent infected cell death rate, δ′T*ω, and virus is produced at a constant rate, pv, from each infected cell.
The dynamics of antigen were not modeled explicitly as in [101]. Instead, a basic “on-off” model that has successfully describe the immune response to lymphocytic choriomeningitis virus (LCMV) infection [39, 40] was adopted to approximate antigenic stimulation of latently infected cells. The activation function f(t) takes on the value 0 when there is no activation and 1 when there is full activation [39], i.e.,
| (25) |
where Ton is the time at which activation starts and Toff is the time when the activation ends. Δt = Toff − Ton is the duration of the activation.
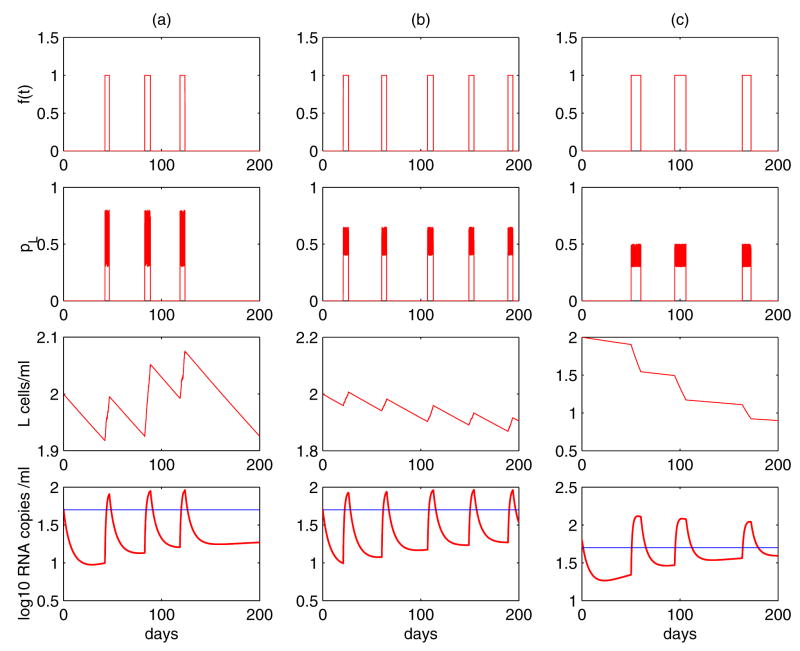
Figure 7 shows the results of stochastic simulations of Eq. (24). The timing, frequency and duration of the blips are determined by when, how often and for how long the activation occurs. Several factors, including the probability that a daughter cell remains in the latent state, the level of latently infected cells, and the drug efficacy, all impact the amplitude of viral blips in the simulation. An interesting result is that occasional replenishment of the latent reservoir induced by asymmetric division of latently infected cells upon intermittent antigenic stimulation is able to explain the differences between the divergent estimates of the half-life of the reservoir. With different distributions of the probability pL, Fig. 7 exhibits three distinct decay profiles of the latent reservoir. In column (a), a statistically significant decay of the reservoir was not seen. This is consistent with the clinical observations in some patients [55]. In column (b), although occasional activation replenishes the latently infected cell pool, the size of the reservoir diminishes gradually. However, the decay is extremely slow due to the occasional replenishment. This scenario might correspond to a long half-life of ~44 months estimated in references [55, 202]. Column (c) is with a small probability pL, which implies that the renewal ability of the reservoir is poor. Occasional encounters with antigen activate latently infected cells to produce virus and consequently consume the latent reservoir more quickly than in previous scenarios. This case might explain the short half-life (for example, ~6 months) estimated in other references [27, 185, 235]. Why pL would differ between patient populations is unclear and needs further clarification.
Figure 7.
Simulations of Eq. (24) show that it is able to generate intermittent viral blips. (a) The interval between two consecutive activations, ΔT, obeys a normal distribution N (50, 10). The duration each activation lasts, Δt, obeys a uniform distribution U (4, 6). The probability pL obeys a uniform distribution U (0.3, 0.8). The decay rate of the latent reservoir is not significantly different from zero. (b) ΔT ~ N (40, 10), Δt ~ U (4, 6), pL ~ U (0.4, 0.65). The latent cell pool size decreases, but the decay rate is very small, representing a very long half-life, for example, 44 months. (c) ΔT ~ N (50, 10), Δt ~ U (7, 14), pL ~ U (0.3, 0.5). The latent reservoir shows a quicker decay than in the scenario (b), corresponding to a shorter half-life, such as 6 months. The other parameter values used in the simulations are [194]: λ = 104 mL−1 day−1, dT = 0.01 day−1, ε = 0.85, k = 2.4 × 10−8 mL day−1, η = 0.001, d0 = 0.001 day−1, aL = 0.1 day−1, δ′ = 0.7863 day−1(mL/cell)ω, ω = 0.44, pv = 2000 day−1, c = 23 day−1.
Although the model presented above can explain both the occurrence of intermittent viral blips and a stable latent reservoir in patients on HAART, we do not know whether latently infected CD4+ T cells undergo asymmetric division like stem cells or T cells as shown in [15]. This awaits direct experimental verification in the future.
5.3.3 Latently infected cell activation: programmed expansion and contraction
Both CD4+ and CD8+ T cell responses to infectious agents, e.g., LCMV [95], consist of three distinct phases: initial antigen-driven expansion and differentiation into effector cells, followed by rapid contraction of activated cells and formation of a small number of memory cells [2]. By developing mathematical models that include these phases, De Boer et al. [39, 40] studied CD4+ and CD8+ T cell responses to LCMV. Using a model that accounts for the programmed cascade of divisions during expansion of the CD8+ T cell response (Eq. (22)), Jones and Perelson [102] showed that latent cell activation is able to explain transient episodes of viremia observed in well-suppressed patients on effective treatment. They developed the following model:
| (26) |
where L0 denotes resting latently infected cells that have not divided and Li denotes cells that have been stimulated by antigen and have completed i divisions. p0(A) is the proliferation rate of a cell in its first division, which can be defined as in (23) but with K8 now denoting the half-saturation of antigen that stimulates a latent cell into division. Activated cells proliferate at rate p, die at rate dLA, transition into the productively infected class at rate aL, and revert to resting at rate ρ. Antigen dynamics are described in (22). The model also uses a density-dependent death rate of productively infected cells.
Simulations of Eq. (26) together with (22) demonstrate that latent cell activation caused by sporadic immune activation can generate viral blips [102], as shown in Fig. 6.
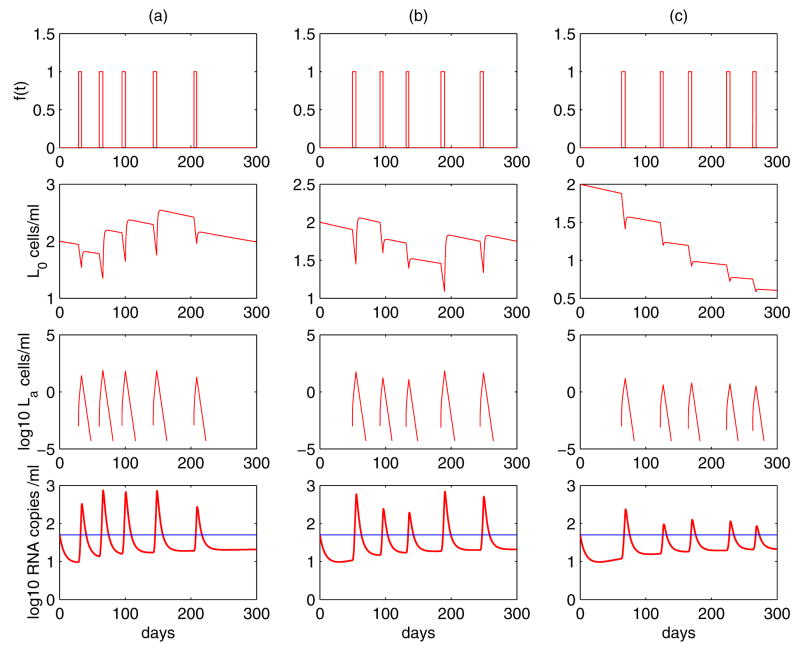
Based on (26), Rong and Perelson [195] developed a new model in which latently infected cells are hypothesized to experience a programmed expansion and contraction in the latently infected CD4+ T cell response to their specific antigen. With the assumption that a small portion of the activated cells revert back to a resting state by the process that normally generates memory CD4+ T cells, the latently infected cell pool can be replenished by occasional activations. Let L0 represent resting latently infected CD4+ T cells, which encounter their relevant antigen and are activated to enter La, the class of activated cells. The model describing the programmed expansion and contraction of latently infected cells upon antigen encounter is as follows:
| (27) |
where the activation function f(t) is given by (25). When the antigen is present, resting latently infected cells, L0, can be activated into the La class with a constant rate a. Activated cells proliferate at rate p. Following the expansion phase, there is a contraction phase in which activated cells have death rate σ, and can revert to the resting state at rate ρ. In addition, activated cells transition into productively infected cells at rate aL. A density-dependent death rate of productively infected cells is employed again to maintain the robust low steady state viral load.
Simulations of Eq. (27) show that it is able to generate viral blips with amplitude and duration consistent with the findings of Di Mascio et al. [45] (Fig. 8). The antigen induces temporary substantial proliferation of activated cells, which then reseed the latent reservoir. The extent to which the latent cell pool is replenished depends on the proliferative ability of activated cells. In Fig. 8(a), the proliferation rate is chosen to be p = 1.4 day−1, which implies that cell divisions occur 8 – 12 times over an interval of 4 – 6 days. In this case, the activation induces a high level of activated cells, and hence the amplitude of viral blips remains relatively high. Accordingly, there are many activated cells going back to the resting state. Thus, no statistically significant decay of the latent reservoir was observed, suggesting that the viral reservoir can be extremely stable. Figure 8(b) shows an example with a slightly smaller proliferation rate, p = 1.35 day−1. Although activated cells and the viral load increase to high levels, there is still a decay of the latent reservoir. However, the decay is very slow, with a half-life of approximately 44 months. In Fig. 8(c), the proliferation rate is chosen to be p = 1 day−1, which means cells divide 6 – 8 times during 4 – 6 days. In this situation, fewer activated cells are produced, and the reservoir replenishment is limited. Consequently, the latent reservoir decays relatively quickly because cell activation consumes latently infected cells. Figure 8(c) shows a realization of Eq. (27) in which the half-life of the reservoir decay is about 6 months.
Figure 8.
Simulations of Eq. (27) show that it can robustly generate viral blips. The decay of the latent reservoir is primarily determined by p, which represents the potential of resulting activated cells to proliferate during the latently infected cell response. (a) p = 1.4 day−1. No statistically significant decay of latently infected cells was observed. (b) p = 1.35 day−1. The latent reservoir decays very slowly. This realization shows a half-life of ~ 44 months. (c) p = 1 day−1. The latent reservoir decays more quickly than in (b), representing a half-life of about 6 months. The parameter values used are [195]: ΔT ~ N(50, 10), Δt ~ U(4, 6), a = 0.05 day−1, σ = 0.8 day−1, ρ = 0.01 day−1. The other parameter values are the same as in Fig. 7.
A density-dependent infected cell death rate has been exploited in the above model to generate a low steady state viral load in the absence of antigen. Equation (27) can be modified to show that occasional activation of latently infected cells is able to generate low-level persistent viremia as well as intermittent viral blips during HAART [195]. Without assuming density-dependent cell death, the viral load in the above model decreases very quickly in the absence of activation because activated cells decline rapidly to an extremely low level after the contraction phase, with not enough cells entering the productively infected class. If activated cells are maintained at a low level rather than decreasing to zero quickly during the contraction phase, then low steady state viral loads are possible. In fact, in the study of Chun et al. [29], high levels of HIV proviral DNA were found in activated CD4+ T cells in maximally suppressed patients. Although a fraction of these cells could harbor defective provirus, the evidence of spontaneous release of virus during overnight culture without any activating stimuli would argue for the persistence of infectious virus in activated CD4+ T cells.
Motivated by the observation of a multiphasic contraction phase in the CD4+ T cell response during acute LCMV infection [39, 95], a model with a biphasic contraction phase in the latent CD4+ T cell response was developed [195]. Following the expansion phase as described in (27), there is a biphasic contraction: a rapid contraction phase, during which activated cells die rapidly by apoptosis or activation-induced cell death, and a slower phase where activated cells die at their base mortality rate.
With different proliferation rates chosen to characterize the different potentials of activated cells to proliferate during expansion, simulations of the model with a biphasic contraction phase also exhibit three distinct decay profiles of the latent reservoir [195]. The viral load does not decline to an unreasonably low level in the absence of antigenic stimulation because it is maintained by a small number of activated cells that transition into the productive stage after the rapid contraction phase. Relative contributions of ongoing viral replication to both viral persistence and the latent reservoir persistence were also examined quantitatively with this model. The result showed that residual ongoing replication during HAART is only a minor factor [195], consistent with the conclusions in recent studies [132, 199].
To summarize, occasional activation of latently infected cells by antigen can produce a large number of activated cells temporarily, and thereby generate intermittent viral blips. Different potentials of activated cells to proliferate during the initial clonal expansion phase are able to explain the different decay characteristics of the latent reservoir observed in different studies. The levels of persistent viremia and latently infected cells are not correlated with treatment potency, suggesting that the stability of the latent reservoir may not arise from ongoing residual replication during HAART.
