Abstract
Chronic hyperglycemia causes oxidative stress, which contributes to damage in various tissues and cells, including pancreatic β-cells. The expression levels of antioxidant enzymes in the islet are low compared with other tissues, rendering the β-cell more susceptible to damage caused by hyperglycemia. The aim of this study was to investigate whether increasing levels of endogenous glutathione peroxidase-1 (GPx-1), specifically in β-cells, can protect them against the adverse effects of chronic hyperglycemia and assess mechanisms that may be involved. C57BLKS/J mice overexpressing the antioxidant enzyme GPx-1 only in pancreatic β-cells were generated. The biological effectiveness of the overexpressed GPx-1 transgene was documented when β-cells of transgenic mice were protected from streptozotocin. The transgene was then introgressed into the β-cells of db/db mice. Without use of hypoglycemic agents, hyperglycemia in db/db-GPx(+) mice was initially ameliorated compared with db/db-GPx(−) animals and then substantially reversed by 20 wk of age. β-Cell volume and insulin granulation and immunostaining were greater in db/db-GPx(+) animals compared with db/db-GPx(−) animals. Importantly, the loss of intranuclear musculoaponeurotic fibrosarcoma oncogene homolog A (MafA) that was observed in nontransgenic db/db mice was prevented by GPx-1 overexpression, making this a likely mechanism for the improved glycemic control. These studies demonstrate that enhancement of intrinsic antioxidant defenses of the β-cell protects it against deterioration during hyperglycemia.
β-cell-specific GPx-1 overexpression in db/db mice protects the β-cell from oxidative stress and progression in dysfunction associated with chronic hyperglycemia.
Chronic hyperglycemia caused by β-cell dysfunction leads to secondary complications in eyes, nerves, kidneys, vascular tissue, and the β-cell itself. Proposed mechanisms for the effects of glucose toxicity on β-cell failure include excessive levels of reactive oxygen species (ROS) and consequent oxidative stress (1,2), which can lead to cellular damage of macromolecules including proteins, lipids, carbohydrates, and nucleic acids. Cellular defenses against ROS include the activation of several antioxidant enzymes, such as superoxide dismutases (SOD), catalase, and glutathione peroxidase (GPx). The expression levels of antioxidant enzymes in the islet are low compared with other tissues (3,4,5,6), rendering the β-cell less resistant to oxidative stress. ROS also participate in the destruction of pancreatic β-cells that leads to autoimmune diabetes (7).
These studies were designed to ascertain whether enrichment of antioxidant protection of the β-cell ameliorates the morphologic changes and cellular dysfunction it experiences during the natural history of diabetes in db/db mice. We chose to overexpress GPx-1 because of its ability to metabolize both H2O2 and lipid peroxides. Cu/Zn-SOD and Mn-SOD catabolize superoxide radicals but in this process generate another oxidant, H2O2. Whereas catalase is capable of metabolizing H2O2, it does not catabolize lipid peroxides. We previously observed that adenoviral overexpression of GPx-1 in isolated rat islets decreased ROS levels and protected β-cells from oxidative stress (8). There have been no published studies in genetic models of diabetes examining whether increasing levels of endogenous GPx-1 specifically in β-cells will protect them against the adverse effects of chronic hyperglycemia.
This study addresses three questions: 1) will enrichment of endogenous GPx-1 expression in β-cells ameliorate the degree of hyperglycemia experienced by db/db mice during the course of their natural history of developing diabetes; 2) if so, are the beneficial effects related to changes in total β-cell volume and insulin granulation; and 3) are the critical insulin gene transcription factors musculoaponeurotic fibrosarcoma oncogene homolog A (MafA) and pancreatic duodenal homeobox (PDX)-1 involved in the molecular mechanisms mediating the beneficial effects of GPx-1 overexpression?
Materials and Methods
Animals and diet
Male and female C57BLKS/J (stock no. 000662) and heterozygote C57BLKS/J-m+/+Leprdb/+ (stock no. 000642) (db/+) mice were obtained from the Jackson Laboratory (Bar Harbor, ME; note: 12% of db/db mice do not become hyperglycemic by 18 wk of age). Control and transgenic C57BLKS/J mice were fed a rodent diet of 8604 meal with added sodium selenite (0.0445% Na2SeO3 in sucrose, T.05443; Harlan Teklad, Indianapolis, IN). Control and transgenic C57BLKS/J-m+/+Leprdb mice were fed modified LabDiet JL mouse/auto 6F 5K52 with added 0.6 ppm selenium (catalog no. 1811272; TestDiet, Richmond, IN). Mice were kept on a 12-h light, 12-h dark cycle and fed ad libitum. All procedures were approved by the Pacific Northwest Diabetes Research Institute’s Animal Care and Use Committee.
