Abstract
The inwardly rectifying potassium channel (Kir) regulates resting membrane potential, K+ homeostasis, heart rate, and hormone secretion. The outward current is blocked in a voltage-dependent manner, upon the binding of intracellular polyamines or Mg2+ to the transmembrane pore domain. Meanwhile, electrophysiological studies have shown that mutations of several acidic residues in the intracellular regions affected the inward rectification. Although these acidic residues are assumed to bind polyamines, the functional role of the binding of polyamines and Mg2+ to the intracellular regions of Kirs remains unclear. Here, we report thermodynamic and structural studies of the interaction between polyamines and the cytoplasmic pore of mouse Kir3.1/GIRK1, which is gated by binding of G-protein βγ-subunit (Gβγ). ITC analyses showed that two spermine molecules bind to a tetramer of Kir3.1/GIRK1 with a dissociation constant of 26 μm, which is lower than other blockers. NMR analyses revealed that the spermine binding site is Asp-260 and its surrounding area. Small but significant chemical shift perturbations upon spermine binding were observed in the subunit-subunit interface of the tetramer, suggesting that spermine binding alters the relative orientations of the four subunits. Our ITC and NMR results postulated a spermine binding mode, where one spermine molecule bridges two Asp-260 side chains from adjacent subunits, with rearrangement of the subunit orientations. This suggests the functional roles of spermine binding to the cytoplasmic pore: stabilization of the resting state conformation of the channel, and instant translocation to the transmembrane pore upon activation through the Gβγ-induced conformational rearrangement.
The inwardly rectifying K+ channel (Kir)3 plays a pivotal role in controlling resting membrane potential, K+ homeostasis, heart rate, and hormone secretion (1). The inward rectification property of Kir is reportedly due to the voltage-dependent blockade of the outward current by intracellular polyamines and Mg2+ (2–6). The importance of the electrostatic interactions of polyamines and Mg2+ with the acidic residues in Kirs has been indicated by electrophysiological studies in combination with site-directed mutagenesis, which have been accelerated by the recent progress in the structural analyses of Kirs (7–10). The crystal structures revealed that they form a symmetric tetramer, in which the transmembrane pore, containing the K+-selective filter and the cytoplasmic pore consisting of the N- and C-terminal regions form a long pore for the K+ pathway.
In Kir2.1/IRK1, the strongest inward rectifier in the Kir family, negatively charged acidic residues that influence the inward rectification have been identified, including Asp-172 (11) in the transmembrane pore and Glu-224, Asp-255, Asp-259, and Glu-299 in the cytoplasmic pore (9, 12–19). These acidic residues are assumed to be responsible for the electrostatic interaction with polyamines, in which the nitrogen atoms are positively charged at neutral pH (20).
In other members of the Kir family, some of the electronegative residues are replaced by neutral ones, such as Gly and Ser. Although the total number of acidic residues correlates with the strength of inward rectification, the correlation cannot be completely explained for the strong rectifiers (9). In Kir3.1/GIRK1, which exhibits relatively strong inward rectification among the Kir family proteins, two of the four important acidic residues in Kir2.1 (Glu-224 and Asp-255) are replaced by Ser (Ser-225 and Ser-256 in Kir3.1, respectively). This suggests that the binding site(s) and stoichiometry of polyamine binding differ among the Kir proteins.
Although the binding of polyamines to the cytoplasmic pore of Kirs is considered to be required for the blockade of the transmembrane pore to enable the inward rectification (16, 19, 21), the functional role of the polyamine binding to the cytoplasmic pore is still unclear. In particular, Kir3/GIRK is activated by the binding of G-protein βγ-subunits (Gβγ) to the cytoplasmic pore through the conformational rearrangement of the channel (22). Thus, the elucidation of the binding mode of Kir and polyamines based on the detection of their direct interaction as well as the effects of the binding on the protein conformation provides insights into the functional roles of the cytoplasmic pore in the gating and the inward rectifying property of the channel.
Here, we report the thermodynamic and structural analyses of the interaction between spermine and the intracellular regions of Kir3.1/GIRK1. Our isothermal titration calorimetry (ITC) results indicated that two spermine molecules bind to a tetramer of Kir3.1/GIRK1, with a dissociation constant (Kd) value of 26 μm. NMR analyses together with ITC results revealed the spermine binding mode, in which one spermine molecule bridges two Asp-260 side chains of two adjacent subunits, with the alteration of the relative orientations of the four subunits. The spermine binding mode revealed here suggests the functional roles of the spermine binding to the cytoplasmic pore: in the resting state of the channel, spermine stabilizes a certain channel conformation, and upon activation, spermine is dissociated from the cytoplasmic pore through the Gβγ-induced conformational rearrangement, leading to rapid translocation to the transmembrane pore to block the outward K+ current.
EXPERIMENTAL PROCEDURES
Sample Preparation
The N- and C-terminal intracellular regions (residues 41–63 and 190–371) of mouse Kir3.1/GIRK1 were fused into a single polypeptide, which is hereafter referred to as GIRKCP (cytoplasmic pore). The sample preparation of GIRKCP is described elsewhere (23). Briefly, the DNA encoding GIRKCP with ten N-terminal His residues, followed by the PreScission protease cleavage site, was inserted into the pET24d(+) plasmid, which was transformed into the Escherichia coli C41 strain. Mutations were introduced by the QuikChange® (Stratagene) method. GIRKCP and its mutants were overexpressed in the C41 strain and were purified to homogeneity by His-select Ni affinity chromatography, His tag cleavage by PreScission protease, and then gel filtration using Superdex 200 PG (GE Healthcare). The elution profile of the gel-filtration column indicated that GIRKCP forms a tetramer, which was confirmed by a calibration curve made by standard proteins for molecular mass as well as the SEC-MALS experiments (see supplemental Fig. S1 for details). We prepared uniformly 2H,15N-labeled GIRKCP, whose amide hydrogen atoms were fully exchanged to 1H by unfolding with 6 m guanidine and subsequent refolding.