It is difficult to investigate the dynamics of viral loads and the latent reservoir in patients on HAART due to the detection limits of assays. Although a set of models reviewed in this section are consistent with much of our knowledge about low level viremic persistence, the latent reservoir, and viral blips, we cannot exclude one versus the other hypothesis. More experimental data, particularly those on the activation of latently infected CD4+ T cells, are needed to determine which mechanism(s) would provide more biologically reasonable explanations for those phenomena observed in HIV patients on long-term effective therapy.
6 Treatment implications
Low-level persistent viremia, a stable latent reservoir, and intermittent viral blips observed in infected individuals on potent combination treatment all suggest that current HAART regimens are unable to eradicate HIV-1 from patients. Studies of the dynamics, mechanisms and relationships between them could have important treatment implications.
6.1 Treatment intensification
New classes of antiretroviral drugs have been developed and approved by the US Food and Drug Administration (FDA), e.g., the HIV entry inhibitor, maraviroc [130], the fusion inhibitor, enfuvirtide [183], and the integrase inhibitor, raltegravir [32]. These new drugs can be used in highly antiretroviral treatment-experienced patients and work against viral variants that are resistant to reverse transcriptase inhibitors and protease inhibitors. Mathematical models have been used to study the effects of the integrase inhibitor raltegravir on virus dynamics [152, 198]. Interestingly, the model analysis suggests that the later in the life cycle an inhibitor acts, the more rapid the decay in plasma viremia [198]. This may explain that patients taking a raltegravir-based HAART regimen have a faster time to suppression of viremia than patients taking a HAART regimen based on the RT inhibitor efavirenz [135, 152].
As more anti-HIV drugs appear, treatment intensification would be thought likely to eliminate the virus. Although treatment intensification was shown to decrease the viral load to a lower level even in some patients with years of highly suppressive therapy [82], and in some cases accelerate the decay of the latent reservoir [186], its long-term effects have not been clearly documented [71, 132]. A recent study by Dinoso et al. [50] showed that treatment intensification with any of three different antiretroviral drugs (efavirenz, lopinavir/ritonavir, or atazanavir/ritonavir) could not reduce residual HIV-1 viremia in patients on HAART.
Given that the latent reservoir has been identified as a major barrier to virus eradication, elimination of the reservoir is necessary before the ultimate goal of viral eradication can be achieved. Experimental evidence [149, 196, 215] and modeling results, as we reviewed here [194, 195, 199], have both suggested that the remarkable stability of the latent reservoir is very likely due to the intrinsic stability of resting memory CD4+ T cells and/or occasional replenishment by antigenic stimulation or homeostatic proliferation. If this is the case, then simply intensifying current HAART regimens is unlikely to have an influential impact on the long-term decay of the latent reservoir.
6.2 Early therapy
The best time to initiate antiretroviral therapy remains controversial. In order to prevent progressive immune damage, treatment ideally should be started as early as possible following infection [163]. The impetus for early therapy also comes from the initial prediction that prolonged potent combination therapy would be able to eradicate the virus from infected individuals. However, the attractiveness of early treatment has to be balanced against the drawbacks of prolonged overall duration of therapy. The discovery that integrated virus can hide in latently infected cells and persist in the presence of immune responses and potent therapy also tempers the enthusiasm for the initial early and aggressive approach. Once the reservoir of latently infected cells is established, it becomes very difficult to eradicate.
Chun et al. [22] and Finzi et al. [56] showed that initiation of HAART as early as 10 days after the onset of symptoms of primary infection could not prevent generation of latently infected CD4+ T cells even though the plasma viremia could be successfully controlled shortly after the adminstration of HAART. However, in other studies, early treatment was shown to some extent to reduce the frequency of latently infected cells, confine the size of the latent reservoir [7, 128], limit the evolution of HIV-1 in viral reservoirs [175], and lower viral blip frequency [46]. Strain et al. [216] reassessed the impact of initiating early treatment on the latent reservoir size in 27 patients who initiated therapy before or < 6 months after seroconversion and whose viremia was successfully suppressed to below 50 copies/mL. The total cell-associated infectivity, which measures the population size of cellular reservoirs, could not be detected in most patients after one year of treatment. In contrast, replication-competent virus could be recovered from all 17 control patients who initiated treatment during chronic infection. These results show that early therapy may reduce the size and/or accelerate the decay of the latent reservoir. However, failure to recover replication-competent virus does not necessarily imply that latently infected cells are completely eradicated as the frequency of latently infected cells may simply fall below the detection limit of the assay employed [9]. Furthermore, partial reduction in the latent reservoir size may not be of significant clinical benefit [9, 77] because theoretically one infectious virion released from latently infected cells could reignite infection quickly. This notion is supported by the rapid HIV rebound to the pretreatment viral level when treatment is interrupted in patients with sustained viral suppression [97, 134, 233].
Recently, the shortest half-life of latently infected CD4+ T cells (~ 4.6 months) has been reported by Chun et al. [27] in patients who initiated antiviral therapy early after infection. This raises the hope that < 10 years of continuous therapy would possibly eliminate latently infected cells in infected individuals who undergo early treatment. However, a close examination of the data shows that in at least 4 out of 7 patients the reservoir decay appears to exhibit a second, slower phase [133], which is consistent with the result that the latent pool has a heterogeneous and dynamic composition [215]. The possibility that HIV-1 may persist in multiple cellular or anatomical reservoirs makes viral eradication even more difficult.
Due to lack of long-term follow-up studies, there are very few mathematical models, if any, that have been developed to assess whether initiating antiretroviral therapy early has antiviral and immunological benefits for HIV-1 patients. This evaluation also involves considering the early treatment-associated side-effects and cost, and awaits more future studies.
6.3 Treatment interruptions
Currently, lifelong treatment may be required for most HIV-1 patients. However, lifelong antiretroviral therapy is largely limited by toxicities, emergence of drug resistance, and cost. In an attempt to reduce drug exposure without compromising efficacy, investigators have proposed structured treatment interruptions (STIs) (see reviews in [5, 70, 127]). In patients who initiate treatment during acute infection, STI that allows viral rebound is hypothesized to preserve or boost cellular immune responses. In patients who receive therapy in chronic infection with controlled viremia, STI may enhance immune responses as in the scenario of acute infection, and minimize drug-related adverse effects and cost. In patients with chronic treated infection but with virologic failure because of multi-drug resistance, STI has been proposed as a way of generating a reversion from resistant to wild-type virus, improving virological responses to subsequent (salvage) therapy.
However, the available evidence does not support that STI is associated with better immunologic, virologic or clinical outcomes than continuous antiretroviral therapy, although short-term STI may have the potential to allow patients drug holidays in carefully controlled clinical trials [5]. Interruptions of antiretroviral drugs have often been accompanied by a decline in CD4+ T cell counts and an exponential growth of plasma HIV-1 RNA levels [58, 233], which is likely to repopulate HIV cellular reservoirs and sometimes can result in severe AIDS-related clinical events, particularly when treatment interruption and resumption cycles are not adequately controlled [129]. The intention that STI would restore drug sensitivity and thereby preserve subsequent treatment options in those patients who developed multi-drug resistance has been difficult to realize because the latent reservoir can serve as a lifelong archive for all forms of viral strains that ever evolved and replicated [159, 120, 177, 196, 205]. Recent studies have suggested that both the wild-type virus that circulated before the onset of HAART and different strains of drug-resistant variants that evolved during non-suppressive periods of therapy remain detectable even after a long period of successful HAART [117, 196, 220]. All of the virus in the latent reservoir has the potential to reemerge when therapy is stopped or selective drug pressure is exerted. In this regard, even if there is a (temporary) shift from multiply-resistant to wild-type virus in some cohort studies during treatment interruptions [43, 146], drug resistant variants can reemerge when therapy is reinitiated. Therefore, failure to respond better to salvage therapy for those patients who initiated STI than patients who directly switched to salvage therapy is not surprising [120, 178]. Due to these interruption-associated risks, the use of STI has not been recommended outside the setting of rigorously controlled clinical trials with frequent monitoring and close follow-up.
Mathematical models have also been developed to assess possible therapeutic outcomes of STIs [1, 4, 113]. For example, Bajaria et al. [4] simulated the effects of different STI regimens and predicted that responses to STIs could be affected by a few factors, such as the duration of the interruption, disease stage at initiation of treatment, pretreatment viral load, and strength of the immune response. Krakovska and Wahl [113] showed that STI patterns with long therapy interruptions, e.g., weeks or months, are rarely optimal, whereas STI with very short drug-free periods can improve the benefit of treatment, particularly using those antiretroviral drugs with long half-lives. Adams et al. [1] derived continuous and suboptimal STI therapy protocols based on a viral dynamic model with immune responses, and illustrated a possible scenario in which STIs could lead to long-term control of HIV-1 by the immune system after discontinuation of therapy. These results are mainly theoretical. Moreover, the fact that the latent reservoir can archive drug resistant viral quasispecies, as discussed above, need also be considered when evaluating the effects of STIs.
6.4 Activation strategies
In an attempt to flush out the latent reservoir, immune stimulation with different activating agents has been proposed. It was expected that reactivated infected cells would be vulnerable to the attack by viral cytopathic effects and host immune responses, and that de novo infection could be largely prevented by potent antiretroviral therapy. However, such activation strategies to date have shown only limited success. For example, combination of proinflammatory cytokines interleukin-2 (IL-2), IL-6 and TNF-α was shown to increase the proliferation of resting CD4+ T cells [219] and HIV-1 replication in latently infected CD4+ cells [23]. Intermittent administration of only IL-2 with continuous HAART resulted in a greater reduction in the pool of resting CD4+ T cells containing replication-competent HIV-1 compared with patients who received HAART alone [24]. However, when therapy was interrupted, high levels of viral RNA were observed in most patients [20, 36], suggesting that long-term suppressive HAART in combination with IL-2 did not eliminate HIV-1 infection. Besides the reservoir of latently infected CD4+ T cells, viral rebound was believed to come from other unidentified reservoirs, such as tissue-bound macrophages [65].
The monocyte/macrophage activator IFN-γ along with IL-2 administered to HAART-treated patients during early infection was associated with a proviral DNA decrease and immune reconstitution, but was still unable to induce HIV-1 remission [116]. When a broad-spectrum T cell stimulator OKT3 and IL-2 were added to the immune activation therapy, patients failed to achieve measurable purging of the cellular HIV reservoir despite apparent T cell activation and proliferation [184]. Furthermore, side-effects of high drug doses were serious and antibodies against OKT3 developed rapidly in all patients [184].
Some other activation strategies, such as HAART supplemented with a histone deacetylase (HDAC) inhibitor valproic acid [124] or the nontumorigenic phorbol ester prostratin [112, 114], have been proposed to purge the latent virus out of the reservoir. Although valproic acid induced latent viral expression from resting CD4+ T cells in vitro [232] and a small clinical trial suggested a possible effect of valproic acid in accelerating clearance of HIV from resting CD4+ T cells [124], its clinical effect on the decay of the latent reservoir was cast in doubt by a later study [203]. Prostratin might be a promising activating agent that induces latent viral expression in vivo with minimum activation effects on the immune system [112, 114], but its effects still await more future studies.
Several issues need to be kept in mind when developing activation strategies. First, global T cell activation has to be avoided since it would significantly increase the number of susceptible T cells and might induce viral replication beyond the threshold that can be contained by current antiretroviral treatment. Further, the cytokines released by massive T cell activation would prove harmful as has been observed with activation via bacterial superantigens [61, 140]. Second, if latently infected cells can proliferate then activation of these cells may also induce the renewal of the latent reservoir, as suggested by models in Section 5.3. Third, partial reduction of the size of the latent reservoir might not be of clinical significance if activating agents in combination with potent antiretroviral drugs cannot lead to a complete eradication of all cellular/anatomical reservoirs for HIV-1.
7 Concluding remarks
Mathematical modeling, in conjunction with experimental data, has yielded quantitative results that have greatly improved our understanding of AIDS pathogenesis and facilitated the management of patients with HIV-1 infection. Since HIV-1 takes about 10 years, on average, to progress from initial infection to full-blown AIDS, its replication was thought to be a slow process. A simple model used to interpret clinical data from Phase I/II drug trials showed that virus is produced and cleared rapidly. From the large quantity of virions that are produced every day, one can estimate that, on average, every single possible point mutation of the viral genome and a fraction of all possible double mutations will occur each day. Thus, drug resistance could develop quickly to any single drug, particularly those that have a low resistance barrier. These results provide a reason for treating HIV-1 patients with multiple drugs since the mid-1990s.
Although HAART is able to suppress viral loads in many patients to below the detection limits of current assays, HIV-1 cannot be eradicated with current treatment regimens. Mathematical models have provided explanations for some important features in patients on effective combination therapy. Both residual ongoing viral replication and virus released from viral reservoirs may contribute to the persistence of low-level viremia during suppressive HAART. Viral evolution seems to be largely halted in patients who have optimal adherence and viral loads below the detection limit. Long-term control of viral replication is possible in these patients if drug toxicities can be overcome and patients adhere well to the therapy. The remarkable stability of the latent reservoir appears to be maintained mainly by the intrinsic stability of latently infected resting CD4+ T cells and/or renewal due to a few factors, such as residual ongoing replication, homeostatic proliferation and latently infected cell activation. Treatment intensification in combination with activating agents at the current time cannot eradicate the latent reservoir. Intermittent viral blips may represent enhanced viral replication or increased viral release from the latent reservoir due to occasional activation of target CD4+ T cells or latently infected cells, or when of low amplitude just reflect normal biological or statistical variations around the detection limit of current assays.
