Abstract
Two major functions of the mammalian ovary are the production of germ cells (oocytes), which allow continuation of the species, and the generation of bioactive molecules, primarily steroids (mainly estrogens and progestins) and peptide growth factors, which are critical for ovarian function, regulation of the hypothalamic-pituitary-ovarian axis, and development of secondary sex characteristics. The female germline is created during embryogenesis when the precursors of primordial germ cells differentiate from somatic lineages of the embryo and take a unique route to reach the urogenital ridge. This undifferentiated gonad will differentiate along a female pathway, and the newly formed oocytes will proliferate and subsequently enter meiosis. At this point, the oocyte has two alternative fates: die, a common destiny of millions of oocytes, or be fertilized, a fate of at most approximately 100 oocytes, depending on the species. At every step from germline development and ovary formation to oogenesis and ovarian development and differentiation, there are coordinated interactions of hundreds of proteins and small RNAs. These studies have helped reproductive biologists to understand not only the normal functioning of the ovary but also the pathophysiology and genetics of diseases such as infertility and ovarian cancer. Over the last two decades, parallel progress has been made in the assisted reproductive technology clinic including better hormonal preparations, prenatal genetic testing, and optimal oocyte and embryo analysis and cryopreservation. Clearly, we have learned much about the mammalian ovary and manipulating its most important cargo, the oocyte, since the birth of Louise Brown over 30 yr ago.
This review provides a comprehensive and current summary of mammalian ovarian biology from formation and function to cancer and advances in the reproductive clinic.
I. Introduction
- II. Ovarian Development and Differentiation
- A. Primordial germ cell formation and migration
- B. Formation of the bipotential gonad
- C. The XX gonad is not an innocent bystander in sex determination
- D. Sexually dimorphic changes in the initiation of meiosis
- III. Ovarian Folliculogenesis
- A. Formation of an ovarian follicle—oocyte survival vs. primordial follicle formation
- B. Maintenance of primordial follicles and initial recruitment
- C. Preantral folliculogenesis
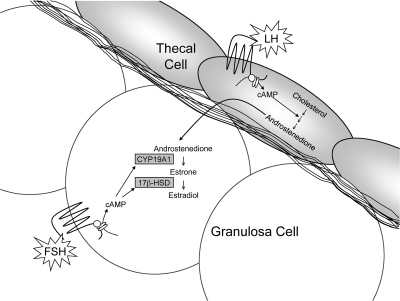
- D. Theca formation and physiology
- E. Antral follicle formation, FSH, and estradiol
- F. The preovulatory follicle, cumulus expansion, ovulation, and luteinization
- G. Regulation of meiotic arrest and reentry
- IV. Ovarian Cancer
- A. Epithelial ovarian cancer
- B. Sex cord-stromal ovarian cancer
- C. Germ cell ovarian cancer
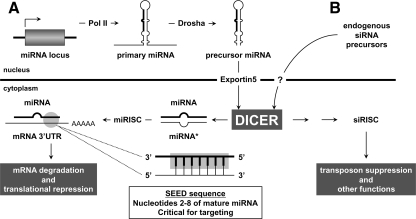
- D. Small RNAs in ovarian physiology and cancer
- V. The Assisted Reproductive Technology Laboratory
- A. Hormonal preparations
- B. In vitro fertilization and intracytoplasmic sperm injection
- C. Advances in cryopreservation
- D. Choosing the best oocyte—morphological and molecular analysis
- E. Stem cells and nuclear cloning
VI. Future Perspectives
I. Introduction
The word “ovary” is derived from the Latin word “ovum,” meaning egg. The mammalian ovary is not only the female gonad, containing the supply of germ cells to produce the next generation, but also the female reproductive gland, controlling many aspects of female development and physiology. After the union of an oocyte and a spermatozoon to become a zygote, all cells up to the eight-cell stage of embryogenesis appear to have similar totipotency (potential to become any lineage), because these cells all appear morphologically identical. However, with the formation of a 16-cell morula, the cells begin the process of differentiation with cells being allocated to either the inside or outside of the embryo. This process is exaggerated further at the blastocyst stage in which three lineages are defined: trophectoderm (future placenta), epiblast (future embryo), and primitive endoderm (future yolk sac). After implantation and further differentiation, cells within the epiblast eventually form the precursors of the primordial germ cells (PGCs), the first cells of the future ovary to be defined. The PGCs enter the indifferent gonad, and eventually the ovary forms and permits the PGCs to differentiate into oocytes, which enter meiosis and subsequently arrest; this differentiation step and entry into meiosis suggest that the last of the oocyte “stem cells” (i.e., the PGCs) likely disappear at this stage of fetal life. The meiotically arrested oocytes eventually become surrounded by pre-granulosa cells and form individual primordial follicles, the resting pool of oocytes that have the potential to be recruited into the growing follicle pool in the postpubertal mammal, to be fertilized, and to contribute to the next generation (Fig. 1).
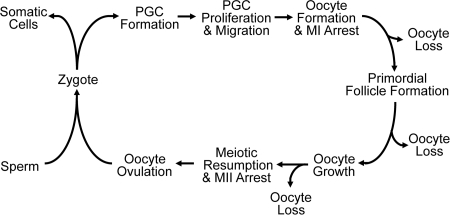
Figure 1.
Reproductive life cycle of a mammalian female. An oocyte and a spermatozoon will fuse to form a zygote and undergo multiple steps in embryogenesis. At about E6.5 in mouse, the PGC will be allocated and go through multiple steps to reach the genital ridge. In an XX mammal, the germ cell will form an oocyte that arrests at meiosis I (MI). During prenatal life in women, and in the perinatal period in mice, the oocyte will be encased in somatic cells to become primordial follicles. Upon recruitment into the growing pool, the oocyte increases in size during folliculogenesis. The LH surge will induce resumption of meiosis, release of the first body, arrest at MII, and subsequent ovulation of the oocyte into the fallopian tube. Fertilization with a spermatozoon will induce the completion of meiosis and release of the second polar body. The cycle continues in the next generation of females. During the reproductive cycle, there are multiple steps where significant oocyte loss is observed.
Through various types of developmental, genetic, physiological, and hormonal analyses, the above steps in the reproductive cycle of a mammalian female have begun to be understood in great detail. Studies in mice have proven invaluable for identifying genes critical to normal ovarian development and function. Mutations in many of the human homologs of these genes often contribute to infertility in women (Table 1). In the process of deconstructing the female reproductive life cycle, techniques for manipulation of the human (and nonhuman) oocyte have been developed to more effectively create “test tube” babies. In addition, we have begun to understand conditions in which these well-orchestrated events of female reproductive development and physiology go awry, leading to diseases that range from psychologically distressing, such as infertility, to life-threatening, such as ovarian cancer. In this review, we describe the development, physiology, and pathology of the mammalian ovary from its formation to all the wondrous details that have been discovered about it in vivo, in the test tube, and in the clinical reproductive setting.
Table 1.
Mutations associated with infertility in women
| Gene | Phenotype | OMIM gene [OMIM infertility] |
|---|---|---|
| Bone morphogenetic protein 15 (BMP15) | Hypergonadotropic ovarian failure (POF4) | 300247 [300510] |
| Bone morphogenetic protein receptor 1B (BMPR1B) | Ovarian dysfunction, hypergonadotropic hypogonadism and acromesomelic chondrodysplasia | 603248 |
| Chromobox homolog 2, Drosophila polycomb class (CBX2; M33) | Autosomal 46,XY, male-to-female sex reversal (phenotypically perfect females) | 602770 (67) |
| Chromodomain helicase DNA-binding protein 7 (CHD7) | CHARGE syndrome and Kallmann syndrome (KAL5) | 608892 [612370] |
| Diaphanous homolog 2 (DIAPH2) | Hypergonadotropic, premature ovarian failure (POF2A) | 300108 [300511] |
| Fibroblast growth factor 8 (FGF8) | Normosmic hypogonadotropic hypogonadism and Kallmann syndrome (KAL6) | 600483 [612702] |
| Fibroblast growth factor receptor 1 (FGFR1) | Kallmann syndrome (KAL2) | 136350 [147950] |
| FSH receptor (FSHR) | Hypergonadotropic hypogonadism and ovarian hyperstimulation syndrome | 136435 |
| FSH β (FSHB) | Deficiency of FSH, primary amenorrhea and infertility | 136530 [229070] |
| Forkhead box L2 (FOXL2) | Isolated POF (POF3) associated with BPES type I; FOXL2 402C→G mutations associated with human granulosa cell tumors | 605597 [608996] |
| Fragile X mental retardation 1 (FMR1) | Premature ovarian failure (POF1) associated with premutations | 309550 [311360] |
| GnRH receptor (GNRHR) | Hypogonadotropic hypogonadism | 138850 |
| GnRH 1 (GNRH1) | Normosmic hypogonadotropic hypogonadism | 152760 (769, 770) |
| Kallmann syndrome 1 (KAL1) | Hypogonadotropic hypogonadism and anosmia, X-linked Kallmann syndrome (KAL1) | 308700 |
| KISS1 receptor (KISS1R; GPR54) | Hypogonadotropic hypogonadism | 604161 |
| LH β (LHB) | LHB G102S mutations associated with infertility | 152780 |
| LH/choriogonadotropin receptor (LHCGR) | Hypergonadotropic hypogonadism (LH resistance) | 152790 |
| Nuclear receptor subfamily 0, group B, member 1 (NROB1; DAX1) | X-linked congenital adrenal hypoplasia with hypogonadotropic hypogonadism; dosage-sensitive male-to-female sex reversal | 300473 [300200; 300018] |
| Nuclear receptor subfamily 5, group A, member 1 (NR5A1; SF1) | 46,XY male-to-female sex reversal and streak gonads and congenital lipoid adrenal hyperplasia; 46,XX gonadal dysgenesis and 46,XX primary ovarian insufficiency | 184757 (771) |
| Premature ovarian failure 1B (POF1B) | Hypergonadotropic, primary amenorrhea (POF2B) | 300603 [300604] |
| Prokineticin 2 (PROK2) | Normosmic hypogonadotropic hypogonadism and Kallmann syndrome (KAL4) | 607002 [610628] |
| Prokineticin receptor 2 (PROKR2) | Kallmann syndrome (KAL3) | 607123 [244200] |
| R-spondin family, member 1 (RSPO1) | 46,XX, female-to-male sex reversal (individuals contain testes) | 609595 |
| Sex-determining region Y (SRY) | Mutations lead to 46,XY females; translocations lead to 46,XX males | 480000 |
| SRY-related HMG-box gene 9 (SOX9) | Autosomal 46,XY male-to-female sex reversal (campomelic dysplasia) | 608160 |
| Tachykinin 3 (TAC3) | Normosmic hypogonadotropic hypogonadism | 162330 |
| Tachykinin receptor 3 (TACR3) | Normosmic hypogonadotropic hypogonadism | 162332 |
Because of space limitations, most cases associated with female-to-male sex reversal due to steroidogenesis defects, syndromes, and chromosomal abnormalities are excluded from the table. The primary reference (in parentheses) is included for work not yet described in OMIM.
II. Ovarian Development and Differentiation
Future components of the mammalian ovary develop long before a distinct ovary-like organ can be discerned. In this section, we describe how the germ cells arise and reach the undifferentiated gonad, the factors involved in formation of the ovary, and the early steps that distinguish the female germline from the male germline.
A. Primordial germ cell formation and migration
Despite the early descriptions of mouse PGCs over 50 yr ago (1), the last decade has continued to see dramatic advances in our understanding of the molecular mechanisms of PGC formation and migration (current knowledge summarized in Table 2). Chiquoine (1) had initially shown that the putative PGC stained strongly for alkaline phosphatase as early as embryonic day (E) 8.5. Consistent with these findings, these alkaline phosphatase-positive cells were shown to be depleted in the classic white spotting (KIT) and steel (KIT ligand) mouse mutants that are known to lack germ cells in their gonads (2,3). By 1967, Ozdzenski (4) was able to identify these putative PGCs at the base of the allantois as early as E8.0. Additional microscopic studies in the 1970s (5,6) were extremely helpful in characterizing these cells and their migration (see below). However, it was not until 1990 that additional experimental proof confirmed that these alkaline phosphatase-positive cells were in fact PGCs. First, at E7.25, a cluster of cells were observed containing a “spot” in their cytoplasm that stained intensely for alkaline phosphatase activity; these cells were present at the base of the yolk sac before formation of the allantois (7). Second, follow-up studies confirmed that these cells were in fact the only PGCs because ablation of the cells resulted in embryos without germ cells whereas transplantation of these cells leads to their proliferation. Thus, using alkaline phosphatase as a marker, the female and male (mouse) germline was thought to be specified by at least E7.25.
Table 2.
PGC events and pathways in the mouse
| PGC event | Timepoint | Major pathways and genes (nonessential markers) |
|---|---|---|
| Induction/competence | E6.0–6.5 | BMP2/4/8 signaling through SMAD1/5 (IFITM3) |
| Early specification | E6.5 | PRDM1 and PRDM14 induced; LIN28 induction suppresses let-7 maturation, allowing PRDM1 protein to be expressed; HOXB1 suppressed and restricted at somatic lineage |
| Late specification | E7.5 | PRDM1 and PRDM14 mark all future PGCs; pluripotency markers POU5F1, SOX2, and NANOG turned on (DPPA3, ALPL) |
| Chromatin reprogramming (similar to ES cells) | E8.5 | H3K27me3 induced; H3K9me2 erased; H2/H4 RMC2 induced |
| Migration and entry into genital ridge | E8.5–11.5 | KIT ligand/KIT; NANOS3; DND1 |
| Loss of imprinting and reversal of chromatin reprogramming | E10.5–12.5 | PRDM1-PRMT5 translocation out of nucleus |
| Meiosis competent state | E11.5 | DAZL |
| Meiotic entry | E12.5 | CYP26B1 down-regulated in XX germ cells |
One enigma was that alkaline phosphatase was not required for this process; mutation of ALPL (alkaline phosphatase, liver/bone/kidney) does not alter the number of PGCs or their migration (8). This indicated that ALPL marked these cells but was not involved in either the formation or function of PGCs.
What then are the factors involved in formation of PGC precursors and their specification, and when do these factors act? Knockout models have helped greatly to define members of the bone morphogenetic protein (BMP) family as major extrinsic factors that are key to the early development of PGC precursors (reviewed in Refs. 9,10,11 and summarized in Table 3). BMP4 and BMP8B, secreted from the extraembryonic ectoderm (12,13), and BMP2, secreted from the visceral endoderm (14), are required for the early discrimination of PGC precursors from the somatic cells of the embryo. These BMPs signal in a dosage-dependent manner to the epiblast cells through a BMP receptor cascade that involves phosphorylation of the BMP SMADs, SMAD1 and SMAD5, both of which, along with their common SMAD partner, SMAD4, have been shown to function in this pathway (15,16,17,18). In contrast, the other BMP-signaling SMAD, SMAD8, is dispensable for this process (19). It is believed that BMPs begin to act on the pluripotent proximal epiblast cells between E5.5 and E6.0 to allow them to be “competent” to become a PGC precursor. Signals (some of which are likely BMPs) from the extraembryonic ectoderm and the visceral endoderm result in restriction and formation of PGC precursors only from epiblast cells in the posterior of the embryo (20). One of the earliest genes induced by BMPs is Ifitm3 (interferon-induced transmembrane protein 3; Fragilis), an excellent early marker for the competence step as well as the further differentiation of the PGC (21). However, like ALPL, absence of IFITM3 and its related family members does not alter PGC formation (22), making it a functionally dispensable but key marker protein.
Table 3.
Phenotypes of mice with mutations in PGC markers and pathway components (order based on expression and/or function)
| Gene (pseudonym) | Phenotype/findings | Ref. |
|---|---|---|
| Bmp2 | Embryonic lethal; reduced PGCs | 14,772 |
| Bmp4 | Embryonic lethal; no PGCs | 12,773 |
| Bmp8b | Viable; male infertility; reduced PGCs | 13,774 |
| Smad1 | Embryonic lethal; reduced PGCs | 16,17,775 |
| Smad5 | Embryonic lethal; reduced PGCs | 15,776,777 |
| Smad4 | Embryonic lethal; absent PGCs | 18,778,779 |
| Ifitm3 (Fragilis) | Not essential for PGC function | 22 |
| Prdm1 (Blimp1) | Embryonic lethal; PGC specification defect | 23 |
| Prdm14 | Infertility; PGC specification defect | 29 |
| Ehmt1 (Glp) | Unknown PGC function | |
| Ehmt2 (G9a) | Unknown PGC function | |
| Dppa3 (Stella) | Not essential for PGC function | 24,25 |
| Alpl (Alkaline phosphatase) | Not essential for PGC function | 8 |
| Pou5f1 (Oct4) | Pluripotency marker | 780,781 |
| Nanog | Pluripotency marker | 781,782,783 |
| Sox2 | Pluripotency marker | 781,784 |
| Nanos3 | Infertile; PGC migration defect | 44 |
| Dnd1 (Ter) | Infertile; PGC migration defect | 45 |
| Kitl | Variable phenotypes depending on mutation; PGC migration defect | 785 |
| Kit | Variable phenotypes depending on mutation; PGC migration defect | 785 |
| Tgfbr1 (Alk5) | Embryonic lethal; enhanced PGC migration | 42,786 |
At approximately E6.25, six of the IFITM3-positive epiblast cells adjacent to the extraembryonic ectoderm express the protein PRDM1 (PRDI- BF1- RIZ domain containing 1; BLIMP1); these cells are the first PGC precursors and the first cells of the mammalian embryo for which their fate is committed (23). PRDM1 is a transcriptional repressor that contains a PRDI-BF1-RIZ domain and five Krüppel-like C2H2 zinc finger (DNA binding) domains. PRDM1 was first identified in a screen for gene products differentially expressed at E7.5 in founder PGC but not adjacent somatic cells (21). Within 1 d, there are 20–28 PRDM1-positive tightly clustered cells, and by E7.5, 40 PRDM1-positive cells are also positive for alkaline phosphatase and show nearly 100% concordance with DPPA3 (developmental pluripotency-associated 3; STELLA), another nonessential marker of PGCs (24,25). Lineage tracing experiments confirmed that the PRDM1-positive cells were the germline-restricted PGC progenitors, and that by E7.5, these 40 cells were the founder population of PGCs.
In parallel with the expression and lineage-tracing studies, confirmation that PRDM1 was essential for PGC specification came from several additional mouse knockout studies (23). First, Bmp4 null mice that lack PGCs also lack PRDM1-positive cells. Second, null mutations of Prdm1 show that heterozygotes have a reduction in the number of PGCs at E7.5, whereas homozygous mutants have near zero PGCs (23,26). The few “PGC-like” cells that were observed at the base of the allantois in the null mutants failed to increase in number, and none of these cells migrated normally. High levels of PRDM1 expression are also required for PGC specification as determined by the reduction in PGCs in heterozygous mutants and the absence of germ cells in mice carrying a hypomorphic PRDM1-green fluorescent protein reporter allele (27).
Although ALPL, DPPA3, and IFITM3 are important nonessential markers for the lineage restricted PGC, PRDM14 was identified as a relative of PRDM1 that is not only expressed but required in PGCs (28,29). PRDM14 is expressed at least as early as PRDM1 (i.e., ∼E6.5) in PGC precursors, and similar to PRDM1, expression of PRDM14 is dependent on BMP4 signaling through at least SMAD1 (i.e., no PRDM14-positive cells are observed in mice null for these genes). Phenotypically, absence of PRDM14 results in few PRDM1-positive cells, with only a few of these cells observed to migrate. However, unlike Prdm1 null mice, which die during embryogenesis (23,26), Prdm14 null female and male mice are viable but infertile. Thus, Prdm14 is the second identified gene that is essential for specification of the mammalian germline.
Because the germline fails to develop in the absence of PRDM1 and PRDM14, what is the relationship of these two PRDI- BF1- RIZ domain-containing proteins? Based on analysis of Prdm1 null embryos and Prdm14 null embryos, it is clear that PRDM1 continues to be expressed in Prdm14 null embryos and vice versa, indicating that these proteins function in independent pathways to specify the germline (29). However, continued PRDM14 expression requires PRDM1 and vice versa. Although the direct regulators of PRDM14 and PRDM1 are unknown, let-7 family microRNAs are important modulators of PRDM1 expression, and LIN28 is also required in PGC specification. During an in vitro screen for genes involved in PGC specification, small interfering RNA (siRNA) knockdown of Lin28 was found to reduce the number of PGC-positive colonies (30). LIN28 is known to suppress the maturation of let-7 microRNAs (31,32,33,34,35). Furthermore, the Prdm1 3′ untranslated region (UTR) contains an important let-7 binding site. Thus, LIN28 induction suppresses the levels of let-7 microRNAs, which relieves the inhibition of PRDM1 synthesis and allows PGC specification to proceed.
How do PRDM1 and PRDM14 function to establish the mammalian germline? To address this question, the Surani and Saitou laboratories (21,23,29) have performed detailed single cell quantitative analysis of gene expression using cells from wild-type, PRDM1 mutant, and PRDM14 mutant embryos. Analysis of wild-type embryos had first identified PRDM1 and PRDM14. It also became clear that major (parallel) events in PGC specification are repression of somatic cell gene expression, induction of PGC-enriched gene expression, and reexpression of pluripotency genes. In particular, the Hoxa1 and Hoxb1 genes, which are highly expressed in the somatic epiblast cells in the posterior portion of the developing embryo at E7.25-E7.5, are never synthesized in the founder PGCs. Likewise, other mesodermal genes [e.g., brachyury (T), Fgf8, and Snai1] are also suppressed. However, in the absence of PRDM1, the majority of PGC-like cells are positive for Hoxa1 and/or Hoxb1. This somatic cell repression program is still intact in the absence of PRDM14. Absence of PRDM14, but not PRDM1, results in failed induction of the lineage restricted DPPA3, whereas absence of PRDM1, but not PRDM14, leads to absence of the PGC-specific gene Nanos3 (see below). PGCs also show induction of several pluripotency “master regulatory” genes including Sox2, Pou5f1 (Oct4), and Nanog. Absence of PRDM14 leads to repression of Sox2, whereas Prdm1 null cells show variable expression of Sox2. Thus, the somatic cell repression function is unique to PRDM1, whereas both PRDM14 and PRDM1 regulate some PGC-specific transcripts as well as the pluripotency-associated protein SOX2.
Since PRDM1 was identified almost two decades ago, much more data have been accumulated on it than PRDM14, which was only presented in publications 2 yr ago. As mentioned earlier, PRDM1 functions in multiple tissues, and its absence leads to embryonic lethality, whereas PRDM14 is only required in the mammalian germline. In addition to their roles in PGC specification, both PRDM1 and PRDM14 have been implicated in cancer. As implied by its pseudonym (BLIMP1, B-lymphocyte-induced maturation protein 1), PRDM1 is a master regulator of the terminal differentiation of B cells into Ig-producing plasma cells through its ability to act as a transcriptional repressor, blocking the transcription of a diverse set of genes such as Myc and p53. Loss of function mutations in human chromosome 6q21, the location where PRDM1 maps, are implicated to cause B and T cell lymphomas, whereas other studies have demonstrated key tumor suppressor roles of PRDM1 in these lineages. Alternatively, gene amplification of 8q13, where PRDM14 maps, are observed in multiple cancers including breast cancer, which demonstrates increased expression of Prdm14 mRNA and protein (36). Increased expression of PRDM14 in breast cancer cells stimulates growth, whereas knockdown induces apoptosis. Retroviral insertion into the Prdm14 locus in mice results in its overexpression and consequent B cell lymphomas (37). These studies suggest that PRDM14 functions as an oncogene. In human ES cells, PRDM14 maintains cell renewal (38). This information on their tumor suppressor vs. oncogenic roles confirms that PRDM1 and PRDM14 appear to exert their effects in different manners.
At E7.5, a time in which PGC specification has occurred, PGCs express markers of pluripotency including POU5F1, SOX2, NANOG, as well as DPPA3 and ALPL. At this time point, the methylation pattern of a PGC is predominantly dimethylated at histone 3 lysine 9 (H3K9me2), whereas there are low levels of trimethylated histone 3 lysine 27 (H3K27me3). As their symbols imply, both PRDM1 and PRDM14 have PRDI-BF1-RIZ domains (also called SET domains) that have structural similarity to histone methyltransferases. In B cells, where PRDM1 is a master regulator, PRDM1 interacts with euchromatic histone lysine N-methyltransferase 2 (EHMT2) (39), which performs dimethylation mainly at histone 3 lysine 9. Between E7.5 and E8.5, PGCs demonstrate major chromatin changes, increasing the levels of H3K27me3 and erasing the H3K9me2 methylation marks (40,41), patterns that resemble the chromatin patterns of pluripotent stem cells. Similar to ES cells, the H3K27me3 marks also appear to be involved in the repression of the “somatic cell” gene expression program. H3K9me2 erasure occurs despite the presence of EHMT2, likely because euchromatic histone methyltransferase 1 (EHMT1), which complexes with EHMT2 for methylation of H3K9me2, is down-regulated by E7.25 (28). Although it is unclear how PRDM1 and PRDM14 directly influence these lysine methylation changes, chromatin changes in H3K27me3 and H3K9me2 from E7.5 to E8.5 do occur. Furthermore, additional data are evident on the roles of PRDM1 in arginine methylation. PRDM1 complexes with protein arginine N-methyltransferase 5 (PRMT5) to dimethylate histone 2A and histone 4 at arginine 3 by E8.5. Along with these changes, the PGCs arrest at the G2 stage of the cell cycle and transiently become transcriptionally silent as they migrate from the base of the yolk sac along the hindgut to the genital ridge (40).
The exact trigger that initiates PGC migration to the genital ridge and the chemoattractants that are required for directional movement toward the genital ridge are slowly beginning to be understood. The trigger(s) could be the expression of a key receptor on the PGC and/or the expression of the secreted chemoattractant from the genital ridge. An extracellular matrix gradient along the path of migration is important, and if too much matrix is laid down, PGCs show reduced migration. For example, suppression of TGFβ signaling by knocking out Tgfbr1 (Alk5) leads to enhanced migration due to reduction in the levels of TGFβ-induced collagen type 1 in the extracellular matrix (42). One of the best candidates to function as a chemoattractant for PGCs is KIT ligand. Using PGCs obtained from E10.5 and E11.5 embryos, in vitro migration assays demonstrated that KIT ligand could function as an effective chemoattractant for the PGCs and that the phosphatidylinositol 3-kinase (PI3K)/AKT and SRC kinase pathways were involved downstream of KIT in the PGC (43). Although it is not clear whether KIT ligand/KIT signaling is involved in the earliest steps of activation and migration, these data support a late role for this pathway in PGC migration into the genital ridge.
Although not involved in PGC migration, several additional gene products are necessary for PGC survival during their migration. For example, two RNA binding proteins, NANOS3 and DND1 (dead end homolog 1; TER), are expressed in PGCs from E7.5 onward and protect the PGCs from apoptosis; mutations in either Nanos3 or Dnd1 lead to few germ cells in the genital ridge and germ cell deficiency (44,45). Consistent with the pleiotropic roles of the KIT ligand/KIT pathway, these proteins not only function in PGC migration but also aid in PGC survival and proliferation.
During the migration stage, the histone marks and the expression of the major pluripotency genes are maintained. However, beginning at approximately E10.5-E12.5 and coinciding with entry of the PGCs into the gonadal ridge, chromatin and gene expression changes are observed. At approximately E11.5, the PRDM1-PRMT5 complex, which is observed in the nucleus from the specification stage through PGC migration, translocates to the cytoplasm. In parallel, the pluripotency-associated genes also begin to be down-regulated, whereas the RNA helicase DHX38 [DEAH (Asp-Glu-Ala-His) box polypeptide 38], which is normally repressed through arginine methylation of histones by the PRDM1-PRMT5 complex, begins to be expressed (46). The E8.5-E11.5 period, when the PGCs show highest expression of the pluripotency-associated genes, is also the only window for production of embryonic germ cells from the PGC. It is postulated that secreted molecules and proteins from the somatic cells in the genital ridge directly influence these major reprogramming events. Among the changes that are observed are genome-wide demethylation, erasure of imprinting, and reactivation of the inactive X chromosome in females. Recent transgene studies have helped to explain X chromosome reactivation and the role of the female gonadal environment in this process (47). The erasure of histone 2A/4 arginine 3 methylation is consistent with the aforementioned translocation of PRDM1-PRMT5 complex out of the nucleus. Lastly, once PGCs enter the genital ridge, they appear to lose their ability to migrate.
B. Formation of the bipotential gonad
Similar to the PGC, the sex of the gonadal ridge initially is irrelevant; PGCs are attracted equally to an XX or an XY gonadal ridge. The undifferentiated or bipotential gonadal ridge arises at approximately E10.5 between the coelomic epithelium and the mesonephros, the two distinct tissues along with the PGCs that contribute most of the cells of the future ovary or testis during the subsequent sexual differentiation stages. The initial steps in the process appear to be a thickening of the coelomic epithelium. Several factors have been identified to play key roles in formation of the genital ridge with so-called bipotential possibilities (i.e., the potential to develop into a testis or an ovary depending on the genetic makeup of the somatic cells in and surrounding the genital ridge; see Sections II.C and II.D).
Several of the major “bipotential gonad” gene products also set the stage for the upcoming differentiation into either a testis or an ovary (Fig. 2 and Table 4). Probably the first key gene in the development of the bipotential gonad is the homeobox gene empty spiracles homolog 2 (Emx2). In the absence of EMX2, which is expressed in the epithelium, the thickening of the coelomic epithelium is not obvious, resulting in sex-independent absence of the gonads as well as absence of the Müllerian duct and Wolffian duct derivatives (48). Thus, EMX2 appears to be a transcriptional regulator of subsequent events leading to gonad and urogenital system formation.
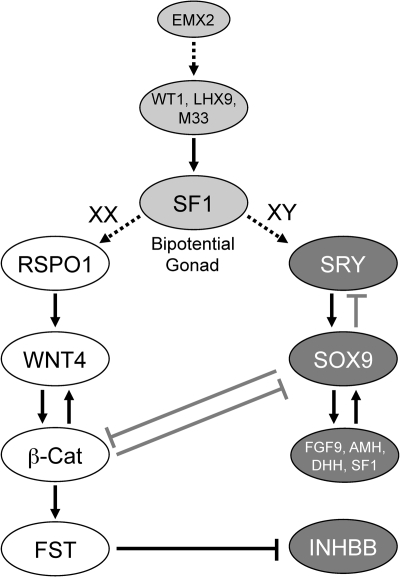
Figure 2.
Gonad function and sexual differentiation. As shown, several major gene products influence the formation of the bipotential gonad (light gray), the development of the ovary (white), and the development of the testis (dark gray). SF1 is a central player in the bipotential gonad, being regulated by WT1, LHX9, and M33 (CBX2), and at other steps in gonadal differentiation. In XY gonads, SRY functions in a short window in pre-Sertoli cells to up-regulate the transcription of SOX9 that is already expressed at low levels through the action of SF1. This higher SOX9 expression then suppresses SRY in a negative feedback loop and also up-regulates itself through the combined actions of SF1 and SOX9 on the SOX9 promoter. SOX9 also up-regulates FGF9 that signals back through FGFR2 to maintain/increase SOX9 expression. The ovarian differentiation pathway involves RSPO1 increasing the signaling of WNT4, which up-regulates β-catenin. β-catenin acts to up-regulate WNT4 and other proteins such as FST. The testis pathway appears to mainly antagonize this pathway through decreasing β-catenin levels. Likewise, β-catenin antagonizes the testis pathway by destabilizing SOX9.
Table 4.
Mouse mutants with defects in the formation of the gonad
| Gene | Phenotype | Ref. |
|---|---|---|
| Emx2 | Midgestation embryonic lethality; absence of gonads due to defects in the coelomic epithelium | 48 |
| Wt1 | Midgestation embryonic lethality; lack of gonads by E14 | 51 |
| Wt1 (−KTS splice variant) | Streak gonads in males and females | 53 |
| Wt1 (+KTS splice variant) | Male-to-female sex reversal | 53 |
| Lhx9 | Viable mice; male-to-female sex reversal | 56 |
| Cbx2 (M33) | Lethality (60%) between birth and 21 d; male-to-female sex reversal | 65 |
| Pod1 | Neonatal lethality; disorganized gonads | 68 |
| Nr5a1 (Sf1) | Early postnatal lethality; absence of both gonads and Müllerian duct derivatives | 59 |
Wilms tumor 1 homolog (Wt1) is the second gene important for formation of the bipotential gonad. WT1 mutations or deletions (e.g., 11p13) in patients are associated with several human syndromes that include genitourinary abnormalities. 46,XY male-to-female sex reversal is observed typically in Frasier syndrome (49), occasionally in Denys-Drash syndrome (50), and in one patient with WAGR syndrome (50). WT1 is a transcription factor with four zinc finger domains. Although knockout of Wt1 in mice leads to midgestation embryonic lethality, it was noted that male and female knockout mice lacked gonads by E14, indicating that WT1 is required for early formation of the bipotential gonad (51). WT1 is expressed in the coelomic epithelium during gonad formation, and in the Sertoli cells and granulosa cells during their formation. Consistent with its expression during gonadal development, analysis of the Wt1 mutants at E11 and E12 showed that the thickening of the coelomic epithelium was markedly reduced compared with wild-type embryos but that PGC migration into these genital ridges still occurred. These findings indicate that WT1 may not be required for the initial step in gonad formation but becomes essential soon after, at least for further development and/or maintenance of these cells.
WT1 acts at several points in the pathways of bipotential gonad formation and sex determination. There are two alternative splice variants of WT1 that include or exclude the lysine-threonine-serine (KTS) amino acids between the third and fourth zinc fingers; functionally, the presence of the KTS amino acids prevents the fourth zinc finger from binding to DNA, resulting in lower transcriptional activity (52). To study the relative significance of these two isoforms, mutations were made to disrupt one or the other form (53). The absence of the WT1(−KTS) isoform results in streak gonads in both male and female gonads secondary to increased cell death during gonad formation. These results are consistent with data demonstrating that the WT1(−KTS) isoform functions to regulate the expression of steroidogenic factor 1 (SF1; NR5A1, nuclear receptor subfamily 5, group A, member 1) (54). Alternatively, absence of the WT1(+KTS) form of WT1 (mimicking the mutation seen in Frasier syndrome in which there is less of the +KTS variant) leads to male-to-female sex reversal and reduced SRY (sex-determining region of chromosome Y) and SOX9 (SRY-box containing gene 9) expression, consistent with a later role of WT1 in sex determination (see Section II.C). In addition, WT1 and GATA4 transcriptionally cooperate on the mouse, pig, and human SRY promoters, and the synergy is strongest with the WT1(+KTS) isoform (55), which is also consistent with the above in vivo data.
LIM homeobox protein 9 (LHX9) is another key regulator that functions in the development of the bipotential gonad. Absence of LHX9 does not alter viability; however, all postnatal mice are phenotypically female with no gonads (56). Lhx9 is expressed in the coelomic epithelium of the genital ridge at E9.5, and by E11.5, Lhx9 is expressed highly in the coelomic epithelium and at lower levels in the developing gonad. Analysis of the Lhx9 null gonads at earlier embryonic stages demonstrates no morphological differences from wild type at E11.5, with normal migration of the PGC into the genital ridge, but no further development thereafter, and a complete loss of any defined gonad-like structures by E13.5. The absence of any gonad results in a lack of testosterone and anti-Müllerian hormone (AMH) synthesis in XY embryos, leading to no development of the Wolffian duct and failure of the Müllerian duct to regress, respectively. This situation phenocopies the experiments that Alfred Jost performed over 50 yr earlier in which removal of embryonic rabbit gonads results in a ductal system that resembles a female (57).
Because the embryonic phenotype of the Lhx9 null mouse resembles the Wt1 null mouse, what is the pathway relationship of these two transcription factors and what regulates Lhx9? Although transcriptional regulators of Lhx9 in the genital ridge have not been reported, another study has demonstrated that Lhx9 is regulated in the developing heart by a transcriptional complex of GATA4 and FOG2 (friend of GATA2; ZFPM2, zinc finger protein, multitype 2) (58). This is important because the GATA4/FOG2 complex is also involved in the regulation of SRY (see Section II.C). LHX9 subsequently functions along with WT1 in the regulation of SF1, which is first expressed in the coelomic epithelium and also in the daughter cells that migrate into the urogenital ridge to become either Sertoli cells or granulosa cells. Analysis of the Lhx9−/− urogenital ridge at E11.5 shows very low levels of SF1, whereas Sf1−/− urogenital ridges have normal levels of LHX9 (56). Likewise, SF1 is not expressed in the Wt1−/− urogenital ridges (54). Furthermore, in addition to the four binding sites for WT1 in the Sf1 promoter, there also is one binding site for LHX9, and both LHX9 and WT1(−KTS) synergize to regulate transcription of Sf1 (54). Consistent with these findings and the function of SF1 as the key gene downstream of WT1 and LHX9, absence of SF1 leads to failure of development of the bipotential gonad and absence of gonads at birth (59), phenocopying the WT1 and LHX9 knockout mice. These findings are recapitulated in human patients that have SF1 (NR5A1) mutations, resulting in XY male-to-female sex reversal (Table 1) (60,61,62,63,64).
Further evidence for the central role of SF1 in bipotential gonad formation comes from analysis of additional knockout mice. Mice lacking CBX2 (chromobox homolog 2; M33) also display XY male-to-female sex reversal and defects in ovarian development (65). CBX2 is a polycomb gene homolog that likely functions through effects on chromatin structure. Absence of CBX2 leads to variable gonadal phenotypes, including many cases of XY null mice in which the gonad appeared to be an ovary and where external genitalia were feminized. Cbx2−/− XX offspring were all sterile, ovaries were always smaller, and in two of 16 cases they were absent. Examination of E13.5 null males revealed an absence of testis cords. Many of these findings are consistent with CBX2 playing a role upstream of SF1, a finding consistent with nearly identical defects that are observed in the spleens and adrenal glands of Cbx2 and Sf1 knockout mice (66). SF1 was decreased at the mRNA and protein levels in the CBX2 knockout spleen and adrenal gland, and at least in Y1 mouse adrenocortical cells, chromatin immunoprecipitation studies showed that CBX2 binds to the Sf1 locus. Consistent with these findings, a mutation in human CBX2 was discovered to play an important role in sex determination (67). Because of maternal age, prenatal karyotype analysis was performed, and the fetus was shown to be 46,XY. However, the child that was born was phenotypically female with normal ovaries and a female reproductive tract. After analysis of several sex determination genes, compound heterozygous mutations (inherited from each parent) were found in the CBX2 alleles, resulting in P98L and R443P alterations in each of the CBX2 proteins. These mutations were at evolutionarily conserved amino acid positions. Whereas transfection of a wild-type CBX2 construct into H295R cells resulted in induction of the endogenous SF1 gene or SF1-luciferase constructs, the two CBX2 mutant constructs showed minimal induction of SF1 or luciferase. Thus, CBX2 positively regulates SF1 in the bipotential gonad to influence downstream expression of SRY, SOX9, or both in the sex determination cascade. Mutations in CBX2 and other genes that affect sex determination and female fertility are presented in Table 1.
Whereas absence of the above factors leads to suppressed levels of SF1, a different scenario is observed in mice null for Pod1 (also known as Tcf21, transcription factor 21) (68). POD1 is a basic helix-loop-helix (bHLH) transcription factor and is expressed at E11.5 in the coelomic epithelium and the region between the gonad and mesonephros with persistence of expression in both glands at E12.5 and through birth. In the absence of POD1, external genitalia are feminized and the gonads of both sexes are disorganized. Beginning at E11.0, the knockout gonads were shorter, and by E12.5, testes resembled ovaries with absence of testis cords and lack of formation of the male-specific coelomic vessel, whereas the ovary lacked a mesenchymal zone. In both cases, the gonads remained close to the adrenal glands. Similar to the above mutants, PGCs migrate normally into the genital ridges. Although SRY and SOX9 were expressed in presumptive pre-Sertoli cells and differentiated Sertoli cells, levels of SOX9 were suppressed initially and disappeared by E18.5. The major cause of the gonadal defects appears to originate in the steroidogenic interstitial cell population. Normally, wild-type embryonic ovaries do not express CYP11A1 (cytochrome P450 side-chain cleavage enzyme), a downstream target of SF1, and in the male gonad CYP11A1 is not expressed in Leydig cells until after E12.5. However, CYP11A1 is expressed at earlier timepoints (E11.5) in POD1-deficient gonads of both sexes, and there is a delay in expression of SF1. At E12.5, SF1 is expressed in the developing testis, but not the coelomic epithelium, and POD1 and SF1 do not colocalize. However, in the Pod1 knockout, the same cells in the coelomic epithelium and gonad that normally express POD1 (marked by a lacZ reporter) are now SF1-positive. Other studies suggest that POD1 acts indirectly to repress Sf1 at the transcriptional level. Thus, POD1 appears to act in an interstitial cell progenitor to suppress SF1, and in the absence of POD1, promiscuous expression of SF1 and its downstream target, CYP11A1, disrupt testis and ovary development. Therefore, not only does absence of SF1 result in defects in the bipotential gonad and sex determination, but ectopic production of SF1 is also detrimental to ovarian and testicular differentiation programs.
C. The XX gonad is not an innocent bystander in sex determination
In mammals, the sex chromosomes, in particular the presence of a Y chromosome, determine whether the undifferentiated gonad will differentiate into a testis (in the case of an XY mammal such as a man) or an ovary (in the case of an XX mammal such as a woman) (69). One fewer X chromosome (i.e., XO observed in Turner’s syndrome, in which the woman has streak ovaries) or two or more copies of the X chromosome in the presence of a Y chromosome (e.g., XXY as observed in Klinefelter’s syndrome, in which the man has testes) does not alter the sex differentiation of the bipotential gonad (70,71). These studies, along with genetic analysis of sex-reversed patients (XX males or XY females), in which a portion of the Y chromosome was either translocated to another chromosome or was deleted from the Y chromosome, respectively, helped to identify the SRY gene in mice and men (72,73). Because this review centers on the ovary, we will focus our discussion on how SRY prevents the development of an ovary and directs the bipotential gonad to form a testis. More detailed discussions of the history of SRY and its role in sex determination are reviewed by leading groups in this field (74,75,76).
Based on all of the above studies, it was believed that the presence of SRY actively caused testis development to occur and that in the absence of SRY, the ovary passively developed (i.e., the so-called “default” pathway). The Sry gene encodes a high-mobility group (HMG) box motif that is responsible for its DNA binding characteristics and its ability to bind DNA. The HMG box, which is the most conserved SRY sequence between mammals, is most prone to mutations that cause male-to-female sex reversal in patients. Based on studies in the mouse, the SRY protein is expressed in each pre-Sertoli cell during a narrow window of several hours in the period of gonadal differentiation (E10.5-E12.5), resulting in up-regulation of Sox9, the major (if not the only) gene transcriptionally downstream of SRY (77). In addition to their roles in the bipotential gonad, several of the same transcription factors act on the major genes involved in sex determination. SF1, WT1, and the GATA4/FOG2 complex are required for the transcription of Sry (55). Furthermore, the initial low level of expression of Sox9 in the bipotential gonad requires SF1 (59), and the subsequent high level induction of Sox9 that is required for testis formation is regulated first by both SRY and SF1 and then by SF1 and SOX9 as part of a feedback loop (78). Because only 10% of cases of male-to-female sex reversal are due to mutations in SRY, many genetic studies were helpful in identifying mutations of gene products involved in sex determination that are downstream of SRY, such as SOX9. For example, loss of function mutations in SOX9 in humans causes the severe bone disease, campomelic dysplasia, in which XY male-to-female sex reversal is also observed (79,80), whereas duplication of the region encoding SOX9 (i.e., gain of function mutation) has been shown to cause XX female-to-male sex reversal (81). SOX9 not only results in a positive feedback on itself but also up-regulates SF1 to positively regulate Sox9 (82) and also induces fibroblast growth factor 9 (FGF9) that signals back through FGF receptor 2 (FGFR2) to increase Sox9 levels (83,84,85,86,87). These studies continued to support the idea that induction of the “male” genes Sry or Sox9 positively causes the undifferentiated gonad to develop into a testis.
In 1993, both McElreavey et al. (88) and Goodfellow and Lovell-Badge (89) proposed the “Z” model for sex determination. In this model, the XX gonad produces a factor “Z” that actively stimulates the ovarian differentiation cascade, and SRY or some downstream target of SRY inhibited this cascade. As shown in Fig. 2, there is an interplay of gene products in the testis and ovarian differentiation cascades that functions to suppress key proteins in the opposing pathway. A significant amount of data have begun to accumulate in support of the Z model. Along with the identification of the genes mutated or duplicated in cases of XX female-to-male and XY male-to-female sex reversal, several mouse models have been created to understand the ovarian differentiation pathway (Table 5). Loss of function mutations in R-spondin homolog 1 (RSPO1) were identified as the cause of the recessive disorder palmoplantar hyperkeratosis. All individuals with this syndrome are phenotypic males (either XY or XX), the first such case of complete XX female-to-male sex reversal (90). RSPO1 is a secreted protein that acts extracellularly to increase the signaling of WNT4. The extracellular protein dickkopf homolog 1 (DKK1) binds to a cell surface complex of kringle-containing transmembrane protein 1 (KREMEN1) and low-density lipoprotein receptor-related protein 6 (LRP6) to cause internalization of this ternary complex. However, RSPO1 binds to KREMEN1, dislodging DKK1, and allowing LRP6 access to FRIZZLED (FZD) for binding of WNT4 to the LRP6-FZD coreceptor complex and signaling to increase β-catenin (CTNNB1) levels (91). An alternative model suggests that RSPO1 functions directly to stimulate β-catenin signaling by binding to LRP6 (92), although the activity of RSPO1 alone is much less than in the presence of a WNT ligand (91).
Table 5.
Transgenic animals with alterations in sex determination
| Transgenic model | Phenotype | Ref. |
|---|---|---|
| Rspo1 KO | Development of male-specific coelomic vessel in XX gonad | 93,94 |
| Gata4 KI | No coelomic vessel defects | 99 |
| Fog2 KO | No coelomic vessel defects | 99 |
| Wnt4 KO | Development of male-specific coelomic vessel in XX gonad | 95 |
| Ctnnb1 (β-catenin) cKO (Nr5a1-Cre) | Development of male-specific coelomic vessel in XX gonad | 97,99 |
| Ctnnb1 (β-catenin) Tg | XY, male-to-female sex reversal | 96 |
| Fst KO | Development of male-specific coelomic vessel in XX gonad | 98 |
| Fgfr2 cKO (Heat shock-Cre; Sf1-Cre) | XY, male-to-female sex reversal | 86 |
| Sox9 Tg | XX, female-to-male sex reversal | 787 |
| Fgf9 KO | XY, male-to-female sex reversal | 83 |
| Sry Tg | XX, female-to-male sex reversal | 788 |
| Pdgfra KO | Disrupted testis cord formation and abnormal Leydig cells | 789 |
KO, knockout; cKO, conditional knockout (Cre transgenic); KI, knockin; Tg, transgenic.
As presented in Table 5, mutations or overexpression of multiple components of the ovary determination cascade [RSPO1, WNT4, β-catenin, or follistatin (FST)] will result in variable degrees of female-to-male sex reversal (93,94,95,96,97,98). The developing testis contains cords and a coelomic vessel, structures that are absent in the ovary. Mutations in ovary determination cascade genes (Rspo1, Wnt4, and Fst) lead to the presence of a coelomic vessel in the ovary. Besides its role in the regulation of Sry expression and testis determination, the GATA4/FOG2 complex also suppresses the expression of Dkk1, thereby altering the downstream components of the WNT4 signaling pathway in the ovary (99). Not only WNT4 levels, but also FST levels are suppressed. FST was initially detected as a differentially expressed gene in the developing ovary compared with the testis (100), and absence of FST leads to the development of a coelomic vessel in the developing ovary (98). These findings are milder forms of sex reversal compared with mice lacking RSPO1 or WNT4 where additional alterations are observed, including 17α-hydroxylase/17,20-lyase cytochrome P450 (CYP17A1)-positive, cytochrome P450 aromatase (CYP19A1)-positive adrenal-like cells that produce androgens.
Where do these ovarian and testicular factors converge to influence sex determination? Much data have accumulated to indicate that β-catenin is a central downstream signaling protein in the sex determination pathway. Activation of the WNT4 pathway allows β-catenin to translocate to the nucleus to interact with hepatocyte nuclear factor 1 homeobox A (HNF1A; TCF1) and regulate transcription. The testis differentiation pathway regulates the ovarian differentiation pathway in two ways: 1) human SRY inhibits β-catenin-mediated transcription by direct interaction with β-catenin (101); and 2) SOX9 interacts with β-catenin to cause their mutual degradation. These studies suggest that β-catenin may be the infamous Z factor that is regulated by the testis differentiation pathway. Thus, in the ovarian differentiation pathway, increased levels of β-catenin will result in degradation of SOX9, preventing it from inducing itself or other genes, such as Fgf9. Indeed, studies in both humans and mice support a role for β-catenin as a major pro-ovary and anti-testis factor. In humans, a duplication of the region encoding WNT4 and RSPO1 has been shown to cause XY male-to-female sex reversal (102). In mice, overexpression of a stable allele of β-catenin causes XY male-to-female sex reversal (96), whereas conditional knockout (cKO) of β-catenin (97,99) leads to the presence of a coelomic vessel in XX gonads.
D. Sexually dimorphic changes in the initiation of meiosis
Before entry of PGCs into the urogenital ridge, XX (“female”) and XY (“male”) PGCs appear to function identically in all aspects. This suggests that there are no sexually dimorphic products for formation, migration, and entry of the PGCs into the genital ridge and that the sex chromosomes are not influencing this process. The first mechanistic difference between an XX and an XY germ cell in the genital ridge is reactivation of the inactive X in the female PGC. Soon after this point, there is a major change in the fate of these PGCs. Whereas XY germ cells arrest in mitosis and do not divide again until postnatally as spermatogonia, the XX germ cells continue to divide and then enter meiosis at approximately E12.5. Subsequently, the female germ cells arrest at the diplotene stage of meiosis I and do not resume meiosis until postnatally during ovarian folliculogenesis. As mentioned in Section I, this indicates that the last known dividing oocyte “stem cell” must also disappear at this time. Elegant studies from several laboratories have been able to piece together the molecular details of these important sexual dimorphic processes (Fig. 3).
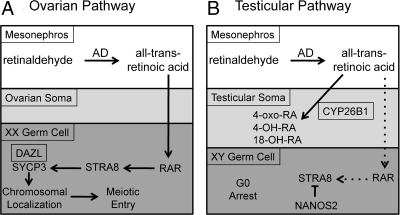
Figure 3.
Sexually dimorphic initiation of meiosis in the embryonic ovary. During embryogenesis, the mesonephroi adjacent to the developing ovary (A) and testes (B) contain several aldehyde dehydrogenases that convert retinaldehyde to all-trans-retinoic acid (RA). The somatic cells of the developing testes contain the enzyme CYP26B1, which degrades RA to pass freely to the germ cell to bind to retinoic acid receptors (RAR). In the developing ovary, RA induces STRA8, which induces SYCP3, which is stably translated in the presence of DAZL and becomes chromosomally localized as the XX germ cell becomes an oocyte and enters meiosis. In male germ cells, the absence of high enough levels of RA early and under the repressive actions of NANOS at later time points, STRA8 is not synthesized and the XY germ cell becomes mitotically arrested.
The first gene necessary to lay the groundwork for these changes is Dazl (deleted in azoospermia-like). Dazl is expressed in both XX and XY PGCs at the time of arrival at the genital ridge (103). Normally, both XX and XY germ cells begin to express Sycp3 (synaptonemal complex protein 3) but in the absence of DAZL, SYCP3 is essentially absent, thereby defining a “meiosis-competent” germ cell (104). In the presence of DAZL, this intermediate premeiotic germ cell is responsive to retinoic acid, the meiotic initiation molecule derived from mesonephroi of both sexes (105,106). In addition, DAZL is an RNA binding protein that specifically binds to the 3′ UTR of the Sycp3 mRNA to positively regulate its translation (107). Thus, DAZL acts both upstream in the PGC as well as downstream to regulate meiosis.
If both male and female germ cells are “primed” for meiosis and bathed in a similar retinoid acid environment, what causes the sexually dimorphic paths? The gene product that is responsible for these changes is cytochrome P450 26B1 (CYP26B1), the major protein that degrades retinoic acid. CYP26B1 is down-regulated in somatic cells of the ovary at E12.5 but up-regulated in the testis, thus allowing accessibility of the XX germ cells, but not the XY germ cells, to retinoic acid (105). In the absence of CYP26B1, the XY germ cells of the embryonic testes initiate meiosis, mimicking the XX germ cells. Furthermore, the synthetic retinoid Am580, which is not degraded by CYP26B1, has a similar induction of meiosis in the germ cells of wild-type testes (108), yielding further evidence of the interrelationships of retinoic acid, CYP26B1, and the initiation of meiosis.
How is retinoic acid acting in the germ cell to regulate meiosis? The Page laboratory (109) had previously shown that a known retinoic acid target, Stra8 (stimulated by retinoic acid gene 8), a bHLH transcription factor, was expressed in the embryo exclusively in XX germ cells before meiotic entry. Using a knockout approach, their laboratory showed that absence of STRA8 blocked entry of the XX germ cells into meiosis, and the meiotic markers Dmc1 and Spo11 were undetectable in the E14.5 ovaries (110). Consistent with retinoic acid as the major inducer of Stra8 in embryonic germ cells, absence of CYP26B1 also leads to increased Stra8 in the embryonic testes after E12.5 (111) paralleling the Stra8 rise seen in females. As the levels of CYP26B1 wane after E13.5 in the testes with increased exposure of the XY germ cells to retinoic acid, a “backup” protein, NANOS2, is expressed and represses Stra8; absence of NANOS2 in the males leads to induction of Stra8 (after E13.5), whereas expression of NANOS2 in embryonic female germ cells suppresses Stra8 (111). Thus, a retinoic acid to Stra8 pathway results in sexually dimorphic differences in meiotic entry in females vs. males. Additional signaling pathways that regulate meiotic arrest and reentry postnatally are discussed in the following section.
III. Ovarian Folliculogenesis
Autocrine, paracrine, juxtacrine, and endocrine factors are essential for ovarian folliculogenesis. Besides the oocyte, the reproductive cargo of the follicle, the somatic cells of the ovarian follicle, namely the granulosa cells that function as the ovarian “nurse” cells and thecal cells that function to supply the granulosa cells with the estrogenic precursor, androstenedione, are recruited to the oocyte and are directly or indirectly necessary for oocyte development, physiology, and survival. As shown in Fig. 4, the major stages of ovarian folliculogenesis are formation of the primordial follicle; recruitment into the growing pool to form a primary, secondary, and tertiary follicle; and lastly ovulation and subsequent formation of a corpus luteum (CL). In this section, we will describe these steps in greater detail. Several of the mutations that cause infertility in humans are also summarized in Table 1.
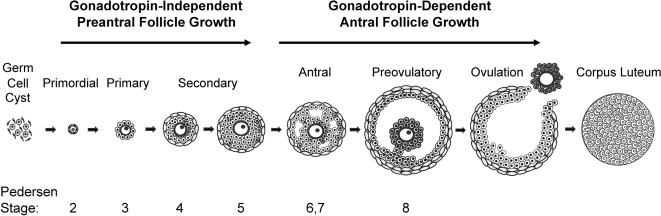
Figure 4.
Classification of the major stages of mammalian folliculogenesis. Primordial follicles form 1–2 d after birth in mice and in utero in humans. Preantral follicles begin to develop prenatally in humans, whereas in mice this occurs postnatally. In both mice and humans, preantral follicular development does not require stimulation by the pituitary gonadotropins. By the secondary stage, an additional layer of somatic cells, the theca, forms outside the basement membrane of the follicle. At puberty, FSH secreted by the pituitary promotes further granulosa cell proliferation and survival. Ovulation of the dominant follicle occurs in response to a rise in the other pituitary gonadotropin, LH. After ovulation, the remaining granulosa and theca cells undergo terminal differentiation to form the CL. In most cases, primordial, primary, secondary, preantral, and antral are names commonly used to refer to the different stages of folliculogenesis; however, a classification system described by Pedersen and Peters (768) is also used. Pedersen stages are determined based on the size of the oocyte and the number of granulosa cells in cross-section for any given follicle. Although not shown, certain Pedersen stages are subdivided (i.e., 3a, 3b, 5a, 5b) depending on the number of granulosa cells surrounding the oocyte.
A. Formation of an ovarian follicle—oocyte survival vs. primordial follicle formation
Before formation of an ovarian follicle, oocytes are present within germ cell clusters, also referred to as germ cell cysts or nests. Primordial follicle formation occurs when oocytes that survive the process of germ cell cluster breakdown are individually surrounded with squamous pre-granulosa cells. This represents the first stage of folliculogenesis, and it takes place during the latter half of fetal development in humans and in the days immediately following birth in mice (112,113). In mammals, the population of primordial follicles serves as a resting and finite pool of oocytes available during the female reproductive life span. Although germ cell cluster breakdown, primordial follicle formation, and subsequent recruitment remain the least understood steps of folliculogenesis, key regulators of these initial stages of follicle development continue to be identified. Furthermore, despite many unanswered questions during this crucial period, the concept of ovarian cross talk between oocytes and somatic cells is apparent from the formation of primordial follicles onward (114,115).
After differentiation of PGCs, oogonia undergo mitotic proliferation with incomplete cytokinesis, leaving daughter cells connected by intercellular bridges. The majority of germ cells in a cluster divide synchronously such that a single germ cell cluster contains 2n germ cells (116). Germ cells subsequently enter meiosis, becoming oocytes. Individual oocytes within these nests lack surrounding somatic cells, and the majority of the oocytes will undergo apoptosis as the germ cell clusters break down to give rise to primordial follicles (117).
Interestingly, although intercellular bridges appear to be evolutionarily conserved structures connecting germ cells from insects to mammals, they do not appear to be essential for follicle formation or female fertility in mice. Testis-expressed gene 14 (TEX14) was discovered as the first essential protein in the postnatal intercellular bridge that interconnects differentiating male germ cells during spermatogenesis (118). Whereas male mice lacking TEX14 are sterile due to postnatal defects in spermatogenesis (118), TEX14 null females have normal fertility over a 6-month breeding period and follicles in all stages of folliculogenesis at 1 yr of age, suggesting that intercellular bridge formation during embryonic germ cell development is not essential for female fertility (119). Nevertheless, intercellular bridges may have a role in determining the initial pool of oocytes because postnatal day (P) 2.5 Tex14−/− ovaries have fewer oocytes relative to control ovaries.
Members of the B cell lymphoma/leukemia (BCL) protein family have opposing functions in regulating germ cell apoptosis (120). Although BCL2 and BCLX protect against apoptosis, BAX promotes cell death. Deletion of antiapoptotic BCL2 results in fewer oocytes and primordial follicles at 6 wk of age, with no differences in the number of primary and preantral follicles (121). Neonatal ovaries, however, have not been examined; this would be useful to determine the initial reserve of primordial follicles. As has been observed for BCLX (122), BCL2 may influence survival during PGC development rather than, or in addition to, during germ cell cluster breakdown and primordial follicle endowment. For example, loss of proapoptotic BCL member Bax increases the number of germ cells at E13.5, before the start of meiosis and germ cell apoptosis (123), but its role during primordial follicle formation is controversial. Although 6-wk-old Bax−/− females have increased nonatretic primordial follicles compared with wild-type controls and, accordingly, a prolonged reproductive life span (124), there are conflicting reports regarding the roles of BAX postnatally during primordial follicle endowment. Postnatal day 4 (P4) Bax−/− females were originally reported to have similar numbers of primordial follicles; however, others documented an increase in primordial follicles at P4 (123). This discrepancy may be due to criteria used in classifying primordial follicles because Greenfeld et al. (123) counted follicles with a mixture of squamous and cuboidal granulosa cells as primordial, rather than as transitioning into primary follicles.
One regulator of BAX is the aryl hydrocarbon receptor (AHR), a ligand-activated member of the PER-ARNT-SIM family of transcription factors that is activated by polycyclic aromatic hydrocarbons (PAHs). The mouse Bax promoter contains two consensus AHR response elements, and exposure of female mice to PAHs induces Bax and subsequently apoptosis in primordial and primary oocytes (125). In support of a role for BAX in establishing primordial follicles, ovaries from Ahr null mice have approximately a 2-fold increase in primordial follicles at P2-P4 (126,127), but no differences in germ cell number before birth on E18 (126). Interestingly, in ovaries from Ahr−/− mice, there are no differences in the number of primordial follicles by P8, and there are actually fewer antral follicles at 8 wk (126), whereas ovaries from Bax−/− females at P7 still contain significantly more primordial follicles (123), suggesting that additional regulators of BAX are active in oocytes during this period. The AHR-BAX pathway is required for PAH-induced oocyte death in mice (125), suggesting that activation of AHR in humans exposed to environmental toxins may contribute to premature ovarian failure (POF), also known as primary ovarian insufficiency.
A second apoptotic pathway that operates in cells involves activation of caspases, which are proteases that upon activation cleave a number of cellular proteins leading to apoptosis. Targeted disruption of the caspase 2 gene (Casp2) resulted in significantly more primordial follicles at P4 in Casp2−/− ovaries, and oocytes from Casp2−/− mice were resistant to the chemotherapeutic agent doxorubicin (128). Thus, during fetal development and in the perinatal period, apoptosis is important in establishing the primordial pool, and apoptotic cell death continues throughout folliculogenesis in oocytes and granulosa cells during follicular atresia.
FIGLA (factor in the germline α) is a germ cell-specific bHLH transcription factor that is required for initial follicle formation. The bHLH family of transcription factors functions by forming homo- or heterodimers that bind to gene regulatory regions containing E-box consensus sequences (CANNTG). Female mice lacking Figla are sterile secondary to a complete absence of follicles and oocytes (129). Upon closer inspection, although ovaries from E18 Figla null and wild-type females have similar numbers of germ cell clusters in the perinatal period, primordial follicles fail to form, and oocytes are depleted by P2 in Figla null ovaries. FIGLA was first identified through in vitro studies as a regulator of all three zona pellucida genes, Zp1, Zp2, and Zp3 (130). The zona pellucida proteins comprise the glycoprotein-rich matrix that surrounds developing oocytes and is essential for fertilization. Although mice deficient in Zp1, Zp2, or Zp3 are either infertile or subfertile (131,132,133), follicles lacking any of the zona pellucida genes can progress through all stages of folliculogenesis. Furthermore, the zona pellucida matrix does not form until oocytes begin to grow, suggesting that FIGLA has additional downstream targets whose misregulation prevents early oocyte-somatic cell interactions. Human FIGLA is also expressed only in female germ cells and, like mouse FIGLA, can heterodimerize with the ubiquitous E12 bHLH transcription factor and bind E-box consensus elements in the human ZP2 promoter (134,135). Human FIGLA transcripts are detectable by 14 wk gestation and dramatically increase at midgestation (19 wk), corresponding to the time of human primordial follicle formation.
Gene expression studies comparing ovaries from Figla−/− and wild-type mice at four different time points showed the greatest number of differentially expressed genes when comparing newborn ovaries, consistent with the onset of primordial follicle formation (136). Of the altered genes, 165 were decreased, and 38 were increased in Figla−/− ovaries, and a large percentage of these genes code for transcription factors or proteins with nucleic acid binding functions. Interestingly, many genes normally expressed in the testis were up-regulated in Figla−/− ovaries, suggesting a role for FIGLA in repressing male germ cell-specific genes in oocytes. Pou5f1, which postnatally is germ cell-specific and expressed in growing oocytes, was decreased, and its postnatal up-regulation occurs just after FIGLA is expressed (137), suggesting that FIGLA is one regulator of this important transcription factor during this key time period. Members of the oocyte-specific NLRP (NACHT, leucine-rich repeat and pyrin domain containing) gene family were also decreased or absent in Figla null ovaries. Despite shared structural motifs in the NLRP family, individual proteins do not appear to be functionally redundant; inactivation of a single Nlrp gene, Nlrp5, also known as Mater, prevents embryo development beyond the two-cell stage (138). Thus, in addition to identifying target genes and pathways during primordial follicle formation, the results of these studies suggest that FIGLA might directly or indirectly be important during the later stages of oocyte development.
