Abstract
Aspirin (ASA) and dexamethasone (DEX) are widely used anti-inflammatory agents yet their mechanism(s) for blocking polymorphonuclear neutrophil (PMN) accumulation at sites of inflammation remains unclear. Here, we report that inhibition of PMN infiltration by ASA and DEX is a property shared by aspirin-triggered lipoxins (ATL) and the glucocorticoid-induced annexin 1 (ANXA1)-derived peptides that are both generated in vivo and act at the lipoxin A4 receptor (ALXR/FPRL1) to halt PMN diapedesis. These structurally diverse ligands specifically interact directly with recombinant human ALXR demonstrated by specific radioligand binding and function as well as immunoprecipitation of PMN receptors. In addition, the combination of both ATL and ANXA1-derived peptides limited PMN infiltration and reduced production of inflammatory mediators (that is, prostaglandins and chemokines) in vivo. Together, these results indicate functional redundancies in endogenous lipid and peptide anti-inflammatory circuits that are spatially and temporally separate, where both ATL and specific ANXA1-derived peptides act in concert at ALXR to downregulate PMN recruitment to inflammatory loci.
Inflammation plays a key role in many prevalent diseases such as arthritis, and there is increased appreciation that atherosclerosis and Alzheimer disease also share uncontrolled inflammation as part of their etiology1. The anti-inflammatory and analgesic actions of ASA have been well known for more than 100 years2-7. At low doses, ASA displays newly recognized beneficial actions including prevention of cardiovascular diseases not shared by other non-steroidal anti-inflammatory drugs3, that may also extend to cancer treatments4,5. Dating from the early work of Hench et al.8, glucocorticoids are also widely used to treat inflammatory diseases. Although prolonged treatment with either ASA or glucocorticoids is widely used, each is associated with an unacceptably high level of adverse side effects9,10. Thus it is important to understand their action in order to have new approaches to controlling unwanted inflammation. Control of PMN functions is critical in the amplification of inflammation. ASA and glucocorticoids each independently share the ability to inhibit a key first step in inflammation, namely leukocyte-endothelial adhesion11. The molecular basis, however, underlining their anti-PMN activity remains of interest because their elucidation could shed light on novel pathways that might be useful in sparing unwanted side effects of these agents.
Impact of combined ASA and DEX on neutrophil extravasation
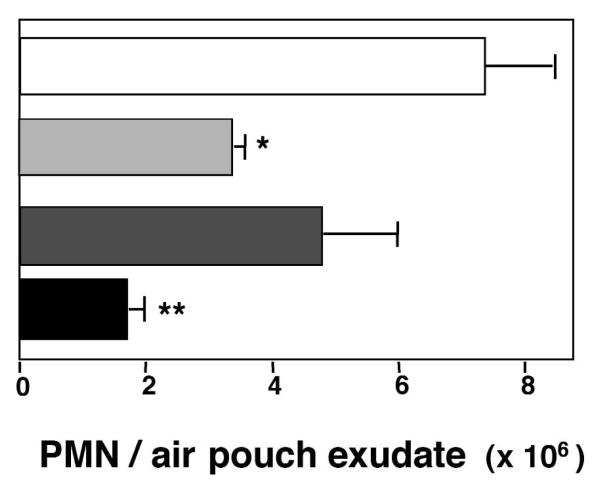
To this end, we addressed whether ASA and glucocorticoids have overlapping components in their therapeutic actions, namely regulation of PMN trafficking (Fig. 1). Using a murine dorsal air pouch inflammation that is widely studied and likened to features of rheumatoid synovium12, we evaluated the combined impact of ASA and DEX in regulating PMN recruitment to inflammatory sites and exudate formation with the microbial particle Zymosan A. Administration of both agents (10 μg DEX and 1 mg ASA) into pouches gave statistically significant inhibition of Zymosan A-driven PMN infiltration (~76%) (Fig. 1). For direct comparison, when administrated alone, DEX (10 μg) gave 53% and ASA (1 mg) 36% inhibition (Fig. 1). This is consistent with results of early studies with human rheumatoid arthritis patients where low-dose DEX and ASA in combination was found to be a highly effective anti-inflammatory therapy13. Also, the results in Fig. 1 suggest that the inhibitory actions of DEX and ASA in combination on PMN trafficking in vivo may involve pathways in addition to those already appreciated for these agents when used independently8-11.
Fig. 1.
Additive actions with DEX and ASA on PMN extravasation. DEX or ASA were injected into murine air pouches 15 min before Zymosan A (1 mg) injection. PMN accumulation was measured 4 h later by light microscopy. Results are the mean ± s.e.m of n = 8 per group. *, P < 0.05; **, P < 0.01 versus Zymosan A alone. □, Zymosan A alone;  , +DEX;
, +DEX;  , +ASA; ■, +DEX & ASA.
, +ASA; ■, +DEX & ASA.
Of the various hypotheses proposed for glucocorticoid's anti-inflammatory action, attention initially focused on annexin 1 (denoted earlier as lipocortin 1)14. Annexin 1 (ANXA1) is a protein of 346 amino acids and a member of the annexin superfamily that is a highly abundant PMN-derived protein induced by glucocorticoids in vivo. Both ANXA1 as well as its enzymatically generated ANXA1-derived peptide (for example. peptide Ac2-26, or Ac-AMVSEFLKQAWFIENEEQEYVQTVK) are potent inhibitors of PMN transmigration and phagocytosis in vitro and in vivo15,16. Along these lines, in addition to inhibiting formation of both prothrombotic and pro-inflammatory eicosanoids6,7, ASA treatment was more recently shown to trigger formation of endogenous anti-inflammatory lipid mediators17. These mediators are generated, for example, when ASA acetylates cyclooxygenase-2 and initiates the generation of 15-epimeric lipoxin A4 (LXA4), termed aspirin-triggered LXA4 (ATL). Like other autacoids, both ATL and their lipoxygenase-derived carbon 15 epimers, the native lipoxins, are generated and act locally, followed by rapid inactivation via metabolic conversion. Metabolically stable analogs of LXA4 and ATL were designed to resist rapid enzymatic inactivation that prolongs their actions. These compounds display potent anti-PMN actions and act with high affinity (Kd ~0.5 nM) at a G protein-coupled receptor, LXA4 receptor (ALXR; also referred to as formyl peptide receptor-like 1 or FPRL1)17. Along with its endogenous anti-inflammatory lipid ligands, ALXR/FPRL1 also directly interacts with selective peptides that are revealed during tissue damage in situ to evoke PMN necrotaxis18. These independent observations raised the possibility that DEX- and ASA-generated endogenous mediators might share some common pathways governing leukocytes in inflammation. To test this, we first questioned whether ANXA1-derived peptides themselves might interact with human PMN ALXR/FPRL1. The two pathways converge at a shared receptor.