At present, many new antiviral drugs have been developed. Some of them may have an improved penetration ability and have a higher barrier to drug resistance. Further knowledge of the persistence of all possible cellular and anatomical reservoirs is crucial in the management of HIV-1 infection. Greater efforts are being put on new activation strategies that can purge latent virus from HIV-1 reservoirs. Mathematical models may help evaluate the effectiveness of new drugs (and drug combinations), and as shown here, test more possible mechanisms of viral persistence, HIV-1 latency, reservoir stability and viral blips. They can be used to explore why the immune system cannot induce long-term viral control, and provide more insights into the slow depletion of CD4+ T cells, viral evolution, and disease progression. We believe that mathematical modeling would provide more valuable information for future research of HIV-1 treatment.
Acknowledgments
Portions of this work were performed under the auspices of the U.S. Department of Energy under contract DE-AC52-06NA25396. This work was supported by NIH grants AI28433 and RR06555. We thank three reviewers for their constructive comments and suggestions that improved this manuscript.
Footnotes
Plasma viral load: the quantity of cell-free HIV-1 measured in the plasma of HIV-infected individuals. It is normally expressed as HIV-1 RNA copies per millilitre.
CD4+ T cells: a subpopulation of lymphocytes that express the CD4 receptor. CD4+ T cells are the main target cells of HIV-1 infection. They play an important role in the induction of antibody and other immune responses. The progressive decline of the CD4+ T cell level is a hallmark of HIV disease progression.
Ongoing viral replication: the processes of viral infection and reproduction that are continuing even in the presence of effective combination drug therapy.
Memory T cells: a subset of antigen-specific T cells that persist for a long time after an infection has resolved. Upon a second encounter with their cognate antigen, they can reproduce to mount a faster and stronger immune response than during their first encounter with the antigen.
Cytopathic effect: structural changes in a host cell as a result of viral infection. Examples include cell rounding, disorientation, swelling or shrinking, or even death.
Reservoir: any cell compartment that can serve as a source of replicating virus. It can be a specific cell population or an anatomical site.
Half-life: the time required for a quantity to decay to half of its initial value.
Viral set-point: a relatively constant viral load level during the asymptomatic period of HIV-1 infection
Parameter identifiability of the three-dimensional viral dynamic model (1) has been investigated in a few studies. Identifiability determines whether all model parameters can be uniquely estimated from measured outputs. Jeffrey and Xia [99] showed that all six parameters are identifiable if the measurements of the viral load and the total number of CD4+ T cells are both available. If only the viral load data are available, Wu et al. [231] showed that only four parameters and the product of two parameters (N and λ) are identifiable.
Reverse transcription: the process of converting viral RNA into DNA by the HIV-1 enzyme reverse transcriptase. Reverse transcriptase sometimes makes mistakes reading the RNA sequence.
PBMC: peripheral blood mononuclear cell. It is a blood cell, such as a lymphocyte or a monocyte.
Unintegrated proviral DNA: viral DNA that has not been integrated into the genome of the host cell.
Cell-associated HIV RNA: viral RNA generated from the transcription of the integrated viral DNA.
Replication-competent virus: virus harbored in cells that can infect other cells when released and start new viral replication.
Drug sanctuary: any site, for example, the central nervous system and the testes, in which some antiretroviral drugs may have reduced effects due to the poor penetration.
Integrated HIV-1 DNA: viral DNA that has been integrated into the genome of the host cell. HIV-1 has its own enzyme, integrase, that facilitates this insertion. The integrated DNA is usually called a provirus. Integrated proviruses may never be activated. Some can be activated and transcribed, resulting in production of cell-associated HIV RNA and plasma virus budded from the host cell. A large fraction of plasma virus released is noninfectious [49, 121].
Quasispecies: a group of viruses related by mutation(s), competing within an environment of high mutation rate, where a sizeable fraction of offspring are expected to contain one or more mutations.
Bystander proliferation: proliferation that is not driven by the cell engaging its antigen-specific receptor, and for example could be due to cytokines in the environment.
Opportunistic infections: infections caused by pathogens that normally do not cause disease in an individual with an intact immune system. Such infections, however, are a particular danger for people with AIDS because of a compromised immune system, which presents an “opportunity” for infections.
CD8+ T cells: T cells with a CD8 receptor that can recognize antigens on the surface of virus-infected cells and induce immune responses to kill them. Naive CD8+ T cells are CD8+ T cells that have never been stimulated by antigen.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adams BM, Banks HT, Davidian M, Rosenberg ES. Estimation and prediction with HIV-treatment interruption data. Bull Math Biol. 2007;69:563–584. doi: 10.1007/s11538-006-9140-6. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed R, Gray D. Immunological memory and protective immunity: understanding their relation. Science. 1996;272:54–60. doi: 10.1126/science.272.5258.54. [DOI] [PubMed] [Google Scholar]
- 3.Bailey JR, Sedaghat AR, Kieffer T, Brennan T, Lee PK, Wind-Rotolo M, Haggerty CM, Kamireddi AR, Liu Y, Lee J, Persaud D, Gallant JE, Cofrancesco J, Jr, Quinn TC, Wilke CO, Ray SC, Siliciano JD, Nettles RE, Siliciano RF. Residual human immunodeficiency virus type 1 viremia in some patients on antiretroviral therapy is dominated by a small number of invariant clones rarely found in circulating CD4+ T cells. J Virol. 2006;80:6441–6457. doi: 10.1128/JVI.00591-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bajaria SH, Webb G, Kirschner DE. Predicting differential responses to structured treatment interruptions during HAART. Bull Math Biol. 2004;66:1093–1118. doi: 10.1016/j.bulm.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 5.Benson CA. Structured treatment interruptions–new findings. Top HIV Med. 2006;14:107–111. [PubMed] [Google Scholar]
- 6.Birk M, Aleman S, Visco-Comandini U, Sonnerborg A. Proviral HIV-1 dynamics and evolution in patients receiving efficient long-term antiretroviral combination therapy. HIV Med. 2000;1:205–211. doi: 10.1046/j.1468-1293.2000.00030.x. [DOI] [PubMed] [Google Scholar]
- 7.Blankson JN, Finzi D, Pierson TC, Sabundayo BP, Chadwick K, Margolick JB, Quinn TC, Siliciano RF. Biphasic decay of latently infected CD4+ T cells in acute human immunodeficiency virus type 1 infection. J Infect Dis. 2000;182:1636–1642. doi: 10.1086/317615. [DOI] [PubMed] [Google Scholar]
- 8.Blankson JN, Persaud D, Siliciano RF. The challenge of viral reservoirs in HIV-1 infection. Annu Rev Med. 2002;53:557–593. doi: 10.1146/annurev.med.53.082901.104024. [DOI] [PubMed] [Google Scholar]
- 9.Blankson JN, Siliciano JD, Siliciano RF. The effect of early treatment on the latent reservoir of HIV-1. J Infect Dis. 2005;191:1394–1396. doi: 10.1086/428783. [DOI] [PubMed] [Google Scholar]
- 10.Bonhoeffer S, Coffin JM, Nowak MA. Human immunodeficiency virus drug therapy and virus load. J Virol. 1997;71:3275–3278. doi: 10.1128/jvi.71.4.3275-3278.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonhoeffer S, May RM, Shaw GM, Nowak MA. Virus dynamics and drug therapy. Proc Natl Acad Sci USA. 1997;94:6971–6976. doi: 10.1073/pnas.94.13.6971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonhoeffer S, Nowak MA. Pre-existence and emergence of drug resistance in HIV-1 infection. Proc R Soc Lond B. 1997;264:631–637. doi: 10.1098/rspb.1997.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bucy RP. Immune clearance of HIV type 1 replication-active cells: a model of two patterns of steady state HIV infection. AIDS Res Hum Retroviruses. 1999;15:223–227. doi: 10.1089/088922299311394. [DOI] [PubMed] [Google Scholar]
- 14.Callaway DS, Perelson AS. HIV-1 infection and low steady state viral loads. Bull Math Biol. 2002;64:29–64. doi: 10.1006/bulm.2001.0266. [DOI] [PubMed] [Google Scholar]
- 15.Chang JT, Palanivel VR, Kinjyo I, Schambach F, Intlekofer AM, Banerjee A, Longworth SA, Vinup KE, Mrass P, Oliaro J, Killeen N, Orange JS, Russell SM, Weninger W, Reiner SL. Asymmetric T lymphocyte division in the initiation of adaptive immune responses. Science. 2007;315:1687–1691. doi: 10.1126/science.1139393. [DOI] [PubMed] [Google Scholar]
- 16.Chen HY, Di Mascio M, Perelson AS, Ho DD, Zhang L. Determination of virus burst size in vivo using a single-cycle SIV in rhesus macaques. Proc Natl Acad Sci USA. 2007;104:19079–19084. doi: 10.1073/pnas.0707449104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ching N, Yang OO, Deville JG, Nielsen-Saines K, Ank BJ, Sim MS, Bryson YJ. Pediatric HIV-1-specific cytotoxic T-lymphocyte responses suggesting ongoing viral replication despite combination antiretroviral therapy. Pediatr Res. 2007;61:692–697. doi: 10.1203/pdr.0b013e31805365ef. [DOI] [PubMed] [Google Scholar]
- 18.Chomont N, et al. HIV reservoir size and persistence are driven by T-cell survival and homeostatic proliferaiton. Nat Med. 2009 doi: 10.1038/nm.1972. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chun TW, Carruth L, Finzi D, Shen X, DiGiuseppe JA, Taylor H, Hermankova M, Chadwick K, Margolick J, Quinn TC, Kuo YH, Brookmeyer R, Zeiger MA, Barditch-Crovo P, Siliciano RF. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature. 1997;387:183–188. doi: 10.1038/387183a0. [DOI] [PubMed] [Google Scholar]
- 20.Chun TW, Davey RT, Jr, Engel D, Lane HC, Fauci AS. Re-emergence of HIV after stopping therapy. Nature. 1999;401:874–875. doi: 10.1038/44755. [DOI] [PubMed] [Google Scholar]
- 21.Chun TW, Davey RT, Jr, Ostrowski M, Shawn Justement J, Engel D, Mullins JI, Fauci AS. Relationship between pre-existing viral reservoirs and the re-emergence of plasma viremia after discontinuation of highly active anti-retroviral therapy. Nat Med. 2000;6:757–761. doi: 10.1038/77481. [DOI] [PubMed] [Google Scholar]
- 22.Chun TW, Engel D, Berrey MM, Shea T, Corey L, Fauci AS. Early establishment of a pool of latently infected, resting CD4(+) T cells during primary HIV-1 infection. Proc Natl Acad Sci USA. 1998;95:8869–8873. doi: 10.1073/pnas.95.15.8869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chun TW, Engel D, Mizell SB, Ehler LA, Fauci AS. Induction of HIV-1 replication in latently infected CD4+ T cells using a combination of cytokines. J Exp Med. 1998;188:83–91. doi: 10.1084/jem.188.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chun TW, Engel D, Mizell SB, Hallahan CW, Fischette M, Park S, Davey RT, Jr, Dybul M, Kovacs JA, Metcalf JA, Mican JM, Berrey MM, Corey L, Lane HC, Fauci AS. Effect of interleukin-2 on the pool of latently infected, resting CD4+ T cells in HIV-1-infected patients receiving highly active anti-retroviral therapy. Nat Med. 1999;5:651–655. doi: 10.1038/9498. [DOI] [PubMed] [Google Scholar]
