Abstract
Objective
The goal of this pilot study was to evaluate the effects of testosterone co-therapy on mammary gland and endometrial measures in a postmenopausal primate model.
Design
Twenty-five surgically postmenopausal cynomolgus monkeys were randomized by social group to receive daily treatment with (1) placebo, (2) oral micronized 17β-estradiol (1 mg/day equivalent in women) + progesterone (200 mg/day equivalent in women) (E+P), or (3) E+P with testosterone administered via subcutaneous pellets for 8 weeks at a high dose (15 mg) followed by 8 weeks at a low dose (1.5 mg) (E+P+T). The main outcome measures were breast and endometrial epithelial proliferation, as measured by Ki67 / MIB1 immunolabeling.
Results
Intralobular breast proliferation did not differ significantly among groups after 8 weeks of treatment but was marginally higher (P = 0.03) in the E+P+T group after 16 weeks of treatment. No significant increase in proliferation was seen for E+P alone. Comparable changes in mammary gland markers of estrogen receptor activity were seen for E+P and E+P+T groups. In the endometrium, the addition of T did not increase endometrial glandular proliferation or estrogen receptor activity or result in any distinct histologic changes.
Conclusions
Findings of this study do not support the idea that testosterone antagonizes effects of combined hormone therapy on breast proliferation or markers of estrogen receptor activity. Overall short-term effects of testosterone co-therapy on the mammary gland and endometrium were minimal.
Keywords: testosterone, hormone therapy, estradiol, progesterone, breast, endometrium, proliferation
Introduction
Androgens have emerged in recent years as an important issue in menopausal women's health.1-3 The postmenopausal period is associated with decreased levels of not only ovarian estrogen and progesterone but also endogenous androgens,4 and age-related changes in circulating androgens have been associated with several chronic diseases.5-8 Limited evidence also suggests that exogenous androgen therapy may have potential benefits on various health parameters, including sexual function, bone density, and lean body mass.1,9,10 Nevertheless, androgens have not been a part of traditional postmenopausal hormone therapy (HT) regimens due in large part to concerns over safety and lack of efficacy data for specific menopausal indications.1-2
Androgen effects on breast cancer risk are particularly controversial.1,3,9,11-13 With some exceptions, in vitro and rodent studies have generally found that androgens attenuate estrogen-induced proliferation in breast cancer cells or induced mammary tumors.9,13,14 These findings are supported by two small studies in postmenopausal macaques showing moderate antagonism of combined estrogen+progestogen therapy on breast proliferation by testosterone (T).15-16 Similar results were also reported in a recent trial of postmenopausal women which found increased breast cell proliferation following combined HT alone but not after the addition of a T patch.17
In contrast to these findings, epidemiologic studies of postmenopausal women point to potential adverse effects of androgens on the breast. Several prospective observational studies have shown a positive association between endogenous serum androgens and breast cancer risk,3,11,12 with ∼2 to 3 times greater risk for postmenopausal women in the upper quartile of serum T concentrations compared to those in the lower quartile.18-23 A recent study also found higher risk of relapse and lower event-free survival in postmenopausal breast cancer patients with high endogenous T compared to those with low T.24 Other studies have found no significant relationship between serum androgens and breast cancer risk, particularly after adjusting for serum estrogen and other risk factors.25-27 In one of the few epidemiologic reports to examine the effects of exogenous androgen therapy on breast cancer risk, a 2.48 relative risk for breast cancer was found in women using esterified estrogens + methyltestosterone (mT) compared with never-users of HT.28 This increase in risk with E+mT was significantly greater compared with estrogen-only therapy and marginally greater compared with estrogen+progestogen therapy (but was only significant during the first 5 years of therapy). Such data have contributed to concerns that androgens may actually augment estrogen effects on the breast epithelium.
Recent evidence indicates that certain types of long-term combined HT may increase the risk of breast cancer in postmenopausal women.30-32 These findings have led to uncertainty regarding the safest types of HT and increased interest in alternative types of HT that may provide a better safety profile. Such alternatives include androgen co-therapies such as T. The goal of the current study was to investigate the effects of adding T to combination estrogen + progestogen therapy on the breast and endometrium in a postmenopausal primate model. We hypothesized that a combination of estradiol (E) + progesterone (P) + T would result in less proliferation and markers of estrogen receptor activity compared with E+P alone.
Materials and Methods
Animal subjects
For this study 25 adult female surgically menopausal cynomolgus monkeys (Macaca fascicularis) with an average age of 8.8 ± 0.3 years were used. All animals had been ovariectomized for 2.3 years and housed since this time in stable social groups of 3 - 5 animals each. Prior studies in these animals are described elsewhere.33-36 Macaques are Old World primates with >90% overall genetic coding sequence identity to humans,37 including important genes related to breast cancer risk.38 Female macaques have a 28-day menstrual cycle and ovarian hormone profile highly similar to that of women,39 and prior work from our laboratory and others have demonstrated similarities between macaque and human mammary gland biology, including cytokeratin expression,40 sex steroid receptor expression,41 responses to endogenous and exogenous sex steroids,33,42-44 and the presence of hyperplastic and neoplastic mammary gland lesions.45-46 All procedures in this study were conducted in compliance with State and Federal laws, standards of the U.S. Department of Health and Human Services, and guidelines established by the Wake Forest University Animal Care and Use Committee. The facilities and laboratory animal program of Wake Forest University are fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care.
Study design and treatments
The animals received no hormone treatment for 6 weeks prior to the start of the current experiment. Animals were randomized by social group to the following treatments: (1) placebo control (Con) (n = 7); (2) oral micronized 17β-estradiol (E, 1 mg/day) (Estrace®; Bristol-Myers Squibb, New York, NY, USA) + oral micronized progesterone (P, 200 mg/day) (Prometrium®; Solvay Pharmaceuticals, Marietta, GA, USA) (n = 8); or (3) E+P+testosterone (T), delivered via 90-day release subcutaneous pellets containing either 15 mg or 1.5 mg of testosterone (Innovative Research of America, Sarasota, FL, USA) (n = 10). Total treatment time was 16 wks, divided into 8 wk phases for high and low dose T. After 8 wks, the animals were anesthetized with ketamine and buprenorphine; high-dose pellets were removed (from the interscapular subcutis) and low-dose pellets were implanted (into the caudal aspect of the left brachium). Doses of T were designed to represent high-dose supraphysiologic (15 mg) and low-dose physiologic (1.5 mg) concentrations. Daily doses are expressed in human equivalents; absolute daily doses (in mg/kg body weight) were 0.05 for E, 11.1 for P, and an estimated ∼0.06 (high dose) and ∼0.006 (low dose) for T. Throughout the experiment the animals were fed a standard control diet with casein / lactalbumin as the protein source. The diet contained 17.8% of calories from protein, 34.5% from fat, 47.7% from carbohydrates, and 0.20 mg cholesterol/kcal.
The animals were dosed with E and P each morning between 9:00 and 11:00 am. Estradiol was administered within a fruit punch (Crystal Lite®) vehicle, while P was injected into a small marshmallow or piece of banana or tangerine (to minimize parenteral absorption). Control animals received a placebo fruit punch and fruit piece. For dosing, all animals were previously trained to enter a catch cage, drink the fruit punch from a syringe, and then eat the marshmallow or fruit. Individual oral drug doses were calculated based on body weight.
Tissue collection and processing
Mammary gland samples were collected following 8 wks (high-dose T) and 16 wks (low-dose T) of treatment. For interim biopsies, the animals were anesthetized with ketamine and buprenorphine and a small (∼0.4 gram) sample of mammary gland was removed from a preselected breast quadrant. Biopsies were performed by an experienced veterinary surgeon (CJL), and the animals were monitored and given analgesia during recovery following approved clinical procedures. Following 16 wks of treatment, animals were sedated with ketamine and euthanized using sodium pentobarbital (100 mg/kg, intravenous), as recommended by the Panel on Euthanasia of the American Veterinary Medical Association. Following collection, half of each mammary gland sample was fixed at 4°C in 4% paraformaldehyde for 24 hrs, transferred to 70% ethanol, trimmed, embedded in paraffin, and sectioned to 5 μm in thickness for hematoxylin and eosin (H&E) and immunohistochemical staining. The other half was snap-frozen in liquid nitrogen for later use in gene expression assays. At necropsy uteri were also collected, weighed, and sectioned transversely at the point of greatest diameter. Half was fixed for histologic, morphometric, and immunohistochemical evaluations while the endometrium from the other half was trimmed and snap-frozen. Histopathologic assessments were made blinded to treatment group.
