Abstract
IGF-I is expressed in virtually every tissue of the body, but with much higher expression in the liver than in any other tissue. Studies using mice with liver-specific IGF-I knockout have demonstrated that liver-derived IGF-I, constituting a major part of circulating IGF-I, is an important endocrine factor involved in a variety of physiological and pathological processes. Detailed studies comparing the impact of liver-derived IGF-I and local bone-derived IGF-I demonstrate that both sources of IGF-I can stimulate longitudinal bone growth. We propose here that liver-derived circulating IGF-I and local bone-derived IGF-I to some extent have overlapping growth-promoting effects and might have the capacity to replace each other (= redundancy) in the maintenance of normal longitudinal bone growth. Importantly, and in contrast to the regulation of longitudinal bone growth, locally derived IGF-I cannot replace (= lack of redundancy) liver-derived IGF-I for the regulation of a large number of other parameters including GH secretion, cortical bone mass, kidney size, prostate size, peripheral vascular resistance, spatial memory, sodium retention, insulin sensitivity, liver size, sexually dimorphic liver functions, and progression of some tumors. It is clear that a major role of liver-derived IGF-I is to regulate GH secretion and that some, but not all, of the phenotypes in the liver-specific IGF-I knockout mice are indirect, mediated via the elevated GH levels. All of the described multiple endocrine effects of liver-derived IGF-I should be considered in the development of possible novel treatment strategies aimed at increasing or reducing endocrine IGF-I activity.
I. Introduction
- II. Liver-Derived IGF-I and Longitudinal Bone Growth
- A. Background, including the original somatomedin hypothesis
- B. Tissue-specific manipulation of IGF-I expression
- C. Target cells for GH and IGF-I in the growth plate
- D. Human genetic disorders and skeletal growth
- E. Mechanism of action for GH and IGF-I in the regulation of bone growth—an update
- III. Effects of Liver-Derived vs. Locally Produced IGF-I on Bone Mass
- A. In vivo and in vitro studies on IGF-I expression and action in bone
- B. Transgenic overexpression in bone
- C. Conditional knockout in bone
- D. Liver-derived IGF-I and bone mass
- IV. Liver-Derived IGF-I and GH Secretion
- A. Pulsatile GH secretion
- B. Negative feedback of liver-derived IGF-I on GH secretion
- C. Liver-derived IGF-I and sexually dimorphic effects of GH
- D. Human studies
- V. The Role of Liver-Derived IGF-I for Metabolism and Body Composition
- A. Carbohydrate metabolism
- B. Fat mass
- C. Lipid metabolism
- VI. Liver-Derived IGF-I and Other Tissues
- A. Brain
- B. Cardiovascular system
- C. Kidney
- D. Liver
- E. Prostate
- VII. Clinical Implications
- A. Indications for the use of IGF-I and adverse effects
- B. Potential indications for combined GH and IGF-I treatment
- C. Diabetes mellitus
- D. Other potential future indications
VIII. Summary: The Role of Liver-Derived IGF-I
IX. General Conclusions
I. Introduction
This review examines the physiological role of liver-produced IGF-I, a controversial topic addressed or touched upon in several earlier articles in Endocrine Reviews (1,2,3,4,5). We aim to summarize present knowledge regarding the role of liver-derived IGF-I in different organ systems, focusing on information that has appeared in the literature since the previous review in 2001 (4).
Although there are several biological effects of IGF-I, they essentially are related to either its status as a growth factor or the structural similarity between the IGF-I and insulin systems. Comparisons of the chemical structures of IGF-I and proinsulin show large (about 40%) amino acid sequence homology. The type I IGF receptor (subsequently referred to as the IGF-I receptor), which mediates the biological effects of IGF-I, has a 60% amino acid sequence homology with the insulin receptor (5,6). Indeed, the IGF-I receptor and insulin receptor can form heterotetramers consisting of one α- and one β-chain from the IGF-I receptor complex and one α- and one β-chain from the insulin receptor (5,6). The secretion of both IGF-I and insulin is stimulated by food intake and inhibited by fasting, a major biological similarity between these systems. This review covers the metabolic effects of liver IGF-I on carbohydrate and fat metabolism as well as its possible effects on body fat accumulation.
In addition to its metabolic and partly insulin-like effects, IGF-I is a growth factor and a prime mediator of the growth-promoting effects of GH. This review discusses the role of liver-derived IGF-I for the anabolic and growth-promoting effect of GH, and especially considers the relation between hepatic and extrahepatic IGF-I production. We also discuss the origin and effects of circulating IGF-I and describe the interaction between IGF-I and GH in the classic negative feedback loop formed by the hypothalamus-pituitary and liver, in relation to the well-known pulsatility of GH secretion (7).
The effect of IGF-I is modulated by its association with six IGF binding proteins (IGFBPs) (8,9). IGFBPs regulate the biological actions of IGF-I (and also IGF-II) in several ways. They transport IGFs from the circulation to peripheral tissues (e.g., IGFBP-1, -2, and -4), maintain a reservoir of IGFs in the circulation (mainly IGFBP-3), potentiate or inhibit IGF action, and may also exert IGF-independent effects (9). Furthermore, in addition to binary complexes with IGFBPs in biological fluids, IGF-I is sequestered into ternary complexes of one molecule each of IGF-I, IGFBP-3 (or IGFBP-5), and an acid-labile subunit (ALS) (10). The present review discusses mouse models with knockout (KO) of liver-derived or locally produced IGF-I as well as models with KO of liver-derived IGF-I combined with KO of total ALS and/or total IGFBP-3.
There are some limitations on the content of this review. We do not provide a detailed discussion of the effects of IGF-I on development and prenatal growth or the effects of the IGF system on tumor growth and metastasis, a topic recently described in Endocrine Reviews (5). Furthermore, we do not discuss the effects of IGF-II in detail because it is mainly important in prenatal and tumor growth (although it is produced in large quantities in humans, but not rodents, after birth) (5). In the mouse models used to investigate the effects of liver-derived IGF-I, no evidence suggests compensatory effects by IGF-II (11,12). However, IGF-II may be of importance for kidney growth during adulthood (see Section VI.C).
II. Liver-Derived IGF-I and Longitudinal Bone Growth
A. Background, including the original somatomedin hypothesis
Although several hormones and nutritional factors participate importantly in normal postnatal longitudinal bone growth, it is generally accepted that GH is the most important hormone in this respect (3,13,14,15). However, GH is not required for normal intrauterine growth, a finding supported by evidence that GH deficiency and/or GH insensitivity do not associate with reduced birth size (15,16,17,18). Mouse models with GH deficiency or GH insensitivity suggest that the major effect of GH on postnatal growth does not occur until after 2 wk of age (15,19,20). In contrast, KO studies in mice and case reports of patients with inactivating mutations of IGF-I or the IGF-I receptor have established clearly that IGF-I is a major regulator of intrauterine growth (19,21,22,23,24,25). Evidence that birth weight and size associate with polymorphisms in the IGF-I promoter region further supports a role of IGF-I for intrauterine growth (26,27,28). Importantly, the stimulatory role of IGF-I on intrauterine growth is independent of GH. An important role of IGF-I in body growth is supported by evidence that low serum IGF-I associates with low body weight in dogs (29,30), and a single IGF-I allele is a major determinant of small size in dogs (31).
A widely discussed question involves whether the stimulatory effect of GH on postnatal growth acts directly on tissues or is mediated by a liver-derived growth factor, initially called sulfation factor but later renamed somatomedin and subsequently shown to be identical to IGF-I. According to the original somatomedin hypothesis by Daughaday et al. (1,32,33), GH stimulates skeletal growth by stimulating liver production of IGF-I, which in turn stimulates longitudinal bone growth in an endocrine manner (Fig. 1A).
Figure 1.
Hypotheses of GH-mediated regulation of postnatal longitudinal bone growth. The different hypotheses of the mode of action for GH on longitudinal bone growth are described in detail in the text of Section II. A, Hypothesis proposed 1950–1980; B, hypothesis proposed 1980–2000; and C, currently proposed hypothesis.
During the 1980s and 1990s, the original somatomedin hypothesis was challenged by some key findings. The first experiments suggesting a role of locally produced IGF-I came from studies of D’Ercole et al. (34,35), who showed that multiple tissues produce IGF-I and that GH treatment of hypophysectomized rats increased IGF-I content of many organs. Several studies have confirmed a stimulatory role of GH on IGF-I expression in multiple nonhepatic tissues (36,37,38,39,40). Consistent with the idea that GH may mediate its effects in part by increasing local production of IGF-I, we demonstrated that local injection of human GH directly into the cartilage growth plates of the hind limbs of hypophysectomized rats produced significantly increased length of injected limbs compared with the control, contralateral limbs (41). Other investigators have confirmed our results, clearly establishing a direct effect in the growth plate region of GH on bone growth (2,42,43,44,45,46). Furthermore, the findings that simultaneous infusion of IGF-I antiserum and GH blocked the local effect of GH on cartilage growth (47) and that local administration of GH stimulated linear growth of hypophysectomized rats and GH-deficient mice with little or no increase in serum IGF-I provided indirect evidence that GH’s effect on cartilage growth may be mediated in part via increased local IGF-I production and/or action (2,3,46). In addition, the original somatomedin hypothesis was challenged by the finding that treatment of GH-deficient patients with GH resulted in a better growth response than treating GH-insensitive patients with recombinant human IGF-I (rhIGF-I) (48,49). These results indicated that GH has local effects that may be independent of increased levels of the circulating “endocrine” form of IGF-I, thereby introducing an alternative to the original somatomedin hypothesis. We proposed that the local effect of GH included both an effect that could not be replaced by IGF-I and an effect mediated by increased local IGF-I (Fig. 1B) (2,3). In addition, although believed to be of minor importance, a role for circulating liver-derived IGF-I could not be excluded (Fig. 1B). Importantly, until a decade ago, techniques were not available to evaluate the relative importance of liver-derived endocrine IGF-I vs. locally produced IGF-I for the regulation of bone growth.
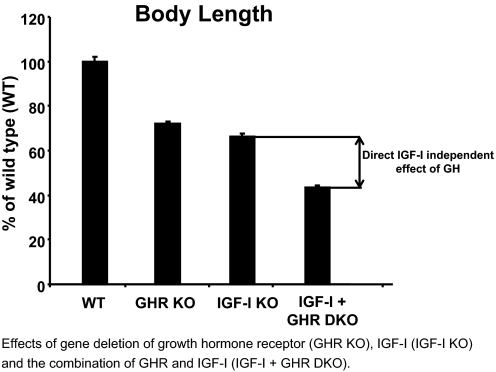
An important study by Lupu et al. (19) established an IGF-I-independent effect of GH on body growth. They compared the body length of mice with GH receptor inactivation (GHR KO), IGF-I inactivation (IGF-I KO) and IGF-I GHR double inactivation (IGF-I + GHR DKO; Fig. 2). Importantly, GHR-IGF-I double KO mice had a more severe reduction in body length than IGF-I KO mice, demonstrating that GH exerts IGF-I-independent effects on body growth (Fig. 2). The authors concluded that GH and IGF-I promote postnatal growth by both independent and common functions because the growth retardation of double GHR-IGF-I KO is more severe than that observed with either class of single mutants (19). Although it is clear that IGF-I is the major regulator of intrauterine growth, our comparison of GH-deficient and total IGF-I KO mice revealed that GH contributed more to longitudinal bone growth than IGF-I during postpubertal growth (50).
Figure 2.
GH exerts direct effects not dependent on IGF-I on body length. Comparison of body length in GH receptor KO (GHR KO), IGF-I KO, IGF-I/GHR double KO (IGF-I + GHR DKO), and wild-type (WT) mice at 130 d of age. The figure is adapted from Table 3 in Lupu et al. (19). Importantly, these data demonstrate that IGF-I and GH exert at least partly independent and additive stimulatory effects on body length. Values are given as percentage of WT and are means ± sem.
B. Tissue-specific manipulation of IGF-I expression
Several elegant mouse models with tissue-specific manipulation of IGF-I expression have been developed and characterized during the last 10 yr to evaluate the relative importance of liver-derived endocrine IGF-I vs. locally produced IGF-I for the regulation of bone growth. The cre/loxP recombination system was used for all of these mouse models (51).
First, two mouse models with liver-specific IGF-I inactivation were developed in 1999 (Fig. 3B). Both models had exon 4 of the IGF-I gene completely inactivated in hepatocytes using either an albumin-Cre-mediated (12) or an inducible Mx-1-Cre-mediated (11) DNA excision. The growth phenotypes and most of the other phenotypes described during the last 10 yr (i.e., bone parameters, GH secretion, and carbohydrate metabolism) of these two mouse models with liver-specific IGF-I inactivation are rather similar, which we believe strengthens the validity of the findings.
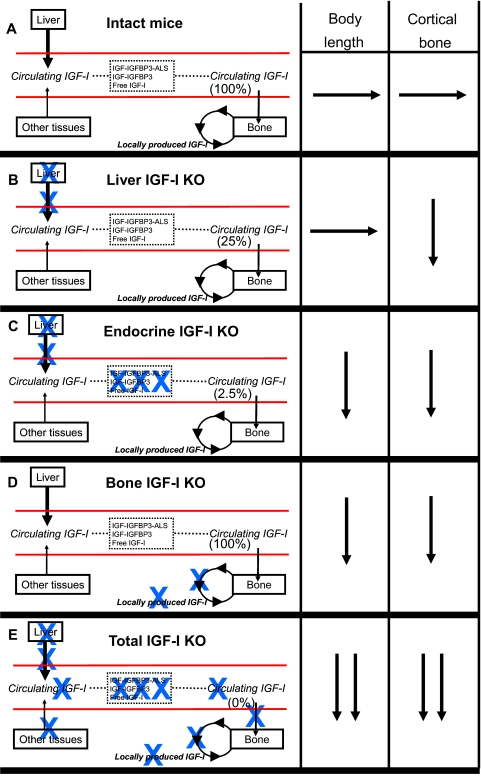
Figure 3.
The role of liver-derived IGF-I, the total pool of circulating endocrine IGF-I, and bone-derived IGF-I for body length and cortical bone mass. The bone length and the cortical bone mass in various IGF-I KO mouse models in relation to intact mice (A) and mice with total IGF-I inactivation (E, total IGF-KO) are summarized. The IGF-I KO models given in panels B–D are mice with liver-specific IGF-I inactivation (B, liver IGF-I KO); mice with dramatically reduced circulating endocrine IGF-I levels due to triple inactivation of liver IGF-I, total ALS, and total IGFBP-3 (C, endocrine IGF-I KO); and mice with bone-specific IGF-I inactivation (D, bone IGF-I KO). The total pool of circulating “endocrine” IGF-I in serum expressed as percentage of intact mice is given within parentheses for each mouse model. Blue X indicates inactivation/lack of this component. →, Unchanged compared with intact mice; ↓, reduced compared with intact mice but less reduced than in mice with total IGF-I KO; ↓↓, substantially reduced to the level seen in total IGF-I KO.
Second, to determine the role of bone-derived IGF-I for bone growth, we recently developed two different mouse models with inactivated IGF-I in chondrocytes and osteoblasts, respectively (52,53). These two conditional KO models disrupt local but not circulating endocrine IGF-I action (Fig. 3D).
Third, Stratikopoulos et al. (54) recently presented a complex but elegant model in which they reexpressed IGF-I specifically in the liver of mice with a totally inactivated IGF-I [i.e., liver IGF-I producer (LIP) mice]. The two mouse models with liver-specific IGF-I inactivation were aimed to determine whether liver-derived IGF-I is required for normal bone growth, whereas the experiments using the LIP mice were designed to determine whether liver-derived IGF-I has the capacity to stimulate bone growth in the absence of local bone-derived IGF-I and, therefore, should be regarded as complementary. Although each mouse model is informative, their growth phenotypes indicate that a full understanding of liver IGF-I action cannot be achieved by looking at only one model.
1. Lessons from liver-specific IGF-I inactivation (Fig. 3, B and C)
The role of liver-derived IGF-I for the regulation of longitudinal bone growth was evaluated, as described above, by using two different mouse models (11,12). Both mouse models demonstrated that inactivation of liver-derived IGF-I resulted in an approximately 75% reduction in serum IGF-I with essentially unaffected body length (Fig. 3B) (11,12). Based on these findings, it was proposed that GH stimulates longitudinal bone growth both via a direct effect not involving modulation of IGF-I and via local bone-derived IGF-I, but not liver-derived IGF-I (4,11,12,55). A recent finding showing that mice with liver-specific (using albumin-driven Cre) GH receptor inactivation have normal body length and tibial length despite substantially decreased serum IGF-I levels supports the hypothesis that GH-stimulated growth does not require GH stimulation of IGF-I synthesis in the liver (56).
Because GH levels increased due to lack of IGF-I-mediated negative feedback in the mouse models with liver-specific IGF-I inactivation (for further details, see Section IV and Fig. 4), one might speculate that the growth of these mice could be compensated by enhanced growth stimulatory effect of GH on nonhepatic tissues (4,11,12,55,57). As investigated in several nonhepatic tissues, however, IGF-I mRNA levels did not increase in the two mouse models with liver-specific IGF-I inactivation (11,12).
Figure 4.
Proposed model for hypothalamic-pituitary-liver feedback axis of GH secretion and how it is affected by liver-specific IGF-I deletion. Mice with liver-specific IGF-I KO (right panel) have increased GH secretion. Increased GH levels in turn enhance the liver weight. In male mice with depletion of liver-derived IGF-I, the enhanced GH trough levels feminize liver functions regulated by the sexual dimorphism of GH secretion in rodents. The mechanism by which lack of liver-derived IGF-I increases GH secretion seems to involve increased expression of GHRH and ghrelin receptors (right panel) and augmented responsiveness to these ligands at the level of the pituitary. There is no evidence that lack of liver-derived IGF-I enhances GH secretion via an effect on the hypothalamus, possibly because enhanced pituitary GH and local hypothalamic IGF-I secretion partly counteract the effects of lack of liver-derived IGF-I on the hypothalamus.
Another potential explanation for the lack of effect on body growth in mice with inactivated liver-derived IGF-I is that the remaining 25% of circulating IGF-I might be more bioavailable and, therefore, sufficient for skeletal growth because IGFBP-3 levels clearly decrease and ALS levels increase in these mice (11,12,58). In normal mice, 70–80% of IGF-I in the circulation exists in a ternary complex consisting of IGF-I, IGFBP-3, and ALS. This complex has a relatively long half-life (t1/2 = 10–16 h). A smaller complex (50 kDa), comprising IGF-I and serum IGFBPs (mainly IGFBP-3), comprises about 15–20% of the circulating pool; the remainder (<5%) is free IGF-I (7.5 kDa), with an extremely short half-life (59). Analyses of mouse models with inactivated ALS and/or IGFBP-3 demonstrated that the total pool of circulating IGF-I depends on the amount of circulating ALS and IGFBP-3, which contributes to the stability of circulating IGF-I (60,61). In addition, circulating IGFBPs (mainly IGFBP-3) might either reduce or enhance the biological activity of IGF-I in the target tissues (in this case, bone).
Yakar et al. (61,62) investigated body growth in mice with very low circulating IGF-I levels resulting from double KO of liver-derived IGF-I and total ALS (62) and of triple KO of liver-derived IGF-I, total ALS and total IGFBP-3 [= endocrine IGF-I KO, Fig. 3C, (61)]. Both mouse models have very low serum IGF-I levels (−97.5% in triple KO mice), most probably as a combined result of deficient IGF-I secretion from the liver and reduced stability of the remaining IGF-I in the circulation. In contrast to mouse models with isolated liver-specific IGF-I KO (Fig. 3B), both the double and triple KO mice displayed a modest but significant reduction in total body length (Fig. 3C) (61,62), clearly demonstrating that endocrine IGF-I is indeed essential for normal body growth. Based on a comparison between the essentially normal body length in mice with liver-specific IGF-I KO, with approximately 25% serum IGF-I remaining, and the reduced body length in the double and triple-inactivated mice, with 2–10% remaining serum IGF-I, we propose that a serum IGF-I threshold in the range of 10–25% of normal serum IGF-I exists, below which serum IGF-I levels associate with body growth (11,12,61,62). In contrast, normal body growth is seen above this serum IGF-I threshold.
Importantly, reduced body length in the triple KO mice (−6%) with extremely low circulating IGF-I levels is much lower compared with mice with total IGF-I inactivation (−40%; Table 1), suggesting that a substantial proportion of the body growth depends on local IGF-I expression. Although the mouse models with combined liver IGF-I KO and total ALS KO/IGFBP-3 KO, with extremely low serum IGF-I, have been very informative in elucidating the role of endocrine IGF-I, one must consider that both ALS and IGFBP-3 might exert effects other than contributing to the stability of circulating IGF-I. Although the liver is the principal source of circulating IGFBP-3 and ALS, others have reported expression of both IGFBP-3 and ALS in bone and other nonhepatic tissues (60,61,63). Furthermore, IGFBP-3 acts independently of IGF-I to regulate growth, apoptosis, and metabolism of target cells (64,65,66,67,68,69). Therefore, the expression of ALS and IGFBP-3 in nonhepatic tissues and possible IGF-I-independent effects of IGFBP-3 should be considered while interpreting findings using mice with combined liver IGF-I KO and ALS KO/IGFBP-3 KO.
Table 1.
Magnitude of skeletal changes in mice lacking total, local bone, liver-derived, and endocrine IGF-I actions
| Total IGF-I KOa | Local bone IGF-I KO
|
Liver IGF-I KOd | Endocrine IGF-I KOe | ||
|---|---|---|---|---|---|
| Chondrocyte IGF-I KOb | Osteoblast IGF-I KOc | ||||
| Serum IGF-I | 100% ↓ | Normal | Normal | 75% ↓ | 97.5% ↓ |
| Length | 40% ↓ | 7% ↓ | 14% ↓ | Normal | 6% ↓ |
| Cortical bone width | 38% ↓ | 7% ↓ | 20% ↓ | 9% ↓ | 18% ↓ |
| Cortical vBMD | 30% ↓ | 4% ↓ | 5% ↓ | 7% ↓ | 11% ↓ |
| Trabecular BV/TV | 55% ↑ | ND | 20% ↓f | 10–20% ↓ | 8% ↓f |
| Bone formation rate | 70% ↓ | ND | 48% ↓ | ND | ND |
| Mineral apposition rate | 40% ↓ | ND | 38% ↓ | ND | ND |
ND, Not determined; vBMD, volumetric BMD; BV/TV, bone volume/ total volume.
Data for length, bone width, and cortical vBMD were derived from Mohan et al. (50), whereas data for trabecular BV/TV, bone formation rate, and mineral apposition rate were derived from Bikle et al. (153).
Data for chondrocyte IGF-I KO mice were derived from Govoni et al. (52).
Data for osteoblast IGF-I KO mice were derived from Govoni et al. (53).
Data for liver-specific IGF-I KO are summaries of the main findings from several studies using mice with liver-specific IGF-I KO (11,12,58,61,62,155).
Data for length and bone width of endocrine IGF-I KO were derived from triple KO mice lacking liver-derived IGF-I, total IGFBP-3, and total ALS (61), whereas data for cortical vBMD and trabecular BV/TV were derived from double KO mice lacking liver-derived IGF-I and ALS (62).
Not significant.
2. Lessons from IGF-I inactivation in bone (bone IGF-I KO; Fig. 3D)
Several different types of cells in the bone microenvironment might contribute to local autocrine/paracrine produced IGF-I in bone. However, the relative contribution of IGF-I produced by various cell types has been unclear. To determine whether IGF-I produced locally by chondrocytes exerts a significant role in regulating bone growth, we used the Cre/loxP approach to disrupt IGF-I production in cells that express collagen type II α-1 chain because type II collagen is predominantly expressed in chondrocytes (= chondrocyte IGF-I KO) (52). In these studies, we found that conditional disruption of IGF-I in chondrocytes led to significant reductions in body length (−7%). Interestingly, postnatal gain in body length (between 4 and 12 wk of age) decreased by as much as 27%. Because disruption of IGF-I in type II collagen producing cells did not influence liver IGF-I expression or circulating levels of IGF-I, these data clearly establish a role for local chondrocyte-produced IGF-I in regulating bone growth. However, skeletal growth of the chondrocyte-specific IGF-I KO mice was clearly less affected compared with mice with total IGF-I inactivation (−40%; Table 1), demonstrating that IGF-I from either the circulation or from other cells in the bone microenvironment can maintain, at least partly, body growth in the absence of chondrocyte-derived IGF-I.
To further explore the role of autocrine/paracrine IGF-I for bone growth, we recently investigated the relative contribution of IGF-I produced by cells of osteoblastic lineage in regulating skeletal growth (53). Because both immature and mature osteoblasts express IGF-I, we used transgenic Cre mice in which Cre expression is driven by entire regulatory regions of the collagen type I α-2 chain for disruption of IGF-I gene in type I collagen producing mesenchymal cells (collagen type I α-2 IGF KO). IGF-I expression in bone and muscle but not liver and brain decreased significantly in the conditional mutants. Importantly, disruption of IGF-I in type I collagen-producing mesenchymal cells did not influence circulating levels of IGF-I, whereas body and femur length decreased by 14–15%, thus supporting the notion that local IGF-I is essential for normal bone growth. However, similar to the chondrocyte-specific IGF-I KO mice, skeletal growth was clearly less affected than in mice with total IGF-I inactivation (−40%; Table 1). These data indicate that local IGF-I from both chondrocytes and osteoblasts participates in the regulation of bone growth. If one assumes that deficient body length in mice with inactivated IGF-I in osteoblasts (−14%) is additive to that seen in mice with chondrocyte-specific IGF-I KO (−7%), combined inactivation would lead to 21% reduction in body length, more pronounced than that seen in mice with extremely low circulating endocrine IGF-I resulting from triple inactivation of liver-derived IGF-I, total ALS, and total IGFBP-3 (−6%; Table 1). However, validation of these theoretical calculations requires development of the combined osteoblast-chondrocyte IGF-I KO mouse model. Due to technical reasons, it remains complicated to inactivate IGF-I simultaneously in all different cell types in the bone microenvironment (not only including osteoblasts and chondrocytes) without affecting liver IGF-I expression, making it difficult to accurately determine the entire proportion of bone growth that is dependent on local IGF-I expression. Nevertheless, the two described mouse models with osteoblast/chondrocyte IGF-I KO with unaffected serum IGF-I levels have clearly established a role for local IGF-I in the regulation of longitudinal bone growth that cannot be replaced by endocrine IGF-I under normal physiological circumstances.
3. Lessons from reexpression of IGF-I specifically in the liver in mice with total IGF-I inactivation
The role of liver-derived IGF-I for bone growth was recently investigated further by the research group of Efstratiadis (54) using an advanced and elegant mouse model with reexpression of IGF-I specifically in the liver in mice with total IGF-I inactivation. They generated double transgenic mice carrying in an Igf1 null background, 1) a Igf1 cDNA placed downstream of a transcriptional “stop” DNA sequence flanked by loxP sites (floxed); and 2) a cre transgene driven by a liver-specific promoter (α-1 antitrypsin). The Igf1 cDNA, which was inserted by knockin into the mutated and inactive Igf1 locus itself to ensure proper transcriptional regulation, was expressed exclusively in the liver after Cre-mediated excision of the floxed block (= LIP mice).
The study demonstrated that approximately 30% of the body growth could be achieved by liver-specific IGF-I reexpression, whereas a substantial proportion of the body growth also required nonhepatic IGF-I expression. Thus, this study clearly demonstrates that liver-derived IGF-I can enhance bone growth in the absence of local IGF-I but not sufficiently for normal bone growth. Further research is required to elucidate whether the mechanisms for the effects of liver-derived IGF-I and local IGF-I on longitudinal bone growth differ. However, due to breeding difficulties, only three LIP mice were evaluated and, therefore, the absolute magnitude of the growth-promoting capacity of liver-derived IGF-I should be interpreted with caution. Future studies using similar methodology for the reexpression of IGF-I specifically in bone in mice with total IGF-I inactivation will be useful to determine the proportion of body growth that can be achieved by local bone-specific IGF-I expression in the absence of liver-derived IGF-I.
A capacity of pharmacologically elevated IGF-I expression in the liver to stimulate body growth in mice with normal endogenous IGF-I expression in nonhepatic tissues is demonstrated by evidence that mice with transthyretin-driven liver IGF-I overexpression, resulting in increased serum IGF-I levels (50–60%), display slightly but significantly increased body length (70). Thus, genetically elevated liver IGF-I expression results in increased body growth in mice with no IGF-I expression in nonhepatic tissues (54) and also in mice with normal IGF-I expression in nonhepatic tissues (70), demonstrating that liver-derived IGF-I has the capacity to stimulate body growth.
4. Conclusions regarding regulation of body growth from the studies using mouse models with tissue-specific manipulation of IGF-I
The experiments using different mouse models with tissue-specific IGF-I inactivation alone or in combination with total ALS and IGFBP-3 KO clearly demonstrate that both endocrine IGF-I (Fig. 3C) and bone-derived IGF-I (Fig. 3D) are required for normal longitudinal bone growth (52,53,61,62). Because the two mouse models with liver-specific IGF-I inactivation but with normal nonhepatic IGF-I expression have serum IGF-I levels above the threshold (<25% of serum IGF-I in intact mice) required for essentially normal body length, liver-derived IGF-I is not required for essentially normal body length in the presence of normal IGF-I expression in nonhepatic tissues (Fig. 3B) (11,12). However, as demonstrated in the LIP mouse model, liver-derived IGF-I clearly can increase longitudinal body growth in the absence of nonhepatic IGF-I expression (54). Furthermore, the longitudinal bone growth of both the mouse models with endocrine IGF-I inactivation and the mouse models with IGF-I inactivation in bone is clearly less severely affected compared with mice with total IGF-I inactivation. We propose that all of these findings can be explained, because endocrine IGF-I and local bone-derived IGF-I to some extent have overlapping growth-promoting effects and might partly but not completely have the capacity to replace each other (= redundancy) in the maintenance of normal longitudinal bone growth.
