Abstract
Background
Children with severe allergic asthma have persistent airway inflammation and oxidant stress.
Objectives
We hypothesized that children with severe allergic asthma would have increased concentrations of the NO oxidation products nitrite, nitrate, and nitrotyrosine in the proximal and distal airway epithelial lining fluid (ELF). We further hypothesized that NO oxidation products would be associated with higher exhaled nitric oxide (FENO), greater allergic sensitization, and lower pulmonary function.
Methods
Bronchoalveolar lavage (BAL) was obtained from 15 children with mild-to-moderate asthma, 30 children with severe allergic asthma, 5 non-asthmatic children and 20 non-smoking adults. The BAL was divided into proximal and distal portions and nitrite, nitrate, and nitrotyrosine were quantified.
Results
Children with mild-to-moderate and severe allergic asthma had increased concentrations of nitrite (adult control: 15 ± 3; pediatric control: 23 ± 4; mild-to-moderate asthma: 56 ± 26; severe asthma: 74 ± 18 µM), nitrate (37 ± 13 vs. 145 ± 38 vs. 711 ± 155 vs. 870 ± 168 µM) and nitrotyrosine (2 ± 1 vs. 3 ± 1 vs. 9 ± 3 vs. 10 ± 4 µM) in the proximal ELF. Similar results were seen in the distal ELF although the concentrations were significantly lower (p < 0.05 for each). Although univariate analyses revealed no associations between NO oxidation products and clinical features, multivariate analyses revealed FENO to be a significant predictor of NO oxidation in asthmatic children.
Conclusions
NO oxidation products are increased in the ELF of asthmatic children. The relationship between FENO and airway nitrosative stress is complicated and requires further study.
Keywords: Asthma, Children, Nitric oxide, Nitrogen oxides, Nitrosation, Nitrosative Stress, Reactive nitrogen species
INTRODUCTION
Severe allergic asthma in school-age children is a complex disorder characterized by persistent airway inflammation, ongoing symptoms, and increased exhaled nitric oxide (FENO) concentrations despite treatment with high doses of inhaled and oral corticosteroids.1,2 Although airway nitric oxide (NO) is essential for epithelial signaling and host defense, excessive NO production results in NO oxidation and potential toxicity.3 This process of excessive NO oxidation is commonly referred to as “nitrosative stress”4 and ultimately promotes protein nitration, resulting in structural and functional protein alterations that may enhance the inflammatory response.5 Thus, excessive airway NO concentrations in children with severe allergic asthma may contribute to an ongoing cycle of airway destruction with airway injury.6
In the human airway, the most readily detectable NO oxidation products include nitrite (NO2−) and nitrate (NO3−), which can be derived from NO through a series of reactions involving superoxide anion (O·2−) and oxygen (Figure 1). Nitrotyrosine is also easily measured in airway samples and reflects the overall degree of protein nitration.7 Indeed, previous studies have noted increased nitrite, nitrate and nitrotyrosine concentrations in the exhaled breath condensate of asthmatic children8–10 and in the epithelial lining fluid (ELF) of adults with mild-to-moderate and severe asthma.11,12 However, no study to date has examined NO oxidation products in the ELF of asthmatic children. Because children with severe asthma have profound airway oxidant stress,13 the purpose of this study was to quantify NO oxidation products in the ELF of children with mild-to-moderate and severe allergic asthma. The secondary purpose of this study was to determine the association between increased ELF NO oxidation products and clinical features of asthma severity in children. We hypothesized that children with severe allergic asthma would have increased concentrations of the NO oxidation products nitrite, nitrate, and nitrotyrosine in the proximal and distal airway epithelial lining fluid (ELF). We further hypothesized that these increased NO oxidation products would be associated with increased FENO, greater allergic sensitization, and lower pulmonary function.
Figure 1.
Diagram of nitric oxide (NO) metabolite formation in the airways.
METHODS
Sample
Children 5–17 years of age with symptomatic asthma attending an asthma clinic at Emory University were invited to participate in this study. Asthmatic children met published criteria for persistent asthma14 and had a history of at least a 12% change in the forced expiratory volume in one second (FEV1) after albuterol administration.15 Severe asthma was diagnosed according to criteria developed by the NIH/NHLBI Severe Asthma Research Program,1 which were adapted from the American Thoracic Society’s Consensus Panel Report (Online Repository, Table E1).16 Thresholds for high-dose inhaled corticosteroids (ICS) were defined as ≥ 440 mcg of fluticasone equivalent per day for children less than 12 years and ≥ 880 mcg for children 12–17 years of age.14 Children with severe allergic asthma were treated with a stable dose of ICS or oral corticosteroids for at least 6 months prior to recruitment. Adherence to ICS therapy was monitored by an analysis of prescription refills. Informed consent was obtained from all caregivers. Children also provided verbal and written assent.
Children who fit criteria for severe allergic asthma underwent flexible bronchoscopy with bronchoalveolar lavage (BAL) as indicated for persistent asthma symptoms despite appropriate treatment with high-dose inhaled and systemic corticosteroids.17 Children with mild-to-moderate asthma underwent bronchoscopy for suspected foreign body aspiration, recurrent pneumonia, persistent cough, and suspected congenital anomalies. Controls for this study included children with psychogenic (habit) cough or vocal cord dysfunction undergoing bronchoscopy for definitive diagnosis and healthy, non-smoking adult volunteers. Control subjects were nonsmokers with no family history of asthma and a negative bronchodilator response.
Procedures
Spirometry was performed before and after 2 inhalations of albuterol sulfate (90 µg/inhalation) with a portable spirometer (KoKo® Legend, Ferraris, Louisville, CO). The results fulfilled ATS criteria for reproducibility18 and were interpreted according to reference standards.19 Atopic sensitization was assessed by skin prick testing using a standard kit (Multi-Test® II, Lincoln Diagnostics, Decatur, IL) containing tree pollen, grass pollen, ragweed pollen, weed pollen, dog hair, cat epithelium, alternaria, cladosporidium, aspergillus, Dermatophagoides pteronyssinus, Dermatophagoides farinae, cockroach, normal saline, and histamine extracts (Greer Laboratories, Lenoir, NC). The application site was examined 15 minutes after application and considered positive if both a wheal ≥3 mm diameter and erythema ≥ 10 mm diameter were present.