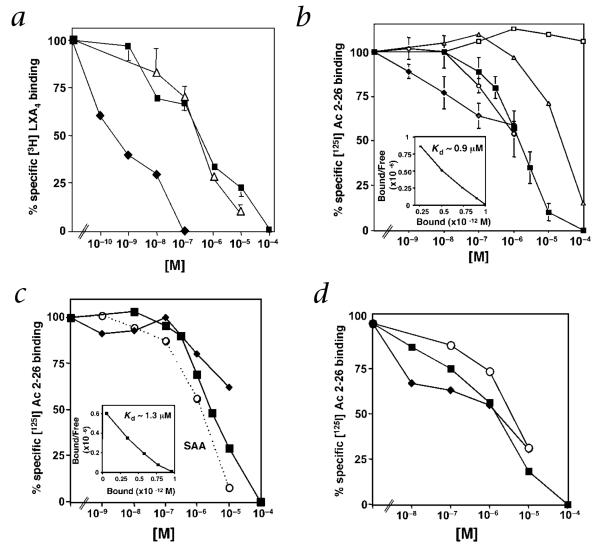
[3H]LXA4 was prepared for direct ligand binding with human embryonic kidney (HEK) 293 cells expressing human ALXR. Both ANXA1-derived peptide Ac2-26 and a shorter peptide denoted Ac2-12 (AcAMVSEFLKQAW) competed for specific [3H]LXA4 binding with similar affinities (50% inhibitory concentration (IC50) ~0.3 μM) (Fig. 2a). To determine whether ANXA1-derived peptides directly bind recombinant ALXR/FPRL1, we prepared [125I-Tyr] Ac2-26 peptide (corresponding to the major portion of the N-terminus of ANXA1), which gave specific and saturable binding with a Kd value of ~0.9 μM (Fig. 2b, inset). The full-length ANXA1 protein itself also competed for [125I-Tyr]Ac2-26 binding with similar affinity, whereas the Ac2-12 peptide gave an IC50 of ~20 μM. In contrast, a scrambled sequence peptide Ac2-6 did not compete for specific [125I-Tyr]Ac2-26 binding (Fig. 2b). The structurally unrelated lipid mediator LXA4 also specifically competed for [125I-Tyr]Ac2-26 binding with HEK293 cells expressing ALXR/FPRL1 (Fig. 2b). These ligands are selective for ALXR/FPRL1, as neither LXA4 (ref. 18) nor Ac2-26 peptide competed for [3H]LTB4 binding to human LTB4 receptor (BLT1), a related lipid mediator G protein-coupled receptor (GPCR) (ref. 19), with BLT1-transfected HEK293 cells (Supplementary Fig. A online).
Fig. 2.
Direct interaction of ANXA1 with ALXR: competitive [125I-Tyr]Ac2-26 and [3H]LXA4 binding. a and b, Human ALXR-transfected HEK293 cells (0.5 × 106) were incubated with [3H]LXA4 (a) or [125I-Tyr]Ac2-26 (b) in the presence of an increasing concentration of unlabeled compounds: LXA4 (◆), Ac2-26 (■), Ac2-12 (△), ANXA1 (○) or scrambled Ac2-6 (□). c and d, Suspension (c) or adherent human PMNs (d) were incubated with [125I-Tyr]Ac2-26 in the presence of increasing concentration of unlabeled compounds: LXA4 (◆), Ac2-26 (■) or SAA (□). Insets in b and c, Scatchard plots of specific [125I-Tyr]Ac2-26 binding. Results represent the mean ± s.e.m. from duplicates of n =3.
For the purpose of direct comparison, isolated human PMNs in suspension gave a Kd of ~1.3 μM for [125I-Tyr]Ac2-26, a value comparable to that obtained with recombinant ALXR/FPRL1, and LXA4 competed for [125I-Tyr]Ac2-26 peptide binding to human PMN (Fig. 2c). Serum amyloid protein A (SAA), a proteolytic product of the acute phase response20, was also tested because, at much higher concentrations than LXA4, it competes for specific [3H]LXA4 binding18, activates HEK293 cells expressing ALXR/FPRL1 (ref. 21) and inhibits human PMN oxidative burst22. SAA competed for specific [125I-Tyr]Ac2-26 binding with an apparent IC50 of ~1 μM with human PMNs (Fig. 2c); this value is comparable to that of ANXA1, suggesting that SAA and ANXA1 share recognition sites on PMNs for their action. As PMN adhesion is a key event that externalizes ANXA1, permitting its inhibitory actions in PMN recruitment23,24, both lipid (LXA4) and protein (SAA and ANXA1) ligand interactions with endogenous ALXR/FPRL1 were evaluated with adherent PMNs and compared with those of PMNs in suspension. Adherent PMNs gave specific [125I-Tyr]Ac2-26 peptide binding with an apparent Kd of ~0.9 μM (Fig. 2d), and both LXA4 and SAA retained their ability to compete for specific [125I-Tyr]Ac2-26 peptide binding. Taken together, these results provide the first direct evidence that ANXA1 as well as specific ANXA1-derived peptides directly interact with human PMNs as well as recombinant ALXR.