Transgenic mice
We obtained a cDNA clone for human GPx-1 from ResGen Invitrogen Corp. (clone 5428866; Carlsbad, CA) in the pOTB7 vector. The cDNA was excised using BglII and BamHI and religated into vector 4158 between the promoter for rat insulin and the major histocompatibility complex poly A tail. The 4158 plasmid was linearized using BglII. Plasmids were amplified in DH5α competent cells. PCR primers were designed to allow DNA sequencing across ligation sites to confirm cDNA orientation and fidelity of the new construct. A 2051-bp clone containing the rat insulin II promoter, human GPx-1 cDNA, and major histocompatibility complex poly A tail (9) was excised, linearized, cleaned using standard methods and a QIAGEN column (Germantown, MD), and used for injection into recently fertilized oocytes from C57BLKS/J mice for the generation of transgenic mice.
GPx-1 genotyping
DNA was extracted from ear punches using the Puregene DNA purification system, cell and tissue kit (Gentra Systems, Minneapolis, MN) according to the manufacturer’s instructions. Amplification of genomic DNA was performed using Platinum PCR SuperMix 96 (Invitrogen). Two separate PCRs were run for each sample, which contained 1–2 μl DNA and 50 ng each of the forward and reverse primers. The first reaction consisted of human GPx-1 forward primer, 5′-CCAGTCGGTGTATGCCTTCT, and poly A tail reverse primer, 5′-CTCAGAAAGTTCCACAGCGGAG. The second reaction consisted of rat insulin promoter forward primer, 5′-CTGATCCACCCTTAATGGGA, and human GPx-1 reverse primer, 5′-GTTCACCTCGCACTTCTCG. PCR cycling conditions were as follows: one 2-min denaturation step at 94 C; 30 cycles of 95 C for 30 sec, 62 C for 50 sec, and 68 C for 30 sec; and one final step at 72 C for 10 min. PCR products were separated on a 1% agarose gel. A 1095-bp fragment was visualized from the first reaction and a 709-bp fragment was visualized from the second reaction.
Pancreatic islet isolation
Islets were isolated from mouse pancreata as described by Tanaka et al. (8) with the following modifications: use of 0.75 mg/ml collagenase and omission of the histopaque gradient step.
Western analysis
Whole-cell extracts were obtained by harvesting the tissues in lysis buffer [140 mm NaCl, 10 mm Tris (pH 7.4), 1 mm CaCl2, 1 mm MgCl2, 10% glycerol, 1% Nonidet P-40, 1 mm dithiothreitol, 0.5 mm phenylmethylsulfonyl fluoride, 2 ng/μl aprotinin, 10 ng/μl leupeptin]. Protein concentrations were measured by the BCA assay (Pierce, Rockford, IL). Cellular proteins (10 μg for kidney, liver and hypothalamus; 25 μg for islets) were separated on 10% polyacrylamide Ready gels (Bio-Rad, Hercules, CA) and electrotransferred onto Trans-blot nitrocellulose membranes (Bio-Rad). Membranes were immunoblotted with GPx-1 antisera (Lab Frontiers, Seoul Korea; 1:1000, cross-reacts to human and mouse) overnight at 4 C in 5% nonfat dry milk in PBS with 0.5% Tween 20. The membranes were washed and immunoblotted with antirabbit IgG (1:4000; Jackson ImmunoResearch Laboratories, West Grove, PA) for 1 h at room temperature. GPx-1 protein was detected using the Amersham ECL Western blotting detection kit (GE Healthcare, Piscataway, NJ). Membranes were stripped and reprobed with anti-TFIID (N-12, 1:200; Santa Cruz Biotechnology, Santa Cruz, CA) to control for protein loading. OptiQuant image analysis software (Packard, Meriden, CT) was used for densitometry measurements of the autoradiograms.
Streptozotocin treatment
Streptozotocin (Sigma, Milwaukee, WI) was dissolved in sterile citrate buffer [0.05 m sodium citrate (pH 4.5)] and used within 5 min of preparation. Six-week-old male wild-type and GPx-1 transgenic mice were injected once daily ip (40 mg/kg) for 5 consecutive days. Nonfasting blood glucose levels were monitored over 8 wk with measurements taken from the tail vein using an Accu-Chek glucometer (Roche Diagnostics, Indianapolis, IN).
Cross-breeding GPx-1 transgenic line with db/db mice
GPx-1 transgenic mice were backcrossed with C57BLKS/ J-m+/+Leprdb/+ mice. The resulting offspring were crossed for up to three generations with C57BLKS/J-m+/+Leprdb/+ mice (db/+) to produce offspring with the following genotypes: wild-type [wt-GPx(−)], wt-GPx(+), db/+-GPx(−), db/+-GPx(+), db/db-GPx(−), and db/db-GPx(+). Genotyping for Leprdb was performed as described by the Jackson Laboratory. Both male and female mice were used for subsequent analyses.
Blood glucose measurements
Weekly glucose levels were measured in nonfasted mice in blood taken from the tail vein using an Accu-Chek glucometer. The limitations of this method are such that values greater than 599 mg/dl cannot be determined; thus the samples that registered a “hi” readout were arbitrarily assigned a value of 600 mg/dl.
