Abstract
Hemoglobin (Hb) degradation is essential for the growth of the intraerythrocytic stages of malarial parasites. This process, which occurs inside an acidic digestive vacuole (DV), is thought to involve the action of four aspartic proteases, termed plasmepsins (PMs). These enzymes have received considerable attention as potential antimalarial drug targets. Leveraging the availability of a set of PM-knockout lines generated in Plasmodium falciparum, we report here that a wide range of previously characterized or novel aspartic protease inhibitors exert their antimalarial activities independently of their effect on the DV PMs. We also assayed compounds previously shown to inhibit cysteine proteases residing in the DV. The most striking observation was a ninefold increase in the potency of the calpain inhibitor N-acetyl-leucinyl-leucinyl-norleucinal (ALLN) against parasites lacking all four DV PMs. Genetic ablation of PM III or PM IV also decreased the level of parasite resistance to the β-hematin binding antimalarial chloroquine. On the basis of the findings of drug susceptibility and isobologram assays, as well as the findings of studies of the inhibition of Hb degradation, morphological analyses, and stage specificity, we conclude that the DV PMs and falcipain cysteine proteases act cooperatively in Hb hydrolysis. We also identify several aspartic protease inhibitors, designed to target DV PMs, which appear to act on alternative targets early in the intraerythrocytic life cycle. These include the potent diphenylurea compound GB-III-32, which was found to be fourfold less potent against a P. falciparum line overexpressing plasmepsin X than against the parental nontransformed parasite line. The identification of the mode of action of these inhibitors will be important for future antimalarial drug discovery efforts focusing on aspartic proteases.
Plasmodium falciparum malaria continues to exert a tremendous burden on communities in tropical and subtropical regions of the world. Efforts to control this disease have been stymied by the acquisition of resistance by this parasite to key antimalarials, including chloroquine (CQ) and pyrimethamine-sulfadoxine (60). Hemoglobin (Hb) degradation plays an essential role in malarial parasite development within infected red blood cells. As such, the parasite proteases involved in Hb degradation appear to be attractive targets for the development of novel antimalarials that are unaffected by the existing mechanisms of drug resistance.
Hb digestion takes place in an acidic compartment, referred to as the digestive vacuole (DV; also known as the food vacuole) (23). This degradative process provides a source of amino acids and is thought to help maintain intracellular osmolarity during rapid parasite growth (40). Biochemical studies have implicated the DV aspartic proteases, termed plasmepsin (PMs), and the cysteine proteases, termed falcipains, as key mediators of this degradative process (24, 51, 55). Because of their ability to initiate the degradation of native Hb, PMs have long been considered important candidate drug targets (4).
The four highly homologous DV PMs (PMs I, II, III [histidine aspartic protease {HAP}], and IV; gene identifiers, PF14_0076, PF14_0077, PF14_0078, and PF14_0075, respectively) are located as a contiguous set on chromosome 14. PM IV is the sole DV-specific PM found in all Plasmodium species sequenced and is the original DV PM ortholog that gave rise to P. falciparum paralogs PM I, II, and III through gene duplications (13, 16). PMs are translated as type II integral membrane proenzymes (5). Following trafficking to the DV, the N-terminal prodomain, which encompasses the cytosolic and transmembrane regions, is cleaved to release the mature enzyme into the DV lumen (25). This process has been shown to be inhibited by the calpain inhibitor N-acetyl-leucinyl-leucinyl-norleucinal (ALLN), suggesting that calpain might act as a maturase for PM activation (3). Drew et al. (18) recently showed that PM activation could occur via the falcipains. At low pH, PM processing can also occur autocatalytically (3, 18, 32).
Two separate studies have now shown that no single DV PM is essential for the in vitro propagation of P. falciparum intraerythrocytic stages (38, 48). However, some of the PM-disrupted lines displayed reduced growth rates when they were cultured in complete medium. This was particularly pronounced with the PM IV-knockout (KO) line (38, 48). The slow growth of these slow-growth phenotypes was exacerbated in amino acid-limited medium (38, 39). Recently, a double-crossover integration strategy was successfully used to obtain parasites lacking PM I, PM II, and PM III (the triple-PM-KO line, denoted Δ3pfpm) or all four DV PMs (the quadruple-PM-KO line, denoted Δ4pfpm) (8). The Δ3pfpm parasite showed small decreases in growth rates and susceptibility to inhibitors compared to those of parental strain 3D7. Greater differences were observed with the Δ4pfpm parasite line, which had a lower rate of growth in complete medium, was severely hindered in its growth in amino acid-limited medium, and produced less of the Hb degradation by-product hemozoin. Δ4pfpm parasites also demonstrated condensed DVs by microscopic analysis (8; P. Moura, unpublished observations). Those parasites also contained numerous multimembrane vesicles in their DVs, suggesting that the growth defect of these parasites might have an etiology beyond impaired Hb digestion.
In the study described here, we have leveraged the availability of these DV PM-KO lines to assess the specificity of putative DV protease inhibitors and probe their modes of action. Our data reveal that the disruption of PMs significantly enhances parasite susceptibility to several cysteine protease inhibitors and can also influence the potency of CQ. We also found that several PM aspartic protease inhibitors, previously thought to be specific to PM I or PM II, do not appear to act primarily on those targets in cultured parasites and appear to kill early-stage parasites prior to DV formation. The data suggest that these aspartic protease inhibitors exert their antimalarial activities primarily on one or more non-DV PMs and secondarily on the DV PMs.
MATERIALS AND METHODS
Parasite cultures.
The P. falciparum lines were cultured in human erythrocytes in RPMI 1640 medium (Invitrogen) supplemented with 0.5% Albumax II (Invitrogen), 50 mg/liter hypoxanthine, 25 mM HEPES, 0.225% NaHCO3, and 10 mg/liter of gentamicin, as described previously (21). The Dd2, GCO3, and 3D7 lines were obtained from Thomas Wellems (Laboratory of Malaria and Vector Research, NIAID, NIH). The PM I-, PM II-, PM III (HAP)-, and PM IV-KO clones, termed Δpfpm1, Δpfpm2, Δpfpm3, and Δpfpm4, respectively, were generated in the CQ-resistant Dd2 line by the use of a single-site crossover that leads to the disruption of the functional locus, as reported previously (48). The triple-PM-KO line, previously termed B12, lacks PM I, PM II, and PM III, whereas the quadruple-PM-KO line, previously termed C10, lacks all four DV PMs known to date. Both lines were generated in the CQ-sensitive 3D7 line by double crossover, as reported previously (8), and are referred to herein as Δ3pfpm (B12) and Δ4pfpm (C10), respectively.
Plasmid construction and transfections.
Parasite transfection vectors, derived from the pPM plasmids previously reported from our laboratory (35), were used in the present study to express PM V (PF13_0133) or PM X (PF08_0108). These proteins were fused at their C termini to monomeric red fluorescent protein (mRFP). PM V and PM X were amplified from 3D7 line genomic DNA by using the primer pairs 5′-TACCCTAGGATGAATAATTATTTTTTAAGGAAAGAAAATTTTTTTATATTG plus 5′-TACAGATCTTGTTGATTCCTGTATGGGAGATTTATTTTG and 5′-TACCCTAGGATGAAACGCATTAGCCCTCTAAAC plus 5′-TACAGATCTGTTTTTACTTTTTGCTCTTGCTACTCC, respectively (the AvrII and BglII sites are underlined in the first and second sequences of each primer pair, respectively). Inserts were subcloned into pGEM-T (Promega), the sequences were verified, and the inserts were subcloned into plasmid pPM-BSD-hrp3pro-crt(GCO3)-mRFP (35) in the place of the pfcrt allele (which was excised by using AvrII and BglII). The PM-mRFP fusions were expressed under the control of the hrp3 (MAL13P1.480) 1.3-kb promoter, which is maximally active in early ring stages (consistent with the timing of expression of PM V and PM X). The resulting plasmids were named pPM-BSD-hrp3pro-pfpmV-mRFP and pPM-BSD-hrp3pro-pfpmX-mRFP.
Parasites of the Dd2 line were transfected with plasmids pPM-BSD-hrp3pro-pfpmV-mRFP and pPM-BSD-hrp3pro-pfpmX-mRFP as described previously (22). At approximately 24 h postelectroporation, the parasite cultures were treated with 2 μg/ml blasticidin S hydrochloride (Invitrogen) and thereafter were maintained at that concentration.
In vitro drug susceptibility assays.
In vitro drug responses were measured by using 64- to 68-h [3H]hypoxanthine incorporation assays, as described previously (21). In these assays, cells were maintained in medium containing 2.5 mg/liter hypoxanthine (low hypoxanthine medium), and 0.7 μCi of [3H]hypoxanthine was added per well for the final 16 to 20 h. We prefer this version of the assay to the 42- to 48-h version (17) because in our experience, the longer assays are less dependent on the initial stage specificity of the parasite culture and thus provide more reproducible 50% inhibitory concentrations (IC50s) between assays. We note that several other groups routinely perform these 64- to 68-h assays (14, 20). In our assays, microtiter plates were prepared for each drug by using a Precision 2000 automated pipetting system (Bio-Tek Instruments Inc.). Two days prior to use, ring-stage parasites were synchronized with 5% sorbitol. For the assays, these were plated at a 0.4% final parasitemia and a 1.6% final hematocrit (21).