Immunohistochemistry
Immunostaining procedures were performed on fixed mammary gland and uterine tissues using commercially-available primary monoclonal antibodies for the proliferation marker Ki67 (Ki67/MIB1; Dako, Carpinteria, CA, USA; 1:50 dilution), progesterone receptor (PGR) (NCL-PGR; Novocastra, Newcastle-upon-Tyne, UK; 1:100 dilution), estrogen receptor alpha (NCL-ER-6F11; Novocastra; 1:100 dilution), and androgen receptor (AR-2F12 and AR-318, Novocastra; 1:10 dilution). Staining methods included antigen-retrieval with citrate buffer (pH 6.0), biotinylated rabbit anti-mouse Fc antibody as a linking reagent, alkaline phosphatase-conjugated streptavidin as the label, and Vector Red as the chromogen (Vector Laboratories, Burlingame, CA, USA). Cell labeling was quantified by a computer-assisted manual counting technique using a digital grid filter to select cells for counting across at least 3 microscopic fields47 and our modified procedure of cell selection, described previously.48 This technique provides more systematic and less biased cell counts and covers a larger area of tissue than counting a greater number of cells sequentially. Each immunostain batch included negative control slides which used the same protocol as for study slides except that non-immune serum (from the same species as primary antibody) was used in place of the primary antibody. Numbers of positively stained cells were measured as a percentage of the total number examined (100 cells) for each anatomic compartment (lobular epithelium and extralobular ducts for mammary gland, superficial / deep glands and stroma for endometrium). All measurements were made blinded to treatment group.
Quantitative gene expression
Expression levels of mRNA transcripts for genes associated with cellular proliferation (Ki67 antigen, MKI67), estrogen / progesterone action (estrogen receptor alpha, ESR1; progesterone receptor, PGR; signal transducer and activator of transcription 5A, STAT5A), and androgen action (androgen receptor, AR; kallikrein 3, KLK3) were determined using quantitative real-time reverse transcriptase polymerase chain reaction (qRT-PCR). Mammary gland and endometrial total RNA was extracted, purified, quantitated, qualitatively evaluated for intactness, and reverse-transcribed using techniques described previously.49 Cynomolgus macaque-specific primer-probe sets for internal control genes (glyceraldehyde-3-phosphate dehydrogenase, GAPDH; β-Actin, ACTB), ESR1, AR, and KLK3 were generated through the Applied Biosystems (ABI) Taqman Assay-by-Design service (Foster City, CA), while commercially available rhesus (STAT5A) or human (PGR, MKI67) Taqman assays were used for remaining assays (Supplementary Table 1). All probes were designed to span an exon-exon junction to eliminate genomic DNA amplification. Normal premenopausal breast tissue and hormone receptor negative tumor tissue were run as positive and/or negative controls on each plate. Reactions were performed on an ABI Prism 7000 using standard reagents and thermocycling protocol.49 Relative expression levels were determined using the ΔΔCt method described in ABI User Bulletin #2 (available online). The Ct values for GAPDH and ACTB were averaged for use in internal calibration, while reference breast tissue RNA was run in parallel as an external calibrator.
Uterine ultrasound and histomorphometry
Uterine area was determined by trans-abdominal ultrasound using a Sonosite 180 portable ultrasound machine with a 5.0 MHz linear transducer (Sonosite, Bothell, WA, USA). Maximal transverse cross-sectional area was measured on static representative digital images using public domain software (NIH ImageJ 1.33j, available online). Endometrial thickness and glandular area were quantified by histomorphometry, as described previously.50 Briefly, H&E-stained slides were digitized using an Infinity 3 digital camera (Lumenera, North Andover, MA), and measurements were taken with Image-Pro Plus software version 5.1 (Media Cybernetics, Silver Spring, MD). For endometrial thickness and glandular area, microscopic fields (3 for thickness, 6 for area) were randomly selected at an objective magnification of 20× and measurements were taken at the point of greatest perpendicular depth. Area was determined by manual tracing of glandular endometrial units and expressed as a percentage of the total area examined. All measurements were made blinded to treatment group.
Vaginal cytology and epithelial thickness
To evaluate treatment effects on vaginal maturation, vaginal keratinocytes were collected with a cotton swab, rolled onto a glass slide, fixed, and stained using a modified Papanicolau method. Maturation value was calculated as follows: (0.2 × % parabasal cells) + (0.6 × % intermediate cells) + (% superficial cells). Vaginal epithelial thickness was quantified by histomorphometry at the end of the study on H&E-stained slides, using techniques as for endometrial thickness.
Serum hormones
Serum concentrations of E, P, and T were measured from samples collected 2-4 hrs (acute) and 20-28 hrs (lag) after oral EP dosing. Blood was collected by femoral venipuncture following sedation with ketamine, and serum concentrations were quantitated by radioimmunoassay using commercially available kits from Diagnostic Systems Laboratories (Webster, TX, USA) (E, DSL-4800 ultra-sensitive; P, Coat-A-Count) and Diagnostic Products Corp. (Los Angeles, CA, USA) (total T, Coat-A-Count). For E and P assays, serum was extracted with ethyl ether using standard procedures.
Serum lipids
Blood was collected at baseline, after 8 wks (high dose T), and after 16 wks (low dose T) of treatment for measurement of serum lipid/lipoprotein concentrations. Samples were collected after food had been withheld for >18 hrs. Total cholesterol (TC), high density lipoprotein cholesterol (HDL), and triglyceride (TG) concentrations were measured using enzymatic methods on the COBAS FARA II analyzer (Roche Diagnostics Inc., Montclair, NJ, USA), with protocols and reagents supplied by Boehringer Mannheim. Serum samples from all timepoints were run at the same time. Lipid assays were run in a clinical chemistry laboratory at Wake Forest University School of Medicine which is fully standardized with this method and is in the continuing surveillance phase of the Centers for Disease Control (Atlanta, GA, USA) Lipid Standardization Program. HDL concentrations were measured using the heparin-manganese precipitation procedure.51 Low density lipoprotein cholesterol (LDL) plus very low density lipoprotein cholesterol (VLDL) was calculated as the difference between TPC and HDL.
Statistics
This study was designed as a pilot investigation. Statistical power was calculated based on prior mammary lobular epithelial proliferation data, determined by Ki67 immunolabeling, using conjugated equine estrogens (CEE) + medroxyprogesterone acetate (MPA) as the hormone treatment.43 For this measure, we estimated the minimum difference between control and hormone therapy means to be 14% positive cells with an overall standard deviation of 12.0%. The sample size in each group providing >70% chance at a 0.05 significance level to detect a statistically significant increase in proliferation was 8 per group. Data were analyzed using analysis of variance for breast and uterine endpoints, body weight, and serum hormones. A mixed general linear model with baseline covariance was used for serum lipid data. All variables were evaluated for their distribution and equality of variances between diets. Due to non-normal distribution, immunohistochemistry and serum hormone data were evaluated using a nonparametric Kruskal-Wallis test followed by two-sided Wilcoxon Rank Sum pairwise analysis. All qRT-PCR data were log-transformed to improve distribution and then retransformed to original scale and reported as percent control with 90% confidence interval. Data are otherwise reported as mean (± standard error). A two-tailed Fisher's exact test was used to evaluate treatment group differences in lesion prevalence on histology. Missing datapoints included a subset of mammary gland biopsies (8 wk) lacking lobuloalveolar (n = 5, 1 control and 4 E+P) or ductal (n = 9, 2 Con, 6 E+P, and 1 E+P+T) epithelium on sectioning and unmeasurable uterine ultrasound images (n = 5, 1 Con and 1 E+P+T at 0 wks, 1 Con at 8 wks, 2 Con at 16 wks). All pairwise P-values were adjusted for the number of pairwise tests (3) using a Bonferroni correction. Data were analyzed using the SAS statistical package (version 8; SAS Institute, Cary, NC). A two-tailed significance level of 0.05 was chosen for all comparisons.