On the day of bronchoscopy, participants submitted FENO samples and underwent venipuncture. FENO was collected with a reservoir bag within 1 hour prior to bronchoscopy. For this procedure, subjects took two tidal breaths of NO-free air through a scrubbing filter, followed by a 6-second exhalation at a fixed flow rate of 0.35 L/second.20 The first 150 mL exhaled were discarded. Subjects repeated this procedure three times. The resulting samples were analyzed offline by chemiluminescence (Sievers NOA™ 280-I, Ionic Instruments, Boulder, CO) within 1 hour of collection. The data were averaged to reflect mean FENO. Serum immunoglobulin (IgE) concentrations and plasma urea were determined after venipuncture.
Bronchoscopy in pediatric participants was performed by pediatric pulmonologists using a laryngeal mask airway. BAL fluid was collected from the right middle lobe with three 1 mL/kg (50 mL maximum) saline lavages flushed through the suction channel of a flexible bronchoscope (Olympus BF-3C160 [3.7 mm] or BF-P160 [4.9 mm], Olympus America Inc., Melville, NY). Bronchoscopy was performed in adults using a flexible bronchoscope (Olympus BF-1T20D) passed trans-nasally into the right middle lobe. Three 50 mL saline aliquots were instilled and immediately aspirated. The first lavage from all participants was reserved for evaluation of proximal airway constituents.21 The second and third lavages were pooled for distal airway constituent analysis. In children, the BAL return volume was divided between the research and clinical laboratories.
BAL was centrifuged at 1200 rpm within 1 hour of collection for 7 minutes at 4° C to separate the supernatant and cellular fractions. Given the limited number of cells present in the proximal airway lavage, the cell pellets from the proximal and distal airway lavages were pooled and resuspended in 10 mL of Dulbecco’s Modified Eagles Medium with 10% fetal calf serum for cell counting. Total cell counts were performed manually with a hemocytometer and cellular differentials were determined from 300 consecutive cells after Wright staining.
The protein content of the BAL supernatant was assessed using a Coomassie (Bradford) protein assay (Pierce Biotechnology, Rockford, IL) read at an absorbance of 595 nm with a detection limit 1 µg/mL. Urea nitrogen was measured in plasma and BAL supernatant using a quantitative colorimetric assay (Pointe Scientific, Canton, MI) with sensitivity of 0.05 to 150 mg/dL. The dilution of the proximal and distal BAL was calculated from [urea]plasma/[urea]BAL.22
Nitrite and total nitrite + nitrate concentrations were determined from the BAL supernatant using a colorimetric assay (Cayman Chemical, Ann Arbor, MI) analyzed at 540 nM with a lower detection limit of 0.1 uM. All samples were analyzed in duplicate. For this assay, nitrate was converted to nitrite with nitrite reductase, followed by the addition of Griess reagent. Nitrate concentrations were determined by subtracting the concentration of nitrite from total nitrite + nitrate. To minimize false nitrate and nitrate readings during the assay, samples were analyzed immediately after thawing. The background nitrite and nitrate content in the saline lavage fluid was pre-determined and subtracted from the final concentration values.
Nitrotyrosine concentrations were determined spectrophotometrically using a microplate sandwich ELISA (Oxis International, Foster City, CA) with sensitivity of 2.0 nM and inter-assay precision of 11%. Samples were analyzed in duplicate and corrected for the background levels of nitrotyrosine in the saline lavage fluid. Absorbance was measured at 450 nm.
Statistical analysis
Data were analyzed with SPSS® software (Version 15, SPSS Inc., Chicago, IL). Nitrite, nitrate, and nitrotyrosine from the proximal and distal airway lavage were adjusted according to the urea dilution22 and were logarithmically transformed. Nitrotyrosine concentrations were further adjusted for the total protein content of the BAL supernatant. Differences between groups and post-hoc tests were assessed by Kruskal-Wallis tests and Mann-Whitney U tests, respectively. Pearson correlations were used to examine associations between NO oxidation products and clinical features. To evaluate factors that might affect NO oxidation in the ELF of asthmatic children, multivariate backward elimination linear regression was performed using total nitrite + nitrate concentrations in the proximal and distal ELF as dependent variables and age, gender, ethnicity, ICS dose, FEV1, FEV1 bronchodilator reversibility, serum IgE, history of hospitalization, FENO, and the percentage of airway eosinophils and neutrophils as predictors. Multicollinearity between predictors was assessed with tolerance statistics. Entry and removal probabilities were set at 0.05 and 0.10, respectively. Significance was defined as a two-tailed α ≤ 0.05 for all tests.
RESULTS
Initially, 49 asthmatic children (severe asthma, n = 32), 7 pediatric controls, and 20 healthy adult controls were recruited for this study. However, five children, including 2 pediatric controls, 2 mild-to-moderate asthmatics, and 2 severe asthmatics were infected with Streptococcus pneumoniae, Haemophilus influenzae, and/or Moraxella catarrhalis and were excluded from data analysis due to potential denitrification.23 The features of the excluded children appear in the online repository (Online repository, Tables E2–E3). Thus the final sample included in data analysis contained 30 children with severe allergic asthma, 15 children with mild-to-moderate asthma, 5 pediatric controls, and 20 adult controls.
