Abstract
 Detailed studies of the thermal conversion of 1-azidohepta-3,4,6-trienes into cyclopentennelated dihydropyrroles are presented. High levels of diastereoselectivity and regioselectivity are documented. A mechanistic proposal that accounts for all of the diverse results is developed through the use of density functional calculations. These calculations provide support for the intervention of unexpected mechanistic subtleties, such as the planarity of an azatrimethylenemethane diyl intermediate and an apparent Woodward–Hoffmann-type electrocyclization of a five-atom diyl array.
Detailed studies of the thermal conversion of 1-azidohepta-3,4,6-trienes into cyclopentennelated dihydropyrroles are presented. High levels of diastereoselectivity and regioselectivity are documented. A mechanistic proposal that accounts for all of the diverse results is developed through the use of density functional calculations. These calculations provide support for the intervention of unexpected mechanistic subtleties, such as the planarity of an azatrimethylenemethane diyl intermediate and an apparent Woodward–Hoffmann-type electrocyclization of a five-atom diyl array.
Introduction
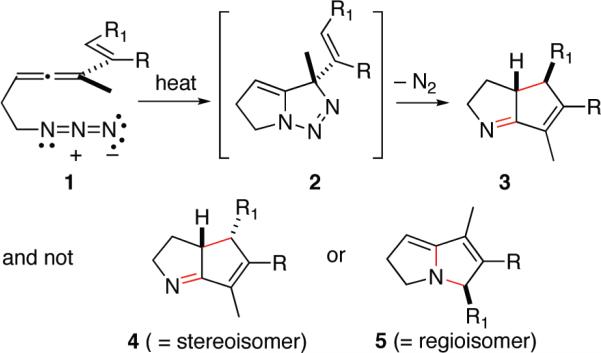
The cascade cyclization of 1-azidohepta-3,4,6-trienes 1 to furnish cyclopentennelated dihydropyrrole products 3 holds great promise for the efficient synthesis of cognate alkaloids. This transformation, as exemplified by the generic conversion illustrated in Scheme 1, is initiated thermally, and the structural and electronic features that determine both the stereochemical and the regiochemical outcome of this cascade sequence favor formation of 3 and not 4 or 5.1 The mechanistic intricacies of this multistep reaction sequence have been probed by density functional theory, and the computational results are consistent with the intermediacy of conformationally related singlet diyls whose behavior in electrocyclizations parallels that expected by their closed-shell cousins.2a In this full accounting of these developmental studies, the scope of the cyclization sequence is explored through reaction of several new allenyl azide substrates, and new computational results are brought to bear on emerging issues of regioselectivity upon cyclization of reactive intermediates.
Scheme 1.
Allenyl Azide Cycloaddition Cascade; New C–C and C–N Bonds Indicated in Red
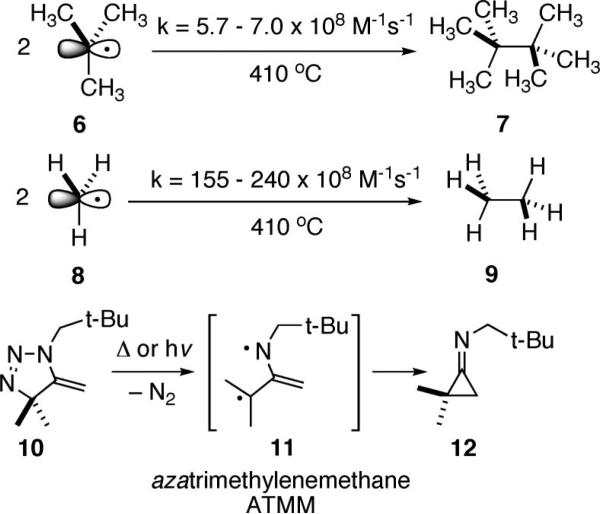
The impetus for this work can be traced to the disclosure that the rate of tert-butyl radical 6 dimerization is exceedingly fast, in actuality only about 30 times slower than the rate of methyl radical 8 dimerization,3 Scheme 2. Extrapolating from this observation, we speculated that combination of two highly substituted carbon radicals may provide a facile means to forge a C–C bond in an extremely sterically hindered environment. In particular, an interest in the synthesis of certain structural classes of alkaloids focused our attention on di (carbon) radical generation from substrates bearing strategically placed nitrogens, and that restriction led to the work of Quast,4 who provided evidence for the intermediacy of a nitrogen version of the classic trimethylenemethane diyl, azatrimethylenemethane (ATMM) 11, upon either thermolysis or photolysis of the triazoline 10.4b This diyl may have been implicated but unrecognized in allene/azide cycloaddition chemistry reported earlier by Shechter5 and later it was posited as a reactive intermediate by Gilbert,6 but no deliberate effort to exploit ATMM chemistry in reaction design has appeared prior to our work. We envisioned that this reactive diyl might participate in a range of useful bond forming reactions by analogy with trimethylenemethane chemistry, such as (1) [3 + 2] cycloadditions with alkenes and (2) electrocyclization through additional and adjacent unsaturation. It is this latter thrust that will be detailed in this work.
SCHEME 2.
Precedents for Diyl Combination and Generation
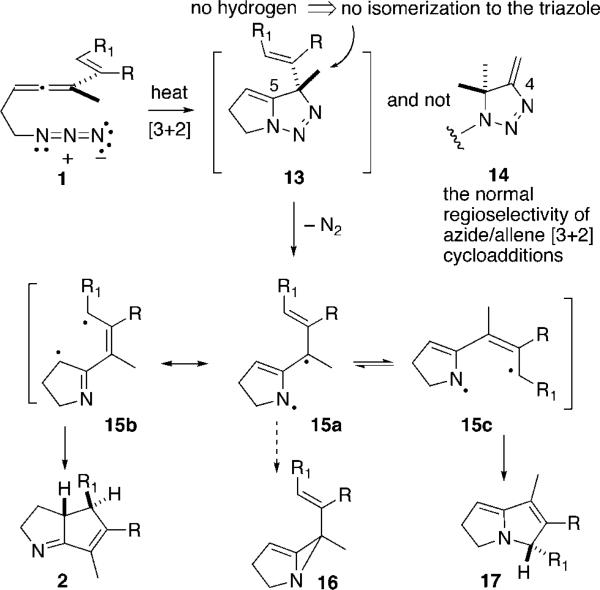
At the outset of our studies, we recognized that utilizing allene-azide [3 + 2] cycloaddition chemistry as an entry into methylene triazoline formation and hence ATMM generation/reaction (1 → 13 → 15, Scheme 3) had to overcome three intrinsic problems: (1) the normal regiochemistry of allene-azide cycloaddition is reported to provide the undesired 4-methylene triazoline 14 and not the requisite 5-methylene isomer,4a,7 (2) 5-methylene triazolines are prone to isomerization to afford the aromatic triazoles,8 and (3) any ATMM 15 so formed might simply cyclize to form an iminocyclopropane as per the work of Quast, 11 → 12, faster than it might be steered toward other more desirable processes. Fortunately, all of these issues can be addressed satisfactorily by the simple expediency of linking the azide and allene by a two-atom tether as shown with 1. This intramolecular version of an azide-allene [3 + 2] cycloaddition substrate cannot engage in reaction to form 4-methylene triazolines. In addition this substrate circumvents isomerization of 13 into a triazole since there is no C(4) hydrogen to tautomerize, and any cyclization of the diyl 15a into the alkylideneaziridine-containing species 16 is likely to be thwarted by the significant energetic cost of forming a bicyclic product bearing a trans alkene within a six-membered ring.9 Thus, we set out to test the premise that 1-azido-3,4,6-heptadienes 1 can serve as effective precursors of the ATMM diyl 15. A priori, it was unclear if the desired regiochemistry of bond formation, C–C as per 15bf2, would be favored over C–N bond formation as per 15cf17. The role that structural and electronic factors play in defining the yield, stereoselectivity, and regioselectivity of bond formation upon diyl connection within 15 will be detailed below.
SCHEME 3.
Development of an Intramolecular Allenyl Azide Cycloaddition Cascade
Results and Discussion
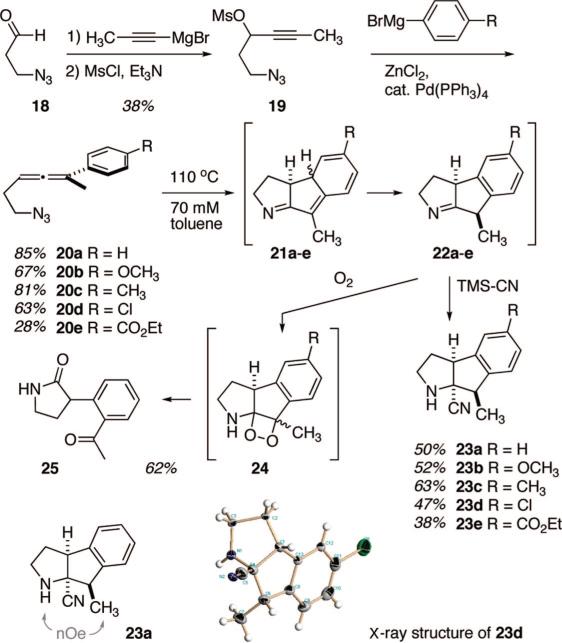
Feasibility studies commenced with the preparation of the simple aryl-substituted allenyl azides 20a–20e, Scheme 4. These species were selected for the initial studies based upon their ease of synthesis via well-precedented routes and the expectation that the strategically placed aryl substituent might promote formation of (and/or help stabilize) the incipient diyl that is central to the cascade reorganization detailed in Scheme 3. The known azide/acrolein adduct 1810 served as an entry point into the desired allenyl azides via application of Konno's allene synthesis procedure.11b The aryl nucleophiles were introduced as their zinc salts under palladium mediation. The terminal methyl group was incorporated to prevent tautomerization of any intermediate triazoline into a triazole. The yields of allene formation seemed insensitive to the electronic character of the aryl nucleophile, with the exception of the p-ester-substituted version 20e, which may have suffered from reduced nucleophilicity of the corresponding zincate.
