SUMMARY
Heterozygous mutations of KCNA1, the gene encoding potassium channel Kv1.1 subunits, cause episodic ataxia type 1 (EA1), which is characterized by paroxysmal cerebellar incoordination and interictal myokymia. Some mutations are also associated with epilepsy. Although Kv1.1-containing potassium channels play important roles in neuronal excitability and neurotransmitter release, it is not known how mutations associated with different clinical features affect the input-output relationships of individual neurons. We transduced rat hippocampal neurons, which were cultured on glial micro-islands, with lentiviruses expressing wild-type or mutant human KCNA1, and injected either depolarizing currents to evoke action potentials or depolarizing voltage commands to evoke autaptic currents. α-Dendrotoxin and tetraethylammonium allowed a pharmacological dissection of potassium currents underlying excitability and neurotransmission. Overexpression of wild-type Kv1.1 decreased both neuronal excitability and neurotransmitter release. By contrast, the C-terminus-truncated R417stop mutant, which is associated with severe drug-resistant EA1, had the opposite effect: increased excitability and release probability. Another mutant, T226R, which is associated with EA1 that is complicated by contractures and epilepsy, had no detectable effect on neuronal excitability; however, in common with R417stop, it markedly enhanced neurotransmitter release. The results provide direct evidence that EA1 mutations increase neurotransmitter release, and provide an insight into mechanisms underlying the phenotypic differences that are associated with different mutations.
INTRODUCTION
Heterozygous mutations of KCNA1, which encodes the potassium channel subunit Kv1.1, cause episodic ataxia type 1 (EA1) (Browne et al., 1994), a dominantly inherited disorder that is characterized by paroxysmal cerebellar incoordination and interictal myokymia (Rajakulendran et al., 2007). Distinct mutations are associated with variable manifestations including cramps, contractures, titubation and seizures (Zuberi et al., 1999; Eunson et al., 2000). Kv1.1 is widely expressed throughout the nervous system (Vacher et al., 2008) and assembles with other Kv1 members (Wang et al., 1993). Kv1.1-containing channels exhibit variable kinetics depending on the other pore-forming subunits and associated cytoplasmic β subunits (Rettig et al., 1994; Lai and Jan, 2006). The functional consequences of KCNA1 mutations have been examined by expression of mutant and/or wild-type (WT) Kv1.1 in non-neuronal cells (Adelman et al., 1995; D’Adamo et al., 1998; Zerr et al., 1998a; Zerr et al., 1998b; Zuberi et al., 1999; Eunson et al., 2000; Bretschneider et al., 1999; Boland et al., 1999; Rea et al., 2002). Mutations confer a loss of Kv1.1 function through altered kinetics or reduced current density, in some cases with impaired trafficking (Rea et al., 2002; Manganas et al., 2001). The variable clinical severity of distinct mutations correlates imperfectly with their in vitro consequences (Eunson et al., 2000; Rea et al., 2002). It is not known how mutations affect the normal role of Kv1.1 in modulating neuronal firing and neurotransmitter release (Geiger and Jonas, 2000; Kole et al., 2007). A mouse knock-in model of EA1 exhibits stress-induced incoordination and increased γ-aminobutyric acid (GABA) release at interneuron-Purkinje cell synapses (Herson et al., 2003). Although this suggests that cerebellar dysfunction in EA1 is related to enhanced neurotransmission, it remains to be determined whether this is a feature of all EA1 mutations.
In order to compare the effects of overexpressing WT or mutant Kv1.1 on neuronal functions, we used lentiviral particles to express heterologous Kv1.1 channels in hippocampal neurons that were grown on glial micro-islands. This well-characterized preparation allows neurons to form synapses back on themselves (autapses), and consequently permits neurotransmitter release to be studied. We compared the consequences of overexpression of WT Kv1.1 with that of the R417stop mutation (RX), which is associated with severe drug-resistant EA1 (Eunson et al., 2000), and T226R (TR), which is associated with severe muscle contractures and, in some affected individuals, epilepsy (Zuberi et al., 1999; Kinali et al., 2004).
RESULTS
Previous heterologous expression studies have shown dominant negative effects of both RX and TR (Rea et al., 2002). Moreover, it has been argued that RX may prevent the surface expression of co-expressed WT subunits (Manganas et al., 2001; Rea et al., 2002) (although see Zhu et al., 2007). We therefore asked whether the different lentivirus-delivered constructs altered the subcellular distribution of Kv1.1. Because the protein sequences for rat and human Kv1.1 are 98% identical, the interactions between the mutant and endogenous WT subunits are unlikely to differ appreciably from those that occur in heterozygous individuals. We used an antibody that recognizes a cytoplasmic C-terminal epitope (409–495) that extends beyond the last amino acid in the truncated RX mutant. We first verified that the antibody detects full-length Kv1.1, but not RX (supplementary material Fig. S1). Hippocampal neurons transduced with WT, TR or RX, as well as control (CT) untransduced neurons, showed indistinguishable Kv1.1 immunofluorescence (Fig. 1A). Notably, intracellular retention of endogenous Kv1.1 was not detected with RX expression, suggesting that lentiviral expression does not lead to the pathological inclusions that have been detected with other methods (Manganas et al., 2001).