Although upstream regulators of FIGLA are unknown, studies have identified a number of signaling pathways important in germ cell cluster breakdown and primordial follicle formation that could potentially regulate FIGLA or other mediators of early folliculogenesis. Previous observations supported a role for maternal hormones in the maintenance of germ cell clusters during mouse fetal development. Multi-oocyte follicles containing two or more oocytes within a follicle boundary might arise from oocyte clusters that fail to separate. Multi-oocyte follicles occur more frequently in mice exposed to estrogens or estrogenic compounds prenatally or in the neonatal period (139,140). In an in vitro ovarian culture system, ovaries from newborn mice cultured over the course of 7 d in the presence of estradiol, progesterone, or genistein, a phytoestrogen, exhibit fewer single oocytes and more germ cell clusters, supporting a role for these steroids in blocking cluster breakdown and primordial follicle formation (141). Newborn rat ovaries cultured in the presence of progesterone or newborn rats injected with progesterone show a similar reduction in primordial follicle formation (142). The authors propose that in developing mice, high maternal steroids provide an inhibitory signal that prevents cluster breakdown, whereas at birth, the drop in these steroids allows clusters to break apart (141,142). However, whereas mice lacking Cyp19a1, which are deficient in estradiol, have a reduction in primordial follicles at 10 wk of age (143), ovaries in the neonatal period have not been examined, and the chief defect in folliculogenesis in CYP19A1-deficient females is a block at the antral follicle stage (144). Likewise, mice lacking the progesterone receptor (PR) have normal follicular development, and the primary ovarian defect is inability to ovulate (145). Furthermore, in humans, primordial follicle formation occurs during the last half of fetal development when maternal estradiol and progesterone are high, suggesting a different mechanism controlling primordial follicle formation in humans. Studies in nonhuman primates suggest that estradiol actually promotes primordial follicle formation. Late gestation fetuses from baboons treated with an aromatase inhibitor show a reduction in primordial follicles and an increase in germ cell clusters (146). Thus, the effects and physiological relevance of estradiol and progesterone during follicle formation are unclear, and further studies are warranted. It is possible that the effects of progesterone in rodents are partly due to signaling through a membrane progesterone receptor, rather than the classical nuclear receptor. In rats, the inhibitory effect of progesterone on primordial follicle assembly is not completely reversed when the nuclear PR antagonist, RU-486, is also added (142).
Whereas estradiol and progesterone may partially inhibit primordial follicle formation in rodents, NOTCH signaling appears to promote it. There are four NOTCH receptors in mammals that interact with two families of ligands, DELTA-like and JAGGED, on neighboring cells. Ligand binding of NOTCH leads to proteolytic cleavage by the ADAM-family of metalloproteases, followed by cleavage by γ-secretase to free the NOTCH intracellular domain (NICD). The NICD translocates to the nucleus and interacts with the DNA-binding CSL [CBF1, Su(H), and Lag-1] transcription factor and its coactivator, Mastermind, to promote transcription (147). In neonatal mouse ovaries, the NOTCH2 receptor is expressed in pre-granulosa cells, the NOTCH ligand JAGGED1 is expressed in germ cells, and NOTCH target genes Hes1 and Hey2 are in both cell types. Using a similar in vitro ovarian culture system, blocking NOTCH signaling with γ-secretase inhibitors decreases primordial follicle formation and increases the germ cells remaining in clusters (148). Further support for NOTCH signaling during primordial follicle formation and later stages of folliculogenesis is evident in lunatic fringe (Lfng) null mice. Lunatic fringe is a modulator of the NOTCH pathway and is expressed in granulosa and thecal cells of developing follicles. Although most Lfng null mice die shortly after birth, females that survive are infertile with follicular defects that include multi-oocyte follicles (149).
B. Maintenance of primordial follicles and initial recruitment
Follicle recruitment is generally subdivided into two broad categories: initial activation of primordial follicles, which occurs throughout life until menopause; and, after puberty, cyclic recruitment of a limited number of small follicles from the growing cohort, from which a subset is selected for dominance and ovulation (150). Although the initial recruitment of follicles from the primordial into the growing pool remains a poorly understood process, in recent years mutant mouse models have led to the identification of several key transcription factors and signaling pathways that regulate this early step in folliculogenesis. The transition from primordial to primary follicle is marked histologically by a morphological change in granulosa cells from squamous to cuboidal.
NOBOX (newborn ovary homeobox), SOHLH1 (spermatogenesis and oogenesis helix-loop-helix 1), and SOHLH2 are critical transcription factors during the transition from primordial to primary follicles. Nobox and Sohlh1 were both identified using an in silico subtraction strategy to identify expressed sequence tags that are preferentially expressed in oocytes but not in other mouse cDNA libraries (151). Sohlh2 was subsequently discovered using the BLAST program of the National Center for Biotechnology Information to search for bHLH domains that share homology with Sohlh1 (152). All three genes are expressed in germ cell clusters, primordial follicles, and primary follicles in females, whereas Sohlh1 and Sohlh2 are also expressed in spermatogonia. Whereas SOHLH1 and SOHLH2 disappear rapidly as oocytes reach the secondary follicle stage, NOBOX continues to be expressed throughout folliculogenesis. Mice lacking any of these three transcription factors are sterile (153,154,155,156). Although ovaries from newborn Nobox−/− or Sohlh1−/− mice contain similar numbers of germ cell clusters and primordial follicles relative to controls, progression beyond the primordial follicle stage is disrupted. By P3, control mice have formed primary follicles, but Nobox−/− and Sohlh1−/− mice lack primary follicles and begin to demonstrate an early postnatal loss of oocytes. Ovaries from Sohlh2−/− are remarkably similar to Sohlh1−/− and Nobox−/− ovaries, although occasional follicles escape early postnatal death and progress to multilayered follicles. Thus, mice deficient in Nobox, Sohlh1, or Sohlh2 have defects in the primordial to primary follicle transition, and these genes do not appear to function redundantly during early folliculogenesis.
Molecular analysis of Nobox−/− ovaries shows that expression levels of key oocyte-specific genes, including Gdf9, Bmp15, Mos, Pou5f1, and several Nlrp family members, are dramatically decreased (157). Furthermore, Gdf9 and Pou5f1 appear to be directly regulated by NOBOX, and several other down-regulated genes also contain putative NOBOX consensus binding elements in their promoters (158). Although Figla expression and some FIGLA targets, such as Zp1, Zp2, and Zp3, are not altered in Nobox−/− ovaries, many of the gene changes observed in Nobox−/− ovaries were also found in Figla−/− ovaries (136), including an up-regulation of several testis-specific genes, which suggests that these transcription factors function in both individual and redundant regulatory pathways during oogenesis.
Ovaries from Sohlh1−/− mice have gene changes similar to those observed in Nobox−/− ovaries, but also show a significant reduction in Nobox, Figla, Zp1, and Zp3 (155). In contrast, Sohlh1 is not significantly affected in Nobox−/− ovaries, suggesting that SOHLH proteins function upstream of Nobox and Figla. The ovarian physiology in Sohlh1−/− mice, however, is less severe than in Figla−/− mice, which may be due to persistent low-level expression of Figla in these mice. An additional transcription factor, Lhx8, which encodes a LIM homeodomain protein, is also down-regulated in Sohlh1−/− ovaries and the ovarian phenotype in Lhx8−/− mice phenocopies Sohlh1−/− mice (155,159). By chromatin immunoprecipitation and reporter assays, SOHLH1 appears to directly regulate Lhx8, Zp1, and Zp3 through conserved E-box promoter elements, but not Nobox or Zp2. Newborn ovaries from Sohlh2−/− mice have very similar molecular changes as those from Sohlh1−/− mice, consistent with data suggesting that SOHLH1 and SOHLH2 form heterodimers (160). Interestingly, Sohlh2 is down-regulated in Sohlh1−/− ovaries, and likewise, Sohlh1 is down-regulated in Sohlh2−/− ovaries, supporting a further role for transcriptional cross-regulation (153). Unlike the findings in Nobox−/− ovaries, in newborn ovaries from Sohlh1−/− or Sohlh2−/− mice, Kit receptor is down-regulated (153). Kit is also decreased in newborn Figla−/− ovaries (136), and in Lhx8−/− newborn ovaries, both Kit and its ligand, Kitl, are reduced (159).
Interactions between KIT ligand and the KIT tyrosine kinase receptor appear to be critical in early folliculogenesis. During postnatal ovarian development, KIT is expressed in oocytes and KIT ligand is expressed in pre-granulosa and granulosa cells throughout folliculogenesis (161,162,163,164,165). The importance of KIT/KIT ligand signaling during folliculogenesis was first identified in mutant mice and later extended by in vivo function blocking and in vitro culture studies. As mentioned in Section II, many different mutations of Kit or Kitl, encoded by the W and Sl loci, respectively, result in defects in PGC development. A number of alleles have been identified in both loci that have differential effects on female fertility. In particular, the Steel Panda (Slpan) and Steel Contrasted (Slcon) mutations, which result in reduced expression of normal Kitl transcript in the gonads (166,167), provide insight into the roles of KIT/KIT ligand during early folliculogenesis. In addition to a reduction in the number of germ cells, mice homozygous for the Slpan and Slcon alleles have fewer oocytes in the growing pool, and the majority of those that develop arrest at the primary follicle stage. Female mice with the Steel Transfer (Slt) mutation display a similar arrest of folliculogenesis with many primordial follicles present, but few growing follicles (168). Because Slcon females might have a single litter and have occasional follicles that progress beyond the primary stage, despite increased atresia at the antral follicle stage, there may be a threshold level of KIT ligand necessary for primordial follicle recruitment and later follicular development. The Slpan, Slcon, and Slt mutant mice might have sufficient levels of KIT ligand produced for some follicles to reach the primary stage, but insufficient levels of KIT ligand lead to early arrest or increased atresia.
In vivo and in vitro studies further support a role for KIT/KIT ligand interactions during initiation of follicular growth from the primordial pool. Newborn mice injected with an antibody to KIT (ACK2) that blocks interaction with KIT ligand have a block at the primordial stage of follicle development. When P2 mice, which have formed primordial follicles, are injected with ACK2 antibodies, primary follicle development is only slightly interrupted, although these mice show a delay in antral follicle development (165). Similar inhibition of primordial follicle development is seen in an in vitro neonatal rat ovary organ culture system. In contrast, treatment of neonatal rat ovaries with recombinant KIT ligand accelerates the primordial to primary follicle transition, resulting in an increased number of growing follicles (169). These studies and the Kitl mutant mice suggest that intact KIT signaling is not essential during germ cell cluster breakdown, but it is necessary during the transition from primordial to primary follicles and in later stages of follicle development.
The mechanisms by which KIT ligand/KIT signaling contribute to the primordial to primary follicle transition are not entirely known; however, evidence suggests that the KIT ligand/KIT pathway induces the PI3K/AKT pathway, leading to phosphorylation and inactivation of forkhead box O3 (FOXO3; FKHRL1), an inhibitor of primordial follicle activation. PI3K catalyzes the conversion of the membrane phospholipid phosphatidylinositol 4,5-bisphosphate (PIP2) to the second messenger, phosphatidylinositol 3,4,5-trisphosphate (PIP3), which leads to AKT activation. FOXO3 is a member of the FOXO subfamily of forkhead transcription factors, which are downstream targets of the PI3K/AKT pathway; activation of the PI3K/AKT pathway functionally suppresses FOXO transcription factors secondary to phosphorylation and nuclear exclusion (170). When mouse or rat oocytes are treated with KIT ligand, FOXO3 is phosphorylated in a PI3K/AKT-dependent manner because treatment with a PI3K inhibitor prevents AKT activation and subsequent FOXO3 phosphorylation (171).
The functional consequence of FOXO3 inactivation is illustrated in mice lacking Foxo3. Although Foxo3 is expressed in multiple tissues, the chief phenotypic defect in Foxo3−/− mice is a rapid decline in fertility and ultimately sterility by 15 wk of age. Histologically, by 2 wk of age, ovaries from Foxo3−/− mice are enlarged relative to controls, have a marked increase in growing and atretic follicles, and have an absence of primordial follicles (172). By 8.5–9.5 wk of age, ovaries from Foxo3−/− mice have abundant zona pellucida remnants (172,173), suggesting widespread follicular activation, followed secondarily by atresia. Because forkhead box O1 (FOXO1) mRNA and protein are expressed in mouse oocytes (174,175), it appears that FOXO1 regulates different functions or is not expressed in sufficient quantities to substitute for FOXO3. Thus, deletion of Foxo3 removes an oocyte activation brake, leading to premature recruitment of follicles and a complete depletion of the primordial pool before sexual maturity. Further support for the inhibitory role of FOXO3 on follicular activation is seen in transgenic mice expressing constitutively active Foxo3 under control of the Zp3 promoter, which is active beginning at the primary stage of folliculogenesis. Zp3-Foxo3 transgenic females are severely infertile due to impeded follicular development beyond primary and secondary follicles (176).
Additional genetic studies support a critical role for the PI3K/AKT pathway upstream of FOXO3. Oocyte-specific deletion of Pten (phosphatase and tensin homolog deleted on chromosome 10), which opposes the actions of PI3K by converting PIP3 to PIP2, causes premature activation of primordial follicles, with an ovarian phenotype nearly identical to Foxo3 deletion (177,178). Loss of Pten in oocytes results in enhanced PI3K activity, AKT hyperactivation, and functional suppression of Foxo3 secondary to hyperphosphorylation and nuclear export. Concurrent loss of Pten and Foxo3 in oocytes does not have a synergistic effect on follicle activation (178), and the PI3K inhibitor LY294002 suppresses primordial follicle activation in ovaries with Pten-deficient oocytes but has no effect in Foxo3 mutant ovaries (177), suggesting a linear PTEN-PI3K-AKT-FOXO3 pathway. The initial fertility of Foxo3 and conditional Pten mutant females suggests that this pathway is not essential for later steps of folliculogenesis, ovulation, or fertilization. The critical role for this pathway in regulating primordial follicle activation throughout the reproductive life span has been demonstrated using a tamoxifen-inducible germ cell-specific Cre mouse model (Vasa-CreERT2). Administration of tamoxifen to adult Vasa-CreERT2 Foxo3flox/flox or Vasa-CreERT2 Ptenflox/flox mice causes the same global activation of primordial follicles that is seen in the ubiquitous Foxo3 and Pten knockout or conditional models (177). It is unknown whether KIT is an upstream modulator of this pathway in vivo, or at least one of many receptor tyrosine kinases that might initiate this signaling cascade to promote primordial follicle recruitment. However, female mice with a mutated KIT receptor (KitY719F) that prevents binding and activation of PI3K have a retardation of folliculogenesis beyond the primary stages of development (179), similar to mice with constitutively active FOXO3 (176). It would be interesting to determine whether FOXO3 is predominantly nuclear in these mice and whether loss of Foxo3 would rescue the observed histological findings.
How nuclear FOXO3 prevents primordial follicle activation is unknown. It has been proposed to arrest growth through increased expression of cyclin-dependent kinase inhibitor 1B (CDKN1B; also known as p27Kip1) (180), which is retained in the nuclei of 20-d-old mice expressing constitutively active Foxo3 (176), and FOXO3 regulation of p27Kip1 has been documented in other systems (181). Indeed, p27Kip1 also functions as a suppressor of primordial follicle activation because Cdkn1b−/− females exhibit premature activation of primordial follicles (182). However, whereas Cdkn1b−/− and Foxo3−/− both have increased recruitment from and ultimate depletion of the primordial follicle pool, mice lacking both genes show a synergistic acceleration of follicle activation, suggesting that they can function independently to suppress primordial follicle activation. Furthermore, by Western blot analysis, p27Kip1 levels are normal in oocytes from Foxo3−/− mice, and p27Kip1-deficient oocytes have normal total and phospho-FOXO3 (182), although immunolocalization of each protein in the respective mutants may have been more conclusive. It is likely that FOXO3 has multiple targets in primordial oocytes that contribute to the quiescent state until the appropriate signal initiates activation and oocyte growth.
Finally, recent work has identified an additional critical mediator of PI3K activation, 3-phosphoinositide dependent protein kinase-1 (PDPK1; PDK1). Binding of PIP3 leads to activation of PDK1 and subsequent phosphorylation of AKT and other kinases of the AGC family (protein kinases A, G, and C) (183). Oocyte-specific deletion of Pdk1 caused infertility; despite no difference in ovarian morphology and follicle count through P23, pubertal ovaries were smaller and contained fewer follicles at all stages, secondary to depletion of primordial follicles (184). Whereas KIT ligand stimulated AKT phosphorylation in cultured wild-type oocytes, it did not in Pdk1 cKO oocytes, thereby preventing FOXO3 phosphorylation (and thus presumably primordial follicle activation). However, phosphorylation of ribosomal protein S6 kinase (RPS6KB1; S6K1), an additional PDK1 target downstream of PI3K activation, was also disrupted. This prevented phosphorylation and activation of the 40S ribosomal protein, RPS6, which is necessary for ribosome biogenesis and protein translation (185). Liu and colleagues (184) predicted that RPS6 may be downstream of PI3K-AKT-S6K1 and important in oocyte growth after primordial follicle activation. Indeed, conditional deletion of Rps6 resulted in a more profound ovarian defect with Rsp6 cKO ovaries already smaller by P23 and completely devoid of follicles by 8 wk of age (184). Thus, the suppressed AKT signaling in PDK1-deficient oocytes appears to both prevent primordial follicle activation through retained nuclear FOXO3 and negatively impact oocyte survival through decreased RPS6 activity. This also suggests tight control over the PI3K-PDK1-AKT signaling pathway in maintaining and activating the pool of primordial follicles.
Whereas FOXO3 is the key oocyte factor critical for suppressing primordial follicle activation, another forkhead domain transcription factor, forkhead box L2 (FOXL2), is crucial in the transition from squamous to cuboidal granulosa cells that occurs during the primordial to primary transition. Nonsense mutations in FOXL2 cause type I blepharophimosis/ptosis/epicanthus inversus syndrome (BPES) and FOXL2 duplications cause type II BPES (186). Type I BPES is also associated with POF. In the ovary, FOXL2 is expressed in pre-granulosa cells surrounding primordial follicles and in granulosa cells throughout folliculogenesis. Foxl2−/− mice form primordial follicles, but differentiation of granulosa cells from the squamous to cuboidal state is blocked, granulosa cell proliferation is interrupted, oocyte growth is retarded, and secondary follicles fail to form (187,188). At 2 wk of age, the majority of primordial follicles are activated in Foxl2−/− ovaries, as demonstrated by expression of TGFβ family member growth differentiation factor 9 (Gdf9), a marker of oocyte activation (see Section III. C). However, this activation is accompanied by widespread follicular atresia and a near absence of primordial follicles by 8 wk of age because the defective granulosa cells fail to support growing oocytes (187). Another TGFβ superfamily member, AMH (Müllerian-inhibiting substance, MIS), shows reduced expression in Foxl2−/− compared with wild-type ovaries (187); however, this is likely secondary to the general perturbation of folliculogenesis in Foxl2−/− ovaries.
AMH induces regression of the Müllerian ducts during male fetal sex differentiation (189). In the ovary, AMH produced by granulosa cells of growing follicles also appears to suppress primordial follicle recruitment. In the rodent and human ovary, AMH and its type II receptor, AMHR2, are expressed in granulosa cells of primary and growing preantral follicles (190,191). Although female mice lacking AMH are fertile, Amh−/− juveniles show an increase in growing follicles, and by 4 months this increase is reflected in a reduction of primordial follicles compared with wild-type littermates. By 13 months of age, Amh−/− females have few remaining primordial follicles and correspondingly few growing follicles (192). In vitro studies support the in vivo findings because neonatal ovaries cultured in the presence of recombinant AMH show fewer growing follicles (193). Thus, AMH appears to inhibit the growth of primordial follicles, and in its absence, there is a faster depletion of growing follicles, although it is unknown how AMH functions to repress primordial follicle recruitment (i.e., whether this is a direct or indirect effect of AMH).
Clinically, serum AMH may be a useful biomarker of ovarian reserve (190). In women and mice, serum AMH declines with increasing age. Whereas it is difficult to establish a direct link between serum AMH and the primordial follicle pool in humans, antral follicle number is positively correlated with AMH (194). In mice, there is a strong correlation between serum AMH and the number of primordial follicles (195).
Neurotrophins are soluble growth factors whose functions in development extend beyond the nervous system and include regulation of early folliculogenesis. At least four of the five neurotrophins are expressed in the ovary, including nerve growth factor (Ngf), brain-derived neurotrophic factor (Bdnf), neurotrophin 3 (Ntf3), and neurotrophin 5 (Ntf5). In addition, all four neurotrophin receptors are present in the ovary, including neurotrophic tyrosine kinase receptor types 1 through 3 (Ntrk1, Ntrk2, and Ntrk3; also known as TrkA, TrkB, and TrkC, respectively), and Ngfr (p75), which recognizes all neurotrophins with low affinity (Ref. 196; and reviewed in Ref. 197). Ngf expression in the somatic cells and oocytes precedes follicle formation, and ovaries from 7-d-old Ngf−/− mice contain mostly primordial follicles with few primary follicles, whereas wild-type ovaries show numerous primary and secondary follicles at this age (198). Similar to observations in Foxl2-deficient ovaries (187,188), Ngf-deficient ovaries have reduced somatic cell proliferation, suggesting that NGF signaling is also important in the differentiation of squamous pre-granulosa cells to cuboidal granulosa cells during primordial follicle activation. Although mice lacking the common p75 NGF receptor are fertile with no defects in follicle formation (199), mice lacking the high-affinity NGF receptor, NTRK1, are perinatal lethal (200,201), and the ovarian effects of Ntrk1 deficiency remain unknown, although it would be possible to transplant Ntrk1-deficient ovaries under the kidney capsule of wild-type mice to assess postnatal follicular development, as described with the Ntrk2-deficient ovaries (202).
In addition to the above genetic models and the in vitro studies that complement the in vivo findings, multiple other in vitro studies suggest that several pathways converge to activate or repress primordial follicle recruitment. Ovaries from 4-d-old rats cultured in vitro show an increase in growing follicles and fewer primordial follicles when basic FGF2, keratinocyte growth factor (FGF7), BMP4, leukemia inhibitory factor (LIF), platelet-derived growth factor (PDGF), or glial-derived neurotrophic factor (GDNF) are added to the culture medium (Refs. 203 and 204; and reviewed in Ref. 205). Injection of BMP7 into the ovarian bursa of rats also results in fewer primordial follicles and, correspondingly, more growing follicles (206). At least some of these factors, including FGF2, PDGF, and LIF, may promote the primordial to primary transition by up-regulation of Kitl in granulosa cells. In primordial follicles, FGF2 and PDGF are primarily expressed in oocytes, whereas LIF is found in pre-granulosa and somatic cells (207). Besides AMH, the only other factor shown to inhibit primordial follicle recruitment is the chemoattractive cytokine, CXCL12. Both CXCL12 and its receptor, CXCR4, are predominantly expressed in primordial and activated oocytes. When neonatal mouse ovaries are treated with CXCL12, there is a reduction in growing follicles and a higher density of primordial follicles (208). CXCL12/CXCR4 interactions are also important during PGC migration when CXCR4 is expressed in migrating germ cells and CXCL12 is expressed in the dorsal body wall. In CXCR4-deficient embryos, fewer PGCs reach the genital ridge (209).
Whether any of the above observations with added growth factors have physiological relevance in vivo remains to be determined. For example, Fgf2−/− mice are viable and fertile (210); Fgf7−/− mice have abnormal hair development, but fertility defects have not been reported (211); Lif−/− mice have defects in implantation, but normal folliculogenesis and ovulation (212). Disruption of the genes encoding many of the other putative regulators of primordial follicle activation results in embryonic or perinatal lethality. Furthermore, given the ability of many of these growth factors to influence Kitl expression, it is possible that redundant pathways have evolved so that one factor may compensate for deficiency of others. Thus, validation of the in vitro findings would require cKO models, as well as generation of mice lacking two or more genes in the appropriate ovarian cell type.
Although the molecular events that control primordial follicle formation and maintenance in vivo remain poorly defined, a number of mouse models in recent years have helped to identify master regulators of this critical period in folliculogenesis. The findings in these mutant mice are summarized in Table 6. Moreover, essential mediators of these processes are candidate genes for POF. The clinical criteria for POF are amenorrhea for at least 4 months before 40 yr of age with two serum FSH measurements in the menopausal range (213). In addition to patients with type I BPES that have nonsense mutations in FOXL2 (186), rare function-disrupting mutations in NOBOX and FIGLA have been observed in Caucasian and Chinese women, respectively, with nonsyndromic ovarian failure (214,215). Because these women were heterozygous for NOBOX or FIGLA mutations, further work is needed to determine whether these mutations are sufficient to cause ovarian failure. In vitro studies suggest that at least one of the missense mutations in NOBOX had a dominant negative effect on the ability of wild-type NOBOX to bind DNA.
Table 6.
Mouse models with defects in early folliculogenesis
| Gene | Reproductive phenotype | Fertility status | Ref. |
|---|---|---|---|
| Folliculogenesis-specific basic helix-loop-helix (Figla; FIGa) | No primordial follicles develop at birth and oocytes die | Decreased reproductive lifespan | 129 |
| LIM homeobox protein 8 (Lhx8) | Primordial to primary follicle block and oocyte loss | Infertile | 155 |
| NOBOX oogenesis homeobox (Nobox) | Primordial to primary follicle block and oocyte loss | Infertile | 156 |
| Spermatogenesis and oogenesis-specific basic helix-loop-helix 1 (Sohlh1) | Primordial to primary follicle block and oocyte loss | Infertile | 155,790 |
| Spermatogenesis and oogenesis-specific basic helix-loop-helix 2 (Sohlh2) | Primordial to primary follicle block and oocyte loss | Infertile | 153,160 |
| B-cell leukemia/lymphoma 2 (Bcl2) | Fewer oocytes/primordial follicles in the postnatal ovary | Subfertile | 121 |
| Aryl-hydrocarbon receptor (Ahr) | Increased primordial follicles early; decreased numbers of antral follicles in adults | Subfertile | 126,127 |
| Bcl2-associated X protein (Bax) | Increased oocytes and primordial follicles | Prolonged reproductive lifespan | 123,124 |
| Kit ligand (Kitl; Steel) | Panda, contrasted, and transfer mutants have reduced germ cells and block in folliculogenesis at primary stage | Infertile | 166,167,168 |
| Anti-Müllerian hormone (Amh) | Early depletion of primordial follicles | Decreased reproductive lifespan | 192,193 |
| Forkhead box O3 (Foxo3a) | Global follicular activation and early follicular depletion | Progressive infertility | 172 |
| Forkhead box L2 (Foxl2) | Ovarian failure due to absence of germ cell proliferation and differentiation | Infertile | 187,188 |
| Phosphatase and tensin homolog deleted on chromosome 10 (Pten) (cKO) | Global follicular activation and early follicular depletion | Progressive infertility | 177,178 |
| 3-Phosphoinositide-dependent protein kinase-1 (Pdpk1; Pdk1) (cKO) | Ovarian failure due to depletion of primordial follicles, presumably through decreased survival | Infertile | 184 |
| Ribosomal protein S6 (Rps6) (cKO) | Ovarian failure due to depletion of primordial follicles, presumably through decreased survival | Infertile | 184 |
| Growth differentiation factor-9 (Gdf9) | Folliculogenesis arrest at the one-layer follicle stage | Infertile | 223,225 |
| Nerve growth factor (Ngf) | Fewer primary and secondary follicles; reduced granulosa cell proliferation | Infertile | 198 |
C. Preantral folliculogenesis
Preantral folliculogenesis is characterized by oocyte growth, granulosa cell proliferation, and acquisition of an additional somatic cell layer, the theca. Preantral follicle growth in mice begins 10–12 d after birth when a cohort of developing follicles reaches the secondary stage of folliculogenesis. Secondary follicles contain oocytes in midgrowth stages surrounded by two or more layers of granulosa cells. Growth of preantral follicles is dependent on autocrine and paracrine regulatory factors but appears to be gonadotropin-independent. Mice deficient in the FSHβ subunit (216) or the FSH receptor (FSHR) (217,218), as well as hypogonadal mice with naturally occurring mutations in GnRH, which results in a marked reduction in synthesis of FSH and LH from the anterior pituitary (219), have normal preantral follicle growth despite defective antral folliculogenesis.
During preantral folliculogenesis, the complex bidirectional communication between the oocyte and the somatic compartments of the follicle becomes more apparent. Although the oocyte relies on surrounding somatic cells to support its growth and development, the rate of follicular development is critically dependent on the oocyte. This dominant role for the oocyte in directing folliculogenesis has been demonstrated in elegant reaggregation experiments performed by Eppig et al. (220) where oocytes isolated from secondary follicles of P12 mice were combined with somatic cells from newborn ovaries. The reaggregated ovaries exhibited accelerated folliculogenesis and contained antral follicles 9 d after grafting beneath the renal capsule. Furthermore, the granulosa cells from these antral follicles underwent cumulus expansion when recovered cumulus-oocyte complexes were treated with FSH, and the oocytes from these isolated cumulus-oocyte complexes could resume meiosis and undergo fertilization. In contrast, reaggregated control ovaries in which both the oocytes and somatic cells were from newborn mice contained only secondary follicles 9 d after grafting. Although the precise mechanism by which the oocyte orchestrates follicular development is not known, oocyte-secreted factors appear to have crucial roles.
The first oocyte-derived growth factor demonstrated to be critical for somatic cell function in vivo was GDF9. In mice, GDF9 is first expressed in the oocytes of primary follicles with persistent expression until after ovulation (221,222). Consistent with this expression pattern, Gdf9 null mice form primordial and primary follicles, but have a block in follicular development at the primary stage of folliculogenesis (223). Histologically, whereas the oocytes in Gdf9−/− ovaries grow more rapidly compared with controls (224), the granulosa cells show reduced proliferation and defects in differentiation with eventual development of an abnormal steroidogenic phenotype (225), and a theca layer fails to develop (223). In addition, very few granulosa cells in Gdf9−/− ovaries undergo apoptosis. Despite similar levels of Kit expression in the oocyte, granulosa cell levels of Kitl and inhibin α are dramatically increased (225), suggesting that GDF9 from the oocyte negatively regulates granulosa cell production of these growth factors. The increase in inhibin α could prevent proliferation of granulosa cells at the primary follicle stage because mice lacking both inhibin α and GDF9 develop multilayered follicles (226). The up-regulation of Kitl may lead to enhanced signaling through oocyte-expressed KIT, contributing to the increased oocyte size observed in Gdf9−/− ovaries (224).
An oocyte-granulosa cell regulatory loop has been postulated where KIT ligand from granulosa cells promotes oocyte growth until a specific size is reached, upon which time GDF9 secretion from enlarged oocytes suppresses Kitl expression in cumulus cells to slow or stop further oocyte growth (114). As previously mentioned, in addition to the role of KIT/KIT ligand signaling during initial recruitment of primordial follicles, mutations affecting KIT or KIT ligand often show defects at the primary follicle stage, similar to what is observed in GDF9 null ovaries. For example, the Slpan mutant mice, with a hypomorphic Kitl allele, exhibit decreased oocyte recruitment, and the majority of follicles fail to develop beyond the early primary stage. Furthermore, whereas addition of KIT ligand to follicles growing in culture enhances oocyte growth (227), treatment of granulosa cells isolated from preantral and antral follicles with recombinant GDF9 suppresses Kitl expression (228). However, Gdf9 is not misexpressed (i.e., decreased) in the oocytes of mice with a mutated KIT receptor (179), suggesting that KIT signaling does not directly regulate Gdf9 in such a feedback loop.
BMP15 is another oocyte-secreted TGFβ superfamily member that was identified using a homology-based cloning strategy to identify BMP homologs (229). In addition to 52% amino acid identity, BMP15 and GDF9 share interesting features. Bmp15 mRNA has an expression pattern identical to Gdf9 in mouse oocytes, and BMP15 and GDF9 proteins lack a conserved cysteine residue found in other TGFβ superfamily members. This cysteine is required for intermolecular disulfide bond formation that occurs during dimerization of other family members, indicating that BMP15 and GDF9 form noncovalent homo- and/or heterodimers. Despite these characteristics, unlike GDF9, BMP15 is not required during preantral folliculogenesis in mice. Bmp15−/− are subfertile; however, this is secondary to decreased ovulation and fertilization rates, rather than disrupted folliculogenesis (230). GDF9 and BMP15 may, however, have redundant roles during folliculogenesis, with GDF9 being the dominant growth factor in mice. Ovaries from Gdf9+/− Bmp15−/− mice have more defects than Bmp15−/− mice, including fewer late-stage follicles and CLs and increased oocyte loss.
There appear to be species-specific differences as to the importance of oocyte-secreted GDF9 and BMP15 during folliculogenesis. To date, five polymorphisms causing nonsense or missense mutations in BMP15 and one causing a missense mutation in GDF9 have been identified in several breeds of sheep. These mutations are associated with increased ovulation in heterozygous carriers, but sterility in homozygous carriers (231,232). The ovarian phenotype of sheep with homozygous X-linked mutations [Fecundity X (FecX)] is similar to that of Gdf9 null mice with failure of follicles to develop beyond the primary follicle stage and uncoupling of oocyte growth relative to granulosa cell proliferation (233). Linkage analysis, however, mapped the FecX allele to the BMP15 gene locus (234), which is in an orthologous location on mouse and human X chromosomes (229). In vitro studies suggest that certain BMP15 missense mutations, such as the V31D substitution found in the Inverdale strain of sheep (234), may impair proteolytic processing and secretion of GDF9 (235). However, despite oocyte and granulosa cell abnormalities, antral follicles are present in ovaries from sheep homozygous for a point mutation in the GDF9 gene, which causes a nonconservative amino acid substitution in a region of the mature protein that is predicted to interact with its type I receptor (232). Thus, whereas mouse BMP15 does not appear to have a critical function in early folliculogenesis, in sheep it is essential for early follicular development, and GDF9 may be more important in later stages. Although they are rare, mutations in BMP15 and GDF9 that affect secretion or function when evaluated in in vitro assays have been reported in women with POF (236,237,238,239,240).
The neurotrophins NTF5 and BDNF, which signal through NTRK2 on the oocyte, are expressed in human and mouse primordial follicles (202,241) and appear to have redundant roles in preantral folliculogenesis. Loss of Ntf5 alone does not alter follicle number through the secondary stage; however, concomitant loss of Ntf5 and Bdnf causes a significant reduction in the number of secondary follicles, which is also seen in Ntrk2 null ovaries. Although not significant, the number of primary follicles is also decreased, whereas the population of primordial follicles is unaffected in P7 Ntrk2−/− mice (202). When Ntrk2 null ovaries from P4-P5 mice are transplanted under the renal capsule of adult wild-type females, after a period of 2 wk the ovaries are nearly depleted of oocytes and those that remain are degenerating, in contrast to transplanted control ovaries, which contained follicles that have reached the antral stage (202). Thus, although NTF5, BDNF, and NTRK2 do not appear to be critical for initial follicle recruitment, progression beyond the primary stage is interrupted. Because Gdf9 and Kitl levels are unchanged in Ntrk2 null ovaries, NTF5 and BDNF appear to affect the transition to secondary follicles independent of these pathways.
In addition to the multiple paracrine factors involved in the intricate dialogue between the somatic cells and oocyte of a developing follicle, direct connections via intercellular membrane gap junction channels are also essential during folliculogenesis. Gap junctions allow the transfer of ions, metabolites, and small molecules between neighboring cells. Connexins are the core proteins that make up gap junctions. Several connexins are expressed in the mammalian ovary (reviewed in Refs. 242 and 243), and at least two, connexin 43 (CX43; GJA1) and connexin 37 (CX37; GJA4), have essential and distinct roles during folliculogenesis. CX43 forms gap junctions between granulosa cells throughout folliculogenesis (244), whereas CX37 localizes to oocyte-granulosa cell gap junctions beginning in the primary follicle stage (245). Gja1 knockout mice die in the early postnatal period due to severe cardiac malformations (246). Neonatal Gja1−/− ovaries are small secondary to germ cell deficiency that occurs as early as E11.5 (244). To determine whether the remaining germ cells could participate in folliculogenesis, ovaries from fetal and newborn Gja1−/− mice were cultured in vitro or transplanted under the kidney capsule of wild-type mice (244,247). These CX43-deficient ovaries showed a block at the primary follicle stage with impaired granulosa cell proliferation and retardation of oocyte growth. The oocytes were also morphologically abnormal with defects in meiotic maturation.
Oocytes in mice lacking CX37 also have defects in meiotic competence and do not grow to a normal size (248), but follicular development progresses to the later preantral stage (245). Despite a near-complete absence of large antral (Graafian) follicles, CX37-deficient ovaries have numerous small CL-like structures, suggesting that communication via gap junctions is a major mechanism regulating CL formation; when oocyte-granulosa cell coupling is disrupted, premature luteinization occurs. This is perhaps logical, given that luteinization normally occurs after ovulation, a natural disruptor of oocyte-granulosa cell gap junctions. Thus, whereas CX37 gap junctions are essential for the preantral to antral follicle transition, CX43 gap junctions are required for granulosa cell proliferation earlier in folliculogenesis to form multilayered follicles, and both types of junctions support proper oocyte development.
Although we have highlighted a few growth factors and signaling pathways as well as the importance of intercellular connections during preantral folliculogenesis, numerous mouse models with defects in preantral follicular development have been characterized. These mutant mice are summarized in Table 7.
Table 7.
Mouse models with defects in preantral folliculogenesis
| Gene | Reproductive phenotype | Fertility status | Ref. |
|---|---|---|---|
| FSH receptor (Fshr) | Preantral block in folliculogenesis | Infertile | 218 |
| FSH β (Fshb) | Preantral block in folliculogenesis; rescued by exogenous gonadotropins | Infertile | 216 |
| Cyclin D2 (Ccnd2) | Failure of granulosa cell proliferation | Infertile | 292 |
| Discoidin domain receptor family, member 2 (Ddr2; slie) | Spontaneous mutant; smaller pituitaries and gonadal dysfunction, dwarfism | Infertile | 791 |
| IGF-I (Igf1) | Hypogonadal; impaired antral follicle formation | Infertile | 290 |
| Nitric oxide synthase 1, neuronal (Nos1) | Impaired central hormonal regulation of reproductive function; decreased ovary weight, decreased CLs | Infertile | 792 |
| Phosphate cytidylyltransferase 1, choline, b isoform (Pcyt1b) | Multiple follicular defects; reduced ovarian follicles and CLs | Subfertile | 793 |
| SH2B adaptor protein 1 (Sh2b1) | Small, anovulatory ovaries with reduced numbers of developing follicles | Subfertile | 794 |
| Rous sarcoma oncogene (Src) | Defect in antral follicle development; anovulation | Infertile | 795 |
| TAF4B RNA polymerase II, TATA box binding protein-associated factor (Taf4b; TAFII105) | Defects in follicular development, oocyte maturation and fertilization | Infertile | 308,309,310 |
| Thrombospondin 1 (Thbs1) | Increased VEGF and ovarian hypervascularization, increased follicle numbers but decreased size of preantral and antral follicles | Subfertile | 796 |
| Ubiquitin protein ligase E3A (Ube3a; E6-AP) | Ovarian hypoplasia; defects in ovulation | Subfertile | 797 |
VEGF, Vascular endothelial growth factor.
D. Theca formation and physiology
Once the follicle achieves two layers of granulosa cells, an additional morphologically distinct layer of somatic cells, the theca, differentiates as the outermost layer of the follicle (113). Cells of the theca interna layer, which forms just outside the basement membrane surrounding the granulosa cells, have ultrastructural features, including numerous mitochondria with tubular cristae, smooth endoplasmic reticulum, and abundant lipid vesicles, that correspond with their principal function as a source of androgens for neighboring granulosa cells to convert to estrogens (249). The theca externa, composed of fibroblasts, smooth muscle-like cells, and macrophages, is important during ovulation. Cells that contribute to the theca differentiate from mesenchymal precursor cells present in the ovarian stroma, adjacent to developing follicles (250). Like preantral folliculogenesis, theca formation is gonadotropin-independent because thecal precursor cells lack LH receptors and the theca layer still forms in the ovaries of FSH-deficient mice (216). Upon formation of a discernible theca interna layer, however, LH principally controls thecal cell androgen production.
Although the factors that regulate thecal cell differentiation are unknown, they appear to be small molecules in the 20- to 25-kDa range secreted by growing follicles. When undifferentiated theca-interstitial cells were cultured in conditioned medium from rat preantral follicles with two to five layers of granulosa cells, markers of theca differentiation, including mRNAs for LH/choriogonadotropin receptor (Lhcgr) and the Cyp17a1 family member, were expressed and androgens were produced (251). Candidate factors that may contribute to thecal cell differentiation include IGF, KIT ligand, and GDF9. In cultured rat thecal cells, IGF-I increased expression of Lhcgr, Cyp11a1, and 3β-hydroxysteroid dehydrogenase (Hsd3b1), but mRNAs for other thecal cell markers, including steroidogenic acute regulatory protein (Star) and Cyp17a1, were only up-regulated when KIT ligand was also added (252). As mentioned in Section III. E, a thecal layer fails to form in Gdf9−/− ovaries (223), which show a lack of thecal cell markers Cyp17a1, Lhcgr, and Kit, despite an abundance of presumed thecal cell precursors in the interstitium and increased circulating FSH and LH (225). Whether GDF9 regulates thecal cell recruitment and/or differentiation directly or indirectly through regulation of preantral granulosa cell development is unknown. Recombinant mouse GDF9 has been shown to up-regulate Igf1 in cultured granulosa cells (253,254), suggesting that at least some of the effects of GDF9 on theca development are indirect. Furthermore, GDF9 may be more important in thecal differentiation because Inha Gdf9 double knockout mice form a morphological theca layer, but thecal cell markers are not expressed in this layer (226).
Although long believed to exist, putative thecal stem cells have recently been isolated from neonatal mouse ovaries. Using a procedure that had previously been successful in isolating male germline stem (GS) cells from neonatal testes (255), Honda et al. (256) hoped to isolate putative and controversial female GS cells. Instead, the authors isolated somatic cell colonies that were positive for alkaline phosphatase (a stem cell marker) and proliferated and remained in an undifferentiated state in serum-free GS cell media. When the cells were treated with serum, LH, IGF-I, and KIT ligand, or with conditioned medium from granulosa cells, they underwent cytological changes consistent with steroidogenic ability and secreted androstenedione into the culture medium. Transplantation of EGFP-positive, undifferentiated stem cells into host ovaries showed colonization of interstitial areas and inner and outer theca layers around fully grown follicles.
Members of the hedgehog family of secreted morphogens are also candidate regulators of early thecal cell differentiation and may be especially important in regulating differentiation of smooth muscle cells in the theca externa during ovulation. In mammals, there are three secreted hedgehog ligands, including Indian hedgehog, desert hedgehog, and sonic hedgehog (IHH, DHH, and SHH). Hedgehog ligands bind and functionally inactivate transmembrane patched (PTCH1 and PTCH2) receptors on responsive cells, causing derepression of the Smoothened (SMO) seven pass transmembrane receptor, ultimately regulating the activity or levels of Gli family transcription factors (GLI1, GLI2, and GLI3) (reviewed in Ref. 257). Beginning in the primary follicle stage, Ihh and Dhh mRNAs localize to granulosa cells of growing follicles, decreasing before ovulation, whereas Ptch1 and Gli1 are in the mesenchymal stromal cells surrounding primary follicles and increased in the theca layer of larger follicles (258). Although Dhh knockout mice do not have an ovarian phenotype, Ihh may have redundant or dominant roles during follicular development. To study constitutive activation of hedgehog signaling, a transgenic mouse (SmoM2) has been developed that conditionally expresses a dominant SMO protein that is not inhibited by patched receptors (259). Dominant activation of SMO in granulosa and thecal cells using Amhr2-Cre (260,261) caused defective formation of the smooth muscle layer found in the theca externa and severely impaired ovulation (262). The observation that excess hedgehog signaling prevented smooth muscle differentiation is in agreement with other organ systems where mesenchymal cells furthest from an epithelial hedgehog source differentiate into smooth muscle (262). Thus, in wild-type mice, higher levels of PTCH1 in thecal cells may limit hedgehog signaling in the follicle, allowing differentiation of the theca externa. Because there is extensive cross talk between TGFβ and hedgehog pathways in several cell types and tissues (263), it would be interesting to determine whether components of hedgehog signaling are altered in Gdf9 null mice, contributing to the absence of thecal differentiation as a result of GDF9 deficiency.
Granulosa cells of antral follicles are the chief source of estradiol production, yet they lack the biosynthetic enzyme (CYP17A1) necessary to produce the aromatizable androgen and estradiol precursor, androstenedione. Although cells of the theca interna express CYP17A1, they are deficient in CYP19A1, the key enzyme in the conversion of androstenedione to estrogens that is expressed by granulosa cells of later-stage follicles (264). Expression of each of these enzymes is controlled by pituitary gonadotropins, forming the basis of the two-cell, two-gonadotropin concept of estradiol production (reviewed in Ref. 249, and summarized in Fig. 5). In response to LH stimulation, thecal cells express key steroidogenic enzymes, including CYP11A1, HSD3B1, and CYP17A1. LH also promotes up-regulation of STAR, which facilitates delivery of cholesterol to the inner mitochondrial membrane where CYP11A1 is located. Granulosa cells respond to FSH by up-regulating CYP19A1 and 17β-hydroxysteroid dehydrogenase (HSD17B1).
Figure 5.
The two-cell, two-gonadotropin concept of follicular steroid production. The main function of thecal cells during folliculogenesis is the production of steroids. Although thecal cells are capable of de novo production of androgens, they lack aromatase (CYP19A1), which is required to convert androgens into estradiol. Thecal cells respond to basal levels of LH by up-regulating biosynthetic enzymes involved in steroid production, including STAR, CYP11A1, CYP17A1, and 3β-hydroxysteroid dehydrogenase (3β-HSD). STAR facilitates the transport of cholesterol to the inner mitochondrial membrane, where it is converted to pregnenolone by CYP11A1. Pregnenolone is converted to dehydroepiandrosterone (DHEA) by CYP17A1. Finally, 3β-HSD converts DHEA into androstenedione, which diffuses across the basement membrane to granulosa cells. In response to stimulation by FSH, granulosa cells up-regulate CYP19A1 and 17β-hydroxysteroid dehydrogenase (17β-HSD), which convert androstenedione into estradiol (249).
Because estradiol is not essential until later stages of folliculogenesis and ovulation (see Section III. E), but thecal cells express LH receptor starting at the secondary follicle stage, it is important to suppress excess androgen biosynthesis in preantral and small antral follicles. To modulate the stimulatory effect of LH on theca androgen production in smaller follicles, granulosa cells secrete factors, such as activins, that inhibit androstenedione production (265,266). KIT ligand from granulosa cells may also up-regulate factors in thecal cells, including TGFβ, TGFα, FGF7, and hepatocyte growth factor, that have autocrine inhibitory effects on androstenedione production (reviewed in Ref. 267). On the other hand, a threshold level of androgens from thecal cells may be necessary for preantral follicular growth, and production may be controlled by GDF9 (268). In a rat follicle culture system, intra-oocyte injection of Gdf9 morpholino antisense oligonucleotides suppressed preantral follicle growth, Cyp17a1 expression, and testosterone production, and these effects were attenuated by exogenous GDF9 (268). The androgen receptor antagonist flutamide also blocked GDF9-induced follicle growth. There are, however, conflicting reports on the effects of recombinant GDF9. Treatment of rat theca-interstitial cells with recombinant GDF9 stimulates androstenedione production (269), whereas in bovine thecal cell culture, GDF9 increased thecal cell proliferation but decreased IGF-I-induced steroidogenesis (270). Many of these observations may be indirect effects of GDF9 and could also be due to differences in culture conditions.
Although we have focused our discussion on factors involved in normal theca formation and function, thecal cells, through excess androgen biosynthesis, contribute to polycystic ovarian syndrome. In addition to clinical features of hyperandrogenism due to excess ovarian and adrenal androgen production, this heterogeneous disorder is accompanied by ovarian dysfunction, including ovulatory defects and/or polycystic ovaries (271). For a more extensive review of the pathophysiology and potential genes involved in polycystic ovarian syndrome, the reader is referred to previous work in this journal (272,273).
E. Antral follicle formation, FSH, and estradiol
A number of important changes take place in the follicle during formation of the antrum. During antral folliculogenesis, multiple small, fluid-filled spaces eventually coalesce to form a single antral cavity that separates two functionally distinct granulosa cell populations. The newly formed mural granulosa cells line the wall of the follicle and are critical for steroidogenesis and ovulation, whereas the cumulus granulosa cells surround the oocyte, promoting its growth and developmental competence. These two cell types appear to be defined by opposing gradients of FSH from outside the follicle and oocyte-secreted factors from within (274). The transition from preantral to antral follicle marks a change from principally intraovarian to extraovarian regulation of folliculogenesis as the hypothalamic-pituitary-gonadal (HPG) axis starts functioning. Although preantral follicles are responsive to FSH, during antral folliculogenesis, FSH becomes essential (216) not only to prevent granulosa cell apoptosis and follicular atresia (275), but also for granulosa cell proliferation, estradiol production, and LH receptor expression (276).
FSH and LH are the pituitary gonadotropins that coordinate antral follicle development and ovulation. These heterodimeric glycoprotein hormones have a unique β-subunit and a common α-subunit that is also shared with TSH and chorionic gonadotropin. A number of positive and negative feedback loops in the HPG axis coordinate follicle maturation and dominant follicle selection with sexual behavior and preparation for pregnancy. In addition to the negative feedback of estradiol on the HPG axis, the ovary also produces growth factors, including activins, inhibins, and FSTs, that modulate pituitary FSH secretion (277), but also act locally to regulate follicular development.
To circumvent the multiple defects observed in mice lacking the common α-subunit (and thus lacking FSH, LH, and TSH) (278), and the difficulty in studying FSH function independent of LH in hypogonadal mutants (219), mice lacking the FSHβ subunit and therefore circulating FSH were generated (216). Whereas FSH is not essential for male fertility, FSH-deficient females were infertile due to a block in folliculogenesis before antral follicle formation. Despite the absence of antral follicles and no ovulation in FSH-deficient mice, ovaries at 6 wk contained all earlier stages of follicles, including primordial, primary, and multilayered preantral follicles, supporting the notion that early folliculogenesis is gonadotropin-independent. The lack of large antral follicles and CLs probably accounts for the smaller-sized ovaries. Juvenile FSH-deficient mice responded to exogenous administration of pregnant mare serum gonadotropin (PMSG) and human chorionic gonadotropin (hCG), with similar numbers of oocytes recovered from control and knockout females, suggesting that ovulatory competence was not affected by the absence of FSH. Mice lacking FSHR are also infertile with very similar ovarian and uterine findings (217,218). Although mutations in FSH and FSHR in humans are rare, the clinical features in humans are similar to the defects observed in mouse models (279,280,281,282,283,284).
The regulation and functions of FSH in mammals appear to be evolutionarily conserved. When a 10-kb human FSHβ transgene that contained gonadotrope-specific, GnRH-responsive, and steroid-responsive elements was introduced into the FSHβ knockout mice to create an interspecies FSH heterodimer hybrid (mouse α:human FSHβ), defects in folliculogenesis were rescued and fertility of transgenic mice was restored to wild-type levels (285). Bitransgenic mice were also engineered that expressed the α-subunit of hCG and the FSHβ subunit under the control of the metallothionein 1 (MT1) promoter, resulting in expression of human FSH from multiple tissues in FSHβ-deficient mice. Although fertility was restored in some females (30%), litters were smaller and two out of three females died in the postpartum period. The different phenotypes in the two transgenic mice may be due to the nature of FSH secretion, which is constitutive (nonpulsatile) when under control of MT1 but more physiological (pulsatile) in the human FSHβ transgenic model.
The classical signaling cascade activated by binding of FSH to the G protein-coupled FSHR is a linear adenylyl cyclase (AC)/cAMP/protein kinase A (PKA) pathway that results in phosphorylation and activation of the transcription factor cAMP-response element-binding protein to regulate a number of target genes, including aromatase, the α- and β-subunits of inhibin, LH receptor, and many more. In recent years, however, a number of additional intracellular signaling pathways, some of which are PKA-independent, have also been identified (reviewed in Refs. 286 and 287). Although FSH up-regulates serum and glucocorticoid-induced kinase 1 (SGK1) at the transcriptional level through the classical PKA pathway, phosphorylation and activation of SGK1 and AKT also occurs in a PKA-independent, PI3K-dependent fashion that still requires cAMP (288). Although activation of PI3K in this setting was proposed to be mediated by cAMP-regulated guanine nucleotide exchange factors that activate RAS-like small GTPases upstream of PI3K, more recent studies point to FSHR activation of a SRC tyrosine kinase-dependent pathway (289).
IGF-I also activates the PI3K pathway in granulosa cells. FSH and IGF-I signaling pathways impact proliferation, differentiation, and survival of granulosa cells, in part by distinct regulation of the levels of FOXO1 mRNA and protein (174,286). Igf1 null mice are infertile, with an arrest at the preantral follicle stage similar to FSHβ- and FSHR-deficient ovaries (290,291). Interestingly, although Igf1 and Fshr mRNAs colocalize in healthy gonadotropin-responsive follicles, Igf1 is not altered in ovaries from FSH-deficient females, but Fshr and Cyp19a1 are reduced in IGF-I knockout mice (291). Thus, IGF-I appears to enhance granulosa cell responsiveness to FSH by augmenting levels of FSHR.
FSH, IGF-I, and estradiol signaling cascades control granulosa cell proliferation through modulation of the cell cycle. The D- and E-type cyclins positively regulate entry into the cell cycle by binding cyclin-dependent kinases (CDK4/6 and CDK2, respectively) and activating a cascade that promotes the G1/S transition. CDK inhibitors, such as p27Kip1, block cell cycle progression by inactivating cyclin-CDK complexes. Mice null for cyclin D2 (Ccnd2) have impaired granulosa cell proliferation and demonstrate an arrest in folliculogenesis at the preantral stage (292), similar to Fshb−/− and Igf1−/− mice. Treatment of rat granulosa cells with PMSG up-regulates Ccnd2 mRNA in a cAMP-dependent manner, and Ccnd2 null ovaries show a minimal response to exogenous FSH, in contrast to rapid granulosa cell proliferation observed in controls. Surprisingly, in FSHβ null mice, Ccnd2 is only modestly decreased (and cell cycle inhibitor mRNAs are not up-regulated) (293), whereas Igf1 is unchanged in granulosa cells (291) and serum estradiol is not altered (216). Ccnd2 also showed little change in FSHR knockout mice (218). Thus, despite the obvious requirement for cyclin D2 during antral follicle formation, these findings suggest a dynamic interdependence of FSH, IGF-I, estradiol, and other pathways in regulation of granulosa cell proliferation. For example, in vitro studies in rat granulosa cells suggest that both FSH signaling to remove FOXO1 repression of Ccnd2 and SMAD2/3 signaling are required to up-regulate cyclin D2 (294).
As shown in Fig. 5, estradiol production in the ovary relies on an interplay between thecal and granulosa cells, and the final biosynthetic step requires aromatase to convert androgens to estrogens. Cyp19a1 null mice are unable to produce estradiol (144), and therefore provide insight into the role of this sex steroid on folliculogenesis. Ovaries from 12- to 14-wk-old Cyp19a1 null mice contained follicles of all types; however, the mice were infertile and CLs were absent, suggesting impaired ovulation. In addition, many antral follicles were histologically abnormal with uneven granulosa cell layers and increased apoptosis. Follicular atresia increased with age, and many antral follicles that remained were cystic and hemorrhagic (295). Consistent with a role for estradiol in the negative feedback regulation of gonadotropin production, serum FSH and LH were elevated in Cyp19a1 null mice, and the high LH likely contributed to hyperplastic ovarian stroma, as well as markedly increased serum testosterone levels. A second Cyp19a1 knockout mouse model had similar findings and suggested that the increased atresia could be due to up-regulation of proapoptotic genes, including p53 and Bax (296).
The effects of estradiol on folliculogenesis are mediated by two estrogen receptors, ERα (Esr1) and ERβ (Esr2). These classical ERs are members of the nuclear receptor superfamily of ligand-activated transcription factors. A membrane-bound G protein-coupled receptor, GPR30 (Gper), might mediate rapid, nongenomic estradiol signaling (297) and is implicated in maintenance of meiotic arrest in fish oocytes (298). In ovaries from 5-month-old GPR30-deficient mice, however, fertility is unimpaired and folliculogenesis appears normal, with follicles in all stages of development and CLs present (299). In contrast, mice lacking ERα (300), ERβ (301), or both (302,303) have several ovarian findings consistent with their patterns of expression. ERβ is expressed in granulosa cells of growing follicles and is regulated by gonadotropins, whereas ERα is predominantly expressed in thecal and interstitial cells (304). Absence of ERα causes infertility, and mutant ovaries contain enlarged, cystic, and hemorrhagic follicles and no CLs or evidence of ovulation, similar to Cyp19a1 null mice and the findings in polycystic ovarian syndrome. The elevated androgen, estradiol, and LH levels in the serum of Esr1 null mice indicate that loss of ERα significantly affects the negative feedback of estradiol on the HPG axis. Intraovarian feedback of estradiol on thecal cell androgen production also appears to be disrupted in Esr1 null follicles, which occurs through loss of repression of Cyp17a1 by ERα, and consequently increased androstenedione production (305). Mice lacking ERβ are subfertile, with the principal defect being reduced ovulation, which may be attributed to reduced expression of LHCGR in granulosa cells (306) because induction of LHCGR in these cells is highest in preovulatory follicles and depends on the synergistic interactions of FSH and estradiol, as shown originally in hormonally primed hypophysectomized immature rats (307). As discussed in Section III. F, LH is a critical mediator of events in the periovulatory period. Ovaries from young adult ERβ knockout mice have follicles at all stages of development, but fewer CLs and more atretic follicles. As might be expected based on the individual ER knockouts, mice lacking both ERα and ERβ (αβERKO) are anovulatory, but they also exhibit a phenotype distinct from each single ER knockout. Ovaries from prepubertal αβERKO mice exhibit precocious maturation with adult-like antral follicles. In adult αβERKO ovaries, however, most follicles only reach the small antral stage, CLs are absent, and there are many sex-reversed follicles with degenerating or absent oocytes and the presence of Sertoli-like cells. Similar Sertoli-like and Leydig-like cells are observed in aromatase knockout mice (144). In summary, the ER and CYP19A1 knockout models suggest that unlike FSH, estradiol is not essential for antral follicle formation but is critical for granulosa cell growth and differentiation to maintain antral follicles and promote ovulation.
TAF4B is a gonad-enriched transcriptional coactivator subunit of the TFIID core transcriptional complex that may be an important cofactor in regulation of FSH target genes. The TFIID complex consists of the TATA-binding protein and a number of TATA-binding protein-associated factors (TAFs) that function as coactivators to recruit RNA polymerase II to specific gene promoters. In the ovary, TAF4B primarily localizes to granulosa cells of large preantral follicles (308,309) in adult mice, but it is also detected in the oocytes of embryonic and prepubertal mice (310). Taf4b null females are infertile with elevated serum FSH and multiple defects throughout folliculogenesis, including a reduction in primordial, preantral, and antral follicles in prepubertal and adult TAF4B-deficient ovaries, as well as less proliferation and increased apoptosis of granulosa cells (309,310). Ccnd2 and Cyp19a1 were also reduced in Taf4b null adult ovaries (308), but these findings and the elevated serum FSH could be secondary to a widespread decrease in granulosa cells and ovarian failure that prevents feedback of granulosa cell-produced estradiol and inhibin on pituitary FSH production. To identify TAF4B-dependent promoters, TAF4B was overexpressed in a spontaneously immortalized rat granulosa cell line (311). In this context, TAF4B activated Ccnd2, Inha, Inhba, and Fst promoters and also up- regulated the transcription factor c-Jun, which is rapidly induced by FSH (312). These effects were through direct association of TAF4B with target gene promoters. In a human granulosa cell line, increased cAMP leads to PKA-dependent phosphorylation of TAF4B (313), which might affect its function, and in pig granulosa cells FSH increases Taf4b expression, which is required for maximal induction of IGF binding protein 3 (Igfbp3) by FSH (314). Taken together, these in vitro studies and the granulosa cell proliferation and survival defects in TAF4B-deficient ovaries suggest that although TAF4B appears critical during multiple stages of folliculogenesis, it is both a target and a mediator of FSH signaling in later stages.
Androgens may also have a role in priming granulosa cells to respond to gonadotropins because ovaries from 10-d-old androgen receptor (Ar) null females have a reduction in Fshr, and Ar null adults are subfertile, with decreased ovulation rates and defects in cumulus-oocyte complexes (315). In another Ar−/− model (316), Fshr trended lower but was not significantly decreased in 8-wk-old ovaries, although by this age a number of hormones and growth factors, including FSH, estradiol, and IGF-I, could have compensated for the absence of AR. Ar and Fshr mRNAs colocalize to granulosa cells, and testosterone up-regulates granulosa cell FSHR in primate ovaries (317). Further work is needed to determine whether androgens influence follicular response to FSH.
Although FSH signaling is essential for antral follicle formation and survival and we have focused on FSH- regulated pathways in this section, a number of other factors have also been identified that are critically important during this stage of folliculogenesis. Mutant mouse models with alterations in antral follicle development are summarized in Table 8. In addition, mutations in multiple genes have been identified that cause hypogonadism and infertility in women secondary to disruption of gonadotropin-signaling pathways (Ref. 284, and summarized in Table 1).
Table 8.
Mouse models with defects in antral follicle development
| Gene | Reproductive phenotype | Fertility status | Ref. |
|---|---|---|---|
| A disintegrin-like and metalloprotease with thrombospondin type 1 motif, 1 (Adamts1) | Defects in preovulatory follicle development | Subfertile | 341 |
| Inner mitochondrial membrane peptidase 2-like (Immp2l) (transgenic insertion) | Folliculogenesis and ovulation defects | Infertile | 798 |
| Insulin receptor substrate 2 (Irs2) | Small, anovulatory ovaries with reduced numbers of follicles | Infertile | 799 |
| Estrogen receptor α (Esr1; ERα) | Enlarged, cystic and hemorrhagic follicles, and no CLs or evidence of ovulation | Infertile | 300 |
| Estrogen receptor β (Esr2; ERβ) | Block in late antral folliculogenesis and decreased ovulation | Subfertile | 301 |
| Superoxide dismutase 1 (Sod1) | Folliculogenesis defect; failure to maintain pregnancy | Subfertile | 800,801 |
| SRY-box containing gene 3 (Sox3) | Follicular atresia and oogenesis defects | Subfertile | 802 |
| TNF type I receptor superfamily, member 1a (Tnfrsf1a) | Enhanced prepubertal response to gonadotropins; early ovarian senescence | Subfertile | 803 |
F. The preovulatory follicle, cumulus expansion, ovulation, and luteinization
Although a majority of follicles in the growing pool will undergo atresia, a select few antral follicles (the number varies by species) in a developing cohort reach the preovulatory stage. Those follicles that survive to this stage were likely most responsive (because of higher relative FSHR expression) to decreasing serum FSH that occurs through negative feedback of estradiol and inhibin on the pituitary. Whereas rising serum estradiol functions to suppress pituitary FSH secretion, increased follicular estradiol production enhances pituitary LH production, resulting in the LH surge. Preovulatory follicles express LHCGR at high concentrations in granulosa cells, enabling them to respond to the LH surge, which initiates a cascade of events leading to oocyte meiotic resumption, cumulus expansion, follicle rupture, and finally terminal differentiation of the remaining granulosa and thecal cells to create the CL.
Consistent with the patterns of expression of LHCGR in developing follicles and the cyclic rise in LH that precedes ovulation in mammals, mice deficient in LH (Lhb null) (318) or LH receptor (Lhcgr null) (319,320) are infertile with defects in steroidogenesis. As mentioned in Section III. C, Lhcgr is a marker for thecal cells, and LH induces many enzymes involved in steroid biosynthesis. Lhb null females have decreased serum estradiol and progesterone and a reduction in Cyp11a1, Cyp17a1, and Cyp19a1, despite an intact thecal layer. Folliculogenesis is blocked at the early antral follicle stage; healthy large antral and preovulatory follicles and CLs are absent. However, similar to Fshb null mice, exogenous gonadotropin administration rescues the follicular defects, and comparable numbers of oocytes are recovered from LH-deficient and control-stimulated females. The histological defects in Lhcgr null ovaries mirror those observed in Lhb null ovaries, and serum estradiol and progesterone are also decreased in Lhcgr null females. Hormone replacement therapy with estradiol and progesterone failed to restore fertility or reverse the follicular defects (319); however, this may partially be attributed to the absence of LH receptor in antral follicles because one of the effects of estradiol, as shown by ERβ-deficient mice, is up-regulation of Lhcgr expression (306). Nevertheless, additional ER-independent targets downstream of LH signaling are likely responsible for growth beyond the early antral stage and for triggering ovulation. In recent years, a number of factors that influence the response of follicles to the LH surge have been identified.