SCHEME 4.
Syntheses and Preliminary Studies with Saturated-Tether Aryl-Substituted Allenyl Azides
Preliminary scouting studies on the thermolysis of allenyl azide 20a led to the observation that substrate consumption was rapid above ~80 °C, and therefore refluxing toluene was chosen as a convenient reaction medium. Surprisingly, the initial isolate from this thermolysis sequence was not a dihydropyrrole-containing species as anticipated by 1 → 2/17 (Scheme 3), but rather a compound whose spectral data argued for the pyrrolidone derivative 25. Thus, the allenyl azide 20a formally lost one molecule of N2 and gained one molecule of O2 upon formation of 25. The emergence of two carbonyl functions and the ortho-aromatic attachment points suggested that 25 originated though the expected intermediates 21a and 22a, but apparently 22a was not stable enough in air (O2) to survive exposure during workup and chromatography. Evidently, oxygenation of 22a, through some as yet undefined mechanism, intervened. A putative intermediate cyclic peroxide 24 could serve as a precursor to 25 via simple fragmentation. The formation of this dicarbonyl product was encouraging, as it hinted at the formation of an imine 22a through the anticipated ATMM chemistry.
The challenge then became devising an experimental procedure that permitted isolation of 22a, or a stable derivative thereof, prior to exposure to air. After screening several possible additives (nucleophiles = TMS-CN, NaBH4, TMSCH2CH=CH2, BrMgCH2CH=CH2, indole, PhSH; electrophiles = Ac2O, AcCl) with the crude thermosylate prior to air exposure, the best results were obtained with TMS-CN. Sodium borohydride did provide amine from imine reduction, but at a rather lower yield (~30%). Cyanide addition followed by aqueous acid workup furnished consistently good yields of the cyanopyrrolidines 23a–23e as single stereoisomers. The structural assignments of these cascade cyclization products rests on NOE analysis of 23a (see Scheme 4) and a single crystal X-ray determination of 23d (Scheme 4; see Supporting Information for details). The relative stereochemistries of the remaining cyanopyrrolidines were assigned by correlation of spectral data with 23a and 23d. The yields of the differing aryl substrates are similar, but the more electron deficient aromatic rings (20d and 20e) proceeded to product with the lowest efficiency. These observations are consistent with a model in which the diyl itself is electron-deficient (vide infra), and hence having electron-rich radical stabilizing aryl groups (i.e., 20b–20c) may promote higher yields compared to the electron-deficient analogues.
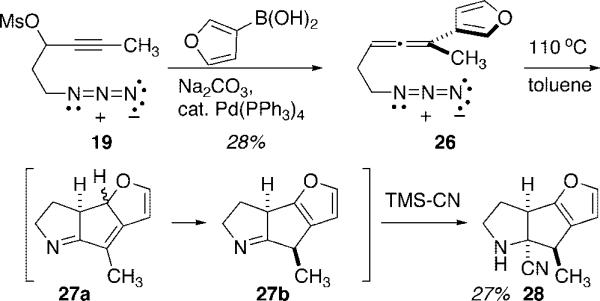
A brief foray into alternative aryl-substituted allenyl azides focused on the 3-furyl species 26, Scheme 5. Thermolysis of 26 under the conditions established above, followed by TMS-CN quench of the presumably sensitive imine intermediate, delivered the tricyclic product 28 in modest yield. The initial diyl cyclization occurred completely regioselectively at C(2) of the furan nucleus with no evidence for cyclization at the alternative C(4) site detected. The basis for this selectivity can be appreciated by considering resonance forms for the intermediate ATMM diyl, which places spin density on C(2) but not C(4) of the furan nucleus.
SCHEME 5.
Extension of the Allenyl Azide Cyclization Cascade to a Furan-Containing Substrate
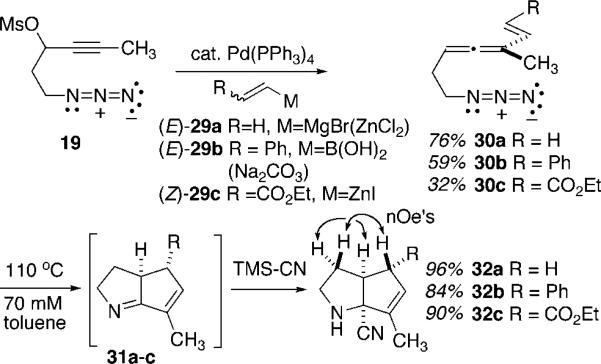
This thermal cyclization cascade of allenyl azides could be expanded to include simple vinyl-substituted substrates, Scheme 6. The syntheses of the vinyl, β-styryl, and β-acryloyl allenyl azide substrates 30a–30c, respectively, were accomplished through Konno's propargyl mesylate-to-allene methodology in strict analogy with the aryl series of Scheme 4. Note that the use of the (Z)-acryloyl zincate 29c afforded the (E)-vinyl allene product 30c. Thermolysis of the vinyl-substituted allenyl azides 30a–30c under the optimized conditions (110 °C, toluene, 70 mM) led to clean conversion into the expected dihydropyrrole products 32a–32c following TMS-CN addition to the crude reaction mixture. In each case, the cyanopyrrolidine product was isolated in excellent yield, with only insignificant yield variation in response to the electronic character of the vinyl substituent. NOE measurements established the stereochemistry as shown in 32, and in no case was there any evidence for formation of a minor stereoisomer. The formation of a cis ring juncture upon cyanide addition to the putative imine intermediates 31a–31c is no surprise, given the large energetic bias for cis-bicyclo-[3.3.0]octane skeleta over the trans alternative. However, the clean evolution of a cis relationship between the vinyl appendage R and the ring juncture hydrogen in 31 upon diyl cyclization warrants further analysis. Arguments based on differential steric interactions and also arguments based on subtle electronic effects revealed through computational analysis of the diyl closure will be presented along with Scheme 9. At this early juncture in the allenyl azide cyclization studies, however, it was sufficient to realize that complete control of stereochemistry was achievable through this chemistry and that this observation might translate to useful diastereoselectivity in related and more complex systems of interest in alkaloid synthesis.
SCHEME 6.
Synthesis and Thermal Cascade Cyclization of Vinyl-Substituted Allenyl Azides
SCHEME 9.
Rationale for the Stereochemical Outcome of ATMM Diyl Cyclization
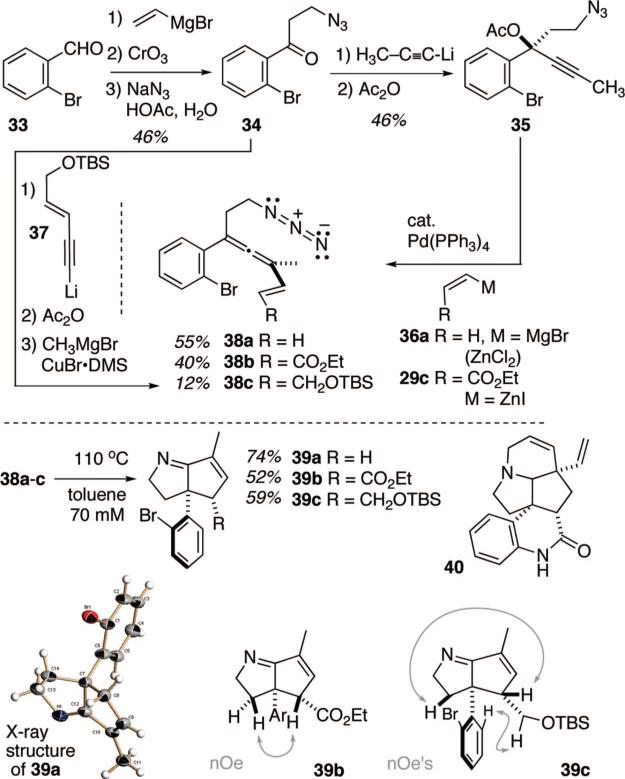
The naturally occurring alkaloid meloscine (40)12 represented one such target of opportunity, wherein the core azabicyclo-[3.3.0]octane structural unit is embedding in a complex penta-cyclic framework. In fact, the stereochemical relationship between the ring juncture aryl appendage and the lactam's carbonyl functionality maps directly onto the stereochemical outcome of the vinyl allenyl azide cyclization cascade. However, an approach to this complex target that productively exploits the ATMM chemistry would out of necessity require a tetra-substituted allene substrate, a species heretofore unexplored in our studies. Initial feasibility experiments were conducted with the tetrasubstituted allenyl azide substrates 38a–38c to probe this point, Scheme 7. These species were readily available through a minor adaptation of the earlier allene-forming chemistry, in these instances extending from the azido propiophenone species 34. The mesylate prepared from the first-formed alcohol derived from 34 and propynyl lithium was not stable enough to be isolated; instead it readily suffered elimination to form an alkene-containing species among several other uncharacterized decomposition products. Hence, the more stable acetate 35 was substituted without incident in the palladium-mediated allene-forming transform with both the vinyl and acryloyl zincates.11a In addition, an allylic silyl ether variant 38c was prepared by different chemistry (34 + 37 →→ 38c) in modest yield. The choice of the o-bromoaryl ring was predicated on the desire to incorporate the most inert functionality possible that still might be used to forge the Ar–N bond required for meloscine. Preliminary scouting experiments with o-NO2- or o-NHBOC-aryl rings led to complications in the substrate synthesis. Thermolysis of these tetrasubstituted allene substrates all proceeded smoothly to produce bicycles 39a–39c in good yield under the standard reaction conditions. In a departure from all previous substrates, these tetrasubstituted allenes afforded imine products that could be isolated via chromatography in the presence of air (O2) without the oxidation problems discussed earlier (Scheme 4). The structure of bicyclic imine 39a was secured by single crystal X-ray analysis (see Supporting Information for details). The structures of 39b and 39c followed from analysis of the spectral data using the data of 39a as a comparison point. The stereochemical assignment of these two species rested on the NOE correlations shown in Scheme 7.
