Abstract
The ductus arteriosus (DA), an essential vascular shunt for fetal circulation, begins to close immediately after birth. Although Ca2+ influx through several membrane Ca2+ channels is known to regulate vasoconstriction of the DA, the role of the T-type voltage-dependent Ca2+ channel (VDCC) in DA closure remains unclear. Here we found that the expression of α1G, a T-type isoform that is known to exhibit a tissue-restricted expression pattern in the rat neonatal DA, was significantly up-regulated in oxygenated rat DA tissues and smooth muscle cells (SMCs). Immunohistological analysis revealed that α1G was localized predominantly in the central core of neonatal DA at birth. DA SMC migration was significantly increased by α1G overexpression. Moreover, it was decreased by adding α1G-specific small interfering RNAs or using R(−)-efonidipine, a highly selective T-type VDCC blocker. Furthermore, an oxygenation-mediated increase in an intracellular Ca2+ concentration of DA SMCs was significantly decreased by adding α1G-specific siRNAs or using R(−)-efonidipine. Although a prostaglandin E receptor EP4 agonist potently promoted intimal thickening of the DA explants, R(−)-efonidipine (10−6 m) significantly inhibited EP4-promoted intimal thickening by 40% using DA tissues at preterm in organ culture. Moreover, R(−)-efonidipine (10−6 m) significantly attenuated oxygenation-induced vasoconstriction by ∼27% using a vascular ring of fetal DA at term. Finally, R(−)-efonidipine significantly delayed the closure of in vivo DA in neonatal rats. These results indicate that T-type VDCC, especially α1G, which is predominantly expressed in neonatal DA, plays a unique role in DA closure, implying that T-type VDCC is an alternative therapeutic target to regulate the patency of DA.
The ductus arteriosus (DA)2 is an essential vascular shunt between the aortic arch and the pulmonary trunk during a fetal period (1). After birth, the DA closes immediately in accordance with its smooth muscle contraction and vascular remodeling, whereas the connecting vessels such as the aorta and pulmonary arteries remain open. When the DA fails to close after birth, the condition is known as patent DA, which is a common form of congenital heart defect. Patent DA is also a frequent problem with significant morbidity and mortality in premature infants. Investigating the molecular mechanism of DA closure is important not only for vascular biology but also for clinical problems in pediatrics.
Voltage-dependent Ca2+ channels (VDCCs) consist of multiple subtypes, named L-, N-, P/Q-, R-, and T-type. L-type VDCCs are known to play a primary role in regulating Ca2+ influx and thus vascular tone in the development of arterial smooth muscle including the DA (2–4). Our previous study demonstrated that all T-type VDCCs were expressed in the rat DA (5). α1G subunit, especially, was the most dominant isoform among T-type VDCCs. The abundant expression of α1G subunit suggests that it plays a role in the vasoconstriction and vascular remodeling of the DA. In this regard, Nakanishi et al. (6) demonstrated that 0.5 mm nickel, which blocks T-type VDCC, inhibited oxygen-induced vasoconstriction of the rabbit DA. On the other hand, Tristani-Firouzi et al. (7) demonstrated that T-type VDCCs exhibited little effect on oxygen-sensitive vasoconstriction of the rabbit DA. Thus, the role of T-type VDCCs in DA vasoconstriction has remained controversial.
In addition to their role in determining the contractile state, a growing body of evidence has demonstrated that T-type VDCCs play an important role in regulating differentiation (8, 9), proliferation (10–12), migration (13, 14), and gene expression (15) in vascular smooth muscle cells (SMCs). Hollenbeck et al. (16) and Patel et al. (17) demonstrated that nickel inhibited platelet-derived growth factor-BB-induced SMC migration. Rodman et al. (18) demonstrated that α1G promoted SMC proliferation in the pulmonary artery. The DA dramatically changes its morphology during development. Intimal cushion formation, a characteristic feature of vascular remodeling of the DA (19–21), involves many cellular processes: an increase in SMC migration and proliferation, production of hyaluronic acid under the endothelial layer, impaired elastin fiber assembly, and so on (1, 19, 21–23). Although our previous study demonstrated that T-type VDCCs are involved in smooth muscle cell proliferation in the DA (5), the role of T-type VDCCs in vascular remodeling of the DA has remained poorly understood.
In the present study, we hypothesized that T-type VDCCs, especially α1G subunit, associate with vascular remodeling and vasoconstriction in the DA. To test our hypothesis, we took full advantage of recent molecular and pharmacological developments. We chose the recently developed, highly selective T-type VDCC blocker R(−)-efonidipine instead of low dose nickel for our study. Selective inhibition or activation of α1G subunit was also obtained using small interfering RNA (siRNA) technology or by overexpression of the α1G subunit gene, respectively. We found that Ca2+ influx through T-type VDCCs promoted oxygenation-induced DA closure through SMC migration and vasoconstriction.
EXPERIMENTAL PROCEDURES
Animals
Timed pregnant Wistar rats were purchased from Japan SLC, Inc. (Shizuoka, Japan). The DA was obtained from rat fetuses on the 19th day of gestation (preterm) and on the 21st day of gestation (term) and from neonates within at least 6 h after delivery. The DAs on the 19th day of gestation remained immature, whereas they became mature on the 21st day of gestation. All of the animals were cared for in compliance with the guiding principles of the American Physiologic Society. The experiments were approved by the Ethical Committees on Animal Experiments of Waseda University and Yokohama City University School of Medicine.
Reagents
R(−)-Efonidipine, a highly selective T-type VDCC blocker, and ONO-AE1–329, a selective prostaglandin E receptor EP4 agonist, were provided by Nissan Chemical Industries, Ltd. (Saitama, Japan) and ONO Pharmaceutical Co. (Osaka, Japan), respectively. Nitrendipine, a selective L-type VDCC blocker, was purchased from Sigma-Aldrich. α1G plasmid was kindly provided by Dr. Lory at Université Montpellier (Montpellier, France). Collagenase II was from the Worthington Biochemical Corp. (Lakewood, NJ). Collagenase/dispase was purchased from Roche Applied Science. Fetal calf serum (FCS) was purchased from Invitrogen. Platelet-derived growth factor-BB and 10% buffered formalin were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). Elastase type II-A, trypsin inhibitor type I-S, bovine serum albumin V, penicillin-streptomycin solution, Dulbecco's modified Eagle's medium, and Hanks' balanced salt solution were purchased from Sigma-Aldrich. Anti-α1G and anti-α-smooth muscle actin antibodies were purchased from Sigma-Aldrich. Anti-Ki-67 antibody was purchased from Dako.
Immunoblotting
To examine the expression of α1G protein after birth, we used pooled tissues obtained from one littermate of Wistar rat neonates on the day of birth. After excision, the tissues were immediately frozen in liquid nitrogen and stored at −80 °C until use. Immunoblotting was performed as described previously (5).