Because bronchoscopy was performed only for clinical indications, all of the asthmatics were symptomatic. None of the children with mild-to-moderate asthma had evidence of airway infection or chronic aspiration syndromes. The features of the final sample are presented in Table I. Whereas all (100%) children with severe asthma had allergic sensitization, allergic sensitization was present in only half (53%) of the children with mild-to-moderate asthma (Online repository Table E4). Children with severe allergic asthma were also treated with higher doses of ICS but had significantly lower baseline pulmonary function and increased bronchodilator reversibility. Whereas FENO was elevated in both groups of asthmatics, there were no differences in FENO between children with mild-to-moderate and severe allergic asthma (Table I).
Table I.
Features of the sample. Data represent the mean ± SD or the frequency (%).
| Adult control (n = 20) |
Pediatric control (n = 5) |
Mild-to-Moderate asthma (n = 15) |
Severe asthma (n = 30) |
|
|---|---|---|---|---|
| Age (in years) | 39 ± 10 | 11 ± 4a | 10 ± 4a | 10 ± 4a |
| Male gender | 8 (40) | 3 (60) | 10 (67) | 15 (50) |
| Caucasian | 8 (40) | 4 (80) | 14 (93)a | 9 (30)b,c |
| African-American | 11 (55) | 1 (20) | 1 (7)a | 20 (67)b,c |
| ICS dose (µg fluticasone/day) | 0 | 0 | 262 ± 189a,b | 917 ± 236a,b,c |
| Asthma medications | ||||
| Budesonide | 0 | 0 | 3 (20) | 7 (23) |
| Fluticasone | 0 | 0 | 1 (7) | 1 (3) |
| Fluticasone/salmeterol | 0 | 0 | 8 (53)a,b | 22 (73)a,b,c |
| Montelukast | 0 | 0 | 10 (67)a,b | 28 (93)a,b,c |
| Prednisone | 0 | 0 | 0 | 11 (37)a,b,c |
| Emergency room visit (previous year) | 0 | 0 | 3 (20) | 28 (93)a,b,c |
| Hospitalization (previous year) | 0 | 0 | 1 (7) | 26 (87)a,b,c |
| Intensive Care Unit admission (ever) | 0 | 0 | 0 | 14 (47)a,b,c |
| Intubation (ever) | 0 | 0 | 0 | 6 (20)a,b,c |
| FVC (% predicted) | 98 ± 16 | 102 ± 18 | 102 ± 15 | 87 ± 19a,b,c |
| FEV1 (% predicted) | 103 ± 16 | 101 ± 15 | 100 ± 15 | 73 ± 20a,b,c |
| FEV1 FVC | 0.86 ± 0.07 | 0.89 ± 0.03 | 0.87 ± 0.06 | 0.74 ± 0.12a,b,c |
| FEF25–75 (% predicted) | 121 ± 32 | 92 ± 16a | 94 ± 23a | 51 ± 25a,b,c |
| FEV1 bronchodilator reversibility (%)1 | 3 ± 6 | 6 ± 5 | 9 ± 11 | 23 ± 17 |
| FENO (offline, ppb) | 5 ± 3 | 7 ± 4 | 11 ± 12a | 13 ± 10a,b |
| Elevated baseline FENO (> 10 ppb) | 4 (20) | 2 (40) | 3 (20) | 20 (67)a,b,c |
| Reported allergies | Not assessed | 2 (40) | 9 (60) | 25 (83) |
| Reported atopic dermatitis | Not assessed | 0 | 5 (33) | 21 (70) |
| Number of skin prick responses | Not assessed | 0 | 2 ± 2 | 5 ±3b,c |
| Serum IgE (kU/L) | 100 ± 194 | 80 ± 64 | 94 ± 139 | 487 ± 730a,b,c |
Calculated by: [(FEV1; post-bronchodilator – FEV1 pre-bronchodilator)/predicted FEV1]*100
p < 0.05 vs. adult control
p < 0.05 vs. pediatric control
p < 0.05 vs. mild-to-moderate asthma
The characteristics of the BAL fluid are presented in the online repository (Table E5). Although larger lavage volumes were used for adult controls, the percentage of BAL return was similar between adult and pediatric controls (proximal lavage: 23 vs. 27%; distal lavage: 49 vs. 35% for adult vs. pediatric controls). However, the BAL samples from adult controls were characterized by higher total cell counts (adult control: 7.81 ± 3.61; pediatric control: 3.53 ± 2.32; mild-to-moderate asthma: 3.81 ± 3.06; severe asthma: 3.32 ± 2.02 × 106, p < 0.01). Whereas severe asthmatics had the highest percentage of BAL eosinophils (adult control: 0.4 ± 0.5; pediatric control: 0.3 ± 0.5; mild-to-moderate asthma: 0.7 ± 0.7; severe asthma: 1.9 ± 3.2%, p = 0.03), mild-to-moderate and severe asthmatics had higher percentages of neutrophils compared to both groups of controls (adult control: 3.5 ± 3.2; pediatric control: 3.2 ± 1.4; mild-to-moderate asthma: 5.3 ± 3.9; severe asthma: 5.2 ± 3.2, p = 0.04).