Results
Body weight and reproductive tract measures
No baseline differences in body weight, uterine area, or vaginal maturation index were noted among treatment groups (P > 0.1 for all) (Table 1). Increases in uterine area, vaginal maturation index, and vaginal epithelial thickness were seen for E+P and E+P+T (both high and low T doses) compared to control (P < 0.05 for all), while no significant effects on body weight were observed (Table 1). Endometrial thickness was also greater in the E+P and E+P+T groups following the low dose T phase (P < 0.001), while endometrial glandular area was only marginally higher (ANOVA P = 0.051) compared to control. E+P and E+P+T groups did not differ significantly for any of these measures.
Table 1. Treatment effects on body weight and reproductive tract measures.1-3.
| Con | E+P | E+P+T | ANOVA P value | |
|---|---|---|---|---|
| Body weight (kg) | ||||
| Baseline (0 wk) | 4.57 ± 0.35 | 4.54 ± 0.32 | 4.46 ± 0.29 | 0.97 |
| High-dose T (8 wk) | 4.69 ± 0.39 | 4.59 ± 0.37 | 4.59 ± 0.33 | 0.98 |
| Low-dose T (16 wk) | 4.68 ± 0.37 | 4.67 ± 0.35 | 4.54 ± 0.31 | 0.94 |
| Uterine area (cm2) | ||||
| Baseline (0 wk) | 0.79 ± 0.12 | 0.88 ± 0.10 | 0.72 ± 0.09 | 0.53 |
| High-dose T (8 wk) | 0.63 ± 0.21 | 1.57 ± 0.18b | 1.38 ± 0.16a | 0.008 |
| Low-dose T (16 wk) | 0.68 ± 0.19 | 1.88 ± 0.15c | 1.72 ± 0.14c | <0.001 |
| Endometrial thickness (mm) | ||||
| Low-dose T (16 wk) | 0.95 ± 0.16 | 2.34 ± 0.15c | 2.02 ± 0.14c | <0.001 |
| Endometrial glandular area (%) | ||||
| Low-dose T (16 wk) | 9.6 ± 1.5 | 14.8 ± 1.4 | 12.7 ± 1.2 | 0.051 |
| Vaginal maturation index | ||||
| Baseline (0 wk) | 54.3 ± 5.6 | 51.4 ± 5.3 | 55.4 ± 4.7 | 0.85 |
| High-dose T (8 wk) | 47.5 ± 5.5 | 78.5 ± 5.1c | 73.7 ± 4.6b | <0.001 |
| Low-dose T (16 wk) | 48.3 ± 3.2 | 80.9 ± 3.0c | 83.0 ± 2.7c | <0.001 |
| Vaginal epithelial thickness (μm) | ||||
| Low-dose T (16 wk) | 190 ± 73 | 615 ± 68c | 560 ± 61c | <0.001 |
Control = placebo; E = oral 17β-Estradiol; P = oral micronized progesterone; T = testosterone administered via subcutaneous pellets at high (8 wk) and low (16 wk) doses. n = 7, 8, and 10 for Con, E+P, and E+P+T groups, respectively, for all measures except uterine area; for this latter measure n = 6, 8, and 9 at baseline, n = 6, 8, 10 at 8 wk, and n = 5, 8, and 10 at 16 wk for Con, E+P, and E+P+T groups, respectively.
Values represent mean ± standard error.
Letters indicate significant differences with control group (a P < 0.05, b P < 0.01, c P < 0.001). No significant differences were noted between E+P and E+P+T groups.
Serum estrogens, progesterone, and testosterone
The oral E dose resulted in acute and lag serum E concentrations of ∼200 - 500 pg/ml (734 - 1835 pmol/L) and ∼15 - 30 pg/ml (55 - 110 pmol/L), respectively. The oral P dose provided acute and lag serum P concentrations of ∼10 - 40 ng/ml (42 - 127 nmol/L) and ∼2 - 10 ng/ml (6 - 32 nmol/L), respectively (Table 2). These concentration ranges are within the daily range for postmenopausal women taking oral micronized E and P.52,53 Serum E and P were significantly higher at acute and lag timepoints for both E+P and E+P+T groups compared to control (P < 0.05 for all); the only difference between these groups was acute serum P, which was higher for E+P+T (high dose T) compared to E+P at 2 wks but not thereafter. The high-dose T pellet resulted in serum T concentrations of ∼500 - 750 ng/ml (1735 - 2603 nmol/L) at 2 wks and ∼150 - 250 ng/ml (521 - 868 nmol/L) at 8 wks, while the low-dose T pellet dose resulted in serum T concentrations of ∼75 - 150 ng/ml (260 - 521 nmol/L) at 10 wks and ∼10 - 25 ng/ml (35 - 87 nmol/L) at 16 wks.
Table 2. Treatment effects on serum hormone concentrations.1-4.
| Con | E+P | E+P+T | ANOVA P value | ||
|---|---|---|---|---|---|
| Estradiol (pg/ml) | |||||
| 2 wk: | 2-4hr PD | <5 | 428.1 ± 64.6b | 303.0 ± 57.8b | <0.001 |
| 8 wk: | 20-28hr PD | <5 | 25.3 ± 4.9a | 16.9 ± 4.4a | 0.01 |
| 10 wk: | 2-4hr PD | <5 | 366.4 ± 78.8b | 239.0 ± 70.5b | <0.001 |
| 10 wk: | 20-28hr PD | <5 | 23.3 ± 4.9b | 18.6 ± 4.4b | <0.001 |
| 16 wk: | 20-28hr PD | <5 | 24.9 ± 5.8b | 36.3 ± 5.2b | <0.001 |
| Progesterone (ng/ml) | |||||
| 2 wk: | 2-4hr PD | <1 | 13.2 ± 3.6b | 33.7 ± 3.2b,d | <0.001 |
| 8 wk: | 20-28hr PD | <1 | 2.8 ± 0.2b | 2.6 ± 0.2b | <0.001 |
| 10 wk: | 2-4hr PD | <1 | 22.4 ± 2.7b | 24.8 ± 2.4b | <0.001 |
| 10 wk: | 20-28hr PD | <1 | 3.4 ± 0.4b | 3.9 ± 0.3b | <0.001 |
| 16 wk: | 20-28hr PD | <1 | 6.6 ± 2.0b | 8.9 ± 1.7b | <0.001 |
| Testosterone (ng/dl) | |||||
| 0 wk: | na | 12.8 ± 2.7 | 11.2 ± 2.5 | 12.8 ± 2.3 | 0.90 |
| 2 wk: | 2-4hr PD | 6.5 ± 56.0 | 2.5 ± 52.4 | 669.1 ± 46.8b,d | <0.001 |
| 8 wk: | 20-28hr PD | 11.4 ± 17.8 | 8.8 ± 16.6 | 196.4 ± 14.9b,d | <0.001 |
| 10 wk: | 2-4hr PD | 10.8 ± 19.6 | 12.8 ± 18.3 | 128.3 ± 16.4b,d | <0.001 |
| 10 wk: | 20-28hr PD | 7.4 ± 14.8 | 1.7 ± 13.9 | 98.1 ± 12.4b,d | <0.001 |
| 16 wk: | 20-28hr PD | 14.5 ± 3.4 | 11.5 ± 3.2 | 17.3 ± 2.8 | 0.47 |
Control = placebo; E = oral 17β-Estradiol; P = oral micronized progesterone; T = testosterone administered via subcutaneous pellets. n = 7, 8, and 10 for Con, E+P, and E+P+T groups, respectively, for all measures.