Isothermal Titration Calorimetry (ITC)
The binding of spermine and Mg2+ to GIRKCP and its mutants was measured by ITC (24), using a MicroCal VP-ITC MicroCalorimeter (MicroCal Inc.). The protein samples were dialyzed against a buffer containing 10 mm HEPES·NaOH (pH 7.0), 0–150 mm KCl, and 5 mm dithiothreitol. 900 μm spermine and 1.4 mm MgCl2 in the dialysis buffer were prepared as the injection solutions. Experiments were performed at 15–30 °C. A 30–47 μm solution of GIRKCP tetramer or its mutants was titrated by the injection of a total of 290 μl of 900 μm spermine or 1.4 mm MgCl2 in 29 aliquots. Because essentially no heat of dilution was observed by titrating the spermine solution into the dialysis buffer, the raw titration data were analyzed using the MicroCal Origin software version 5.0, provided by the manufacturer. The heat of dilution of MgCl2 was subtracted from the data for the MgCl2 titration into GIRKCP. The thermodynamic parameters are listed in Tables 1 and 2.
TABLE 1.
Thermodynamic parameters for the interaction of GIRKCP with polyamines and MgCl2
| Injectant | Temperature | KCl | Model | Stoichiometry per tetramer | Kd | ΔH | ΔS |
|---|---|---|---|---|---|---|---|
| K | mm | μm | kcal/mol | kcal/mol K | |||
| Spermine | 303 | 0 | Two sitesa | First site 1 (fixed) | 0.06 ± 0.01 | −6.00 (fixed) | 0.013 |
| Second site 0.99 ± 0.01 | 0.22 ± 0.04 | −2.33 ± 0.06 | 0.23 | ||||
| 303 | 50 | Single sites | 1.835 ± 0.007 | 0.49 ± 0.04 | −2.54 ± 0.02 | 0.020 | |
| 303 | 100 | Single sites | 1.784 ± 0.007 | 6.8 ± 0.4 | −2.10 ± 0.01 | 0.017 | |
| 303 | 150 | Single sites | 1.772 ± 0.004 | 25.9 ± 0.04 | −1.809 ± 0.006 | 0.015 | |
| 283 | 50 | Single sites | 1.82 ± 0.01 | 0.34 ± 0.04 | 0.839 ± 0.007 | 0.033 | |
| 288 | 50 | Single sites | 1.2 ± 0.2 | 3 ± 3 | 0.23 ± 0.04 | 0.026 | |
| 293 | 50 | Single sites | 1.60 ± 0.01 | 0.66 ± 0.09 | −0.97 ± 0.01 | 0.025 | |
| 298 | 50 | Single sites | 1.54 ± 0.01 | 0.82 ± 0.09 | −1.93 ± 0.02 | 0.021 | |
| Spermidine | 303 | 50 | Single sites | 1.57 ± 0.08 | 16 ± 3 | −2.0 ± 0.1 | 0.015 |
| Putrescine | 303 | 50 | N/A | ||||
| MgCl2 | 298 | 50 | Single sites | 2.4 ± 0.2 | 101 ± 17 | −0.7 ± 0.1 | 0.016 |
a A two-site model was applied, in which the stoichiometry and the ΔH value for the first binding site are fixed as one and the value of the y-section of the isotherm, respectively.
TABLE 2.
Thermodynamic parameters for the interactions of GIRKCP mutants with spermine
| Mutant | Model | Stoichiometry per tetramer | Kd | ΔH | ΔS |
|---|---|---|---|---|---|
| μm | kcal/mol | kcal/mol K | |||
| Wild typea | Single sites | 1.54 ± 0.01 | 0.82 ± 0.09 | −1.93 ± 0.02 | 0.021 |
| E250N | N/A | ||||
| D260N | N/A | ||||
| D275N | Single sites | 1.74 ± 0.01 | 1.4 ± 0.1 | −2.09 ± 0.02 | 0.02 |
| E300N | N/A | ||||
| Q227A/Q261A | Single sites | 1.656 ± 0.003 | 0.29 ± 0.01 | −6.75 ± 0.02 | 0.0073 |
| F255A | Single sites | 1.194 ± 0.005 | 3.0 ± 0.2 | −0.845 ± 0.05 | 0.022 |
a Data for the wild type GIRKCP are presented for reference.
NMR Analyses
All NMR experiments were performed at 35 °C on a Bruker Avance-800 or Avance-600 spectrometers equipped with cryogenic probes. The sample concentrations of GIRKCP and its mutants were 100–200 μm as the tetramer.
To observe intermolecular NOE signals between spermine and GIRKCP, 15N-edited NOESY HSQC spectra were observed for uniformly 2H,15N-labeled GIRKCP, and the mutants Q271A, Q261A, and Q227A/Q261A, in 50 mm HEPES·NaOH buffer (pH 7.0), containing 50 mm KCl, 5 mm dithiothreitol, and 8%(v/v) D2O. A two-dimensional NOESY spectrum was observed for the uniformly 2H-labeled GIRKCP, in which only isoleucine, methionine, or phenylalanine residues were selectively 1H,15N-labeled. The F255A mutant was also phenylalanine-selectively 1H,15N-labeled in a 2H-background, to confirm the assignments of the intermolecular NOE signals between Phe-255 and spermine. Each sample contained spermine at a 5 equiv. amount relative to the protein. The NOE mixing time was set at 300 ms.
