Abstract
The pleiotropic receptor tyrosine kinase Kit can provide cytoskeletal signals that define cell shape, positioning, and migration, but the underlying mechanisms are less well understood. In this study, we provide evidence that Kit signals through Wiskott-Aldrich syndrome protein (WASP), the central hematopoietic actin nucleation-promoting factor and regulator of the cytoskeleton. Kit ligand (KL) stimulation resulted in transient tyrosine phosphorylation of WASP, as well as interacting proteins WASP-interacting protein and Arp2/3. KL-induced filopodia in bone marrow–derived mast cells (BMMCs) were significantly decreased in number and size in the absence of WASP. KL-dependent regulation of intracellular Ca2+ levels was aberrant in WASP-deficient BMMCs. When BMMCs were derived from WASP-heterozygous female mice using KL as a growth factor, the cultures eventually developed from a mixture of WASP-positive and -negative populations into a homogenous WASP-positive culture derived from the WASP-positive progenitors. Thus, WASP expression conferred a selective advantage to the development of Kit-dependent hematopoiesis consistent with the selective advantage of WASP-positive hematopoietic cells observed in WAS-heterozygous female humans. Finally, KL-mediated gene expression in wild-type and WASP-deficient BMMCs was compared and revealed that approximately 30% of all Kit-induced changes were WASP dependent. The results indicate that Kit signaling through WASP is necessary for normal Kit-mediated filopodia formation, cell survival, and gene expression, and provide new insight into the mechanism in which WASP exerts a strong selective pressure in hematopoiesis.
Introduction
Kit is a member of the type III subclass of receptor tyrosine kinases (RTKs), which also includes the platelet-derived growth factor receptors α and β, the FMS-like tyrosine kinase 3, and the colony-stimulating factor 1 receptor (c-fms, macrophage colony-stimulating factor receptor). The Kit protein is organized as an N-terminal extracellular ligand-binding domain, a transmembrane domain, and a cytoplasmic kinase domain, which is activated by ligand-mediated receptor dimerization. Murine loss-of-function mutations of Kit result in the dominant W spotting defect, which is marked by defective pigmentation, hematopoiesis, and gametogenesis, as well as a growing list of neurologic defects.1–5 The severity of the phenotype of W mice is related to the degree of loss of Kit expression or function. Kit-null mutants die of severe anemia shortly after birth. Kit ligand (KL; also known as stem cell factor or Steel factor) is produced by stromal cells in either membrane-bound or soluble isoforms.6 Loss-of-function mutations of the Steel (Sl) locus result in a phenotype closely resembling that of W mouse strains.7
In addition to its role as a hematopoietic maintenance, growth, and differentiation factor, Kit regulates cell shape, motility, and adhesion via cytoskeletal changes,8–12 but the underlying molecular mechanisms are less well understood. In the present study, we define a role for the Wiskott-Aldrich syndrome (WAS) protein (WASP) in Kit-mediated signaling, cytoskeletal changes, and gene expression.
The hematopoietic-specific WASP and its ubiquitously expressed homologue neuronal WASP (N-WASP) have emerged as key proteins connecting signaling cascades to actin polymerization.13,14 WASP and N-WASP form a core family of nucleation-promoting factors that interact with and regulate the actual nucleators of new actin filaments, above all the Arp2/3 complex.15,16 An additional subfamily of WASP proteins consists of the suppressor of cyclic adenosine 5′-monophosphate receptor/WASP-family verprolin-homology proteins.17,18 Loss of functional WASP in humans results in Wiskott-Aldrich syndrome (WAS),19–22 an X-linked recessive immunodeficiency disorder marked by eczema, thrombocytopenia, and increased susceptibility to autoimmune disorders and cancers.20,23,24 Deformation and deregulation of the actin cytoskeleton in hematopoietic cells from WAS patients are most likely the basis for many of the clinical defects.25–27 WASP interacts directly with the ubiquitously expressed WASP-interacting protein (WIP), the Rho-GTPase Cdc42, and the Arp2/3 complex.13,28–30 Formation of this multiprotein complex in combination with conformational changes of WASP regulates actin nucleation through Arp2/3.31 More recently, a role for WASP in the formation/aggregation of lipid rafts or the immunologic synapse has been described.32,33
Whereas most of the knowledge of WASP function originates from studies comparing normal with WASP-deficient cells, an important clue for a broader role of WASP in the maintenance of precursor or hematopoietic stem cells (HSCs) came from the observation of nonrandom X-inactivation in all peripheral blood or CD34+ progenitor cells from heterozygous female carriers.34,35 Because such progenitor populations are dependent on the KL/Kit pathway, we hypothesized that WASP acts downstream of Kit in the hematopoietic niche, thereby providinga selective advantage of progenitor cells with intact Kit-WASP signaling.
In this study, we show evidence that WASP and WIP are part of Kit-mediated signal transduction and that WASP is necessary for Kit-induced filopodia formation, cellular Ca2+ signaling, survival, and gene expression.
Methods
Reagents and antibodies
Recombinant mouse KL, mouse interleukin-3 (IL-3), human KL, and human IL-3 were purchased from BioSource International. Polyclonal antibodies against WASP (H-250), WIP (H-224), and Arp2 (H-84) were obtained from Santa Cruz Biotechnology. The antibody against N-WASP was obtained from Cell Signaling Technology. The antiphosphotyrosine antibodies, clones 4G10 and PY20, were purchased from Upstate Biotechnology and BD Biosciences, respectively. Phosphospecific antibodies against signal transducer and activator of transcription 3 (Stat3) and Stat5 were obtained from BD Biosciences, and against c-Jun N-terminal kinase and p38 from Cell Signaling Technology. Anti-Stat3 and anti-Stat5 antibodies were from Santa Cruz Biotechnology.
Mice, cell lines, cell and bone marrow–derived mast cell culture, and KL stimulation
The 129S6/SvEvTac-Wastm1Sbs/J mice were originally obtained from Frederick Alt, Children's Hospital (Boston, MA). Congenic C57BL/6 strains were obtained by backcrossing for more than 10 generations. All procedures involving animals were performed under the Institutional Animal Care and Use Committee approved by the Administrative Panel on Laboratory Animal Care at Stanford University. M-07e cells36 were obtained from the German Collection of Microorganisms and Animal Cell Cultures (DSMZ) and cultured in RPMI-1640 (Invitrogen/Gibco) with 10% fetal calf serum (FCS; Omega Scientific) in the presence of human IL-3 (20 ng/mL). Bone marrow (BM)–derived mast cell (BMMC) cultures were established, as previously reported,9 with some minor modifications. BM cells obtained from femur and tibia of 4- to 8-week-old mice (WASP-deficient or wild-type [wt] littermate controls) were depleted of red cells (red cell lysis buffer; eBioscience), washed, and then cultured in BMMC medium in the presence of murine IL-3 and KL (20 ng/mL each) for 4 to 6 weeks. BMMC medium consisted of RPMI-1640, 20% FCS, 1 mM sodium pyruvate (Gibco), 0.1 mM nonessential amino acids supplement (Gibco), and 50 μM 2-mercaptoethanol (Thermo Fisher Scientific). Cultures were depleted of adherent cells by transferring the nonadherent cells into new flasks approximately once per week during the first 3 weeks of culture, and dead cells were removed from the cultures by density centrifugation (Histopaque-1083; Sigma-Aldrich) when necessary. For KL stimulation, M-07e cells were growth factor as well as serum deprived for 12 hours before stimulation. BMMCs were growth factor deprived for 12 hours, and then incubated in BMMC medium containing no or 2% FCS for additional 4 hours. Stimulation of cells was performed with concentrations of KL, as indicated.
