Abstract
Heterotrimeric G proteins mediate the earliest step in cell responses to external events by linking cell surface receptors to intracellular signaling pathways. Gz is a member of the Gi family of G proteins that is prominently expressed in platelets and brain. Here, we show that deletion of the α subunit of Gz in mice: (i) impairs platelet aggregation by preventing the inhibition of cAMP formation normally seen at physiologic concentrations of epinephrine, and (ii) causes the mice to be more resistant to fatal thromboembolism. Loss of Gzα also results in greatly exaggerated responses to cocaine, reduces the analgesic effects of morphine, and abolishes the effects of widely used antidepressant drugs that act as catecholamine reuptake inhibitors. These changes occur despite the presence of other Giα family members in the same cells and are not accompanied by detectable compensatory changes in the level of expression of other G protein subunits. Therefore, these results provide insights into receptor selectivity among G proteins and a model for understanding platelet function and the effects of psychoactive drugs.
Heterotrimeric guanine nucleotide binding proteins (G proteins) comprised of α, β, and γ subunits mediate diverse cellular responses by linking receptors on the cell surface to intracellular signaling pathways. At least 20 human genes are known to encode GTP-binding α subunits. Half of these are members of the Giα family, including the ubiquitously expressed and nearly identical Gi1α, Gi2α, and Gi3α, as well as several with restricted expression, such as Gzα, Goα, and transducin. The best described effector for most Gi family members is adenylyl cyclase. However, others exist as well, including cGMP phosphodiesterase, ion channels, phospholipase C, and Rap1GAP (1–5). One unresolved issue is the need for such a multiplicity of Giα family members, many of which are commonly expressed within the same cell. One approach to addressing this question has been the development of mice that lack individual family members. Of the three broadly expressed members of the family, mice lacking Gi2α have the most striking phenotype with abnormalities of T cell function and thymocyte maturation, as well as an increased susceptibility to develop inflammatory bowel disease (6–8). Deletion of Gi1α or Gi3α, on the other hand, has not been reported to produce an obvious effect. Clearly, however, if there are functional differences among these and the other Gi family members, then receptor selection among them is potentially a major determinant for cellular responses.
Of the 10 known members of the Giα family, the sequence of Gzα bears the least similarity to the others. Gzα has a limited distribution in humans with prominent expression in blood platelets and selected areas of the brain. Like other members of the family, Gzα has been shown to inhibit cAMP formation by adenylyl cyclase when over-expressed (9), but it is not known whether this is part of its role in vivo. Similarly, in over-expression systems, Gzα has been shown to couple to a wide variety of receptors, including some that are present in human neurons and platelets, but it is unclear which are its preferred partners in vivo. At the molecular level, Gzα is notable for having a relatively slow intrinsic rate of GTP hydrolysis (10), for binding to RGSZ1 (11, 12) and Rap1GAP (4, 5), and for serving as a substrate for protein kinase C (13) and PAK (14). However, it is not clear how (or if) any of these properties render Gz functionally distinct, nor it is known whether Gzα fills a unique niche in receptor-initiated signaling that requires its expression in platelets and neurons in particular.
To address these issues, we have developed mice in which Gzα was replaced with green fluorescent protein and examined the consequences in vitro and in vivo, concentrating on potential effects on platelet and central nervous system function. The Gzα(−/−) mice show complete loss of expression of Gzα without evident compensatory changes in the level of expression of other Giα family members. Their platelets show strikingly impaired responses to epinephrine, a failure to suppress cAMP formation when adenylyl cyclase is stimulated by PGI2, and resistance to otherwise lethal disseminated thromboembolism initiated by injection of epinephrine plus collagen, all of which help to define the role of epinephrine in platelet activation in vivo. In behavioral studies, Gzα(−/−) mice show enhanced locomotor responses to cocaine, reduced analgesia from morphine, and complete loss of responses to antidepressants that are norepinephrine reuptake inhibitors. Taken together, these results demonstrate a clear preference among receptors for different members of the Gi family, define a unique role for Gzα in platelets and the central nervous system, and provide a model for platelet and antidepressant function in vivo.
Methods
Generation of Knockout Mice.
Clones encoding mouse Gzα were isolated from a 129Sv genomic DNA library (Stratagene) by using a human Gzα cDNA probe. Sequences flanking the first coding exon were subcloned and used to generate the knockout construct. The coding region was replaced with an enhanced green fluorescent protein (EGFP) promoter trap cassette containing the EGFP ORF (CLONTECH) amplified by using GFP1 (SalI tag GTCGAC CA TGG TGA GCA AGG GCG) and GFP2 (XbaI tag TCTAGA CTC TAC TTG TAC AGC TCG TCC) primers and a 3′ polyadenylation sequence from the PGK gene amplified by using PGK1 (GFP2 tag GGACGAGCTGTACAA GTAGAGTCTAGA GCC TGA GAA AGG AAG TGA GC) and PGK2 (XhoI tag CTCGAG AT CTA TAG ATC ATG AGT GGA) primers. The resulting cassette was inserted into the knockout vector, pNT (B. M. Spiegelman, Dana–Farber Cancer Institute, Boston, MA). The 5′ homologous sequence was amplified by using K1 (NotI tag GCGGCCGC CAA GCT CAG AGA TGT GGA GT) and K2 (XhoI tag CTCGAG CT TTG CCG ACA TCC CAT G) primers and subcloned into pNT. The 3′ homologous sequence was amplified by using K3 (BamHI tag GGATCC AG TGG CTA TGA CCT GAA GCT) and K4 (EcoRI tag GAATTC TG AAC CAC AGG ATA GAC AGG) primers. The targeting construct was transfected into WW6 ES cells (15). Homologous recombination events were confirmed by Southern blot analysis using probes from 5′ and 3′ flanking regions of Gzα gene and from EGFP sequence. Chimeric mice derived from W64 and W89 ES lines were generated by the Transgenic and Chimeric Mouse Facility at University of Pennsylvania and back-crossed once with C57BL/6. The experiments were done with mice from the W64 ES line.