An aliquot of 700 μm spermine solution dissolved in the NMR sample buffer was used for the titration experiments, in which 0.25, 0.50, 0.75, 1.0, 2.0, 3.0, 4.0, and 5.0 equivalents of a tetramer of uniformly 2H,15N-labeled GIRKCP was added. After the addition of each aliquot of spermine, one-dimensional 1H and two-dimensional 1H-15N TROSY spectra were acquired. The titration was performed up to a maximum spermine:tetramer of GIRKCP molar ratio of 5:1. It should be noted that GIRKCP exhibits no chemical shift change at the concentration range from 20 to 300 μm as a tetramer, and thus, the chemical shift changes through the spermine titration is not due to the sample dilution but the effects arisen from the interaction with spermine. The assignments of the backbone NMR signals from GIRKCP were reported (BioMagResBank accession number: 11067 (23)). The assignments for the spermine-bound form of GIRKCP were transferred from those in the absence of spermine by tracing the chemical shift perturbation (CSP) upon spermine addition. The assignments for the spermine-bound form were confirmed by 15N-edited NOESY TROSY of the partially protonated GIRKCP, which was expressed in D2O-based M9 medium containing 1 g/liter 1H-glucose. The normalized CSP values (Δδ) were obtained using the formula, Δδ = {(Δδ1H)2+(Δδ15N/6.5)2}0.5 (25). The error values were calculated by the formula, 2 ·( Δδ1H ·R1H + Δδ15N ·R15N/6.52)/Δδ, where R1H and R15N are the digital resolutions in ppm in the 1H and 15N dimensions, respectively.
1H-15N TROSY spectra were observed for the E250N, D260N, D275N, and E300N mutants, and were compared with the spectrum of GIRKCP, to ascertain the structural effects of the mutations.
RESULTS
Two Spermine Molecules Bind to the Intracellular Regions of Kir3.1/GIRK1
In this study, we used the cytoplasmic pore of mouse Kir3.1/GIRK1, referred to as GIRKCP, which consists of residues 41–63 and 190–371 as a single polypeptide. The validity of this construct was indicated by its crystal structure (7, 9), which was essentially identical to the structures of the cytoplasmic pores in the full-length Kir channels, such as KirBac1.1 and a chimera of KirBac1.3 and Kir3.1 (8, 10). GIRKCP was expressed in E. coli, and was purified to homogeneity. Size exclusion chromatography analyses in combination with multiple-angle light scattering (SEC-MALS) indicated that the average molecular mass was 92 K (supplemental Fig. S1), corresponding to a tetramer of the theoretical mass of 24 K protein.
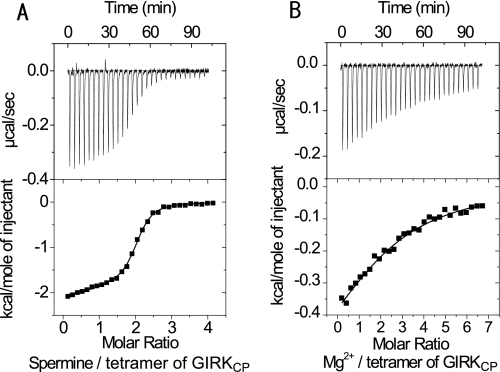
ITC experiments were carried out to compare the binding properties of GIRKCP with spermine, spermidine, putrescine, and Mg2+. Fig. 1A shows the isotherms upon the titration of spermine into the GIRKCP solution. The best fit of the integrated isotherms using a single-site model indicated that two spermine molecules bind to a GIRKCP tetramer with a dissociation constant (Kd) value of 500 nm, in 50 mm KCl at 303 K. Spermidine and Mg2+ exhibited significantly lower affinities to GIRKCP (Fig. 1B and Table 1), which was 30- and 120-fold lower than spermine, respectively. A titration curve was not obtained for putrescine, although a certain amount of heat exchange was observed, suggesting that the binding is weak (Kd > 1 mm). Because the intracellular concentrations of spermine and spermidine are 0.1–1.0 mm, and 1.0–10 mm for Mg2+, spermine is the major binder to the intracellular regions of Kir3.1/GIRK. Therefore, our further analyses were focused on spermine as a Kir blocker.
FIGURE 1.
Isothermal titration microcalorimetric analyses of the interactions between GIRKCP and spermine or MgCl2. Upper panel, trace of the calorimetric titration of 29 × 10 μl aliquots of spermine (A) and MgCl2 (B) into the cell containing GIRKcp. Lower panel, integrated binding isotherm obtained from the experiment. A, two-site model with the fixed binding stoichiometry of 1 for the binding of the first spermine molecule was applied to fit the data. B, single site model was applied to the MgCl2 binding. Parameters obtained from the best fit (solid line) are summarized in Table 1.
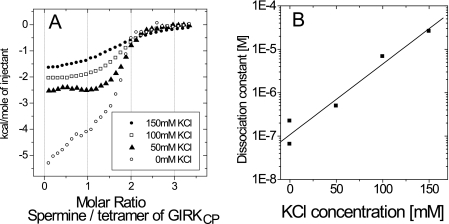
To estimate the electrostatic contribution to the spermine binding, ITC experiments were performed at various KCl concentrations, from 0 to 150 mm. While the binding stoichiometry was unchanged, the Kd values increased as the KCl concentration increased (Fig. 2 and Table 1). At the nearly physiological KCl concentration of 150 mm, the Kd value was 26 μm.
FIGURE 2.
Salt concentration dependence of the GIRKCP-spermine interaction. A, binding isotherms at different concentrations of KCl for the GIRKCP-spermine interaction. B, plot of the dissociation constant value (Kd) against the KCl concentration.
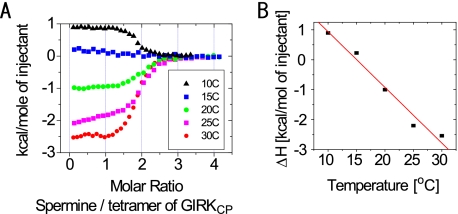
In addition, the heat capacity change upon binding (ΔCp), i.e. the temperature dependence of the enthalpy change (ΔH), was evaluated by performing the ITC experiment at various temperatures (Fig. 3). At the KCl concentration of 50 mm, ΔCp was −0.19 kcal/mol K. The negative ΔCp value suggests that desolvation from the surface of GIRKCP and/or spermine occurs upon binding.