Flow cytometric analysis
To analyze surface expression of Kit and Fcϵ receptor I (FcϵRI), growth factor–deprived BMMCs were labeled with allophycocyanin-conjugated rat anti-Kit antibody (clone 2B8; BD Biosciences) and with murine anti-dinitrophenyl immunoglobulin (Ig)E (clone SPE-7; Sigma-Aldrich), followed by fluorescein isothiocyanate–conjugated rat anti–mouse IgE antibody (clone R35-72; BD Biosciences), respectively. Detection of intracellular WASP was performed after permeabilization (Cytofix/Cytoperm; BD Biosciences) and staining of the cells with phycoerythrin-conjugated mouse anti-WASP antibody (clone B-9; Santa Cruz Biotechnology). All labeling procedures included a step for blocking of murine FcγRIII/II (mouse BD Fc Block; BD Biosciences) and were controlled by staining of the cells with nonspecific antibodies of the same isotype. Antibody-labeled cells were analyzed by flow cytometry using a FACS-Vantage flow cytometer (BD Biosciences).
Immunoprecipitations and immunoblotting
After stimulation with KL, cells were collected in ice-cold phosphate-buffered saline containing 1 mM sodium orthovanadate (Sigma-Aldrich) and lysed in lysis buffer (1-2 × 107 cells/mL) containing 10 mM Tris-HCl (pH 7.4), 150 mM NaCl, 20 mM sodium phosphate (pH 7.4), 10 mM sodium pyrophosphate (pH 7.4), 5 mM EDTA (ethylenediaminetetraacetic acid), 1 mM sodium orthovanadate, 1 mM glycerophosphate (Sigma-Aldrich), and 1% Triton X-100. Proteinase inhibitors (Complete; Roche) were added, according to the manufacturer's recommendations. Postnuclear supernatants were subjected to 1 round of preclearing with protein A-Sepharose (Amersham/Pharmacia). A total of 3 to 6 μg of antibody was used per immunoprecipitation (IP), and antibody-protein complexes were collected with 50 to 70 μL protein A-Sepharose. Western blotting was performed, as previously described.37
Measurement of Kit-induced changes in the intracellular Ca2+ concentration
For analysis of Kit-induced Ca2+ signals, indo-1 was used, an ultraviolet light–excitable Ca2+-binding protein.38 Growth factor-deprived BMMCs were incubated with a cell-permeable ester of Indo-1 (Indo-1 AM; Invitrogen/Molecular Probes) in loading buffer (1 mM calcium, 1 mM magnesium, 1% FCS in HBSS) at 37°C for 30 minutes. Cells were washed with loading buffer containing 2.5 mM probenecid (Invitrogen/Molecular Probes) and resuspended in loading buffer containing 2.5 mM probenecid and propidium iodide at room temperature. Before stimulation, BMMCs loaded with indo-1 were incubated at 37°C for 5 minutes, and baseline emissions were measured for 1 minute on a FACSVantageSE cytometer (BD Biosciences) using a 350 nm ultraviolet laser. For stimulation, KL was added to a final concentration of 100 ng/mL, and the response was recorded for additional 4 minutes at a flow rate of 106 cells/minute. Immobilization of extracellular Ca2+ was achieved by adding EDTA (ethylenediaminetetraacetic acid) and EGTA (ethyleneglycoltetraacetic acid) into the assay buffer (2 mM) before stimulation. Changes of Ca2+ concentration ([Ca2+]i) were calculated after exclusion of nonviable cells and via the 405 nm/485 nm emission ratio using the FlowJo analysis software (TreeStar). Data were displayed as relative changes in [Ca2+]i after baseline adjustment.
Analysis of filopodia formation
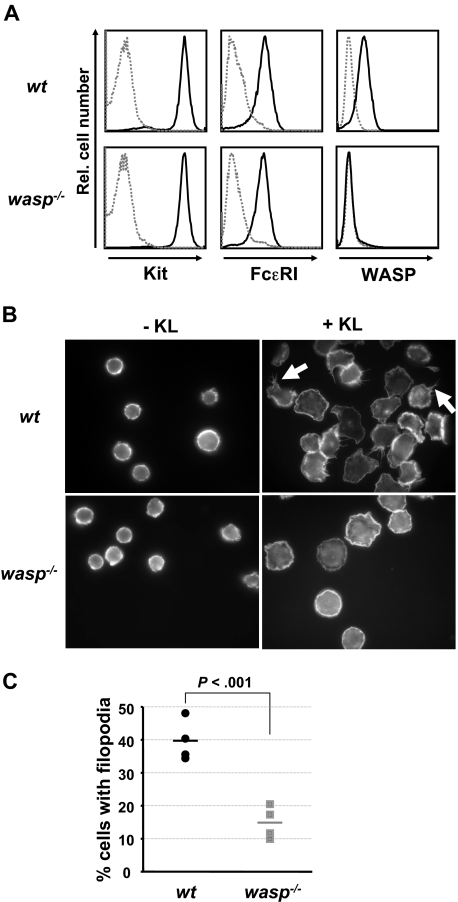
Growth factor–deprived BMMCs were resuspended in BMMC medium without growth factors or serum, seeded onto glass slides coated with bovine fibronectin (20 μg/mL; Sigma-Aldrich), and allowed to adhere for 1 hour at 37°C. Adherent cells were either stimulated with KL (20 ng/mL), or left unstimulated for 30 minutes at 37°C. After fixation and permeabilization of the cells in the presence of 3.7% paraformaldehyde/phosphate-buffered saline, pH 7.4, containing 1-palmitoyl-sn-glycero-3-phosphocholine (100 μg/mL; Sigma-Aldrich), actin filaments were labeled using alexa fluor 488–conjugated phalloidin (Invitrogen/Molecular Probes). Cells were analyzed using a fluorescent microscope at a magnification of ×63, oil immersion (Zeiss), and images were acquired using an Axiocam digital camera and Axiovision software (Zeiss). A total of 1237 wt and 1509 wasp−/− cells from 4 BMMC cultures of each genotype was analyzed in blinded studies.