Western Blot Analysis.
Platelets or homogenized brain lysates in hypotonic buffer (25 mM Hepes, pH 7.5, 1 mM EDTA, 1 mM DTT) plus a protease inhibitor mixture (Sigma) were centrifuged at 660 × g for 10 min at 4°C to remove cell debris, and then frozen and thawed three times. Membrane proteins were pelleted at 10,000 × g for 30 min at 4°C and resuspended in RIPA buffer (1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS in PBS). Protein samples (40 μg of brain, 20 μg of platelets) were loaded on 12% SDS-PAGE gels, separated, and then transferred to poly(vinylidene difluoride) (PVDF) membranes. The membranes were blocked with 5% nonfat dry milk in Tris-buffered saline (TBS) for 1 h at room temperature, and then incubated with the appropriate antisera (1:200 dilution) in TBS with 1% gelatin for 2 h at room temperature. Afterward, the PVDF membranes were washed three times with 0.2% Tween 20 in TBS for 10 min, and then incubated with horseradish peroxidase-conjugated anti-rabbit immunoglobulin (1:2000 dilution; Amersham Pharmacia) for 1 h at room temperature. The blot was then washed three times with TBS/Tween and analyzed by using an ECL Western blot detection kit (Amersham Pharmacia). G protein subunits were detected with rabbit antisera 2919 (Gzα), 116 (Gi1α/i2α), 0945 (Gqα), 1191 (Gsα), 9072 (Goα), and Q8136 (Gβγ) (13, 16, 17). ERK-1 was detected by using a rabbit polyclonal antiserum purchased from Santa Cruz Biotechnology.
Platelet Aggregation and Secretion.
Blood was collected from the inferior vena cava of anesthetized mice (100 mg/kg pentobarbital) by using a heparinized syringe (15 units/ml blood). Samples from four mice were pooled and diluted with 3 ml of Hepes/Tyrode buffer (129 mM NaCl/8.9 mM NaHCO3/2.8 mM KCl/0.8 mM KH2PO4/10 mM Hepes/0.8 mM MgCl2/5.6 mM dextrose, pH 7.4). Red cells were removed by centrifugation and the final platelet count was adjusted to 2–3 × 108/ml using Hepes/Tyrode buffer. Aggregation and dense granule ATP release were measured in a lumi-aggregometer (Chrono-Log, Havertown, PA). Collagen used was from Sigma.
Thromboembolism.
Male Gzα(−/−) mice that were 2–3 months old were compared with their wild-type littermates. Each mouse received 100 μl of either (i) collagen (150 μg/ml; Chrono-Log) plus epinephrine (300 μM) or (ii) collagen (150 μg/ml) plus ADP (170 mM) by tail vein injection. The lungs from mice challenged by collagen plus epinephrine were dissected immediately after death or as indicated, fixed with 4% paraformaldehyde, embedded in paraffin, and sectioned. The sections were stained with hematoxylin and eosin.
cAMP.
Washed platelets were stimulated with PGI2 (10 nM) in the presence of 7 mM theophylline to inhibit cAMP phosphodiesterase. After a 5-min incubation, epinephrine was added for an additional 3 min at the concentrations indicated in the text. The reaction was then stopped by adding ice-cold trichloroacetic acid (10%). cAMP was measured by RIA (NEN).
Analgesia Assay.
Mice were tested on a Hotplate Analgesia Meter (Columbus Instruments, Columbus, OH) 15 min after i.p. injection of morphine. The testing device provides a constant 58°C surface. The time required for the mice to jump off the hotplate was recorded. The maximum time the animals were left on the test plate was 120 s.
Locomotor Activity Assay.
Mice were placed in their home cages within a 30 × 24 × 8-cm frame with infrared sources and detectors in an 8-beam array strip with 1.25 inches of spacing that allowed for detection of horizontal and vertical movements. Beam break data were recorded. For the first 3 days, the mice were placed in chambers immediately after saline injections to measure locomotor activity associated with a novel environment. On days 4–9, the mice were given daily injections of either saline or cocaine (20 mg/kg) and then placed in the locomotor activity chambers. The expression of behavioral sensitization was seen throughout the time course (days 5–7) as well as on day 33, when they were given a reduced dose of cocaine (10 mg/kg).
Swimming Test.
