Abstract
The γ134.5 protein, a virulence factor of herpes simplex viruses, redirects protein phosphatase 1 to dephosphorylate the α subunit of translation initiation factor 2 (eIF2α). Additionally, it inhibits the induction of antiviral genes by TANK-binding kinase 1. Nevertheless, its precise role in vivo remains to be established. Here we show that eIF2α dephosphorylation by γ134.5 is crucial for viral neuroinvasion. V193E and F195L substitutions in γ134.5 abrogate viral replication in the eye and spread to the trigeminal ganglia and brain. Intriguingly, inhibition of antiviral gene induction by γ134.5 is not sufficient to exhibit viral virulence.
Herpes simplex viruses (HSV) are human pathogens responsible for a variety of diseases, including genital herpes, keratitis, and encephalitis (28). It has been established that γ134.5 is essential for viral virulence (7, 19). HSV γ134.5 is a multifunctional protein involved in different processes of HSV infection, such as dephosphorylation of the α subunit of translation initiation factor 2 (eIF2α), major histocompatibility complex class II expression, autophagy, and virus egress (2, 14, 22, 25). We have noted that γ134.5 suppresses the maturation of dendritic cells and the induction of antiviral genes, where it targets TANK-binding kinase 1 (TBK1), a key component of Toll-like receptor (TLR)-related pathways (17, 26). Notably, in HSV-infected cells, γ134.5 also prevents translation arrest mediated by the double-stranded RNA-dependent protein kinase (PKR) (6, 8). This is accomplished by γ134.5 recruiting protein phosphatase 1 (PP1) to dephosphorylate eIF2α (13, 14). In this context, it has been demonstrated that the γ134.5 null mutant is virulent in PKR−/− mice but not in wild-type mice (7, 18). PKR is a component integrating innate signaling pathways leading to translation arrest and the expression of proinflammatory cytokines (11, 12, 16, 21). In addition to eIF2α phosphorylation, PKR has a broad range of regulatory functions, which include the activation of NF-κB and interferon regulatory factor 3 (IRF3) in response to signals of TLRs or cytosolic RNA sensors (11, 12, 16, 20, 21, 30). Accordingly, deletion of PKR not only impairs eIF2α phosphorylation but also has a compounding effect on the aforementioned events. Further, removal of γ134.5 from HSV may disrupt more than one viral function. This study was undertaken to further define the role of γ134.5 in HSV neuroinvasion.
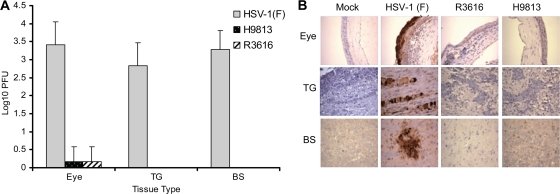
Early studies revealed that γ134.5 is a corneal virulence factor (1, 27). Since γ134.5 is thought to function via the PP1 binding and effector domains (3), we asked whether the PP1 binding domain had a role in HSV infection in vivo. We focused on a recombinant virus, H9813, which bears V193E and F195L substitutions in γ134.5 (4). Such mutations disrupt the interaction of γ134.5 and PP1 (29). As controls, we included wild-type HSV-1(F) and R3616, which has deletion of the entire γ134.5 gene (7). Mice were infected with 4 × 105 PFU of HSV-1(F), R3616, or H9813 through bilateral corneal scarification. At 5 days postinfection, viral yields in different tissues were determined. Figure 1A shows that HSV-1(F) replicated efficiently in the eye, with a titer of 2.6 × 103 PFU. Additionally, the virus was able to travel to the trigeminal ganglia and brain stem, reaching titers of 6.7 × 102 PFU and 1.9 × 103 PFU, respectively. In contrast, R3616 failed to replicate in the eye, with a titer of 1.5 PFU. Infectious viruses were not detectable in the trigeminal ganglia and brain stem. A similar phenotype was seen for H9813. The results of immunohistochemical staining of tissue sections correlated with these phenotypes (Fig. 1B). All mice infected with HSV-1(F) had positive staining in the three tissue types tested. For the R3616 and H9813 viruses, positive staining was observed only in the eye, for two and one out of six mice, respectively. Trigeminal ganglia and brain stem tissues were negative for both viruses.
FIG. 1.
