Abstract
Tocopherols (vitamin E) comprise a class of lipid-soluble antioxidants synthesized only in plants, algae, and some cyanobacteria. The majority of tocopherols in photosynthetic cells is in the α form, which has the highest vitamin E activity in humans, whereas the β, γ, and δ forms normally account for a small percentage of total tocopherols. The antioxidant activities of these forms of tocopherol differ depending on the experimental system, and their relative activities in vivo are unclear. In a screen for suppressors of the xanthophyll-deficient npq1 lor1 double mutant of Chlamydomonas reinhardtii, we isolated a vte3 mutant lacking α-tocopherol but instead accumulating β-tocopherol. The vte3 mutant contains a mutation in the homolog of a 2-methyl-6-phytyl-1,4-benzoquinone methyltransferase gene found in plants. The vte3 npq1 lor1 triple mutant with β-tocopherol survived better under photooxidative stress than did the npq1 lor1 mutant, but the vte3 mutant on its own did not have an obvious phenotype. Following transfer from low light to high light, the triple mutant showed a higher efficiency of photosystem II, a higher level of cell viability, and a lower level of lipid peroxide, a marker for oxidative stress, than did the npq1 lor1 mutant. After high-light transfer, the level of the photosystem II reaction center protein, D1, was also higher in the vte3 npq1 lor1 mutant, but the rate of D1 photodamage was not significantly different from that of the npq1 lor1 mutant. Taken together, these results suggest that the replacement of α-tocopherol by β-tocopherol in a xanthophyll-deficient strain of Chlamydomonas reinhardtii contributes to better survival under conditions of photooxidative stress.
Tocopherols (vitamin E) are amphipathic molecules (Fig. 1) synthesized exclusively in oxygenic photosynthetic organisms. Because tocopherols are essential nutrients in the human diet, their function and chemistry both in vitro and in animal systems have been studied extensively. Tocopherols have been linked to the prevention of diseases such as cancer, atherosclerosis, and neuronal degeneration (41, 52, 56). They function as potent lipid-soluble antioxidants in both plants and animals (44). There are two main antioxidant functions of tocopherols. One function is the scavenging of harmful radicals, especially lipid peroxyl radicals (42). In this reaction, tocopherols donate an electron from the chromanol head group to a lipid peroxyl radical and stop membrane lipid peroxidation chain reactions. Tocopherol itself becomes a radical and is thought to be regenerated by interacting with ascorbate (48). The second antioxidant function of tocopherol is the quenching of singlet oxygen. In animals, singlet oxygen can be generated, for example, by UV-activated endogenous photosensitizers in the skin (39). In plants, singlet oxygen is generated mostly by triplet chlorophyll in photosystem II (25). Aside from being an antioxidant, tocopherol is also involved in non-antioxidant regulatory functions that affect membrane rigidity, transcription, intracellular signaling, photosynthesis, macronutrient starvation, and carbohydrate metabolism (13, 20, 28, 33, 38, 40, 44).
FIG. 1.
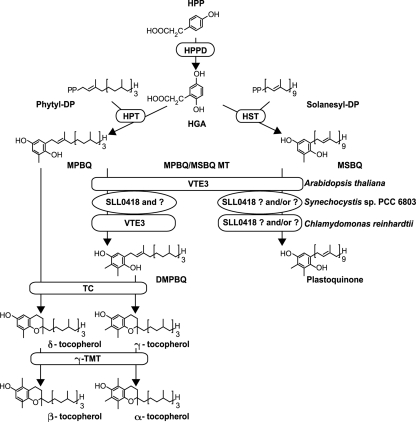
Tocopherol and plastoquinone biosynthetic pathways in A. thaliana, Synechocystis sp. strain PCC 6803, and C. reinhardtii. Differences in the methylation step of MPBQ and MSBQ are shown. Question marks indicate unknown enzymes or that the function of a specific enzyme has not be proven, and the “and/or” indicates a possibility of functional redundancy or that another enzyme is responsible for the function. TMT, γ-tocopherol methyltransferase; HPP, p-hydroxyphenylpyruvate; HPPD, p-hydroxyphenylpyruvate dioxygenase; HPT, homogentisate phytyltransferase; HST, homogentisate solanesyltransferase; MPBQ/MSPQ MT, MPBQ/MSBQ methyltransferase; phytyl-DP, phytyldiphosphate; solanesyl-DP, solanesyldiphosphate; TC, tocopherol cyclase.
Tocopherol comes in four different forms: α, β, γ, and δ. All forms are composed of a hydrophilic chromanol head group synthesized from the shikimate pathway and a lipophilic phytyl side chain synthesized from the isoprenoid pathway (Fig. 1). The differences among the four forms are the numbers and positions of the methyl groups on the head group. α-Tocopherol has three methyl groups, β- and γ-tocopherol have two, and δ-tocopherol has one. The tocopherol biosynthetic pathway has been studied for both the model plant Arabidopsis thaliana and a cyanobacterium, Synechocystis sp. strain PCC 6803 (11). The synthesis of tocopherol is initiated by the addition of a phytyl side chain to the homogentisic acid (HGA) head group, creating 2-methyl-6-phytyl-1,4-benzoquinol (MPBQ) (Fig. 1). The addition of a different side chain to the HGA head group results in the production of 2-methyl-6-solanesyl-1,4-benzoquinol (MSBQ), an intermediate in plastoquinone biosynthesis. In A. thaliana, the methylation of MPBQ and MSBQ is catalyzed by the same enzyme, MPBQ/MSBQ methyltransferase, generating 2,3-dimethyl-5-phytyl-1,4-benzoquinol (DMPBQ) and plastoquinone, respectively. The cyclization of MPBQ and DMPBQ by tocopherol cyclase produces δ- and γ-tocopherol, which are methylated to form β-tocopherol and α-tocopherol, respectively.
α-Tocopherol is the most studied form of tocopherol, because it has the highest vitamin E activity due to its preferential retention by the tocopherol transfer protein in animals (21). α-Tocopherol is also the predominant form in cells of photosynthetic organisms, except in seeds, which contain mostly the γ form. To increase vitamin E content, the tocopherol composition of soybean seeds has been altered by the overexpression of A. thaliana MPBQ/MSBQ methyltransferase together with γ-tocopherol methyltransferase, resulting in the accumulation of α- instead of γ-tocopherol (54). Increasing evidence has led to a better understanding of other forms of tocopherols in biological systems. For instance, it was previously shown that γ-tocopherol is a better antioxidant than α-tocopherol against reactive nitrogen species in liposomes (8). Transgenic tobacco accumulating γ-tocopherol showed a higher tolerance toward sorbitol and methyl viologen but was more sensitive to salt stress than the wild-type strain, which accumulates α-tocopherol (1). The relative antioxidant activity of tocopherols has been shown to be α > β > γ > δ according to data from liposome studies (15). On the other hand, in vitro studies examining the antioxidant activity in fats and oils showed the relative antioxidant activity to be δ > β > γ > α (23). Additionally, the relative physical quenching of singlet oxygen by tocopherols is α ≥ β > γ > δ, whereas the relative chemical quenching is α > γ > δ > β (22). Due to its low chemical reactivity with singlet oxygen, it was previously suggested that β-tocopherol might be a suitable form under conditions where harmful oxidation products may not be readily eliminated (22).