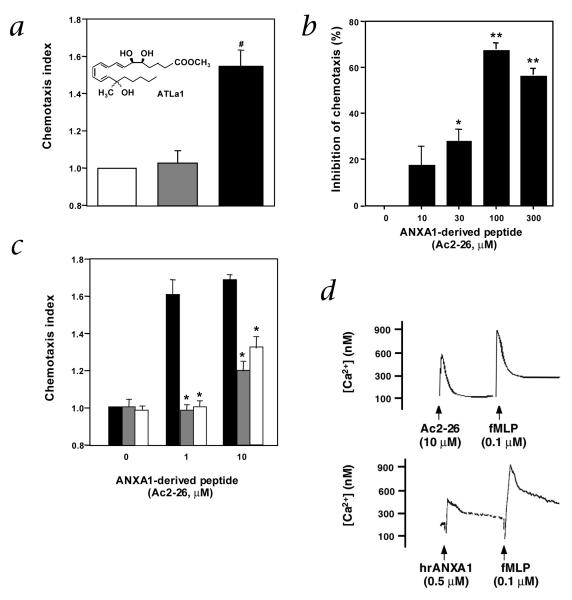
Next, we determined whether these ligands could evoke cellular responses via recombinant ALXR/FPRL1. We examined chemotaxis with cells expressing ALXR/FPRL1 together with Gqo chimera18. Although ATL analogs inhibit PMN chemotaxis in vivo and in vitro, the presence of Gqo chimera enables this ligand-receptor interaction to drive chemotaxis as a reporter system in vitro. A stable analog of both LXA4 and ATL, denoted ATL analog 1 (ATLa1; 15(R/S)-methyl-LXA4) (Fig. 3a inset) gave clear chemotaxis responses (Fig. 3a), whereas the Ac2-26 peptide at concentrations greater than 1,000 times that of ATLa1 did not. This peptide markedly inhibited ATLa1-evoked reporter cell (Gqo chimera-ALXR) chemotaxis in a concentration-dependent manner (Fig. 3b). These findings indicate that the ANXA1-derived peptide and ATLa1 can act at the same recombinant ALXR/FPRL1, yet each ligand evoked different responses using this Gqo reporter system. This was also demonstrable with human PMNs. With the primary cells in vitro, the peptide Ac2-26 displays a chemotactic activity (Fig. 3c), and checkerboard analysis indicated a chemokinetic activity as well (data not shown). In contrast, ATLa1 alone at 10–100 nM did not evoke chemotaxis, but clearly blocked Ac2-26 evoked chemotaxis. Together with results with recombinant receptors (Fig. 3a and b), they indicated that Ac2-26 and ATLa1 interact with the same PMN receptor (Fig. 3c).
Fig. 3.
ANXA1 peptides directly interact with recombinant as well as endogenous PMN ALXR. a, CHO-Gqo-ALXR cells were added to the upper compartment of a microchamber (5 × 104 cells per well). Chemotaxis was initiated by addition of Ac2-26 peptide (100 μM;  ) or aspirin-triggered lipoxin A4 analog 1 (ATLa1; 100 nM; ■) to the lower compartment. □, vehicle. b, CHO-Gqo-ALXR cells were pretreated with Ac2-26 peptide for 30 min at 37 °C and added to the upper compartment of a microchamber (5 × 104 cells per well). Chemotaxis was initiated by addition of ATLa1 (100 nM) to the lower compartment. c, Human PMNs were added to the upper compartment of a microchamber (5 × 104 cells per well). Chemotaxis was initiated by addition of Ac2-26 peptide (1–10 μM; ■) to the lower compartment. In some cases, cells were treated with 10 (
) or aspirin-triggered lipoxin A4 analog 1 (ATLa1; 100 nM; ■) to the lower compartment. □, vehicle. b, CHO-Gqo-ALXR cells were pretreated with Ac2-26 peptide for 30 min at 37 °C and added to the upper compartment of a microchamber (5 × 104 cells per well). Chemotaxis was initiated by addition of ATLa1 (100 nM) to the lower compartment. c, Human PMNs were added to the upper compartment of a microchamber (5 × 104 cells per well). Chemotaxis was initiated by addition of Ac2-26 peptide (1–10 μM; ■) to the lower compartment. In some cases, cells were treated with 10 ( ) or 100 nM (□) ATLa1 for 30 min at 37 °C. #, P = 0.01, versus vehicle; *, P < 0.01, versus Ac2-26 alone (a and c) or % inhibition of ATLa1- evoked chemotaxis (*, P = 0.02; **, P < 0.01, versus ATLa1 alone in b). Data represent the mean ± s.e.m. from n = 3 experiments. d, Fura2-AM-loaded human PMNs (5 × 106 cells/incubation) were incubated with the different stimuli, and rapid changes in intracellular [Ca2+] measured by fluorimetry. Additions of agonists are denoted by arrows. hrANXA1: human recombinant annexin 1. Traces are representative of 3 independent experiments.
) or 100 nM (□) ATLa1 for 30 min at 37 °C. #, P = 0.01, versus vehicle; *, P < 0.01, versus Ac2-26 alone (a and c) or % inhibition of ATLa1- evoked chemotaxis (*, P = 0.02; **, P < 0.01, versus ATLa1 alone in b). Data represent the mean ± s.e.m. from n = 3 experiments. d, Fura2-AM-loaded human PMNs (5 × 106 cells/incubation) were incubated with the different stimuli, and rapid changes in intracellular [Ca2+] measured by fluorimetry. Additions of agonists are denoted by arrows. hrANXA1: human recombinant annexin 1. Traces are representative of 3 independent experiments.
Endogenous ANXA1/ALXR association in activated PMN
Recently, results from studies on the ANXA1-derived peptides and inhibition of PMN chemotaxis in vitro implicated that these peptides could interact with GPCRs related at the nucleotide sequence level to ALXR/FPRL1—namely one of the formyl peptide receptors (FPRs)25. However, direct evidence for specific binding of the ANXA1 peptides or full-length protein to either human25 or mouse26 FPRs was not demonstrated, and ANXA1 interactions with circulating leukocytes or peritoneal macrophages was only partially (~40%) diminished in FPR-deficient mice26. Thus, the involvement of FPRs in the inhibitory actions of ANXA1 and its bioactive peptides remained indirect because the conclusions drawn relied heavily on the use of FPR receptor antagonist: the Boc compounds that can also partially compete at ALXR/FPRL1 (see Supplementary Fig. B online; panels a and b showing that Boc1 compound blocked the effect of SAA, a selective ALXR/FPRL1 ligand). Also, results from FPR-deficient mice may not be conclusive because they were not characterized for potential compensatory changes in related murine receptors or direct ligand binding. Moreover, these findings suggested that additional, possibly related, receptors were responsible for most of the inhibitory actions of ANXA1 in vivo and in human disease. Along these lines, the ANXA1 peptide Ac2-26 as well as human recombinant ANXA1 (hrANXA1) even at high concentrations (for example, 10 μM and 0.5 μM, respectively) did not affect the cellular response of human PMNs produced by subsequent addition of fMLP (85 ± 4% of the fMLP response; n = 4, P > 0.05) (Fig. 3d). Thus, ANXA1 and fMLP each evoke responses via distinct and separate receptors on human PMNs. Notably, the Ac2-26 peptide response with PMNs (that is, calcium mobilization) (Supplementary Fig. B online; panel c) was partially blocked by genistein, a tyrosine kinase inhibitor that also modulates LXA4-regulated PMN transmigration and adhesion27. The Ac2-26 response further suggests that ANXA1 and LXA4 can share some intracellular signaling pathway(s) in PMNs (Figs. 2 and 3).