(A) Viral replication in the eye, trigeminal ganglia, and brain. Groups of 6-week-old female BALB/c mice were mock infected or infected with HSV-1(F), R3616, or H9813 at 4 × 105 PFU through corneal scarification. At 5 days postinfection, eye, trigeminal ganglia (TG), and brain stem (BS) tissues were collected to determine virus yields. Data are expressed as means ± standard deviations for six mice for each group. (B) Immunohistochemistry staining of mouse tissues. The sections from eye, trigeminal ganglia, and brain stem tissues described above were reacted with anti-HSV-1 antibody, and immunohistochemistry was performed. Specific HSV-1 staining is shown in brown. Representative images from each mouse group were chosen for the panels.
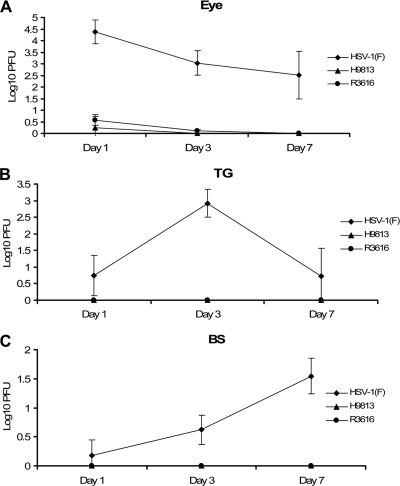
We further examined the kinetics of in vivo viral replication on days one, three, and seven. Data in Fig. 2A show that HSV-1(F) replicated efficiently in the eye on day one, reaching a titer of 2.4 × 104 PFU. As infection continued, HSV-1(F) maintained viral yields at 1.1 × 103 PFU and 3.2 × 102 PFU in the eye on days three and seven. The gradual reduction of viral replication probably resulted from the activation of host responses as infection continued. In this period, neither R3616 nor H9813 replicated at an appreciable level from the onset, reaching a meager titer of 1.8 PFU over the course of infection. In the trigeminal ganglia (Fig. 2B), HSV-1(F) appeared on day one with a titer of 5.6 PFU and replicated to a peak titer of 8.3 × 102 PFU on day three, indicating that wild-type virus spread efficiently to the trigeminal ganglia. By day seven, the viral titer was brought down to 5.2 PFU. Similar to results for R3616, H9813 was unable to reach detectable levels in this tissue. In the brain (Fig. 2C), HSV-1(F) had average viral titers of 1.5, 4.2, and 3.5 × 101 PFU, respectively, on days one, three, and seven. In contrast, neither R3616 nor H9813 was detectable in the brain. Therefore, subtle mutations in γ134.5 prevented viral replication in the peripheral tissue and subsequent spread to the central nervous system. Our results provided evidence that V193 and F195 of γ134.5 indeed were essential for HSV neuroinvasion in vivo.
FIG. 2.
Kinetics of viral replication in vivo. Groups of 6-week-old female BALB/c mice were mock infected or infected with HSV-1(F), R3616, or H9813 via bilateral corneal scarification with 4 × 105 PFU of virus. Mice from each group were sacrificed on days 1, 3, 5, and 7. At this time, tissue samples from the eye (A), trigeminal ganglia (TG) (B), or brain stem (BS) (C) were used for titration on Vero cells. Data are expressed as means ± standard deviations for five mice for each group.