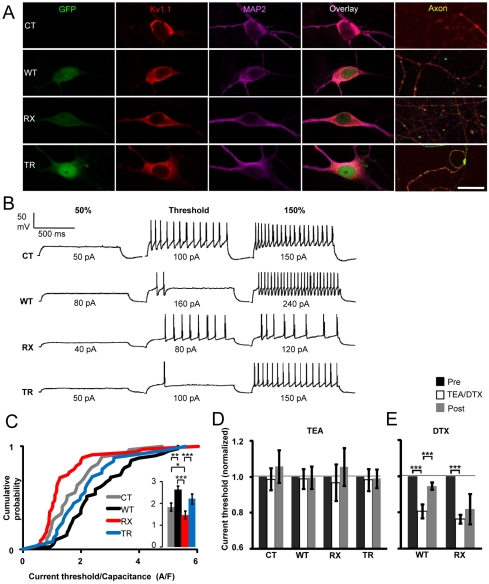
Fig. 1.
Kv1.1 manipulation affects excitability. (A) Lentiviral expression of Kv1.1 does not alter overall Kv1.1 distribution in neurons after 15 days in vitro. CT, WT, RX and TR neurons stained for Kv1.1 (red, second column) and the dendrite marker microtubule associated protein 2 (MAP2) (magenta, third column). Transduction was verified by green fluorescent protein (GFP) expression (first column), which was driven by a separate promoter. Kv1.1 was primarily present in the perikaryal and somato-dendritic compartments but was also found in axons (defined by the absence of MAP2 staining, fifth column). Aggregation of endogenous Kv1.1 in the endoplasmic reticulum (ER) was not observed in any group. Bar, 25 μ m (somato-dendritic panels), 120 μ m (axonal panels). (B) WT and RX Kv1.1 have opposite effects on neuronal excitability. Example traces from CT neurons and neurons expressing WT, RX and TR Kv1.1 at 50%, 100% and 150% of the current threshold. The scale bar applies to all traces. (C) Cumulative probability plot showing an increase in the current threshold (normalized by capacitance) for neurons expressing WT Kv1.1 (black, n=43) compared with CT neurons (gray, n=29), and a reduction in the current threshold for RX-expressing neurons (red, n=36) compared with CT, WT and TR (blue, n=27) neurons. Inset: mean±s.e.m. current threshold/capacitance (using the same color code), based on the results of the Mann-Whitney U-test (*P≤0.05, **P≤0.01, ***P≤0.001). (D) TEA (1 mM) had no consistent effect on the current threshold in any group. (E) α-DTX (100 nM) reduced the current threshold (normalized by capacitance) in both WT- and RX-expressing neurons (paired t-tests).
We examined action potential generation by injecting 1-second-long depolarizing currents of increasing amplitudes (Fig. 1B). No significant difference was seen between CT neurons and the neurons expressing different Kv1.1 constructs for any of: resting membrane potential, capacitance, input resistance, voltage threshold or spike amplitude (supplementary material Table S1). However, the minimal current required to fire neurons varied systematically: the current threshold, which was normalized by cell capacitance to control for differences in cell size, was larger in WT-expressing neurons (2.62±0.18 A/F, n=43, P<0.01, Mann-Whitney U-test) and smaller in RX-expressing neurons (1.47±0.18 A/F, n=36, P<0.05) than in CT untransduced neurons (1.84±0.18 A/F, n=29) (Fig. 1C). Surprisingly, in contrast to RX, cells expressing TR showed no difference in current threshold from WT-expressing cells (2.21±0.23 A/F, n=27). Thus, the two EA1 mutations have discordant effects on neuronal excitability.
A possible interpretation of the above results is that Kv1.1 subunits contribute to setting the current threshold (Brew et al., 2003), and that WT increases their abundance, whereas RX (but not TR) exerts a dominant negative effect (Rea et al., 2002). The preserved Kv1.1 staining in RX-transduced neurons (Fig. 1A) would be consistent with this interpretation if much of the endogenous Kv1.1 is intracellular, and/or if heteromeric channels containing RX and endogenous Kv1.1 subunits at the surface are dysfunctional. We examined the role of Kv1.1 in neuronal excitability by applying tetraethylammonium (TEA), which blocks homomeric Kv1.1 channels. The WT, RX and TR transgenes all carried the Y379V mutation, which reduces TEA sensitivity by approximately 600-fold (Rea et al., 2002), to discriminate between endogenous and lentivirus-expressed Kv1.1 subunits. TEA (1 mM) had no effect on the current threshold in any group, including CT neurons (Fig. 1D), although it profoundly affected spike width (see below). This implies that homomeric Kv1.1 channels do not contribute substantially to neuronal excitability. However, the results do not exclude a role for heteromeric channels that contain Kv1.1 together with other Kv1 subunits, which normally lack the extracellular tyrosine residue required for TEA binding. Indeed, 1 mM TEA should only reduce the current carried by a channel containing two Kv1.1 subunits by about 10% (Rea et al., 2002).
We next examined the effect of α-dendrotoxin (α-DTX), which blocks channels containing Kv1.1, Kv1.2 or Kv1.6 (Grupe et al., 1990; Grissmer et al., 1994). We restricted attention to WT- and RX-transduced neurons, because these exhibited the lowest and highest excitability, respectively. α-DTX (100 nM) reduced the current threshold, normalized by capacitance, in both groups of neurons [P<0.001, paired t-test, for both WT (0.81±0.04 of control, n=11) and RX (0.76±0.02, n=6)] (Fig. 1E). Taken together, these results suggest that Kv1.1 affects neuronal excitability through interaction with other Kv1 subunits, including Kv1.2 and Kv1.6, which co-assemble with Kv1.1 (Vacher et al., 2008; Wang et al., 1999).