- 25.Chun TW, Fauci AS. Latent reservoirs of HIV: Obstacles to the eradication of virus. Proc Natl Acad Sci USA. 1999;96:10958–10961. doi: 10.1073/pnas.96.20.10958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chun TW, Finzi D, Margolick J, Chadwick K, Schwartz D, Siliciano RF. In vivo fate of HIV-1-infected T cells: quantitative analysis of the transition to stable latency. Nat Med. 1995;1:1284–1290. doi: 10.1038/nm1295-1284. [DOI] [PubMed] [Google Scholar]
- 27.Chun TW, Justement JS, Moir S, Hallahan CW, Maenza J, Mullins JI, Collier AC, Corey L, Fauci AS. Decay of the HIV reservoir in patients receiving antiretroviral therapy for extended periods: implications for eradication of virus. J Infect Dis. 2007;195:1762–1764. doi: 10.1086/518250. [DOI] [PubMed] [Google Scholar]
- 28.Chun TW, Justement JS, Pandya P, Hallahan CW, McLaughlin M, Liu S, Ehler LA, Kovacs C, Fauci AS. Relationship between the size of the human immunodeficiency virus type 1 (HIV-1) reservoir in peripheral blood CD4+ T cells and CD4+:CD8+ T cell ratios in aviremic HIV-1-infected individuals receiving long-term highly active antiretroviral therapy. J Infect Dis. 2002;185:1672–1676. doi: 10.1086/340521. [DOI] [PubMed] [Google Scholar]
- 29.Chun TW, Nickle DC, Justement JS, Large D, Semerjian A, Curlin ME, O’Shea MA, Hallahan CW, Daucher M, Ward DJ, Moir S, Mullins JI, Kovacs C, Fauci AS. HIV-infected individuals receiving effective antiviral therapy for extended periods of time continually replenish their viral reservoir. J Clin Invest. 2005;115:3250–3255. doi: 10.1172/JCI26197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chun TW, Stuyver L, Mizell SB, Ehler LA, Mican JA, Baseler M, Lloyd AL, Nowak MA, Fauci AS. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc Natl Acad Sci USA. 1997;94:13193–13197. doi: 10.1073/pnas.94.24.13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clavel F, Hance AJ. HIV drug resistance. N Engl J Med. 2004;350:1023–1035. doi: 10.1056/NEJMra025195. [DOI] [PubMed] [Google Scholar]
- 32.Cocohoba J, Dong BJ. Raltegravir: the first HIV integrase inhibitor. Clin Ther. 2008;30:1747–1765. doi: 10.1016/j.clinthera.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 33.Coffin JM. HIV population dynamics in vivo: implications for genetic variation, pathogenesis, and therapy. Science. 1995;267:483–489. doi: 10.1126/science.7824947. [DOI] [PubMed] [Google Scholar]
- 34.Cohen Stuart JW, Wensing AM, Kovacs C, Righart M, de Jong D, Kaye S, Schuurman R, Visser CJ, Boucher CA. Transient relapses (“blips”) of plasma HIV RNA levels during HAART are associated with drug resistance. J Acquir Immune Defic Syndr. 2001;28:105–113. doi: 10.1097/00042560-200110010-00001. [DOI] [PubMed] [Google Scholar]
- 35.Collier AC, Coombs RW, Schoenfeld DA, Bassett RL, Timpone J, Baruch A, Jones M, Facey K, Whitacre C, McAuliffe VJ, Friedman HM, Merigan TC, Reichman RC, Hooper C, Corey L. Treatment of human immunodeficiency virus infection with saquinavir, zidovudine, and zalcitabine. N Engl J Med. 1996;334:1011–1017. doi: 10.1056/NEJM199604183341602. [DOI] [PubMed] [Google Scholar]
- 36.Davey RT, Jr, Bhat N, Yoder C, Chun TW, Metcalf JA, Dewar R, Natarajan V, Lempicki RA, Adelsberger JW, Miller KD, Kovacs JA, Polis MA, Walker RE, Falloon J, Masur H, Gee D, Baseler M, Dimitrov DS, Fauci AS, Lane HC. HIV-1 and T cell dynamics after interruption of highly active antiretroviral therapy (HAART) in patients with a history of sustained viral suppression. Proc Natl Acad Sci USA. 1999;96:15109–15114. doi: 10.1073/pnas.96.26.15109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Boer RJ. Understanding the failure of CD8+ T-cell vaccination against simian/human immunodeficiency virus. J Virol. 2007;81:2838–2848. doi: 10.1128/JVI.01914-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Boer RJ, Ganusov VV, Milutinovic D, Hodgkin PD, Perelson AS. Estimating lymphocyte division and death rates from CFSE data. Bull Math Biol. 2006;68:1011–1031. doi: 10.1007/s11538-006-9094-8. [DOI] [PubMed] [Google Scholar]
- 39.De Boer RJ, Homann D, Perelson AS. Different dynamics of CD4+ and CD8+ T cell responses during and after acute lymphocytic choriomeningitis virus infection. J Immunol. 2003;171:3928–3935. doi: 10.4049/jimmunol.171.8.3928. [DOI] [PubMed] [Google Scholar]
- 40.De Boer RJ, Oprea M, Antia R, Murali-Krishna K, Ahmed R, Perelson AS. Recruitment times, proliferation, and apoptosis rates during the CD8(+) T-cell response to lymphocytic choriomeningitis virus. J Virol. 2001;75:10663–10669. doi: 10.1128/JVI.75.22.10663-10669.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De Boer RJ, Perelson AS. Target cell limited and immune control models of HIV infection: a comparison. J Theor Biol. 1998;190:201–214. doi: 10.1006/jtbi.1997.0548. [DOI] [PubMed] [Google Scholar]
- 42.De Boer RJ, Perelson AS. Estimating division and death rates from CFSE data. J Comp Appl Math. 2005;184:140–164. doi: 10.1007/s11538-006-9094-8. [DOI] [PubMed] [Google Scholar]
- 43.Deeks SG, Wrin T, Liegler T, Hoh R, Hayden M, Barbour JD, Hellmann NS, Petropoulos CJ, McCune JM, Hellerstein MK, Grant RM. Virologic and immunologic consequences of discontinuing combination antiretroviral-drug therapy in HIV-infected patients with detectable viremia. N Engl J Med. 2001;344:472–480. doi: 10.1056/NEJM200102153440702. [DOI] [PubMed] [Google Scholar]
- 44.Devereux HL, Youle M, Johnson MA, Loveday C. Rapid decline in detectability of HIV-1 drug resistance mutations after stopping therapy. AIDS. 1999;13:123–127. [PubMed] [Google Scholar]
- 45.Di Mascio M, Markowitz M, Louie M, Hogan C, Hurley A, Chung C, Ho DD, Perelson AS. Viral blip dynamics during highly active antiretroviral therapy. J Virol. 2003;77:12165–12172. doi: 10.1128/JVI.77.22.12165-12172.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Di Mascio M, Markowitz M, Louie M, Hurley A, Hogan C, Simon V, Follmann D, Ho DD, Perelson AS. Dynamics of intermittent viremia during highly active antiretroviral therapy in patients who initiate therapy during chronic versus acute and early human immunodeficiency virus type 1 infection. J Virol. 2004;78:10566–10573. doi: 10.1128/JVI.78.19.10566-10573.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Di Mascio M, Percus JK, Percus OE, Markowitz M, Ho DD, Perelson AS. Duration of an intermittent episode of viremia. Bull Math Biol. 2005;67:885–900. doi: 10.1016/j.bulm.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 48.Di Mascio M, Ribeiro RM, Markowitz M, Ho DD, Perelson AS. Modeling the long-term control of viremia in HIV-1 infected patients treated with antiretroviral therapy. Math Biosci. 2004;188:47–62. doi: 10.1016/j.mbs.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 49.Dimitrov DS, Willey RL, Sato H, Chang LJ, Blumenthal R, Martin MA. Quantitation of human immunodeficiency virus type 1 infection kinetics. J Virol. 1993;67:2182–2190. doi: 10.1128/jvi.67.4.2182-2190.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dinoso JB, et al. Treatment intensification does not reduce residual HIV-1 viremia in patients on highly active antiretroviral therapy. Proc Natl Acad Sci USA. 2009 doi: 10.1073/pnas.0903107106. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dornadula G, Zhang H, VanUitert B, Stern J, Livornese L, Jr, Ingerman MJ, Witek J, Kedanis RJ, Natkin J, DeSimone J, Pomerantz RJ. Residual HIV-1 RNA in blood plasma of patients taking suppressive highly active antiretroviral therapy. JAMA. 1999;282:1627–1632. doi: 10.1001/jama.282.17.1627. [DOI] [PubMed] [Google Scholar]
- 52.Duh EJ, Maury WJ, Folks TM, Fauci AS, Rabson AB. Tumor necrosis factor alpha activates human immunodeficiency virus type 1 through induction of nuclear factor binding to the NF-kappa B sites in the long terminal repeat. Proc Natl Acad Sci USA. 1989;86:5974–5978. doi: 10.1073/pnas.86.15.5974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dybul M, Daucher M, Jensen MA, Hallahan CW, Chun TW, Belson M, Hidalgo B, Nickle DC, Yoder C, Metcalf JA, Davey RT, Ehler L, Kress-Rock D, Nies-Kraske E, Liu S, Mullins JI, Fauci AS. Genetic characterization of rebounding human immunodeficiency virus type 1 in plasma during multiple interruptions of highly active antiretroviral therapy. J Virol. 2003;77:3229–3237. doi: 10.1128/JVI.77.5.3229-3237.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ferguson NM, deWolf F, Ghani AC, Fraser C, Donnelly CA, Reiss P, Lange JM, Danner SA, Garnett GP, Goudsmit J, Anderson RM. Antigen-driven CD4+ T cell and HIV-1 dynamics: residual viral replication under highly active antiretroviral therapy. Proc Natl Acad Sci USA. 1999;96:15167–15172. doi: 10.1073/pnas.96.26.15167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Finzi D, Blankson J, Siliciano JD, Margolick JB, Chadwick K, Pierson T, Smith K, Lisziewicz J, Lori F, Flexner C, Quinn TC, Chaisson RE, Rosenberg E, Walker B, Gange S, Gallant J, Siliciano RF. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat Med. 1999;5:512–517. doi: 10.1038/8394. [DOI] [PubMed] [Google Scholar]
- 56.Finzi D, Hermankova M, Pierson T, Carruth LM, Buck C, Chaisson RE, Quinn TC, Chadwick K, Margolick J, Brookmeyer R, Gallant J, Markowitz M, Ho DD, Richman DD, Siliciano RF. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997;278:1295–1300. doi: 10.1126/science.278.5341.1295. [DOI] [PubMed] [Google Scholar]
- 57.Finzi D, Siliciano R. Viral dynamics in HIV-1 infection. Cell. 1998;93:665–671. doi: 10.1016/s0092-8674(00)81427-0. [DOI] [PubMed] [Google Scholar]
- 58.Fischer M, Hafner R, Schneider C, Trkola A, Joos B, Joller H, Hirschel B, Weber R, Gunthard HF Swiss HIV Cohort Study. HIV RNA in plasma rebounds within days during structured treatment interruptions. AIDS. 2003;17:195–199. doi: 10.1097/00002030-200301240-00009. [DOI] [PubMed] [Google Scholar]
- 59.Fraser C, Ferguson NM, Anderson RM. Quantification of intrinsic residual viral replication in treated HIV-infected patients. Proc Natl Acad Sci USA. 2001;98:15167–15172. doi: 10.1073/pnas.261283598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fraser C, Ferguson NM, de Wolf F, Anderson RM. The role of antigenic stimulation and cytotoxic T cell activity in regulating the long-term immunopathogenesis of HIV: mechanisms and clinical implications. Proc Biol Sci. 2001;268:2085–2095. doi: 10.1098/rspb.2001.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fraser JD, Proft T. The bacterial superantigen and superantigen-like proteins. Immunol Rev. 2008;225:226–243. doi: 10.1111/j.1600-065X.2008.00681.x. [DOI] [PubMed] [Google Scholar]
- 62.Frenkel LM, Wang Y, Learn GH, McKernan JL, Ellis GM, Mohan KM, Holte SE, De Vange SM, Pawluk DM, Melvin AJ, Lewis PF, Heath LM, Beck IA, Mahalanabis M, Naugler WE, Tobin NH, Mullins JI. Multiple viral genetic analyses detect low-level human immunodeficiency virus type 1 replication during effective highly active antiretroviral therapy. J Virol. 2003;77:5721–5730. doi: 10.1128/JVI.77.10.5721-5730.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Furtado MR, Callaway DS, Phair JP, Kunstman KJ, Stanton JL, Macken CA, Perelson AS, Wolinsky SM. Persistence of HIV-1 transcription in peripheral-blood mononuclear cells in patients receiving potent antiretroviral therapy. N Engl J Med. 1999;340:1614–1622. doi: 10.1056/NEJM199905273402102. [DOI] [PubMed] [Google Scholar]
- 64.Gallant JE. Antiretroviral drug resistance and resistance testing. Top HIV Med. 2005;13:138–142. [PubMed] [Google Scholar]
- 65.Geeraert L, Kraus G, Pomerantz RJ. Hide-and-seek: the challenge of viral persistence in HIV-1 infection. Annu Rev Med. 2008;59:215–229. doi: 10.1146/annurev.med.59.062806.123001. [DOI] [PubMed] [Google Scholar]