We isolated a Chlamydomonas reinhardtii mutant that accumulates β-tocopherol instead of α-tocopherol. In A. thaliana, defects in MPBQ/MSBQ methyltransferase cause a similar tocopherol phenotype (7). However, because this enzyme is also responsible for the synthesis of plastoquinone in A. thaliana, comparison of the role of β- and α-tocopherol in vivo has been difficult due to the plastoquinone deficiency (7, 32). In Synechocystis sp. strain PCC 6803, a mutant of MPBQ methyltransferase (sll0418) showed no effect on the plastoquinone level (4), but both the tocopherol content and composition were only slightly affected, with only a 35% reduction in the amount of total tocopherol, the majority of which remained in the α form (40). Interestingly, our C. reinhardtii mutant completely lacks α-tocopherol and synthesizes normal levels of plastoquinone. This mutant provides us with an in vivo system to investigate the role of β-tocopherol compared to α-tocopherol in survival under oxidative stress conditions without affecting photosynthesis.
MATERIALS AND METHODS
Strains and growth conditions.
Wild-type Chlamydomonas reinhardtii strain 4A+ (137c background) was used in this work (12). The npq1 lor1 mutant was previously described (35). All strains were maintained on Tris-acetate-phosphate plates (17) at 10 μmol photons m−2 s−1 before being transferred to appropriate conditions for experiments. For all high-performance liquid chromatography (HPLC) analyses and physiological characterizations, cells were grown photoautotrophically in 100 ml liquid high-salt minimal medium (17) to a density of 1 × 106 to 2 × 106 cells/ml in continuous low light (50 μmol photons m−2 s−1) and, when indicated, shifted to high light (500 μmol photons m−2 s−1). Cell viability was measured by plating a known number of cells and counting the number of colonies after 10 days in low light.
HPLC analysis.
Tocopherols were extracted from 2 to 4 ml of cell culture by vortexing the cell pellet in 200 μl acetone at maximum speed for 30 s. Normal-phase HPLC was used to identify the forms of tocopherols that accumulated. The acetone extract was evaporated under N2 gas, and the pellet was resuspended in 200 μl hexane. The hexane extract was filtered through a 2-μm nylon filter, and 25 μl of the extract was subjected to normal-phase HPLC on a 4.6- by 250-mm Luna 5-μm silica column (Phenomenex, Torrance, CA) as described previously (46). Reverse-phase HPLC was used routinely to quantify the amounts of chlorophyll a and tocopherols in all strains. The acetone extract was filtered and subjected to HPLC on a 4.6- by 250-mm Spherisorb S5 ODS1 cartridge column (Waters, Milford, MA) at 30°C as previously described (5). Tocopherols were detected by fluorescence at 325 nm (295-nm excitation). Tocopherol and chlorophyll a concentrations were calculated from standard curves generated from known concentrations of standards.
The same reverse-phase HPLC system was used to detect plastoquinone by reading the absorbance at 255 nm. Cells harvested from 100 ml of culture were lyophilized, and plastoquinone was extracted from 10 to 15 mg of dry pellet in the same manner as described above for tocopherol extraction except that the extraction was done three times. The supernatants were pooled, evaporated under N2 gas, and redissolved in 200 μl acetone. Pure plastoquinone used to generate a standard curve was a gift from D. Creed (University of Southern Mississippi).
Chlorophyll fluorescence and lipid peroxidation measurements.
Samples were taken at the indicated time points for chlorophyll a measurement by spectrophotometry (37). Chlorophyll fluorescence was measured using an FMS2 pulse-amplitude-modulation fluorometer (Hansatech, King's Lynn, United Kingdom) as previously described (6). The number of cells corresponding to 5 μg chlorophyll a were deposited onto a 25-mm-diameter, 12-μm-pore-size nitrocellulose filter (Millipore, Bedford, MA) by filtration and were dark adapted in a moist petri dish for 15 min prior to measurements. Thiobarbituric acid-reactive substances were measured as previously described (5) except that 1 ml of trichloroacetic acid-thiobarbituric acid solution was used per sample.
Genetic analysis.
Genetic crosses and tetrad analysis were performed according to established methods (17). For dominance testing, the allelic arg7-1 and arg7-8 mutations, which exhibit intragenic complementation, were used to select for stable diploid strains on TAP agar medium without arginine (17). For linkage analysis, the mutant was crossed to polymorphic wild-type strain S1-D2 (mt−) (16). An insertion-deletion polymorphism in VTE3 was scored by PCR using a forward primer, 5′-AACCACCTTGAGGTTGGGGTCGTC-3′, and a reverse primer, 5′-CAATGCGAAAGGAGTCGCAACCAT-3′, followed by agarose gel electrophoresis. A marker for the sll0418 gene product homolog was scored by PCR using a forward primer, 5′-GAGCCAACGCGATGGGTGCTAGATG-3′, and a reverse primer, 5′-ATCATGGCGCCTCACAGATTGCATT-3′, followed by digestion with ScrFI and agarose gel electrophoresis.
Complementation with wild-type VTE3.
Plasmid vector pGenD-Ble was used to generate constructs for complementation (14). Full-length VTE3 cDNA was amplified using a forward primer, 5′-GGAATTCCATATGCTTGGGCAATCCCTGCGAGGC-3′, engineered with an NdeI site (underlined) and a reverse primer, 5′-CGGAATTCTTACATCTCCCAGTTCTTGGGCCA-3′, engineered with an EcoRI site (underlined). Similarly, a forward primer, 5′-GGAATTCCATATGCTTGGGCAATCCCTGCGAGGC-3′, and a reverse primer, 5′-CGGAATTCCATCTAGCACCCATCGCGTTGGCTC-3′ (restriction sites are underlined), were used to amplify full-length cDNA of the sll0418 gene product homolog. Vector pMS188, carrying a zeocin resistance gene, was cotransformed to select for transformants (45). Nuclear transformation was carried out as previously described (30). The amounts of vector pGenD-Ble and vector pMS188 used were 1 μg and 300 ng, respectively. Transformants were selected on TAP plates containing 5 μM zeocin, and their tocopherol phenotype was scored by HPLC. Both complemented and noncomplemented transformants were checked by PCR for the presence of the PSAD-VTE3 construct, and only the complemented transformants showed a PCR product on an agarose gel (data not shown).
Isolation of nucleic acids and DNA sequencing.
DNA was isolated from cells grown in 10 ml TAP using DNAzol reagent (Invitrogen) according to the manufacturer's instructions. RNA was isolated as previously described (27). Sequencing of PCR fragments and constructs for transformation was performed as previously described (5). All sequence analyses and alignments were performed using Lasergene software (DNASTAR, Madison, WI).
Preparation of protein extracts, sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and immunoblot analysis.
When indicated, lincomycin was added to cultures to a final concentration of 500 μg/ml. Cells were harvested from 10-ml culture aliquots. Protein extracts were prepared as previously described (5). A sample volume corresponding to 0.5 × 106 cells was loaded into each well of Novex precast 10 to 20% polyacrylamide Tris-glycine gradient gels (Invitrogen, Carlsbad, CA). Proteins were blotted onto a nitrocellulose membrane and probed with anti-D1 antibody, which was a gift from A. Melis (University of California, Berkeley, CA). The reactive protein bands were detected with peroxidase-linked secondary antibody, and the signals were detected by enhanced chemiluminescence (Pierce, Rockford, IL). Signal strength was quantified by use of the ImageJ program (2).
Nucleotide sequence accession numbers.
The GenBank accession numbers for the sequence data reported in this article are EU391265 (C. reinhardtii γ-tocopherol methyltransferase genomic DNA), EU391266 (C. reinhardtii cyanobacterium-type MPBQ/MSBQ methyltransferase complete cDNA), and EU391267 (C. reinhardtii plant-type MPBQ/MSBQ methyltransferase complete cDNA).
RESULTS
Isolation and genetic characterization of vte3.