Serum was collected 2-4 hrs and/or 20-28 hrs post-dosing (PD) after 2 and 8 weeks of the low-dose T (wk 1-8) and high-dose T (wk 9-16) treatment phases.
For conversion to SI units, multiply by the following conversion factors: 3.67 for estradiol (pmol/l), 3.18 for progesterone (nmol/l), and 0.035 for testosterone (nmol/l).
Values represent means ± standard error.
Letters indicate significant differences with control group (a P < 0.05, b P < 0.01, c P < 0.001) or with E+P group (d P < 0.01).
Mammary gland Ki67, PGR, and ESR1 expression
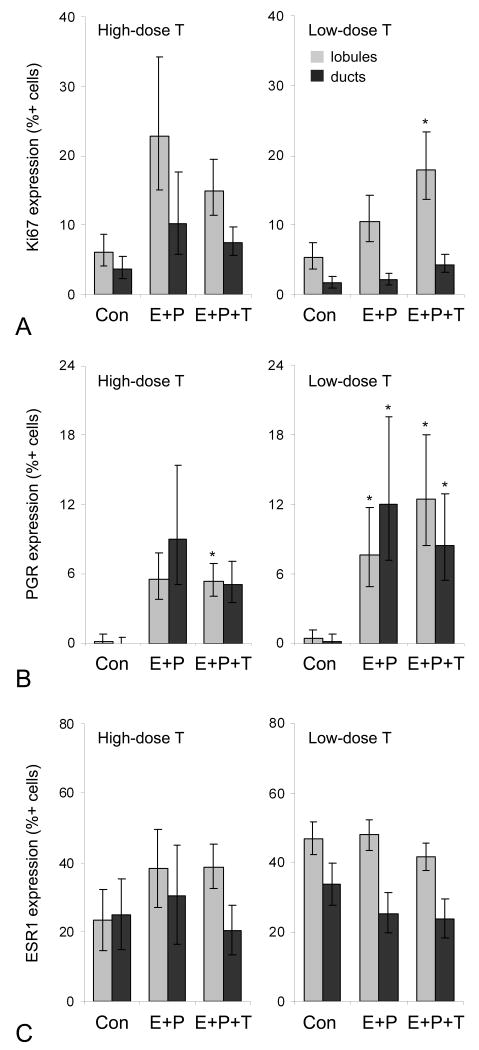
The proliferation marker Ki67 is widely used as a prognostic indicator in human breast cancer54 and a risk marker of hormone exposure in preclinical studies.33,42,43,46 At 8 wks, E+P and E+P+T (high dose T) had similar levels of proliferation (lobular and ductal epithelium). Neither treatment group differed significantly from the control group, although a trend was noted for increased proliferation in the E+P group (ANOVA P = 0.05) (Figure 1A). Comparable changes were also seen between E+P and E+P+T groups in PGR expression (Figure 1B). At 16 wks, significantly greater lobular proliferation was present for E+P+T (low dose T) (P = 0.03) but not E+P treatment compared to control (Figure 1A), while lobular and ductal PGR expression was higher in both hormone treatment groups (P < 0.05 for all) (Figure 1B). No significant group differences were noted for ESR1 expression at either timepoint (Figure 1C). Immunolabeling for AR was not quantified due to lack of staining in mammary gland lobular and ductal epithelium in all treatment groups, in contrast to distinct nuclear staining in positive control human and macaque prostate gland epithelium (Supplementary Figure 1).
Fig. 1.
Mammary gland effects of high-dose and low-dose testosterone co-therapy (T) given with oral estradiol and progesterone (E+P) on expression of markers of proliferation (Ki67) (A), estrogen receptor activation (progesterone receptor, PGR) (B), and estrogen receptor alpha (ESR1) (C) as determined by immunolabeling. At 8 wk n = 6, 4, and 10 for lobular epithelium and n = 5, 2, and 9 for ductal epithelium for Con, E+P, and E+P+T groups, respectively. At 16 wk n = 7, 8, and 10 for Con, E+P, and E+P+T groups, respectively, for both lobular and ductal epithelium. *P < 0.05 compared to control group (Con).
Mammary gland gene expression
We next measured mRNA expression of several intramammary markers related to proliferation (MKI67) and estrogen (ESR1), progesterone (PGR), and androgen (AR) receptor signaling. In the mammary gland, expression of the estrogen-sensitive PGR was significantly higher with E+P (P = 0.02) but not E+P+T treatment following the low-dose T phase (Table 3). Expression of progestogen-sensitive STAT5A was higher in the E+P and E+P+T groups following the low-dose T phase and in the E+P+T (but not E+P) group following the high-dose phase (P < 0.05 for all). No statistically significant treatment effects were seen on mammary expression of ESR1, MKI67, AR, or the androgen-sensitive KLK3 (Table 3).
Table 3. Treatment effects on mammary gland and endometrial gene expression.1-3.
| Con | E+P | E+P+T | |
|---|---|---|---|
| Mammary gland: High-dose T (8wk) | |||
| ESR1 | 1.0 (0.8 - 1.2) | 0.7 (0.5 - 0.8) | 1.0 (0.7 - 1.0) |
| PGR | 1.0 (0.8 - 1.3) | 1.2 (1.0 - 1.5) | 1.5 (1.2 - 1.8) |
| AR | 1.0 (0.8 - 1.2) | 0.8 (0.7 - 0.9) | 0.6 (0.5 - 0.7) |
| MKI67 | 1.0 (0.6 - 1.4) | 1.4 (1.0 - 1.8) | 0.8 (0.4 - 1.2) |
| KLK3 | 1.0 (0.7 - 1.4) | 2.0 (1.5 - 2.6) | 1.7 (1.3 - 2.2) |
| STAT5A | 1.0 (0.8 - 1.3) | 1.4 (1.2 - 1.8) | 2.2 (1.8 - 2.7)a |
| Mammary gland: Low-dose T (16wk) | |||
| ESR1 | 1.0 (0.8 - 1.3) | 1.6 (1.3 - 2.0) | 1.1 (0.9 - 1.4) |
| PGR | 1.0 (0.7 - 1.4) | 4.2 (3.0 - 5.8)a | 2.2 (1.7 - 3.0) |
| AR | 1.0 (0.9 - 1.1) | 1.5 (1.4 - 1.7) | 1.4 (1.2 - 1.5) |
| MKI67 | 1.0 (0.0 - 2.2) | 3.1 (1.9 - 4.2) | 1.9 (0.9 - 2.9) |
| KLK3 | 1.0 (0.7 - 1.4) | 1.3 (0.9 - 1.7) | 1.7 (1.3 - 2.2) |
| STAT5A | 1.0 (0.7 - 1.3) | 3.6 (2.7 - 4.8)a | 3.2 (2.5 - 4.1)a |
| Endometrium: Low-dose T (16wk) | |||
| ESR1 | 1.0 (0.7 - 1.4) | 0.6 (0.5 - 0.8) | 0.4 (0.3 - 0.5) |
| PGR | 1.0 (0.7 - 1.4) | 1.8 (1.3 - 2.4) | 1.7 (1.3 - 2.2) |
| AR | 1.0 (0.8 - 1.3) | 0.6 (0.5 - 0.7) | 0.8 (0.6 - 0.9) |
| MKI67 | 1.0 (0.7 - 1.4) | 1.6 (1.2 - 2.3) | 0.4 (0.3 - 0.5)b |
| KLK3 | 1.0 (0.6 - 1.6) | 5.4 (3.5 - 8.2)a | 1.6 (1.1 - 2.3) |
| STAT5A | 1.0 (0.8 - 1.2) | 0.6 (0.5 - 0.7) | 0.6 (0.5 - 0.7) |
Con = Control (placebo); E = oral 17β-Estradiol; P = oral micronized progesterone; T = testosterone administered via subcutaneous pellets; ESR1 = estrogen receptor alpha; PGR = progesterone receptor; AR = androgen receptor; MKI67 = gene for the proliferation marker Ki67 antigen; STAT5A, signal transducer and activator of transcription 5A; KLK3, kallikrein 3. n = 7, 8, and 10 for Con, E+P, and E+P+T groups, respectively, for all measures.