Does Kv1.1 only shunt the injected current? If this hypothesis were correct, injecting 150% of the threshold current should cause neurons in all groups to fire at similar rates. However, neurons that overexpressed WT Kv1.1 fired almost twofold faster (28.0±2.9 Hz, n=16) than CT (16.6±2.4 Hz, n=15) or TR-transduced neurons (15.9±3.4 Hz, n=7) (Fig. 2A). RX-transduced neurons, by contrast, fired at a lower frequency (12.3±1.4 Hz, n=15), although this did not reach significance. The higher firing rate of WT-overexpressing neurons was accompanied by a shorter delay to the first spike (Fig. 2B) and a shorter afterhyperpolarization (AHP) decay time (supplementary material Fig. S2). RX-expressing neurons again exhibited a trend in the opposite direction, with a lower firing rate, longer delay to the first spike (Fig. 2A,B), significantly lower AHP amplitude and longer AHP decay time (supplementary material Fig. S2). These effects can be explained by a faster rate of membrane charging in WT-transduced neurons and a slower rate of charging in RX-transduced neurons. Alternatively, Kv1.1-mediated repolarization may limit Na+ channel inactivation, analogous to the role of Kv3 channels in fast-spiking interneurons (Martina et al., 1998; Lau et al., 2000). However, α-DTX failed to affect spike frequency, spike delay or AHP duration, arguing against a privileged role of Kv1.1 in allowing rapid spiking.
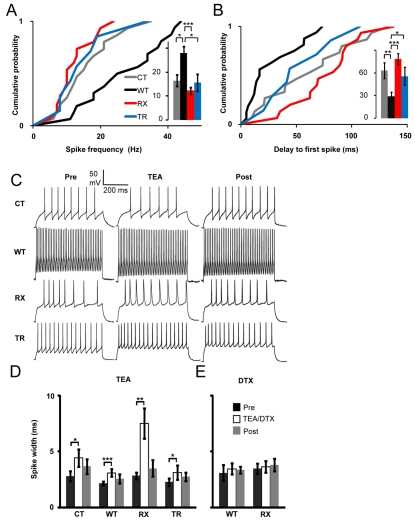
Fig. 2.
Effects of Kv1.1 manipulation on action potentials. (A) Cumulative probability plot showing the distribution of firing rates measured at 150% of the current threshold (inset: mean±s.e.m.). WT-expressing neurons (n=16) showed an increased spike frequency compared with CT (n=15), RX (n=15) and TR (n=7) neurons. (B) WT-expressing neurons showed a decreased delay to the first spike (29±6 ms) compared with RX (78±7 ms, P<0.001), CT (63±11 ms, P=0.01) and TR (56±12 ms, P=0.04) neurons (Mann-Whitney U-tests; *P≤0.05, **P≤0.01, ***P≤0.001). (C) Sample traces from CT, WT, RX and TR neurons before, during and after TEA (1 mM) application. (D) Application of TEA increased the action potential duration in CT (n=10), WT (n=20), TR (n=8) and especially RX (n=16) neurons. (E) α-DTX (100 nM) did not affect spike width (Student’s t-tests; *P≤0.05, **P≤0.01, ***P≤0.001).
Neurotransmitter release is exquisitely sensitive to spike width, and Kv1.1 plays a role in presynaptic action potential repolarization (Geiger and Jonas, 2000; Bischofberger et al., 2002; Kole et al., 2007; Southan and Robertson, 1998). The action potential duration at the soma (measured at 0 mV, and averaged from all spikes recorded at 150% of current threshold) was smaller in neurons overexpressing WT Kv1.1 than in control neurons, although the difference was not significant. However, TEA (1 mM) reversibly increased spike width in all four groups of neurons (Fig. 2C,D). Notably, the degree of spike broadening was approximately twofold greater in RX-expressing neurons than in the other groups. Because the lentivirus-expressed Kv1.1 subunits were TEA insensitive, this result indicates an enhanced dependence of repolarization on other TEA-sensitive channels, notably Kv3 (Rudy and McBain, 2001) and BK channels (Sah and Faber, 2002). Consistent with this model, application of α-DTX, which does not act on these channels, did not affect spike width (Fig. 2D).
The robust effect of RX on neuronal excitability provides a candidate disease mechanism. However, TR, which is associated with EA1 that is complicated by contractures and epilepsy, had no effect on any spiking parameter. We therefore examined the effects of WT and mutant Kv1.1 on neurotransmitter release.
We evoked autaptic excitatory postsynaptic currents (EPSCs) with trains of 40 escape currents (Fig. 3A,B), and measured spontaneous events (‘minis’) to estimate the quantal charge (Q). EPSCs typically decreased in amplitude during the trains, which was accounted for by depletion of the readily-releasable pool (RRP) of vesicles. Because, at a high stimulation rate, the EPSC amplitude is determined principally by the rate of vesicle replenishment, the RRP can be estimated by back-extrapolating the steady-state replenishment phase of a cumulative plot of autaptic responses (expressed as charge, and normalized by Q) to the ordinate (Fig. 3C) (Schneggenburger et al., 1999). The vesicular release probability (PRves) was obtained from the ratio of the first autaptic EPSC (also scaled by Q) to the RRP. The paired-pulse ratio (PPR), which typically scales inversely with PRves, was obtained from the ratio of the second autaptic EPSC to the first (denoted PPR50). PPR was also estimated from two escape currents delivered at 10 and 5 Hz (PPR100 and PPR200, respectively).