NO oxidation products in the proximal and distal airway lavage
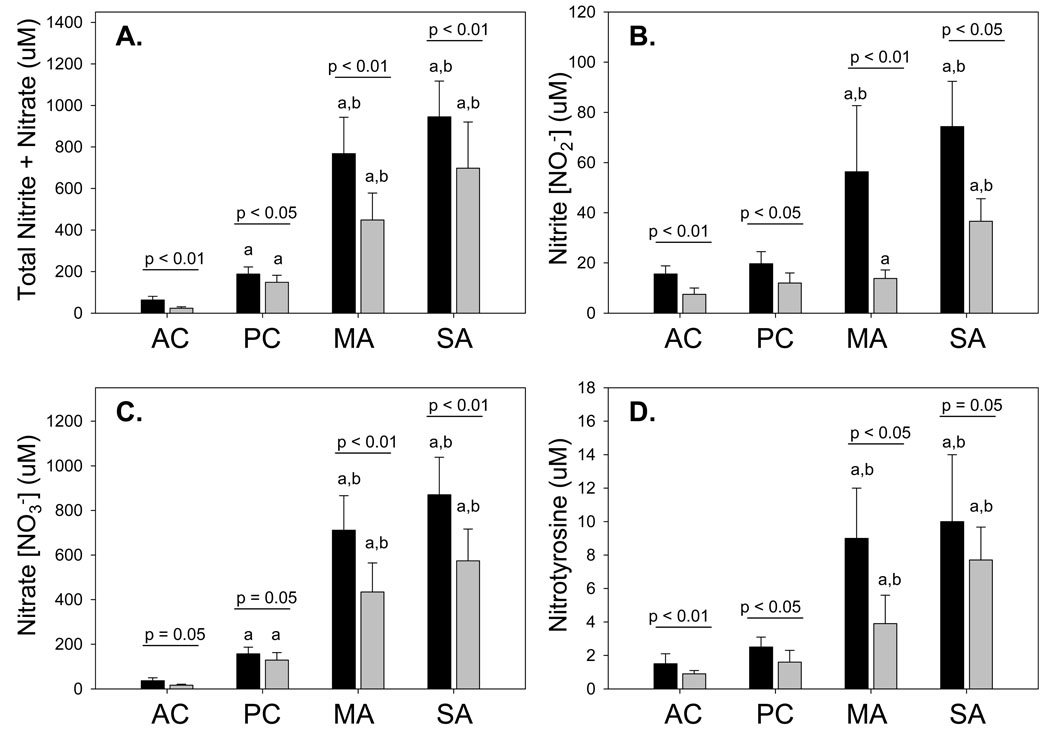
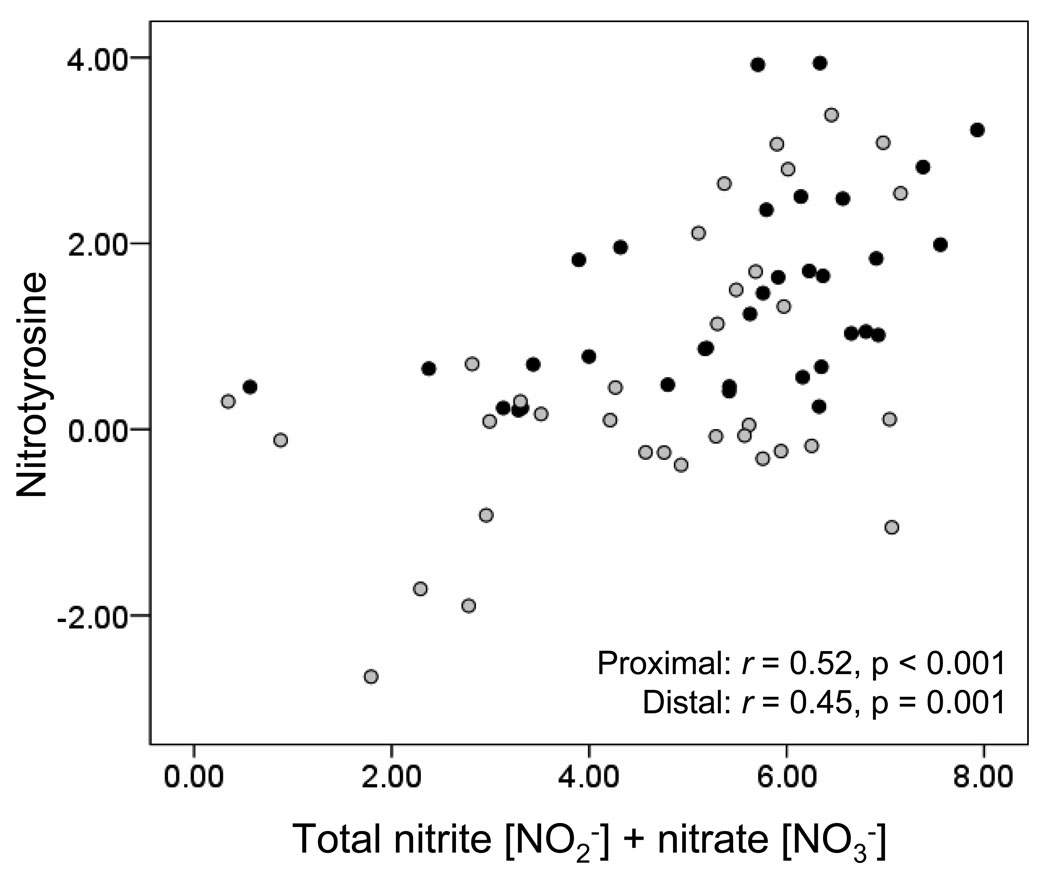
Compared to controls, children with mild-to-moderate and severe allergic asthma had significantly higher concentrations of nitrite, nitrate and nitrotyrosine in the ELF (Figure 2). However, no significant differences in NO oxidation products were observed between children with mild-to-moderate and severe allergic asthma. In each group, nitrate was the most abundant NO oxidation product measured, with concentrations nearly 10-fold higher than those of nitrite. Furthermore, nitrite, nitrate and nitrotyrosine concentrations were also consistently higher in the proximal versus the distal airway ELF (Figure 2). Similar increases in NO oxidation products were also apparent in the raw BAL samples without adjustment for the urea dilution (Online repository Figure E1). Analysis of the entire sample (all asthmatics and controls) revealed strong correlations between proximal and distal airway ELF concentrations of total nitrite + nitrate (r = 0.76, p < 0.01), nitrite (r = 0.50, p < 0.01), nitrate (r = 0.76, p < 0.01), and nitrotyrosine (r = 0.34, p = 0.02). When this analysis was restricted only to asthmatic children, similar correlations between the proximal and distal ELF NO oxidation products were observed (total nitrite + nitrate: r = 0.44, p = < 0.01; nitrite: r = 0.58, p < 0.01; nitrate: r = 0.31, p = 0.05; nitrotyrosine: r = 0.35, p = 0.05). Within the proximal and distal airway ELF, high agreement was further observed between the measured concentrations of total nitrite + nitrate and nitrotyrosine (Figure 3).