C. Target cells for GH and IGF-I in the growth plate
As described in Section II.A, it is clear that both GH and IGF-I exert a direct growth stimulatory effect in the growth plate region (3,41,42,45,47). However, the primary target cells for GH and IGF-I in the growth plate during postnatal growth remain unclear. Based on in vitro studies of cultured growth plate chondrocytes performed 20 yr ago, we proposed earlier that GH within the growth plate acts primarily on growth plate precursors in the resting zone, followed by an IGF-I-mediated clonal expansion of chondrocytes in the proliferative layer of the growth plate (2,3,71,72). However, we believe that the validity of our proposed mode of action remains unproven in animal studies in vivo. Some in vivo analyses have suggested that GH but not IGF-I can increase cell divisions within the resting zone of the growth plate and can also increase its size (73,74). An effect of GH specifically in the resting zone is supported by the recent in vivo finding that GH induced rapid STAT5 phosphorylation in the resting zone but not in the proliferative or hypertrophic zones of the growth plate (75). However, a study by Hunziker et al. (76) indicated that not only GH but also IGF-I can increase cell divisions in the resting zone to some extent. Furthermore, Lupu et al. (19) suggested that it is not yet proven that cells in the resting zone are the immediate precursors of proliferative chondrocytes. When analyzing the growth plates of total IGF-I-inactivated mice with elevated GH levels, Wang et al. (74,77) found that chondrocyte hypertrophy was affected, whereas chondrocyte numbers and proliferation were unaffected, suggesting that hypertrophic chondrocytes are important target cells for IGF-I. They also found that GH receptor-inactivated mice had a hypoplastic resting zone, whereas IGF-I-inactivated mice displayed an enlarged resting zone. Therefore, they proposed a dual role for GH in promoting longitudinal bone growth; an IGF-I-independent role in growth plate chondrocyte generation, and an IGF-I-dependent role in promoting chondrocyte hypertrophy (74). In contrast, Lupu et al. (19) found that both GH receptor-inactivated and IGF-I-inactivated mice had affected chondrocyte proliferation and that both IGF-I and the GH receptors are expressed in the proliferative layer of the growth plate, suggesting that proliferative chondrocytes are primary target cells for both GH and IGF-I. These conflicting in vivo results suggest that further studies are required to definitely identify the primary target cells for both GH and IGF-I in the growth plate region.
D. Human genetic disorders and skeletal growth
Several recent clinical case reports describing the growth phenotype of patients with genetic disorders affecting different GH/IGF-I related components have given important information about the effect of GH and IGF-I on skeletal growth (28). The growth phenotype of patients with genetic disorders, resulting in GH deficiency, GH insensitivity, IGF-I deficiency, IGF-I insensitivity, and ALS deficiency, are discussed below. In contrast, human mutations in any of the IGFBPs remain undescribed.
1. GH deficiency and GH insensitivity
Patients with genetic disorders resulting in GH deficiency (mutations in GH, GHRH receptor, Pit-1, or Prop-1) or GH insensitivity [mutations in GH receptor or signal transducers and activators of transcription protein 5b (STAT5b)] have normal birth size but a pronounced reduction of final height (18,78,79,80,81,82,83,84,85,86,87). Patients with GH insensitivity have low IGF-I levels and, due to a loss of negative feedback, increased GH secretion (see Section IV). Because GH signaling induces IGFBP-3, these patients also have reduced IGFBP-3 levels (84,85,86). Treatment of GH-deficient patients with GH results in a more pronounced postnatal growth response than treatment of GH-insensitive patients with IGF-I, supporting the notion that GH exerts IGF-I independent effects on body growth (48,49).
2. IGF deficiency as a result of IGF-I gene deletion/inactivating mutation
Woods et al. (25) first described a patient with a homozygous IGF-I gene deletion in 1996. Subsequently, a patient with inactivating mutation of IGF-I was presented by Walenkamp et al. (28,88), and a patient with a mutation resulting in altered amino acid sequence for the E domain of the IGF-I precursor associated with very low IGF-I levels was presented by Bonapace et al. (89). In these three patients, IGF-I deficiency resulted in severe intrauterine and postnatal growth retardation as well as sensorineural deafness, microcephaly, and mental retardation. Interestingly, IGF-I haploinsufficiency resulted in subtle inhibition of intrauterine and postnatal growth (88). IGF-I treatment resulted in increased linear bone growth in the first patient with a homozygous IGF-I gene deletion (90).
3. IGF-I insensitivity
Abuzzahab et al. (24) first presented a patient with heterozygous mutation of the IGF-I receptor, resulting in intrauterine and postnatal growth retardation, in 2003. Subsequently, several patients with heterozygous IGF-I receptor mutations and growth deficiency have been presented, but the magnitude of growth deficiency has varied from modest to severe, probably because the different described mutations resulted in variable degrees of remaining IGF-I signaling (24,91,92,93,94). Patients with IGF-I insensitivity are characterized by elevated IGF-I levels resulting from increased GH secretion due to a lack of IGF-I receptor-mediated negative GH feedback (28). In addition, they often have microcephaly. A gene dose effect of IGF-I receptor for human skeletal growth is supported by evidence that patients with IGF-I receptor haploinsufficiency resulting from terminal 15q deletion, including the IGF-I receptor, display intrauterine and postnatal growth failure (95,96). In addition, trisomy of terminal 15q, resulting in duplication of the IGF-I receptor, associates with increased height (97). Thus far, no case has been found with a homozygous IGF-I receptor mutation, and observations in mice suggest that this defect is lethal (22,94). In a patient with persistent postnatal growth retardation associated with haploinsufficiency of the IGF-I receptor due to a deletion of one copy of the gene, GH treatment yielded growth acceleration and resulted in normal adult height (96).
4. ALS deficiency
In 2004, Domené et al. (98,99,100) reported the first patient with a homozygous inactivating mutation of the ALS gene, followed by descriptions of several other cases of ALS deficiency (101,102,103). The first case had a minimal growth phenotype, but some later cases displayed a slightly more advanced growth phenotype (98,99,100,101,102,103). Importantly, follow-up of the first reported patient with ALS deficiency demonstrated normal growth spurt and final height (99). Because patients with ALS deficiency cannot form ternary complexes, they have very low IGF-I and IGFBP-3 levels, similar to those seen in ALS KO mice (60,98,99,100,101,102,103). The extremely low circulating IGF-I levels associated with only a modest reduction of growth rates and final height in patients with ALS deficiency support the hypothesis of peripheral IGF-I and direct GH action (but not endocrine IGF-I) as the main promoters of longitudinal bone growth (103). Actually, the circulating IGF-I levels in patients with total ALS deficiency are comparable to the levels found in classical GH deficiency and GH insensitivity, conditions that cause much more severe growth deficiency than ALS deficiency (99).
In conclusion, human genetic disorders causing IGF-I insensitivity and total IGF-I deficiency associate with reduced intrauterine and postnatal growth, whereas GH deficiency and GH insensitivity specifically result in severe postnatal growth deficiency. The growth deficiency in patients with total IGF-I deficiency is severe, whereas patients with partial IGF-I resistance due to heterozygous mutations in the IGF-I receptor gene have a variable but often moderate growth deficiency (102). Patients with total ALS deficiency show modest growth deficiency that is clearly less pronounced compared with patients having total IGF-I deficiency and generally is less severe compared with patients having IGF-I receptor mutations. Compared with patients with total IGF-I deficiency, the much less pronounced growth deficiency in patients with total ALS deficiency, associated with extremely low circulating IGF-I levels, supports the hypothesis that bone-derived IGF-I can maintain an essentially normal postnatal longitudinal bone growth in situations with severe deficiency of endocrine IGF-I.
E. Mechanism of action for GH and IGF-I in the regulation of bone growth—an update
Figure 1C summarizes our current proposed mechanism of action for GH- and IGF-I-mediated regulation of longitudinal bone growth. Compared with previous hypotheses (Fig. 1, A and B), the proposed modifications/similarities are based mainly on new data presented after 2001 including: 1) the finding that double GH receptor/IGF-I KO mice have a more severe reduction in bone length than IGF-I KO mice clearly establishes a direct effect of GH not dependent on IGF-I (Fig. 1Ci) (19); 2) a key role of local bone-derived IGF-I for bone growth is established by the finding that mouse models with IGF-I inactivation in bone (52,53), but with normal circulating IGF-I levels, display reduced longitudinal bone growth (Fig. 1Cii); and 3) a role of circulating endocrine IGF-I was demonstrated by the result that triple KO mice, devoid of the major part of the circulating endocrine IGF-I, displayed reduced bone length and by the result that reexpression of IGF-I in the liver in total IGF-I-deficient mice resulted in growth stimulation (Fig. 1Ciii) (54,61).
Some previously proposed models for the mode of action of GH and IGF-I on longitudinal bone growth (Fig. 1, A and B) have considered modulation of liver IGF-I synthesis as identical to circulating endocrine IGF-I. Recent advances in the field have clearly shown that besides liver IGF-I secretion, the serum levels of ALS and IGFBP-3 have to be considered because these are of importance for the stability of IGF-I (Fig. 1C). Two independent mouse models with no remaining liver IGF-I expression showed that 75% of serum IGF-I is liver-derived, suggesting that 25% of circulating IGF-I derives from nonhepatic tissues; therefore, this nonhepatic contribution to circulating IGF-I levels should be considered as well. However, further studies are required to determine the exact origin and contribution of non-liver-derived IGF-I to serum IGF-I levels. Thus, the GH effect mediated via the circulating endocrine pool of IGF-I (Figs. 1C and 3C) is complex and might include modulation of: 1) liver IGF-I production; 2) IGF-I secretion from nonhepatic tissues to the circulation; and 3) the amount of ALS/IGFBP-3 in the circulation. Loss of one of these components (e.g., in single liver-specific IGF-I KO or total ALS KO mice) does not essentially affect body length (11,12,60). However, when both liver-derived IGF-I and total ALS are inactivated, the remaining circulating IGF-I has a very short half-life, and the nonhepatic tissues consequently lack the capacity to maintain the total circulating IGF-I pool above a threshold level (around 10–25% of intact mice) required for normal longitudinal bone growth (61).
In conclusion, it is clear that GH stimulates longitudinal bone growth by both IGF-I-independent mechanisms (Fig. 1Ci) and IGF-I-dependent mechanisms (Fig. 1C, ii and iii). We propose that endocrine IGF-I (Fig. 1Ciii) and local bone-derived IGF-I (Fig. 1Cii) have some overlapping growth-promoting effects and can partly (= redundancy) but not completely replace each other in the maintenance of normal longitudinal bone growth.
III. Effects of Liver-Derived vs. Locally Produced IGF-I on Bone Mass
In addition to IGF-I from the circulation (see Section III.D), IGFs are available to skeletal tissues through de novo synthesis by osteoblasts and osteoclasts and also by release of stored IGFs from bone matrix during osteoclastic bone resorption (104,105,106,107,108). This section discusses the relative importance of bone-derived vs. liver-derived IGF-I for bone mass.
A. In vivo and in vitro studies on IGF-I expression and action in bone
We and others have shown that IGFs are the most abundant growth factors produced by bone cells and stored in bone matrix (104,105,106,107,108,109). The finding that 40–50% of basal osteoblast cell proliferation can be blocked by inhibiting the actions of endogenously produced IGFs in serum-free cultures of osteoblasts in vitro provides evidence that locally produced IGFs contribute importantly to basal bone cell proliferation (110,111). In addition, osteoblast production of IGF-I is known to be regulated by agents that influence bone formation. For example, osteoblasts contain GH receptors, and GH treatment increases production of IGF-I in osteoblasts (3,112,113). The finding that the growth-promoting effects of GH on osteoblasts can be abolished by coincubation with IGF-I-neutralizing antibodies attests to the importance of IGF-I in mediating GH effects in osteoblasts (114). Thus, the effects of GH not only on growth plate cartilage but also on bone may involve local production and/or actions of IGF-I.
In vitro studies demonstrate that the regulatory effects of many systemic hormones (e.g., PTH, thyroid hormone, glucocorticoids) and local effectors (bone morphogenetic proteins, mechanical strain) may mediate their effects on osteoblasts in part by controlling local production of IGFs (105,107,115,116,117,118,119). For example, PTH treatment increases both proliferation and matrix production in serum-free cultures of bone cells (120,121,122). Findings that PTH treatment also increases IGF-I expression and that biological effects of PTH on osteoblasts in vitro can be blocked by the addition of IGF-I-neutralizing antibodies suggest that PTH effects on osteoblasts may involve increased production and/or actions of IGF-I (123,124). Accordingly, two independent studies have provided direct evidence that the anabolic effects of PTH on bone formation in vivo require IGF-I action in growing mice (125,126,127). Another study demonstrated that PTH administration increased bone density in the femur and vertebra of wild-type mice without altering the serum IGF-I levels and that the skeletal anabolic effects were lost in mice lacking insulin receptor substrate-1, a signal downstream of the IGF-I receptor (128). Thus, these data provide convincing evidence for a role of locally produced IGF-I in mediating anabolic effects of PTH on bone. However, using several different mouse strains with liver-specific IGF-I inactivation, global deletion of ALS, and both liver-specific IGF-I and total ALS inactivated genes, it was found that the PTH response on trabecular bone was genotype dependent (129).
Another important mediator of skeletal growth is thyroid hormone (130,131,132). Huang et al. (133) demonstrated that exogenous addition of T3 increased indices of osteoblast cell activity in serum-free cultures and that neutralization of IGF-I action blocked the biological effects of T3 on osteoblasts, thus suggesting that T3 effects on bone may in part depend on local production of IGF-I in bone. In contrast to T3, glucocorticoids exert negative effects on the skeleton by inhibiting bone formation and stimulating bone resorption (107,134,135,136,137,138,139,140,141). Studies in several laboratories have shown that glucocorticoid actions on bone formation parameters involve regulation of production of IGF-I and/or their binding proteins both in vitro and in vivo (107,134,135,136,137,138,139,140,141).
Mechanical strain is the only true negative feedback system regulating bone formation, and evidence suggests that mechanical strain’s ability to increase bone formation is mediated, at least in part, by locally produced IGFs (107,125). Compared with contralateral unloaded bone, mechanical loading resulted in rapid induction of IGF-I mRNA levels in the loaded limb within 4 h (142). Consistent with these data, a single 10-min episode of mechanical stimulation increased IGF-I expression in osteocytes of loaded bone within 6 h (143). In contrast to loading that increases IGF-I expression and bone formation, skeletal unloading yielded decreased proliferation of osteoblasts and their progenitors resulting from skeletal unloading-induced inhibition of the IGF-I signaling pathway. In terms of the mechanism by which mechanical strain and IGF-I interact to regulate osteoblast proliferation, studies have shown that IGF-I and mechanical strain interact synergistically to increase IGF-I receptor phosphorylation in an integrin-dependent manner involving recruitment of SH domain containing protein tyrosine phosphatase (SHP1) and/or SHP2 to IGF-I receptors and inhibition of SHP-mediated IGF-I receptor phosphorylation (144,145,146).
In conclusion, there is considerable experimental evidence to support a role for locally produced IGF-I in mediating the effects of systemic regulators on the bone formation process, and mechanical strain and IGF-I interact to regulate osteoblast proliferation.
B. Transgenic overexpression in bone
If locally produced IGFs are important regulators of bone formation, modulation of local IGF expression in bone should lead to corresponding changes in the bone formation process. Indeed, the following transgenic studies support a role for locally produced IGF-I in mediating bone formation changes (Table 2). To examine the influence of GH on bone deposition, Saban et al. (147) generated lines of transgenic mice expressing the GH gene driven by β-globin regulatory elements. GH synthesis in the transgenic lines has been shown to be erythroid-specific by RNAse protection experiments and by in situ hybridization of proximal tibia. Findings that serum levels of GH did not differ significantly in transgenic lines compared with control mice suggested that local but not systemic levels of GH increased in transgenic mice. Bone mineral density (BMD) was 30–40% higher in transgenic mice compared with control mice. Accordingly, histological cross-sections of tibia showed that adult transgenic mice had 20–45% increased cortical thickness compared with their controls. These findings suggest that GH released from erythroid cells increases bone deposition in part by stimulating local production of IGF-I in the bone marrow. In another study, Zhao et al. (148) created transgenic mice that overexpressed IGF-I specifically in mature osteoblasts by driving transgene expression using osteocalcin-specific promoter. In these studies, overexpression of IGF-I in its normal paracrine environment increased cancellous bone volume as determined by histomorphometric analyses. Consistent with these data, Jiang et al. (149) demonstrated that transgenic overexpression of IGF-I using 3.6 kb of 5′ upstream regulatory sequence and most of the first intron of the rat Col1a1 gene resulted in transgenic calvaria that were wider and had greater marrow and bone areas. These transgenic overexpression studies indicated that increased expression of IGF-I in cells of osteoblastic lineage or in bone microenvironment increased indices of bone formation presumably via increased autocrine/paracrine actions of locally produced IGF-I.
Table 2.
Genetically altered mouse models to evaluate local IGF-I actions in bone
| Model | Promoter used | IGF-I alteration | Skeletal phenotype | Ref. |
|---|---|---|---|---|
| hGH transgenic | β-globin | Overexpression in erythroid tissue | Increased bone density and cortical thickness | 147 |
| IGF-I transgenic | Human osteocalcin | Overexpression in mature osteoblasts | Increased bone formation rate, trabecular and cortical bone volume | 148 |
| IGF-I transgenic | 3.6-kb rat collagen 1α1 | Overexpression in immature and mature osteoblasts | Increased femur length, cortical width and cross- sectional area; increased calvarial thickness | 149 |
| PAPP-A transgenic | 2.3-kb rat collagen 1α1 | Increased free IGF in bone microenvironment | Increased calvarial thickness and BMD; increased size of long bones | 151 |
| Chondrocyte IGF-I KO | 3.0-kb mouse collagen 2α1 | IGF-I disruption in chondrocytes | Decreased length, bone size, and BMD | 52 |
| Osteoblast IGF-I KO | Entire mouse collagen 1α2 gene | IGF-I disruption in cells of osteoblast lineage | Decreased length, bone size, and BMD | 53 |
PAPP-A, An IGFBP-specific protease; hGH, human GH.
If locally produced IGFs in bone exert significant biological effects on cells of osteoblastic lineage, then inhibition of IGF action in the bone microenvironment should lead to inhibition of bone formation. To evaluate this prediction, Zhang et al. (150) produced transgenic mice with targeted expression of inhibitory IGFBP-4 using a human osteocalcin promoter to direct transgene expression specifically in osteoblasts. Their results showed that several indices related to bone formation decreased in the transgenic mice. Although the transgenic mice were of normal size and weight at birth, they exhibited striking growth retardation postnatally. In addition to the anticipated reduction in bone weights, there was also a modest reduction in the weight of other organs, raising the possibility that osteoblast-produced IGFBP-4 could have inhibited IGF actions in other tissues via paracrine and/or endocrine manner. In a recent study, Qin et al. (151) evaluated the consequence of transgenic overexpression of PAPP-A, an IGFBP-specific protease, on bone metabolism in mice and determined that overexpression of PAPP-A in osteoblasts using 3.6 kb of 5′ upstream regulatory sequence and most of the first intron of the rat type I collagen α1 gene produced anabolic effects on bone in mice. PAPP-A overexpression was found to increase IGF bioavailability by inducing proteolysis of inhibitory IGFBPs in bone microenvironment. Thus, the transgenic studies involving modulation of IGF action via increasing expression of IGFBP-4 or its protease, PAPP-A, exert opposite effects on bone formation, suggesting that locally produced IGF-I participates in bone formation.
In conclusion, several different transgenic overexpression studies affecting local IGF-I levels or bioavailability support a role of bone-derived IGF-I for bone formation.
C. Conditional knockout in bone
Although the transgenic studies provide evidence for the participation of locally produced IGF-I in regulating bone formation, one caveat with transgenic approaches is that the levels expressed by transgenic overexpression often are too high to represent normal physiological conditions. To overcome this drawback, the Cre/loxP approach has been used to specifically disrupt IGF-I or its receptor in bone cell types. Zhang et al. (152) generated osteoblast-specific KO of the IGF-I receptor gene by crossing IGF-I receptor loxP mice with transgenic mice in which Cre expression was driven by the human osteocalcin promoter. The mice carrying osteoblast-specific disruption of the IGF-I receptor were of normal size and weight but demonstrated a 24% decrease in cancellous bone volume at the distal femur caused by decreased trabecular number and increased trabecular separation as measured by micro-computed tomography. Histomorphometric analyses of bone formation indices at the epiphysis revealed significant decreases in bone formation rate, mineral apposition rate, and osteoblast number at 3 wk of age. The rate of osteoid mineralization decreased significantly in the mutant mice compared with control littermate mice. These findings suggest that IGF-I is essential for coupling matrix biosynthesis to sustained mineralization, particularly during pubertal growth spurt.
In contrast, neither cortical bone volume nor cortical thickness was significantly affected in the mutant mice. The lack of significant cortical bone phenotype in this mouse model can be explained by the fact that the osteocalcin promoter, used to drive Cre expression in this study, disrupts IGF-I receptor expression in terminally differentiated mature osteoblasts only. Because IGF-I is expressed and acts on stromal cells as well as on immature and mature osteoblasts, it is not surprising that the observed skeletal changes in mice in which IGF-I receptor is disrupted only in mature osteoblasts is less pronounced compared with mice with conditional disruption of IGF-I in both immature and mature osteoblasts (see below, collagen type I α2 IGF-I KO mice) (53).
Because disruption of the IGF-I receptor in osteoblasts would eliminate not only the actions of locally produced IGF-I but also endocrine IGF-I, this model did not distinguish the role of endocrine vs. local IGF-I in regulating bone formation in vivo.
We recently found that conditional disruption of IGF-I in chondrocytes resulted not only in reduced bone length (see Section II.B) but also in reduced total body areal BMD (−5%) and bone width (−7%) in the femur and vertebrae (Table 1). Expression levels of PTHrP, Dlx-5, and Sox-9 decreased by 30–40% in the conditional mutants, suggesting that IGF-I produced by chondrocytes may regulate longitudinal growth and bone width, in part via regulating expression of one or more messenger molecules involved in chondrocyte proliferation and/or differentiation. Because disruption of IGF-I in type II collagen-producing cells did not influence circulating levels of IGF-I, these data establish a role for chondrocyte-produced IGF-I in regulating not only longitudinal bone growth but also bone width and bone mass accrual.
In addition, we investigated the relative contribution of IGF-I produced by cells of osteoblastic lineage in regulating bone mass accrual and bone metabolism (53). For these experiments, we used collagen type I α2 IGF-I KO mice (described in Section II.B), which have significantly reduced IGF-I expression in bone but normal levels of circulating IGF-I. Importantly, total body (−13%), femur (−41%), and vertebrae (−16%) areal BMD as measured by dual-energy x-ray absorptiometry decreased significantly in the mutant mice. Peripheral quantitative computer tomography analyses demonstrated a reduced cortical bone mass in both femur and vertebra. Histomorphometric studies revealed significant decreases in bone formation rate and mineral apposition rate in the conditional mutants, thus suggesting that loss of local IGF-I resulted in impaired differentiation and/or function of osteoblasts (Table 1). Three independent studies have now shown that disruption of the IGF-I receptor or IGF-I gene in osteoblasts resulted in a significant deficit in the mineralization rate (50,152,153,154).
IGF-I effect on bone formation is dependent on the number and activity of osteoblasts. The finding that mineral apposition rate was significantly compromised in total IGF-I KO and osteoblast-specific IGF-I KO mice (Table 1) suggests that locally derived IGF-I contributes predominantly to osteoblast activity.
In conclusion, the studies using mice with conditional KO of IGF-I or the IGF-I receptor in osteoblasts, support the notion that locally produced IGF-I is critical for optimal bone development and subsequent mineralization.
D. Liver-derived IGF-I and bone mass
1. Cortical bone
To evaluate the relative contribution of endocrine IGF-I action in bone mass accrual, mouse models with disruption of liver-derived IGF-I (11,12), total IGFBP-3 (61), and total ALS (60,62) have been used in the past. As described in detail in Section II, the two mouse models with liver-specific IGF-I inactivation had largely unaffected bone length. In contrast, both mouse models displayed a significant reduction in cortical bone mass (58,62), clearly establishing a role of liver-derived IGF-I for cortical bone mass (Fig. 3B and Table 1). However, the magnitude of the cortical bone deficit in these mice was less pronounced than that seen in mice with total inactivation of IGF-I (50). To rule out the possibility that the remaining 25% of circulating IGF-I in mice with liver-specific IGF-I KO is more readily bioavailable and thus sufficient for most of the bone mass accrual, Yakar et al. (61) generated triple KO mice lacking liver-specific expression of IGF-I, total ALS, and total IGFBP-3 (see Section II), resulting in a more than 97% reduction of total serum IGF-I levels (Table 1). Although the triple KO mice also exhibited a significant deficit in bone mass accrual, mainly resulting from reduced cortical bone mass, it was still less severe than that seen in mice with total IGF-I KO (Table 1). Thus, both liver-derived IGF-I and local IGF-I are required for normal accrual of cortical bone. Importantly, because both cortical bone mass and longitudinal bone growth were affected in the endocrine IGF-I KO mice (2.5% remaining serum IGF-I; Fig. 3C), whereas cortical bone mass but not longitudinal bone growth was affected in mice with liver-specific IGF-I inactivation (25% remaining serum IGF-I; Fig. 3B), we propose that the serum IGF-I threshold below which serum IGF-I influences cortical bone mass (>25% of normal IGF-I) is higher than the corresponding threshold for longitudinal bone growth (<25% of normal IGF-I).
2. Trabecular bone
Trabecular bone analysis by micro-computed tomography revealed a small but not statistically significant decrease in bone volume/total volume both in mice lacking endocrine IGF-I and in mice devoid of osteoblast-derived IGF-I. Similar analyses in mice with liver-specific IGF-I KO demonstrated a slight reduction of bone volume/total volume, which reached statistical significance in one study (155) but not in another study (61). In contrast, bone volume/total volume was 55% higher in mice with total disruption of IGF-I (Table 1). The reason for the differences in trabecular bone volume between total and osteoblast IGF-I KO remains undetermined. The complex role of IGF-I for trabecular bone is further illustrated by the results that mice with liver-specific IGF-I KO were protected from ovariectomy-induced trabecular bone loss (155).
In conclusion, comparisons of skeletal deficits of mouse models lacking total, liver-derived, endocrine, and bone IGF-I reveal the following:
a. Although disruption of endocrine or local IGF-I clearly resulted in reduced cortical bone mass, the magnitude of skeletal changes in mice lacking either of these IGF-I actions does not reach the severity seen in the total IGF-I KO mice. The mechanisms behind the more pronounced bone deficit in total IGF-I KO mice than in mice with endocrine or bone IGF-I KO might, in addition to lack of direct IGF-I effects on bone, include inadequate nutrition or altered serum levels of hormones such as vitamin D, PTH, and sex steroids. We have shown that serum levels of 1,25-dihydroxyvitamin-D were significantly reduced in the total IGF-I KO mice (154). Accordingly, serum calcium levels were slightly reduced in the total IGF-I KO mice, resulting in increased serum PTH levels. The elevated PTH levels in total IGF-I KO mice could be rescued by correcting IGF-I deficiency in these mice. Furthermore, a low calcium diet fed to these mice increased bone resorption and reduced bone formation to a greater extent than in wild-type mice. Because bone formation and bone resorption were uncoupled in total IGF-I KO mice, bone accretion took place at a reduced rate during the pubertal growth period. These data are consistent with the idea that lack of IGF-I could influence bone mass accrual in part via inducing changes in the PTH-vitamin D-calcium axis.
b. The serum IGF-I threshold (>25% of normal IGF-I) below which serum IGF-I influences cortical bone mass is higher than the corresponding threshold (<25% of normal IGF-I) for longitudinal bone growth. In general, it seems as if liver-derived/endocrine IGF-I affects cortical bone mass more than longitudinal bone growth (Table 1 and Fig. 3). In contrast, local IGF-I seems equally important for cortical bone and longitudinal bone growth (Fig. 3). Future studies to determine the mechanisms by which endocrine and local IGF-I regulate skeletal growth and potential interactions between these mechanisms will provide a better understanding of the mechanism of IGF-I action in bone.
IV. Liver-Derived IGF-I and GH secretion
Studies using mice with liver-specific IGF-I inactivation demonstrate that depletion of liver-derived IGF-I enhances GH secretion from the pituitary (57). This section reviews the importance of liver-derived IGF-I for the regulation of spontaneous pulsatile GH secretory pattern, especially in terms of the sexual dimorphism of the GH secretory pattern. Furthermore, the physiological importance of changes in GH secretory pattern induced by deficiency of liver-derived IGF-I will be discussed.
A. Pulsatile GH secretion
1. Biological effects of sexually dimorphic pulsatile GH secretion
GH secretion is pulsatile in rodents as well as humans. In rodents, there is also a clear sexual dimorphism (7,156,157). Male rats have episodic bursts of GH secretion and low GH levels between pulses, and female rats have higher basal interpulse GH levels and more frequent but lower amplitude pulses (157). The sexual dimorphism of GH secretion is best characterized in rats, from which repeated blood samples can be obtained to analyze the GH secretory pattern, but available results indicate similar gender differences in mice (7,158). The GH secretion pattern in turn regulates several sexually dimorphic liver functions, including expression of P450 enzymes (159). Another liver function regulated by the GH secretory pattern relates to the number of TGFα/epidermal growth factor (EGF) receptors induced by a pulsatile plasma GH pattern in male rats (160,161,162,163). GH-regulated sexually dimorphic liver functions also include expression of major urinary protein (MUP) and prolactin receptors (7,164).