Mice were transported to the testing room at least 1 h before testing. Sessions were conducted between noon and 6 PM, with animals randomly assigned to treatment conditions and tested in counterbalanced order. The mice were placed in individual glass cylinders as described by Porsolt et al. (18), except that a swim cylinder of larger diameter (46 × 21 cm) was used. Videotapes of the sessions were scored for the duration of immobility during the last 4 min of a 6-min session. Mice were judged to be immobile when the only movements they made was to keep their head above water. Desipramine (Sigma) and reboxetine (Pharmacia & Upjohn, Kalamazoo, MI) were administered i.p. at 10 ml/kg 30 min before testing. Data were analyzed by two-factor ANOVA, with Newman–Keul's as the follow-up test.
Results
Generation of Gzα(−/−) Mice.
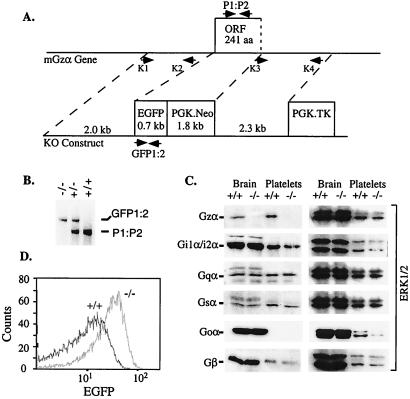
Mice in which Gzα was replaced by EGFP were generated by homologous recombination in embryonic stem cells followed by intercrosses between heterozygous mice (Fig. 1). Western blots of brain and platelet lysates confirmed that Gzα is no longer expressed and also showed that there are no compensatory changes in the level of expression of other forms of Gα and Gβ. Message encoding EGFP was present in brain lysates from Gzα(−/−) mice. EGFP fluorescence was readily detectable in platelets (Fig. 1) and megakaryocytes (not shown). Development appeared to be normal and the number of Gzα(−/−) mice produced by breeding heterozygous mice was not substantially different from what would be predicted by simple Mendelian genetics. Gzα(−/−) mice appeared healthy and were fertile, but were initially somewhat smaller than their wild-type littermates. Blood counts, including platelet counts, were within normal limits, as were tail bleeding times. There was no apparent tendency for increased bleeding, either spontaneously or from surgical sites.
Figure 1.
Targeted disruption of the mouse Gzα gene. (A) Knockout construct. Primers K1, K2, K3, and K4 were used to generate the targeting vector by PCR. The positive selection marker (PGK.Neo) and the negative selection marker (PGK.TK) are indicated. The ORF containing the first two-thirds of the coding region is replaced in-frame by EGFP. (B) PCR genotyping of mouse genomic DNA using the primer pairs indicated. (C) Immunoblots with rabbit polyclonal antibodies that recognize Gzα, Gi1α/Gi2α, Gqα, Gsα, Goα, and four isoforms of Gβ. Note that Goα is normally expressed in brain, but not in platelets. The blots were stripped and reprobed with anti-ERK1/2 as a loading control. (D) FACS analysis of platelet-rich plasma.
Abnormal Platelet Responses in Gzα(−/−) Mice.
Platelets are normally activated at sites of vascular injury by a combination of collagen and thrombin with support from secreted, circulating, or locally generated agonists such as ADP, thromboxane A2 (TxA2), and epinephrine. Collectively, these agonists help to arrest bleeding by initiating platelet adhesion to the injured surface and by promoting the formation of platelet aggregates. With the exception of collagen, each of these agonists activates phospholipase Cβ primarily via the G protein, Gq (19). Many of them inhibit cAMP formation by adenylyl cyclase as well, either directly or indirectly via secreted ADP. Both processes are believed to contribute to platelet activation. Elevated cAMP levels, which inhibit platelet activation, are believed to occur in vivo when endothelium-derived PGI2 activates platelet adenylyl cyclase. Agonists that just activate phospholipase C or just suppress cAMP formation have also been shown to cause platelet aggregation when added in combination. Epinephrine is one such agonist, facilitating platelet activation by suppressing the increase in cAMP formation otherwise caused by PGI2 released from endothelial cells (and perhaps doing other things as well), although causing little, if any, activation of phospholipase C. Although platelets express Gz, its role in any of these events is unclear.
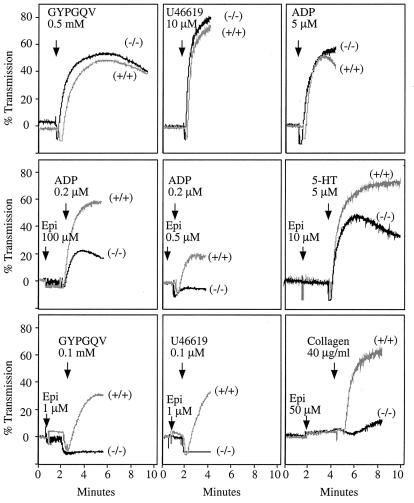
When tested ex vivo with the PAR4 (thrombin receptor) peptide agonist, GYPGQV, or the TxA2 receptor agonist, U46619, platelets from the Gzα(−/−) mice showed the same profile of aggregation (Fig. 2) and dense granule secretion (not shown) as their wild-type littermates at both maximally effective and submaximal concentrations of each agonist. Loss of Gzα had no effect on platelet shape change. Aggregation responses were also generally normal with collagen, ADP and arachidonate (Fig. 2, and data not shown). However, there was a striking difference in platelet responses to epinephrine in the wild-type and Gzα(−/−) mice (Fig. 2). In both the wild-type mice and the Gzα(−/−) mice, epinephrine was unable to cause platelet aggregation on its own. However, when added to wild-type platelets, epinephrine caused enhanced aggregation in response to all of the other agonists that were tested, even when the other agonist was added at a concentration too low to cause aggregation on its own and regardless of whether epinephrine was added before or after the other agonist. This potentiating effect of epinephrine, which is thought to be its role in vivo, was greatly diminished or absent in platelets from the Gzα(−/−) mice.