CQ dihydrochloride was procured from Winthrop-Breon. Mono-desethylchloroquine (m-dCQ) was a generous gift from William Ellis (Walter Reed Army Institute of Research, Silver Spring, MD). Verapamil hydrochloride (VP), leupeptin, ALLN, N-(trans-epoxysuccinyl)-l-leucine 4-guanidinobutyl-amide (E-64), and pepstatin A (a new lot) were purchased from Sigma. Wherever VP was included, it was used at a final concentration of 0.8 μM. An earlier Sigma stock of pepstatin A (an old lot, dating back more than 6 years) was a kind gift from Philip Rosenthal (University of California at San Francisco). The Roche compound Ro 40-4388 was kindly provided by Patrick Bray (Liverpool School of Tropical Medicine, Liverpool, United Kingdom). Atovaquone was a kind gift from Akhil Vaidya (Drexel University College of Medicine, Philadelphia, PA). Compounds 11b, 13, 15, and 19 were provided by Karolina Ersmark (Institut European de Chimie et Biologie, Bordeaux, France). Compounds GB-III-24, GB-III-28, and GB-III-32 were kindly provided by Karl Werbovetz (Ohio State University, Columbus). The structures of all these compounds are presented in Fig. S1 in the supplemental material. The IC50s (i.e., the drug concentrations required for 50% inhibition of [3H]hypoxanthine uptake in drug-treated wells compare with that in non-drug-treated control wells) were calculated by regression analysis of the dose-response curves. For each compound and parasite line, assays were performed in duplicate on four to eight separate occasions. One-way analysis of variance (nonparametric) and Bonferroni's multiple-comparison posttest were used to monitor for statistical significance.
Isobologram analyses.
Antimalarial interactions were assessed over a range of concentrations by a fixed-ratio method based on the IC50s (23, 47). Briefly, IC50s were determined for each compound. Stock solutions were then prepared at 16 times the IC50 of each compound. Solutions were combined at fixed ratios of 10:0, 9:1, 7:3, 5:5, 3:7, 1:9, and 0:10 for compound A-compound B. These seven starting solutions were serially diluted across eight twofold dilutions. For each plate, one row was dedicated to no-drug controls containing just low-hypoxanthine medium. Microtiter plates were processed by 72-h [3H]hypoxanthine drug susceptibility assays, as described above. Fractional IC50s (FIC50s) were calculated on the basis of the IC50s obtained per assay for each compound (the FIC50 is equal to the IC50 of drug A in combination with drug B/IC50 of drug A alone). The average FIC50s were calculated from at least four separate assays (except for assays with compound GB-III-32, which, due to its limited availability, was tested only twice) and were plotted as isobolograms for both compounds. The mean sums of the FIC50s were calculated for all of the combinations and lines tested and were based on the results obtained with fixed ratios of 7:3, 5:5, and 3:7. These were tested for statistical significance by a nonparametric one-way analysis of variance test and by use of Bonferroni's multiple-comparison posttest.
Quantification of undigested Hb.
Sorbitol-synchronized parasite cultures were harvested at the mid-trophozoite stage (30 h postinvasion) and were lysed with 0.15% saponin. The parasite pellets were washed three times in phosphate-buffered saline and were resuspended in phosphate-buffered saline containing a protease inhibitor cocktail (Complete minitablets; Roche), 0.1% sodium dodecyl sulfate (SDS), and 0.05% sodium deoxycholate. Each sample was mixed with loading buffer and electrophoresed on 15% SDS-polyacrylamide gels. These were either stained with Coomassie or transferred onto polyvinylidene difluoride (PVDF) membranes (Bio-Rad). The membranes were cut along the 20-kDa marker and probed with rabbit antisera directed to either human Hb (diluted 1:5,000; Sigma) (29) or F-actin (diluted 1:10,000; Abcam). Following the washes, the membranes were incubated with horseradish peroxidase-conjugated donkey anti-rabbit immunoglobulin G (diluted 1:7,000; GE Healthcare). The bands were visualized by using an enhanced chemiluminescence detection kit (Amersham). The protein levels were quantified by densitometric analysis of the autoradiographic data by using an AlphaEaseFC apparatus (Alpha Innotech) and were further validated by the used of Coomassie-stained gels. Undigested Hb band intensities were normalized against the F-actin band intensities to correct for loading differences. For each parasite line, the normalized Hb band intensities were expressed as a percentage of the control Dd2 line band intensities.
Parasite imaging.
Blood smears from the Hb degradation assay were fixed with methanol, dried, stained in 10% Giemsa stain (Sigma) for 30 min, washed, and air dried. Color images were captured from each slide by using a ×100 objective on an Olympus CX 41 microscope with an Olympus DP12 digital camera.
For the imaging of live cells, the cells were adhered to poly-l-lysine-coated glass-bottom culture dishes (MatTek). Adherent cells were overlaid with 1 ml of RPMI medium containing 2 μg/ml Hoechst 33342 (Sigma) to stain the nuclei and were immediately imaged at room temperature on a Nikon TE300 inverted microscope with ×100 1.4-numerical-aperture PlanApo optics and a Hamamatsu Orca-ERG charge-coupled-device camera. Images were collected with Openlab software systems (version 5.0; Improvision) and were analyzed by using Photoshop software (Adobe Systems).
RESULTS
Genetic disruption of DV PMs increases parasite susceptibility to cysteine protease inhibitors.
Recent studies have suggested that PMs and falcipains can act cooperatively to mediate the degradation of Hb and that certain hemoglobinase inhibitors might act on both classes of proteases (8, 39, 53). To further address these relationships, we tested the activities of the well-known cysteine protease inhibitors E-64, ALLN, and leupeptin against P. falciparum clones disrupted in one or more PM genes.
When they were treated with E-64, the Δpfpm2, Δpfpm3, and Δpfpm4 lines showed a significant 33 to 38% decrease in IC50s compared to the IC50 for the Dd2 parental line (P < 0.001 for Δpfpm2 and Δpfpm3 and P < 0.01 for Δpfpm4; Table 1; assay details are provided in Table S1 in the supplemental material). This finding concurred with the results that we obtained using the Δpfpm4 line, which was generated in the 3D7 line (P < 0.001; Table 1). For ALLN, the potency did not differ substantially between the single-PM-KO mutant lines and the Dd2 parental line. However, both of the multiple-PM-KO mutants, Δ3pfpm and Δ4pfpm, demonstrated significantly increased susceptibilities; and this was notably the case for the latter mutant, which showed a ninefold decrease in the IC50 compared to that of the 3D7 parental line (P < 0.001; Table 1). These findings support the hypothesis that the PMs and some cysteine proteases play redundant or complementary roles in the DV and that the absence of all PMs in the Δ4pfpm line renders these parasites highly susceptible to ALLN-mediated inhibition of falcipains. Leupeptin demonstrated a detectable alteration in IC50s only in the Δpfpm3 line, which was 40% more susceptible than the Dd2 parental line (P < 0.01; Table 1), and in none of the multiple-PM-KO lines, suggesting that the proteases specifically inhibited by leupeptin differ from the set targeted by ALLN in terms of how their functions overlap with those of the PMs.
TABLE 1.
Susceptibility of PM-KO lines to known protease inhibitors
| Parasite line | IC50a |
||||
|---|---|---|---|---|---|
| E-64 (μM) | ALLN (nM) | Leupeptin (μM) | Ro 40-4388 (nM) | Pepstatin A new (μM) | |
| Dd2 | 4.5 ± 0.6 | 104.7 ± 13.5 | 7.5 ± 0.6 | 208.8 ± 16.1 | 25.0 ± 3.0 |
| Δpfpm1 | 3.7 ± 0.2 | 94.5 ± 9.6 | 6.8 ± 0.6 | 197.9 ± 16.0 | 27.5 ± 2.1 |
| Δpfpm2 | 3.0 ± 0.2*** | 86.4 ± 9.8 | 5.2 ± 0.4 | 178.6 ± 12.7 | 23.5 ± 1.3 |
| Δpfpm3 | 2.8 ± 0.1*** | 61.8 ± 7.8* | 4.4 ± 0.7** | 180.9 ± 14.3 | 20.0 ± 3.7 |
| Δpfpm4 | 3.0 ± 0.1** | 84.5 ± 14.3 | 6.7 ± 0.7 | 201.8 ± 18.5 | 23.4 ± 2.6 |
| GC03 | 3.2 ± 0.2** | 97.9 ± 10.3 | 10.1 ± 0.7* | 162.4 ± 11.7 | 36.0 ± 2.8* |
| 3D7 | 3.4 ± 0.2 | 72.4 ± 7.8 | 6.8 ± 0.2 | 187.1 ± 9.4 | 16.4 ± 1.1 |
| Δ3pfpm | 3.0 ± 0.2 | 44.1 ± 7.9* | 17.3 ± 2.5* | 175.6 ± 11.0 | 11.5 ± 1.3* |
| Δ4pfpm | 2.1 ± 0.2*** | 7.9 ± 0.7*** | 7.8 ± 1.4 | 187.6 ± 11.6 | 18.4 ± 1.3 |
IC50s are expressed as means ± SEMs. Each value was determined from four to nine separate drug assays performed in duplicate. The degree of statistical significance is indicated with an asterisk(s): *, P < 0.05; **, P < 0.01; ***, P < 0.001.
We also tested the KO lines with atovaquone as a control to evaluate whether the effects that we observed with the cysteine and aspartic protease inhibitors were specific for the targeted genes or, alternatively, reflected a general impairment in growth that would nonspecifically skew the IC50s for any antimalarial compound. Assays with the mitochondrial electron transport chain inhibitor atovaquone revealed no differences in the IC50s between the PM-KO lines and the Dd2 parental line (see Table S1 in the supplemental material).
CQ resistance is attenuated in parasites lacking DV PMs.