Gene expression profiling
After KL stimulation of BMMCs in replicates (n = 4 for wt, n = 3 for wasp−/−), total RNA was extracted from stimulated or untreated control cells (RNeasy Mini Kit; Qiagen), and quality was assessed by agarose gel electrophoresis and spectrometrically quantified (NanoDrop; Thermo Fisher Scientific). From each sample, 100 ng of total RNA was subjected to 2 rounds of cDNA amplification, followed by in vitro transcription into biotinylated complementary RNA (Affymetrix), according to the manufacturer's protocol (GeneChip Expression Analysis Technical Manual; Affymetrix). The labeled complementary RNAs were hybridized to a mouse gene array (GeneChip Mouse Genome 430 2.0 Array; Affymetrix) and analyzed by laser scanning (GeneChip Scanner; Affymetrix). Data were analyzed using “R” (http://cran.r-project.org/) and Bioconductor (www.bioconductor.org) software. Background correction, normalization, and calculation of gene expression values by model fitting were performed using the Robust Multiarray Average procedure.39 Genes that were differentially expressed in response to KL were identified using Significance Analysis of Microarrays40 with a fold-change cutoff of 2 for up-regulated and 0.5 for down-regulated genes. The false-discovery rate was less than 0.1%. Gene lists were generated by analysis of up- or down-regulated genes for the enrichment of functional categories using DAVID (http://david.abcc.ncifcrf.gov/home.jsp). Heat maps were generated using “R.” All microarray data have been deposited into the ArrayExpress public database under accession number E-MEXP-2282.
Results
KL stimulation results in tyrosine phosphorylation of WASP, WIP, and Arp2
To exert its function as a nucleation-promoting factor, WASP undergoes secondary modifications in form of tyrosine phosphorylation and interacts with WIP and the Arp2/3 complex.29,30,41 To test for a role for WASP in Kit signaling, we analyzed tyrosine phosphorylation of immunoprecipitated WASP and WIP during a time course of Kit stimulation, and probed the presence of interaction partners (Figure 1). Kit-induced tyrosine phosphorylation of WASP was detected after 1 minute of KL stimulation, was maximal at 5 minutes, and gradually decreased after 10 and 15 minutes (Figure 1A). Kit stimulation also resulted in tyrosine phosphorylation of WIP with similar kinetics (Figure 1B), and the same was true for Arp2 (Figure 1C). Coimmunoprecipitation analysis revealed that WASP was constitutively associated with WIP and that both proteins formed a complex in their tyrosine-phosphorylated state (Figure 1B). The corresponding result was obtained when the immunoprecipitated WASP was analyzed for complex formation with WIP (data not shown). Arp2 was found in a complex with WASP or WIP (Figure 1C). KL-induced tyrosine phosphorylation of WASP and interaction partners WIP and Arp2 as well as reciprocal association of these molecules suggest that WASP is functionally involved in Kit signaling. To our knowledge, this is the first report of transient tyrosine phosphorylation of WIP.
Figure 1.
KL stimulation results in tyrosine phosphorylation of WASP, WIP, and Arp2. M-07e cells were stimulated with KL (250 ng/mL) for the times indicated, and WASP and WIP were immunoprecipitated. Bound fractions of the immunoprecipitations (IPs) were analyzed for tyrosine phosphorylation (PY) and protein amounts of the primary and associated proteins by immunoblot (IB). WASP IPs (A) were analyzed for PY (top panel) and protein amounts of WASP (bottom panel). WIP IPs (B) were analyzed for PY (top panel; short exposure showing phospho [p] WIP), protein amounts of WIP (second panel), PY (third panel; long exposure showing pWASP), and protein amounts of WASP (fourth panel). Please note that both WASP and WIP are tyrosine phosphorylated and form a complex as phosphoproteins. In the longer exposure showing pWASP in complex with pWIP, the chemoluminescence signals from pWIP and Ig are not separated due to close migration on sodium dodecyl sulfate–polyacrylamide gel electrophoresis. Both WASP and WIP IPs (C) were analyzed for PY of Arp2 (top panel) and protein amounts of Arp2 (bottom panel). Experiments were performed twice, and comparable results were obtained.
WASP is required for normal Kit-induced formation of filopodia
To identify and study the biologic functions of Kit that are dependent on WASP, we generated BMMCs from normal (wt) and WASP-deficient mice (wasp−/−). Consistent with the findings of others, wasp−/− BMMC cultures matured normally and with similar kinetics (data not shown).42 BMMCs from either genomic background showed comparable amounts of Kit and FcϵRI surface expression after 6 weeks of culture as signs of maturity of the mast cells (Figure 2A). Both Kit and WASP have independently been implicated in the regulation of cell shape and the formation of membrane protrusions in BMMCs.12,42 We compared KL-induced cell spreading and filopodia formation in normal and WASP-deficient BMMCs (Figure 2B). Whereas the nonstimulated cells of either genotype appeared similar in size and shape and the extent of KL-mediated cell spreading was comparable in wt and WASP-deficient BMMCs, KL-induced filopodia formation was significantly reduced in the absence of WASP (Figure 2B-C). This suggests that WASP is critically involved in the transduction of Kit signals that regulate the actin cytoskeleton and promote filopodia formation.
Figure 2.
WASP is required for normal Kit-induced formation of filopodia. BMMC cultures from wt or WASP-deficient mice were obtained, as described in “Mice, cell lines, cell and bone marrow–derived mast cell culture, and KL stimulation.” Mature BMMCs were analyzed for FcϵRI and Kit surface expression as well as for intracellular expression of WASP by flow cytometry. (A) BMMCs were seeded onto fibronectin-coated slides, and cell morphology in absence or presence of KL (45 minutes, 20 ng/mL) was examined by fluorescence microscopy after phalloidin staining of intracellular actin. The images show representative examples of 4 independent experiments. (B) Filopodia formation in wt BMMCs is indicated by white arrows. A summary of the results is shown in a vertical scatter plot indicating the individual data points of the percentage of cells displaying filopodia formation. Bars indicate the mean values. (C) A total of 1237 wt and 1509 wasp−/− cells in 4 independent experiments was analyzed. P was calculated using an unpaired Student t test.
KL-induced Ca2+ influx is impaired in the absence of WASP
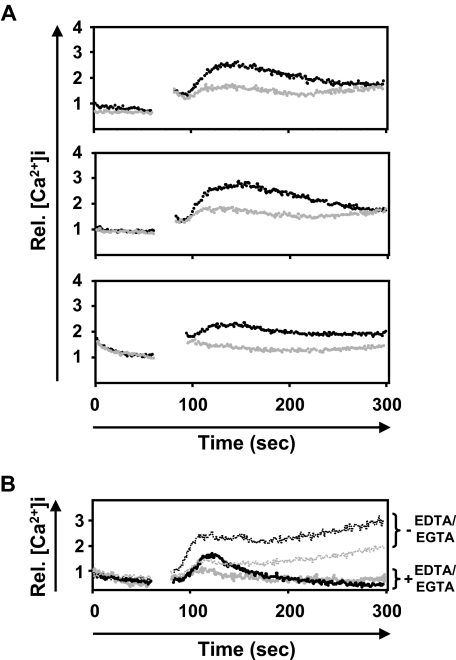
BMMCs have successfully been used to investigate Kit-dependent regulation of Ca2+ signaling,43,44 and T and B cells from WAS patients display defective regulation of [Ca2+]i through their respective antigen receptors.45,46 We investigated WASP function in KL-induced Ca2+ signaling using wt and WASP-deficient BMMCs (Figure 3). Elevation of [Ca2+]i is thought to result from 2 subsequent and connected events, as follows: the initial emptying of intracellular stores, most notably the endoplasmic reticulum, and the subsequent voltage-operated channel (VOC)–mediated Ca influx from the extracellular space into the cell.47 KL-induced increase of [Ca2+]i in WASP-deficient BMMCs was significantly reduced compared with that of wt BMMCs (Figure 3A). Addition of extracellular EDTA/EGTA immobilizes exterior Ca2+, inhibits the VOC-mediated response, and thus allowed us to separately analyze KL-mediated Ca2+ signaling through emptying of intracellular stores alone. This approach revealed that the initial Ca2+ response was also reduced in the absence of WASP (Figure 3B).