Values represent relative mean mRNA fold change (90% confidence interval) from control group, as measured by quantitative RT-PCR.
Letters indicate significant differences with control group (a P < 0.05) or with E+P group (bP < 0.05).
Mammary gland histology
Mammary gland tissues were evaluated on routine histology for evidence proliferative changes. Lobules were qualitatively more apparent in the E+P and E+P+T groups (Supplemental Figure 2A-2C). At 8 wks, findings included columnar cell change (a benign proliferative lesion46) in three biopsies (1 Con, 1 E+P, 1 E+P+T) and atypical ductal hyperplasia in one biopsy (E+P+T) (Supplemental Figure 2D). At 16 wks, findings include columnar cell change in two cases (both E+P) and columnar cell hyperplasia in one case (Con).
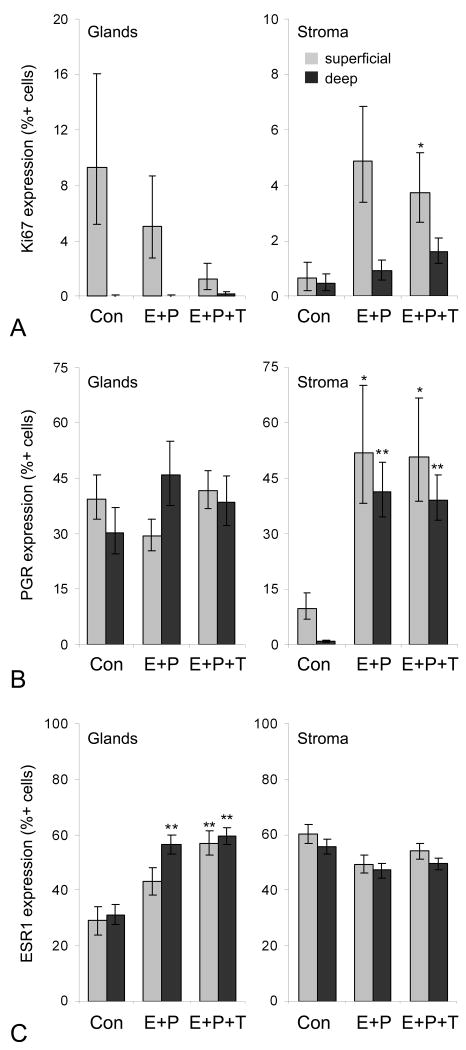
Endometrial Ki67, PGR, and ESR1 expression
Endometrial gland Ki67 immunolabeling (assessed after low-dose T phase only) tended to be lower in the E+P+T group although this effect was not significant (ANOVA P = 0.10). Both E+P and E+P+T treatments were associated with greater stromal Ki67 (significant for E+P+T at P = 0.02 but not E+P at P = 0.12) (Figure 2A). PGR expression did not differ significantly among treatments in glands but was higher in stroma (superficial and deep) in both E+P and E+P+T groups (P < 0.05 for both) (Figure 2B). Glandular ESR1 labeling was higher in both E+P and E+P+T groups (P < 0.01 for both), while no significant group differences were noted for stromal ESR1 (Figure 2C). No significant group differences were seen for endometrial PGR, ESR1, AR, and STAT5A gene expression, while MKI67 expression was lower in the E+P+T compared to E+P group (P = 0.04) and KLK3 expression was higher in the E+P group (P = 0.04) but not E+P+T group (Table 3).
Fig. 2.
Endometrial effects of oral estradiol and progesterone given alone (E+P) or with testosterone co-therapy (E+P+T). Data correspond to low dose T phase only. Figures indicate nuclear expression of Ki67 (A), progesterone receptor (PGR) (B), and estrogen receptor alpha (ESR1) (C) as determined by immunolabeling. n = 7, 8, and 10 for Con, E+P, and E+P+T groups, respectively, for all measures. *P < 0.05 and **P < 0.01 compared to control group (Con).
Endometrial histology
General histologic findings included diffuse glandular and stromal atrophy in all control endometria and varying degrees of stromal edema and expansion, glandular elongation, and spiral arteriolar development in E+P and E+P+T endometria. Lesions included simple glandular hyperplasia in one case (E+P), decidual stromal change in five cases (three E+P, two E+P+T), and superficial hemorrhage in one case (E+P).
Serum lipids
Serum lipid parameters did not differ among groups at baseline (Table 4). Total plasma cholesterol was significantly lower in the E+P group compared to both control and E+P+T groups (P < 0.05 for both) following the high but not the low T dose phase. A similar pattern was noted for LDL+VLDL. In contrast, serum TG was significantly increased in both E+P and E+P+T groups (P < 0.05 for both) following the high but not the low T dose phase Serum HDL concentrations were marginally lower in E+P and E+P+T groups after the high dose (ANOVA P = 0.13) and low dose T phases (ANOVA P = 0.05), while no group differences were observed for TC to HDL ratio.
Table 4. Treatment effects on serum lipids.1-4.
| Con | E+P | E+P+T | ANOVA P value | |
|---|---|---|---|---|
| TC (mg/dl) | ||||
| Baseline (0 wk) | 253.0 ± 20.6 | 274.3 ± 19.3 | 223.3 ± 17.3 | 0.16 |
| High-dose T (8 wk) | 227.1 ± 11.2 | 175.3 ± 10.9b | 215.1 ± 9.8c | 0.009 |
| Low-dose T (16 wk) | 171.0 ± 11.9 | 153.7 ± 11.6 | 153.2 ± 10.4 | 0.47 |
| LDL+VLDL (mg/dl) | ||||
| Baseline (0 wk) | 150.9 ± 24.0 | 188.9 ± 22.5 | 119.8 ± 20.1 | 0.10 |
| High-dose T (8 wk) | 125.0 ± 8.2 | 92.9 ± 8.1a | 128.4 ± 7.2c | 0.01 |
| Low-dose T (16 wk) | 170.3 ± 6.8 | 186.0 ± 6.8 | 184.8 ± 6.0 | 0.20 |
| HDL (mg/dl) | ||||
| Baseline (0 wk) | 102.1 ± 8.6 | 85.4 ± 8.0 | 103.5 ± 7.2 | 0.22 |
| High-dose T (8 wk) | 100.9 ± 6.7 | 88.7 ± 6.5 | 82.5 ± 5.6 | 0.13 |
| Low-dose T (16 wk) | 79.8 ± 5.9 | 59.6 ± 5.8 | 63.4 ± 5.0 | 0.052 |
| Triglycerides (mg/dl) | ||||
| Baseline (0 wk) | 53.1 ± 8.9 | 39.6 ± 8.3 | 37.7 ± 7.4 | 0.39 |
| High-dose T (8 wk) | 24.4 ± 11.2 | 51.9 ± 7.0a | 62.2 ± 6.3b | 0.004 |
| Low-dose T (16 wk) | 44.0 ± 8.6 | 37.9 ± 7.9 | 51.2 ± 7.1 | 0.46 |
| TC:HDL | ||||
| Baseline (0 wk) | 2.64 ± 0.47 | 3.56 ± 0.44 | 2.27 ± 0.39 | 0.11 |
| High-dose T (8 wk) | 2.31 ± 0.21 | 2.15 ± 0.21 | 2.74 ± 0.18 | 0.12 |
| Low-dose T (16 wk) | 2.25 ± 0.35 | 2.74 ± 0.35 | 2.93 ± 0.30 | 0.34 |
Con = Control (placebo); E = oral 17β-Estradiol; P = oral micronized progesterone; T = testosterone administered via subcutaneous pellets; TC, total cholesterol; LDL, low density lipoprotein; VLDL, very low density lipoprotein; HDL, high density lipoprotein. n = 7, 8, and 10 for Con, E+P, and E+P+T groups, respectively, for all measures.
Values represent mean ± standard error.
For conversion to SI units (mmol/l), divide by 38.67 for total cholesterol, LDL+VLDL, and HDL, and by 88.57 for triglycerides.