Immunohistochemistry
Pancreata were fixed in 4% formalin and paraffin embedded. Sections were incubated overnight with the following primary antibodies: synaptophysin (1:500; Dako, Carpinteria, CA), insulin (1:1000; Dako), PDX-1 (1:1000; Chemicon, Temecula, CA), MafA (1:1000; Bethyl Laboratories, Montgomery, TX), and GPx-1 (1:500; Lab Frontier). After the sections were washed in PBS (pH 7.4), they were incubated with peroxidase-labeled anti-guinea pig or antirabbit IgG (1:200; Vector Laboratories, Burlingame, CA) secondary antibody for 30 min at room temperature. The peroxidase reaction was developed by incubating sections in 0.3% H2O2 and 0.15% 3,3′-diaminobenzidine tetrachloride (Sigma). For the double-labeling immunohistochemistry, fluorescein isothiocyanate -labeled anti-guinea pig IgG and Cy3-labeled antirabbit IgG secondary antibodies (1:200; Jackson ImmunoResearch Laboratories) were used.
Morphometry
The size of endocrine cell populations was determined according to Cavalieri’s principle. Total volumes of synaptophysin- and insulin-positive cells were estimated by point counting. Pancreatic tissues were cut in 5-μm-thick paraffin sections. Every 40th section was immunostained and stereologically examined using a light microscope that projected the image onto a 600-point grid at a final magnification of ×270, with an area per point of 5220 μm2. For each pancreas, 700-2000 points hitting the immunostained cells were counted. The total volume of immunopositive cells was calculated by multiplying the total number of points overlaying the stained cells per section with the area per point and the distance between subsequent immunostained sections.
Electron microscopy
Pancreatic tissue was fixed in cacodylate-buffered glutaraldehyde, postfixed in osmium tetroxide, and embedded in Spurr’s resin. Ultrathin sections were stained with uranylacetate and lead citrate and examined using an EM109 electron microscope (Zeiss, New York, NY).
Statistical analysis
Results are presented as mean ± sem. Differences were determined by using parametric and nonparametric unpaired t testing as well as ANOVA where appropriate with a P < 0.05 considered to be statistically significant. P values are provided in the legend of the figures and are marked by asterisks within the figures.
Results
GPx-1 protein is overexpressed only in the β-cells of transgenic mice
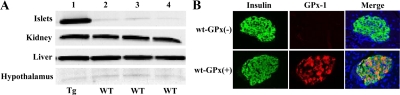
GPx-1 protein expression in isolated pancreatic islets of transgenic mice was 12.6-fold greater than that found in the islets of wild-type mice. Transgene overexpression was specific to islets because there were no changes in GPx-1 levels in kidney, liver, or hypothalamus (Fig. 1A). Double-staining immunohistochemistry for insulin and GPx-1 was performed on pancreas sections (Fig. 1B). Whereas insulin staining was present in islets from both wt-GPx(−) and wt-GPx(+), only wt-GPx(+) islets stained positively for GPx-1. The merged image indicates that the GPx-1 protein localizes to the β-cells.
Figure 1.
GPx-1 protein is overexpressed only in islets from transgenic mice. A, Western analysis of GPx-1 protein levels in islets, kidney, liver, and hypothalamus from a C57BLKS/J GPx-1 transgenic (Tg) mouse in lane 1 and tissues from three wild-type (wt) C57BLKS/J mice in lanes 2–4. Islet GPx-1 expression is 12.6-fold higher in transgenic mice compared with wild-type mice. B, Immunohistochemistry for insulin (green) and GPx-1 (red) protein in the islets of wt-GPx(−) and wt-GPx(+) mice (magnification, ×40).
GPx-1 provides protection against oxidative stress-induced β-cell loss
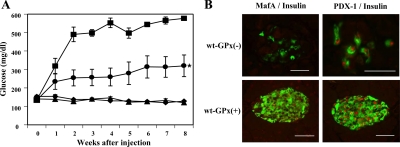
To test the biological effectiveness of the overexpressed GPx-1 enzyme, we administered streptozotocin (STZ), an agent that selectively destroys β-cells. STZ treatment has been shown to stimulate superoxide anion (10) and H2O2 generation (11) and hydroxyl radical formation (12) and increases the levels of malonyl dialdehyde as well as nitric acid and peroxynitrate in pancreatic islets (13). Six-week-old male wt-GPx(+) and wt-GPx(−) mice were given multiple injections of low-dose STZ, and plasma glucose levels were monitored weekly over a period of 8 wk (Fig. 2A). STZ-treated wt-GPx(−) mice exhibited marked hyperglycemia (577 ± 6 mg/dl), whereas wt-GPx(+) mice had significantly lower glucose levels (320 ± 59 mg/dl, P < 0.003). The glucose levels of the wt-GPx(+) mice were not expected to reach that of nontreated mice because the major mechanism of action for STZ is that this agent is an alkylating compound in addition to being an oxidant (14). A dramatic reduction in β-cell immunostaining positively for insulin was observed in the islets of wt-GPx(−) mice compared with wt-GPx(+) mice (Fig. 2B). In the few remaining β-cells of wt-GPx(−) islets, staining for MafA was absent but staining for PDX-1 was positive.
Figure 2.