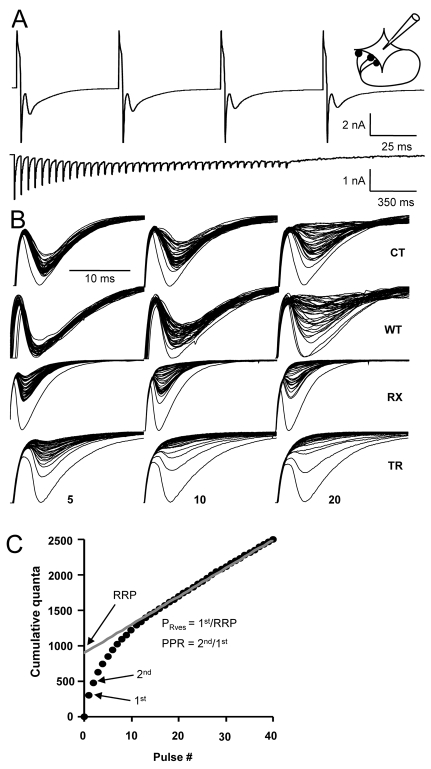
Fig. 3.
Effects of Kv1.1 manipulation on neurotransmitter release. (A) Upper trace: a 20-Hz spike train elicited in an autaptic neuron, showing four depolarizing stimulation artifacts (upward deflections) followed by escape action currents and EPSCs, respectively (downward deflections). Lower trace: a full 20-Hz train with stimulation artifacts and escape currents removed, showing 40 consecutive EPSCs. Inset: a schematic representation of an autaptic neuron. (B) Superimposed, successive autaptic responses from individual neurons obtained during a 40-pulse train at 5, 10 and 20 Hz (left, middle and right columns, respectively). At 5 Hz, the CT autapses (top row) showed relatively little synaptic rundown, but at 10 and 20 Hz, autaptic responses become gradually smaller owing to depletion of the RRP. WT neurons (second row) showed less rundown. RX (third row) and TR (fourth row) neurons showed increased rundown. (C) The RRP was estimated by back-extrapolating the steady-state replenishment phase to the ordinate. PRves was estimated as the ratio of the first response to the RRP. PPR was calculated as the ratio of the second to the first response.
Neither the absolute size of the RRP, nor the mean amplitude of minis, differed significantly between CT neurons and neurons expressing exogenous Kv1.1 constructs. However, the use-dependent decrease in amplitude was more marked in neurons transduced with RX or TR, and less pronounced in WT-expressing neurons (Fig. 3B). Estimated from 20-Hz trains, PRves was lower in WT-expressing neurons than in CT neurons (P<0.05, unpaired t-test). By contrast, both TR and RX increased PRves (P=0.05). Consistent with these effects, PPR was increased in WT-expressing neurons, but decreased in TR- and RX-transduced neurons. These effects on PPR were, as expected, most robust at short inter-pulse intervals (Table 1). Thus, two independent methods reveal systematic and opposite effects of WT and mutant Kv1.1 on exocytosis.
Table 1.
Effects of Kv1.1 manipulations and effects of TEA or DTX on PRves and PPR
| Release parameters | Release parameters and effect of TEA | |||
|---|---|---|---|---|
| CT | WT | RX | TR | |
| PRves | 0.21±0.02 (18) | 0.15±0.01 (10)* | 0.27±0.04 (13)† | 0.28± 0.03 (9)†† |
| PRves (1 mM TEA) | 0.18±0.01 (4) | 0.17±0.02 (3) | 0.40±0.05 (14)** ,†,‡ | 0.29±0.04 (7) |
| PPR50 | 0.67±0.03 (18) | 0.97±0.1 (10)** | 0.63±0.04 (13)†† | 0.68±0.05 (7)†† |
| PPR50 (TEA) | 0.77±0.03 (4) | 0.85±0.03 (3) | 0.52±0.04 (14)*,† | 0.70±0.04 (6)* |
| PPR100 | 0.75±0.03 (8) | 0.85±0.06 (9) | 0.67±0.05 (14)† | 0.67±0.05 (9)† |
| PPR100 (TEA) | 0.71±0.05 (4) | 0.79±0.06 (3) | 0.53±0.03 (15)*,† | 0.64±0.04 (7) |
| PPR200 | 0.73±0.05 (6) | 0.78±0.04 (6) | 0.68±0.03 (11) | 0.56±0.04 (9) |
| PPR200 (TEA) | 0.69±0.01 (2) | 0.90±0.08 (3) | 0.59±0.04 (14)† | 0.57±0.04 (7) |
| Release parameters | Release parameters and effect of DTX | |||
| WT | RX | |||
| PRves | 0.14±0.04 (4) | 0.32±0.02 (5)†† | ||
| PRves (100 nM-DTX) | 0.16±0.04 (4) | 0.33±0.02 (5)† | ||
| PPR50 | 0.94±0.10 (4) | 0.64±0.01 (5)† | ||
| PPR50 (DTX) | 0.81±0.12 (4) | 0.52±0.02 (5)†,‡ | ||
| PPR100 | 0.88±0.08 (4) | 0.60±0.02 (5)† | ||
| PPR100 (DTX) | 0.79±0.09 (4) | 0.58±0.03 (5) | ||
| PPR200 | 0.88±0.05 (4) | 0.65±0.04 (5)† | ||
| PPR200 (DTX) | 0.76±0.07 (4) | 0.56±0.07 (5) | ||
The effects of TEA or DTX on PRves and PPR were measured with 50, 100 and 200 ms inter-stimulus intervals. Data are shown as mean±s.e.m. (n).