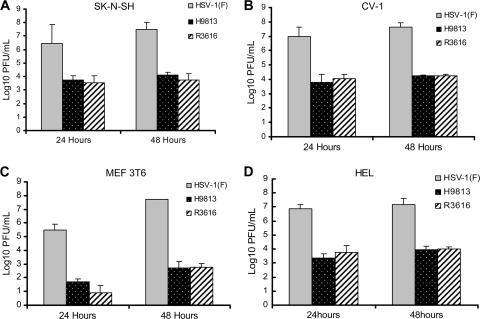
It has been reported that in response to HSV infection, a number of antiviral mechanisms operate in a cell type- and time-dependent manner (24). Remarkably, TLR3 and the cytosolic sensors RIG-I, MDA5, and DAI relay signals to induce the interferon response (15, 23, 31). In this process, TBK1 activates IRF3 to induce antiviral responses. In addition, PKR, whose expression is elevated by interferon, is activated during HSV infection (6). To further characterize γ134.5, we examined viral growth in different mammalian cell lines. Cells were infected with HSV-1(F), R3616, or H9813 (0.05 PFU/ml), and virus yields were determined. The results displayed in Fig. 3A show that HSV-1(F) replicated to a titer of 3.0 × 106 PFU/ml 24 h after infection in SK-N-SH human neural cells. High levels of viral replication were maintained at 48 h, reaching 3.0 × 107 PFU/ml. Replication of R3616 was drastically reduced, with titers of 3.6 × 103 PFU/ml and 5.8 × 103 PFU/ml at 24 and 48 h, respectively. H9813 replicated similarly to R3616 over the same time period, reaching titers of 5.4 × 103 PFU/ml at 24 h and 1.2 × 104 PFU/ml at 48 h. Similar growth patterns were observed in CV-1 kidney epithelial cells (Fig. 3B), 3T6 mouse embryonic fibroblasts (Fig. 3C), and human embryonic lung (HEL) fibroblasts (Fig. 3D). In each case, H9813 replicated poorly, mirroring the growth patterns of R3616 virus. Thus, V193 and F195 in γ134.5 are required to overcome the inhibitory effect of host cells on HSV replication. Given that valine and phenylalanine in the PP1 binding motif are conserved among other PP1 binding proteins (9), these results suggest that the PP1 binding motif in γ134.5 may represent a functional module which dictates the outcome of HSV infection and pathogenesis.
FIG. 3.
Viral growth properties in mammalian cell lines. Confluent monolayers of SK-N-SH (A), CV-1 (B), MEF 3T6 (C), or HEL (D) cells were infected with HSV-1(F), R3616, or H9813 at 0.05 PFU per cell and incubated at 37°C. Viruses were harvested at 24 and 48 h postinfection. Samples were then freeze-thawed three times and titrated on Vero cells. Representative experiments done in triplicate are shown.
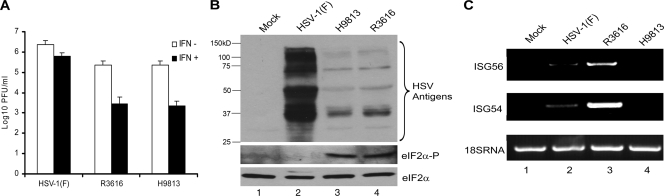
To define the mechanistic basis of γ134.5 action, we analyzed viral growth and response to type I interferon (IFN) in Vero cells, which are deficient in alpha/beta IFN (IFN-α/β) and IRF3 production (5, 10). Cells, untreated or pretreated with IFN-α (200 U/ml), were infected with viruses at 0.05 PFU, and viral yields were determined. As shown in Fig. 4A, in the absence of IFN all viruses replicated efficiently, with titers ranging from 2.3 × 105 to 2.3 × 106 PFU/ml. V193E and F195L substitutions in γ134.5 or removal of γ134.5 had a marginal effect on HSV replication. This phenotype likely resulted from a defect in type I IFN or IRF3 production in Vero cells. When treated with type I IFN, only wild-type HSV-1(F) was able to replicate efficiently, reaching a titer of 6.2 × 105 PFU/ml. In sharp contrast, R3616 and H9813 viruses were highly sensitive to IFN treatment, with a titer reduced to 2.8 × 103 PFU/ml. Thus, the PP1 binding motif contributes to HSV resistance to type I IFN.
FIG. 4.
(A) Viral response to IFN. Monolayers of Vero cells were either untreated or pretreated with IFN-α (200 U/ml; Sigma) for 20 h. Cells were then infected with indicated viruses at 0.05 PFU per cell and incubated at 37°C. At 24 h postinfection, cells were harvested and freeze-thawed three times, and virus yields were determined by titration. (B) Synthesis of viral proteins and eIF2α dephosphorylation. Monolayers of HEL fibroblasts were either mock infected or infected with viruses as indicated (5 PFU/cell). At 18 h after infection, lysates of cells were subjected to electrophoresis and reacted with antibodies against mixed HSV-1 antigens (Dako Coporation), eIF2α, and phosphorylated eIF2α (Cell signaling Tech). Size markers are listed on the left. (C) Viral induction of antiviral genes. HEL fibroblast cells were mock infected or infected with HSV-1(F), R3616, or H9813 (5 PFU/cell) as indicated. At 6 h postinfection, total RNA was extracted from cells and subjected to reverse transcription-PCR amplification and electrophoresis for ISG56, ISG54, and 18s rRNA.