To assess whether the disruption of DV PMs could affect the potency of drugs that act downstream of Hb degradation, we tested the activity of CQ against our lines. This agent is believed to act by binding to the Hb degradation product β-hematin and inhibiting heme detoxification (11). The assays revealed a significant 25% decrease in the CQ IC50s for Δpfpm3 and Δpfpm4 compared to that for the CQ-resistant Dd2 parental line (P < 0.01; Table 2). These results were confirmed in independent tests with the CQ metabolite m-dCQ. We also tested the CQ response in the presence of VP, which is known to reverse CQ resistance in a pfcrt allele-specific manner (34, 41). No significant difference in the degree of reversibility of CQ resistance by VP in the KO lines versus the parental line was observed. We also assayed the CQ response in the Δ3pfpm and Δ4pfpm lines, which were generated in the CQ-sensitive 3D7 background (8). No significant differences in response between either of these two lines and the 3D7 parental strain were observed with CQ or m-dCQ, although Δ3pfpm was noted to have a 25% lower IC50 (Table 2; details are provided in Table S2 in the supplemental material). We note that 3D7 is highly sensitive to CQ and has a very steep kill curve (IC90/IC50 ratios, ∼1.25 for 3D7 and ∼1.8 for Dd2; data not shown), and thus, it is not surprising that any subtle effect of PM gene disruptions on the mode of action of CQ would not be evident in a CQ-sensitive background.
TABLE 2.
Susceptibility of PM-KO lines to CQ with or without VP
| Parasite line | IC50 (nM)a |
||
|---|---|---|---|
| CQ | m-dCQ | CQ + VP | |
| Dd2 | 194.4 ± 6.8 | 1,596 ± 81.4 | 79.4 ± 9.7 |
| Δpfpm1 | 177.4 ± 12.2 | 1,494 ± 87.9 | 55.8 ± 10.7 |
| Δpfpm2 | 175.0 ± 11.1 | 1,409 ± 28.6 | 59.9 ± 7.7 |
| Δpfpm3 | 145.0 ± 5.6** | 1,180 ± 116.3** | 47.4 ± 8.0 |
| Δpfpm4 | 144.2 ± 9.9** | 1,165 ± 142.5* | 63.0 ± 13.0 |
| GC03 | 23.9 ± 0.5*** | 29.6 ± 0.7*** | 19.5 ± 2.2*** |
| 3D7 | 22.6 ± 0.9 | 25.1 ± 0.7 | ND |
| Δ3pfpm | 17.0 ± 2.6 | 19.0 ± 2.9 | ND |
| Δ4pfpm | 24.7 ± 2.3 | 23.5 ± 0.6 | ND |
IC50s are expressed as means ± SEMs. Each value was determined from six to eight separate drug assays performed in duplicate. The degree of statistical significance is indicated with an asterisk(s): *, P < 0.05; **, P < 0.01; ***, P < 0.001. ND, not determined.
Evidence that selected novel aspartic protease inhibitors do not exert antimalarial activity through inhibition of DV PMs.
The design of novel DV PM protease inhibitors with potent enzyme and whole-cell activities has benefited substantially from the elucidation of the crystal structure of PM II bound to pepstatin A (56, 57). That analysis demonstrated that the cleavage of the α33Phe—34Leu Hb peptide bond proceeds via a tetrahedral intermediate bound to a protonated form of one of the aspartic acids in the PM II active site and revealed notable and potentially exploitable differences between PM II and the human cathepsin D homolog. Studies with purified PM I or PM II have discovered potent inhibitors with activities that can descend to the low nanomolar range (9, 15, 27, 31, 45, 46, 57). Additionally, some aspartic protease inhibitors can reduce the levels of parasitemia in rodent models (1, 28), allowing for optimism that PM inhibitors effective against the malaria parasite could be developed.
Several types of transition-state aspartic protease inhibitors which mimic the tetrahedral intermediate formed during PM II cleavage of Hb have now been reported (27). These lack a large aliphatic substituent group found in the statin-based drugs and contain a hydroxyethylamine core structure, which had previously been used as a transition-state mimic in the design of human immunodeficiency virus protease inhibitors (59). These compounds also contain a basic secondary amine, one prime side amino acid residue to minimize the size and peptidic nature of the inhibitor, and a large P1′ substituent group (see Fig. S1 in the supplemental material). Such compounds have been touted to be potential therapeutics because of their high degrees of potency and their selectivities for purified PM II rather than the human cathepsin D homolog. Among inhibitors of this class, we tested compound 11b, described by Noteberg et al. (46). Results from dose-response assays with the single- or multiple-PM-KO lines produced similar IC50s for all lines and the parental controls, indicating that the antimalarial activity of this transition-state mimic was not due to the direct inhibition of the DV PMs (Table 3; see Tables S1 and S2 in the supplemental material).
TABLE 3.
Activities of novel structure-guided inhibitors of PMs on PM-KO lines
| Parasite line | IC50a |
||||||
|---|---|---|---|---|---|---|---|
| 11b (μM) | 13 (μM) | 15 (μM) | 19 (μM) | GB-III-24 (nM) | GB-III-28 (nM) | GB-III-32 (nM) | |
| Dd2 | 4.4 ± 0.2 | 3.2 ± 0.4 | 5.6 ± 1.1 | 7.3 ± 1.9 | 787.5 ± 41.7 | 824.7 ± 72.7 | 19.8 ± 2.1 |
| Δpfpm1 | 5.3 ± 0.7 | 2.9 ± 0.9 | 4.4 ± 0.6 | 8.3 ± 1.4 | 918.5 ± 80.3 | 1,074 ± 150.6 | 20.8 ± 3.2 |
| Δpfpm2 | 4.3 ± 0.2 | 3.8 ± 0.4 | 3.0 ± 0.6 | 3.3 ± 1.5 | 831.8 ± 77.0 | 892.5 ± 48.5 | 14.2 ± 3.7 |
| Δpfpm3 | 4.5 ± 0.2 | 3.4 ± 0.5 | 5.6 ± 0.9 | 5.5 ± 1.0 | 705.5 ± 39.9 | 615.4 ± 102.5 | 10.3 ± 1.7 |
| Δpfpm4 | 4.7 ± 0.4 | 3.2 ± 0.3 | 3.2 ± 0.3 | 7.2 ± 1.2 | 879.9 ± 146.1 | 874.1 ± 105.0 | 31.3 ± 4.7* |
| GC03 | 3.3 ± 0.5 | 1.7 ± 0.5 | 2.4 ± 0.5* | 10.1 ± 2.0 | 853.4 ± 27.8 | 796.0 ± 50.4 | 15.1 ± 2.4 |
| 3D7 | 4.2 ± 0.2 | 2.9 ± 0.3 | 6.2 ± 0.5 | 5.0 ± 0.4 | 1,364 ± 41.4 | 1,094 ± 77.1 | 19.9 ± 2.6 |
| Δ3pfpm | 4.3 ± 0.3 | 3.3 ± 0.5 | 5.7 ± 0.4 | 5.0 ± 0.4 | 1,240 ± 87.4 | 1,095 ± 91.8 | 14.6 ± 1.3 |
| Δ4pfpm | 3.8 ± 0.4 | 2.4 ± 0.2 | 6.0 ± 0.7 | 4.2 ± 0.5 | 1,088 ± 109.7 | 776.6 ± 86.5* | 15.3 ± 0.8 |
| PM V-mRFP | ND | ND | ND | ND | 1,706 ± 100.6 | 1,588 ± 76.0 | 27.6 ± 2.1 |
| PM X-mRFP | ND | ND | ND | ND | 1,477 ± 117.3 | 1,125 ± 145.4 | 72.5 ± 2.2*** |
IC50s are expressed as means ± SEMs. Each value was determined from three to six separate drug assays performed in duplicate. The degree of statistical significance in comparison to the result for the parental line (Dd2 for the single PM-KO lines and 3D7 for the triple and quadruple KO lines) is indicated with an asterisk(s): *, P < 0.05; ***, P < 0.001. ND, not determined.
We also assessed compounds 13, 15, and 19, which were the most potent of a series of inhibitors of purified PM I and PM II reported by Ersmark et al. (19), with their Ki values being in the low nanomolar range (see Fig. S1 in the supplemental material). These C-2-symmetric linear inhibitors are P1/P1′ side chain derivatives of 1,2-hydroxyethylene, an effective inhibitor of the human immunodeficiency virus type 1 aspartic protease. In molecular modeling studies with PM II, these inhibitors revealed an alternative tetrahedral intermediate scaffold for inhibitor design (19). In our assays, none of these compounds displayed a significant difference in potency in any PM-KO line (Table 3). We note, however, that the IC50 of compound 15 for the CQ-sensitive GC03 line was significantly different from the IC50s for the Dd2 line as well as the 3D7 line (P < 0.05), illustrating the strain-dependent differences in susceptibility to protease inhibitors.
We also investigated a distinct chemical series identified during an enzyme inhibition-based screen of putative protease inhibitors that had been selected from the Walter Reed chemical inventory. This screen had identified WR268961, a nonpeptidyl diphenylurea compound, to be a potent and selective inhibitor of PM II (31). This discovery was followed up through the generation of a series of amidine-containing derivatives, from which we selected compounds GB-III-24 and GB-III-28 (see reference 7, in which these compounds are referred to as compounds 4c and 4e, respectively). These compounds differed in their p-position placement of a trifluoromethyl group on the 4-phenoxy ring (see Fig. S1 in the supplemental material). Both compounds had IC50s comparable to the IC50 of the parent compound, WR268961, indicating no effect of the trifluoromethyl group on whole-cell potency. Among the KO lines, only the Δ4pfpm line demonstrated a significantly altered susceptibility, with a 30% decrease in the GB-III-28 IC50 compared to that for the 3D7 line (P < 0.05; Table 3). The IC50s for the unmodified lines (the Dd2, GC03, and 3D7 lines) were 800 to 1,400 nM for both compounds. We also investigated GB-III-32 (7), which has an additional amidine group at the p position of the 4-phenoxy ring (see Fig. S1 in the supplemental material). This compound had a 30-fold increase in whole-cell activity compared to the activity of WR268961 and was exquisitely potent, with IC50s of 15 to 20 nM for the Dd2, GC03, and 3D7 lines (Table 3). The assays revealed a significant decrease in the susceptibility of the Δpfpm4 line, suggesting that PM IV might act as a target for GB-III-32 (P < 0.05; Table 3).