SCHEME 7.
Allenyl Azide Cyclization Cascade with Tetrasubstituted Allene Precursors
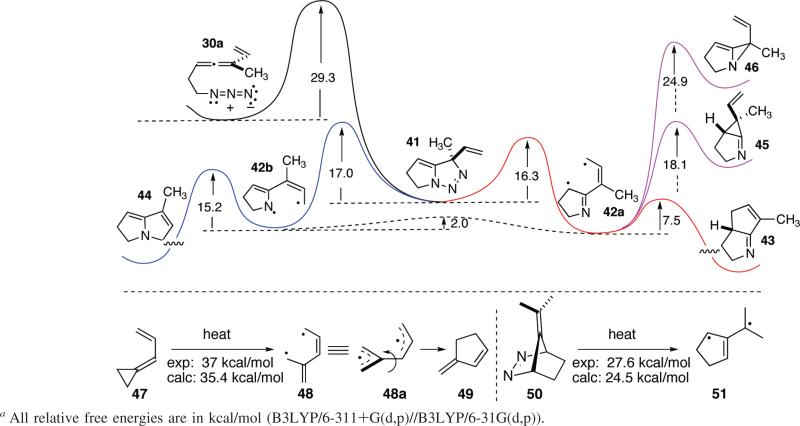
The observation of cyclization products consistent with the predictions of our ATMM diyl-based mechanistic hypothesis (Scheme 3) in no way validates that hypothesis; for example, the reaction could proceed through facile [3,3]-sigmatropic reorganization of the highly strained alkylideneaziridine 16, a species dismissed as unlikely earlier. In order to gain more insight into this complex process, we turned to density functional theory in its Kohn–Sham formulation, using the B3LYP/6-311+G(d,p)//B3LYP/6-31G(d,p) computational approach.13 This choice of basis set/calculational protocol was predicated on its successful application in earlier computational studies on diradical systems. Thus, the near coincidence between experimental and computed values for the activation barriers of the conversion of 47 into the vinyl trimethylenemethane diyl 48 related to 15 (Scheme 3)14 and the formation of the trimethylenemethane diyl 51 from N2 extrusion within 5015 provide validation for this computational approach (Scheme 8). A complete mechanistic profile is illustrated in Scheme 8 for azido allene 30a. These calculations suggest that the slowest step in the reaction sequence is initial azide-allene [3 + 2] cycloaddition to form the triazoline 41. Loss of nitrogen (N2) from this triazoline appears to be a concerted process. Forced attempts to preferentially cleave either the C–N bond or the N–N bond of 41 led to concomitant stretching of the remaining scissile bond such that, at the transition state, both bonds are severed in a concerted but nonsynchronous manner. Depending upon the direction of rotation of the exocyclic C–C bond, two geometrically distinct ATMM diyls can be generated, 42a and 42b. The rotamer 42a appears to be slightly more stable (~0.2 kcal/mol) than 42b, but the barrier to interconversion between them is no larger than 2 kcal/mol, ensuring that a Curtin–Hammet situation prevails (i.e., rapid interconversion of 42a and 42b followed by slower reaction of either species). The key to understanding the observation that C–C bond formation (42a → 43) and not C–N bond formation (42b → 44) prevails lies in the calculated activation barrier for the two closures. The barrier to C–C bond formation is on the order of 8 kcal/mol lower than the C–N forming alternative, and that difference, coupled with the low barrier to 42a/42b interconversions, ensures that only the C–C bonded product 43 is formed.
SCHEME 8.
Calculated Cyclization Cascade Reaction Profile for Model Allenyl Azide 30a, and Related Systems for Method Validationa
These calculations also address the possibility that an alkylideneaziridine (cf. 16, Scheme 3) or an iminocyclopropane (cf. Scheme 2, 11 → 12) might be accessible through these diyl intermediates. In fact, the calculated activation barrier for closure of 42a into the alkylideneaziridine 46 and the barrier for formation of iminocyclopropane 45 from this same diyl are higher than direct C–C bond-forming cyclization (→ 43) by at least 10 kcal/mol. Thus, these calculations suggest that the overall conversion of 30a into 43 can be interpreted as proceeding through the triazoline 41 and the ATMM diyl 42a without intervention of either strained 3-membered ring species 45 or 46.
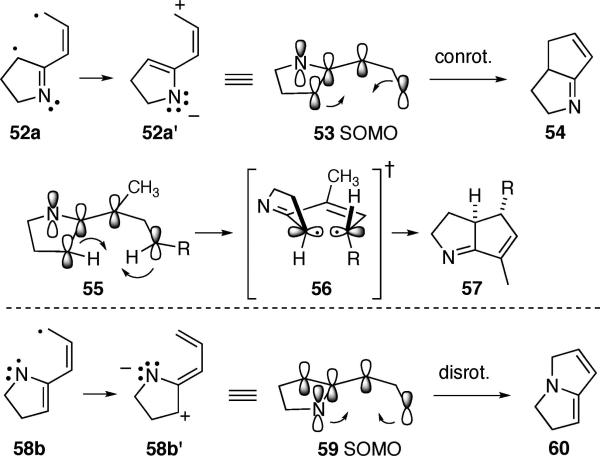
One of the more surprising results to emerge from these calculations involves the geometry and underlying mechanistic course of diyl cyclization within 42a to deliver 43. Much prior work has focused on evaluating the structure and cyclization pathway for the all-carbon analogue, the vinyl trimethylenemethane 48.16 The upshot of those studies appears to be that singlet diyl 48 preferentially exists in an orthogonal equilibrium geometry (48a), which proceeds to product 49 via rotation of the indicated C–C bond. In contrast, the calculated results with the aza system 52a indicate that a planar and not an orthogonal geometry of the diyl is the most stable arrangement, Scheme 9. Furthermore, this planar arrangement of the 6-atom/6-electron array within 52a is conducive to an electrocyclization via termini rotation related to the familiar Woodward–Hoffmann-esque conrotatory/disrotatory motions. The nitrogen atom within 52a′ polarizes the π-system to the extent that it can be approximated by the resonance form 52a′. The contribution of this dipolar depiction to the overall electron distribution of the SOMOs (singly occupied molecular orbitals) can be estimated by natural orbital population analysis, and this approach reveals that approximately 60% of the electron distribution can be viewed as the dipolar form 52a′, with 40% remaining as the diradical 52a. The consequences of skewing the electron distribution in this way are 2-fold: (1) the planar geometry is more stable than the orthogonal geometry (similar charge-separated resonance forms scarcely contribute to the electronic structure of the all-carbon case 48), and (2) the cyclic array of electrons in 52a′ approximates a 4π-electron system, which is constrained energetically to participate in a conrotatory cyclization of the termini under thermal conditions. Although the simple calculational model 52a → 54 has no stereochemical marker at the alkene terminus, the actual experimental systems 32b/32c and 38b/38c do. Conrotatory cyclization of the termini in the generic ATMM diyl substrate 55 should occur through a postulated transition state 56 to furnish the strictly syn product 57, a prediction completely consistent with the experimental observations. Steric factors may also play a role in promoting facile termini rotation in this manner, as the smaller H rather than the larger R group swings under the dihydropyrrole ring during this process. Interestingly, this computational model makes the opposite prediction for the (unobserved) cyclization of 58b into 60. In this instance, the electronegativity of the nitrogen enhances the electron density of the cyclic 5-atom array (= 58b′), and it should behave more like a 6-π-electron electro-cyclization in the Woodward–Hoffmann sense and proceed through a disrotatory cyclization to give the opposite stereochemical outcome compared with 52a.
The thermally initiated cascade cyclization of 1-azido-hepta-3,4,6-trienes is an effective methodology for the efficient assembly of cyclopentennelated dihydropyrroles. The products are formed with complete regiochemical control for the C–C bonded isomer and with complete stereocontrol for the syn disposition of adjacent groups spanning the bond forming site. The underlying control elements for both of these reaction characteristics are revealed through density functional calculations that identify and place putative reactive intermediates along the reaction coordinate. Regioselectivity appears to be favored by the greater spin density on carbon (vs nitrogen) in the ATMM diyl intermediate, whereas stereoselectivity derives from a 5-electron electrocyclization that follows the predictions of the Woodward–Hoffmann rules. The application of this methodology in the total synthesis of alkaloid targets is ongoing and will be reported in due course.
Experimental Section
Computational Methodology
All of the stationary points were located with the B3LYP13 density functional as implemented in Gaussian0317 in conjunction with the 6-31G(d,p) basis set. To further improve the electronic energy values, an energy refinement was computed with the larger triple-ζ quality 6-311+G(d,p) basis set. This scheme usually is noted as B3LYP/6-311+G(d,p)//B3LYP/6-31G(d,p). Second derivatives of the energy with respect to the Cartesian nuclear coordinates were computed for all stationary points and subsequent harmonic analysis confirmed the nature (minimum or transition state) of each structure. Unscaled frequency values obtained from the second derivatives were employed for thermochemical analysis.