In HSV-infected cells, γ134.5 recruits PP1 to form a high-molecular-weight complex that dephosphorylate eIF2α (13). We evaluated eIF2α phosphorylation and viral protein synthesis. HEL fibroblasts were mock infected or infected with viruses. At 18 h postinfection, lysates of cells were subjected to Western blot analysis with antibodies against eIF2α, phosphorylated eIF2α, and HSV antigens. As indicated in Fig. 4B, HSV-1(F)-infected cells exhibited efficient viral polypeptide synthesis without detectable eIF2α phosphorylation. In contrast, both H9813- and R3616-infected cells had little viral protein synthesis, which paralleled increased eIF2α phosphorylation. Hence, Val193 and F195 in γ134.5 mediate eIF2α dephosphorylation, which is linked to viral protein synthesis and resistance to IFN-α/β.
Because γ134.5 also blocks the induction of antiviral genes by targeting TBK1 (26), we asked whether the interaction of γ134.5 with PP1 contributed to this process during HSV infection. HEL fibroblasts were mock infected or infected with viruses. At 6 h postinfection, reverse transcription-PCR analysis was performed to evaluate the expression of ISG54, ISG56, and 18S rRNA. As seen in Fig. 4C, R3616 significantly increased both ISG54 and ISG56 mRNA compared to HSV-1(F). H9813 was able to block the induction of these antiviral genes. There was no detectable accumulation of ISG54 or ISG56 mRNA. Consistently, like wild-type virus, H9813 also inhibited IRF3 nuclear translocation of IRF3, whereas R3616 stimulated IRF3 nuclear translocation (data not shown). Herein, perturbation of Val193 and F195 did not affect the ability of γ134.5 to block the induction of antiviral genes. We conclude that γ134.5 inhibits the induction of antiviral genes mediated by TBK1 independently of PP1. In this regard, it is interesting that H9813 was unable to replicate in the eye, trigeminal ganglia, and brain. This phenotype correlated with defective viral growth, eIF2α dephosphorylation, and resistance to IFN-α/β. Thus, suppression of virus-induced antiviral gene expression alone did not restore viral virulence. HSV γ134.5 appears to function in a temporal manner (8, 26). At the early stage of infection, it functions to inhibit or alleviate the induction of antiviral genes. As virus infection proceeds, it precludes eIF2α phosphorylation triggered by the onset of viral DNA replication. We speculate that γ134.5 may promote viral virulence in vivo by coordinately modulating PP1 and TBK1. Consistent with this idea, we demonstrate that eIF2α dephosphorylation by γ134.5 is essential to display HSV virulence.
Acknowledgments
We thank Bernard Roizman and Nancy Reich for providing valuable reagents.
This work was supported in part by grants from the National Institute of Allergy and Infectious Diseases (grant no. AI081711 to B.H.) and the National Natural Science Foundation of China (grant no. 30670080 to Y.C.).
Footnotes
Published ahead of print on 16 September 2009.
REFERENCES
- 1.Bolovan, C. A., N. M. Sawtell, and R. L. Thompson. 1994. ICP34.5 mutants of herpes simplex virus type 1 strain 17syn+ are attenuated for neurovirulence in mice and for replication in confluent primary mouse embryo cell cultures. J. Virol. 68:48-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown, S. M., A. R. MacLean, J. D. Aitken, and J. Harland. 1994. ICP34.5 influences herpes simplex virus type 1 maturation and egress from infected cells in vitro. J. Gen. Virol. 75:3679-3686. [DOI] [PubMed] [Google Scholar]
- 3.Cerveny, M., S. Hessefort, K. Yang, G. Cheng, M. Gross, and B. He. 2003. Amino acid substitutions in the effector domain of the γ134.5 protein of herpes simplex virus 1 have differential effects on viral response to interferon-α. Virology 307:290-300. [DOI] [PubMed] [Google Scholar]