Expansion of the cumulus cells on a hyaluronan-rich extracellular matrix surrounding the oocyte is initiated by the LH surge and is required for normal ovulation and fertilization. Regulation of this process is multifactorial and dependent on the activation of MAPK signaling (321,322), as well as oocyte-secreted paracrine factors (323). After the LH surge, a number of genes involved in formation and stabilization of the extracellular matrix of the cumulus oophorus are up-regulated (324). Although a number of genes are induced in cumulus-oocyte complexes in the periovulatory period (325), we will focus on those that are essential for proper formation of the cumulus matrix; these genes include Has2, Ptgs2, Tnfaip6, and Ptx3. As shown in Fig. 6, hyaluronan synthase 2 (HAS2) is required for the production of hyaluronan, which forms the structural backbone of the cumulus matrix. Hyaluronan chains are stabilized through interactions with additional matrix proteins. TNFα-induced protein 6 (TNFAIP6) catalyzes the formation of covalent crosslinks between hyaluronan and the heavy chain of serum-derived inter-α-trypsin inhibitor (IαI) (326). Pentraxin 3 (PTX3) appears to stabilize the cumulus matrix through interactions with IαI (327). Prostaglandin synthase 2 (PTGS2; also known as COX2) is the rate-limiting enzyme in the synthesis of prostaglandins, and genetic studies suggest that prostaglandin signaling through the prostaglandin E receptor 2, subtype EP2 (PTGER2) functions upstream of TNFAIP6 (328). Although it is not entirely understood how these proteins interact to organize the extracellular matrix of the cumulus oophorus, each of them is essential for normal cumulus expansion. Targeted disruption of Ptgs2 (329,330), Ptger2 (331,332,333), Tnfaip6 (334), or Ptx3 (254) results in abnormal or absent cumulus expansion and extreme subfertility (in Ptgs2, Ptger2, and Ptx3 null females) or sterility (in Tnfaip6 null females). Has2 null mice are embryonic lethal (335), but RNA interference (RNAi)-mediated silencing of Has2 in cultured cumulus-oocyte complexes reduces cumulus expansion (336). IαI biosynthesis in the liver requires the α-1-microglobulin/bikunin light chain (encoded by Ambp) for proper assembly and secretion. Ambp null female mice also demonstrate severely impaired cumulus expansion and a marked reduction in fertility, which is rescued by administration of IαI (337,338). Finally, many additional factors are in the cumulus matrix. Chondroitin sulfate proteoglycan 2 (CSPG2; also known as versican), which can bind hyaluronan, is induced by LH and detected in cumulus-oocyte complexes in the periovulatory follicle (325,339). Cspg2 null mice are embryonic lethal (340), so the physiological role of CSPG2 in cumulus expansion has not been determined. However, ADAMTS1 (a disintegrin-like and metallopeptidase with thrombospondin type 1 motif, 1) is a protease known to cleave CSPG2, and Adamts1 null females are subfertile with defects in preovulatory follicle development (341), including less extensive cumulus expansion (342).
Figure 6.
Hyaluronan-IαI-PTX3 interactions stabilize the cumulus matrix. GDF9 and BMP15 secreted by the oocyte stimulate cumulus cells to produce HAS2, TNFAIP6, and PTX3. HAS2 catalyzes the synthesis of hyaluronan (HA; curved line), the structural backbone of the cumulus matrix. Hyaluronan is covalently linked to the heavy chain of IαI (white box) by the catalytic activity of TNFAIP6. Multimers of PTX3 (black trapezoids) stabilize the hyaluronan matrix by interacting with IαI.
As previously mentioned, two functionally distinct granulosa cell populations exist in the antral follicle. Interestingly, the mural granulosa cells lining the follicle express high levels of LHCGR, whereas the cumulus cells surrounding the oocyte do not (at least in mice) (274,343). How then does the LH surge lead to induction of target genes in cumulus cells that are critical for cumulus expansion? Conti and colleagues (344) have shown that the LH surge causes a rapid increase in epidermal growth factor (EGF)-like family members, Areg, Ereg, and Btc (encoding amphiregulin, epiregulin, and betacellulin, respectively), specifically in mural granulosa cells of preovulatory follicles. These ligands are synthesized as integral membrane proteins and are released from the cell surface by proteolytic cleavage of the ectodomain. They then bind and activate EGF tyrosine kinase receptors (EGFRs). All three of these growth factors stimulate cumulus expansion and oocyte maturation in vitro in an EGFR-dependent manner. The effect of these EGF-like factors on cumulus expansion occurs through up-regulation of Ptgs2, Has2, and Tnfaip6 genes (344), whose products are essential for formation and stabilization of the extracellular matrix of the cumulus oophorus. Within 4 h of hCG stimulation, transcripts for Areg, Ereg, and Btc are also detected in cumulus-oocyte complexes, suggesting that an autocrine regulatory loop is established to maintain EGF-like growth factor expression in cumulus cells (325).
The in vitro findings have been verified by in vivo analysis in Areg−/− or Ereg−/− mice, which show reduced cumulus expansion in response to exogenous gonadotropins (345). Although Egfr−/− mice are embryonic lethal, mice with a hypomorphic Egfr allele (Egfrwa2) are viable, and Areg−/− Egfrwa2/wa2 double mutant females showed more profound defects in induction of Ptgs2, Has2, and Tnfaip6, cumulus expansion, and ovulation after gonadotropin administration (345). In support of an evolutionarily conserved role for EGF-like growth factors in cumulus expansion, amphiregulin is abundant in human follicular fluid obtained from patients undergoing in vitro fertilization (IVF) (346,347).
As mentioned previously, the LH surge leads to activation of MAPK signaling, which was first implicated in an in vitro culture assay to be an important mediator of cumulus expansion. The UO126 inhibitor of MAPK signaling prevented gonadotropin, EGF, and cAMP analog stimulation of cumulus expansion (321). Recently, Richards and colleagues (322) validated these findings in vivo. Binding of EGF-like growth factors to EGFR leads to activation of MAPK3 and MAPK1; also known as extracellular signal-regulated kinases 1 and 2 (ERK1/2), respectively. Both Mapk3 null mice and Mapk1 cKO mice [using granulosa cell-specific Cyp19a1-Cre (264)] are fertile. Mapk1 Mapk3 double mutant mice (Mapk1/3 dKO), however, are sterile with defects not only in cumulus expansion but also in ovulation, luteinization, and oocyte meiotic maturation (322). In response to hCG, granulosa cells normally stop dividing and terminally differentiate, resulting in decreased estradiol production and increased progesterone. However, when Mapk1/3 dKO mice were given hCG, granulosa cells continued to proliferate, and serum estradiol remained elevated because Cyp19a1 continued to be expressed at a high level, and Sult1e1, an estradiol-metabolizing enzyme (348), was not induced, whereas progesterone did not increase due to the lack of induction of Cyp11a1, Star, and other genes. Thus, MAPK signaling is a critical target and effector of several events triggered by the LH surge in preovulatory follicles. Of further interest, disruption of MAPK1/3 in the pituitary completely blocks the LH surge mode in females but has no effect on basal LH production, thereby rendering the females infertile, whereas the males are fertile (349).
Despite convincing in vitro and genetic evidence for EGF-like factors and MAPK signaling as mediators of cumulus expansion in response to the LH surge, the oocyte also has an obligatory role in this process. To circumvent the absence of LH receptors on cumulus cells, in vitro expansion of isolated cumulus-oocyte complexes relies on the addition of FSH, cAMP analogs, or EGF. Nearly 20 yr ago, two groups demonstrated that the oocyte is also required to achieve expansion. Microsurgical removal of the oocyte from isolated cumulus-oocyte complexes [referred to as oocytectomized (OOX) complexes] prevented cumulus expansion when FSH or EGF was added to the culture medium (323). Expansion of OOX complexes could be rescued by coculture with denuded oocytes or conditioned medium from denuded oocytes. A similar experiment in which cumulus cells were mechanically dissociated from oocytes and cultured separately showed that these cells failed to synthesize a mucinous matrix in response to FSH, but oocyte coculture or oocyte-conditioned medium restored matrix production (350). These experiments suggested that oocytes secrete a cumulus expansion-enabling factor (CEEF) that allowed oocytes to respond to FSH. Although follicular development in GDF9 knockout mice is arrested at the primary follicle stage, in vitro studies have identified critical functions for GDF9 in preovulatory granulosa cells and suggest that it may be the primary CEEF secreted by fully grown oocytes. Recombinant GDF9 up-regulates Has2, Ptgs2, Ptger2, Tnfaip6, and Ptx3 in granulosa cell culture systems (221,254). Using an RNAi approach, injection of mouse oocytes with Gdf9 double-stranded RNA, but not Bmp15 double-stranded RNA, resulted in lower Has2 and Ptgs2 expression as well as limited cumulus expansion when OOX cumulus complexes were cocultured with Gdf9 knockdown oocytes (351).
GDF9 signals through an unusual heterodimeric complex of a type I TGFβ family receptor, and the type II BMP receptor, BMPRII, to activate SMAD2/3 (352). In vitro and in vivo evidence suggests that cumulus expansion is dependent on SMAD2/3 signaling. The ALK4/5/7 inhibitor, SB-431542, prevents SMAD2/3 activation and cumulus expansion of OOX complexes cultured in the presence of FSH and GDF9 or oocytes (353), suggesting that one or more of these type I receptors is involved. Conditional deletion of Smad2 and Smad3 in granulosa cells using Amhr2-Cre (Smad2/3 cKO) disrupts cumulus expansion in vivo (354). Interestingly, although treatment of Smad2/3 cKO granulosa cells with GDF9 failed to induce Has2 and Ptx3, the other cumulus expansion-related transcripts, Ptgs2 and Tnfaip6, were attenuated but still up-regulated in response to GDF9. These results suggest that GDF9 may function through both SMAD2/3-dependent and SMAD2/3-independent pathways to regulate cumulus expansion. It is plausible that SMAD-independent signaling by GDF9 could involve activation of MAPK pathways because TGFβ family members have been shown to activate MAPK pathways (263), and activation of these pathways is required for cumulus expansion (321,322).
Although there is convincing evidence that GDF9 is sufficient to function as a CEEF, there is, however, controversy as to whether GDF9 is the sole CEEF (355). BMP15 has also been implicated in the regulation of cumulus expansion and up-regulates expression of EGF-like growth factors in cumulus cells in vitro (356). Although the fertility defects in Bmp15−/− mice are subtle and Gdf9+/− mice are phenotypically normal, Gdf9+/− Bmp15−/− double mutant mice on a 129SvEV inbred background are infertile with impaired cumulus expansion (230). Furthermore, coculture of Gdf9+/− Bmp15−/− oocytes with OOX cumulus complexes fails to enable cumulus expansion in the presence of FSH, and the cumulus cells have less activation of MAPK, suggesting that BMP15 acts synergistically with GDF9 as a CEEF (357). BMP15 is believed to signal through the type I activin receptor-like kinase ALK6 (also known as BMPR1B) to activate SMAD1/5/8 pathways (358). Bmpr1b−/− female mice are infertile secondary to defects in cumulus expansion, despite a paradoxical increase in Ptgs2 (359). Finally, BMP15 is found in the follicular fluid of patients undergoing IVF and is associated with increased fertilization and embryo development (360). Although a direct link between BMP15 and cumulus expansion in human cumulus-oocyte complexes was not made, other studies have associated higher levels of HAS2 and PTGS2 in human cumulus cells with higher quality embryos (361,362). Because both BMP15 and GDF9 are expressed in human cumulus-oocyte complexes (363), the relative contribution of each factor to human cumulus expansion and developmental potential remains to be determined. Studies in Bmp15−/− and Gdf9+/− Bmp15−/− mice, however, suggest that both growth factors influence developmental competence of oocytes after fertilization (357).
Cumulus expansion and ovulation are not mutually exclusive, as demonstrated by many of the mutant mouse models discussed thus far that have defects in both processes. Ovulation has been compared with an inflammatory-like process, based on the follicular hyperemia, large amount of prostaglandin production, and synthesis of the hyaluronan-rich extracellular matrix that occurs during this stage of follicular development (364). Indeed, many of the matrix-associated genes that are up-regulated in the follicle after the LH surge are also found at sites of inflammation.
A number of transcriptional regulators induced downstream of LH receptor activation are necessary for ovulation. After the LH surge, progesterone receptor (PR) is rapidly induced in the mural granulosa cells of the preovulatory follicle (365,366). Like ERα and ERβ, PR is a member of the nuclear receptor superfamily of transcription factors. Although progesterone has classically been associated with pregnancy, PR-deficient mice illustrate the importance of progesterone in ovulation as well (145). There are two PR isoforms, PR-A and PR-B, that arise from a single gene (Pgr) as a result of transcription from alternative promoters and translation initiation at two alternative start codons in the Pgr transcript (367). The original Pgr null mouse model eliminated production of either PR isoform, and females were infertile secondary to anovulation, despite cumulus expansion in unruptured follicles (145). CLs were absent; however, subsequent studies showed that granulosa cells in unruptured follicles expressed markers of ovulation and luteinization, including Ptgs2 and Cyp11a1, suggesting that the differentiation response of granulosa cells to the LH surge was intact in Pgr null mice (368). Selective ablation of each PR isoform by point mutation of each alternative start codon demonstrated that PR-A, but not PR-B, mediates the ovulatory response to the LH surge (369,370).
Although it is not entirely understood how PR regulates ovulation, misregulation of a number of gene targets downstream of PR has been identified (371). Genes for the Adamts1 and cathepsin L (Ctsl) proteases are down-regulated, and although only Adamts1 null mice have impaired ovulation (341), cathepsin L may function redundantly because a number of proteases are expressed in the periovulatory follicle and many individual knockout models are fertile (372). The vasoactive molecule endothelin 2 (Edn2) is induced in preovulatory follicles in a PR-dependent manner (373), and mice treated with endothelin receptor antagonists have a dramatic decline in ovulation secondary to impaired smooth muscle contraction that normally drives follicular rupture (374). Edn2 and another PR target gene, cGMP-dependent protein kinase (Prkg2) (375), may be indirectly regulated by PR through peroxisome proliferator-activated receptor γ (PPARγ, encoded by Pparg). Pparg is also decreased in Pgr null ovaries and conditional deletion of Pparg (Pparg cKO) in the periovulatory follicle using PR-Cre (376) caused subfertility secondary to impaired follicle rupture (377). A subset of PR target genes, including Edn2, Prkg2, and Il6, were decreased in Pparg cKO ovaries, and a PPARγ-specific antagonist, GW9662, markedly attenuated induction of these genes in cultured granulosa cells. Another PR target gene is Snap25, which regulates vesicle secretion and appears to be important for the release of potent cytokines from granulosa cells as part of the inflammatory and immune-like response of ovulation (364,378). Highlighting the diverse functions of PR downstream of the LH surge in the periovulatory period, components of many other signaling pathways, including EGF-like growth factors and members of the WNT signaling family, are also altered in PR-deficient mice (371).
Other transcriptional regulators that mediate the ovulatory response to the LH surge include C/EBPβ (CCAAT/enhancer-binding protein β, encoded by Cebpb) and several members of the nuclear receptor family (discussed in detail below). C/EBPβ is a member of a family of basic leucine zipper proteins that recognize similar DNA motifs and dimerize with themselves or other basic leucine zipper transcription factors to activate or repress gene transcription. Cebpb is strongly up-regulated in periovulatory follicles after hCG administration, and similar to Pgr null mice, Cebpb−/− females are sterile and their ovaries contain many unruptured follicles but lack CLs (379). Less is known about how C/EBPβ functions downstream of the LH surge during ovulation; however, both Cyp19a1 and inhibin α mRNAs fail to be down-regulated after hCG administration to Cebpb−/− females, suggesting that C/EBPβ may be an important transcriptional repressor during the granulosa cell differentiation process initiated by the LH surge (379,380).
Interestingly, the ovarian phenotype of Cebpb−/− females is similar to Mapk1/3 dKO females. In response to amphiregulin, C/EBPβ has been shown to induce expression of genes up-regulated in the periovulatory period, including, Ptgs2, Tnfaip6, Pgr, and Star, and the up- regulation of these genes is dependent on MAPK activity (322). However, conditional deletion of Cebpb in granulosa cells results in subfertility, and CLs are present in some adult ovaries, suggesting that C/EBPβ is not the only transcription factor downstream of MAPK signaling in granulosa cells (322).
Conditional deletion of two related members of the NR5A subfamily, liver receptor homolog 1 (LRH1, encoded by Nr5a2) and SF1 (encoded by Nr5a1) leads to infertility secondary to anovulation, despite distinct molecular and hormonal changes in each mutant (381,382). In Lrh1 cKO mice, there is increased CYP19A1 expression and enhanced follicular estradiol production secondary to a decrease in nitric oxide synthase 3 (NOS3), an inhibitor of CYP19A1 expression and activity in mice and humans (383,384). Chromatin immunoprecipitation analysis identified Nos3 as a direct target of LRH1 (381). Ptgs2 and Tnfaip6 were substantially reduced after hCG treatment, which may partially be due to the enhanced estradiol signaling because this has previously been shown to reduce PTGS2 in mice lacking the estradiol-metabolizing enzyme SULT1E1 (348). Finally, Lrh1 cKO mice also exhibited impaired progesterone synthesis secondary to a decrease in scavenger receptor B1 (Scarb1), Star, and Cyp11a1 after hCG administration (381).
Although Sf1 cKO mice are also anovulatory, adult ovaries are substantially smaller compared with wild-type ovaries, which was not readily apparent in Lrh1 cKO mice. In addition, despite similar basal serum estradiol levels in Sf1 cKO vs. control mice, the increase in estradiol and progesterone after PMSG was blunted in Sf1 cKO mice. Furthermore, in contrast to Lrh1 cKO mice, CYP19A1 was slightly decreased in Sf1 cKO mice, and there was no difference in Cyp11a1 in ovaries from untreated or PMSG-stimulated females (382). CYP11A1 did, however, appear to be ectopically expressed in the granulosa cells of large follicles, rather than only the thecal layer or CL. Although mRNA levels for other steroidogenic enzymes critical for progesterone synthesis were not reported, it appears that SF1 and LRH1 have unique roles in the regulation of some ovarian target genes.
Nuclear receptor interacting protein 1 (NRIP1; also known as RIP140), functions as a coregulator of the nuclear receptor superfamily. Nrip1−/− female mice are also infertile secondary to anovulation; however, the unruptured follicles in these mice go on to form CLs with trapped oocytes (385). More detailed histological analysis of Nrip1 null ovaries demonstrated defects in cumulus expansion, suggesting that NRIP1 is also an important mediator of this process, as well as ovulation. Gene expression profiling showed that after gonadotropin stimulation, Nrip1 null mice fail to induce many genes already discussed that are important for cumulus expansion, including EGF-like growth factors (Areg, Ereg, and Btc), Has2, Ptgs2, Tnfaip6, and Cspg2, whereas many genes involved in cell-cell adhesion are up-regulated and might, therefore, impede expansion. Nrip1 is most dramatically up-regulated in mural granulosa cells after PMSG administration, whereas Cebpb increases after PMSG plus hCG administration (379,380,385). Because both FSH and LH activate cAMP/PKA/cAMP response element-binding protein pathways to influence gene expression, the increase in NRIP1 and C/EBPβ in the periovulatory period may function to modulate transcription of the many target genes with cAMP response elements.
In contrast to evidence supporting a role for NRIP1 and C/EBPβ downstream of LH, the testicular orphan nuclear receptor 4 (TR4; also known as nuclear receptor subfamily 2, group C, member 2, Nr2c2) may be important in preparing preovulatory follicles for the LH surge. Nr2c2−/− mice (386) exhibit subfertility, with smaller ovaries containing fewer preovulatory follicles and CLs, as well as a muted response to superovulation protocols (387). These defects, however, appear to be secondary to a decrease in LH receptor. Furthermore, TR4 appears to regulate Lhcgr expression through direct binding to a TR4 response element in the Lhcgr promoter, suggesting that TR4 activity is important before the LH surge, perhaps to prepare granulosa cells to respond to the rise in LH that triggers ovulation.
After follicle rupture and release of the cumulus-oocyte complex, the final fate of the remaining granulosa and thecal cells is terminal differentiation to form the CL, a highly differentiated endocrine structure responsible for secreting progesterone to stimulate the uterus and maintain pregnancy. The processes controlling the formation, function, and regression of the CL are complex and have recently been reviewed in this journal (388) and elsewhere (389). Therefore, we will focus on the key regulators of CL formation and maintenance.
The process of CL formation requires the granulosa cells to exit the cell cycle. Although FSH is critical for promoting granulosa cell proliferation through up-regulation of cyclin D2, the LH surge, via obligatory MAPK1/3 activation (322), causes cell cycle arrest by shifting the balance of cell cycle regulators to favor inhibitors of cell cycle progression, such as the CDK inhibitor p27Kip1. In particular, using a rat model system, cyclin D2 is rapidly and robustly down-regulated in the preovulatory follicle after hCG administration (390). Although there is also a transient decrease in p27Kip1, by 24 h after hCG, p27Kip1 is markedly increased in terminally differentiated luteal cells. An additional CDK inhibitor, p21Cip1 (CDKN1A), is induced within 2–4 h of hCG administration. Loss of function studies suggest that p27Kip1 is the principal regulator of luteinization, although p21Cip1 likely has a cooperative role. Cdkn1b−/− female mice are sterile, and their ovaries exhibit granulosa cell hyperplasia and impaired differentiation to form CLs (391,392,393). In further support of a luteinization defect, ovulation in response to exogenous gonadotropins is unimpaired, but pregnancy is not maintained (391). Although Cdkn1a−/− female mice are fertile (394,395), in contrast to observations in Cdkn1b−/− mice, their ovaries are not enlarged compared with wild-type ovaries. Cdkn1b−/− Cdkn1a−/− ovaries were even larger than Cdkn1b−/− ovaries and had more profound granulosa cell hyperproliferation, suggesting that p27Kip1 and p21Cip1 function synergistically to promote cell cycle exit in differentiating granulosa cells (396).
An additional cell cycle regulator, CDK4, is indirectly involved in maintaining the CL once it has formed. Cdk4−/− female mice (397,398) are infertile because of insufficient progesterone production by the CL to allow embryo implantation. This is due to a failure of pituitary lactotropes to proliferate and produce prolactin (PRL) (399). Although progesterone is considered the hormone of pregnancy, in rodents PRL has a critical role in CL maintenance and progesterone production. Both isoforms of the PRL receptor (PRLR) are up-regulated during luteinization in an LH-dependent manner (400). Prlr−/− mice are sterile secondary to insufficient progesterone production to support implantation and pregnancy (401). A more detailed characterization of Prlr−/− ovaries revealed that CLs initially form, but they were disorganized, failed to show signs of neovascularization, and had increased apoptosis (402). Although Cdkn1b levels increased as expected in Prlr−/− ovaries at 1.5 d post-coitus (dpc), they were not maintained at elevated levels by 2.5 dpc, which may be attributed to a relative decrease in Lhcgr expression over the same time period. Transcripts for numerous enzymes involved in steroidogenesis that are known to be induced by LH, including Star, Cyp11a1, and Hsd3b1, were also reduced. Conversely, Prlr−/− ovaries showed an increase in the progesterone-metabolizing enzyme 20α-hydroxysteroid dehydrogenase (AKR1C18, aldo-keto reductase family 1, member C18) in regressing CLs. Although their ovaries were not examined, Prl−/− mice are also infertile with an impaired pseudopregnancy response and irregular estrous cycles (403). PRL signaling activates a Janus kinase/signal transducer and activation of transcription (STAT) pathway, specifically STAT5A/B. Stat5a−/− Stat5b−/− mice are also infertile, and similar to Prlr−/− mice, their ovaries show reduced Cdkn1b and increased AKR1C18 (404). These mouse models suggest that PRL is important in maintaining CLs and progesterone production through positive regulation of LH receptor and negative regulation of AKR1C18. In women, hCG secretion by the embryonic trophoblast serves a function analogous to PRL in sustaining the CL during early pregnancy (389).
What might regulate PRLR to prevent premature regression of CLs? Components of the WNT/FZD signaling pathways, including Wnt4, Fzd1, and Fzd4, are highly expressed in periovulatory follicles, and Fzd4 is particularly increased in CLs. The functional significance of this is demonstrated by Fzd4−/− mice, which are infertile with histological and molecular defects in their ovaries that are quite similar to Prlr−/− mice (405). Moreover, Prlr was also reduced at 1.5 and 5.5 dpc, suggesting that WNT signaling is necessary for cells of the CL to be able to respond to PRL. It is also possible that PTEN impacts the response of cells to PRL because in Ptenflox/flox Cyp19a1-Cre mice that have a conditional deletion of Pten in granulosa/luteal cells, the life span of CLs is extended (406).
The timing of luteinization is important for normal reproductive function, and the fact that it occurs after ovulation supports a role for oocyte-derived inhibitors that prevent premature luteinization. As mentioned, oocyte-secreted factors oppose FSH signaling to define two distinct granulosa cell populations in the antral follicle. Specifically, oocyte-secreted proteins signal through Smad2/3 to oppose FSH induction of ovulatory (Lhcgr) and steroidogenic (Cyp11a1) markers in the cumulus cells close to the oocyte (407), suggesting that a TGFβ family member, such as GDF9, may be an important inhibitor of luteinization. Support for this is seen in mice with conditional deletion of the common SMAD, Smad4, in granulosa cells of preantral follicles (408). Although Smad4 cKO ovaries exhibit a number of follicular defects, including impaired cumulus expansion and ovulation, there were a number of small and large follicles with luteinizing cells surrounding oocytes, and these findings were accentuated after administration of PMSG alone in 3-wk-old mice. Molecularly, in contrast to control mice, granulosa cells from PMSG-stimulated Smad4 cKO mice had significantly higher levels of genes related to steroidogenesis and luteinization, including Lhcgr, Cyp11a1, Hsd17b7, Star, secreted frizzled-related protein (Sfrp4), and the prostaglandin F receptor (Ptgfr). Although it is also possible that the loss of SMAD4 prevents granulosa cells from responding appropriately to BMP/TGFβ/activin signals from the surrounding stroma that might also inhibit luteinization, Eppig and colleagues (407) provide convincing evidence that activation of SMAD signaling by oocyte-secreted factors is key to preventing premature differentiation of granulosa cells.
Although we have tried to highlight critical mediators of ovulation, cumulus expansion, and luteinization, many additional factors are involved in these processes in the periovulatory follicle. Mutant mouse models illustrating the diverse number of factors that regulate these events are summarized in Table 9.
Table 9.
Mouse models with defects in the periovulatory period
| Gene | Reproductive phenotype | Fertility status | Ref. |
|---|---|---|---|
| Cumulus expansion and ovulation
|
|||
| LH β (Lhb) | Block in folliculogenesis at the early antral stage | Infertile | 318 |
| LH/choriogonadotropin receptor (Lhcgr) | Block in folliculogenesis at the early antral stage | Infertile | 319,320 |
| Prostaglandin-endoperoxide synthase 2 (Ptgs2; Cox2) | Defects in ovulation, cumulus expansion, and implantation | Mostly infertile | 329,330 |
| Prostaglandin E receptor 2, subtype EP2 (Ptger2) | Defects in ovulation and cumulus expansion; decreased fertilization and preimplantation defects | Subfertile | 331,332,333 |
| Pentraxin 3 (Ptx3) | Defects in cumulus-oocyte complex integrity and ovulation | Subfertile | 254,804 |
| TNFα−induced protein 6 (Tnfaip6) | Impaired cumulus matrix formation resulting in failed cumulus-oocyte complex expansion | Infertile | 334 |
| CCAAT/enhancer-binding protein β (Cebpb) | Reduced ovulation; fail to form CL | Infertile | 379 |
| IL-6 signal transducer (Il6st; gp130) | Defect in oocyte maturation/ovulation | Subfertile | 805 |
| LFNG O-fucosylpeptide 3-β-N-acetylglucosaminyltransferase (Lfng; lunatic fringe homolog) | Multiple defects in folliculogenesis; luteinized follicles with trapped oocytes; defect in meiotic maturation of oocytes | Infertile | 149 |
| Nitric oxide synthase 3, endothelial cell (Nos3; eNos) | Compromised ovulation, delayed meiotic progression from metaphase I | Subfertile | 806 |
| Nuclear receptor interacting protein (Nrip1; RIP40) | Ovulation defect; ovaries accumulate luteinized and unruptured follicles | Infertile | 385 |
| Nuclear receptor subfamily 2, group C, member 2 (Nr2c2; TR4) | Small ovaries with few preovulatory follicles and CLs; decreased ovulation | Variable lethality; subfertile | 386,387 |
| 2′-5′ Oligoadenylate synthetase 1D (Oas1d) | Defects in folliculogenesis and ovulatory efficiency | Subfertile | 807 |
| Phosphodiesterase type 4, cAMP specific (Pde4d) | Impaired ovulation; luteinized follicles with trapped oocytes | Subfertile | 808 |
| Progesterone receptor (Pgr; PR) | Anovulation; CLs absent | Infertile | 145 |
| Sirtuin 1 (Sirt1; SIR2a) | Small ovaries; anovulation; CLs absent | Variable lethality; extremely subfertile | 809 |
| Sulfotransferase family 1E, member 1 (Sult1e1) | Abnormal ovulation and cumulus expansion | Subfertile | 348,810 |
| Transformation-related protein 73 (Trp73) | TAp73 isoform deficiency; defective ovulation, oocyte meiotic defects, and maternal effects | Variable lethality; infertile | 791 |
| Y box protein 2 (Ybx2; Msy2) | Follicular atresia, oocyte loss, anovulation | Infertile | 811 |
| Peroxisome proliferator activated receptor γ (Pparg) (cKO) | Impaired ovulation | Subfertile | 377 |
| α1 Microglobulin/bikunin (Ambp) | Defects in ovulation and cumulus-oocyte complex integrity | Subfertile | 337,338 |
| Amphiregulin (Areg) | Suppressed cumulus expansion, delayed meiotic reentry | Subfertile | 345 |
| Bone morphogenetic protein 15 (Bmp15) | Defects in cumulus expansion and ovulation | Subfertile | 230 |
| Bone morphogenetic protein receptor, type IB (Bmpr1b) | Defects in estrous cyclicity, cumulus expansion, and endometrial gland development | Subfertile | 359 |
| Epiregulin (Eregwa2/wa2; hypomorph) | Suppressed cumulus expansion, defective meiotic resumption, ovulation defect | Subfertile | 345 |
| MAPKs 3 and 1 (Mapk3−/−Mapk1 cKO) | Defects in cumulus expansion and oocyte meiotic maturation; anovulation and absent CLs | Infertile | 322 |
| Nuclear receptor subfamily 5, group 2, member 1 (Nr5a1; Sf1, steroidogenic factor 1) (cKO) | Small ovaries with few resting and growing follicles; defects in estrous cyclicity, cumulus expansion, and ovulation; CLs absent | Infertile | 382 |
| Nuclear receptor subfamily 5, group 2, member 2 (Nr5a2; Lrh1, liver receptor homolog 1) (cKO) | Defects in estrous cyclicity, cumulus expansion, and ovulation; CLs absent | Infertile | 381 |
| Phospholipase C-β1 (Plcb1) | Decreased ovulation with trapped oocytes; abnormal mating behavior | Subfertile | 812 |
| Corpus luteum formation | |||
| Cyclin-dependent kinase 4 (Cdk4) | Defects in the hypothalamic-pituitary-gonadal axis disrupts maintenance of CL during pregnancy | Infertile | 397,398 |
| (Continued) | |||
Table 9A.
(Continued)
| Gene | Reproductive phenotype | Fertility status | Ref. |
|---|---|---|---|
| Cyclin-dependent kinase inhibitor 1B (Cdkn1b; p27Kip1) | Corpus luteum differentiation failure and granulosa cell hyperplasia | Infertile | 391,392,393 |
| Early growth response 1 (Egr1; NGFI-A); (targeted lacZ insertion) | Down-regulation of LH receptor, not remedied with gonadotropin treatment | Infertile | 813 |
| Early growth response 1 (Egr1; NGFI-A); (targeted neo insertion) | LH insufficiency, loss of estrous cyclicity, no CLs; rescued by treatment with gonadotropins | Infertile | 814 |
| Aminopeptidase puromycin-sensitive (Npepps; Psa) | Lack of CL formation and PRL production causes early pregnancy loss | Infertile | 815 |
| Prostaglandin F receptor (Ptgfr) | Do not undergo parturition, failed luteolysis | Infertile | 816 |
| Prolactin (Prl) | Irregular estrous cycles and impaired pseudopregnancy response | Infertile | 403 |
| Prolactin receptor (Prlr) | Compromised ovulation, fertilization, and preimplantation development in of Prlr null embryos | Infertile | 401,402 |
| MAD homolog 4 (Smad4) (cKO) | Premature luteinization; decreased ovulation with trapped oocytes | Subfertile | 408 |
Although many genes are critical for multiple events during this period, mouse models are categorized based on the principal findings.
G. Regulation of meiotic arrest and reentry
Oocytes start meiosis in the embryonic gonad and progress through the diplotene stage of the first meiotic prophase. Meiosis is arrested by E14.5 in mouse oocytes and does not resume until the LH surge, when a number of changes take place in the somatic cells and the oocyte to facilitate meiotic maturation. The resumption of meiosis can be recognized morphologically by dissolution of the nuclear envelope, referred to as germinal vesicle breakdown (GVBD).
In the course of follicular development, oocytes acquire the competence to resume meiosis such that about the time of antrum formation they have synthesized a threshold level of maturation-promoting factor (MPF), which is a complex consisting of CDK1 and cyclin B that regulates the G2/M transition of the cell cycle in mitosis and meiosis (409,410). However, MPF promotes meiosis, so to maintain arrest at prophase I until the LH surge, MPF must be kept in an inactive state. It has been known for decades that once mammalian oocytes attain meiotic competence, they rely on the follicular milieu to prevent premature resumption of meiosis. Removal of the oocyte from antral follicles causes spontaneous maturation, as evidenced by GVBD (411,412). Importantly, this inhibitory signal appears to originate from mural granulosa cells because the oocytes were isolated with their surrounding cumulus cells. Likewise, the requirement of high levels of cAMP in the oocyte to maintain meiotic arrest has been dogma for many years, and a decrease in oocyte cAMP levels is associated with the resumption of meiosis (reviewed in Refs. 409 and 410). For years, however, two questions remained unanswered: how does high cAMP maintain meiotic arrest, and how are cAMP levels controlled within the oocyte?
The net effect of elevated cAMP is activation of PKA signaling pathways that keep MPF in its inactive state, and although the targets of PKA were postulated to be regulators of CDK phosphorylation (409), whether this was by direct or indirect means has only recently been established. CDK1 is negatively regulated through phosphorylation on Thr14 and Tyr15. The WEE1/MYT1 protein kinases phosphorylate and inactivate CDK1, whereas CDC25 phosphatases activate CDK1 by dephosphorylating Thr14 and Tyr15 (413). Using a novel strategy to isolate PKA substrates involved in regulating meiotic maturation, Conti and colleagues (414) performed a small pool expression screen of a mouse oocyte cDNA library and identified Wee2 (WEE1 homolog 2; Wee1b). In vitro assays showed that PKA could phosphorylate WEE2, and in vivo reduction of WEE2 by the generation of transgenic mice expressing a short hairpin RNA (shRNA) corresponding to Wee2 induced GVBD in about 25% of oocytes recovered after PMSG stimulation, despite genetic [i.e., in Pde3a−/− oocytes (415), see below] or chemical maintenance of high cAMP conditions. In vitro injection of Wee2 shRNA showed a similar rate of GVBD in the presence of high cAMP. That not all oocytes in which Wee2 was knocked down underwent GVBD suggests that either compensatory WEE1/MYT1 family members may function in the oocyte, or the high cAMP conditions prevented CDC25 phosphatases from activating MPF. Although all three CDC25 phosphatase family members (CDC25A, CDC25B, and CDC25C) are expressed in mouse oocytes (416), only CDC25B appears to be essential for activating CDK1. Cdc25b null mice are sterile, and their oocytes cannot activate CDK1 to resume meiosis, but the meiotic arrest defect is rescued by microinjection of Cdc25b mRNA (417). Cdc25c−/− mice are fertile (418), and although Cdc25a−/− mice are embryonic lethal (419), RNAi experiments suggest that CDC25A may have a supportive role in meiotic resumption (420). CDC25 was first identified as a PKA substrate in Xenopus oocytes. Duckworth et al. (421) demonstrated that PKA phosphorylates CDC25C, leading to binding and sequestration in the cytoplasm by 14-3-3. The signal to resume meiosis (in this case, progesterone) led to dephosphorylation of CDC25, followed by dephosphorylation and activation of CDK1. Two groups have recently shown that similar regulation of CDC25B by PKA occurs in mouse oocytes (422,423), thus establishing that PKA directly targets CDC25B for cytoplasmic sequestration in prophase-arrested oocytes. Hence, despite acquiring meiotic competence by the early antral follicle stage, high cAMP concentrations in the oocytes of preovulatory follicles keep PKA active, thereby inhibiting the CDK1 activator, CDC25B, and activating the CDK1 inhibitor, WEE2.
What controls cAMP levels upstream of PKA? Early studies suggested that oocytes have an endogenous means by which they control intracellular cAMP homeostasis. Inhibitors of cAMP phosphodiesterases (PDEs), such as 3-isobutyl-1-methylxanthine (IBMX), prevent metabolism of cAMP and therefore spontaneous maturation of isolated oocytes (424). Stimulation of ACs or heterotrimeric G proteins that activate ACs with forskolin or cholera toxin, respectively, prevents or attenuates meiotic resumption, and this effect is potentiated in the presence of small concentrations of IBMX (425,426). Diffusion of cAMP from granulosa cells to the oocyte by gap junctions has been proposed as a second means by which meiotic arrest is maintained (409,410). Work in recent years has solidified the hypothesis that cAMP homeostasis in the oocyte is under the control of endogenous factors; however, there also appears to be a role for gap junctional communication between follicular cells in the regulation of meiotic arrest.
After initially establishing a role for stimulatory G proteins (GαS) (427) within the oocyte in maintaining meiotic arrest, Mehlmann et al. (428) searched an expressed sequence tag database from a prophase I-arrested oocyte cDNA library and identified the orphan G protein-coupled receptor, GPR3, as a candidate activator of GαS. GPR3 was particularly interesting because of its constitutive activation of AC activity when overexpressed in HEK293 cells (429). The majority of oocytes within antral follicles of Gpr3−/− females demonstrate premature resumption of meiosis before the LH surge (428,430). As a consequence, Gpr3−/− females are subfertile and develop POF (430). Taking advantage of the turnover properties of GαS and a green fluorescent protein-linked GαS molecule, GPR3 was shown to directly activate GαS (431). The putative AC downstream of GPR3/GαS in the oocyte was determined using a degenerate cloning strategy. AC3 (Adcy3) was identified as the predominant oocyte AC in rats, whereas AC1 (Adcy1) and AC9 (Adcy9) were most frequently amplified in mouse oocytes (432). However, AC3 was also abundant in mouse oocytes, and although most Adcy3−/− mice died soon after birth (433), those females that reached maturity exhibited reduced fertility and fecundity. Histologically, Adcy3−/− ovaries showed premature meiotic resumption in about 50% of the oocytes (432). The incomplete penetrance of this phenotype could be due to the other ACs present in oocytes. Nevertheless, there is strong evidence that GPR3 signals through GαS to activate an AC that keeps cAMP elevated before the rise in LH.
If GPR3 is truly a constitutively active G protein-coupled receptor, how is the cAMP signal terminated to allow activation of MPF? In in vitro follicle culture, LH does not change the localization of GαS from the oocyte plasma membrane to the cytoplasm, suggesting that GPR3/GαS signaling is not inhibited in response to LH. The other ways to regulate the cAMP signal could be increasing cAMP turnover or targeting the cAMP effector PKA. PDEs catalyze the hydrolysis of cAMP, and they appear to have an evolutionarily conserved function in vertebrate oocytes. PDE3-specific inhibitors block meiotic resumption and oocyte maturation in Xenopus oocytes and cultured murine and human oocytes (434,435,436,437). Although there are two PDE3 isoforms, PDE3A and PDE3B, PDE3A appears to be the major form expressed in mammalian oocytes (435,436) and an essential function for PDE3A in vivo is seen in Pde3a−/− female mice, which are sterile because ovulated oocytes remain arrested in the germinal vesicle (GV) stage of meiosis I (415). Inhibition of PKA, however, restored meiotic maturation in Pde3a−/− oocytes in vitro. These studies indicated that one means by which oocytes resume meiosis is through PDE3A-mediated cAMP hydrolysis, leading to a reduction in PKA activity.
PKA activity can also be mediated by localization away from substrates by A-kinase anchoring proteins (AKAPs). AKAP1 localizes primarily to the mitochondria in wild-type oocytes; whereas the RIIα PKA subtype is primarily cytoplasmic in GV-stage oocytes, in meiosis II (MII) oocytes, AKAP1 and RIIα PKA colocalize to the mitochondria (438). Akap1−/− females are severely subfertile with ovulated oocytes that remain arrested in the GV-stage and maintain a cytoplasmic distribution of RIIα PKA. This suggests that upon receiving the signal to resume meiosis, PKA is sequestered to the mitochondria by AKAP1, allowing meiotic maturation to proceed. There also appears to be an additional unidentified AKAP that facilitates the cytoplasmic distribution of PKA in GV-stage oocytes. Injecting an inhibitor (HT31) that disrupts PKA/AKAP interactions into GV-stage oocytes caused GVBD, despite maintaining high levels of cAMP with the PDE inhibitor IBMX. Taken together, the resumption of meiosis after a rise in LH is facilitated by at least two mechanisms that reduce PKA activity: a reduction in cAMP by PDE3A, and redistribution of PKA away from its targets by AKAP1. The mediators of this critical stage in oocyte development are summarized in Table 10.
Table 10.
Mouse models with defects in meiotic arrest or reentry
| Gene | Reproductive phenotype | Fertility status | Ref. |
|---|---|---|---|
| G protein-coupled receptor 3 (Gpr3) | Abnormal resumption of meiosis in oocytes | Subfertile | 428,430 |
| Adenylate cyclase 3 (Adcy3) | Premature meiotic resumption in 50% of oocytes | Subfertile | 432 |
| Phosphodiesterase 3A, cGMP inhibited (Pde3a) | Presence of trapped oocytes; failure of ovulated oocytes to progress beyond GV stage | Infertile | 415 |
| Cell division cycle 25 homolog B (Cdc25b) | Oocytes are arrested in meiotic prophase with defects in MPF activity | Infertile | 417 |
| Moloney sarcoma oncogene (Mos) | Parthenogenetic activation, cysts, and teratomas | Subfertile | 453,454 |
A second path to meiotic resumption after the LH surge appears to involve the regulation of gap junctions. Although it is clear that oocytes are capable of endogenous cAMP production, it is unclear whether somatic cells also contribute cAMP to the oocyte by way of gap junctions, and that the two sources of cAMP are required for preventing premature MPF activation. The disruption of gap junctions with chemical inhibitors has demonstrated that they are important in preventing meiotic maturation (439). It is known that oocyte maturation depends on cumulus cell MAPK activity (321,322), and Jaffe and colleagues (440) recently demonstrated that LH causes a rapid MAPK-dependent phosphorylation and closure of the CX43 gap junctions between the follicular somatic cells, whereas the CX37 junctions between oocytes and cumulus cells remain open. Importantly, inhibition of MAPK activation (using low doses of the U0126 MAP Kinase Kinase inhibitor) prevented LH-induced channel closure, but not GVBD in response to LH, suggesting that gap junction closure is one of two redundant mechanisms by which LH signaling causes reentry into meiosis.
There are still a number of unanswered questions regarding the reinitiation of meiosis I, namely, how is the LH signal to mural granulosa cells propagated to the oocyte to allow maturation? What are the molecules that pass between gap junctions to maintain arrest? The EGF-like growth factors that are critical for ovulation and cumulus expansion also appear to be involved in promoting oocyte maturation in vitro and in vivo (344,345). However this is likely an indirect effect because although they induce oocyte maturation in cumulus-oocyte complexes, they do not have an effect on denuded oocytes (344,441). Steroids are known to influence meiotic maturation in lower vertebrates and have been implicated as mediators downstream of EGFR activation in cultured mouse cumulus-oocyte complexes. EGFR activation up-regulates steroid production, and with the exception of ERα, the other classical steroid receptors (AR, ERβ, PR) are detected in mouse oocytes, and treatment with their respective ligand agonists induced oocyte maturation (442). However, the physiological significance of steroid receptors in mammalian oocyte maturation remains to be determined, and maturation defects have not been reported in Ar, Esr2, or Pgr null oocytes. Moreover, others have reported inhibitory effects of steroids on oocyte maturation (Refs. 443 and 444; and reviewed in Ref. 410). Follicular fluid-meiosis-activating sterol (FF-MAS), first isolated from human follicular fluid (445), has also been proposed as an inducer of oocyte maturation; however, the timing of the rise in FF-MAS in vivo appears later than the onset of GVBD, and FF-MAS may be more important in the meiosis I to metaphase II transition (reviewed in Ref. 410).
In addition to the possibility that cAMP passes through gap junctions into the oocyte, cGMP has been proposed as a competitive inhibitor of PDE3A that enters the oocyte through gap junctions (446). Although inhibiting gap junctional communication did not alter PDE3 activity, the methods for quantifying PDE3 activity may have diluted cGMP, thereby preventing competitive inhibition during in vitro assays (439,440). Intraoocyte cGMP levels decrease during spontaneous maturation of rat oocytes, microinjection of cGMP prevents resumption of meiosis (447), and inhibiting guanylate cyclase activity in rat ovarian follicles induces oocyte maturation (448). More sensitive Förster resonance energy transfer-based cyclic nucleotide assays have recently been used to quantify cAMP and cGMP levels in follicle-enclosed mouse oocytes (449). Exposure of these follicles to LH resulted in a decrease in cGMP in both the oocyte and somatic cells, as well as a decrease in oocyte cAMP. The decrease in cGMP occurred by both gap junction-dependent and -independent mechanisms, resulting in increased PDE3A activity and a corresponding decrease in cAMP, followed by GVBD. Thus, although much work remains to identify the intermediate steps from the LH surge and the elusive in vivo inducer of oocyte maturation, the numerous mouse models generated in recent years have established a role for intra-oocyte regulation of cAMP homeostasis as essential for meiotic arrest and reentry. Likewise, there is sufficient evidence that gap junctional communication throughout the somatic compartment and between somatic cells and oocytes is also important.
After activation of MPF and reentry into meiosis, chromosomal condensation and segregation occurs, the nuclear envelope breaks down, and asymmetric division of the cytoplasm results in extrusion of the first polar body. Unlike mitosis, there is no intervening S-phase, and instead, meiosis progresses to a second division to reduce the genome from diploid to haploid. Whereas maintaining MPF in an inactive stage until the LH surge keeps oocytes arrested in prophase I, the activity of a cytostatic factor (CSF) (450) causes metaphase II arrest in the oocyte by stabilization of MPF, and the completion of MII does not occur until fertilization. CSF stabilizes MPF by inhibiting the anaphase-promoting complex/cyclosome (APC/C), an E3 ubiquitin ligase that targets cyclin B for 26S proteosomal degradation to allow the metaphase-anaphase transition (451). Although the existence of CSF was proposed in 1971 (450), it took almost two decades to identify MOS, a serine-threonine kinase, encoded by the Moloney sarcoma oncogene (Mos), as a critical component of CSF, first in Xenopus laevis oocytes (452), then in the mid-1990s in mouse oocytes. Disruption of the mouse Mos gene caused female subfertility, and Mos knockout oocytes underwent parthenogenetic activation secondary to a failure of metaphase II arrest (453,454). MOS may also have a function in the transition to MII because emission of the first polar body is delayed (454) and it is enlarged, which appears to result from failure of the spindle apparatus to translocate to the cortex, resulting in an altered cleavage plane. The enlarged polar bodies frequently went through another round of division, rather than degrading, which is the normal fate in wild-type oocytes (455). Although MOS satisfies the criteria of a CSF component (450), how it functions to inhibit APC/C activity in mammalian oocytes is currently unknown.
More recently, endogenous meiotic inhibitor 2 (EMI2; also known as F-box protein 43, FBXO43) has been identified as an additional protein with CSF activity in Xenopus eggs (456) and mouse oocytes (457). FBXO43 functions by inhibiting the APC/C, which in mouse oocytes may bethrough direct interaction with the APC/C activator CDC20 (457). RNAi-mediated knockdown of Fbxo43 in mouse oocytes causes progression through the cell cycle without arresting at metaphase II and results in aberrant cytokinesis. Expressing a stable form of FBXO43 and treating oocytes with a chemical inducer of parthenogenesis (SrCl2) resulted in stable MII arrest. Alternatively, knocking down both Fbxo43 and its presumed target, Cdc20, maintains meiotic arrest. Moreover, knockdown of Cdc20 alone prevents egg activation after SrCl2 treatment or sperm head injection (457). Thus, FBXO43 also has CSF activity that is manifest through inhibition of CDC20, thereby preventing APC/C activation to allow degradation of cyclin B and progression through the cell cycle. FBXO43 has also been implicated in the entry into MII by stabilizing cyclin B in interkinesis to allow formation of a metaphase II spindle (458).
Because both MOS and FBXO43 have CSF activity and FBXO43 specifically inhibits APC/C activity, could FBXO43 be the missing link between MOS and metaphase II arrest? Although Xenopus eggs have been a good model system for studying MOS signaling pathways, there appear to be differences in MOS targets in mammals (459). MOS is an activator of MAPK-signaling pathways, and in Xenopus, MOS activation of MAPK leads to activation of the 90-kDa ribosomal protein S6 kinase (p90RSK) (460,461), which was recently shown to phosphorylate Xenopus FBXO43 on Ser335/Thr336, causing increased stability and activity, thereby contributing to metaphase II arrest (462,463). However, p90RSK does not appear to be the downstream mediator of MOS in maintaining metaphase II arrest because oocytes from triple Rsk null mice (Rsk1, Rsk2, Rsk3) have a stable CSF arrest, and injection of constitutively active Rsk1 or Rsk2 RNA into Mos−/− oocytes does not restore MII arrest (464). Thus, in mammals, a direct linear pathway connecting MOS activation of MAPK signaling to phosphorylation and stabilization of FBXO43 cannot currently be drawn. Generation of Fbxo43−/− mice or overexpression of a stable FBXO43 protein in Mos−/− oocytes may help clarify any connection between FBXO43 and MOS as components of CSF.
The oocyte completes the final stages of meiosis after fertilization. The signal to resume meiosis comes from the sperm, which introduces phospholipase C ζ into the oocyte cytoplasm (465), triggering calcium oscillations that activate APC/C, leading to cyclin B degradation and the completion of meiosis (466). Thus, after being formed in the embryo and nurtured postnatally by granulosa cells as it progressed through multiple stages of folliculogenesis, the oocyte’s journey ends with fertilization, and the cycle begins anew.
IV. Ovarian Cancer
Ovarian cancer is the most lethal gynecological malignancy in the United States and the fifth leading cause of cancer death in women, with an estimated 21,650 new cases and 15,520 deaths in 2008 (467). The 5-yr survival rate for women diagnosed with cancer localized to the ovary exceeds 90%; however, nearly 70% of cases are diagnosed after the cancer has metastasized to distant sites, and the 5-yr survival rate for these patients is only 30%. These statistics reflect the lack of an effective screening test for early stage diagnosis and highlight difficulties in the successful treatment of advanced-stage disease. In the following sections, we discuss the three major classes of ovarian cancer: epithelial, sex cord-stromal, and germ cell.
A. Epithelial ovarian cancer
1. Origins and genetics
Approximately 90% of ovarian cancers are epithelial (carcinomas) and are classified by their histological features as serous (50% of ovarian cancers), endometrioid (20%), mucinous (10%), clear cell (5%), transitional, mixed, and undifferentiated (468,469,470). These cancers are thought to arise from the ovarian surface epithelium (OSE), the single layer of squamous-to-cuboidal epithelial cells that comprises the ovarian surface (reviewed in Ref. 471). Unlike most adult epithelia, the OSE has both epithelial and mesenchymal characteristics that are advantageous for participating in ovarian rupture and repair through repeated ovulatory cycles. These functions may also increase the susceptibility of OSE to genetic mutation and malignant transformation (472,473,474). Putative precursor lesions are hypothesized to originate in OSE-lined clefts and inclusion cysts in the ovarian cortex, two irregular morphological structures that become more prevalent with age (470,471,475). In the progression to malignancy, the OSE differentiates and resembles the lining of different Müllerian duct-derived regions of the female reproductive tract (471). For example, serous, endometrioid, and mucinous ovarian carcinomas are histologically similar to normal epithelia of the fallopian tube, endometrium, and endocervix, respectively. One decade ago, this observation raised an alternative hypothesis that epithelial ovarian cancers do not develop from metaplastic changes in the OSE but instead arise directly from the fallopian tube and secondary Müllerian system, which is composed of microscopic paraovarian and paratubal structures lined by Müllerian epithelium, namely endosalpingiosis, endometriosis, and endocervicosis (476,477). Although this hypothesis has not gained widespread approval, recent studies suggest that some tumors diagnosed as serous “ovarian” carcinoma more likely originated in the distal fallopian tube and then implanted on the ovarian surface, particularly in women with BRCA1 (breast cancer 1) or BRCA2 (breast cancer 2) mutations (478,479,480). Additionally, the association between endometriosis and ovarian carcinomas of the endometrioid and clear cell subtypes is well documented (reviewed in Refs. 481,482,483).
The different histological subtypes and grades of epithelial ovarian cancer are genetically distinct disease entities with unique defects in specific signaling pathways (reviewed in Refs. 470 and 484). Approximately 50–80% of high-grade serous carcinomas demonstrate mutations in the tumor suppressor p53 (470,484). On the other hand, low-grade serous carcinomas and serous borderline tumors frequently have mutations in KRAS, BRAF, or ERBB2 that constitutively activate MAPK signaling and lead to abnormal regulation of downstream target genes, including overexpression of cyclin D1 (470,484,485). The rarity of KRAS, BRAF, or ERBB2 mutations in high-grade serous carcinomas has challenged the dogma that these tumors naturally progress from low-grade serous carcinomas. A growing body of molecular evidence now supports the hypothesis that benign serous cystadenomas transform into serous borderline tumors that are the likely precursors of low-grade serous carcinomas, but that high-grade serous carcinomas develop independently and more rapidly from unknown precursors that undergo distinct genetic alterations. Similar to high-grade serous carcinomas, the majority of high-grade endometrioid ovarian cancers have p53 mutations and arise from unidentified precursor lesions (470,484). In contrast, low-grade endometrioid carcinomas may come from endometrioid borderline tumors with endometriosis as the likely precursor. Low-grade endometrioid carcinomas are characterized by aberrant activation of the canonical WNT signaling pathway, specifically through activating mutations in β-catenin and less commonly through inactivating mutations in negative regulators of β-catenin such as APC, AXIN1, and AXIN2 (470,484). Deregulation of the PI3K signaling pathway has also been described secondary to activating mutations in PIK3CA and inactivating mutations in the tumor suppressor PTEN (470,484). Likewise, PIK3CA mutations have been reported in 20–25% of clear cell ovarian carcinomas, with multiple studies showing a strong association with endometriosis (470,484). Finally, over 75% of mucinous ovarian cancers demonstrate mutations in KRAS (470,484).
Five years ago, Shih and Kurman proposed a new classification scheme for ovarian carcinomas based on their hypothesized patterns of tumor progression (484,486,487,488). In this model, tumors are designated as type I or type II. Type I tumors are generally slow growing and localized to the ovary at diagnosis, whereas type II tumors are more aggressive and present as advanced-stage metastatic disease. Type I tumors are composed of genetically distinct low-grade serous and mucinous cancers that develop in an adenoma-borderline-carcinoma sequence, as well as low-grade endometrioid and clear cell cancers that progress from endometriosis. Type II tumors are high-grade serous, endometrioid, and undifferentiated carcinomas that do not have recognizable precursor lesions but commonly harbor p53 mutations and high levels of chromosomal instability.
2. Modeling epithelial ovarian cancer
Understanding the fundamental mechanisms governing the pathogenesis of epithelial ovarian cancers depends on the ability to develop robust models in vitro and in vivo. Over the past 30 yr, important advances have been made using rodent and human OSE cultures, human ovarian cancer cell lines, xenograft models, and genetically engineered mouse models (summarized in Table 11). Auersperg and colleagues (471) were pioneers in the isolation, culture, and genetic manipulation of rodent and human OSE, including OSE from women with family histories of ovarian cancer. In 1981, they introduced Kirsten murine sarcoma virus into cultured rat OSE and observed that transformed cells formed endometrioid tumors within 1 wk of sc or ip injection into immunosuppressed female rats (489). These early experiments established a framework for further studying the potential of individual oncogenes to immortalize and transform OSE and promote tumorigenesis. Of note, there are important structural and physiological differences between rodent and human OSE that should be considered when extrapolating results between species. Repeated passaging of rodent OSE cultures results in spontaneous immortalization and malignant transformation based on loss of contact inhibition, substrate-independent growth, and tumor formation in nude mice (473,474,490). In contrast, human OSE cultures are more prone to undergo senescence (471), consistent with the observation that human cells generally require more mutations to bypass senescence and growth arrest compared with murine cells (491). This interspecies variation suggests that experiments with cultured human OSE might be more relevant than with cultured rodent OSE. Immortalized ovarian surface epithelial (IOSE) cell lines have been derived from human OSE by transfection with simian virus 40 (SV40) early genes alone or with the catalytic subunit of telomerase (hTERT) (492,493,494,495,496). Subsequent transfection of SV40-immortalized cells with E-cadherin or transfection of SV40/hTERT-immortalized cells with ERBB2, activated HRAS, or activated KRAS causes transformation and tumor development in immunocompromised mice (496,497,498). Immortalization and malignant transformation of human OSE cells has also been achieved by transfection with human papilloma virus type 16 E6/E7 genes (499,500).
Table 11.
Mouse models of epithelial ovarian cancer
| Model | Phenotype | Ref. |
|---|---|---|
| p53−/− ovarian explants transduced with Myc, activated Kras, or activated Akt, followed by injection of cells into nude mice sc, ip, or intrabursally | Infection with at least two oncogenes causes poorly differentiated carcinomas 8 wk after injection; infection with one oncogene has longer latency of 12 wk to 6 months; intrabursal injection leads to ip and retroperitoneal metastases | 503 |
| p53flox/flox Brca1flox/flox ovarian explants transduced with Cre and Myc, followed by ip injection into immunodeficient or immunocompetent mice | Serous carcinomas with hemorrhagic ascites and peritoneal metastases | 524 |
| Amhr2-TAg (transgenic; mouse Amhr2 promoter driving expression of SV40 T antigen) | Bilateral, poorly differentiated ovarian carcinomas with serous components and ip metastases; tumors present in newborn ovaries | 504,509 |
| AdCre-infected p53flox/flox Rb1flox/flox (double cKO) | Serous, poorly differentiated, and undifferentiated metastatic ovarian carcinomas; median survival of 227 d after AdCre injection | 510 |
| LSL-KrasG12D/+ Ptenflox/flox Amhr2cre/+ (activation of oncogenic Kras allele and conditional deletion of Pten) | Serous ovarian carcinomas | 513 |
| AdCre-infected LSL-KrasG12D/+ Ptenflox/flox (activation of oncogenic Kras allele and conditional deletion of Pten) | Endometrioid ovarian carcinomas with peritoneal dissemination | 511,512 |
| AdCre-infected Apcflox/flox Ptenflox/flox (double cKO) | Endometrioid ovarian carcinomas with peritoneal dissemination | 515 |
AdCre, ovarian intrabursal injection of recombinant adenovirus expressing Cre recombinase; LSL, LoxP-Stop-LoxP.
Although OSE cultures are useful for modeling early steps in cancer formation, cell lines established from naturally occurring human ovarian cancers are valuable for exploring advanced-stage disease. Numerous cell lines have been studied in vitro and injected sc, ip, and intrabursally (orthotopically) into rodents to compare tumor behavior between various histological subtypes and to analyze the efficacy of novel therapeutics. Advanced noninvasive imaging technologies facilitate in vivo visualization of tumor progression and metastasis (501). One caveat to these xenograft models is that the tumors formed in vivo may be histologically different from the original cancer that gave rise to the cell line. For example, ES-2 cells were reportedly derived from ovarian clear cell carcinoma but form undifferentiated carcinomas in xenografted mice (502). These incongruities may be secondary to subtle heterogeneity in the parental cancer, variations in tumor microenvironment, or genetic aberrations that accumulate in culture, thus raising the general question of whether culture-based cancer models accurately recapitulate tumorigenesis in vivo. Furthermore, xenograft models using either human ovarian cancer cell lines or human OSE cultures require immunodeficient mice and neglect potential roles for the immune system in tumor progression.
Orsulic et al. (503) established one of the earliest mouse models for ovarian cancer. They used transgenic mice expressing avian retroviral receptor under control of the keratin 5 promoter, which drives expression in epithelial cells including OSE. Ovarian explants from these mice were cultured transiently and infected with retroviral vectors carrying the oncogenes Myc, activated Kras, or activated Akt. Mutations in all of these genes have been reported in human ovarian cancers. Transduced ovarian cell aggregates were injected sc into nude mice. Eight weeks after injection, recipient mice had poorly differentiated carcinomas when ovarian cells were infected with at least two oncogenes and only on a p53−/− background. Infection with one oncogene on a p53−/− background was also sufficient for tumor formation, but with a longer latency of 12 wk to 6 months, perhaps allowing time for additional genetic mutations to occur. Moreover, when infected cells were implanted under the ovarian bursa of nude mice, widespread ip and retroperitoneal metastases were observed. These features make it a convenient model for testing therapies that may prevent dissemination; however, this system still depends on manipulation of cells in culture and does not model the natural progression of disease from intact ovaries. To overcome these potential confounding factors, stronger genetic approaches were required.
Connolly et al. (504) created transgenic mice expressing SV40 T antigen (TAg) under control of the promoter for Amhr2. Amhr2 is expressed in the developing Müllerian ducts, OSE, ovarian granulosa cells, oviducts, and uterus (260,504,505,506,507). In addition, Amhr2 expression has been reported in human ovarian cancer cell lines and ascites cells isolated from ovarian cancer patients (508). Fifty percent of Amhr2-TAg transgenic females developed bilateral ovarian carcinomas with ip metastases by 6–13 wk of age. The tumors were mostly poorly differentiated, with some regions resembling human serous ovarian cancers. They were also positive for epithelial markers cytokeratins 8 and 19 but negative for the granulosa cell marker inhibin α, suggesting that they were derived from OSE cells and not granulosa cells. On closer examination, ovarian tumors were already present in newborn females, consistent with the embryonic expression of Amhr2 (509). This makes it difficult to study the earliest steps of cancer initiation using the Amhr2-TAg transgenic model and highlights the need for inducible systems in which disease onset can be controlled.
In rodents, encapsulation of the ovary by the bursal membrane creates a protected cavity for the selective delivery of inducing agents to the OSE. Intrabursal injection of recombinant adenovirus-expressing Cre recombinase (AdCre) drives Cre-mediated recombination specifically in the OSE and not in other stromal cells or oocytes. This approach has been effective for conditional deletion of floxed tumor suppressor genes and conditional activation of oncogenes preceded by a floxed stop sequence. Using this technology, Flesken-Nikitin et al. (510) deleted the tumor suppressors p53 and Rb1 in the OSE. p53 mutations are common in high-grade human ovarian carcinomas, and involvement of RB1 has also been described. Targeted inactivation of both genes in murine OSE resulted in serous ovarian cancers as well as poorly differentiated and undifferentiated tumors with metastases. Double knockout mice had a median survival of 227 d after AdCre injection. Deletion of Rb1 alone did not cause ovarian tumors, whereas deletion of p53 alone rarely caused ovarian cancer, consistent with the fact that these tumor suppressors regulate interconnected signaling pathways.
The mouse models described above exhibit ovarian carcinomas that are either poorly differentiated or with serous histology, the most common subtype in women. Despite these groundbreaking advances, decoding the morphological and genetic diversity of epithelial ovarian cancers requires models that faithfully represent each major histological subtype. Dinulescu et al. (511,512) used the intrabursal AdCre-loxP system to simultaneously activate an oncogenic Kras allele and delete the Pten tumor suppressor in murine OSE. Double mutant females developed invasive endometrioid ovarian carcinomas with peritoneal dissemination as early as 7 wk after AdCre injection. The tumors showed activation of the MAPK and AKT pathways, so this model may be instrumental in testing targeted therapies that specifically inhibit these signaling cascades. Interestingly, activation of Kras alone was insufficient to provoke tumor formation but instead resulted in peritoneal endometriosis and benign endometriosis-like lesions on the ovary. Deletion of Pten alone produced similar benign epithelial ovarian lesions but did not cause endometriosis, although one of 13 Pten cKO females demonstrated endometrioid ovarian cancer at 26 wk, raising the possibility that the endometriosis-like lesions on the ovary were potential precursors to ovarian cancer. More recently, Richards and colleagues (513) generated similar Kras and Pten mutations in OSE cells using mice expressing Amhr2-Cre rather than the intrabursal administration of AdCre used by Dinulescu et al. (511). Curiously, these mutants developed serous, rather than endometrioid, OSE adenocarcinomas with 100% penetrance. Reasons for the phenotypic differences in these two models expressing the same mutant genes remain to be determined but could be dependent on the stage and/or OSE cell context-specific events initiated by AdCre compared to Amhr2-Cre. In women, loss of heterozygosity at the 10q23.3 PTEN locus and somatic mutations in PTEN have been observed in endometriosis and ovarian carcinomas of the endometrioid and clear cell subtypes (514). In contrast, KRAS mutations have not been reported in endometriosis and are rare in endometrioid ovarian cancer, with no evidence to support coexistence or cooperativity with PTEN inactivation.
