Abstract
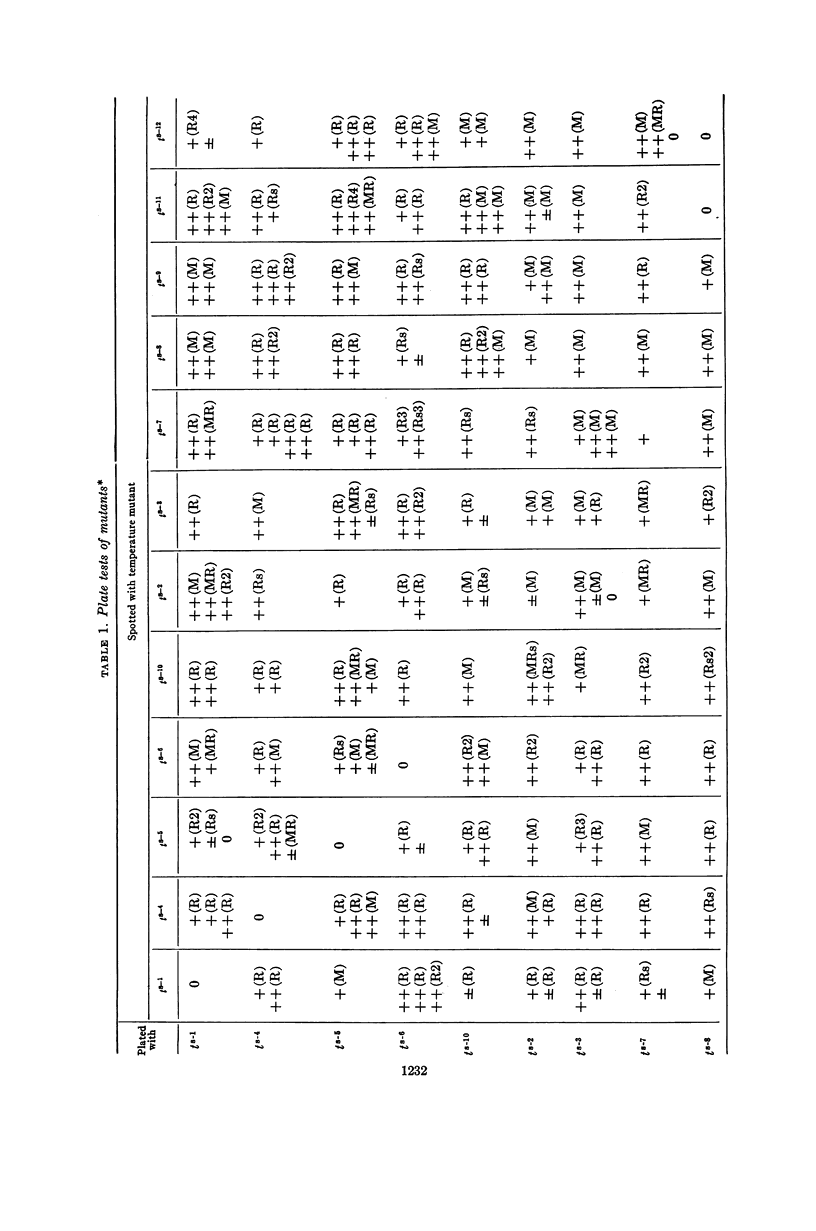
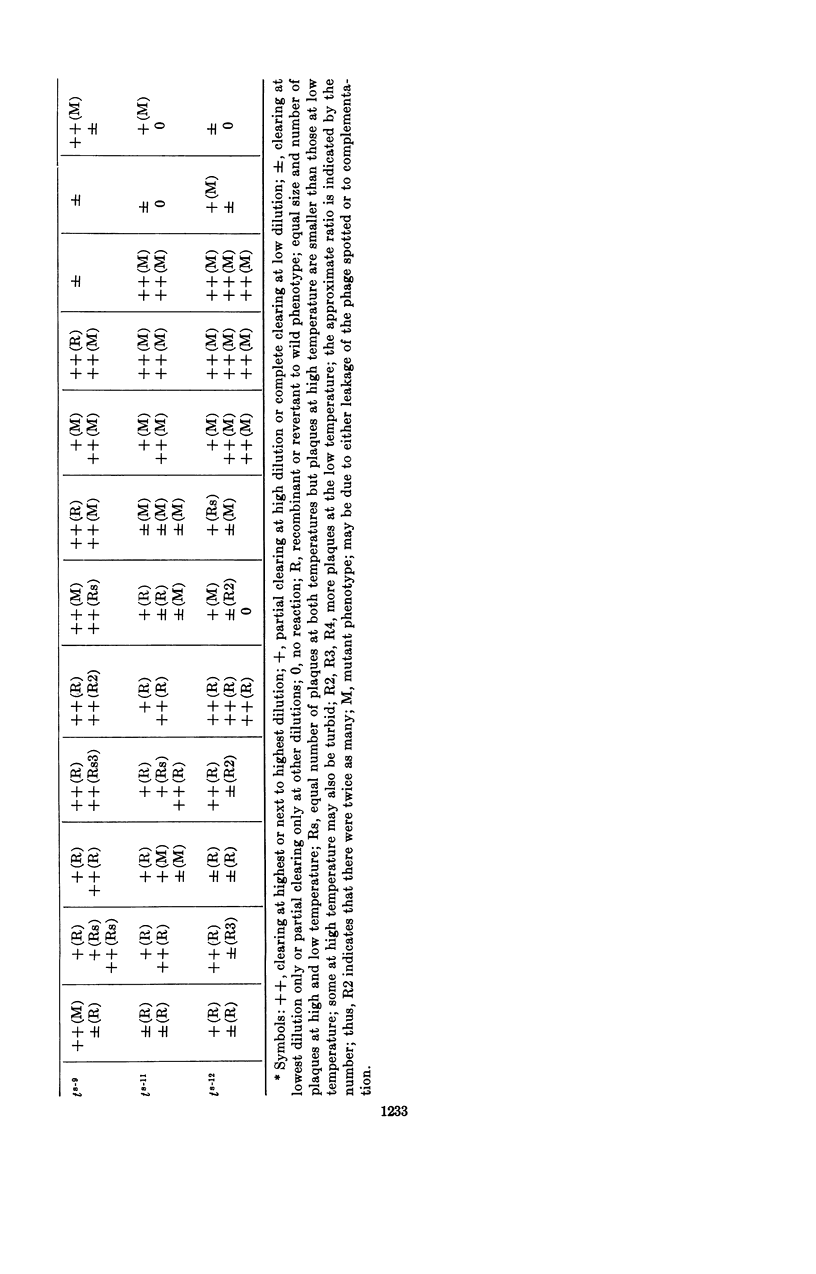
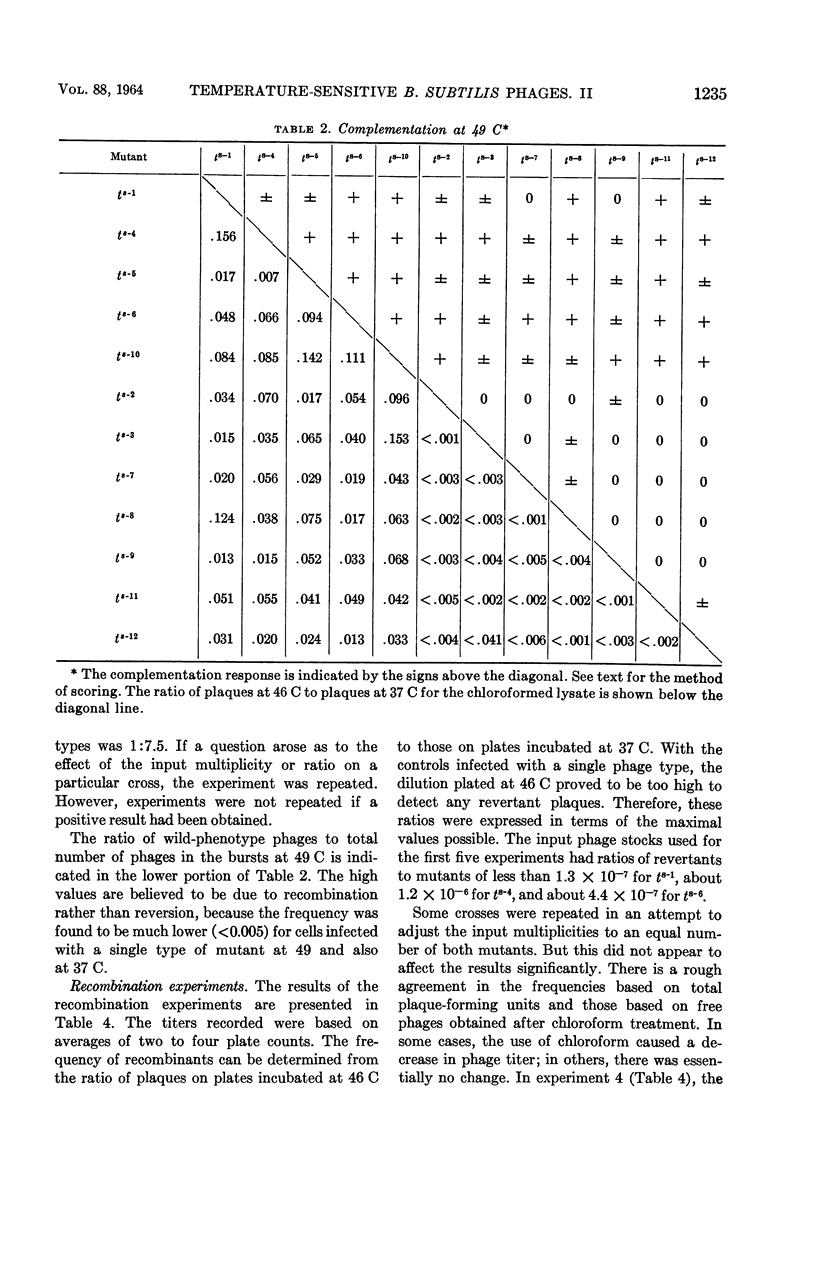
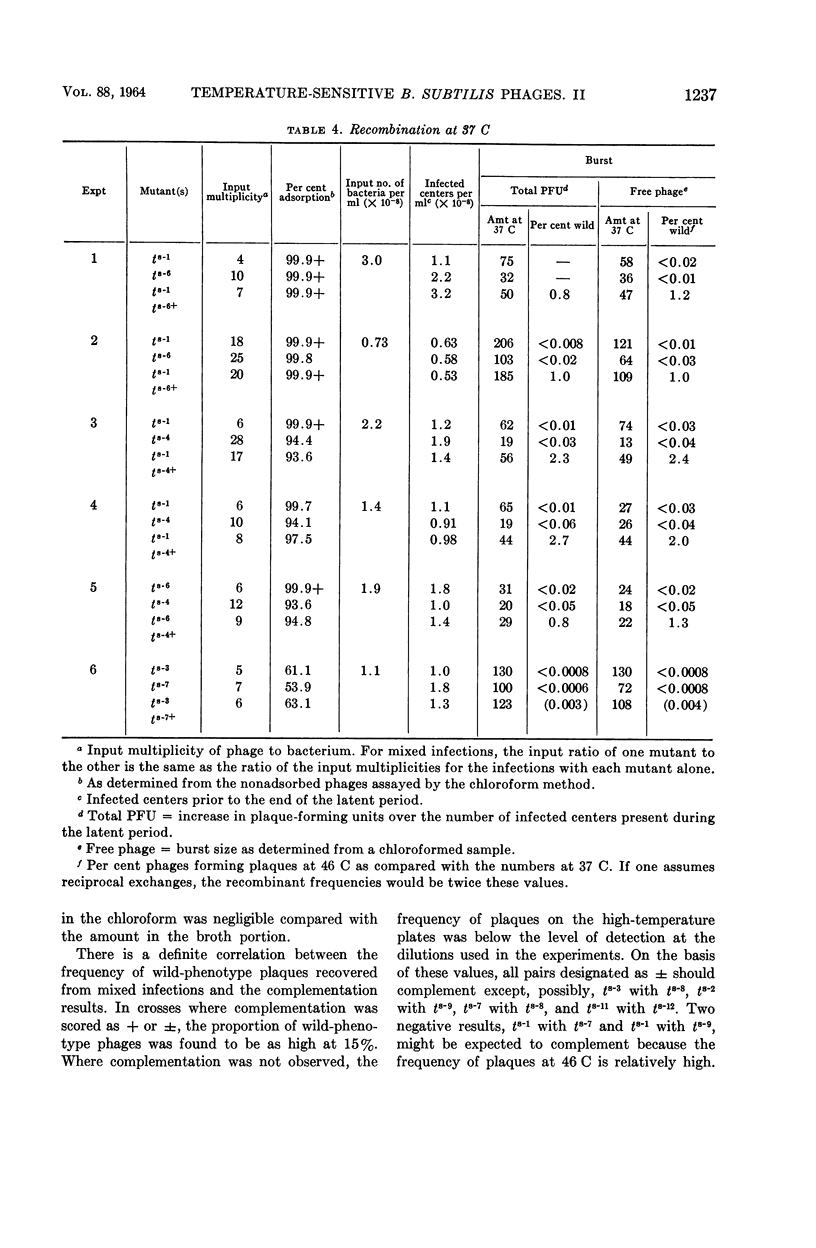
Nishihara, Mutsuko (University of California, Los Angeles), and W. R. Romig. Temperature-sensitive mutants of Bacillus subtilis bacteriophage SP3. II. In vivo complementation studies. J. Bacteriol. 88:1230–1239. 1964.—A plate-spotting procedure was used in initial attempts to group the temperature-sensitive Bacillus subtilis phage SP3 mutants by complementation. The results obtained did not show any clear patterns of reactions among the mutants. Crosses were, therefore, repeated in broth at a temperature of 49 C, which greatly reduced the extent of replication of each mutant type alone. The data on mixed infections indicated that there was a minimum of six complementation groups. Of the 12 isolates, 7 did not seem to complement with each other; the rest complemented with each other and with the seven noncomplementing mutants. There was a positive correlation between the complementation reaction of a pair and the recovery of wild-phenotype phages from a 49 C broth lysate. The relative proportion of phages capable