The secretory pattern of GH is important for body growth in rats. A certain daily dose of GH induces larger body growth if it is given in a pulsatile manner than if it is given continuously (7,165). A pulsatile GH secretory pattern induces IGF-I expression more effectively in rib growth plate and skeletal muscle, whereas there is no clear effect by GH pulsatility on liver IGF-I expression or serum IGF-I levels (36,166).
2. Regulation of pulsatile GH secretion
GH secretion from the pituitary is regulated by two major hypothalamic peptides: GHRH, which induces GH secretion; and somatostatin, which inhibits GH secretion. Low basal GH levels between pulses in male rats probably result from suppression of GH by surges of hypothalamic somatostatin, which may inhibit release of hypothalamic GHRH as well as its action on the pituitary (Fig. 4, left panel) (7,167,168,169). Activation of the ghrelin receptor by treatment with analogs of the stomach-produced hormone ghrelin induces GH secretion (170,171), involving direct stimulation at the pituitary level (172) as well as stimulation of GHRH release and possibly also inhibition of somatostatin release at the hypothalamic level (173,174,175). However, endogenous ghrelin may have little effect on GH secretion in mice, given that body growth and serum IGF-I levels are largely unaffected in ghrelin and ghrelin receptor KO mice (176,177). This is in marked contrast to the profound effects of disruption of the GHRH system on GH secretion and body growth in mice (178,179). On the other hand, selective lack of ghrelin receptor constitutive signaling in humans, due to ghrelin receptor mutations, may lead to a syndrome characterized by short stature (180). Furthermore, the ghrelin analog MK-677 has been shown to be effective in enhancing serum IGF-I levels in humans (181,182). In conclusion, GHRH and somatostatin regulate GH secretion in humans and experimental animals, whereas endogenous ghrelin may be more important for growth promotion in humans than in rodents.
An additional level of regulation of GH secretion derives from the fact that GH can inhibit its own secretion via a short loop feedback effect at the hypothalamic level (183). The negative feedback effect of a spontaneous GH pulse in a male rat lasts about 3 h, causing the following GH trough (184,185). Moreover, this effect seems to be dependent on the fact that each GH pulse with a certain time delay induces a somatostatin surge from the hypothalamus during the next GH trough (Fig. 5) (184,185). Therefore, a dynamic interaction between the hypothalamus and the pituitary, and not only a biological clock function in the hypothalamus itself, could be of importance for the regular GH secretory pattern in male rats. This assumption also seems to be in line with mathematical predictions of how the GH secretory pattern is regulated (186).
Figure 5.
Both hypothalamic somatostatin and liver-derived IGF-I reduce basal pituitary GH secretion. Proposed model is shown for the regulation of basal GH release from the pituitary by both intermittent somatostatin release from the hypothalamus and continuous IGF-I release from the liver in male rodents. The GH secretion in male rodents is intermittent, with low basal levels between pulses. In the normal situation (left panels), the low basal GH levels are due to suppression of GH release from the pituitary by pulses of hypothalamic somatostatin (upper left) that coincide with the low basal GH levels (middle left). In addition, basal GH levels are suppressed by continuous release of liver-derived IGF-I (lower left). Loss of either hypothalamic somatostatin (somatostatin depletion; central panels) or liver-derived IGF-I (liver IGF-I depletion; right panels) causes enhanced basal GH levels. Therefore, the effects of both somatostatin and IGF-I seem necessary to maintain low GH trough levels and thereby the masculinizing effect of pulsatile GH secretion in rodents. Arrows depict the effect of a GH pulse to initiate the somatostatin pulse during the next coming GH trough. Therefore, GH can inhibit its own secretion via a short loop feedback effect at the hypothalamic level, suggesting that the pulsatility of GH secretion is due to a reciprocal interplay between the hypothalamus and the pituitary and is not only due to an intrinsic rhythm of the hypothalamus itself.
Sexual dimorphism in rodents seems to be regulated by estrogen secretion in adult females and by androgen secretion neonatally and during adulthood in males. Essentially, estrogen increases and androgen decreases basal GH levels. These effects seem to be mediated by changes in hypothalamic release of somatostatin and GHRH (7,187).
B. Negative feedback of liver-derived IGF-I on GH secretion
Specific depletion of liver-derived IGF-I causes a compensatory general increase in serum GH levels in mice (Fig. 4, right panel) (11,12). This finding indicates that endogenous IGF-I secretion from the liver tonically suppresses GH secretion from the pituitary and concurs with earlier studies that pharmacological treatment with IGF preparations suppresses GH secretion (188,189).
Depletion of liver-derived IGF-I in male mice causes a feminization of some of the GH-regulated sexually dimorphic markers of liver functions (see Section IV.A), demonstrated by decreased levels of liver-derived MUP in urine and increased expression of prolactin receptors in the liver in male mice with liver-specific IGF-I KO (Fig. 4) (57). Due to the small blood volume in mice, it is difficult to measure the GH secretion pattern, but the indirect measurements of MUP and prolactin receptors indicate that the increased GH secretion in mice with liver-specific IGF-I KO results partly from more continuous GH secretion with increased baseline levels of GH. Thus, liver-derived IGF-I may tonically suppress basal GH secretion in male rodents and contribute to masculinization of various liver functions (Figs. 4 and 5). Both male and female mice with liver-specific IGF-I KO had increased relative liver weights (Fig. 4) (57), probably due to increased GH levels (see Section VI.D).
To investigate the mechanism behind the increased GH levels in liver IGF-I-deficient mice, the mRNA levels of several regulatory factors that govern GH secretion were measured in the hypothalamus and the pituitary. Liver IGF-I-deficient mice showed increased expression of the receptors for GHRH and ghrelin/GH secretagogue (GHS) in the pituitary (Fig. 4). In accord with this finding, a GHRH antagonistic effect of IGF-I has earlier been demonstrated in rat pituitary cells in vitro (188). An inhibitory effect of IGF-I on GHRH receptor mRNA levels has also been reported in vivo after IGF-I replacement to the GH-deficient spontaneous dwarf rat (190). The increased pituitary GHRH and ghrelin/GHS mRNA levels in mice with liver-specific IGF-I depletion associated with increased responsiveness to systemically injected GHRH and ghrelin analog/GHS (57), suggesting an increase in the number of bioactive receptors in these animals (Fig. 4). As discussed above (see Section IV.A), endogenous GHRH likely is more important than endogenous ghrelin in stimulating GH secretion in mice (176,177,178,179), and the inhibition of GHRH effects by IGF-I may, therefore, be more important than that of ghrelin for GH secretion in rodents.
Depletion of liver-derived IGF-I or systemic IGF-I treatment to GH-deficient rats did not affect expression of hypothalamic GHRH, somatostatin, or neuropeptide Y, arguing against a negative feedback effect by endogenous liver-derived IGF-I at the hypothalamic level (57,190). It has been reported that IGF-I can decrease hypothalamic GHRH expression and enhance somatostatin expression when given intracerebroventricularly, but not systemically (191). It was suggested that those results reflect a possible physiological effect by local IGF-I expression in the hypothalamus on GH secretion. Moreover, intracerebroventricularly administered IGF-I, at least when given in conjunction with IGF-II, seems to suppress GH secretion via effects on the central nervous system (192). IGF-I has been shown to have other effects in line with a regulation of GH secretion at the hypothalamic level, e.g., enhanced somatostatin release in vitro (7,169). However, these findings probably have implications for effects exerted by local hypothalamic IGF-I rather than by endogenous liver-derived IGF-I. In the liver-specific IGF-I KO mice, Wallenius et al. (57) observed a slight, probably GH-dependent, compensatory increase in hypothalamic IGF-I expression that partly might have reversed the effect of liver-derived IGF-I deficiency on GH secretion (Fig. 4).
In summary, loss of the feedback effect exerted by liver-derived IGF-I on the hypothalamic pituitary system results in increased GH secretion, including elevated baseline GH levels between pulses and increased expression and responsiveness of pituitary GHRH and ghrelin receptors. Therefore, the major site of action of endogenous liver-derived IGF-I in the regulation of GH secretion seems to be at the pituitary rather than at the hypothalamic level.
C. Liver-derived IGF-I and sexually dimorphic effects of GH
1. Liver
During the last decade, several studies have clarified the mechanisms mediating the effects of GH secretion on sexually dimorphic liver functions. The latent cytoplasmic transcription factor STAT5b is activated and translocated to the nucleus in rat liver in response to male pulsatile GH stimulation, but much less so after continuous GH treatment (193). Moreover, results from experiments with STAT5b KO mice indicate that STAT5b is responsible for the masculinization of the male liver (194,195). The finding that liver-specific IGF-I KO feminizes GH-regulated liver functions (Fig. 4) (57) could be regarded as support for a mediator role of IGF-I in masculinization, as previously suggested (164). On the other hand, there are findings that do not support this hypothesis. For example, there was no difference in hepatic IGF-I mRNA expression after pulsatile and continuous GH treatment (36), and the levels of serum IGF-I were even somewhat higher after continuous GH treatment (166). This is in contrast to the observation that the established masculinizing factor STAT5b is activated more effectively by a pulsatile pattern (193). Moreover, there are few IGF-I receptors on the hepatocytes of the intact liver of male mice that could mediate a putative masculinizing effect (196,197,198). In conclusion, the most likely explanation for the finding that sexually dimorphic liver functions are feminized in male mice with liver-specific IGF-I KO (57) is that these mice exhibit enhanced basal GH levels. However, to finally elucidate the possible role of IGF-I as a hepatic mediator of masculinization, it is necessary to investigate whether GH-deficient liver-specific IGF-I KO mice are resistant to the masculinizing effect of pulsatile GH treatment in a way similar to that shown for STAT5b KO mice (199).
2. Body growth
There is emerging evidence that the GH-induced gender differences in body growth in rodents are mediated by nonhepatic, peripheral mechanisms involving STAT5b and local IGF-I. Global STAT5b KO markedly decreased body weight gain in male but not in female mice, whereas combined KO of STAT5b and the closely related STAT5a significantly reduced body weight gain in females and suppressed body growth more than KO of STAT5b alone in males (194,200). Therefore, STAT5b may be important for male-specific body growth, whereas STAT5a participates importantly in body growth in both sexes. Muscle-specific STAT5a/STAT5b KO suppresses IGF-I mRNA levels, decreases longitudinal bone growth, and decreases body weight by 20% and 12% in male and female mice, respectively (201). Pulsatile GH causes a larger increase in muscle and rib cartilage IGF-I mRNA levels compared with continuous GH treatment (36), whereas liver IGF-I mRNA and serum IGF-I levels are stimulated at least as well by continuous as by pulsatile GH treatment (36,166). Taken together, these data support the notion that larger body growth in male compared with female rodents could be due to effects exerted outside the liver, involving STAT5b activation and more effective stimulation of locally produced IGF-I, whereas liver-derived IGF-I is less important.
D. Human studies
GH secretion is pulsatile in all studied mammalian species, including humans (7,169,202). Additionally, sexual dimorphism of GH secretion occurs in humans, although it is less obvious than that in rodents (see Section IV.A). The results of several careful studies show that the GH secretory pattern is less regular in women than in men (202). Barkan and co-workers (203,204) reported that trough GH levels are higher in young women than in young men, at least when the measurements are done during the follicular phase in the women, and the GH pulses are more frequent and the large nocturnal GH pulse is lower in women. Interestingly, the effects of IGF-I on GH secretion seem to be influenced by gender and sex steroids in humans. IGF-I suppresses both spontaneous and GHRH-stimulated GH secretion more effectively in men than in women, whereas men are less sensitive than women to the GH-suppressive effect of a GHRH antagonist (203,204). Moreover, Veldhuis et al. (205,206) observed recently that sex steroids influence the effects of IGF-I on GH secretion in older men and women. In addition, oral estrogens can suppress IGF-I production by the liver in humans (207). Therefore, there are indications that the regulation of GH secretion is sexually dimorphic and regulated by sex steroids in humans and that there are interactions between sex steroids and IGF-I in regulation of GH secretion in humans as well as in rodents.
In a clinical study, healthy adult subjects were treated with a selective GH receptor antagonist, which reduced plasma IGF-I levels by approximately 30%. Similar to liver IGF-I-deficient mice, the decrease in serum IGF-I was accompanied by an increase in mean GH levels in serum, due to augmented amplitude of GH secretory bursts as well as elevated basal GH release in both women and men (208). In that study, it was difficult to discern whether the enhanced endogenous GH secretion resulted from decreased liver IGF-I secretion and/or was caused by suppression of the short loop negative feedback at the hypothalamic level that has been described for GH (7,184,185).
A physiological function of IGF-I feedback on GH secretion was demonstrated by Hartman et al. (209), who showed that the enhanced GH secretion in humans during fasting is caused by a decrease in circulating IGF-I levels, even in the absence of decreased blood glucose, presumably due to decreased hepatic IGF-I production. Increases in GH production induced by low IGF-I production could be of importance for the adaptation to fasting with lipolysis and insulin antagonism. Interestingly, recent studies in rodents suggest that fibroblast growth factor 21, a hormone produced during fasting, may specifically induce GH resistance in the liver and decrease hepatic IGF-I production by specific inhibition of STAT5 (210).
In summary, IGF-I seems to interact with sex steroids in the regulation of GH secretion in humans, as also shown for experimental animals. Moreover, suppressed liver IGF-I production and enhanced GH levels seem to be of importance for the physiological response to fasting with lipolysis and insulin resistance in humans.
V. The Role of Liver-Derived IGF-I for Metabolism and Body Composition
A. Carbohydrate metabolism
Given the large homologies between the insulin and IGF-I systems, it is not surprising that IGF-I exerts profound effects on carbohydrate metabolism. IGF-I may induce insulin-like effects on glucose uptake directly in tissues with IGF-I receptors. Alternatively, IGF-I may enhance insulin sensitivity by suppressing GH release, via its well-known negative feedback (see Section IV.B).
Based on studies of liver-specific IGF-I KO mice, it has become clear that specific elimination of liver-derived IGF-I results in increased insulin levels and decreased insulin sensitivity (211,212). Moreover, using the hyperinsulinemic-euglycemic clamp, insulin insensitivity was demonstrated in muscle, liver, and fat tissues (213,214). The mice with liver-specific IGF-I KO also are more sensitive to streptozotocin-induced diabetes, due to decreased insulin sensitivity (215), or to loss of the antiapoptotic effect that IGF-I exerts on β-cells (216), as discussed below. Strong evidence suggests that decreased insulin sensitivity is secondary to decreased negative feedback by liver-derived IGF-I at the pituitary level, which in turn leads to enhanced secretion of GH, a hormone with well-known diabetogenic effects (Fig. 6) (214). However, it has been proposed that IGF-I has direct effects on glucose metabolism (217,218), and therefore, it cannot be ruled out fully that IGF-I also can have some effects of its own on glucose metabolism and insulin sensitivity (69).
Figure 6.
Effects of depletion of liver IGF-I on carbohydrate metabolism. Lack of liver-derived IGF-I (right panel) causes enhanced GH secretion from the pituitary, which in turn results in insulin insensitivity in the liver, skeletal muscle, and adipose tissue. Therefore, more insulin is needed to prevent glucose secretion from the liver and to stimulate glucose uptake by skeletal muscle and adipose tissue. The mice with liver-specific IGF-I inactivation are normoglycemic but hyperinsulinemic.
The effects of ALS and IGF binding proteins on carbohydrate metabolism are complex. ALS KO does not seem to affect insulin sensitivity or GH secretion significantly (213). Surprisingly, combined KO of liver IGF-I and ALS resulted in improved insulin sensitivity compared with wild-type controls (213), despite a large increase in plasma GH levels in these double KO animals. One possible explanation is that these mice have increased levels of bioactive IGF-I in some tissues, high enough to affect insulin sensitivity directly but still not over the threshold level needed to have a substantial negative feedback effect on GH secretion. However, clarifying this issue will require further studies.
The KO of IGFBP-3, the third component of the ternary IGF-I:IGFBP-3:ALS complex that constitutes most of IGF-I in plasma, does not substantially affect insulin sensitivity or glucose metabolism (61,219), and induction of supraphysiological levels of IGFBP-3 has variable effects on glucose metabolism (220). In a very recent thorough study, Yakar et al. (61) investigated the effect of triple KO of liver-derived IGF-I, total IGFBP-3, and total ALS, resulting in extremely low levels of circulating IGF-I. Serum insulin levels were higher in triple KO mice compared with controls, but if anything, the levels were lower than those in mice with liver-specific IGF-I KO only.
The above-mentioned data indicate that liver-derived IGF-I can modulate insulin resistance (probably largely mediated by increased GH secretion) and thereby cause compensatory changes in insulin secretion. However, other data suggest a direct effect of IGF-I on insulin release from pancreatic β-cells, although the importance of these effects for glucose metabolism in mice with liver-specific IGF-I KO is not fully clear. It has been demonstrated that β-cell-specific depletion of IGF-I receptors causes decreased glucose-stimulated insulin secretion but no changes in β-cell mass (221,222). If IGF-I receptor deficiency in β-cells combines with insulin receptor depletion, the mice develop overt diabetes (223). Some evidence suggests that IGF-I, like insulin, can prevent apoptosis of β-cells via the insulin receptor substrate-2 pathway (216). In contrast, others have reported that pancreas-specific inactivation of the IGF-I gene leads to increased insulin secretion and islet cell mass, suggesting that pancreatic IGF-I may instead inhibit the islet cells (224).
In conclusion, liver-derived IGF-I seems to increase insulin sensitivity, largely by suppressing GH secretion from the pituitary. Consequently, lack of liver-derived IGF-I results in increased GH secretion and decreased insulin sensitivity in skeletal muscle, liver, and fat (Fig. 6). These effects of IGF-I can also be modulated by IGFBP-3 and ALS, opening the possibility that further research might identify methods to modulate the circulating levels of IGF-I system components for the treatment of diabetes (see Section VII).
B. Fat mass
According to several reports, liver-specific KO of IGF-I in mice enhances serum leptin levels at 2–4 months of age (61,211,214). High serum leptin levels could reflect increased fat mass after liver-specific IGF-I KO (61), but may also be secondary to the increased serum insulin levels discussed above (Section V.A) because insulin is known to stimulate leptin secretion (211,225,226). A third possible explanation is a direct effect by IGF-I on leptin production because it has been shown that injection of IGF-I acutely decreases leptin mRNA in fat as well as serum leptin levels in rodents (227,228). Later in life, however, mice with liver-specific IGF-I KO are protected against the increase in body fat mass that occurs at an older age in wild-type mice (211,229).
In accord with the finding that old mice with liver-specific IGF-I KO have reduced fat mass, it has been reported that mice overexpressing IGFBP-1, a well-known inhibitor of the metabolic effects of IGF-I, also become leaner (230). Although the reason for the fat-reducing effect of decreased levels of bioavailable IGF-I in different mouse models is unknown, these mice may acquire some protection from the age-related decrease in plasma GH levels, and GH in turn reduces fat mass. In support of this notion, it is well-known that liver-specific IGF-I KO increases serum GH levels (11,12), and it has been proposed that the decreased GH secretion that occurs with age in both humans and rodents contributes to age-related obesity (231,232). Alternatively, liver-derived IGF-I may be required for proliferation and differentiation of preadipocytes, although the expression of IGF-I locally in fat seems unaffected in mice with liver-specific IGF-I KO (11). It has been shown that overexpression of both IGFBP-1 and IGFBP-2, the principal binding proteins of differentiating preadipocytes, decreases adipocyte differentiation in mice (230,233). To our knowledge, no studies have examined the effect of liver-derived IGF-I on body fat mass in animals given a high-fat diet.
With respect to the elimination of endogenous binding proteins, body fat increases markedly in young ALS KO mice, but only slightly in young IGFBP-3 KO mice. As expected, young adult triple gene KO mice lacking liver-derived IGF-I, total ALS, and total IGFBP-3 also show increased adiposity (61). In contrast, triple gene KO of IGFBP-3, -4, and -5 resulted in decreased fat mass and markedly decreased adipocyte size (219).
In conclusion, lack of endogenous liver-derived IGF-I seemingly causes a transient increase in body fat and serum leptin levels in young mice, followed by decreased fat mass in older mice. It seems likely that the suppression of fat mass in older mice with liver-specific IGF-I KO results, at least partly, from enhanced GH secretion. Finally, as discussed in Section IV.D, decreased release of liver-derived IGF-I in response to absence of food intake may contribute to fasting-induced GH release in humans. This provides an important part of adaptation to fasting by inducing insulin resistance and lipolysis (209).
C. Lipid metabolism
There are few reports on the effects of liver-derived IGF-I on serum lipids. We observed enhanced levels of both total cholesterol and low-density lipoprotein cholesterol in male and female mice with liver-specific IGF-I KO (211). These findings concur with the observation that IGF-I treatment can decrease serum cholesterol in humans (234,235). The role of GH regarding increased cholesterol levels in mice with liver-specific IGF-I KO remains unclear. GH deficiency associates with elevated serum cholesterol, and GH replacement therapy normalizes those levels (236,237,238,239). Moreover, GH treatment reverses the increase of serum cholesterol levels that occurs in old rodents (240). Therefore, although GH has been reported to increase cholesterol levels in young rodents (240), the increased cholesterol levels in mice with liver-specific IGF-I KO probably did not result from increased GH levels in these mice. Some, but not all, studies have reported that liver-specific IGF-I KO enhances serum triglyceride levels (211,214). However, the accumulation of triglycerides in muscle and liver appears unchanged by lack of liver-derived IGF-I (214). Thus, further studies are needed to fully evaluate the role of circulating IGF-I on lipid metabolism.
VI. Liver-Derived IGF-I and Other Tissues
A. Brain
It is well established that IGF-I is of major importance for normal brain development, which will be briefly discussed. Importantly, during the last 5–10 yr, a series of studies have shown that liver-derived circulating IGF-I is an important regulator of adult brain function, which will be reviewed in this section.
1. Developmental effects of IGF-I
IGF-I receptors are widely distributed in the brain (241), and IGF-I is essential for the development of the brain (241,242,243,244). In contrast to GH overexpression, which results in a smaller increase in brain size than body size (245,246), brain size is comparatively more increased than body size in transgenic mice with lifelong, global IGF-I overexpression (246). IGF-I overexpression increases myelination and the number of neurons and synapses (246,247,248,249,250), and overexpression of IGF-I protects granule neurons from apoptosis and improves ataxia in weaver mice (251). Inactivation of IGF-I or its receptor reduces brain size as well as the granule cell layer in the dentate gyrus and the numbers of oligodendrocytes and myelinated axons (252,253,254). Thus, IGF-I regulates the growth of new brain cells including neurons and glia, reduces developmental apoptosis, and increases synaptogenesis during early postnatal development.
2. Inactivation of the IGF-I receptor in the central nervous system
Using the cre-loxP recombinase system (nestin promoter), a mouse model with homozygous inactivation of the IGF-I receptor in neurons or glia was recently created (255). These mice were microcephalic and developed a complex phenotype involving severe growth retardation, infertility, and abnormal behavior (255). Mice with heterozygous inactivation of the IGF-I receptor in the central nervous system, including the hypothalamus, were also created (255). The phenotype in terms of central nervous function was not evaluated, but these mice were long-lived (255).
3. IGF-I affects behavior in adult mice
In adult life, IGF-I has been shown to be of importance for behavior. Intracerebroventricular administration of IGF ameliorated age-related behavioral deficits (256). In a later study in old mice with liver-specific IGF-I KO, both horizontal and rearing activity levels decreased when the mice were placed in a new environment (257). These results, combined with the compensatory high circulating GH in mice with liver-specific IGF-I KO (11,57), indicate that liver-derived circulating IGF-I mediates at least some of the effects of GH on exploratory activity previously observed in mice with overexpression of GH both globally (258) and in the central nervous system only (259).
4. Circulating IGF-I mediates exercise-induced effects on the adult brain
In older animals, it has been shown that an increase in circulating GH levels, by treatment with GH or GHRH, improves vascular microvasculature in the brain (260) and can prevent the age-related decrement in spatial learning and reference memory as assessed using the Morris water maze test (261). Studies have now investigated the extent to which these effects are mediated by the concomitant increase in circulating IGF-I. Circulating IGF-I mediates the exercise-induced increase in the number of new neurons in the adult hippocampus (262), and liver-derived IGF-I is a necessary factor in mediating the beneficial effects of exercise on brain vessel growth (263), anxiety, and spatial learning (264). The proangiogenic activity of liver-derived circulating IGF-I likely contributes to its neuroprotective actions by means of proper nutrient and oxygen supply to neurons (263).
5. Circulating IGF-I regulates brain amyloid-β (Aβ) levels in adult mice
A major finding is that liver-derived circulating IGF-I is one of the factors that regulates the clearance of brain Aβ (265). Systemic treatment with IGF-I can reduce the Aβ burden in Tg2576 mice overexpressing a mutant form of human amyloid precursor protein (APP695) (265). The mechanism underlying this increased clearance of brain Aβ by liver-derived circulating IGF-I, at the level of the blood-brain barrier, was speculated to involve enhanced transport of Aβ carrier proteins such as albumin and transthyretin into the brain (265). In a later study, the choroid plexus endocytic receptor megalin, a multicargo transporter known to participate in brain uptake of Aβ carriers, was identified as a potential mediator of IGF-I-induced clearance of Aβ and was also involved in IGF-I transport into the brain (266). Furthermore, treatment with IGF-I ameliorates cognitive dysfunction in double mutant amyloid precursor protein/presenilin 2 mice, counteracting vascular dysfunction by reducing vascular endothelial growth factor levels and endothelial cell proliferation and normalizing vascular density (267). IGF-I treatment also normalized abnormally elevated brain IGF-I receptor levels in amyloid precursor protein/presenilin 2 mice (267).
6. Circulating IGF-I regulates hippocampal function and spatial memory in adult mice
Using the Morris water maze test, mice with liver-specific IGF-I KO display reduced spatial learning and memory (268) (Fig. 7). Histochemical analyses revealed an increase in immunoreactivities but a decrease in mRNA levels of dynorphin and enkephalin in the dental gyrus of the hippocampus in old mice with liver-specific IGF-I KO (268). The intracellular accumulation of dynorphin and enkephalin might reflect decreased release of the peptide, probably related to reduced transcription of the two opioid peptides (268). Old mice with liver-specific IGF-I KO also displayed astrocytosis and increased metabotropic glutamate receptor 7a-immunoreactivity (268). These neurochemical disturbances suggest synaptic dysfunction and early neurodegeneration in old mice with liver-specific IGF-I KO (268). In addition to these characteristics, mice with liver-specific IGF-I KO exhibit disrupted long-term potentiation (a measure of synaptic plasticity) in the hippocampus, but not in cortex (269), and a reduction in the density of glutamatergic boutons leading to an imbalance in the glutamatergic/GABAergic synapse ratio in the hippocampus (269). The cognitive and synaptic deficits in mice with liver-specific IGF-I KO were ameliorated by prolonged systemic administration of IGF-I that normalized the density of glutamatergic boutons in the hippocampus (269), further demonstrating the importance of liver-derived circulating IGF-I for brain function.
Figure 7.
Reduced spatial learning (A) and memory (B) in mice with liver-specific IGF-I inactivation. Reference memory was measured using the water maze test. During this test, the latency time for the mice to find a platform hidden in water is recorded. The release point, where the mice are let into the water, is randomly shifted. A, Latency time to find the escape platform in 15-month-old mice with liver-specific IGF-I KO (n = 9) and control mice (n = 9) during the first 5 d of the water maze. A two-way ANOVA for repeated measurements was performed, followed by Student Newman Keul’s post hoc test. B, Results of the spatial reference memory (probe) test at day 6. The reference memory (probe) test was performed in the absence of the platform, and percentage of time spent in the trained quadrant was recorded. In B, the given P value is based on an unpaired t test. Values are given as means ± sem. Adapted from Ref. 268.
7. Mechanisms showing how circulating IGF-I affects the function of the adult brain
As described above, liver-derived IGF-I exerts multiple effects on the brain, but it is yet not fully clear whether these effects of IGF-I can be explained only by effects at the blood-brain barrier. IGF-I can pass the blood-brain barrier (270), and IGF-I has the direct capacity to modulate synaptic efficacy in several in vitro studies (271,272,273). Therefore, there are several possible explanations regarding how deficiency of liver-derived circulating IGF-I impairs spatial memory learning and synaptic function. At the current stage of knowledge, it is difficult to establish which mechanism is most important. Furthermore, deficiency of liver-derived circulating IGF-I results in unchanged circulating glucose but increased circulating insulin levels, indicating an adequately compensated insulin resistance (211,214). Because systemic levels of insulin correlate with Alzheimer’s disease (AD) neuropathology (274), the increased insulin levels observed in mice with liver-specific IGF-I KO might contribute to reduced spatial learning and memory in these mice.