As a result of the potential diradical character of some of the structures considered in this work, the internal and external stability of the wave functions was computed via the Hermitian stability matrices A and B in all cases.18 For all of the structures exhibiting unstable restricted wave functions, the spin symmetry constraint of the wave function was released (i.e., expanding the SCF calculation to an unrestricted space, UB3LYP), leading to stable unrestricted wave functions.
This methodology has been tested extensively and compared against very robust and specialized methodology (CASSCF, CASPR2) for the study of oxa and aza derivatives of the trimethylenemethane motif.19
General Procedure 1. Allenylazide Synthesis
To a solution of ZnCl2 (3.3 equiv) in THF (0.33 M solution) was added the appropriate Grignard reagent RMgBr (3.3 equiv), and the mixture was stirred at room temperature for 30 min. The reaction mixture was cooled to –50 °C and Pd(PPh3)4 (5 mol%) in 2 mL of THF, and the propargylic azidomesylate 19 (1 equiv) in THF (0.18 M solution) were added sequentially. The reaction mixture was allowed to warm to room temperature over the course of 10 h. After addition of an equal volume of a saturated NH4Cl solution, the organic layer was extracted into Et2O and washed with water and brine. Drying the organic phase over Na2SO4 and removal of solvent under reduced pressure resulted in a yellow oil. This crude product was purified by chromatography using 5% Et2O in hexane as the eluant.
General Procedure 2. Cyclization and Trapping with TMS-CN
A deoxygenated solution of allenylazide in toluene-d8 (0.06 M) was refluxed in a flame-dried Schlenk flask for 5 h, after which time the reaction mixture was cooled to room temperature and cannulated into a flask containing a deoxygenated 0 °C solution of TMS-CN (2 equiv) in CH2Cl2 (0.05 M). After stirring the mixture for 12 h at room temperature, water was added and the mixture was extracted with an equal volume of CH2Cl2, washed with brine, and dried over Na2SO4. Evaporation of the organic phase in vacuo gave a brown oil. Purification of this crude material by flash chromatography (1:1 hexanes/Et2O) furnished the cyanopyrrolidine product as a pale yellow film.
1-Azido-hex-4-yn-3-yl Methanesulfonate (19)
To an ice-cold solution of 3-azidopropionaldehyde10 (1.1 g, 12 mmol) in 20 mL of THF was added 1-propynyl magnesium bromide (0.50 M in THF, 24 mL, 12 mmol) dropwise under a nitrogen atmosphere. The reaction mixture was held at 0 °C for 1.5 h and then slowly warmed to room temperature over an additional 1 h. The reaction mixture was poured into 30 mL of saturated NH4Cl solution, and the organic layer was extracted into Et2O, washed with water and then brine. Drying the organic phase over Na2SO4, followed by evaporation of the solvent in vacuo, gave a yellow oil. Purification of this crude material through a small silica gel plug with Et2O as the eluant gave 1-azido-hex-4-yn-3-ol as a pale yellow oil (1.1 g, 65%). IR (neat) 3390, 2099 cm−1; 1H NMR (400 MHz, CDCl3) δ 4.46 (bs, 1H), 3.52–3.42 (m, 2H), 2.64 (d, J = 4.4 Hz, 1H), 1.94–1.86 (m, 2H), 1.84 (d, J = 2.1 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 82.3, 79.6, 60.3, 48.2, 37.1, 3.9. APCIMS m/z relative intensity 140 (MH+ 55); HRMS (+ESI) calcd for C6H10N3O 140.0824, found 140.0828.
Methanesulfonyl chloride (1.2 mL, 16 mmol) and triethylamine (2.8 mL, 20 mmol) were added to a solution of 1-azido-hex-4-yn-3-ol (1.1 g, 7.9 mmol) in 40 mL of CH2Cl2 at –50 °C, and the mixture was stirred for 2.5 h at that temperature. The reaction mixture was poured into 30 mL of saturated NaHCO3 solution. The mixture was then extracted with 30 mL of CH2Cl2 and the organic layer was then washed with water and brine, dried over Na2SO4 and concentrated in vacuo. This crude material was purified by column chromatography (1:1 hexane/Et2O) to give the azidopropargylic mesylate 19 as a pale yellow oil (1.0 g, 58%). IR (neat) 2099 cm−1; 1H NMR (400 MHz, CDCl3) δ 5.24 (m, 1H), 3.49 (t, J = 6.6 Hz, 2H), 3.1 (s, 3H), 2.14–2.00 (m, 2H), 1.89 (d, J = 2.1 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 87.0, 74.4, 69.9, 47.3, 39.5, 35.6, 4.0. ESIMS m/z relative intensity 140 (MH+ – N2); HRMS (+ESI) calcd for C7H12NO3S 190.0538, found 190.0536.
1-Azido-5-(phenyl)hexa-3,4-diene (20a)
Following General Procedure 1, azidomesylate 19 (200 mg, 0.92 mmol) was converted into azidoallene 20a (155 mg, 85%). IR (neat) 2096, 1950 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.44 (d, J = 7.4 Hz, 2H), 7.36 (t, J = 7.5, 2H), 7.23 (t, J = 7.2 Hz, 1H), 5.5 (m, 1H), 3.42 (t, J = 7.1 Hz, 2H), 2.42 (q, J = 6.7 Hz, 2H), 2.14 (d, J = 3.0 Hz, 3H); 13C NMR (75 MHz, CDCl3) δ 205.2, 137.4, 128.8, 127.2, 126.1, 102.2, 89.8, 51.2, 29.0, 17.5; APCIMS m/z relative intensity 172 (MH+ – N2 50); HRMS (+ESI) calcd for C12H13N3Na 222.0998, found 222.1007.
1-Azido-5-(4′-methoxyphenyl)hexa-3,4-diene (20b)
Following General Procedure 1, azidomesylate 19 (200 mg, 0.92 mmol) was converted into azidoallene 20b (140 mg, 67%). IR (neat) 2094, 1960 cm−1; 1H NMR (300 MHz, CDCl3) δ 7.35 (d, J = 8.8 Hz, 2H), 6.88 (d, J = 8.9 Hz, 2H), 5.47 (m, 1H), 3.82, (s, 3H), 3.41 (t, J = 6.9 Hz, 2H), 2.40 (q, J = 6.6 Hz, 2H), 2.11 (d, J = 2.9 Hz, 3H); 13C NMR (75 MHz, CDCl3) δ 204.6, 159.0, 129.6, 127.2, 114.2, 101.7, 89.6, 55.7, 51.2, 29.1, 17.6; APCIMS m/z relative intensity 202 (MH+ – N2 45); HRMS (+ESI) calcd for C13H16NO 202.1232, found 202.1219.
1-Azido-5-(4′-methylphenyl)hexa-3,4-diene (20c)
Following General Procedure 1, azidomesylate 19 (500 mg, 2.3 mmol) was converted into azidoallene 20c (400 mg, 81%). IR (neat) 2095 1951 cm−1; 1H NMR (360 MHz, CDCl3) δ 7.21 (d, J = 8.2 Hz, 2H), 7.05 (d, J = 8.0 Hz, 2H), 5.4 (m, 1H), 3.3 (t, J = 6.9 Hz, 2H), 2.32–2.27 (m, 2H), 2.25 (s, 3H), 2.02 (d, J = 2.9 Hz, 3H); 13C NMR (90 MHz, CDCl3) δ 204.9, 136.8, 134.4, 129.5, 126.0, 102.1, 89.5, 51.2, 29.0, 21.5, 17.5; APCIMS m/z relative intensity 186 (MH+ – N2 100); HRMS (+ESI) calcd for C13H16N 186.1283, found 186.1286.
1-Azido-5-(4′-chlorophenyl)hexa-3,4-diene (20d)
Following General Procedure 1, azidomesylate 19 (200 mg, 0.92 mmol) was converted into azidoallene 20d (165 mg, 77%). IR (neat) 2096, 1952 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.36–7.20 (m, 4H), 5.55–5.48 (m, 1H), 3.42 (t, J = 6.8 Hz, 2H), 2.44–2.38 (q, J = 6.6 Hz, 2H), 2.12 (d, J = 2.9 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 205.2, 135.9, 132.8, 128.8, 127.4, 101.4, 90.2, 51.1, 28.9, 17.4; APCIMS m/z relative intensity 206 (MH+ – N2 100); HRMS (+APPI) calcd for C12H13ClN 206.0737, found 206.0743.
1-Azido-5-(4′-carbethoxyphenyl)hexa-3,4-diene (20e)
A solution of propargyl azidomesylate 19 (200 mg, 0.92 mmol) and Pd(PPh3)4 (53 mg, 0.05, 5 mol%) in 15 mL of THF was cooled to –50 °C. Commercially available 4-(ethoxycarbonylphenyl) zinc iodide (0.50 M in THF, 5.6 mL, 2.8 mmol) was added to the reaction mixture dropwise and the solution was allowed to warm to room temperature over the course of 12 h. The reaction mixture was then poured into 20 mL of saturated NH4Cl solution and extracted with 30 mL of Et2O. The organic phase was then washed with water and brine and dried over Na2SO4, and the solvent was removed in vacuo. Purification of the crude oil by column chromatography using 5% Et2O in hexane resulted in 20e as a pale yellow oil (154 mg, 61%). IR (neat) 2099, 1950 cm−1; 1H NMR (400 MHz, CDCl3) δ 8.01 (d, J = 8.6 Hz, 2H), 7.47 (d, J = 8.4 Hz, 2H), 5.5 (m, 1H), 4.40 (q, J = 7.1 Hz, 2H), 3.43 (t, J = 6.8 Hz, 2H), 2.42 (q, J = 6.2 Hz, 2H), 2.15 (d, J = 2.9 Hz, 3H), 1.41 (t, J = 7.1 Hz, 3H); 13C NMR (75 MHz, CDCl3) δ 206.1, 166.9, 142.2, 130.0, 128.0, 125.9, 101.9, 90.2, 61.3, 51.1, 28.8, 17.3, 14.7; APCIMS m/z relative intensity 244 (MH+ – N2 100); HRMS (+APPI) calcd for C15H18NO2 244.1338, found 244.1353.