The npq1 lor1 double mutant is unable to synthesize the carotenoids lutein and zeaxanthin, resulting in photobleaching and cell death in high light (6, 35). Several suppressors of the npq1 lor1 double mutant were identified by their ability to survive in high light (5). HPLC analysis showed that one of the suppressors lacked α-tocopherol and accumulated a different form of tocopherol. The mutation responsible for this phenotype was named vte3. Backcrosses to the npq1 lor1 parent revealed that vte3 was not sufficient for the suppressor phenotype and that a fourth mutation was primarily responsible for the ability to grow in high light. The fourth mutation was eliminated during subsequent backcrossing, and the vte3 mutant in the npq1 lor1 background was then crossed to a wild-type strain five times to isolate isogenic vte3, npq1 lor1, and vte3 npq1 lor1 strains for further characterization.
A normal-phase HPLC column that separates all four forms of tocopherol was used for tocopherol identification and quantitation. Wild-type and npq1 lor1 strains accumulated α-tocopherol with only a small percentage of other tocopherols (Table 1). In contrast, the vte3 and vte3 npq1 lor1 strains completely lacked α-tocopherol but accumulated β-tocopherol with a small amount of δ-tocopherol (Table 1). The total amounts of tocopherol in these strains were similar (Table 1).
TABLE 1.
HPLC analysis of tocopherols and plastoquinonea
| Strain | Mean amt (pmol/106 cells) ± SD (%)b |
Mean plastoquinone level (nmol/g [dry weight]) ± SDc | ||||
|---|---|---|---|---|---|---|
| Total tocopherol | α-Tocopherol | β-Tocopherol | γ-Tocopherol | δ-Tocopherol | ||
| Wild type | 10.1 ± 1.7 | 8.2 ± 1.4 (81) | 0.4 ± 0.1 (4) | 1.0 ± 0.5 (10) | 0.5 ± 0.1 (5) | 294 ± 24 |
| vte3 mutant | 14.0 ± 1.9 | 0 (0) | 13.7 ± 1.8 (98) | 0 (0) | 0.3 ± 0.1 (2) | 318 ± 32 |
| Complemented vte3 mutant | 27.0 ± 5.5 | 25 ± 5.4 (92) | 0.2 ± 0.0 (1) | 1.7 ± 0.1 (6) | 0.1 ± 0.0 (1) | NA |
| npq1 lor1 mutant | 10.0 ± 0.2 | 9.0 ± 0.2 (90) | 0.3 ± 0.1 (4) | 0.5 ± 0.1 (4) | 0.2 ± 0.1 (2) | NA |
| vte3 npq1 lor1 mutant | 10.1 ± 1.0 | 0 (0) | 9.9 ± 1.0 (98) | 0 (0) | 0.2 ± 0.1 (2) | NA |
| VTE3/VTE3 diploid | 29.6 ± 4.3 | 24.3 ± 4.1 (82) | 2.7 ± 1.7 (9) | 2.0 ± 1.3 (7) | 0.6 ± 0.3 (2) | NA |
| vte3/vte3 diploid | 12.3 ± 3.4 | 0 (0) | 11.7 ± 3.1 (95) | 0 (0) | 0.6 ± 0.6 (5) | NA |
| VTE3/vte3 diploid | 36.5 ± 11.3 | 25.5 ± 5.8 (70) | 7.3 ± 4.5 (20) | 2.1 ± 1.5 (6) | 1.6 ± 1.2 (4) | NA |
The data are means ± SD (n = 3 to 6).
The percentage of each tocopherol in the total pool is given in parentheses.
NA, not applicable.
Tetrad analysis of the progeny from backcrosses of vte3 strains to the wild type showed a 2:2 segregation of β-tocopherol and α-tocopherol accumulation based on HPLC of over 50 complete tetrads (data not shown), indicating that the tocopherol phenotype of vte3 is due to a single nuclear mutation. Similar to haploid strains, a wild-type diploid strain accumulated α-tocopherol, and a vte3/vte3 diploid accumulated β-tocopherol (Table 1). A heterozygous vte3/VTE3 strain accumulated mostly α-tocopherol, but a significant amount of β-tocopherol was observed (Table 1), indicating that vte3 is semidominant.
vte3 contains a mutation in a plant-type MPBQ methyltransferase homolog.
The accumulation of β-tocopherol in vte3 suggested a defect in MPBQ methyltransferase. Previously reported studies showed that divergent genes encode this enzyme in plants and cyanobacteria (7). C. reinhardtii is the only known organism so far that contains orthologs of both the plant-type (VTE3) and cyanobacterium-type (sll0418) MPBQ methyltransferase genes. Before the C. reinhardtii genome draft sequence was completed, the two orthologs of MPBQ methyltransferase were found by a similarity search of the EST Database (47). The sequences of the expressed sequence tags were used to design primers to amplify and sequence full-length cDNAs of both genes from wild-type C. reinhardtii. The deduced protein sequences were used to generate alignments with A. thaliana and Synechocystis sp. strain PCC 6803 MPBQ methyltransferases (Fig. 2).
FIG. 2.
Sequence alignment of MPBQ/MSBQ methyltransferases from A. thaliana and Synechocystis sp. strain PCC 6803, their orthologs in C. reinhardtii, and γ-tocopherol methyltransferase from C. reinhardtii. Residues that are identical in at least two of the sequences are shaded in black. Underlined residues indicate the positions of the three S-adenosylmethionine binding domains. The asterisk indicates the position of the vte3 mutation in C. reinhardtii VTE3. γ-TMT, γ-tocopherol methyltransferase.
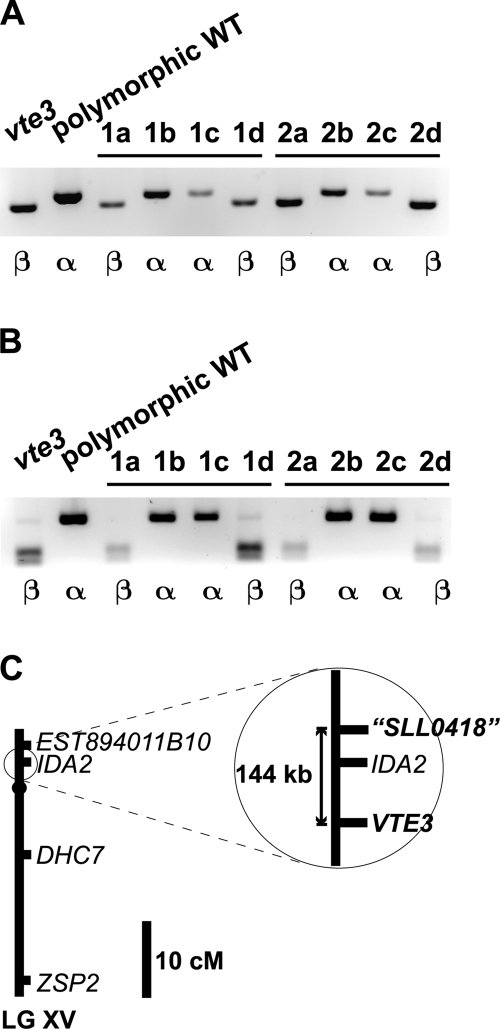
Linkage analysis was performed to determine if either of the two orthologs might be affected in the vte3 mutant. A PCR-based marker was developed for each gene, and over 50 progeny from a cross between the vte3 mutant and a polymorphic wild-type strain were scored for the cosegregation of the markers with the β-tocopherol accumulation phenotype. Unexpectedly, both markers cosegregated with the β-tocopherol accumulation phenotype in all of the progeny (Fig. 3), indicating that the phenotype was closely linked to both orthologs. The two genes were subsequently found to be 144 kb apart on linkage group XV (Fig. 3C). Sequencing cDNAs of both genes from vte3 revealed that the plant-type ortholog carried an A-to-C point mutation in the sixth exon of the gene. This mutation translates into a change of a conserved threonine residue at position 137 to a proline residue (Fig. 2). The cyanobacterium-type ortholog was unaffected.
FIG. 3.