Figure 2.
(A) Total nitrite + nitrate, (B) nitrite, (C) nitrate, and (D) nitrotyrosine concentrations (µM) in the proximal (dark bars) and distal (light bars) airway ELF. Data represent the mean ± SEM with AC = adult control, PC = pediatric control, MA= mild-to-moderate asthma, and SA = severe asthma. ap < 0.05 versus AC, bp < 0.05 versus PC.
Figure 3.
Scatterplot depicting the relationship between total nitrite + nitrate and nitrotyrosine concentrations (µM) in the proximal (dark circles) and distal (light circles) airway ELF. Data were logarithmically transformed.
Relationship of NO oxidation products to FENO and other clinical features in asthmatic children
To determine the clinical implications of increased ELF oxidation products in children with mild-to-moderate and severe asthma, correlational analysis was first performed between NO oxidation products and clinical features of asthma severity, including FENO, serum IgE, the number of skin prick responses, FEV1, FEV1 bronchodilator reversibility, and the percentage of BAL eosinophils and neutrophils. This analysis was restricted to children with mild-to-moderate and severe asthma and did not include controls. No significant correlations were observed between NO oxidation products and any the clinical features measured, including FENO (Online repository Table E6). However, FENO was significantly associated with the percentage of BAL eosinophils (r = 0.35, p = 0.04) and serum IgE (r = 0.30, p = 0.02).
To further evaluate factors that might affect NO oxidation in the ELF of asthmatic children, multivariate backward elimination linear regression was performed using total nitrite + nitrate concentrations in the proximal and distal ELF as the dependent variables and age, gender, ethnicity, ICS dose, FEV1, FEV1 bronchodilator reversibility, serum IgE, history of hospitalization, FENO, and the percentage of airway eosinophils and neutrophils as predictors. Control data was excluded. In the proximal ELF, age (p < 0.01), gender (p = 0.02), and FENO (p = 0.06) were significant predictors of nitrite + nitrate concentrations (final model R2 = 0.49, p = 0.01, online repository Table E7). Likewise, gender (p = 0.05) and FENO (p = 0.05) were significant predictors of total nitrite + nitrate concentrations in the distal ELF of mild-to-moderate and severe asthmatic children (final model R2 = 0.25, p = 0.05, online repository Table E8). In both the proximal and distal airway ELF, the relationship between FENO and NO oxidation was negative, such that higher FENO concentrations were associated with lower NO oxidation product formation.
DISCUSSION
To our knowledge, this is the first study to directly measure NO oxidation products in the ELF of children with persistent asthma. Compared to controls, children with mild-to-moderate and severe allergic asthma had increased concentrations of nitrite, nitrate, and nitrotyrosine in the ELF which were consistently higher in the proximal versus the distal airways. Contrary to our hypothesis, we failed to detect significant differences in NO oxidation products between mild-to-moderate and severe asthmatic children. Furthermore, no associations between NO oxidation products and clinical features such as FENO were detected using univariate analyses. However, with multivariate modeling to control for the potential confounding effects of ICS and atopy on NO synthesis, FENO was identified as a modest predictor of NO oxidation product formation. While the clinical relevance of this finding is yet unclear, these data highlight the complexity of NO biology in children with asthma and suggest that the relationship between FENO and NO oxidation is not directly proportional. Thus in children with severe asthma, lower FENO concentrations may not necessarily indicate the absence of airway inflammation, but instead may reflect decreased NO bioavailability from increased NO oxidation.
Airway NO biochemistry is complex and the exact contribution of NO to the pathogenesis of asthma is not fully understood. NO is produced by nitric oxide synthases (NOS) in a variety of cell types and serves as an important signaling molecule both within and outside of the cell.3 NO production is also vital to the epithelial antiviral and immune defenses of the airways.24 While the generation of NO oxidation products from NO is important for transcription factor activation and the regulation of airway inflammation,3 excessive airway NO production from altered NOS isoforms or lack of endogenous NOS inhibition can lead to the oxidation of NO and potential nitrogen oxide toxicity.25 The resulting nitrosative stress may ultimately contribute to protein dysfunction and airway cellular destruction.26 Our findings of increased nitrite, nitrate and nitrotyrosine in the ELF of asthmatic children confirm that nitrosative stress is a distinguishing feature of the asthmatic airway. However, the underlying mechanisms responsible for this finding are unclear and warrant further study.
Although this is the first study to directly measure NO oxidation products in the ELF of children with mild-to-moderate and severe allergic asthma, our findings support previously-reported observations in the exhaled breath condensate. In these previous studies, baseline concentrations of nitrite, nitrate and nitrotyrosine were significantly higher in the exhaled breath condensate of asthmatic children.8,10,27 Whereas others have shown reductions in nitrite and nitrate after 8 weeks of ICS therapy,28 we observed NO oxidation in the ELF of children with mild-to-moderate and severe allergic asthma despite ICS treatment. This observation is intriguing and may reflect decreased sensitivity to ICS in this population. Alternatively, NO oxidation products in the ELF may reflect complex biochemical abnormalities that are distinct from other types of airway inflammation and are not necessarily influenced by ICS treatment.29
While there is increasing evidence of distal airway inflammation in human30 and experimental31 models of asthma, our results show that airway nitrosative stress is consistently higher in the proximal versus the distal airways. For this study, we performed sequential BAL of the right middle lobe to separate proximal and distal airway constituents.21 Because this method of lavage was adapted for children to account for different body weights, our data may not accurately reflect nitrosative stress in the bronchial versus alveolar airspace. Thus our distal airway samples may have contained a pooling of bronchial and alveolar NO oxidation products. However, our findings are similar to those of others showing increased inflammation in the bronchial versus alveolar space in asthmatic adults32 and lend support to the more proximal involvement of the airways in asthmatic children.