Figure 2.
Platelet aggregation in Gzα(−/−) mice. Platelets in plasma were stimulated with each of the agonists indicated. The results with the Gzα(−/−) are indicated as (−/−). The results obtained with their wild-type littermates are indicated as (+/+). The tracings shown represent data from at least three experiments. Epi, epinephrine; U46619, a TxA2 analog; GYP, PAR4 peptide agonist GYPGQV; 5-HT, serotonin.
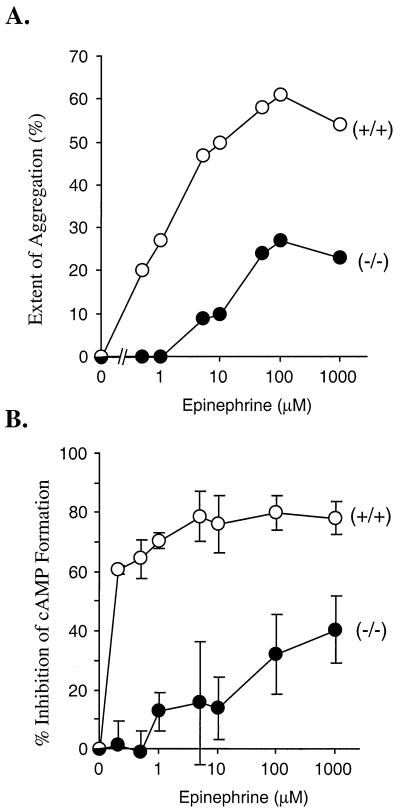
Fig. 3A shows dose/response curves for platelet aggregation caused by adding increasing concentrations of epinephrine in the presence of a fixed, subthreshold concentration of ADP (0.2 μM). With platelets that contain Gzα, aggregation was readily detectable at epinephrine concentrations below 1 μM. With platelets that lacked Gzα, higher concentrations of epinephrine were required to cause aggregation and the maximum response observed was less than half that seen with the wild-type platelets—even at epinephrine concentrations as high as 1 mM. To determine whether the failure of epinephrine to promote platelet responses to other agonists was associated with a failure to inhibit adenylyl cyclase, cAMP formation was compared in intact platelets from wild-type mice and their Gzα(−/−) littermates. The basal cAMP concentration was the same in both, as were the increase in cAMP levels caused by PGI2 and the inhibitory effects of thrombin and ADP at high and low concentrations (not shown). However, in the Gzα(−/−) mice, the ability of epinephrine to suppress PGI2-stimulated cAMP formation was markedly impaired, with an IC50 at least 2 orders of magnitude higher in the knockouts than the wild-type mice (Fig. 3B). Even at 1 mM (the highest concentration tested), epinephrine inhibited cAMP formation in the knockout platelets only half as well as in the wild-type platelets. Like the results in the aggregation assays, this suggests that α2A-adrenergic receptors prefer Gz to the other Gi family members expressed in platelets and that the presence of Gzα greatly reduces the concentration at which epinephrine evokes responses from platelets.
Figure 3.
Stimulation of platelet aggregation and inhibition of cAMP formation by epinephrine. (A) Aggregation was measured in response to a combination of 0.2 μM ADP plus epinephrine at the final concentrations indicated. As is shown in Fig. 2, epinephrine did not stimulate aggregation when added alone. The results shown are representative of two experiments. (B) The ability of epinephrine to inhibit PGI2-stimulated cAMP formation was measured. The results are expressed as percent inhibition of cAMP formation in the absence of epinephrine. The results shown are the mean ± SEM from two experiments.
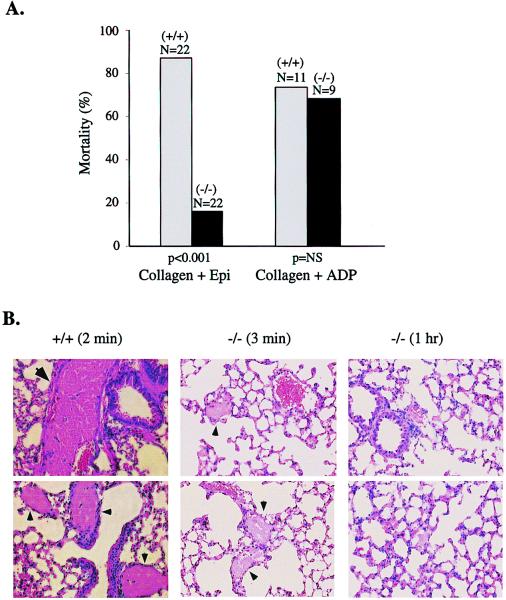
To see whether the difference in platelet function observed ex vivo translated into impaired platelet function in vivo, Gzα(−/−) mice and their wild-type littermates were injected intravenously with collagen plus epinephrine, a combination shown previously to induce disseminated thromboembolism (20). Substantial differences were observed among the mice. Under conditions in which nearly all of the wild-type mice succumbed to pulmonary thrombosis, most of the Gzα(−/−) mice survived (Fig. 4A). There were no marked gender differences. Postmortem examination of lung sections showed massive thrombus formation in the wild-type mice (Fig. 4B). In contrast, there was little thrombus formation in the lungs from Gzα(−/−) mice that were killed 3 min or 1 h after injection. This suggests that they had a reduced clot burden to begin with and that the impairment of platelet function caused by deleting Gzα had a meaningful effect on platelet activation in vivo. In contrast to these results, there was no difference in mortality rates between wild-type and Gzα(−/−) mice when thrombosis was initiated with collagen plus ADP (Fig. 4A). By using a similar technique, Fabre et al. (21) found improved survival in mice lacking the P2Y1 ADP receptor when they were challenged with ADP plus collagen, and Offermanns et al. (19) found improved survival in Gqα(−/−) mice challenged with epinephrine plus collagen.