3-(2-Acetyl-phenyl)-pyrrolidin-2-one (25)
A deoxygenated solution of allenylazide 20a (30 mg, 0.15 mmol) in toluene-d8,(2mL) was heated in a sealed tube at 100 °C for 5 h. The reaction mixture was cooled to room temperature and solvent evaporated to give a brown film. Purification of this crude oil by flash chromatography (9:1 EtOAc/Et3N) resulted in 25 as a yellow film (19 mg, 62%). IR (neat) 3297, 1684 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.73 (d, J = 7.7 Hz, 1H) 7.49 (t, J = 6.4 Hz, 1H), 7.36 (q, J = 7.7 Hz, 2H), 4.31 (t, J = 9.4 Hz, 1H), 3.49 (dd, J = 8.9, 4.8 Hz, 2H), 2.77 (m, 1H), 2.63 (s, 3H) 2.22 (m 1H); 13C NMR (75 MHz, CDCl3) δ 202.6, 179.1, 139.2, 139.0, 132.5, 130.3, 129.6, 127.3, 45.6, 40.8, 32.0, 30.0; APCIMS m/z relative intensity 204 (MH+ 40); HRMS (+APCI) calcd for C12H14NO2 204.1025, found 204.1033.
Phenyl Substrate (23a)
Following General Procedure 2, azidoallene 20a (45 mg, 0.23 mmol) was converted into cyanopyrrolidine 23a (23 mg, 50%). IR (neat) 3342, 2097 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.29–7.27 (m, 2H), 7.14–7.19 (m, 2H), 4.05 (dd, J = 8.7, 2.7 Hz, 1H), 3.64 (q, J = 7.2 Hz, 1H), 3.24 (apparent q, J = 7.3 Hz, 1H), 3.12–3.07 (m, 1H), 2.56–2.48 (m, 1H), 2.15–2.07 (m 1H), 1.51 (d, J = 7.1 Hz, 3H); 13C NMR (75 MHz, CDCl3) δ 144.0, 143.2, 128.2, 128.1, 124.4, 124.0, 123.2, 68.3, 55.5, 47.5, 46.7, 31.3, 12.5; APCIMS m/z relative intensity 199 (MH+ 100); HRMS (+APCI) calcd for C13H14N2 199.1241, found 199.1229.
Methoxyphenyl Substrate (23b)
Following General Procedure 2, azidoallene 20b (70 mg, 0.31 mmol) was converted into cyanopyrrolidine 23b (36 mg, 52%). IR (neat) 3441, 2220 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.04 (d, J = 8.3 Hz, 1H), 6.81 (dd, J = 8.9, 1.9 Hz, 1H), 6.70 (s, 1H), 3.99 (dd, J = 6.7, 2.2 Hz, 1H), 3.81 (s, 3H), 3.59 (q, J = 7.1, 1H), 3.24 (apparent q, J = 9.2 Hz, 1H), 3.12 (ddd, J = 8.3, 8.3, 4.3 Hz, 1H), 2.53–2.45 (m, 1H), 2.13–2.06 (m, 1H), 1.76 (bs, 1H), 1.47 (d, 7.1 Hz, 3H); 13C NMR (75 MHz, CDCl3) δ 160.3, 145.5, 135.3, 124.6, 123.2, 113.8, 110.0, 68.5, 55.9, 55.4, 46.8, 31.1, 12.8; APCIMS m/z relative intensity 229 (MH+ 100); HRMS (+APCI) calcd for C14H17N2O 229.1335, found 229.1335.
Methylphenyl Substrate (23c)
Following General Procedure 2, azidoallene 20c (35 mg, 0.16 mmol) was converted into cyanopyrrolidine 23c (22 mg, 63%). IR (neat) 3337, 2220 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.09 (d, J = 7.7 Hz, 1H), 7.02 (d, J = 7.7 Hz, 1H), 6.98 (s, 1H), 4.01 (dd, J = 8.7, 2.4 Hz, 1H), 3.60 (q, J = 7.0 Hz, 1H), 3.25 (apparent q, J = 8.5 Hz, 1H), 3.10 (ddd, J = 8.0, 8.0, 3.6 Hz, 1H), 2.45−2.53 (m, 1H), 2.36 (s, 3H), 2.06−2.13 (m, 1H), 1.85 (bs, 1H), 1.48 (d, J = 7.1 Hz, 3H); 13C NMR (75 MHz, CDCl3) δ 144.1, 140.2, 138.1, 128.9, 125.1, 123.7, 123.9, 68.4, 55.4, 47.1, 46.7, 31.1, 21.7, 12.6; APCIMS m/z relative intensity 213 (MH+ 100); HRMS (+APCI) calcd for C14H17N2 213.1381, found 213.1386.
Chlorophenyl Substrate (23d)
Following General Procedure 2, azidoallene 20d (35 mg, 0.15 mmol) was converted into cyanopyrrolidine 23d (16 mg, 47%). Crystals suitable for X-ray crystallographic analysis were obtained by slow evaporation of an Et2O solution of 23d over a period of 48 h at 25 °C. Mp: 92−98 °C. IR (neat) 3338, 2220 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.24 (dd, J = 8.1, 1.3 Hz, 1H), 7.15 (s, 1H), 7.06 (d, J = 8.1 Hz, 1H), 4.01 (dd, J = 8.9, 2.7 Hz, 1H), 3.58 (q, J = 7.0 Hz, 1H), 3.24 (apparent q, J = 8.3 Hz, 1H), 3.12 (ddd, J = 8.1, 8.1, 4.3 Hz, 1H), 2.55−2.47 (m, 1H), 2.12−2.04 (m, 1H), 1.88 (bs, 1H), 1.48 (d, J = 7.1 Hz, 3H); 13C NMR (75 MHz, CDCl3) δ 146.0, 141.7, 133.9, 128.4, 125.2, 124.8, 122.8, 68.4, 55.1, 47.1, 46.6, 31.1, 12.5; APCIMS m/z relative intensity 233 (MH+ 100); HRMS (+APCI) calcd for C13H14N2Cl 233.0845, found 233.0840.
Phenylester Substrate (23e)
Following General Procedure 2, azidoallene 20e (30 mg, 0.11 mmol) was converted into cyanopyrrolidine 23e (11 mg, 37%). IR (neat) 3339, 2225, 1714 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.98 (d, J = 7.9 Hz, 1H), 7.85 (s, 1H), 7.20 (d, J = 7.9 Hz, 1H), 4.38 (q, J = 7.1 Hz, 2H), 4.06 (dd, J = 8.7, 2.5 Hz, 1H), 3.65 (q, J = 6.9 Hz, 1H), 3.27 (apparent q, J = 8.5 Hz, 1H), 3.14−3.07 (m, 1H), 2.60−2.51 (m, 1H), 2.20−2.12 (m, 1H), 1.69 (bs, 1H), 1.52 (d, J = 7.1 Hz, 3H), 1.41 (t, J = 7.1 Hz, 3H); 13C NMR (75 MHz, CDCl3) δ 166.8, 148.5, 144.5, 130.8, 129.9, 125.8, 123.9, 122.8, 68.3, 61.5, 55.1, 47.6, 46.6, 31.1, 14.8, 12.5; APCIMS m/z relative intensity 271 (MH+ 100); HRMS (+APCI) calcd for C16H19N2O2 271.1423, found 271.1441.
Furanyl Substrate 26
To a solution of propargyl azidomesylate 19 (0.14 g, 0.65 mmol) and Pd(PPh3)4 (36 mg, 0.03 mmol, 5 mol%) in 10 mL of THF were added commercially available 3-furanyl boronic acid (0.17 g, 1.5 mmol) and sodium carbonate (95 mg, 0.9 mmol). After the reaction mixture was refluxed for 4 h, the solution was poured into water and the mixture was extracted with 15 mL of Et2O. Drying over Na2SO4, followed by evaporation of solvent and chromatography on silica gel using 5% Et2O in hexane afforded 26 as a pale yellow oil (35 mg, 28%). IR (neat) 2098, 1948 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.38 (t, J = 1.8 Hz, 1H), 7.37 (s, 1H), 6.4(d, J = 1.73 Hz, 1H), 5.42 (m, 1H), 3.40 (t, J = 6.9 Hz, 2H), 2.37 (q, J = 6.6 Hz, 2H), 2.01 (d, J = 2.9 Hz, 3H); 13C NMR (75 MHz, CDCl3) δ 204.4, 143.7, 138.8, 124.9, 109.4, 95.3, 89.3, 51.1, 29.0, 17.6; TOFMSES m/z relative intensity (MH+ 20); HRMS (+ESMS) calcd for C10H12N3O 190.0980, found 190.0987.