Cho and colleagues (515) examined a large number of primary human endometrioid ovarian cancers and found a significant frequency of coexisting mutations in WNT/β-catenin and PI3K/PTEN signaling pathways. This occurred almost exclusively in tumors that were low-grade and lacking p53 mutations. To investigate whether these pathways cooperate to drive the malignant transformation of OSE, they used the intrabursal AdCre-loxP system to conditionally inactivate the tumor suppressors Apc and Pten, which are negative regulators of β-catenin and PI3K, respectively. Whereas no abnormalities were reported for either single mutant, concomitant deregulation of both pathways induced endometrioid ovarian carcinomas with 100% penetrance within 6 wk of AdCre injection. The tumors were accompanied by hemorrhagic ascites, occasional peritoneal dissemination to the liver, and areas of spindle cell morphology that were negative for cytokeratin and E-cadherin immunoreactivity, suggesting epithelial-to-mesenchymal transition. Despite the strong association between endometrioid ovarian cancer and endometriosis in women, there was no evidence of endometriosis-like lesions in the Apc Pten cKO. However, alterations in gene expression between the mouse tumors and control ovaries were highly correlated with aberrations in human endometrioid ovarian cancers, in particular to those harboring mutations in WNT/β-catenin and PI3K/PTEN signaling. Hence, this mouse model may be extremely valuable for identifying therapies specifically tailored to endometrioid ovarian cancer. Furthermore, the phenotype reveals that genetic interactions between canonical WNT signaling and the PI3K/PTEN pathway promote ovarian tumorigenesis. Molecular intersections between these pathways have been described. For example, AKT directly phosphorylates β-catenin, consequently increasing β-catenin transcriptional activity and promoting tumor cell invasion in vitro (516). Nevertheless, it remains unknown which mechanisms of cross talk are relevant to ovarian cancer.
Approximately 10% of epithelial ovarian cancers are hereditary with evidence of autosomal dominant genetic susceptibility (reviewed in Ref. 517). Loss of function germline mutations in the tumor suppressor BRCA1 account for 90% of these cases and are highly penetrant, conferring a 40–60% lifetime risk of developing ovarian cancer, compared with 1–2% in the general population (517,518). The tumors are typically serous ovarian carcinomas. BRCA1 inactivation also occurs in the majority of sporadic ovarian tumors but usually through mechanisms other than somatic mutation, for instance, epigenetic silencing due to promoter hypermethylation (reviewed in Ref. 519). BRCA1 has critical functions in the DNA damage response and the maintenance of genomic integrity; therefore, defects in BRCA1 have widespread cellular consequences that presumably enhance vulnerability to genomic instability and oncogenic transformation. Indeed, familial ovarian cancers develop at a younger age compared with sporadic ovarian cancers (517). Ovaries removed prophylactically from BRCA1 carriers and women with a family history of ovarian cancer displayed a higher frequency of morphological alterations in the OSE that may reflect preneoplastic lesions (520,521,522). Similar trends were observed in murine OSE after targeted deletion of Brca1 using intrabursal AdCre administration (523). Despite these findings, Brca1 cKO females did not develop ovarian tumors even 1 yr after injection. BRCA1 inactivation in primary cultures of murine OSE suppressed proliferation, increased apoptosis, and heightened sensitivity to cisplatin (523,524). On the other hand, concomitant deletion of BRCA1 and p53 enhanced proliferation, whereas inactivation of p53 alone had no effect, suggesting that p53 dysfunction or other oncogenic events are essential mediators of BRCA1-associated tumorigenesis (523). Accordingly, p53 mutations are more common in hereditary than sporadic ovarian cancers and may be an early initiating event in BRCA1 carriers (525,526). Although other players in BRCA1-mediated ovarian cancer remain to be identified, retroviral infection with the Myc oncogene (but not Kras, Erbb2, or Akt) was sufficient for transforming OSE cells with combined deficiency of Brca1 and p53 (524). BRCA1 binds MYC and represses its transcriptional and transforming activity (229). MYC is amplified in BRCA1-associated breast cancer, but its role in BRCA1-associated ovarian cancer is unclear (527,528).
B. Sex cord-stromal ovarian cancer
Tumors emanating from the gonadal sex cords and stroma comprise about 7% of ovarian cancers and include granulosa-theca cell tumors, thecoma-fibromas, Sertoli-Leydig cell tumors, sex cord tumors with annular tubules, and gynandroblastomas (468,529). Hormone secretion is a salient feature of these cancers, which often present as a large, unilateral adnexal mass confined to the ovary with signs and symptoms of estrogen or androgen excess. The mainstay of treatment is surgery to remove the tumor, followed by adjuvant chemotherapy in selected patients. Granulosa cell tumors account for approximately 70% of sex cord-stromal ovarian cancers and are classified as adult (95%) or juvenile (5%) (529). The adult variant is more common in postmenopausal women and associated with abnormal vaginal bleeding, endometrial hyperplasia, and endometrial carcinoma, whereas the juvenile type frequently entails gonadotropin-independent isosexual precocious puberty in prepubertal girls (i.e., secondary to estrogen secretion by the tumor). In contrast to epithelial ovarian cancers, the majority of which are diagnosed at advanced stages with poor prognoses, about 80% of adult granulosa cell tumors are diagnosed at stage I and have 5- and 10-yr survival rates exceeding 90% (529). Nevertheless, adult granulosa cell tumors are slow-growing and tend toward late recurrence; although the median time to relapse is around 5 yr after initial diagnosis, recurrences have been reported after 40 yr, and recurrent disease bears a somber prognosis (529,530).
Sex cord-stromal ovarian cancers are rare, and this is most likely explained by the highly orchestrated removal of apoptotic granulosa cells in follicles undergoing atresia that occurs in most (99%) of all growing follicles. The rarity of these cancers has hindered comprehensive study of the genetic aberrations responsible for tumor formation and progression in women. On the other hand, granulosa cell tumors are the most common spontaneous ovarian tumors in mice (531). Beamer and colleagues have extensively investigated the pathogenesis of spontaneous granulosa cell tumors in SWR and SWXJ mouse strains. Tumors in these mice are grossly visible by 6 wk of age and histologically resemble human juvenile granulosa cell tumors (531,532). The window of tumor initiation is between 3 and 5 wk of age; beyond this timeframe, unaffected females are no longer at risk. Incomplete penetrance of the phenotype during a restricted period of ovarian maturation indicates a complex interplay between multiple genetic and environmental factors. Detailed linkage studies in these spontaneous mouse models have identified nine granulosa cell tumor (Gct) susceptibility loci (532,533,534,535,536). The Gct1 locus on distal mouse chromosome 4 surfaced in three independent mapping crosses using divergent mouse strains (532). Loss of this genomic region is a secondary aberration in transgenic mouse models of oligodendroglioma and mammary carcinoma (532,537,538). The orthologous region on human chromosome 1p is also deleted in various cancers, strongly suggesting the presence of as yet unidentified tumor suppressor genes (532,539,540,541). Additionally, Gct4 is an X-linked tumor susceptibility locus that modulates responsiveness to androgenic steroids and shows a strong parent-of-origin effect associated with paternal inheritance, raising the possibility that epigenetic effects such as imprinting might also influence tumor formation (534,535,536).
Further insight into the pathways involved in ovarian sex cord-stromal tumorigenesis stems from a variety of transgenic mouse models (summarized in Table 12). TGFβ superfamily members are critical regulators of ovarian folliculogenesis and granulosa cell function, and include inhibins, activins, BMPs, growth and differentiation factors, and anti-Müllerian hormone. Granulosa cells and Sertoli cells secrete inhibins (α:βA, α:βB) and activins (βA:βA, βB:βB, βA:βB) that suppress (i.e., inhibins) or stimulate (i.e., activins) pituitary production and secretion of FSH. Mice null for the inhibin α-subunit gene (Inha−/−), and therefore completely inhibin-deficient, develop bilateral, multifocal, mixed, invasive sex cord-stromal tumors with 100% penetrance in both sexes as early as 4 wk of age (542,543). The mice eventually die secondary to a cancer cachexia syndrome marked by severe weight loss and multiple extragonadal defects (544). Hence, inhibins are secreted tumor suppressors with gonadal specificity. For more than 15 yr, inhibin-deficient mice have been a valuable model system to investigate modifiers of gonadal tumorigenesis, including gonadotropins, sex steroid hormones and receptors, cell cycle regulators, and activin signaling (summarized in Table 13).
Table 12.
Mouse models of sex cord-stromal ovarian cancer
| Model | Phenotype | Ref. |
|---|---|---|
| SWR/SWXJ strains | Spontaneous granulosa cell tumors by 6 wk of age; incomplete penetrance | 531 |
| Inha−/− (inhibin α knockout)a | Bilateral, mixed sex cord-stromal ovarian tumors with 100% penetrance as early as 4 wk of age; cancer cachexia syndrome; elevated FSH, estradiol, and activins A and B | 542,543,544 |
| Fshr−/− (FSH receptor knockout) | Unilateral Sertoli-Leydig cell tumors by 1 yr of age; cachexia; elevated FSH and LH; low estradiol | 817 |
| bLHβ-CTP (transgenic; chronic hypersecretion of LH) | Strain-dependent formation of granulosa cell tumors with 100% penetrance by 5 months of age; polycystic ovaries | 550,551 |
| Inhα-TAg (transgenic; mouse inhibin α promoter driving expression of SV40 T antigen) | Granulosa cell tumors with 100% penetrance by 6 months of age | 552 |
| bLHβ-CTP/Inhα-TAg (double transgenic) | Earlier formation, more rapid progression, and enhanced aggressiveness of granulosa cell tumors compared to either single transgenic model; occasional metastases to lungs and liver | 553 |
| MT-hCG (transgenic; mouse metallothionein 1 promoter fused to coding sequences of human chorionic gonadotropin α- and β-subunits) | Hemorrhagic and cystic ovaries; thecomas and stromal cell expansion; enlarged uterine horns; urinary tract abnormalities; elevated estradiol | 554 |
| Smad1 Smad5 (double cKO using Amhr2-Cre) | Unilateral or bilateral poorly differentiated granulosa cell tumors with 100% penetrance by 3 months of age; metastases to lymph nodes and peritoneum | 576 |
| Smad1 Smad5 Smad8 (triple cKO using Amhr2-Cre) | Similar to Smad1 Smad5 double cKO | 576 |
| Ctnnb1flox(ex3)/+ Amhr2cre/+ (conditional expression of dominant stable form of β-catenin) | Unilateral or bilateral, hemorrhagic, cystic granulosa cell tumors; detected in mice older than 5 months and prevalence increased with age; precursor lesions; osseous metaplasia | 583,584 |
| Ptenflox/flox Amhr2cre/+ (cKO) | Unilateral or bilateral granulosa cell tumors with low penetrance; ossification and cysts; metastases to lungs | 585 |
| Ptenflox/flox Ctnnb1flox(ex3)/+ Amhr2cre/+ (double conditional mutant) | Bilateral granulosa cell tumors with 100% penetrance; dysplastic cells in newborn ovaries; death prior to 9 wk of age | 585 |
| Men1+/− (multiple endocrine neoplasia 1 heterozygote) | Granulosa cell tumors; multiple endocrine tumors; tumors show loss of heterozygosity at Men1 locus | 818,819,820 |
| OSP1-TAg (transgenic; rat ovarian-specific promoter 1 driving expression of SV40 T antigen) | Unilateral granulosa cell tumors; tumors in multiple other tissues | 821 |
Mouse models related to the inhibin α knockout are described in Table 13.
Table 13.
Mouse models related to the inhibin α knockout
| Model | Phenotype | Endocrine abnormalitiesa | Survival relative to Inha−/− | Ref. |
|---|---|---|---|---|
| Inha−/− (inhibin α knockout) | Bilateral, mixed sex cord-stromal ovarian tumors with 100% penetrance as early as 4 wk of age; cancer cachexia syndrome | Elevated FSH, estradiol, and activins A and B | n/a | 542,543,544 |
| Gonadotropins | ||||
| Inha−/− Gnrh1hpg/hpg (gonadotropin releasing hormone) | No tumors, only premalignant ovarian lesions (e.g., seminiferous tubule-like structures without germ cells) | Suppressed FSH and LH | ↑↑↑ | 545 |
| Inha−/− Fshb−/− (FSH β subunit) | Slower growing and less invasive tumors; delayed onset of cancer cachexia syndrome | FSH absent; decreased estradiol and activin A compared to Inha−/− | ↑↑ | 549 |
| Inha−/− Lhb−/− (LH β subunit) | Delayed tumor progression | Lower FSH compared to Inha−/− at 6 wk of age | ↑ | 555 |
| Sex steroid hormones and receptors | ||||
| Inha−/− females treated with flutamide, an androgen antagonist | Delayed tumor development and less hemorrhagic tumors | Decreased FSH compared to untreated Inha−/− | ↑ | 558 |
| Inha−/− Esr1−/− (estrogen receptor α) | More rapid tumor development and earlier onset of cancer cachexia syndrome | Elevated estradiol; suppressed FSH and LH compared to Inha−/− | ↓↓ | 558 |
| Inha−/− Esr2−/− (estrogen receptor β) | Similar to Inha−/− | Estradiol and gonadotropin levels similar to Inha−/− | ↓ | 558 |
| Inha−/− Esr1−/− Esr2−/− (estrogen receptors α and β) | Accelerated tumor development and earlier onset of wasting syndrome | Elevated FSH | ↓↓↓ | 558 |
| Cell cycle regulators | ||||
| Inha−/− Ccnd2−/− (cyclin D2) | Delayed onset and slower progression of tumors and cancer cachexia syndrome | Elevated FSH | ↑↑ | 562 |
| Inha−/− Cdkn1b−/− (cyclin-dependent kinase inhibitor 1B, also known as p27Kip1) | Accelerated tumor development and earlier onset of wasting syndrome | Elevated FSH and activin A | ↓↓↓ | 557,562 |
| Inha−/− Rb1−/− (retinoblastoma 1) | Accelerated tumor progression | Higher LH and progesterone compared to Inha−/− | ↓ | 563 |
| Activin signaling | ||||
| Inha−/− Acvr2a−/− (activin receptor IIA) | Similar to Inha−/− with notable absence of cancer cachexia syndrome | Elevated activins A and B | ↑↑↑ | 567 |
| Inha−/− MT-Fst (mouse metallothionein 1 promoter fused to coding sequence of mouse follistatin) | Less hemorrhagic tumors and less severe wasting syndrome | Elevated FSH compared to MT-Fst controls; decreased activin A compared to Inha−/− | ↑ | 571 |
| Inha−/− treated with ACVR2A-mFc, a chimeric activin antagonist | Slower tumor progression and absence of wasting syndrome | Trend toward lower FSH compared to PBS-treated Inha−/− females | ↑↑↑ | 572 |
| Inha−/− Smad3−/− (MAD homolog 3, also known as Madh3) | Slower tumor progression and delayed onset of wasting syndrome | Decreased activin A and trend toward lower estradiol compared to Inha−/− at 15 wk of age | ↑↑↑ | 573,574 |
| Miscellaneous | ||||
| Inha−/− Gdf9−/− (growth differentiation factor 9) | Similar to Inha−/− | Not determined | Unchanged | 226 |
n/a, Not applicable.
Serum levels as compared to wild-type mice, unless otherwise specified.
FSH and LH are pituitary gonadotropins composed of a common α-subunit and a hormone-specific β-subunit, the synthesis of which is regulated in part by hypothalamic GnRH. When inhibin α mutants were crossed to mice deficient in GnRH [i.e., the hypogonadal (Gnrh1hpg) model], in which FSH and LH levels are suppressed, the resulting double homozygous mutants were strikingly free of tumors and the cancer cachexia syndrome (545). Although the ovaries of Inha−/− Gnrh1hpg/hpg mice contain seminiferous tubule-like structures similar to lesions in Inha−/− females, these structures do not evolve beyond the premalignant stage. In an analogous experiment, Beamer and colleagues (546,547) showed that an intact hypothalamic-pituitary axis is essential for granulosa cell tumor development in genetically susceptible SWR/SWXJ female mice. These data suggest that gonadotropins are key regulators of ovarian tumorigenesis, consistent with the dramatic rise in the incidence of ovarian cancer around menopause, when gonadotropin levels are elevated (reviewed in Ref. 548).
Further experiments were necessary to delineate the individual contributions of FSH and LH. Inhibin-deficient mice have increased serum FSH levels compared with wild-type mice, consistent with the known function of inhibins to suppress FSH (542). As mentioned earlier, binding of FSH to its receptor on granulosa cells activates cAMP-dependent PKA signaling and other pathways, ultimately promoting the expression of genes that orchestrate granulosa cell proliferation and differentiation. Female mice deficient in inhibins and FSH (Inha−/− Fshb−/−) have increased survival because of slower growing, less invasive tumors along with delayed onset of the wasting syndrome (549). In a complementary gain of function experiment, human FSH α- and β-subunits were simultaneously overexpressed from the mouse MT1 promoter on a wild-type background (549). Transgenic female mice with elevated human FSH do not develop cancer but instead have hemorrhagic and cystic ovaries in addition to urinary tract dysfunction, features reminiscent of human ovarian hyperstimulation and polycystic ovarian syndromes. Together, these models demonstrate that FSH signaling promotes sex cord-stromal tumor progression but is not necessary for tumor formation.
Corroborating evidence from several different mouse models emphasizes the contribution of LH to granulosa cell tumorigenesis. Chronic hypersecretion of LH in transgenic female mice (bLHβ-CTP) causes polycystic ovaries and strain-dependent formation of granulosa cell tumors with 100% penetrance by 5 months of age (550,551). In another transgenic model, granulosa cell tumors invariably develop by 6 months of age when the inhibin α promoter drives SV40 TAg expression (Inhα-TAg) (552). Notably, double transgenic bLHβ-CTP/Inhα-TAg females display earlier formation and more rapid progression of tumors (553). Related to the bLHβ-CTP model, the mouse MT1 promoter was used to simultaneously overexpress α- and β-subunits of hCG, a placental glycoprotein hormone and LH analog that acts through the LH receptor (554). Like MT-hFSH mice, MT-hCG transgenic females develop hemorrhagic and cystic ovaries coincident with urinary tract defects; thecomas and marked stromal cell expansion are also observed in the ovaries.
In light of these observations and to further understand the distinct phenotypes of Inha−/− Gnrh1hpg/hpg mice and Inha−/− Fshb−/− mice, we recently characterized mice deficient in both inhibins and LH (555). Inha−/− Lhb−/− females develop ovarian cancer but have increased survival because of delayed tumor progression, an effect correlated with elevated tumor expression of p15INK4b (Cdkn2b) and a trend toward higher levels of p27Kip1, both genes that repress cell proliferation. Loss of expression of CDK inhibitors p15INK4b and p16INK4a (Cdkn2a) have been reported in adult granulosa cell tumors in women, and the loss of p27Kip1 exacerbates tumorigenesis in inhibin-deficient mice (556,557). Inha−/− Lhb−/− ovarian tumors also have decreased expression of Cyp17a1, the LH-induced thecal cell enzyme that converts pregnenolone and progesterone to dehydroepiandrosterone and androstenedione, respectively (555). Lower Cyp17a1 expression might reduce the levels of these androgens, consequently slowing tumor growth in Inha−/− Lhb−/− females. This explanation is based on the observation that treatment of inhibin-deficient females with the androgen antagonist flutamide prolongs life span by diminishing tumor progression (558). Conversely, exogenously supplied androgens increase the frequency of granulosa cell tumors in genetically susceptible SWR/SWXJ female mice (534,546,559). Importantly, treatment of SWR/SWXJ females with hCG, but not FSH, also induces tumors (547). These experiments collectively suggest that LH signaling is sufficient for ovarian sex cord-stromal tumor formation, in part by triggering androgen production.
Consistent with the fact that granulosa cells are the principal site of estradiol synthesis, granulosa cell tumors in women and mice secrete estradiol. To investigate the role of estrogen signaling in sex cord-stromal ovarian cancers, our group generated mice lacking inhibin α, ERα (Esr1), and ERβ (Esr2) (558). Inha−/− Esr1−/− females exhibit a substantial decrease in survival attributed to more rapid tumor progression and earlier onset of the cancer cachexia syndrome. Although Inha−/− Esr2−/− females are similar to Inha−/− single mutants, triple knockout Inha−/− Esr1−/− Esr2−/− females demonstrate the most severe phenotype with 100% death by 9 wk of age, a timepoint at which the majority of Inha−/− mice are still alive. These data indicate that in the absence of inhibins, ER signaling is protective in females. Accordingly, estradiol treatment suppresses granulosa cell tumor incidence in genetically susceptible SWR/SWXJ mice (559). Because ESR1 and ESR2 are expressed in human granulosa cell cancers, the protective effect of estradiol may occur through direct action on the tumor by unknown mechanisms (560). Estradiol also imparts negative feedback on the hypothalamic-pituitary axis that is predominantly mediated by ERα (561). Therefore, an alternative explanation is that estradiol reduces LH synthesis and release from the pituitary in an ER-intact mouse, which in turn mitigates tumorigenesis as described earlier.
Signaling cascades activated by gonadotropins and sex steroid hormones ultimately converge on a final common pathway: the cell cycle. The G1/S transition is regulated by D- and E-type cyclins that complex with CDKs (CDK4/6 and CDK2, respectively) to promote cell proliferation, whereas CDK inhibitors such as p27Kip1 block cell cycle progression. The absence of CCND2 causes female sterility because of impaired granulosa cell proliferation in response to FSH (292). On the other hand, sterility in p27Kip1 null females is secondary to defects in cell cycle withdrawal and luteinization in response to LH (391,392,393). Ccnd2 expression is higher in human granulosa cell tumors compared with other ovarian cancers and normal ovaries (292). Moreover, sex cord-stromal cancers in inhibin-deficient females have elevated Ccnd2 and Cdk4 mRNA and lower p27Kip1 protein compared with wild-type ovaries, leading to the hypothesis that these alterations might foster tumor growth (557). Indeed, the loss of CCND2 prolonged survival, and the loss of p27Kip1 decreased survival in inhibin-deficient mice, correlating with changes in the rate of tumor development and onset of the associated wasting syndrome (557,562). Granulosa cell-specific deletion of Rb1 (retinoblastoma 1) on the inhibin α null background also accelerates ovarian tumorigenesis (563). When unphosphorylated and active, RB1 binds to E2F transcription factors and prevents the expression of genes required for cell cycle progression (reviewed in Ref. 564). RB1 also blocks cell proliferation by inhibiting the ubiquitination and degradation of p27Kip1 (565,566). Accordingly, ovarian tumors lacking inhibins and RB1 have lower p27Kip1 levels and increased expression of mitotic markers such as CCND2, PCNA (proliferating cell nuclear antigen), and CCNB1 (cyclin B1) compared with tumors from Inha−/− females. Despite these aberrations, double mutants showed only a modest rise in mortality, likely because there was also increased apoptosis that may have limited tumor growth and higher expression of related family members Rbl1 (retinoblastoma-like 1; p107) and Rbl2 (retinoblastoma-like 2; p130) that might have partially compensated for loss of Rb1.
Along with their endocrine actions as regulators of FSH levels, inhibins and activins secreted from granulosa cells function in autocrine and paracrine fashions to balance growth and differentiation within the maturing follicle. TGFβ superfamily ligands signal through transmembrane serine/threonine kinase receptor complexes that phosphorylate and activate intracellular SMAD proteins, which then translocate to the nucleus to control gene expression. Activins bind ACVR2A and ACVR2B (type II receptors) that recruit activin receptor-like kinases (type I receptors), which signal through SMAD2 and SMAD3. Gonadal tumors in inhibin-deficient mice secrete large amounts of activins into the circulation (544). Because inhibins antagonize activins at the receptor level, inhibin-deficient mice are a useful model for studying the consequences of unchecked activin signaling on tumorigenesis. Deletion of Acvr2a on the inhibin α null background does not abolish tumor development but instead prolongs life span by preventing the cancer cachexia syndrome (567). This wasting syndrome is characterized by severe weight loss, lethargy, hepatocellular inflammation and necrosis, and atrophy of the glandular stomach (544). Acvr2a is expressed in mouse liver and glandular stomach, and infusion of recombinant activin A in rodents induces apoptosis of hepatocytes around the central vein, a pattern similar to the liver pathology in cachectic Inha−/− mice (567,568,569). These data indicate that increased activin signaling specifically through ACVR2A in hepatocytes and the glandular stomach causes the wasting syndrome in inhibin-deficient mice.
Although absence of ACVR2A does not affect tumor growth, activin signaling through ACVR2B or other unidentified receptors may promote tumor progression. Activin A stimulates the growth of gonadal tumor cell lines derived from inhibin α- and p53-deficient mice, whereas FST inhibits tumor cell proliferation (570). FST binds to activin β-subunits and interferes with the binding of activins to ACVR2A and ACVR2B. Transgenic overexpression of FST in inhibin-deficient mice expectedly decreases the severity of the wasting syndrome but also produces less hemorrhagic ovarian tumors (571). Similar outcomes were observed when Inha−/− mice were injected with a chimeric activin antagonist consisting of the ACVR2A extracellular domain fused to the Fc region of murine IgG2a (ACVR2A-mFc) (572). Treatment with ACVR2A-mFc completely prevented the wasting syndrome and delayed tumor progression.
The models described thus far modulate activin signaling at the ligand or receptor level. To investigate downstream components of the pathway, two independent groups generated Inha−/− Smad3−/− double knockout mice that have increased survival because of diminished ovarian tumor progression (573,574). SMAD3 regulates Ccnd2 expression through transcriptional activation at the Ccnd2 promoter and through potentiation of FSH signaling (294,574,575). Double mutant ovarian tumors have lower CCND2 and partial insensitivity to FSH signaling, specifically through PI3K/AKT, leading to a phenotype reminiscent of both Inha−/− Ccnd2−/− mutants and Inha−/− Fshb−/− mutants (574). Notably, loss of SMAD3 attenuates tumor development to a greater degree in inhibin-deficient males than females, supporting functional redundancy with SMAD2 in ovarian granulosa cells (354,573).
Recent work has shown that the oncogenic effects of activin signaling through SMAD2 and SMAD3 are balanced by the tumor suppressive roles of BMP signaling through SMAD1, SMAD5, and SMAD8. Conditional deletion of Smad1/5 or Smad1/5/8 in ovarian granulosa cells causes progressive infertility and granulosa cell tumors that metastasize to the lymph nodes and peritoneum (576). The tumors and metastases express inhibin α, and therefore activin signaling is either normal or suppressed because the mutants do not develop elevated activin levels or cachexia. However, the nuclear localization of phospho-SMAD2/3 immunoreactivity indicates activation of SMAD2/3 by the physiologically normal levels of activins or by another ligand such as TGFβ. Multiple TGFβ-induced genes (e.g., Hmga2, Mmp2, Tgfbi) are up-regulated in Smad1/5 knockout tumors compared with wild-type granulosa cells, and overexpression of TGFβ promotes tumor invasion and metastasis in a variety of human cancers. Overall, this model emphasizes that growth and differentiation of granulosa cells are determined by complex interactions between BMP and TGFβ/activin pathways.
Similar to granulosa cell tumors in women, the ovarian cancers in Smad1/5 cKO mice and in genetically susceptible SWR/SWXJ mice are inhibin-positive (576,577). Throughout the literature, this has raised questions about the relevance of the inhibin-deficient mouse model to human ovarian cancers. If inhibins are markers for human granulosa cell tumors, how can they be tumor suppressors? The recently described phenotypes of ACVR2A-mFc-treated inhibin-deficient mice, Inha−/− Smad3−/− mutants, and in particular the Smad1/5 cKO have shed light on this apparent paradox. Activation of SMAD2/3 promotes tumorigenesis upon stimulation by TGFβ or activin signaling; hence, inhibins are clearly tumor suppressors in part because they function as activin antagonists. The strong evidence implicating TGFβ superfamily signaling in granulosa cell tumorigenesis and the fact that granulosa cell tumors in women are inhibin-positive (578) suggest that these tumors have mutations that circumnavigate the inhibins, effectively making them inhibin-resistant and ultimately leading to SMAD2/3 activation, SMAD1/5 inactivation, or aberrations in the expression or function of their respective target genes. Validation of this hypothesis awaits investigation of TGFβ superfamily signaling in human granulosa cell cancers. Furthermore, although inhibin α mutations may not be a common initiating event in human granulosa cell tumors, the TGFβ model predicts that the loss of inhibins would exacerbate tumorigenesis by unleashing activin (and FSH) signaling. Indeed, Ala-Fossi et al. (579) reported that in contrast to early-stage granulosa cell tumors in women, advanced-stage tumors were negative for inhibin α immunostaining and correlated with shorter survival.
Aside from TGFβ superfamily signaling, WNT pathway components are also expressed in ovarian granulosa cells (580,581,582), and alterations in WNT signaling have been implicated in granulosa cell tumorigenesis. Richards and colleagues (583) observed nuclear localization of β-catenin in human and equine granulosa cell tumors, indicating aberrant activation of the canonical WNT pathway. To model this scenario, they generated Ctnnb1flox(ex3)/+ Amhr2cre/+ mice expressing a dominant stable form of β-catenin in granulosa cells. Mutant females developed unilateral or bilateral tumors composed of sheets of granulosa cells and hemorrhagic cysts lined with granulosa cells. Tumors were only detected in mice older than 5 months, and the prevalence increased with age because eight of 14 females were affected by 7.5 months and two others developed tumors after 1 yr. The tumors apparently evolve from precursor lesions that are present at 6 wk of age and characterized by vascular follicle-like nests of disorganized, pleiomorphic granulosa cells in addition to cavitary and cystic structures. Gene expression analyses revealed that some of these pretumoral lesions progress through an intermediate stage resembling osseous metaplasia, consistent with known roles for WNT signaling in cell fate determination and differentiation (583,584). These unique observations suggest that metaplastic transformation might be an important premalignant step for human granulosa cell tumors, although this has not yet been described.
Regions of ossification and cystic structures were also reported in granulosa cell tumors of Ptenflox/flox Amhr2cre/+ cKO females (585). Only 7% of these mutants developed tumors between 7 wk and 7 months, but the tumors were particularly aggressive and metastasized to the lungs. In contrast, the Ptenflox/flox Cyp19a1-Cre mice do not develop tumors but do exhibit enhanced ovulation rates related to reduced follicular apoptosis (406). Thus, there appears to be a stage-dependent effect of disrupting Pten in granulosa cells. The tumor suppressor PTEN represses the PI3K/AKT pathway, a major effector of FSH signaling in granulosa cells (586). FSH activation of PI3K/AKT triggers mammalian target of rapamycin (mTOR) signaling and attenuates FOXO1 repression at the Ccnd2 promoter (294,586) by rapidly turning off expression of the Foxo1 gene in granulosa cells (174). Accordingly, tumors resulting from deletion of PTEN in granulosa cells demonstrated loss of FOXO1 expression and elevated mTOR and phospho-mTOR levels compared with Cre-negative granulosa cells (585). Unexpectedly, the tumors also showed loss of phospho-AKT expression, an incongruent finding that may be the end result of negative feedback loops induced by early constitutive activation of the PI3K/AKT pathway. Human and equine granulosa cell tumors do not have alterations in PTEN expression but instead show perinuclear and nuclear localization of phospho-AKT, the significance of which remains unclear.
As mentioned earlier, there are several avenues of cross talk between PI3K/AKT and canonical WNT signaling, and these pathways cooperatively induce endometrioid tumors in a mouse model of epithelial ovarian cancer (515). Hence, simultaneous activation of both pathways in granulosa cells might also have synergistic effects on sex cord-stromal tumorigenesis. Boerboom and colleagues (585) tested this hypothesis by generating Ptenflox/flox Ctnnb1flox(ex3)/+ Amhr2cre/+ mice, which developed bilateral granulosa cell tumors with enhanced severity compared with either single conditional mutant. The phenotype was fully penetrant with dysplastic cells present in newborn ovaries and death occurring before 9 wk of age, a rapid course that likely precluded metastasis. Indeed, surgical removal of the tumors from 6-wk-old females prolonged survival and allowed for the emergence of pulmonary metastases, indicating that the tumors were capable of malignant progression. Future dissection of the precise mechanisms regulating tumorigenesis in these models may shed new light on the pathogenesis of human granulosa cell tumors.
Finally, in an exciting and provocative study, Huntsman and colleagues (587) used next generation sequencing technology to comprehensively profile the transcriptomes of four human adult granulosa cell tumors. They identified a single recurrent somatic missense mutation (402C→G, C134W) in the FOXL2 gene. This variant was confirmed to be present in 86 of 89 independent adult granulosa cell tumors, three of 14 thecomas, and one of 10 juvenile granulosa cell tumors, but absent in multiple sex cord-stromal tumors of other subtypes and numerous unrelated ovarian and breast cancers. As described earlier in Section III, FOXL2 is a forkhead transcription factor that is expressed in granulosa cells and essential for their differentiation from squamous to cuboidal morphology during the primordial to primary follicle transition. Previously characterized germline mutations in FOXL2 cause type I BPES with POF (186), a phenotype recapitulated in Foxl2 null female mice, which are sterile (187,188). Although the somatic mutation in the current study occurs on the surface of the forkhead DNA-binding domain, the impact on FOXL2 function remains unclear. Nonetheless, the specificity of the mutation for adult granulosa cell tumors holds promise for future investigation.
C. Germ cell ovarian cancer
Germ cell tumors represent 20–25% of ovarian neoplasms but only 1–2% of ovarian cancers (588). They account for the majority of ovarian tumors in women younger than 20 yr old. Unlike epithelial ovarian cancers, germ cell tumors are frequently diagnosed at stage I disease, perhaps because they grow rapidly and provoke symptoms early, secondary to capsular distension, hemorrhage, or necrosis (589). Also, women with advanced-stage germ cell tumors often respond well to treatment. From 1973 to 2002, the age-adjusted incidence of germ cell ovarian cancer declined, and survival rates improved significantly, with 5-yr relative survival exceeding 80% (589).
Ovarian germ cell tumors are classified as teratomas, dysgerminomas (the female counterpart to the male seminoma), endodermal sinus (yolk sac) tumors, choriocarcinomas, embryonal carcinomas, and mixed (468,588). Teratomas (from the Greek word “teras,” or monster) are the most common type and are further divided into mature (benign), immature (malignant), and monodermal or highly specialized (struma ovarii and carcinoid tumors). Mature cystic teratomas are pathologically intriguing because they contain adult tissues from all three germ layers (ectoderm, mesoderm, endoderm), for example, skin, hair, sebaceous glands, teeth, cartilage, bone, thyroid, and neural tissue. Immature teratomas similarly contain derivatives of all three germ layers, but with less differentiation and closer resemblance to embryonic than adult tissues (468). The majority of malignant ovarian germ cell tumors are immature teratomas, dysgerminomas, or endodermal sinus tumors (589).
Most basic research on germ cell tumorigenesis has centered around mature and immature teratomas. In women and mice, ovarian teratomas arise from parthenogenetic cleavage of oocytes; in other words, oocytes that begin to form embryos in the absence of fertilization by sperm. Cytogenetic analyses have revealed five mechanisms of origin for human ovarian teratomas: meiosis I error; MII error; duplication of the genome of a mature ovum; mitotic division of a premeiotic germ cell; and fusion of two ova (590,591). Scattered reports of spontaneous ovarian teratomas in mice began as early as 1920 (592). Although the scarcity of these tumors has precluded detailed study in mouse models, there are a few exceptions (summarized in Table 14). Stevens and Varnum (592) observed that strain LT/Sv females develop spontaneous ovarian teratomas as early as 30 d of age, with 50% of females affected by the time they are 90 d old. The penetrance jumps to more than 80% in recombinant inbred lines LTXBO and LTXBJ that were generated from intercrossing strains LT/Sv and C57BL/6J (593,594). This is curious because C57BL/6J females are not predisposed to teratoma formation. The phenotype is likely multigenic and includes a semidominant LT/Sv allele at ovarian teratoma susceptibility locus (Ots1) on chromosome 6 and modifier alleles from C57BL/6J (593,595).
Table 14.
Mouse models of germ cell ovarian cancer
| Model | Phenotype | Ref. |
|---|---|---|
| LT/Sv and LT-related strains (e.g., LTXBO, LTXBJ) | Abnormal metaphase I arrest; spontaneous ovarian teratomas as early as 30 d of age; 50–80% penetrance by 90 d of age | 592,593 |
| Mos−/− (Moloney sarcoma oncogene knockout) | Destabilization of metaphase II arrest; 30% have teratomas between 4 and 8 months of age | 453,454,603,604 |
| Inhα-Bcl2 (transgenic; mouse inhibin α promoter driving expression of Bcl2) | Mature cystic teratomas in 4 of 20 females | 607 |
| TG.KD (transgenic; imprinted transgene integrated on chromosome 8 of FVB/N strain) | Unilateral or bilateral mature and immature teratomas with occasional metastases to lymph nodes and lungs; 15–20% of hemizygous females affected; dominant effect secondary to alterations of endogenous gene(s) at insertion locus | 822 |
| Ubiquitin C-hCGαβ (double transgenic; human ubiquitin C promoter driving expression of human chorionic gonadotropin α- and β-subunits) | Teratomas; precocious puberty; enhanced steroidogenesis; infertility | 823 |
The teratomas in LT/Sv and LT-related strains (hereafter referred to as LT) are mostly benign and stem from parthenotes in the ovary that resemble normal embryos until the blastocyst stage when they become disorganized and form tumors (592). Eppig and colleagues (593,596,597) outlined the sequence of events leading from oocyte to tumor in LT females, namely metaphase I arrest, parthenogenetic activation, completion of the first meiotic division, development to the blastocyst stage, and teratoma formation. The mammalian oocyte normally advances without interruption from prophase I to metaphase II by the time of ovulation, remaining arrested in metaphase II until fertilization activates the egg and triggers completion of the second meiotic division. LT oocytes frequently deviate from this paradigm and arrest at metaphase I instead of metaphase II; when this occurs in the ovary (i.e., before ovulation), these oocytes are vulnerable to tumorigenesis (593). Metaphase I arrest in LT oocytes is initiated by sustained activation of the spindle assembly checkpoint, possibly due to meiotic spindle abnormalities or intrinsic defects in the checkpoint machinery (598). Although the checkpoint is eventually inactivated, the delay allows for premature buildup of CSF to levels that maintain metaphase I arrest (599,600,601,602). In normal oocytes that are not delayed, CSF does not reach adequate levels to induce arrest until metaphase II. The mechanisms responsible for parthenogenetic activation and teratoma development subsequent to metaphase I arrest in LT oocytes remain unknown.
As mentioned, in normal mammalian oocytes CSF is essential for metaphase II arrest by the time of ovulation. An integral component of CSF is MOS, a serine/threonine kinase with MAPK-stimulatory activity. Whereas metaphase I arrest predisposes LT oocytes to form teratomas, destabilization of metaphase II arrest leads to parthenogenetic activation of oocytes and teratoma development in Mos knockout females (453,454,603,604). The teratomas are usually benign and histologically similar to those in LT mice but appear with lower frequency and later onset; specifically, 30% of Mos−/− females have tumors between 4 and 8 months of age (604). Hirao and Eppig (594) noted that this incidence is peculiarly low given the large proportion of mutant oocytes that become parthenogenetically activated. However, in contrast to LT parthenotes, Mos−/− parthenotes rarely progress to the blastocyst stage, and this might explain their decreased propensity to form tumors (594).
In light of the substantial cross talk between oocytes and somatic cells, it is not surprising that several pieces of evidence have revealed connections between granulosa cells and the development of teratomas. In LT strains, the primary lesions responsible for tumor formation are intrinsic to the oocyte; nonetheless, cumulus cells contribute to the maintenance of metaphase I arrest, induction of parthenogenesis, and eventual progression to metaphase II (605). LT ovaries also contain numerous abnormal follicles comprised of a single layer of granulosa cells surrounding a disproportionately large oocyte (606). Parthenogenetic embryos that form teratomas are usually found in these granulosa cell-deficient follicles, and the presence of these follicles is necessary but insufficient for germ cell tumorigenesis in LT females. In another mouse model, overexpression of the antiapoptotic protein BCL2 in ovarian somatic cells resulted in mature cystic teratomas in four of 20 transgenic females (607). The predominant phenotype of these transgenic mice is prolonged somatic cell survival and enhanced folliculogenesis, and it was hypothesized that paracrine signals from mutant somatic cells influence oocyte differentiation and in some instances steer oocytes toward tumorigenesis. On the other hand, overexpression of BCL2 in mouse oocytes delays spontaneous apoptosis of oocytes in culture, but does not cause germ cell tumors (608).
D. Small RNAs in ovarian physiology and cancer
The discovery of small noncoding RNAs that direct gene silencing has unveiled new dimensions in reproductive physiology and disease. There are three major classes of silencing RNAs encoded in the mammalian genome: microRNAs (miRNAs), Piwi-interacting RNAs (piRNAs), and siRNAs. Each of these small RNA categories has unique structural and functional characteristics (summarized in Table 15).
Table 15.
Characteristics of mammalian small RNAs
| Class | Length (nucleotides) | Synthesis | Function |
|---|---|---|---|
| miRNA | 21–23 | DICER-dependent | Regulation of translation and stability of target mRNAs |
| siRNA | 21–23 | DICER-dependent | Transposon suppression and pseudogene regulation of founding source mRNAs |
| piRNA | 24–31 | DICER-independent feed-forward amplification loop | Transposon regulation and unknown functions |
1. Small RNAs in ovarian physiology
As illustrated in Fig. 7, miRNAs are transcribed by RNA polymerase II as long primary transcripts that are processed into approximately 70-nt stem-loop precursors by a complex that includes the ribonuclease (RNase) III DROSHA and the RNA-binding protein DGCR8. Precursor miRNAs are exported from the nucleus to the cytoplasm where they are cleaved by a second RNase III, DICER (Dicer1), to produce a 21- to 23-nt duplex structure, one strand of which is preferentially incorporated into the RNA-induced silencing complex (RISC). The RISC guides pairing of the mature miRNA with complementary sequences in the 3′ UTR of target mRNA transcripts. Perfect complementarity between a miRNA and its mRNA target causes mRNA degradation through endonucleolytic cleavage (reviewed in Ref. 609). Although this is often the scenario in plants, target pairing in animal cells is frequently imperfect and also down-regulates gene expression by repressing translation, possibly at the initiation or elongation steps, and by accelerating mRNA decay through removal of the poly(A) tail (609). In a complex twist to the miRNA dogma, recent evidence indicates that miRNAs may also activate translation under rare conditions (610). To date, there are approximately 700 human and 500 mouse miRNAs, and many are evolutionarily conserved. Collectively, miRNAs are predicted to directly regulate over 60% of human protein-coding genes (611). For several years, they were the only small RNAs that had been identified in mammals.
Figure 7.
Dicer-dependent synthesis of miRNAs and endogenous siRNAs. A, miRNA genes are transcribed by RNA polymerase II to generate primary transcripts. These transcripts are cropped by the DROSHA/DGCR8 Microprocessor complex to yield approximately 70-nt precursor miRNAs, which are exported from the nucleus to the cytoplasm by Exportin 5. There, the DICER complex processes the precursor into a 21- to 23-nt duplex consisting of the mature miRNA and its antisense. The mature miRNA is preferentially loaded into the miRNA-induced silencing complex (miRISC), which mediates pairing with complementary sequences in the 3′ UTR of target mRNA transcripts, leading to mRNA degradation and translational repression. The specificity of targeting is especially dependent on nucleotides 2–8 of the mature miRNA, known as the seed sequence. B, Endogenous siRNAs are synthesized from long, double-stranded RNA precursors derived from repetitive sequences, sense-antisense pairs, or inverted repeats that form hairpins. It is unknown whether endogenous siRNA precursors use Exportin 5 to translocate to the nucleus; however, once in the cytoplasm they are processed by the DICER complex into 21- to 23-nt duplexes. Mature siRNAs are incorporated into the siRNA-induced silencing complex (siRISC), which is similar to the miRISC but may also have unique components. Endogenous siRNAs function in transposon suppression and other functions such as pseudogene regulation of founding source mRNAs.
In 2006, five laboratories independently reported the discovery of mammalian piRNAs in mouse and rat testes (612,613,614,615,616). PiRNAs (24–31 nt) are longer than miRNAs and siRNAs (21–23 nt), consistent with the observation that piRNAs are generated without DICER through a feed-forward amplification loop that is still under investigation (reviewed in Refs. 617,618,619). There are estimated to be over 100,000 PiRNAs in the mammalian genome; they are encoded in relatively few genomic clusters and in a strand-specific manner within each cluster. Although the genomic locations of piRNA clusters are conserved between species, specific piRNA sequences are poorly conserved. Unlike miRNAs and siRNAs, which interact with Argonaute family proteins in the RISC, piRNAs interact with Piwi family proteins (MILI, MIWI, and MIWI2; also known as PIWIL2, PIWIL1, and PIWIL4, respectively) (reviewed in Ref. 620). In mice, homozygous deletion of individual Piwi proteins causes spermatogenesis arrest and male sterility (621,622,623). Furthermore, Mili and Miwi2 knockout males demonstrate activation of transposable elements, supporting the hypothesis that piRNAs suppress transposon mobilization in the male germline (623,624). Interestingly, the majority of mammalian piRNAs do not map to annotated repeats such as transposons and retrotransposons, suggesting other potential functions of the piRNA pathway including epigenetic regulation and translational control (617).
If piRNAs guard the male germline against transposon activation, what mechanisms similarly protect the female germline? Initially, only a handful of piRNAs had been cloned from whole mouse ovary, and it was unknown which were expressed in oocytes (625). Moreover, MILI is the only Piwi family protein detected in oocytes (626), but Mili knockout females are fertile (622), implying that piRNAs are not the only factors that maintain stability of the oocyte genome. Watanabe et al. (616) identified a novel class of oocyte-expressed small RNAs known as endogenous siRNAs. Similar to miRNAs, siRNAs are processed by DICER into 21- to 23-nt species that function in the RISC. However, unlike miRNAs, which have short stem-loop precursors, siRNAs are synthesized from longer double-stranded RNA precursors that are derived from retrotransposons and other sources.
The initial data provided a glimpse into the siRNA population in the female germline and motivated two independent laboratories to comprehensively profile the gamut of small RNAs in mouse oocytes using deep sequencing technology (626,627). In addition to detecting annotated miRNAs, they uncovered a broad MILI-bound piRNA population resembling piRNAs expressed in early-stage spermatocytes. Even more compelling was the identification of new endogenous siRNAs that are abundantly expressed. Most of these siRNAs are predicted to target specific retrotransposons that are also suppressed by piRNAs, a functional redundancy that could explain the absence of a phenotype in Mili knockout females, although retrotransposons preferentially targeted by either piRNAs or siRNAs were observed as well. Apart from retrotransposons, double-stranded RNA precursors of siRNAs are also generated from inverted repeat structures that form hairpins, the pairing of overlapping transcripts that are oppositely oriented, and the pairing of a protein-coding mRNA and an antisense transcript from its corresponding pseudogene. This latter example is most intriguing and suggests that mammalian pseudogenes, previously relegated to the domain of nonfunctional “junk DNA,” instead regulate the expression of their founding source mRNAs through the siRNA pathway.
Because DICER is required for the synthesis of both siRNAs and miRNAs, conditional deletion of Dicer1 in mouse oocytes offered additional insight into the functional roles of these small RNA pathways in the female germline (628,629). Dicer1 Zp3-Cre cKO females are sterile despite the observation that their ovaries are histologically normal and responsive to gonadotropins. Further investigation of mutant oocytes highlighted defects in spindle organization and chromosome cohesion that block completion of meiosis I. Microarray profiling of DICER cKO oocytes revealed up-regulation of genes involved in microtubule dynamics and increased expression of maternal transcripts that are normally degraded during meiotic maturation, suggesting that DICER-dependent small RNA pathways foster oocyte maturation by accelerating mRNA turnover and regulating genes essential for spindle integrity. MiRNAs predicted to target genes involved in microtubule-based processes include mir-103, let-7d, mir-16, mir-30b, and mir-30c. Also, the complete set of up-regulated genes was significantly enriched in putative binding sites for mir-495, mir-126, and mir-302c*.
Transposon levels and oocyte transcripts with transposon sequences embedded in their 3′ UTRs are increased in DICER cKO oocytes; in particular, repetitive sequences such as short interspersed nuclear elements (SINEs) and mouse transcript subfamilies B and C (MTB/MTC) are elevated (626,627,628). MTB and MTC are almost exclusively regulated by endogenous siRNAs and not piRNAs, indicating that transposon activation in mutant oocytes is secondary to loss of DICER-dependent siRNA biogenesis (627). Putative targets of pseudogene-derived siRNAs are also increased in the absence of DICER, for example, oogenesin 4 (Oog4), histone deacetylase 1 (Hdac1), and Ran GTPase activating protein 1 (Rangap1). The regulatory target of RANGAP1 is the RAS oncogene family member RAN, which is important for microtubule organization in mouse oocytes (630). Interestingly, mir-30b and mir-30c are predicted to target Ran, and accordingly, Ran expression is increased in the DICER knockout (628). The putative silencing of Ran by miRNAs and Rangap1 by siRNAs leads to speculation that miRNA and siRNA pathways may coordinately regulate related functional networks that are essential for oocyte maturation.
Whereas current evidence suggests that mammalian piRNAs are critical primarily in the male germline, and roles for endogenous siRNAs may be restricted to oocytes and ES cells, miRNA expression is ubiquitous and essential for the proper function of germ cells and somatic cells (summarized in Table 16). Conditional deletion of Dicer1 in various somatic cells and tissues of mice results in dramatic phenotypes that are predominantly attributed to loss of specific miRNAs, albeit dysfunction caused by disruption of siRNA biogenesis cannot be definitively excluded. To investigate global roles for miRNA and siRNA pathways in somatic cells of the female reproductive tract, our laboratory and others generated Dicer1 cKO mice using Amhr2-Cre (631,632,633,634). Although Amhr2-Cre is commonly used for studying gene function in ovarian granulosa cells, it is also expressed postnatally in the smooth muscle and stromal cells of the oviducts and uterus, and embryonically in the mesenchyme of the developing Müllerian ducts that give rise to these structures (260,505,506,507). Dicer1 Amhr2-Cre females are sterile and have multiple reproductive defects, including prominent bilateral oviductal cysts that act as a reservoir for spermatozoa and oocytes and prevent embryos from transiting the oviduct to enter the uterus for implantation. The uteri of mutant females demonstrate normal decidualization (631); however, they are shorter and have fewer endometrial glands, an observation that correlates with implantation defects after embryo transfer experiments (633). Mutant ovaries are histologically normal except for a subtle but significant increase in granulosa cell apoptosis. In response to gonadotropin stimulation, immature cKO females ovulate significantly fewer oocytes, have oocytes that are trapped in luteinized follicles, and show a marked decreased in the proportion of oocytes that progress to the two-cell stage after overnight culture. Taken together, these observations suggest that DICER is essential for proper development of the oviducts and uterus, and that DICER expression in granulosa cells regulates ovulation and indirectly contributes to oocyte quality because of communication between somatic and germ cell compartments of the ovary.
Table 16.
Dicer mutations with female reproductive phenotypes
| Mouse model | Phenotype | Ref. |
|---|---|---|
| Dicer1 Zp3-Cre (Dicer cKO in oocytes) | Sterile; defects in spindle organization and chromosome cohesion block completion of meiosis I; increased expression of genes involved in microtubule dynamics, maternal transcripts normally degraded during meiotic maturation, transposons, and putative targets of pseudogene-derived siRNAs | 626,627,628,629 |
| Dicer1 Amhr2-Cre (Dicer cKO in ovary, oviduct, uterus) | Sterile; bilateral oviductal cysts sequester embryos and prevent transit to the uterus; decreased ovulation; uteri decidualize normally but are shorter with fewer endometrial glands and demonstrate implantation defects after embryo transfer experiments | 631,632,633,634 |
| Dicer1 hypomorphic mutation | Sterile; luteal insufficiency due to diminished vascularity in CLs | 636 |
One possible explanation for the ovulatory defects we observed may be aberrant LH signaling in granulosa cells upon loss of DICER. Fiedler et al. (635) compared miRNA expression profiles in granulosa cells isolated from wild-type mice pharmacologically stimulated with PMSG alone or PMSG followed by hCG. Of the 212 miRNAs detected, 13 miRNAs were differentially expressed as early as 4 h after hCG treatment. The expression levels of two up-regulated miRNAs, mir-132 and mir-212, were also increased in granulosa cell cultures treated with cAMP, a principal second messenger downstream of LH. Notably, these two miRNAs share identical seed sequences and hence may target some of the same transcripts. Although specific interactions between granulosa cell miRNAs and mRNAs remain to be identified and validated, these data demonstrate that LH signaling alters granulosa cell miRNA expression, which in turn might influence the regulation of genes that govern ovulation.
It appears that granulosa cell miRNA expression may be dispensable for luteinization because we did not observe luteinization defects after deleting DICER in granulosa cells (631). However, miRNAs may indirectly affect CL function through roles in angiogenesis. Otsuka et al. (636) created a viable mouse model with hypomorphic DICER expression and reduced miRNA production. They reported female sterility secondary to luteal insufficiency, characterized by lower serum progesterone levels and decreased ovarian expression of Lhcgr, Cyp11a1, and Prlr after copulation. Mutant females showed diminished vascularity in their CLs, along with up-regulation of the antiangiogenic factors tissue inhibitor of metalloproteinase 1 (TIMP1) and platelet factor 4 (PF4). The Timp1 3′ UTR has binding sites for two miRNAs, mir-17–5p and let-7b, which decreased expression of a luciferase reporter fused to the Timp1 3′ UTR and also reduced TIMP1 activity in cultured endothelial cells. Intrabursal injection of wild-type mice with inhibitors against these two miRNAs increased TIMP1 levels and impaired CL angiogenesis, whereas combined overexpression of mir-17–5p and let-7b in hypomorphic DICER females suppressed TIMP1 levels and improved CL vascularity. Nonetheless, restoration of these two miRNAs failed to maintain pregnancy in DICER mutants, thus highlighting potential roles for other miRNAs.
2. Misregulation of miRNAs in ovarian cancer
The precise functions of ovarian miRNAs remain to be delineated; however, studies in a wide range of tissues and cell types indicate that miRNAs guide fundamental cellular processes such as proliferation, differentiation, and apoptosis. Hence, it is not surprising that aberrations in miRNA expression have been observed in a variety of human cancers. MiRNA profiles reflect the developmental lineages and differentiation states of tumors, sometimes with greater accuracy than mRNA profiles (637). During mammalian development, a global rise in miRNA levels correlates with increasing cellular differentiation. In contrast, cancers generally demonstrate an overall reduction in miRNA expression that corresponds to a less differentiated state. Searching for a causal relationship between these observations, Kumar et al. (638) globally repressed miRNA levels in mouse and human cancer cell lines using shRNA-mediated knockdown of three factors required for miRNA processing: DROSHA, DGCR8, and DICER. With knockdown of these gene products, the cells showed increased proliferation, improved colony formation, and better migration, and also formed tumors more rapidly and with greater invasive properties in nude mice. Notably, impaired miRNA processing enhanced tumorigenesis only in cells that were already transformed but was insufficient to cause de novo transformation, suggesting that decreases in miRNA expression modify rather than initiate carcinogenesis. Although these experiments broadly implicate miRNAs as tumor suppressors, either through direct targeting or indirect regulation of oncogenes, other studies highlight roles for specific miRNAs as oncogenes such that their overexpression accelerates tumorigenesis, clearly indicating that these small RNAs have dual functions in cancer.
Potential mechanisms of miRNA deregulation in cancer are diverse and include defects in miRNA processing (638), DNA copy number abnormalities (639,640,641), epigenetic alterations (641,642), mutations in the miRNA or its mRNA binding site (643), and aberrant transcription (644). Using microarray profiling, a handful of studies have identified genome-wide alterations in miRNA expression in primary epithelial ovarian tumors and established ovarian cancer cell lines. This work has focused predominantly on the most common subtype, serous ovarian carcinoma, presumably because these tumors are more readily procured, although a few endometrioid and clear cell ovarian cancers have also been profiled. Both Iorio et al. (645) and Nam et al. (646) found up-regulation of mir-141, mir-200a, mir-200b, mir-200c, and mir-21 in ovarian cancers compared with normal ovary, down-regulation of mir-99a, mir-100, mir-125a, mir-125b, mir-143, mir-145, mir-214, and let-7 family members, and aberrations unique to their respective studies. Similarly, Yang et al. (647) observed up-regulation of mir-200a and down-regulation of mir-100, mir-125b, and let-7 family members in their analysis of serous ovarian cancers compared with HIOSE118, a human OSE cell line immortalized with SV40 large TAg (648). On the other hand, Dahiya et al. (649) reported changes in the opposite direction for mir-99a, mir-100, mir-141, mir-200a, and mir-21 when comparing ovarian cancers to HOSE-B, a human OSE cell line immortalized with human papillomavirus genes E6 and E7. These discrepancies underscore a major challenge in dissecting the molecular signature of ovarian cancer: the choice of “normal” control dramatically influences the outcome of genome-wide expression studies.
Zorn et al. (650) systematically illustrated this problem by comparing the gene expression profiles between a common set of serous ovarian carcinomas and five different control groups: OSE brushings; whole ovary; short-term cultures of normal OSE (NOSE); SV40 large TAg-immortalized OSE cell lines (IOSE); and telomerase-immortalized OSE cell lines (TIOSE). For each cancer and normal pairing, the majority of differentially expressed genes were unique to that particular comparison, with no gene present in all five comparisons. Additionally, hierarchical clustering of the controls revealed distinct profiles for each group, with the first major branch point separating the cultured samples (NOSE, IOSE, TIOSE) from those that were not cultured (OSE brushings and whole ovary), pointing to the substantial impact of in vitro manipulation on OSE-derived cells. Taken together, these data emphasize that candidate genes and miRNAs that emerge from profiling experiments should be meticulously examined in independent functional assays to evaluate their actual relevance to cancer formation and progression. To date, specific roles for only a few miRNAs have been described in epithelial ovarian cancer (summarized in Table 17).
Table 17.
MiRNAs with putative functions in epithelial ovarian cancer
| MiRNA | Select validated targets | Potential role of miRNA | Ref. |
|---|---|---|---|
| mir-34a; mir-34b; mir-34c | BCL2; CDK4; CDK6; CCNE2; MET | Activated by p53; loss of function mutations in p53 may decrease mir-34 expression | 651 |
| mir-200a; mir-200b; mir-200c; mir-141; mir-429; mir-205 | ZEB1; ZEB2 | Repress epithelial-to-mesenchymal transition | 659 |
| mir-214 | PTEN | Overexpression promotes chemoresistance | 647 |
| mir-199a-5p | IKKβ (IKBKB) | Loss of function activates NF-κB pathway, which may foster a protumor microenvironment | 667 |
| let-7 family | KRAS; HRAS; MYC; HMGA2 | Loss of function may promote tumorigenesis; let-7i knockdown increases chemoresistance | 666,677,678,679,680 |
Given the frequent mutation of p53 in high-grade human ovarian carcinomas, Corney et al. (651) compared miRNA expression profiles between primary cultures of wild-type and p53-deficient mouse OSE cells to screen for miRNAs that are regulated in a p53-dependent manner. Mir-34b and mir-34c were significantly down-regulated upon p53 inactivation and are transcribed from a single locus containing an upstream p53 binding site that is conserved between mouse and human. Both miRNAs are markedly decreased in a p53 null human ovarian cancer cell line (SKOV-3) compared with short-term cultures of human OSE cells, and two profiling studies reported lower levels of mir-34c in primary serous ovarian carcinomas compared with normal ovary (645) or an immortalized OSE cell line (649). Overexpression of mir-34b and mir-34c in p53 null mouse OSE cell lines suppressed their proliferation and anchorage-independent growth (651). These data are consistent with five independent studies demonstrating activation of mir-34 family members by p53, with mir-34 overexpression inducing cell cycle arrest or apoptosis depending on the cellular context (652,653,654,655,656). Accordingly, predicted mir-34 targets are involved in cell cycle control, apoptosis, and DNA repair, and validated targets include BCL2, CDK4/6, cyclin E2 (CCNE2), and met proto-oncogene (MET) (652,654,657). Aside from ovarian cancer, reduced mir-34 expression has been reported in neuroblastoma (658), pancreatic cancer (653), and non-small cell lung cancer (652). Thus, the mir-34 family is an integral effector of the p53 tumor suppressor network, and the loss of mir-34 expression, either secondary to p53 mutation or independently in the case of p53 wild-type tumors, may be a driving force in ovarian and other cancers.
One of the hallmarks of tumor progression is epithelial-to-mesenchymal transition (EMT), a complex process characterized by loss of E-cadherin-mediated cell-cell adhesion and polarity, and by acquisition of vimentin expression and the ability to migrate and invade, ultimately facilitating metastasis. The zinc finger E-box binding homeobox transcription factors ZEB1 and ZEB2 promote EMT by repressing expression of E-cadherin and other master regulators of epithelial polarity (659,660). The ratio of ZEB2/E-cadherin expression is higher in stage IV compared with stage III ovarian carcinomas and may predict poor overall survival (661). Several independent studies demonstrated that mir-200 family members (mir-200a, mir-200b, mir-200c, mir-141, mir-429) and mir-205 directly target ZEB1 and ZEB2 and prevent EMT induction (659,662,663). In the National Cancer Institute panel of human cancer cell lines (NCI60), which includes several ovarian cancer lines, mir-200 members are selectively expressed in E-cadherin-positive and vimentin-negative cells, further indicating that these miRNAs are strong determinants of the epithelial phenotype (659). Moreover, Park et al. (659) found a significant correlation between mir-200c and E-cadherin expression in primary serous ovarian carcinomas. These data raise the possibility that loss of mir-200 family members may contribute to ovarian cancer metastasis, but counterintuitive to this hypothesis is the observation that higher levels of mir-200 family miRNAs were significantly correlated with shorter overall survival in women with serous ovarian carcinoma (646). A formal comparison of mir-200 expression and function between primary and metastatic ovarian lesions has not been done.
Loss of function mutations in PTEN are characteristic of endometrioid ovarian carcinomas (664); however, reduced or absent PTEN expression has been described in other ovarian cancer subtypes with intact PTEN alleles (665), hinting at alternate mechanisms of PTEN inactivation. Yang et al. (647) showed that the PTEN 3′ UTR is targeted by mir-214, which is up-regulated in ovarian cancers [although two other studies (645,646) reported decreased mir-214 expression] and inversely correlated with PTEN protein levels. Overexpression of mir-214 in human ovarian cancer cell lines reduced PTEN expression and conferred resistance to cisplatin-induced apoptosis by activating the AKT pathway, whereas knockdown of mir-214 sensitized cells to cisplatin (647). Although many women with ovarian cancer are initially responsive to adjuvant chemotherapy with cisplatin, the majority of patients develop recurrent disease that is drug-resistant. Out of 11 patients with recurrent ovarian cancer, eight had low or undetectable mir-214 levels in their primary tumors but elevated mir-214 expression in their recurrent lesions, suggesting that mir-214 may promote chemoresistance in ovarian cancer. Notably, an independent analysis detected higher mir-214 expression in primary ovarian carcinomas from women with poor responses to chemotherapy compared to those with greater chemosensitivity (666).
Chen et al. (667) identified another miRNA-mediated pathway that may be a critical regulator of chemoresponsiveness in ovarian cancer. Nuclear factor-κB (NF-κB) activation directs the constitutive secretion of proinflammatory cytokines that promote tumor progression by stimulating cell proliferation, inducing antiapoptotic proteins, and enhancing chemoresistance. Using ovarian cancer cell lines and primary cells isolated from malignant ovarian cancer ascites and solid tumors, they demonstrated that NF-κB pathway activity is directly dependent on the expression of inhibitor of kappa light polypeptide gene enhancer in B-cells, kinase beta (IKKβ), part of a protein complex that phosphorylates and degrades an inhibitor of NF-κB. Whereas NF-κB activity varied with IKKβ protein levels, IKKβ mRNA expression was relatively constant, indicating posttranscriptional regulation of IKKβ. Microarray profiling revealed that the expression of mir-199a was inversely correlated with NF-κB activity, and further experiments showed direct targeting of IKKβ by mir-199a. Therefore, low mir-199a expression leads to up-regulation of IKKβ and contributes to NF-κB pathway activation, potentially fostering a protumor microenvironment. Interestingly, mir-199a levels were lower in ovarian cancers compared with normal ovary (645), but higher in ovarian cancers compared with an IOSE cell line (649).