FIGURE 3.
Temperature dependence of the GIRKCP-spermine interaction. A, binding isotherms at different temperatures for the GIRKCP-spermine interaction. B, plot of the enthalpy change (ΔH) against the experimental temperature yielded a ΔCp value of −0.19 kcal/mol·K as the slope of the fitted line.
Residues Proximal to Spermine Based on Intermolecular NOEs
The recently reported crystal structure of the cytoplasmic pore of Kir2.1 as well as the site-directed mutagenesis identified four acidic residues (Glu-224, Asp-255, Asp-259, and Glu-299) in the intracellular region that influence inward rectification (9). Two of the corresponding residues are conserved in Kir3.1/GIRK1 (Asp-260 and Glu-300). Although the mutations of these residues impaired the inward rectification in Kir2.1 (9, 13, 14), the residues that directly bind remained unknown. To identify the spermine-interacting residues in Kir3.1/GIRK1, we obtained intermolecular NOEs between GIRKCP and spermine, which in principle are observed between two protons existing within 5 Å.
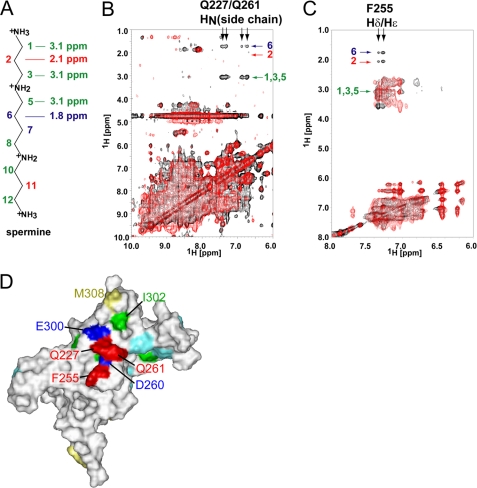
The proton resonances from spermine were assigned by the combination of NOESY and DQF-COSY experiments. The protons at positions 2 and 6 resonate at 2.1 and 1.7 ppm, respectively, and the signals from positions 1, 3, and 5 are overlapped at 3.1 ppm (Fig. 4A). Only one set of NMR signals for spermine was observed. When the spermine concentration was successively increased in the presence of a constant amount of GIRKCP, the chemical shift values for the spermine NMR signals gradually shifted up to those in the free-state, indicating that spermine undergoes fast exchange on the NMR time scale between the protein-bound state and the free state. Therefore, we observed the transferred NOE signals as follows, using a sample containing 5 equivalent amount of spermine per GIRKCP tetramer, in which the molar ratio of the bound spermine and free spermine is 2:3.
FIGURE 4.
Intermolecular NOE signals between GIRKCP and spermine. A, chemical structure of spermine, labeled the positions of the methylene groups and their chemical shifts. The chemical shift values are shown for positions 1–3 and 5–6, due to the symmetry of the molecule. B, overlay of the two-dimensional 15N-edited NOESY HSQC spectra for uniformly 2H,15N-labeled GIRKCP in complex with spermine. The spectra for wild type GIRK-spermine and that for the mutant Q227A/Q261A-spermine are shown in black and red, respectively. The assignments of the intermolecular NOE signals are indicated. C, overlay of the NOESY spectra for phenylalanine-selective 1H,15N-labeled GIRKCP in a 2H-background in complex with spermine. The spectra for wild type GIRK-spermine and that for the mutant F255A-spermine are shown in black and red, respectively. The assignments of the intermolecular NOE signals are indicated. D, mapping of the residues with the intermolecular NOEs. The residues with the intermolecular NOEs, Gln-227, Phe-255, and Gln-261 are mapped in red on a surface representation of one of the subunits in the GIRKCP tetramer, which is viewed from the inside of the pore. The amino acid selectively 1H-labeled residues, Ile, Met, and Phe (except for Phe-255), which were used to survey the intermolecular NOE with spermine, are colored green, yellow, and cyan, respectively. The positions of Asp-260 and Glu-300 are also indicated in blue.
The 15N-edited NOESY HSQC spectrum for uniformly 2H,15N-labeled GIRKCP mixed with a saturating amount of spermine exhibited only a few NOE signals between the spermine protons at positions 1/3/5 and 6 and the side chain amide protons of two of the Asn and/or Gln residues (Fig. 4B, black). The absence of an NOE at position 2 strongly suggests that the NOE signals at 3.1 ppm are probably from position 5, and not from 1 or 3. A closer look at the crystal structure of GIRKCP revealed that there are only two Gln residues (Gln-227 and Gln-261) and no Asn residue among the residues exposed to the cytoplasmic pore. We prepared three mutants (Q227A, Q261A, and Q227A/Q261A), and confirmed their binding affinities for GIRKCP by ITC (Table 2). We then performed 15N-edited NOESY HSQC experiments under the same conditions as those for the wild-type GIRKCP. The intermolecular NOE signals for only one Gln residue were observed for the mutants, Q227A and Q261A (supplemental Fig. S2), while no intermolecular NOE signal was observed for the Q227A/Q261A mutant (Fig. 4B, red). Meanwhile, the ITC experiment indicated that the spermine binding affinity was essentially unchanged by the mutation of Q227A/Q261A (Table 2). Thus, the intermolecular NOEs were assigned between the side chain amide protons of Gln-227 and Gln-261 and the spermine protons at positions 5 and 6. No other NOE signals between the amide protons of the protein and spermine were observed.