of forming wild-phenotype plaques on plates incubated at 46 C to the total number of plaque-forming units was higher in a lysate of a mixed infection with two mutants than in lysates of each mutant alone. Moreover, this frequency was higher for a mixed lysate made at 49 C than for a lysate of the same two mutants made at 37 C. These observations suggested that genetic recombination might occur at 49 C, and that the increased recovery of wild-phenotype phages in lysates made at this temperature might be due to a selective advantage for these phages. Recombination experiments at 37 C with some complementing pairs gave frequencies of 2.0 to 4.8%. The ratio of wild-phenotype revertants to total phages in the stock lysates used for these crosses at 37 C was less than 10−6. The noncomplementing mutants were not conclusively shown to be nonidentical.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CAMPBELL A., DELCAMPILLO-CAMPBELL A. MUTANT OF LAMBDA BACTERIOPHAGE PRODUCING A THERMOLABILE ENDOLYSIN. J Bacteriol. 1963 Jun;85:1202–1207. doi: 10.1128/jb.85.6.1202-1207.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase M, Doermann A H. High Negative Interference over Short Segments of the Genetic Structure of Bacteriophage T4. Genetics. 1958 May;43(3):332–353. doi: 10.1093/genetics/43.3.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIRKSEN M. L., HUTSON J. C., BUCHANAN J. M. HOST-DEPENDENT SYNTHESIS OF ALTERED DEOXYCYTIDYLATE HYDROXYMETHYLASE AFTER INFECTION OF ESCHERICHIA COLI WITH CERTAIN AMBER MUTANTS OF BACTERIOPHAGE T4. Proc Natl Acad Sci U S A. 1963 Sep;50:507–513. doi: 10.1073/pnas.50.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDGAR R. S., FEYNMAN R. P., KLEIN S., LIELAUSIS I., STEINBERG C. M. Mapping experiments with r mutants of bacteriophage T4D. Genetics. 1962 Feb;47:179–186. doi: 10.1093/genetics/47.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HELINSKI D. R., YANOFSKY C. Correspondence between genetic data and the position of amino acid alteration in a proein. Proc Natl Acad Sci U S A. 1962 Feb;48:173–183. doi: 10.1073/pnas.48.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz N. H., Fling M. Genetic Determination of Tyrosinase Thermostability in Neurospora. Genetics. 1953 Jul;38(4):360–374. doi: 10.1093/genetics/38.4.