To further explore the mode of action of these potent amidine-containing diphenylureas, we tested their potencies against parasite lines engineered to overexpress PM V or PM X, which we postulated might represent alternative targets. These proteins were fused to mRFP to facilitate their detection in the recombinant lines. The PM V-mRFP-expressing parasite line showed no difference in susceptibility to compounds GB-III-24, GB-III-28, and GB-III-32 compared to that for its Dd2 parental strain. In contrast, the IC50 of GB-III-32 for the PM X-mRFP parasite line demonstrated a fourfold increase compared to that for its Dd2 parental strain (Table 3). This suggests that PM X might be a primary target for GB-III-32. In preliminary live cell imaging studies, we observed PM V-mRFP fluorescence in the perinuclear space of asexual blood-stage parasites (see Fig. S2A and B in the supplemental material), suggestive of localization in the endoplasmic reticulum. This location is consistent with that identified in an earlier study (33). The fluorescence of PM X-mRFP was putatively localized primarily to the DV and secondarily to the parasitophorous vacuolar space (see Fig. S2C and D in the supplemental material).
Having found that our subset of structure-guided inhibitors lacked specificity for the individual DV PMs, PMs I to IX, we turned our attention to the better-known aspartic protease inhibitors Ro 40-4388 and pepstatin A. Ro 40-4388 is an aspartic protease peptidomimetic compound that has activity against P. falciparum at submicromolar concentrations and that potently inhibits purified PM I yet that poorly inhibits PM II (42). This compound also reduces the level of accumulation of CQ in the DV. This is thought to result from the inhibition of Hb degradation by Ro 40-4388, which consequently reduces the levels of β-hematin in the DV and which leads to the retention of reduced levels of CQ (11). In contrast to the long-standing interpretation that this inhibitor acts through the specific inhibition of PM I, our assays revealed no difference in the susceptibilities of the Dd2 line, the Δpfpm1 parasite line, or any other PM-KO line to this compound (Table 1).
A similar result was obtained with pepstatin A, whose affinity for PM II has been mapped at the X-ray structural level (57). We found the whole-cell activity of this inhibitor to be unaltered in lines disrupted for PM II or any other DV PM (Table 1). During these studies, we also observed that pepstatin A from the original source (old pepstatin A), which was partially purified from a microbial extract, was 10-fold more potent than the newer chemically synthesized source used to obtain the data presented in Table 1 (the data for old pepstatin A is provided in Table S1 in the supplemental material). Liu et al. recently reported a similar observation and attributed this to a potent contaminant in the original pepstatin A preparation (39). Our studies with the newer pepstatin A revealed an increased susceptibility of the Δ3pfpm line (P < 0.05; Table 1), yet no single DV PM appeared to be the most probable and important target.
Aspartic protease inhibitors and other DV-targeting agents display complex in vitro interactions that can be altered by the loss of PM IV.
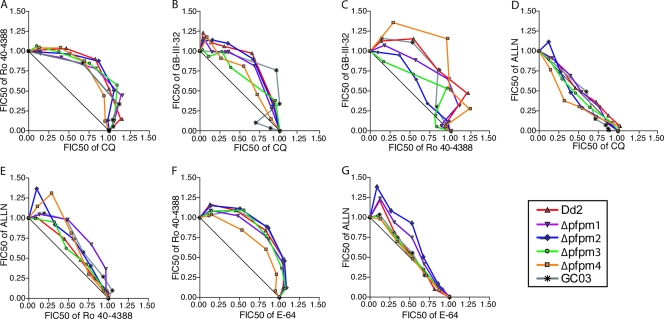
We extended these in vitro susceptibility assays to investigate the interactions between compounds believed to target processes inside the DV (23). For this, we utilized a fixed-ratio method to construct isobolograms and performed drug combination assays on two to four separate occasions with each single-PM-KO line and as well as the Dd2 and GC03 control lines. The FIC50s were calculated on the basis of the IC50s of each compound per assay (the FIC50 is equal to the IC50 of drug A in combination with drug B/IC50 of drug A alone). The mean FIC50s for each fixed ratio are plotted in Fig. 1 as isobolograms for each drug combination. When compounds displayed a purely additive effect, the FIC50 of drug A plus the FIC50 of drug B was equal to 1 and the values from the different combinations plot as a straight line. Synergy can be defined as a drug-drug interaction whereby the combination has a greater effect when the agents are tested together rather than as individual agents and where the sum of the FIC50s is reproducibly <1, creating a concave curve. Antagonism can be defined as an interaction whereby the two-drug combination has less of an effect than the each agent alone, producing a sum of FIC50s that is reproducibly >1 and that plots as a convex curve (6). The mean sums of the FIC50s of each combination at fixed ratios of 7:3, 5:5, and 3:7 for all lines tested are reported in Table 4. Mean values ± standard errors of the means (SEMs) for all the different combinations are plotted for the Δpfpm4 and the parental lines in Fig. S3 in the supplemental material.
FIG. 1.
Isobologram analyses of interactions between antimalarial compounds that are postulated to inhibit either aspartic proteases (Ro 40-4388, GB-III-32) or cysteine proteases (ALLN, E-64) or that target β-hematin (CQ). Panels A to G represent data from selected combinations discussed in the text. Additional pairwise combinations are presented in Fig. S3 in the supplemental material. Assays were performed by a fixed-ratio method based on the IC50s, with the combinations being tested at constant ratios of 10:0, 9:1, 7:3, 5:5, 3:7, 1:9, and 0:10 (47). FIC50s were calculated from the results of two to four separate assays, and the plots were compared to a theoretical line that produced a sum of the FIC50s of 1 at all ratios tested, which represents an additive effect of both compounds. Substantial deviations above and below this line of additivity reflect antagonistic and synergistic effects, respectively.
TABLE 4.
Mean sums of FIC50sa
| Parasite line | Sum of FIC50s for: |
||||||
|---|---|---|---|---|---|---|---|
| Ro 40-4388-CQ | GB-III-32-CQ | GB-III-32-Ro 40-4388 | ALLN-CQ | ALLN-Ro 40-4388 | Ro 40-4388-E-64 | ALLN-E-64 | |
| Dd2 | 1.59 | 1.41 | 1.67 | 1.05 | 1.13 | 1.58 | 1.06 |
| Δpfpm1 | 1.51 | 1.33 | 1.50 | 1.06 | 1.42 | 1.52 | 1.21 |
| Δpfpm2 | 1.60 | 1.44 | 1.20 | 0.91 | 1.24 | 1.62 | 1.33* |
| Δpfpm3 | 1.55 | 1.16 | 1.25 | 0.94 | 1.17 | 1.55 | 1.05 |
| Δpfpm4 | 1.46 | 1.24 | 1.72 | 0.80** | 1.33 | 1.36 | 1.01 |
| GCO3 | 1.25 | 1.28 | 1.46 | 0.89 | 1.31 | 1.60 | 1.05 |
The sums of the FIC50s are expressed as the means for fixed ratios of 7:3, 5:5, and 3:7. The degree of statistical significance is indicated with an asterisk(s): *, P < 0.05; **, P < 0.01.
Combination assays with the aspartic protease inhibitor Ro 40-4388 and CQ revealed antagonism in all PM KO lines, in addition to Dd2 and GC03 (Fig. 1A). A similar finding of antagonism was previously reported for the K1, HB3, and NF54 lines and had been attributed to Ro 40-4388 inhibiting the liberation of heme moieties that act as a receptor for CQ, resulting in reduced CQ accumulation in the DV (10, 12, 42, 43). Between the Dd2-based lines, the sum of the FIC50s of Ro 40-4388 and CQ showed no apparent differences in the degree of antagonism (sum of FIC50s, >1) (Table 4). We also observed antagonism between the aspartic protease inhibitor GB-III-32 and CQ (Fig. 1B; Table 4). Interaction studies between GB-III-32 and Ro 40-4388 revealed various degrees of antagonism, with the least being observed in Δpfpm2 and Δpfpm3 (Fig. 1C and Table 4). The mean sum of the FIC50s was >1 for all lines tested, although there was a lower sum for Δpfpm2, Δpfpm3, and GC03 (Table 4). These findings correlate with the increased sensitivity of these three lines to Ro 40-4388 and GB-III-32 (Tables 1 and 3).
In contrast to these antagonistic interactions, the maturase inhibitor ALLN combined with CQ produced an additive combination for all lines except Δpfpm4 (Fig. 1D; Table 4; see Fig. S3F in the supplemental material). This is interesting, as ALLN would be predicted to inhibit the maturation of all DV PMs. If the model that has been proposed to account for antagonism between Ro 40-4388 and CQ is correct (12), we would predict that ALLN should also reduce the amounts of free heme available for CQ binding, thereby causing an antagonistic relationship. ALLN was also tested in combination with Ro 40-4388, revealing weakly antagonistic relationships (Fig. 1E; Table 4).
To investigate the interactions between the different classes of protease inhibitors, we also tested the aspartic protease inhibitor Ro 40-4388 or the maturase inhibitor ALLN in combination with the cysteine protease inhibitor E-64. Notably, the combination of Ro 40-4388 and E-64 had a highly antagonistic effect for all lines (Fig. 1F; Table 4). The responses to the ALLN and E-64 combination were mostly additive. However, Δpfpm1 and Δpfpm2 showed evidence of some antagonism at relatively high ALLN concentrations and low E-64 concentrations (Fig. 1G; Table 4).