- 4.Cheng, G., M. Gross, M. E. Brett, and B. He. 2001. AlaArg motif in the carboxyl terminus of the γ134.5 protein of herpes simplex virus type 1 is required for the formation of a high-molecular-weight complex that dephosphorylates eIF-2α. J. Virol. 75:3666-3674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chew, T., R. Noyce, S. E. Collins, M. H. Hancock, and K. L. Mossman. 2009. Characterization of the interferon regulatory factor 3-mediated antiviral response in a cell line deficient for IFN production. Mol. Immunol. 46:393-399. [DOI] [PubMed] [Google Scholar]
- 6.Chou, J., J. J. Chen, M. Gross, and B. Roizman. 1995. Association of a M(r) 90,000 phosphoprotein with protein kinase PKR in cells exhibiting enhanced phosphorylation of translation initiation factor eIF-2α and premature shutoff of protein synthesis after infection with γ134.5− mutants of herpes simplex virus 1. Proc. Natl. Acad. Sci. USA 92:10516-10520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chou, J., E. R. Kern, R. J. Whitley, and B. Roizman. 1990. Mapping of herpes simplex virus-1 neurovirulence to γ134.5, a gene nonessential for growth in culture. Science 250:1262-1266. [DOI] [PubMed] [Google Scholar]
- 8.Chou, J., and B. Roizman. 1992. The γ134.5 gene of herpes simplex virus 1 precludes neuroblastoma cells from triggering total shutoff of protein synthesis characteristic of programed cell death in neuronal cells. Proc. Natl. Acad. Sci. USA 89:3266-3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen, P. T. 2002. Protein phosphatase 1-targeted in many directions. J. Cell Sci. 115:241-256. [DOI] [PubMed] [Google Scholar]
- 10.Diaz, M. O., S. Ziemin, M. M. Le Beau, P. Pitha, S. D. Smith, R. R. Chilcote, and J. D. Rowley. 1988. Homozygous deletion of the alpha- and beta 1-interferon genes in human leukemia and derived cell lines. Proc. Natl. Acad. Sci. USA 85:5259-5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gil, J., M. A. Garcâia, P. Gomez-Puertas, S. Guerra, J. Rullas, H. Nakano, J. Alcamí, and M. Esteban. 2004. TRAF family proteins link PKR with NF-κB activation. Mol. Cell. Biol. 24:4502-4512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goh, K. C., M. J. deVeer, and B. R. Williams. 2000. The protein kinase PKR is required for p38 MAPK activation and the innate immune response to bacterial endotoxin. EMBO J. 19:4292-4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He, B., M. Gross, and B. Roizman. 1998. The γ134.5 protein of herpes simplex virus 1 has the structural and functional attributes of a protein phosphatase 1 regulatory subunit and is present in a high molecular weight complex with the enzyme in infected cells. J. Biol. Chem. 273:20737-20743. [DOI] [PubMed] [Google Scholar]
- 14.He, B., M. Gross, and B. Roizman. 1997. The γ134.5 protein of herpes simplex virus 1 complexes with protein phosphatase 1α to dephosphorylate the α subunit of the eukaryotic translation initiation factor 2 and preclude the shutoff of protein synthesis by double-stranded RNA-activated protein kinase. Proc. Natl. Acad. Sci. USA 94:843-848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ishii, K. J., C. Coban, H. Kato, K. Takahashi, Y. Torii, F. Takeshita, H. Ludwig, G. Sutter, K. Suzuki, H. Hemmi, S. Sato, M. Yamamoto, S. Uematsu, T. Kawai, O. Takeuchi, and S. Akira. 2006. A Toll-like receptor-independent antiviral response induced by double-stranded B-form DNA. Nat. Immunol. 7:40-48. [DOI] [PubMed] [Google Scholar]
- 16.Jiang, Z., M. Zamanian-Daryoush, H. Nie, A. M. Silva, B. R. Williams, and X. Li. 2003. Poly(I-C)-induced Toll-like receptor 3 (TLR3)-mediated activation of NFkappa B and MAP kinase is through an interleukin-1 receptor-associated kinase (IRAK)-independent pathway employing the signaling components TLR3-TRAF6-TAK1-TAB2-PKR. J. Biol. Chem. 278:16713-16719. [DOI] [PubMed] [Google Scholar]