To pinpoint additional miRNAs that modulate chemoresponsiveness, Sorrentino et al. (668) analyzed differences in miRNA expression between various human ovarian cancer cell lines that are sensitive or resistant to cisplatin and paclitaxel. Three miRNAs (mir-30c, mir-130a, mir-335) were consistently down-regulated in the drug-resistant cell lines, and each miRNA has a unique set of predicted target genes. Although the levels of these miRNAs were not manipulated to show a causative role in drug response, reporter assays demonstrated that mir-130a targets colony stimulating factor 1 (CSF1). The overexpression of CSF1 and its receptor denotes poor prognosis in ovarian and breast cancers (669,670,671). CSF1 mediates chemoresistance of breast cancer cells and promotes invasion and metastasis of ovarian cancer cells in culture and in a xenograft model (672,673,674). Although it was previously demonstrated that the CSF1 3′ UTR contains an AU-rich element that binds glyceraldehyde-3-phosphate dehydrogenase and controls mRNA stability (675,676), this is the first report indicating posttranscriptional regulation of CSF1 by the miRNA pathway.
Extending beyond the profiles of drug-resistant cell lines, Zhang and colleagues (666) identified miRNAs that are differentially expressed between primary ovarian cancers from women with chemosensitive and chemoresistant disease. Among the top miRNAs comprising a distinct signature, let-7i was dramatically reduced in chemoresistant tumors, and lower let-7i levels were significantly correlated with shorter progression-free survival. Knockdown of let-7i in human ovarian cancer cell lines increased resistance to cisplatin, whereas let-7i overexpression enhanced chemosensitivity. These data are corroborated by a wealth of evidence indicating that let-7 family members are potent tumor suppressors in a variety of human cancers. Let-7 is a chief regulator of cell proliferation pathways and directly represses known oncogenes such as KRAS (677), HRAS (677), MYC (678), and HMGA2 (HMG AT-hook 2) (679,680). HMGA2 is an architectural transcription factor that is undetectable in normal OSE but is expressed in ovarian cancer (681,682,683). Silencing of HMGA2 in ovarian cancer cell lines suppressed growth and increased apoptosis in culture and significantly reduced tumor burden in nude mice (683). HMGA2 expression is inversely correlated with let-7 expression in the NCI60 cancer cell lines and in primary ovarian carcinomas (681,682). Patients with high HMGA2 and low let-7 in their tumors have worse progression-free survival compared to women with a low HMGA2/let-7 expression ratio, suggesting that this ratio may be an important predictor of outcome (681,682).
Let-7 is among a group of miRNAs that are absent in the early embryo but then induced in later stages of development and in adult tissues (682). It has been hypothesized that these miRNAs in part control the onset and maintenance of cell differentiation by suppressing the expression of embryonic proteins, including HMGA2. On the other hand, the loss of let-7 might allow reexpression of the embryonic program and transformation to a de-differentiated state that drives tumorigenesis. Indeed, reduced let-7 levels determine key properties of breast cancer stem cells, namely self-renewal (through the regulation of HRAS) and multipotent differentiation (through the regulation of HMGA2), and these findings may have broad implications for other human cancers (684). As mentioned in Section II, the posttranscriptional maturation of let-7 primary transcripts is suppressed by the pluripotent factor LIN28 and its homolog, LIN28B (31,32,33,34,35). In agreement with the discussion above, high LIN28B expression is significantly associated with increased risk of disease progression and death in women with epithelial ovarian cancer (685). Furthermore, consistent with roles for LIN28 in PGC development and oncogenesis, LIN28/LIN28B expression is a reliable marker for testicular germ cell tumors (30), and these proteins may likewise be involved in the pathogenesis of germ cell ovarian cancers, although this has not yet been explored.
3. MiRNAs as prognostic and diagnostic biomarkers
The aforementioned studies highlight emerging roles for miRNAs in the pathogenesis of ovarian cancer, including regulating the response to chemotherapy and the potential to metastasize. If aberrations in miRNA function significantly influence tumor behavior, it follows that the expression patterns of specific miRNAs may be powerful prognostic indicators for cancer patients. For women with ovarian cancer, the identification of miRNA-related biomarkers is still in its infancy, but the early data have revealed several candidates whose expression in ovarian tumors portends prolonged (+) or diminished (−) survival, for example, let-7(+), mir-200(−), mir-93(−), and the eight miRNAs(+) clustered in the Dlk1-Gtl2 domain, a putative tumor suppressor locus on human chromosome 14 (641,646,666). These eight miRNAs are mir-337, mir-368, mir-376a, mir-376b, mir-377, mir-410, mir-432, and mir-495 (641).
As described earlier, there is a global reduction in miRNA expression in a variety of human cancers, suggesting deregulation of the miRNA processing machinery. Lower mRNA and protein levels of DROSHA and DICER in advanced-stage, poorly differentiated epithelial ovarian carcinomas compared with benign OSE specimens have been reported (686). Low DROSHA expression significantly correlated with suboptimal surgical cytoreduction, whereas low DICER1 expression significantly associated with advanced tumor stage and was an independent predictor of shorter survival. These data conflict with other evidence demonstrating similar mRNA and protein levels of DROSHA and DICER between early- and late-stage epithelial ovarian cancers, without any correlation to patient survival (641). A third study found that there were no significant differences in the expression of miRNA processing enzymes in multiple types of human cancer (637). Hence, the utility of these factors as prognostic markers remains inconclusive. Finally, a fourth study identified lower expression of eukaryotic translation initiation factor 6 as an independent predictor of reduced disease-free survival, but not overall survival, in women with serous ovarian carcinoma (687). Eukaryotic translation initiation factor 6 associates with the RISC complex and is a key mediator of miRNA-dependent gene silencing (688), suggesting that global impairment of miRNA function may contribute to tumor recurrence.
The stark contrast in 5-yr survival rates for women diagnosed with advanced stage ovarian cancer (30%) compared with localized disease (90%) emphasizes the crucial importance of early detection (467). Recently, several laboratories demonstrated that tumor-derived miRNAs in serum or plasma are stable markers for cancer detection (689,690), an exciting discovery considering that miRNA expression profiles classify human cancers with high accuracy (637). The mechanisms by which circulating miRNAs are protected from endogenous RNase activity remain unknown, but may involve the packaging of miRNAs into exosomes, vesicles of endocytic origin that are released into the extracellular environment (691,692). A pilot comparison of serum miRNA expression between healthy women and patients newly diagnosed with epithelial ovarian cancer before treatment revealed up-regulation (mir-21, mir-92, mir-93, mir-126, mir-29a) and down-regulation (mir-155, mir-127, mir-99b) of specific miRNAs in cancer patients (693). These preliminary observations are encouraging and warrant further investigation on a larger scale, especially to determine whether differentially expressed miRNAs are clinically informative in early stage disease. Indeed, the development of minimally invasive miRNA screens to detect and monitor ovarian cancer would be revolutionary.
V. The Assisted Reproductive Technology Laboratory
Infertility is a worldwide problem for individuals wishing to reproduce, affecting about 15% of couples and causing significant economic, social, and psychological distress for the childless couple (694,695). Approximately 9% of women in the 20- to 44-yr-old age group experience a 12-month period where they are unable to become pregnant (696). Luckily for these women, assisted reproductive technologies (ART) have been developed to help them achieve their dreams of becoming mothers. In this section, we will briefly describe the advances that have been made in the ART laboratory and clinic that have revolutionized reproduction. For a historical perspective on gonadotropin use in the clinic, the reader is referred to a 2004 article by Lunenfeld (697). For a more extensive review of the development of ovarian stimulation agents and their use in the clinic, the reader is referred to a 2006 review in this journal by Macklon et al. (698). In the current review, we will give sufficient background to bring the reader up to speed and give an update on key advances in the last few years that we believe will have an important impact on the advancement of female fertility.
A. Hormonal preparations
The first “test tube baby,” Louise Brown, celebrated her 31st birthday this year (699). During the past three decades, there have been many procedural changes that have come about to enhance the success rate of ART. Whereas Louise Brown was conceived through a natural ovulatory cycle (699), within a few years, clomiphene citrate and urinary gonadotropins were being used to hyperstimulate the ovaries (700). Clomiphene citrate, a selective ER modulator, inhibits the feedback of estrogen on the pituitary leading to increased release of FSH (a noncovalent α:β heterodimer; the α-subunit is common to the four glycoprotein hormones, whereas the β-subunit is unique to FSH, LH, hCG, and TSH) and stimulation of follicle recruitment. Since the early days of ART, there have been multiple advances in the hormones, their concentrations, and the preparations that are given to women to stimulate their ovaries to produce mature oocytes that can be fertilized in vitro. Although clomiphene citrate continues to be used in the setting of controlled ovarian stimulation, multiple preparations of human postmenopausal gonadotropins and recombinant preparations of human FSH, LH, and hCG (viewed as “safer” because they are not prone to any possible transfer of infectious agents such as viruses or prions) have been effectively developed and are being used successfully in the ART clinic. Furthermore, these recombinant preparations are not only used in the ART clinic; in 2008, Pergoveris, a mixture of recombinant FSH and LH, was approved in Europe for the treatment of severe combined FSH and LH deficiencies.
To more closely mimic the longer continuous levels of FSH seen in normal cycles, several recombinant long-acting FSH analogs have been created including FSH-CTP (addition of one or more O-linked C-terminal extensions of hCGβ to the FSHβ C terminus), tandem FSH-CTP (fusion of FSHβ-CTP and the common α-subunit), an N-linked tandem FSH variant (FSHβ-α subunit fusion linked by an N-linked peptide), single-chain FSH-Fc (a human FSHβ-α subunit-linker sequence-IgG1 Fc domain fusion), and heterodimeric FSH-Fc fusion (human FSHβ-linker sequence-IgG1 Fc domain-6 His and human α- subunit-linker sequence-IgG1 Fc domain covalent dimer) (701,702,703). FSH-CTP (corifollitropin alfa), the first of this new generation of long-acting gonadotropin analogs (also referred to as sustained follicular stimulants) to be tested in feasibility studies in the clinic, has an approximately 2- to 3-fold longer half-life (t½ ≈ 65 h) and essentially identical biopotency compared with FSH, producing similar numbers of oocytes (704). Most importantly, corifollitropin alfa yielded equal numbers of good quality embryos to transfer, the incidence of ovarian hyperstimulation syndrome was low, and only a single initial injection of corifollitropin alfa was required, compared to multi-daily injections of recombinant FSH injections. Results from a randomized phase II trial (NCT00598208) showed that corifollitropin alfa was highly effective in a 1-wk regimen (705). Patients were given 60, 120, or 180 mg of corifollitropin alfa on d 1, a GnRH antagonist on d 5 through the end of the cycle, and daily injections of recombinant FSH (Puregon) beginning 1 wk later until it was time to induce oocyte maturation with hCG. Compared with daily injections of Puregon for the first 7 d of the cycle, there was a statistically significant increase in the number of cumulus-oocyte complexes retrieved when patients were treated with 120 mg and 180 mg of corifollitropin alfa. Likewise, there was an increase in the mean number of good quality embryos obtained, and cumulative pregnancy rate was also higher in these same groups. In July 2008, Schering announced the results of its first phase III trial (NCT00696800, called ENGAGE), the largest double-blind fertility trial ever. In this trial, 1509 women received either a single 150 mg dose of corifollitropin alfa or daily injections of 200 IU of Puregon for 7 d. Other aspects of the study (the GnRH antagonist and follow-up injections of FSH and hCG) were the same as the phase II trial. The findings indicated that the ongoing pregnancy rate with corifollitropin alfa (38.9%) vs. Puregon (38.1%) was similar. If results of the additional phase III trials are as promising as the above studies, then corifollitropin alfa protocols could replace many of the older protocols in the clinic with added benefits to the patient. In January 2009, the European Medicine Agency decided to review Schering-Plough’s Marketing Authorization Application for corifollitropin alfa. For an extensive review on corifollitropin alfa, the reader should see the recent paper by Fauser et al. (706).
One goal of the pharmaceutical companies who are working in the ART area has been to develop orally bioavailable small molecule (low molecular weight) FSH and LH agonists that could replace the injectable glycoproteins. The early days of development of these small molecules have been reviewed by Lunenfeld (697), and the more recent advances have been reviewed by Arey (707). An amazing aspect regarding the development of these small molecular analogs is that they can “substitute” for the large bulky glycosylated LH or FSH protein dimers. It is believed that these small molecules bind to allosteric pockets in the FSH or LH receptors that are distinct from the orthostatic binding sites of the endogenous ligands. Thus, these small molecules are not mimicking the FSH or LH ligand binding, but instead activate the receptor through an independent site to induce a conformational change in the receptor that mimics endogenous ligand binding to the receptor. This feature of the small molecules has made it easier to find and develop both more potent and specific FSH and LH agonists.
There are three classes of small molecule glycoprotein hormone receptor agonists: the thiazolidine (FSHR agonists), the pyrazole (LH receptor agonists), and the thienopyrimidine (TSH receptor/LH receptor agonist) classes. Members of the thiazolidine class were the first reported small molecule glycoprotein hormone agonists that were shown to act allosterically (708). Although the pyrazole class was shown to be selective for LH receptor agonist activity with an EC50 in the low micromolar range (709), discovery and optimization studies resulted in a specific thienopyrimidine analog (Org 43553) with an EC50 of 3.7 nm for LH receptor activation (710). Org 43553 is the first orally active LH receptor agonist, is able to induce ovulation in mice and rats when given orally (710), and has been developed as a good radioligand for the identification of more potent analogs (711). It will be interesting to see whether similar orally efficacious FSHR analogs can also be developed, possibly following the leads of Org 43553.
B. In vitro fertilization and intracytoplasmic sperm injection
It is estimated that 3.5 million babies have been born through the use of ART. Although children have been born through the injection of sperm into a woman’s reproductive tract (intrauterine or intracervical insemination), the most common procedures used in the ART clinic are IVF and intracytoplasmic sperm injection (ICSI). IVF, as its name implies, allows oocytes to be fertilized by spermatozoa in vitro and requires the donor’s spermatozoa to have all of the necessary characteristics for fertilization (i.e., the spermatozoa must be motile, have the capability to bind to the oocyte zona pellucida, and be able to fuse with the oocyte membrane). ICSI was first reported in 1992, and involves the injection of a single sperm into the cytoplasm of an MII oocyte. Because ICSI bypasses a number of steps in the fertilization process, normal spermatozoa and abnormal (e.g., immotile) spermatozoa or earlier stage elongation and elongated spermatids can be used for the procedure, allowing some men with infertility to pass their genetic material to offspring. In 2008, the International Committee for Monitoring Assisted Reproductive Technologies reported that about 200,000 babies are born annually from ART and the total number of procedures are about 1.3 million. In addition, approximately 50% of ART procedures performed in 2008 were in seven countries (Japan, the United States, France, Germany, Spain, the United Kingdom, and Australia). Based on 2004 data, Denmark has the highest incidence of ART babies born (4.2% of all births) (705). Furthermore, most ART laboratories now use ICSI (63% of cycles) as the routine procedure.
Despite the growing use of ICSI worldwide, there are reports of increased malformations in children born from ICSI including imprinting errors such as Beckwith-Wiedemann syndrome and Angelman syndrome (reviewed in Refs. 712 and 713). However, these malformations are less likely to be associated with the ICSI procedure per se than the transfer of sperm containing defective genetic material or, alternatively, the use of oocytes from women who are transmitting genetic abnormalities to their offspring. The Practice Committee of the American Society for Reproductive Medicine and the Practice Committee of the Society for Assisted Reproductive Technology have formulated a number of recommendations on ICSI (714).
In addition to the above studies on ICSI, there are also reports that ART (IVF or ICSI) is associated with birth defects. Using data from the National Birth Defects Prevention Study for babies born in the United States between October 1997 and December 2003, a number of structural birth defects were found to be increased in children whose mothers used ART to become pregnant (715). Among the defects observed in singleton births are septal heart defects, cleft lip with or without cleft palate, esophageal atresia, and anorectal atresia. Similar to ICSI itself, the etiologies of the increase in these birth defects are not clear, although an increase in transmission of defective genetic material and/or epigenetic alterations associated with culturing of embryos under nonphysiological conditions could be causal.
C. Advances in cryopreservation
Freezing of spermatozoa for IVF or ICSI is relatively straightforward. Likewise, excellent strategies have been developed for cryopreserving human and nonhuman embryos. Although challenges remain for the preservation of ovarian tissue (see below), a major breakthrough has been made for cryopreserving oocytes.
Why is the cryopreservation of oocytes (or ovarian tissue) important? Oocytes are highly sensitive to toxins, including polycyclic aromatic hydrocarbons (120,716), as well as drugs used for cancer chemotherapy and radiation treatment. Furthermore, in some countries, there are strict guidelines for human embryo freezing. For example, the German embryo protection law that was passed in 1991 states that no more than three embryos can be produced in each IVF cycle and all embryos, irrespective of their quality, must be transferred, cannot be frozen, and cannot be discarded. However, this has led to an increased incidence of fetal reductions. Italy subsequently enacted nearly identical legislation restricting embryo freezing and limiting the number of oocytes fertilized to three. Thus, there are practical and ethical reasons why oocyte cryopreservation should be used.
In 1986, the first baby was born from a frozen human oocyte (717). Despite the millions of children that have been born through ART procedures, only a few hundred have been born through fertilization of a frozen oocyte. This low incidence is secondary to the poor survival of oocytes cryopreserved with the typical slow-freezing cryoprotectant solutions. Both the slow speed of the freezing methods and the long-term exposure of oocytes to the solutions used for cryoprotection are associated with significant trauma to the human oocyte, making this methodology impractical in the ART laboratory (reviewed in Ref. 718).
Fortunately, scientists and clinicians have been able to take an alternative approach for oocyte cryopreservation which involves vitrification, the rapid freezing of specimens (e.g., oocytes or embryos) in ultrasmall volumes (719,720,721,722,723,724,725,726). The original study by Kuwayama et al. (719) demonstrated higher oocyte survival, fertilization, and pregnancy outcome when a slightly lower concentration of ethylene glycol (5.0 mol/liter) was used and when cumulus cells were not removed from the oocytes, suggesting that this method could also be used in the future for freezing GV oocytes. In the 4 yr since this initial report, the vitrification approach for freezing human oocytes has become remarkably efficient in multiple ART laboratories (725). One possible reason for the success of the vitrification method over slow freezing is the more rapid recovery of the meiotic spindle with vitrification (727,728), allowing fertilization after thawing to proceed in a more timely manner. In addition, various groups have also begun to use straws or CryoLoops that are closed to the liquid nitrogen (729,730), thereby avoiding any possible contamination (infectious or otherwise) of the vitrified human oocytes. Likewise, vitrification has also been shown to be highly efficient for the cryopreservation of d 3 human embryos (731); 94.8% of vitrified embryos survived the procedure compared with slow freezing (88.7%), and significantly more developed to the blastocyst stage with vitrification (60.3%) vs. slow freezing (49.5%). These vitrification strategies are also beginning to be used for freezing human ovarian cortex (732) for the preservation of fertility in women who are subjected to chemotherapy or radiation therapy for cancer treatment. However, with this cohort of women, a strategy for the in vitro culture of human primordial follicles to fertilizable MII oocytes is a necessary first step to ensuring future fertility.
D. Choosing the best oocyte—morphological and molecular analysis
In vivo or in vitro, the best embryo will obviously develop from the best oocyte. Abnormalities in oocytes, whether genetic or metabolic, are not beneficial to a healthy embryo or a healthy offspring. A major problem for the ART laboratory is how to decide which oocyte is “best.” Unlike chorionic villus biopsies or amniocentesis, where there are an abundance of cells available for molecular and chromosomal analysis, the genetic status of the oocyte DNA that joins with the sperm cannot be evaluated. Over the last few years, there have been several advances made for noninvasive analysis of the oocyte, and these strategies are discussed below.
In theory, all oocytes that come from a chromosomally normal female should be capable of progressing through meiosis to become a normal haploid gamete that is capable of fusing with a haploid sperm to become a normal diploid embryo. Meiosis in females has several stages as follows: oocytes enter meiosis during embryogenesis and arrest in diplotene of meiosis I; after follicle recruitment, oocytes in antral follicles will reenter meiosis after the LH surge, release the first polar body but subsequently arrest at metaphase of MII; meiosis is completed upon fertilization with a sperm and release of the second polar body (Fig. 1). This abrupt stop-and-go pattern in female meiosis would not be considered advantageous to producing a normal haploid germ cell. With age, the oocytes in women are more likely to demonstrate chromosomal defects (733), increasing the risk of miscarriage, newborn death, or the birth of a child with chromosomal abnormalities. Although it is impossible to evaluate these possibilities at the time of oocyte-sperm fusion in vivo in the fallopian tube, it has become possible to perform some analysis of the oocytes in vitro in the ART clinic. Because the majority of aneuploidies arise in oocytes, the polscope has been used in some ART laboratories for noninvasive analysis of human oocytes over the last decade (reviewed in Ref. 734). The polscope is a polarized light microscope that can visualize birefringent structures in the oocyte. The two major structures that can be analyzed are the meiotic spindle, an important component necessary for producing a normal chromosomal complement, and the zona pellucida, the large, abundant, glycoprotein coat that surrounds the oocyte. By visualizing these two structures, the polscope allows a clinician to make judgments about the “normality” of an oocyte, and several groups have been able to make generalities about the best oocyte. In the original publication by Keefe and colleagues (735,736), 61.8% of oocytes were fertilized when obvious meiotic spindles were observed with the polscope vs. 44.2% fertilized when the spindles were not observed with the polscope. Unlike fluorescent labeling techniques, the short time of exposure (10–20 sec) of the oocytes to the polarized light does not have any detrimental effects on the oocyte and its ability to produce viable embryos. Other studies have demonstrated that oocytes with longer spindle lengths (>12 nm) and wider inner zona pellucida layers (10–12 nm) resulted in more ICSI fertilized oocytes that progressed to the blastocyst stage (737). Meta-analysis from multiple studies also confirmed the above findings that visualization of the meiotic spindle resulted in higher fertilization rates and better embryos based on multiple parameters (738). Surprisingly, there was no correlation with clinical pregnancy or implantation rate, although the polscope may be useful when there is a governmental limit to the number of oocytes that can be fertilized in one cycle.
A second method that has been used to make predictions about oocyte chromosomal quality is polar body biopsy (reviewed in Ref. 739). Polar body biopsy can be viewed as noninvasive because the removal of the first polar body does not alter the oocyte. Removal of the polar body can subsequently permit the analysis of the chromosomal remains after the first meiotic division by methods such as fluorescent in situ hybridization or comparative genomic hybridization. Thus, in situations where prenatal genetic diagnosis of a blastomere is either not permitted or is unwanted, this could be a viable noninvasive option for the couple. Similar to other noninvasive methodologies, this method cannot detect any meiotic defects that result at the second meiotic division, although biopsies of the second polar body are also possible, nor can it evaluate chromosomal defects that may arise from the paternal side at fertilization. However, first polar body biopsy has been used in combination with oocyte vitrification, and in this scenario may allow clinicians to make additional judgments about which oocyte is best (740).
Based on studies of cumulus cell-oocyte interactions in the mouse, our group and others have evaluated gene expression patterns of human cumulus cells as another noninvasive strategy to predict oocyte quality. Our studies were directed at three of the major targets of the oocyte-secreted growth factors GDF9 and BMP15, namely HAS2, PTGS2, and the BMP antagonist gremlin 1 (GREM1) (361). Because cumulus cells are stripped off the oocyte before ICSI and their gene expression is regulated by these oocyte-secreted growth factors, we hypothesized that poor quality or immature oocytes would not regulate expression of these cumulus cell genes to the same extent as high quality or mature oocytes. Our findings showed that expression of PTGS2, HAS2, and GREM1 in cumulus cells derived from oocytes that were fertilized and developed into morphologically high-grade embryos (grades 3, 4, and 5) were 6-fold, 6-fold, and 15-fold higher, respectively, compared with cumulus cells derived from oocytes that gave rise to low-grade embryos (grades 1 and 2). A follow-up study by Cillo et al. (362) confirmed the HAS2 and GREM1 findings. Several groups have taken these studies one step further. Sirard’s group (741) used cDNA microarrays and Affymetrix oligonucleotide microarrays to correlate gene expression in mural granulosa cells with oocyte quality and pregnancy. These studies identified 115 genes associated with competent follicles. Hamamah and colleagues (742) used Affymetrix microarrays to correlate cumulus cell gene expression with pregnancy outcome. These authors identified two genes, BCL2L11 (BCL2-like 11) and PCK1 (phosphoenolpyruvate carboxykinase 1), as being up-regulated and NFIB (nuclear factor I/B) as being down-regulated in cumulus cells of oocytes that led to a positive pregnancy outcome.
In summary, we believe that all of the above methods could be used to make the best predictions of oocyte quality, especially in environments where there are restrictions on embryo freezing or where all fertilized eggs, regardless of quality, must be transferred to the mother.
E. Stem cells and nuclear cloning
As mentioned in Section I. D, during embryogenesis there are sexually dimorphic differences in the pathways that male and female germ cells take; whereas male PGCs in the current somatic cell environment will arrest in mitosis, female PGCs will go through one last mitotic division and then enter and arrest in meiosis. Male germ cells will eventually become spermatogonial stem cells that can be isolated and shown to propagate in culture (although not yet successful for humans). However, because female germ cells enter meiosis, it makes it difficult to imagine a scenario where these differentiated cells can be dedifferentiated to become oocyte stem cells capable of replenishing a new ovarian environment. This may be one reason for the rapid depletion of oocytes over time and the eventual entry into menopause, which is in contrast to spermatogonial stem cells that remain throughout the lifetime of most male mammals.
Alternative strategies to reproduce “oocytes” in vitro have now been described (743). These studies demonstrate that follicle-like structures can form in vitro during mouse ES cell cultures and that cells that mimic oocytes (i.e., express several oocyte markers such as GDF9, FIGLA, ZP1, ZP2, and ZP3 and can enter meiosis) are observed to form. Although fertilization and key events to recapitulate normal oocyte physiology have not been observed, these observations hold promise for future endeavors into this area including nuclear cloning (see below). At this point, studies have not been reported where human “oocytes” can be derived from human ES cells.
In parallel with the above situation, at least in mice, it has been possible for many years to convert PGCs into pluripotent embryonic germ cells (744,745,746). The growth factors LIF, KIT ligand, and FGF2 appear to be critical for this dedifferentiation event (747). Key reprogramming events during the formation of embryonic germ cells are the suppression of PRDM1 and the subsequent derepression of Myc and Klf4 (Krüppel-like factor 4), the movement of PRMT5 out of the nucleus and into the cytoplasm, and the early activation of the LIF/STAT3 pathway, with translocation of STAT3 into the nucleus. These studies not only make it possible to understand how PGCs form from embryonic germ cells by studying the process in reverse, but now also make it possible to go all the way back to totipotency by starting out with PGCs. Interestingly, it is now possible to produce pluripotent stem cells from mouse testis (748,749) and adult human testis (750). Although not described so far, it is also possible that these XY stem cells could be used to make “oocytes” for additional manipulation in vitro.
Major advances in nuclear cloning have occurred recently, and it has even become possible to produce induced pluripotent stem (iPS) cells from multiple lineages (751,752). One shortfall for mammalian nuclear cloning studies and in particular the production of designer ES cells for each individual has been the scarcity of oocytes for these studies. The new advances in the development of iPS cells may help to overcome this deficit. However, if iPS cells are not viewed as “replacement” for ES cells, the advent of the above nuclear cloning involves the injection of a diploid nucleus into an oocyte in which the nucleus was removed. This strategy was first used to create the sheep Dolly, the first mammal created via cloning (753). However, described techniques for oocyte cryopreservation may now make it possible to address the availability of oocytes as a limiting factor for nuclear cloning (754). Thus, continued advances in the ART laboratory may again help advance broad areas of medicine and healthcare.
VI. Future Perspectives
This review has focused on genetic models of ovarian development and folliculogenesis, and along the way, we have touched on numerous growth factors and signaling pathways implicated in these processes. Mouse ES cell technology has made it possible to study hundreds of genes in the ovary. By designing conditional alleles and taking advantage of the numerous transgenic Cre recombinase lines now available for studying gene function during formation of the germline and the gonad, and in oocytes and somatic cells of the ovary at different stages of postnatal development, we will continue to expand our knowledge of normal and pathological ovarian biology. Using in silico strategies and online databases to identify candidate genes with similar expression patterns to known ovarian genes is one way to find novel genes that may influence follicular development (755). The Ovarian Kaleidoscope Database is a useful online resource with up-to-date information on the expression, function, and regulation of genes in the ovary (756).
The prevalence of POF increases with age such that one in 100 women are affected by the time they are 40 yr old (213,757). Frustratingly, the causes of POF in the vast majority of affected women remain unknown. Although women who are FMR1 (fragile X mental retardation 1) premutation carriers make up the largest identified group with POF, they account for only 2% of nonfamilial cases (758), and the pathogenesis of ovarian failure in this cohort is still unknown. It is not even known whether the abnormalities in the FMR1 premutation carriers leading to POF are primarily due to hormonal, somatic cell, or germ cell defects, or a combination of the three.
As reproductive biologists continue to identify genes with critical functions in folliculogenesis, we may also gain insight into additional candidate genes that contribute to POF, which may help physicians diagnose and treat women with this condition, as well as idiopathic infertility. Furthermore, the sequencing of the human genome, the follow-up International HapMap Project that identified polymorphisms within distinct ethnic populations (759), and the availability of multiple other tools for genome-wide analysis of patient DNA will help researchers and clinicians hone in on genomic regions associated with fertility disorders. This information, in conjunction with already available or easily generated mouse models, should contribute to a better understanding of the etiology of human infertility and potentially identify therapeutic targets for treating this condition. For example, the HapMap consortium identified several single nucleotide polymorphisms (SNPs) in ACVR1, which encodes a type I receptor (ALK2) for some TGFβ family members, in over 5% of Caucasians. Although there was no difference in the frequency of ACVR1 SNPs between normovulatory women and women with polycystic ovarian syndrome, the SNPs were associated with elevated levels of AMH and follicle number in the women with polycystic ovarian syndrome (195). Thus, although the current approach to determining the etiology of nonsyndromic POF includes karyotype analysis, testing for premutations in FMR1, and testing for autoimmune disorders (including adrenal and thyroid) (213), additional genetic tests may become available in this postgenomics era.
Although there are many agents that reduce reproductive life span and induce reproductive senescence (e.g., cigarette smoking), one of the major hurdles for reproductive biologists and clinicians is how to prolong and enhance the reproductive life span of a woman. Although dietary restriction and low body fat shut down reproductive cycling, as observed in long-distance runners, it is possible that these effects may not only prolong somatic life span but also reproductive (ovarian) life span as recently suggested (760,761). Thus, these transient halts may actually be advantageous to the body and ovary alike. An intriguing article by Schmidt et al. (762) showed that male mice housed with females had a 20% longer reproductive life span than bachelor males. The reverse experiment has not been reported, so the question remains: Does the presence of a man extend a woman’s reproductive life span?
In addition to employing genomic tools when evaluating fertility disorders, the advent of high-throughput genomic technologies, particularly next generation sequencing, will also broaden our knowledge of genetic events that lead to ovarian cancer. In a search for novel mutations, The Cancer Genome Atlas has sequenced 6000 candidate oncogenes and tumor suppressors, including miRNA-encoding loci, in an extensive panel of human serous epithelial ovarian tumors and matched normal controls (http://cancergenome.nih. gov). Large-scale collaborations are also under way to identify chromosomal aberrations, gene fusions, and cancer-initiating “stem” cells in serous and other ovarian carcinoma histotypes. These data will inspire the creation of novel mouse models to investigate tumorigenic mechanisms, especially for the less common mucinous and clear cell subtypes for which there are no genetic models. In parallel to mining the cancer genome, the merits of sequencing the cancer transcriptome are exemplified by the recent report that FOXL2 mutations are pathognomonic for human adult granulosa cell tumors (587). Relatively few tumors need to be sequenced in the discovery phase of these studies, suggesting that a similar strategy may lead to the identification of specific mutations in human juvenile granulosa cell tumors, conceivably in TGFβ pathway genes or regions orthologous to the Gct susceptibility loci, based on insight gleaned from mouse models.
The discovery of specific functions for miRNAs in ovarian cancer has made them enticing therapeutic targets, especially as sensitizing agents for existing treatments considering their ability to modulate chemosensitivity. Although overexpression of miRNAs in vivo might be accomplished using shRNA constructs along with conventional gene delivery methods (e.g., viral and liposomal), the inhibition of specific cancer-associated miRNAs may be achieved using modified oligonucleotides with high affinity for complementary RNA (antimiRs). Several groups have demonstrated efficient silencing of liver-specific mir-122 in mice using independently designed antimiRs (763,764,765). Importantly, Kauppinen and colleagues (766) reported similar results in nonhuman primates without any associated toxicities. By antagonizing mir-122, hepatitis C replication can be inhibited, thereby uncovering a novel approach to prevent chronic hepatitis C infection, a major cause of hepatocellular carcinoma (767). These groundbreaking studies suggest that miRNAs are promising and tangible molecular targets for the treatment of human cancers, including ovarian cancer.
Advances in the ART laboratory continue to occur. The rapidity with which recombinant FSH, LH, and hCG have become available for clinical use is remarkable, especially because the first reports demonstrating that these dimers can be effectively secreted were published in the mid-1980s. These recombinant proteins have played a major role in the fertility clinic, and the next generation of long-acting FSH analogs are making their way onto the scene, with corifollitropin alfa leading the way. The recent successes with the identification and synthesis of orally active small molecule LH receptor agonists and the findings of specific small molecule FSHR agonists suggest that a new arsenal of allosteric drugs may be available in the next decade to complement and expand existing treatment options for infertile couples. Future goals of ART should be to increase births of singletons and reduce multigestation pregnancies. New noninvasive methodologies could include identification of cumulus cell gene expression signatures that differentiate good and bad oocytes and the development of advanced metabolic and proteomic assays for oocyte- and embryo-secreted substances. All of this progress will undoubtedly increase the chances of a couple bringing a healthy newborn into their lives.
Acknowledgments
We are grateful to our colleagues, JoAnne Richards, Daniel de Matos, and Stephen Palmer, for their outstanding insights and suggestions on this review. We thank Roopa Nalam for providing invaluable support in preparing tables and figures, and Shirley Baker for expert assistance in preparation of the manuscript.
Footnotes
Reproductive biology and cancer studies in the Matzuk laboratory have been supported by National Institutes of Health Grants R01HD32067, R01CA60651, R37HD33437, P01HD36289, and U01HD60496 (to M.M.M.); the Specialized Cooperative Centers in Reproduction and Infertility (U54HD07495) (to M.M.M.), T32DK00763, K12DK083014, T32GM07730 (to M.A.E.), T32HD007165 (to M.A.E.), T32GM008307 (to A.K.N.); and by the Ovarian Cancer Research Fund (to M.M.M.), the Joseph and Matilda Melnick Endowed Fund (to A.K.N.), and Baylor Research Advocates for Student Scientists (to M.A.E. and A.K.N.).
Disclosure Summary: The authors have nothing to disclose.
First Published Online September 23, 2009
Abbreviations: AC, Adenylyl cyclase; ADAMTS1, a disintegrin-like and metallopeptidase with thrombospondin type 1 motif, 1; AdCre, adenovirus expressing Cre recombinase; AHR, aryl hydrocarbon receptor; AKAP, A-kinase anchoring protein; AKR1C18, aldo-keto reductase family 1, member C18; ALPL, alkaline phosphatase, liver/bone/kidney; AMH, anti-Müllerian hormone; AMHR2, AMH type II receptor; APC/C, anaphase-promoting complex/cyclosome; AR, androgen receptor; Areg, amphiregulin; ART, assisted reproductive technology; BCL, B cell lymphoma/leukemia; BDNF, brain-derived neurotrophic factor; bHLH, basic helix-loop-helix; BMP, bone morphogenetic protein; BPES, blepharophimosis/ptosis/epicanthus inversus syndrome; BRCA1, breast cancer 1; Btc, betacellulin; CBX2, chromobox homolog 2; CDK, cyclin-dependent kinase; C/EBPβ, CCAAT/enhancer-binding protein β; CEEF, cumulus expansion-enabling factor; cKO, conditional knockout; CL, corpus luteum; CSF, cytostatic factor; CSF1, colony stimulating factor 1; CSPG2, chondroitin sulfate proteoglycan 2; CTNNB1, β-catenin; CX43, connexin 43; CYP11A1, cytochrome P450 side-chain cleavage enzyme; CYP17A1, cytochrome P450 17α-hydroxylase/17,20-lyase; CYP19A1, cytochrome P450 aromatase; CYP26B1, cytochrome P450 26B1; DAZL, deleted in azoospermia-like; DHH, desert hedgehog; DKK1, dickkopf homolog 1; DND1, dead end homolog 1; dpc, days post-coitus; DPPA3, developmental pluripotency-associated 3; E, embryonic day; EGF, epidermal growth factor; EGFR, EGF tyrosine kinase receptor; EHMT2, euchromatic histone lysine N-methyltransferase 2; EMT, epithelial-to-mesenchymal transition; EMX2, empty spiracles homolog 2; ER, estrogen receptor; Ereg, epiregulin; αβERKO, ERα and ERβ knockout; FBXO43, F-box protein 43; FF-MAS, follicular fluid-meiosis-activating sterol; FGF, fibroblast growth factor; FGFR2, FGF receptor 2; FIGLA, factor in the germline α; FMR1, fragile X mental retardation 1; FOG2, friend of GATA2; FOX, forkhead box; FSHR, FSH receptor; FST, follistatin; FZD, FRIZZLED; Gct, granulosa cell tumor; GDF9, growth differentiation factor 9; GREM1, gremlin 1; GS, germline stem (cells); GV, germinal vesicle; GVBD, GV breakdown; HAS2, hyaluronan synthase 2; hCG, human chorionic gonadotropin; H3K9me2, dimethylated histone 3 lysine 9; H3K27me3, trimethylated histone 3 lysine 27; HMG, high-mobility group; HMGA2, HMG AT-hook 2; HPG, hypothalamic-pituitary-gonadal; Hsd3b1, 3β-hydroxysteroid dehydrogenase; IBMX, 3-isobutyl-1-methylxanthine; ICSI, intracytoplasmic sperm injection; IFITM3, interferon-induced transmembrane protein 3; IHH, Indian hedgehog; IKKβ, inhibitor of kappa light polypeptide gene enhancer in B-cells, kinase beta; IαI, inter-α-trypsin inhibitor; IOSE, immortalized OSE; iPS, induced pluripotent stem (cells); IVF, in vitro fertilization; KTS, lysine-threonine-serine; Lfng, lunatic fringe; LHCGR, LH/choriogonadotropin receptor; LHX9, LIM homeobox protein 9; LIF, leukemia inhibitory factor; LRH1, liver receptor homolog 1; LRP6, low-density lipoprotein receptor-related protein 6; Mapk1/3 dKO, Mapk1 Mapk3 double mutant mice; MII, meiosis II; miRNA, microRNA; MOS, Moloney sarcoma oncogene; MPF, maturation-promoting factor; MT1, metallothionein 1; mTOR, mammalian target of rapamycin; NF-κB, nuclear factor-κB; NGF, nerve growth factor; NLRP, leucine-rich repeat and pyrin domain containing; NOBOX, newborn ovary homeobox; NOS3, nitric oxide synthase 3; NR5A, nuclear receptor subfamily 5, group A; Nr2c2, nuclear receptor subfamily 2, group C, member 2; NRIP1, nuclear receptor interacting protein 1; NTF5, neurotrophin 5; NTRK, neurotrophic tyrosine kinase receptor; OOX, oocytectomized; OSE, ovarian surface epithelium; P, postnatal day; PAH, polycyclic aromatic hydrocarbon; PDE, phosphodiesterase; PDGF, platelet-derived growth factor; PGC, primordial germ cell; PI3K, phosphatidylinositol 3-kinase; PIP2, phosphatidylinositol 4,5-bisphosphate; piRNA, Piwi-interacting RNA; PKA, protein kinase A; PMSG, pregnant mare serum gonadotropin; POF, premature ovarian failure; PPARγ, peroxisome proliferator-activated receptor γ; PR, progesterone receptor; PRDM, PRDI- BF1- RIZ domain containing 1; PRL, prolactin; PRLR, PRL receptor; PRMT5, protein arginine N-methyltransferase 5; PTCH, patched; PTEN, phosphatase and tensin homolog; PTGER2, prostaglandin E receptor 2, subtype EP2; PTGS2, prostaglandin synthase 2; PTX3, pentraxin 3; Rangap1, Ran GTPase activating protein 1; RISC, RNA-induced silencing complex; RNAi, RNA interference; RSPO1, R-spondin homolog 1; SF1, steroidogenic factor 1; shRNA, short hairpin RNA; siRNA, small interfering RNA; SMO, Smoothened; SNP, single nucleotide polymorphism; SOHLH1, spermatogenesis and oogenesis helix-loop-helix 1; SOX9, SRY-box containing gene 9; SRY, sex-determining region of chromosome Y; STAR, steroidogenic acute regulatory protein; STAT, signal transducer and activation of transcription; Stra8, stimulated by retinoic acid gene 8; SV40, simian virus 40; SYCP3, synaptonemal complex protein 3; TAF, TATA-binding protein-associated factor; TAg, T antigen; TEX14, testis-expressed gene 14; TIMP1, tissue inhibitor of metalloproteinase 1; TNFAIP6, TNFα-induced protein 6; TR4, testicular orphan nuclear receptor 4; UTR, untranslated region; WEE2, WEE1 homolog 2; WT1, Wilms tumor 1 homolog.
References
- Chiquoine AD 1954 The identification, origin and migration of the primordial germ cells in the mouse embryo. Anat Rec 118:135–146 [DOI] [PubMed] [Google Scholar]
- Mintz B, Russell ES 1957 Gene-induced embryological modifications of primordial germ cells in the mouse. J Exp Zool 134:207–237 [DOI] [PubMed] [Google Scholar]
- McCoshen JA, McCallion DJ 1975 A study of the primordial germ cells during their migratory phase in Steel mutant mice. Experientia 31:589–590 [DOI] [PubMed] [Google Scholar]
- Ozdzenski W 1967 Observations on the origin of the primordial germ cells in the mouse. Zool Polon 17:367–379 [Google Scholar]
- Spiegelman M, Bennett D 1973 A light- and electron-microscopic study of primordial germ cells in the early mouse embryo. J Embryol Exp Morphol 30:97–118 [PubMed] [Google Scholar]
- Clark JM, Eddy EM 1975 Fine structural observations on the origin and associations of primordial germ cells of the mouse. Dev Biol 47:136–155 [DOI] [PubMed] [Google Scholar]
- Ginsburg M, Snow MH, McLaren A 1990 Primordial germ cells in the mouse embryo during gastrulation. Development 110:521–528 [DOI] [PubMed] [Google Scholar]
- MacGregor GR, Zambrowicz BP, Soriano P 1995 Tissue non-specific alkaline phosphatase is expressed in both embryonic and extraembryonic lineages during mouse embryogenesis but is not required for migration of primordial germ cells. Development 121:1487–1496 [DOI] [PubMed] [Google Scholar]
- Chang H, Brown CW, Matzuk MM 2002 Genetic analysis of the mammalian TGF-β superfamily. Endocr Rev 23:787–823 [DOI] [PubMed] [Google Scholar]
- Hayashi K, de Sousa Lopes SM, Surani MA 2007 Germ cell specification in mice. Science 316:394–396 [DOI] [PubMed] [Google Scholar]
- Saga Y 2008 Mouse germ cell development during embryogenesis. Curr Opin Genet Dev 18:337–341 [DOI] [PubMed] [Google Scholar]
- Lawson KA, Dunn NR, Roelen BA, Zeinstra LM, Davis AM, Wright CV, Korving JP, Hogan BL 1999 Bmp4 is required for the generation of primordial germ cells in the mouse embryo. Genes Dev 13:424–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying Y, Liu XM, Marble A, Lawson KA, Zhao GQ 2000 Requirement of BMP8b for the generation of primordial germ cells in the mouse. Mol Endocrinol 14:1053–1063 [DOI] [PubMed] [Google Scholar]
- Ying Y, Zhao GQ 2001 Cooperation of endoderm-derived BMP2 and extraembryonic ectoderm-derived BMP4 in primordial germ cell generation in the mouse. Dev Biol 232:484–492 [DOI] [PubMed] [Google Scholar]
- Chang H, Matzuk MM 2001 Smad5 is required for mouse primordial germ cell development. Mech Dev 104:61–67 [DOI] [PubMed] [Google Scholar]
- Tremblay KD, Dunn NR, Robertson EJ 2001 Mouse embryos lacking Smad1 signals display defects in extra-embryonic tissues and germ cell formation. Development 128:3609–3621 [DOI] [PubMed] [Google Scholar]
- Hayashi K, Kobayashi T, Umino T, Goitsuka R, Matsui Y, Kitamura D 2002 SMAD1 signaling is critical for initial commitment of germ cell lineage from mouse epiblast. Mech Dev 118:99–109 [DOI] [PubMed] [Google Scholar]
- Chu GC, Dunn NR, Anderson DC, Oxburgh L, Robertson EJ 2004 Differential requirements for Smad4 in TGFβ-dependent patterning of the early mouse embryo. Development 131:3501–3512 [DOI] [PubMed] [Google Scholar]
- Arnold SJ, Maretto S, Islam A, Bikoff EK, Robertson EJ 2006 Dose-dependent Smad1, Smad5 and Smad8 signaling in the early mouse embryo. Dev Biol 296:104–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Sousa Lopes SM, Hayashi K, Surani MA 2007 Proximal visceral endoderm and extraembryonic ectoderm regulate the formation of primordial germ cell precursors. BMC Dev Biol 7:140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitou M, Barton SC, Surani MA 2002 A molecular programme for the specification of germ cell fate in mice. Nature 418:293–300 [DOI] [PubMed] [Google Scholar]
- Lange H, Holec S, Cognat V, Pieuchot L, Le Ret M, Canaday J, Gagliardi D 2008 Degradation of a polyadenylated rRNA maturation by-product involves one of the three RRP6-like proteins in Arabidopsis thaliana. Mol Cell Biol 28:3038–3044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohinata Y, Payer B, O'Carroll D, Ancelin K, Ono Y, Sano M, Barton SC, Obukhanych T, Nussenzweig M, Tarakhovsky A, Saitou M, Surani MA 2005 Blimp1 is a critical determinant of the germ cell lineage in mice. Nature 436:207–213 [DOI] [PubMed] [Google Scholar]
- Payer B, Saitou M, Barton SC, Thresher R, Dixon JP, Zahn D, Colledge WH, Carlton MB, Nakano T, Surani MA 2003 Stella is a maternal effect gene required for normal early development in mice. Curr Biol 13:2110–2117 [DOI] [PubMed] [Google Scholar]
- Bortvin A, Goodheart M, Liao M, Page DC 2004 Dppa3/Pgc7/stella is a maternal factor and is not required for germ cell specification in mice. BMC Dev Biol 4:2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent SD, Dunn NR, Sciammas R, Shapiro-Shalef M, Davis MM, Calame K, Bikoff EK, Robertson EJ 2005 The zinc finger transcriptional repressor Blimp1/Prdm1 is dispensable for early axis formation but is required for specification of primordial germ cells in the mouse. Development 132:1315–1325 [DOI] [PubMed] [Google Scholar]
- Robertson EJ, Charatsi I, Joyner CJ, Koonce CH, Morgan M, Islam A, Paterson C, Lejsek E, Arnold SJ, Kallies A, Nutt SL, Bikoff EK 2007 Blimp1 regulates development of the posterior forelimb, caudal pharyngeal arches, heart and sensory vibrissae in mice. Development 134:4335–4345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabuta Y, Kurimoto K, Ohinata Y, Seki Y, Saitou M 2006 Gene expression dynamics during germline specification in mice identified by quantitative single-cell gene expression profiling. Biol Reprod 75:705–716 [DOI] [PubMed] [Google Scholar]
- Yamaji M, Seki Y, Kurimoto K, Yabuta Y, Yuasa M, Shigeta M, Yamanaka K, Ohinata Y, Saitou M 2008 Critical function of Prdm14 for the establishment of the germ cell lineage in mice. Nat Genet 40:1016–1022 [DOI] [PubMed] [Google Scholar]
- West JA, Viswanathan SR, Yabuuchi A, Cunniff K, Takeuchi A, Park IH, Sero JE, Zhu H, Perez-Atayde A, Frazier AL, Surani MA, Daley GQ 2009 A role for Lin28 in primordial germ-cell development and germ-cell malignancy. Nature 460:909–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo I, Joo C, Cho J, Ha M, Han J, Kim VN 2008 Lin28 mediates the terminal uridylation of let-7 precursor microRNA. Mol Cell 32:276–284 [DOI] [PubMed] [Google Scholar]
- Newman MA, Thomson JM, Hammond SM 2008 Lin-28 interaction with the Let-7 precursor loop mediates regulated microRNA processing. RNA 14:1539–1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybak A, Fuchs H, Smirnova L, Brandt C, Pohl EE, Nitsch R, Wulczyn FG 2008 A feedback loop comprising lin-28 and let-7 controls pre-let-7 maturation during neural stem-cell commitment. Nat Cell Biol 10:987–993 [DOI] [PubMed] [Google Scholar]
- Viswanathan SR, Daley GQ, Gregory RI 2008 Selective blockade of microRNA processing by Lin28. Science 320:97–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piskounova E, Viswanathan SR, Janas M, LaPierre RJ, Daley GQ, Sliz P, Gregory RI 2008 Determinants of microRNA processing inhibition by the developmentally regulated RNA-binding protein Lin28. J Biol Chem 283:21310–21314 [DOI] [PubMed] [Google Scholar]
- Nishikawa N, Toyota M, Suzuki H, Honma T, Fujikane T, Ohmura T, Nishidate T, Ohe-Toyota M, Maruyama R, Sonoda T, Sasaki Y, Urano T, Imai K, Hirata K, Tokino T 2007 Gene amplification and overexpression of PRDM14 in breast cancers. Cancer Res 67:9649–9657 [DOI] [PubMed] [Google Scholar]
- Dettman EJ, Justice MJ 2008 The zinc finger SET domain gene Prdm14 is overexpressed in lymphoblastic lymphomas with retroviral insertions at Evi32. PLoS ONE 3:e3823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuneyoshi N, Sumi T, Onda H, Nojima H, Nakatsuji N, Suemori H 2008 PRDM14 suppresses expression of differentiation marker genes in human embryonic stem cells. Biochem Biophys Res Commun 367:899–905 [DOI] [PubMed] [Google Scholar]
- Gyory I, Wu J, Fejér G, Seto E, Wright KL 2004 PRDI-BF1 recruits the histone H3 methyltransferase G9a in transcriptional silencing. Nat Immunol 5:299–308 [DOI] [PubMed] [Google Scholar]
- Seki Y, Yamaji M, Yabuta Y, Sano M, Shigeta M, Matsui Y, Saga Y, Tachibana M, Shinkai Y, Saitou M 2007 Cellular dynamics associated with the genome-wide epigenetic reprogramming in migrating primordial germ cells in mice. Development 134:2627–2638 [DOI] [PubMed] [Google Scholar]
- Hajkova P, Ancelin K, Waldmann T, Lacoste N, Lange UC, Cesari F, Lee C, Almouzni G, Schneider R, Surani MA 2008 Chromatin dynamics during epigenetic reprogramming in the mouse germ line. Nature 452:877–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuva de Sousa Lopes SM, van den Driesche S, Carvalho RL, Larsson J, Eggen B, Surani MA, Mummery CL 2005 Altered primordial germ cell migration in the absence of transforming growth factor β signaling via ALK5. Dev Biol 284:194–203 [DOI] [PubMed] [Google Scholar]
- Farini D, La Sala G, Tedesco M, De Felici M 2007 Chemoattractant action and molecular signaling pathways of Kit ligand on mouse primordial germ cells. Dev Biol 306:572–583 [DOI] [PubMed] [Google Scholar]
- Tsuda M, Sasaoka Y, Kiso M, Abe K, Haraguchi S, Kobayashi S, Saga Y 2003 Conserved role of nanos proteins in germ cell development. Science 301:1239–1241 [DOI] [PubMed] [Google Scholar]
- Youngren KK, Coveney D, Peng X, Bhattacharya C, Schmidt LS, Nickerson ML, Lamb BT, Deng JM, Behringer RR, Capel B, Rubin EM, Nadeau JH, Matin A 2005 The Ter mutation in the dead end gene causes germ cell loss and testicular germ cell tumours. Nature 435:360–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ancelin K, Lange UC, Hajkova P, Schneider R, Bannister AJ, Kouzarides T, Surani MA 2006 Blimp1 associates with Prmt5 and directs histone arginine methylation in mouse germ cells. Nat Cell Biol 8:623–630 [DOI] [PubMed] [Google Scholar]
- Chuva de Sousa Lopes SM, Hayashi K, Shovlin TC, Mifsud W, Surani MA, McLaren A 2008 A X chromosome activity in mouse XX primordial germ cells. PLoS Genet 4:e30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto N, Yoshida M, Kuratani S, Matsuo I, Aizawa S 1997 Defects of urogenital development in mice lacking Emx2. Development 124:1653–1664 [DOI] [PubMed] [Google Scholar]
- Barbaux S, Niaudet P, Gubler MC, Grünfeld JP, Jaubert F, Kuttenn F, Fékété CN, Souleyreau-Therville N, Thibaud E, Fellous M, McElreavey K 1997 Donor splice-site mutations in WT1 are responsible for Frasier syndrome. Nat Genet 17:467–470 [DOI] [PubMed] [Google Scholar]
- Le Caignec C, Delnatte C, Vermeesch JR, Boceno M, Joubert M, Lavenant F, David A, Rival JM 2007 Complete sex reversal in a WAGR syndrome patient. Am J Med Genet A 143A:2692–2695 [DOI] [PubMed] [Google Scholar]
- Kreidberg JA, Sariola H, Loring JM, Maeda M, Pelletier J, Housman D, Jaenisch R 1993 WT-1 is required for early kidney development. Cell 74:679–691 [DOI] [PubMed] [Google Scholar]