Tissue Staining and Immunohistochemistry
To demonstrate the immunoperoxidase of α1G subunit and Ki-67 in the rat DA, tissue staining and immunohistochemistry were carried out as previously explained (5, 21).
Primary Culture of Rat DA SMCs
Vascular SMCs in primary culture were obtained from the DAs of Wistar rat embryos on embryonic day 21, as described previously (5, 21). The confluent cells for four to six passages were used in the experiments. We checked that these cells were in a more differentiated state than the cells of primary isolations, although the cells for four to six passages were in a less differentiated state than DA tissues at term and adult aortic artery, when the differentiation state was determined by the expression of marker genes of SMC differentiation such as SM1, SM2, and SMemb (supplemental Fig. S1). Therefore, it should be noted that the phenotype of cultured DA SMCs was not completely the same as the contractile phenotype of tissues of the DA and aorta. To examine the effect of a change in oxygen tension, DA SMCs were cultured in a hypoxic chamber (1% O2, 5% CO2) and then transferred to a normoxic chamber (21% O2, 5% CO2).
SMC Migration Assay
The migration assay was performed using 24-well transwell culture inserts with polycarbonate membranes (8-μm pores; Corning Inc.) as described previously with some minor modifications (21). Using a fibronectin-coated membrane of a Boyden chamber, we examined an acute (∼4 h) effect of T-type VDCC on SMC migration by overexpression of a α1G protein or by inhibition with R(−)-efonidipine or with α1G-specific siRNAs. To examine the effect of T-type VDCC on actively migrating SMCs, 10% FCS was used as a potent stimulator for SMC migration.
Transfection of Plasmid DNAs and siRNAs in DA SMCs
A full-length of rat α1G cDNA was ligated to pcDNA3.1 plasmid vector. Plasmid DNAs were purified using a High Pure plasmid isolation kit (Roche Applied Science) according to the manufacturer's instructions. Genome-ONE NEO, an inactivated, column-purified HVJ-E vector, was purchased from Ishihara Sangyo Co. Ltd.(Osaka, Japan). Plasmid DNAs were transfected according to the manufacturer's instructions.
Two double-standard 21-bp siRNAs to the selected region of α1G subunit cDNA were purchased from Qiagen. The antisense siRNA sequences targeting α1G subunit were 5′-UAGCAUUGGACAGGAAUCGdTdA-3′ and 5′-UAAUGUGACGAGAAUGCGCdAdT-3′. AllStars negative control siRNA purchased from Qiagen was used as a control nonsilencing siRNA. siRNAs were transfected as previously described (21).
Quantitative Reverse Transcription-PCR Analysis
Both isolation of total RNA from pooled tissues or cultured SMCs and generation of cDNA and reverse transcription-PCR analysis for α1G subunit were carried out as described previously (5). The forward primer specific for α1G subunit was 5′-cctgatttcttttcgcccag-3′. The reverse primer specific for α1G subunit was 5′-tggcaaaaggctctttcgtag-3′. The 5′ and 3′ primers specific for glyceraldehyde-3-phosphate dehydrogenase were 5′-cccatcaccatcttccaggagcg-3′ and 3′-gcagggatgatgttctgggctgcc-5′. The abundance of each gene was determined relative to internal control using a Quantitect primer assay (Qiagen). For each reverse transcription-PCR experiment that included a reverse transcription negative control, we confirmed that there was no amplification in any reaction.
Intracellular Ca2+ Concentration in DA SMCs
DA SMCs were loaded with fura-2/AM (Dojindo, Kumamoto, Japan) in a Tyrode solution (137 mm NaCl, 2.7 mm KCl, 1.4 mm CaCl2, 5.6 mm glucose, 0.5 mm MgCl2, 0.3 mm NaH2PO4, 12 mm NaHCO3, pH7.4). The DA SMCs on a 96-well microplate (Nunc) were superfused with the solution containing 2.5 μm fura-2/AM for 20 min at 37 °C. After the dye loading, the loading buffer was then removed, and the cells were washed twice with loading buffer. DA SMCs were incubated for 24 h under the following conditions within the dye loading time: a humidified chamber, 5% CO2, and 1% O2 at 37 °C. Intracellular Ca2+ concentration of DA SMCs was performed using the ARVOTMMX fluorescence microplate reader (PerkinElmer Life Sciences) from 5 min at room air after the hypoxic incubation. Fura-2/AM was excited at 340 and 390 nm with fluorescence emission detected using a 510-nm band pass filter. We evaluated intracellular Ca2+ concentration with the use of the observed fluorescence ratio 340/390 nm.
Organ Culture
Ex vivo DA organ culture was prepared as described previously (21). Briefly, fetal arteries including the DA on the 19th day of gestation were stimulated for 2 days by R(−)-efonidipine under the following conditions: a humidified chamber, a concentration of 10−6 m in 0.5% FCS containing Dulbecco's modified Eagle's medium, 5% CO2, and 95% ambient mixed air at 37 °C. The segments were fixed in 10% buffered formalin and embedded in paraffin. Morphometric analyses were conducted using Win Roof version 5.0 software (Mitani Corp., Tokyo, Japan). Intimal cushion formation was defined as [intimal area]/[medial area].
Isometric Tension of the DA Vascular Rings
After the maternal rats were anesthetized on the 21st day of gestation with an overdose of pentobarbital (100 mg/kg), the fetuses at embryonic day 21 were delivered by cesarean section. Either the vascular ring of the DA or the descending aorta was placed in a tissue bath and kept at 37 °C. Two tungsten wires (30 μm in diameter) were threaded into the lumen, and the preparation was mounted in a two-channel myograph (Dual Wire myograph system 410A; Unique Medical, Tokyo, Japan). One tungsten wire was connected to a micro-manipulator, and the other was connected to a force transducer. All of the vascular rings were initially stabilized for at least 60 min with a modified Krebs-Henseleit solution (Sigma-Aldrich) that was equilibrated with room air and whose temperature was maintained at 37 °C by a heated water jacket. Isometric tension was continuously monitored using a PowerLab/8 SP system (ADInstruments, Inc., Colorado Springs, CO). After stabilization, the vascular ring was exposed to 1 μm of indomethacin-containing Krebs-Henseleit solution for at least 10 min at 37 °C. After the vascular ring was washed with modified Krebs-Henseleit solution and relaxed, the resting tension was adjusted to 0.30 mN. After a plateau vasoconstriction had been attained using oxygen exposure (95% O2, 5% CO2), R(−)-efonidipine or the L-type Ca2+ channel blocker, nitrendipine, was added to stimulate vasodilatation. After the vasoconstriction reached a new steady state, the concentration of R(−)-efonidipine was increased from 10−7 m to 10−5 m. At the end of all experiments, vasoconstriction of the DA was induced by potassium-enriched solutions (22 mm NaCl, 120 mm KCl, 1.5 mm CaCl2, 6 mm glucose, 1 mm MgCl2, 5 mm HEPES, pH 7.4).