Figure 4.
Thromboembolism model. (A) Thromboembolic mortality was observed following the injection of collagen plus epinephrine (Epi) or collagen plus ADP as described in Methods. The P values (NS, statistically nonsignificant) shown in the figure were calculated by the χ2 test. (B) Lung sections from mice challenged with collagen plus epinephrine are shown. The figure represents data obtained from three animals of each genotype or time point. Large thrombi in large vessels were found only in the wild-type mice (indicated by large arrows). Smaller thrombi that are widespread in the (+/+) samples, but are much less frequent in (−/−) samples, are also indicated (small arrows). The (−/−) mice killed 1 h after challenge are clear of obvious clots.
Altered Behavioral Responses in Gzα(−/−) Mice.
Turning from platelets to the brain, signaling through G protein-coupled receptors is involved in many well known drug-induced behavioral changes, and some of the receptors are known to be able to couple to Gz, at least when over-expressed. For instance, studies with knockout mice show that dopamine receptors are required for locomotor response to cocaine (22–24) and opioid receptors, particularly μ-opioid receptors, mediate morphine-induced analgesic response (25). In brain, the two isoforms of Goα (GoαA and GoαB) are the most abundant G proteins, but other Giα family members are also present, including Gzα (Fig. 1). Deletion of Goα in mice has been shown to produce severe defects in the central nervous system, behavioral abnormalities, and reduced survival (8, 26, 27).
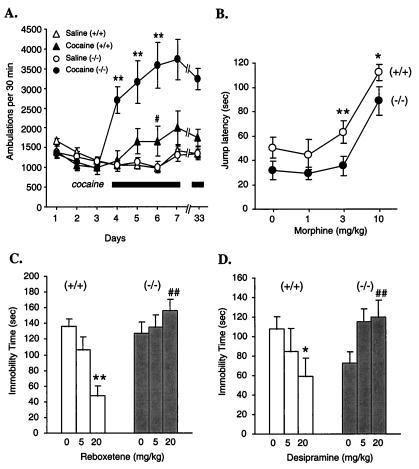
The contribution of Gzα to signaling in the brain was tested by observing responses to cocaine, morphine and two antidepressants that are catecholamine reuptake inhibitors, desipramine and reboxetine. All four of these drugs have been associated, either directly or indirectly, with receptors that are potentially coupled to Gz. Acute administration of cocaine in rodents normally produces motor stimulant effects that increase with repeated administration of the drug, a phenomenon known as behavioral sensitization (28, 29). These effects are thought to be mediated via dopamine D1 and D2 receptors (22–24). There were no differences in baseline locomotor activity between wild-type and Gzα(−/−) mice. However, acute responses to cocaine were greatly enhanced in the absence of Gzα, and this augmented response was maintained throughout the development of behavioral sensitization (Fig. 5A). This suggests that there is a central role for Gz in the pathways that suppress cocaine-induced locomotor response, leading to an increased response in the absence of Gzα.
Figure 5.
Altered behavioral responses. (A) Locomotor activity. Twelve mice of each genotype were injected with saline (i.p.) on days 1–3. On days 4–7, half of the mice in each group received cocaine injections (D, 20 mg/kg, i.p.), followed by a repeat injection on day 33 (10 mg/kg). The other half received saline. ANOVA results: **, P < 0.01 Gzα(−/−) vs. wild-type; #, P < 0.05 for wild-type day 6 vs. day 4. (B) Morphine analgesia. Gzα(+/+) and (−/−) mice were divided into four groups: saline (n = 20), morphine (1.0 mg/kg, n = 14), morphine (3.0 mg/kg, n = 20), and morphine (10 mg/kg, n = 12). Student's t test for Gzα(+/+) vs. Gzα(−/−): *, P < 0.05; **, P < 0.01. (C and D) Antidepressant effects of desipramine and reboxetine in the forced swimming test. The data are presented as mean ± SEM. For the desipramine study: Gzα(+/+), saline n = 15, 5.0 mg/kg, n = 10, 20.0 mg/kg, n = 15; Gzα(−/−), saline n = 14, 5.0 mg/kg, n = 10, 20 mg/kg, n = 14. For the reboxetine study, n = 10 for all groups. *, P < 0.05; **, P < 0.01 vs. respective saline control; ##, P < 0.01 vs. wild-type group under the same treatment condition. ANOVA yielded significant gene × drug interaction terms for desipramine [F(2, 72) = 6.16, P < 0.005] and reboxetine [F(2, 54) = 8.51, P < 0.01].