- Laity JH, Dyson HJ, Wright PE 2000 Molecular basis for modulation of biological function by alternate splicing of the Wilms’ tumor suppressor protein. Proc Natl Acad Sci USA 97:11932–11935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammes A, Guo JK, Lutsch G, Leheste JR, Landrock D, Ziegler U, Gubler MC, Schedl A 2001 Two splice variants of the Wilms’ tumor 1 gene have distinct functions during sex determination and nephron formation. Cell 106:319–329 [DOI] [PubMed] [Google Scholar]
- Wilhelm D, Englert C 2002 The Wilms tumor suppressor WT1 regulates early gonad development by activation of Sf1. Genes Dev 16:1839–1851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto Y, Taniguchi H, Hamel F, Silversides DW, Viger RS 2008 A GATA4/WT1 cooperation regulates transcription of genes required for mammalian sex determination and differentiation. BMC Mol Biol 9:44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birk OS, Casiano DE, Wassif CA, Cogliati T, Zhao L, Zhao Y, Grinberg A, Huang S, Kreidberg JA, Parker KL, Porter FD, Westphal H 2000 The LIM homeobox gene Lhx9 is essential for mouse gonad formation. Nature 403:909–913 [DOI] [PubMed] [Google Scholar]
- Jost A 1947 Recherches sur la differenciation sexuelle de l’ embryon de lapin. Arch Anat Microsci Morphol Exp 36:271–315 [Google Scholar]
- Smagulova FO, Manuylov NL, Leach LL, Tevosian SG 2008 GATA4/FOG2 transcriptional complex regulates Lhx9 gene expression in murine heart development. BMC Dev Biol 8:67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X, Ikeda Y, Parker KL 1994 A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell 77:481–490 [DOI] [PubMed] [Google Scholar]
- Achermann JC, Ito M, Ito M, Hindmarsh PC, Jameson JL 1999 A mutation in the gene encoding steroidogenic factor-1 causes XY sex reversal and adrenal failure in humans. Nat Genet 22:125–126 [DOI] [PubMed] [Google Scholar]
- Achermann JC, Ozisik G, Ito M, Orun UA, Harmanci K, Gurakan B, Jameson JL 2002 Gonadal determination and adrenal development are regulated by the orphan nuclear receptor steroidogenic factor-1, in a dose-dependent manner. J Clin Endocrinol Metab 87:1829–1833 [DOI] [PubMed] [Google Scholar]
- Correa RV, Domenice S, Bingham NC, Billerbeck AE, Rainey WE, Parker KL, Mendonca BB 2004 A microdeletion in the ligand binding domain of human steroidogenic factor 1 causes XY sex reversal without adrenal insufficiency. J Clin Endocrinol Metab 89:1767–1772 [DOI] [PubMed] [Google Scholar]
- Schlaubitz S, Yatsenko SA, Smith LD, Keller KL, Vissers LE, Scott DA, Cai WW, Reardon W, Abdul-Rahman OA, Lammer EJ, Lifchez CA, Magenis E, Veltman JA, Stankiewicz P, Zabel BU, Lee B 2007 Ovotestes and XY sex reversal in a female with an interstitial 9q33.3-q34.1 deletion encompassing NR5A1 and LMX1B causing features of Genitopatellar syndrome. Am J Med Genet A 143A:1071–1081 [DOI] [PubMed] [Google Scholar]
- Lin L, Philibert P, Ferraz-de-Souza B, Kelberman D, Homfray T, Albanese A, Molini V, Sebire NJ, Einaudi S, Conway GS, Hughes IA, Jameson JL, Sultan C, Dattani MT, Achermann JC 2007 Heterozygous missense mutations in steroidogenic factor 1 (SF1/Ad4BP, NR5A1) are associated with 46,XY disorders of sex development with normal adrenal function. J Clin Endocrinol Metab 92:991–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh-Fukui Y, Tsuchiya R, Shiroishi T, Nakahara Y, Hashimoto N, Noguchi K, Higashinakagawa T 1998 Male-to-female sex reversal in M33 mutant mice. Nature 393:688–692 [DOI] [PubMed] [Google Scholar]
- Katoh-Fukui Y, Owaki A, Toyama Y, Kusaka M, Shinohara Y, Maekawa M, Toshimori K, Morohashi K 2005 Mouse Polycomb M33 is required for splenic vascular and adrenal gland formation through regulating Ad4BP/SF1 expression. Blood 106:1612–1620 [DOI] [PubMed] [Google Scholar]
- Biason-Lauber A, Konrad D, Meyer M, DeBeaufort C, Schoenle EJ 2009 Ovaries and female phenotype in a girl with 46,XY karyotype and mutations in the CBX2 gene. Am J Hum Genet 84:658–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui S, Ross A, Stallings N, Parker KL, Capel B, Quaggin SE 2004 Disrupted gonadogenesis and male-to-female sex reversal in Pod1 knockout mice. Development 131:4095–4105 [DOI] [PubMed] [Google Scholar]
- Painter T 1923 Studies in mammalian spermatogenesis. II. The spermatogenesis of man. J Exp Zool 37:291–336 [Google Scholar]
- Ford CE, Jones KW, Polani PE, de Almeida JC, Briggs JH 1959 A sex-chromosome anomaly in a case of gonadal dysgenesis (Turner’s syndrome). The Lancet 1:711–713 [DOI] [PubMed] [Google Scholar]
- Jacobs PA, Strong JA 1959 A case of human intersexuality having a possible XXY sex-determining mechanism. Nature 183:302–303 [DOI] [PubMed] [Google Scholar]
- Sinclair AH, Berta P, Palmer MS, Hawkins JR, Griffiths BL, Smith MJ, Foster JW, Frischauf AM, Lovell-Badge R, Goodfellow PN 1990 A gene from the human sex-determining region encodes a protein with homology to a conserved DNA-binding motif. Nature 346:240–244 [DOI] [PubMed] [Google Scholar]
- Gubbay J, Collignon J, Koopman P, Capel B, Economou A, Münsterberg A, Vivian N, Goodfellow P, Lovell-Badge R 1990 A gene mapping to the sex-determining region of the mouse Y chromosome is a member of a novel family of embryonically expressed genes. Nature 346:245–250 [DOI] [PubMed] [Google Scholar]
- Wilhelm D, Palmer S, Koopman P 2007 Sex determination and gonadal development in mammals. Physiol Rev 87:1–28 [DOI] [PubMed] [Google Scholar]
- DiNapoli L, Capel B 2008 SRY and the standoff in sex determination. Mol Endocrinol 22:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekido R, Lovell-Badge R 2009 Sex determination and SRY: down to a wink and a nudge? Trends Genet 25:19–29 [DOI] [PubMed] [Google Scholar]
- Sekido R, Bar I, Narváez V, Penny G, Lovell-Badge R 2004 SOX9 is up-regulated by the transient expression of SRY specifically in Sertoli cell precursors. Dev Biol 274:271–279 [DOI] [PubMed] [Google Scholar]
- Sekido R, Lovell-Badge R 2008 Sex determination involves synergistic action of SRY and SF1 on a specific Sox9 enhancer. Nature 453:930–934 [DOI] [PubMed] [Google Scholar]
- Foster JW, Dominguez-Steglich MA, Guioli S, Kwok C, Weller PA, Stevanoviæ M, Weissenbach J, Mansour S, Young ID, Goodfellow PN 1994 Campomelic dysplasia and autosomal sex reversal caused by mutations in an SRY-related gene. Nature 372:525–530 [DOI] [PubMed] [Google Scholar]
- Wagner T, Wirth J, Meyer J, Zabel B, Held M, Zimmer J, Pasantes J, Bricarelli FD, Keutel J, Hustert E, Wolf U, Tommerup N, Schemp W, Scherer G 1994 Autosomal sex reversal and campomelic dysplasia are caused by mutations in and around the SRY-related gene SOX9. Cell 79:1111–1120 [DOI] [PubMed] [Google Scholar]
- Huang B, Wang S, Ning Y, Lamb AN, Bartley J 1999 Autosomal XX sex reversal caused by duplication of SOX9. Am J Med Genet 87:349–353 [DOI] [PubMed] [Google Scholar]
- Shen JH, Ingraham HA 2002 Regulation of the orphan nuclear receptor steroidogenic factor 1 by Sox proteins. Mol Endocrinol 16:529–540 [DOI] [PubMed] [Google Scholar]
- Colvin JS, Green RP, Schmahl J, Capel B, Ornitz DM 2001 Male-to-female sex reversal in mice lacking fibroblast growth factor 9. Cell 104:875–889 [DOI] [PubMed] [Google Scholar]
- Schmahl J, Kim Y, Colvin JS, Ornitz DM, Capel B 2004 Fgf9 induces proliferation and nuclear localization of FGFR2 in Sertoli precursors during male sex determination. Development 131:3627–3636 [DOI] [PubMed] [Google Scholar]
- Kim Y, Kobayashi A, Sekido R, DiNapoli L, Brennan J, Chaboissier MC, Poulat F, Behringer RR, Lovell-Badge R, Capel B 2006 Fgf9 and Wnt4 act as antagonistic signals to regulate mammalian sex determination. PLoS Biol 4:e187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Bingham N, Sekido R, Parker KL, Lovell-Badge R, Capel B 2007 Fibroblast growth factor receptor 2 regulates proliferation and Sertoli differentiation during male sex determination. Proc Natl Acad Sci USA 104:16558–16563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagheri-Fam S, Sim H, Bernard P, Jayakody I, Taketo MM, Scherer G, Harley VR 2008 Loss of Fgfr2 leads to partial XY sex reversal. Dev Biol 314:71–83 [DOI] [PubMed] [Google Scholar]
- McElreavey K, Vilain E, Cotinot C, Payen E, Fellous M 1993 Control of sex determination in animals. Eur J Biochem 218:769–783 [DOI] [PubMed] [Google Scholar]
- Goodfellow PN, Lovell-Badge R 1993 SRY and sex determination in mammals. Annu Rev Genet 27:71–92 [DOI] [PubMed] [Google Scholar]
- Parma P, Radi O, Vidal V, Chaboissier MC, Dellambra E, Valentini S, Guerra L, Schedl A, Camerino G 2006 R-spondin1 is essential in sex determination, skin differentiation and malignancy. Nat Genet 38:1304–1309 [DOI] [PubMed] [Google Scholar]
- Binnerts ME, Kim KA, Bright JM, Patel SM, Tran K, Zhou M, Leung JM, Liu Y, Lomas 3rd WE, Dixon M, Hazell SA, Wagle M, Nie WS, Tomasevic N, Williams J, Zhan X, Levy MD, Funk WD, Abo A 2007 R-Spondin1 regulates Wnt signaling by inhibiting internalization of LRP6. Proc Natl Acad Sci USA 104:14700–14705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Q, Yokota C, Semenov MV, Doble B, Woodgett J, He X 2007 R-spondin1 is a high affinity ligand for LRP6 and induces LRP6 phosphorylation and β-catenin signaling. J Biol Chem 282:15903–15911 [DOI] [PubMed] [Google Scholar]
- Chassot AA, Ranc F, Gregoire EP, Roepers-Gajadien HL, Taketo MM, Camerino G, de Rooij DG, Schedl A, Chaboissier MC 2008 Activation of β-catenin signaling by Rspo1 controls differentiation of the mammalian ovary. Hum Mol Genet 17:1264–1277 [DOI] [PubMed] [Google Scholar]
- Tomizuka K, Horikoshi K, Kitada R, Sugawara Y, Iba Y, Kojima A, Yoshitome A, Yamawaki K, Amagai M, Inoue A, Oshima T, Kakitani M 2008 R-spondin1 plays an essential role in ovarian development through positively regulating Wnt-4 signaling. Hum Mol Genet 17:1278–1291 [DOI] [PubMed] [Google Scholar]
- Vainio S, Heikkilä M, Kispert A, Chin N, McMahon AP 1999 Female development in mammals is regulated by Wnt-4 signalling. Nature 397:405–409 [DOI] [PubMed] [Google Scholar]
- Maatouk DM, DiNapoli L, Alvers A, Parker KL, Taketo MM, Capel B 2008 Stabilization of β-catenin in XY gonads causes male-to-female sex-reversal. Hum Mol Genet 17:2949–2955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CF, Bingham N, Parker K, Yao HH 2009 Sex-specific roles of β-catenin in mouse gonadal development. Hum Mol Genet 18:405–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao HH, Matzuk MM, Jorgez CJ, Menke DB, Page DC, Swain A, Capel B 2004 Follistatin operates downstream of Wnt4 in mammalian ovary organogenesis. Dev Dyn 230:210–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuylov NL, Smagulova FO, Leach L, Tevosian SG 2008 Ovarian development in mice requires the GATA4-FOG2 transcription complex. Development 135:3731–3743 [DOI] [PubMed] [Google Scholar]
- Menke DB, Page DC 2002 Sexually dimorphic gene expression in the developing mouse gonad. Gene Expr Patterns 2:359–367 [DOI] [PubMed] [Google Scholar]
- Bernard P, Sim H, Knower K, Vilain E, Harley V 2008 Human SRY inhibits β-catenin-mediated transcription. Int J Biochem Cell Biol 40:2889–2900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan BK, Mohammed M, Ching ST, Délot E, Chen XN, Dewing P, Swain A, Rao PN, Elejalde BR, Vilain E 2001 Up-regulation of WNT-4 signaling and dosage-sensitive sex reversal in humans. Am J Hum Genet 68:1102–1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seligman J, Page DC 1998 The Dazh gene is expressed in male and female embryonic gonads before germ cell sex differentiation. Biochem Biophys Res Commun 245:878–882 [DOI] [PubMed] [Google Scholar]
- Lin Y, Gill ME, Koubova J, Page DC 2008 Germ cell-intrinsic and -extrinsic factors govern meiotic initiation in mouse embryos. Science 322:1685–1687 [DOI] [PubMed] [Google Scholar]
- Bowles J, Knight D, Smith C, Wilhelm D, Richman J, Mamiya S, Yashiro K, Chawengsaksophak K, Wilson MJ, Rossant J, Hamada H, Koopman P 2006 Retinoid signaling determines germ cell fate in mice. Science 312:596–600 [DOI] [PubMed] [Google Scholar]
- Anderson EL, Baltus AE, Roepers-Gajadien HL, Hassold TJ, de Rooij DG, van Pelt AM, Page DC 2008 Stra8 and its inducer, retinoic acid, regulate meiotic initiation in both spermatogenesis and oogenesis in mice. Proc Natl Acad Sci USA 105:14976–14980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds N, Collier B, Bingham V, Gray NK, Cooke HJ 2007 Translation of the synaptonemal complex component Sycp3 is enhanced in vivo by the germ cell specific regulator Dazl. RNA 13:974–981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean G, Li H, Metzger D, Chambon P, Petkovich M 2007 Apoptotic extinction of germ cells in testes of Cyp26b1 knockout mice. Endocrinology 148:4560–4567 [DOI] [PubMed] [Google Scholar]
- Menke DB, Koubova J, Page DC 2003 Sexual differentiation of germ cells in XX mouse gonads occurs in an anterior-to-posterior wave. Dev Biol 262:303–312 [DOI] [PubMed] [Google Scholar]
- Baltus AE, Menke DB, Hu YC, Goodheart ML, Carpenter AE, de Rooij DG, Page DC 2006 In germ cells of mouse embryonic ovaries, the decision to enter meiosis precedes premeiotic DNA replication. Nat Genet 38:1430–1434 [DOI] [PubMed] [Google Scholar]
- Suzuki A, Saga Y 2008 Nanos2 suppresses meiosis and promotes male germ cell differentiation. Genes Dev 22:430–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker TG 1963 A quantitative and cytological study of germ cells in human ovaries. Proc R Soc Lond B Biol Sci 158:417–433 [DOI] [PubMed] [Google Scholar]
- Hirshfield AN 1991 Development of follicles in the mammalian ovary. Int Rev Cytol 124:43–101 [DOI] [PubMed] [Google Scholar]
- Eppig JJ 2001 Oocyte control of ovarian follicular development and function in mammals. Reproduction 122:829–838 [DOI] [PubMed] [Google Scholar]
- Matzuk MM, Burns KH, Viveiros MM, Eppig JJ 2002 Intercellular communication in the mammalian ovary: oocytes carry the conversation. Science 296:2178–2180 [DOI] [PubMed] [Google Scholar]
- Pepling ME, Spradling AC 1998 Female mouse germ cells form synchronously dividing cysts. Development 125:3323-3328 [DOI] [PubMed] [Google Scholar]
- Pepling ME, Spradling AC 2001 Mouse ovarian germ cell cysts undergo programmed breakdown to form primordial follicles. Dev Biol 234:339–351 [DOI] [PubMed] [Google Scholar]
- Greenbaum MP, Yan W, Wu MH, Lin YN, Agno JE, Sharma M, Braun RE, Rajkovic A, Matzuk MM 2006 TEX14 is essential for intercellular bridges and fertility in male mice. Proc Natl Acad Sci USA 103:4982–4987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenbaum MP, Iwamori N, Agno JE, Matzuk MM 2009 Mouse TEX14 is required for embryonic germ cell intercellular bridges but not female fertility. Biol Reprod 80:449–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzuk MM 2001 Eggs in the balance. Nat Genet 28:300–301 [DOI] [PubMed] [Google Scholar]
- Ratts VS, Flaws JA, Kolp R, Sorenson CM, Tilly JL 1995 Ablation of bcl-2 gene expression decreases the numbers of oocytes and primordial follicles established in the postnatal female mouse gonad. Endocrinology 136:3665–3668 [DOI] [PubMed] [Google Scholar]
- Rucker 3rd EB, Dierisseau P, Wagner KU, Garrett L, Wynshaw-Boris A, Flaws JA, Hennighausen L 2000 Bcl-x and Bax regulate mouse primordial germ cell survival and apoptosis during embryogenesis. Mol Endocrinol 14:1038–1052 [DOI] [PubMed] [Google Scholar]
- Greenfeld CR, Pepling ME, Babus JK, Furth PA, Flaws JA 2007 BAX regulates follicular endowment in mice. Reproduction 133:865–876 [DOI] [PubMed] [Google Scholar]
- Perez GI, Robles R, Knudson CM, Flaws JA, Korsmeyer SJ, Tilly JL 1999 Prolongation of ovarian lifespan into advanced chronological age by Bax-deficiency. Nat Genet 21:200–203 [DOI] [PubMed] [Google Scholar]
- Matikainen T, Perez GI, Jurisicova A, Pru JK, Schlezinger JJ, Ryu HY, Laine J, Sakai T, Korsmeyer SJ, Casper RF, Sherr DH, Tilly JL 2001 Aromatic hydrocarbon receptor-driven Bax gene expression is required for premature ovarian failure caused by biohazardous environmental chemicals. Nat Genet 28:355–360 [DOI] [PubMed] [Google Scholar]
- Benedict JC, Lin TM, Loeffler IK, Peterson RE, Flaws JA 2000 Physiological role of the aryl hydrocarbon receptor in mouse ovary development. Toxicol Sci 56:382–388 [DOI] [PubMed] [Google Scholar]
- Robles R, Morita Y, Mann KK, Perez GI, Yang S, Matikainen T, Sherr DH, Tilly JL 2000 The aryl hydrocarbon receptor, a basic helix-loop-helix transcription factor of the PAS gene family, is required for normal ovarian germ cell dynamics in the mouse. Endocrinology 141:450–453 [DOI] [PubMed] [Google Scholar]
- Bergeron L, Perez GI, Macdonald G, Shi L, Sun Y, Jurisicova A, Varmuza S, Latham KE, Flaws JA, Salter JC, Hara H, Moskowitz MA, Li E, Greenberg A, Tilly JL, Yuan J 1998 Defects in regulation of apoptosis in caspase-2-deficient mice. Genes Dev 12:1304–1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soyal SM, Amleh A, Dean J 2000 FIGα, a germ cell-specific transcription factor required for ovarian follicle formation. Development 127:4645–4654 [DOI] [PubMed] [Google Scholar]
- Liang L, Soyal SM, Dean J 1997 FIGα, a germ cell specific transcription factor involved in the coordinate expression of the zona pellucida genes. Development 124:4939–4947 [DOI] [PubMed] [Google Scholar]
- Rankin TL, O'Brien M, Lee E, Wigglesworth K, Eppig J, Dean J 2001 Defective zonae pellucidae in Zp2-null mice disrupt folliculogenesis, fertility and development. Development 128:1119–1126 [DOI] [PubMed] [Google Scholar]
- Rankin T, Talbot P, Lee E, Dean J 1999 Abnormal zonae pellucidae in mice lacking ZP1 result in early embryonic loss. Development 126:3847–3855 [DOI] [PubMed] [Google Scholar]
- Rankin T, Familari M, Lee E, Ginsberg A, Dwyer N, Blanchette-Mackie J, Drago J, Westphal H, Dean J 1996 Mice homozygous for an insertional mutation in the Zp3 gene lack a zona pellucida and are infertile. Development 122:2903–2910 [DOI] [PubMed] [Google Scholar]
- Bayne RA, Martins da Silva SJ, Anderson RA 2004 Increased expression of the FIGLA transcription factor is associated with primordial follicle formation in the human fetal ovary. Mol Hum Reprod 10:373–381 [DOI] [PubMed] [Google Scholar]
- Huntriss J, Gosden R, Hinkins M, Oliver B, Miller D, Rutherford AJ, Picton HM 2002 Isolation, characterization and expression of the human factor in the germline α (FIGLA) gene in ovarian follicles and oocytes. Mol Hum Reprod 8:1087–1095 [DOI] [PubMed] [Google Scholar]
- Joshi S, Davies H, Sims LP, Levy SE, Dean J 2007 Ovarian gene expression in the absence of FIGLA, an oocyte-specific transcription factor. BMC Dev Biol 7:67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesce M, Gross MK, Schöler HR 1998 In line with our ancestors: Oct-4 and the mammalian germ. Bioessays 20:722–732 [DOI] [PubMed] [Google Scholar]
- Tong ZB, Gold L, Pfeifer KE, Dorward H, Lee E, Bondy CA, Dean J, Nelson LM 2000 Mater, a maternal effect gene required for early embryonic development in mice. Nat Genet 26:267–268 [DOI] [PubMed] [Google Scholar]
- Iguchi T, Fukazawa Y, Uesugi Y, Takasugi N 1990 Polyovular follicles in mouse ovaries exposed neonatally to diethylstilbestrol in vivo and in vitro. Biol Reprod 43:478–484 [DOI] [PubMed] [Google Scholar]
- Iguchi T, Takasugi N, Bern HA, Mills KT 1986 Frequent occurrence of polyovular follicles in ovaries of mice exposed neonatally to diethylstilbestrol. Teratology 34:29–35 [DOI] [PubMed] [Google Scholar]
- Chen Y, Jefferson WN, Newbold RR, Padilla-Banks E, Pepling ME 2007 Estradiol, progesterone, and genistein inhibit oocyte nest breakdown and primordial follicle assembly in the neonatal mouse ovary in vitro and in vivo. Endocrinology 148:3580–3590 [DOI] [PubMed] [Google Scholar]
- Kezele P, Skinner MK 2003 Regulation of ovarian primordial follicle assembly and development by estrogen and progesterone: endocrine model of follicle assembly. Endocrinology 144:3329–3337 [DOI] [PubMed] [Google Scholar]
- Britt KL, Saunders PK, McPherson SJ, Misso ML, Simpson ER, Findlay JK 2004 Estrogen actions on follicle formation and early follicle development. Biol Reprod 71:1712–1723 [DOI] [PubMed] [Google Scholar]
- Fisher CR, Graves KH, Parlow AF, Simpson ER 1998 Characterization of mice deficient in aromatase (ArKO) because of targeted disruption of the cyp19 gene. Proc Natl Acad Sci USA 95:6965–6970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery Jr CA, Shyamala G, Conneely OM, O'Malley BW 1995 Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev 9:2266–2278 [DOI] [PubMed] [Google Scholar]
- Zachos NC, Billiar RB, Albrecht ED, Pepe GJ 2002 Developmental regulation of baboon fetal ovarian maturation by estrogen. Biol Reprod 67:1148–1156 [DOI] [PubMed] [Google Scholar]
- Bray SJ 2006 Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol 7:678–689 [DOI] [PubMed] [Google Scholar]
- Trombly DJ, Woodruff TK, Mayo KE 2009 Suppression of Notch signaling in the neonatal mouse ovary decreases primordial follicle formation. Endocrinology 150:1014–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn KL, Johnson J, Beres BJ, Howard S, Wilson-Rawls J 2005 Lunatic fringe null female mice are infertile due to defects in meiotic maturation. Development 132:817–828 [DOI] [PubMed] [Google Scholar]
- McGee EA, Hsueh AJ 2000 Initial and cyclic recruitment of ovarian follicles. Endocr Rev 21:200–214 [DOI] [PubMed] [Google Scholar]
- Rajkovic A, Yan M S C, Klysik M, Matzuk M 2001 Discovery of germ cell-specific transcripts by expressed sequence tag database analysis. Fertil Steril 76:550–554 [DOI] [PubMed] [Google Scholar]
- Ballow DJ, Xin Y, Choi Y, Pangas SA, Rajkovic A 2006 Sohlh2 is a germ cell-specific bHLH transcription factor. Gene Expr Patterns 6:1014–1018 [DOI] [PubMed] [Google Scholar]
- Choi Y, Yuan D, Rajkovic A 2008 Germ cell-specific transcriptional regulator sohlh2 is essential for early mouse folliculogenesis and oocyte-specific gene expression. Biol Reprod 79:1176–1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao J, Yamamoto M, Richardson TE, Chapman KM, Denard BS, Hammer RE, Zhao GQ, Hamra FK 2008 Sohlh2 knockout mice are male-sterile because of degeneration of differentiating type A spermatogonia. Stem Cells 26:1587–1597 [DOI] [PubMed] [Google Scholar]
- Pangas SA, Choi Y, Ballow DJ, Zhao Y, Westphal H, Matzuk MM, Rajkovic A 2006 Oogenesis requires germ cell-specific transcriptional regulators Sohlh1 and Lhx8. Proc Natl Acad Sci USA 103:8090–8095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkovic A, Pangas SA, Ballow D, Suzumori N, Matzuk MM 2004 NOBOX deficiency disrupts early folliculogenesis and oocyte-specific gene expression. Science 305:1157–1159 [DOI] [PubMed] [Google Scholar]
- Choi Y, Qin Y, Berger MF, Ballow DJ, Bulyk ML, Rajkovic A 2007 Microarray analyses of newborn mouse ovaries lacking Nobox. Biol Reprod 77:312–319 [DOI] [PubMed] [Google Scholar]
- Choi Y, Rajkovic A 2006 Characterization of NOBOX DNA binding specificity and its regulation of Gdf9 and Pou5f1 promoters. J Biol Chem 281:35747–35756 [DOI] [PubMed] [Google Scholar]
- Choi Y, Ballow DJ, Xin Y, Rajkovic A 2008 Lim homeobox gene, lhx8, is essential for mouse oocyte differentiation and survival. Biol Reprod 79:442–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoda S, Miyazaki T, Miyazaki S, Yoshimura T, Yamamoto M, Tashiro F, Yamato E, Miyazaki J 2009 Sohlh2 affects differentiation of KIT positive oocytes and spermatogonia. Dev Biol 325:238–248 [DOI] [PubMed] [Google Scholar]
- Horie K, Takakura K, Taii S, Narimoto K, Noda Y, Nishikawa S, Nakayama H, Fujita J, Mori T 1991 The expression of c-kit protein during oogenesis and early embryonic development. Biol Reprod 45:547–552 [DOI] [PubMed] [Google Scholar]
- Joyce IM, Pendola FL, Wigglesworth K, Eppig JJ 1999 Oocyte regulation of kit ligand expression in mouse ovarian follicles. Dev Biol 214:342–353 [DOI] [PubMed] [Google Scholar]
- Manova K, Huang EJ, Angeles M, De Leon V, Sanchez S, Pronovost SM, Besmer P, Bachvarova RF 1993 The expression pattern of the c-kit ligand in gonads of mice supports a role for the c-kit receptor in oocyte growth and in proliferation of spermatogonia. Dev Biol 157:85–99 [DOI] [PubMed] [Google Scholar]
- Manova K, Nocka K, Besmer P, Bachvarova RF 1990 Gonadal expression of c-kit encoded at the W locus of the mouse. Development 110:1057–1069 [DOI] [PubMed] [Google Scholar]
- Yoshida H, Takakura N, Kataoka H, Kunisada T, Okamura H, Nishikawa SI 1997 Stepwise requirement of c-kit tyrosine kinase in mouse ovarian follicle development. Dev Biol 184:122–137 [DOI] [PubMed] [Google Scholar]
- Bedell MA, Brannan CI, Evans EP, Copeland NG, Jenkins NA, Donovan PJ 1995 DNA rearrangements located over 100 kb 5′ of the Steel (Sl)-coding region in Steel-panda and Steel-contrasted mice deregulate Sl expression and cause female sterility by disrupting ovarian follicle development. Genes Dev 9:455–470 [DOI] [PubMed] [Google Scholar]
- Huang EJ, Manova K, Packer AI, Sanchez S, Bachvarova RF, Besmer P 1993 The murine steel panda mutation affects kit ligand expression and growth of early ovarian follicles. Dev Biol 157:100–109 [DOI] [PubMed] [Google Scholar]
- Kuroda H, Terada N, Nakayama H, Matsumoto K, Kitamura Y 1988 Infertility due to growth arrest of ovarian follicles in Sl/Slt mice. Dev Biol 126:71–79 [DOI] [PubMed] [Google Scholar]
- Parrott JA, Skinner MK 1999 Kit-ligand/stem cell factor induces primordial follicle development and initiates folliculogenesis. Endocrinology 140:4262–4271 [DOI] [PubMed] [Google Scholar]
- Accili D, Arden KC 2004 FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell 117:421–426 [DOI] [PubMed] [Google Scholar]
- Reddy P, Shen L, Ren C, Boman K, Lundin E, Ottander U, Lindgren P, Liu YX, Sun QY, Liu K 2005 Activation of Akt (PKB) and suppression of FKHRL1 in mouse and rat oocytes by stem cell factor during follicular activation and development. Dev Biol 281:160–170 [DOI] [PubMed] [Google Scholar]
- Castrillon DH, Miao L, Kollipara R, Horner JW, DePinho RA 2003 Suppression of ovarian follicle activation in mice by the transcription factor Foxo3a. Science 301:215–218 [DOI] [PubMed] [Google Scholar]
- Hosaka T, Biggs 3rd WH, Tieu D, Boyer AD, Varki NM, Cavenee WK, Arden KC 2004 Disruption of forkhead transcription factor (FOXO) family members in mice reveals their functional diversification. Proc Natl Acad Sci USA 101:2975–2980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JS, Sharma SC, Falender AE, Lo YH 2002 Expression of FKHR, FKHRL1, and AFX genes in the rodent ovary: evidence for regulation by IGF-I, estrogen, and the gonadotropins. Mol Endocrinol 16:580–599 [DOI] [PubMed] [Google Scholar]
- Liu Z, Rudd MD, Hernandez-Gonzalez I, Gonzalez-Robayna I, Fan HY, Zeleznik AJ, Richards JS 2009 FSH and FOXO1 regulate genes in the sterol/steroid and lipid biosynthetic pathways in granulosa cells. Mol Endocrinol 23:649–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Rajareddy S, Reddy P, Du C, Jagarlamudi K, Shen Y, Gunnarsson D, Selstam G, Boman K, Liu K 2007 Infertility caused by retardation of follicular development in mice with oocyte-specific expression of Foxo3a. Development 134:199–209 [DOI] [PubMed] [Google Scholar]
- John GB, Gallardo TD, Shirley LJ, Castrillon DH 2008 Foxo3 is a PI3K-dependent molecular switch controlling the initiation of oocyte growth. Dev Biol 321:197–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy P, Liu L, Adhikari D, Jagarlamudi K, Rajareddy S, Shen Y, Du C, Tang W, Hämäläinen T, Peng SL, Lan ZJ, Cooney AJ, Huhtaniemi I, Liu K 2008 Oocyte-specific deletion of Pten causes premature activation of the primordial follicle pool. Science 319:611–613 [DOI] [PubMed] [Google Scholar]
- Kissel H, Timokhina I, Hardy MP, Rothschild G, Tajima Y, Soares V, Angeles M, Whitlow SR, Manova K, Besmer P 2000 Point mutation in kit receptor tyrosine kinase reveals essential roles for kit signaling in spermatogenesis and oogenesis without affecting other kit responses. EMBO J 19:1312–1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenkman AB, Burgering BM 2003 FoxO3a eggs on fertility and aging. Trends Mol Med 9:464–467 [DOI] [PubMed] [Google Scholar]
- Dijkers PF, Medema RH, Pals C, Banerji L, Thomas NS, Lam EW, Burgering BM, Raaijmakers JA, Lammers JW, Koenderman L, Coffer PJ 2000 Forkhead transcription factor FKHR-L1 modulates cytokine-dependent transcriptional regulation of p27(KIP1). Mol Cell Biol 20:9138–9148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajareddy S, Reddy P, Du C, Liu L, Jagarlamudi K, Tang W, Shen Y, Berthet C, Peng SL, Kaldis P, Liu K 2007 p27kip1 (cyclin-dependent kinase inhibitor 1B) controls ovarian development by suppressing follicle endowment and activation and promoting follicle atresia in mice. Mol Endocrinol 21:2189–2202 [DOI] [PubMed] [Google Scholar]
- Mora A, Komander D, van Aalten DM, Alessi DR 2004 PDK1, the master regulator of AGC kinase signal transduction. Semin Cell Dev Biol 15:161–170 [DOI] [PubMed] [Google Scholar]
- Reddy P, Adhikari D, Zheng W, Liang S, Hämäläinen T, Tohonen V, Ogawa W, Noda T, Volarevic S, Huhtaniemi I, Liu K 2009 PDK1 signaling in oocytes controls reproductive aging and lifespan by manipulating the survival of primordial follicles. Hum Mol Genet 18:2813–2824 [DOI] [PubMed] [Google Scholar]
- Volarevic S, Stewart MJ, Ledermann B, Zilberman F, Terracciano L, Montini E, Grompe M, Kozma SC, Thomas G 2000 Proliferation, but not growth, blocked by conditional deletion of 40S ribosomal protein S6. Science 288:2045–2047 [DOI] [PubMed] [Google Scholar]
- Crisponi L, Deiana M, Loi A, Chiappe F, Uda M, Amati P, Bisceglia L, Zelante L, Nagaraja R, Porcu S, Ristaldi MS, Marzella R, Rocchi M, Nicolino M, Lienhardt-Roussie A, Nivelon A, Verloes A, Schlessinger D, Gasparini P, Bonneau D, Cao A, Pilia G 2001 The putative forkhead transcription factor FOXL2 is mutated in blepharophimosis/ptosis/epicanthus inversus syndrome. Nat Genet 27:159–166 [DOI] [PubMed] [Google Scholar]
- Schmidt D, Ovitt CE, Anlag K, Fehsenfeld S, Gredsted L, Treier AC, Treier M 2004 The murine winged-helix transcription factor Foxl2 is required for granulosa cell differentiation and ovary maintenance. Development 131:933–942 [DOI] [PubMed] [Google Scholar]
- Uda M, Ottolenghi C, Crisponi L, Garcia JE, Deiana M, Kimber W, Forabosco A, Cao A, Schlessinger D, Pilia G 2004 Foxl2 disruption causes mouse ovarian failure by pervasive blockage of follicle development. Hum Mol Genet 13:1171–1181 [DOI] [PubMed] [Google Scholar]
- Behringer RR, Finegold MJ, Cate RL 1994 Mullerian-inhibiting substance function during mammalian sexual development. Cell 79:415–425 [DOI] [PubMed] [Google Scholar]
- Visser JA, de Jong FH, Laven JS, Themmen AP 2006 Anti-Mullerian hormone: a new marker for ovarian function. Reproduction 131:1–9 [DOI] [PubMed] [Google Scholar]
- Mishina Y, Rey R, Finegold MJ, Matzuk MM, Josso N, Cate RL, Behringer RR 1996 Genetic analysis of the Mullerian-inhibiting substance signal transduction pathway in mammalian sexual differentiation. Genes Dev 10:2577–2587 [DOI] [PubMed] [Google Scholar]
- Durlinger AL, Kramer P, Karels B, de Jong FH, Uilenbroek JT, Grootegoed JA, Themmen AP 1999 Control of primordial follicle recruitment by anti-Mullerian hormone in the mouse ovary. Endocrinology 140:5789–5796 [DOI] [PubMed] [Google Scholar]
- Durlinger AL, Gruijters MJ, Kramer P, Karels B, Ingraham HA, Nachtigal MW, Uilenbroek JT, Grootegoed JA, Themmen AP 2002 Anti-Mullerian hormone inhibits initiation of primordial follicle growth in the mouse ovary. Endocrinology 143:1076–1084 [DOI] [PubMed] [Google Scholar]
- de Vet A, Laven JS, de Jong FH, Themmen AP, Fauser BC 2002 Antimullerian hormone serum levels: a putative marker for ovarian aging. Fertil Steril 77:357–362 [DOI] [PubMed] [Google Scholar]
- Kevenaar ME, Meerasahib MF, Kramer P, van de Lang-Born BM, de Jong FH, Groome NP, Themmen AP, Visser JA 2006 Serum anti-mullerian hormone levels reflect the size of the primordial follicle pool in mice. Endocrinology 147:3228–3234 [DOI] [PubMed] [Google Scholar]
- Dissen GA, Hirshfield AN, Malamed S, Ojeda SR 1995 Expression of neurotrophins and their receptors in the mammalian ovary is developmentally regulated: changes at the time of folliculogenesis. Endocrinology 136:4681–4692 [DOI] [PubMed] [Google Scholar]
- Dissen GA, Garcia-Rudaz C, Ojeda SR 2009 Role of neurotrophic factors in early ovarian development. Semin Reprod Med 27:24–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dissen GA, Romero C, Hirshfield AN, Ojeda SR 2001 Nerve growth factor is required for early follicular development in the mammalian ovary. Endocrinology 142:2078–2086 [DOI] [PubMed] [Google Scholar]
- Lee KF, Li E, Huber LJ, Landis SC, Sharpe AH, Chao MV, Jaenisch R 1992 Targeted mutation of the gene encoding the low affinity NGF receptor p75 leads to deficits in the peripheral sensory nervous system. Cell 69:737–749 [DOI] [PubMed] [Google Scholar]
- Liebl DJ, Klesse LJ, Tessarollo L, Wohlman T, Parada LF 2000 Loss of brain-derived neurotrophic factor-dependent neural crest-derived sensory neurons in neurotrophin-4 mutant mice. Proc Natl Acad Sci USA 97:2297–2302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeyne RJ, Klein R, Schnapp A, Long LK, Bryant S, Lewin A, Lira SA, Barbacid M 1994 Severe sensory and sympathetic neuropathies in mice carrying a disrupted Trk/NGF receptor gene. Nature 368:246–249 [DOI] [PubMed] [Google Scholar]
- Paredes A, Romero C, Dissen GA, DeChiara TM, Reichardt L, Cornea A, Ojeda SR, Xu B 2004 TrkB receptors are required for follicular growth and oocyte survival in the mammalian ovary. Dev Biol 267:430–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dole G, Nilsson EE, Skinner MK 2008 Glial-derived neurotrophic factor promotes ovarian primordial follicle development and cell-cell interactions during folliculogenesis. Reproduction 135:671–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson EE, Detzel C, Skinner MK 2006 Platelet-derived growth factor modulates the primordial to primary follicle transition. Reproduction 131:1007–1015 [DOI] [PubMed] [Google Scholar]
- Skinner MK 2005 Regulation of primordial follicle assembly and development. Hum Reprod Update 11:461–471 [DOI] [PubMed] [Google Scholar]
- Lee WS, Otsuka F, Moore RK, Shimasaki S 2001 Effect of bone morphogenetic protein-7 on folliculogenesis and ovulation in the rat. Biol Reprod 65:994–999 [DOI] [PubMed] [Google Scholar]
- Nilsson EE, Kezele P, Skinner MK 2002 Leukemia inhibitory factor (LIF) promotes the primordial to primary follicle transition in rat ovaries. Mol Cell Endocrinol 188:65–73 [DOI] [PubMed] [Google Scholar]
- Holt JE, Jackson A, Roman SD, Aitken RJ, Koopman P, McLaughlin EA 2006 CXCR4/SDF1 interaction inhibits the primordial to primary follicle transition in the neonatal mouse ovary. Dev Biol 293:449–460 [DOI] [PubMed] [Google Scholar]
- Molyneaux KA, Zinszner H, Kunwar PS, Schaible K, Stebler J, Sunshine MJ, O'Brien W, Raz E, Littman D, Wylie C, Lehmann R 2003 The chemokine SDF1/CXCL12 and its receptor CXCR4 regulate mouse germ cell migration and survival. Development 130:4279–4286 [DOI] [PubMed] [Google Scholar]
- Ortega S, Ittmann M, Tsang SH, Ehrlich M, Basilico C 1998 Neuronal defects and delayed wound healing in mice lacking fibroblast growth factor 2. Proc Natl Acad Sci USA 95:5672–5677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Degenstein L, Fuchs E 1996 Keratinocyte growth factor is required for hair development but not for wound healing. Genes Dev 10:165–175 [DOI] [PubMed] [Google Scholar]
- Stewart CL, Kaspar P, Brunet LJ, Bhatt H, Gadi I, Köntgen F, Abbondanzo SJ 1992 Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature 359:76–79 [DOI] [PubMed] [Google Scholar]
- Nelson LM 2009 Clinical practice. Primary ovarian insufficiency. N Engl J Med 360:606–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y, Choi Y, Zhao H, Simpson JL, Chen ZJ, Rajkovic A 2007 NOBOX homeobox mutation causes premature ovarian failure. Am J Hum Genet 81:576–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Chen ZJ, Qin Y, Shi Y, Wang S, Choi Y, Simpson JL, Rajkovic A 2008 Transcription factor FIGLA is mutated in patients with premature ovarian failure. Am J Hum Genet 82:1342–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar TR, Wang Y, Lu N, Matzuk MM 1997 Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility. Nat Genet 15:201–204 [DOI] [PubMed] [Google Scholar]
- Abel MH, Wootton AN, Wilkins V, Huhtaniemi I, Knight PG, Charlton HM 2000 The effect of a null mutation in the follicle-stimulating hormone receptor gene on mouse reproduction. Endocrinology 141:1795–1803 [DOI] [PubMed] [Google Scholar]
- Dierich A, Sairam MR, Monaco L, Fimia GM, Gansmuller A, LeMeur M, Sassone-Corsi P 1998 Impairing follicle-stimulating hormone (FSH) signaling in vivo: targeted disruption of the FSH receptor leads to aberrant gametogenesis and hormonal imbalance. Proc Natl Acad Sci USA 95:13612–13617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattanach BM, Iddon CA, Charlton HM, Chiappa SA, Fink G 1977 Gonadotrophin-releasing hormone deficiency in a mutant mouse with hypogonadism. Nature 269:338–340 [DOI] [PubMed] [Google Scholar]
- Eppig JJ, Wigglesworth K, Pendola FL 2002 The mammalian oocyte orchestrates the rate of ovarian follicular development. Proc Natl Acad Sci USA 99:2890–2894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elvin JA, Clark AT, Wang P, Wolfman NM, Matzuk MM 1999 Paracrine actions of growth differentiation factor-9 in the mammalian ovary. Mol Endocrinol 13:1035–1048 [DOI] [PubMed] [Google Scholar]
- McGrath SA, Esquela AF, Lee SJ 1995 Oocyte-specific expression of growth/differentiation factor-9. Mol Endocrinol 9:131–136 [DOI] [PubMed] [Google Scholar]
- Dong J, Albertini DF, Nishimori K, Kumar TR, Lu N, Matzuk MM 1996 Growth differentiation factor-9 is required during early ovarian folliculogenesis. Nature 383:531–535 [DOI] [PubMed] [Google Scholar]
- Carabatsos MJ, Elvin J, Matzuk MM, Albertini DF 1998 Characterization of oocyte and follicle development in growth differentiation factor-9-deficient mice. Dev Biol 204:373–384 [DOI] [PubMed] [Google Scholar]
- Elvin JA, Yan C, Wang P, Nishimori K, Matzuk MM 1999 Molecular characterization of the follicle defects in the growth differentiation factor 9-deficient ovary. Mol Endocrinol 13:1018–1034 [DOI] [PubMed] [Google Scholar]
- Wu X, Chen L, Brown CA, Yan C, Matzuk MM 2004 Interrelationship of growth differentiation factor 9 and inhibin in early folliculogenesis and ovarian tumorigenesis in mice. Mol Endocrinol 18:1509–1519 [DOI] [PubMed] [Google Scholar]
- Packer AI, Hsu YC, Besmer P, Bachvarova RF 1994 The ligand of the c-kit receptor promotes oocyte growth. Dev Biol 161:194–205 [DOI] [PubMed] [Google Scholar]
- Joyce IM, Clark AT, Pendola FL, Eppig JJ 2000 Comparison of recombinant growth differentiation factor-9 and oocyte regulation of KIT ligand messenger ribonucleic acid expression in mouse ovarian follicles. Biol Reprod 63:1669–1675 [DOI] [PubMed] [Google Scholar]
- Dube JL, Wang P, Elvin J, Lyons KM, Celeste AJ, Matzuk MM 1998 The bone morphogenetic protein 15 gene is X-linked and expressed in oocytes. Mol Endocrinol 12:1809–1817 [DOI] [PubMed] [Google Scholar]
- Yan C, Wang P, DeMayo J, DeMayo FJ, Elvin JA, Carino C, Prasad SV, Skinner SS, Dunbar BS, Dube JL, Celeste AJ, Matzuk MM 2001 Synergistic roles of bone morphogenetic protein 15 and growth differentiation factor 9 in ovarian function. Mol Endocrinol 15:854–866 [DOI] [PubMed] [Google Scholar]
- Hanrahan JP, Gregan SM, Mulsant P, Mullen M, Davis GH, Powell R, Galloway SM 2004 Mutations in the genes for oocyte-derived growth factors GDF9 and BMP15 are associated with both increased ovulation rate and sterility in Cambridge and Belclare sheep (Ovis aries). Biol Reprod 70:900–909 [DOI] [PubMed] [Google Scholar]
- McNatty KP, Smith P, Moore LG, Reader K, Lun S, Hanrahan JP, Groome NP, Laitinen M, Ritvos O, Juengel JL 2005 Oocyte-expressed genes affecting ovulation rate. Mol Cell Endocrinol 234:57–66 [DOI] [PubMed] [Google Scholar]
- Braw-Tal R, McNatty KP, Smith P, Heath DA, Hudson NL, Phillips DJ, McLeod BJ, Davis GH 1993 Ovaries of ewes homozygous for the X-linked Inverdale gene (FecXI) are devoid of secondary and tertiary follicles but contain many abnormal structures. Biol Reprod 49:895–907 [DOI] [PubMed] [Google Scholar]
- Galloway SM, McNatty KP, Cambridge LM, Laitinen MP, Juengel JL, Jokiranta TS, McLaren RJ, Luiro K, Dodds KG, Montgomery GW, Beattie AE, Davis GH, Ritvos O 2000 Mutations in an oocyte-derived growth factor gene (BMP15) cause increased ovulation rate and infertility in a dosage-sensitive manner. Nat Genet 25:279–283 [DOI] [PubMed] [Google Scholar]
- Liao WX, Moore RK, Otsuka F, Shimasaki S 2003 Effect of intracellular interactions on the processing and secretion of bone morphogenetic protein-15 (BMP-15) and growth and differentiation factor-9. Implication of the aberrant ovarian phenotype of BMP-15 mutant sheep. J Biol Chem 278:3713–3719 [DOI] [PubMed] [Google Scholar]
- Di Pasquale E, Beck-Peccoz P, Persani L 2004 Hypergonadotropic ovarian failure associated with an inherited mutation of human bone morphogenetic protein-15 (BMP15) gene. Am J Hum Genet 75:106–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovanci E, Rohozinski J, Simpson JL, Heard MJ, Bishop CE, Carson SA 2007 Growth differentiating factor-9 mutations may be associated with premature ovarian failure. Fertil Steril 87:143–146 [DOI] [PubMed] [Google Scholar]
- Laissue P, Christin-Maitre S, Touraine P, Kuttenn F, Ritvos O, Aittomaki K, Bourcigaux N, Jacquesson L, Bouchard P, Frydman R, Dewailly D, Reyss AC, Jeffery L, Bachelot A, Massin N, Fellous M, Veitia RA 2006 Mutations and sequence variants in GDF9 and BMP15 in patients with premature ovarian failure. Eur J Endocrinol 154:739–744 [DOI] [PubMed] [Google Scholar]
- Rossetti R, Di Pasquale E, Marozzi A, Bione S, Toniolo D, Grammatico P, Nelson LM, Beck-Peccoz P, Persani L 2009 BMP15 mutations associated with primary ovarian insufficiency cause a defective production of bioactive protein. Hum Mutat 30:804–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Qin Y, Kovanci E, Simpson JL, Chen ZJ, Rajkovic A 2007 Analyses of GDF9 mutation in 100 Chinese women with premature ovarian failure. Fertil Steril 88:1474–1476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RA, Robinson LL, Brooks J, Spears N 2002 Neurotropins and their receptors are expressed in the human fetal ovary. J Clin Endocrinol Metab 87:890–897 [DOI] [PubMed] [Google Scholar]
- Gershon E, Plaks V, Dekel N 2008 Gap junctions in the ovary: expression, localization and function. Mol Cell Endocrinol 282:18–25 [DOI] [PubMed] [Google Scholar]
- Kidder GM, Mhawi AA 2002 Gap junctions and ovarian folliculogenesis. Reproduction 123:613–620 [DOI] [PubMed] [Google Scholar]
- Juneja SC, Barr KJ, Enders GC, Kidder GM 1999 Defects in the germ line and gonads of mice lacking connexin43. Biol Reprod 60:1263–1270 [DOI] [PubMed] [Google Scholar]
- Simon AM, Goodenough DA, Li E, Paul DL 1997 Female infertility in mice lacking connexin 37. Nature 385:525–529 [DOI] [PubMed] [Google Scholar]
- Reaume AG, de Sousa PA, Kulkarni S, Langille BL, Zhu D, Davies TC, Juneja SC, Kidder GM, Rossant J 1995 Cardiac malformation in neonatal mice lacking connexin43. Science 267:1831–1834 [DOI] [PubMed] [Google Scholar]
- Ackert CL, Gittens JE, O'Brien MJ, Eppig JJ, Kidder GM 2001 Intercellular communication via connexin43 gap junctions is required for ovarian folliculogenesis in the mouse. Dev Biol 233:258–270 [DOI] [PubMed] [Google Scholar]
- Carabatsos MJ, Sellitto C, Goodenough DA, Albertini DF 2000 Oocyte-granulosa cell heterologous gap junctions are required for the coordination of nuclear and cytoplasmic meiotic competence. Dev Biol 226:167–179 [DOI] [PubMed] [Google Scholar]
- Magoffin DA 2005 Ovarian theca cell. Int J Biochem Cell Biol 37:1344–1349 [DOI] [PubMed] [Google Scholar]
- Hirshfield AN 1991 Theca cells may be present at the outset of follicular growth. Biol Reprod 44:1157–1162 [DOI] [PubMed] [Google Scholar]
- Magarelli PC, Zachow RJ, Magoffin DA 1996 Developmental and hormonal regulation of rat theca-cell differentiation factor secretion in ovarian follicles. Biol Reprod 55:416–420 [DOI] [PubMed] [Google Scholar]
- Huang CT, Weitsman SR, Dykes BN, Magoffin DA 2001 Stem cell factor and insulin-like growth factor-I stimulate luteinizing hormone-independent differentiation of rat ovarian theca cells. Biol Reprod 64:451–456 [DOI] [PubMed] [Google Scholar]
- Pangas SA, Jorgez CJ, Matzuk MM 2004 Growth differentiation factor 9 regulates expression of the bone morphogenetic protein antagonist gremlin. J Biol Chem 279:32281–32286 [DOI] [PubMed] [Google Scholar]
- Varani S, Elvin JA, Yan C, DeMayo J, DeMayo FJ, Horton HF, Byrne MC, Matzuk MM 2002 Knockout of pentraxin 3, a downstream target of growth differentiation factor-9, causes female subfertility. Mol Endocrinol 16:1154–1167 [DOI] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M, Miki H, Inoue K, Ogonuki N, Toyokuni S, Ogura A, Shinohara T 2005 Long-term culture of mouse male germline stem cells under serum- or feeder-free conditions. Biol Reprod 72:985–991 [DOI] [PubMed] [Google Scholar]
- Honda A, Hirose M, Hara K, Matoba S, Inoue K, Miki H, Hiura H, Kanatsu-Shinohara M, Kanai Y, Kono T, Shinohara T, Ogura A 2007 Isolation, characterization, and in vitro and in vivo differentiation of putative thecal stem cells. Proc Natl Acad Sci USA 104:12389–12394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King PJ, Guasti L, Laufer E 2008 Hedgehog signalling in endocrine development and disease. J Endocrinol 198:439–450 [DOI] [PubMed] [Google Scholar]
- Wijgerde M, Ooms M, Hoogerbrugge JW, Grootegoed JA 2005 Hedgehog signaling in mouse ovary: Indian hedgehog and desert hedgehog from granulosa cells induce target gene expression in developing theca cells. Endocrinology 146:3558–3566 [DOI] [PubMed] [Google Scholar]
- Xie J, Murone M, Luoh SM, Ryan A, Gu Q, Zhang C, Bonifas JM, Lam CW, Hynes M, Goddard A, Rosenthal A, Epstein Jr EH, de Sauvage FJ 1998 Activating Smoothened mutations in sporadic basal-cell carcinoma. Nature 391:90–92 [DOI] [PubMed] [Google Scholar]
- Jamin SP, Arango NA, Mishina Y, Hanks MC, Behringer RR 2002 Requirement of Bmpr1a for Mullerian duct regression during male sexual development. Nat Genet 32:408–410 [DOI] [PubMed] [Google Scholar]
- Jorgez CJ, Klysik M, Jamin SP, Behringer RR, Matzuk MM 2004 Granulosa cell-specific inactivation of follistatin causes female fertility defects. Mol Endocrinol 18:953–967 [DOI] [PubMed] [Google Scholar]
- Ren Y, Cowan RG, Harman RM, Quirk SM 2009 Dominant activation of the hedgehog signaling pathway in the ovary alters theca development and prevents ovulation. Mol Endocrinol 23:711–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Wang XF 2009 Signaling cross-talk between TGF-β/BMP and other pathways. Cell Res 19:71–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan HY, Shimada M, Liu Z, Cahill N, Noma N, Wu Y, Gossen J, Richards JS 2008 Selective expression of KrasG12D in granulosa cells of the mouse ovary causes defects in follicle development and ovulation. Development 135:2127–2137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillier SG, Yong EL, Illingworth PJ, Baird DT, Schwall RH, Mason AJ 1991 Effect of recombinant activin on androgen synthesis in cultured human thecal cells. J Clin Endocrinol Metab 72:1206–1211 [DOI] [PubMed] [Google Scholar]
- Zachow RJ, Magoffin DA 1995 Granulosa cell modulation of luteinizing hormone-dependent androgen production by ovarian theca-interstitial cells: a temporal switch from suppression to augmentation stimulated by follicle-stimulating hormone in vitro. Biol Reprod 53:758–765 [DOI] [PubMed] [Google Scholar]
- Magoffin DA 2002 The ovarian androgen-producing cells: a 2001 perspective. Rev Endocr Metab Disord 3:47–53 [DOI] [PubMed] [Google Scholar]
- Orisaka M, Jiang JY, Orisaka S, Kotsuji F, Tsang BK 2009 Growth differentiation factor 9 promotes rat preantral follicle growth by up-regulating follicular androgen biosynthesis. Endocrinology 150:2740–2748 [DOI] [PubMed] [Google Scholar]
- Solovyeva EV, Hayashi M, Margi K, Barkats C, Klein C, Amsterdam A, Hsueh AJ, Tsafriri A 2000 Growth differentiation factor-9 stimulates rat theca-interstitial cell androgen biosynthesis. Biol Reprod 63:1214–1218 [DOI] [PubMed] [Google Scholar]
- Spicer LJ, Aad PY, Allen DT, Mazerbourg S, Payne AH, Hsueh AJ 2008 Growth differentiation factor 9 (GDF9) stimulates proliferation and inhibits steroidogenesis by bovine theca cells: influence of follicle size on responses to GDF9. Biol Reprod 78:243–253 [DOI] [PubMed] [Google Scholar]
- Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, Janssen OE, Legro RS, Norman RJ, Taylor AE, Witchel SF 2009 The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril 91:456–488 [DOI] [PubMed] [Google Scholar]
- Palomba S, Falbo A, Zullo F, Orio Jr F 2009 Evidence-based and potential benefits of metformin in the polycystic ovary syndrome: a comprehensive review. Endocr Rev 30:1–50 [DOI] [PubMed] [Google Scholar]
- Escobar-Morreale HF, Luque-Ramírez M, San Millán JL 2005 The molecular-genetic basis of functional hyperandrogenism and the polycystic ovary syndrome. Endocr Rev 26:251–282 [DOI] [PubMed] [Google Scholar]
- Diaz FJ, Wigglesworth K, Eppig JJ 2007 Oocytes determine cumulus cell lineage in mouse ovarian follicles. J Cell Sci 120:1330–1340 [DOI] [PubMed] [Google Scholar]
- Chun SY, Eisenhauer KM, Minami S, Billig H, Perlas E, Hsueh AJ 1996 Hormonal regulation of apoptosis in early antral follicles: follicle-stimulating hormone as a major survival factor. Endocrinology 137:1447–1456 [DOI] [PubMed] [Google Scholar]
- Richards JS 1994 Hormonal control of gene expression in the ovary. Endocr Rev 15:725–751 [DOI] [PubMed] [Google Scholar]
- Bernard D, Woodruff TK 2001 Glycoprotein hormones: transgenic mice as tools to study regulation and function. Totowa, NJ: Humana Press [Google Scholar]
- Kendall SK, Samuelson LC, Saunders TL, Wood RI, Camper SA 1995 Targeted disruption of the pituitary glycoprotein hormone α-subunit produces hypogonadal and hypothyroid mice. Genes Dev 9:2007–2019 [DOI] [PubMed] [Google Scholar]
- Aittomäki K, Lucena JL, Pakarinen P, Sistonen P, Tapanainen J, Gromoll J, Kaskikari R, Sankila EM, Lehväslaiho H, Engel AR, Nieschlag E, Huhtaniemi I, de la Chapelle A 1995 Mutation in the follicle-stimulating hormone receptor gene causes hereditary hypergonadotropic ovarian failure. Cell 82:959–968 [DOI] [PubMed] [Google Scholar]
- Doherty E, Pakarinen P, Tiitinen A, Kiilavuori A, Huhtaniemi I, Forrest S, Aittomäki K 2002 A Novel mutation in the FSH receptor inhibiting signal transduction and causing primary ovarian failure. J Clin Endocrinol Metab 87:1151–1155 [DOI] [PubMed] [Google Scholar]
- Jiang M, Aittomäki K, Nilsson C, Pakarinen P, Iitiä A, Torresani T, Simonsen H, Goh V, Pettersson K, de la Chapelle A, Huhtaniemi I 1998 The frequency of an inactivating point mutation (566C→T) of the human follicle-stimulating hormone receptor gene in four populations using allele-specific hybridization and time-resolved fluorometry. J Clin Endocrinol Metab 83:4338–4343 [DOI] [PubMed] [Google Scholar]
- Layman LC, Lee EJ, Peak DB, Namnoum AB, Vu KV, van Lingen BL, Gray MR, McDonough PG, Reindollar RH, Jameson JL 1997 Delayed puberty and hypogonadism caused by mutations in the follicle-stimulating hormone β-subunit gene. N Engl J Med 337:607–611 [DOI] [PubMed] [Google Scholar]
- Matthews CH, Borgato S, Beck-Peccoz P, Adams M, Tone Y, Gambino G, Casagrande S, Tedeschini G, Benedetti A, Chatterjee VK 1993 Primary amenorrhoea and infertility due to a mutation in the β-subunit of follicle-stimulating hormone. Nat Genet 5:83–86 [DOI] [PubMed] [Google Scholar]
- Themmen APN, Huhtaniemi IT 2000 Mutations of gonadotropins and gonadotropin receptors: elucidating the physiology and pathophysiology of pituitary-gonadal function. Endocr Rev 21:551–583 [DOI] [PubMed] [Google Scholar]
- Kumar TR, Low MJ, Matzuk MM 1998 Genetic rescue of follicle-stimulating hormone β-deficient mice. Endocrinology 139:3289–3295 [DOI] [PubMed] [Google Scholar]
- Richards JS, Russell DL, Ochsner S, Hsieh M, Doyle KH, Falender AE, Lo YK, Sharma SC 2002 Novel signaling pathways that control ovarian follicular development, ovulation, and luteinization. Recent Prog Horm Res 57:195–220 [DOI] [PubMed] [Google Scholar]
- Ulloa-Aguirre A, Zariñán T, Pasapera AM, Casas-González P, Dias JA 2007 Multiple facets of follicle-stimulating hormone receptor function. Endocrine 32:251–263 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Robayna IJ, Falender AE, Ochsner S, Firestone GL, Richards JS 2000 Follicle-stimulating hormone (FSH) stimulates phosphorylation and activation of protein kinase B (PKB/Akt) and serum and glucocorticoid-induced kinase (Sgk): evidence for A kinase-independent signaling by FSH in granulosa cells. Mol Endocrinol 14:1283–1300 [DOI] [PubMed] [Google Scholar]
- Wayne CM, Fan HY, Cheng X, Richards JS 2007 Follicle-stimulating hormone induces multiple signaling cascades: evidence that activation of Rous sarcoma oncogene, RAS, and the epidermal growth factor receptor are critical for granulosa cell differentiation. Mol Endocrinol 21:1940–1957 [DOI] [PubMed] [Google Scholar]
- Baker J, Hardy MP, Zhou J, Bondy C, Lupu F, Bellvé AR, Efstratiadis A 1996 Effects of an Igf1 gene null mutation on mouse reproduction. Mol Endocrinol 10:903–918 [DOI] [PubMed] [Google Scholar]
- Zhou J, Kumar TR, Matzuk MM, Bondy C 1997 Insulin-like growth factor I regulates gonadotropin responsiveness in the murine ovary. Mol Endocrinol 11:1924–1933 [DOI] [PubMed] [Google Scholar]
- Sicinski P, Donaher JL, Geng Y, Parker SB, Gardner H, Park MY, Robker RL, Richards JS, McGinnis LK, Biggers JD, Eppig JJ, Bronson RT, Elledge SJ, Weinberg RA 1996 Cyclin D2 is an FSH-responsive gene involved in gonadal cell proliferation and oncogenesis. Nature 384:470–474 [DOI] [PubMed] [Google Scholar]
- Burns KH, Yan C, Kumar TR, Matzuk MM 2001 Analysis of ovarian gene expression in follicle-stimulating hormone β knockout mice. Endocrinology 142:2742–2751 [DOI] [PubMed] [Google Scholar]
- Park Y, Maizels ET, Feiger ZJ, Alam H, Peters CA, Woodruff TK, Unterman TG, Lee EJ, Jameson JL, Hunzicker-Dunn M 2005 Induction of cyclin D2 in rat granulosa cells requires FSH-dependent relief from FOXO1 repression coupled with positive signals from Smad. J Biol Chem 280:9135–9148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britt KL, Drummond AE, Cox VA, Dyson M, Wreford NG, Jones ME, Simpson ER, Findlay JK 2000 An age-related ovarian phenotype in mice with targeted disruption of the Cyp 19 (aromatase) gene. Endocrinology 141:2614–2623 [DOI] [PubMed] [Google Scholar]
- Toda K, Takeda K, Okada T, Akira S, Saibara T, Kaname T, Yamamura K, Onishi S, Shizuta Y 2001 Targeted disruption of the aromatase P450 gene (Cyp19) in mice and their ovarian and uterine responses to 17β-oestradiol. J Endocrinol 170:99–111 [DOI] [PubMed] [Google Scholar]
- Prossnitz ER, Arterburn JB, Smith HO, Oprea TI, Sklar LA, Hathaway HJ 2008 Estrogen signaling through the transmembrane G protein-coupled receptor GPR30. Annu Rev Physiol 70:165–190 [DOI] [PubMed] [Google Scholar]
- Pang Y, Dong J, Thomas P 2008 Estrogen signaling characteristics of Atlantic croaker G protein-coupled receptor 30 (GPR30) and evidence it is involved in maintenance of oocyte meiotic arrest. Endocrinology 149:3410–3426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto C, Fuchs I, Kauselmann G, Kern H, Zevnik B, Andreasen P, Schwarz G, Altmann H, Klewer M, Schoor M, Vonk R, Fritzemeier KH 2009 GPR30 does not mediate estrogenic responses in reproductive organs in mice. Biol Reprod 80:34–41 [DOI] [PubMed] [Google Scholar]
- Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O 1993 Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci USA 90:11162–11166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, Sar M, Korach KS, Gustafsson JA, Smithies O 1998 Generation and reproductive phenotypes of mice lacking estrogen receptor β. Proc Natl Acad Sci USA 95:15677–15682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couse JF, Hewitt SC, Bunch DO, Sar M, Walker VR, Davis BJ, Korach KS 1999 Postnatal sex reversal of the ovaries in mice lacking estrogen receptors α and β. Science 286:2328–2331 [DOI] [PubMed] [Google Scholar]
- Dupont S, Krust A, Gansmuller A, Dierich A, Chambon P, Mark M 2000 Effect of single and compound knockouts of estrogen receptors α (ERα) and β (ERβ) on mouse reproductive phenotypes. Development 127:4277–4291 [DOI] [PubMed] [Google Scholar]
- Britt KL, Findlay JK 2003 Regulation of the phenotype of ovarian somatic cells by estrogen. Mol Cell Endocrinol 202:11–17 [DOI] [PubMed] [Google Scholar]
- Taniguchi F, Couse JF, Rodriguez KF, Emmen JM, Poirier D, Korach KS 2007 Estrogen receptor-α mediates an intraovarian negative feedback loop on thecal cell steroidogenesis via modulation of Cyp17a1 (cytochrome P450, steroid 17α-hydroxylase/17,20 lyase) expression. FASEB J 21:586–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couse JF, Yates MM, Deroo BJ, Korach KS 2005 Estrogen receptor-β is critical to granulosa cell differentiation and the ovulatory response to gonadotropins. Endocrinology 146:3247–3262 [DOI] [PubMed] [Google Scholar]
- Richards JS, Ireland JJ, Rao MC, Bernath GA, Midgley Jr AR, Reichert Jr LE 1976 Ovarian follicular development in the rat: hormone receptor regulation by estradiol, follicle stimulating hormone and luteinizing hormone. Endocrinology 99:1562–1570 [DOI] [PubMed] [Google Scholar]
- Freiman RN, Albright SR, Zheng S, Sha WC, Hammer RE, Tjian R 2001 Requirement of tissue-selective TBP-associated factor TAFII105 in ovarian development. Science 293:2084–2087 [DOI] [PubMed] [Google Scholar]
- Voronina E, Lovasco LA, Gyuris A, Baumgartner RA, Parlow AF, Freiman RN 2007 Ovarian granulosa cell survival and proliferation requires the gonad-selective TFIID subunit TAF4b. Dev Biol 303:715–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falender AE, Shimada M, Lo YK, Richards JS 2005 TAF4b, a TBP associated factor, is required for oocyte development and function. Dev Biol 288:405–419 [DOI] [PubMed] [Google Scholar]
- Geles KG, Freiman RN, Liu WL, Zheng S, Voronina E, Tjian R 2006 Cell-type-selective induction of c-jun by TAF4b directs ovarian-specific transcription networks. Proc Natl Acad Sci USA 103:2594–2599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma SC, Richards JS 2000 Regulation of AP1 (Jun/Fos) factor expression and activation in ovarian granulosa cells. Relation of JunD and Fra2 to terminal differentiation. J Biol Chem 275:33718–33728 [DOI] [PubMed] [Google Scholar]
- Wu Y, Lu Y, Hu Y, Li R 2005 Cyclic AMP-dependent modification of gonad-selective TAF(II)105 in a human ovarian granulosa cell line. J Cell Biochem 96:751–759 [DOI] [PubMed] [Google Scholar]