Our data do not show clear linear associations between ELF NO oxidation products and clinical features of asthma in children, which may be a function of our limited sample size or our patient selection. In addition, it is possible that our measurements of FENO and NO oxidation products were confounded by ICS and atopy. In steroid-naïve asthmatics, FENO falls in a dose-dependent manner after the initiation of ICS.33 Allergic sensitization is also associated with increased FENO independent of asthma,34 a finding which may be attributable to a late-phase influx of eosinophils. In the present study, all of the children with severe asthma were treated with ICS and had objective evidence of aeroallergen sensitization. Furthermore, 80% (n= 12) of the children mild-to-moderate asthma were taking daily ICS and 53% (n = 8) had positive skin prick responses. Whereas NO metabolites were not associated with any clinical features, like others,35 we did observe an association between FENO and airway eosinophils. This finding may explain the utility of FENO in guiding ICS reduction and evaluating asthma control.36 Because there may also be neutrophilic or other patterns of airway inflammation in children with severe asthma, our findings also may reflect the marked heterogeneity of this group of patients. Alternatively, the differences in FENO among asthmatics may be due to airway pH37 or altered s-nitrosothiol metabolism38 and not NO oxidation.
This study had a number of limitations. Because bronchoscopy cannot be ethically performed in healthy children, our pediatric control group was limited to non-asthmatic children with significant respiratory symptoms. The inclusion of these children may have resulted in inadvertent selection of a group of children with significant nitrosative stress. It is also possible that some of our mild-to-moderate asthmatics were under-treated. Thus, the NO oxidation products measured in our group of children with mild-to-moderate asthma may not be reflective of the larger population and may have been reduced with more aggressive ICS treatment.
In summary, we have demonstrated significant increases in the formation of NO oxidation products in the proximal and distal airway ELF of children with persistent asthma. Contrary to our hypothesis, NO oxidation products did not differ between children with mild-to-moderate and severe allergic asthma. While these data highlight the magnitude of oxidant stress that is present in the airways of children with symptomatic asthma, the relationship of this nitrosative stress to asthma severity is yet unclear. Additional studies are warranted to determine the clinical utility of measuring NO oxidation products in asthmatic children, particularly given the marked heterogeneity of the disease. It may be that targeted interventions to reduce nitrosative stress are indicated in children with significant nitrosative stress despite ICS treatment.
ACKNOWLEDGMENTS
**The Severe Asthma Research Program is a multicenter asthma research group funded by the National Heart Lung Blood Institute (NHLBI) and consists of the following contributors (* = Steering Committee members): Brigham and Women's Hospital, Boston, MA: Elliot Israel*, Bruce D. Levy, Gautham Marigowda; Cleveland Clinic, Cleveland, OH: Serpil C. Erzurum*, Raed A. Dweik, Suzy A. A. Comhair, Abigail R. Lara, Sumita Khatri, Marcelle Baaklini, Daniel Laskowski, Jacqueline Sharp; Emory University, Atlanta, GA: W. Gerald Teague*, Anne M. Fitzpatrick; Imperial College School of Medicine, London, UK: Kian F. Chung*, Mark Hew, Sally Meah; National Jewish Medical and Research Center, Denver, CO: Sally E. Wenzel*,; University of Pittsburgh, Pittsburgh, PA: William J. Calhoun*,; Bill T. Ameredes; University of Virginia, Charlottesville, VA: Benjamin Gaston*,; University of Wisconsin, Madison, WI: William W. Busse*, Nizar Jarjour, Cheri Swenson; Wake Forest University, Winston-Salem, NC: Eugene R. Bleecker*, Deborah Meyers, Wendy Moore, Stephen Peters, Annette Hastie, Gregory Hawkins; Washington University in St. Louis, St. Louis, MO: Mario Castro*, Leonard Bacharier, Iftikhar Hussain, Jaime Tarsi; Data Coordinating Center, Denver, CO: Douglas Curran-Everett*; NHLBI, Bethesda, MD: Patricia Noel.
The authors would like to acknowledge Dr. Benjamin Gaston and Dr. Serpil Erzurum for their thoughtful assistance and careful review of this manuscript. The authors would also like to thank Eric Hunter for his assistance with subject recruitment and Meredith Brown for her assistance with sample preparation and laboratory analyses.
This study was supported with funds from NIH/NINR KO1 NR010548, NIH/NCRR K12 RR017643, and NIH/NHLBI SARP RO1 HL69170.
ABBREVIATIONS
- BAL
Bronchoalveolar lavage
- ELF
Epithelial lining fluid
- FENO
Fraction of exhaled nitric oxide
- FEF25–75
Forced expiratory flow
- FEV1
Forced expiratory volume in one second
- FVC
Forced vital capacity
- ICS
Inhaled corticosteroid
- IgE
Immunoglobulin E
- NO
Nitric oxide
- NO2−
Nitrite
- NO3−
Nitrate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This manuscript has an on-line data repository
CLINICAL IMPLICATIONS
Symptomatic children with mild-to-moderate and severe allergic asthma have significant nitrosative stress despite corticosteroid treatment. Additional therapies to decrease airway nitrosative stress may be warranted in these children.
CAPSULE SUMMARY
Symptomatic children with persistent asthma have significant oxidation of nitric oxide (i.e.,“nitrosative stress”) in the airways despite corticosteroid treatment. Nitrosative stress may account for ongoing symptoms in this group of children.