Furthermore, we explored the intermolecular NOEs with hydrophobic residues in the vicinity of Gln-227 and Gln-261, which are located between Asp-260 and Glu-300. We prepared the protein samples with Phe, Ile, or Met-selective 1H,15N-labeling in a 2H-background, since Phe-255 and Ile-302 are proximal to Asp-260 and Glu-300, respectively, and Met-308 exists at the exit of the cytoplasmic pore on the membrane side (see Fig. 4D). While no intermolecular NOEs were observed for Ile- or Met-selectively 1H-labeled GIRKCP, the Phe-selectively labeled protein showed NOE peaks between the aromatic protons of a phenylalanine residue and positions 1/3/5, 2 and 6 of spermine (Fig. 4C, black). These NOEs were assigned by the fact that the F255A mutant did not exhibit any intermolecular NOEs from the aromatic proton of the mutants (Fig. 4C, red), while the mutant retained the spermine binding affinity (Table 2).
In summary, Gln-227, Gln-261, and Phe-255 were identified to exist in the proximity (within 5 Å) of the bound spermine molecules. The side chain amide protons of Gln-227 and Gln-261 are close to positions 5 and 6 of the spermine protons, while the aromatic protons of Phe-255 are close to all of the protons of the spermine methylene groups (Fig. 4D).
Spermine Binding Induces a Conformational Rearrangement of the Cytoplasmic Pore of Kir3.1/GIRK1
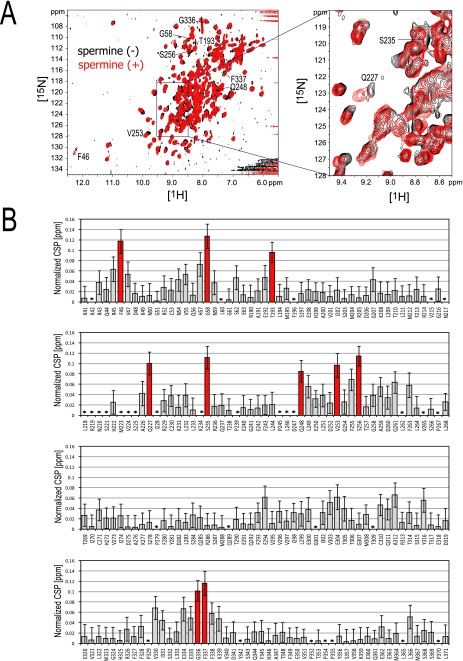
The spermine binding effect on GIRKCP was also investigated by CSP, which reflects the direct spermine-binding as well as the conformational change of the protein. A number of the 1H-15N TROSY signals for the backbone amide groups of GIRKCP changed their chemical shifts upon spermine binding. Fig. 5A shows the spectral overlay of the TROSY spectra in the absence and presence of spermine, and a plot of the normalized CSP values (Δδ) against the residue numbers of GIRKCP. The residues with significant CSPs (Δδ ≥ 0.08 ppm) are Phe-46, Gly-58, Thr-193, Gln-227, Ser-235, Gln-248, Val-253, Ser-256, Gly-336, and Phe-337 (Fig. 5B). The mapping of these residues on the structures (Fig. 5, panels C and D) indicates that Gln-227, Val-253, and Ser-256 exist at the inner pore site, where the intermolecular NOEs were observed (residues Gln-227, Phe-255, and Gln-261), and that the rest of the perturbed residues are located at the subunit-subunit interface of the tetramer, which is distal to the spermine binding site (Fig. 5D). In particular, Phe-46 and Gln-248 form hydrogen bonds with the Glu-294 and Phe-263 of the adjacent subunit, respectively, in the crystal structure of the spermine-free form of the GIRKCP (PDB code: 1N9P). Therefore, the CSPs for the residues at the subunit-subunit interface should be interpreted as the rearrangement of the relative orientations of the subunits.
FIGURE 5.
Chemical shift perturbation of the backbone NMR signals of GIRKCP upon spermine binding. A, overlay of the TROSY spectra in the presence (red) and absence (black) of spermine. The samples contain 100 μm GIRKCP tetramer and 200 μm spermine (red), or 137 μm GIRKCP (black), respectively. The signals with CSPs larger than 0.08 ppm are labeled. B, CSP upon spermine binding. The x and y axes depict the residues of GIRKCP and the normalized CSP values: {(Δδ1H)2 + (Δδ15N/6.5)2}0.5 (25). The CSPs larger than 0.08 ppm are indicated as red bars. The error bars were calculated based on the digital resolution. Asterisks indicate the residues with no data. C, residues with significant CSPs in B are colored red, with their names on the surface of the single subunit of the crystal structure (PDB code: 1N9P), viewed from the center of the tetramer pore (left) and from the outside (right). Because the coordinates of Gly-58 are missing in the PDB file, Gly-58 was not able to be mapped on the structure. D, mapping of the residues with CSPs (red) on the two adjacent subunits in the GIRKCP tetramer (white and cyan). The residues on the cyan and white subunits are labeled with and without a prime (′). Adjacent residues in other subunits, which are proximal to the residues with CSPs, are shown in parentheses with dotted lines, where double and triple primes indicate the different subunits. The dotted circle depicts the spermine binding site.
Effects of the Mutation of Conserved Acidic Residues on the Spermine Binding and the Conformation of GIRKCP
Asp-260 and Asp-275, respectively, exist at the center and the exit on the intracellular side of the pore and lack intersubunit contacts, while Glu-250 and Glu-300 are located at the subunit-subunit interface. To evaluate the contribution of the two acidic residues, Asp-260 and Glu-300, to the spermine binding, the mutants that neutralize their negative charge (D260N and E300N) were characterized by ITC and NMR. In addition, we investigated two other acidic residues, Glu-250 and Asp-275, using the E250N and D275N mutants.
ITC experiments revealed no heat exchange upon spermine injections to E250N, D260N, or E300N (data not shown). The affinity of D275N for spermine was essentially identical to that of the wild-type protein (Table 2).