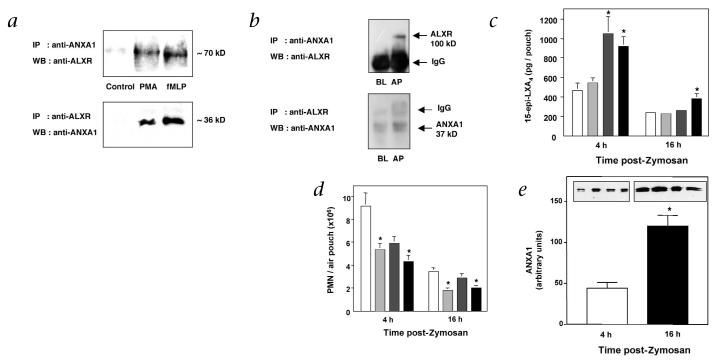
The direct interaction between ANXA1 and ALXR/FPRL1 was further substantiated via immunoprecipitation with human PMNs. PMNs were activated with either PMA or fMLP to adhere cells and bring ANXA1 on the cell surface24 to detect ANXA1 interaction with its putative receptor. With adherent PMNs, immunoreactive bands of ~70 kD, corresponding to the mass of human ALXR/FPRL1 (ref. 28), was co-immunoprecipitated by specific antibodies against ANXA1, demonstrated by western-blot analysis using specific antibody against ALXR (Fig. 4a, upper panel). Also, immunoreactive bands of ~36 kD, consistent with molecular masses of ANXA1, were obtained by co-immunoprecipitation with anti-ALXR, followed by western blot using anti-ANXA1 (Fig. 4a, lower panel). In comparison, ANXA1 and ALXR/FPRL1 were not co-immunoprecipitated with non-adherent PMNs (control; see Fig. 4a). The need for PMN activation to bring about ANXA1–ALXR/FPRL1 interaction was also seen with murine cells, as a much stronger signal was detected in inflamed air-pouch cells compared with blood-borne PMNs (Fig. 4b). Thus, these results indicate direct protein–protein interaction between endogenous ANXA1 and ALXR/FPRL1 when PMNs adhere in vitro and extravasate in vivo.
Fig. 4.
Both ATL and ANXA1 are generated in vivo and interact with ALXR. a, Adherent human PMNs (5 × 106) incubated (in 6-well plates) in presence of 0.1 μM PMA or 0.1 μM fMLP for 30 min at 37 °C. b, Murine blood-borne PMNs (BL) or air pouch (AP) cells (>95% PMNs) collected 4 h after injection of 1 mg Zymosan A were immunoprecipitated (IP) with either the monoclonal antibody against ANXA1 (top) or polyclonal antibody against ALXR peptide (bottom). Immunoprecipitated proteins were probed with anti-ALXR (top) or anti-ANXA1 (bottom) by western blotting (WB). Immunoblots represent 3 independent experiments. DEX or ASA were injected into murine air pouches with the indicated doses 15 min before Zymosan A (1 mg) injection. c–e, After 4 or 16 h, PMN accumulation (c), 15-epi-LXA4 (d) and ANXA1 generation (e) were determined. Insets, Representative western blot exudates at 4 and 16 h. Results are the mean ± s.e.m of n = 8 per group. *, P < 0.05 versus Zymosan A alone. For c–e: □, Zymosan alone;  , + 3 μg DEX;
, + 3 μg DEX;  , + 300 μg ASA; ■, + 3 μg DEX + 300 μg ASA.
, + 300 μg ASA; ■, + 3 μg DEX + 300 μg ASA.
Generation of endogenous ATL and ANXA1
Next, we examined whether these anti-inflammatory mediators (for example, ANXA1 and ATL) are generated in murine air-pouch exudates when DEX and ASA are administered. In combination, low doses of DEX (3 μg) and ASA (300 μg) gave 42% inhibition of Zymosan A-initiated PMN infiltration at both 4 and 16 hours (Fig. 4c). 15-epi-LXA4 (ATL) was generated and present in ASA-treated animals at 4 hours that diminished by 16 hours (Fig. 4d). Without administration of ASA, animals gave very low 15-epi-LXA4 (~500 and ~200 pg per pouch at 4 and 16 hours, respectively). In this regard, Zymosan A can stimulate some 15-epi-LXA4 generation via ASA-independent pathways, as its precursor 15R-HETE is also produced by p450-dependent mechanisms, and lipoxygenases can produce low levels of the R epimers29. In comparison, ANXA1 generation was low at 4 hours and much higher at 16 hours (Fig. 4e). Together, these results indicate different time courses for the generation of lipid (that is, ATL) versus protein (ANXA1) mediators during the host inflammatory response, and they suggest that ALXR/FPRL1 could regulate PMNs by interacting with each class of ligands within specific phases of the inflammatory response. This spatial and temporal difference between the two ligand-receptor pairs in vivo likely explains the Kd values for each observed in vitro (Figs. 2 and 4). As previously suggested24,25, a further consideration is needed regarding the bioactive levels of ANXA1 and its peptides in vivo: it is still speculative but highly likely that the actual amounts of ANXA1 and its peptides within the microenvironment where they are generated (that is, by the adherent PMNs and surrounding tissue matrix) may be much higher than that measured within the solubilized materials quantified in biological fluids.