Time Course of DA Closure after Birth
After the maternal rats were anesthetized on the 21st day of gestation with an overdose of pentobarbital (100 mg/kg), rat newborns were obtained by cesarean section. Immediately after cesarean section, the neonates were given intraperitoneal injections either with R(−)-efonidipine at a concentration of 10 μg/g of body weight or 100 μg/g of body weight or with saline. After injection, the neonates were incubated in room air at 33 °C for 30 or 90 min, and then the DA segments were fixed and analyzed by the same method used in the organ culture. The degrees of vasoconstriction and intimal cushion formation were estimated by [open area]/[total vessel area] and [intimal area]/[medial area], respectively.
Statistical Analysis
All of the data are presented as the means ± S.E. Student's unpaired t tests were used to compare mRNA expression in the DA and other tissues. Comparisons between data from multiple groups were performed by unpaired analysis of variance followed by the Student's-Newmann-Keuls test. p values <0.05 were considered statistically significant.
RESULTS
α1G Protein Was Up-regulated and Localized in the Cells Sealing the Lumen of the DA after Birth
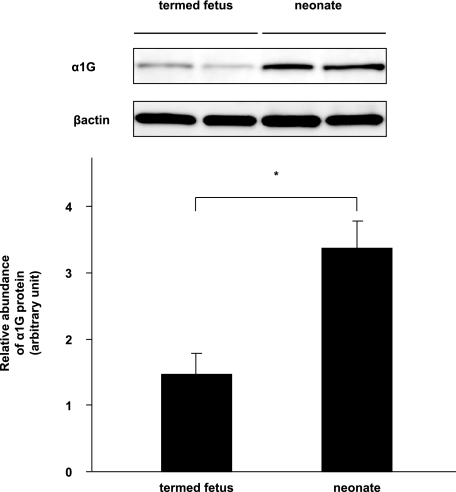
Our previous study demonstrated that α1G mRNA was predominantly expressed and were significantly up-regulated in the DA during vascular development (5). Then we examined the expression of α1G protein in the DA tissue during the perinatal period. Consistent with the change in the expression of mRNA, the expression levels of α1G protein were significantly up-regulated by 2.3-fold in the neonatal DA just after birth from what they were in the DA on embryonic day 21 (Fig. 1).
FIGURE 1.
Western blot analysis of the rat perinatal DA. The expression levels of α1G protein were significantly up-regulated by 2.3-fold in the neonatal DA relative to that in the DA of termed fetuses. The values are expressed as the means ± S.E. (n = 4). * indicates p < 0.01.
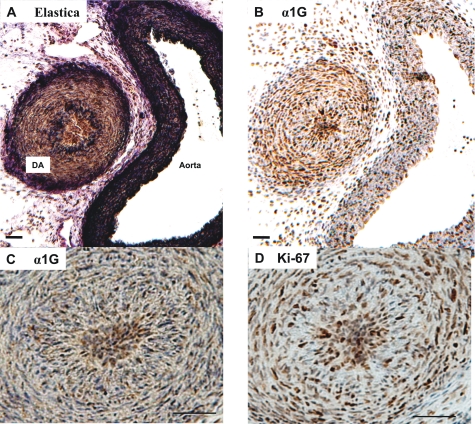
Because our previous study had demonstrated that α1G protein was strongly expressed in the region of intimal thickening of the mature DA at embryonic day 21 (5), we further investigated the localization of α1G protein in the DA after birth. We found that the lumen of the rat neonatal DA was filled with densely packed intimal cells within 1 h after birth, whereas the lumen of the aorta was widely open (Fig. 2, A and B). The immunoreaction of α1G protein was strongly stained in these cells of the central core of the DA lumen (Fig. 2C). Ki-67, a nuclear antigen associated with cell proliferation, was also strongly stained in the packed intimal cells (Fig. 2D), suggesting that α1G is profoundly expressed in the proliferative cells.
FIGURE 2.
Immunohistochemistry of rat neonatal DA. A, elastica staining of the DA and the aorta. B, α1G subunit staining of the DA and the aorta. Strong immunoreaction was found in the DA. C, enlarged image of the DA stained by anti-α1G subunit antibody. D, Ki-67 staining. Strong immunoreaction was found in the overlapping region of the α1G subunit staining. Scale bars, 50 μm.
Oxygenation Up-regulated the Expression of α1G in DA Smooth Muscle Cells
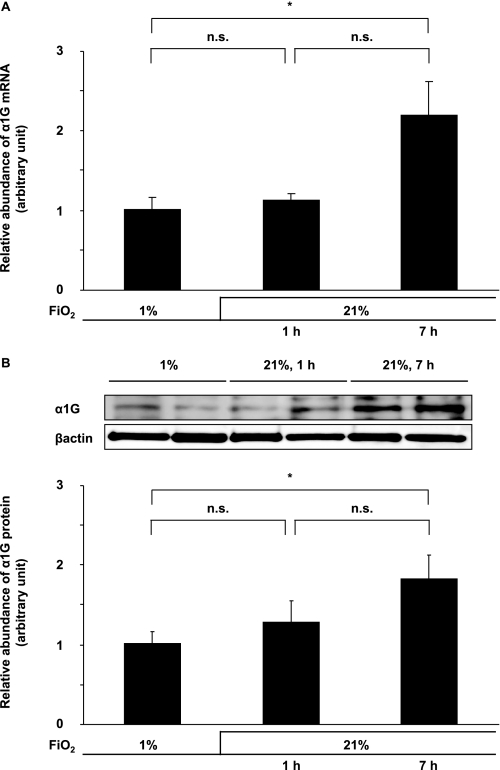
We then examined the effect of oxygenation on the expression of α1G in DA SMCs. When the condition of the culture medium was changed from hypoxia (1% oxygen) to normoxia (21% oxygen) in the DA SMCs after 7 h, the expression levels of α1G mRNA were up-regulated by 2.2-fold, and those of protein were up-regulated by 1.9-fold (Fig. 3), although they were unchanged after 1 h. These data may explain the association between the up-regulation of α1G in the rat neonatal DA and an increase in oxygen tension in the circulation after birth.
FIGURE 3.
Effects of oxygenation on α1G expression. When the condition of the culture medium was changed from hypoxia (1% oxygen) to normoxia (21% oxygen), the expression levels of both α1G mRNA (A) and protein (B) were significantly up-regulated after 7 h by 2.2- and 1.9-fold, respectively. The values are expressed as the means ± S.E. (n = 6). * indicates p < 0.01. n.s., not significant.