Molecular genetic analysis of vte3. (A and B) Cosegregation analysis of vte3 and the ortholog of A. thaliana VTE3 (A) and the ortholog of the Synechocystis sp. strain PCC 6803 sll0418 gene product (B). The vte3 mutant was crossed to a polymorphic wild-type strain (WT) to obtain haploid progeny. A single-nucleotide polymorphism was scored for each gene. The form of tocopherol accumulated in each strain is indicated by α or β. 1a and 2d are progeny of two complete tetrads. A total of 45 progeny were tested. (C) Relative positions of both orthologs on linkage group XV. The orthologs are located 144 kb apart on linkage group XV. EST894011B10, IDA2, DHC7, and ZSP2 are chromosomal markers. VTE3, A. thaliana ortholog; sll0418, Synechocystis sp. strain PCC 6803 gene product ortholog.
To confirm that the mutation in the plant-type VTE3 gene was responsible for the phenotype observed, a wild-type copy of the gene was transformed into the mutant. The expression of VTE3 cDNA under the control of the PSAD promoter (14) complemented the vte3 mutation, as shown by the restoration of the wild-type tocopherol phenotype albeit with a higher total tocopherol level (Table 1). The higher tocopherol level could be due to the expression vector used, or VTE3 might be a limiting enzyme for α-tocopherol production. No transformants with a construct carrying the cyanobacterium-type gene were complemented. Altogether, these results show that the accumulation of β-tocopherol in vte3 is caused by a mutation in the plant-type MPBQ methyltransferase gene, VTE3.
Analysis of plastoquinone in vte3.
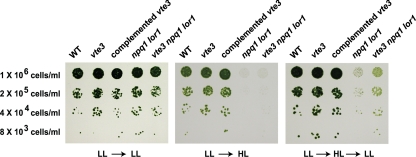
Plant-type MPBQ methyltransferase mutants that lack α-tocopherol and/or accumulate β-tocopherol have been isolated in other model organisms such as A. thaliana and maize (7, 9, 32). Because this enzyme catalyzes both the conversion of MPBQ to DMPBQ in the tocopherol biosynthetic pathway and the conversion of MSBQ to plastoquinone, an electron carrier in the photosynthetic membrane, the mutants also exhibit a decrease or absence of plastoquinone, which leads to a seedling-lethal phenotype. In contrast, the Chlamydomonas vte3 mutant did not show any obvious differences in photoautotrophic growth compared to that of the wild type (Fig. 4), implying that plastoquinone must be present in the mutant. Plastoquinone measurement by HPLC confirmed that the vte3 mutation of C. reinhardtii does not affect plastoquinone synthesis (Table 1).
FIG. 4.
Growth phenotype of C. reinhardtii mutants under different light conditions. Serial dilutions of cells were spotted onto minimal agar medium and grown under conditions of low light for 1 week before being shifted to the indicated conditions. Cells were incubated for 72 h in low light (LL) (left) and high light (HL) (middle). For light shift conditions (right), cells were treated in the same way as they were for high light except that they were later transferred back to low light for 5 days. Low light, 50 μmol photons m−2 s−1; high light, 500 μmol photons m−2 s−1. WT, wild type.
Phenotypes of vte3 under conditions of photooxidative stress.
To investigate the antioxidant function of β-tocopherol in vivo, we examined the growth of vte3 strains under oxidative stress conditions. The vte3 and complemented vte3 strains showed no growth phenotype relative to the wild type in continuous low light or high light or following a shift from low light to high light (Fig. 4). Both the npq1 lor1 and vte3 npq1 lor1 strains were able to grow in low light but underwent photooxidative bleaching in high light. Even though both strains completely bleached in high light, only the vte3 npq1 lor1 strain survived and resumed growth when the plate was shifted back to low light (Fig. 4). Even when left longer in low light, the npq1 lor1 strain never recovered to the same extent as the vte3 npq1 lor1 strain (data not shown).
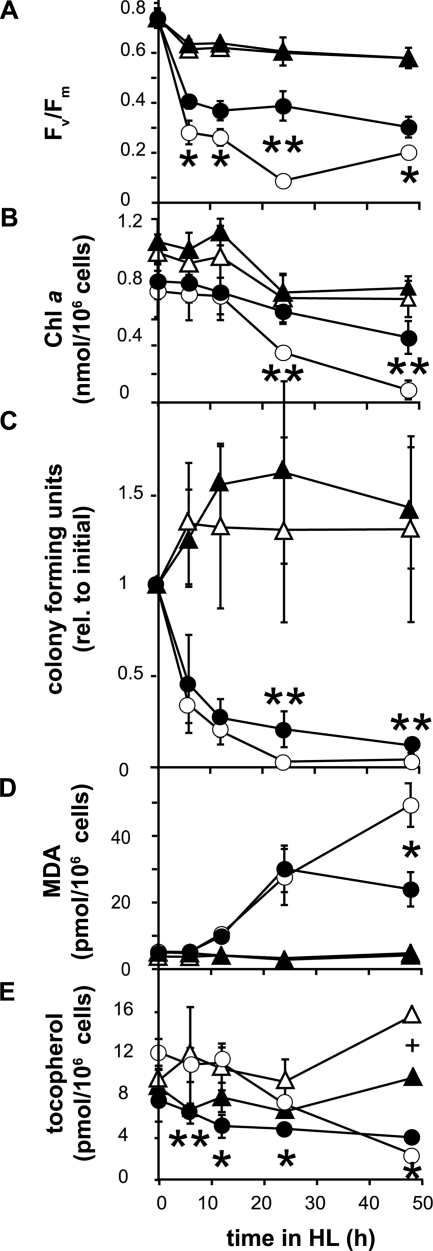
We further explored the underlying basis for the difference in growth phenotypes between the npq1 lor1 and vte3 npq1 lor1 strains. One proposed function of tocopherol is to protect the photosynthetic membrane from photooxidative damage, which can be monitored by photosynthesis parameters and the amount of lipid peroxide produced. Fv/Fm values (a measurement of photosystem II efficiency), the lipid peroxidation level, and cell viability were determined, along with chlorophyll a and tocopherol contents, by using cultures that had been exposed to high-light stress. The wild-type and vte3 strains did not exhibit differences in any of the parameters measured except for the tocopherol content at 48 h. In contrast, the vte3 npq1 lor1 strain showed significant differences in most of the parameters compared to the npq1 lor1 strain. All strains exhibited similar Fv/Fm values before high-light transfer, and they showed an initial decrease in Fv/Fm values during the first 6 h (Fig. 5A). Subsequently, the Fv/Fm value for the vte3 npq1 lor1 strain remained relatively constant, whereas the Fv/Fm value for the npq1 lor1 mutant decreased further, reaching a minimum at 24 h. A loss of chlorophyll a was observed for both the double and the triple mutants upon high-light exposure. Nevertheless, the triple mutant was able to retain a significantly higher chlorophyll a content, especially at later time points (Fig. 5B). The vte3 npq1 lor1 strain also exhibited slightly but significantly higher cell viability than the npq1 lor1 strain (Fig. 5C). Thiobarbituric acid-reactive substances were used to measure the degree of lipid peroxidation of all strains under stress. The wild-type and vte3 strains exhibited a low and constant level of lipid peroxidation (Fig. 5D). The npq1 lor1 double mutant showed a level of lipid peroxidation that was similar to that of the vte3 npq1 lor1 triple mutant at up to 24 h in high light. At 48 h, however, the lipid peroxidation level continued to increase in the double mutant but stayed constant in the triple mutant. The tocopherol content in the wild-type and vte3 strains stayed relatively constant for the first 24 h. At 48 h, however, the levels of tocopherol increased in both strains, with the wild type having a significantly higher tocopherol level. A difference in tocopherol levels between the double and the triple mutants was observed at as early as 6 h until the end of the experiment. The α-tocopherol level in the npq1 lor1 strain followed the same trend as that of chlorophyll a, staying at a constant level for the first 12 h but exhibiting a dramatic decrease at 24 and 48 h. In contrast, the β-tocopherol level in the vte3 npq1 lor1 strain decreased in the first 12 h before remaining relatively constant.