Synergism between ATLa and ANXA1 peptide
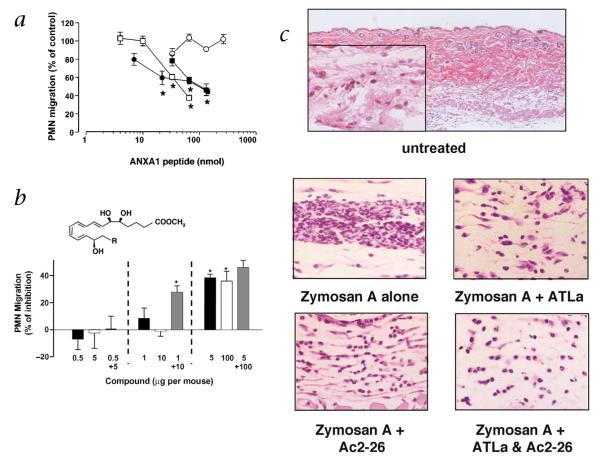
In view of these considerations, we tested in vivo whether administration of exogenous ATL could inhibit Zymosan A-initiated PMN infiltration and if ANXA1-derived peptides share this inhibitory property. Intravenous delivery of ANXA1-derived peptides (for example, Ac2-26, Ac2-12 and Ac2-6) inhibited PMN infiltration in a dose-dependent fashion whereas the non-physiologically scrambled Ac2-6 peptide sequence, which did not compete at ALXR/FPRL1 (Fig. 2b), did not share this action (Fig. 5a). Intravenous delivery of the stable analog of ATL (5 μg per mouse; ~12 nmol), denoted ATL analog (ATLa; 15-epi-16-(para-fluoro)-phenoxy-LXA4) gave ~40% inhibition comparable with that obtained with 100 μg (~33 nmol) peptide Ac2-26 (see Fig. 5b) (compare inhibition of TNF-α-induced PMN infiltration30). Functional synergism was observed with lower doses of ATLa (1 μg or 2.4 nmol) and peptide Ac2-26 (10 μg or 3.3 nmol) (Fig. 5b). Results in Fig. 5b indicate that when administered, ATLa and Ac2-26 were each potent inhibitors of PMN infiltration, and synergism was achieved with a combination of sub-threshold doses of both agents. Inhibition with ATL and ANXA1-derived peptides was also demonstrated with other markers of inflammation including macrophage inflammatory protein-1α (MIP-1α) and prostaglandin E2 (PGE2), which were both inhibited in these mice (Supplementary Fig. C online).
Fig. 5.
Synergism with ANXA1-derived peptides and ATLa in vivo. a, ANXA1-derived peptides were administered at the reported doses via the tail vein 15 min prior to injection of 1 mg Zymosan A into 6-d-old air pouches. PMN accumulation was measured 4 h later. The number of migrated PMNs in the control group (vehicle-treated mice) was 7.5 ± 0.5 × 106 PMNs per mouse. Data are mean ± s.e.m of 6–12 mice; *, P < 0.05 versus control migration as calculated on original values. □, Ac2-26; ●, Ac2-12; ■, Ac2-6; ○, Ac2-6 scramble. b, Animals were treated i.v. with the reported doses of ATLa (■; 0.5–5 μg, 1.2–12 nmol; chemical structure shown, R = para-fluoro-phenoxy), Ac2-26 (□; 5–100 μg, corresponding to ~1.5–33 nmol) or both ATLa and Ac2-26 ( ) 15 min before local injection of Zymosan A. PMN accumulation was measured after 4 h. The number of migrated PMNs in the control group (mice treated with Zymosan A alone) was 6.5 ± 0.55 × 106 PMNs per mouse. Data are mean ± s.e.m of n = 6–8 mice per group; *, P < 0.05 versus control migration as calculated on original values. c, Air-pouch biopsies. Sections from the top dose groups (that is, 5 μg of ATLa and 100 μg of Ac2-26; see b) were prepared and were stained with H&E. Magnifications, ×10 for the top panel and ×40 for the inset and lower panels.
) 15 min before local injection of Zymosan A. PMN accumulation was measured after 4 h. The number of migrated PMNs in the control group (mice treated with Zymosan A alone) was 6.5 ± 0.55 × 106 PMNs per mouse. Data are mean ± s.e.m of n = 6–8 mice per group; *, P < 0.05 versus control migration as calculated on original values. c, Air-pouch biopsies. Sections from the top dose groups (that is, 5 μg of ATLa and 100 μg of Ac2-26; see b) were prepared and were stained with H&E. Magnifications, ×10 for the top panel and ×40 for the inset and lower panels.
Histological analysis showed that Zymosan A gave a markedly increased number of PMNs in the skin-tissue linings surrounding the pouch cavity (Fig. 5c); this number was reduced by ATLa, Ac2-26 and their combination. The degree of PMN tissue trafficking was reduced to the level observed without Zymosan A challenge. These findings, together with the radioligand binding and immunoprecipitation results (Fig. 2 and 4), indicate that a shared site of action on PMNs is responsible for the anti-PMN properties of ATL and ANXA1 as well as their mimetics. Recently it is reported that a phosphatidylserine-specific receptor was critical for the clearance of apoptotic cells and that this may be a critical point for modulating resolution31. Along these lines, lipoxin A4 stimulates monocytes/macrophages in a nonphlogistic fashion to enhance the uptake of apoptotic neutrophils32, an activity also shared by glucocorticoids33.
Discussion
Production of ANXA1-derived peptides is activated in vivo during the process of PMN extravasation23, and within bronchoalveolar lavage fluids from patients with cystic fibrosis, where processing of ANXA1 at its N-terminal portion seems to be cleaved by PMN-derived elastase34. Notably, several other naturally occurring peptides also exhibit inhibitory actions in cell adhesion and migration with other leukocyte subclasses. Interleukin-2 (IL-2) peptides, for example, generated by PMN elastase inhibit IL-2 induced T-cell adhesion and migration35. Thus, endogenously generated peptides derived during inflammation or from inflammation-induced proteins may serve as feedback inhibitors to terminate and/or halt the amplification of inflammation and could work together with lipid mediators such as LXA4 and aspirin-triggered lipid mediators to promote resolution.