- Ongeri EM, Verderame MF, Hammond JM 2007 The TATA binding protein associated factor 4b (TAF4b) mediates FSH stimulation of the IGFBP-3 promoter in cultured porcine ovarian granulosa cells. Mol Cell Endocrinol 278:29–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu YC, Wang PH, Yeh S, Wang RS, Xie C, Xu Q, Zhou X, Chao HT, Tsai MY, Chang C 2004 Subfertility and defective folliculogenesis in female mice lacking androgen receptor. Proc Natl Acad Sci USA 101:11209–11214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiina H, Matsumoto T, Sato T, Igarashi K, Miyamoto J, Takemasa S, Sakari M, Takada I, Nakamura T, Metzger D, Chambon P, Kanno J, Yoshikawa H, Kato S 2006 Premature ovarian failure in androgen receptor-deficient mice. Proc Natl Acad Sci USA 103:224–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil S, Vendola K, Zhou J, Bondy CA 1999 Androgen and follicle-stimulating hormone interactions in primate ovarian follicle development. J Clin Endocrinol Metab 84:2951–2956 [DOI] [PubMed] [Google Scholar]
- Ma X, Dong Y, Matzuk MM, Kumar TR 2004 Targeted disruption of luteinizing hormone β-subunit leads to hypogonadism, defects in gonadal steroidogenesis, and infertility. Proc Natl Acad Sci USA 101:17294–17299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei ZM, Mishra S, Zou W, Xu B, Foltz M, Li X, Rao CV 2001 Targeted disruption of luteinizing hormone/human chorionic gonadotropin receptor gene. Mol Endocrinol 15:184–200 [DOI] [PubMed] [Google Scholar]
- Zhang FP, Poutanen M, Wilbertz J, Huhtaniemi I 2001 Normal prenatal but arrested postnatal sexual development of luteinizing hormone receptor knockout (LuRKO) mice. Mol Endocrinol 15:172–183 [DOI] [PubMed] [Google Scholar]
- Su YQ, Wigglesworth K, Pendola FL, O'Brien MJ, Eppig JJ 2002 Mitogen-activated protein kinase activity in cumulus cells is essential for gonadotropin-induced oocyte meiotic resumption and cumulus expansion in the mouse. Endocrinology 143:2221–2232 [DOI] [PubMed] [Google Scholar]
- Fan HY, Liu Z, Shimada M, Sterneck E, Johnson PF, Hedrick SM, Richards JS 2009 MAPK3/1 (ERK1/2) in ovarian granulosa cells are essential for female fertility. Science 324:938–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buccione R, Vanderhyden BC, Caron PJ, Eppig JJ 1990 FSH-induced expansion of the mouse cumulus oophorus in vitro is dependent upon a specific factor (s) secreted by the oocyte. Dev Biol 138:16–25 [DOI] [PubMed] [Google Scholar]
- Richards JS 2005 Ovulation: new factors that prepare the oocyte for fertilization. Mol Cell Endocrinol 234:75–79 [DOI] [PubMed] [Google Scholar]
- Hernandez-Gonzalez I, Gonzalez-Robayna I, Shimada M, Wayne CM, Ochsner SA, White L, Richards JS 2006 Gene expression profiles of cumulus cell oocyte complexes during ovulation reveal cumulus cells express neuronal and immune-related genes: does this expand their role in the ovulation process? Mol Endocrinol 20:1300–1321 [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay D, Asari A, Rugg MS, Day AJ, Fülöp C 2004 Specificity of the tumor necrosis factor-induced protein 6-mediated heavy chain transfer from inter-α-trypsin inhibitor to hyaluronan: implications for the assembly of the cumulus extracellular matrix. J Biol Chem 279:11119–11128 [DOI] [PubMed] [Google Scholar]
- Scarchilli L, Camaioni A, Bottazzi B, Negri V, Doni A, Deban L, Bastone A, Salvatori G, Mantovani A, Siracusa G, Salustri A 2007 PTX3 interacts with inter-α-trypsin inhibitor: implications for hyaluronan organization and cumulus oophorus expansion. J Biol Chem 282:30161–30170 [DOI] [PubMed] [Google Scholar]
- Ochsner SA, Russell DL, Day AJ, Breyer RM, Richards JS 2003 Decreased expression of tumor necrosis factor-α-stimulated gene 6 in cumulus cells of the cyclooxygenase-2 and EP2 null mice. Endocrinology 144:1008–1019 [DOI] [PubMed] [Google Scholar]
- Dinchuk JE, Car BD, Focht RJ, Johnston JJ, Jaffee BD, Covington MB, Contel NR, Eng VM, Collins RJ, Czerniak PM, Gorry SA, Trzaskos JM 1995 Renal abnormalities and an altered inflammatory response in mice lacking cyclooxygenase II. Nature 378:406–409 [DOI] [PubMed] [Google Scholar]
- Lim H, Paria BC, Das SK, Dinchuk JE, Langenbach R, Trzaskos JM, Dey SK 1997 Multiple female reproductive failures in cyclooxygenase 2-deficient mice. Cell 91:197–208 [DOI] [PubMed] [Google Scholar]
- Hizaki H, Segi E, Sugimoto Y, Hirose M, Saji T, Ushikubi F, Matsuoka T, Noda Y, Tanaka T, Yoshida N, Narumiya S, Ichikawa A 1999 Abortive expansion of the cumulus and impaired fertility in mice lacking the prostaglandin E receptor subtype EP(2). Proc Natl Acad Sci USA 96:10501–10506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy CR, Zhang Y, Brandon S, Guan Y, Coffee K, Funk CD, Magnuson MA, Oates JA, Breyer MD, Breyer RM 1999 Salt-sensitive hypertension and reduced fertility in mice lacking the prostaglandin EP2 receptor. Nat Med 5:217–220 [DOI] [PubMed] [Google Scholar]
- Tilley SL, Audoly LP, Hicks EH, Kim HS, Flannery PJ, Coffman TM, Koller BH 1999 Reproductive failure and reduced blood pressure in mice lacking the EP2 prostaglandin E2 receptor. J Clin Invest 103:1539–1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fülöp C, Szántó S, Mukhopadhyay D, Bárdos T, Kamath RV, Rugg MS, Day AJ, Salustri A, Hascall VC, Glant TT, Mikecz K 2003 Impaired cumulus mucification and female sterility in tumor necrosis factor-induced protein-6 deficient mice. Development 130:2253–2261 [DOI] [PubMed] [Google Scholar]
- Camenisch TD, Spicer AP, Brehm-Gibson T, Biesterfeldt J, Augustine ML, Calabro Jr A, Kubalak S, Klewer SE, McDonald JA 2000 Disruption of hyaluronan synthase-2 abrogates normal cardiac morphogenesis and hyaluronan-mediated transformation of epithelium to mesenchyme. J Clin Invest 106:349–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura K, Su YQ, Eppig JJ 2009 Targeted suppression of Has2 mRNA in mouse cumulus cell-oocyte complexes by adenovirus-mediated short-hairpin RNA expression. Mol Reprod Dev 76:537–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H, Kajikawa S, Kuroda S, Horisawa Y, Nakamura N, Kaga N, Kakinuma C, Kato K, Morishita H, Niwa H, Miyazaki J 2001 Impaired fertility in female mice lacking urinary trypsin inhibitor. Biochem Biophys Res Commun 281:1154–1160 [DOI] [PubMed] [Google Scholar]
- Zhuo L, Yoneda M, Zhao M, Yingsung W, Yoshida N, Kitagawa Y, Kawamura K, Suzuki T, Kimata K 2001 Defect in SHAP-hyaluronan complex causes severe female infertility. A study by inactivation of the bikunin gene in mice. J Biol Chem 276:7693–7696 [DOI] [PubMed] [Google Scholar]
- Russell DL, Ochsner SA, Hsieh M, Mulders S, Richards JS 2003 Hormone-regulated expression and localization of versican in the rodent ovary. Endocrinology 144:1020–1031 [DOI] [PubMed] [Google Scholar]
- Mjaatvedt CH, Yamamura H, Capehart AA, Turner D, Markwald RR 1998 The Cspg2 gene, disrupted in the hdf mutant, is required for right cardiac chamber and endocardial cushion formation. Dev Biol 202:56–66 [DOI] [PubMed] [Google Scholar]
- Shindo T, Kurihara H, Kuno K, Yokoyama H, Wada T, Kurihara Y, Imai T, Wang Y, Ogata M, Nishimatsu H, Moriyama N, Oh-hashi Y, Morita H, Ishikawa T, Nagai R, Yazaki Y, Matsushima K 2000 ADAMTS-1: a metalloproteinase-disintegrin essential for normal growth, fertility, and organ morphology and function. J Clin Invest 105:1345–1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittaz L, Russell DL, Wilson T, Brasted M, Tkalcevic J, Salamonsen LA, Hertzog PJ, Pritchard MA 2004 Adamts-1 is essential for the development and function of the urogenital system. Biol Reprod 70:1096–1105 [DOI] [PubMed] [Google Scholar]
- Peng XR, Hsueh AJ, LaPolt PS, Bjersing L, Ny T 1991 Localization of luteinizing hormone receptor messenger ribonucleic acid expression in ovarian cell types during follicle development and ovulation. Endocrinology 129:3200–3207 [DOI] [PubMed] [Google Scholar]
- Park JY, Su YQ, Ariga M, Law E, Jin SL, Conti M 2004 EGF-like growth factors as mediators of LH action in the ovulatory follicle. Science 303:682–684 [DOI] [PubMed] [Google Scholar]
- Hsieh M, Lee D, Panigone S, Horner K, Chen R, Theologis A, Lee DC, Threadgill DW, Conti M 2007 Luteinizing hormone-dependent activation of the epidermal growth factor network is essential for ovulation. Mol Cell Biol 27:1914–1924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh M, Zamah AM, Conti M 2009 Epidermal growth factor-like growth factors in the follicular fluid: role in oocyte development and maturation. Semin Reprod Med 27:52–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue Y, Miyamoto S, Fukami T, Shirota K, Yotsumoto F, Kawarabayashi T 2009 Amphiregulin is much more abundantly expressed than transforming growth factor-α and epidermal growth factor in human follicular fluid obtained from patients undergoing in vitro fertilization-embryo transfer. Fertil Steril 91:1035–1041 [DOI] [PubMed] [Google Scholar]
- Gershon E, Hourvitz A, Reikhav S, Maman E, Dekel N 2007 Low expression of COX-2, reduced cumulus expansion, and impaired ovulation in SULT1E1-deficient mice. FASEB J 21:1893–1901 [DOI] [PubMed] [Google Scholar]
- Bliss SP, Miller A, Navratil AM, Xie J, McDonough SP, Fisher PJ, Landreth GE, Roberson MS 2009 ERK signaling in the pituitary is required for female but not male fertility. Mol Endocrinol 23:1092–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salustri A, Yanagishita M, Hascall VC 1990 Mouse oocytes regulate hyaluronic acid synthesis and mucification by FSH-stimulated cumulus cells. Dev Biol 138:26–32 [DOI] [PubMed] [Google Scholar]
- Gui LM, Joyce IM 2005 RNA interference evidence that growth differentiation factor-9 mediates oocyte regulation of cumulus expansion in mice. Biol Reprod 72:195–199 [DOI] [PubMed] [Google Scholar]
- Mazerbourg S, Hsueh AJ 2006 Genomic analyses facilitate identification of receptors and signalling pathways for growth differentiation factor 9 and related orphan bone morphogenetic protein/growth differentiation factor ligands. Hum Reprod Update 12:373–383 [DOI] [PubMed] [Google Scholar]
- Dragovic RA, Ritter LJ, Schulz SJ, Amato F, Thompson JG, Armstrong DT, Gilchrist RB 2007 Oocyte-secreted factor activation of SMAD 2/3 signaling enables initiation of mouse cumulus cell expansion. Biol Reprod 76:848–857 [DOI] [PubMed] [Google Scholar]
- Li Q, Pangas SA, Jorgez CJ, Graff JM, Weinstein M, Matzuk MM 2008 Redundant roles of SMAD2 and SMAD3 in ovarian granulosa cells in vivo. Mol Cell Biol 28:7001–7011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangas SA, Matzuk MM 2005 The art and artifact of GDF9 activity: cumulus expansion and the cumulus expansion-enabling factor. Biol Reprod 73:582–585 [DOI] [PubMed] [Google Scholar]
- Yoshino O, McMahon HE, Sharma S, Shimasaki S 2006 A unique preovulatory expression pattern plays a key role in the physiological functions of BMP-15 in the mouse. Proc Natl Acad Sci USA 103:10678–10683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su YQ, Wu X, O'Brien MJ, Pendola FL, Denegre JN, Matzuk MM, Eppig JJ 2004 Synergistic roles of BMP15 and GDF9 in the development and function of the oocyte-cumulus cell complex in mice: genetic evidence for an oocyte-granulosa cell regulatory loop. Dev Biol 276:64–73 [DOI] [PubMed] [Google Scholar]
- Moore RK, Otsuka F, Shimasaki S 2003 Molecular basis of bone morphogenetic protein-15 signaling in granulosa cells. J Biol Chem 278:304–310 [DOI] [PubMed] [Google Scholar]
- Yi SE, LaPolt PS, Yoon BS, Chen JY, Lu JK, Lyons KM 2001 The type I BMP receptor BmprIB is essential for female reproductive function. Proc Natl Acad Sci USA 98:7994–7999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YT, Tang L, Cai J, Lu XE, Xu J, Zhu XM, Luo Q, Huang HF 2007 High bone morphogenetic protein-15 level in follicular fluid is associated with high quality oocyte and subsequent embryonic development. Hum Reprod 22:1526–1531 [DOI] [PubMed] [Google Scholar]
- McKenzie LJ, Pangas SA, Carson SA, Kovanci E, Cisneros P, Buster JE, Amato P, Matzuk MM 2004 Human cumulus granulosa cell gene expression: a predictor of fertilization and embryo selection in women undergoing IVF. Hum Reprod 19:2869–2874 [DOI] [PubMed] [Google Scholar]
- Cillo F, Brevini TA, Antonini S, Paffoni A, Ragni G, Gandolfi F 2007 Association between human oocyte developmental competence and expression levels of some cumulus genes. Reproduction 134:645–650 [DOI] [PubMed] [Google Scholar]
- Assou S, Anahory T, Pantesco V, Le Carrour T, Pellestor F, Klein B, Reyftmann L, Dechaud H, De Vos J, Hamamah S 2006 The human cumulus–oocyte complex gene-expression profile. Hum Reprod 21:1705–1719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JS, Liu Z, Shimada M 2008 Immune-like mechanisms in ovulation. Trends Endocrinol Metab 19:191–196 [DOI] [PubMed] [Google Scholar]
- Natraj U, Richards JS 1993 Hormonal regulation, localization, and functional activity of the progesterone receptor in granulosa cells of rat preovulatory follicles. Endocrinology 133:761–769 [DOI] [PubMed] [Google Scholar]
- Park OK, Mayo KE 1991 Transient expression of progesterone receptor messenger RNA in ovarian granulosa cells after the preovulatory luteinizing hormone surge. Mol Endocrinol 5:967–978 [DOI] [PubMed] [Google Scholar]
- Mulac-Jericevic B, Conneely OM 2004 Reproductive tissue selective actions of progesterone receptors. Reproduction 128:139–146 [DOI] [PubMed] [Google Scholar]
- Robker RL, Russell DL, Espey LL, Lydon JP, O'Malley BW, Richards JS 2000 Progesterone-regulated genes in the ovulation process: ADAMTS-1 and cathepsin L proteases. Proc Natl Acad Sci USA 97:4689–4694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulac-Jericevic B, Lydon JP, DeMayo FJ, Conneely OM 2003 Defective mammary gland morphogenesis in mice lacking the progesterone receptor B isoform. Proc Natl Acad Sci USA 100:9744–9749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulac-Jericevic B, Mullinax RA, DeMayo FJ, Lydon JP, Conneely OM 2000 Subgroup of reproductive functions of progesterone mediated by progesterone receptor-B isoform. Science 289:1751–1754 [DOI] [PubMed] [Google Scholar]
- Richards JS 2007 Genetics of ovulation. Semin Reprod Med 25:235–242 [DOI] [PubMed] [Google Scholar]
- Richards JS, Russell DL, Ochsner S, Espey LL 2002 Ovulation: new dimensions and new regulators of the inflammatory-like response. Annu Rev Physiol 64:69–92 [DOI] [PubMed] [Google Scholar]
- Palanisamy GS, Cheon YP, Kim J, Kannan A, Li Q, Sato M, Mantena SR, Sitruk-Ware RL, Bagchi MK, Bagchi IC 2006 A novel pathway involving progesterone receptor, endothelin-2, and endothelin receptor B controls ovulation in mice. Mol Endocrinol 20:2784–2795 [DOI] [PubMed] [Google Scholar]
- Ko C, Gieske MC, Al-Alem L, Hahn Y, Su W, Gong MC, Iglarz M, Koo Y 2006 Endothelin-2 in ovarian follicle rupture. Endocrinology 147:1770–1779 [DOI] [PubMed] [Google Scholar]
- Sriraman V, Rudd MD, Lohmann SM, Mulders SM, Richards JS 2006 Cyclic guanosine 5′-monophosphate-dependent protein kinase II is induced by luteinizing hormone and progesterone receptor-dependent mechanisms in granulosa cells and cumulus oocyte complexes of ovulating follicles. Mol Endocrinol 20:348–361 [DOI] [PubMed] [Google Scholar]
- Soyal SM, Mukherjee A, Lee KY, Li J, Li H, DeMayo FJ, Lydon JP 2005 Cre-mediated recombination in cell lineages that express the progesterone receptor. Genesis 41:58–66 [DOI] [PubMed] [Google Scholar]
- Kim J, Sato M, Li Q, Lydon JP, Demayo FJ, Bagchi IC, Bagchi MK 2008 Peroxisome proliferator-activated receptor γ is a target of progesterone regulation in the preovulatory follicles and controls ovulation in mice. Mol Cell Biol 28:1770–1782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada M, Yanai Y, Okazaki T, Yamashita Y, Sriraman V, Wilson MC, Richards JS 2007 Synaptosomal-associated protein 25 gene expression is hormonally regulated during ovulation and is involved in cytokine/chemokine exocytosis from granulosa cells. Mol Endocrinol 21:2487–2502 [DOI] [PubMed] [Google Scholar]
- Sterneck E, Tessarollo L, Johnson PF 1997 An essential role for C/EBPβ in female reproduction. Genes Dev 11:2153–2162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkart AD, Mukherjee A, Sterneck E, Johnson PF, Mayo KE 2005 Repression of the inhibin α-subunit gene by the transcription factor CCAAT/enhancer-binding protein-β. Endocrinology 146:1909–1921 [DOI] [PubMed] [Google Scholar]
- Duggavathi R, Volle DH, Mataki C, Antal MC, Messaddeq N, Auwerx J, Murphy BD, Schoonjans K 2008 Liver receptor homolog 1 is essential for ovulation. Genes Dev 22:1871–1876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelusi C, Ikeda Y, Zubair M, Parker KL 2008 Impaired follicle development and infertility in female mice lacking steroidogenic factor 1 in ovarian granulosa cells. Biol Reprod 79:1074–1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder GD, Holmes RW, Bates JN, Van Voorhis BJ 1996 Nitric oxide inhibits aromatase activity: mechanisms of action. J Steroid Biochem Mol Biol 58:63–69 [DOI] [PubMed] [Google Scholar]
- Kagabu S, Kodama H, Fukuda J, Karube A, Murata M, Tanaka T 1999 Inhibitory effects of nitric oxide on the expression and activity of aromatase in human granulosa cells. Mol Hum Reprod 5:396–401 [DOI] [PubMed] [Google Scholar]
- White R, Leonardsson G, Rosewell I, Ann Jacobs M, Milligan S, Parker M 2000 The nuclear receptor co-repressor nrip1 (RIP140) is essential for female fertility. Nat Med 6:1368–1374 [DOI] [PubMed] [Google Scholar]
- Collins LL, Lee YF, Heinlein CA, Liu NC, Chen YT, Shyr CR, Meshul CK, Uno H, Platt KA, Chang C 2004 Growth retardation and abnormal maternal behavior in mice lacking testicular orphan nuclear receptor 4. Proc Natl Acad Sci USA 101:15058–15063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LM, Wang RS, Lee YF, Liu NC, Chang YJ, Wu CC, Xie S, Hung YC, Chang C 2008 Subfertility with defective folliculogenesis in female mice lacking testicular orphan nuclear receptor 4. Mol Endocrinol 22:858–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocco C, Telleria C, Gibori G 2007 The molecular control of corpus luteum formation, function, and regression. Endocr Rev 28:117–149 [DOI] [PubMed] [Google Scholar]
- Devoto L, Fuentes A, Kohen P, Cespedes P, Palomino A, Pommer R, Munoz A, Strauss 3rd JF 2009 The human corpus luteum: life cycle and function in natural cycles. Fertil Steril 92:1067–1079 [DOI] [PubMed] [Google Scholar]
- Robker RL, Richards JS 1998 Hormone-induced proliferation and differentiation of granulosa cells: a coordinated balance of the cell cycle regulators cyclin D2 and p27Kip1. Mol Endocrinol 12:924–940 [DOI] [PubMed] [Google Scholar]
- Fero ML, Rivkin M, Tasch M, Porter P, Carow CE, Firpo E, Polyak K, Tsai LH, Broudy V, Perlmutter RM, Kaushansky K, Roberts JM 1996 A syndrome of multiorgan hyperplasia with features of gigantism, tumorigenesis, and female sterility in p27(Kip1)-deficient mice. Cell 85:733–744 [DOI] [PubMed] [Google Scholar]
- Kiyokawa H, Kineman RD, Manova-Todorova KO, Soares VC, Hoffman ES, Ono M, Khanam D, Hayday AC, Frohman LA, Koff A 1996 Enhanced growth of mice lacking the cyclin-dependent kinase inhibitor function of p27(Kip1). Cell 85:721–732 [DOI] [PubMed] [Google Scholar]
- Nakayama K, Ishida N, Shirane M, Inomata A, Inoue T, Shishido N, Horii I, Loh DY, Nakayama K 1996 Mice lacking p27(Kip1) display increased body size, multiple organ hyperplasia, retinal dysplasia, and pituitary tumors. Cell 85:707–720 [DOI] [PubMed] [Google Scholar]
- Brugarolas J, Chandrasekaran C, Gordon JI, Beach D, Jacks T, Hannon GJ 1995 Radiation-induced cell cycle arrest compromised by p21 deficiency. Nature 377:552–557 [DOI] [PubMed] [Google Scholar]
- Deng C, Zhang P, Harper JW, Elledge SJ, Leder P 1995 Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell 82:675–684 [DOI] [PubMed] [Google Scholar]
- Jirawatnotai S, Moons DS, Stocco CO, Franks R, Hales DB, Gibori G, Kiyokawa H 2003 The cyclin-dependent kinase inhibitors p27Kip1 and p21Cip1 cooperate to restrict proliferative life span in differentiating ovarian cells. J Biol Chem 278:17021–17027 [DOI] [PubMed] [Google Scholar]
- Rane SG, Dubus P, Mettus RV, Galbreath EJ, Boden G, Reddy EP, Barbacid M 1999 Loss of Cdk4 expression causes insulin-deficient diabetes and Cdk4 activation results in β-islet cell hyperplasia. Nat Genet 22:44–52 [DOI] [PubMed] [Google Scholar]
- Tsutsui T, Hesabi B, Moons DS, Pandolfi PP, Hansel KS, Koff A, Kiyokawa H 1999 Targeted disruption of CDK4 delays cell cycle entry with enhanced p27(Kip1) activity. Mol Cell Biol 19:7011–7019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jirawatnotai S, Aziyu A, Osmundson EC, Moons DS, Zou X, Kineman RD, Kiyokawa H 2004 Cdk4 is indispensable for postnatal proliferation of the anterior pituitary. J Biol Chem 279:51100–51106 [DOI] [PubMed] [Google Scholar]
- Russell DL, Richards JS 1999 Differentiation-dependent prolactin responsiveness and STAT (signal transducers and activators of transcription) signaling in rat ovarian cells. Mol Endocrinol 13:2049–2064 [DOI] [PubMed] [Google Scholar]
- Ormandy CJ, Camus A, Barra J, Damotte D, Lucas B, Buteau H, Edery M, Brousse N, Babinet C, Binart N, Kelly PA 1997 Null mutation of the prolactin receptor gene produces multiple reproductive defects in the mouse. Genes Dev 11:167–178 [DOI] [PubMed] [Google Scholar]
- Grosdemouge I, Bachelot A, Lucas A, Baran N, Kelly PA, Binart N 2003 Effects of deletion of the prolactin receptor on ovarian gene expression. Reprod Biol Endocrinol 1:12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horseman ND, Zhao W, Montecino-Rodriguez E, Tanaka M, Nakashima K, Engle SJ, Smith F, Markoff E, Dorshkind K 1997 Defective mammopoiesis, but normal hematopoiesis, in mice with a targeted disruption of the prolactin gene. EMBO J 16:6926–6935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teglund S, McKay C, Schuetz E, van Deursen JM, Stravopodis D, Wang D, Brown M, Bodner S, Grosveld G, Ihle JN 1998 Stat5a and Stat5b proteins have essential and nonessential, or redundant, roles in cytokine responses. Cell 93:841–850 [DOI] [PubMed] [Google Scholar]
- Hsieh M, Boerboom D, Shimada M, Lo Y, Parlow AF, Luhmann UF, Berger W, Richards JS 2005 Mice null for Frizzled4 (Fzd4−/−) are infertile and exhibit impaired corpora lutea formation and function. Biol Reprod 73:1135–1146 [DOI] [PubMed] [Google Scholar]
- Fan HY, Liu Z, Cahill N, Richards JS 2008 Targeted disruption of Pten in ovarian granulosa cells enhances ovulation and extends the life span of luteal cells. Mol Endocrinol 22:2128–2140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz FJ, Wigglesworth K, Eppig JJ 2007 Oocytes are required for the preantral granulosa cell to cumulus cell transition in mice. Dev Biol 305:300–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangas SA, Li X, Robertson EJ, Matzuk MM 2006 Premature luteinization and cumulus cell defects in ovarian-specific Smad4 knockout mice. Mol Endocrinol 20:1406–1422 [DOI] [PubMed] [Google Scholar]
- Eppig JJ, Viveiros MM, Marin-Bivens C, De La Fuente R 2004 The ovary. Amsterdam: Elsevier Academic Press [Google Scholar]
- Mehlmann LM 2005 Stops and starts in mammalian oocytes: recent advances in understanding the regulation of meiotic arrest and oocyte maturation. Reproduction 130:791–799 [DOI] [PubMed] [Google Scholar]
- Edwards RG 1965 Maturation in vitro of mouse, sheep, cow, pig, rhesus monkey and human ovarian oocytes. Nature 208:349–351 [DOI] [PubMed] [Google Scholar]
- Pincus G, Enzmann EV 1935 The comparative behavior of mammalian eggs in vivo and in vitro. I. The activation of ovarian eggs. J Exp Med 62:665–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malumbres M, Barbacid M 2005 Mammalian cyclin-dependent kinases. Trends Biochem Sci 30:630–641 [DOI] [PubMed] [Google Scholar]
- Han SJ, Chen R, Paronetto MP, Conti M 2005 Wee1B is an oocyte-specific kinase involved in the control of meiotic arrest in the mouse. Curr Biol 15:1670–1676 [DOI] [PubMed] [Google Scholar]
- Masciarelli S, Horner K, Liu C, Park SH, Hinckley M, Hockman S, Nedachi T, Jin C, Conti M, Manganiello V 2004 Cyclic nucleotide phosphodiesterase 3A-deficient mice as a model of female infertility. J Clin Invest 114:196–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Wolgemuth DJ 1995 The distinct and developmentally regulated patterns of expression of members of the mouse Cdc25 gene family suggest differential functions during gametogenesis. Dev Biol 170:195–206 [DOI] [PubMed] [Google Scholar]
- Lincoln AJ, Wickramasinghe D, Stein P, Schultz RM, Palko ME, De Miguel MP, Tessarollo L, Donovan PJ 2002 Cdc25b phosphatase is required for resumption of meiosis during oocyte maturation. Nat Genet 30:446–449 [DOI] [PubMed] [Google Scholar]
- Chen MS, Hurov J, White LS, Woodford-Thomas T, Piwnica-Worms H 2001 Absence of apparent phenotype in mice lacking Cdc25C protein phosphatase. Mol Cell Biol 21:3853–3861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray D, Terao Y, Nimbalkar D, Hirai H, Osmundson EC, Zou X, Franks R, Christov K, Kiyokawa H 2007 Hemizygous disruption of Cdc25A inhibits cellular transformation and mammary tumorigenesis in mice. Cancer Res 67:6605–6611 [DOI] [PubMed] [Google Scholar]
- Solc P, Saskova A, Baran V, Kubelka M, Schultz RM, Motlik J 2008 CDC25A phosphatase controls meiosis I progression in mouse oocytes. Dev Biol 317:260–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckworth BC, Weaver JS, Ruderman JV 2002 G2 arrest in Xenopus oocytes depends on phosphorylation of cdc25 by protein kinase A. Proc Natl Acad Sci USA 99:16794–16799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirino G, Wescott MP, Donovan PJ 2009 Protein kinase A regulates resumption of meiosis by phosphorylation of Cdc25B in mammalian oocytes. Cell Cycle 8:665–670 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zhang Z, Xu XY, Li XS, Yu M, Yu AM, Zong ZH, Yu BZ 2008 Protein kinase A modulates Cdc25B activity during meiotic resumption of mouse oocytes. Dev Dyn 237:3777–3786 [DOI] [PubMed] [Google Scholar]
- Schultz RM, Montgomery RR, Belanoff JR 1983 Regulation of mouse oocyte meiotic maturation: implication of a decrease in oocyte cAMP and protein dephosphorylation in commitment to resume meiosis. Dev Biol 97:264–273 [DOI] [PubMed] [Google Scholar]
- Urner F, Herrmann WL, Baulieu EE, Schorderet-Slatkine S 1983 Inhibition of denuded mouse oocyte meiotic maturation by forskolin, an activator of adenylate cyclase. Endocrinology 113:1170–1172 [DOI] [PubMed] [Google Scholar]
- Vivarelli E, Conti M, De Felici M, Siracusa G 1983 Meiotic resumption and intracellular cAMP levels in mouse oocytes treated with compounds which act on cAMP metabolism. Cell Differ 12:271–276 [DOI] [PubMed] [Google Scholar]
- Mehlmann LM, Jones TL, Jaffe LA 2002 Meiotic arrest in the mouse follicle maintained by a Gs protein in the oocyte. Science 297:1343–1345 [DOI] [PubMed] [Google Scholar]
- Mehlmann LM, Saeki Y, Tanaka S, Brennan TJ, Evsikov AV, Pendola FL, Knowles BB, Eppig JJ, Jaffe LA 2004 The Gs-linked receptor GPR3 maintains meiotic arrest in mammalian oocytes. Science 306:1947–1950 [DOI] [PubMed] [Google Scholar]
- Uhlenbrock K, Gassenhuber H, Kostenis E 2002 Sphingosine 1-phosphate is a ligand of the human gpr3, gpr6 and gpr12 family of constitutively active G protein-coupled receptors. Cell Signal 14:941–953 [DOI] [PubMed] [Google Scholar]
- Ledent C, Demeestere I, Blum D, Petermans J, Hämäläinen T, Smits G, Vassart G 2005 Premature ovarian aging in mice deficient for Gpr3. Proc Natl Acad Sci USA 102:8922–8926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freudzon L, Norris RP, Hand AR, Tanaka S, Saeki Y, Jones TL, Rasenick MM, Berlot CH, Mehlmann LM, Jaffe LA 2005 Regulation of meiotic prophase arrest in mouse oocytes by GPR3, a constitutive activator of the Gs G protein. J Cell Biol 171:255–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner K, Livera G, Hinckley M, Trinh K, Storm D, Conti M 2003 Rodent oocytes express an active adenylyl cyclase required for meiotic arrest. Dev Biol 258:385–396 [DOI] [PubMed] [Google Scholar]
- Wong ST, Trinh K, Hacker B, Chan GC, Lowe G, Gaggar A, Xia Z, Gold GH, Storm DR 2000 Disruption of the type III adenylyl cyclase gene leads to peripheral and behavioral anosmia in transgenic mice. Neuron 27:487–497 [DOI] [PubMed] [Google Scholar]
- Sadler SE 1991 Inhibitors of phosphodiesterase III block stimulation of Xenopus laevis oocyte ribosomal S6 kinase activity by insulin-like growth factor-I. Mol Endocrinol 5:1947–1954 [DOI] [PubMed] [Google Scholar]
- Shitsukawa K, Andersen CB, Richard FJ, Horner AK, Wiersma A, van Duin M, Conti M 2001 Cloning and characterization of the cyclic guanosine monophosphate-inhibited phosphodiesterase PDE3A expressed in mouse oocyte. Biol Reprod 65:188–196 [DOI] [PubMed] [Google Scholar]
- Richard FJ, Tsafriri A, Conti M 2001 Role of phosphodiesterase type 3A in rat oocyte maturation. Biol Reprod 65:1444–1451 [DOI] [PubMed] [Google Scholar]
- Nogueira D, Albano C, Adriaenssens T, Cortvrindt R, Bourgain C, Devroey P, Smitz J 2003 Human oocytes reversibly arrested in prophase I by phosphodiesterase type 3 inhibitor in vitro. Biol Reprod 69:1042–1052 [DOI] [PubMed] [Google Scholar]
- Newhall KJ, Criniti AR, Cheah CS, Smith KC, Kafer KE, Burkart AD, McKnight GS 2006 Dynamic anchoring of PKA is essential during oocyte maturation. Curr Biol 16:321–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sela-Abramovich S, Edry I, Galiani D, Nevo N, Dekel N 2006 Disruption of gap junctional communication within the ovarian follicle induces oocyte maturation. Endocrinology 147:2280–2286 [DOI] [PubMed] [Google Scholar]
- Norris RP, Freudzon M, Mehlmann LM, Cowan AE, Simon AM, Paul DL, Lampe PD, Jaffe LA 2008 Luteinizing hormone causes MAP kinase-dependent phosphorylation and closure of connexin 43 gap junctions in mouse ovarian follicles: one of two paths to meiotic resumption. Development 135:3229–3238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs SM, Daniel SA, Eppig JJ 1988 Induction of maturation in cumulus cell-enclosed mouse oocytes by follicle-stimulating hormone and epidermal growth factor: evidence for a positive stimulus of somatic cell origin. J Exp Zool 245:86–96 [DOI] [PubMed] [Google Scholar]
- Jamnongjit M, Gill A, Hammes SR 2005 Epidermal growth factor receptor signaling is required for normal ovarian steroidogenesis and oocyte maturation. Proc Natl Acad Sci USA 102:16257–16262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eppig JJ, Freter RR, Ward-Bailey PF, Schultz RM 1983 Inhibition of oocyte maturation in the mouse: participation of cAMP, steroid hormones, and a putative maturation-inhibitory factor. Dev Biol 100:39–49 [DOI] [PubMed] [Google Scholar]
- Kaji E, Bornslaeger EA, Schultz RM 1987 Inhibition of mouse oocyte cyclic AMP phosphodiesterase by steroid hormones: a possible mechanism for steroid hormone inhibition of oocyte maturation. J Exp Zool 243:489–493 [DOI] [PubMed] [Google Scholar]
- Byskov AG, Andersen CY, Nordholm L, Thøgersen H, Xia G, Wassmann O, Andersen JV, Guddal E, Roed T 1995 Chemical structure of sterols that activate oocyte meiosis. Nature 374:559–562 [DOI] [PubMed] [Google Scholar]
- Törnell J, Billig H, Hillensjö T 1991 Regulation of oocyte maturation by changes in ovarian levels of cyclic nucleotides. Hum Reprod 6:411–422 [DOI] [PubMed] [Google Scholar]
- Törnell J, Billig H, Hillensjö T 1990 Resumption of rat oocyte meiosis is paralleled by a decrease in guanosine 3′,5′-cyclic monophosphate (cGMP) and is inhibited by microinjection of cGMP. Acta Physiol Scand 139:511–517 [DOI] [PubMed] [Google Scholar]
- Sela-Abramovich S, Galiani D, Nevo N, Dekel N 2008 Inhibition of rat oocyte maturation and ovulation by nitric oxide: mechanism of action. Biol Reprod 78:1111–1118 [DOI] [PubMed] [Google Scholar]
- Vaccari S, Weeks Ii JL, Hsieh M, Menniti FS, Conti M 27 May 2009 Cyclic GMP signaling is involved in the luteinizing hormone-dependent meiotic maturation of mouse oocytes. Biol Reprod 81:595–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masui Y, Markert CL 1971 Cytoplasmic control of nuclear behavior during meiotic maturation of frog oocytes. J Exp Zool 177:129–145 [DOI] [PubMed] [Google Scholar]
- Peters JM 2006 The anaphase promoting complex/cyclosome: a machine designed to destroy. Nat Rev Mol Cell Biol 7:644–656 [DOI] [PubMed] [Google Scholar]
- Sagata N, Watanabe N, Vande Woude GF, Ikawa Y 1989 The c-mos proto-oncogene product is a cytostatic factor responsible for meiotic arrest in vertebrate eggs. Nature 342:512–518 [DOI] [PubMed] [Google Scholar]
- Colledge WH, Carlton MB, Udy GB, Evans MJ 1994 Disruption of c-mos causes parthenogenetic development of unfertilized mouse eggs. Nature 370:65–68 [DOI] [PubMed] [Google Scholar]
- Hashimoto N, Watanabe N, Furuta Y, Tamemoto H, Sagata N, Yokoyama M, Okazaki K, Nagayoshi M, Takeda N, Ikawa Y, et al 1994 Parthenogenetic activation of oocytes in c-mos-deficient mice. Nature 370:68–71 [DOI] [PubMed] [Google Scholar]
- Choi T, Fukasawa K, Zhou R, Tessarollo L, Borror K, Resau J, Vande Woude GF 1996 The Mos/mitogen-activated protein kinase (MAPK) pathway regulates the size and degradation of the first polar body in maturing mouse oocytes. Proc Natl Acad Sci USA 93:7032–7035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A, Duncan PI, Rauh NR, Sauer G, Fry AM, Nigg EA, Mayer TU 2005 Xenopus polo-like kinase Plx1 regulates XErp1, a novel inhibitor of APC/C activity. Genes Dev 19:502–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoji S, Yoshida N, Amanai M, Ohgishi M, Fukui T, Fujimoto S, Nakano Y, Kajikawa E, Perry AC 2006 Mammalian Emi2 mediates cytostatic arrest and transduces the signal for meiotic exit via Cdc20. EMBO J 25:834–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madgwick S, Hansen DV, Levasseur M, Jackson PK, Jones KT 2006 Mouse Emi2 is required to enter meiosis II by reestablishing cyclin B1 during interkinesis. J Cell Biol 174:791–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry AC, Verlhac MH 2008 Second meiotic arrest and exit in frogs and mice. EMBO Rep 9:246–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt RR, Ferrell Jr JE 1999 The protein kinase p90 rsk as an essential mediator of cytostatic factor activity. Science 286:1362–1365 [DOI] [PubMed] [Google Scholar]
- Gross SD, Schwab MS, Lewellyn AL, Maller JL 1999 Induction of metaphase arrest in cleaving Xenopus embryos by the protein kinase p90Rsk. Science 286:1365–1367 [DOI] [PubMed] [Google Scholar]
- Inoue D, Ohe M, Kanemori Y, Nobui T, Sagata N 2007 A direct link of the Mos-MAPK pathway to Erp1/Emi2 in meiotic arrest of Xenopus laevis eggs. Nature 446:1100–1104 [DOI] [PubMed] [Google Scholar]
- Nishiyama T, Ohsumi K, Kishimoto T 2007 Phosphorylation of Erp1 by p90rsk is required for cytostatic factor arrest in Xenopus laevis eggs. Nature 446:1096–1099 [DOI] [PubMed] [Google Scholar]
- Dumont J, Umbhauer M, Rassinier P, Hanauer A, Verlhac MH 2005 p90Rsk is not involved in cytostatic factor arrest in mouse oocytes. J Cell Biol 169:227–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders CM, Larman MG, Parrington J, Cox LJ, Royse J, Blayney LM, Swann K, Lai FA 2002 PLC ζ: a sperm-specific trigger of Ca(2+) oscillations in eggs and embryo development. Development 129:3533–3544 [DOI] [PubMed] [Google Scholar]
- Nixon VL, Levasseur M, McDougall A, Jones KT 2002 Ca(2+) oscillations promote APC/C-dependent cyclin B1 degradation during metaphase arrest and completion of meiosis in fertilizing mouse eggs. Curr Biol 12:746–750 [DOI] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ 2008 Cancer statistics, 2008. CA Cancer J Clin 58:71–96 [DOI] [PubMed] [Google Scholar]
- Crum CP 1999 The female genital tract. In: Cotran RS, Kumar V, Collins T, eds. Robbins pathologic basis of disease. 6th ed. Philadelphia: W.B. Saunders Company; 1066–1079 [Google Scholar]
- Seidman JD, Horkayne-Szakaly I, Haiba M, Boice CR, Kurman RJ, Ronnett BM 2004 The histologic type and stage distribution of ovarian carcinomas of surface epithelial origin. Int J Gynecol Pathol 23:41–44 [DOI] [PubMed] [Google Scholar]
- Bell DA 2005 Origins and molecular pathology of ovarian cancer. Mod Pathol 18(Suppl 2):S19–S32 [DOI] [PubMed] [Google Scholar]
- Auersperg N, Wong AS, Choi KC, Kang SK, Leung PC 2001 Ovarian surface epithelium: biology, endocrinology, and pathology. Endocr Rev 22:255–288 [DOI] [PubMed] [Google Scholar]
- Fathalla MF 1971 Incessant ovulation—a factor in ovarian neoplasia? Lancet 2:163 [DOI] [PubMed] [Google Scholar]
- Godwin AK, Testa JR, Handel LM, Liu Z, Vanderveer LA, Tracey PA, Hamilton TC 1992 Spontaneous transformation of rat ovarian surface epithelial cells: association with cytogenetic changes and implications of repeated ovulation in the etiology of ovarian cancer. J Natl Cancer Inst 84:592–601 [DOI] [PubMed] [Google Scholar]
- Testa JR, Getts LA, Salazar H, Liu Z, Handel LM, Godwin AK, Hamilton TC 1994 Spontaneous transformation of rat ovarian surface epithelial cells results in well to poorly differentiated tumors with a parallel range of cytogenetic complexity. Cancer Res 54:2778–2784 [PubMed] [Google Scholar]
- Feeley KM, Wells M 2001 Precursor lesions of ovarian epithelial malignancy. Histopathology 38:87–95 [DOI] [PubMed] [Google Scholar]
- Dubeau L 1999 The cell of origin of ovarian epithelial tumors and the ovarian surface epithelium dogma: does the emperor have no clothes? Gynecol Oncol 72:437–442 [DOI] [PubMed] [Google Scholar]
- Dubeau L 2008 The cell of origin of ovarian epithelial tumours. Lancet Oncol 9:1191–1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch A, Shaw P, Rosen B, Murphy J, Narod SA, Colgan TJ 2006 Clinical and pathologic findings of prophylactic salpingo-oophorectomies in 159 BRCA1 and BRCA2 carriers. Gynecol Oncol 100:58–64 [DOI] [PubMed] [Google Scholar]
- Kindelberger DW, Lee Y, Miron A, Hirsch MS, Feltmate C, Medeiros F, Callahan MJ, Garner EO, Gordon RW, Birch C, Berkowitz RS, Muto MG, Crum CP 2007 Intraepithelial carcinoma of the fimbria and pelvic serous carcinoma: Evidence for a causal relationship. Am J Surg Pathol 31:161–169 [DOI] [PubMed] [Google Scholar]
- Crum CP, Drapkin R, Kindelberger D, Medeiros F, Miron A, Lee Y 2007 Lessons from BRCA: the tubal fimbria emerges as an origin for pelvic serous cancer. Clin Med Res 5:35–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Gorp T, Amant F, Neven P, Vergote I, Moerman P 2004 Endometriosis and the development of malignant tumours of the pelvis. A review of literature. Best Pract Res Clin Obstet Gynaecol 18:349–371 [DOI] [PubMed] [Google Scholar]
- Somigliana E, Vigano' P, Parazzini F, Stoppelli S, Giambattista E, Vercellini P 2006 Association between endometriosis and cancer: a comprehensive review and a critical analysis of clinical and epidemiological evidence. Gynecol Oncol 101:331–341 [DOI] [PubMed] [Google Scholar]
- Nezhat F, Datta MS, Hanson V, Pejovic T, Nezhat C, Nezhat C 2008 The relationship of endometriosis and ovarian malignancy: a review. Fertil Steril 90:1559–1570 [DOI] [PubMed] [Google Scholar]
- Cho KR, Shih IeM 2009 Ovarian cancer. Annu Rev Pathol 4:287–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anglesio MS, Arnold JM, George J, Tinker AV, Tothill R, Waddell N, Simms L, Locandro B, Fereday S, Traficante N, Russell P, Sharma R, Birrer MJ, deFazio A, Chenevix-Trench G, Bowtell DD 2008 Mutation of ERBB2 provides a novel alternative mechanism for the ubiquitous activation of RAS-MAPK in ovarian serous low malignant potential tumors. Mol Cancer Res 6:1678–1690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih IeM, Kurman RJ 2004 Ovarian tumorigenesis: a proposed model based on morphological and molecular genetic analysis. Am J Pathol 164:1511–1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer G, Stöhr R, Cope L, Dehari R, Hartmann A, Cao DF, Wang TL, Kurman RJ, Shih IeM 2005 Patterns of p53 mutations separate ovarian serous borderline tumors and low- and high-grade carcinomas and provide support for a new model of ovarian carcinogenesis: a mutational analysis with immunohistochemical correlation. Am J Surg Pathol 29:218–224 [DOI] [PubMed] [Google Scholar]
- Kurman RJ, Shih IeM 2008 Pathogenesis of ovarian cancer: lessons from morphology and molecular biology and their clinical implications. Int J Gynecol Pathol 27:151–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams AT, Auersperg N 1981 Transformation of cultured rat ovarian surface epithelial cells by Kirsten murine sarcoma virus. Cancer Res 41:2063–2072 [PubMed] [Google Scholar]
- Roby KF, Taylor CC, Sweetwood JP, Cheng Y, Pace JL, Tawfik O, Persons DL, Smith PG, Terranova PF 2000 Development of a syngeneic mouse model for events related to ovarian cancer. Carcinogenesis 21:585–591 [DOI] [PubMed] [Google Scholar]
- Hahn WC, Weinberg RA 2002 Modelling the molecular circuitry of cancer. Nat Rev Cancer 2:331–341 [DOI] [PubMed] [Google Scholar]
- Maines-Bandiera SL, Kruk PA, Auersperg N 1992 Simian virus 40-transformed human ovarian surface epithelial cells escape normal growth controls but retain morphogenetic responses to extracellular matrix. Am J Obstet Gynecol 167:729–735 [DOI] [PubMed] [Google Scholar]
- Leung EH, Leung PC, Auersperg N 2001 Differentiation and growth potential of human ovarian surface epithelial cells expressing temperature-sensitive SV40 T antigen. In Vitro Cell Dev Biol Anim 37:515–521 [DOI] [PubMed] [Google Scholar]
- Nitta M, Katabuchi H, Ohtake H, Tashiro H, Yamaizumi M, Okamura H 2001 Characterization and tumorigenicity of human ovarian surface epithelial cells immortalized by SV40 large T antigen. Gynecol Oncol 81:10–17 [DOI] [PubMed] [Google Scholar]
- Davies BR, Steele IA, Edmondson RJ, Zwolinski SA, Saretzki G, von Zglinicki T, O'Hare MJ 2003 Immortalisation of human ovarian surface epithelium with telomerase and temperature-sensitive SV40 large T antigen. Exp Cell Res 288:390–402 [DOI] [PubMed] [Google Scholar]
- Liu J, Yang G, Thompson-Lanza JA, Glassman A, Hayes K, Patterson A, Marquez RT, Auersperg N, Yu Y, Hahn WC, Mills GB, Bast Jr RC 2004 A genetically defined model for human ovarian cancer. Cancer Res 64:1655–1663 [DOI] [PubMed] [Google Scholar]
- Ong A, Maines-Bandiera SL, Roskelley CD, Auersperg N 2000 An ovarian adenocarcinoma line derived from SV40/E-cadherin-transfected normal human ovarian surface epithelium. Int J Cancer 85:430–437 [PubMed] [Google Scholar]
- Kusakari T, Kariya M, Mandai M, Tsuruta Y, Hamid AA, Fukuhara K, Nanbu K, Takakura K, Fujii S 2003 C-erbB-2 or mutant Ha-ras induced malignant transformation of immortalized human ovarian surface epithelial cells in vitro. Br J Cancer 89:2293–2298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao SW, Mok SC, Fey EG, Fletcher JA, Wan TS, Chew EC, Muto MG, Knapp RC, Berkowitz RS 1995 Characterization of human ovarian surface epithelial cells immortalized by human papilloma viral oncogenes (HPV-E6E7 ORFs). Exp Cell Res 218:499–507 [DOI] [PubMed] [Google Scholar]
- Gregoire L, Rabah R, Schmelz EM, Munkarah A, Roberts PC, Lancaster WD 2001 Spontaneous malignant transformation of human ovarian surface epithelial cells in vitro. Clin Cancer Res 7:4280–4287 [PubMed] [Google Scholar]
- Lyons SK 2005 Advances in imaging mouse tumour models in vivo. J Pathol 205:194–205 [DOI] [PubMed] [Google Scholar]
- Shaw TJ, Senterman MK, Dawson K, Crane CA, Vanderhyden BC 2004 Characterization of intraperitoneal, orthotopic, and metastatic xenograft models of human ovarian cancer. Mol Ther 10:1032–1042 [DOI] [PubMed] [Google Scholar]
- Orsulic S, Li Y, Soslow RA, Vitale-Cross LA, Gutkind JS, Varmus HE 2002 Induction of ovarian cancer by defined multiple genetic changes in a mouse model system. Cancer Cell 1:53–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly DC, Bao R, Nikitin AY, Stephens KC, Poole TW, Hua X, Harris SS, Vanderhyden BC, Hamilton TC 2003 Female mice chimeric for expression of the simian virus 40 TAg under control of the MISIIR promoter develop epithelial ovarian cancer. Cancer Res 63:1389–1397 [PubMed] [Google Scholar]
- Jeyasuria P, Ikeda Y, Jamin SP, Zhao L, De Rooij DG, Themmen AP, Behringer RR, Parker KL 2004 Cell-specific knockout of steroidogenic factor 1 reveals its essential roles in gonadal function. Mol Endocrinol 18:1610–1619 [DOI] [PubMed] [Google Scholar]
- Deutscher E, Hung-Chang Yao H 2007 Essential roles of mesenchyme-derived β-catenin in mouse Mullerian duct morphogenesis. Dev Biol 307:227–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arango NA, Kobayashi A, Wang Y, Jamin SP, Lee HH, Orvis GD, Behringer RR 2008 A mesenchymal perspective of Mullerian duct differentiation and regression in Amhr2-lacZ mice. Mol Reprod Dev 75:1154–1162 [DOI] [PubMed] [Google Scholar]
- Masiakos PT, MacLaughlin DT, Maheswaran S, Teixeira J, Fuller Jr AF, Shah PC, Kehas DJ, Kenneally MK, Dombkowski DM, Ha TU, Preffer FI, Donahoe PK 1999 Human ovarian cancer, cell lines, and primary ascites cells express the human Mullerian inhibiting substance (MIS) type II receptor, bind, and are responsive to MIS. Clin Cancer Res 5:3488–3499 [PubMed] [Google Scholar]
- Garson K, Shaw TJ, Clark KV, Yao DS, Vanderhyden BC 2005 Models of ovarian cancer–are we there yet? Mol Cell Endocrinol 239:15–26 [DOI] [PubMed] [Google Scholar]
- Flesken-Nikitin A, Choi KC, Eng JP, Shmidt EN, Nikitin AY 2003 Induction of carcinogenesis by concurrent inactivation of p53 and Rb1 in the mouse ovarian surface epithelium. Cancer Res 63:3459–3463 [PubMed] [Google Scholar]
- Dinulescu DM, Ince TA, Quade BJ, Shafer SA, Crowley D, Jacks T 2005 Role of K-ras and Pten in the development of mouse models of endometriosis and endometrioid ovarian cancer. Nat Med 11:63–70 [DOI] [PubMed] [Google Scholar]
- Matzuk MM 2005 Gynecologic diseases get their genes. Nat Med 11:24–26 [DOI] [PubMed] [Google Scholar]
- Fan HY, Liu Z, Paquet M, Wang J, Lydon JP, DeMayo FJ, Richards JS 2009 Cell type-specific targeted mutations of Kras and Pten document proliferation arrest in granulosa cells versus oncogenic insult to ovarian surface epithelial cells. Cancer Res 69:6463–6472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato N, Tsunoda H, Nishida M, Morishita Y, Takimoto Y, Kubo T, Noguchi M 2000 Loss of heterozygosity on 10q23.3 and mutation of the tumor suppressor gene PTEN in benign endometrial cyst of the ovary: possible sequence progression from benign endometrial cyst to endometrioid carcinoma and clear cell carcinoma of the ovary. Cancer Res 60:7052–7056 [PubMed] [Google Scholar]
- Wu R, Hendrix-Lucas N, Kuick R, Zhai Y, Schwartz DR, Akyol A, Hanash S, Misek DE, Katabuchi H, Williams BO, Fearon ER, Cho KR 2007 Mouse model of human ovarian endometrioid adenocarcinoma based on somatic defects in the Wnt/β-catenin and PI3K/Pten signaling pathways. Cancer Cell 11:321–333 [DOI] [PubMed] [Google Scholar]
- Fang D, Hawke D, Zheng Y, Xia Y, Meisenhelder J, Nika H, Mills GB, Kobayashi R, Hunter T, Lu Z 2007 Phosphorylation of β-catenin by AKT promotes β-catenin transcriptional activity. J Biol Chem 282:11221–11229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo A, Calò V, Bruno L, Rizzo S, Bazan V, Di Fede G 2009 Hereditary ovarian cancer. Crit Rev Oncol Hematol 69:28–44 [DOI] [PubMed] [Google Scholar]
- King MC, Marks JH, Mandell JB 2003 Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science 302:643–646 [DOI] [PubMed] [Google Scholar]
- Weberpals JI, Clark-Knowles KV, Vanderhyden BC 2008 Sporadic epithelial ovarian cancer: clinical relevance of BRCA1 inhibition in the DNA damage and repair pathway. J Clin Oncol 26:3259–3267 [DOI] [PubMed] [Google Scholar]
- Salazar H, Godwin AK, Daly MB, Laub PB, Hogan WM, Rosenblum N, Boente MP, Lynch HT, Hamilton TC 1996 Microscopic benign and invasive malignant neoplasms and a cancer-prone phenotype in prophylactic oophorectomies. J Natl Cancer Inst 88:1810–1820 [DOI] [PubMed] [Google Scholar]
- Werness BA, Afify AM, Bielat KL, Eltabbakh GH, Piver MS, Paterson JM 1999 Altered surface and cyst epithelium of ovaries removed prophylactically from women with a family history of ovarian cancer. Hum Pathol 30:151–157 [DOI] [PubMed] [Google Scholar]
- Schlosshauer PW, Cohen CJ, Penault-Llorca F, Miranda CR, Bignon YJ, Dauplat J, Deligdisch L 2003 Prophylactic oophorectomy: a morphologic and immunohistochemical study. Cancer 98:2599–2606 [DOI] [PubMed] [Google Scholar]
- Clark-Knowles KV, Garson K, Jonkers J, Vanderhyden BC 2007 Conditional inactivation of Brca1 in the mouse ovarian surface epithelium results in an increase in preneoplastic changes. Exp Cell Res 313:133–145 [DOI] [PubMed] [Google Scholar]
- Xing D, Orsulic S 2006 A mouse model for the molecular characterization of brca1-associated ovarian carcinoma. Cancer Res 66:8949–8953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramus SJ, Bobrow LG, Pharoah PD, Finnigan DS, Fishman A, Altaras M, Harrington PA, Gayther SA, Ponder BA, Friedman LS 1999 Increased frequency of TP53 mutations in BRCA1 and BRCA2 ovarian tumours. Genes Chromosomes Cancer 25:91–96 [DOI] [PubMed] [Google Scholar]
- Werness BA, Parvatiyar P, Ramus SJ, Whittemore AS, Garlinghouse-Jones K, Oakley-Girvan I, DiCioccio RA, Wiest J, Tsukada Y, Ponder BA, Piver MS 2000 Ovarian carcinoma in situ with germline BRCA1 mutation and loss of heterozygosity at BRCA1 and TP53. J Natl Cancer Inst 92:1088–1091 [DOI] [PubMed] [Google Scholar]
- Grushko TA, Dignam JJ, Das S, Blackwood AM, Perou CM, Ridderstråle KK, Anderson KN, Wei MJ, Adams AJ, Hagos FG, Sveen L, Lynch HT, Weber BL, Olopade OI 2004 MYC is amplified in BRCA1-associated breast cancers. Clin Cancer Res 10:499–507 [DOI] [PubMed] [Google Scholar]
- Rhei E, Bogomolniy F, Federici MG, Maresco DL, Offit K, Robson ME, Saigo PE, Boyd J 1998 Molecular genetic characterization of BRCA1- and BRCA2-linked hereditary ovarian cancers. Cancer Res 58:3193–3196 [PubMed] [Google Scholar]
- Colombo N, Parma G, Zanagnolo V, Insinga A 2007 Management of ovarian stromal cell tumors. J Clin Oncol 25:2944–2951 [DOI] [PubMed] [Google Scholar]
- East N, Alobaid A, Goffin F, Ouallouche K, Gauthier P 2005 Granulosa cell tumour: a recurrence 40 years after initial diagnosis. J Obstet Gynaecol Can 27:363–364 [DOI] [PubMed] [Google Scholar]
- Beamer WG, Hoppe PC, Whitten WK 1985 Spontaneous malignant granulosa cell tumors in ovaries of young SWR mice. Cancer Res 45:5575–5581 [PubMed] [Google Scholar]
- Dorward AM, Shultz KL, Horton LG, Li R, Churchill GA, Beamer WG 2005 Distal Chr 4 harbors a genetic locus (Gct1) fundamental for spontaneous ovarian granulosa cell tumorigenesis in a mouse model. Cancer Res 65:1259–1264 [DOI] [PubMed] [Google Scholar]
- Beamer WG, Tennent BJ, Shultz KL, Nadeau JH, Shultz LD, Skow LC 1988 Gene for ovarian granulosa cell tumor susceptibility, Gct, in SWXJ recombinant inbred strains of mice revealed by dehydroepiandrosterone. Cancer Res 48:5092–5095 [PubMed] [Google Scholar]
- Tennent BJ, Shultz KL, Beamer WG 1993 Genetic susceptibility for C19 androgen induction of ovarian granulosa cell tumorigenesis in SWXJ strains of mice. Cancer Res 53:1059–1063 [PubMed] [Google Scholar]
- Beamer WG, Shultz KL, Tennent BJ, Nadeau JH, Churchill GA, Eicher EM 1998 Multigenic and imprinting control of ovarian granulosa cell tumorigenesis in mice. Cancer Res 58:3694–3699 [PubMed] [Google Scholar]
- Dorward AM, Shultz KL, Ackert-Bicknell CL, Eicher EM, Beamer WG 2003 High-resolution genetic map of X-linked juvenile-type granulosa cell tumor susceptibility genes in mouse. Cancer Res 63:8197–8202 [PubMed] [Google Scholar]
- Weiss WA, Burns MJ, Hackett C, Aldape K, Hill JR, Kuriyama H, Kuriyama N, Milshteyn N, Roberts T, Wendland MF, DePinho R, Israel MA 2003 Genetic determinants of malignancy in a mouse model for oligodendroglioma. Cancer Res 63:1589–1595 [PubMed] [Google Scholar]
- Montagna C, Andrechek ER, Padilla-Nash H, Muller WJ, Ried T 2002 Centrosome abnormalities, recurring deletions of chromosome 4, and genomic amplification of HER2/neu define mouse mammary gland adenocarcinomas induced by mutant HER2/neu. Oncogene 21:890–898 [DOI] [PubMed] [Google Scholar]
- Benn DE, Dwight T, Richardson AL, Delbridge L, Bambach CP, Stowasser M, Gordon RD, Marsh DJ, Robinson BG 2000 Sporadic and familial pheochromocytomas are associated with loss of at least two discrete intervals on chromosome 1p. Cancer Res 60:7048–7051 [PubMed] [Google Scholar]
- Murakami M, Hashimoto N, Takahashi Y, Hosokawa Y, Inazawa J, Mineura K 2003 A consistent region of deletion on 1p36 in meningiomas: identification and relation to malignant progression. Cancer Genet Cytogenet 140:99–106 [DOI] [PubMed] [Google Scholar]
- Rajgopal A, Carr IM, Leek JP, Hodge D, Bell SM, Roberts P, Horgan K, Bonthron DT, Selby PJ, Markham AF, MacLennan KA 2003 Detection by fluorescence in situ hybridization of microdeletions at 1p36 in lymphomas, unidentified on cytogenetic analysis. Cancer Genet Cytogenet 142:46–50 [DOI] [PubMed] [Google Scholar]
- Matzuk MM, Finegold MJ, Su JG, Hsueh AJ, Bradley A 1992 α-Inhibin is a tumour-suppressor gene with gonadal specificity in mice. Nature 360:313–319 [DOI] [PubMed] [Google Scholar]
- Matzuk MM, Kumar TR, Shou W, Coerver KA, Lau AL, Behringer RR, Finegold MJ 1996 Transgenic models to study the roles of inhibins and activins in reproduction, oncogenesis, and development. Recent Prog Horm Res 51:123–154; discussion 155–157 [PubMed] [Google Scholar]
- Matzuk MM, Finegold MJ, Mather JP, Krummen L, Lu H, Bradley A 1994 Development of cancer cachexia-like syndrome and adrenal tumors in inhibin-deficient mice. Proc Natl Acad Sci USA 91:8817–8821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar TR, Wang Y, Matzuk MM 1996 Gonadotropins are essential modifier factors for gonadal tumor development in inhibin-deficient mice. Endocrinology 137:4210–4216 [DOI] [PubMed] [Google Scholar]
- Beamer WG, Shultz KL, Tennent BJ, Shultz LD 1993 Granulosa cell tumorigenesis in genetically hypogonadal-immunodeficient mice grafted with ovaries from tumor-susceptible donors. Cancer Res 53:3741–3746 [PubMed] [Google Scholar]
- Dorward AM, Shultz KL, Beamer WG 2007 LH analog and dietary isoflavones support ovarian granulosa cell tumor development in a spontaneous mouse model. Endocr Relat Cancer 14:369–379 [DOI] [PubMed] [Google Scholar]
- Choi JH, Wong AS, Huang HF, Leung PC 2007 Gonadotropins and ovarian cancer. Endocr Rev 28:440–461 [DOI] [PubMed] [Google Scholar]
- Kumar TR, Palapattu G, Wang P, Woodruff TK, Boime I, Byrne MC, Matzuk MM 1999 Transgenic models to study gonadotropin function: the role of follicle-stimulating hormone in gonadal growth and tumorigenesis. Mol Endocrinol 13:851–865 [DOI] [PubMed] [Google Scholar]
- Risma KA, Clay CM, Nett TM, Wagner T, Yun J, Nilson JH 1995 Targeted overexpression of luteinizing hormone in transgenic mice leads to infertility, polycystic ovaries, and ovarian tumors. Proc Natl Acad Sci USA 92:1322–1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keri RA, Lozada KL, Abdul-Karim FW, Nadeau JH, Nilson JH 2000 Luteinizing hormone induction of ovarian tumors: oligogenic differences between mouse strains dictates tumor disposition. Proc Natl Acad Sci USA 97:383–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kananen K, Markkula M, Rainio E, Su JG, Hsueh AJ, Huhtaniemi IT 1995 Gonadal tumorigenesis in transgenic mice bearing the mouse inhibin α-subunit promoter/simian virus T-antigen fusion gene: characterization of ovarian tumors and establishment of gonadotropin-responsive granulosa cell lines. Mol Endocrinol 9:616–627 [DOI] [PubMed] [Google Scholar]
- Mikola M, Kero J, Nilson JH, Keri RA, Poutanen M, Huhtaniemi I 2003 High levels of luteinizing hormone analog stimulate gonadal and adrenal tumorigenesis in mice transgenic for the mouse inhibin-α-subunit promoter/Simian virus 40 T-antigen fusion gene. Oncogene 22:3269–3278 [DOI] [PubMed] [Google Scholar]
- Matzuk MM, DeMayo FJ, Hadsell LA, Kumar TR 2003 Overexpression of human chorionic gonadotropin causes multiple reproductive defects in transgenic mice. Biol Reprod 69:338–346 [DOI] [PubMed] [Google Scholar]
- Nagaraja AK, Agno JE, Kumar TR, Matzuk MM 2008 Luteinizing hormone promotes gonadal tumorigenesis in inhibin-deficient mice. Mol Cell Endocrinol 294:19–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcellana-Panlilio MY, Egeler RM, Ujack E, Magliocco A, Stuart GC, Robbins SM, Coppes MJ 2002 Evidence of a role for the INK4 family of cyclin-dependent kinase inhibitors in ovarian granulosa cell tumors. Genes Chromosomes Cancer 35:176–181 [DOI] [PubMed] [Google Scholar]
- Cipriano SC, Chen L, Burns KH, Koff A, Matzuk MM 2001 Inhibin and p27 interact to regulate gonadal tumorigenesis. Mol Endocrinol 15:985–996 [DOI] [PubMed] [Google Scholar]
- Burns KH, Agno JE, Chen L, Haupt B, Ogbonna SC, Korach KS, Matzuk MM 2003 Sexually dimorphic roles of steroid hormone receptor signaling in gonadal tumorigenesis. Mol Endocrinol 17:2039–2052 [DOI] [PubMed] [Google Scholar]
- Beamer WG, Shultz KL, Tennent BJ 1988 Induction of ovarian granulosa cell tumors in SWXJ-9 mice with dehydroepiandrosterone. Cancer Res 48:2788–2792 [PubMed] [Google Scholar]
- Chu S, Mamers P, Burger HG, Fuller PJ 2000 Estrogen receptor isoform gene expression in ovarian stromal and epithelial tumors. J Clin Endocrinol Metab 85:1200–1205 [DOI] [PubMed] [Google Scholar]
- Couse JF, Yates MM, Walker VR, Korach KS 2003 Characterization of the hypothalamic-pituitary-gonadal axis in estrogen receptor (ER) null mice reveals hypergonadism and endocrine sex reversal in females lacking ERα but not ERβ. Mol Endocrinol 17:1039–1053 [DOI] [PubMed] [Google Scholar]
- Burns KH, Agno JE, Sicinski P, Matzuk MM 2003 Cyclin D2 and p27 are tissue-specific regulators of tumorigenesis in inhibin α knockout mice. Mol Endocrinol 17:2053–2069 [DOI] [PubMed] [Google Scholar]
- Andreu-Vieyra C, Chen R, Matzuk MM 2007 Effects of granulosa cell-specific deletion of Rb in Inha-α null female mice. Endocrinology 148:3837–3849 [DOI] [PubMed] [Google Scholar]
- Burkhart DL, Sage J 2008 Cellular mechanisms of tumour suppression by the retinoblastoma gene. Nat Rev Cancer 8:671–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji P, Jiang H, Rekhtman K, Bloom J, Ichetovkin M, Pagano M, Zhu L 2004 An Rb-Skp2–p27 pathway mediates acute cell cycle inhibition by Rb and is retained in a partial-penetrance Rb mutant. Mol Cell 16:47–58 [DOI] [PubMed] [Google Scholar]
- Ji P, Zhu L 2005 Using kinetic studies to uncover new Rb functions in inhibiting cell cycle progression. Cell Cycle 4:373–375 [DOI] [PubMed] [Google Scholar]
- Coerver KA, Woodruff TK, Finegold MJ, Mather J, Bradley A, Matzuk MM 1996 Activin signaling through activin receptor type II causes the cachexia-like symptoms in inhibin-deficient mice. Mol Endocrinol 10:534–543 [DOI] [PubMed] [Google Scholar]
- Schwall RH, Robbins K, Jardieu P, Chang L, Lai C, Terrell TG 1993 Activin induces cell death in hepatocytes in vivo and in vitro. Hepatology 18:347–356 [DOI] [PubMed] [Google Scholar]
- Hully JR, Chang L, Schwall RH, Widmer HR, Terrell TG, Gillett NA 1994 Induction of apoptosis in the murine liver with recombinant human activin A. Hepatology 20:854–862 [DOI] [PubMed] [Google Scholar]