P<0.05,
P<0.01 vs CT;
P<0.05,
P<0.01 vs WT;
P<0.05 vs baseline (before drug application).
TEA (1 mM) had little effect on PRves and PPR in CT-, WT- or TR-transduced neurons. However, TEA further increased PRves in RX-transduced neurons and this effect was accompanied by decreases in PPR (Table 1). This is consistent with the greater effect of TEA on spike width in RX-transduced neurons than in other groups (Fig. 2C,D), and implies redundancy between Kv1.1 and other TEA-sensitive channels. The more pronounced effect on RX could also reflect an altered subunit preference during the assembly of heteromeric channels (Hopkins et al., 1994).
Finally, α-DTX application reduced PPR, although this only reached significance for a 50-ms inter-pulse interval in RX-transduced neurons (Table 1).
Taken together, these data argue that both the RX and TR mutant Kv1.1 channels that are associated with EA1 increase neurotransmitter release, and that these phenomena are incompletely compensated for by other channels that contribute to action potential repolarization.
DISCUSSION
This study reveals a striking dissociation between the two EA1 mutations. RX, which is associated with severe EA1 (Eunson et al., 2000), robustly decreased the current threshold and increased neurotransmitter release. By contrast, TR, which is associated with EA1 complicated by contractures and, in some individuals, epilepsy (Zuberi et al., 1999; Kinali et al., 2004), only affected exocytosis and had no detectable effect on spiking.
A limitation of the methods used here is that developmental or cell-type differences in the expression of Kv1.1 and other channel subunits cannot be recapitulated faithfully. Furthermore, gene dosage is difficult to control. Nevertheless, the preservation of Kv1.1 staining and the absence of intracellular inclusions with RX argue that some pathological consequences of overexpression were avoided. Indeed, the expression system is sufficiently sensitive to detect bidirectional effects of manipulating Kv1.1 on spiking and exocytosis.
The increase in vesicular release probability observed with transduction of either RX or TR subunits provides direct evidence for enhanced neurotransmission in EA1 (Herson et al., 2003). Because presynaptic calcium influx, the trigger for exocytosis, is proportional to spike duration, a compelling mechanism underlying this increase in neurotransmission is an impairment of presynaptic action potential repolarization (Geiger and Jonas, 2000). If this, in turn, led to inactivation of axonal sodium channels, the resultant change in spike shape could further compound the effect of vesicle depletion on the time-course of the cumulative autaptic EPSC amplitude. Although WT Kv1.1 overexpression showed a trend towards shortened action potentials measured at the soma, no consistent effect was seen for either the RX or TR mutation prior to adding TEA. A possible explanation is that Kv1.1-containing channels have a greater influence on spike shape at presynaptic boutons (and/or in axons) than at the soma, consistent with evidence that genetic deletion of Kv1.1 disrupts the temporal fidelity of transmission in the axons of the auditory pathway (Kopp-Scheinpflug et al., 2003).
In contrast to spike width, neither TR expression nor TEA application had a detectable effect on the current threshold, which was, however, increased robustly by WT, and decreased by RX expression and α-DTX treatment. The absence of an effect of TEA can be explained by postulating that Kv1.1 exists, together with other Kv1 subunits, in relatively TEA-insensitive heteromultimers. Instead, the finding that α-DTX reduces current threshold in all groups is consistent with an important role of Kv1.2, or more likely Kv1.6 (Ramaswami et al., 1990), in the subthreshold conductance.
Why did the TR mutation have no effect on the current threshold, even though it robustly affected neurotransmission? TR exerts a dominant negative effect in Xenopus oocytes (Zuberi et al., 1999; Rea et al., 2002). Three other amino acid substitutions have been reported at this residue in EA1 (Comu et al., 1996; Scheffer et al., 1998), at least one of which also confers a dominant negative effect (Chen et al., 2007). However, no evidence for intracellular aggregation of TR (or of T226A or T226M) was found in COS cell expression (Manganas et al., 2001). Indeed, both homomeric and heteromeric TR-containing channels can be detected in heterologous expression, albeit with a depolarized activation threshold and slowed kinetics (Zuberi et al., 1999; Rea et al., 2002). Thus, a possible explanation as to why TR did not affect the current threshold is that residual heteromeric channels mediate a sufficient conductance to regulate excitability.
The lower current threshold observed for RX, but not for TR, may explain why the two mutations are associated with distinct phenotypes. If Kv1.1 plays a disproportionately important role in regulating cortical interneuron excitability (Goldberg et al., 2008), their decreased threshold may dampen circuit excitability, partially compensating for enhanced neurotransmitter release. This protective effect may not occur for TR, providing a possible explanation for the epilepsy that is seen in association with this mutation. As for the more severe neuromyotonia and contractures associated with the TR mutation, this might reflect the ability of mutant Kv1.1-containing heterotetramers to traffic to sites in the distal axon that are normally occupied by WT channels. A direct test of these hypotheses will require the methods that have been applied to hippocampal cultures in the present study to be extended to different neuronal types that are implicated in episodic ataxia, myokymia and seizures.