Letters indicate significant differences with control group (a P < 0.05, b P < 0.01) or with E+P group (cP < 0.05).
Discussion
The use of T-containing agents as hormone co-therapies has increased in recent years,28 despite lack of specific use guidelines and mixed evidence regarding T effects on breast cancer risk. In this pilot study we found a small increase in intralobular breast proliferation following the addition of low-dose T but no effects on histology or markers of estrogen receptor activity. We found no evidence that T antagonizes the effects of combined HT given in the form of E and P. These findings suggest that the short-term effects of T co-therapy on breast proliferation and hormonally-mediated markers are limited, even at higher doses. In addition, we found little evidence that T markedly affects proliferation or estrogen-related responses in the endometrium.
Previous data regarding androgen effects on the breast are mixed. Some concerns with T relate to the potential for its conversion to E within the mammary gland and other tissues, thereby contributing to postmenopausal estrogen exposure.4 Mammary gland epithelial cells (at least in tumors) may express ARs,55 and prior studies in monkeys and women have suggested that activation of ARs by T may antagonize estrogen effects.15-17 Results of the current study do not support these latter findings. Possible reasons for this discrepancy may relate to the types of hormones and relative doses used in the respective studies. The previous monkey studies15,16 were short pilot studies (3 days, n = 4-5 group) that used a much higher sustained dose of E (2.5 mg subcutaneous pellets), resulting in a greater effect on proliferation (+284%) than seen with lower dose E+P in the current study. The recent trial in postmenopausal women also used a higher oral E dose (2.0 mg/d), a different progestogen (noresthisterone acetate at 1.0 mg/d), and a transdermal T patch rather than subcutaneous implant. A more stimulatory combined HT formulation may thus be required for any potential antagonistic effects of T to be seen. It is also possible that the current study, as a pilot investigation, was underpowered to detect any such effects if modest. As indicated in the methods, power was calculated using prior data from CEE+MPA rather than E+P. More recent data indicate that standard doses of E+P may have less stimulatory effects on mammary proliferation than CEE+MPA.33 This difference, along with missing mammary proliferation datapoints for the high-dose T phase (related to an unexpected high degree of adiposity of the animals), further limited power to detect group differences in proliferation. An additional factor related to T (as well as E and P) effects is route of administration. T therapy may be given orally (mT, T undeconate), transdermally (T patches, gels, and creams), intramuscularly (T enanthate), and via micronized T implants,3 and it is currently unknown how mode of delivery may influence effects on breast, uterus, and other target tissues.
Few prior studies have examined testosterone effects on the endometrium.3 In the current study E+P and E+P+T treatments resulted in stromal changes characteristic of combined HT formulations (edema, conspicuous arterioles, decidual change) without an increase in glandular proliferation, indicating that the P dose adequately antagonized potential stimulatory effects of E on the epithelium. The addition of T did not increase endometrial glandular proliferation or estrogen receptor activity or result in any detectable histologic changes. For some markers the changes in the E+P+T group were marginally less than those in the E+P group (e.g. MKI67 gene expression), suggesting that certain doses of T co-therapy may exert a mild antagonistic effect on E effects. This pattern is consistent with a recent study in postmenopausal women which found no evidence of histologic endometrial changes or increased endometrial proliferation following 3 months of treatment with oral T undecanoate given alone and modest antagonism of estrogen effects when given alongside E valerate.56 The lack of stimulatory effect of testosterone on endometrial epithelium is also consistent with prior rodent and cell culture studies,57-58 although it remains unclear whether T given alone may increase estrogen exposure via local aromatization.
An important issue involving T therapy is the lack of clear guidelines for diagnosing androgen insufficiency.1 This problem is due in large part to the lack of reliable methods for detecting and validating low physiologic concentrations of serum T in postmenopausal women. The variation in serum T values in control and E+P groups in this study reflect this issue and highlight the inherent clinical challenge of individually calibrating T doses from a low to a high physiologic level. We also observed a marked decrease in serum T over time for high and low T doses, which was likely due to local fibrosis around the pellets. It is unclear how this decline in T over the course of the each phase may have affected the results. Also of note is the lack of increased serum E concentrations in the E+P+T group, suggesting that aromatization of T was minimal (at the systemic level at least). This latter point is consistent with evidence from a number of T studies in women3 which found no evidence of increased serum E following T therapy.
Conclusions
In this pilot study we found limited overall effects of T co-therapy on breast and endometrial parameters. In the mammary gland, greater lobular epithelial proliferation was seen following low-dose but not high-dose E+P+T. While this change may indicate a potential mild stimulatory effect of T, the effect was small and not significantly different from E+P alone. The lack of effects on PGR expression in the breast suggests that T does not exert robust effects on estrogen receptor activity. Nuclear AR expression was not detected in mammary gland lobular and ductal epithelium. Treatment with E+P resulted in significantly higher uterine area, vaginal maturation, and endometrial thickness, and neither low nor high dose T altered these effects. The addition of T did not increase endometrial glandular proliferation or estrogen receptor activity or result in any distinct histologic changes. Addition of high dose but not low dose T resulted in modest attenuation of E+P effects on select serum cholesterol measures.
Supplementary Material
Supplemental FIG. 1. Lack of androgen receptor (AR) expression within mammary gland epithelium. Representative images of positive control human (A) and macaque (B) prostate glands showing positive AR immunolabeling (red nuclei), in contrast to mammary gland lobular epithelium (C).
Supplemental FIG. 2. Treatment effects on mammary gland histology. Representative mammary gland images following treatment with placebo (A), oral estradiol and progesterone, (B) or oral estradiol and progesterone with testosterone co-therapy (C). Arrowheads in (A) indicate lobular (black) and extralobular duct (white) compartments. An example of ductal hyperplasia with luminal micropapillae and columnar cell change (inset) is shown in (D). H&E stain.
Supplementary Table 1. Primer / probe sets for target genes evaluated by qRT-PCR.
Acknowledgments
The investigators thank Jean Gardin, Chuck Boyd, Matthew Dwyer, Hermina Borgerink, Joseph Finley, Lisa O'Donnell, and Maryanne Post for technical assistance. Statistical advice was provided by Dr. Haiying Chen in the Department of Biostatistical Sciences, Wake Forest University School of Medicine. We also thank Dr. Donna Perry for providing prostate control tissues. Funding for this work was provided by the Martin & Sharleen Cohen Foundation for Biomedical Research (JMC / CEW) and the National Institutes of Health (NIH) National Center for Research Resources (NCRR) Grant K01RR021322 (CEW). The contents are solely the responsibility of the authors and do not necessarily represent the view of the NCRR or NIH.
This work was supported by the Martin & Sharleen Cohen Foundation for Biomedical Research (JMC / CEW) and the National Institutes of Health (NCRR K01RR021322) (CEW).