Evaluation of malarial Hb degradation in the PM-KO lines.
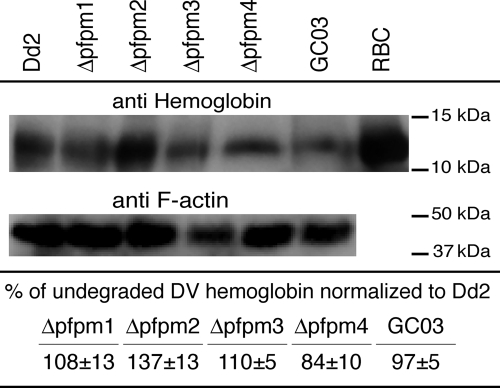
We were interested in assessing whether the loss of any DV PMs caused a measurable difference in the amount of undegraded Hb in the DV. To test this, parasites were synchronized and harvested at 30 h postinvasion (i.e., at the mid-trophozoite stage) on three separate occasions. Saponin-lysed parasite protein extracts were subjected to SDS-polyacrylamide gel electrophoresis, transferred to PVDF membranes, and probed with antibodies that recognize either intact Hb (but not digested heme fragments or hemozoin) or parasite F-actin. For each line, F-actin was used to normalize the protein loading and to derive the relative amounts of undigested Hb calculated from five separate gels. The normalized Hb amounts for each line were then compared to the amount of Hb present in the Dd2 control parental line. The biggest difference that we observed was with the Δpfpm2 line, which had ∼35% more undegraded Hb than the Dd2 line (Fig. 2). Δpfpm4 was also estimated to contain ∼15% less undegraded Hb relative to that in Dd2 (Fig. 2). We note that electron microscopy studies earlier revealed an abundance of multiple translucent vesicles inside the DV in Δpfpm4, which may reflect the reduced kinetics of Hb degradation in this line (48).
FIG. 2.
Western blot analysis of the levels of undigested Hb in PM-KO lines compared to the levels in control nonrecombinant lines and uninfected red blood cells. The Hb levels in the individual lines were normalized to the F-actin levels. The table at the bottom presents the mean Hb levels normalized to the F-actin levels, and the Hb levels are listed as a percentage of the levels present in the Dd2 parental line. Values were calculated from five separate Western blots and are shown as the means ± SEMs.
Effects of protease inhibitors on Hb degradation.
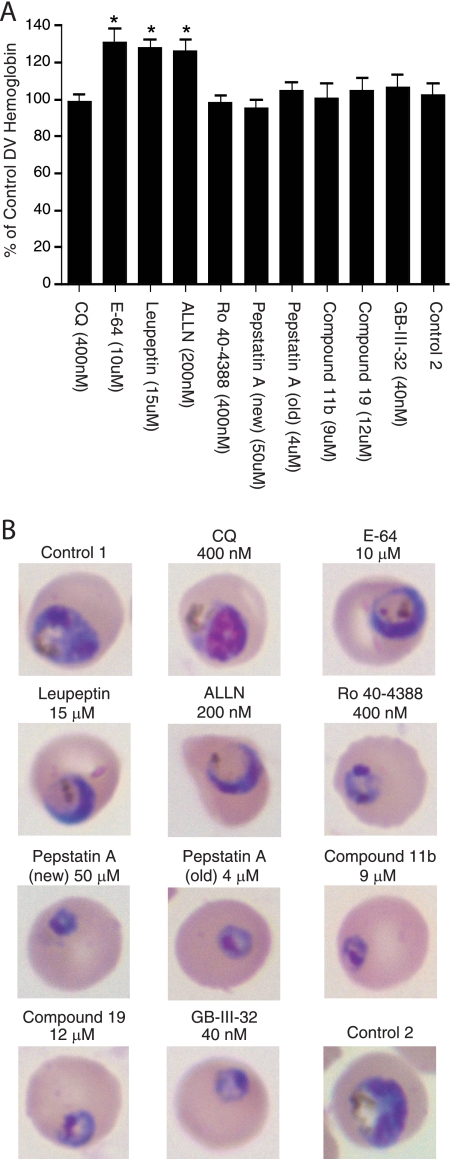
Earlier studies have shown that cysteine protease inhibitors can inhibit Hb degradation (50, 52). In the present study, we investigated whether this also applied to our panel of aspartic protease inhibitors. For this, we doubly synchronized CQ-resistant Dd2 parasites and inoculated identical cultures with late-ring-stage cultures (at ∼18 h postinvasion). The cultures were exposed to 2× the IC50 of selected compounds (see below) or were maintained as drug-free controls. The cultures were smeared 24 h later for imaging, and the parasites were lysed with saponin and harvested to obtain protein samples. We tested CQ, E-64, leupeptin, ALLN, Ro 40-4388, pepstatin A (new and old), compounds 11b and 19, and compound GB-III-32. Samples were electrophoresed on four separate occasions, transferred to PVDF membranes, and probed with anti-Hb or anti-F-actin antibodies. For each drug treatment, the mean amounts of Hb were calculated as the percentage of the total amount of Hb in parasites from one of the untreated wells (control 1) (Fig. 3). CQ treatment produced no inhibition of Hb proteolysis, which was the same result achieved with no treatment, which has been reported previously and which is consistent with its later time of action (29). In contrast, E-64, leupeptin, and ALLN all caused an ∼30% increase in the amount of undigested Hb (P < 0.05 compared to the results for the control 2 untreated well). This finding is consistent with the findings of earlier studies (32, 49). Under our conditions, none of the aspartic protease inhibitors tested affected Hb degradation, suggesting alternative modes of action.
FIG. 3.
(A) Effects of CQ and aspartic or cysteine protease inhibitors on the levels of undegraded Hb in the Dd2 parental line. The results show a significant increase (P < 0.05) in Hb levels following treatment of the parasites with E-64, leupeptin, or ALLN at the concentrations indicated. (B) Giemsa-stained images of parasites harvested after 24 h of treatment with the indicated compounds.
We also collected images of Giemsa-stained parasites collected after 24 h of incubation with each compound. Those shown in Fig. 3B were chosen as the most representative of ∼40 images taken for each treatment (additional examples are provided in Fig. S4 in the supplemental material). All images for the no-drug control show early to mid-schizont stages (∼40 h postinvasion), as evidenced by the multinucleated parasites in the two control samples. The CQ-treated Dd2 parasites progressed to late trophozoite and early schizont stages, by which time these CQ-resistant parasites had adopted a distinct cellular morphology characterized by dark staining and prominent nuclei. Striking morphological changes were associated with E-64- and leupeptin-treated parasites, which progressed to the mid- to late-trophozoite stages with enlarged DVs and reduced amounts of hemozoin, as reported previously (49). The findings for the ALLN-treated parasites were similar to those for parasites treated with E-64 and leupeptin, although ALLN appeared to additionally exhibit an effect at earlier stages that included early trophozoites with swollen DVs and, occasionally, late rings that did not progress in development. The aspartic protease inhibitors Ro 40-4388, pepstatin A (new and old), compounds 11b and 19, and compound GB-III-32 all prevented the parasites from progressing beyond the late ring or early trophozoite stage, with the parasites appearing compact with dark staining. Relatively large amounts of cell lysis and debris were often observed in the aspartic protease inhibitor-treated cultures. Our data agree with those in earlier reports that E-64, leupeptin, and ALLN directly inhibit Hb degradation and the DV function. Our data also suggest that the aspartic protease inhibitors do not primarily act upon DV PMs because they killed the parasites before most developed a visible DV, as had previously been reported with pepstatin A (old) (2). This leads us to postulate that these aspartic protease inhibitors kill parasites by targeting an essential non-DV PM that is expressed early during intraerythrocytic development.
DISCUSSION
In this study, we have leveraged the availability of P. falciparum lines genetically disrupted in one or more DV PM genes to probe the specificities of compounds identified as aspartic protease inhibitors on the basis of structural studies or enzymatic screens. This was examined for Ro 40-4388 and pepstatin A, as well as compound 11 and 19, and compound GB-III-32 (7, 19). Our results show that, in contrast to the data acquired with purified recombinant enzymes, the cellular activity of these inhibitors appears to be directed primarily against distinct protein targets not encompassed by the four DV PMs. Evidence that the true target(s) of many of these aspartic protease inhibitors may nevertheless be functionally related to the DV PMs comes from the in vitro enzyme inhibition data. In addition, a number of PM-KO lines also displayed enhanced susceptibility, suggesting that those PMs might be able to functionally complement the primary targets.
By analyzing the effects of these designated aspartic protease inhibitors on Hb degradation and assessing their impacts on the morphology and development of treated parasites, we conclude that many of these inhibitors do not affect the degradation of Hb. Instead, these compounds appear to act early, even prior to the visible formation of the DV, suggesting alternative modes of action. The challenge is now to identify the essential target(s). We note that of the six non-DV localized PMs in the P. falciparum genome, PM V, PM IX (PF14_0281), and PM X are expressed during the asexual blood stage (5, 33, 37). This creates the interesting and testable hypothesis that these aspartic protease inhibitors might be targeting one or more of these alternative PMs.
Our initial inhibitor assays focused on compounds that were shown to have inhibitory enzymatic activity against PM II in vitro. These compounds, named 11b, 13, 15, and 19, exhibited whole-cell inhibition at low-μM to double-digit-nM concentrations; and their activities were not significantly modified by the absence of any particular DV PM (Table 3). The results obtained with compounds GB-III-24, GB-III-28, and GB-III-32 confirm the potency of amidine-containing compounds against P. falciparum at nanomolar concentrations (30, 58). The overexpression of PM X resulted in a significant decrease in parasite susceptibility to GB-III-32, suggesting that this relatively unexplored PM might constitute a primary target (Table 3). Our findings for Ro 40-4388 and pepstatin A illustrate that enzyme screens and structural studies are not completely predictive of the specificity of a compound against the whole organism. Neither compound displayed a significant change in potency in any individual PM-KO line (Table 1), even though Ro 40-4388 was identified from a screen for compounds active against purified recombinant PM I (42) and the in vitro binding of pepstatin A to PM II has been defined at the structural level (57).