α1G Promoted SMC Migration of the DA
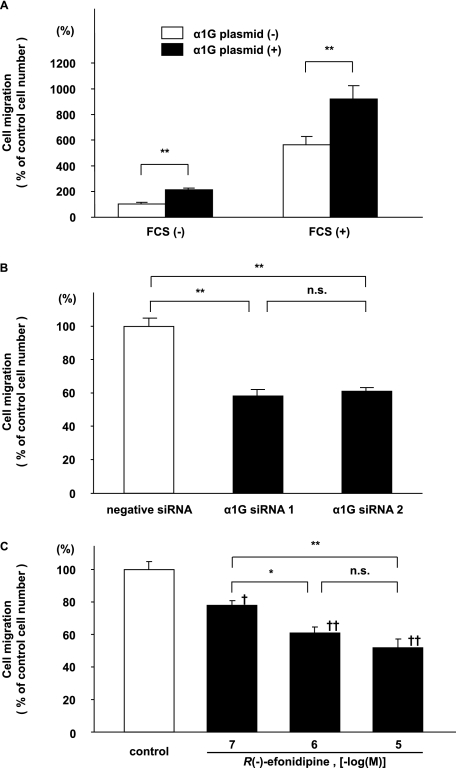
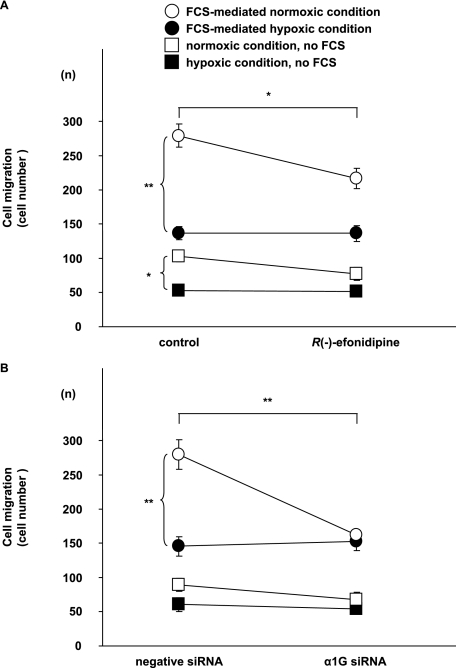
Progressive SMC migration from vascular media into the endothelial layer is an important vascular remodeling process of the DA at birth (1, 19, 23). These findings regarding the localization of α1G protein that was observed suggest that α1G regulated the SMC migration of the DA. First, when the expression of α1G mRNA was up-regulated by ∼2.5-fold by transfection of α1G plasmids in DA SMCs (supplemental Fig. S2A), SMC migration was significantly increased by 2.1-fold (p < 0.01) (Fig. 4A). In the presence of 10% FCS, SMC migration was increased by more than 5-fold, probably because of hormones and/or cytokines that were contained in FCS. It should be noted that FCS did not significantly affect the expression of α1G mRNA (supplemental Fig. S3). α1G overexpression further increased FCS-mediated SMC migration by 1.6-fold (p < 0.01) (Fig. 4A). Next, we examined the effect of genetic and pharmacological inhibition of T-type VDCC on DA SMC migration. Two different siRNAs for α1G significantly decreased the expression of α1G mRNA and protein in DA SMCs by ∼60% (supplemental Fig. S2, B and C), whereas they did not significantly change the expression of α1C, α1C splice variant, and α1D mRNAs that was abundantly detected in the rat DA (supplemental Fig. S4) After these α1G siRNAs were used, FCS-mediated SMC migration was significantly decreased by ∼42% when compared with that of a nontargeting negative control siRNA (Fig. 4B). Furthermore, R(−)-efonidipine, a recently developed, highly selective T-type VDCC blocker, significantly attenuated FCS-mediated SMC migration by ∼48% in a dose-dependent manner (p < 0.01) (Fig. 4C). Mibefradil, another selective T-type VDCC blocker, also significantly attenuated FCS-mediated SMC migration by ∼17% in a dose-dependent manner (p < 0.05), although its inhibitory effect was weaker than that of R(−)-efonidipine (supplemental Fig. S5).
FIGURE 4.
DA SMC migration assay. A, effects of α1G overexpression on DA SMC migration. Migration was significantly increased in α1G overexpressed SMCs when compared with that of control SMCs (n = 7). 10% FCS increased SMC migration by more than 5-fold. In the α1G-overexpressed cells, SMC migration was further increased by 1.6-fold (n = 7). B, effects of α1G-specific siRNAs on DA SMC migration. Two different α1G-specific siRNAs significantly decreased FCS-mediated SMC migration by ∼58% when compared with a nontargeting negative control siRNA (n = 11). C, effects of R(−)-efonidipine on DA SMC migration. R(−)-efoidipine significantly attenuated DA SMC migration by ∼52% in a dose-dependent manner (n = 8). The values are expressed as the means ± S.E. * and ** indicate p < 0.05 and p < 0.01, and † and †† indicate p < 0.05 and p < 0.01 versus control, respectively. n.s., not significant.
Oxygenation Was Required for α1G-mediated SMC Migration
Because vascular remodeling of the DA, including SMC migration, dramatically progresses after birth, we hypothesized that oxygenation might play a critical role in regulating this remodeling through an increase in Ca2+ influx via T-type VDCCs. When cultured DA SMCs were used and when the condition of culture medium was changed from hypoxia to normoxia, SMC migration was increased by ∼2.0-fold in the normoxic group when compared with that in the hypoxic group, either in the absence or presence of 10% FCS (p < 0.01), indicating that oxygenation promoted SMC migration of the DA. Furthermore, we examined the effect of T-type VDCCs on oxygenation-mediated migration. We found that R(−)-efonidipine at a concentration of 10−6 m significantly attenuated DA SMC migration by ∼23% when the culture medium was changed from hypoxia to normoxia (p < 0.05), although it exhibited no effect on SMC migration in a continuously hypoxic medium (Fig. 5A). In addition, using one of the α1G-specific siRNAs that significantly decreased the expression of α1G mRNA as presented above, we found that the α1G-specific siRNA significantly decreased DA SMC migration by ∼42% when compared with a nontargeting negative control siRNA in a culture medium changed from hypoxia to normoxia (p < 0.01) (Fig. 5B). However, the α1G-specific siRNA did not suppress SMC migration in a continuously hypoxic medium. It should be noted that FCS was still capable of stimulating DA SMC migration even in the presence of R(−)-efonidipine and α1G-specific siRNA, suggesting that FCS contains some cell migrating factors that are independent of the effect of T-type VDCCs on SMC migration. Taken together, these independent experiments indicated that T-type VDCCs, most likely α1G, played an important role in promoting oxygenation-mediated SMC migration in the DA.
FIGURE 5.
Effects of oxygenation on DA SMC migration. A, effects of R(−)-efoidipine on oxygenation-mediated DA SMC migration. R(−)-Efonidipine significantly attenuated DA SMC migration by ∼77% in a culture medium changed from hypoxia to normoxia (n = 8). It should be noted that no effect of the α1G-specific siRNA and R(−)-efonidipine on SMC migration was observed in the hypoxic culture medium. B, effects of α1G-specific siRNA on oxygenation-mediated DA SMC migration. When the condition of the culture medium was changed from hypoxia to normoxia, SMC migration was significantly increased in the normoxic group when compared with that in the hypoxic group in the presence of 10% FCS (n = 5). DA SMC migration was significantly decreased by ∼58% when compared with that of a nontargeting negative control siRNA in a culture medium changed from hypoxia to normoxia (n = 5). The values are expressed as the means ± S.E. * and ** indicate p < 0.05 and p < 0.01, respectively.