References
- 1.NAMS expert panel. The role of testosterone therapy in postmenopausal women: position statement of The North American Menopause Society. Menopause. 2005;12:497–511. doi: 10.1097/01.gme.0000177709.65944.b0. [DOI] [PubMed] [Google Scholar]
- 2.Wierman ME, Basson R, Davis SR, et al. Androgen therapy in women: an Endocrine Society Clinical Practice guideline. J Clin Endocrinol Metab. 2006;91:3697–3710. doi: 10.1210/jc.2006-1121. [DOI] [PubMed] [Google Scholar]
- 3.Braunstein GD. Safety of testosterone treatment in postmenopausal women. Fertil Steril. 2007;88:1–17. doi: 10.1016/j.fertnstert.2007.01.118. [DOI] [PubMed] [Google Scholar]
- 4.Simpson ER. Aromatization of androgens in women: current concepts and findings. Fertil Steril. 2002;77 4:S6–S10. doi: 10.1016/s0015-0282(02)02984-9. [DOI] [PubMed] [Google Scholar]
- 5.Bachmann G, Bancroft J, Braunstein G, et al. Female androgen insufficiency: the Princeton consensus statement on definition, classification, and assessment. Fertil Steril. 2002;77:660–665. doi: 10.1016/s0015-0282(02)02969-2. [DOI] [PubMed] [Google Scholar]
- 6.Greendale GA, Edelstein S, Barrett-Connor E. Endogenous sex steroids and bone mineral density in older women and men: the Rancho Bernardo Study. J Bone Miner Res. 1997;12:1833–1843. doi: 10.1359/jbmr.1997.12.11.1833. [DOI] [PubMed] [Google Scholar]
- 7.Barrett-Connor E, Goodman-Gruen D. Cognitive function and endogenous sex hormones in older women. J Am Geriatr Soc. 1999;47:1289–1293. doi: 10.1111/j.1532-5415.1999.tb07427.x. [DOI] [PubMed] [Google Scholar]
- 8.Ding EL, Song Y, Manson JE, Rifai N, Buring JE, Liu S. Plasma sex steroid hormones and risk of developing type 2 diabetes in women: a prospective study. Diabetologia. 2007;50:2076–2084. doi: 10.1007/s00125-007-0785-y. [DOI] [PubMed] [Google Scholar]
- 9.Labrie F, Luu-The V, Labrie C, et al. Endocrine and intracrine sources of androgens in women: inhibition of breast cancer and other roles of androgens and their precursor dehydroepiandrosterone. Endocr Rev. 2003;24:152–182. doi: 10.1210/er.2001-0031. [DOI] [PubMed] [Google Scholar]
- 10.Somboonporn W, Davis S, Seif MW, Bell R. Testosterone for peri- and postmenopausal women. Cochrane Database Syst Rev. 2005;4:CD004509. doi: 10.1002/14651858.CD004509.pub2. [DOI] [PubMed] [Google Scholar]
- 11.Hankinson SE, Eliassen AH. Endogenous estrogen, testosterone and progesterone levels in relation to breast cancer risk. J Steroid Biochem Mol Biol. 2007;106:24–30. doi: 10.1016/j.jsbmb.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.von Schoultz B. Androgens and the breast. Maturitas. 2007;57:47–49. doi: 10.1016/j.maturitas.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 13.Somboonporn W, Davis SR, National Health and Medical Research Council Testosterone effects on the breast: implications for testosterone therapy for women. Endocr Rev. 2004;25:374–388. doi: 10.1210/er.2003-0016. [DOI] [PubMed] [Google Scholar]
- 14.Jayo MJ, Register TC, Hughes CL, et al. Effects of an oral contraceptive combination with or without androgen on mammary tissues: a study in rats. J Soc Gynecol Investig. 2000;7:257–265. [PubMed] [Google Scholar]
- 15.Dimitrakakis C, Zhou J, Wang J, et al. A physiologic role for testosterone in limiting estrogenic stimulation of the breast. Menopause. 2003;10:292–298. doi: 10.1097/01.GME.0000055522.67459.89. [DOI] [PubMed] [Google Scholar]
- 16.Zhou J, Ng S, Adesanya-Famuiya O, Anderson K, Bondy CA. Testosterone inhibits estrogen-induced mammary epithelial proliferation and suppresses estrogen receptor expression. FASEB J. 2000;14:1725–1730. doi: 10.1096/fj.99-0863com. [DOI] [PubMed] [Google Scholar]
- 17.Hofling M, Hirschberg AL, Skoog L, Tani E, Hägerström T, von Schoultz B. Testosterone inhibits estrogen/progestogen-induced breast cell proliferation in postmenopausal women. Menopause. 2007;14:183–190. doi: 10.1097/01.gme.0000232033.92411.51. [DOI] [PubMed] [Google Scholar]
- 18.Key T, Appleby P, Barnes I, Reeves G, Endogenous Hormones and Breast Cancer Collaborative Group Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J Natl Cancer Inst. 2002;94:606–616. doi: 10.1093/jnci/94.8.606. [DOI] [PubMed] [Google Scholar]
- 19.Missmer SA, Eliassen AH, Barbieri RL, Hankinson SE. Endogenous estrogen, androgen, and progesterone concentrations and breast cancer risk among postmenopausal women. J Natl Cancer Inst. 2004;96:1856–1865. doi: 10.1093/jnci/djh336. [DOI] [PubMed] [Google Scholar]
- 20.Tamimi RM, Byrne C, Colditz GA, Hankinson SE. Endogenous hormone levels, mammographic density, and subsequent risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 2007;99:1178–1187. doi: 10.1093/jnci/djm062. [DOI] [PubMed] [Google Scholar]
- 21.Dorgan JF, Longcope C, Stephenson HE, Jr, et al. Relation of prediagnostic serum estrogen and androgen levels to breast cancer risk. Cancer Epidemiol Biomarkers Prev. 1996;5:533–539. [PubMed] [Google Scholar]
- 22.Berrino F, Muti P, Micheli A, et al. Serum sex hormone levels after menopause and subsequent breast cancer. J Natl Cancer Inst. 1996;88:291–296. doi: 10.1093/jnci/88.5.291. [DOI] [PubMed] [Google Scholar]
- 23.Yu H, Shu XO, Shi R, et al. Plasma sex steroid hormones and breast cancer risk in Chinese women. Int J Cancer. 2003;105:92–97. doi: 10.1002/ijc.11034. [DOI] [PubMed] [Google Scholar]
- 24.Micheli A, Meneghini E, Secreto G, et al. Plasma testosterone and prognosis of postmenopausal breast cancer patients. J Clin Oncol. 2007;25:2685–2690. doi: 10.1200/JCO.2006.09.0118. [DOI] [PubMed] [Google Scholar]
- 25.Hankinson SE, Willett WC, Manson JE, et al. Plasma sex steroid hormone levels and risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 1998;90:1292–1299. doi: 10.1093/jnci/90.17.1292. [DOI] [PubMed] [Google Scholar]
- 26.Zeleniuch-Jacquotte A, Bruning PF, Bonfrer JM, et al. Relation of serum levels of testosterone and dehydroepiandrosterone sulfate to risk of breast cancer in postmenopausal women. Am J Epidemiol. 1997;145:1030–1038. doi: 10.1093/oxfordjournals.aje.a009059. [DOI] [PubMed] [Google Scholar]
- 27.Beattie MS, Costantino JP, Cummings SR, et al. Endogenous sex hormones, breast cancer risk, and tamoxifen response: an ancillary study in the NSABP Breast Cancer Prevention Trial (P-1) J Natl Cancer Inst. 2006;98:110–115. doi: 10.1093/jnci/djj011. [DOI] [PubMed] [Google Scholar]
- 28.Tamimi RM, Hankinson SE, Chen WY, Rosner B, Colditz GA. Combined estrogen and testosterone use and risk of breast cancer in postmenopausal women. Arch Intern Med. 2006;166:1483–1489. doi: 10.1001/archinte.166.14.1483. [DOI] [PubMed] [Google Scholar]
- 29.Suthers K. Estratest is not approved by the Food and Drug Administration. Arch Intern Med. 2007;167:205–206. doi: 10.1001/archinte.167.2.205-c. [DOI] [PubMed] [Google Scholar]
- 30.Chlebowski RT, Hendrix SL, Langer RD, et al. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: the Women's Health Initiative Randomized Trial. JAMA. 2003;289:3243–3253. doi: 10.1001/jama.289.24.3243. [DOI] [PubMed] [Google Scholar]
- 31.Ross RK, Paganini-Hill A, Wan PC, Pike MC. Effect of hormone replacement therapy on breast cancer risk: estrogen versus estrogen plus progestin. J Natl Cancer Inst. 2000;92:328–332. doi: 10.1093/jnci/92.4.328. [DOI] [PubMed] [Google Scholar]