FIG. 5.
Chlorophyll fluorescence, chlorophyll a content, cell viability, lipid peroxidation, and tocopherol content upon high-light (HL) exposure of C. reinhardtii strains. (A) The chlorophyll fluorescence parameter Fv/Fm, representing the maximal photosynthetic efficiency of photosystem II in the dark-adapted state. (B) Chlorophyll (Chl) a content per cell. (C) Cell viability expressed as CFU/ml of culture relative to the initial value (rel. to initial). (D) Lipid peroxidation quantified as malondialdehyde (MDA) equivalents per cell. (E) Tocopherol content per cell. Data shown are means ± standard deviations (n = 4 to 7). Error bars are shown where they are larger than symbols. The differences between the npq1 lor1 and vte3 npq1 lor1 strains were statistically significant (Student's t test) (* indicates a P value of <0.05, and ** indicates a P value of <0.01). The difference in tocopherol contents between the wild-type and vte3 strains at 48 h was statistically significant (Student's t test) (+ indicates a P value of <0.05). Open circles, npq1 lor1 mutant; filled circles, vte3 npq1 lor1 mutant; open triangles, wild type; filled triangles, vte3 mutant.
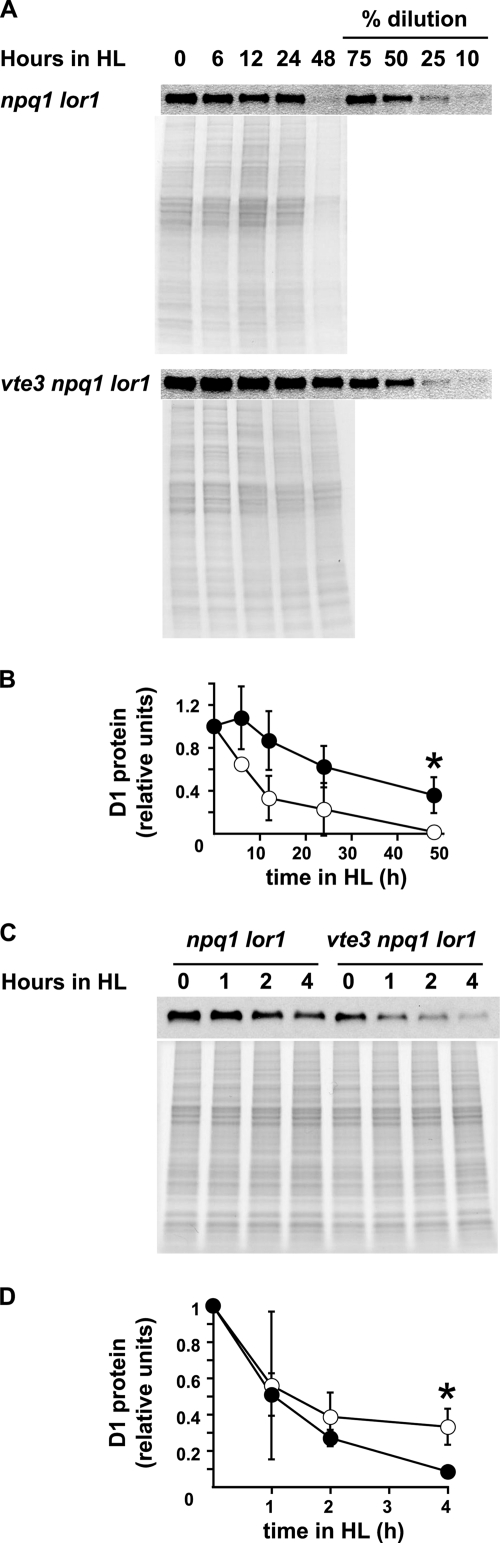
Because tocopherol has been shown to protect the D1 reaction center protein from degradation by singlet oxygen generated in photosystem II during oxidative stress (26, 53), we investigated D1 protection by different forms of tocopherol in the npq1 lor1 and vte3 npq1 lor1 strains. Cultures exposed to high light were used for immunoblotting and quantitation of total D1 (Fig. 6A and B). The double mutant immediately exhibited a steady decrease in levels of total D1 protein after high-light transfer (Fig. 6B). In contrast, the triple mutant showed a delay in the decrease in the D1 level in the first 6 h. After 6 h, the level of D1 in the triple mutant also decreased steadily, as in the double mutant. After 48 h in high light, the steady-state level of D1 was significantly lower in the double mutant than in the triple mutant. To assay D1 photodamage and degradation, a similar experiment was performed with lincomycin added to inhibit the synthesis of new D1 after degradation (Fig. 6C and D). The initial rates of D1 photodamage and degradation were not significantly different between the two strains (Fig. 6D). However, 4 h after lincomycin was added, the amount of D1 was significantly higher in the npq1 lor1 strain than in the vte3 npq1 lor1 strain.
FIG. 6.
Total amount of the D1 protein and its turnover after high-light (HL) transfer. (A) Immunoblot analysis of total amounts of the D1 protein after high-light transfer. Coomassie-stained polyacrylamide gels are shown as loading controls. Samples were taken at the indicated time points. Dilutions were made from the samples at time zero. (B) Quantification of the D1 protein from data shown in A. (C) Immunoblot analysis of the D1 protein after high-light transfer in the presence of lincomycin. A Coomassie-stained polyacrylamide gel is shown as a loading control. (D) Quantification of the D1 protein from data in C. Data shown are means ± standard deviations (n = 3). Error bars are shown where they are larger than the symbols. The differences between the npq1 lor1 and vte3 npq1 lor1 strains were statistically significant (Student's t test) (* indicates a P value of <0.05). Open circles, npq1 lor1 mutant; filled circles, vte3 npq1 lor1 mutant.
DISCUSSION
In A. thaliana, the enzyme MPBQ/MSBQ methyltransferase catalyzes both the conversion of MPBQ to DMPBQ, a precursor of γ- and α-tocopherols, and the conversion of MSBQ to plastoquinone. A mutation in the gene encoding this enzyme therefore affects the synthesis of both molecules. Plastoquinone-deficient mutants of maize and A. thaliana exhibit severe growth defects and seedling lethality (7, 9, 32). They are also α-tocopherol deficient. For cyanobacteria, the situation is different. First, the cyanobacterial enzyme is very diverged from the plant version, with less than 20% amino acid identity between VTE3 from A. thaliana and the sll0418 gene product from Synechocystis sp. strain PCC 6803 (7). Second, Synechocystis sp. strain PCC 6803 sll0418 mutants did not completely lack α-tocopherol or plastoquinone. Instead, the tocopherol content decreased to 35% of the wild-type level, and its composition was only slightly affected, with a small increase in β- and δ-tocopherol levels (40). The plastoquinone level appeared to be unaffected (4). These phenotypes suggest that Synechocystis must possess additional methyltransferases that have activity toward MPBQ and MSBQ. There is also evidence supporting the existence of a second plastoquinone biosynthetic pathway in Synechocystis sp. strain PCC 6803 (10).