α1G Regulated Oxygenation-induced Intracellular Ca2+ Increases
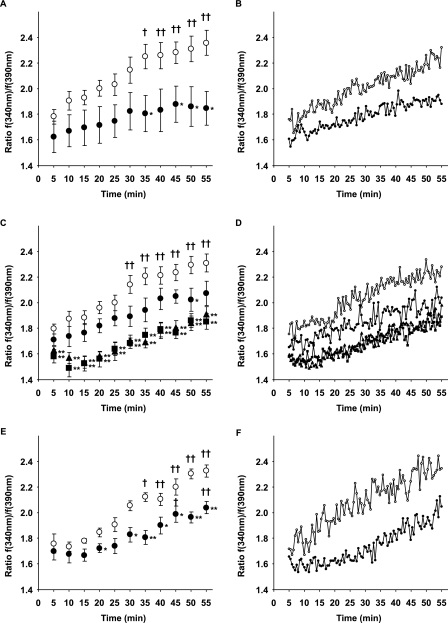
It has been known that oxygenation increases intracellular Ca2+ concentration of the DA to consequently initiate smooth muscle contraction of the DA (6, 24). Accordingly, using fura-2/AM fluorescence assay, we also found that intracellular Ca2+ concentration of cultured DA SMCs was significantly increased by ∼1.3-fold by oxygenation when the condition of culture medium was changed from hypoxia to normoxia (Fig. 6, A and B). Importantly, we found that R(−)-efonidipine significantly inhibited the oxygenation-mediated increase in an intracellular Ca2+ concentration of cultured DA SMCs by ∼12 or ∼20% at a concentration of 10−7 or 10−6 m, respectively (Fig. 6, C and D). In addition, using one of the α1G-specific siRNAs that significantly decreased the expression of α1G mRNA as presented above, we found that the α1G-specific siRNA significantly decreased the oxygenation-mediated increase in intracellular Ca2+ concentration of cultured DA SMCs by ∼15% (Fig. 6, E and F). Taken together, these data indicated that T-type VDCCs, most likely α1G, played an important role in the oxygenation-mediated increases in intracellular Ca2+ concentration of the DA.
FIGURE 6.
Effects of oxygenation on intracellular Ca2+ concentration in DA SMCs. A, effects of oxygenation on the fura-2/AM fluorescence ratio. When the condition of the culture medium was changed from hypoxia to normoxia, the fluorescence ratio 340/390 nm was significantly increased from 35 min after oxygenation (open circle, n = 6). The fluorescence ratio 340/390 nm was not significantly increased when the condition of the culture medium stayed in the normoxia (closed circle, n = 6). B, traces of the ratio of representative A. C, effects of R(−)-efonidipine on the oxygenation-mediated increase in the fura-2/AM fluorescence ratio. R(−)-Efonidipine at a concentration of 10−7 m significantly decreased the ratio by ∼12% in only 50 min after oxygenation (closed circle, n = 6), when compared with controls (open circle, n = 6). R(−)-Efonidipine at a concentration of 10−6 m significantly decreased the ratio from 5 min after oxygenation, which decreased by ∼20% (closed triangle, n = 6). R(−)-Efonidipine at a concentration of 10−5 m significantly decreased the ratio from 5 min after oxygenation, which decreased by at most 21% (closed square, n = 6). D, traces of the ratio of representative C. E, effects of α1G siRNA on oxygenation-mediated increase of the ratio. α1G siRNA significantly suppressed the oxygenation-mediated increases in the ratio by 15% from 20 min (closed circle, n = 6) when compared with a nontargeting negative control siRNA (open circle, n = 6). F, traces of the ratio of representative E. The values are expressed as the means ± S.E. † and †† indicate p < 0.05 and p < 0.01 versus 5 min after oxygenation, respectively, and * and ** indicate p < 0.05 and p < 0.01 versus the normoxic condition, respectively.
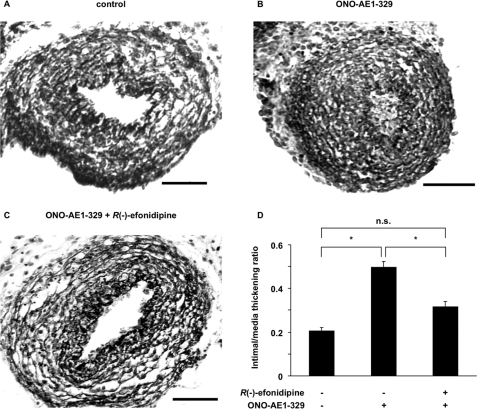
R(−)-Efonidipine Inhibited EP4-mediated Intimal Thickening in the Rat DA
Our previous study had demonstrated that when immature rat DA explants were exposed to an EP4-specific agonist, ONO-AE1–329, for 48 h in an organ culture, intimal cushion formation was fully developed in DA explants (21). To investigate whether T-type VDCC indeed plays an important role in promoting intimal cushion formation, we examined the effect of R(−)-efonidipine on ONO-AE1–329-mediated intimal cushion formation in the rat DA explants (Fig. 7). The intimal thickening was 2.5-fold greater in the presence of ONO-AE1–329 than it was in the control (Fig. 7, B and D), which was consistent with our previous findings (21). R(−)-Efonidipine significantly inhibited ONO-AE1–329-mediated intimal cushion formation by 40% (Fig. 7, C and D). The immunostaining of Ki-67 of ONO-AE1–329-mediated intimal cushion formation was similar in the presence or absence of R(−)-efonidipine (supplemental Fig. S6).
FIGURE 7.
Effects of R(−)-efonidipine on EP4-mediated intimal thickening of immature rat DA explants. A, control. B, ONO-AE1–329 (10−6 m). C, ONO-AE1–329 (10−6 m) plus R(−)-efonidipine (10−6 m). Scale bars, 50 μm. D, the ratio of intimal and medial thickening. Intimal thickening was 2.5-fold greater in the presence of ONO-AE1–329 than it was in the control. R(−)-Efonidipine significantly inhibited ONO-AE1–329-mediated intimal cushion formation by 60%. The values are expressed as the means ± S.E. (n = 5). * indicates p < 0.01. n.s., not significant.