To further define how and when several of the aspartic protease inhibitors exhibit their antimalarial action, we carried out Hb degradation inhibition assays with synchronized parasites. The results suggest that these compounds do not impair Hb degradation but, rather, inhibit parasite development at an earlier stage of development, corresponding to the period of late rings to early trophozoites (Fig. 3). These studies suggest that these inhibitors might target a separate PM that is essential for survival and that is expressed earlier than the DV PMs. In our assays, only the cysteine protease inhibitors E-64, leupeptin, and ALLN inhibited Hb degradation and noticeably affected DV morphology (Fig. 3). We also used microscopy to assess the stage at which parasite development was arrested following treatment with the various inhibitors. CQ exhibited its effect later than the aspartic protease inhibitors, as evidenced by the ability of the parasites to proceed further in development when they were treated with CQ. The aspartic protease inhibitors caused substantial host cell and parasite lysis and inhibited parasites from progressing beyond late ring stages, and there was no evidence of DV formation. In contrast, treatment with the cysteine protease inhibitors E-64, leupeptin, and ALLN resulted in parasite arrest later, at the trophozoite stage. The most notable effect was the appearance of a swollen DV, comprising a large percentage of the parasite cell volume and apparently fewer hemozoin crystals (Fig. 3; see Fig. S4 in the supplemental material).
The results of the inhibition of Hb degradation studies combined with those of the isobologram analyses lead us to reconsider earlier interpretations about the mode of action of these aspartic protease inhibitors. Previously, the antagonism detected between Ro 40-4388 and CQ was proposed to result from the former inhibiting PM I-mediated heme degradation, resulting in the availability of less β-hematin receptor for the sequestration of CQ in the DV and leading to fewer CQ-β-hematin complexes and reduced CQ toxicity (10, 42, 43). We also observed antagonism between CQ and Ro 40-4388 and found a similar interaction between CQ and GB-III-32 (Fig. 1A and B). Nonetheless, our data suggest an alternative explanation, as antagonism was also observed in the Δpfpm1 line. The results of our time course studies, discussed above, lead us to propose instead that this antagonism might result from these protease inhibitors acting prior to the period of maximal DV development and CQ action. This differed from the results obtained with ALLN, an inhibitor of PM activation in mid- to late trophozoites, which was found to have a largely additive effect with CQ (Fig. 1D). In parallel studies, we also observed an additive effect between CQ and old pepstatin A (see Fig. S3A in the supplemental material). Our assays also revealed clear antagonism between Ro 40-4388 and E-64 (Fig. 1F). This finding is in contrast to that presented in an earlier report that indicated synergy between pepstatin A (old) and E-64 (24). One possibility that ties together these findings is that the effect observed between pepstatin A (old) and CQ in our study and between pepstatin A (old) and E-64 in the earlier work is due to a contaminant in the pepstatin A (old) preparation and not the aspartic protease inhibitor itself (39).
A striking finding from our studies was that the PM-KO lines displayed a greater change in their response to the cysteine protease inhibitors than to the aspartic protease inhibitors. The largest change was observed with the calpain inhibitor ALLN, which reportedly inhibits the maturase activity responsible for activating the DV PMs (18). ALLN became nearly twice as potent in the Δ3pfpm line (which retained only PM IV) and almost 10-fold more potent in the mutant with quadruple mutations, the Δ4pfpm line (Table 1). This strongly suggests that ALLN inhibits an additional function, for if its only biological effect were to inhibit processing of the four DV PMs, then Δ4pfpm parasites should be impervious to its action. E-64, leupeptin, and ALLN all demonstrated significant increases in potency in several of the PM-KO lines. One interpretation is that the PMs and falcipains share some cooperative functions in the DV and that the loss of PMs renders falcipain inhibition by cysteine protease inhibitors more effective. Evidence of interrelationships between the aspartic and cysteine proteases, as well as their inhibitors, also comes from earlier studies indicating that falcipain-2-KO parasites became highly susceptible to old or new preparations of the aspartic protease inhibitor pepstatin A (39, 54).
CQ accumulates in the DV of CQ-sensitive parasites in larger amounts than in CQ-resistant parasites and is believed to kill parasites through the inhibition of heme detoxification (10, 34, 36). The significant, 25% decrease in CQ and m-dCQ IC50s seen with the Δpfpm3 and Δpfpm4 parasite lines (Table 2), generated in the CQ-resistant Dd2 parasite background, demonstrates a close relationship between the role of those two PMs in Hb degradation and the mode of action of CQ. Their loss might alter the kinetics of heme release, its oxidation and conversion to β-hematin, or hemozoin biomineralization or might otherwise compromise the CQ resistance mechanism. Alternatively, the loss of PM III or PM IV might result in liberated globin chains being inefficiently processed into amino acids that can be transported out of the DV and alter the amounts and properties of di- or oligopeptide moieties in ways that enhance the uptake of CQ into the DV. We note that these differences in the individual CQ-resistant PM-KO lines were not observed in the multiple-PM- and falcipain-KO lines created in other studies, including Δ3pfpm and Δ4pfpm, all of which were generated in the CQ-sensitive 3D7 parasite (8, 38, 39). We also observed that the CQ-resistant Dd2 line and the CQ-sensitive 3D7 and GC03 lines differed in their responses to several of the selected aspartic and cysteine protease inhibitors (Tables 1 to 3; see Tables S1 and S2 in the supplemental material), presumably reflecting differences in their intracellular and/or DV physiology.
Our studies also provide new insights into the family of DV PMs. The data strongly suggest that PM IV (the original ortholog of the four DV PMs) and PM III (HAP) are functionally the most important (even though the majority of PM inhibitor screens and structure-guided syntheses have been directed toward PM I or PM II), because the genetic ablation of PM IV or PM III most often led to increased susceptibility to the protease inhibitors tested. The Δpfpm4 parasite was earlier reported to be the most difficult of all four DV PM-KO lines to generate and had the slowest growth phenotype (48). Slow growth was also reported in a separate double PM I- and PM IV-KO line (38). Both these lines that included a PM IV KO displayed increased sensitivity to E-64. The response of Δpfpm4 to drug combinations also frequently differed from the responses of the other PM-KO lines (Fig. 1; Table 4; see Fig. S1 in the supplemental material). The genetic ablation of PM III led to the most significant decreases in IC50s in response to the greatest number of compounds tested, with this being observed with E-64, leupeptin, ALLN, CQ, and m-dCQ (Tables 1 and 2).
Δpfpm2 clearly demonstrated the overlapping modes of action of the aspartic and cysteine protease inhibitors, as this line showed a significantly increased sensitivity to the cysteine protease E-64 (Table 1). Δpfpm2 also accumulated the largest amount of undegraded Hb (Fig. 2). These data support earlier findings that purified PM II protein can initiate Hb degradation in an acidic environment similar to that of the DV (26). The Δpfpm1 parasite had no significant responses to any of the drugs tested, suggesting that PM I may be the least important of all the DV PMs (Tables 1, 2, and 3). Our findings that individual PM-KO lines differ quite markedly in their susceptibilities to protease inhibitors and CQ suggest that DV PMs have evolved some unique functions that are not fully compensated for by other PMs or falcipains. Only for E-64 and ALLN were the Δ3pfpm and Δ4pfpm parasites (generated in 3D7 parasites) similar to certain individual PM-KO lines (generated in Dd2 parasites) in terms of showing altered levels of susceptibility. Differences in the genetic backgrounds and the DV physiologies of the Dd2 and 3D7 lines may, in part, account for this, in addition to their defined differences in DV PM content. Morphologically, we noticed that the Δ3pfpm and Δ4pfpm parasites had substantially reduced DV volumes and to have seemingly normal amounts of hemozoin compared to the DV volumes and amounts of hemozoin in nontransformed 3D7 parasites (data not shown), yet their growth rates were clearly different, with the relatively normal growth in Δ3pfpm contrasting with poor growth in Δ4pfpm, even in normal (amino acid-rich) medium (8). This supports the idea that PM IV is able to maintain a normal DV function in the absence of the other three PMs.
On the basis of the findings of our studies, we propose that the aspartic protease inhibitors tested in the present study act against the parasite by blocking the action of one or more PMs distinct from PM I to PM IV. The major targets are likely to be expressed in early developmental stages, as suggested by the lack of an effect on Hb degradation, frequent antagonism with CQ, and the killing of parasites in those stages that precede the formation of mature DVs (Fig. 1 and 3). This leads us to suspect a role for PM V, PM IX, or PM X and to highlight their potential as putative candidate targets. The identification of specific and potent PM inhibitors remains an important goal that can benefit from research into adaptive inhibitors that have a high affinity for an important PM (e.g., a non-DV PM such as PM V, PM IX, or PM X) and that can also inhibit other PM family members (44). Clearly, a detailed understanding of the structural and functional differences between the individual PMs and of the mode of action of PM inhibitors will be imperative for future antimalarial drug discovery efforts that target this family of aspartic proteases.
Supplementary Material
Acknowledgments
We extend our sincere thanks to Eric Ekland for his thoughtful and constructive comments on the manuscript and Celeste Li for help with manuscript preparation. We gratefully acknowledge Patrick Bray, Akhil Vaidya, Karolina Ersmark, Karl Werbovetz, Gautam Bhattacharya, Ben Dunn, and Thomas Wellems for providing compounds or parental lines. We thank Fred Bonilla for providing us with the Δ3pfpm and Δ4pfpm lines.