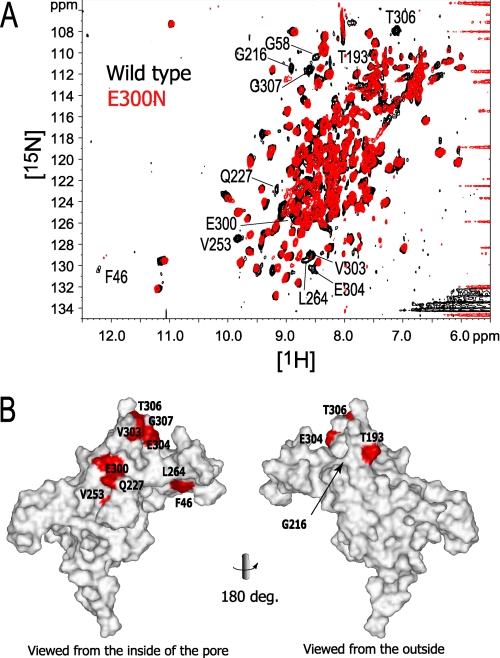
The chemical shift differences between these mutants and the wild type were then investigated by 1H-15N TROSY spectra. The Asn mutations of Glu-250 and Glu-300, which are located at the subunit-subunit interface, generated TROSY spectra different from that of the wild type. The spectral overlay between the E300N mutant and the wild type is shown in Fig. 6. Eleven signals were shifted by the E300N mutation to the extent that they are not overlapped with the corresponding signals from the wild type protein: Phe-46, Gly-58, Thr-193, Gly-216, Gln-227, Val-253, E300N, Val-303, Glu-304, Thr-306, and Gly-307 (Fig. 6). These residues are located in the region around Glu-300 to the subunit-subunit interface, and the upper part (membrane side) of the protein including G-loop (residues 303–307). The E250N mutant also showed a large spectral difference (data not shown). Because Glu-250 and Glu-300 are involved in direct contacts between two subunits, the wide distribution of the residues affected by the mutations indicates that the relative orientation of the subunits was altered by these mutations.
FIGURE 6.
The effect of the E300N mutation on the chemical shift. A, The 1H-15N TROSY signals of the E300N mutant that do not overlap with the corresponding signals from the wild-type protein are labeled. B, mapping of the affected residues on the surface of a subunit, viewed from the center of the tetramer pore (left) and from the outside (right). The buried residue (Gly-216) is indicated with an arrow. Because the coordinates of Gly-58 are missing in the PDB file, they were not able to be mapped on the structure.
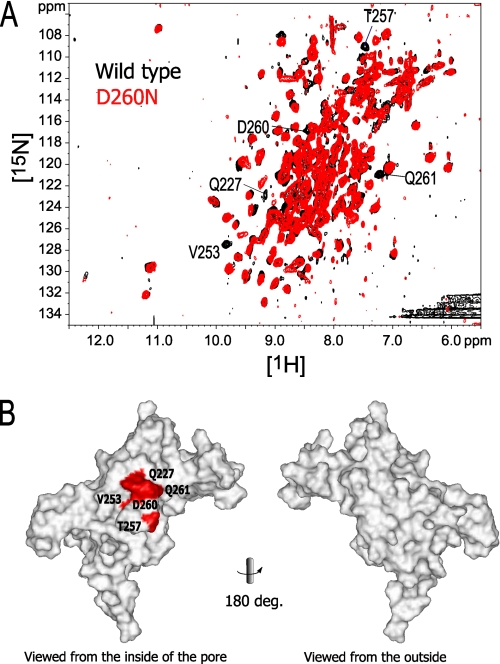
On the other hand, the charge-neutralizing mutation of Asp-260, which does not contact any atom of the other subunit, introduced chemical shift changes to the NMR signals from the following residues: Gln-227, Val-253, Thr-257, D260N, and Gln-261 (Fig. 7). The five affected residues are all adjacent to Asp-260, indicating that the effect of the D260N mutation is local effect and does not lead to the relative orientation of the subunits.
FIGURE 7.
The effect of the D260N mutation on the chemical shift. A, 1H-15N TROSY signals with chemical shift change larger than the signal line width between the wild type and D260N are labeled. B, mapping of the affected residues on the surface of a subunit, viewed from the center of the tetramer pore (left) and from the outside (right). Buried residues are indicated with an arrow. Because the coordinates of Gly-58 are missing in the PDB file, Gly-58 was not able to be mapped on the structure.
The spectral difference of the D275N mutant from the wild type was limited to Asp-275 and its neighborhood (data not shown). The CSP upon the addition of spermine to D275N was similar to that of the wild type. Considering the result that the spermine binding affinity of D275N is comparable to that of the wild type (Table 2), Asp-275 is involved in neither the direct binding to spermine nor the conformational rearrangement upon spermine binding.
DISCUSSION
Plasticity in the Relative Subunit Orientation of the GIRKCP Tetramer
The ITC results indicated that two spermine molecules bind to a tetramer of GIRKCP, with a dissociation constant (Kd) value of 26 μm at the KCl concentration of 150 mm. The KCl concentration dependence of the Kd values (Fig. 2) shows the significant contribution of the electrostatic interaction to the binding between the positive charges of spermine and the negative charges of the acidic residues in GIRKCP.
Small but significant CSPs induced by the binding of spermine were observed for the residues at the subunit-subunit interface of the GIRKCP tetramer (Fig. 5). It should be noted that the extent of CSP was relatively small, which might be accounted for by the small structural alteration, or by the averaging of the CSP for the corresponding residues of four subunits of the GIRKCP tetramer. The binding stoichiometry of two molecules of spermine per a tetramer of GIRKCP (Fig. 1 and Table 1) strongly suggests that the tetramer is formed by a dimer of the spermine-bound dimers, producing two distinct types of the subunit-subunit interface: interfaces within and between the spermine-bound dimer. Because the observed CSP is the average of the corresponding residues, which may have different CSPs, in the fast exchange regime on the NMR timescale, it tends to be smaller than the typical CSPs for the conformational change. The residues with the small but significant CSPs distribute widely at the subunit-subunit interfaces, strongly suggesting that spermine binding altered the relative subunit orientation of the tetramer.