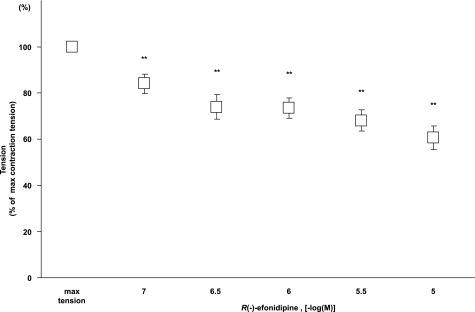
R(−)-Efonidipine Attenuated Oxygenation-induced Vasoconstriction of the Rat DA
Because vasoconstriction is another important factor of DA closure, we examined the role of T-type VDCCs in DA vasoconstriction by exposing a vascular ring of the fetal DA at embryonic day 21 to oxygenating Krebs-Henseleit solution. R(−)-Efonidipine attenuated oxygenation-induced vasoconstriction in a dose-dependent manner (Fig. 8). Isometric tension induced by oxygen was attenuated by ∼27% or by 40% in the presence of R(−)-efonidipine at a concentration of 10−6 or 10−5 m, respectively. R(−)-Efonidipine exhibits selective inhibition of T-type VDCCs at a concentration of 10−6 m, whereas it inhibits not only T-type but also L-type VDCCs at a concentration of 10−5 m (25). The data indicated that not only T-type but also L-type VDCCs contributed to oxygenation-induced vasoconstriction. In this regard, nitrendipine, a selective L-type VDCC blocker, additively inhibited oxygenation-induced vasoconstriction in the presence of R(−)-efonidipine (10−6 m) (data not shown).
FIGURE 8.
Effects of R(−)-efonidipine on vasoconstriction of rat DA. DA tension as a function of R(−)-efonidipine. R(−)-Efonidipine significantly attenuated oxygen-induced vasoconstriction in a dose-dependent manner (n = 18). The values are expressed as the means ± S.E. ** indicates p < 0.01 versus maximal vasoconstriction.
R(−)-Efonidipine Delayed Closure of the Rat Neonatal DA
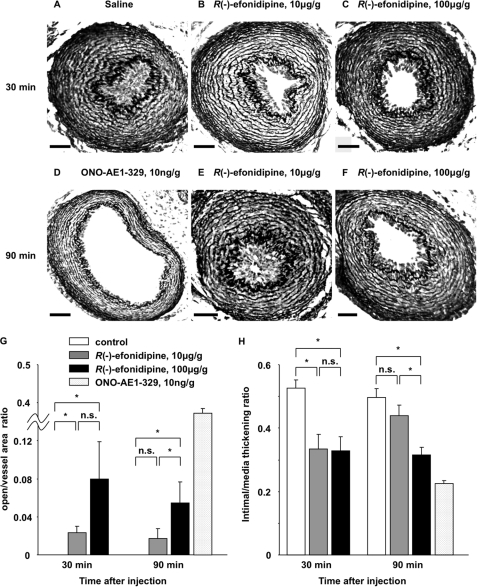
The present study demonstrated that Ca2+ influx via T-type VDCCs, especially α1G, stimulated SMC migration, intimal cushion formation, and vasoconstriction in the cultured DA SMCs or DA explants. It would be important to determine whether blockade of Ca2+ influx via T-type VDCCs indeed prevents in vivo DA closure after birth. To test this possibility, R(−)-efonidipine was intraperitoneally injected into rat newborns just after delivery by cesarean section. The data obtained is summarized in Table 1. All of the control DAs closed at 30 min after saline injection (Fig. 9A), whereas all of the DAs remained open at 90 min after ONO-AE1–329 injection (Fig. 9D). After injection of R(−)-efonidipine at a concentration of 10 μg/g of body weight, most (71%, five of seven neonatal rats) of the DAs remained open at 30 min, whereas less than half (43%, three of seven neonatal rats) remained open at 90 min (Fig. 9, B and E). Furthermore, when the dose of R(−)-efonidipine was increased to 100 μg/g of body weight, the percentage of patent DA was increased to 86% at both 30 and 90 min after injection of R(−)-efonidipine (Fig. 9, C and F). The ratio of [open area]/[total vessel area] was significantly increased by administration of R(−)-efonidipine (n = 6–7; Fig. 9G), suggesting that it attenuated vasoconstriction of the neonatal rat DA. The ratio of [intimal area]/[medial area] was significantly decreased by administration of R(−)-efonidipine (n = 6–7; Fig. 9H), suggesting that it inhibited intimal cushion formation. These data indicated that blockade of T-type VDCCs indeed delays closure of the rat neonatal DA through vasodilation and intimal cushion formation.
TABLE 1.
Patent DA ratio and DA patency ratio when two different substances were intraperitoneally injected into rat newborns at two different times just after delivery by cesarean section
| Substance injected (dosage) | Time after injection | DA patency ratioa |
|---|---|---|
| min | % | |
| Saline | 30 | 0/6 (0) |
| R(−)-Efonidipine (10 μg/g of body weight) | 30 | 5/7 (71) |
| R(−)-Efonidipine (100 μg/g of body weight) | 30 | 6/7 (86) |
| Saline | 90 | 0/6 (0) |
| R(−)-Efonidipine (10 μg/g of body weight) | 90 | 3/7 (43) |
| R(−)-Efonidipine (100 μg/g of body weight) | 90 | 6/7 (86) |
| ONO-AE1–329 (10 ng/g of body weight) | 90 | 3/3 (100) |
a Number of experiments in which DA patency was observed per number of experiments. The numbers in parentheses indicate the percentages of patent DA.
FIGURE 9.
Effects of R(−)-efonidipine on the patency of in vivo rat neonatal DA. A–F, representative morphology of the neonatal DA 30 min after peritoneal injection of saline (A), of R(−)-efonidipine (10 μg/g of body weight) (B), and of R(−)-efonidipine (100 μg/g of body weight) (C), and 90 min after injection of ONO-AE1–329 (10 ng/g of body weight) (D), of R(−)-efonidipine (10 μg/g of body weight) (E), and of R(−)-efonidipine (100 μg/g of body weight) (F). Scale bars, 50 μm. G, the ratio of [open area]/[total vessel area] versus time after injection. The ratio was significantly increased by administration of R(−)-efonidipine. H, the ratio of [intimal area]/[medial area] versus time after injection. The ratio was significantly decreased by administration of R(−)-efonidipine. The values are expressed as the means ± S.E. (n = 6–7). * indicates p < 0.05. n.s., not significant.
DISCUSSION
The present study revealed that T-type VDCCs, especially α1G subunit, play a role in promoting oxygenation-induced DA closure through vasoconstriction (functional closure) and intimal cushion formation (anatomical closure) in the rat neonatal DA. Accordingly, blockade of T-type VDCC using R(−)-efonidipine indeed retarded closure of the rat neonatal DA (Fig. 9 and Table 1).
In terms of functional DA closure, we found that blockade of T-type VDCC resulted in vasodilation of ex vivo rat DA (Fig. 8), using R(−)-efonidipine that exhibits more selective inhibition of T-type VDCC than that exhibited in other previous studies (6, 7). Although the effect of R(−)-efonidipine on vasorelaxation seemed relatively modest when compared with that on intracellular Ca2+ concentration, it should be noted that the protocols of the experiments were different for measurement of contractility and intracellular Ca2+ concentration. In the experiment for measurement of contractility, R(−)-efonidipine was added after oxygenation-induced vasoconstriction had already occurred. Because vasorelaxation is a relatively slow process, the inhibitory effect of R(−)-efonidipine on vasoconstriction seemed rather less than expected. In addition to T-type VDCC, other channels such as L-type VDCC and transient receptor potential channels are also involved in Ca2+ influx in the DA (6, 26) and should be taken into consideration.