Figure 3.
KL-induced Ca2+ influx is impaired in the absence of WASP. Three independent BMMC cultures from wt or WASP-deficient mice were obtained, and cells were prepared and labeled for measurement of [Ca2+]i, as described in “Measurement of Kit-induced changes in the intracellular Ca2+ concentration.” The real-time KL-induced changes of [Ca2+]i in 3 wt (■) or 3 WASP-deficient BMMC cultures (▩) were analyzed by flow cytometry over 5 minutes (A). In a variant experiment, KL-induced changes of [Ca2+]i were analyzed in wt or WASP-deficient BMMCs either in the presence (bold lines) or absence of EDTA/EGTA (dotted thin lines; B). Immobilization of extracellular Ca2+ by EDTA/EGTA allows the separate analysis of changes in [Ca2+]i through the emptying of intracellular stores alone.
KL-driven generation of wasp+/− BMMCs reveals a selective advantage for WASP-expressing cells
The observation of nonrandom inactivation of the X-chromosome in obligate human female carriers of WAS suggests that WASP confers a selective advantage to early hematopoietic progenitors.35 Such progenitors also express Kit, and the selection process could at least in part be dependent on Kit-WASP signals. Because KL alone can promote the maturation of BMMCs from hematopoietic stem or progenitor cells, although at a slower rate and with lower yield, we tested whether or not a selective advantage through WASP could be observed in the generation of BMMCs from heterozygous murine BM when cultured solely in the presence of KL.
In 2 independent experiments with corresponding results (Figure 4; supplemental Figure 1C, available on the Blood website; see the Supplemental Materials link at the top of the online article), BM from heterozygous female mice was used to establish 3 independent BMMC cultures, and cultures were grown in the presence of KL for 90 days. Kit-positive cells were analyzed on days 0, 10, and 90 for intracellular expression of WASP and compared with simultaneously grown BMMC control cultures derived from wt or wasp−/− female mice. We tested the validity of the antibody used for the flow cytometry analysis by intracellular staining and immunfluorescence analysis of Kit-positive BM cells from wt, wasp−/−, or wasp+/− mice. Overall, the data indicated that approximately 30% to 35% of Kit-positive BMMCs from heterozygous mice expressed WASP (supplemental Figure 1A-B). The WASP-positive fraction of Kit+ cells in BM from heterozygous animals as determined by flow cytometry at day 0 was estimated to be less than 25% (Figure 4) or approximately 30% (supplemental Figure 1C). At day 10, heterozygous cultures showed an increase of the WASP+ fraction up to 30% to 50% of the Kit+ cells, and at day 90 (at the stage of mature mast cells), the cells derived from heterozygous animals were entirely positive for WASP expression. These results indicate that Kit-WASP signaling confers a selective advantage in this BMMC model of hematopoietic lineage maturation.
Figure 4.
KL-driven generation of wasp+/− BMMCs reveals a selective advantage for WASP-expressing cells. In 2 separate experiments that delivered corresponding results (1 shown), 3 cultures from wasp+/− BM were established at day 0 and maintained in the presence of KL as growth factor (40 ng/mL) for 90 days. Intracellular WASP expression was evaluated in the Kit-positive populations of the wasp+/− cultures (blue) and compared with that of the control wt (green) or wasp−/− (red) cultures by flow cytometry at the culture days indicated.
Comparison of KL-mediated gene expression profiles in wt and wasp−/− BMMCs establishes a profound genetic effect of Kit-WASP signaling
The importance of WASP in Kit-mediated survival was suggestive of genetic effects of WASP in Kit biology; hence, we compared Kit-induced gene expression in wt BMMCs and WASP-deficient BMMCs (Figure 5; supplemental Tables 1-4). To reduce overlay of possible secondary indirect genetic changes, we chose to perform the array analysis after a relatively short exposure to KL for 12 hours.
Figure 5.
Comparison of KL-mediated gene expression profiles in wt and wasp−/− BMMCs indicates a profound genetic effect through Kit-WASP signaling. Wt or WASP-deficient BMMCs were mock treated or stimulated with KL (500 ng/mL) for 12 hours in replicates (n = 4, wt; n = 3, wasp−/−). Gene expression analysis was performed and analyzed, as described in “Gene expression profiling.” The effects of WASP on the Kit gene expression profile were either independent, permissive (up- or down-regulation requiring the presence of WASP), or blocking (up- or down-regulation only in the absence of WASP; A, Venn diagram and B, heat map). The Venn diagram was generated comparing genes with a fold-change cutoff of 2 for up-regulated and 0.5 for down-regulated genes. Two heat maps with selected genes that encode for proteins with either actin- or Ca2+-binding function are shown (C). Heat map shows the relative expression levels of selected survival/apoptosis-related genes of wt or WASP-deficient BMMCs when exposed to KL (D).
Of approximately 1500 genes that were up- or down-regulated in response to Kit stimulation, approximately two-thirds were WASP independent and one-third was WASP dependent (Figure 5A). WASP-independent effects are Kit-mediated changes in expression of genes that are common to both wt and WASP-deficient cells. Many of the WASP-independent effects were attenuated or accentuated in WASP-negative cells (supplemental Tables 1-4). The WASP-dependent changes in Kit-regulated gene expression can be divided into permissive and blocking effects (Figure 5A-B). Permissive effects were those requiring WASP, that is they only occurred in wt, but not in wasp−/− cells. Blocking effects were those in which WASP prevented Kit-mediated regulation of genes; changes were present in wasp−/−, but not in wt cells.
Consistent with a major role for Kit as a hematopoietic growth factor, genes highly enriched in the KL up-regulated gene pools of wt or WASP-deficient cells are involved in cell cycle regulation, DNA/RNA replication/production, or cellular metabolic processes, whereas down-regulated genes were, for example, enriched for genes with function in apoptosis (supplemental Lists 1-2 and 5-6). Differentially regulated genes showed some interesting additional functional clustering, as follows: for instance, genes that were up-regulated through Kit only in the presence of WASP showed some enrichment in the lectins, annexins, and genes with phospholipid-binding function, whereas the gene pool that was down-regulated only in absence of WASP was enriched for genes with function in chemotaxis or locomotion (supplemental Lists 3,8). Interestingly, several genes that are involved in actin or Ca2+ regulation were significantly up-regulated only in the presence of WASP (Figure 5C). In addition, selected genes involved in the regulation of survival and apoptosis were differentially expressed when cells were exposed to KL (Figure 5D). Taken together, these findings indicate that WASP has profound effects on Kit-mediated gene regulation.
Discussion
The observation of positive selection of hematopoietic progenitors through WASP in the hematopoietic compartment led us to hypothesize that a functional Kit-WASP pathway may be involved. Using hematopoietic cell lines and BMMCs, a primary cellular background relevant for the analysis of Kit biology, we show that WASP plays important roles in Kit function and biology.