The adverse effect of STZ treatment is ameliorated in wt-GPx(+) mice. A, Nonfasting plasma glucose levels were significantly greater after multiple low-dose STZ treatment in male wt-GPx(−) (▪, n = 5) compared with wt-GPx(+) (•, n = 7) mice. *, P < 0.003. Levels in both genotypes were higher than those observed in non-STZ-treated male wt-GPx(−) (▴, n = 8) and wt-GPx(+) (♦, n = 6) mice. B, Immunohistochemistry of islets from STZ-treated mice. Double immunostaining for insulin (green) and MafA (red, first set of panels) and PDX-1 (red, second set of panels) in islets from wt-GPx(−) and wt-GPx(+) mice 8 wk after STZ treatment. The marked changes in wt-GPx(−) animals were not observed in wt-GPx(+) animals (magnification, ×80 for the wt-GPx(−) PDX-1 image and ×40 for all other images; bar, 50 μm).
GPx-1 overexpression ameliorates hyperglycemia in db/db mice
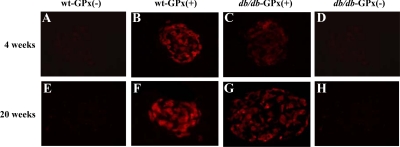
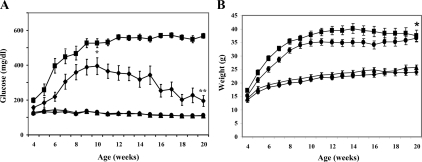
GPx-1 transgenic mice were backcrossed with db/db mice to examine whether the antioxidant enzyme would provide protection against type 2 diabetes. GPx-1 immunostaining was more intense in the islets of transgenic mice [wt-GPx(+) and db/db-GPx(+)] and essentially undetectable in β-cells from the nontransgenic mice [wt-GPx(−) and db/db-GPx(−)] (Fig. 3). Glucose levels in nonfasting mice were measured weekly until 20 wk of age (Fig. 4A). Onset of hyperglycemia was both delayed and attenuated in the db/db-GPx(+) mice compared with their db/db-GPx(−) littermates. After reaching maximum glucose levels of 395 ± 48 mg/dl at 10 wk of age, compared with 526 ± 25 mg/dl (P < 0.009) in the db/db-GPx(−) mice, the glucose levels of the db/db-GPx(+) mice gradually declined by the 20th week to 195 ± 31 mg/dl (vs. 156 ± 12 at the fourth week), whereas the glucose levels in db/db-GPx(−) mice remained elevated (567 ± 14 mg/dl, P < 0.0001). Glucose levels of the wt-GPx(+) mice did not differ from that of their wild-type littermates (111 ± 3 vs. 109 ± 3 mg/dl, respectively). The body weights (Fig. 4B) of the db/db-GPx(+) mice were slightly lower at 4 wk of age but rose at a rate similar to the db/db-GPx(−) animals. From 10 to 20 wk of age, the body weights of db/db-GPx(+) mice did not differ from their db/db-GPx(−) littermates. Both groups of db/db animals had body weights significantly greater than their wt-GPx(−) and wt-GPx(+) littermates (P < 0.0001).
Figure 3.
GPx-1 immunohistochemistry in pancreatic islets of mice at 4 and 20 wk of age. GPx-1 immunostaining was localized to the β-cells of transgenic, GPx(+) mice (B, C, F, and G). In contrast, no GPx-1 staining was observed in the β-cells of nontransgenic, GPx(−) mice (A, D, E, and H) (magnification, ×40).
Figure 4.
Hyperglycemia was reduced and nearly normalized in db/db-GPx(+) mice. A, Plasma glucose levels were measured weekly in the nonfasted state from 4 to 20 wk of age in db/db-GPx(−) (▪, n = 19), db/db-GPx(+) (•, n = 13), wt-GPx(−) (▴, n = 14), and wt-GPx(+) (♦, n = 11) mice. The mean peak glucose value in db/db-GPx(+) animals occurred at 10 wk of age and was significantly lower than the 10-wk value in db/db-GPx(−) animals. *, P < 0.009. The glucose value at the end of the study (20 wk) was also significantly different. **, P < 0.0001. Additionally, the levels for db/db-GPx(+) animals significantly declined from 10 to 20 wk of age (P < 0.04). B, Body weights were measured weekly. At 20 wk of age, body weights of db/db-GPx(−) mice (▪) did not differ from db/db-GPx(+) mice (•); however, both groups of db/db mice had greater weights than wt-GPx(−) (▴) and wt-GPx(+) (♦) mice. *, P < 0.0001.