In terms of anatomical DA closure, we found that R(−)-efonidipine inhibited intimal cushion formation in the rat DA explants. Schmitt et al. (27) have demonstrated that blockade of T-type VDCC using mibefradil prevents pathological neointimal formation after vascular injury. Although the mechanism has not been well understood, several studies, including our previous one, have indicated that T-type VDCC promotes both proliferation (5, 18, 28) and migration of vascular SMCs (16). However, T-type VDCC may not significantly increase cell proliferation and thus EP4-mediated intimal cushion formation, because R(−)-efonidipine did not affect the expression of Ki-67 in the DA explants in the presence of ONO-AE1–329 (supplemental Fig. S6) Therefore, SMC migration must be involved in T-type VDCC-mediated intimal cushion formation. Accordingly, using both molecular and pharmacological approaches in our present study, we found that Ca2+ influx through T-type VDCC promotes SMC migration when cells were harvested in a normoxic condition and that DA SMC migration is promoted in accordance with an increase in extracellular Ca2+ concentration (supplemental Fig. S7). It should be noted that several studies have demonstrated that L-type VDCC also promotes SMC migration and thus neointima formation in other vessels (14, 17, 29), although the findings are more controversial than those on T-type VDCC. In our unpublished experiments, we found that nitrendipine, a selective L-type VDCC blocker, also inhibits FCS-mediated SMC migration in the rat DA. A further study warrants examination of which type of Ca2+ channels plays a more significant role in neointima formation of the DA.
Interestingly, the effect of T-type VDCC on SMC migration was not observed in the hypoxic condition of the culture medium (Fig. 5). Importantly, oxygenation increased intracellular Ca2+ concentration that was significantly inhibited by R(−)-efonidipine (Fig. 6). These results suggested that oxygenation activates T-type VDCC, increases intracellular Ca2+ concentration, and then promotes migration of DA SMCs. In this sense, several studies have demonstrated that reactive oxygen species enhance the activity of T-type VDCC (30) and that hypoxia inhibits the activity (31). Furthermore, we found that oxygenation up-regulated the expression levels of α1G subunit in DA SMCs. Consistently, the expression levels of α1G subunit protein in the DA were significantly up-regulated after birth, which happens to be when oxygen tension in circulatory blood is dramatically increased. Although several studies have demonstrated that hypoxia up-regulated the expression of T-type VDCCs (32, 33), to our knowledge, our report is the first one showing that oxygenation up-regulated the expression of α1G mRNA and protein. Because oxygenation is known to promote DA closure after birth, the present data highlight another important role of oxygenation in the vascular remodeling through T-type VDCC. We, however, could not define the mechanism by which oxygenation activates T-type VDCC and up-regulates the expression of α1G in the present study. This important question should be addressed in our future study.
Although T-type VDCC is not the only factor (channel) in DA closure, T-type VDCC, especially α1G, plays a unique role in DA closure for the following reasons: 1) the expression of α1G is highly restricted in the DA, especially after birth, whereas that of other Ca2+ channels may not be, and 2) T-type VDCC promotes both functional and anatomical closure of the DA. These characters of α1G T-type VDCC are important, particularly for clinical applications. For example, blockade of T-type VDCC does not have serious adverse effects such as hypotension or cardiac dysfunction (34), whereas blockade of L-type VDCC may induce hypotension or cardiac dysfunction (35), especially in neonates. These adverse events are critical to keep the DA open in patients with congenital heart diseases involving right- or left-sided obstruction, because their circulatory systems are usually less compliant. For these patients, prostaglandin E1 administration is commonly used as the most potent vasodilator of the DA (1). However, our previous study supports the notion that chronic prostaglandin E1 stimulation may have an adverse effect on such patients because it also promotes anatomical DA closure. On the contrary, blockade of T-type VDCC prevents both vasoconstriction and EP4-mediated intimal cushion formation of the DA. Therefore, blockade of T-type VDCC may be an alternative therapeutic target to maintain the patency of the DA.
In conclusion, T-type Ca2+ channels, especially α1G subunit, promote oxygenation-induced DA closure through SMC migration and vasoconstriction in rats. Our present study implies that selectively blocking T-type Ca2+ channels is an effective alternative strategy to prostaglandin E2 analogs for keeping the DA open in patients with a DA-dependent congenital heart defect.
Supplementary Material
Acknowledgments
We are grateful to Dr. Robert DiGiovanni at the Institute for Biomedical Engineering, Consolidated Research Institute for Advanced Science and Medical Care, Waseda University for critical reading and English editing of the manuscript. R(−)-Efonidipine was kindly provided by Nissan Chemical Industries, Ltd. (Saitama, Japan).
This work was supported, in whole or in part, by National Institutes of Health Grant RO1 GM067773 (to Y. I.). This work was also supported by grants from the Yokohama Foundation for Advanced Medical Science (to T. A., U. Y., Y. I., and S. M.), the Ministry of Education, Culture, Sports, Science and Technology of Japan (to S. I., U. Y., Y. I., and S. M.), the Special Coordination Funds for Promoting Science and Technology, MEXT (to S. M.), “High-Tech Research Center” Project for Private Universities: matching fund subsidy from MEXT (to S. M.), Waseda University Grant for Special Research Projects (to S. M.), the Mother and Child Health Foundation (to S. M.), Miyata Cardiology Research Promotion Founds (to U. Y. and S. M.), Takeda Science Foundation (to S. M.), Foundation for Growth Science (to S. M.), Japan Cardiovascular Research Foundation (to S. M.), Mitsubishi Pharma Research Foundation (to S. M.), Yokohama Academic Foundation (to T. A. and S. I.), Inoue Foundation for Science (to T. A.), the Naito Foundation (to T. A.), the Uehara Memorial Foundation (to U. Y.), the Kitsuen Research Foundation (to Y. I.), and the Japan Space Forum (to Y. I.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S7.
- DA
- ductus arteriosus
- VDCC
- voltage-dependent Ca2+ channel
- SMC
- smooth muscle cell
- siRNA
- small interfering RNA
- FCS
- fetal calf serum
- fura-2/AM
- fura-2 acetoxymethyl ester.