The change in the relative orientations of the subunits was also indicated by the NMR spectral differences introduced by the E300N mutation (Fig. 6). Because Glu-300 possesses the contacts with adjacent subunit, the E300N mutation might have caused the subunit rearrangement. These data consistently indicated that GIRKCP possesses the plasticity in the intersubunit orientation of the tetramer.
The plasticity was also suggested by the crystal structures of a Kir3.1-KirBac1.3 chimera, where two channels in the same crystal adopt different conformations through rigid body displacements of the subunits (10). Their relative orientations also differ from those in the previous structures of the cytoplasmic pore of Kir3.1/GIRK1 (7, 9). By focusing on Asp-260 in these structures, we noticed that the distances between the oxygen atoms of the Asp-260 carboxyl group from the adjacent subunit range from 9.3 Å (PDB code: 1N9P) to 11.3 Å (PDB code: 2QKS, molecule B).
The Spermine Binding Site on Kir3.1/GIRK1
To date, the results of electrophysiological studies using mutants of Kir2.1 (9, 13, 14) have suggested that the intracellular acidic residues in Kir3.1, Asp-260 and Glu-300, are critical residues for the inward rectification. Our ITC results also indicated that D260N and E300N do not bind to spermine. However, our NMR data consistently support that Asp-260, not Glu-300, interacts directly with spermine: (1) Intermolecular transferred NOE signals were observed for Gln-227, Gln-261, and Phe-255, which surround Asp-260 (Fig. 4), while no NOE signals with spermine were observed for the Ile or Met side chains, which are located in the proximity of Glu-300 (2). CSPs upon the addition of spermine were not observed for the site around Glu-300, but were found for the residues surrounding Asp-260 (Fig. 5D) (3). While the residues with remarkable chemical shift changes by the D260N mutation is limited to the proximity (Fig. 7), those by the E300N mutation are distributed widely at the subunit-subunit interface of the tetramer (Fig. 6). Therefore, the impaired spermine affinity of the E300N mutant might be due to the structural rearrangement of the four subunits.
Based on these results, we conclude that Asp-260, not Glu-300, is the negatively charged residue that is critical for the direct binding of spermine. This is also consistent with the recent electrostatics calculation report, indicating that Asp-260 is the strongest negatively charged contributor to the electrostatics of the cytoplasmic regions of Kir3.1 (26).
The aspartate residue corresponding to Asp-260 in Kir3.1/GIRK1 is highly conserved in other human Kirs except for Kir6; Kir2.1–2.4, 3.1–3.4, and 4.1, and is substituted by another acidic residue, glutamate, in Kir1.1 and 1.3 and 7.1. Therefore, the binding site identified here in Kir3.1/GIRK1 might exist in other Kirs. However, the numbers and positions of the acidic residues in the cytoplasmic pore differ among the Kirs, suggesting that alternative binding sites might be found in other Kirs.
The Spermine Binding Mode
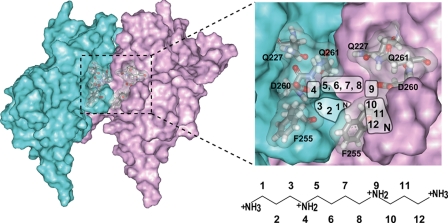
We have proposed the spermine binding mode to Kir3.1/GIRK1, based on the pattern of the intermolecular transferred NOEs, the binding stoichiometry, and the electrostatic interaction with Asp-260 (Fig. 8). It should be noted that the protein structure used for the schematic illustration in Fig. 8 represents the spermine-free state, which is different from the spermine-bound state. While all of the proton sites of spermine exhibited NOEs with the aromatic protons of Phe-255, only positions 5 and 6 of the spermine protons showed NOEs with the side chain amide protons of Gln-227 and Gln-261 (Fig. 4). Because the NOE signals from position 2 of spermine were observed only with Phe-255, and not with Gln-227/Gln-261, both termini of the spermine should be close to Phe-255 and away from Gln-227/Gln-261, while the central methylene groups at positions 5 and 6 (and the symmetrical positions 7 and 8) should point upward toward Gln-227/Gln-261. Because Asp-260 exists between Phe-255 and Gln-227/Gln-261, the positive charges on the nitrogen atoms of spermine at positions 4 and 9 interact with the negative charge of the Asp-260 side chain. The spermine binding stoichiometry of two per tetramer of GIRKCP, suggests that one spermine molecule bridges the two negative charges of the Asp-260 residues of adjacent subunits in the GIRK tetramer.
FIGURE 8.
The postulated spermine binding mode. Two adjacent subunits of the tetramer of GIRKCP are shown as the surfaces colored cyan and magenta, in which the spermine binding site is indicated by the dotted rectangle (left). The postulated areas for the spermine atoms in the binding site are illustrated (right). The residues identified in this study are labeled.
The Role of Spermine Binding to the Cytoplasmic Pore
As shown in Fig. 5, spermine binding changed the chemical shifts of the residues at the subunit-subunit interface, in addition to those of the direct binding site. This seems to be related to the spermine binding mode; a molecule of spermine bridges two Asp-260 side chains, stabilizing a certain orientation of the four subunits of the tetramer.
Kir3/GIRK is activated by the binding of G-protein βγ subunits (Gβγ) to the intracellular regions. FRET microscopy indicated that the intracellular regions of the GIRK1/GIRK4 channel exhibit conformational rearrangements upon Gβγ binding (22). Our data revealed that spermine binding stabilizes a certain intersubunit orientation, which is probably different from that of the Gβγ-bound state. Therefore, the spermine binding to the cytoplasmic pore might contribute to the stabilization of the resting state of the channel.
Conversely, the Gβγ-induced rearrangement of the four subunits of Kir3.1/GIRK1 upon its activation could disrupt the ability of spermine to bridge the two Asp-260 side chains, leading to impaired affinity for spermine. Therefore, the spermine binding mode revealed here suggests that spermine is dissociated from the cytoplasmic pore of the channel upon its activation. Because the spermine binding site is at the center of the cytoplasmic pore, the dissociation from the site would be beneficial for the access to the final binding site in the transmembrane region, to accomplish the blockade of the outward K+ current.