Responses to morphine were also abnormal, although not as dramatically. Previous studies have suggested that Gzα can mediate the effects of μ-opioid receptors, although it was not entirely clear from those studies how specific and selective this interaction is (30, 31). To test this directly, morphine was administered to wild-type and Gzα(−/−) mice and its analgesic effects were assessed. In wild-type mice, morphine caused a dose-dependent increase in the time that the mice would willingly remain on a 58°C surface. Deletion of Gzα caused a modest shift in the dose/response curve to higher concentrations of morphine (Fig. 5B). This is in contrast to the complete loss of analgesia seen in μ-receptor knockout mice (25) and suggests that μ-opioid receptors either may interact with other G proteins as well or Gz is linked to only a subset of the receptors that are involved in this response.
Finally, because of the diminished response to epinephrine observed in the platelet studies, the behavioral effects of drugs that increase central catecholamine transmission were examined by using a swimming test. Immobility during this test has been shown to be reduced by antidepressant drugs (32). That is, mice given antidepressants persist longer in their attempts to escape from the swimming pool. None of the mice were allowed to drown and the test was ended after 6 min. Two different drugs that are selective norepinephrine reuptake inhibitors were tested. Both desipramine and reboxetine caused a dose-dependent decrease in immobility time in the wild-type mice, but there was a strikingly complete loss of antidepressant effects in the Gzα(−/−) mice (Fig. 5 C and D). This suggests that the receptors which mediate the antidepressant effects of the increased levels of norepinephrine are dependent on Gzα for signaling.
Discussion
Most agonists that initiate platelet activation at sites of vascular injury or serve as neurotransmitters in the brain do so by binding to G protein-coupled receptors, as do many of the drugs that interfere with or promote these processes. Once activated, receptors can potentially interact with any of the heterotrimeric G proteins that are expressed in platelets and neurons. Because G proteins vary in the repertoire of effectors to which they can couple, receptor selectivity among G proteins becomes a major determinant of the events that will occur in response to extracellular events. The ten known members of the Giα family vary in their degree of similarity to each other from approximately 60% for Gzα to >90% for Gi1α, Gi2α, and Gi3α. Presumably, the separate gene products are maintained to serve distinguishable roles or provide distinct patterns of expression. However, it is not always possible to recapitulate these differences in over-expression studies. Aside from dissimilarities in sequence, Gzα is distinguishable from other Giα family members by its limited intrinsic rate of GTP hydrolysis and by its prominent expression in platelets and neurons. Until recently, little has been known about its role in vivo in the cells in which it is normally expressed. To better understand its role and its relationship to the receptors with which it could potentially interact, we have studied platelet function and the effects of psychoactive drugs in mice that lack Gz because the gene for Gzα has been deleted.
Because platelet counts were normal in the Gzα(−/−) mice, Gz does not appear to be required for normal megakaryocyte maturation and platelet formation. However, Gz clearly does play a role in platelet activation by at least one agonist, epinephrine. In platelets from the knockout mice, the ability of epinephrine to inhibit cAMP formation was greatly impaired compared with wild-type littermates. This did not cause an increase in bleeding times in the Gzα(−/−) mice or cause an increased tendency to bleed spontaneously, but it did protect the mice from disseminated thromboembolism when they were injected with collagen plus epinephrine. By comparison, there was no apparent effect on platelet activation by ADP, collagen, U46619, or GYPGQV, and no protection from thrombosis when the mice were injected with collagen plus ADP. It is of interest to compare this result with those obtained with platelets from mice lacking Gi2α or Gqα. A preliminary report shows that loss of Gi2α impairs platelet responses to ADP, another relatively mild phenotype (33). Loss of Gqα, on the other hand, causes a markedly increased bleeding tendency and a failure to respond to most agonists ex vivo—presumably because Gqα is the primary mediator of phospholipase C activation in mouse platelets (19). Notably, the Gqα(−/−) mice, like the Gzα(−/−) mice in the present study, also showed increased resistance to the thromboembolic effects of collagen plus epinephrine.
The requirement of Gz for platelet responses to epinephrine is particularly interesting. The ability of epinephrine to potentiate platelet activation is thought to be due in large part to its ability to inhibit adenylyl cyclase via α2A-adrenergic receptors (34) and small increases in cAMP concentrations have been shown to greatly diminish platelet responses to agonist (35). Yet even under conditions of stress, circulating epinephrine concentrations are not very high. The presence of Gzα in mouse platelets appears to shift the dose/response curve for epinephrine toward the range that platelets are likely to encounter at sites of vascular damage, increasing their sensitivity to epinephrine by two or more orders of magnitude. Because there are no other known receptors for epinephrine on platelets, this shift in the dose/response curve suggests that the selectivity of platelet α2A-adrenergic receptors for G proteins may be determined in part by agonist concentration, and not solely by sequence. It may also explain why in previous experiments with platelet membranes inhibition of cAMP formation by epinephrine was shown to be suppressed by pertussis toxin (36). Under those conditions when high concentrations of epinephrine were used, the physiological effect of Gzα, which is not a pertussis toxin substrate, would be masked by the presence of other pertussis toxin sensitive Giα family members, including, in the case of platelets, Gi2α. Those other family members appear able to substitute for Gzα at high agonist concentrations.