In summary, our results are the first to indicate that both ANXA1 and ATL directly interact with human ALXR/FPRL1. By convergence at the same anti-inflammatory receptor, these two structurally distinct endogenous systems, namely lipid-derived (for example, ATL) and protein-derived (for example, ANXA1) mediators, limit PMNs in vivo. This overlap may have evolved to ensure that inflammation loci remain local, walled off and self-limited, as well as to protect from self-damage from within. Because several related sequences are present in this cluster of GPCRs (ref. 36) (for example, FPRL2 etc.), it is likely in view of these results and those of others that additional endogenous ligands may share these functions. Moreover, impingement of these natural endogenous systems by popular therapies such as ASA and DEX may, at least in part, underlie the proven efficacy of the combination of DEX and ASA in controlling rheumatic diseases13 and the success of their individual use this past century. These systems likely represent functional redundancies in endogenous anti-inflammation circuits that unveil presently un-appreciated mechanisms operative in governing PMN responses in host defense, and they now open new avenues for approaches such as combination therapies.
Methods
Inflammation in vivo
PMN migration and mediator formation were assessed using 6-day-old murine air pouches in Swiss Albino mice (n = 8 per group). After Harvard Medical Area IRB approval (Protocol #02570), and approval by the Home Office UK Project License (PPL70/4804), compounds (for example, ASA, DEX, ATLa, ANXA1 and ANXA1 mimetics) were delivered as bolus injections either into the tail vein in 100 μl PBS (ATLa, ANXA1 and its mimetics) or locally into air pouches in 100 μl PBS (ASA and DEX). Zymosan A (1 mg/0.5 ml saline) was locally injected into the air pouches 15 min later to induce inflammation37. 4 or 16 h later, air pouches were washed with 2 ml PBS containing 25 U/ml heparin, and PMNs were counted by light microscopy following staining in Turk's solution. Cell-free lavage fluids were used for quantification of mouse MIP-1α and PGE2 using available ELISAs (R&D System, Oxford, UK and Amersham, Buckinghamshire, UK). 15-epi-LXA4 generation was also determined using an ELISA specific for the 15R epimer as described29. ANXA1 generation was determined by western-blot analysis using specific antibodies to ANXA1. For microscopic analysis, tissues were obtained with a 6-mm tissue biopsy punch and fixed in 10% buffered formaldehyde. Samples were then embedded in paraffin, sliced and stained with H&E.
Preparation of labeled ligands
[11,12-3H]LXA4-methyl ester was prepared with Schering AG (Berlin, Germany) using acetylenic-LXA4-methyl ester as precursor (prepared with N.A. Petasis) essentially as described18, and was a gift of H.D. Perez. [11,12-3H]LXA4-methyl ester was isolated and purified using a Hewlett Packard (San Fernando, California) 1100 Series Diode Array Detector (DAD) equipped with a binary pump and eluted on a Beckman (Fullerton, California) Ultrasphere C18 column (250 × 4.5 mm, 5 μm)18. To minimize chemical degradation of [11,12-3H]LXA4-methyl ester, it was used immediately after HPLC chromatography for binding experiments. [125I-Tyr]Ac2-26 was prepared by custom synthesis with Phoenix Pharmaceuticals (Belmont, California).
Radioligand binding
Human PMNs were obtained from donors according to the Brigham and Women's Hospital Human Research Committee Protocol #88-02642 and the Ethical Committee (UK) ELCHAP/00/029. Informed consent was obtained from all subjects. [3H]LXA4, [3H]LTB4 and [125I-Tyr]Ac2-26 binding was performed with freshly isolated human PMNs or HEK293 cells (American Type Culture Collection, Rockville, Maryland) transfected with human recombinant ALXR (GenBank Accession #U81501) or BLT18. Cells were suspended in Dulbecco's PBS with CaCl2 and MgCl2 (DPBS++). Aliquots (0.5 × 106/ml HEK293 or 5 × 106/ml PMNs) were incubated with 1 nM of [11,12-3H]LXA4 (~60,000 c.p.m., specific activity ~10 Ci/mmol), [5,6,8,9,11,12,14,15-3H]LTB4 (~20,000 c.p.m., specific activity ~200 Ci/mmol, from NEN, Boston, Massachusetts) or 30 nM [125I-Tyr]Ac2-26 (~80,000 c.p.m., specific activity ~1,171.5 Ci/mmol), in the absence or presence of increasing concentrations of unlabeled compounds for 40 min at 4 °C. The bound and unbound radioligands were separated by filtration through Whatman GF/C glass microfiber filters (Fisher, Pittsburgh, Pennsylvania)18. Non-specific binding was determined in the presence of 1 μM of unlabeled homoligands. To obtain adherent PMNs, freshly isolated PMNs were plated in collagen-coated 6-well plates (5 × 106/ml) in the presence of low-dose (1 nM) PMA for 30 min. Cells were washed with DPBS and [125I-Tyr]Ac2-26 binding was carried out with adherent cells using the conditions described above. The unbound radioligands were aspirated and cells washed with DPBS. The adherent cells were then lysed with scintillation mixture and radioactivity determined.
Chemotaxis
Chemotaxis was carried out using a microchamber technique (chemotaxis chamber from Neuro Probe, Cabin John, Maryland)18. Chinese hamster ovary (CHO) cells expressing ALXR and Gqo chimera (from B. Conklin) or freshly prepared human PMNs (ref. 24) were incubated with vehicle or peptide Ac2-26 for 30 min at 37 °C and added to the upper wells of chemotaxis chamber (5 × 104 cells per well). After incubation for 4 h at 37 °C, polycarbonate membrane was removed, stained with a Diff-Quick staining kit (Dade Behring, Newark, Delaware) and migrated cells enumerated. Chemotaxis index was calculated as the mean number of cells that migrated toward medium containing chemoattractant solution divided by the mean number of cells migrated toward the medium containing vehicle only.
Ca2+ mobilization
Changes in the cytosolic-free calcium concentration were monitored in human PMNs loaded with 1 mμM fura 2/acetoxymethylester (Molecular Probes, Leiden, Holland) at 37 °C for 1 h. Fura 2 fluorescence was performed with aliquots of 5 × 106 PMNs in 2 ml HBSS, using a fluorimeter (Jobin Yvon 3D, Lonjumeau, France), equipped with a thermally controlled cuvette holder and a magnetic stirrer.