GPx-1 overexpression prevents the loss of β-cell volume and insulin granulation
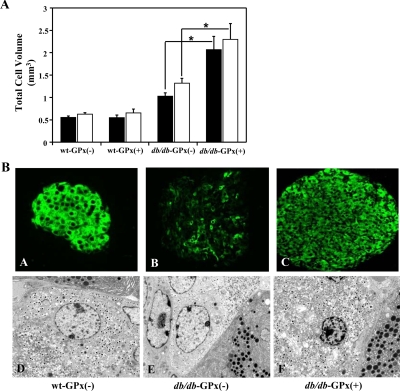
The sizes of the endocrine and β-cell populations were determined in 20-wk-old mice by estimating the volume of total synaptophysin- and insulin-positive cells (Fig. 5A). Both the total islet and β-cell volumes from db/db-GPx(+) mice (2.28 ± 0.36 and 2.06 ± 0.30 mm3, respectively) exceeded (P < 0.01) the volumes observed in the islets from wt-GPx(−) mice (0.61 ± 0.04 and 0.54 ± 0.04 mm3, respectively) and db/db-GPx(−) mice (1.30 ± 0.12 and 1.01 ± 0.08 mm3, respectively). As anticipated (see Materials and Methods), two of the db/db-GPx(−) mice did not become hyperglycemic and provided an obese, nondiabetic mouse comparison. Islets from these mice also had considerable increases in total islet and β-cell volumes (3.17 ± 0.8 and 2.88 ± 0.7 mm3, respectively). Insulin immunostaining and electron microscopy imaging (Fig. 5B) revealed attenuated insulin staining and insulin granulation in the β-cells of db/db-GPx(−) mice, whereas β-cells from db/db-GPx(+) mice had insulin staining and granulation similar to that of wt-GPx(−) mice.
Figure 5.
Islets from db/db-GPx(+) mice had larger β-cell volume and an increase in insulin staining and granulation at 20 wk of age compared with that of db/db-GPx(−) mice. A, Total islet (open bars) and β-cell (solid bars) volume measurements. Compared with wt-GPx(−) and wt-GPx(+) mice (n = 5), both db/db-GPx(−) (n = 5) and db/db-GPx(+) (n = 3) mice had greater cell volumes, and the volumes in db/db-GPx(+) were greater than those in db/db-GPx(−) mice. *, P < 0.01. B, Insulin immunohistochemistry (magnification, ×40) and electron microscopy images (magnification, ×3700) of β-cells. Islets of diabetic db/db-GPx(−) mice showed weak insulin immunostaining in most of the β-cells (B) and a reduced number of insulin granules (E). In contrast, the transgenic db/db-GPx(+) mouse islets displayed an insulin staining intensity (C) and insulin granule density (F) similar to that of the wild-type, wt-GPx(−) islets (A and D).
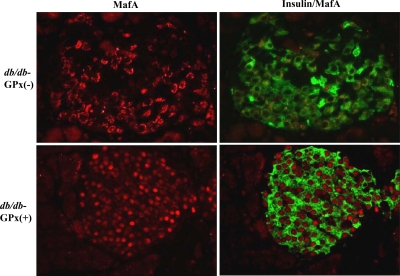
GPx-1 overexpression prevents the loss of intranuclear MafA
To assess whether MafA, a critically important activator of insulin gene transcription, was affected in the transgenic mice, immunohistochemical analysis of MafA expression was performed. In hyperglycemic db/db-GPx(−) mice, MafA was virtually absent in the nucleus and located only in the cytoplasmic/perinuclear space. However, MafA was present only in the nucleus of β-cells in db/db-GPx(+) (Fig. 6), wt-GPx(−), and wt-GPx(+) mice (supplemental Fig. S1 published on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org). In contrast, intranuclear expression of PDX-1, another important regulator of the insulin gene, was not adversely affected in db/db-GPx(−) mice. Immunostaining of pancreatic sections for PDX-1 revealed β-cell nuclear localization at 20 wk of age in all genotypes (supplemental Fig. S2).
Figure 6.
Immunohistochemistry for insulin and MafA in the islets of 20-wk-old mice. Double immunostaining for insulin (green) and MafA (red). MafA was observed only in the nucleus of β-cells in normoglycemic db/db-GPx(+) mice, whereas it was only in the cytoplasm of β-cells in hyperglycemic db/db-GPx(−) mice (n = 3) (magnification, ×40).
Discussion
These studies were designed to ascertain whether overexpression of GPx-1, specifically in β-cells, would ameliorate hyperglycemia and, if so, what mechanisms might be involved. GPx-1 overexpression in wild-type animals protected them from STZ treatment, a chemical model of diabetes. Mice that overexpressed GPx-1 were protected from STZ-induced β-cell damage and loss of intranuclear MafA. Complete normalization of glycemia was not expected due to the dual action of STZ whereby its major action is as an alkylating compound (14). GPx-1 overexpression in db/db mice, a model of increased genetic diabetes susceptibility, led to initial amelioration and then reversal of hyperglycemia without use of hypoglycemic agents. This outcome was accompanied by significant increases in β-cell volume as well as increased insulin staining and granulation. MafA was located in the cytoplasm only of β-cells in db/db-GPx(−) mice, whereas MafA was located within the nucleus (its site of action) in the β-cells of db/db-GPx(+) mice. These findings in a genetic model of diabetes are novel and support the concept that progressive deterioration in the function of β-cells via continually elevated glucose levels and oxidative stress can be compensated for by enhancing endogenous antioxidant protection of the β-cell with GPx-1.
The behavior of blood glucose levels during the first 10 wk was anticipated based on our previous work with antioxidant treatment in the Zucker diabetic fatty rat (15). In those studies n-acetylcysteine (NAC) and aminoguanidine individually ameliorated the degree of hyperglycemia developed by the animals. NAC treatment has also been shown to reduce plasma glucose levels in db/db mice (16). The current study is unique in that the glycemic levels nearly returned to 4-wk-old levels by 20 wk of age, even after a period of elevated glucose levels at 8–12 wk of age, and points to the effectiveness of enhancing GPx-1 levels within the β-cell. The GPx-1 transgene is driven by the insulin promoter, which is glucose responsive. Therefore, we speculate that as glucose levels gradually increased, stimulation of the insulin promoter led to greater expression of GPx-1 and thus a higher degree of antioxidant protection and reversal of β-cell dysfunction.