- 66.Gett AV, Hodgkin PD. A cellular calculus for signal integration by T cells. Nat Immunol. 2000;1:239–244. doi: 10.1038/79782. [DOI] [PubMed] [Google Scholar]
- 67.Greub G, Cozzi-Lepri A, Ledergerber B, Staszewski S, Perrin L, Miller V, Francioli P, Furrer H, Battegay M, Vernazza P, Bernasconi E, Gunthard HF, Hirschel B, Phillips AN, Telenti A Frankfurt HIV Clinic Cohort and the Swiss HIV Cohort Study. Intermittent and sustained low-level HIV viral rebound in patients receiving potent antiretroviral therapy. AIDS. 2002;16:1967–1969. doi: 10.1097/00002030-200209270-00017. [DOI] [PubMed] [Google Scholar]
- 68.Grossman Z, Feinberg M, Kuznetsov V, Dimitrov D, Paul W. HIV infection: how effective is drug combination treatment? Immunol Today. 1998;19:528–532. doi: 10.1016/s0167-5699(98)01353-x. [DOI] [PubMed] [Google Scholar]
- 69.Grossman Z, Polis M, Feinberg MB, Grossman Z, Levi I, Jankelevich S, Yarchoan R, Boon J, de Wolf F, Lange JM, Goudsmit J, Dimitrov DS, Paul WE. Ongoing HIV dissemination during HAART. Nat Med. 1999;5:1099–1104. doi: 10.1038/13410. [DOI] [PubMed] [Google Scholar]
- 70.Gulick RM. Structured treatment interruption in patients infected with HIV. Drugs. 2002;62:245–253. doi: 10.2165/00003495-200262020-00001. [DOI] [PubMed] [Google Scholar]
- 71.Gulick RM, Lalama CM, Ribaudo HJ, Shikuma CM, Schackman BR, Schouten J, Squires KE, Koletar SL, Pilcher CD, Reichman RC, Klingman KL, Kuritzkes DR. Intensification of a triple-nucleoside regimen with tenofovir or efavirenz in HIV-1-infected patients with virological suppression. AIDS. 2007;21:813–823. doi: 10.1097/QAD.0b013e32805e8753. [DOI] [PubMed] [Google Scholar]
- 72.Gulick RM, Mellors JW, Havlir D, Eron JJ, Gonzalez C, McMahon D, Richman DD, Valentine FT, Jonas L, Meibohm A, Emini EA, Chodakewitz JA. Treatment with indinavir, zidovudine, and lamivudine in adults with human immunodeficiency virus infection and prior antiretroviral therapy. N Engl J Med. 1997;337:734–739. doi: 10.1056/NEJM199709113371102. [DOI] [PubMed] [Google Scholar]
- 73.Gunthard HF, Frost SD, Leigh-Brown AJ, Ignacio CC, Kee K, Perelson AS, Spina CA, Havlir DV, Hezareh M, Looney DJ, Richman DD, Wong JK. Evolution of envelope sequences of human immunodeficiency virus type 1 in cellular reservoirs in the setting of potent antiviral therapy. J Virol. 1999;73:9404–9412. doi: 10.1128/jvi.73.11.9404-9412.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gunthard HF, Wong JK, Ignacio CC, Guatelli JC, Riggs NL, Havlir DV, Richman DD. Human immunodeficiency virus replication and genotypic resistance in blood and lymph nodes after a year of potent antiretroviral therapy. J Virol. 1998;72:2422–2428. doi: 10.1128/jvi.72.3.2422-2428.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gunthard HF, Wong JK, Spina CA, Ignacio C, Kwok S, Christopherson C, Hwang J, Haubrich R, Havlir D, Richman DD. Effect of influenza vaccination on viral replication and immune response in persons infected with human immunodeficiency virus receiving potent antiretroviral therapy. J Infect Dis. 2000;181:522–531. doi: 10.1086/315260. [DOI] [PubMed] [Google Scholar]
- 76.Haase AT, Henry K, Zupancic M, Sedgewick G, Faust RA, Melroe H, Cavert W, Gebhard K, Staskus K, Zhang ZQ, Dailey PJ, Balfour HH, Jr, Erice A, Perelson AS. Quantitative image analysis of HIV-1 infection in lymphoid tissue. Science. 1996;274:985–989. doi: 10.1126/science.274.5289.985. [DOI] [PubMed] [Google Scholar]
- 77.Haggerty CM, Pitt E, Siliciano RF. The latent reservoir for HIV-1 in resting CD4+ T cells and other viral reservoirs during chronic infection: insights from treatment and treatment-interruption trials. Curr Opin HIV AIDS. 2006;1:62–68. doi: 10.1097/01.COH.0000191897.78309.70. [DOI] [PubMed] [Google Scholar]
- 78.Hall PA, Watt FM. Stem cells: the generation and maintenance of cellular diversity. Development. 1989;106:619–633. doi: 10.1242/dev.106.4.619. [DOI] [PubMed] [Google Scholar]
- 79.Hammer SM, Squires KE, Hughes MD, Grimes JM, Demeter LM, Currier JS, Eron JJ, Jr, Feinberg JE, Balfour HH, Jr, Deyton LR, Chodakewitz JA, Fischl MA. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. AIDS Clinical Trials Group 320 Study Team. N Engl J Med. 1997;337:725–733. doi: 10.1056/NEJM199709113371101. [DOI] [PubMed] [Google Scholar]
- 80.Han Y, Lassen K, Monie D, Sedaghat AR, Shimoji S, Liu X, Pierson TC, Margolick JB, Siliciano RF, Siliciano JD. Resting CD4+ T cells from human immunodeficiency virus type 1 (HIV-1)-infected individuals carry integrated HIV-1 genomes within actively transcribed host genes. J Virol. 2004;78:6122–6133. doi: 10.1128/JVI.78.12.6122-6133.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Havlir DV, Bassett R, Levitan D, Gilbert P, Tebas P, Collier AC, Hirsch MS, Ignacio C, Condra J, Günthard HF, Richman DD, Wong JK. Prevalence and predictive value of intermittent viremia with combination hiv therapy. JAMA. 2001;286:171–179. doi: 10.1001/jama.286.2.171. [DOI] [PubMed] [Google Scholar]
- 82.Havlir DV, Strain MC, Clerici M, Ignacio C, Trabattoni D, Ferrante P, Wong JK. Productive infection maintains a dynamic steady state of residual viremia in human immunodeficiency virus type 1-infected persons treated with suppressive antiretroviral therapy for five years. J Virol. 2003;77:11212–11219. doi: 10.1128/JVI.77.20.11212-11219.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hawkins ED, Turner ML, Dowling MR, van Gend C, Hodgkin PD. A model of immune regulation as a consequence of randomized lymphocyte division and death times. Proc Natl Acad Sci USA. 2007;104:5032–5037. doi: 10.1073/pnas.0700026104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hermankova M, Ray SC, Ruff C, Powell-Davis M, Ingersoll R, D’Aquila RT, Quinn TC, Siliciano JD, Siliciano RF, Persaud D. HIV-1 drug resistance profiles in children and adults with viral load of <50 copies/ml receiving combination therapy. JAMA. 2001;286:196–207. doi: 10.1001/jama.286.2.196. [DOI] [PubMed] [Google Scholar]
- 85.Hermankova M, Siliciano JD, Zhou Y, Monie D, Chadwick K, Margolick JB, Quinn TC, Siliciano RF. Analysis of human immunodeficiency virus type 1 gene expression in latently infected resting CD4+ T lymphocytes in vivo. J Virol. 2003;77:7383–7392. doi: 10.1128/JVI.77.13.7383-7392.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hlavacek WS, Stilianakis NI, Notermans DW, Danner SA, Perelson AS. Influence of follicular dendritic cells on decay of HIV during antiretroviral therapy. Proc Natl Acad Sci USA. 2000;97:10966–10971. doi: 10.1073/pnas.190065897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hlavacek WS, Stilianakis NI, Perelson AS. Influence of follicular dendritic cells on HIV dynamics. Philos Trans R Soc Lond B Biol Sci. 2000;355:1051–1058. doi: 10.1098/rstb.2000.0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hlavacek WS, Wofsy C, Perelson AS. Dissociation of HIV-1 from follicular dendritic cells during HAART: mathematical analysis. Proc Natl Acad Sci USA. 1999;96:14681–14686. doi: 10.1073/pnas.96.26.14681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ho DD. Viral counts count in HIV infection. Science. 1996;272:1124–1125. doi: 10.1126/science.272.5265.1124. [DOI] [PubMed] [Google Scholar]
- 90.Ho DD. Toward HIV eradication or remission: the tasks ahead. Science. 1998;280:1866–1867. doi: 10.1126/science.280.5371.1866. [DOI] [PubMed] [Google Scholar]
- 91.Ho DD, Neumann AU, Perelson AS, Chen W, Leonard JM, Markowitz M. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature. 1995;373:123–126. doi: 10.1038/373123a0. [DOI] [PubMed] [Google Scholar]
- 92.Ho D, Rota T, Hirsch M. Infection of monocyte/macrophages by human T lymphotropic virus type III. J Clin Invest. 1986;77:1712–1715. doi: 10.1172/JCI112491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hockett RD, Kilby JM, Derdeyn CA, Saag MS, Sillers M, Squires K, Chiz S, Nowak MA, Shaw GM, Bucy RP. Constant mean viral copy number per infected cell in tissues regardless of high, low, or undetectable plasma HIV RNA. J Exp Med. 1999;189:1545–1554. doi: 10.1084/jem.189.10.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Holte SE, Melvin AJ, Mullins JI, Tobin NH, Frenkel LM. Density-dependent decay in HIV-1 dynamics. J Acquir Immune Defic Syndr. 2006;41:266–276. doi: 10.1097/01.qai.0000199233.69457.e4. [DOI] [PubMed] [Google Scholar]
- 95.Homann D, Teyton L, Oldstone MB. Differential regulation of antiviral T-cell immunity results in stable CD8+ but declining CD4+ T-cell memory. Nat Med. 2001;7:913–919. doi: 10.1038/90950. [DOI] [PubMed] [Google Scholar]
- 96.Horvitz HR, Herskowitz I. Mechanisms of asymmetric cell division: two Bs or not two Bs, that is the question. Cell. 1992;68:237–255. doi: 10.1016/0092-8674(92)90468-r. [DOI] [PubMed] [Google Scholar]
- 97.Imamichi H, Crandall KA, Natarajan V, Jiang MK, Dewar RL, Berg S, Gaddam A, Bosche M, Metcalf JA, Davey RT, Jr, Lane HC. Human immunodeficiency virus type 1 quasi species that rebound after discontinuation of highly active antiretroviral therapy are similar to the viral quasi species present before initiation of therapy. J Infect Dis. 2001;183:36–50. doi: 10.1086/317641. [DOI] [PubMed] [Google Scholar]
- 98.Jan YN, Jan LY. Asymmetric cell division. Nature. 1998;392:775–778. doi: 10.1038/33854. [DOI] [PubMed] [Google Scholar]
- 99.Jeffrey AM, Xia X. Identifiability of HIV/AIDS model. In: Tan WY, Wu H, editors. Deterministic and Stochastic Models of AIDS Epidemics and HIV Infections with Intervention. World Scientific; Singapore: 2005. [Google Scholar]
- 100.Jones LE, Perelson AS. Modeling the effects of vaccination on chronically infected HIV-positive patients. J Acquir Immune Defic Syndr. 2002;31:369–377. doi: 10.1097/00126334-200212010-00001. [DOI] [PubMed] [Google Scholar]
- 101.Jones LE, Perelson AS. Opportunistic infection as a cause of transient viremia in chronically infected HIV patients under treatment with HAART. Bull Math Biol. 2005;67:1227–1251. doi: 10.1016/j.bulm.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 102.Jones LE, Perelson AS. Transient viremia, plasma viral load, and reservoir replenishment in HIV-infected patients on antiretroviral therapy. J Acquir Immune Defic Syndr. 2007;45:483–493. doi: 10.1097/QAI.0b013e3180654836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jordan A, Bisgrove D, Verdin E. HIV reproducibly establishes a latent infection after acute infection of T cells in vitro. EMBO J. 2003;22:1868–1877. doi: 10.1093/emboj/cdg188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kaech SM, Ahmed R. Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naive cells. Nat Immunol. 2001;2:415–422. doi: 10.1038/87720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kao SY, Calman AF, Luciw PA, Peterlin BM. Anti-termination of transcription within the long terminal repeat of HIV-1 by tat gene product. Nature. 1987;330:489–493. doi: 10.1038/330489a0. [DOI] [PubMed] [Google Scholar]
- 106.Kepler TB, Perelson AS. Drug concentration heterogeneity facilitates the evolution of drug resistance. Proc Natl Acad Sci USA. 1998;95:11514–11519. doi: 10.1073/pnas.95.20.11514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kieffer TL, Finucane MM, Nettles RE, Quinn TC, Broman KW, Ray SC, Persaud D, Siliciano RF. Genotypic analysis of HIV-1 drug resistance at the limit of detection: virus production without evolution in treated adults with undetectable HIV loads. J Infect Dis. 2004;189:1452–1465. doi: 10.1086/382488. [DOI] [PubMed] [Google Scholar]
- 108.Kim H, Perelson AS. Viral and latent reservoir persistence in HIV-1-infected patients on therapy. PLoS Comput Biol. 2006;2:e135. doi: 10.1371/journal.pcbi.0020135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kim H, Perelson AS. Dynamic characteristics of HIV-1 reservoirs. Curr Opinion HIV and AIDS. 2006;1:152–156. doi: 10.1097/01.COH.0000203836.08974.96. [DOI] [PubMed] [Google Scholar]