Immunoprecipitation and western blotting
PMNs were freshly prepared from healthy volunteers by histopaque (Sigma-Aldrich Ltd., Poole, UK) gradient separation24. Mouse blood-derived PMNs were obtained by negative immunomagnetic separation38, whereas extravasated cells were collected from the air pouch exudates (see below). Human PMNs were plated in 6-well plates in the absence or presence of 0.1 μM PMA, or 0.1 μM fMLP to produce ~80% adhesion (15 min at 37 °C). In all cases, cell extracts prepared from adherent PMNs were immunoprecipitated with either 10 μl rabbit serum against human ALXR peptide [ASWGGTPEERLK] at the second extracellular loop28 or 5 μl antibody against ANXA1 (ref. 24) in the presence of phosphatase and proteases inhibitors. Immunoprecipitated proteins were subject to electrophoresis on a 12% SDS–PAGE gel and transferred onto ECL Hybond nitrocellulose membrane (Amersham, Buckinghamshire, UK). Membranes were further hybridized for ANXA1 or ALXR detection using the specific antibodies: anti-ANXA1 (1:10,000) or anti-ALXR rabbit serum (1:13,000). Immunoreactive proteins were detected using an enhanced chemiluminescence ECL kit from Amersham (UK).
Statistical analysis
Results were expressed as the mean ± s.e.m. of distinct experiments (performed in triplicate) or in animals. In vitro experiments were analyzed by Student's t-test with P < 0.05 taken as statistically significant. Analysis of in vivo data was done with one-way ANOVA plus Dunnett's test.
Supplementary Material
Acknowledgment
We thank B. Schmidt for microscopic analyses, N. Petasis for synthetic LXA4 and ATL stable analogs (prepared for P01-DE13499), and M.H. Small for expert assistance in manuscript preparation. This work was supported in part by grants GM38765 and P01-DE13499 (to C.N.S.) and by grants P0567 and P0583 of the Arthritis Research Campaign UK (to M.P.).
Footnotes
Note: Supplementary information is available on the Nature Medicine website.
Competing interests statement
The authors declare that they have no competing financial interests.
References
- 1.Cotran RS. Inflammation: Historical perspectives. In: Gallin JI, Snyderman R, Fearon DT, Haynes BF, Nathan C, editors. Inflammation: Basic Principles and Clinical Correlates. 3rd edn Lippincott Williams & Wilkins; Philadelphia: 1999. pp. 5–10. [Google Scholar]
- 2.Weissmann G. Aspirin. Sci. Am. 1991;264:84–90. doi: 10.1038/scientificamerican0191-84. [DOI] [PubMed] [Google Scholar]
- 3.Patrono C. Aspirin: new cardiovascular uses for an old drug. Am. J. Med. 2001;110:62S–65S. doi: 10.1016/s0002-9343(00)00645-8. [DOI] [PubMed] [Google Scholar]
- 4.Barnes CJ, Hardman WE, Cameron IL, Lee M. Aspirin, but not sodium salicylate, indomethacin, or nabumetone, reversibly suppresses 1,2-dimethylhydrazine-induced colonic aberrant crypt foci in rats. Dig. Dis. Sci. 1997;42:920–926. doi: 10.1023/a:1018812430512. [DOI] [PubMed] [Google Scholar]
- 5.Marcus AJ. Aspirin as prophylaxis against colorectal cancer. N. Engl. J. Med. 1995;333:656–658. doi: 10.1056/NEJM199509073331011. [DOI] [PubMed] [Google Scholar]
- 6.Samuelsson B. Les Prix Nobel: Nobel Prizes, Presentations, Biographies and Lectures. Almqvist & Wiksell; Stockholm: 1982. From studies of biochemical mechanisms to novel biological mediators: Prostaglandin endoperoxides, thromboxanes and leukotrienes; pp. 153–174. [DOI] [PubMed] [Google Scholar]
- 7.Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nature (London) New Biol. 1971;231:232–235. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- 8.Hench PS, Kendall EC, Slocumb CH, Polley HE. Proc. Staff Meet. Mayo Clin. 1949;24:181. [PubMed] [Google Scholar]
- 9.Boumpas DT, Wilder RL. Corticosteroids. In: Koopman WJ, editor. Arthritis and Allied Conditions: A Textbook of Rheumatology. 14th Lippincott Williams & Wilkins; Philadelphia: 2001. pp. 827–847. [Google Scholar]
- 10.Derry S, Loke YK. Risk of gastrointestinal haemorrhage with long term use of aspirin: meta-analysis. Br. Med. J. 2000;321:1183–1187. doi: 10.1136/bmj.321.7270.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cronstein BN, Weissmann G. Targets for antiinflammatory drugs. Annu. Rev. Pharmacol. Toxicol. 1995;35:449–462. doi: 10.1146/annurev.pa.35.040195.002313. [DOI] [PubMed] [Google Scholar]
- 12.Sin YM, Sedgwick AD, Chea EP, Willoughby DA. Mast cells in newly formed lining tissue during acute inflammation: A six day air pouch model in the mouse. Ann. Rheum. Dis. 1986;45:873–877. doi: 10.1136/ard.45.10.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jick H, Pinals RS, Ullian R, Slone D, Muench H. Dexamethasone and dexamethasone-aspirin in the treatment of chronic rheumatoid arthritis. Lancet. 1965;2:1203–1205. doi: 10.1016/s0140-6736(65)90632-x. [DOI] [PubMed] [Google Scholar]
- 14.Flower RJ, Rothwell NJ. Lipocortin-1: Cellular mechanisms and clinical relevance. Trends Pharmacol. Sci. 1994;15:71–76. doi: 10.1016/0165-6147(94)90281-x. [DOI] [PubMed] [Google Scholar]