REFERENCES
- 1.Fitzpatrick AM, Gaston BM, Erzurum SC, Teague WG. Features of severe asthma in school-age children: Atopy and increased exhaled nitric oxide. J Allergy Clin Immunol. 2006;118:1218–1225. doi: 10.1016/j.jaci.2006.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Payne DNR, Adcock IM, Wilson NM, Oates T, Scallan M, Bush A. Relationship between exhaled nitric oxide and mucosal eosinophilic inflammation in children with difficult asthma, after treatment with oral prednisolone. Am J Respir Crit Care Med. 2001;164:1376–1381. doi: 10.1164/ajrccm.164.8.2101145. [DOI] [PubMed] [Google Scholar]
- 3.Bove PF, van der Vliet A. Nitric oxide and reactive nitrogen species in airway epithelial signaling and inflammation. Free Radical Biol Med. 2006;41:515–527. doi: 10.1016/j.freeradbiomed.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 4.Hausladen A, Privalle CT, Keng T, DeAngelo J, Stamler JS. Nitrosative stress: activation of the transcription factor OxyR. Cell. 1996:719–729. doi: 10.1016/s0092-8674(00)80147-6. [DOI] [PubMed] [Google Scholar]
- 5.Ghosh S, Janocha AJ, Aronica MA, Swaidani S, Comhair SA, Xu W, Zheng L, Kaveti S, Kinter M, Hazen SL, Erzurum SC. Nitrotyrosine proteome survey identifies oxidative mechanism of catalase inactivation. J Immunol. 2006;176:5587–5597. doi: 10.4049/jimmunol.176.9.5587. [DOI] [PubMed] [Google Scholar]
- 6.Saleh D, Ernst P, Lim S, Barnes PJ, Giaid A. Increased formation of the potent oxidant peroxynitrite in the airways of asthmatic patients is associated with induction of nitric oxide synthase: effect of inhaled glucocorticoid. FASEB J. 1998;12:929–937. [PubMed] [Google Scholar]
- 7.van der Vliet A, Eiserich JP, O'Neill CA, Halliwell B, Cross CE. Tyrosine modification by reactive nitrogen species: a closer look. Arch Biochem Bioophys. 1995;319:341–349. doi: 10.1006/abbi.1995.1303. [DOI] [PubMed] [Google Scholar]
- 8.Ratnawati, Morton J, Henry RL, Thomas PS. Thomas PS. Exhaled breath condensate nitrite/nitrate and pH in relation to pediatric asthma control and exhaled nitric oxide. Pediatr Pulmonol. 2006;41:929–936. doi: 10.1002/ppul.20469. [DOI] [PubMed] [Google Scholar]
- 9.Robroeks CMHHT, van de Kant KDG, Jobsis Q, Hendriks HJE, van Gent R, Wouters EFM, Damoiseaux JGMC, Bast A, Wodzig WKWH, Dompeling E. Exhaled nitric oxide and biomarkers of exhaled breath condensate indicate the presence, severity and control of childhood asthma. Clin Exp Allergy. 2007;37:1303–1311. doi: 10.1111/j.1365-2222.2007.02788.x. [DOI] [PubMed] [Google Scholar]
- 10.Baraldi E, Giordano G, Pasquale MF, Carraro S, Mardegan A, Bonetto G, Bastardo C, Zacchello F, Zanconato S. 3-Nitrotyrosine, a marker of nitrosative stress, is increased in breath condensate of allergic asthmatic children. Allergy. 2006;61:90–96. doi: 10.1111/j.1398-9995.2006.00996.x. [DOI] [PubMed] [Google Scholar]
- 11.Dweik RA, Comhair SA, Gaston B, Thunnissen FB, Farver C, Thomassen MJ, Kavuru M, Hammel J, Abu-Soud HM, Erzurum SC. NO chemical events in the human airway during the immediate and late antigen-induced asthmatic response. Proc Natl Acad Sci U.S.A. 2001;98:2622–2627. doi: 10.1073/pnas.051629498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.MacPherson JPC, Comhair SA, Erzurum SC, Klein DF, Lipscomb MF, Kavuru MS, Samoszuk MK, Hazen SL. Eosinophils are a major source of nitric oxide-derived oxidants in severe asthma: characterization of pathways available to eosinophils for generating reactive nitrogen species. J Immunol. 2001;166:5763–5772. doi: 10.4049/jimmunol.166.9.5763. [DOI] [PubMed] [Google Scholar]
- 13.Fitzpatrick AM, Teague WG, Holguin F, Yeh M, Brown LAS. Airway glutathione homeostasis is altered in children with severe asthma: evidence for oxidant stress. J Allergy Clin Immunol. 2009;123:146–152. doi: 10.1016/j.jaci.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The NAEPP Expert Panel Report: Guidelines for the Diagnosis and Management of Asthma - Update on Selected Topics. Bethesda, MD: National Institutes of Health; 2002. National Heart, Lung and Blood Institute, National Asthma Education and Prevention Program (2002) Publication no. 02–5075. [Google Scholar]
- 15.American Thoracic Society. Lung function testing: selection of reference values and interpretive strategies. Am Rev Respir Dis. 1991;144:1202. doi: 10.1164/ajrccm/144.5.1202. [DOI] [PubMed] [Google Scholar]
- 16.American Thoracic Society. Proceedings of the American Thoracic Society Workshop on Refractory Asthma: current understanding, recommendations, and unanswered questions. Am J Respir Crit Care Med. 2000;162:2341–2351. doi: 10.1164/ajrccm.162.6.ats9-00. [DOI] [PubMed] [Google Scholar]
- 17.Payne D, McKenzie SA, Stacey S, Misra D, Haxby E, Bush A. Safety and ethics of bronchoscopy and endobronchial biopsy in difficult asthma. Arch Dis Child. 2001;84:423–426. doi: 10.1136/adc.84.5.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J. ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 19.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 20.American Thoracic Society and the European Respiratory Society. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide,2005. Am J Respir Crit Care Med. 2005;171:912–930. doi: 10.1164/rccm.200406-710ST. [DOI] [PubMed] [Google Scholar]