The demand for insulin production in mice with a db/db background was met much more successfully by β-cells that overexpressed GPx-1 as evidenced by the increase in β-cell volume and insulin granulation observed in the islets of db/db-GPx(+) mice. Similarly, treatment of db/db mice with a combination of NAC, vitamin C, and vitamin E increased β-cell mass and insulin content (16).
Preservation of intranuclear MafA is one mechanism involved in the protection of β-cells by GPx-1 overexpression. This conclusion is strongly supported by evidence from previously published studies from our laboratory and others. MafA has been shown to be a critical transcription factor in the regulation of the insulin gene (17,18). Mutations within the MafA binding site of the insulin promoter virtually abolish insulin promoter activity (19,20), and its absence in nuclear extracts seriously disrupts insulin gene expression (15,20). Reconstitution of glucotoxic HIT-T15 cells devoid of intranuclear MafA with exogenous MafA significantly restores the loss of insulin promoter activity (21). It has been demonstrated that MafA is sensitive to proteosomal degradation (21,22), especially under conditions of oxidative stress (15,21). Additionally, we have shown that treatment with the antioxidant NAC prevented the loss of MafA protein and its binding to the insulin promoter under hyperglycemic conditions (21). Reducing oxidative stress in the β-cell with GPx-1 preserves intranuclear MafA in which it is able to transactivate the insulin gene. That islet staining for PDX-1 protein did not differ between the hyperglycemic and normoglycemic mice in the current study points to the specificity and importance of the intranuclear MafA defect in the islets of db/db mice. This is similar to observations made in βTC-6 cells in which PDX-1 binding to the insulin promoter was not altered under chronic hyperglycemic conditions, whereas the binding of MafA was reduced (20). Alternatively, timing may be a factor such that MafA protein stability is affected by oxidative stress early in the manifestation of diabetes, whereas PDX-1 protein is more stable and may be affected later in the disease. In HIT-T15 cells cultured under high glucose conditions, the reduction of MafA binding to the insulin promoter occurred much earlier than the loss of PDX-1 binding (23) and more closely correlated with decreases in insulin mRNA and content levels (24). Therefore, a mechanism for protection of β-cells in db/db mice by GPx-1 likely involves preservation of MafA localization to the nucleus.
The scientific literature in this area of research is large and consistent with the concept of beneficial effects of antioxidants and antioxidant enzyme expression on β-cells undergoing oxidative stress. Cheng et al. (25) reported that reducing ROS levels in the islets of db/db mice with drugs improved β-cell structure and function. Additionally, treatment of db/db mice with NAC improved nonfasting glucose levels, and β-cell mass was increased after treatment with a combination of NAC, vitamin C, and vitamin E (16). Studies on the effects of antioxidant overexpression on β-cell function have also been reported. Whereas overexpression of Cu/Zn-SOD has been shown to protect against nitric oxide cytotoxicity (26) and alloxan and streptozotocin-induced diabetes (7,27), others have found that it leads to an increased sensitivity to oxidative stress (28). Combinatorial overexpression of catalase (29) or GPx (28,30) was necessary to improve the protective effect of Cu/Zn-SOD or MnSOD. β-Cell-specific overexpression of catalase alone resulted in a partial protection of mouse β-cells from H2O2 and streptozotocin treatment but offered no protection against the cytokine IL-1β (31). Whereas overexpression of GPx-1 in cell lines is generally beneficial, global overexpression in animals is not always as favorable (for review see Ref. 32). McClung et al. (33) generated transgenic mice that overexpressed GPx throughout all tissues. These animals became insulin resistant and developed both hyperglycemia and hyperinsulinemia.
Our study is unique in that it establishes the first animal model of β-cell-specific overexpression of GPx-1. Similar to the db/db-TRX(+) transgenic mice, in which thioredoxin is overexpressed in the β-cell (34), we observed a delay in the progression of hyperglycemia in db/db-GPx(+) mice. The peak glucose values in both transgenic lines were significantly attenuated compared with nontransgenic db/db mice. In distinction, however, the glycemic levels of db/db-GPx(+) mice nearly returned to the 4 wk level by 20 wk of age, whereas the glycemic levels of the db/db-TRX(+) mice did not reverse. The difference in effectiveness between overexpression of GPx-1 in our study and TRX in the previous study might be due to the levels of overexpressed proteins, specific efficacy for β-cells, or mechanisms and targets of action (35). Furthermore, db/db-GPx(+) β-cells were positive for intranuclear MafA expression, whereas db/db-TRX(+) mouse β-cells were not. The difference between these two transgenic models with regard to the MafA intranuclear location is likely to be relevant to the mechanism by which the db/db-GPx(+) mice have markedly better control of glycemia.