Deletion of Gzα affected not only platelet activation but also the behavioral responses of the mice to psychoactive drugs. All comparisons were made between littermates produced by mating heterozygous mice. In the absence of Gzα, cocaine caused a much more pronounced increase in locomotor activity than in its presence. Studies from knockout mice of D2-dopamine receptor suggest that these receptors play a role in initiating and maintaining normal locomotor behavior (24, 37). The locomotor effects of cocaine have not been reported in D2 receptor knockout mice. However, deletion or blockade of D1 receptors abolishes behavioral sensitization (22, 23). The present results suggest that the Gzα(−/−) mice will provide a good model for understanding the signaling events altered by cocaine. The effects of deleting Gzα on morphine responses were more subtle, but included a rightward shift in the analgesia dose/response curve. Hendry and coworkers (38) have observed an increased tolerance to morphine in Gzα(−/−) mice of a different strain, an observation compatible with ours. No changes were observed in the density of μ and δ opioid receptors or affinity of morphine for its receptors. Finally, one especially striking phenotype of Gzα(−/−) mice is the complete loss of the antidepressant effect to catecholamine reuptake inhibitors. Based on this result, signaling through Gz is required for the antidepressant effect of these drugs, an observation that raises the possibility that patients who fail to respond to antidepressant drugs of this class have defects in signaling through Gz.
Collectively, these observations provide several insights into the role of Gz and the relationships between the members of the Gi family. Gz preferential couples adenylyl cyclase to a biologically and pharmacologically relevant set of G protein-coupled receptors and, at least in the case of platelet α2A-adrenergic receptors, may allow receptors to respond to agonists at far lower concentrations than would otherwise occur. Under baseline conditions, Gzα(−/−) mice were indistinguishable from their wild-type littermates. It was only following specific challenges that differences attributable to the loss of Gzα emerged, protecting them from the effects of prothrombotic agents and altering their responses to behavior-modifying drugs.
Acknowledgments
We thank Danny Liang and Elizabeth Belmonte for their excellent technical assistance and Dr. Mark Kahn for his valuable suggestions. We also thank Dr. Ian Hendry and Kim Kelleher for sharing the results of their own studies on Gzα(−/−) mice before publication. This study was supported by grants from the National Institutes of Health (HL45181, MH36262, and DA11649).
Abbreviations
- EGFP
enhanced green fluorescent protein
- TBS
Tris-buffered saline
- TxA2
thromboxane A2
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.180194597.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.180194597
References
- 1.Arshavsky V Y, Bownds M D. Nature (London) 1992;357:416–417. doi: 10.1038/357416a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee S B, Rhee S G. Curr Opin Cell Biol. 1995;7:183–189. doi: 10.1016/0955-0674(95)80026-3. [DOI] [PubMed] [Google Scholar]
- 3.Hille B, Armstrong C M, MacKinnon R. Nat Med. 1999;5:1105–1109. doi: 10.1038/13415. [DOI] [PubMed] [Google Scholar]
- 4.Meng J, Glick J L, Polakis P, Casey P J. J Biol Chem. 1999;274:36663–36669. doi: 10.1074/jbc.274.51.36663. [DOI] [PubMed] [Google Scholar]
- 5.Mochizuki N, Ohba Y, Kiyokawa E, Kurata T, Murakami T, Ozaki T, Kitabatake A, Nagashima K, Matsuda M. Nature (London) 1999;400:891–894. doi: 10.1038/23738. [DOI] [PubMed] [Google Scholar]
- 6.Rudolph U, Finegold M J, Rich S S, Harriman G R, Srinivasan Y, Brabet P, Boulay G, Bradley A, Birnbaumer L. Nat Genet. 1995;10:143–150. doi: 10.1038/ng0695-143. [DOI] [PubMed] [Google Scholar]
- 7.Hornquist C E, Lu X, Rogers-Fani P M, Rudolph U, Shappell S, Birnbaumer L, Harriman G R. J Immunol. 1997;158:1068–1077. [PubMed] [Google Scholar]
- 8.Jiang M, Boulay G, Spicher K, Peyton M J, Brabet P, Birnbaumer L, Rudolph U. Recept Channels. 1997;5:187–192. [PubMed] [Google Scholar]
- 9.Wong Y H, Conklin B R, Bourne H R. Science. 1992;255:339–342. doi: 10.1126/science.1347957. [DOI] [PubMed] [Google Scholar]