KL stimulation led to tyrosine phosphorylation of WASP, WIP, and associated Arp2. As shown in other systems, tyrosine phosphorylation of WASP regulates activity and affinity for downstream molecules such as WIP and Arp2/3.48,49 Tyrosine phosphorylation of WASP has been observed in response to RTK signaling50 or through receptors with associated tyrosine kinase activity,51–53 and Src family kinases (SFKs) have been suggested to directly phosphorylate WASP.51,54 Kit-dependent activation of SFKs is well established,55–57 and in the current study tyrosine phosphorylation of WASP could be a result of Kit-induced activation of SFKs. Alternatively, WASP could be a direct substrate of Kit. Complex formation between WASP/WIP and Arp2 was constitutive, confirming previous observations of a functional multiprotein complex of WASP/WIP/Arp2/3.29,30,58,59 Like WASP, WIP and Arp2 were also tyrosine phosphorylated in response to KL. Tyrosine phosphorylation of Arp2 has recently been shown to be required for its activity.60 This implies that additional control mechanisms for the activity of the Arp2/3 complex exist, other than the regulation through nucleation-promoting factors such as WASP.
Our finding that KL-induced generation of membrane protrusions in the form of filopodia was defective in WASP-deficient BMMCs indicates that WASP is necessary for the correct transduction of Kit signals to the cytoskeleton. Actin reorganization in response to KL was described in the early literature on Kit function.8 Since then, several proteins have been demonstrated to be effectors of Kit-mediated actin reorganization. Studies performed in BMMCs showed that the pleckstrin domain-containing Rac effector SWAP-70 plays a role in Kit-dependent actin reorganization and filopodia formation through Kit, whereas the SFK fyn is required for normal Kit-induced spreading and lamellipodia formation.12,44 WASP has been previously demonstrated to be necessary for normal FcϵRI-mediated cell spreading in BMMCs.42 Although our data demonstrate loss of filopodia formation, Kit-mediated cell spreading seemed normal in WASP-deficient cells. In addition, real-time actin reorganization as analyzed by flow cytometry after phalloidin staining of Kit-stimulated BMMCs was comparable between wt and WASP-deficient cells (data not shown). This suggests that WASP mediates localized effects of Kit stimulation that are independent of overall actin reorganization. One explanation for this dichotomy may be that in hematopoietic cells, where WASP, N-WASP, and other WASP-subfamily members are coexpressed,42,61 the WASP defect may be partially complemented. It is noteworthy, however, that we were unable to detect KL-induced tyrosine phosphorylation of N-WASP in BMMCs. In addition, N-WASP protein levels in wt and WASP-deficient BMMCs were similar, which argues against a compensatory up-regulation of N-WASP in the absence of WASP (supplemental Figure 1).
Our current findings introduce WASP as a positive regulator of RTK-mediated Ca2+ signaling. Ca2+ signals through Kit are a known phenomenon and have recently become of particular interest for investigations of Kit function in nociception or in pacemaker activity of Cajal cells.5,62,63 Few molecular regulators of Kit-Ca2+ signals have been investigated in detail. The phosphatase Src homology 2-containing inositol 5′ phosphatase, for example, was shown to have a negative regulatory effect on Kit-mediated Ca2+ signals, most likely through a general negative feedback regulation of Kit signaling, whereas SWAP-70 seems to play a positive regulatory role.43,44 WASP-dependent regulation of Ca2+ levels has been shown in a variety of receptor systems with associated tyrosine kinase activity, such as the T-cell receptor, B-cell receptor, and FcϵRI.42,45,46 Those results are in agreement with our current finding of WASP as a positive regulator of Ca2+ signaling. According to our observations, WASP could be involved in both phases of KL-induced Ca2+ signaling, the initial emptying of the intracellular stores, and the subsequent influx of extracellular Ca2+ through VOCs. Alternatively, the reduction of the first phase Ca2+ response through KL could result in a reduced trigger for the subsequent influx of Ca2+. Because Ca2+ is involved in nearly every aspect of cellular control, including gene expression, intact Kit–WASP-Ca2+ signals are expected to be crucial for Kit-mediated maintenance of the hematopoietic compartment.
The KL-dependent generation of BMMCs from WASP-heterozygous BM revealed a selective advantage of WASP-expressing over WASP-deficient cells reminiscent of the selective advantage of WASP-positive hematopoietic cells observed in WAS-heterozygous female humans. In mice, a defect in chemotaxis was suggested to be responsible for a disadvantage of WASP-deficient HSCs,64 and in the natural competitive setting of wasp+/− mice, regulatory T cells, natural killer T cells, CD4 and CD8 T lymphocytes, marginal zone B cells, and macrophages demonstrate a selective advantage through WASP.65 In humans, the selective advantage that corrects for the presence of the dysfunctional allele is already present at the stage of CD34+ progenitors.35 As such progenitors are also dependent on Kit, we postulate a selective advantage through intact Kit-WASP signaling. It is unlikely that the observed skewing of the BMMC cultures toward WASP-positive populations was due to a basic cell cycle difference, because the control cultures of wt or wasp−/− BM matured at similar rates and efficiencies. In addition, thymidine incorporation in wt and WASP-deficient BMMCs was not significantly different (data not shown), and total tyrosine phosphorylation, activation of p38 mitogen-activated protein kinase, c-Jun N-terminal kinase/stress-activated protein kinase, as well as Stat3 and Stat5, as analyzed by Western blotting of total lysates, were comparable (supplemental Figure 2). As discussed below, a difference in the relative expression levels of survival and apoptotic-related genes might explain the selective advantage of WASP-expressing cells. Because gene therapy approaches greatly depend on a selective advantage conferred by the gene of interest, better understanding of a Kit-WASP–dependent selective advantage may be relevant for WAS gene therapy.66,67
Comparison of the genetic profiles induced by KL in the presence or absence of WASP revealed a strong influence of WASP on KL up- or down-regulated genes. There are changes in gene expression downstream of Kit that were independent of WASP, suggesting either Kit-signaling pathways that do not require WASP or pathways that are complemented by the presence of another WASP family member, for example, N-WASP. The observation of both permissive and blocking effects suggests that WASP can either promote or suppress some Kit-mediated changes in gene expression. Although Kit is expressed in both hematopoietic and nonhematopoietic progenitors, WASP is exclusively expressed in the hematopoietic lineage. The presence of both permissive and blocking effects of WASP downstream of Kit suggests the interesting possibility that the permissive effects might be Kit genetic effects specific to hematopoietic cells, whereas the changes in gene expression blocked in the presence of WASP might occur in other Kit-positive progenitors that lack WASP, for example, embryonic or neural stem cells.
The other interesting observation was the WASP-dependent regulation of genes with potential function in actin reorganization or Ca2+ signaling. WASP appeared to regulate the expression of proteins that are directly downstream of Kit-WASP signaling. Thus, the immediate defect in Kit-mediated actin reorganization or Ca2+ signaling might be amplified by secondary changes in gene expression.