METHODS
Forebrains from 1-day-old rats were dissected and collected in Hank’s buffered salts solution (HBSS, Sigma), and buffered with 7 mM HEPES (pH 7.30). Hippocampi and overlying cortices were minced separately and incubated for 20 minutes in 0.25% trypsin in HBSS at 37°C, washed three times in HBSS, and triturated with fire-polished Pasteur pipettes. Cortical cells were plated in T75 flasks (Sigma) in Dulbecco’s modified Eagle’s medium (DMEM), supplemented with 10% fetal bovine serum (FBS) and 0.5% penicillin/streptomycin to favor glial growth. Glass cover slips (Menzel, Germany) were etched with 65% nitric acid (Sigma), coated with 0.15% agarose (Sigma) and sprayed with 0.25 mg/ml of rat tail collagen (BD Biosciences, Bedford, MA). After drying and UV sterilization, glial cells were plated at 750 cells/cm2. On day in vitro (DIV) 7, hippocampal neurons were added at 1500 cells/cm2. After attachment, the culture medium was changed to Neurobasal medium supplemented with 2% B27, 2% FBS and 0.25% glutamax. Virus particles were added within 24 hours at 50 μ l per 500 μ l of medium (in 2 cm2 wells) yielding transduction efficiencies of approximately 80%. At neuronal DIV3, 0.6 μ M of cytosine arabinoside (Sigma) was added to prevent glial overgrowth and 50% of the medium was changed every week. Unless otherwise stated, all chemicals were from Invitrogen.
Human wild-type Kv1.1 (WT), and the R417stop (RX) and T226R (TR) mutations (Zuberi et al., 1999; Eunson et al., 2000), were subcloned into pCDH1-copGFP lentivectors (System Biosciences, CA) using standard techniques (supplementary material Fig. S3). In addition, the Y379V mutation was introduced into each construct to reduce the sensitivity of the expressed Kv1.1 channels to bath-applied TEA (Sigma): Ki<250 mM instead of 0.4 mM (Rea et al., 2002). Primers and sequences are available upon request. (Because WT and mutant Kv1.1 expression had opposite effects on the examined parameters, we did not systematically study an additional group of neurons transduced with an empty vector.) Expression and packaging vectors were co-transfected into HEK293FT cells using Lipofectamine 2000. After 24 hours, the transfected cells were incubated in pure OptiMEM. After 48–72 hours, the supernatant was centrifuged for 3 minutes at 1000 rpm, passed through a 0.45 μ m filter to remove any remaining debris, and then applied directly to the hippocampal cultures.
Whole cell patch-clamp recordings were performed on 8–24 DIV neurons. The patch pipette solution contained: 125 mM K-gluconate, 10 mM NaCl, 4.6 mM MgCl2, 4 mM Na2-ATP, 15 mM creatine phosphate, 1 mM EGTA and 20 U/ml phosphocreatine kinase (pH 7.3). The external medium contained: 140 mM NaCl, 2.4 mM KCl, 4 mM CaCl2, 4 mM MgCl2, 10 mM HEPES and 10 mM glucose (pH 7.3, chemicals from Sigma). GABAA and N-methyl-D-aspartate (NMDA) receptors were blocked with 100 μ M picrotoxin (Tocris, UK) and 50 μ M D-aminophosphonovaleric acid (Ascent, UK), respectively. Signals were recorded with Axopatch 1D or 700B amplifiers using Clampex 9 (Axon Instruments). Patch pipettes had a resistance of 5–7 MΩ and in the voltage clamp, access resistance was compensated to 7–15 MΩ. After membrane rupture, cells were held at −70 mV and screened for autapses with 1-ms depolarizing voltage commands to between −30 and +30 mV. For cells exceeding a 500 pA autaptic current, spontaneous events were recorded for 1 minute to estimate the quantal amplitude, and then trains of 40 1-ms voltage command pulses above threshold were delivered at 5, 10 and 20 Hz to generate unclamped action currents (escape currents). These trains were repeated in 1 mM TEA or 100 nM α-DTX (Alomone labs, Israel) and after washout. In parallel, cells were recorded in current clamp to determine action potential characteristics. Where necessary, AMPA receptors were blocked with 25 μ M of NBQX (Ascent, UK). After setting the bridge balance and adjusting the holding current to keep the cell at −70 mV, the current threshold was estimated with 1-second-long current injections, with 10 pA incremental steps. Action potential characteristics, before, during and after 1 mM TEA or 100 nM α-DTX were determined at 150% of the current threshold.
Clampfit 9.2, Matlab and KyPlot were used for offline analysis. Averages are given ± the standard error of the mean (s.e.m.). Statistical significance was tested using Mann-Whitney U-tests and Student’s t-tests.
DIV15 cultures were fixed for 5 minutes consecutively in 2% and 4% paraformaldehyde. After washing in 0.01 M PBS, cells were permeabilized in 0.1% Triton X-100 for 5 minutes and pre-incubated for 20 minutes with 4% normal goat serum. Cells were incubated for 1 hour in a mixture of 0.2% rabbit anti-human Kv1.1 (ab65790), raised against amino acids 409–495, 0.01% chicken anti-MAP2 (ab5392; Abcam, UK) and 0.1% Triton X-100 in PBS. After washing, cells were incubated for 1 hour in 0.1% goat anti-rabbit Alexa Fluor 546 (Invitrogen) and 0.2% goat anti-chicken Cy5 (ab6569; Abcam), washed, and mounted in polyvinyl alcohol with DABCO antifading (10981; Sigma). All incubations were at room temperature. Images were acquired with a Zeiss 510 confocal microscope.
Supplementary Material
Acknowledgments
This work was supported by the Medical Research Council , The Wellcome Trust , Action Medical Research and the Worshipful Company of Pewterers. We are grateful to D. A. Rusakov and M. C. Walker for comments on the manuscript, and to M. Shah for helpful discussions. Deposited in PMC for release after 6 months.