Furanyl Cyanopyrrolidine 28
Following General Procedure 2, azidoallene 26 (30 mg, 0.16 mmol) was converted into cyanopyrrolidine 28 (8 mg, 27%). IR (neat) 3346, 2098 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.34 (d, J = 1.0 Hz, 1H), 6.2 (d, J = 1.8 Hz, 1H), 3.97 (dd, J = 8.4, 4.0 Hz, 1H), 3.46 (q, J = 7.1, 1H), 3.22 (m, 1H), 3.11−3.18 (m, 1H), 2.16−2.24 (m, 1H), 1.93−2.04 (m, 1H), 1.34 (d, 7.1 Hz, 3H); 13C NMR (75 MHz, CDCl3) δ 157.3, 147.6, 127.0, 123.0, 107.6, 72.6, 50.7, 48.0, 41.6, 29.2, 13.6; TOFMSES m/z relative intensity 189 (MH+ 100); HRMS (+ES) calcd for C11H13N2O 189.1028, found 189.1019.
1-Azido-6-methylhepta-3,4,7-triene (30a)
Following General Procedure 1, azidomesylate 19 (200 mg, 0.92 mmol) was converted into azidoallene 30a (105 mg, 76%). IR (neat) 2097, 1948 cm−1; 1H NMR (360 MHz, CDCl3) δ 6.25 (dd, J = 17.4, 10.6 Hz, 1H) 5.16 (m, 1H), 5.04 (d, J = 17.4 Hz, 1H), 4.96 (d, J = 10.6 Hz, 1H), 3.26 (t, J = 6.9 Hz, 2H), 2.22 (q, J = 6.7 Hz, 2H), 1.75 (d, J = 2.7 Hz, 3H); 13C NMR (90 MHz, CDCl3) δ 207.9, 136.0, 113.0, 101.9, 87.2, 51.0, 28.8, 15.1; APCIMS m/z relative intensity 244 (MH+-N2 100); HRMS (+ESI) calcd for C16H N6 (M2H+) 299.1984, found 299.1976.
(E)-1-Azido-6-methyl-8-(phenyl)octa-3,4,7-triene (30b)
To a solution of propargyl azidomesylate 19 (55 mg, 0.25 mmol) and Pd(PPh3)4 (15 mg, 0.01, 5 mol%) in 10 mL of THF were added commercially available trans-2-phenylvinylboronic acid (67 mg, 0.45 mmol) and sodium carbonate (95 mg, 0.9 mmol). After the reaction mixture was refluxed for 4 h, the solution was poured into water and the mixture was extracted with 15 mL of Et2O. Drying over Na2SO4, followed by evaporation of solvent and chromatography on silica gel using 5% Et2O in hexane afforded 30b as a pale yellow oil (33 mg, 59%). IR (neat) 2096, 1942 cm−1; 1H NMR (360 MHz, CDCl3) δ 7.11−7.38 (m, 5H), 6.68 (d, J = 16.1 Hz, 1H), 6.35 (d, J = 16.4 Hz, 1H), 5.24 (bs, 1H), 3.29 (t, J = 6.7 Hz, 2H), 2.76 (q, J = 6.7 Hz, 2H), 1.87 (d, J = 2.1 Hz, 3H); 13C NMR (90 MHz, CDCl3) δ 208.9, 137.9, 129.0, 128.1, 128.0, 127.7, 126.7, 102.3, 87.4, 51.3, 29.0, 15.9; APCIMS m/z relative intensity 198 (MH+ – N2 100); HRMS (+ESI) calcd for C14H16N 198.1283, found 198.1292.
Ethyl (E)-1-Azido-4-(methyl)oct-2,4,5-trienoate (30c)
To a solution of propargyl azidomesylate 19 (108 mg, 0.50 mmol) and Pd(PPh3)4 (30 mg, 0.02, 5 mol%) in 10 mL of THF was added (Z)-ethoxycarbonylethenylzinc iodide20 (1.5 mmol), and the mixture was heated at 50 °C for 3 h. After the reaction solution was cooled to room temperature, water was added and the mixture was extracted with 15 mL of Et2O. The organic layer was dried over Na2SO4 and solvent was evaporated in vacuo. The crude product was purified by chromatography using 8% Et2O in hexane as the eluant to give 30c as a yellow oil (35 mg, 32%). IR (neat) 2099, 1941 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.32 (d, J = 15.7 Hz, 1H), 5.83 (d, J = 15.7 Hz, 1H), 5.38 (bs, 1H), 4.22 (q, J = 7.2 Hz, 2H), 3.38 (t, J = 6.7 Hz, 2H), 2.37 (q, J = 6.7 Hz, 2H), 1.87 (d, J = 2.6 Hz, 3H), 1.37 (t, J = 7.2 Hz, 3H) ; 13C NMR (100 MHz, CDCl3) δ 211.3, 167.1, 144.9, 118.2, 101.2, 87.8, 60.7, 50.9, 28.4, 15.4, 14.7; APCIMS m/z relative intensity 194 (MH+ – N2 100); HRMS (+ESI) calcd for C11H16NO2 194.1181, found 194.1179.
Vinyl Substrate (32a)
Following General Procedure 2, azidoallene 30a (20 mg, 0.10 mmol) was converted into cyanopyrrolidine 32a (19 mg, 96%). IR (neat) 3426, 2097 cm−1; 1H NMR (400 MHz, CDCl3) δ 5.5 (d, J = 1.3 Hz, 1H), 3.20 (dddd, J = 11.5, 8.7, 5.9, 2.7 Hz, 1H), 3.07 (dt, J = 10.4, 6.3, 6.3 Hz, 1H), 2.92 (dt, J = 10.4, 6.2, 6.2 Hz, 1H), 2.76 (dddd, J = 11.2, 8.8,4.9, 2.5 Hz, 1H), 2.22−2.16 (m, 1H), 2.16−2.09 (m, 1H), 1.85 (bs, 1H) 1.54 (d, J = 7.3 Hz, 3H), 1.57−1.49 (m, 1H); 13C NMR (100 MHz, CDCl3) δ 137.2, 129.8, 122.5, 72.8, 49.0, 47.3, 38.5, 36.0, 12.9; APCIMS m/z relative intensity 149 (MH+ 100); HRMS (+APCI) calcd for C9H13N2 149.1075, found 149.1073.
(E)-Styryl Substrate (32b)
Following General Procedure 2, azidoallene 30b (18 mg, 0.08 mmol) was converted into cyanopyrrolidine 32b (15 mg, 84%). IR (neat) 3331, 2098 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.37−7.17 (m, 5H), 5.59 (s, 1H), 3.6 (s, 1H), 3.17−3.14 (m, 2H), 3.04−2.98 (m, 1H), 2.28−2.20 (m, 1H), 1.96 (d, J = 1.27 Hz, 3H), 1.89−1.78 (m, 1H); 13C NMR (100 MHz, CDCl3) δ 144.1, 138.3, 133.5, 129.3, 127.6, 127.3, 122.3, 72.8, 59.1, 58.4, 47.2, 35.4, 13.1; APCIMS m/z relative intensity 225 (MH+ 100); HRMS (+APCI) calcd for C15H17N2 225.1390, found 225.1386.
(E)-Acryloyl Substrate (32c)
Following General Procedure 2, azidoallene 30c (18 mg, 0.08 mmol) was converted into cyanopyrrolidine 32c (16 mg, 90%). IR (neat) 3250, 2090, 1731 cm−1; 1H NMR (300 MHz, CDCl3) δ 5.57(s, 1H), 4.19 (q, J = 7.1 Hz, 2H) 3.53 (m, 1H), 3.30 (d, J = 2.3 Hz, 1H), 3.17−3.09 (m, 1H), 2.96−2.88 (m, 1H), 2.31−2.19 (m, 1H), 1.89 (t, J = 1.6 Hz, 3H), 1.89−1.58 (m, 1H) 1.30 (t, J = 7.2 Hz, 3H); 13C NMR (75 MHz, CDCl3) δ 172.7, 140.7, 127.5, 121.5, 72.4, 61.8, 57.0, 51.8, 47.2, 34.8, 14.6, 13.1; APCIMS m/z relative intensity 225 (MH+ 100); HRMS (+ESI) calcd for C12H17N2O2 221.1289, found 221.1284.
3-Azido-1-(2-bromophenyl)-propan-1-one (34)
To a solution of (o-bromophenyl)vinyl ketone21 (16 g, 76 mmol) in AcOH and water (1:1 v/v) sodium azide (19.5 g, 300 mmol) was added and the reaction solution was allowed to stir at room temperature. After 24 h, the excess AcOH was neutralized carefully with solid sodium carbonate. The solution was extracted three times with Et2O (300 mL), the combined organic extracts were washed with brine, dried over Na2SO4 and the solvent was evaporated. The dark oil was purified by column chromatography using 25% ether in hexanes to give the keto azide 34 (14.4 g, 74%). IR (neat) 2097, 1694 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.60 (d, J = 7.9 Hz, 1H), 7.42 (dd, J = 7.7, 1.7 Hz, 1H), 7.36 (dt, J = 7.8, 1.8 Hz, 1H), 7.29 (dd, J = 7.5, 1.8 Hz, 1H), 3.69 (t, J = 6.4 Hz, 2H), 3.20 (t, J = 6.4 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ 201.4, 141.1, 134.3, 132.5, 129.2, 128.0, 119.2, 46.5, 42.0; TOFMSES m/z relative intensity 253.99 (MH+ 50); HRMS (+TOFMSAP) calcd for C9H9N3OBr 253.9929, found 253.9937.