When BMMCs were exposed to KL, some selected survival-related genes such as Bcl2 or phosphatidylinositol 3-kinase–related genes were relatively higher expressed in wt BMMCs, whereas proapoptotic factors such as caspases showed a lower expression in wt BMMCs compared with WASP-deficient BMMCs. In addition, we observed that some genes of the tumor necrosis factor–receptor subfamilies are relatively higher expressed in the absence of WASP. Ligands for these receptors might be secreted by other cells during BMMC generation, or receptors may be activated via autocrine mechanisms. Such differences in survival and apoptosis-regulating genes may contribute to the selective advantage of WASP-expressing cells during the generation of BMMCs in vitro or hematopoiesis in vivo.27
The KL concentrations used to test the role of WASP in Kit-mediated biologic responses were in some cases the result of optimal dose-response analysis (ie, in the KL-induced filopodia formation) or deliberately determined (ie, in the analysis of the Kit-mediated genetic profile). We cannot exclude that especially the high KL dose used for the gene array analysis may create some artifacts, and therefore, may be considered with some caution. However, Kit-expressing cells in the hematopoietic niche could be exposed to very similar KL concentrations, depending on their distance from KL-producing stromal cells. In addition, KL is active not only in its soluble, but also in its membrane-bound form. The direct contact between a stromal cell and an effector cell such as HSC could provide a local KL stimulation effect that is comparable with the high KL concentration used in the analysis of the Kit genetic profile.
Taken together, our data show that WASP plays important roles in Kit function and biology: cytoskeletal changes, regulation of intracellular calcium levels, cell survival, and gene expression through Kit are either entirely or to a significant extent dependent on WASP. The profound effect of WASP on Kit-mediated gene expression is most likely the basis for the selective advantage seen in the BMMC culture system and could further explain the strong selective pressure WASP exerts in early hematopoiesis. Besides hematopoietic stem cells, mast cells, and lymphoid and erythroid progenitors, Kit is also expressed by multiple other types of stem cells and progenitors, including germ cells, embryonic stem cells, and neural stem cells. Further work is required to determine how the pleiotropic RTK Kit regulates actin reorganization, Ca2+ signaling, and survival in nonhematopoietic precursors, and how N-WASP might mediate signaling in the absence of WASP.
Supplementary Material
Acknowledgments
We thank YuJun Yang for excellent array sample preparation, Jing Huang for animal care, and Shannon Phan and Kefu Liu for enthusiastic technical support during the summer of 2007.
This work was supported by National Institutes of Health grants AI50765, HL54729, and 5PO1 CA049605-2O.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: M.M. performed research, analyzed data, and wrote the paper; S.V. analyzed data; M.S. performed research and analyzed data; S.L. and A.B. analyzed data; K.W. designed experiments and wrote the paper; and T.J. designed experiments, performed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Thomas Jahn, Stanford University School of Medicine, Stanford University Medical Center, 300 Pasteur Dr, Rm H306B, Mailcode 5208, Stanford, CA 94305-5208; e-mail: tjahn@stanford.edu.
References
- 1.Geissler EN, Ryan MA, Housman DE. The dominant-white spotting (W) locus of the mouse encodes the c-kit proto-oncogene. Cell. 1988;55:185–192. doi: 10.1016/0092-8674(88)90020-7. [DOI] [PubMed] [Google Scholar]
- 2.Chabot B, Stephenson DA, Chapman VM, Besmer P, Bernstein A. The proto-oncogene c-kit encoding a transmembrane tyrosine kinase receptor maps to the mouse W locus. Nature. 1988;335:88–89. doi: 10.1038/335088a0. [DOI] [PubMed] [Google Scholar]
- 3.Cable J, Huszar D, Jaenisch R, Steel KP. Effects of mutations at the W locus (c-kit) on inner ear pigmentation and function in the mouse. Pigm Cell Res. 1994;7:17–32. doi: 10.1111/j.1600-0749.1994.tb00015.x. [DOI] [PubMed] [Google Scholar]
- 4.Gore BB, Wong KG, Tessier-Lavigne M. Stem cell factor functions as an outgrowth-promoting factor to enable axon exit from the midline intermediate target. Neuron. 2008;57:501–510. doi: 10.1016/j.neuron.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 5.Milenkovic N, Frahm C, Gassmann M, et al. Nociceptive tuning by stem cell factor/c-Kit signaling. Neuron. 2007;56:893–906. doi: 10.1016/j.neuron.2007.10.040. [DOI] [PubMed] [Google Scholar]
- 6.Matsui Y, Zsebo KM, Hogan BL. Embryonic expression of a haematopoietic growth factor encoded by the Sl locus and the ligand for c-kit. Nature. 1990;347:667–669. doi: 10.1038/347667a0. [DOI] [PubMed] [Google Scholar]
- 7.Copeland NG, Gilbert DJ, Cho BC, et al. Mast cell growth factor maps near the steel locus on mouse chromosome 10 and is deleted in a number of steel alleles. Cell. 1990;63:175–183. doi: 10.1016/0092-8674(90)90298-s. [DOI] [PubMed] [Google Scholar]
- 8.Blume-Jensen P, Claesson-Welsh L, Siegbahn A, Zsebo KM, Westermark B, Heldin CH. Activation of the human c-kit product by ligand-induced dimerization mediates circular actin reorganization and chemotaxis. EMBO J. 1991;10:4121–4128. doi: 10.1002/j.1460-2075.1991.tb04989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Serve H, Yee NS, Stella G, Sepp-Lorenzino L, Tan JC, Besmer P. Differential roles of PI3-kinase and Kit tyrosine 821 in Kit receptor-mediated proliferation, survival and cell adhesion in mast cells. EMBO J. 1995;14:473–483. doi: 10.1002/j.1460-2075.1995.tb07023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scott G, Liang H, Luthra D. Stem cell factor regulates the melanocyte cytoskeleton. Pigm Cell Res. 1996;9:134–141. doi: 10.1111/j.1600-0749.1996.tb00101.x. [DOI] [PubMed] [Google Scholar]
- 11.Vosseller K, Stella G, Yee NS, Besmer P. c-kit receptor signaling through its phosphatidylinositide-3′-kinase-binding site and protein kinase C: role in mast cell enhancement of degranulation, adhesion, and membrane ruffling. Mol Biol Cell. 1997;8:909–922. doi: 10.1091/mbc.8.5.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Samayawardhena LA, Kapur R, Craig AW. Involvement of Fyn kinase in Kit and integrin-mediated Rac activation, cytoskeletal reorganization, and chemotaxis of mast cells. Blood. 2007;109:3679–3686. doi: 10.1182/blood-2006-11-057315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Symons M, Derry JM, Karlak B, et al. Wiskott-Aldrich syndrome protein, a novel effector for the GTPase CDC42Hs, is implicated in actin polymerization. Cell. 1996;84:723–734. doi: 10.1016/s0092-8674(00)81050-8. [DOI] [PubMed] [Google Scholar]