Footnotes
COMPETING INTERESTS
The authors declare no competing financial interests.
AUTHOR CONTRIBUTIONS
J.H.H. and D.M.K. conceived and designed the experiments; J.H.H. performed the experiments; S.S., J.H.H. and S.R. designed and made the lentiviruses; J.H.H., C.H. and D.M.K. analyzed the data; J.H.H., C.H., S.R., M.G.H., S.S. and D.M.K. wrote the paper.
SUPPLEMENTARY MATERIAL
Supplementary material for this article is available at http://dmm.biologists.org/lookup/suppl/doi:10.1242/dmm.003582/-/DC1
REFERENCES
- Adelman JP, Bond CT, Pessia M, Maylie J. (1995). Episodic ataxia results from voltage-dependent potassium channels with altered functions. Neuron 15, 1449–1454 [DOI] [PubMed] [Google Scholar]
- Bischofberger J, Geiger JRP, Jonas P. (2002). Timing and efficacy of Ca2+ channel activation in hippocampal mossy fiber boutons. J Neurosci. 22, 10593–10602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boland LM, Price DL, Jackson KA. (1999. Episodic ataxia/myokymia mutations functionally expressed in the Shaker potassium channel. Neuroscience 91, 1557–1564 [DOI] [PubMed] [Google Scholar]
- Bretschneider F, Wrisch A, Lehmann-Horn F, Grissmer S. (1999). Expression in mammalian cells and electrophysiological characterization of two mutant Kv1.1 channels causing episodic ataxia type 1 (EA-1). Eur J Neurosci. 11, 2403–2412 [DOI] [PubMed] [Google Scholar]
- Brew HM, Hallows JL, Tempel BL. (2003). Hyperexcitability and reduced low threshold potassium currents in auditory neurons of mice lacking the channel subunit Kv1.1. J Physiol. 548, 1–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne DL, Gancher ST, Nutt JG, Brunt ER, Smith EA, Kramer P, Litt M. (1994). Episodic ataxia/myokymia syndrome is associated with point mutations in the human potassium channel gene, KCNA1. Nat Genet. 8, 136–140 [DOI] [PubMed] [Google Scholar]
- Chen H, von Hehn C, Kaczmarek LK, Ment LR, Pober BR, Hisama FM. (2007). Functional analysis of a novel potassium channel (KCNA1) mutation in hereditary myokymia. Neurogenetics 8, 131–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comu S, Giuliani M, Narayanan V. (1996). Episodic ataxia and myokymia syndrome: a new mutation of potassium channel gene Kv1.1. Ann Neurol. 40, 684–687 [DOI] [PubMed] [Google Scholar]
- D’Adamo MC, Liu Z, Adelman JP, Maylie J, Pessia M. (1998). Episodic ataxia type-1 mutations in the hKv1.1 cytoplasmic pore region alter the gating properties of the channel. EMBO J. 17, 1200–1207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eunson LH, Rea R, Zuberi SM, Youroukos S, Panayiotopoulos CP, Liguori R, Avoni P, McWilliam RC, Stephenson JB, Hanna MG, et al. (2000). Clinical, genetic, and expression studies of mutations in the potassium channel gene KCNA1 reveal new phenotypic variability. Ann Neurol. 48, 647–656 [PubMed] [Google Scholar]
- Geiger JR, Jonas P. (2000). Dynamic control of presynaptic Ca(2+) inflow by fast-inactivating K(+) channels in hippocampal mossy fiber boutons. Neuron 28, 927–939 [DOI] [PubMed] [Google Scholar]
- Goldberg EM, Clark BD, Zagha E, Nahmani M, Erisir A, Rudy B. (2008). K+ channels at the axon initial segment dampen near-threshold excitability of neocortical fast-spiking GABAergic interneurons. Neuron 58, 387–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grissmer S, Nguyen AN, Aiyar J, Hanson DC, Mather RJ, Gutman GA, Karmilowicz MJ, Auperin DD, Chandy KG. (1994). Pharmacological characterization of five cloned voltage-gated K+ channels, types Kv1.1, 1.2, 1.3, 1.5, and 3.1, stably expressed in mammalian cell lines. Mol Pharmacol. 45, 1227–1234 [PubMed] [Google Scholar]
- Grupe A, Schröter KH, Ruppersberg JP, Stocker M, Drewes T, Beckh S, Pongs O. (1990). Cloning and expression of a human voltage-gated potassium channel: a novel member of the RCK potassium channel family. EMBO J. 9, 1749–1756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herson PS, Virk M, Rustay NR, Bond CT, Crabbe JC, Adelman JP, Maylie J. (2003). A mouse model of episodic ataxia type-1. Nat Neurosci. 6, 378–383 [DOI] [PubMed] [Google Scholar]
- Hopkins WF, Demas V, Tempel BL. (1994). Both N- and C-terminal regions contribute to the assembly and functional expression of homo- and heteromultimeric voltage-gated K+ channels. J Neurosci. 14, 1385–1393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinali M, Jungbluth H, Eunson LH, Sewry CA, Manzur AY, Mercuri E, Hanna MG, Muntoni F. (2004). Expanding the phenotype of potassium channelopathy: severe neuromyotonia and skeletal deformities without prominent Episodic Ataxia. Neuromuscul Disord. 14, 689–693 [DOI] [PubMed] [Google Scholar]
- Kole MHP, Letzkus JJ, Stuart GJ. (2007). Axon initial segment Kv1 channels control axonal action potential waveform and synaptic efficacy. Neuron 55, 633–647 [DOI] [PubMed] [Google Scholar]