8. Human studies
In humans, IGF-I gene mutations associate with sensorineural deafness, microcephaly, and mental retardation (25,88,89). In adult hypopituitary patients with severe GH deficiency and low circulating IGF-I levels, the impairment of cognitive function is less distinct, but a recent meta-analysis showed that in GH-deficient adult hypopituitary patients not receiving GH replacement, most cognitive domains display moderate to large impairments compared with matched controls (275). After GH replacement, cognitive performance improved moderately, particularly in terms of attention and memory (275). In older subjects (65 yr and over) lower circulating levels of IGF-I associated with lower Mini-Mental State Examination performance (276). Two studies showed that patients with AD or vascular dementia had lower circulating IGF-I concentrations than controls without dementia (277,278). In contrast, total and free circulating IGF-I levels increased in patients with late-onset AD (279), consistent with the hypothesis that the early phase of AD may be a state of resistance to IGF-I signaling (279). There are few data regarding IGF-I levels in cerebrospinal fluid in subjects with AD. In one study, the cerebrospinal fluid level of immunoreactive IGF-I did not differ between subjects with dementia of the Alzheimer type and healthy subjects (280).
In conclusion, IGF-I is of major importance for brain development. In adult life, circulating IGF-I participates importantly in reactive vessel remodeling in the brain, the clearance of brain Aβ, cognitive function, and behavior. Reduced spatial learning and memory in mice with liver-specific IGF-I KO are not due to changes in GH secretion because these mice have increased circulating GH levels. The relative importance of liver-derived circulating IGF-I vs. brain IGF-I is presently unclear. Global deficiency of IGF-I produces a more severe central nervous system phenotype than deficiency of liver-derived IGF-I. However, the global IGF-I deficiency model includes developmental effects, whereas the liver-specific IGF-I KO model does not, rendering direct comparisons between the models difficult. In mice, systemic IGF-I treatment improves central nervous functions including spatial retention memory; it remains undetermined whether systemic IGF-I treatment will be a useful treatment option for diseases like AD in humans.
B. Cardiovascular system
Both the myocardium and blood vessels express IGF-I (35,281), and IGF-I mRNA transcripts have been found in both aortic smooth muscle cells and endothelial cells (282). Moreover, functional receptors for GH (283,284,285) and IGF-I (286,287) are present in the heart and vasculature. In addition, IGF-I production in the heart increases in response to GH (35,281). Hence, there are possibilities of direct actions of GH as well as endocrine or autocrine/paracrine effects of IGF-I on the cardiovascular system.
Apart from stimulating cardiac growth and possibly also contractility, GH/IGF-I may also participate in the regulation of vascular tone and thereby peripheral resistance (288,289). Studies indicate that IGF-I is a potent vasodilator and that this effect may be partly mediated by increased nitric oxide release from the endothelium (290,291,292). Accumulating evidence would also suggest that IGF-I plays a role in vascular diseases such as atherosclerosis and restenosis (293). IGF-I is a potent stimulator of vascular smooth muscle cell (VSMC) proliferation and migration, and this may play a role in the early development of atherosclerotic plaque formation (293). Providing evidence suggesting the involvement of locally produced IGF-I in this setting, Grant et al. (294) observed expression of IGF-I in VSMC derived from coronary atherectomy of both de novo and restenotic plaques, as opposed to VSMC from normal coronary arteries, where IGF-I expression could not be detected. In further support of a role of local IGF-I in the progression of atherosclerosis, genetic deletion of the IGFBP-specific protease PAPP-A, which results in reduced local IGF-I bioavailability, associates with resistance to atherosclerotic lesion development in apolipoprotein E-deficient mice challenged with a high-fat diet (295).
In addition, circulating IGF-I may participate importantly in the development of atherosclerosis because it has been reported that individuals with low serum IGF-I have significantly higher risk of developing atherosclerosis and coronary heart disease during a 15-yr follow-up period (296). Moreover, a polymorphism of the IGF-I gene linked to lower levels of serum IGF-I may associate with increased carotid intima-media thickness and aortic pulse wave velocity, which are considered to be early markers of atherosclerosis (297). In analogy, GH-deficient adult patients with low serum IGF-I values have been shown to have signs of early-onset atherosclerosis (298,299), and this may be reversed by GH substitution (300,301). Thus, it has been proposed that circulating IGF-I, largely regulated by GH, would have a protective role against atherosclerosis and cardiovascular disease, whereas IGF-I produced locally in blood vessels may actually promote atherogenesis by different mechanisms (302).
Several studies attempting to describe the consequences of cardiac overexpression of IGF-I have been published, although results are complex to interpret due to leakage of cardiac IGF-I to the circulation. Reiss et al. (303) reported a time-dependent increase in heart weight with a concomitant increase in cardiomyocyte cell number in mice with cardiac-specific IGF-I overexpression. However, it was also observed in this experimental model that body weight and levels of circulating IGF-I were clearly increased compared with wild-type mice, making additional endocrine effects of IGF-I probable. In more recent studies, cardiac overexpression of IGF-I was reported to protect against aging-related alterations in diastolic function (304), intracellular Ca2+ homeostasis, and apoptosis (305), although cardiac overexpression of IGF-I was again accompanied by a substantial increase in circulating IGF-I. Cardiac-specific overexpression of IGF-I also increased median survival (305). Somewhat conflicting results of cardiac IGF-I overexpression have also been reported, showing initially beneficial effects on systolic function followed by pathological cardiac hypertrophy and deterioration of systolic function in aging mice (306).
The KO models have also been used to clarify the importance of IGF-I for the regulation of cardiovascular function. Signaling through the IGF-I receptor in the heart has been believed to play a role during adaptive cardiac hypertrophy after hemodynamic load (307), and cardiomyocyte-specific KO of the IGF-I receptor with preserved serum IGF-I levels impairs physiological cardiac hypertrophy in response to exercise (308). Mice carrying a mutant IGF-I allele, with 30% of wild-type IGF-I levels present in all tissues and serum (309), showed a significant elevation of mean arterial pressure compared with wild-type controls. Somewhat surprisingly, these partially IGF-I-deficient mice had increased cardiac contractility, possibly due to a compensatory β-adrenergic coupling. When subjected to experimental myocardial infarction, the same mouse model of general partial IGF-I deficiency displayed altered cardiac remodeling, despite the preservation of cardiac function (310). However, due to the general lowering of IGF-I in both tissues and serum, as well as the fact that IGF-I deficiency was present since the beginning of lifespan in this experimental model, the role of endocrine vs. autocrine/paracrine IGF-I was not clarified.
A more recent study in mice with liver-specific IGF-I KO that resulted in an 80% decrease of circulating IGF-I showed an elevation of blood pressure, similar to that in the above described IGF-I mutant mice, as well as a comparable decrease in serum IGF-I, suggesting that decreased endocrine-acting IGF-I is responsible for this blood pressure-elevating effect (311). A decrease in circulating IGF-I also associated with endothelial dysfunction of mesenteric resistance vessels and increased expression of endothelin-1 in the aorta. In contrast to generally IGF-I-deficient mice, cardiac contractility remained unchanged in mice with liver-specific IGF-I KO compared with controls. Stroke volume and cardiac output decreased in mice with liver-specific IGF-I KO, although fractional shortening, velocity of circumferential shortening, and functional cardiac reserve, measured with stress echocardiography, remained unaffected, suggesting that reduced cardiac output was secondary to increased peripheral vascular resistance rather than decreased contractility per se. Although this experimental model associates with increased levels of circulating GH, it is less likely that increased GH activity is the explanation for increased vascular resistance because bovine GH transgenic mice have unchanged blood pressure compared with controls (312) and a number of both experimental studies and clinical studies show decreased peripheral resistance after GH administration (288,289).
Mice with a specific KO of liver-produced IGF-I have been reported to have improved survival compared with wild-type controls when subjected to oxidative stress (313) and to have attenuated aging-related deterioration in contractile function and intracellular Ca2+ handling when isolated cardiomyocytes were studied in vitro (314).
In summary, different transgenic models with KO or overexpression of IGF-I, general or local, clearly demonstrate a wide range of cardiovascular effects that can be attributed to IGF-I signaling. However, the exact role of endocrine vs. locally acting IGF-I is difficult to elucidate in many models due to either spillover of IGF-I into the circulation from cardiac overexpression or IGF-I deficiency in both tissues and circulation due to a general KO. Nevertheless, results from mice with liver-specific IGF-I KO with decreased levels of circulating IGF-I and generally unchanged tissue IGF-I levels suggest a role of liver-derived IGF-I in the regulation of blood pressure and peripheral resistance.
C. Kidney
Both GH and IGF-I receptors are expressed in the kidney (315,316). Rodent models illustrate the importance of the GH/IGF-I system for kidney size. Mice with global overexpression of GH and/or IGF-I have renal and glomerular hypertrophy (317,318,319). In IGF-I transgenic as well as IGF-I-treated rodents, IGF-I stimulates renal growth to an approximately similar extent as it stimulates overall body growth (286,317). In mice with global inactivation of the IGF-I gene that survive the postnatal period, the proportionally reduced kidney size associates with reduced glomerular size and decreased numbers of nephrons (320). GH receptor KO mice with very low IGF-I levels also display a proportional decrease in kidney weight (14,15). The role of liver-derived circulating IGF-I for kidney size has been investigated in mice with liver-specific IGF-I KO (11,321). Despite their secondary high circulating GH levels (57), mice with liver-specific IGF-I KO had an absolute and relative decrease in kidney weight, however, with no alterations in kidney morphology including the size, number, and distribution of renal structures. A microarray analysis of the kidneys of mice with liver-specific IGF-I KO revealed normal IGF-I mRNA levels, but a pronounced and tissue-specific decrease in renal IGF-II mRNA levels (321). Given the facts that mice overexpressing IGF-II have increased relative kidney weight (322,323,324), and that postnatally elevated IGF-II selectively increased the kidney weight of total IGF-I KO mice (324), it may be hypothesized that liver-derived circulating IGF-I increases renal IGF-II expression, resulting in symmetrical renal growth.
IGF-I has rapid effects on renal hemodynamics, including an increased renal blood flow and glomerular filtration rate (325,326,327). GH treatment has similar, but slower, effects on kidney function, and these are, therefore, considered to be mainly mediated by IGF-I (315). GH-deficient rats have a reduced glomerular filtration rate, which is restored by IGF-I treatment, supporting the notion that the low levels of local and/or circulating IGF-I in this model are responsible for this phenotype (328). However, the finding of unchanged creatinine clearance in mice with liver-specific IGF-I KO does not support a major role of liver-derived IGF-I for glomerular filtration (321). Thus, it may be hypothesized that local IGF-I, rather than liver-derived circulating IGF-I, participates in the maintenance of a normal glomerular filtration rate.
Administration of IGF-I causes dose-dependent retention of sodium and water (329), possibly through activation of distal tubular sodium channels (330). However, the role of IGF-I in the physiological regulation of water and sodium balance has been less clear (315). The role of liver-derived IGF-I for renal sodium handling was therefore investigated in mice with liver-specific IGF-I KO, which displayed increased urinary loss of sodium (321). In humans, GH treatment increases serum IGF-I levels, sodium reabsorption, and fluid retention. Therefore, it is likely that the increased urinary loss of sodium in mice with liver-specific IGF-I KO is caused by the deficiency of circulating IGF-I and that the sodium retention seen during GH treatment is mediated by the concomitant increase in circulating IGF-I.
Evidence suggests a role for the GH/IGF-I axis in the development of progressive glomerular sclerosis (315). Mice with overexpression of GH, but not mice with overexpression of IGF-I, develop progressive glomerulosclerosis (318,319,331), despite higher levels of circulating (and presumably renal) IGF-I in the IGF-I transgenic mice. Consequently, a role of GH, rather than IGF-I, has been emphasized for the development of glomerular sclerosis (315). This is in accord with results showing that mice with GH receptor gene disruption are protected against diabetes-induced renal damage (332). A direct effect of GH on the glomerular podocyte, with increased levels of reactive oxygen species and reorganization of the actin cytoskeleton in these cells in response to GH, has been proposed as one possible mechanism underlying the development of progressive glomerulosclerosis in transgenic mouse models with high GH levels (333). However, despite the fact that mice with liver-specific IGF-I KO are insulin resistant, although not diabetic (211), and have elevated levels of GH (57), there were no signs of glomerulopathy in older mice with liver-specific IGF-I KO (321). The absence of renal structural, vascular, and/or glomerular complications in the mice with liver-specific IGF-I KO (321) suggests that high circulating GH and low circulating IGF-I in itself does not cause glomerular sclerosis/renal failure. However, in diseases such as type 1 diabetes mellitus (202), where circulating GH is high and circulating IGF-I is low, the possibility cannot be fully excluded that high circulating GH levels combined with low circulating IGF-I levels can accelerate an already existing renal disease.
Taken together, available data suggest that liver-derived IGF-I is a regulator of kidney size and the renal excretion of sodium. In contrast, there is no support for a role of liver-derived circulating IGF-I in the regulation of glomerular filtration rate, where local renal IGF-I expression may be more important for the physiological effects. Although direct effects of GH may be less important in the regulation of kidney size and function, data suggest a role of GH, rather than IGF-I, in the development of progressive glomerular sclerosis in rodent models. Importantly, key pieces of knowledge are still lacking, and models with kidney-specific inactivation and overexpression of IGF-I would provide valuable new information.
D. Liver
1. IGF-I and the size of the intact liver
The liver is a major target organ for GH, and GH is an important regulator of hepatic IGF-I production. It is believed that IGF-I does not affect the function of hepatocytes directly because there are few IGF-I receptors on these cells in the intact liver (196,197,198). This is in accord with results indicating that enhanced hepatic glucose production in mice with liver-specific IGF-I KO is not due to decreased direct effects of hepatic IGF-I on hepatocytes, but it might be secondary to enhanced GH secretion. This notion is supported by the results of crossbreeding liver-specific IGF-I KO mice with GH antagonist transgenic mice, showing that depletion of GH can reverse this effect of decreased liver IGF-I production (see Section V.A) (Fig. 6) (214). The lack of IGF-I receptors on hepatocytes would also mean that liver-derived IGF-I, as well as nonliver IGF-I, would be unable to stimulate liver growth during adulthood. Accordingly, mice with liver-specific IGF-I KO did not display decreased growth of the intact liver, but the liver instead became disproportionally large, likely due to direct stimulation by enhanced GH secretion (see Section IV.A) (Fig. 4) (11,12). In line with this, GH receptor-deficient mice have reduced relative liver weight (14). Moreover, overexpression of GH in transgenic mice causes a disproportional growth of the liver, whereas this is less apparent in mice overexpressing IGF-I (245,318).
2. IGF-I and liver regeneration
The comparatively slow liver growth in normal healthy mice differs in many ways from the very rapid liver growth occurring after hepatectomy. In rodents, this is an established model, where almost normal liver size is achieved again about 7–10 d after two thirds partial hepatectomy. The regulation of liver regeneration has been studied extensively, and it has been shown to be dependent on the cytokines IL-6 and TNF-α (334,335), which also seem to stimulate the growth of the intact liver (336). In addition, growth factors, such as hepatocyte growth factor and TGF-α/EGF, have been implicated in liver growth (334). It has been shown in various models that GH is important for liver regeneration in both rats and mice (198,337,338,339). IGF-I treatment, like GH treatment, to hypophysectomized rats accelerates DNA synthesis during liver regeneration. Later, it was reported that depletion of liver IGF-I and ALS, which reduces serum IGF-I levels to about 10% of normal intact mice, delays hepatocyte proliferation with about 12 h (198). Moreover, depletion of IGF-I receptors normally present in hepatocytes and epithelial cholangial cells of regenerating liver reduces hepatocyte proliferation in regenerating liver by about half in male but not female mice (340). It remains unknown why IGF-I stimulates liver regeneration more effectively than the growth of intact liver, but there are some possible mechanisms. First, IGF-I effects may be potentiated by EGF/TGF-α (341), and this growth factor is more active during liver regeneration (334). Second, IGF-I has been shown to increase the production of the potent liver growth factor hepatocyte growth factor from hepatic stellate cells, one of the cell types besides hepatocytes in the liver, providing a possible alternative route for how IGF-I indirectly stimulates hepatocyte proliferation and thereby liver growth (342). Third, Alternatively, the stimulatory effect by IGF-I on liver regeneration may be exerted directly on hepatocytes because the expression of IGF-I receptors as well as IGF-I binding increases in the remaining liver after partial hepatectomy (196,197,340), although this effect has not been seen in all studies (198). The levels of the intracellular signaling molecule insulin receptor substrate-1 also increase in regenerating liver, providing another possible mechanism for enhanced IGF-I sensitivity (340). And finally, IGFBPs may play a role for IGF-I effects on liver regeneration. IGFBP-1, one of the IGFBPs that also may exert IGF-I independent effects (343), increases rapidly after partial hepatectomy (344). Targeted disruption of the IGFBP-1 gene causes delayed and decreased DNA synthesis in hepatocytes of regenerating liver (345).
In conclusion, endogenous GH is needed for the growth of both intact and regenerating liver in rodents. Liver-derived IGF-I also stimulates liver regeneration, but it does not seem to stimulate growth of the intact liver directly. Liver-derived IGF-I may instead decrease relative liver size indirectly by suppressing GH secretion (Fig. 4).
E. Prostate
In this section, only the effect of IGF-I on prostate size will be reviewed. The role of IGF-I in relation to prostate cancer will not be described because this has recently been reviewed in Endocrine Reviews (5).
The prostate is composed of two compartments, glandular and fibromuscular. In primary culture, prostatic epithelial cells express the IGF-I receptor, and IGF-I stimulates them to proliferate and produce IGFBPs (346,347). Prostatic stromal cells also express the IGF-I receptor (348,349,350). There is local production of IGF-I in the prostatic stromal cells (351,352), and locally produced IGF-I can act in a paracrine fashion on prostatic epithelial cells (352). Thus, both endocrine and locally derived IGF-I may have the capacity to regulate the growth of prostate-derived cells.
Mice with overexpression of bovine GH display increased ventral prostate weight (353). GH treatment of hypophysectomized rats increased the expression of the androgen receptor (AR) as well as the expression of IGF-I and the IGF-I receptor in prostate (354).Transgenic mice with global inactivation of the IGF-I gene, as well as mice overexpressing a GH antagonist, have decreased prostate size (355). Administration of bovine GH had no effect on restoring prostate development in transgenic mice with global inactivation of the IGF-I gene (355), suggesting that reduced prostate size in these mice was due mainly to low IGF-I activity. Transgenic mice expressing human IGF-I in basal epithelial cells of prostate had leakage of human IGF-I into the circulation and prostate hyperplasia in early life (and later neoplastic changes in prostate) (356). Another study, which focused mainly on prostate cancer development, examined transgenic (PB-Des) mice with specific overexpression in prostate epithelial cells of human IGF-Ides, which encodes a mature isoform of IGF-I with decreased affinity for IGFBPs due to a 3-amino acid deletion in the N terminus (357). IGF-Ides was not detected in the serum of PB-Des mice, confirming that the expression of the exogenous IGF-I was confined to the prostate, and no difference vs. controls was seen in terms of genitourinary tract weight normalized to body weight (357). Finally, conditional deletion of the IGF-I receptor in prostate epithelium did not affect total body or prostate weight (358). Therefore, taken together, overexpression or inactivation of IGF-I or its receptor specifically in prostate epithelium does not appear to result in any major change of total prostate weight.
Mice with adult liver-specific inactivation of IGF-I displayed clearly reduced prostate weight, with a more marked effect on the ventral prostate (VP) lobe than that on the dorsolateral prostate (DLP) and anterior prostate lobes (359). Reduced AR mRNA and AR protein levels were observed in the VP lobe of mice with liver-specific IGF-I KO, and immunohistochemistry revealed reduced intracellular AR immunoreactivity in the VP and DLP lobes (359). The nonaromatizable androgen dihydrotestosterone increased VP weight to a lesser extent in orchidectomized mice with liver-specific IGF-I KO compared with orchidectomized controls, indicating that the down-regulation of AR expression in the prostate of mice with liver-specific IGF-I KO was functionally important (Fig. 8).
Figure 8.
Mice with liver-specific inactivation of IGF-I displayed reduced prostate weight. The clearly decreased prostate weight in mice with liver-specific IGF-I KO associated with decreased mRNA and protein levels of the AR in the ventral prostate. Thus, liver-specific IGF-I deletion reduces the number of ARs expressed in the prostate (right panel). Testosterone from the testicles is then less effective in increasing prostate size. This could, hence, be a mechanism for circulating IGF-I to converge with the androgenic pathway in the regulation of prostate size.
Mice with global inactivation of the IGF-I gene displayed impaired development of the glandular prostate compartment (355). It was later shown that IGF-I can affect the development of both prostate compartments (360). In mice with liver-specific IGF-I KO, analysis of prostate morphology showed reductions of both the glandular and the fibromuscular compartments of the VP and DLP lobes that were proportional to the reductions of the weights of these lobes (359). It is unlikely that the reduction of the fibromuscular prostate compartment of the mice with liver-specific IGF-I KO were due to reduced AR expression in these mice because previous studies show that androgen inhibition or treatment primarily acts on the prostate glandular epithelium, whereas the effect on the fibromuscular tissue is relatively small (360,361,362,363). It could, therefore, be hypothesized that liver-derived IGF-I regulates the glandular prostate compartment by affecting AR mRNA and protein levels in the prostate, whereas the effects on the fibromuscular compartment are due to other androgen-independent mechanisms.
In humans, GH excess (acromegaly) results in high serum IGF-I values and increased prostate size (364). Prostate size decreases in GH-deficient adults with low IGF-I values (365), and GH replacement therapy increases both serum IGF-I levels and prostate size in such patients (365). Furthermore, combined GH and testosterone treatments have additive affects on prostate size in adults with GH deficiency and low serum IGF-I values (365). Thus, in accord with findings in liver-specific IGF-I KO mice, human data provide support for the notion that IGF-I and androgens interact in the regulation of prostate size (Fig. 8).
In conclusion, IGF-I regulates prostate size and probably mediates the effect of GH on prostate size. Although the relative importance of circulating IGF-I vs. that of locally produced IGF-I is not fully clear, the fact that mice with liver-specific IGF-I KO display clearly reduced prostate weight demonstrates that liver-derived circulating IGF-I is important for the regulation of prostate weight. We propose that liver-derived IGF-I increases prostate size, at least partly, by increasing the number of ARs and thereby androgen responsiveness (Fig. 8). This could, hence, be a mechanism for circulating IGF-I to converge with the androgenic pathway in the regulation of prostate size.
VII. Clinical Implications
A. Indications for the use of IGF-I and adverse effects
IGF-I treatment increases linear growth in patients with severe IGF-I deficiency resulting from IGF-I gene mutations or GH insensitivity (see Section II.D) (48,49,85,90,366). However, treatment of GH-deficient patients with GH results in a more pronounced postnatal growth response than treatment of GH-insensitive patients with IGF-I, supporting the notion that GH exerts IGF-I-independent effects on body growth (48,49,366). In 2005, the United States Food and Drug Administration approved the use of rhIGF-I for long-term treatment of children with short stature resulting from severe primary IGF-I deficiency, defined as height and IGF-I sd scores below/equal to −3 and normal or elevated GH levels. In 2007, similar approval was granted in Europe by the European Medicines Agencies. The cause of IGF-I deficiency was defined as various mutations in the GH receptor, defects in the post-GH receptor signaling pathway, and/or IGF-I gene defects. Treatment was also approved for children with GH deficiency who had developed neutralizing antibodies to GH.
Information about the long-term efficacy and dose effect of IGF-I treatment in children with various severities and forms of IGF-I deficiency is still lacking. However, results from the Increlex (Tercica) Growth Forum Database show that treatment with rhIGF-I for a period of 1 yr stimulates height velocity in prepubertal children with moderate IGF-I deficiency (height sd score, −2.5), in a dose-dependent manner (367). Chernausek et al. (366) previously reported a similar dose-dependent effect in children with severe primary IGF-I deficiency. Chernausek et al. (366) reported the adverse events in 76 children with severe IGF-I deficiency due to GH insensitivity treated with rhIGF-I for up to 12 yr. The most common adverse events were hypoglycemia (49%), injection site lipohypertrophy (32%), and tonsillar/adenoidal hypertrophy (22%). Other adverse events included benign increase in intracranial pressure and coarsening of facial features (366). The authors concluded that adverse events are common but rarely of sufficient severity to interrupt or modify treatment. Other side effects, e.g., myalgia and arthralgia, may be transient, and the frequency and severity of side effects of IGF-I treatment have been suggested to be less severe in patients with mild IGF-I deficiency compared with those with severe IGF-I deficiency (368).
B. Potential indications for combined GH and IGF-I treatment
It is quite clear that both GH and IGF-I are critical components for stimulation of statural growth and that GH stimulates the production of IGF-I in various organs including liver and bone (see Section II). As summarized in Fig. 1C, GH stimulates longitudinal bone growth by both IGF-I-dependent and IGF-I-independent mechanisms. It seems that endocrine IGF-I and local bone-derived IGF-I have to some extent overlapping growth-promoting effects and can partly (= redundancy) replace each other in the maintenance of normal longitudinal bone growth (see Section II.B). Therefore, it is logical that pharmacological administration of IGF-I can replace not only liver-derived IGF-I but also the effect of locally produced IGF-I on bone growth. A major finding supporting the hypothesis that both normal IGF-I and GH actions are required for normal bone growth is the finding by Lupu et al. (19) that GHR-IGF-I double KO mice had a more severe reduction in bone length than mice with IGF-I KO or GHR KO. These experimental findings suggest that some patients with both affected GH and IGF-I action might benefit from combined GH and IGF-I treatment. In the clinical setting, it seems quite logical that patients with growth disorders that have pronounced GH deficiency will benefit from GH replacement. Likewise, it appears clear that patients with pronounced primary IGF-I deficiency will benefit from IGF-I treatment/replacement therapy. In clinical practice, however, patients with growth disorders often do not exhibit complete deficiencies of GH and/or IGF-I, respectively, but rather partial deficiencies. The potential efficacy of IGF-I treatment in large groups of children with growth disorders that exhibit partial hormone deficiencies, currently labeled as GH deficiency, intrauterine growth retardation, idiopathic short stature, GH insensitivity, and IGF-I deficiency is presently unclear. Because the majority of the patients in these quite large and heterogeneous groups of patients do not have a defined geno- and phenotype, it appears that patient groups with these labels overlap to some extent and that the most obvious common denominator is a poor growth velocity. Therefore, clinical studies are needed in children with growth disorders exhibiting partial hormone deficiencies. Such studies should aim to explore whether the combined effect of GH and IGF-I stimulates statural growth more effectively than monotherapy of IGF-I and GH, respectively, and whether side effects during combined GH and IGF-I treatment will be acceptable compared with those seen during monotherapy with IGF-I or GH.
One further potential indication for combined GH and IGF-I treatment is growth retardation in chronic renal failure (CRF). CRF in children is associated with growth retardation despite normal or elevated GH levels, consistent with a state of GH resistance (369,370). The IGF-binding capacity of CRF serum is increased by 7- to 10-fold due to alterations in IGFBP concentrations, resulting in decreased IGF bioactivity of CRF serum despite normal total IGF-I levels (370,371,372). Thus, CRF in children is associated with a functional IGF-I deficiency (370). GH treatment improves catch-up growth and allows most children with CRF to achieve normal adult height (373), but final adult height is still below the genetic target (373). In uremic rats, GH or IGF-I treatment alone induced an approximately similar increase in body growth, and an additive effect on longitudinal growth was demonstrated when both agents were administered together (374,375). In addition, concomitant GH treatment prevented the hypoglycemia that was noted with IGF-I treatment alone (375). It remains, however, to be determined whether combined GH and IGF-I treatment will be more effective than GH alone in children with CRF and whether side effects will be acceptable for the combined treatment.
C. Diabetes mellitus
Available clinical data show elevated 24-h GH release in both untreated and treated type 1 diabetic patients; exaggerated GH release in response to GHRH occurs consistently (202). Main regulators of GH secretion in patients with type 1 diabetes are nutritional intake, metabolic factors, and serum IGF-I concentration (202,217). Several lines of evidence suggest that insulin deficiency in the portal vein causes, at least partly, the GH hypersecretion observed in type 1 diabetic patients by regulating hepatic IGF-I regeneration (217). In short, in type 1 diabetic patients, the lack of insulin in the portal vein, combined with deteriorated nutritional status, results in reduced liver production of IGF-I, and as a consequence, low serum IGF-I levels (217). The low serum IGF-I levels, by lack of negative feedback action (see Section IV.B), result in compensatory high GH secretion. The GH hypersecretion then results in reduced insulin sensitivity due to the well-known diabetogenic effects of GH (see Section V.A). Furthermore, GH hypersecretion in type 1 diabetic patients may accelerate diabetic microvascular complications because there is an association between GH secretion and diabetic retinopathy (376,377,378). Treatment with sc injected insulin does not fully normalize the aberrations in the GH/IGF-I axis in type 1 diabetic patients, at least partly because sc injected insulin reaches the portal vein only to a small extent (217). It could, therefore, be hypothesized that addition of IGF-I treatment to insulin treatment in type 1 diabetic patients may reduce GH hypersecretion, thereby improving insulin sensitivity, retinopathy, and possibly also other diabetic microvascular complications in these patients (217,379). Because KO of IGF-I receptors in vascular endothelial cells protects against retinal neovascularization, this hypothesis is not without controversy (380).
Treatment trials performed thus far indicate that administration of a single dose of IGF-I or IGF-I treatment can reduce GH hypersecretion, improve insulin sensitivity, and decrease the requirement of insulin in adolescents and adults with type 1 diabetes mellitus for up to 3 months (218,381,382,383,384,385). Moreover, combined administration of IGF-I and IGFBP-3 for 2 wk induced similar types of improvements (235). However, beneficial effects did not last beyond 6 months of IGF-I treatment, likely due to poor compliance with the multi-injection regime required rather than a reduced effect of IGF-I (217,382).