- 14.Moreau V, Frischknecht F, Reckmann I, et al. A complex of N-WASP and WIP integrates signalling cascades that lead to actin polymerization. Nat Cell Biol. 2000;2:441–448. doi: 10.1038/35017080. [DOI] [PubMed] [Google Scholar]
- 15.Yarar D, To W, Abo A, Welch MD. The Wiskott-Aldrich syndrome protein directs actin-based motility by stimulating actin nucleation with the Arp2/3 complex. Curr Biol. 1999;9:555–558. doi: 10.1016/s0960-9822(99)80243-7. [DOI] [PubMed] [Google Scholar]
- 16.Rohatgi R, Ma L, Miki H, et al. The interaction between N-WASP and the Arp2/3 complex links Cdc42-dependent signals to actin assembly. Cell. 1999;97:221–231. doi: 10.1016/s0092-8674(00)80732-1. [DOI] [PubMed] [Google Scholar]
- 17.Miki H, Suetsugu S, Takenawa T. WAVE, a novel WASP-family protein involved in actin reorganization induced by Rac. EMBO J. 1998;17:6932–6941. doi: 10.1093/emboj/17.23.6932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Machesky LM, Mullins RD, Higgs HN, et al. Scar, a WASp-related protein, activates nucleation of actin filaments by the Arp2/3 complex. Proc Natl Acad Sci U S A. 1999;96:3739–3744. doi: 10.1073/pnas.96.7.3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wiskott A. Familial congenital Morbus Werlhofii [German]? Monatsschr Kinderheilkd. 1937;68:212–216. [Google Scholar]
- 20.Aldrich RA, Steinberg AG, Campbell DC. Pedigree demonstrating a sex-linked recessive condition characterized by draining ears, eczematoid dermatitis and bloody diarrhea. Pediatrics. 1954;13:133–139. [PubMed] [Google Scholar]
- 21.Derry JM, Ochs HD, Francke U. Isolation of a novel gene mutated in Wiskott-Aldrich syndrome. Cell. 1994;79:922ff. [PubMed] [Google Scholar]
- 22.Derry JM, Kerns JA, Weinberg KI, et al. WASP gene mutations in Wiskott-Aldrich syndrome and X-linked thrombocytopenia. Hum Mol Genet. 1995;4:1127–1135. doi: 10.1093/hmg/4.7.1127. [DOI] [PubMed] [Google Scholar]
- 23.Cooper MD, Chae HP, Lowman JT, Krivit W, Good RA. Wiskott-Aldrich syndrome: an immunologic deficiency disease involving the afferent limb of immunity. Am J Med. 1968;44:499–513. doi: 10.1016/0002-9343(68)90051-x. [DOI] [PubMed] [Google Scholar]
- 24.Binder V, Albert MH, Kabus M, Bertone M, Meindl A, Belohradsky BH. The genotype of the original Wiskott phenotype. N Engl J Med. 2006;355:1790–1793. doi: 10.1056/NEJMoa062520. [DOI] [PubMed] [Google Scholar]
- 25.Ochs HD, Slichter SJ, Harker LA, Von Behrens WE, Clark RA, Wedgwood RJ. The Wiskott-Aldrich syndrome: studies of lymphocytes, granulocytes, and platelets. Blood. 1980;55:243–252. [PubMed] [Google Scholar]
- 26.Snapper SB, Rosen FS, Mizoguchi E, et al. Wiskott-Aldrich syndrome protein-deficient mice reveal a role for WASP in T but not B cell activation. Immunity. 1998;9:81–91. doi: 10.1016/s1074-7613(00)80590-7. [DOI] [PubMed] [Google Scholar]
- 27.Rawlings SL, Crooks GM, Bockstoce D, Barsky LW, Parkman R, Weinberg KI. Spontaneous apoptosis in lymphocytes from patients with Wiskott-Aldrich syndrome: correlation of accelerated cell death and attenuated bcl-2 expression. Blood. 1999;94:3872–3882. [PubMed] [Google Scholar]
- 28.Kolluri R, Tolias KF, Carpenter CL, Rosen FS, Kirchhausen T. Direct interaction of the Wiskott-Aldrich syndrome protein with the GTPase Cdc42. Proc Natl Acad Sci U S A. 1996;93:5615–5618. doi: 10.1073/pnas.93.11.5615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramesh N, Anton IM, Hartwig JH, Geha RS. WIP, a protein associated with Wiskott-Aldrich syndrome protein, induces actin polymerization and redistribution in lymphoid cells. Proc Natl Acad Sci U S A. 1997;94:14671–14676. doi: 10.1073/pnas.94.26.14671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Machesky LM, Insall RH. Scar1 and the related Wiskott-Aldrich syndrome protein, WASP, regulate the actin cytoskeleton through the Arp2/3 complex. Curr Biol. 1998;8:1347–1356. doi: 10.1016/s0960-9822(98)00015-3. [DOI] [PubMed] [Google Scholar]
- 31.Kim AS, Kakalis LT, Abdul-Manan N, Liu GA, Rosen MK. Autoinhibition and activation mechanisms of the Wiskott-Aldrich syndrome protein. Nature. 2000;404:151–158. doi: 10.1038/35004513. [DOI] [PubMed] [Google Scholar]
- 32.Rozelle AL, Machesky LM, Yamamoto M, et al. Phosphatidylinositol 4,5-bisphosphate induces actin-based movement of raft-enriched vesicles through WASP-Arp2/3. Curr Biol. 2000;10:311–320. doi: 10.1016/s0960-9822(00)00384-5. [DOI] [PubMed] [Google Scholar]
- 33.Dupré L, Aiuti A, Trifari S, et al. Wiskott-Aldrich syndrome protein regulates lipid raft dynamics during immunological synapse formation. Immunity. 2002;17:157–166. doi: 10.1016/s1074-7613(02)00360-6. [DOI] [PubMed] [Google Scholar]
- 34.Fearon ER, Kohn DB, Winkelstein JA, Vogelstein B, Blaese RM. Carrier detection in the Wiskott Aldrich syndrome. Blood. 1988;72:1735–1739. [PubMed] [Google Scholar]
- 35.Wengler G, Gorlin JB, Williamson JM, Rosen FS, Bing DH. Nonrandom inactivation of the X chromosome in early lineage hematopoietic cells in carriers of Wiskott-Aldrich syndrome. Blood. 1995;85:2471–2477. [PubMed] [Google Scholar]
- 36.Avanzi GC, Brizzi MF, Giannotti J, et al. M-07e human leukemic factor-dependent cell line provides a rapid and sensitive bioassay for the human cytokines GM-CSF and IL-3. J Cell Physiol. 1990;145:458–464. doi: 10.1002/jcp.1041450310. [DOI] [PubMed] [Google Scholar]
- 37.Jahn T, Leifheit E, Gooch S, Sindhu S, Weinberg K. Lipid rafts are required for Kit survival and proliferation signals. Blood. 2007;110:1739–1747. doi: 10.1182/blood-2006-05-020925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 39.Irizarry RA, Hobbs B, Collin F, et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4:249–264. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- 40.Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci U S A. 2001;98:5116–5121. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miki H, Nonoyama S, Zhu Q, Aruffo A, Ochs HD, Takenawa T. Tyrosine kinase signaling regulates Wiskott-Aldrich syndrome protein function, which is essential for megakaryocyte differentiation. Cell Growth Differ. 1997;8:195–202. [PubMed] [Google Scholar]