1-Acetoxy-1-(azidoethyl)-1-(2-bromophenyl)but-2-yne (35)
The keto azide 34 (1.0 g, 3.9 mmol) was dissolved in 39 mL of THF and treated with propynyl lithium (235 mg, 5.1 mmol) at room temperature. After 20 min, the dark solution was treated with 15 mL of aqueous NH4Cl and the mixture was extracted with 50 mL of Et2O. The combined extracts are washed with brine and dried over Na2SO4 to give a viscous oil which was taken to the next step without purification.
The crude alcohol was taken up in 40 mL of CH2Cl2 and treated with DMAP (618 mg, 5.1 mmol) and Ac2O (444 μL, 4.7 mmol). After complete consumption of the starting material was indicated by TLC analysis, the reaction mixture was treated with a saturated solution of NaHCO3, extracted with 50 mL of CH2Cl2 and washed with brine. Evaporation of the combined organic phases gave a dark oil. The crude acetate was purified by column chromatography (30% ether in hexanes) to give the acetate 35 (0.60 g, 46%). IR (neat) 2099, 1731 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.99 (dd, J = 7.9, 1.6 Hz, 1H), 7.57 (dd, J = 8.0, 1.3 Hz, 1H), 7.34 (dt, J = 7.9, 1.2 Hz, 1H), 7.15 (dt, J = 7.8, 1.6 Hz, 1H), 3.57 (m, 1H), 3.39 (m, 1H), 2.79 (m, 1H), 2.36 (m, 1H), 2.13 (s, 3H), 2.02 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 168.8, 138.5, 135.9, 130.8, 130.0, 127.8, 119.1, 86.7, 78.5, 76.8, 47.9, 39.6, 21.6, 4.3; TOFMSES m/z relative intensity 358 (MNa+ 90); HRMS (+MSES) calcd for C14H14N3O2BrNa 358.0167, found 358.0190.
Tetrasubstituted Allene Substrate 38a
Following General Procedure 1, propargyl acetate 35 (0.50 g, 1.5 mmol) and vinyl magnesium bromide were converted to the allene 38a (0.25 g, 55%). IR (neat) 2096, 1946 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.60 (d, J = 7.5 Hz, 1H), 7.31 (m, 1H), 7.30 (m, 1H), 7.14 (m, 1H), 6.48 (dd, J = 17.4, 10.6 Hz, 1H), 5.20 (d, J = 17.4 Hz, 1H), 5.11 (d, J = 10.6 Hz, 1H), 3.41 (t, J = 6.9 Hz, 2H), 2.68 (t, J = 6.9 Hz, 2H), 1.93 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 206.3, 138.8, 135.4, 133.6, 130.8, 129.2, 127.9, 123.4, 114.1, 103.2, 102.2, 47.8, 33.2, 14.9; TOFMSES m/z relative intensity 276 (MH+ – N2 100); HRMS (+MSEI) calcd for C14H14N3Br 303.0371, found 303.0344.
Tetrasubstituted Allene Substrate 38b
To a solution of propargyl acetate 35 (0.20 g, 0.60 mmol) and Pd(PPh3)4 (69 mg, 0.06, 10 mol%) in 10 mL of THF was added (Z)-ethoxycarbonylethenylzinc iodide 29c20 (2.5 mmol) in 5.0 mL of THF and the mixture was stirred at room temperature for 2 h, after which water was added and the mixture was extracted with 15 mL of Et2O. The organic layer was dried over Na2SO4 and solvent was evaporated in vacuo. The crude product was purified by chromatography using 8% Et2O in hexane as the eluent to give 38b as a yellow oil (90 mg, 40%). IR (neat) 2098, 1944, 1715 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.61 (d, J = 8.2, 0.9 Hz, 1H), 7.42 (d, J = 15.7 Hz, 1H), 7.31 (m, 2H), 7.18 (m, 1H), 5.89 (d, J = 15.8 Hz, 1H), 4.23 (q, J = 7.1 Hz, 2H), 3.42 (t, J = 6.9 Hz, 2H), 2.70 (t, J = 7.0 Hz, 2H), 1.93 (s, 3H), 1.32 (t, J = 7.1 Hz, 3H); 13C NMR (90 MHz, CDCl3) δ 209.2, 167.0, 143.8, 137.2, 133.3, 130.3, 129.2, 127.6, 123.0 118.5 102.6, 101.9, 60.4, 49.2, 32.7, 14.8, 14.3; TOFMSES m/z relative intensity 348 (MH+ – N2 50); HRMS (+MSEI) calcd for C17H19NBrO2 348.0599, found 348.0600.
Tetrasubstituted Allene Substrate 38c
The keto azide 34 (0.66 g, 2.6 mmol) was dissolved in 26 mL of THF and treated with 1-(tert-butyldimethylsilyloxy)pent-2-ene-4-ynyl lithium22 (2.6 mmol) at room temperature. After 20 min, the dark solution was treated with 15 mL of aqueous NH4Cl and the mixture was extracted with 50 mL of Et2O. The combined extracts are washed with brine and dried over Na2SO4 to give a viscous oil which was taken to the next step without purification.
The crude alcohol was taken up in 40 mL of CH2Cl2 and treated with DMAP (618 mg, 5.1 mmol) and Ac2O (444 μL, 4.7 mmol). After complete consumption of the starting material was indicated by TLC analysis, the reaction mixture was treated with a saturated solution of NaHCO3, extracted with 50 mL of CH2Cl2 and washed with brine. Evaporation of the combined organic phases gave a dark oil. Chromatographic purification of this residue on silica gel (30% Et2O in hexanes) furnished the propargyl acetate (0.70 g, 55%). IR (neat) 2097, 1751 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.95 (d, J = 7.9 Hz, 1H), 7.59 (d, J = 8.0 Hz, 1H), 7.35 (t, J = 7.6 Hz, 1H), 7.16 (t, J = 7.9 Hz, 1H), 6.35 (td, J = 15.8, 3.9 Hz, 1H), 5.94 (td, J = 15.8, 2.0 Hz, 1H), 4.29 (dd, J = 3.6, 2.2 Hz, 2H), 3.58 (m, 1H), 3.41 (m, 1H), 2.83 (m, 1H), 2.43 (m, 1H), 2.13 (s, 3H), 0.95 (s, 9H), 0.11 (s, 6H); 13C NMR (75 MHz, CDCl3) δ 168.7, 144.9, 138.2, 135.9, 130.8, 130.0, 127.8, 119.1, 107.7, 88.4, 86.3, 78.6, 63.1, 47.8, 39.5, 26.3, 21.6, 18.8, –5.0; TOFMSES m/z relative intensity 492 (MH+ 75); HRMS (+MSES) calcd for C22H31N3O3SiBr 492.1318, found 492.1309.
To a solution of CuBr • Me2S (3.7 g, 18 mmol) in 20 mL of THF at –40 °C was added MeMgBr (6.0 mL of a 3 M solution in Et2O, 18 mmol) and the mixture was stirred for 1 h, after which the propargyl acetate (0.73 g, 1.5 mmol) in 5 mL of THF was cannulated into the reaction mixture at –40 °C. The reaction mixture was warmed to room temperature over a period of 8 h to allow complete consumption of starting material. The excess cuprate was then destroyed with dropwise addition of saturated NH4Cl solution. The organic layer was extracted with 3 × 50 mL of Et2O and washed with water and brine. Drying the combined organic extracts over Na2SO4 and removal of solvent under reduced pressure resulted in a brown oil. This crude product was purified by column chromatography using pure hexanes as the eluent to provide the allene 38c (0.20 g, 30%). IR (neat) 2097, 1957 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.59 (d, J = 7.7 Hz, 1H), 7.29 (m, 2H), 7.13 (m, 1H), 6.33 (dd, J = 15.7, 1.3 Hz, 1H), 5.74 (td, J = 15.6, 5.2 Hz, 1H), 4.22 (d, J = 5.2 Hz, 2H), 3.40 (t, J = 7.0 Hz, 2H), 2.66 (t, J = 7.1 Hz, 2H), 1.92 (s, 3H), 0.94 (s, 9H), 0.11 (s, 6H); 13C NMR (75 MHz, CDCl3) δ 206.3, 139.0, 133.6, 130.8, 129.4, 129.1, 128.1, 127.9, 123.4, 102.6, 102.1, 64.3, 49.8, 33.3, 26.4, 18.9, 15.7, –4.7; TOFMSES m/z relative intensity 420 (MH+-N2 100); HRMS (+MSES) calcd for C21H31NOSiBr 420.1358, found 420.1354.
H-Substituted Bicycle 39a
Following General Procedure 2 without the TMS-CN addition step, allenylazide 38a (0.20 g, 0.66 mmol) gave 39a as the product (134 mg, 74%). Crystals suitable for X-ray crystallographic analysis were obtained by slow evaporation of an Et2O/pentane (1:1) solution of 39a over a period of 48 h at 25 °C. Mp: 100–105 °C. 1H NMR (400 MHz, CDCl3) δ 7.64 (dd, J = 7.8, 1.4 Hz, 1H), 7.17 (dt, J = 7.5, 1.4 Hz, 1H), 7.11 (dt, J = 7.9, 1.9 Hz, 1H), 6.92 (dd, J = 7.6, 1.8 Hz, 1H), 6.38 (s, 1H), 4.11 (q, J = 7.2 Hz, 1H), 3.70 (m, 1H), 2.88 (dd, J = 17.3, 1.7 Hz, 1H), 2.60 (d, J = 16.4 Hz, 1H), 2.56 (dd, J = 12.7, 4.4 Hz, 1H), 2.10 (m, 1H), 2.06 (d, J = 1.7 Hz, 3H); 13C NMR (75 MHz, CDCl3) δ 191.2, 146.1, 143.0, 135.9, 135.0, 128.8, 128.3, 127.3, 124.1, 64.4, 64.1, 43.1, 40.8, 12.3; TOFMSES m/z relative intensity 276 (MH+ – N2 100); HRMS (+MSEI) calcd for C14H15NBr 276.0388, found 276.0391.