In patients with type 2 diabetes mellitus, GH secretion is dependent on age and body composition, but clearly elevated GH levels are usually not seen (202). This gives further support for the notion that deficiency of insulin in the portal vein, via a reduction of circulating IGF-I, at least partly causes GH hypersecretion in type 1 diabetic patients. However, also in type 2 diabetic patients, short-term IGF-I treatment (234,386) or combined IGF-I and IGFBP-3 treatment (387,388) can improve glycemic control and reduce the requirement of insulin.
In conclusion, GH hypersecretion in type 1 diabetes mellitus associates with insulin resistance and retinopathy progression. Treatment studies performed thus far indicate that IGF-I administration can reduce GH hypersecretion, improve insulin sensitivity, and decrease the dose of insulin required. However, additional long-term IGF-I treatment studies are needed to evaluate whether addition of IGF-I treatment to insulin treatment can affect diabetic microvascular complications. Furthermore, it needs to be thoroughly evaluated whether possible beneficial effects of IGF-I treatment to patients with diabetes mellitus type 1 or 2 will be meaningful when taking into account side effects of IGF-I.
D. Other potential future indications
Based on experimental animal studies, blockade of endocrine IGF-I might speculatively be beneficial for treatments of some types of tumors (see recent review in Ref. 5) and for the reduction of prostate size in patients with benign prostate hyperplasia, but might result in detrimental effects on brain function as well as insulin resistance, hypertension, and cortical bone osteoporosis. Although IGF-I treatment could be hypothesized to be beneficial for cortical bone mass and memory function, it might also result in benign prostate hyperplasia, increased risk of progression of some tumors, hypoglycemia, and other well-known side effects of IGF-I. Therefore, an overall evaluation of positive and negative effects of IGF-I on various organ systems must be performed in these patient groups before determining the usefulness of IGF-I treatment.
VIII. Summary: The Role of Liver-Derived IGF-I
Studies using mice with liver-specific IGF-I inactivation have clearly established that the major part of circulating IGF-I is liver-derived. This indicates that liver-derived IGF-I might exert important endocrine effects. The experiments using different mouse models with tissue-specific IGF-I inactivation indicate that endocrine IGF-I and local bone-derived IGF-I to some extent have overlapping growth-promoting effects and might partly but not completely have the capacity to replace each other (= redundancy) in the maintenance of normal longitudinal bone growth. In contrast to the moderate importance of liver-derived IGF-I for the maintenance of normal longitudinal bone growth, it is now well established that liver-derived IGF-I is a major regulator of several other phenotypes. Importantly, and in contrast to the regulation of bone growth, locally derived IGF-I cannot replace liver-derived IGF-I for the regulation of these phenotypes (= lack of redundancy).
Loss of liver-derived IGF-I feedback on the hypothalamic pituitary system increases GH levels, an effect that seems to be exerted at the pituitary rather than the hypothalamic level. It is clear that some of the phenotypes in the liver-specific IGF-I KO mice are indirect, mediated via the elevated GH levels (Fig. 9). The phenotypes mediated indirectly via elevated GH levels include insulin resistance, increased liver size, and affected sexually dimorphic liver functions (Fig. 9, right column), whereas others including reduced cortical bone mass, reduced kidney size, reduced prostate size, increased peripheral vascular resistance, reduced sodium retention, reduced tumor progression (of some but not all tumors evaluated; see recent review in Ref. 5), and several of the affected brain functions are probably direct IGF-I effects (Fig. 9, left column) that are not mediated via the elevated GH levels.
Figure 9.
Proposed role of liver-derived IGF-I. This figure shows the authors’ proposed endocrine effects of liver-derived IGF-I discussed in this review. It is clear that a major role of liver-derived IGF-I is to regulate GH secretion and that some of the phenotypes in the liver-specific IGF-I KO mice are indirect via reduced GH feedback, resulting in elevated pituitary GH secretion. The phenotypes proposed to be mediated via the altered GH feedback (right column) include insulin resistance, increased liver size, and affected sexually dimorphic liver functions, whereas other effects such as reduced cortical bone mass, reduced kidney size, reduced prostate size, increased peripheral vascular resistance, reduced spatial memory, reduced sodium retention, and reduced tumor progression (of some but not all tumors evaluated, see Ref. 5 for recent review; not discussed in this review) are proposed to be direct IGF-I effects (left column). In addition, liver-derived IGF-I has the capacity to stimulate longitudinal bone growth directly (left column), but it is not required for essentially normal bone growth in the presence of normal IGF-I expression in bone (= redundancy). Importantly, and in contrast to the regulation of longitudinal bone growth, locally derived-IGF-I cannot replace liver-derived IGF-I for the regulation of the other described phenotypes in this figure (= lack of redundancy).
Some of the physiological effects of liver-derived IGF-I are summarized below. However, it should be emphasized that it is only possible to directly evaluate the relative importance of liver-derived IGF-I vs. local IGF-I for bone- and pancreas-related phenotypes because bone and pancreas are the only nonhepatic tissue in which IGF-I, thus far, has been successfully inactivated (52,53,224). Nevertheless, the role of liver-derived IGF-I can be determined for all evaluated phenotypes. Liver-derived IGF-I is a major stimulator of cortical bone size. Importantly, the serum IGF-I threshold below which IGF-I influences cortical bone mass is higher than that for body length. In contrast, local bone-derived IGF-I seems equally important for cortical bone and longitudinal bone growth. Specific elimination of liver-derived IGF-I results in increased insulin levels as well as decreased insulin sensitivity in muscle, liver, and fat tissues. It is now well established that liver-derived IGF-I regulates specific brain functions in adult mice. Circulating IGF-I is required to mediate the effect of exercise on anxiety and spatial learning, for reactive vessel modeling, and for clearance of brain amyloid β. Mice with liver-specific IGF-I KO demonstrate reduced exploratory activity as well as impaired spatial learning and memory.
Mice with liver-specific IGF-I inactivation display elevated blood pressure, endothelial dysfunction in resistance vessels, and increased peripheral vascular resistance. These data suggest that liver-derived IGF-I regulates blood pressure by modulating peripheral vascular resistance. Analyses of the kidneys in mice with liver-specific IGF-I inactivation demonstrated that liver-derived IGF-I is a specific stimulator of kidney size associated with major alterations in kidney IGF-II expression. In addition, liver-derived IGF-I reduces renal excretion of sodium.
Liver-specific IGF-I KO results in larger livers, probably due to decreased feedback inhibition by IGF-I on GH secretion and thereby resulting in GH-stimulated liver growth. Finally, adult mice with liver-specific inactivation of IGF-I display reduced prostate weight, prostate AR expression, and androgen-induced weight gain. We propose that liver-derived IGF-I, at least partly, increases prostate size by increasing the number of ARs and thereby androgen responsiveness. These experimental findings clearly establish liver-derived IGF-I as an endocrine factor with a variety of important physiological functions.
IX. General Conclusions
Studies comparing the impact of liver-derived IGF-I and local bone-derived IGF-I demonstrate that both sources of IGF-I have the capacity to stimulate longitudinal bone growth, although liver-derived IGF-I is not required for an essentially normal longitudinal bone growth. We propose that liver-derived circulating IGF-I and local bone-derived IGF-I have some overlapping growth-promoting effects and might have the capacity to replace each other (= redundancy) in the maintenance of normal longitudinal bone growth. Importantly, and in contrast to the regulation of longitudinal bone growth, locally derived IGF-I cannot replace (= lack of redundancy) liver-derived IGF-I for the regulation of a large number of other physiological functions including cortical bone mass, kidney size, prostate size, peripheral vascular resistance, spatial memory, sodium retention, insulin sensitivity, liver size, progression of some tumors, and sexually dimorphic liver functions. It is clear that a major role of liver-derived IGF-I is to regulate GH secretion and that some, but not all, of the phenotypes in liver-derived IGF-I KO mice are indirect, mediated via the elevated GH levels.
Future studies developing and evaluating several mouse models with local disruption of IGF-I in different IGF-I-dependent tissues are required for direct evaluation of the role of local IGF-I. However, these studies might be difficult to perform and/or evaluate because: 1) there are some limitations in the tissue specificity and efficiency for the different available tissue-specific promoters driving Cre expression in mice; 2) most tissues are heterogeneous, including several different cell types that might contribute to the local pool of IGF-I by paracrine mechanisms; and 3) liver-derived IGF and local IGF-I might exert redundant effects in some tissues.
We believe that all of the described multiple endocrine effects of liver-derived IGF-I should be considered in the development of possible novel treatment strategies aimed at increasing or reducing endocrine IGF-I activity. The substantially increased knowledge about the endocrine role of liver-derived IGF-I might result in improved/novel treatment strategies of IGF-I-dependent diseases in the future, but it is clear that further research in this area is required.
Footnotes
This work was supported by the Swedish Research Council, the Swedish Foundation for Strategic Research, The ALF/LUA research grant in Gothenburg, the Lundberg Foundation, the Torsten and Ragnar Söderberg Foundation, the Novo Nordisk Foundation, and National Institute of Arthritis and Musculoskeletal and Skin Diseases Grants AR-048139 and AR-031062 (to S.M.).
Disclosure Summary: C.O., S.M., K.S., Å.T., J.I., J.-O.J., and J.S. have nothing to declare. O.I. was cofounder of Tercica Inc., a company that produced IGF-I for use in children of short stature. Tercica Inc. was acquired by Ipsen June 2008. O.I. has no current ownership of stocks or options in Tercica or Ipsen.
First Published Online July 9, 2009
Abbreviations: AD, Alzheimer’s disease; ALS, acid-labile subunit; AR, androgen receptor; BMD, bone mineral density; CRF, chronic renal failure; DLP, dorsolateral prostate; EGF, epidermal growth factor; GHS, GH secretagogue; IGFBP, IGF binding protein; KO, knockout; LIP, liver IGF-I producer; MUP, major urinary protein; rhIGF-I, recombinant human IGF-I; SHP, SH domain containing protein tyrosine phosphatase; STAT5b, signal transducers and activators of transcription protein 5b; VP, ventral prostate; VSMC, vascular smooth muscle cell.
References
- Daughaday WH, Rotwein P 1989 Insulin-like growth factors I and II. Peptide, messenger ribonucleic acid and gene structures, serum, and tissue concentrations. Endocr Rev 10:68–91 [DOI] [PubMed] [Google Scholar]
- Isaksson OG, Lindahl A, Nilsson A, Isgaard J 1987 Mechanism of the stimulatory effect of growth hormone on longitudinal bone growth. Endocr Rev 8:426–438 [DOI] [PubMed] [Google Scholar]
- Ohlsson C, Bengtsson BA, Isaksson OG, Andreassen TT, Slootweg MC 1998 Growth hormone and bone. Endocr Rev 19:55–79 [DOI] [PubMed] [Google Scholar]
- Le Roith D, Bondy C, Yakar S, Liu JL, Butler A 2001 The somatomedin hypothesis: 2001. Endocr Rev 22:53–74 [DOI] [PubMed] [Google Scholar]
- Samani AA, Yakar S, LeRoith D, Brodt P 2007 The role of the IGF system in cancer growth and metastasis: overview and recent insights. Endocr Rev 28:20–47 [DOI] [PubMed] [Google Scholar]
- Yakar S, Sun H, Zhao H, Pennisi P, Toyoshima Y, Setser J, Stannard B, Scavo L, Leroith D 2005 Metabolic effects of IGF-I deficiency: lessons from mouse models. Pediatr Endocrinol Rev 3:11–19 [PubMed] [Google Scholar]
- Jansson JO, Edén S, Isaksson O 1985 Sexual dimorphism in the control of growth hormone secretion. Endocr Rev 6:128–150 [DOI] [PubMed] [Google Scholar]
- Rajaram S, Baylink DJ, Mohan S 1997 Insulin-like growth factor-binding proteins in serum and other biological fluids: regulation and functions. Endocr Rev 18:801–831 [DOI] [PubMed] [Google Scholar]
- Jones JI, Clemmons DR 1995 Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev 16:3–34 [DOI] [PubMed] [Google Scholar]
- Boisclair YR, Rhoads RP, Ueki I, Wang J, Ooi GT 2001 The acid-labile subunit (ALS) of the 150 kDa IGF-binding protein complex: an important but forgotten component of the circulating IGF system. J Endocrinol 170:63–70 [DOI] [PubMed] [Google Scholar]
- Sjögren K, Liu JL, Blad K, Skrtic S, Vidal O, Wallenius V, LeRoith D, Törnell J, Isaksson OG, Jansson JO, Ohlsson C 1999 Liver-derived insulin-like growth factor I (IGF-I) is the principal source of IGF-I in blood but is not required for postnatal body growth in mice. Proc Natl Acad Sci USA 96:7088–7092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakar S, Liu JL, Stannard B, Butler A, Accili D, Sauer B, LeRoith D 1999 Normal growth and development in the absence of hepatic insulin-like growth factor I. Proc Natl Acad Sci USA 96:7324–7329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims NA, Clément-Lacroix P, Da Ponte F, Bouali Y, Binart N, Moriggl R, Goffin V, Coschigano K, Gaillard-Kelly M, Kopchick J, Baron R, Kelly PA 2000 Bone homeostasis in growth hormone receptor-null mice is restored by IGF-I but independent of Stat5. J Clin Invest 106:1095–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjögren K, Bohlooly-Y M, Bohlooly YM, Olsson B, Coschigano K, Törnell J, Mohan S, Isaksson OG, Baumann G, Kopchick J, Ohlsson C 2000 Disproportional skeletal growth and markedly decreased bone mineral content in growth hormone receptor −/− mice. Biochem Biophys Res Commun 267:603–608 [DOI] [PubMed] [Google Scholar]
- Zhou Y, Xu BC, Maheshwari HG, He L, Reed M, Lozykowski M, Okada S, Cataldo L, Coschigamo K, Wagner TE, Baumann G, Kopchick JJ 1997 A mammalian model for Laron syndrome produced by targeted disruption of the mouse growth hormone receptor/binding protein gene (the Laron mouse). Proc Natl Acad Sci USA 94:13215–13220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wit JM, van Unen H 1992 Growth of infants with neonatal growth hormone deficiency. Arch Dis Child 67:920–924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage MO, Blum WF, Ranke MB, Postel-Vinay MC, Cotterill AM, Hall K, Chatelain PG, Preece MA, Rosenfeld RG 1993 Clinical features and endocrine status in patients with growth hormone insensitivity (Laron syndrome). J Clin Endocrinol Metab 77:1465–1471 [DOI] [PubMed] [Google Scholar]
- Wajnrajch MP, Gertner JM, Harbison MD, Chua Jr SC, Leibel RL 1996 Nonsense mutation in the human growth hormone-releasing hormone receptor causes growth failure analogous to the little (lit) mouse. Nat Genet 12:88–90 [DOI] [PubMed] [Google Scholar]
- Lupu F, Terwilliger JD, Lee K, Segre GV, Efstratiadis A 2001 Roles of growth hormone and insulin-like growth factor 1 in mouse postnatal growth. Dev Biol 229:141–162 [DOI] [PubMed] [Google Scholar]
- Efstratiadis A 1998 Genetics of mouse growth. Int J Dev Biol 42:955–976 [PubMed] [Google Scholar]
- Baker J, Liu JP, Robertson EJ, Efstratiadis A 1993 Role of insulin-like growth factors in embryonic and postnatal growth. Cell 75:73–82 [PubMed] [Google Scholar]
- Liu JP, Baker J, Perkins AS, Robertson EJ, Efstratiadis A 1993 Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r). Cell 75:59–72 [PubMed] [Google Scholar]
- Powell-Braxton L, Hollingshead P, Warburton C, Dowd M, Pitts-Meek S, Dalton D, Gillett N, Stewart TA 1993 IGF-I is required for normal embryonic growth in mice. Genes Dev 7:2609–2617 [DOI] [PubMed] [Google Scholar]
- Abuzzahab MJ, Schneider A, Goddard A, Grigorescu F, Lautier C, Keller E, Kiess W, Klammt J, Kratzsch J, Osgood D, Pfäffle R, Raile K, Seidel B, Smith RJ, Chernausek SD 2003 IGF-I receptor mutations resulting in intrauterine and postnatal growth retardation. N Engl J Med 349:2211–2222 [DOI] [PubMed] [Google Scholar]
- Woods KA, Camacho-Hübner C, Savage MO, Clark AJ 1996 Intrauterine growth retardation and postnatal growth failure associated with deletion of the insulin-like growth factor I gene. N Engl J Med 335:1363–1367 [DOI] [PubMed] [Google Scholar]
- Arends N, Johnston L, Hokken-Koelega A, van Duijn C, de Ridder M, Savage M, Clark A 2002 Polymorphism in the IGF-I gene: clinical relevance for short children born small for gestational age (SGA). J Clin Endocrinol Metab 87:2720 [DOI] [PubMed] [Google Scholar]
- Vaessen N, Janssen JA, Heutink P, Hofman A, Lamberts SW, Oostra BA, Pols HA, van Duijn CM 2002 Association between genetic variation in the gene for insulin-like growth factor-I and low birthweight. Lancet 359:1036–1037 [DOI] [PubMed] [Google Scholar]
- Walenkamp MJ, Wit JM 2007 Genetic disorders in the GH IGF-I axis in mouse and man. Eur J Endocrinol 157(Suppl 1):S15–S26 [DOI] [PubMed] [Google Scholar]
- Eigenmann JE, Amador A, Patterson DF 1988 Insulin-like growth factor I levels in proportionate dogs, chondrodystrophic dogs and in giant dogs. Acta Endocrinol (Copenh) 118:105–108 [DOI] [PubMed] [Google Scholar]
- Tryfonidou MA, Holl MS, Vastenburg M, Oosterlaken-Dijksterhuis MA, Birkenhäger-Frenkel DH, van den Brom WE, Hazewinkel HA 2003 Hormonal regulation of calcium homeostasis in two breeds of dogs during growth at different rates. J Anim Sci 81:1568–1580 [DOI] [PubMed] [Google Scholar]
- Sutter NB, Bustamante CD, Chase K, Gray MM, Zhao K, Zhu L, Padhukasahasram B, Karlins E, Davis S, Jones PG, Quignon P, Johnson GS, Parker HG, Fretwell N, Mosher DS, Lawler DF, Satyaraj E, Nordborg M, Lark KG, Wayne RK, Ostrander EA 2007 A single IGF1 allele is a major determinant of small size in dogs. Science 316:112–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daughaday WH, Hall K, Raben MS, Salmon Jr WD, van den Brande JL, van Wyk JJ 1972 Somatomedin: proposed designation for sulphation factor. Nature 235:107 [DOI] [PubMed] [Google Scholar]
- Daughaday W 1981 Growth hormone and the somatomedins. In: Daughaday WH, ed. Endocrine control of growth. New York: Elsevier; 1–24 [Google Scholar]
- D'Ercole AJ, Applewhite GT, Underwood LE 1980 Evidence that somatomedin is synthesized by multiple tissues in the fetus. Dev Biol 75:315–328 [DOI] [PubMed] [Google Scholar]
- D'Ercole AJ, Stiles AD, Underwood LE 1984 Tissue concentrations of somatomedin C: further evidence for multiple sites of synthesis and paracrine or autocrine mechanisms of action. Proc Natl Acad Sci USA 81:935–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isgaard J, Carlsson L, Isaksson OG, Jansson JO 1988 Pulsatile intravenous growth hormone (GH) infusion to hypophysectomized rats increases insulin-like growth factor I messenger ribonucleic acid in skeletal tissues more effectively than continuous GH infusion. Endocrinology 123:2605–2610 [DOI] [PubMed] [Google Scholar]
- Isgaard J, Möller C, Isaksson OG, Nilsson A, Mathews LS, Norstedt G 1988 Regulation of insulin-like growth factor messenger ribonucleic acid in rat growth plate by growth hormone. Endocrinology 122:1515–1520 [DOI] [PubMed] [Google Scholar]
- Lowe Jr WL, Roberts Jr CT, Lasky SR, LeRoith D 1987 Differential expression of alternative 5′ untranslated regions in mRNAs encoding rat insulin-like growth factor I. Proc Natl Acad Sci USA 84:8946–8950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe Jr WL, Lasky SR, LeRoith D, Roberts Jr CT 1988 Distribution and regulation of rat insulin-like growth factor I messenger ribonucleic acids encoding alternative carboxyterminal E-peptides: evidence for differential processing and regulation in liver. Mol Endocrinol 2:528–535 [DOI] [PubMed] [Google Scholar]
- Roberts Jr CT, Lasky SR, Lowe Jr WL, Seaman WT, LeRoith D 1987 Molecular cloning of rat insulin-like growth factor I complementary deoxyribonucleic acids: differential messenger ribonucleic acid processing and regulation by growth hormone in extrahepatic tissues. Mol Endocrinol 1:243–248 [DOI] [PubMed] [Google Scholar]
- Isaksson OG, Jansson JO, Gause IA 1982 Growth hormone stimulates longitudinal bone growth directly. Science 216:1237–1239 [DOI] [PubMed] [Google Scholar]
- Russell SM, Spencer EM 1985 Local injections of human or rat growth hormone or of purified human somatomedin-C stimulate unilateral tibial epiphyseal growth in hypophysectomized rats. Endocrinology 116:2563–2567 [DOI] [PubMed] [Google Scholar]
- Isgaard J, Nilsson A, Lindahl A, Jansson JO, Isaksson OG 1986 Effects of local administration of GH and IGF-1 on longitudinal bone growth in rats. Am J Physiol 250:E367–E372 [DOI] [PubMed] [Google Scholar]
- Nilsson A, Isgaard J, Lindahl A, Dahlström A, Skottner A, Isaksson OG 1986 Regulation by growth hormone of number of chondrocytes containing IGF-I in rat growth plate. Science 233:571–574 [DOI] [PubMed] [Google Scholar]
- Schlechter NL, Russell SM, Greenberg S, Spencer EM, Nicoll CS 1986 A direct growth effect of growth hormone in rat hindlimb shown by arterial infusion. Am J Physiol 250:E231–E235 [DOI] [PubMed] [Google Scholar]
- Isaksson OG, Ohlsson C, Nilsson A, Isgaard J, Lindahl A 1991 Regulation of cartilage growth by growth hormone and insulin-like growth factor I. Pediatr Nephrol 5:451–453 [DOI] [PubMed] [Google Scholar]
- Schlechter NL, Russell SM, Spencer EM, Nicoll CS 1986 Evidence suggesting that the direct growth-promoting effect of growth hormone on cartilage in vivo is mediated by local production of somatomedin. Proc Natl Acad Sci USA 83:7932–7934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage MO, Attie KM, David A, Metherell LA, Clark AJ, Camacho-Hübner C 2006 Endocrine assessment, molecular characterization and treatment of growth hormone insensitivity disorders. Nat Clin Pract Endocrinol Metab 2:395–407 [DOI] [PubMed] [Google Scholar]
- Backeljauw PF, Underwood LE 2001 Therapy for 6.5–7.5 years with recombinant insulin-like growth factor I in children with growth hormone insensitivity syndrome: a clinical research center study. J Clin Endocrinol Metab 86:1504–1510 [DOI] [PubMed] [Google Scholar]
- Mohan S, Richman C, Guo R, Amaar Y, Donahue LR, Wergedal J, Baylink DJ 2003 Insulin-like growth factor regulates peak bone mineral density in mice by both growth hormone-dependent and -independent mechanisms. Endocrinology 144:929–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn R, Schwenk F, Aguet M, Rajewsky K 1995 Inducible gene targeting in mice. Science 269:1427–1429 [DOI] [PubMed] [Google Scholar]
- Govoni KE, Lee SK, Chung YS, Behringer RR, Wergedal JE, Baylink DJ, Mohan S 2007 Disruption of insulin-like growth factor-I expression in type IIαI collagen-expressing cells reduces bone length and width in mice. Physiol Genomics 30:354–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govoni KE, Wergedal JE, Florin L, Angel P, Baylink DJ, Mohan S 2007 Conditional deletion of insulin-like growth factor-I in collagen type 1α2-expressing cells results in postnatal lethality and a dramatic reduction in bone accretion. Endocrinology 148:5706–5715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratikopoulos E, Szabolcs M, Dragatsis I, Klinakis A, Efstratiadis A 2008 The hormonal action of IGF1 in postnatal mouse growth. Proc Natl Acad Sci USA 105:19378–19383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlsson C, Sjögren K, Jansson JO, Isaksson OG 2000 The relative importance of endocrine versus autocrine/paracrine insulin-like growth factor-I in the regulation of body growth. Pediatr Nephrol 14:541–543 [DOI] [PubMed] [Google Scholar]
- Fan Y, Menon RK, Cohen P, Hwang D, Clemens T, Digirolamo DJ, Kopchick JJ, Leroith D, Trucco M, Sperling MA 2009 Liver-specific deletion of the growth hormone receptor reveals essential role of GH signaling in hepatic lipid metabolism. J Biol Chem 284:19937–19944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallenius K, Sjögren K, Peng XD, Park S, Wallenius V, Liu JL, Umaerus M, Wennbo H, Isaksson O, Frohman L, Kineman R, Ohlsson C, Jansson JO 2001 Liver-derived IGF-I regulates GH secretion at the pituitary level in mice. Endocrinology 142:4762–4770 [DOI] [PubMed] [Google Scholar]
- Sjögren K, Sheng M, Movérare S, Liu JL, Wallenius K, Törnell J, Isaksson O, Jansson JO, Mohan S, Ohlsson C 2002 Effects of liver-derived insulin-like growth factor I on bone metabolism in mice. J Bone Miner Res 17:1977–1987 [DOI] [PubMed] [Google Scholar]
- LeRoith D 2008 Clinical relevance of systemic and local IGF-I: lessons from animal models. Pediatr Endocrinol Rev 5(Suppl 2):739–743 [PubMed] [Google Scholar]
- Ueki I, Ooi GT, Tremblay ML, Hurst KR, Bach LA, Boisclair YR 2000 Inactivation of the acid labile subunit gene in mice results in mild retardation of postnatal growth despite profound disruptions in the circulating insulin-like growth factor system. Proc Natl Acad Sci USA 97:6868–6873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakar S, Rosen CJ, Bouxsein ML, Sun H, Mejia W, Kawashima Y, Wu Y, Emerton K, Williams V, Jepsen K, Schaffler MB, Majeska RJ, Gavrilova O, Gutierrez M, Hwang D, Pennisi P, Frystyk J, Boisclair Y, Pintar J, Jasper H, Domene H, Cohen P, Clemmons D, LeRoith D 2009 Serum complexes of insulin-like growth factor-1 modulate skeletal integrity and carbohydrate metabolism. FASEB J 23:709–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakar S, Rosen CJ, Beamer WG, Ackert-Bicknell CL, Wu Y, Liu JL, Ooi GT, Setser J, Frystyk J, Boisclair YR, LeRoith D 2002 Circulating levels of IGF-1 directly regulate bone growth and density. J Clin Invest 110:771–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin E, Zhou J, Dai J, Baxter RC, Bondy CA 1994 Cellular localization and regulation of gene expression for components of the insulin-like growth factor ternary binding protein complex. Endocrinology 134:2498–2504 [DOI] [PubMed] [Google Scholar]