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas W. K., Davis B. D. Production of an Altered Pantothenate-Synthesizing Enzyme by a Temperature-Sensitive Mutant of Escherichia Coli. Proc Natl Acad Sci U S A. 1952 Sep;38(9):785–797. doi: 10.1073/pnas.38.9.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NISHIHARA M., ROMIG W. R. TEMPERATURE-SENSTIVIE MUTANTS OF BACILLUS SUBTILIS BACTERIOPHAGE SP3. I. ISOLATION AND CHARACTERIZATION. J Bacteriol. 1964 Nov;88:1220–1229. doi: 10.1128/jb.88.5.1220-1229.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROMIG W. R., BRODETSKY A. M. Isolation and preliminary characterization of bacteriophages for Bacillus subtilis. J Bacteriol. 1961 Jul;82:135–141. doi: 10.1128/jb.82.1.135-141.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STREISINGER G., MUKAI F., DREYER W. J., MILLER B., HORIUCHI S. Mutations affecting the lysozyme of phage T4. Cold Spring Harb Symp Quant Biol. 1961;26:25–30. doi: 10.1101/sqb.1961.026.01.007. [DOI] [PubMed] [Google Scholar]
- YANOFSKY C., HELINSKI D. R., MALING B. D. The effects of mutation on the composition and properties of the A protein of Escherichia coli tryptohan synthetase. Cold Spring Harb Symp Quant Biol. 1961;26:11–24. doi: 10.1101/sqb.1961.026.01.006. [DOI] [PubMed] [Google Scholar]