C. reinhardtii is the only known organism so far that carries orthologs of both the plant and cyanobacterial MPBQ methyltransferase genes (7). A previously reported study showed that the C. reinhardtii cyanobacterium-type enzyme can use both MPBQ and MSBQ as substrates in vitro (7). Therefore, it seemed likely that this enzyme has a role in both tocopherol and plastoquinone syntheses in this organism. For our study, however, the C. reinhardtii plant-type VTE3 enzyme appears to be the only enzyme functioning as an MPBQ methyltransferase in tocopherol synthesis in vivo, because the vte3 mutant does not accumulate any trace amount of α-tocopherol. The fact that the level of plastoquinone is unaffected in this mutant (Table 1) indicates that a methyltransferase other than VTE3 functions in plastoquinone synthesis or that there is a functional redundancy of methyltransferases in this step of plastoquinone synthesis. One obvious candidate for MSBQ methyltransferase activity is the cyanobacterium-type enzyme. In the Chlamydomonas vte3 background, we generated RNA interference lines targeting the gene encoding the cyanobacterium-type enzyme, but they showed no reduction of the plastoquinone level (data not shown). Three scenarios are then possible. First, it is possible that neither VTE3 nor the cyanobacterium-type enzyme is involved in plastoquinone synthesis. Second, both VTE3 and the cyanobacterium-type enzyme might be involved, but this particular vte3 allele might not affect plastoquinone synthesis. Last, either VTE3 or the cyanobacterium-type enzyme (or both) are normally used for plastoquinone synthesis; however, one or more other methyltransferases can function in plastoquinone synthesis if the other enzyme(s) is absent. Resolution of this issue will require further investigation.
It is not surprising that the vte3 mutation on its own shows no growth phenotype even under conditions of high light. Results from previous studies of other organisms support this observation. Synechocystis sp. strain PCC 6803 and A. thaliana tocopherol mutants lacking all tocopherols or having a reduced level of α-tocopherol also showed no growth phenotype under conditions of high-light treatment (19, 29, 40). Only under extreme conditions, such as a combination of very high light and very low temperature or high light with linoleic or linolenic acid treatment, did the phenotypes emerge. Because tocopherol-deficient mutants showed no phenotype, it was previously suggested that other mechanisms such as the presence of other antioxidants can compensate for the loss of tocopherol (19, 36).
The most plausible mechanism of compensation for a tocopherol deficiency is the antioxidant activity of carotenoids. Both tocopherols and carotenoids have been shown to be essential for preventing lipid peroxidation and protecting photosystem II from singlet-oxygen damage (29, 43). Several studies have demonstrated interactions or overlapping roles of these two groups of lipid-soluble antioxidants. For example, α-tocopherol and zeaxanthin were previously shown to have a synergistic effect on protection against lipid peroxidation in liposomes (55). The A. thaliana npq1 mutant, lacking zeaxanthin and antheraxanthin, is able to grow in high light, but it accumulates larger amounts of α-tocopherol during photoacclimation, suggesting that tocopherol can compensate for the missing carotenoid (18). Conversely, the A. thaliana vte1 mutant, which lacks tocopherol, accumulates larger amounts of zeaxanthin (19). Synechocystis sp. strain PCC 6803 tocopherol mutants, which showed no phenotype in high light, were sensitive to a combination of high light and treatment with a carotenoid synthesis inhibitor, norflurazon (29). The previously characterized C. reinhardtii npq1 lor1 mutant, which lacks the carotenoids lutein and zeaxanthin and is sensitive to photooxidative stress (6, 35), is therefore an ideal background in which to study vte3. The isolation of vte3 in a screen for suppressors of npq1 lor1 and the demonstration of vte3 phenotypes in this background provide further evidence of the relationship between tocopherol and carotenoids.
When exposed to high light, the npq1 lor1 mutant exhibits severe photobleaching and a loss of cell viability, photosystem II function, and the D1 protein, along with increased levels of lipid peroxidation (6). The substitution of α-tocopherol with β-tocopherol in the vte3 npq1 lor1 strain resulted in a partial rescue of these phenotypes (Fig. 4 to 6). The maintenance of photosystem II activity seems to be very sensitive to the form of tocopherol present, as the differences in Fv/Fm values between the npq1 lor1 and vte3 npq1 lor1 strains appeared as early as 6 h after high-light transfer. The Fv/Fm value remained lower in the double mutant than in the triple mutant at all time points, indicating higher photosystem II activity in the triple mutant.
Photosystem II constantly undergoes a damage-and-repair cycle, even under normal light conditions, which does not cause oxidative stress (24). This cycle involves the proteolytic degradation of the damaged D1 protein, followed by the synthesis and assembly of new D1 along with other proteins into photosystem II (3, 31). Under oxidative stress conditions such as high light, the rate of repair cannot keep up with the rate of damage, leading to a process called photoinhibition (50). Several previously reported studies have shown that various stresses, including oxidative stress, inhibit photosystem II repair but do not accelerate the rate of photosystem II damage (34, 49-51). When the npq1 lor1 and vte3 npq1 lor1 strains were exposed to high light in the presence of lincomycin, there was no significant difference in the initial rate of D1 degradation (Fig. 6D). Four hours after lincomycin was added, the vte3 npq1 lor1 strain showed a significantly higher level of D1 degradation than did the npq1 lor1 strain. However, the steady-state level of D1 after high-light exposure in the absence of lincomycin was higher in the vte3 npq1 lor1 mutant (Fig. 6B). Thus, in order to have a higher steady-state level of D1, the accumulation of β-tocopherol in the triple mutant must somehow allow the triple mutant to maintain a higher rate of new D1 synthesis. Although D1 degradation in the presence of lincomycin seemed to be higher in the vte3 npq1 lor1 strain at 4 h than in the npq1 lor1 strain, we cannot conclude that β-tocopherol is a less efficient singlet-oxygen quencher than α-tocopherol because there might be other factors that affect D1 degradation. Moreover, we tested the growth of these two strains in the presence of rose bengal, a singlet-oxygen generator, and found no difference in their sensitivities (data not shown).
Differences in photosystem II inactivation were followed by differences in the loss of chlorophyll, cell viability, lipid peroxidation, and tocopherol (Fig. 5). Interestingly, the loss of tocopherol generally did not follow the same trend as other parameters. The steady-state level of β-tocopherol in the vte3 npq1 lor1 strain decreased initially and then remained constant, whereas the α-tocopherol pool in the npq1 lor1 strain was relatively constant initially and decreased only after 12 h as the cells were bleaching. Assuming that the rate of tocopherol synthesis does not differ between the two strains, the initial decline in β-tocopherol levels in the vte3 npq1 lor1 strain would indicate a higher rate of turnover of tocopherol. Even though the level of β-tocopherol in the vte3 npq1 lor1 strain was much lower than that of α-tocopherol in the npq1 lor1 strain at 12 h, the triple mutant was better able to withstand photooxidative stress than the double mutant. When the wild-type and vte3 strains were compared, the level of β-tocopherol was lower in the vte3 strain at 48 h, but there was no difference in any of the parameters measured even though the difference in the tocopherol level was clear.
There are several possibilities that might explain the better survival of the vte3 npq1 lor1 strain than the npq1 lor1 strain in high light. Damaged photosystem II reaction centers might generate free chlorophyll molecules that photosensitize singlet-oxygen production, leading to further oxidative damage and cell death. It is possible that β-tocopherol is a better quencher of singlet oxygen or scavenger of lipid peroxyl radicals than α-tocopherol in vivo. A previous study reported that β-tocopherol physically quenches singlet oxygen in vitro with the same efficiency as that of α-tocopherol but exhibits very little chemical reactivity (22). This might allow β-tocopherol to avoid direct oxidation by singlet oxygen, thereby maintaining its capacity for physical quenching. This could be particularly important in the npq1 lor1 background, which shows an increased generation of singlet oxygen in high light (6, 27). Alternatively, the two forms of tocopherol might activate different gene expressions in response to oxidative stress. We cannot exclude the possibility that it is the absence of α-tocopherol, rather than the presence of β-tocopherol, that contributed to the better survival of the triple mutant. Future experiments will address these possibilities and explore the specific molecular mechanisms involved. Nevertheless, it is clear from our results that the accumulation of β-tocopherol instead of α-tocopherol in a xanthophyll-deficient mutant confers greater resistance to photooxidative stress in vivo.