- Jaques G, Noll K, Wegmann B, Witten S, Kogan E, Radulescu RT, Havemann K 1997 Nuclear localization of insulin-like growth factor binding protein 3 in a lung cancer cell line. Endocrinology 138:1767–1770 [DOI] [PubMed] [Google Scholar]
- Schedlich LJ, Young TF, Firth SM, Baxter RC 1998 Insulin-like growth factor-binding protein (IGFBP)-3 and IGFBP-5 share a common nuclear transport pathway in T47D human breast carcinoma cells. J Biol Chem 273:18347–18352 [DOI] [PubMed] [Google Scholar]
- Yamanaka Y, Fowlkes JL, Wilson EM, Rosenfeld RG, Oh Y 1999 Characterization of insulin-like growth factor binding protein-3 (IGFBP-3) binding to human breast cancer cells: kinetics of IGFBP-3 binding and identification of receptor binding domain on the IGFBP-3 molecule. Endocrinology 140:1319–1328 [DOI] [PubMed] [Google Scholar]
- Schedlich LJ, Le Page SL, Firth SM, Briggs LJ, Jans DA, Baxter RC 2000 Nuclear import of insulin-like growth factor-binding protein-3 and -5 is mediated by the importin β subunit. J Biol Chem 275:23462–23470 [DOI] [PubMed] [Google Scholar]
- Lee KW, Ma L, Yan X, Liu B, Zhang XK, Cohen P 2005 Rapid apoptosis induction by IGFBP-3 involves an insulin-like growth factor-independent nucleomitochondrial translocation of RXRα/Nur77. J Biol Chem 280:16942–16948 [DOI] [PubMed] [Google Scholar]
- Kaplan SA, Cohen P 2007 The somatomedin hypothesis 2007: 50 years later. J Clin Endocrinol Metab 92:4529–4535 [DOI] [PubMed] [Google Scholar]
- Liao L, Dearth RK, Zhou S, Britton OL, Lee AV, Xu J 2006 Liver-specific overexpression of the insulin-like growth factor-I enhances somatic growth and partially prevents the effects of growth hormone deficiency. Endocrinology 147:3877–3888 [DOI] [PubMed] [Google Scholar]
- Ohlsson C, Nilsson A, Isaksson OG, Lindahl A 1992 Effect of growth hormone and insulin-like growth factor-I on DNA synthesis and matrix production in rat epiphyseal chondrocytes in monolayer culture. J Endocrinol 133:291–300 [DOI] [PubMed] [Google Scholar]
- Nilsson A, Ohlsson C, Isaksson OG, Lindahl A, Isgaard J 1994 Hormonal regulation of longitudinal bone growth. Eur J Clin Nutr 48(Suppl 1):S150–S158; discussion S158–S160 [DOI] [PubMed] [Google Scholar]
- Ohlsson C, Nilsson A, Isaksson O, Lindahl A 1992 Growth hormone induces multiplication of the slowly cycling germinal cells of the rat tibial growth plate. Proc Natl Acad Sci USA 89:9826–9830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Zhou J, Cheng CM, Kopchick JJ, Bondy CA 2004 Evidence supporting dual, IGF-I-independent and IGF-I-dependent, roles for GH in promoting longitudinal bone growth. J Endocrinol 180:247–255 [DOI] [PubMed] [Google Scholar]
- Gevers EF, Hannah MJ, Waters MJ, Robinson IC 9 April 2009 Regulation of rapid Stat5 phosphorylation in the resting cells of the growth plate and in the liver by growth hormone and feeding. Endocrinology 150:3627–3636 [DOI] [PubMed] [Google Scholar]
- Hunziker EB, Wagner J, Zapf J 1994 Differential effects of insulin-like growth factor I and growth hormone on developmental stages of rat growth plate chondrocytes in vivo. J Clin Invest 93:1078–1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Zhou J, Bondy CA 1999 Igf1 promotes longitudinal bone growth by insulin-like actions augmenting chondrocyte hypertrophy. FASEB J 13:1985–1990 [DOI] [PubMed] [Google Scholar]
- Pfäffle RW, DiMattia GE, Parks JS, Brown MR, Wit JM, Jansen M, Van der Nat H, Van den Brande JL, Rosenfeld MG, Ingraham HA 1992 Mutation of the POU-specific domain of Pit-1 and hypopituitarism without pituitary hypoplasia. Science 257:1118–1121 [DOI] [PubMed] [Google Scholar]
- Tatsumi K, Miyai K, Notomi T, Kaibe K, Amino N, Mizuno Y, Kohno H 1992 Cretinism with combined hormone deficiency caused by a mutation in the PIT1 gene. Nat Genet 1:56–58 [DOI] [PubMed] [Google Scholar]
- Phillips 3rd JA, Cogan JD 1994 Genetic basis of endocrine disease. 6. Molecular basis of familial human growth hormone deficiency. J Clin Endocrinol Metab 78:11–16 [DOI] [PubMed] [Google Scholar]
- Maheshwari HG, Silverman BL, Dupuis J, Baumann G 1998 Phenotype and genetic analysis of a syndrome caused by an inactivating mutation in the growth hormone-releasing hormone receptor: Dwarfism of Sindh. J Clin Endocrinol Metab 83:4065–4074 [DOI] [PubMed] [Google Scholar]
- Wu W, Cogan JD, Pfäffle RW, Dasen JS, Frisch H, O'Connell SM, Flynn SE, Brown MR, Mullis PE, Parks JS, Phillips 3rd JA, Rosenfeld MG 1998 Mutations in PROP1 cause familial combined pituitary hormone deficiency. Nat Genet 18:147–149 [DOI] [PubMed] [Google Scholar]
- Rosenbloom AL, Guevara-Aguirre J, Rosenfeld RG, Francke U 1999 Growth hormone receptor deficiency in Ecuador. J Clin Endocrinol Metab 84:4436–4443 [DOI] [PubMed] [Google Scholar]
- Kofoed EM, Hwa V, Little B, Woods KA, Buckway CK, Tsubaki J, Pratt KL, Bezrodnik L, Jasper H, Tepper A, Heinrich JJ, Rosenfeld RG 2003 Growth hormone insensitivity associated with a STAT5b mutation. N Engl J Med 349:1139–1147 [DOI] [PubMed] [Google Scholar]
- Laron Z 2004 Laron syndrome (primary growth hormone resistance or insensitivity): the personal experience 1958–2003. J Clin Endocrinol Metab 89:1031–1044 [DOI] [PubMed] [Google Scholar]
- Vidarsdottir S, Walenkamp MJ, Pereira AM, Karperien M, van Doorn J, van Duyvenvoorde HA, White S, Breuning MH, Roelfsema F, Kruithof MF, van Dissel J, Janssen R, Wit JM, Romijn JA 2006 Clinical and biochemical characteristics of a male patient with a novel homozygous STAT5b mutation. J Clin Endocrinol Metab 91:3482–3485 [DOI] [PubMed] [Google Scholar]
- Rosenfeld RG, Rosenbloom AL, Guevara-Aguirre J 1994 Growth hormone (GH) insensitivity due to primary GH receptor deficiency. Endocr Rev 15:369–390 [DOI] [PubMed] [Google Scholar]
- Walenkamp MJ, Karperien M, Pereira AM, Hilhorst-Hofstee Y, van Doorn J, Chen JW, Mohan S, Denley A, Forbes B, van Duyvenvoorde HA, van Thiel SW, Sluimers CA, Bax JJ, de Laat JA, Breuning MB, Romijn JA, Wit JM 2005 Homozygous and heterozygous expression of a novel insulin-like growth factor-I mutation. J Clin Endocrinol Metab 90:2855–2864 [DOI] [PubMed] [Google Scholar]
- Bonapace G, Concolino D, Formicola S, Strisciuglio P 2003 A novel mutation in a patient with insulin-like growth factor 1 (IGF1) deficiency. J Med Genet 40:913–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods KA, Camacho-Hübner C, Bergman RN, Barter D, Clark AJ, Savage MO 2000 Effects of insulin-like growth factor-I (IGF-I) therapy on body composition and insulin resistance in IGF-I gene deletion. J Clin Endocrinol Metab 85:1407–1411 [DOI] [PubMed] [Google Scholar]
- Inagaki K, Tiulpakov A, Rubtsov P, Sverdlova P, Peterkova V, Yakar S, Terekhov S, LeRoith D 2007 A familial insulin-like growth factor-I receptor mutant leads to short stature: clinical and biochemical characterization. J Clin Endocrinol Metab 92:1542–1548 [DOI] [PubMed] [Google Scholar]
- Kawashima Y, Kanzaki S, Yang F, Kinoshita T, Hanaki K, Nagaishi J, Ohtsuka Y, Hisatome I, Ninomoya H, Nanba E, Fukushima T, Takahashi S 2005 Mutation at cleavage site of insulin-like growth factor receptor in a short-stature child born with intrauterine growth retardation. J Clin Endocrinol Metab 90:4679–4687 [DOI] [PubMed] [Google Scholar]
- Raile K, Klammt J, Schneider A, Keller A, Laue S, Smith R, Pfäffle R, Kratzsch J, Keller E, Kiess W 2006 Clinical and functional characteristics of the human Arg59Ter insulin-like growth factor I receptor (IGF1R) mutation: implications for a gene dosage effect of the human IGF1R. J Clin Endocrinol Metab 91:2264–2271 [DOI] [PubMed] [Google Scholar]
- Walenkamp MJ, van der Kamp HJ, Pereira AM, Kant SG, van Duyvenvoorde HA, Kruithof MF, Breuning MH, Romijn JA, Karperien M, Wit JM 2006 A variable degree of intrauterine and postnatal growth retardation in a family with a missense mutation in the insulin-like growth factor I receptor. J Clin Endocrinol Metab 91:3062–3070 [DOI] [PubMed] [Google Scholar]
- Okubo Y, Siddle K, Firth H, O'Rahilly S, Wilson LC, Willatt L, Fukushima T, Takahashi S, Petry CJ, Saukkonen T, Stanhope R, Dunger DB 2003 Cell proliferation activities on skin fibroblasts from a short child with absence of one copy of the type 1 insulin-like growth factor receptor (IGF1R) gene and a tall child with three copies of the IGF1R gene. J Clin Endocrinol Metab 88:5981–5988 [DOI] [PubMed] [Google Scholar]
- Walenkamp MJ, de Muinck Keizer-Schrama SM, de Mos M, Kalf ME, van Duyvenvoorde HA, Boot AM, Kant SG, White SJ, Losekoot M, Den Dunnen JT, Karperien M, Wit JM 2008 Successful long-term growth hormone therapy in a girl with haploinsufficiency of the insulin-like growth factor-I receptor due to a terminal 15q26.2->qter deletion detected by multiplex ligation probe amplification. J Clin Endocrinol Metab 93:2421–2425 [DOI] [PubMed] [Google Scholar]
- Kant SG, Kriek M, Walenkamp MJ, Hansson KB, van Rhijn A, Clayton-Smith J, Wit JM, Breuning MH 2007 Tall stature and duplication of the insulin-like growth factor I receptor gene. Eur J Med Genet 50:1–10 [DOI] [PubMed] [Google Scholar]
- Domené HM, Bengolea SV, Martínez AS, Ropelato MG, Pennisi P, Scaglia P, Heinrich JJ, Jasper HG 2004 Deficiency of the circulating insulin-like growth factor system associated with inactivation of the acid-labile subunit gene. N Engl J Med 350:570–577 [DOI] [PubMed] [Google Scholar]
- Domené HM, Martínez AS, Frystyk J, Bengolea SV, Ropelato MG, Scaglia PA, Chen JW, Heuck C, Wolthers OD, Heinrich JJ, Jasper HG 2007 Normal growth spurt and final height despite low levels of all forms of circulating insulin-like growth factor-I in a patient with acid-labile subunit deficiency. Horm Res 67:243–249 [DOI] [PubMed] [Google Scholar]
- Domené HM, Scaglia PA, Lteif A, Mahmud FH, Kirmani S, Frystyk J, Bedecarrás P, Gutiérrez M, Jasper HG 2007 Phenotypic effects of null and haploinsufficiency of acid-labile subunit in a family with two novel IGFALS gene mutations. J Clin Endocrinol Metab 92:4444–4450 [DOI] [PubMed] [Google Scholar]
- Hwa V, Haeusler G, Pratt KL, Little BM, Frisch H, Koller D, Rosenfeld RG 2006 Total absence of functional acid labile subunit, resulting in severe insulin-like growth factor deficiency and moderate growth failure. J Clin Endocrinol Metab 91:1826–1831 [DOI] [PubMed] [Google Scholar]
- van Duyvenvoorde HA, Kempers MJ, Twickler TB, van Doorn J, Gerver WJ, Noordam C, Losekoot M, Karperien M, Wit JM, Hermus AR 2008 Homozygous and heterozygous expression of a novel mutation of the acid-labile subunit. Eur J Endocrinol 159:113–120 [DOI] [PubMed] [Google Scholar]
- Heath KE, Argente J, Barrios V, Pozo J, Díaz-González F, Martos-Moreno GA, Caimari M, Gracia R, Campos-Barros A 2008 Primary acid-labile subunit deficiency due to recessive IGFALS mutations results in postnatal growth deficit associated with low circulating insulin growth factor (IGF)-I, IGF binding protein-3 levels, and hyperinsulinemia. J Clin Endocrinol Metab 93:1616–1624 [DOI] [PubMed] [Google Scholar]
- Canalis E 1997 Insulin-like growth factors and osteoporosis. Bone 21:215–216 [DOI] [PubMed] [Google Scholar]
- Mohan S, Baylink DJ 1996 Insulin-like growth factor system components and the coupling of bone formation to resorption. Horm Res 45(Suppl 1):59–62 [DOI] [PubMed] [Google Scholar]
- Mohan S, Baylink D 1998 Mechanism of age-related bone loss: growth hormone/IGFs. In: Rosen CJ, ed. The aging skeleton. San Diego: Academic Press; 457–496 [Google Scholar]
- Mohan S, Baylink D 1999 IGF system components and their role in bone metabolism. In: Rosenfeld RG, Roberts C, eds. IGFs in health and diseases. Totowa, NJ: Humana Press; 457–496 [Google Scholar]
- Rosen CJ, Donahue LR 1998 Insulin-like growth factors and bone: the osteoporosis connection revisited. Proc Soc Exp Biol Med 219:1–7 [DOI] [PubMed] [Google Scholar]
- Mohan S, Baylink D 1999 Role of growth hormone/insulin-like growth factor axis. In: Rosen CJ, Bilezikian, JP, eds. The aging skeleton. San Diego: Academic Press; 209–219 [Google Scholar]
- Mohan S, Bautista CM, Wergedal J, Baylink DJ 1989 Isolation of an inhibitory insulin-like growth factor (IGF) binding protein from bone cell-conditioned medium: a potential local regulator of IGF action. Proc Natl Acad Sci USA 86:8338–8342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan S, Nakao Y, Honda Y, Landale E, Leser U, Dony C, Lang K, Baylink DJ 1995 Studies on the mechanisms by which insulin-like growth factor (IGF) binding protein-4 (IGFBP-4) and IGFBP-5 modulate IGF actions in bone cells. J Biol Chem 270:20424–20431 [DOI] [PubMed] [Google Scholar]
- Kasukawa Y, Miyakoshi N, Mohan S 2004 The anabolic effects of GH/IGF system on bone. Curr Pharm Des 10:2577–2592 [DOI] [PubMed] [Google Scholar]
- Nilsson A, Swolin D, Enerback S, Ohlsson C 1995 Expression of functional growth hormone receptors in cultured human osteoblast-like cells. J Clin Endocrinol Metab 80:3483–3488 [DOI] [PubMed] [Google Scholar]
- Ernst M, Froesch ER 1988 Growth hormone dependent stimulation of osteoblast-like cells in serum-free cultures via local synthesis of insulin-like growth factor I. Biochem Biophys Res Commun 151:142–147 [DOI] [PubMed] [Google Scholar]
- Canalis E 1993 Insulin like growth factors and the local regulation of bone formation. Bone 14:273–276 [DOI] [PubMed] [Google Scholar]
- Canalis E, Gabbitas B 1995 Skeletal growth factors regulate the synthesis of insulin-like growth factor binding protein-5 in bone cell cultures. J Biol Chem 270:10771–10776 [DOI] [PubMed] [Google Scholar]
- Conover CA 2000 In vitro studies of insulin-like growth factor I and bone. Growth Horm IGF Res 10(Suppl B):S107–S110 [DOI] [PubMed] [Google Scholar]
- Mohan S, Baylink DJ 1991 Bone growth factors. Clin Orthop Relat Res:30–48 [PubMed] [Google Scholar]
- Rosen CJ, Donahue LR, Hunter SJ 1994 Insulin-like growth factors and bone: the osteoporosis connection. Proc Soc Exp Biol Med 206:83–102 [DOI] [PubMed] [Google Scholar]
- Bikle DD 2008 Growth hormone/insulin-like growth factor-1/PTH axis in bone. J Bone Miner Res 23:581–583 [DOI] [PubMed] [Google Scholar]
- Jilka RL 2007 Molecular and cellular mechanisms of the anabolic effect of intermittent PTH. Bone 40:1434–1446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettway GJ, Meganck JA, Koh AJ, Keller ET, Goldstein SA, McCauley LK 2008 Parathyroid hormone mediates bone growth through the regulation of osteoblast proliferation and differentiation. Bone 42:806–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linkhart TA, Mohan S 1989 Parathyroid hormone stimulates release of insulin-like growth factor-I (IGF-I) and IGF-II from neonatal mouse calvaria in organ culture. Endocrinology 125:1484–1491 [DOI] [PubMed] [Google Scholar]
- McCarthy TL, Centrella M, Canalis E 1989 Parathyroid hormone enhances the transcript and polypeptide levels of insulin-like growth factor I in osteoblast-enriched cultures from fetal rat bone. Endocrinology 124:1247–1253 [DOI] [PubMed] [Google Scholar]
- Bikle DD, Sakata T, Leary C, Elalieh H, Ginzinger D, Rosen CJ, Beamer W, Majumdar S, Halloran BP 2002 Insulin-like growth factor I is required for the anabolic actions of parathyroid hormone on mouse bone. J Bone Miner Res 17:1570–1578 [DOI] [PubMed] [Google Scholar]
- Maulén NP, Henríquez EA, Kempe S, Cárcamo JG, Schmid-Kotsas A, Bachem M, Grünert A, Bustamante ME, Nualart F, Vera JC 2003 Up-regulation and polarized expression of the sodium-ascorbic acid transporter SVCT1 in post-confluent differentiated CaCo-2 cells. J Biol Chem 278:9035–9041 [DOI] [PubMed] [Google Scholar]
- Miyakoshi N, Kasukawa Y, Linkhart TA, Baylink DJ, Mohan S 2001 Evidence that anabolic effects of PTH on bone require IGF-I in growing mice. Endocrinology 142:4349–4356 [DOI] [PubMed] [Google Scholar]
- Yamaguchi M, Ogata N, Shinoda Y, Akune T, Kamekura S, Terauchi Y, Kadowaki T, Hoshi K, Chung UI, Nakamura K, Kawaguchi H 2005 Insulin receptor substrate-1 is required for bone anabolic function of parathyroid hormone in mice. Endocrinology 146:2620–2628 [DOI] [PubMed] [Google Scholar]
- Yakar S, Bouxsein ML, Canalis E, Sun H, Glatt V, Gundberg C, Cohen P, Hwang D, Boisclair Y, Leroith D, Rosen CJ 2006 The ternary IGF complex influences postnatal bone acquisition and the skeletal response to intermittent parathyroid hormone. J Endocrinol 189:289–299 [DOI] [PubMed] [Google Scholar]
- Bassett JH, Williams GR 2008 Critical role of the hypothalamic-pituitary-thyroid axis in bone. Bone 43:418–426 [DOI] [PubMed] [Google Scholar]
- Göthe S, Wang Z, Ng L, Kindblom JM, Barros AC, Ohlsson C, Vennström B, Forrest D 1999 Mice devoid of all known thyroid hormone receptors are viable but exhibit disorders of the pituitary-thyroid axis, growth, and bone maturation. Genes Dev 13:1329–1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlsson C, Nilsson A, Isaksson O, Bentham J, Lindahl A 1992 Effects of tri-iodothyronine and insulin-like growth factor-I (IGF-I) on alkaline phosphatase activity, [3H]thymidine incorporation and IGF-I receptor mRNA in cultured rat epiphyseal chondrocytes. J Endocrinol 135:115–123 [DOI] [PubMed] [Google Scholar]
- Huang BK, Golden LA, Tarjan G, Madison LD, Stern PH 2000 Insulin-like growth factor I production is essential for anabolic effects of thyroid hormone in osteoblasts. J Bone Miner Res 15:188–197 [DOI] [PubMed] [Google Scholar]
- Canalis E, Bilezikian JP, Angeli A, Giustina A 2004 Perspectives on glucocorticoid-induced osteoporosis. Bone 34:593–598 [DOI] [PubMed] [Google Scholar]
- Cheng SL, Zhang SF, Mohan S, Lecanda F, Fausto A, Hunt AH, Canalis E, Avioli LV 1998 Regulation of insulin-like growth factors I and II and their binding proteins in human bone marrow stromal cells by dexamethasone. J Cell Biochem 71:449–458 [DOI] [PubMed] [Google Scholar]
- Mehls O, Himmele R, Homme M, Kiepe D, Klaus G 2001 The interaction of glucocorticoids with the growth hormone-insulin-like growth factor axis and its effects on growth plate chondrocytes and bone cells. J Pediatr Endocrinol Metab 14(Suppl 6):1475–1482 [PubMed] [Google Scholar]
- Robson H, Siebler T, Shalet SM, Williams GR 2002 Interactions between GH, IGF-I, glucocorticoids, and thyroid hormones during skeletal growth. Pediatr Res 52:137–147 [DOI] [PubMed] [Google Scholar]
- Swolin D, Brantsing C, Matejka G, Ohlsson C 1996 Cortisol decreases IGF-I mRNA levels in human osteoblast-like cells. J Endocrinol 149:397–403 [DOI] [PubMed] [Google Scholar]
- van der Eerden BC, Karperien M, Wit JM 2003 Systemic and local regulation of the growth plate. Endocr Rev 24:782–801 [DOI] [PubMed] [Google Scholar]
- Vidal NO, Brändström H, Jonsson KB, Ohlsson C 1998 Osteoprotegerin mRNA is expressed in primary human osteoblast-like cells: down-regulation by glucocorticoids. J Endocrinol 159:191–195 [DOI] [PubMed] [Google Scholar]
- Zhou M, Graham R, Russell G, Croucher PI 2001 MDC-9 (ADAM-9/Meltrin γ) functions as an adhesion molecule by binding the α (v) β(5) integrin. Biochem Biophys Res Commun 280:574–580 [DOI] [PubMed] [Google Scholar]
- Raab-Cullen DM, Thiede MA, Petersen DN, Kimmel DB, Recker RR 1994 Mechanical loading stimulates rapid changes in periosteal gene expression. Calcif Tissue Int 55:473–478 [DOI] [PubMed] [Google Scholar]
- Lean JM, Jagger CJ, Chambers TJ, Chow JW 1995 Increased insulin-like growth factor I mRNA expression in rat osteocytes in response to mechanical stimulation. Am J Physiol 268:E318–E327 [DOI] [PubMed] [Google Scholar]
- Bikle DD 2008 Integrins, insulin like growth factors, and the skeletal response to load. Osteoporos Int 19:1237–1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur S, Mohan S, Baylink DJ, Lau KH 2005 Fluid shear stress synergizes with insulin-like growth factor-I (IGF-I) on osteoblast proliferation through integrin-dependent activation of IGF-I mitogenic signaling pathway. J Biol Chem 280:20163–20170 [DOI] [PubMed] [Google Scholar]
- Sakata T, Wang Y, Halloran BP, Elalieh HZ, Cao J, Bikle DD 2004 Skeletal unloading induces resistance to insulin-like growth factor-I (IGF-I) by inhibiting activation of the IGF-I signaling pathways. J Bone Miner Res 19:436–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saban J, Schneider GB, Bolt D, King D 1996 Erythroid-specific expression of human growth hormone affects bone morphology in transgenic mice. Bone 18:47–52 [DOI] [PubMed] [Google Scholar]
- Zhao G, Monier-Faugere MC, Langub MC, Geng Z, Nakayama T, Pike JW, Chernausek SD, Rosen CJ, Donahue LR, Malluche HH, Fagin JA, Clemens TL 2000 Targeted overexpression of insulin-like growth factor I to osteoblasts of transgenic mice: increased trabecular bone volume without increased osteoblast proliferation. Endocrinology 141:2674–2682 [DOI] [PubMed] [Google Scholar]
- Jiang J, Lichtler AC, Gronowicz GA, Adams DJ, Clark SH, Rosen CJ, Kream BE 2006 Transgenic mice with osteoblast-targeted insulin-like growth factor-I show increased bone remodeling. Bone 39:494–504 [DOI] [PubMed] [Google Scholar]
- Zhang M, Faugere MC, Malluche H, Rosen CJ, Chernausek SD, Clemens TL 2003 Paracrine overexpression of IGFBP-4 in osteoblasts of transgenic mice decreases bone turnover and causes global growth retardation. J Bone Miner Res 18:836–843 [DOI] [PubMed] [Google Scholar]
- Qin X, Wergedal JE, Rehage M, Tran K, Newton J, Lam P, Baylink DJ, Mohan S 2006 Pregnancy-associated plasma protein-A increases osteoblast proliferation in vitro and bone formation in vivo. Endocrinology 147:5653–5661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Xuan S, Bouxsein ML, von Stechow D, Akeno N, Faugere MC, Malluche H, Zhao G, Rosen CJ, Efstratiadis A, Clemens TL 2002 Osteoblast-specific knockout of the insulin-like growth factor (IGF) receptor gene reveals an essential role of IGF signaling in bone matrix mineralization. J Biol Chem 277:44005–44012 [DOI] [PubMed] [Google Scholar]
- Bikle D, Majumdar S, Laib A, Powell-Braxton L, Rosen C, Beamer W, Nauman E, Leary C, Halloran B 2001 The skeletal structure of insulin-like growth factor I-deficient mice. J Bone Miner Res 16:2320–2329 [DOI] [PubMed] [Google Scholar]
- Kasukawa Y, Baylink DJ, Wergedal JE, Amaar Y, Srivastava AK, Guo R, Mohan S 2003 Lack of insulin-like growth factor I exaggerates the effect of calcium deficiency on bone accretion in mice. Endocrinology 144:4682–4689 [DOI] [PubMed] [Google Scholar]
- Lindberg MK, Svensson J, Venken K, Chavoshi T, Andersson N, Movérare Skrtic S, Isaksson O, Vanderschueren D, Carlsten H, Ohlsson C 2006 Liver-derived IGF-I is permissive for ovariectomy-induced trabecular bone loss. Bone 38:85–92 [DOI] [PubMed] [Google Scholar]
- Tannenbaum GS, Martin JB 1976 Evidence for an endogenous ultradian rhythm governing growth hormone secretion in the rat. Endocrinology 98:562–570 [DOI] [PubMed] [Google Scholar]
- Edén S 1979 Age- and sex-related differences in episodic growth hormone secretion in the rat. Endocrinology 105:555–560 [DOI] [PubMed] [Google Scholar]
- MacLeod JN, Pampori NA, Shapiro BH 1991 Sex differences in the ultradian pattern of plasma growth hormone concentrations in mice. J Endocrinol 131:395–399 [DOI] [PubMed] [Google Scholar]
- Gustafsson JA, Mode A, Norstedt G, Skett P 1983 Sex steroid induced changes in hepatic enzymes. Annu Rev Physiol 45:51–60 [DOI] [PubMed] [Google Scholar]
- Jansson JO, Ekberg S, Hoath SB, Beamer WG, Frohman LA 1988 Growth hormone enhances hepatic epidermal growth factor receptor concentration in mice. J Clin Invest 82:1871–1876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekberg S, Carlsson L, Carlsson B, Billig H, Jansson JO 1989 Plasma growth hormone pattern regulates epidermal growth factor (EGF) receptor messenger ribonucleic acid levels and EGF binding in the rat liver. Endocrinology 125:2158–2166 [DOI] [PubMed] [Google Scholar]
- Johansson S, Husman B, Norstedt G, Andersson G 1989 Growth hormone regulates the rodent hepatic epidermal growth factor receptor at a pretranslational level. J Mol Endocrinol 3:113–120 [DOI] [PubMed] [Google Scholar]
- Kashimata M, Hiramatsu M, Minami N 1989 Differential secretory rhythm of growth hormone controls the number of hepatic epidermal growth factor receptors in the rat. J Endocrinol 123:75–81 [DOI] [PubMed] [Google Scholar]
- Norstedt G, Palmiter R 1984 Secretory rhythm of growth hormone regulates sexual differentiation of mouse liver. Cell 36:805–812 [DOI] [PubMed] [Google Scholar]
- Clark RG, Jansson JO, Isaksson O, Robinson IC 1985 Intravenous growth hormone: growth responses to patterned infusions in hypophysectomized rats. J Endocrinol 104:53–61 [DOI] [PubMed] [Google Scholar]
- Bick T, Hochberg Z, Amit T, Isaksson OG, Jansson JO 1992 Roles of pulsatility and continuity of growth hormone (GH) administration in the regulation of hepatic GH-receptors, and circulating GH-binding protein and insulin-like growth factor-I. Endocrinology 131:423–429 [DOI] [PubMed] [Google Scholar]
- Tannenbaum GS, Ling N 1984 The interrelationship of growth hormone (GH)-releasing factor and somatostatin in generation of the ultradian rhythm of GH secretion. Endocrinology 115:1952–1957 [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Vale W 1985 Patterns of growth hormone-releasing factor and somatostatin secretion into the hypophysial-portal circulation of the rat. Science 230:461–463 [DOI] [PubMed] [Google Scholar]
- Frohman LA, Jansson JO 1986 Growth hormone-releasing hormone. Endocr Rev 7:223–253 [DOI] [PubMed] [Google Scholar]
- Smith RG, Van der Ploeg LH, Howard AD, Feighner SD, Cheng K, Hickey GJ, Wyvratt Jr MJ, Fisher MH, Nargund RP, Patchett AA 1997 Peptidomimetic regulation of growth hormone secretion. Endocr Rev 18:621–645 [DOI] [PubMed] [Google Scholar]
- Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K 1999 Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402:656–660 [DOI] [PubMed] [Google Scholar]
- Bowers CY, Momany FA, Reynolds GA, Hong A 1984 On the in vitro and the in vivo activity of a new synthetic hexapeptide that acts on the pituitary to specifically release growth hormone. Endocrinology 114:1537–1545 [DOI] [PubMed] [Google Scholar]
- Dickson SL, Leng G, Robinson IC 1993 Systemic administration of growth hormone-releasing peptide activates hypothalamic arcuate neurons. Neuroscience 53:303–306 [DOI] [PubMed] [Google Scholar]
- Guillaume V, Magnan E, Cataldi M, Dutour A, Sauze N, Renard M, Razafindraibe H, Conte-Devolx B, Deghenghi R, Lenaerts V, Oliver C 1994 Growth hormone (GH)-releasing hormone secretion is stimulated by a new GH-releasing hexapeptide in sheep. Endocrinology 135:1073–1076 [DOI] [PubMed] [Google Scholar]