In conclusion, β-cell-specific GPx-1 overexpression in db/db mice protects the β-cell from oxidative stress and progression in dysfunction associated with chronic hyperglycemia. These results have implications for considering antioxidants as ancillary therapy in situations in which conventional treatment of type 2 diabetes is insufficient to achieve continuous normoglycemia.
Supplementary Material
Acknowledgments
The excellent technical assistance of Mark Caldwell for preparation of the transgenic construct and initial characterization of transgenic mice is appreciated.
Footnotes
This work was supported by National Institutes of Health Grants NIDDK RO1 38325 (to R.P.R.), F32 DK078492 (to M.B.), and NIDDK RO1 DK63159 (to R.C.L.) and American Diabetes Association Mentor Based Fellowship (to R.P.R.).
Current address for S.S.M.M.: Department of Basic Science, University of Texas Southwestern, 5323 Harry Hines Boulevard, Dallas, Texas 75390.
Disclosure Summary: The authors have nothing to declare.
First Published Online October 9, 2009
Abbreviations: GPx, Glutathione peroxidase; MafA, musculoaponeurotic fibrosarcoma oncogene homolog A; NAC, n-acetylcysteine; PDX, pancreatic duodenal homeobox; ROS, reactive oxygen species; SOD, superoxide dismutases; STZ, streptozotocin.
References
- Robertson RP 2004 Chronic oxidative stress as a central mechanism for glucose toxicity in pancreatic islet beta cells in diabetes. J Biol Chem 279:42351–42354 [DOI] [PubMed] [Google Scholar]
- Robertson RP, Harmon JS 2006 Diabetes, glucose toxicity, and oxidative stress: a case of double jeopardy for the pancreatic islet β-cell. Free Radic Biol Med 41:177–184 [DOI] [PubMed] [Google Scholar]
- Grankvist K, Marklund SL, Täljedal IB 1981 CuZn-superoxide dismutase, Mn-superoxide dismutase, catalase and glutathione peroxidase in pancreatic islets and other tissues in the mouse. Biochem J 199:393–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiedge M, Lortz S, Drinkgern J, Lenzen S 1997 Relation between antioxidant enzyme gene expression and antioxidative defense status of insulin-producing cells. Diabetes 46:1733–1742 [DOI] [PubMed] [Google Scholar]
- Lenzen S, Drinkgern J, Tiedge M 1996 Low antioxidant enzyme gene expression in pancreatic islets compared with various other mouse tissues. Free Radic Biol Med 20:463–466 [DOI] [PubMed] [Google Scholar]
- Tonooka N, Oseid E, Zhou H, Harmon JS, Robertson RP 2007 Glutathione peroxidase protein expression and activity in human islets isolated for transplantation. Clin Transplant 21:767–772 [DOI] [PubMed] [Google Scholar]
- Kubisch HM, Wang J, Bray TM, Phillips JP 1997 Targeted overexpression of Cu/Zn superoxide dismutase protects pancreatic β-cells against oxidative stress. Diabetes 46:1563–1566 [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Tran PO, Harmon J, Robertson RP 2002 A role for glutathione peroxidase in protecting pancreatic β cells against oxidative stress in a model of glucose toxicity. Proc Natl Acad Sci USA 99:12363–12368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Alessio DA, Verchere CB, Kahn SE, Hoagland V, Baskin DG, Palmiter RD, Ensinck JW 1994 Pancreatic expression and secretion of human islet amyloid polypeptide in a transgenic mouse. Diabetes 43:1457–1461 [DOI] [PubMed] [Google Scholar]
- Nukatsuka M, Sakurai H, Yoshimura Y, Nishida M, Kawada J 1988 Enhancement by streptozotocin of O2− radical generation by the xanthine oxidase system of pancreatic β-cells. FEBS Lett 239:295–298 [DOI] [PubMed] [Google Scholar]
- Friesen NT, Büchau AS, Schott-Ohly P, Lgssiar A, Gleichmann H 2004 Generation of hydrogen peroxide and failure of antioxidative responses in pancreatic islets of male C57BL/6 mice are associated with diabetes induced by multiple low doses of streptozotocin. Diabetologia 47:676–685 [DOI] [PubMed] [Google Scholar]
- Ohkuwa T, Sato Y, Naoi M 1995 Hydroxyl radical formation in diabetic rats induced by streptozotocin. Life Sci 56:1789–1798 [DOI] [PubMed] [Google Scholar]
- Meghana K, Sanjeev G, Ramesh B 2007 Curcumin prevents streptozotocin-induced islet damage by scavenging free radicals: a prophylactic and protective role. Eur J Pharmacol 577:183–191 [DOI] [PubMed] [Google Scholar]
- Lenzen S 2008 The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia 51:216–226 [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Gleason CE, Tran PO, Harmon JS, Robertson RP 1999 Prevention of glucose toxicity in HIT-T15 cells and Zucker diabetic fatty rats by antioxidants. Proc Natl Acad Sci USA 96:10857–10862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneto H, Kajimoto Y, Miyagawa J, Matsuoka T, Fujitani Y, Umayahara Y, Hanafusa T, Matsuzawa Y, Yamasaki Y, Hori M 1999 Beneficial effects of antioxidants in diabetes: possible protection of pancreatic β-cells against glucose toxicity. Diabetes 48:2398–2406 [DOI] [PubMed] [Google Scholar]