Conclusion
We have described here the affinity, stoichiometry, sites, and mode of spermine binding to the intracellular regions of Kir3.1/GIRK1. The direct binding site of spermine on the Kir channel was first identified, based on the distance information of the intermolecular NOEs. Importantly, spermine bridges two Asp-260 side chains, inducing a change in the relative orientations of the four subunits of the tetramer. Thus, spermine could be dissociated from the cytoplasmic pore upon channel activation by the Gβγ-induced conformational rearrangement. Spermine exerts the voltage-dependent blockade of the outward current, presumably in the transmembrane pore. The activation-dependent dissociation of spermine from the cytoplasmic pore seems to play a role in the rapid and efficient blockade of the transmembrane pore.
Supplementary Material
This work was supported in part by grants from the Japan New Energy and Industrial Technology Development Organization (NEDO) and the Ministry of Economy, Trade, and Industry (METI) (to I. S.), a grant-in-aid for Scientific Research on Priority Areas from the Japanese Ministry of Education, Culture, Sports, Science, and Technology (to M. O. and I. S.), and a grant from Takeda Science Foundation (to M. O.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2.
- Kir
- inwardly rectifying potassium channel
- Gβγ
- G-protein βγ-subunit
- GIRK
- G-protein gated inwardly rectifying potassium channel
- GIRKCP
- cytoplasmic pore domain of GIRK
- ITC
- isothermal titration calorimetry
- CSP
- chemical shift perturbation
- PDB
- Protein Data Bank.
REFERENCES
- 1.Bichet D., Haass F. A., Jan L. Y. (2003) Nat. Rev. Neurosci. 4, 957–967 [DOI] [PubMed] [Google Scholar]
- 2.Ficker E., Taglialatela M., Wible B. A., Henley C. M., Brown A. M. (1994) Science 266, 1068–1072 [DOI] [PubMed] [Google Scholar]
- 3.Lopatin A. N., Makhina E. N., Nichols C. G. (1994) Nature 372, 366–369 [DOI] [PubMed] [Google Scholar]
- 4.Lopatin A. N., Makhina E. N., Nichols C. G. (1995) J. Gen. Physiol. 106, 923–955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fakler B., Brändle U., Glowatzki E., Weidemann S., Zenner H. P., Ruppersberg J. P. (1995) Cell 80, 149–154 [DOI] [PubMed] [Google Scholar]
- 6.Stanfield P. R., Nakajima S., Nakajima Y. (2002) Rev. Physiol. Biochem. Pharmacol. 145, 47–179 [DOI] [PubMed] [Google Scholar]
- 7.Nishida M., MacKinnon R. (2002) Cell 111, 957–965 [DOI] [PubMed] [Google Scholar]
- 8.Kuo A., Gulbis J. M., Antcliff J. F., Rahman T., Lowe E. D., Zimmer J., Cuthbertson J., Ashcroft F. M., Ezaki T., Doyle D. A. (2003) Science 300, 1922–1926 [DOI] [PubMed] [Google Scholar]
- 9.Pegan S., Arrabit C., Zhou W., Kwiatkowski W., Collins A., Slesinger P. A., Choe S. (2005) Nat. Neurosci. 8, 279–287 [DOI] [PubMed] [Google Scholar]
- 10.Nishida M., Cadene M., Chait B. T., MacKinnon R. (2007) EMBO J. 26, 4005–4015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu Z., MacKinnon R. (1994) Nature 371, 243–246 [DOI] [PubMed] [Google Scholar]
- 12.Stanfield P. R., Davies N. W., Shelton P. A., Sutcliffe M. J., Khan I. A., Brammar W. J., Conley E. C. (1994) J. Physiol. 478, 1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang J., Jan Y. N., Jan L. Y. (1995) Neuron 14, 1047–1054 [DOI] [PubMed] [Google Scholar]
- 14.Kubo Y., Murata Y. (2001) J. Physiol. 531, 645–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xie L. H., John S. A., Weiss J. N. (2003) J. Physiol. 550, 67–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo D., Lu Z. (2003) J. Gen. Physiol. 122, 485–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo D., Ramu Y., Klem A. M., Lu Z. (2003) J. Gen. Physiol. 121, 261–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fujiwara Y., Kubo Y. (2006) J. Gen. Physiol. 127, 401–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kurata H. T., Cheng W. W., Arrabit C., Slesinger P. A., Nichols C. G. (2007) J. Gen. Physiol. 130, 145–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frassineti C., Alderighi L., Gans P., Sabatini A., Vacca A., Ghelli S. (2003) Anal. Bioanal. Chem. 376, 1041–1052 [DOI] [PubMed] [Google Scholar]
- 21.Kurata H. T., Phillips L. R., Rose T., Loussouarn G., Herlitze S., Fritzenschaft H., Enkvetchakul D., Nichols C. G., Baukrowitz T. (2004) J. Gen. Physiol. 124, 541–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Riven I., Kalmanzon E., Segev L., Reuveny E. (2003) Neuron 38, 225–235 [DOI] [PubMed] [Google Scholar]
- 23.Yokogawa M., Muramatsu T., Takeuchi K., Osawa M., Shimada I. (2009) Biomol. NMR Assign. 3, 125–128 [DOI] [PubMed] [Google Scholar]
- 24.Wiseman T., Williston S., Brandts J. F., Lin L. N. (1989) Anal. Biochem. 179, 131–137 [DOI] [PubMed] [Google Scholar]
- 25.Mulder F. A., Schipper D., Bott R., Boelens R. (1999) J. Mol. Biol. 292, 111–123 [DOI] [PubMed] [Google Scholar]
- 26.Robertson J. L., Palmer L. G., Roux B. (2008) J. Gen. Physiol. 132, 613–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.