Acknowledgments
We thank David Creed for the gift of plastoquinone, Anastasios Melis for providing the anti-D1 antibody, and Beat Fischer for critical reading of the manuscript.
This work was supported by a grant from the National Institutes of Health (grant GM58799) to K.K.N.
Footnotes
Published ahead of print on 28 August 2009.
REFERENCES
- 1.Abbasi, A. R., M. Hajirezaei, D. Hofius, U. Sonnewald, and L. M. Voll. 2007. Specific roles of α- and γ-tocopherol in abiotic stress responses of transgenic tobacco. Plant Physiol. 143:1720-1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abramoff, M. D., P. J. Magelhaes, and S. J. Ram. 2004. Image processing with ImageJ. Biophoton. Int. 11:36-42. [Google Scholar]
- 3.Aro, E.-M., I. Virgin, and B. Andersson. 1993. Photoinhibition of photosystem II. Inactivation, protein damage and turnover. Biochim. Biophys. Acta 1143:113-134. [DOI] [PubMed] [Google Scholar]
- 4.Backasch, N., R. Schulz-Friedrich, and J. Appel. 2005. Influences on tocopherol biosynthesis in the cyanobacterium Synechocystis sp. PCC 6803. J. Plant Physiol. 162:758-766. [DOI] [PubMed] [Google Scholar]
- 5.Baroli, I., A. D. Do, T. Yamane, and K. K. Niyogi. 2003. Zeaxanthin accumulation in the absence of a functional xanthophyll cycle protects Chlamydomonas reinhardtii from photooxidative stress. Plant Cell 15:992-1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baroli, I., B. L. Gutman, H. K. Ledford, J. W. Shin, B. L. Chin, M. Havaux, and K. K. Niyogi. 2004. Photo-oxidative stress in a xanthophyll-deficient mutant of Chlamydomonas. J. Biol. Chem. 279:6337-6344. [DOI] [PubMed] [Google Scholar]
- 7.Cheng, Z. G., S. Sattler, H. Maeda, Y. Sakuragi, D. A. Bryant, and D. DellaPenna. 2003. Highly divergent methyltransferases catalyze a conserved reaction in tocopherol and plastoquinone synthesis in cyanobacteria and photosynthetic eukaryotes. Plant Cell 15:2343-2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christen, S., A. A. Woodall, M. K. Shigenaga, P. T. Southwell-Keely, M. W. Duncan, and B. N. Ames. 1997. γ-Tocopherol traps mutagenic electrophiles such as NOx and complements α-tocopherol: physiological implications. Proc. Natl. Acad. Sci. USA 94:3217-3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cook, W. B., and D. Miles. 1992. Nuclear mutations affecting plastoquinone accumulation in maize. Photosynth. Res. 31:99-111. [DOI] [PubMed] [Google Scholar]
- 10.Dähnhardt, D., J. Falk, J. Appel, T. A. W. van der Kooij, R. Schulz-Friedrich, and K. Krupinska. 2002. The hydroxyphenylpyruvate dioxygenase from Synechocystis sp. PCC 6803 is not required for plastoquinone biosynthesis. FEBS Lett. 523:177-181. [DOI] [PubMed] [Google Scholar]
- 11.DellaPenna, D. 2005. Progress in the dissection and manipulation of vitamin E synthesis. Trends Plant Sci. 10:574-579. [DOI] [PubMed] [Google Scholar]
- 12.Dent, R. M., C. M. Haglund, B. L. Chin, M. C. Kobayashi, and K. K. Niyogi. 2005. Functional genomics of eukaryotic photosynthesis using insertional mutagenesis of Chlamydomonas reinhardtii. Plant Physiol. 137:545-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dörmann, P. 2007. Functional diversity of tocochromanols in plants. Planta 225:269-276. [DOI] [PubMed] [Google Scholar]
- 14.Fischer, N., and J. D. Rochaix. 2001. The flanking regions of psaD drive efficient gene expression in the nucleus of the green alga Chlamydomonas reinhardtii. Mol. Genet. Genomics 265:888-894. [DOI] [PubMed] [Google Scholar]
- 15.Fukuzawa, K., A. Tokumura, S. Ouchi, and H. Tsukatani. 1982. Antioxidant activities of tocopherols on Fe2+-ascobate-induced lipid-peroxidation in lecithin liposomes. Lipids 17:511-513. [DOI] [PubMed] [Google Scholar]
- 16.Gross, C. H., L. P. W. Ranum, and P. A. Lefebvre. 1988. Extensive restriction fragment length polymorphisms in a new isolate of Chlamydomonas reinhardtii. Curr. Genet. 13:503-508. [DOI] [PubMed] [Google Scholar]
- 17.Harris, E. H. 1989. The Chlamydomonas sourcebook: a comprehensive guide to biology and laboratory use. Academic Press, San Diego, CA. [DOI] [PubMed]
- 18.Havaux, M., J.-P. Bonfils, C. Lütz, and K. K. Niyogi. 2000. Photodamage of the photosynthetic apparatus and its dependence on the leaf developmental stage in the npq1 Arabidopsis mutant deficient in the xanthophyll cycle enzyme violaxanthin de-epoxidase. Plant Physiol. 124:273-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Havaux, M., F. Eymery, S. Porfirova, P. Rey, and P. Dörmann. 2005. Vitamin E protects against photoinhibition and photooxidative stress in Arabidopsis thaliana. Plant Cell 17:3451-3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hofius, D., M.-R. Hajirezaei, M. Geiger, H. Tschiersch, M. Melzer, and U. Sonnewald. 2004. RNAi-mediated tocopherol deficiency impairs photoassimilate export in transgenic potato plants. Plant Physiol. 135:1256-1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hosomi, A., M. Arita, Y. Sato, C. Kiyose, T. Ueda, O. Igarashi, H. Arai, and K. Inoue. 1997. Affinity for α-tocopherol transfer protein as a determinant of the biological activities of vitamin E analogs. FEBS Lett. 409:105-108. [DOI] [PubMed] [Google Scholar]
- 22.Kaiser, S., P. Di Mascio, M. E. Murphy, and H. Sies. 1990. Physical and chemical scavenging of singlet molecular oxygen by tocopherols. Arch. Biochem. Biophys. 277:101-108. [DOI] [PubMed] [Google Scholar]
- 23.Kamal-Eldin, A., and L. A. Appelqvist. 1996. The chemistry and antioxidant properties of tocopherols and tocotrienols. Lipids 31:671-701. [DOI] [PubMed] [Google Scholar]
- 24.Keren, N., H. Gong, and I. Ohad. 1995. Oscillations of reaction center II-D1 protein degradation in vivo induced by repetitive light flashes. J. Biol. Chem. 270:806-814. [DOI] [PubMed] [Google Scholar]
- 25.Krieger-Liszkay, A. 2005. Singlet oxygen production in photosynthesis. J. Exp. Bot. 56:337-346. [DOI] [PubMed] [Google Scholar]
- 26.Kruk, J., H. Hollander-Czytko, W. Oettmeier, and A. Trebst. 2005. Tocopherol as singlet oxygen scavenger in photosystem II. J. Plant Physiol. 162:749-757. [DOI] [PubMed] [Google Scholar]
- 27.Ledford, H. K., I. Baroli, J. W. Shin, B. B. Fischer, R. I. L. Eggen, and K. K. Niyogi. 2004. Comparative profiling of lipid-soluble antioxidants and transcripts reveals two phases of photo-oxidative stress in a xanthophyll-deficient mutant of Chlamydomonas reinhardtii. Mol. Genet. Genomics 272:470-479. [DOI] [PubMed] [Google Scholar]
- 28.Maeda, H., and D. DellaPenna. 2007. Tocopherol functions in photosynthetic organisms. Curr. Opin. Plant Biol. 10:260-265. [DOI] [PubMed] [Google Scholar]