- Matsuoka TA, Kaneto H, Stein R, Miyatsuka T, Kawamori D, Henderson E, Kojima I, Matsuhisa M, Hori M, Yamasaki Y 2007 MafA regulates expression of genes important to islet β-cell function. Mol Endocrinol 21:2764–2774 [DOI] [PubMed] [Google Scholar]
- Olbrot M, Rud J, Moss LG, Sharma A 2002 Identification of β-cell-specific insulin gene transcription factor RIPE3b1 as mammalian MafA. Proc Natl Acad Sci USA 99:6737–6742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Fusco-DeMane D, Henderson E, Efrat S, Stein R 1995 The role of the insulin control element and RIPE3b1 activators in glucose-stimulated transcription of the insulin gene. Mol Endocrinol 9:1468–1476 [DOI] [PubMed] [Google Scholar]
- Poitout V, Olson LK, Robertson RP 1996 Chronic exposure of βTC-6 cells to supraphysiologic concentrations of glucose decreases binding of the RIPE3b1 insulin gene transcription activator. J Clin Invest 97:1041–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon JS, Stein R, Robertson RP 2005 Oxidative stress-mediated, post-translational loss of MafA protein as a contributing mechanism to loss of insulin gene expression in glucotoxic β cells. J Biol Chem 280:11107–11113 [DOI] [PubMed] [Google Scholar]
- Han SI, Aramata S, Yasuda K, Kataoka K 2007 MafA stability in pancreatic β-cells is regulated by glucose and is dependent on its constitutive phosphorylation at multiple sites by glycogen synthase kinase 3. Mol Cell Biol 27:6593–6605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon JS, Tanaka Y, Olson LK, Robertson RP 1998 Reconstitution of glucotoxic HIT-T15 cells with somatostatin transcription factor-1 partially restores insulin promoter activity. Diabetes 47:900–904 [DOI] [PubMed] [Google Scholar]
- Robertson RP, Zhang HJ, Pyzdrowski KL, Walseth TF 1992 Preservation of insulin mRNA levels and insulin secretion in HIT cells by avoidance of chronic exposure to high glucose concentrations. J Clin Invest 90:320–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Q, Law PK, de Gasparo M, Leung PS 2008 Combination of DPP-IV inhibitor LAF237 with AT1 receptor antagonist valsartan enhances pancreatic islet morphology and function in a mouse model of type 2 diabetes. J Pharmacol Exp Ther 327:683–691 [DOI] [PubMed] [Google Scholar]
- Moriscot C, Pattou F, Kerr-Conte J, Richard MJ, Lemarchand P, Benhamou PY 2000 Contribution of adenoviral-mediated superoxide dismutase gene transfer to the reduction in nitric oxide-induced cytotoxicity on human islets and INS-1 insulin-secreting cells. Diabetologia 43:625–631 [DOI] [PubMed] [Google Scholar]
- Kubisch HM, Wang J, Luche R, Carlson E, Bray TM, Epstein CJ, Phillips JP 1994 Transgenic copper/zinc superoxide dismutase modulates susceptibility to type 1 diabetes. Proc Natl Acad Sci USA 91:9956–9959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amstad P, Moret R, Cerutti P 1994 Glutathione peroxidase compensates for the hypersensitivity of Cu,Zn-superoxide dismutase overproducers to oxidant stress. J Biol Chem 269:1606–1609 [PubMed] [Google Scholar]
- Lortz S, Tiedge M 2003 Sequential inactivation of reactive oxygen species by combined overexpression of SOD isoforms and catalase in insulin-producing cells. Free Radic Biol Med 34:683–688 [DOI] [PubMed] [Google Scholar]
- Lepore DA, Shinkel TA, Fisicaro N, Mysore TB, Johnson LE, d'Apice AJ, Cowan PJ 2004 Enhanced expression of glutathione peroxidase protects islet β cells from hypoxia-reoxygenation. Xenotransplantation 11:53–59 [DOI] [PubMed] [Google Scholar]
- Xu B, Moritz JT, Epstein PN 1999 Overexpression of catalase provides partial protection to transgenic mouse β cells. Free Radic Biol Med 27:830–837 [DOI] [PubMed] [Google Scholar]
- Arthur JR 2000 The glutathione peroxidases. Cell Mol Life Sci 57:1825–1835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung JP, Roneker CA, Mu W, Lisk DJ, Langlais P, Liu F, Lei XG 2004 Development of insulin resistance and obesity in mice overexpressing cellular glutathione peroxidase. Proc Natl Acad Sci USA 101:8852–8857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Yamato E, Toyoda S, Tashiro F, Ikegami H, Yodoi J, Miyazaki J 2008 Transgenic expression of antioxidant protein thioredoxin in pancreatic β cells prevents progression of type 2 diabetes mellitus. Antioxid Redox Signal 10:43–49 [DOI] [PubMed] [Google Scholar]
- Nordberg J, Arnér ESJ 2001 Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic Biol Med 31:1287–1312 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.