- 10.Casey P J, Fong H K, Simon M I, Gilman A G. J Biol Chem. 1990;265:2383–2390. [PubMed] [Google Scholar]
- 11.Glick J L, Meigs T E, Miron A, Casey P J. J Biol Chem. 1998;273:26008–26013. doi: 10.1074/jbc.273.40.26008. [DOI] [PubMed] [Google Scholar]
- 12.Wang J, Ducret A, Tu Y, Kozasa T, Aebersold R, Ross E M. J Biol Chem. 1998;273:26014–26025. doi: 10.1074/jbc.273.40.26014. [DOI] [PubMed] [Google Scholar]
- 13.Lounsbury K M, Casey P J, Brass L F, Manning D R. J Biol Chem. 1991;266:22051–22056. [PubMed] [Google Scholar]
- 14.Wang J, Frost J A, Cobb M H, Ross E M. J Biol Chem. 1999;274:31641–31647. doi: 10.1074/jbc.274.44.31641. [DOI] [PubMed] [Google Scholar]
- 15.Ioffe E, Liu Y, Bhaumik M, Poirier F, Factor S M, Stanley P. Proc Natl Acad Sci USA. 1995;92:7357–7361. doi: 10.1073/pnas.92.16.7357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manning D R, Brass L F. Thromb Haemostasis. 1991;66:393–399. [PubMed] [Google Scholar]
- 17.Lounsbury K M, Schlegel B, Poncz M, Brass L F, Manning D R. J Biol Chem. 1993;268:3494–3498. [PubMed] [Google Scholar]
- 18.Porsolt R D, Bertin A, Jalfre M. Arch Int Pharmacodyn Ther. 1977;229:327–336. [PubMed] [Google Scholar]
- 19.Offermanns S, Toombs C F, Hu Y H, Simon M I. Nature (London) 1997;389:183–186. doi: 10.1038/38284. [DOI] [PubMed] [Google Scholar]
- 20.DiMinno G, Silver M J. J Pharmacol Exp Ther. 1983;225:57–60. [PubMed] [Google Scholar]
- 21.Fabre J E, Nguyen M, Latour A, Keifer J A, Audoly L P, Coffman T M, Koller B H. Nat Med. 1999;5:1199–1202. doi: 10.1038/13522. [DOI] [PubMed] [Google Scholar]
- 22.Vezina P, Stewart J. Brain Res. 1989;499:108–120. doi: 10.1016/0006-8993(89)91140-2. [DOI] [PubMed] [Google Scholar]
- 23.Drew K L, Glick S D. Psychopharmacology. 1990;101:465–471. doi: 10.1007/BF02244223. [DOI] [PubMed] [Google Scholar]
- 24.Baik J H, Picetti R, Saiardi A, Thiriet G, Dierich A, Depaulis A, Le Meur M, Borrelli E. Nature (London) 1995;377:424–428. doi: 10.1038/377424a0. [DOI] [PubMed] [Google Scholar]
- 25.Matthes H W D, Maldonado R, Simonin F, Valverde O, Slowe S, Kitchen I, Befort K, Dierich A, Le Meur M, Dolle P, et al. Nature (London) 1996;383:819–823. doi: 10.1038/383819a0. [DOI] [PubMed] [Google Scholar]
- 26.Valenzuela D, Han X, Mende U, Fankhauser C, Mashimo H, Huang P, Pfeffer J, Neer E J, Fishman M C. Proc Natl Acad Sci USA. 1997;94:1727–1732. doi: 10.1073/pnas.94.5.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang M, Gold M S, Boulay G, Spicher K, Peyton M, Brabet P, Srinivasan Y, Rudolph U, Ellison G, Birnbaumer L. Proc Natl Acad Sci USA. 1998;95:3269–3274. doi: 10.1073/pnas.95.6.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robinson T E, Becker J B. Brain Res Rev. 1986;11:157–198. doi: 10.1016/s0006-8993(86)80193-7. [DOI] [PubMed] [Google Scholar]
- 29.Kalivas P W, Stewart J. Brain Res Rev. 1991;16:223–244. doi: 10.1016/0165-0173(91)90007-u. [DOI] [PubMed] [Google Scholar]
- 30.Chan J S, Chiu T T, Wong Y H. J Neurochem. 1995;65:2682–2689. doi: 10.1046/j.1471-4159.1995.65062682.x. [DOI] [PubMed] [Google Scholar]
- 31.Gaibelet G, Meilhoc E, Riond J, Saves I, Exner T, Liaubet L, Nurnberg B, Masson J M, Emorine L J. Eur J Biochem. 1999;261:517–523. doi: 10.1046/j.1432-1327.1999.00301.x. [DOI] [PubMed] [Google Scholar]
- 32.Borsini F, Meli A. Psychopharmacology. 1988;94:147–160. doi: 10.1007/BF00176837. [DOI] [PubMed] [Google Scholar]
- 33.Jantzen H M, Milstone D S, Gousset L, Conley P B, Mortensen R. Blood. 1999;94, Suppl. 1:2749. [Google Scholar]
- 34.Hsu C Y, Knapp D R, Halushka P V. J Pharmacol Exp Ther. 1979;208:366–370. [PubMed] [Google Scholar]
- 35.Keularts I M L W, van Gorp R M A, Feijge M A H, Vuist W M J, Heemskert J W M. J Biol Chem. 2000;275:1763–1772. doi: 10.1074/jbc.275.3.1763. [DOI] [PubMed] [Google Scholar]
- 36.Aktories K, Schultz G, Jakobs K H. Naunyn-Schmiedebergs Arch Pharmacol. 1983;324:196–200. doi: 10.1007/BF00503894. [DOI] [PubMed] [Google Scholar]
- 37.Kelly M A, Rubinstein M, Phillips T J, Lessov C N, Burkhart-Kasch S, Zhang G, Bunzow J R, Fang Y, Gerhardt G A, Grandy D K, Low M J. J Neurosci. 1998;18:3470–3479. doi: 10.1523/JNEUROSCI.18-09-03470.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hendry I A, Kelleher K L, Bartlett S E, Leck K J, Reynolds A J, Heydon K, Mellick A, Megirian D, Matthaei K I. Brain Res. 2000;870:10–19. doi: 10.1016/s0006-8993(00)02387-8. [DOI] [PubMed] [Google Scholar]