REFERENCES
- 1.Smith G. C. (1998) Pharmacol. Rev. 50, 35–58 [PubMed] [Google Scholar]
- 2.Davis M. J., Hill M. A. (1999) Physiol. Rev. 79, 387–423 [DOI] [PubMed] [Google Scholar]
- 3.Moosmang S., Schulla V., Welling A., Feil R., Feil S., Wegener J. W., Hofmann F., Klugbauer N. (2003) EMBO J. 22, 6027–6034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen C. C., Lamping K. G., Nuno D. W., Barresi R., Prouty S. J., Lavoie J. L., Cribbs L. L., England S. K., Sigmund C. D., Weiss R. M., Williamson R. A., Hill J. A., Campbell K. P. (2003) Science 302, 1416–1418 [DOI] [PubMed] [Google Scholar]
- 5.Yokoyama U., Minamisawa S., Adachi-Akahane S., Akaike T., Naguro I., Funakoshi K., Iwamoto M., Nakagome M., Uemura N., Hori H., Yokota S., Ishikawa Y. (2006) Am. J. Physiol. Heart Circ. Physiol. 290, H1660–H1670 [DOI] [PubMed] [Google Scholar]
- 6.Nakanishi T., Gu H., Hagiwara N., Momma K. (1993) Circ. Res. 72, 1218–1228 [DOI] [PubMed] [Google Scholar]
- 7.Tristani-Firouzi M., Reeve H. L., Tolarova S., Weir E. K., Archer S. L. (1996) J. Clin. Invest. 98, 1959–1965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuga T., Kobayashi S., Hirakawa Y., Kanaide H., Takeshita A. (1996) Circ. Res. 79, 14–19 [DOI] [PubMed] [Google Scholar]
- 9.Gollasch M., Löhn M., Furstenau M., Nelson M. T., Luft F. C., Haller H. (2000) J. Hypertens. 18, 989–998 [DOI] [PubMed] [Google Scholar]
- 10.Sperti G., Colucci W. S. (1991) Eur. J. Pharmacol. 206, 279–284 [DOI] [PubMed] [Google Scholar]
- 11.Yang Z., Noll G., Lüscher T. F. (1993) Circulation 88, 832–836 [DOI] [PubMed] [Google Scholar]
- 12.Lijnen P., Fagard R., Petrov V. (1999) J. Cardiovasc. Pharmacol. 33, 595–604 [DOI] [PubMed] [Google Scholar]
- 13.Corsini A., Bonfatti M., Quarato P., Accomazzo M. R., Raiteri M., Sartani A., Testa R., Nicosia S., Paoletti R., Fumagalli R. (1996) J. Cardiovasc. Pharmacol. 28, 687–694 [DOI] [PubMed] [Google Scholar]
- 14.Ruiz-Torres A., Lozano R., Melón J., Carraro R. (2003) Int. J. Clin. Pharmacol. Ther. 41, 386–391 [PubMed] [Google Scholar]
- 15.Wamhoff B. R., Bowles D. K., McDonald O. G., Sinha S., Somlyo A. P., Somlyo A. V., Owens G. K. (2004) Circ. Res. 95, 406–414 [DOI] [PubMed] [Google Scholar]
- 16.Hollenbeck S. T., Nelson P. R., Yamamura S., Faries P. L., Liu B., Kent K. C. (2004) J. Vasc. Surg. 40, 351–358 [DOI] [PubMed] [Google Scholar]
- 17.Patel M. K., Clunn G. F., Lymn J. S., Austin O., Hughes A. D. (2005) Br. J. Pharmacol. 145, 811–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodman D. M., Reese K., Harral J., Fouty B., Wu S., West J., Hoedt-Miller M., Tada Y., Li K. X., Cool C., Fagan K., Cribbs L. (2005) Circ. Res. 96, 864–872 [DOI] [PubMed] [Google Scholar]
- 19.Rabinovitch M. (1996) Semin. Perinatol. 20, 531–541 [DOI] [PubMed] [Google Scholar]
- 20.de Reeder E. G., Poelmann R. E., van Munsteren J. C., Patterson D. F., Gittenberger-de Groot A. C. (1989) Atherosclerosis 79, 29–40 [DOI] [PubMed] [Google Scholar]
- 21.Yokoyama U., Minamisawa S., Quan H., Ghatak S., Akaike T., Segi-Nishida E., Iwasaki S., Iwamoto M., Misra S., Tamura K., Hori H., Yokota S., Toole B. P., Sugimoto Y., Ishikawa Y. (2006) J. Clin. Invest. 116, 3026–3034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yokoyama U., Minamisawa S., Quan H., Akaike T., Suzuki S., Jin M., Jiao Q., Watanabe M., Otsu K., Iwasaki S., Nishimaki S., Sato M., Ishikawa Y. (2008) J. Biol. Chem. 283, 28702–28709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Slomp J., van Munsteren J. C., Poelmann R. E., de Reeder E. G., Bogers A. J., Gittenberger-de Groot A. C. (1992) Atherosclerosis 93, 25–39 [DOI] [PubMed] [Google Scholar]
- 24.Keck M., Resnik E., Linden B., Anderson F., Sukovich D. J., Herron J., Cornfield D. N. (2005) Am. J. Physiol. Lung Cell Mol. Physiol. 288, L917–L923 [DOI] [PubMed] [Google Scholar]
- 25.Tanaka H., Komikado C., Shimada H., Takeda K., Namekata I., Kawanishi T., Shigenobu K. (2004) J. Pharmacol. Sci. 96, 499–501 [DOI] [PubMed] [Google Scholar]
- 26.Hong Z., Hong F., Olschewski A., Cabrera J. A., Varghese A., Nelson D. P., Weir E. K. (2006) Circulation 114, 1372–1379 [DOI] [PubMed] [Google Scholar]
- 27.Schmitt R., Clozel J. P., Iberg N., Bühler F. R. (1995) Arterioscler. Thromb. Vasc. Biol. 15, 1161–1165 [DOI] [PubMed] [Google Scholar]
- 28.Louis H., Lacolley P., Kakou A., Cattan V., Daret D., Safar M., Bonnet J., Daniel Lamazière J. M. (2006) Clin. Exp. Pharmacol. Physiol. 33, 131–138 [DOI] [PubMed] [Google Scholar]
- 29.Fleckenstein-Griin G. (1996) Pflüegers Arch. 432, R53–60 [Google Scholar]
- 30.Tabet F., Savoia C., Schiffrin E. L., Touyz R. M. (2004) J. Cardiovasc. Pharmacol. 44, 200–208 [DOI] [PubMed] [Google Scholar]
- 31.Fearon I. M., Randall A. D., Perez-Reyes E., Peers C. (2000) Pflüegers Arch. 441, 181–188 [DOI] [PubMed] [Google Scholar]
- 32.Del Toro R., Levitsky K. L., López-Barneo J., Chiara M. D. (2003) J. Biol. Chem. 278, 22316–22324 [DOI] [PubMed] [Google Scholar]
- 33.Carabelli V., Marcantoni A., Comunanza V., de Luca A., Díaz J., Borges R., Carbone E. (2007) J. Physiol. 584, 149–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Devlin M. G., Angus J. A., Wilson K. M., Wright C. E. (2002) Clin. Exp. Pharmacol. Physiol. 29, 372–380 [DOI] [PubMed] [Google Scholar]
- 35.Furberg C. D., Psaty B. M., Meyer J. V. (1995) Circulation 92, 1326–1331 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.