- Kopp-Scheinpflug C, Fuchs K, Lippe WR, Tempel BL, Rubsamen R. (2003). Decreased temporal precision of auditory signaling in Kcna1-null mice: an electrophysiological study in vivo. J Neurosci. 23, 9199–9207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai HC, Jan LY. (2006). The distribution and targeting of neuronal voltage-gated ion channels. Nat Rev Neurosci. 7, 548–562 [DOI] [PubMed] [Google Scholar]
- Lau D, Vega-Saenz de Miera EC, Contreras D, Ozaita A, Harvey M, Chow A, Noebels JL, Paylor R, Morgan JI, Leonard CS, et al. (2000). Impaired fast-spiking, suppressed cortical inhibition, and increased susceptibility to seizures in mice lacking Kv3.2 K+ channel proteins. J Neurosci. 20, 9071–9085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manganas LN, Akhtar S, Antonucci DE, Campomanes CR, Dolly JO, Trimmer JS. (2001). Episodic ataxia type-1 mutations in the Kv1.1 potassium channel display distinct folding and intracellular trafficking properties. J Biol Chem. 276, 49427–49434 [DOI] [PubMed] [Google Scholar]
- Martina M, Schultz JH, Ehmke H, Monyer H, Jonas P. (1998). Functional and molecular differences between voltage-gated K+ channels of fast-spiking interneurons and pyramidal neurons of rat hippocampus. J Neurosci. 18, 8111–8125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajakulendran S, Schorge S, Kullmann DM, Hanna MG. (2007). Episodic ataxia type 1, a neuronal potassium channelopathy. Neurotherapeutics 4, 258–266 [DOI] [PubMed] [Google Scholar]
- Ramaswami M, Gautam M, Kamb A, Rudy B, Tanouye MA, Mathew MK. (1990). Human potassium channel genes: molecular cloning and functional expression. Mol Cell Neurosci. 1, 214–223 [DOI] [PubMed] [Google Scholar]
- Rea R, Spauschus A, Eunson LH, Hanna MG, Kullmann DM. (2002. Variable K(+) channel subunit dysfunction in inherited mutations of KCNA1. J Physiol. 538, 5–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettig J, Heinemann SH, Wunder F, Lorra C, Parcej DN, Dolly JO, Pongs O. (1994). Inactivation properties of voltage-gated K+ channels altered by presence of beta-subunit. Nature 369, 289–294 [DOI] [PubMed] [Google Scholar]
- Rudy B, McBain CJ. (2001). Kv3 channels: voltage-gated K+ channels designed for high-frequency repetitive firing. Trends Neurosci. 24, 517–526 [DOI] [PubMed] [Google Scholar]
- Sah P, Faber ESL. (2002). Channels underlying neuronal calcium-activated potassium currents. Prog Neurobiol. 66, 345–353 [DOI] [PubMed] [Google Scholar]
- Scheffer H, Brunt ER, Mol GJ, van der Vlies P, Stulp RP, Verlind E, Mantel G, Averyanov YN, Hofstra RM, Buys CH. (1998). Three novel KCNA1 mutations in episodic ataxia type I families. Hum Genet. 102, 464–466 [DOI] [PubMed] [Google Scholar]
- Schneggenburger R, Meyer AC, Neher E. (1999). Released fraction and total size of a pool of immediately available transmitter quanta at a calyx synapse. Neuron 23, 399–409 [DOI] [PubMed] [Google Scholar]
- Southan AP, Robertson B. (1998). Patch-clamp recordings from cerebellar basket cell bodies and their presynaptic terminals reveal an asymmetric distribution of voltage-gated potassium channels. J Neurosci. 18, 948–955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacher H, Mohapatra DP, Trimmer JS. (2008). Localization and targeting of voltage-dependent ion channels in mammalian central neurons. Physiol Rev. 88, 1407–1447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang FC, Parcej DN, Dolly JO. (1999). alpha subunit compositions of Kv1.1-containing K+ channel subtypes fractionated from rat brain using dendrotoxins. Eur J Biochem. 263, 230–237 [DOI] [PubMed] [Google Scholar]
- Wang H, Kunkel DD, Martin TM, Schwartzkroin PA, Tempel BL. (1993). Heteromultimeric K+ channels in terminal and juxtaparanodal regions of neurons. Nature 365, 75–79 [DOI] [PubMed] [Google Scholar]
- Zerr P, Adelman JP, Maylie J. (1998a). Characterization of three episodic ataxia mutations in the human Kv1.1 potassium channel. FEBS Lett. 431, 461–464 [DOI] [PubMed] [Google Scholar]
- Zerr P, Adelman JP, Maylie J. (1998b). Episodic ataxia mutations in Kv1.1 alter potassium channel function by dominant negative effects or haploinsufficiency. J Neurosci. 18, 2842–2848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Gomez B, Watanabe I, Thornhill WB. (2007). Kv1 potassium channel C-terminus constant HRETE region: arginine substitution affects surface protein level and conductance level of subfamily members differentially. Mol Membr Biol. 24, 194–205 [DOI] [PubMed] [Google Scholar]
- Zuberi SM, Eunson LH, Spauschus A, De Silva R, Tolmie J, Wood NW, McWilliam RC, Stephenson JB, Stephenson JP, Kullmann DM, et al. (1999). A novel mutation in the human voltage-gated potassium channel gene (Kv1.1) associates with episodic ataxia type 1 and sometimes with partial epilepsy. Brain 122, 817–825 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.