Funding for this work was provided in part by an Investigator in Pathogenesis of Infectious Diseases from the Burroughs Wellcome Fund (to D.A.F.). We also gratefully acknowledge financial support for Pedro Moura from the CMBG training grant (grant GM 04791; Pamela Stanley, principal investigator, Albert Einstein College of Medicine, New York, NY).
Footnotes
Published ahead of print on 14 September 2009.
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1.Andrews, K. T., D. P. Fairlie, P. K. Madala, J. Ray, D. M. Wyatt, P. M. Hilton, L. A. Melville, L. Beattie, D. L. Gardiner, R. C. Reid, M. J. Stoermer, T. Skinner-Adams, C. Berry, and J. S. McCarthy. 2006. Potencies of human immunodeficiency virus protease inhibitors in vitro against Plasmodium falciparum and in vivo against murine malaria. Antimicrob. Agents Chemother. 50:639-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bailly, E., R. Jambou, J. Savel, and G. Jaureguiberry. 1992. Plasmodium falciparum: differential sensitivity in vitro to E-64 (cysteine protease inhibitor) and pepstatin A (aspartyl protease inhibitor). J. Protozool. 39:593-599. [DOI] [PubMed] [Google Scholar]
- 3.Banerjee, R., S. E. Francis, and D. E. Goldberg. 2003. Food vacuole plasmepsins are processed at a conserved site by an acidic convertase activity in Plasmodium falciparum. Mol. Biochem. Parasitol. 129:157-165. [DOI] [PubMed] [Google Scholar]
- 4.Banerjee, R., and D. E. Goldberg. 2001. The Plasmodium food vacuole, p. 43-63. In P. J. Rosenthal (ed.), Antimalarial chemotherapy. Humana Press, Totowa, NJ.
- 5.Banerjee, R., J. Liu, W. Beatty, L. Pelosof, M. Klemba, and D. E. Goldberg. 2002. Four plasmepsins are active in the Plasmodium falciparum food vacuole, including a protease with an active-site histidine. Proc. Natl. Acad. Sci. USA 99:990-995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bell, A. 2005. Antimalarial drug synergism and antagonism: mechanistic and clinical significance. FEMS Microbiol. Lett. 253:171-184. [DOI] [PubMed] [Google Scholar]
- 7.Bhattacharya, G. 2005. Activity of amidine-containing diphenylureas against P. falciparum. Lett. Drug Des. Discov. 2:162-164. [Google Scholar]
- 8.Bonilla, J. A., T. D. Bonilla, C. A. Yowell, H. Fujioka, and J. B. Dame. 2007. Critical roles for the digestive vacuole plasmepsins of Plasmodium falciparum in vacuolar function. Mol. Microbiol. 65:64-75. [DOI] [PubMed] [Google Scholar]
- 9.Boss, C., S. Richard-Bildstein, T. Weller, W. Fischli, S. Meyer, and C. Binkert. 2003. Inhibitors of the Plasmodium falciparum parasite aspartic protease plasmepsin II as potential antimalarial agents. Curr. Med. Chem. 10:883-907. [DOI] [PubMed] [Google Scholar]
- 10.Bray, P. G., O. Janneh, K. J. Raynes, M. Mungthin, H. Ginsburg, and S. A. Ward. 1999. Cellular uptake of chloroquine is dependent on binding to ferriprotoporphyrin IX and is independent of NHE activity in Plasmodium falciparum. J. Cell Biol. 145:363-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bray, P. G., M. Mungthin, R. G. Ridley, and S. A. Ward. 1998. Access to hematin: the basis of chloroquine resistance. Mol. Pharmacol. 54:170-179. [DOI] [PubMed] [Google Scholar]
- 12.Bray, P. G., and S. A. Ward. 1998. A comparison of the phenomenology and genetics of multidrug resistance in cancer cells and quinoline resistance in Plasmodium falciparum. Pharmacol. Ther. 77:1-28. [DOI] [PubMed] [Google Scholar]
- 13.Coombs, G. H., D. E. Goldberg, M. Klemba, C. Berry, J. Kay, and J. C. Mottram. 2001. Aspartic proteases of Plasmodium falciparum and other parasitic protozoa as drug targets. Trends Parasitol. 17:532-537. [DOI] [PubMed] [Google Scholar]
- 14.Cooper, R. A., M. T. Ferdig, X. Z. Su, L. M. Ursos, J. Mu, T. Nomura, H. Fujioka, D. A. Fidock, P. D. Roepe, and T. E. Wellems. 2002. Alternative mutations at position 76 of the vacuolar transmembrane protein PfCRT are associated with chloroquine resistance and unique stereospecific quinine and quinidine responses in Plasmodium falciparum. Mol. Pharmacol. 61:35-42. [DOI] [PubMed] [Google Scholar]
- 15.Dahlgren, A., I. Kvarnstrom, L. Vrang, E. Hamelink, A. Hallberg, A. Rosenquist, and B. Samuelsson. 2003. New inhibitors of the malaria aspartyl proteases plasmepsin I and II. Bioorg. Med. Chem. 11:3423-3437. [DOI] [PubMed] [Google Scholar]
- 16.Dame, J. B., C. A. Yowell, L. Omara-Opyene, J. M. Carlton, R. A. Cooper, and T. Li. 2003. Plasmepsin 4, the food vacuole aspartic proteinase found in all Plasmodium spp. infecting man. Mol. Biochem. Parasitol. 130:1-12. [DOI] [PubMed] [Google Scholar]
- 17.Desjardins, R. E., C. J. Canfield, J. D. Haynes, and J. D. Chulay. 1979. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob. Agents Chemother. 16:710-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drew, M. E., R. Banerjee, E. W. Uffman, S. Gilbertson, P. J. Rosenthal, and D. E. Goldberg. 2008. Plasmodium food vacuole plasmepsins are activated by falcipains. J. Biol. Chem. 283:12870-12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ersmark, K., I. Feierberg, S. Bjelic, E. Hamelink, F. Hackett, M. J. Blackman, J. Hulten, B. Samuelsson, J. Aqvist, and A. Hallberg. 2004. Potent inhibitors of the Plasmodium falciparum enzymes plasmepsin I and II devoid of cathepsin D inhibitory activity. J. Med. Chem. 47:110-122. [DOI] [PubMed] [Google Scholar]
- 20.Ferdig, M. T., R. A. Cooper, J. Mu, B. Deng, D. A. Joy, X. Z. Su, and T. E. Wellems. 2004. Dissecting the loci of low-level quinine resistance in malaria parasites. Mol. Microbiol. 52:985-997. [DOI] [PubMed] [Google Scholar]
- 21.Fidock, D. A., T. Nomura, and T. E. Wellems. 1998. Cycloguanil and its parent compound proguanil demonstrate distinct activities against Plasmodium falciparum malaria parasites transformed with human dihydrofolate reductase. Mol. Pharmacol. 54:1140-1147. [DOI] [PubMed] [Google Scholar]
- 22.Fidock, D. A., and T. E. Wellems. 1997. Transformation with human dihydrofolate reductase renders malaria parasites insensitive to WR99210 but does not affect the intrinsic activity of proguanil. Proc. Natl. Acad. Sci. USA 94:10931-10936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fivelman, Q. L., I. S. Adagu, and D. C. Warhurst. 2004. Modified fixed-ratio isobologram method for studying in vitro interactions between atovaquone and proguanil or dihydroartemisinin against drug-resistant strains of Plasmodium falciparum. Antimicrob. Agents Chemother. 48:4097-4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Francis, S. E., I. Y. Gluzman, A. Oksman, A. Knickerbocker, R. Mueller, M. L. Bryant, D. R. Sherman, D. G. Russell, and D. E. Goldberg. 1994. Molecular characterization and inhibition of a Plasmodium falciparum aspartic hemoglobinase. EMBO J. 13:306-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Francis, S. E., D. J. Sullivan, Jr., and D. E. Goldberg. 1997. Hemoglobin metabolism in the malaria parasite Plasmodium falciparum. Annu. Rev. Microbiol. 51:97-123. [DOI] [PubMed] [Google Scholar]
- 26.Gluzman, I. Y., S. E. Francis, A. Oksman, C. E. Smith, K. L. Duffin, and D. E. Goldberg. 1994. Order and specificity of the Plasmodium falciparum hemoglobin degradation pathway. J. Clin. Investig. 93:1602-1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haque, T. S., A. G. Skillman, C. E. Lee, H. Habashita, I. Y. Gluzman, T. J. Ewing, D. E. Goldberg, I. D. Kuntz, and J. A. Ellman. 1999. Potent, low-molecular-weight non-peptide inhibitors of malarial aspartyl protease plasmepsin II. J. Med. Chem. 42:1428-1440. [DOI] [PubMed] [Google Scholar]
- 28.He, Z., L. Qin, L. Chen, N. Peng, J. You, and X. Chen. 2008. Synergy of human immunodeficiency virus protease inhibitors with chloroquine against Plasmodium falciparum in vitro and Plasmodium chabaudi in vivo. Antimicrob. Agents Chemother. 52:2653-2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoppe, H. C., D. A. van Schalkwyk, U. I. Wiehart, S. A. Meredith, J. Egan, and B. W. Weber. 2004. Antimalarial quinolines and artemisinin inhibit endocytosis in Plasmodium falciparum. Antimicrob. Agents Chemother. 48:2370-2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ismail, M. A., R. Brun, J. D. Easterbrook, F. A. Tanious, W. D. Wilson, and D. W. Boykin. 2003. Synthesis and antiprotozoal activity of aza-analogues of furamidine. J. Med. Chem. 46:4761-4769. [DOI] [PubMed] [Google Scholar]