- Shikone T, Matzuk MM, Perlas E, Finegold MJ, Lewis KA, Vale W, Bradley A, Hsueh AJ 1994 Characterization of gonadal sex cord-stromal tumor cell lines from inhibin-α and p53-deficient mice: the role of activin as an autocrine growth factor. Mol Endocrinol 8:983–995 [DOI] [PubMed] [Google Scholar]
- Cipriano SC, Chen L, Kumar TR, Matzuk MM 2000 Follistatin is a modulator of gonadal tumor progression and the activin-induced wasting syndrome in inhibin-deficient mice. Endocrinology 141:2319–2327 [DOI] [PubMed] [Google Scholar]
- Li Q, Kumar R, Underwood K, O'Connor AE, Loveland KL, Seehra JS, Matzuk MM 2007 Prevention of cachexia-like syndrome development and reduction of tumor progression in inhibin-deficient mice following administration of a chimeric activin receptor type II-murine Fc protein. Mol Hum Reprod 13:675–683 [DOI] [PubMed] [Google Scholar]
- Li Q, Graff JM, O'Connor AE, Loveland KL, Matzuk MM 2007 SMAD3 regulates gonadal tumorigenesis. Mol Endocrinol 21:2472–2486 [DOI] [PubMed] [Google Scholar]
- Looyenga BD, Hammer GD 2007 Genetic removal of Smad3 from inhibin-null mice attenuates tumor progression by uncoupling extracellular mitogenic signals from the cell cycle machinery. Mol Endocrinol 21:2440–2457 [DOI] [PubMed] [Google Scholar]
- Tomic D, Miller KP, Kenny HA, Woodruff TK, Hoyer P, Flaws JA 2004 Ovarian follicle development requires Smad3. Mol Endocrinol 18:2224–2240 [DOI] [PubMed] [Google Scholar]
- Pangas SA, Li X, Umans L, Zwijsen A, Huylebroeck D, Gutierrez C, Wang D, Martin JF, Jamin SP, Behringer RR, Robertson EJ, Matzuk MM 2008 Conditional deletion of Smad1 and Smad5 in somatic cells of male and female gonads leads to metastatic tumor development in mice. Mol Cell Biol 28:248–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gocze PM, Beamer WG, de Jong FH, Freeman DA 1997 Hormone synthesis and responsiveness of spontaneous granulosa cell tumors in (SWR × SWXJ-9) F1 mice. Gynecol Oncol 65:143–148 [DOI] [PubMed] [Google Scholar]
- Lappöhn RE, Burger HG, Bouma J, Bangah M, Krans M, de Bruijn HW 1989 Inhibin as a marker for granulosa-cell tumors. N Engl J Med 321:790–793 [DOI] [PubMed] [Google Scholar]
- Ala-Fossi SL, Aine R, Punnonen R, Mäenpää J 2000 Is potential to produce inhibins related to prognosis in ovarian granulosa cell tumors? Eur J Gynaecol Oncol 21:187–189 [PubMed] [Google Scholar]
- Hsieh M, Johnson MA, Greenberg NM, Richards JS 2002 Regulated expression of Wnts and Frizzleds at specific stages of follicular development in the rodent ovary. Endocrinology 143:898–908 [DOI] [PubMed] [Google Scholar]
- Ricken A, Lochhead P, Kontogiannea M, Farookhi R 2002 Wnt signaling in the ovary: identification and compartmentalized expression of wnt-2, wnt-2b, and frizzled-4 mRNAs. Endocrinology 143:2741–2749 [DOI] [PubMed] [Google Scholar]
- Hsieh M, Mulders SM, Friis RR, Dharmarajan A, Richards JS 2003 Expression and localization of secreted frizzled-related protein-4 in the rodent ovary: evidence for selective up-regulation in luteinized granulosa cells. Endocrinology 144:4597–4606 [DOI] [PubMed] [Google Scholar]
- Boerboom D, Paquet M, Hsieh M, Liu J, Jamin SP, Behringer RR, Sirois J, Taketo MM, Richards JS 2005 Misregulated Wnt/β-catenin signaling leads to ovarian granulosa cell tumor development. Cancer Res 65:9206–9215 [DOI] [PubMed] [Google Scholar]
- Boerboom D, White LD, Dalle S, Courty J, Richards JS 2006 Dominant-stable β-catenin expression causes cell fate alterations and Wnt signaling antagonist expression in a murine granulosa cell tumor model. Cancer Res 66:1964–1973 [DOI] [PubMed] [Google Scholar]
- Laguë MN, Paquet M, Fan HY, Kaartinen MJ, Chu S, Jamin SP, Behringer RR, Fuller PJ, Mitchell A, Doré M, Huneault LM, Richards JS, Boerboom D 2008 Synergistic effects of Pten loss and WNT/CTNNB1 signaling pathway activation in ovarian granulosa cell tumor development and progression. Carcinogenesis 29:2062–2072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunzicker-Dunn M, Maizels ET 2006 FSH signaling pathways in immature granulosa cells that regulate target gene expression: branching out from protein kinase A. Cell Signal 18:1351–1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah SP, Köbel M, Senz J, Morin RD, Clarke BA, Wiegand KC, Leung G, Zayed A, Mehl E, Kalloger SE, Sun M, Giuliany R, Yorida E, Jones S, Varhol R, Swenerton KD, Miller D, Clement PB, Crane C, Madore J, Provencher D, Leung P, DeFazio A, Khattra J, Turashvili G, Zhao Y, Zeng T, Glover JN, Vanderhyden B, Zhao C, Parkinson CA, Jimenez-Linan M, Bowtell DD, Mes-Masson AM, Brenton JD, Aparicio SA, Boyd N, Hirst M, Gilks CB, Marra M, Huntsman DG 2009 Mutation of FOXL2 in granulosa-cell tumors of the ovary. N Engl J Med 360:2719–2729 [DOI] [PubMed] [Google Scholar]
- Pectasides D, Pectasides E, Kassanos D 2008 Germ cell tumors of the ovary. Cancer Treat Rev 34:427–441 [DOI] [PubMed] [Google Scholar]
- Smith HO, Berwick M, Verschraegen CF, Wiggins C, Lansing L, Muller CY, Qualls CR 2006 Incidence and survival rates for female malignant germ cell tumors. Obstet Gynecol 107:1075–1085 [DOI] [PubMed] [Google Scholar]
- Surti U, Hoffner L, Chakravarti A, Ferrell RE 1990 Genetics and biology of human ovarian teratomas. I. Cytogenetic analysis and mechanism of origin. Am J Hum Genet 47:635–643 [PMC free article] [PubMed] [Google Scholar]
- Hoffner L, Shen-Schwarz S, Deka R, Chakravarti A, Surti U 1992 Genetics and biology of human ovarian teratomas. III. Cytogenetics and origins of malignant ovarian germ cell tumors. Cancer Genet Cytogenet 62:58–65 [DOI] [PubMed] [Google Scholar]
- Stevens LC, Varnum DS 1974 The development of teratomas from parthenogenetically activated ovarian mouse eggs. Dev Biol 37:369–380 [DOI] [PubMed] [Google Scholar]
- Eppig JJ, Wigglesworth K, Varnum DS, Nadeau JH 1996 Genetic regulation of traits essential for spontaneous ovarian teratocarcinogenesis in strain LT/Sv mice: aberrant meiotic cell cycle, oocyte activation, and parthenogenetic development. Cancer Res 56:5047–5054 [PubMed] [Google Scholar]
- Hirao Y, Eppig JJ 1997 Parthenogenetic development of Mos-deficient mouse oocytes. Mol Reprod Dev 48:391–396 [DOI] [PubMed] [Google Scholar]
- Lee GH, Bugni JM, Obata M, Nishimori H, Ogawa K, Drinkwater NR 1997 Genetic dissection of susceptibility to murine ovarian teratomas that originate from parthenogenetic oocytes. Cancer Res 57:590–593 [PubMed] [Google Scholar]
- Eppig JJ, Kozak LP, Eicher EM, Stevens LC 1977 Ovarian teratomas in mice are derived from oocytes that have completed the first meiotic division. Nature 269:517–518 [DOI] [PubMed] [Google Scholar]
- Eppig JJ 1978 Developmental potential of LT/Sv parthenotes derived from oocytes matured in vivo and in vitro. Dev Biol 65:244–249 [DOI] [PubMed] [Google Scholar]
- Hupalowska A, Kalaszczynska I, Hoffmann S, Tsurumi C, Kubiak JZ, Polanski Z, Ciemerych MA 2008 Metaphase I arrest in LT/Sv mouse oocytes involves the spindle assembly checkpoint. Biol Reprod 79:1102–1110 [DOI] [PubMed] [Google Scholar]
- Hampl A, Eppig JJ 1995 Analysis of the mechanism(s) of metaphase I arrest in maturing mouse oocytes. Development 121:925–933 [DOI] [PubMed] [Google Scholar]
- Hirao Y, Eppig JJ 1997 Analysis of the mechanism(s) of metaphase I arrest in strain LT mouse oocytes: participation of MOS. Development 124:5107–5113 [DOI] [PubMed] [Google Scholar]
- Ciemerych MA, Kubiak JZ 1998 Cytostatic activity develops during meiosis I in oocytes of LT/Sv mice. Dev Biol 200:198–211 [DOI] [PubMed] [Google Scholar]
- Hirao Y, Eppig JJ 1999 Analysis of the mechanism(s) of metaphase I-arrest in strain LT mouse oocytes: delay in the acquisition of competence to undergo the metaphase I/anaphase transition. Mol Reprod Dev 54:311–318 [DOI] [PubMed] [Google Scholar]
- Vande Woude GF 1994 Embryology. On the loss of Mos. Nature 370:20–21 [DOI] [PubMed] [Google Scholar]
- Furuta Y, Shigetani Y, Takeda N, Iwasaki K, Ikawa Y, Aizawa S 1995 Ovarian teratomas in mice lacking the protooncogene c-mos. Jpn J Cancer Res 86:540–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eppig JJ, Wigglesworth K, Hirao Y 2000 Metaphase I arrest and spontaneous parthenogenetic activation of strain LTXBO oocytes: chimeric reaggregated ovaries establish primary lesion in oocytes. Dev Biol 224:60–68 [DOI] [PubMed] [Google Scholar]
- Eppig JJ 1978 Granulosa cell deficient follicles: occurrence, structure, and relationship to ovarian teratocarcinogenesis in strain LT/Sv mice. Differentiation 12:111–120 [PubMed] [Google Scholar]
- Hsu SY, Lai RJ, Finegold M, Hsueh AJ 1996 Targeted overexpression of Bcl-2 in ovaries of transgenic mice leads to decreased follicle apoptosis, enhanced folliculogenesis, and increased germ cell tumorigenesis. Endocrinology 137:4837–4843 [DOI] [PubMed] [Google Scholar]
- Morita Y, Perez GI, Maravei DV, Tilly KI, Tilly JL 1999 Targeted expression of Bcl-2 in mouse oocytes inhibits ovarian follicle atresia and prevents spontaneous and chemotherapy-induced oocyte apoptosis in vitro. Mol Endocrinol 13:841–850 [DOI] [PubMed] [Google Scholar]
- Wu L, Belasco JG 2008 Let me count the ways: mechanisms of gene regulation by miRNAs and siRNAs. Mol Cell 29:1–7 [DOI] [PubMed] [Google Scholar]
- Vasudevan S, Tong Y, Steitz JA 2007 Switching from repression to activation: microRNAs can up-regulate translation. Science 318:1931–1934 [DOI] [PubMed] [Google Scholar]
- Friedman RC, Farh KK, Burge CB, Bartel DP 2009 Most mammalian mRNAs are conserved targets of microRNAs. Genome Res 19:92–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aravin A, Gaidatzis D, Pfeffer S, Lagos-Quintana M, Landgraf P, Iovino N, Morris P, Brownstein MJ, Kuramochi-Miyagawa S, Nakano T, Chien M, Russo JJ, Ju J, Sheridan R, Sander C, Zavolan M, Tuschl T 2006 A novel class of small RNAs bind to MILI protein in mouse testes. Nature 442:203–207 [DOI] [PubMed] [Google Scholar]
- Girard A, Sachidanandam R, Hannon GJ, Carmell MA 2006 A germline-specific class of small RNAs binds mammalian Piwi proteins. Nature 442:199–202 [DOI] [PubMed] [Google Scholar]
- Grivna ST, Beyret E, Wang Z, Lin H 2006 A novel class of small RNAs in mouse spermatogenic cells. Genes Dev 20:1709–1714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau NC, Seto AG, Kim J, Kuramochi-Miyagawa S, Nakano T, Bartel DP, Kingston RE 2006 Characterization of the piRNA complex from rat testes. Science 313:363–367 [DOI] [PubMed] [Google Scholar]
- Watanabe T, Takeda A, Tsukiyama T, Mise K, Okuno T, Sasaki H, Minami N, Imai H 2006 Identification and characterization of two novel classes of small RNAs in the mouse germline: retrotransposon-derived siRNAs in oocytes and germline small RNAs in testes. Genes Dev 20:1732–1743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H 2007 piRNAs in the germ line. Science 316:397 [DOI] [PubMed] [Google Scholar]
- O'Donnell KA, Boeke JD 2007 Mighty Piwis defend the germline against genome intruders. Cell 129:37–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefani G, Slack FJ 2008 Small non-coding RNAs in animal development. Nat Rev Mol Cell Biol 9:219–230 [DOI] [PubMed] [Google Scholar]
- Hutvagner G, Simard MJ 2008 Argonaute proteins: key players in RNA silencing. Nat Rev Mol Cell Biol 9:22–32 [DOI] [PubMed] [Google Scholar]
- Deng W, Lin H 2002 miwi, a murine homolog of piwi, encodes a cytoplasmic protein essential for spermatogenesis. Dev Cell 2:819–830 [DOI] [PubMed] [Google Scholar]
- Kuramochi-Miyagawa S, Kimura T, Ijiri TW, Isobe T, Asada N, Fujita Y, Ikawa M, Iwai N, Okabe M, Deng W, Lin H, Matsuda Y, Nakano T 2004 Mili, a mammalian member of piwi family gene, is essential for spermatogenesis. Development 131:839–849 [DOI] [PubMed] [Google Scholar]
- Carmell MA, Girard A, van de Kant HJ, Bourc'his D, Bestor TH, de Rooij DG, Hannon GJ 2007 MIWI2 is essential for spermatogenesis and repression of transposons in the mouse male germline. Dev Cell 12:503–514 [DOI] [PubMed] [Google Scholar]
- Aravin AA, Sachidanandam R, Girard A, Fejes-Toth K, Hannon GJ 2007 Developmentally regulated piRNA clusters implicate MILI in transposon control. Science 316:744–747 [DOI] [PubMed] [Google Scholar]
- Ro S, Song R, Park C, Zheng H, Sanders KM, Yan W 2007 Cloning and expression profiling of small RNAs expressed in the mouse ovary. RNA 13:2366–2380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Totoki Y, Toyoda A, Kaneda M, Kuramochi-Miyagawa S, Obata Y, Chiba H, Kohara Y, Kono T, Nakano T, Surani MA, Sakaki Y, Sasaki H 2008 Endogenous siRNAs from naturally formed dsRNAs regulate transcripts in mouse oocytes. Nature 453:539–543 [DOI] [PubMed] [Google Scholar]
- Tam OH, Aravin AA, Stein P, Girard A, Murchison EP, Cheloufi S, Hodges E, Anger M, Sachidanandam R, Schultz RM, Hannon GJ 2008 Pseudogene-derived small interfering RNAs regulate gene expression in mouse oocytes. Nature 453:534–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murchison EP, Stein P, Xuan Z, Pan H, Zhang MQ, Schultz RM, Hannon GJ 2007 Critical roles for Dicer in the female germline. Genes Dev 21:682–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang F, Kaneda M, O'Carroll D, Hajkova P, Barton SC, Sun YA, Lee C, Tarakhovsky A, Lao K, Surani MA 2007 Maternal microRNAs are essential for mouse zygotic development. Genes Dev 21:644–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet S, Maro B 2005 Cytoskeleton and cell cycle control during meiotic maturation of the mouse oocyte: integrating time and space. Reproduction 130:801–811 [DOI] [PubMed] [Google Scholar]
- Nagaraja AK, Andreu-Vieyra C, Franco HL, Ma L, Chen R, Han DY, Zhu H, Agno JE, Gunaratne PH, DeMayo FJ, Matzuk MM 2008 Deletion of Dicer in somatic cells of the female reproductive tract causes sterility. Mol Endocrinol 22:2336–2352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong X, Luense LJ, McGinnis LK, Nothnick WB, Christenson LK 2008 Dicer1 is essential for female fertility and normal development of the female reproductive system. Endocrinology 149:6207–6212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez G, Behringer RR 2009 Dicer is required for female reproductive tract development and fertility in the mouse. Mol Reprod Dev 76:678–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastorelli LM, Wells S, Fray M, Smith A, Hough T, Harfe BD, McManus MT, Smith L, Woolf AS, Cheeseman M, Greenfield A 2009 Genetic analyses reveal a requirement for Dicer1 in the mouse urogenital tract. Mamm Genome 20:140–151 [DOI] [PubMed] [Google Scholar]
- Fiedler SD, Carletti MZ, Hong X, Christenson LK 2008 Hormonal regulation of microRNA expression in periovulatory mouse mural granulosa cells. Biol Reprod 79:1030–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka M, Zheng M, Hayashi M, Lee JD, Yoshino O, Lin S, Han J 2008 Impaired microRNA processing causes corpus luteum insufficiency and infertility in mice. J Clin Invest 118:1944–1954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA, Downing JR, Jacks T, Horvitz HR, Golub TR 2005 MicroRNA expression profiles classify human cancers. Nature 435:834–838 [DOI] [PubMed] [Google Scholar]
- Kumar MS, Lu J, Mercer KL, Golub TR, Jacks T 2007 Impaired microRNA processing enhances cellular transformation and tumorigenesis. Nat Genet 39:673–677 [DOI] [PubMed] [Google Scholar]
- Calin GA, Sevignani C, Dumitru CD, Hyslop T, Noch E, Yendamuri S, Shimizu M, Rattan S, Bullrich F, Negrini M, Croce CM 2004 Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc Natl Acad Sci USA 101:2999–3004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Huang J, Yang N, Greshock J, Megraw MS, Giannakakis A, Liang S, Naylor TL, Barchetti A, Ward MR, Yao G, Medina A, O'brien-Jenkins A, Katsaros D, Hatzigeorgiou A, Gimotty PA, Weber BL, Coukos G 2006 microRNAs exhibit high frequency genomic alterations in human cancer. Proc Natl Acad Sci USA 103:9136–9141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Volinia S, Bonome T, Calin GA, Greshock J, Yang N, Liu CG, Giannakakis A, Alexiou P, Hasegawa K, Johnstone CN, Megraw MS, Adams S, Lassus H, Huang J, Kaur S, Liang S, Sethupathy P, Leminen A, Simossis VA, Sandaltzopoulos R, Naomoto Y, Katsaros D, Gimotty PA, DeMichele A, Huang Q, Bützow R, Rustgi AK, Weber BL, Birrer MJ, Hatzigeorgiou AG, Croce CM, Coukos G 2008 Genomic and epigenetic alterations deregulate microRNA expression in human epithelial ovarian cancer. Proc Natl Acad Sci USA 105:7004–7009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang N, Coukos G, Zhang L 2008 MicroRNA epigenetic alterations in human cancer: one step forward in diagnosis and treatment. Int J Cancer 122:963–968 [DOI] [PubMed] [Google Scholar]
- Chin LJ, Ratner E, Leng S, Zhai R, Nallur S, Babar I, Muller RU, Straka E, Su L, Burki EA, Crowell RE, Patel R, Kulkarni T, Homer R, Zelterman D, Kidd KK, Zhu Y, Christiani DC, Belinsky SA, Slack FJ, Weidhaas JB 2008 A SNP in a let-7 microRNA complementary site in the KRAS 3′ untranslated region increases non-small cell lung cancer risk. Cancer Res 68:8535–8540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng S, Calin GA, Croce CM, Coukos G, Zhang L 2008 Mechanisms of microRNA deregulation in human cancer. Cell Cycle 7:2643–2646 [DOI] [PubMed] [Google Scholar]
- Iorio MV, Visone R, Di Leva G, Donati V, Petrocca F, Casalini P, Taccioli C, Volinia S, Liu CG, Alder H, Calin GA, Ménard S, Croce CM 2007 MicroRNA signatures in human ovarian cancer. Cancer Res 67:8699–8707 [DOI] [PubMed] [Google Scholar]
- Nam EJ, Yoon H, Kim SW, Kim H, Kim YT, Kim JH, Kim JW, Kim S 2008 MicroRNA expression profiles in serous ovarian carcinoma. Clin Cancer Res 14:2690–2695 [DOI] [PubMed] [Google Scholar]
- Yang H, Kong W, He L, Zhao JJ, O'Donnell JD, Wang J, Wenham RM, Coppola D, Kruk PA, Nicosia SV, Cheng JQ 2008 MicroRNA expression profiling in human ovarian cancer: miR-214 induces cell survival and cisplatin resistance by targeting PTEN. Cancer Res 68:425–433 [DOI] [PubMed] [Google Scholar]
- Alfonso-De Matte MY, Yang H, Evans MS, Cheng JQ, Kruk PA 2002 Telomerase is regulated by c-Jun NH2-terminal kinase in ovarian surface epithelial cells. Cancer Res 62:4575–4578 [PubMed] [Google Scholar]
- Dahiya N, Sherman-Baust CA, Wang TL, Davidson B, Shih IeM, Zhang Y, Wood 3rd W, Becker KG, Morin PJ 2008 MicroRNA expression and identification of putative miRNA targets in ovarian cancer. PLoS ONE 3:e2436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorn KK, Jazaeri AA, Awtrey CS, Gardner GJ, Mok SC, Boyd J, Birrer MJ 2003 Choice of normal ovarian control influences determination of differentially expressed genes in ovarian cancer expression profiling studies. Clin Cancer Res 9:4811–4818 [PubMed] [Google Scholar]
- Corney DC, Flesken-Nikitin A, Godwin AK, Wang W, Nikitin AY 2007 MicroRNA-34b and MicroRNA-34c are targets of p53 and cooperate in control of cell proliferation and adhesion-independent growth. Cancer Res 67:8433–8438 [DOI] [PubMed] [Google Scholar]
- Bommer GT, Gerin I, Feng Y, Kaczorowski AJ, Kuick R, Love RE, Zhai Y, Giordano TJ, Qin ZS, Moore BB, MacDougald OA, Cho KR, Fearon ER 2007 p53-mediated activation of miRNA34 candidate tumor-suppressor genes. Curr Biol 17:1298–1307 [DOI] [PubMed] [Google Scholar]
- Chang TC, Wentzel EA, Kent OA, Ramachandran K, Mullendore M, Lee KH, Feldmann G, Yamakuchi M, Ferlito M, Lowenstein CJ, Arking DE, Beer MA, Maitra A, Mendell JT 2007 Transactivation of miR-34a by p53 broadly influences gene expression and promotes apoptosis. Mol Cell 26:745–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, He X, Lim LP, de Stanchina E, Xuan Z, Liang Y, Xue W, Zender L, Magnus J, Ridzon D, Jackson AL, Linsley PS, Chen C, Lowe SW, Cleary MA, Hannon GJ 2007 A microRNA component of the p53 tumour suppressor network. Nature 447:1130–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raver-Shapira N, Marciano E, Meiri E, Spector Y, Rosenfeld N, Moskovits N, Bentwich Z, Oren M 2007 Transcriptional activation of miR-34a contributes to p53-mediated apoptosis. Mol Cell 26:731–743 [DOI] [PubMed] [Google Scholar]
- Tarasov V, Jung P, Verdoodt B, Lodygin D, Epanchintsev A, Menssen A, Meister G, Hermeking H 2007 Differential regulation of microRNAs by p53 revealed by massively parallel sequencing: miR-34a is a p53 target that induces apoptosis and G1-arrest. Cell Cycle 6:1586–1593 [DOI] [PubMed] [Google Scholar]
- He X, He L, Hannon GJ 2007 The guardian’s little helper: microRNAs in the p53 tumor suppressor network. Cancer Res 67:11099–11101 [DOI] [PubMed] [Google Scholar]
- Welch C, Chen Y, Stallings RL 2007 MicroRNA-34a functions as a potential tumor suppressor by inducing apoptosis in neuroblastoma cells. Oncogene 26:5017–5022 [DOI] [PubMed] [Google Scholar]
- Park SM, Gaur AB, Lengyel E, Peter ME 2008 The miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes Dev 22:894–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano A, Nieto MA 2008 Non-coding RNAs take centre stage in epithelial-to-mesenchymal transition. Trends Cell Biol 18:357–359 [DOI] [PubMed] [Google Scholar]
- Elloul S, Elstrand MB, Nesland JM, Tropé CG, Kvalheim G, Goldberg I, Reich R, Davidson B 2005 Snail, Slug, and Smad-interacting protein 1 as novel parameters of disease aggressiveness in metastatic ovarian and breast carcinoma. Cancer 103:1631–1643 [DOI] [PubMed] [Google Scholar]
- Gregory PA, Bert AG, Paterson EL, Barry SC, Tsykin A, Farshid G, Vadas MA, Khew-Goodall Y, Goodall GJ 2008 The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol 10:593–601 [DOI] [PubMed] [Google Scholar]
- Korpal M, Lee ES, Hu G, Kang Y 2008 The miR-200 family inhibits epithelial-mesenchymal transition and cancer cell migration by direct targeting of E-cadherin transcriptional repressors ZEB1 and ZEB2. J Biol Chem 283:14910–14914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obata K, Morland SJ, Watson RH, Hitchcock A, Chenevix-Trench G, Thomas EJ, Campbell IG 1998 Frequent PTEN/MMAC mutations in endometrioid but not serous or mucinous epithelial ovarian tumors. Cancer Res 58:2095–2097 [PubMed] [Google Scholar]
- Kurose K, Zhou XP, Araki T, Cannistra SA, Maher ER, Eng C 2001 Frequent loss of PTEN expression is linked to elevated phosphorylated Akt levels, but not associated with p27 and cyclin D1 expression, in primary epithelial ovarian carcinomas. Am J Pathol 158:2097–2106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang N, Kaur S, Volinia S, Greshock J, Lassus H, Hasegawa K, Liang S, Leminen A, Deng S, Smith L, Johnstone CN, Chen XM, Liu CG, Huang Q, Katsaros D, Calin GA, Weber BL, Bützow R, Croce CM, Coukos G, Zhang L 2008 MicroRNA microarray identifies Let-7i as a novel biomarker and therapeutic target in human epithelial ovarian cancer. Cancer Res 68:10307–10314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Alvero AB, Silasi DA, Kelly MG, Fest S, Visintin I, Leiser A, Schwartz PE, Rutherford T, Mor G 2008 Regulation of IKKβ by miR-199a affects NF-κB activity in ovarian cancer cells. Oncogene 27:4712–4723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrentino A, Liu CG, Addario A, Peschle C, Scambia G, Ferlini C 2008 Role of microRNAs in drug-resistant ovarian cancer cells. Gynecol Oncol 111:478–486 [DOI] [PubMed] [Google Scholar]
- Chambers SK, Kacinski BM, Ivins CM, Carcangiu ML 1997 Overexpression of epithelial macrophage colony-stimulating factor (CSF-1) and CSF-1 receptor: a poor prognostic factor in epithelial ovarian cancer, contrasted with a protective effect of stromal CSF-1. Clin Cancer Res 3:999–1007 [PubMed] [Google Scholar]
- Toy EP, Chambers JT, Kacinski BM, Flick MB, Chambers SK 2001 The activated macrophage colony-stimulating factor (CSF-1) receptor as a predictor of poor outcome in advanced epithelial ovarian carcinoma. Gynecol Oncol 80:194–200 [DOI] [PubMed] [Google Scholar]
- Kacinski BM 1997 CSF-1 and its receptor in breast carcinomas and neoplasms of the female reproductive tract. Mol Reprod Dev 46:71–74 [DOI] [PubMed] [Google Scholar]
- Paulus P, Stanley ER, Schäfer R, Abraham D, Aharinejad S 2006 Colony-stimulating factor-1 antibody reverses chemoresistance in human MCF-7 breast cancer xenografts. Cancer Res 66:4349–4356 [DOI] [PubMed] [Google Scholar]
- Chambers SK, Wang Y, Gertz RE, Kacinski BM 1995 Macrophage colony-stimulating factor mediates invasion of ovarian cancer cells through urokinase. Cancer Res 55:1578–1585 [PubMed] [Google Scholar]
- Toy EP, Azodi M, Folk NL, Zito CM, Zeiss CJ, Chambers SK 2009 Enhanced ovarian cancer tumorigenesis and metastasis by the macrophage colony-stimulating factor. Neoplasia 11:136–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonafé N, Gilmore-Hebert M, Folk NL, Azodi M, Zhou Y, Chambers SK 2005 Glyceraldehyde-3-phosphate dehydrogenase binds to the AU-rich 3′ untranslated region of colony-stimulating factor-1 (CSF-1) messenger RNA in human ovarian cancer cells: possible role in CSF-1 posttranscriptional regulation and tumor phenotype. Cancer Res 65:3762–3771 [DOI] [PubMed] [Google Scholar]
- Zhou Y, Yi X, Stoffer JB, Bonafe N, Gilmore-Hebert M, McAlpine J, Chambers SK 2008 The multifunctional protein glyceraldehyde-3-phosphate dehydrogenase is both regulated and controls colony-stimulating factor-1 messenger RNA stability in ovarian cancer. Mol Cancer Res 6:1375–1384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SM, Grosshans H, Shingara J, Byrom M, Jarvis R, Cheng A, Labourier E, Reinert KL, Brown D, Slack FJ 2005 RAS is regulated by the let-7 microRNA family. Cell 120:635–647 [DOI] [PubMed] [Google Scholar]
- Sampson VB, Rong NH, Han J, Yang Q, Aris V, Soteropoulos P, Petrelli NJ, Dunn SP, Krueger LJ 2007 MicroRNA let-7a down-regulates MYC and reverts MYC-induced growth in Burkitt lymphoma cells. Cancer Res 67:9762–9770 [DOI] [PubMed] [Google Scholar]
- Mayr C, Hemann MT, Bartel DP 2007 Disrupting the pairing between let-7 and Hmga2 enhances oncogenic transformation. Science 315:1576–1579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YS, Dutta A 2007 The tumor suppressor microRNA let-7 represses the HMGA2 oncogene. Genes Dev 21:1025–1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shell S, Park SM, Radjabi AR, Schickel R, Kistner EO, Jewell DA, Feig C, Lengyel E, Peter ME 2007 Let-7 expression defines two differentiation stages of cancer. Proc Natl Acad Sci USA 104:11400–11405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SM, Shell S, Radjabi AR, Schickel R, Feig C, Boyerinas B, Dinulescu DM, Lengyel E, Peter ME 2007 Let-7 prevents early cancer progression by suppressing expression of the embryonic gene HMGA2. Cell Cycle 6:2585–2590 [DOI] [PubMed] [Google Scholar]
- Malek A, Bakhidze E, Noske A, Sers C, Aigner A, Schäfer R, Tchernitsa O 2008 HMGA2 gene is a promising target for ovarian cancer silencing therapy. Int J Cancer 123:348–356 [DOI] [PubMed] [Google Scholar]
- Yu F, Yao H, Zhu P, Zhang X, Pan Q, Gong C, Huang Y, Hu X, Su F, Lieberman J, Song E 2007 Let-7 regulates self renewal and tumorigenicity of breast cancer cells. Cell 131:1109–1123 [DOI] [PubMed] [Google Scholar]
- Lu L, Katsaros D, Shaverdashvili K, Qian B, Wu Y, de la Longrais IA, Preti M, Menato G, Yu H 2009 Pluripotent factor lin-28 and its homologue lin-28b in epithelial ovarian cancer and their associations with disease outcomes and expression of let-7a and IGF-II. Eur J Cancer 45:2212–2218 [DOI] [PubMed] [Google Scholar]
- Merritt WM, Lin YG, Han LY, Kamat AA, Spannuth WA, Schmandt R, Urbauer D, Pennacchio LA, Cheng JF, Nick AM, Deavers MT, Mourad-Zeidan A, Wang H, Mueller P, Lenburg ME, Gray JW, Mok S, Birrer MJ, Lopez-Berestein G, Coleman RL, Bar-Eli M, Sood AK 2008 Dicer, Drosha, and outcomes in patients with ovarian cancer. N Engl J Med 359:2641–2650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavin RJ, Smyth PC, Finn SP, Laios A, O'Toole SA, Barrett C, Ring M, Denning KM, Li J, Aherne ST, Aziz NA, Alhadi A, Sheppard BL, Loda M, Martin C, Sheils OM, O'Leary JJ 2008 Altered eIF6 and Dicer expression is associated with clinicopathological features in ovarian serous carcinoma patients. Mod Pathol 21:676–684 [DOI] [PubMed] [Google Scholar]
- Chendrimada TP, Finn KJ, Ji X, Baillat D, Gregory RI, Liebhaber SA, Pasquinelli AE, Shiekhattar R 2007 MicroRNA silencing through RISC recruitment of eIF6. Nature 447:823–828 [DOI] [PubMed] [Google Scholar]
- Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, Peterson A, Noteboom J, O'Briant KC, Allen A, Lin DW, Urban N, Drescher CW, Knudsen BS, Stirewalt DL, Gentleman R, Vessella RL, Nelson PS, Martin DB, Tewari M 2008 Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci USA 105:10513–10518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Ba Y, Ma L, Cai X, Yin Y, Wang K, Guo J, Zhang Y, Chen J, Guo X, Li Q, Li X, Wang W, Zhang Y, Wang J, Jiang X, Xiang Y, Xu C, Zheng P, Zhang J, Li R, Zhang H, Shang X, Gong T, Ning G, Wang J, Zen K, Zhang J, Zhang CY 2008 Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res 18:997–1006 [DOI] [PubMed] [Google Scholar]
- Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO 2007 Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 9:654–659 [DOI] [PubMed] [Google Scholar]
- Taylor DD, Gercel-Taylor C 2008 MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol Oncol 110:13–21 [DOI] [PubMed] [Google Scholar]
- Resnick KE, Alder H, Hagan JP, Richardson DL, Croce CM, Cohn DE 2009 The detection of differentially expressed microRNAs from the serum of ovarian cancer patients using a novel real-time PCR platform. Gynecol Oncol 112:55–59 [DOI] [PubMed] [Google Scholar]
- Matzuk MM, Lamb DJ 2002 Genetic dissection of mammalian fertility pathways. Nat Cell Biol 4 Suppl:S41–S49 [DOI] [PubMed] [Google Scholar]
- Matzuk MM, Lamb DJ 2008 The biology of infertility: research advances and clinical challenges. Nat Med 14:1197–1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boivin J, Bunting L, Collins JA, Nygren KG 2007 International estimates of infertility prevalence and treatment-seeking: potential need and demand for infertility medical care. Hum Reprod 22:1506–1512 [DOI] [PubMed] [Google Scholar]
- Lunenfeld B 2004 Historical perspectives in gonadotrophin therapy. Hum Reprod Update 10:453–467 [DOI] [PubMed] [Google Scholar]
- Macklon NS, Stouffer RL, Giudice LC, Fauser BC 2006 The science behind 25 years of ovarian stimulation for in vitro fertilization. Endocr Rev 27:170–207 [DOI] [PubMed] [Google Scholar]
- Steptoe PC, Edwards RG 1978 Birth after the reimplantation of a human embryo. Lancet 2:366 [DOI] [PubMed] [Google Scholar]
- Trounson AO, Leeton JF, Wood C, Webb J, Wood J 1981 Pregnancies in humans by fertilization in vitro and embryo transfer in the controlled ovulatory cycle. Science 212:681–682 [DOI] [PubMed] [Google Scholar]
- Fares FA, Suganuma N, Nishimori K, LaPolt PS, Hsueh AJ, Boime I 1992 Design of a long-acting follitropin agonist by fusing the C-terminal sequence of the chorionic gonadotropin β subunit to the follitropin β subunit. Proc Natl Acad Sci USA 89:4304–4308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruman JI, Pollak S, Trousdale RK, Klein J, Lustbader JW 2005 Effects of long-acting recombinant human follicle-stimulating hormone analogs containing N-linked glycosylation on murine folliculogenesis. Fertil Steril 83(Suppl 1):1303–1309 [DOI] [PubMed] [Google Scholar]
- Sugahara T, Sato A, Kudo M, Ben-Menahem D, Pixley MR, Hsueh AJ, Boime I 1996 Expression of biologically active fusion genes encoding the common α subunit and the follicle-stimulating hormone β subunit. Role of a linker sequence. J Biol Chem 271:10445–10448 [DOI] [PubMed] [Google Scholar]
- Devroey P, Fauser BC, Platteau P, Beckers NG, Dhont M, Mannaerts BM 2004 Induction of multiple follicular development by a single dose of long-acting recombinant follicle-stimulating hormone (FSH-CTP, corifollitropin alfa) for controlled ovarian stimulation before in vitro fertilization. J Clin Endocrinol Metab 89:2062–2070 [DOI] [PubMed] [Google Scholar]
- Group TCAD-fS 2008 A randomized dose-response trial of a single injection of corifollitropin alfa to sustain multifollicular growth during controlled ovarian stimulation. Hum Reprod 23:2484–2492 [DOI] [PubMed] [Google Scholar]
- Fauser BC, Mannaerts BM, Devroey P, Leader A, Boime I, Baird DT 2009 Advances in recombinant DNA technology: corifollitropin alfa, a hybrid molecule with sustained follicle-stimulating activity and reduced injection frequency. Hum Reprod Update 15:309–321 [DOI] [PubMed] [Google Scholar]
- Arey BJ 2008 Allosteric modulators of glycoprotein hormone receptors: discovery and therapeutic potential. Endocrine 34:1–10 [DOI] [PubMed] [Google Scholar]
- Yanofsky SD, Shen ES, Holden F, Whitehorn E, Aguilar B, Tate E, Holmes CP, Scheuerman R, MacLean D, Wu MM, Frail DE, López FJ, Winneker R, Arey BJ, Barrett RW 2006 Allosteric activation of the follicle-stimulating hormone (FSH) receptor by selective, nonpeptide agonists. J Biol Chem 281:13226–13233 [DOI] [PubMed] [Google Scholar]
- Jorand-Lebrun C, Brondyk B, Lin J, Magar S, Murray R, Reddy A, Shroff H, Wands G, Weiser W, Xu Q, McKenna S, Brugger N 2007 Identification, synthesis, and biological evaluation of novel pyrazoles as low molecular weight luteinizing hormone receptor agonists. Bioorg Med Chem Lett 17:2080–2085 [DOI] [PubMed] [Google Scholar]
- van de Lagemaat R, Timmers CM, Kelder J, van Koppen C, Mosselman S, Hanssen RG 2009 Induction of ovulation by a potent, orally active, low molecular weight agonist (Org 43553) of the luteinizing hormone receptor. Hum Reprod 24:640–648 [DOI] [PubMed] [Google Scholar]
- Heitman LH, Oosterom J, Bonger KM, Timmers CM, Wiegerinck PH, Ijzerman AP 2008 [3H]Org 43553, the first low-molecular-weight agonistic and allosteric radioligand for the human luteinizing hormone receptor. Mol Pharmacol 73:518–524 [DOI] [PubMed] [Google Scholar]
- Alukal JP, Lamb DJ 2008 Intracytoplasmic sperm injection (ICSI)—what are the risks? Urol Clin North Am 35:277–288, ix–x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manipalviratn S, DeCherney A, Segars J 2009 Imprinting disorders and assisted reproductive technology. Fertil Steril 91:305–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Practice Committee of American Society for Reproductive Medicine; Practice Committee of Society for Assisted Reproductive Technology 2008 Genetic considerations related to intracytoplasmic sperm injection (ICSI). Fertil Steril 90:S182–S184 [DOI] [PubMed] [Google Scholar]
- Reefhuis J, Honein MA, Schieve LA, Correa A, Hobbs CA, Rasmussen SA 2009 Assisted reproductive technology and major structural birth defects in the United States. Hum Reprod 24:360–366 [DOI] [PubMed] [Google Scholar]
- Mattison DR 1980 Morphology of oocyte and follicle destruction by polycyclic aromatic hydrocarbons in mice. Toxicol Appl Pharmacol 53:249–259 [DOI] [PubMed] [Google Scholar]
- Chen C 1986 Pregnancy after human oocyte cryopreservation. Lancet 1:884–886 [DOI] [PubMed] [Google Scholar]
- Gardner DK, Sheehan CB, Rienzi L, Katz-Jaffe M, Larman MG 2007 Analysis of oocyte physiology to improve cryopreservation procedures. Theriogenology 67:64–72 [DOI] [PubMed] [Google Scholar]
- Kuwayama M, Vajta G, Kato O, Leibo SP 2005 Highly efficient vitrification method for cryopreservation of human oocytes. Reprod Biomed Online 11:300–308 [DOI] [PubMed] [Google Scholar]
- Vajta G, Nagy ZP 2006 Are programmable freezers still needed in the embryo laboratory? Review on vitrification. Reprod Biomed Online 12:779–796 [DOI] [PubMed] [Google Scholar]
- Lucena E, Bernal DP, Lucena C, Rojas A, Moran A, Lucena A 2006 Successful ongoing pregnancies after vitrification of oocytes. Fertil Steril 85:108–111 [DOI] [PubMed] [Google Scholar]
- Antinori M, Licata E, Dani G, Cerusico F, Versaci C, Antinori S 2007 Cryotop vitrification of human oocytes results in high survival rate and healthy deliveries. Reprod Biomed Online 14:72–79 [DOI] [PubMed] [Google Scholar]
- Barritt J, Luna M, Duke M, Grunfeld L, Mukherjee T, Sandler B, Copperman AB 2007 Report of four donor-recipient oocyte cryopreservation cycles resulting in high pregnancy and implantation rates. Fertil Steril 87:189.e13–e17 [DOI] [PubMed] [Google Scholar]
- Chang CC, Shapiro DB, Bernal DP, Wright G, Kort HI, Nagy ZP 2008 Two successful pregnancies obtained following oocyte vitrification and embryo re-vitrification. Reprod Biomed Online 16:346–349 [DOI] [PubMed] [Google Scholar]
- Chang CC, Shapiro DB, Bernal DP, Wright G, Kort HI, Nagy ZP 2008 Human oocyte vitrification: in-vivo and in-vitro maturation outcomes. Reprod Biomed Online 17:684–688 [DOI] [PubMed] [Google Scholar]
- Cao YX, Xing Q, Li L, Cong L, Zhang ZG, Wei ZL, Zhou P 20 Oct 2008 Comparison of survival and embryonic development in human oocytes cryopreserved by slow-freezing and vitrification. Fertil Steril 10.1016/j.fertnstert.2008.08.069 [DOI] [PubMed] [Google Scholar]
- Larman MG, Minasi MG, Rienzi L, Gardner DK 2007 Maintenance of the meiotic spindle during vitrification in human and mouse oocytes. Reprod Biomed Online 15:692–700 [DOI] [PubMed] [Google Scholar]
- Ciotti PM, Porcu E, Notarangelo L, Magrini O, Bazzocchi A, Venturoli S 2009 Meiotic spindle recovery is faster in vitrification of human oocytes compared to slow freezing. Fertil Steril 91:2399–2407 [DOI] [PubMed] [Google Scholar]
- Isachenko V, Montag M, Isachenko E, Zaeva V, Krivokharchenko I, Shafei R, van der Ven H 2005 Aseptic technology of vitrification of human pronuclear oocytes using open-pulled straws. Hum Reprod 20:492–496 [DOI] [PubMed] [Google Scholar]
- Larman MG, Sheehan CB, Gardner DK 2006 Vitrification of mouse pronuclear oocytes with no direct liquid nitrogen contact. Reprod Biomed Online 12:66–69 [DOI] [PubMed] [Google Scholar]
- Balaban B, Urman B, Ata B, Isiklar A, Larman MG, Hamilton R, Gardner DK 2008 A randomized controlled study of human day 3 embryo cryopreservation by slow freezing or vitrification: vitrification is associated with higher survival, metabolism and blastocyst formation. Hum Reprod 23:1976–1982 [DOI] [PubMed] [Google Scholar]
- Kagawa N, Kuwayama M, Nakata K, Vajta G, Silber S, Manabe N, Kato O 2007 Production of the first offspring from oocytes derived from fresh and cryopreserved pre-antral follicles of adult mice. Reprod Biomed Online 14:693–699 [DOI] [PubMed] [Google Scholar]
- Pellestor F, Anahory T, Hamamah S 2005 Effect of maternal age on the frequency of cytogenetic abnormalities in human oocytes. Cytogenet Genome Res 111:206–212 [DOI] [PubMed] [Google Scholar]
- Keefe D, Liu L, Wang W, Silva C 2003 Imaging meiotic spindles by polarization light microscopy: principles and applications to IVF. Reprod Biomed Online 7:24–29 [DOI] [PubMed] [Google Scholar]
- Wang WH, Meng L, Hackett RJ, Odenbourg R, Keefe DL 2001 The spindle observation and its relationship with fertilization after intracytoplasmic sperm injection in living human oocytes. Fertil Steril 75:348–353 [DOI] [PubMed] [Google Scholar]
- Wang WH, Meng L, Hackett RJ, Keefe DL 2001 Developmental ability of human oocytes with or without birefringent spindles imaged by polscope before insemination. Hum Reprod 16:1464–1468 [DOI] [PubMed] [Google Scholar]
- Rama Raju GA, Prakash GJ, Krishna KM, Madan K 2007 Meiotic spindle and zona pellucida characteristics as predictors of embryonic development: a preliminary study using PolScope imaging. Reprod Biomed Online 14:166–174 [DOI] [PubMed] [Google Scholar]
- Petersen CG, Oliveira JB, Mauri AL, Massaro FC, Baruffi RL, Pontes A, Franco Jr JG 2009 Relationship between visualization of meiotic spindle in human oocytes and ICSI outcomes: a meta-analysis. Reprod Biomed Online 18:235–243 [DOI] [PubMed] [Google Scholar]
- Montag M, van der Ven K, Rösing B, van der Ven H 2009 Polar body biopsy: a viable alternative to preimplantation genetic diagnosis and screening. Reprod Biomed Online 18(Suppl 1):6–11 [DOI] [PubMed] [Google Scholar]
- Keskintepe L, Agca Y, Sher G, Keskintepe M, Maassarani G 20 October 2008 High survival rate of metaphase II human oocytes after first polar body biopsy and vitrification: determining the effect of previtrification conditions. Fertil Steril 10.1016/j.fertnstert.2008.08.114 [DOI] [PubMed] [Google Scholar]
- Hamel M, Dufort I, Robert C, Gravel C, Leveille MC, Leader A, Sirard MA 2008 Identification of differentially expressed markers in human follicular cells associated with competent oocytes. Hum Reprod 23:1118–1127 [DOI] [PubMed] [Google Scholar]
- Assou S, Haouzi D, Mahmoud K, Aouacheria A, Guillemin Y, Pantesco V, Rème T, Dechaud H, De Vos J, Hamamah S 2008 A non-invasive test for assessing embryo potential by gene expression profiles of human cumulus cells: a proof of concept study. Mol Hum Reprod 14:711–719 [DOI] [PubMed] [Google Scholar]
- Hübner K, Fuhrmann G, Christenson LK, Kehler J, Reinbold R, De La Fuente R, Wood J, Strauss 3rd JF, Boiani M, Schöler HR 2003 Derivation of oocytes from mouse embryonic stem cells. Science 300:1251–1256 [DOI] [PubMed] [Google Scholar]
- Matsui Y, Zsebo K, Hogan BL 1992 Derivation of pluripotential embryonic stem cells from murine primordial germ cells in culture. Cell 70:841–847 [DOI] [PubMed] [Google Scholar]
- Resnick JL, Bixler LS, Cheng L, Donovan PJ 1992 Long-term proliferation of mouse primordial germ cells in culture. Nature 359:550–551 [DOI] [PubMed] [Google Scholar]
- Durcova-Hills G, Tang F, Doody G, Tooze R, Surani MA 2008 Reprogramming primordial germ cells into pluripotent stem cells. PLoS ONE 3:e3531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durcova-Hills G, Adams IR, Barton SC, Surani MA, McLaren A 2006 The role of exogenous fibroblast growth factor-2 on the reprogramming of primordial germ cells into pluripotent stem cells. Stem Cells 24:1441–1449 [DOI] [PubMed] [Google Scholar]
- Guan K, Nayernia K, Maier LS, Wagner S, Dressel R, Lee JH, Nolte J, Wolf F, Li M, Engel W, Hasenfuss G 2006 Pluripotency of spermatogonial stem cells from adult mouse testis. Nature 440:1199–1203 [DOI] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M, Inoue K, Lee J, Yoshimoto M, Ogonuki N, Miki H, Baba S, Kato T, Kazuki Y, Toyokuni S, Toyoshima M, Niwa O, Oshimura M, Heike T, Nakahata T, Ishino F, Ogura A, Shinohara T 2004 Generation of pluripotent stem cells from neonatal mouse testis. Cell 119:1001-1012 [DOI] [PubMed] [Google Scholar]
- Conrad S, Renninger M, Hennenlotter J, Wiesner T, Just L, Bonin M, Aicher W, Bühring HJ, Mattheus U, Mack A, Wagner HJ, Minger S, Matzkies M, Reppel M, Hescheler J, Sievert KD, Stenzl A, Skutella T 2008 Generation of pluripotent stem cells from adult human testis. Nature 456:344–349 [DOI] [PubMed] [Google Scholar]
- Park IH, Zhao R, West JA, Yabuuchi A, Huo H, Ince TA, Lerou PH, Lensch MW, Daley GQ 2008 Reprogramming of human somatic cells to pluripotency with defined factors. Nature 451:141–146 [DOI] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA 2007 Induced pluripotent stem cell lines derived from human somatic cells. Science 318:1917–1920 [DOI] [PubMed] [Google Scholar]
- Wilmut I, Schnieke AE, McWhir J, Kind AJ, Campbell KH 1997 Viable offspring derived from fetal and adult mammalian cells. Nature 385:810–813 [DOI] [PubMed] [Google Scholar]
- Chang CC, Sung LY, Amano T, Tian XC, Yang X, Nagy ZP 2009 Nuclear transfer and oocyte cryopreservation. Reprod Fertil Dev 21:37–44 [DOI] [PubMed] [Google Scholar]
- Andreu-Vieyra C, Lin YN, Matzuk MM 2006 Mining the oocyte transcriptome. Trends Endocrinol Metab 17:136–143 [DOI] [PubMed] [Google Scholar]
- Leo CP, Vitt UA, Hsueh AJ 2000 The Ovarian Kaleidoscope database: an online resource for the ovarian research community. Endocrinology 141:3052–3054 [DOI] [PubMed] [Google Scholar]
- Coulam CB, Adamson SC, Annegers JF 1986 Incidence of premature ovarian failure. Obstet Gynecol 67:604–606 [PubMed] [Google Scholar]
- Sherman SL 2000 Premature ovarian failure in the fragile X syndrome. Am J Med Genet 97:189–194 [DOI] [PubMed] [Google Scholar]
- International HapMap Consortium 2003 The International HapMap Project. Nature 426:789–796 [DOI] [PubMed] [Google Scholar]
- Nalam RL, Pletcher SD, Matzuk MM 2008 Appetite for reproduction: dietary restriction, aging and the mammalian gonad. J Biol 7:23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharov AA, Falco G, Piao Y, Poosala S, Becker KG, Zonderman AB, Longo DL, Schlessinger D, Ko MSh 2008 Effects of aging and calorie restriction on the global gene expression profiles of mouse testis and ovary. BMC Biol 6:24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt JA, Oatley JM, Brinster RL 2009 Female mice delay reproductive aging in males. Biol Reprod 80:1009–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krützfeldt J, Rajewsky N, Braich R, Rajeev KG, Tuschl T, Manoharan M, Stoffel M 2005 Silencing of microRNAs in vivo with ‘antagomirs.’ Nature 438:685–689 [DOI] [PubMed] [Google Scholar]
- Esau C, Davis S, Murray SF, Yu XX, Pandey SK, Pear M, Watts L, Booten SL, Graham M, McKay R, Subramaniam A, Propp S, Lollo BA, Freier S, Bennett CF, Bhanot S, Monia BP 2006 miR-122 regulation of lipid metabolism revealed by in vivo antisense targeting. Cell Metab 3:87–98 [DOI] [PubMed] [Google Scholar]
- Elmén J, Lindow M, Silahtaroglu A, Bak M, Christensen M, Lind-Thomsen A, Hedtjärn M, Hansen JB, Hansen HF, Straarup EM, McCullagh K, Kearney P, Kauppinen S 2008 Antagonism of microRNA-122 in mice by systemically administered LNA-antimiR leads to up-regulation of a large set of predicted target mRNAs in the liver. Nucleic Acids Res 36:1153–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmén J, Lindow M, Schütz S, Lawrence M, Petri A, Obad S, Lindholm M, Hedtjärn M, Hansen HF, Berger U, Gullans S, Kearney P, Sarnow P, Straarup EM, Kauppinen S 2008 LNA-mediated microRNA silencing in non-human primates. Nature 452:896–899 [DOI] [PubMed] [Google Scholar]
- Jopling CL, Yi M, Lancaster AM, Lemon SM, Sarnow P 2005 Modulation of hepatitis C virus RNA abundance by a liver-specific microRNA. Science 309:1577–1581 [DOI] [PubMed] [Google Scholar]
- Pedersen T, Peters H 1968 Proposal for a classification of oocytes and follicles in the mouse ovary. J Reprod Fertil 17:555–557 [DOI] [PubMed] [Google Scholar]
- Bouligand J, Ghervan C, Tello JA, Brailly-Tabard S, Salenave S, Chanson P, Lombès M, Millar RP, Guiochon-Mantel A, Young J 2009 Isolated familial hypogonadotropic hypogonadism and a GNRH1 mutation. N Engl J Med 360:2742–2748 [DOI] [PubMed] [Google Scholar]
- Chan YM, de Guillebon A, Lang-Muritano M, Plummer L, Cerrato F, Tsiaras S, Gaspert A, Lavoie HB, Wu CH, Crowley Jr WF, Amory JK, Pitteloud N, Seminara SB 2009 GNRH1 mutations in patients with idiopathic hypogonadotropic hypogonadism. Proc Natl Acad Sci USA 106:11703–11708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lourenço D, Brauner R, Lin L, De Perdigo A, Weryha G, Muresan M, Boudjenah R, Guerra-Junior G, Maciel-Guerra AT, Achermann JC, McElreavey K, Bashamboo A 2009 Mutations in NR5A1 associated with ovarian insufficiency. N Engl J Med 360:1200–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Bradley A 1996 Mice deficient for BMP2 are nonviable and have defects in amnion/chorion and cardiac development. Development 122:2977–2986 [DOI] [PubMed] [Google Scholar]
- Winnier G, Blessing M, Labosky PA, Hogan BL 1995 Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes Dev 9:2105–2116 [DOI] [PubMed] [Google Scholar]
- Zhao GQ, Deng K, Labosky PA, Liaw L, Hogan BL 1996 The gene encoding bone morphogeneetic protein 8B is required for the initiation and maintenance of spermatogenesis in the mouse. Genes Dev 10:1657–1669 [DOI] [PubMed] [Google Scholar]
- Lechleider RJ, Ryan JL, Garrett L, Eng C, Deng C, Wynshaw-Boris A, Roberts AB 2001 Targeted mutagenesis of Smad1 reveals an essential role in chorioallantoic fusion. Dev Biol 240:157–167 [DOI] [PubMed] [Google Scholar]
- Chang H, Huylebroeck D, Verschueren K, Guo Q, Matzuk MM, Zwijsen A 1999 Smad5 knockout mice die at mid-gestation due to multiple embryonic and extraembryonic defects. Development 126:1631–1642 [DOI] [PubMed] [Google Scholar]
- Yang X, Castilla LH, Xu X, Li C, Gotay J, Weinstein M, Liu PP, Deng CX 1999 Angiogenesis defects and mesenchymal apoptosis in mice lacking SMAD5. Development 126:1571–1580 [DOI] [PubMed] [Google Scholar]
- Sirard C, de la Pompa JL, Elia A, Itie A, Mirtsos C, Cheung A, Hahn S, Wakeham A, Schwartz L, Kern SE, Rossant J, Mak TW 1998 The tumor suppressor gene Smad4/Dpc4 is required for gastrulation and later for anterior development of the mouse embryo. Genes Dev 12:107–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Li C, Xu X, Deng C 1998 The tumor suppressor SMAD4/DPC4 is essential for epiblast proliferation and mesoderm induction in mice. Proc Natl Acad Sci USA 95:3667–3672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöler HR, Hatzopoulos AK, Balling R, Suzuki N, Gruss P 1989 A family of octamer-specific proteins present during mouse embryogenesis: evidence for germline-specific expression of an Oct factor. EMBO J 8:2543–2550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer LA, Lee TI, Cole MF, Johnstone SE, Levine SS, Zucker JP, Guenther MG, Kumar RM, Murray HL, Jenner RG, Gifford DK, Melton DA, Jaenisch R, Young RA 2005 Core transcriptional regulatory circuitry in human embryonic stem cells. Cell 122:947–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers I, Colby D, Robertson M, Nichols J, Lee S, Tweedie S, Smith A 2003 Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell 113:643–655 [DOI] [PubMed] [Google Scholar]
- Mitsui K, Tokuzawa Y, Itoh H, Segawa K, Murakami M, Takahashi K, Maruyama M, Maeda M, Yamanaka S 2003 The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell 113:631–642 [DOI] [PubMed] [Google Scholar]
- Botquin V, Hess H, Fuhrmann G, Anastassiadis C, Gross MK, Vriend G, Schöler HR 1998 New POU dimer configuration mediates antagonistic control of an osteopontin preimplantation enhancer by Oct-4 and Sox-2. Genes Dev 12:2073–2090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan P, de Miguel MP 2001 The role of C-Kit/Kit ligand axis in mammalian gametogenesis. Totowa, NJ: Humana Press [Google Scholar]
- Larsson J, Goumans MJ, Sjöstrand LJ, van Rooijen MA, Ward D, Levéen P, Xu X, ten Dijke P, Mummery CL, Karlsson S 2001 Abnormal angiogenesis but intact hematopoietic potential in TGF-β type I receptor-deficient mice. EMBO J 20:1663–1673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal VP, Chaboissier MC, de Rooij DG, Schedl A 2001 Sox9 induces testis development in XX transgenic mice. Nat Genet 28:216–217 [DOI] [PubMed] [Google Scholar]
- Koopman P, Gubbay J, Vivian N, Goodfellow P, Lovell-Badge R 1991 Male development of chromosomally female mice transgenic for Sry. Nature 351:117–121 [DOI] [PubMed] [Google Scholar]
- Brennan J, Tilmann C, Capel B 2003 Pdgfr-α mediates testis cord organization and fetal Leydig cell development in the XY gonad. Genes Dev 17:800–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballow D, Meistrich ML, Matzuk M, Rajkovic A 2006 Sohlh1 is essential for spermatogonial differentiation. Dev Biol 294:161–167 [DOI] [PubMed] [Google Scholar]
- Kano K, Marín de Evsikova C, Young J, Wnek C, Maddatu TP, Nishina PM, Naggert JK 2008 A novel dwarfism with gonadal dysfunction due to loss-of-function allele of the collagen receptor gene, Drd2, in the mouse. Mol Endocrinol 22:1866–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyurko R, Leupen S, Huang PL 2002 Deletion of exon 6 of the neuronal nitric oxide synthase gene in mice results in hypogonadism and infertility. Endocrinology 143:2767–2774 [DOI] [PubMed] [Google Scholar]
- Jackowski S, Rehg JE, Zhang YM, Wang J, Miller K, Jackson P, Karim MA 2004 Disruption of CCTβ2 expression leads to gonadal dysfunction. Mol Cell Biol 24:4720–4733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsuka S, Takaki S, Iseki M, Miyoshi K, Nakagata N, Kataoka Y, Yoshida N, Takatsu K, Yoshimura A 2002 SH2-B is required for both male and female reproduction. Mol Cell Biol 22:3066–3077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roby KF, Son DS, Taylor CC, Montgomery-Rice V, Kirchoff J, Tang S, Terranova PF 2005 Alterations in reproductive function in SRC tyrosine kinase knockout mice. Endocrine 26:169–176 [DOI] [PubMed] [Google Scholar]
- Greenaway J, Lawler J, Moorehead R, Bornstein P, Lamarre J, Petrik J 2007 Thrombospondin-1 inhibits VEGF levels in the ovary directly by binding and internalization via the low density lipoprotein receptor-related protein-1 (LRP-1). J Cell Physiol 210:807–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CL, DeVera DG, Lamb DJ, Nawaz Z, Jiang YH, Beaudet AL, O'Malley BW 2002 Genetic ablation of the steroid receptor coactivator-ubiquitin ligase, E6-AP, results in tissue-selective steroid hormone resistance and defects in reproduction. Mol Cell Biol 22:525–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B, Poirier C, Gaspar T, Gratzke C, Harrison W, Busija D, Matzuk MM, Andersson KE, Overbeek PA, Bishop CE 2008 A mutation in the inner mitochondrial membrane peptidase 2-like gene (Immp2l) affects mitochondrial function and impairs fertility in mice. Biol Reprod 78:601–610 [DOI] [PubMed] [Google Scholar]
- Burks DJ, Font de Mora J, Schubert M, Withers DJ, Myers MG, Towery HH, Altamuro SL, Flint CL, White MF 2000 IRS-2 pathways integrate femal reproduction and energy homeostasis. Nature 407:377–382 [DOI] [PubMed] [Google Scholar]
- Matzuk MM, Dionne L, Guo Q, Kumar TR, Lebovitz RM 1998 Ovarian function in superoxide dismutase 1 and 2 knockout mice. Endocrinology 139:4008–4011 [DOI] [PubMed] [Google Scholar]
- Ho YS, Gargano M, Cao J, Bronson RT, Heimler I, Hutz RJ 1998 Reduced fertility in female mice lacking copper-zinc superoxide dismutase. J Biol Chem 273:7765–7769 [DOI] [PubMed] [Google Scholar]
- Weiss J, Meeks JJ, Hurley L, Raverot G, Frassetto A, Jameson JL 2003 Sox3 is required for gonadal function, but not sex determination, in males and females. Mol Cell Biol 23:8084–8091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roby KF, Son DS, Terranova PF 1999 Alterations of events related to ovarian function in tumor necrosis factor receptor type I knockout mice. Biol Reprod 61:1616–1621 [DOI] [PubMed] [Google Scholar]
- Salustri A, Garlanda C, Hirsch E, De Acetis M, Maccagno A, Bottazzi B, Doni A, Bastone A, Mantovani G, Beck Peccoz P, Salvatori G, Mahoney DJ, Day AJ, Siracusa G, Romani L, Mantovani A 2004 PTX3 plays a key role in the organization of the cumulus oophorus extracellular matrix and in in vivo fertilization. Development 131:1577–1586 [DOI] [PubMed] [Google Scholar]
- Molyneaux KA, Schaible K, Wylie C 2003 GP130, the shared receptor for the LIF/IL6 cytokine family in the mouse, is not required for early germ cell differentiation, but is required cell-autonomously in oocytes for ovulation. Development 130:4287–4294 [DOI] [PubMed] [Google Scholar]
- Jablonka-Shariff A, Olson LM 1998 The role of nitric oxide in oocyte meiotic maturation and ovulation: meiotic abnormalities of endothelial nitric oxide synthase knock-out mouse oocytes. Endocrinology 139:2944–2954 [DOI] [PubMed] [Google Scholar]
- Yan W, Ma L, Stein P, Pangas SA, Burns KH, Bai Y, Schultz RM, Matzuk MM 2005 Mice deficient in oocyte-specific oligoadenylate synthase-like protein, OAS1D display reduced fertility. Mol Cell Biol 25:4615–4624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin SL, Richard FJ, Kuo WP, D'Ercole AJ, Conti M 1999 Impaired growth and fertility of cAMP-specific phosphodiesterase PDE4D- deficient mice. Proc Natl Acad Sci USA 96:11998–12003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBurney MW, Yang X, Jardine K, Hixon M, Boekelheide K, Webb JR, Lansdorp PM, Lemieux M 2003 The mammalian SIR2α protein has a role in embryogenesis and gametogenesis. Mol Cell Biol 23:38–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian YM, Sun XJ, Tong MH, Li XP, Richa J, Song WC 2001 Targeted disruption of the mouse estrogen sulfotransferase gene reveals a role of estrogen metabolism in intracrine and paracrine estrogen regulation. Endocrinology 142:5342–5350 [DOI] [PubMed] [Google Scholar]
- Yang J, Medvedev S, Yu J, Tang LC, Agno JE, Matzuk MM, Schultz RM, Hecht NB 2005 Absence of the DNA-/RNA-binding protein MSY2 results in male and female infertility. Proc Natl Acad Sci USA 102:5755–5760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filis P, Lannagan T, Thomson A, Murray AA, Kind PC, Spears N 2009 Phospholipase C-β1 signaling affects reproductive behavior, ovulation, and implantation. Endocrinology 150:3259–3266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topilko P, Schneider-Maunoury S, Levi G, Trembleau A, Gourdji D, Driancourt MA, Rao CV, Charnay P 1998 Multiple pituitary and ovarian defects in Krox-24 (NGFI-A, Egr-1)-targeted mice. Mol Endocrinol 12:107–122 [DOI] [PubMed] [Google Scholar]
- Lee SL, Sadovsky Y, Swirnoff AH, Polish JA, Goda P, Gavrilina G, Milbrandt J 1996 Luteinizing hormone deficiency and female infertility in mice lacking the transcription factor NGFI-A (Egr-1). Science 273:1219–1221 [DOI] [PubMed] [Google Scholar]
- Osada T, Watanabe G, Sakaki Y, Takeuchi T 2001 Puromycin-sensitive aminopeptidase is essential for the maternal recognition of pregnancy in mice. Mol Endocrinol 15:882–893 [DOI] [PubMed] [Google Scholar]
- Sugimoto Y, Yamasaki A, Segi E, Tsuboi K, Aze Y, Nishimura T, Oida H, Yoshida N, Tanaka T, Katsuyama M, Hasumoto K, Murata T, Hirata M, Ushikubi F, Negishi M, Ichikawa A, Narumiya S 1997 Failure of parturition in mice lacking the prostaglandin F receptor. Science 277:681–683 [DOI] [PubMed] [Google Scholar]
- Danilovich N, Roy I, Sairam MR 2001 Ovarian pathology and high incidence of sex cord tumors in follitropin receptor knockout (FORKO) mice. Endocrinology 142:3673–3684 [DOI] [PubMed] [Google Scholar]
- Bertolino P, Tong WM, Galendo D, Wang ZQ, Zhang CX 2003 Heterozygous Men1 mutant mice develop a range of endocrine tumors mimicking multiple endocrine neoplasia type 1. Mol Endocrinol 17:1880–1892 [DOI] [PubMed] [Google Scholar]
- Loffler KA, Biondi CA, Gartside M, Waring P, Stark M, Serewko-Auret MM, Muller HK, Hayward NK, Kay GF 2007 Broad tumor spectrum in a mouse model of multiple endocrine neoplasia type 1. Int J Cancer 120:259–267 [DOI] [PubMed] [Google Scholar]
- Mould AW, Duncan R, Serewko-Auret M, Loffler KA, Biondi C, Gartside M, Kay GF, Hayward NK 2009 Global expression profiling of sex cord stromal tumors from Men1 heterozygous mice identifies altered TGF-β signaling, decreased Gata6 and increased Csf1r expression. Int J Cancer 124:1122–1132 [DOI] [PubMed] [Google Scholar]
- Garson K, Macdonald E, Dubé M, Bao R, Hamilton TC, Vanderhyden BC 2003 Generation of tumors in transgenic mice expressing the SV40 T antigen under the control of ovarian-specific promoter 1. J Soc Gynecol Investig 10:244–250 [DOI] [PubMed] [Google Scholar]
- Fafalios MK, Olander EA, Melhem MF, Chaillet JR 1996 Ovarian teratomas associated with the insertion of an imprinted transgene. Mamm Genome 7:188–193 [DOI] [PubMed] [Google Scholar]
- Huhtaniemi I, Rulli S, Ahtiainen P, Poutanen M 2005 Multiple sites of tumorigenesis in transgenic mice overproducing hCG. Mol Cell Endocrinol 234:117–126 [DOI] [PubMed] [Google Scholar]