- Dickson SL, Luckman SM 1997 Induction of c-fos mRNA in NPY and GRF neurones in the rat arcleus nucleus following systemic injection of the growth hormone secretagogue, GHRP-6. Endocrinology 138:771–777 [DOI] [PubMed] [Google Scholar]
- Wortley KE, del Rincon JP, Murray JD, Garcia K, Iida K, Thorner MO, Sleeman MW 2005 Absence of ghrelin protects against early-onset obesity. J Clin Invest 115:3573–3578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigman JM, Nakano Y, Coppari R, Balthasar N, Marcus JN, Lee CE, Jones JE, Deysher AE, Waxman AR, White RD, Williams TD, Lachey JL, Seeley RJ, Lowell BB, Elmquist JK 2005 Mice lacking ghrelin receptors resist the development of diet-induced obesity. J Clin Invest 115:3564–3572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eicher EM, Beamer WG 1976 Inherited ateliotic dwarfism in mice. Characteristics of the mutation, little, on chromosome 6. J Hered 67:87–91 [DOI] [PubMed] [Google Scholar]
- Jansson JO, Downs TR, Beamer WG, Frohman LA 1986 Receptor-associated resistance to growth hormone-releasing factor in dwarf “little” mice. Science 232:511–512 [DOI] [PubMed] [Google Scholar]
- Holst B, Schwartz TW 2006 Ghrelin receptor mutations—too little height and too much hunger. J Clin Invest 116:637–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman IM, Pescovitz OH, Murphy G, Treep T, Cerchio KA, Krupa D, Gertz B, Polvino WJ, Skiles EH, Pezzoli SS, Thorner MO 1997 Oral administration of growth hormone (GH) releasing peptide-mimetic MK-677 stimulates the GH/insulin-like growth factor-I axis in selected GH-deficient adults. J Clin Endocrinol Metab 82:3455–3463 [DOI] [PubMed] [Google Scholar]
- Svensson J, Lönn L, Jansson JO, Murphy G, Wyss D, Krupa D, Cerchio K, Polvino W, Gertz B, Boseaus I, Sjöström L, Bengtsson BA 1998 Two-month treatment of obese subjects with the oral growth hormone (GH) secretagogue MK-677 increases GH secretion, fat-free mass, and energy expenditure. J Clin Endocrinol Metab 83:362–369 [DOI] [PubMed] [Google Scholar]
- Tannenbaum GS 1980 Evidence for autoregulation of growth hormone secretion via the central nervous system. Endocrinology 107:2117–2120 [DOI] [PubMed] [Google Scholar]
- Sato M, Chihara K, Kita T, Kashio Y, Okimura Y, Kitajima N, Fujita T 1989 Physiological role of somatostatin-mediated autofeedback regulation for growth hormone: importance of growth hormone in triggering somatostatin release during a trough period of pulsatile growth hormone release in conscious male rats. Neuroendocrinology 50:139–151 [DOI] [PubMed] [Google Scholar]
- Carlsson L, Jansson JO 1990 Endogenous growth hormone (GH) secretion in male rats is synchronized to pulsatile GH infusions given at 3-hour intervals. Endocrinology 126:6–10 [DOI] [PubMed] [Google Scholar]
- Farhy LS, Straume M, Johnson ML, Kovatchev B, Veldhuis JD 2002 Unequal autonegative feedback by GH models the sexual dimorphism in GH secretory dynamics. Am J Physiol Regul Integr Comp Physiol 282:R753–R764 [DOI] [PubMed] [Google Scholar]
- Chowen JA, García-Segura LM, González-Parra S, Argente J 1996 Sex steroid effects on the development and functioning of the growth hormone axis. Cell Mol Neurobiol 16:297–310 [DOI] [PubMed] [Google Scholar]
- Berelowitz M, Szabo M, Frohman LA, Firestone S, Chu L, Hintz RL 1981 Somatomedin-C mediates growth hormone negative feedback by effects on both the hypothalamus and the pituitary. Science 212:1279–1281 [DOI] [PubMed] [Google Scholar]
- Tannenbaum GS, Guyda HJ, Posner BI 1983 Insulin-like growth factors: a role in growth hormone negative feedback and body weight regulation via brain. Science 220:77–79 [DOI] [PubMed] [Google Scholar]
- Kamegai J, Unterman TG, Frohman LA, Kineman RD 1998 Hypothalamic/pituitary-axis of the spontaneous dwarf rat: autofeedback regulation of growth hormone (GH) includes suppression of GH releasing-hormone receptor messenger ribonucleic acid. Endocrinology 139:3554–3560 [DOI] [PubMed] [Google Scholar]
- Sato M, Frohman LA 1993 Differential effects of central and peripheral administration of growth hormone (GH) and insulin-like growth factor on hypothalamic GH-releasing hormone and somatostatin gene expression in GH-deficient dwarf rats. Endocrinology 133:793–799 [DOI] [PubMed] [Google Scholar]
- Harel Z, Tannenbaum GS 1992 Synergistic interaction between insulin-like growth factors-I and -II in central regulation of pulsatile growth hormone secretion. Endocrinology 131:758–764 [DOI] [PubMed] [Google Scholar]
- Tannenbaum GS, Choi HK, Gurd W, Waxman DJ 2001 Temporal relationship between the sexually dimorphic spontaneous GH secretory profiles and hepatic STAT5 activity. Endocrinology 142:4599–4606 [DOI] [PubMed] [Google Scholar]
- Udy GB, Towers RP, Snell RG, Wilkins RJ, Park SH, Ram PA, Waxman DJ, Davey HW 1997 Requirement of STAT5b for sexual dimorphism of body growth rates and liver gene expression. Proc Natl Acad Sci USA 94:7239–7244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman DJ, O'Connor C 2006 Growth hormone regulation of sex-dependent liver gene expression. Mol Endocrinol 20:2613–2629 [DOI] [PubMed] [Google Scholar]
- Caro JF, Poulos J, Ittoop O, Pories WJ, Flickinger EG, Sinha MK 1988 Insulin-like growth factor I binding in hepatocytes from human liver, human hepatoma, and normal, regenerating, and fetal rat liver. J Clin Invest 81:976–981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos A, Yusta B, Fernández-Moreno MD, Blázquez E 1994 Expression of insulin-like growth factor-I (IGF-I) receptor gene in rat brain and liver during development and in regenerating adult rat liver. Mol Cell Endocrinol 101:85–93 [DOI] [PubMed] [Google Scholar]
- Pennisi PA, Kopchick JJ, Thorgeirsson S, LeRoith D, Yakar S 2004 Role of growth hormone (GH) in liver regeneration. Endocrinology 145:4748–4755 [DOI] [PubMed] [Google Scholar]
- Davey HW, Park SH, Grattan DR, McLachlan MJ, Waxman DJ 1999 STAT5b-deficient mice are growth hormone pulse-resistant. Role of STAT5b in sex-specific liver p450 expression. J Biol Chem 274:35331–35336 [DOI] [PubMed] [Google Scholar]
- Teglund S, McKay C, Schuetz E, van Deursen JM, Stravopodis D, Wang D, Brown M, Bodner S, Grosveld G, Ihle JN 1998 Stat5a and Stat5b proteins have essential and nonessential, or redundant, roles in cytokine responses. Cell 93:841–850 [DOI] [PubMed] [Google Scholar]
- Klover P, Hennighausen L 2007 Postnatal body growth is dependent on the transcription factors signal transducers and activators of transcription 5a/b in muscle: a role for autocrine/paracrine insulin-like growth factor I. Endocrinology 148:1489–1497 [DOI] [PubMed] [Google Scholar]
- Giustina A, Veldhuis JD 1998 Pathophysiology of the neuroregulation of growth hormone secretion in experimental animals and the human. Endocr Rev 19:717–797 [DOI] [PubMed] [Google Scholar]
- Jaffe CA, Ocampo-Lim B, Guo W, Krueger K, Sugahara I, DeMott-Friberg R, Bermann M, Barkan AL 1998 Regulatory mechanisms of growth hormone secretion are sexually dimorphic. J Clin Invest 102:153–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessup SK, Dimaraki EV, Symons KV, Barkan AL 2003 Sexual dimorphism of growth hormone (GH) regulation in humans: endogenous GH-releasing hormone maintains basal GH in women but not in men. J Clin Endocrinol Metab 88:4776–4780 [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Keenan DM, Bailey JN, Adeniji A, Miles JM, Paulo R, Cosma M, Soares-Welch C 2008 Estradiol supplementation in postmenopausal women attenuates suppression of pulsatile growth hormone secretion by recombinant human insulin-like growth factor type I. J Clin Endocrinol Metab 93:4471–4478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhuis JD, Keenan DM, Bailey JN, Adeniji A, Miles JM, Paulo R, Cosma M, Soares-Welch C 2009 Testosterone supplementation in older men restrains insulin-like growth factor’s dose-dependent feedback inhibition of pulsatile growth hormone secretion. J Clin Endocrinol Metab 94:246–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung KC, Johannsson G, Leong GM, Ho KK 2004 Estrogen regulation of growth hormone action. Endocr Rev 25:693–721 [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Bidlingmaier M, Anderson SM, Wu Z, Strasburger CJ 2001 Lowering total plasma insulin-like growth factor I concentrations by way of a novel, potent, and selective growth hormone (GH) receptor antagonist, pegvisomant (B2036-peg), augments the amplitude of GH secretory bursts and elevates basal/nonpulsatile GH release in healthy women and men. J Clin Endocrinol Metab 86:3304–3310 [DOI] [PubMed] [Google Scholar]
- Hartman ML, Clayton PE, Johnson ML, Celniker A, Perlman AJ, Alberti KG, Thorner MO 1993 A low dose euglycemic infusion of recombinant insulin-like growth factor I rapidly suppresses fasting-enhanced pulsatile growth hormone secretion in humans. J Clin Invest 91:2453–2462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki T, Lin VY, Goetz R, Mohammadi M, Mangelsdorf DJ, Kliewer SA 2008 Inhibition of growth hormone signaling by the fasting-induced hormone FGF21. Cell Metab 8:77–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjögren K, Wallenius K, Liu JL, Bohlooly-Y M, Pacini G, Svensson L, Törnell J, Isaksson OG, Ahrén B, Jansson JO, Ohlsson C 2001 Liver-derived IGF-I is of importance for normal carbohydrate and lipid metabolism. Diabetes 50:1539–1545 [DOI] [PubMed] [Google Scholar]
- Yakar S, Liu JL, Fernandez AM, Wu Y, Schally AV, Frystyk J, Chernausek SD, Mejia W, Le Roith D 2001 Liver-specific igf-1 gene deletion leads to muscle insulin insensitivity. Diabetes 50:1110–1118 [DOI] [PubMed] [Google Scholar]
- Haluzik M, Yakar S, Gavrilova O, Setser J, Boisclair Y, LeRoith D 2003 Insulin resistance in the liver-specific IGF-1 gene-deleted mouse is abrogated by deletion of the acid-labile subunit of the IGF-binding protein-3 complex: relative roles of growth hormone and IGF-1 in insulin resistance. Diabetes 52:2483–2489 [DOI] [PubMed] [Google Scholar]
- Yakar S, Setser J, Zhao H, Stannard B, Haluzik M, Glatt V, Bouxsein ML, Kopchick JJ, LeRoith D 2004 Inhibition of growth hormone action improves insulin sensitivity in liver IGF-1-deficient mice. J Clin Invest 113:96–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu R, Yakar S, Liu YL, Lu Y, LeRoith D, Miao D, Liu JL 2003 Liver-specific IGF-I gene deficient mice exhibit accelerated diabetes in response to streptozotocin, associated with early onset of insulin resistance. Mol Cell Endocrinol 204:31–42 [DOI] [PubMed] [Google Scholar]
- Hennige AM, Burks DJ, Ozcan U, Kulkarni RN, Ye J, Park S, Schubert M, Fisher TL, Dow MA, Leshan R, Zakaria M, Mossa-Basha M, White MF 2003 Upregulation of insulin receptor substrate-2 in pancreatic β cells prevents diabetes. J Clin Invest 112:1521–1532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt RI, Simpson HL, Sönksen PH 2003 The role of the growth hormone–insulin-like growth factor axis in glucose homeostasis. Diabet Med 20:3–15 [DOI] [PubMed] [Google Scholar]
- Simpson HL, Jackson NC, Shojaee-Moradie F, Jones RH, Russell-Jones DL, Sönksen PH, Dunger DB, Umpleby AM 2004 Insulin-like growth factor I has a direct effect on glucose and protein metabolism, but no effect on lipid metabolism in type 1 diabetes. J Clin Endocrinol Metab 89:425–432 [DOI] [PubMed] [Google Scholar]
- Ning Y, Schuller AG, Bradshaw S, Rotwein P, Ludwig T, Frystyk J, Pintar JE 2006 Diminished growth and enhanced glucose metabolism in triple knockout mice containing mutations of insulin-like growth factor binding protein-3, -4, and -5. Mol Endocrinol 20:2173–2186 [DOI] [PubMed] [Google Scholar]
- Leroith D, Accili D 2008 Mechanisms of disease: using genetically altered mice to study concepts of type 2 diabetes. Nat Clin Pract Endocrinol Metab 4:164–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xuan S, Kitamura T, Nakae J, Politi K, Kido Y, Fisher PE, Morroni M, Cinti S, White MF, Herrera PL, Accili D, Efstratiadis A 2002 Defective insulin secretion in pancreatic β cells lacking type 1 IGF receptor. J Clin Invest 110:1011–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni RN, Holzenberger M, Shih DQ, Ozcan U, Stoffel M, Magnuson MA, Kahn CR 2002 β-Cell-specific deletion of the Igf1 receptor leads to hyperinsulinemia and glucose intolerance but does not alter β-cell mass. Nat Genet 31:111–115 [DOI] [PubMed] [Google Scholar]
- Ueki K, Okada T, Hu J, Liew CW, Assmann A, Dahlgren GM, Peters JL, Shackman JG, Zhang M, Artner I, Satin LS, Stein R, Holzenberger M, Kennedy RT, Kahn CR, Kulkarni RN 2006 Total insulin and IGF-I resistance in pancreatic β-cells causes overt diabetes. Nat Genet 38:583–588 [DOI] [PubMed] [Google Scholar]
- Lu Y, Herrera PL, Guo Y, Sun D, Tang Z, LeRoith D, Liu JL 2004 Pancreatic-specific inactivation of IGF-I gene causes enlarged pancreatic islets and significant resistance to diabetes. Diabetes 53:3131–3141 [DOI] [PubMed] [Google Scholar]
- Saladin R, De Vos P, Guerre-Millo M, Leturque A, Girard J, Staels B, Auwerx J 1995 Transient increase in obese gene expression after food intake or insulin administration. Nature 377:527–529 [DOI] [PubMed] [Google Scholar]
- Leroy P, Dessolin S, Villageois P, Moon BC, Friedman JM, Ailhaud G, Dani C 1996 Expression of ob gene in adipose cells. Regulation by insulin. J Biol Chem 271:2365–2368 [DOI] [PubMed] [Google Scholar]
- Böni-Schnetzler M, Gosteli-Peter MA, Moritz W, Froesch ER, Zapf J 1996 Reduced ob mRNA in hypophysectomised rats is not restored by growth hormone (GH), but further suppressed by exogenously administered insulin-like growth factor (IGF) I. Biochem Biophys Res Commun 225:296–301 [DOI] [PubMed] [Google Scholar]
- Böni-Schnetzler M, Hauri C, Zapf J 1999 Leptin is suppressed during infusion of recombinant human insulin-like growth factor I (rhIGF I) in normal rats. Diabetologia 42:160–166 [DOI] [PubMed] [Google Scholar]
- Tang Z, Yu R, Lu Y, Parlow AF, Liu JL 2005 Age-dependent onset of liver-specific IGF-I gene deficiency and its persistence in old age: implications for postnatal growth and insulin resistance in LID mice. Am J Physiol Endocrinol Metab 289:E288–E295 [DOI] [PubMed] [Google Scholar]
- Rajkumar K, Modric T, Murphy LJ 1999 Impaired adipogenesis in insulin-like growth factor binding protein-1 transgenic mice. J Endocrinol 162:457–465 [DOI] [PubMed] [Google Scholar]
- Sonntag WE, Steger RW, Forman LJ, Meites J 1980 Decreased pulsatile release of growth hormone in old male rats. Endocrinology 107:1875–1879 [DOI] [PubMed] [Google Scholar]
- Rudman D, Feller AG, Nagraj HS, Gergans GA, Lalitha PY, Goldberg AF, Schlenker RA, Cohn L, Rudman IW, Mattson DE 1990 Effects of human growth hormone in men over 60 years old. N Engl J Med 323:1–6 [DOI] [PubMed] [Google Scholar]
- Wheatcroft SB, Kearney MT, Shah AM, Ezzat VA, Miell JR, Modo M, Williams SC, Cawthorn WP, Medina-Gomez G, Vidal-Puig A, Sethi JK, Crossey PA 2007 IGF-binding protein-2 protects against the development of obesity and insulin resistance. Diabetes 56:285–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses AC, Young SC, Morrow LA, O'Brien M, Clemmons DR 1996 Recombinant human insulin-like growth factor I increases insulin sensitivity and improves glycemic control in type II diabetes. Diabetes 45:91–100 [DOI] [PubMed] [Google Scholar]
- Clemmons DR, Moses AC, McKay MJ, Sommer A, Rosen DM, Ruckle J 2000 The combination of insulin-like growth factor I and insulin-like growth factor-binding protein-3 reduces insulin requirements in insulin-dependent type 1 diabetes: evidence for in vivo biological activity. J Clin Endocrinol Metab 85:1518–1524 [DOI] [PubMed] [Google Scholar]
- Merimee TJ, Hollander W, Fineberg SE 1972 Studies of hyperlipidemia in the HGH-deficient state. Metabolism 21:1053–1061 [DOI] [PubMed] [Google Scholar]
- Winter RJ, Thompson RG, Green OC 1979 Serum cholesterol and triglycerides in children with growth hormone deficiency. Metabolism 28:1244–1249 [DOI] [PubMed] [Google Scholar]
- de Boer H, Blok GJ, Van der Veen EA 1995 Clinical aspects of growth hormone deficiency in adults. Endocr Rev 16:63–86 [DOI] [PubMed] [Google Scholar]
- Drake WM, Howell SJ, Monson JP, Shalet SM 2001 Optimizing GH therapy in adults and children. Endocr Rev 22:425–450 [DOI] [PubMed] [Google Scholar]
- Parini P, Angelin B, Rudling M 1999 Cholesterol and lipoprotein metabolism in aging: reversal of hypercholesterolemia by growth hormone treatment in old rats. Arterioscler Thromb Vasc Biol 19:832–839 [DOI] [PubMed] [Google Scholar]
- Russo VC, Gluckman PD, Feldman EL, Werther GA 2005 The insulin-like growth factor system and its pleiotropic functions in brain. Endocr Rev 26:916–943 [DOI] [PubMed] [Google Scholar]
- D'Ercole AJ, Ye P, O'Kusky JR 2002 Mutant mouse models of insulin-like growth factor actions in the central nervous system. Neuropeptides 36:209–220 [DOI] [PubMed] [Google Scholar]
- Messier C, Teutenberg K 2005 The role of insulin, insulin growth factor, and insulin-degrading enzyme in brain aging and Alzheimer’s disease. Neural Plast 12:311–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- JosephD'Ercole A, Ye P 2008 Expanding the mind: insulin-like growth factor I and brain development. Endocrinology 149:5958–5962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea BT, Hammer RE, Brinster RL 1987 Growth allometry of the organs in giant transgenic mice. Endocrinology 121:1924–1930 [DOI] [PubMed] [Google Scholar]
- Chen L, Lund PK, Burgess SB, Rudisch BE, McIlwain DL 1997 Growth hormone, insulin-like growth factor I, and motoneuron size. J Neurobiol 32:202–212 [PubMed] [Google Scholar]
- Carson MJ, Behringer RR, Brinster RL, McMorris FA 1993 Insulin-like growth factor I increases brain growth and central nervous system myelination in transgenic mice. Neuron 10:729–740 [DOI] [PubMed] [Google Scholar]
- Dentremont KD, Ye P, D'Ercole AJ, O'Kusky JR 1999 Increased insulin-like growth factor-I (IGF-I) expression during early postnatal development differentially increases neuron number and growth in medullary nuclei of the mouse. Brain Res Dev Brain Res 114:135–141 [DOI] [PubMed] [Google Scholar]
- O'Kusky JR, Ye P, D'Ercole AJ 2000 Insulin-like growth factor-I promotes neurogenesis and synaptogenesis in the hippocampal dentate gyrus during postnatal development. J Neurosci 20:8435–8442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Kusky JR, Ye P, D'Ercole AJ 2003 Increased expression of insulin-like growth factor augments the progressive phase of synaptogenesis without preventing synapse elimination in the hypoglossal nucleus. J Comp Neurol 464:382–391 [DOI] [PubMed] [Google Scholar]
- Zhong J, Deng J, Phan J, Dlouhy S, Wu H, Yao W, Ye P, D'Ercole AJ, Lee WH 2005 Insulin-like growth factor-I protects granule neurons from apoptosis and improves ataxia in weaver mice. J Neurosci Res 80:481–490 [DOI] [PubMed] [Google Scholar]
- Beck KD, Powell-Braxton L, Widmer HR, Valverde J, Hefti F 1995 Igfl gene disruption results in reduced brain size, CNS hypomyelination, and loss of hippocampal granule and striatal parvalbumin-containing neurons. Neuron 14:717–730 [DOI] [PubMed] [Google Scholar]
- Holzenberger M, Hamard G, Zaoui R, Leneuve P, Ducos B, Beccavin C, Périn L, Le Bouc Y 2001 Experimental IGF-I receptor deficiency generates a sexually dimorphic pattern of organ-specific growth deficits in mice, affecting fat tissue in particular. Endocrinology 142:4469–4478 [DOI] [PubMed] [Google Scholar]
- Vicario-Abejón C, Yusta-Boyo MJ, Fernández-Moreno C, de Pablo F 2003 Locally born olfactory bulb stem cells proliferate in response to insulin-related factors and require endogenous insulin-like growth factor-I for differentiation into neurons and glia. J Neurosci 23:895–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappeler L, Filho CM, Dupont J, Leneuve P, Cervera P, Périn L, Loudes C, Blaise A, Klein R, Epelbaum J, Le Bouc Y, Holzenberger M 2008 Brain IGF-1 receptors control mammalian growth and lifespan through a neuroendocrine mechanism. PLoS Biol 28:e254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowska AL, Mooney M, Sonntag WE 1998 Insulin-like growth factor-I ameliorates age-related behavioral deficits. Neuroscience 87:559–569 [DOI] [PubMed] [Google Scholar]
- Svensson J, Söderpalm B, Sjögren K, Engel J, Ohlsson C 2005 Liver-derived IGF-I regulates exploratory activity in old mice. Am J Physiol Endocrinol Metab 289:E466–E473 [DOI] [PubMed] [Google Scholar]
- Söderpalm B, Ericson M, Bohlooly M, Engel JA, Törnell J 1999 Bovine growth hormone transgenic mice display alterations in locomotor activity and brain monoamine neurochemistry. Endocrinology 140:5619–5625 [DOI] [PubMed] [Google Scholar]
- Bohlooly-Y M, Olsson B, Gritli-Linde A, Brusehed O, Isaksson OG, Ohlsson C, Söderpalm B, Törnell J, Ola B 2001 Enhanced spontaneous locomotor activity in bovine GH transgenic mice involves peripheral mechanisms. Endocrinology 142:4560–4567 [DOI] [PubMed] [Google Scholar]
- Sonntag WE, Lynch CD, Cooney PT, Hutchins PM 1997 Decreases in cerebral microvasculature with age are associated with the decline in growth hormone and insulin-like growth factor 1. Endocrinology 138:3515–3520 [DOI] [PubMed] [Google Scholar]
- Thornton PL, Ingram RL, Sonntag WE 2000 Chronic [D-Ala2]-growth hormone-releasing hormone administration attenuates age-related deficits in spatial memory. J Gerontol A Biol Sci Med Sci 55:B106–B112 [DOI] [PubMed] [Google Scholar]
- Trejo JL, Carro E, Torres-Aleman I 2001 Circulating insulin-like growth factor I mediates exercise-induced increases in the number of new neurons in the adult hippocampus. J Neurosci 21:1628–1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Lopez C, LeRoith D, Torres-Aleman I 2004 Insulin-like growth factor I is required for vessel remodeling in the adult brain. Proc Natl Acad Sci USA 101:9833–9838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trejo JL, Llorens-Martín MV, Torres-Alemán I 2008 The effects of exercise on spatial learning and anxiety-like behavior are mediated by an IGF-I-dependent mechanism related to hippocampal neurogenesis. Mol Cell Neurosci 37:402–411 [DOI] [PubMed] [Google Scholar]
- Carro E, Trejo JL, Gomez-Isla T, LeRoith D, Torres-Aleman I 2002 Serum insulin-like growth factor I regulates brain amyloid-β levels. Nat Med 8:1390–1397 [DOI] [PubMed] [Google Scholar]
- Carro E, Spuch C, Trejo JL, Antequera D, Torres-Aleman I 2005 Choroid plexus megalin is involved in neuroprotection by serum insulin-like growth factor I. J Neurosci 25:10884–10893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Lopez C, Dietrich MO, Metzger F, Loetscher H, Torres-Aleman I 2007 Disturbed cross talk between insulin-like growth factor I and AMP-activated protein kinase as a possible cause of vascular dysfunction in the amyloid precursor protein/presenilin 2 mouse model of Alzheimer’s disease. J Neurosci 27:824–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson J, Diez M, Engel J, Wass C, Tivesten A, Jansson JO, Isaksson O, Archer T, Hökfelt T, Ohlsson C 2006 Endocrine, liver-derived IGF-I is of importance for spatial learning and memory in old mice. J Endocrinol 189:617–627 [DOI] [PubMed] [Google Scholar]
- Trejo JL, Piriz J, Llorens-Martin MV, Fernandez AM, Bolós M, LeRoith D, Nuñez A, Torres-Aleman I 2007 Central actions of liver-derived insulin-like growth factor I underlying its pro-cognitive effects. Mol Psychiatry 12:1118–1128 [DOI] [PubMed] [Google Scholar]
- Reinhardt RR, Bondy CA 1994 Insulin-like growth factors cross the blood-brain barrier. Endocrinology 135:1753–1761 [DOI] [PubMed] [Google Scholar]
- Nilsson L, Sara VR, Nordberg A 1988 Insulin-like growth factor I stimulates the release of acetylcholine from rat cortical slices. Neurosci Lett 88:221–226 [DOI] [PubMed] [Google Scholar]
- Araujo DM, Lapchak PA, Collier B, Chabot JG, Quirion R 1989 Insulin-like growth factor-I (somatomedin-C) receptors in the rat brain: distribution and interaction with the hippocampal cholinergic system. Brain Res 484:130–138 [DOI] [PubMed] [Google Scholar]
- Kar S, Seto D, Doré S, Hanisch U, Quirion R 1997 Insulin-like growth factors-I and -II differentially regulate endogenous acetylcholine release from the rat hippocampal formation. Proc Natl Acad Sci USA 94:14054–14059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns JM, Donnelly JE, Anderson HS, Mayo MS, Spencer-Gardner L, Thomas G, Cronk BB, Haddad Z, Klima D, Hansen D, Brooks WM 2007 Peripheral insulin and brain structure in early Alzheimer disease. Neurology 69:1094–1104 [DOI] [PubMed] [Google Scholar]
- Falleti MG, Maruff P, Burman P, Harris A 2006 The effects of growth hormone (GH) deficiency and GH replacement on cognitive performance in adults: a meta-analysis of the current literature. Psychoneuroendocrinology 31:681–691 [DOI] [PubMed] [Google Scholar]
- Rollero A, Murialdo G, Fonzi S, Garrone S, Gianelli MV, Gazzerro E, Barreca A, Polleri A 1998 Relationship between cognitive function, growth hormone and insulin-like growth factor plasma levels in aged subjects. Neuropsychobiology 38:73–79 [DOI] [PubMed] [Google Scholar]
- Watanabe T, Miyazaki A, Katagiri T, Yamamoto H, Idei T, Iguchi T 2005 Relationship between serum insulin-like growth factor-1 levels and Alzheimer’s disease and vascular dementia. J Am Geriatr Soc 53:1748–1753 [DOI] [PubMed] [Google Scholar]
- Mustafa A, Lannfelt L, Lilius L, Islam A, Winblad B, Adem A 1999 Decreased plasma insulin-like growth factor-I level in familial Alzheimer’s disease patients carrying the Swedish APP 670/671 mutation. Dement Geriatr Cogn Disord 10:446–451 [DOI] [PubMed] [Google Scholar]
- Vardy ER, Rice PJ, Bowie PC, Holmes JD, Grant PJ, Hooper NM 2007 Increased circulating insulin-like growth factor-1 in late-onset Alzheimer’s disease. J Alzheimers Dis 12:285–290 [DOI] [PubMed] [Google Scholar]
- Tham A, Nordberg A, Grissom FE, Carlsson-Skwirut C, Viitanen M, Sara VR 1993 Insulin-like growth factors and insulin-like growth factor binding proteins in cerebrospinal fluid and serum of patients with dementia of the Alzheimer type. J Neural Transm Park Dis Dement Sect 5:165–176 [DOI] [PubMed] [Google Scholar]
- Isgaard J, Nilsson A, Vikman K, Isaksson OG 1989 Growth hormone regulates the level of insulin-like growth factor-I mRNA in rat skeletal muscle. J Endocrinol 120:107–112 [DOI] [PubMed] [Google Scholar]
- Delafontaine P, Bernstein KE, Alexander RW 1991 Insulin-like growth factor I gene expression in vascular cells. Hypertension 17:693–699 [DOI] [PubMed] [Google Scholar]