Ester-Substituted Bicycle 39b
Following General Procedure 2 without the TMS-CN addition step, allenylazide 38b (25 mg, 0.06 mmol) gave 39b as the product (12 mg, 52%). 1H NMR (400 MHz, CDCl3) δ 7.55 (dd, J = 7.8, 1.1 Hz, 1H), 7.21 (dt, J = 7.4, 1.1 Hz, 1H), 7.12 (dt, J = 7.6, 1.6 Hz, 1H), 6.96 (dd, J = 7.5, 1.5 Hz, 1H), 6.30 (s, 1H), 4.08 (dd, J = 15.0, 7.0 Hz, 1H), 4.0 (m, 2H), 3.71 (d, J = 2.6 Hz, 1H), 3.66 (m, 1H), 3.14 (dd, J = 12.5, 4.6 Hz, 1H), 2.20 (dd, J = 12.2, 7.3 Hz, 1H), 2.06 (s, 3H), 1.09 (t, J = 7.2 Hz, 3H); 13C NMR (75 MHz, CDCl3) δ 189.4, 172.5, 144.1, 139.3, 137.8, 134.6, 130.2, 129.1, 127.4, 125.0, 67.9, 64.2, 61.5, 57.9, 41.5, 14.0, 12.1; TOFMSES m/z relative intensity 348 (MH+ 100); HRMS (+MSEI) calcd for C17H19NBrO2 348.0599, found 348.0573.
Methylenesiloxy-Substituted Bicycle 39c
Following General Procedure 2 without the TMS-CN addition step, allenylazide 38c (18 mg, 0.04 mmol) gave 39c (10 mg, 59%). 1H NMR (400 MHz, CDCl3) δ 7.60 (dd, J = 7.8, 1.2 Hz, 1H), 7.16 (dt, J = 7.4, 1.1 Hz, 1H), 7.08 (dt, J = 7.3, 1.4 Hz, 1H), 6.89 (m, 1H), 6.60 (s, 1H), 4.43 (m, 1H), 4.02 (dd, J = 15.0, 7.0 Hz, 1H), 3.61 (ddd, J = 14.6, 14.6, 3.8 Hz, 1H), 3.35 (d, J = 11.4 Hz, 1H), 2.88 (m, 2H) 2.12 (s, 3H), 2.00 (ddd, J = 18.8, 11.3, 7.1 Hz, 1H), 0.85 (s, 9H), 0.00 (s, 3H), –0.03 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 192.2, 147.9, 139.6, 135.9, 135.5, 131.0, 129.0, 127.7, 124.0, 65.0, 63.8, 63.1, 54.7, 40.8, 26.3, 18.6, 12.1, –4.90, –4.97; TOFMSES m/z relative intensity 420 (MH+ 100); HRMS (+MSES) calcd for C21H31NOSiBr 420.1358, found 420.1352.
Supplementary Material
Acknowledgment
Support from the National Institutes of Health, General Medical Sciences division (GM37681) and from the National Science Foundation for the X-Ray Crystallography Facility (CHE 0131112), is gratefully acknowledged. C.S.L. is grateful to CESGA for supercomputer time.
Footnotes
Supporting Information Available: General experimental, details from the X-ray crystallographic determination of 23d and 39a including CIF files, and copies of 1H NMR and 13C NMR spectra for 19, 20a–20e, 23a–23e, 25, 26, 28, 30a–30c, 32a–32c, 34, 35, 38a–38c, and 39a–39c. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Feldman KS, Iyer MR. J. Am. Chem. Soc. 2005;127:4590–4591. doi: 10.1021/ja050757w. [DOI] [PubMed] [Google Scholar]
- 2.López CS, Faza ON, Feldman KS, Iyer MR, Hester DK., II J. Am. Chem. Soc. 2007;129:7638–7646. doi: 10.1021/ja070818l. (b) The reaction profile of the related oxatrimethylenemethane species has been explored via density functional and CASSCF methods; see ref 19a.
- 3.Tsang W, Hampson RF. J. Phys. Chem. Ref. Data. 1986;15:1087–1222. (see pg. 1095). Parkes DA, Quinn CP. J. Chem. Soc. Farad. Soc. I. 1976;72:1952–1970.Tsang W. J. Am. Chem. Soc. 1985;107:2872–2880.Choo KY, Beadle PC, Piszkiewicz W, Golden DM. Int. J. Chem. Kinet. 1976;8:45–58.
- 4.a Quast H, Meichsner G. Chem. Ber. 1987;120:1049–1058. [Google Scholar]; b Quast H, Fuβ A, Heublein A, Jakobi H, Seiferling B. Chem. Ber. 1991;124:2545–2554. [Google Scholar]
- 5.Bleiholder RF, Shechter H. J. Am. Chem. Soc. 1968;90:2131–2137. [Google Scholar]
- 6.Bingham EM, Gilbert JC. J. Org. Chem. 1975;40:224–228. [Google Scholar]
- 7.Barraclough D, Moorhouse NP, Onwuyali EI, Scheinmann F, Hursthouse MB, Galas AMR. J. Chem. Res., Synop. 1984;10:2–103. [Google Scholar]
- 8.Mukai C, Kobayashi M, Kubota S, Takahashi Y, Kitagaki S. J. Org. Chem. 2004;69:2128–2136. doi: 10.1021/jo035729f. [DOI] [PubMed] [Google Scholar]
- 9.a Rule M, Salinaro RF, Pratt DR, Berson JA. J. Am. Chem. Soc. 1982;104:2223–2228. [Google Scholar]; b Feldman KS, Mareska DA. J. Org. Chem. 1999;64:5650–5660. doi: 10.1021/jo9907538. [DOI] [PubMed] [Google Scholar]
- 10.Boyer JHJ. Am. Chem. Soc. 1951;73:5248–5252. [Google Scholar]
- 11.a Jansen A, Krause N. Synthesis. 2002:1987–1992. [Google Scholar]; b Konno T, Tanikawa M, Ishihara T, Yamanaka H. Collect. Czech. Chem. Commun. 2002;67:1421–1435. [Google Scholar]
- 12.Bernauer K, Englert G, Vetter W, Weis E. Helv. Chim. Acta. 1969;52:1886–1905. Synthesis studies: Lévy J, Hugel G. J. Org. Chem. 1986;51:1594–1595.Overman LE, Robertson GM, Robichaud AJ. J. Am. Chem. Soc. 1991;113:2598–2610.Schultz AG, Dai M. Tetrahedron Lett. 1999;40:645–648.
- 13.a Hohenberg P, Kohn W. Phys. Rev. 1964;136:B864–B871. [Google Scholar]; b Kohn W, Sham L. Phys. Rev. A. 1965;140:A1133–A1138. [Google Scholar]; c Stephens PJ, Devlin FJ, Chabalowski CF, Frisch MJ. J. Phys. Chem. 1994;98:11623–11637. [Google Scholar]; d Becke AD. J. Chem. Phys. 1993;98:5648–5652. [Google Scholar]; e Lee C, Yang W, Parr RG. Phys. Rev. B. 1988;37:785–789. doi: 10.1103/physrevb.37.785. [DOI] [PubMed] [Google Scholar]
- 14.Brinker UH, König L. Chem. Ber. 1983;116:882–893. [Google Scholar]
- 15.Cichra DA, Duncan CD, Berson JA. J. Am. Chem. Soc. 1980;102:6527–6533. [Google Scholar]
- 16.a Roth WR, Schmidt T. Tetrahedron Lett. 1971:3639–3642. [Google Scholar]; b Kende AS, Riecke EE. J. Am. Chem. Soc. 1972;94:1397–1399. [Google Scholar]; c Gilbert JC, Higley DP. Tetrahedron Lett. 1973:2075–2078. [Google Scholar]; d Davidson ER, Gajewski JJ, Shook CA, Cohen T. J. Am. Chem. Soc. 1995;117:8495–8501. [Google Scholar]; e Maier G, Senger S. J. Am. Chem. Soc. 1997;119:5857–5861. [Google Scholar]
- 17.Frisch MJ. Gaussian03, revision C.02. Gaussian, Inc.; Wallingford, CT: 2004. [Google Scholar]
- 18.Bauernschmitt R, Ahlrichs R. J. Chem. Phys. 1996;22:9047–9052. [Google Scholar]
- 19.a Hess BA, Jr., Eckart U, Fabian J. J. Am. Chem. Soc. 1998;120:12310–12315. [Google Scholar]; b Hess BA, Jr., Smentek L, Brash AR, Cha JK. J. Am. Chem. Soc. 1999;121:5603–5604. [Google Scholar]; c López CS, Faza ON, York DM, de Lera A. J. Org. Chem. 2004;69:3635–3644. doi: 10.1021/jo049620z. [DOI] [PubMed] [Google Scholar]; d Li J, Worthington SE, Cramer CJ. J. Chem. Soc., Perkin Trans. 2. 1998:1045–1052. [Google Scholar]
- 20.Knochel P, Rao CJ. Tetrahedron. 1993;49:29–48. [Google Scholar]
- 21.Muratake H, Natsume M, Nakai H. Tetrahedron. 2004;60:11783–11803. [Google Scholar]
- 22.Marino JP, McClure MS, Holub DP, Comasseto JV, Tucci FC. J. Am. Chem. Soc. 2002;124:1664–1668. doi: 10.1021/ja017177t. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.