- 17.Jin, H., Y. Ma, B. S. Prabhakar, Z. Feng, T. Valyi-Nagy, Z. Yan, D. Verpooten, C. Zhang, Y. Cao, and B. He. 2009. The γ134.5 protein of herpes simplex virus 1 is required to interfere with dendritic cell maturation during productive infection. J. Virol. 83:4984-4994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leib, D. A., M. A. Machalek, B. R. Williams, R. H. Silverman, and H. W. Virgin. 2000. Specific phenotypic restoration of an attenuated virus by knockout of a host resistance gene. Proc. Natl. Acad. Sci. USA 97:6097-6101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MacLean, A. R., M. ul-Fareed, L. Robertson, J. Harland, and S. M. Brown. 1991. Herpes simplex virus type 1 deletion variants 1714 and 1716 pinpoint neurovirulence-related sequences in Glasgow strain 17+ between immediate early gene 1 and the ‘a’ sequence. J. Gen. Virol. 72:631-639. [DOI] [PubMed] [Google Scholar]
- 20.McAllister, C. S., and C. E. Samuel. 2009. The RNA-activated protein kinase enhances the induction of interferon-beta and apoptosis mediated by cytoplasmic RNA sensors. J. Biol. Chem. 284:1644-1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oganesyan, G., S. K. Saha, B. Guo, J. Q. He, A. Shahangian, B. Zarnegar, A. Perry, and G. Cheng. 2006. Critical role of TRAF3 in the Toll-like receptor-dependent and -independent antiviral response. Nature 439:208-211. [DOI] [PubMed] [Google Scholar]
- 22.Orvedahl, A., D. Alexander, Z. Talloczy, Q. Sun, Y. Wei, W. Zhang, D. Burns, D. A. Leib, and B. Levine. 2007. HSV-1 ICP34.5 confers neurovirulence by targeting the Beclin 1 autophagy protein. Cell Host Microbe 1:23-35. [DOI] [PubMed] [Google Scholar]
- 23.Rasmussen, S. B., S. B. Jensen, C. Nielsen, E. Quartin, H. Kato, Z. J. Chen, R. H. Silverman, S. Akira, and S. R. Paludan. 2009. Herpes simplex virus infection is sensed by both Toll-like receptors and retinoic acid-inducible gene-like receptors, which synergize to induce type I interferon production. J. Gen. Virol. 90:74-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rasmussen, S. B., L. N. Sorensen, L. Malmgaard, N. Ank, J. D. Baines, Z. J. Chen, and S. R. Paludan. 2007. Type I interferon production during herpes simplex virus infection is controlled by cell-type-specific viral recognition through Toll-like receptor 9, the mitochondrial antiviral signaling protein pathway, and novel recognition systems. J. Virol. 81:13315-13324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trgovcich, J., D. Johnson, and B. Roizman. 2002. Cell surface major histocompatibility complex class II proteins are regulated by the products of the γ134.5 and UL41 genes of herpes simplex virus 1. J. Virol. 76:6974-6986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verpooten, D., Y. Ma, S. Hou, Z. Yan, and B. He. 2009. Control of TANK-binding kinase 1-mediated signaling by the γ134.5 protein of herpes simplex virus 1. J. Biol. Chem. 284:1097-1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Whitley, R. J., E. R. Kern, S. Chatterjee, J. Chou, and B. Roizman. 1993. Replication, establishment of latency, and induced reactivation of herpes simplex virus γ134.5 deletion mutants in rodent models. J. Clin. Investig. 91:2837-2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whitley, R. J., and B. Roizman. 2001. Herpes simplex virus infections. Lancet 357:1513-1518. [DOI] [PubMed] [Google Scholar]
- 29.Zhang, C., J. Tang, J. Xie, H. Zhang, Y. Li, J. Zhang, D. Verpooten, B. He, and Y. Cao. 2008. A conserved domain of herpes simplex virus ICP34.5 regulates protein phosphatase complex in mammalian cells. FEBS Lett. 582:171-176. [DOI] [PubMed] [Google Scholar]
- 30.Zhang, P., and C. E. Samuel. 2008. Induction of protein kinase PKR-dependent activation of interferon regulatory factor 3 by vaccinia virus occurs through adapter IPS-1 signaling. J. Biol. Chem. 283:34580-34587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang, S. Y., E. Jouanguy, S. Ugolini, A. Smahi, G. Elain, P. Romero, D. Segal, V. Sancho-Shimizu, L. Lorenzo, A. Puel, C. Picard, A. Chapgier, S. Plancoulaine, M. Titeux, C. Cognet, H. von Bernuth, C. L. Ku, A. Casrouge, X. X. Zhang, L. Barreiro, J. Leonard, C. Hamilton, P. Lebon, B. Heron, L. Vallee, L. Quintana-Murci, A. Hovnanian, F. Rozenberg, E. Vivier, F. Geissmann, M. Tardieu, L. Abel, and J. L. Casanova. 2007. TLR3 deficiency in patients with herpes simplex encephalitis. Science 317:1522-1527. [DOI] [PubMed] [Google Scholar]