- Mathews LS, Enberg B, Norstedt G 1989 Regulation of rat growth hormone receptor gene expression. J Biol Chem 264:9905–9910 [PubMed] [Google Scholar]
- Isgaard J, Wåhlander H, Adams MA, Friberg P 1994 Increased expression of growth hormone receptor mRNA and insulin-like growth factor-I mRNA in volume-overloaded hearts. Hypertension 23:884–888 [DOI] [PubMed] [Google Scholar]
- Wickman A, Friberg P, Adams MA, Matejka GL, Brantsing C, Guron G, Isgaard J 1997 Induction of growth hormone receptor and insulin-like growth factor-I mRNA in aorta and caval vein during hemodynamic challenge. Hypertension 29:123–130 [DOI] [PubMed] [Google Scholar]
- Guler HP, Zapf J, Scheiwiller E, Froesch ER 1988 Recombinant human insulin-like growth factor I stimulates growth and has distinct effects on organ size in hypophysectomized rats. Proc Natl Acad Sci USA 85:4889–4893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickman A, Isgaard J, Adams MA, Friberg P 1997 Inhibition of nitric oxide in rats. Regulation of cardiovascular structure and expression of insulin-like growth factor I and its receptor messenger RNA. J Hypertens 15:751–759 [DOI] [PubMed] [Google Scholar]
- Saccà L, Cittadini A, Fazio S 1994 Growth hormone and the heart. Endocr Rev 15:555–573 [DOI] [PubMed] [Google Scholar]
- Svensson J, Tivesten A, Isgaard J 2005 Growth hormone and the cardiovascular function. Minerva Endocrinol 30:1–13 [PubMed] [Google Scholar]
- Pete G, Hu Y, Walsh M, Sowers J, Dunbar JC 1996 Insulin-like growth factor-I decreases mean blood pressure and selectively increases regional blood flow in normal rats. Proc Soc Exp Biol Med 213:187–192 [DOI] [PubMed] [Google Scholar]
- Walsh MF, Barazi M, Pete G, Muniyappa R, Dunbar JC, Sowers JR 1996 Insulin-like growth factor-I diminishes in vivo and in vitro vascular contractility: role of vascular nitric oxide. Endocrinology 137:1798–1803 [DOI] [PubMed] [Google Scholar]
- Copeland KC, Nair KS 1994 Recombinant human insulin-like growth factor-I increases forearm bloodflow. J Clin Endocrinol Metab 79:230–232 [DOI] [PubMed] [Google Scholar]
- Delafontaine P, Song YH, Li Y 2004 Expression, regulation, and function of IGF-1 IGF-1R and IGF-1 binding proteins in blood vessels. Arterioscler Thromb Vasc Biol 24:435–444 [DOI] [PubMed] [Google Scholar]
- Grant MB, Wargovich TJ, Ellis EA, Tarnuzzer R, Caballero S, Estes K, Rossing M, Spoerri PE, Pepine C 1996 Expression of IGF-I, IGF-I receptor and IGF binding proteins -1, -2, -3, -4 and -5 in human atherectomy specimens. Regul Pept 67:137–144 [DOI] [PubMed] [Google Scholar]
- Harrington SC, Simari RD, Conover CA 2007 Genetic deletion of pregnancy-associated plasma protein-A is associated with resistance to atherosclerotic lesion development in apolipoprotein E-deficient mice challenged with a high-fat diet. Circ Res 100:1696–1702 [DOI] [PubMed] [Google Scholar]
- Juul A, Scheike T, Davidsen M, Gyllenborg J, Jørgensen T 2002 Low serum insulin-like growth factor-I is associated with increased risk of ischemic heart disease: a population-based case-control study. Circulation 106:939–944 [DOI] [PubMed] [Google Scholar]
- Schut AF, Janssen JA, Deinum J, Vergeer JM, Hofman A, Lamberts SW, Oostra BA, Pols HA, Witteman JC, van Duijn CM 2003 Polymorphism in the promoter region of the insulin-like growth factor I gene is related to carotid intima-media thickness and aortic pulse wave velocity in subjects with hypertension. Stroke 34:1623–1627 [DOI] [PubMed] [Google Scholar]
- Markussis V, Beshyah SA, Fisher C, Sharp P, Nicolaides AN, Johnston DG 1992 Detection of premature atherosclerosis by high-resolution ultrasonography in symptom-free hypopituitary adults. Lancet 340:1188–1192 [DOI] [PubMed] [Google Scholar]
- Capaldo B, Patti L, Oliviero U, Longobardi S, Pardo F, Vitale F, Fazio S, Di Rella F, Biondi B, Lombardi G, Saccà L 1997 Increased arterial intima-media thickness in childhood-onset growth hormone deficiency. J Clin Endocrinol Metab 82:1378–1381 [DOI] [PubMed] [Google Scholar]
- Borson-Chazot F, Serusclat A, Kalfallah Y, Ducottet X, Sassolas G, Bernard S, Labrousse F, Pastene J, Sassolas A, Roux Y, Berthezène F 1999 Decrease in carotid intima-media thickness after one-year growth hormone (GH) treatment in adults with GH deficiency. J Clin Endocrinol Metab 84:1329–1333 [DOI] [PubMed] [Google Scholar]
- Pfeifer M, Verhovec R, Zizek B, Prezelj J, Poredos P, Clayton RN 1999 Growth hormone (GH) treatment reverses early atherosclerotic changes in GH-deficient adults. J Clin Endocrinol Metab 84:453–457 [DOI] [PubMed] [Google Scholar]
- Clemmons DR 2007 Modifying IGF1 activity: an approach to treat endocrine disorders, atherosclerosis and cancer. Nat Rev Drug Discov 6:821–833 [DOI] [PubMed] [Google Scholar]
- Reiss K, Cheng W, Ferber A, Kajstura J, Li P, Li B, Olivetti G, Homcy CJ, Baserga R, Anversa P 1996 Overexpression of insulin-like growth factor-1 in the heart is coupled with myocyte proliferation in transgenic mice. Proc Natl Acad Sci USA 93:8630–8635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Wu S, Li SY, Lopez FL, Du M, Kajstura J, Anversa P, Ren J 2007 Cardiac-specific overexpression of insulin-like growth factor 1 attenuates aging-associated cardiac diastolic contractile dysfunction and protein damage. Am J Physiol Heart Circ Physiol 292:H1398–H1403 [DOI] [PubMed] [Google Scholar]
- Li Q, Ren J 2007 Influence of cardiac-specific overexpression of insulin-like growth factor 1 on lifespan and aging-associated changes in cardiac intracellular Ca2+ homeostasis, protein damage and apoptotic protein expression. Aging Cell 6:799–806 [DOI] [PubMed] [Google Scholar]
- Delaughter MC, Taffet GE, Fiorotto ML, Entman ML, Schwartz RJ 1999 Local insulin-like growth factor I expression induces physiologic, then pathologic, cardiac hypertrophy in transgenic mice. FASEB J 13:1923–1929 [DOI] [PubMed] [Google Scholar]
- Guron G, Friberg P, Wickman A, Brantsing C, Gabrielsson B, Isgaard J 1996 Cardiac insulin-like growth factor I and growth hormone receptor expression in renal hypertension. Hypertension 27:636–642 [DOI] [PubMed] [Google Scholar]
- Kim J, Wende AR, Sena S, Theobald HA, Soto J, Sloan C, Wayment BE, Litwin SE, Holzenberger M, LeRoith D, Abel ED 2008 IGF-1 receptor signaling is required for exercise-induced cardiac hypertrophy. Mol Endocrinol 22:2531–2543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lembo G, Rockman HA, Hunter JJ, Steinmetz H, Koch WJ, Ma L, Prinz MP, Ross Jr J, Chien KR, Powell-Braxton L 1996 Elevated blood pressure and enhanced myocardial contractility in mice with severe IGF-1 deficiency. J Clin Invest 98:2648–2655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmen M, Daemen MJ, Bronsaer R, Dassen WR, Zandbergen HR, Kockx M, Smits JF, van der Zee R, Doevendans PA 2001 Cardiac remodelling after myocardial infarction is impaired in IGF-I deficient mice. Cardiovasc Res 50:516–524 [DOI] [PubMed] [Google Scholar]
- Tivesten A, Bollano E, Andersson I, Fitzgerald S, Caidahl K, Sjögren K, Skøtt O, Liu JL, Mobini R, Isaksson OG, Jansson JO, Ohlsson C, Bergström G, Isgaard J 2002 Liver-derived insulin-like growth factor-I is involved in the regulation of blood pressure in mice. Endocrinology 143:4235–4242 [DOI] [PubMed] [Google Scholar]
- Bollano E, Omerovic E, Bohlooly-y M, Kujacic V, Madhu B, Törnell J, Isaksson O, Soussi B, Schulze W, Fu ML, Matejka G, Waagstein F, Isgaard J 2000 Impairment of cardiac function and bioenergetics in adult transgenic mice overexpressing the bovine growth hormone gene. Endocrinology 141:2229–2235 [DOI] [PubMed] [Google Scholar]
- Li Q, Yang X, Sreejayan N, Ren J 2007 Insulin-like growth factor I deficiency prolongs survival and antagonizes paraquat-induced cardiomyocyte dysfunction: role of oxidative stress. Rejuvenation Res 10:501–512 [DOI] [PubMed] [Google Scholar]
- Li Q, Ceylan-Isik AF, Li J, Ren J 2008 Deficiency of insulin-like growth factor 1 reduces sensitivity to aging-associated cardiomyocyte dysfunction. Rejuvenation Res 11:725–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feld S, Hirschberg R 1996 Growth hormone, the insulin-like growth factor system, and the kidney. Endocr Rev 17:423–480 [DOI] [PubMed] [Google Scholar]
- Cingel-Ristiæ V, Flyvbjerg A, Drop SL 2004 The physiological and pathophysiological role of the GH/IGF-axis in the kidney: lessons from experimental rodent models. Growth Horm IGF Res 14:418–430 [DOI] [PubMed] [Google Scholar]
- Mathews LS, Hammer RE, Behringer RR, D'Ercole AJ, Bell GI, Brinster RL, Palmiter RD 1988 Growth enhancement of transgenic mice expressing human insulin-like growth factor I. Endocrinology 123:2827–2833 [DOI] [PubMed] [Google Scholar]
- Quaife CJ, Mathews LS, Pinkert CA, Hammer RE, Brinster RL, Palmiter RD 1989 Histopathology associated with elevated levels of growth hormone and insulin-like growth factor I in transgenic mice. Endocrinology 124:40–48 [DOI] [PubMed] [Google Scholar]
- Doi T, Striker LJ, Gibson CC, Agodoa LY, Brinster RL, Striker GE 1990 Glomerular lesions in mice transgenic for growth hormone and insulin-like growth factor I. I. Relationship between increased glomerular size and mesangial sclerosis. Am J Pathol 137:541–552 [PMC free article] [PubMed] [Google Scholar]
- Rogers SA, Powell-Braxton L, Hammerman MR 1999 Insulin-like growth factor I regulates renal development in rodents. Dev Genet 24:293–298 [DOI] [PubMed] [Google Scholar]
- Svensson J, Tivesten A, Sjögren K, Isaksson O, Bergström G, Mohan S, Mölne J, Isgaard J, Ohlsson C 2007 Liver-derived IGF-I regulates kidney size, sodium reabsorption, and renal IGF-II expression. J Endocrinol 193:359–366 [DOI] [PubMed] [Google Scholar]
- Wolf E, Kramer R, Blum WF, Föll J, Brem G 1994 Consequences of postnatally elevated insulin-like growth factor-II in transgenic mice: endocrine changes and effects on body and organ growth. Endocrinology 135:1877–1886 [DOI] [PubMed] [Google Scholar]
- Blackburn A, Schmitt A, Schmidt P, Wanke R, Hermanns W, Brem G, Wolf E 1997 Actions and interactions of growth hormone and insulin-like growth factor-II: body and organ growth of transgenic mice. Transgenic Res 6:213–222 [DOI] [PubMed] [Google Scholar]
- Moerth C, Schneider MR, Renner-Mueller I, Blutke A, Elmlinger MW, Erben RG, Camacho-Hübner C, Hoeflich A, Wolf E 2007 Postnatally elevated levels of insulin-like growth factor-II (IGF-II) fail to rescue the dwarfism of IGF-I deficient mice except kidney weight. Endocrinology 148:441–451 [DOI] [PubMed] [Google Scholar]
- Hirschberg R, Kopple JD 1989 Evidence that insulin-like growth factor I increases renal plasma flow and glomerular filtration rate in fasted rats. J Clin Invest 83:326–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guler HP, Eckardt KU, Zapf J, Bauer C, Froesch ER 1989 Insulin-like growth factor I increase glomerular filtration rate and renal plasma flow in man. Acta Endocrinol (Copenh) 121:101–106 [DOI] [PubMed] [Google Scholar]
- Guler HP, Schmid C, Zapf J, Froesch ER 1989 Effects of recombinant insulin-like growth factor I on insulin secretion and renal function in normal human subjects. Proc Natl Acad Sci USA 86:2868–2872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschberg R 1993 Effects of growth hormone and IGF-I on glomerular ultrafiltration in growth hormone-deficient rats. Regul Pept 48:241–250 [DOI] [PubMed] [Google Scholar]
- Giordano M, DeFronzo RA 1995 Acute effect of human recombinant insulin-like growth factor I on renal function in humans. Nephron 71:10–15 [DOI] [PubMed] [Google Scholar]
- Blazer-Yost BL, Cox M 1988 Insulin-like growth factor 1 stimulates renal epithelial Na+ transport. Am J Physiol 255:C413–C417 [DOI] [PubMed] [Google Scholar]
- Doi T, Striker LJ, Quaife C, Conti FG, Palmiter R, Behringer R, Brinster R, Striker GE 1988 Progressive glomeruli sclerosis develops in transgenic mice chronically expressing growth hormone and growth hormone releasing factor but not in those expressing insulin-like growth factor-1. Am J Pathol 131:398–403 [PMC free article] [PubMed] [Google Scholar]
- Bellush LL, Doublier S, Holland AN, Striker LJ, Striker GE, Kopchick JJ 2000 Protection against diabetes-induced nephropathy in growth hormone receptor/binding protein gene-disrupted mice. Endocrinology 141:163–168 [DOI] [PubMed] [Google Scholar]
- Reddy GR, Pushpanathan MJ, Ransom RF, Holzman LB, Brosius 3rd FC, Diakonova M, Mathieson P, Saleem MA, List EO, Kopchick JJ, Frank SJ, Menon RK 2007 Identification of the glomerular podocyte as a target for growth hormone action. Endocrinology 148:2045–2055 [DOI] [PubMed] [Google Scholar]
- Fausto N, Laird AD, Webber EM 1995 Liver regeneration. 2. Role of growth factors and cytokines in hepatic regeneration. FASEB J 9:1527–1536 [DOI] [PubMed] [Google Scholar]
- Cressman DE, Greenbaum LE, DeAngelis RA, Ciliberto G, Furth EE, Poli V, Taub R 1996 Liver failure and defective hepatocyte regeneration in interleukin-6-deficient mice. Science 274:1379–1383 [DOI] [PubMed] [Google Scholar]
- Wallenius V, Wallenius K, Hisaoka M, Sandstedt J, Ohlsson C, Kopf M, Jansson JO 2001 Retarded liver growth in interleukin-6-deficient and tumor necrosis factor receptor-1-deficient mice. Endocrinology 142:2953–2960 [DOI] [PubMed] [Google Scholar]
- Uthne K, Uthne T 1972 Influence of liver resection and regeneration on somatomedin (sulphation factor) activity in sera from normal and hypophysectomized rats. Acta Endocrinol (Copenh) 71:255–264 [DOI] [PubMed] [Google Scholar]
- Unterman TG, Phillips LS 1986 Circulating somatomedin activity during hepatic regeneration. Endocrinology 119:185–192 [DOI] [PubMed] [Google Scholar]
- Ekberg S, Luther M, Nakamura T, Jansson JO 1992 Growth hormone promotes early initiation of hepatocyte growth factor gene expression in the liver of hypophysectomized rats after partial hepatectomy. J Endocrinol 135:59–67 [DOI] [PubMed] [Google Scholar]
- Desbois-Mouthon C, Wendum D, Cadoret A, Rey C, Leneuve P, Blaise A, Housset C, Tronche F, Le Bouc Y, Holzenberger M 2006 Hepatocyte proliferation during liver regeneration is impaired in mice with liver-specific IGF-1R knockout. FASEB J 20:773–775 [DOI] [PubMed] [Google Scholar]
- Pietrzkowski Z, Sell C, Lammers R, Ullrich A, Baserga R 1992 Roles of insulinlike growth factor 1 (IGF-1) and the IGF-1 receptor in epidermal growth factor-stimulated growth of 3T3 cells. Mol Cell Biol 12:3883–3889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skrtic S, Wallenius K, Gressner AM, Jansson JO 1999 Insulin-like growth factor signaling pathways in rat hepatic stellate cells: importance for deoxyribonucleic acid synthesis and hepatocyte growth factor production. Endocrinology 140:5729–5735 [DOI] [PubMed] [Google Scholar]
- Lee PD, Giudice LC, Conover CA, Powell DR 1997 Insulin-like growth factor binding protein-1: recent findings and new directions. Proc Soc Exp Biol Med 216:319–357 [DOI] [PubMed] [Google Scholar]
- Mohn KL, Melby AE, Tewari DS, Laz TM, Taub R 1991 The gene encoding rat insulinlike growth factor-binding protein 1 is rapidly and highly induced in regenerating liver. Mol Cell Biol 11:1393–1401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leu JI, Crissey MA, Craig LE, Taub R 2003 Impaired hepatocyte DNA synthetic response posthepatectomy in insulin-like growth factor binding protein 1-deficient mice with defects in C/EBP β and mitogen-activated protein kinase/extracellular signal-regulated kinase regulation. Mol Cell Biol 23:1251–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkel VS, Mohan S, Baylink DJ, Linkhart TA 1990 An inhibitory insulin-like growth factor binding protein (IN-IGFBP) from human prostatic cell conditioned medium reveals N-terminal sequence identity with bone derived IN-IGFBP. J Clin Endocrinol Metab 71:533–535 [DOI] [PubMed] [Google Scholar]
- Cohen P, Peehl DM, Lamson G, Rosenfeld RG 1991 Insulin-like growth factors (IGFs), IGF receptors, and IGF binding proteins in primary culture of prostate epithelial cells. J Clin Endocrinol Metab 73:401–407 [DOI] [PubMed] [Google Scholar]
- Cohen P, Peehl DM, Baker B, Liu F, Hintz RL, Rosenfeld RG 1994 Insulin-like growth factor abnormalities in prostate stromal cells from patients with benign prostatic hyperplasia. J Clin Endocrinol Metab 79:1410–1415 [DOI] [PubMed] [Google Scholar]
- Cohen P, Peehl DM, Rosenfeld RG 1994 The IGF axis in the prostate. Horm Metab Res 26:81–84 [DOI] [PubMed] [Google Scholar]
- Cohen P, Peehl DM, Graves HC, Rosenfeld RG 1994 Biological effects of prostate specific antigen as an insulin-like growth factor binding protein-3 protease. J Endocrinol 142:407–415 [DOI] [PubMed] [Google Scholar]
- Kaplan PJ, Mohan S, Cohen P, Foster BA, Greenberg NM 1999 The insulin-like growth factor axis and prostate cancer: lessons from the transgenic adenocarcinoma of mouse prostate (Tramp) model. Cancer Res 59:2203–2209 [PubMed] [Google Scholar]
- Meinbach DS, Lokeshwar BL 2006 Insulin-like growth factors and their binding proteins in prostate cancer: cause of consequence? Urol Oncol 24:294–306 [DOI] [PubMed] [Google Scholar]
- Ghosh PK, Bartke A 1993 Effects of the expression of bovine growth hormone on the testes and male accessory reproductive glands in transgenic mice. Transgenic Res 2:79–83 [DOI] [PubMed] [Google Scholar]
- Reiter E, Bonnet P, Sente B, Dombrowicz D, de Leval J, Closset J, Hennen G 1992 Growth hormone and prolactin stimulate androgen receptor, insulin-like growth factor-I (IGF-I) and IGF-I receptor levels in the prostate of immature rats. Mol Cell Endocrinol 88:77–87 [DOI] [PubMed] [Google Scholar]
- Ruan W, Powell-Braxton L, Kopchick JJ, Kleinberg DL 1999 Evidence that insulin-like growth factor-I and growth hormone are required for prostate gland development. Endocrinology 140:1984–1989 [DOI] [PubMed] [Google Scholar]
- DiGiovanni J, Kiguchi K, Frijhoff A, Wilker E, Bol DK, Beltrán L, Moats S, Ramirez A, Jorcano J, Conti C 2000 Deregulated expression of insulin-like growth factor 1 in prostate epithelium leads to neoplasia in transgenic mice. Proc Natl Acad Sci USA 97:3455–3460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan-Lefko PJ, Sutherland BW, Evangelou AI, Hadsell DL, Barrios RJ, Foster BA, Demayo F, Greenberg NM 2008 Enforced epithelial expression of IGF-1 causes hyperplastic prostate growth while negative selection is requisite for spontaneous metastogenesis. Oncogene 27:2868–2876 [DOI] [PubMed] [Google Scholar]
- Sutherland BW, Knoblaugh SE, Kaplan-Lefko PJ, Wang F, Holzenberger M, Greenberg NM 2008 Conditional deletion of insulin-like growth factor-I receptor in prostate epithelium. Cancer Res 68:3495–3504 [DOI] [PubMed] [Google Scholar]
- Svensson J, Kindblom J, Shao R, Movérare-Skrtic S, Lagerquist MK, Andersson N, Sjögren K, Venken K, Vanderschueren D, Jansson JO, Isaksson O, Ohlsson C 2008 Liver-derived IGF-I enhances the androgenic response in prostate. J Endocrinol 199:489–497 [DOI] [PubMed] [Google Scholar]
- Kleinberg DL, Ruan W, Yee D, Kovacs KT, Vidal S 2007 Insulin-like growth factor (IGF)-I controls prostate fibromuscular development: IGF-I inhibition prevents both fibromuscular and glandular development in eugonadal mice. Endocrinology 148:1080–1088 [DOI] [PubMed] [Google Scholar]
- Gormley GJ, Stoner E, Bruskewitz RC, Imperato-McGinley J, Walsh PC, McConnell JD, Andriole GL, Geller J, Bracken BR, Tenover JS 1992 The effect of finasteride in men with benign prostatic hyperplasia. N Engl J Med 327:1185–1191 [DOI] [PubMed] [Google Scholar]
- Lepor H 1998 Natural history, evaluation, and nonsurgical management of benign prostatic hyperplasia. In: Walsh PC, Retik AB, Vaughan Jr ED, Wein AJ, eds. Campbell’s urology. Philadelphia: WB Saunders; 1453–1477 [Google Scholar]
- Murakoshi M, Ikeda R, Fukui N, Nakayama T 2001 Relationship between prostatic atrophy and apoptosis in the canine spontaneous benign prostatic hyperplasia (BPH) following chlormadinone acetate (CMA). Tokai J Exp Clin Med 26:71–75 [PubMed] [Google Scholar]
- Colao A, Marzullo P, Ferone D, Spiezia S, Cerbone G, Marinò V, Di Sarno A, Merola B, Lombardi G 1998 Prostate hyperplasia: an unknown feature of acromegaly. J Clin Endocrinol Metab 83:775–779 [DOI] [PubMed] [Google Scholar]
- Colao A, Di Somma C, Spiezia S, Filippella M, Pivonello R, Lombardi G 2003 Effect of growth hormone (GH) and/or testosterone replacement on the prostate in GH-deficient adult patients. J Clin Endocrinol Metab 88:88–94 [DOI] [PubMed] [Google Scholar]
- Chernausek SD, Backeljauw PF, Frane J, Kuntze J, Underwood LE 2007 Long-term treatment with recombinant insulin-like growth factor (IGF)-I in children with severe IGF-I deficiency due to growth hormone insensitivity. J Clin Endocrinol Metab 92:902–910 [DOI] [PubMed] [Google Scholar]
- Cohen A, Levy R, Blethen S, Kuntze J, Hertz J, Frane J, The growth response to insulin-like growth factor-1 treatment during the first year of therapy in prepubertal children is dependent on mean Increlex dose. Program of the 90th Annual Meeting of The Endocrine Society, San Francisco, CA, 2008 (Abstract P2-563) [Google Scholar]
- Rogol A, Frane J, von Stein T, Bright G, Analysis of safety events in clinical trials of rhIGF-1 replacement therapy in children with IGF-1 deficiency. Program of the 90th Annual Meeting of The Endocrine Society, San Francisco, CA, 2008 (Abstract P2-565) [Google Scholar]
- Mahesh S, Kaskel F 2008 Growth hormone axis in chronic kidney disease. Pediatr Nephrol 23:41–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tönshoff B, Kiepe D, Ciarmatori S 2005 Growth hormone/insulin-like growth factor system in children with chronic renal failure. Pediatr Nephrol 20:279–289 [DOI] [PubMed] [Google Scholar]
- Blum WF, Ranke MB, Kietzmann K, Tönshoff B, Mehls O 1991 Growth hormone resistance and inhibition of somatomedin activity by excess of insulin-like growth factor binding protein in uraemia. Pediatr Nephrol 5:539–544 [DOI] [PubMed] [Google Scholar]
- Tönshoff B, Blum WF, Wingen AM, Mehls O 1995 Serum insulin-like growth factors (IGFs) and IGF binding proteins 1, 2, and 3 in children with chronic renal failure: relationship to height and glomerular filtration rate. The European Study Group for Nutritional Treatment of Chronic Renal Failure in Childhood. J Clin Endocrinol Metab 80:2684–2691 [DOI] [PubMed] [Google Scholar]
- Haffner D, Schaefer F, Nissel R, Wühl E, Tönshoff B, Mehls O 2000 Effect of growth hormone treatment on the adult height of children with chronic renal failure. German Study Group for Growth Hormone Treatment in Chronic Renal Failure. N Engl J Med 343:923–930 [DOI] [PubMed] [Google Scholar]
- Hazel SJ, Gillespie CM, Moore RJ, Clark RG, Jureidini KF, Martin AA 1994 Enhanced body growth in uremic rats treated with IGF-I and growth hormone in combination. Kidney Int 46:58–68 [DOI] [PubMed] [Google Scholar]
- Kovács GT, Oh J, Kovács J, Tönshoff B, Hunziker EB, Zapf J, Mehls O 1996 Growth promoting effects of growth hormone and IGF-I are additive in experimental uremia. Kidney Int 49:1413–1421 [DOI] [PubMed] [Google Scholar]
- Poulsen JE 1953 Houssay phenomenon in man: recovery from retinopathy in a case of diabetes with Simmonds disease. Diabetes 2:7–12 [DOI] [PubMed] [Google Scholar]
- Wright AD, Kohner EM, Oakley NW, Hartog M, Joplin GF, Fraser TR 1969 Serum growth hormone levels and the response of diabetic retinopathy to pituitary ablation. Br Med J 2:346–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundbaek K, Christensen N, Jensen V, Johansen K, Olsen T, Hansen A, Orskov H, Osterby R 1970 Diabetes, diabetic angiopathy, and growth hormone. Lancet 2:131–133 [DOI] [PubMed] [Google Scholar]
- Janssen JA, Lamberts SW 2000 Circulating IGF-I and its protective role in the pathogenesis of diabetic angiopathy. Clin Endocrinol (Oxf) 52:1–9 [DOI] [PubMed] [Google Scholar]
- Kondo T, Vicent D, Suzuma K, Yanagisawa M, King GL, Holzenberger M, Kahn CR 2003 Knockout of insulin and IGF-1 receptors on vascular endothelial cells protects against retinal neovascularization. J Clin Invest 111:1835–1842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheetham TD, Jones J, Taylor AM, Holly J, Matthews DR, Dunger DB 1993 The effects of recombinant insulin-like growth factor I administration on growth hormone levels and insulin requirements in adolescents with type 1 (insulin-dependent) diabetes mellitus. Diabetologia 36:678–681 [DOI] [PubMed] [Google Scholar]
- Acerini CL, Patton CM, Savage MO, Kernell A, Westphal O, Dunger DB 1997 Randomised placebo-controlled trial of human recombinant insulin-like growth factor I plus intensive insulin therapy in adolescents with insulin-dependent diabetes mellitus. Lancet 350:1199–1204 [DOI] [PubMed] [Google Scholar]
- Carroll PV, Umpleby M, Ward GS, Imuere S, Alexander E, Dunger D, Sönksen PH, Russell-Jones DL 1997 rhIGF-I administration reduces insulin requirements, decreases growth hormone secretion, and improves the lipid profile in adults with IDDM. Diabetes 46:1453–1458 [DOI] [PubMed] [Google Scholar]
- Acerini CL, Harris DA, Matyka KA, Watts AP, Umpleby AM, Russell-Jones DL, Dunger DB 1998 Effects of low-dose recombinant human insulin-like growth factor-I on insulin sensitivity, growth hormone and glucagon levels in young adults with insulin-dependent diabetes mellitus. Metabolism 47:1481–1489 [DOI] [PubMed] [Google Scholar]
- Carroll PV, Christ ER, Umpleby AM, Gowrie I, Jackson N, Bowes SB, Hovorka R, Croos P, Sönksen PH, Russell-Jones DL 2000 IGF-I treatment in adults with type 1 diabetes: effects on glucose and protein metabolism in the fasting state and during a hyperinsulinemic-euglycemic amino acid clamp. Diabetes 49:789–796 [DOI] [PubMed] [Google Scholar]
- Zenobi PD, Jaeggi-Groisman SE, Riesen WF, Røder ME, Froesch ER 1992 Insulin-like growth factor-I improves glucose and lipid metabolism in type 2 diabetes mellitus. J Clin Invest 90:2234–2241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemmons DR, Moses AC, Sommer A, Jacobson W, Rogol AD, Sleevi MR, Allan G 2005 Rh/IGF-I/rhIGFBP-3 administration to patients with type 2 diabetes mellitus reduces insulin requirements while also lowering fasting glucose. Growth Horm IGF Res 15:265–274 [DOI] [PubMed] [Google Scholar]
- Clemmons DR, Sleevi M, Allan G, Sommer A 2007 Effects of combined recombinant insulin-like growth factor (IGF)-I and IGF binding protein-3 in type 2 diabetic patients on glycemic control and distribution of IGF-I and IGF-II among serum binding protein complexes. J Clin Endocrinol Metab 92:2652–2658 [DOI] [PubMed] [Google Scholar]