- 32.Schairer C, Lubin J, Troisi R, Sturgeon S, Brinton L, Hoover R. Estrogen-progestin replacement and risk of breast cancer. JAMA. 2000;284:691–694. [PubMed] [Google Scholar]
- 33.Wood CE, Register TC, Lees CJ, Chen H, Kimrey S, Cline JM. Effects of estradiol with micronized progesterone or medroxyprogesterone acetate on risk markers for breast cancer in postmenopausal monkeys. Breast Cancer Res Treat. 2007;101:125–134. doi: 10.1007/s10549-006-9276-y. [DOI] [PubMed] [Google Scholar]
- 34.Shively CA, Wood CE, Register TC, et al. Hormone therapy effects on social behavior and activity levels of surgically postmenopausal cynomolgus monkeys. Psychoneuroendocrinology. 2007;32:981–990. doi: 10.1016/j.psyneuen.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 35.Wood CE, Sitruk-Ware RL, Tsong YY, Register TC, Lees CJ, Cline JM. Effects of estradiol with oral or intravaginal progesterone on risk markers for breast cancer in a postmenopausal monkey model. Menopause. 2007;14:639–647. doi: 10.1097/01.gme.0000247017.41007.80. [DOI] [PubMed] [Google Scholar]
- 36.Wood CE, Register TC, Cline JM. Transcriptional profiles of progestogen effects in the postmenopausal breast. Breast Cancer Res Treat. [June 1, 2008];2008 doi: 10.1007/s10549-008-0003-8. Available at: http://www.springerlink.com/content/35547x782u843128/ [DOI] [PubMed]
- 37.Magness CL, Fellin PC, Thomas MJ, et al. Analysis of the Macaca mulatta transcriptome and the sequence divergence between Macaca and human. Genome Biol. 2005;6:R60. doi: 10.1186/gb-2005-6-7-r60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pavlicek A, Noskov VN, Kouprina N, Barrett JC, Jurka J, Larionov V. Evolution of the tumor suppressor BRCA1 locus in primates: implications for cancer predisposition. Hum Mol Genet. 2004;13:2737–2751. doi: 10.1093/hmg/ddh301. [DOI] [PubMed] [Google Scholar]
- 39.Stute P, Wood CE, Kaplan J, Cline JM. Cyclic changes in the mammary gland of cynomolgus macaques. Fertil Steril. 2004;82:1160–1170. doi: 10.1016/j.fertnstert.2004.04.035. [DOI] [PubMed] [Google Scholar]
- 40.Tsubura A, Hatano T, Hayama S, Morii S. Immunophenotypic difference of keratin expression in normal mammary glandular cells from five different species. Acta Anatomica. 1991;140:287–293. doi: 10.1159/000147071. [DOI] [PubMed] [Google Scholar]
- 41.Cheng G, Li Y, Omoto Y, et al. Differential regulation of estrogen receptor (ER)alpha and ERbeta in primate mammary gland. J Clin Endocrinol Metab. 2005;90:435–444. doi: 10.1210/jc.2004-0861. [DOI] [PubMed] [Google Scholar]
- 42.Cline JM, Soderqvist G, von Schoultz E, Skoog L, von Schoultz B. Effects of conjugated estrogens, medroxyprogesterone acetate, and tamoxifen on the mammary glands of macaques. Breast Cancer Res Treat. 1998;48:221–229. doi: 10.1023/a:1005984932268. [DOI] [PubMed] [Google Scholar]
- 43.Cline JM, Register TC, Clarkson TB. Effects of tibolone and hormone replacement therapy on the breast of cynomolgus monkeys. Menopause. 2002;9:422–429. doi: 10.1097/00042192-200211000-00007. [DOI] [PubMed] [Google Scholar]
- 44.Cline JM, Wood CE. Hormonal effects on the mammary gland of postmenopausal nonhuman primates. Breast Dis. 20052006;24:59–70. doi: 10.3233/bd-2006-24105. [DOI] [PubMed] [Google Scholar]
- 45.Wood CE, Usborne A, Tarara R, et al. Hyperplastic and neoplastic lesions of the mammary gland in macaques. Vet Pathol. 2006;43:471–483. doi: 10.1354/vp.43-4-471. [DOI] [PubMed] [Google Scholar]
- 46.Wood CE, Hester J, Appt SE, Geisinger KR, Cline JM. Estrogen effects on epithelial proliferation and benign proliferative lesions in the postmenopausal primate mammary gland. Lab Invest. 2008;88:938–948. doi: 10.1038/labinvest.2008.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lindholm J, van Diest PJ, Haffner D, Mikuz G, Weger AR. A morphometric filter improves the diagnostic value of morphometric analyses of frozen histopathologic sections from mammary tumors. Anal Cell Pathol. 1992;4:443–449. [PubMed] [Google Scholar]
- 48.Cline JM. Assessing the mammary gland of nonhuman primates: effects of endogenous hormones and exogenous hormonal agents and growth factors. Birth Defects Res B Dev Reprod Toxicol. 2007;80:126–146. doi: 10.1002/bdrb.20112. [DOI] [PubMed] [Google Scholar]
- 49.Wood CE, Register TC, Franke AA, Anthony MS, Cline JM. Dietary soy isoflavones inhibit estrogen effects in the postmenopausal breast. Cancer Res. 2006;66:1241–1249. doi: 10.1158/0008-5472.CAN-05-2067. [DOI] [PubMed] [Google Scholar]
- 50.Wood CE, Register TC, Anthony MS, Kock ND, Cline JM. Breast and uterine effects of soy isoflavones and conjugated equine estrogens in postmenopausal female monkeys. J Clin Endocrinol Metab. 2004;89:3462–3468. doi: 10.1210/jc.2003-032067. [DOI] [PubMed] [Google Scholar]
- 51.Appt SE, Clarkson TB, Lees CJ, Anthony MS. Low dose estrogens inhibit coronary artery atherosclerosis in postmenopausal monkeys. Maturitas. 2006;55:187–194. doi: 10.1016/j.maturitas.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 52.Lobo RA, Cassidenti DL. Pharmacokinetics of oral 17 beta-estradiol. J Reprod Med. 1992;37:77–84. [PubMed] [Google Scholar]
- 53.Maxson WS, Hargrove JT. Bioavailability of oral micronized progesterone. Fertil Steril. 1985;44:622–626. [PubMed] [Google Scholar]
- 54.Urruticoechea A, Smith IE, Dowsett M. Proliferation marker Ki-67 in early breast cancer. J Clin Oncol. 2005;23:7212–7220. doi: 10.1200/JCO.2005.07.501. [DOI] [PubMed] [Google Scholar]
- 55.Hall RE, Aspinall JO, Horsfall DJ, et al. Expression of the androgen receptor and an androgen-responsive protein, apolipoprotein D, in human breast cancer. Br J Cancer. 1996;74:1175–1180. doi: 10.1038/bjc.1996.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zang H, Sahlin L, Masironi B, Eriksson E, Lindén Hirschberg A. Effects of testosterone treatment on endometrial proliferation in postmenopausal women. J Clin Endocrinol Metab. 2007;92:2169–2175. doi: 10.1210/jc.2006-2171. [DOI] [PubMed] [Google Scholar]
- 57.Legro RS, Kunselman AR, Miller SA, Satyaswaroop PG. Role of androgens in the growth of endometrial carcinoma: an in vivo animal model. Am J Obstet Gynecol. 2001;184:303–308. doi: 10.1067/mob.2001.109734. [DOI] [PubMed] [Google Scholar]
- 58.Tuckerman EM, Okon MA, Li T, Laird SM. Do androgens have a direct effect on endometrial function? An in vitro study. Fertil Steril. 2000;74:771–779. doi: 10.1016/s0015-0282(00)00711-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental FIG. 1. Lack of androgen receptor (AR) expression within mammary gland epithelium. Representative images of positive control human (A) and macaque (B) prostate glands showing positive AR immunolabeling (red nuclei), in contrast to mammary gland lobular epithelium (C).
Supplemental FIG. 2. Treatment effects on mammary gland histology. Representative mammary gland images following treatment with placebo (A), oral estradiol and progesterone, (B) or oral estradiol and progesterone with testosterone co-therapy (C). Arrowheads in (A) indicate lobular (black) and extralobular duct (white) compartments. An example of ductal hyperplasia with luminal micropapillae and columnar cell change (inset) is shown in (D). H&E stain.
Supplementary Table 1. Primer / probe sets for target genes evaluated by qRT-PCR.