- 42.Pivniouk VI, Snapper SB, Kettner A, et al. Impaired signaling via the high-affinity IgE receptor in Wiskott-Aldrich syndrome protein-deficient mast cells. Int Immunol. 2003;15:1431–1440. doi: 10.1093/intimm/dxg148. [DOI] [PubMed] [Google Scholar]
- 43.Huber M, Helgason CD, Scheid MP, Duronio V, Humphries RK, Krystal G. Targeted disruption of SHIP leads to Steel factor-induced degranulation of mast cells. EMBO J. 1998;17:7311–7319. doi: 10.1093/emboj/17.24.7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sivalenka RR, Jessberger R. SWAP-70 regulates c-kit-induced mast cell activation, cell-cell adhesion, and migration. Mol Cell Biol. 2004;24:10277–10288. doi: 10.1128/MCB.24.23.10277-10288.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Simon HU, Mills GB, Hashimoto S, Siminovitch KA. Evidence for defective transmembrane signaling in B cells from patients with Wiskott-Aldrich syndrome. J Clin Invest. 1992;90:1396–1405. doi: 10.1172/JCI116006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang J, Shehabeldin A, da Cruz LAG, et al. Antigen receptor-induced activation and cytoskeletal rearrangement are impaired in Wiskott-Aldrich syndrome protein-deficient lymphocytes. J Exp Med. 1999;190:1329–1342. doi: 10.1084/jem.190.9.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 48.Cory GO, Garg R, Cramer R, Ridley AJ. Phosphorylation of tyrosine 291 enhances the ability of WASp to stimulate actin polymerization and filopodium formation. J Biol Chem. 2002;277:45115–45121. doi: 10.1074/jbc.M203346200. [DOI] [PubMed] [Google Scholar]
- 49.Cory GO, Cramer R, Blanchoin L, Ridley AJ. Phosphorylation of the WASP-VCA domain increases its affinity for the Arp2/3 complex and enhances actin polymerization by WASP. Mol Cell. 2003;11:1229–1239. doi: 10.1016/s1097-2765(03)00172-2. [DOI] [PubMed] [Google Scholar]
- 50.Tsuboi S, Meerloo J. Wiskott-Aldrich syndrome protein is a key regulator of the phagocytic cup formation in macrophages. J Biol Chem. 2007;282:34194–34203. doi: 10.1074/jbc.M705999200. [DOI] [PubMed] [Google Scholar]
- 51.Guinamard R, Aspenstrom P, Fougereau M, Chavrier P, Guillemot JC. Tyrosine phosphorylation of the Wiskott-Aldrich syndrome protein by Lyn and Btk is regulated by CDC42. FEBS Lett. 1998;434:431–436. doi: 10.1016/s0014-5793(98)01016-3. [DOI] [PubMed] [Google Scholar]
- 52.Baba Y, Nonoyama S, Matsushita M, et al. Involvement of Wiskott-Aldrich syndrome protein in B-cell cytoplasmic tyrosine kinase pathway. Blood. 1999;93:2003–2012. [PubMed] [Google Scholar]
- 53.Badour K, Zhang J, Shi F, Leng Y, Collins M, Siminovitch KA. Fyn and PTP-PEST-mediated regulation of Wiskott-Aldrich syndrome protein (WASp) tyrosine phosphorylation is required for coupling T cell antigen receptor engagement to WASp effector function and T cell activation. J Exp Med. 2004;199:99–112. doi: 10.1084/jem.20030976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Banin S, Truong O, Katz DR, Waterfield MD, Brickell PM, Gout I. Wiskott-Aldrich syndrome protein (WASp) is a binding partner for c-Src family protein-tyrosine kinases. Curr Biol. 1996;6:981–988. doi: 10.1016/s0960-9822(02)00642-5. [DOI] [PubMed] [Google Scholar]
- 55.Timokhina I, Kissel H, Stella G, Besmer P. Kit signaling through PI 3-kinase and Src kinase pathways: an essential role for Rac1 and JNK activation in mast cell proliferation. EMBO J. 1998;17:6250–6262. doi: 10.1093/emboj/17.21.6250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Agosti V, Corbacioglu S, Ehlers I, et al. Critical role for Kit-mediated Src kinase but not PI 3-kinase signaling in pro T and pro B cell development. J Exp Med. 2004;199:867–878. doi: 10.1084/jem.20031983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bromann PA, Korkaya H, Courtneidge SA. The interplay between Src family kinases and receptor tyrosine kinases. Oncogene. 2004;23:7957–7968. doi: 10.1038/sj.onc.1208079. [DOI] [PubMed] [Google Scholar]
- 58.Dong X, Patino-Lopez G, Candotti F, Shaw S. Structure-function analysis of the WIP role in T cell receptor-stimulated NFAT activation: evidence that WIP-WASP dissociation is not required and that the WIP NH2 terminus is inhibitory. J Biol Chem. 2007;282:30303–30310. doi: 10.1074/jbc.M704972200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rodal AA, Sokolova O, Robins DB, et al. Conformational changes in the Arp2/3 complex leading to actin nucleation. Nat Struct Mol Biol. 2005;12:26–31. doi: 10.1038/nsmb870. [DOI] [PubMed] [Google Scholar]
- 60.LeClaire LL, III, Baumgartner M, Iwasa JH, Mullins RD, Barber DL. Phosphorylation of the Arp2/3 complex is necessary to nucleate actin filaments. J Cell Biol. 2008;182:647–654. doi: 10.1083/jcb.200802145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cotta-de-Almeida V, Westerberg L, Maillard MH, et al. Wiskott Aldrich syndrome protein (WASP) and N-WASP are critical for T cell development. Proc Natl Acad Sci U S A. 2007;104:15424–15429. doi: 10.1073/pnas.0706881104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gommerman JL, Rottapel R, Berger SA. Phosphatidylinositol 3-kinase and Ca2+ influx dependence for ligand-stimulated internalization of the c-Kit receptor. J Biol Chem. 1997;272:30519–30525. doi: 10.1074/jbc.272.48.30519. [DOI] [PubMed] [Google Scholar]
- 63.Boddy G, Daniel EE. Role of l-Ca2+ channels in intestinal pacing in wild-type and W/W(V) mice. Am J Physiol. 2005;288:G439–G446. doi: 10.1152/ajpgi.00255.2004. [DOI] [PubMed] [Google Scholar]
- 64.Lacout C, Haddad E, Sabri S, et al. A defect in hematopoietic stem cell migration explains the nonrandom X-chromosome inactivation in carriers of Wiskott-Aldrich syndrome. Blood. 2003;102:1282–1289. doi: 10.1182/blood-2002-07-2099. [DOI] [PubMed] [Google Scholar]
- 65.Westerberg LS, de la Fuente MA, Wermeling F, et al. WASP confers selective advantage for specific hematopoietic cell populations and serves a unique role in marginal zone B-cell homeostasis and function. Blood. 2008;112:4139–4147. doi: 10.1182/blood-2008-02-140715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Santilli G, Thornhill SI, Kinnon C, Thrasher AJ. Gene therapy of inherited immunodeficiencies. Expert Opin Biol Ther. 2008;8:397–407. doi: 10.1517/14712598.8.4.397. [DOI] [PubMed] [Google Scholar]
- 67.Galy A, Roncarolo MG, Thrasher AJ. Development of lentiviral gene therapy for Wiskott Aldrich syndrome. Expert Opin Biol Ther. 2008;8:181–190. doi: 10.1517/14712598.8.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.