- 31.Jiang, S., S. T. Prigge, L. Wei, Y. Gao, T. H. Hudson, L. Gerena, J. B. Dame, and D. E. Kyle. 2001. New class of small nonpeptidyl compounds blocks Plasmodium falciparum development in vitro by inhibiting plasmepsins. Antimicrob. Agents Chemother. 45:2577-2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim, Y. M., M. H. Lee, T. G. Piao, J. W. Lee, J. H. Kim, S. Lee, K. M. Choi, J. H. Jiang, T. U. Kim, and H. Park. 2006. Prodomain processing of recombinant plasmepsin II and IV, the aspartic proteases of Plasmodium falciparum, is auto- and trans-catalytic. J. Biochem. 139:189-195. [DOI] [PubMed] [Google Scholar]
- 33.Klemba, M., and D. E. Goldberg. 2005. Characterization of plasmepsin V, a membrane-bound aspartic protease homolog in the endoplasmic reticulum of Plasmodium falciparum. Mol. Biochem. Parasitol. 143:183-191. [DOI] [PubMed] [Google Scholar]
- 34.Lakshmanan, V., P. G. Bray, D. Verdier-Pinard, D. J. Johnson, P. Horrocks, R. A. Muhle, G. E. Alakpa, R. H. Hughes, S. A. Ward, D. J. Krogstad, A. B. S. Sidhu, and D. A. Fidock. 2005. A critical role for PfCRT K76T in Plasmodium falciparum verapamil-reversible chloroquine resistance. EMBO J. 24:2294-2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee, M. C., P. A. Moura, E. A. Miller, and D. A. Fidock. 2008. Plasmodium falciparum Sec24 marks transitional ER that exports a model cargo via a diacidic motif. Mol. Microbiol. 68:1535-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leed, A., K. DuBay, L. M. Ursos, D. Sears, A. C. De Dios, and P. D. Roepe. 2002. Solution structures of antimalarial drug-heme complexes. Biochemistry 41:10245-10255. [DOI] [PubMed] [Google Scholar]
- 37.Le Roch, K. G., Y. Zhou, P. L. Blair, M. Grainger, J. K. Moch, J. D. Haynes, P. De La Vega, A. A. Holder, S. Batalov, D. J. Carucci, and E. A. Winzeler. 2003. Discovery of gene function by expression profiling of the malaria parasite life cycle. Science 301:1503-1508. [DOI] [PubMed] [Google Scholar]
- 38.Liu, J., I. Y. Gluzman, M. E. Drew, and D. E. Goldberg. 2005. The role of Plasmodium falciparum food vacuole plasmepsins. J. Biol. Chem. 280:1432-1437. [DOI] [PubMed] [Google Scholar]
- 39.Liu, J., E. S. Istvan, I. Y. Gluzman, J. Gross, and D. E. Goldberg. 2006. Plasmodium falciparum ensures its amino acid supply with multiple acquisition pathways and redundant proteolytic enzyme systems. Proc. Natl. Acad. Sci. USA 103:8840-8845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mauritz, J. M., A. Esposito, H. Ginsburg, C. F. Kaminski, T. Tiffert, and V. L. Lew. 2009. The homeostasis of Plasmodium falciparum-infected red blood cells. PLoS Comput. Biol. 5:e1000339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mehlotra, R. K., H. Fujioka, P. D. Roepe, O. Janneh, L. M. Ursos, V. Jacobs-Lorena, D. T. McNamara, M. J. Bockarie, J. W. Kazura, D. E. Kyle, D. A. Fidock, and P. A. Zimmerman. 2001. Evolution of a unique Plasmodium falciparum chloroquine-resistance phenotype in association with pfcrt polymorphism in Papua New Guinea and South America. Proc. Natl. Acad. Sci. USA 98:12689-12694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moon, R. P., L. Tyas, U. Certa, K. Rupp, D. Bur, C. Jacquet, H. Matile, H. Loetscher, F. Grueninger-Leitch, J. Kay, B. M. Dunn, C. Berry, and R. G. Ridley. 1997. Expression and characterisation of plasmepsin I from Plasmodium falciparum. Eur. J. Biochem. 244:552-560. [DOI] [PubMed] [Google Scholar]
- 43.Mungthin, M., P. G. Bray, R. G. Ridley, and S. A. Ward. 1998. Central role of hemoglobin degradation in mechanisms of action of 4-aminoquinolines, quinoline methanols, and phenanthrene methanols. Antimicrob. Agents Chemother. 42:2973-2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nezami, A., and E. Freire. 2002. The integration of genomic and structural information in the development of high affinity plasmepsin inhibitors. Int. J. Parasitol. 32:1669-1676. [DOI] [PubMed] [Google Scholar]
- 45.Nezami, A., T. Kimura, K. Hidaka, A. Kiso, J. Liu, Y. Kiso, D. E. Goldberg, and E. Freire. 2003. High-affinity inhibition of a family of Plasmodium falciparum proteases by a designed adaptive inhibitor. Biochemistry 42:8459-8464. [DOI] [PubMed] [Google Scholar]
- 46.Noteberg, D., E. Hamelink, J. Hulten, M. Wahlgren, L. Vrang, B. Samuelsson, and A. Hallberg. 2003. Design and synthesis of plasmepsin I and plasmepsin II inhibitors with activity in Plasmodium falciparum-infected cultured human erythrocytes. J. Med. Chem. 46:734-746. [DOI] [PubMed] [Google Scholar]
- 47.Ohrt, C., G. D. Willingmyre, P. Lee, C. Knirsch, and W. Milhous. 2002. Assessment of azithromycin in combination with other antimalarial drugs against Plasmodium falciparum in vitro. Antimicrob. Agents Chemother. 46:2518-2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Omara-Opyene, A. L., P. A. Moura, C. R. Sulsona, J. A. Bonilla, C. A. Yowell, H. Fujioka, D. A. Fidock, and J. B. Dame. 2004. Genetic disruption of the Plasmodium falciparum digestive vacuole plasmepsins demonstrates their functional redundancy. J. Biol. Chem. 279:54088-54096. [DOI] [PubMed] [Google Scholar]
- 49.Rosenthal, P. J. 1995. Plasmodium falciparum: effects of proteinase inhibitors on globin hydrolysis by cultured malaria parasites. Exp. Parasitol. 80:272-281. [DOI] [PubMed] [Google Scholar]
- 50.Rosenthal, P. J., J. H. McKerrow, M. Aikawa, H. Nagasawa, and J. H. Leech. 1988. A malarial cysteine proteinase is necessary for hemoglobin degradation by Plasmodium falciparum. J. Clin. Investig. 82:1560-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Salas, F., J. Fichmann, G. K. Lee, M. D. Scott, and P. J. Rosenthal. 1995. Functional expression of falcipain, a Plasmodium falciparum cysteine proteinase, supports its role as a malarial hemoglobinase. Infect. Immun. 63:2120-2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sijwali, P. S., K. Kato, K. B. Seydel, J. Gut, J. Lehman, M. Klemba, D. E. Goldberg, L. H. Miller, and P. J. Rosenthal. 2004. Plasmodium falciparum cysteine protease falcipain-1 is not essential in erythrocytic stage malaria parasites. Proc. Natl. Acad. Sci. USA 101:8721-8726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sijwali, P. S., J. Koo, N. Singh, and P. J. Rosenthal. 2006. Gene disruptions demonstrate independent roles for the four falcipain cysteine proteases of Plasmodium falciparum. Mol. Biochem. Parasitol. 150:96-106. [DOI] [PubMed] [Google Scholar]
- 54.Sijwali, P. S., and P. J. Rosenthal. 2004. Gene disruption confirms a critical role for the cysteine protease falcipain-2 in hemoglobin hydrolysis by Plasmodium falciparum. Proc. Natl. Acad. Sci. USA 101:4384-4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sijwali, P. S., B. R. Shenai, J. Gut, A. Singh, and P. J. Rosenthal. 2001. Expression and characterization of the Plasmodium falciparum haemoglobinase falcipain-3. Biochem. J. 360:481-489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Silva, A. M., A. Y. Lee, J. W. Erickson, and D. E. Goldberg. 1998. Structural analysis of plasmepsin II. A comparison with human aspartic proteases. Adv. Exp. Med. Biol. 436:363-373. [PubMed] [Google Scholar]
- 57.Silva, A. M., A. Y. Lee, S. V. Gulnik, P. Maier, J. Collins, T. N. Bhat, P. J. Collins, R. E. Cachau, K. E. Luker, I. Y. Gluzman, S. E. Francis, A. Oksman, D. E. Goldberg, and J. W. Erickson. 1996. Structure and inhibition of plasmepsin II, a hemoglobin-degrading enzyme from Plasmodium falciparum. Proc. Natl. Acad. Sci. USA 93:10034-10039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stead, A. M., P. G. Bray, I. G. Edwards, H. P. DeKoning, B. C. Elford, P. A. Stocks, and S. A. Ward. 2001. Diamidine compounds: selective uptake and targeting in Plasmodium falciparum. Mol. Pharmacol. 59:1298-1306. [DOI] [PubMed] [Google Scholar]
- 59.Tucker, T. J., W. C. Lumma, Jr., L. S. Payne, J. M. Wai, S. J. de Solms, E. A. Giuliani, P. L. Darke, J. C. Heimbach, J. A. Zugay, W. A. Schleif, J. C. Quintero, E. A. Emini, J. R. Huff, and P. S. Anderson. 1992. A series of potent HIV-1 protease inhibitors containing a hydroxyethyl secondary amine transition state isostere: synthesis, enzyme inhibition, and antiviral activity. J. Med. Chem. 35:2525-2533. [DOI] [PubMed] [Google Scholar]
- 60.World Health Organization. 2003. Africa malaria report. World Health Organization, Geneva, Switzerland.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.