- 21.Rennard SI, Ghafouri M, Thompson AB, Linder J, Vaughan W, Jones K, Ertl RF, Christensen K, Prince A, Stahl MG, Robbins RA. Fractional processing of sequential bronchoalveolar lavage to separate bronchial and alveolar samples. Am Rev Respir Dis. 1990;141:208–217. doi: 10.1164/ajrccm/141.1.208. [DOI] [PubMed] [Google Scholar]
- 22.Rennard SI, Basset G, Lecossier D, O’Donnell KM, Pinkston O, Martin PG, Crystal RG. Estimation of volume of epithelial lining fluid recovered by lavage using urea as a marker of dilution. J Appl Physiol. 1986;60:532–538. doi: 10.1152/jappl.1986.60.2.532. [DOI] [PubMed] [Google Scholar]
- 23.Gaston B, Ratjen F, Vaughan JW, Malhotra NR, Canady RG, Snyder AH, Hunt JF, Gaertig S, Goldberg JB. Nitrogen redox balance in the cystic fibrosis airway: effects of antipseudomonal therapy. Am J Respir Crit Care Med. 2002;165:387–390. doi: 10.1164/ajrccm.165.3.2106006. [DOI] [PubMed] [Google Scholar]
- 24.Xu W, Zheng S, Dweik RA, Erzurum SC. Role of epithelial nitric oxide in airway viral infection. Free Radical Biol Med. 2006;41:19–28. doi: 10.1016/j.freeradbiomed.2006.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andreadis AA, Hazen SL, Comhair SA, Erzurum SC. Oxidative and nitrosative events in asthma. Free Rad Biol Med. 2003;35:213–225. doi: 10.1016/s0891-5849(03)00278-8. [DOI] [PubMed] [Google Scholar]
- 26.Stamler JS, Hausladen A. Oxidative modifications in nitrosative stress. Nat Struct Biol. 1998;5:247–249. doi: 10.1038/nsb0498-247. [DOI] [PubMed] [Google Scholar]
- 27.Fomanek W, Inci D, Lauener RP, Wildhaber JH, Frey U, Hall GL. Elevated nitrite in breath condensates of children with respiratory disease. Eur Respir J. 2002;19:487–491. doi: 10.1183/09031936.02.00101202. [DOI] [PubMed] [Google Scholar]
- 28.Kanazawa H, Nomura S, Hirata K, Yoshikawa J. Effect of inhaled beclomethasone dipropionate on peroxinitrite inhibitory activity in induced sputum from asthmatic patients. Chest. 2003;124:1755–1761. doi: 10.1378/chest.124.5.1755. [DOI] [PubMed] [Google Scholar]
- 29.Gaston B. Inhaled corticosteroid dose reduction in childhood asthma: is nitrosopnea informative? Am J Respir Crit Care Med. 2005;171:1065–1066. doi: 10.1164/rccm.2502003. [DOI] [PubMed] [Google Scholar]
- 30.Berry M, Hargadon B, Morgan A, Shelley M, Richter J, Shaw D, Green RH, Brightling C, Wardlaw AJ, Pavord ID. Alveolar nitric oxide in adults with asthma: evidence of distal lung inflammation in refractory asthma. Eur Respir J. 2005;25:986–991. doi: 10.1183/09031936.05.00132404. [DOI] [PubMed] [Google Scholar]
- 31.Wegmann M, Fehrenbach H, Fehrenbach A, Held T, Schramm C, Garn H, Renz H. Involvement of distal airways in a chronic model of experimental asthma. Clin Exp Allergy. 2005;35:1263–1271. doi: 10.1111/j.1365-2222.2005.02306.x. [DOI] [PubMed] [Google Scholar]
- 32.Shin HW, Shelley DA, Henderson EM, Fitzpatrick A, Gaston B, George SC. Airway nitric oxide release is reduced after PBS inhalation in asthma. J Appl Physiol. 2007;102:1028–1033. doi: 10.1152/japplphysiol.01012.2006. [DOI] [PubMed] [Google Scholar]
- 33.Jones SL, Herbison P, Cowan JO, Flannery EM, Hancox RJ, McLachlan CR, et al. Exhaled NO and assessment of anti-inflammatory effects of inhaled steroid: dose-response relationship. Eur Resp J. 2002;60:601–608. doi: 10.1183/09031936.02.00285302. [DOI] [PubMed] [Google Scholar]
- 34.Travers J, Marsh S, Aldington S, Williams M, Shirtcliffe P, Pritchard A, Weatherall M, Beasley R. Reference ranges for exhaled nitric oxide derived from a random community survey of adults. Am J Respir Crit Care Med. 2007;176:238–242. doi: 10.1164/rccm.200609-1346OC. [DOI] [PubMed] [Google Scholar]
- 35.Lex C, Ferreira F, Zacharasiewicz, Nicholson AG, Haslam PL, Wilson NM, Hansel TT, Payne DNR, Bush A. Airway eosinophilia in children with severe asthma: predictive values of noninvasive tests. Am J Respir Crit Care Med. 2006;174:1286–1291. doi: 10.1164/rccm.200603-352OC. [DOI] [PubMed] [Google Scholar]
- 36.Smith AD, Cowan JO, Brassett KP, Herbison GP, Taylor DR. Use of exhaled nitric oxide measurements to guide treatment in chronic asthma. New Engl J Med. 2005;352:2163–2173. doi: 10.1056/NEJMoa043596. [DOI] [PubMed] [Google Scholar]
- 37.Gaston B, Kelly R, Urban P, Liu L, Henderson EM, Doctor A, Teague WG, Fitzpatrick A, Erzurum S, Hunt JF. Buffering airway acid decreases exhaled nitric oxide in asthma. J Allergy Clin Immunol. 2006;118:817–822. doi: 10.1016/j.jaci.2006.06.040. [DOI] [PubMed] [Google Scholar]
- 38.Que LG, Liu L, Yan Y, Whitehead GS, Gavett SH, Schwartz DA, Stamler JS. Protection from experimental asthma by an endogenous bronchodilator. Science. 2005;308:1618–1621. doi: 10.1126/science.1108228. [DOI] [PMC free article] [PubMed] [Google Scholar]