- 110.Kirschner DE, Webb GF. Understanding drug resistance for montherapy treatment of HIV infection. Bull Math Biol. 1997;59:763–786. doi: 10.1007/BF02458429. [DOI] [PubMed] [Google Scholar]
- 111.Kolber MA, Campo RE, Dickinson GM. Development of anti-retroviral resistance of HIV-1 infected individuals on therapy: is it inevitable? IUBMB Life. 2004;56:301–307. doi: 10.1080/1521-6540400000843. [DOI] [PubMed] [Google Scholar]
- 112.Korin YD, Brooks DG, Brown S, Korotzer A, Zack JA. Effects of prostratin on T-cell activation and human immunodeficiency virus latency. J Virol. 2002;76:8118–8123. doi: 10.1128/JVI.76.16.8118-8123.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Krakovska O, Wahl LM. Drug-sparing regimens for HIV combination therapy: benefits predicted for “drug coasting”, Bull. Math Biol. 2007;69:2627–2647. doi: 10.1007/s11538-007-9234-9. [DOI] [PubMed] [Google Scholar]
- 114.Kulkosky J, Culnan DM, Roman J, Dornadula G, Schnell M, Boyd MR, Pomerantz RJ. Prostratin: activation of latent HIV-1 expression suggests a potential inductive adjuvant therapy for HAART. Blood. 2001;98:3006–3015. doi: 10.1182/blood.v98.10.3006. [DOI] [PubMed] [Google Scholar]
- 115.Kupfer B, Matz B, Daumer MP, Roden F, Rockstroh JK, Qurishi N, Spengler U, Kaiser R. Frequent detection of cell-associated HIV-1 RNA in patients with plasma viral load <50 copies/ml. J Med Virol. 2007;79:1440–1445. doi: 10.1002/jmv.20993. [DOI] [PubMed] [Google Scholar]
- 116.Lafeuillade A, Poggi C, Chadapaud S, Hittinger G, Chouraqui M, Pisapia M, Delbeke E. Pilot study of a combination of highly active antiretroviral therapy and cytokines to induce HIV-1 remission. J Acquir Immune Defic Syndr. 2001;26:44–55. doi: 10.1097/00126334-200101010-00006. [DOI] [PubMed] [Google Scholar]
- 117.Lambotte O, Chaix ML, Gubler B, Nasreddine N, Wallon C, Goujard C, Rouzioux C, Taoufik Y, Delfraissy JF. The lymphocyte HIV reservoir in patients on long-term HAART is a memory of virus evolution. AIDS. 2004;18:1147–1158. doi: 10.1097/00002030-200405210-00008. [DOI] [PubMed] [Google Scholar]
- 118.Langford D, Marquie-Beck J, de Almeida S, Lazzaretto D, Letendre S, Grant I, McCutchan JA, Masliah E, Ellis RJ. Relationship of antiretroviral treatment to postmortem brain tissue viral load in human immunodeficiency virus-infected patients. J Neurovirol. 2006;12:100–107. doi: 10.1080/13550280600713932. [DOI] [PubMed] [Google Scholar]
- 119.Lassen K, Han Y, Zhou Y, Siliciano J, Siliciano RF. The multifactorial nature of HIV-1 latency. Trends Mol Med. 2004;10:525–531. doi: 10.1016/j.molmed.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 120.Lawrence J, Hullsiek KH, Thackeray LM, Abrams DI, Crane LR, Mayers DL, Jones MC, Saldanha JM, Schmetter BS, Baxter JD. Disadvantages of structured treatment interruption persist in patients with multidrug-resistant HIV-1: final results of the CPCRA 064 study. J Acquir Immune Defic Syndr. 2006;43:169–178. doi: 10.1097/01.qai.0000242450.74779.ee. [DOI] [PubMed] [Google Scholar]
- 121.Layne SP, et al. Factors underlying spontaneous inactivation and susceptibility to neutralization of human immunodeficiency virus. Virology. 1992;189:695–714. doi: 10.1016/0042-6822(92)90593-e. [DOI] [PubMed] [Google Scholar]
- 122.Lee HY, Perelson AS. Modeling T cell proliferation and death in vitro based on labeling data: generalizations of the Smith-Martin cell cycle model. Bull Math Biol. 2008;70:21–44. doi: 10.1007/s11538-007-9239-4. [DOI] [PubMed] [Google Scholar]
- 123.Lee PK, Kieffer TL, Siliciano RF, Nettles RE. HIV-1 viral load blips are of limited clinical significance. J Antimicrob Chemother. 2006;57:803–805. doi: 10.1093/jac/dkl092. [DOI] [PubMed] [Google Scholar]
- 124.Lehrman G, Hogue IB, Palmer S, Jennings C, Spina CA, Wiegand A, Landay AL, Coombs RW, Richman DD, Mellors JW, Coffin JM, Bosch RJ, Margolis DM. Depletion of latent HIV-1 infection in vivo: a proof-of-concept study. Lancet. 2005;366:549–555. doi: 10.1016/S0140-6736(05)67098-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Little SJ, McLean AR, Spina CA, Richman DD, Havlir DV. Viral dynamics of acute HIV-1 infection. J Exp Med. 1999;190:841–850. doi: 10.1084/jem.190.6.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Liu SL, Rodrigo AG, Shankarappa R, Learn GH, Hsu L, Davidov O, Zhao LP, Mullins JI. HIV quasispecies and resampling. Science. 1996;273:415–416. doi: 10.1126/science.273.5274.415. [DOI] [PubMed] [Google Scholar]
- 127.Lori F, Foli A, Lisziewicz J. Structured treatment interruptions as a potential alternative therapeutic regimen for HIV-infected patients: a review of recent clinical data and future prospects. J Antimicrob Chemother. 2002;50:155–60. doi: 10.1093/jac/dkf119. [DOI] [PubMed] [Google Scholar]
- 128.Lori F, Jessen H, Lieberman J, Finzi D, Rosenberg E, Tinelli C, Walker B, Siliciano RF, Lisziewicz J. Treatment of human immunodeficiency virus infection with hydroxyurea, didanosine, and a protease inhibitor before seroconversion is associated with normalized immune parameters and limited viral reservoir. J Infect Dis. 1999;180:1827–1832. doi: 10.1086/315113. [DOI] [PubMed] [Google Scholar]
- 129.Lori F, Lisziewicz J. Structured treatment interruptions for the management of HIV infection. JAMA. 2001;286:2981–2987. doi: 10.1001/jama.286.23.2981. [DOI] [PubMed] [Google Scholar]
- 130.MacArthur RD, Novak RM. Reviews of anti-infective agents: maraviroc: the first of a new class of antiretroviral agents. Clin Infect Dis. 2008;47:236–241. doi: 10.1086/589289. [DOI] [PubMed] [Google Scholar]
- 131.Macias J, Palomares JC, Mira JA, Torres MJ, Garcia-Garcia JA, Rodriquez JM, Vergera S, Pineda JA. Transient rebounds of HIV plasma viremia are associated with the emergence of drug resistance mutations in patients on highly active antiretroviral therapy. J Infect. 2005;51:195–200. doi: 10.1016/j.jinf.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 132.Maldarelli F, Palmer S, King MS, Wiegand A, Polis MA, Mican J, Kovacs JA, Davey RT, Rock-Kress D, Dewar R, Liu S, Metcalf JA, Rehm C, Brun SC, Hanna GJ, Kempf DJ, Coffin JM, Mellors JW. ART suppresses plasma HIV-1 RNA to a stable set point predicted by pretherapy viremia. PLoS Pathog. 2007;3:e46. doi: 10.1371/journal.ppat.0030046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Margolis DM, Archin NM. Eliminating persistent HIV infection: getting to the end of the rainbow. J Infect Dis. 2007;195:1734–1736. doi: 10.1086/518254. [DOI] [PubMed] [Google Scholar]
- 134.Markowitz M, Jin X, Hurley A, Simon V, Ramratnam B, Louie M, Deschenes GR, Ramanathan M, Jr, Barsoum S, Vanderhoeven J, He T, Chung C, Murray J, Perelson AS, Zhang L, Ho DD. Discontinuation of antiretroviral therapy commenced early during the course of human immunodeficiency virus type 1 infection, with or without adjunctive vaccination. J Infect Dis. 2002;186:634–643. doi: 10.1086/342559. [DOI] [PubMed] [Google Scholar]
- 135.Markowitz M, et al. Rapid and durable antiretroviral effect of the HIV-1 Integrase inhibitor raltegravir as part of combination therapy in treatment-naive patients with HIV-1 infection: results of a 48-week controlled study. J Acquir Immune Defic Syndr. 2007;46:125–133. doi: 10.1097/QAI.0b013e318157131c. [DOI] [PubMed] [Google Scholar]
- 136.Marsden MD, Zack JA. Eradication of HIV: current challenges and new directions. J Antimicrob Chemother. 2009;63:7–10. doi: 10.1093/jac/dkn455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Martinez MA, Cabana M, Ibanez A, Clotet B, Arno A, Ruiz L. Human immunodeficiency virus type 1 genetic evolution in patients with prolonged suppression of plasma viremia. Virology. 1999;256:180–187. doi: 10.1006/viro.1999.9601. [DOI] [PubMed] [Google Scholar]
- 138.Martinez V, Marcelin AG, Morini JP, Deleuze J, Krivine A, Gorin I, Yerly S, Perrin L, Peytavin G, Calvez V, Dupin N. HIV-1 intermittent viraemia in patients treated by non-nucleoside reverse transcriptase inhibitor-based regimen. AIDS. 2005;19:1065–1069. doi: 10.1097/01.aids.0000174453.55627.de. [DOI] [PubMed] [Google Scholar]
- 139.Martinez-Picado J, DePasquale MP, Kartsonis N, Hanna GJ, Wong J, Finzi D, Rosenberg E, Gunthard HF, Sutton L, Savara A, Petropoulos CJ, Hellmann N, Walker BD, Richman DD, Siliciano R, D’Aquila RT. Antiretroviral resistance during successful therapy of HIV type 1 infection. Proc Natl Acad Sci USA. 2000;97:10948–10953. doi: 10.1073/pnas.97.20.10948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.McCormick JK, Yarwood JM, Schlievert PM. Toxic shock syndrome and bacterial superantigens: an update. Annu Rev Microbiol. 2001;55:77–104. doi: 10.1146/annurev.micro.55.1.77. [DOI] [PubMed] [Google Scholar]
- 141.McLean AR, Michie CA. In vivo estimates of division and death rates of human T lymphocytes. Proc Natl Acad Sci USA. 1995;92:3707–3711. doi: 10.1073/pnas.92.9.3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.McLean AR, Nowak MA. Competition between zidovudine-sensitive and zidovudine-resistant strains of HIV. AIDS. 1992;6:71–79. doi: 10.1097/00002030-199201000-00009. [DOI] [PubMed] [Google Scholar]
- 143.McLean AR, Nowak MA. Models of interactions between HIV and other pathogens. J Theor Biol. 1992;155:69–86. doi: 10.1016/s0022-5193(05)80549-1. [DOI] [PubMed] [Google Scholar]
- 144.Mellors JW, Rinaldo CR, Jr, Gupta P, White RM, Todd JA, Kingsley LA. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science. 1996;272:1167–1170. doi: 10.1126/science.272.5265.1167. [DOI] [PubMed] [Google Scholar]
- 145.Michie CA, Mclean AR, Alcock C, Beverley PC. Lifespan of human lymphocyte subsets defined by CD45 isoforms. Nature. 1992;360:264–265. doi: 10.1038/360264a0. [DOI] [PubMed] [Google Scholar]
- 146.Miller V, Sabin C, Hertogs K, Bloor S, Martinez-Picado J, D’Aquila R, Larder B, Lutz T, Gute P, Weidmann E, Rabenau H, Phillips A, Staszewski S. Virological and immunological effects of treatment interruptions in HIV-1 infected patients with treatment failure. AIDS. 2000;14:2857–2867. doi: 10.1097/00002030-200012220-00007. [DOI] [PubMed] [Google Scholar]
- 147.Mira JA, Macias J, Nogales C, Fernandez-Rivera J, Garcia-Garcia JA, Ramos A, Pineda JA. Transient rebounds of low-level viraemia among HIV-infected patients under HAART are not associated with virological or immunological failure. Antivir Ther. 2002;7:251–256. doi: 10.1177/135965350200700404. [DOI] [PubMed] [Google Scholar]
- 148.Mittler JE, Markowitz M, Ho DD, Perelson AS. Refined estimates for HIV-1 clearance rate and intracellular delay. AIDS. 1999;13:1415–1417. doi: 10.1097/00002030-199907300-00023. [DOI] [PubMed] [Google Scholar]
- 149.Monie D, Simmons RP, Nettles RE, Kieffer TL, Zhou Y, Zhang H, Karmon S, Ingersoll R, Chadwick K, Zhang H, Margolick JB, Quinn TC, Ray SC, Wind-Rotolo M, Miller M, Persaud D, Siliciano RF. A novel assay allows genotyping of the latent reservoir for human immunodeficiency virus type 1 in the resting CD4+ T cells of viremic patients. J Virol. 2005;79:5185–5202. doi: 10.1128/JVI.79.8.5185-5202.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Muller V, Vigueras-Gomez JF, Bonhoeffer S. Decelerating decay of latently infected cells during prolonged therapy for human immunodeficiency virus type 1 infection. J Virol. 2002;76:8963–8965. doi: 10.1128/JVI.76.17.8963-8965.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Murphy EL, Collier AC, Kalish LA, Assmann SF, Para MF, Flanigan TP, Kumar PN, Mintz L, Wallach FR, Nemo GJ Viral Activation Transfusion Study Investigators. Highly active antiretroviral therapy decreases mortality and morbidity in patients with advanced HIV disease. Ann Intern Med. 2001;135:17–26. doi: 10.7326/0003-4819-135-1-200107030-00005. [DOI] [PubMed] [Google Scholar]