- 15.Lim LH, Solito E, Russo-Marie F, Flower RJ, Perretti M. Promoting detachment of neutrophils adherent to murine postcapillary venules to control inflammation: effect of lipocortin 1. Proc. Natl. Acad. Sci USA. 1998;95:14535–14539. doi: 10.1073/pnas.95.24.14535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maridonneau-Parini I, Errasfa M, Russo-Marie F. Inhibition of O2-generation by dexamethasone is mimicked by lipocortin I in alveolar macrophages. J. Clin. Invest. 1989;83:1936–1940. doi: 10.1172/JCI114101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Serhan CN, Takano T, Chiang N, Gronert K, Clish CB. Formation of endogenous “antiinflammatory” lipid mediators by transcellular biosynthesis: Lipoxins and aspirin-triggered lipoxins inhibit neutrophil recruitment and vascular permeability. Am. J. Respir. Crit. Care Med. 2000;161:S95–S101. doi: 10.1164/ajrccm.161.supplement_1.ltta-19. [DOI] [PubMed] [Google Scholar]
- 18.Chiang N, Fierro IM, Gronert K, Serhan CN. Activation of lipoxin A4 receptors by aspirin-triggered lipoxins and select peptides evokes ligand-specific responses in inflammation. J. Exp. Med. 2000;191:1197–1207. doi: 10.1084/jem.191.7.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yokomizo T, Izumi T, Chang K, Takuwa T, Shimizu T. A G-protein-coupled receptor for leukotriene B4 that mediates chemotaxis. Nature. 1997;387:620–624. doi: 10.1038/42506. [DOI] [PubMed] [Google Scholar]
- 20.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N. Engl. J. Med. 1999;340:448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 21.Su SB, et al. A seven-transmembrane, G protein-coupled receptor, FPRL1, mediates the chemotactic activity of serum amyloid A for human phagocytic cells. J. Exp. Med. 1999;189:395–402. doi: 10.1084/jem.189.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Linke RP, Bock V, Valet G, Rothe G. Inhibition of the oxidative burst response of N-formyl peptide-stimulated neutrophils by serum amyloid-A protein. Biochem. Biophys. Res. Commun. 1991;176:1100–1105. doi: 10.1016/0006-291x(91)90397-p. [DOI] [PubMed] [Google Scholar]
- 23.Oliani SM, Paul-Clark MJ, Christian HC, Flower RJ, Perretti M. Neutrophil interaction with inflamed postcapillary venule endothelium alters annexin 1 expression. Am. J. Pathol. 2001;158:603–615. doi: 10.1016/S0002-9440(10)64002-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perretti M, et al. Mobilizing lipocortin 1 in adherent human leukocytes downregulates their transmigration. Nature Med. 1996;2:1259–1262. doi: 10.1038/nm1196-1259. [DOI] [PubMed] [Google Scholar]
- 25.Walther A, Riehemann K, Gerke V. A novel ligand of the formyl peptide receptor: annexin I regulates neutrophil extravasation by interacting with the FPR. Mol. Cell. 2000;5:831–840. doi: 10.1016/s1097-2765(00)80323-8. [DOI] [PubMed] [Google Scholar]
- 26.Perretti M, Getting SJ, Solito E, Murphy PM, Gao JL. Involvement of the receptor for formylated peptides in the in vivo anti-migratory actions of annexin 1 and its mimetics. Am. J. Pathol. 2001;158:1969–1973. doi: 10.1016/S0002-9440(10)64667-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Papayianni A, Serhan CN, Brady HR. Lipoxin A4 and B4 inhibit leukotriene-stimulated interactions of human neutrophils and endothelial cells. J. Immunol. 1996;156:2264–2272. [PubMed] [Google Scholar]
- 28.Kang Y, Taddeo B, Varai G, Varga J, Fiore S. Mutations of serine 236–237 and tyrosine 302 residues in the human lipoxin A4 receptor intracellular domains result in sustained signaling. Biochemistry. 2000;39:13551–13557. doi: 10.1021/bi001196i. [DOI] [PubMed] [Google Scholar]
- 29.Chiang N, et al. Aspirin-triggered 15-epi-lipoxin A4 (ATL) generation by human leukocytes and murine peritonitis exudates: Development of a specific 15-epi-LXA4 ELISA. J. Pharmacol. Exp. Ther. 1998;287:779–790. [PubMed] [Google Scholar]
- 30.Clish CB, et al. Local and systemic delivery of a stable aspirin-triggered lipoxin prevents neutrophil recruitment in vivo. Proc. Natl. Acad. Sci. USA. 1999;96:8247–8252. doi: 10.1073/pnas.96.14.8247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fadok VA, et al. A receptor for phosphatidylserine-specific clearance of apoptotic cells. Nature. 2000;405:28–29. doi: 10.1038/35011084. [DOI] [PubMed] [Google Scholar]
- 32.Godson C, et al. Cutting edge: Lipoxins rapidly stimulate nonphlogistic phagocytosis of apoptotic neutrophils by monocyte-derived macrophages. J. Immunol. 2000;164:1663–1667. doi: 10.4049/jimmunol.164.4.1663. [DOI] [PubMed] [Google Scholar]
- 33.Liu Y, et al. Glucocorticoids promote nonphlogistic phagocytosis of apoptotic leukocytes. J. Immunol. 1999;162:3639–3646. [PubMed] [Google Scholar]
- 34.Tsao FHC, Meyer KC, Chen X, Rosenthal NS, Hu J. Degradation of annexin I in bronchoalveolar lavage fluid from patients with cystic fibrosis. Am. J. Respir. Cell Mol. Biol. 1998;18:120–128. doi: 10.1165/ajrcmb.18.1.2808. [DOI] [PubMed] [Google Scholar]
- 35.Ariel A, et al. IL-2 induces T cell adherence to extracellular matrix: Inhibition of adherence and migration by IL-2 peptides generated by leukocyte elastase. J. Immunol. 1998;161:2465–2472. [PubMed] [Google Scholar]
- 36.Yokomizo T, Izumi T, Shimizu T. Leukotriene B4: Metabolism and signal transduction. Arch. Biochem. Biophys. 2001;385:231–241. doi: 10.1006/abbi.2000.2168. [DOI] [PubMed] [Google Scholar]
- 37.Perretti M, et al. Acute inflammatory response in the mouse: Exacerbation by immunoneutralization of lipocortin 1. Br. J. Pharmacol. 1996;117:1145–1154. doi: 10.1111/j.1476-5381.1996.tb16709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cotter MJ, Norman KE, Hellewell PG, Ridger VC. A novel method for isolation of neutrophils from murine blood using negative immunomagnetic separation. Am. J. Pathol. 2001;159:473–481. doi: 10.1016/S0002-9440(10)61719-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.