- 29.Maeda, H., Y. Sakuragi, D. A. Bryant, and D. DellaPenna. 2005. Tocopherols protect Synechocystis sp. strain PCC 6803 from lipid peroxidation. Plant Physiol. 138:1422-1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCarthy, S. S., M. C. Kobayashi, and K. K. Niyogi. 2004. White mutants of Chlamydomonas reinhardtii are defective in phytoene synthase. Genetics 168:1249-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Melis, A. 1999. Photosystem-II damage and repair cycle in chloroplasts: what modulates the rate of photodamage in vivo? Trends Plant Sci. 4:130-135. [DOI] [PubMed] [Google Scholar]
- 32.Motohashi, R., T. Ito, M. Kobayashi, T. Taji, N. Nagata, T. Asami, S. Yoshida, K. Yamaguchi-Shinozaki, and K. Shinozaki. 2003. Functional analysis of the 37 kDa inner envelope membrane polypeptide in chloroplast biogenesis using a Ds-tagged Arabidopsis pale-green mutant. Plant J. 34:719-731. [DOI] [PubMed] [Google Scholar]
- 33.Munné-Bosch, S., and L. Alegre. 2002. The function of tocopherols and tocotrienols in plants. Crit. Rev. Plant Sci. 21:31-57. [Google Scholar]
- 34.Nishiyama, Y., S. I. Allakhverdiev, and N. Murata. 2006. A new paradigm for the action of reactive oxygen species in the photoinhibition of photosystem II. Biochim. Biophys. Acta 1757:742-749. [DOI] [PubMed] [Google Scholar]
- 35.Niyogi, K. K., O. Björkman, and A. R. Grossman. 1997. The roles of specific xanthophylls in photoprotection. Proc. Natl. Acad. Sci. USA 94:14162-14167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Porfirova, S., E. Bergmüller, S. Tropf, R. Lemke, and P. Dörmann. 2002. Isolation of an Arabidopsis mutant lacking vitamin E and identification of a cyclase essential for all tocopherol biosynthesis. Proc. Natl. Acad. Sci. USA 99:12495-12500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Porra, R. J., W. A. Thompson, and P. E. Kriedemann. 1989. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim. Biophys. Acta 975:384-394. [Google Scholar]
- 38.Provencher, L. M., L. Miao, N. Sinha, and W. J. Lucas. 2001. Sucrose export defective1 encodes a novel protein implicated in chloroplast-to-nucleus signaling. Plant Cell 13:1127-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Redmond, R. W., and I. E. Kochevar. 2006. Spatially resolved cellular responses to singlet oxygen. Photochem. Photobiol. 82:1178-1186. [DOI] [PubMed] [Google Scholar]
- 40.Sakuragi, Y., H. Maeda, D. DellaPenna, and D. A. Bryant. 2006. α-Tocopherol plays a role in photosynthesis and macronutrient homeostasis of the cyanobacterium Synechocystis sp. PCC 6803 that is independent of its antioxidant function. Plant Physiol. 141:508-521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saldeen, K., and T. Saldeen. 2005. Importance of tocopherols beyond alpha-tocopherol: evidence from animal and human studies. Nutr. Res. 25:877-889. [Google Scholar]
- 42.Sattler, S. E., Z. G. Cheng, and D. DellaPenna. 2004. From Arabidopsis to agriculture: engineering improved vitamin E content in soybean. Trends Plant Sci. 9:365-367. [DOI] [PubMed] [Google Scholar]
- 43.Sattler, S. E., L. U. Gilliland, M. Magallanes-Lundback, M. Pollard, and D. DellaPenna. 2004. Vitamin E is essential for seed longevity, and for preventing lipid peroxidation during germination. Plant Cell 16:1419-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schneider, C. 2005. Chemistry and biology of vitamin E. Mol. Nutr. Food Res. 49:7-30. [DOI] [PubMed] [Google Scholar]
- 45.Schroda, M., C. F. Beck, and O. Vallon. 2002. Sequence elements within an HSP70 promoter counteract transcriptional transgene silencing in Chlamydomonas. Plant J. 31:445-455. [DOI] [PubMed] [Google Scholar]
- 46.Shintani, D. K., Z. G. Cheng, and D. DellaPenna. 2002. The role of 2-methyl-6-phytylbenzoquinone methyltransferase in determining tocopherol composition in Synechocystis sp. PCC6803. FEBS Lett. 511:1-5. [DOI] [PubMed] [Google Scholar]
- 47.Shrager, J., C. Hauser, C.-W. Chang, E. H. Harris, J. Davies, J. McDermott, R. Tamse, Z. Zhang, and A. R. Grossman. 2003. Chlamydomonas reinhardtii genome project. A guide to the generation and use of cDNA information. Plant Physiol. 131:401-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smirnoff, N., and G. L. Wheeler. 2000. Ascorbic acid in plants: biosynthesis and function. Crit. Rev. Plant Sci. 19:267-290. [DOI] [PubMed] [Google Scholar]
- 49.Takahashi, S., H. Bauwe, and M. Badger. 2007. Impairment of the photorespiratory pathway accelerates photoinhibition of photosystem II by suppression of repair but not acceleration of damage processes in Arabidopsis. Plant Physiol. 144:487-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Takahashi, S., and N. Murata. 2008. How do environmental stresses accelerate photoinhibition? Trends Plant Sci. 13:178-182. [DOI] [PubMed] [Google Scholar]
- 51.Takahashi, S., T. Nakamura, M. Sakamizu, R. van Woesik, and H. Yamasaki. 2004. Repair machinery of symbiotic photosynthesis as the primary target of heat stress for reef-building corals. Plant Cell Physiol. 45:251-255. [DOI] [PubMed] [Google Scholar]
- 52.Terasawa, Y., Z. Ladha, S. W. Leonard, J. D. Morrow, D. Newland, D. Sanan, L. Packer, M. G. Traber, and R. V. Farese. 2000. Increased atherosclerosis in hyperlipidemic mice deficient in α-tocopherol transfer protein and vitamin E. Proc. Natl. Acad. Sci. USA 97:13830-13834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Trebst, A., B. Depka, and H. Hollander-Czytko. 2002. A specific role for tocopherol and of chemical singlet oxygen quenchers in the maintenance of photosystem II structure and function in Chlamydomonas reinhardtii. FEBS Lett. 516:156-160. [DOI] [PubMed] [Google Scholar]
- 54.Van Eenennaam, A. L., K. Lincoln, T. P. Durrett, H. E. Valentin, C. K. Shewmaker, G. M. Thorne, J. Jiang, S. R. Baszis, C. K. Levering, E. D. Aasen, M. Hao, J. C. Stein, S. R. Norris, and R. L. Last. 2003. Engineering vitamin E content: from Arabidopsis mutant to soy oil. Plant Cell 15:3007-3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wrona, M., M. Różanowska, and T. Sarna. 2004. Zeaxanthin in combination with ascorbic acid or α-tocopherol protects ARPE-19 cells against photosensitized peroxidation of lipids. Free Radic. Biol. Med. 36:1094-1101. [DOI] [PubMed] [Google Scholar]
- 56.Yokota, T., K. Igarashi, T. Uchihara, K. Jishage, H. Tomita, A. Inaba, Y. Li, M. Arita, H. Suzuki, H. Mizusawa, and H. Arai. 2001. Delayed-onset ataxia in mice lacking α-tocopherol transfer protein: model for neuronal degeneration caused by chronic oxidative stress. Proc. Natl. Acad. Sci. USA 98:15185-15190. [DOI] [PMC free article] [PubMed] [Google Scholar]