- 152.Murray JM, Emery S, Kelleher AD, Law M, Chen J, Hazuda DJ, Nguyen BY, Teppler H, Cooper DA. Antiretroviral therapy with the integrase inhibitor raltegravir alters decay kinetics of HIV, significantly reducing the second phase. AIDS. 2007;21:2315–2321. doi: 10.1097/QAD.0b013e3282f12377. [DOI] [PubMed] [Google Scholar]
- 153.Murray JM, Perelson AS. Human immunodeficiency virus: quasi-species and drug resistance. Multiscale Model Simul. 2005;3:300–311. [Google Scholar]
- 154.Nabel G, Baltimore D. An inducible transcription factor activates expression of human immunodeficiency virus in T cells. Nature. 1987;326:711–713. doi: 10.1038/326711a0. [DOI] [PubMed] [Google Scholar]
- 155.Natarajan V, Bosche M, Metcalf JA, Ward DJ, Lane HC, Kovacs JA. HIV-1 replication in patients with undetectable plasma virus receiving HAART. Lancet. 1999;353:119–120. doi: 10.1016/s0140-6736(05)76156-0. [DOI] [PubMed] [Google Scholar]
- 156.Nelson PW, Perelson AS. Mathematical analysis of delay differential equation models of HIV-1 infection. Math Biosci. 2002;179:73–94. doi: 10.1016/s0025-5564(02)00099-8. [DOI] [PubMed] [Google Scholar]
- 157.Nettles RE, Kieffer TL, Kwon P, Monie D, Han Y, Parsons T, Cofrancesco J, Jr, Gallant JE, Quinn TC, Jackson B, Flexner C, Carson K, Ray S, Persaud D, Siliciano RF. Intermittent HIV-1 viremia (blips) and drug resistance in patients receiving HAART. JAMA. 2005;293:817–829. doi: 10.1001/jama.293.7.817. [DOI] [PubMed] [Google Scholar]
- 158.Nettles RE, Kieffer TL, Simmons RP, Cofrancesco J, Jr, Moore RD, Gallant JE, Persaud D, Siliciano RF. Genotypic resistance in HIV-1-infected patients with persistently detectable low-level viremia while receiving highly active antiretroviral therapy. Clin Infect Dis. 2004;39:1030–1037. doi: 10.1086/423388. [DOI] [PubMed] [Google Scholar]
- 159.Noë A, Plum J, Verhofstede C. The latent HIV-1 reservoir in patients undergoing HAART: an archive of pre-HAART drug resistance. J Antimicrob Chemother. 2005;55:410–412. doi: 10.1093/jac/dki038. [DOI] [PubMed] [Google Scholar]
- 160.Nowak MA, Bangham CR. Population dynamics of immune responses to persistent viruses. Science. 1996;272:74–79. doi: 10.1126/science.272.5258.74. [DOI] [PubMed] [Google Scholar]
- 161.Nowak MA, Bonhoeffer S, Shaw GM, May RM. Anti-viral drug treatment: dynamics of resistance in free virus and infected cell populations. J Theor Biol. 1997;184:203–217. doi: 10.1006/jtbi.1996.0307. [DOI] [PubMed] [Google Scholar]
- 162.Nowak MA, May RM. Virus Dynamics: Mathematical Principles of Immunology and Virology. Oxford University Press; 2000. [Google Scholar]
- 163.Oxenius A, Price DA, Easterbrook PJ, O’Callaghan CA, Kelleher AD, Whelan JA, Sontag G, Sewell AK, Phillips RE. Early highly active antiretroviral therapy for acute HIV-1 infection preserves immune function of CD8+ and CD4+ T lymphocytes. Proc Natl Acad Sci USA. 2000;97:3382–3387. doi: 10.1073/pnas.97.7.3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Palmer S, Maldarelli F, Wiegand A, Bernstein B, Hanna GJ, Brun SC, Kempf DJ, Mellors JW, Coffin JM, King MS. Low-level viremia persists for at least 7 years in patients on suppressive antiretroviral therapy. Proc Natl Acad Sci USA. 2008;105:3879–3884. doi: 10.1073/pnas.0800050105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Palmer S, Wiegand AP, Maldarelli F, Bazmi H, Mican JM, Polis M, Dewar RL, Planta A, Liu S, Metcalf JA, Mellors JW, Coffin JM. New real-time reverse transcriptase-initiated PCR assay with single-copy sensitivity for human immunodeficiency virus type 1 RNA in plasma. J Clin Microbiol. 2003;41:4531–4536. doi: 10.1128/JCM.41.10.4531-4536.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Parera M, Ibanez A, Clotet B, Martinez MA. Lack of evidence for protease evolution in HIV-1-infected patients after 2 years of successful highly active antiretroviral therapy. J Infect Dis. 2004;189:1444–1451. doi: 10.1086/382485. [DOI] [PubMed] [Google Scholar]
- 167.Pariente N, Pernas M, de la Rosa R, Gomez-Mariano G, Fernandez G, Rubio A, Lopez M, Benito JM, Lopez-Galindez C, Leal M, Domingo E, Martinez MA, Mas A. Long-term suppression of plasma viremia with highly active antiretroviral therapy despite virus evolution and very limited selection of drug-resistant genotypes. J Med Virol. 2004;73:350–361. doi: 10.1002/jmv.20098. [DOI] [PubMed] [Google Scholar]
- 168.Patterson BK, McCallister S, Schutz M, Siegel JN, Shults K, Flener Z, Landay A. Persistence of intracellular HIV-1 mRNA correlates with HIV-1-specific immune responses in infected subjects on stable HAART. AIDS. 2001;15:1635–1641. doi: 10.1097/00002030-200109070-00005. [DOI] [PubMed] [Google Scholar]
- 169.Percus JK, Percus OE, Markowitz M, Ho DD, Di Mascio M, Perelson AS. The distribution of viral blips observed in HIV-1 infected patients treated with combination antiretroviral therapy. Bull Math Biol. 2003;65:263–277. doi: 10.1016/S0092-8240(02)00095-2. [DOI] [PubMed] [Google Scholar]
- 170.Perelson AS. Modelling viral and immune system dynamics. Nat Rev Immunol. 2002;2:28–36. doi: 10.1038/nri700. [DOI] [PubMed] [Google Scholar]
- 171.Perelson AS, Essunger P, Cao Y, Vesanen M, Hurley A, Saksela K, Markowitz M, Ho DD. Decay characteristics of HIV-1-infected compartments during combination therapy. Nature. 1997;387:188–191. doi: 10.1038/387188a0. [DOI] [PubMed] [Google Scholar]
- 172.Perelson AS, Essunger P, Ho DD. Dynamics of HIV-1 and CD4+ lymphocytes in vivo. AIDS. 1997;11(Suppl A):S17–S24. [PubMed] [Google Scholar]
- 173.Perelson AS, Nelson PW. Mathematical analysis of HIV-1 dynamics in vivo. SIAM Rev. 1999;41:3–44. [Google Scholar]
- 174.Perelson AS, Neumann AU, Markowitz M, Leonard JM, Ho DD. HIV-1 dynamics in vivo: virion clearance rate, infected cell life-span, and viral generation time. Science. 1996;271:1582–1586. doi: 10.1126/science.271.5255.1582. [DOI] [PubMed] [Google Scholar]
- 175.Persaud D, Ray SC, Kajdas J, Ahonkhai A, Siberry GK, Ferguson K, Ziemniak C, Quinn TC, Casazza JP, Zeichner S, Gange SJ, Watson DC. Slow human immunodeficiency virus type 1 evolution in viral reservoirs in infants treated with effective antiretroviral therapy. AIDS Res Hum Retroviruses. 2007;23:381–390. doi: 10.1089/aid.2006.0175. [DOI] [PubMed] [Google Scholar]
- 176.Persaud D, Siberry GK, Ahonkhai A, Kajdas J, Monie D, Hutton N, Watson DC, Quinn TC, Ray SC, Siliciano RF. Continued production of drug-sensitive human immunodeficiency virus type 1 in children on combination antiretroviral therapy who have undetectable viral loads. J Virol. 2004;78:968–979. doi: 10.1128/JVI.78.2.968-979.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Persaud D, Zhou Y, Siliciano JM, Siliciano RF. Latency in human immunodeficiency virus type 1 infection: no easy answers. J Virol. 2003;77:1659–1665. doi: 10.1128/JVI.77.3.1659-1665.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Peterson S, Reid AP, Kim S, Siliciano RF. Treatment implications of the latent reservoir for HIV-1. Adv Pharmacol. 2007;55:411–425. doi: 10.1016/S1054-3589(07)55012-X. [DOI] [PubMed] [Google Scholar]
- 179.Phillips AN. Reduction of HIV concentration during acute infection: independence from a specific immune response. Science. 1996;271:497–499. doi: 10.1126/science.271.5248.497. [DOI] [PubMed] [Google Scholar]
- 180.Piatak M, Jr, Saag MS, Yang LC, Clark SJ, Kappes JC, Luk KC, Hahn BH, Shaw GM, Lifson JD. High levels of HIV-1 in plasma during all stages of infection determined by competitive PCR. Science. 1993;259:1749–1754. doi: 10.1126/science.8096089. [DOI] [PubMed] [Google Scholar]
- 181.Pierson T, McArthur J, Siliciano RF. Reservoirs for HIV-1: mechanisms for viral persistence in the presence of antiviral immune responses and antiretroviral therapy. Annu Rev Immunol. 2000;18:665–708. doi: 10.1146/annurev.immunol.18.1.665. [DOI] [PubMed] [Google Scholar]
- 182.Pilyugin SS, Ganusov VV, Murali-Krishna K, Ahmed R, Antia R. The rescaling method for quantifying the turnover of cell populations. J Theor Biol. 2003;225:275–283. doi: 10.1016/s0022-5193(03)00245-5. [DOI] [PubMed] [Google Scholar]
- 183.Poveda E, Briz V, Soriano V. Enfuvirtide, the first fusion inhibitor to treat HIV infection. AIDS Rev. 2005;7:139–147. [PubMed] [Google Scholar]
- 184.Prins JM, et al. Immuno-activation with anti-CD3 and recombinant human IL-2 in HIV-1-infected patients on potent antiretroviral therapy. AIDS. 1999;13:2405–2410. doi: 10.1097/00002030-199912030-00012. [DOI] [PubMed] [Google Scholar]
- 185.Ramratnam B, Mittler JE, Zhang L, Boden D, Hurley A, Fang F, Macken CA, Perelson AS, Markowitz M, Ho DD. The decay of the latent reservoir of replication-competent HIV-1 is inversely correlated with the extent of residual viral replication during prolonged anti-retroviral therapy. Nat Med. 2000;6:82–85. doi: 10.1038/71577. [DOI] [PubMed] [Google Scholar]
- 186.Ramratnam B, Ribeiro R, He T, Chung C, Simon V, Vanderhoeven J, Hurley A, Zhang L, Perelson AS, Ho DD, Markowitz M. Intensification of antiretroviral therapy accelerates the decay of the HIV-1 latent reservoir and decreases, but does not eliminate, ongoing virus replication. J Acquir Immune Defic Syndr. 2004;35:33–37. doi: 10.1097/00126334-200401010-00004. [DOI] [PubMed] [Google Scholar]
- 187.Regoes RR, Wodarz D, Nowak MA. Virus dynamics: the effect of target cell limitation and immune responses on virus evolution. J Theor Biol. 1998;191:451–462. doi: 10.1006/jtbi.1997.0617. [DOI] [PubMed] [Google Scholar]
- 188.Ribeiro RM, Bonhoeffer S. Production of resistant HIV mutants during antiretroviral therapy. Proc Natl Acad Sci USA. 2000;97:7681–7686. doi: 10.1073/pnas.97.14.7681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Richman DD. Antiviral drug resistance. Antiviral Res. 2006;71:117–121. doi: 10.1016/j.antiviral.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 190.Richman DD, Margolis DM, Delaney M, Greene WC, Hazuda D, Pomerantz RJ. The challenge of finding a cure for HIV infection. Science. 2009;323:1304–1307. doi: 10.1126/science.1165706. [DOI] [PubMed] [Google Scholar]
- 191.Rong L, Feng Z, Perelson AS. Emergence of HIV-1 drug resistance during antiretroviral treatment. Bull Math Biol. 2007;69:2027–2060. doi: 10.1007/s11538-007-9203-3. [DOI] [PubMed] [Google Scholar]
- 192.Rong L, Feng Z, Perelson AS. Mathematical analysis of age-structured HIV-1 dynamics with combination antiretroviral therapy. SIAM J Appl Math. 2007;67:731–756. [Google Scholar]
- 193.Rong L, Gilchrist MA, Feng Z, Perelson AS. Modeling within-host HIV-1 dynamics and the evolution of drug resistance: trade-offs between viral enzyme function and drug susceptibility. J Theor Biol. 2007;247:804–818. doi: 10.1016/j.jtbi.2007.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Rong L, Perelson AS. Asymmetric division of activated latently infected cells may explain the decay kinetics of the HIV-1 latent reservoir and intermittent viral blips. Math Biosci. 2009;217:77–87. doi: 10.1016/j.mbs.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Rong L, Perelson AS. Modeling the effects of latently infected cell activation on low-level viral persistence, slow decay of latent reservoir, and intermittent viral blips in HIV-infected patients on HAART. Preprint 2009 [Google Scholar]
- 196.Ruff CT, Ray SC, Kwon P, Zinn R, Pendleton A, Hutton N, Ashworth R, Gange S, Quinn TC, Siliciano RF, Persaud D. Persistence of wild-type virus and lack of temporal structure in the latent reservoir for human immunodeficiency virus type 1 in pediatric patients with extensive antiretroviral exposure. J Virol. 2002;76:9481–9492. doi: 10.1128/JVI.76.18.9481-9492.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Schrager LK, D’Souza MP. Cellular and anatomical reservoirs of HIV-1 in patients receiving potent antiretroviral combination therapy. JAMA. 1998;280:67–71. doi: 10.1001/jama.280.1.67. [DOI] [PubMed] [Google Scholar]
- 198.Sedaghat AR, Dinoso JB, Shen L, Wilke CO, Siliciano RF. Decay dynamics of HIV-1 depend on the inhibited stages of the viral life cycle. Proc Natl Acad Sci USA. 2008;105:4832–4837. doi: 10.1073/pnas.0711372105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Sedaghat AR, Siliciano JD, Brennan TP, Wilke CO, Siliciano RF. Limits on replenishment of the resting CD4+ T cell reservoir for HIV in patients on HAART. PLoS Pathog. 2007;3:e122. doi: 10.1371/journal.ppat.0030122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Sharkey ME, Teo I, Greenough T, Sharova N, Luzuriaga K, Sullivan JL, Bucy RP, Kostrikis LG, Haase A, Veryard C, Davaro RE, Cheeseman SH, Daly JS, Bova C, Ellison RT, 3rd, Mady B, Lai KK, Moyle G, Nelson M, Gazzard B, Shaunak S, Stevenson M. Persistence of episomal HIV-1 infection intermediates in patients on highly active anti-retroviral therapy. Nat Med. 2000;6:76–81. doi: 10.1038/71569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Sharkey M, Triques K, Kuritzkes DR, Stevenson M. In vivo evidence for instability of episomal human immunodeficiency virus type 1 cDNA. J Virol. 2005;79:5203–5210. doi: 10.1128/JVI.79.8.5203-5210.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Siliciano JD, Kajdas J, Finzi D, Quinn TC, Chadwick K, Margolick JB, Kovacs C, Gange SJ, Siliciano RF. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nat Med. 2003;9:727–728. doi: 10.1038/nm880. [DOI] [PubMed] [Google Scholar]
- 203.Siliciano JD, Lai J, Callender M, Pitt E, Zhang H, Margolick JB, Gallant JE, Cofrancesco J, Jr, Moore RD, Gange SJ, Siliciano RF. Stability of the latent reservoir for HIV-1 in patients receiving valproic acid. J Infect Dis. 2007;195:833–836. doi: 10.1086/511823. [DOI] [PubMed] [Google Scholar]
- 204.Siliciano JD, Siliciano RF. A long-term latent reservoir for HIV-1: discovery and clinical implications. J Antimicrob Chemother. 2004;54:6–9. doi: 10.1093/jac/dkh292. [DOI] [PubMed] [Google Scholar]
- 205.Siliciano RF. Scientific rationale for antiretroviral therapy in 2005: viral reservoirs and resistance evolution. Top HIV Med. 2005;13:96–100. [PubMed] [Google Scholar]
- 206.Simon V, Ho DD. HIV-1 dynamics in vivo: implications for therapy. Nat Rev Microbiol. 2003;1:181–190. doi: 10.1038/nrmicro772. [DOI] [PubMed] [Google Scholar]
- 207.Sklar PA, Ward DJ, Baker RK, Wood KC, Gafoor Z, Alzola CF, Moorman AC, Holmberg SD HIV Outpatient Study (HOPS) Investigators. Prevalence and clinical correlates of HIV viremia (‘blips’) in patients with previous suppression below the limits of quantification. AIDS. 2002;16:2035–2041. doi: 10.1097/00002030-200210180-00008. [DOI] [PubMed] [Google Scholar]
- 208.Smith RJ. Adherence to antiretroviral HIV drugs: how many doses can you miss before resistance emerges? Proc R Soc B. 2006;273:617–624. doi: 10.1098/rspb.2005.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Smith RJ, Wahl LM. Drug resistance in an immunological model of HIV-1 infection with impulsive drug effects. Bull Math Biol. 2005;67:783–813. doi: 10.1016/j.bulm.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 210.Sprent J, Surh CD. T cell momory. Annu Rev Immunol. 2002;20:551–579. doi: 10.1146/annurev.immunol.20.100101.151926. [DOI] [PubMed] [Google Scholar]
- 211.Stafford MA, Corey L, Cao Y, Daar ES, Ho DD, Perelson AS. Modeling plasma virus concentration during primary HIV infection. J Theor Biol. 2000;203:285–301. doi: 10.1006/jtbi.2000.1076. [DOI] [PubMed] [Google Scholar]
- 212.Stanley SK, Ostrowski MA, Justement JS, Gantt K, Hedayati S, Mannix M, Roche K, Schwartzentruber DJ, Fox CH, Fauci AS. Effect of immunization with a common recall antigen on viral expression in patients infected with human immunodeficiency virus type 1. N Engl J Med. 1996;334:1222–1230. doi: 10.1056/NEJM199605093341903. [DOI] [PubMed] [Google Scholar]
- 213.Staszewski S, Morales-Ramirez J, Tashima KT, Rachlis A, Skiest D, Stanford J, Stryker R, Johnson P, Labriola DF, Farina D, Manion DJ, Ruiz NM. Efavirenz plus zidovudine and lamivudine, efavirenz plus indinavir, and indinavir plus zidovudine and lamivudine in the treatment of HIV-1 infection in adults. N Engl J Med. 1999;341:1865–1873. doi: 10.1056/NEJM199912163412501. [DOI] [PubMed] [Google Scholar]
- 214.Stevenson M. HIV-1 pathogenesis. Nat Med. 2003;9:853–860. doi: 10.1038/nm0703-853. [DOI] [PubMed] [Google Scholar]
- 215.Strain MC, Gunthard HF, Havlir DV, Ignacio CC, Smith DM, Leigh-Brown AJ, Macaranas TR, Lam RY, Daly OA, Fischer M, Opravil M, Levine H, Bacheler L, Spina CA, Richman DD, Wong JK. Heterogeneous clearance rates of long-lived lymphocytes infected with HIV: intrinsic stability predicts lifelong persistence. Proc Natl Acad Sci USA. 2003;100:4819–4824. doi: 10.1073/pnas.0736332100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Strain MC, Little SJ, Daar ES, Havlir DV, Gunthard HF, Lam RY, Daly OA, Nguyen J, Ignacio CC, Spina CA, Richman DD, Wong JK. Effect of treatment, during primary infection, on establishment and clearance of cellular reservoirs of HIV-1. J Infect Dis. 2005;191:1410–1418. doi: 10.1086/428777. [DOI] [PubMed] [Google Scholar]
- 217.Tobin NH, Learn GH, Holte SE, Wang Y, Melvin AJ, McKernan JL, Pawluk DM, Mohan KM, Lewis PF, Mullins JI, Frenkel LM. Evidence that low-level viremias during effective highly active antiretroviral therapy result from two processes: expression of archival virus and replication of virus. J Virol. 2005;79:9625–9634. doi: 10.1128/JVI.79.15.9625-9634.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Tough DF, Sprent J. Turnover of naive- and memory-phenotype T cells. J Exp Med. 1994;179:1127–1135. doi: 10.1084/jem.179.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Unutmaz D, Pileri P, Abrignani S. Antigen-independent activation of naive and memory resting T cells by a cytokine combination. J Exp Med. 1994;180:1159–1164. doi: 10.1084/jem.180.3.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Verhofstede C, Noë A, Demecheleer E, De Cabooter N, Van Wanzeele F, Van Der Gucht B, Vogelaers D, Plum J. Drug-resistant variants that evolve during nonsuppressive therapy persist in HIV-1-infected peripheral blood mononuclear cells after long-term highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2004;35:473–483. doi: 10.1097/00126334-200404150-00005. [DOI] [PubMed] [Google Scholar]
- 221.Verhofstede C, Wanzeele FV, Van Der Gucht B, De Cabooter N, Plum J. Interruption of reverse transcriptase inhibitors or a switch from reverse transcriptase to protease inhibitors resulted in a fast reappearance of virus strains with a reverse transcriptase inhibitor-sensitive genotype. AIDS. 1999;13:2541–2546. doi: 10.1097/00002030-199912240-00007. [DOI] [PubMed] [Google Scholar]
- 222.Wahl LM, Nowak MA. Adherence and drug resistance: predictions for therapy outcome. Proc R Soc Lond B. 2000;267:835–843. doi: 10.1098/rspb.2000.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Wei X, Ghosh SK, Taylor ME, Johnson VA, Emini EA, Deutsch P, Lifson JD, Bonhoeffer S, Nowak MA, Hahn BH, Saag MS, Shaw GM. Viral dynamics in human-immunodeficiency-virus type-1 infection. Nature. 1995;373:117–122. doi: 10.1038/373117a0. [DOI] [PubMed] [Google Scholar]
- 224.Weinberger LS, Burnett JC, Toettcher JE, Arkin AP, Schaffer DV. Stochastic gene expression in a lentiviral positive-feedback loop: HIV-1 Tat fluctuations drive phenotypic diversity. Cell. 2005;122:169–182. doi: 10.1016/j.cell.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 225.Wodarz D. Killer Cell Dynamics: Mathematical and Computational Approaches to Immunology. Springer; 2007. [Google Scholar]
- 226.Wodarz D, May RM, Nowak MA. The role of antigen-independent persistence of memory cytotoxic T lymphocytes. Int Immunol. 2000;12:467–477. doi: 10.1093/intimm/12.4.467. [DOI] [PubMed] [Google Scholar]
- 227.Wodarz D, Nowak MA. CD8 memory, immunodominance, and antigenic escape. Eur J Immunol. 2000;30:2704–2712. doi: 10.1002/1521-4141(200009)30:9<2704::AID-IMMU2704>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 228.Wodarz D, Nowak MA. Mathematical models of HIV pathogenesis and treatment. Bioessays. 2002;24:1178–1187. doi: 10.1002/bies.10196. [DOI] [PubMed] [Google Scholar]
- 229.Wong JK, Hezareh M, Günthard HF, Havlir DV, Ignacio CC, Spina CA, Richman DD. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science. 1997;278:1291–1295. doi: 10.1126/science.278.5341.1291. [DOI] [PubMed] [Google Scholar]
- 230.Wu H, Huang Y, Acosta EP, Rosenkranz SL, Kuritzkes DR, Eron JJ, Perelson AS, Gerber JG. Modeling long-term HIV dynamics and antiretroviral response: effects of drug potency, pharmacokinetics, adherence, and drug resistance. J Acquir Immune Defic Syndr. 2005;39:272–283. doi: 10.1097/01.qai.0000165907.04710.da. [DOI] [PubMed] [Google Scholar]
- 231.Wu H, Zhu H, Miao H, Perelson AS. Parameter identifiability and estimation of HIV/AIDS dynamic models. Bull Math Biol. 2008;70:785–799. doi: 10.1007/s11538-007-9279-9. [DOI] [PubMed] [Google Scholar]
- 232.Ylisastigui L, Archin NM, Lehrman G, Bosch RJ, Margolis DM. Coaxing HIV-1 from resting CD4 T cells: histone deacetylase inhibition allows latent viral expression. AIDS. 2004;18:1101–1108. doi: 10.1097/00002030-200405210-00003. [DOI] [PubMed] [Google Scholar]
- 233.Zhang L, Chung C, Hu BS, He T, Guo Y, Kim AJ, Skulsky E, Jin X, Hurley A, Ramratnam B, Markowitz M, Ho DD. Genetic characterization of rebounding HIV-1 after cessation of highly active antiretroviral therapy. J Clin Invest. 2000;106:839–845. doi: 10.1172/JCI10565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Zhang H, Dornadula G, Beumont M, Livornese L, Jr, Van Uitert B, Henning K, Pomerantz RJ. Human immunodeficiency virus type 1 in the semen of men receiving highly active antiretroviral therapy. N Engl J Med. 1998;339:1803–1809. doi: 10.1056/NEJM199812173392502. [DOI] [PubMed] [Google Scholar]
- 235.Zhang L, Ramratnam B, Tenner-Racz K, He Y, Vesanen M, Lewin S, Talal A, Racz P, Perelson AS, Korber BT, Markowitz M, Ho DD. Quantifying residual HIV-1 replication in patients receiving combination antiretroviral therapy. N Engl J Med. 1999;340:1605–1613. doi: 10.1056/NEJM199905273402101. [DOI] [PubMed] [Google Scholar]
- 236.Zhang Z, et al. Sexual transmission and propagation of SIV and HIV in resting and activated CD4+ T cells. Science. 1999;286:1353–1357. doi: 10.1126/science.286.5443.1353. [DOI] [PubMed] [Google Scholar]
- 237.Zhang ZQ, Notermans DW, Sedgewick G, Cavert W, Wietgrefe S, Zupancic M, Gebhard K, Henry K, Boies L, Chen Z, Jenkins M, Mills R, McDade H, Goodwin C, Schuwirth CM, Danner SA, Haase AT. Kinetics of CD4+ T cell repopulation of lymphoid tissues after treatment of HIV-1 infection. Proc Natl Acad Sci USA. 1998;95:1154–1159. doi: 10.1073/pnas.95.3.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Zhu T, Muthui D, Holte S, Nickle D, Feng F, Brodie S, Hwangbo Y, Mullins JI, Corey L. Evidence for human immunodeficiency virus type 1 replication in vivo in CD14(+) monocytes and its potential role as a source of virus in patients on highly active antiretroviral therapy. J Virol. 2002;76:707–16. doi: 10.1128/JVI.76.2.707-716.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]